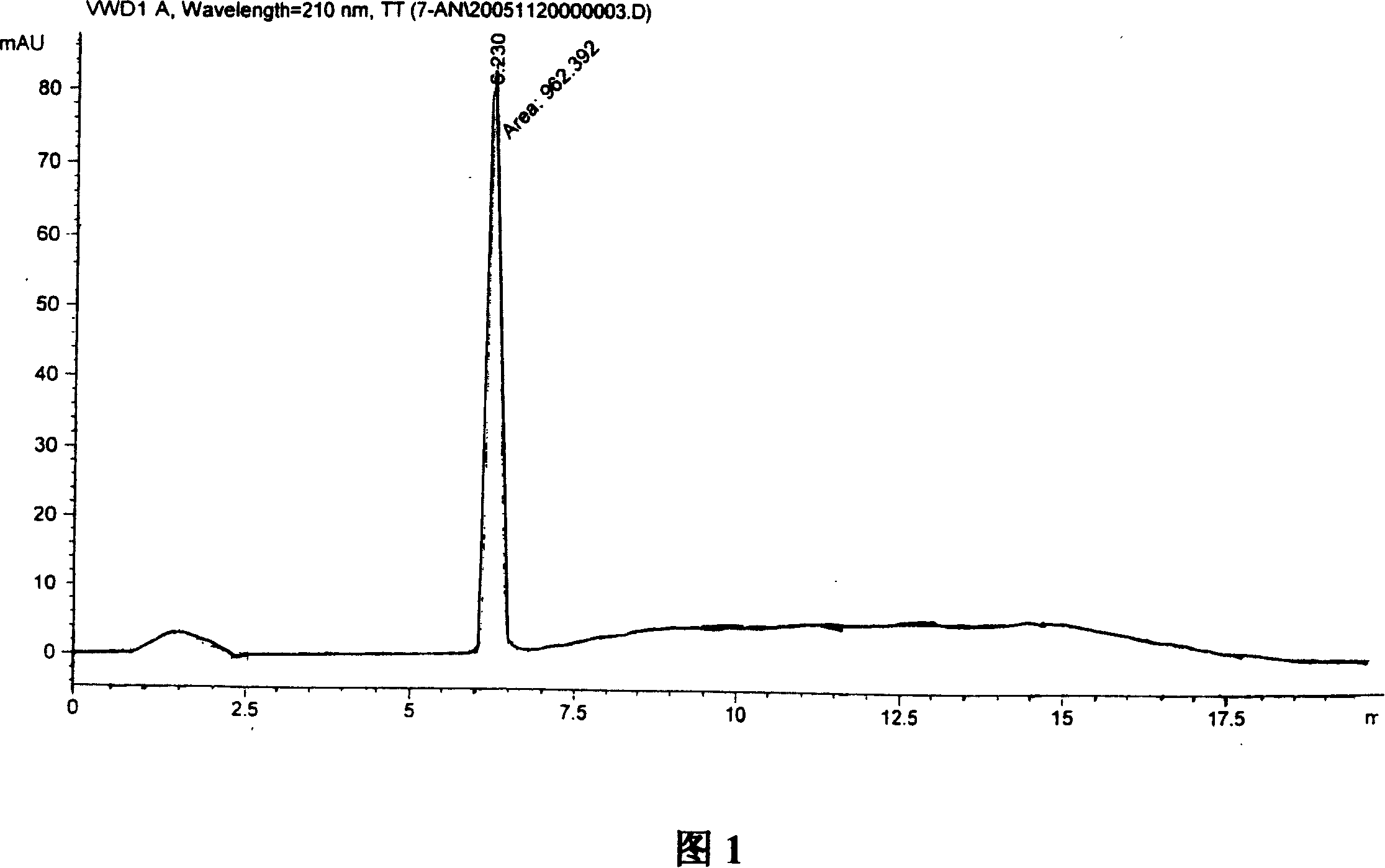
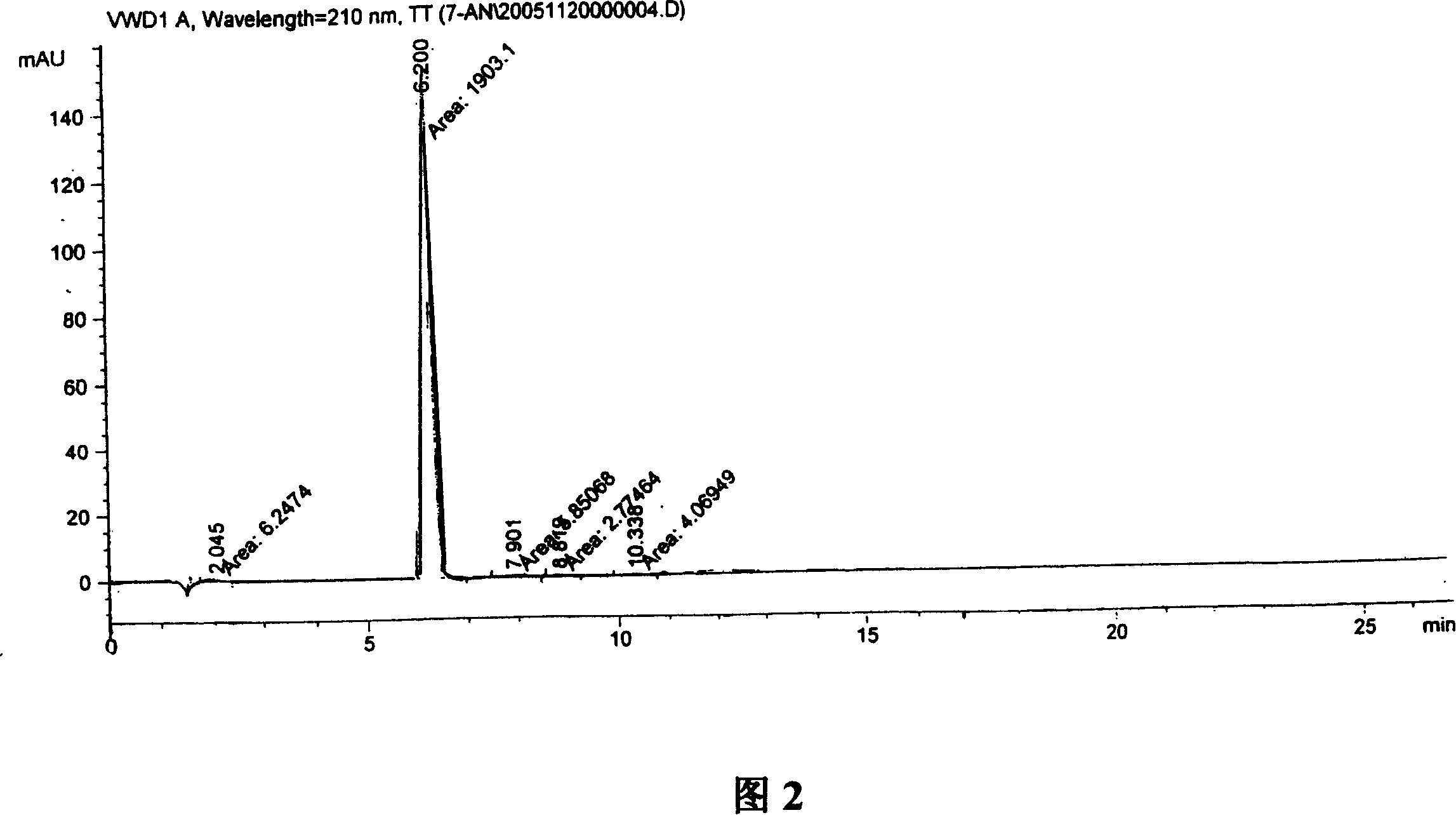
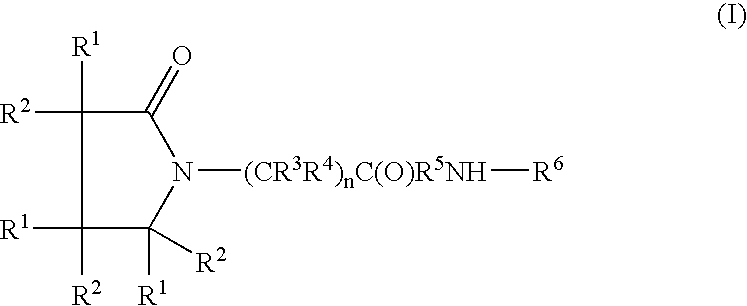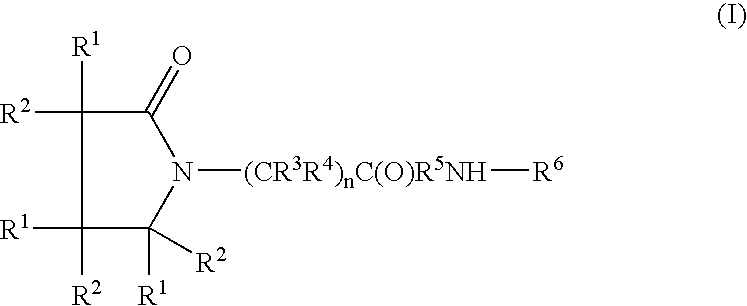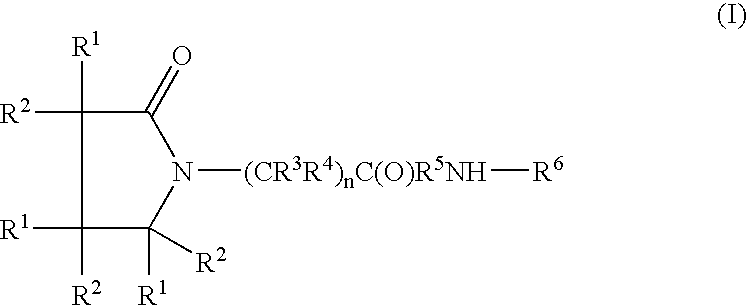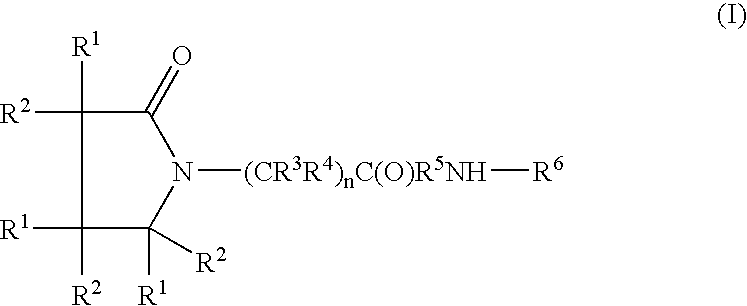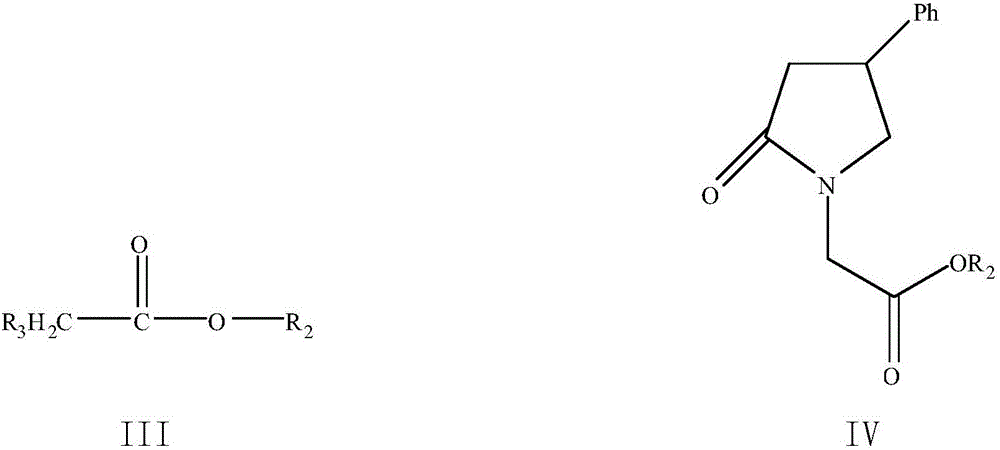Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57 results about "Piracetam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
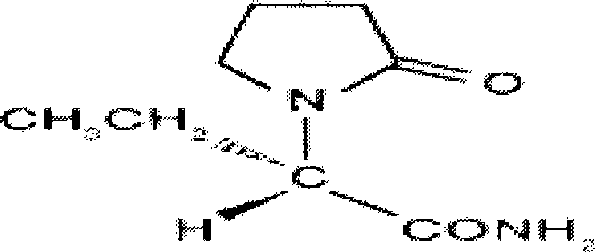
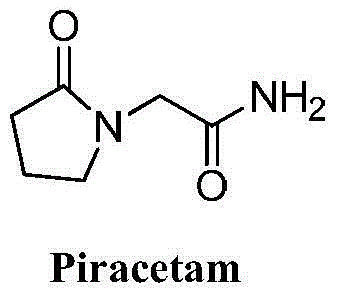
Piracetam (sold under many brand names) is a medication in the racetams group, with chemical name 2-oxo-1-pyrrolidine acetamide. Evidence to support its use for many conditions is unclear, although it is marketed as a nootropic (cognitive enhancer). Studies of piracetam's cognitive effects have had equivocal results, sometimes showing modest benefits in specific populations and sometimes showing minimal or no benefit.
