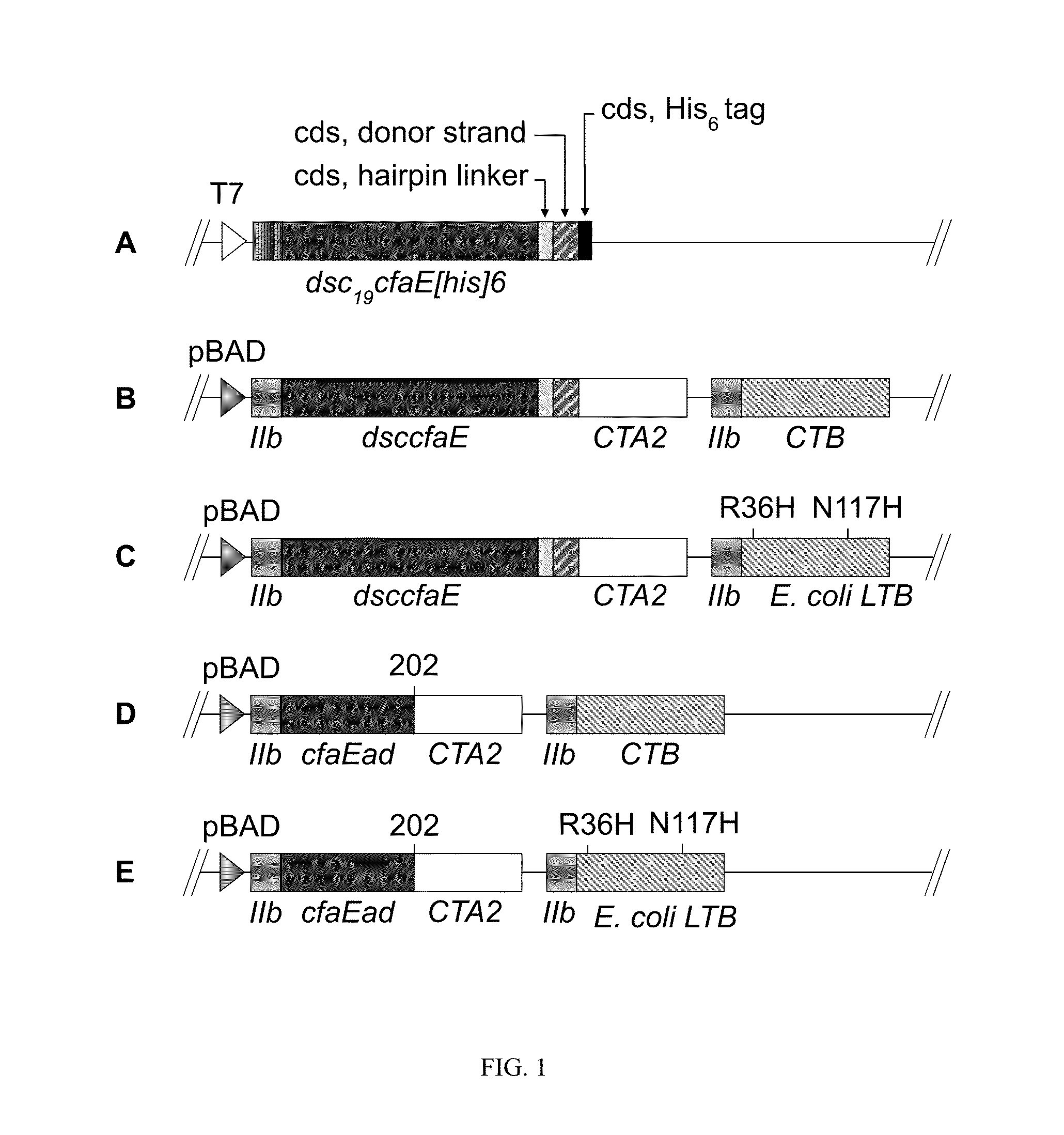
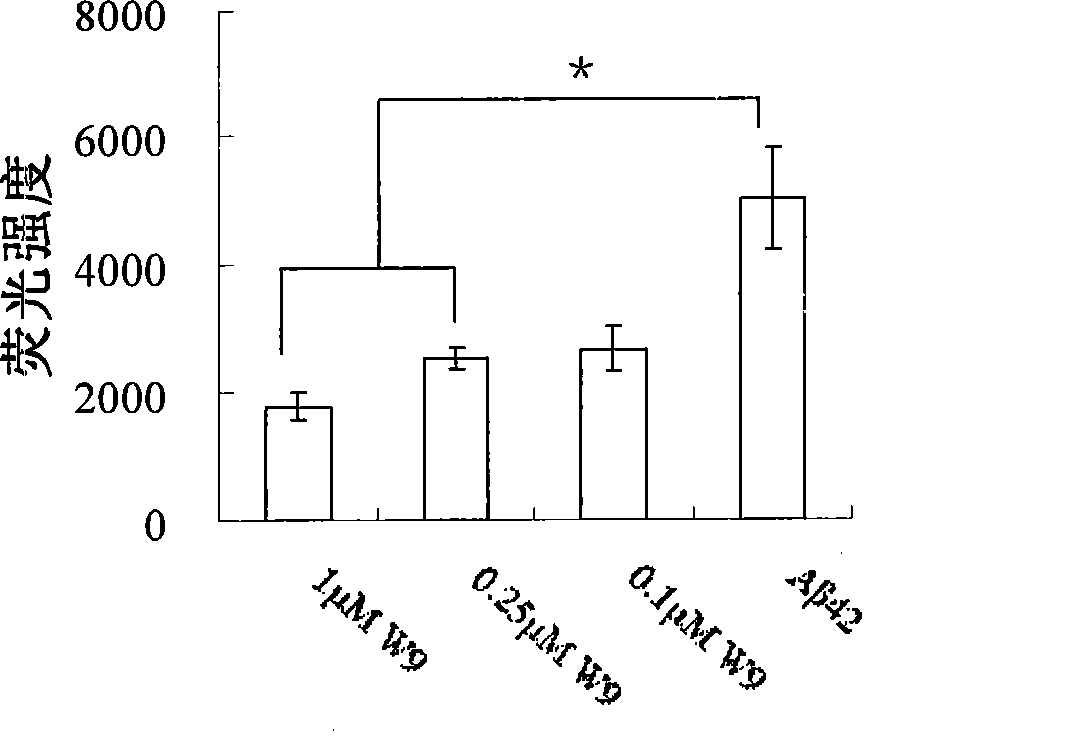Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
71 results about "Enterotoxigenic Escherichia coli" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enterotoxigenic Escherichia coli (ETEC) is a type of Escherichia coli and one of the leading bacterial causes of diarrhea in the developing world, as well as the most common cause of travelers' diarrhea. Insufficient data exist, but conservative estimates suggest that each year, about 157,000 deaths occur, mostly in children, from ETEC. A number of pathogenic isolates are termed ETEC, but the main hallmarks of this type of bacteria are expression of one or more enterotoxins and presence of fimbriae used for attachment to host intestinal cells. The bacteria was identified by the Bradley Sack lab in Kolkata in 1968.
