HIV related peptides combination or fusion for use in HIV vaccine composition or as diagnostic means
A technology of action and immunogen, applied in the field of identifying and providing useful agents for the treatment and diagnosis, diagnostic and predictive reagents, capable of solving problems such as designing drugs and diagnostic/predictive methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0318] Combine or peptide using conventional methods for linear sequences
[0319] Preparation of APTKAKRRVVQREKR
[0320] The peptides were synthesized in amide form from the corresponding starting points according to the general description (hereinafter "Synthetic Overview") of F-moc synthesis (Atherton et al.
[0321] Purity (HPLC): greater than 90%.
[0322] Mass Spectrometry: Theoretical Molecular Weight: 1822.2
[0323] Experimental molecular weight: 1823.0ES+
[0324] Preparation of APTKAKR
[0325] The peptides were synthesized in amide form from the corresponding starting points according to the synthetic overview.
[0326] Purity (HPLC): greater than 90%.
[0327] Mass Spectrometry: Theoretical Molecular Weight: 769.6
[0328] Experimental molecular weight: 760.7ES+
[0329] Preparation of APTRAKR
[0330] The peptides were synthesized in amide form from the corresponding starting points according to the synthesis outline.
[0331] Purity (HPLC): greater tha...
Embodiment 2
[0444] Synthesis of complex peptides
[0445] Preparation of CGGAKRRVVGGAKRRVVGQREKRAV
[0446] |
[0447] CGGGDQQLLGGAEEEIVGGIEEEGGERDRD
[0448] Purity (HPLC): greater than 90%.
[0449] Theoretical molecular weight: 5750.4
[0450] Preparation of CGGAKRRVVGGAKRRVVGGQREKR
[0451] |
[0452] CGGGDQQLLGGAEEEIVGGIEEEGG
[0453] Purity (HPLC): greater than 90%.
[0454] Theoretical molecular weight: 4965.6
[0455] Preparation of CGGAEEEVVGGDQQLL
[0456] |
[0457] GCGGAKRRVVGGAKRRVV
[0458] Purity (HPLC): greater than 90%.
[0459] Theoretical molecular weight: 3410.9
[0460] Preparation:
[0461] GAKRRVVGG C GGAKRRVVQREKRAGEREKRA (SEQ ID NO: 38)
[0462] |
[0463] G K GGIEEEGGRDRDRGGEQDRDR (SEQ ID NO: 39)
[0464] Purity (HPLC): greater than 94%.
[0465]Theoretical molecular weight: 5876.93
Embodiment 3
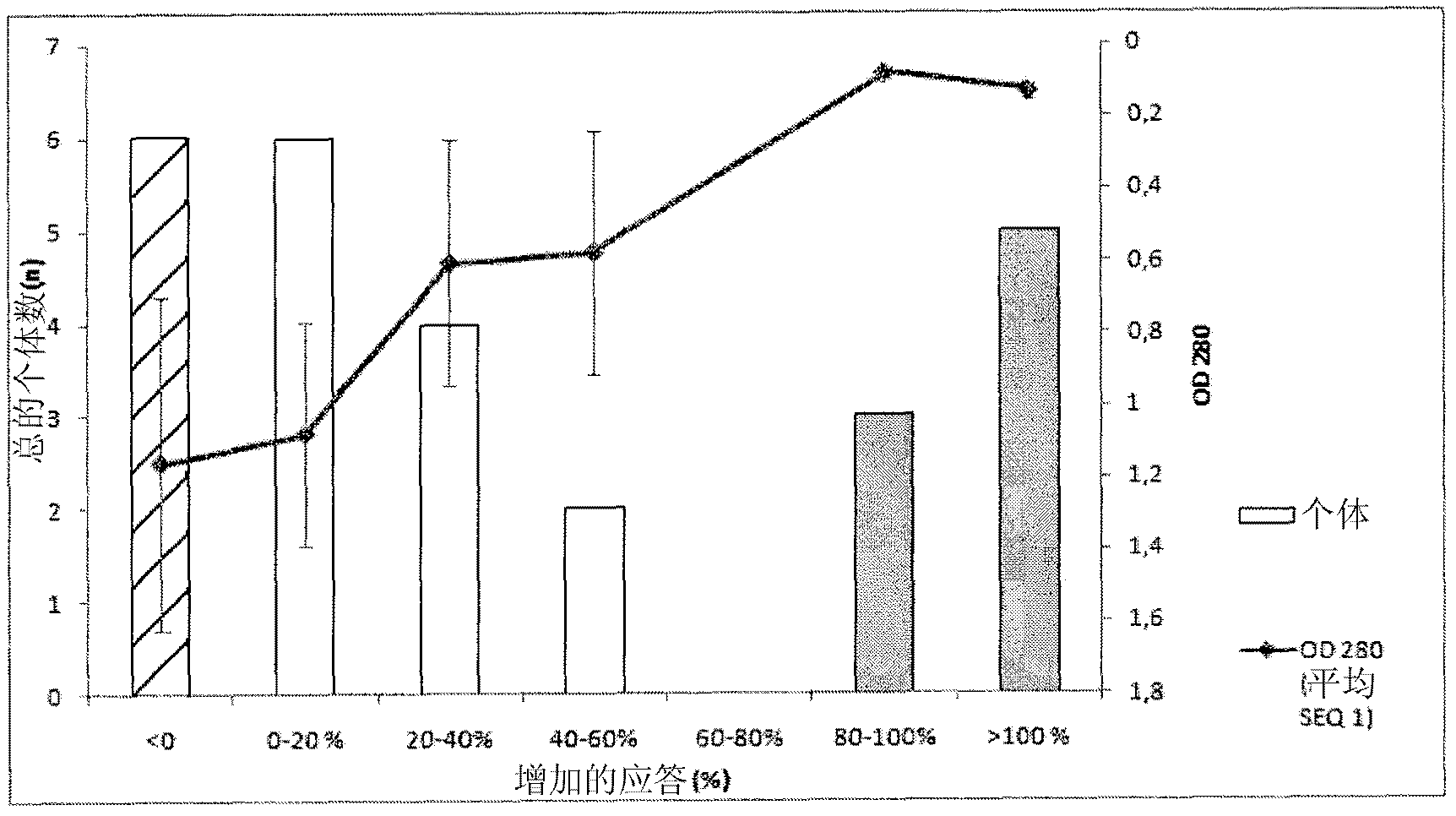
