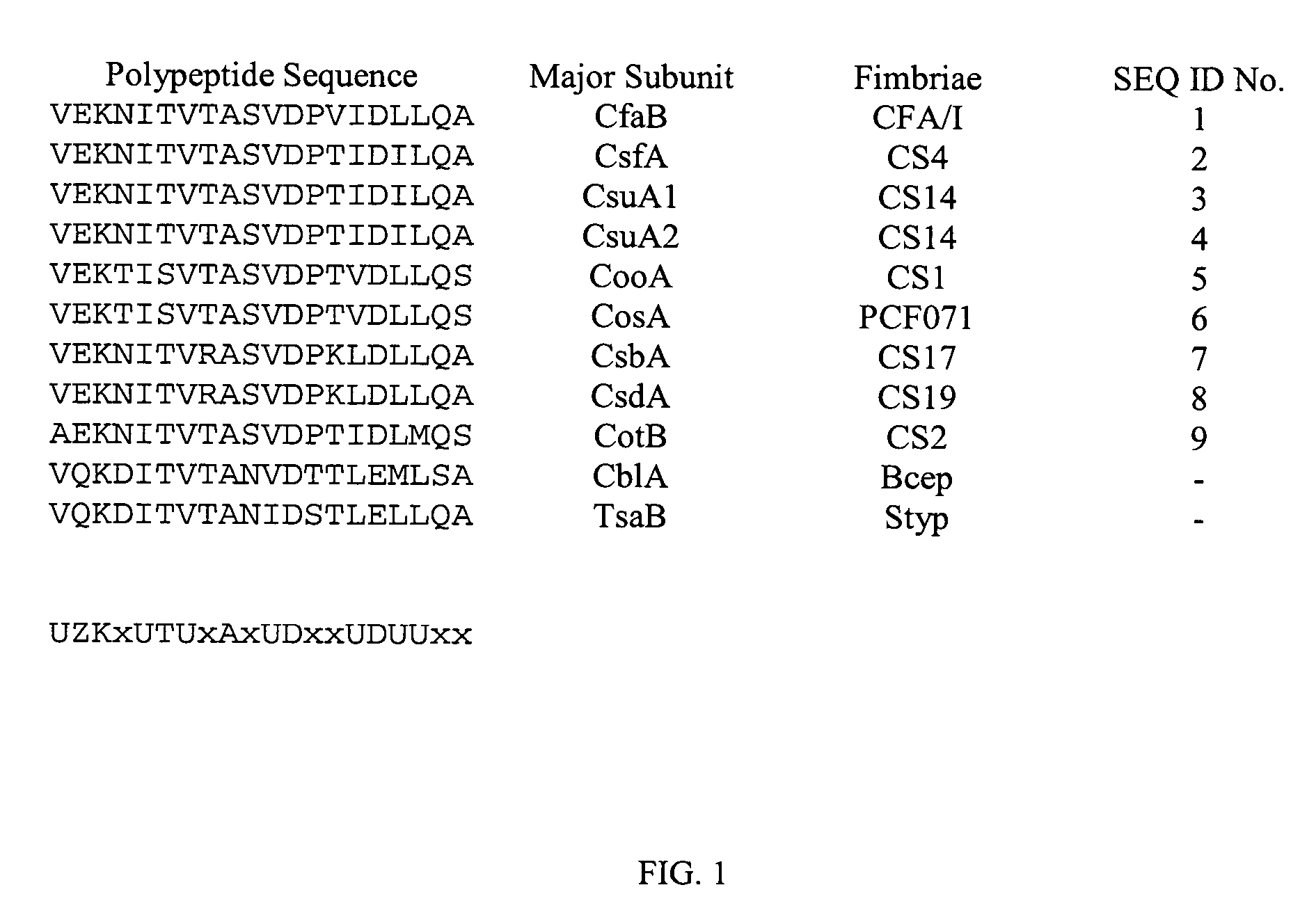
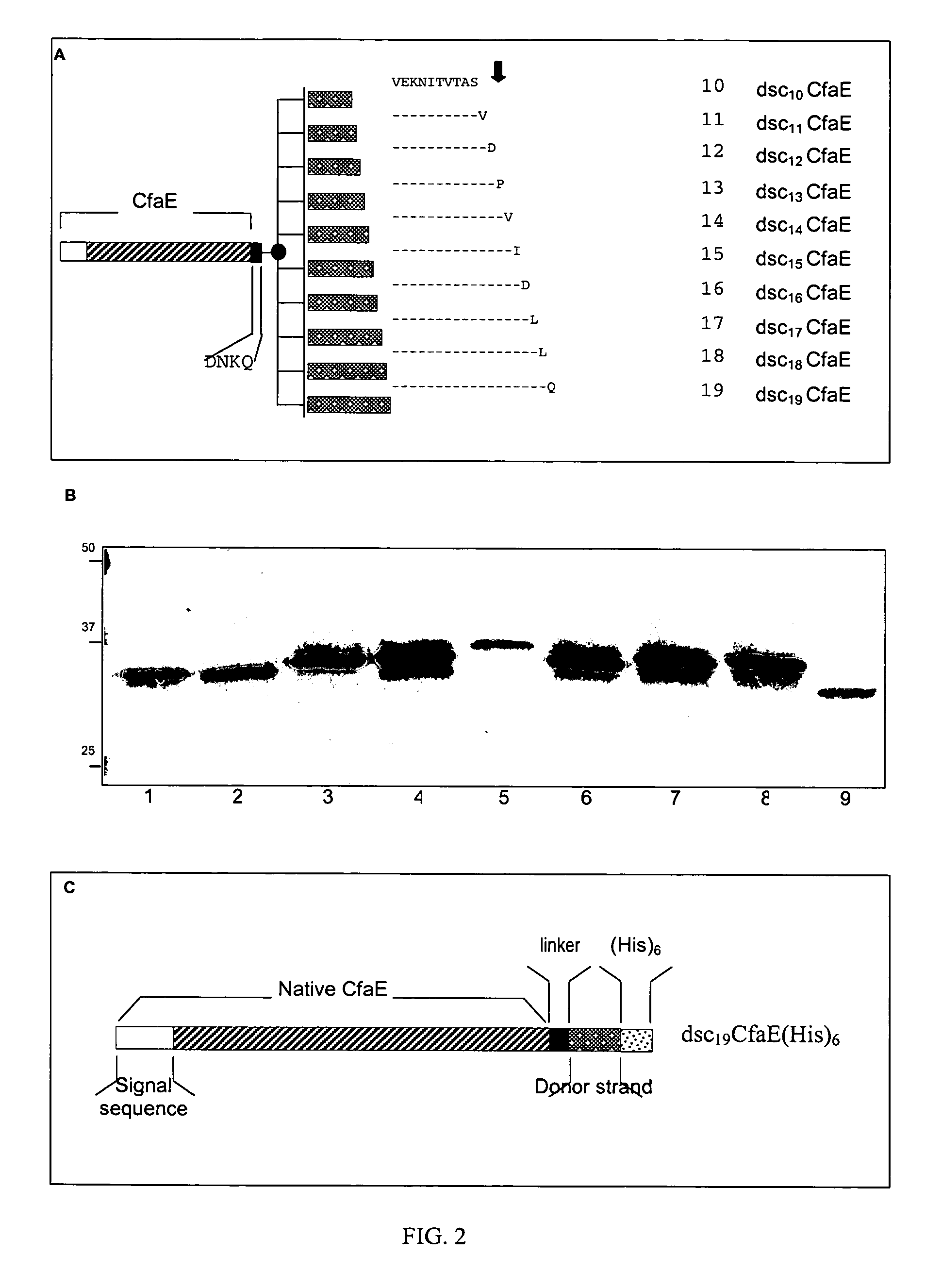
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
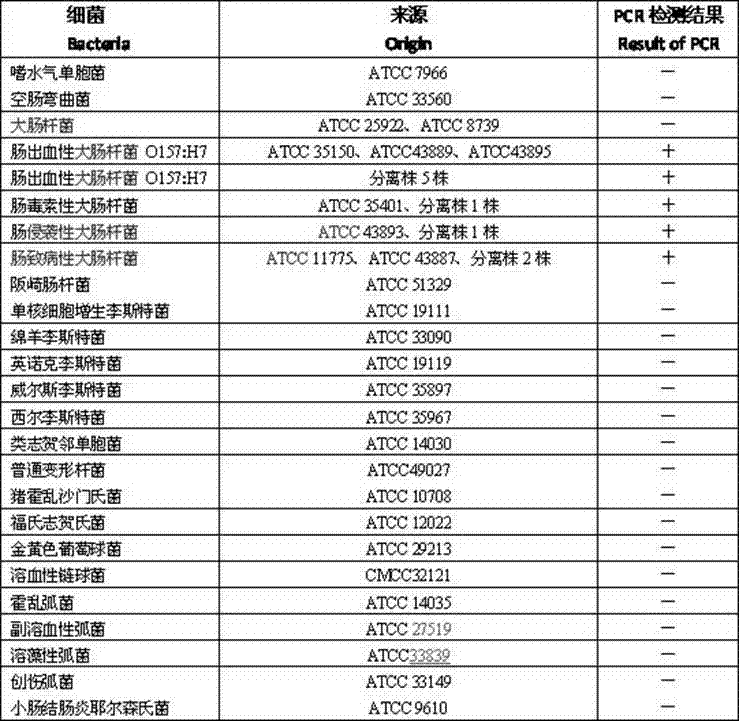
157 results about "Enterotoxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
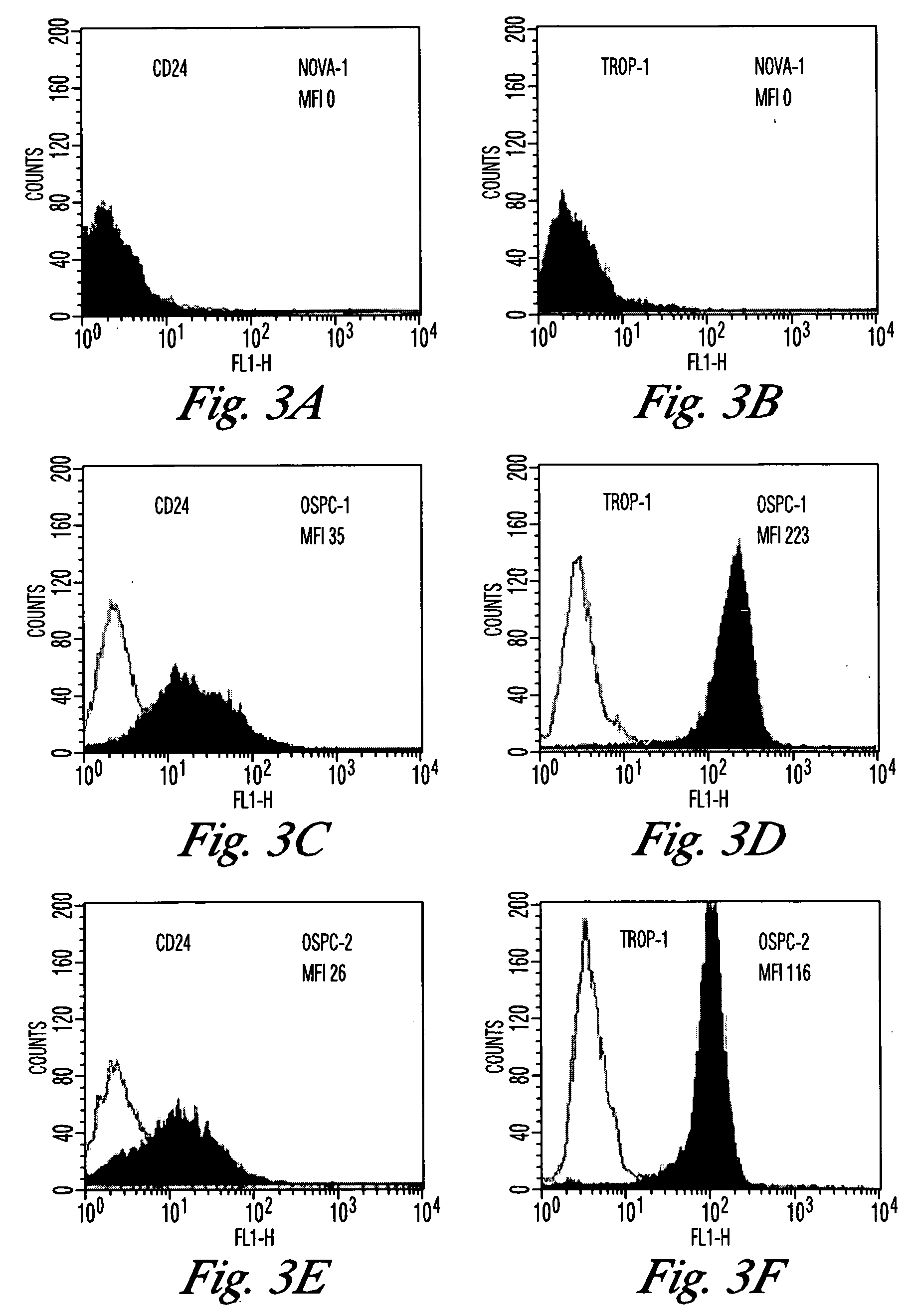
An enterotoxin is a protein exotoxin released by a microorganism that targets the intestines. Enterotoxins are chromosomally encoded or plasmid encoded exotoxins that are produced and secreted from several bacterial organisms. They are often heat-stable, and are of low molecular weight and water-soluble. Enterotoxins are frequently cytotoxic and kill cells by altering the apical membrane permeability of the mucosal (epithelial) cells of the intestinal wall. They are mostly pore-forming toxins (mostly chloride pores), secreted by bacteria, that assemble to form pores in cell membranes. This causes the cells to die.
Methods of promoting immunopotentiation and preparing antibodies with anti-CD3 antibodies
Disclosed are immunopotentiating agents, and vaccines thereof, which enhance and / or otherwise modify immune responses, and method for their preparation and use in vivo. Immunopotentiating agents can be single agents that act directly, adjuvants added concurrently with the agents, or heteroconjugates wherein the immunopotentiating agent is chemically coupled to the compound against which an immune response is desired. Examples of immunopotentiating agents include monoclonal antibodies, such as anti-CD3, anti-CD2) and anti-CD5 antibodies, and proteins derived from microorganisms (e.g., enterotoxins) which activate T cells. The compounds against which an immune response can be generated, which may be the second component in a heteroconjugate, include compound from abnormal or diseased tissues such as tumors, or infectious agents, such as viruses, bacteria, fungi, protozoal or metozoal parasites, and can be obtained by natural or recombinant means. Methods of using the invention to prepare monoclonal antibodies are particularly disclosed.