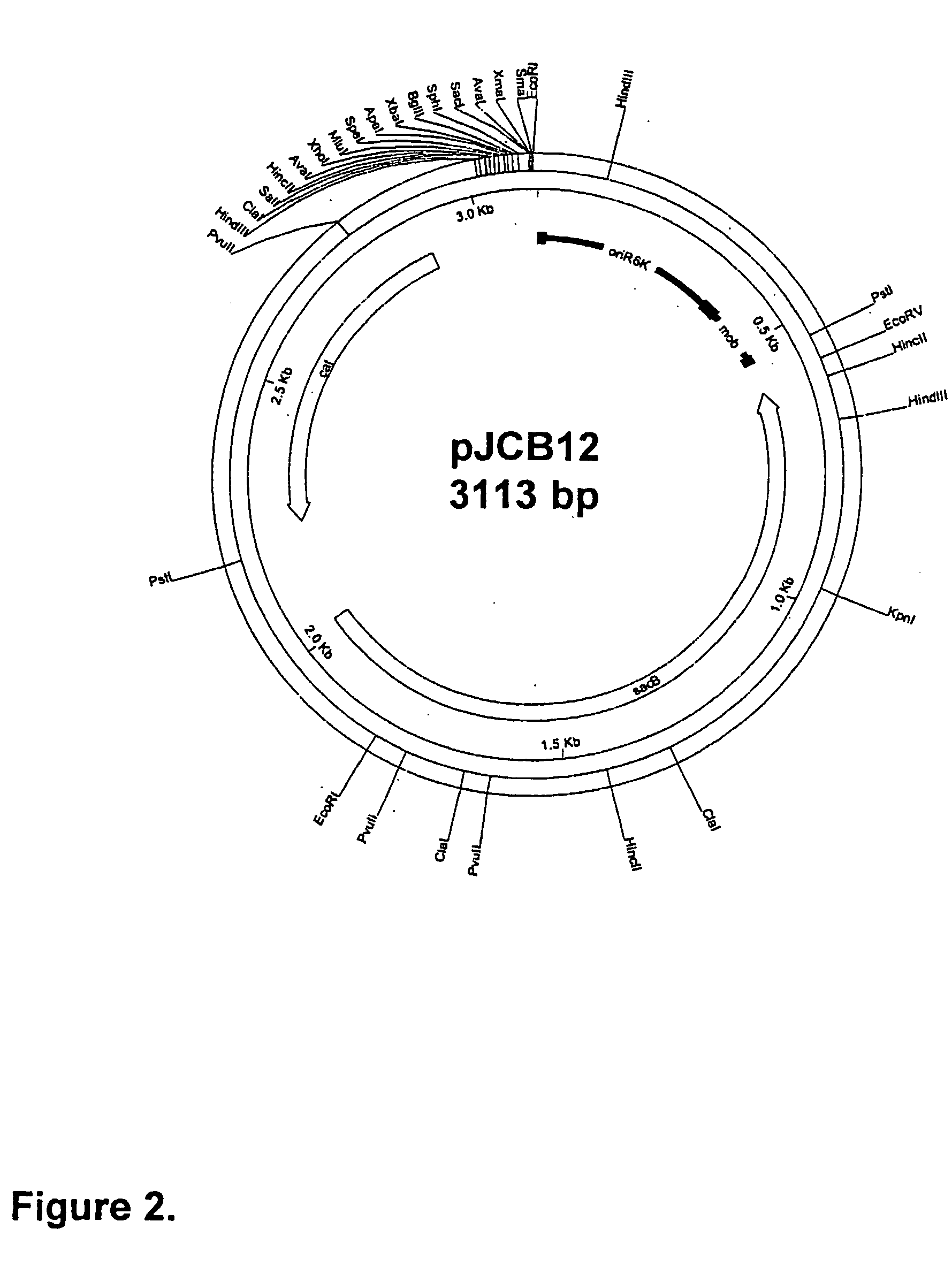
Bacteriophage EK99-C of enterotoxigenic Escherichia coil ETEC-K99 and application thereof
InactiveCN101724607ASafety precautionsSafe therapeuticAntibacterial agentsDigestive systemEnterotoxigenic Escherichia coliBiology
The invention relates to a bacteriophage, in particular to a strong-lytic bacteriophage EK99-C of an enterotoxigenic Escherichia coil K99 (ETEC-K99). The invention discloses the strong-lytic bacteriophage of the enterotoxigenic Escherichia coil K99 obtained by separation and application thereof in the aspects of young animal diarrhoea treatment, bacterial infection and environment purification and sterilization. The bacteriophage (named EK99-C) obtained by separation is a bacteriophage monomer which has strong lytic activity to a pathogenic E. coli K99 bacterial strain (ETEC-K99) and can be applied to the treatment of animal diarrhoea caused by the K99 bacterial strain of young animals (such as pigs, cattle and sheep), sterilization of environments of animal house breeding, slaughtering and the like, and the sterilization of the K99 bacterial strain on apparatuses. The invention also provides a new method and a new idea for treating the animal diarrhoea and sterilizing the environments and the apparatuses, namely biological prevention and control technology. The bacteriophage is preserved in the CCTCC and the preservation number is CCTCC NO: M209208.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Attenuated bacteria useful in vaccines
InactiveUS20050054075A1Improve protectionReliable and rapid isolationAntibacterial agentsBiocideBacterial strainImmunogenicity
The invention provides strains of bacteria, especially enterotoxigenic E. coli, attenuated by mutations in the genes encoding enterotoxins (LT, ST, EAST1) and optionally further attenuated by deletion of additional chromosomal genes. In addition the invention provides strains of attenuated bacteria expressing immunogenic but non-toxic variants of one or more of these enterotoxins. These bacteria are useful as a vaccine against diarrhoeal disease.
Owner:ACAMBIS RES LTD
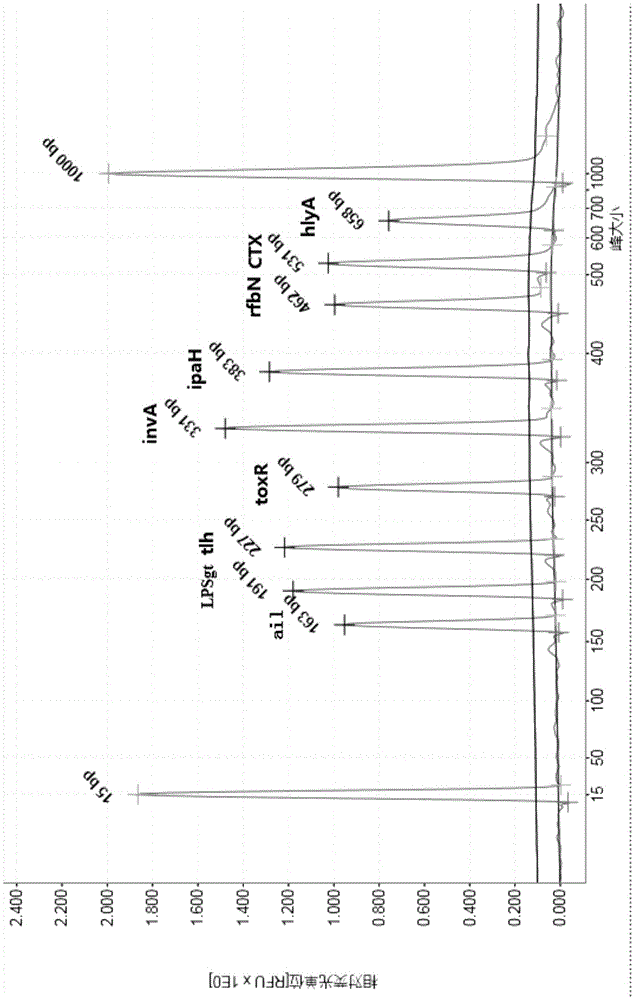
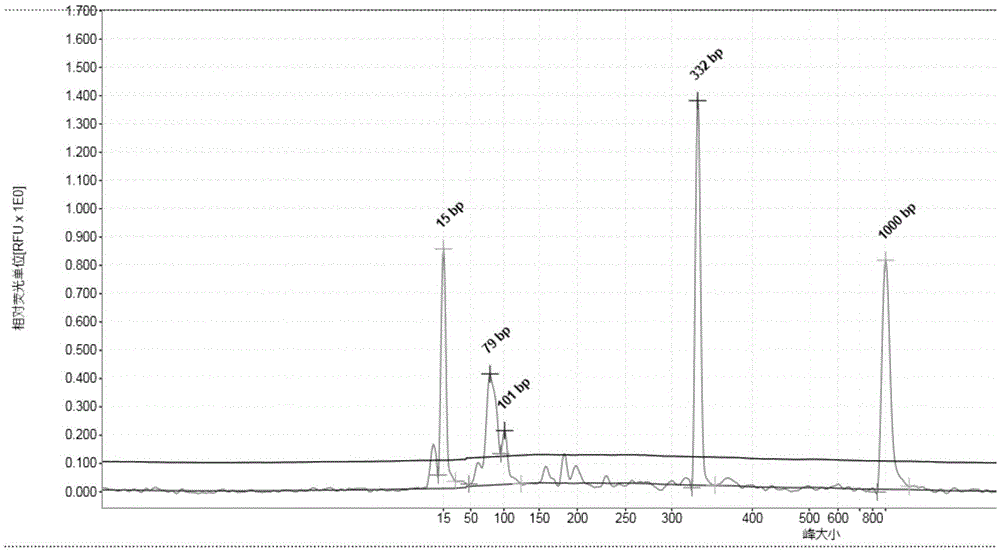
Multiple PCR detection kit for 11 intestinal pathogen nucleic acid and application of detection kit
ActiveCN105525031ASuitable for emergency testingReduce testing costsMicrobiological testing/measurementMicroorganism based processesEscherichia coliVibrio parahaemolyticus
The invention discloses a multiple PCR detection kit for 11 intestinal pathogen nucleic acid and application of the detection kit. The detection kit comprises primers for amplifying 11 intestinal pathogens which include vibrio cholerae serotype O1, vibrio cholerae serotype O139, salmonella, Shigella, vibrio parahaemolyticus, Yersinia enterocolitica, enterophathogenic escherichia coli (EPEC), enteroinvasive escherichia coli (EIEC), enteroaggregative escherichia coli (EAEC), enterotoxigenic escherichia coli (ETEC) and escherichia coli O157:H7. The multiple PCR detection kit has the advantages that the kit is capable of performing multiple detection, high in sensitivity and fast and convenient to use; specific primer sequences are used to guarantee detecting result reliability; the detection method is simple to operate, time saving, labor saving, high in detection throughput, low in reagent consumable cost, capable of directly detecting the nucleic acid extracted from encephalitis pathogens, low in detection platform and staff technical level requirements and capable of being widely popularized in conventional detection.
Owner:NANJING MOKOBIO BIOTECH +2
Transformed bacteria producing CS6 antigens as vaccines
Disclosed herein are antigens that stimulate protective antibodies against enterotoxigenic Escherichia coli. Also disclosed herein are proteins encoded by cssA and cssB genes as well as constructs containing the genes and methods of using thereof.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Gene engineering monoclonal antibody combined with A-beta oligomer specificity
ActiveCN101463082AStrong binding specificityImprove playbackNervous disorderBacteriaEscherichia coliSingle-Chain Antibodies
The invention relates to the technical field of genetic engineering antibody and provides a monoclonal antibody. The variable region of heavy chain of the monoclonal antibody contains the amino acid sequences shown in SEQ ID NO.1, SEQ ID NO.2 and SEO ID NO.3; the variable region of light chain of the monoclonal antibody contains the amino acid sequences shown in SEQ ID NO.4, SEQ ID NO.5 and SEQ ID NO.6. The invention also specifically provides a humanization single-chain antibody generated by the recombinant strain of enterotoxigenic Escherichia coli with the accession number CGMCC No.2821 and the amino acid sequence of the humanization single-chain antibody is shown in SEQ ID NO.7. The antibody of the invention can be specifically bound with the A-beta oligomer, effectively inhibit the fibrosis aggregation of A-beta and obviously alleviate the toxic effect of the A-beta on cells. The invention also relates to a pharmaceutical composite containing the antibody. The antibody of the invention has strong activity, good specificity, easy preparation and wide prospect of experiment application and clinical application.
