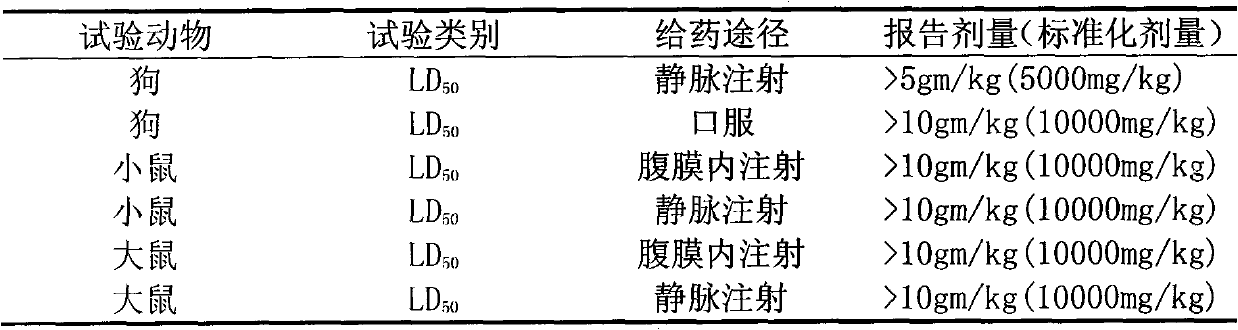
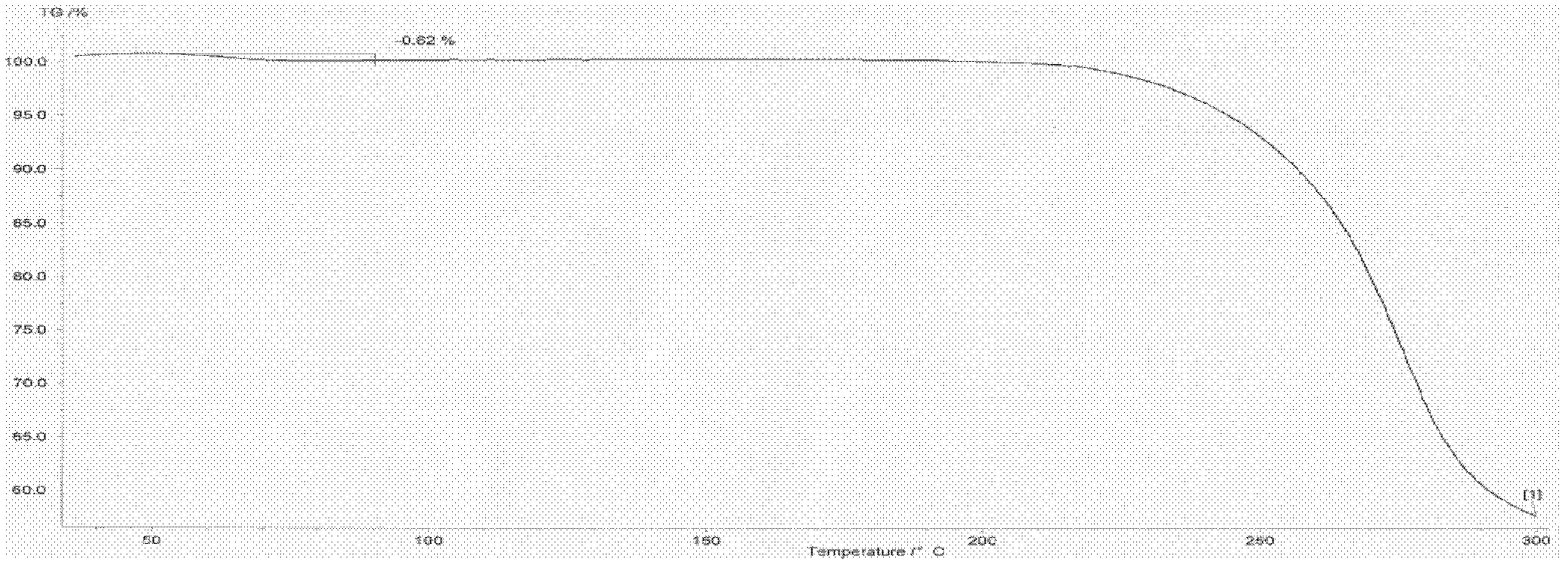
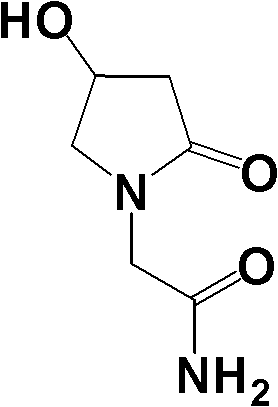
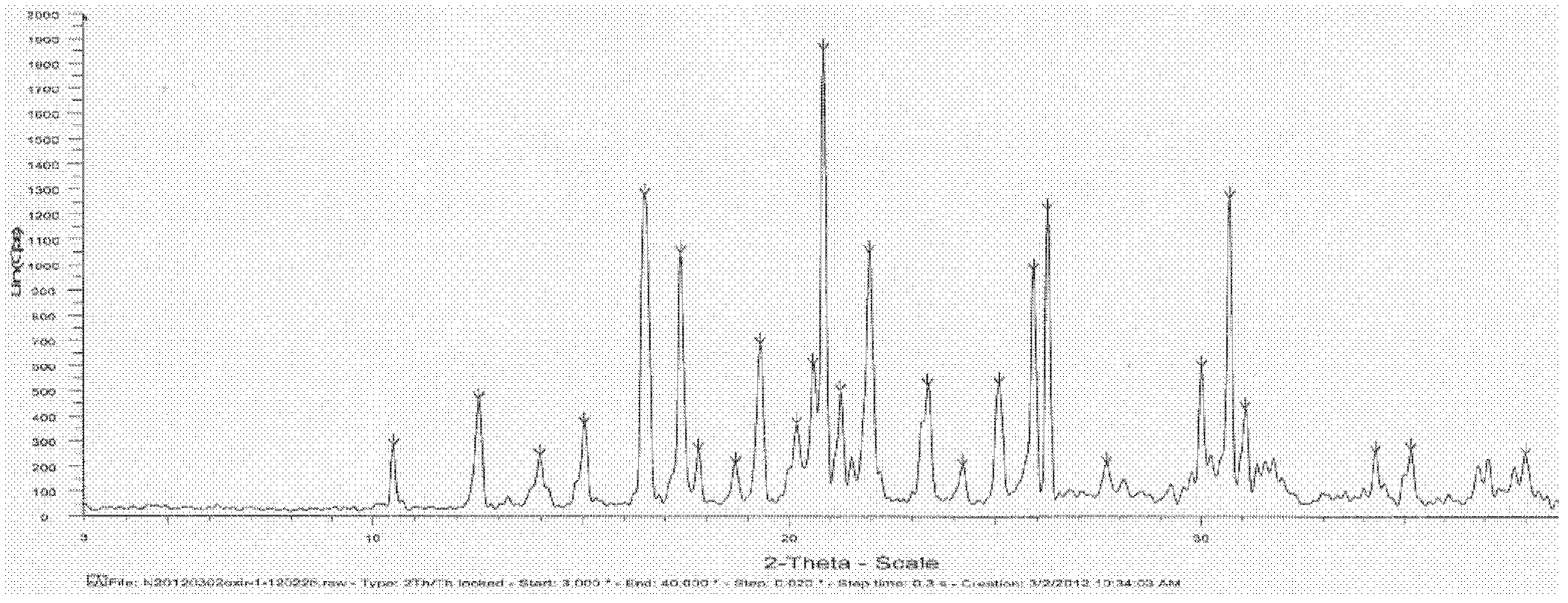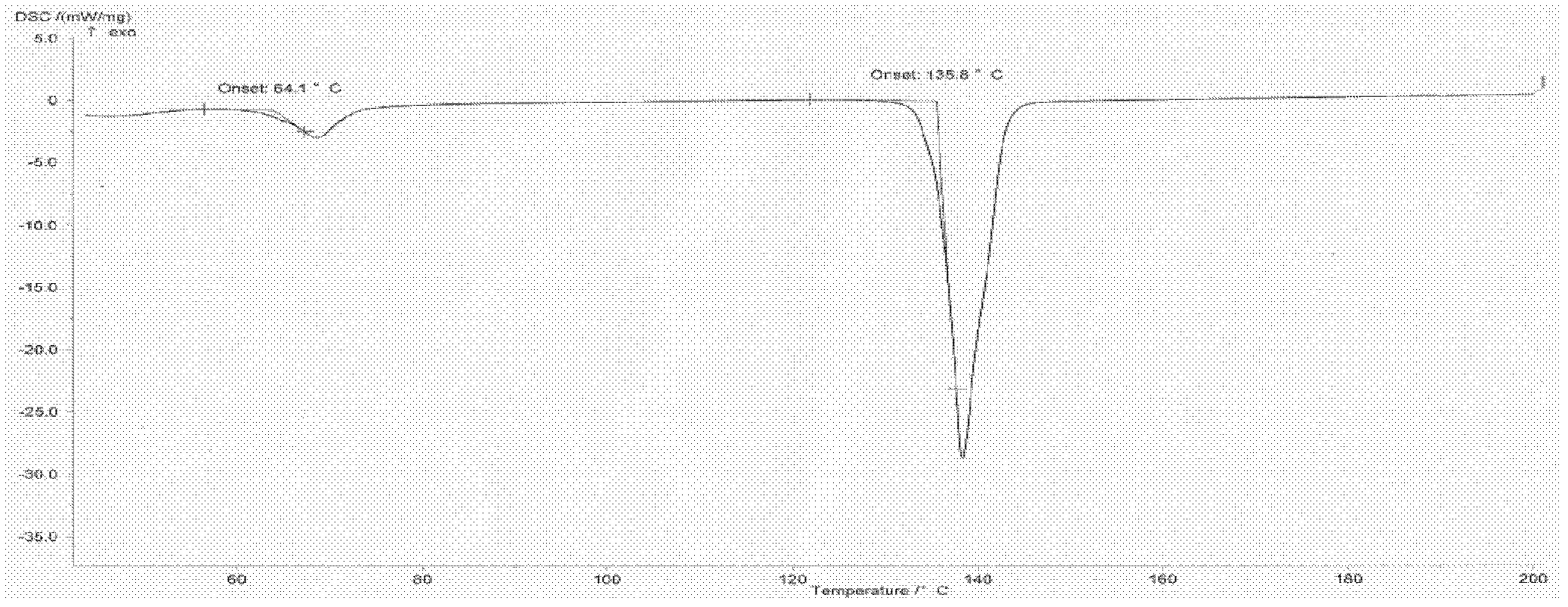Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
301 results about "Oxiracetam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
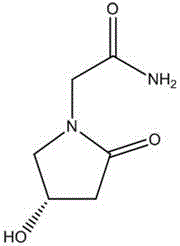
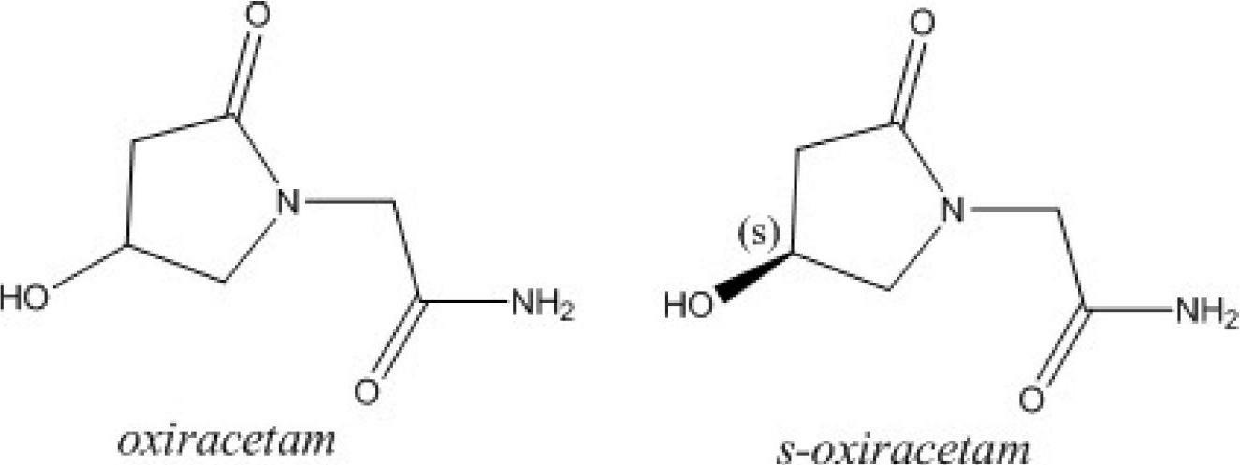
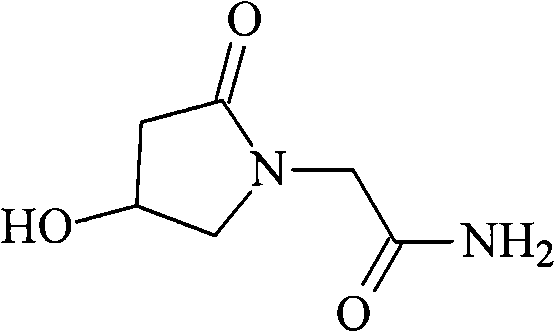
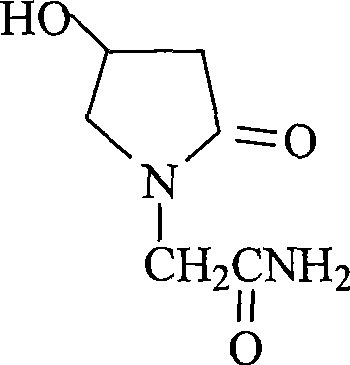
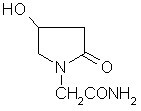
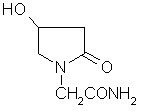
Oxiracetam (developmental code name ISF 2522) is a nootropic drug of the racetam family and very mild stimulant. Several studies suggest that the substance is safe even when high doses are consumed for a long period of time. However, the mechanism of action of the racetam drug family is still a matter of research. Oxiracetam is not approved by Food and Drug Administration for any medical use in the United States.
