Antigen and TLR agonist targeting co-loaded cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant, and preparation method and application thereof
A vaccine adjuvant and agonist technology, applied in the direction of cancer antigen components, vertebrate antigen components, and resistance to vector-borne diseases, etc., can solve problems such as hindering the clinical manifestations of vaccine adjuvants, weak immune response, and difficult application of adjuvants. Achieve the effects of enhancing cellular and humoral immune responses, strong lymphocyte activation, and enhancing antigen uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
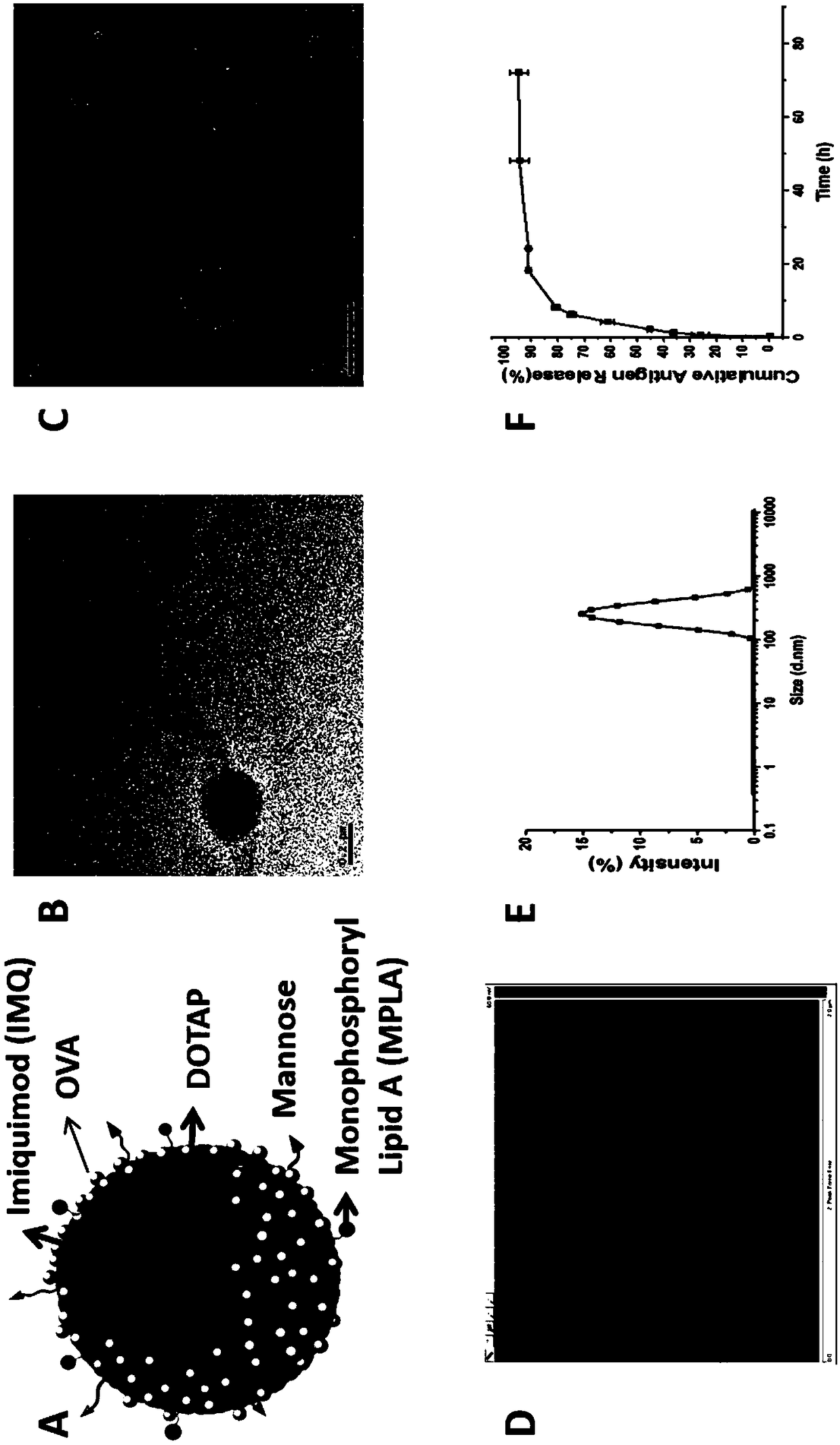
[0047] This example provides a cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant co-loaded with an antigen and a TLR agonist. The preparation method includes the steps:
[0048] S1: Dissolve 20 mg amphiphilic triblock copolymer PCL-b-PEG-b-PCL with 1 mg cationic phospholipid DOTAP, 100 μg TLR7 agonist IMQ, 10 μg TLR4 agonist MPLA in dichloromethane, then remove the organic solvent by rotary evaporation , form a uniform film on the bottle wall, dry the residual solvent with nitrogen, put it in a vacuum drying oven, and dry it in vacuum for 12 hours; among them, the amphiphilic triblock copolymer PCL-b-PEG-b-PCL The molecular weight is 16000, and the mass percentage of the PEG hydrophilic segment is greater than 45%.