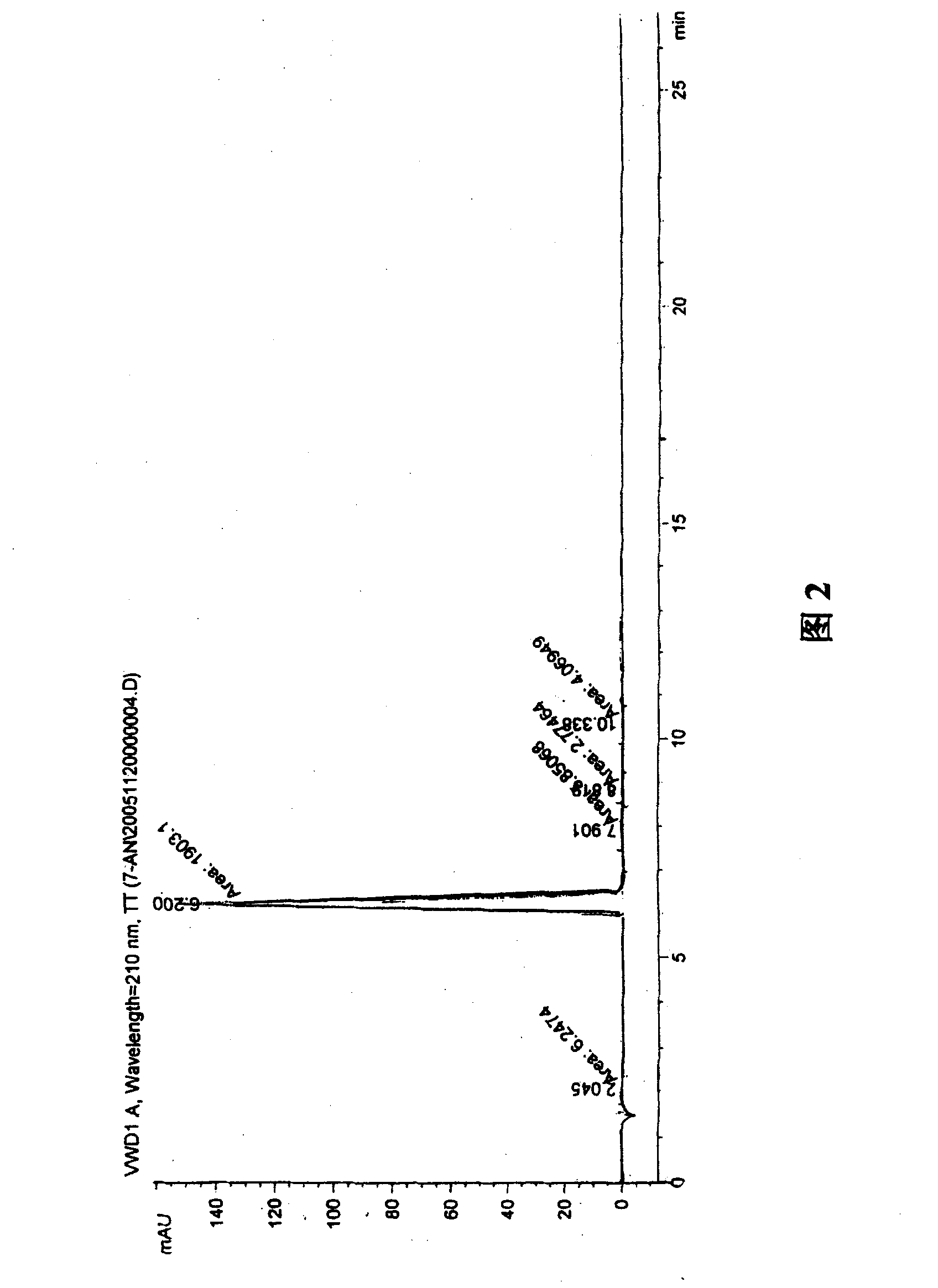
Synthesis, split and racemization of chirality medicament levetiracetam midbody (S)-(+)-2-amido butyramide hydrochlorate
ActiveCN101130504AReduce dosageLow costOrganic compound preparationCarboxylic acid amides optical isomer preparationButyramidePyrrolidine
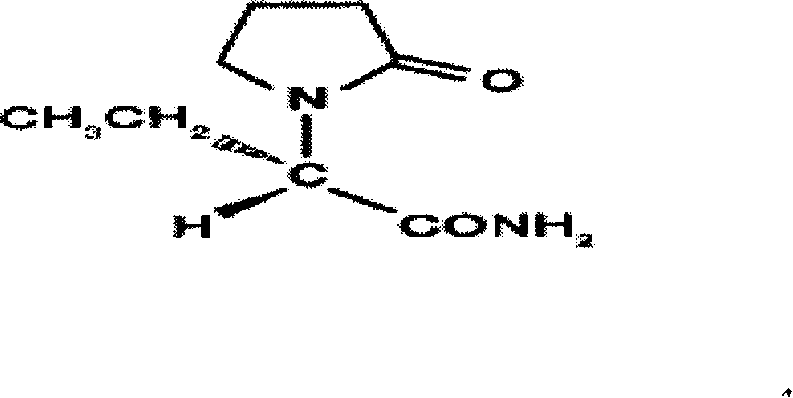
The invention discloses a new synthesizing technique of chiral drug (S)-alpha-ethyl-2-oxo-1-pyrrolidine acetamide ( left Piracetam) intermediate (S)-(+)-2-aminobutanamide hydrochlorate, which comprises the following steps: adopting 2-brobutyrate as initial raw material; aminating; esterifying; ammonolyzing; detaching; looping; obtaining the object compound; making mixed rotary free alkaline (+-)-2-aminobutanamide; adopting half-quantum resolution method to connect chemical detaching salt to evolve salt; removing the detaching agent through alkalization; obtaining the (S)-(+)-2-aminobutanamide hydrochlorate with optical activity; using the mother liquor to make the product. The invention improves the receiving rate and saves the cost of raw material with simply technical operation and low cost, which resolves the resolved mother liquor after racemic action again to reduce the pollution of environment, therefore fitting for industrialized manufacturing.
Owner:ABA CHEM CORP
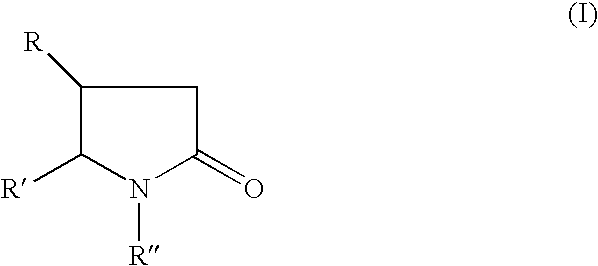
Piracetam and piracetam analog conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound of the formulawhere R1 is H, C1–C4 alkyl and OH; R2 in is H, C1–C4 alkyl and OH; R3 is H and C1–C4 alkyl; R4 is H and C1–C4 alkyl; n is an integer between 0 and 2 inclusive; R5 is a nullity, NHR7C(O)—, C6H4—, C6H4—O—; R7 is C2–C6 alkyl; and R6 is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine, blood brain barrier (BBB) peptide, membrane translocating peptide, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, transferrin, glucosylamine, amino saccharin, saccharin ester, lactylamine, leucine, tryptophan, amino glutamate and amino cholines.
Owner:MILLER LANDON C G
Piracetam and piracetam analog conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound of the formula where R1 is H, C1-C4 alkyl and OH; R2 in is H, C1-C4 alkyl and OH; R3 is H and C1-C4 alkyl; R4 is H and C1-C4 alkyl; n is an integer between 0 and 2 inclusive; R5 is a nullity, NHR7C(O)—, C6H4—, C6H4—O—; R7 is C2-C6 alkyl; and R6 is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine, blood brain barrier (BBB) peptide, membrane translocating peptide, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, transferrin, glucosylamine, amino saccharin, saccharin ester, lactylamine, leucine, tryptophan, amino glutamate and amino cholines.
Owner:MILLER LANDON C G
Oral Chinese herbal preparation for treating post-traumatic brain syndrome
InactiveCN102631579AReduce dosageFast absorptionNervous disorderInanimate material medical ingredientsLicorice rootsTherapeutic effect
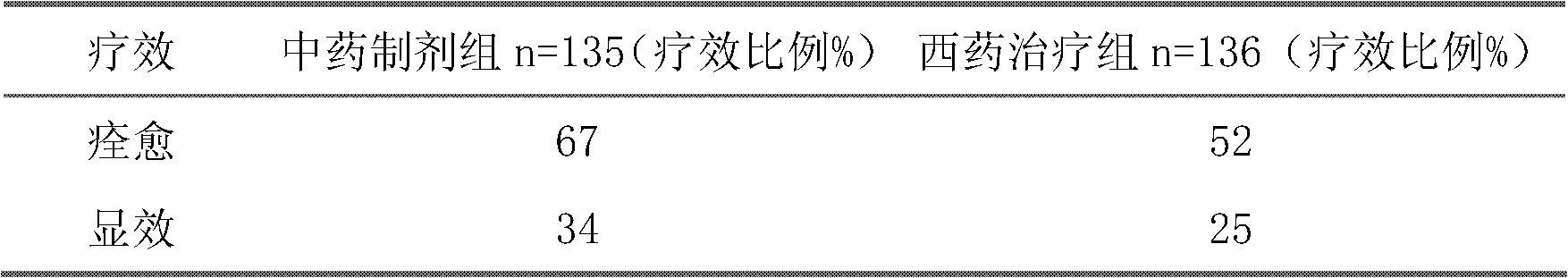
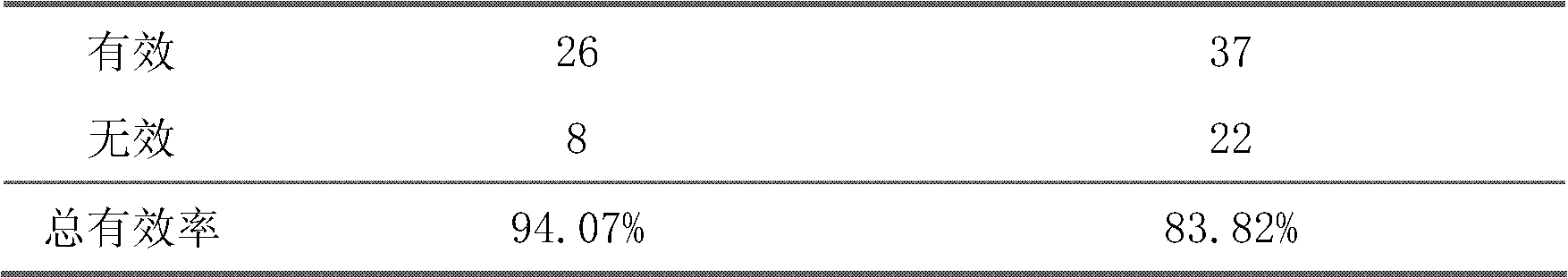
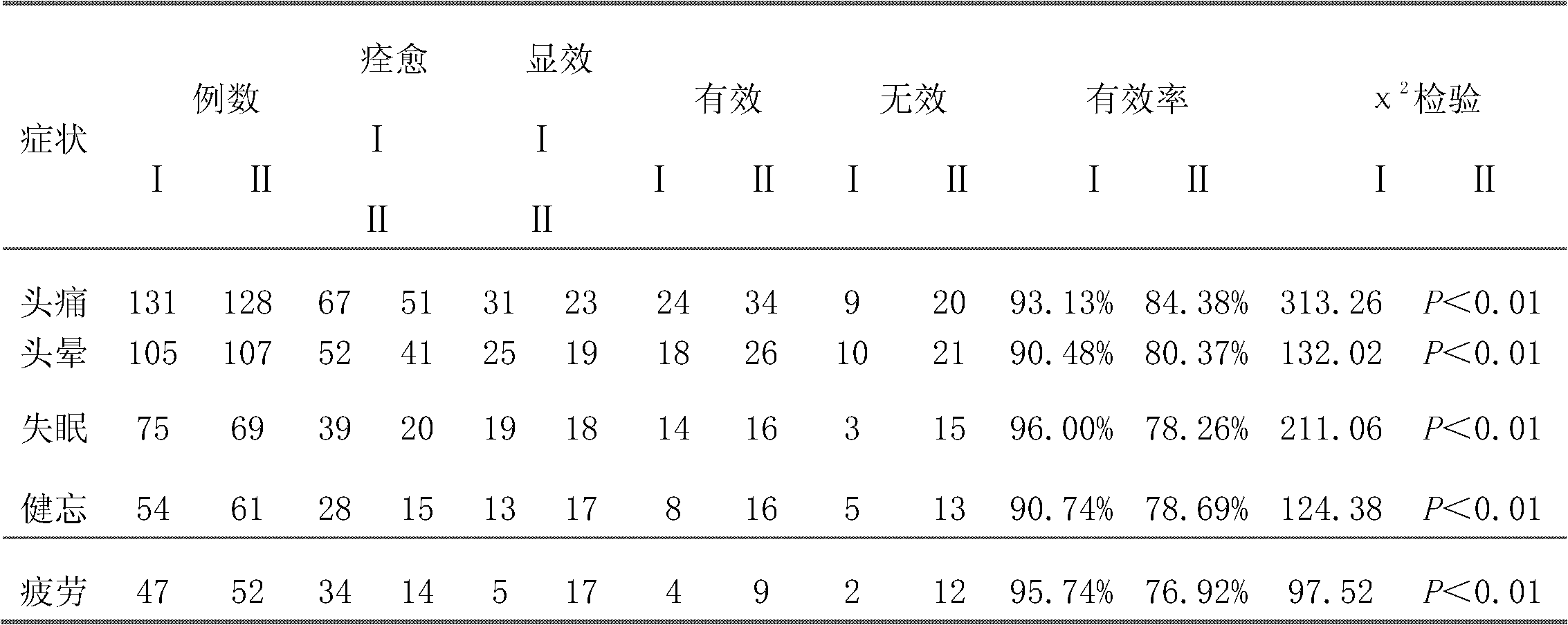
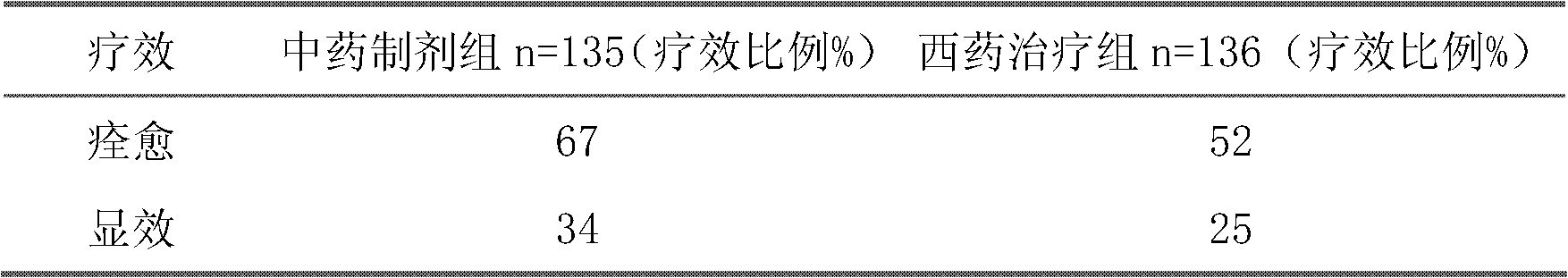
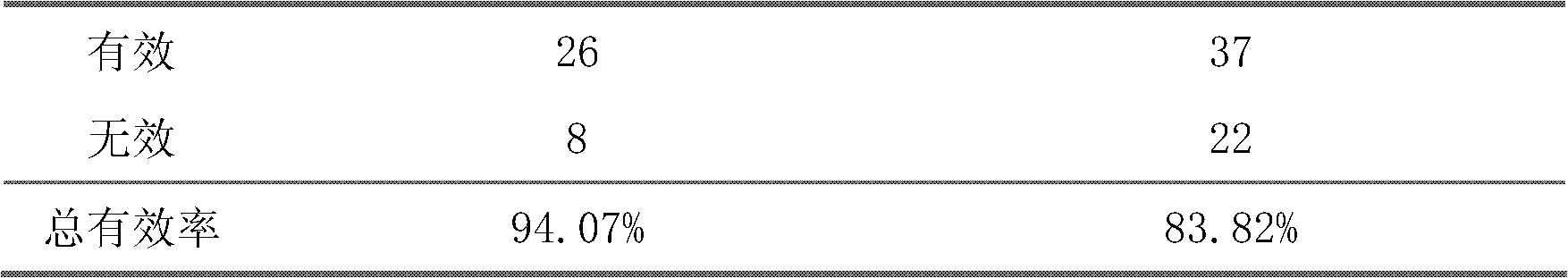
The invention belongs to an oral Chinese herbal preparation for treating post-traumatic brain syndrome, and the oral Chinese herbal preparation is a human medicinal product. The preparation is prepared from the following Chinese herbs in parts by weight: 10+ / -2 parts of Astragalus mongholicus, 10+ / -2 parts of American ginseng, 10+ / -2 parts of Angelica sinensis, 10+ / -2 parts of medlar, 10+ / -2 parts of the root of red-rooted salvia, 10+ / -2 parts of ligusticum wallichii, 10+ / -2 parts of root of common peony, 10+ / -2 parts of peach kernel, 10+ / -2 parts of Poria cocos, 10+ / -2 parts of pericarpium citri reticulatae, 10+ / -2 parts of plantain seed, 10+ / -2 parts of tabasheer, 10+ / -2 parts of rhizoma acori graminei, 10+ / -2 parts of gastrodia elata, 10+ / -2 parts of uncaria, 10+ / -2 parts of spina date seed, 10+ / -2 parts of amber powder and 6+ / -1.2 parts of honey-fried licorice root. Clinical effect observation proves that the total effective rate of the oral Chinese herbal preparation to the oral traditional Chinese medicine preparation is 94.07%, and has better treatment effect (P<0.01) compared with Western medicines such as Rotundine, piracetam, oryzanol and nimodipine for treating post-traumatic brain syndrome. Moreover, the Chinese herbal preparation has no obvious toxic or side effect, and can be taken for a long time. The dose of the preparation is small, and effective medicinal components are easy to release, can be absorbed quickly and can fully play the pharmaceutical effect. The preparation is convenient to carry, easy to take and beneficial to large-scale production.