Owner:MACROGENICS INC
Therapy with clostridium perfringens enterotoxin to treat ovarian and uterine cancer
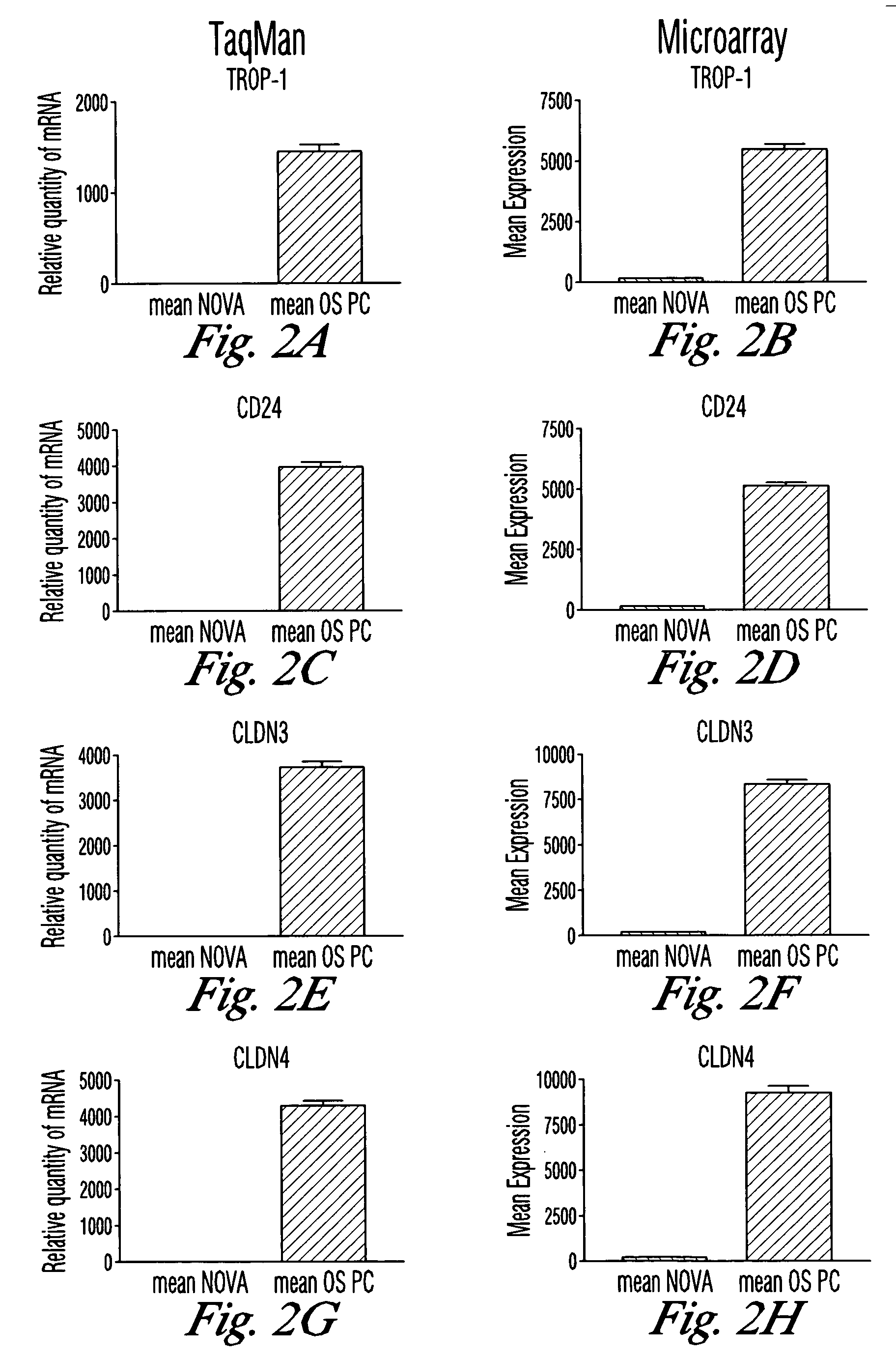
ActiveUS20060084594A1Low toxicityGood curative effectBacterial antigen ingredientsIn-vivo radioactive preparationsIntraperitoneal routeCancer cell
The invention discloses high levels of receptors for Clostridium perfringens enterotoxin (CPE) have been found in ovarian cancer and uterine cancer tissue samples. In addition, successful in vivo treatment of a mouse model of ovarian cancer with intraperitoneal injection of CPE is disclosed. High levels of Ep-CAM protein is also disclosed in ovarian cancer tissue samples. Thus, the invention provides a method of treating ovarian cancer and uterine cancer by administering CPE. The invention also provides a method of treating cancer in a mammal involving intraperitoneal administration of CPE, where at least some cancerous cells are located in or adjacent to the peritoneal cavity of the mammal. The invention also provides a method of treating ovarian cancer involving administering an anti-Ep-CAM antibody. The invention also provides a method of treating cancers expressing claudin-3 or claudin-4 by administering an antibody against claudin-3 and / or an antibody against claudin-4. The invention also provides a method of protecting a mammal from CPE toxicity involving administering a protective agent that binds to claudin-3 and / or claudin-4 and inhibits CPE binding to claudin-3 and / or claudin-4.
Owner:YALE UNIV
Amino acid sequences for therapeutic and prophylactic use against diseases due to clostridium difficile toxins
InactiveUS7151159B2Antibacterial agentsFungiClostridium difficile toxin AClostridium difficile (bacteria)
The invention relates to monoclonal antibodies capable of recognizing and neutralizing epitopes from the ligand domain, the translocation domain or the catalytic domain of the enterotoxin (toxin A) and cytotoxin (toxin B) from Clostridium difficile, as well as their production and therapeutic and prophylactic applications to diseases caused by said toxins.
Owner:VON EICHEL STREIBER CHRISTOPH
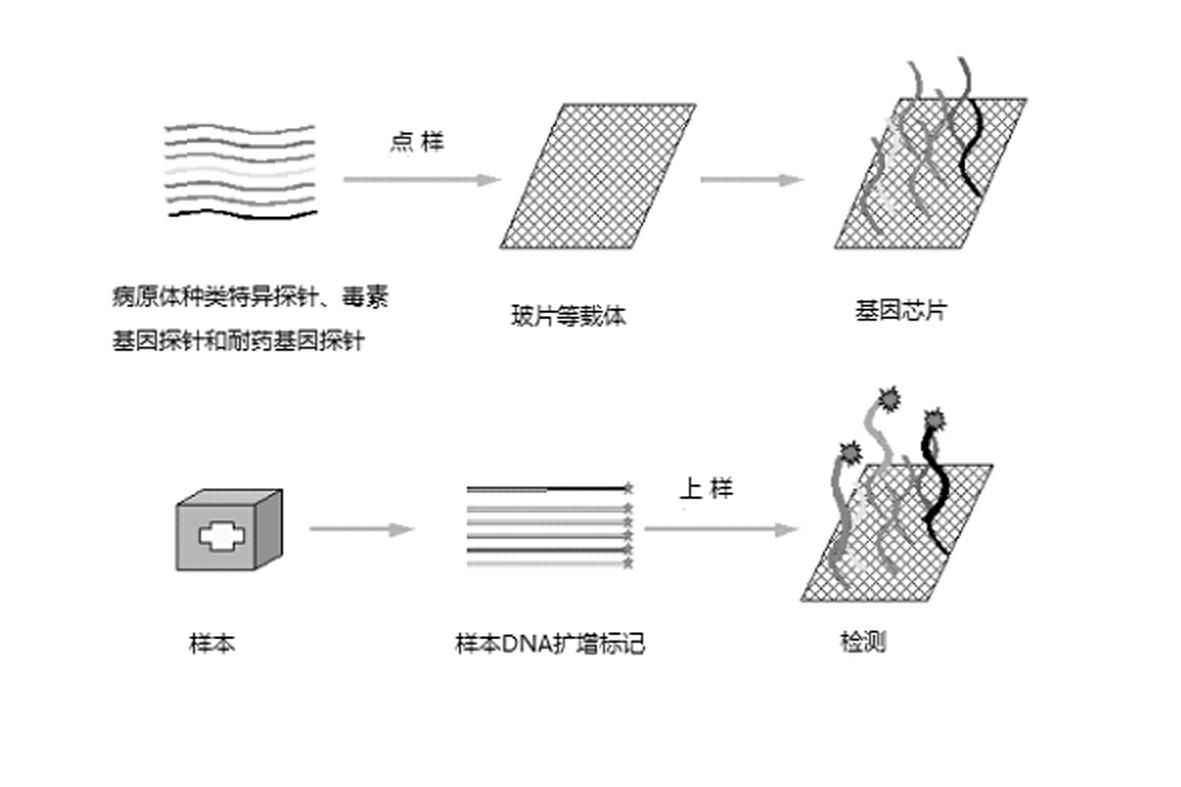
Gene chip for high-flux detection of pathogens and application thereof
InactiveCN102534013AStrong specificityDetermine the typeMicrobiological testing/measurementAgainst vector-borne diseasesYersinia pestisBrucella
The invention relates to a gene chip for high-flux detection of pathogens and application thereof. The gene comprises (1) a combination of 174 oligonucleotide probes of pathogen variety specific genes, toxin genes and drug-resistant genes; and (2) a probe array, which is formed by curing the oligonucleotide probes on a carrier material by arm molecules. The gene chip comprises 174 gene probes, namely 32 pathogen variety specific gene probes of the following 8 pathogens of Burkholderia mallei, Burkholderia pseudomallei, Brucella, salmonella, Yersinia pestis, Bacillus anthracis, comma bacillus and the like, 25 toxin gene probe of the following 7 toxins of diphtheria toxin, Shiga toxin, staphylococcus enterotoxin, choleratoxin and the like, and 117 drug-resistant gene probes of 17 drug-resistant genes of extended-spectrum beta-lactamase, cephalosporinase, carbapenemase, integrase gene, common gene engineering carrier drug-resistant gene and the like. The gene chip can be used to detect multiple pathogen variety specific genes, toxin genes and drug-resistant genes.
