Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
83 results about "Therapeutic evaluation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Breast cancer detecting marker as well as detecting method, kit and biological chip thereof
InactiveCN101988061AEasy to storeWide detection rangeMicrobiological testing/measurementDNA/RNA fragmentationHuman bodyEarly breast cancer
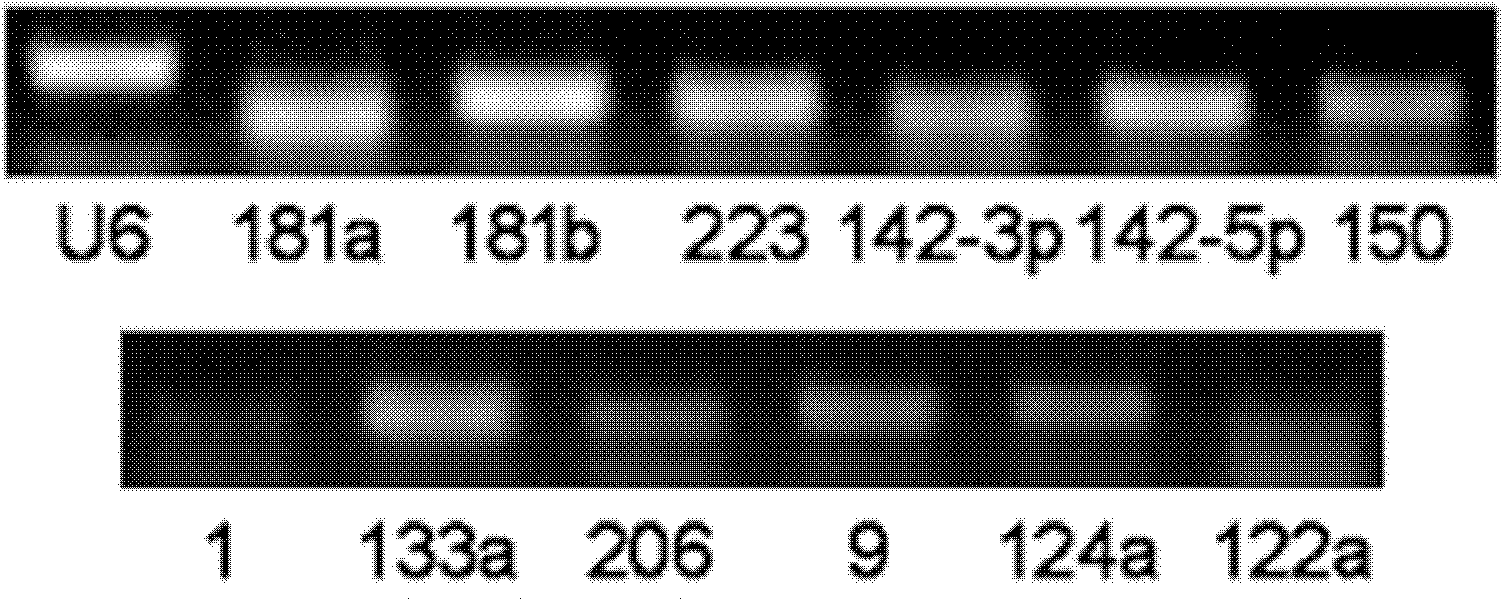
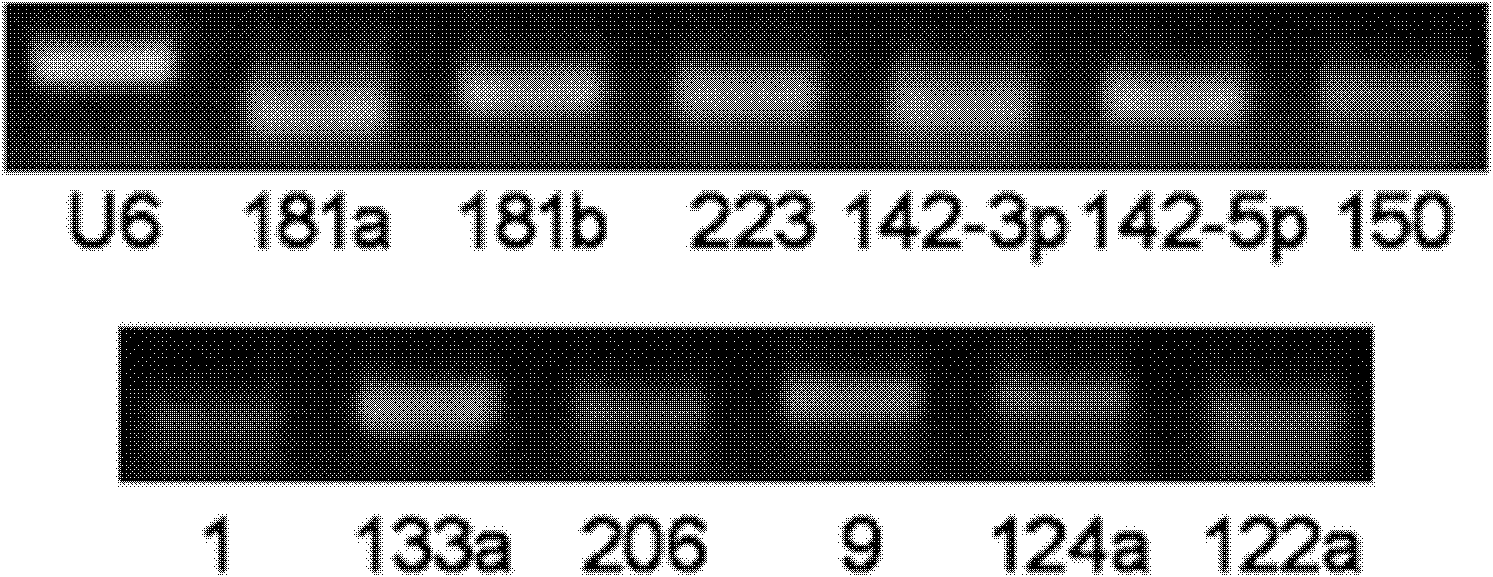
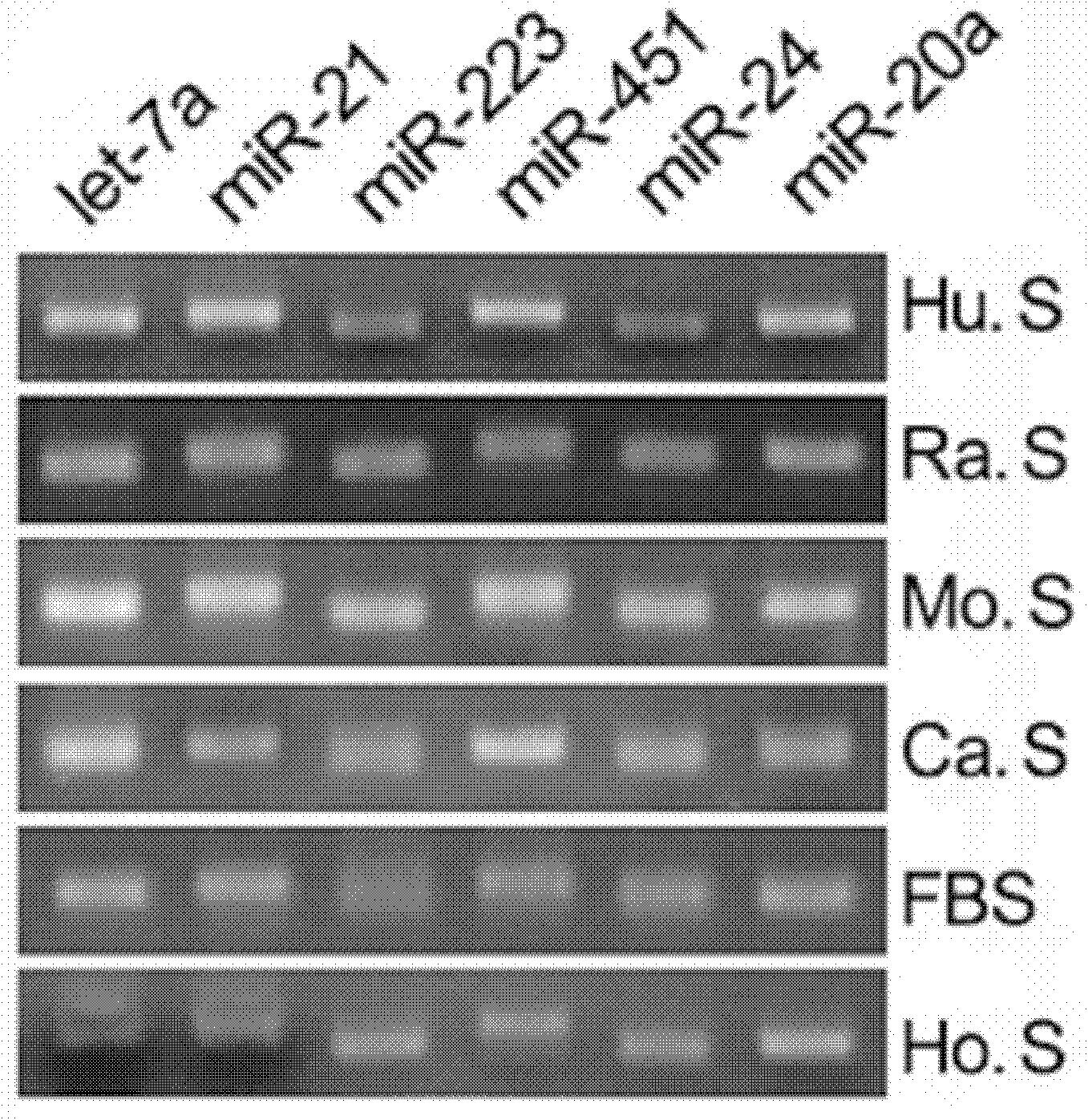
The invention relates to a breast cancer detecting marker by utilizing 16 specific tiny ribonucleic acids steadily existing in blood serum and blood plasma of a human body as well as a detecting method, a kit and a biological chip thereof, and the detecting marker can be used in the aspects of diagnosis and differential diagnosis of breast cancer, occurrence and recurrence forecast as well as efficacy assessment of disease complications, screening and therapeutic evaluation of medicinal active ingredients and the like, and has the advantages of wide detection pedigree, high sensitivity and low detection cost, the materials are conveniently taken, the samples are easy to store and the like. The method can be widely used in related work such as breast cancer census, the defects of low specificity and low sensitivity caused by insuperable individual difference of a single marker can be improved and the clinical detectable rate of the breast cancer is notably improved, and is an effective mean of early breast cancer diagnosis.
Owner:JIANGSU MICROMEDMARK BIOTECH
Wearable intelligent monitoring system for cardiovascular and cerebrovascular diseases and method
PendingCN107960990ATake advantage ofEasy to useEvaluation of blood vesselsCatheterTherapeutic evaluationDisease
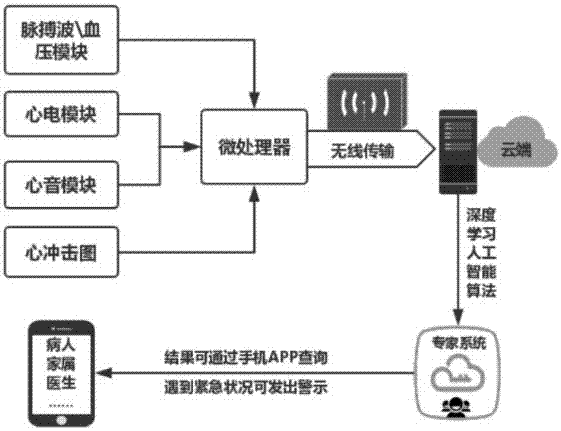
The invention discloses a wearable intelligent monitoring system for cardiovascular and cerebrovascular diseases and a method. The system comprises a signal detection module, a signal transmission module, a cloud intelligent expert system and a client which are successively connected, wherein the signal detection module is used for collecting and processing pulse wave, blood pressure wave, electrocardiogram, phonocardiogram and ballistocardiogram; the signal transmission module is used for wirelessly transmitting a monitoring signal, which is collected and processed by the signal detection module, to the cloud intelligent expert system; the cloud intelligent expert system is a cloud server system based on a CNN depth network and is used for quantitative forecast of the cardiovascular and cerebrovascular diseases; the client is used for displaying the forecast results of the cardiovascular and cerebrovascular diseases. By continuous data collection and big data integration application,the system can be used for giving the loop parameter of the local area of a body and the quantitative evaluation of the cardiovascular and cerebrovascular diseases, and has important significance in early warning, therapeutic evaluation, prevention and cure drug selection and the like for a patient suffering from the cardiovascular and cerebrovascular diseases.
Owner:SHANGHAI UNIV OF MEDICINE & HEALTH SCI
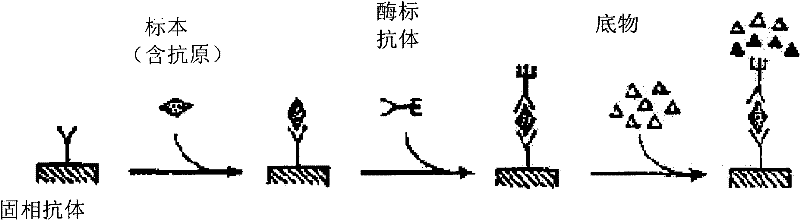
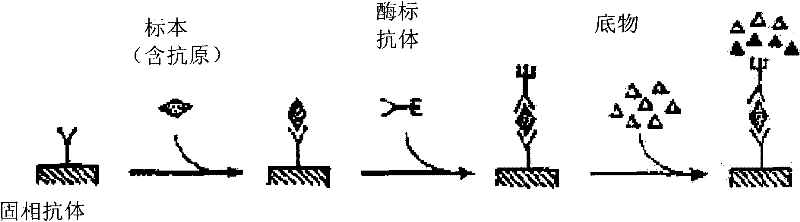
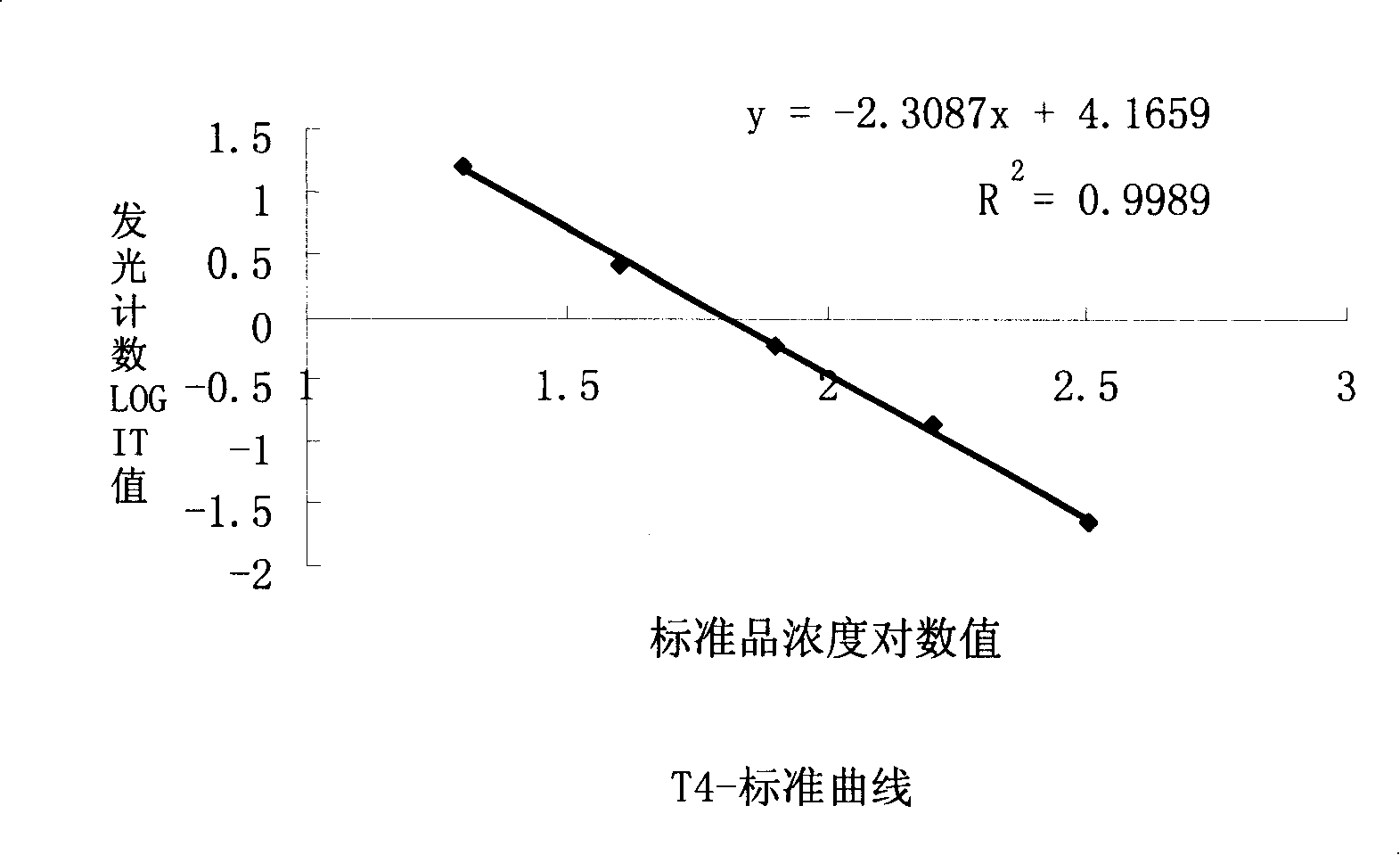
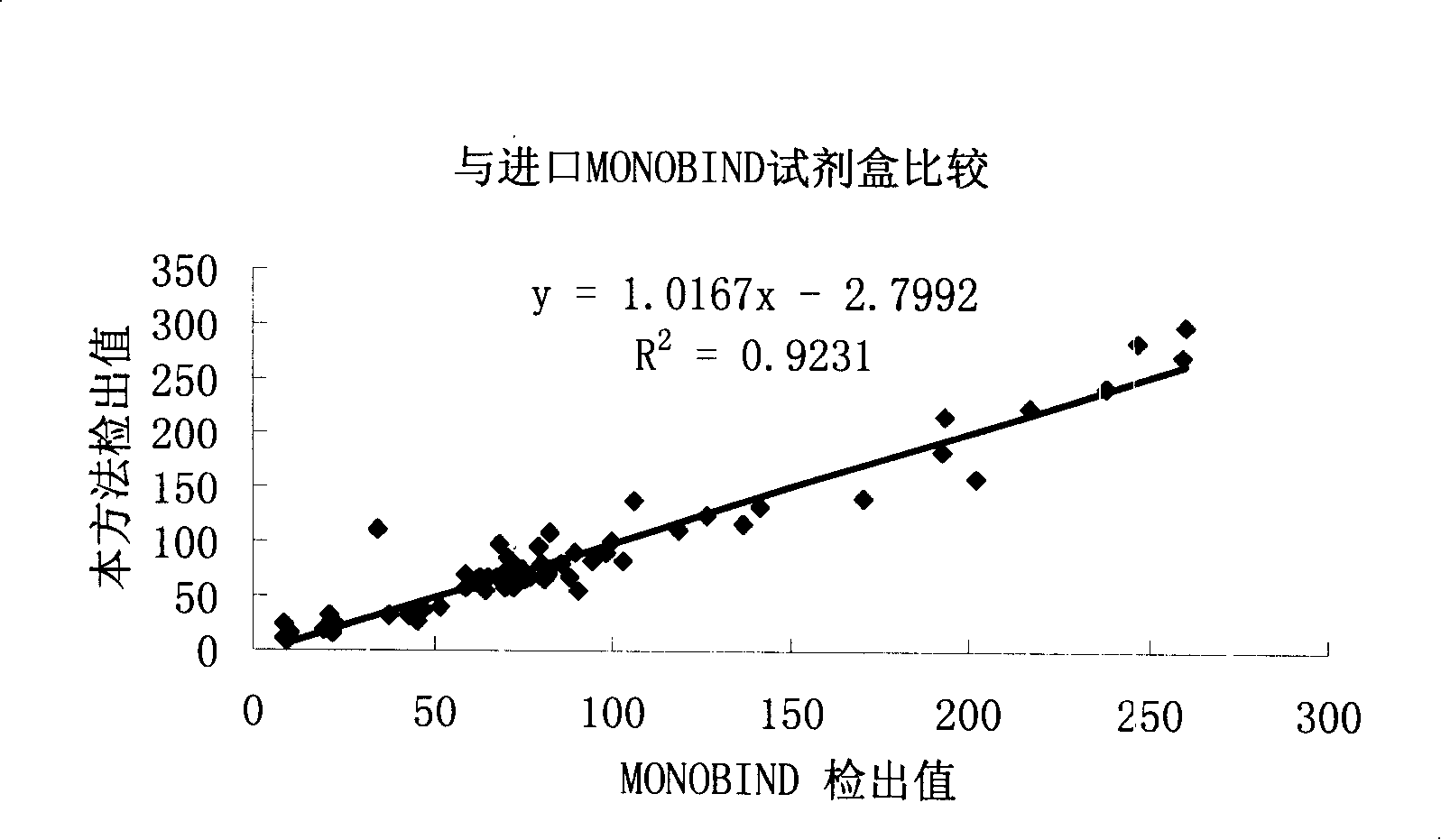
Thyroxine chemiluminescence immune analysis quantitative measuring reagent kit and method for preparing the same
ActiveCN101201354ALow feesEasy to operateChemiluminescene/bioluminescenceSevere hypothyroidismChemiluminescence
The invention belongs to the technical field of immunodiagnosis, and discloses a thyroxine (T4) enzymatic chemiluminescence quantitative determination kit. The kit disclosed by the invention consists of a T4 standard product, an antibody pre-coated reaction plate, a horseradish peroxidase marker, diluent, cleaning mixture, and chemiluminescence substrate liquid. The kit disclosed by the invention has the advantages of quickness, handiness, sensitiveness, cheapness and high repeatability, and is an important index for diagnosis and therapeutic evaluation of hyperthyroidism and hypothyroidism. The invention has high clinic use value. The invention also provides a method to prepare the kit.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
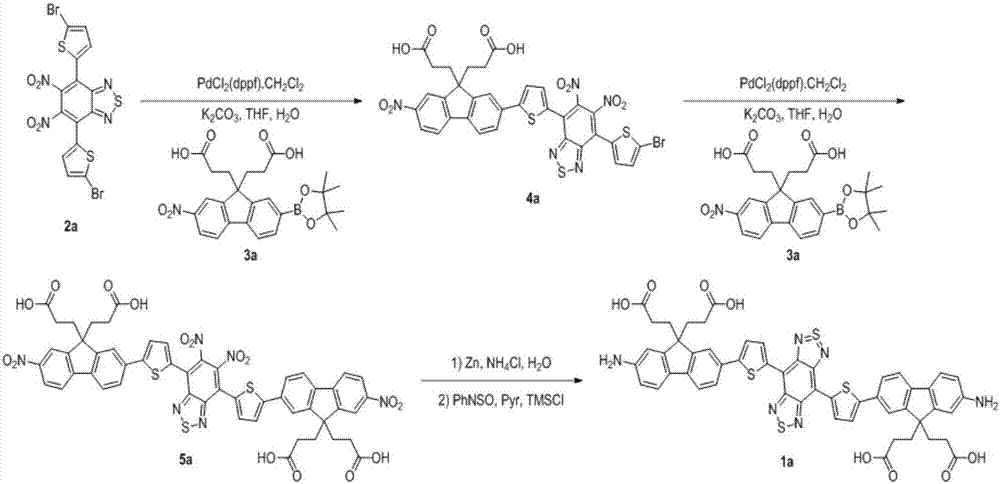
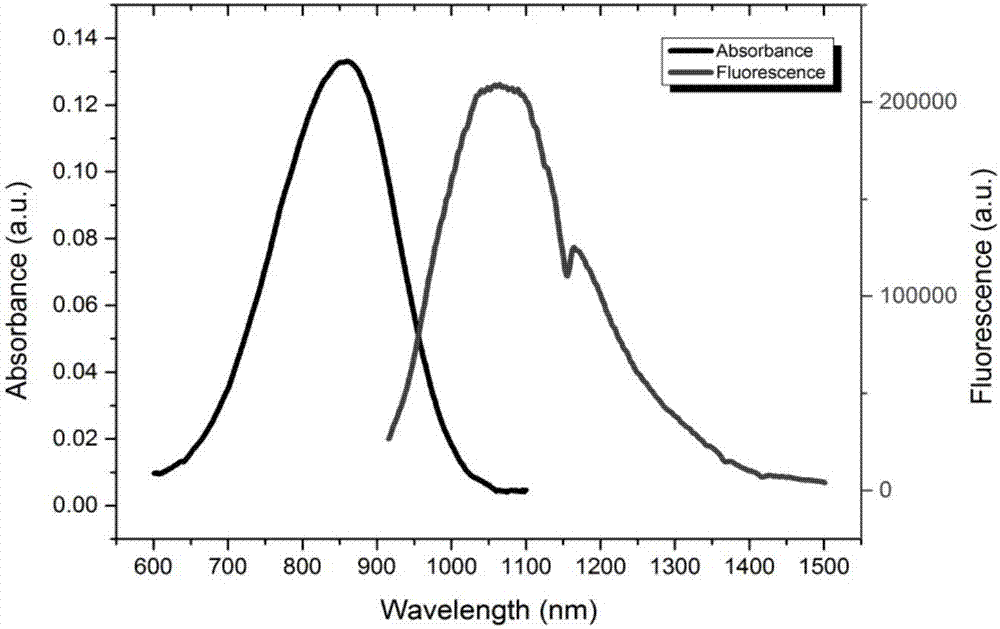
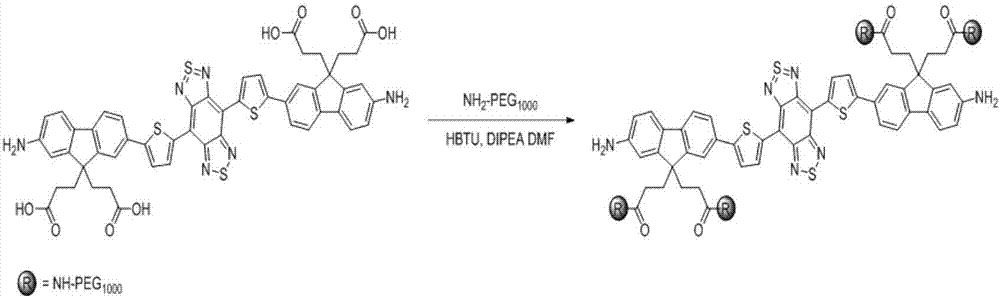
Modifiable second near-infrared window fluorescent imaging probe and preparation method and application thereof
ActiveCN106977529AHigh quantum yieldGood water solubilityOrganic chemistryIn-vivo testing preparationsSolubilityWhole body
The invention discloses a near-infrared window fluorescent imaging agent containing diazosulfide and a fluorene ring and a preparation method thereof. A modifiable group is introduced into the fluorene ring of the fluorescent compound, increased modifiable sites can be used for connection of different bioactive substances, water solubility and biological compatibility can be improved, and the application range in the biomedical field can be expanded. The fluorescent imaging agent has the advantages of high fluorescence intensity, no toxicity, good biocompatibility, and the like, and has excellent application prospects. The invention also discloses the application of the fluorescent imaging agent in the field of brain glioma, systemic angiography and sentinel lymph node dissection. In addition, the imaging agent has good modificability, and also can be used for in-vitro detection of various disease markers, in-vivo diagnosis and surgical navigation treatment of breast cancer, prostate cancer and colon cancer and other cancer, evaluation of curative effect after tumorectomy, and the like.
