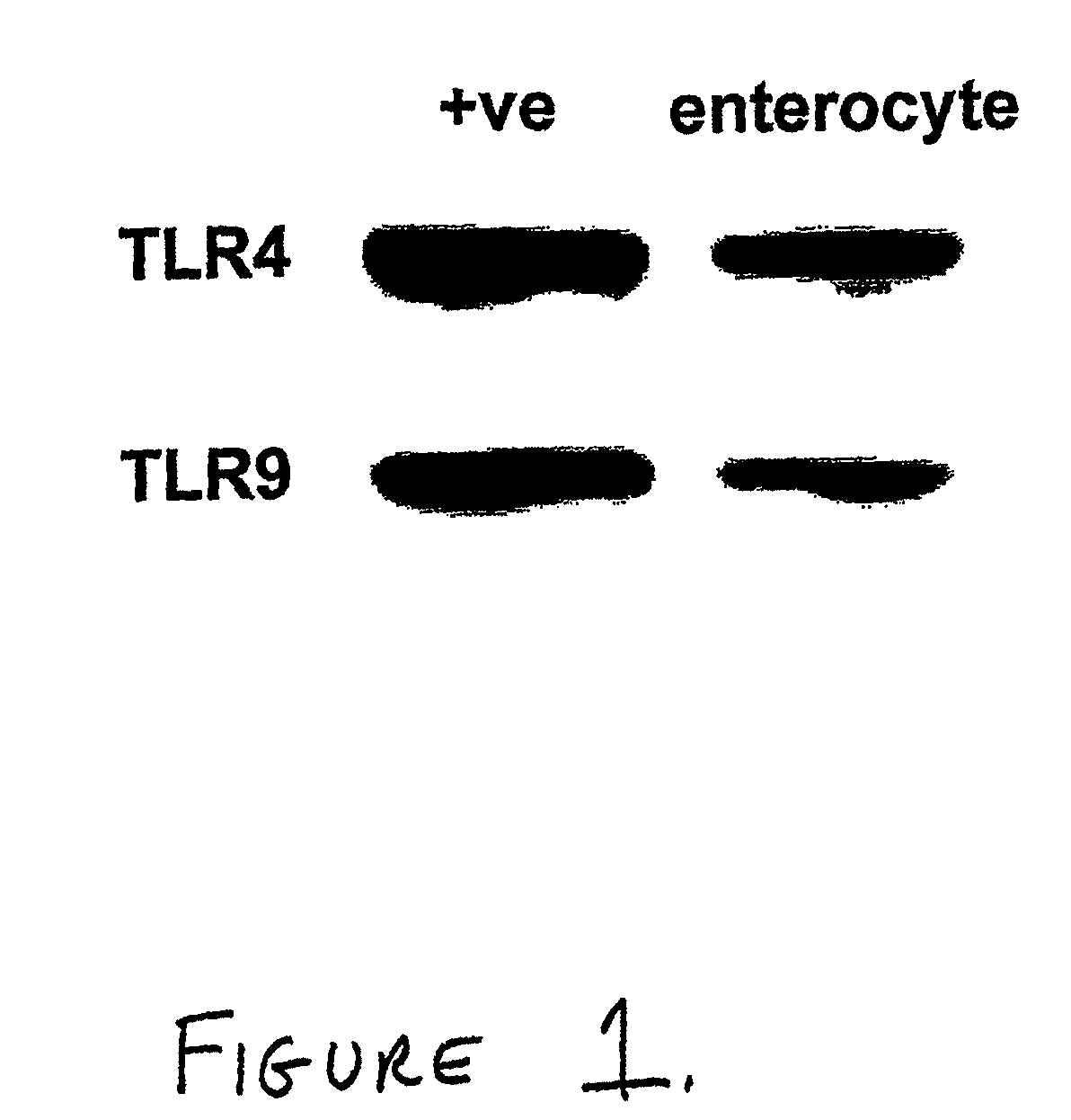
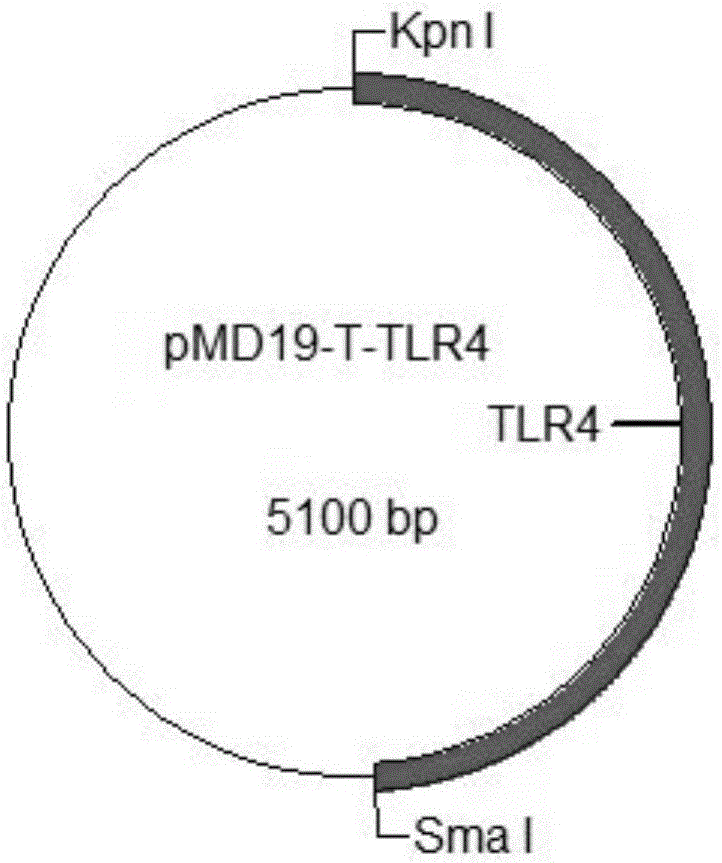
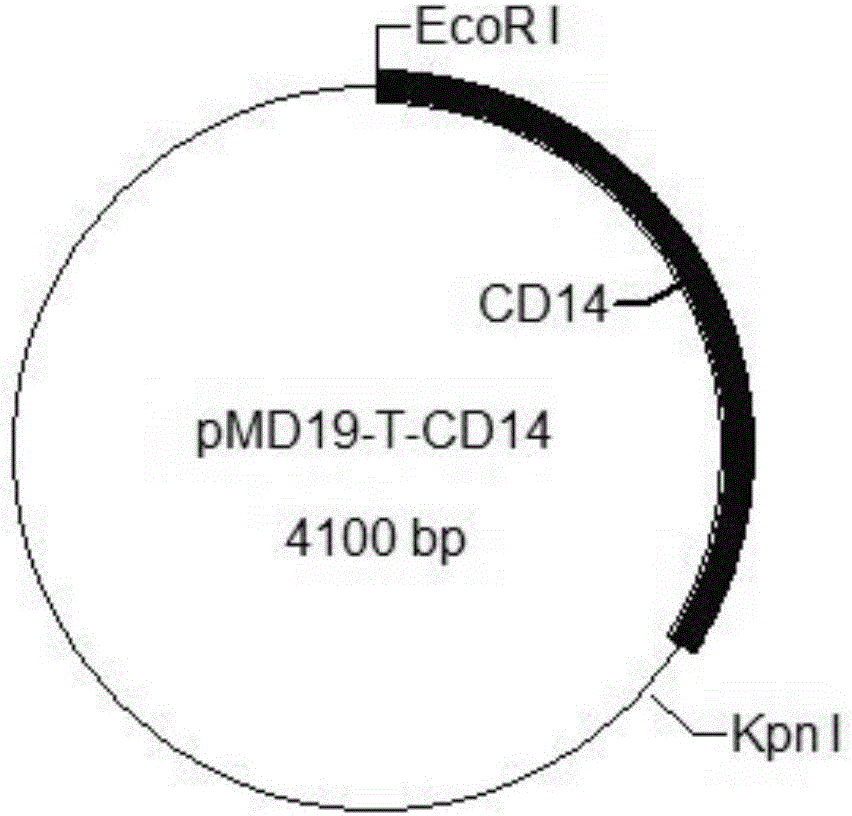
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
195 results about "TLR4" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
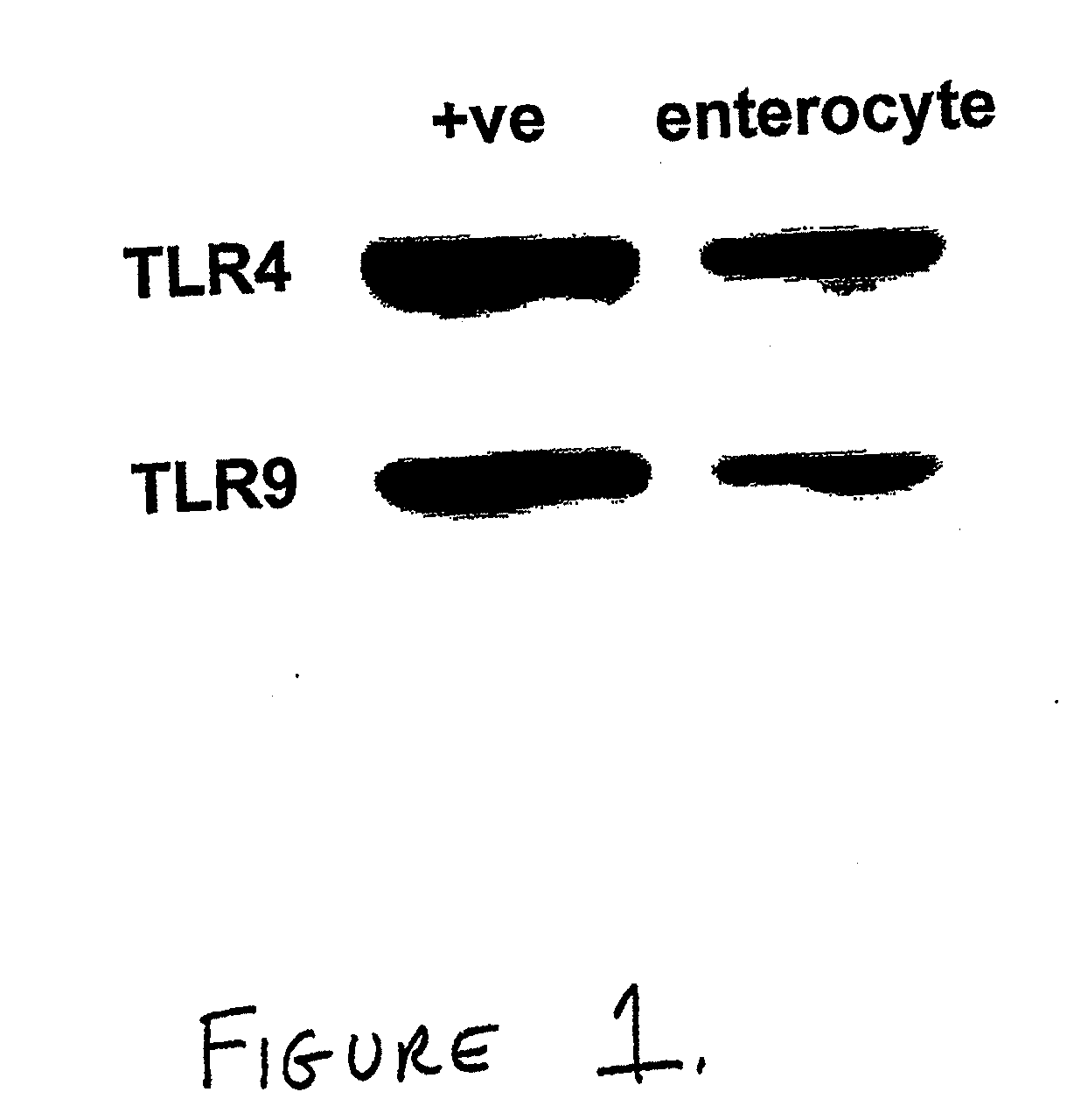
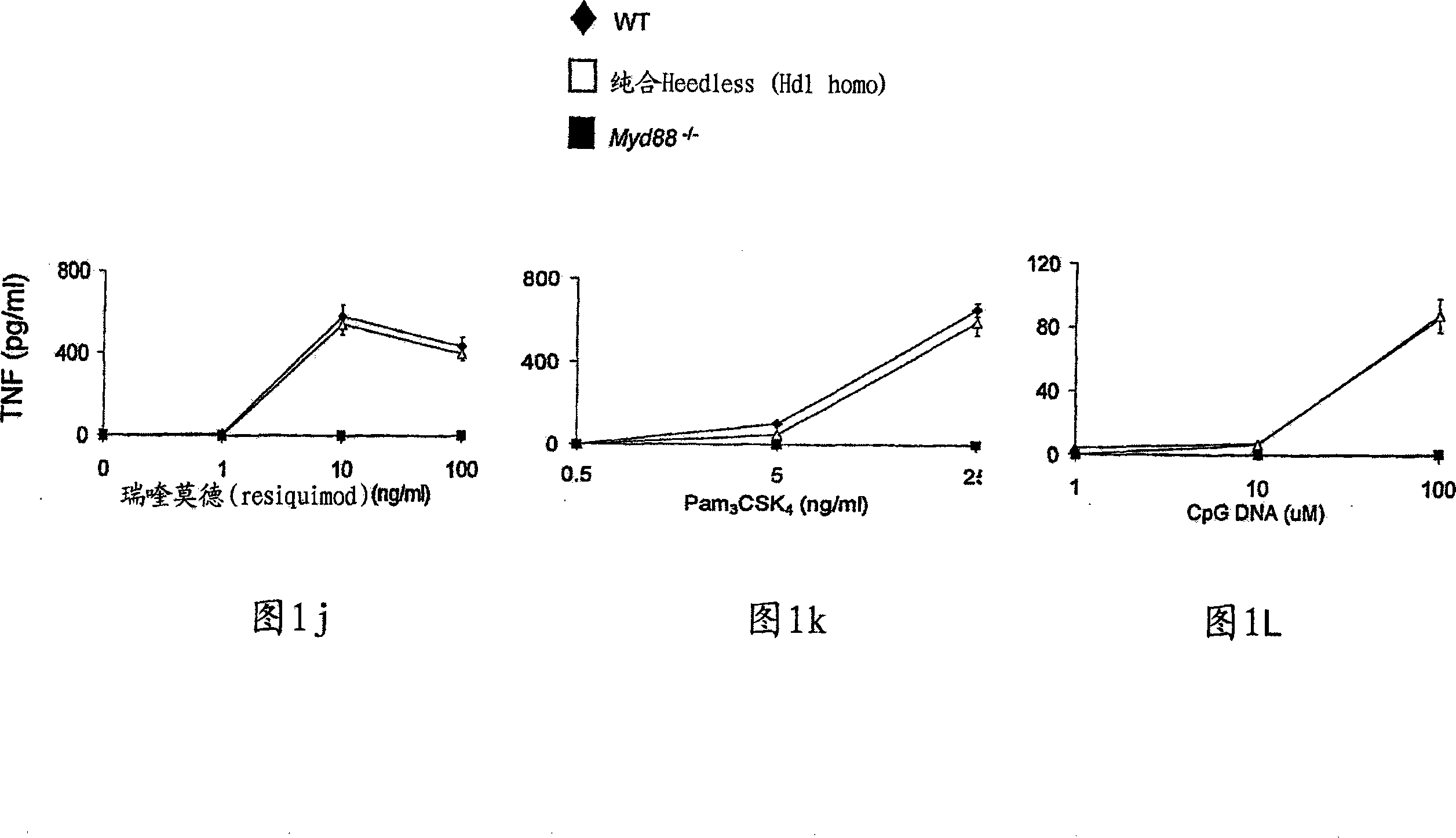
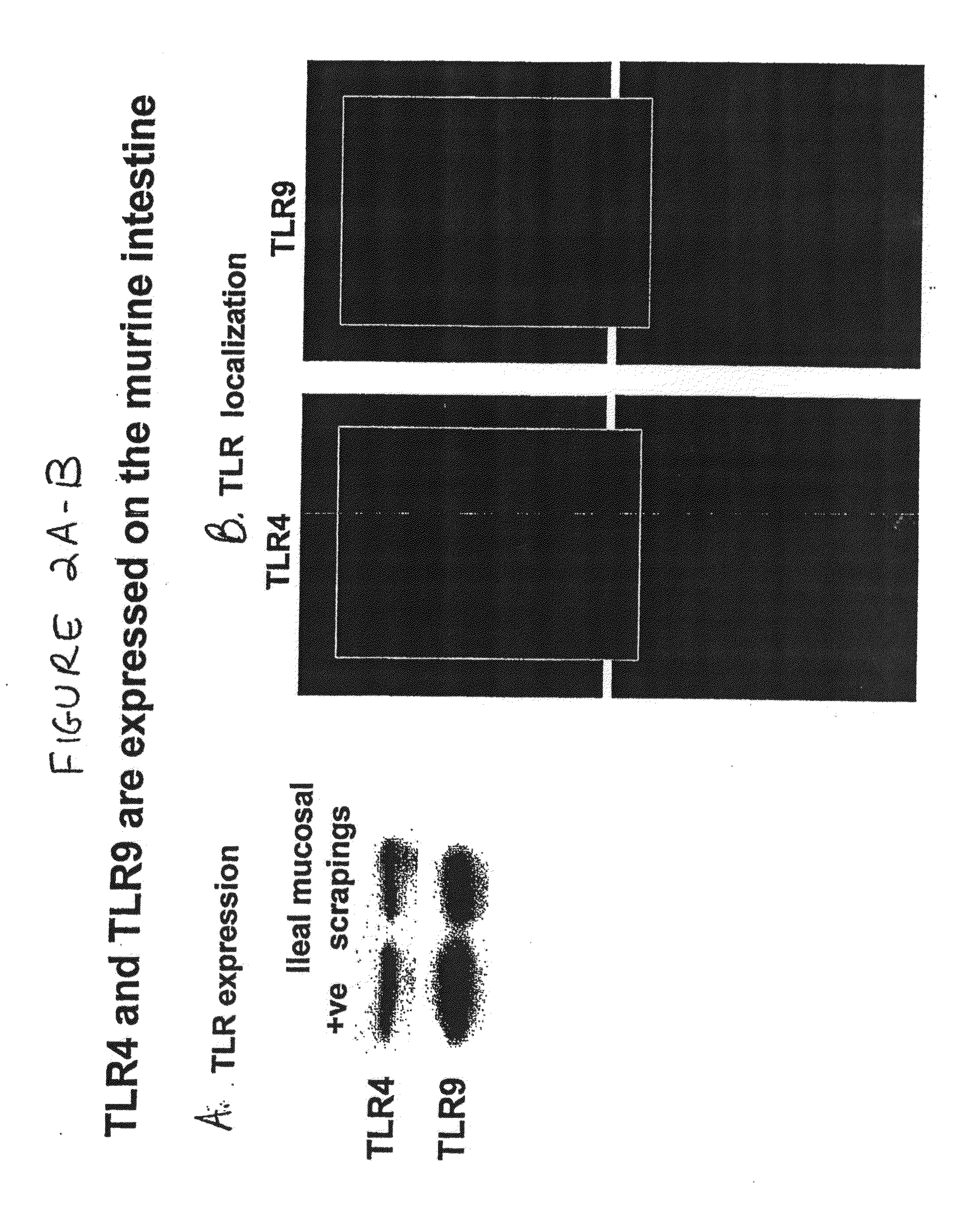
Toll-like receptor 4 is a protein that in humans is encoded by the TLR4 gene. TLR4 is a transmembrane protein, member of the toll-like receptor family, which belongs to the pattern recognition receptor (PRR) family. Its activation leads to an intracellular signaling pathway NF-κB and inflammatory cytokine production which is responsible for activating the innate immune system. It is most well-known for recognizing lipopolysaccharide (LPS), a component present in many Gram-negative bacteria (e.g. Neisseria spp.) and select Gram-positive bacteria. Its ligands also include several viral proteins, polysaccharide, and a variety of endogenous proteins such as low-density lipoprotein, beta-defensins, and heat shock protein.
Use of toll-like receptor-9 agonists, toll-like receptor-4 antagonists, and/or nuclear oligomerization domain-2 agonists for the treatment or prevention of toll-like receptor-4-associated disorders
ActiveUS8188058B2Reduce marksReduce signalingAntibacterial agentsBiocideDiseaseNK1 receptor antagonist
The present invention relates to the use of a TLR9 agonist and / or a TLR4 antagonist and / or a NOD2 agonist for treatment or prevention of disorders involving TLR4 activation, such as systemic sepsis and necrotizing enterocolitis.
Owner:UNIVERSITY OF PITTSBURGH
Construction method of cell model for detecting pyrogens, cell model and pyrogen detection kit
ActiveCN106148286AImprove stabilityIncreased sensitivityCell receptors/surface-antigens/surface-determinantsCulture processWestern blotCytokine
The invention provides a construction method of a cell model for detecting pyrogens, the cell model and a pyrogen detection kit. The cell model utilizes specific locations of CRISPR / CAS9 induced genomes to form double-bond fission, TLR4 and CD14-MD2 are knocked into two chromosomes of a cell line respectively by the aid of the homologous recombination repair principle, green fluorescence GFP and red fluorescence RFP are respectively used for tracing finally successfully constructed TLR4 / CD14 / MD2 fixed-point knocked-in fluorescent tracer cell models, and the LPS stimulating cell model can detect release of IL-6 and TNF-a cytokines by means of ELISA, Western Blot, mass spectrum and immunomagnetic beads. The cell model is good in stability and high in sensitivity, and the lowest detectable limit can reach 0.005EU / mL and is far lower than 0.025EU / mL of the Tachypleus Amebocyte Lysate method.