Owner:李越希
Anti-adhesin based passive immunoprophlactic
The invention relates to an immunogenic composition and method of the immunogenic composition for the production and administration of a passive immunoprophylactic against enterotoxigenic Escherichia coli. The immunoprophylactic is made collecting anti-adhesin in the colostrum or milk of vaccinated domesticated animals such as cows. The immunoprophylactic is administered either as a dietary supplement or in capsular or tablet form.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Escherichia coli strain for an oral vaccine against post-weaning diarrhea in pigs
The invention relates to a strain suitable for producing a live, orally applicableEscherichia coli vaccine for the prevention of post-weaning diarrhoea in pigs, and the procedure suitable for producing that strain. The essence of the strain is that the enterotoxin-free and originally wild-type Escherichia coli strain simultaneously produces two adhesive fimbriae (F4 and F18), whereas the essence of the procedure is that the enterotoxin-producing ability if the wild, pathogenic, enterotoxigenic Escherichia coli strain originally capable of producing enterotoxins and F18 fimbriae is abolished by a genetic intervention while retaining the ability of the strain to produce F18 fimbria facilitating adhesion to the small intestinal wall of weaned piglets, and subsequently the strain thus modified is rendered capable of producing a further surface adhesion fimbria (F4).
Owner:MTA ALLATORVOS TUDOMANYI K I
Bacillus Licheniformis and Method for Detoxification of Zearalenone
ActiveUS20110171722A1Good detoxification effectIncrease cellulase activityBacteriaSolid waste disposalBiotechnologyBacillus licheniformis
The present invention discloses an isolated pure culture of a novel strain CK1 of Bacillus licheniformis, BCRC 910458, capable of detoxification of zearalenone (ZEN). Using physiological, biochemical, morphological identification and 16S rRNA gene sequence analysis methods, the strain CK1 was identified as Bacillus licheniformis. Through extracellular xylanase, CMCase protease assays and evaluations for zearalenone detoxification, the strain CK1 strain was identified to possess good ZEN-detoxifying ability, to be non-hemolytic, non-enterotoxin producing, and displayed high levels of extracellular xylanase, cellulase, and protease activities. Accordingly, Bacillus licheniformis CK1 can be applied as food and feed supplement for bio-detoxification of ZEN.