[0049]S2: Add 10 mL of double-distilled water to the dried product, hydrate at 65°C for 5 hours, oscillate and mix evenly, and then sonicate for 10 minutes in an ice bath to form a stable emulsion, filter the stable emulsion with a 0.45 μm filter ...
Embodiment 2
[0057] This example provides a cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant that targets mannose and co-loads antigens and TLR agonists (TLR4 agonist MPLA, TLR7 agonist IMQ and antigen OVA). The preparation method includes steps:
[0058] S1: Mix 20 mg amphiphilic triblock copolymer PCL-b-PEG-b-PCL with 1 mg cationic phospholipid DOTAP, 100 μg TLR7 agonist IMQ, 10 μg TLR4 agonist MPLA, 0.4 mg DSPE-PEG-NH 2 Dissolve in dichloromethane, then remove the organic solvent by rotary evaporation, and form a uniform film on the wall of the bottle, dry the residual solvent with nitrogen, then put it in a vacuum drying oven, and dry it in vacuum for 12 hours; among them, the amphiphilic triembed The molecular weight of segment copolymer PCL-b-PEG-b-PCL is 16000, and the mass percentage of PEG hydrophilic segment is greater than 45%.
[0059] S2: Add 10 mL of double-distilled water to the dried product, hydrate at 65°C for 5 hours, oscillate and mix evenly, and then ...
