Combination vaccine against various HIVs and combination method thereof
A technology for HIV and combination vaccines, used in antiviral agents, pharmaceutical formulations, antibody medical ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
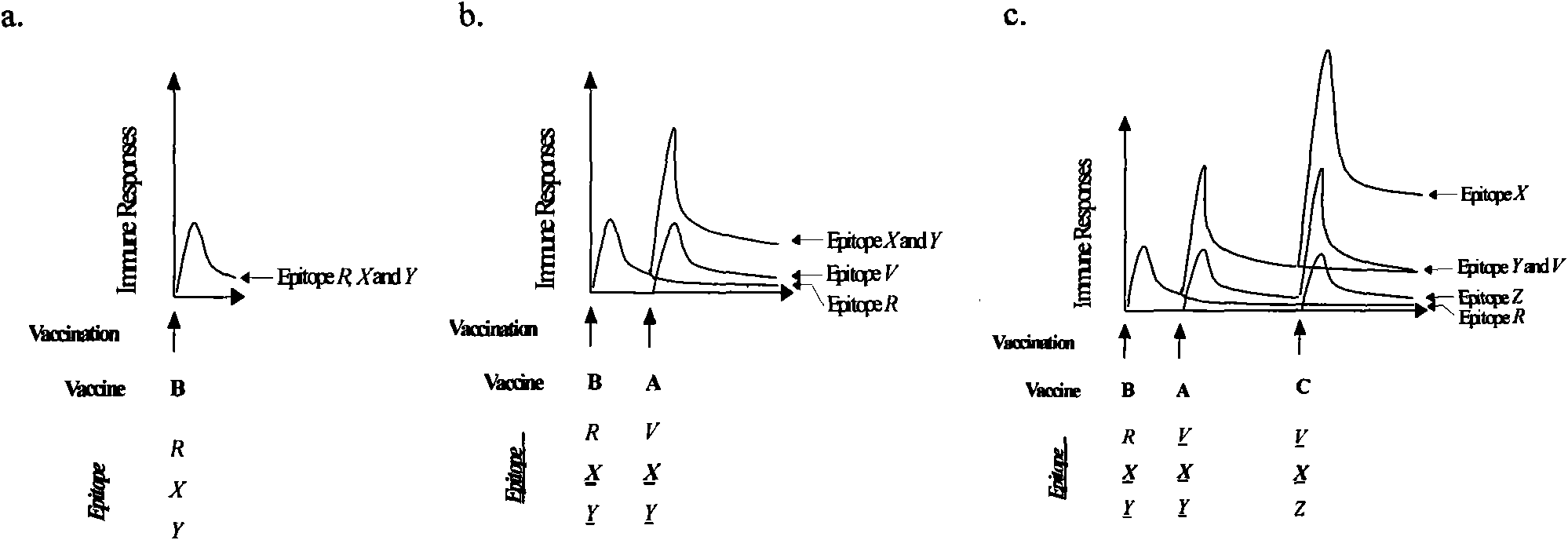
[0038] In a mouse model, the combination of gp140 and gp145 derived from the B' subtype activated a broad spectrum of neutralizing antibodies against multiple subtypes of HIV. After the recombinant DNA vaccine (named sv140 or sv145, inoculated three times) and recombinant vaccinia (named rTTV140 or rTTV145, inoculated once) were constructed, female BALB / c mice were immunized according to the procedures in Table 1, and 3 weeks after the immunization Neutralizing antibody testing was performed. In order to ensure the repeatability of the mouse neutralization experiment, equal volumes of serum from all mice in each vaccine immunization group were mixed for detection. Self-isolated HIV-1 virus strains (1 laboratory strain SF33, 1 HIV-1B` clinical isolate, 3 HIV-1B` / C clinical isolates) were used for neutralization test detection. The method refers to the operation procedure of Professor David C. Montefiori's laboratory at Duke University (Protocol for Measuring Neutralizing Antib...
Embodiment 2
[0043] Combining immunization with subtype C-derived gp140 and gp145 activated a broad spectrum of neutralizing antibodies against multiple subtypes of HIV in a mouse model. The method is as described in Example 1, and the results are shown in Table 2. The sv140+rTTV140 immunization group has no neutralizing activity against any HIV-1 virus strain; Neutralizing activity of subtype C isolate BC-1. The mouse sera from the two sequence cross-immunization groups showed high and broad-spectrum neutralizing antibody activity, especially the serum from the sv145+rTTV140 immunization group could neutralize the three viruses of laboratory strains SF33, BC-1, and BC-4 strain, and its titer can reach more than 1:60.
[0044] Table 2. Titers of sera of mice immunized with different combinations of vaccines at 50% virus suppression
[0045] HIV-1 strain
Embodiment 3
[0047] Combining gp140 and gp145 derived from subtype C immunization activated a broad spectrum of neutralizing antibodies against multiple subtypes of HIV in a guinea pig model. Method is as described in embodiment one, and result is as follows figure 2 As shown, the corresponding test results of serum collected at 6 weeks showed that all immunization groups had a certain neutralization effect, and half of the guinea pigs in all experimental groups could neutralize all isolates of HIV-1; but the sequential immunization group still neutralized The activity was better, about 3 / 4 guinea pigs in each group could neutralize all clinical isolates.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com