Owner:牛刚
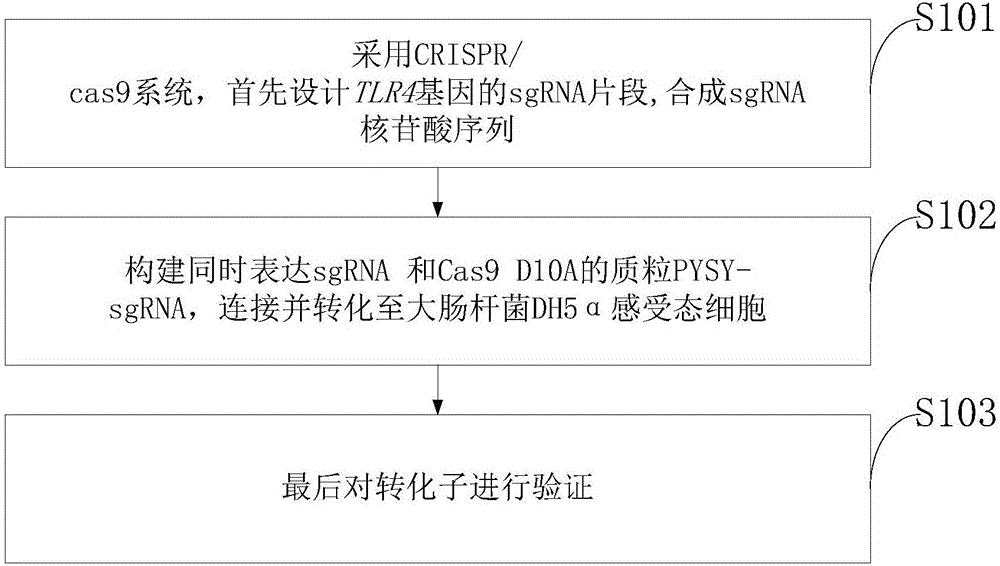
Goat TLR4 gene knock-out vector and construction method thereof
InactiveCN106755097AThe method is simple and fastHigh knockout efficiencyNucleic acid vectorVector-based foreign material introductionEscherichia coliCompetent cell
The invention discloses a goat TLR4 gene knock-out vector and a construction method thereof. The construction method comprises the following steps: firstly, designing an sgRNA fragment of the TLR4 gene by adopting a CRISPR / cas9 system, synthesizing an sgRNA nucleotide sequence, constructing and simultaneously expressing the sgRNA and plasmid PYSY-sgRNA of Cas9 D10A, connecting and transforming to an Escherichia coli DH5 alpha competent cell, and verifying the transformant; and judging and proving by enzyme digestion and sequencing that the TLR4 gene knock-out vector is constructed correctly. The invention adopts the CRISPR / cas9 for constructing the vector, and provides a theoretical basis for subsequently acquiring a goat TLR4 gene deletion type alveolar epithelial cell system, and studying the immune response molecular mechanism of mycoplasma pneumonia infection.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE ANHUI ACAD OF AGRI SCI
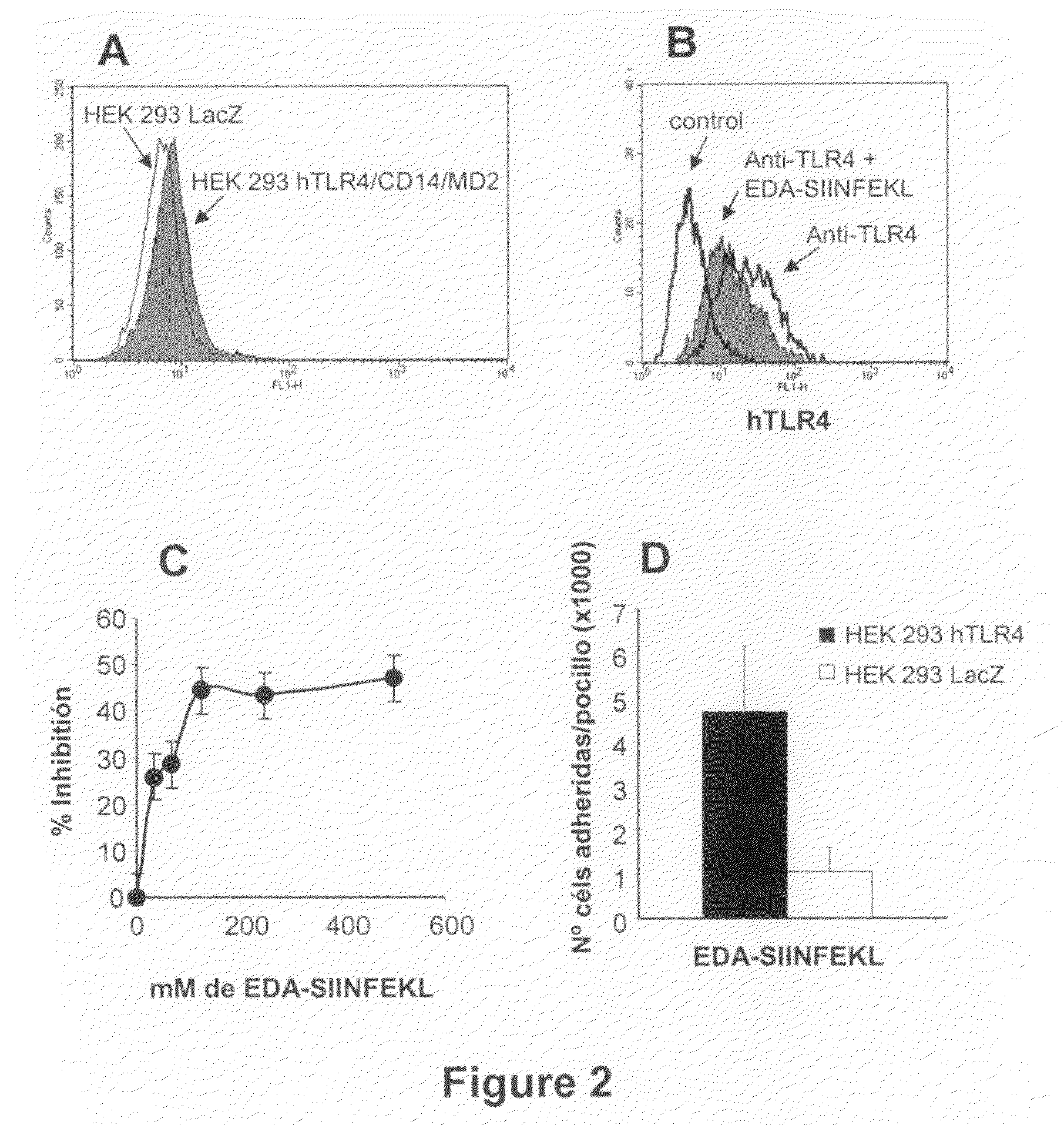
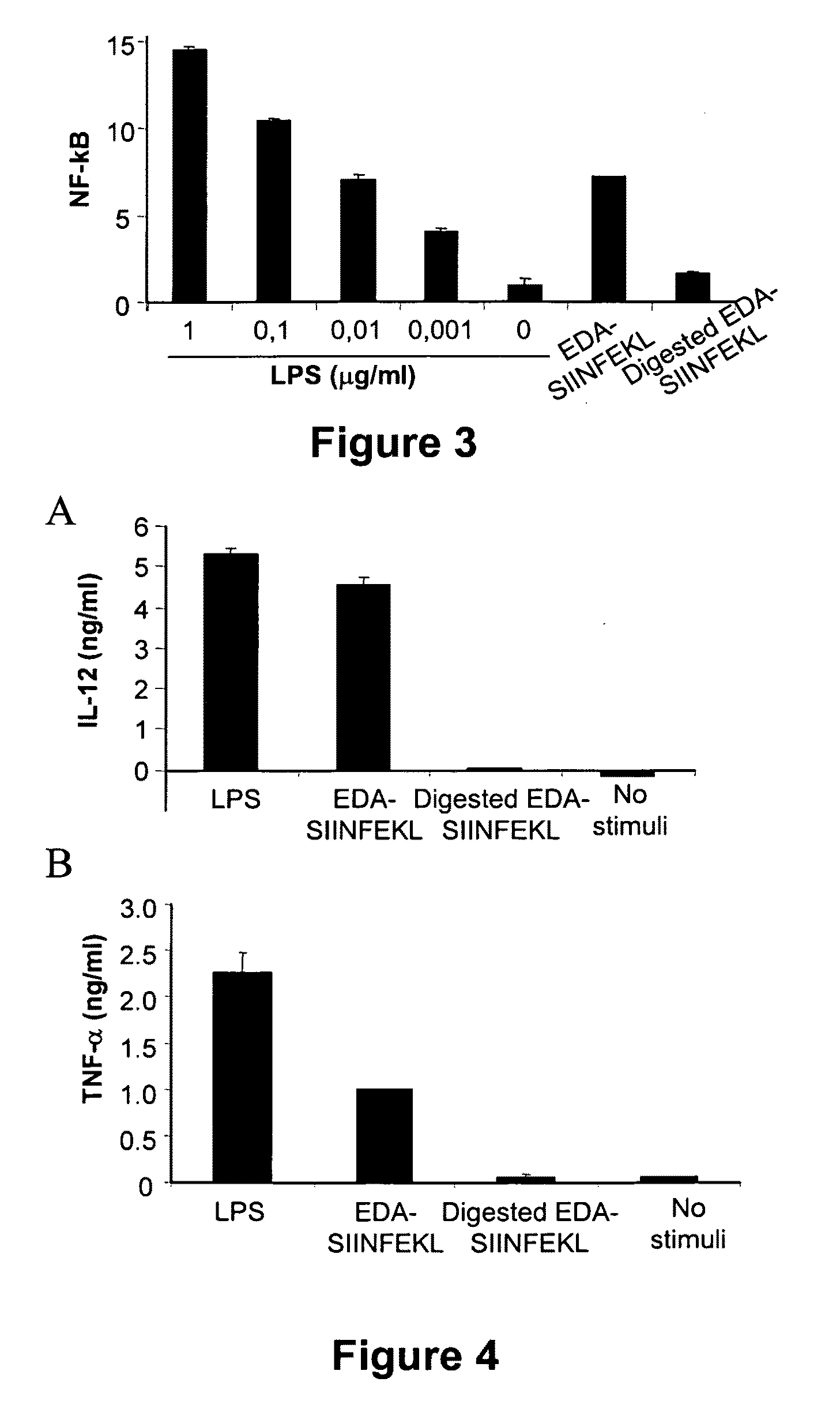
Agents and methods based on the use of the eda domian of fibronectin
ActiveUS20090220532A1Facilitate its translocationHigh expressionAntibacterial agentsOrganic active ingredientsTLR4Biology