Owner:HOSPITAL NO 3 CPLA
Medicinal composition for preventing and curing intracranial hypertension
InactiveCN1111405CHeterocyclic compound active ingredientsCardiovascular disorderControlled hypotensionHypotension shock
This invention relates to the medicinal composition for prevention and cure of intracranial hypertension, which contains piracetam (1-acetamido-2ketone-pyrrolidine) and the carrier tolerable to pharmaceutics. It can further contain osmotic diuretic such as mannitol,etc.; or anti-tissue exuedative medicine such as esculin sodium; or the medicine such as nitroglycerin, sodium nitroprusside, etc. used for resulting in controllable hypotension.
Owner:ZHUHAI HEFAN MEDICINE
Piracetam medicine composition with function of promoting thinking and memory and its prepn
InactiveCN1398635AEnsure the quality of preparationsGood curative effectNervous disorderDipeptide ingredientsSide effectMedicine
The present invention is Piractetam medicine composition with the function of promoting thinking and memory and its preparation process. The composition consists of Piracetam and reductive glutathione in the weight ratio of 10-100 to 6-12 as well as pharmaceutically acceptable supplementary material. It has high curative effect, high stability, less adverse reaction and high safety.
Owner:湖南康源制药有限公司
Piracetam orally disintegrating tablets
InactiveCN102125524AReasonable formulaGood compatibilityNervous disorderPill deliveryOrally disintegrating tabletMagnesium stearate
The invention discloses piracetam orally disintegrating tablets and a preparation method thereof. The main drug of the piracetam orally disintegrating tablets is piracetam, and the auxiliary materials comprise microcrystalline cellulose, mannitol, lactose, crosslinked polyvinylpyrrolidone, sodium cyclamate, menthol, silica gel micropowder and magnesium stearate. The piracetam orally disintegrating tablets can effectively treat senile dementia, have convenience in taking, good taste, rapid disintegration, quick absorption and high bioavailability, and provide convenience for patients with senile dementia who have inconvenience in taking medicine.
Owner:QINGDAO UNIV OF SCI & TECH
Encephalic proteolytic products of compound Piracetam and its preparation
ActiveCN1546063AImprove metabolism regulation functionSignificant effectOrganic active ingredientsNervous disorderHydrolysatePhysiology
The invention relates to a Piracetam brain proteolytic preparation for treating brain neurological disorders and its preparing process, wherein the preparation comprises the following raw materials (by weight portion), Piracetam 35-70%, brain proteolytic product 25-50%, glutacid 2-10%, chondroitin sulfate 2-10%, vitamin B1 0.05-0.5%, vitamin B2 0.05-0.5%, vitamin B6 0.03-0.3%, and vitamin E 0.3-1.0%.
Owner:LIAOYUAN YULONG YADONG PHARMA
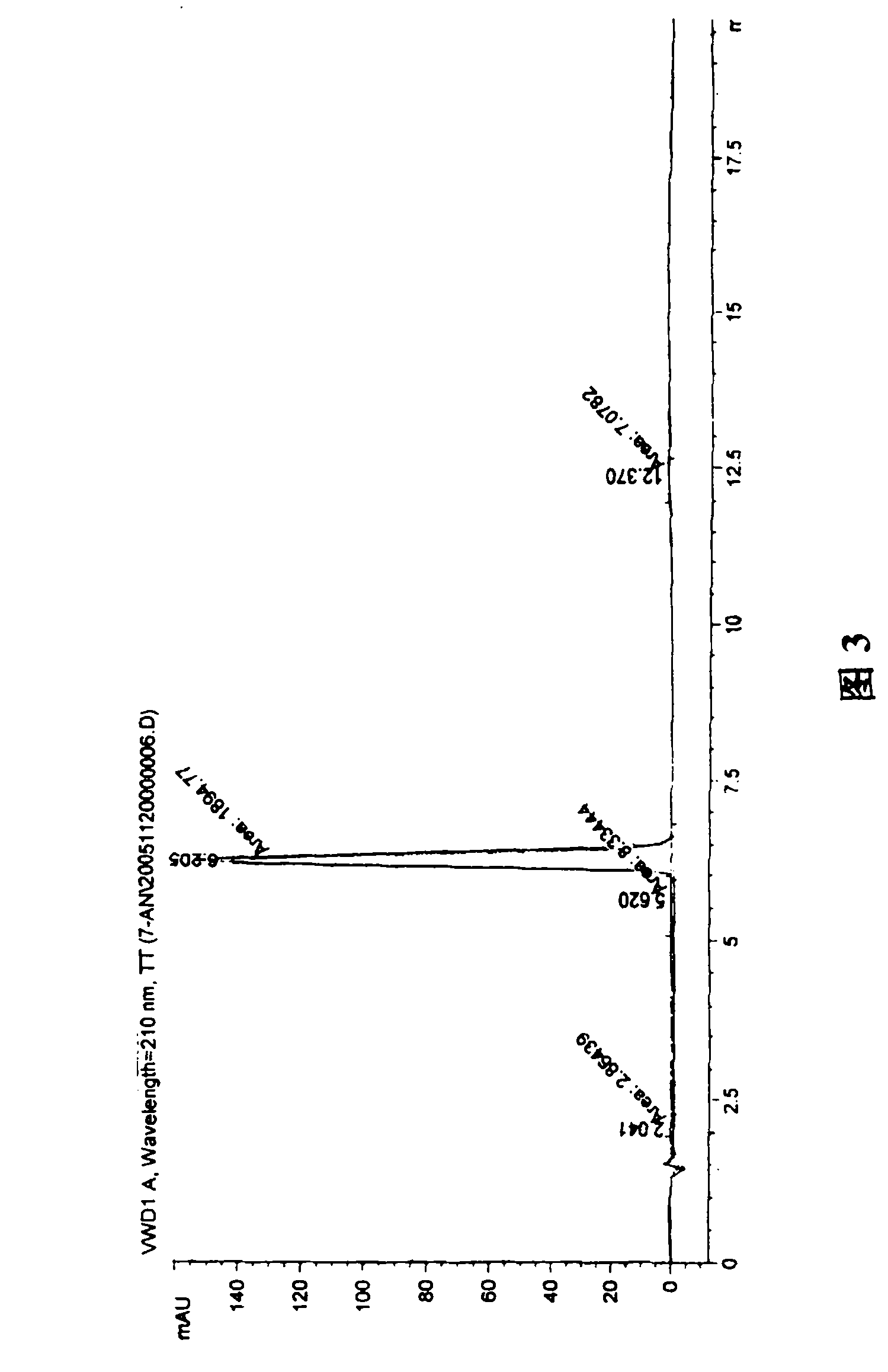
Synthesis, split and racemization method for preparing chirality medicament levetiracetam midbody (S)-(+)-2-amido butyramide hydrochlorate
ActiveCN101130504BReduce dosageHigh purityOrganic compound preparationCarboxylic acid amides optical isomer preparationEthyl groupRacemization
Owner:ABA CHEM CORP
Method for treating apathy syndrome
InactiveUS20100048634A1Improve frontal and executive cognitive functionBiocideNervous disorderChronic viral hepatitis CRisk stroke
The present invention provides a method of treating apathy syndrome in a human subject. The human subject is first evaluated to determine whether one or more behavioral characteristics of apathy are observed. If such characteristics are observed, the subject is treated with a 2-oxopyrrolidine compound, such as nefiracetam, piracetam, aniracetam, pramiracetam, nebracetam, fasoracetam, levetiracetam, or oxiracetam, in an amount effective to produce an improvement in such apathy characteristics. The present invention is useful in treating apathy in a subject suffering from conditions associated with or characterized by frontal-subcortical dysfunction. The present invention is also useful in treating apathy in a subject suffering from a stroke, Alzheimer's disease, Parkinson's disease, traumatic brain injury, depression, schizophrenia, chronic hepatitis C infection, or HIV infection.