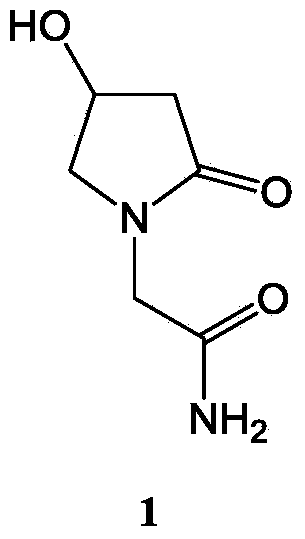
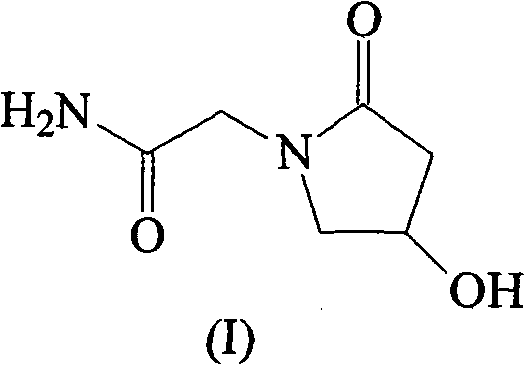
Application of levo-oxiracetam in preparation of medicine for treating memory and intelligence disturbance
The invention relates to new use of levo-oxiracetam in pharmaceutical field, and in particular relates to application of levo-oxiracetam in preparation of a medicine for treating memory and intelligence disturbance. The experiment result shows that the levo-oxiracetam is a main active ingredient for playing efficacy in oxiracetam, the clinical dosage can be greatly reduced by singly using the levo-oxiracetam, and the potential toxic and side effect is reduced. According to the invention, the levo-oxiracetam is a single active ingredient, the raw material purity is greater than 99.5% so as to effectively avoid the toxicity risk caused by other impurities in the medicine, the pharmacy is safer, the medicine quality is more controllable, and the curative effect is more precise.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +2
Applications of levorotary oxiracetam and oxiracetam in preparing drugs for preventing or treating coma
The invention provides applications of levorotary oxiracetam and oxiracetam in preparing drugs for preventing or treating coma. Experimental results indicate that levorotary oxiracetam has obvious awaking effect on coma caused by alcoholism, but dextrorotary oxiracetam basically has no effect, and the awaking effect of levorotary oxiracetam is two times of that of dextrorotary / levorotary mixed oxiracetam; levorotary oxiracetam has remarkable awaking effect on comas caused by both trauma and anesthesia.
Owner:CHONGQING RUNZE PHARM CO LTD
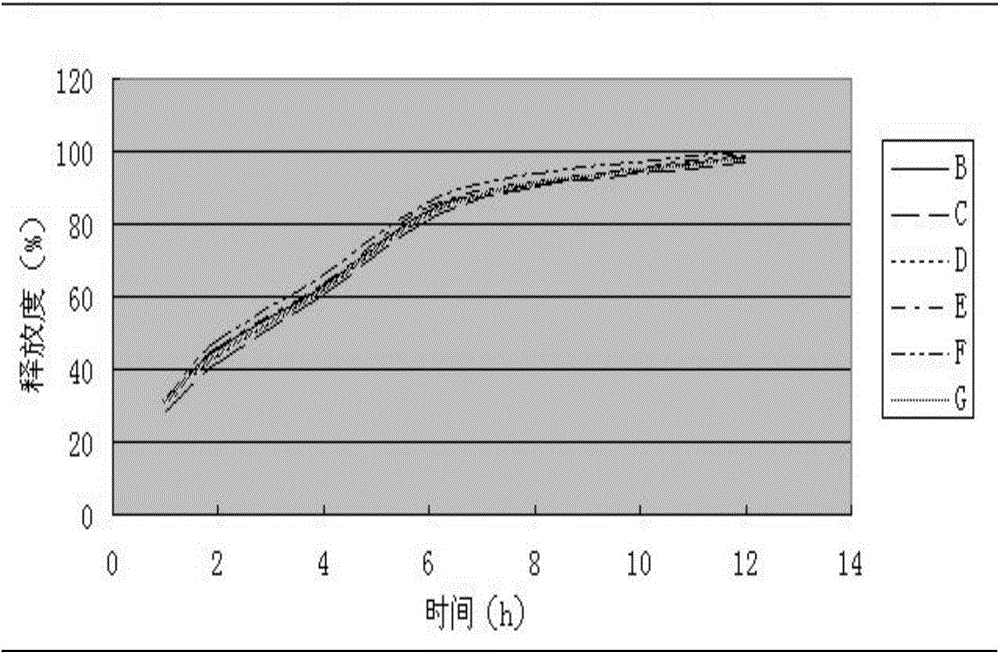
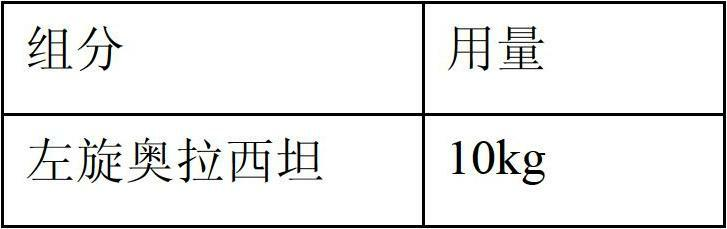
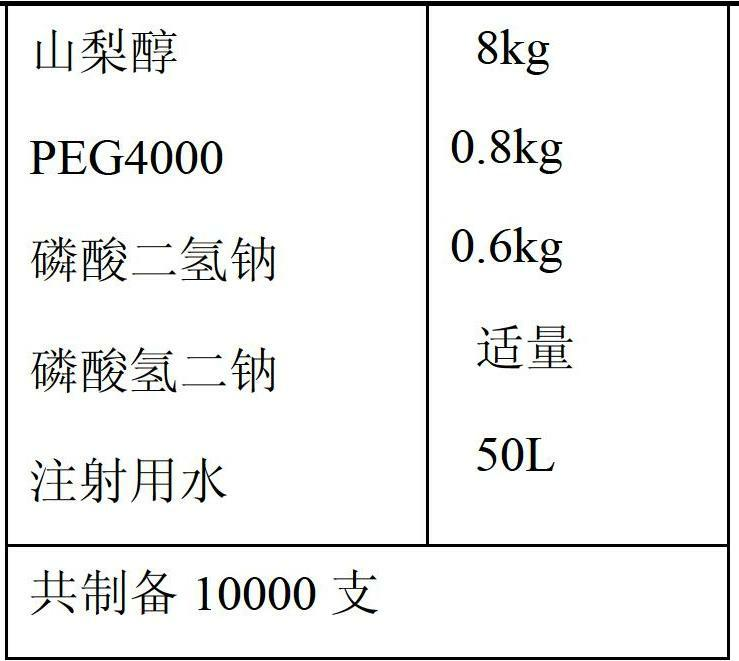
Levo-oxiracetam slow-release tablet and preparation method thereof
ActiveCN103599083AReduce adverse reactionsImprove securityOrganic active ingredientsNervous disorderInjury brainBlood concentration
The invention relates to a levo-oxiracetam slow-release tablet mainly prepared from levo-oxiracetam, which is prepared from the following raw and auxiliary materials in parts by weight: 0.8-1.2 parts of slow-release framework material, 0.06-0.12 part of flow aid, 0.02-0.05 part of lubricant, 0.02-0.05 part of antisticking agent and 1-1.5 parts of adhesive. The levo-oxiracetam slow-release tablet is used for treating brain injury, and neural afunction and disturbance of memory and intelligence caused by brain injury, and has a smooth surface; the detection proves that the release behavior of the main drug levo-oxiracetam satisfies the requirements for the slow-release tablet; and the main drug levo-oxiracetam is slowly released, so the levo-oxiracetam slow-release tablet can be taken less frequently than the traditional preparation. The main drug levo-oxiracetam in the levo-oxiracetam slow-release tablet is slowly released, and can provide steady and enduring effective blood concentration, thereby avoiding or reducing the phenomena of peaks and troughs of blood concentration, and being beneficial to enhancing the medicine application safety and reducing the untoward reaction of the medicine.
Owner:CHONGQING RUNZE PHARM CO LTD
Freeze-dried powder injection of L-oxiracetam and process for preparing freeze-dried powder injection
ActiveCN102670527AFast sublimationShorten freeze-drying timePowder deliveryOrganic active ingredientsPolyethylene glycolEngineering
The invention relates to a freeze-dried powder injection of L-oxiracetam and a process for preparing the freeze-dried powder injection. The freeze-dried powder injection comprises, by weight, 1 part of the L-oxiracetam, 0.1 to 10 parts of an excipient, 0.01 to 1 part of an antisticking agent and 0.01 to 0.5 part of a pH regulator, wherein polyethylene glycol (PEG) series of products serve as the antisticking agent preferably. The freeze-dried powder injection which is prepared on the basis of a prescription according to the process has the advantages of being short in freeze-drying time, attractive in appearance, short in redissolving time and high in stability.
Owner:南京博德生物制药有限公司
Oxiracetam liposome injection
InactiveCN101732251AUnexpected effectNo change in colorOrganic active ingredientsNervous disorderSide effectMedicine
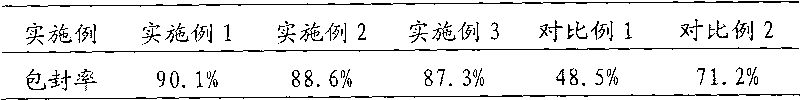
The invention discloses an oxiracetam liposome injection which is characterized by comprising the following components in parts by weight: 1 part of oxiracetam, 3-18 parts of phospholipid, 1-12 parts of cholesterol, 0.5-7 parts of tween 80 and an appropriate amount of osmotic-pressure regulating agent and buffering solution. The oxiracetam liposome injection has the advantages of good stability, high entrapment rate, small toxic and side effects and simple preparation, and is suitable for clinical requirements.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Oxiracetam compound and new method thereof
InactiveCN102050774AHigh purityIncrease contentNervous disorderOrganic chemistryActivated carbonSide effect
The invention provides an Oxiracetam compound and a new method thereof. The method comprises the following steps: firstly dissolving Oxiracetam in water, carrying out adsorption and purification with activated carbon, precipitating with an organic solvent, separating and purifying with a chromatographic column, and finally carrying out crystallization with an organic solvent, thus the high purity Oxiracetam compound is obtained. The purity of the final product is greatly improved compared with the existing product, the quality of preparation is improved, the toxic and side effects are reduced, and the safety in the application of preparing a drug used for treating senile dementia is ensured; and meanwhile, compared with the prior art, the process in the invention is simple and feasible, the reaction conditions are mild, the cost is low, the yield is high, and the product purity is high, thus being applicable to industrialized production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Preparing method of oxiracetam injection and products thereof
InactiveCN1424034AReduce workloadThe preparation process is feasibleOrganic active ingredientsDrug compositionsActivated carbonMedical prescription
An oxiracetam injection is prepared from oxiracetam, gluclose (or sodium chloride) for injection and water for injection through proportioning, dissolving the glucose or sodium chloride for injection in the water for injection, adding activated carbon, heating, holding the temp. filtering, adding oxiracetam to the filtrate, stirring for dissolving, adding the water for injection, regulating pH value, fine filtering, bottling and sterilizing at 105-126.5 deg.C.
Owner:诸葛华明 +1
Oxiracetam injection
ActiveCN101396358AGood storage stabilityQuality improvementOrganic active ingredientsNervous disorderInjection solutionIntermediate product
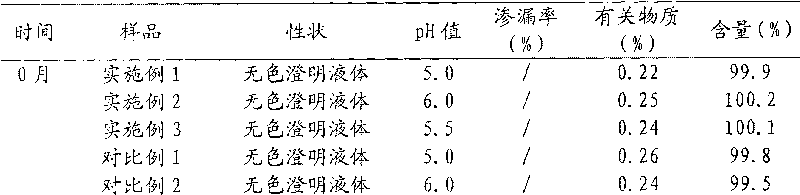
The invention relates to an oxiracetam injection solution which comprises 1 weight portion of oxiracetam, 1.5 to 5.5 weight portions of glucose and 50 to 100 weight portions of injection water, wherein, the pH value of the injection solution is 3.8 to 4.5. The invention also provides a preparation method of the oxiracetam injection solution which comprises the steps as follows: a) the bulk drug oxiracetam and the glucose or sodium chloride are solved in the injection water under the temperature of 40 DEG C to 70 DEG C to obtain dissolved solution; b) the dissolved solution is cooled to the room temperature, active carbon is added for decolorizing, the active carbon is removed by filtering, proper quantity of water is complemented, and citric acid and sodium citrate are added to adjust the pH value to 3.8 to 4.5 to obtain the dissolved solution with adjusted pH value; c) an intermediate product after encapsulation is sterilized; and d) the product after the sterilization is packed. The oxiracetam injection solution manufactured by the prescription and the preparation method of the invention has good storage stability.
