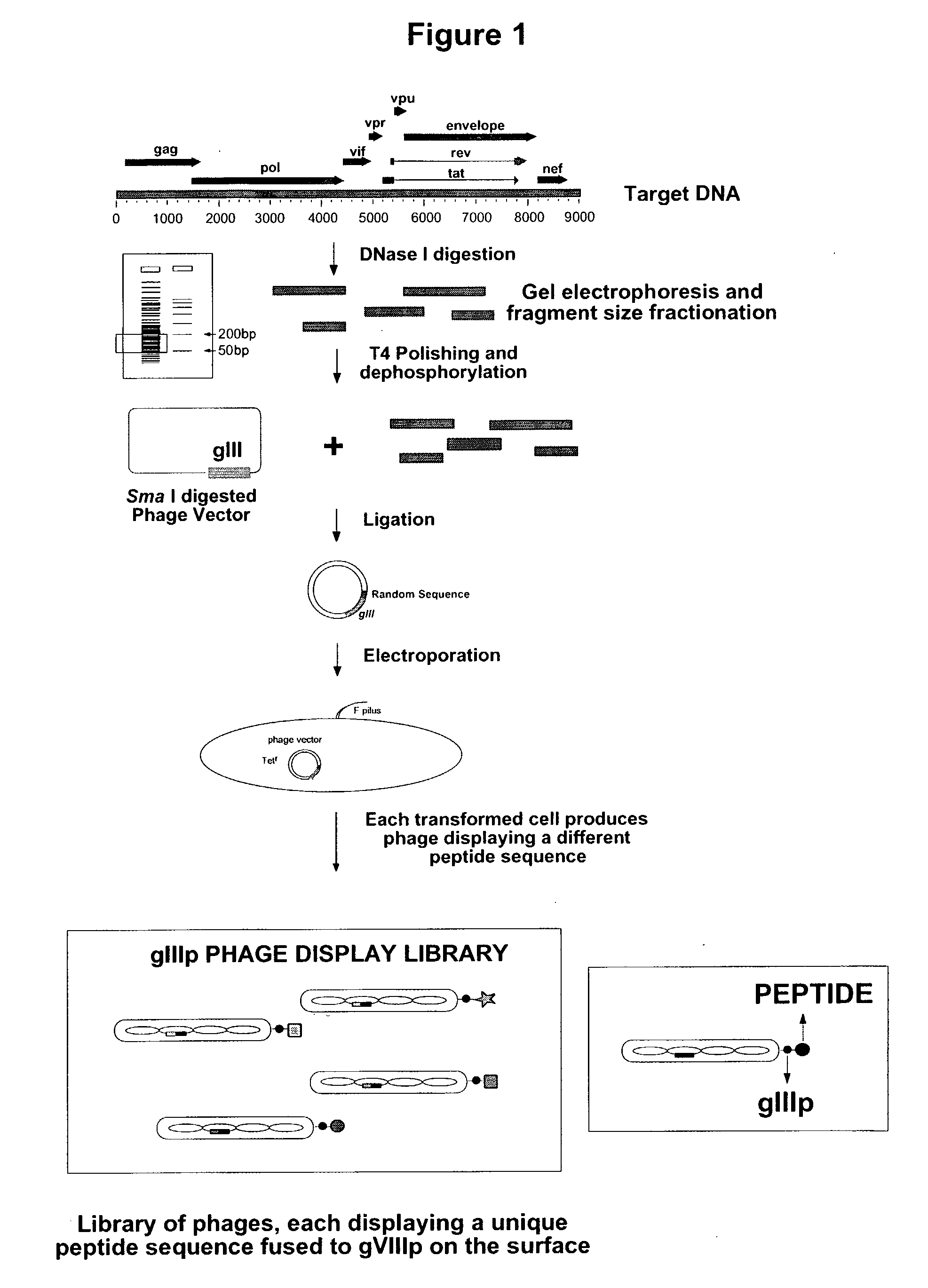
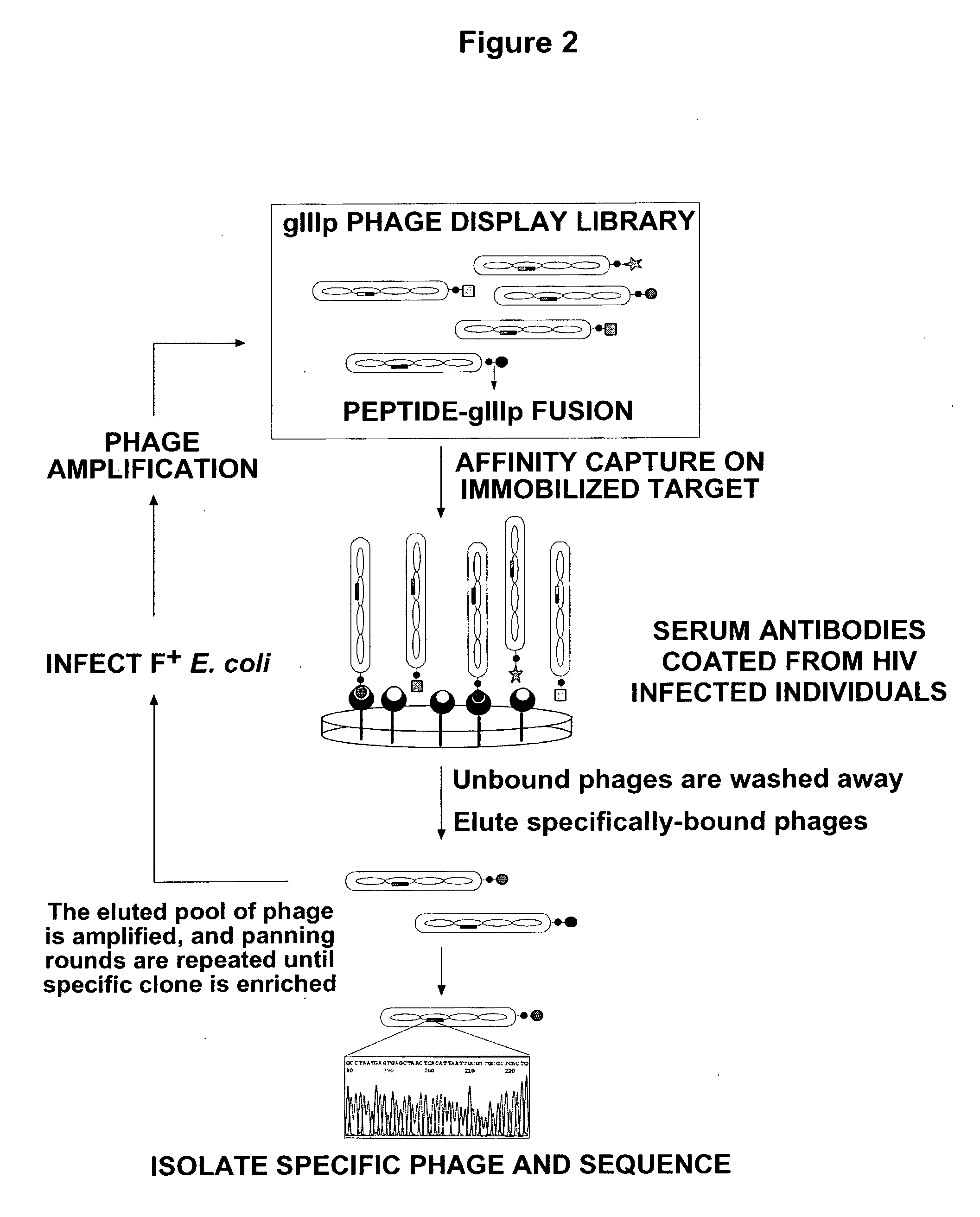
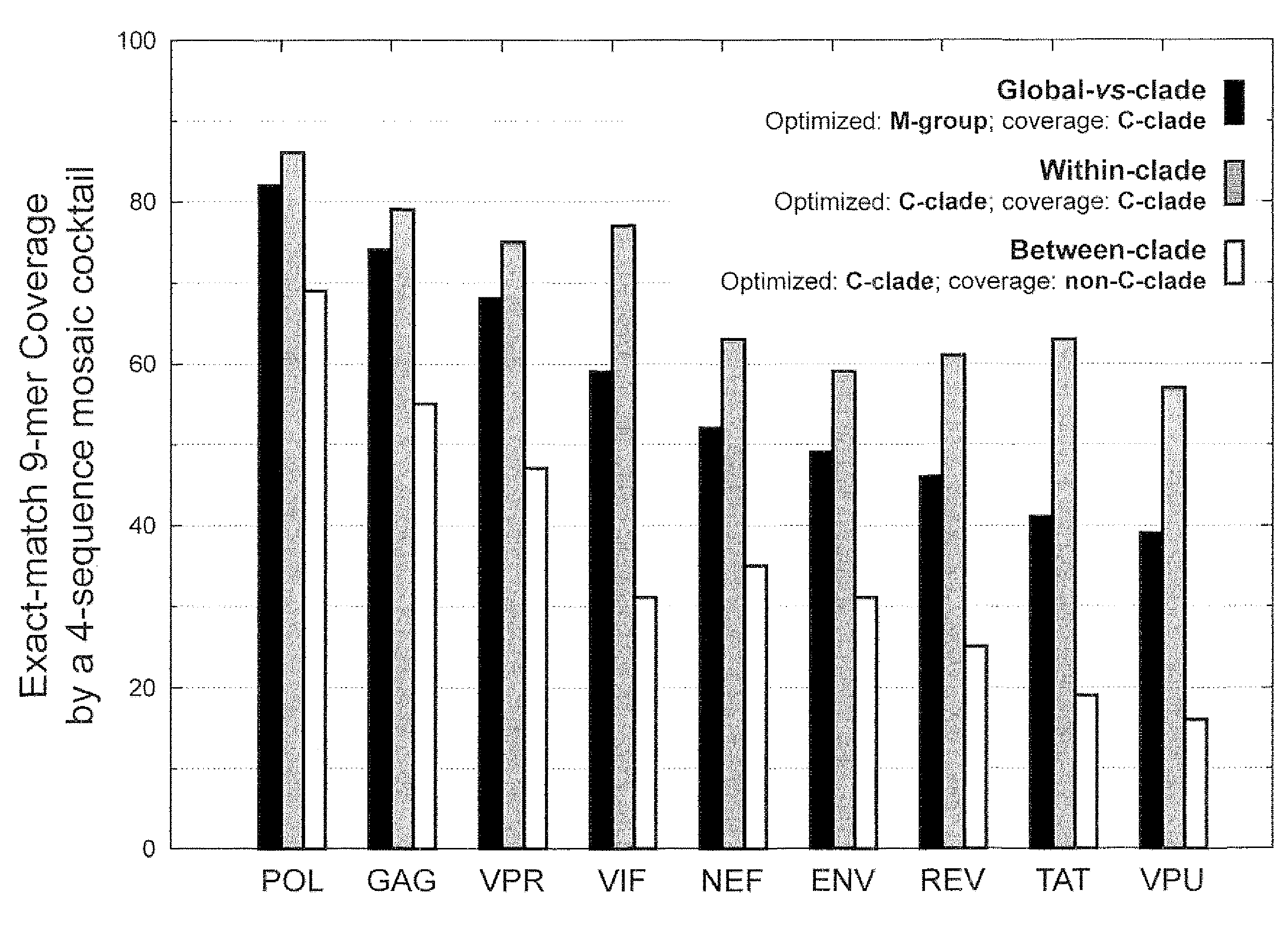
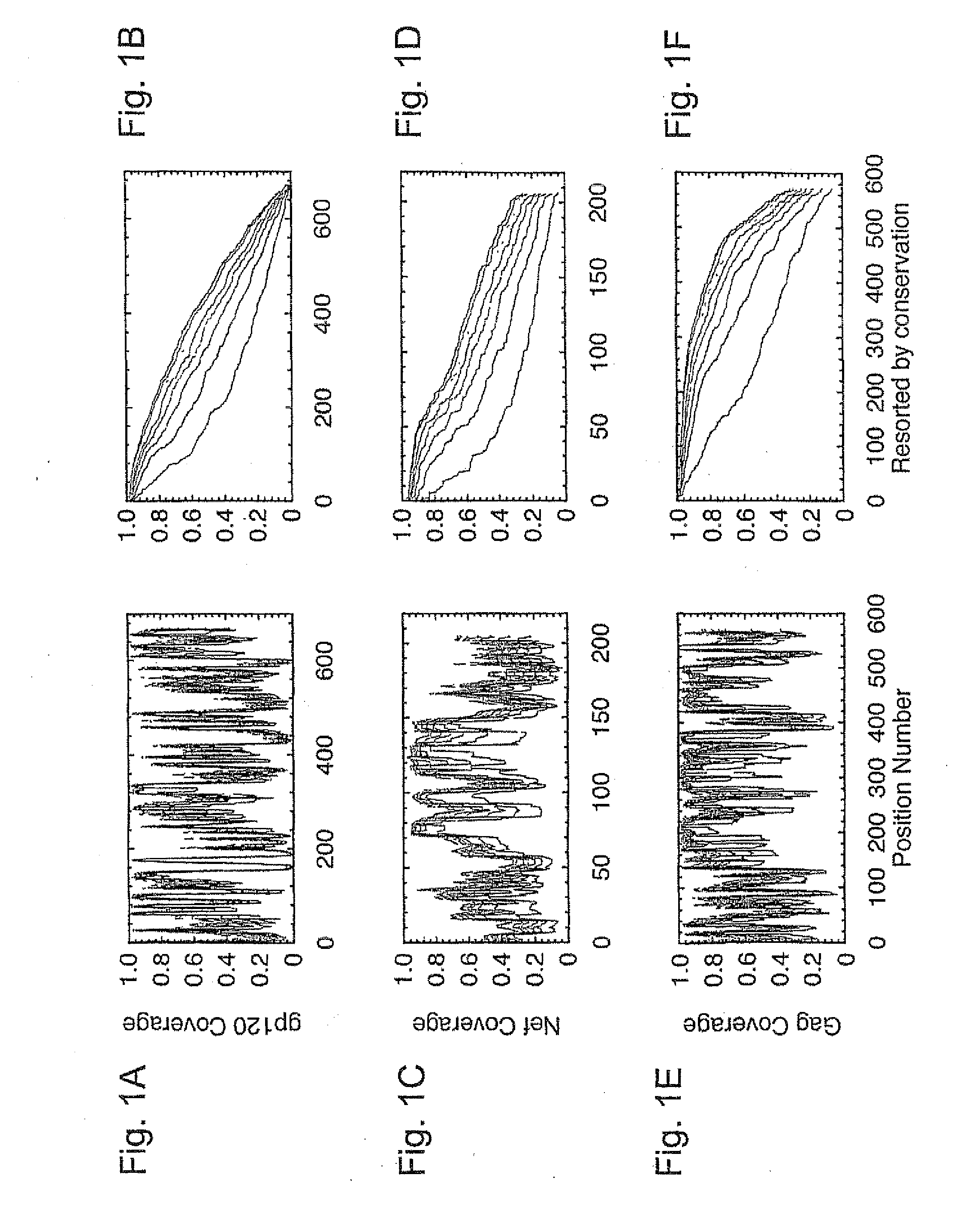
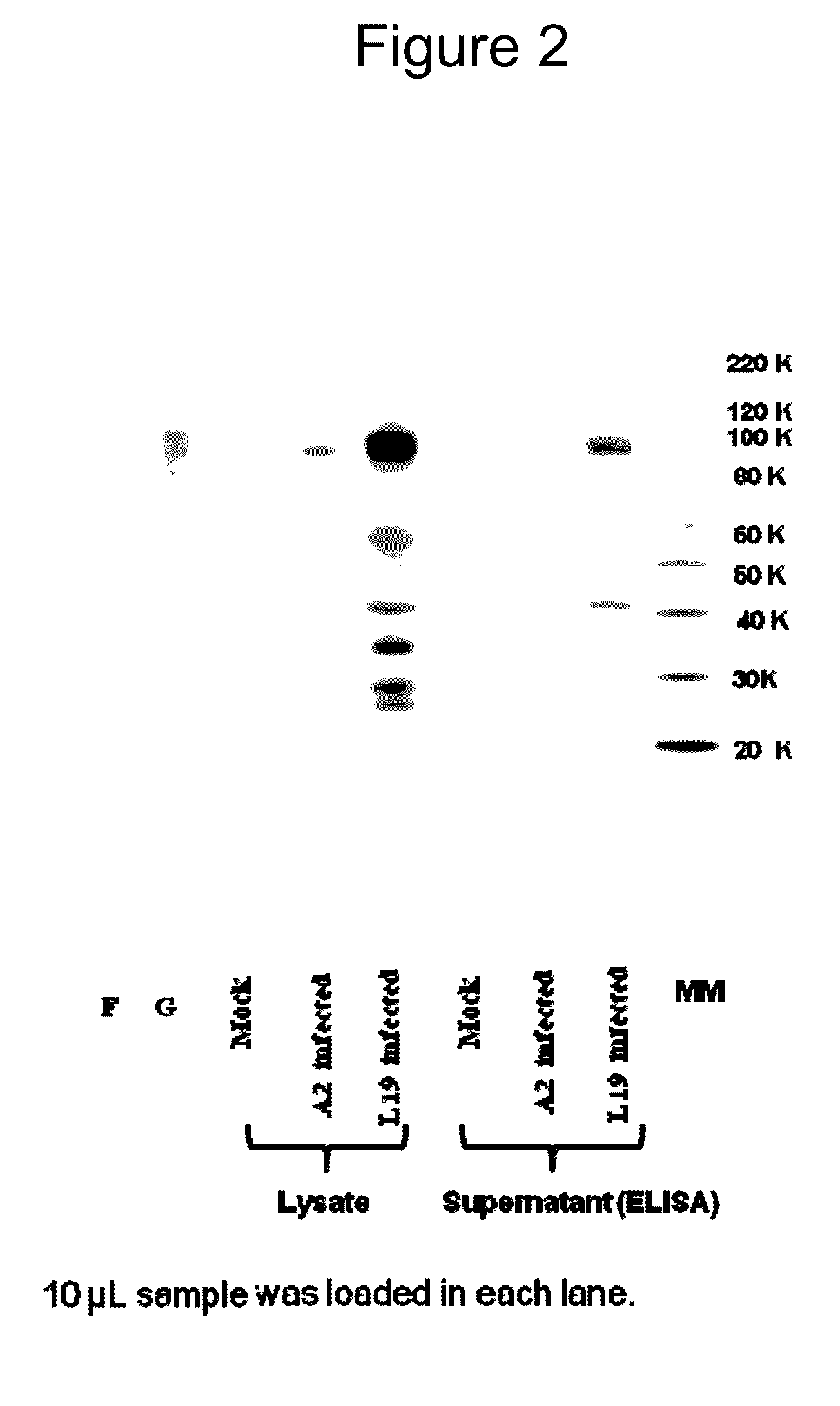
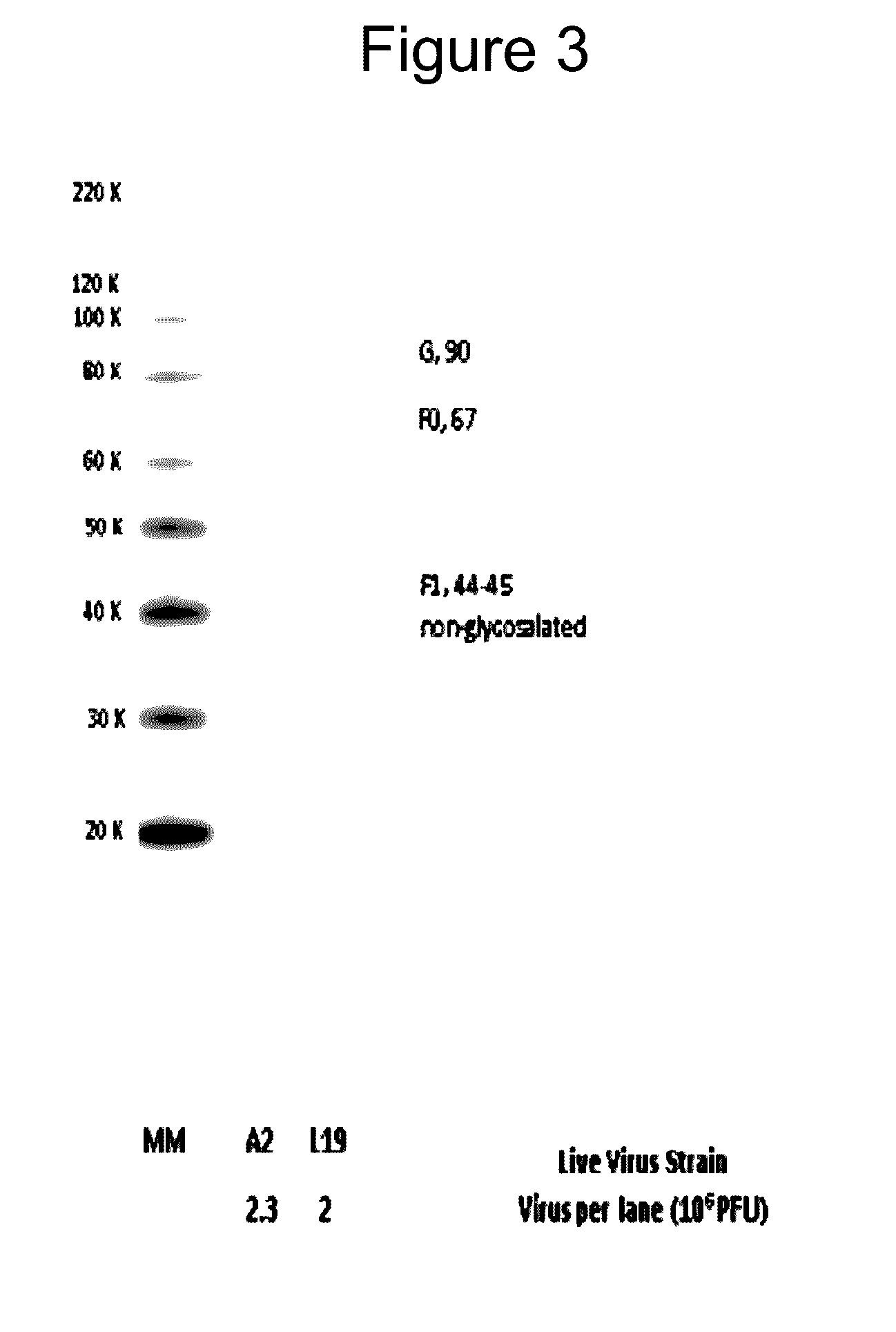
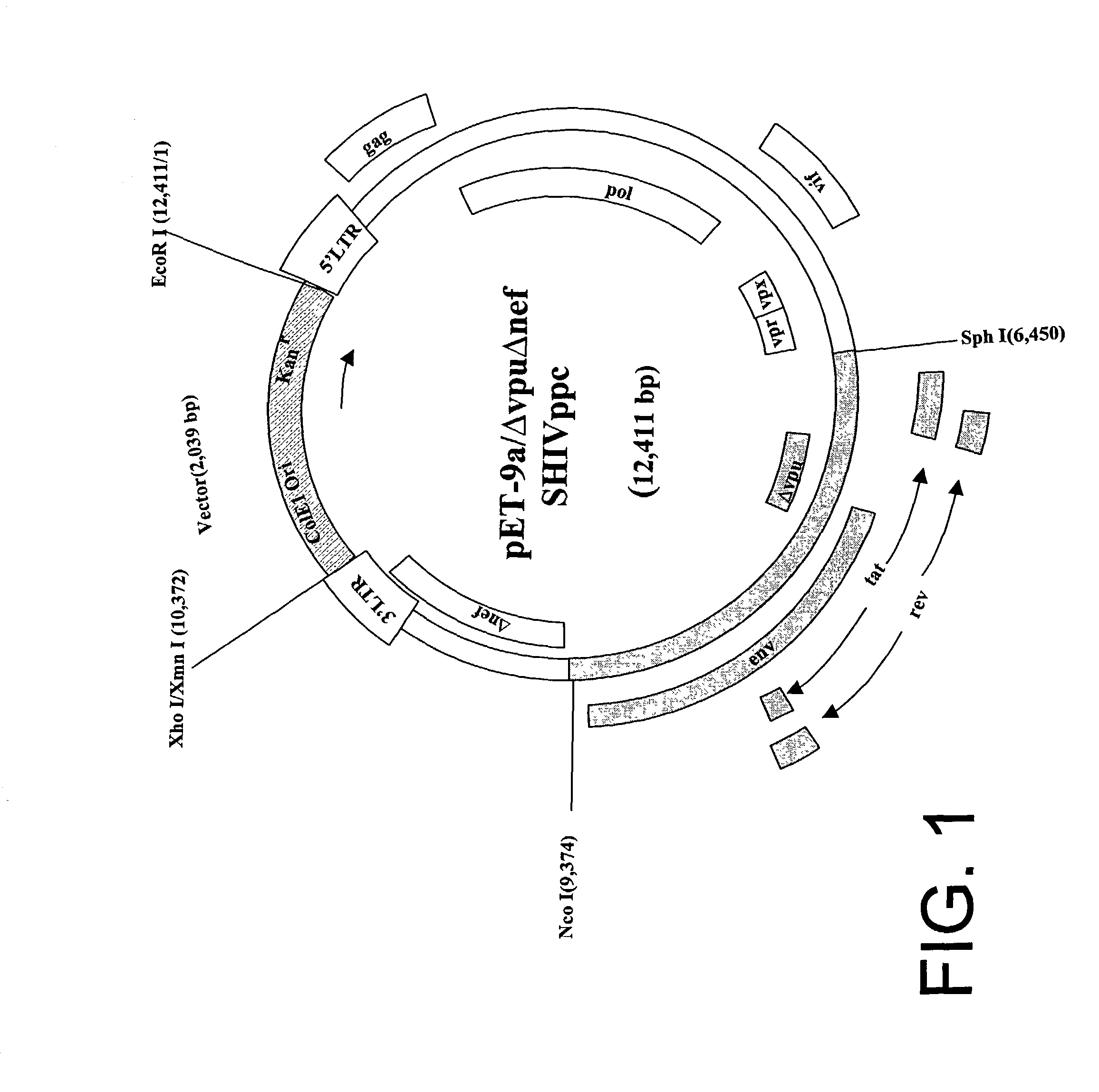
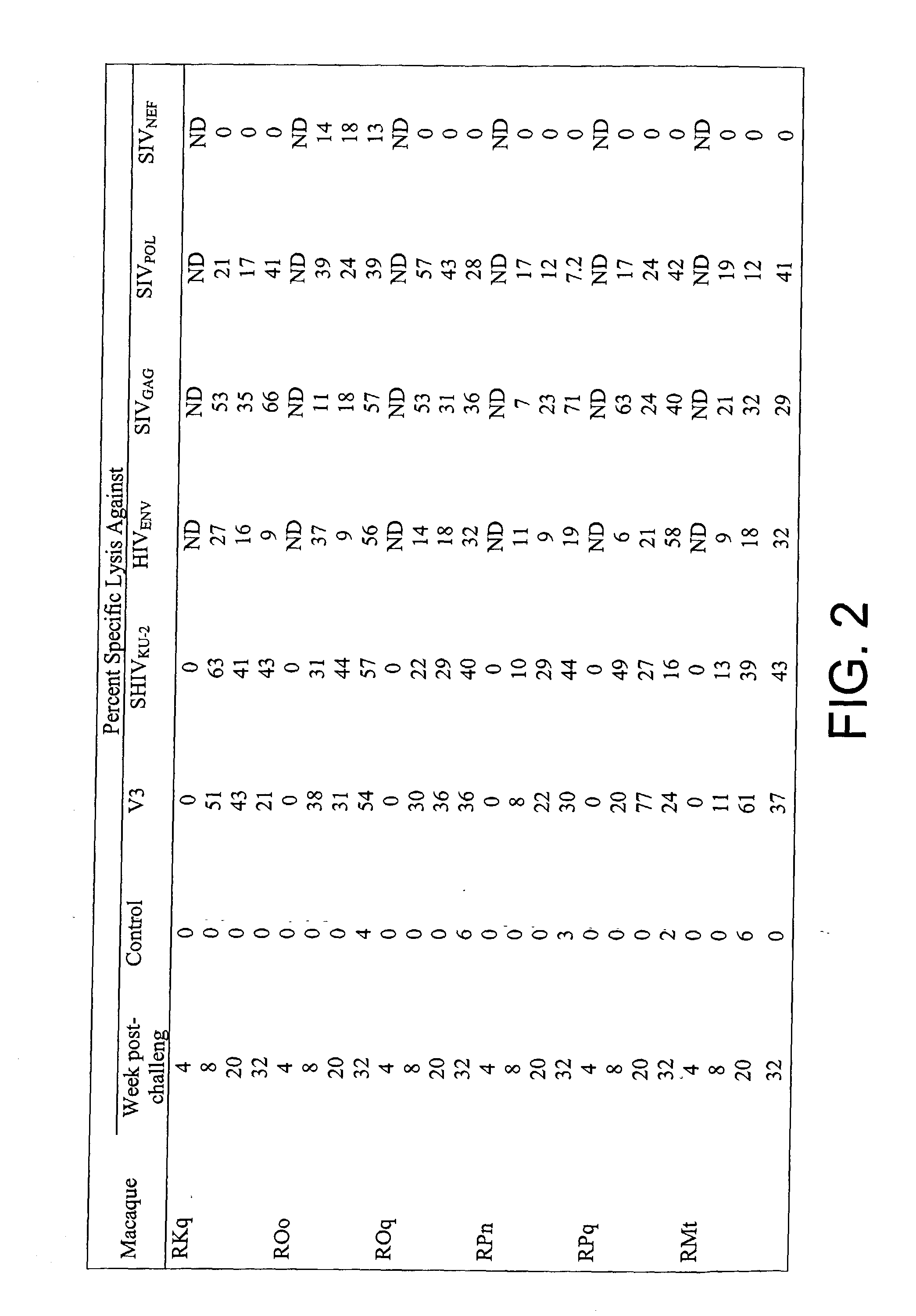
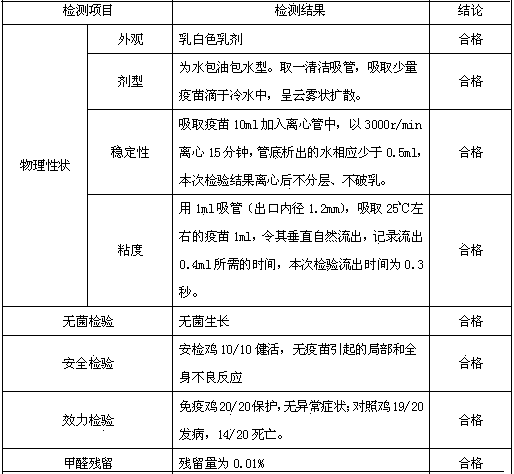
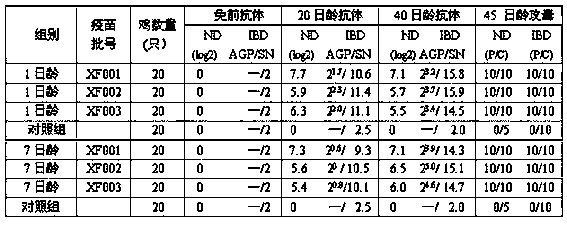
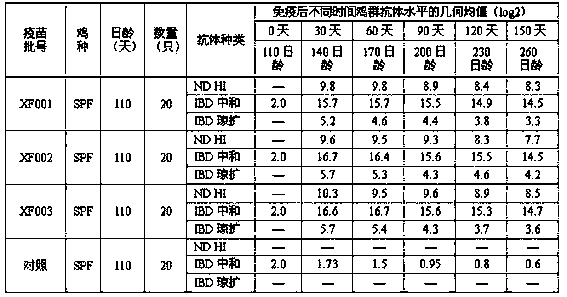
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
117 results about "HIV vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An HIV vaccine may have the purpose of protecting individuals who do not have HIV from being infected with the virus (a preventive vaccine), or treating an HIV-infected person (a therapeutic vaccine). There are two approaches to an HIV vaccine: an active vaccination approach in which a vaccine aims to induce an immune response against HIV; and a passive vaccination approach in which preformed antibodies against HIV are administered.