Owner:CHA ALBERT
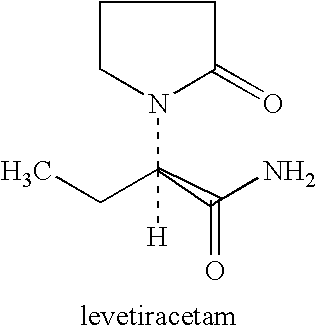
Use of levetiracetam in preparing intelligence benefiting medicament
InactiveCN101172105ASymptoms improve with treatmentNervous disorderHeterocyclic compound active ingredientsPharmacyDisease
The invention relates to the purpose of Levetiracetam during the process of preparing galangal medicine, the Levetiracetam is anti-epilepsia medicine with good curative effect at present, because the Levetiracetam and the structure of the naoyizhi medicine piracetam have large similarity, the invention aims at exploring the function of the Levetiracetam on the aspect of Naoyizhi. The invention is to find out therapeutic medicine with more obvious, safer and more reliable curative effect for intelligence damage caused by a cerebrovascular disease. The normal taking dosage of the Levetiracetam is 500 to 1500 mg / time. 2 to 3 times for one day, the Levetiracetam and auxiliary materials which can be accepted on the pharmacy can be produced into oral tablets, capsules and pelletized granules.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
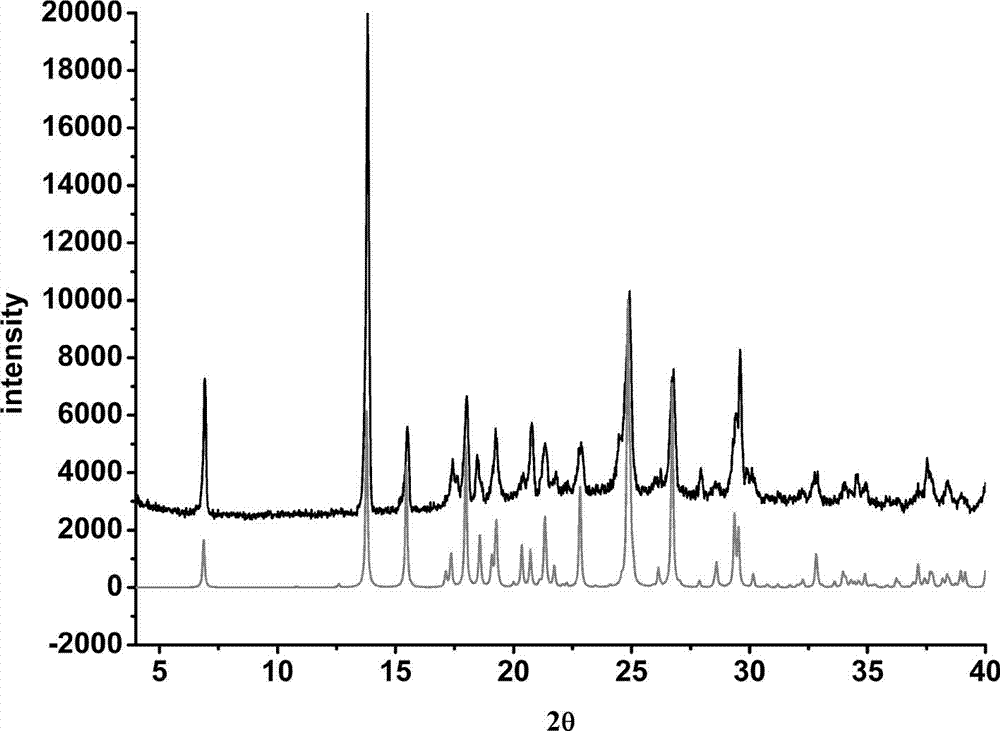
Febuxostat pharmaceutical co-crystal and preparation method thereof
The invention belongs to the technical field of a pharmaceutical co-crystal, and particularly relates to a novel febuxostat pharmaceutical co-crystal and a preparation method thereof. The space group of piracetam pharmaceutical co-crystal prepared by the invention is a monoclinic system, one isonicotinic acid molecule and two febuxostat molecules are combined together through a hydrogen bond respectively to form a basic structural unit of the febuxostat pharmaceutical co-crystal. A solvent selected in a preparation process of pharmaceutical co-crystal is ethyl acetate, and a solvent room temperature volatilization method is adopted. Due to a relatively low boiling point of the selected organic solvent, crystal is separated out during the process of solvent volatilization. The pharmaceutical co-crystal prepared by the invention inherits characteristics of traditional medicaments in preparing hyperuricemia of gout patients, and also has obvious change on solubility, stability and bioavailability.
Owner:吉林三善恩科技开发有限公司
Oral Chinese herbal preparation for treating post-traumatic brain syndrome
InactiveCN102631579BReduce dosageFast absorptionNervous disorderInanimate material medical ingredientsLicorice rootsTherapeutic effect
Owner:HOSPITAL NO 3 CPLA
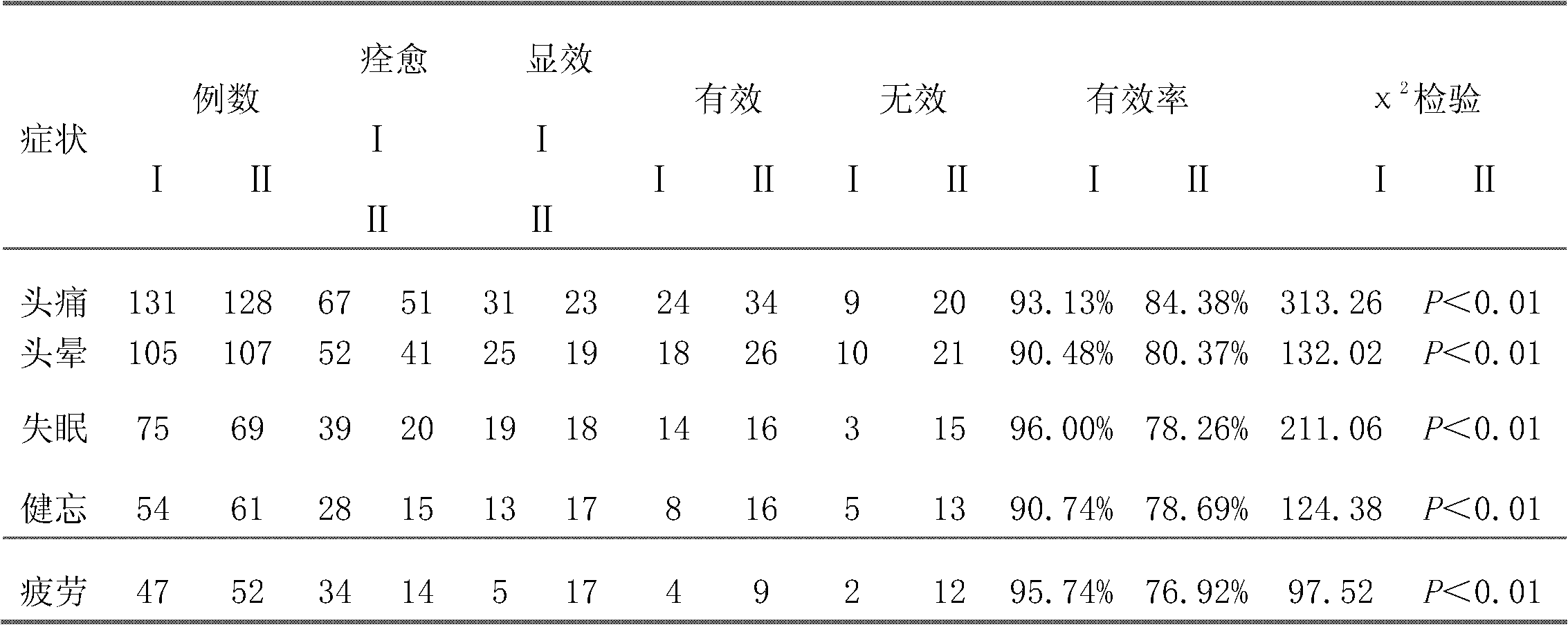
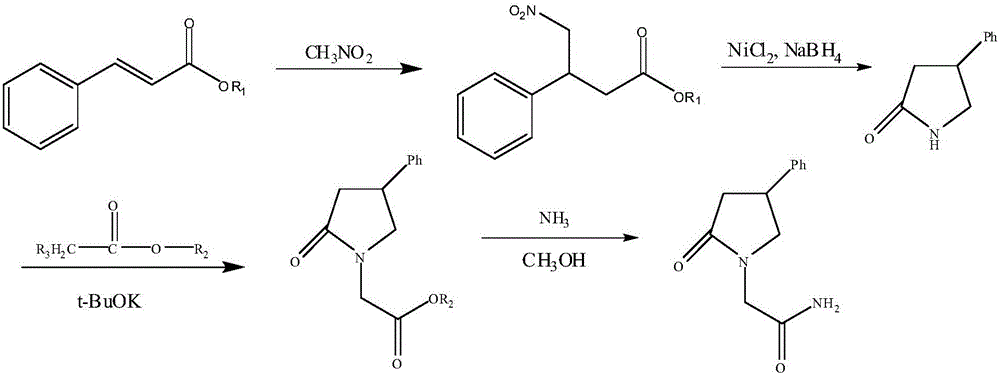
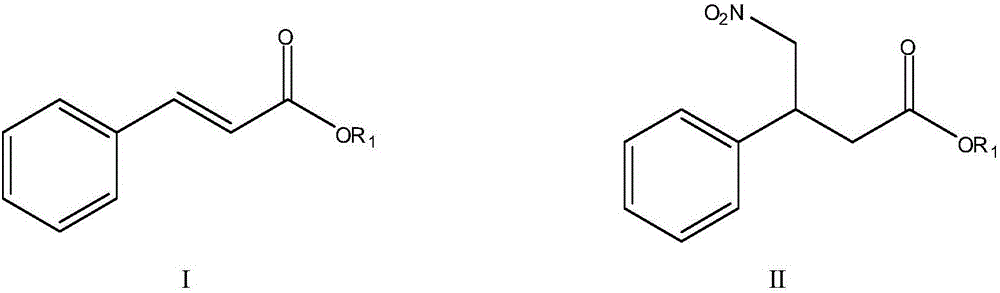
Method for preparing phenyl piracetam
The invention discloses a method for preparing phenyl piracetam, and belongs to the field of compound preparing. The method includes the following steps that alkali, cinnamic acid alkyl ester and nitromethane are subjected to an addition reaction to obtain 4-nitryl-3-phenylbutyric acid alkyl ester; after nitryl of the 4-nitryl-3-phenylbutyric acid alkyl ester is reduced with a reducing agent, the reduced nitryl and carbonyl are cyclized, and 4-phenyl-2-pyrrolidone is obtained; alkali, haloacetic acid alkyl ester and the 4-phenyl-2-pyrrolidone are subjected to an alkylation reaction to obtain 4-phenyl-2-pyrrolidone-1-acetic acid alkyl ester; the 4-phenyl-2-pyrrolidone-1-acetic acid alkyl ester and ammonia gas are reacted to obtain the phenyl piracetam. The method has the advantages of being short in reaction step, high in atom utilization, more environmentally friendly, safe in operation, high in reaction yield and purity, beneficial for achieving industrialization an the like.
