Humanization modified rat ING4 gene and adenovirus expression vectors thereof
A humanized, adenovirus technology, applied in the fields of biotechnology and medicine, can solve problems such as reports about the application of adenovirus vectors in anti-tumor gene therapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Example 1 Point mutation method of humanized transformation mouse ING4 gene
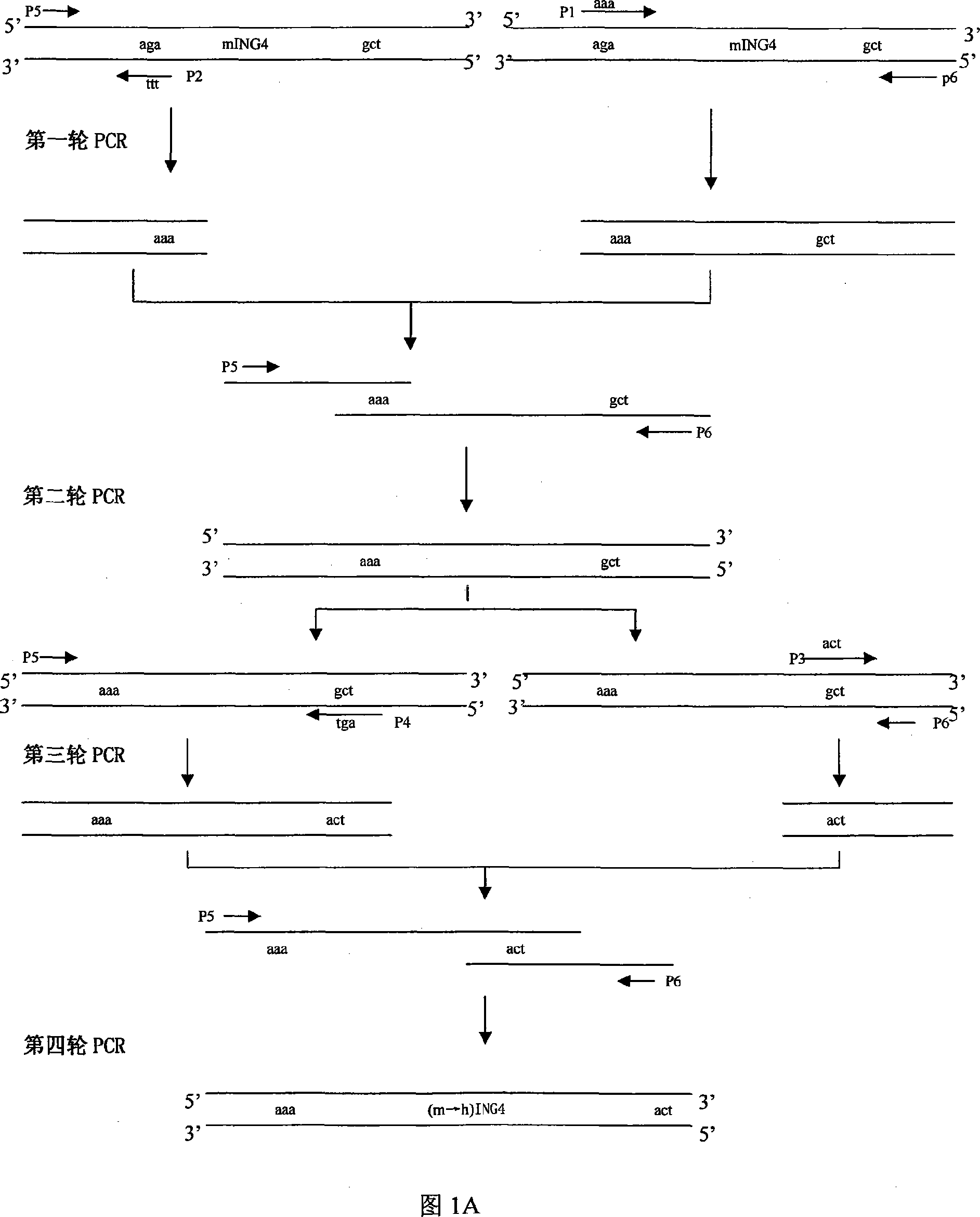
[0122] According to the amino acid sequence of human ING4, the mouse ING4 gene was humanized by point mutation technology (Fig. 1A). Design two pairs of mutant primers P1, P2, P3, P4 and full-length ING4 upstream and downstream primers P5, P6, which change the 66th amino acid coding sequence aga into aaa, and the 156th amino acid coding sequence gct into act in mouse ING4; use pcDNA3.0 -mING-4 plasmid as a template, the first round of PCR was performed with P1 and P6, P2 and P5 respectively, and mixed after gel recovery, then the mixed PCR product was used as a template, and the second PCR was performed with primers P5 and P6, after gel recovery Then use this PCR product as a template, carry out the third round of PCR with primers P3 and P6, P4 and P5 respectively, mix after gel recovery, and then use this mixed PCR product as a template to carry out the fourth round of PCR with primers P5 and...
Embodiment 2
[0124] Example 2 Construction of recombinant adenovirus vector
[0125] (1) Construction of pAdTrack-CMV-ING4 (with GFP marker)
[0126] After the purified fourth-round PCR product and the pAdTrack-CMV plasmid with the GFP marker gene were digested by SalI and HindIII, the target fragment was recovered by the gel extraction kit, and then ligated by T4 DNA ligase overnight, and the calcium chloride method was used. The DH5α competent cells were transformed, and the positive clones of the pAdTrack-CMV-ING4 transfer plasmid were screened and identified by sequencing. The correctly sequenced pAdTrack-CMV-ING4 plasmid and the pAdTrack-CMV empty plasmid were linearized with PmeI single enzyme digestion, gel recovery and pAdEasy-1 adenovirus vector calcium chloride method were used to co-transform BJ5183 competent, and positive clones were selected Plasmids were extracted, and after identification by enzyme digestion, a large number of transformants were amplified.
[0127] Plasmid...
Embodiment 3
[0131] Example 3 Obtaining and Identification of Recombinant Viruses Ad-ING4-GFP and Ad-ING4-ΔGFP
[0132] The pAd-ING4, pAd-ING4-ΔGFP and pAd homologous recombinant adenoviral plasmids constructed above were linearized with Pac I, and then 70% of adherent QBI-293A cells were transfected according to the operation instructions of Lipofectamine Reagent. Observe the fluorescence under a fluorescent microscope for 3-5 days, collect the cell suspension on 7-10 days, centrifuge at 2000r / min for 5min, suspend the cell pellet with sterile PBS, freeze and thaw the cell suspension three times, centrifuge at 2000r / min for 5min, and take In the supernatant, high-titer Ad-ING4-GFP, Ad-ING4-ΔGFP and Ad-GFP adenoviruses obtained after multiple rounds of infection and amplification were stored at -80°C. The light microscope morphology and fluorescence observation results of QBI-293A cells infected with ING4 recombinant adenovirus are shown in Figures 5A-5D.
[0133] Trypsinize the QBI-293A ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com