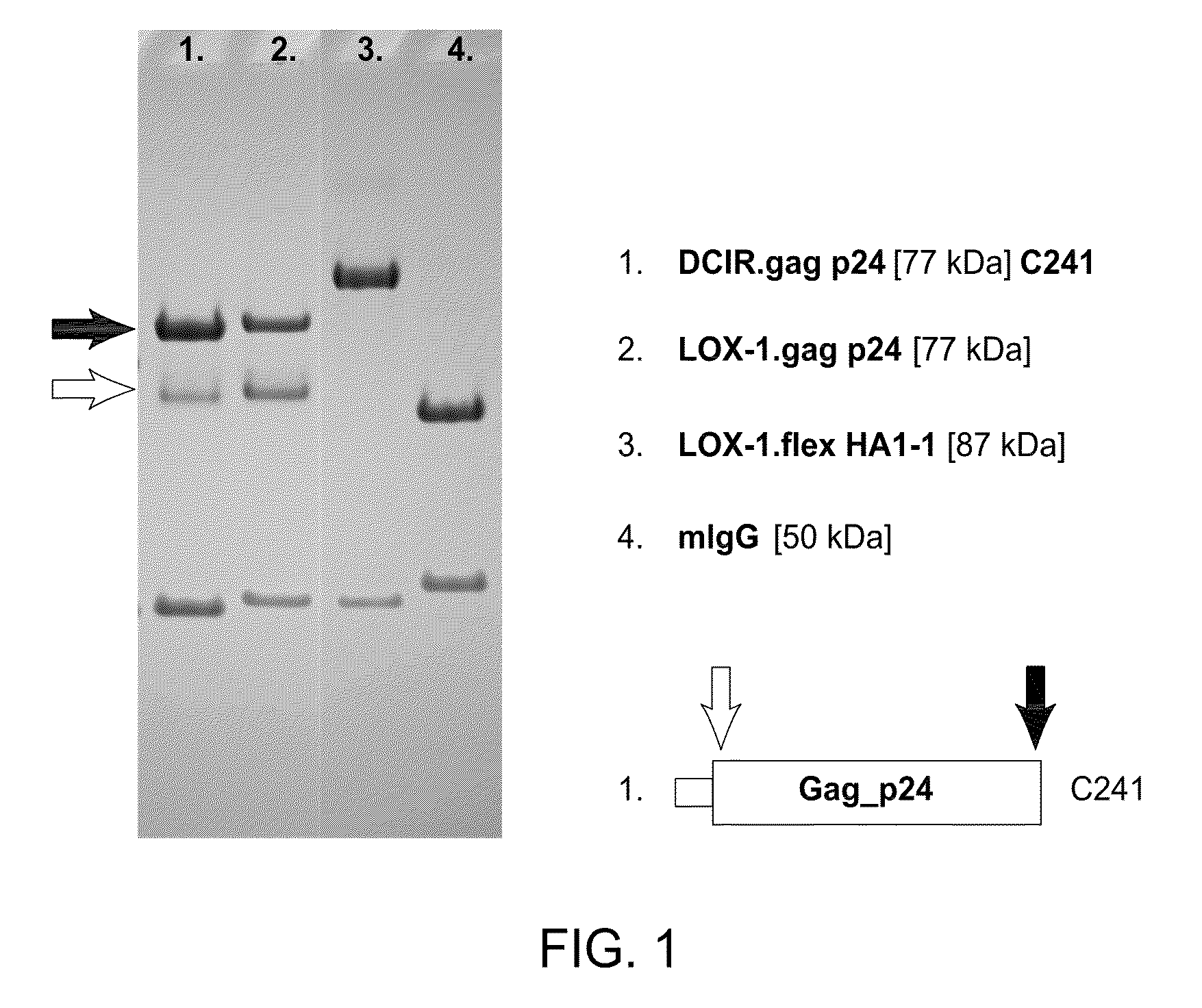
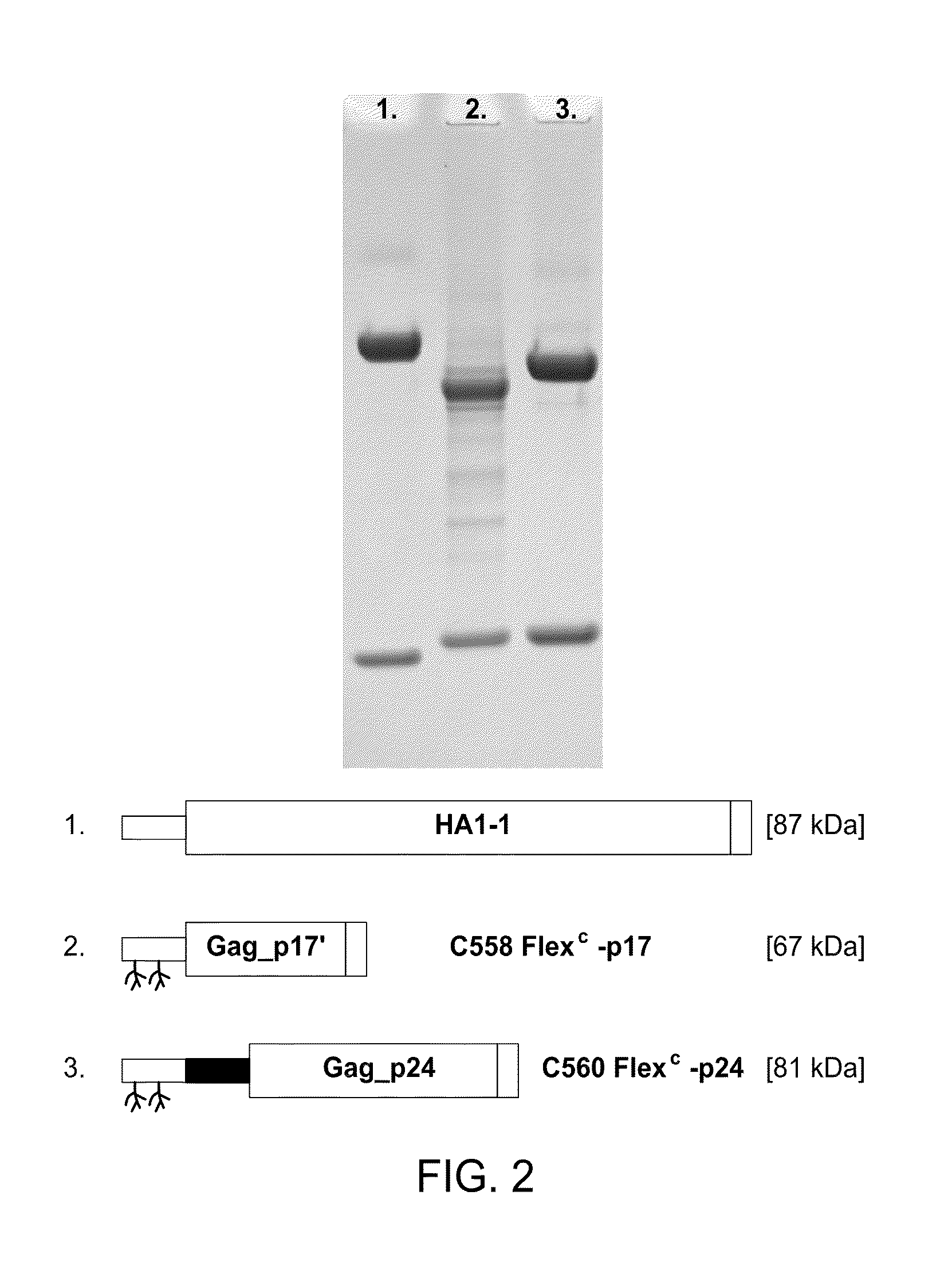
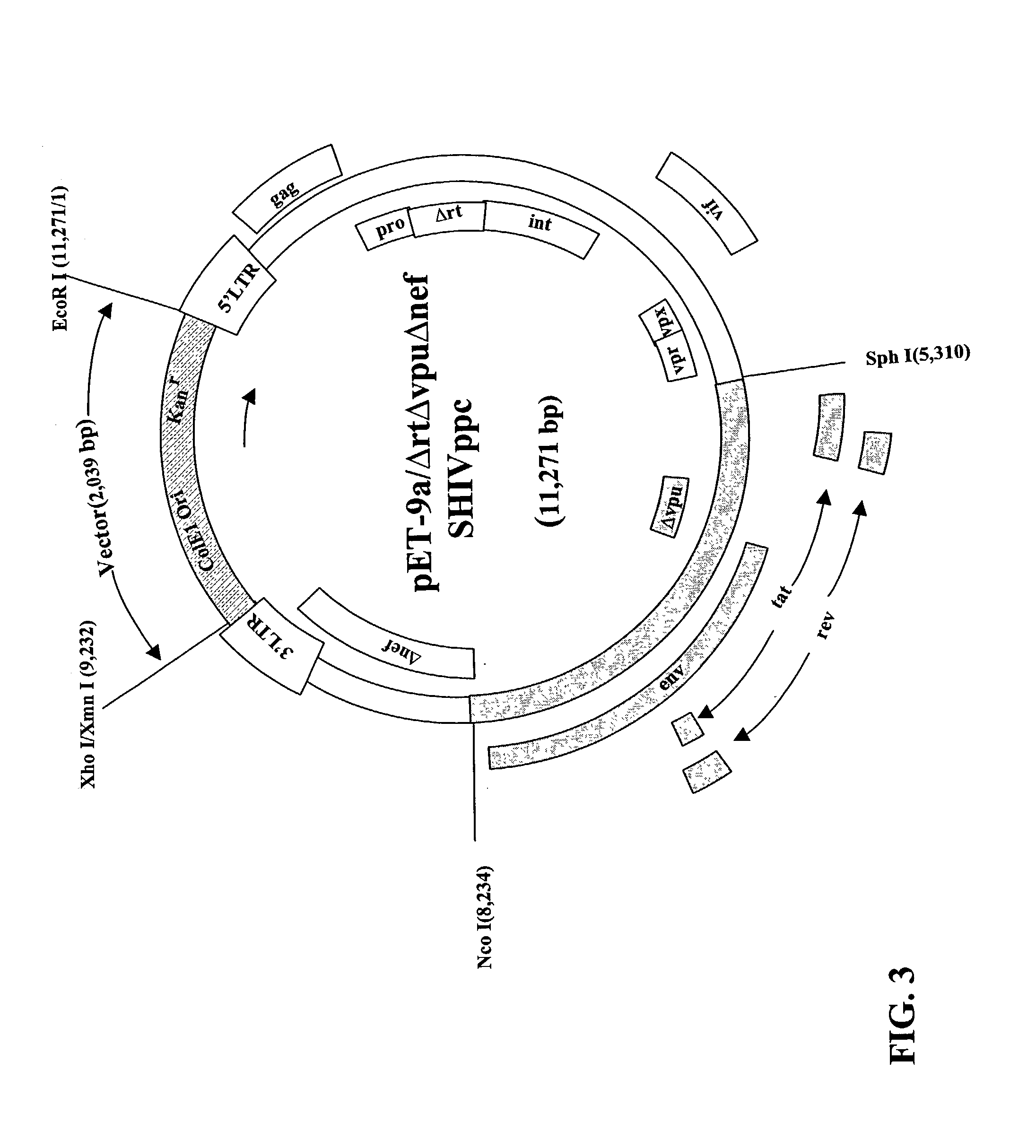
HIV vaccine based on targeting maximized gag and nef to dendritic cells
ActiveUS20100135994A1Improve efficiencyIncrease flexibilityBiocidePeptide/protein ingredientsCyclin D1Dendritic cell
The present invention includes compositions and methods for making and using a vaccine that includes a DC-specific antibody or fragment thereof to which an engineered Gag antigen is attached to form an antibody-antigen complex, wherein the Gag antigen is less susceptible to proteolytic degradation by eliminating one or more proteolytic sites or a DC-specific antibody or fragment thereof to which an engineered Nef antigen is attached to form an antibody-antigen complex, wherein the Nef antigen comprises one or more codon usage optimization that increase antibody-antigen complex secretion, or both, wherein the vaccine is able to elicit an HIV-specific T cell immune response to Gag p17, Gag p24, Nef and / or Cyclin D1.
Owner:BAYLOR RES INST
Method for the development of an HIV vaccine
InactiveUS6383806B1Effective immune responseInhibiting infectivityAnimal cellsMicrobiological testing/measurementReverse transcriptaseImmunodeficiency virus
Human immunodeficiency virus (HIV) comprising reverse transcriptase inactivated by photoinactivation used to evoke an immune response. The immune response may protect an individual from challenges with live virus. Alternatively, the inactivated HIV particles may be used to augment the immune response to HIV in an infected individual.
Owner:PHOTOIMMUNE BIOTECH
HIV vaccine
InactiveUS7067134B1Efficient replicationAvoid cell damageViral antigen ingredientsInactivation/attenuationHIV vaccineFhit gene
A novel HIV vaccine is provided. In particular, the vaccine comprises an avirulent and non-cytolytic recombinant HIV wherein the NSS of the virus' envelope glycoprotein is replaced with a non-cytolytic signal sequence and nef gene of the virus is deleted which renders the virus avirulent.
Owner:UNIV OF WESTERN ONTARIO
Method for the development of an HIV vaccine
InactiveUS6503753B1Reduce riskSource securityAnimal cellsViral antigen ingredientsImmunodeficiency virusReverse transcriptase
Human immunodeficiency virus (HIV) comprising reverse transcriptase inactivated by photoinactivation. The inactivated virus may be more safely handled, stored, and analyzed, used in diagnostic procedures and kits, and may be used as an immunogen to evoke an immune response. The immune response may protect an individual from challenges with live virus. Alternatively, the inactivated HIV particles may be used to augment the immune response to HIV in an infected individual.
Owner:PHOTOIMMUNE BIOTECH
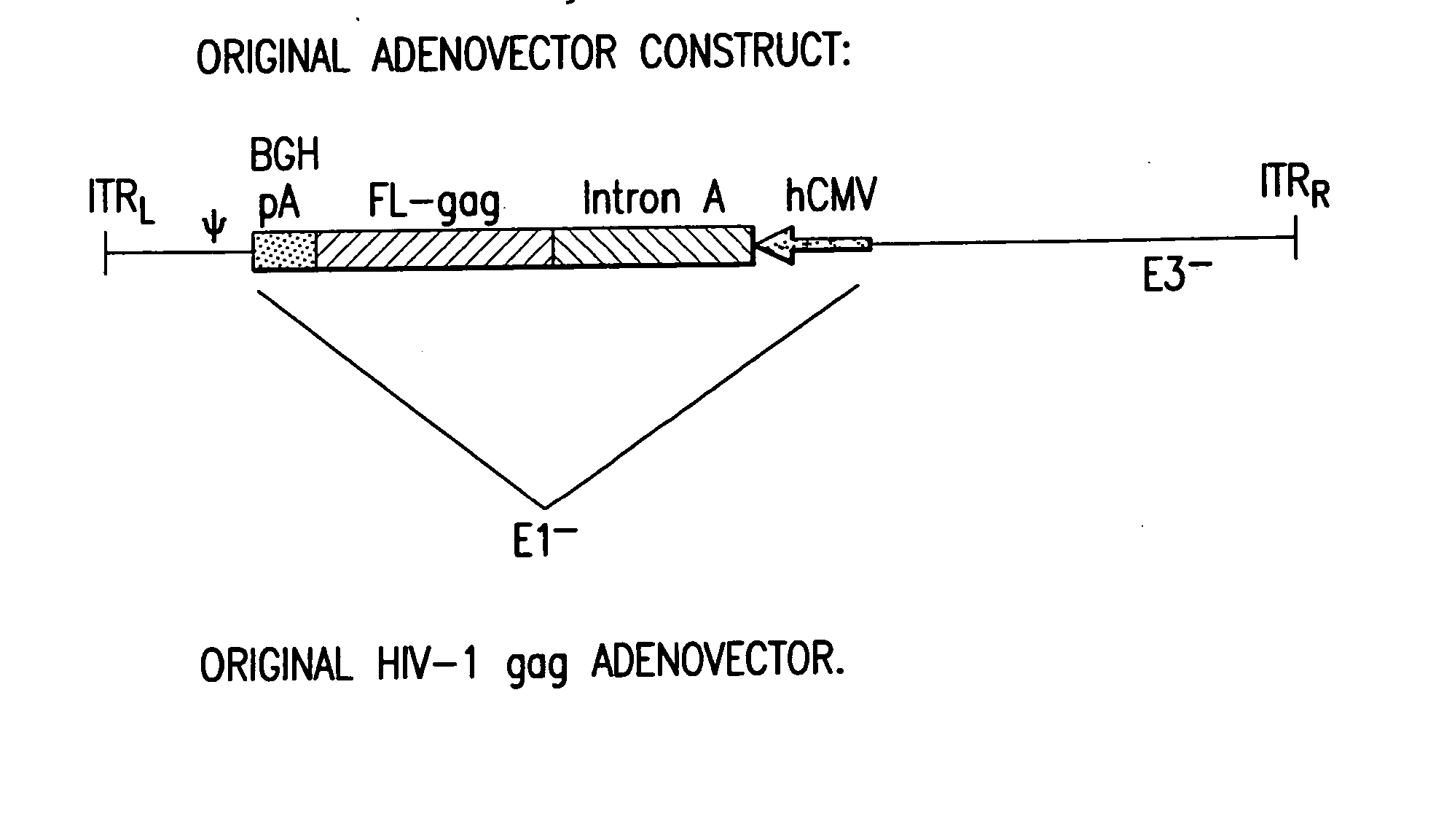
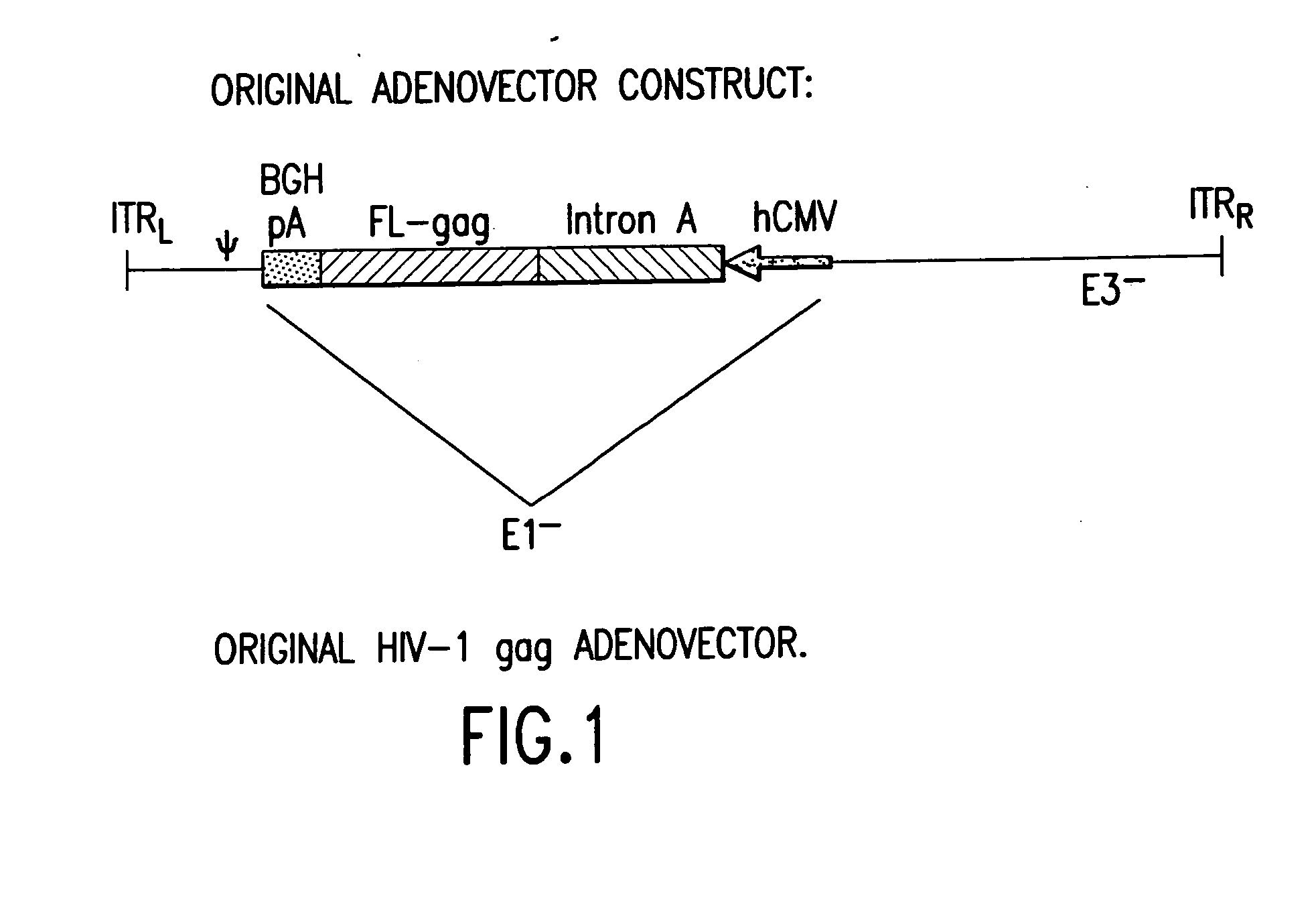
Enhanced first generation adenovirus vaccines expressing codon optimized HIV1-Gag, Pol, Nef and modifications
InactiveUS20070054395A1Genetically stableImprove featuresVectorsViral antigen ingredientsMammalReverse transcriptase
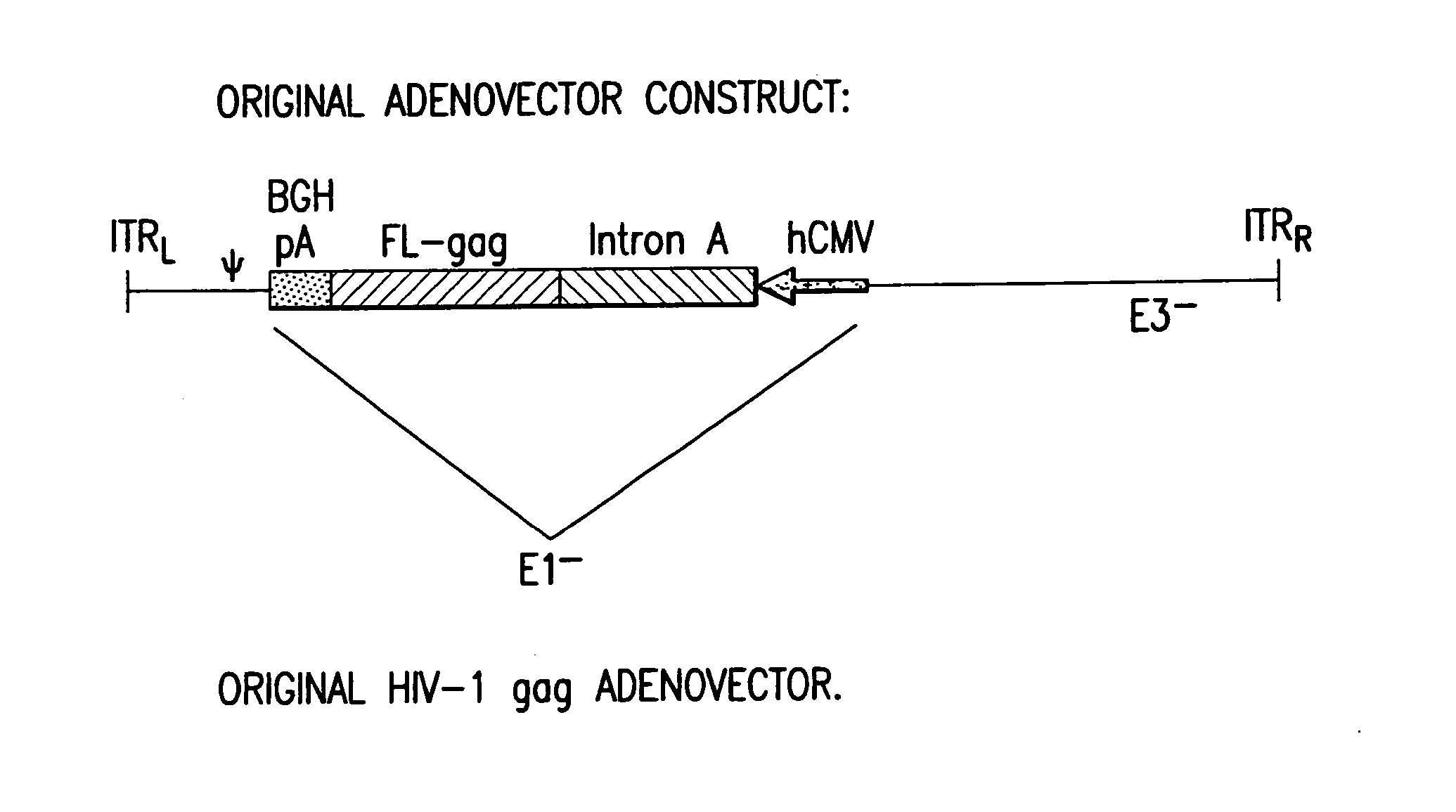
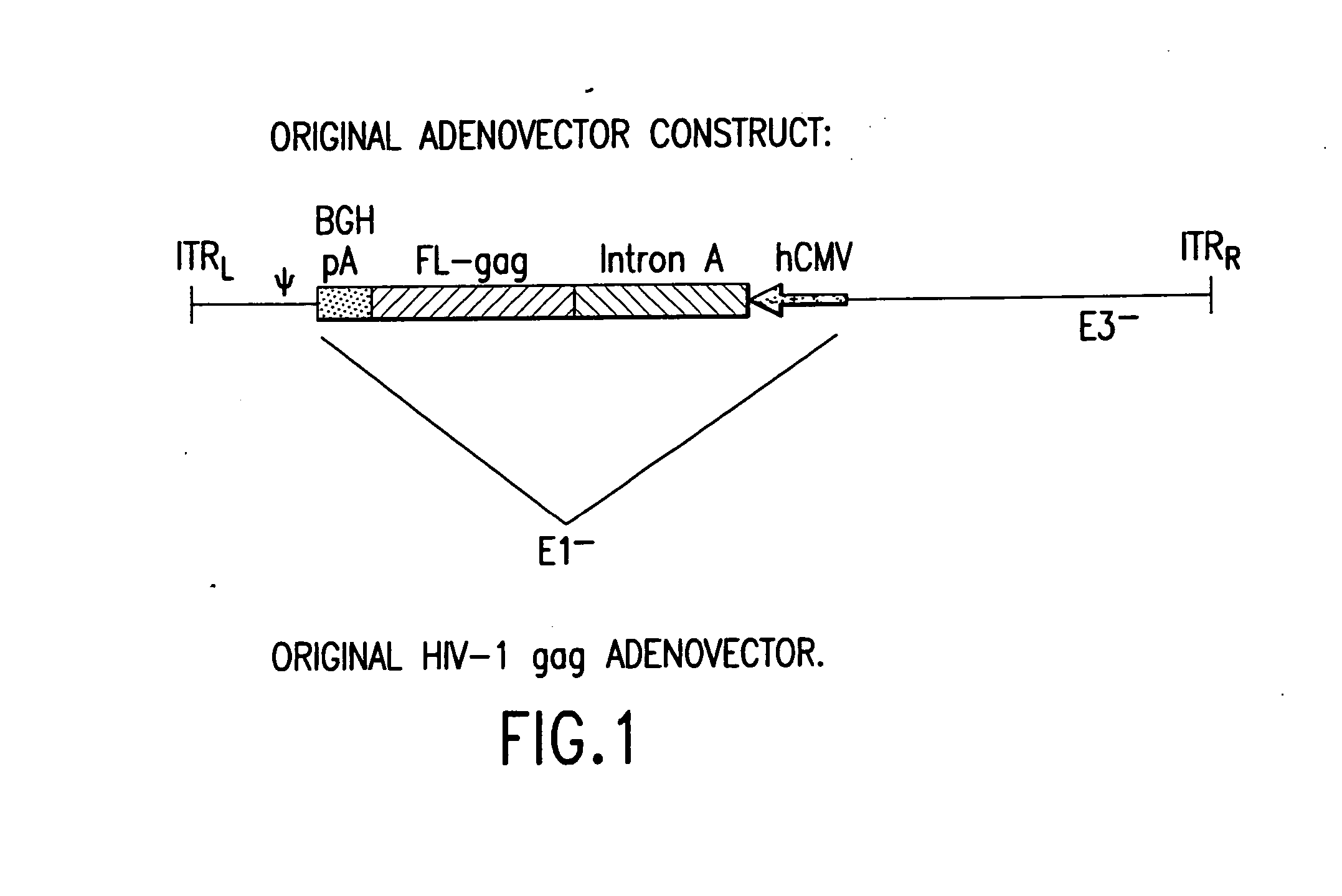
First generation adenoviral vectors and associated recombinant adenovirus-based HIV vaccines which show enhanced stability and growth properties and greater cellular-mediated immunity are described within this specification. These adenoviral vectors are utilized to generate and produce through cell culture various adenoviral-based HIV-1 vaccines which contain HIV-1 gag, HIV-1 pol and / or HIV-1 nef polynucleotide pharmaceutical products, and biologically relevant modifications thereof. These adenovirus vaccines, when directly introduced into living vertebrate tissue, preferably a mammalian host such as a human or a non-human mammal of commercial or domestic veterinary importance, express