This invention relates to the use of a polypeptide in the production of an immunostimulatory agent, said polypeptide comprising a sequence corresponding to the EDA domain of fibronectin, a fragment of the EDA domain which can bind to TLR4 or a variant of said EDA domain or a fragment which can bind to TLR4 and which has a homology of more than 70% with any form or natural fragment of the EDA domain. The invention also relates to the production methods and applications of said agent.
Owner:PROYECTO DE BIOMEDICINA CIMA
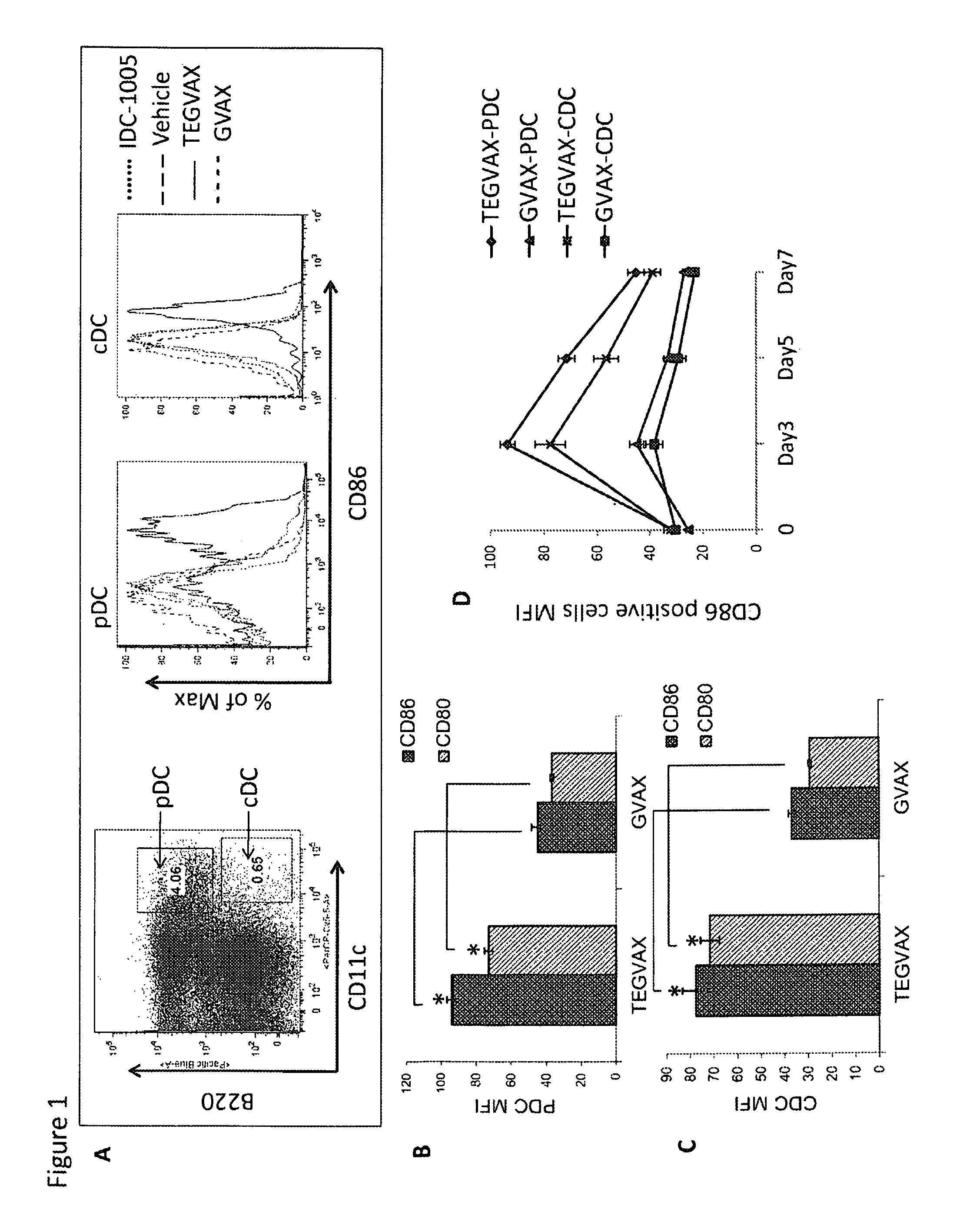
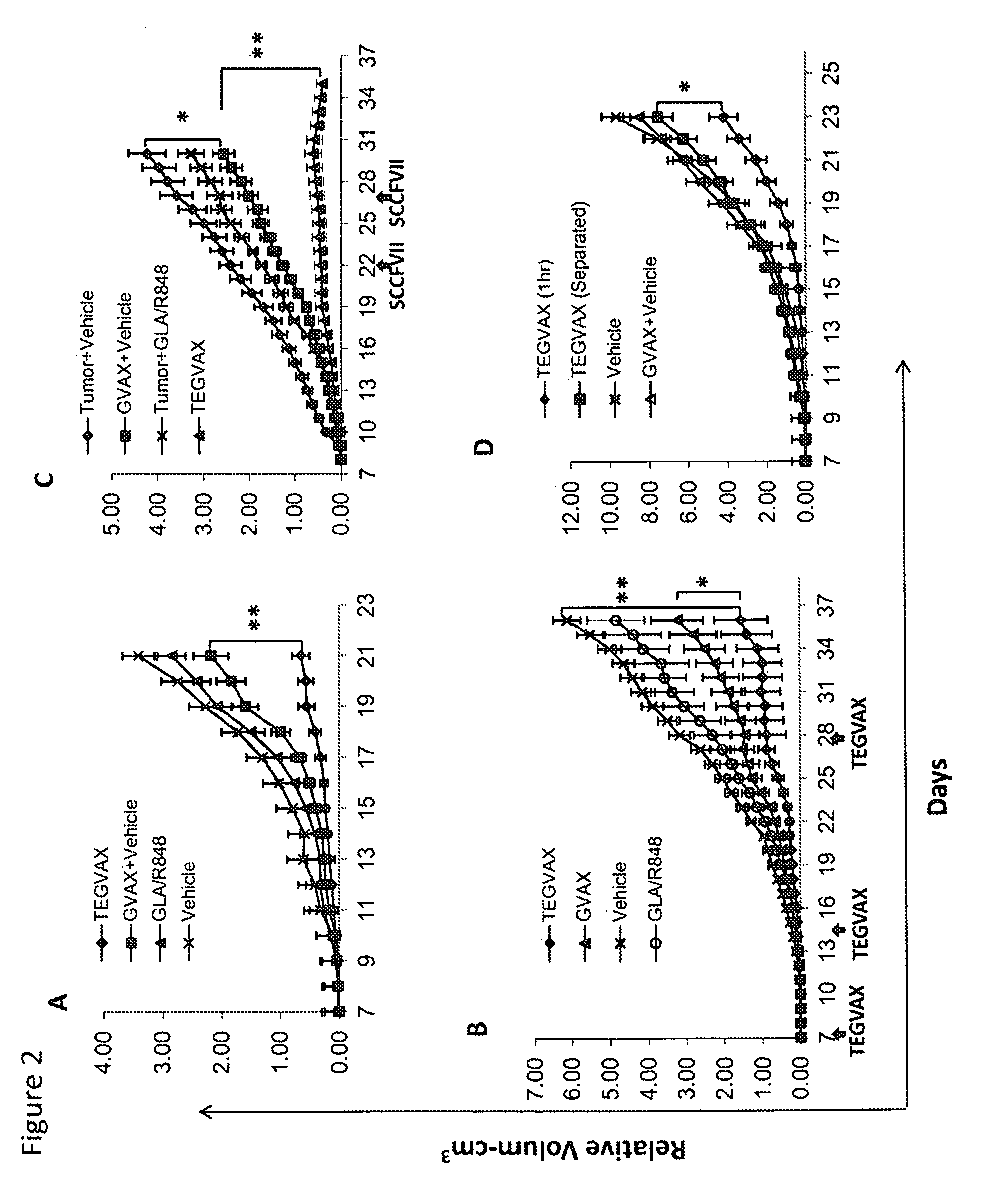
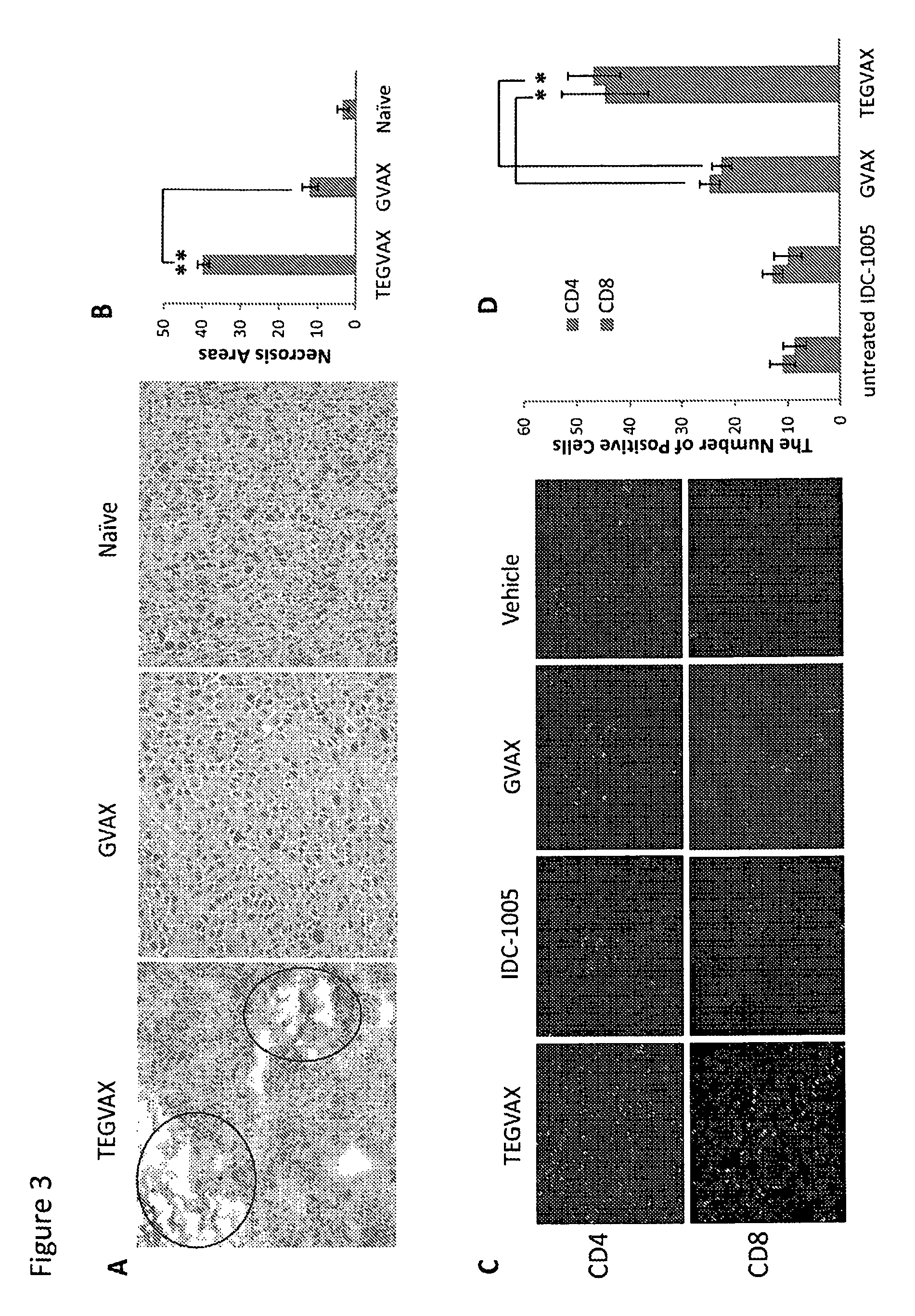
Cancer immunotherapy
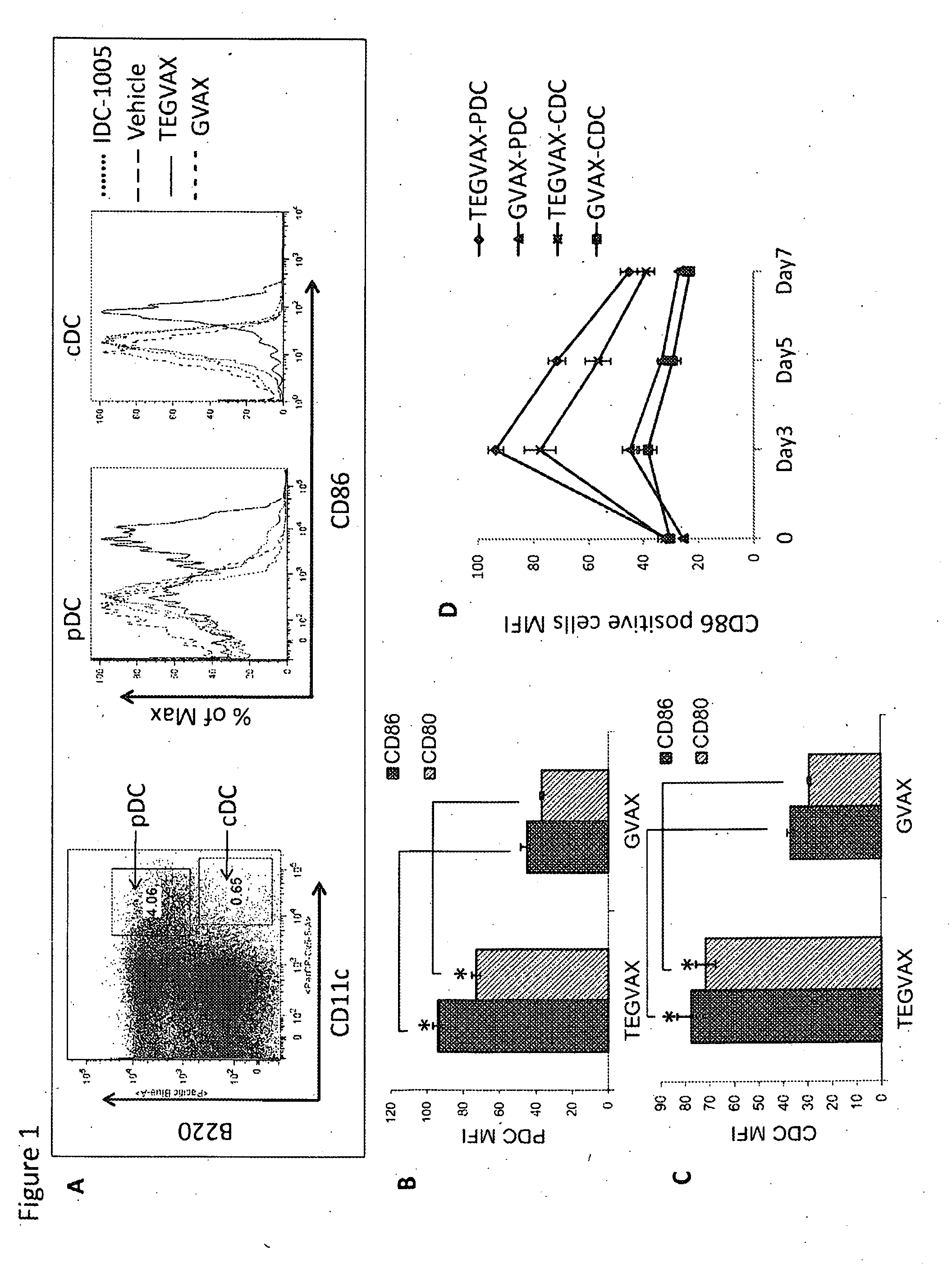
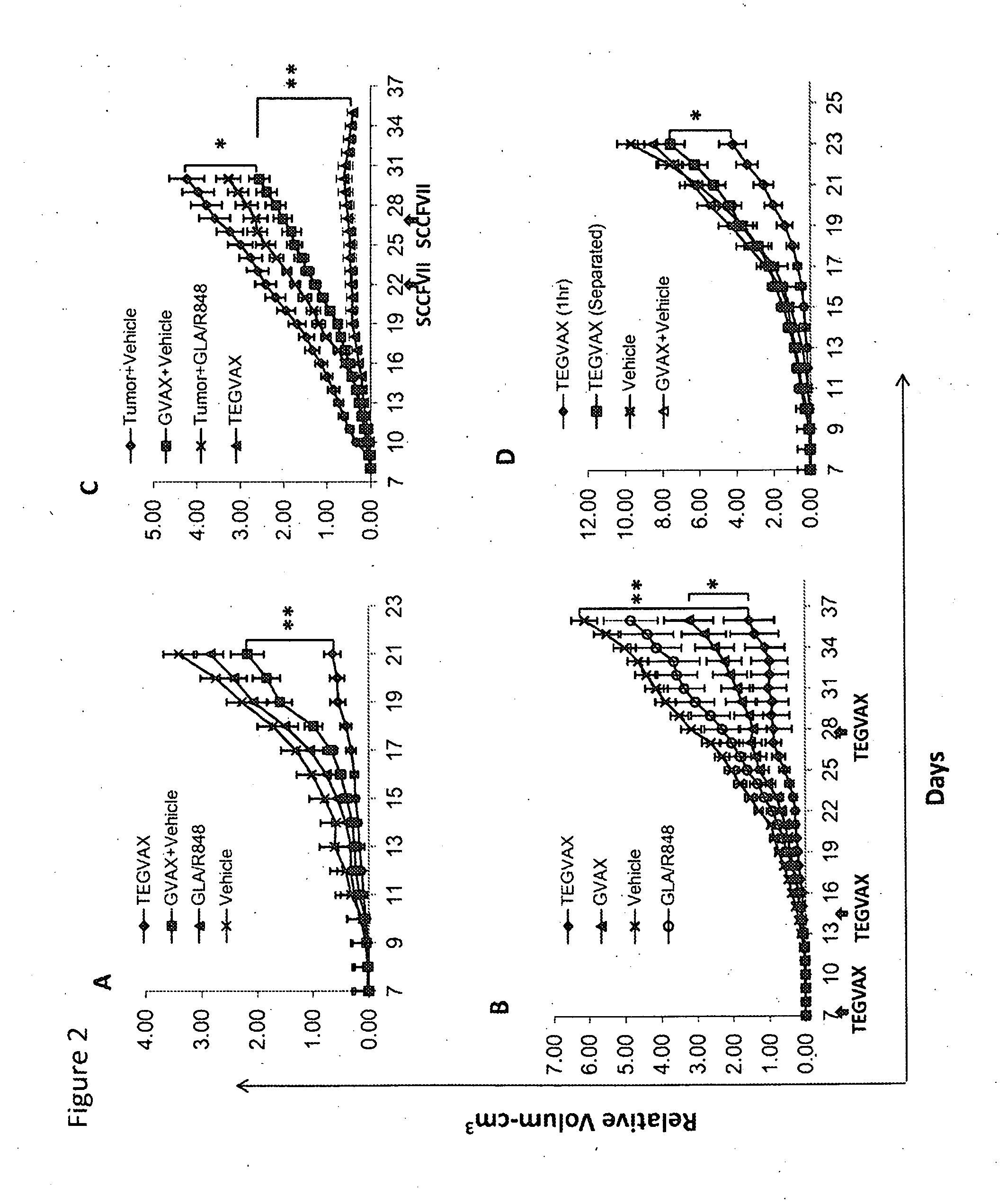
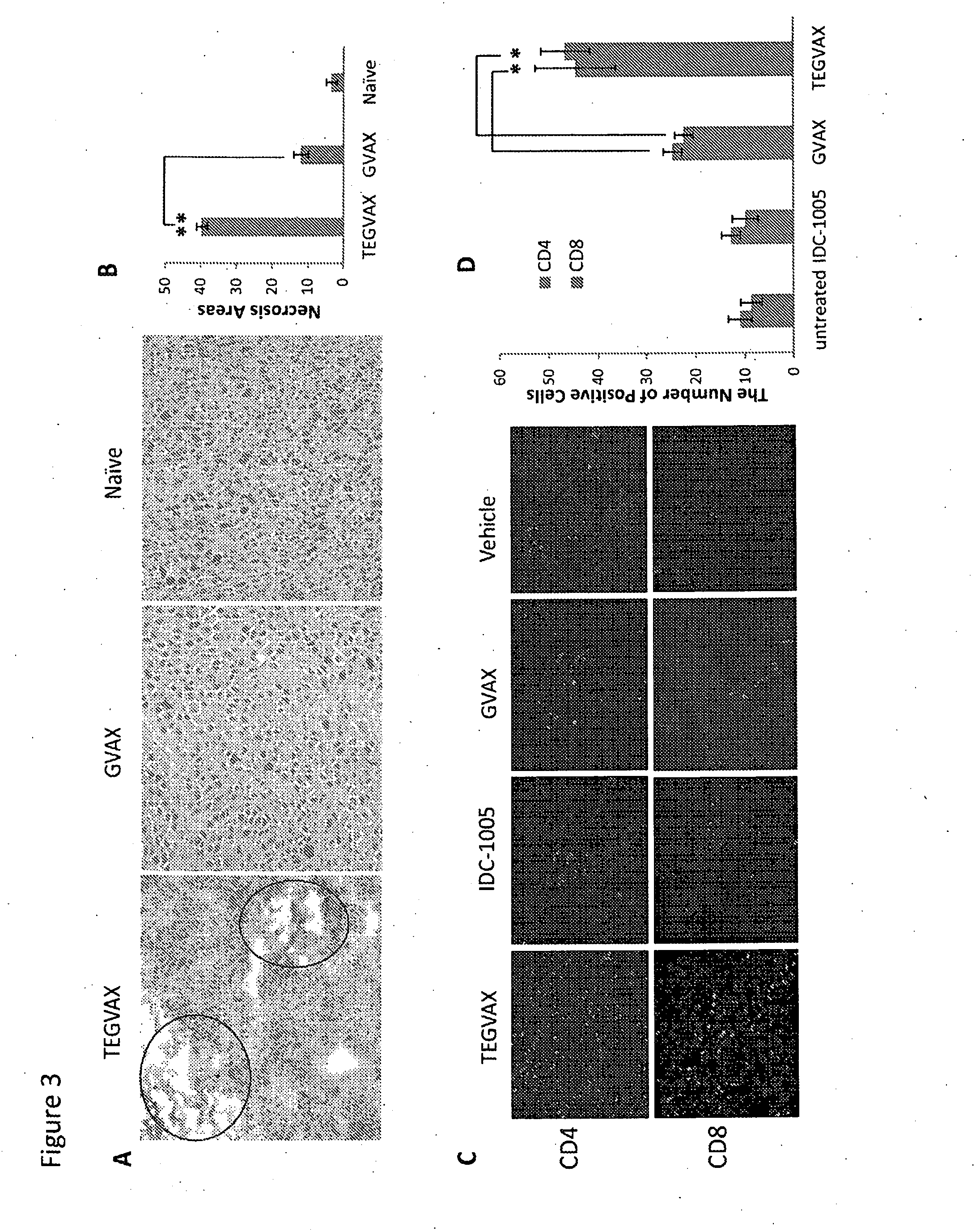
We formulated multiple TLR agonists into GVAX (lethally irradiated tumor cell vaccines engineered to secrete GM-CSF). Specifically, GLA and R848, TLR4 and TLR7 / 8 agonists found to be safe in patients, were formulated with GVAX (TEGVAX—for TLR agonists enhanced GVAX), and this formulation was effective in producing anti-tumor responses in 3 different preclinical models, including palpable B16. These anti-tumor responses were correlated with increased CD4 and CD8 T-cells that can secrete IFNγ circulating in the tumor microenvironment as well as significantly higher level of p15E specific CTL mediated cell killing in mice treated with TEGVAX in comparison to controls. When combined with anti-PD-1 antibody, TEGVAX was able to induce regression of established B16 tumors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Use of toll-like receptor-9 agonists, toll-like receptor-4 antagonists, and/or nuclear oligomerization domain-2 agonists for the treatment or prevention of toll-like receptor-4-associated disorders
ActiveUS20080311112A1Reduce marksReduce signalingAntibacterial agentsBiocideDiseaseNK1 receptor antagonist
