Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
70 results about "Human respiratory virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Page issues. Human respiratory syncytial virus (HRSV) is a syncytial virus that causes respiratory tract infections. It is a major cause of lower respiratory tract infections and hospital visits during infancy and childhood.
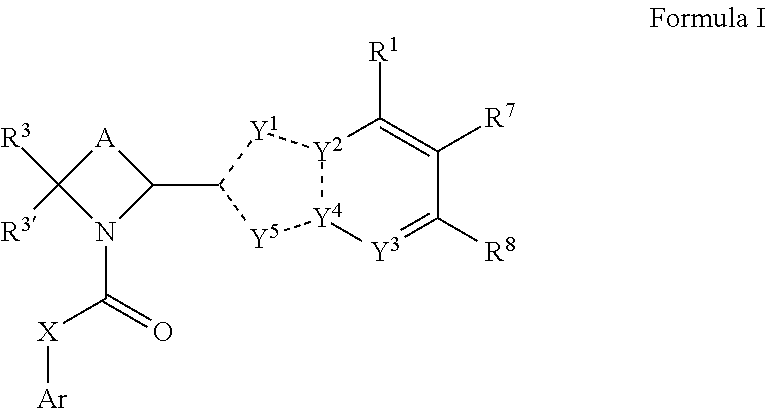
Methods and compounds for treating paramyxoviridae virus infections
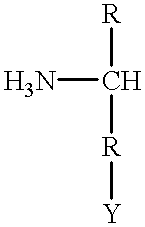
Provided are methods for treating Paramyxoviridae virus infections by administering ribosides, riboside phosphates and prodrugs thereof, of Formula I:wherein the 1′ position of the nucleoside sugar is substituted. The compounds, compositions, and methods provided are particularly useful for the treatment of Human parainfluenza and Human respiratory syncytial virus infections.
Owner:GILEAD SCI INC
Methods and compounds for treating paramyxoviridae virus infections
Provided are methods for treating Paramyxoviridae virus infections by administering ribosides, riboside phosphates and prodrugs thereof, of Formula I:wherein the 1′ position of the nucleoside sugar is substituted. The compounds, compositions, and methods provided are particularly useful for the treatment of Human parainfluenza and Human respiratory syncytial virus infections.
Owner:GILEAD SCI INC
Compounds and methods for antiviral treatment
Compounds and pharmaceutically acceptable salts and esters and compositions thereof, for treating viral infections are provided. The compounds and compositions are useful for treating Pneumovirinae virus infections. The compounds, compositions, and methods provided are particularly useful for the treatment of Human respiratory syncytial virus infections.
Owner:GILEAD SCI INC
Compounds and methods for antiviral treatment
Compounds and pharmaceutically acceptable salts and esters and compositions thereof, for treating viral infections are provided. The compounds and compositions are useful for treating Pneumovirinae virus infections. The compounds, compositions, and methods provided are particularly useful for the treatment of Human respiratory syncytial virus infections.
Owner:GILEAD SCI INC
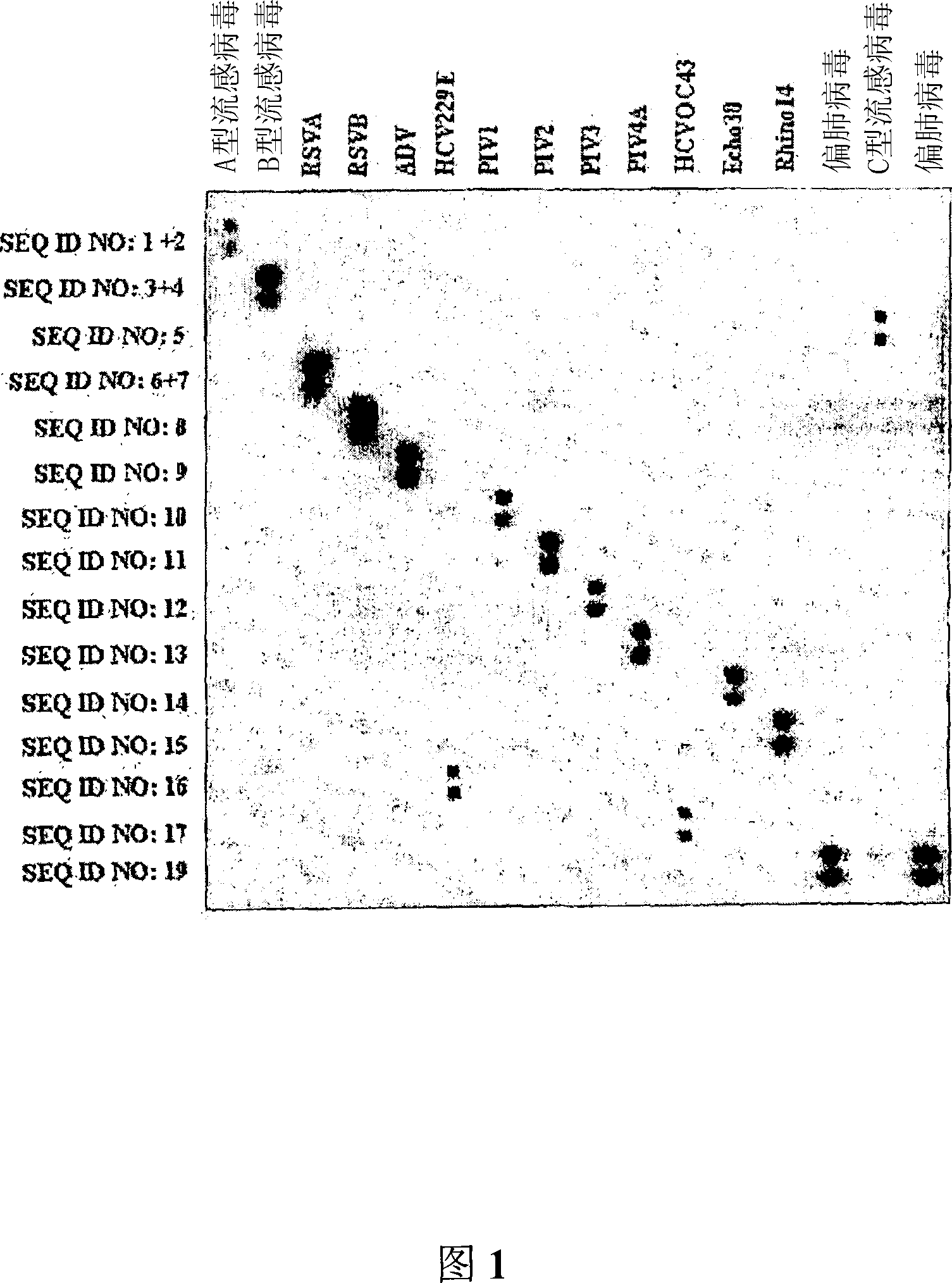
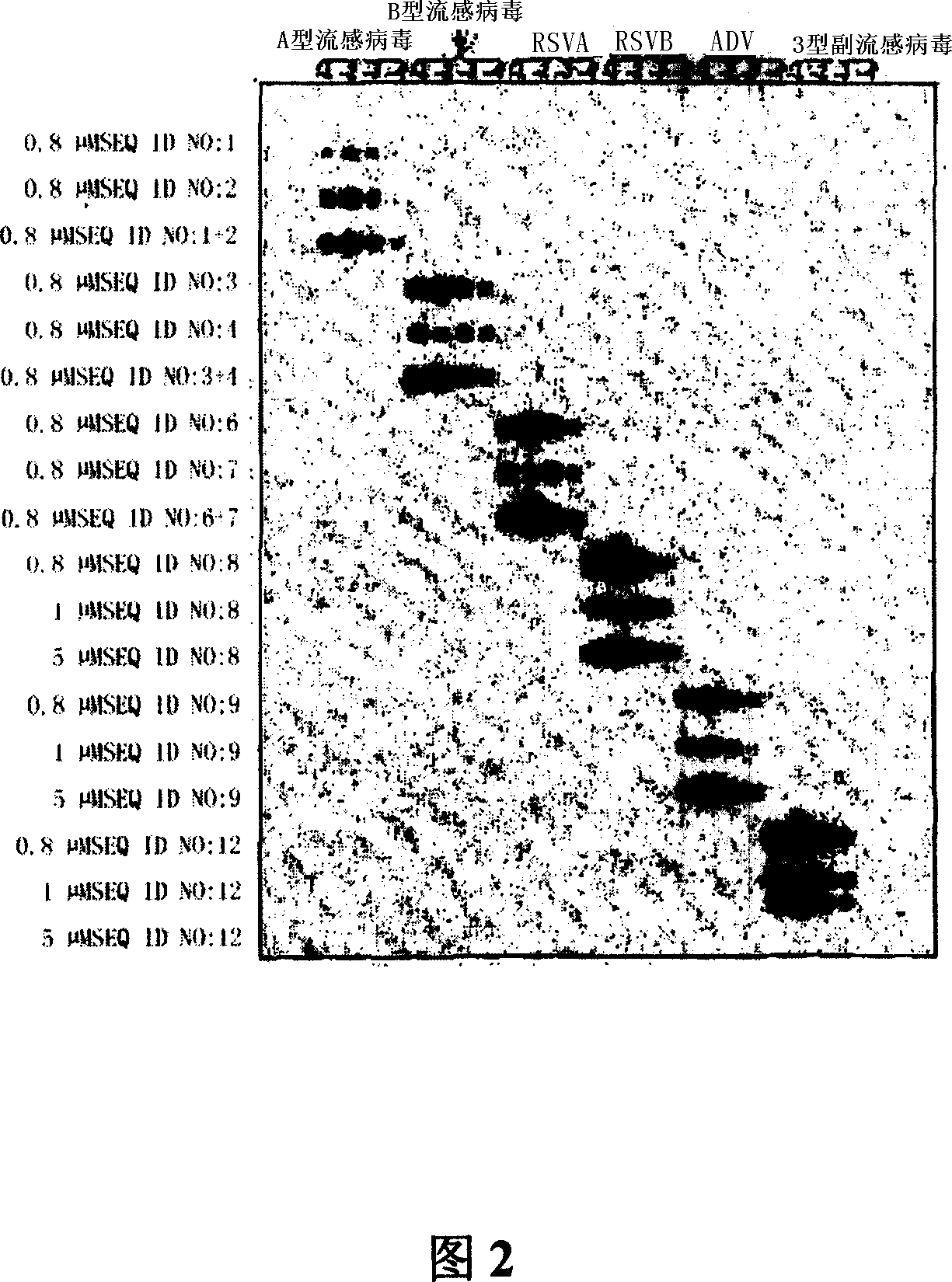
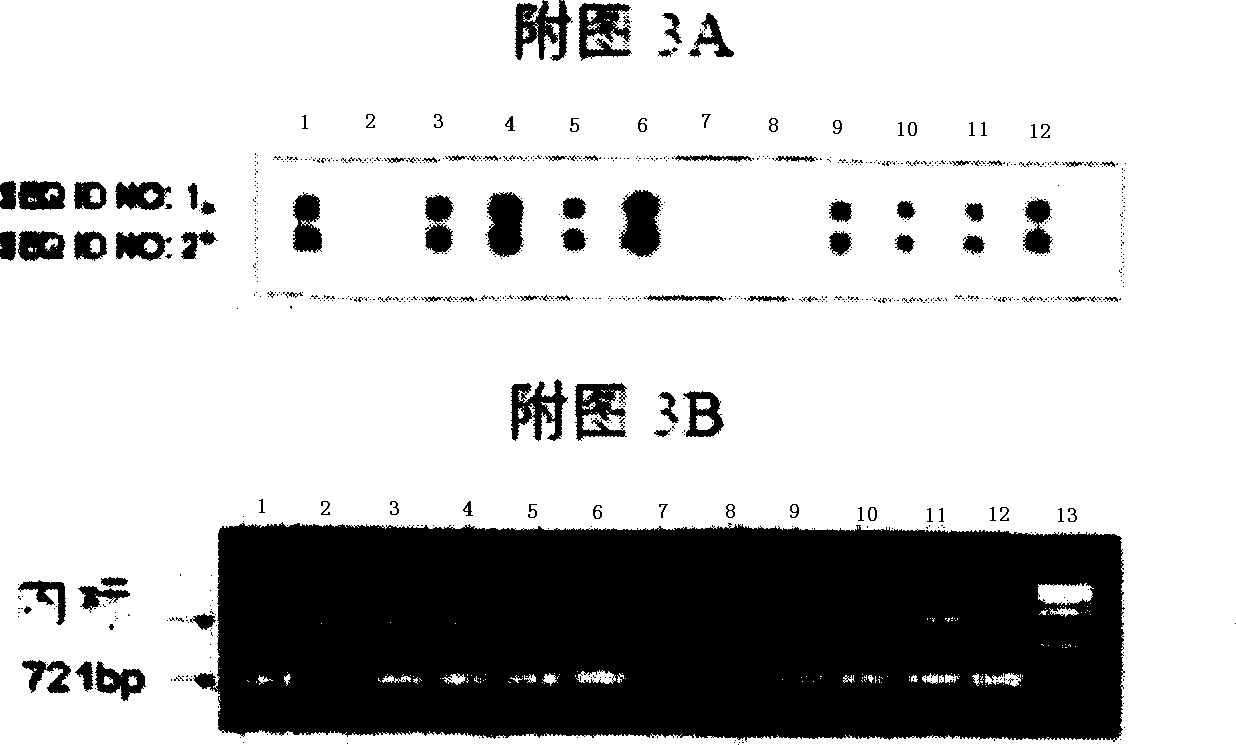
Nucleic acid sequences for the amplification and detection of respiratory viruses
InactiveUS20100279273A1Improve the situationSsRNA viruses negative-senseSsRNA viruses positive-senseSpecific detectionHuman respiratory virus
The present invention relates to methods of detection, as well as assays, reagents and kits for the specific detection of 15 clinically important respiratory viruses including influenza A and B viruses, human respiratory syncytial viruses, human metapneumoviruses, human enteroviruses, all serotypes of rhinoviruses, 7 serotypes of adenoviruses, parainfluenza viruses types 1, 2, 3, and 4, as well as coronaviruses NL, 229E, OC43, and SARS-CoV. The present invention allows for the detection of each of these respiratory viruses in a single assay.