the HIV1-Gag, Pol and / or Nef protein or biologically modification thereof, inducing a cellular immune response which specifically recognizes HIV-1. The exemplified polynucleotides of the present invention are synthetic DNA molecules encoding HIV-1 Gag, encoding codon optimized HIV-1 Pol, derivatives of optimized HIV-1 Pol (including constructs wherein protease, reverse transcriptase, RNAse H and integrase activity of HIV-1 Pol is inactivated), HIV-1 Nef and derivatives of optimized HIV-1 Nef, including nef mutants which effect wild type characteristics of Nef, such as myristylation and down regulation of host CD4. The adenoviral vaccines of the present invention, when administered alone or in a combined modality regime, will offer a prophylactic advantage to previously uninfected individuals and / or provide a therapeutic effect by reducing viral load levels within an infected individual, thus prolonging the asymptomatic phase of HIV-1 infection.
Owner:EMINI EMILIO A +7
Canine distemper attenuated vaccine strain and application thereof
InactiveCN101914503ACharacteristics of typical paramyxovirionsImproving immunogenicityInactivation/attenuationMicroorganism based processesMicroorganismCanine distemper virus CDV
The invention discloses a canine distemper attenuated vaccine strain and application thereof. In the invention, passing and cloning are performed on a separated canine distemper virulent strain to culture the canine distemper attenuated vaccine strain, and the microorganism collection number is CGMCC No.3810. The canine distemper attenuated vaccine strain of the invention can provide better protection for the canines suffering homological virulent attack, has perfect immunogenicity and can provide relatively good immune protection for the canines infected with the canine distemper virus. The attenuated vaccine strain can be prepared into single vaccine or united vaccine (live vaccine or inactivated vaccine) and can effectively prevent or cure the canine distemper. The attenuated vaccine strain of the invention has the advantages of stable transmissibility, lasting immunity, good effect, safety, reliability, long storage time and the like.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for the development of an HIV vaccine
InactiveUS20030104011A1Reduce riskSeriousness of riskAnimal cellsViral antigen ingredientsReverse transcriptaseHIV vaccine
Owner:PHOTOIMMUNE BIOTECH
Polyvalent Vaccine
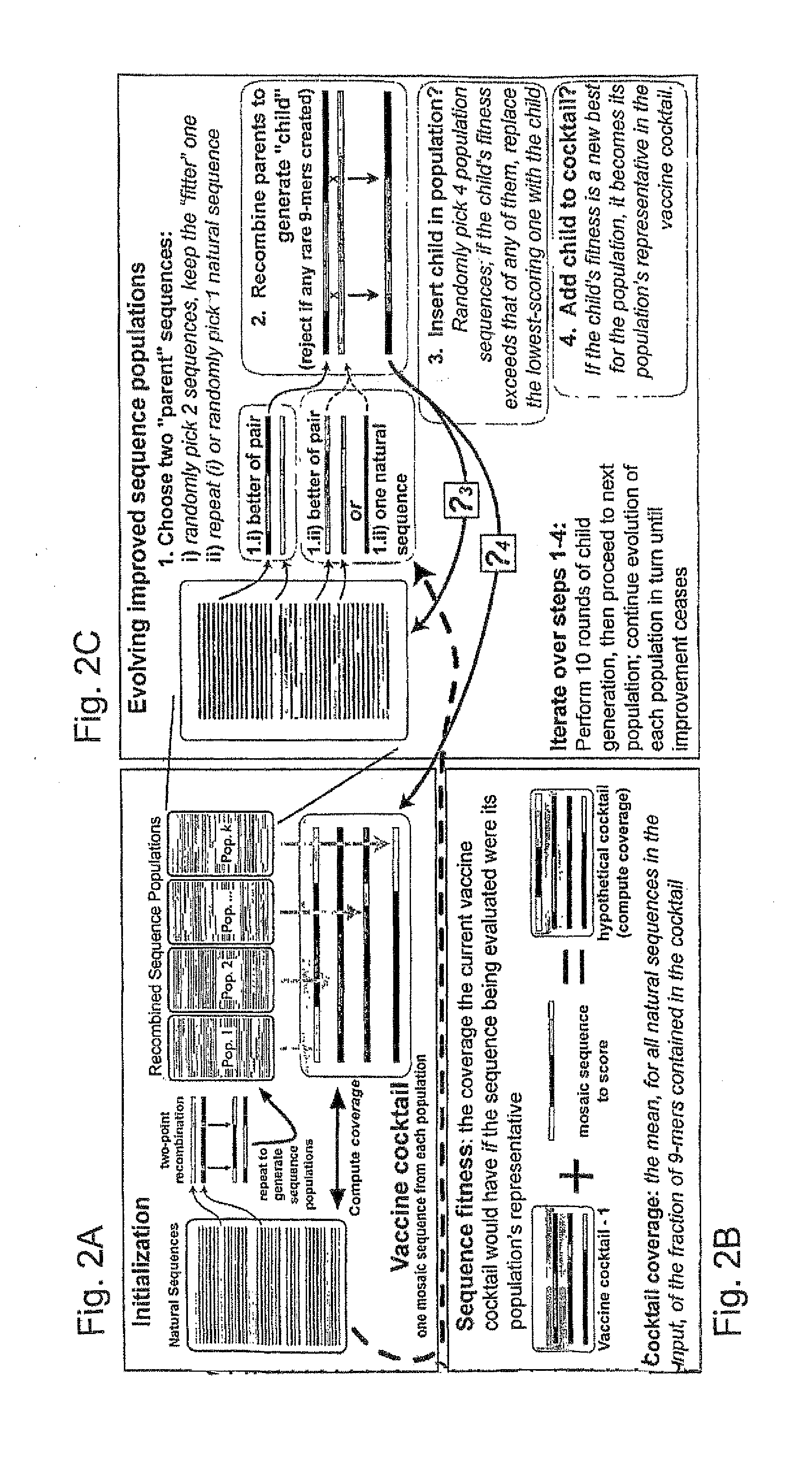
ActiveUS20090324631A1Organic active ingredientsPeptide/protein ingredientsVaccinationGenetic algorithm
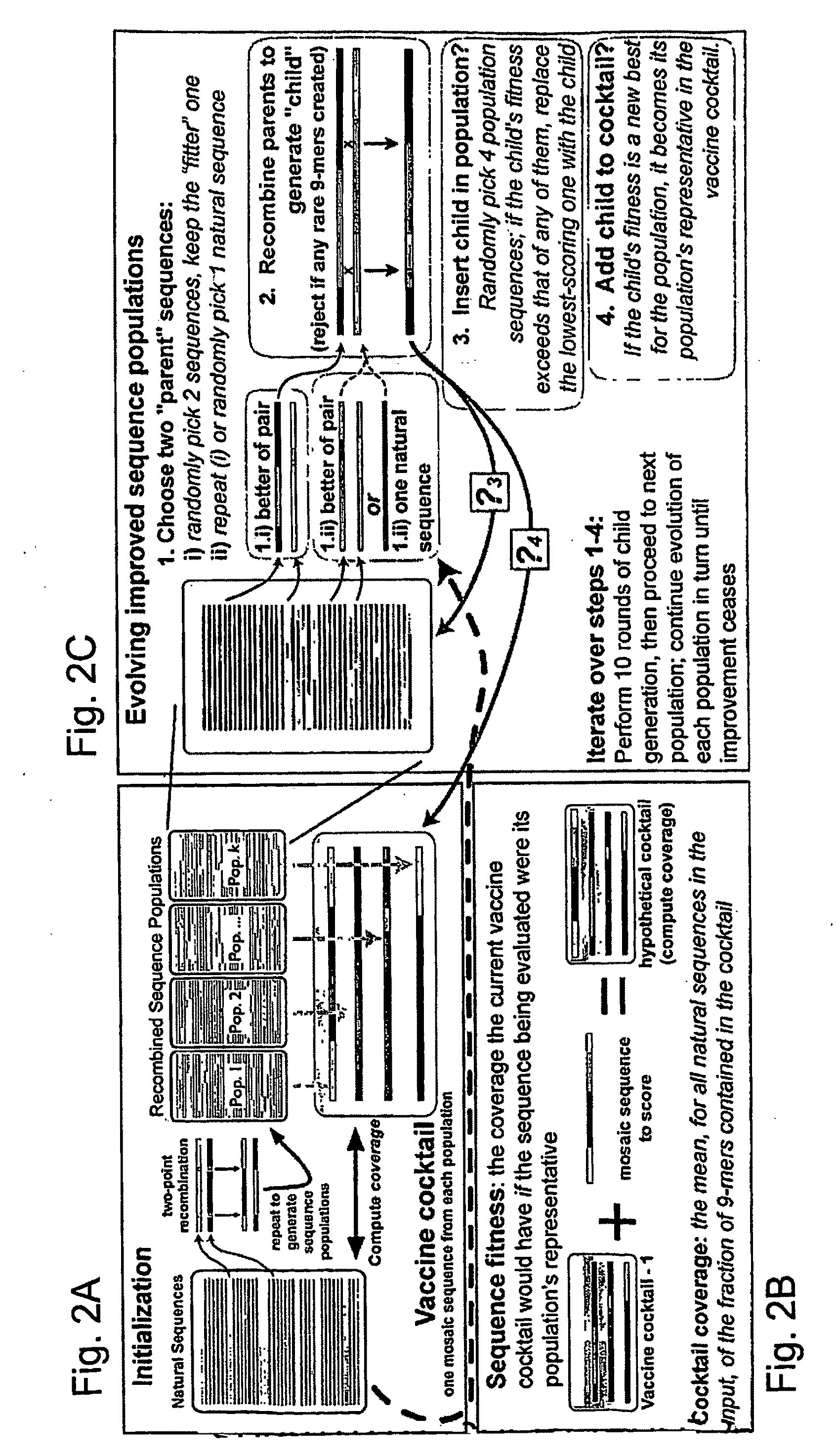
The present invention relates, in general, to an immunogenic composition (e.g., a vaccine) and, in particular, to a polyvalent immunogenic composition, such as a polyvalent HIV vaccine, and to methods of using same. The invention further relates to methods that use a genetic algorithm to create sets of polyvalent antigens suitable for use, for example, in vaccination strategies.
Owner:DUKE UNIV +3
HIV vaccine formulation
ActiveUS20170362280A1Improve stabilityGood for clinical useHydroxy compound active ingredientsViral antigen ingredientsAdjuvantEngineering
Immunogenic compositions containing a human immunodeficiency virus (HIV) gp140 protein, sorbitol, polysorbate 20, and histidine buffer are described. The described immunogenic compositions are advantageous in that they are stable at refrigerated temperature for extended periods of time, and are compatible with an adjuvant. Also described are methods of using the immunogenic compositions to induce an immune response against an HIV in a subject. The immunogenic compositions can be administered alone, or in combination with one or more additional HIV antigens, or one or more adenovirus vectors encoding the one or more additional HIV antigens.