Owner:NORTHEAST PHARMA GRP
Preparation method for cerebroprotein hydrolysate in piracetam cerebroprotein hydrolysate tablets
InactiveCN102188447AEasy to synthesizePromote repairNervous disorderPeptide/protein ingredientsHydrolysateTotal nitrogen
The invention provides a preparation method for cerebroprotein hydrolysate in piracetam cerebroprotein hydrolysate tablets. The cerebroprotein hydrolysate is extracted from brain tissues of mammals except for human being and contains specific polypeptide and various amino acids including free lysine and free glutamic acid, and molecular weight of all compositions is below 5,000-10,000 daltons. The weight proportion of polypeptide to free lysine to free glutamic acid in the cerebroprotein hydrolysate is (1-200):(0.1-100):(0.1-80). According to the invention, the pharmaceutical composition contains active substances capable of treating cranial vascular disease and cerebral metabolism disorder sequela caused thereby, the active substances can regulate and improve cerebral metabolism. After determination, the content of amino nitrogen in the cerebroprotein hydrolysate achieves 40-50 percent of total nitrogen content, so that the cerebroprotein hydrolysate can be used for preparing compound piracetam cerebroprotein hydrolysate tablets.
Owner:HONGMEI PHARMA CHINA
Traditional Chinese medical composition used for treatment of vascular dementia and preparation and application thereof
InactiveCN102579934AR&D safetyEffectively developedNervous disorderPill deliveryWater vaporFiltration
The invention relates to a traditional Chinese medical composition used for treatment of vascular dementia and a preparation and application thereof. The traditional Chinese medical composition is prepared with the following components by mass: 20-50 parts of polygonum multiflorum, 15-50 parts of alpinia oxyphylla, 15-50 parts of prepared polygala tenuifolia, 10-15 parts of prepared leech, 10-40 parts of ligusticum wallichii and 1-3 parts of borneol. The preparation method comprises: extracting medicinal materials respectively with water vapor and in a boiling method, using ethanol for precipitation after filtration of water extract, and decompressing and concentrating ethanol extract, spraying, drying, adding additives and carrying out dry granulation to prepare particles, capsules and tablets; and adding additives, adding water for dilution, and preparing orally taken solution, admixture and water aqua. 31 preliminary clinical tests show that the efficacy is obvious, the curing efficiency is 22.6 percent, the total efficiency is 80.6 percent and is significantly higher than 8 percent and 56 percent of piracetam control groups, and P is less than 0.05.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Use of levetiracetam in preparing intelligence-benefiting medicaments
InactiveCN101239059AExtended swim timeEasy to learnOrganic active ingredientsNervous disorderPharmacyDisease
The invention relates to a usage of levetiracetam in preparing a medicine helpful for intelligence. Levetiracetam is a good medicine in curing epilepsy at present. Due to the great similarity in terms of structure with piracetam which is helpful for intelligence, the invention aims to further discover the function of levetiracetam in improving the intelligence, and to provide a medicine which is safer and more remarkable and reliable in curing the intelligence damage caused by cerebrovascular diseases. The normal dose of levetiracetam is 500mg to 1500mg at one time, two times per day. Levetiracetam can be made into orally taken troches, capsules and grains, together with accessories medically acceptable.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Novel synthesis method of nootropic piracetam
InactiveCN104478779AMild reaction conditionsEasy to operateOrganic chemistrySodium methoxideChemical synthesis
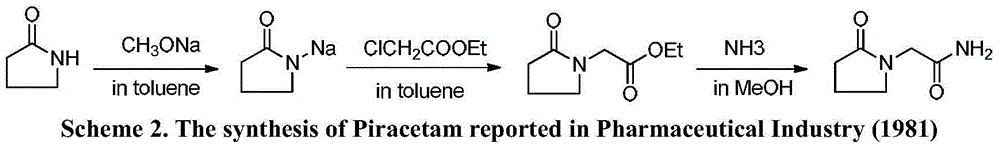
The invention belongs to the field of chemical synthesis and relates to a synthesis method of piracetam. The method comprises the following steps: by taking alpha-pyrrolidone as raw material, slowly dropwise adding a methanol solution (28.4% w / w) of sodium methoxide at reduced pressure; distilling the methanol solvent at reduced pressure and normal pressure; after methanol is evaporated completely, slowly dropwise adding a methylbenzene solution of methyl chloroacetate, controlling the reaction temperature to be 20 to 110 DEG C and reacting for 5.0 hours under heat-preserved condition; cooling to room temperature; carrying out suction filtering; distilling the filtrate at reduced pressure; collecting fractions with the temperature of 100 to 105 DEG C; mixing the fractions with ammonia gas / a methanol solution; reacting at the temperature of 50 to 70 DEG C for 3-18 hours; thermally filtering to obtain a primary crude product; concentrating the filtrate at reduced pressure to obtain a secondary crude product; mixing the crude products; recrystallizing by using an alcohol solvent; filtering and drying to obtain white crystals to obtain piracetam, wherein the mole ratio of alpha-pyrrolidone to methyl chloroacetate is 1:(1.0-2.0). The method is mild in reaction condition, simple and convenient in production and operation; synthesis raw materials and solvents are cheap and easily available, and therefore the method is suitable for large-scale industrial production; after the recrystallization, the yield is as high as 82.4% (metered based on the alpha-pyrrolidone), and the purity is higher than 99.9%.
