Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "PDGFRA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
PDGFRA, i.e. platelet-derived growth factor receptor A, also termed PDGFRα, i.e. platelet-derived growth factor receptor α, is a receptor located on the surface of a wide range of cell types. This receptor binds to certain isoforms of platelet-derived growth factors (PDGFs) and thereby becomes active in stimulating cell signaling pathways that elicit responses such as cellular growth and differentiation. The receptor is critical for the development of certain tissues and organs during embryogenesis and for the maintenance of these tissues and organs, particularly hematologic tissues, throughout life. Mutations in the gene which codes for PDGFRA, i.e. the PDGFRA gene, are associated with an array of clinically significant neoplasms.
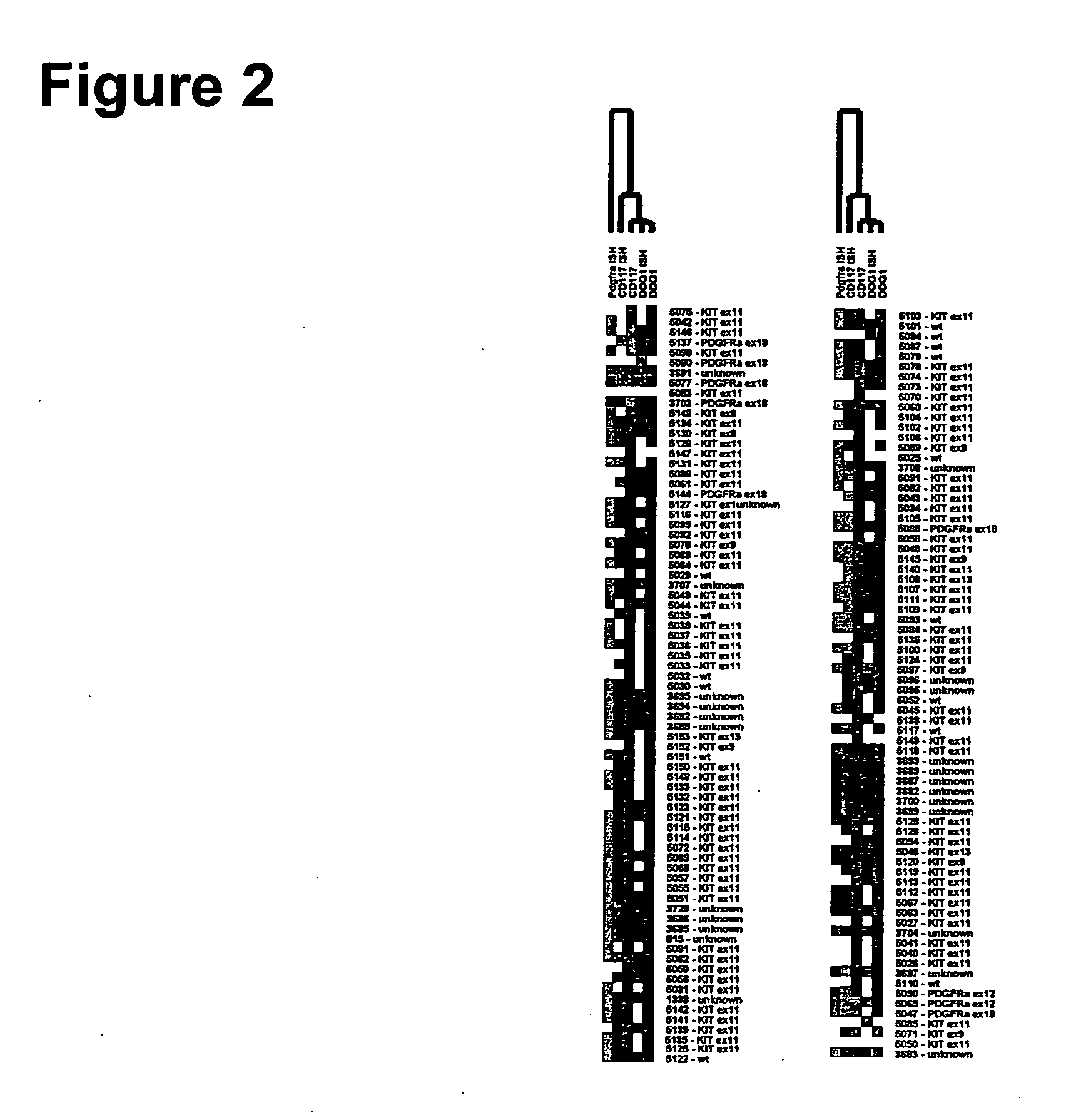
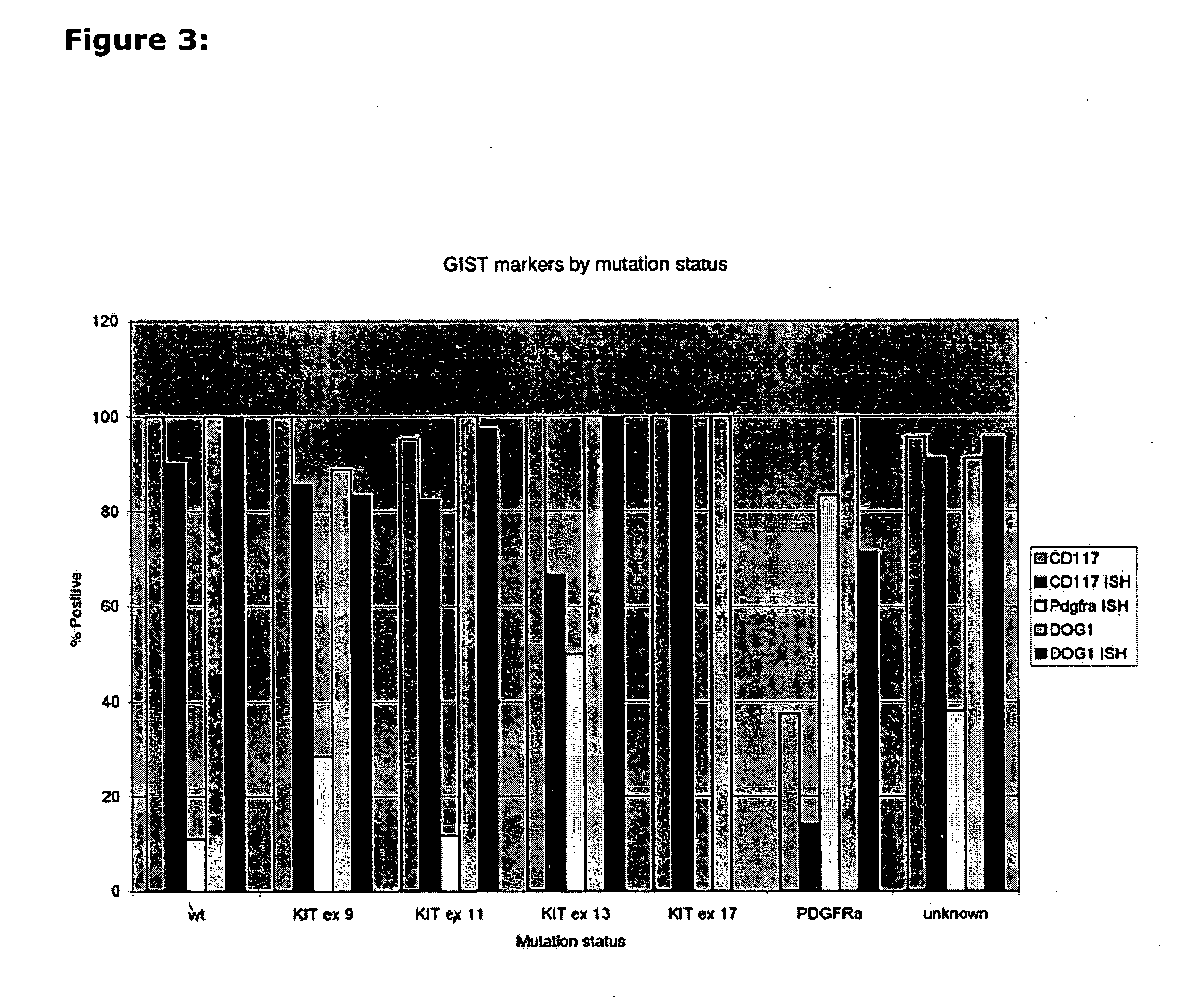
Methods and compositions for diagnosing gastrointestinal stromal tumors
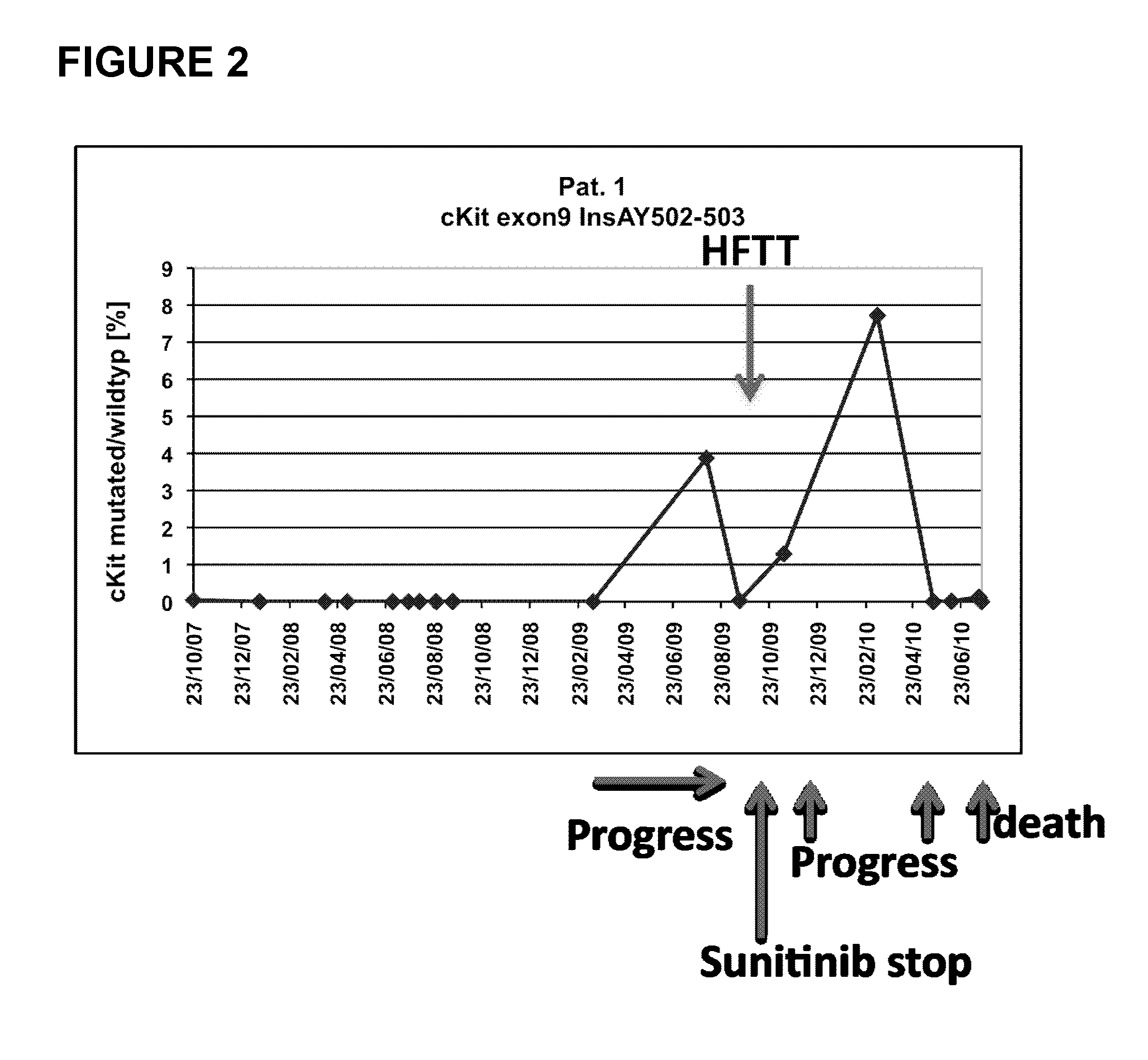
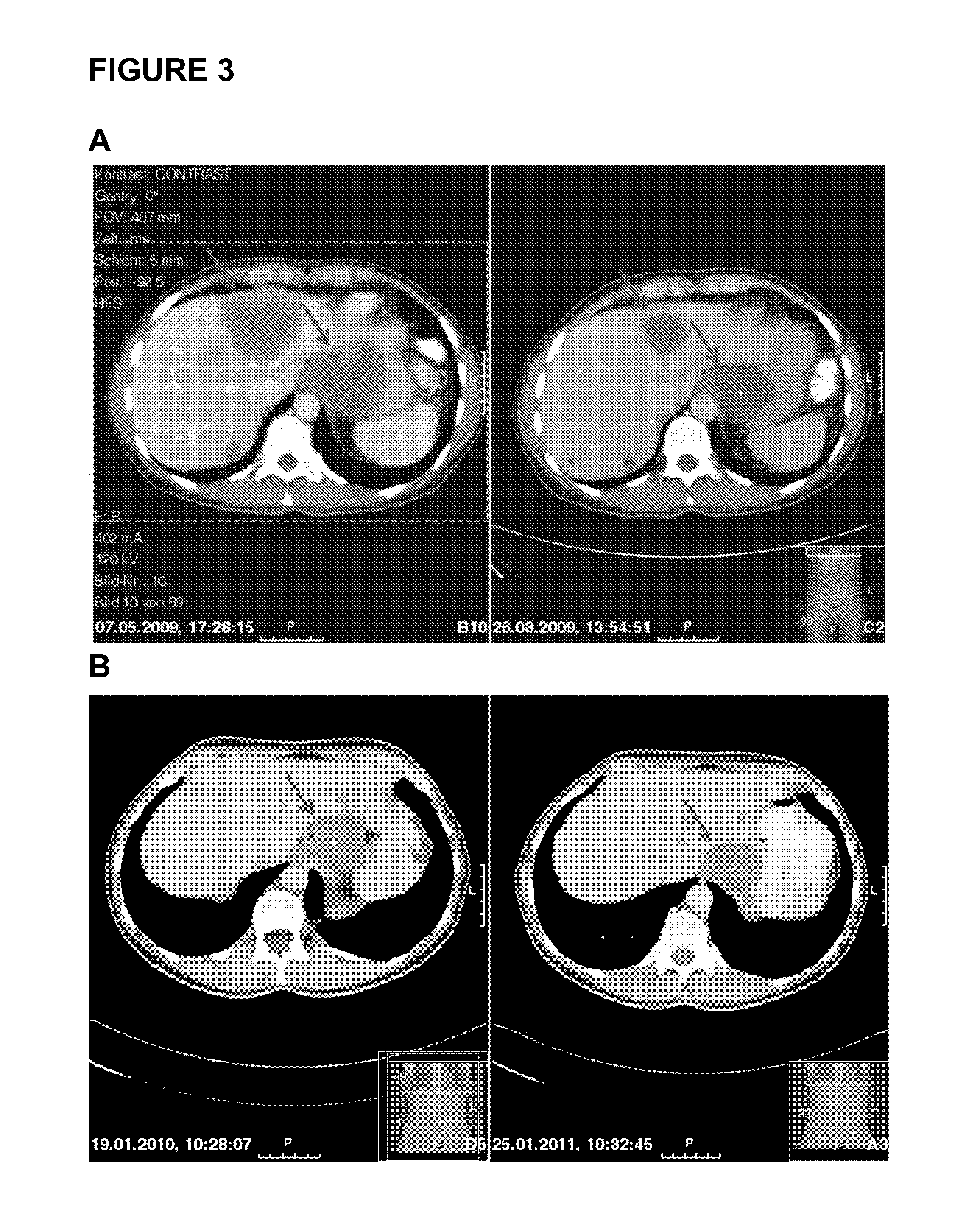
The present invention relates to an in vitro method for diagnosing and / or monitoring in a subject a gastrointestinal stromal tumor or a predisposition to develop a gastrointestinal stromal tumor, comprising detecting and / or analyzing in a test sample derived from the subject one or more mutations at the DNA level in any one or both of the marker genes cKIT (GenBank acc. no. NM_000222.2) and PDGFRA (GenBank acc. no. NM_006206.4), wherein the DNA is circulating DNA, and wherein the presence of any one of the mutations detected in the test sample is indicative of a gastrointestinal stromal tumor or a predisposition to develop a gastrointestinal stromal tumor in the subject. The present invention is also directed to a corresponding kit-of-parts for diagnosing and / or monitoring a gastrointestinal stromal tumor or a predisposition to develop a gastrointestinal stromal tumor, comprising means for detecting and / or analyzing one or more mutations as defined herein, as well as to the use of one or more mutations as defined herein as a panel of molecular markers for diagnosing and / or monitoring a gastrointestinal stromal tumor or a predisposition to develop a gastrointestinal stromal tumor.