The present invention relates to the use of a TLR9 agonist and / or a TLR4 antagonist and / or a NOD2 agonist for treatment or prevention of disorders involving TLR4 activation, such as systemic sepsis and necrotizing enterocolitis.
Owner:UNIVERSITY OF PITTSBURGH
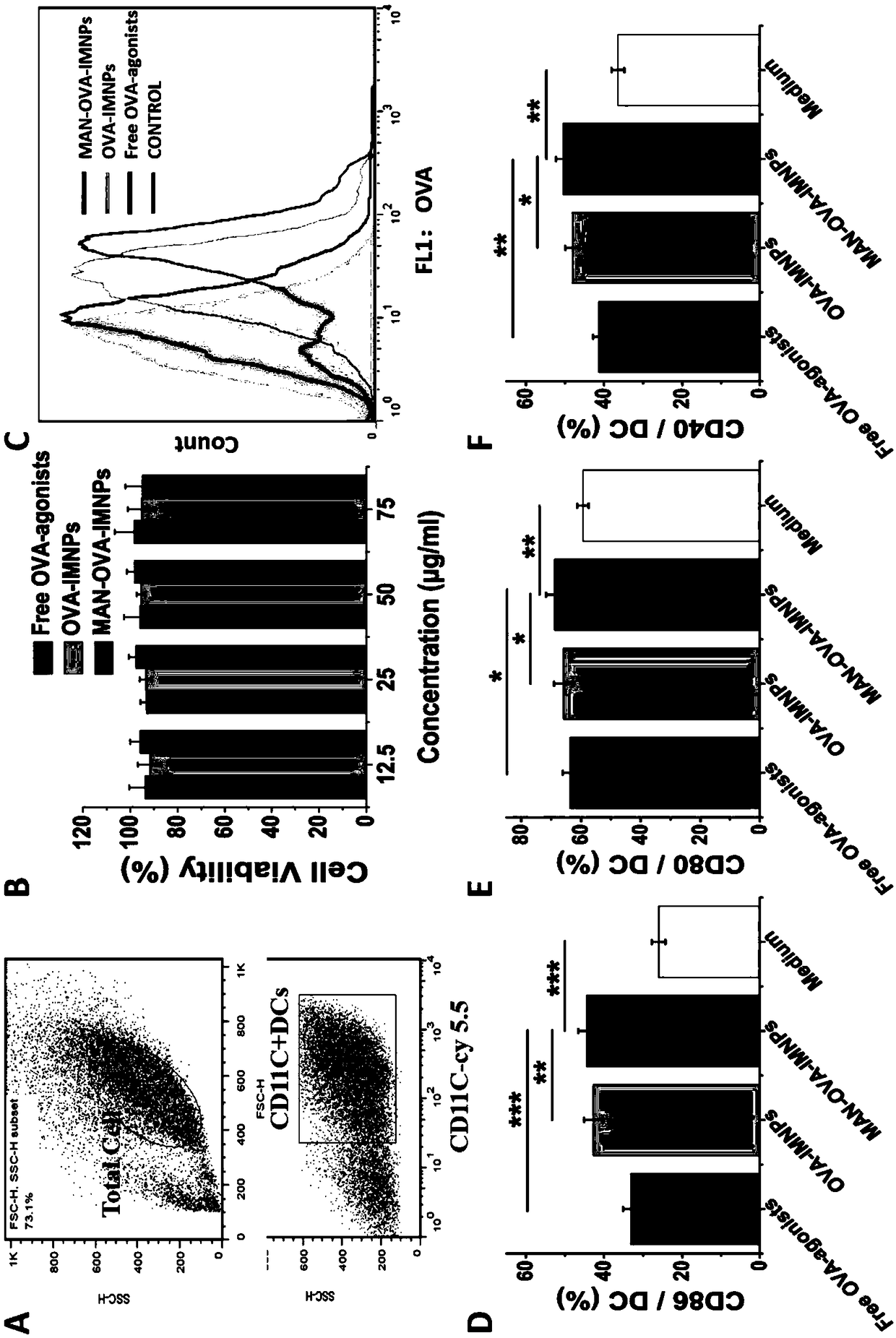
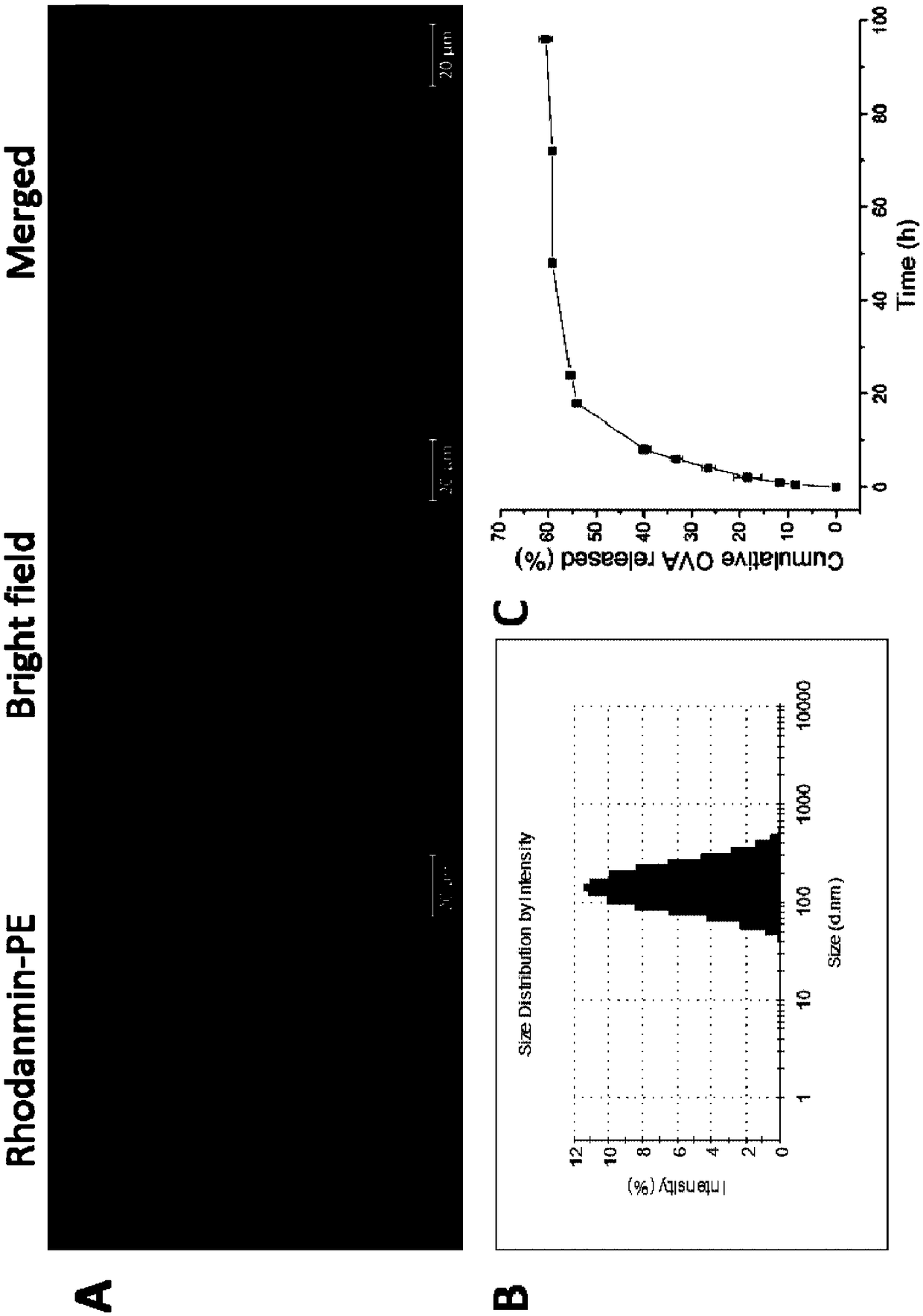
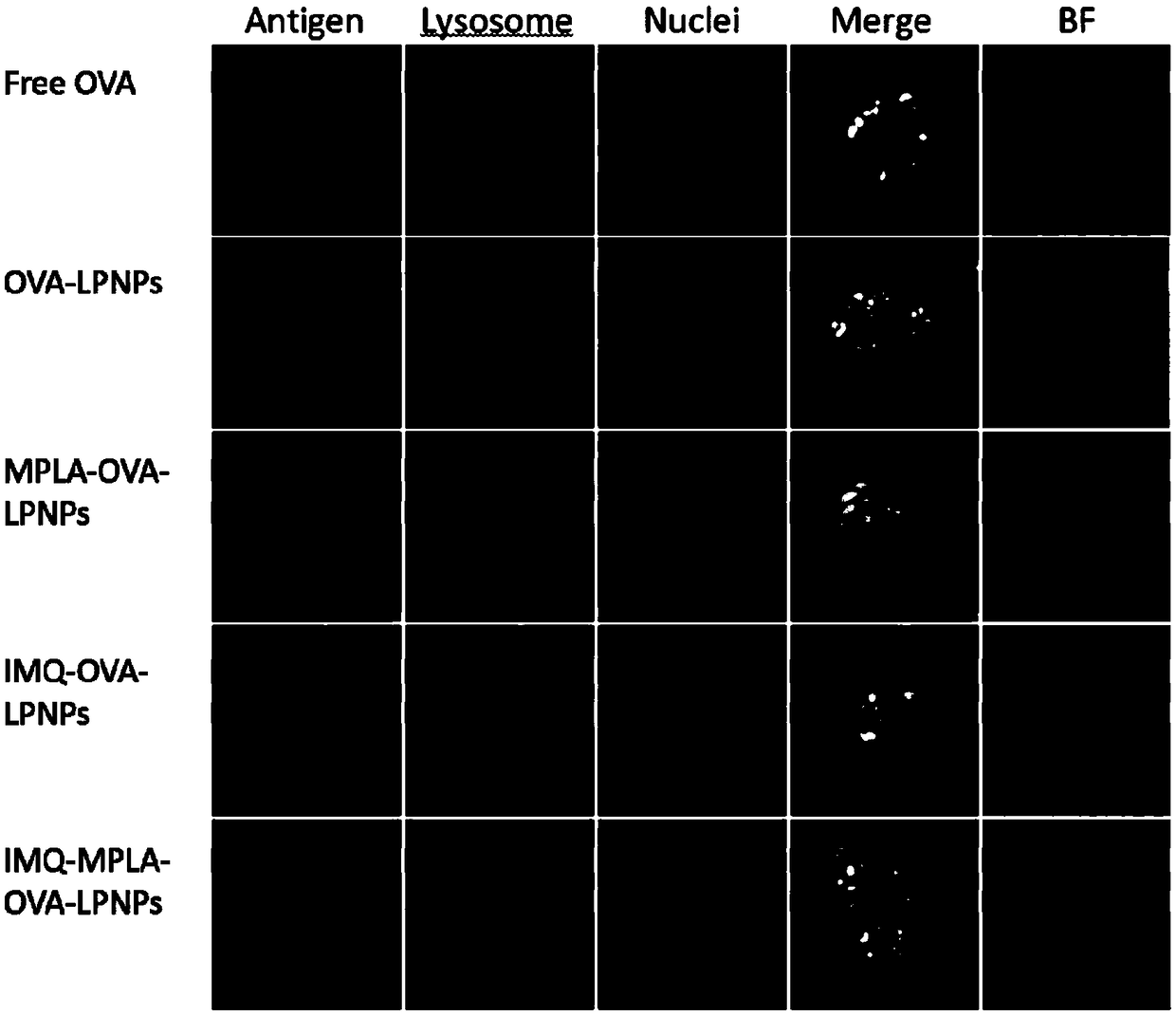
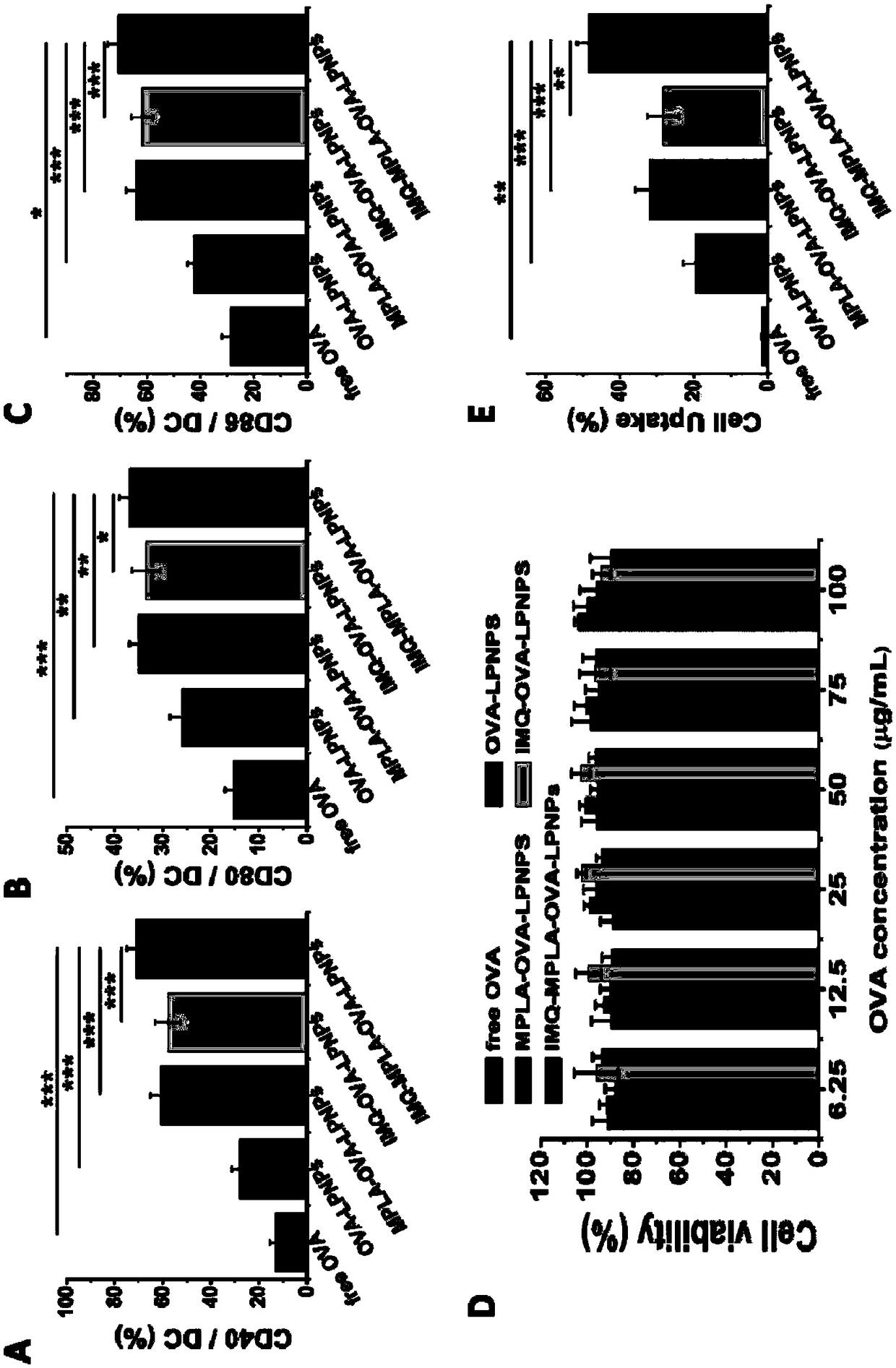
Antigen and TLR agonist targeting co-loaded cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant, and preparation method and application thereof
ActiveCN108992666AStrong activationRealize the loadCancer antigen ingredientsPharmaceutical non-active ingredientsDendritic cellPhospholipid
The invention relates to an antigen and TLR agonist targeting co-loaded cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant, and a preparation method and an application thereof. A hydrophobic inner core is encapsulated with a TLR7 agonist, a phospholipid layer is encapsulated with a TLR4 agonist, an antigen is adsorbed by cationic phospholipids in the phospholipid layer, and ligation of a mannose ligand makes the vaccine adjuvant like a specific targeting antigen to have the dendritic cell presenting ability, so the DC cell intake and the maturation promoting effect are enhanced; and the antigen is protected by hybridized nanoparticles, the antigen uptake by DC cells is improved, immune response after antigen stimulation is significantly enhanced by the TLR agonist, and thecross-presentation of the antigen is significantly improved, so vaccine adjuvant has a strong potent T cell killing effect, can induce cytokine secretion, has a long-acting memory T cell response, andhas an excellent prevention effect on tumors.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