Owner:TSINGHUA UNIV
Panda source lactobacillus plantarum and application
InactiveCN106434411AProtection from damageReduce percentageAntibacterial agentsBacteriaEscherichia coliTLR4
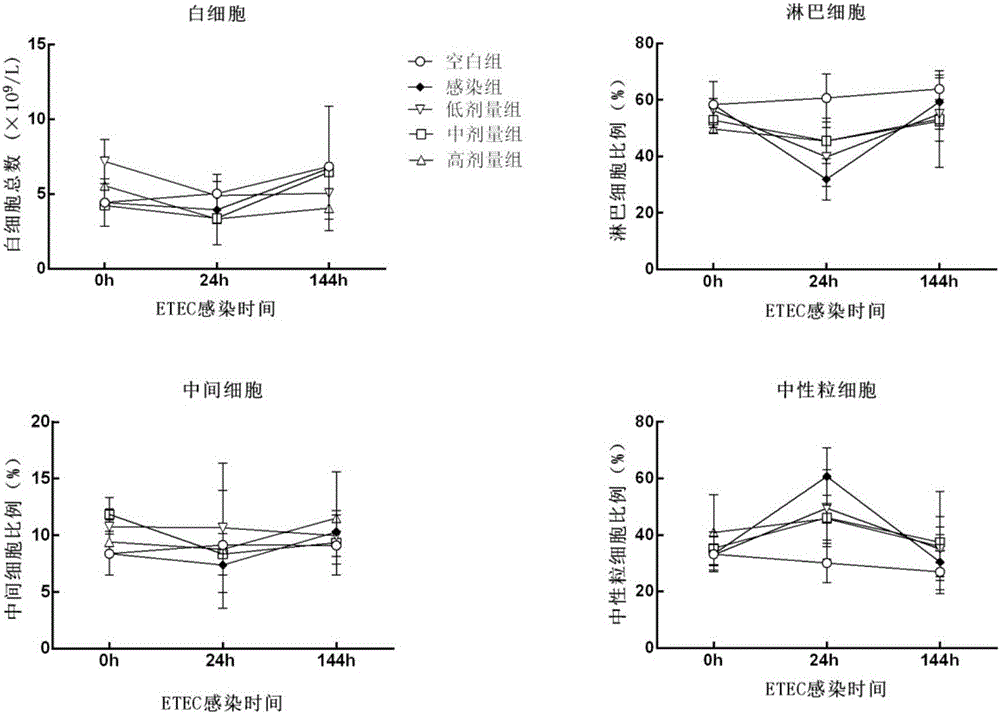
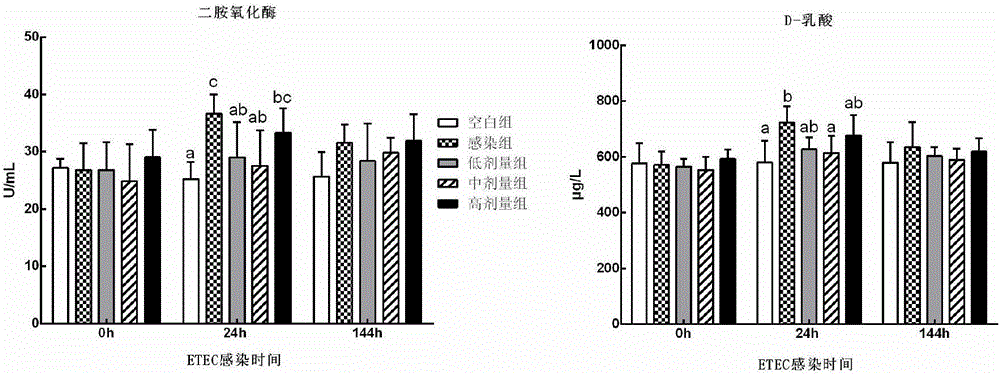
The invention discloses a panda source lactobacillus plantarum and application. The strain was preserved in China Center for Type Culture Collection on May 4, 2016 and the preservation number is CCTCC NO: M2016245. The panda source lactobacillus plantarum provided by the invention can be used for improving antibody levels of IgA, IgM and IgG immune globulins of blood serum; a condition that the percent of lymphocytes is reduced and the percent of neutrophils is increased, caused by infection and induction of enterotoxigenic escherichia coli, is inhibited; expression levels of inflammation related factors IL-1beta, IL-8, IL-6, TLR4 and MyD88mRNA (messenger Ribonucleic Acid) are remarkably down-regulated, and intestinal acute inflammation response caused by the enterotoxigenic escherichia coli is alleviated.
Owner:SICHUAN AGRI UNIV +1
Optimized enterotoxigenic escherichia coli-producing polyvalent antigen gene sequence and application thereof in preventing weaned piglet diarrhea
InactiveCN104593397AFull protective responseHigh expressionAntibacterial agentsBacterial antigen ingredientsEscherichia coliInclusion bodies
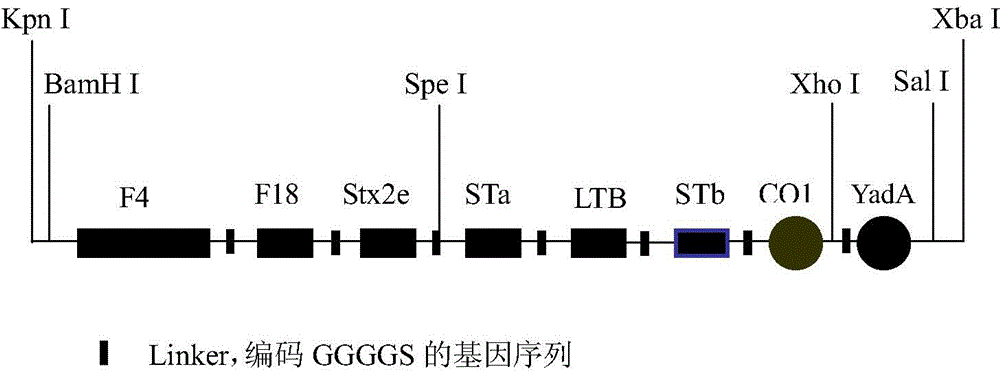
The invention discloses an optimized enterotoxigenic escherichia coli (ETEC)-producing polyvalent antigen gene sequence and the application thereof in preventing weaned piglet diarrhea, and belongs to the technical field of biology. The polyvalent antigen gene sequence is synthesized by a large fragment according to the preference of the lactococcus lactis codon, and