Owner:NAT TAIWAN UNIV
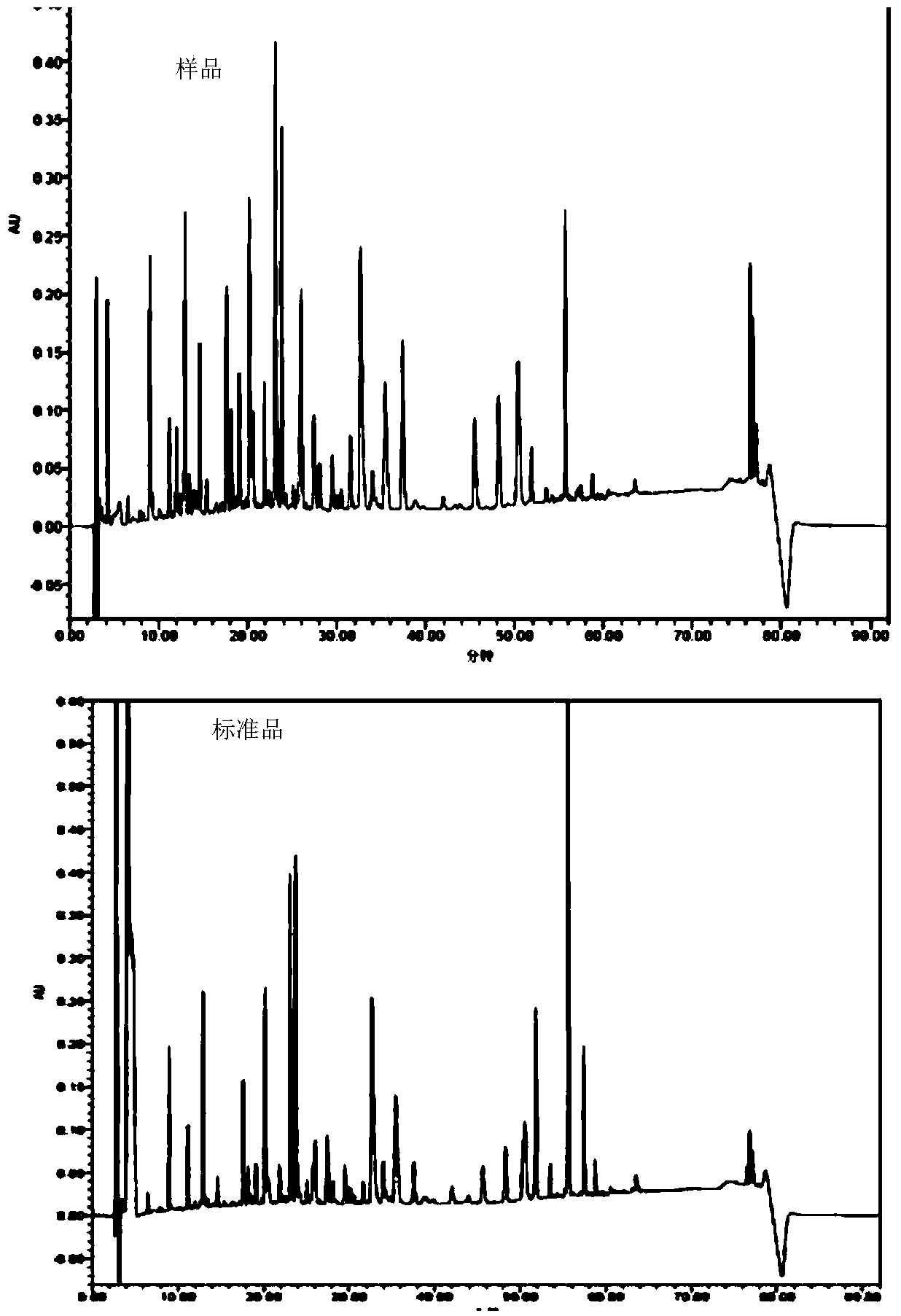
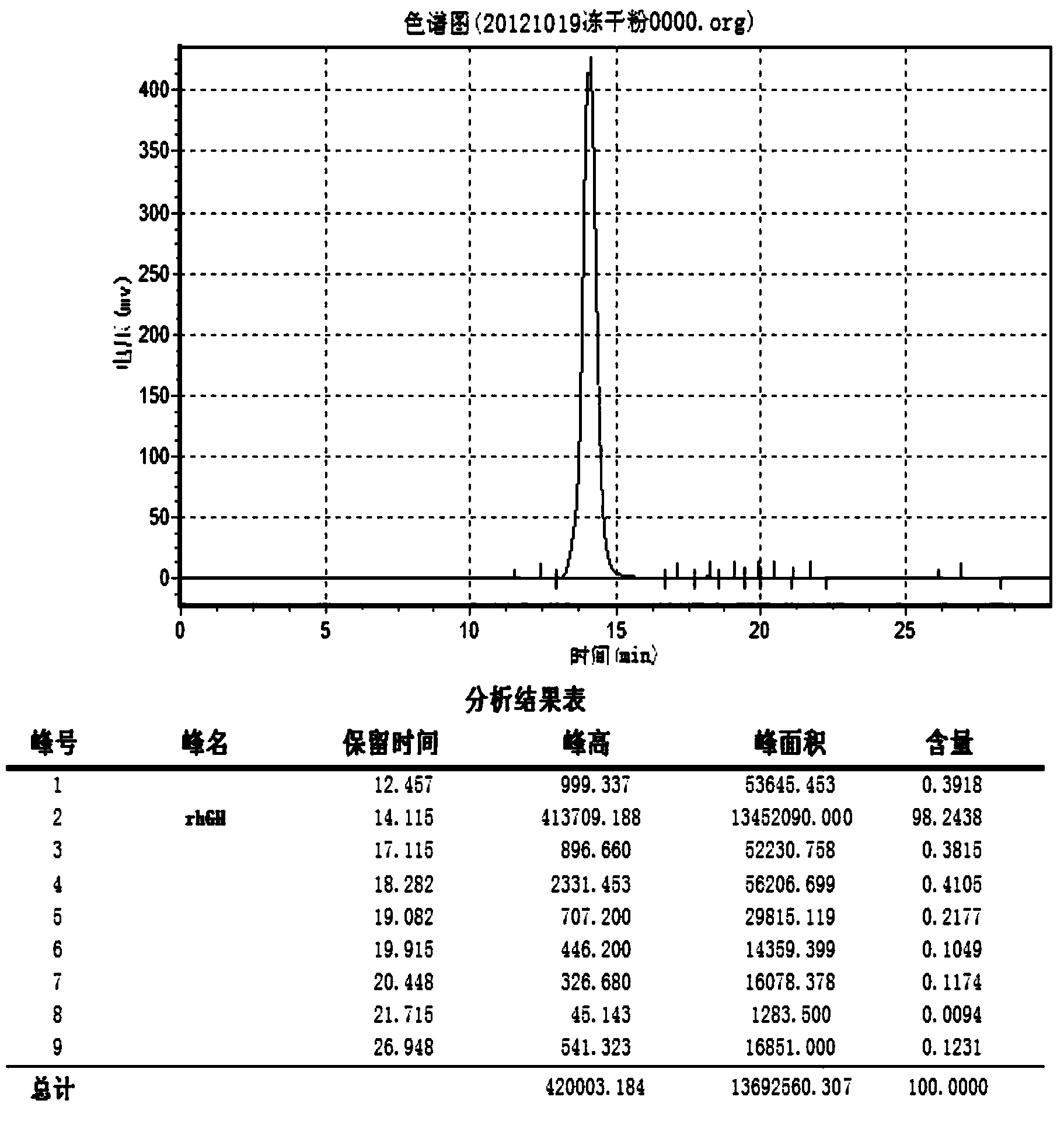
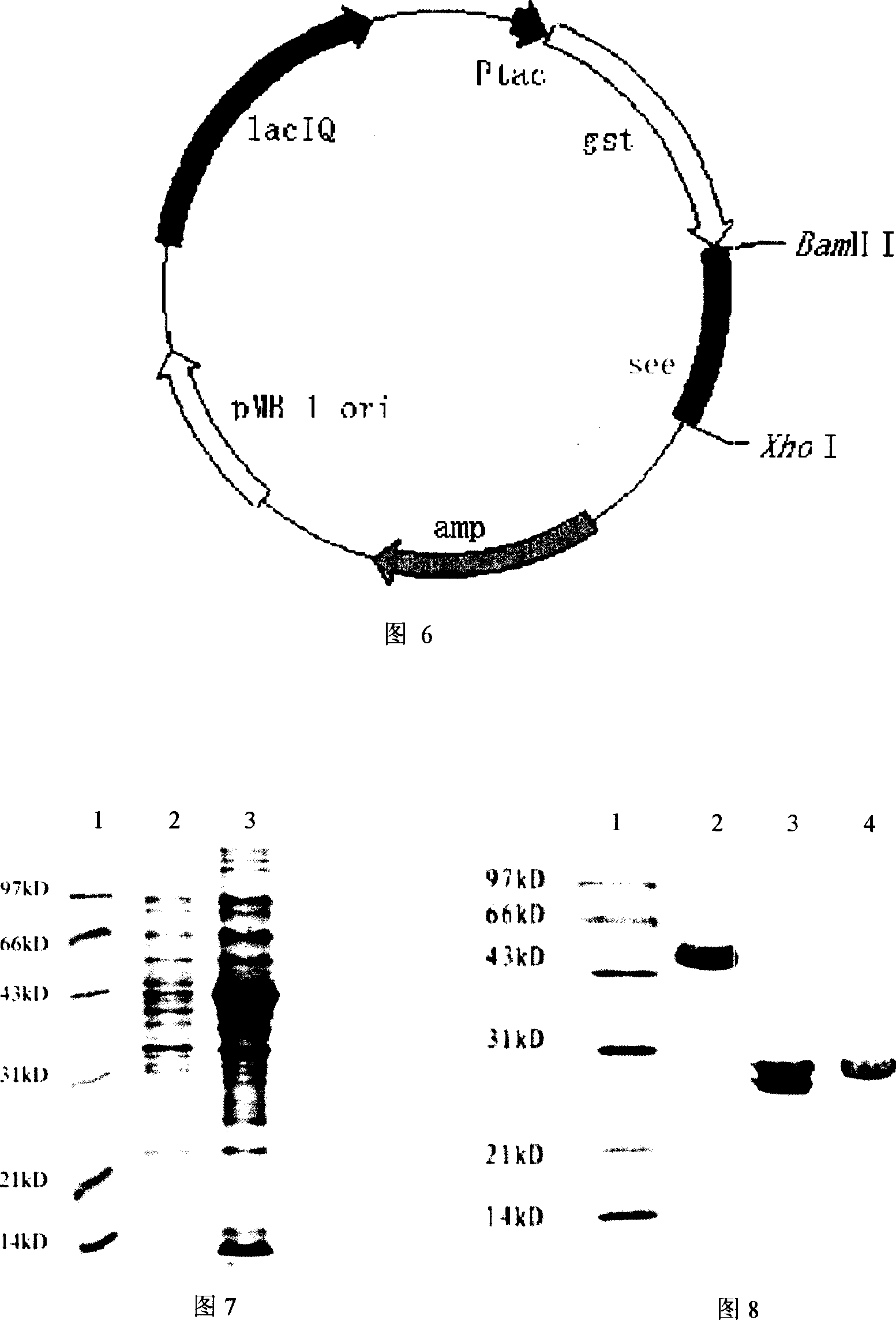
Recombinant engineering bacteria for efficiently expressing human growth hormone, construction method and application
ActiveCN103882015AEfficient secretionGood secretory expressionBacteriaMicroorganism based processesPeptideToxin
The invention discloses recombinant engineering bacteria for efficiently expressing human growth hormone (hGH), a construction method and an application and provides an escherichia coli operon for expressing recombinant hGH, an expression plasmid containing an operon sequence and engineering bacteria QJSW-SZ01 (CGMCC No:7258) used for secretory expression of the recombinant hGH and obtained by transformation of the expression plasmid containing the operon. The recombinant engineering bacteria are characterized in that a coding sequence of a signal peptide of an escherichia coli heat-stable enterotoxin is changed, and rare codons of escherichia coli in the coding sequence are mutated so as to avoid formation of a secondary structure; meanwhile, an amino acid is altered on the terminal of the coding sequence so that the coding sequence is more beneficial to guidance of secretory expression of hGH; the signal peptide, an escherichia coli alkaline phosphatsae promoter (phoA promoter) and an escherichia coli T7 terminator are combined to be used as an expression control element so that the recombinant hGH is efficiently secretory-expressed in the escherichia coli in a soluble form. The recombinant engineering bacteria lay the foundation of finally developing a low-cost hGH pharmaceutical product.
Owner:吉林省奇健生物技术有限公司
Staphylococcus aureus enterotoxin E and its preparation and uses
InactiveCN1962692ATypical superantigen activityGrowth inhibitionBacteriaDepsipeptidesAntigenStaphylococcus aureus enterotoxin E
The invention discloses a recombination Staphylococcus aureus enteritis toxin E with SEQ ID NO.1 amino acid sequence, which is composed of pGEX-4T-1 and SEQ ID NO.2 nucleotide sequence. The invention utilizes hyperantigen activity to accelerate the breeding of splenocyte and inhibit tumour from growing, which is fit for preparing high-purity enterotoxin and hyperantigen agent.