Owner:ALBERT LUDWIGS UNIV FREIBURG
Markers identified for liver fibrosis and cirrhosis and the microarray panel thereof
InactiveUS20080161203A1Cure and slow down liver fibrosis progressionSlow curingPeptide librariesHydrolasesCFHR5Biology
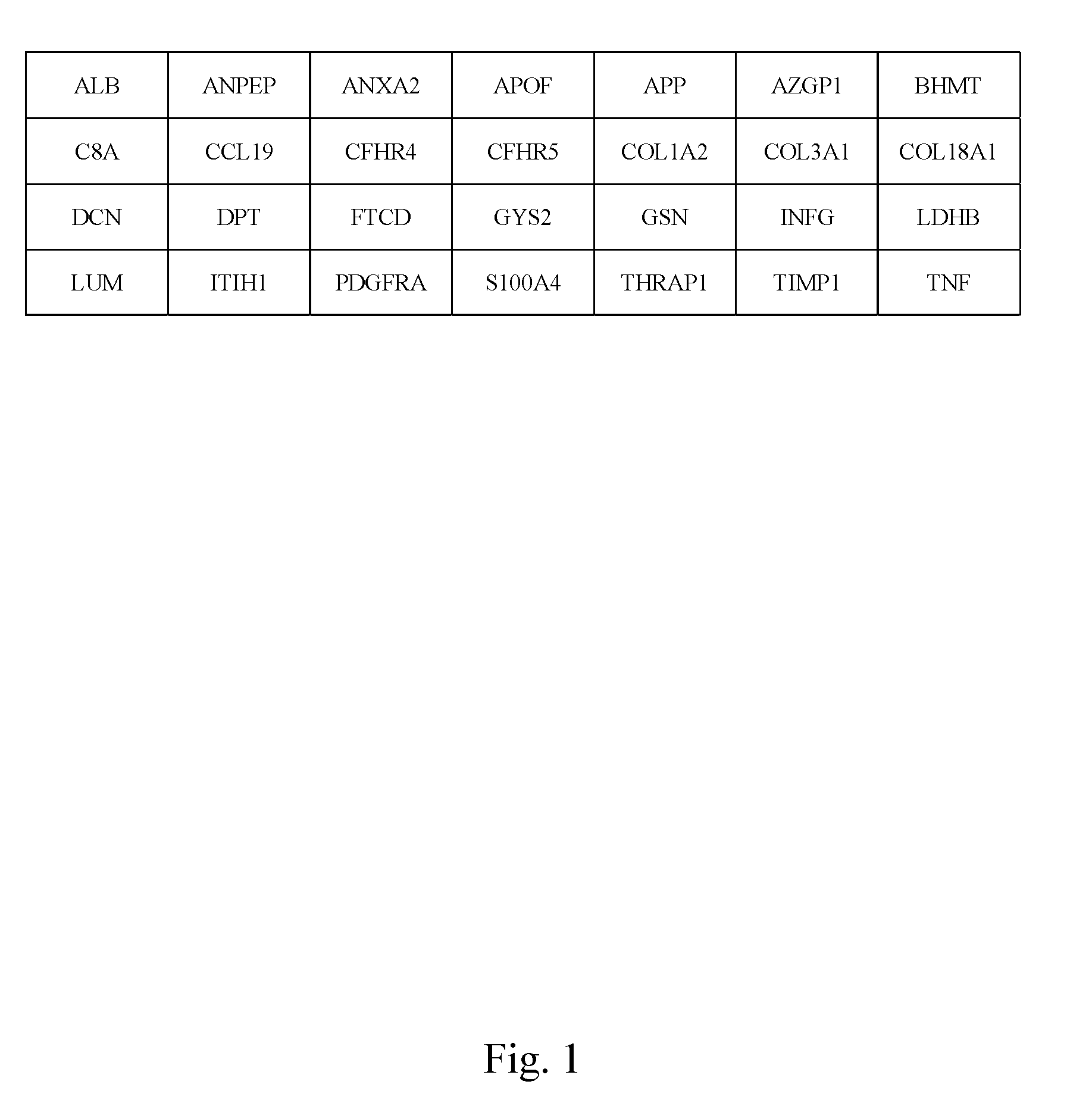
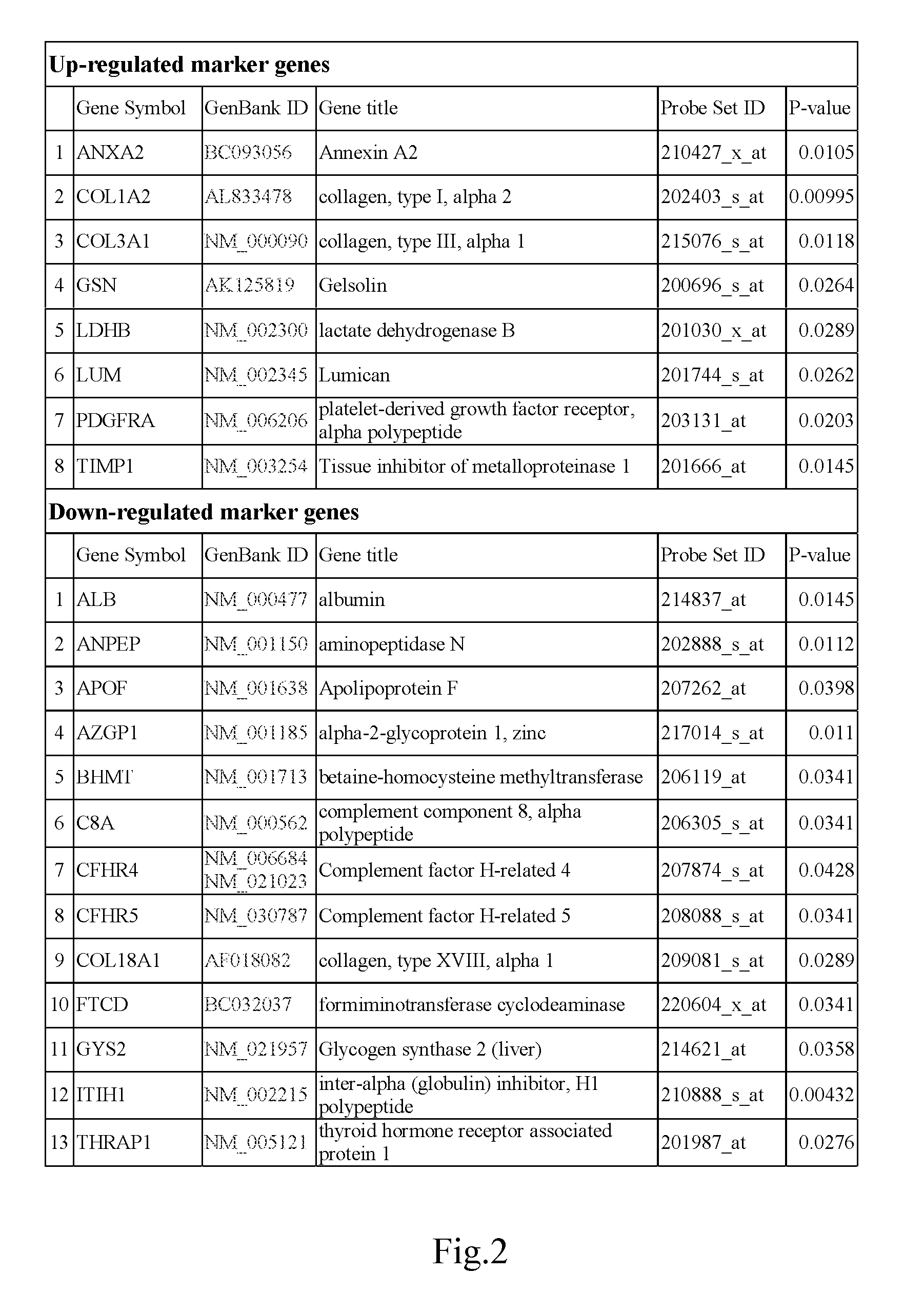
In the present invention, the markers identified for liver fibrosis and cirrhosis and the microarray panel thereof comprise at least one of the following proteins / genes:8 up-regulated genes such asANXA2; COL1A2; COL3A1; GSN; LDHB; LUM; PDGFRA and TIMP1.13 down-regulated genes such asALB; ANPEP; APOF; AZGP1; BHMT C8A; CFHR4; CFHR5; COL18A1; FTCD; GYS2; ITIH1, and THRAP1.9 therapeutic targets such as PDGFRA; S100A4; COL3A1; CCL19; DCN; DPT; APP; TNF; and INFG.The present invention is capable of screening markers for the early warning of the occurrence for severe fibrosis or cirrhosis and potentially targets for drug design.
Owner:VITA GENOMICS
Methods and compositions for the diagnosis for early hepatocellular carcinoma
ActiveUS20080038736A1Microbiological testing/measurementBiological testingCyclin D1Early Hepatocellular Carcinoma
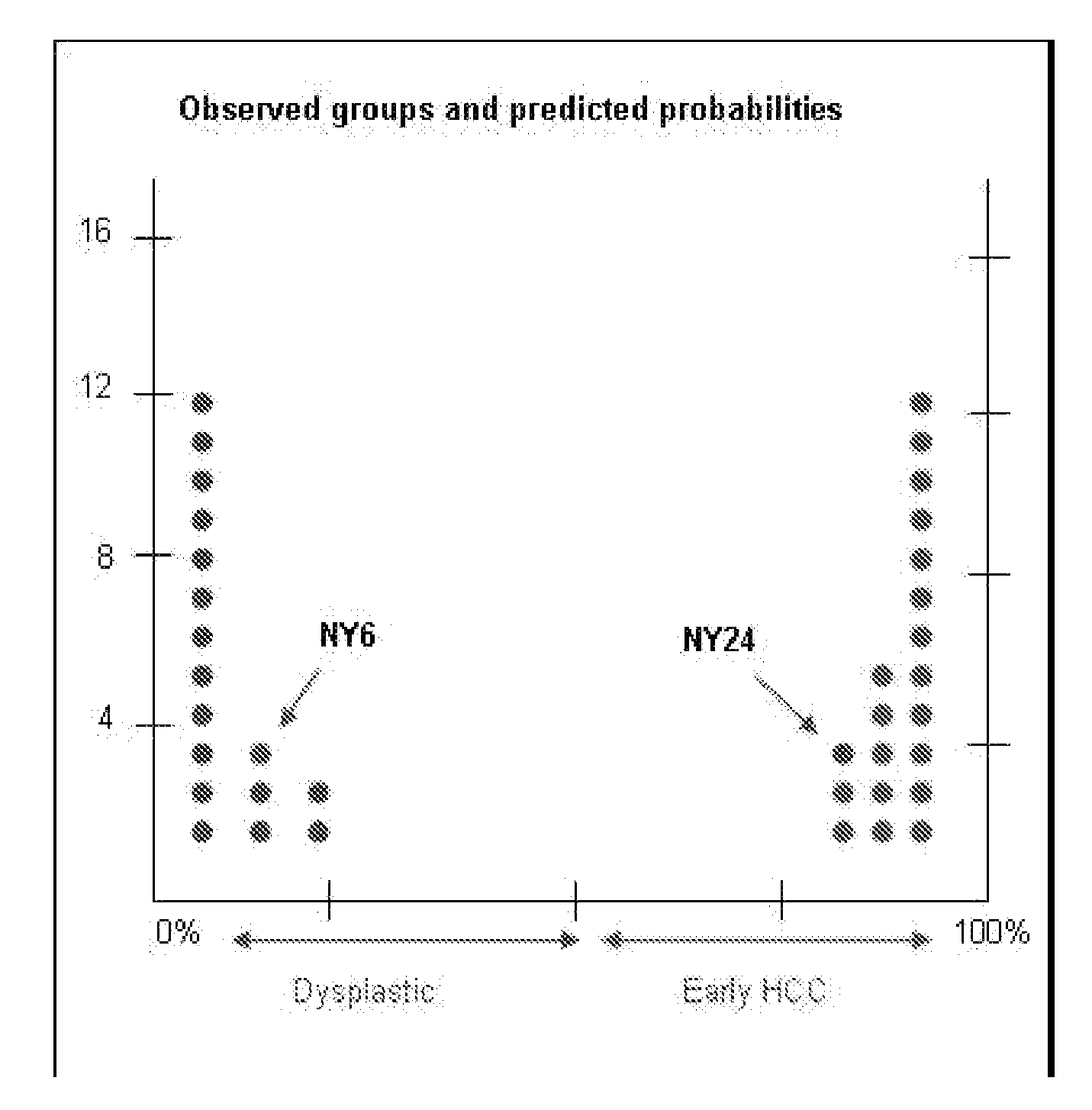
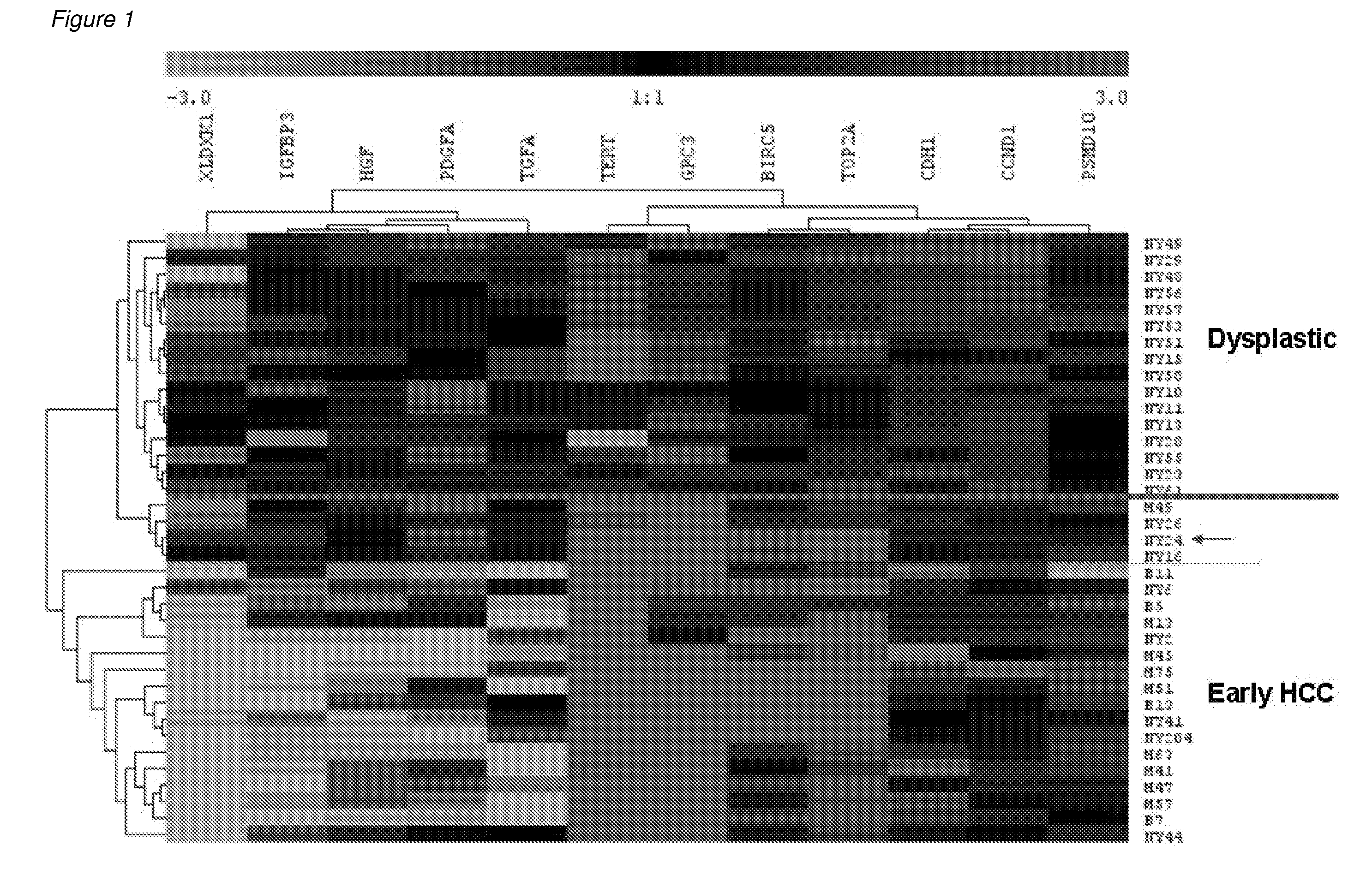
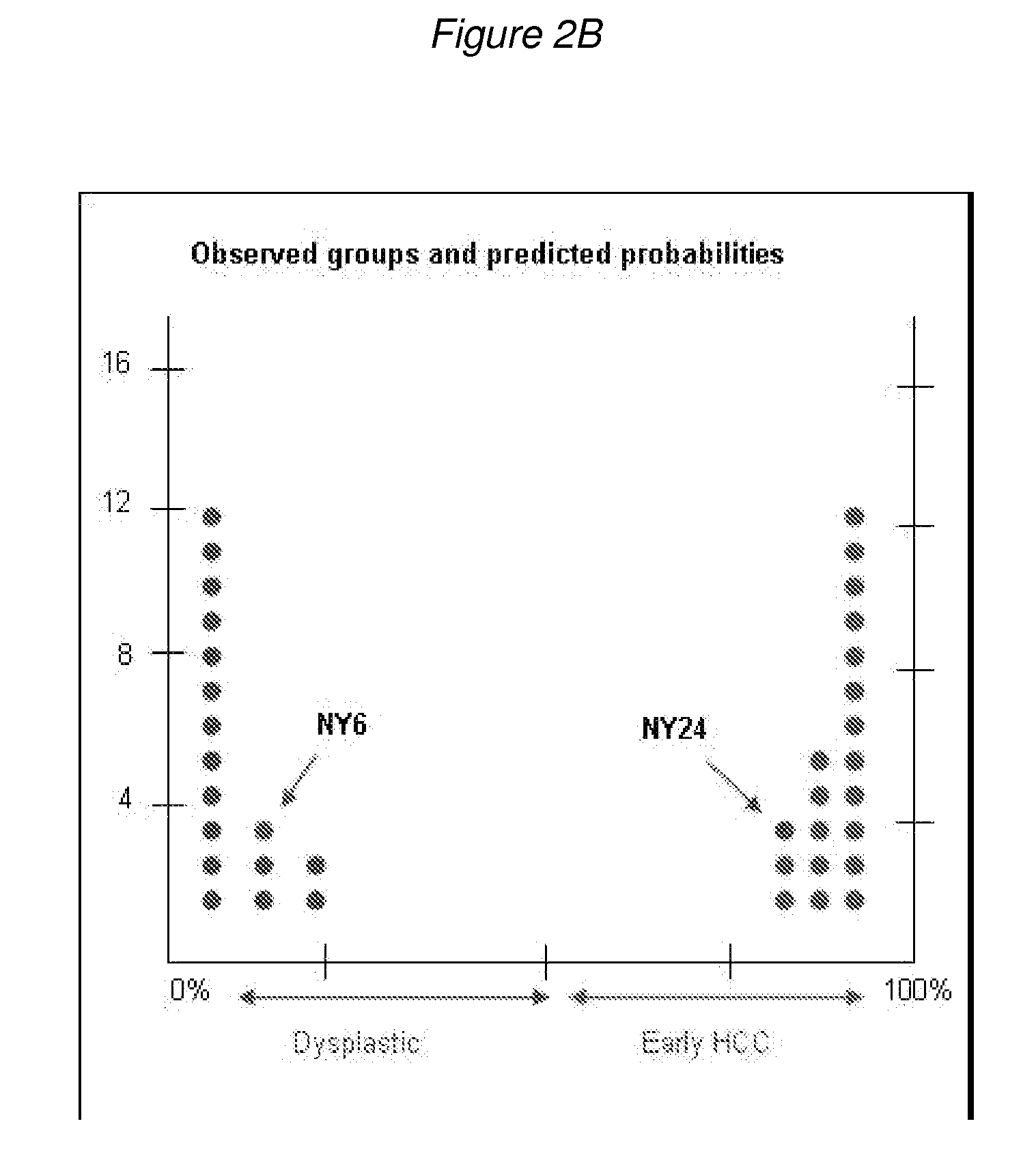
Methods and compositions are provide to allow discrimination of dysplastic nodules from early HCC nodules. More specifically, it has been determined that TERT, GPC3, gankyrin, survivin, TOP2A, LYVE1, Ecadherin, IGFBP3, PDGFRA, TGFA, cyclin D1 and HGF are differentially expressed in HCC as compared to normal liver cells and liver cells that have dysplastic, non-cancerous nodules.
Owner:MT SINAI SCHOOL OF MEDICINE
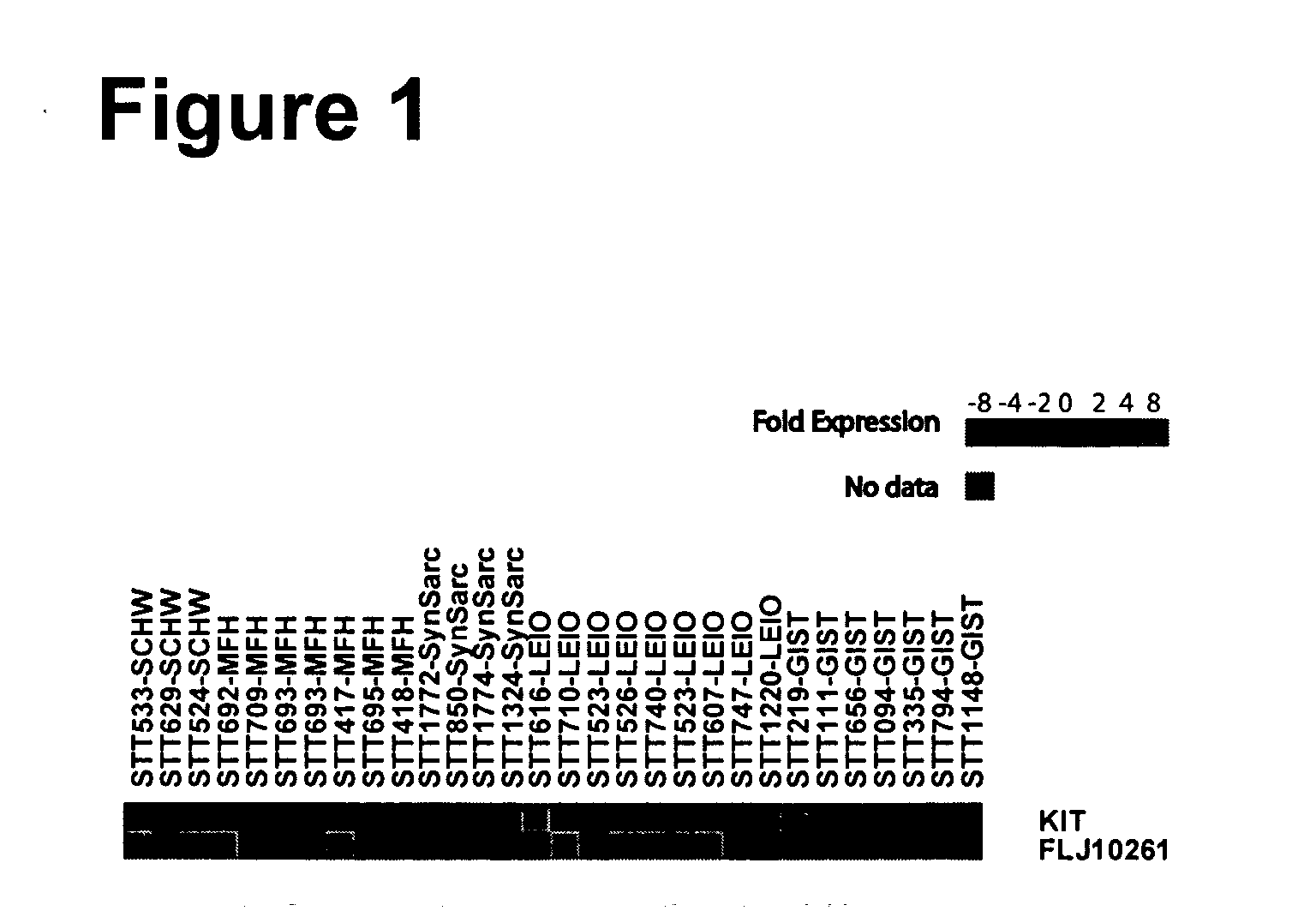
Glioma molecule typed gene group and application thereof
ActiveCN104178556AAccurately judge the prognostic survival timeOvercome limitationsMicrobiological testing/measurementMorphologic diagnosisWilms' tumor
Provided in the present invention is a set of gene groups for predicting or for the auxiliary prediction of the survival time prognosis of patients with neuroglioma, consisting of a PM gene group and an EM gene group with a total of 68 genes. A subtype marking gene group consisting of two major gene groups co-expressed with PDGFRA and EGFR can stably divide neuroglioma samples derived from different database sources into three specific subtypes, and can be applied to clinical diagnosis for neuroglioma and judging the survival time prognosis of patients with neuroglioma.
Owner:北京金岱生物科技有限公司
Serum protein marker for early screening and diagnosis of esophageal squamous carcinoma, kit and detection method
ActiveCN110716044AGuaranteed specificityGood reference valueColor/spectral properties measurementsOncologyBiomedicine
