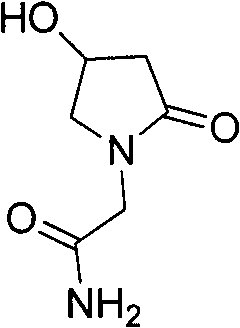
Method for preparing oxiracetam
A chloroacetoacetate and pressure technology, applied in the field of chemical raw material preparation, can solve problems such as high yield and short steps, and achieve the effects of improving reaction yield, fast reaction speed, and increasing effective collision probability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
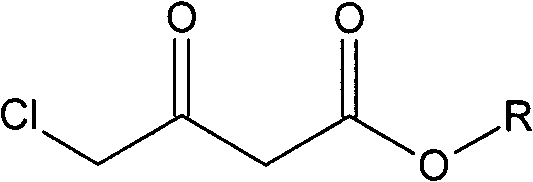
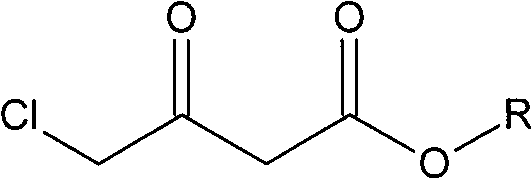
[0027] Take a mixture of 49.38g (300mmol) ethyl 4-chloroacetoacetate and 100ml ethanol and pour it into a 200ml single-necked bottle, and degas it with ultrasonic waves under reduced pressure. Quickly weigh 10.64mg (0.06mmol) of palladium chloride and 29.9mg (0.06mmol) of DIOP ligand with a microbalance, and then add them, degassed substrate and solvent into a 500ml autoclave under nitrogen protection. At room temperature, the air in the autoclave was replaced with nitrogen, then replaced with hydrogen three times, and filled with hydrogen to the pressure required for the reaction. Allow it to react at the set temperature for a certain period of time until the pressure does not change and stop the reaction. Pass circulating water to cool the temperature of the kettle body to room temperature, release hydrogen carefully, take out the reactor, evaporate the solvent on a rotary evaporator, and finally use an oil pump to distill under reduced pressure at 15 mm Hg and collect the f...
Embodiment 2
[0030] Take a mixture of 45.18g (300mmol) methyl 4-chloroacetoacetate and 100ml methanol and pour it into a 200ml single-necked bottle, and use ultrasonic degassing under reduced pressure. Quickly weigh 10.64mg (0.06mmol) of palladium chloride and 29.9mg (0.06mmol) of DIOP ligand with a microbalance, and then add them, degassed substrate and solvent into a 500ml autoclave under nitrogen protection. At room temperature, the air in the autoclave was replaced with nitrogen, then replaced with hydrogen three times, and filled with hydrogen to the pressure required for the reaction. Allow it to react at the set temperature for a certain period of time until the pressure does not change and stop the reaction. Pass circulating water to cool the temperature of the kettle body to room temperature, release hydrogen carefully, take out the reactor, evaporate the solvent on a rotary evaporator, and finally use an oil pump to distill under reduced pressure at 15 mm Hg and collect the fract...
Embodiment 3
[0033] Take a mixture of 49.38g (300mmol) ethyl 4-chloroacetoacetate and 100ml methanol and pour it into a 200ml single-necked bottle, and use ultrasonic degassing under reduced pressure. Quickly weigh 12.56mg (0.06mmol) of rhodium trichloride and 27.51mg (0.06mmol) of the DIPAMP ligand with a microbalance, then add it and degassed substrate and solvent to a 500ml autoclave under nitrogen protection . At room temperature, the air in the autoclave was replaced with nitrogen, then replaced with hydrogen three times, and filled with hydrogen to the pressure required for the reaction. Allow it to react at the set temperature for a certain period of time until the pressure does not change and stop the reaction. Pass circulating water to cool the temperature of the kettle body to room temperature, carefully release hydrogen, take out the reactor, evaporate the solvent on a rotary evaporator, and finally use an oil pump to distill under reduced pressure at 6 mm Hg and collect the fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com