Owner:UNIV LAVAL
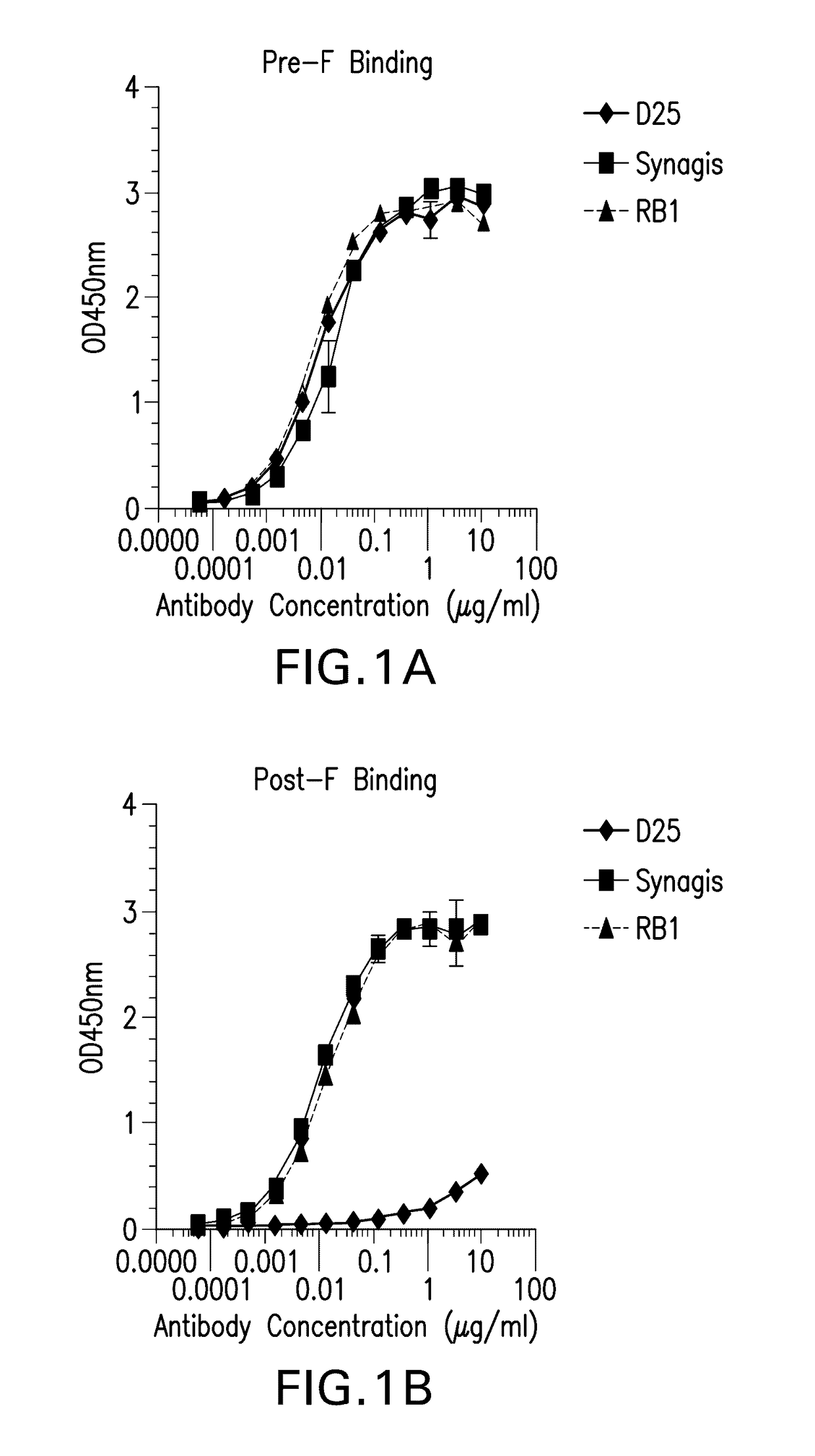
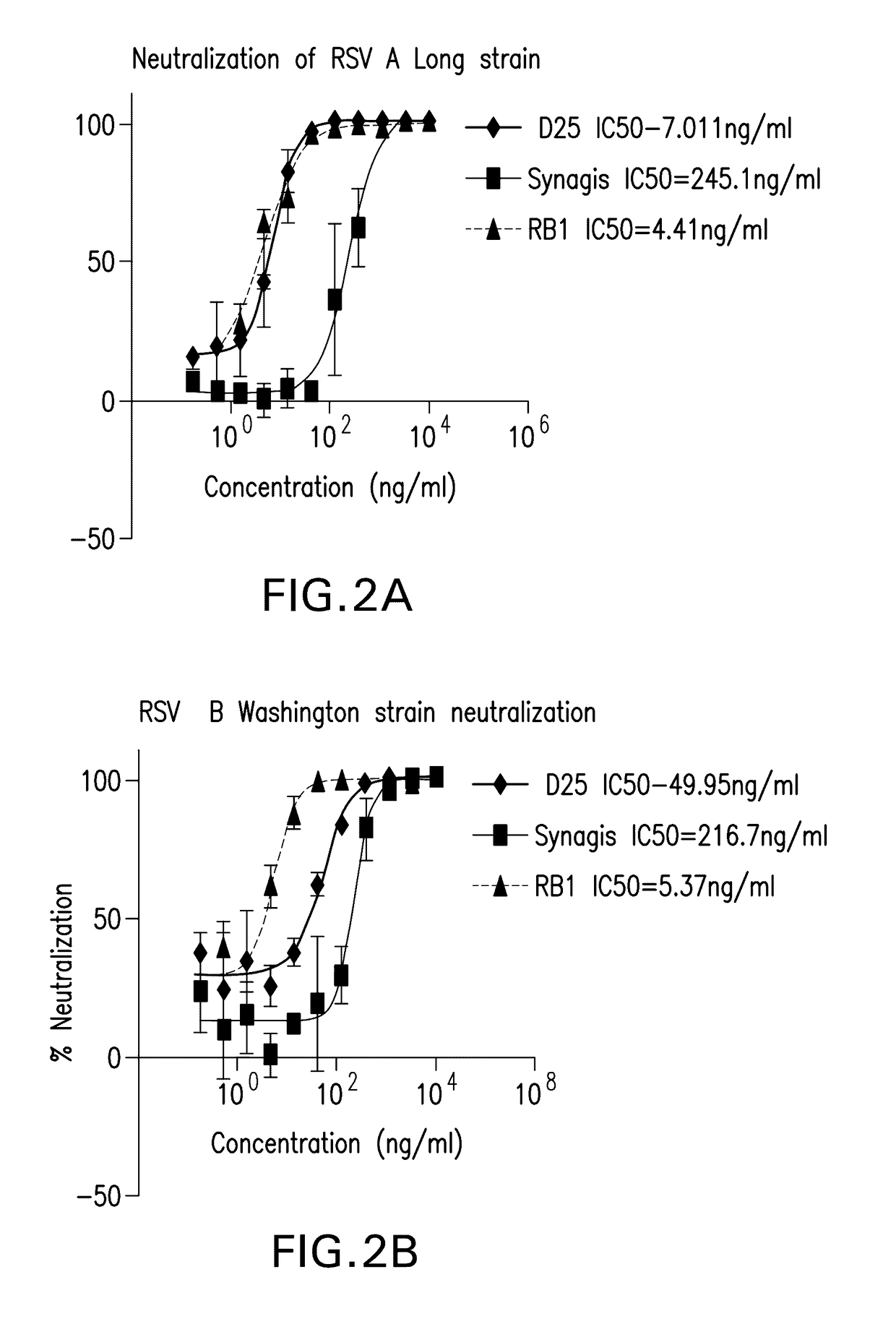
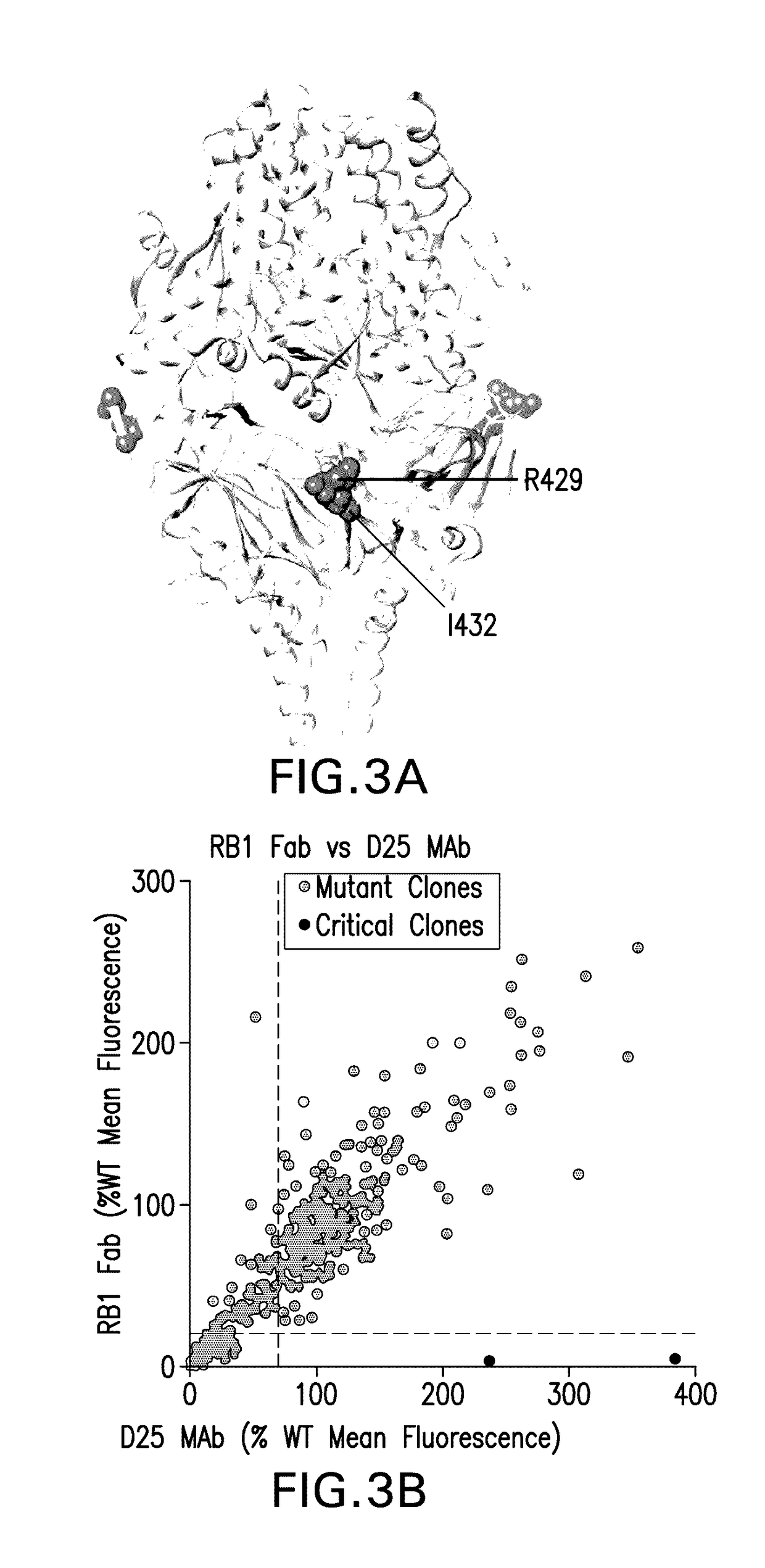
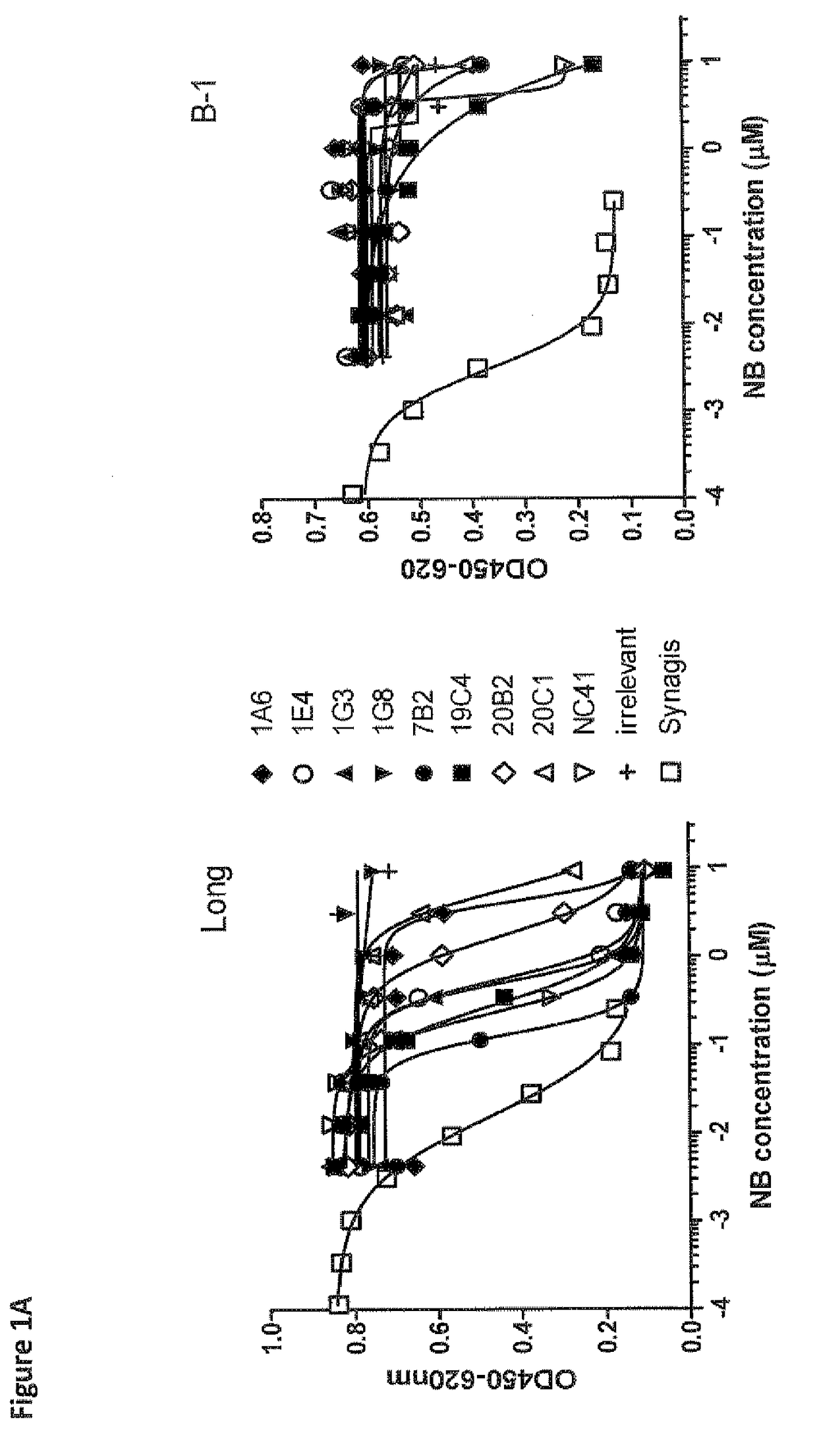
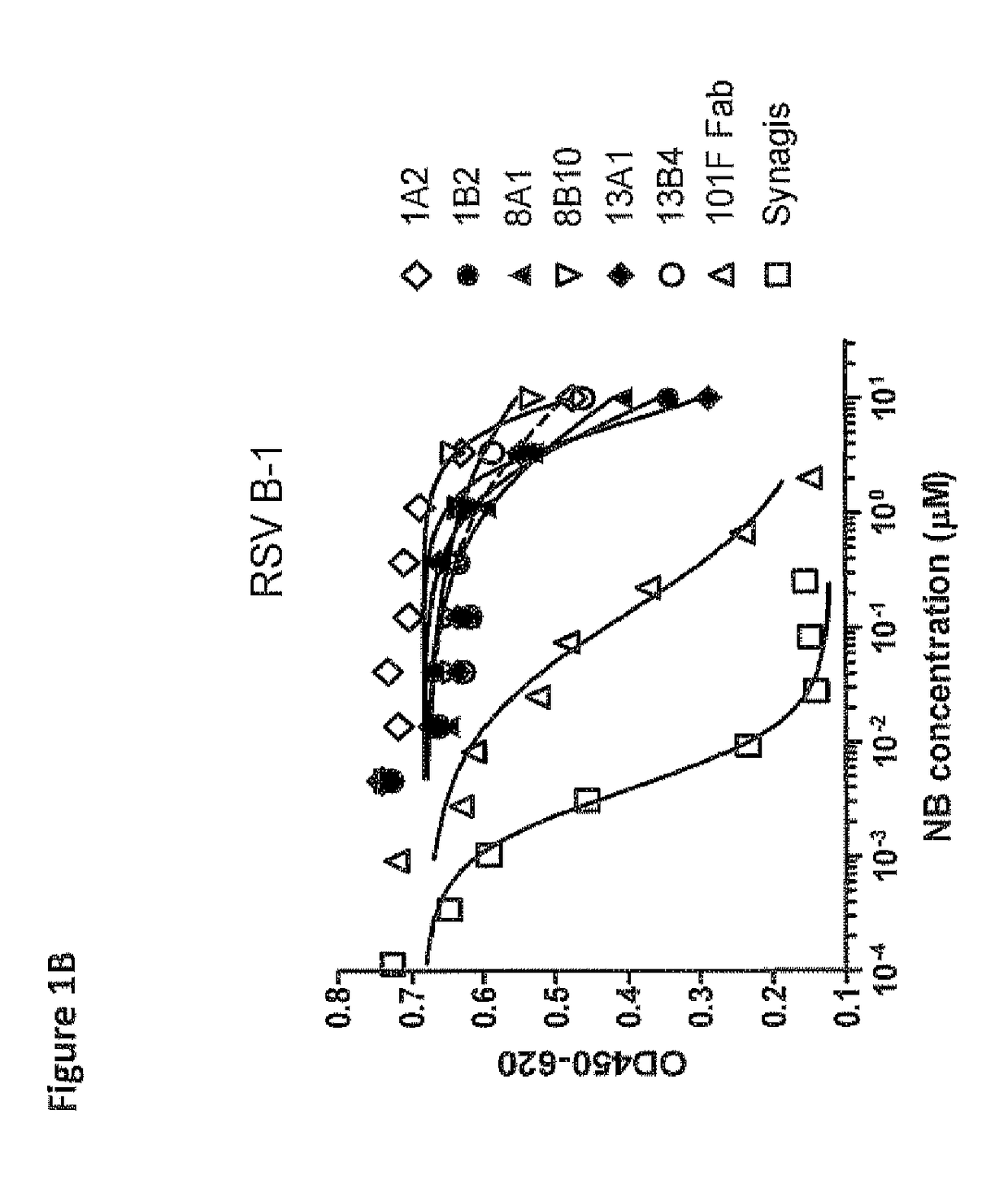
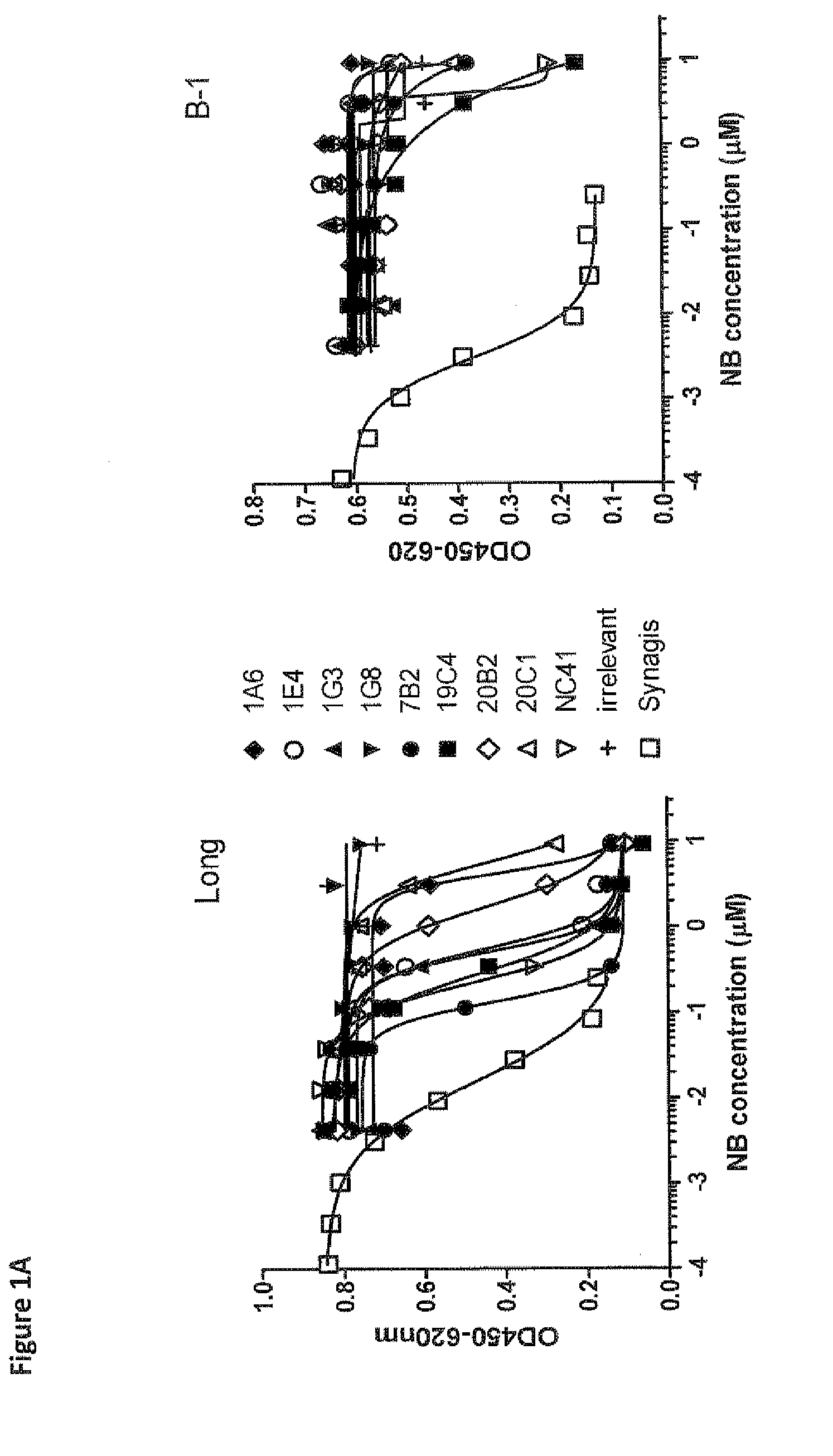
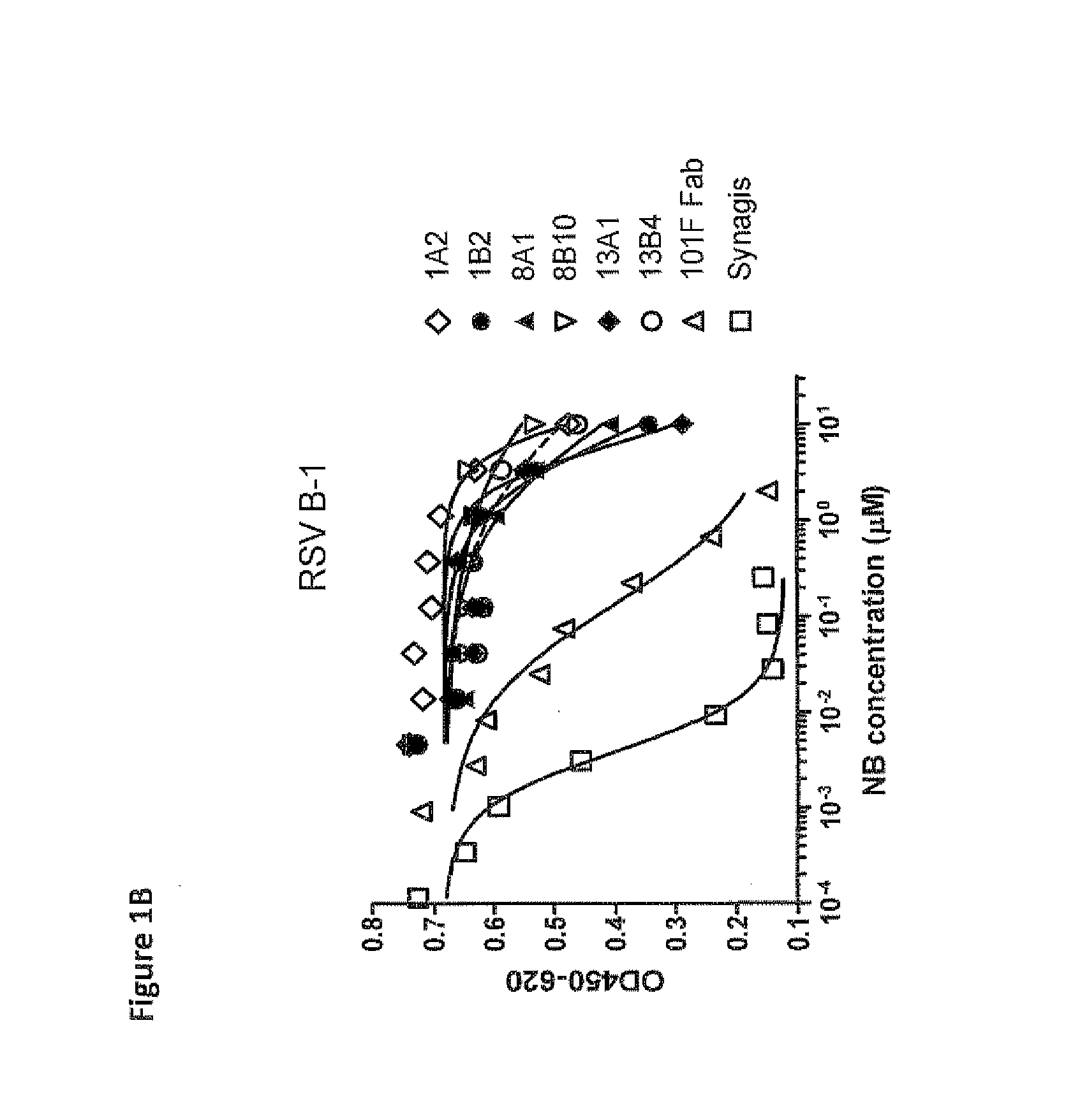
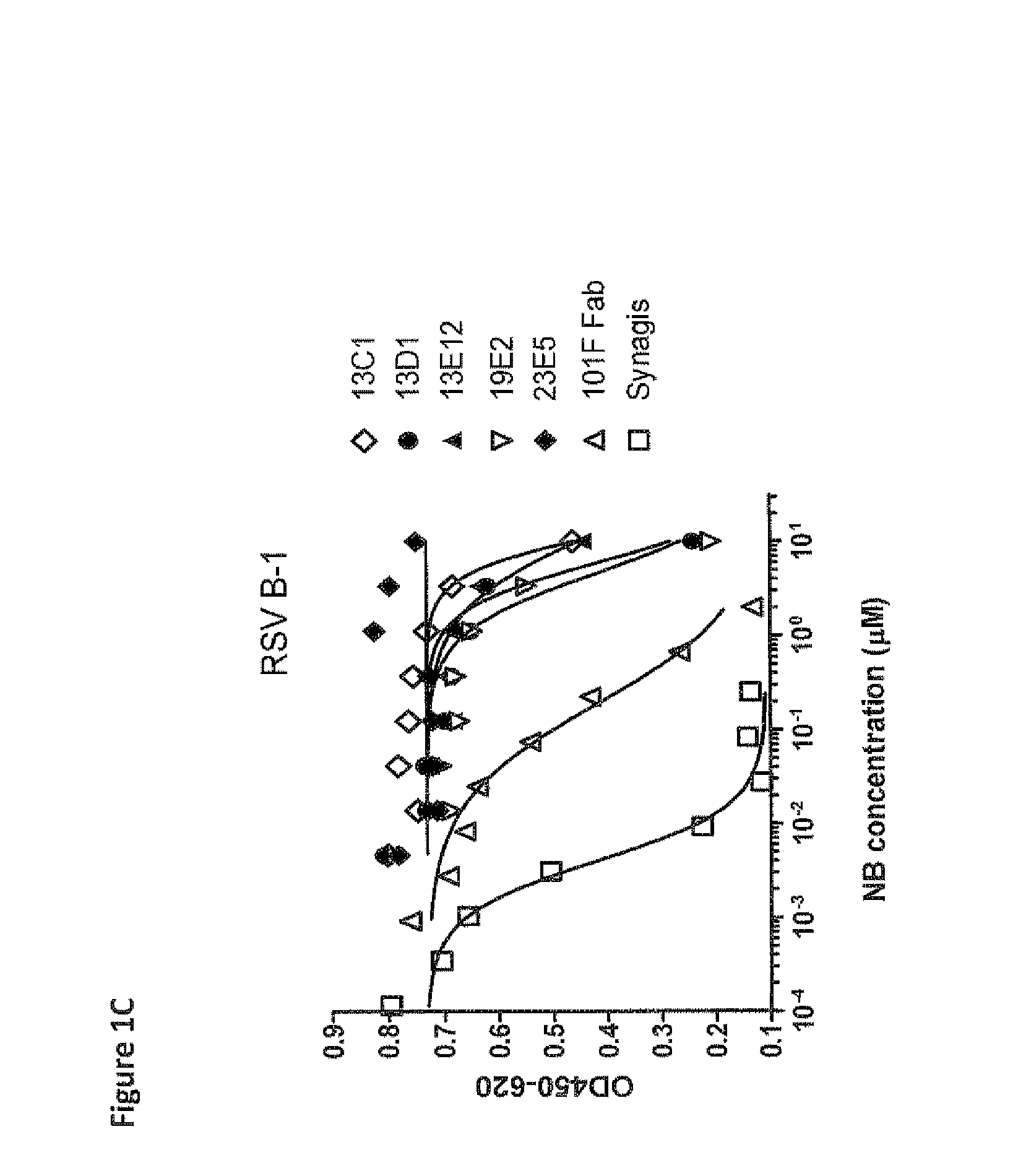
Antibodies against human respiratory syncytial virus (RSV) and methods of use
InactiveCN102656189AOrganic active ingredientsPeptide/protein ingredientsDiseaseAntigen Binding Fragment
Provided herein are antibodies or antigen-binding fragments thereof that immunospecifically bind to the fusion (F) protein of Respiratory Syncytial Virus (RSV). Also provided are methods for of prevention, treatment and diagnosis of viral infection and / or the treatment of