Owner:武汉振豪生物科技有限公司
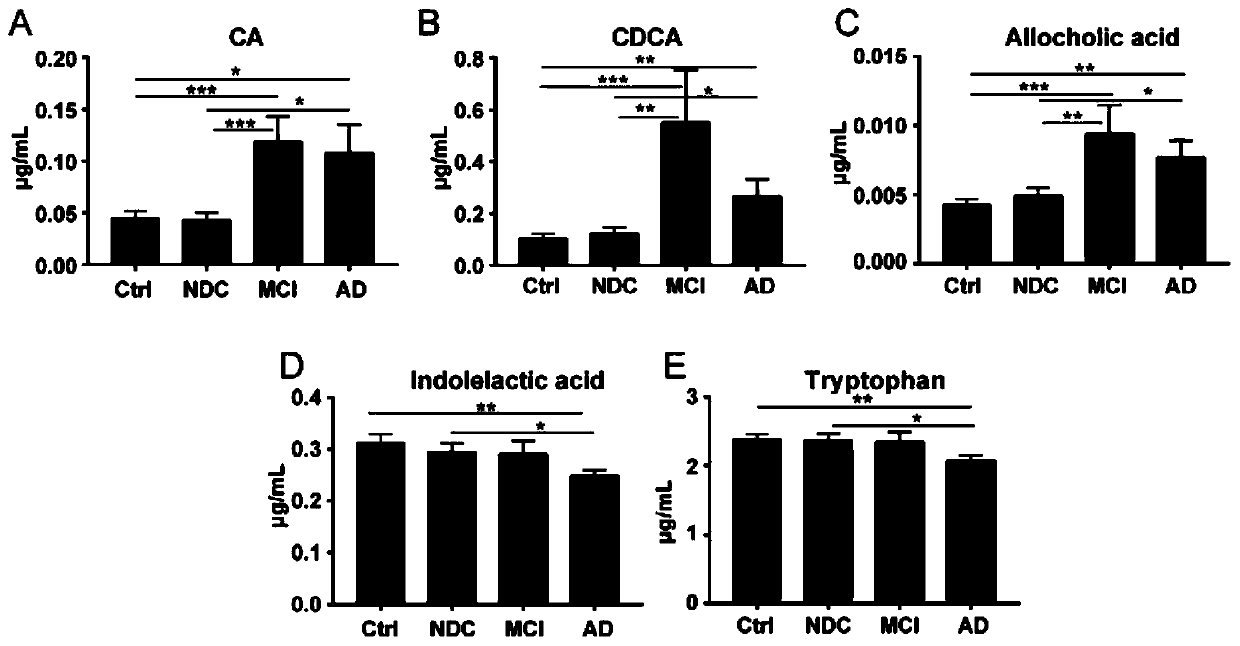
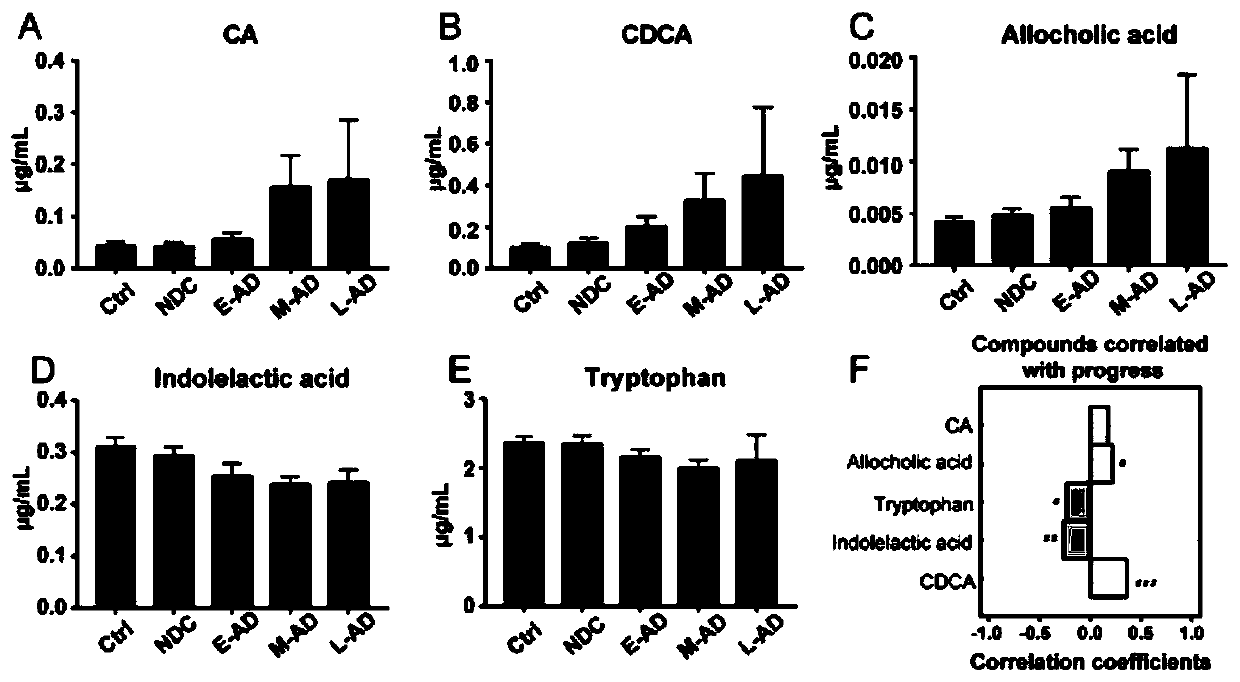
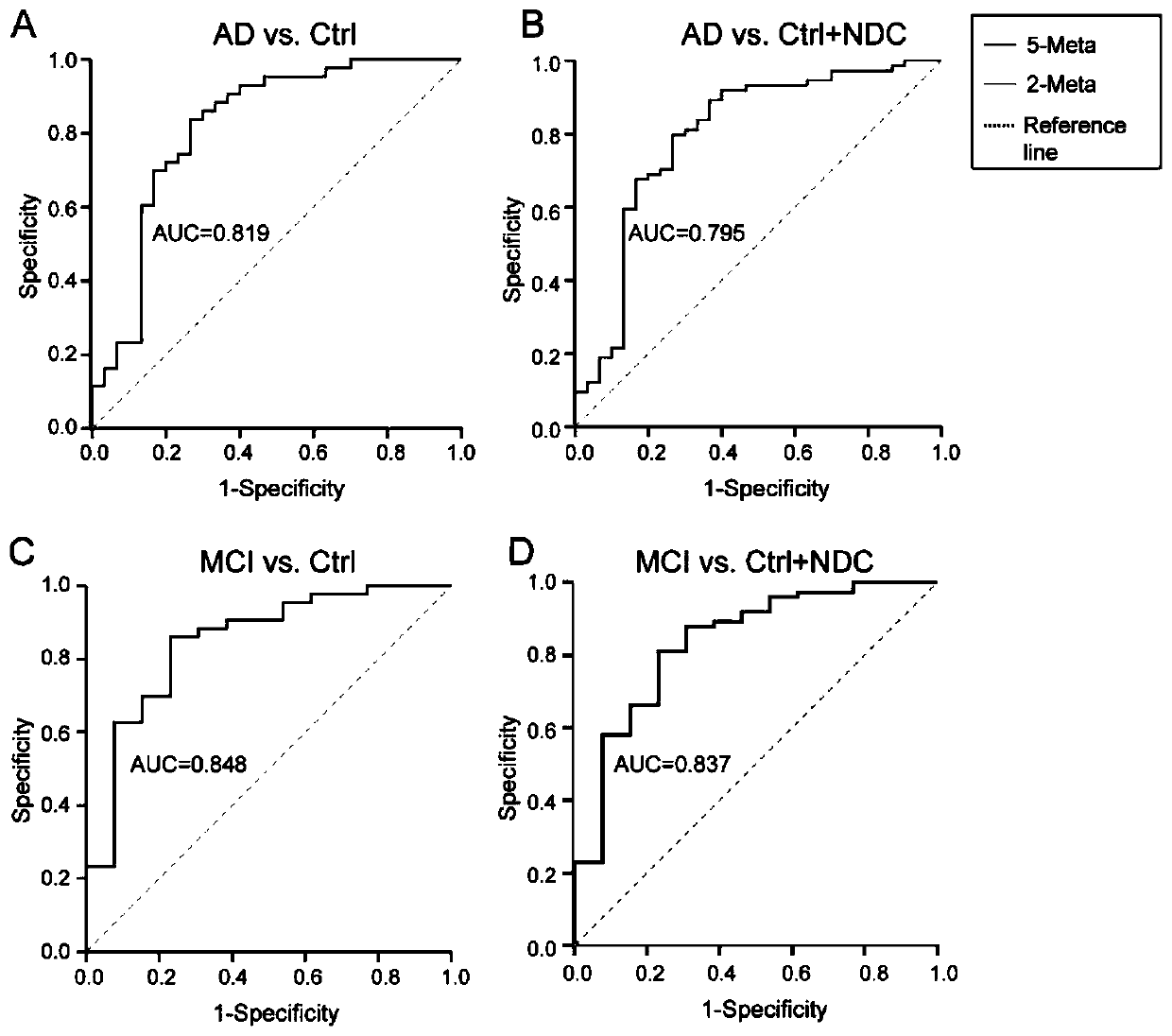
A set of biomarkers for diagnosing AD in a subject or determining a risk of the AD in the subject and an application thereof
The invention relates to a set of biomarkers for diagnosing AD in a subject or determining a risk of the AD in the subject. Diagnostic markers are a cholic acid, a chenodeoxycholic acid, an allocholicacid, a benzpyrole-3-lactic acid and tryptophan. The invention also provides the application of the above diagnostic markers in preparation of a differential diagnostic reagent for an Alzheimer's disease and a kit. Plasma fingerprint spectrum analysis of AD patients, MCI patients, Ctrl and NDC populations is performed through a plasma sample collection-plasma sample pretreatment-ultra-high performance liquid chromatography and mass spectrometry analysis method, and contents of the above five diagnostic markers are detected so that the markers are applied to preparation of products related todiagnosis of the Alzheimer's disease, and therapeutic evaluation. The markers can evaluate an early phase of the Alzheimer's disease, accuracy is high, a detection speed is fast, cost is low, traumasare small, and patients can easily accept. A scientific and effective treatment plan is provided for the Alzheimer's disease and a good application prospect is possessed.
Owner:FIRST AFFILIATED HOSPITAL OF DALIAN MEDICAL UNIV
Detection system for detecting human immune state
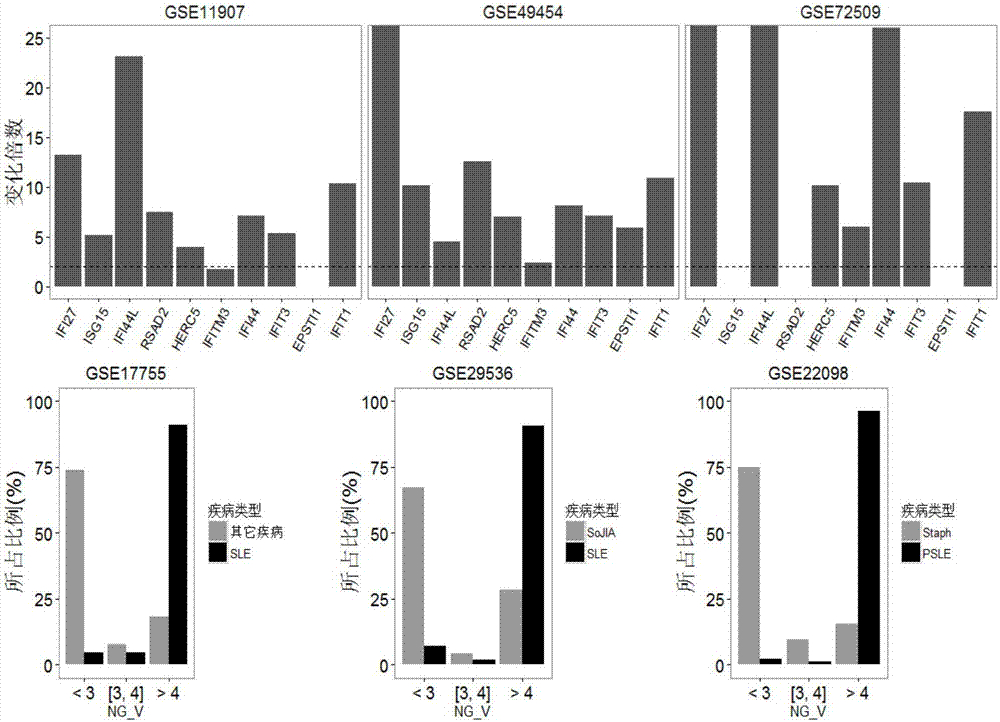
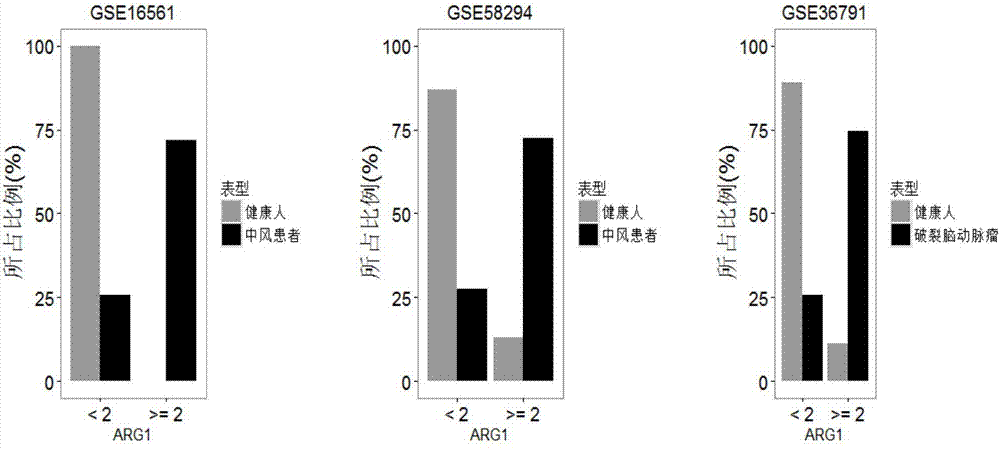
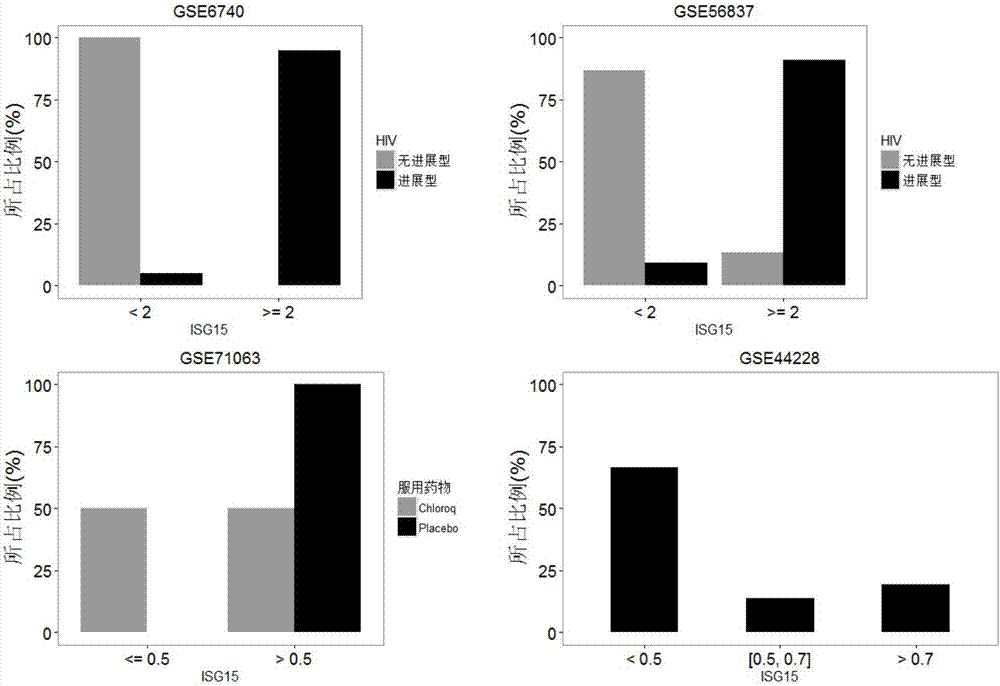
The invention relates to a detection system for detecting a human immune state and belongs to the technical field of gene detection. The detection system provided by the invention is used for detecting 20 genes, including IFI27, ISG15, IFI44L, RSAD2, HERC5, IFITM3, IFI44, IFIT3, EPSTI1, IFIT1, HP, ANXA3, ARG1, FCGR1B, S100A12, MMP9, IL18R1, TLR3, GYG1 AND FCGR1A in peripheral blood; the assessment for the human immune state can be realized in the manner of increasing or reducing the expression level of each gene; and the genes are utilized to guide accurate medical treatment, including disease accurate parting, prognosis, treatment tracking and therapeutic evaluation. The detection system has excellent clinic application values and wide application prospects.
