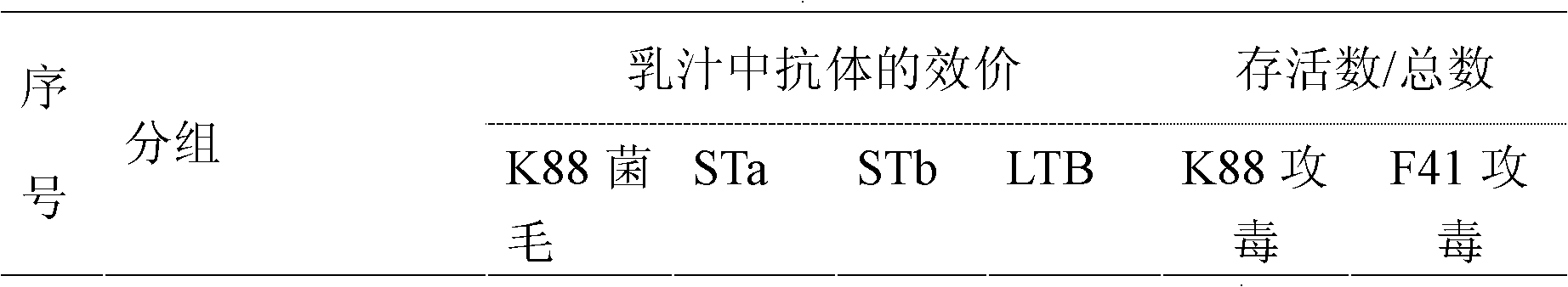
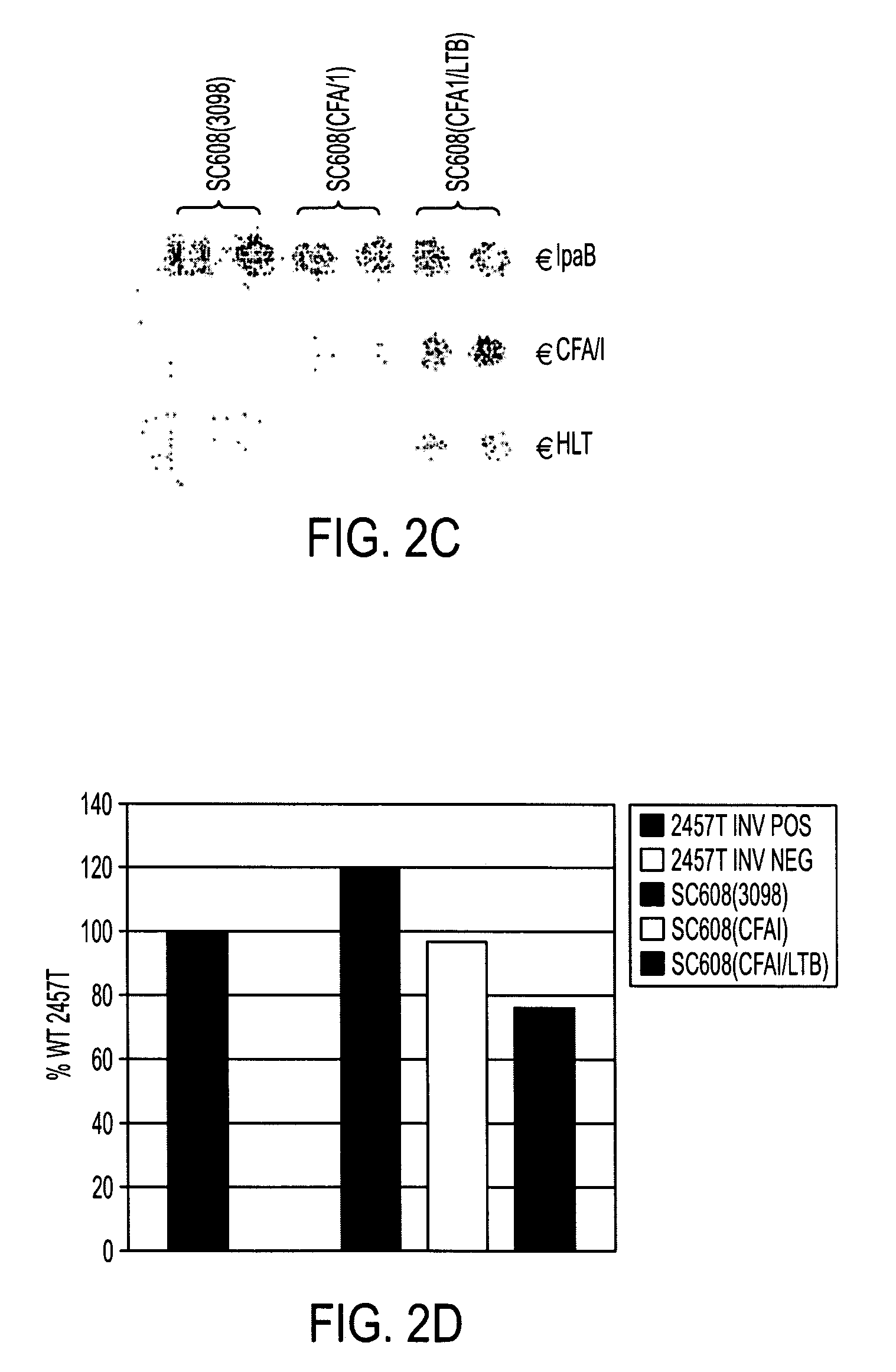
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
108 results about "Bacterial enterotoxins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An enterotoxin is a protein exotoxin released by a microorganism that targets the intestines. Enterotoxins are chromosomally encoded or plasmid encoded exotoxins that are produced and secreted from several bacterial organisms.
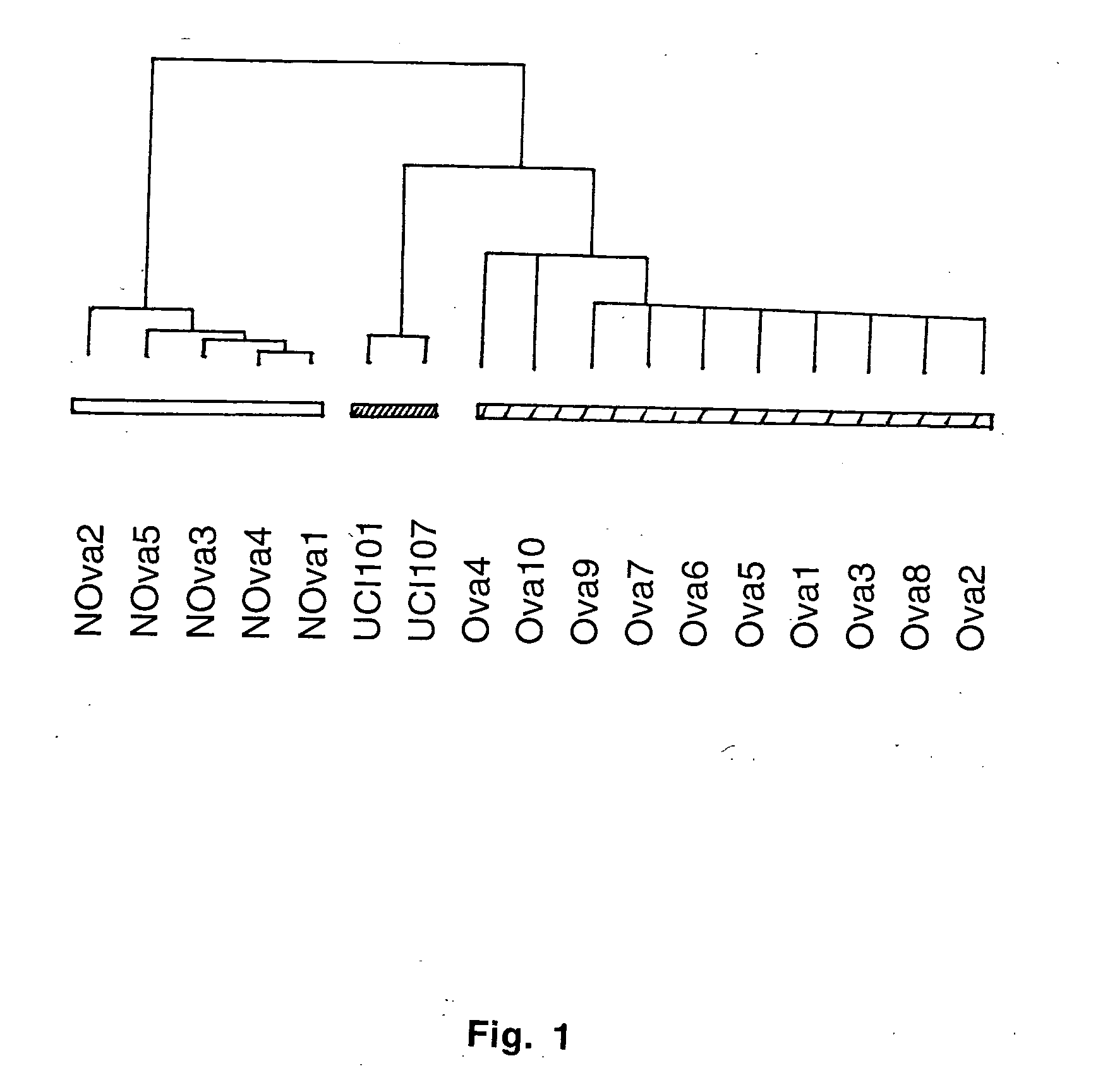
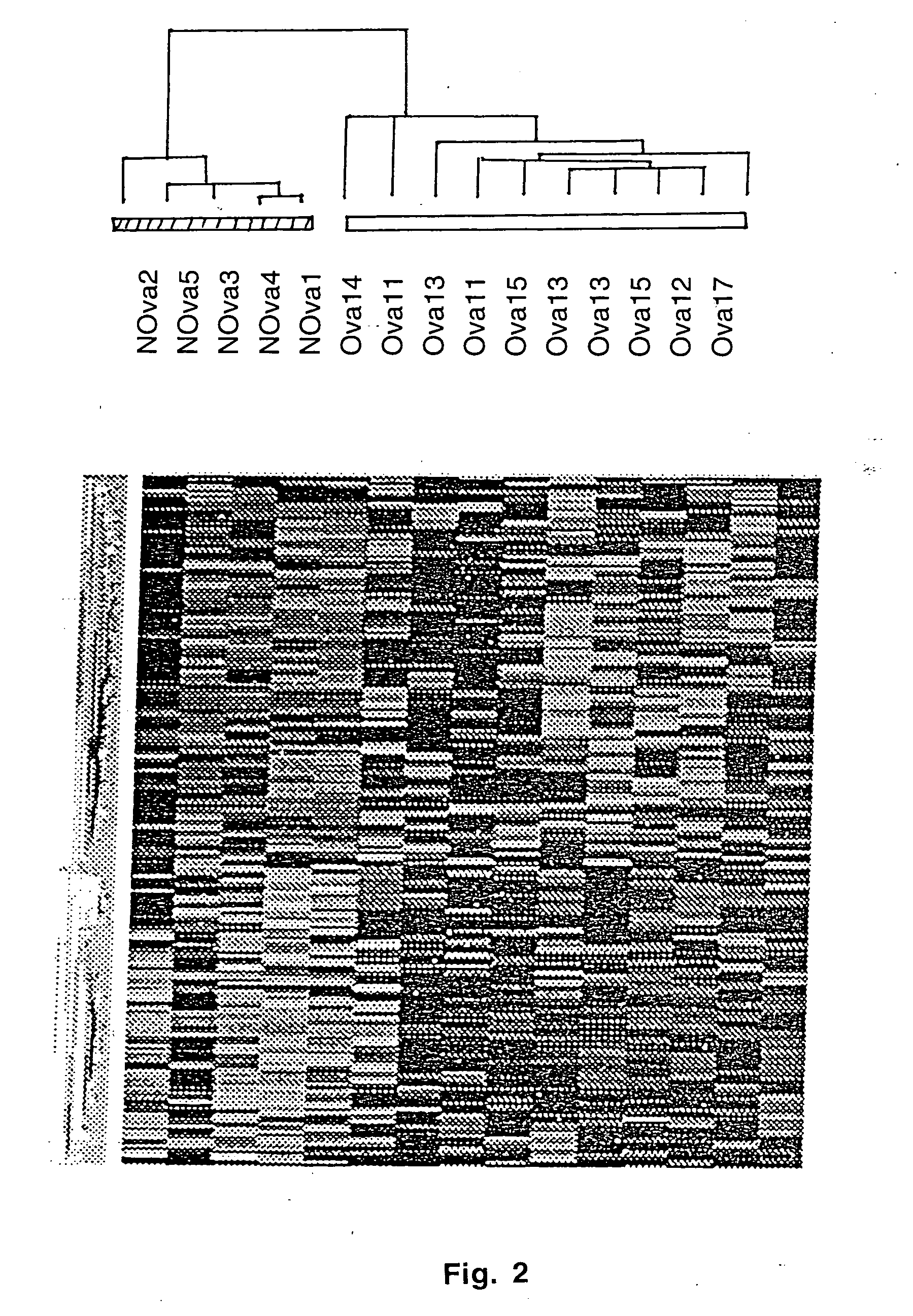
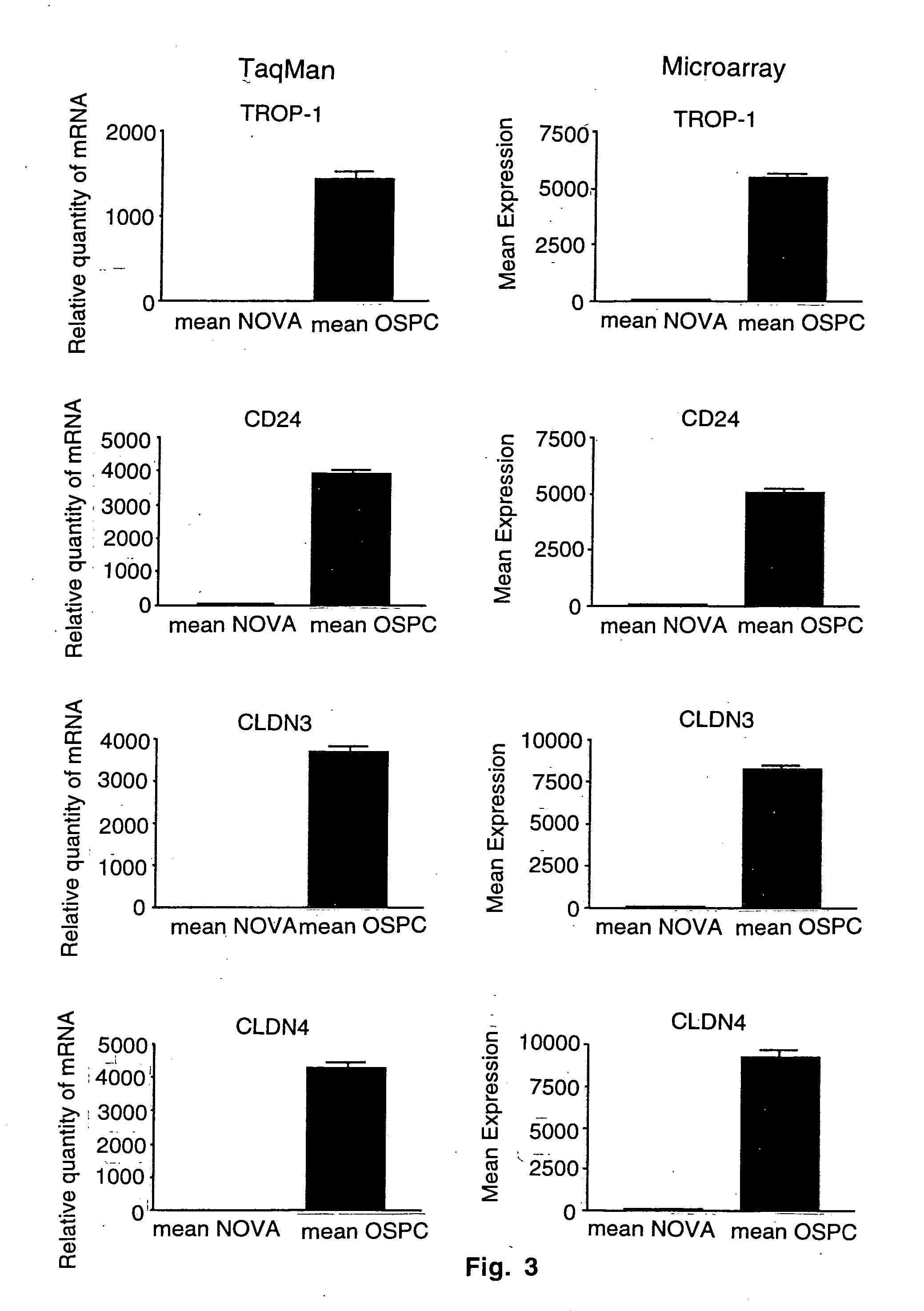
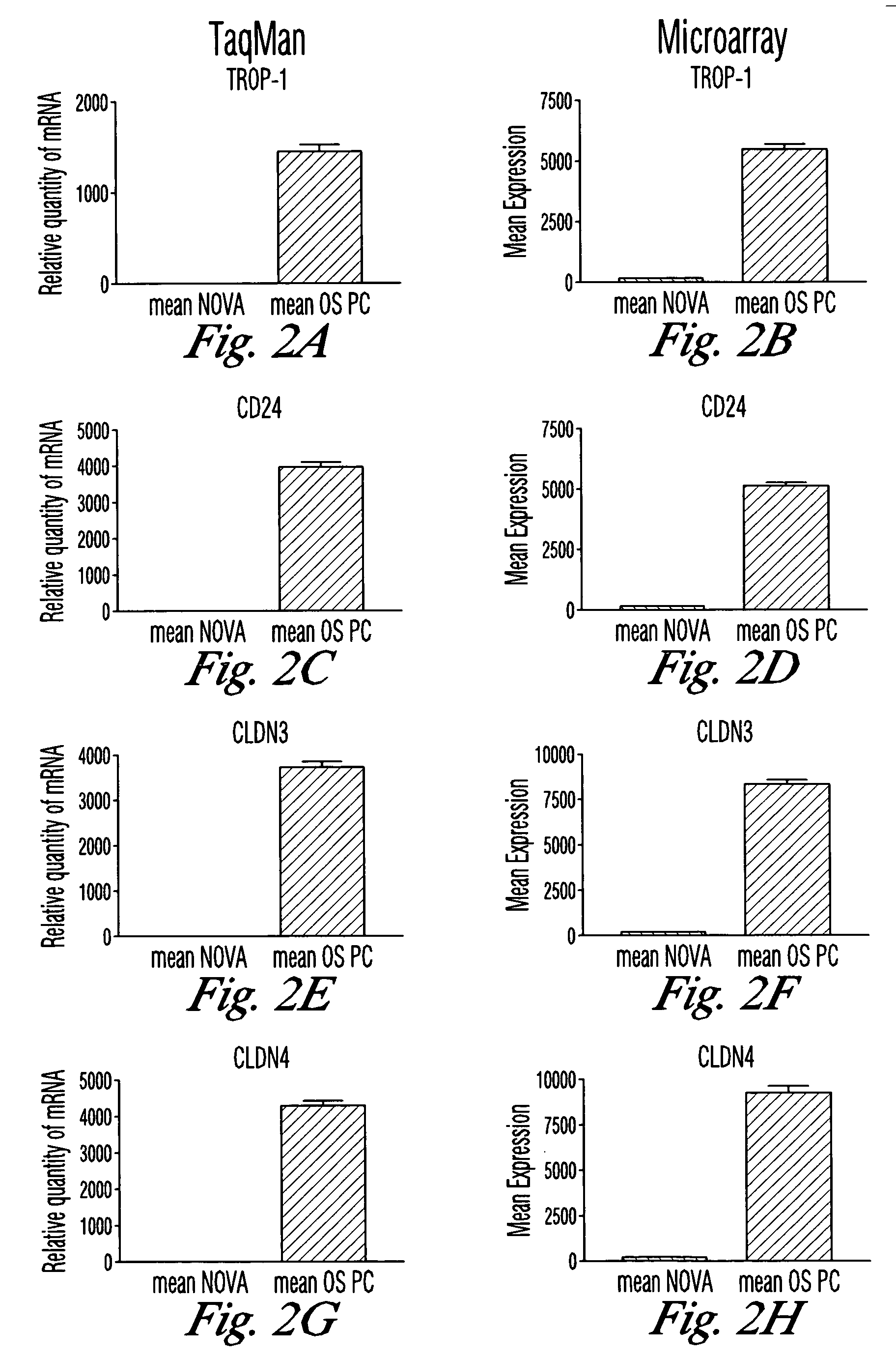
Gene expression profiling in primary ovarian serous papillary tumors and normal ovarian epithelium
InactiveUS20060078941A1Highligthing the divergence of gene expressionMicrobiological testing/measurementBiological testingKallikrein-10Gene family
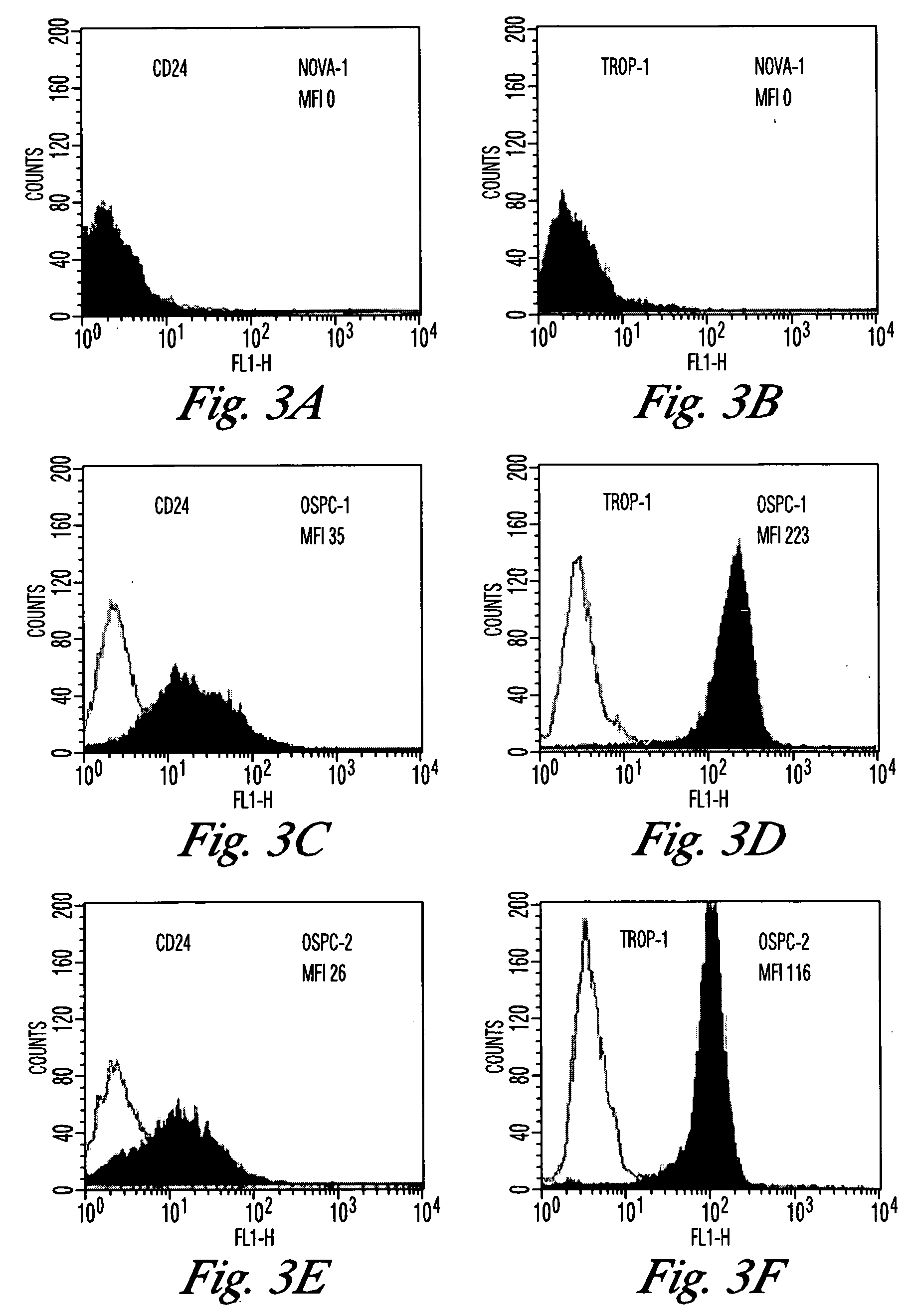
Gene expression profiling and hierarchial clustering analysis readily distinguish normal ovarian epithelial cells from primary ovarian serous papillary carcinomas. Laminin, tumor-associated calcium signal transducer 1 and 2 (TROP-1 / Ep-CAM; TROP-2), claudin 3, claudin 4, ladinin 1, S100A2, SERPIN2 (PAI-2), CD24, lipocalin 2, osteopontin, kallikrein 6 (protease M), kallikrein 10, matriptase and stratifin were found among the most highly overexpressed genes in ovarian serous papillary