Owner:广东世信药业有限公司 +1
Oxiracetam freeze-drying preparation for injection and preparation method thereof
ActiveCN103446067AStable in natureLong validity periodOrganic active ingredientsPowder deliveryFreeze-dryingImpurity
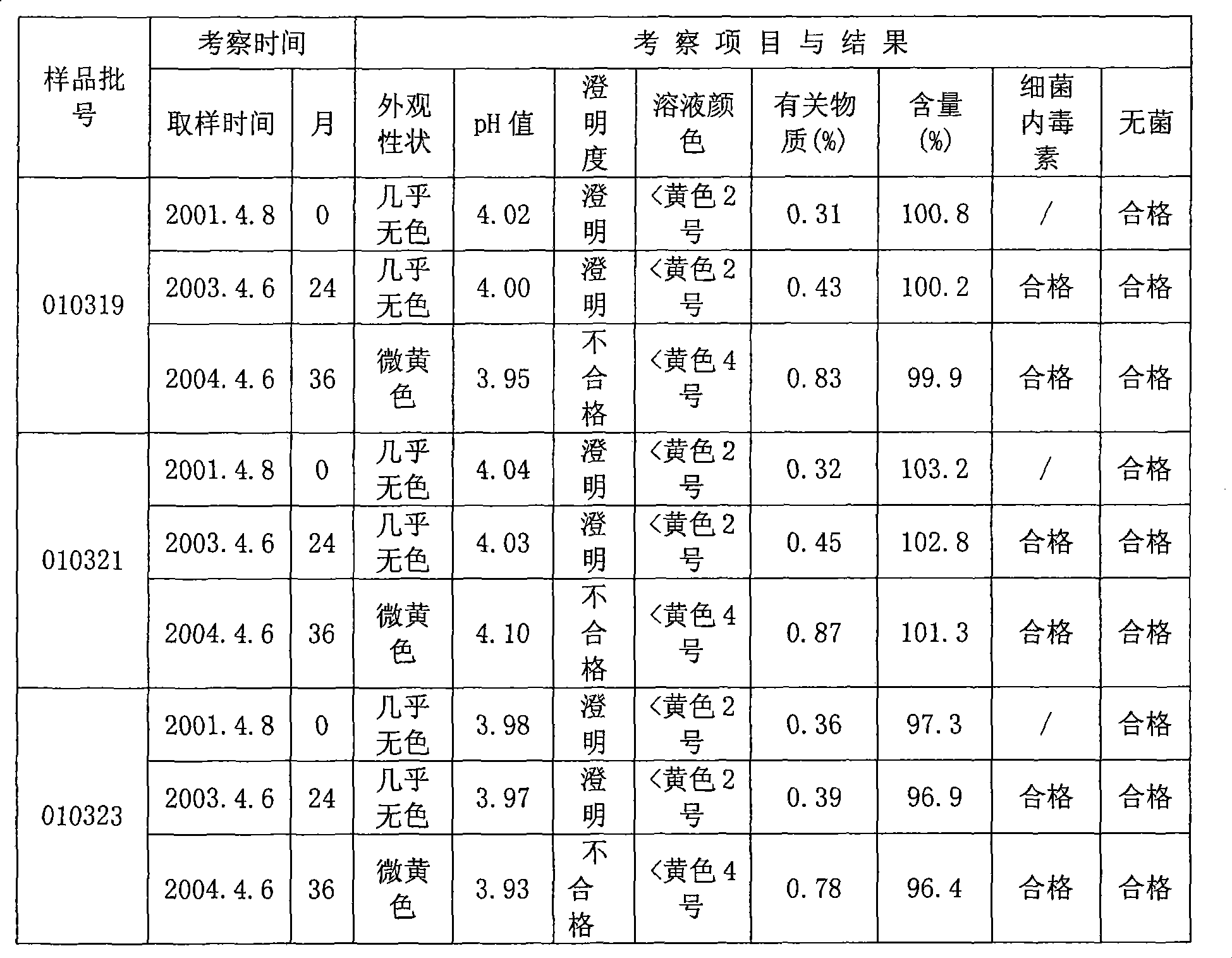
The invention relates to an oxiracetam freeze-drying preparation for injection and a preparation method thereof, belonging to the technical field of medicines. The freeze-drying preparation is a crystal form compound formed by oxiracetam, D-sorbitol and mannitol in a weight ratio of 1: (0.2-0.25): (0.05-0.1). The oxiracetam freeze-drying preparation has the beneficial effects that the production cycle is shortened by about 20 hours, the rate of finished products is increased by above 99%, the phenomena such as the upward shifting and the eruption of the product are avoided, medicine molecules are constrained in crystal lattices, the water content is extremely low and can be controlled below 0.1%, the preparation is difficult to degrade and has relatively good stability, the period of validity is up to 36 months, the impurity content is greatly reduced, the single maximum impurity is less than 0.1%, and the total impurities are less than 0.5%; no organic solvents are involved in the production process, the energy consumption and the cost are substantially reduced, and the technology has environmentally-friendly effect and is suitable for large-scale industrialization production. Compared with the prior art, the oxiracetam freeze-drying preparation provided by the invention has obviously improved curative effect and is suitable for clinical application.
Owner:CSPC OUYI PHARM CO LTD
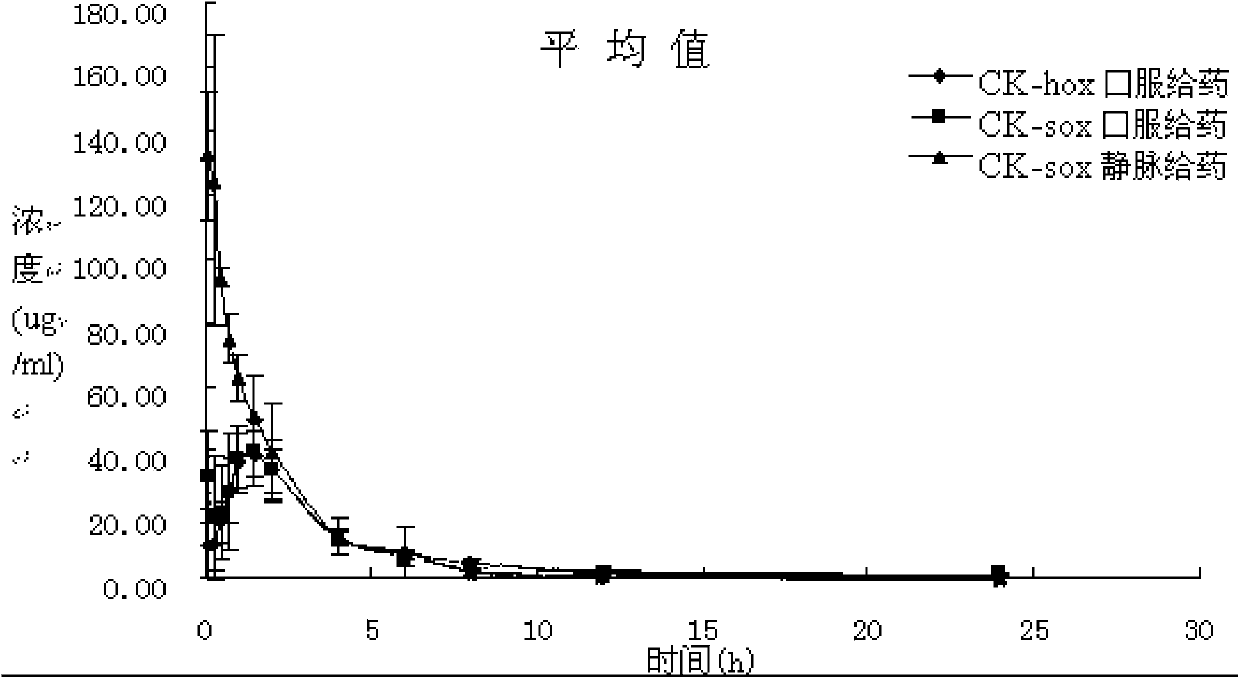
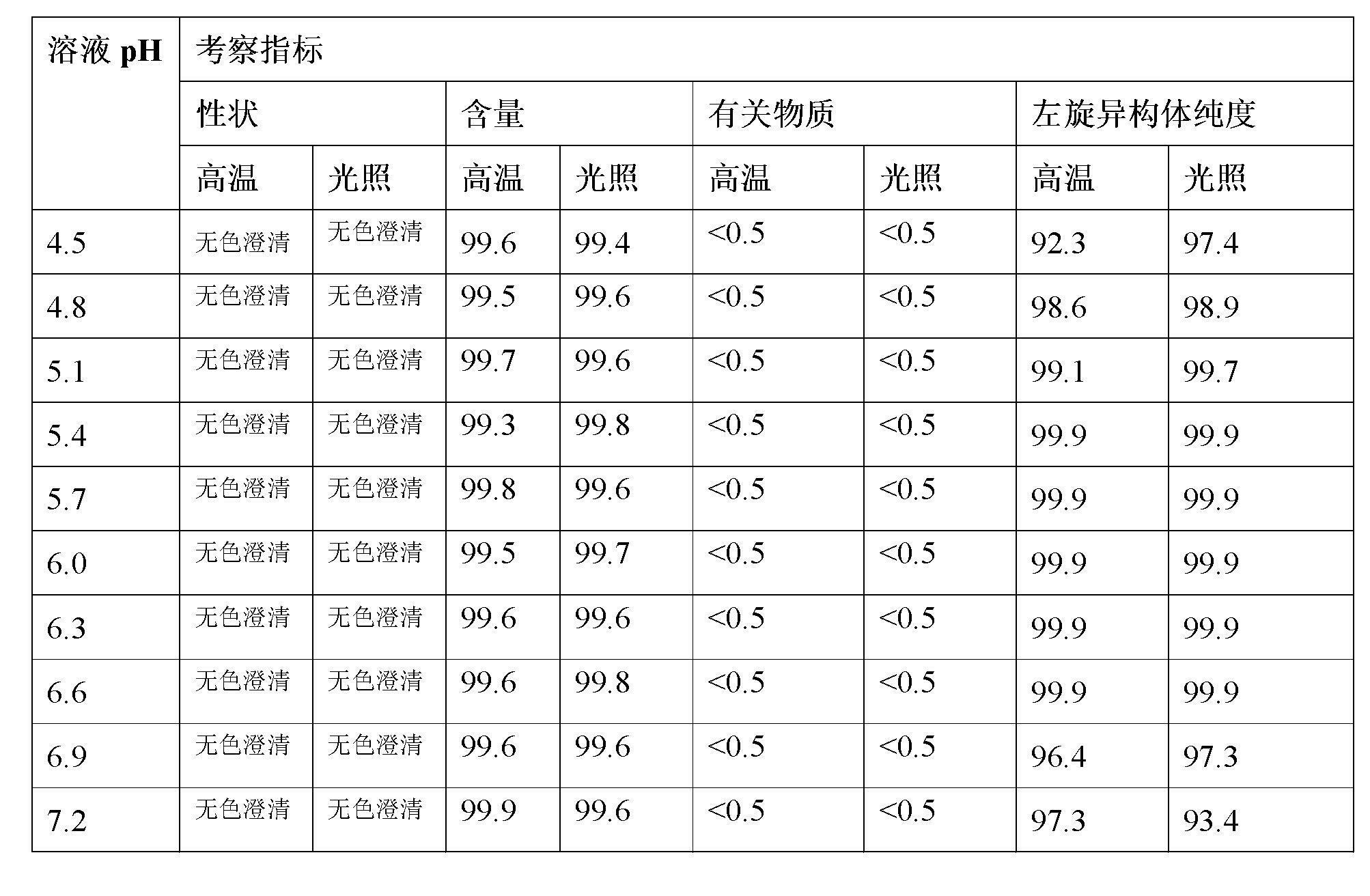
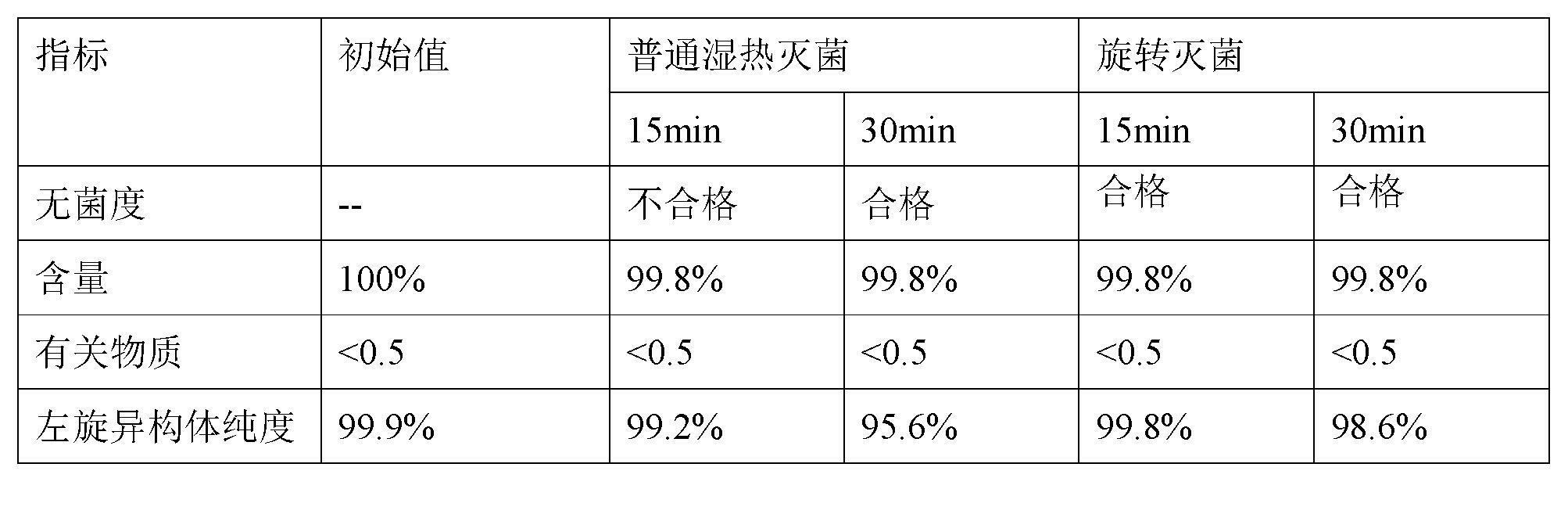
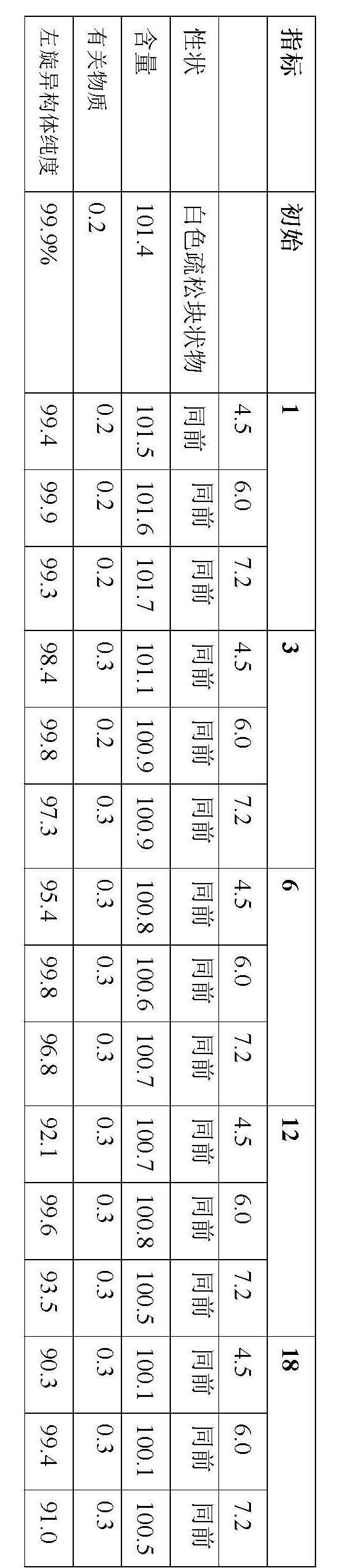
Stable S-oxiracetam preparation for injection and preparation method of same
The invention relates to a stable preparation for injection by taking S-oxiracetam as active ingredient. The preparation is a composition for injection, and is formed by the S-oxiracetam or salts thereof serving as active ingredient and pharmaceutically acceptable auxiliary material. To restrain the racemization of the S-oxiracetam, when the powder injection with the active ingredient is prepared, and only the pH value of the S-oxiracetam medicament solution ranges from 4.5 to 7.0, the pH value is between 5.4 to 6.6 preferably, so that the S-oxiracetam medicament solution can be produced, and the final freeze-dried product has acceptable long stability; and moreover, when injection is produced, a rotary steam sterilization method needs to be adopted, terminal rotating sterilization is performed for 15 to 45 minutes under the temperature of 121 DEG C, and the terminal rotating sterilization is performed for 15 to 20 minutes preferably, so that the S-oxiracetam injection with high purity can be obtained and has acceptable long stability.
