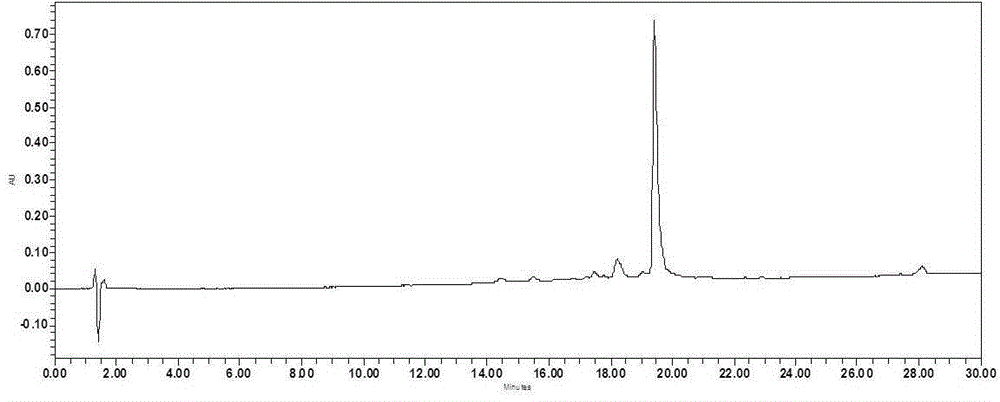
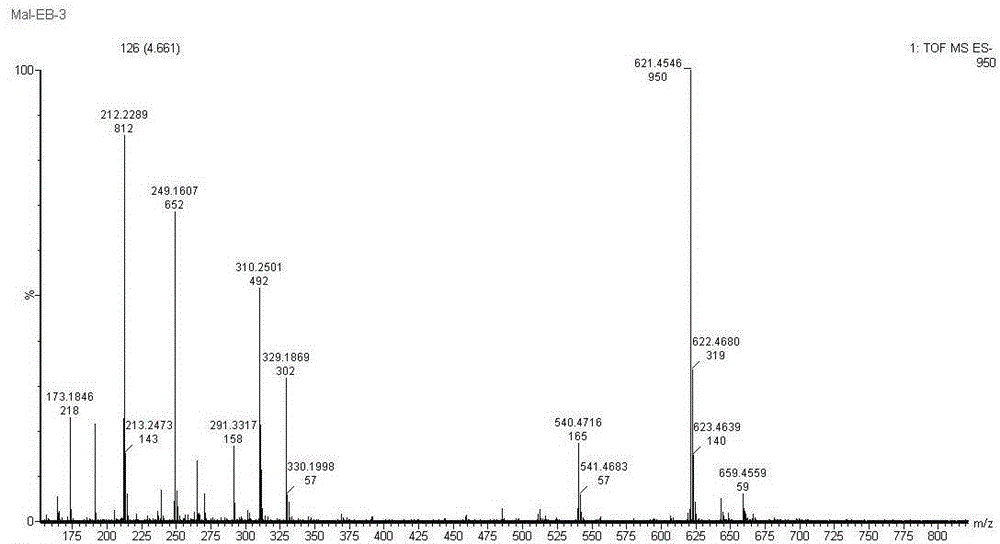
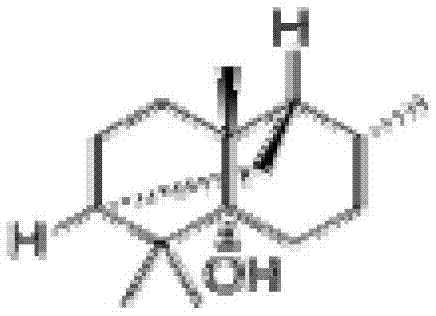
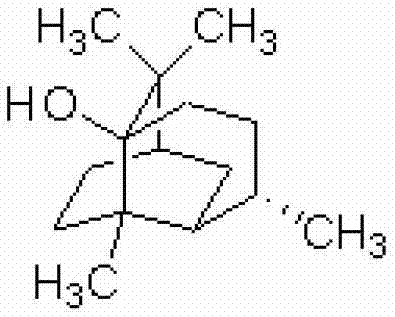
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2208 results about "Drug efficiency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of Efficacy. The ability of a drug to produce a predictable effect in the body. Many factors influence a drug’s efficacy, from foods and other drugs to health conditions and a person’s metabolic characteristics. An individual’s age, weight, gender, and level of activity also may affect the rate at which a drug enters,...
Application of levo-oxiracetam in preparation of medicine for treating memory and intelligence disturbance
The invention relates to new use of levo-oxiracetam in pharmaceutical field, and in particular relates to application of levo-oxiracetam in preparation of a medicine for treating memory and intelligence disturbance. The experiment result shows that the levo-oxiracetam is a main active ingredient for playing efficacy in oxiracetam, the clinical dosage can be greatly reduced by singly using the levo-oxiracetam, and the potential toxic and side effect is reduced. According to the invention, the levo-oxiracetam is a single active ingredient, the raw material purity is greater than 99.5% so as to effectively avoid the toxicity risk caused by other impurities in the medicine, the pharmacy is safer, the medicine quality is more controllable, and the curative effect is more precise.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +2
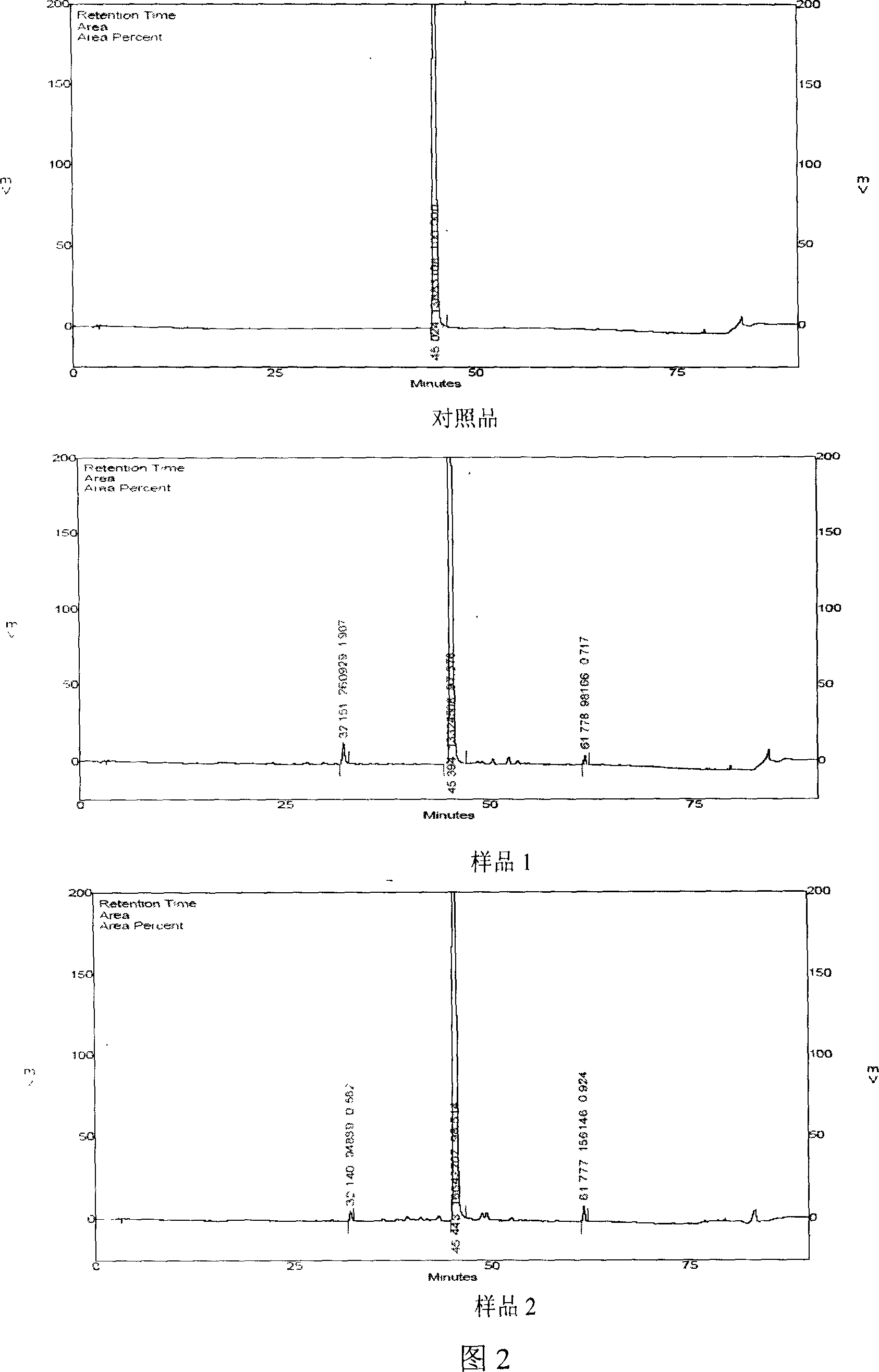
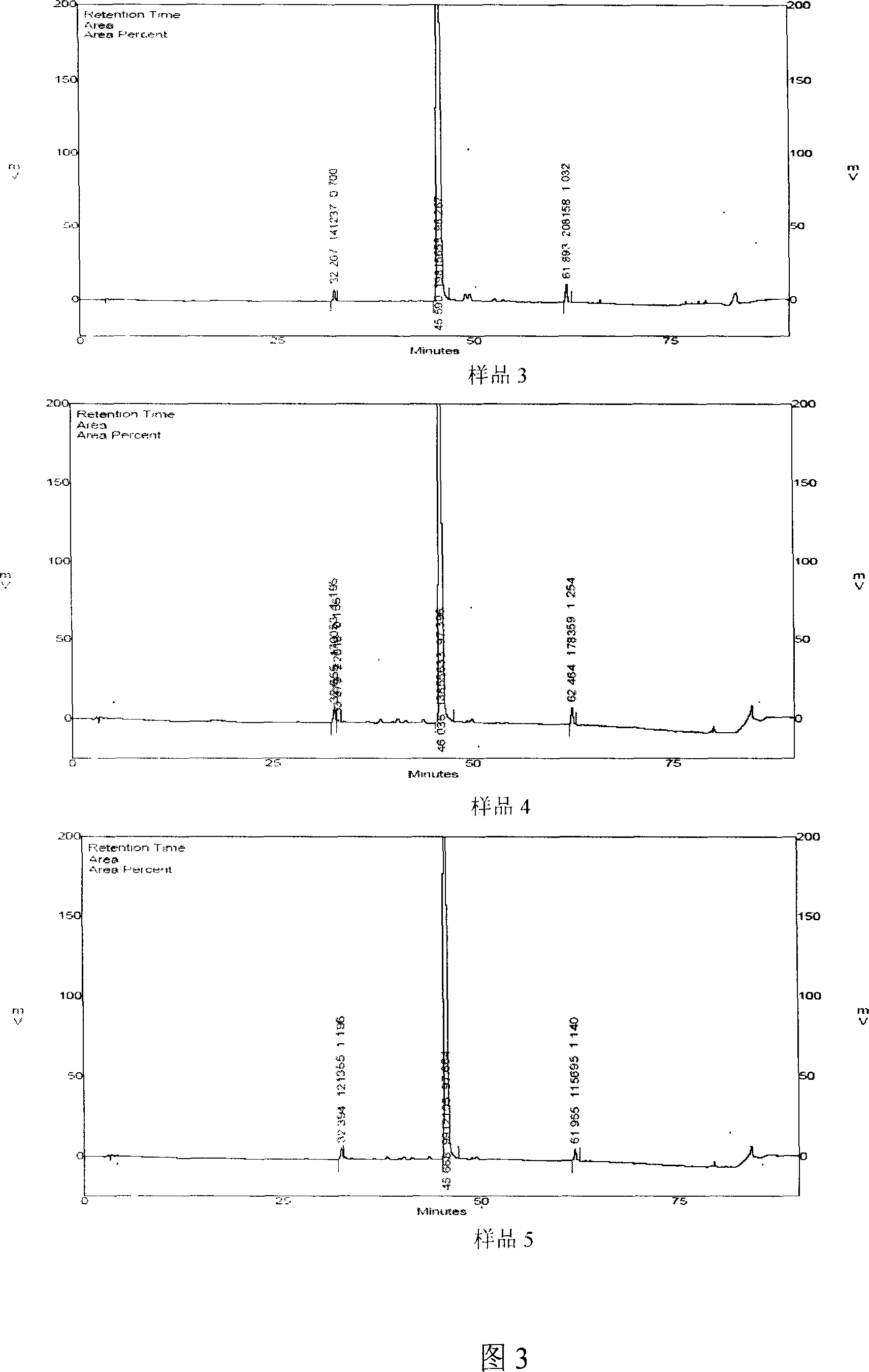
Salvia minium phenolic acid A and process of preparing preparation and use
InactiveCN100999470AImprove conversion rateGood repeatabilityOrganic active ingredientsOrganic chemistryMedicineCurative effect
This invention concerns the method of extracting salvianolic acid A from Chinese crude drug: danshen root, and the quality control methods and drug combinations, and the application of this drug. It can be used in the preparation of the prevention drugs for cardiovascular disease, liver damage, liver fibrosis, pulmonary fibrosis and other.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
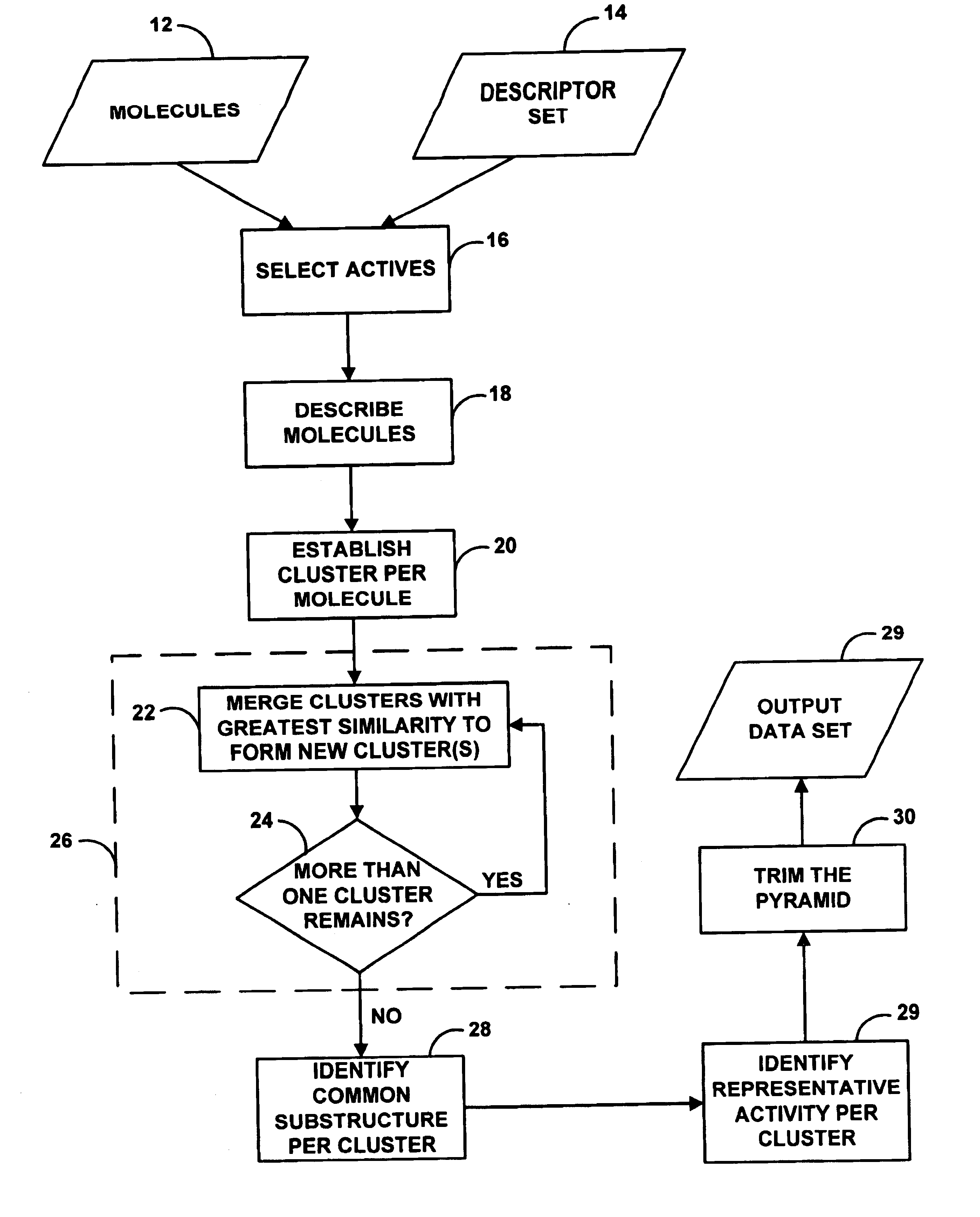
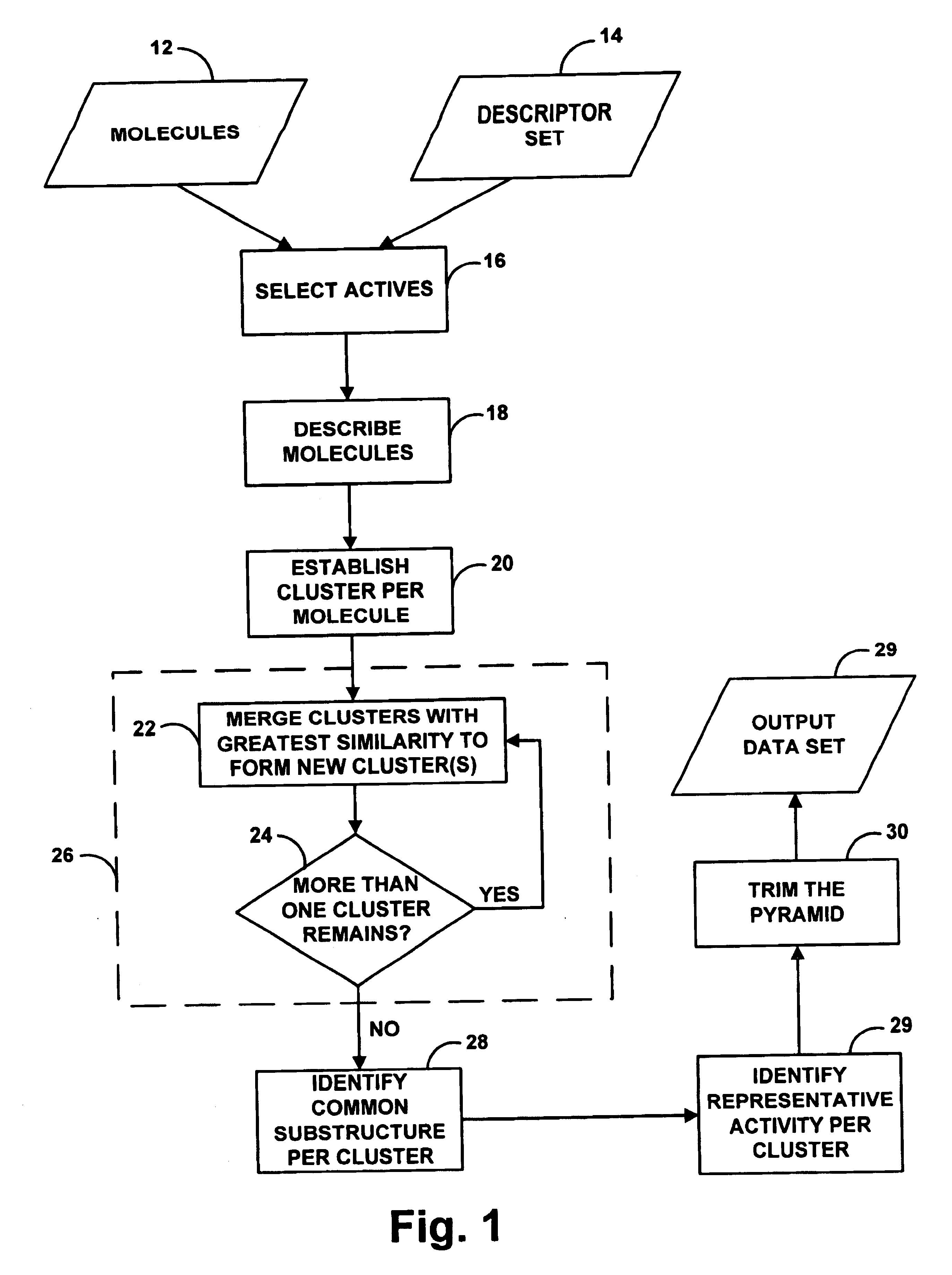
Method and system for artificial intelligence directed lead discovery though multi-domain agglomerative clustering
A system for helping a chemist to identify pharmacophoric mechanisms, based on a set of input data representing many chemical compounds. Given an input data set defining for each compound a feature characteristic and an activity characteristic, a computer agglomeratively clusters representations of the molecules based on their feature characteristics. The result of this process is a multi-domain pyramid structure, made up of a number of nodes each representing one or more molecules. For each node, the computer identifies a representative feature set (such as a largest substructure common among the molecules in the node) and a representative activity level (such as an average of the activity levels of the molecules in the node). The computer then provides as output to a chemist a description of all or part of the pyramid. This process thus converts a large set of raw data into an understandable and commercially useful form, which can assist the chemist in developing beneficial new pharmaceuticals.