carcinomas, whereas transforming growth factor beta receptor III, platelet-derived growth factor receptor alpha, SEMACAP3, ras homolog gene family, member I (ARHI), thrombospondin 2 and disabled-2 / differentially expressed in ovarian carcinoma 2 (Dab2 / DOC2) were significantly down-regulated. Therapeutic strategy targeting TROP-1 / Ep-CAM by monoclonal chimeric / humanized antibodies may be beneficial in patients harboring chemotherapy-resistant ovarian serous papillary carcinomas. Claudin-3 and claudin-4 being receptors for Clostridium Perfringens enterotoxin, this toxin may be used as a novel therapeutic agent to treat ovarian serous papillary tumors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
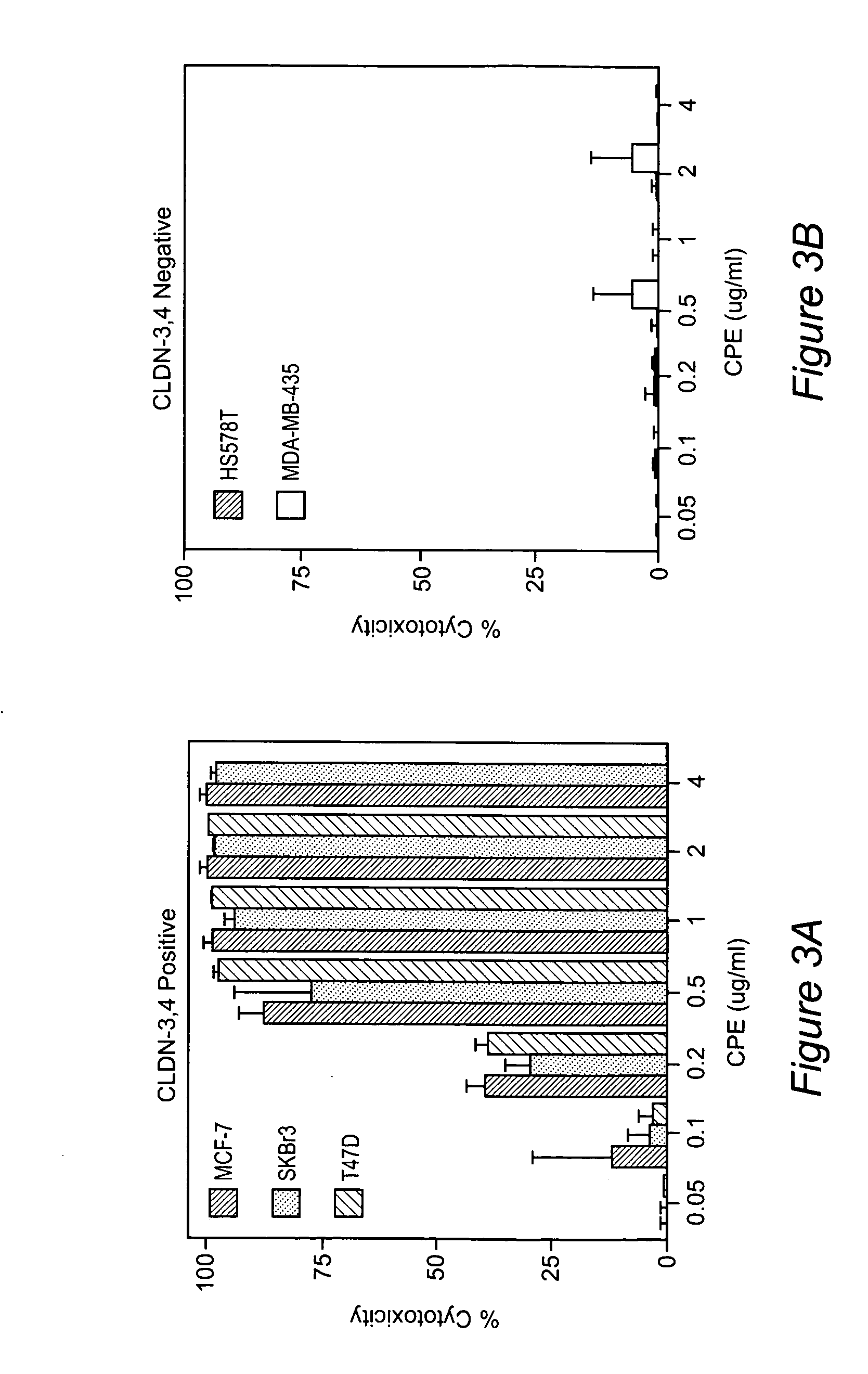
Therapy with clostridium perfringens enterotoxin to treat ovarian and uterine cancer
ActiveUS20060084594A1Low toxicityGood curative effectBacterial antigen ingredientsIn-vivo radioactive preparationsIntraperitoneal routeCancer cell
The invention discloses high levels of receptors for Clostridium perfringens enterotoxin (CPE) have been found in ovarian cancer and uterine cancer tissue samples. In addition, successful in vivo treatment of a mouse model of ovarian cancer with intraperitoneal injection of CPE is disclosed. High levels of Ep-CAM protein is also disclosed in ovarian cancer tissue samples. Thus, the invention provides a method of treating