the synthesized polyvalent antigen gene sequence contains six antigen genes, namely common ETEC dominant serotype F4<+> primary structure protein FaeG causing the weaned piglet diarrhea, the receptor binding domain RBD of F18<+>, and toxins Stx2e and STa mutant, LTB subunit and STb, and molecular peptides CO1 and YadA31 genes targeting the M cells and the intestinal cells, respectively; the genes are connected by GGGGS. The genes are applicable to constructing a lactic acid bacterium living-vector vaccine and remarkably improving the secretory expression quantity of the target protein, and have excellent immunogenicity and protection effect; the genes also are suitable for high-efficiency expression in the escherichia coli; experiments prove that the inclusion body of the genes has excellent immunocompetence and can be taken as the vaccine for preventing the weaned piglets from F4<+> and F18<+> ETEC infection.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
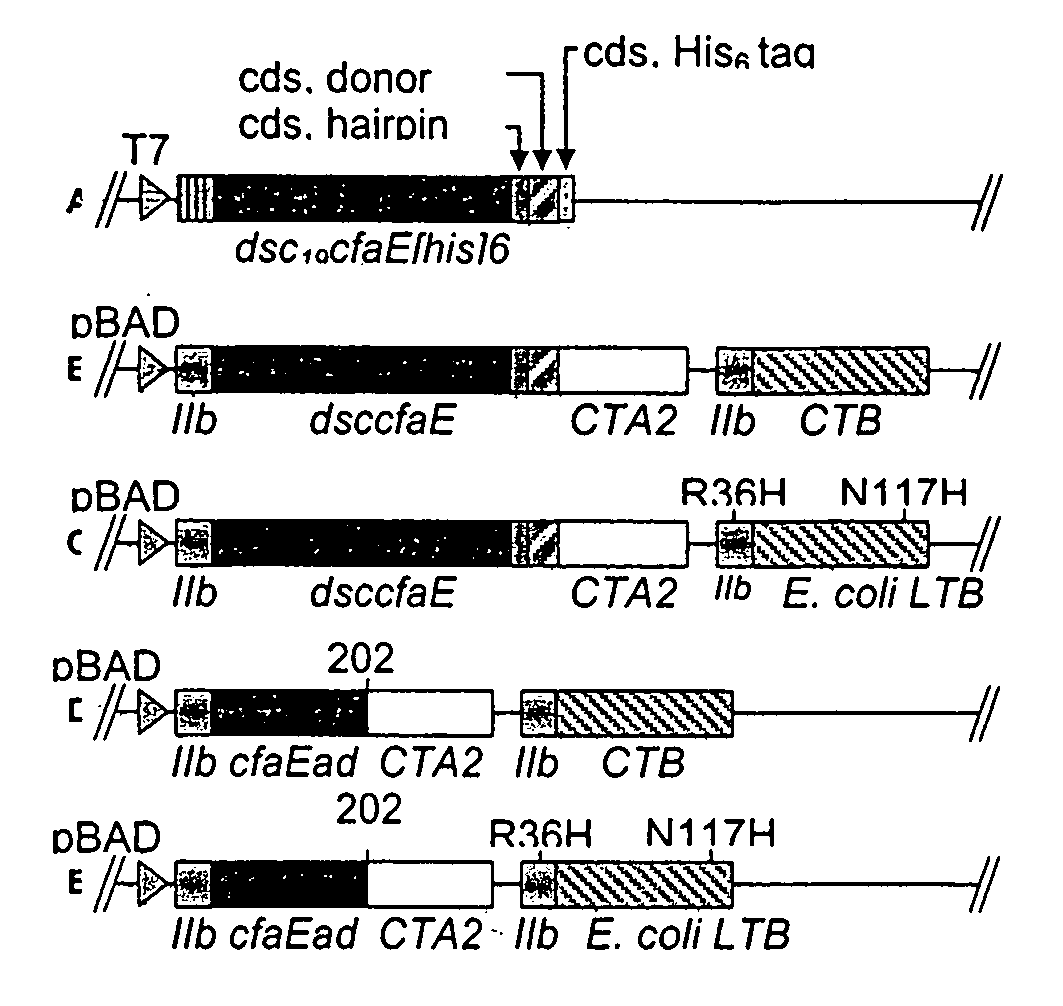
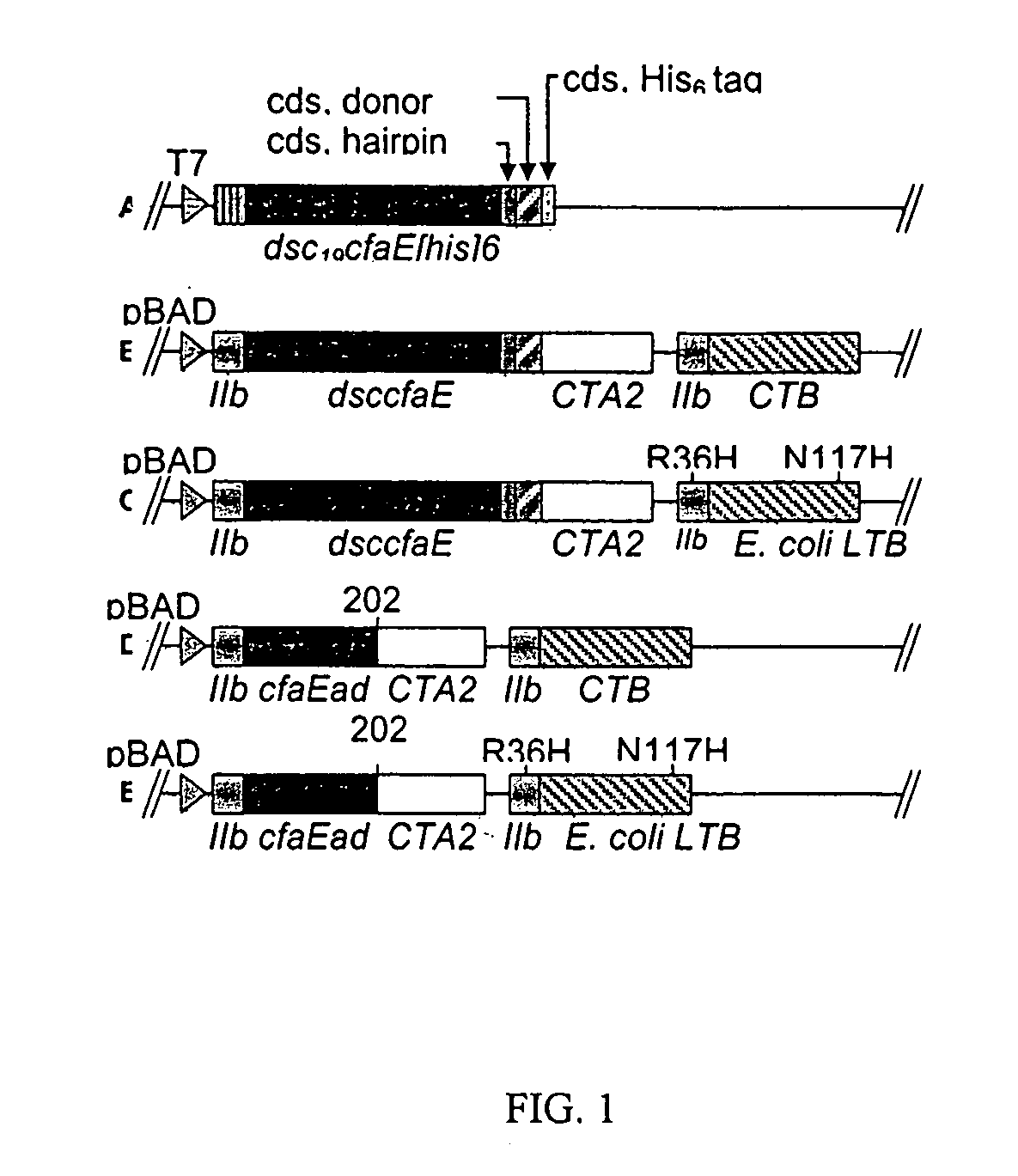
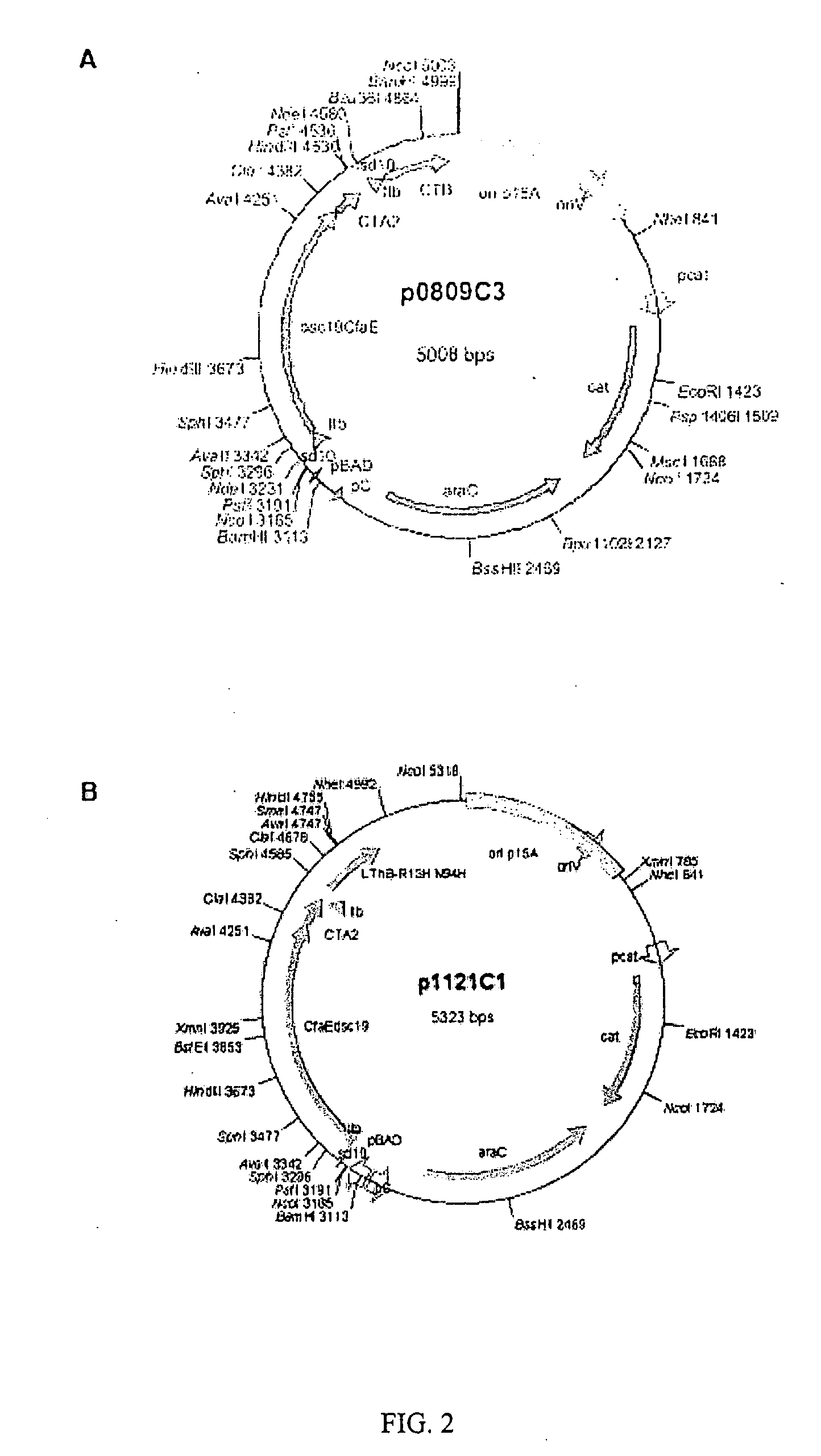
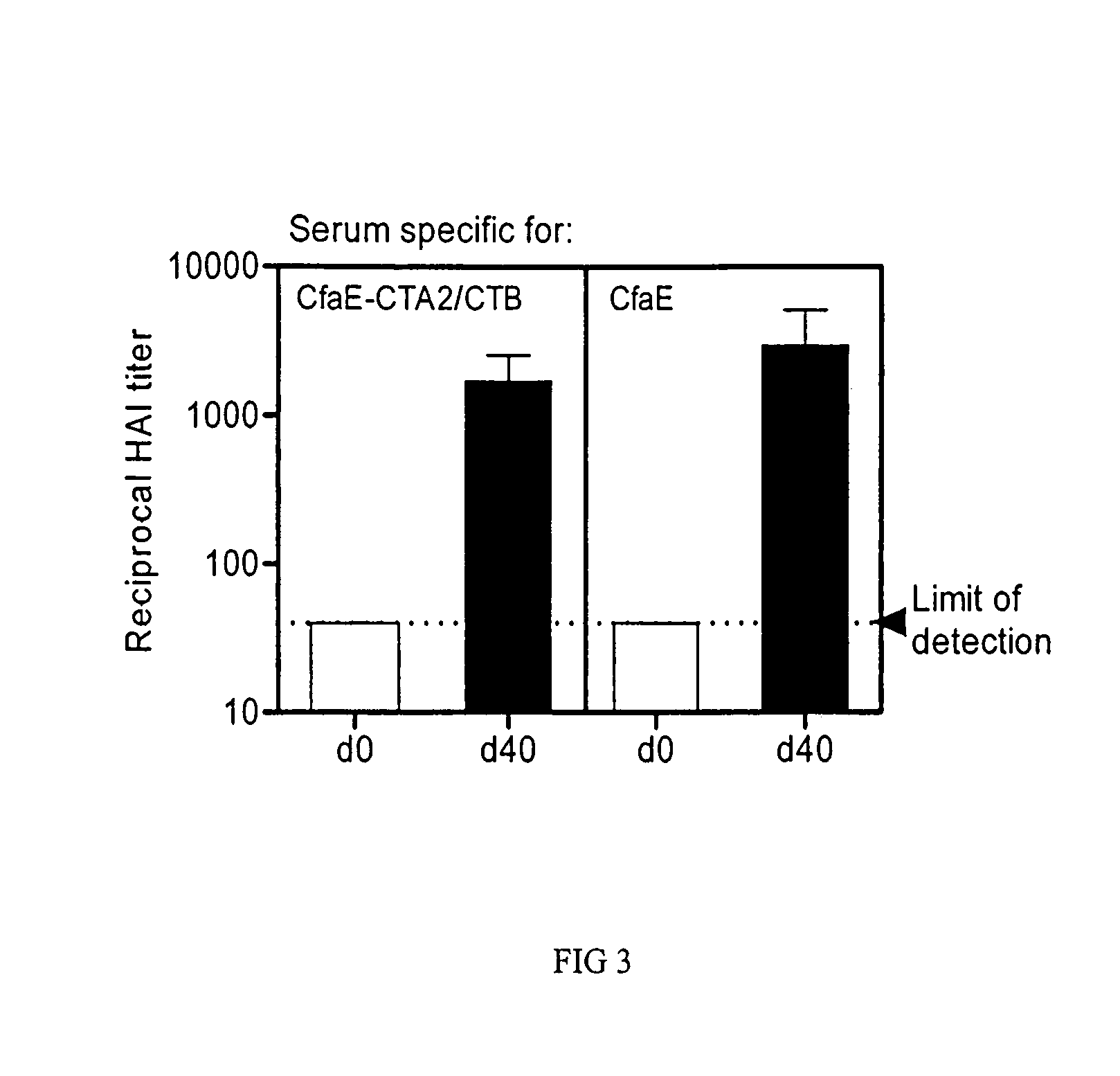
Adhesin-enterotoxin chimera based immunongenic composition against enterotoxigenic Escherichia Coli
The inventive subject matter relates to an immunogenic composition composed of a chimeric molecule including a conformationally stable adhesin from Escherichia coli fused to a bacterial toxin A subunit, such as cholera toxin A subunit or heat-labile Escherichia coli toxin A subunit. The invention also relates to the adhesin-toxin chimera noncovalently associated with a toxin B subunit of the same or different species as the A subunit. The invention also relates to a method of utilizing an adhesin / toxin fusion composition to elicit an immune response.