Embodiment 3
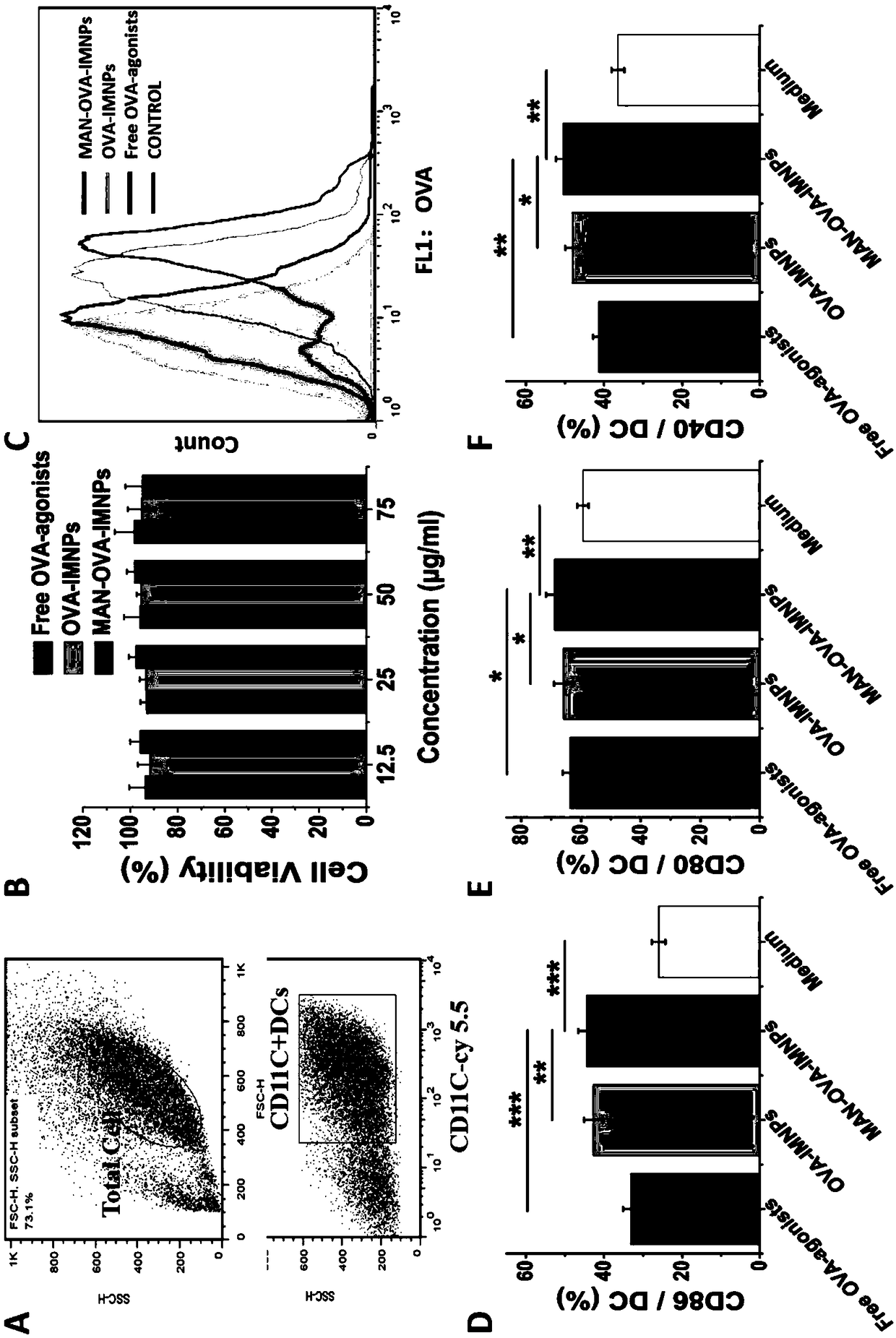
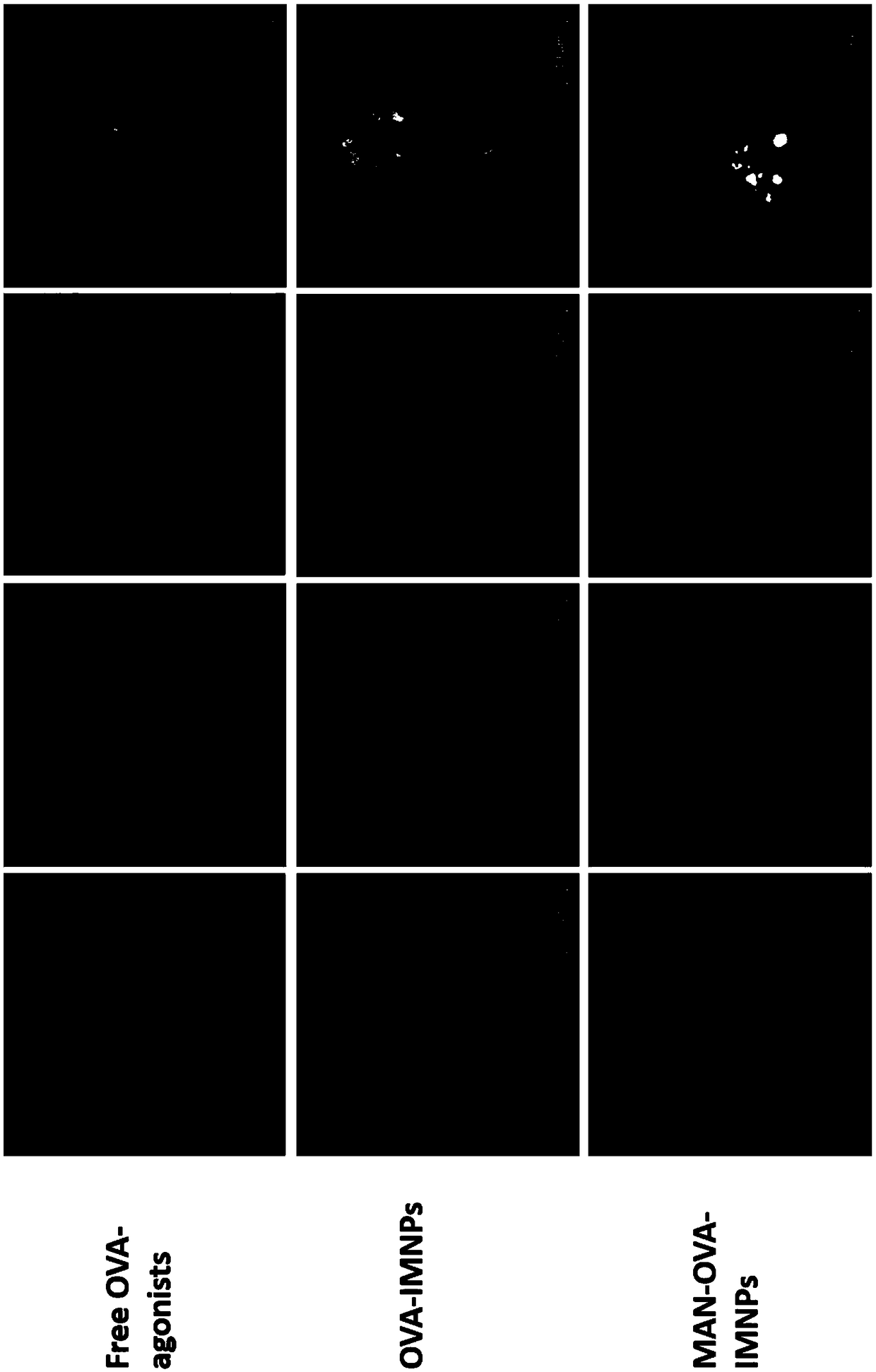
[0063] The mannose-targeted cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant (MAN-OVA-IMNPs) prepared in Example 2 of the present invention, which co-loads antigen and TLR agonist, was tested for promoting BMDCs maturation and cytotoxicity. The OVA-IMNPs in the following experiments were prepared in Example 1.
[0064] Immature BMDCs were stimulated with free OVA-agonists, OVA-IMNPs or MAN-OVA-IMNPs vaccine adjuvant for 24 hours. Subsequently, BMDCs were collected and co-incubated with 100 μL diluted cy5.5-anti-mouse CD11c, PE-anti-mouse CD40, FITC-anti-mouse CD86, and APC-anti-mouse CD80 for 30 min at 4°C. CD11c was detected by flow cytometry (FACS, BD FACSCalibur, US) + Expression of CD40, CD86 and CD80 on BMDCs.
[0065] MTS reagent (MTS, Promega, Madison, WI) was used to detect the viability of BMDCs after co-incubation with free OVA-agonists or OVA-containing cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvants. The immature BMDCs cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com