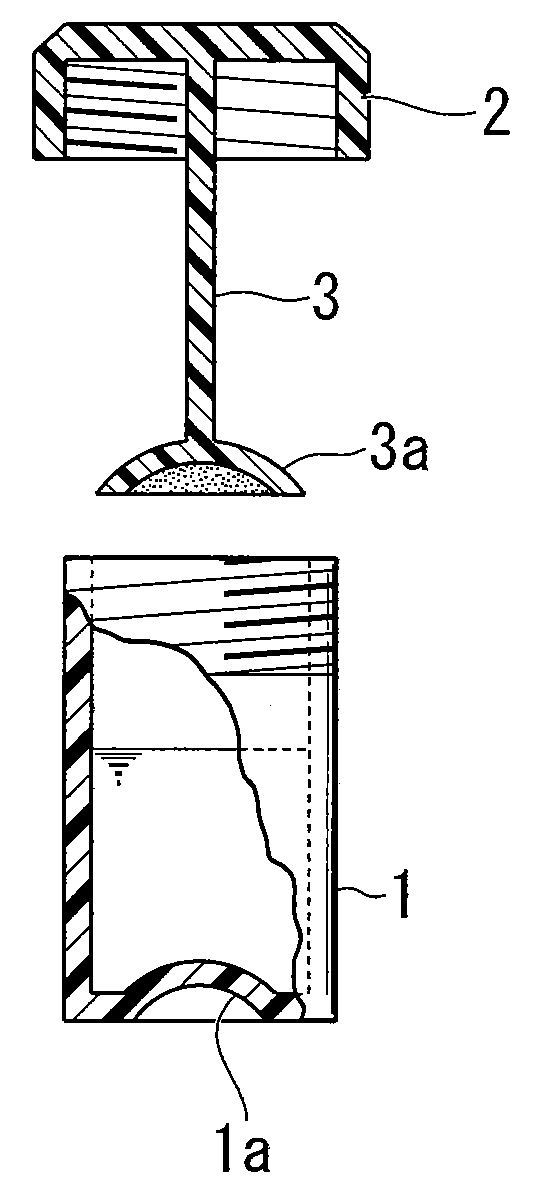
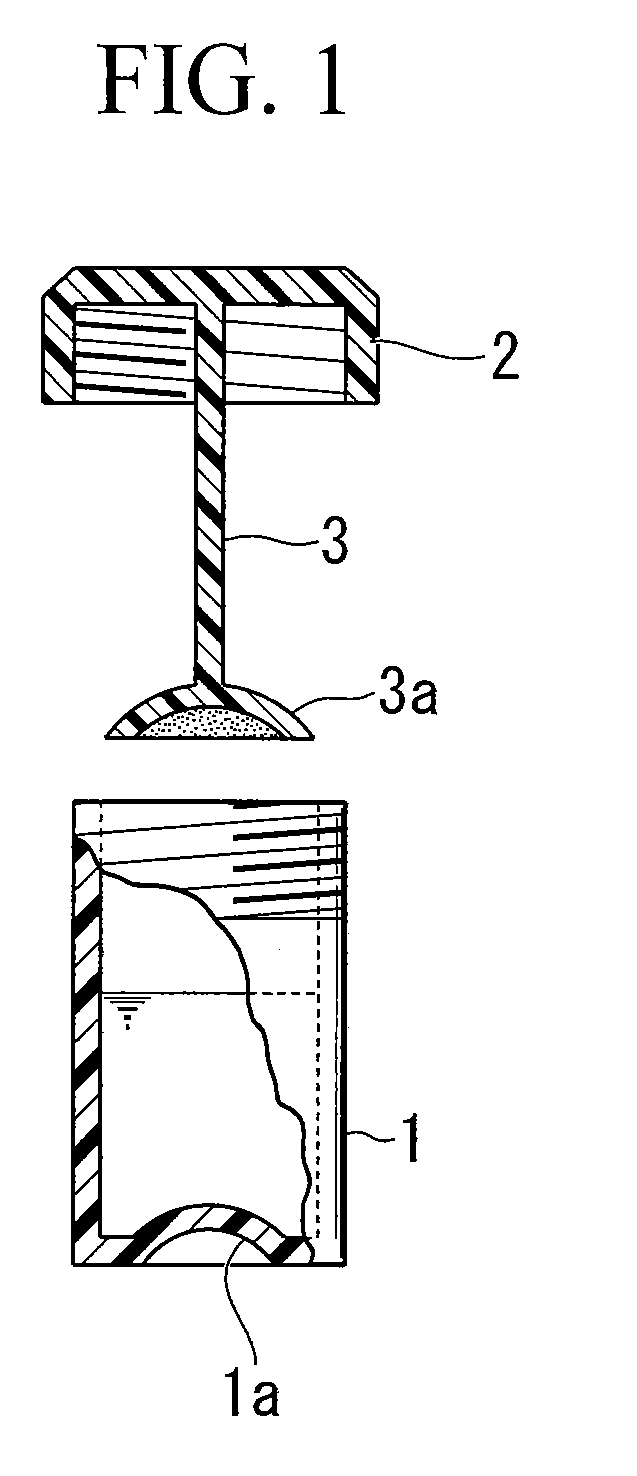
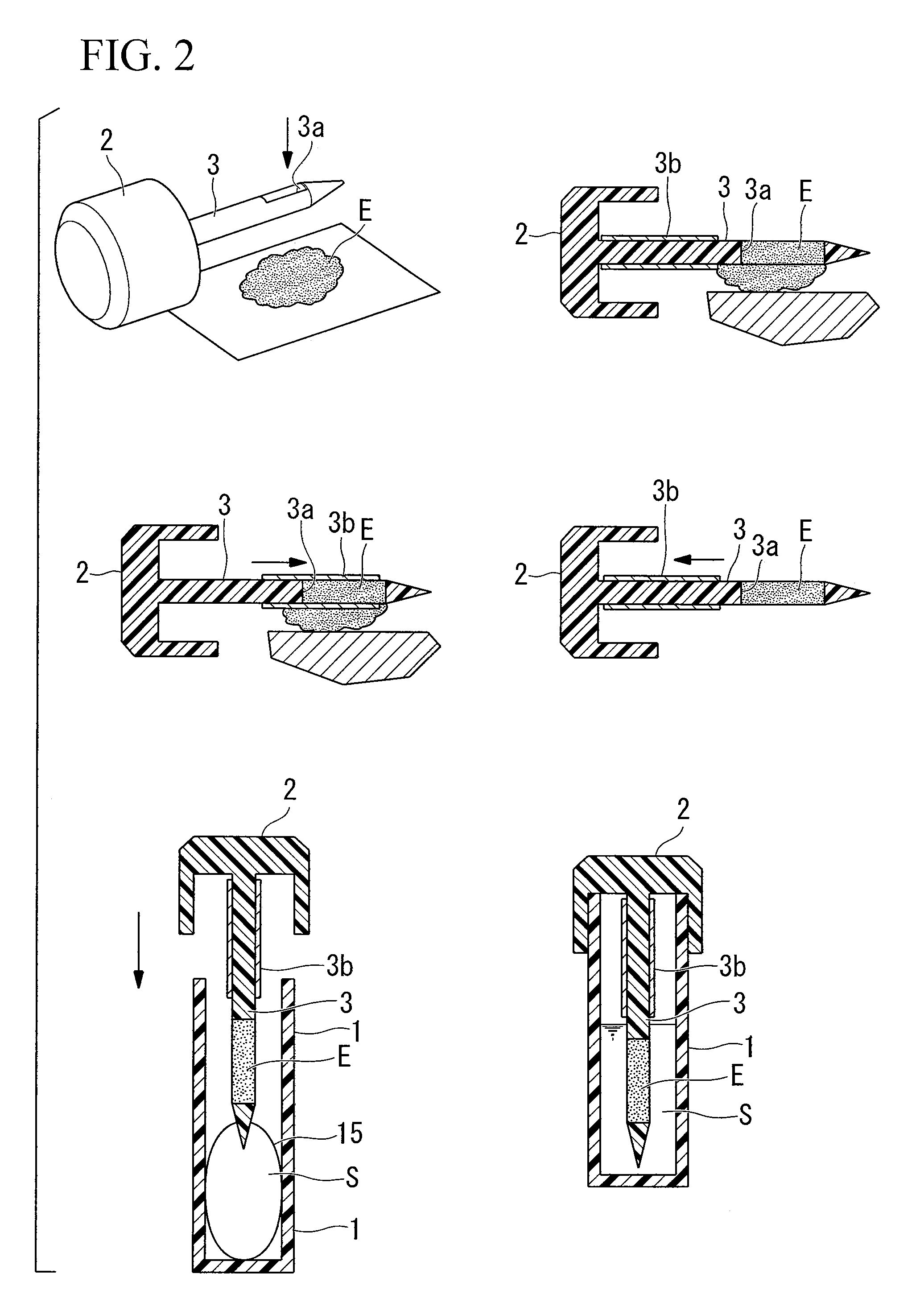
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
165 results about "Stool sample" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
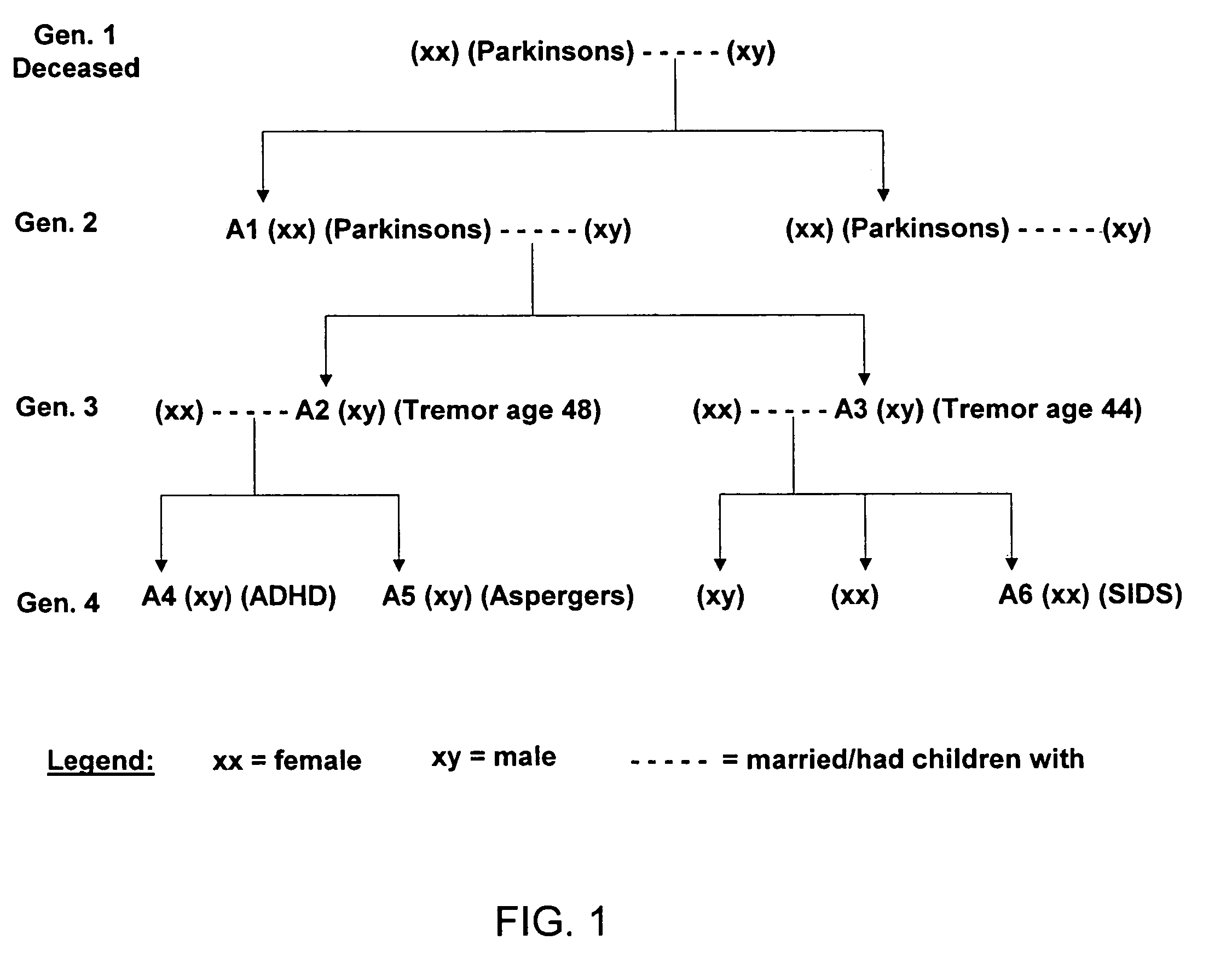
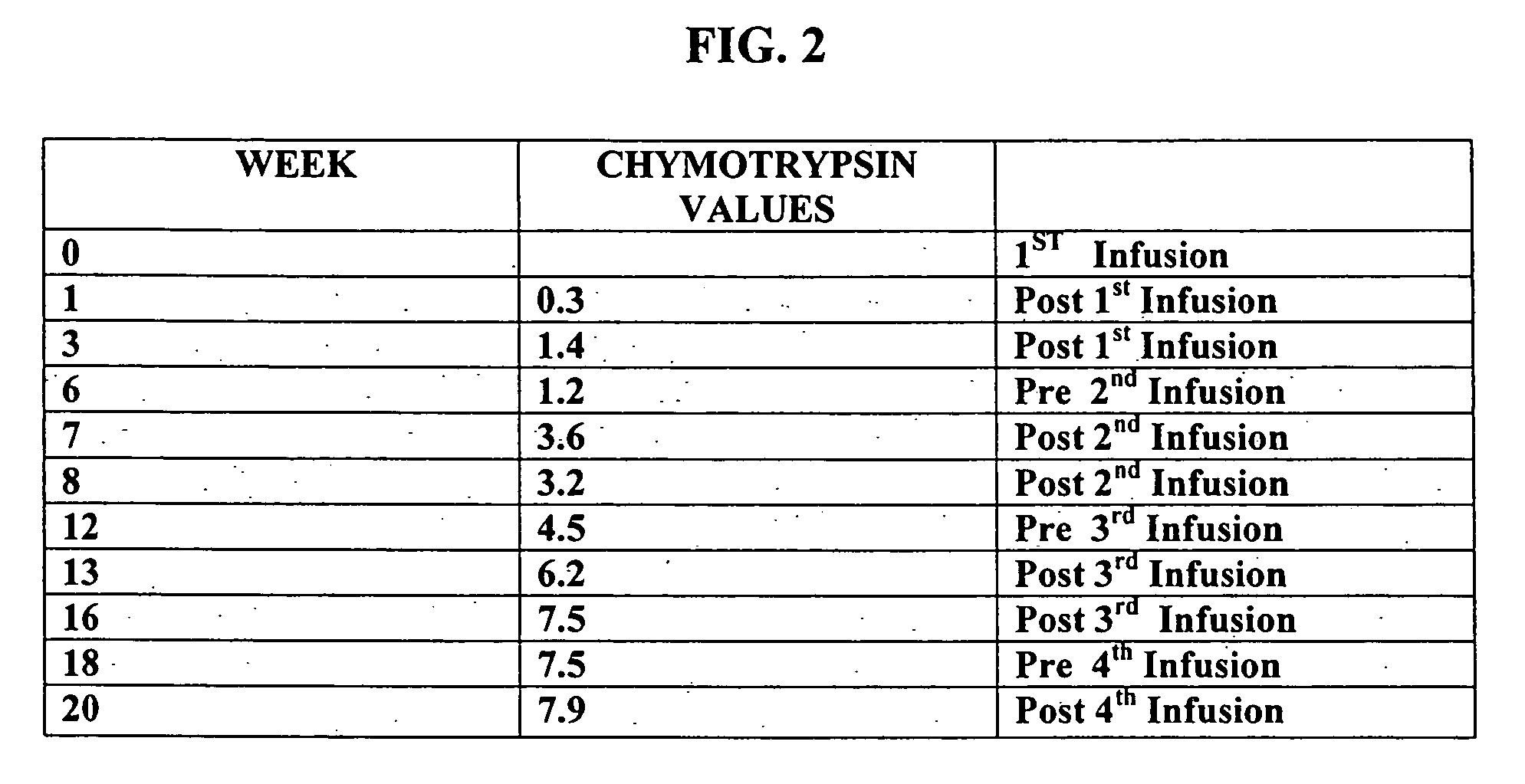
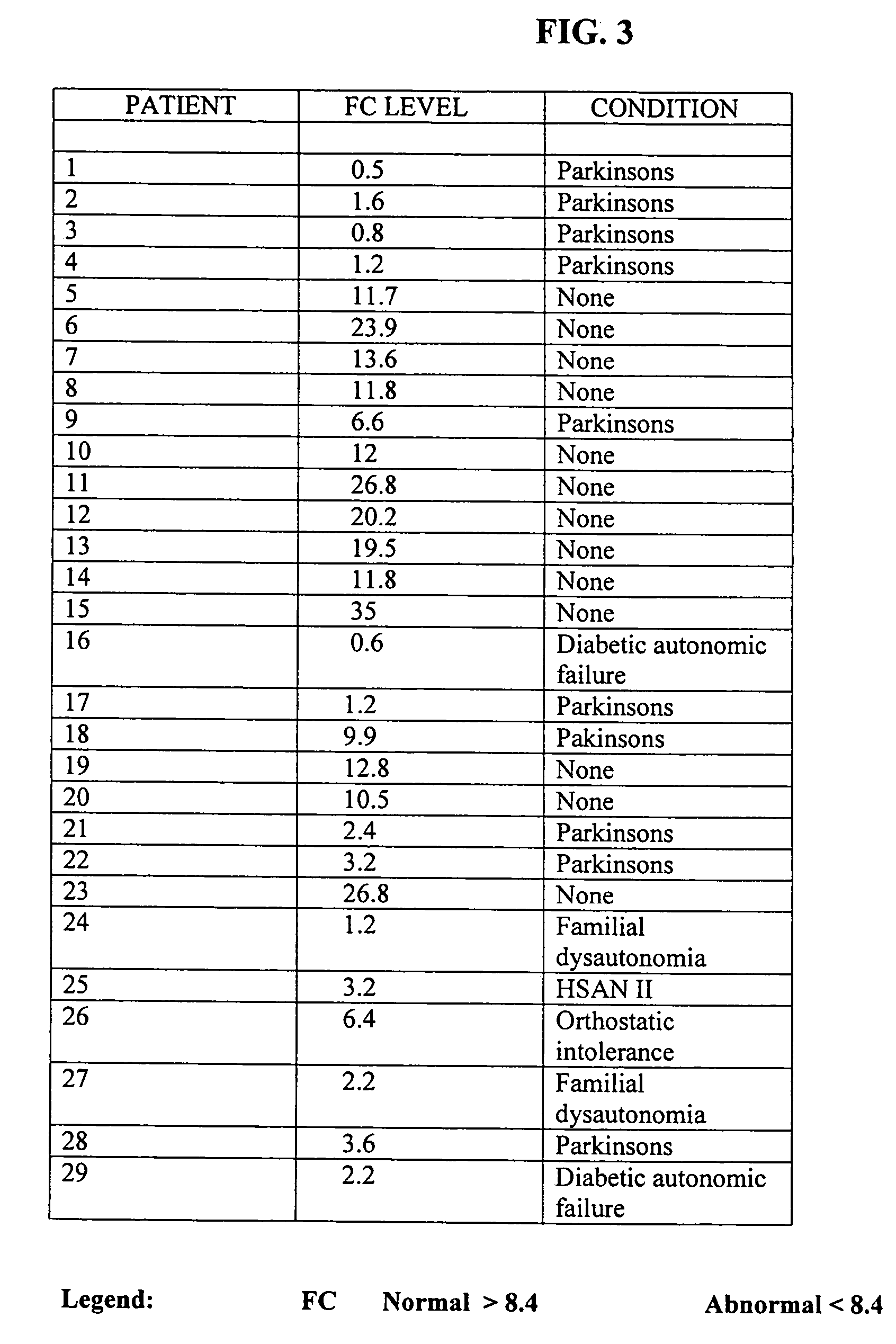
Methods for diagnosing and treating dysautonomia and other dysautonomic conditions
Methods for aiding in the diagnosis of dysautonomic disorders and dysautonomic conditions and methods for treating individuals diagnosed as having a dysautonomic disorder or a dysautonomic condition. In one aspect, a diagnosis method comprising analyzing a stool sample of an individual for the presence of a biological marker wherein the quantity of the biological marker is an indication of whether the individual has, or can develop, a dysautonomic disorder or dysautonomic condition, as well as a therapeutic method for treating a dysautonomic disorder or dysautonomic condition by administration of, e.g., secretin, neuropeptides, peptides and / or digestive enzymes.
Owner:CUREMARK
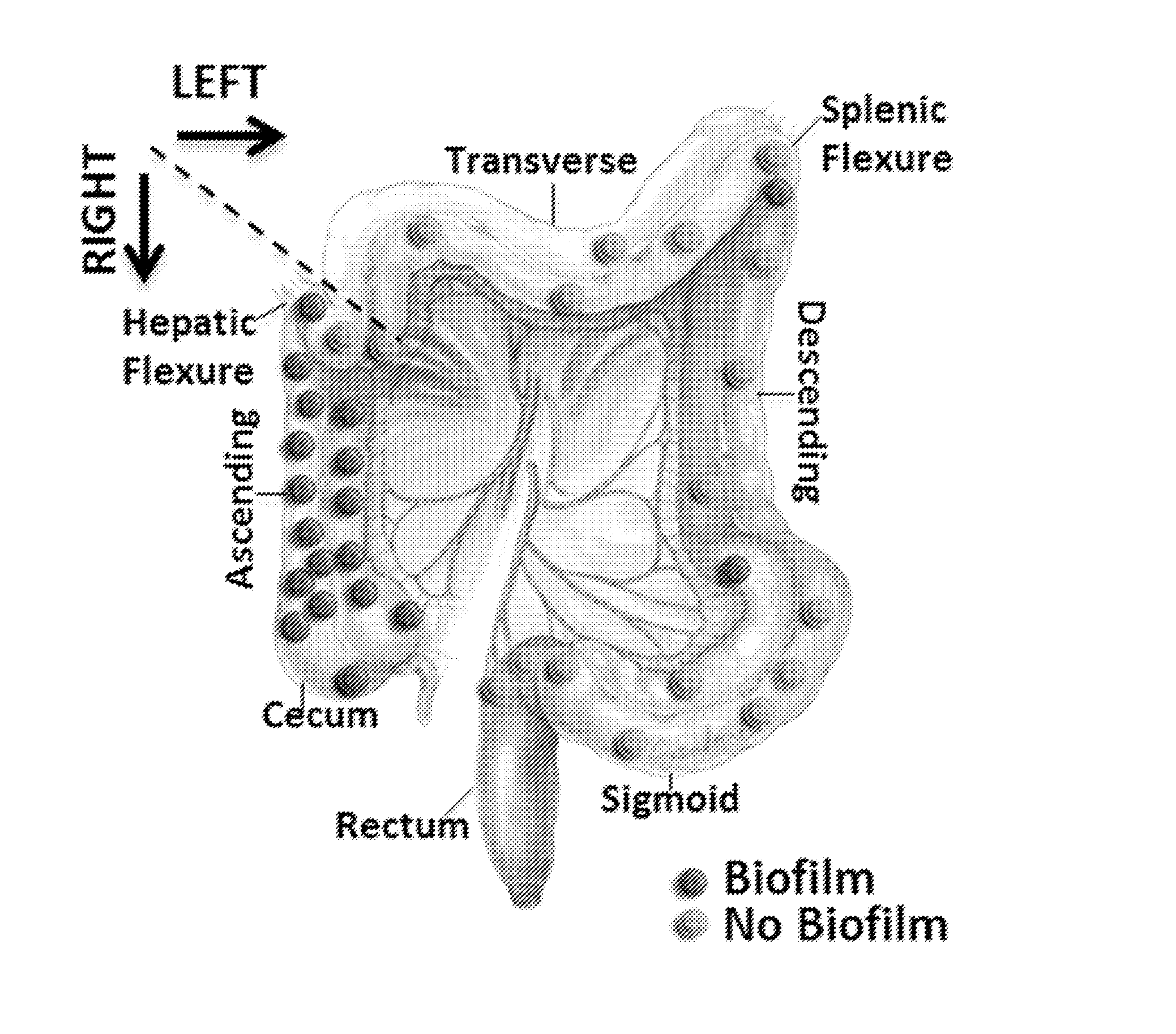
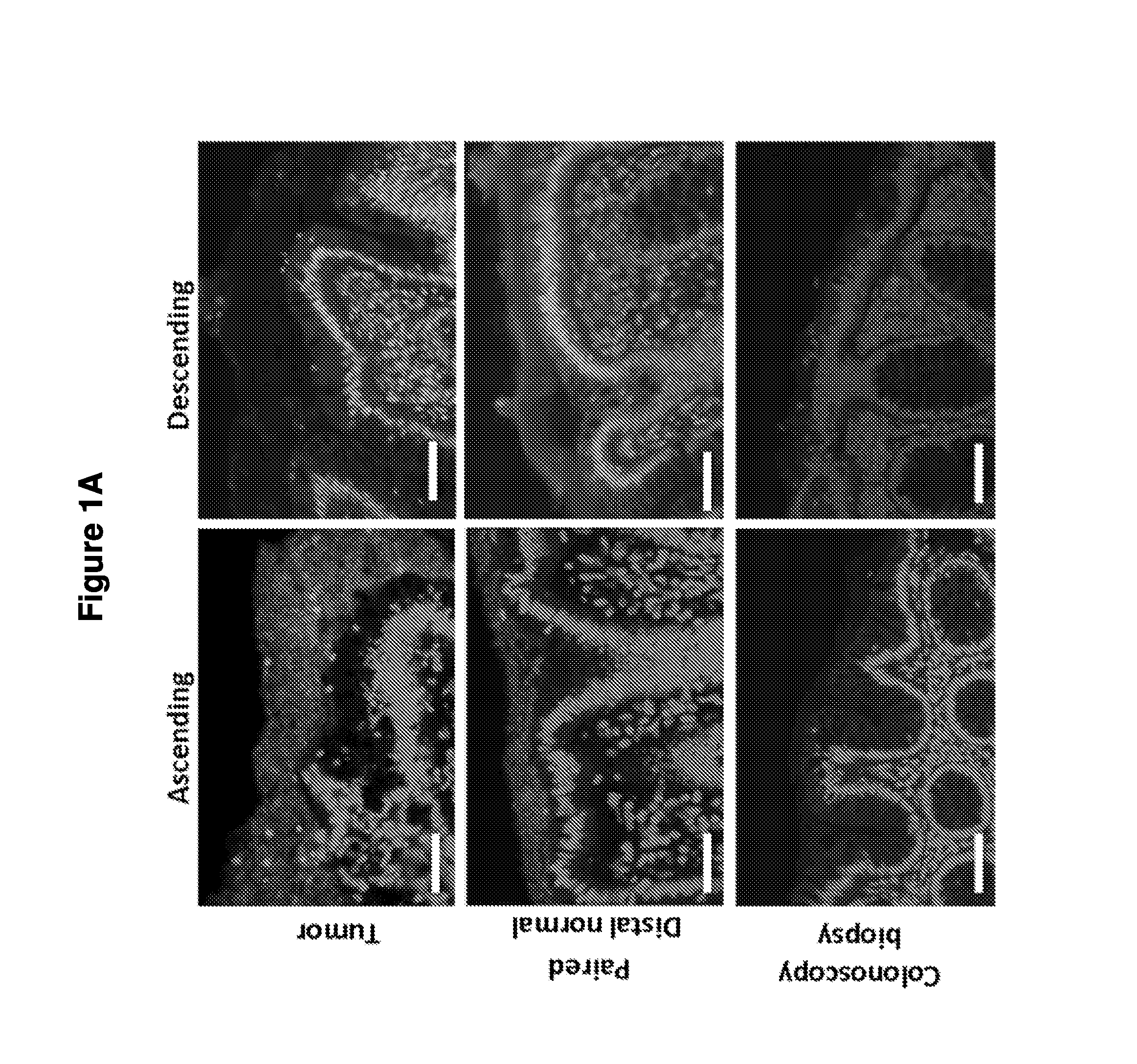
