Cationic phospholipid-polymer hybridized nanoparticle vaccine adjuvant of common-carrier antigen, MPLA (Monophosphoryl Lipid A) and IMQ (Imiquimod) as well as preparation method and application thereof
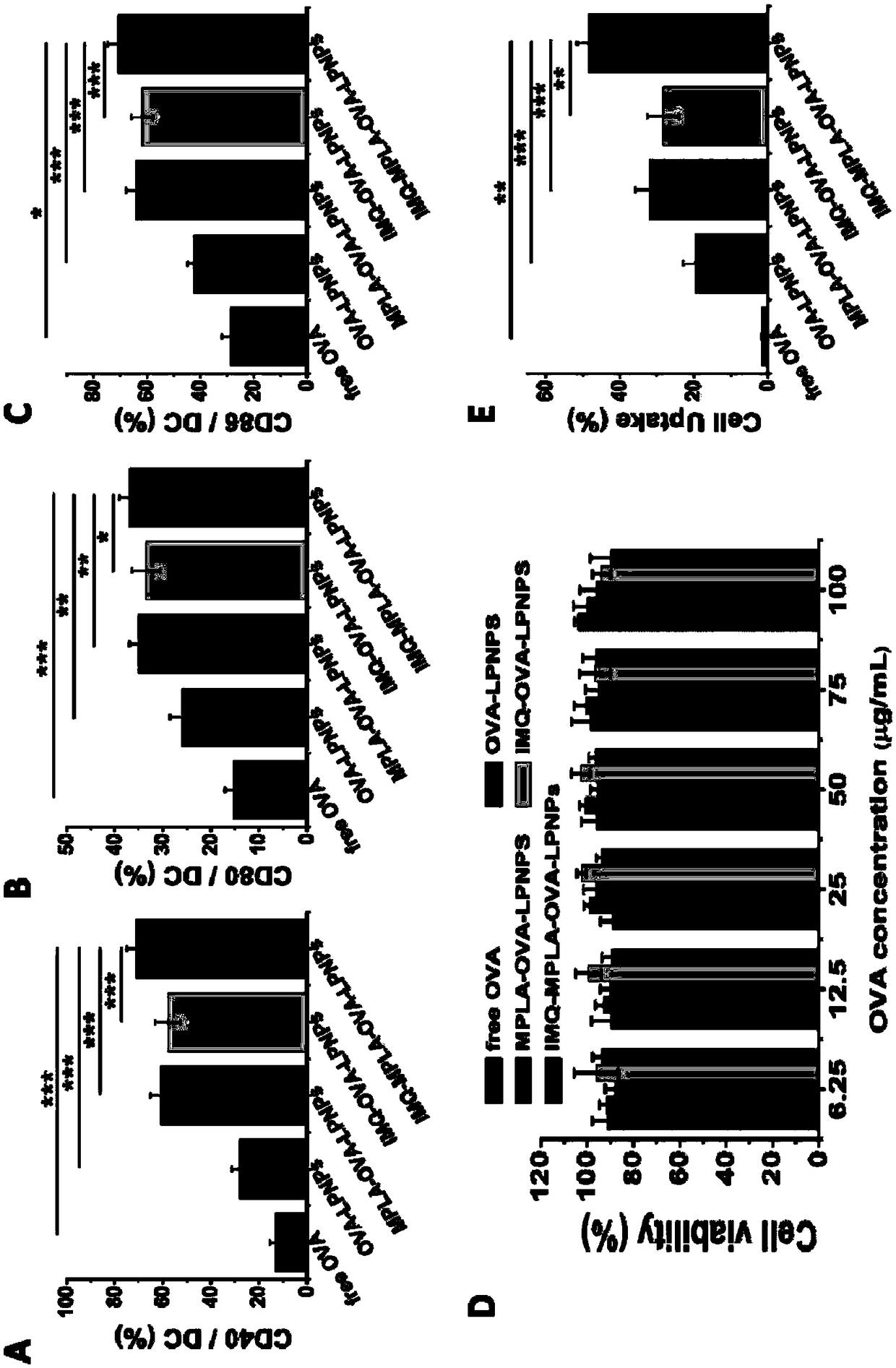
A vaccine adjuvant and cationic technology, which is applied in the field of cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant and preparation, can solve the problems of low drug loading, low biocompatibility, and rapid drug leakage, so as to promote DCs Mature, good adjuvant effect, effect of improving antigen uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The invention provides a preparation method of an antigen-loaded cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant, comprising the steps of:
[0035] S1: Dissolve the amphiphilic triblock copolymer PCL-b-PEG-b-PCL and the cationic phospholipid DOTAP in an organic solvent, then remove the organic solvent by rotary evaporation, and form a uniform film on the bottle wall, blow with nitrogen Dry the residual solvent, then put it in a vacuum drying oven, and dry it in vacuum for 12-24h; wherein, the molecular weight of the amphiphilic triblock copolymer PCL-b-PEG-b-PCL is 10000-24000, preferably 16000, wherein PEG The mass percentage of the hydrophilic segment is greater than 45%, the mass ratio of the amphiphilic triblock copolymer PCL-b-PEG-b-PCL to the cationic phospholipid DOTAP is 20 mg: (0.5-2) mg; the organic solvent is selected from acetonitrile, A mixture of one or more of dichloromethane and chloroform;
[0036] Preferably, it also includes the st...