Owner:BEIJING INST OF GENOMICS CHINESE ACAD OF SCI CHINA NAT CENT FOR BIOINFORMATION
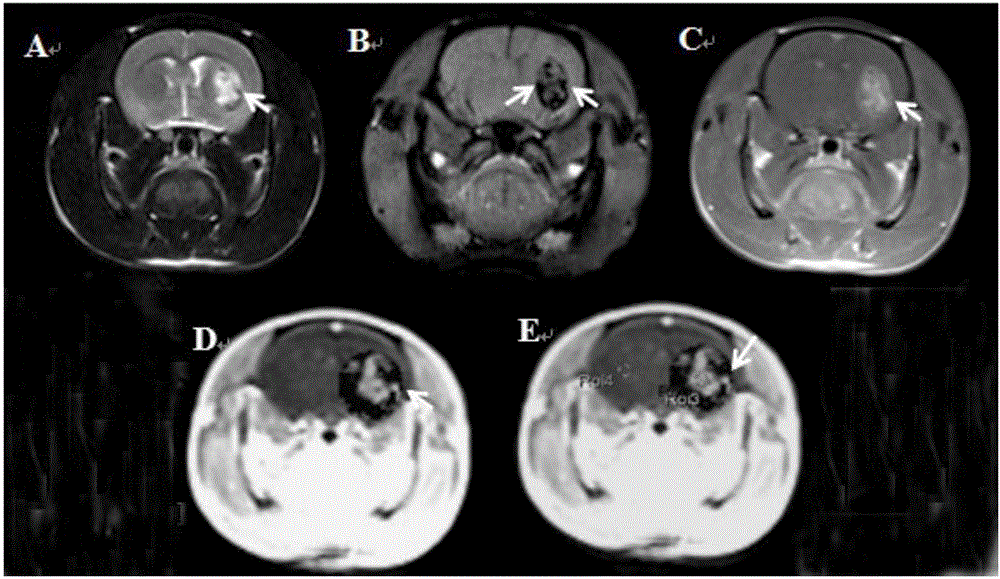
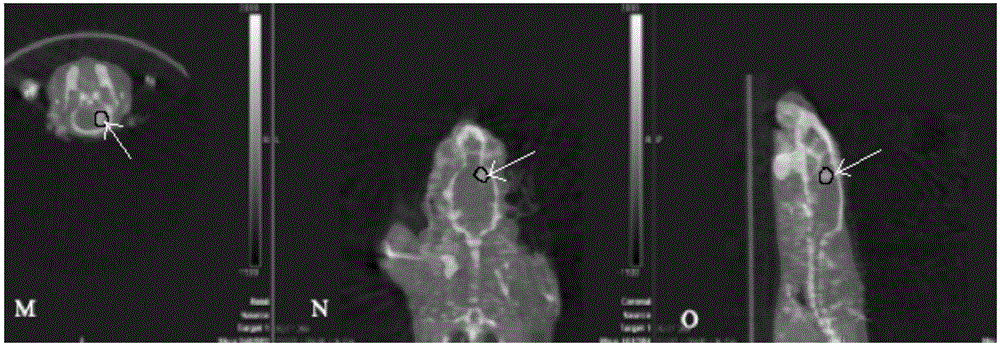
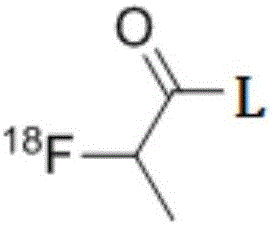
[<18>F] AlF marked positron emission tomography (PET) polypeptide probe and preparation method thereof
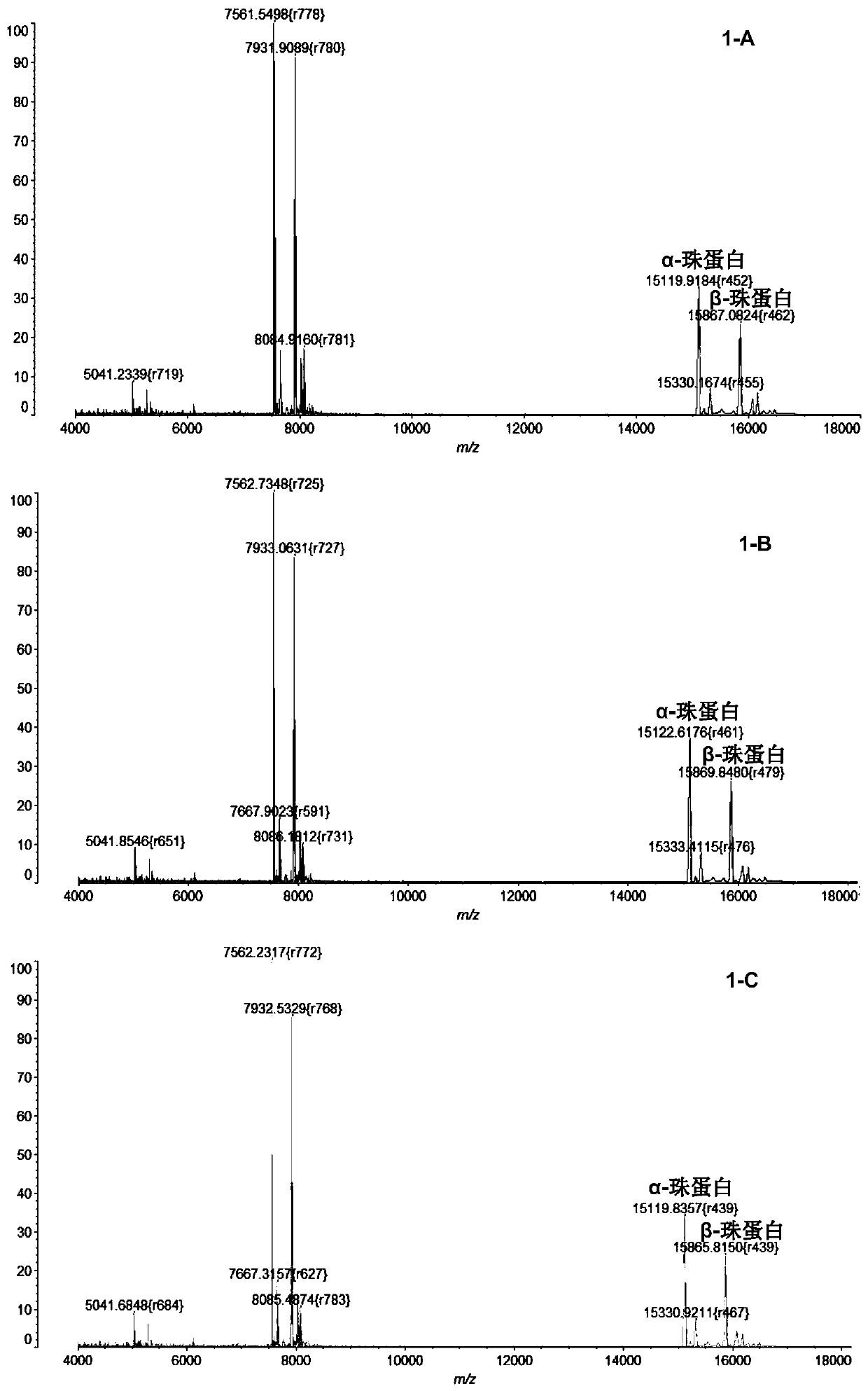
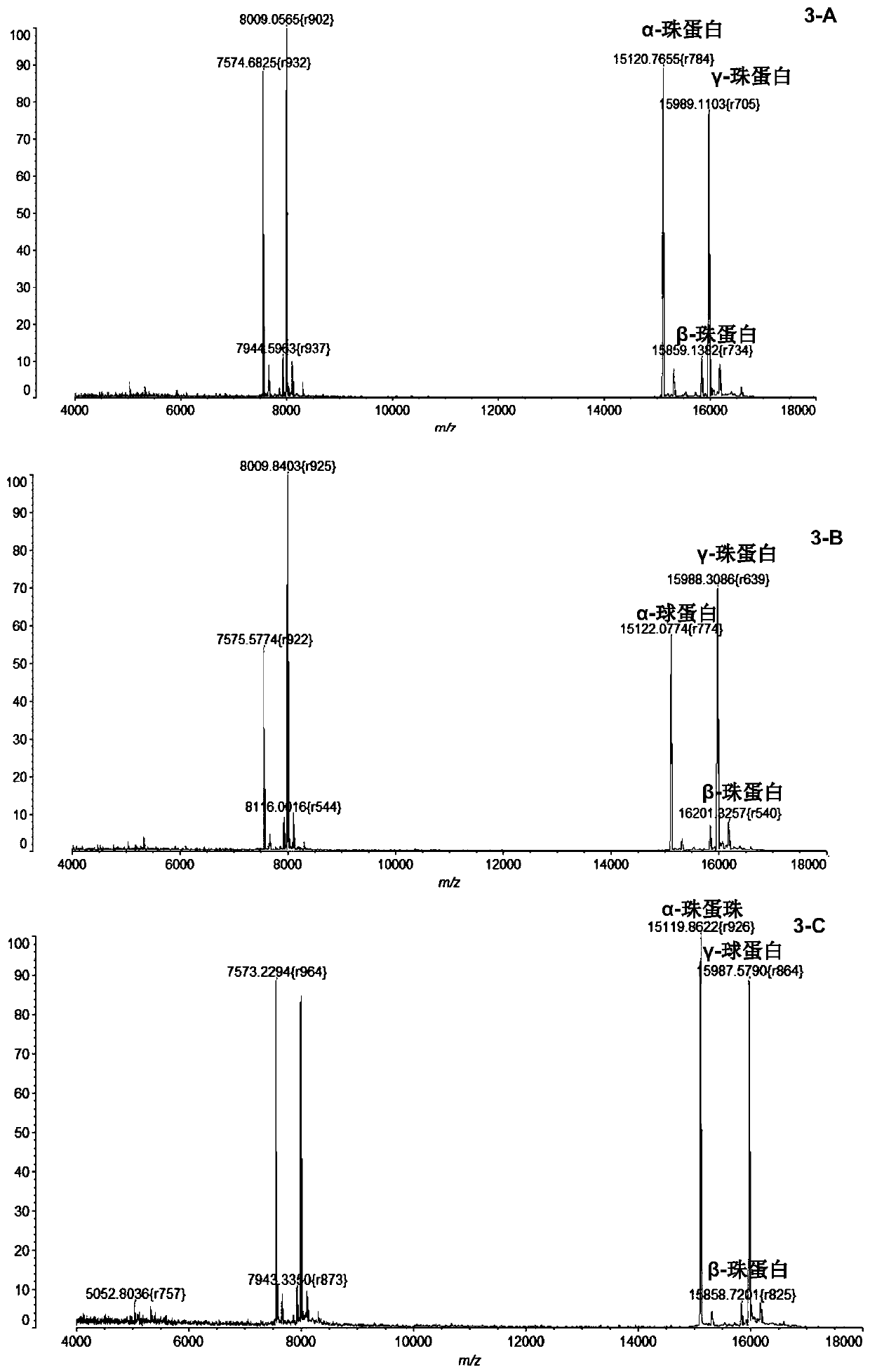
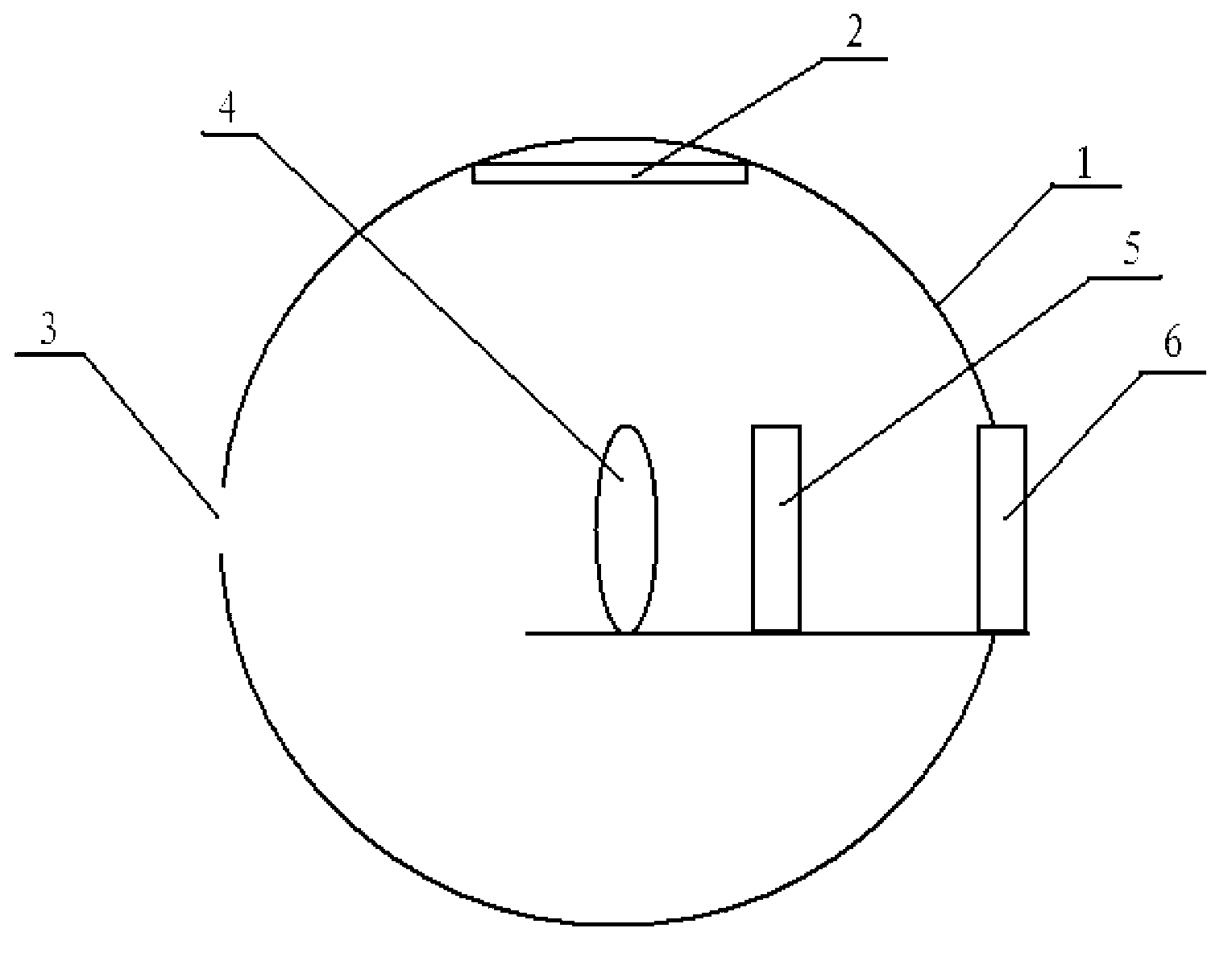
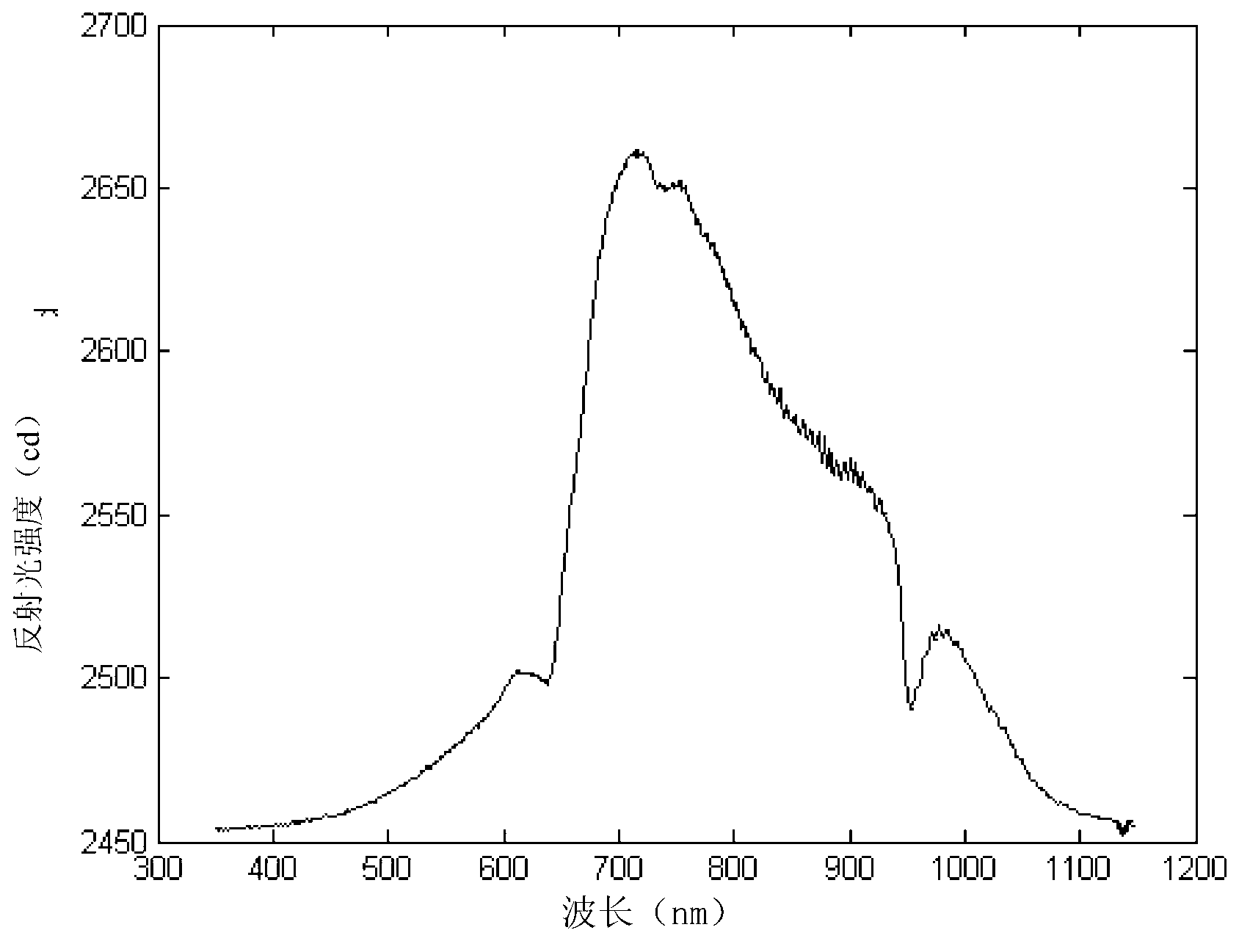
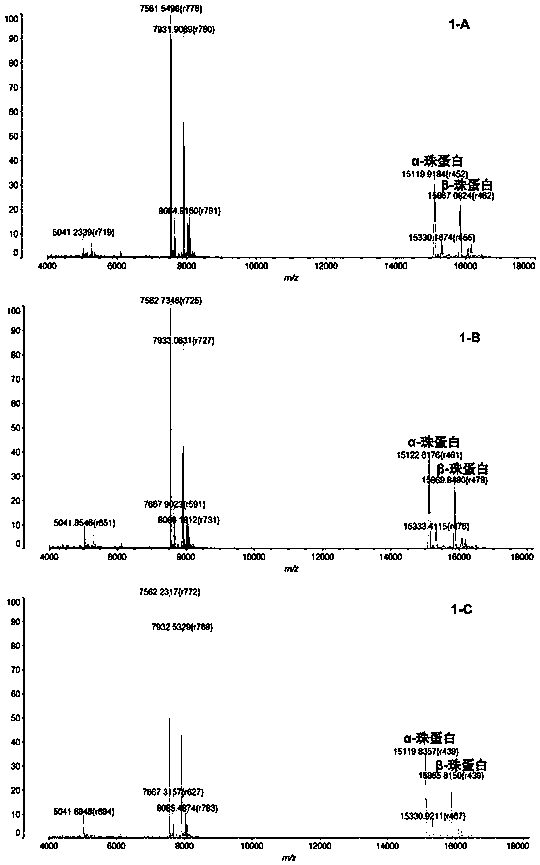
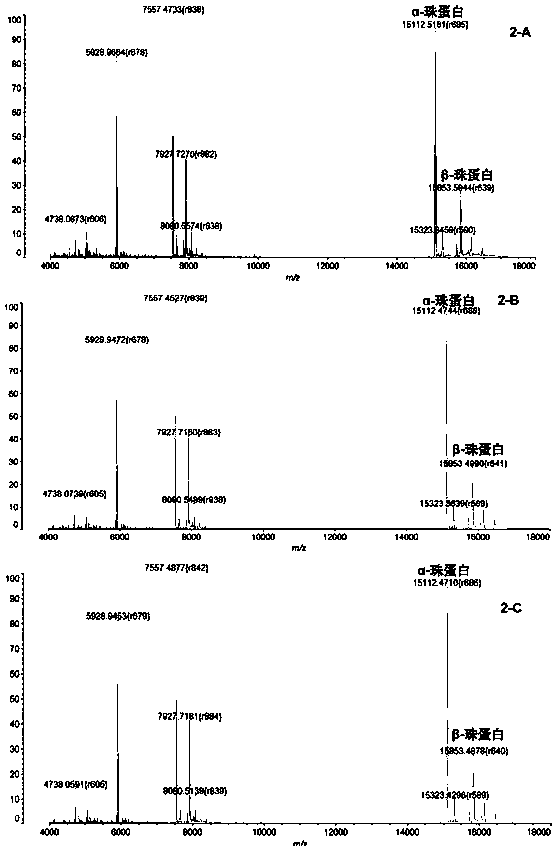
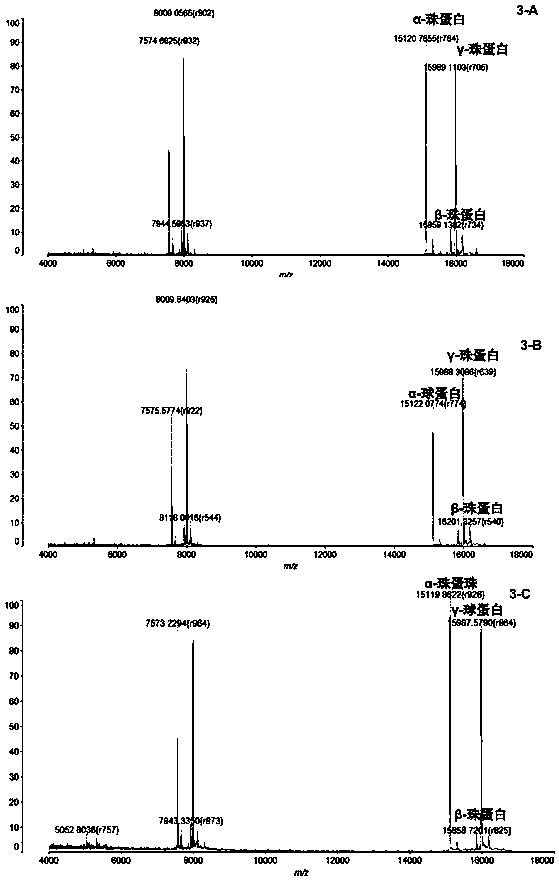
InactiveCN104725473ADoes not affect biological activityDoes not affect pharmacokinetic propertiesPeptide preparation methodsIn vivoStructural formula
The invention discloses a [<18>F] AlF marked positron emission tomography (PET) polypeptide probe and a preparation method thereof. The probe comprises TMTP1 polypeptide and NOTA, which are connected together, and the structural formulae of the TMTP1 polypeptide and the NOTA are shown in the description. According to the [<18>F] AlF marked positron emission tomography (PET) polypeptide probe and the preparation method thereof, a glycine is added to the TMTP1, and a G-TMTP1 polypeptide is designed, so that the polypeptide does not influence the biological activity after being radioactively marked; furthermore, 18F is taken as a radionuclide, and the G-TMTP1 polypeptide is marked by utilizing a [<18>F] AlF-NOTA method, so that the obtained [<18>F] AlF-marked PET polypeptide probe has excellent pharmacokinetics properties, the background cleaning rate is high, the liver uptake rate is low, in-vivo stability is good, the uptake rate of tumor site is high, highly metastatic tumor can be diagnosed specifically, and the polypeptide probe can be applied to diagnosis or therapeutic evaluation of highly metastatic malignant tumor.
Owner:THE FIRST AFFILIATED HOSPITAL OF XIAMEN UNIV +1
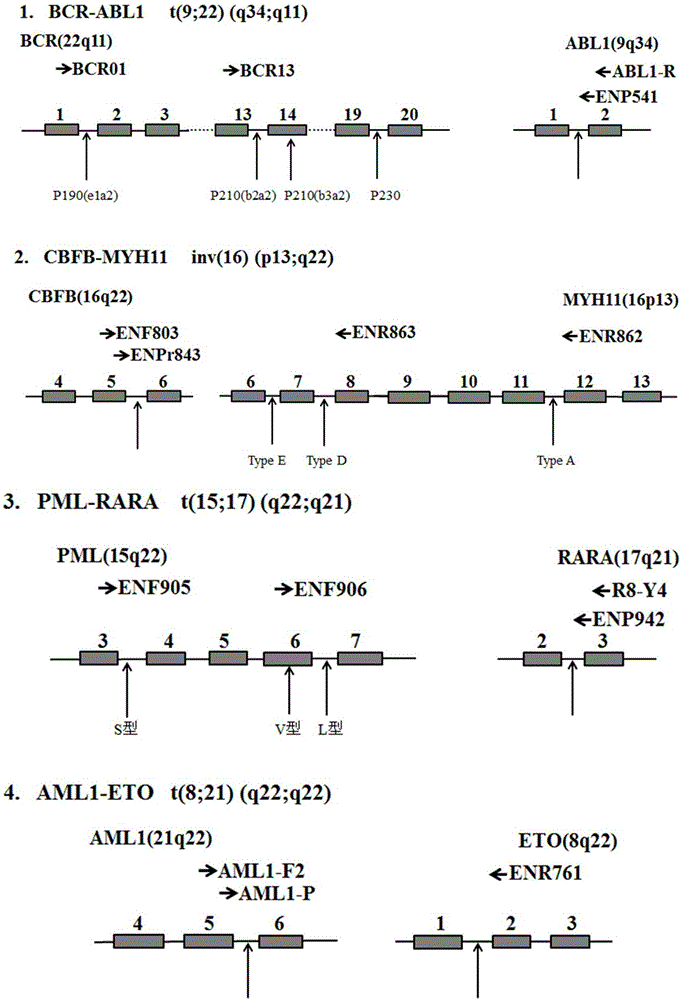
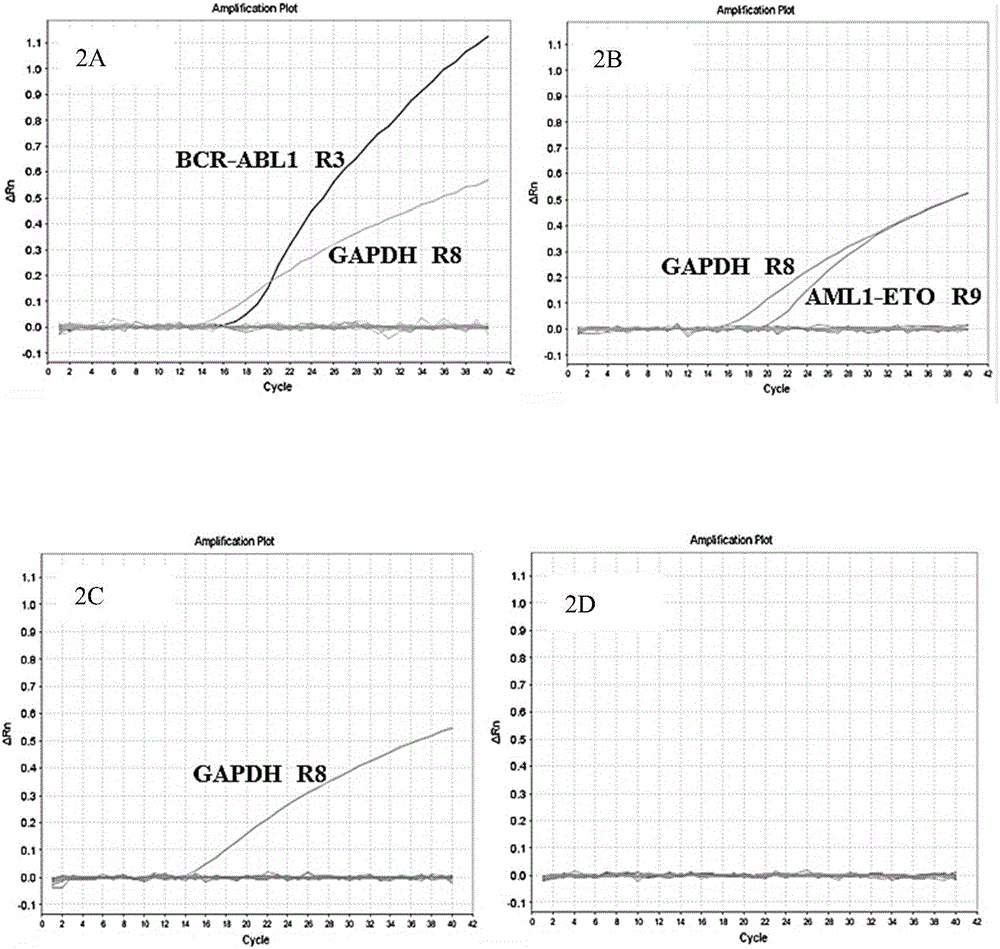
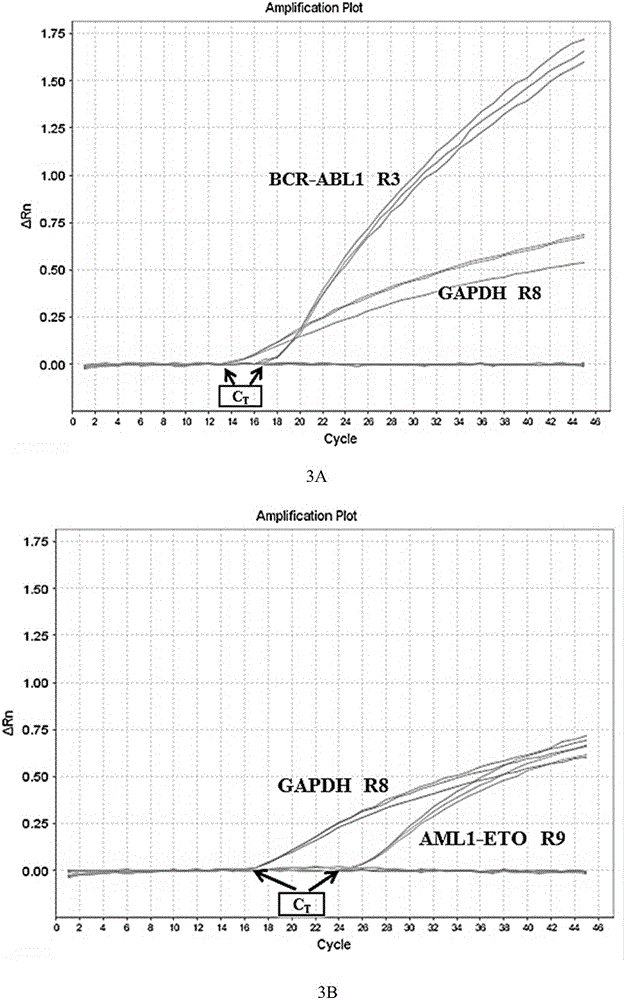
Primer, probe, kit and method for qualitatively detecting fusion genes of leukemia
ActiveCN105838792AImprove featuresImprove efficiencyMicrobiological testing/measurementDNA/RNA fragmentationPcr methodGene engineering
The invention belongs to the technical field of gene engineering and discloses a prime-probe combination for detecting fusion genes of leukemia, a kit containing the prime-probe combination and a multiplex fluorescent RT-PCR method for detecting the fusion genes of the leukemia by virtue of the prime-probe combination or the kit. Based on a multiplex fluorescent RT-PCR technique, the method is simple, rapid and high in sensitivity. Besides, by virtue of the reasonable prime-probe combination, the mutual action between the two primers, the prime and the probe as well as two probes are effectively avoided, and the detection error is reduced. By virtue of the method, 45 fusion genes of the leukemia can be comprehensively and qualitatively detected, the detection time is effectively shortened, and very important detection measures are provided for the evaluation of clinical diagnosis and treatment and the prognosis of the leukemia.