Owner:JANSSEN VACCINES & PREVENTION BV
HIV vaccine composition
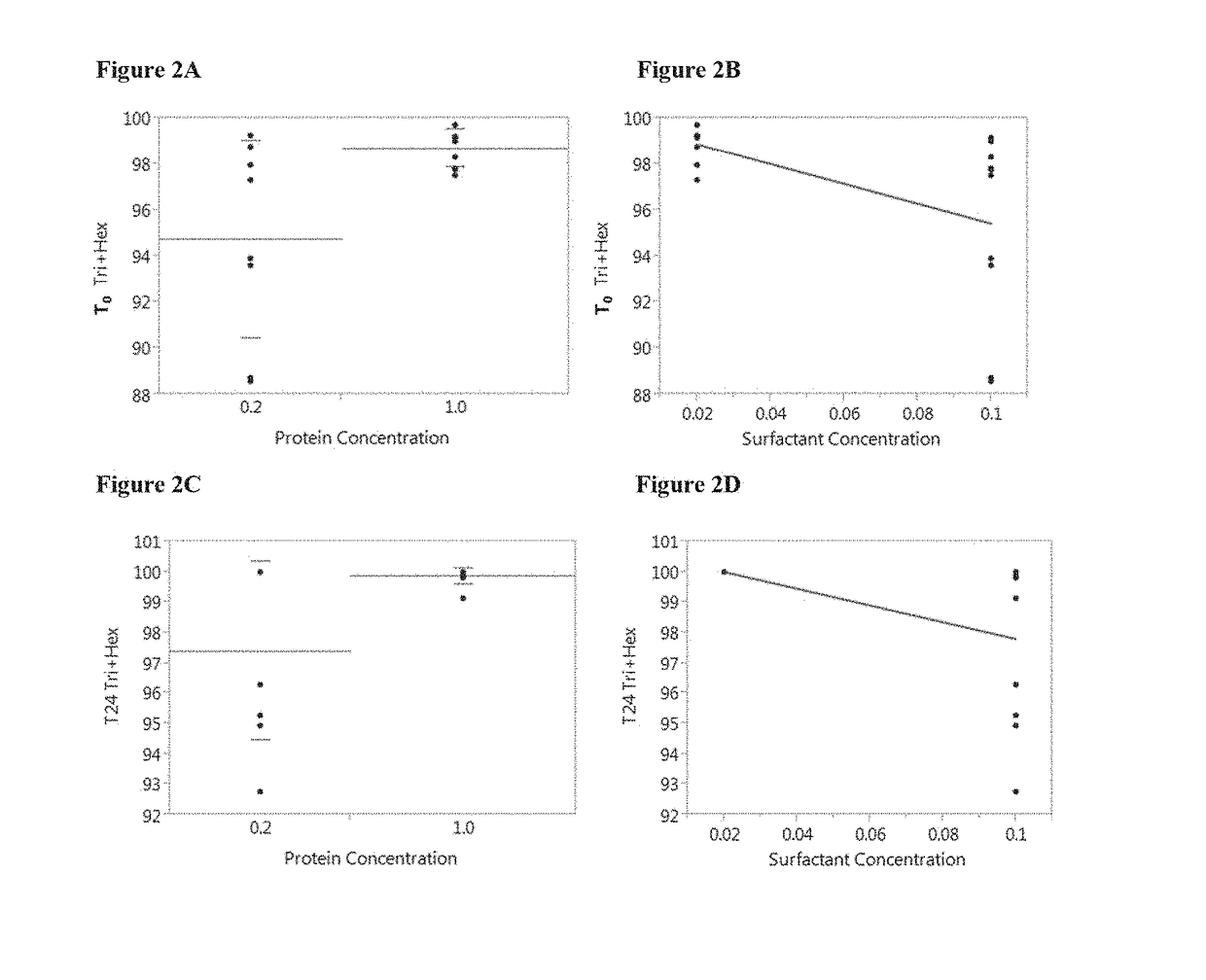
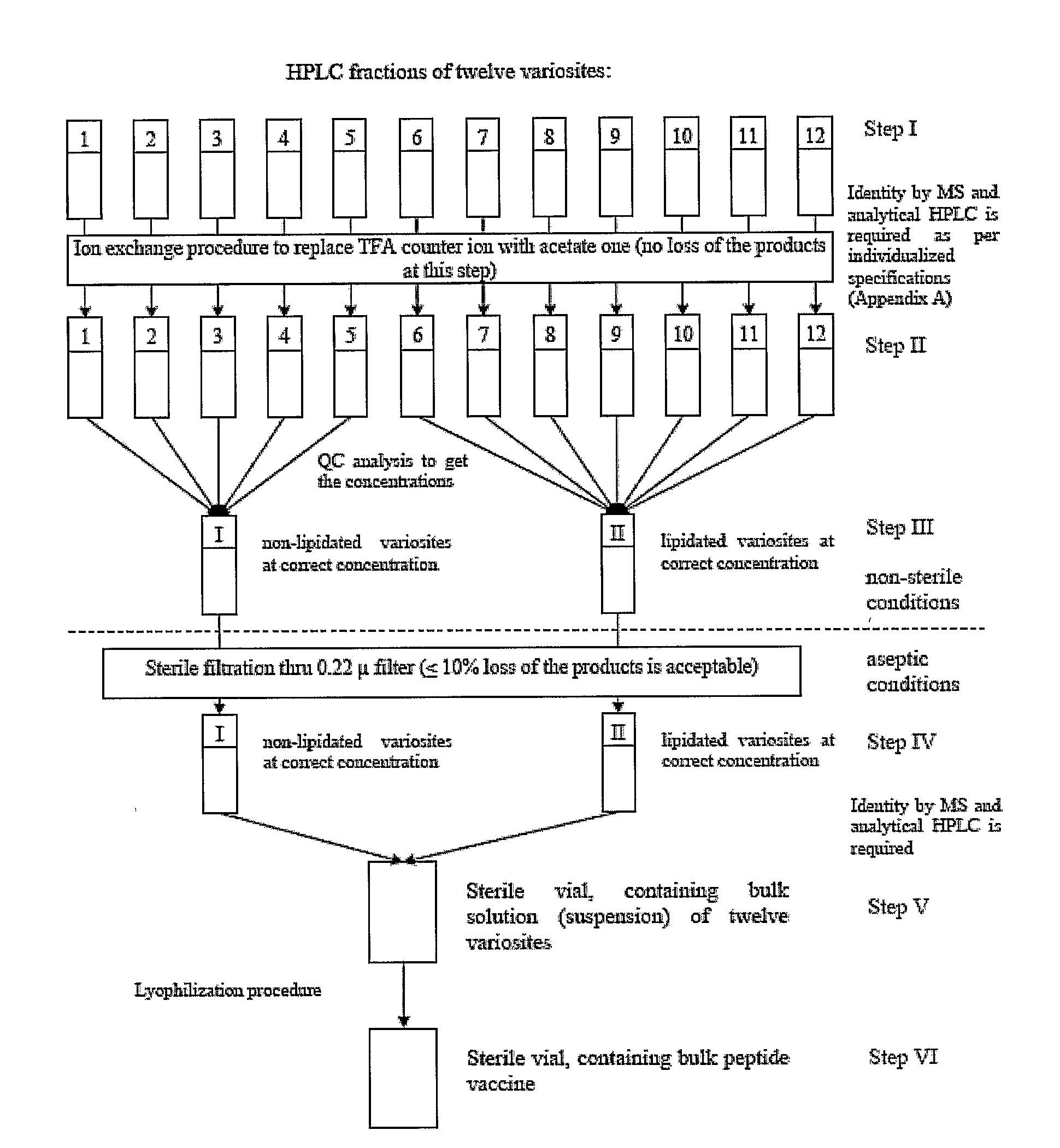
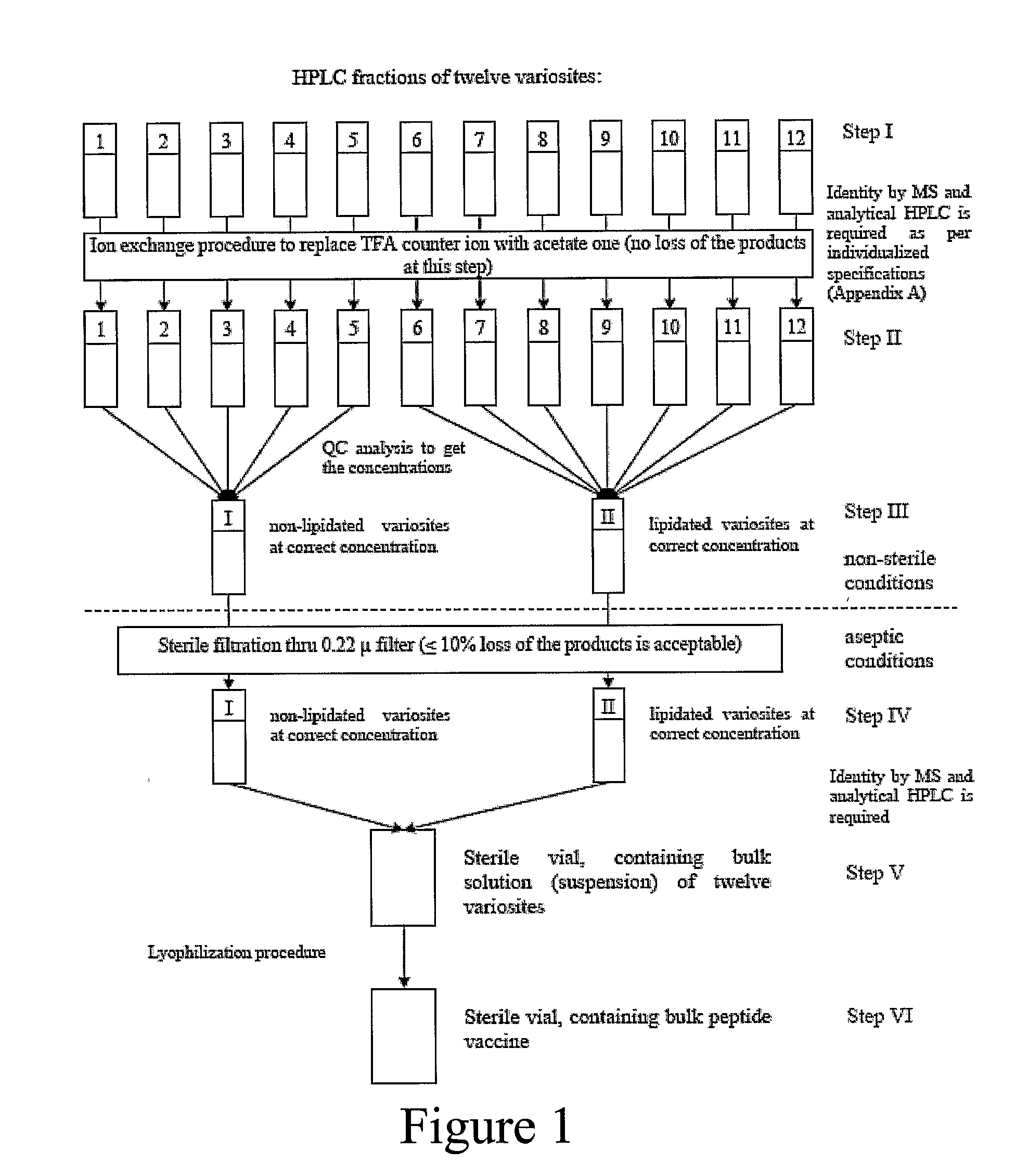
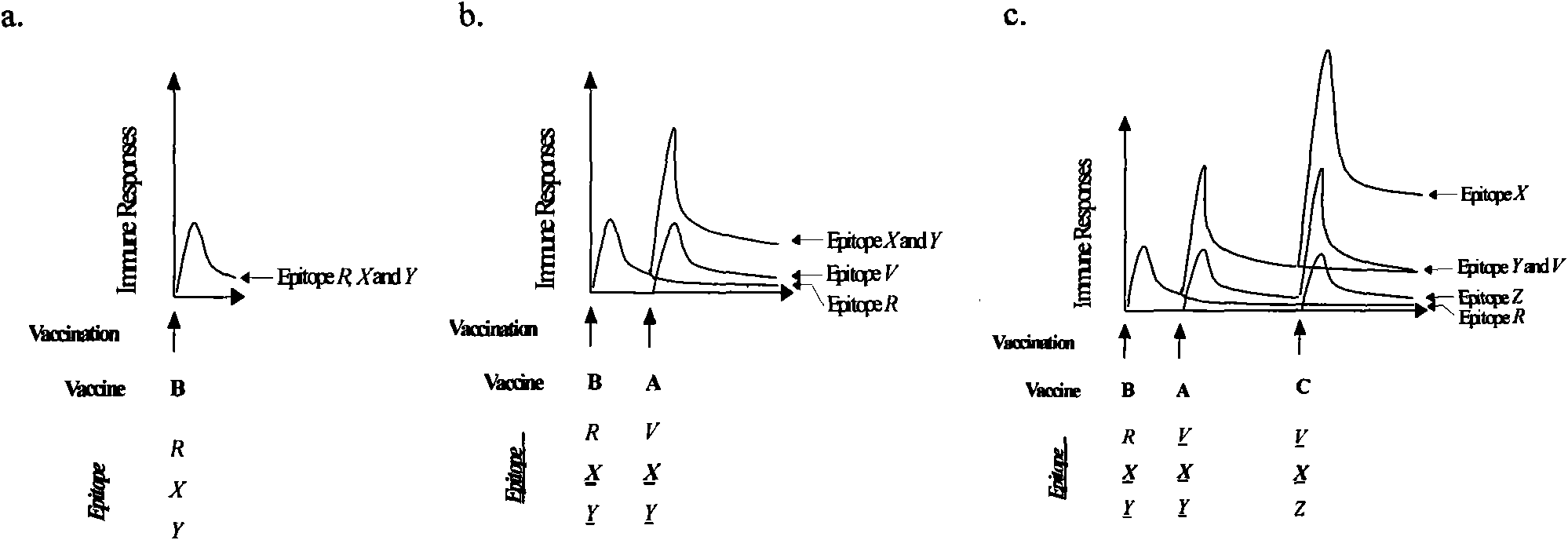
An anti-HIV vaccine composition is disclosed. The vaccine comprises an combination of immunogenic peptide mixtures, which mixtures may be prepared in a single synthesis. The composition collectively represents the in vivo variability seen in immunogenic epitopes from highly variable regions of HIV. Immunization with the vaccine elicits broadly reactive immunity (CTL and T helper cell responses) against the divergent strains of HIV upon which it is based. The vaccine may be formulated to target regionally distinct variability based on an HIV clade predominant in a geographical region.
Owner:VARIATION BIOTECHNOLOGIES INC
Combination vaccine against various HIVs and combination method thereof
InactiveCN101569745ANew Vaccine FeaturesGenetic material ingredientsAntiviralsHIV vaccineCell Membrane Proteins
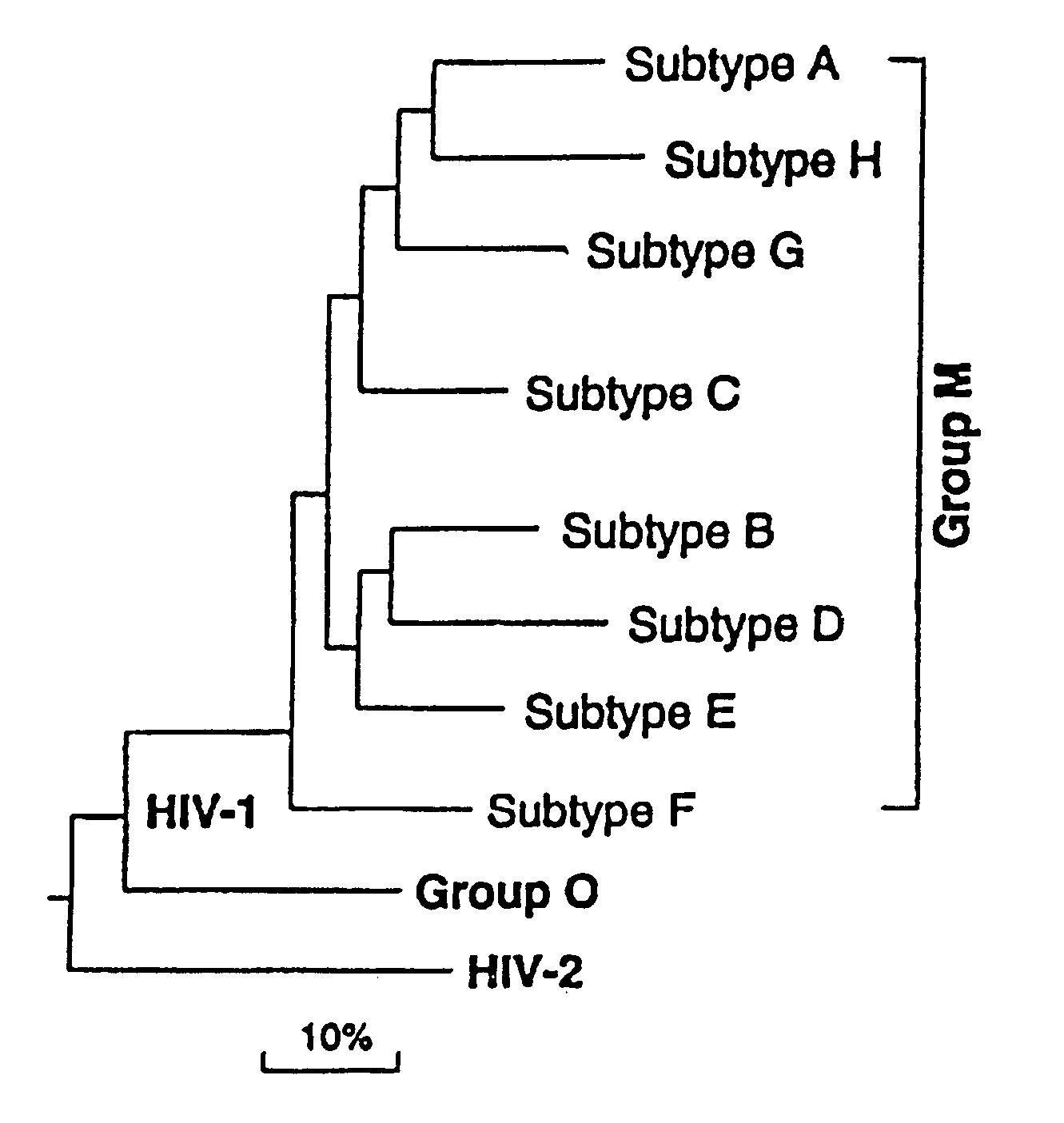
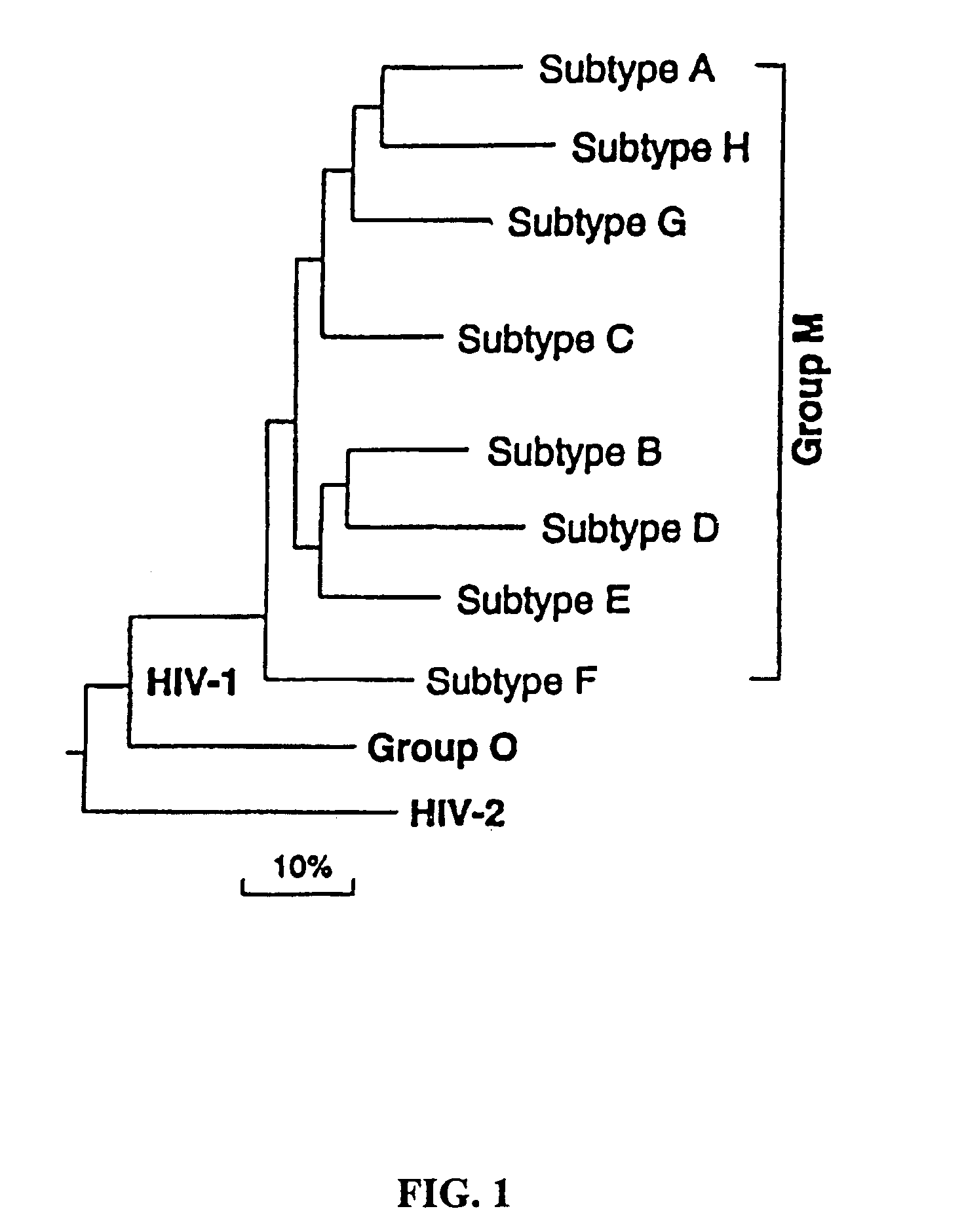
The invention discloses a combination vaccine against various HIVs and a combination method thereof. The combination vaccine consists of two or more HIV vaccines, different AIDS vaccines contain different HIV membrane proteins or membrane protein encoding genes, and each HIV vaccine is independently inoculated and inoculated at least one time, namely the total times of inoculation are at least twice. The core of the technology is that the different HIV membrane proteins are used to carry out sequential immunization, namely in sequential repeated immune processes of the vaccines, the HIV membrane proteins used in the immunization at different times are different so as to obtain a broad-spectrum neutral antibody against the HIVs with high titer. The HIV preventing vaccine developed by the method can be used for preventing various subtype HIV infections.