The invention discloses a serum protein marker for early screening and diagnosis of esophageal squamous carcinoma, and belongs to the field of biomedical technology. The serum protein marker is any one or a combination of two or more of proteins encoded by P53, GNA11, GNAS, PTEN, ACVR1B, FBXW7, EGFR, PDGFRA, SRSF2, MEN1, DAXX or CASP8 genes. Based on the role played by cancer driving genes in tumorigenesis and development, a human protein chip encoded by 138 cancer driving genes is customized, the human protein chip contains 180 human-derived recombinant proteins for screening out potential markers capable of diagnosing caners or other markers capable of characterizing cancers, early detection serum markers of the esophageal squamous carcinoma are primarily screened out by the protein chip, and then are verified by an ELISA indirect method experiment, a group of joint serum protein markers of esophageal squamous carcinoma for the early screening and diagnosis of the esophageal squamouscarcinoma is screened out at last for assisting the clinical diagnosis of the esophageal squamous carcinoma, so that the serum protein marker has better reference value.
Owner:ZHENGZHOU UNIV
Biomarker combination for molecular typing and/or prognosis prediction of muscle-invasive bladder cancer and application of biomarker combination
PendingCN109797221AMeet analysis needsEffectively assess the risk of adverse prognosisMicrobiological testing/measurementHybridisationGAS6Screening method
The invention relates to a biomarker combination for molecular typing and / or prognosis prediction of the muscle-invasive bladder cancer, and a screening method and application thereof. The biomarker combination comprises the following genes: FGF10, TP53INP1, DDR2, MYC, CDC73, IGF1, PLA2G1B, SKI, FN1, EGFR, PPARG, PDGFRA, PDGFD, GAS6, PDGFC, FNTB and CCNB1. Through non-negative matrix factorizationclustering analysis based on transcription data of the biomarker combination, the muscle-invasive bladder cancer can be classified to respectively correspond to different expression characteristic spectrums. The classification corresponds to significantly different overall survival statuses, thus being used for survival prognosis assessment. The transcription data analysis method adopted has theadvantages that the number of biomarker combinations is small, the analysis steps are simple, the requirements of large sample analysis are met, the requirements for the calculation ability are low, and the method is applicable to standardized transcription data; the transcription data can be a transcriptomic data sub set or a set of genetic transcription data for individual detection.