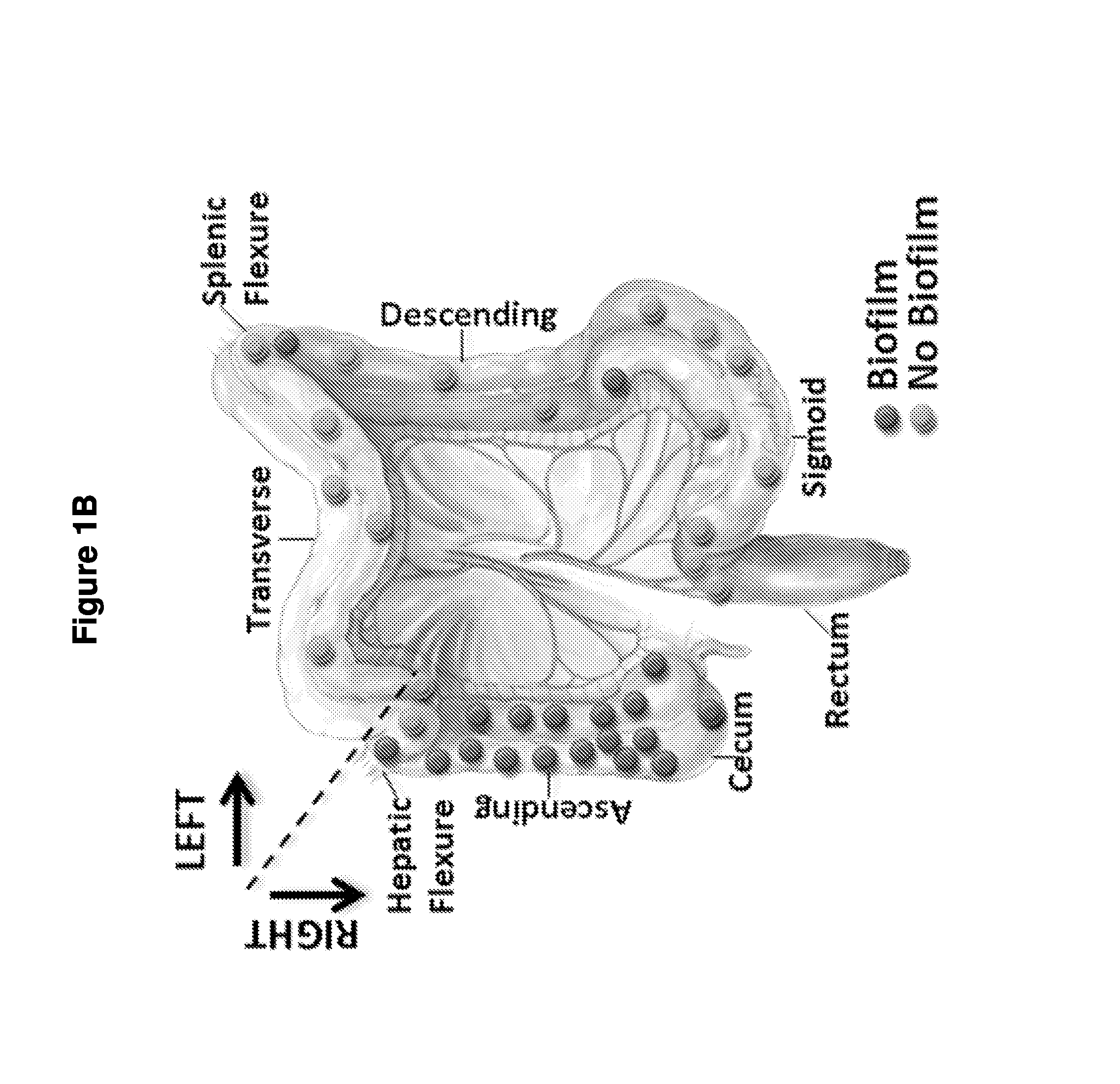
Biofilm formation to define risk for colon cancer
ActiveUS20160223553A1Preventing and diminishing developmentIncreased proliferationMicrobiological testing/measurementUnknown materialsFecesIncreased risk
Methods of identifying subjects at increased risk of cancer, based upon detection of biofilms and / or biofilm-associated microbes within a subject, are disclosed. Therapies designed to prevent formation and / or reduce the size of biofilms in a subject identified to be at increased risk of cancer based upon detection of biofilms and / or biofilm-associated microbes are disclosed. In particular embodiments, the invention provides for identification of a subject at elevated risk of developing or having colorectal cancer and / or a colorectal adenoma, based upon detection of a biofilm and / or biofilm-associated bacteria within the gastrointestinal tract of the subject (optionally, within a biopsy specimen and / or stool sample of such subject). Therapies involving administration of an antibiotic agent and / or a probiotic agent to a subject, to prevent or reduce biofilm formation within the gastrointestinal tract of the subject, optionally provided in combination with additional cancer therapy, are also disclosed.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