Owner:VACDIAGN BIOTECH
Immunogens for HIV vaccination
ActiveUS9988425B2Reducing viral burdenIncrease infectionAntibody mimetics/scaffoldsViral antigen ingredientsVaccinationHIV vaccine
Owner:FUNDACIO PRIVADA INST DE RECERCA DE LA SIDA CAIXA +1
Polyvalent vaccine
The present invention relates, in general, to an immunogenic composition (e.g., a vaccine) and, in particular, to a polyvalent immunogenic composition, such as a polyvalent HIV vaccine, and to methods of using same. The invention further relates to methods that use a genetic algorithm to create sets of polyvalent antigens suitable for use, for example, in vaccination strategies.
Owner:LOS ALAMOS NATIONAL SECURITY +3
Enhanced first generation adenovirus vaccines expressing condon optimized HIV1-Gag, Pol, Nef and modifications
InactiveUS20070077257A1Improved cellular-mediated immune responseLow transmission rateViral antigen ingredientsVirus peptidesTreatment effectNucleotide
First generation adenoviral vectors and-recombinant adenovirus-based HIV vaccines which contain HIV-1 gag, HIV-1 pol and / or HIV-1 nef polynucleotide pharmaceutical products, and biologically relevant modifications thereof are described. The adenovirus vaccines, when directly introduced into living vertebrate tissue, express the relevant proteins, inducing a cellular immune response which specifically recognizes HIV-1. The exemplified polynucleotides of the present invention are synthetic DNA molecules encoding HIV-1 Gag, HIV-1 Pol, HIV-1 Nef, and derivatives thereof. The adenoviral vaccines of the present invention, alone or in combination, will offer a prophylactic advantage to previously uninfected individuals and / or provide a therapeutic effect by reducing viral load levels within an infected individual, thus prolonging the asymptomatic phase of HIV-1 infection.
Owner:EMINI EMILIO A +7
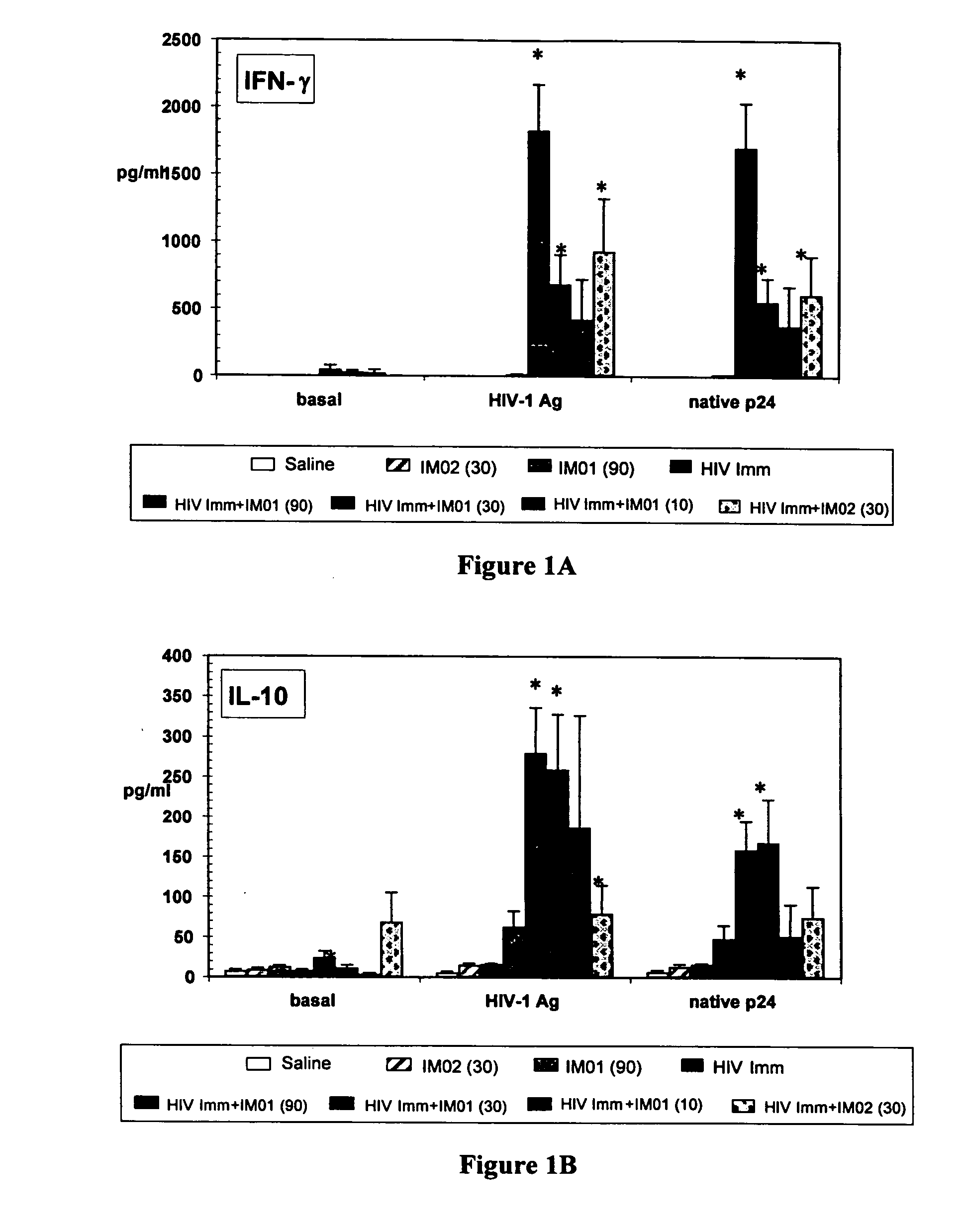
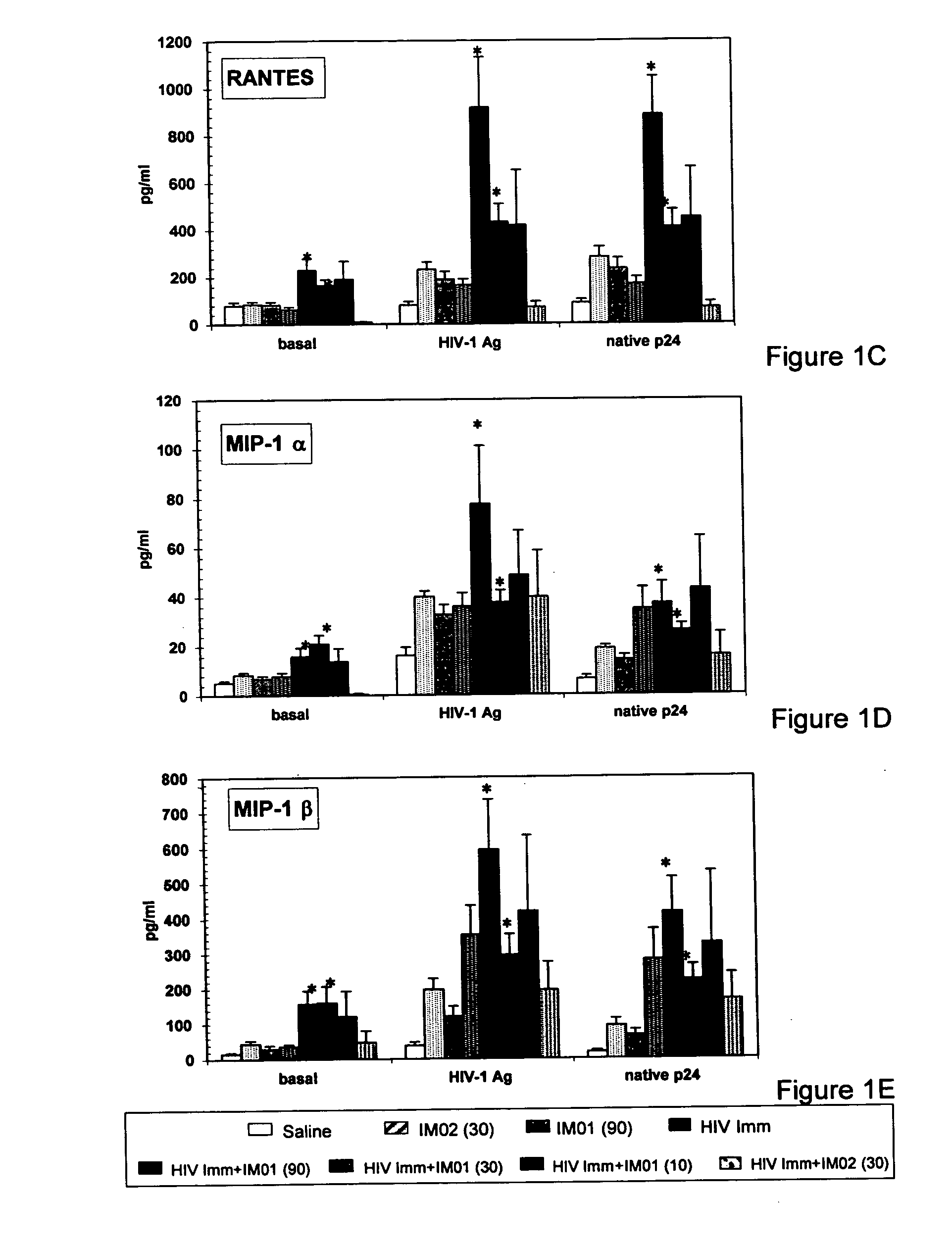
Enhanced activity of HIV vaccine using a second generation immunomodulatory oligonucleotide
InactiveUS20050266015A1Prolongation in time to progressionAvoid infectionViral antigen ingredientsGenetic material ingredientsInfection riskHIV vaccine
The invention relates to the therapeutic use of a second generation immunomodulatory oligonucleotide in combination with HIV-1 antigen or immunogen to enhance the ability to reduce the risk HIV infection and to control the progression of HIV infection to prevent AIDS Related Complex (ARC) and AIDS.
Owner:THE IMMUNE RESPONSE +1
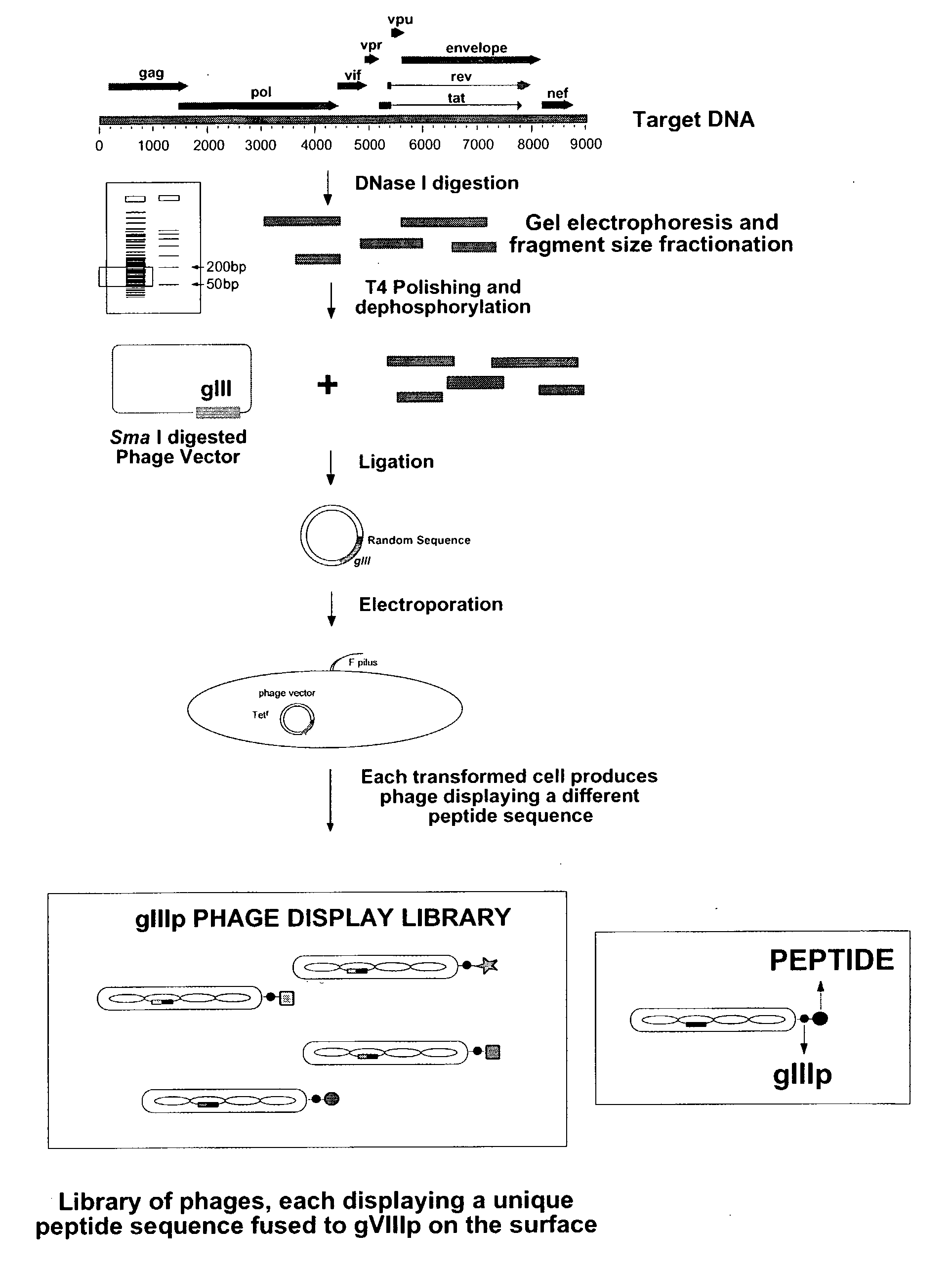
Molecular Scaffolds for HIV-1 Epitopes
InactiveUS20080096187A1Altering neutralization potential of CD4-inducedImprove accessibilitySugar derivativesMicrobiological testing/measurementDiseaseEpitope
Methods and compositions are provided for the use of an envelope polypeptide or a functional variant thereof from a lentivirus that is not HIV-1 as a molecular scaffold for HIV-1 epitopes. The HIV-1 epitopes can be recognized by HIV-1 binding antibodies, HIV-1 neutralizing antibodies and / or CD4-induced antibodies. Thus, methods are provided for detecting HIV-1 binding antibodies in a subject infected with HTV-1. Further provided are methods to determine an epitope for an HIV-1 binding antibody; methods to assay for an HIV-1 binding antibody; methods to identify a soluble CD4 mimic; methods to neutralize an non-HIV-1 virus; diagnostic assays to monitor HIV disease in a subject or to monitor the subject's response to immunization by a HIV vaccine; and methods to alter the neutralization potential of an HIV-1 derived CD4-induced antibody. Chimeric polypeptides, chimeric polynucleotides, kits, cells and viruses are also provided.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERVICES HEREINAFTER THE GOVERNMENT +2
Inactivated vaccines for aids and other infectious diseases
InactiveUS7033813B2Bioreactor/fermenter combinationsBiological substance pretreatmentsManufacturing technologyBACTERIAL INFECTIOUS DISEASES
Presented herein is a description for the manufacturing of inactivated HIV for use in vaccines against AIDS, as well as other inactivated viruses for other infectious diseases. This invention incorporates methods for inactivating infectious virus particles while retaining protein integrity and antigenicity. The methods utilize critical, near-critical or supercritical fluids with or without polar cosolvents. This invention would allow for the creation of HIV vaccines from genetically attenuated HIV strains for a greater degree of product safety, and from combinations of different HIV strains for broader protection. This HIV vaccine manufacturing technology is inexpensive, amenable to large-scale processing and portable, i.e. it can be readily implemented in a host country site. This invention can be utilized for other viral and bacterial infectious diseases, such as influenza and hepatitis.
Owner:APHIOS
Compositions and methods for the detection of hiv-1/hiv-2 infection
InactiveUS20090023164A1Negative impactContribute to other social harmsBioreactor/fermenter combinationsBiological substance pretreatmentsSerum igeImmunodeficiency virus
This invention relates to compositions and methods or the detection of immunodeficiency virus infection, especially immunodeficiency virus-1 (HIV-1) infection. The invention particularly concerns compositions and methods that may be used in HIV vaccine recipients whose sera may contain vaccine-generated anti-HIV-1 antibodies.