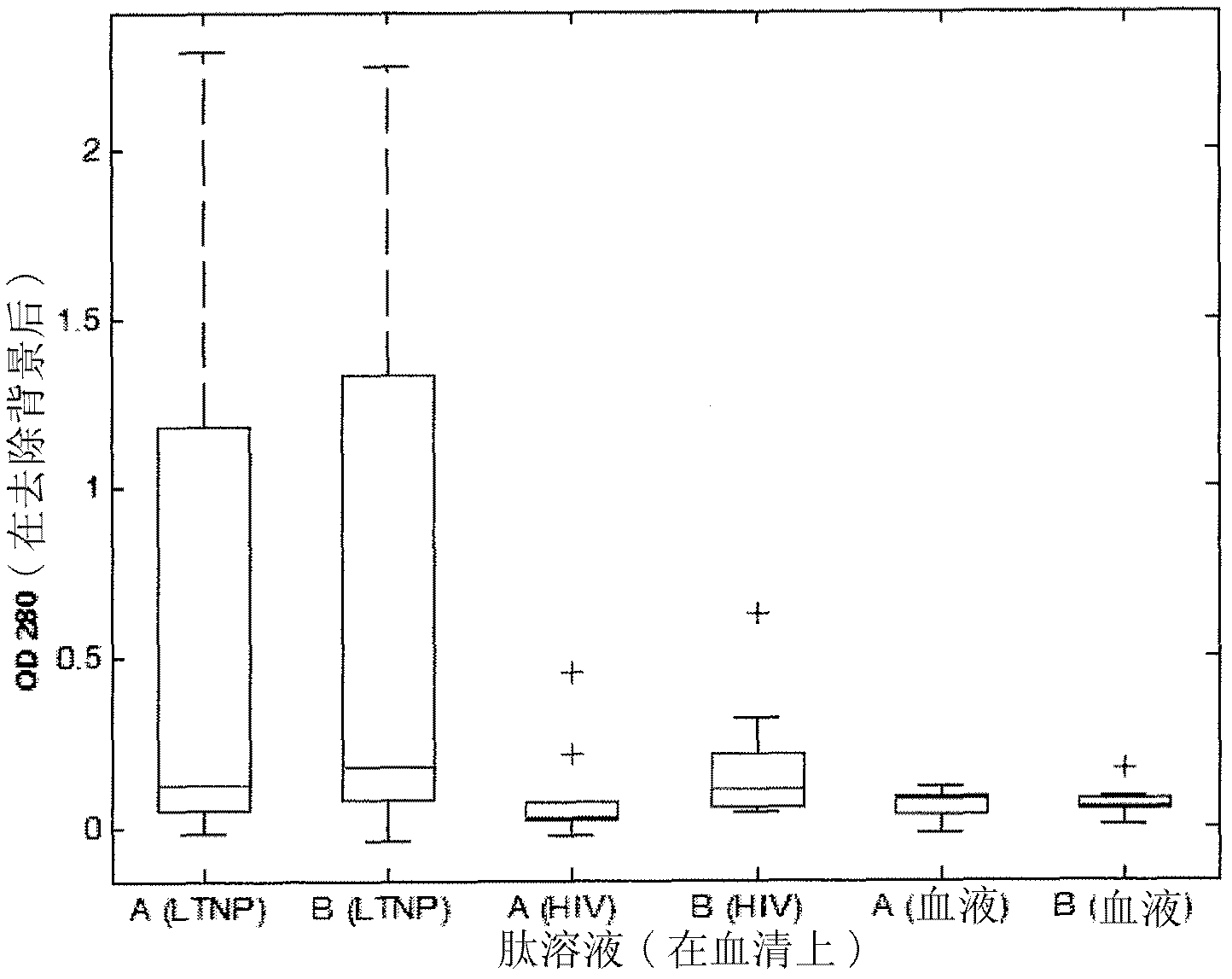
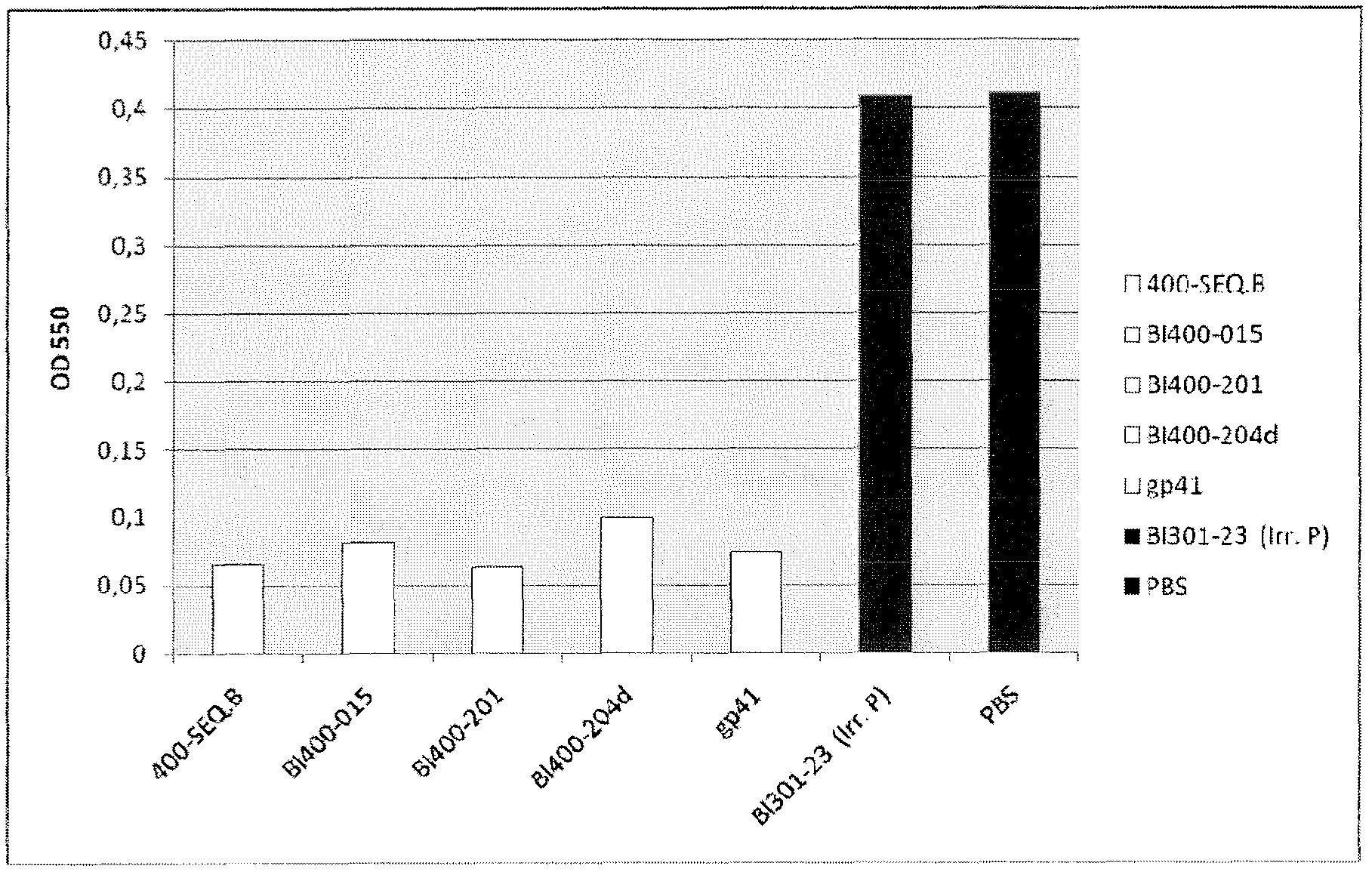
[0467] Recognition of SEQ ID NO: 1 alone and in combination with SEQ ID NO: 6, 8 and 9 by pooled human sera from HIV chronically infected individuals, LTNP and non-infected blood donors
[0468] Sera of SEQ ID NO: 1 alone or in combination with SEQ ID NO: 6 (having the sequence DRPEGIEEEEGGERDR), 8 and 9 were determined according to general ELISA principles using magnetic particles as solid support or immobilization of peptides to 96-well plates Reactivity (seroreactivity).
[0469] method:
[0470] In the system described below, peptides are coated on magnetic particles using recognized techniques. For all peptides, 300 μg was coated on the particles except for SEQ ID NO: 1 where 600 μg was used. SEQ ID NO: 1 (from C5) and SEQ ID NO: 6, 8 and 9 (from gp41) were pre-incubated overnight at 4°C to allow interactions between the C5 and gp41 sequences, respectively, and all combinatorially. Sera were then incubated with peptide-coated beads according to established protocols. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com