Embodiment 1
[0041] This embodiment provides a cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant loaded with antigen OVA. The preparation method comprises steps:
[0042] S1: Dissolve 20mg of amphiphilic triblock copolymer PCL-b-PEG-b-PCL and 1mg of cationic phospholipid DOTAP in dichloromethane, then remove the organic solvent by rotary evaporation, and form a uniform film on the bottle wall, Dry the residual solvent with nitrogen, then put it in a vacuum drying oven, and dry it in vacuum for 12-24 hours; among them, the molecular weight of the amphiphilic triblock copolymer PCL-b-PEG-b-PCL is 16000, and the PEG hydrophilic chain Segment mass percentage greater than 45%.
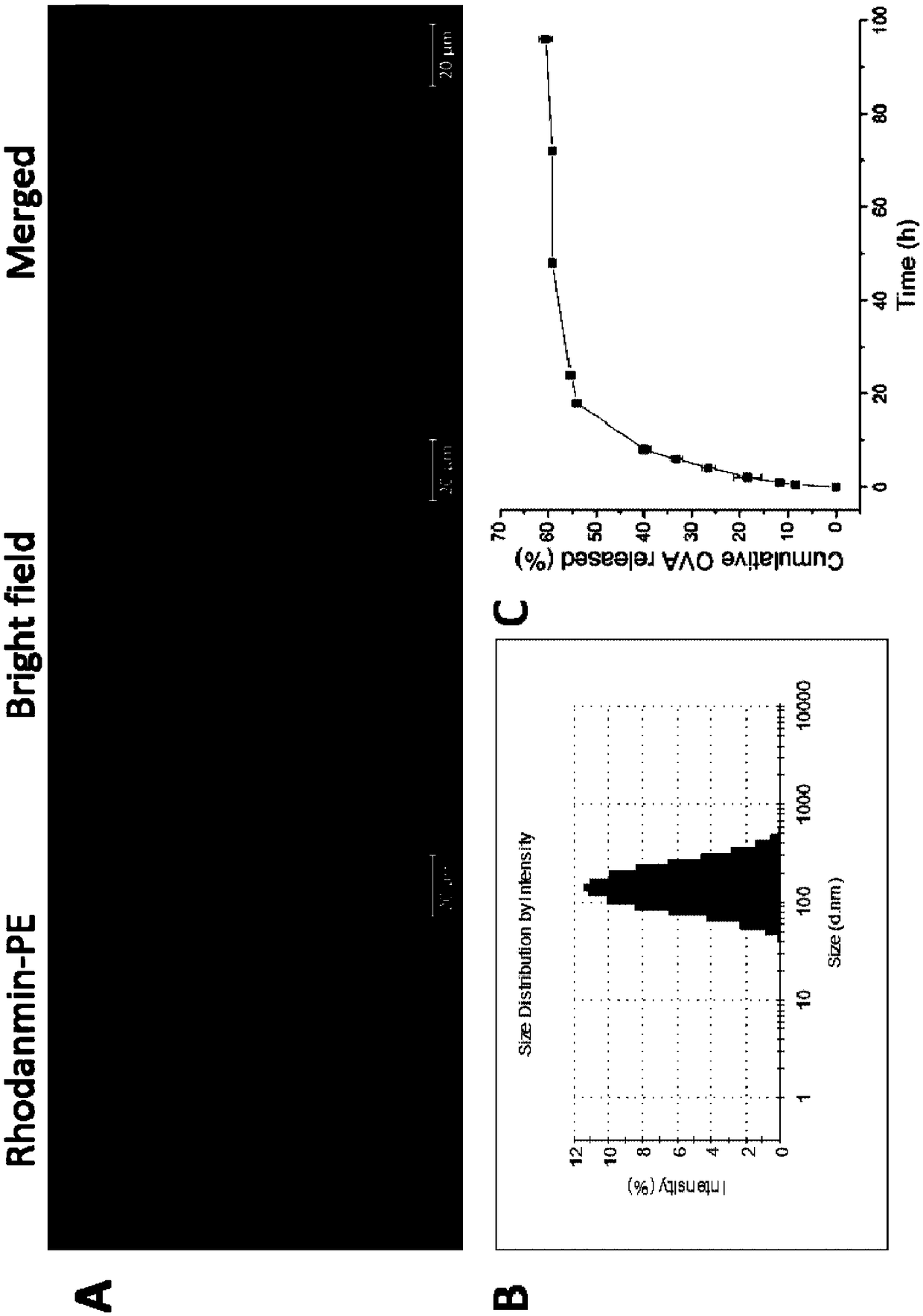
[0043] S2: Add 10 mL of double-distilled water to the dried product, hydrate at 65°C for 5 hours, oscillate and mix evenly, and then sonicate for 10 minutes in an ice bath to form a stable emulsion, filter the stable emulsion with a 0.45 μm filter membrane, collect the filtrate, and obtain Cationic pho...
Embodiment 2
[0047] This embodiment provides a cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant loaded with TLR7 agonist IMQ and antigen OVA. The preparation method includes the steps:
[0048] S1: 20 mg amphiphilic triblock copolymer PCL-b-PEG-b-PCL, 1 mg cationic phospholipid DOTAP, and 100 μg TLR7 agonist IMQ were dissolved in dichloromethane, and then the organic solvent was removed by rotary evaporation, forming a layer a uniform film, dry the residual solvent with nitrogen, put it in a vacuum drying oven, and dry it in vacuum for 12 hours; wherein, the molecular weight of the amphiphilic triblock copolymer PCL-b-PEG-b-PCL is 16000, of which PEG The mass percentage of the hydrophilic segment is greater than 45%.
[0049] S2: Add 10 mL of double-distilled water to the dried product, hydrate at 65°C for 5 hours, oscillate and mix evenly, and then sonicate for 10 minutes in an ice bath to form a stable emulsion, filter the stable emulsion with a 0.45 μm filter membrane,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com