Owner:SHANGHAI TISSUEBANK BIOTECH +3
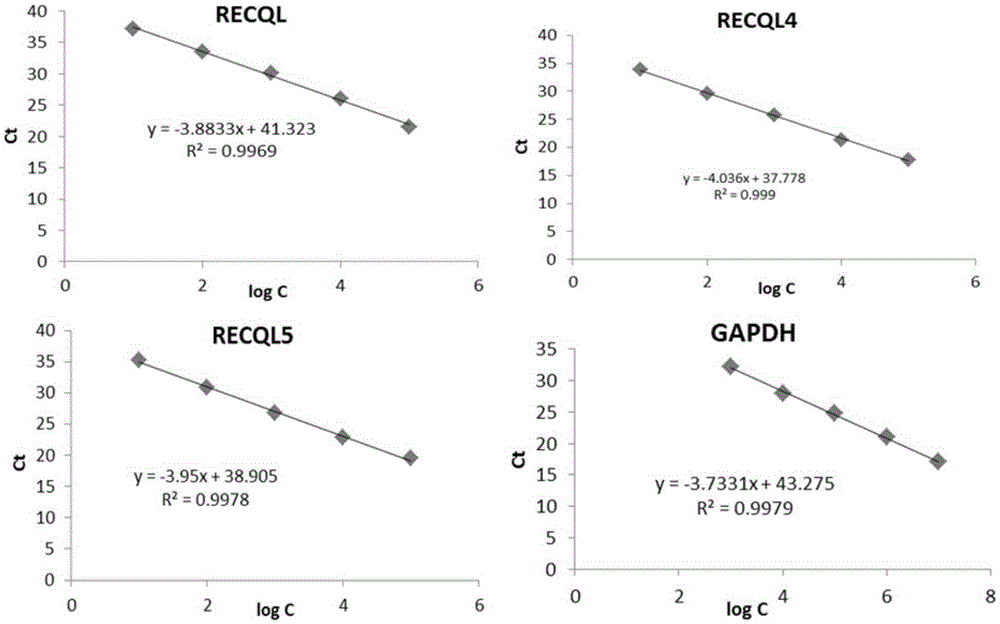
Breast cancer combined diagnosis markers and detecting kit
ActiveCN105256014AImprove featuresHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationReference genesFluorescence
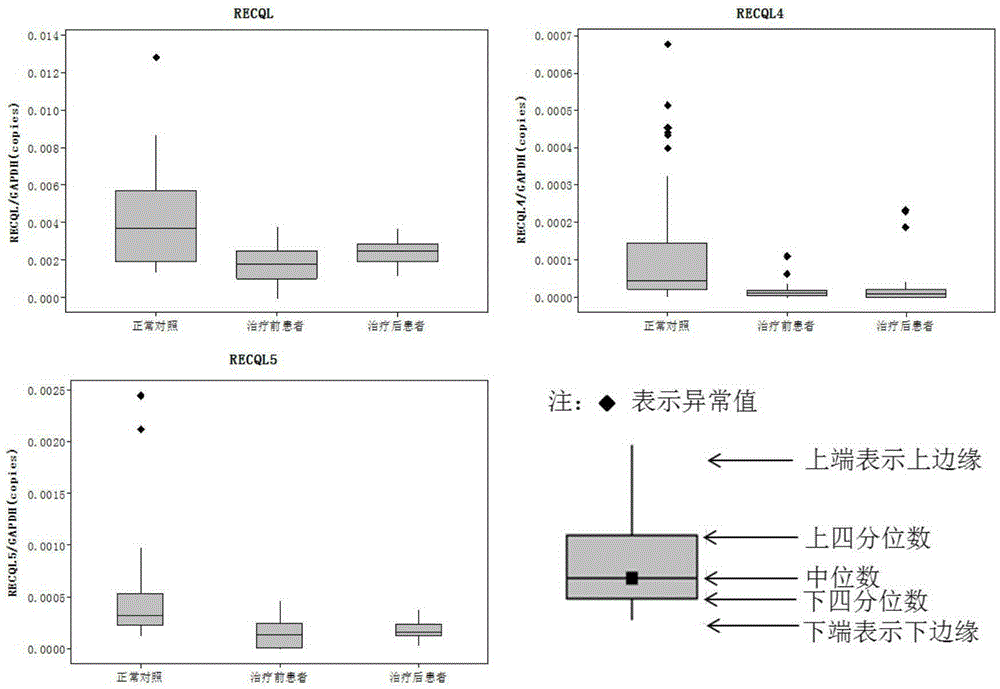
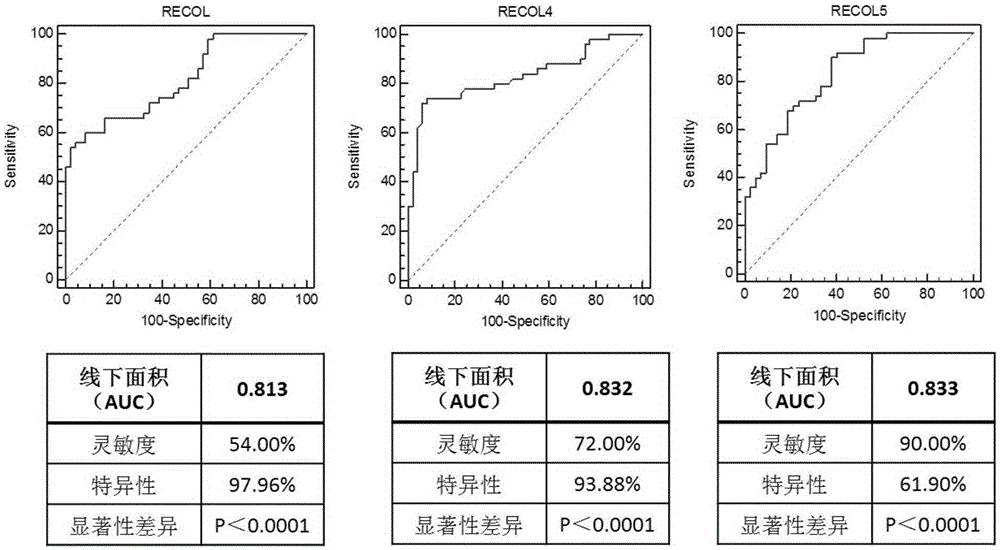
The invention discloses a breast cancer combined diagnosis markers and a detecting kit. Studies show that RECQL, RECQL4 and RECQL5 genes can serve as the combined markers and used for preparing breast cancer detection and / or therapeutic evaluation reagents. According to the kit formed by the markers, a specificity RT-PCR primer designed with GAPDH serving as a reference gene, and a probe, a real-time fluorescence quantitative PCR method is used for measuring the content of mRNA of the RECQL, RECQL4 and RECQL5 genes drifting away in peripheral blood monouclear cells and urine of a patient, and diagnosis and therapeutic effect evaluation can be carried out on breast cancer. Compared with a conventional diagnosis method, the method has the advantages that trauma is small, use is fast, throughput is high, sensitivity and specificity are good, and early diagnosis and therapeutic evaluation can be carried out on the breast cancer better.
Owner:王义明
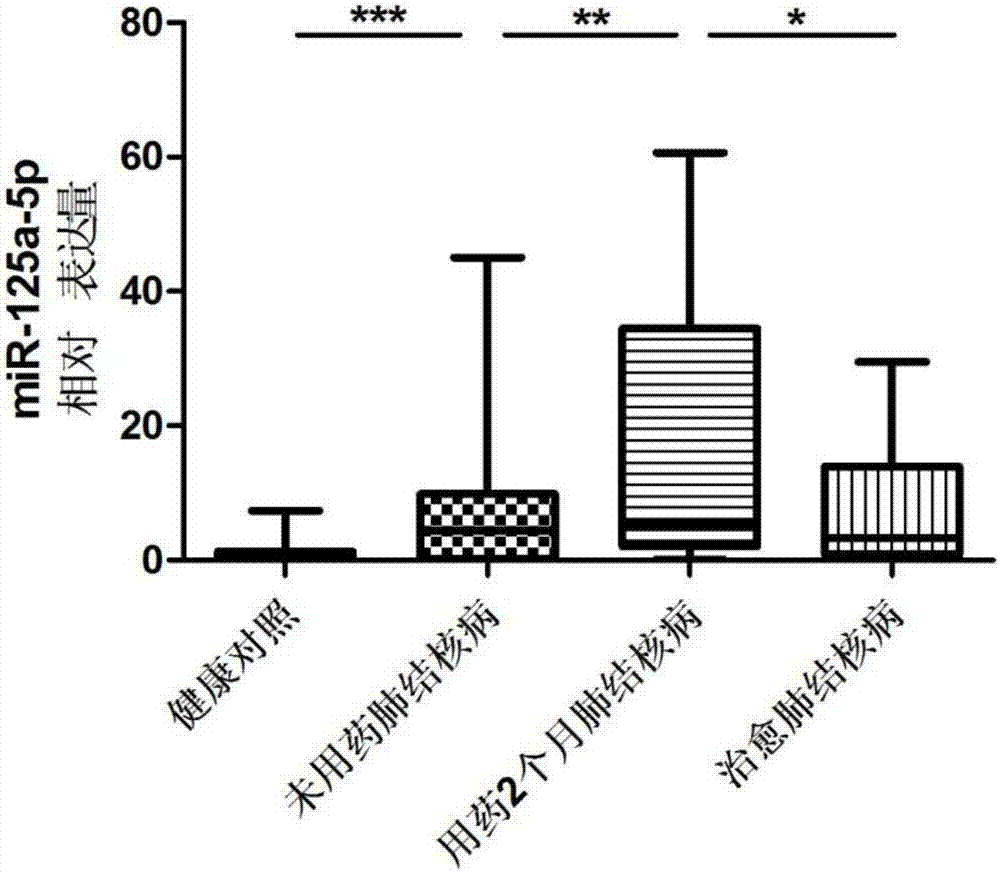
Serum miRNA combination-based pulmonary tuberculosis therapeutic effect evaluation kit and applications thereof
InactiveCN106884052AReliable resultsStrong specificityMicrobiological testing/measurementRNA extractionCurative effect
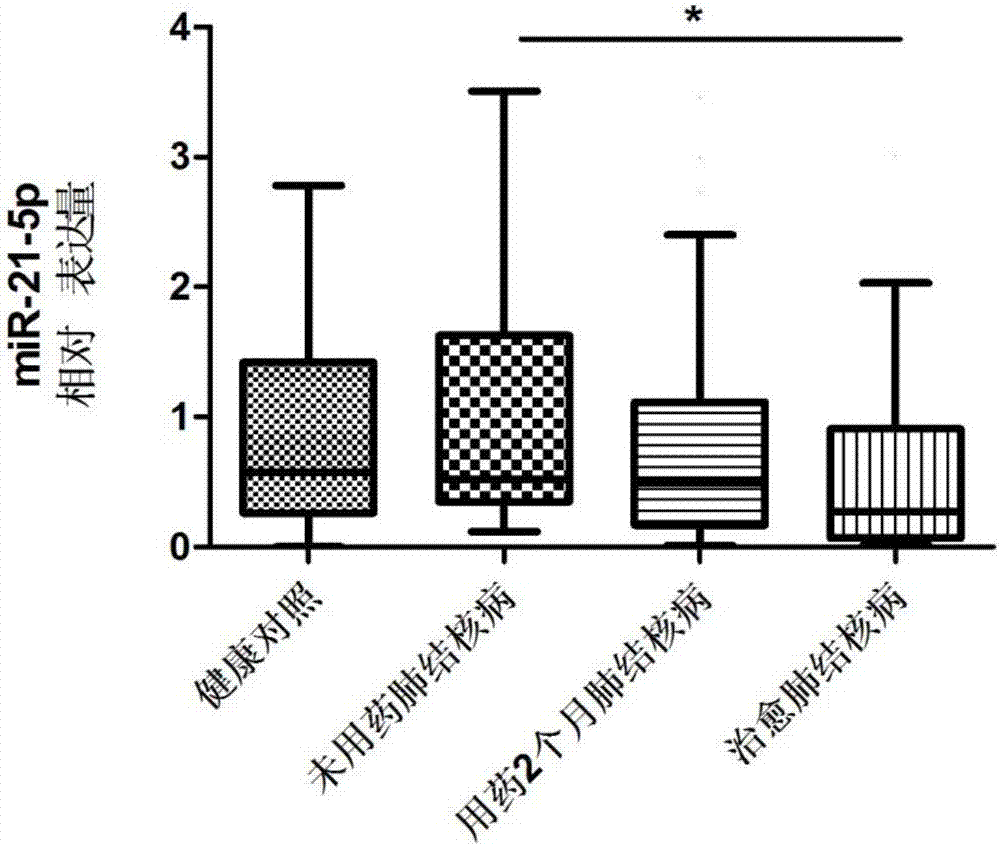
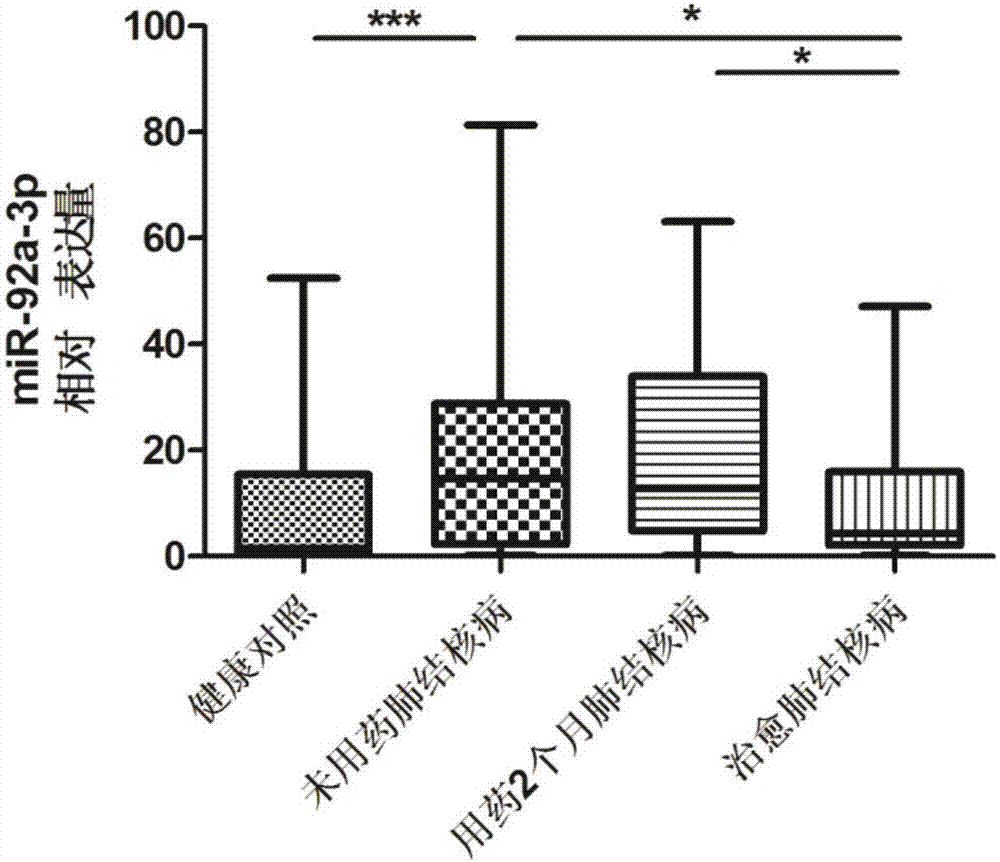
The invention discloses a serum miRNA combination-based pulmonary tuberculosis therapeutic effect evaluation kit and applications thereof. The kit comprises an RNA extraction buffering solution, a Poly (A)-containing reaction solution, a reverse transcription reaction solution, a serum miRNA upstream primer combination, an internal reference upstream primer, a universal downstream primer and a fluorescent quantitation RT-PCR reaction solution, wherein the serum miRNA upstream primer combination comprises four differentially expressed serum miRNA: hsa-miR-21-5p, hsa-miR-92a-3p, hsa-miR-125a-5p and hsa-miR-148b-3p, the sequences of which are respectively SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3 and SEQ ID NO:4; the internal reference is hsa-miR-16, the sequence of which is SEQ ID NO:5. The detection sensitivity of the kit on treatment of pulmonary tuberculosis is 65.38%, and the specificity is 80.77%, so that the specificity is strong and the sensitivity is high, and the kit has higher accuracy, and is simple and convenient to operate, high in efficiency, small in specimen state limit, and superior to the sputum culture currently clinically adopted, thus providing a new evaluation method for the evaluation on the therapeutic efficacy of pulmonary tuberculosis.