Owner:ZHEJIANG UNIV
Immunogenic detoxified mutant e. coli lt-a toxin
InactiveUS20030113338A1Maximise adjuvanticityMaximise immunogenicityAntibacterial agentsBiocideEscherichia coliAdjuvant
An immunogenic detoxified protein is provided which comprises the amino acid sequence of subunit A of an E. coli heat labile toxin (LT-A) or a fragment thereof in which at least amino acid Ala-72 of the A subunit is mutated, preferably by substitution with Arg. The toxoid is useful as vaccine against an enterotoxigenic strain of E. coli and is produced by recombinant DNA means by site-directed mutagenesis. It is also an effective adjuvant.
Owner:CHIRON CORP
Oral staphylococcus aureus filtrate preparation for improving immune function
InactiveCN1509725AHigh activityImprove leaching ratePeptide/protein ingredientsBacteria material medical ingredientsMedicineWhite blood cell
An orally applied filtrate of staphylococcus aureus for improving immunity and increasing the number of white cells and white platelets in human body contains enterotoxin SEA, SEB, SEC, SED and TSST-1. Its advantages are sure curative effect and low toxic by-effect.
Owner:SHENYANG XIEHE GRP LTD
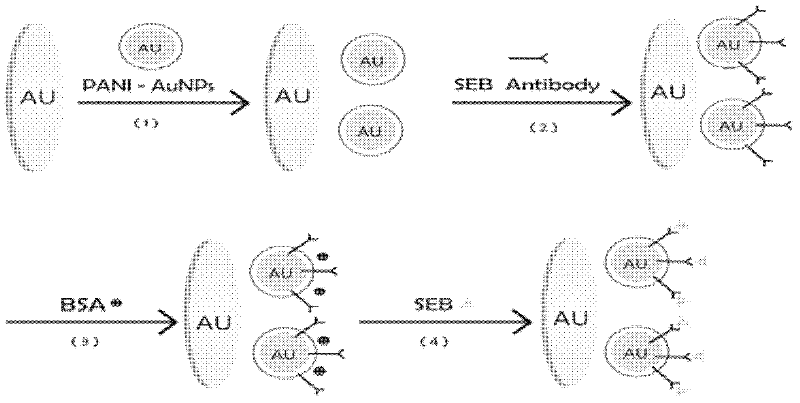
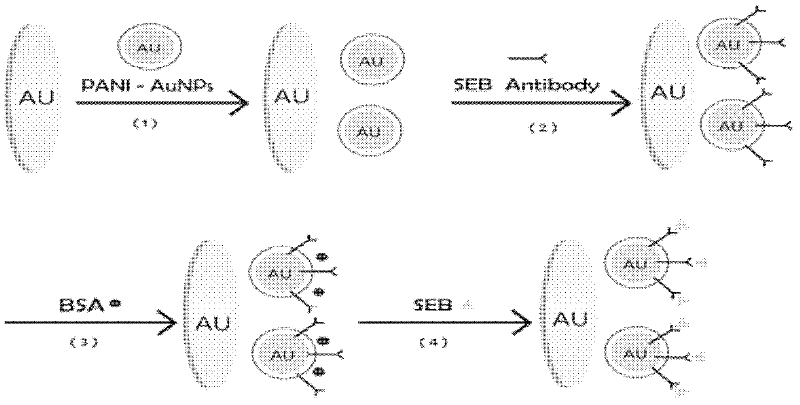
Manufacture method and application of immune sensor based on polyaniline nano-particle composite membrane
InactiveCN102520187AGood dispersionScattered blockingMaterial analysis by electric/magnetic meansBiological testingCorrelation coefficientStaphylococcus aureus enterotoxin B
The invention relates to a manufacture method and application of an immune sensor based on a polyaniline nano-particle composite membrane. By manufacturing the polyaniline nano-particle composite membrane, self-conductivity of materials is improved, and the membrane can be successfully applied to the immune sensor so as to greatly reduce detection limits of the sensor and achieve better stability. Representation and measurement of the sensor are performed through a cyclic voltammetry method and an alternate current impedance method, a staphylococcus aureus enterotoxin B detection standard curve is built, the linear range ranges from 0.1ng / ml to 8ng / ml, correlation coefficient R2=0.9932, and detection limit is 0.033ng / ml(S / N=3). The manufacture method and the application are good in specificity, can be used repeatedly, are good in stability, can be applied to fast detection of staphylococcus aureus enterotoxin B in dairy products and has wide application prospect, and dairy product detection recovery rate ranges from 84% to 111%.
Owner:上海雄图生物科技有限公司
Methicillin-resistant staphylococcus aureus (MRSA) recombinant multivalent subunit genetic engineering vaccine and method for preparing same
The invention discloses methicillin-resistant staphylococcus aureus (MRSA) recombinant multivalent subunit genetic engineering vaccine and a method for preparing the same. The recombinant multivalent subunit genetic engineering vaccine is prepared by recombining, fussing and expressing the active and functional fragments of two antigen molecules of ClfA and IsdB and the specific enterotoxin C mutant for the MRSA to construct recombinant multivalent genetic engineering antigen protein under the assistance of aluminum adjuvant. The recombinant multivalent subunit genetic engineering vaccine is constructed with a unique method and prepared with simple process, is easy to be amplified and has good repeatability and high protein purity. The experiment on the animal shows that the recombinant multivalent subunit genetic engineering vaccine can effectively stimulate the body to produce higher humoral immune response and have good immune protection function.
Owner:CHENGDU OLYMVAX BIOPHARM +1
Antibodies for the treatment of clostridium difficile-associated infection and disease
ActiveUS20130202618A1Improve the quality of lifeConvenient treatmentAntibacterial agentsPeptide/protein ingredientsClostridial infectionBacteroides
Provided herein are reagents, compositions, and therapies with which to treat Clostridium difficile infection and related disease conditions and pathologies, such as Clostridium difficile-associated diarrhea, resulting from infection by Clostridium difficile bacteria and the enterotoxins produced by these bacteria. In particular, antibodies or antigen-binding fragments thereof that bind specifically to toxin A and / or toxin B of C difficile and neutralize the activities of these toxins; compositions comprising such antibodies; and methods of using the antibodies and the compositions are provided.
Owner:PROGENICS PHARMA INC
Rebuild golden staphylococcus enterotoxin N and preparation and application thereof
InactiveCN101037478ATypical superantigen activityGrowth inhibitionPeptide/protein ingredientsDepsipeptidesAntigenStaphylococcus cohnii
A recombinant Methicillin-resistant Staphylococcus aureus enterotoxin N belongs to a super antigen with SEQ ID NO.1 amino acid sequence, which is expressed by a recombination of a gene coding SEN from the Staphylococcus aureus and a plasmid vector, and a transformation to the proper host and is obtained a recombinant a highly purified SEN protein by affinity purification. The invention proves the recombinant protein has a typical super antigen activity better than SEC and can be applied in the preparation of activating the lymphocytes multiplication and inhibiting the tumor cell proliferation. The product is suitable for preparing the highly purified enterotoxin with the super antigen activity and exploiting super antigen agent. The invention has a wise design, purifies the target protein with affinity chromatography, has a simple process and a high purification speed.
Owner:ZHEJIANG UNIV
Staphylococcus aureus metabolic product medicine oral preparation and its use
ActiveCN1283264CEasy to takeEasy to storeAntibacterial agentsNervous disorderMetaboliteStaphylococcus cohnii
The present invention relates to orally taken medicine preparation for treating tumor, AIDS, diabetes and hepatitis B, stopping drug addiction, resisting virus and raising immunity and with less toxic side effect. The preparation contains original metabolic product liquid obtained with staphylococcus aureus through culture, sterilizing and filtering; or contains metabolic product of staphylococcus aureus with one or more of enterotoxin A, B, C1, C2, C3 and D and TSST-1 as the effective components in the content of 1-50 ng / ml. The preparation of the present invention has high curative effect.