Owner:SIMULATIONS PLUS +6
Method of evaluating drug efficacy for treating atherosclerosis
A method of evaluating drug efficacy of experimental pharmaceutical agents or biological agents proposed for the treatment of arterial plaque burden is described herein. Arterial plaque from a first appendage is removed and analyzed for the content of one or more markers. One or more test pharmaceutical agent or biological agent alone or in combination are administered to the patient for a specified period of time equal to a period of time after which it is supposed that a marker level will have changed in response to the drug. Arterial plaque is then removed from a second appendage and analyzed for the same marker or markers as the first plaque tissue in order to determine whether the test pharmaceutical agent or biological agent was efficacious in altering the production of the marker in the patient.
Owner:TYCO HEALTHCARE GRP LP
Human-body absorbable trauma dressing containing Yunnan white drug powder or Yunnan white drug powder extractive
InactiveCN101804218AIncrease usageImprove usabilityAbsorbent padsBandagesDressing changeCurative effect
The invention discloses a human-body absorbable trauma dressing containing Yunnan white drug powder or Yunnan white drug powder extractive, which is a novel medicine-carried dressing or a novel formulation of Yunnan white drug powder. The invention has the following remarkable characteristics: (1) the dressing can be absorbed by human bodies to lessen the pain added by dressing change and reduce the treatment cost; (2) the dressing can be made into a film solid dressing or an aquagel dressing so as to expand the use modes, the scope of applications and the drug effect of the Yunnan white drug powder; and (3) the curative effect of the dressing is enhanced by selecting a carrier material, auxiliary medicaments and functional accessories and adjusting the microstructure structure. The novel absorbable Yunnan white drug powder dressing overcomes the defects of the traditional Yunnan white drug powder in use and has economic and social values.
Owner:王艳
Methods for improving drug efficacy
ActiveUS20140186341A1Reduce airway obstructionImprove efficacyBiocideElectrotherapyNervous systemObstructive Pulmonary Diseases
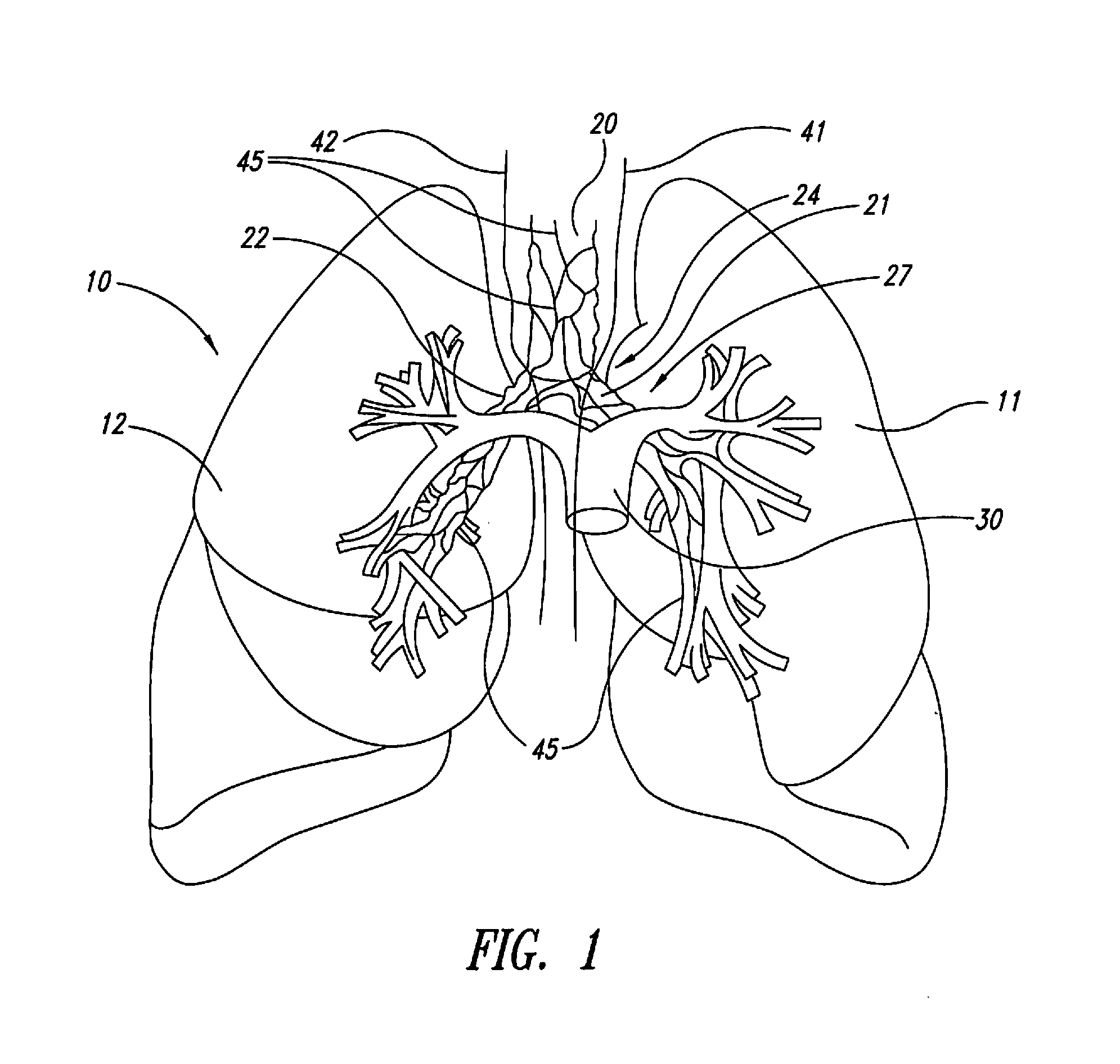
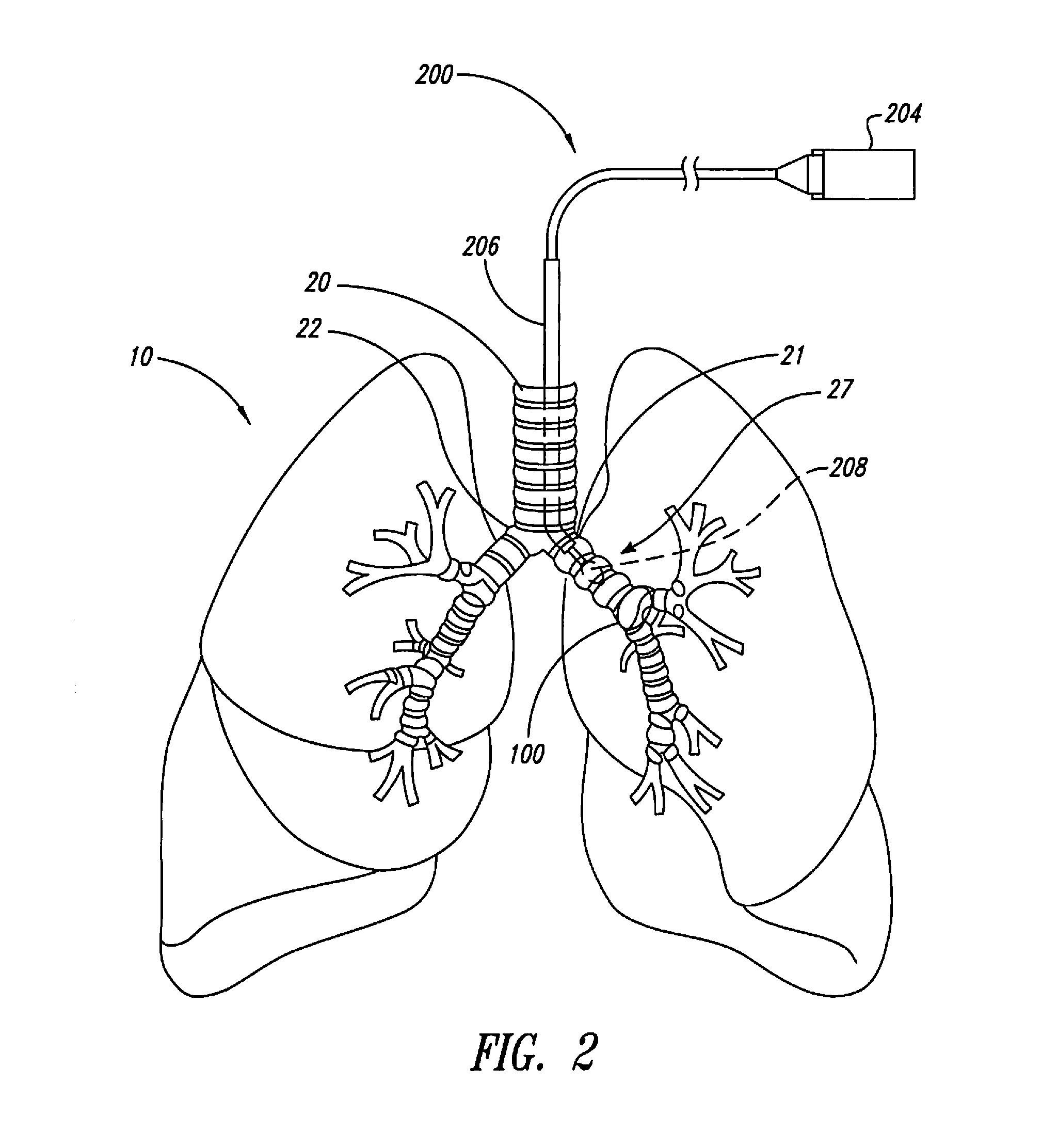
The present disclosure provides methods for improving drug efficacy in a patient having an obstructed airway in a lung. Such methods modulate nerve activity in the autonomic nervous system of a patient to reduce obstruction of an airway in a lung of the patient prior to administering a drug to the patient. These methods are especially useful in improving efficacies of bronchodilators in treating obstructive lung diseases, such as chronic obstructive pulmonary disease.