one more symptoms of RSV-mediated disease. Methods of generating antibodies that immunospecifically bind RSV F protein also are provided.
Owner:JANSSEN VACCINES & PREVENTION BV
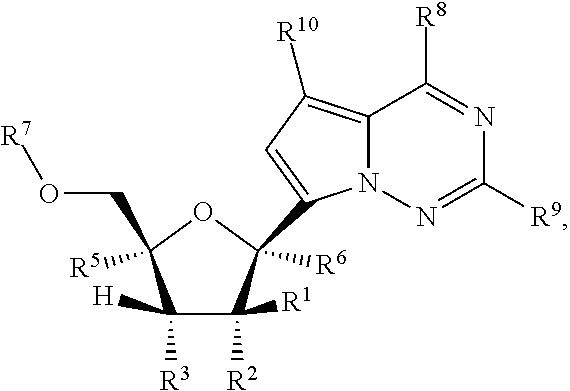
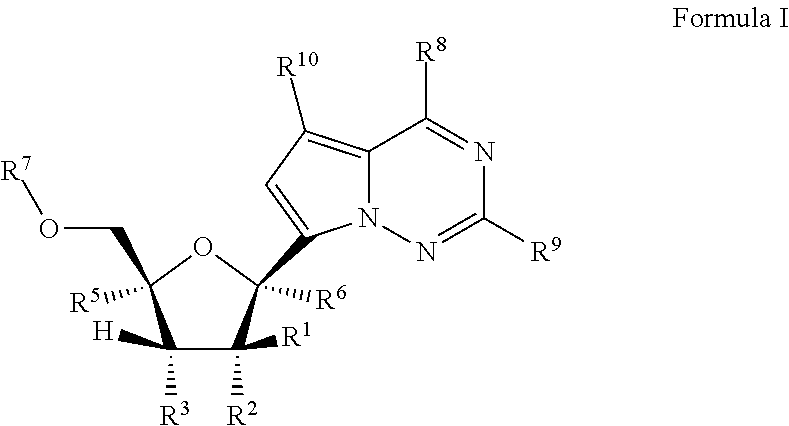
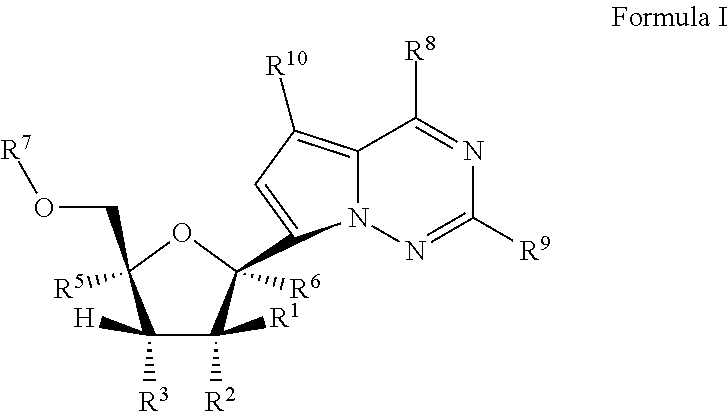
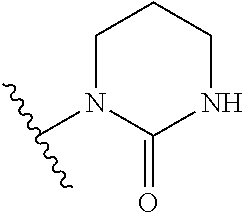
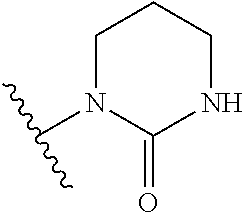
Pyrazolo[1,5-a]pyrimidines for antiviral treatment
The invention provides compounds of Formula I or Formula II:or a pharmaceutically acceptable salt or ester, thereof, as described herein. The compounds and compositions thereof are useful for treating Pneumovirinae virus infections. The compounds, compositions, and methods provided are particularly useful for the treatment of Human respiratory syncytial virus infections.