Owner:XIEHE PHARMA FACTORY SHENYANG
Recombinant strain for expression of enterotoxin colibacillus adhesin gene and its application in vitelline antibody fodder
InactiveCN101113428AReduce diarrhea rateImprove feed conversionBacteriaAnimal feeding stuffBacterial AdhesinsRecombinant escherichia coli
The invention pertains to the scientific technical field of animal nutrition and feed, meanwhile, relates to application of animal molecular biologic technology. Particularly, the invention relates to an expression for constructing escherichia coli K88 of enterotoxin of chitterlings and recomposed escherichia coli of F18 adhesion gene faeG and fedF and application thereof in preparation of egg-yolk antibody feed. The escherichia coli expressed the recomposed plasmids of escherichia coli K88ac of the enterotoxin and the adhesion genes faeG, F18ac and fedF is constructed by constructing fusion gene of faeG and fedF by using connecting peptid, and inserting the fusion gene into a prokaryotic expression carrier pET28a. The recomposed escherichia coli BL21 (DE3) / pET-8818 of the invention are preserved in the China Center for Type Culture Collection (CCTCC) with a preservation number of CCTCC NO: M207090. The invention also discloses application of the recomposed escherichia coli with the preservation number of CCTCC NO: M207090 in the preparation of the egg-yolk antibody feed.
Owner:HUAZHONG AGRI UNIV
Gene recombinant vaccine for preventing enterovirus 71 infection and preparation method thereof
The invention discloses a gene recombinant vaccine for preventing enterovirus 71 (EV71) infection and a preparation method thereof. The inflection comprises multiple diseases related with the nervous system such as hand-foot-and-mouth disease, paralytic diseases of sterile meningitis, cephalitis and poliomyelitis and the like. Escherichia coli labile enterotoxin B subunit (LTB) is used as an immunological enhancement adjuvant, two fragments of linear neutralizing epitope SP55 and SP70 in EV71 virus coat protein VP1 are used as antigens, prokaryotic expression plasmids of LTB-SP55-SP70 fusion genes are constructed by using gene engineering technology, the plasmids are expressed in escherichia coli, and a recombinant expression product is purified for preparing the EV71 virus gene engineering vaccine.
Owner:中国疾病预防控制中心病毒病预防控制所 +1
Multi-PCR (polymerase chain reaction) detection method of four diarrhoeic Escherichia coli and primer group thereof
InactiveCN102851384AVerify reliabilityVerify availabilityMicrobiological testing/measurementDNA/RNA fragmentationEnteroinvasive E. coliDiarrheagenic Escherichia coli
The invention relates to a multi-PCR (polymerase chain reaction)-based method for quickly detecting four diarrhoeic Escherichia coli and special primers thereof. The method comprises the following steps: respectively selecting enterohemorrhagic Escherichia coli O157:H7O-antigen gene, enterotoxigenic Echerichia coli LT gene, enteropathogenic Echerichia coli bfpA gene and enteroinvasive Echerichia coli invasion plasmid gene as target genes, carrying out comparative analysis on the gene sequences, selecting the conserved region of the target gene sequence to design and synthesize the primers which are disclosed as SEQ ID NO.1-8 in the sequence table, and establishing a multi-PCR detection method to carry out qualitative detection on the four diarrhoeic Escherichia coli. The method provided by the invention has strong detection specificity. The invention also relates to a kit for detection. The invention is quick and simple and has the advantages of high accuracy and high sensitivity.
Owner:哈尔滨海关技术中心
Antigenically-changed enterotoxin C2 mutant, coding gene thereof, preparation thereof and application thereof
InactiveCN102633867AGood superantigen activityHigh tumor suppressor activityPeptide/protein ingredientsDepsipeptidesAgricultural scienceEnterotoxin
An antigenically-changed enterotoxin C2 mutant, a coding gene thereof, preparation thereof and application thereof relate to the technical field of gene engineering, in particular to an antigenically-changed enterotoxin C2 mutant with officinal prospect, a coding gene thereof, preparation thereof and application thereof. An amino acid sequence of the antigenically-changed enterotoxin C2 mutant is shown in a sequence listing SEQ ID NO: 3, and a base sequence of the coding gene of the enterotoxin C2 mutant is shown in a sequence listing SEQ ID NO: 1.
Owner:SHENYANG INST OF APPL ECOLOGY CHINESE ACAD OF SCI
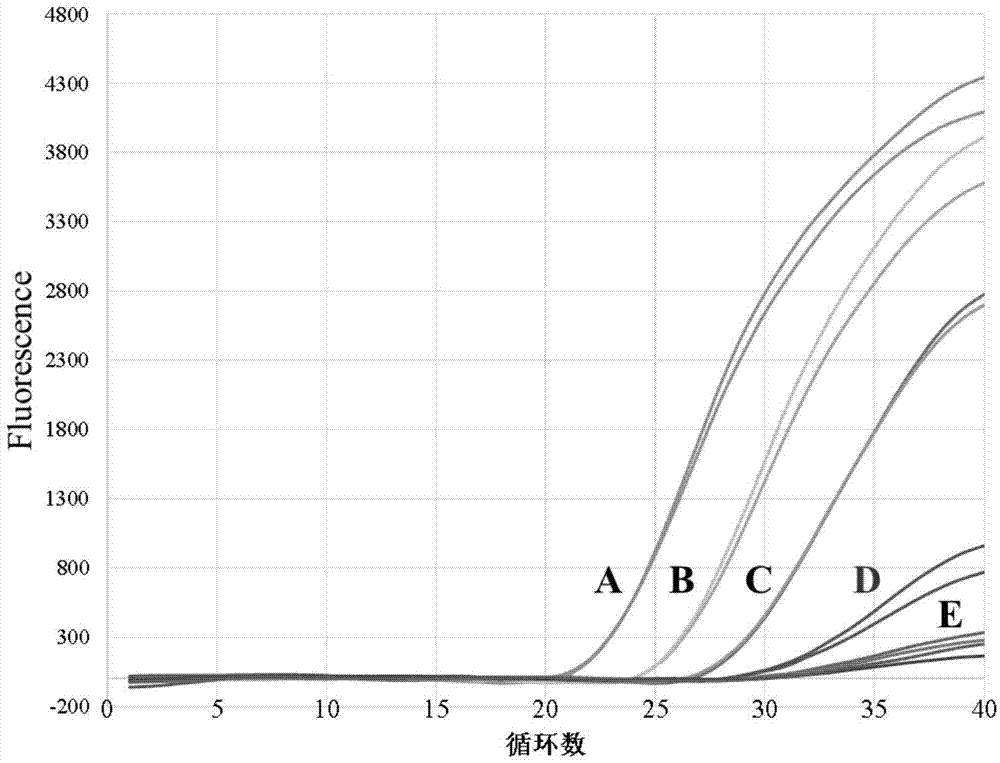
Dual fluorescence quantitative PCR (polymerase chain reaction) detection method and detection kit for clostridium difficile enterotoxin A and B
InactiveCN102952886AReduce serious infectionsSimple and fast operationMicrobiological testing/measurementMicroorganism based processesClostridium difficile toxin BBiology
The invention discloses a dual fluorescence quantitative PCR (polymerase chain reaction) detection method and a detection kit for clostridium difficile enterotoxin A and B. The method comprises the steps that 1) the DNA (deoxyribonucleic acid) of a sample to be detected is extracted; 2) the DNA (deoxyribonucleic acid) of the sample to be detected is used as a template and is subjected to fluorescence quantitative PCR reaction; 3) the fluorescence of each cyclic product in the PCR reaction is subjected to fluorescence detection, and whether the sample to be detected contains clostridium difficile enterotoxin A and B or not is judged according to the lowest Ct value and the highest fluorescence value in the fluorescence detection. The invention also discloses a specific primer, a fluorescence probe and the detection kit. The dual fluorescence quantitative PCR detection method is simple, convenient and quick to operate, can detect the clostridium difficile enterotoxin A and B simultaneously, is high in detection sensitivity and specificity and can be applicable to the laboratory emergency detection of outburst epidemic caused by clostridium difficile.
Owner:ZHANGJIAGANG EENTRY EXIT INSPECTION & QUARANTINE BUREAU
Kit and method for quickly detecting enterotoxin generating escherichia coli
InactiveCN101974616AMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementEnterotoxinGene
The invention discloses a kit and a detection method for quickly detecting enterotoxin generating escherichia coli. The kit contains primers, of which the gene sequences are expressed as SEQ ID No: 1-4; and the kit can specifically amplify genes from the enterotoxin generating escherichia coli. The kit can quickly and accurately detect the food polluted by the enterotoxin generating escherichia coli, and a simple, convenient and reliable method for food production and selling supervision is provided.