Owner:FUKANGREN BIO PHARMA
Oxiracetam injection and preparation method thereof
The invention provides an oxiracetam injection, characterized in that 1000 ml of the injection contains 100-250 g of oxiracetam, and the pH value is adjusted to 4.0-5.5 by using 0.01-0.1 mol / L of a NaOH solution, and the balance of the injection is injection water. The invention also provides a preparation method of the oxiracetam injection. The oxiracetam injection has the advantages of low content of impurities and good stability, and can be stored at room temperature.
Owner:YAOPHARMA CO LTD
Prepn and product of oxiracetam powder for injection
InactiveCN1486693AEasy to shapeSimple preparation processOrganic active ingredientsPowder deliveryAlcoholFreeze-drying
The present invention provides one kind of Oxiracetam freeze dried powder for injection and its preparation and product. It is prepared with Oxiracetam, sorbic alcohol or citric acid or lactobionic acid or sodium lactobionate, and water in the weight ratio of 1 to 0.1-2.0 to 1-10 and through weighing Oxiracetam and sorbic alcohol or citric acid or lactobionic acid or sodium lactobionate, dissolving in some container, adding active carbon, heating, filtering to eliminate carbon, regulating pH value, microfiltering, bottling in bacteria-free glass bottle, low temperature freeze drying and sealing.
Owner:HARBIN SANLIAN PHARMA CO LTD
Synthetic method of oxiracetam
ActiveCN104230777AEasy to separate and purifyRaw materials are cheap and easy to getOrganic chemistry methodsAlcoholEsterification reaction
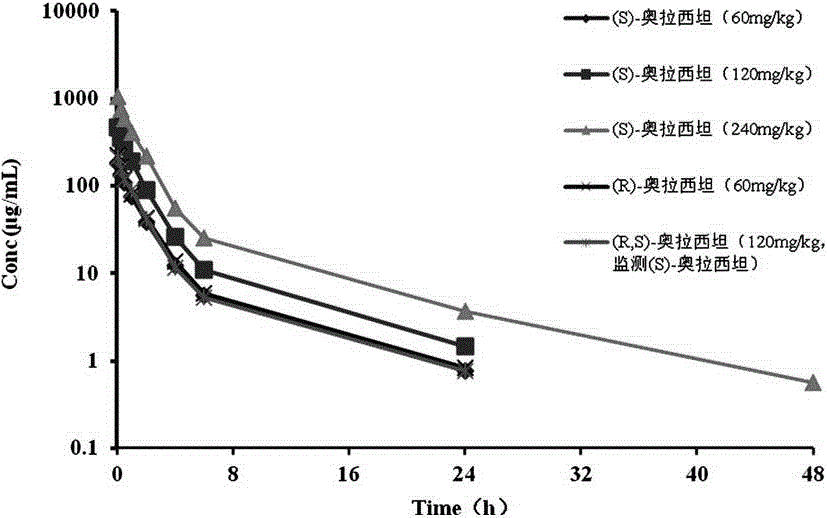
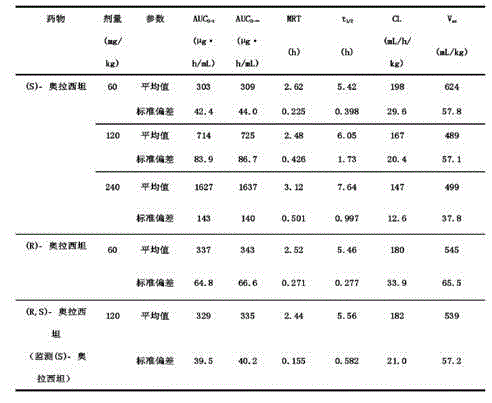
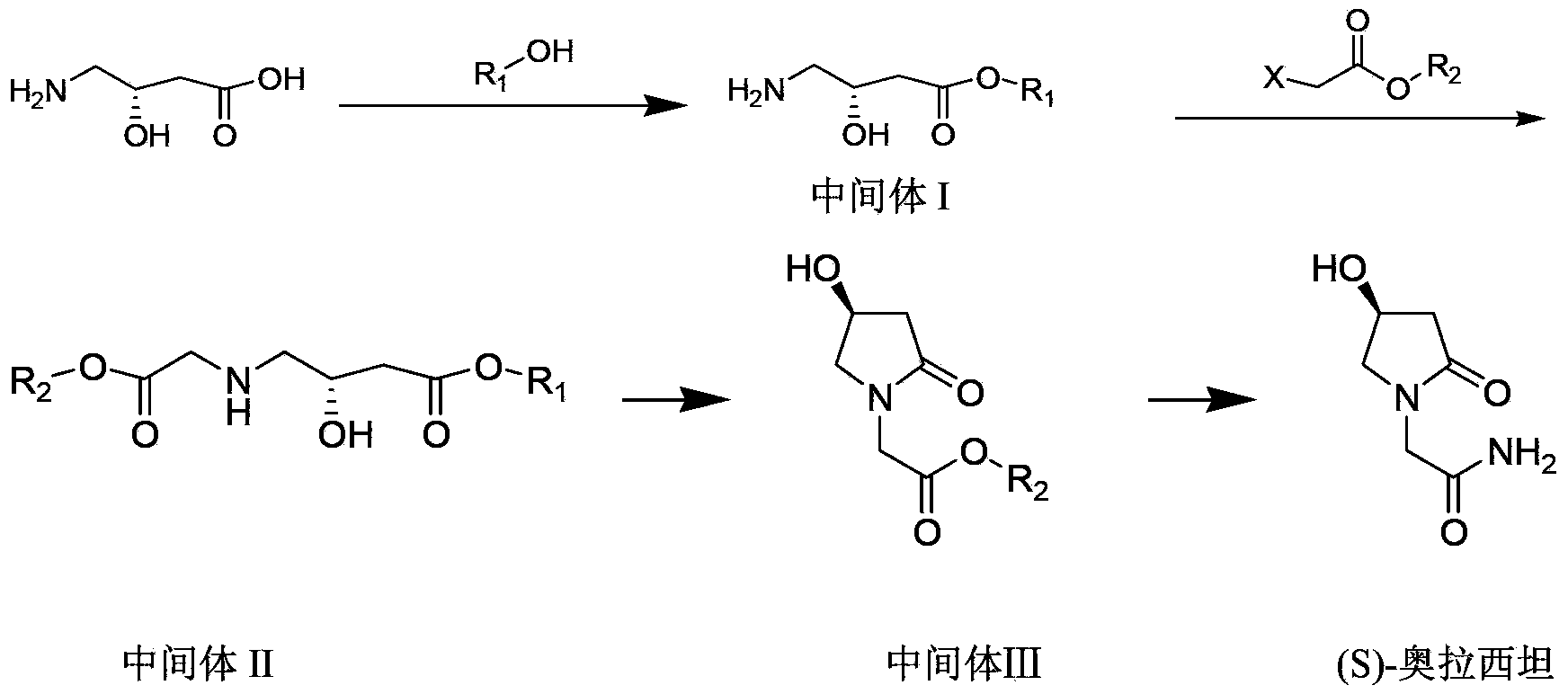
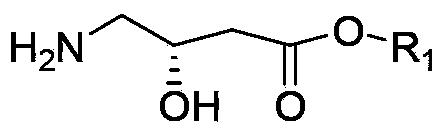
The invention discloses a synthetic method of (S)-oxiracetam. The synthetic method comprises the following steps: (1) by adopting S-4-amino-3-hydroxybutyric acid as a starting material, performing esterification reaction with alcohol to obtain an intermediate I; (2) performing condensation reaction between the intermediate I and halogenated acetic ester to obtain an intermediate II; (3) performing ring closing reaction on the intermediate II to obtain an intermediate III; and (4) performing ammonolysis reaction on the intermediate III to obtain a target product namely (S)-oxiracetam. By adopting a synthetic route of oxiracetam disclosed by the invention, at least more than 20% of an (S)-oxiracetam product with relatively ideal yield can be obtained, and a new oxiracetam synthetic route can be created.
Owner:CHONGQING RUNZE PHARM CO LTD +1
Application of dextro-oxiracetam in pharmaceutical field
InactiveCN106166150AHas antiepileptic activityImprove bioavailabilityOrganic active ingredientsNervous disorderPartial epilepsyTherapeutic effect
The invention provides application of dextro-oxiracetam in preparation of drugs for prevention or treatment of epilepsy. Experimental results show that dextro-oxiracetam has obvious treatment effect on generalized epilepsy seizure, partial epilepsy seizure and status epilepticus.