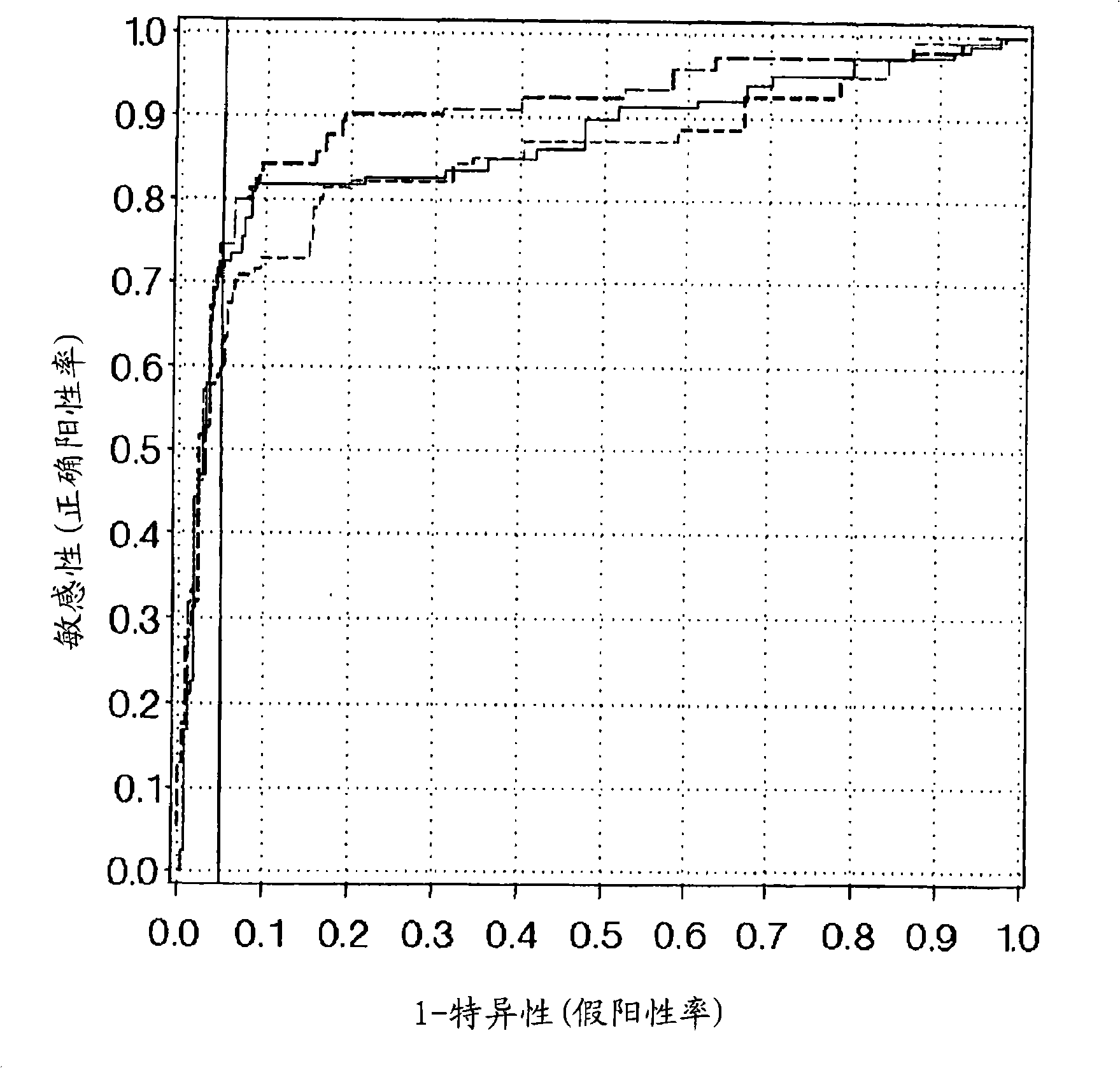
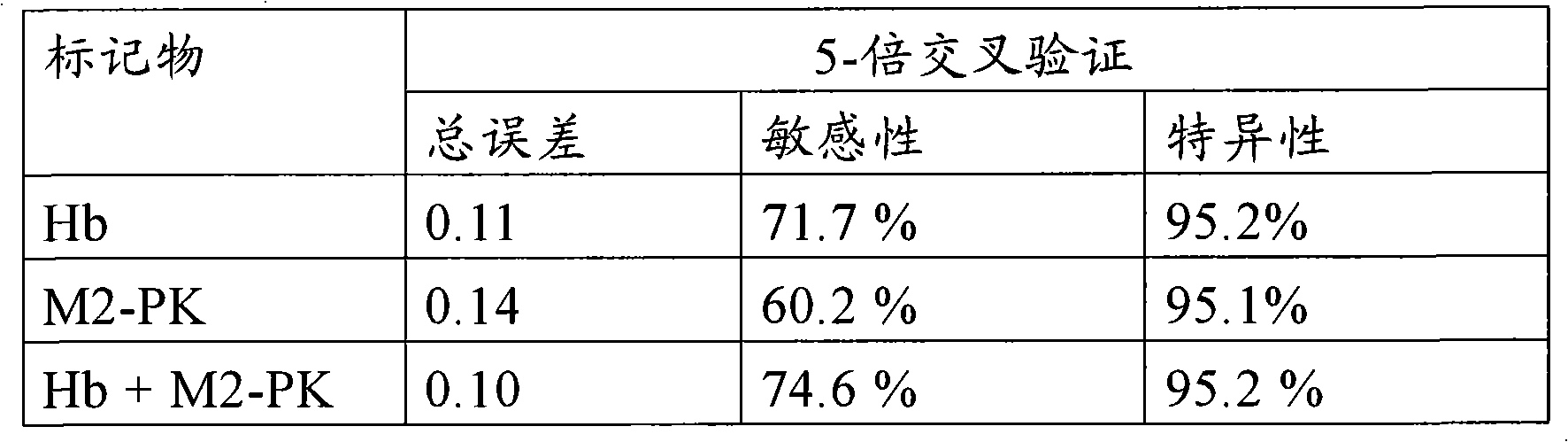
Assessing colorectal cancer by measuring hemoglobin and m2-pk in a stool sample
InactiveUS20090075311A1Microbiological testing/measurementBiological material analysisMedicineBiochemical markers
The present invention relates to a method aiding in the assessment of colorectal cancer. The method especially is used in assessing the absence or presence of colorectal cancer in vitro. The method is, for example, practiced by analyzing biochemical markers, comprising measuring in a stool sample the concentration of hemoglobin and M2-PK and correlating the concentrations determined to the absence or presence of colorectal cancer. To further improve the assessment of colorectal cancer in a method of this invention the level of one or more additional marker may be determined together with hemoglobin and M2-PK in a stool sample and be correlated to the absence or presence of colorectal cancer. The invention also relates to the use of a marker panel comprising hemoglobin and M2-PK in the early diagnosis of colorectal cancer, and it teaches a kit for performing the method of the invention.