Owner:UNIV OF COLORADO THE REGENTS OF
Lactobacillus reuteri having probiotic effect and resisting ETEC (Enterotoxigenic Escherichia Coli) and application
InactiveCN108004179AReduce load rateGrowth inhibitionBacteriaMicroorganism based processesLaboratory cultureEnterotoxigenic Escherichia coli
The invention belongs to the technical field of microorganisms, and particularly relates to lactobacillus reuteri from piglet intestines. The lactobacillus reuteri has strong stress resistance, can inhibit ETEC (Enterotoxigenic Escherichia Coli) from growing and the load rate of the ETEC in mice intestines. The invention specifically relates to lactobacillus reuteri having a probiotic effect and resisting ETEC and application thereof. The lactobacillus reuteri is preserved in the China General Microbiological Culture Collection Center, the preservation number is CGMCC 14598, and the lactobacillus reuteri can be applied to feed additives.
Owner:LONGDA FOODSTUFF GRP +1
Gene chip and kit for detecting diarrheagenic escherichia coli in food and clinical samples
InactiveCN101760514AImprove accuracyGood repeatabilityMicrobiological testing/measurementEnteroinvasive Escherichia coliSolid phases
The invention provides a gene chip and a kit for detecting diarrheagenic escherichia coli in food and clinical samples. The gene chip comprises a solid-phase carrier and an oligonucleotide probe fixed on the solid-phase carrier, wherein the oligonucleotide probe contains DNA fragments and the complementary DNA or RNA sequence selected from eae genes of enteropathogenic escherichia coli (EPEC), lt, STap and STah genes of enterotoxigenic escherichia coli (ETEC), stx1 and stx2 of enterohaemorrhagic escherichia coli (EHEC), ipaH genes of enteroinvasive escherichia coli (EIEC), escherichia coli O157wzy genes and escherichia coli 16s genes. The invention further provides the kit containing the gene chip. The utilization of the gene chip and the kit for detecting the diarrheagenic escherichia coli has the advantages of simple operation, high accuracy and strong repeatability.
Owner:TIANJIN BIOCHIP TECH CO LTD +1
Method for fast detecting enterotoxigenic Escherichia coli K88
InactiveCN101968447AHigh sensitivityStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceAptamerEscherichia coli
The invention provides a method for fast detecting enterotoxigenic Escherichia coli K88. In the method, a fluorophore-labeled group (FAM) aptamer is selected to combine with the enterotoxigenic Escherichia coli K88, then a fluorescent signal on the aptamer is captured; an enterotoxigenic Escherichia coli K88 system to be detected is prepared, wherein the concentration of the enterotoxigenic Escherichia coli K88 is given, a fluorescence value with emission wavelength nm is detected at 455nm of excitation wavelength, and a standard curve is drawn; and a sample system to be detected is prepared,and the standard curve is checked in accordance with a fluorescence value thereof to solve the enterotoxigenic Escherichia coli K88 content in the sample to be detected. The method of the invention has the advantages of high sensitivity, strong specificity, wide measurement range, simple devices, and convenient operation, is suitable for detecting the enterotoxigenic Escherichia coli K88 content in various samples containing the enterotoxigenic Escherichia coli K88, and having very important application value in environmental monitoring, food analysis and other aspects.
Owner:邓乐
Colonization factor (CF) antigens of enterotoxigenic escherichia coli in recombinant bacteria
Escherichia coli strains, such as enterotoxigenic E. coli strains, genetically engineered to express from recombinant plasmids one or more colonization factors (CFs) associated with enterotoxigenic Escherichia coli bacteria (ETEC) in an increased amount compared to said CFs expressed by ETEC wild-type reference strains, as well as a method of producing such strains, and vaccine compositions against diarrhea comprising such strains, are described. Further, E. coli strains expressing unnatural combination of at least two different CFs, e.g., CFA / I+CS2, CFA / I+CS6, or CS2+CS4 are disclosed.
Owner:GOTOVAX AB
Vaccine for porcine post-weaning diarrhea caused by enterotoxigenic escherichia coli
Vaccines and methods for making and using the same. An example vaccine may be a vaccine against enterotoxigenic Escherichia coli. The vaccine may include an Escherichia coli strain. The Escherichia coli strain may produce K88 fimbria and a fusion protein including a mutant LT enterotoxin linked with a STb enterotoxin. An example method for producing a vaccine for porcine post-weaning diarrhea may include providing a first strain of Escherichia coli. The strain may include the eltAB gene and the estB gene. The method may also include amplifying the eltAB gene, mutating the eltAB gene, generating a genetic fusion of the mutant eltAB gene with the estB gene, and transforming a second strain of Escherichia coli with the genetic fusion.
Owner:SOUTH DAKOTA STATE UNIVERSITY
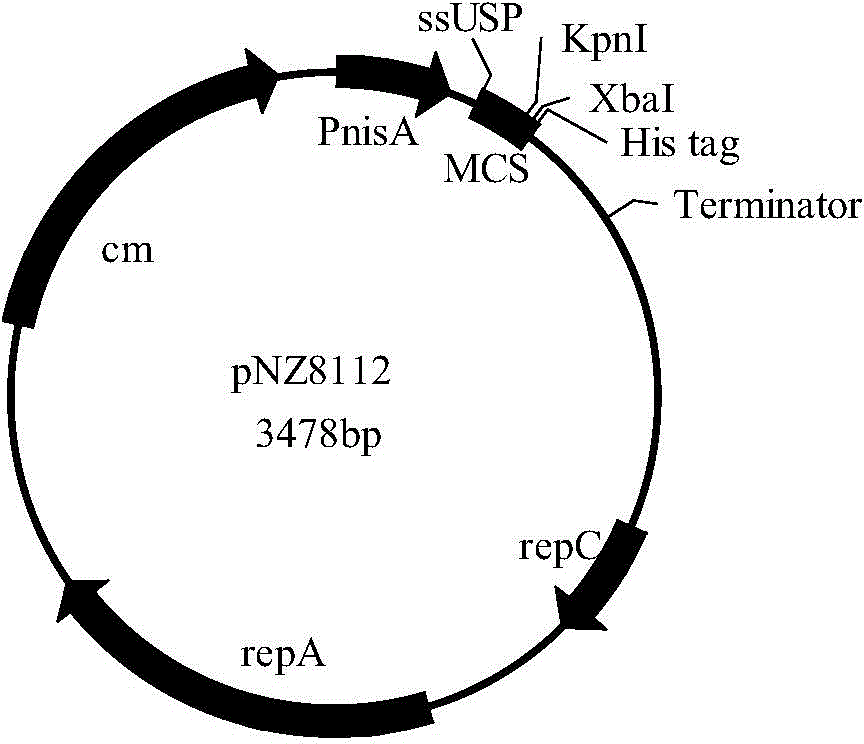
Construction method and application of faeG expressing gene engineering probiotic
InactiveCN102031262AEasy to manufactureImprove immunityAntibacterial agentsBacteriaWestern blotDiarrhea
The invention discloses a construction method and application of a faeG expressing gene engineering probiotic. The method comprises the following steps of: according to the gene sequence and the expression vector plasmid characteristic of a disclosed F4 escherichia coli pilus adhesin faeG, designing a primer which contains a specific endonuclease site; performing polymerase chain reaction (PCR) amplification by taking deoxyribonucleic acid (DNA) of an enterotoxigenic escherichia coli plasmid as a template to obtain a faeG gene-containing segment, linking the segment with an expression vector plasmid pNZ8148 to obtain a recombinant plasmid pNZ8148-faeG, transferring the recombinant plasmid into lactobacillus or a galactococcus cell by using an electrotransformation method to obtain gene engineering probiotic which expresses enterotoxigenic escherichia coli pilus adhesin faeG, expressing under the induction of nisin and analyzing and proving a target gene by SDS-PAGE and Western blot so as to express correctly. A recombinant strain can be used for preventing colibacillus diarrhea of suckling pigs and weanling pigs.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
STa-LTB-STb fusion protein of enterotoxin of escherichia coli and encoding gene and application thereof
InactiveCN109467606APreserve immunogenicityAvoid inclusion body formsAntibacterial agentsFungiAntigenAnimal husbandry
The invention discloses a STa-LTB-STb fusion protein of enterotoxin of escherichia coli. The amino acid sequence of the fusion protein is as shown in SEQ ID NO.2 and the gene sequence of the fusion protein is as shown in SEQ ID NO.1. The invention also discloses the application of the Ta-LTB-STb fusion protein of enterotoxin in preparing a protein vaccine for preventing or treating escherichia coli induced diarrhea. The STa-LTB-STb fusion protein of enterotoxin has thermal stable enterotoxin and thermosensitive enterotoxin immunogenicity simultaneously. The STa-LTB-STb fusion protein of enterotoxin is prepared into a vaccine as an antigen to immune a mammal, high level thermal stable enterotoxin and thermosensitive enterotoxin antibodies can be generated for the body, so that the protecting effect of the vaccine is improved, and harms of enterotoxigenic escherichia coli to production of animal husbandry are prevented effectively.