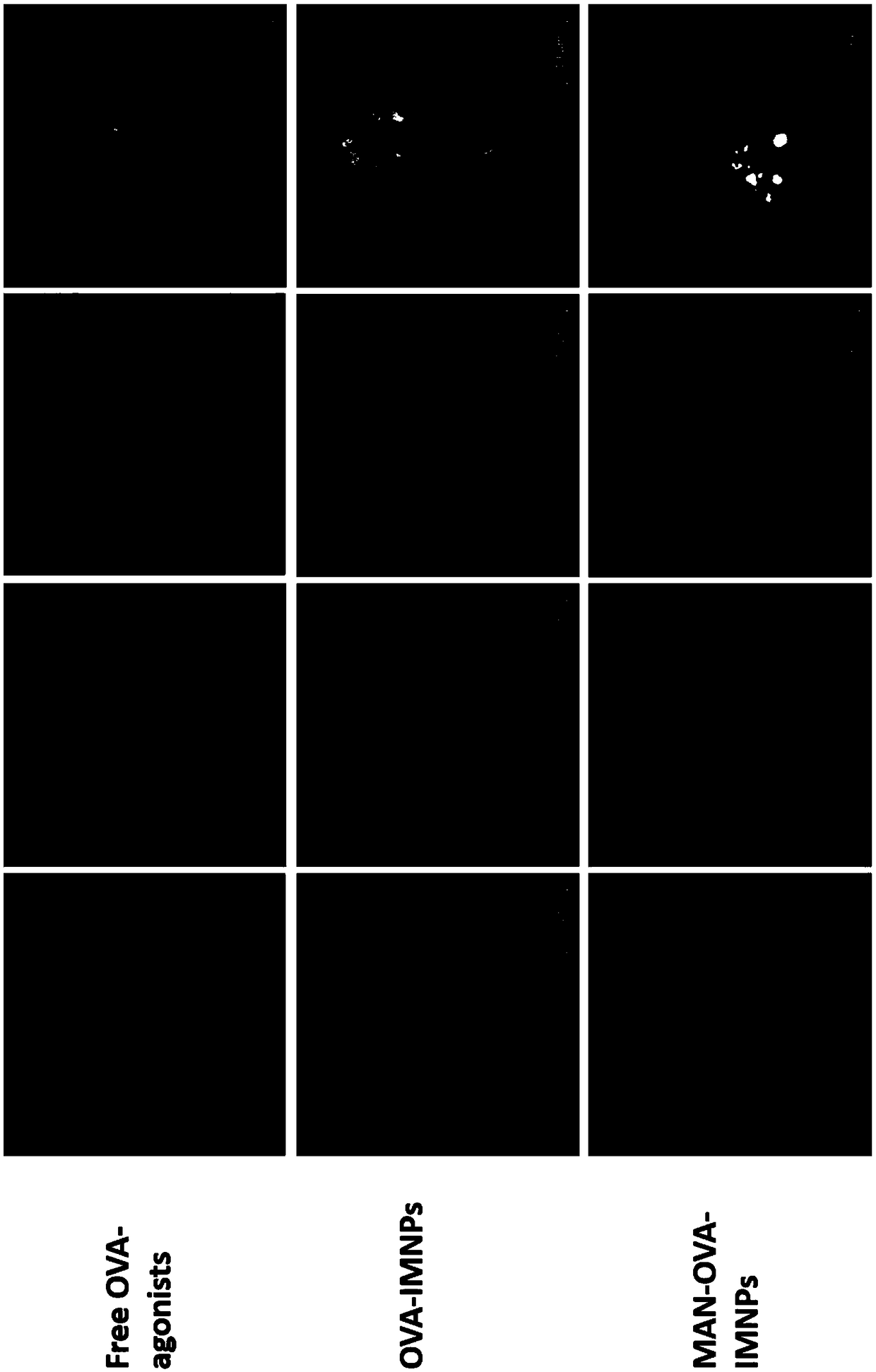
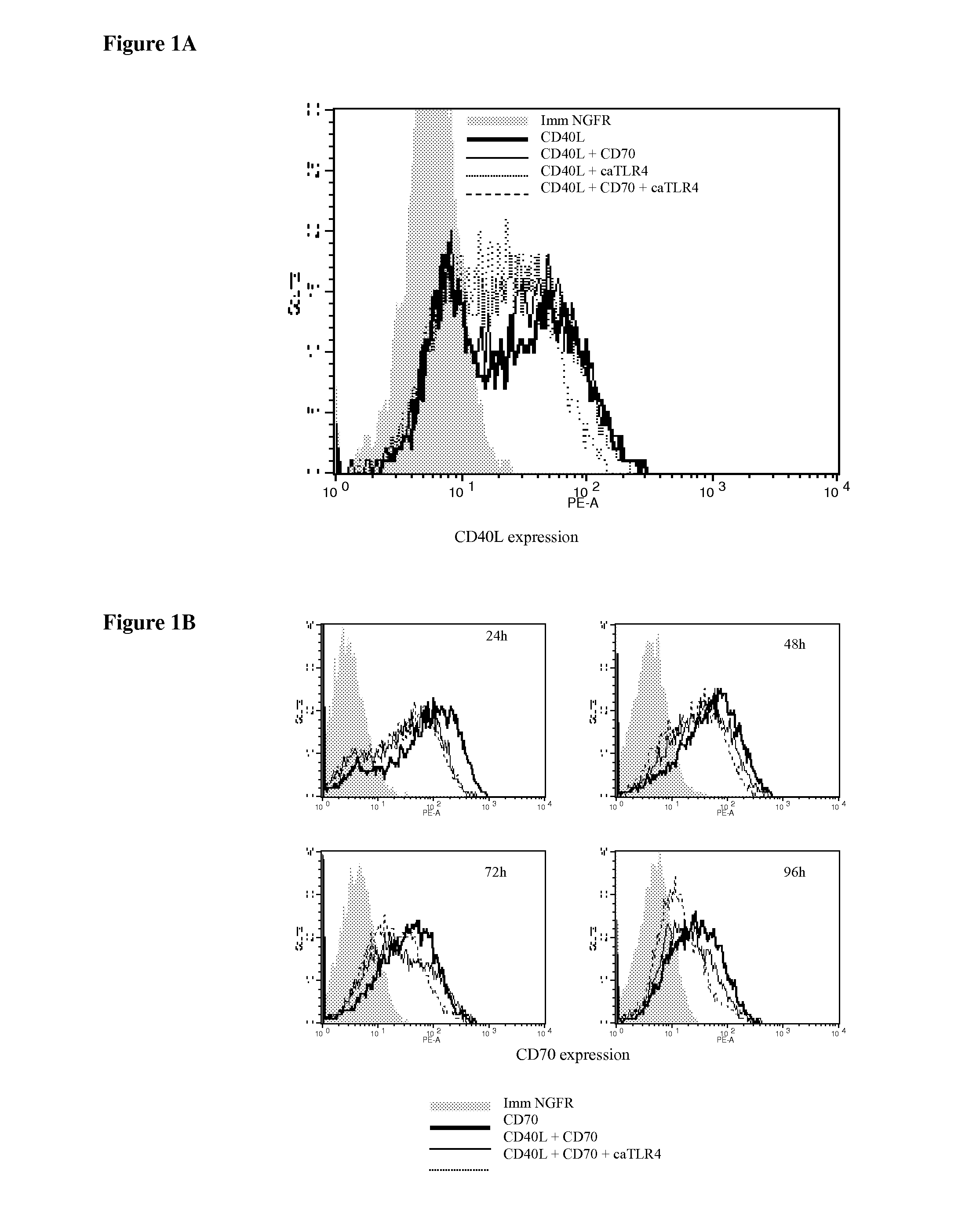
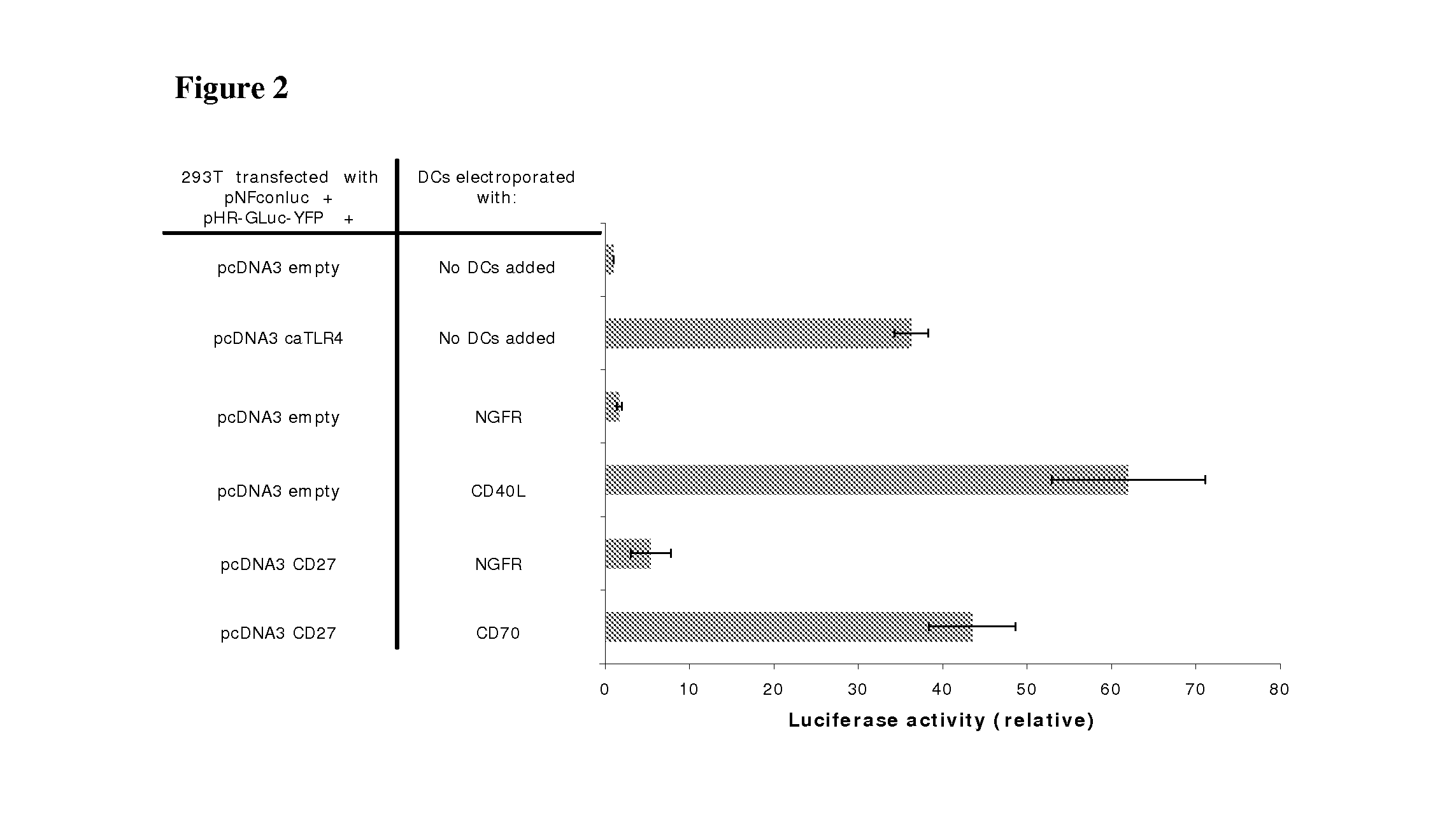
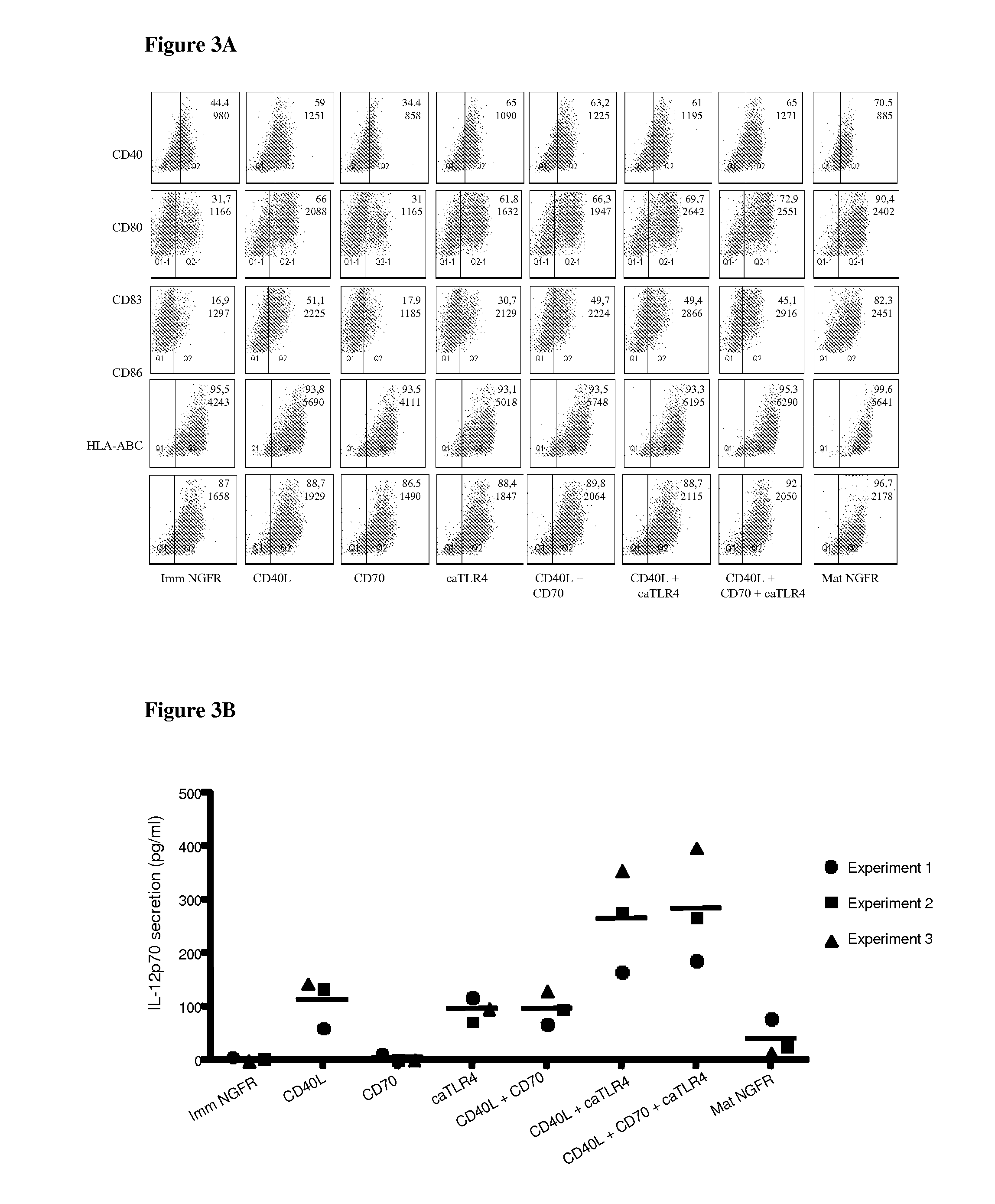
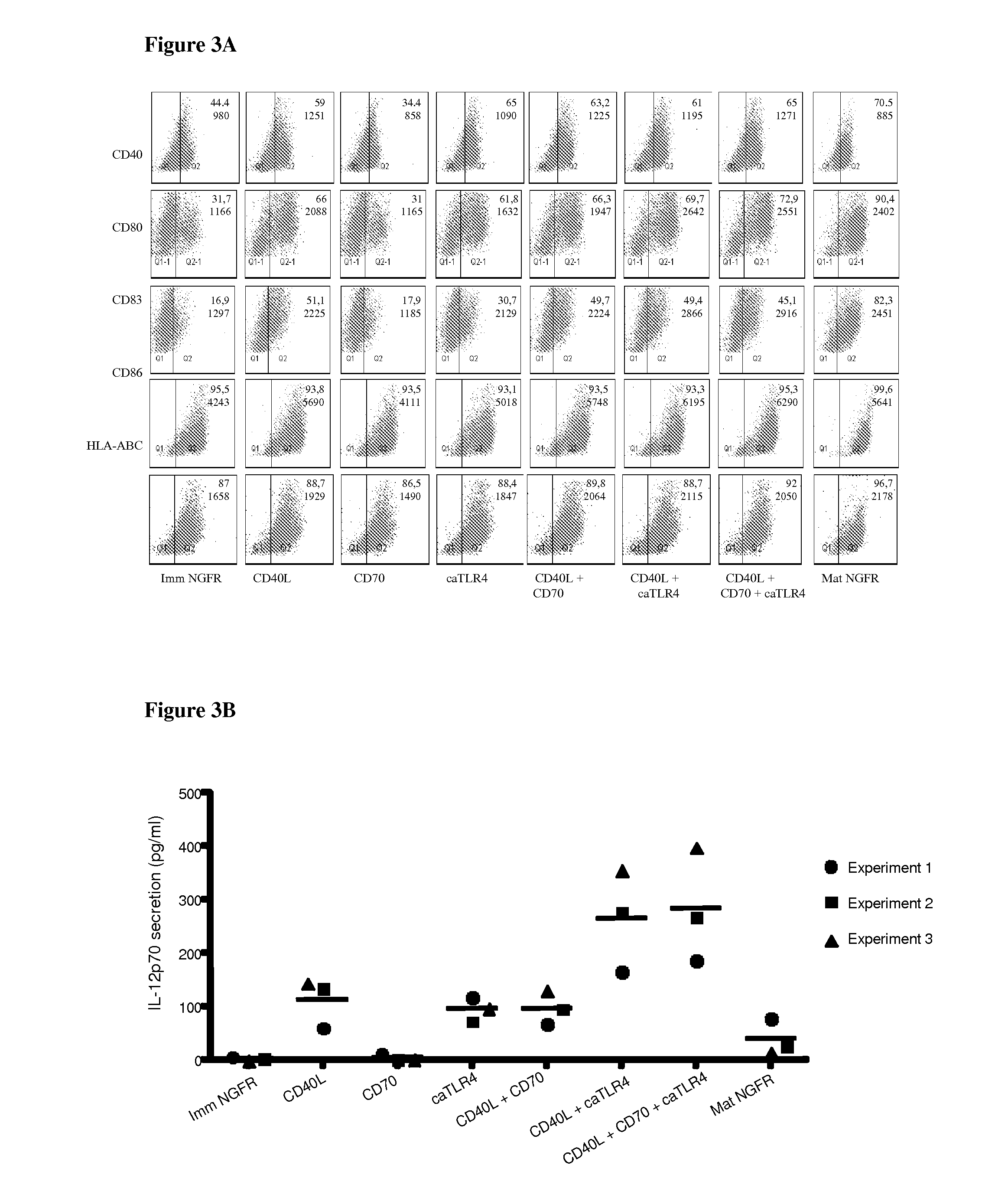
Enhancing the t-cells stimulatory capacity of human antigen presenting cells and their use in vaccination
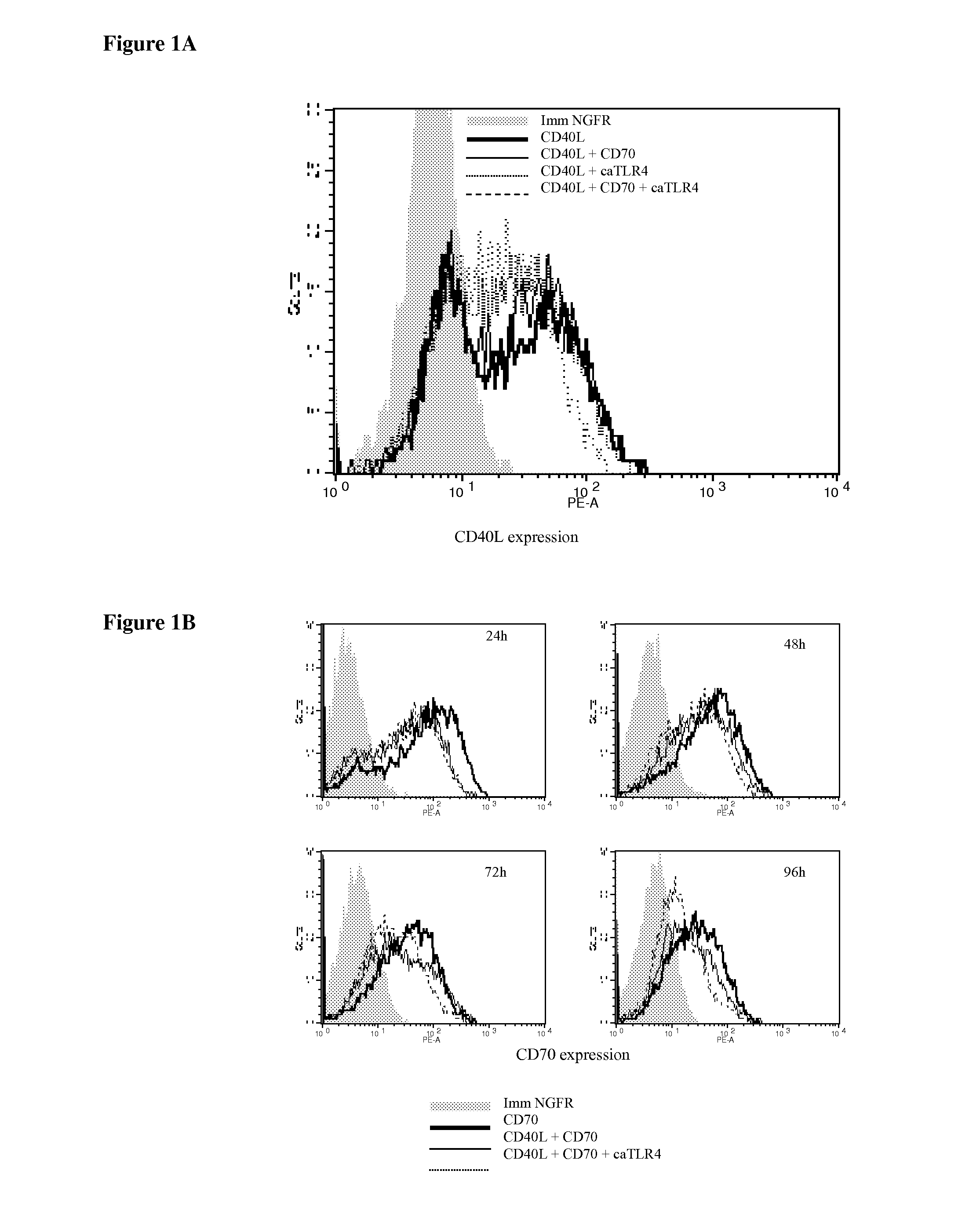
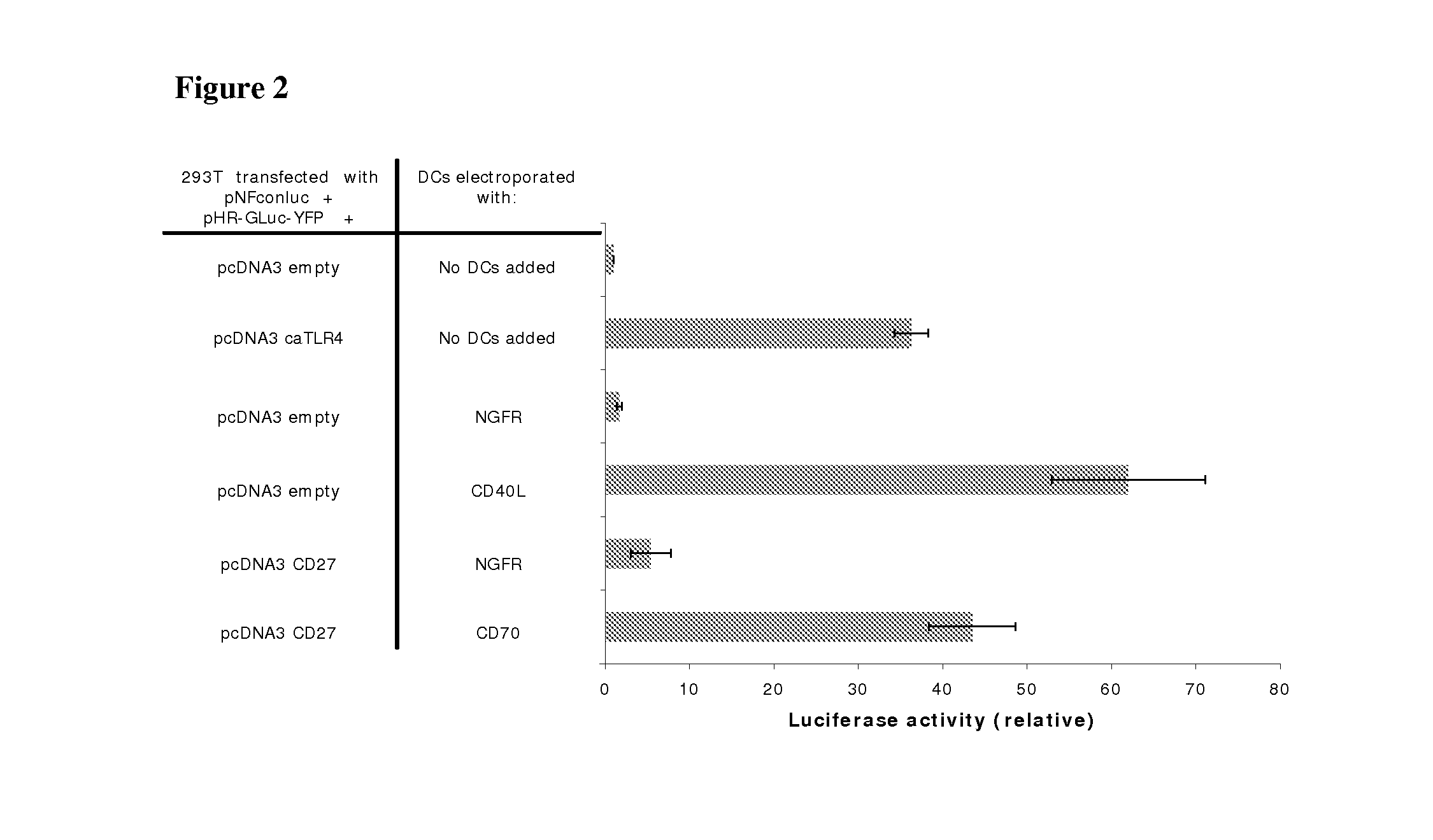
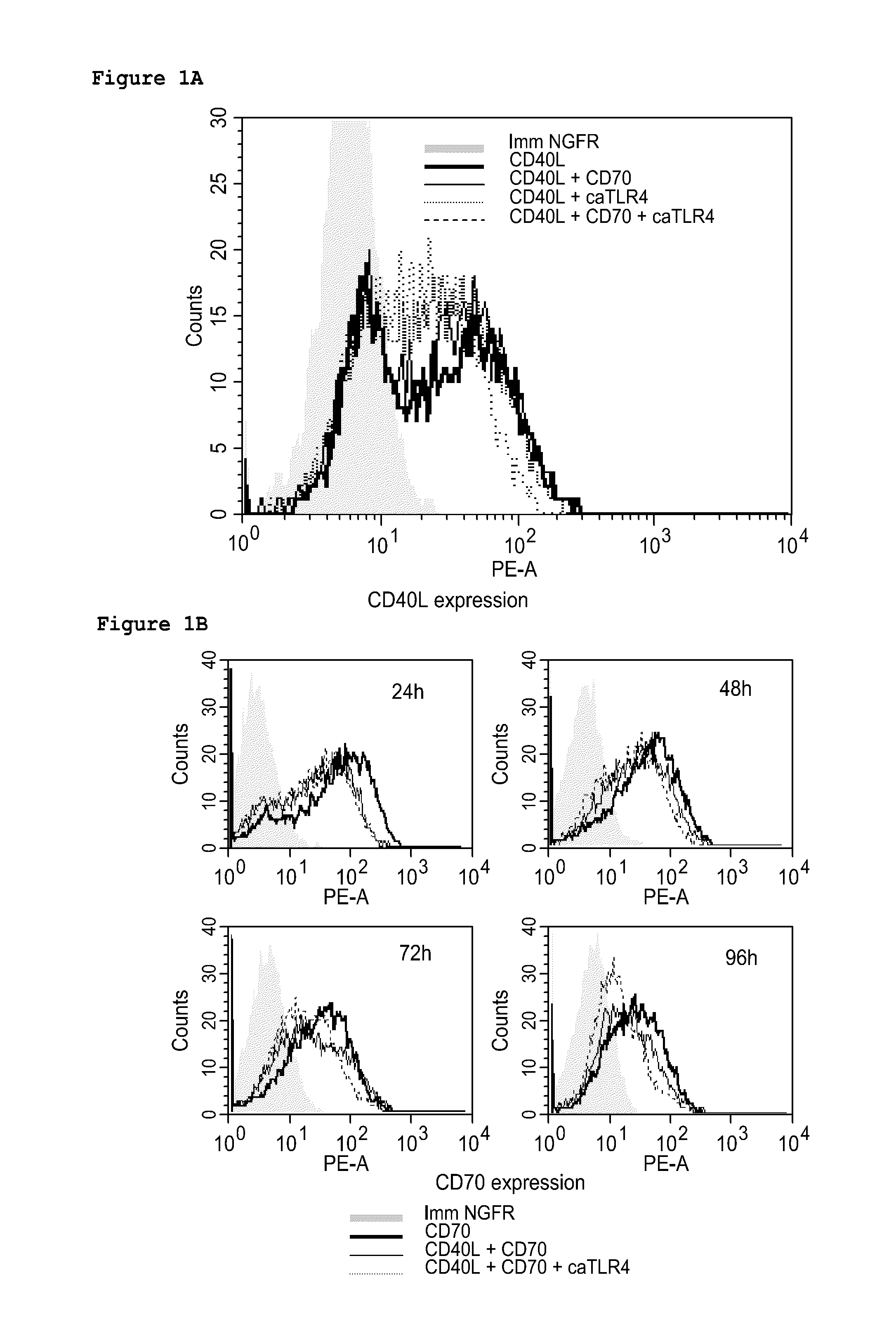
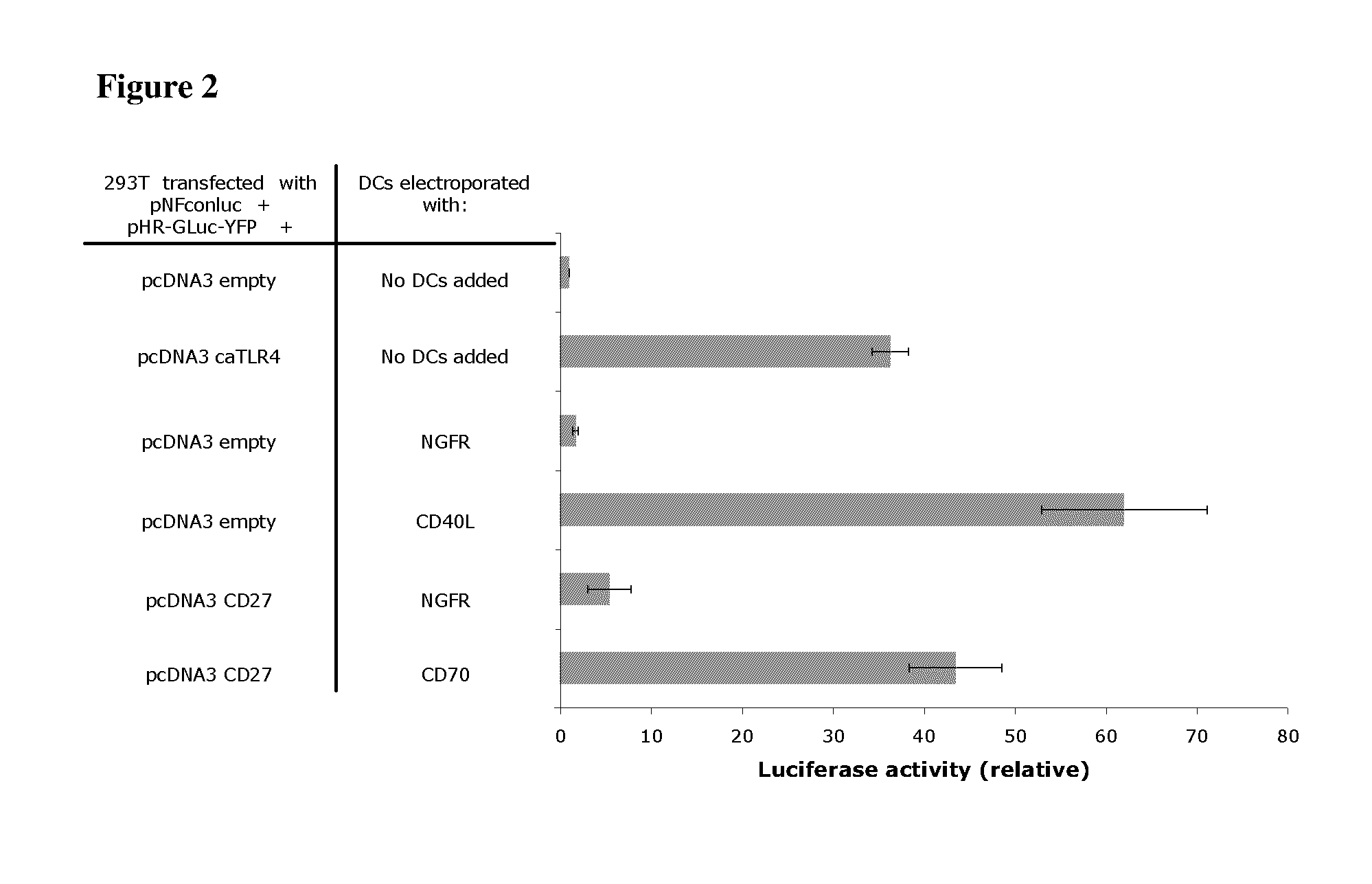
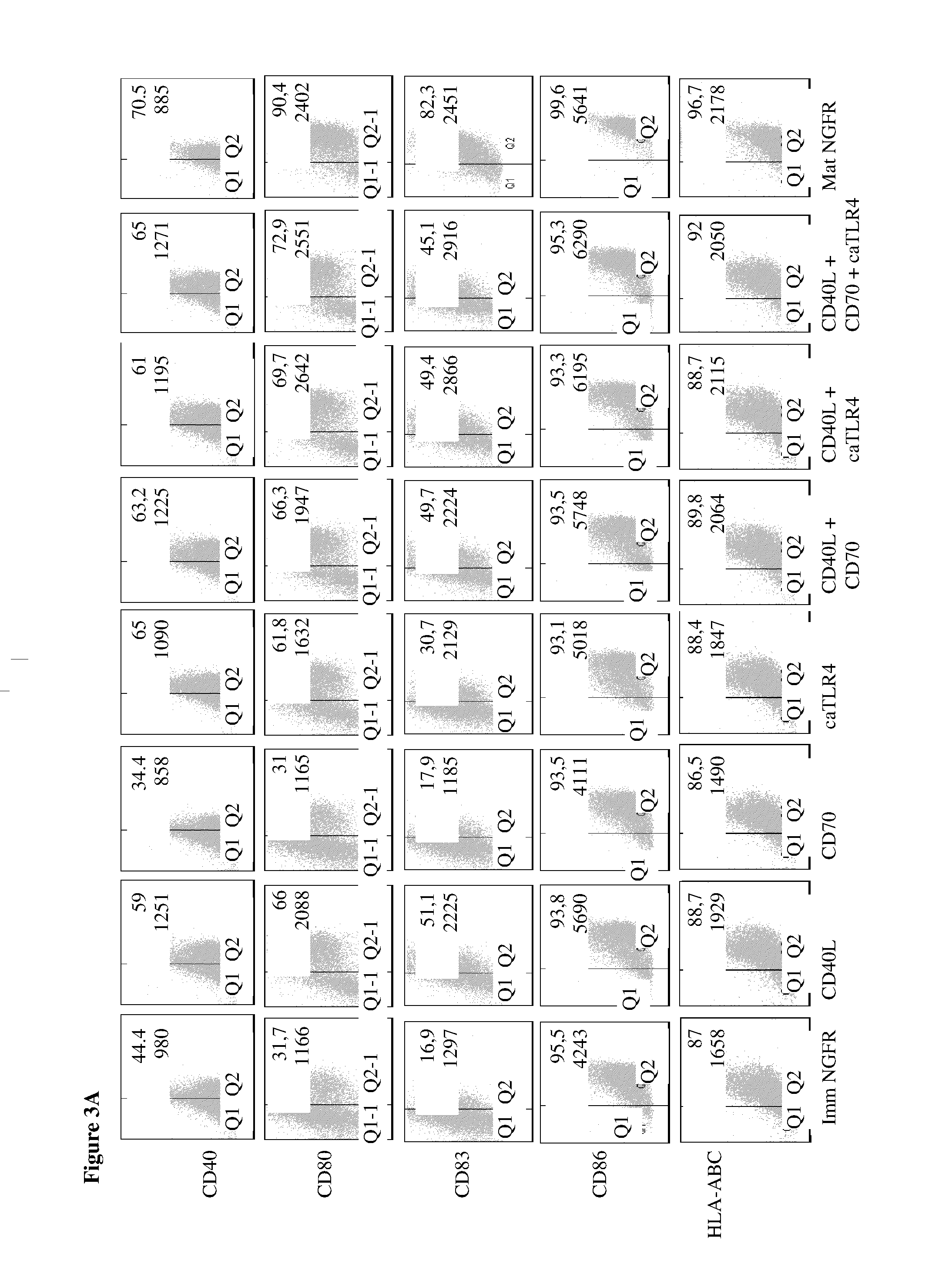
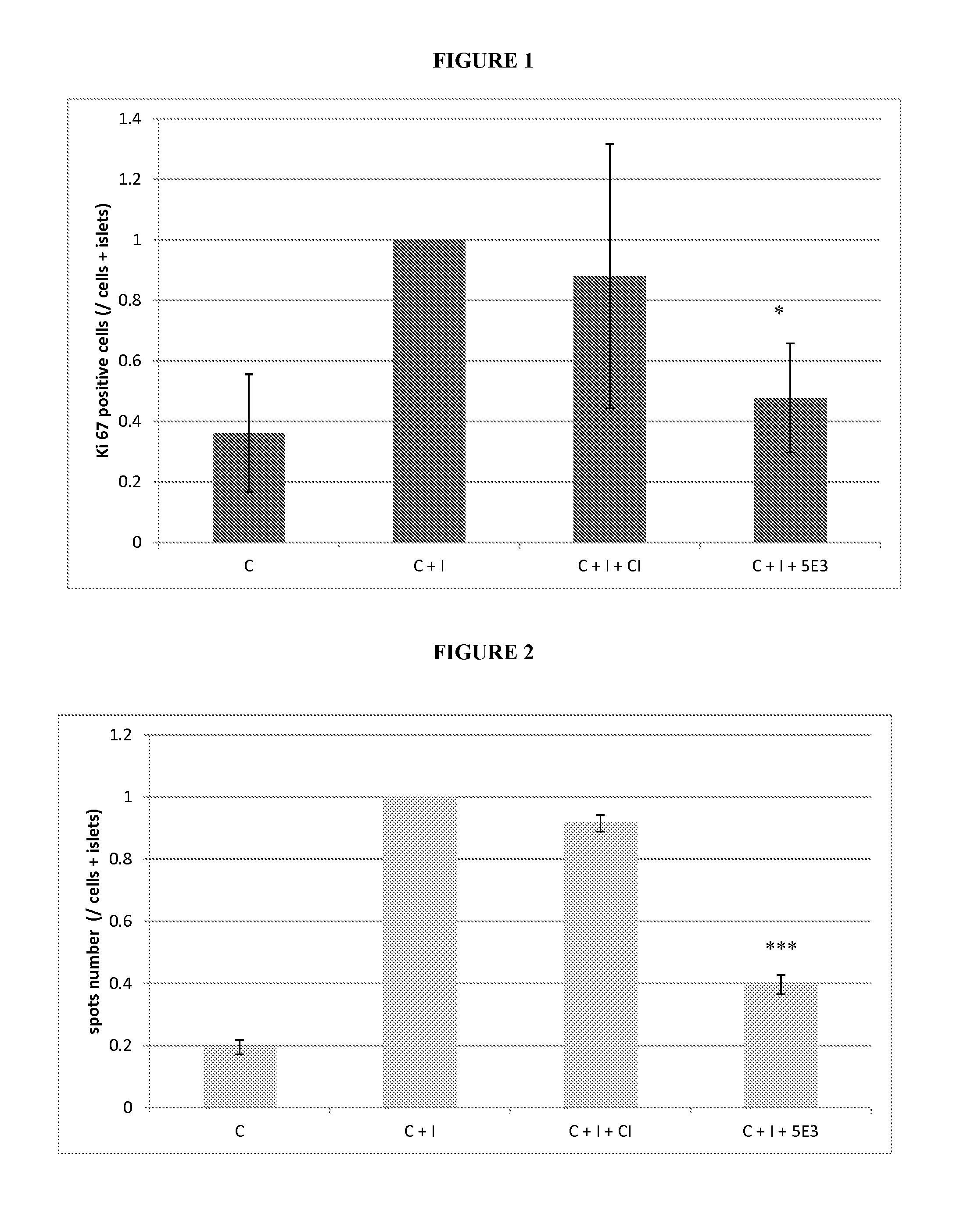
With the current invention, we provide new methods of enhancing the T-cell stimulatory capacity of human dendritic cells (DCs) and their use in cancer vaccination. The method comprises the introduction of different molecular adjuvants to human DCs through transfection with at least two mRNA or DNA molecules encoding markers selected from the group of: CD40L, CD70, constitutively active TLR4 (caTLR4), IL-12p70, EL-selectin, CCR7 and / or 4-1 BBL; or in combination with inhibition of SOCS, A20, PD-L1 and / or STAT3 expression, for example through siRNA transfection. We could show a clear increase in the immunostimulatory capacity of DCs obtained in this way, enabling them to elicit an unexpectedly high T-cell immune response in vitro. Introduction of at least two of the above molecules, in combination with a tumor-specific antigen enables the DCs to elicit a significant host-mediated T-cell immune response in vivo against the tumor antigen and thus makes them very attractive in the manufacturing of anti-cancer vaccines.