Owner:SHENYANG PHARMA UNIVERSITY
Effervescent tablet containing piracetam
InactiveCN102018683AEasy to storeImprove stabilityOrganic active ingredientsNervous disorderEffervescent tabletSodium bicarbonate
The invention relates to an effervescent tablet containing piracetam, which comprises piracetam, citric acids, sodium bicarbonates, oligosaccharides or other edible sugars, and the weight ratio of the piracetam to the citric acid to the sodium bicarbonate to the oligosaccharide or other edible sugars is (10 to 20): 30: 7: 200. The effervescent tablet in the invention has the advantages that the piracetam used as a functional composition is prepared into the effervescent tablet, therefore, compared with other medicament forms, the piracetam-containing effervescent tablet has the advantages of good stability, easy absorption, good taste, and capability of being directly soaked in water for drinking without mixing, and is easy to keep and dissolve.
Owner:张家港市华菱化工机械有限公司
Chinese-Western compound preparation for treating senile dementia and preparation method thereof
InactiveCN108904739AImprove circulationImprove insomnia and forgetfulnessNervous disorderCapsule deliveryAmnesiaSide effect
The invention discloses a Chinese-Western compound preparation for treating senile dementia and a preparation method thereof. The Chinese-Western compound preparation is formed by compounding traditional Chinese medicine components and Western medicine components. The traditional Chinese medicine components are prepared from the following raw materials in parts by weight: 10-20 parts of rhizoma gastrodiae, 10-15 parts of radix salviae miltiorrhizae, 10-15 parts of folium ginkgo, 5-10 parts of cortex albiziae, 5-10 parts of fructus alpiniae oxyphyllae, 2-5 parts of dark plum, and 5-10 parts oflumbricus; the western medicines are composed of the following components: 1-1.5 parts of piracetam, 1-1.5 parts of donepezil hydrochloride and 0.5-1 part of vitamin B1. The method of combining Chinese and Western medicines can effectively improve the circulation functions of brain nerves, brain blood vessels and brain blood circulation, improves and treats insomnia, amnesia, hypomnesia and cognitive decline caused by senile dementia, has the advantages of short course of treatment, quick effect and low side effects, and has broad medical application prospects.
Owner:SHAANXI UNIV OF CHINESE MEDICINE
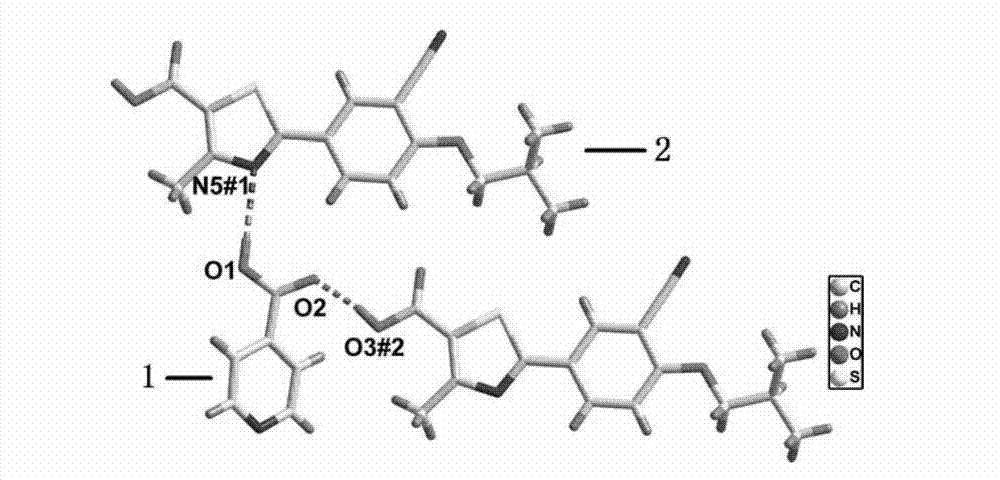
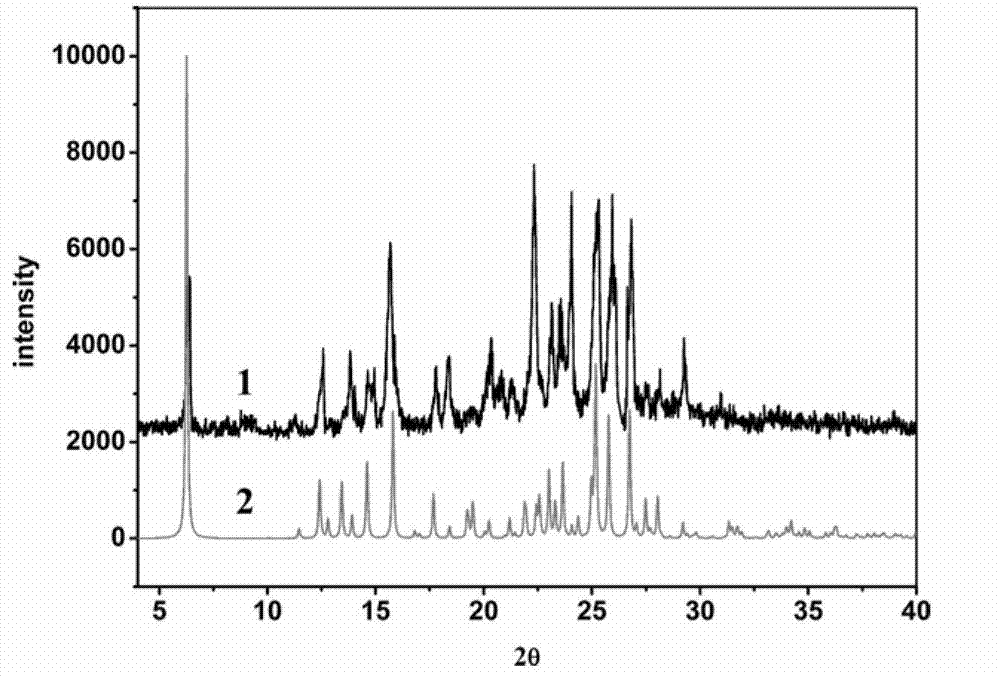
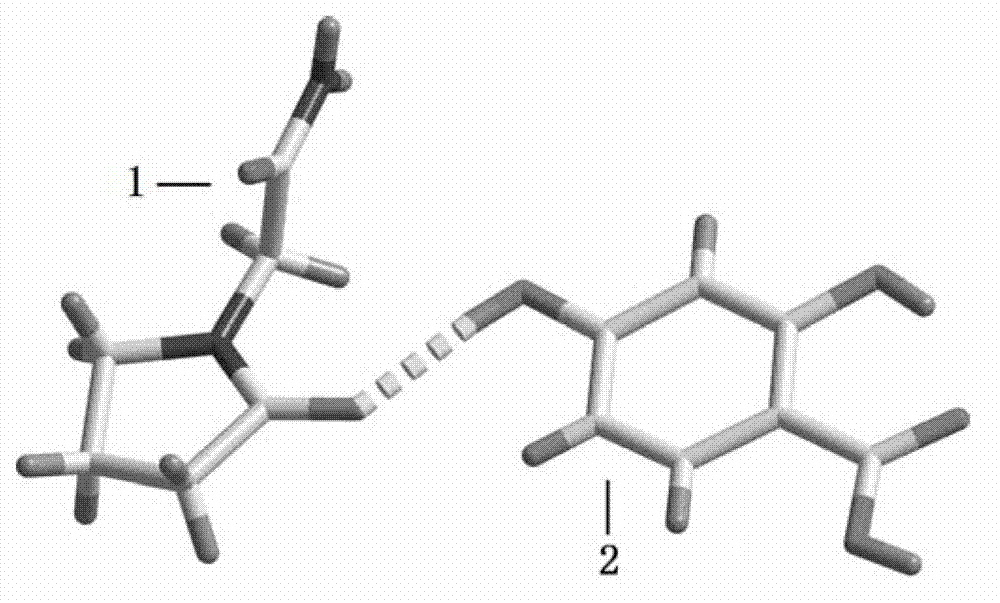
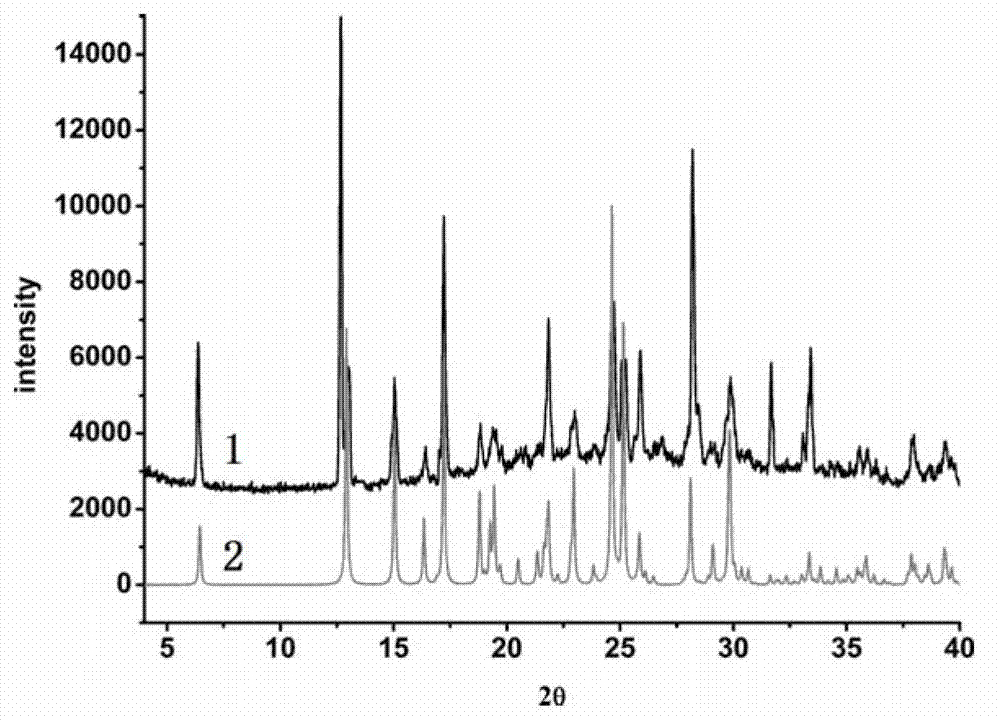
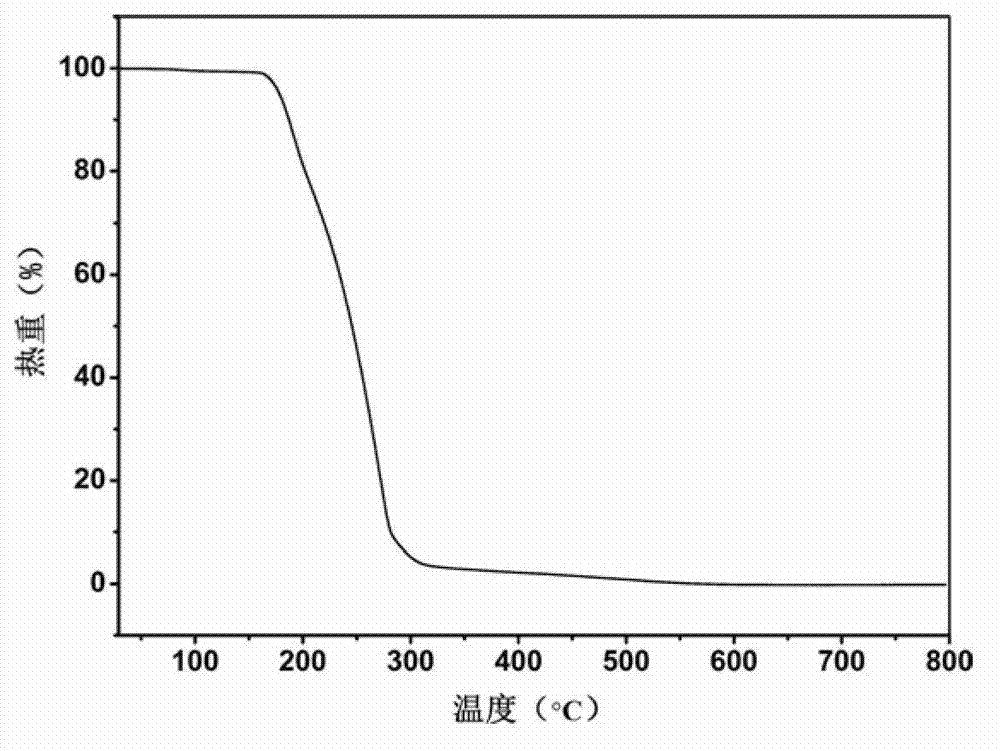
Piracetam pharmaceutical co-crystal using 2,4-dihydroxy-benzoic acid as precursor and preparation method of co-crystal
The invention belongs to the technical field of pharmaceutical co-crystals and in particular relates to a piracetam pharmaceutical co-crystal and a preparation method thereof. The space group of the prepared piracetam pharmaceutical co-crystal is a monoclinic system; one piracetam moleculre (1) and one 2,4-dihydroxy-benzoic acid molecule (2) are combined together by a hydrogen bond to form a basic structure unit of the piracetam pharmaceutical co-crystal, wherein the O atom on the hydroxyl group of the 2,4-dihydroxy-benzoic acid molecule is uses as a hydrogen bond donator, and the O atom on the hydroxyl group of the piracetam molecule is used a hydrogen bond acceptor. The solvent selected in the preparation process of the pharmaceutical co-crystal is methanol; and the adopted method is a solvent room-temperature evaporation method. The boiling point of the selected organic solvent is relatively low, and so crystals are separated out in the evaporation process of the solvent. According to the pharmaceutical co-crystal prepared by the preparation method disclosed by the invention, the characteristics of the traditional material medicines for restoring nerve cells are inherited for treating a patient with brain damages; and the solubility, stability and bioavailability of the pharmaceutical co-crystal are remarkably improved.
Owner:吉林三善恩科技开发有限公司
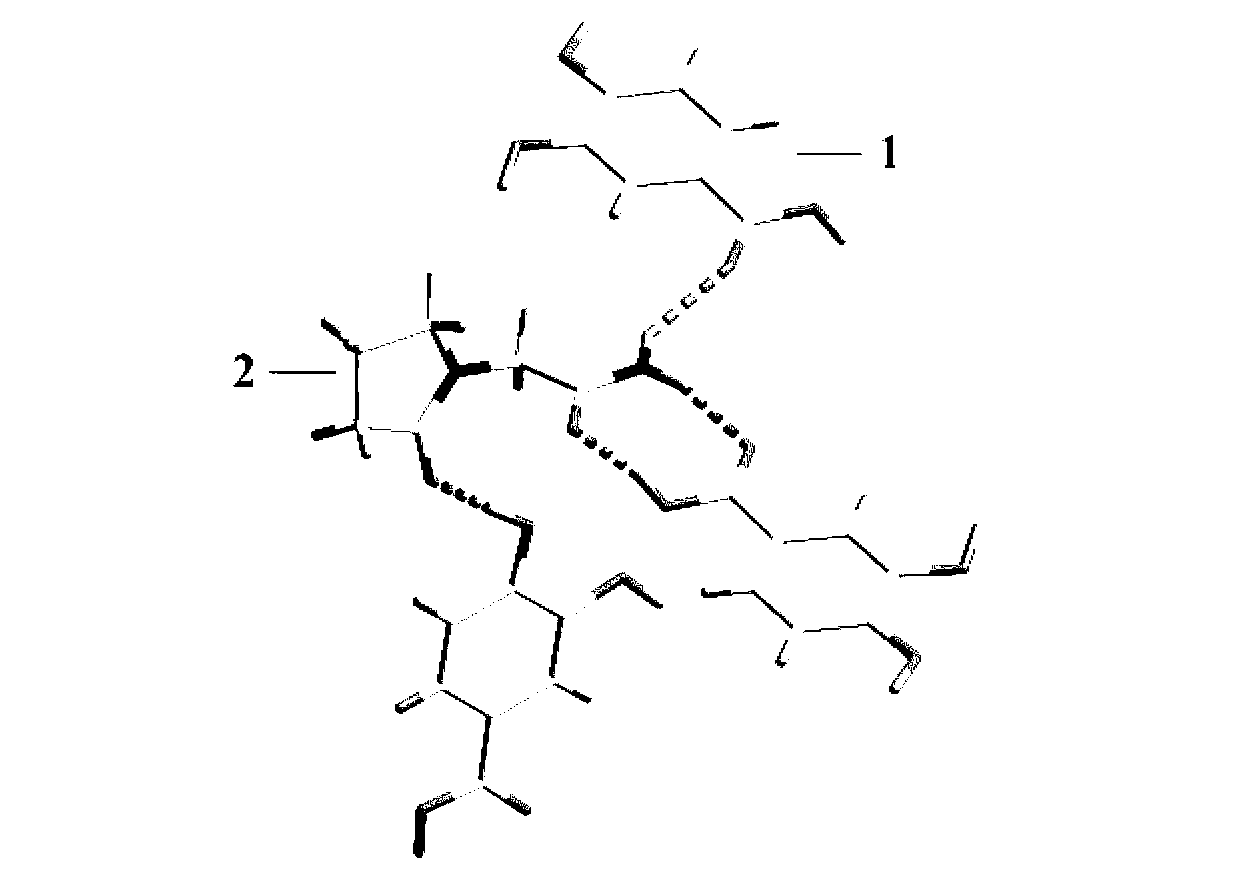
Piracetam pharmaceutical co-crystal taking 3,4-dihydroxy-benzoic acid as precursor and preparation method of piracetam pharmaceutical co-crystal
The invention belongs to the technical field of pharmaceutical co-crystals, and particularly relates to a piracetam pharmaceutical co-crystal taking 3,4-dihydroxy-benzoic acid as a precursor and a preparation method of the piracetam pharmaceutical co-crystal. A space group of the pharmaceutical co-crystal is a monoclinic system, and three 3,4-dihydroxy-benzoic acid molecules (1) and a piracetam molecule (2) are combined through hydrogen bonds to form a basic structural unit of the piracetam pharmaceutical co-crystal. In a pharmaceutical co-crystal preparation process, a selected solvent is methanol; an adopted method is a solvent room-temperature volatilization method; and as a boiling point of the selected organic solvent is lower, a crystal is precipitated in a solvent volatilization process. The prepared pharmaceutical co-crystal inherits the characteristic of the traditional bulk pharmaceutical for repairing nerve cells of a brain function injured patient, and the dissolvability, the stability and the bioavailability of the pharmaceutical co-crystal are improved obviously.