ovarian cancer and uterine cancer by administering CPE. The invention also provides a method of treating cancer in a mammal involving intraperitoneal administration of CPE, where at least some cancerous cells are located in or adjacent to the peritoneal cavity of the mammal. The invention also provides a method of treating ovarian cancer involving administering an anti-Ep-CAM antibody. The invention also provides a method of treating cancers expressing claudin-3 or claudin-4 by administering an antibody against claudin-3 and / or an antibody against claudin-4. The invention also provides a method of protecting a mammal from CPE toxicity involving administering a protective agent that binds to claudin-3 and / or claudin-4 and inhibits CPE binding to claudin-3 and / or claudin-4.
Owner:YALE UNIV
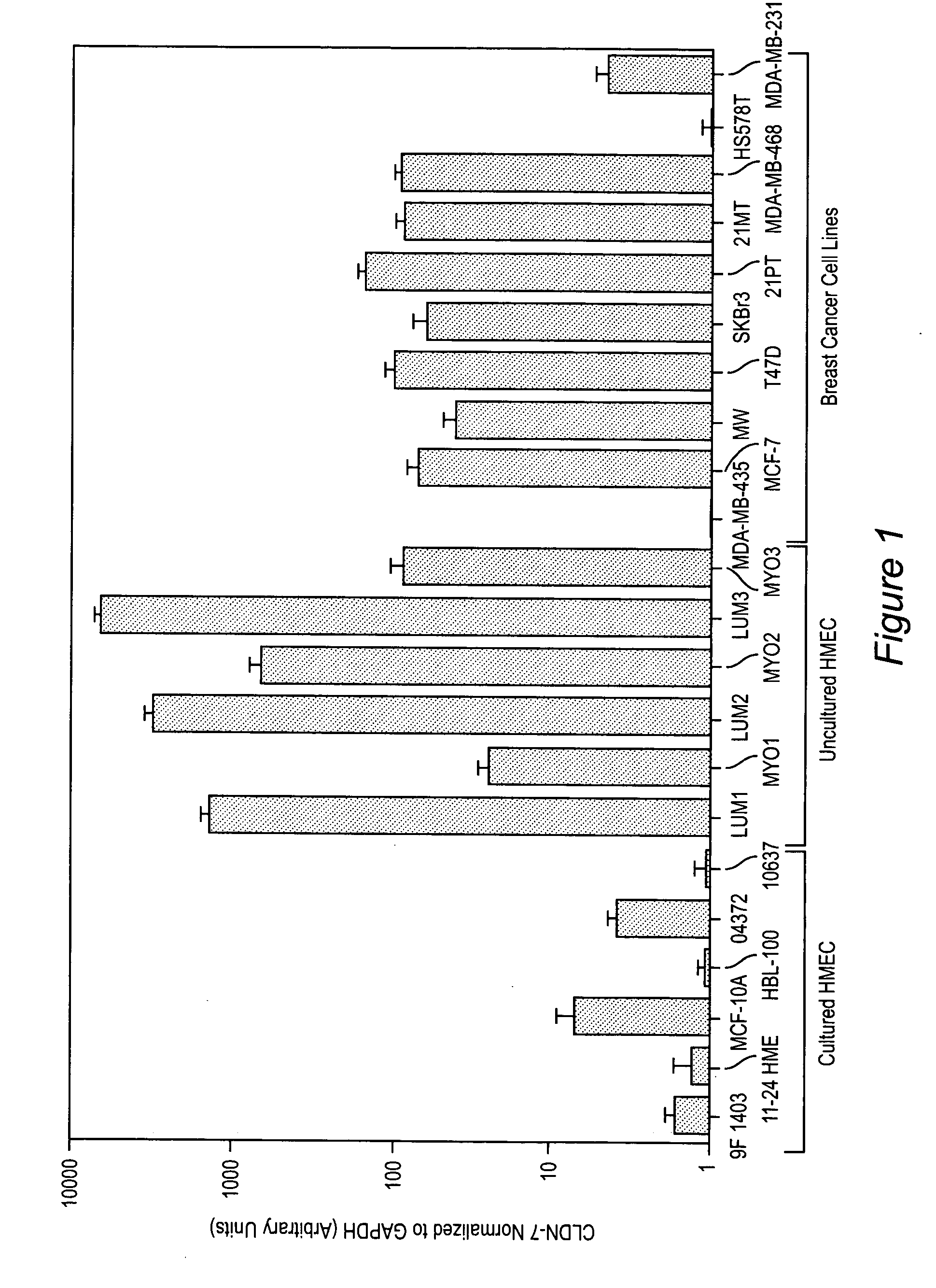
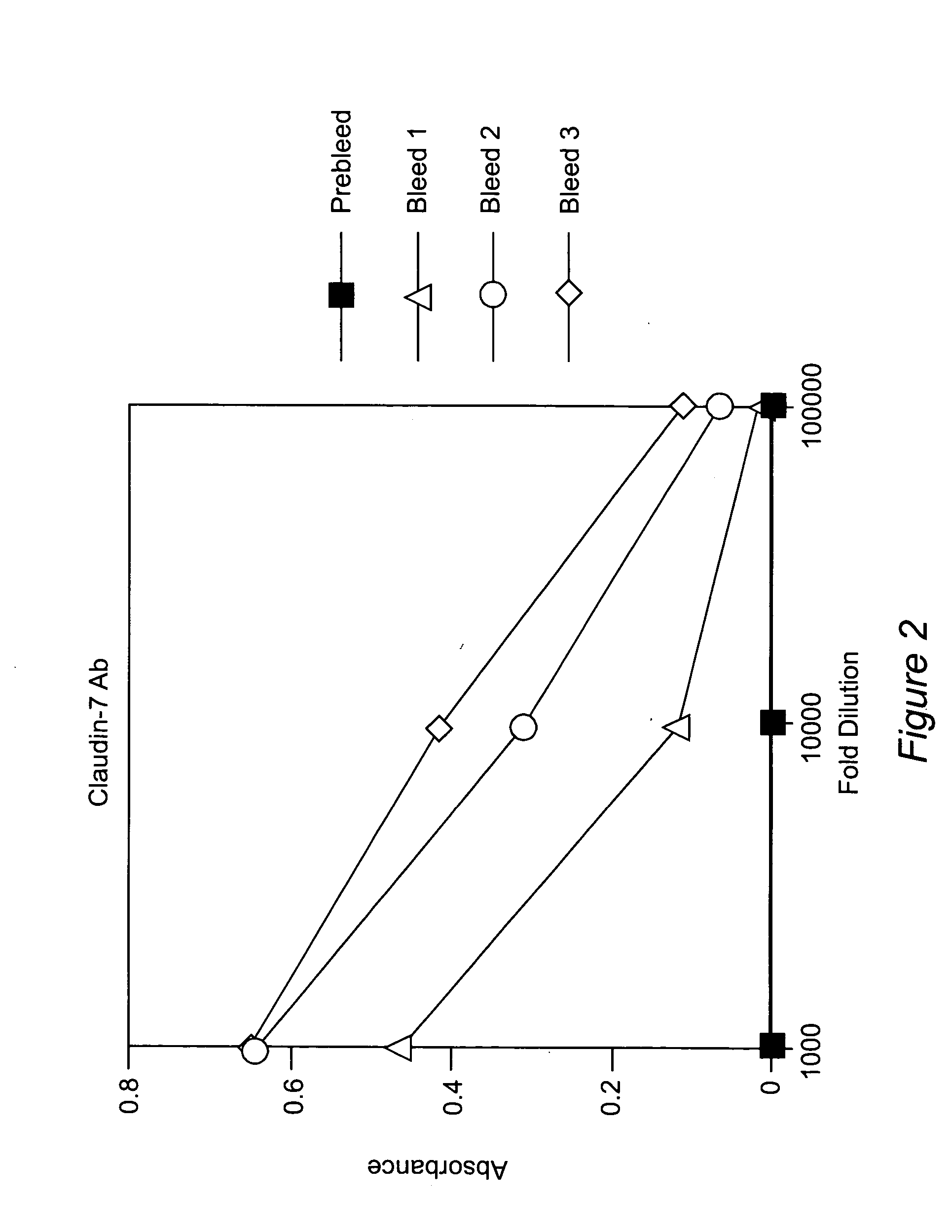
Claudins as markers for early detection, diagnosis, prognosis and as targets of therapy for breast and metastatic brain or bone cancer
InactiveUS20050074798A1Decrease cell-to-cell adhesionPromote cell migrationPeptide/protein ingredientsMicrobiological testing/measurementCancer cellBacterial enterotoxins
Methods of diagnosis, prognosis, and treatment of breast cancer, and of metastatic brain cancer, are provided The diagnostic and prognostic methods involve the immunohistochemical detection of the level of expression