Owner:SHANGHAI TENTH PEOPLES HOSPITAL
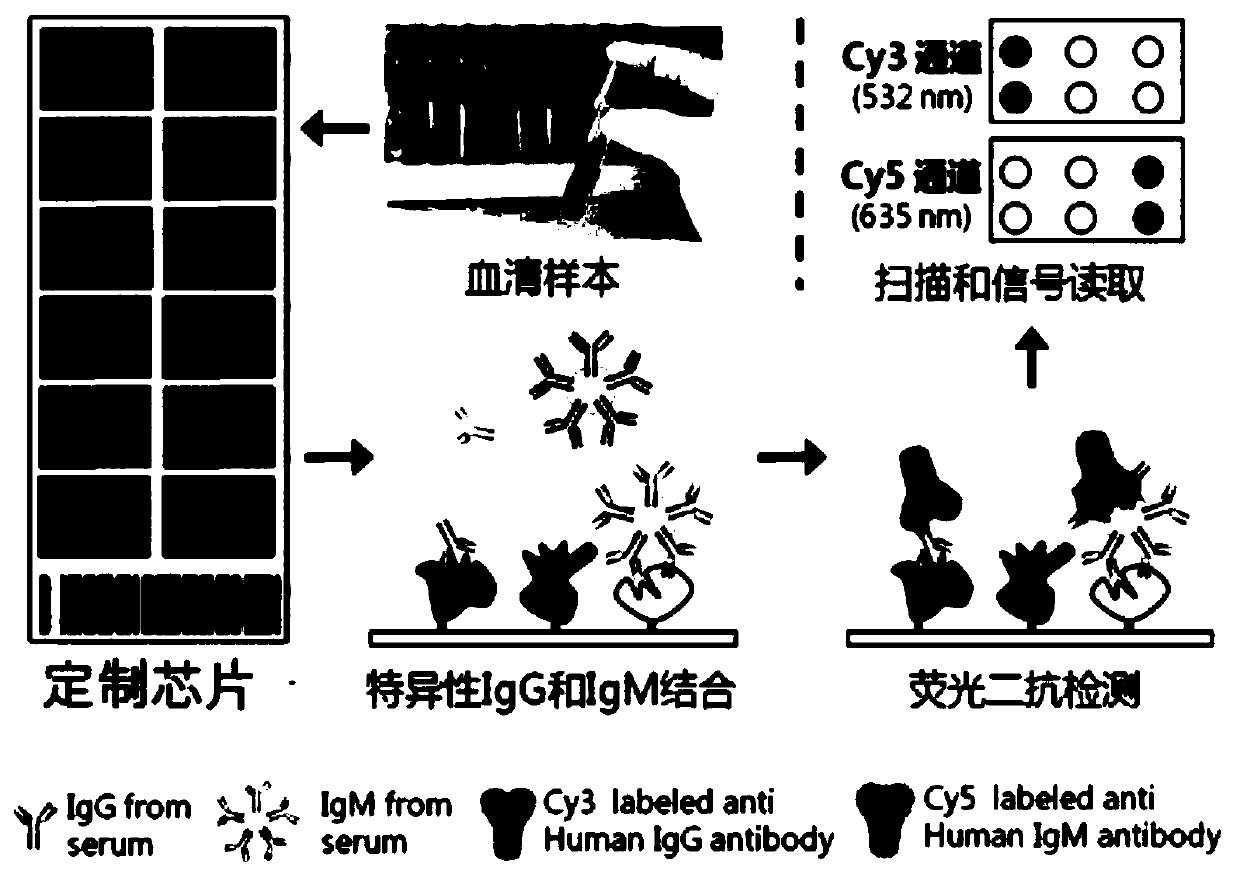
Topical administration of therapeutic agents and oligonucleotide formulations
Aspects of the invention relate to topical and ocular formulations of spherical nucleic acids (SNA), as well as methods of use thereof and compositions thereof. The formulations may include an inhibitor such as an inhibitor of tumour necrosis factor alpha (TNFa), platelet-derived growth factor subunit A (PDGFA), platelet-derived growth factor subunit B (PDGFB), platelet-derived growth factor subunit C (PDGFC), platelet-derived growth factor subunit D (PDGFD), platelet-derived growth factor receptor alpha (PDGFRA), platelet-derived growth factor receptor beta (PDGFRB), platelet-derived growth factor receptor like (PDGFRL), vascular endothelial growth factor A (VEGFA), vascular endothelial growth factor B (VEGFB), vascular endothelial growth factor C (VEGFC) vascular endothelial growth factor D (VEGFD), vascular endothelial growth factor receptor-1 (VEGFR1), vascular endothelial growth factor receptor-2 (VEGFR2), vascular endothelial growth factor receptor-3 (VEGFR3), beta-2 adrenergic receptor (ADRB2), connective tissue growth factor (CTGF), interleukin 1 beta (IL1 β), interleukin 1 receptor-1 (IL1 R1), interleukin 1 receptor-2 (IL1R2), and interleukin 1 receptor-3 (IL1R3). Aspects of the invention further relate to nanostructures comprising self-assembling therapeutic oligonucleotides, such as antisense oligonucleotides, that are linked to a molecular species, wherein the molecular species is positioned in a core of the nanostructure and the oligonucleotides extend radially from the core.
Owner:EXICURE INC
Construction method and applications of tumor gene variation library for high-throughput sequencing detection
InactiveCN107312822ALow costWith amplificationMicrobiological testing/measurementLibrary creationPDGFRASomatic cell
The present invention discloses a construction method of a tumor gene variation library for high-throughput sequencing detection, wherein the tumor gene variation library covers 389 somatic cell mutations on human genes such as EGFR, K-Ras, B-raf, PIK3CA, N-ras, c-MET, AKT1, HER2, c-KIT, PDGFRA, ALK-EML4, ROS1 and RET. According to the present invention, the plurality of the target sequences are subjected to single-tube amplification to rapidly complete the construction of the library, wherein the construction time of the whole library is only 2-3 h, and the manual time is only 15-30 min; and by combining the obtained library and the high-throughput sequencing platform, the difficult problem that the multi-gene and multi-target detection of the somatic cells is required on the basis of the small amount of the clinical samples in tumors, genetic diseases and other diseases in the clinic can be effectively solved, and the cost is low.
Owner:XIAMEN SPACEGEN BIOTECH CO LTD
Tumor markers and uses thereof
InactiveUS20060040292A1Useful for ClassificationMicrobiological testing/measurementMaterial analysisStromal tumorAbnormal tissue growth
The present invention relates to three gene markers that are useful in classifying tumors. The markers are known herein as DOG1 (GenBank GeneID 55107), KIT (GenBank GeneID 3815) and PDGFRA (GenBank GeneID 5156) and can be used alone or in combination to identify subclasses of tumors. The classification methods are exemplified herein using gastrointestinal stromal tumors (GISTs). It is to be understood and will be appreciated that the same markers may be used in the classification of tumors other than GISTs. The methods may further comprise providing diagnostic, prognostic, or predictive information based on the classifying step. Classifying may include stratifying the tumor (and thus stratifying a subject having the tumor), e.g., for a clinical trial. The methods may further comprise selecting a treatment based on the classifying step.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Multi-target kinase inhibitor, pharmaceutical composition and preparation method and application of multi-target kinase inhibitor
ActiveCN109776432AStrong inhibitory activityGood antitumor effectOrganic active ingredientsPowder deliveryDiseaseTyrosine-kinase inhibitor
The invention belongs to the technical field of biological medicines, in particular relates to a multi-target kinase inhibitor and a pharmaceutical composition containing the multi-target kinase inhibitor, and also relates to a preparation method and application of the multi-target kinase inhibitor. The structural general formula of the multi-target kinase inhibitor provided by the invention is shown in a formula (I), wherein the structure of R is selected from a formula (a), a formula (b), a formula (c), a formula (d), a formula (f) and the formula (d); the multi-target kinase inhibitor can effectively inhibit the enzymatic activity of RET, VEGFR3 and PDGFRA, and can effectively treat diseases which are regulated and controlled by multi-target kinase and are related to the abnormal signaltransduction pathway of the multi-target kinase, such as cancers of breast, respiratory tract, brain, reproductive organ, digestive tract, urinary tract, eye, liver, skin, head and / or neck, as well as distal metastatic cancers, such as lymphoma, sarcoma, leukemia and the like. The active ingredient of the pharmaceutical composition comprises the multi-target kinase inhibitor, and the weight percentage of the multi-target kinase inhibitor in the composition is 1-50%. (See the instruction for specific formulas.).
Owner:GUANGZHOU LIUSHUN BIO TEC CO LTD +1
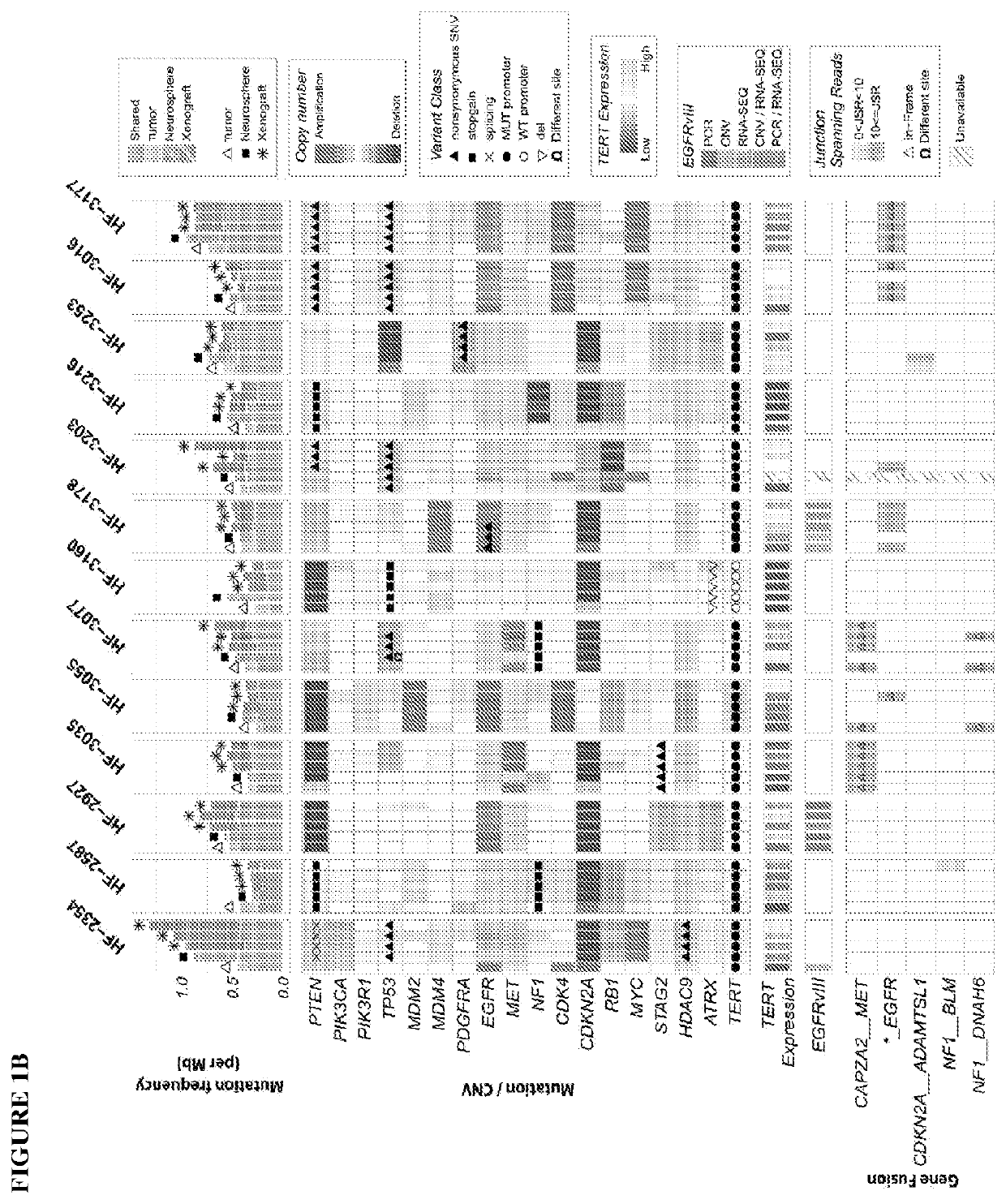
A method of targeting patient-specific oncogenes in extrachromosomal DNA to treat glioblastoma
PendingUS20190360029A1Inhibits tumor glioma growthCompounds screening/testingMicrobiological testing/measurementBAP1Present method
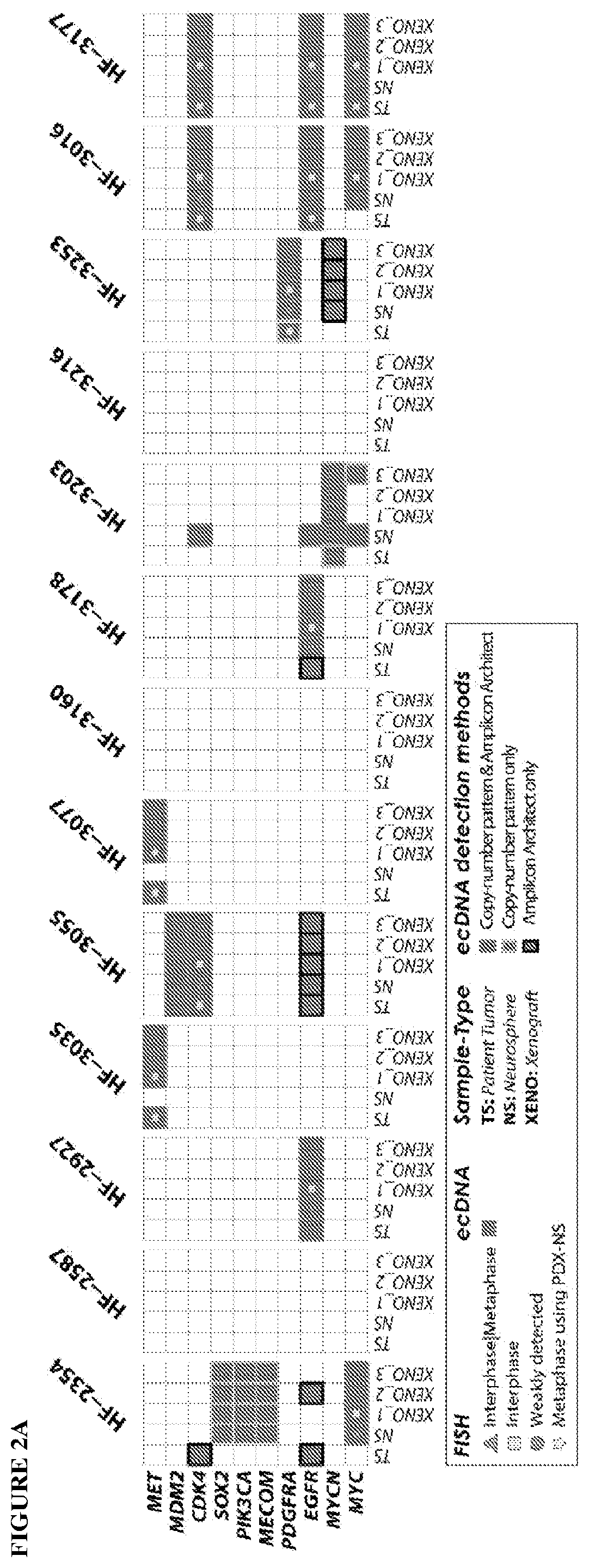
Provided are methods of targeting patient-specific oncogenes in extrachromosomal DNA (ecDNA) to treat glioma in a human. The present methods include identifying a drug that targets against an oncogene present in ecDNA of a human suffering from glioma, such as glioblastoma. The identified oncogenes present in ecDNA include MET, MET / CAPZA2, MDM2, CDK4, SOX2, PIK3CA, MECOM, PDGFRA, EGFR, MYCN, MYC, TERT, SMARCA4, RP56, FBXW7, CDK6, CCND2, ERBB2, BRCA1, and BAP1. The present methods include identifying a drug targeted against the ecDNA oncogene, which drug inhibits the function of the identified oncogene, so as to inhibit tumor growth or progression of the glioma in the human. Also provided are PDX mouse models to further identify and / or confirm patient-specific drugs that target the identified oncogene(s) present in ecDNA. Also provided are methods of diagnosing gliomas or recurrent gliomas and methods of screening or monitoring for recurrence of gliomas. Further provided are methods of validating a predicted presence of ecDNA in a brain tumor using fluorescence in situ hybridization (FISH). Also provided are methods of screening drug candidates for a patient by implanting different identified drugs that target an identified oncogene into PDX mouse models.