Owner:李继承
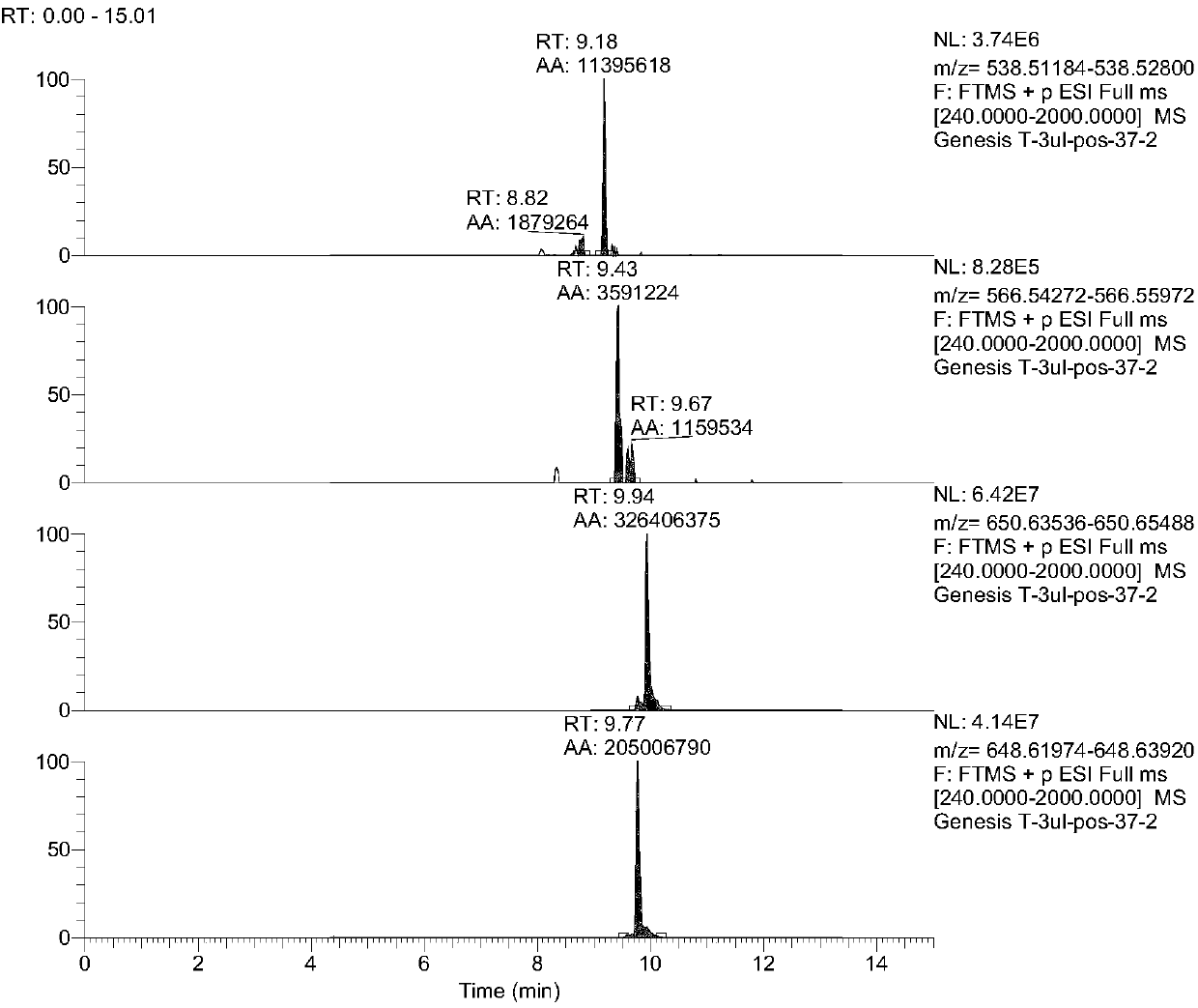
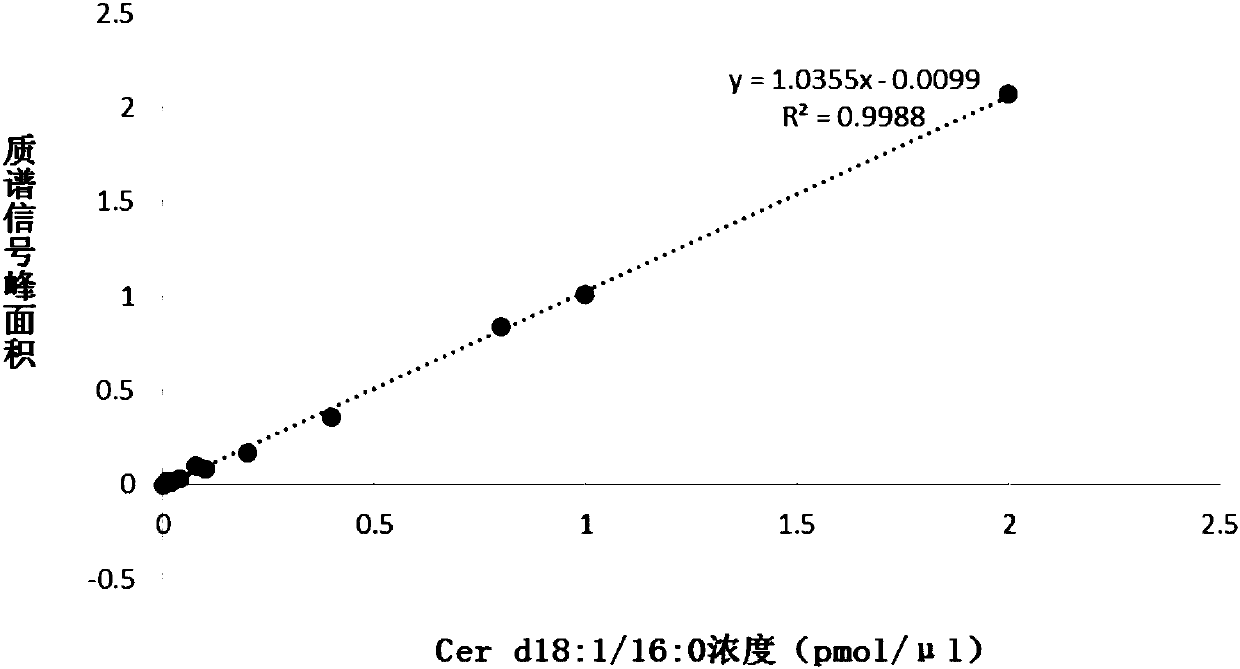
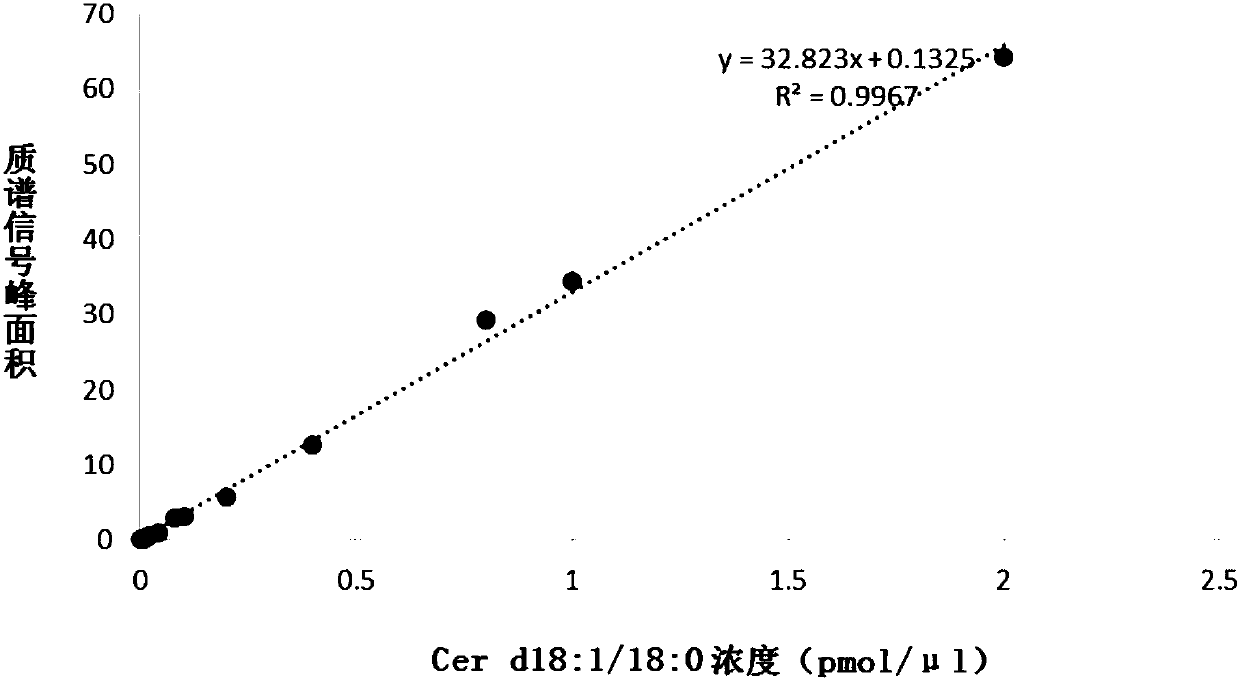
Kit for estimating coronary artery diseases
PendingCN108037275AEffective diagnosisAccurate diagnosisComponent separationBiological testingCoronary arteriesTherapeutic evaluation
The invention provides a kit for estimating coronary artery diseases. The kit comprises a detection reagent for detecting concentrations of Cer(d18:1 / 16:0), Cer(d18:1 / 18:0), Cer(d18:1 / 24:0) and Cer(d18:1 / 24) in a sample of a study subject and a specification. The kit can be efficiently and accurately used for the diagnosis, risk stratification, illness monitoring and curative effect evaluation ofthe coronary artery diseases, is good in sensitivity and specificity and can meet the clinical diagnosis and treatment requirement of the coronary artery diseases.
Owner:江苏豪思生物科技有限公司
Characteristic protein maker composition for mass spectroscopic diagnosis of thalassemia and diagnostic products thereof
PendingCN110632326AIncreased sensitivityImprove featuresMaterial analysis by electric/magnetic meansBiological testingMedicineCurative effect
The invention provides a characteristic protein fragment composition for detecting thalassemia and a mass spectrometry model for evaluating therapeutic effect of thalassemia medicine or evaluating effect of a treatment method, wherein the sequence of the characteristic protein fragment composition is shown in SEQ ID No.1-3, respectively. The characteristic protein fragment composition or mass spectrometry model provided by the invention can be used for the diagnosis and screening of thalassemia, as well as the evaluation of the treatment method and drug efficacy of thalassemia. The method is simple and easy to operate, and high in accuracy, and provides new methods and ideas for the evaluation of diagnosis and screening, treatment methods and drug efficacy of thalassemia.
Owner:BIOYONG TECH +1
Multispectral tongue picture collecting device
InactiveCN103222856AImprove accuracyDiagnostic recording/measuringSensorsOphthalmologyLighting spectrum
The invention discloses a multispectral tongue picture collecting device which comprises a box body, wherein a tongue body detection opening is formed in one side of the box body; a digital camera is arranged on the other side of the box body through penetrating through the box body wall; a light source is arranged on the inner surface of the box body; an optical lens is arranged inside the box body; the tongue body detection opening and the digital camera are positioned in the image conjugate positions on the two sides of the optical lens respectively; and a light splitting device is arranged between the optical lens and the digital camera. The multispectral tongue picture collecting device provided by the invention acquires the multispectral information of the detected tongue body at last through acquiring multispectral images of the tongue body; through combination of three dimensional information of a first dimensional light spectrum and a second dimensional image of the tongue body, physiological and pathological information brought by the tongue body can be reflected, so that more objective information can be provided for doctors of Chinese medicine for tongue diagnosis, and the accuracy in clinical diagnosis of the doctors of Chinese medicine can be improved; and meanwhile through the comparison of the tongue body three dimensional information of a patient in different stages, objective references can be provided for evaluation of clinical curative effect of doctors of Chinese medicine.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Blood vessel photodynamic therapeutic dose evaluation method
The invention relates to a method for evaluating and optimizing blood vessel photodynamic therapeutic effects of different light dosages on the same experimental animal, in particular to therapeutic effects of a photodynamic method is used for treating vascular disease, such as nevus flammeus and choroidal vessels, and the method can be used for evaluating and optimizing illumination parameters of a pulse dye laser and other photo-thermal therapy for treating the vascular disease. According to the method, structured light irradiation with preset dosage is performed on the same experimental animal, and the method comprises the main steps of sample dissecting and dyeing, sample slicing and three-dimensional reconstructing, and experimental data analyzing and evaluating, thereby obtaining the therapeutic evaluations of different light dosages on the same experimental animal. Through the method, clinical experiment animals can be greatly reduced, the evaluations of therapeutic effects of blood vessels in different depths and diameters can be provided, and the determination of the optimal illumination dosage is accelerated.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
VEGF (vascular endothelial growth factor) monoclonal antibody and fracture healing evaluation antibody chip with same
InactiveCN102863529AHigh potencyHigh-throughput detectionPeptide librariesImmunoglobulins against growth factorsDrug biological activityStandardization
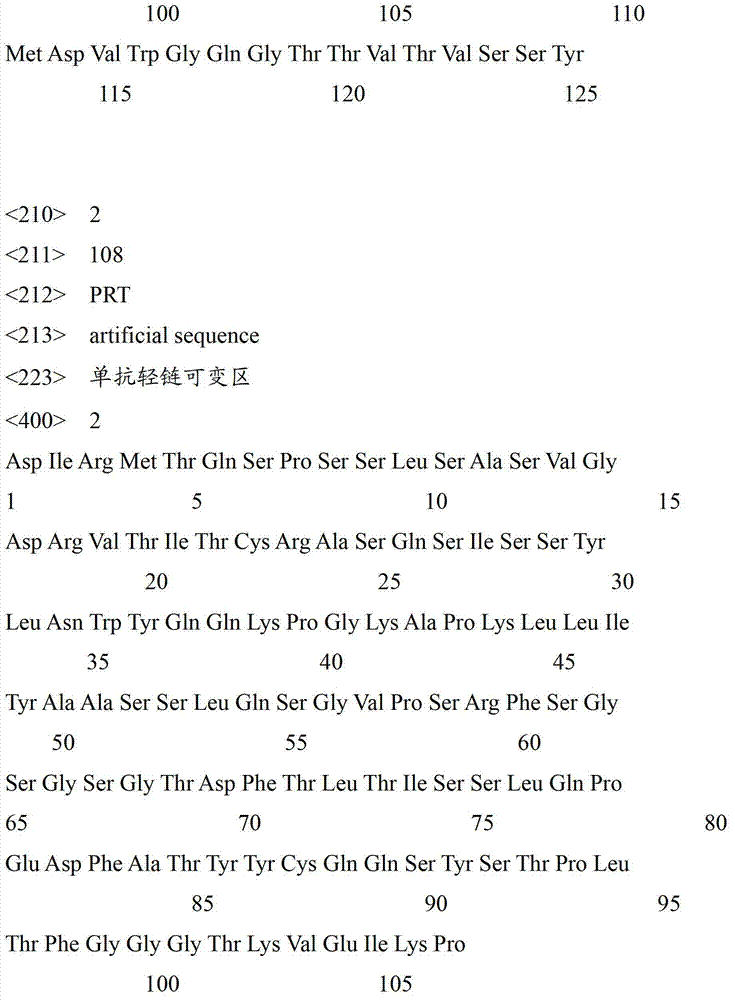
The invention relates to the technical field of bioengineering and discloses a VEGF (vascular endothelial growth factor) monoclonal antibody and a fracture healing evaluation antibody chip with the same. The amino acid sequence of a heavy chain variable region of the monoclonal antibody is shown as SEQ ID NO.1, and the amino acid sequence of a light chain variable region of the monoclonal antibody is shown as SEQ ID NO.2. Homogeneity and biological activity unicity of the monoclonal antibody enables antigen antibody reaction results to be convenient for quality control and beneficial to standardization and normalization. The chip with the monoclonal antibody can be directly applied to clinical therapeutic evaluation on fracture healing, solves the technical problems of time and labor consumption, poor result repeatability and the like of the prior art, has extremely high application value in the field of clinical treatment and has wide application prospect.
Owner:李彬
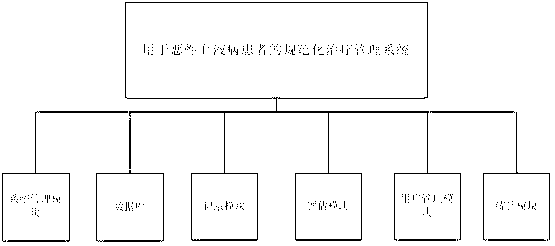
Standardized therapy management system used for hematological malignancy patients
InactiveCN103020778AImprove the ability to standardize treatment managementReduce work omissionsResourcesNODALDisease
A standardized therapy management system used for hematological malignancy patients comprises a data base, a recording module, an evaluation module and a statistics module. The standardized therapy management system used for hematological malignancy patients comprehensively records disease information of the patients; provides standardized therapy, evaluation procedure and examination and testing methods; and manages therapeutic evaluation time and reexamination time nodes of the patients in standardized mode. Doctors can improve the capability of clinician standardized therapy management through the statistics module, work omissions caused by inadequate experience and negligence can be reduced, and state, curative effect and prognoses of the hematological malignancy patients can be evaluated through the evaluation module.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
A diagnostic kit for detecting paragonimiasis and its preparation method
InactiveCN102279259AFacilitate early diagnosisAids in prognosisMaterial analysis by observing effect on chemical indicatorDiseaseNitrocellulose
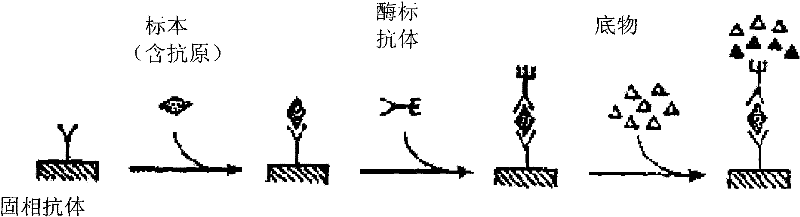
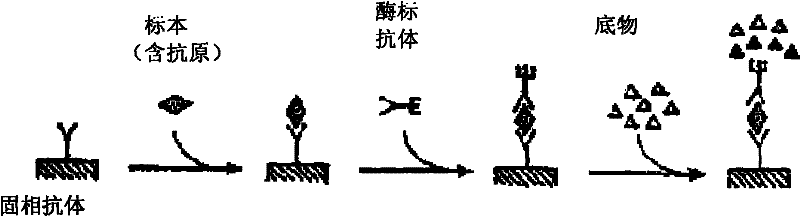
The invention provides a diagnostic kit for detecting Paragonimiasis, which contains a rabbit polyclonal antibody against the soluble antigen of Paragonia wescheri adult worm, an enzyme-labeled rabbit polyclonal antibody against the soluble antigen of Paragonimus westermani adult worm, Nitrocellulose membrane, bovine serum albumin, PBST washing solution, substrate chromogenic solution, positive control sample, negative control sample and polyethylene reaction plate. The invention also provides a preparation method of a diagnostic kit for detecting paragonimiasis. The invention adopts the double-antibody sandwich method Dot-ELISA to detect the circulating antigen of Paragonimus westermani, has high sensitivity and strong specificity, and can realize rapid diagnosis and curative effect assessment of Paragonimiasis.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
A kit for detecting circulating antigens of Agkistrodon acutina using specific polyclonal antibodies
InactiveCN102288754AFacilitate early diagnosisAids in prognosisMaterial analysis by observing effect on chemical indicatorSerum immunoglobulinsNitrocellulosePentastomiasis
The invention provides a kit for detecting circulating antigens of Agkistrodon acutinae using specific polyantibody, comprising rabbit anti-Aag-IgG, enzyme-labeled antibody (HRP-Aag-IgG), nitrocellulose film, 1 % bovine serum albumin in PBST solution, PBST washing solution, substrate chromogenic solution, positive and negative control sample composition and polyethylene reaction plate. The invention also provides a preparation method of a kit for detecting circulating antigens of Agkistrodon acutina by using specific polyantibody. The invention adopts the polyclonal antibody to measure the circulating antigen, which is beneficial to making early diagnosis and helping to judge the prognosis. When the parasites in the body die, the circulating antigens disappear quickly, which can be used for efficacy assessment. The kit of the invention has high sensitivity and strong specificity, and can realize rapid diagnosis, drug screening and curative effect assessment of Agkistrodon acutinae ligamentiworm disease.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Online analysis system
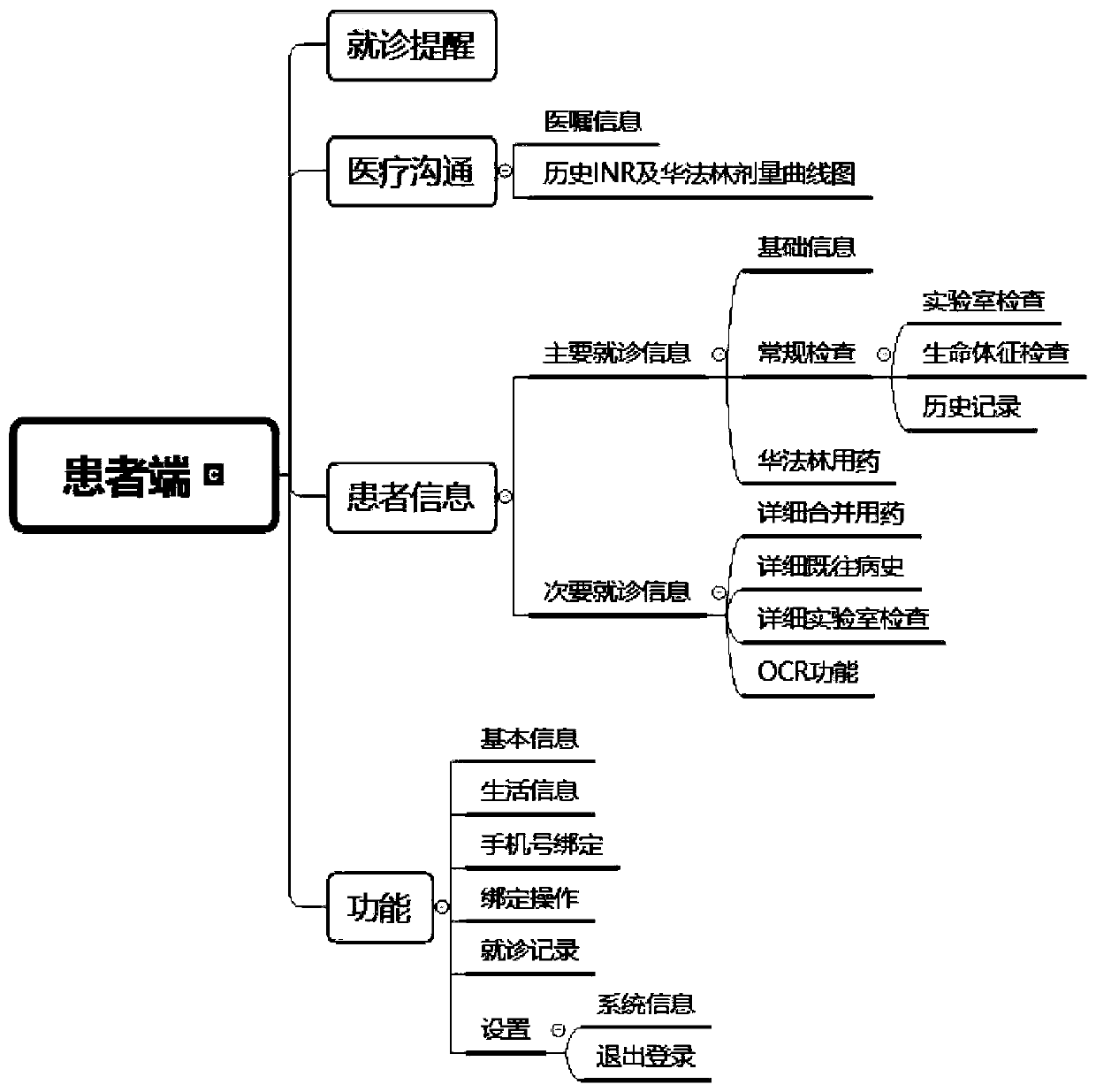
PendingCN111243704AEffective complianceEffective anticoagulationMedical communicationDrug and medicationsTherapeutic evaluationPatient compliance
The invention relates to an online analysis system, and the system comprises a curative effect evaluation module. The curative effect evaluation module comprises a data obtaining unit which is used for extracting data corresponding to a doctor recommended dose, an actual medicine taking dose and an INR from a database; a curve graph drawing unit which is used for drawing a curve graph by taking the dosage recommended by the doctor, the actual medicine taking dosage and the INR as vertical coordinates and taking time as horizontal coordinates, and obtaining a patient compliance index through the difference of the two generated curves; and a data annotation unit which is used for calculating the time percentage of the part not exceeding the target INR in the INR curve graph. According to theonline analysis system, the INR curve graph is obtained, the part, not exceeding the target INR, in the INR curve graph is marked, the marked curve graph and the calculated time percentage are displayed on a doctor terminal and a patient terminal, and the intelligent level can be improved by adopting the online analysis system.