of the proteins claudin 1, 3, 4, and 7 in tissue or cell samples. Claudins 1 and 7 are underexpressed in the majority of breast cancers, and claudins 3 and 4 are overexpressed. The methods of treatment involve the use of Clostridium perfringens enterotoxin (or a variant thereof) to lyse metastatic cancer cells in the brain and bone that overexpress claudins 3 and 4.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
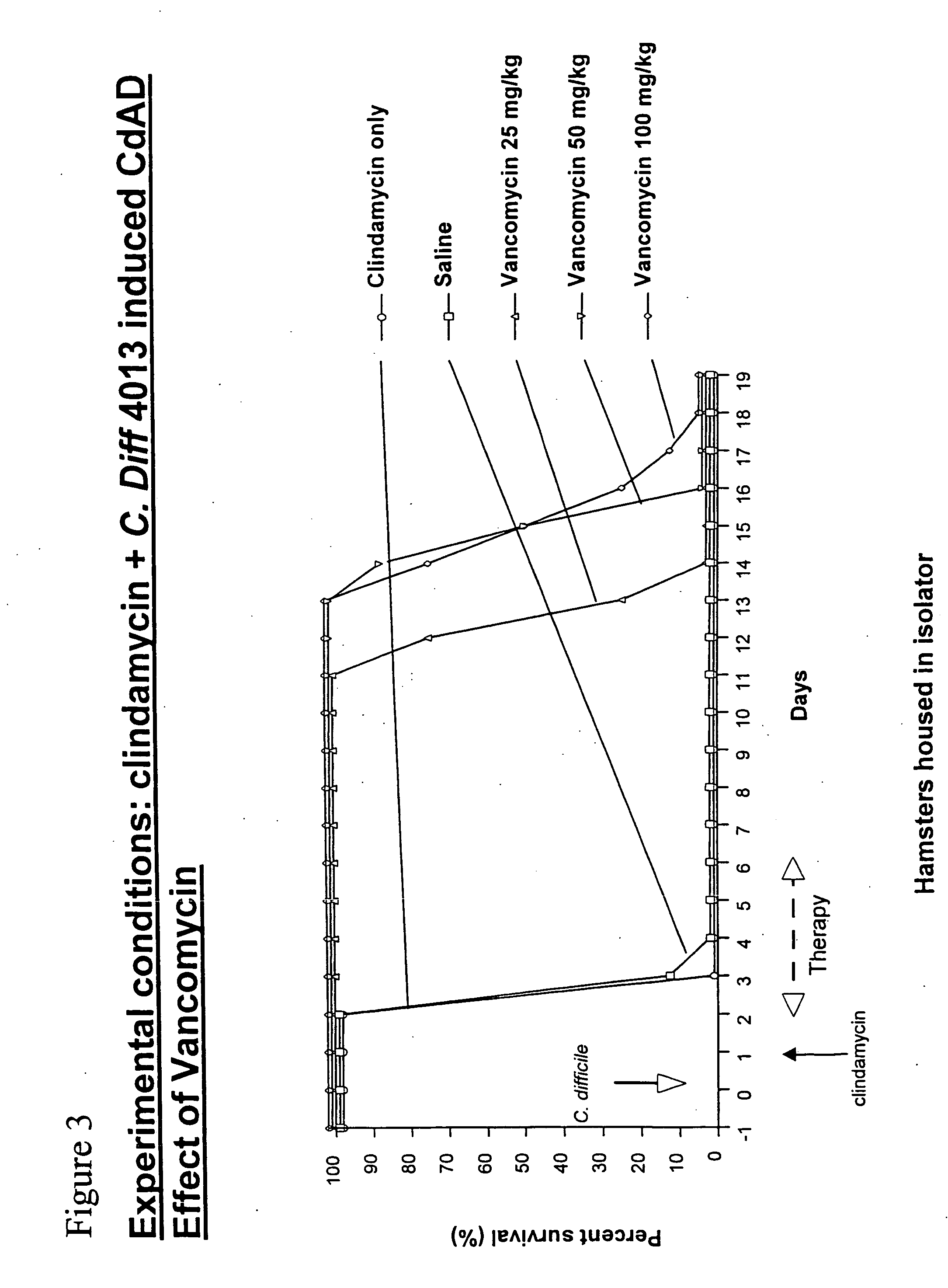
Antibodies for the treatment of Clostridium difficile-associated infection and disease
ActiveUS8986697B2Improve the quality of lifeImprove survivabilityAntibacterial agentsPeptide/protein ingredientsAntigenDisease
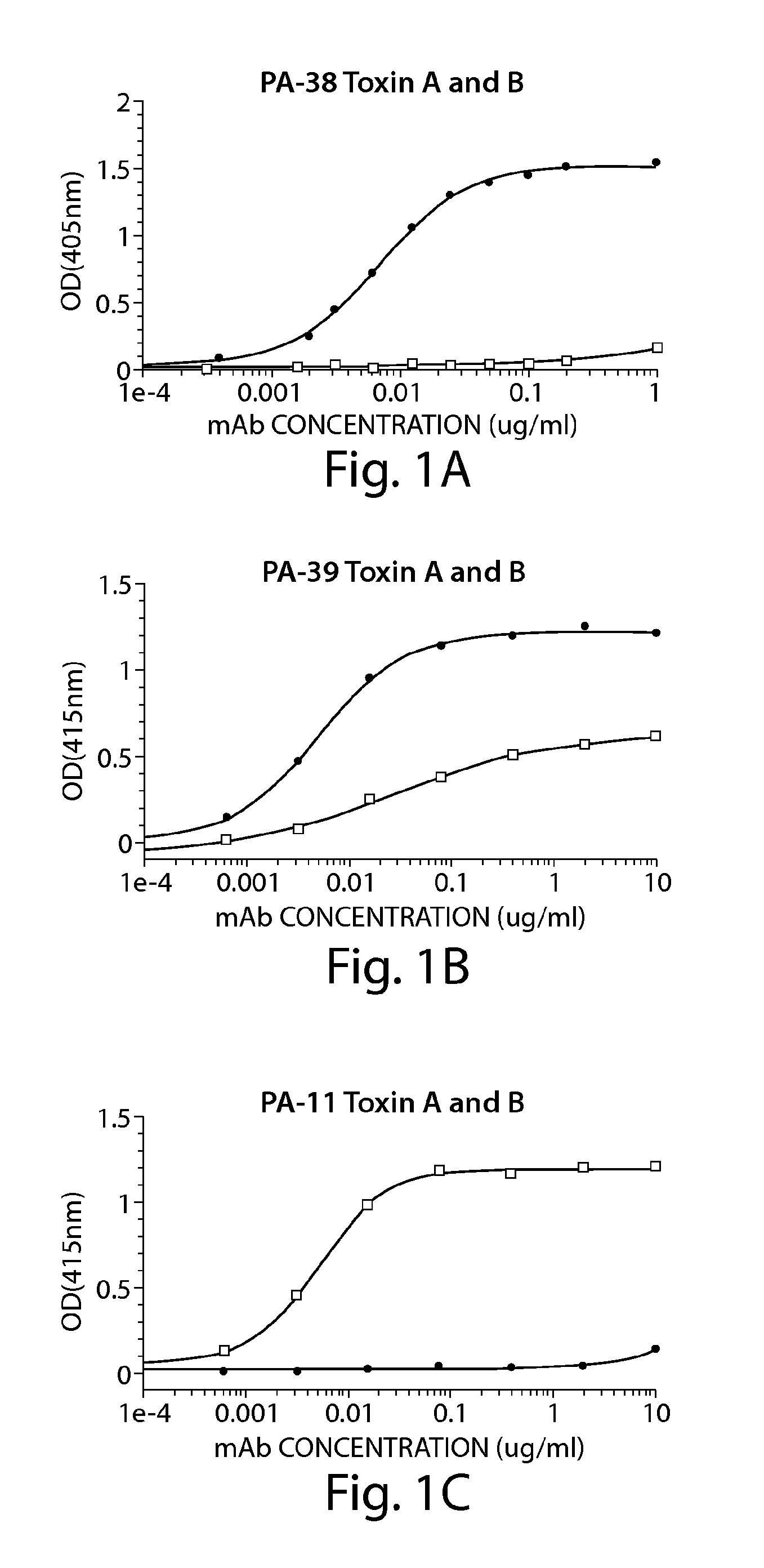
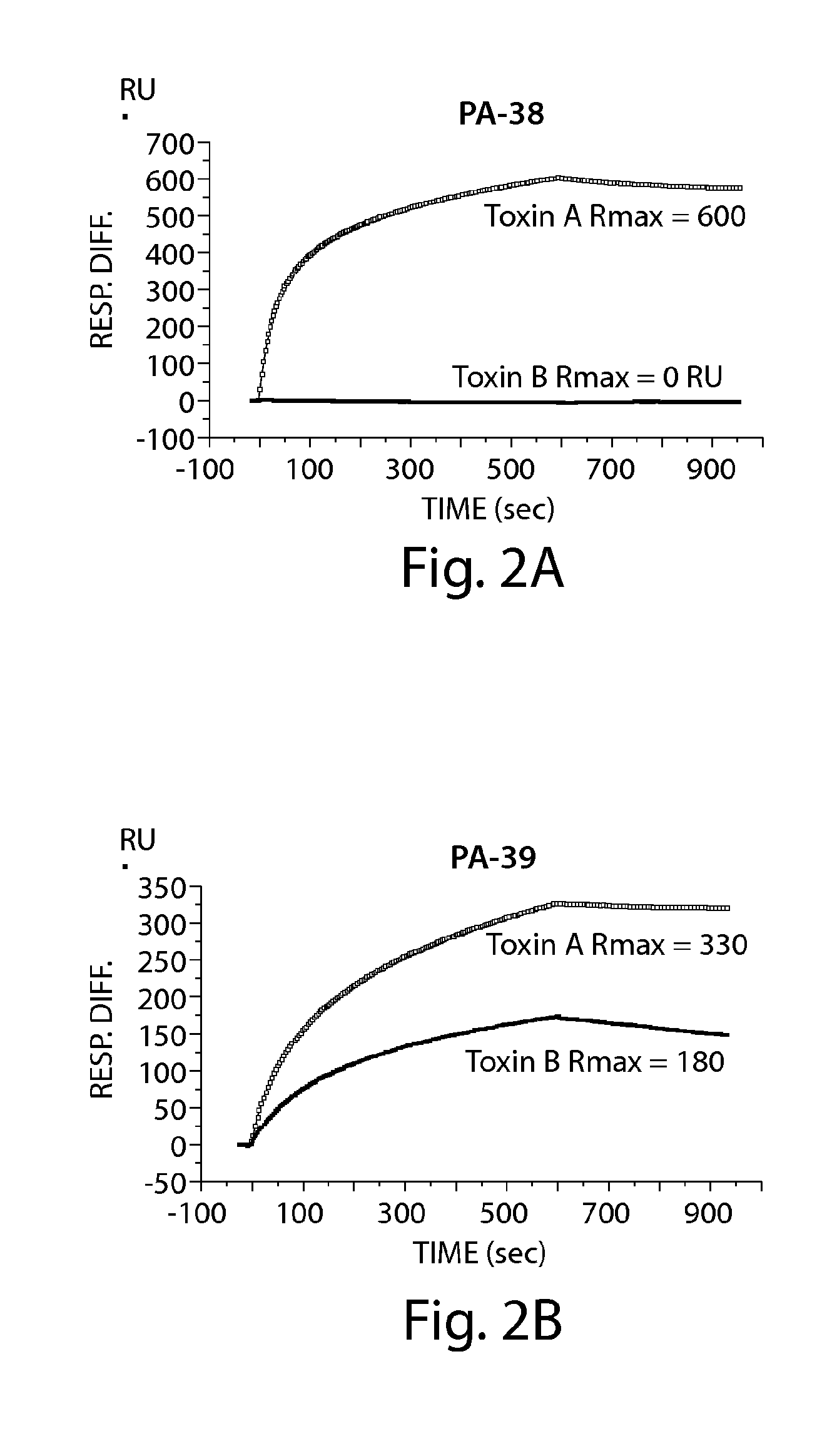
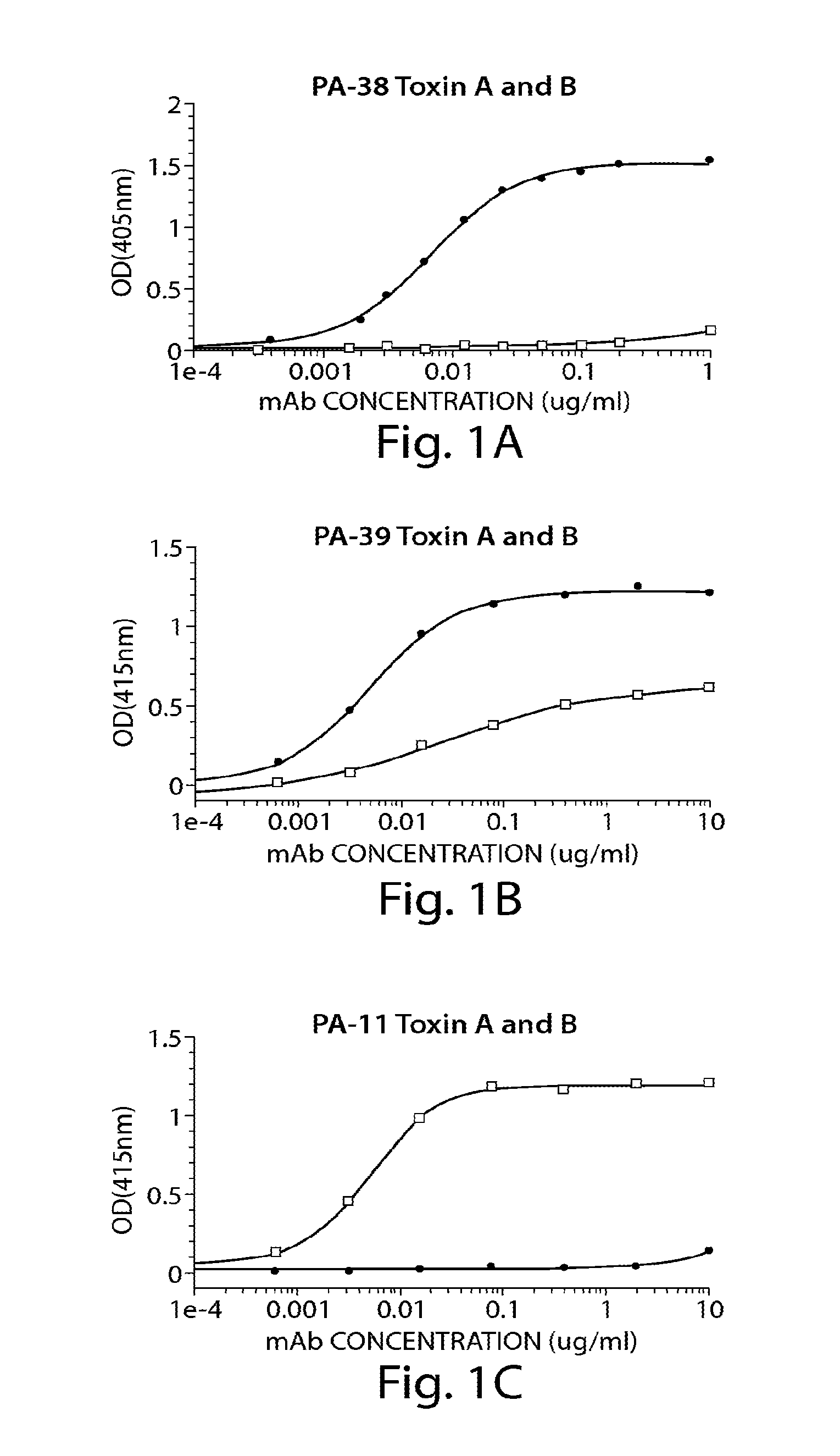
Provided herein are reagents, compositions, and therapies with which to treat Clostridium difficile infection and related disease conditions and pathologies, such as Clostridium difficile-associated diarrhea, resulting from infection by Clostridium difficile bacteria and the enterotoxins produced by these bacteria. In particular, antibodies or antigen-binding fragments thereof that bind specifically to toxin A and / or toxin B of C difficile and neutralize the activities of these toxins; compositions comprising such antibodies; and methods of using the antibodies and the compositions are provided.