Owner:GILEAD SCI INC
Multiplex fluorescence quantitative PCR kit for detecting 19 human respiratory viruses
PendingCN110468234AStrong specificityAccurate Diagnostic InformationMicrobiological testing/measurementMicroorganism based processesFluorescenceViral Respiratory Tract Infection
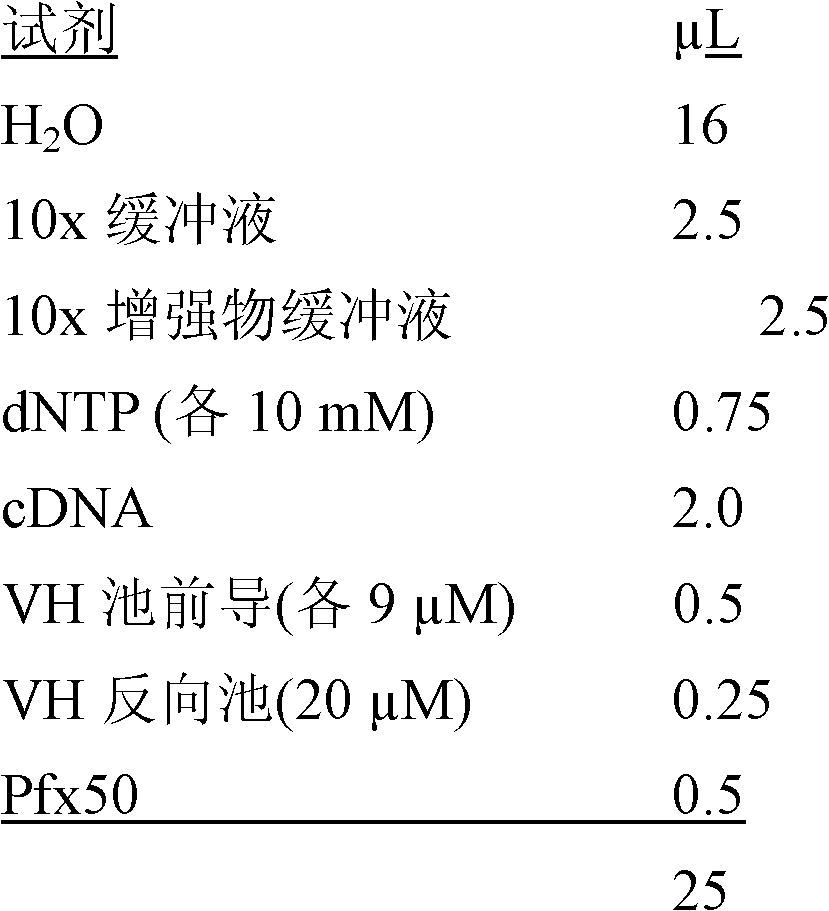
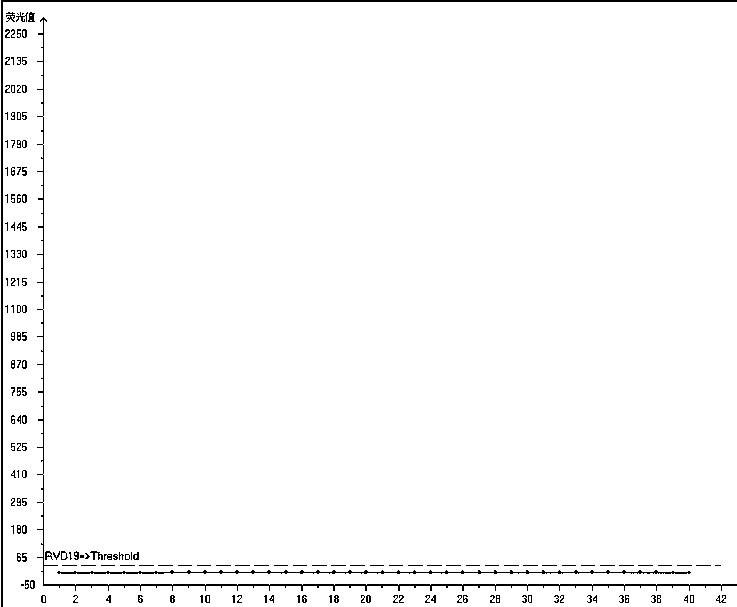
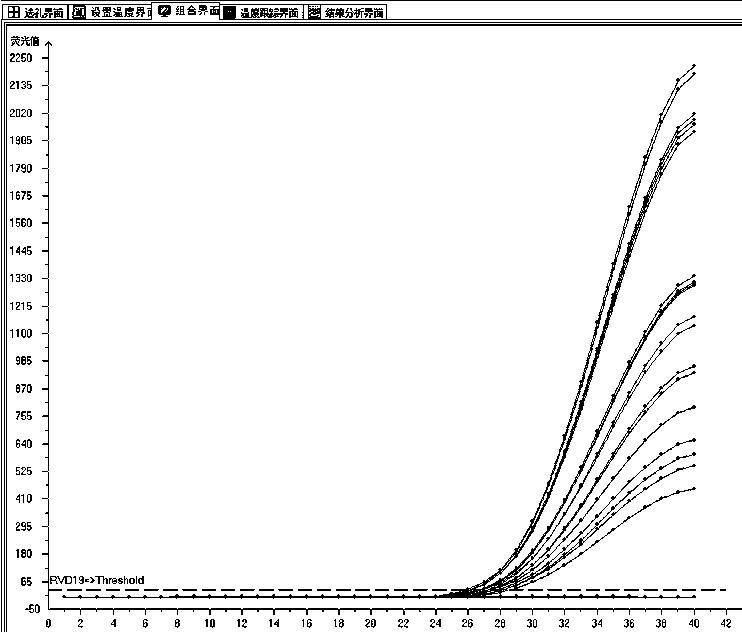
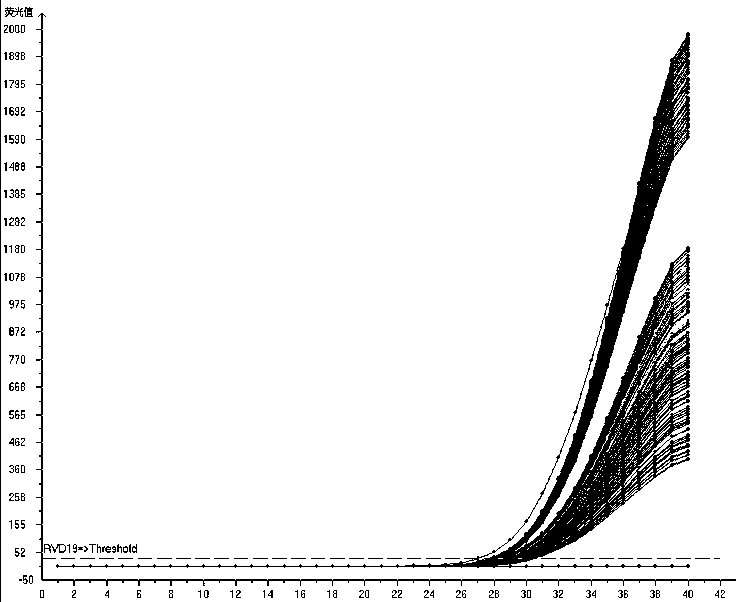
The invention relates to a multiplex fluorescence quantitative PCR kit for detecting 19 human respiratory viruses. The multiplex fluorescence quantitative PCR kit comprises a PCR amplification kit anda reference kit, wherein the PCR amplification kit comprises RVD19 amplification solutions 1#-8# . The kit adopts an 8-hole combined detection method, wherein each hole comprises three fluorescence channels, and each channel corresponds to one pathogen, so that the 19 human respiratory viruses can be qualitatively detected and distinguished simultaneously, which is currently the most comprehensive viral respiratory tract infection pathogen detection method, and provides a basis for comprehensive judgment of the viral respiratory tract infection pathogens and laboratory-assisted diagnosis forclinical examination.
Owner:厦门安普利生物工程有限公司 +1
Probes and methods for the simultaneous detection and identification of multiple viruses that cause respiratory infections in humans
InactiveCN101107366AShorten the lengthSynthetic economyMicrobiological testing/measurementAgainst vector-borne diseasesEnterovirusSevere acute respiratory syndrome
The invention relates to probes and assays which are used for the simultaneous detection, in a single assay sample, of a plurality of nucleic acid sequences of viruses that cause respiratory infections in humans, selected from among influenza virus type A, influenza virus type B, influenza virus type C, human respiratory syncytial virus type A, human respiratory syncytial virus type B, human adenovirus, human parainfluenza virus type 1, human parainfluenza virus type 2, human parainfluenza virus type 3, human parainfluenza virus types 4A and 4B, enterovirus, rhinovirus, human coronavirus type 229E, human coronavirus type OC43, coronavirus that causes severe acute respiratory syndrome (SARS), human metapneumovirus and combinations thereof.
Owner:INST DE SALUD CARLOS III
Rapid human respiratory syncytial virus detection method and kit based on magnetic separating and quantum dot labeling
ActiveCN105319373AIt has the effect of synergistic amplification of multiple signalsFast separationBiological testingEtiologyFluorescence
The invention provides a human respiratory syncytial virus antigen detection method based on magnetic separating and quantum dot labeling. The method comprises the steps that 1, immune nano magnetic beads resisting a human respiratory syncytial virus are prepared; 2, quantum-dot-labeled nano-probes resisting the human respiratory syncytial virus are prepared; 3, after a sample to be detected is dissolved with a sample processing solution, the immune nano magnetic beads resisting the human respiratory syncytial virus are added in a dissolved solution, magnetic separating is performed after full mixing and reacting are performed, washing is performed with a PBST buffer solution, the quantum-dot-labeled nano-probes resisting the human respiratory syncytial virus are added in obtained precipitates, magnetic separating is performed after reacting is performed, and after washing is performed with the PBST buffer solution, a fluorescence value is detected with a fluorescence microplate reader. Accordingly, the accurate, rapid and high-sensitivity method for detecting the human respiratory syncytial virus is established, and the very high practical value in the aspects such as clinical diagnosis, etiology identification and epidemiological investigation of the human respiratory syncytial virus is achieved.
Owner:湖北诺美华抗体药物技术有限公司
Amino acid sequences directed against human respiratory syncytial virus (HRSV) and polypeptides comprising the same for the prevention and/or treatment of respiratory tract infections
ActiveUS20120301469A1Improve propertiesLow costFungiLiquid surface applicatorsHuman respiratory virusADAMTS Proteins
Amino acid sequences are provided that are directed against / and or that can specifically bind protein F of hRSV, as well as to compounds or constructs, and in particular proteins and polypeptides, that comprise or essentially consist of one or more such amino acid sequences. The amino acid sequences, polypeptides and therapeutic compounds and compositions provided by the invention show an improved stability, less immunogenicity and / or improved affinity and / or avidity for protein F of hRSV. The invention also relates to the uses of such amino acid sequences, polypeptides, compounds or constructs for prophylactic and / or therapeutic purposes.