Owner:长沙四维医疗器械科技有限责任公司
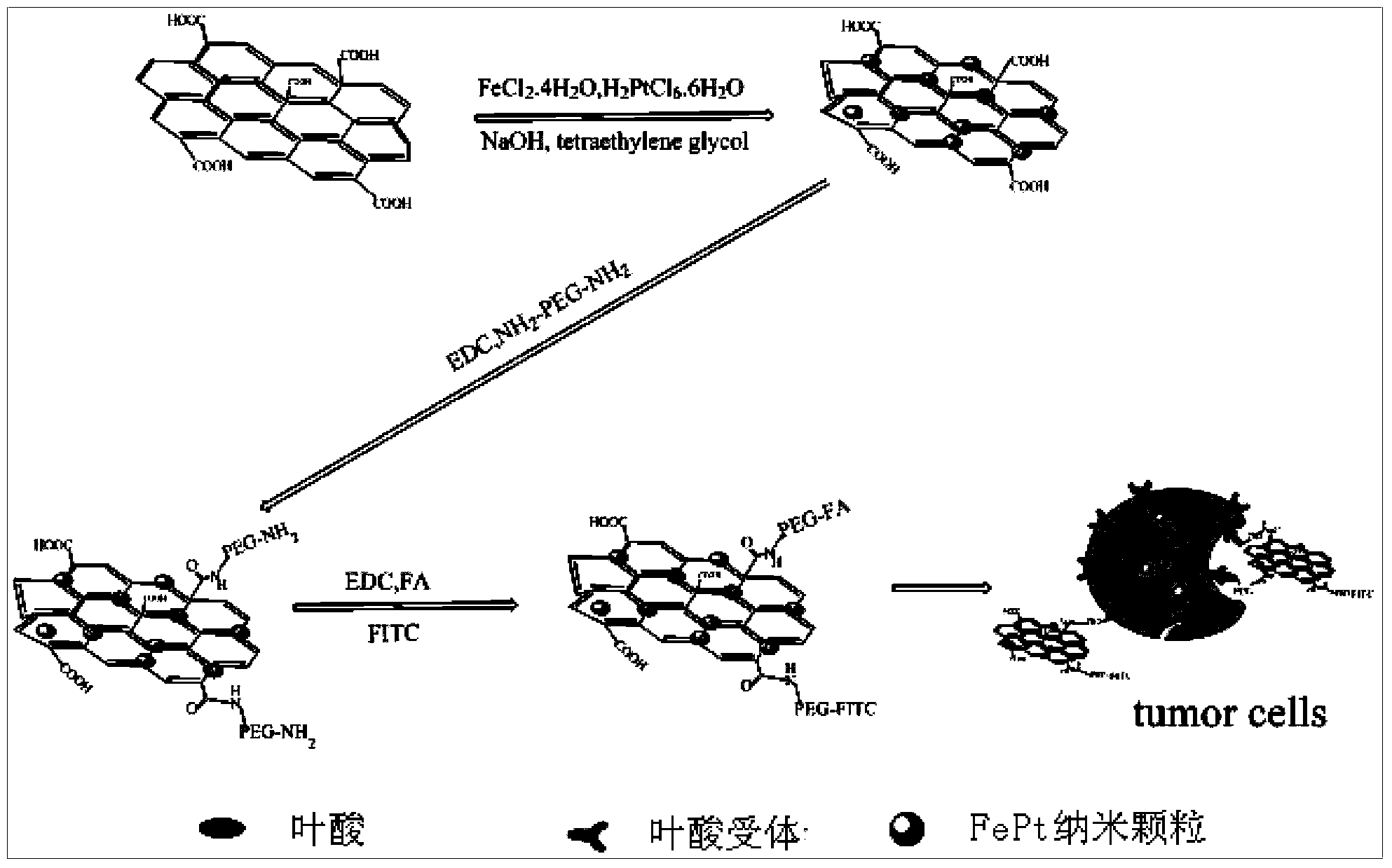
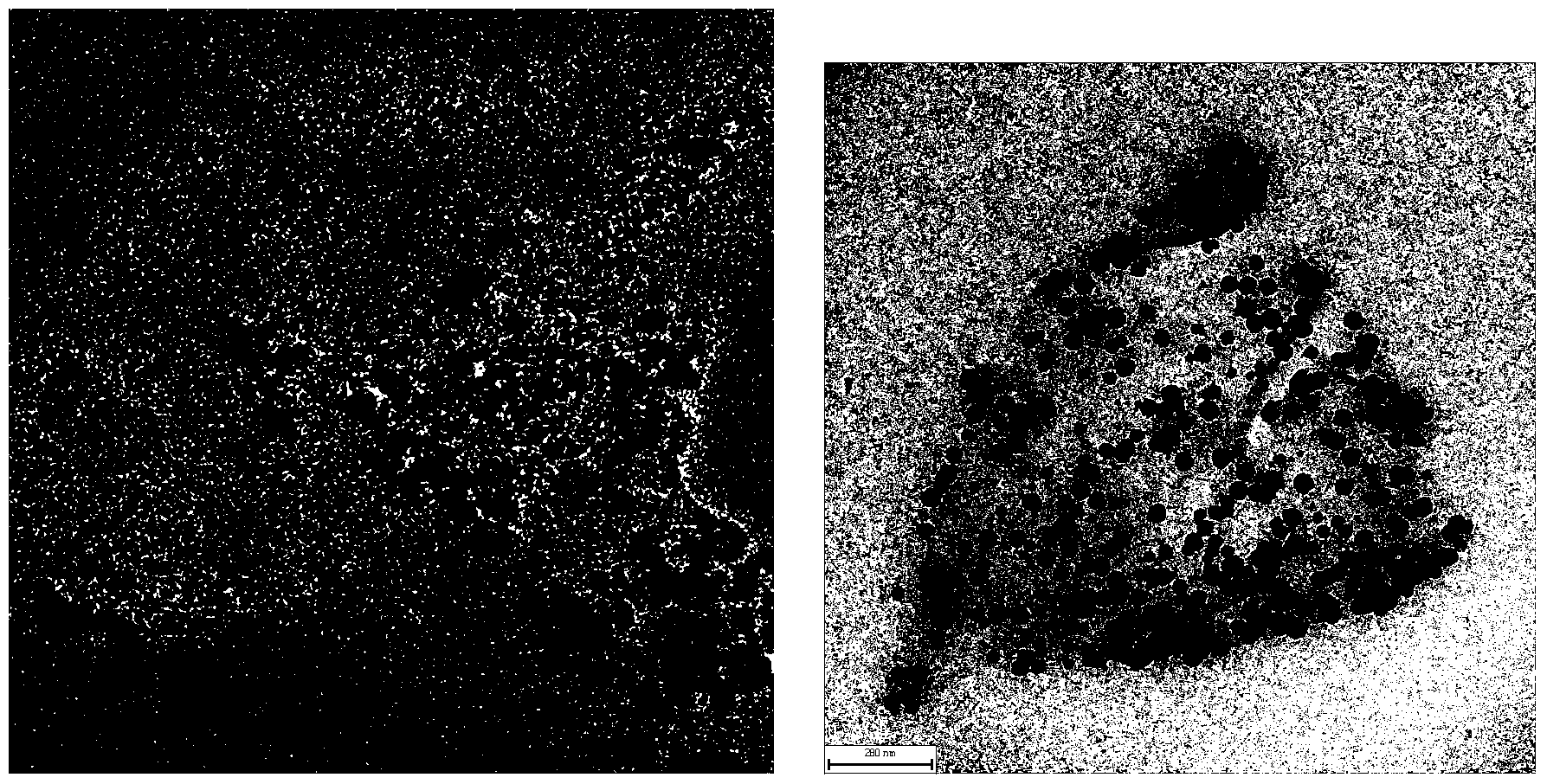
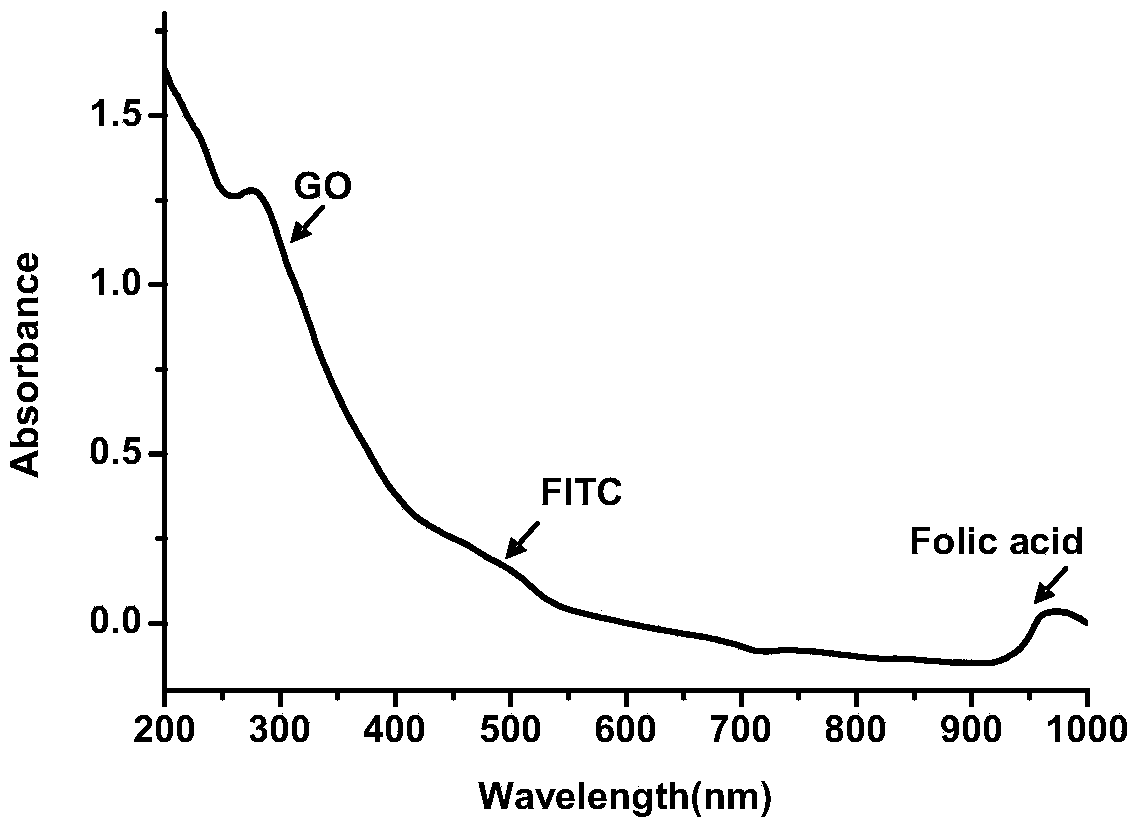
FePt/GO nanocomposite based diagnosis and treatment reagent and application thereof
InactiveCN104069514AEnergy modified materialsNMR/MRI constrast preparationsPolyethylene glycolMolecular level
The invention discloses synthesis and an application of a FePt / GO nanocomposite based diagnosis and treatment reagent. A nano FePt / GO composite is taken as the core, and fluorescein isothiocyanate (FITC) and folic acid are connected by polyethylene glycol (PEG). Based on the design, the invention constructs a diagnosis and treatment nano probe which integrates multi-target (molecular level and external magnetic field), bimodal imaging diagnosis (MRI and fluorescence) and dual treatment (chemotherapy and thermal therapy) to treat the selected tumor, thereby achieving the central goal of early target diagnosis and synchronous in situ treatment of tumors. According to the nano probe, the main synthesis manner comprises low temperature microwave radiation-liquid phase synthesis, and graphene is taken as a carrier to control the synthesis of the FePt / GO nanocomposite. The core relates to controlled synthesis of FePt / GO nanocomposite, physicochemical property research, surface finish, target bimodal imaging in cellular level (invitro) and synchronous in situ treatment bimodule imaging location, and visualization treatment and curative effect evaluation.
Owner:LINYI UNIVERSITY
Application of characteristic protein composition or mass spectrum model for representing thalassemia
InactiveCN110658251AIncreased sensitivityImprove featuresCompounds screening/testingBiological material analysisTherapeutic evaluationProtein composition
The invention provides a mass spectrum model for detecting a characteristic protein fragment composition of thalassemia and evaluating a therapeutic effect of a thalassemia drug or evaluating an effect of a treatment method. A sequence of the characteristic protein fragment composition is shown as SEQ ID No.1-3. The characteristic protein fragment composition or the mass spectrum model can be usedfor diagnosis and screening of the thalassemia and evaluation of the treatment method of the thalassemia and the therapeutic effect of the drug. The method is simple and easy to operate and is high in accuracy, and a new method and idea is provided for diagnosis and screening of the thalassemia, the treatment method and drug therapeutic effect evaluation.
Owner:长沙湘华质谱医学科技有限公司 +1
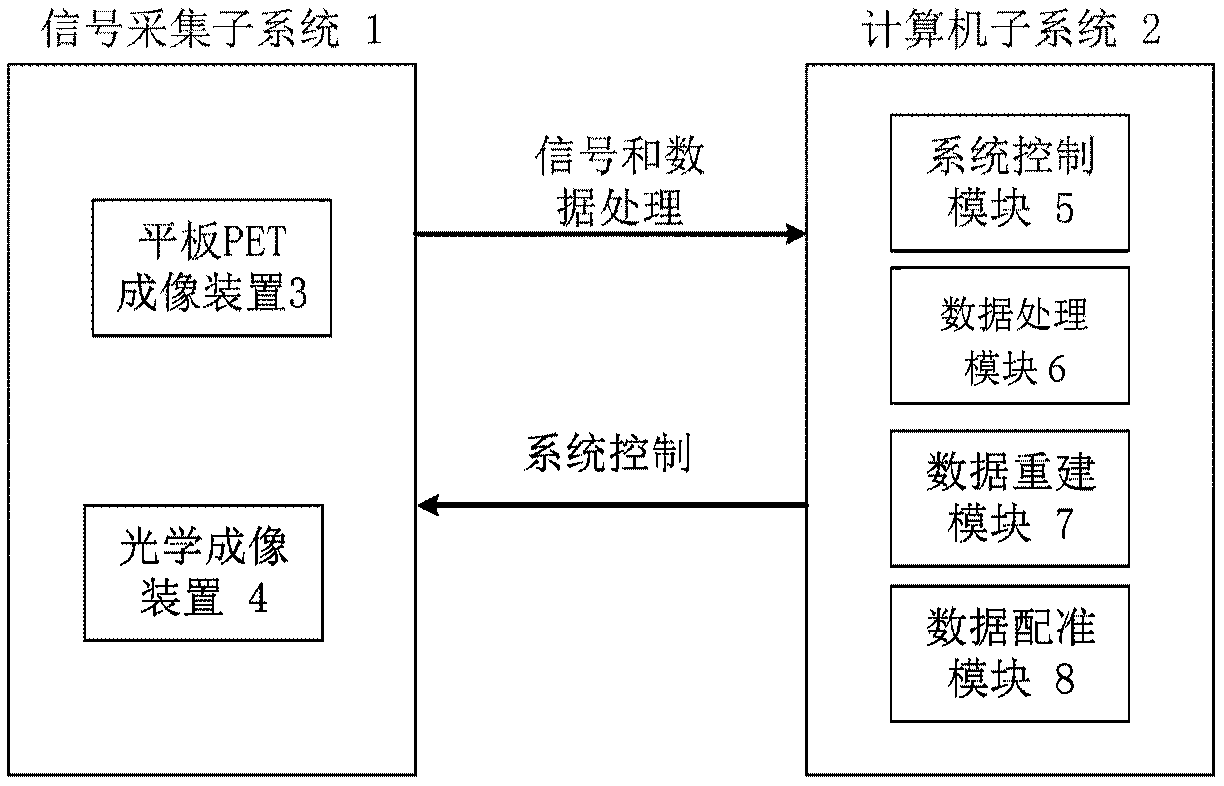
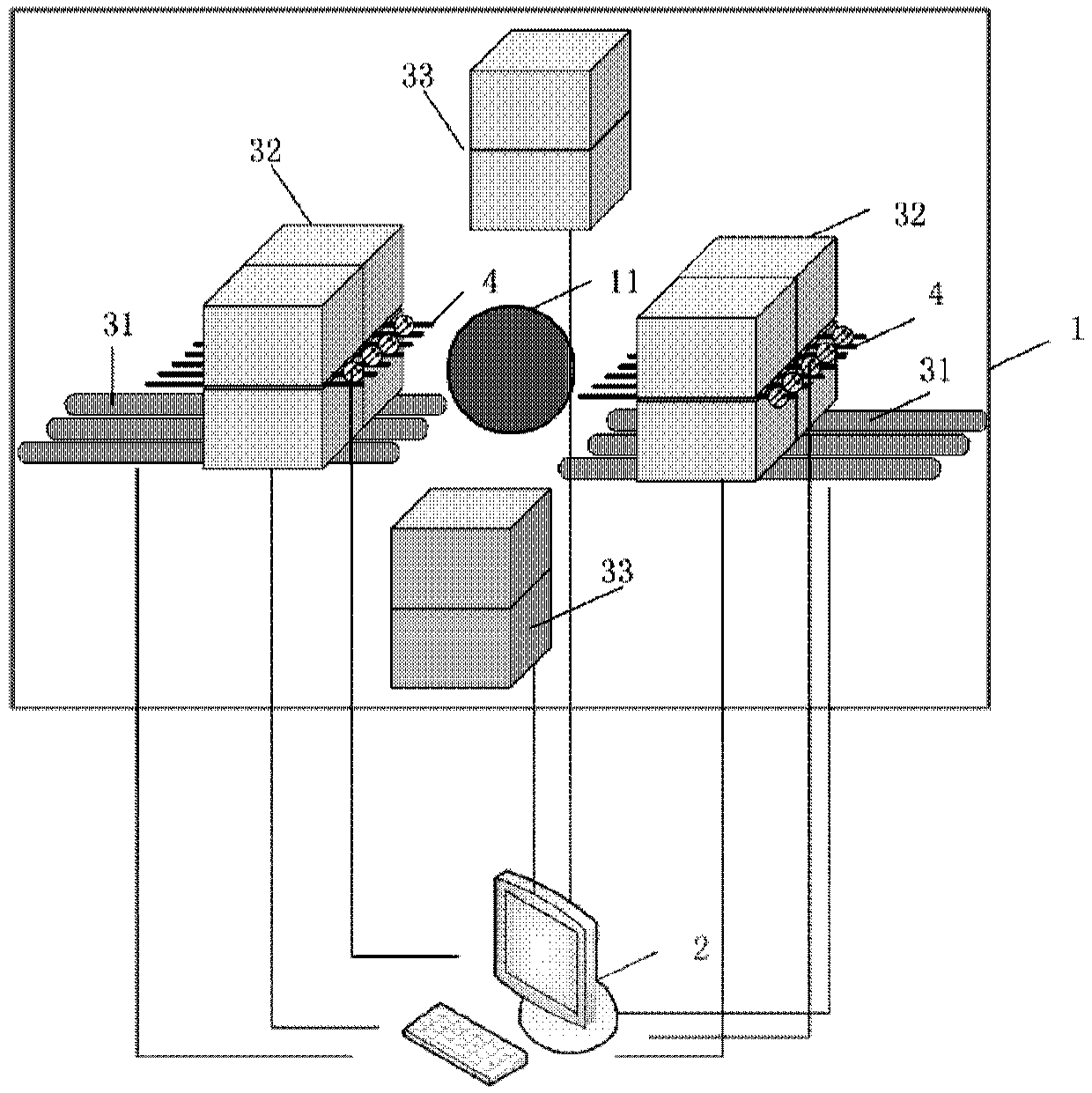
Panel PET and optical dual-mode fusion imaging system and method based on breast cancer detection
ActiveCN109589128AGuaranteed Spatial ResolutionGuaranteed Simultaneous ImagingComputerised tomographsDiagnostics using tomographyDual modeImagery technique
The invention discloses a panel PET and optical dual-mode fusion imaging system and method based on breast cancer detection. The system comprises a signal acquisition subsystem and a computer subsystem, wherein the signal acquisition subsystem comprises a panel PET imaging device and an optical imaging device, and is used for obtaining PET data and optical data; the computer subsystem comprises asystem control module, a data processing module, a data reconstruction module and a data registration module; the data processing module can preprocess the PET data and optical data; the data reconstruction module can reconstruct the preprocessed PET data and optical data respectively to obtain reconstructed images; the data registration module is used for performing registration and fusion on thetwo reconstructed images, so as to realize accurate positioning of breast cancer. The system can perform PET imaging and optical imaging at the same time on a breast, and provides a powerful image technology support for the early accurate diagnosis and prognosis therapeutic evaluation of breast cancer.
Owner:INST OF AUTOMATION CHINESE ACAD OF SCI
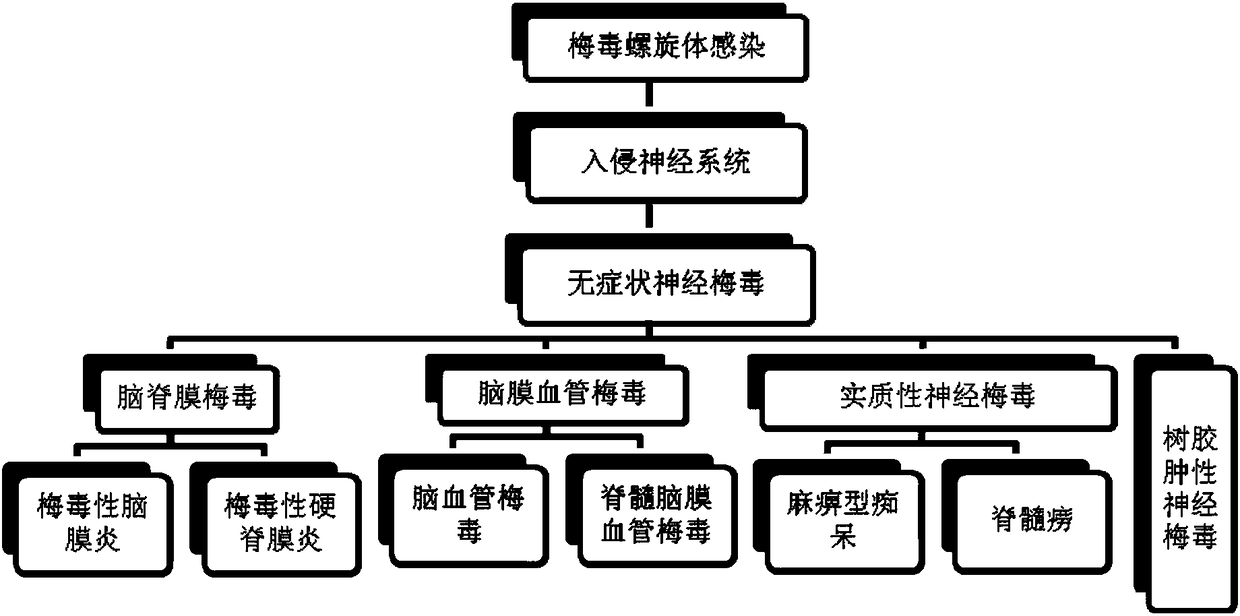
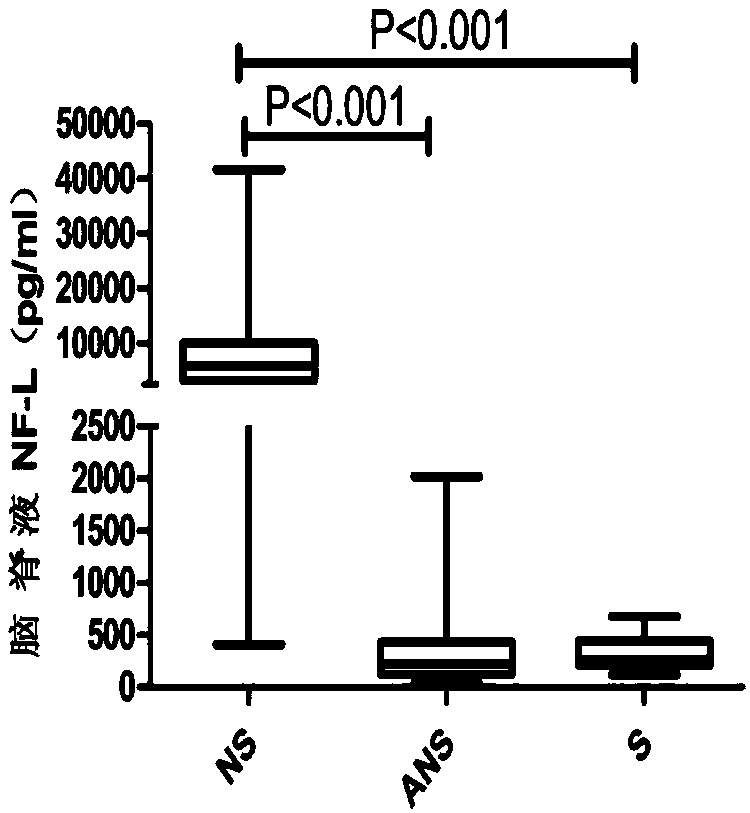
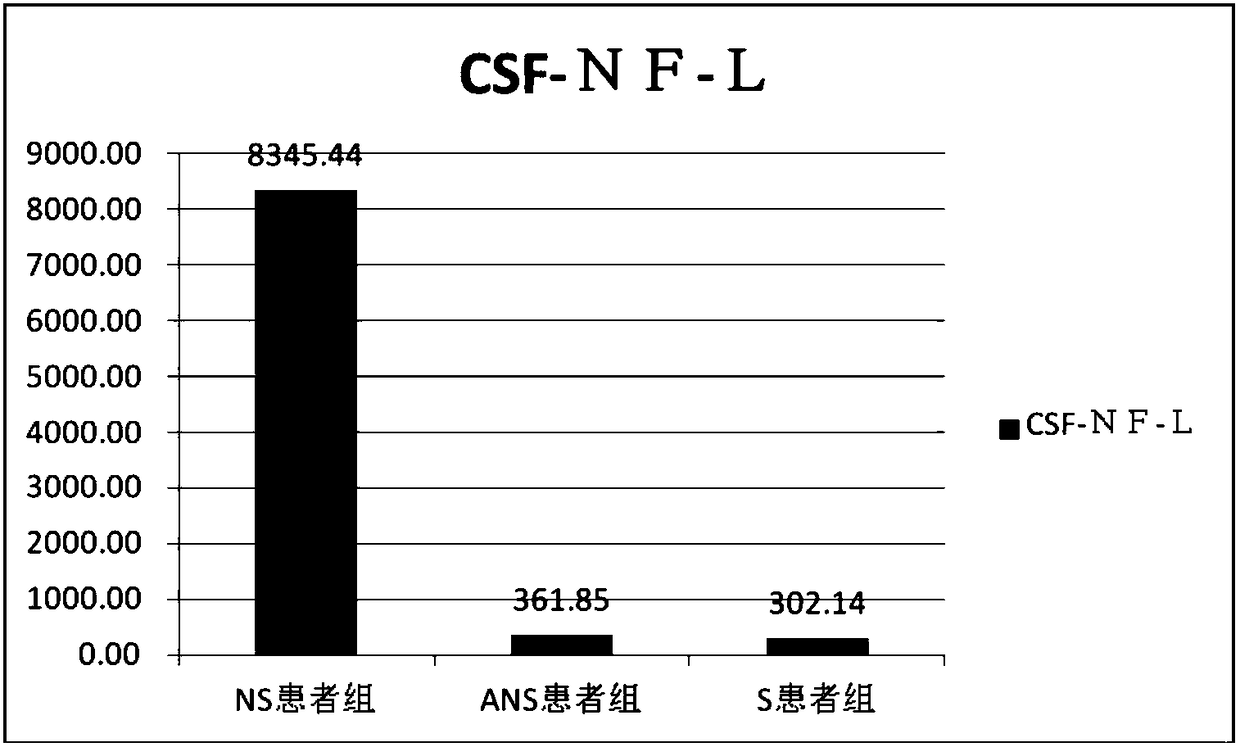
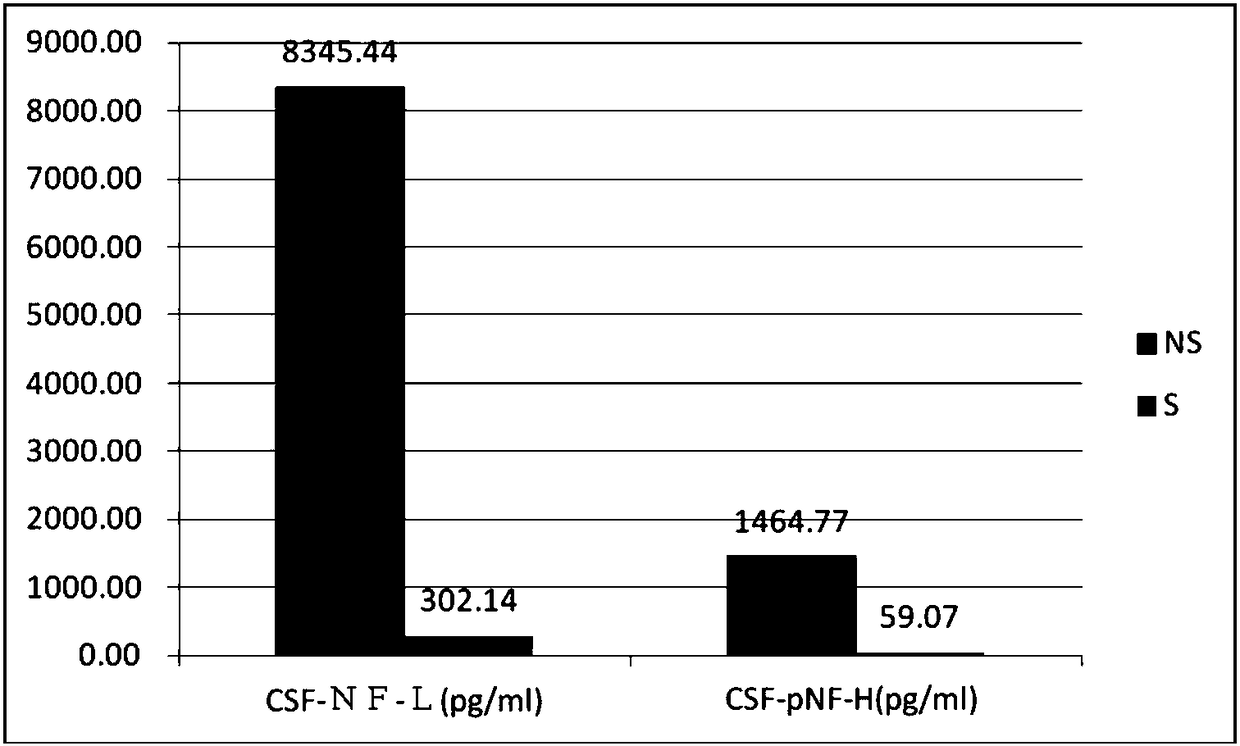
Application of NF-L in neurosyphilis cerebrospinal fluid detection
The invention relates to application of NF-L in neurosyphilis cerebrospinal fluid detection, and specifically provides a neurosyphilis detection kit which comprises a detection reagent for detecting level of neurosyphilis cerebrospinal fluid NF-L. The invention further provides application of a first detection reagent for detecting neurosyphilis cerebrospinal fluid NF-L in preparing the neurosyphilis detection kit. The kit can be used for early diagnosis, typing and staging, therapeutic evaluation and / or prognosis evaluation for neurosyphilis, and has the advantages of being high in reliability, high in sensitivity and the like.