Owner:DALIAN UNIV OF TECH
Multiple PCR primer group for detecting five fimbrial genes of enterotoxigenic escherichia coli, kit and detection method
ActiveCN108588248AReliable detectionStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationEscherichia coliSpecific detection
The invention discloses a multiple PCR primer group for detecting five fimbrial genes of enterotoxigenic escherichia coli, a kit and a detection method. The enterotoxigenic escherichia coli causing diarrhea of young livestock is usually provided with F4, F5, F6, F18 and F41 fimbria and encoding genes thereof. A set of specific detection primer group is designed and synthesized by comparing and analyzing five fimbrial gene sequences, a multiple PCR detection method for detecting the five fimbrial genes for the enterotoxigenic escherichia coli is established, and a kit containing the specific primer group is assembled. When being used for detecting the five fimbrial genes of the enterotoxigenic escherichia coli, the kit has the advantages of high specificity, rapidness, simplicity, high sensitivity and the like, is suitable for rapidly detecting the fimbrial genes of escherichia coli separated from diarrhea samples of sick livestock, and is effective technological means of screening andauthenticating the serotype of the fimbria of the enterotoxigenic escherichia coli.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Genetic vaccines for the production of chicken egg-yolk antibodies against enterotoxigenic Escherichia coli and other pathogens
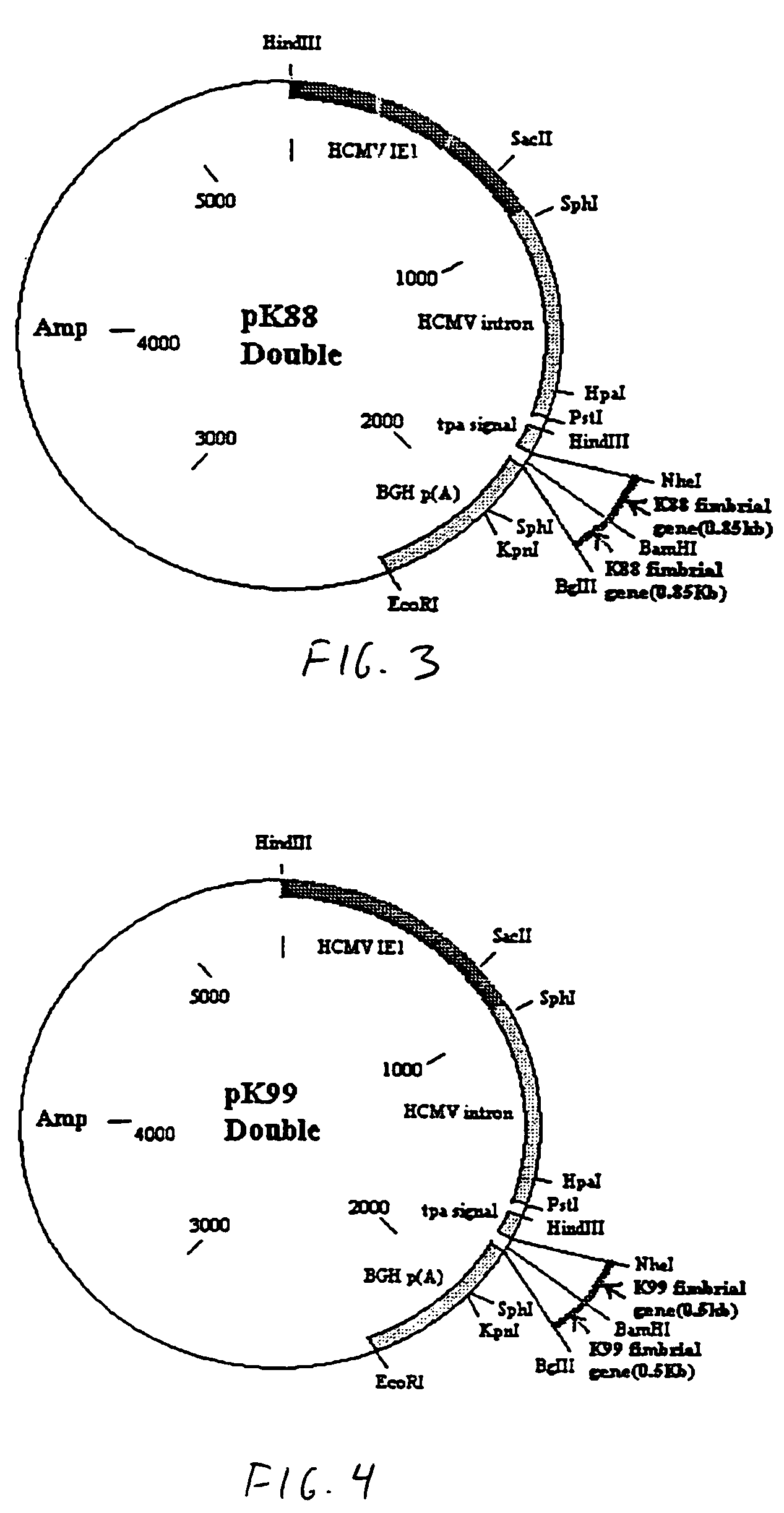
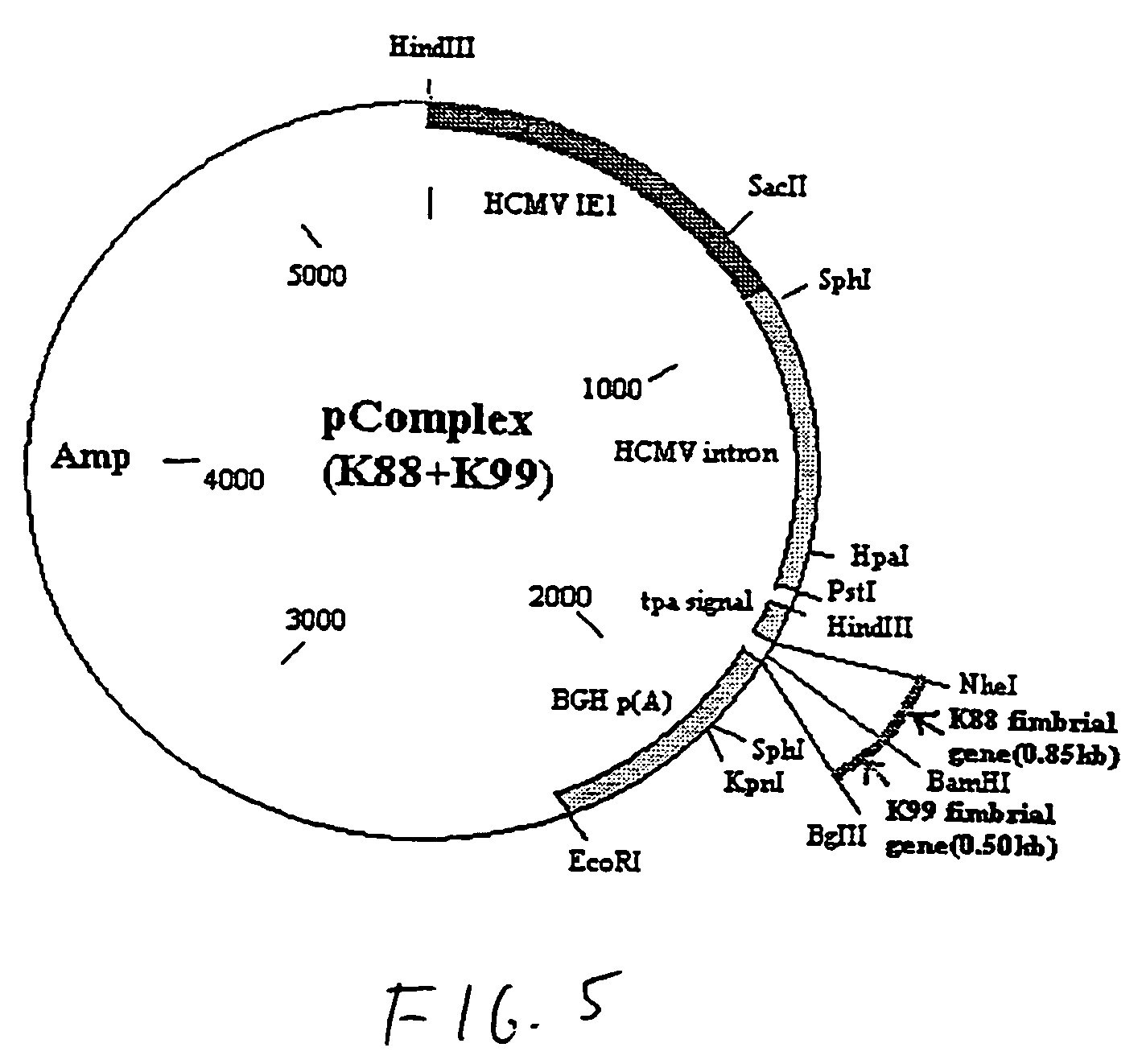
Genetic vaccine which comprises plasmid(s) containing genes coding for antigens of enterotoxigenic Escherichia coli (ETEC) strains is disclosed. Additionally, plasmids may consist of multiple copies of the same antigen (i.e. K88 or K99 fimbrial antigen) or multiple antigens (ie. K88 and K99 fimbrial antigens) and genetic adjuvants such as cytokines (IL-2, IL-4 & GM-CSF), costimulatory molecules (CD80 & CD86) or chemokines or immunostimulatory sequences. A method for isolating antibodies from chicken egg yolk for passive immunization of animals, as well as humans to control diarrhoeal diseases using the genetic vaccines is also disclosed.