Owner:NUVAIRA INC
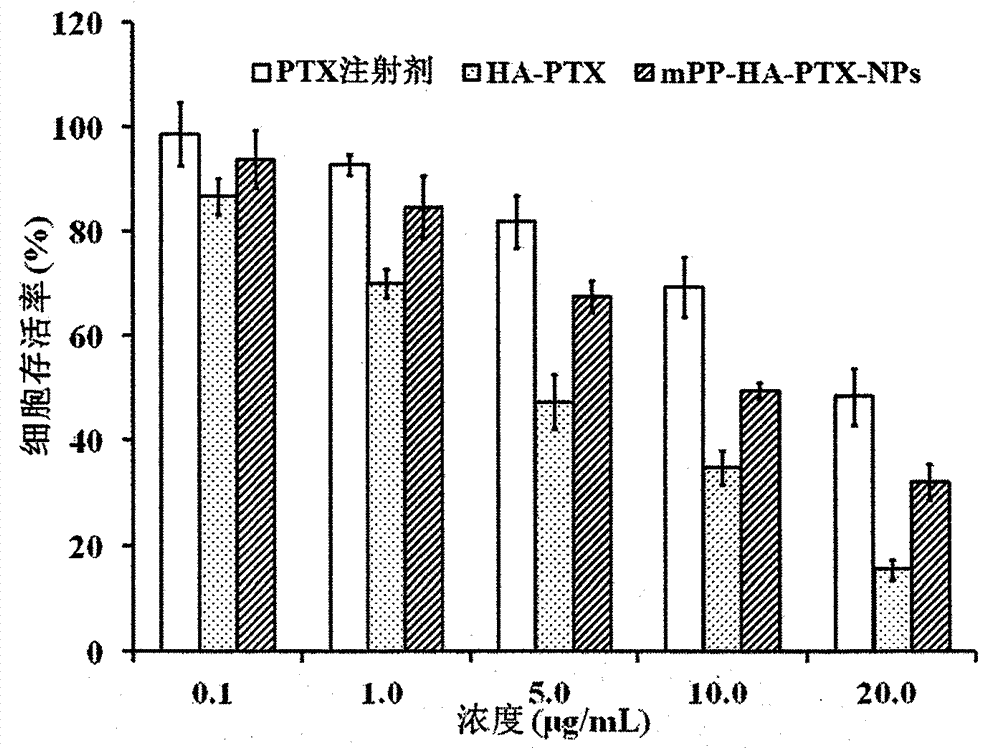
Preparation and application of hyaluronic acid-antitumor drug conjugate and composite nanoparticle composition
ActiveCN103751795AImprove solubilityAvoid devouringOrganic active ingredientsPharmaceutical non-active ingredientsTumor targetingEfficacy
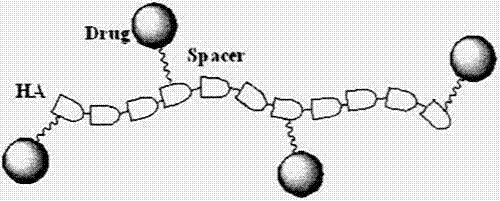
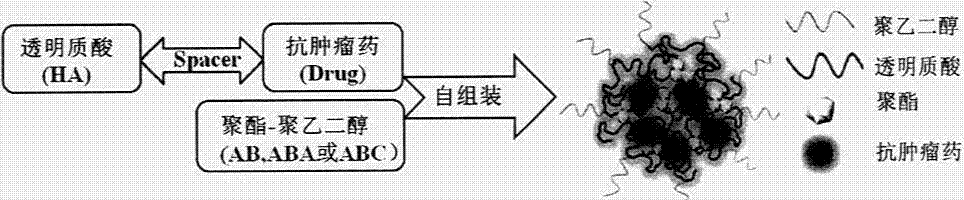
The present invention relates to a preparation method and an application of a hyaluronic acid-antitumor drug conjugate and composite nanoparticle composition with characteristics of active targeting antitumor effect and biodegradability. The preparation method is characterized by comprising: (1) a synthesis method for conjugating an antitumor drug and a spacer and conjugating a targeting ligand hyaluronic acid or an ammonium salt thereof and the antitumor drug-spacer; and (2) a new technology for assembling the hyaluronic acid-antitumor drug conjugate and amphiphilic polyester block copolymer composite nanoparticles. The composition has effects of substantially increased drug loading, efficacy improving, in vivo long-circulating effect achievement, active tumor targeting property and drug toxic-side effect reduction. The macromolecular conjugate and complex nanoparticle composition can be used for injection administration, oral administration or mucosal administration. In addition, the preparation method has characteristics of mature process and high yield, and is suitable for industrial production.
Owner:CHINA PHARM UNIV
Marker for detecting colon and rectum cancer as well as detection method, kit and biological chip thereof
InactiveCN101988060AEasy to storeWide detection rangeMicrobiological testing/measurementDNA/RNA fragmentationBULK ACTIVE INGREDIENTBlood plasma
The invention relates to a marker for detecting colon and rectum cancer by utilizing 86 specific ribonucleic acids stably existing in the serum / plasma of a human body, as well as a method, a relevant kit and a biological chip for detecting the marker. The method can be used in the aspects of diagnosing and differentially diagnosing the colon and rectum cancer and predicting the generation and the recurrence of the complications of diseases, evaluating the curative effect, screening the active ingredients in drugs, evaluating the drug effect and the like, and has the advantages of wide detection pedigree, high sensitivity and low detection cost, material is convenient for obtainment, samples are easy to store and the like. The method can be widely used for generally surveying the colon and rectum cancer and relevant work and improving low specificity and low sensitivity which are brought by the individual difference difficultly and are difficult to overcome by the single marker, thereby obviously enhancing the clinical detection rate of the colon and rectum cancer and becoming an effective means for early diagnosing the colon and rectum cancer.
Owner:JIANGSU MICROMEDMARK BIOTECH
Amino-modified mesoporous silica with dual drug-loading effects
InactiveCN104027814AIncrease load factorImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsMicrosphereUrsolic acid
The invention relates to the technical field of nano materials and specifically relates to amino-modified silica with dual drug-loading effects. According to the technical scheme, firstly, mesoporous silica nanoparticles are prepared by a template method; secondly, surface amino modification is carried out onto the mesoporous silica nanoparticles by 3-aminopropyltriethoxy silane; thirdly, ursolic acid is chemically coupled to the spherical mesoporous silica nanoparticles by amido bonds; and fourthly, the coupled nanoparticles are dispersed in DMF (dimethyl formamide) solution; a proper amount of ursolic acid is added, mixed and stirred; and finally vacuum drying is carried out to obtain the amino-modified silica with dual drug-loading effects. The mesoporous nano material with dual drug-loading effects prepared by the mesoporous silica disclosed by the invention is high in loading rate, can achieve controlled release effect for the loading drug ursolic acid, so that drug effect lasts for a long period time, and therefore, bioavailability of the ursolic acid is greatly improved.
Owner:FUZHOU UNIV
Pancreatic cancer marker, and detection method, kit and biochip thereof
ActiveCN101942502AEasy to storeWide detection rangeMicrobiological testing/measurementDNA/RNA fragmentationBlood plasmaBULK ACTIVE INGREDIENT
The invention provides a pancreatic cancer marker, and a detection method, a kit and a biochip thereof. The pancreatic cancer marker provided by the invention contains 36 kinds of micro ribonucleic acids which stably exist in the serum / plasma of a person receiving the test and can be detected. The invention also provides a kit and a biochip of tools or elements for detecting the pancreatic cancer marker. The combination, the method, the kit and the biochip provided by the invention can be used for auxiliary diagnosis and differential diagnosis of pancreatic cancers, predication of occurrence and recrudescence of disease complications, evaluation of the curative effect, screening of active ingredients of medicaments, evaluation of pesticide effectiveness and the like, and has the advantages of wide detection range, high sensitivity, low detection cost, readily available raw materials, easy storage of samples and the like; and the method is widely applied to work related to the general survey of pancreatic cancers, improves the specificity and sensitivity which are low in the single marker due to individual difference, obviously increases clinical detection rate of pancreatic cancers and becomes an effective method for early diagnosis of pancreatic cancers.
Owner:JIANGSU MICROMEDMARK BIOTECH
Construction and prediction method of integrated drug target prediction system
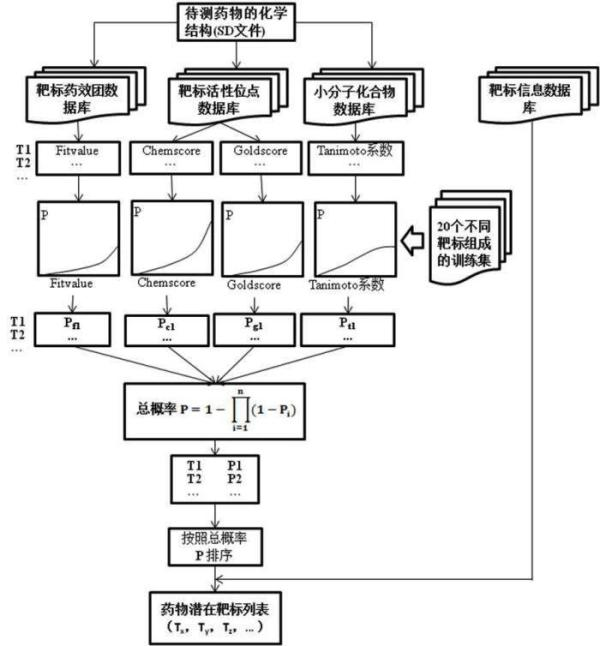
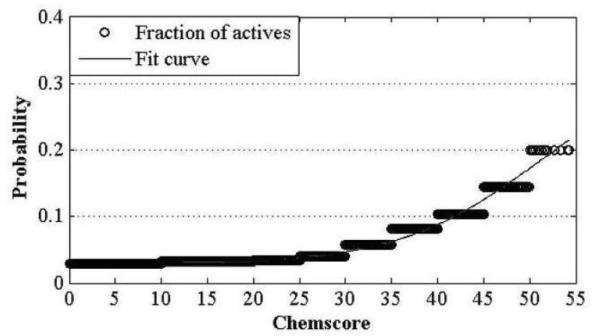
InactiveCN102663214AOvercoming the problem of low forecasting accuracySpecial data processing applicationsPharmacophoreEngineering
The invention discloses a construction and prediction method of an integrated drug target prediction system. The method comprises the following steps of: analyzing a protein crystal structure database; selecting the protein bound with the drug-like ligand small molecule or the protein with the small molecule ligand binding potential as a target point, and building a crystal structure database of targets; for these targets, collecting the information of the targets related to diseases, the biology type and the active small molecule ligand information, and integrating a comprehensive target screening database composed of an active site database, a pharmacophore database, a small molecular compound database and a target basic information database. Based on the comprehensive target screening database, the construction of the integrated drug target prediction system is realized through a script program or a PipelinePilot flow, and the probability of target prediction accuracy of the method is provided. The method provided by the invention exerts the three above technical advantages to provide the probability of target prediction, thereby providing effective basis for further experimental verification.
Owner:SICHUAN UNIV
Tandospirone citrate, preparation method thereof, formulations and quality control method
ActiveCN101362751ARaise quality standardsImprove clinical anxiolytic effectNervous disorderOrganic chemistryCITRATE ESTERQuality control
The invention provides citrate tandospirone which is characterized by comprising a compound I with the weight percent content not more than 0.5 percent and a compound II with the weight percent content not more than 0.5 percent. The structure formulas of the compound I and the compound II are as the right formulas. The invention also provides a preparation method and a quality control method of the citrate tandospirone. The results of a pharmacodynamic test and clinical observation show that after the quality standard of the citrate tandospirone is improved, the effect of clinical antianxiety is obviously improved and untoward effect is obviously reduced.
Owner:SICHUAN CREDIT PHARMA
Composite biological agent of crusta oligosaccharide and alga fertilizer, and preparation method
ActiveCN101092315ADoes not affect absorptionImprove immunityBiocideAnimal repellantsBiological agentAlginic acid
This invention discloses a method for preparing chito-oligosaccharide / seaweed fertilizer composite biological agent. The composite biological agent is composed of: chito-oligosaccharide 0.2-3 wt. %, seaweed fertilizer 0.5-3 wt. % (according to alginic acid weight), buffer solution (pH = 5-8) 1-30 wt. % (according to solute weight), and water as balance. The composite biological agent has improved drug efficiency and fertilizer efficiency, and can prevent and treat plant disease. Besides, the composite biological agent is environmentally friendly.
