Small molecule inhibitor for preventing Alzheimer's disease Abeta polypeptide from fiberizing and its preparation method, pharmaceutical composition and application
A compound and pharmaceutical technology, applied in the field of medicinal chemistry, can solve the problems of protease degradation, impermeability of the blood-brain barrier, toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
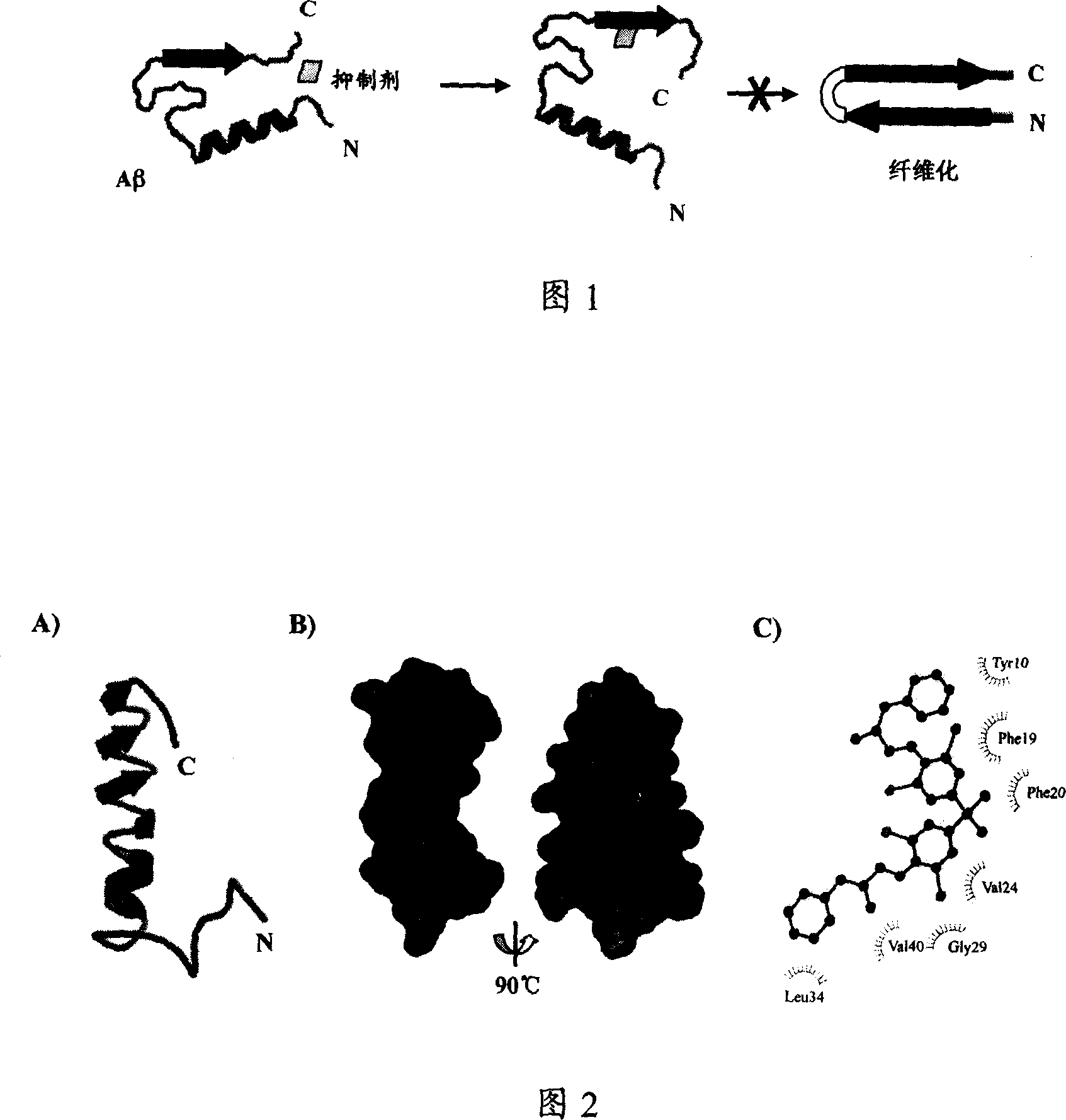
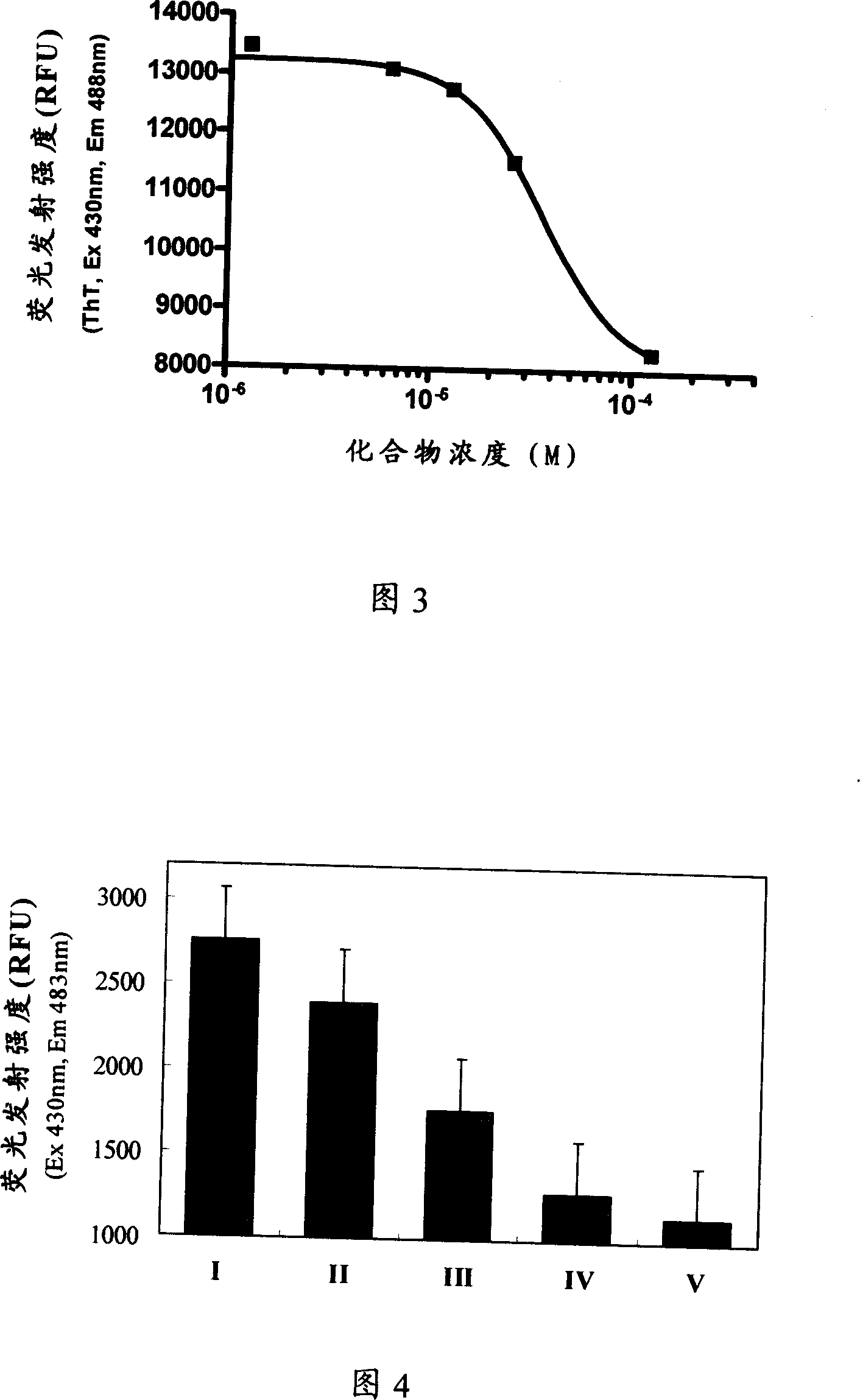
Image
Examples
Embodiment 1
[0097] Preparation of 2,2-bis[3,5-dibromo-4-(2-hydroxy)propoxyphenyl]propane
[0098]
[0099] 1.1.1 Preparation of 2,2-bis(4-hydroxyphenyl)propane (method 1)
[0100] Put 1.16 g (0.02 mol) of acetone and 5.64 g. (0.06 mol) of phenol in a 100 mL flask, stir, add 12 mL of concentrated hydrochloric acid, and pass through dry hydrogen chloride gas, stop the reaction after one week of reaction. Add water, crush the solid reactant, distill off excess phenol, and filter with suction to obtain an almost white solid, dry it, and then recrystallize it with 20 mL of ethanol to give 4.1 g of a white needle-like product (yield 90%), melting point 151-153 ℃ (reported in literature 151 ℃).
[0101] 1.1.2 Preparation of 2,2-bis(4-hydroxyphenyl)propane (method 2)