Owner:KARL JOHANN
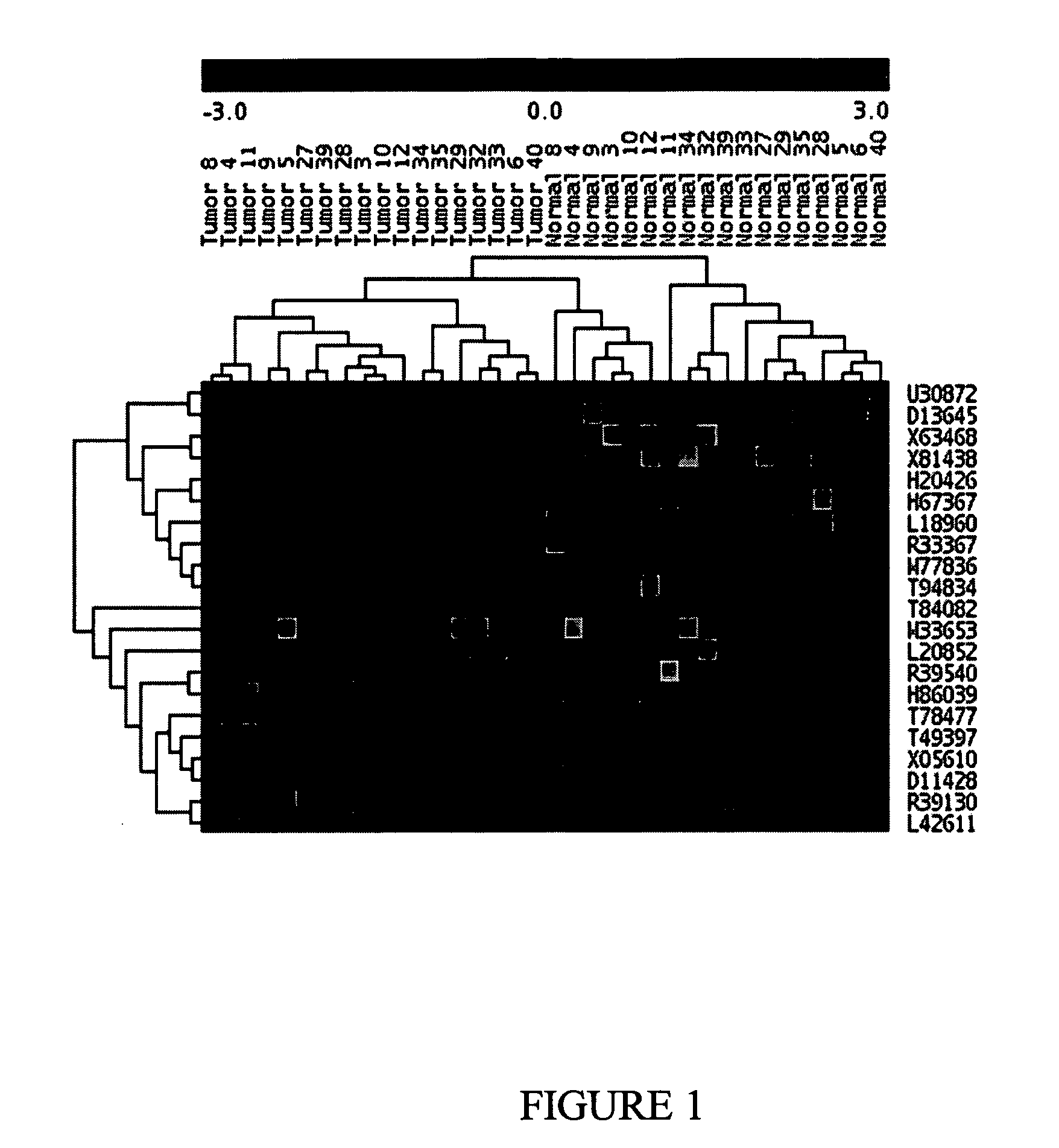
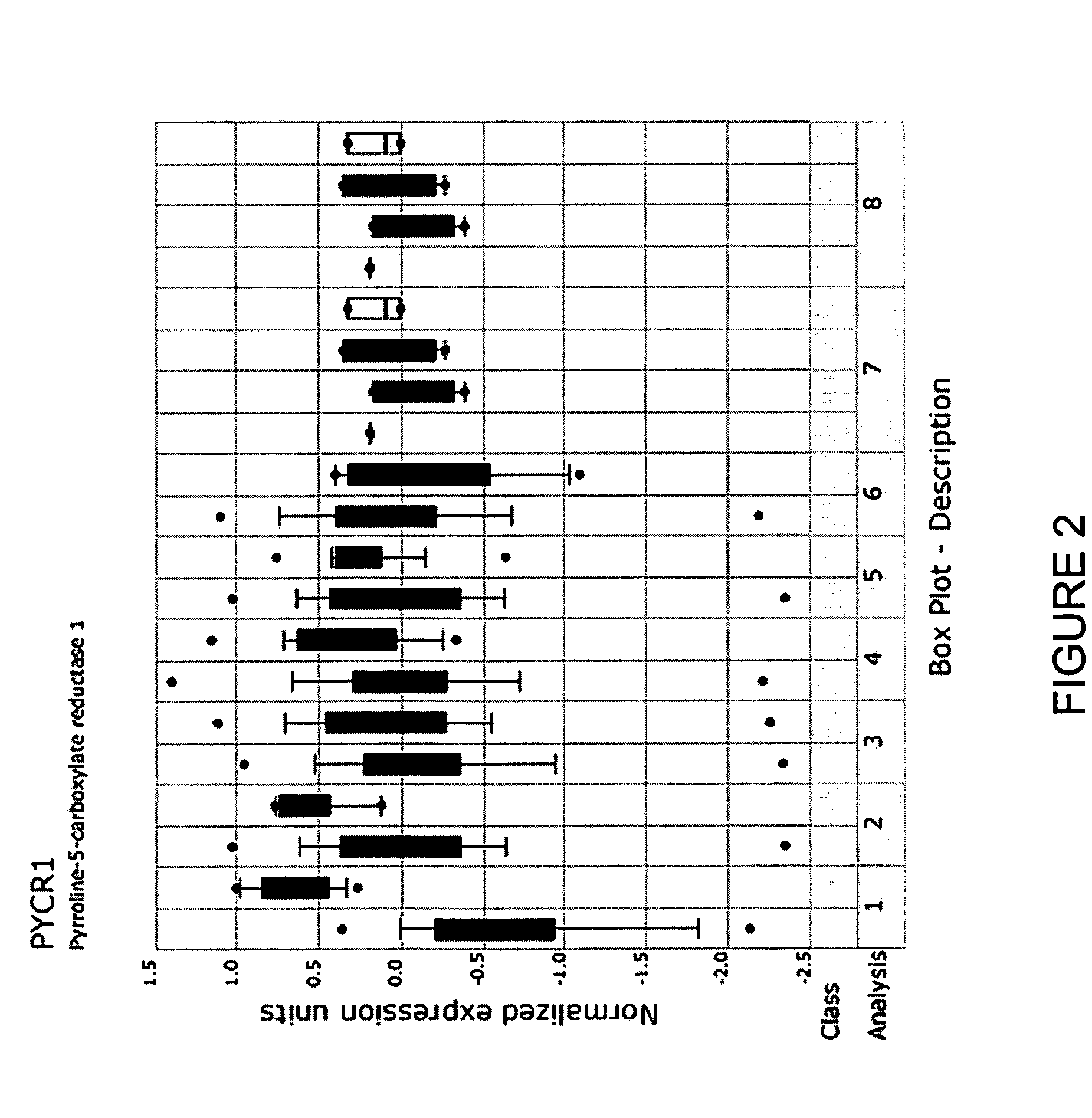
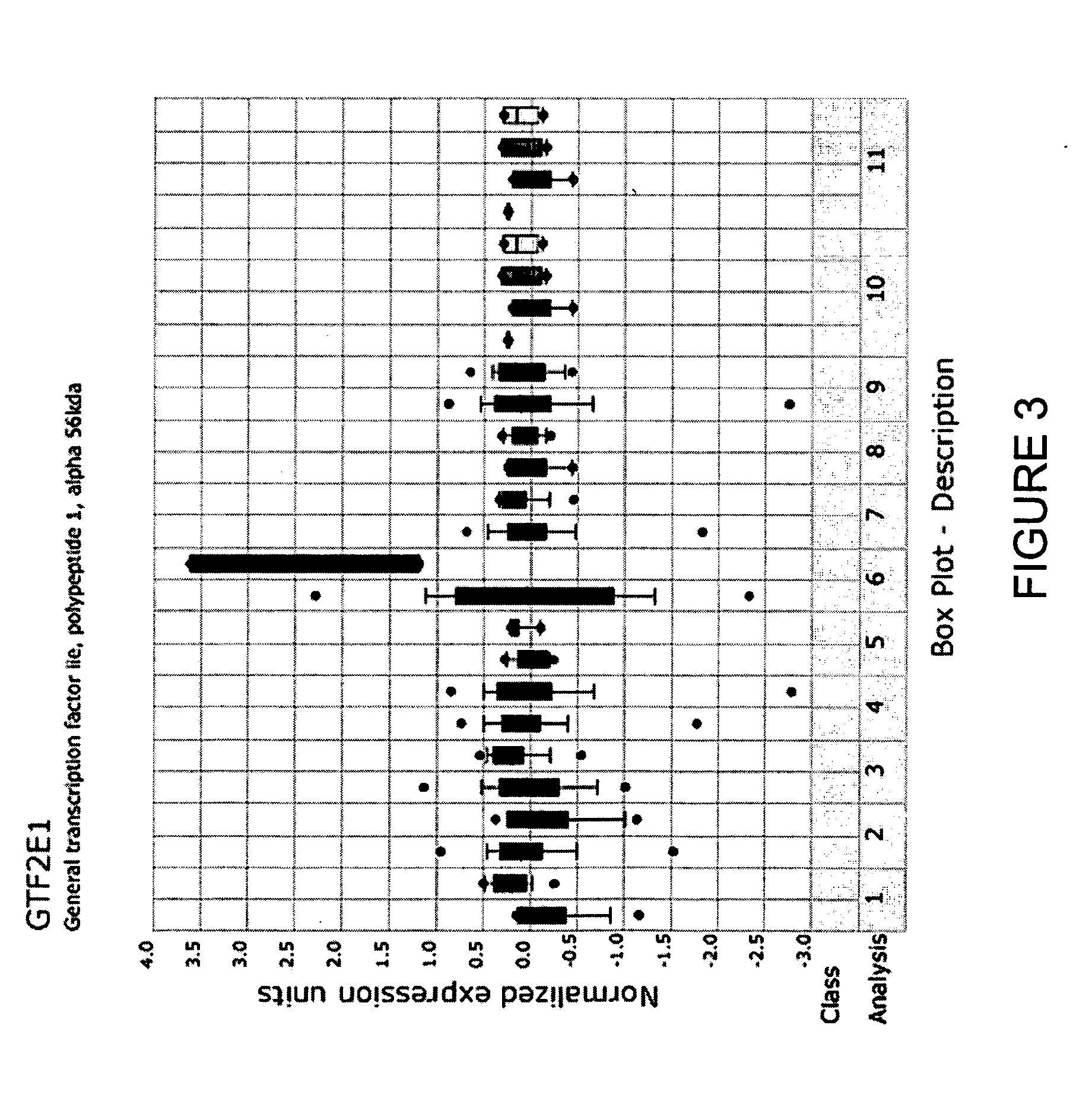
Molecular method for diagnosis of colon cancer
Methods for diagnosing or detecting cancerous colon tissue. A panel of 21 specific marker genes are provided. The overexpression of some of these marker genes compared to their expression in normal colon tissue and the underexpression of the rest of these marker genes are indicative of cancerous colon tissue. By using these 21 marker genes as a diagnostic tool, smaller tissue samples, such as those obtained by core needle biopsies and from patient stool samples, can be used.
Owner:NAT RES COUNCIL OF CANADA
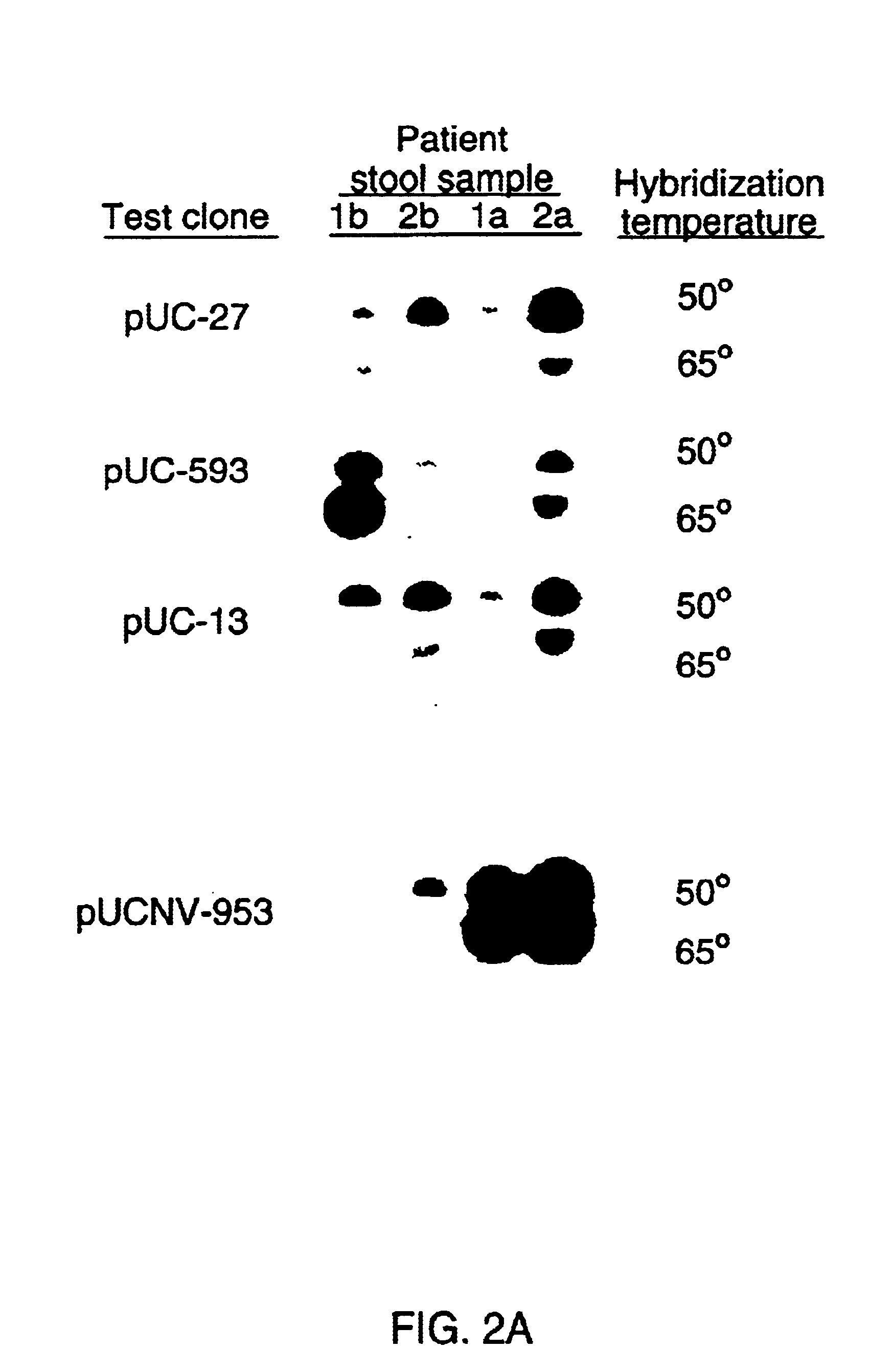
Methods and reagents to detect and characterize norwalk and related viruses
Double-stranded cDNA was synthesized from nucleic acid extracted from Norwalk virus purified from stool specimens of volunteers. One clone was isolated from a cDNA library constructed in a pUC-13 vector after amplification of the cDNA. The specificity of this cDNA (pUCNV-953) was shown by hybridization assays. The cDNA reacted with post (but not pre-) infection stool samples from Norwalk volunteers and with highly purified Norwalk virus, but not with other common enteric viruses such as hepatitis A virus and rotavirus. Finally, the probe detected virus in the same fractions of CsCl gradients in which viral antigen was detected using a specific Norwalk virus radioimmunoassay, and particles were detected by immune electron microscopy. Single-stranded RNA probes derived from the DNA clone after subcloning into an in vitro transcription vector were also used to show that the Norwalk virus contains a ssRNA genome of about 8 kb in size. The original clone was also used to detect additional cDNAs which represent at least 7 kb of nucleic acid of the Norwalk genome. The availability of a Norwalk-specific cDNA and the first partial genome sequence information allow rapid cloning of the entire genome and of establishment of sensitive diagnostic assays. Such assays can be based on detection of Norwalk virus nucleic acid or Norwalk viral antigen using polyclonal or monoclonal antibodies to proteins expressed from the cDNA or to synthetic peptides made based on the knowledge of the genome sequence. Assays using proteins deduced from the Norwlk virus genome and produced in expression systmes can measure antibody responses. Vaccines made by recombinant DNA technology are now feasible.