Owner:QINGDAO SONTI BIOTECH
Fusion protein for treating diabetes, and its preparing method and use
InactiveCN1935846AImprove efficacyEasy to separate and purifyOrganic active ingredientsMetabolism disorderHalf-lifeFactor ii
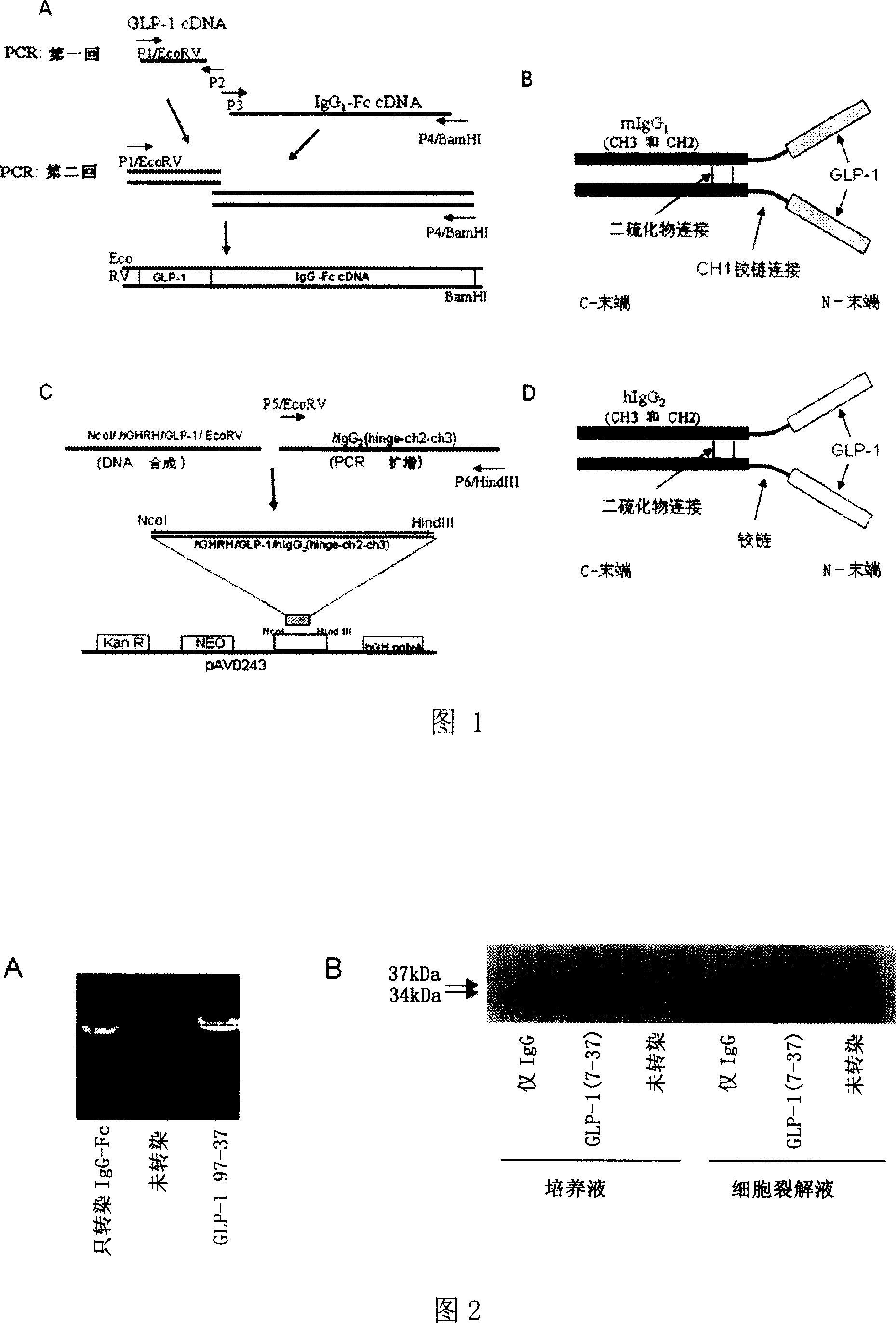
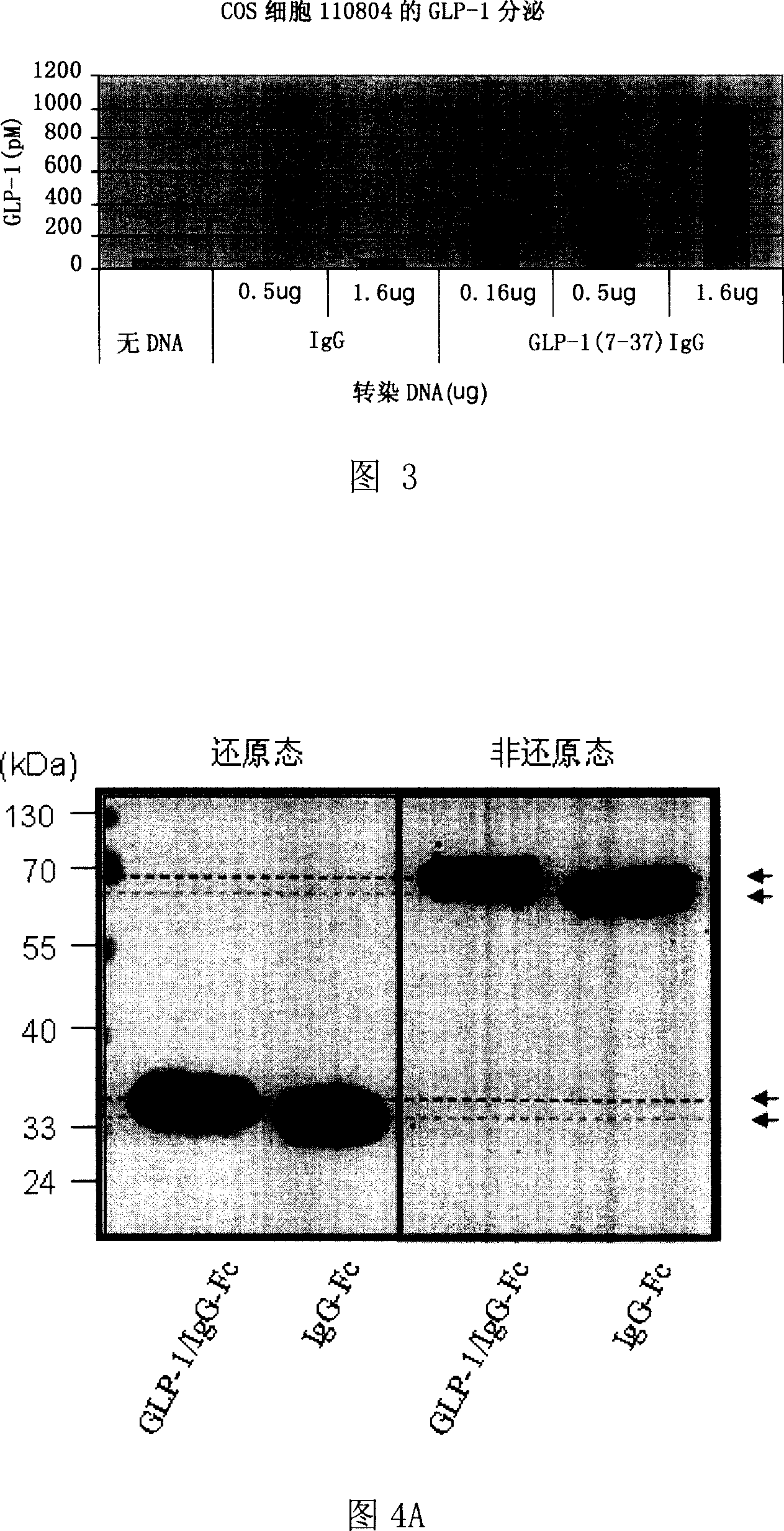
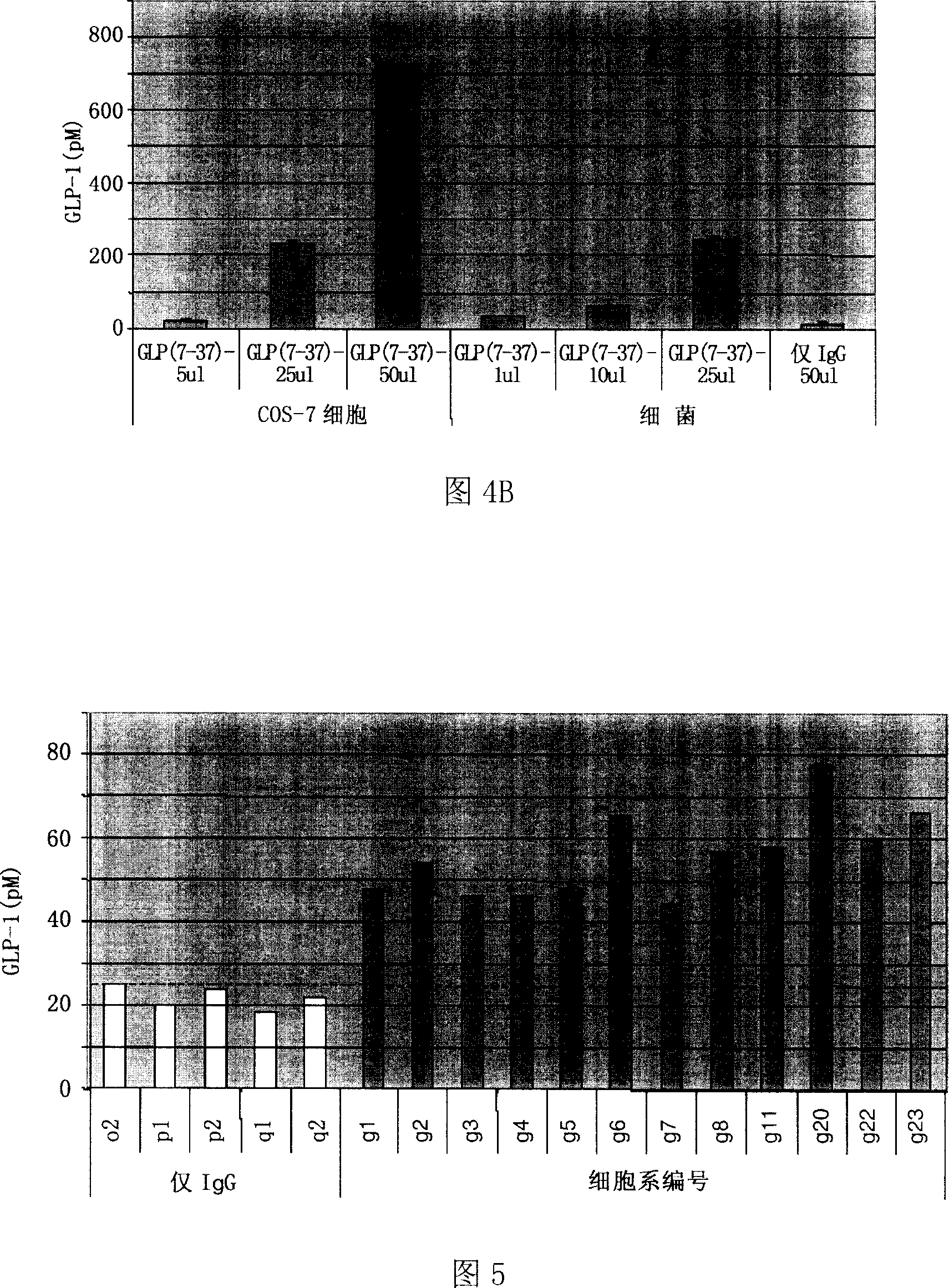
The invention relates to fusion protein used to prevent and cure I type and II type diabetes and its preparation method and application. It supplies the fusion protein which is formed by glucagon analogy peptide GLP-1, GLP-1 mutant and IgG-Fc, its analogy factor lizard salivary gland polypeptide extendin-4 and IgG-Fc, and the application of the above fusion protein and its DNA used in diabetes prevention and therapy. The fusion protein can increase not only GLP-1 effect, but also the affinity and immunological tolerance of ligand, which is excreted with the form of homogeneity dimmer to improve polypeptide drug effect, overcomes the defect of the GLP-1 for short half-life, and simplifies purification process.
Owner:王庆华 +1
Compound liposome containing anti-tumor drugs and preparation method and application thereof
InactiveCN102091036ASolve the problem of poor wettabilityReduce harmMacromolecular non-active ingredientsAntineoplastic agentsDiseaseLiposome
The invention belongs to a liposome, in particular relates to a compound liposome preparation with high targeting property of tumor tissues and efficient penetrability of tumor cells. The invention is characterized in that hyaluronic acid or sodium hyaluronate, tumor cell selective penetrating peptides and a liposome are used for together constructing a compound liposome. The compound liposome is characterized by utilizing the dual-tumor cell targeting property of the hyaluronic acid and tumor cell selective penetrating peptides to effectively solve the problem that because a conventional liposome and traditional cell penetrating peptides have no selectivity on normal cells and tumor cells, the toxicity is increased when the drug effect is increased in the therapeutic process, thereby fully meeting the clinical requirements of high efficiency and low toxicity when anti-tumor drugs are prepared into preparations for treating diseases.
Owner:CHINA PHARM UNIV
Quality control method of liuwei wuling tablets
InactiveCN105758962AReduce wear and tearShort analysis timeComponent separationBiological testingMedicinal herbsHplc fingerprint
The invention relates to the technical field of compound traditional Chinese preparations, and in particular relates to a quality control method of liuwei wuling tablets. The quality control method is characterized in that 20 different proportion groups are formed by randomly sampling six medicinal materials of the liuwei wuling tablets; a corresponding HPLC fingerprint spectrum is established, and in-vitro anti-hepatic fibrosis activity is measured; and an SPSS software is utilized to analyze, so that a pectrum-effect relationship of in-vitro anti-hepatic fibrosis effect of the liuwei wuling tablets is established. The relatively perfect quality control method is established on the basis of pharmacodynamic substances of the liuwei wuling tablets. Compared with a conventional single-index content measuring method, the quality control method is more comprehensive, scientific and standard, and has the advantages that the content of multiple components is simultaneously measured, so that not only the analysis time is shortened, but also the loss of a solvent is saved.