Owner:UNITED STATES OF AMERICA
Polyvalent vaccine
The present invention relates, in general, to an immunogenic composition (e.g., a vaccine) and, in particular, to a polyvalent immunogenic composition, such as a polyvalent HIV vaccine, and to methods of using same. The invention further relates to methods that use a genetic algorithm to create sets of polyvalent antigens suitable for use, for example, in vaccination strategies.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +3
Omv vaccine against burkholderia infections
InactiveUS20140004178A1Provide protectionAvoid infectionAntibacterial agentsBacterial antigen ingredientsMedicineGram-negative bacterial infections
The present disclosure relates to vaccine compositions and methods of using the vaccine compositions to provide protection against various Gram-negative bacterial infections, including Burkholderia infections.
Owner:THE ADMINISTRATORS OF THE TULANE EDUCATIONAL FUND
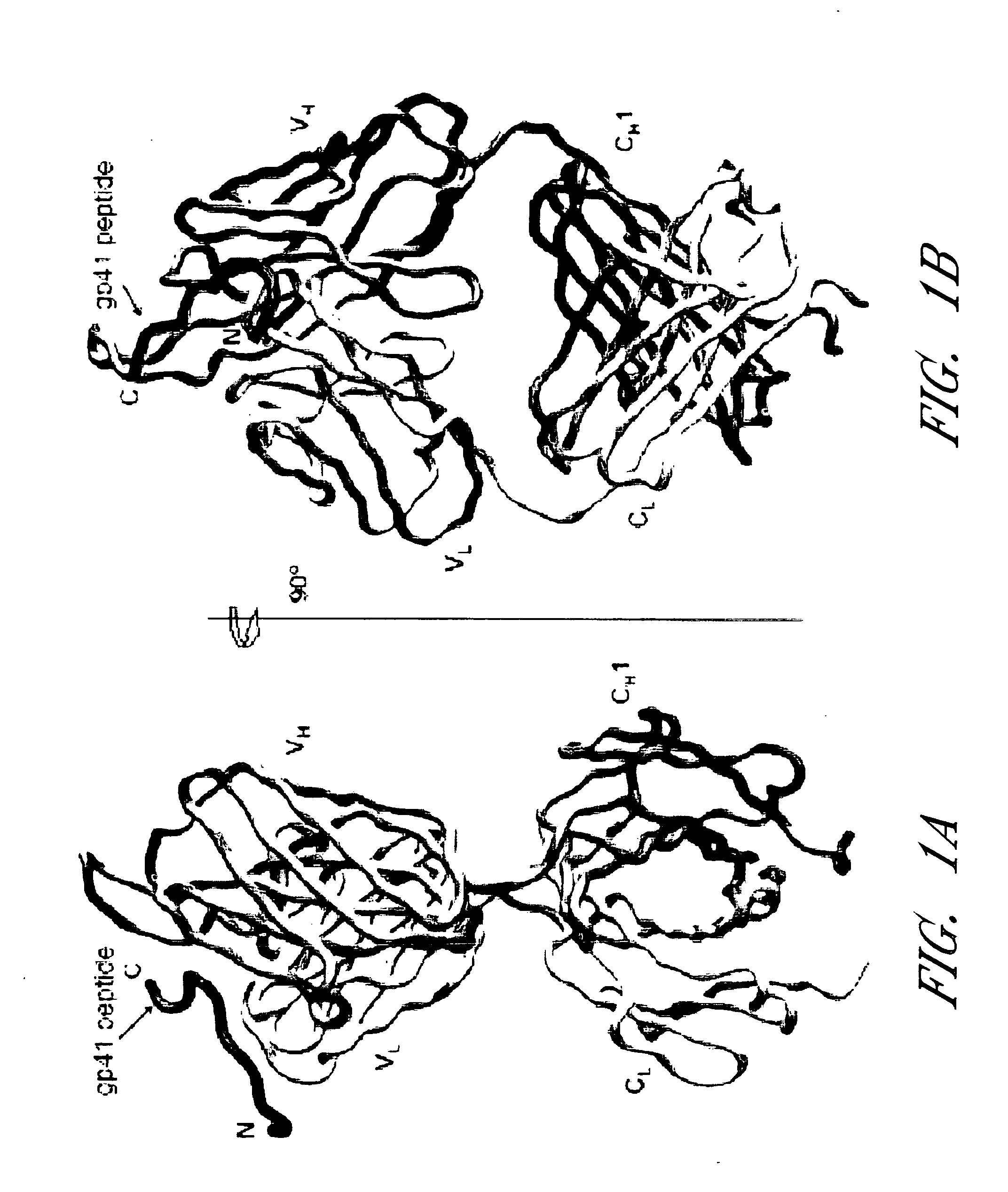
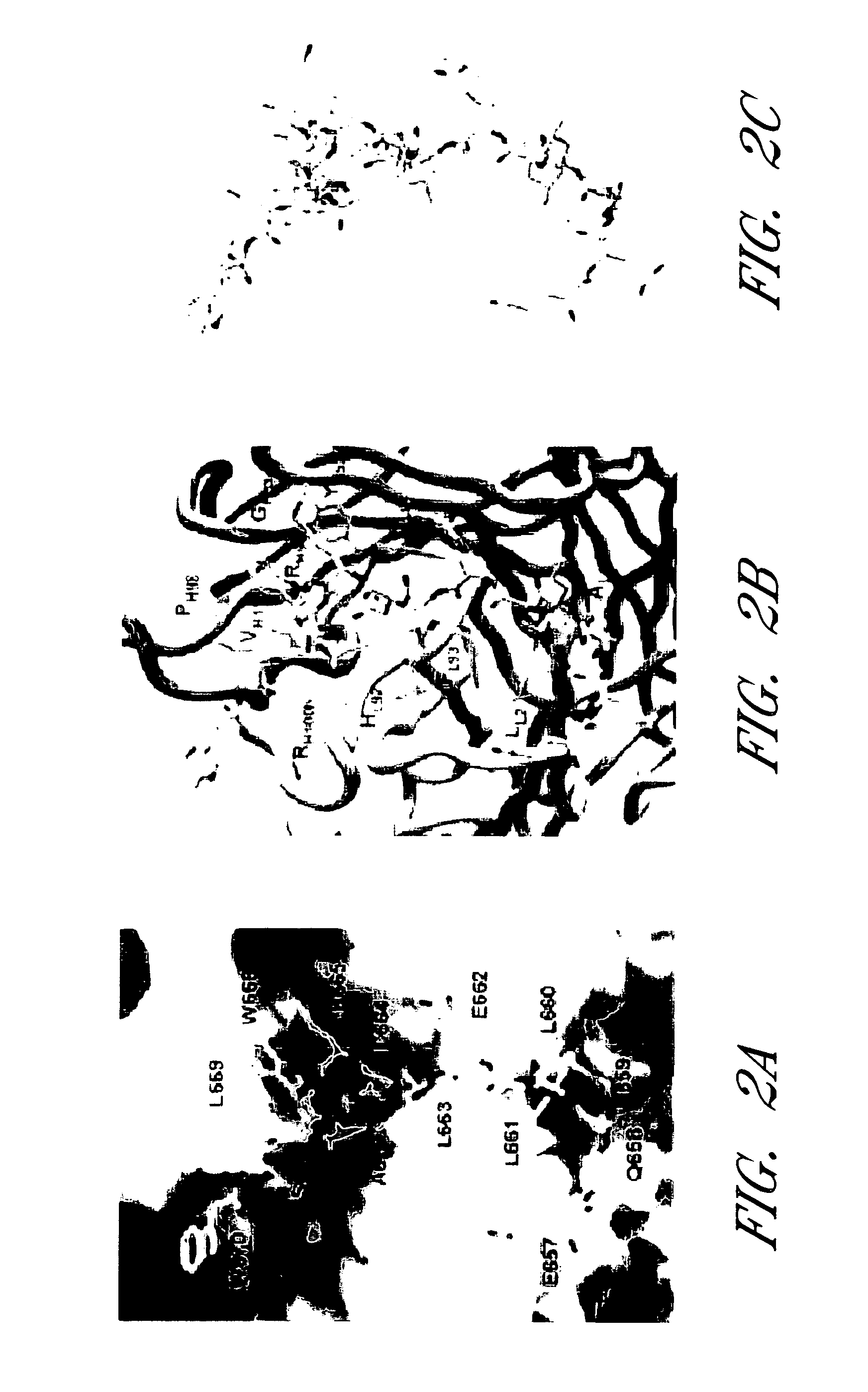
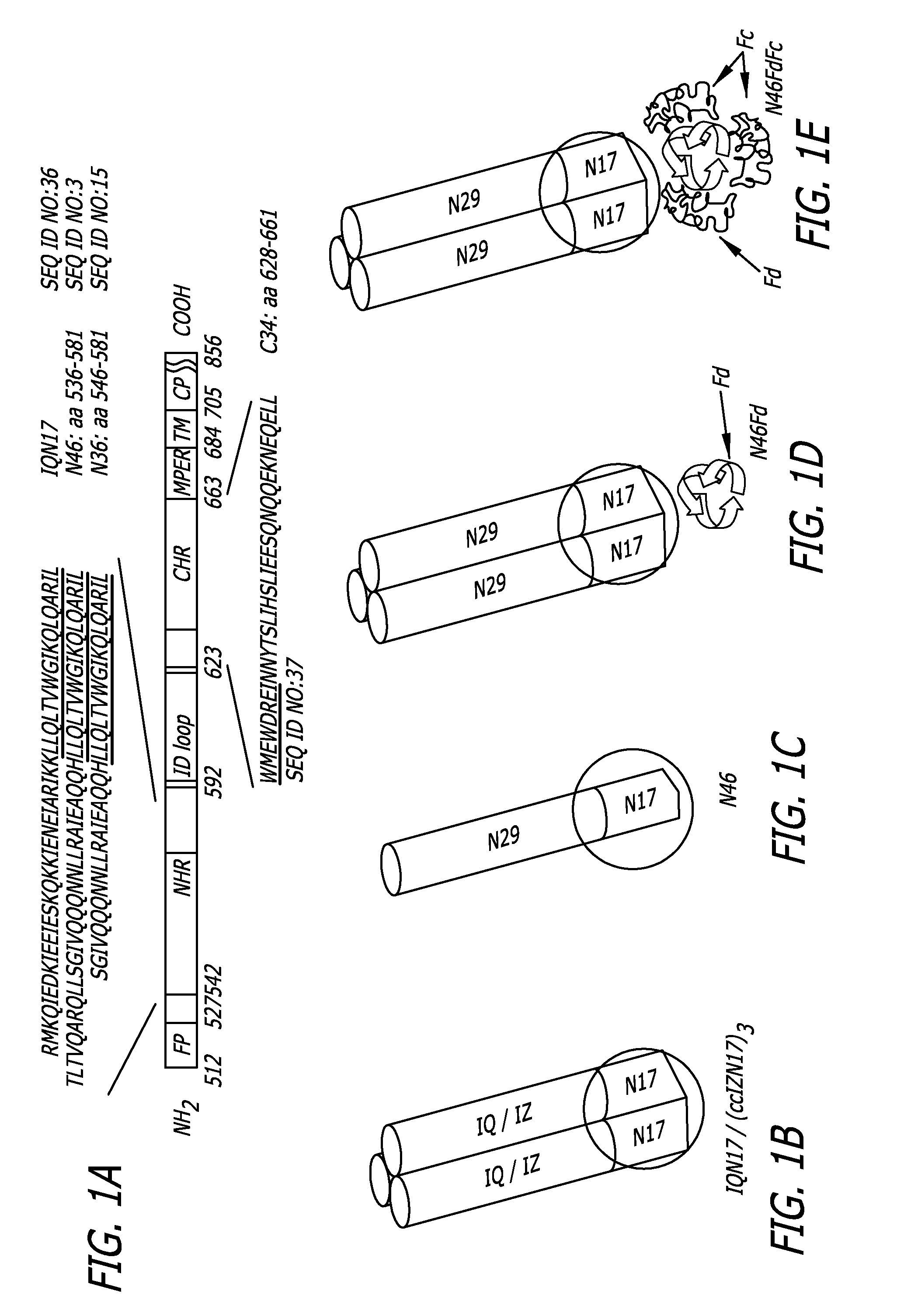
HIV vaccine immunogens and immunization strategies to elicit broadly-neutralizing anti-HIV-1 antibodies against the membrane proximal domain of HIV GP41
This invention relates to novel peptide immunogens that generate an immune response in mammals against HIV gp41, to pharmaceutical compositions that comprise such immunogens, and to methods of treating Immunodeficiency disease, especially HIV infection and AIDS, that employ such pharmaceutical compositions.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Immunoenhacer-linked oligomeric HIV vaccines
Provided herein are immunogenic compositions comprising fusion proteins, the fusion proteins comprising lentivirus gp41 or a fragment thereof, a trimerization or oligomerization motif and an immunoenhancer that elicit potent and broad HIV neutralizing antibody responses in the immunized hosts. Also disclosed are methods of making and using the immunogenic compositions.
Owner:NEW YORK BLOOD CENT
Porcine parvnvirus living vaccine and preparation method thereof
InactiveCN101745106AImproving immunogenicityExtended shelf lifeAntiviralsAntibody medical ingredientsRetention periodCost burden
The invention relates to a porcine parvnvirus living vaccine and a preparation method thereof. The living vaccine is prepared by adopting a PPVS-1A strain (the HA valence is not less than 29, and the TCID50 is not less than 107.0 / ml) to obtain a cell culture through being inoculated with a swine testis (ST) subculture cell growing well, adding a suitable stabilizing agent and carrying out freezing vacuum drying. The virus content of each first part of the living vaccine is not less than 105.0TCID50. Compared with the prior art, the porcine parvnvirus living vaccine has good immunogenicity, rapid antibody generation after immunization, high titer and long maintenance time of the generated antibody, long retention period and small immunizing dose. When the porcine parvnvirus living vaccine is used to carry out vaccine injection a plurality of weeks before hybridization, the pregnant sow can maintain strong immunizing power in the easy infection period. The porcine parvnvirus living vaccine is the excellent choice for preventing the porcine parvnvirus. The adopted preparation method has reasonable process and lower cost and greatly reduces the cost burden of the livestock breeding.