Owner:PROGENICS PHARMA INC
Attenuated bacteria useful in vaccines
InactiveUS20050054075A1Improve protectionReliable and rapid isolationAntibacterial agentsBiocideBacterial strainImmunogenicity
The invention provides strains of bacteria, especially enterotoxigenic E. coli, attenuated by mutations in the genes encoding enterotoxins (LT, ST, EAST1) and optionally further attenuated by deletion of additional chromosomal genes. In addition the invention provides strains of attenuated bacteria expressing immunogenic but non-toxic variants of one or more of these enterotoxins. These bacteria are useful as a vaccine against diarrhoeal disease.
Owner:ACAMBIS RES LTD
Novel isolated bacteriophage having e. coli-specific bactericidal activity and antibacterial composition comprising the same
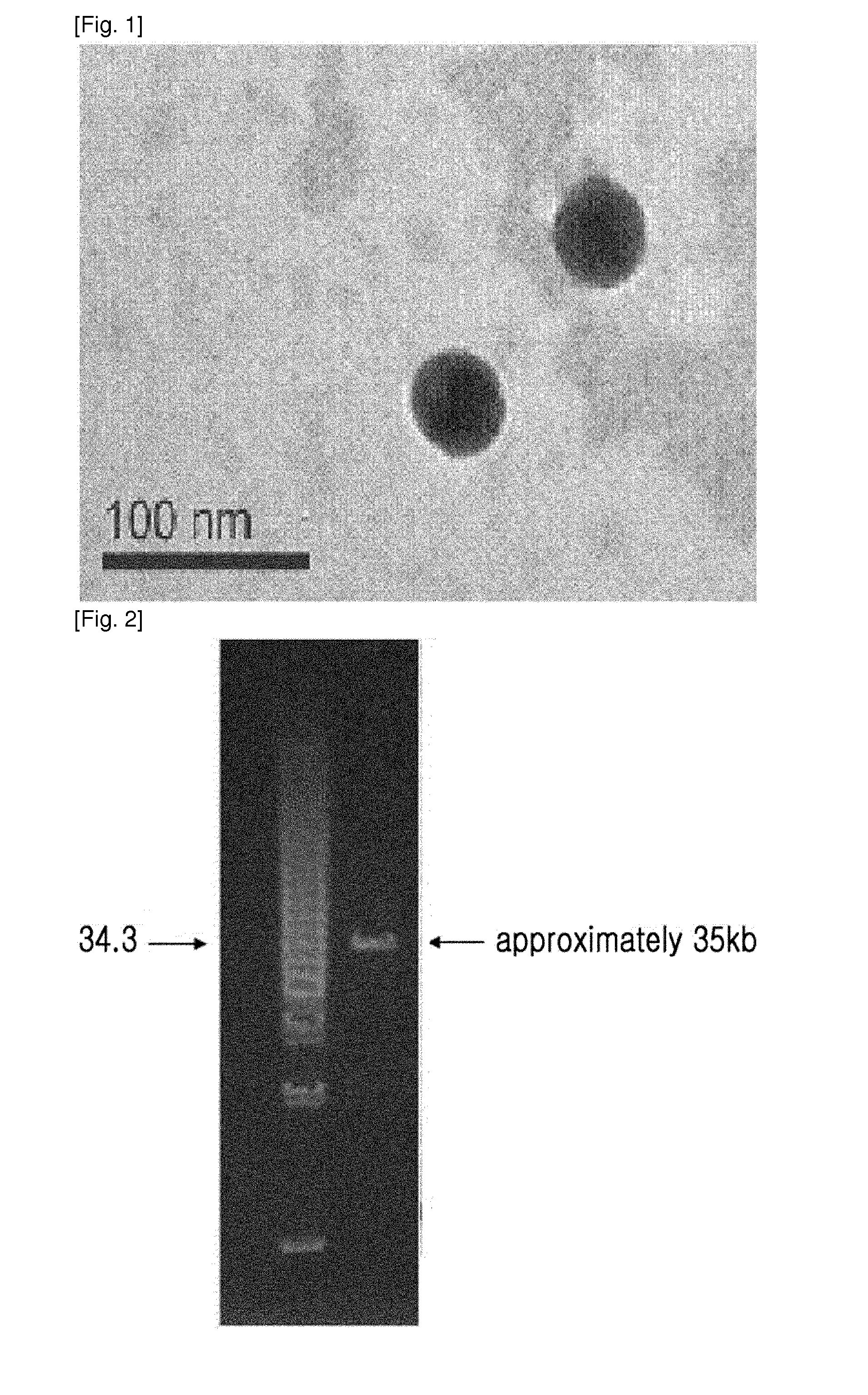
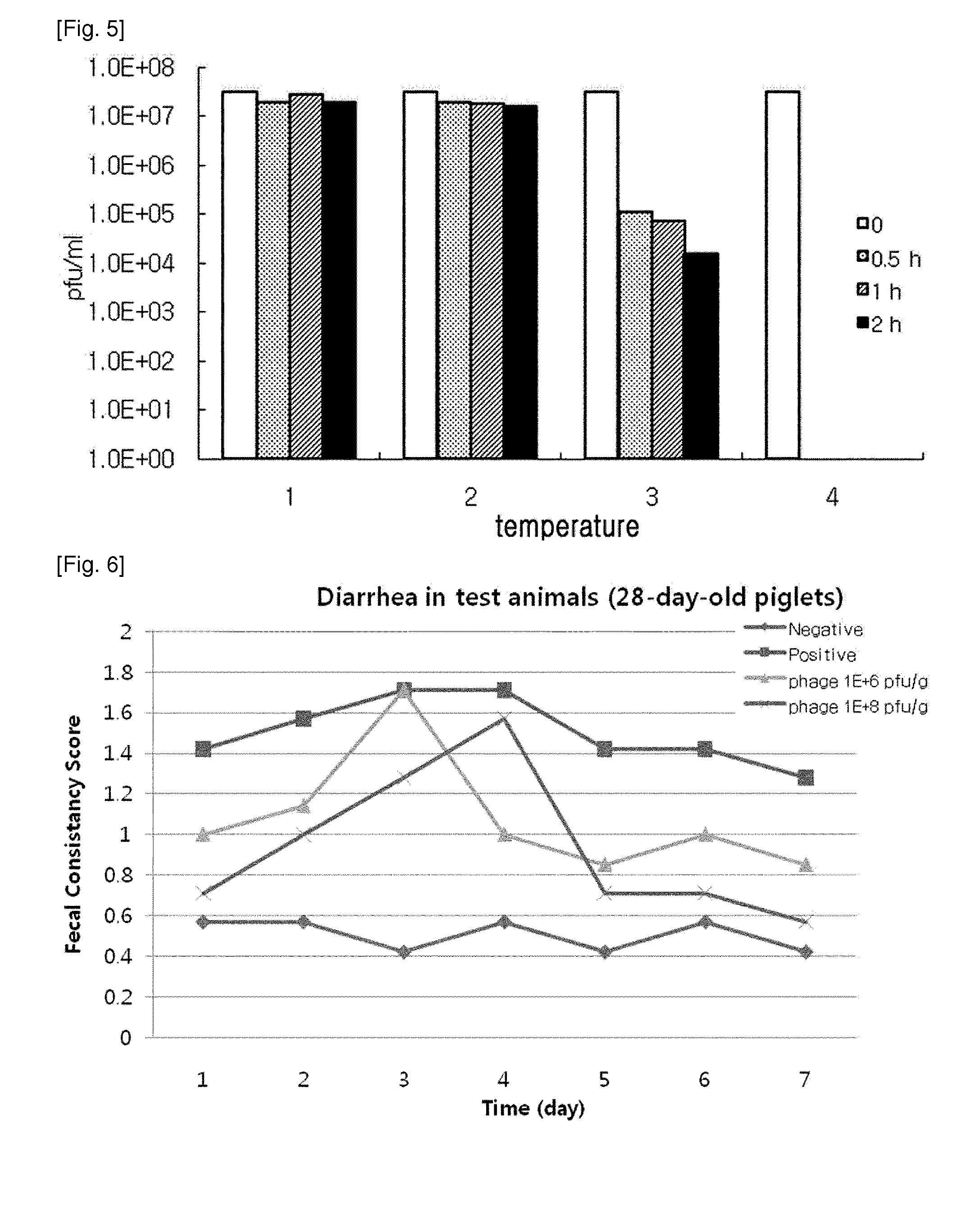
ActiveUS20140356330A1Good acidExcellent heat-resistanceAntibacterial agentsBiocideEscherichia coliPhage therapy
The present invention relates to a novel bacteriophage having an E.-coli-specific bactericidal activity, a composition for the prevention or treatment of infectious diseases caused by Enterotoxigenic E.-coli comprising the bacteriophage as an active ingredient, an antibiotic comprising the bacteriophage as an active ingredient, a feed additive composition comprising the bacteriophage as an active ingredient, a sanitizer or cleaner comprising the bacteriophage as an active ingredient, and a method for treating colibacillosis using the bacteriophage. The novel bacteriophage of the present invention has a specific bactericidal activity against pathogenic E. coli, and excellent acid- and heat-resistance. Therefore, the novel bacteriophage can be used for the prevention or treatment of swine colibacillosis, which is an infectious disease caused by pathogenic E.-coli, and can also be widely used in animal feed additive compositions, sanitizers, and cleaners.
Owner:CJ CHEILJEDANG CORP
Enterotoxin adsorbent, method of adsorptive removal, and adsorption apparatus
InactiveUS6969616B2Improve adsorption capacityEfficient removalBacterial antigen ingredientsOther blood circulation devicesBacterial enterotoxinsSorbent
The present invention is directed to an enterotoxin adsorbent comprising a compound having a log P (P denotes a partition coefficient in an octanol-water system) value of not less than 2.50 as immobilized on a water-insoluble carrier.