Owner:BAYLOR COLLEGE OF MEDICINE
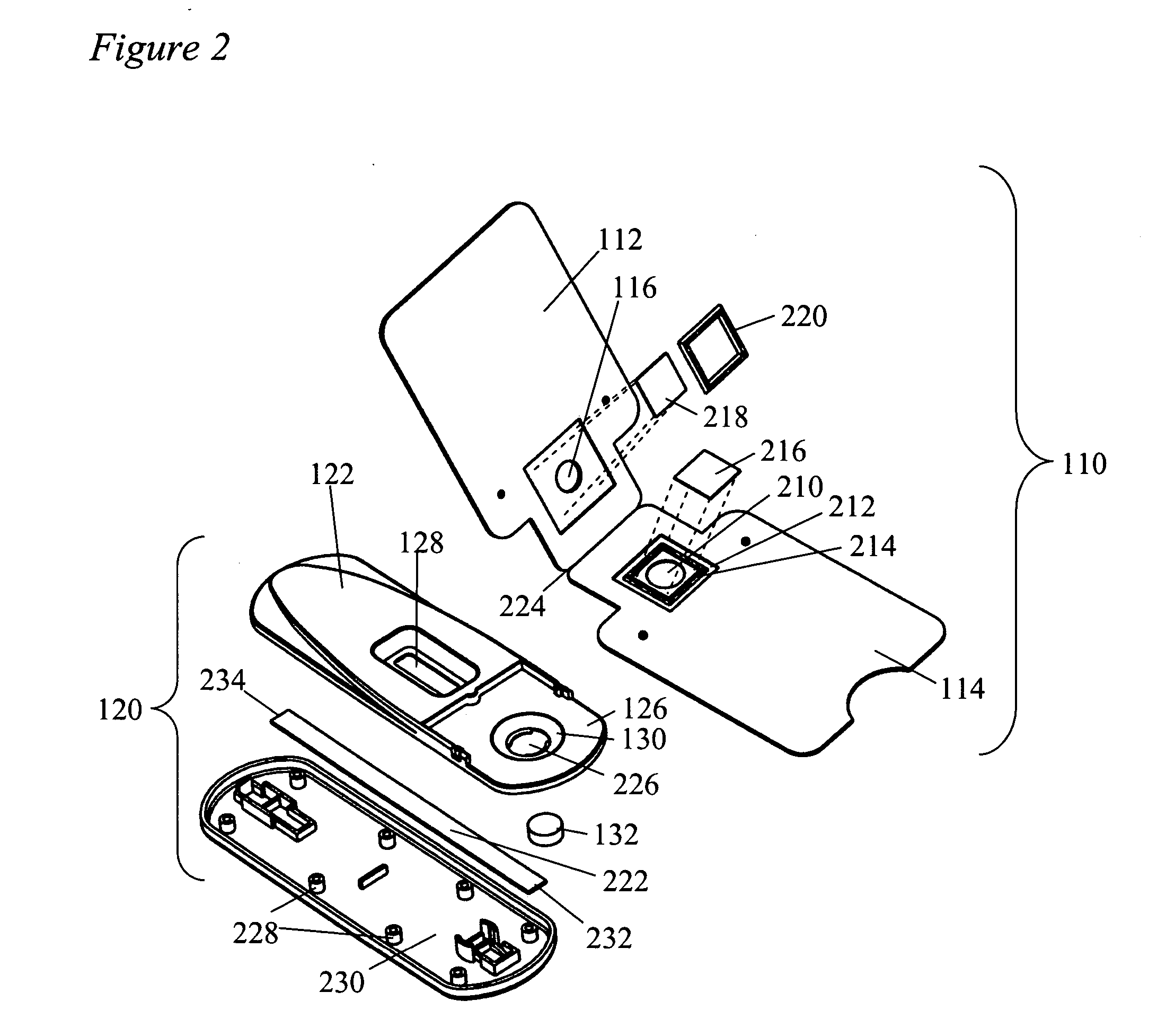
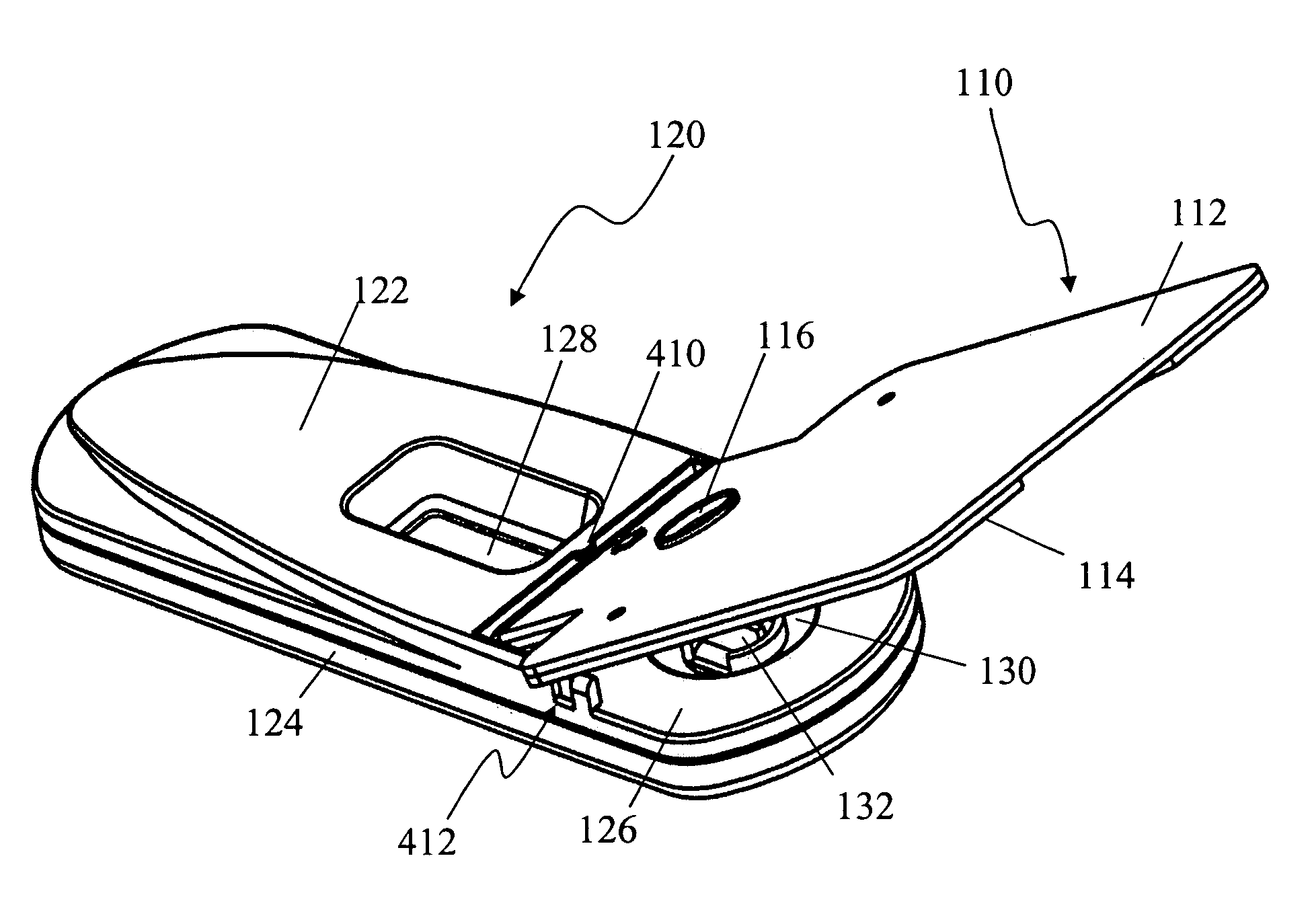
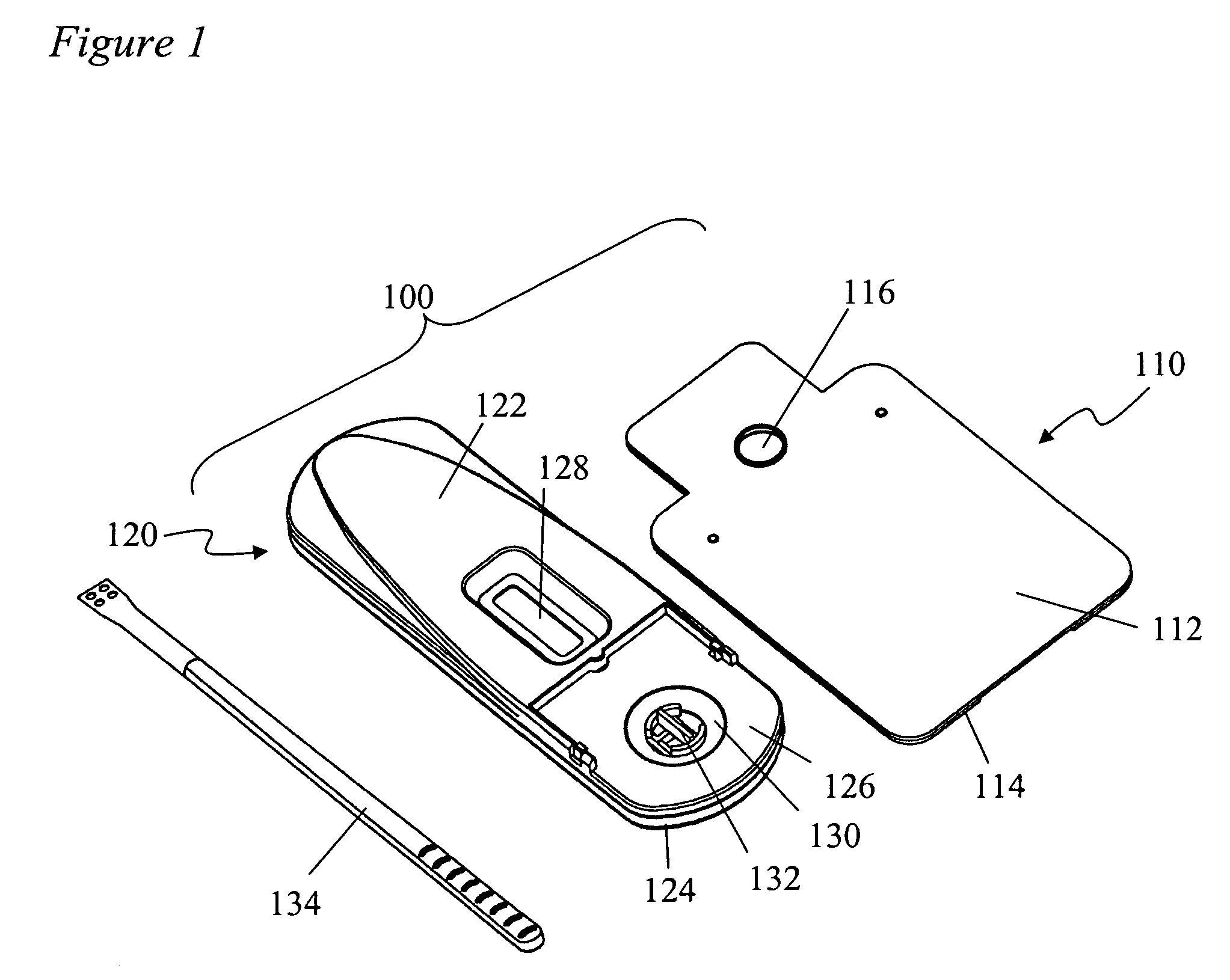
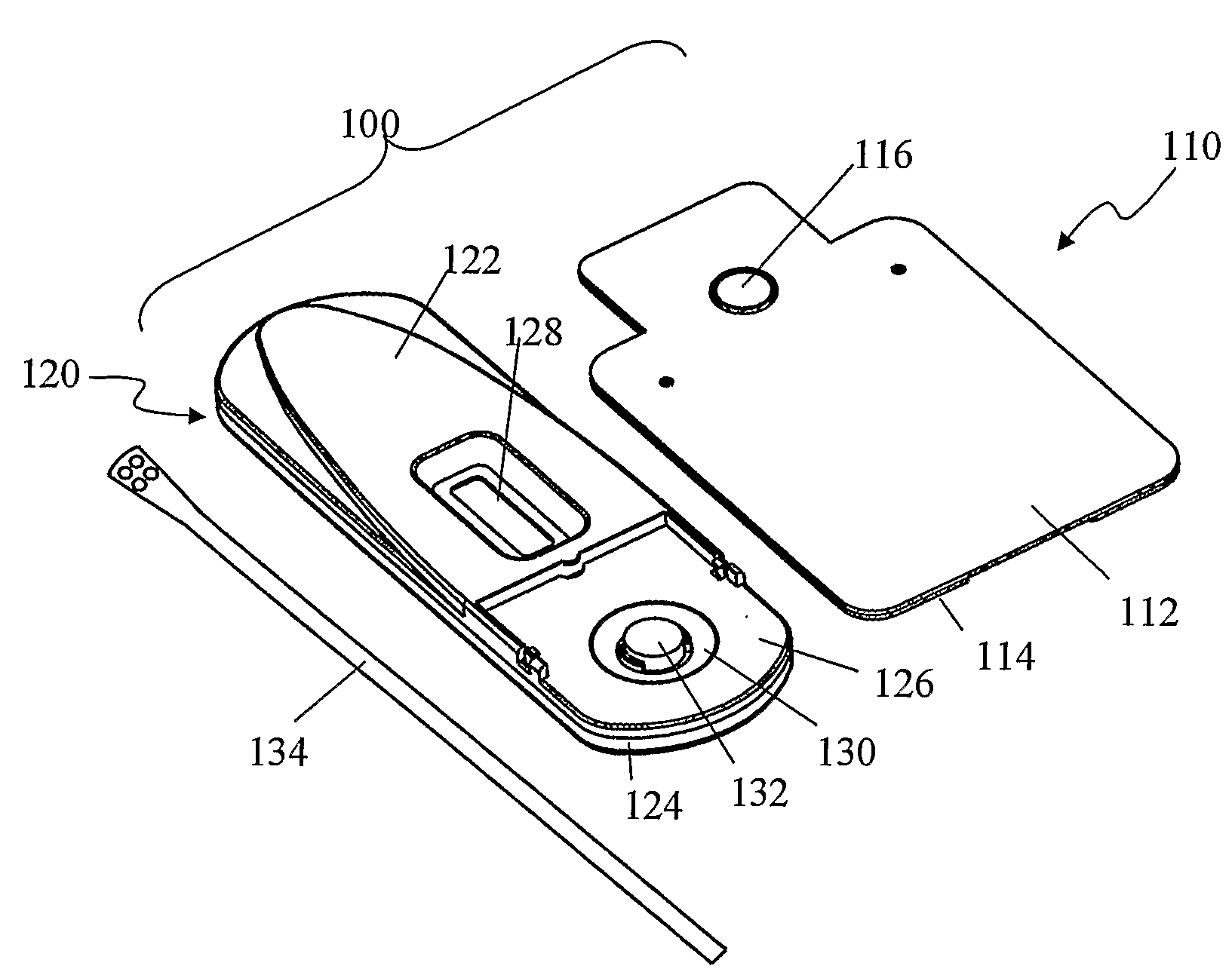
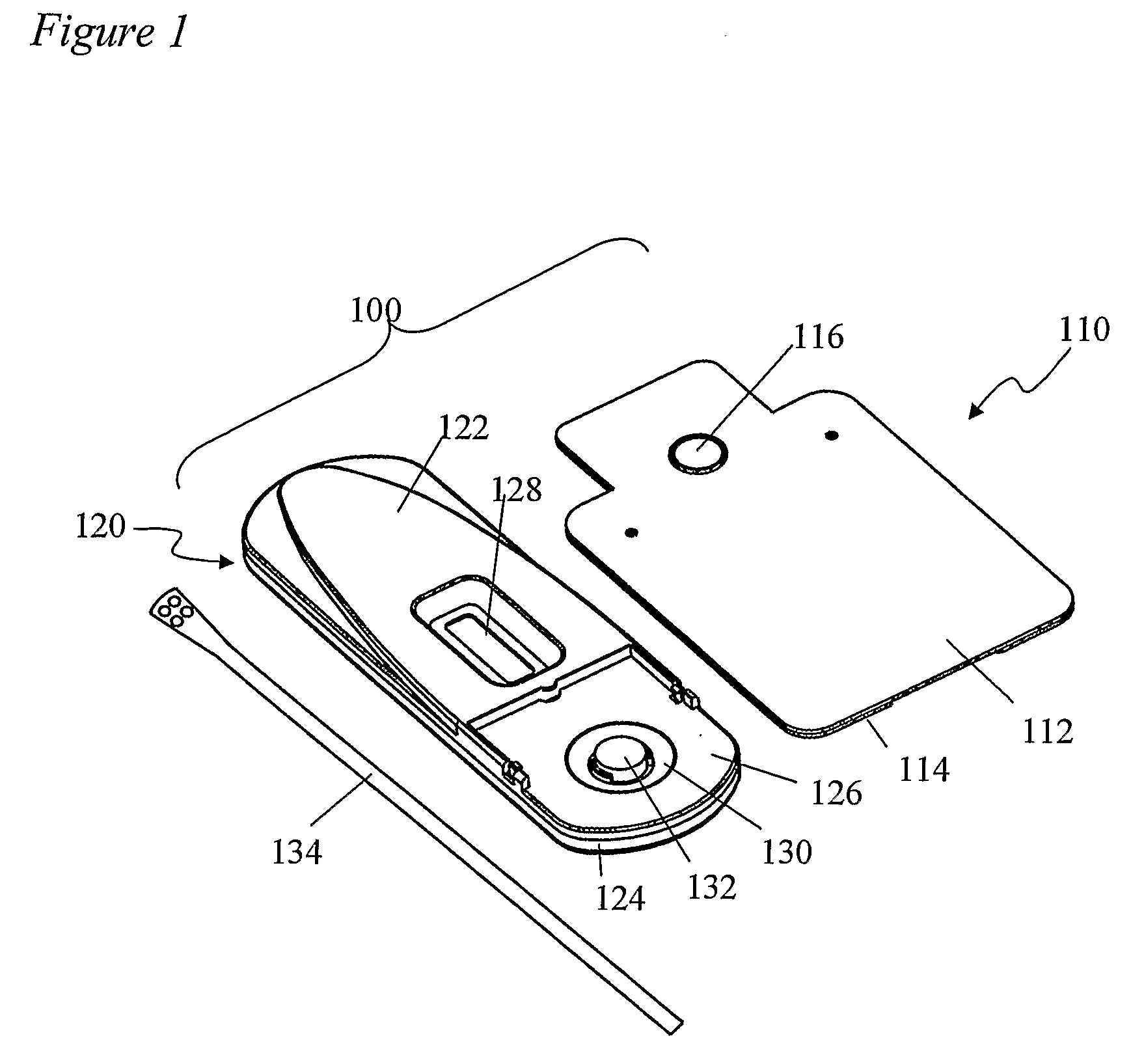
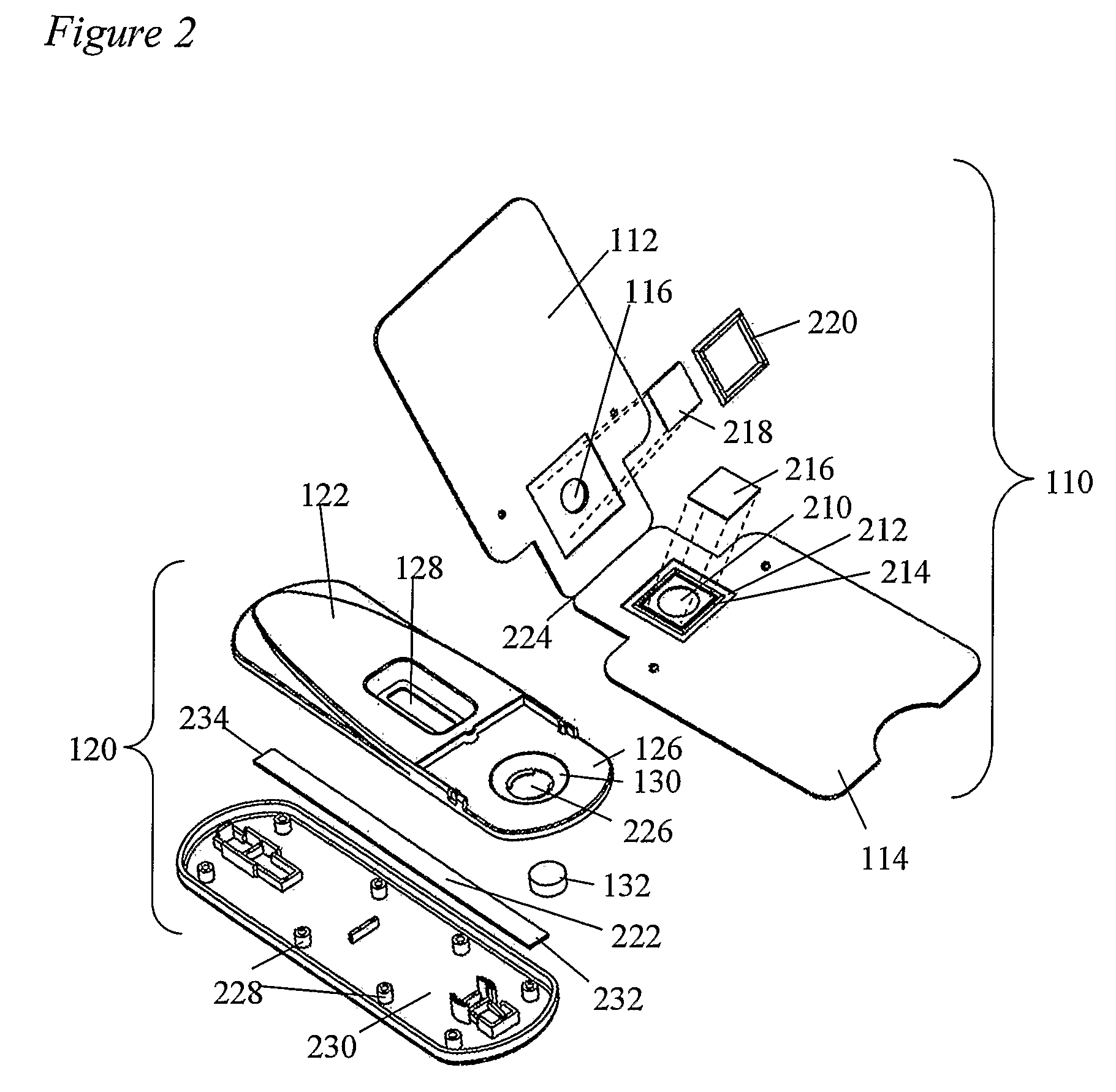
Devices and methods for sample collection and analysis
ActiveUS20060246598A1Reduce surface tensionRaise transfer toAnalysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorAnalyteSemi solid
The present invention provides devices, methods, and kits for the collection of a solid or semi-solid sample and analysis for the presence, absence, or quantity of an analyte. The invention provides a collection slide having a first card and a second card. The first card has a sample collection area. The first and second cards have orifices allowing the passage of fluid through the sample collection area, and the cards are hingeably connected to each other. The invention also provides an assay device having a housing with a test element, a results window, and a docking area for receiving and engaging the collection slide. In one embodiment the collection slide and device can be used to detect the presence of fecal occult blood (human hemoglobin) in a stool sample. Many other embodiments are described herein.