Owner:ABLYNX NV
Respiratory syncytial virus sub-units vaccine, preparation and application
InactiveCN101264323ALimit developmentEnhance cellular immune responseDepsipeptidesAntiinfectivesEscherichia coliCtl epitope
The invention relates to a respiratory syncytial virus vaccine, and the preparation method and application, in particular to an application of escherichia coli to express and recombinant respiratory syncytial virus G protein and mutation G protein, and preparation vaccine with nontoxic typed escherichia coli heat labile enterotoxin, for human or animal preventive inoculation, and for respiratory syncytial virus resistance, belonging to the field of biotechnology. The respiratory syncytial virus vaccine is characterized in that: the respiratory syncytial virus subunits vaccine comprises lopped respiratory syncytial virus RSV protein G, and further comprises nontoxic typed escherichia coli heat labile enterotoxin LT adjuvant. The vaccines are lopped respiratory syncytial virus RSV protein G containing amino acid between aa130 and 230 of the original G protein, and substitutes the amino acid CAWIC (CX3C module ordered) between aa182 and 186 with the amino acid YLEKESIYY (CTL epitope) on the RSV M protein, forming the GCIL protein. The respiratory syncytial virus vaccine has the advantages of remarkable practical significance for preventing human respiratory syncytial virus infection.
Owner:KUNMING UNIV OF SCI & TECH
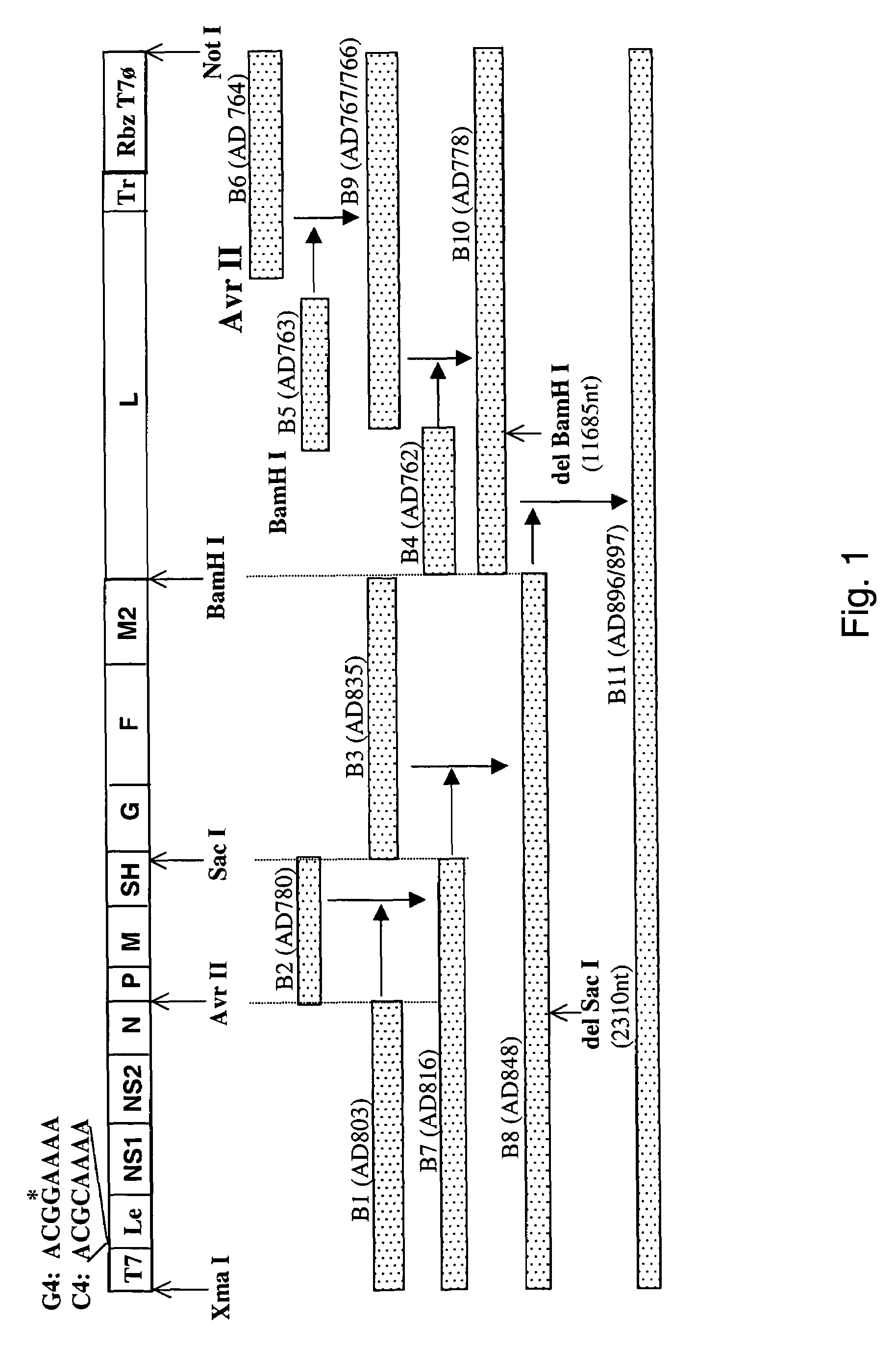
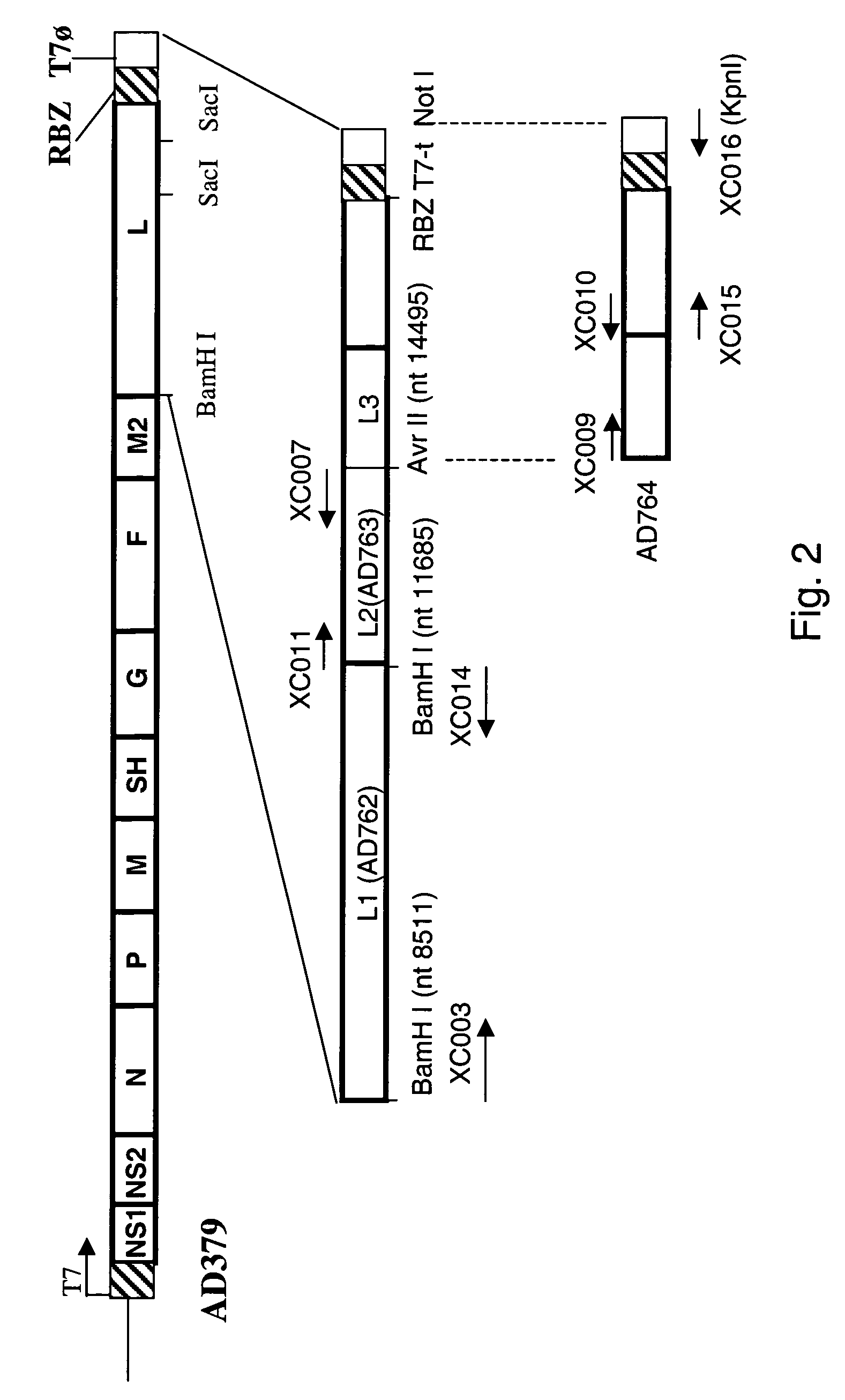
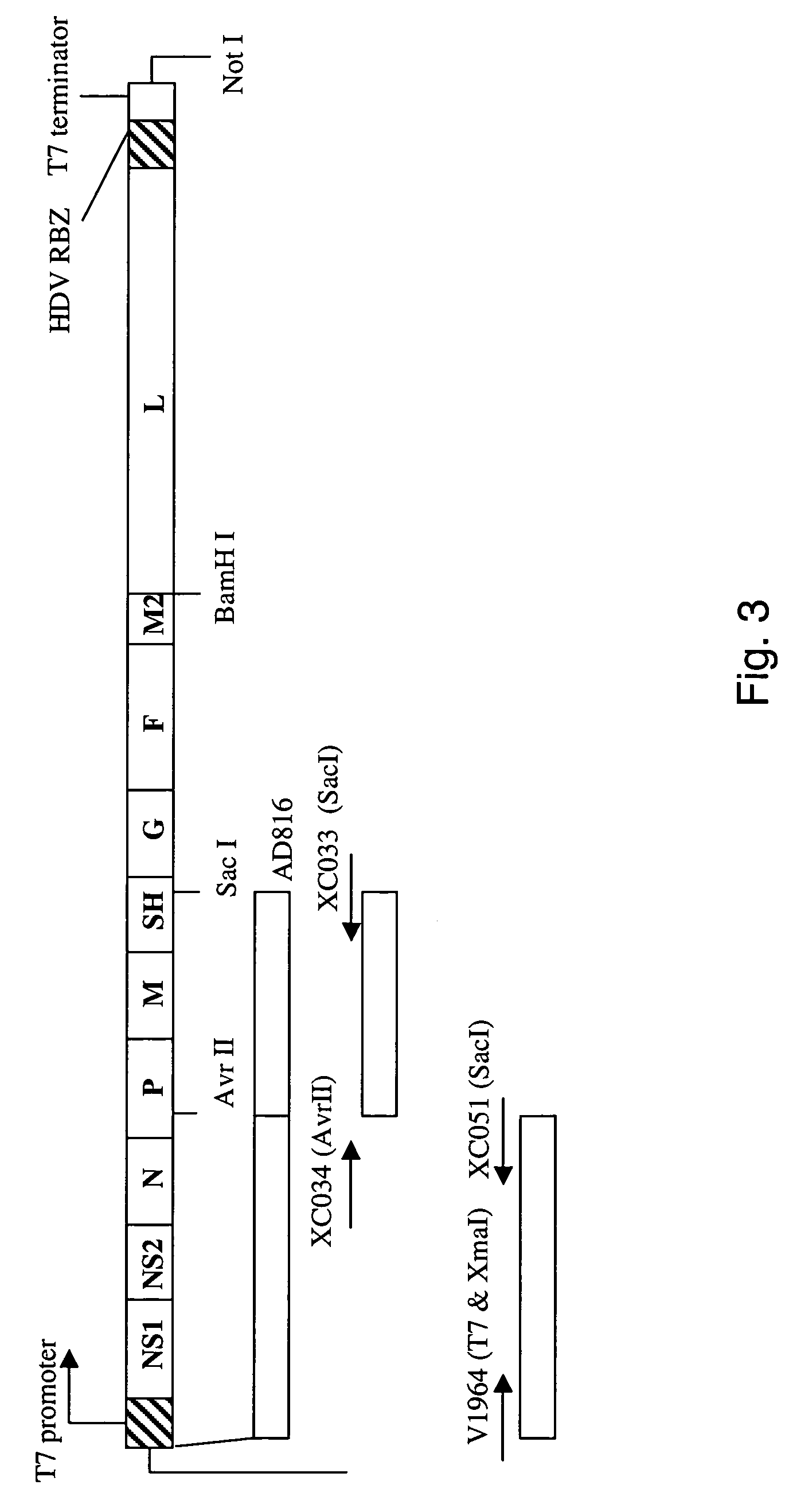
Nucleic acids encoding respiratory syncytial virus subgroup B strain 9320
ActiveUS7572904B2SsRNA viruses negative-senseVirus peptidesAttachment proteinRespiratory syncytial virus (RSV)
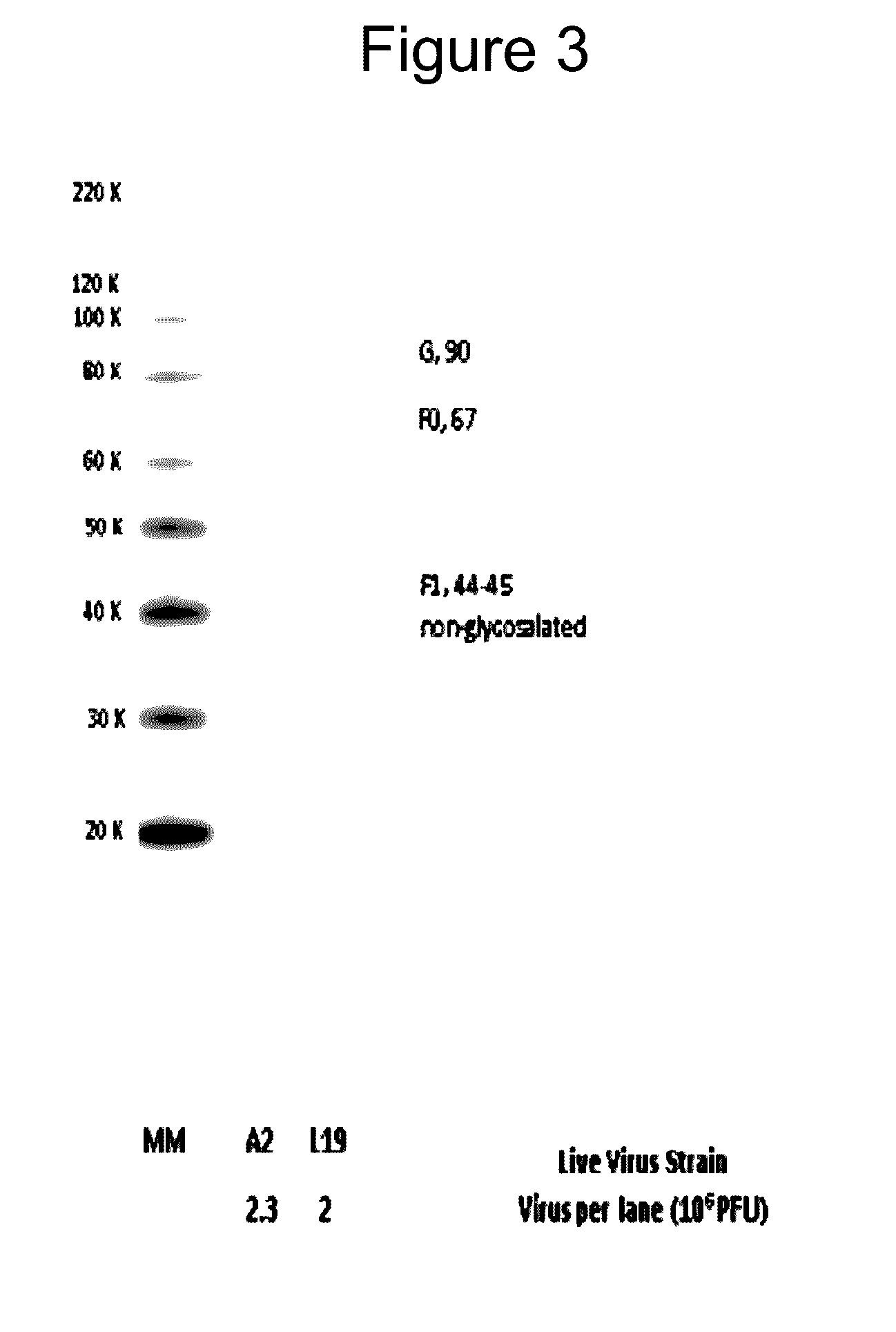
The complete polynucleotide sequence of the human respiratory syncytial virus subgroup B strain 9320 genome is provided. Proteins encoded by this polynucleotide sequence are also provided. Isolated or recombinant RSV (e.g., attenuated recombinant RSV), nucleic acids, and polypeptides, e.g., comprising mutations in the attachment protein G, are also provided, as are immunogenic compositions comprising such isolated or recombinant RSV, nucleic acids, and polypeptides. Related methods are also described.
Owner:MEDIMMUNE LLC
Fluorescence quantitative PCR reagent kit and detection method for human respiratory syncytial virus
InactiveCN1544656AAvoid subjectivityStrong specificityMicrobiological testing/measurementBiological testingRNA extractionFluorescence
The invention discloses a Human respiratory syncytial virus (HRSV) fluorescent quantitative PCR reagent box and detecting method, the reagent box including RNA extract, reverse transcription enzyme, RNA enzyme inhibitor, reverse transcription reacting solution, standard positive template, Taq DNA polyase, fluorescent quantitative reacting solution and standard negative reference and control solution. Using the reagent box to firstly extract virus total RNA from the sample to be detected to make reverse transcription into cDNA, where cDNA and the standard positive template act as fluorescent quantitative PCR, and calculating the initial HRSV concentration by the software in fluorescent quantitative PCR apparatus. It has fast detection, convenience and safety of operation, time saving and high efficiency and can implement early diagnosis and effective prevention of HRSV.
Owner:WUHAN UNIV +1
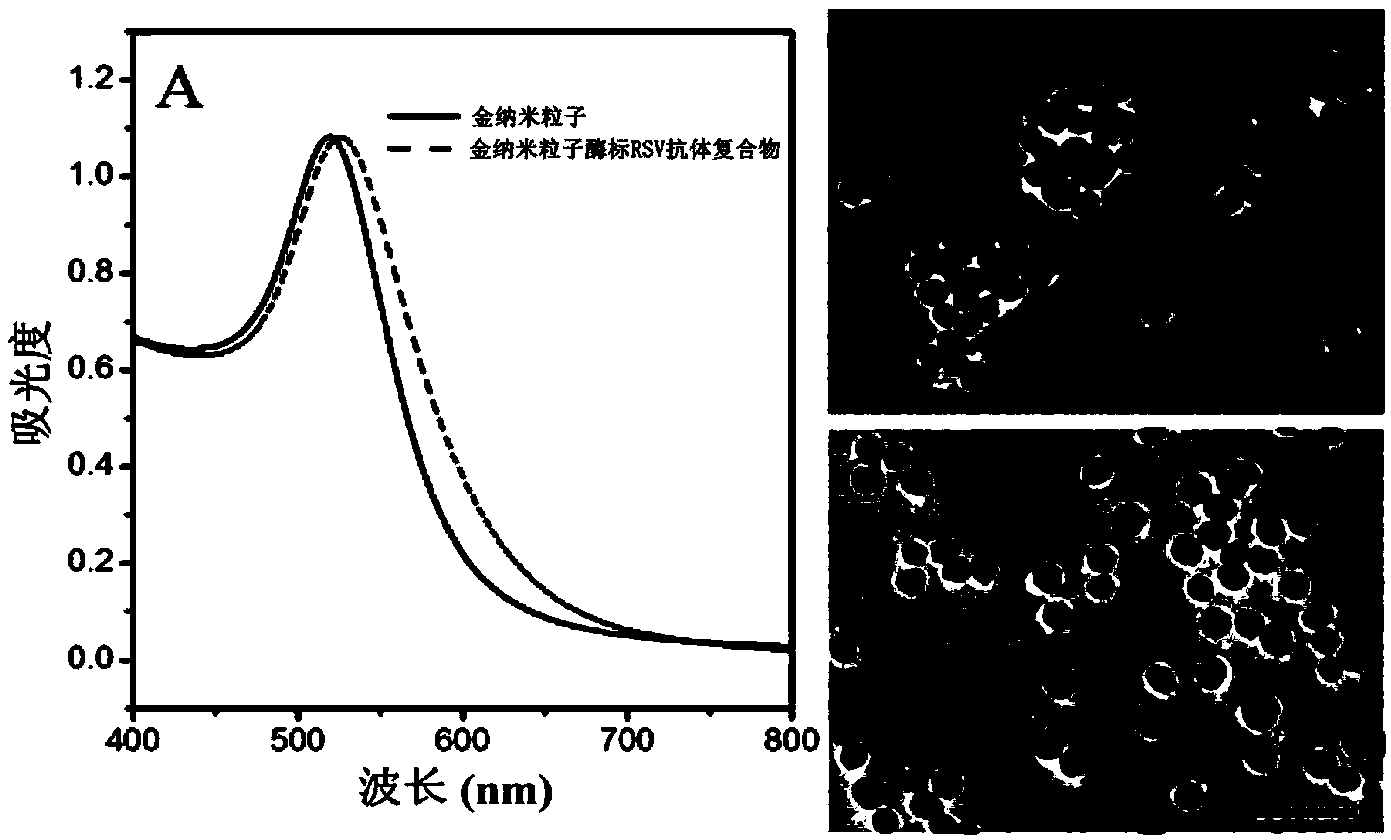
Human respiratory syncytial virus detection kit based on nanogold signal amplification
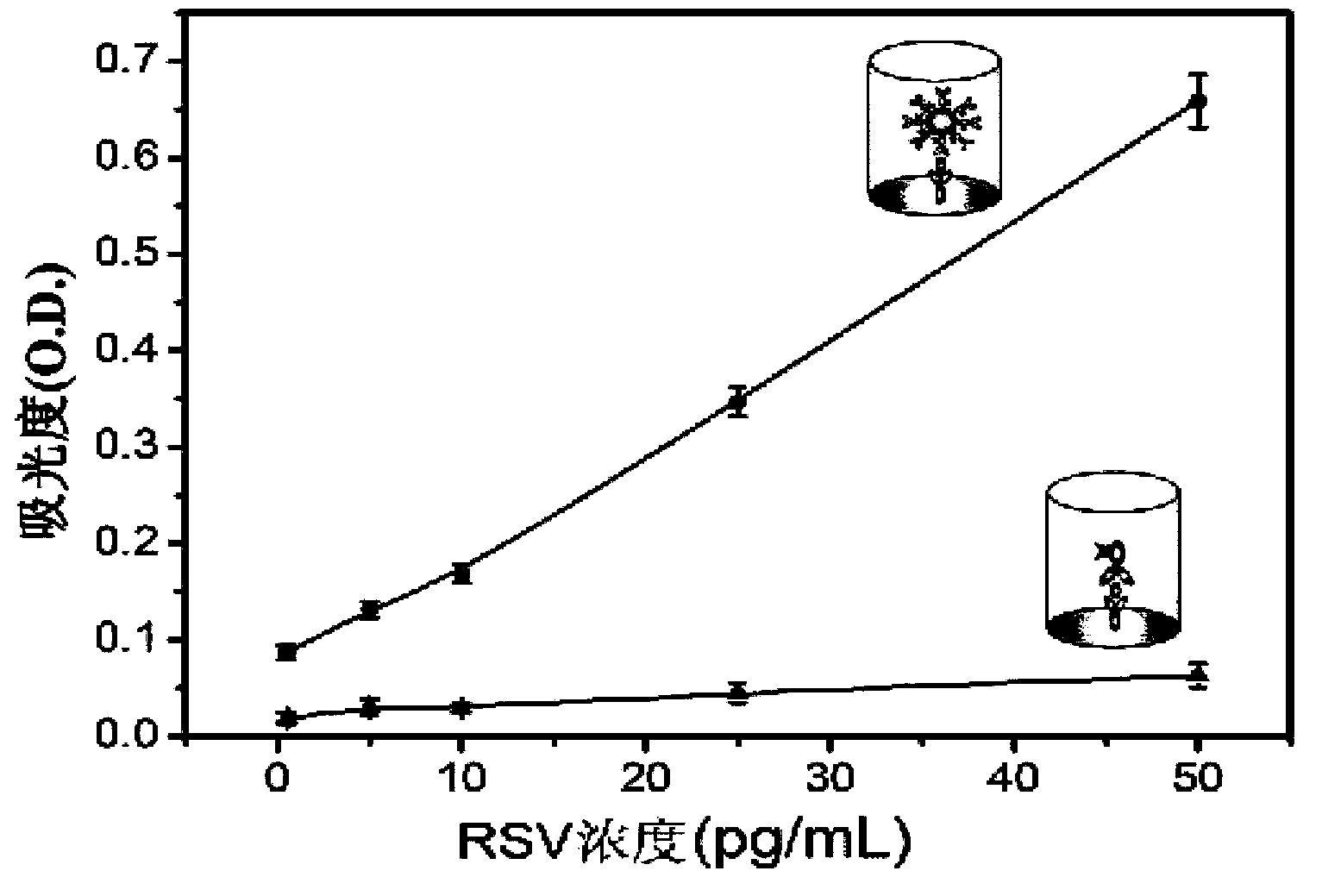
Belonging to the technical field of medical test, the invention in particular relates to a human respiratory syncytial virus (RSV) detection kit based on nanogold signal amplification. Technically, the invention aims to provide a new choice for human respiratory syncytial virus detection. According to a technical scheme adopted by the invention, the human respiratory syncytial virus detection kit based on nanogold signal amplification comprises an RSV reference, an RSV polyclonal antibody, and a gold nanoparticle enzyme labeled RSV antibody complex. The RSV reference is an RSV viral antigen, and the antigen concentration is 60-80pg / mL. The gold nanoparticle enzyme labeled RSV antibody complex is a horse radish peroxidase labeled RSV polyclonal antibody with gold nanoparticles as the carrier, and the antibody concentration is 0.83-1.67 microgram / mL. The kit provided in the invention provides a new choice for RSV detection.
