Magnetic particle chemiluminescence assay kit and method for detecting staphylococcal enterotoxin A (SEA)
A chemiluminescence immunoassay, kit technology, applied in chemiluminescence/bioluminescence, analysis by chemical reaction of materials, measurement devices, etc. problems such as the use of radioimmunoassay to achieve the effects of high precision, high sensitivity and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Construction of Magnetic Particle Chemiluminescence Immunoassay Kit for Quantitative Detection of Enterotoxin SEA
[0025] A magnetic particle chemiluminescent immunoassay kit for the quantitative detection of enterotoxin SEA was constructed to include the following components:
[0026] (1) Alkaline phosphatase (ALP) labeled mouse anti-Staphylococcus aureus enterotoxin SEA monoclonal antibody marker;
[0027] (2) Mouse anti-Staphylococcus aureus enterotoxin SEA monoclonal antibody labeled with fluorescein isothiocyanate;
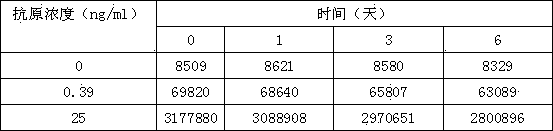
[0028] (3) The concentrations of SEA standard solutions are: 25ng / ml, 10ng / ml, 3ng / ml, 0.9ng / ml, 0.3ng / ml, 0ng / ml;
[0029] (4) Rat anti-FITC monoclonal antibody labeled magnetic microsphere separation reagent;
[0030] (5) The washing solution is phosphate buffer containing 0.5% Tween-20 and 0.15M pH7.4;
[0031] (6) The sample diluent is 0.05% Tween-20 and 1% BSA 20mM pH7.2 phosphate buffer;
[0032] (7) Alkaline phosphatase chemilum...
Embodiment 2
[0033] Example 2 Preparation of Staphylococcus aureus enterotoxin SEA monoclonal antibody labeled with fluorescein isothiocyanate
[0034] 2.1 Preparation of reagents required for SEA monoclonal antibody labeling with fluorescein isothiocyanate
[0035] Coating buffer: pH 9.5, 0.1mol / L carbonate buffer;
[0036] 2.2 Preparation of SEA monoclonal antibody labeled fluorescein isothiocyanate
[0037] Dilute the SEA monoclonal antibody to 3-5mg / ml with pH 9.5, 0.1mol / L carbonate buffer, add 0.1-0.3ml of 1mg / ml FITC solution to each mg of antibody, and incubate at 37°C for 2-4h; After the reaction, put it into a dialysis bag and dialyze overnight at 2-8°C in pH 9.5, 0.1mol / L carbonate buffer solution. During this process, replace the buffer solution 1-2 times to make it 0.1-5ug / ml , and then stored at 2-8°C.
Embodiment 3
[0038] Example 3 Preparation of Staphylococcus aureus enterotoxin SEA monoclonal antibody-labeled alkaline phosphatase (ALP) label
[0039] 3.1 Reagents required for the activation of Staphylococcus aureus enterotoxin SEA monoclonal antibody
[0040] Reaction buffer: 0.1mol / L phosphate, 0.15mol / L sodium chloride, pH 7.3;
[0041] Stop buffer: 0.1mol / L phosphate, 0.5mol / L hydroxylamine, 25mM EDTA, pH 7.4
[0042] 3.2 Preparation of activated SEA monoclonal antibody
[0043] Dialyze the SEA monoclonal antibody in the reaction solution at room temperature for 2-4min, replace the buffer solution 1-2 times in the middle, then dilute the SEA monoclonal antibody to 2-5mg / ml with the reaction buffer; weigh a certain amount of activator SATA , diluted to 13mg / ml with DMSO, and mixed; add 5-30ul 13mg / ml SATA solution to each ml of antibody, and react at room temperature for 10-30min. Then dialyze in the stop buffer at room temperature for 2-4 hours, and replace the buffer 1-2 times...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com