Owner:山东世博金都药业有限公司
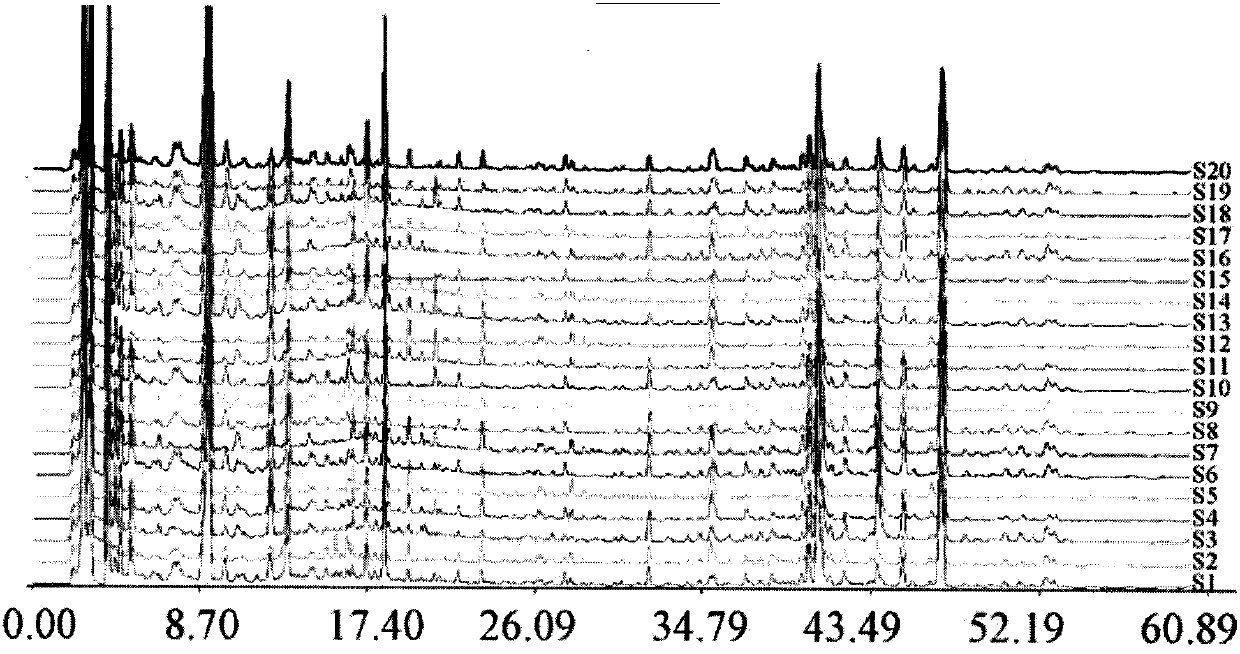
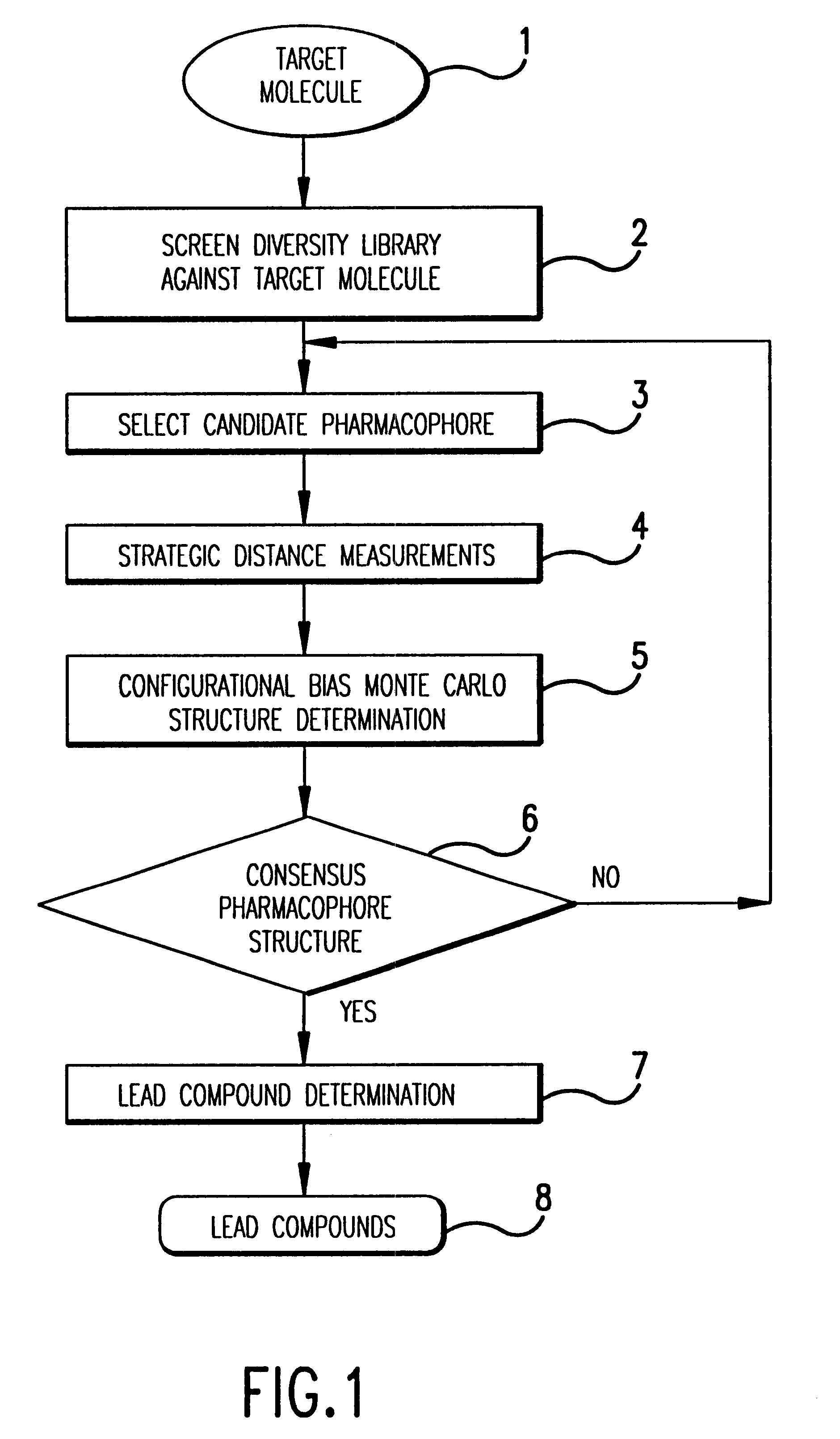
Consensus configurational bias Monte Carlo method and system for pharmacophore structure determination
InactiveUS6341256B1Accurate distance measurementMake a pharmacophore structure determinationPeptide librariesNanotechNMR - Nuclear magnetic resonancePeptide
In a specific embodiment, this invention includes a method for determining an accurate, consensus pharmacophore structure shared by compounds that bind selectively to a target molecule. Optionally, the method begins with screening a diversity library against the target molecule of interest to pick the selectively binding members. Next the structure of the selected members is examined and a candidate pharmacophore responsible for the binding to the target molecule is determined. Next, preferably by REDOR nuclear magnetic resonance, several highly accurate interatomic distances are determined in certain of the selected members which are related to the candidate pharmacophore. A highly accurate consensus, configurational bias, Monte Carlo method determination of the structure of the candidate pharmacophore is made using the structure of the selected members and incorporating as constraints the shared candidate pharmacophore and the several measured distances. This determination is adapted to efficiently examine only relatively low energy configurations while respecting any structural constraints present in the organic diversity library. If the diversity library contains short peptides, the determination respects the known degrees of freedom of peptides as well as any internal constraints, such as those imposed by disulfide bridges. Finally, the highly accurate pharmacophore so determined is used to select lead organics for drug development targeted at the initial target molecule.
Owner:CURAGEN CORP
Ginsenoside Rg3 liposome and preparation method thereof
InactiveCN103417479AOrganic active ingredientsPharmaceutical non-active ingredientsInhalationEfficacy
The invention provides ginsenoside Rg3 liposome and a preparation method thereof, a drug is encapsulated by using the manner of liposome and proliposome, the obtained liposome has a high encapsulation rate and a stable property, and can significantly improve the absorbable degree and bioavailability of the ginsenoside Rg3, meanwhile enhance the targeting property thereof to a tumor tissue, and improve drug efficacy. At the same time, the obtained liposome can be dosed in a plurality of manners such as oral taking, injection, inhalation by lung, and the like, and can improve the application scope of drugs and the compliance of patients.
Owner:JILIN UNIV
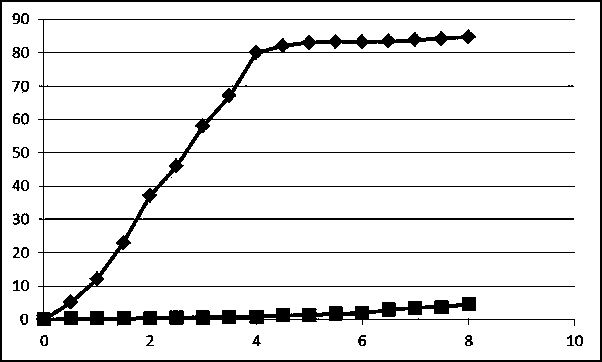
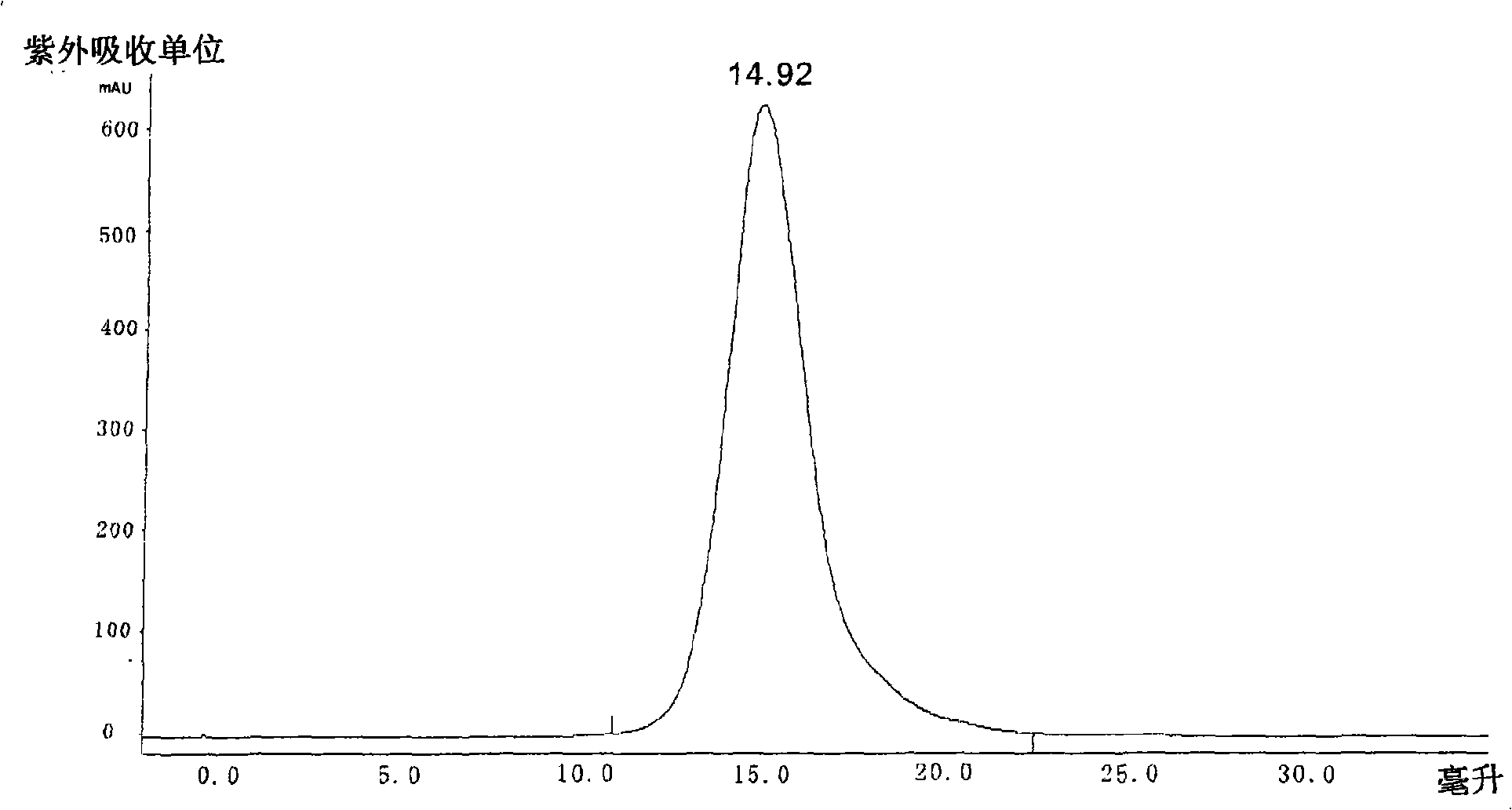
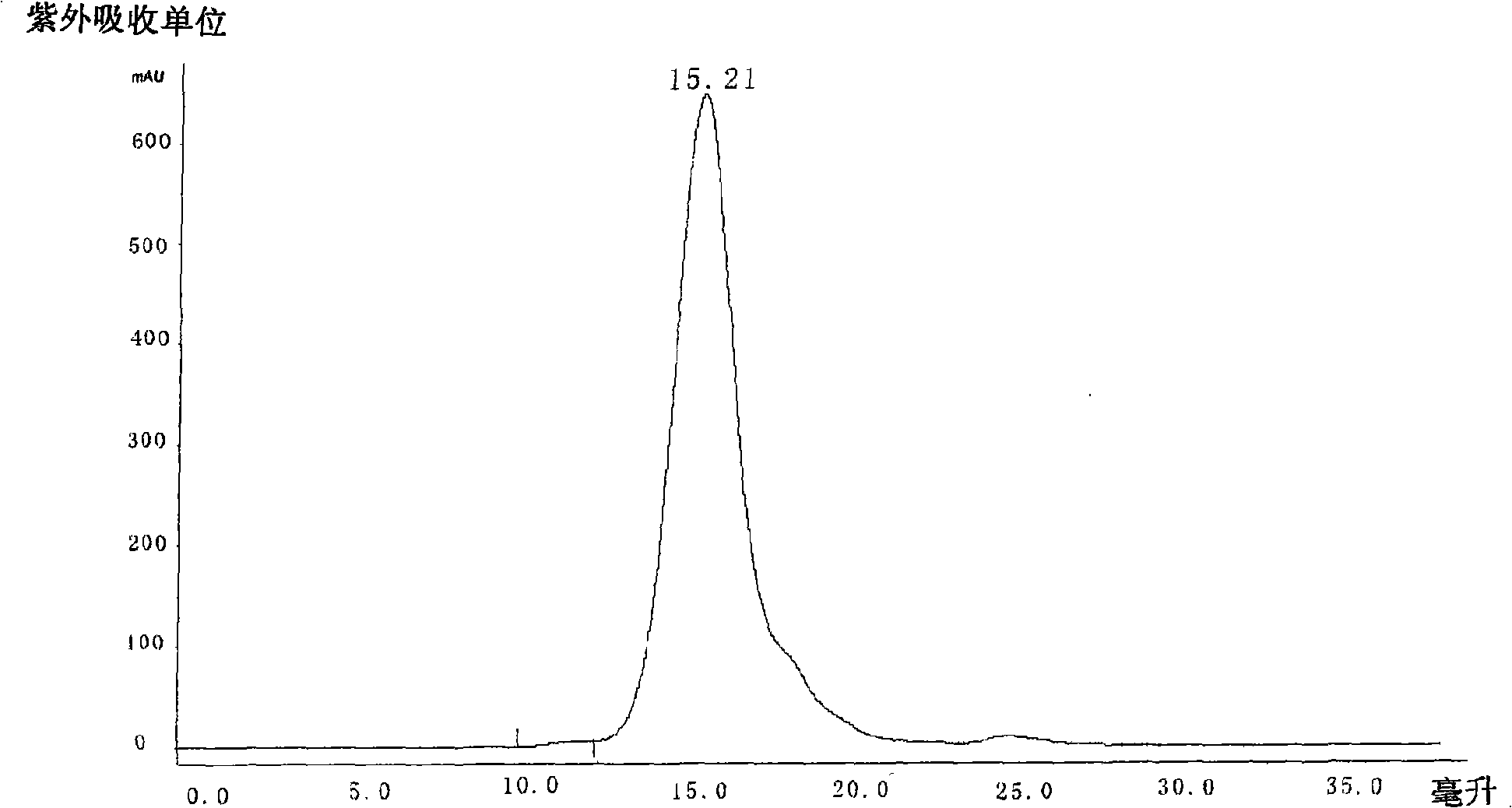
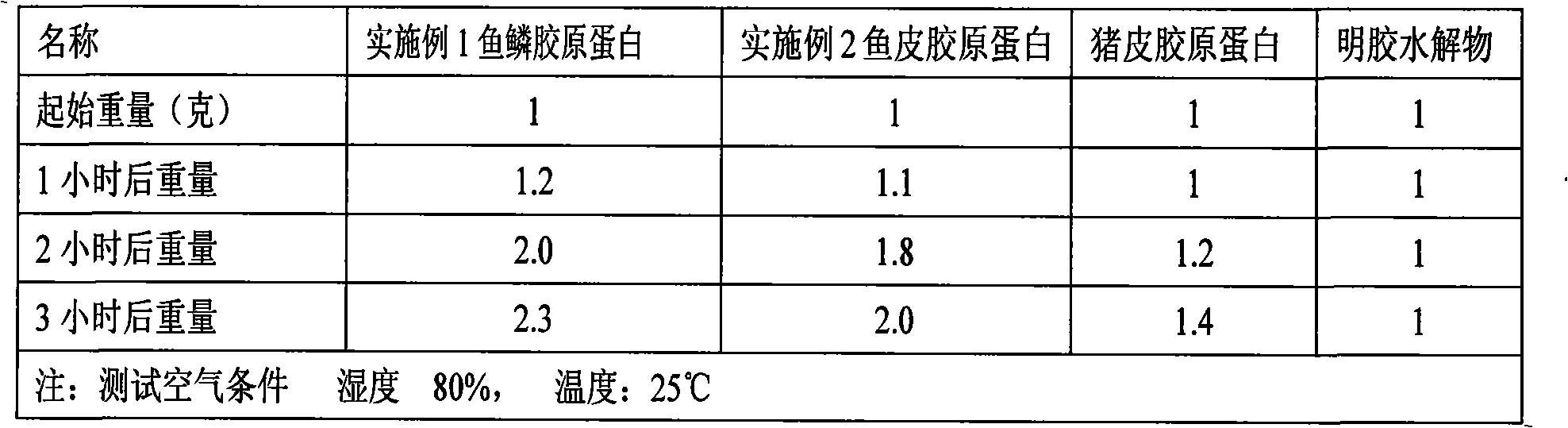
Collagen protein and collagen polypeptides, preparation thereof and applications
ActiveCN101289507AMolecular weight can be controlledControllable distributionOrganic active ingredientsCosmetic preparationsProcess equipmentArthritis
The invention provides a collagen and a collagen polypeptide, wherein, the weight percentage of hydroxylysine is more than 1.3 percent. The invention also provides the preparation technology and the application of the collagen and the collagen polypeptide. The invention can make full use of inexpensive raw materials including the fish skin and fish scale of tilapias to produce products with high added value. The collagen polypeptide is used for treating arthritis with a definite drug effect, and the clinical dose is considerably less than other collagen polypeptides. The collagen polypeptide can solve the pollution problem of fish skin wastes, and at the same time avoid the risk of infectious diseases which can be caused by the collagen of land animals. The collagen and the collagen polypeptide have the advantages of considerable economic benefits, energy conservation and environmental protection, simple process equipment, low cost and easy industrialization.