Owner:HENRY FORD HEALTH SYST
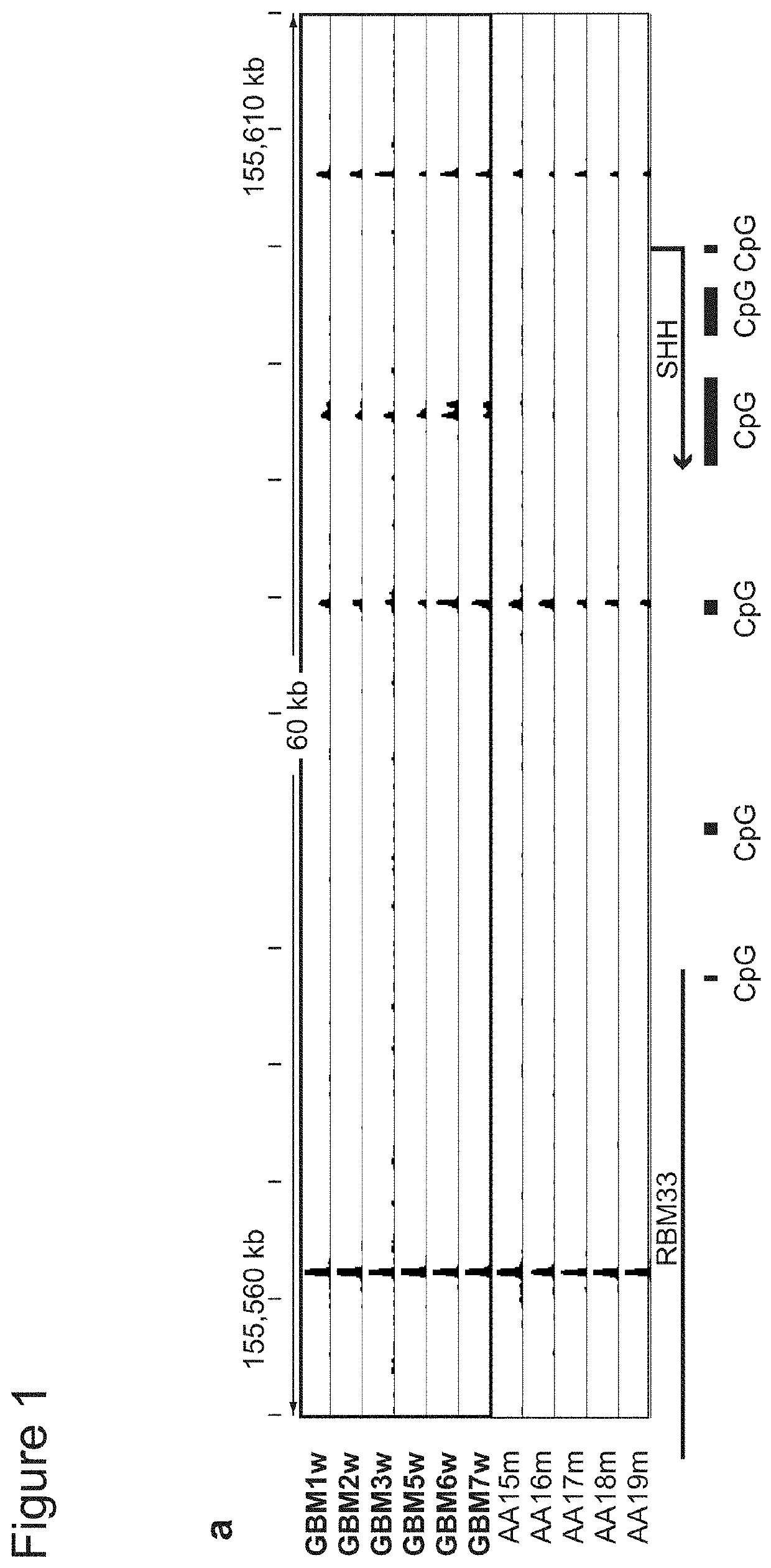
Methods of detecting insulator dysfunction and oncogene activation for screening, diagnosis and treatment of patients in need thereof
ActiveUS20200224274A1Reduced CTCF bindingLoss of insulation functionMicrobiological testing/measurementDiseaseBinding site
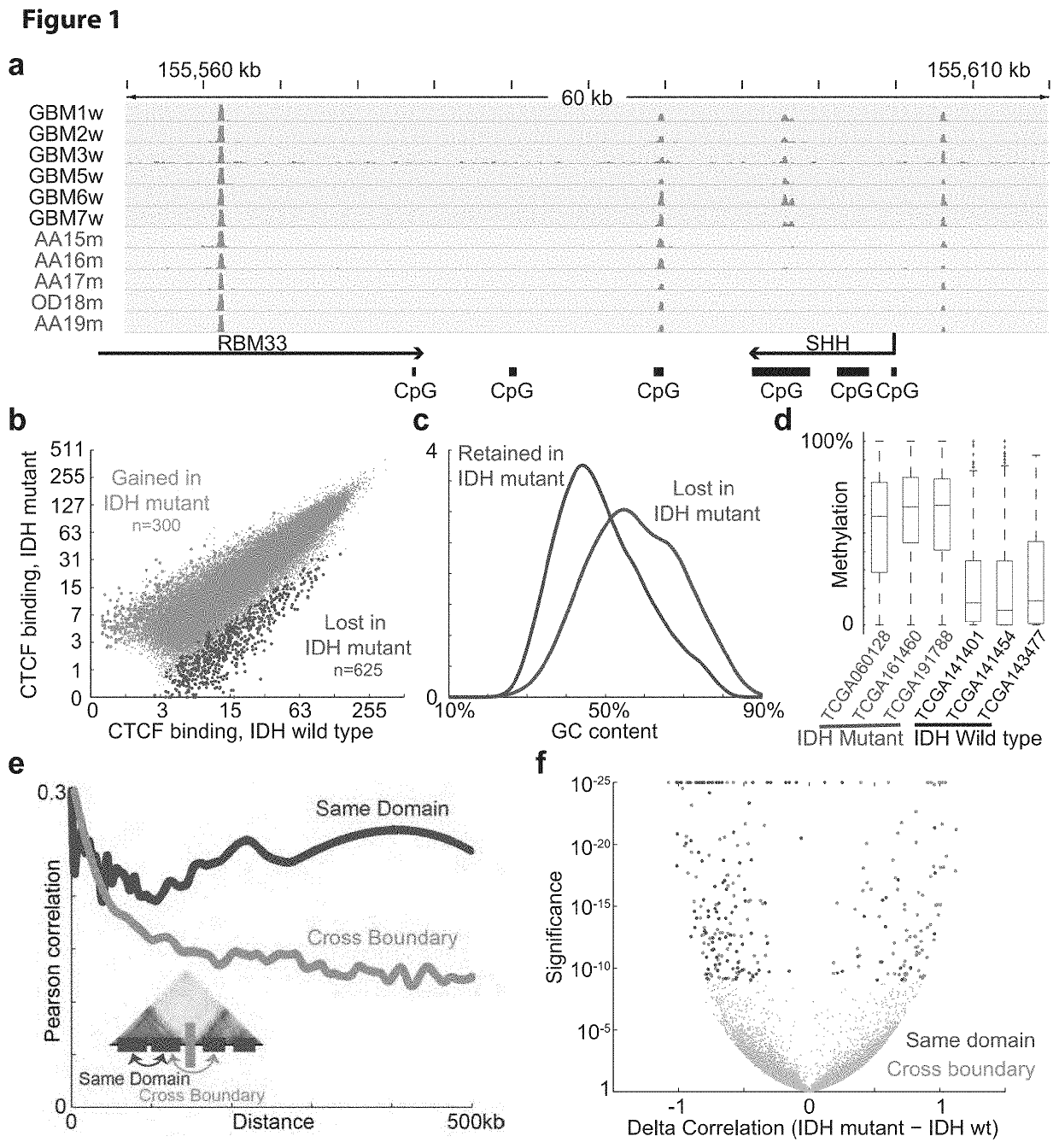
The present application generally to the diagnosis and treatment of diseases resulting from the alteration of chromatin boundaries between topologically-associated domains. In particular, the present application relates to detection of mutations causing DNA hypermethylation phenotypes, CpG methylation within CTCF binding motifs, and aberrant gene expression caused by altered chromatin topology. Applicants show that IDH mutant gliomas exhibit hyper-methylation at CTCF binding sites, compromising binding of this methylation-sensitive insulator protein. Applicants also demonstrate that loss of CTCF at a domain boundary permits a constitutive enhancer to aberrantly interact with the receptor tyrosine kinase gene PDGFRA, a prominent glioma oncogene. Thus, Applicants have uncovered that IDH mutations may promote gliomagenesis by disrupting chromosomal topology and allowing aberrant regulatory interactions that induce oncogene expression.
Owner:THE GENERAL HOSPITAL CORP
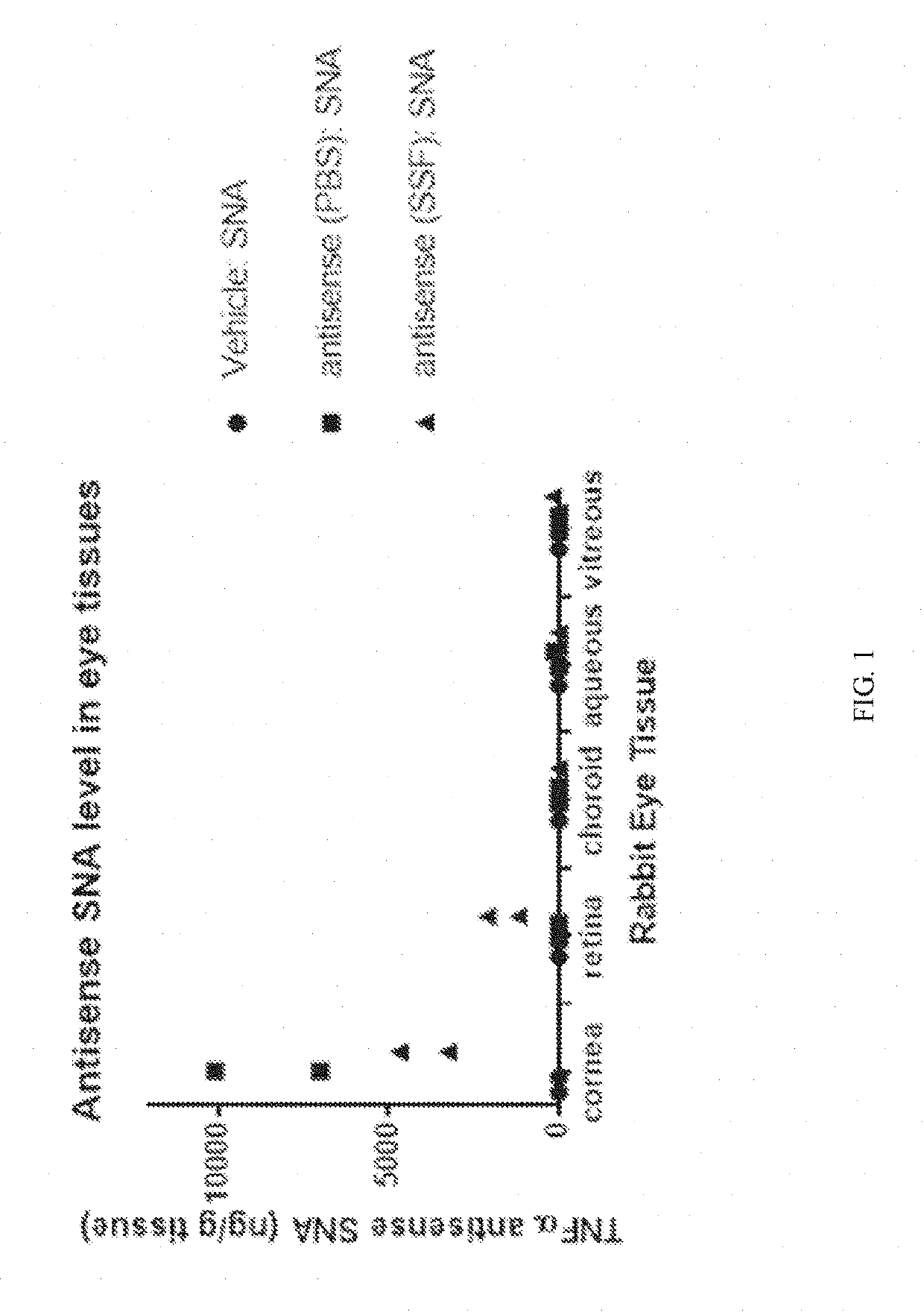
Rapid single-tube detection method and rapid single-tube detection kit for multipoint mutation of PDGFRA (platelet-derived growth factor receptor alpha) gene
InactiveCN105087792AReduce design difficultyLow costMicrobiological testing/measurementEnzyme systemPlatelet
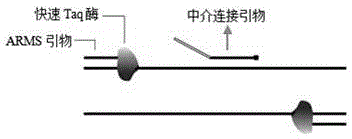
The invention discloses a rapid single-tube detection method and a rapid single-tube detection kit for multipoint mutation. The rapid single-tube detection method for multipoint mutation includes: 1) designing ARMS primers as well as downstream primers, mediated ligation primers, a universal fluorescence probe and a quenching probe corresponding to the ARMS primers; 2) mixing the ARMS primers as well as the downstream primers, the mediated ligation primers, the universal fluorescence probe and the quenching probe corresponding to the ARMS primers with a rapid hot-start Taq enzyme system prior to amplification; 3) detecting fluorescence change of a reaction system and determining whether point mutation exists or not. The rapid single-tube detection method has the advantages that the rapid single-tube detection method is simple to operate and capable of detecting existence of multipoint mutation qualitatively; the primers used in the detection method are low in design difficulty, so that primer synthesis cost is reduced substantially; limitations of an existing ARMS technology are overcome, and detection throughput is improved greatly; substituting single-tube reaction for previous multi-tube detection is achieved, and accordingly, manpower and material resources are saved.