Owner:KANEKA CORP
Adhesin-enterotoxin chimera based immunongenic composition against enterotoxigenic Escherichia Coli
The inventive subject matter relates to an immunogenic composition composed of a chimeric molecule including a conformationally stable adhesin from Escherichia coli fused to a bacterial toxin A subunit, such as cholera toxin A subunit or heat-labile Escherichia coli toxin A subunit. The invention also relates to the adhesin-toxin chimera noncovalently associated with a toxin B subunit of the same or different species as the A subunit. The invention also relates to a method of utilizing an adhesin / toxin fusion composition to elicit an immune response.
Owner:UNIV OF COLORADO THE REGENTS OF
Antibody Protective Agent And Methods Of Using Same
ActiveUS20110262626A1Improve accuracyImprove stabilityImmunoglobulins against bacteriaBiological material analysisAntibodyEnvironmentally friendly
The invention provides an effective and environmentally friendly antibody protective agent and the methods of using it in immunological detection. The antibody protective agent helps antibody to maintain relatively high immunological activity at room temperature. Working electrodes coated with antibodies and the antibody protective agent are installed in immunological detection devices to enhance stability and accuracy of immunological detection. The antibody protective agent is effectively used in the detection of a variety of toxins, for example, aflatoxin, staphylococcal enterotoxin, algae toxin, and vomitoxin.
Owner:JIANGNAN UNIV +1
Staphylococcal enterotoxin gene engineering reshaped antibody and its preparation method and use
InactiveCN103224560AOvercome the defects in the detection meansImmunoglobulins against bacteriaBiological testingAntiendomysial antibodiesGenetic engineering
The invention discloses a staphylococcal enterotoxin gene engineering reshaped antibody and its preparation method and use. The staphylococcal enterotoxin gene engineering reshaped antibody has an amino acid sequence shown in the formula of SEQ ID No.1 in the sequence table. Through construction of light and heavy chain eukaryotic co-expression vectors of the staphylococcal enterotoxin monoclonal antibody, a high-efficiency expression and stable-secretion mammalian cell line and the gene engineering reshaped antibody having high singularity and strong affinity are obtained. The staphylococcal enterotoxin gene engineering reshaped antibody can be used in staphylococcal enterotoxin detection, cell indirect immunofluorescence detection and flow cytometry detection.
Owner:TIANJIN UNIV
Saccharide derivatives
Disclosed are novel saccharide derivatives which inhibit binding of toxins, such as heat-labile enterotoxin or cholera toxin, to their receptors either in vitro or in vivo. Additionally, disclosed are compounds which inhibit binding of enterovirulent organisms (e.g., bacteria, virus, fungi, and the like), such as Vibrio cholerae and enterotoxigenic strains of Escherichia coli, to their cell surface receptors.
Owner:SYNSORB BIOTECH INC
PCR (polymerase chain reaction) synchronous detection kit for staphylococcus aureus enterotoxin A and B genes
InactiveCN102181547ASensitive and accurate detectionReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationNucleotideInverse polymerase chain reaction
The invention discloses a PCR (polymerase chain reaction) synchronous detection primer for staphylococcus aureus enterotoxin A and B genes, a detection kit thereof and a detection method. The nucleotide sequence of the synchronous detection primer is as shown in SEQ (sequence) ID (identity) No. 1 and 2. The invention further provides the PCR detection kit containing the primer. By adopting the detection kit, the staphylococcus aureus enterotoxin A and B in a food can be accurately and sensitively detected, and the lowest detection concentration of DNA (deoxyribonucleic acid) is 3.58ng; furthermore, the detection kit has no cross reaction with other bacteria, and the specificity is good; simultaneously, the pretreatment process of samples is simple, the consumed time is short, a large number of the samples can be detected simultaneously, and the cost is low.
Owner:BEIJING SANYUAN FOOD
Fusion protein for detecting anti-ETEC (enterotoxigenic escherichia coil) antibody of pigs, as well as preparation method and application thereof
ActiveCN105061602AGood immunoreactivityIncrease productionBacteriaBiological testingAmino acidRapid detection
The invention provides a fusion protein for detecting an anti-ETEC (enterotoxigenic escherichia coil) antibody of pigs, as well as a preparation method and application thereof, and belongs to the technical field of biology. The fusion protein has an amino acid sequence as shown in SEQ ID NO:1. The method for preparing the fusion protein comprises the following steps: inserting a coding gene of the fusion protein into a pCold I plasmid to obtain a recombinant vector; transforming a host cell by the recombinant vector to obtain a positive recombinant bacterium; and inducing the positive recombinant bacterium to express the fusion protein, and purifying to obtain the fusion protein. The fusion protein has excellent immunoreactivity and high yield, can be used for simultaneously detecting the anti-F4 type ETEC and anti-F5 type ETEC antibody of pigs. A kit for detecting the anti-ETEC antibody is exact in detection, simple and convenient to operate and excellent in specificity, sensibility and repeatability, is applicable to popularization in clinical application, and provides a reliable means to rapid detection of the anti-ETEC antibody.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
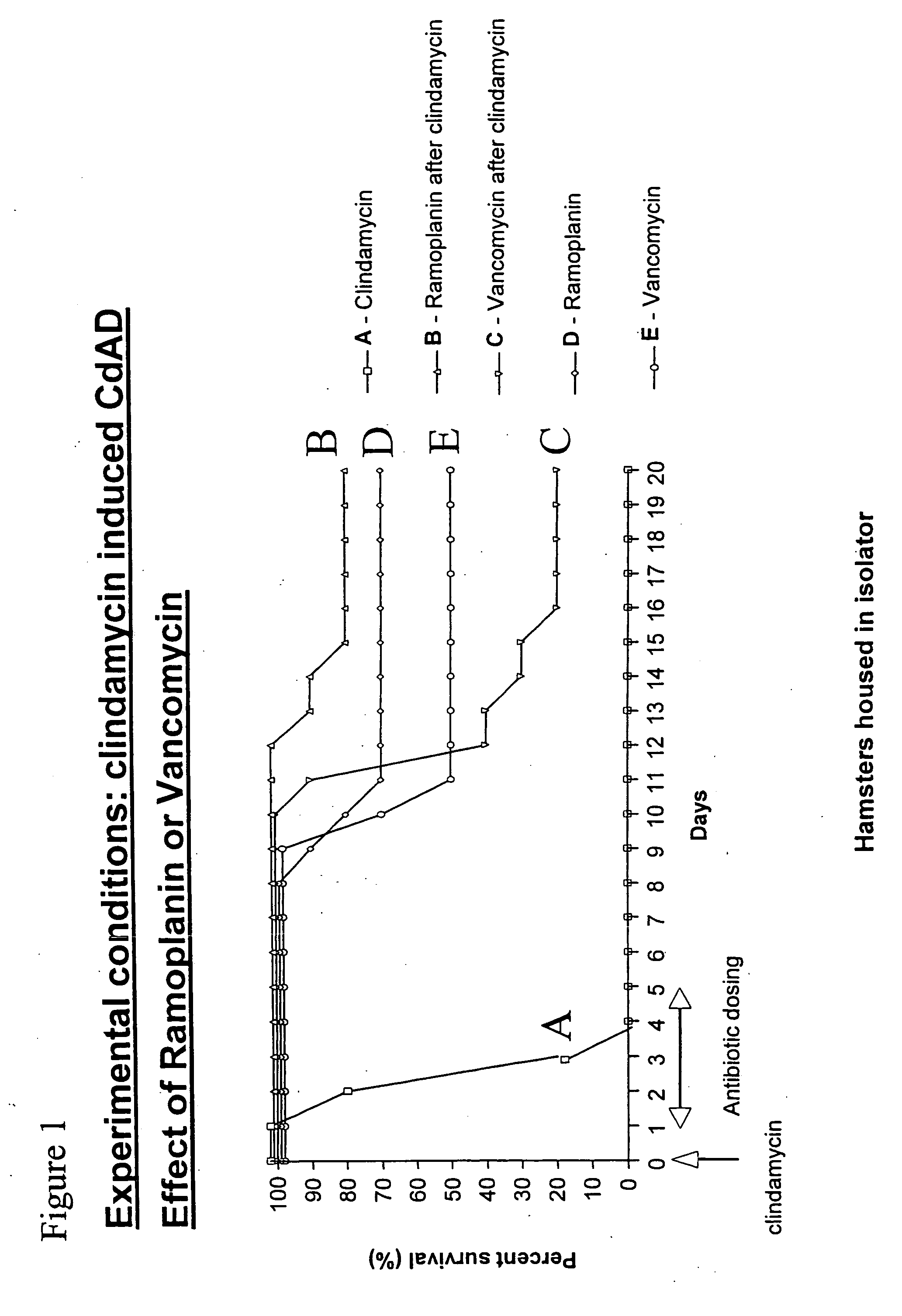
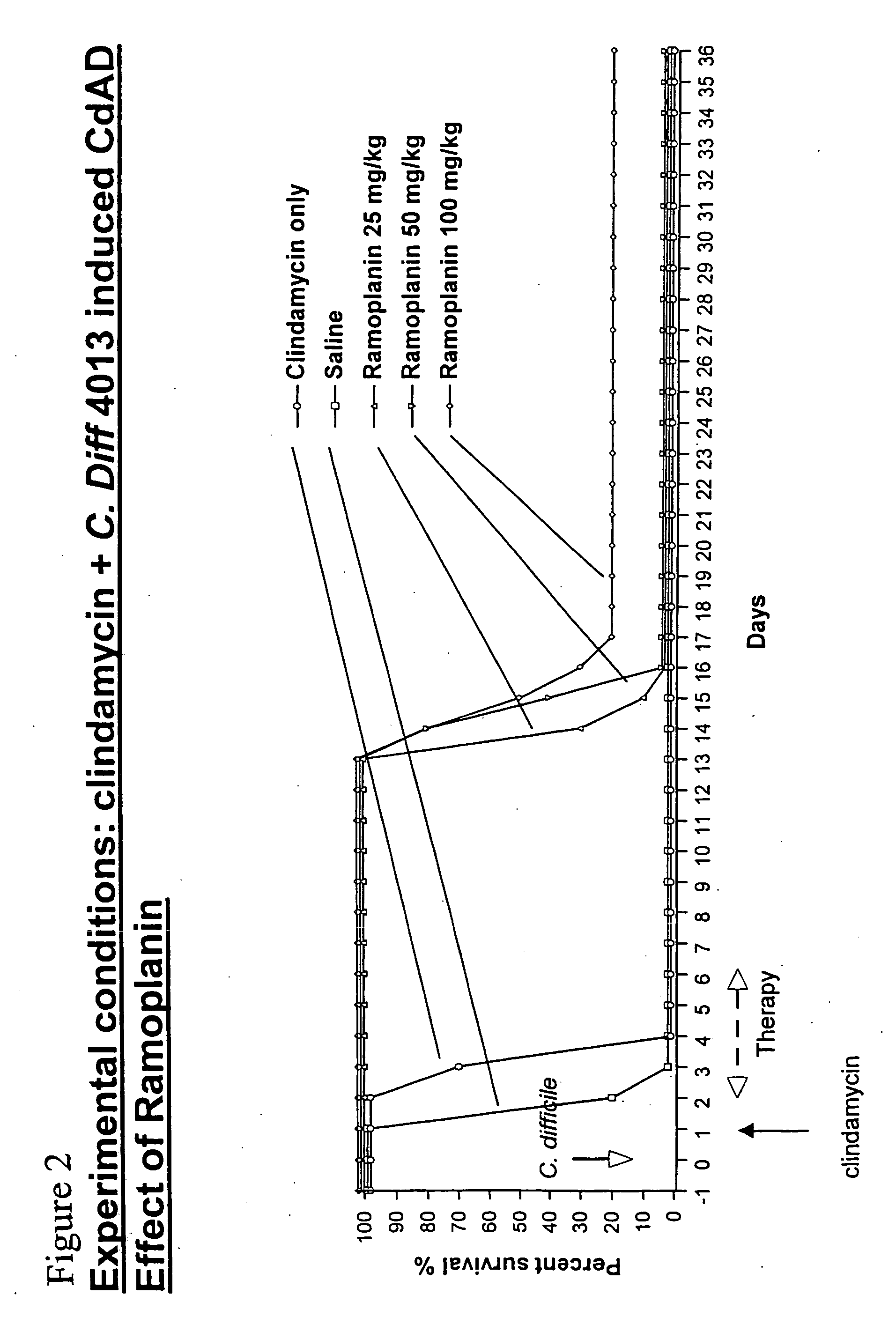
Use of ramoplanin to treat diseases associated with the use of antibiotics
InactiveUS20070014849A1Prevent relapseInhibit relapse of antibiotic-associated diarrheaAntibacterial agentsBiocideDiseaseBacteroides
The invention features a method of treating or preventing a disease associated with the use of antibiotics in a patient in need thereof by administering to the patient ramoplanin in an amount and for a duration effective to treat said disease. The disease may be caused, for example, by the presence of a bacterium such as enterotoxin producing strains of C. difficile, C. perfringens, or S. aureus.
