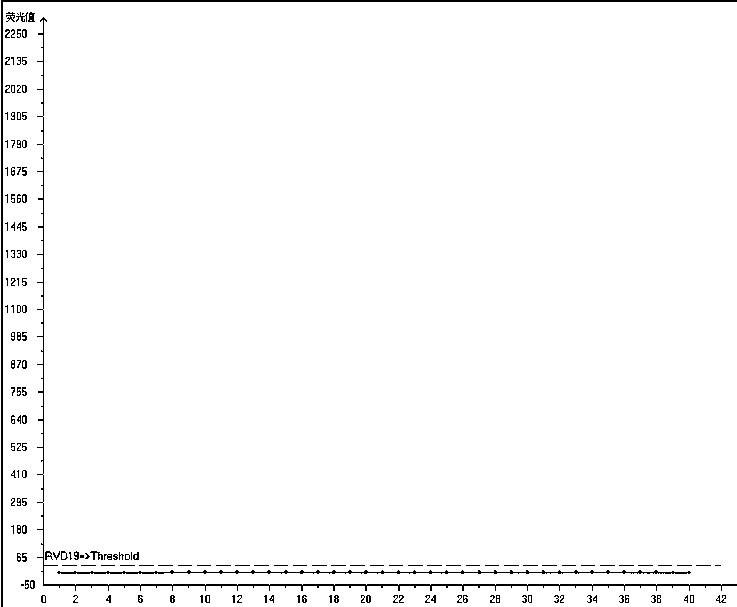
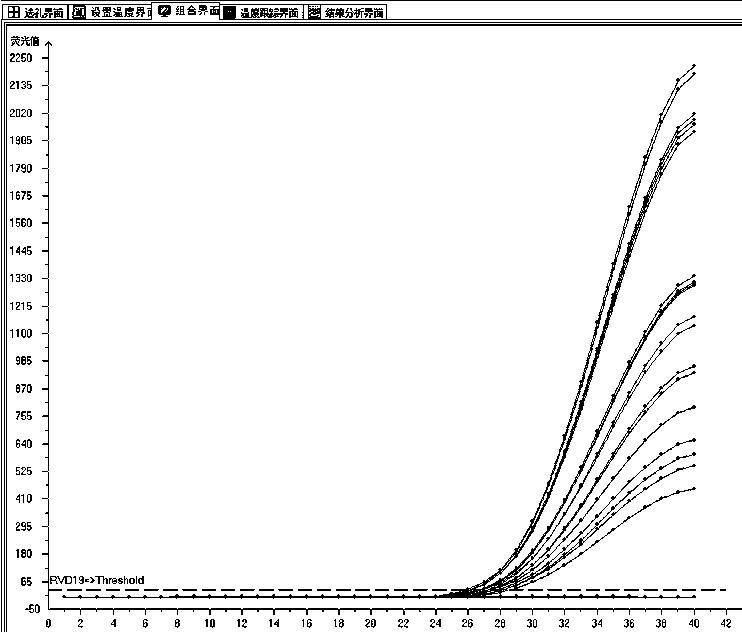
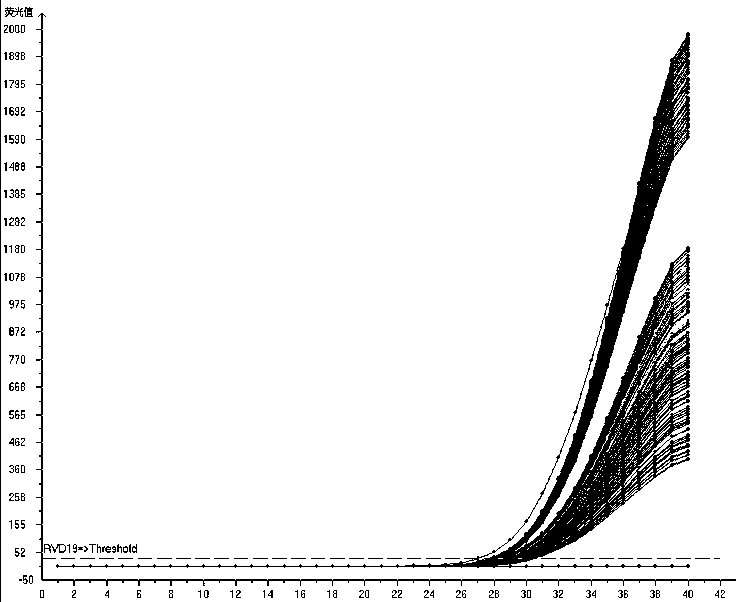
Multiplex fluorescence quantitative PCR kit for detecting 19 human respiratory viruses
A multiple fluorescence quantitative and virus detection technology, applied in the biological field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1: Preparation of a multiplex fluorescent quantitative PCR kit for the detection of 19 human respiratory viruses
[0108] The present invention is used for the detection of 19 kinds of human respiratory tract viruses multiplex fluorescent quantitative PCR kit comprises PCR amplification kit and reference product kit, wherein, the composition of PCR amplification kit is shown in Table 1, and the composition of reference product kit is shown in Table 1 2.
[0109] Table 1 Composition list of PCR amplification kit
[0110]
[0111] Table 2 List of components of the reference kit
[0112]
[0113] The kit of the present invention can be used to detect 19 kinds of human respiratory viruses: influenza A virus (IAV), influenza B virus (IBV), human parainfluenza virus type Ⅰ (HPIVⅠ), human parainfluenza virus type Ⅱ (HPIVⅡ), Human parainfluenza virus type Ⅲ (HPIVⅢ), human adenovirus (HADV), human respiratory syncytial virus (HRSV), human coronavirus 229E group (...
Embodiment 2
[0153] Embodiment 2: the detection method of multiplex fluorescent quantitative PCR kit
[0154] 1. Detection Kit
[0155] The multiplex fluorescence quantitative PCR kit prepared in Example 1 for the detection of 19 kinds of human respiratory viruses.
[0156] Detection object
[0157] 2.1 Preparation of internal standard granular saline containing the housekeeping gene β-actin in human cells (hereinafter referred to as "internal standard saline"):
[0158] In the reference substance preparation room in the clean work area, measure 800mL of normal saline (0.9% sodium chloride solution) into a 1000mL volumetric flask, add 5.0×10 9 Copies / mL pseudovirus particles (RNA virus) 1mL, dilute to 1000mL with normal saline, turn over to mix well, so that the final concentration is 5.0×10 6 copies / mL. Transfer the above solution to a 1000mL beaker and divide it for use.
[0159] 6 samples tested negative
[0160] Collect respiratory tract samples such as Staphylococcus aureus, St...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com