Owner:CHONGQING RUNZE PHARM CO LTD
Oxiracetam medicinal composition, and preparation method and application thereof
InactiveCN103301114AImprove securityOrganic active ingredientsNervous disorderSide effectChemical compound
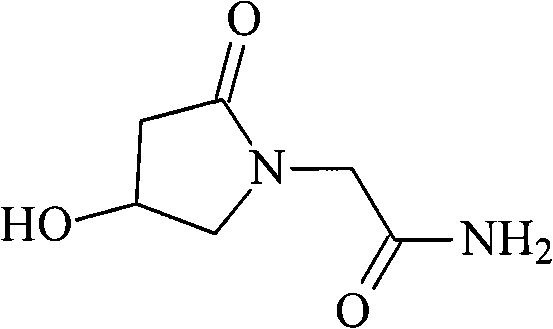
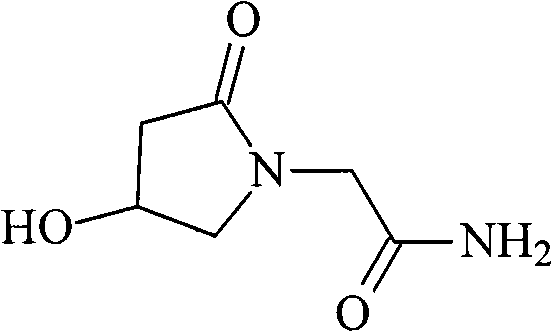
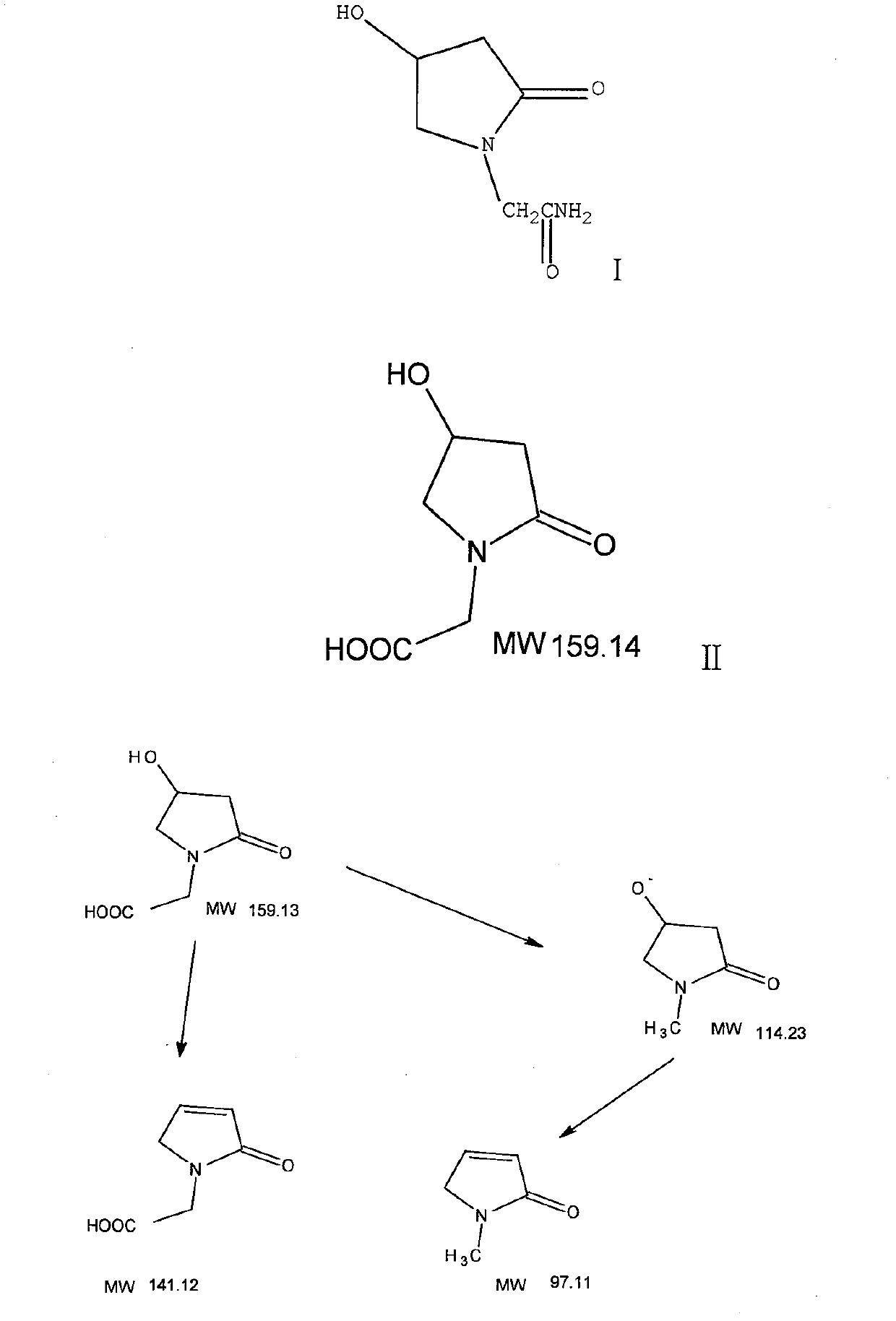
The invention discloses an oxiracetam medicinal composition having a high safety, and a preparation method and an application thereof. The medicinal composition contains oxiracetam and below 0.5% of a compound having a structure represented by formula II. A case that 4-hydroxy-2-oxo-1-pyrrolidineaceticacid is the largest-content impurity in the oxiracetam compound is proved for the first time, purifying characterization and relevant researches on 4-hydroxy-2-oxo-1-pyrrolidineaceticacid are carried out, and the researches find that 4-hydroxy-2-oxo-1-pyrrolidineaceticacid is related with the side effects of the oxiracetam medicine and can cause a series of untoward effects. The oxiracetam medicinal composition has a high safety. The invention further provides the preparation method of the oxiracetam medicinal composition.
Owner:LIAONING YILING KECHUANG BIOLOGICAL MEDICAL TECH
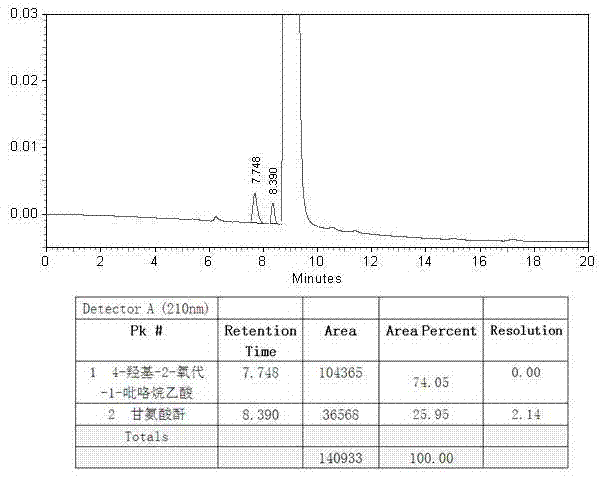
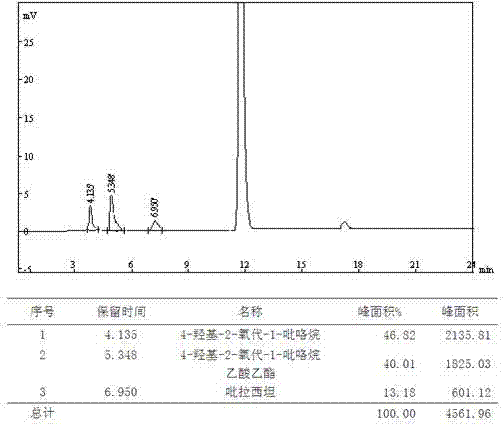
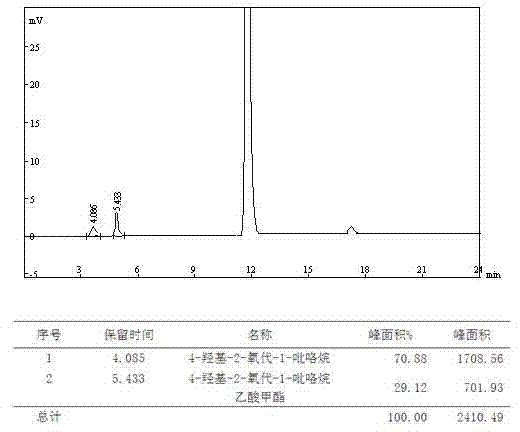
Analysis and detection method for oxiracetam and impurities thereof
ActiveCN103076409AQuality improvementHigh selectivityComponent separationAnalysis methodChromatography column
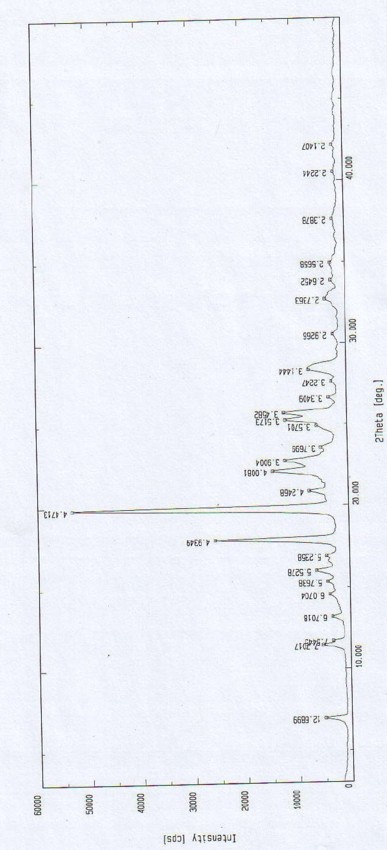
The invention relates to an analysis and detection method for materials related to oxiracetam, in particular to an oxiracetam quality analysis method. The method comprises the steps of adopting a high performance liquid chromatography to perform quality analysis for oxiracetam drug substances or pharmaceutical preparations containing the oxiracetam. According to the invention, the adopted chromatographic column is a C18 or C8 chromatographic column at the temperature of 30-40 DEG C. Besides, the adopted high performance liquid chromatography can effectively separate the oxiracetam and the impurities thereof under a certain chromatographic condition. Therefore, through the method, the content of each impurity in the oxiracetam can be accurately measured.
Owner:北京元延医药科技股份有限公司
Oxiracetam compound with steady crystal form
The invention belongs to the technical field of medicine, and particularly relates to an oxiracetam compound. The oxiracetam compound provided by the invention contains semi-crystalline water. The oxiracetam compound has the advantages that related substances are small, the purity is high, the stability is good, and a moisture-absorption and weight-gaining effect is not obvious even if under a high humidity condition.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Oxiracetam preparation and method of preparing the same
ActiveCN101152175ALarge doseExtend cycle timeOrganic active ingredientsNervous disorderTreatment effectPrill
The invention provides an oxiracetam agent and the preparation method, which relates to medical technical field and is used to induce the drug to penetrate through blood-brain barrier and to improve treatment effects. The agent contains oxiracetam nano-particle and excipientpharmaceutical excipient. After improved, the oxiracetam nano-particle and excipientpharmaceutical excipient contains drug core and a modified layer. The drug core mainly comprises principal agent and carrier, wherein the carrier is polymer material containing amino polysaccharide with a content of 70 to 95 percent, and the principal agent is oxiracetam with a content of 5 to 30 percent. The invention adopts polymer material containing amino polysaccharide as carrier to carry the oxiracetam and endow the oxiracetam with good brain targeting function. And the invention adopts surfactant to modify the nano surface of the carrier and lengthens the recycling time of the carrier nano-granule and the nano-particle in the recycling system. Tests show that the blood-brain barrier transmittance is 24h, which is 36.75 percent higher than ordinary oxiracetam agent and greatly enhances the dosage of oxiracetam passing through the blood-brain barrier.
Owner:CSPC OUYI PHARM CO LTD
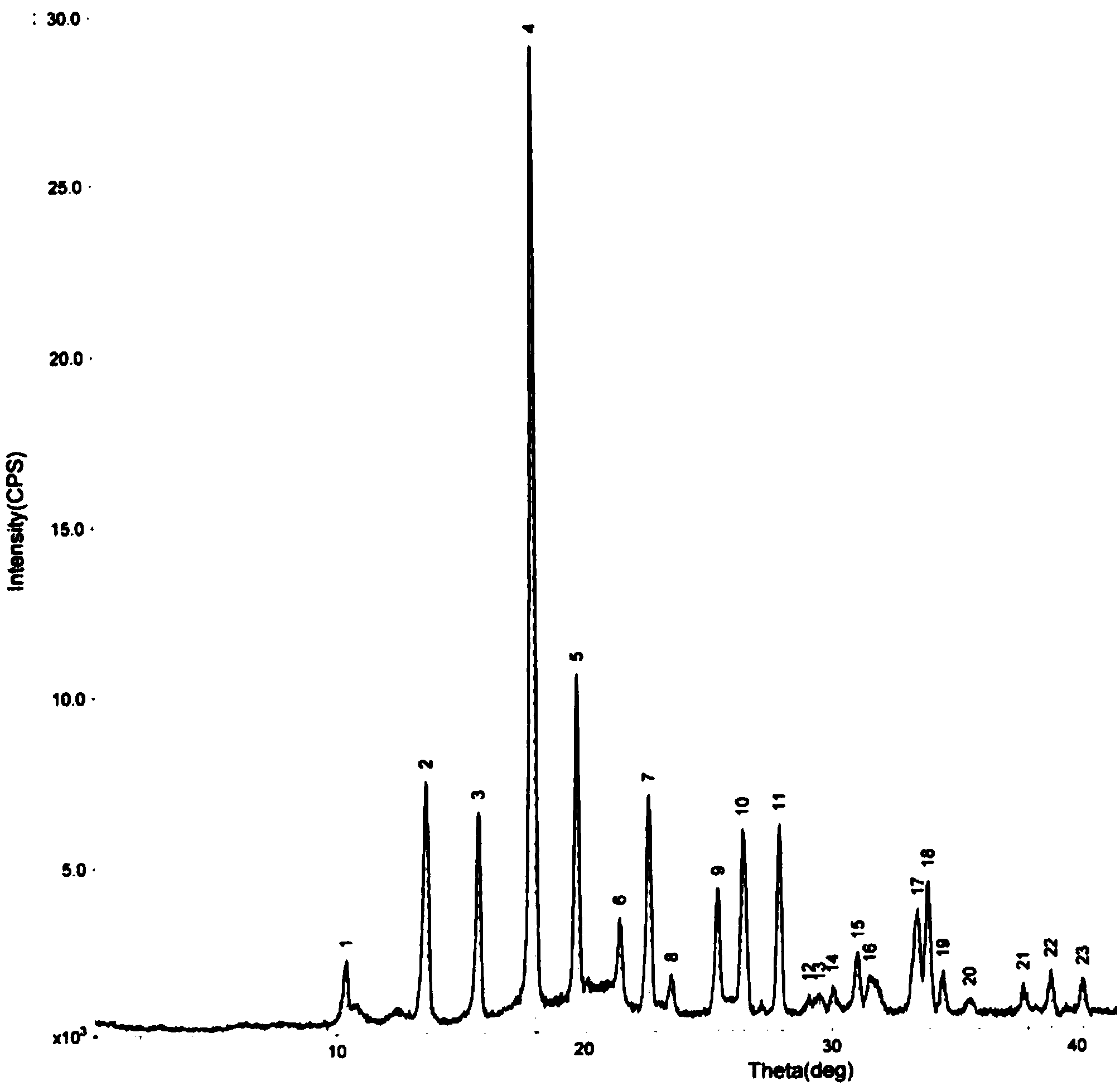
Oxiracetam crystal form and preparation method thereof
ActiveCN103342673ANo residueEasy to operateOrganic active ingredientsNervous disorderOrganic solventCurative effect
The invention relates to an oxiracetam crystal form and a preparation method thereof. The oxiracetam crystal form is fewer in impurity and high in purity, especially is stable in crystal form, good in quality stability and excellent in particle fluidity, is beneficial to transportation, storage and production of pharmaceutical preparations and also can guarantee the curative effect and the safety of the pharmaceutical preparations in clinical application. The invention also provides the preparation method of the oxiracetam crystal form. The method is an environmentally-friendly process, only uses water as a solvent, and is simple to operate and low in production cost; and the prepared product has no residue of an organic solvent, is good in safety and is especially suitable for industrial production.