Owner:GUANGZHOU HEAS BIOTECH CO LTD
Marker molecule for predicting sensitivity of patient to preoperative chemoradiotherapy and surgical mesorectal resection and derivative product of marker molecule
InactiveCN113846164AIncreased sensitivityImprove responseHealth-index calculationMicrobiological testing/measurementPreoperative chemoradiotherapyPharmaceutical drug
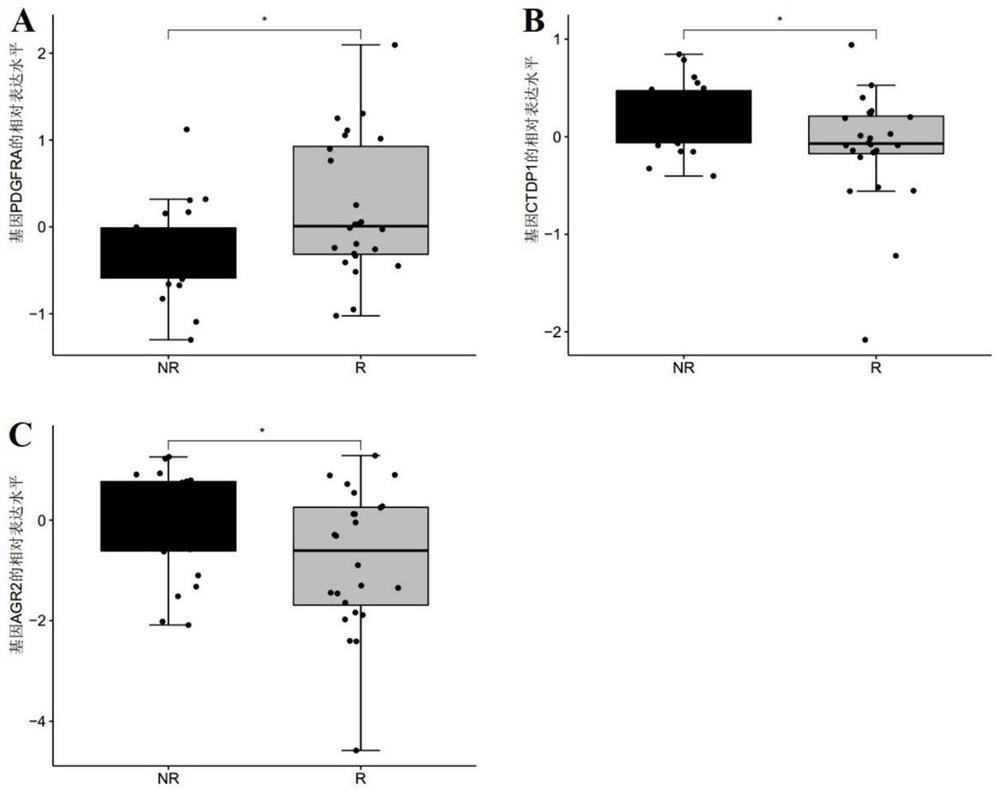
The invention discloses a marker molecule for predicting the sensitivity of a patient to preoperative chemoradiotherapy and surgical mesorectal resection and a derivative product of the marker molecule. The marker molecule is PDGFRA, CTDP1 and / or AGR2; and verification shows that the marker molecule has good diagnostic efficiency for predicting the sensitivity or reactivity of a rectal cancer patient to surgery treatment, and can be widely applied to prediction of the sensitivity of the rectal cancer patient to surgery treatment. The invention further discloses a pharmaceutical composition for improving the sensitivity of the rectal patient to the preoperative chemoradiotherapy and surgical mesorectal resection.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Detection kit for oral squamous cell carcinoma lymph node metastasis prediction
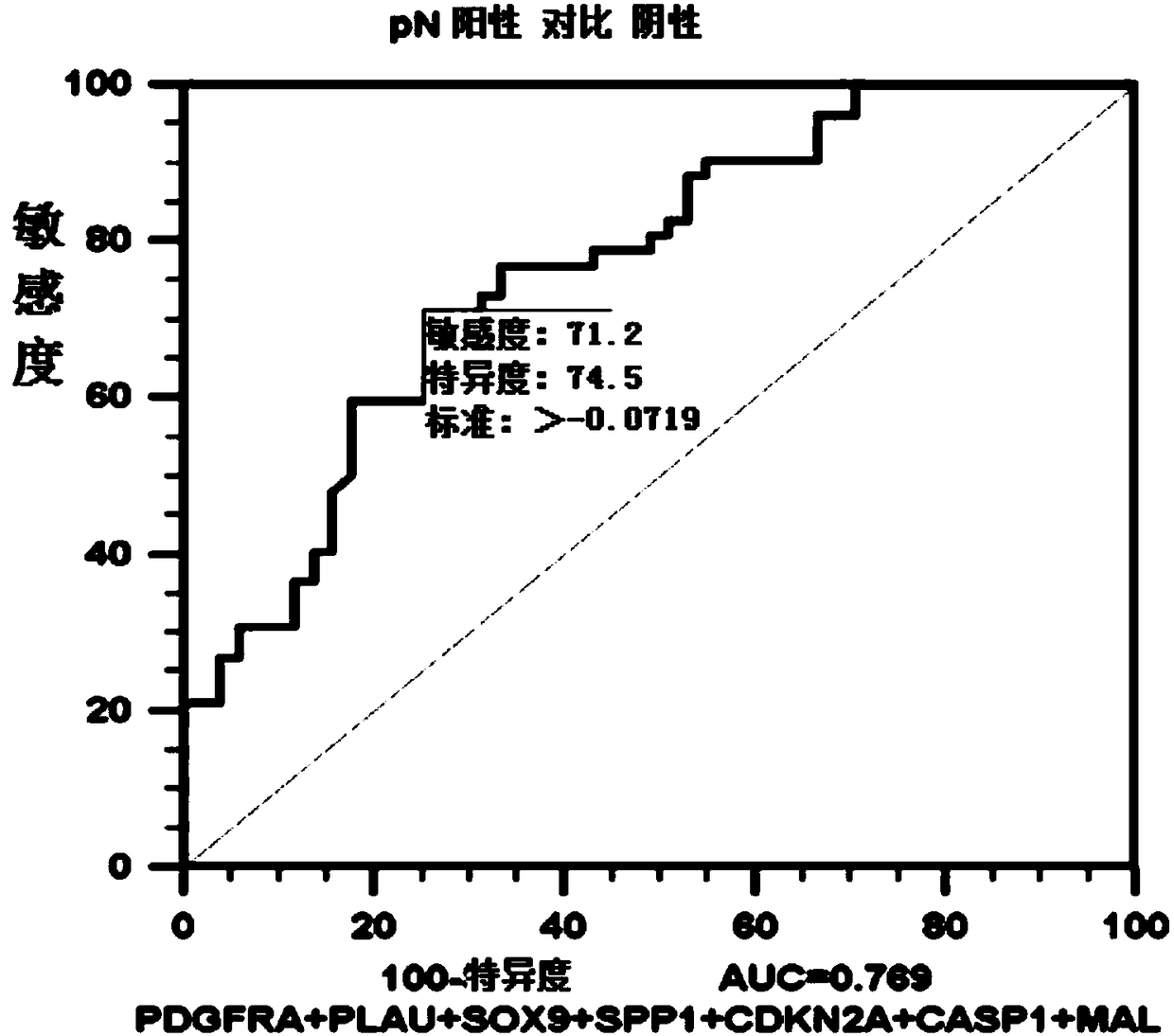
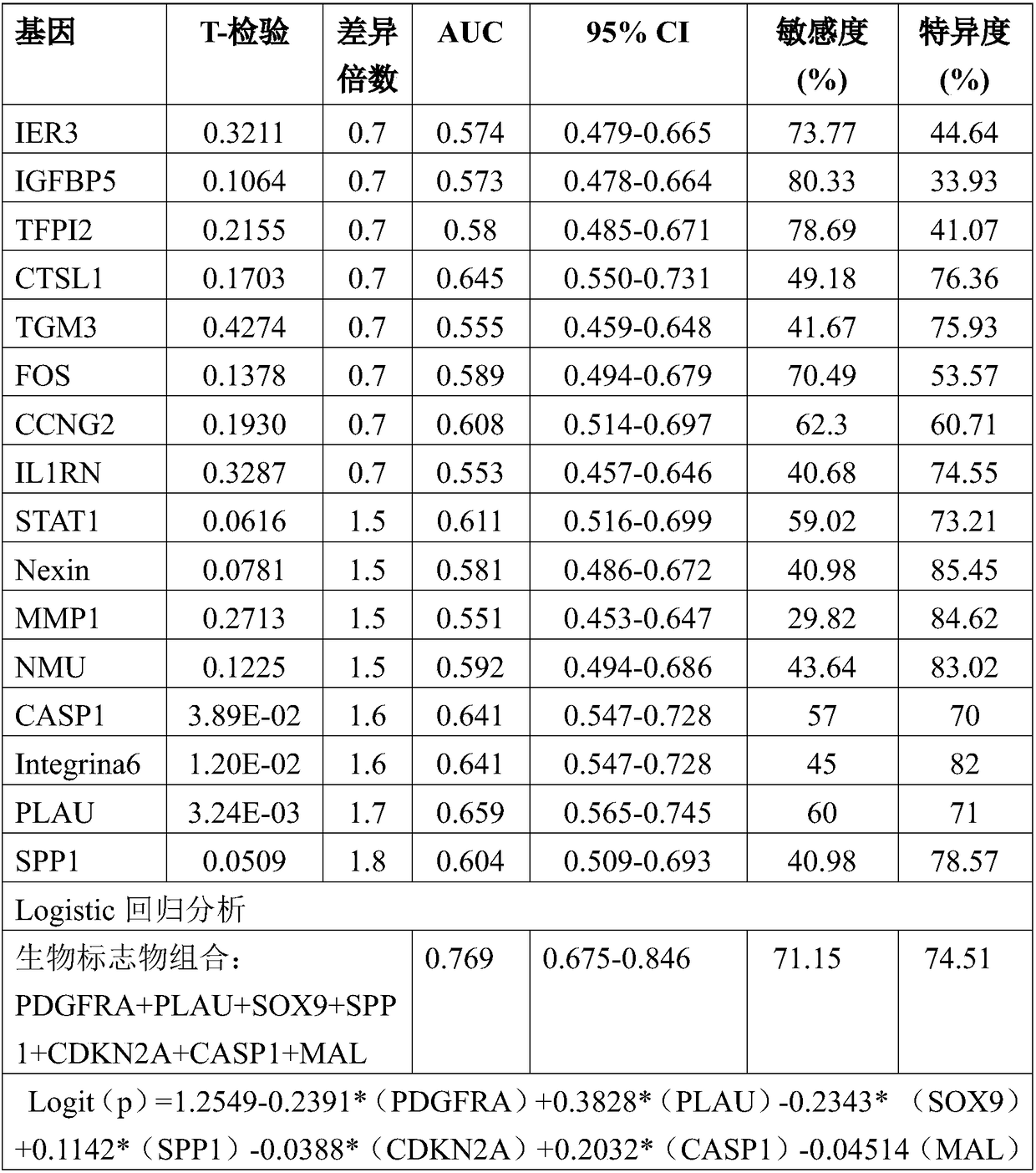
ActiveCN108546761AObjective predictionAccurate predictionMicrobiological testing/measurementForward primerSquamous Carcinomas
The invention relates to the technical field of gene detection, and discloses a detection kit for oral squamous cell carcinoma lymph node metastasis prediction. The kit comprises forward primer and reverse primer sequences of 7 kinds of genes including PDGFRA, PLAU, SOX9, SPP1, CDKN2A, CASP1 and MAL. The invention also discloses a method for screening the gene or the gene combination for the oralsquamous cell carcinoma lymph node metastasis prediction. The detection kit can be used for objectively, accurately and sensitively predicting the oral squamous cell carcinoma lymph node metastasis condition, so that the survival rate of the oral squamous cell carcinoma patients can be favorably improved.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Method for constructing lung cancer multi-gene mutation library
The invention discloses a method for constructing a lung cancer multi-gene mutation library. The method can realize mutation of 1435 somatic cells of human genes EGFR, K-ras, B-raf, N-ras, PIK3CA, c-MET, TP53, c-Kit, PDGFRA, ALK, ROS1, RET, ERBB2, NTRK1 and NTRK3. The method can individually manage multiple target sequences and fast finish library construction. The whole library construction process is finished in 2-3h and the manual operation time is only 45min. The method can be used for Illumina and Ion torrent platforms. Through combination of the method and a high-throughput sequencing platform, the problem that a small amount of paraffin tissue slices and peripheral blood samples of a lung cancer patient need multi-gene and multi-target detection of somatic cells is solved and a costis low.
Owner:CHONGQING CANCER INST
Application of miRNA
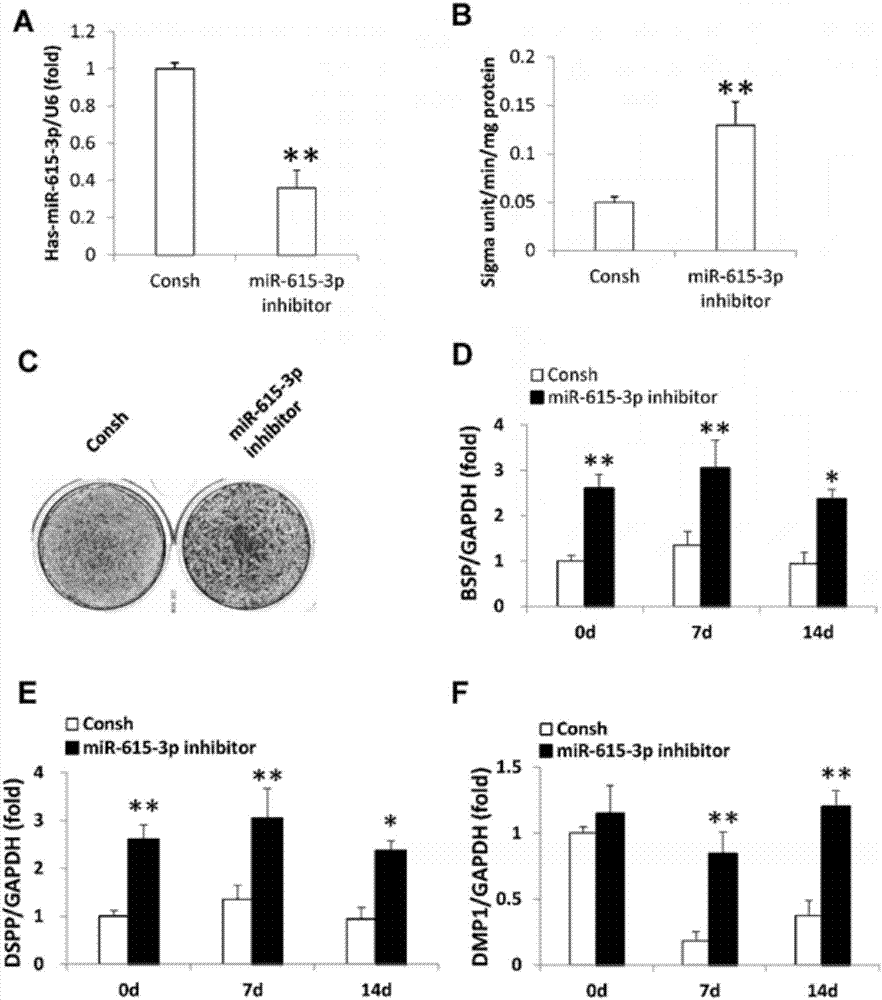
The invention relates to the field of molecular biology, and particularly relates to an application of miRNA. 15 miRNAs non-odontogenic tissue derived mesenchymal stem cells and odontogenic tissue derived mesenchymal stem cells are differentially expressed, wherein compared with odontogenic tissue derived mesenchymal stem cells, 9 non-odontogenic tissue derived mesenchymal stem cells are highly expressed, while 6 odontogenic tissue derived mesenchymal stem cells are lowly expressed. Inhibition of candidate miRNAs(hsa-miR-615-3p, hsa-miR-196b-5p) can be used for improving the dentin differentiation potential of WJCMSCs. Furthermore, seven key target genes which are highly expressed in dental tissues derived from mesenchymal stem cells can be obtained, including TFAP2C, SEMA6D, PDGFRA, PAX9, NR4A3, SATB2 and ZNF367.
Owner:BEIJING STOMATOLOGY HOSPITAL CAPITAL MEDICAL UNIV
Detection kit for combined detection of chronic myeloid leukemia
ActiveCN112266963AAchieving Simultaneous DetectionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDimerABL
The invention provides a kit for combined detection of chronic myeloid leukemia. The kit comprises a special linker for DNA library construction, a specific composite primer for detecting gene mutation, fusion and deletion, and a composite primer for library amplification. The special linker is eight dimers formed by a linker primer 1 and eight different i5-terminal primers respectively; the typesof gene mutation, fusion and deletion are BCR / ABL fusion, FGFR1 rearrangement, JAK2 gene V617F mutation, JAK2 rearrangement, NUP98 rearrangement, PDGFRA rearrangement and PDGFRB rearrangement; and the library amplification composite primer has different i7 ends, and the sequence of the primer is SEQ ID NO:39-46. The kit can be used for detecting a plurality of common molecular genetic variationsof chronic myeloid leukemia at one time, and is simple to operate, short in time and high in detection efficiency.