Owner:BEIJING DITAN HOSPITAL CAPITAL MEDICAL UNIV
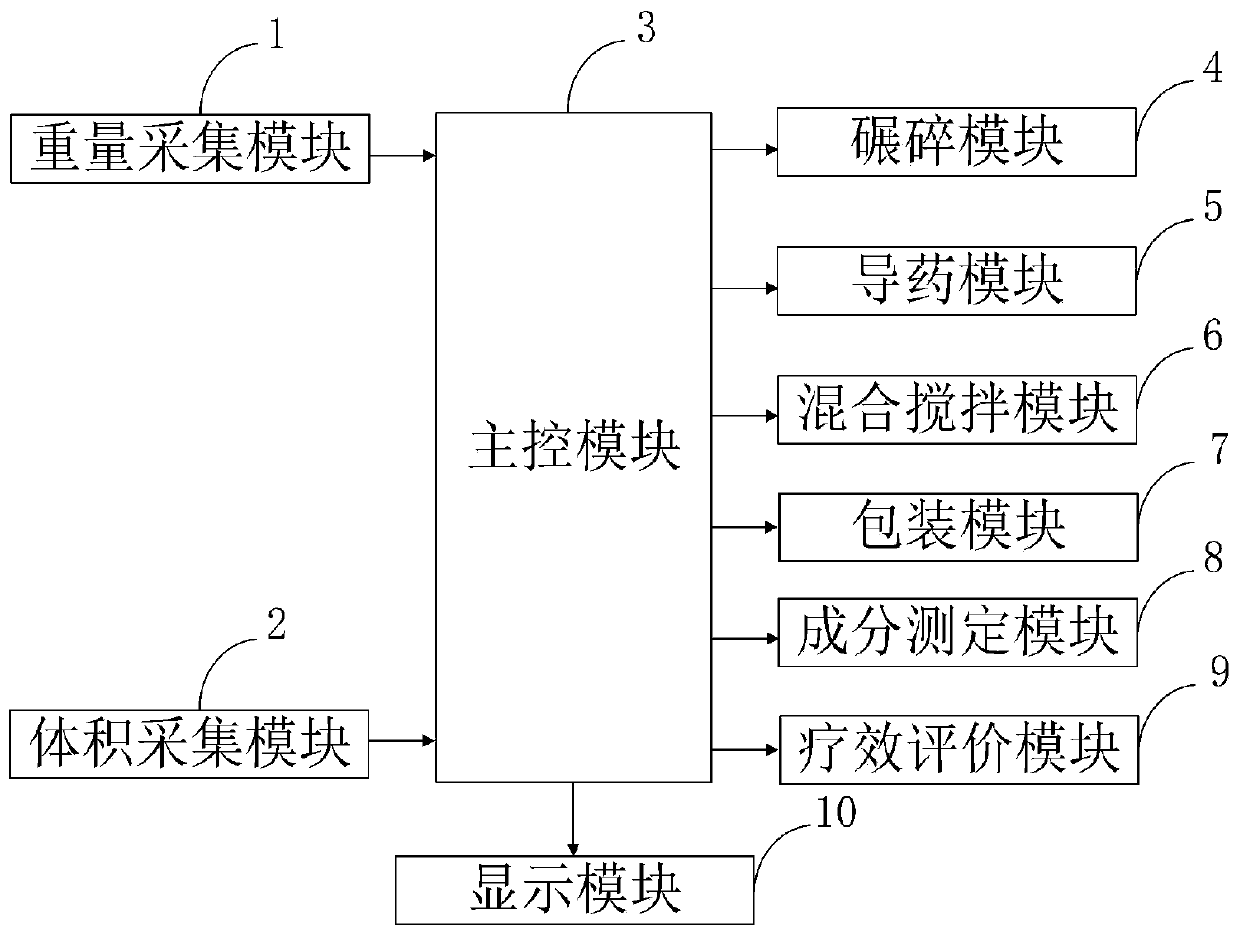
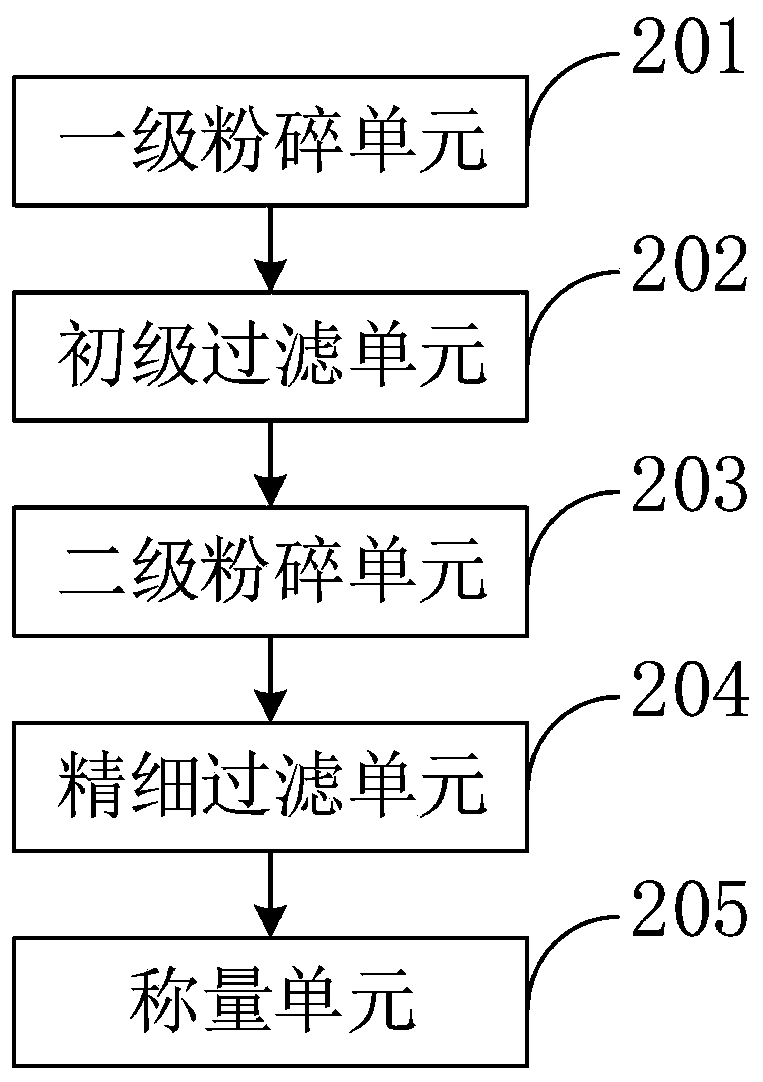
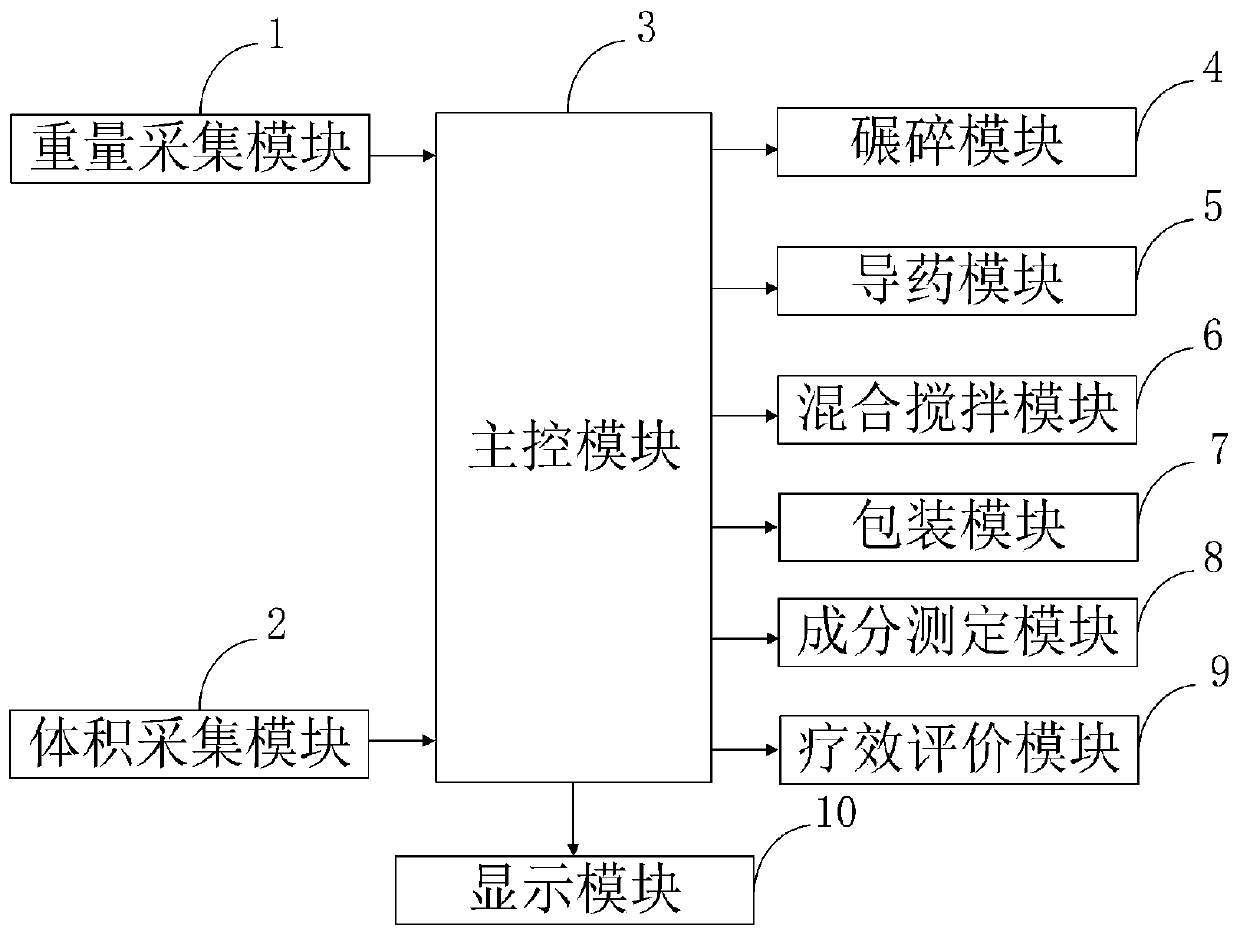
Intelligent hospital pharmaceutical dispensing information processing system and method
InactiveCN111180022AQuick evaluationEasy to detectPhase-affecting property measurementsWeighing apparatus for materials with special property/formInformation processingTherapeutic evaluation
The invention belongs to the technical field of hospital pharmaceutical dispensing, and discloses an intelligent hospital pharmaceutical dispensing information processing system and method, and the method comprises the steps: collecting the weight data of a medicine through a weight collection module by utilizing a weighing device; measuring the volume data of the liquid medicine by using a volumetric device through a volume acquisition module; a main control module performing crushing operation on the solid medicine by using a crushing machine through a crushing module; guiding liquid medicine in through a medicine guide module by utilizing a medicine guide pipe; stirring the mixed medicine through a mixing and stirring module by utilizing a stirrer; packaging the prepared medicines by using a packaging machine through a packaging module; determining medicine components by using determination equipment through a component determination module; evaluating the curative effect of the medicine by utilizing an evaluation program through a curative effect evaluation module; and displaying the collected weight and volume of the medicine, the measured components and the evaluation resultby utilizing a display through a display module. The method can be used for rapidly, simply and accurately detecting the content of the components in the mixed medicine in a lossless manner.
Owner:李东方
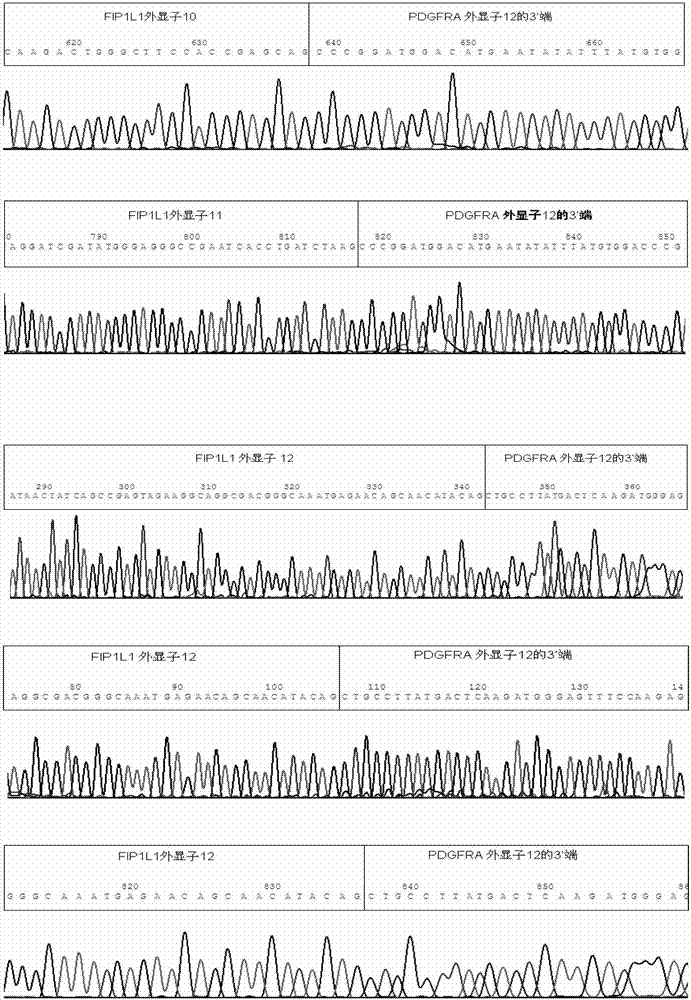
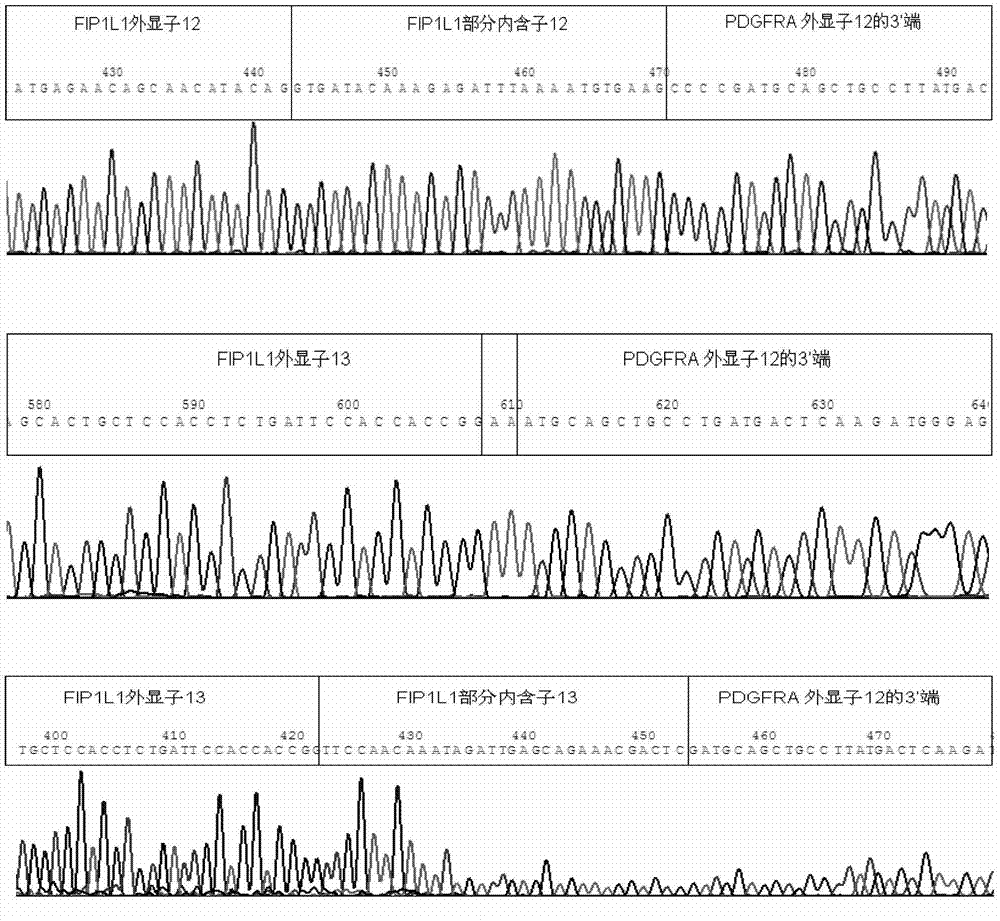
Reagent kid for quantitatively testing mRNA (messenger ribonucleic acid) level of FIP1L1-PDGFRA (feline infectious peritonitis 1 like 1-platelet-derived growth factor receptor alpha) fusion genes
InactiveCN102827935AGuaranteed specific amplificationAccurately reflect tumor burdenMicrobiological testing/measurementFluorescence/phosphorescenceFip1l1 pdgfraPlatelet-Derived Growth Factor Receptor Alpha
The invention discloses a reagent kit for quantitatively testing mRNA (messenger ribonucleic acid) level of FIP1L1-PDGFRA (feline infectious peritonitis 1 like 1-platelet-derived growth factor receptor alpha) fusion genes. The test reagent contains upstream primers I, downstream primers I and TaqMan probes I which are used for real-time quantitative PCR (polymerase chain reaction) testing for mRNA of the FIP1L1-PDGFRA fusion genes. The upstream primers I include at least one of single-chain DNA (deoxyribose nucleic acid) shown as sequences 1, 2, 3, 4 and 5 in a sequence table, the downstream primers I include at least one of two single-chain DNA as shown in sequence 9 and 10 in the sequence table, and the TaqMan probes I include at least one of two single-chain DNA as shown in sequences 9 and 10 in the sequence table. The reagent kit has the advantages of speediness, simplicity, convenience and the like in testing, common types of FIP1L1-PDGFRA fusion genes can be covered in one experiment, and the mRNA level of the FIP1L1-PDGFRA fusion genes can be tested. The reagent kit can be used for FIP1L1-PDGFRA fusion gene screening and therapeutic evaluation for eosinophilia patients, and further can be used for monitoring minimal residual diseases.