Owner:NANOTHERAPEUTICS INC
Gene recombinant vaccine for preventing enterovirus 71 infection and preparation method thereof
The invention discloses a gene recombinant vaccine for preventing enterovirus 71 (EV71) infection and a preparation method thereof. The inflection comprises multiple diseases related with the nervous system such as hand-foot-and-mouth disease, paralytic diseases of sterile meningitis, cephalitis and poliomyelitis and the like. Escherichia coli labile enterotoxin B subunit (LTB) is used as an immunological enhancement adjuvant, two fragments of linear neutralizing epitope SP55 and SP70 in EV71 virus coat protein VP1 are used as antigens, prokaryotic expression plasmids of LTB-SP55-SP70 fusion genes are constructed by using gene engineering technology, the plasmids are expressed in escherichia coli, and a recombinant expression product is purified for preparing the EV71 virus gene engineering vaccine.
Owner:中国疾病预防控制中心病毒病预防控制所 +1
Use of slaphylococcus aureus enterotoxins in preparing drugs for treating or preventing A1DS
InactiveCN1509762AGood antiviral effectRaise the ratioPeptide/protein ingredientsAntiviralsBacterial enterotoxinsMedicine
An application of staphylococcus aureus enterotoxin in preparing the medicines for preventing and treating AIDS is disclosed. Said enterotoxin may be SEA, SEB, SEC1, SEC2, and SED. Its advantages are low dosage and high curative effect.
Owner:SHENYANG XIEHE GRP LTD
Protein G staphylococcus aureus enterotoxin kit and preparation method thereof
InactiveCN105353129AIncrease profitReduce dosageMaterial analysisAbzymeStaphylococcus aureus enterotoxin B
The invention relates to a protein G staphylococcus aureus enterotoxin kit and a preparation method thereof. Protein G is adopted to preprocess an enzyme labeling plate, and quick and accurate detection on staphylococcus aureus enterotoxin A / B is realized by combining with an ELISA double-antibody sandwich method. The kit consists of the 96-hole enzyme labeling plate, a staphylococcus aureus enterotoxin A-resistant protein G recombinant protein monoclonal antibody, a staphylococcus aureus enterotoxin B-resistant monoclonal antibody, a staphylococcus aureus enterotoxin antibody enzyme labeling object containing a horseradish peroxidase label, a sample diluent, a cleaning solution and a stop buffer. The protein G staphylococcus aureus enterotoxin kit can quickly and effectively detect the residual level of tony red in a to-be-detected sample, and also has the characteristics of simple operation, good specificity, high sensitivity, and the like.
Owner:HUAAN MAGNECH BIO TECH
Fusion of multiple enterotoxin genes of escherichia coli and application thereof
ActiveCN101914564AImproving immunogenicityImprove protectionAntibacterial agentsBacterial antigen ingredientsEscherichia coliInfant animal
The invention relates to an escherichia coli (E. coli) trivalent enterotoxin fusion gene and application thereof, and belongs to the field of genetic engineering subunit vaccines. The enterotoxigenic E. coli (ETEC) is a main pathogen causing baby-animal and infantile diarrhea. Aiming at the defects that the conventional TEC vaccine has narrow protection range and cannot induce high-level antitoxin, the invention designs a trivalent E. coli enterotoxin fusion with the fusion mode of 5'-STa-LT-STb-3' or 5'-STb-LT-STa-3'. After the multivalent enterotoxin gene or protein immunizes animals, high-level STa, STb and LT resistant antibodies can be induced to be generated. Therefore, the escherichia coli (E. coli) trivalent enterotoxin fusion gene can neutralize the toxicity of a key pathogenic factor enterotoxin, improve the immunity protection rate and widen the protection range without being limited by E. coli pilus types so as to effectively control ETEC diarrhea.
Owner:DALIAN UNIV OF TECH
Methods and compositions for promoting immunopotentiation
InactiveUS20060078557A1Increasing cell proliferationRapid rise in intracellular calciumImmunoglobulin superfamilyVirus peptidesAbnormal tissue growthFungal microorganisms
This invention discloses immunopotentiating agents which stimulate an immune response. These agents are categorized into single agents that act directly, adjuvants added concurrently with the agents, or heteroconjugates. Heteroconjugate agents elicit or enhance a cellular or humoral immune response which may be specific for an epitope contained within an amino acid sequence. Enhanced hematopoieses by bone marrow stem cell recruitment was also a result of administering some of these agents. Examples of immunopotentiating agents include monoclonal antibodies and proteins derived from microorganisms (e.g., enterotoxins) which activate T cells. One method of treatment disclosed uses only the immunopotentiating agent to stimulate the immune system. Another uses adjuvants in combination with the agent. A third method employs heteroconjugates. Heteroconjugates comprise: (a) an immunopotentiating protein which is characterized as having an ability to stimulate T cells; and (b) a second protein having an amino acid sequence which includes an epitope against which a cellular or humoral response is desired. This invention also relates to a method of preparing the heteroconjugate, and to a method of stimulating the immune system in vivo in a novel way. One route of stimulation is to activate T cells, in some instances, specific subsets of T cells, by administering heteroconjugates containing an immunopotentiating protein and a second protein, to mammals. For this method of treatment, the second protein in the heteroconjugate is derived from abnormal or diseased tissue, or from an infectious agent; alternatively, the second protein is produced synthetically by standard methods of molecular biology. Sources of the second protein include tumors, viruses, bacteria, fungi, protozoal or metozoal parasites. Monoclonal antibodies or T cells prepared from mammals whose immune systems have responded to administration of the heteroconjugate may be produced and administered to induce passive immunity. A method of preparing a hybridoma which secretes said monoclonal antibodies and use of these monoclonal antibodies and T cells, are also disclosed. This invention is also directed to a vaccine comprising the heteroconjugate.
Owner:BLUESTONE JEFFERY A
Colloidal gold immuno-chromatography test paper strip used for detecting escherichia coil F5 pilus and preparation method thereof
The invention discloses a colloidal gold immuno-chromatography test paper strip used for detecting escherichia coil F5 pilus and a preparation method thereof, which relate to a test paper strip for detecting escherichia coil pilus and a preparation method thereof. The invention provides the colloidal gold immuno-chromatography test paper strip used for detecting escherichia coil F5 pilus and the preparation method thereof. The test paper strip consists of a PVC back lining, a sample pad, a combination pad, a cellulose nitrate membrane and a water absorbent pad. The preparation method comprises the following steps of: preparing an immunogen; coating an anti-F5 pilus polyclonal antibody and a sheep anti-mouse IgG on the cellulose nitrate membrane; preparing an anti-F5 pilus monoclonal antibody; coating the anti-F5 pilus monoclonal antibody and a colloidal gold marker on the combination pad; and finally, continuously adhering the sample pad, the combination pad, the cellulose nitrate membrane and the water absorbent pad on the PVC back lining in turn, and cutting the PVC back lining into the test paper strip. The colloidal gold immuno-chromatography test paper strip has high specificity, stability and sensibility, can be used for identifying enterotoxigenic escherichia coil F5 pilus, has an intuitive result, and is convenient to be conserved.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Enterotoxin C2 superantigen mutant proteins, and coding gene and preparation and application thereof
InactiveCN102060916AHigh tumor suppressor activityReduce emeticPeptide/protein ingredientsDepsipeptidesEnterotoxinAntigen
The invention relates to enterotoxin C2 superantigen mutant proteins, and a coding gene and preparation and application thereof. The enterotoxin C2 superantigen mutant proteins SAM-1, SAM-2 and SAM-3 respectively have amino acid sequences of SEQID NO:2, SEQID NO:4 and SEQID NO:6 shown in a sequence table; the preparation process comprises the following steps of: performing overlap PCR amplification by taking a DNA segment having a base sequence of SEQID NO:7 in the sequence table as a template; performing PCR amplification by taking sec2-F and sec2-R as primers and an overlap PCR product as a template to obtain a mutant gene; cloning the mutant gene in an expression vector pET-28a, and constructing into a gene engineering bacterium for heterologously expressing each enterotoxin C2 superantigen mutant protein by taking escherichia coli BL21(DE3) as a host bacterium; and inducing and heterologously expressing to obtain each enterotoxin C2 superantigen mutant protein. The mutant proteins keep higher super-antigen activity and tumor suppressing activity, have obviously reduced emetic activity and febrifacient activity compared with the wild enterotoxin C2 protein, and have better clinical medication prospect.
Owner:SHENYANG XIEHE GRP
Weight losing prescription
InactiveCN103263582AEliminate intestinal toxinsNo side effectsMetabolism disorderPlant ingredientsMedicinal herbsBacterial enterotoxins
The invention discloses a weight losing prescription, and relates to a Chinese medicinal formula. The weight losing prescription is prepared from the following Chinese medicinal herbs by weight: 40 grams of oriental wormwood, 20 grams of fleece-flower root, 30 grams of cherokee rose fruit, 30 grams of many-flower solomonseal rhizome, 15 grams of raw hawthorn, 20 grams of root of red-rooted salvia, 10 grams of rhubarb, 5 grams of pseudo-ginseng powder, 20 grams of kudzuvine root, 30 grams of medlar, and 15 grams of white atractylodes rhizome. A preparation method for the prescription comprises the following step of: decocting the Chinese medicinal herbs by adding water in a weight ratio, wherein the decoction is taken twice every day. The prescription can clear enterotoxin, is naturally mild, does not have side effect, and has a good weight losing effect.
Owner:陈静静
Novel prophylactics/remedies for immunopathy
InactiveUS20060024322A1Low toxicityBacterial antigen ingredientsPeptide/protein ingredientsWild typeBULK ACTIVE INGREDIENT
A prophylactic / remedy for immunopathy for immunopathy comprising, as an active ingredient, modifications of Staphylococcal enterotoxin B (SEB) with substitution of at least one amino acid residues within the amino acid sequence of natural type SEB, or derivatives thereof, wherein the SEB modifications or derivatives thereof have inhibitory activity on T cell activation wherein they interact with specific Vβ component of T cell receptor (TCR) but are reduced in their immunological responsiveness to SEB without inducing elimination of T cells having specific Vβ component, the elimination being normally induced by natural type SEB or recombinant wild-type SEB.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES & KOWA COMPANY
Duck Tembusu virus E protein-LTB fusion protein and application thereof
The invention aims to provide a duck Tembusu virus gene engineering subunit vaccine which is prepared by the following steps: screening to obtain duck Tembusu virus E protein with Domain III structure field of dominant antigen epitope, constituting a novel fusion protein LTB-Es from Lingker and enterotoxin LTB, and preparing the duck Tembusu virus gene engineering subunit vaccine by using the fusion protein as the antigen. The amino acid sequence of the coding protein of the duck Tembusu virus novel fusion protein LTB-Es is SEQ ID NO:1, and one of the nucleotide sequences is SEQ ID NO:2. The prokaryotic expression vector is utilized to construct the Escherichia coli BL21(DE3) host bacterium capable of expressing the duck Tembusu virus novel fusion protein LTB-Es. The gene engineering subunit vaccine prepared from the purified recombinant expressed protein can enable the immunized duck group to obtain immunoprotection.
Owner:QINGDAO AGRI UNIV
Quick detection agent of staphylococcus intestinal toxin
InactiveCN1955739AShorten detection timeSimplify operating proceduresBiological testingAntigenBacterial enterotoxins
A reagent bar used for quickly detecting staphylococcus enterotoxin consists of reagent, reagent bar and micro-scale pipet. It is featured as using cell clasmatosis solution as reagent and carrying 20 micron L of specific single clone antibody of anti-staphylococcus enterotoxin, collaurum labeled single clone antibody of anti-staphylococcus enterotoxin, collaurum labeled quality control antigen and antibody of relevant antigen on reagent bar.