Owner:苏州科贝生物技术有限公司
Methods and compositions for diagnosing gastrointestinal stromal tumors
Owner:ALBERT LUDWIGS UNIV FREIBURG
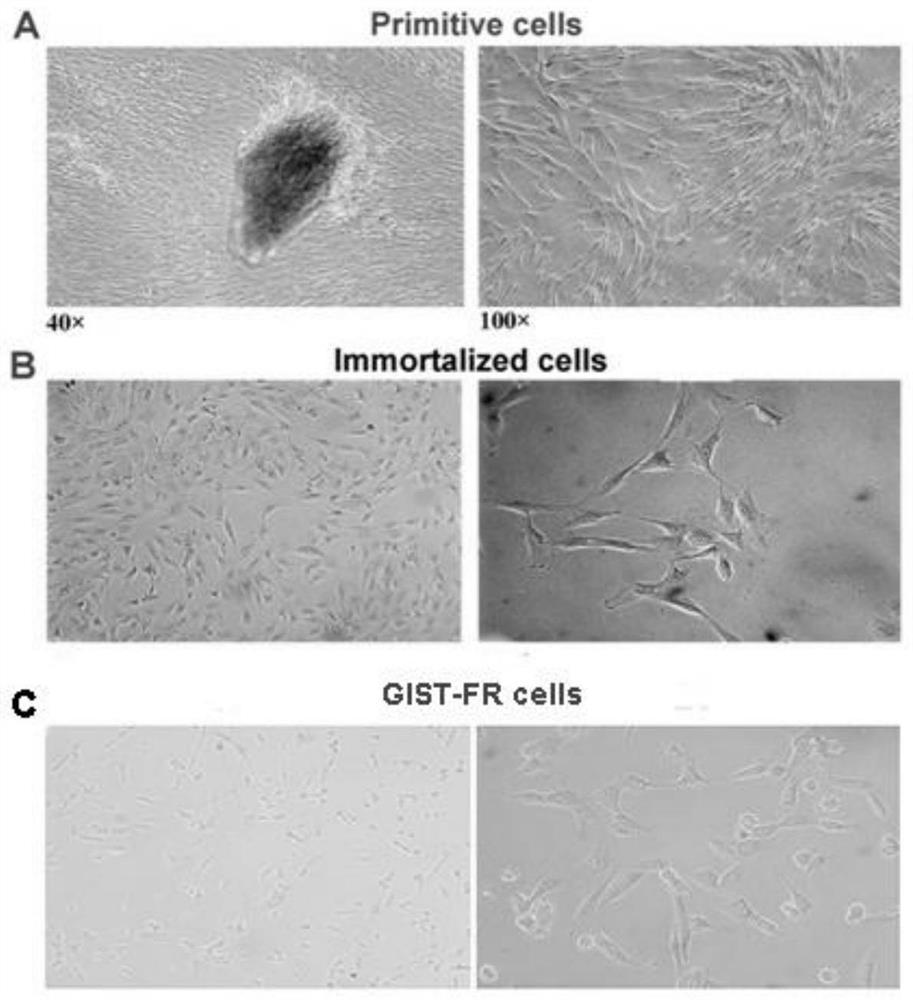
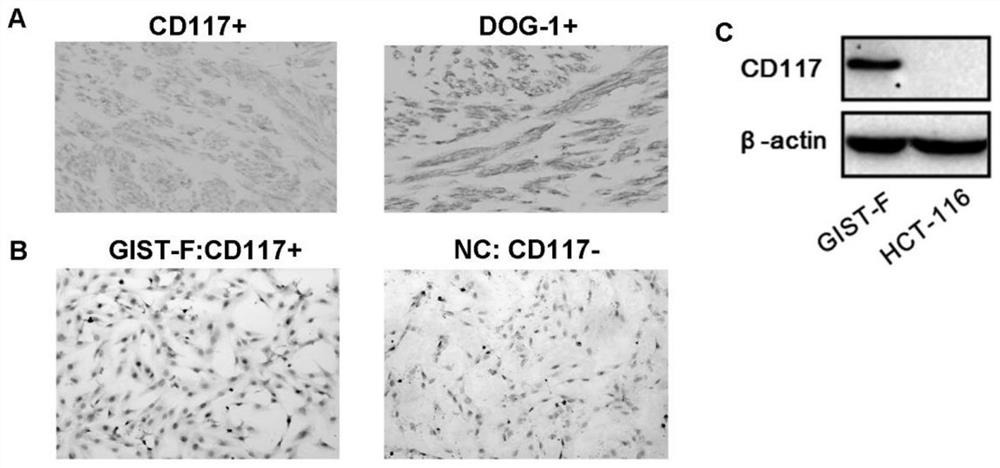
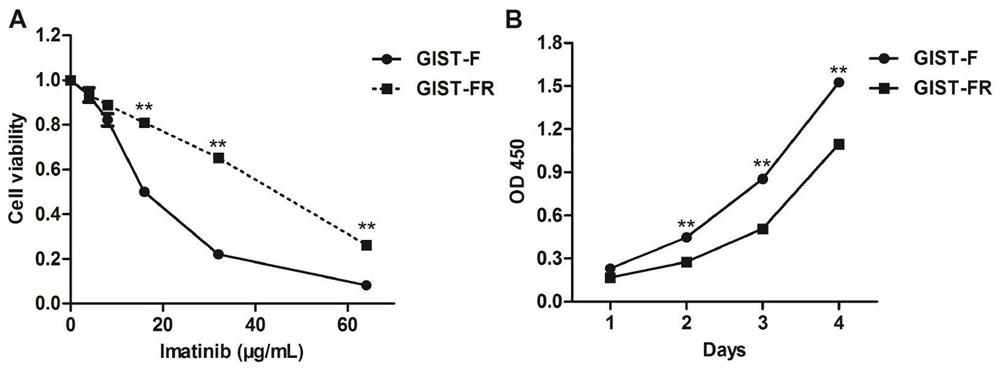
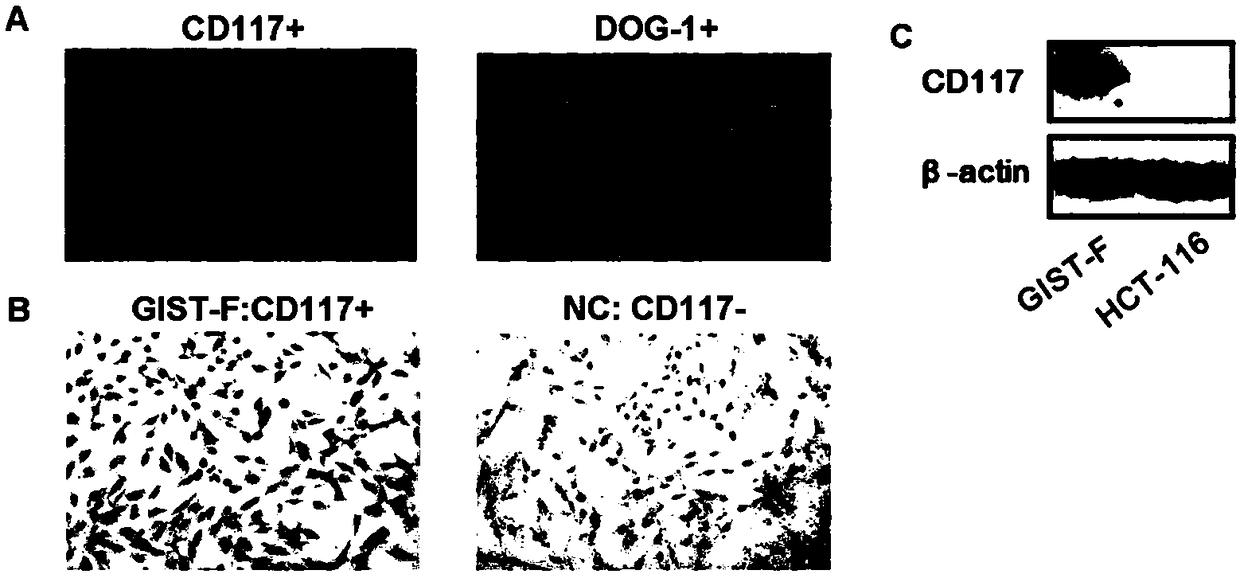
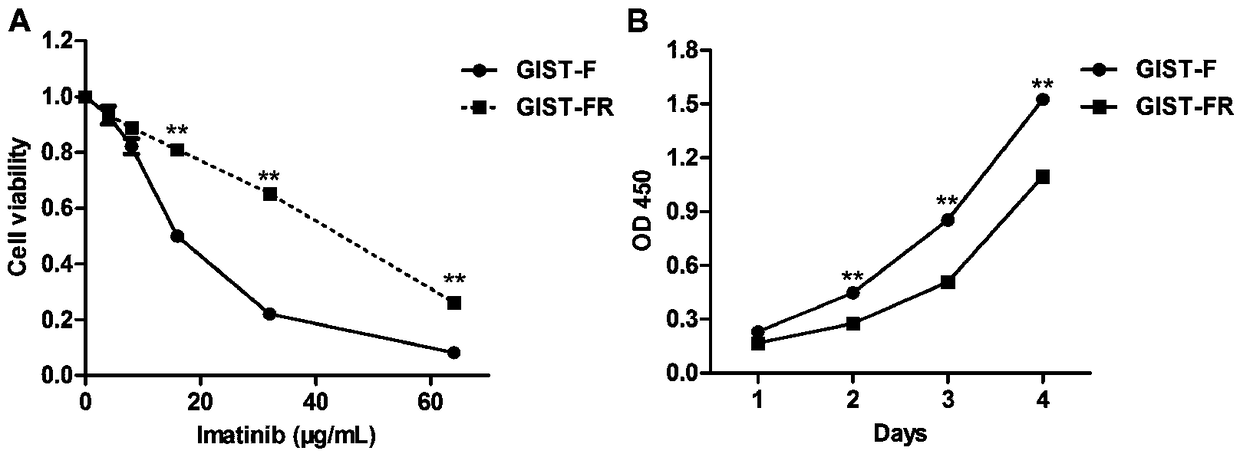
Imatinib-drug-resistance KIT and PDGFRA wild type GIST cell strain and establishment method and application thereof
The invention provides an Imatinib-drug-resistance KIT and PDGFRA wild type GIST cell strain which is named as GIST-FR and preserved in the China Center for Type Culture Collection with the preservation number being CCTC NO:C2017111. The invention further provides an establishment method of the cell strain and application of the cell strain as a cell model for discussing the human GIST drug resistance mechanism and researching relevant signal channels thereof. A tissue block culture method is adopted to obtain GIST primary cells, immortalization is carried out, and a GIST-F cell line is established; then Imatinib drug resistance induction is carried out on the GIST-F cell line, and the Imatinib-drug-resistance GIST cell strain GIST-FR is established. The GIST-FR cells are subjected to in-vitro passage for more than 40 generations, growth is slow compared with GIST-F cells, IC50 of Imatinib is remarkably increased, and the drug resistance index is 3.52 (P is less than 0.01); and a wholeexon group sequencing result shows that KIT and PDGFRA of the GIST-FR cells are negative. The GIST cell line is successfully built from human GIST primary tissue, and further induction is carried outto obtain the Imatinib-drug-resistance cells thereof, so that a foundation is laid for the GIST pathogenesis and drug resistance related research.
Owner:AFFILIATED HOSPITAL OF JIANGNAN UNIV
Neuroglioma Molecular Subtyping Gene Group and Use Thereof
InactiveUS20160102364A1Effective predictionMicrobiological testing/measurementProtein nucleotide librariesClinical diagnosisGenome
A set of gene groups for predicting or for the auxiliary prediction of the survival time prognosis of patients with neuroglioma consists of a PM gene group and an EM gene group with a total of 68 genes. A subtype marking gene group consisting of two major gene groups co-expressed with PDGFRA and EGFR can stably divide neuroglioma samples from different database sources into three specific subtypes, and can be applied to clinical diagnosis for neuroglioma and judging the survival time prognosis of patients with neuroglioma.
Owner:BEIJING NORMAL UNIVERSITY
N-(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methylphenyl)-5-phenyl-4,5-dihydro-1h-pyrazole-1-carboxamide derivative, and pharmaceutical composition containing same as active ingredient for treating kinase-related diseases
The N-(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methylphenyl)-5-phenyl-4,5-dihydro-1H-pyrazole-1-carboxamide derivative exhibits excellent inhibitory activity against at least one kinase selected from the group consisting of ABL1, ABL2, AURKB, BRK, CDK11, CDK8, CDK9, CDKL2, CIT, DDR1, FLT3, HIPK4, HUNK, JAK3, KIT, LOK, LTK, MET, MLK2, MUSK, MYO3A, PAK3, PCTK3, PDGFRA, PDGFRB, RIPK1, TIE1 and ZAK, and thus being useable as a therapeutic agent for kinase-related disease.
Owner:VORONOIBIO INC
Kit for detecting mutation of gastrointestinal stromal tumor-related genes and application thereof
ActiveCN107419009AAccurate and Effective MonitoringMutations are accurate and effectiveMicrobiological testing/measurementStromal tumorHigh flux
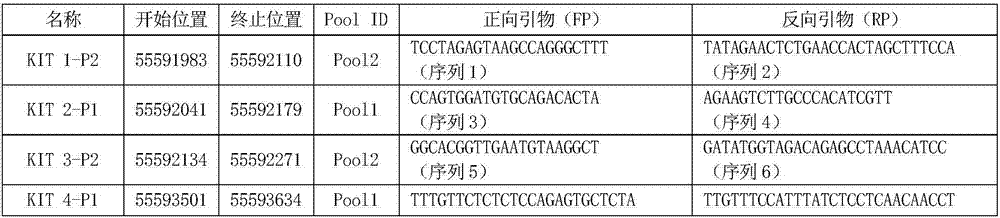
The invention discloses a kit for detecting the mutation of gastrointestinal stromal tumor-related genes and application thereof. The invention provides application of a primer pair group (as shown in sequence 1 to sequence 40) and a readable vector recording a method for detecting the mutation of the gastrointestinal stromal tumor-related genes of a to-be-detected person to preparation of the kit used for detecting the mutation of the gastrointestinal stromal tumor-related genes. According to the invention, instead of traditional tumor tissue or section, blood plasma is use as a detection sample in the invention; 20 specific primer pairs are designed directed at the gastrointestinal stromal tumor-related genes, i.e., a KIT gene and a PDGFRA gene; a high-flux sequencing detection method is employed; so the data volume of detection is increased, the accuracy of experimental results is improved, and the mutation of the gastrointestinal stromal tumor-related genes can be accurately and effectively monitored.
Owner:上海讯迈丰生物科技有限责任公司
Clonal eosinophilia fusion gene detection probe composition, kit and application thereof
ActiveCN113249469ALower Sequencing CostsHigh sensitivityMicrobiological testing/measurementDNA preparationBase JTarget gene
The invention provides a clonal eosinophilia fusion gene detection probe composition, a kit and application thereof. According to the invention, firstly, related fusion genes are classified, wherein core genes FGFR1, PDGFRA and PDGFRB for clonal eosinophilia fusion detection are I-type genes, so that full-length transcripts of the genes are used as target regions; and other genes are II-type genes, so that only fragments near the fusion breakpoint are used as target regions. According to a base complementary pairing principle, complementary sequences of target regions are designed as probes to form a 'liquid phase chip', the probes relate to 39 target genes, eosinophilia fusion genes are detected by capturing corresponding target sequences in a transcriptome library and combining high-throughput sequencing, multiple fusion can be detected at a time, new fusion can be discovered, the detection cost is reduced, and the accuracy of a detection result is improved.
Owner:QIAGEN SUZHOU TRANSLATIONAL MEDICINE CO LTD
Lung cancer targeted drug and chemotherapy drug genomes and application thereof in lung cancer clinical drug treatment
InactiveCN112121170AGood treatment effectSmall toxicityAntineoplastic agentsPharmaceutical active ingredientsSide effectROS1
The invention discloses lung cancer targeted drug and chemotherapy drug genomes and an application thereof in lung cancer clinical drug treatment. The targeted drug genome comprises AKT1, ALK, BRAF, CCND1, CDKN2A, DDR2, EGFR, ERBB2, ERBB4, FBXW7, FGFR1, FGFR2, FGFR3, HRAS, IGF1R, KDR, KIT, KRAS, MAP2K1, MET, NF1, NRAS, NTRK1, NTRK3, PDGFRA, PIK3CA, PTEN, RET, ROS1, STK11 and TP53; and the chemotherapy drug genome comprises ABCB1, CDA, CEP72, CYP1B1, CYP2C8, DPYD, ERCC1, ERCC2, GSTM, GSTP1, MTHFR, RRM1, SLCO1B1, TEKT4, TPMT, TYMS, UMPS, XPC and XRCC1. The lung cancer targeted drug and chemotherapy drug genomes disclosed by the invention are applied to guidance of clinical precise chemotherapy of the lung cancer or auxiliary diagnosis of the lung cancer and curative effect and after-healingmonitoring, can provide the most effective drug selection for lung cancer patients with drug resistance or toxic and side effect intolerance caused by conventional drugs and targeted and chemotherapydrug requirements, improve the drug treatment effect, and reduce the toxic and side effects of the drugs.