Owner:VRIJE UNIV BRUSSEL
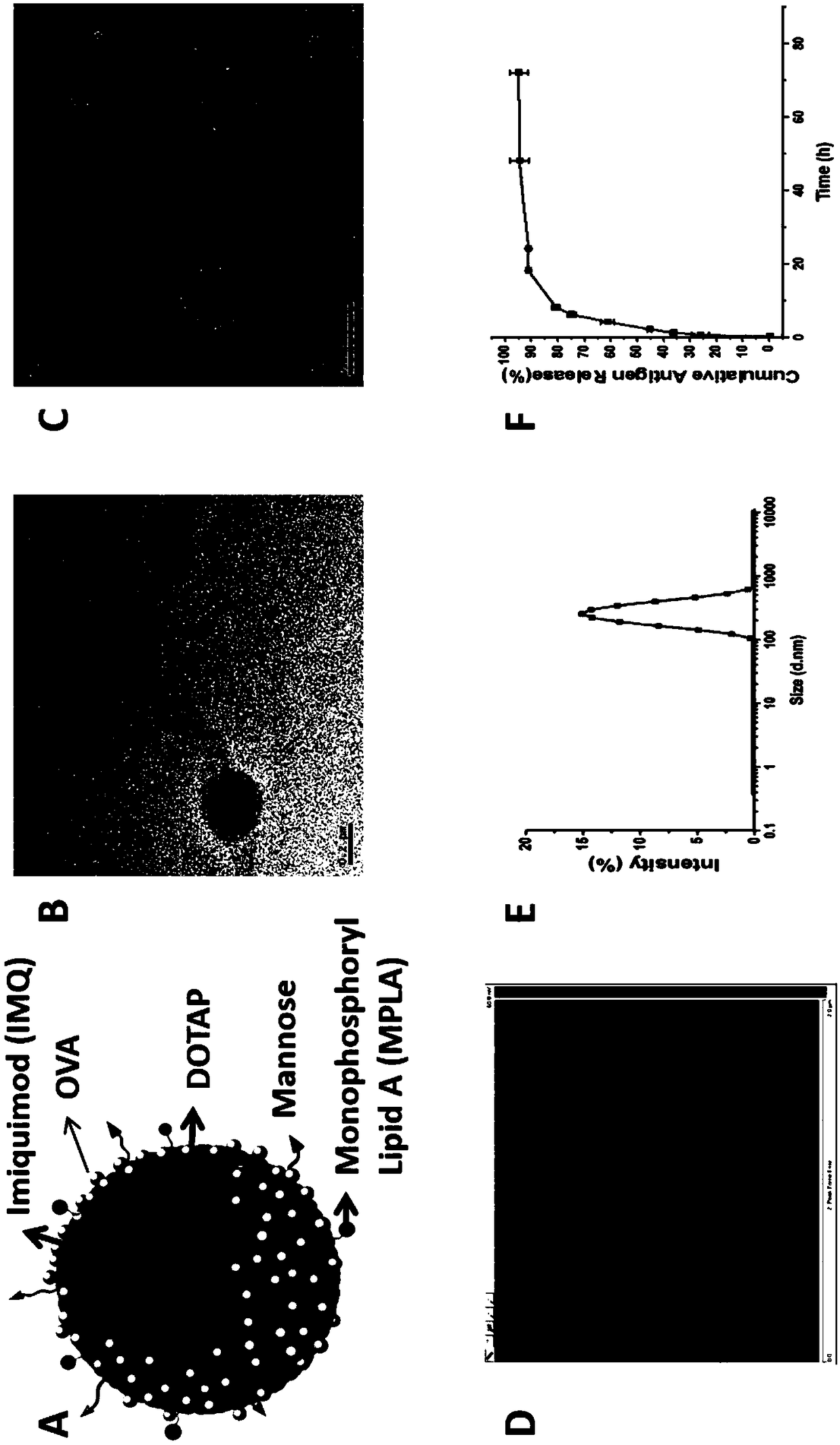
Cationic phospholipid-polymer hybridized nanoparticle vaccine adjuvant of common-carrier antigen, MPLA (Monophosphoryl Lipid A) and IMQ (Imiquimod) as well as preparation method and application thereof
ActiveCN108743939AGood biocompatibilityPromote degradationPharmaceutical non-active ingredientsAntibody medical ingredientsDendritic cellPhospholipid
The invention relates to a cationic phospholipid-polymer hybridized nanoparticle vaccine adjuvant of a common-carrier antigen, MPLA (Monophosphoryl Lipid A) and IMQ (Imiquimod) as well as a preparation method and application thereof. The vaccine adjuvant is characterized in that the IMQ as a TLR7 agonist is loaded on a hydrophobic core; the MPLA as a TLR4 agonist is loaded in a phopholipid layer;cationic phospholipid DOTAP (1,2-dioleoy-3-trimethylammonium-propane) in the phopholipid layer is used for adsorbing an antigen; the antigen is protected through hybridized nanoparticles, and the ingestion of the antigen by dendritic cells is improved; immune response after antigen stimulation is improved remarkably through the TLR agonist, and cross-presentation of the antigen is improved remarkably. The hybridized nanoparticles as the vaccine adjuvant can load the antigen and different types of TLR agonists simultaneously, can deliver the antigen through a plurality of immune paths, and promotes the DC activation and maturation. The cross-presentation level is raised, a strong and powerful T-cell killing effect is achieved, cell factor secretion is induced, a long-term memory T-cell reaction is generated, and higher prevention capability for tumors is achieved.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Purified Ethyl Ester Sophorolipid for the Treatment of Sepsis
InactiveUS20120142621A1High purityEfficient productionBiocideCarbohydrate active ingredients[Candida] apicolaChemical transformation
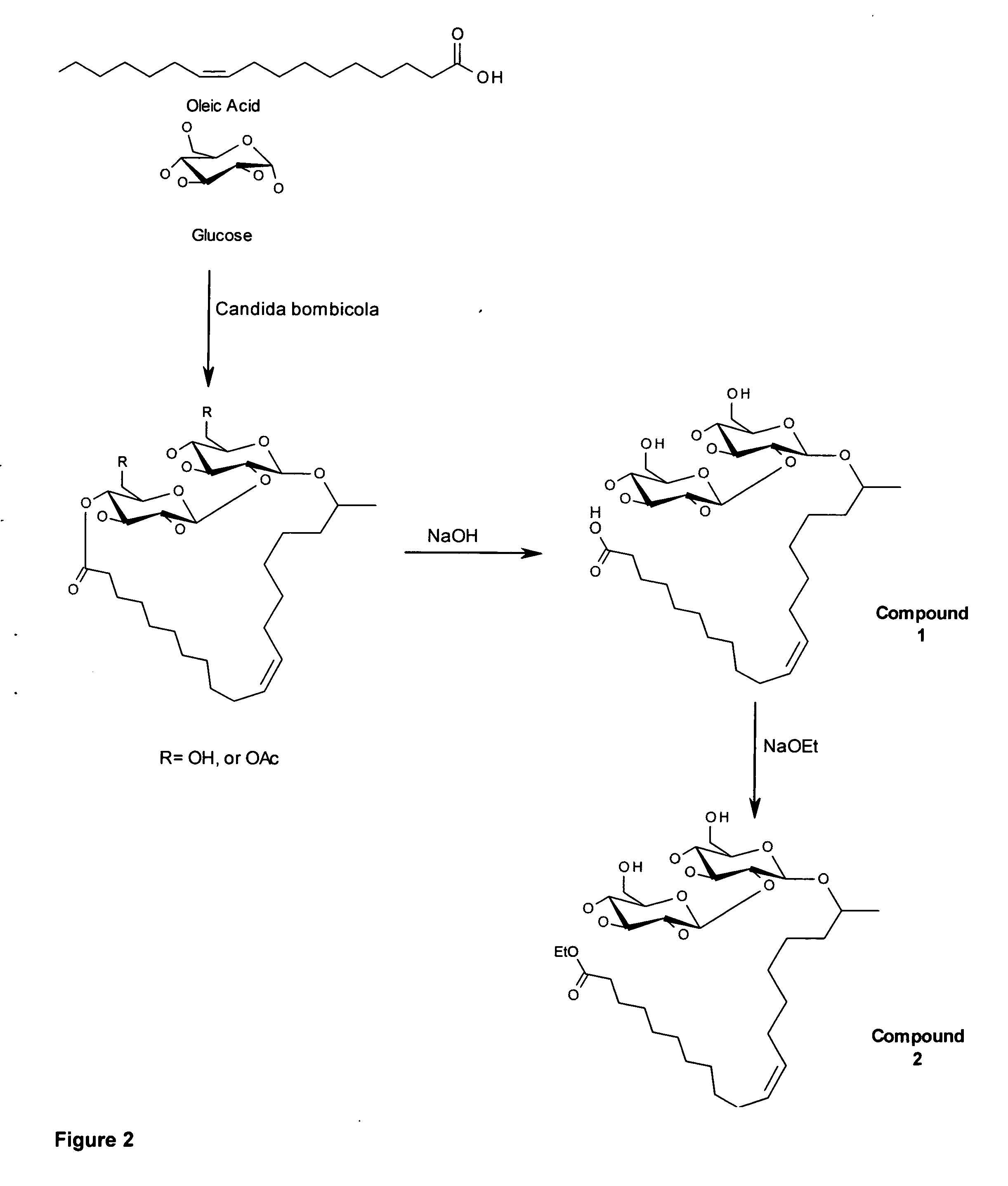
A microbial ethyl esther sophorolipid derivative with no acetylated groups produced by Candida species, for treating and preventing sepsis / septic shock. The method of producing sophorolipids is through microbial resting cells of Candida bombicola. The sophorolipids obtained from resting state cultures are isolated as a complex mixture of compounds and then decanted as a dense oil from the culture broth, subsequently washed to remove free fatty acids. Secondary chemical transformation via base catalyzed hydrolysis is used to reduce the 8 possible structural sophorolipid species to a single moiety, the 17-L-[(2′-O-b-D-glucopyranosyl-b-D-glucopyranosyl)-oxy]-cis-9-octadecenoate de-acetylated free acid. The compound acts primarily through decreasing inflammatory cytokines and eliciting other synergistic anti-inflammatory mechanisms by blocking TLR4-CD14 upstream of the inflammatory signaling cascade. The compound can be administered either intraperitoneally or intravenously at single or multiple doses of 5-30 mg / kg of weight in solvent media or in capped nanoparticles, preferably within 48 hours of sepsis inception.
Owner:STREETCAREC SLOAN
Enhancing the T-cells stimulatory capacity of human antigen presenting cells and their use in vaccination
With the current invention, we provide new methods of enhancing the T-cell stimulatory capacity of human dendritic cells (DCs) and their use in cancer vaccination. The method comprises the introduction of different molecular adjuvants to human DCs through transfection with at least two mRNA or DNA molecules encoding markers selected from the group of: CD40L, CD70, constitutively active TLR4 (caTLR4), IL-12p70, EL-selectin, CCR7 and / or 4-1 BBL; or in combination with inhibition of SOCS, A20, PD-L1 and / or STAT3 expression, for example through siRNA transfection. We could show a clear increase in the immunostimulatory capacity of DCs obtained in this way, enabling them to elicit an unexpectedly high T-cell immune response in vitro. Introduction of at least two of the above molecules, in combination with a tumor-specific antigen enables the DCs to elicit a significant host-mediated T-cell immune response in vivo against the tumor antigen and thus makes them very attractive in the manufacturing of anti-cancer vaccines.
Owner:VRIJE UNIV BRUSSEL
Panda source lactobacillus plantarum and application
InactiveCN106434411AProtection from damageReduce percentageAntibacterial agentsBacteriaEscherichia coliTLR4
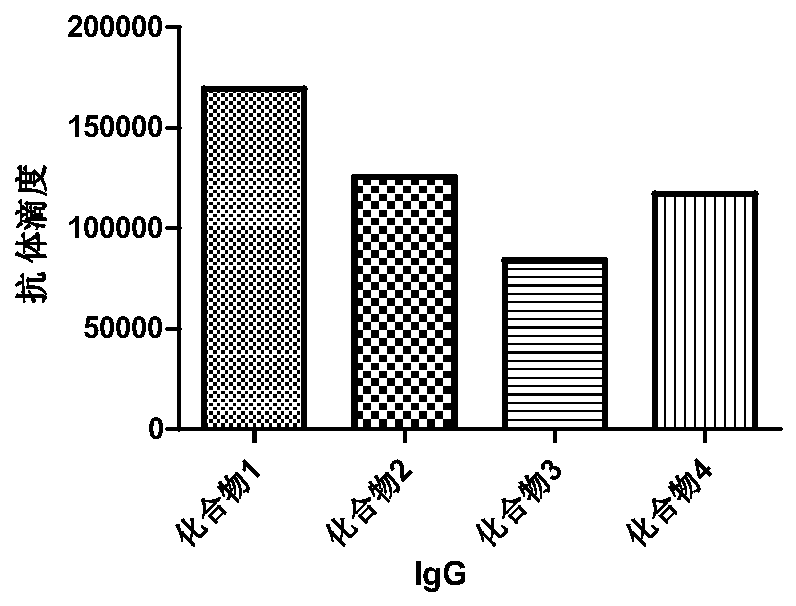
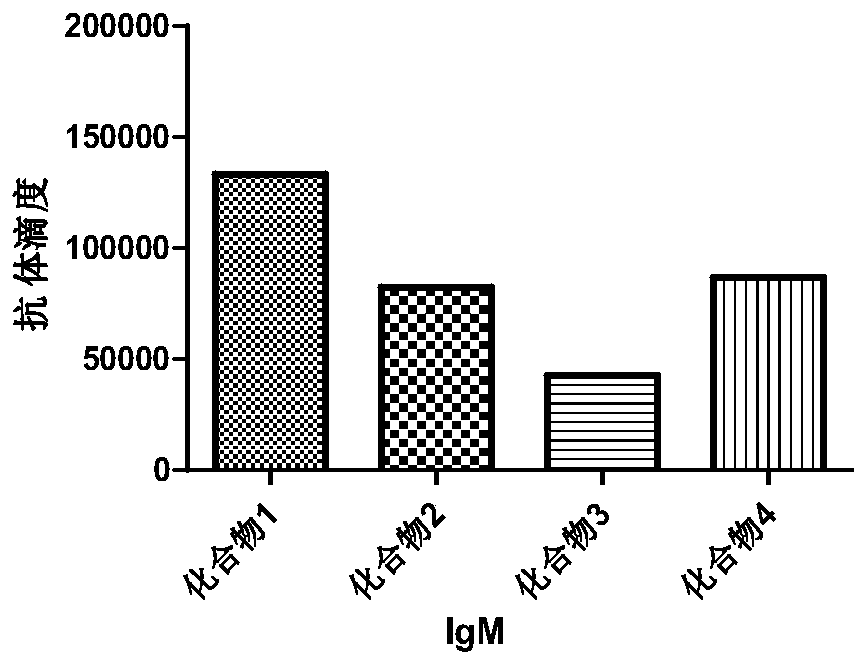
The invention discloses a panda source lactobacillus plantarum and application. The strain was preserved in China Center for Type Culture Collection on May 4, 2016 and the preservation number is CCTCC NO: M2016245. The panda source lactobacillus plantarum provided by the invention can be used for improving antibody levels of IgA, IgM and IgG immune globulins of blood serum; a condition that the percent of lymphocytes is reduced and the percent of neutrophils is increased, caused by infection and induction of enterotoxigenic escherichia coli, is inhibited; expression levels of inflammation related factors IL-1beta, IL-8, IL-6, TLR4 and MyD88mRNA (messenger Ribonucleic Acid) are remarkably down-regulated, and intestinal acute inflammation response caused by the enterotoxigenic escherichia coli is alleviated.
Owner:SICHUAN AGRI UNIV +1
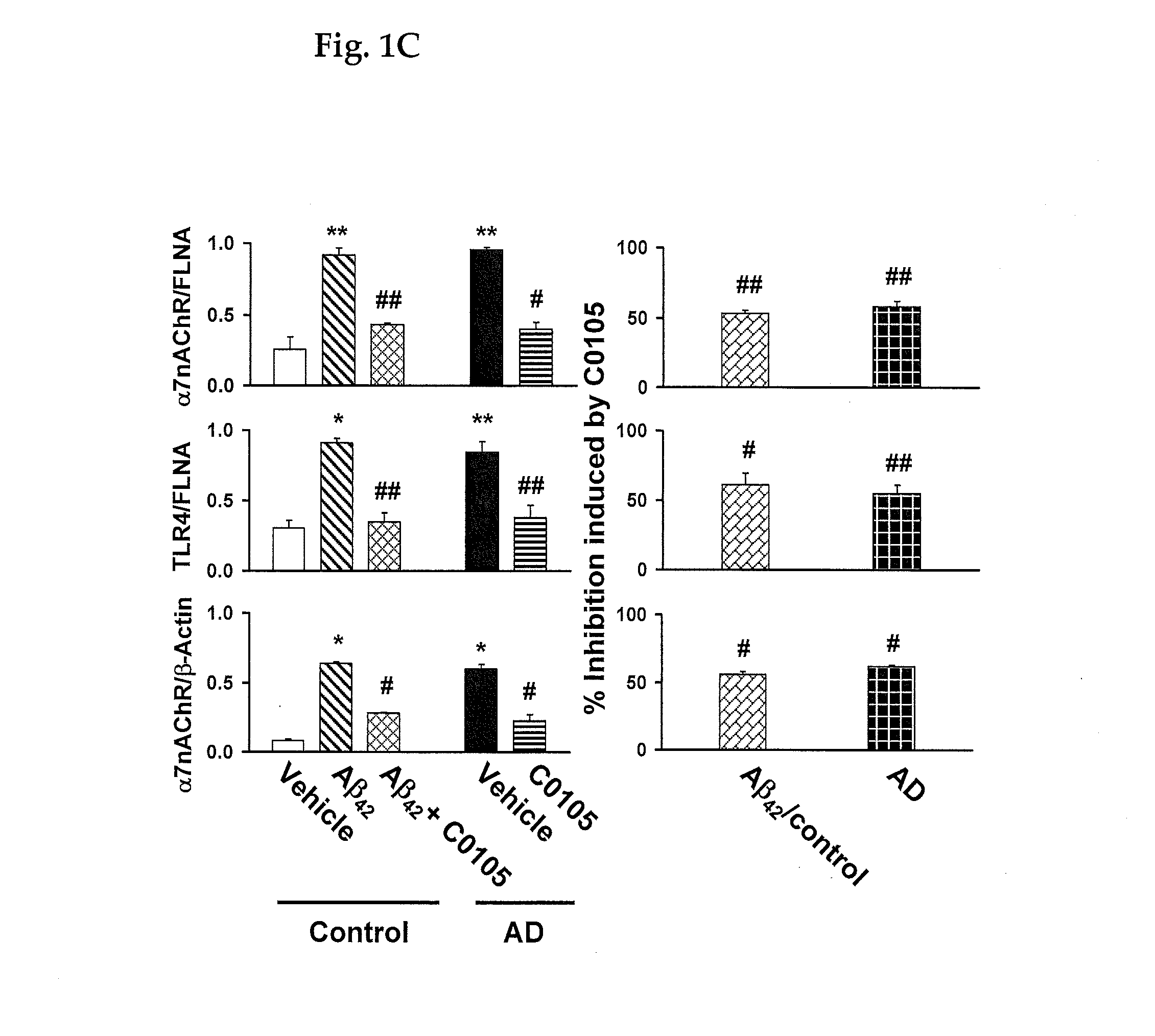
Alzheimer's disease assay in a living patient
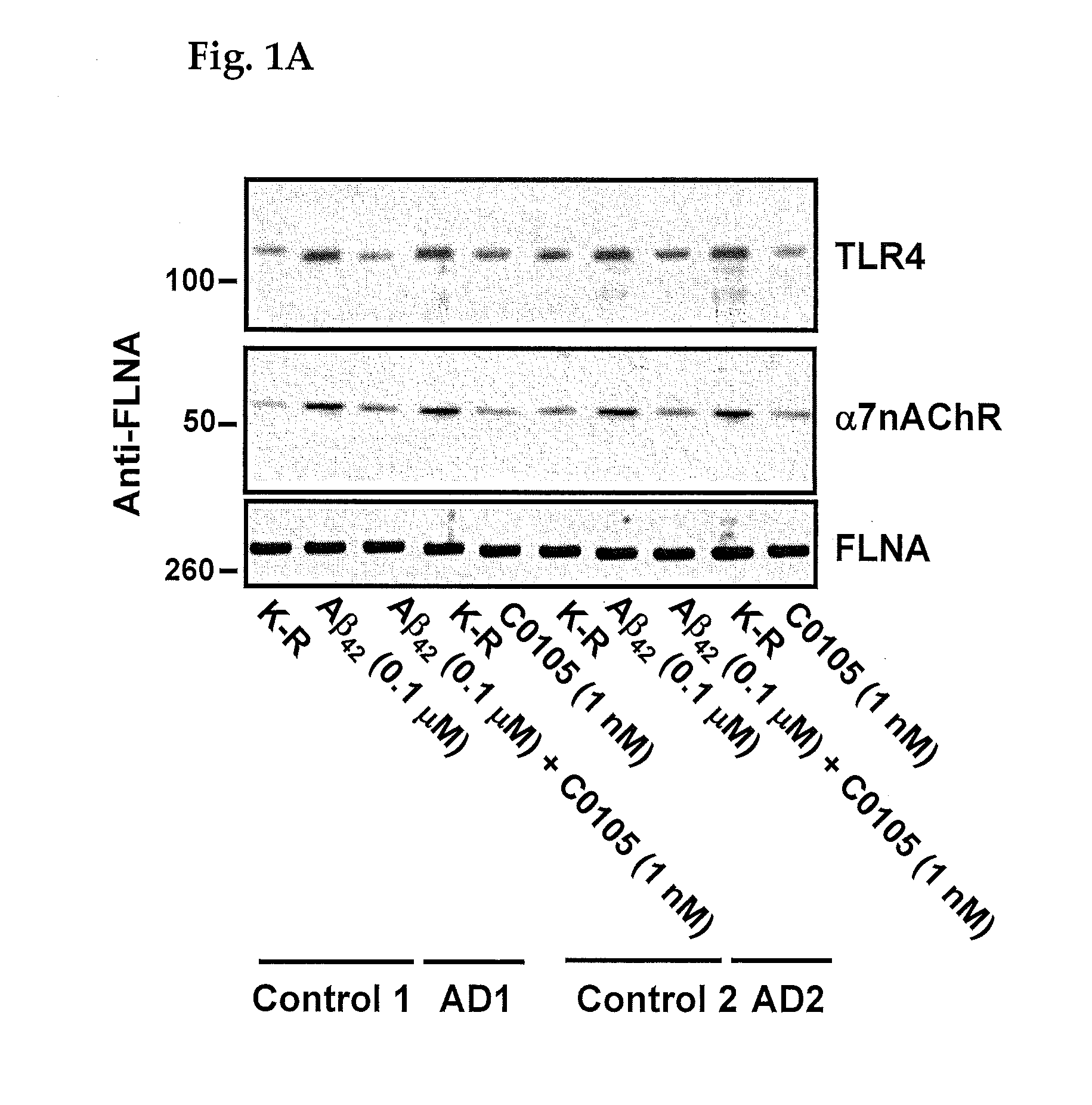
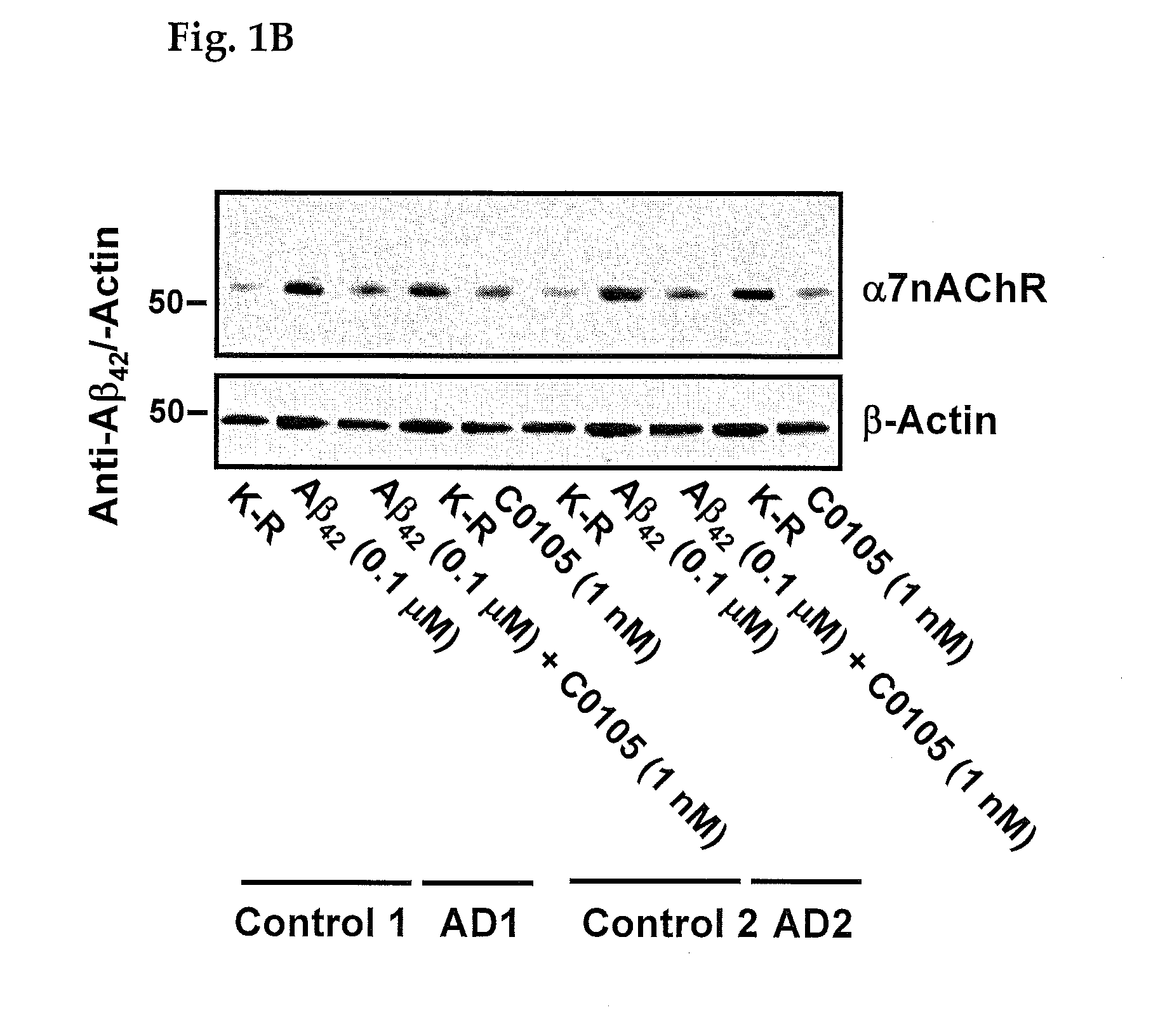
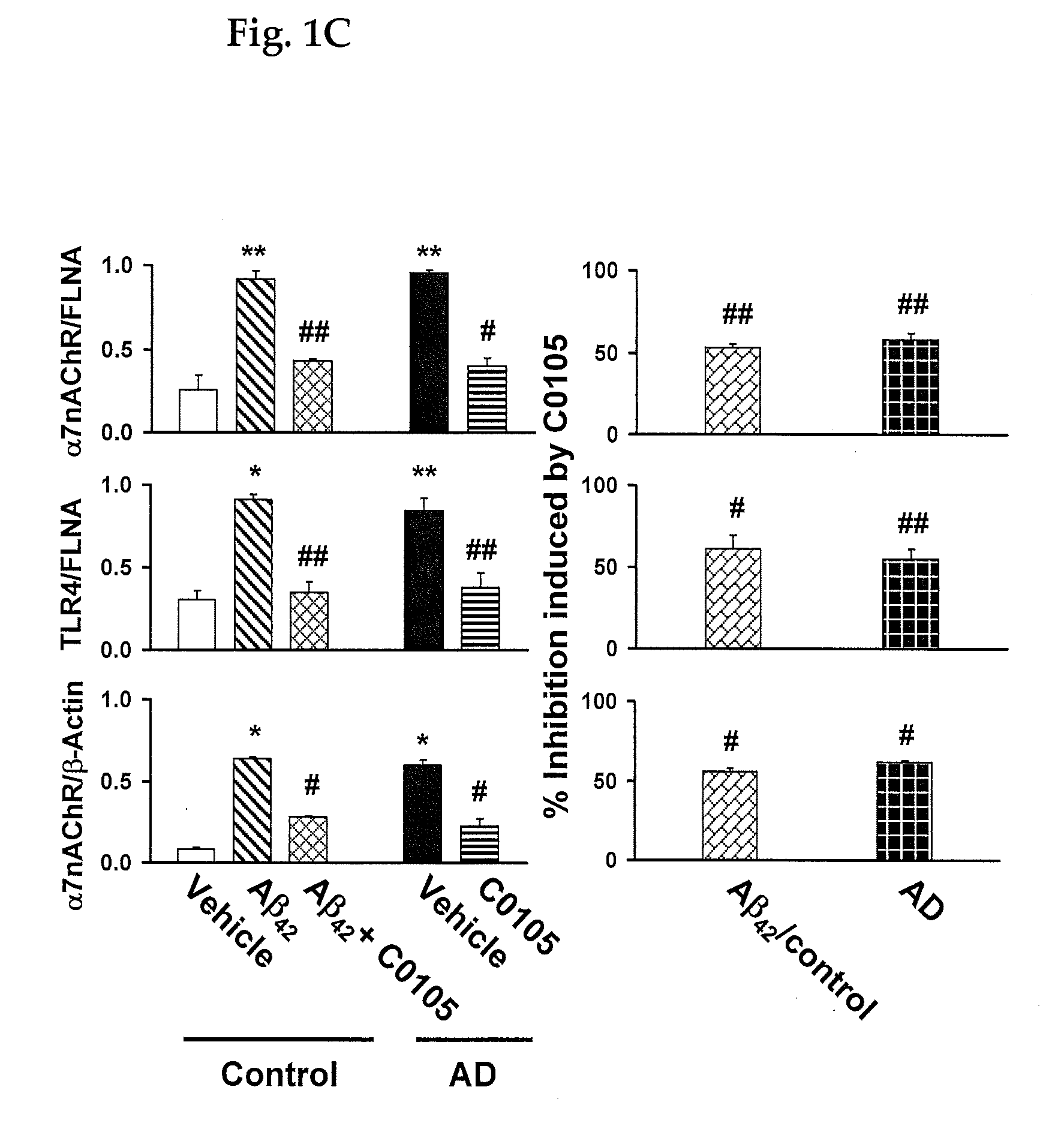
An assay for Alzheimer's disease (AD) pathology in a living patient is disclosed wherein an amount of α7nAChR or TLR4 in a FLNA-captured protein complex or α7nAChR in an Aβ-captured protein complex or α7nAChR-FLNA, or TLR4-FLNA and / or α7nAChR-Aβ42 complex present as a protein-protein complex in a sample is compared to the amount in a standard sample from a person free of AD pathology. An amount greater than in the standard sample indicates AD pathology. Also disclosed is an assay predictive of prognosis for treatment with a medicament in which the amount of an above protein or protein complex is compared to an amount in the presence of a medicament that binds to a FLNA pentapeptide and contains at least four pharmacophores of FIGS. 7-12. An amount of protein or protein complex determined in the presence of medicament that is less than the first amount indicates a favorable treatment prognosis.