Owner:UNIVERSITY OF MANITOBA
Single-chain antibody of swine-origin enterotoxigenic Escherichia coli K88 FaeG resistant protein and preparation method of single-chain antibody
ActiveCN106008710AImmunoglobulins against bacteriaFermentationEscherichia coliSingle-Chain Antibodies
The invention discloses a single-chain antibody of swine-origin enterotoxigenic Escherichia coli K88 FaeG resistant protein and a preparation method of the single-chain antibody. The single-chain antibody is formed by connecting a heavy-chain variable region VH and a light-chain variable region VL of an antibody through short connecting peptide, an amino acid sequence of the light-chain variable region VL is shown as SEQ ID No:1, and an amino acid sequence of the heavy-chain variable region is shown as SEQ ID No:2. Molecular weight of the single-chain antibody is about 28 kDa, the single-chain antibody can specifically recognizes enterotoxigenic Escherichia coli K88 and can be used for blocking infection and adhesion of the enterotoxigenic Escherichia coli K88.
Owner:SHANGHAI JIAO TONG UNIV
K99-987P-F41 recombinant protein and application thereof
InactiveCN103275228AAffecting functions that avoid interactionsStrong specificityAntibacterial agentsBacterial antigen ingredientsAntigenEscherichia coli
The invention relates to a K99-987P-F41 recombinant protein, wherein the amino acid sequence thereof is shown by SEQ ID No.2. The invention also provides a coding gene of the recombinant protein, a preparation method and an application in preparing an enterotoxigenic escherichia coli (ETEC) vaccine. The three-section target genes ETEC adhesins K99, 987P and F41 are serially connected and sub-cloned to an escherichia coli prokaryotic expression vector pET30a(+), and can be used for preparing an escherichia coli K99-987-F41 series connection expression subunit vaccine; multiple antibodies can be obtained by immunizing once, and the vaccine is convenient to use and overcomes the shortcomings of a monovalent vaccine; meanwhile, since a few surface proteins are contained, many unrelated antigenic determinants of bacteria and side reaction caused by crude extraction or semi-purification preparation are eliminated; and the K99-987P-F41 recombinant protein has the characteristics of good safety and good stability, can overcome the shortcomings of whole-cell inactivated vaccines, and has the advantage of high specificity of subunit vaccines.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Primer for quickly determining enterotoxigenic eschericha coli in feed sample and application for primer
ActiveCN102559892AImprove detection efficiencyHigh detection efficiencyMicrobiological testing/measurementFluorescence/phosphorescenceAgricultural scienceFeed additive
The invention belongs to the technical field of feed science and detection of feed additives, and particularly relates to a primer for quickly determining enterotoxigenic eschericha coli K88 in a feed sample and application for the primer. A pair of primers is designed aiming at the pilus specific gene of the enterotoxigenic eschericha coli K88, the gradient dilution of deoxyribonucleic acid (DNA) is used as an external standard substance; and an SYBRGreen I real-time quantitative polymerase chain reaction (PCR) detection method for the enterotoxigenic eschericha coli K88 is established; by the method, the enterotoxigenic eschericha coli K88 in the feed sample can be quantified quickly and specifically; more than or equal to 2*10<2> CFU / g of enterotoxigenic eschericha coli K88 can be detected in the feed sample, and only 5 hours is required by the whole operation process; and compared with the conventional detection method, the method has the advantages of simple detection process, high detection efficiency and accuracy, short detection period and the like, and lays the foundation for development of a kit for quickly and accurately detecting the enterotoxigenic eschericha coli.
Owner:河北方田农牧科技有限公司 +1
Preparation method and use of enterotoxigenic Escherichia coli egg yolk antibody powder
ActiveCN102304182AReduce manufacturing costReduce stressEgg immunoglobulinsFood processingBiotechnologyEscherichia coli
The invention provides a preparation method and use of safe and environment-friendly enterotoxigenic Escherichia coli (ETEC) egg yolk antibody powder. The preparation method comprises: selecting a healthy commercial egg-laying chicken, immunizing the egg-laying chicken by using pig ETEC triple vaccine as an antigen and injecting through muscle according to a ratio of 0.5 to 2ml / feather, obtaining specific IgY, immunizing for 50 days for the first time, starting to collect eggs, and preparing Escherichia coli egg yolk antibody powder, wherein immunization is performed again every other 40 days for reinforcement, and high-titer IgY eggs can be obtained continuously. Fresh egg yolk pulp is quickly dewatered and dried in several seconds by a spray drying technique to form Escherichia coli egg yolk antibody powder, the spray drying temperature at an inlet is 120 to 155 DEG C, the spray drying temperature at an outlet is 60 to 80 DEG C, and the Escherichia coli egg yolk antibody powder is kept in a drying tower for 2 to 3 hours. The preparation method has the advantages that: the Escherichia coli egg yolk antibody powder can be used as a feed additive for various kinds of livestock and poultry and widely used in livestock and poultry production; and the Escherichia coli egg yolk antibody powder can improve the production performance of piglets in early weanling period, has obvious effects on prevention and treatment of diarrhea in piglets and overcomes the drawbacks of coarse egg yolk antibody.
Owner:TWINS FEED
Adhesin-enterotoxin chimera based immunongenic composition against enterotoxigenic Escherichia coli
The inventive subject matter relates to an immunogenic composition composed of a chimeric molecule including a conformationally stable adhesin from Escherichia coli fused to a bacterial toxin A subunit, such as cholera toxin A subunit or heat-labile Escherichia coli toxin A subunit. The invention also relates to the adhesin-toxin chimera noncovalently associated with a toxin B subunit of the same or different species as the A subunit. The invention also relates to a method of utilizing an adhesin / toxin fusion composition to elicit an immune response.
Owner:UNIV OF COLORADO THE REGENTS OF
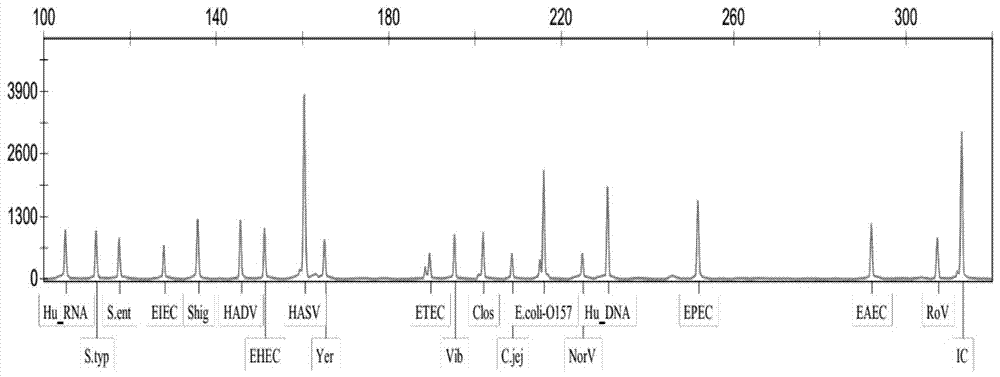
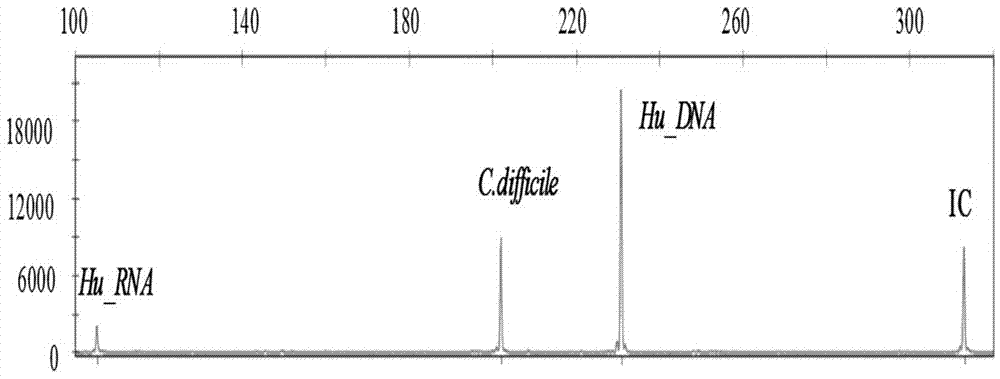
Diarrhea pathogenic bacteria multi-gene detection system and its kit and application
ActiveCN107083446AEliminate pollutionOptimize the reaction systemMicrobiological testing/measurementAgainst vector-borne diseasesEscherichia coliHuman DNA sequencing
The invention relates to a diarrhea pathogenic bacteria multi-gene detection system and its kit and application. The detection system has 15 pairs of primers, wherein 13 pairs of diarrhea pathogens primers, one pair of human genome beta-actin primers and one pair of system quality controlled beta-actin primers are included. Diarrhea pathogens refer to campylobacter jejuni, shigella, clostridium difficile, salmonella enteritidis, salmonella typhimurium, enterotoxigenic escherichia coli, escherichia coli O157, vibriones, yersinia enterocolitica and the like. The diarrhea pathogenic bacteria multi-gene detection system and its kit do not require routine culture and other steps, synchronous detection and analysis of the various diarrhea pathogens can be directly conducted on a stool sample in the same reaction system, the shortcomings that a routine detection method is low in flux and low in detection rate and takes much time are made up for, a comprehensive, accurate and low-cost pathogen diagnosis is provided for clinical use for the first time, and an important reference is provided for individualized medication and accurate medical treatment.