Owner:史宗洁
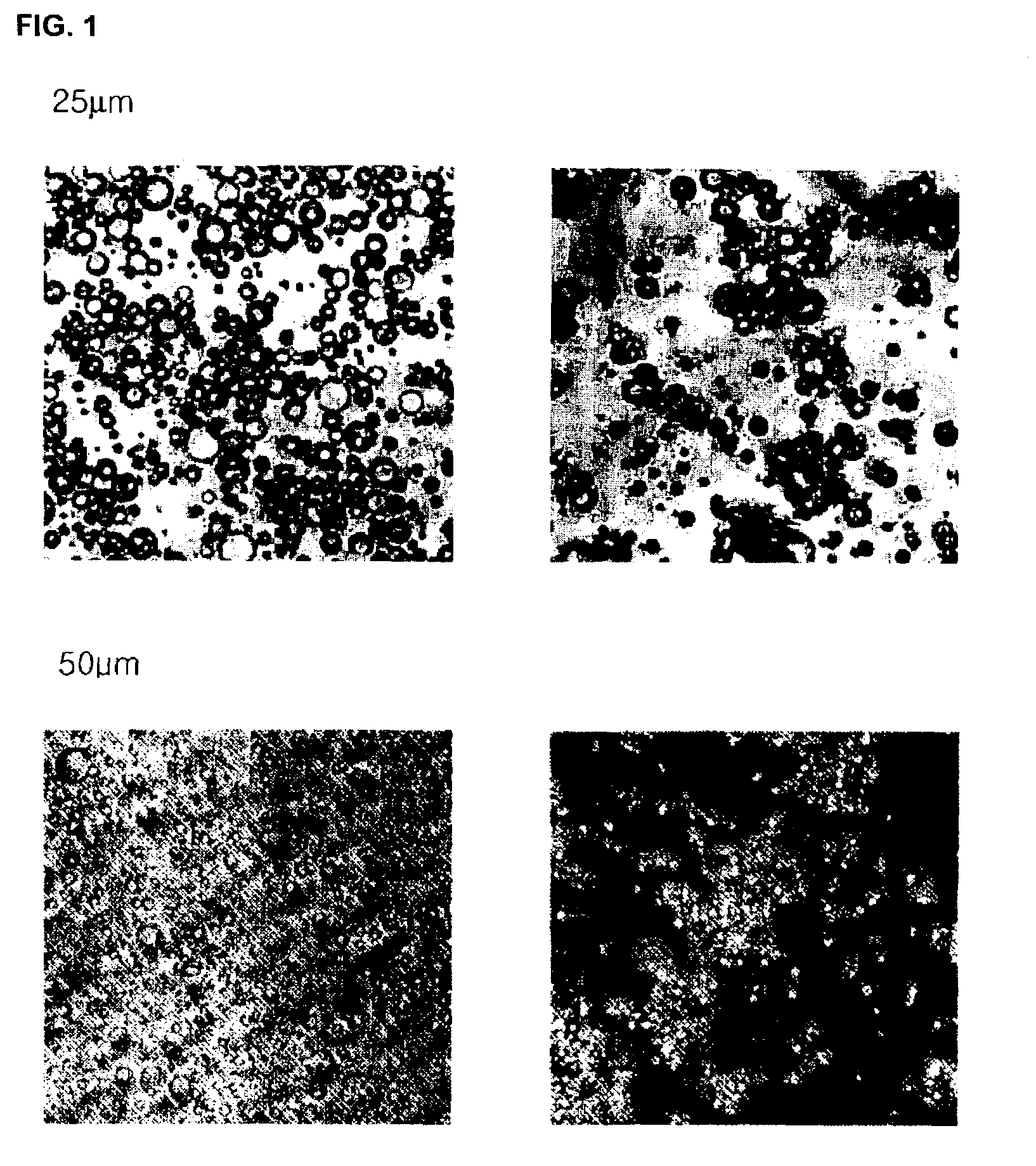
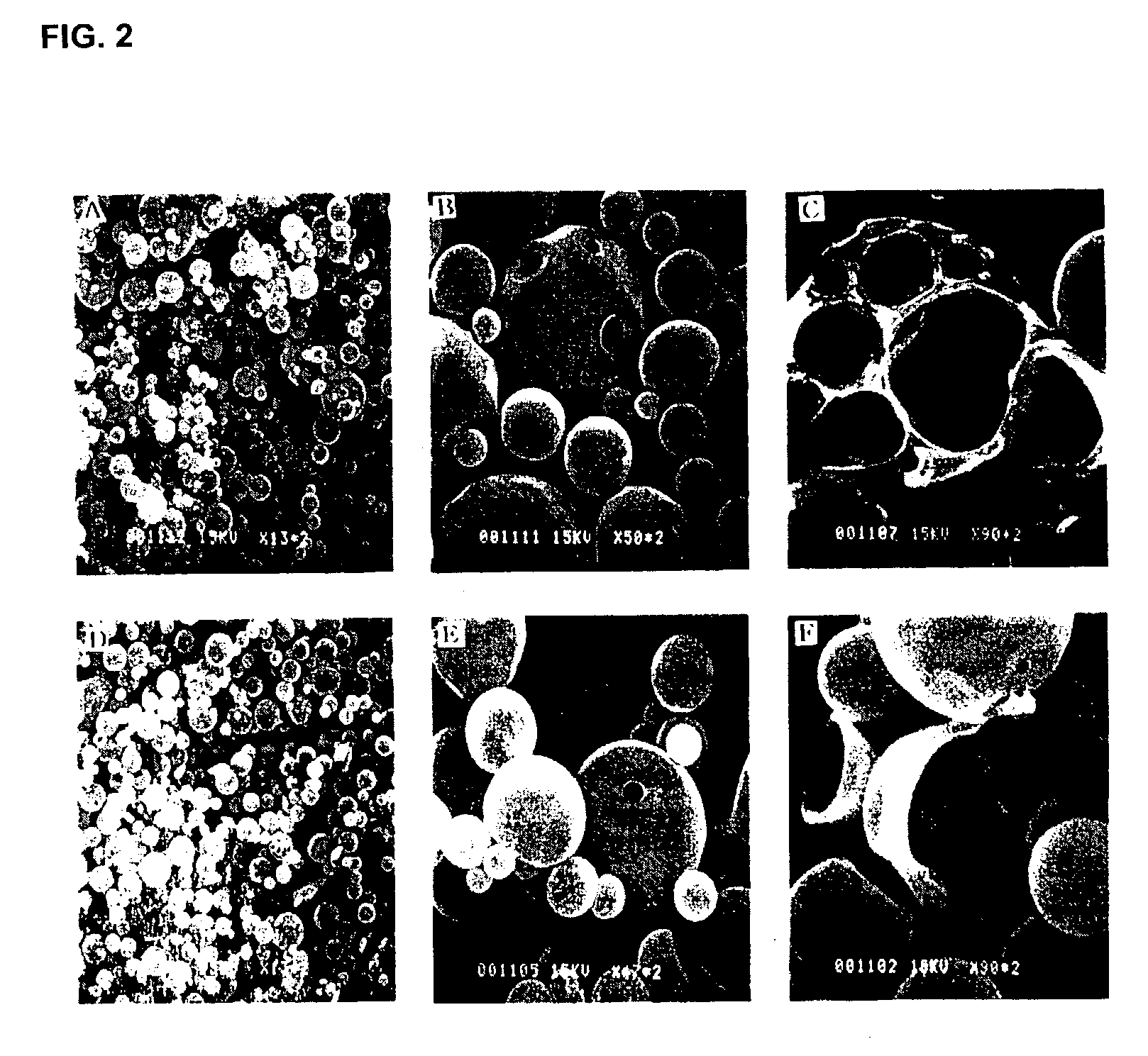
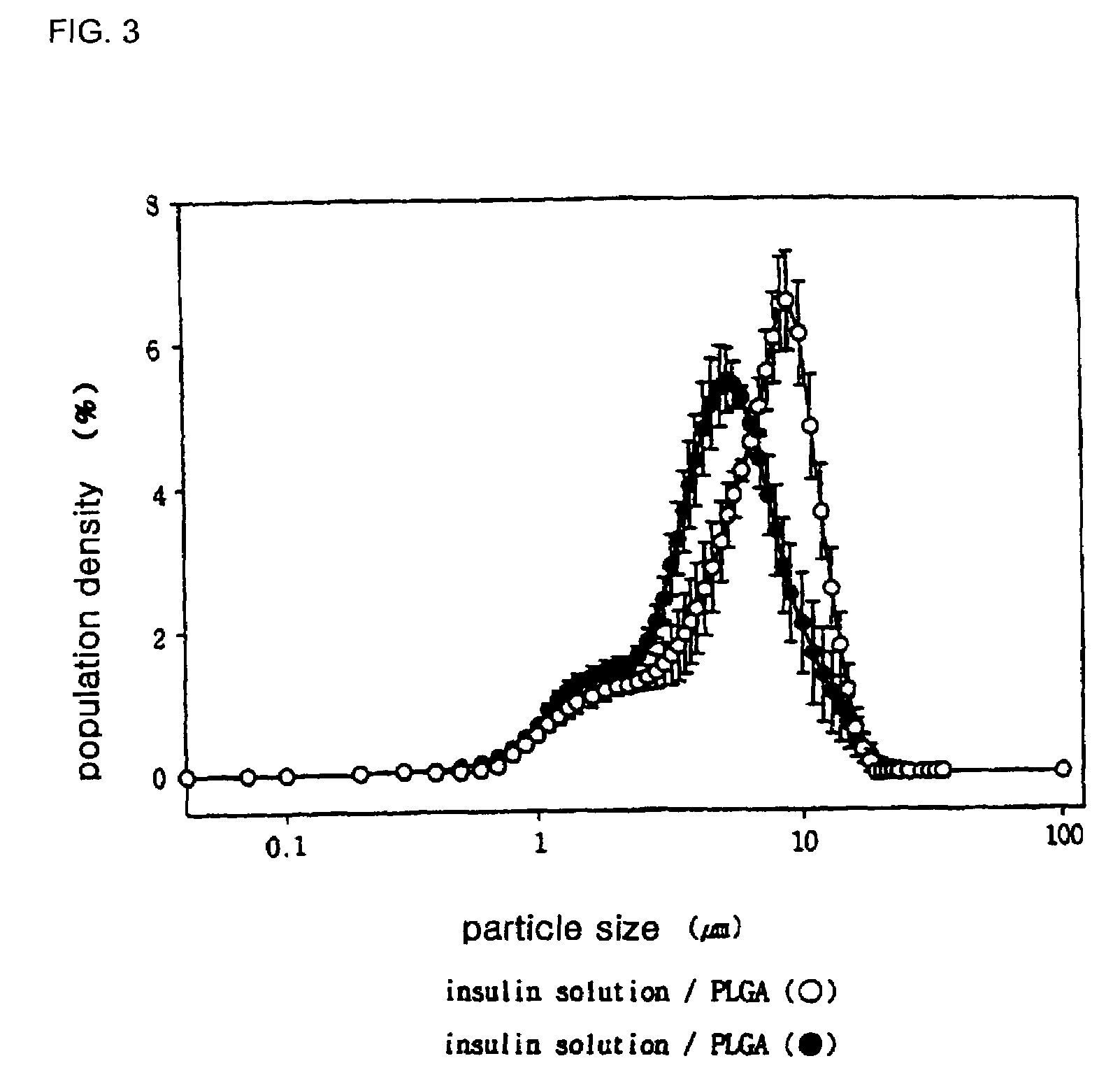
Controlled release preparation of insulin and its method
InactiveUS7087246B2Easily prone to denaturationLarge molecular weightPowder deliveryBiocideDrugMicroencapsulations
A controlled release preparation of insulin and its method are provided. The controlled release preparation of insulin contains microparticles obtained by microencapsulation of uniform microcystals of insulin using biodegradable polymeric materials. Since the denaturation of insulin that may occur during microencapsulation is reduced, the stability of the preparation can be increased. Also, the ratio of insulin to a polymer carrier is increased, which is suitable for pulmonary delivery. Further, the controlled release preparation of insulin can continuously exhibit pharmaceutical efficacy in vivo in a stable manner for an extended period of time.
Owner:APROTECH
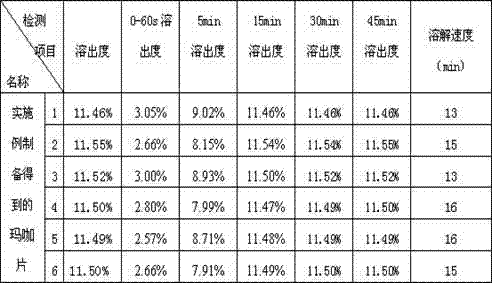
Maca tablet and preparation method thereof
InactiveCN102823798AImprove bioavailabilityRapid dissolutionFood preparationMagnesium stearateStearic acid
The invention provides a maca tablet and a preparation method thereof and relates to the technical field of health care products. The maca tablet is prepared by the following raw materials by weight: 581.84 g of maca powder, 56.48 g of microcrystalline cellulose, 30.4 g of lactose, 78.56 g of povidone, 15.12 g of magnesium stearate and 37.6 g of starch; and the average relative molecular weight of the povidone is 2000. The preparation method of the maca tablet comprises the following steps of: uniformly mixing all the raw materials, spraying 90 percent of ethanol solution to moisten, palletizing, drying, carrying out intermediate inspection, granulating and tabletting; and spraying 456.5 g of ethanol solution on every 800 g of raw materials. The maca tablet has the advantages that the effective components are quickly dissolved out, the biological availability is high, and the medical effects of effective component maca can be better realized, and the cost for drug consumption is reduced. The maca tablet has the effects of relieving the physical fatigue, improving the sexual function, improving the fertility, adjusting the internal secretion and the like, and particularly relieving the physical fatigue and the improving the sexual function.
Owner:SHANDONG YIBAO BIOLOGICS
Breast cancer detecting marker as well as detecting method, kit and biological chip thereof
InactiveCN101988061AEasy to storeWide detection rangeMicrobiological testing/measurementDNA/RNA fragmentationHuman bodyEarly breast cancer
The invention relates to a breast cancer detecting marker by utilizing 16 specific tiny ribonucleic acids steadily existing in blood serum and blood plasma of a human body as well as a detecting method, a kit and a biological chip thereof, and the detecting marker can be used in the aspects of diagnosis and differential diagnosis of breast cancer, occurrence and recurrence forecast as well as efficacy assessment of disease complications, screening and therapeutic evaluation of medicinal active ingredients and the like, and has the advantages of wide detection pedigree, high sensitivity and low detection cost, the materials are conveniently taken, the samples are easy to store and the like. The method can be widely used in related work such as breast cancer census, the defects of low specificity and low sensitivity caused by insuperable individual difference of a single marker can be improved and the clinical detectable rate of the breast cancer is notably improved, and is an effective mean of early breast cancer diagnosis.