Owner:CSPC OUYI PHARM CO LTD
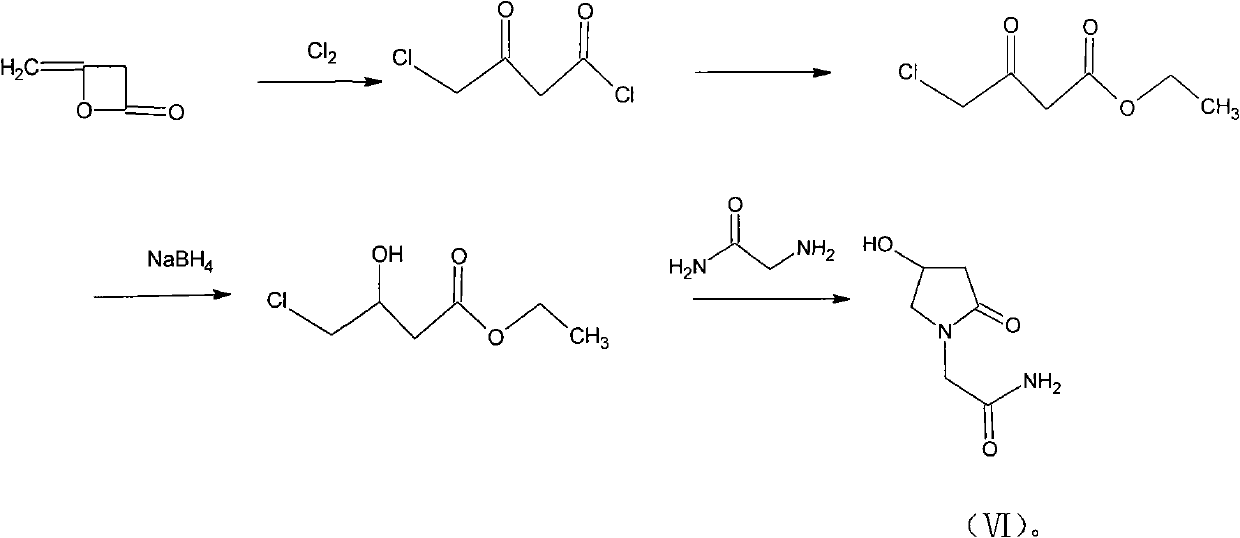
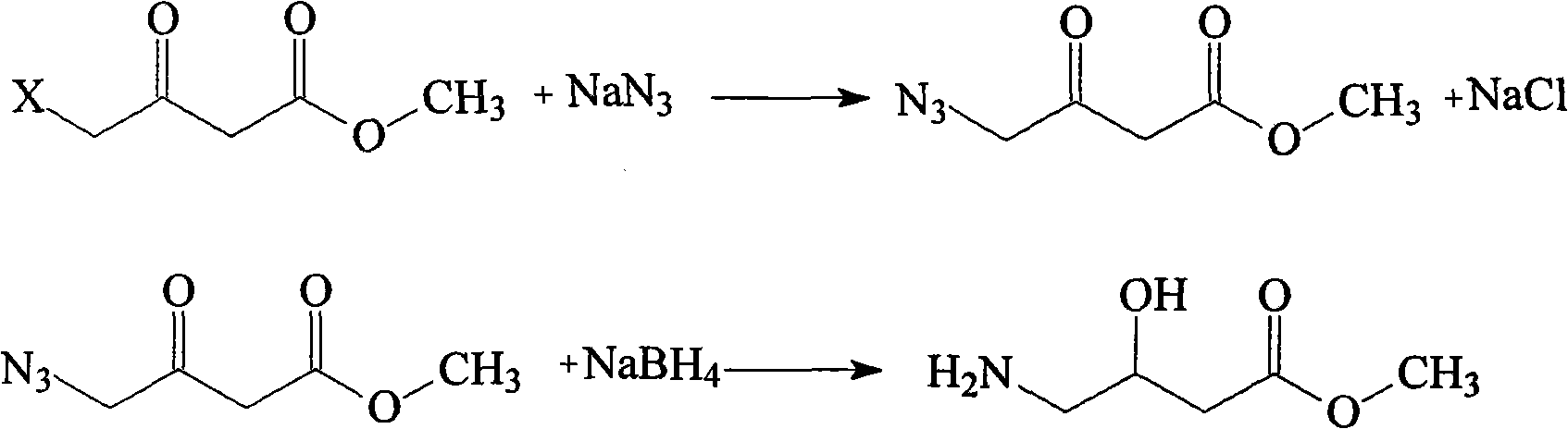
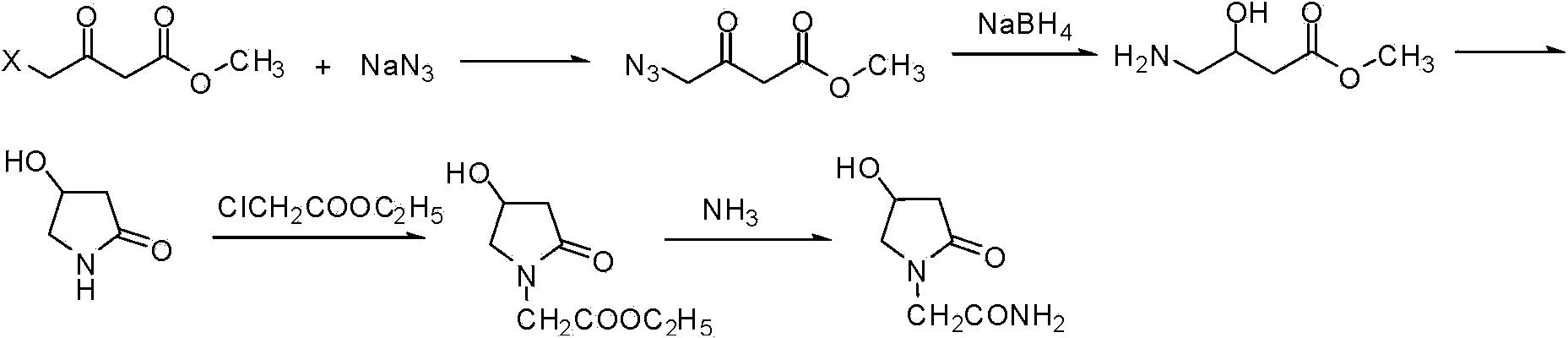
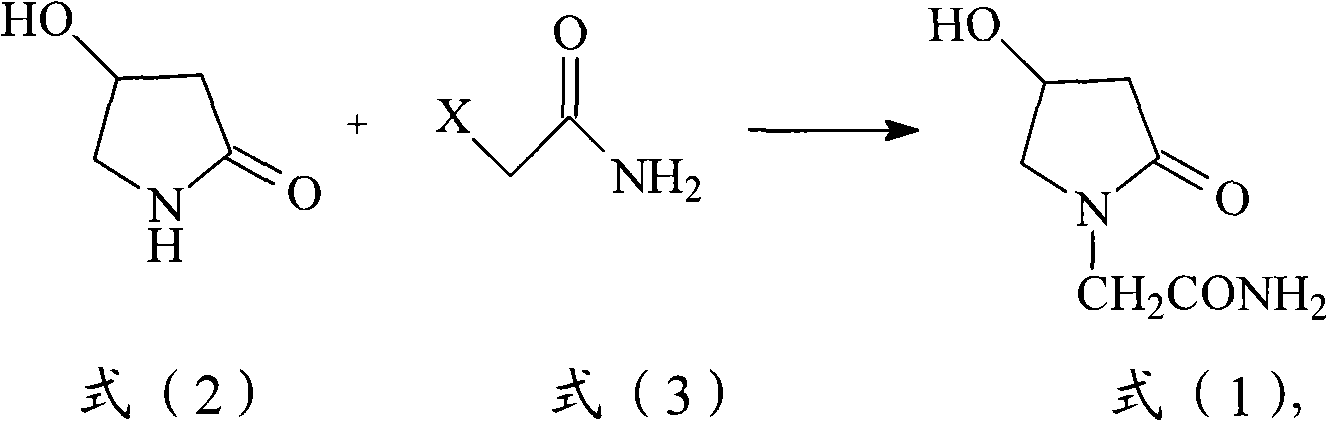
Preparation method of Oxiracetam
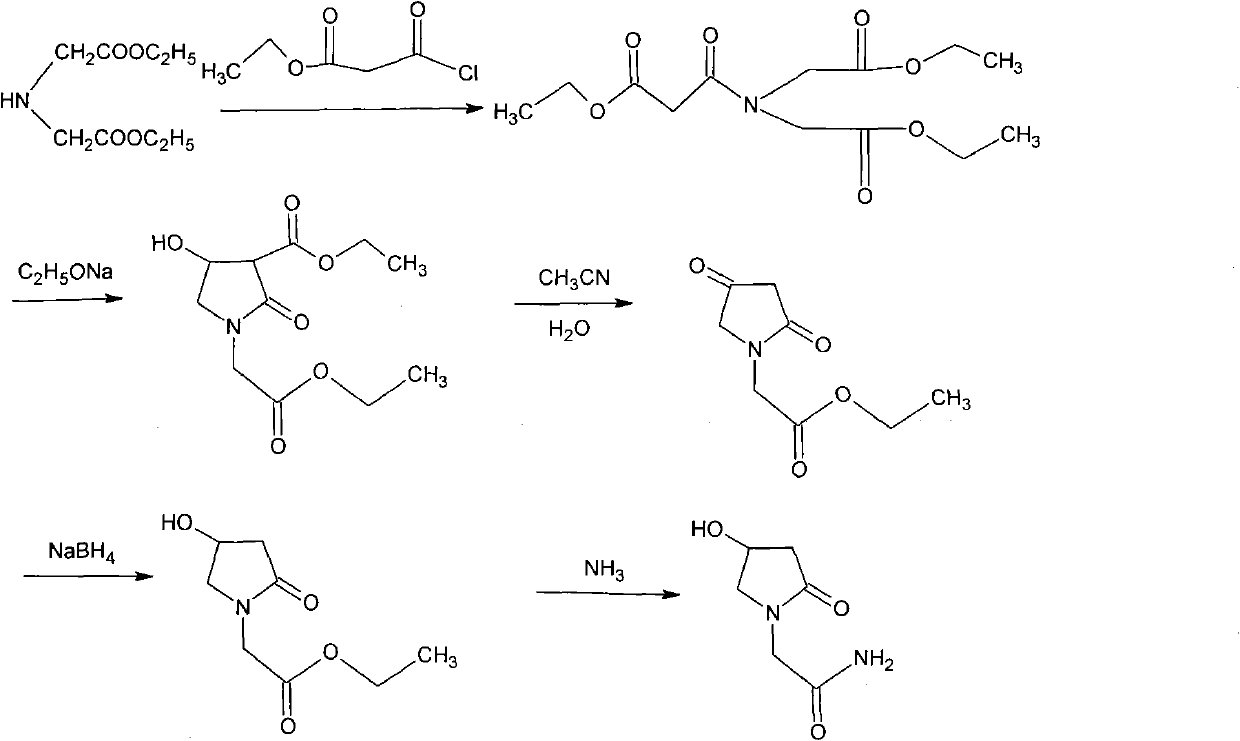
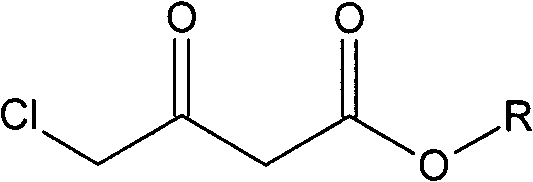
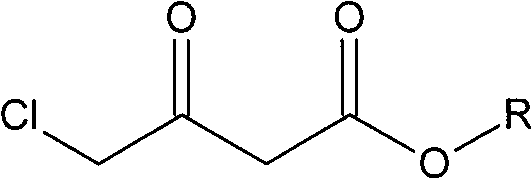
The invention relates to a preparation method of Oxiracetam, which takes 4-chlorine acetoacetic ester (1) as raw material, and prepared by the following synthetic route. The preparation method is simple and convenient, and has short route, low raw material, higher product yield, low cost and good purity.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
Injection composition containing oxiracetam and preparation method and application thereof
ActiveCN102552125AImprove stabilitySimple recipePowder deliveryOrganic active ingredientsChemical compositionOxiracetam
The invention provides an injection composition containing oxiracetam and a preparation method and an application thereof. The composition contains sodium hydroxide. The invention further provides a preparation method and an application of the medicinal composition. The composition is mainly used for improving memory and the memory learning function of a mentally retarded patient, and is suitablefor memory and amentia caused by symptoms such as mild or moderate vascular dementia, senile dementia, brain trauma and the like, or is taken as an auxiliary treatment medicament.