Owner:ABBOTT RAPID DIAGNOSTICS INT UNLTD
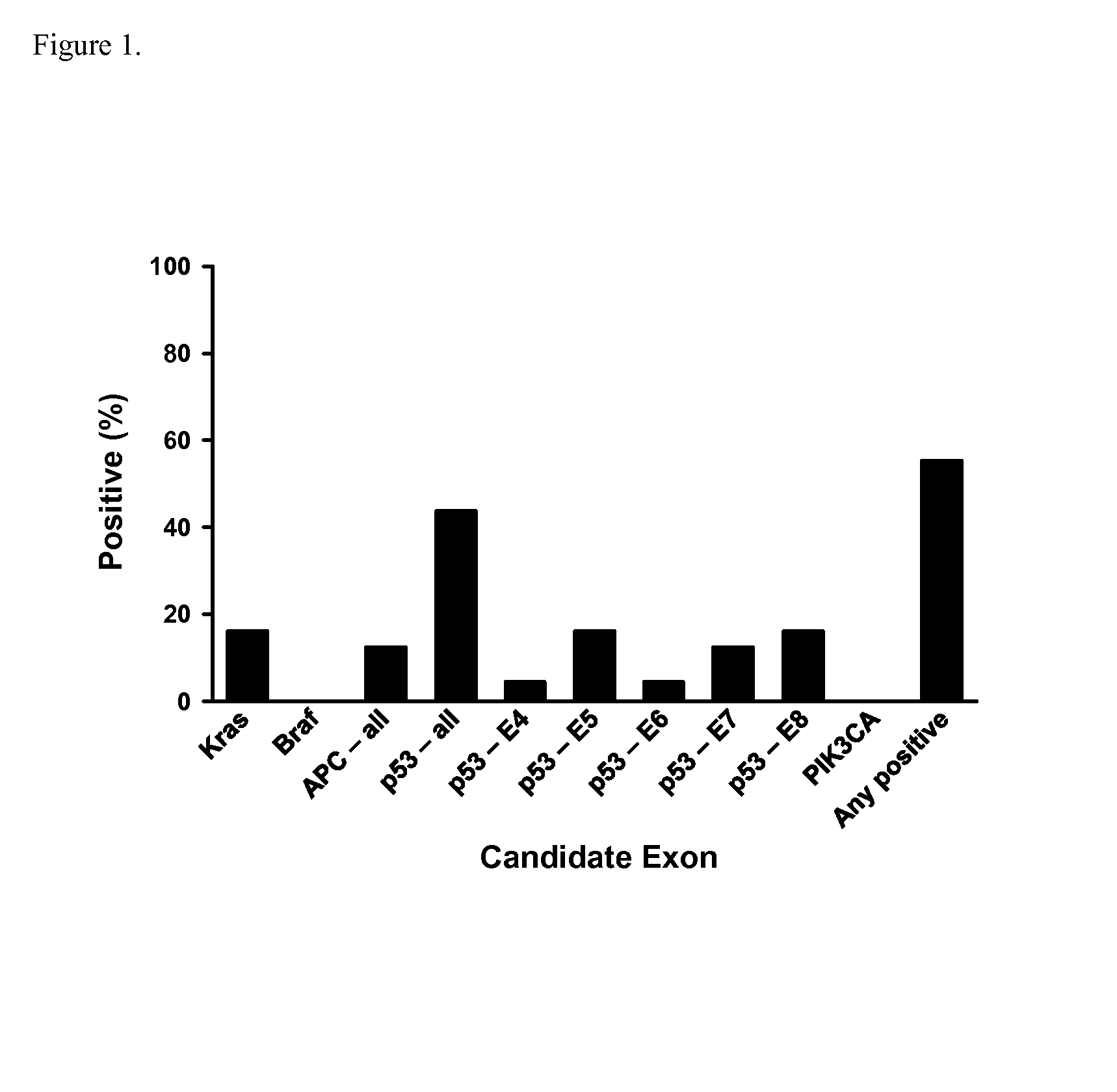
Methods and materials for detecting colorectal cancer and adenoma
InactiveUS20130012410A1High detectionHigh level of sensitivityMicrobiological testing/measurementLibrary screeningFOXE1Mammal
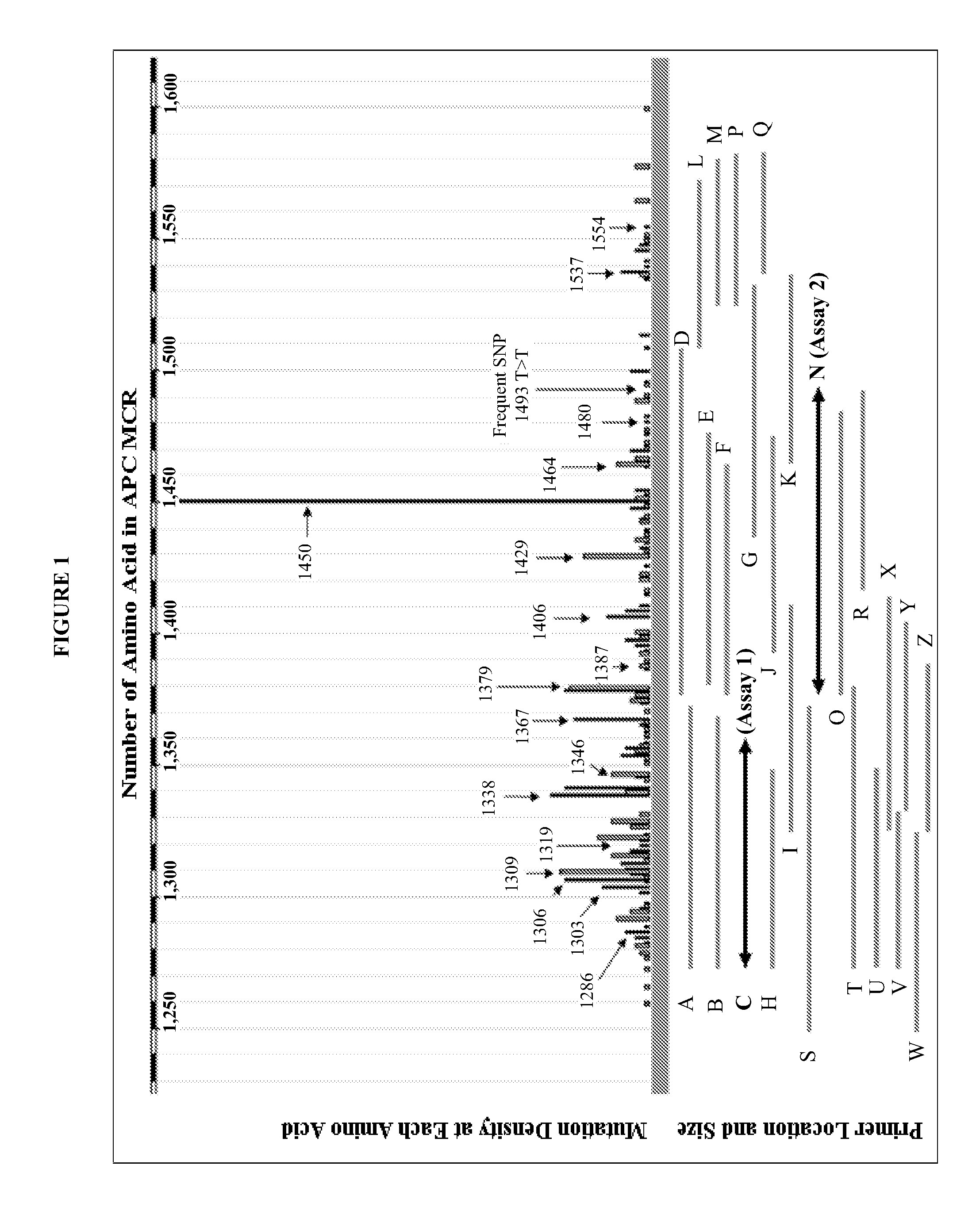
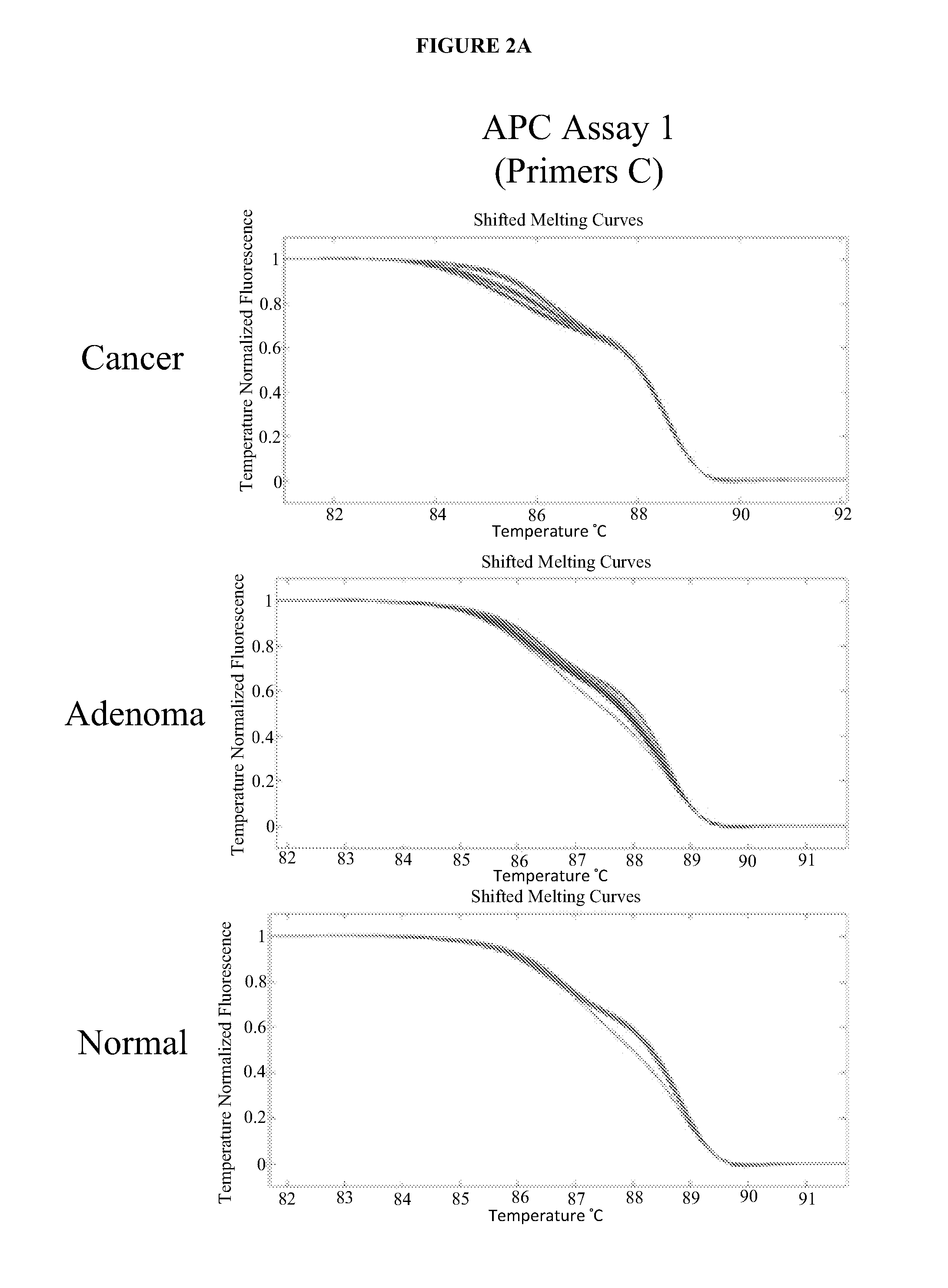
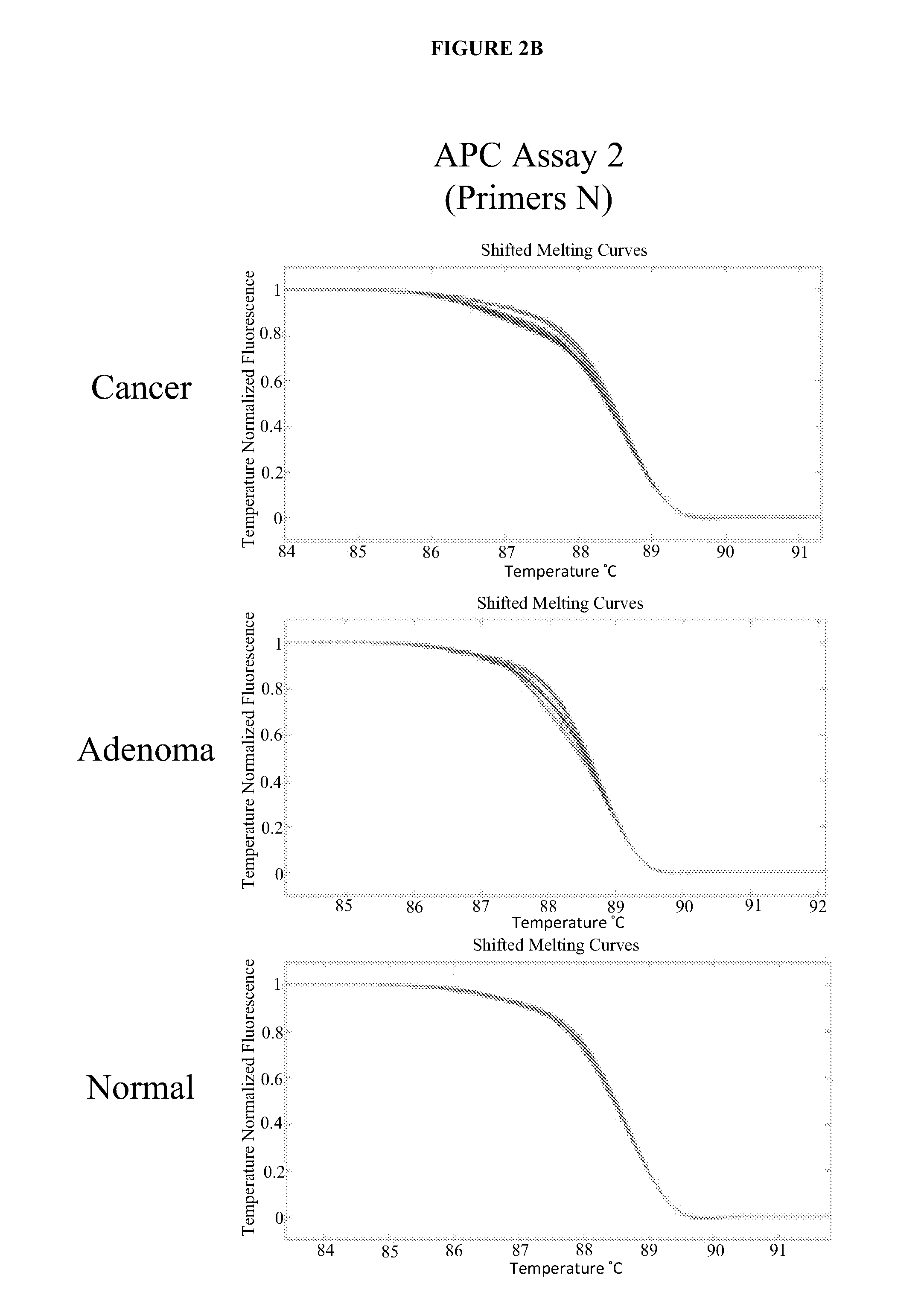
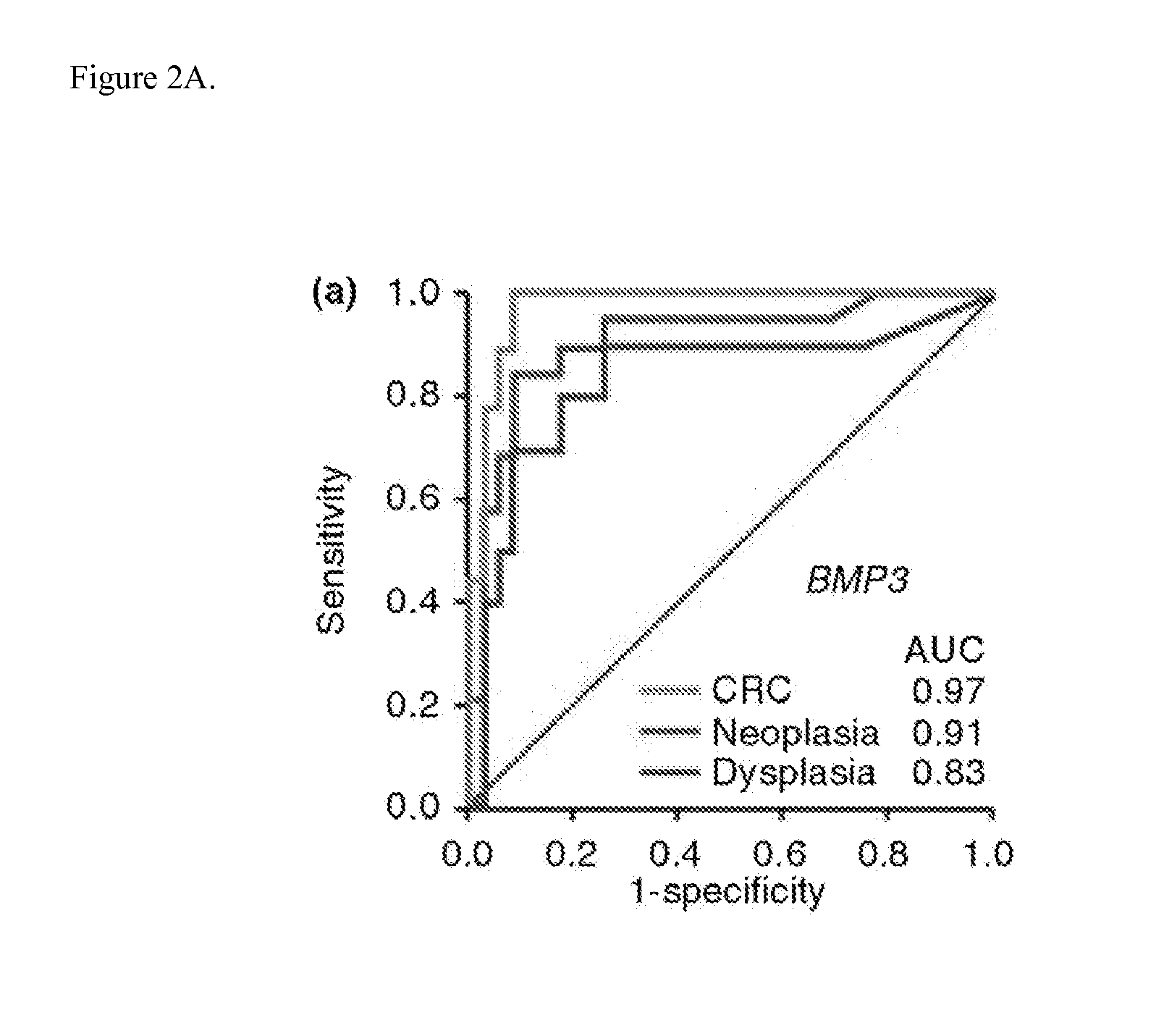
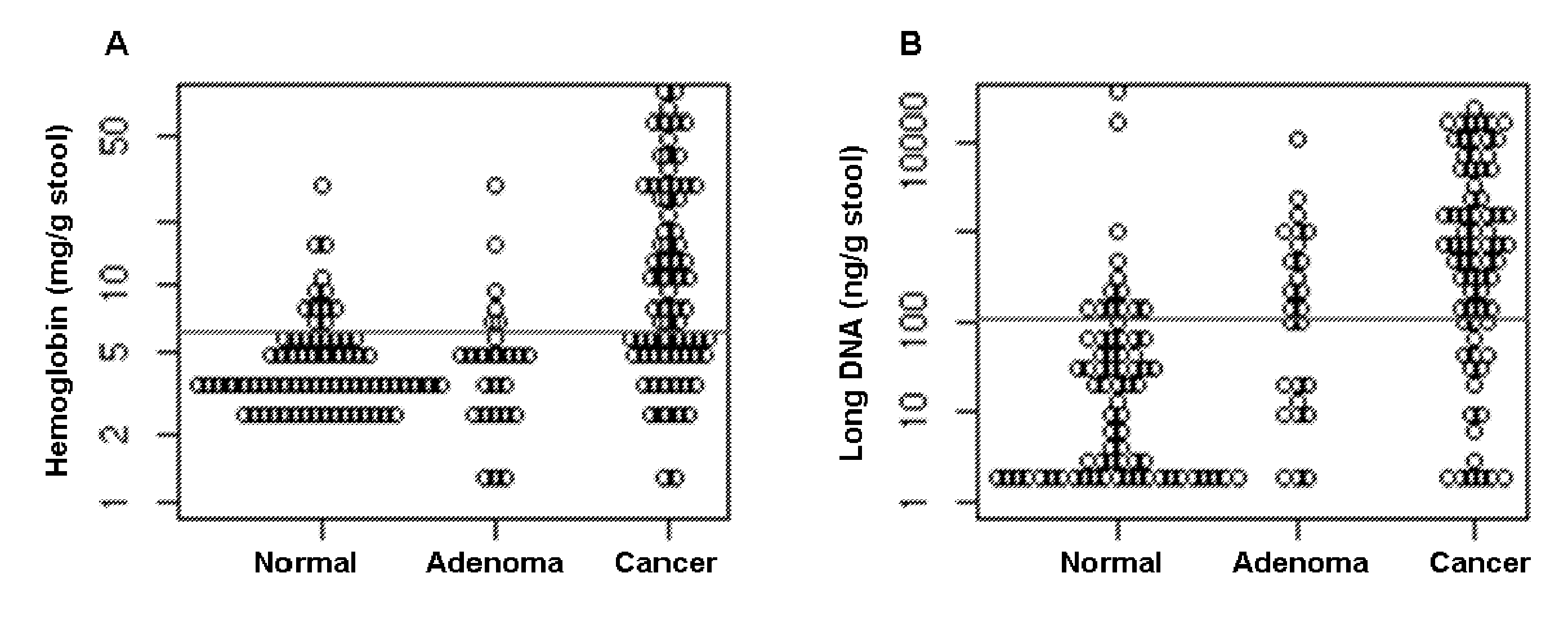
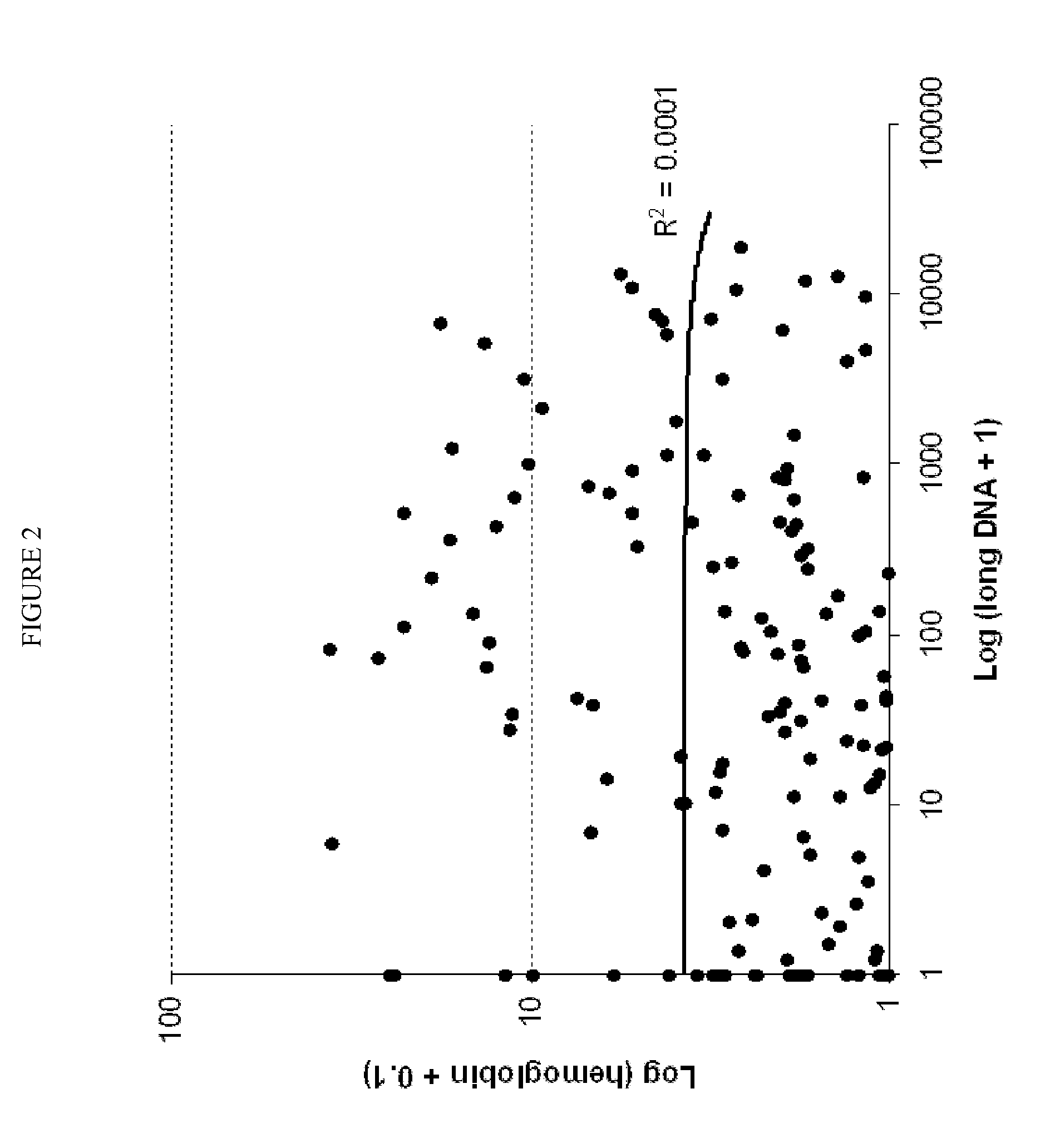
The present invention provides methods and materials related to the detection of colorectal neoplasm-specific markers (e.g., markers associated with colorectal cancer, markers associated with adenoma) in or associated with a subject's stool sample. In