Owner:JIANGSU MICROMEDMARK BIOTECH
Exendin-4 modified by Evans blue or derivatives of Evans blue and preparation method and application of Exendin-4
ActiveCN104650217AProlong half-life in vivoEasy to manufactureHormone peptidesPeptide/protein ingredientsDiseaseHalf-life
The invention relates to a preparation method and application of Exendin-4 biologically located and modified by Evans blue (Evans Blue) or derivatives of Evans blue. The product generated after the C terminal of Exendin-4 is specifically modified with Evans blue or derivatives of Evans blue has biological activity similar to Exendin-4 which is not modified but has longer in vivo half-life than the Exendin-4 which is not modified. Based on the significance of the Exendin-4 in treatment of diseases, the invention also discloses application of the Exendin-4 modified by Evans blue or derivatives of Evans blue in preparation of medicines for treating type-II diabetes and myocardial infarction. The Exendin-4 has the advantages of simplicity and convenience in preparation, obvious curative effect, long-lasting and stable drug effect, convenience in storage and the like and has significance in research and development of new drugs for promoting anti-diabetic and anti-infarction high-efficiency treatment.
Owner:SHANGHAI THERANOSTICS BIOTECH CO LTD
New application of patchouli alcohol
ActiveCN103156826AStrong inhibitory activityEffective treatmentAntibacterial agentsCosmetic preparationsBiotechnologyAntibacterial activity
The invention relates to an application of patchouli alcohol in preparation of antibacterial drugs, healthcare food, food, cosmetics, disinfectors or daily chemical articles. The invention also provides the antibacterial drugs, healthcare food, food, cosmetics, disinfectors or daily chemical articles. The patchouli alcohol has good antibacterial activity on pathogenic bacteria or conditional pathogen, can be used for effectively treating bacterium infectious diseases and simultaneously can also effectively antagonize methicillin-resistant staphylococcus epidermidis drug-resistance bacteria; and the pharmacodynamics activity of the patchouli alcohol is even equivalent to that of vancomycin, thus the possibility is provided for slowing down or avoiding the occurrence of the drug-resistance bacteria.
Owner:CHENGDU HUASUN GRP INC LTD +1
Establishment of self-assembly nanoparticles of redox hypersensitive disulfide bond bridged prodrug
InactiveCN108478803AImprove anti-tumor effectPromote enrichmentPowder deliveryHydroxy compound active ingredientsSynthesis methodsCarbon chain
The invention belongs to the technical field of medicine, and designs and synthesizes a series of disulfide bond-containing micromolecular prodrugs in which carbon chains in different lengths are linked (sulfur atoms in the disulfide bond are respectively located alpha, beta and gamma sites of an ester bond). PTX (Paclitaxel)-CIT (Citronellol) is used as a sample, and a synthesis method is simpleand easy. On the basis, a micromolecular prodrug self-assembly nano-drug delivery system is prepared. The preparation method is simple and convenient, the stability is high, and efficient encapsulation and delivery of drugs are realized. The invention discovers that the disulfide bond has redox dual sensitivity, can be fractured under the action of high-expression ROS (Reactive Oxygen Species) andGSH (Glutathione) of tumor cells, and PTX can be released; particularly, redox dual hypersensitivity is shown by PTX-CIT prodrugs (alpha-PTX-SS-CIT), which are located in the alpha site of a carbonylgroup, of the disulfide bond, the alpha-PTX-SS-CIT prodrugs can be quickly fractured to release the PTX and take effects, an anti-tumor effect of the PTX is remarkably improved, and a wide development prospect is obtained.
Owner:SHENYANG PHARMA UNIVERSITY
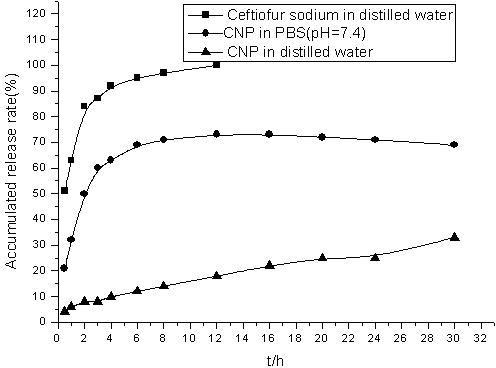
Chitosan nanoparticle preparation of ceftiofur sodium, and preparation method thereof
InactiveCN102319219AAchieve sustained releaseProlong the action timeAntibacterial agentsPowder deliveryChitosan nanoparticlesCeftiofur sodium
The invention belongs to the field of drug nanopreparation, especially relates to a chitosan nanoparticle preparation of ceftiofur sodium, and a preparation method thereof. The chitosan nanoparticle preparation is characterized in that: the preparation comprises 3-7 mg / mL of chitosan, 1.13-2.63 mg / mL of sodium tripolyphosphate and 2-5 mg / mL of ceftiofur sodium. The preparation method adopts an ionic crosslinking method and comprises the following steps: dissolving the chitosan, dissolving the ceftiofur sodium in the sodium tripolyphosphate solution, adding the sodium tripolyphosphate solution containing the ceftiofur sodium to the chitosan solution under magnetic stirring, and the like. According to the present invention, the ceftiofur sodium chitosan nanoparticles have target distribution and slow release property, such that the action time of the drug can be prolonged, the efficacy can be increased, the toxic and side-effect of the drug can be reduced, the antibacterial stability of the drug can be improved; the preparation method has characteristics of simpleness, reliability and controllable quality, and is suitable for the industrial production.
Owner:SICHUAN JINRUIKE ANIMAL PHARMA
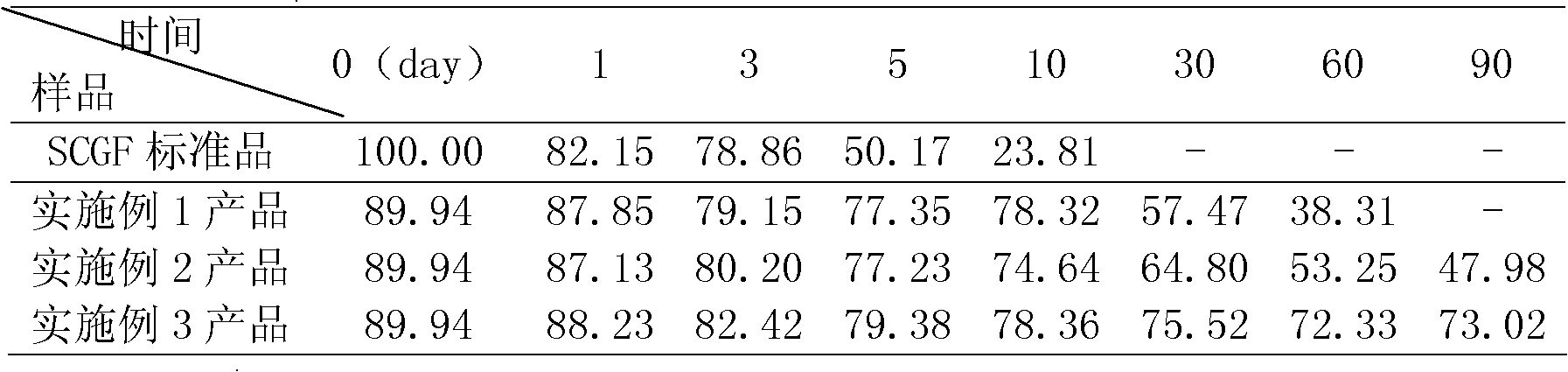
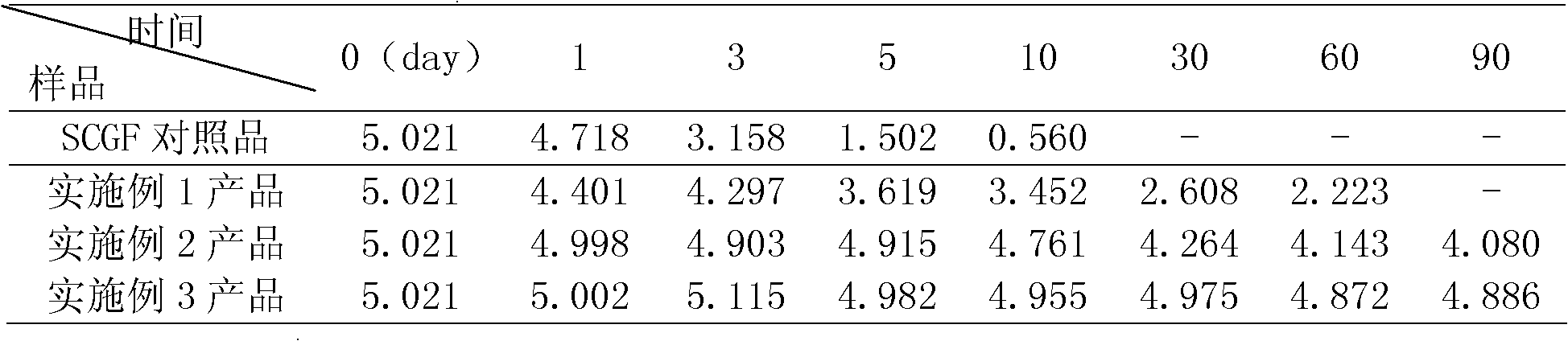
HSCGF liposome as well as preparation and application thereof
ActiveCN102512370AKeep aliveImprove stabilityCosmetic preparationsHair removalCholesterolPhospholipid
The invention discloses an HSCGF (Human Stem Cell Growth Factor) liposome as well as the preparation and the application thereof. The HSCGF liposome comprises 2.0 to 4.0 percent of HSCGF, 8.0 to 10.0 percent of phosphatide, 2.5 to 3.5 percent of cholesterol and 10 to 25 percent of protecting agents. The HSCGF is prepared into liposome, the activity of the HSCGF is kept effectively, the stability of SCGF (Stem Cell Growth Factor) can be improved obviously, skin can be promoted to absorb and utilize the SCGF, and the HSCGF liposome has the advantages of good affinity, no toxicity or side effects, and wide application range. The anti-wrinkle performance of cosmetics containing the HSCGF coated with the liposome can still be kept well after the stability is examined for three months. The HSCGF liposome is used as raw material and other active materials and auxiliary materials are used as supplements so as to prepare frequently-used medicine forms to illustrate the drug effect of the SCGF, so that the bioavailability can be improved.
Owner:GUANGZHOU SALIAI BIOLOGICAL GENETIC ENG CO LTD
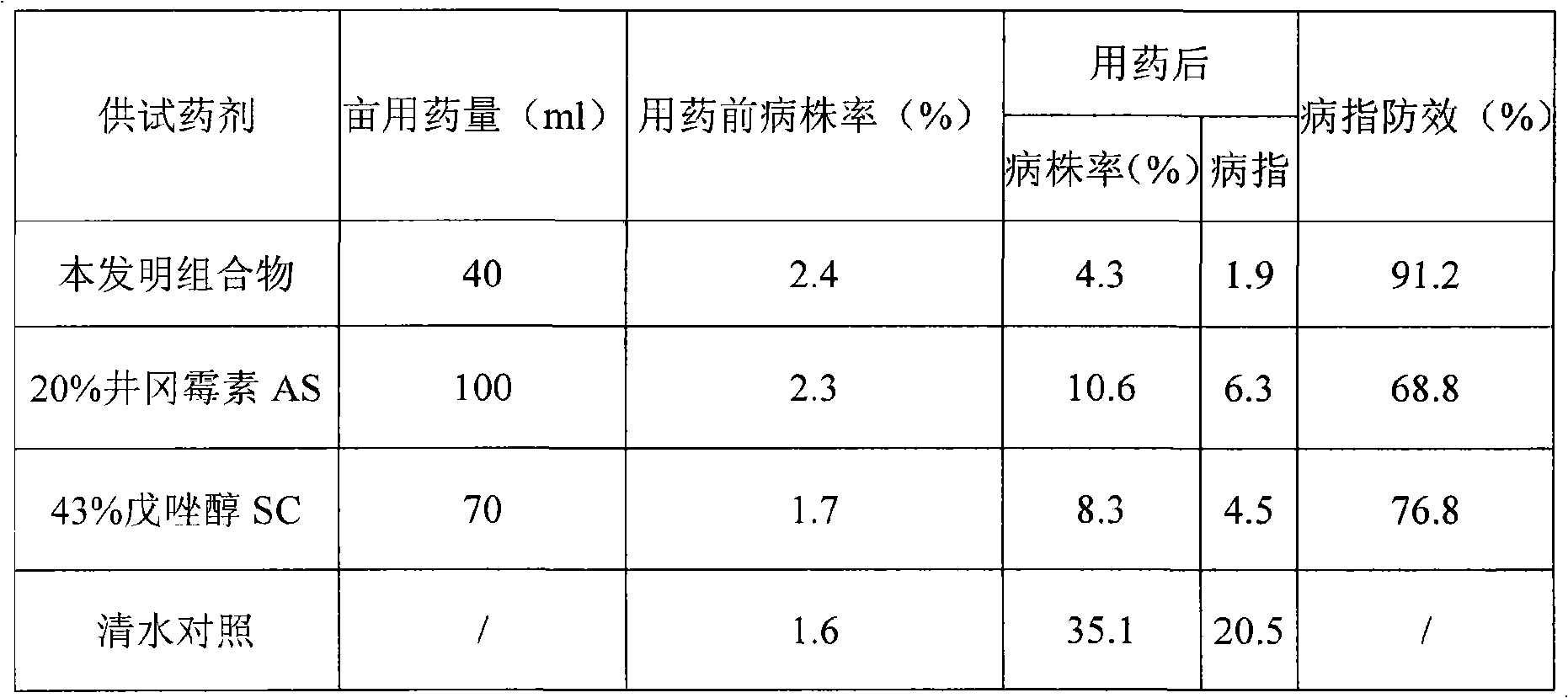
Sterilization composition containing Tebuconazole and Validamycin
InactiveCN101554168AReduce the burden onPromote production and incomeBiocideFungicidesEcological environmentSuspending Agents
The invention provides a sterilization composition containing Tebuconazole and Validamycin, which is aided with solvent and emulsifier to form suspending agent or form wettable powder agent with assistant and carriers. The composite preparation of Tebuconazole and Validamycin can generate higher synergistic effect, expand the scope of disease prevention and control, delay the generation of drug resistance, remarkably improve the drug efficiency, reduce the load of farmers and guarantee the safe production and ecological environment of agriculture. The invention can be used in agricultural plant chemical protection field, and play an outstanding and effective role in protecting and treating germ harmfulness of fruit trees, vegetables, wheat, rice and the like.