Owner:ORIGISSAY BIOLOGICS TECH
Gene chip for detecting enterohemorrhagic escherichia coli, use method and detection kit
InactiveCN101760517AAmplification synchronization is validOvercoming Primer Interference ProblemsMicrobiological testing/measurementAgainst vector-borne diseasesAntigenEnzyme Gene
The invention relates to a gene chip for detecting enterohemorrhagic escherichia coli, comprising a solid-phase carrier and an oligonucleotide specific probe fixed on the solid-phase carrier, wherein the oligonucleotide specific probe fixed on the solid-phase carrier has at least one of the following gene sequences: at least one DNA fragment selected from oligosaccharide unit treatment enzyme gene sequences in O-antigen gene clusters of different sero-group escherichia coli; a DNA fragment selected from coding genes of enterohemorrhagic escherichia coli enterotoxin; a DNA fragment selected from shigella dysenteriae type-1 genes; and a complementary DNA or RNA fragment of the previous DNA fragments. The invention also relates to a use method of the gene chip and a kit comprising the gene chip. The gene chip and the kit can achieve the purpose of detecting the enterohemorrhagic escherichia coli and have simple and convenient operation, high accuracy and strong repeatability.
Owner:TIANJIN BIOCHIP TECH CO LTD
Kit and method for simultaneously detecting Staphylococcus aureus and five enterotoxins thereof
ActiveCN106520923AShort detection cycleImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesStaphylococcus cohniiFluorescence
The present invention provides a kit and a method for simultaneously detecting Staphylococcus aureus and five enterotoxins thereof. The kit comprises a pair of universal primers, a fluorescent probe and a hybridization linking probe designed according to the specific genes of Staphylococcus aureus and five enterotoxins thereof. According to the detection method, the DNA of the suspected bacteria extracted from a sample to be detected is adopted as a template, detection is performed by combining a multiple linking probe amplification technology and a fluorescent probe melting curve technology, and the conditions of the Staphylococcus aureus and the five enterotoxins thereof in the sample to be detected are analyzed and treated through the melting curve.
Owner:SHENZHEN CENT FOR DISEASE CONTROL & PREVENTION
A set of oligonucleotide probe for detecting intestinal hemorrhage type colibacillus and vibrio cholerae
InactiveCN1661113AAccurate detectionEfficient detectionMicrobiological testing/measurementAgainst vector-borne diseasesDiseaseFood poisoning
A set of oligonucleotide probe for detecting the enterohemorrhagic E. coli O157:H7 and cholera vibrio O139 is designed on the Shiga-like toxin generating gene stx1 or stx2 and the beta-glucuronidase gene uidA of said enterohemorrhagic E coli O157:H7 and the enterotoxin A subset (ctxA), toxicity coordinate pilus A subset (tcpA) and glucosyltransferase (LPS gt) genes of cholera vibrio O139, and has high sensibility and specificity. It can be used to detect the enterohemorrhagic E. coli O157:H7, cholera vibrio O139 and other pathogenic genes from the specimen.
Owner:RADIOLOGY INST ACAD OF MILITARY MEDICINE SCI PLA
Method for preparing Chinese medicinal veterinary medicament for enhancing non-specific immune function
InactiveCN101926861AEnhance non-specific immune functionDecreased growth performanceImmunological disordersPlant ingredientsDiseaseBaical Skullcap Root
The invention relates to a veterinary Chinese medicinal composition for improving growth performance and enhancing the non-specific immune function of an organism. The composition is prepared from the following raw material medicaments: Chinese pulsatilla root, astragalus, large head atractylodes rhizome, indigowoad root, baical skullcap root, bupleurum and rhubarb. The invention also relates to a veterinary medicament prepared from the veterinary Chinese medicinal composition, a method for preparing the veterinary medicament and the application of the veterinary Chinese medicinal composition. The veterinary Chinese medicinal composition has the effects of strengthening body resistance, eliminating evil, clearing away heat and toxic material and enhancing the non-specific immune function of the organism and can treat various atypical symptoms such as low immune function of the organism, immune tolerance, endogenous toxin, stress and the like, the decrease of the growth performance and damp-heat symptoms such as anemopyretic cold, flu, gumboro disease, enterotoxin syndrome and the like.
Owner:四川华神农大动物保健药品有限公司
Method for detecting staphylococcus aureus and enterotoxin A gene in food
ActiveCN104726576ATo make up for the lack of specificityPracticalMicrobiological testing/measurementStaphylococcus cohniiStaphylococcus pseudintermedius
The invention discloses a method for detecting staphylococcus aureus and enterotoxin A gene in food, as well as a detecting target gene, a primer and a probe thereof. The method comprises the following steps: processing a food sample to be tested by using the PMA to eliminate the over high quantification problem caused by the dead bacterium DNA; creating a detecting method for rapidly and quantitatively detecting the staphylococcus aureus in the food, screening the enterotoxin A gene and indicating the false-negative PMA-qPCR of PCR reaction. By adopting the detecting method, the staphylococcus aureus with 10<3>-10<8> cfu / g of pollution load in the food can be accurately quantified within 5-6h and the enterotoxin A gene can be screened. The detecting method provided by the invention is strong in specificity, accurate in quantification and rapid in detecting speed and the viable bacteria also can be detected.
Owner:SHANGHAI JIAO TONG UNIV
Method for detoxifying zearalenone
The present invention provides a novel Bacillus amyloliquefaciens and a method for detoxifying zearalenone. The Bacillus amyloliquefaciens of the present invention shows high zearalenone-degrading activity, non-hemolytic and non-enterotoxin producing properties, and acidic and bile salt resistant. Moreover, B. amyloliquefaciens has the abilities of xylanase, carboxyl-methyl cellulase, amylase, and protease.
Owner:NAT TAIWAN UNIV
Cloning and heterologous expression method of enterotoxin C2 ultra-antigen mutation protein gene
InactiveCN101423834ABiologically activeRetain superantigen activityBacteriaRecombinant DNA-technologyAntigenHeterologous
The invention relates to a genetic engineering technology, in particular to a superantigen mutant protein gene of enterotoxin C2 and a method for cloning, expressing and preparing the same. The superantigen mutant protein gene of the enterotoxin C2 comprises base sequences in tables of SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3 and SEQ ID NO: 4. Allogenetic expression of the superantigen mutant protein gene provides development potential of the enterotoxin C2 in the anti-tumor aspect; and the superantigen mutant protein gene performs deletion mutant on an amino terminal thereof on the basis of SEC2, the amino terminal affects the biological activity, and mutant protein kept with the superantigen activity is expected to be obtained on the basis simultaneously. The superantigen mutant protein gene of the enterotoxin C2 applied to preparing a superantigen anti-tumor biological agent has the characteristics of high yield, stable production, convenient purification and the like.
Owner:SHENYANG INST OF APPLIED ECOLOGY - CHINESE ACAD OF SCI
Mucosal membrane receptor and uses thereof
ActiveUS20110129525A1Beneficial effectPowder deliveryCompound screeningBacterial diarrheaReceptor for activated C kinase 1
The invention is based on the identification of aminopeptidase N (APN) as the receptor for F4 fimbriae of enterotoxigenic E. coli (ETEC). Based on the observation that oral administration of F4 fimbriae induces a protective intestinal mucosal immune response against a subsequent challenge with F4 ETEC, and the observation that the internalization of said F4 fimbriae is clathrin-mediated, the present invention provides the characterization of APN as a target useful in: in an in vitro assay to screen for molecules that are capable to mimic the clathrin-mediated F4 endocytosis; in an in vitro assay to screen for molecules that are capable to modulate the binding of F4 fimbriae with APN; in the development of a carrier for the delivery of antigens / therapeutics, i.e. immunomodulators to the intestinal submucosa or the intestinal mucosa-associated lymphoid tissue, wherein said carrier comprises an APN specific target molecule that mimics the clathrin-mediated F4 endocytosis. The use of the carriers thus identified or the treatments thus identified, in a method of inducing an antigen specific intestinal mucosal immune response, and / or in the treatment of bacterial diarrhea, is a further aspect of the present invention.
Owner:UNIV GENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com