Clonal eosinophilia fusion gene detection probe composition, kit and application thereof
An eosinophil detection kit technology, applied in recombinant DNA technology, microbial assay/inspection, biochemical equipment and methods, etc., can solve the problems of adding new fusions, poor scalability, and missed detection of new fusions, etc. Achieve the effect of low sequencing cost, reliable results and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] In this embodiment, fusion detection is performed on the bone marrow samples of patients with clonal hypereosinophilia.
[0063] (1) Nucleic acid extraction
[0064] Add the sample and EL buffer at a ratio of 1:5, and incubate on ice, briefly vortex and mix twice during the incubation period to lyse the red blood cells, and then incubate at 4°C at 400 ×g Centrifuge for 10 minutes, discard the supernatant; add EL buffer (2 times the volume of the sample) at a ratio of 1:2, vortex briefly to mix, 400 at 4°C ×g Centrifuge for 10 min, discard the supernatant;
[0065] Add appropriate RLT buffer solution according to the sample volume to dissolve leukocytes, vortex or pipette to mix, transfer to the lysis column, centrifuge at the maximum speed for 2 minutes, and collect the filtrate; add 1 volume of 70% ethanol to the filtrate and pipette to mix. Transfer to filter column, 8000 ×g Centrifuge for 15s, discard the collection tube; transfer the filter column to a new collec...
Embodiment 2
[0093] Example 2 Capture of related fragments of the target fusion gene
[0094] (1) Capture target fragments
[0095] Put the above total amount of 2 μg pre-library into a new 1.5 mL centrifuge tube, add 5 μL HumanCot-1 DNA and 2 μL Universal Blockers to it, cover the tube cap, and pierce a hole on the tube cap with a disposable syringe Put it into a vacuum concentrator, and dry at a constant temperature of 60°C until there is no liquid;
[0096] Add a total volume of 17 μL hybridization solution (including 2×HybridizationBuffer, Hybridization Buffer Enhancer, probe composition and Nuclease-Free Water to the dry centrifuge tube, where the concentration of the probe composition in the reaction system is 400 amol / probe / rxn), mix well, place at room temperature for 5 min, transfer to a PCR tube, and run the HYB program (hot lid at 100°C, 95°C for 30 s, 65°C for 4 h, 65°C Hold);
[0097] After the HYB program runs, transfer the washed 17 μL M-270 magnetic bead solution to the ...
Embodiment 3
[0110] In this embodiment, bone marrow samples from 6 patients with clinically suspected hypereosinophilia were used for detection.
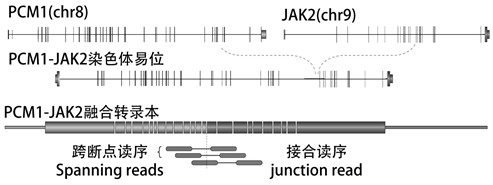
[0111] The detection method comprises steps such as image 3 Shown: first extract the RNA in the sample, then carry out mRNA enrichment, and then carry out pre-library construction, including: RNA thermal interruption, reverse transcription of fragmented RNA into cDNA, end repair, adding A tail, adding Connecting adapters and PCR amplification; then, using the probe composition of the present invention for hybridization capture, including: blocking library DNA, washing magnetic beads, hybridization, and obtaining a capture library after elution, followed by amplification and purification to obtain On-board inspection;
[0112] The specific implementation steps are shown in Example 1 and Example 2.
[0113] The test results are shown in Table 6. Among the 6 patients with clinically suspected hypereosinophilia, MYO18A-PDGFRB fusion was detected ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com