Owner:SOUTHWEST UNIVERSITY
Biological preparation for preventing and controlling human respiratory syncytial virus infection and preparation method
ActiveCN104353063AImprove stabilityLow costPeptide/protein ingredientsAntiviralsHuman respiratory virusViral infection
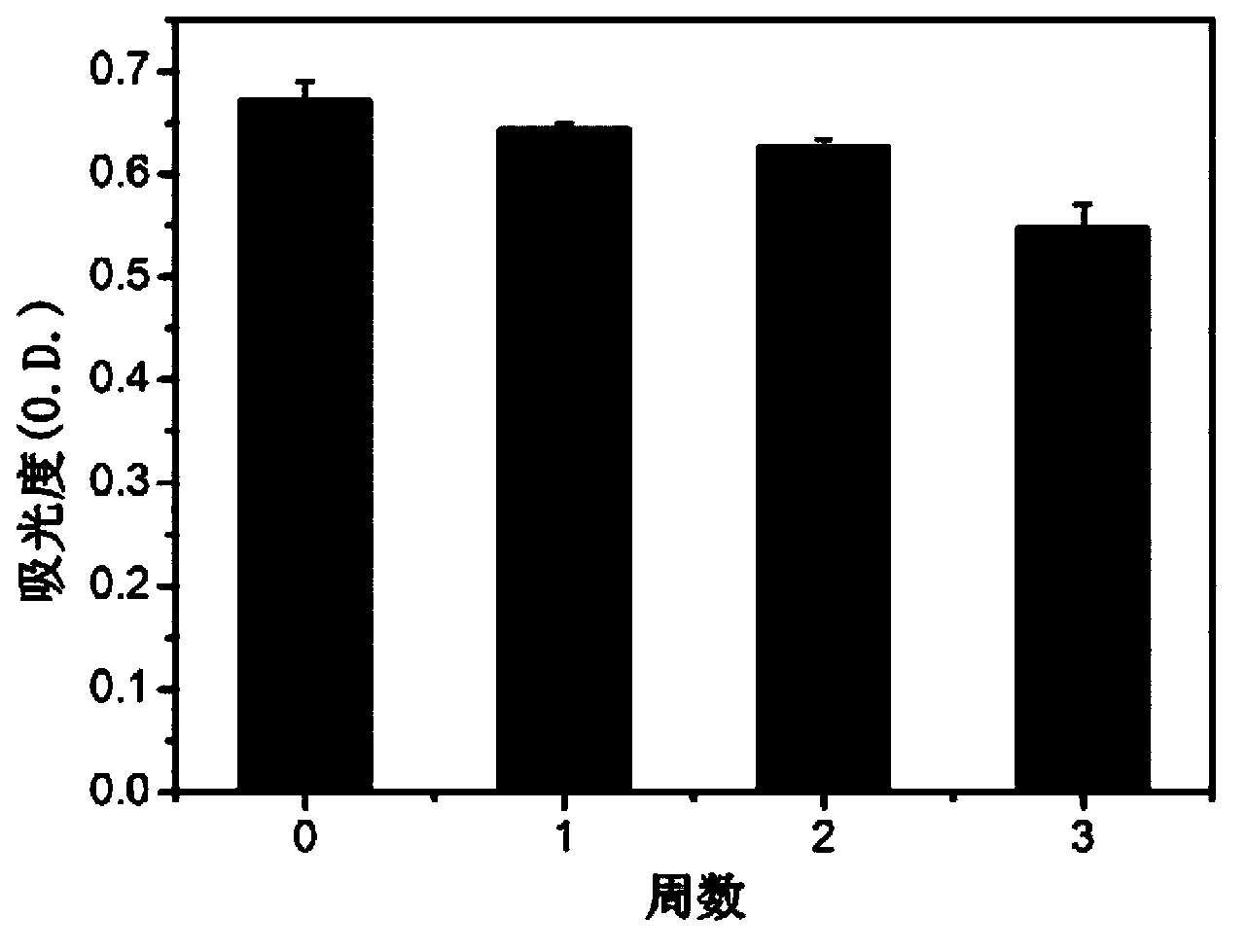
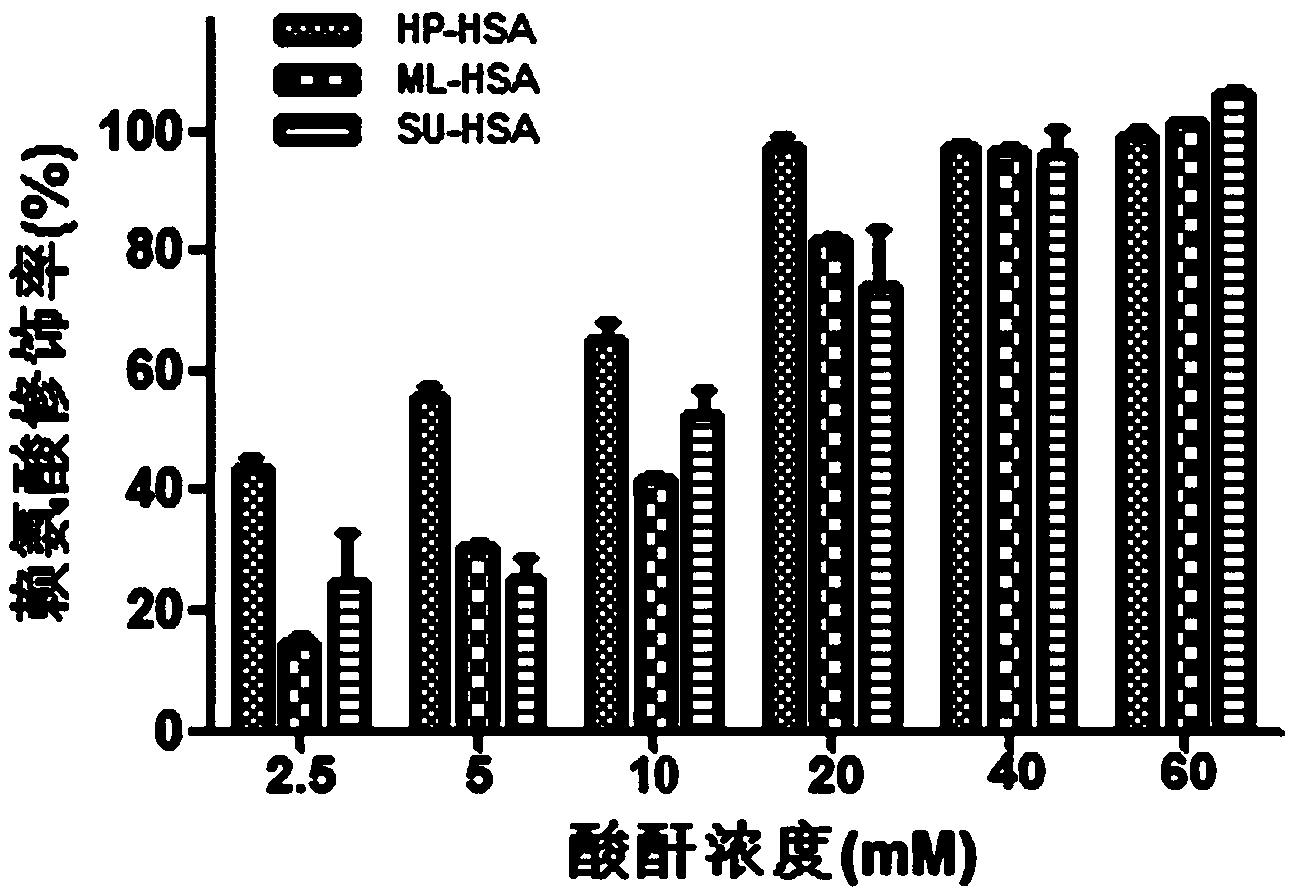
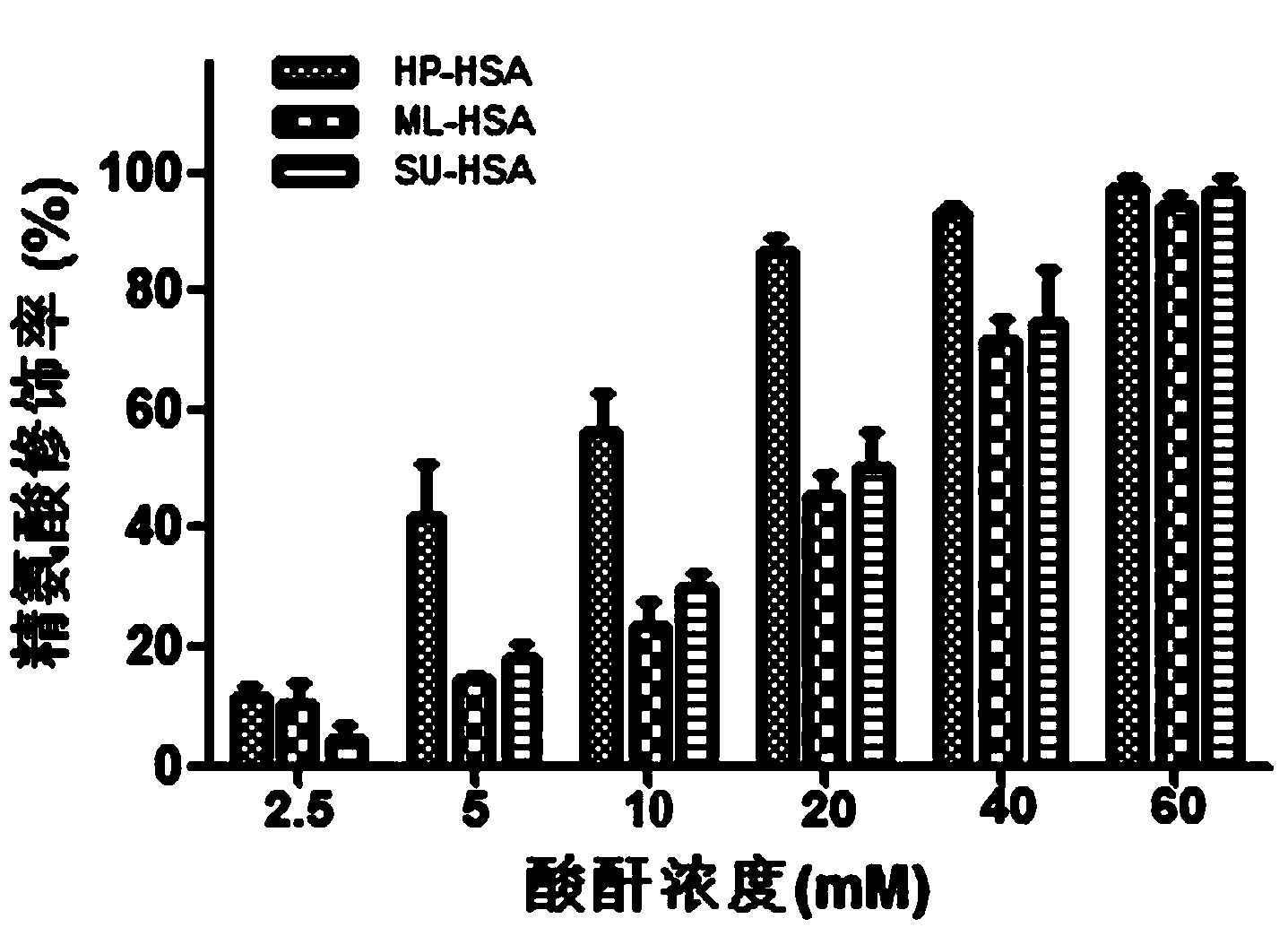
The invention relates to a novel antiviral biological preparation in the technical field of medicines, in particular to a biological preparation for preventing and controlling human respiratory syncytial virus infection and a preparation method. The biological preparation adopts globulin virus entry or fusion inhibitors. The biological preparation is applied to the first stage that virus invades target cells, and blocks the infection of virus to the cells, so as to achieve the effect of preventing and controlling the virus. The biological preparation adopts active anhydride to modify separated and purified amino acid with positive charge on the surface, thereby having the function of preventing the human RSV (respiratory syncytial virus) from entering and infecting the target cells.
Owner:SHANXI JINBO BIO PHARMA CO LTD
Human respiratory syncytial virus vaccine
ActiveUS9492525B2Improve immunityPromotes the normal antigen conformationSsRNA viruses negative-senseOrganic active ingredientsHuman respiratory virusHIV vaccine
Owner:NANOBIO CORP
Anti-human respiratory syncytial virus (RSV) antibodies and methods of use
ActiveCN103097412ASsRNA viruses negative-senseOrganic active ingredientsDiseaseAntigen Binding Fragment
Provided herein are antibodies or antigen-binding fragments thereof that immunospecifically bind to the fusion (F) protein of Respiratory Syncytial Virus (RSV). Also provided are methods for of prevention, treatment and diagnosis of viral infection and / or the treatment of one more symptoms of RSV-mediated disease. Methods of generating antibodies that immunospecifically bind RSV F protein also are provided.
Owner:JANSSEN VACCINES & PREVENTION BV
Pyrazolo[1,5-a]pyrimidines for antiviral treatment
The invention provides compounds of Formula I or Formula II:or a pharmaceutically acceptable salt or ester, thereof, as described herein. The compounds and compositions thereof are useful for treating Pneumovirinae virus infections. The compounds, compositions, and methods provided are particularly useful for the treatment of Human respiratory syncytial virus infections.