Owner:北京爱力佳医药科技有限公司
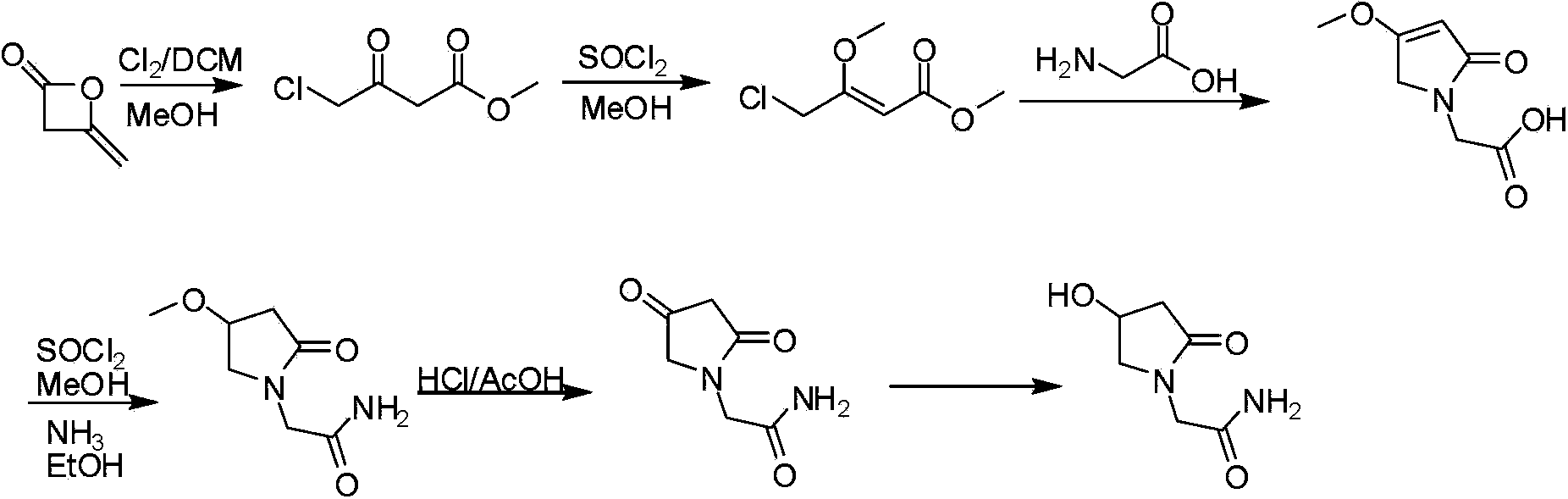
Oxiracetam compound and new method thereof
The invention relates to an oxiracetam compound and a new method thereof. In the method, 3-chloro-2-hydroxy propionitrile is used as an initial raw material. The method comprises the following steps of: synthesizing 4-hydroxyl-2-pyrrolidone through 4-chloro-3-hydroxyl-butylamide; and then synthesizing oxiracetam. The invention overcomes the defects of complicated preparation process, trouble steps, high cost, low product purity and difficult purification of the prior art.
Owner:HAINAN LINGKANG PHARMA CO LTD
Synthetic method of oxiracetam
The invention discloses a synthetic method of oxiracetam. The synthetic method is characterized in that acety ketene is used as a raw material, and four steps of bromination, esterification, reduction and ring closure are carried out to obtain the oxiracetam. The synthetic method provided by the invention has the advantages of easy operation, high safety, environmental protection, cheap and easily available raw materials, mild reaction conditions, high yield and substantially reduced product cost, thereby being more suitable for industrial production.
Owner:广安凯特制药有限公司 +2
Oxiracetam drug activity composition and preparation method thereof
ActiveCN102846600AComply with medicinal requirementsQuality improvementOrganic active ingredientsNervous disorderClinical efficacyEthyl acetate
The present invention provides an oxiracetam drug activity composition, which comprises the following components: a component I, a component II and a component III, wherein the component I is oxiracetam, the component I content is more than or equal to 98.0%, the component II is glycine anhydride, the component II content is more than 0 and is less than or equal to 0.3%, the component III is one or a plurality materials selected from 4-hydroxy-2-oxo-1-pyrrolidineacetic acid, 4-hydroxy-2-oxo-1-pyrrolidine, ethyl 4-hydroxy-2-oxopyrrolidine-1-acetate, methyl 4-hydroxy-2-oxopyrrolidine-1-acetate, and piracetam, the component III content is more than 0 and is less than or equal to 1.5%, the 4-hydroxy-2-oxo-1-pyrrolidineacetic acid content is more than 0 and is less than or equal to 0.5%, and the total content of the component II and the component III is less than or equal to 1.5%. The drug activity composition of the present invention has stable quality, and can completely meet quality requirements on the drug activity composition by oxiracetam preparations. In addition, the prepared preparation has characteristics of safety, effectiveness, and controllable quality, and clinical therapy effects and medication safety of the oxiracetam preparation are ensured.
Owner:CSPC OUYI PHARM CO LTD
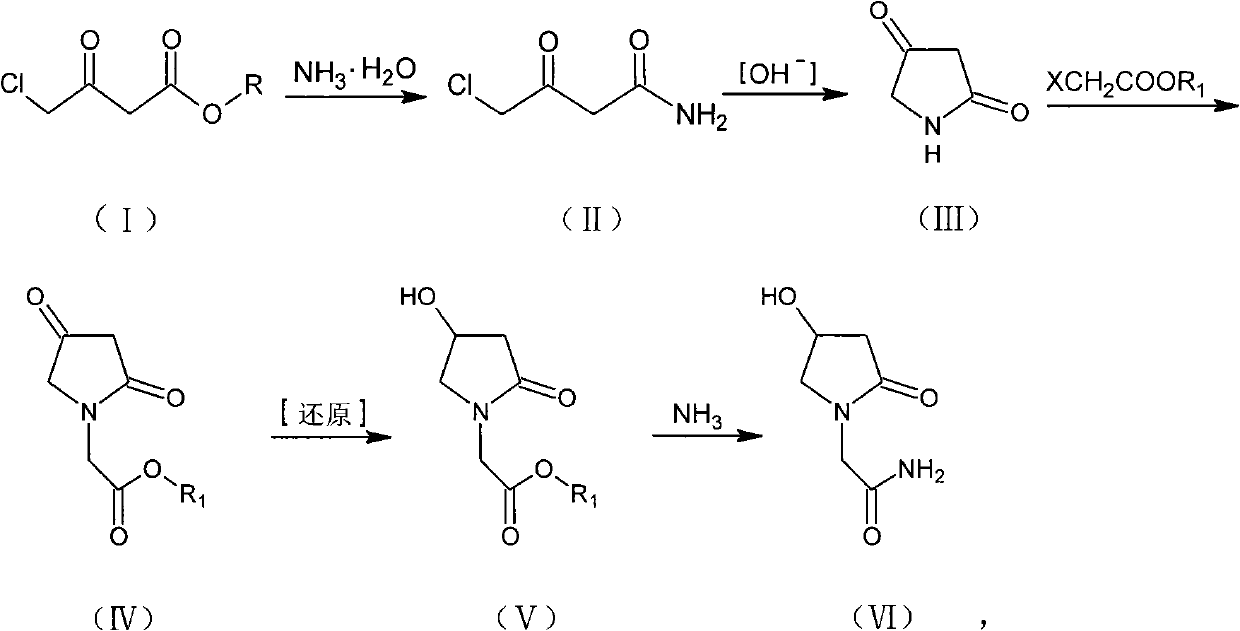
Preparation method of Oxiracetam, product of Oxiracetam and use of product
InactiveCN101885697AThe reaction steps are simpleRaw materials are easy to getOrganic active ingredientsNervous disorderTreatment effectDistillation
The invention provides a method for preparing Oxiracetam, which comprises the following steps: performing alkaline treatment of 4-hydroxy-2-pyrrolidone under a water-free condition; reacting the treated 4-hydroxy-2-pyrrolidone with 2-chloroacetamide in an organic solvent in the presence of a catalyst; and after the reaction is finished, performing filtration, distillation and recrystallization to obtain the Oxiracetam. In the method, the final product can be obtained by a one-step reaction, the operation is simple and convenient, and large scale production can be realized. The invention also provides the Oxiracetam obtained by the method, which has the characteristics of high yield, high purity and good treatment effect. The invention also provides the use of the Oxiracetam obtained by the method in the preparation of medicaments for treating brain dysfunction, impaired memory and senile dementia.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Novel crystal formations of levogyration oxiracetam and preparation method thereof
The invention relates to novel crystal formations of levogyration oxiracetam, including a crystal formation A, a crystal formation B and a crystal formation C, which have excellent stability and are very suitable for the preparation of common formulations of levogyration oxiracetam. The invention further discloses a preparation method and characterization of the crystal formations, as well as a pharmaceutical composition containing the crystal formations.
Owner:南京斯帕克医药科技有限公司
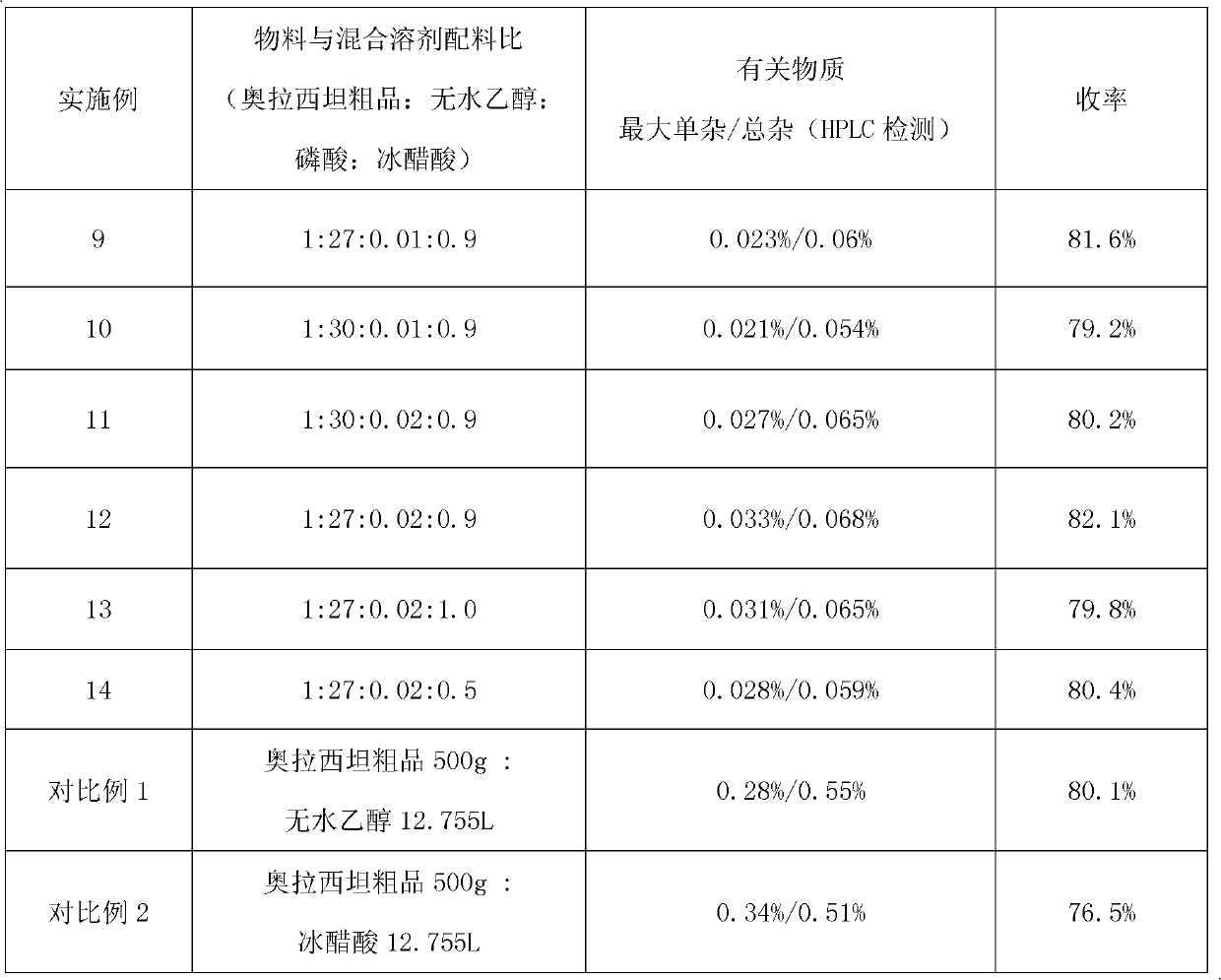
Method for refining oxiracetam
The invention relates to a method for refining oxiracetam, which comprises the following steps: adding a crude oxiracetam product into a mixed solvent of anhydrous alcohol, phosphoric acid and glacial acetic acid, heating to 80+ / -2 DEG C under reflux, adding activated carbon and continuing reflux; and filtering while the solution is hot to obtain a colorless clear solution, stirring at 5-10 DEG Cto crystallize for 10-12 hours, centrifugalizing, and drying to obtain the fine oxiracetam product. The purity of the fine oxiracetam product is higher than 99.9%, the maximum single impurity contentis less than 0.05%, and the total impurity content is less than 0.1%. The invention is simple to operate, environment-friendly, and applicable to industrial mass production.
Owner:SHANDONG JINHE DRUG RES DEV
Purification method for sinistrogyration oxiracetam
InactiveCN102531988AGood water solubilityHigh purityOrganic chemistryOrganic solventPurification methods
Provided is a purification method for sinistrogyration oxiracetam. The method comprises the steps of adding sinistrogyration oxiracetam coarse products in water for dissolving, dropping organic solvent in solution to enable the solution to become turbid just, standing at 0-18 DEG C for 1-3 days, separating out colorless transparent crystals, filtering, washing by using ice water at the temperature of 0-5 DEG C in a top mode, and drying in a vacuumizing mode to obtain the sinistrogyration oxiracetam with high purity. By means of the corresponding purification process using water as the organic solvent in the purification method, 89% of purity of the sinistrogyration oxiracetam coarse products is effectively increased to 97.5-98.2% of high performance liquid chromatography (HPLC) purity, and the purity of the sinistrogyration oxiracetam is greatly improved. Simultaneously, the purification method is simple, mild in control conduction, low in cost of production and applicable to large-scale industrial production.