Owner:上海佳牧生物制品有限公司 +1
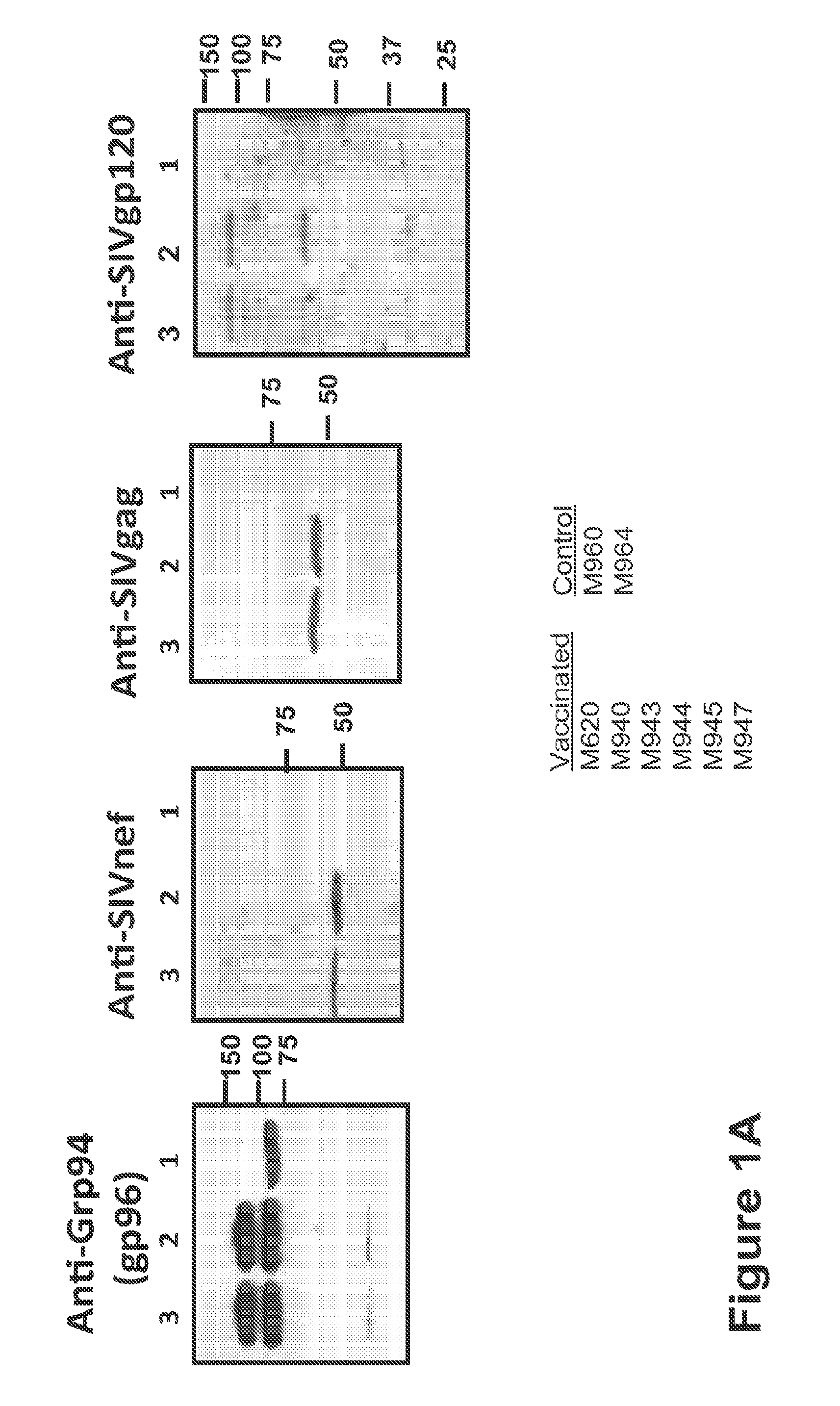
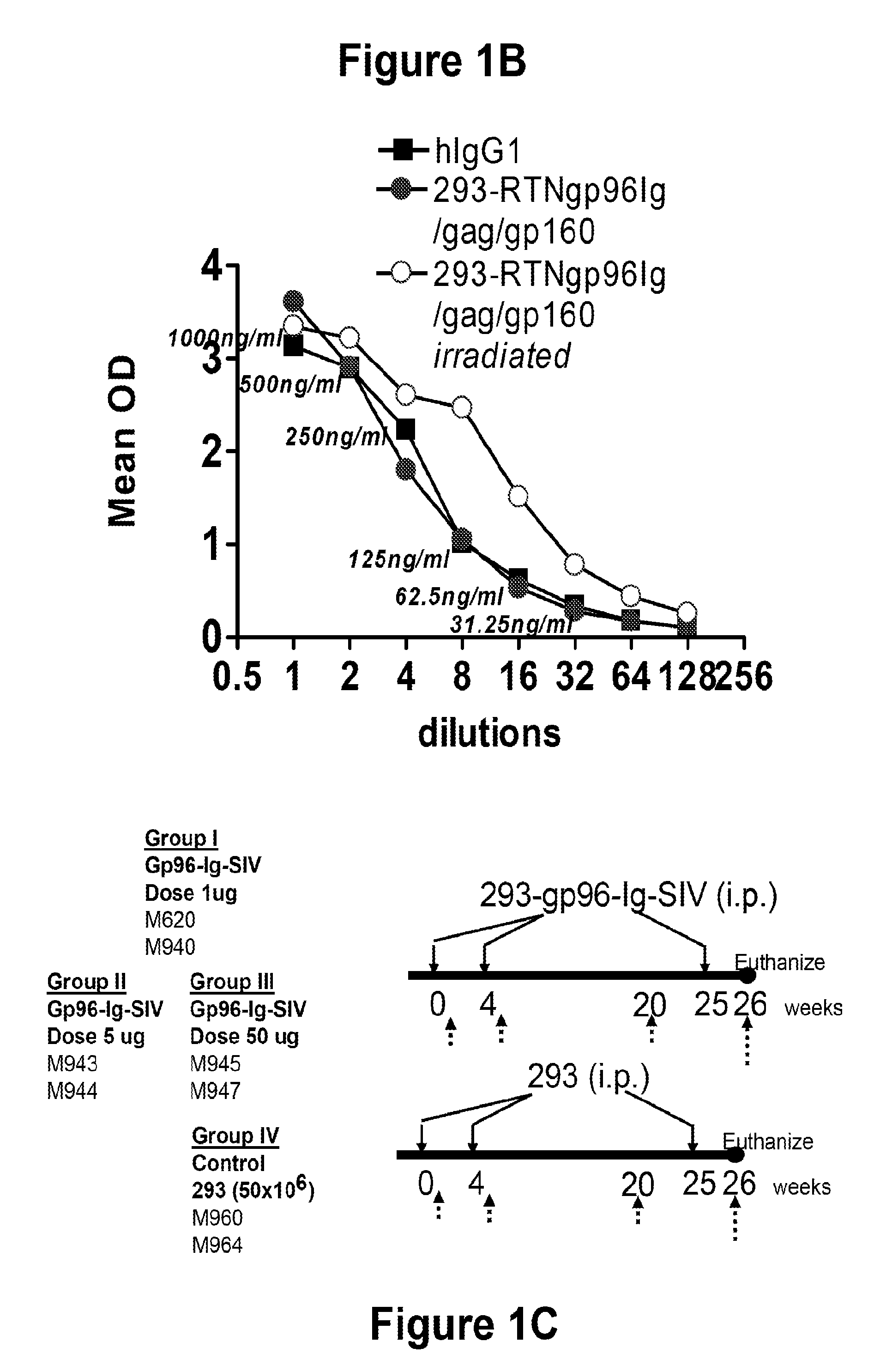
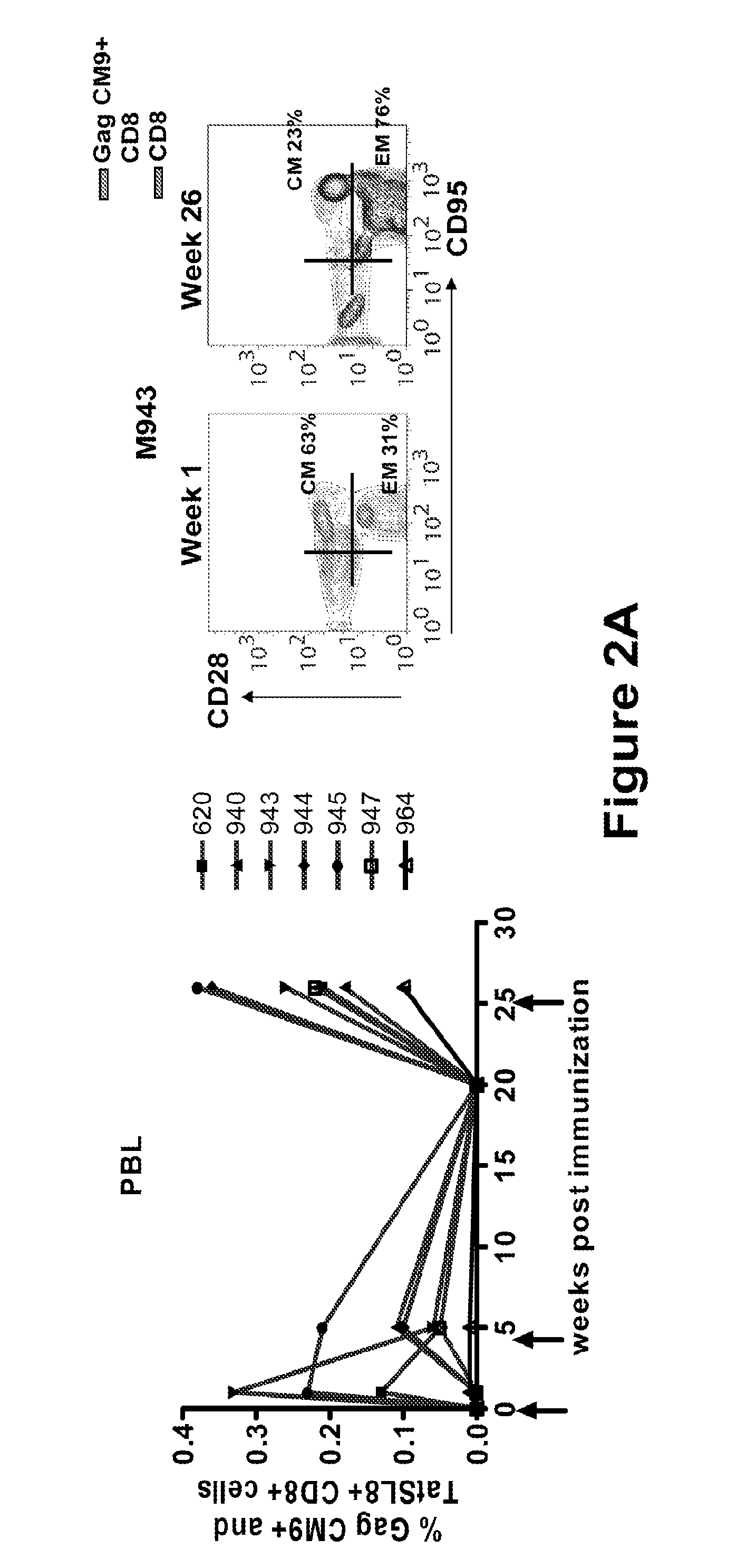
Hiv/siv vaccines for the generation of mucosal and systemic immunity
Compositions of genetically engineered, secreted gp96 (gp69-Ig) induced strong mucosal and systemic immune responses and CD8 expansion that was independent of CD4 help. Immunization of patients with gp96-Ig immunization is especially attractive for induction of mucosal and systemic immunity to SIV / HIV and other diseases.
Owner:UNIV OF MIAMI
Combined live vaccine against porcine reproductive and respiratory syndrome, swine fever and pseudorabies, and preparation method thereof
ActiveCN102727882AImprove immune efficiencyIncrease productionViral antigen ingredientsAntiviralsDiseaseHIV vaccine
The invention provides a combined live vaccine for preventing porcine reproductive and respiratory syndrome, swine fever and pseudorabies, and a preparation method and application thereof. According to the invention, no immunosuppression occurs among three vaccine strains of the combined live vaccine; the combined live vaccine is identical with each single vaccine in the aspects of security, immunogenicity, immunity duration and immuno-protective effects, but in the aspect of convenience, the combined live vaccine is more convenient than each single vaccine since prevention of three diseases is realized through only one immunization; thus, work load of immunization and inoculation is mitigated, stress on swinery is reduced, and immunological paralysis and immunological failure caused by frequent immunization are avoided, thereby achieving the effect of preventing porcine reproductive and respiratory syndrome, swine fever and pseudorabies.
Owner:华威特(江苏)生物制药有限公司
Human respiratory syncytial virus vaccine
ActiveUS9492525B2Improve immunityPromotes the normal antigen conformationSsRNA viruses negative-senseOrganic active ingredientsHuman respiratory virusHIV vaccine
Owner:NANOBIO CORP
HIV vaccine and method of use
InactiveUS20030220276A1Induce immune responseAntibody mimetics/scaffoldsGenetic material ingredientsReverse transcriptaseHIV vaccine
The present invention relates to a vaccine for immunization against HIV. The vaccine has DNA sequences encoding a plurality of viral proteins, including NEF, VPU and reverse transcriptase. The vaccine is rendered nonpathogenic by the disruption of the gene(s) encoding for at least one of these proteins.
Owner:UNIV KANSAS MEDICAL CENT
Preparation method for newcastle disease and infectious bursal disease bigeminal composite inactivated vaccine
ActiveCN103585626AImprove immunityImprove ability to prevent diseaseViral antigen ingredientsAntiviralsAntigenDisease
The invention discloses a preparation method for a newcastle disease and infectious bursal disease bigeminal composite inactivated vaccine and belongs to the technical field of biological products for veterinary use. The preparation method comprises preparation of a newcastle disease antigen liquid, preparation of an infectious bursal disease antigen liquid and preparation of the newcastle disease and infectious bursal disease bigeminal composite inactivated vaccine. The vaccine prepared by the invention has a dual characteristic of water in oil and oil in water and has the advantages of integration of a slow release function of the conventional vaccine and the jointed functions of a water-soluble immunopotentiator, Chinese herbal medicinal polysaccharide, an analgesic and the like. According to the preparation method, the immunity of an organism is greatly improved, and the humoral immunity is improved; the newcastle disease and infectious bursal disease bigeminal composite inactivated vaccine can stimulate the organism to generate efficient neutralizing antibodies, and the disease prevention ability of the organism is improved. According to the newcastle disease and infectious bursal disease bigeminal composite inactivated vaccine, adverse reactions of chickens caused by fractional immunization of multiple vaccines are avoided, the immunization procedure is simplified, and the investment cost is reduced. The vaccine preparation by the invention is suitable for chickens of all ages; one-day chicks are immunized, so that immunity failures of a live vaccine caused by maternal interference resistance or immunosuppression caused by high toxicity can be avoided; the newcastle disease and infectious bursal disease bigeminal composite inactivated vaccine is safely and effectively used.
Owner:浙江美保龙生物技术有限公司
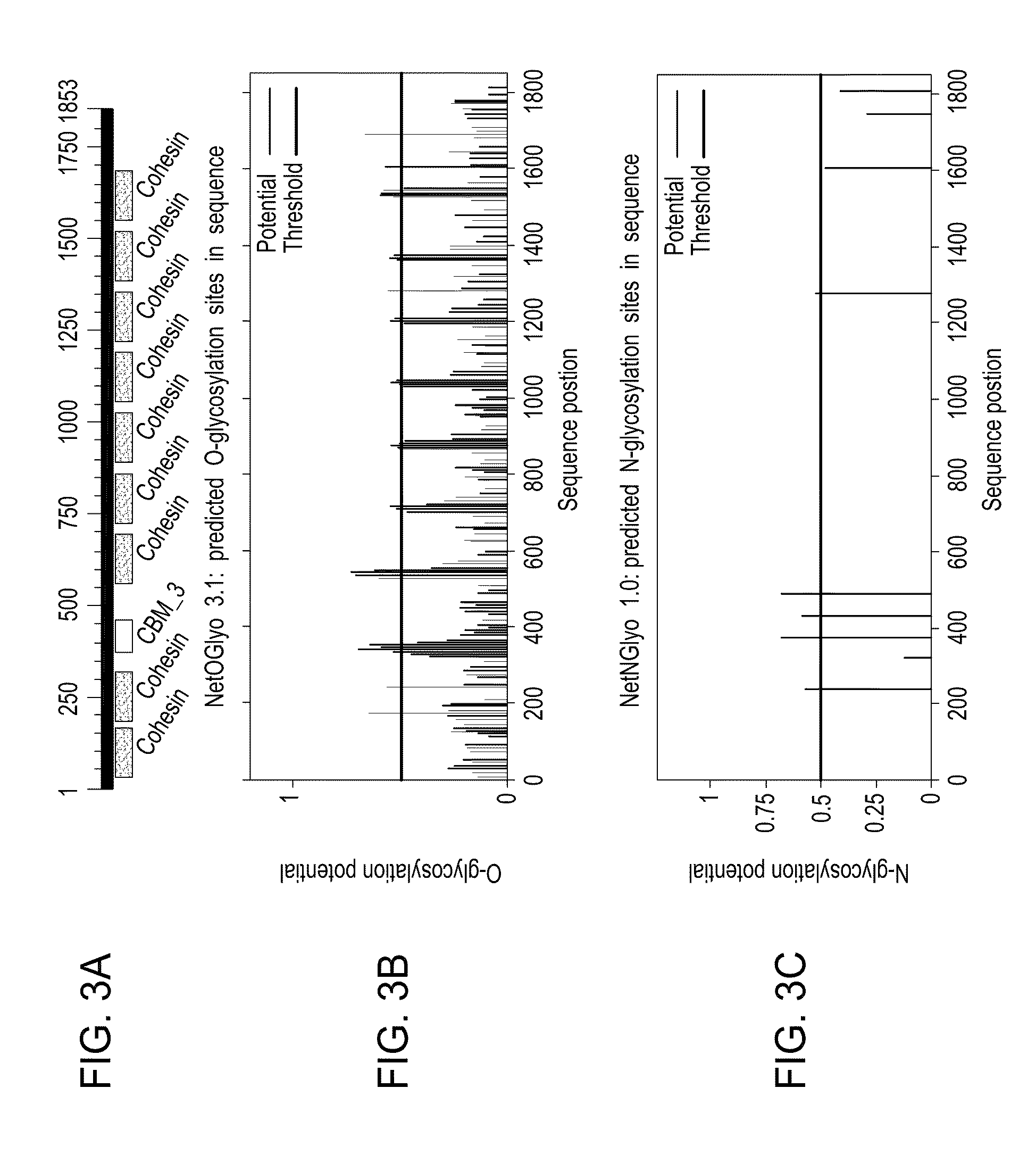
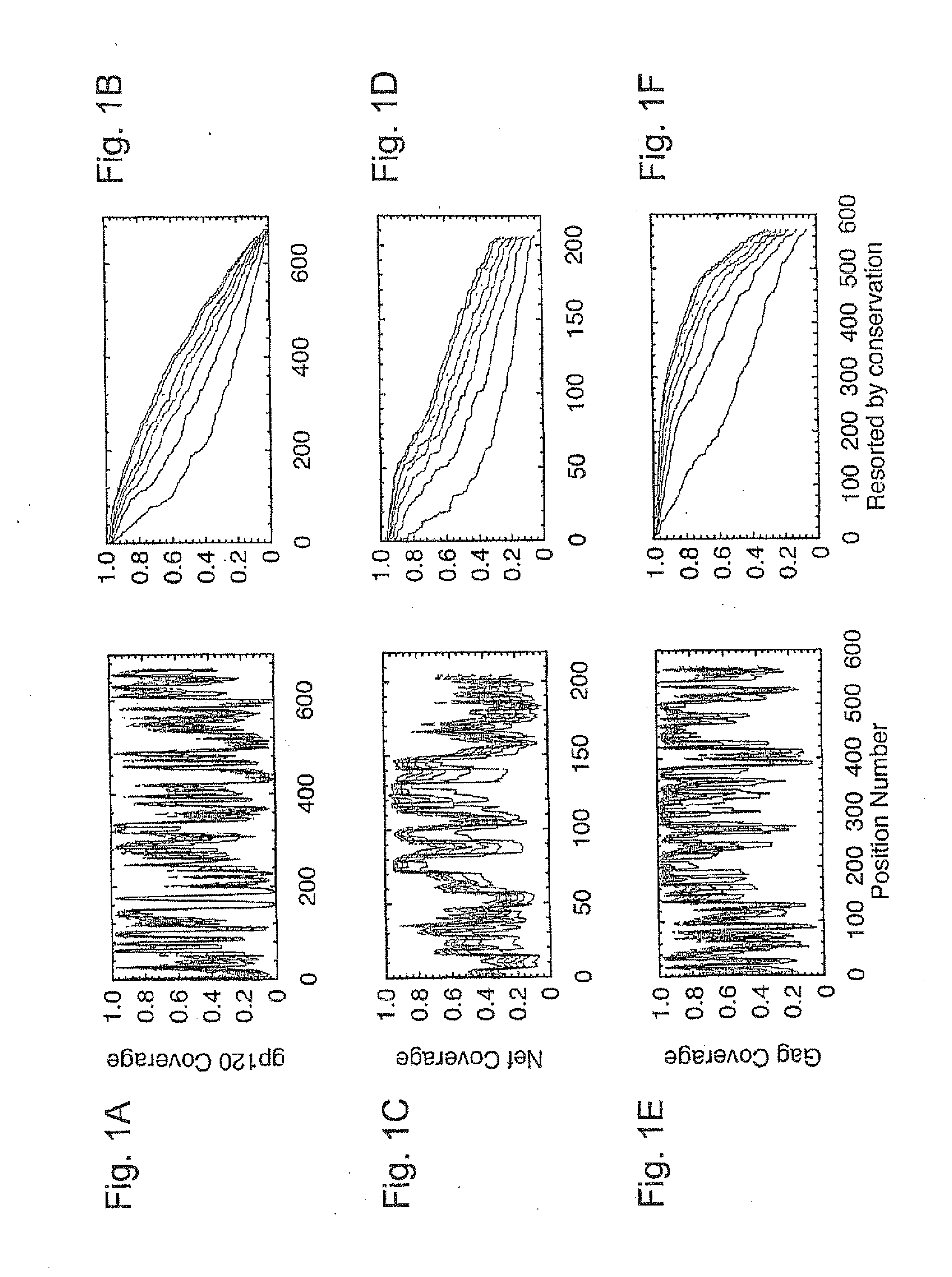
Broadly Representative Antigen Sequences and Method for Selection
InactiveUS20100183651A1Maximize overlapEliminate needPeptide/protein ingredientsVirus peptidesEpitopeNucleotide
A novel method for generating vaccine sequences is disclosed herein that preserves contiguous epitope length stretches of amino acids or nucleotides from an input pool of sequences. The method generates continuous, stepwise epitope consensus that together provides for a single globally optimized sequence. The end sequences are designed to maximize overlap between any potential epitope length sequence extract from a natural antigen sequence. The disclosed method, thus, allows one to maximize the number of potential natural epitopes that are mimicked in a resultant vaccine sequence. Various representative HIV vaccine sequences have been generated and are disclosed herein.
Owner:MERCK SHARP & DOHME CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com