Owner:湖南万家丰科技有限公司
Puerarin nanocrystalline medical composition and preparation method thereof
InactiveCN103211759AImprove stabilityImprove solubilityOrganic active ingredientsMetabolism disorderSolubilityFOOD EFFECT
The invention belongs to the technical field of medicines and relates to a puerarin nanocrystalline medical composition and a preparation method and application thereof. Specifically, the puerarin composition is a puerarin nanocrystalline composition. The puerarin composition provided by the invention remarkably improves the solubility and bioavailabilityof puerarin and reduces or eliminates the food effect of the medicine, and is good in stability and can be prepared into various common preparation forms easily, the stability and effect of the medicine are improved, so that the composition has good prospect.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
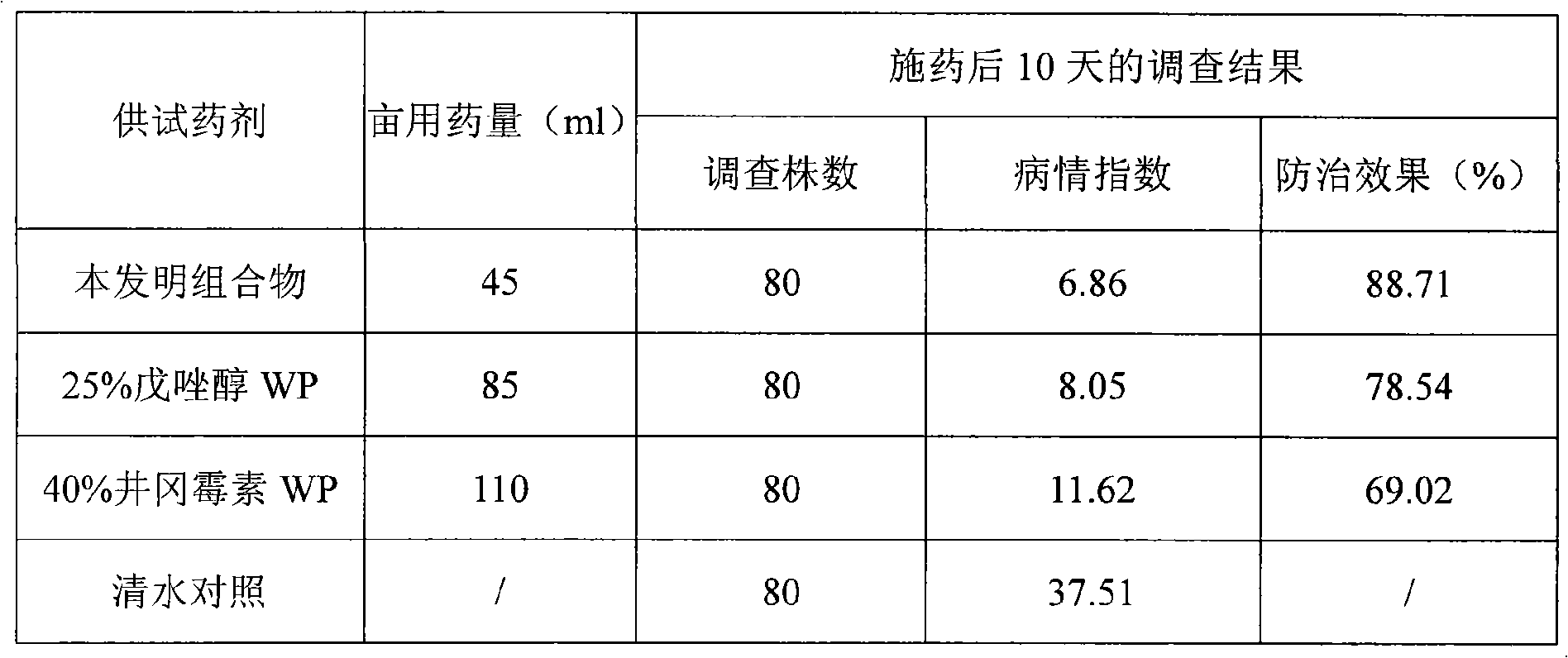
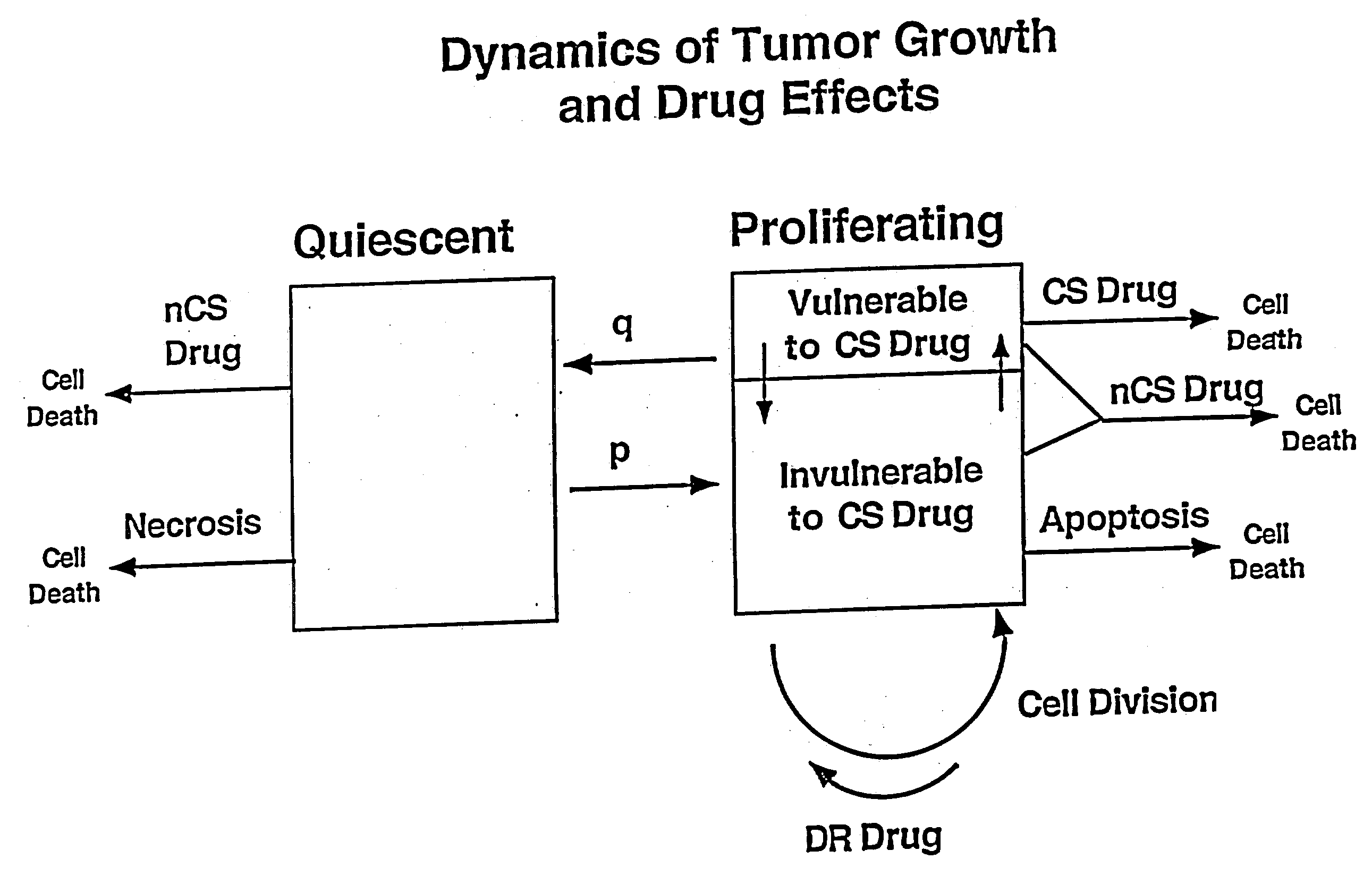
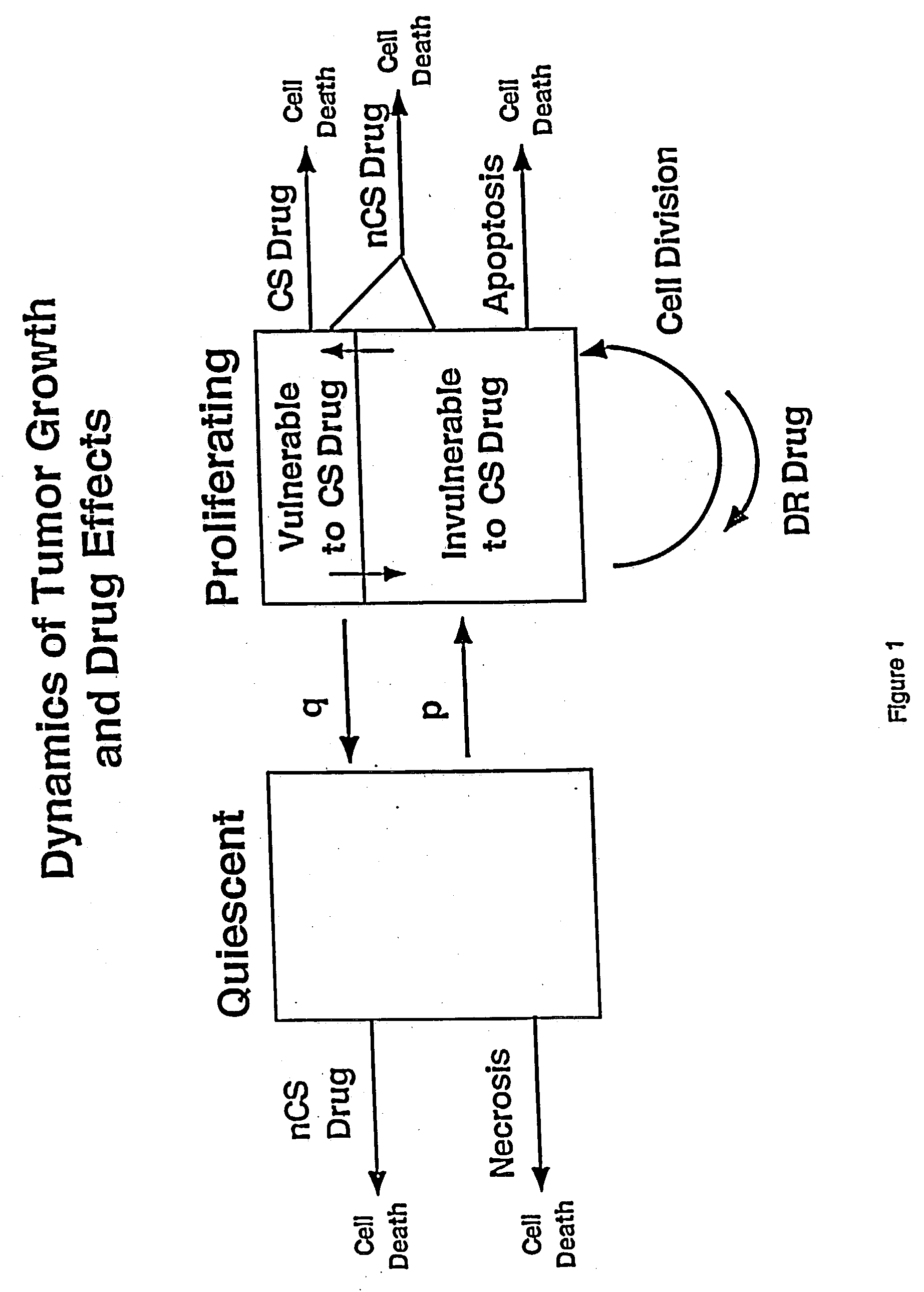
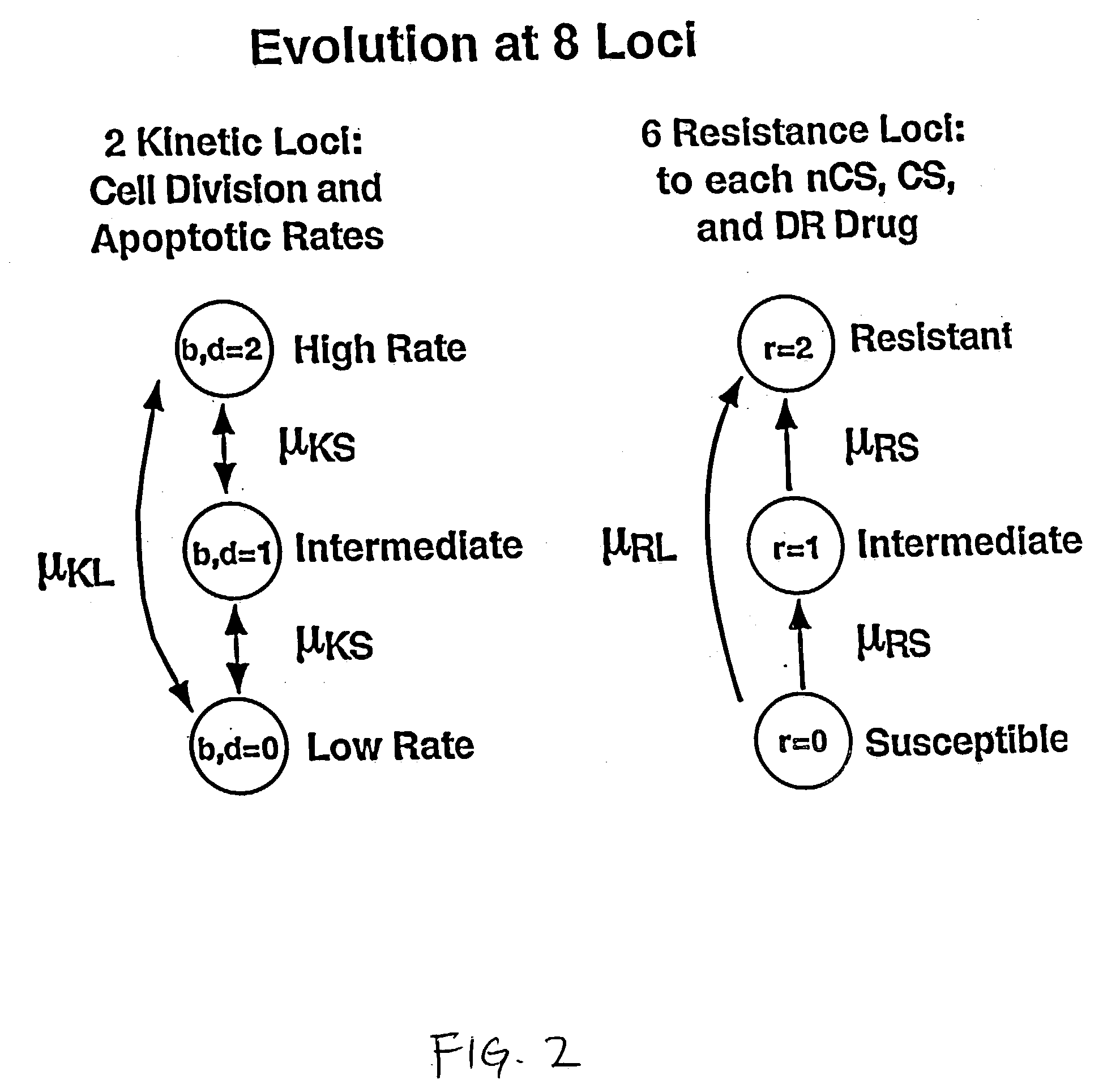
Computational model, method, and system for kinetically-tailoring multi-drug chemotherapy for individuals
InactiveUS20030144798A1Medical simulationMechanical/radiation/invasive therapiesComputational problemMedicine
A method and system for tailoring treatment regimens to individual patients with diseased cells exhibiting evolution of resistance to such treatments. A mathematical model is provided which models rates of population change of proliferating and quiescent diseased cells using cell kinetics and evolution of resistance of the diseased cells, and pharmacokinetic and pharmacodynamic models. Cell kinetic parameters are obtained from an individual patient and applied to the mathematical model to solve for a plurality of treatment regimens, each having a quantitative efficacy value associated therewith. A treatment regimen may then be selected from the plurlaity of treatment options based on the efficacy value.
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com