Owner:GILEAD SCI INC
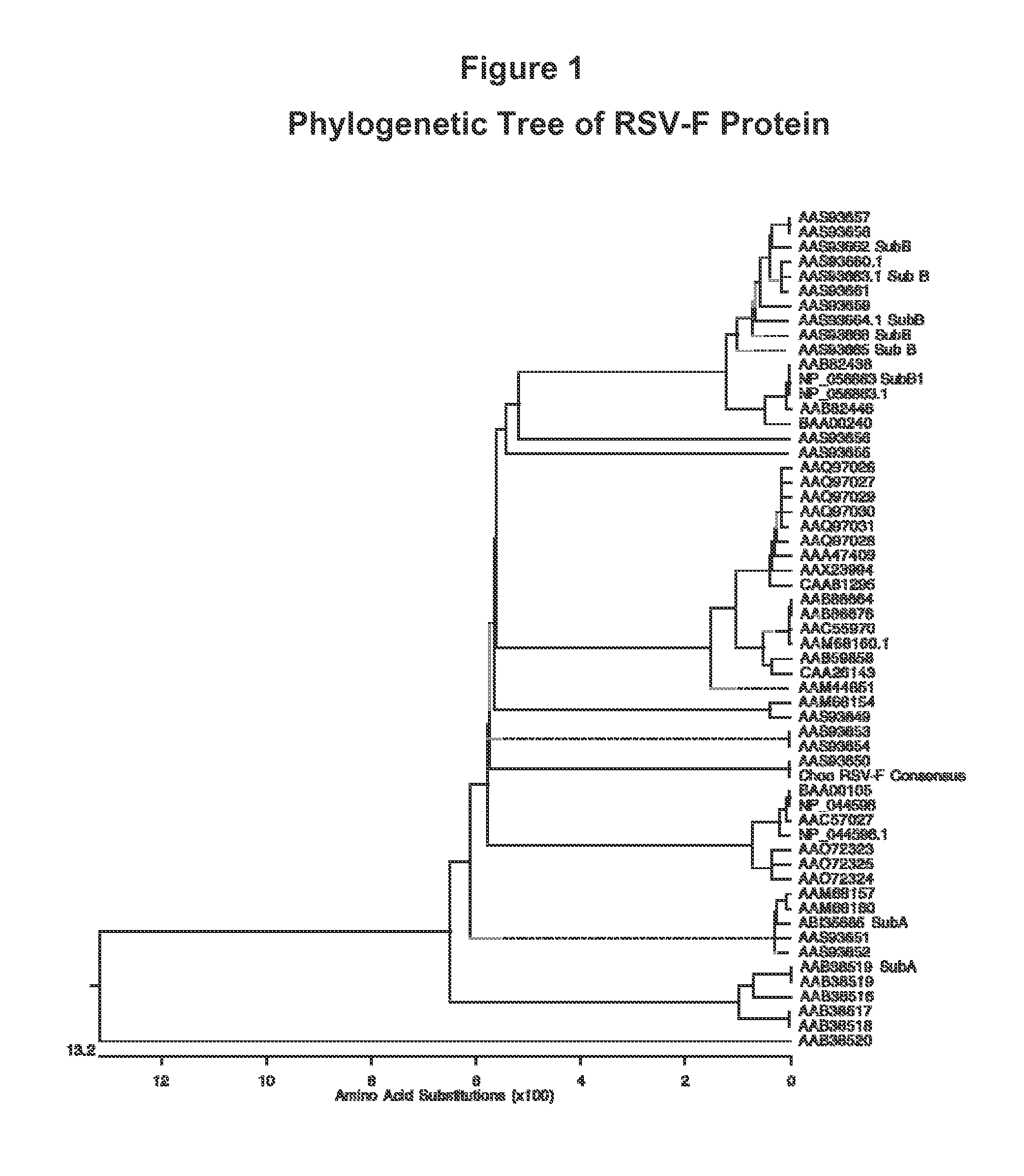
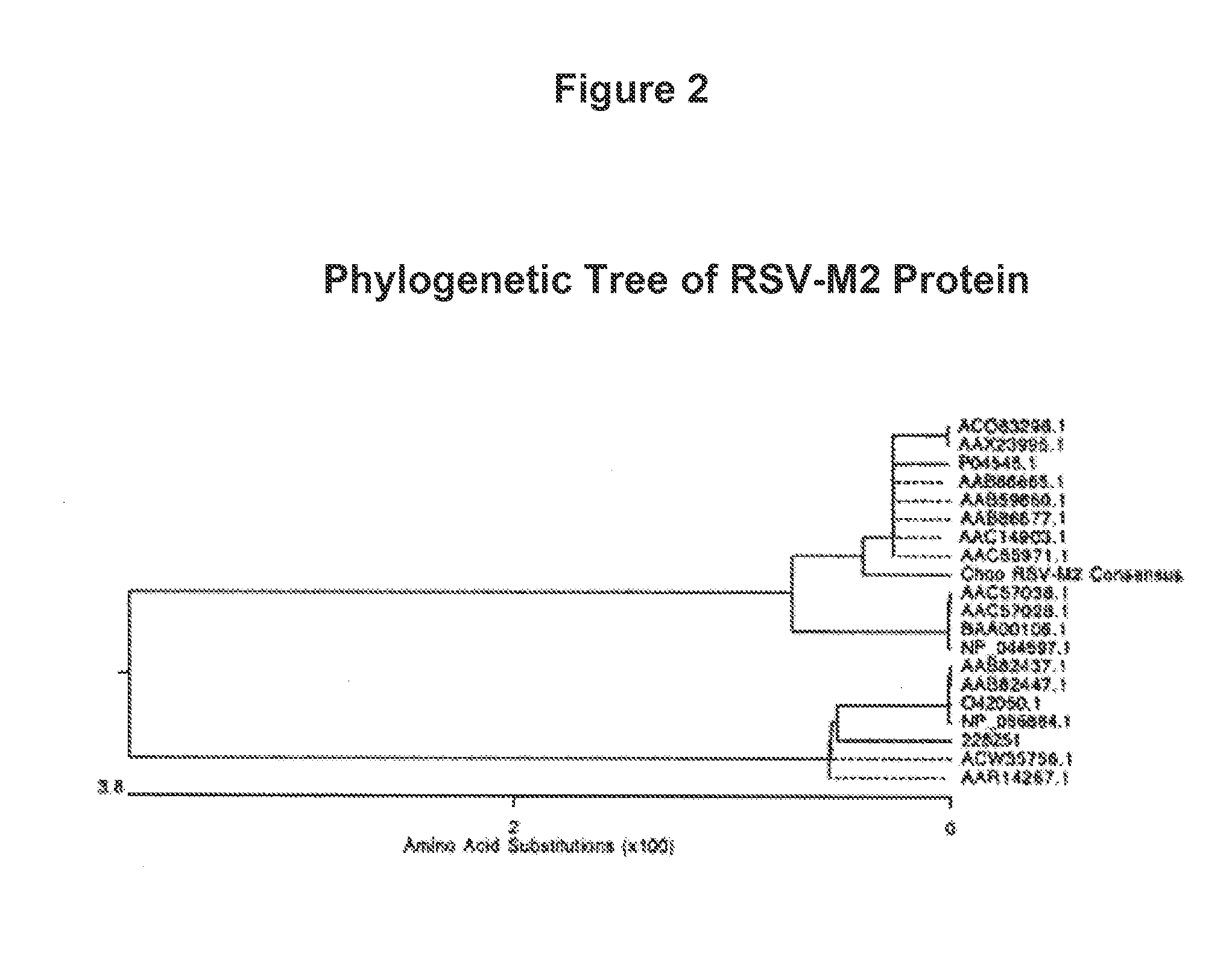
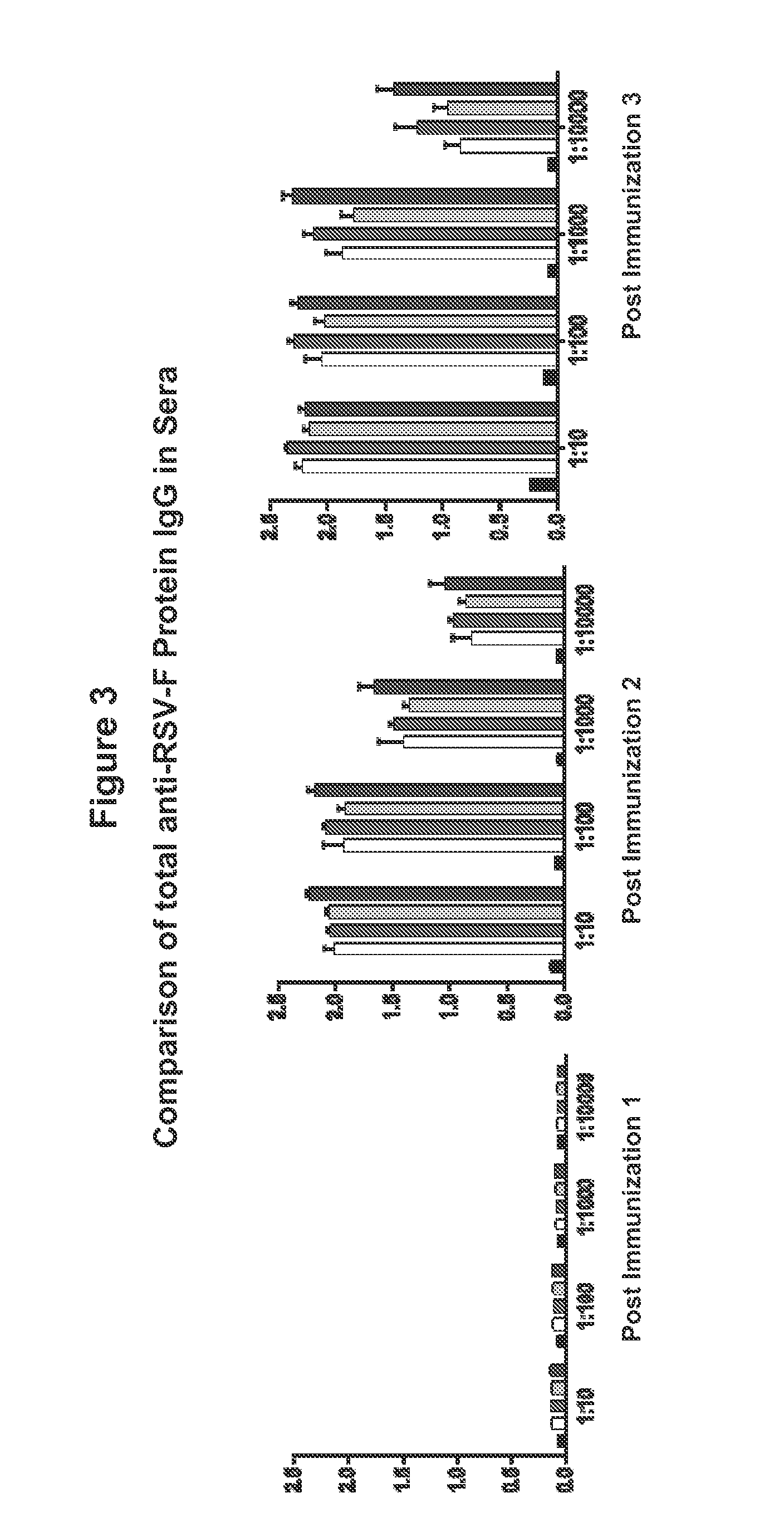
Human Respiratory Syncytial Virus Consensus Antigens, Nucleic Acid Constructs And Vaccines Made Therefrom, And Methods Of Using Same
ActiveUS20150079121A1SsRNA viruses negative-senseGenetic material ingredientsSequence signalHuman respiratory virus
Nucleic acid molecules and compositions comprising one or more nucleic acid sequences that encode an RSV immunogen are disclosed. Nucleic acid are disclosed that comprise the sequences that encodes consensus RSV F protein or immunogenic fragment thereof, sequences that encodes an RSV G(A) protein or immunogenic fragment thereof and sequences that encodes an RSV G(B) protein or immunogenic fragment thereof. Compositions comprising one, combinations of two or all three sequences are disclosed. The coding sequences optionally include operable linked coding sequence that encode a signal peptide. Nucleic acid molecules and compositions comprising the chemokine CC20 and / or a consensus RSV M2-1 protein or immunogenic fragment thereof are also disclosed. Immunomodulatory methods and methods of inducing an immune response against RSV are disclosed. Method of preventing RSV infection and methods of treating individuals infected with RSV are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Purification method of F protein of human respiratory syncytial virus
InactiveCN102199198AIncrease concentrationHigh purityPeptide preparation methodsDepsipeptidesSucrosePurification methods
The invention discloses a purification method of F protein of human respiratory syncytial virus. The purification method comprises the following steps of: infecting HEp-2 cells or Vero cells with RSV (Respiratory Syncytial Virus), collecting the cells and a culture medium after complete pathological changes occur in the cells, centrifuging, taking supernatant, precipitating by using PEG6000, further purifying through sucrose density gradient centrifugation after the obtained precipitation is dissolved; subjecting a protein crude extracting solution to ion exchange chromatography and gel filtration chromatography in sequence for purifying the F protein after the envelope protein of the purified virus is dissolved in a lysis solution. The high-purified F protein can be obtained through two-step chromatography; the antigenicity of the F protein is kept in the purification process; and the purified F protein can be still detected by a group of monoclinic antibodies.
Owner:BEIJING JIAOTONG UNIV
Polynucleotides and Uses Thereof
InactiveUS20100068226A1Simple and inexpensive to produceHigh temperature-stableSsRNA viruses negative-senseOrganic active ingredientsNucleotideHuman respiratory virus
The present invention provides an isolated polynucleotide comprising or consisting of the nucleotide sequence encoding the G protein of human respiratory syncytial virus (RSV), wherein the nucleotide sequence is codon optimised for expression in mammalian cells and wherein the polynucleotide provides increased expression of the G protein in mammalian cells relative to expression of the wildtype RSV-G gene. Preferably, the polynucleotide comprises or consists of the nucleotide sequence of SEQ ID NO:2. Further aspects of the invention provide pharmaceutical compositions, in particular vaccines, for use in methods of immunising a subject against RSV infection.
Owner:TAYLOR GERALDINE +1
Nucleic Acids Encoding Respiratory Syncytial Virus Subgroup B strain 9320
ActiveUS20090285853A1SsRNA viruses negative-senseVirus peptidesAttachment proteinRespiratory syncytial virus (RSV)
The complete polynucleotide sequence of the human respiratory syncytial virus subgroup B strain 9320 genome is provided. Proteins encoded by this polynucleotide sequence are also provided. Isolated or recombinant RSV (e.g., attenuated recombinant RSV), nucleic acids, and polypeptides, e.g., comprising mutations in the attachment protein G, are also provided, as are immunogenic compositions comprising such isolated or recombinant RSV, nucleic acids, and polypeptides. Related methods are also described.
Owner:MEDIMMUNE LLC
Cloning, overexpression and therapeutic use of bioactive histidine ammonia lyase
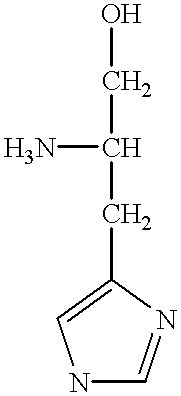
InactiveUS6939541B2Not decreaseOrganic active ingredientsPeptide/protein ingredientsDrug biological activityCombination therapy
Histidine ammonia lyase (HAL) isolated from Corynebacteriaceae can decrease serum histidine levels, induce accumulation of urocanic acid, and is not inhibited by L-histidinol. As a result, histidine ammonia lyases similar to the one isolated from Corynebacteriaceae are uniquely suitable for combination therapy with L-histidinol to treat histidine- and / or histamine-dependent pathologies, for example, infectious viruses, such as human Respiratory Syncytial Virus (RSV), Herpes Simplex Virus (HSV), and Human Immunodeficiency Virus (HIV), as well as cancers.
Owner:SOUTH CAROLINA UNIV OF
Anti-human respiratory syncytial virus N protein antibodies and immunochromatographic kit using the same
ActiveCN105753981AHigh potencyReduce manufacturing costImmunoglobulins against virusesMaterial analysisLinear epitopeHuman respiratory virus
The present invention relates to anti-human respiratory syncytial virus N protein antibodies and immunochromatographic kit for detection of human respiratory syncytial virus by using the same. The anti-human respiratory syncytial virus N protein antibodies separately recognize two linear epitopes consisting of No.21-34 amino acids and No.226-239 of human respiratory syncytial virus N protein; the human respiratory syncytial virus N protein has sequence number of AAB59852.1 in GenBank; amino acid sequence of sites No.21-34 and No.226-239 of the human respiratory syncytial virus N protein are respectively SKYTIQRSTGDSID and FGIAQSSTRGGSRV. The two kinds of rabbit anti-human respiratory syncytial virus N protein antibodies have the characteristics of good specificity, high purity, high titer and low preparation cost.
Owner:HUBEI UNIV OF TECH +1
Antibody neutralizing human respiratory syncytial virus
ActiveUS20170121394A1High activityFunction increaseImmunoglobulins against virusesAntiviralsPassive ImmunotherapyHuman respiratory virus
The present invention relates to monoclonal antibodies which have high anti-RSV neutralizing titers. The invention further provides for isolated nucleic acids encoding the antibodies of the invention and host cells transformed therewith. The invention yet further provides for diagnostic, prophylactic and therapeutic methods employing the antibodies and nucleic acids of the invention, particularly as a passive immunotherapy agent in infants and the elderly.