Owner:PAIN THERAPEUTICS INC
Compositions and methods for modulating cells via CD14 and toII-like receptor 4 signaling pathway
Compositions and methods for screening and identifying compounds that modulate Toll-like receptor 4 (TLR4) pathway signaling via CD14 and ligands are provided. Provided are methods of treating various disease states, such as inflammatory or autoimmune diseases, in mammalian subjects by modulating Toll-like receptor 4 (TLR4) pathway signaling through CD14 and ligands. Transgenic non-human animals and methods of developing transgenic non-human animals are provided, wherein the transgenic non-human animals contain a loss-of-function mutation in the CD14 gene.
Owner:THE SCRIPPS RES INST
Enhancing the t-cell stimulatory capacity of human antigen presenting cells in vitro and in vivo and its use in vaccination
ActiveUS20140056939A1Reduced activityAvoid it happening againBacterial antigen ingredientsPeptide/protein ingredientsAntigenConstitutively active
Owner:VRIJE UNIV BRUSSEL
Anti-tlr4 antibodies and methods of use thereof
ActiveUS20120177648A1Prevent rejectionAvoid survivalMammal material medical ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsTransplant rejectionToll-like receptor
This invention relates generally to antibodies that specifically bind Toll-like Receptor 4 (TLR-4), and to methods of using the anti-TLR4 antibodies as therapeutics and to methods of using the anti-TLR4 antibodies in methods of preventing transplant rejection and / or prolonging survival of transplanted biological material.
Owner:NOVIMMUNE
Method for preventing or treating neuropathic pain
InactiveUS20070244066A1Reduce expressionReduced activityBiocideGenetic material ingredientsMedicineToll-like receptor
The present invention is a method for preventing or treating neuropathic pain. Using an agent to decrease the expression or activity of Toll-like receptor 4 (TLR4), behavioral hypersensitivity is attenuated thereby preventing or treating neuropathic pain in a subject in need of such treatment.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Toll-LIke Receptor 4 Deficiency and Downstream Effectors Cause Pulmonary Emphysema
The present invention provides compositions and methods for the detection, treatment, and prevention of emphysema / COPD. Compositions of the present invention comprise TLR4 activators, Nox3 inhibitors, and Cathepsin E inhibitors useful in the treatment or prevention of emphysema / COPD. Cathepsin E is a downstream effector of TLR4, wherein when cathepsin E is overexpressed in lung of an individual, the individual is at higher risk of developing emphysema / COPD. Cathepsin E is further identified as a biomarker useful in the identification of an individual with, or at-risk of developing emphysema / COPD.
Owner:YALE UNIV
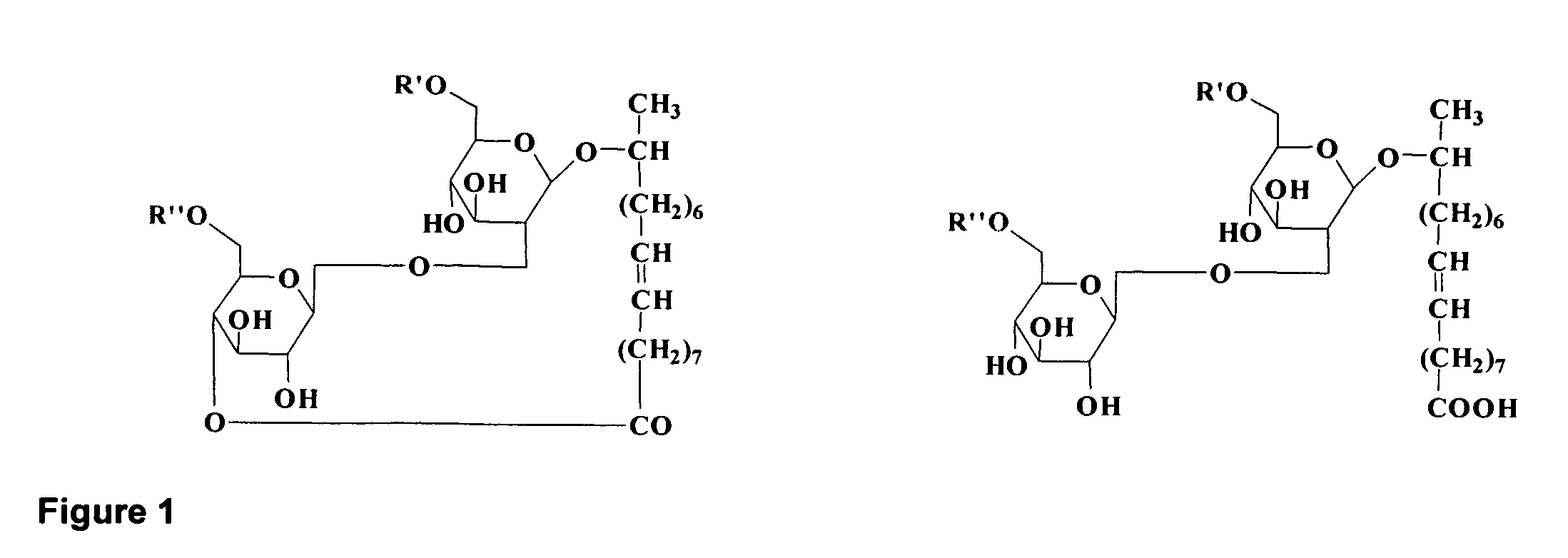
Monophosphate A conjugated-Tn anti-tumor vaccine and application thereof
ActiveCN110075291AClear structureGood prospects for anti-tumor applicationCancer antigen ingredientsCarrier-bound antigen/hapten ingredientsWilms' tumorAntineoplastic Vaccine
The invention provides a monophosphate A conjugated-Tn anti-tumor vaccine. The monophosphate A conjugated-Tn anti-tumor vaccine is a compound shown in a formula (I), namely Y-L-X(I). The monophosphateA conjugated-Tn anti-tumor vaccine is characterized in that a compound of the second generation of TLR4 ligand I of wholly new structure in the formula (I) is used for replacing MPLA (modified polylactic acid) and coupling with a compound (Tn) with clinical development potential in a formula (II), so as to obtain the two-component vaccine with a clear structure and a stronger anti-tumor function;the anti-tumor application prospect is better.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Use of toll-like receptor-9 agonists
The present invention relates to the use of a TLR9 agonist and / or a TLR4 antagonist and / or a NOD2 agonist for treatment or prevention of disorders involving TLR4 activation, such as systemic sepsis and necrotizing enterocolitis.
Owner:UNIVERSITY OF PITTSBURGH
Cancer immunotherapy
InactiveUS20140341978A1Skin cancer vaccineMammal material medical ingredientsAbnormal tissue growthTumor response
We formulated multiple TLR agonists into GVAX (lethally irradiated tumor cell vaccines engineered to secrete GM-CSF). Specifically, GLA and R848, TLR4 and TLR7 / 8 agonists found to be safe in patients, were formulated with GVAX (TEGVAX—for TLR agonists enhanced GVAX), and this formulation was effective in producing anti-tumor responses in 3 different preclinical models, including palpable B16. These anti-tumor responses were correlated with increased CD4 and CD8 T-cells that can secrete IFNγ circulating in the tumor microenvironment as well as significantly higher level of p15E specific CTL mediated cell killing in mice treated with TEGVAX in comparison to controls. When combined with anti-PD-1 antibody, TEGVAX was able to induce regression of established B16 tumors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
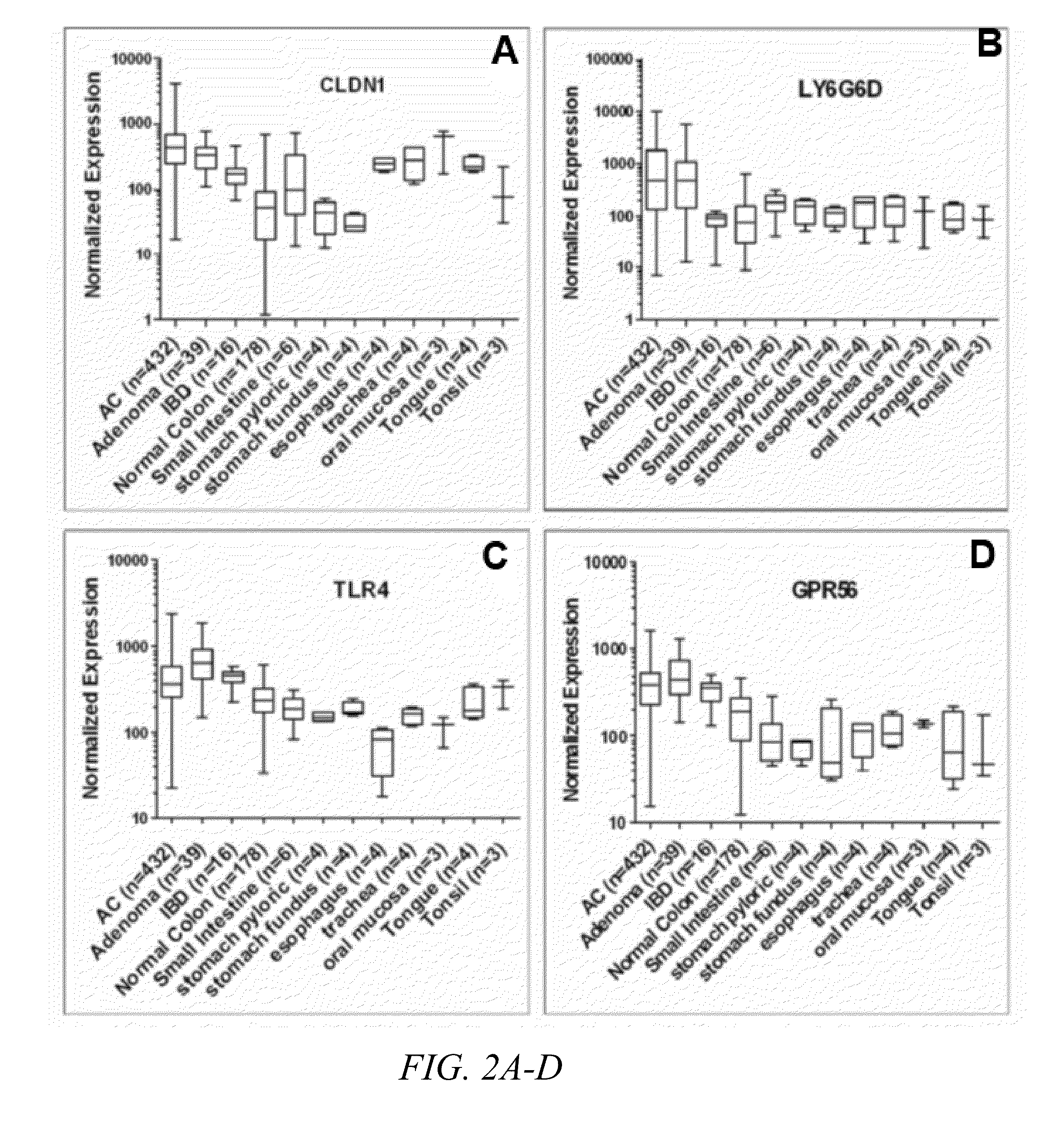
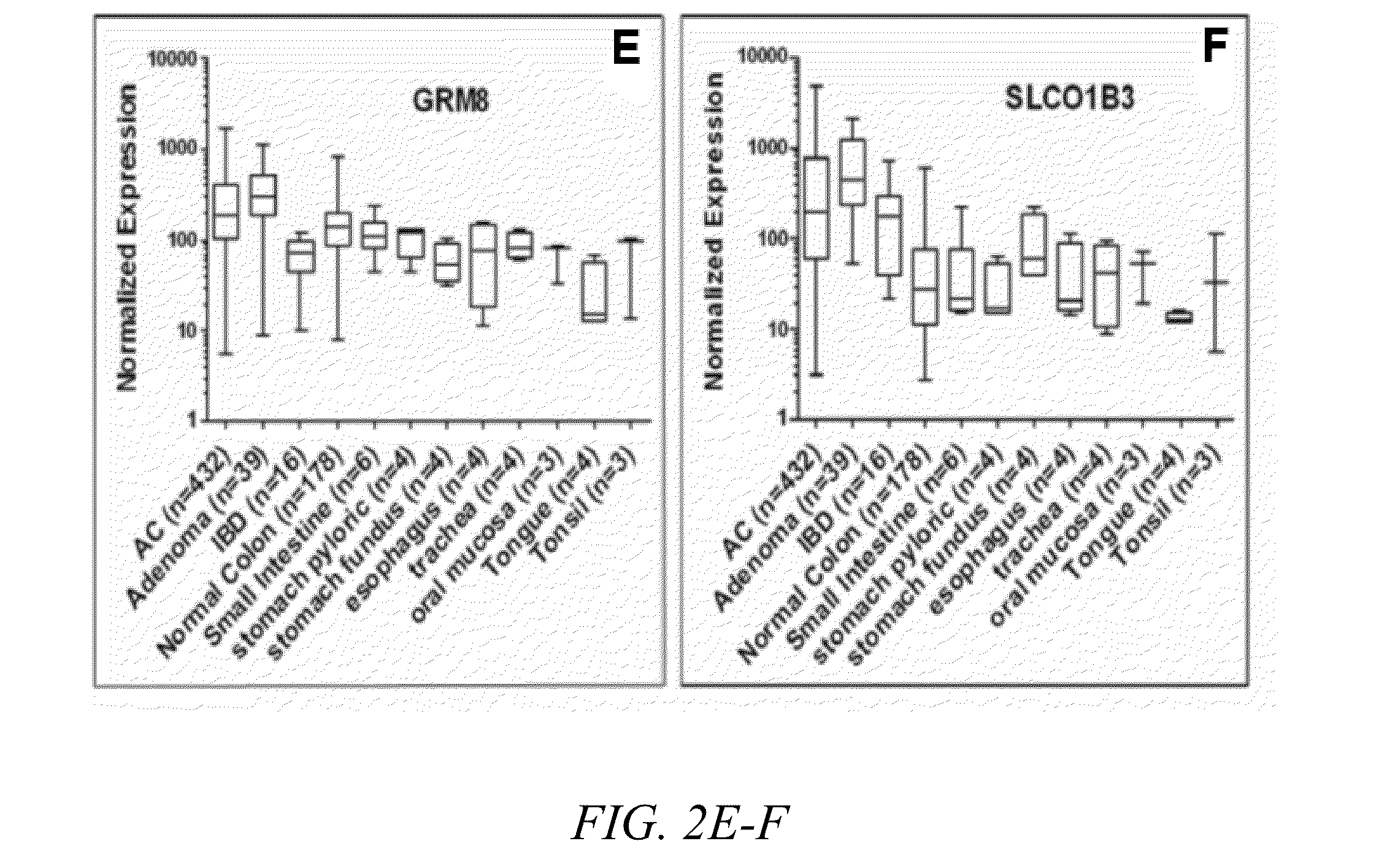
Method of screening for colon cancer using biomarkers
ActiveUS20140105827A1Strong specificityHigh sensitivityMicrobiological testing/measurementLibrary screeningAdenocarcinomaMolecular imaging
The present invention relates to biomarkers for colon cancers, specifically adenomas and adenocarcinomas in the GI tract. The inventors have discovered that the expression and / or overexpression of biomarkers such as CLDN1, GPR56, GRM8, LY6G6D, TLR4 and SLCO1B3 are indicative of adenomas and adenocarcinomas. A method of detecting colon cancer using targeted molecular imaging agents is also presented.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA +1
Use of Toll-like receptor-9 agonists, Toll-like receptor-4 antagonists, and/or nuclear oligomerization domain-2 agonists for the treatment or prevention of Toll-like receptor-4-associated disorders
ActiveUS20120077868A1Reduce marksReduce signalingAntibacterial agentsBiocideDiseaseNK1 receptor antagonist
The present invention relates to the use of a TLR9 agonist and / or a TLR4 antagonist and / or a NOD2 agonist for treatment or prevention of disorders involving TLR4 activation, such as systemic sepsis and necrotizing enterocolitis.