Owner:吉林三善恩科技开发有限公司
Novel piracetam drug co-crystal and preparation method thereof
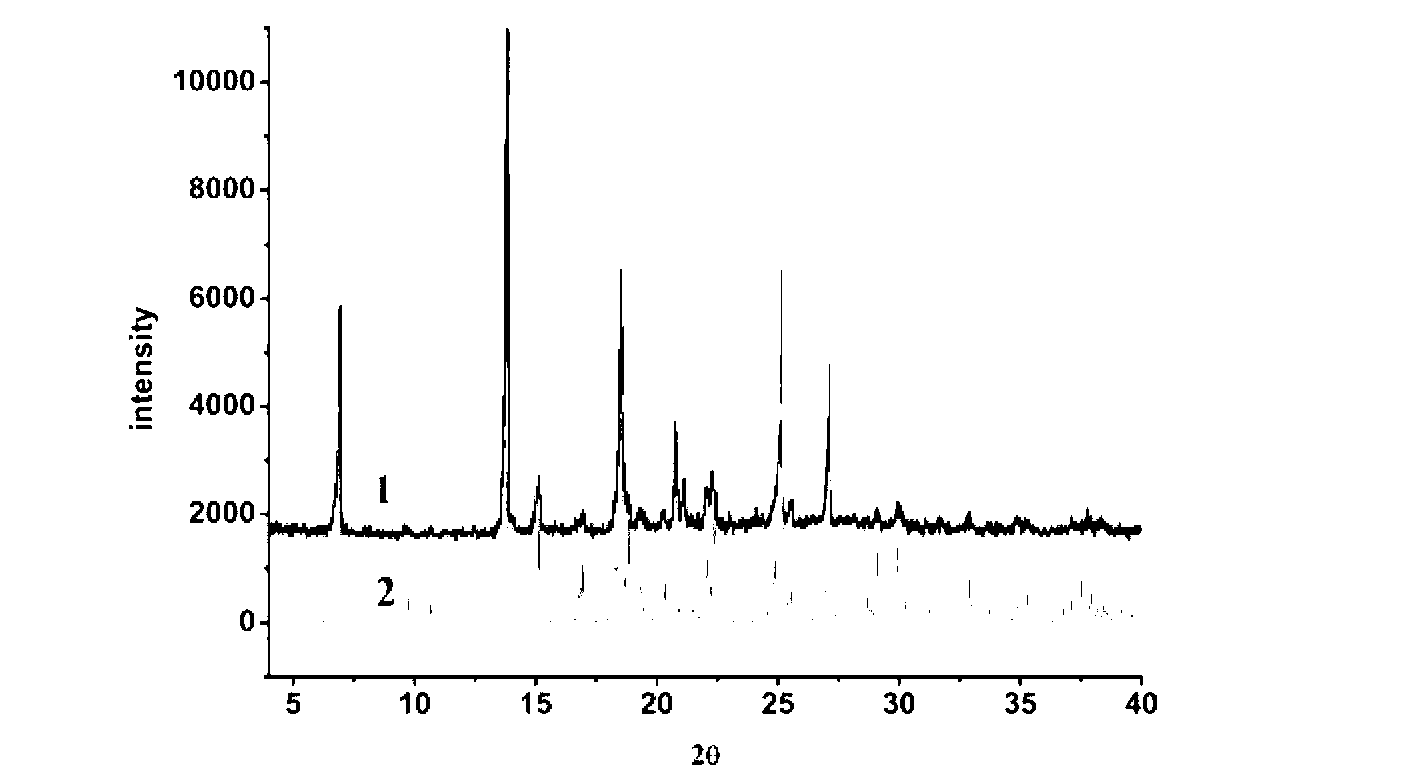
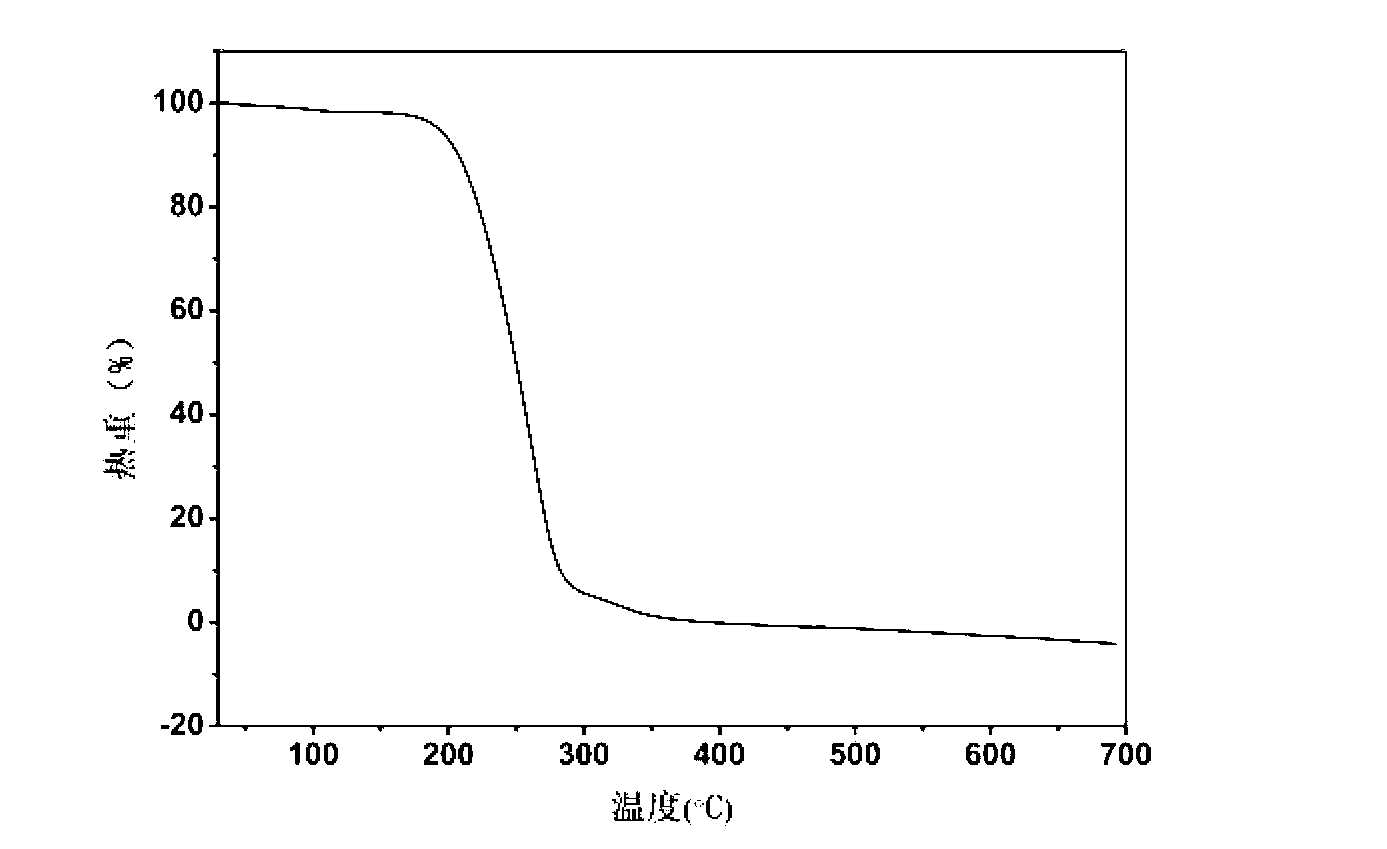
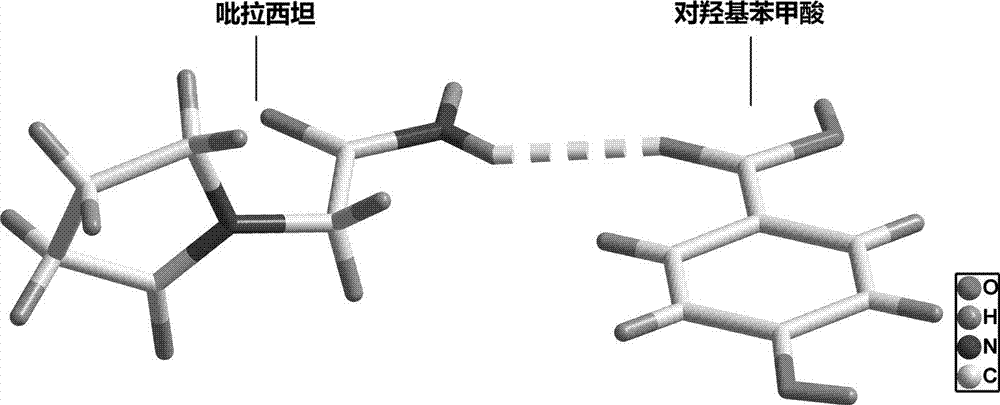
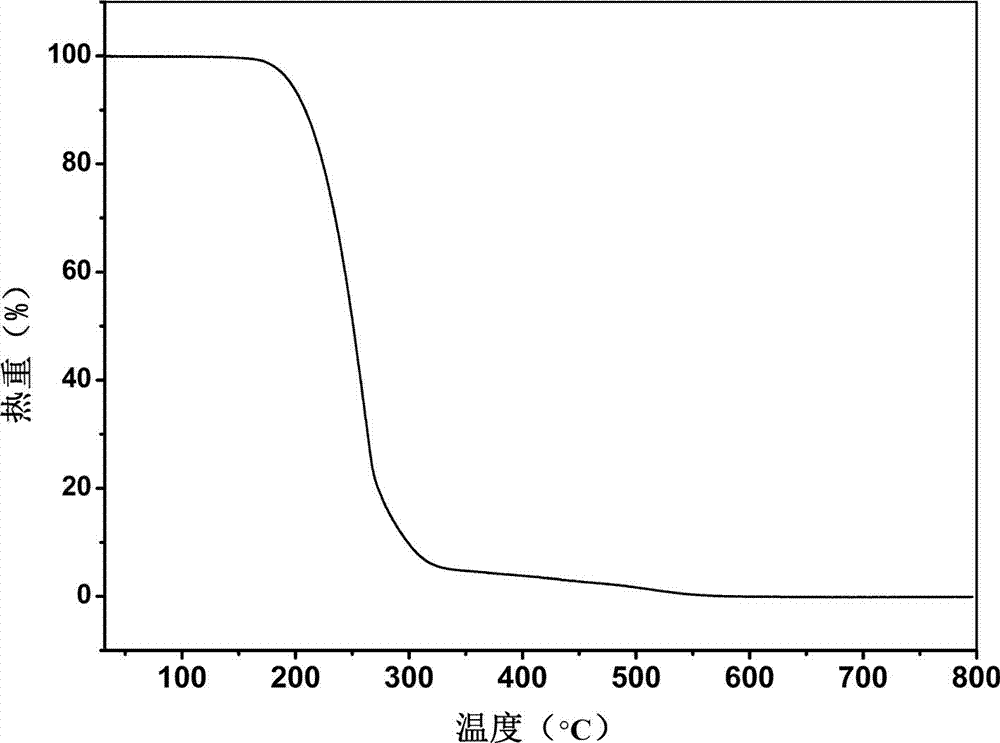
The invention belongs to the technical field of drug co-crystals and in particular relates to a novel piracetam drug co-crystal and a preparation method thereof. An N atom on an amino group in one piracetam molecule is taken as a hydrogen bond donor, an O atom on a p-hydroxybenzoic acid carboxyl is taken as a hydrogen bond acceptor, and the N atom and the O atom form a basic structural unit of the piracetam co-crystal by forming hydrogen bonds. The preparation method of the piracetam drug co-crystal is a solvent room temperature volatilization method. The drug co-crystal prepared by the invention inherits the characteristic of the traditional bulk drug of treating cerebral injury and cerebrovascular diseases, and is obviously improved in the aspects of dissolubility, stability and bioavailability.
Owner:JILIN INST OF CHEM TECH
Cerebroprotein hydrolysate in piracetam and cerebroprotein hydrolysate tablets and preparation method of cerebroprotein hydrolysate
ActiveCN103656607AEasy to operateSuitable for large-scale industrial productionNervous disorderHydrolysed protein ingredientsHydrolysatePepsin
The invention belongs to the field of biological agents, and relates to cerebroprotein hydrolysate in piracetam and cerebroprotein hydrolysate tablets and a preparation method of the cerebroprotein hydrolysate, in particular to cerebroprotein hydrolysate which is extracted from brain tissues of mammals excluding human and contains polypeptide and multiple amino acids including free amino acids and free glutamic acid, as well as a preparation method of the cerebroprotein hydrolysate. The provided method comprises steps such as homogenate, two times of degreasing of acetone, enzymolysis of trypsin and pepsin and the like. The content of amino acids in the cerebroprotein hydrolysate prepared with the method is remarkably higher than that in the prior art.