Owner:CHONGQING RUNZE PHARM CO LTD
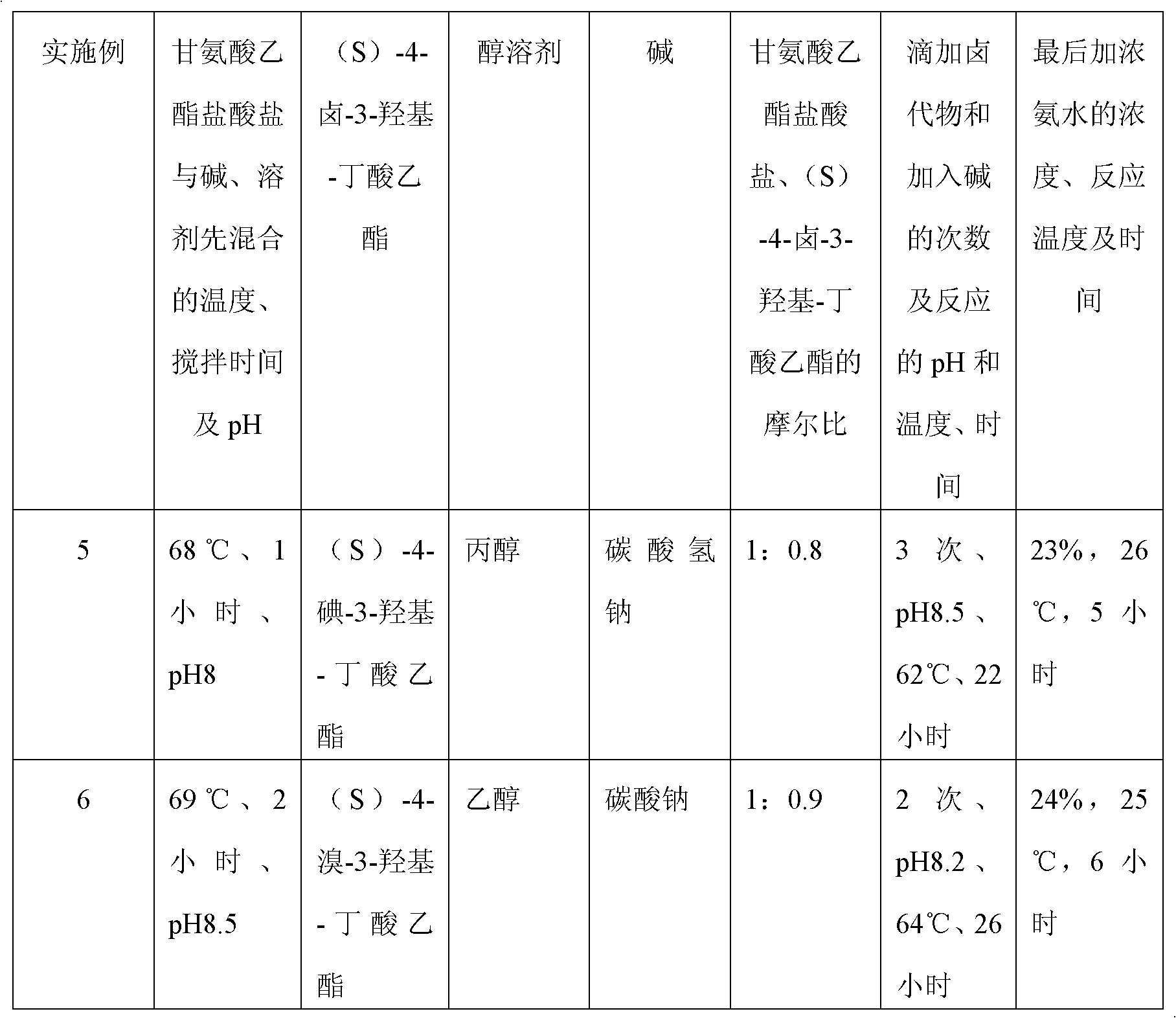
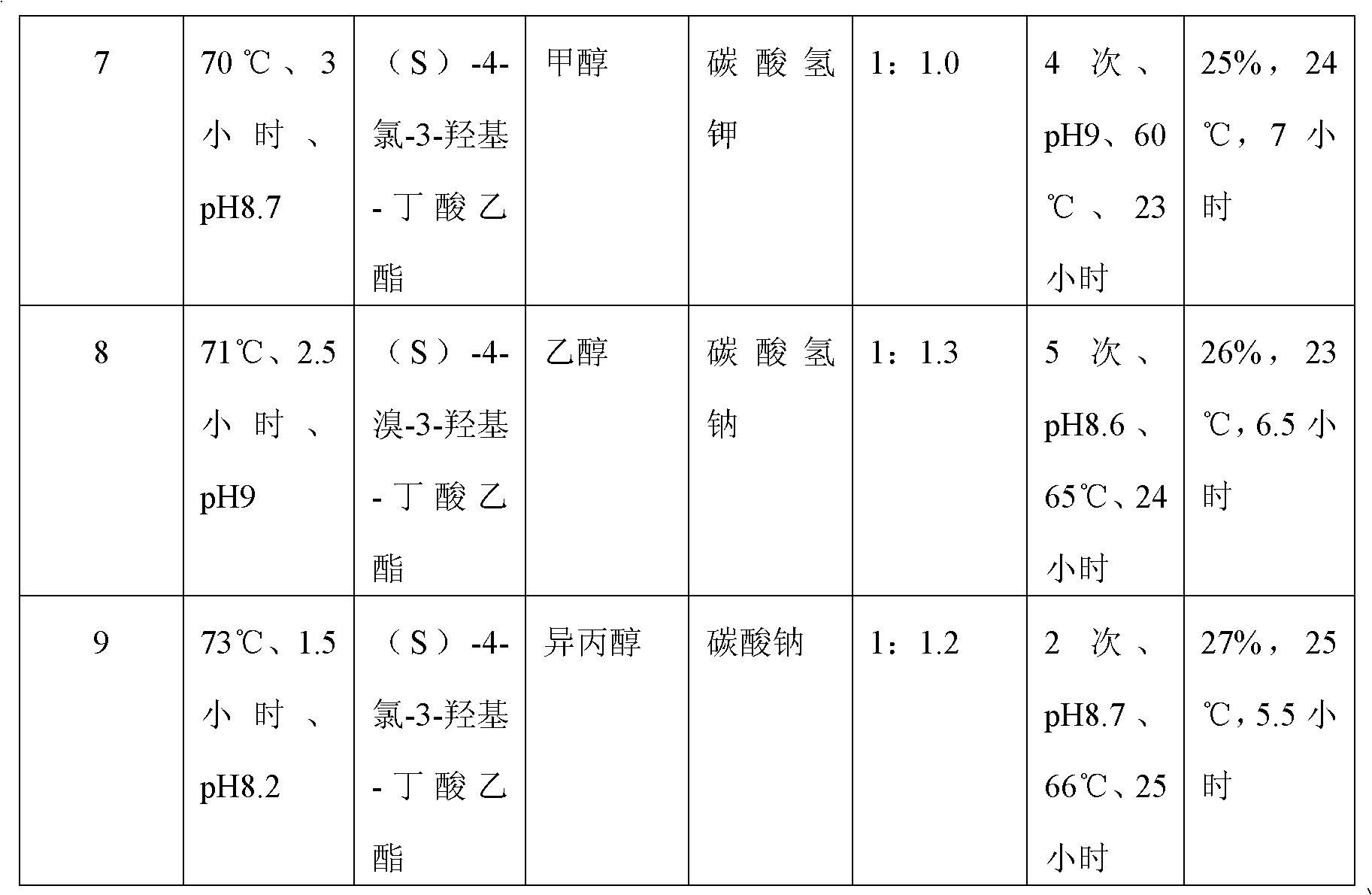
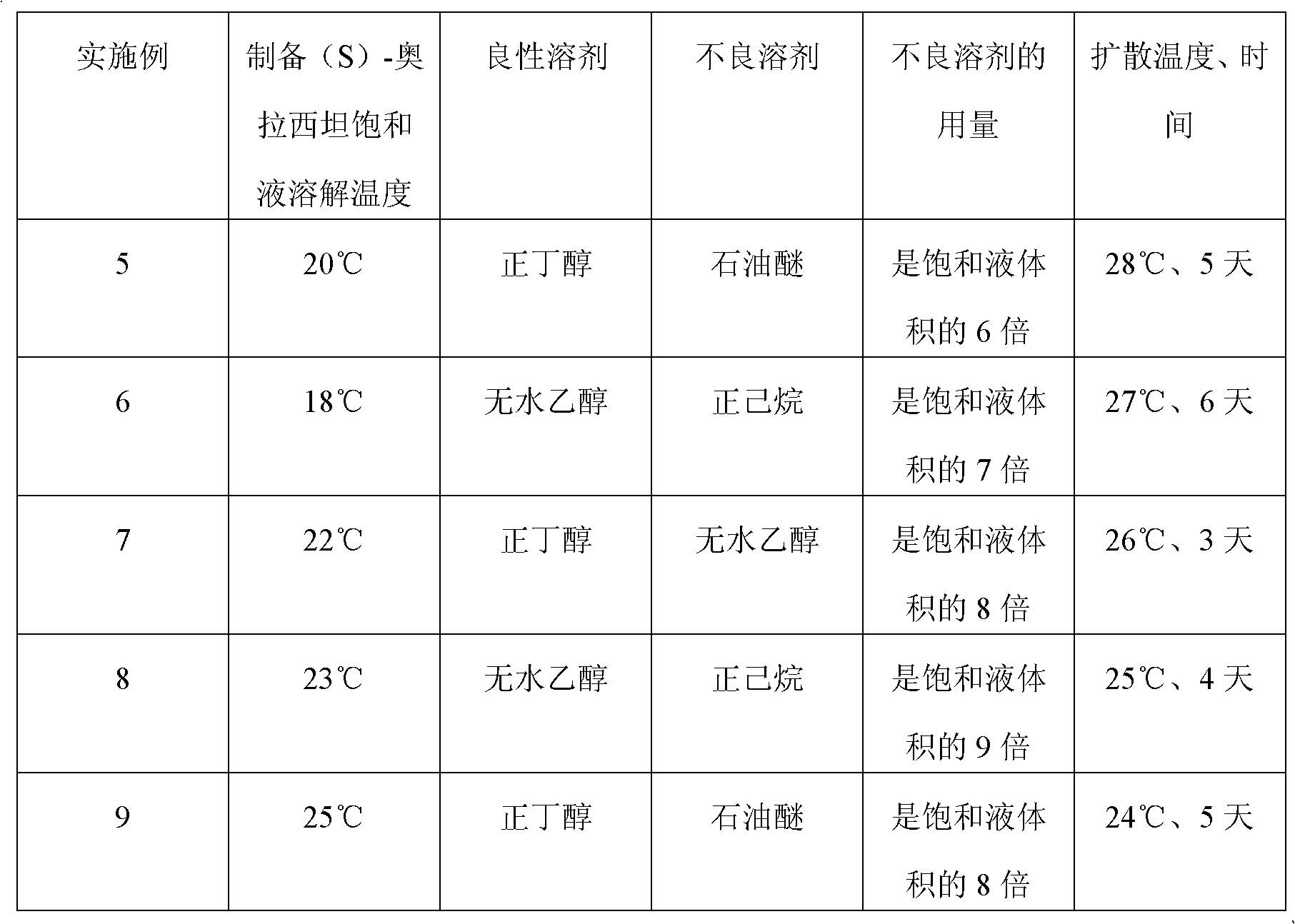
Preparation method of (S)-oxiracetam
A preparation method of (S)-oxiracetam comprises the steps of conducting a reaction of a glycine ethyl ester hydrochloride with (S)-4-halgen-3-hydroxy-ethyl butyrate in an alcohol solvent under an alkaline condition, wherein the glycine ethyl ester hydrochloride and the (S)-4-halgen-3-hydroxy-ethyl butyrate are adopted as raw materials, filtering, washing with an inorganic alcohol, concentrating, then extracting, conducting water-phase concentration, introducing stronger ammonia water for a reaction so as to prepare a crude product of (S)-oxiracetam, and purifying the crude product; and mixing the glycine ethyl ester hydrochloride with alkali and the alcohol solvent firstly, then dropping the (S)-4-halgen-3-hydroxy-ethyl butyrate raw material in the mixture, and adding the alkali in times so as to control the pH value in the reaction to be 8-9. Purification treatment comprises the steps that the crude product of (S)-oxiracetam is dissolved in a benign solvent, a saturated solution is prepared at the room temperature, and then the saturated solution is dispersed in a closed environment by a poor solvent. The HPLC (high-performance liquid chromatography) purity of the prepared (S)-oxiracetam reaches up to more than 99.0%, the yield is high and reaches up to 33%, the reaction condition is moderate, the operation is simple, and the industrialized scale production is facilitated.
Owner:CHONGQING RUNZE PHARM CO LTD
Method for preparing oxiracetam
InactiveCN102633705ANo pollution in the processReduce lossOrganic chemistryReaction rateRates reactions
The invention discloses a method for preparing oxiracetam. The method comprises the following step of carrying out catalytic hydrogenation by taking 4-chloro-acetylacetic ester as a raw material under the action of certain temperature, certain pressure and a catalyst to obtain 4-cholor-3-hydroxyl butyrate. According to the method, three wastes can be avoided, the environmental pollution can be avoided, the raw material 4-chloro-acetylacetic ester is low in price and easy to obtain, the yield is high, and a product can be almost quantitatively obtained. A microwave reaction method is used in the step of preparing the oxiracetam by cyclization, so that the unnecessary loss of heat energy can be reduced, the reaction yield and the reaction rate are improved, and the product is easy to separate and purify.
Owner:SHIJIAZHUANG LICKON PHARMATECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com