Owner:吴斌 +2
Expression vector for secreting antibody fragment using e. coli signal sequence and method for mass-producing antibody fragment
InactiveUS20090104660A1Polypeptide with localisation/targeting motifBacteriaMicroorganismAntibody fragments
A recombinant expression vector capable of expressing and secreting an antibody fragment fused with E. coli thermostable enterotoxin signal sequence derivative or E. coli outer membrane protein A (Omp A) signal sequence in the form of a soluble heterozygote protein is used to mass-produce the antibody fragment by culturing a microorganism transformed with the expression vector in a medium and collecting the antibody fragment secreted from the transformed microorganism into the medium
Owner:HANMI SCI CO LTD
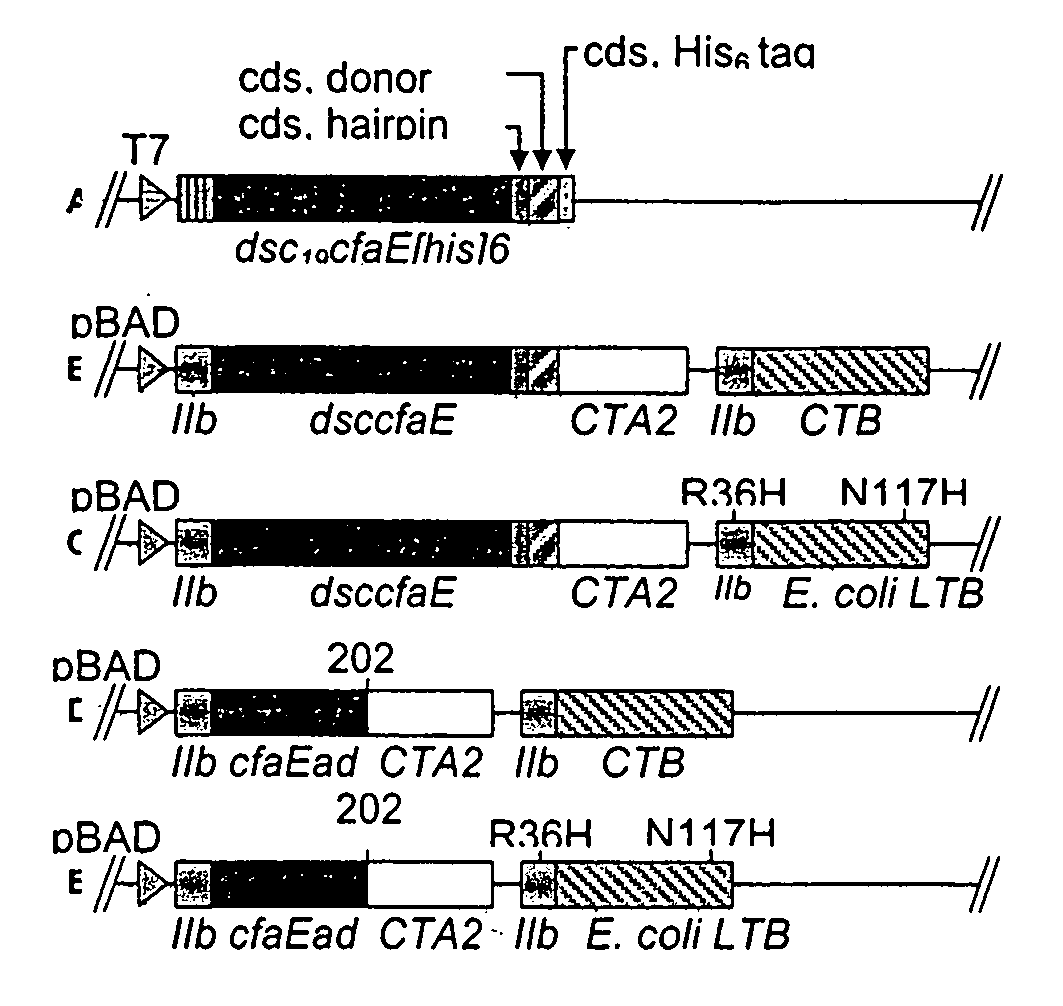
Construction of live attenuated Shigella vaccine strains that express CFA/I antigens (CfaB and CfaE) and the B subunit of heat-labile enterotoxin (LTB) from enterotoxigenic E. coli
ActiveUS7759106B2Reduce intrusionBacterial antigen ingredientsBacteriaHeterologousMucosal Immune Responses
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Novel bacteriophage and antibacterial composition comprising the same
ActiveUS20160083696A1Excellent acid resistance and heat resistanceImprove the heating effectAntibacterial agentsBiocideBiologyHuman use
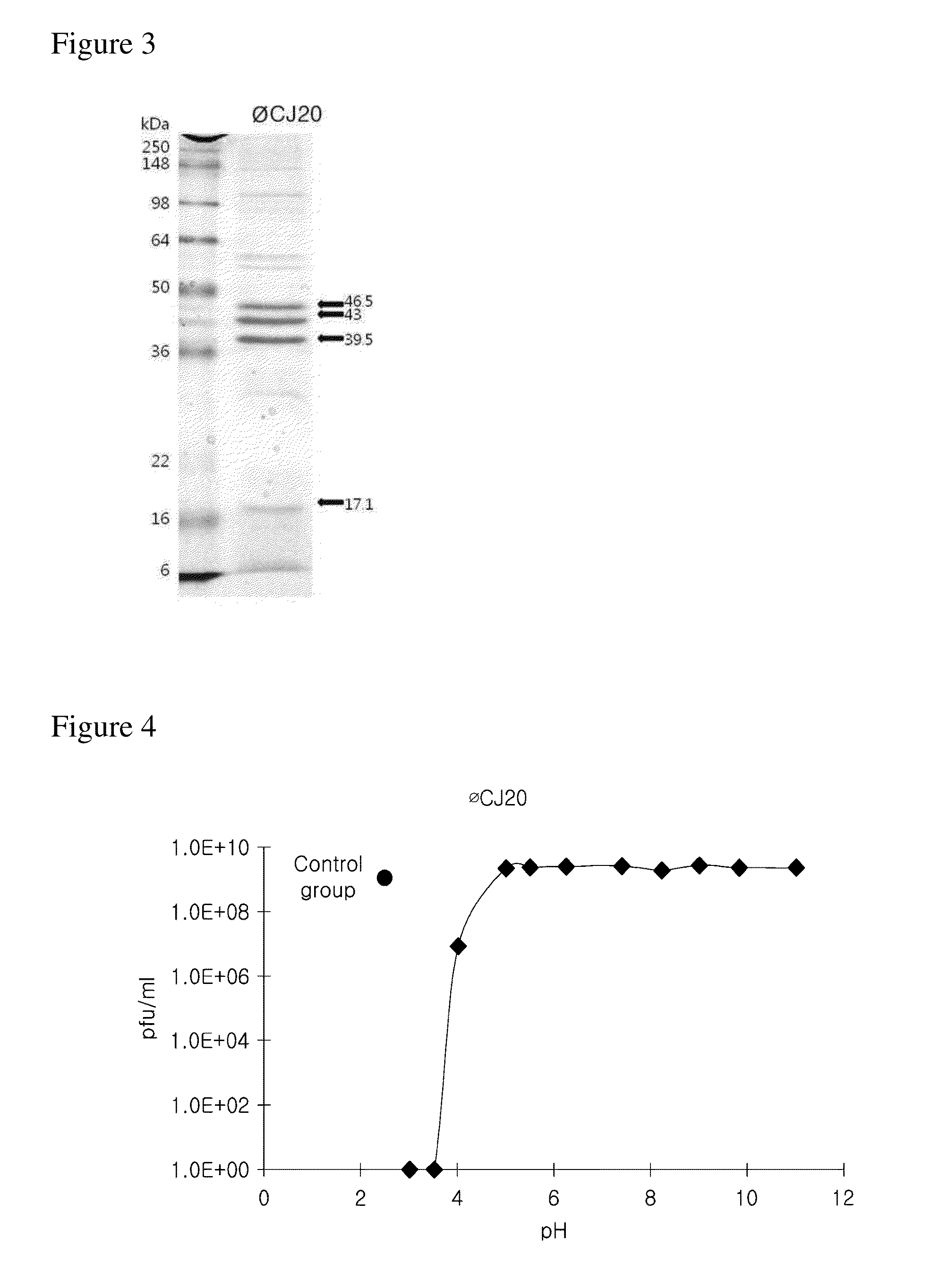
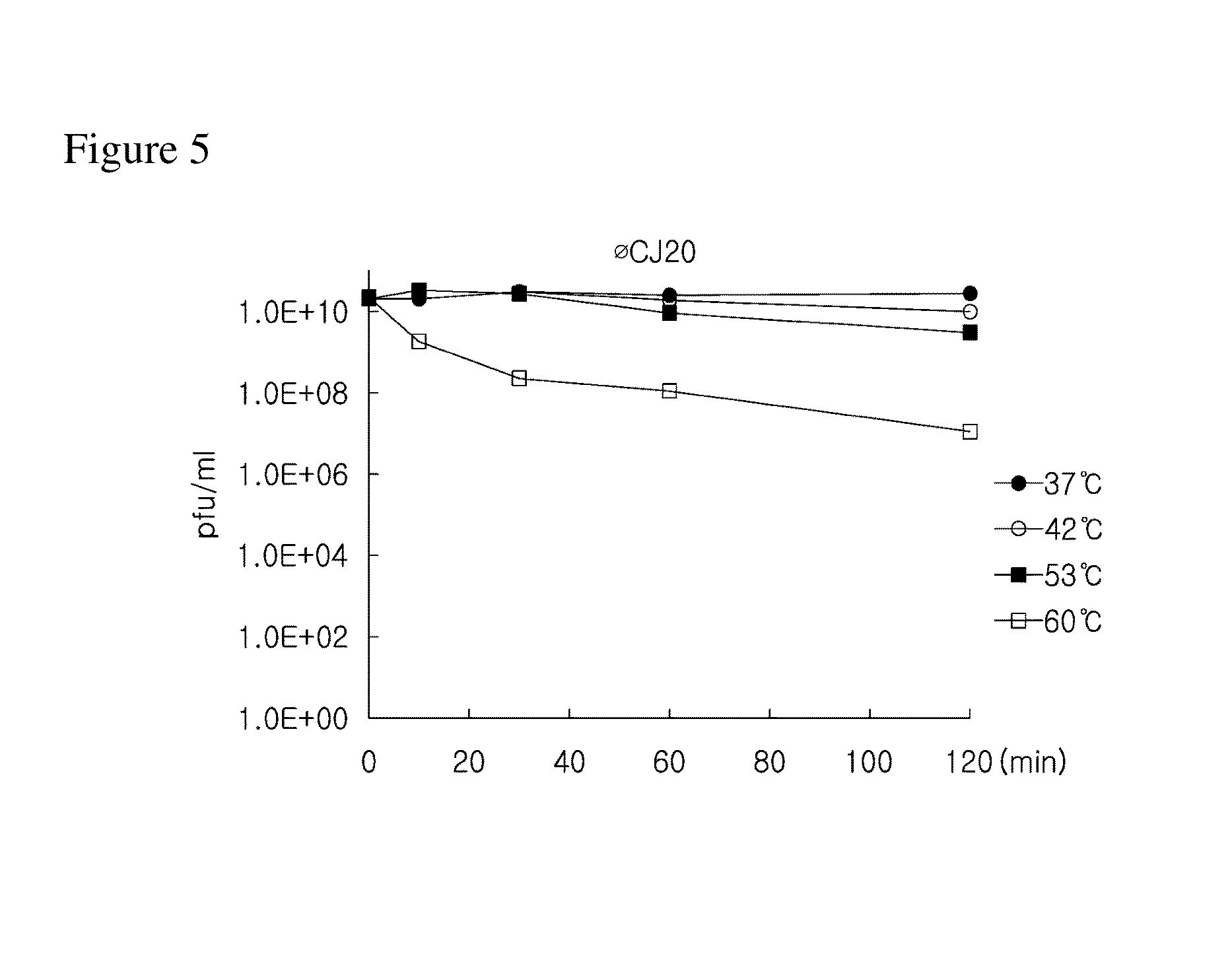
Provided is a novel bacteriophage ΦCJ20 (KCCM11362P). In addition, the present invention relates to an antibacterial composition including the bacteriophage ΦCJ20 (KCCM11362P) as an active ingredient. Further, the present invention is a method of preventing and / or treating infectious diseases by enterotoxigenic Escherichia coli in animals except for humans using the bacteriophage ΦCJ20 (KCCM11362P) or the antibacterial composition containing the bacteriophage ΦCJ20 (KCCM11362P) as an active ingredient.
Owner:CJ CHEILJEDANG CORP
Magnetic particle chemiluminescence assay kit and method for detecting staphylococcal enterotoxin A (SEA)
ActiveCN103383354AEasy to useStrong specificityChemiluminescene/bioluminescenceBacterial enterotoxinsStaphylococcus
The invention discloses a magnetic particle chemiluminescence assay kit and a method for detecting SEA, and belongs to the technical field of food safety detection. The method is a magnetic particle chemiluminescence assay method for fast detecting SEA in foods and preparations. The method comprises the following steps of diluting a standard substance, adding a marker into the dilute standard substance, carrying out uniform mixing, warm bath, sedimentation and washing, adding a luminous substrate into the mixture, determining chemiluminescence intensity, drawing a standard curve, wherein an OD value of a detected sample is in the range of the standard curve. The magnetic particle chemiluminescence assay kit has the advantages of simple operation, fast speed, high sensitivity, strong singularity and low cost.
Owner:北京泽诚生物技术有限公司
Antibodies for the treatment of clostridium difficile-associated infection and disease
InactiveUS20150175681A1Improve the quality of lifeImprove survivabilityAntibacterial agentsPeptide/protein ingredientsAntigenDisease
Provided herein are reagents, compositions, and therapies with which to treat Clostridium difficile infection and related disease conditions and pathologies, such as Clostridium difficile-associated diarrhea, resulting from infection by Clostridium difficile bacteria and the enterotoxins produced by these bacteria. In particular, antibodies or antigen-binding fragments thereof that bind specifically to toxin A and / or toxin B of C difficile and neutralize the activities of these toxins; compositions comprising such antibodies; and methods of using the antibodies and the compositions are provided.
Owner:PROGENICS PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com