Owner:HONGMEI PHARMA CHINA
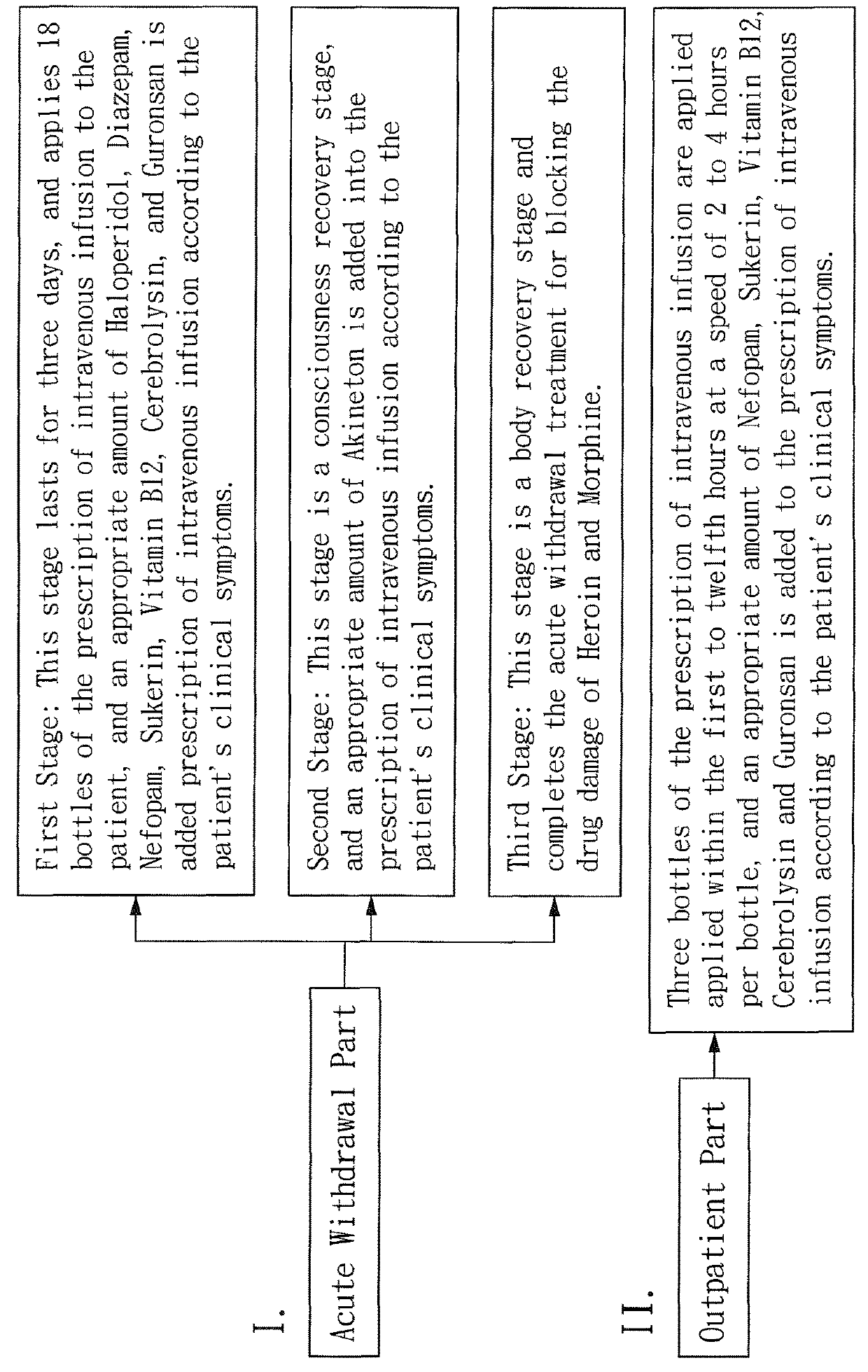
Prescription of intravenous medication for blocking heroin or morphine intoxication path and using thereof
ActiveUS10004781B2Enhancing withdrawalPrevent addictionNervous disorderPeptide/protein ingredientsMorphine poisoningBlock drugs
Owner:CHENG EN CHE
Prescription of Intravenous Medication for Blocking Heroin or Morphine Intoxication Path and Using Thereof
ActiveUS20170157209A1Enhancing withdrawalReduce usageNervous disorderPeptide/protein ingredientsMorphine poisoningBlock drugs
Disclosed are a prescription of intravenous medication for blocking a heroin or morphine intoxication path and its using. The prescription of intravenous infusion includes Cerebrolysin, Piracetam, Cimetidine, Scopolamine Butylbromide, Nefopam, B.C Complex, Vitamin B1, Ascorbic Acid, Ketorolac, Guronsan, Hyoscine Butylbromide and Sukerin. The prescription activates a patient's liver and circulatory system to block drug intoxication. The using for blocking drugs includes an acute withdrawal part, wherein the prescription of intravenous infusion is injected and the timing of drug administration and the safe dosage are controlled according to clinical symptoms, and an appropriate amount of a supplementary medicine such as Haloperidol is added, and the processes of detoxication, relieving symptoms, suppressing restlessness, and sobering are conducted to block acute withdrawal symptoms of an acute withdrawal addict quickly and successfully.
Owner:CHENG EN CHE
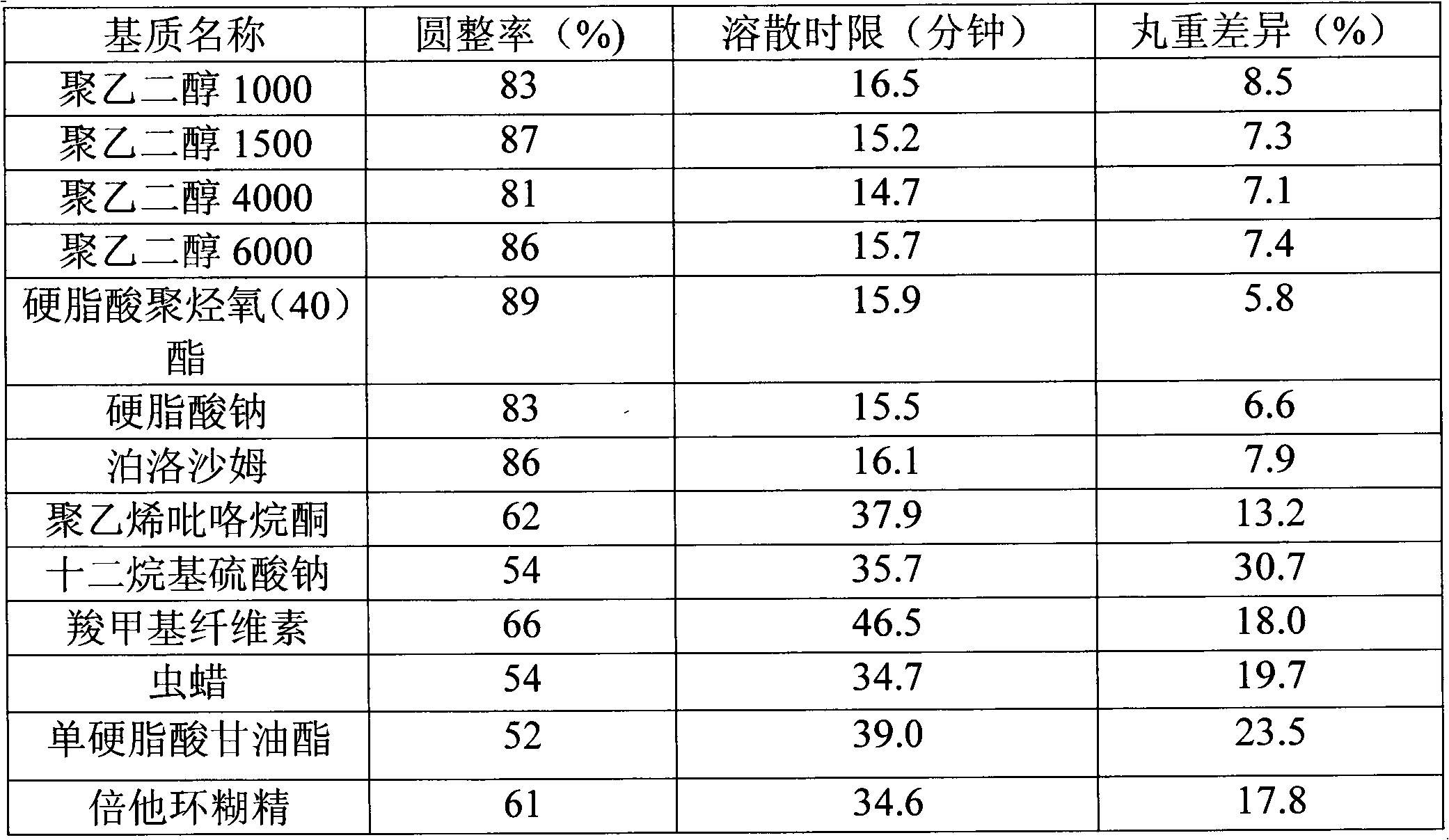
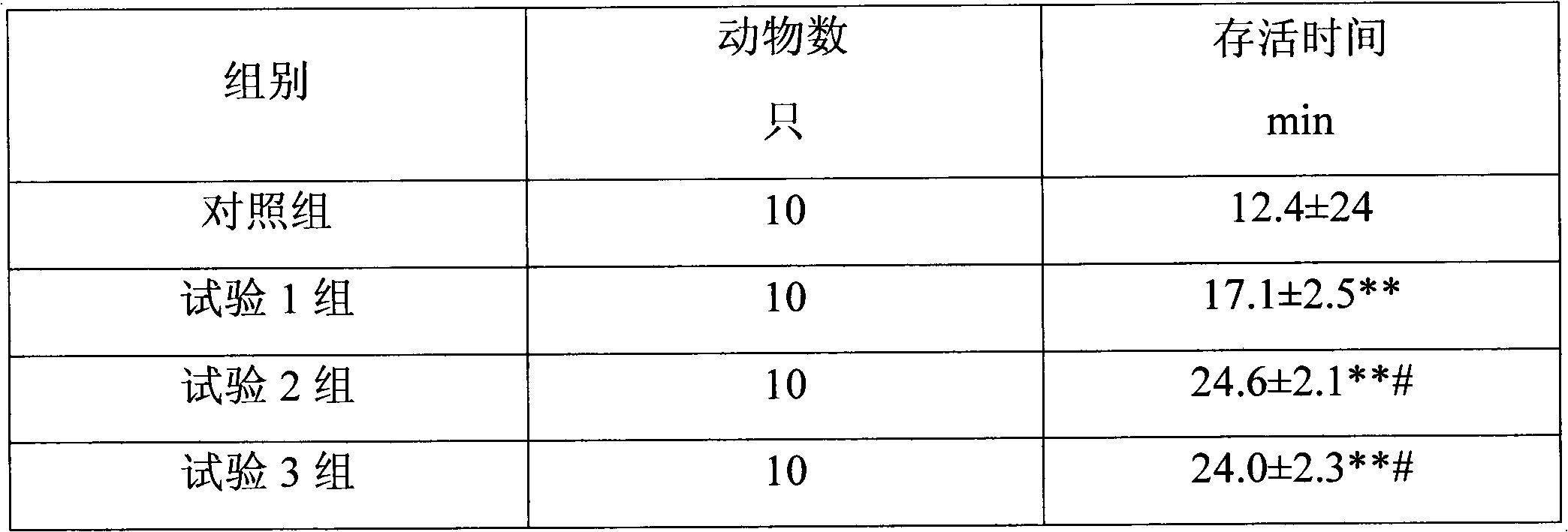
Piracetam cerebroprotein hydrolysate dropping pill and preparation method thereof
The invention belongs to the technical field of medicines and discloses a piracetam cerebroprotein hydrolysate dropping pill and a preparation method thereof. The dropping pill comprises active ingredients and a substrate, wherein the active ingredients are composed of piracetam, cerebroprotein hydrolysate, and the like. The prescription of the dropping pill provided by the invention is confirmed according to a scientific screening test. A pharmacologic test proves that the dropping pill provided by the invention has better pharmacologic effect than that of the marketed preparation taking compound piracetam and cerebroprotein hydrolysate as raw materials.
Owner:张睿
Compound preparation of piracetam and its use
InactiveCN1679611AImprove functional statusShort reaction timeOrganic active ingredientsNervous disorderDiseaseHigh energy
A high-energy composite medicine for treating acute or chronic cerebrovascular disease, cerebral trauma, toxipathic cerebrosis, hypomneis and cerebral disfunction contains proportionally inosine, ATP, VC and piracetam.
Owner:FUKANGREN BIO PHARMA
Eye drop for treatment of nearsightedness and preparation method of eye drop
InactiveCN109908327AOvercome prone side effectsOvercome relapse-prone defectsSenses disorderAnthropod material medical ingredientsVitamin CSide effect
The invention belongs to the field of medication and particularly relates to an eye drop for treatment of nearsightedness and a preparation method of the eye drop. The eye drop comprises, by weight, 5-15 parts of silkworm larva, 5-15 parts of caulis dendrobii powder, 70-79 parts of fructus ligustri lucidi, 90-100 parts of semen cassiae, 50-80 parts of Vitamin B1, 50-70 parts of Vitamin C, 5-15 parts of borneol, 5-15 parts of mouse nerve growth factors, 50-80 parts of Vitamin B12, 5-15 parts of sodium chloride and 60-80 parts of piracetam. By the arrangement, problems that drug therapy is proneto side effects, defects prone to recurrence, risks and sequelae of surgical treatment and wearing optical glasses can only overcome the shortcoming of daily vision of patients with visual impairmentbut not the root cause are solved, and the eye drop adopts natural raw materials is developed into a safe, efficient and high-quality product with reasonable compatibility and synergistic effect, remarkable effect in the treatment of nearsightedness and safety and efficiency.
Owner:陈锦济生物医学技术(广州)有限公司
Method for treating apathy syndrome
InactiveUS20060241144A1Improve frontalImprove executive cognitive functionBiocideNervous disorderChronic hepatitisRisk stroke
The present invention provides a method of treating apathy syndrome in a human subject. The human subject is first evaluated to determine whether one or more behavioral characteristics of apathy are observed. If such characteristics are observed, the subject is treated with a 2-oxopyrrolidine compound, such as nefiracetam, piracetam, aniracetam, pramiracetam, nebracetam, fasoracetam, levetiracetam, or oxiracetam, in an amount effective to produce an improvement in such apathy characteristics. The present invention is useful in treating apathy in a subject suffering from conditions associated with or characterized by frontal-subcortical dysfunction. The present invention is also useful in treating apathy in a subject suffering from a stroke, Alzheimer's disease, Parkinson's disease, traumatic brain injury, depression, schizophrenia, chronic hepatitis C infection, or HIV infection.
Owner:HAMILTON PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com