Owner:HUADONG HOSPITAL +1
Gene engineering monoclonal antibody combined with A-beta oligomer specificity
InactiveCN101463084AStrong binding specificityImprove playbackNervous disorderBacteriaEscherichia coliSingle-Chain Antibodies
The invention relates to the technical field of engineered antibody and provides a monoclonal antibody. The variable region of heavy chain of the monoclonal antibody contains the amino acid sequences shown in SEQ ID NO.1, SEQ ID NO.2 and SEO ID NO.3; the variable region of light chain of the monoclonal antibody contains the amino acid sequences shown in SEQ ID NO.4, SEQ ID NO.5 and SEQ ID NO.6. The invention also specifically provides a humanization single-chain antibody generated by the recombinant strain of enterotoxigenic Escherichia coli with the accession number CGMCC No.2820 and the amino acid sequence of the humanization single-chain antibody is shown in SEQ ID NO.7. The antibody of the invention can be specifically bound with A-beta oligomer, effectively inhibit the fibrosis aggregation of A-beta and obviously alleviate the toxic effect of the A-beta on cells. The invention also relates to a pharmaceutical composite containing the antibody. The antibody of the invention has strong activity, good specificity, easy preparation and wide prospect of experiment application and clinical application.
Owner:TSINGHUA UNIV
Bacillus amyloliquefaciens exopolysaccharide for inhibiting growth of enterotoxin-producing escherichia coli
The invention discloses bacillus amyloliquefaciens exopolysaccharide for inhibiting growth of enterotoxin-producing escherichia coli and belongs to the technical field of bioengineering. The molecularweight of the exopolysaccharide is 8005 Da, and the exopolysaccharide is prepared from fructose and glucose and is a levan with a special structure, and the molecular structure of the exopolysaccharide has 7 specific repeating units. Each repeating unit contains 7 fructosyls, uses 6 beta-(2,6)-fructosyl as a framework, and uses one beta-(1,2)-fructosyl as a branched chain. The bacillus amyloliquefaciens exopolysaccharide EPS-JN4 has the effect of inhibiting the growth of enterotoxigenic escherichia coli, has the lowest inhibitory concentration of 5 * 10-7 mg polysaccharide / CFU escherichia coli and has good potential of replacing antibiotics to treat diarrhea caused by escherichia coli.
Owner:JIANGNAN UNIV
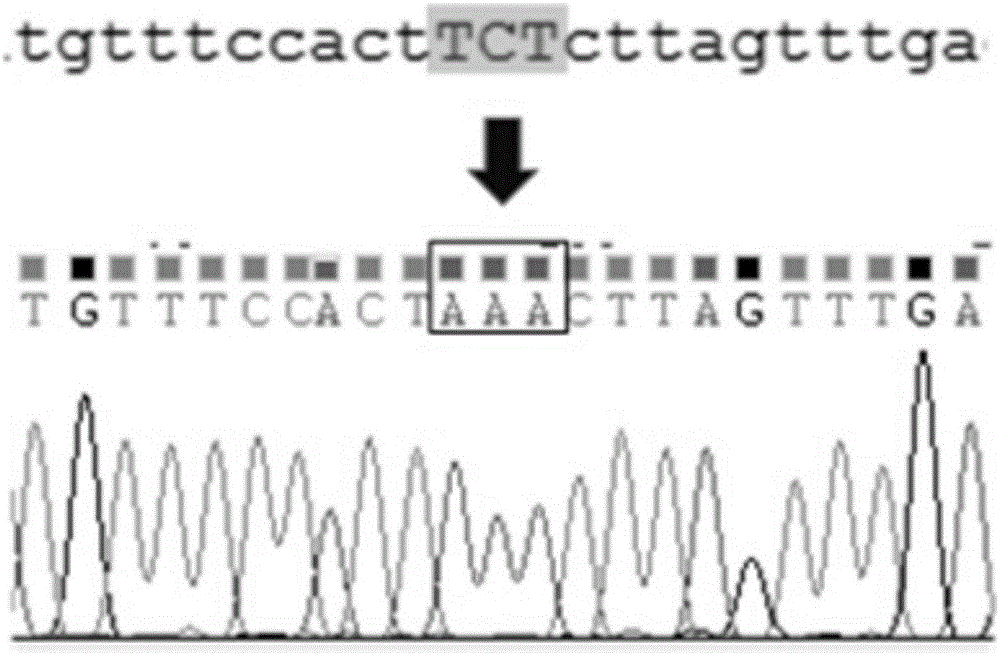
Method for constructing K88ac<+> enterotoxigenic escherichia coli attenuated virus strain and application
ActiveCN106148376AAvoid Easy Lost SituationsDoes not affect treatmentAntibacterial agentsBacteria material medical ingredientsEscherichia coliAntibiotic Y
The invention provides a method for constructing a K88ac<+> enterotoxigenic escherichia coli attenuated virus strain. The method includes the following steps that 1, K88ac<+> enterotoxigenic escherichia coli enterotoxin genes are subjected to traceless point mutation, wherein TCT on the LT I gene of K88ac<+> enterotoxigenic escherichia coli without antibiotics resistance genes is mutated into AAA, and correspondingly-encoded No. 63 serine is mutated into lysine (K); 2, the K88ac<+> enterotoxigenic escherichia coli enterotoxin genes are knocked out, wherein on the basis of the first step, the ST II genes in the K88ac<+> enterotoxigenic escherichia coli are knocked out, and therefore the aim of attenuating the K88ac<+> enterotoxigenic escherichia coili is achieved accordingly. The attenuated virus strain constructed with the method is high in adhesivity and long in in-vivo planting time, infection of the pathopoiesia stain can be prevented, and the method can be used for biological preventing of colibacillosis caused by K88ac<+> ETEC.
Owner:YANGZHOU UNIV
Gene engineering monoclonal antibody combined with A-beta oligomer specificity
ActiveCN101463085AStrong binding specificityImprove playbackNervous disorderBacteriaEscherichia coliSingle-Chain Antibodies
The invention relates to the technical field of engineered antibody and provides a monoclonal antibody. The variable region of heavy chain of the monoclonal antibody contains the amino acid sequences shown by SEQ ID NO.1, SEQ ID NO.2 and SEO ID NO.3; the variable region of light chain of the monoclonal antibody contains the amino acid sequences shown by SEQ ID NO.4, SEQ ID NO.5 and SEQ ID NO.6. The invention also specifically provides a humanization single-chain antibody generated by the recombinant strain of enterotoxigenic Escherichia coli with the accession number CGMCC No.2819 and the amino acid sequence of the humanization single-chain antibody is shown in SEQ ID NO.7. The antibody of the invention can be specifically bound with the A-beta oligomer, effectively inhibit the fibrosis aggregation of A-beta and obviously alleviate the toxic effect of the A-beta on cells. The invention also relates to a pharmaceutical composite containing the antibody. The antibody of the invention has strong activity, good specificity, easy preparation and wide prospect of experiment application and clinical application.
Owner:TSINGHUA UNIV
Use of modified hollow fiber materials for removing exotoxins produced by escherichia coli from liquids, preferably from blood and plasma as well as their use for treating concomitant diseases
ActiveCN103842083AAvoid the disadvantages of treatment measuresIon-exchange process apparatusSemi-permeable membranesFiberESCHERICHIA COLI ANTIGEN
Owner:SAFE BT INC
Specific marking method for enterotoxigenic escherichia coli
ActiveCN109486844ALong wavelengthStrong penetrating powerBacteriaMicroorganism based processesESCHERICHIA COLI ANTIGENIn vivo
The invention relates to a specific marking method for enterotoxigenic escherichia coli. The method includes taking a red fluorescent protein gene as a reporter gene, and utilizing a CRISPR / Cas9 system to knock a RFP gene into a genome of the enterotoxigenic escherichia coli at a fixed point. The specifically-marked enterotoxigenic escherichia coli constructed by the method can realize the specific identification and monitoring of pathogenic escherichia coli, and provide a methodological basis for studying the infection path and pathogenic mechanism of the enterotoxigenic escherichia coli in vivo.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Primer and method for identifying bovine rotavirus and enterotoxigenic escherichia coli
ActiveCN107699639AHigh sensitivityEfficient amplificationMicrobiological testing/measurementMicroorganism based processesEscherichia coliBovine rotavirus
The invention provides a visual multiplex fluorescent LAMP detection method which can detect bovine rotavirus (BRV) and enterotoxigenic escherichia coli (ETEC). According to the detection method, twofluorophores are introduced into an LAMP method, a result is directly judged by virtue of colors of a reaction product, for example, red is namely bovine diarrhea caused by the BRV, and green is namely bovine diarrhea caused by the ETEC. The detection method provided by the invention has the advantage that the two viruses can be identified and detected at the same time through once LAMP reaction in one reaction tube. The primer designed by the invention is high in sequence sensitivity, and each reaction can detect 100 copied mixed templates at least; and specificity is good, and a target genecan be efficiently amplified; and clinical detection effect is good. The detection method provided by the invention is convenient and rapid, does not need to use an expensive instrument, is low in cost and can realize field pathogeny detection.
Owner:GUANGXI VETERINARY RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com