Owner:PEOPLES HOSPITAL PEKING UNIV
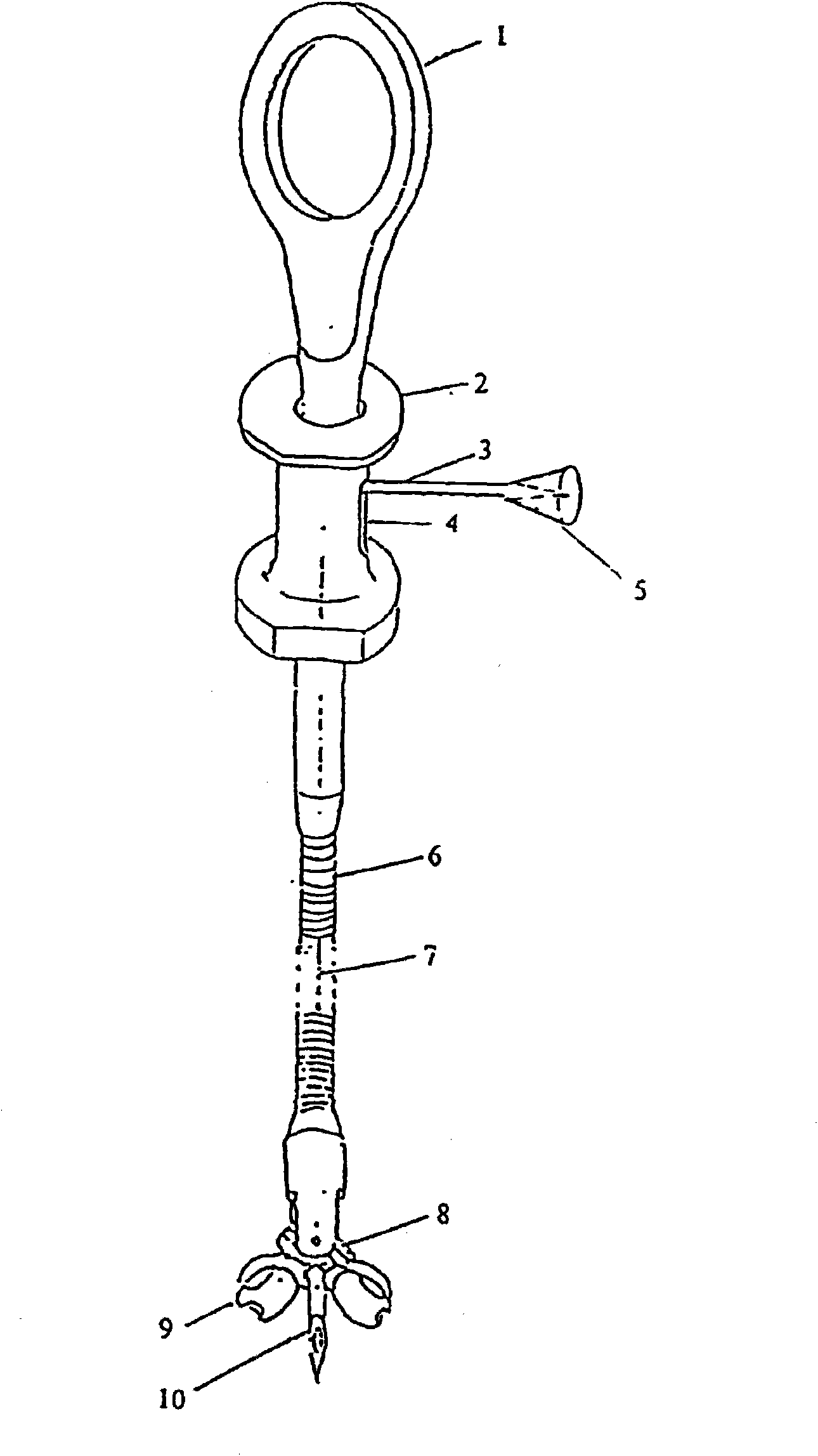
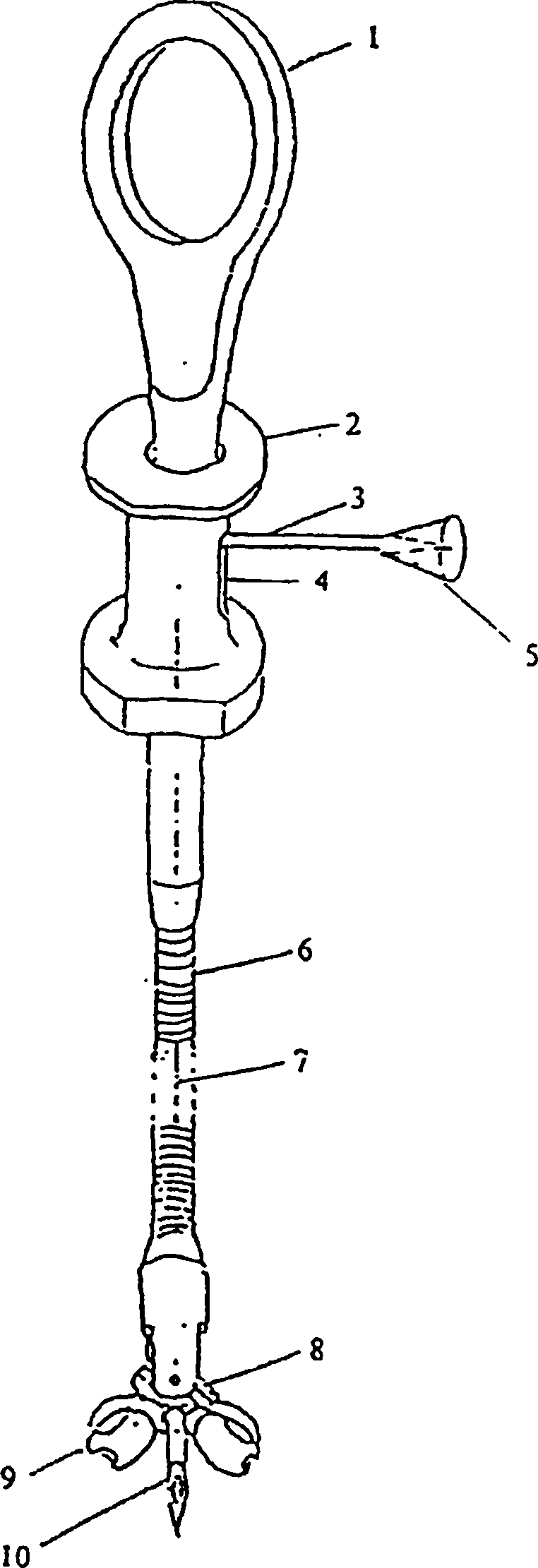
Coelomic mucosa calibrating biopsy forceps
InactiveCN102309346APrevent unmarkedCorrect efficacy evaluationSurgeryVaccination/ovulation diagnosticsTherapeutic evaluationCoelom
The invention relates to a pair of coelomic mucosa calibrating biopsy forceps, which comprise a handle. A sliding groove is arranged in the lateral direction of the handle. A soft tube is connected to one end of the handle. The other end of the soft tube is connected with the biopsy forceps through a biopsy joint. An injection needle is arranged in the middle of the biopsy forceps. The injection needle passes through the biopsy joint and the soft pipe and is connected with a needle pushing rod. An injection interface is communicated with the needle pushing rod. A handlebar is arranged at the other end of the handle. A steel wire on the handlebar is connected with the biopsy joint. The coelomic mucosa calibrating biopsy forceps disclosed by the invention have the benefits that: the coelomic mucosa calibrating biopsy forceps are used for pre-cancerous pathological examination of various coelomic mucosa and long-term follow up and preventing from not calibrating after minute lesion biopsy, therefore, the effective treatment cannot be carried out in the operation because of not judging focus; and the correct therapeutic evaluation can be given on prognosis of pre-cancerous lesion pharmacotherapy.
Owner:陈卫
Targeted-imaging agent used for detecting glioma and preparation method and application of targeted-imaging agent
InactiveCN106344938APrecise boundariesPrecisely defined boundariesRadioactive preparation carriersImaging agentImage detection
The invention relates to the technical field of medical imaging technology, in particular to a targeted-imaging agent used for detecting glioma and preparation method and application of the targeted-imaging agent. The targeted-imaging agent refering to <18>F-fluorobenzoic acid-chlorotoxin has the advantages that boundaries of the glioma can be accurately defined; high sensitivity is achieved due to the fact that the chlorotoxin marked with <18>F can be subjected to imaging detection via PET (positron emission tomography) and focus 6mm in size can be detected with the PET which is much higher than SPECT (single photon emission computed tomography) in image resolution; a brand new direction is provided for diagnosis the glioma, and important clinical value in aspects of early diagnosis of the glioma, boundary define of the glioma, therapeutic evaluation, targeted drug delivery and the like is achieved; the targeted-imaging agent can be applied to the PET, the boundaries of the glioma can be accurately defined, high image resolution can be realized, the focus 6mm in size can be detected, and the PET is much higher than the SPECT in the image resolution in the prior art.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
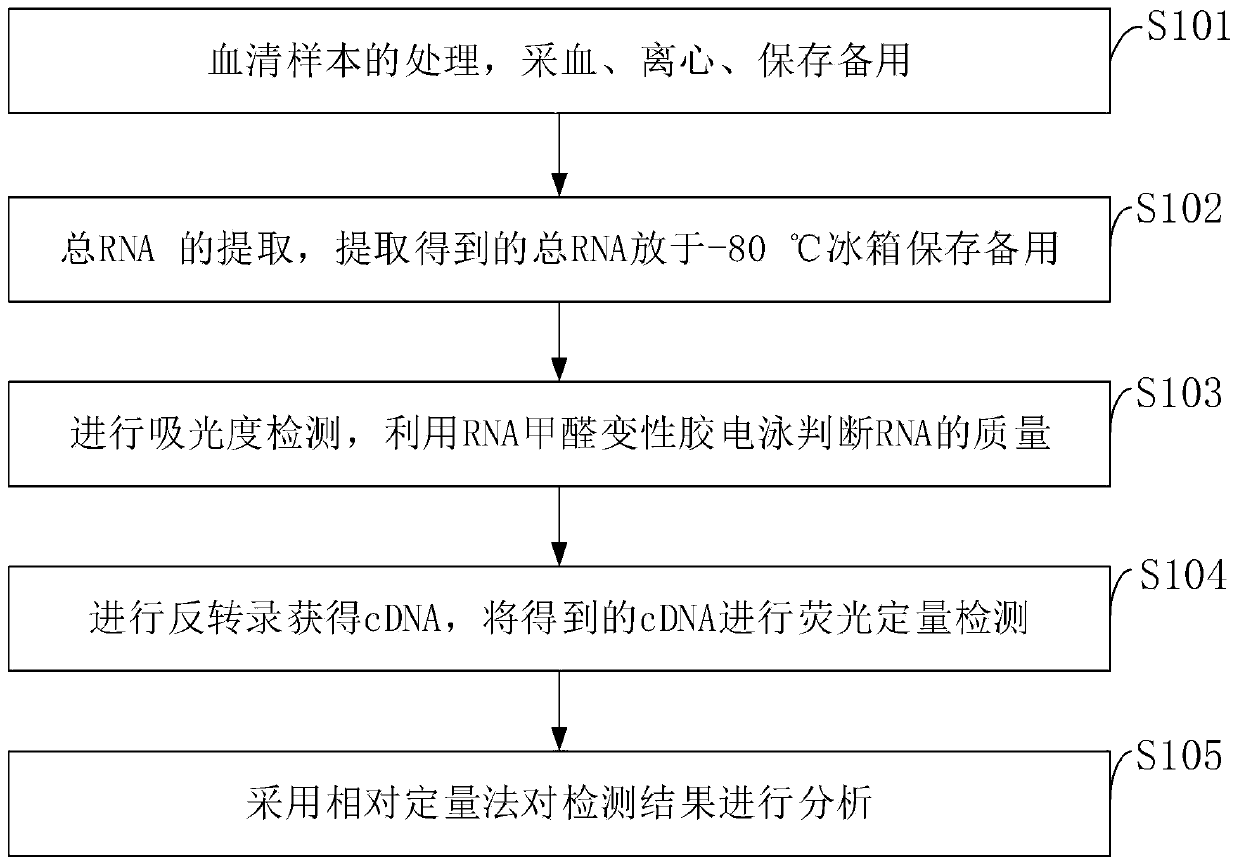
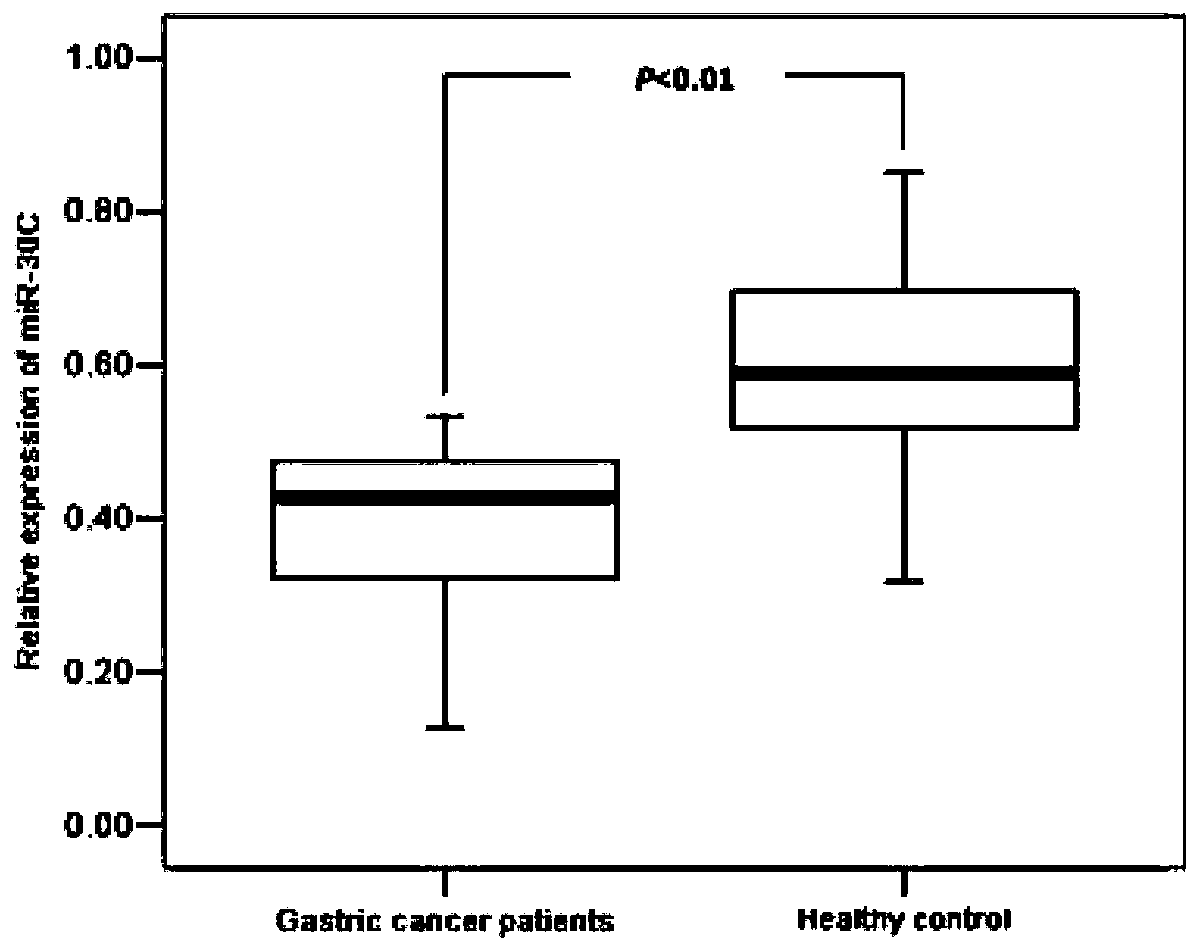
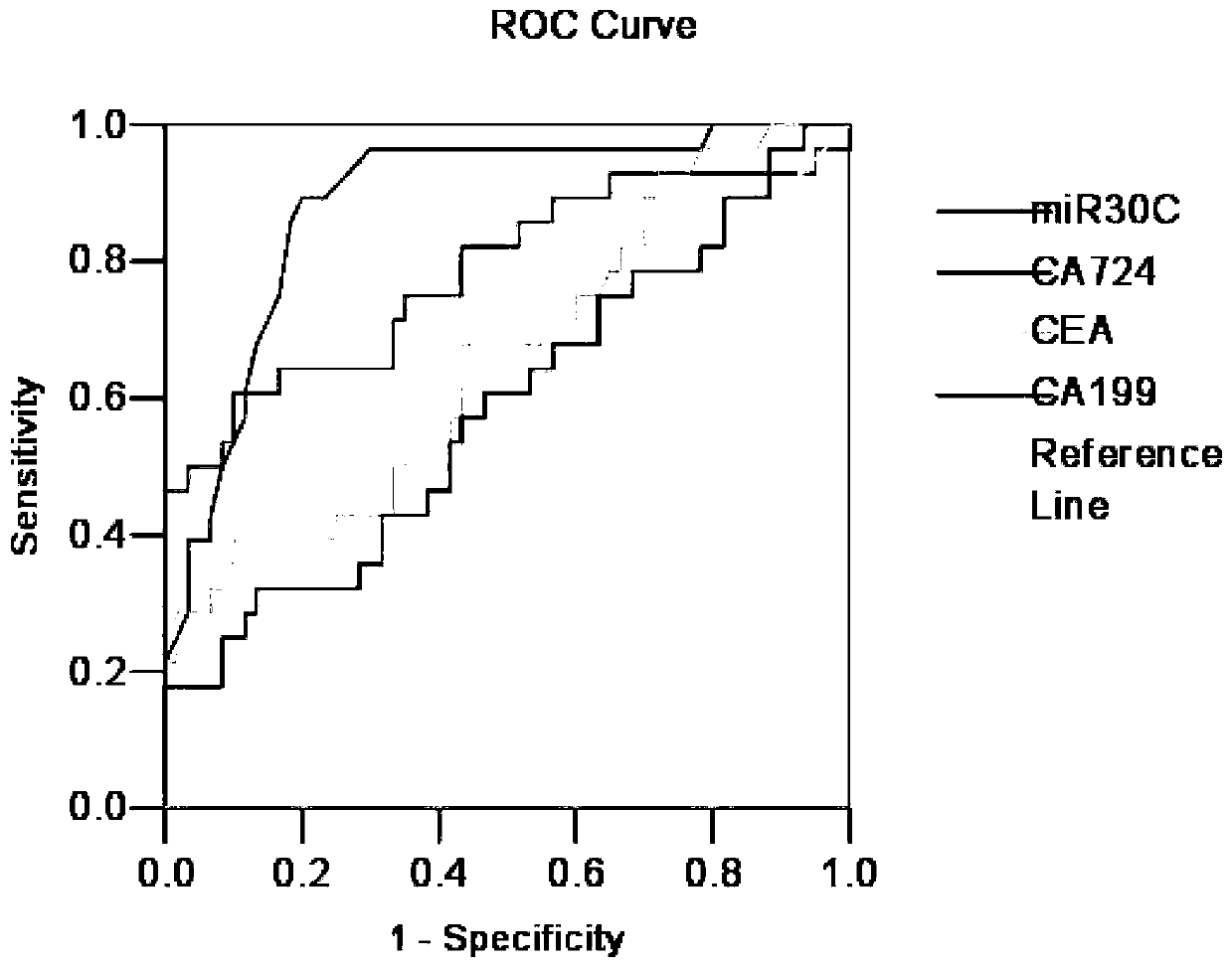
Blood circulation miRNA biomarker assay kit for diagnosis and prognosis evaluation of gastric cancer
PendingCN110229907AGood diagnostic performanceGood application prospectMicrobiological testing/measurementRefrigerated temperatureTumor marker
The invention belongs to the technical field of kits, and discloses a blood circulation miRNA biomarker assay kit for diagnosis and prognosis evaluation of gastric cancer. The assay method with the blood circulation miRNA biomarker assay kit for diagnosis and prognosis evaluation of gastric cancer comprises steps of processing serum samples, blood collection, centrifugation, storage for later use;extracting total RNA, storing the extracted total RNA in a refrigerator at 80 DEG C for later use; conducting absorbance detection, determining the quality of the RNA by RNA formaldehyde degradationgel electrophoresis; obtaining cDNA by reverse transcription; conducting fluorescence quantitative detection for the obtained cDNA; and analyzing the detected results by relative quantitative method.Through evaluation of efficacy in diagnosis compared with traditional tumor markers CEA, CA199 and CA724, the blood circulation miRNA biomarker assay kit of the invention is superior to the traditional tumor markers. The miR-30c is suitable for detection of gastric cancer as a diagnostic marker, and can be developed into a novel tumor detection marker diagnosis kit for diagnosis of gastric cancer.The blood circulation miRNA biomarker assay kit is used for evaluation of curative effect and prognosis estimation, and has a good application prospect.
Owner:内蒙古医科大学附属人民医院
Near-infrared fluorescent small molecular probe and preparation and application thereof
ActiveCN111072584ARealize Visual ImagingOrganic chemistryFluorescence/phosphorescenceMyelin sheathNear infrared imaging
The invention relates to the field of near-infrared imaging, in particular to a near-infrared fluorescent small molecular probe and preparation thereof and application of the near-infrared fluorescentsmall molecular probe in the field of myelin sheath imaging. The structural formula of the small molecular probe is shown in the following figure, and fluorescence performance tests show that the emission wavelength of the small molecular probe is greater than 650nm, and the small molecular probe enters a near-infrared region. Meanwhile, the small molecular probe can be effectively combined witha myelin sheath part, the imaging effect of the myelin sheath part is remarkably enhanced, the small molecular probe can be applied to myelin sheath imaging under the near-infrared condition, and a brand-new method can be provided for diagnosis and curative effect evaluation of central nervous system diseases such as multiple sclerosis.
Owner:NANJING POLYTECHNIC INSITUTE
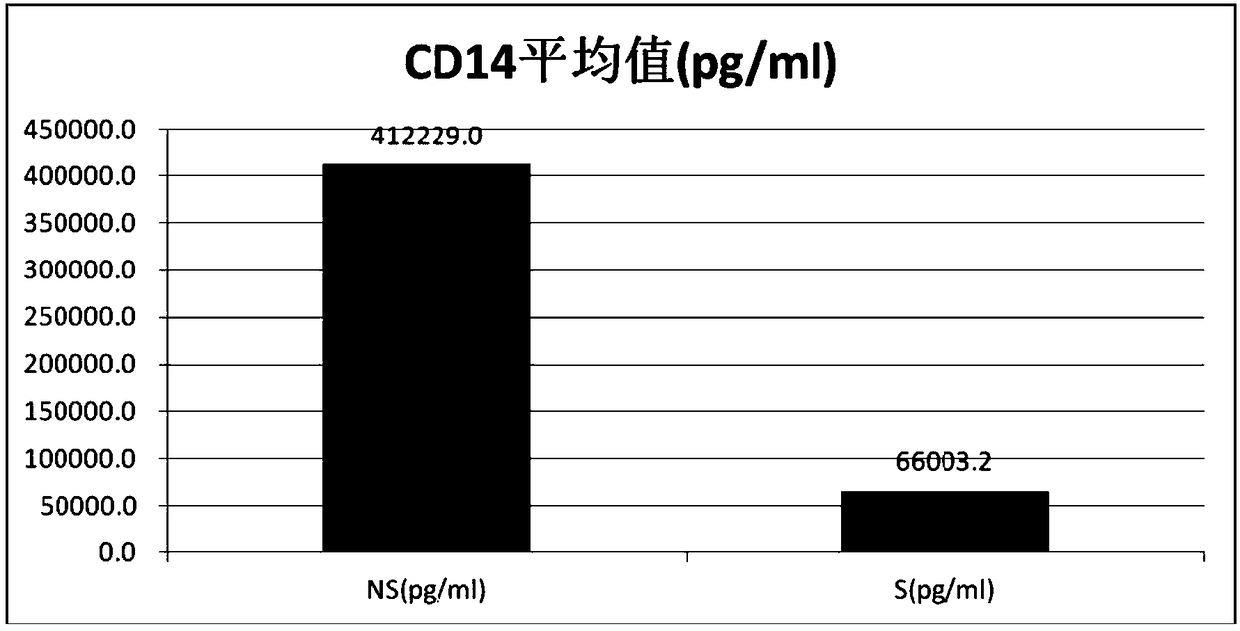
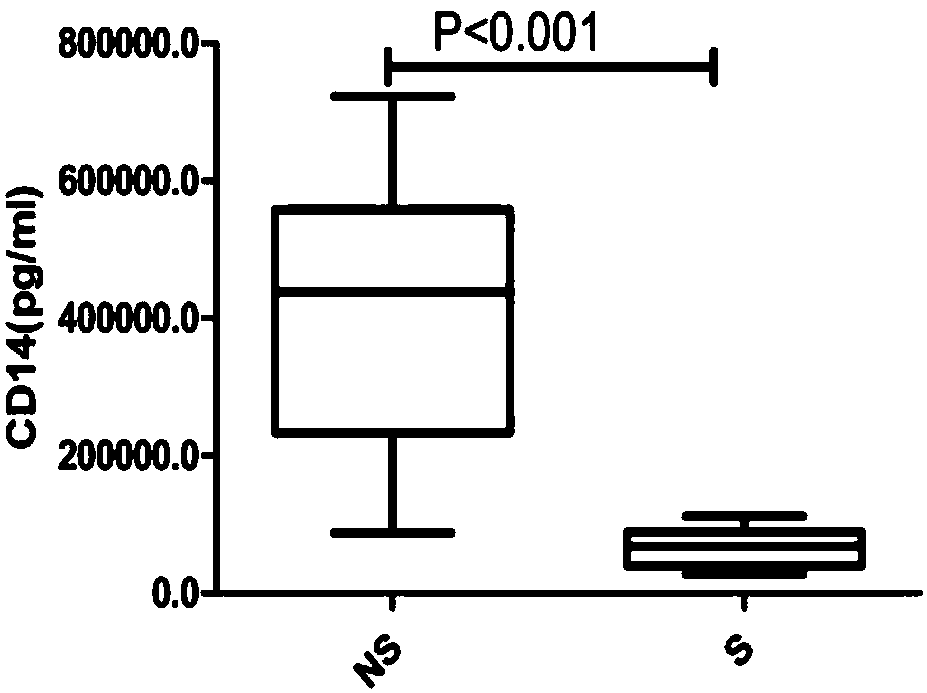
Syphilis detection kit containing CD14 detection reagent and application of reagent in syphilis detection
The invention relates to application of CD14 in syphilis detection, and particularly provides a syphilis detection kit. The kit comprises a detection reagent such as a CD14-resistant antibody for detecting the CD14 level in a biological sample. The invention further provides application of the first detection reagent for detecting CD14 to preparation of the syphilis detection kit. The syphilis detection kit can be used for early-stage diagnosis, parting and staging, therapeutic evaluation and / or prognosis evaluation of syphilis especially neurosyphilis, and has the advantages of being reliable, sensitive, convenient to use and rapid.
Owner:BEIJING DITAN HOSPITAL CAPITAL MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![[<18>F] AlF marked positron emission tomography (PET) polypeptide probe and preparation method thereof [<18>F] AlF marked positron emission tomography (PET) polypeptide probe and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/13785fee-f1d4-4334-9714-3e2e39f3ae96/HDA0000568632230000011.PNG)
![[<18>F] AlF marked positron emission tomography (PET) polypeptide probe and preparation method thereof [<18>F] AlF marked positron emission tomography (PET) polypeptide probe and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/13785fee-f1d4-4334-9714-3e2e39f3ae96/HDA0000568632230000012.PNG)
![[<18>F] AlF marked positron emission tomography (PET) polypeptide probe and preparation method thereof [<18>F] AlF marked positron emission tomography (PET) polypeptide probe and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/13785fee-f1d4-4334-9714-3e2e39f3ae96/HDA0000568632230000021.PNG)