Owner:MERCK SHARP & DOHME LLC
Amino acid sequences directed against human respiratory syncytial virus (HRSV) and polypeptides comprising the same for the prevention and/or treatment of respiratory tract infections
ActiveUS9644022B2Low costImprove stabilityLiquid surface applicatorsPowdered material dispensingWAS PROTEINHuman respiratory virus
Amino acid sequences are provided that are directed against / and or that can specifically bind protein F of hRSV, as well as to compounds or constructs, and in particular proteins and polypeptides, that comprise or essentially consist of one or more such amino acid sequences. The amino acid sequences, polypeptides and therapeutic compounds and compositions provided by the invention show an improved stability, less immunogenicity and / or improved affinity and / or avidity for protein F of hRSV. The invention also relates to the uses of such amino acid sequences, polypeptides, compounds or constructs for prophylactic and / or therapeutic purposes.
Owner:ABLYNX NV
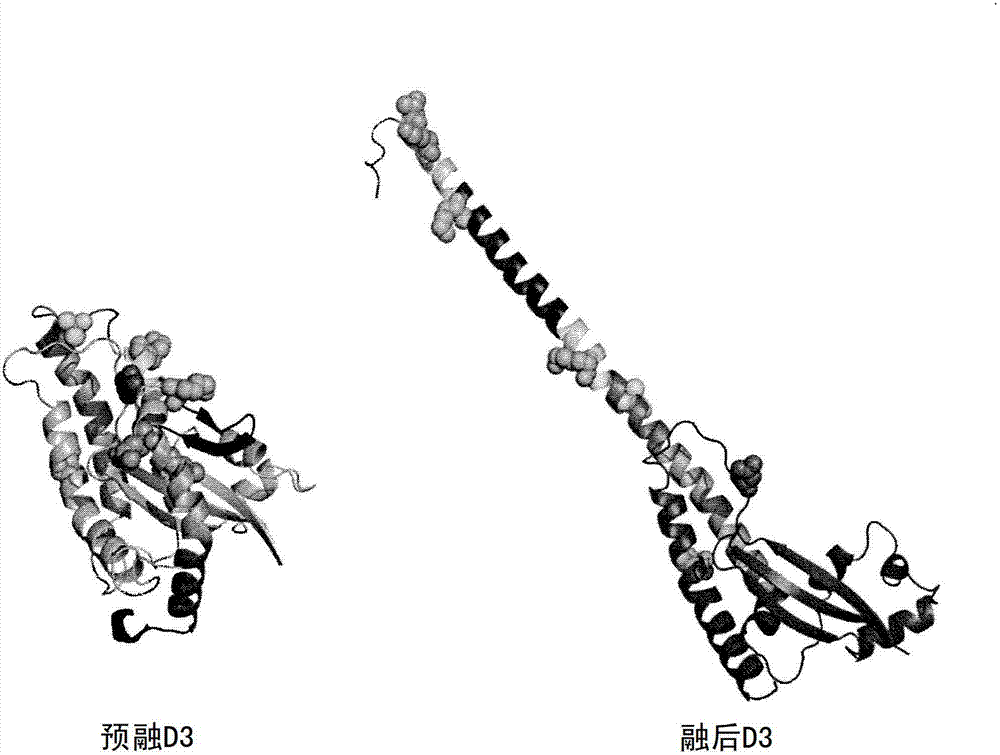
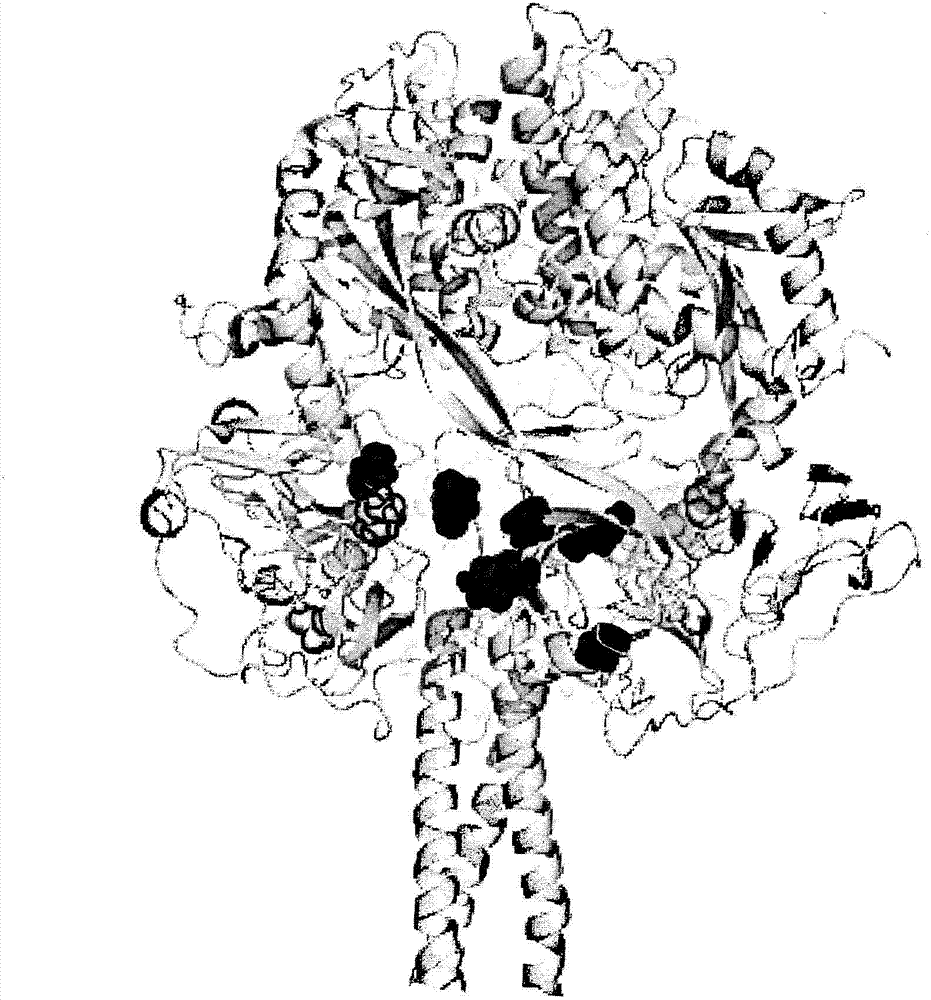
Vaccine candidates for human respiratory syncytial virus (RSV) having attenuated phenotypes
ActiveUS20190233476A1Suitable for useSsRNA viruses negative-senseVirus peptidesUltrasound attenuationWild type
Reported herein are presumptively de-attenuating mutations that are useful, either individually or in combinations that may include other known mutations, in producing recombinant strains of human respiratory syncytial virus (RSV) exhibiting attenuation phenotypes. Also described herein is a novel RSV construct, Min_L-NPM2-1(N88K)L, which exhibits an attenuated phenotype, is stable and is as immunogenic as wild type RSV. The recombinant RSV strains described here are suitable for use as live-attenuated RSV vaccines. Exemplary vaccine candidates are described. Also provided are polynucleotide sequences capable of encoding the described viruses, as well as methods for producing and using the viruses.
Owner:CODAGENIX INC +1
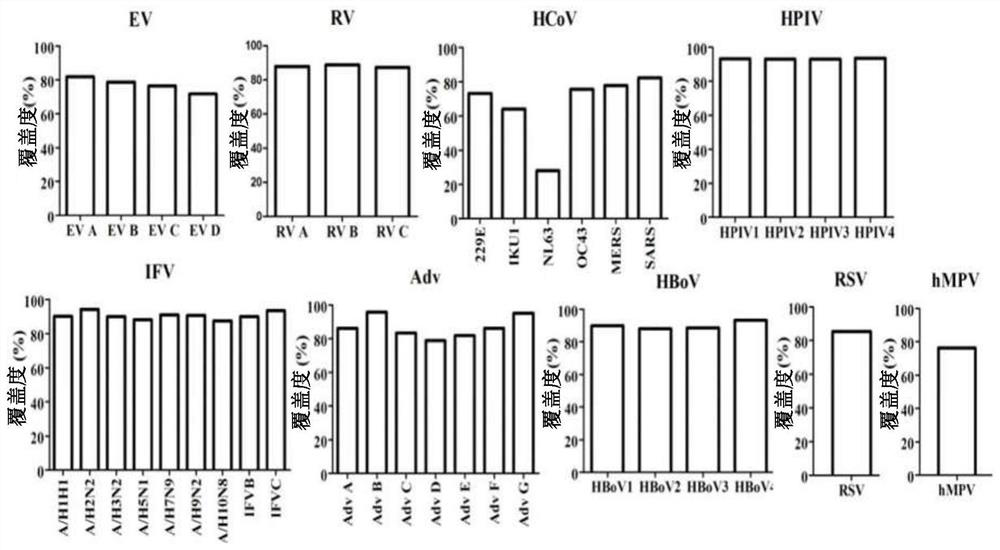
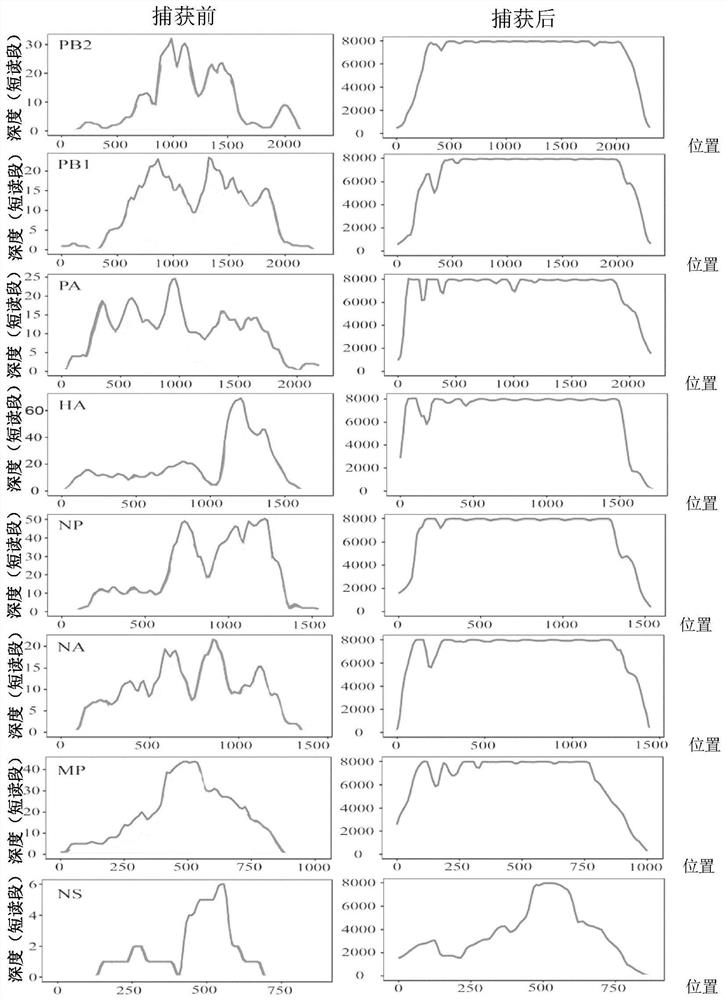
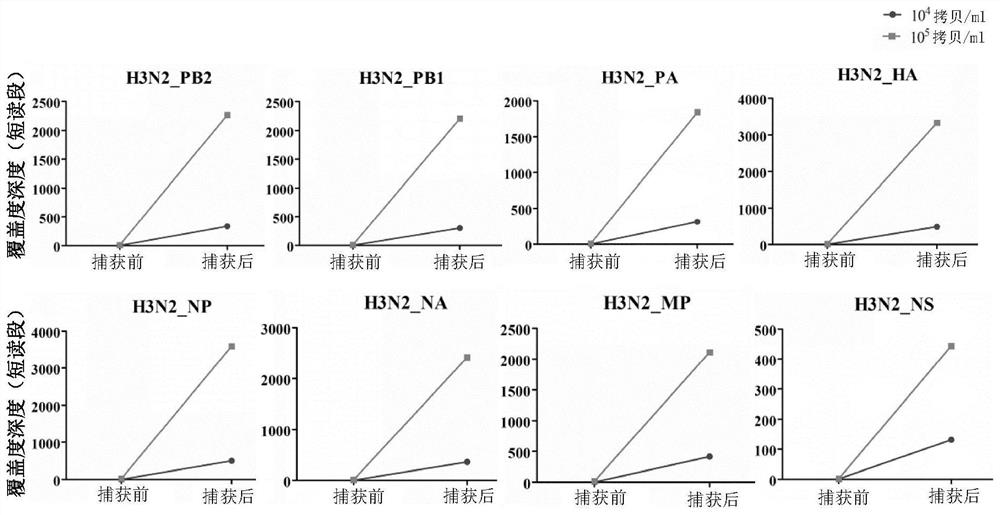
Human respiratory virus targeted enrichment capture probe set and application thereof
The invention relates to the field of human respiratory virus diagnosis, and provides a method for generating a targeted enrichment capture probe set for human respiratory viruses. The method comprises the following steps of acquiring a gene sequence of a virus infecting a human respiratory tract; designing a probe through a sliding window method, and performing exhaustion of all possible nucleicacid sequences; filtering the obtained nucleic acid sequences; filtering out sequences highly homologous with the human genome in a nucleic acid sequence library; and clustering the probe sequences. The invention also relates to application of the probe set obtained through the method to preparation of a next-generation sequencing library of human respiratory viruses, so that whole genome sequenceinformation is obtained, the genome variation is identified, potential epidemic propagation characteristics of viruses are evaluated, and early warning and prevention and control of respiratory diseases are guided.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Method for screening siRNA on basis of bioinformatics
InactiveCN104419702AAvoid spreadingSmall toxicityBioreactor/fermenter combinationsBiological substance pretreatmentsHuman respiratory virusRespiratory virus
The invention provides a method for screening siRNA on basis of bioinformatics. The siRNA obtained through the method is capable of silencing or suppressing the human respiratory virus genes without silencing or suppressing the human genes. The method comprises the following steps: 1) providing a complete genome sequence of the human respiratory viruses; 2) designing first candidate siRNA sequences by taking the complete genome sequence of the human respiratory viruses as a template, and ensuring that the first candidate siRNA sequences are capable of silencing or suppressing the human respiratory virus genes; and 3) excluding the first candidate siRNA sequences which are capable of generating off-target effect to the human genes through comparative analysis so as to obtain second siRNA sequences which do not silence or suppress the human genes. According to the method, rapid response can be carried out in allusion to the new burst respiratory virus epidemic situations, so that the spreading of the acute epidemic situations can be avoided; and the siRNA type drugs obtained through the method are suitable for mucosal immunity.
Owner:HANGZHOU CONVERD CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
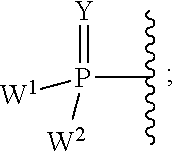





![Pyrazolo[1,5-a]pyrimidines for antiviral treatment Pyrazolo[1,5-a]pyrimidines for antiviral treatment](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/65ebc2a7-5baa-4fc2-ae18-dcd04adfaafa/US08486938-20130716-C00001.png)
![Pyrazolo[1,5-a]pyrimidines for antiviral treatment Pyrazolo[1,5-a]pyrimidines for antiviral treatment](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/65ebc2a7-5baa-4fc2-ae18-dcd04adfaafa/US08486938-20130716-C00002.png)
![Pyrazolo[1,5-a]pyrimidines for antiviral treatment Pyrazolo[1,5-a]pyrimidines for antiviral treatment](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/65ebc2a7-5baa-4fc2-ae18-dcd04adfaafa/US08486938-20130716-C00003.png)





![Pyrazolo[1,5-a]pyrimidines for antiviral treatment Pyrazolo[1,5-a]pyrimidines for antiviral treatment](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/abfc2c6b-0ff1-4dad-968a-4f7e8c3aaa50/US20120003215A1-20120105-C00001.png)
![Pyrazolo[1,5-a]pyrimidines for antiviral treatment Pyrazolo[1,5-a]pyrimidines for antiviral treatment](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/abfc2c6b-0ff1-4dad-968a-4f7e8c3aaa50/US20120003215A1-20120105-C00002.png)
![Pyrazolo[1,5-a]pyrimidines for antiviral treatment Pyrazolo[1,5-a]pyrimidines for antiviral treatment](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/abfc2c6b-0ff1-4dad-968a-4f7e8c3aaa50/US20120003215A1-20120105-C00003.png)