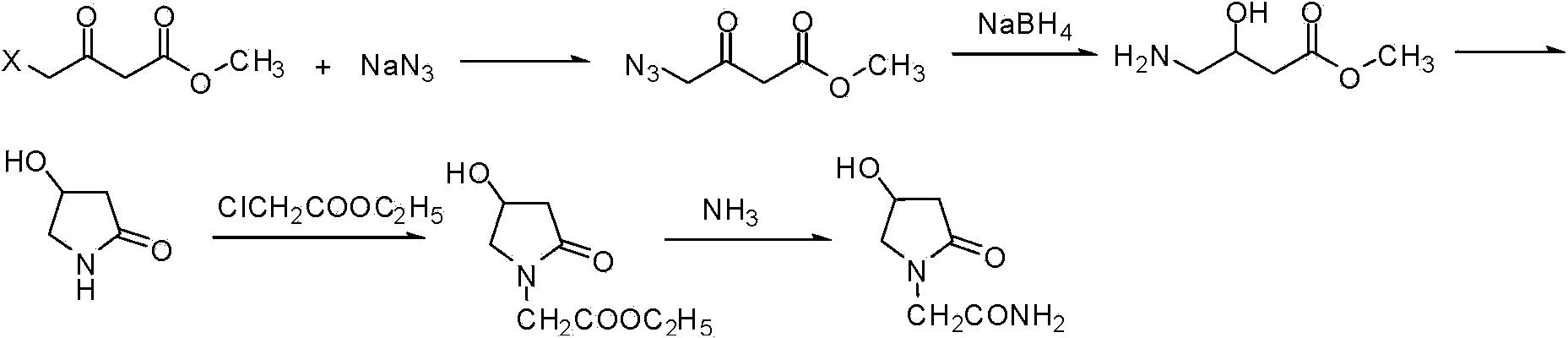
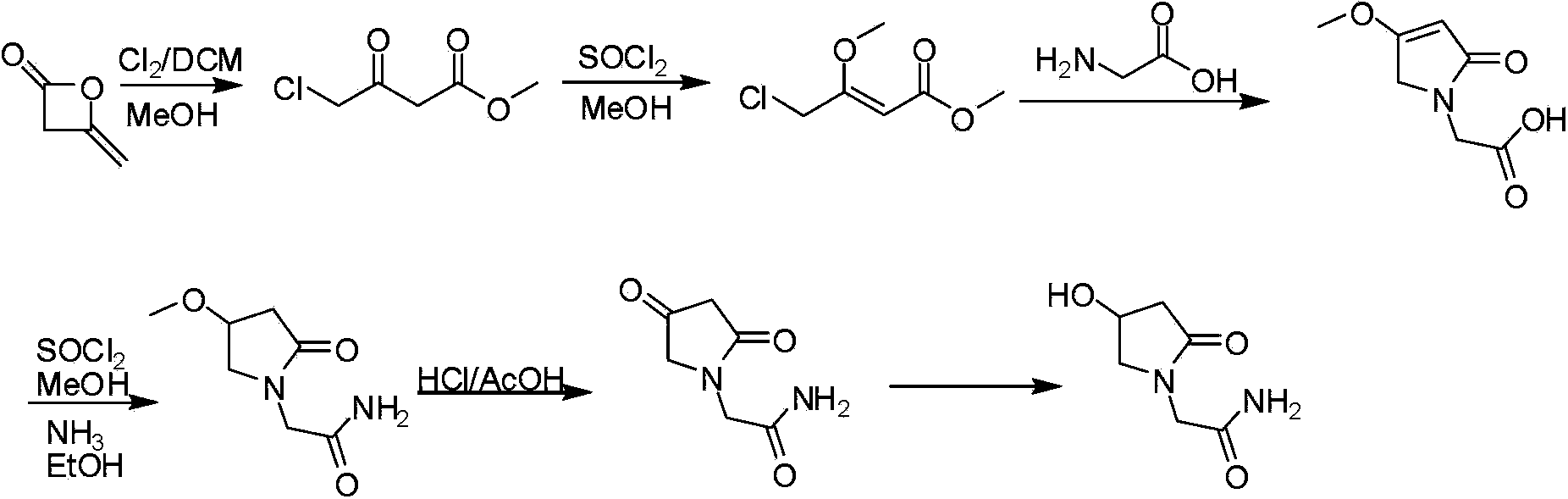
Synthetic method of oxiracetam
A synthetic method and compound technology, applied in the field of medicine, can solve the problems of yield and finished product quality, salt is not easy to remove, etc., and achieve the effect of reducing production cost, simple operation, and suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Add 100 g of diketene and 500 g of dichloromethane into the reaction flask, and control the temperature at 20-30° C. Slowly add 476g of bromine dropwise, maintaining the temperature at 20-30°C. After the drop is complete, the reaction solution is stirred for 6 hours. The water pump decompresses and recovers dichloromethane until no obvious liquid evaporates (control temperature ≤ 30°C).
[0042] (2) Control the temperature of 4-bromo-3-oxobutyryl bromide obtained in the above step (1) at 20-30° C., add 45 g of methanol dropwise to the residual liquid, and after the addition is complete, the reaction liquid is stirred for 30 minutes. Control temperature ≤ 50°C, -0.08MPa, evaporate methanol to dryness under reduced pressure to obtain 204g of methyl 4-bromo-3-oxobutanoate, two-step yield 87.9%.
[0043] (3) Put 200g of methyl 4-bromo-3-oxobutanoate and 400g of methanol into a 2000ml reaction bottle, cool down to 15-17°C, control the temperature at 15-20°C, slowly add ...
Embodiment 2
[0046] (1) Add 420 g of diketene and 2730 g of dichloromethane into the reaction flask, and control the temperature at 20-30° C. Slowly add bromine 1490g dropwise, maintain the temperature at 20-30°C, pass through for about 8 hours, and stir the reaction solution for 1 hour. The water pump decompresses and recovers dichloromethane until no obvious liquid evaporates (control temperature ≤ 30°C). The steps for preparing oxiracetam refined product from 4-bromo-3-oxobutyryl bromide are the same as (2), (3) and (4) in Example 1, and the total yield is 49.2%. ( 1 H-NMR (D 2 O) δ2.82 (1H, dd, J = 17.8Hz), 2.34 (1H, dd, 17.8Hz), 4.58 (1H, d, J = 6.0Hz), 3.45 (1H, d, J = 11.4Hz), 3.84(1H,dd,J=11.4Hz), 3.93(1H,d,J=16.0Hz), 4.06(1H,d,J=16.0Hz)
Embodiment 3
[0048] (1) (2) (3) the synthesis steps are all with example 1.
[0049] (4) Add 73g of glycinamide hydrochloride, 500g of anhydrous methanol, and 41g of sodium hydroxide into the reaction flask, heat up to reflux, and slowly add 100g of methyl 4-bromo-3-hydroxybutyrate dropwise (about 1.0 hour dropwise), after the dropwise addition, continue the reflux reaction for 24.0 hours, after the reaction, filter while hot, and wash the filter cake once with 100g hot methanol. Methanol was distilled off under reduced pressure to obtain a solid viscous substance, and 400 g of methanol was added, stirred and crystallized to obtain crude oxiracetam. Add the crude product to 400°C of methanol and raise the temperature to about 50°C, add 10 g of activated carbon to keep it warm for decolorization, and cool down to crystallize to obtain 53 g of refined oxiracetam with a yield of 66.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com