Owner:UNIVERSITY OF PITTSBURGH
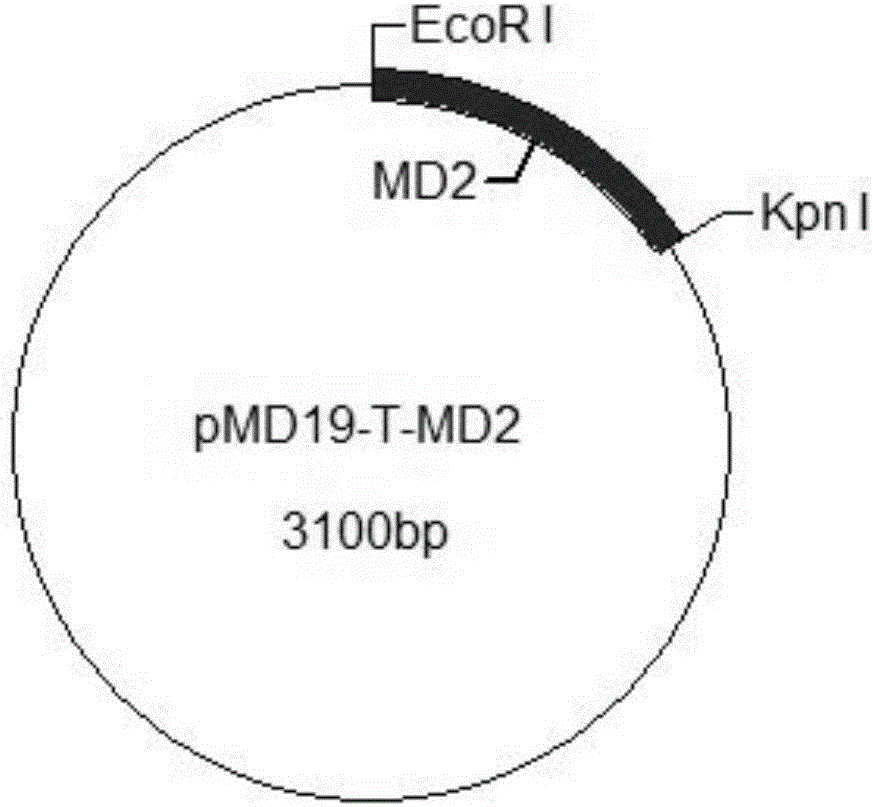
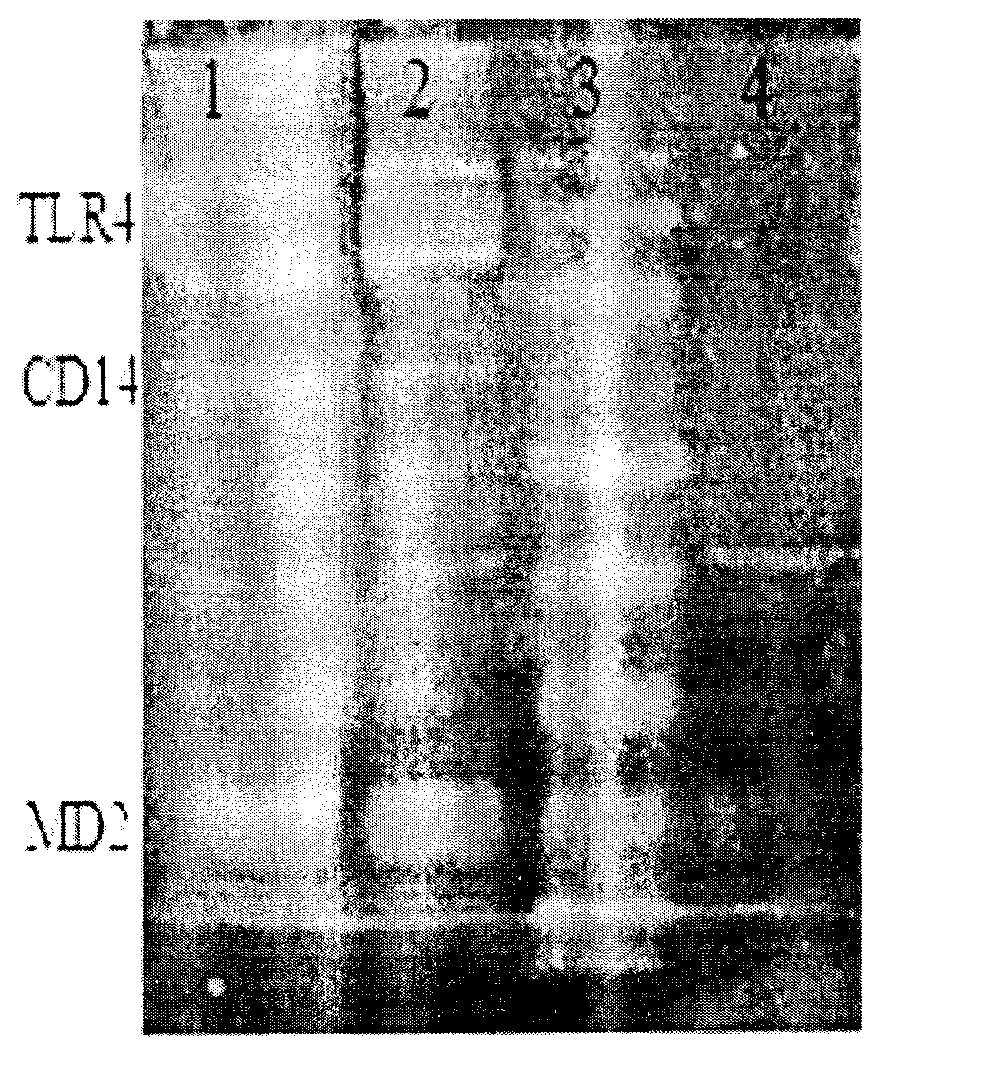
Cell model having TLR4 immune response function and method for constructing same
ActiveCN102796706AVector-based foreign material introductionForeign genetic material cellsMechanism of actionCD15
The invention discloses a cell model having TLR4 immune response function and a method for constructing the cell model. The cell model and the method are characterized in that: three different bovine genes MD2, CD15 and TLR4 are connected and assembled to a vector by 2A sequence, the vector is delivered into a commercial cell line, and the constructed cell model has the function of LPS-mediated immune response. The cell model constructed in the invention and the construction method provide technical supports for the study of functions of the bovine TLR4 gene and the elucidation of the bovine TLR4 gene mechanism, therefore are valuable tool for studying bovine TLR4 gene functions.
Owner:新疆维吾尔自治区畜牧科学院中国-澳大利亚绵羊育种研究中心
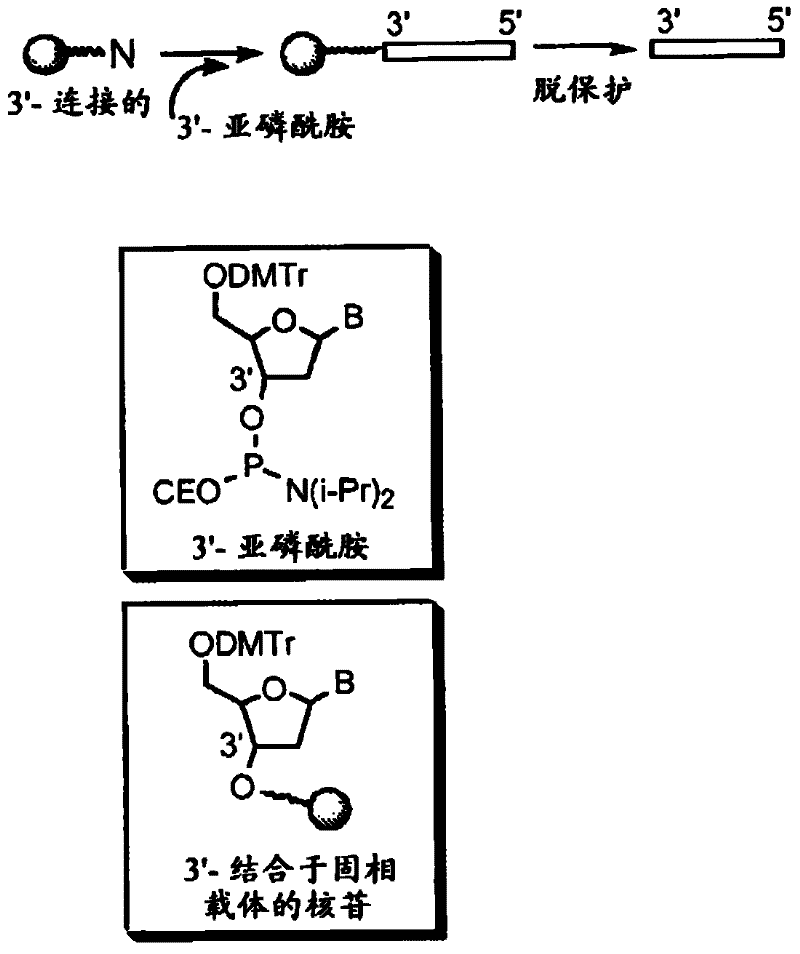
Modulation of Toll-Like Receptor 4 Expression by Antisense Oligonucleotides
InactiveUS20100111936A1Efficiently inhibit expressionEfficient expressionAntibacterial agentsSenses disorderDiseaseToll-like receptor
Antisense oligonucleotide compounds, compositions and methods are provided for down regulating the expression of TLR4. The compositions comprise antisense oligonucleotides targeted to nucleic acids encoding TLR4. The compositions may also comprise antisense oligonucleotides targeted to nucleic acids encoding TLR4 in combination with other therapeutic and / or prophylactic compounds and / or compositions. Methods of using these compounds and compositions for down-regulating TLR4 expression and for prevention or treatment of diseases wherein modulation of TLR4 expression would be beneficial are provided.
Owner:IDERA PHARMA INC
Alzheimer's disease assay in a living patient
ActiveUS20140017698A1Increase awarenessImprove effectivelyNervous disorderDisease diagnosisDiseaseAssay
An assay for Alzheimer's disease (AD) pathology in a living patient is disclosed wherein an amount of α7nAChR or TLR4 in a FLNA-captured protein complex or α7nAChR in an Aβ-captured protein complex or α7nAChR-FLNA, or TLR4-FLNA and / or α7nAChR-Aβ42 complex present as a protein-protein complex in a sample is compared to the amount in a standard sample from a person free of AD pathology. An amount greater than in the standard sample indicates AD pathology. Also disclosed is an assay predictive of prognosis for treatment with a medicament in which the amount of an above protein or protein complex is compared to an amount in the presence of a medicament that binds to a FLNA pentapeptide and contains at least four pharmacophores of FIGS. 7-12. An amount of protein or protein complex determined in the presence of medicament that is less than the first amount indicates a favorable treatment prognosis.
Owner:PAIN THERAPEUTICS INC
Application of hordenine in preparing drug for treating hypophysoma
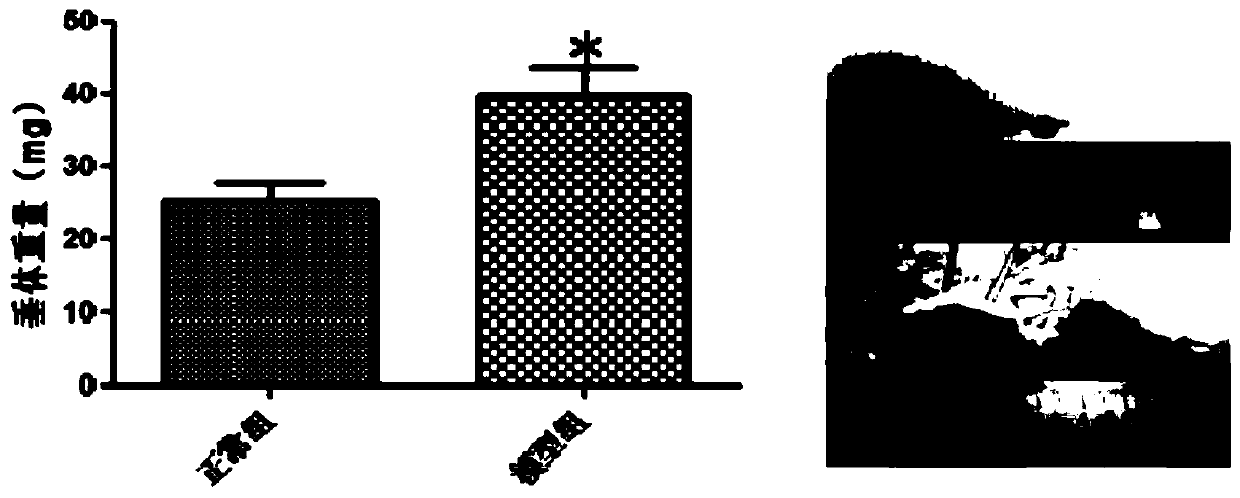
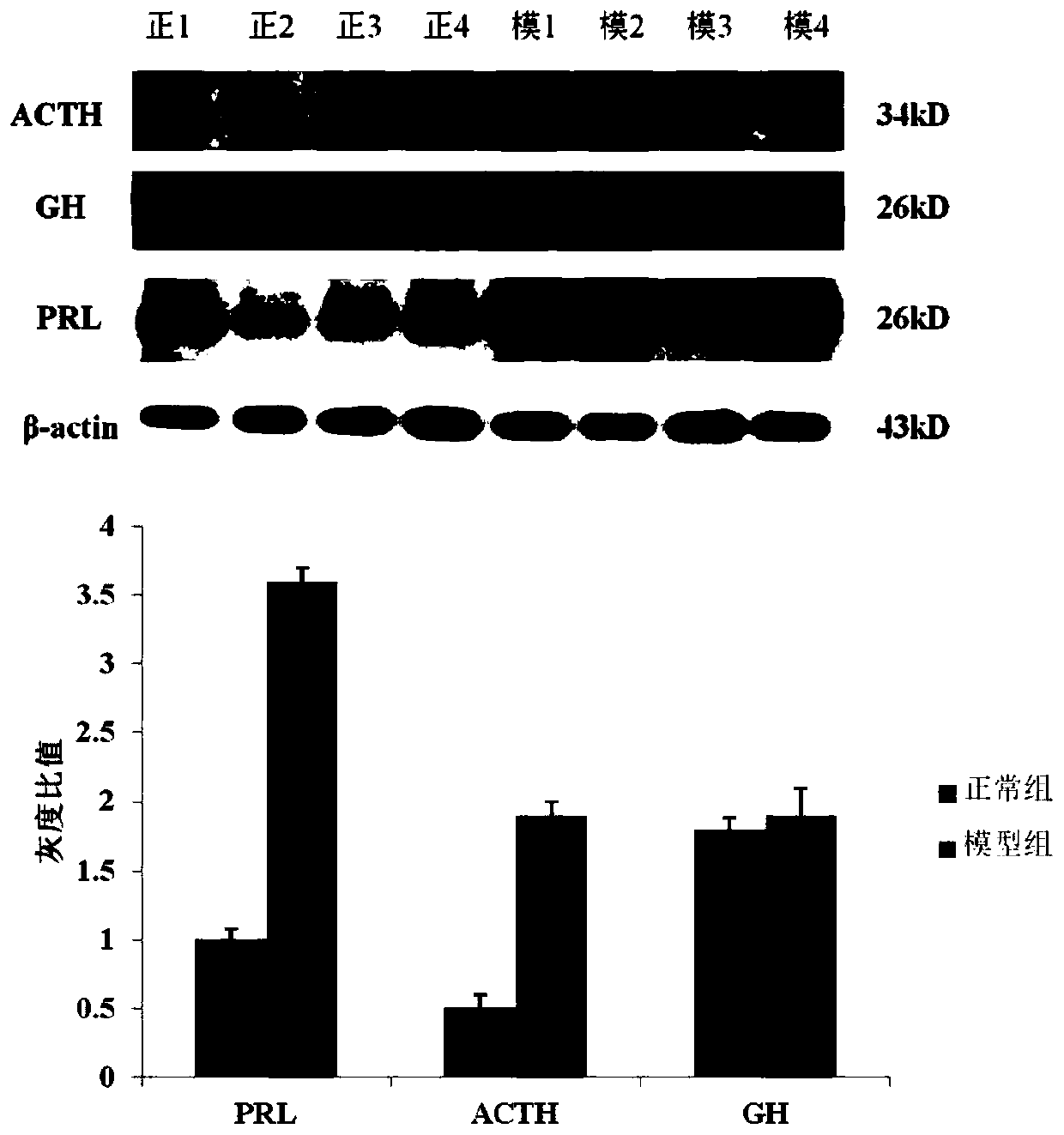
ActiveCN110151742ASolving the plight of drug shortagesEnhanced inhibitory effectOrganic active ingredientsAntineoplastic agentsAdrenocorticotropic hormoneTLR4
The invention discloses application of hordenine in preparing a drug for treating hypophysoma, and relates to the technical field of biopharmacy. Specifically, in the application, the inventor finds that the hordenine can inhibit a TLR4 / NF-kB / MAPK signaling pathway, thereby being conductive to exerting the pharmaceutical effect on treating prolactinoma and adrenocorticotropic hormone adenoma, andsolving the problem of drug shortage for patients with the prolactinoma and the adrenocorticotropic hormone adenoma.
Owner:王雄 +1
Application of icarisid II in preparation of sensitizer for chemotherapic medicine
InactiveCN103239464AIncreased sensitivitySmall doseOrganic active ingredientsAntineoplastic agentsSide effectMelanoma
The invention belongs to the field of biological medicines, and relates to application of icarisid II in preparation of a sensitizer for a chemotherapic medicine. Shown in the experimental results, the icarisid II can obviously improve the inhibition effect of the taxol to the cancer cell proliferation, the taxol-induced tumor cell apoptosis, and the effect of inhibiting a TLR4 (Toll-Like Receptors 4) patch in order to improve the sensitivity of a chemotherapeutic medicine; the icarisid II can be used as the sensitizer for the chemotherapic medicine, and is applied to the tumor chemotherapy process, in order to obviously promote the medicine sensitivity of different tumor cells to the chemotherapic medicine, therefore, the dosage of the chemotherapic medicines is decreased, and the toxic and side effects of the chemotherapic medicine to normal cells of a human body are reduced. The icarisid II can be used as an active ingredient to be used together with a pharmaceutically acceptable carrier in order to prepare a pharmaceutical composition performing sensibilization in tumor chemotherapy, and the pharmaceutical composition is applied to chemosensitization for a plurality of tumors, including but not limited to melanoma and cutaneous squamous cell carcinoma and the like.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
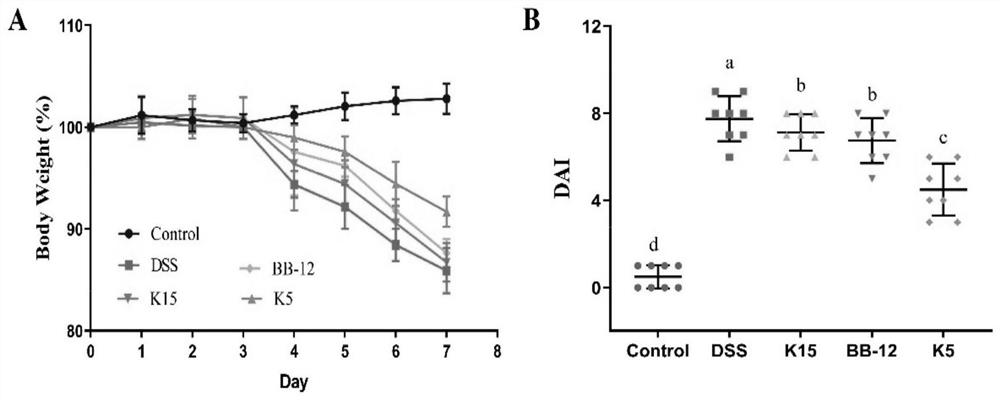
Bifidobacterium longum subsp. Longum capable of preventing and relieving colitis symptoms and application thereof
ActiveCN114164134AEasily damagedEasy to changeBacteriaDigestive systemBiotechnologyUlcerative colitis
The invention provides bifidobacterium longum subsp. Longum KLDS K5 capable of preventing and relieving symptoms of colitis, and belongs to the technical field of microorganisms. The invention further provides a preparation method of the bifidobacterium longum subsp. Longum KLDS K5. Experiments show that the bifidobacterium longum provided by the invention can tolerate the human gastrointestinal environment, relieve weight loss in the ulcerative colitis disease period, improve colonic mucosa injury and reduce MPO activity, and inhibit oxidation-related factors through iNOS and COX-2 signal pathways so as to relieve inflammatory bowel diseases; nF-kappa B p65 nuclear transfer is inhibited through a TLR4 / MyD88 / NF-kappa B signal channel, the expression quantity of proinflammatory factors TNF-alpha, IL-1beta, IL-6 and IL-10 in colon is reduced, the transcriptional level of colon tight connection related proteins Claudin-1, ZO-1 and Occludin and mucoprotein MUC2 is up-regulated, the intestinal flora change after DSS induction can be improved in the genus level, and the richness and diversity of the intestinal flora are improved.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Modulation of toll-like receptor 4 expression by antisense oligonucleotides
Owner:IDERA PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com