Owner:合肥金域医学检验实验室有限公司
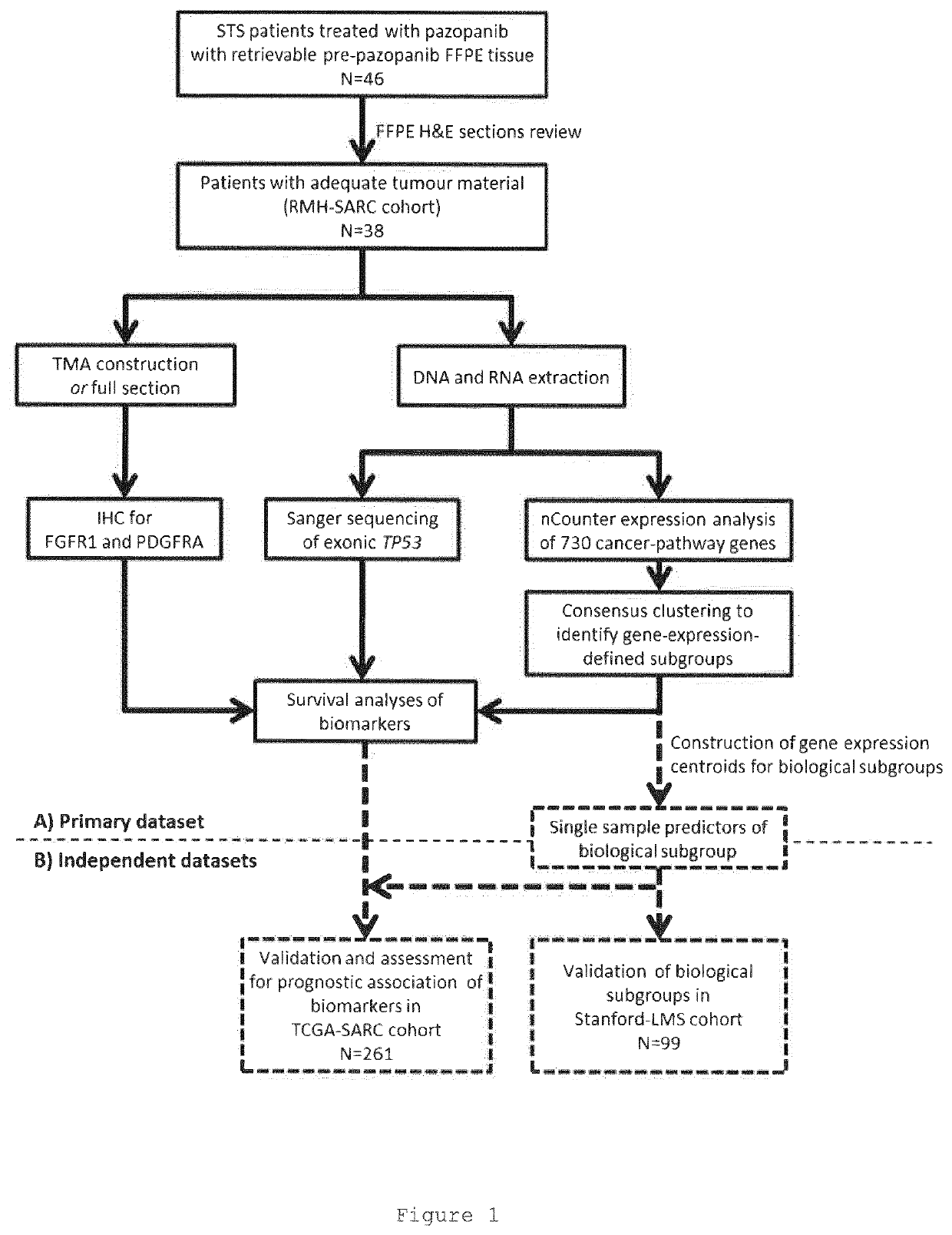
Materials and methods for stratifying and treating cancers
PendingUS20210010085A1Shrink tumorAddress bad outcomesOrganic active ingredientsTherapiesTyrosine-kinase inhibitorTyrosine
The present invention relates to materials and methods for stratifying and treating cancers and to methods of identifying / selecting patients for treatment of cancer with tyrosine kinase inhibitors. Gene expression profiles, TP53 mutations and FGFR1 and PDGFRA expression are used to identify / select / stratify the cancers and patients.
Owner:THE INST OF CANCER RES ROYAL CANCER HOSPITAL +1
Imatinib-resistant kit and pdgfra wild-type gist cell lines and their construction methods and applications
ActiveCN108642016BGenetically modified cellsCell culture supports/coatingSignalling pathwaysImatinib
The Imatinib-resistant KIT and PDGFRA wild-type GIST cell line provided by the present invention is named GIST-FR, and is preserved in the China Center for Type Culture Collection with the preservation number CCTC NO: C2017111. At the same time, the present invention also provides the construction method of this cell line and the use of the cell line as a cell model for the investigation of the drug resistance mechanism of human gastrointestinal stromal tumors and the study of related signaling pathways. The cells were then immortalized to establish the GIST-F cell line, which was then induced with Imatinib resistance to establish the Imatinib-resistant GIST cell line GIST-FR. GIST‑FR cells have been passaged in vitro for more than 40 generations. Compared with GIST‑F cells, GIST‑FR cells grow slower, and their IC50 to Imatinib is significantly higher, with a drug resistance index of 3.52 (P<0.01). and PDGFRA were negative. The present invention successfully establishes GIST cell lines from human GIST primary tissues, and further induces its Imatinib-resistant cells, laying a foundation for the research on GIST pathogenesis and drug resistance.
Owner:AFFILIATED HOSPITAL OF JIANGNAN UNIV
Methods of detecting insulator dysfunction and oncogene activation for screening, diagnosis and treatment of patients in need thereof
ActiveUS11339442B2Avoids off-target binding and its resulting side effectHigh degreeMicrobiological testing/measurementDiseaseBinding site
The present application generally to the diagnosis and treatment of diseases resulting from the alteration of chromatin boundaries between topologically-associated domains. In particular, the present application relates to detection of mutations causing DNA hypermethylation phenotypes, CpG methylation within CTCF binding motifs, and aberrant gene expression caused by altered chromatin topology. Applicants show that IDH mutant gliomas exhibit hyper-methylation at CTCF binding sites, compromising binding of this methylation-sensitive insulator protein. Applicants also demonstrate that loss of CTCF at a domain boundary permits a constitutive enhancer to aberrantly interact with the receptor tyrosine kinase gene PDGFRA, a prominent glioma oncogene. Thus, Applicants have uncovered that IDH mutations may promote gliomagenesis by disrupting chromosomal topology and allowing aberrant regulatory interactions that induce oncogene expression.
Owner:THE GENERAL HOSPITAL CORP
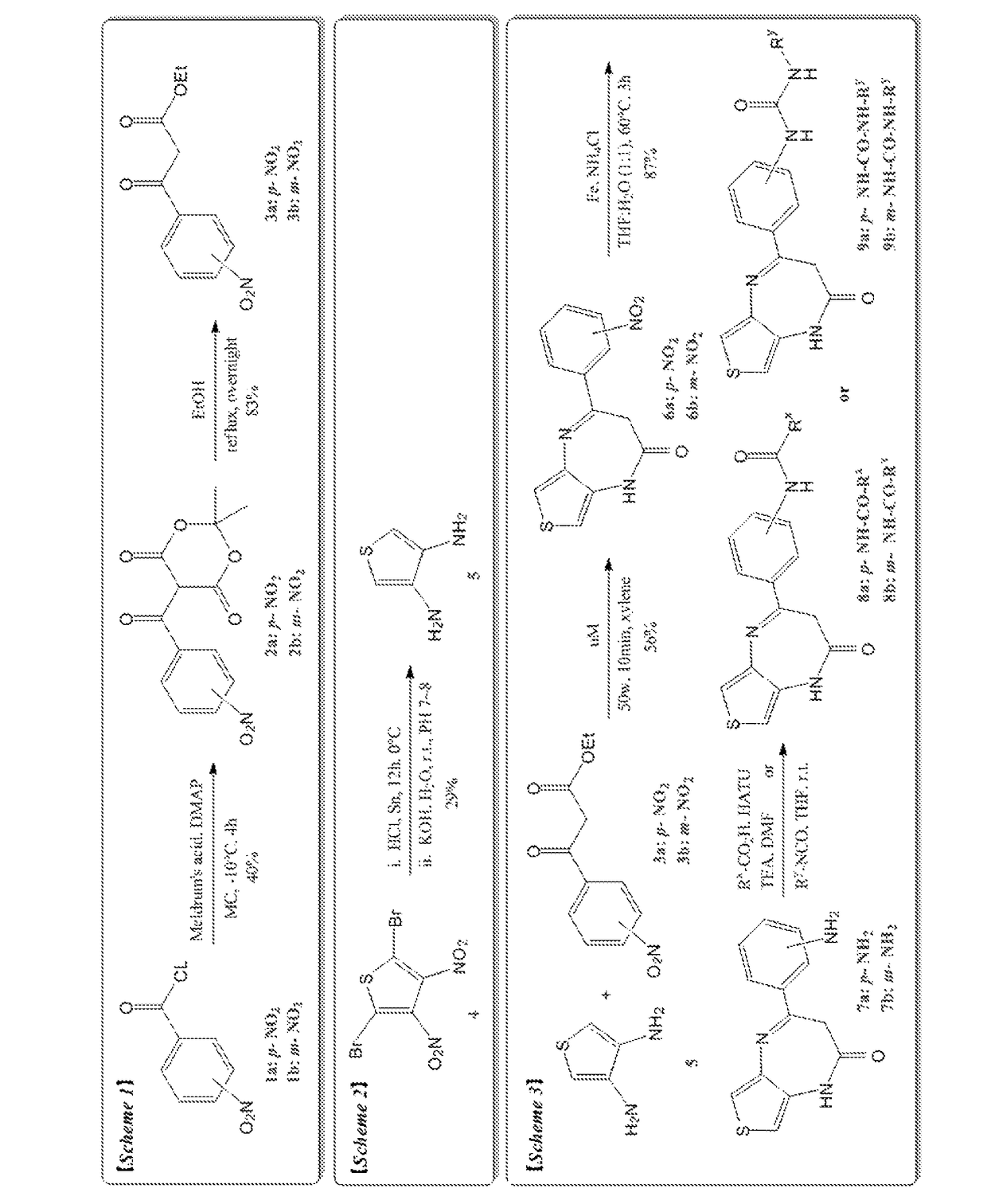
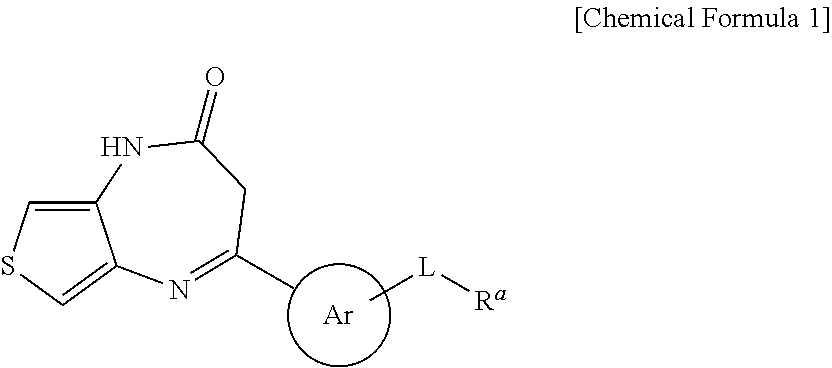
Thienodiazepine derivatives or pharmaceutically acceptable salts thereof, and pharmaceutical composition including the same as an active ingredient
ActiveUS10174047B2Prevent proliferationHigh inhibition rateOrganic active ingredientsOrganic chemistryDiseaseAbnormal cell
The present invention relates to novel thienodiazepine derivatives or pharmaceutically acceptable salts thereof, and a pharmaceutical composition including the same. The thienodiazepine derivatives or pharmaceutically acceptable salts thereof exhibit selective inhibition activities against protein kinases such as c-Kit, FLT3, FMS, LYN, RAF1, VEGFR3, PDGFRa, PDGFRb, RET, etc., and thus can be used as a pharmaceutical composition for prevention or treatment of abnormal cell growth diseases.
Owner:IND UNIV COOPERATION FOUND HANYANG UNIV ERICA CAMPUS
Primers, kit and pcr method for detecting d842v polymorphic site of pdgfra gene
ActiveCN104830991BImprove abilitiesStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceType specific
The invention discloses a primer and a kit for detecting PDGFRA (platelet-derived growth factor receptor alpha) gene D842V polymorphic sites and a PCR (polymerase chain reaction) method of the primer and the kit. The primer comprises a wild specific sense primer, a mutant type specific sense primer, and a reverse primer shared by the wild specific sense primer and the mutant type specific sense primer. The wild specific sense primer has an SEQ No.17 sequence, the mutant type specific sense primer has an SEQ No.14 sequence, and the shared reverse primer has an SEQ No.16 sequence. The kit has the advantages of simplicity in detection, quickness, accuracy, low cost and the like, and powerful tools are provided for scientific research and clinic analysis of the PDGFRA gene D842V polymorphic sites.
Owner:沈阳优吉诺生物科技有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



































![N-(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methylphenyl)-5-phenyl-4,5-dihydro-1h-pyrazole-1-carboxamide derivative, and pharmaceutical composition containing same as active ingredient for treating kinase-related diseases N-(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methylphenyl)-5-phenyl-4,5-dihydro-1h-pyrazole-1-carboxamide derivative, and pharmaceutical composition containing same as active ingredient for treating kinase-related diseases](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/77c3e28b-f209-43fe-882b-1922a0990f85/US20210284647A1-C00001.png)
![N-(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methylphenyl)-5-phenyl-4,5-dihydro-1h-pyrazole-1-carboxamide derivative, and pharmaceutical composition containing same as active ingredient for treating kinase-related diseases N-(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methylphenyl)-5-phenyl-4,5-dihydro-1h-pyrazole-1-carboxamide derivative, and pharmaceutical composition containing same as active ingredient for treating kinase-related diseases](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/77c3e28b-f209-43fe-882b-1922a0990f85/US20210284647A1-C00002.png)
![N-(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methylphenyl)-5-phenyl-4,5-dihydro-1h-pyrazole-1-carboxamide derivative, and pharmaceutical composition containing same as active ingredient for treating kinase-related diseases N-(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methylphenyl)-5-phenyl-4,5-dihydro-1h-pyrazole-1-carboxamide derivative, and pharmaceutical composition containing same as active ingredient for treating kinase-related diseases](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/77c3e28b-f209-43fe-882b-1922a0990f85/US20210284647A1-C00003.png)