[0102] Put 0.02 mole of phenol and 0.02 mole of 2,2-diethylthiopropane in a 100mL flask, stir, feed hydrogen chloride gas, and continue the reaction at room temperature for 6.5 hours; then, stop the reaction, distill, and dry ...
Embodiment 2
[0109] Preparation of 2,2-bis[3,5-dibromo-4-furyl-2-formyloxyphenyl]propane
[0110]
[0111] The 1-chloro-2-propanol in Step 1.3 of Example 1 was replaced with furan-2-formyl chloride, and the rest of the required raw materials, reagents and preparation methods were the same as in Example 1 to obtain the product 2,2-bis[3,5 -Dibromo-4-furyl-2-formyloxyphenyl]propane.
[0112] 1 HNMR (CDCl 3 )ppm: δ1.68(s, 6H, CH 3 ), δ6.63(m, 2H, ArH), δ7.42(s, 4H, ArH), δ7.49(m, 2H, ArH), δ7.73(m, 2H, ArH).EI-MS m / s 732[M+].
Embodiment 3
[0114] Preparation of 2,2-bis[3,5-dibromo-4-(5-bromo-furyl)-2-formyloxyphenyl]propane
[0115]
[0116] The 1-chloro-2-propanol in Step 1.3 of Example 1 is replaced by 5-bromo-furan-2-formyl chloride, and the rest of the required raw materials, reagents and preparation methods are the same as in Example 1 to obtain the product 2,2-di [3,5-Dibromo-4-(5-bromo-furyl)-2-formyloxyphenyl]propane.
[0117] 1 HNMR (CDCl 3 )ppm: δ1.71(s, 6H, CH 3 ), δ6.55(m, 2H, ArH), δ7.12(s, 3H, ArH), δ7.31(m, 3H, ArH).EI-MS m / s 889[M+].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com