particular, the present invention provides methods and materials for identifying mammals (e.g., humans) having a colorectal neoplasm by detecting the presence and level of indicators of colorectal neoplasia such as, for example, long DNA (e.g., quantified by Alu PCR) and the presence and level of tumor-associated gene alterations (e.g., mutations in KRAS, APC, melanoma antigen gene, p53, BRAF, BAT26, PIK3CA) or epigenetic alterations (e.g., DNA methylation) (e.g., CpG methylation) (e.g., CpG methylation in coding or regulatory regions of bmp-3, bmp-4, SFRP2, vimentin, septin9, ALX4, EYA4, TFPI2, NDRG4, FOXE1) in DNA from a stool sample obtained from the mammal.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
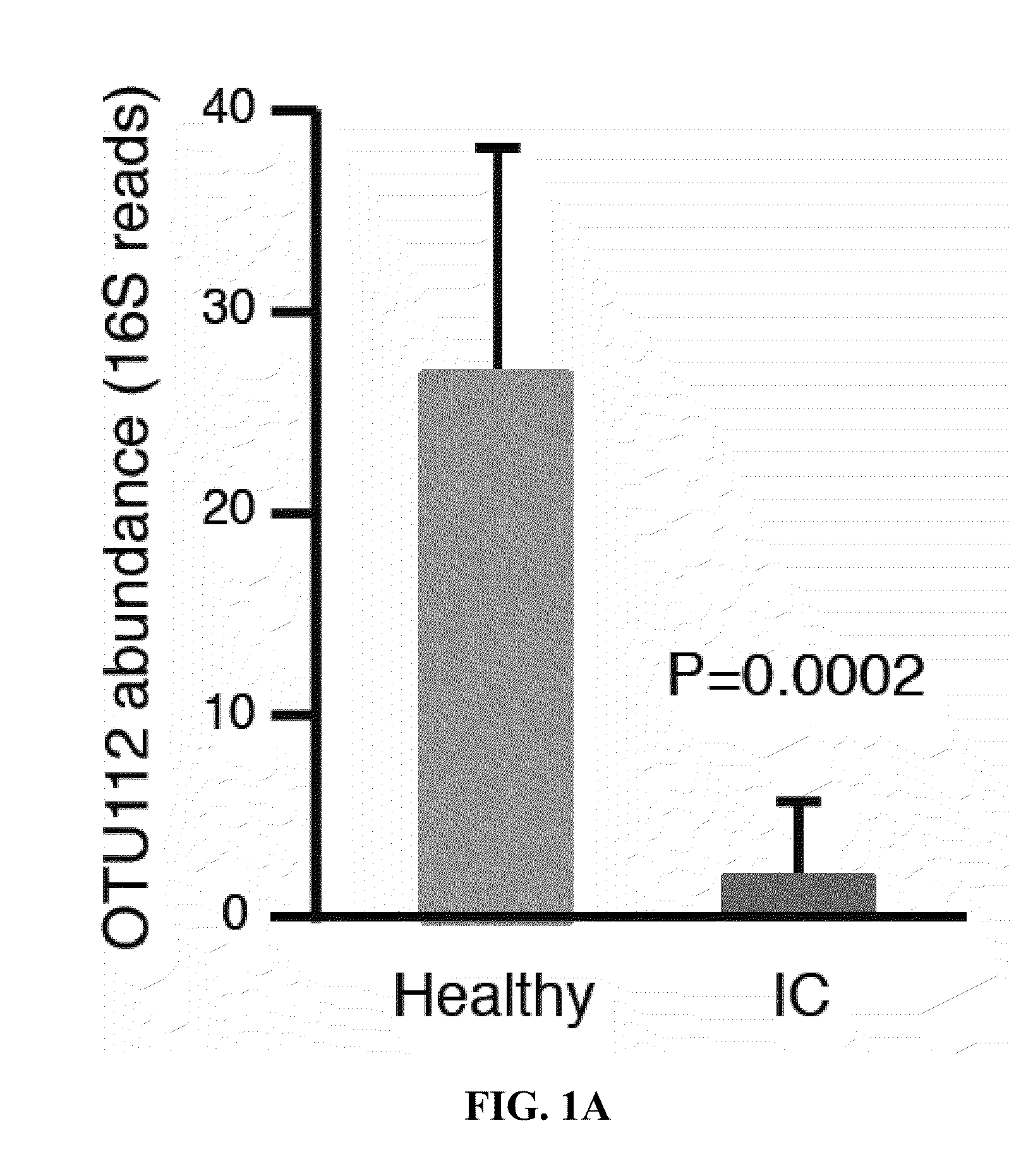
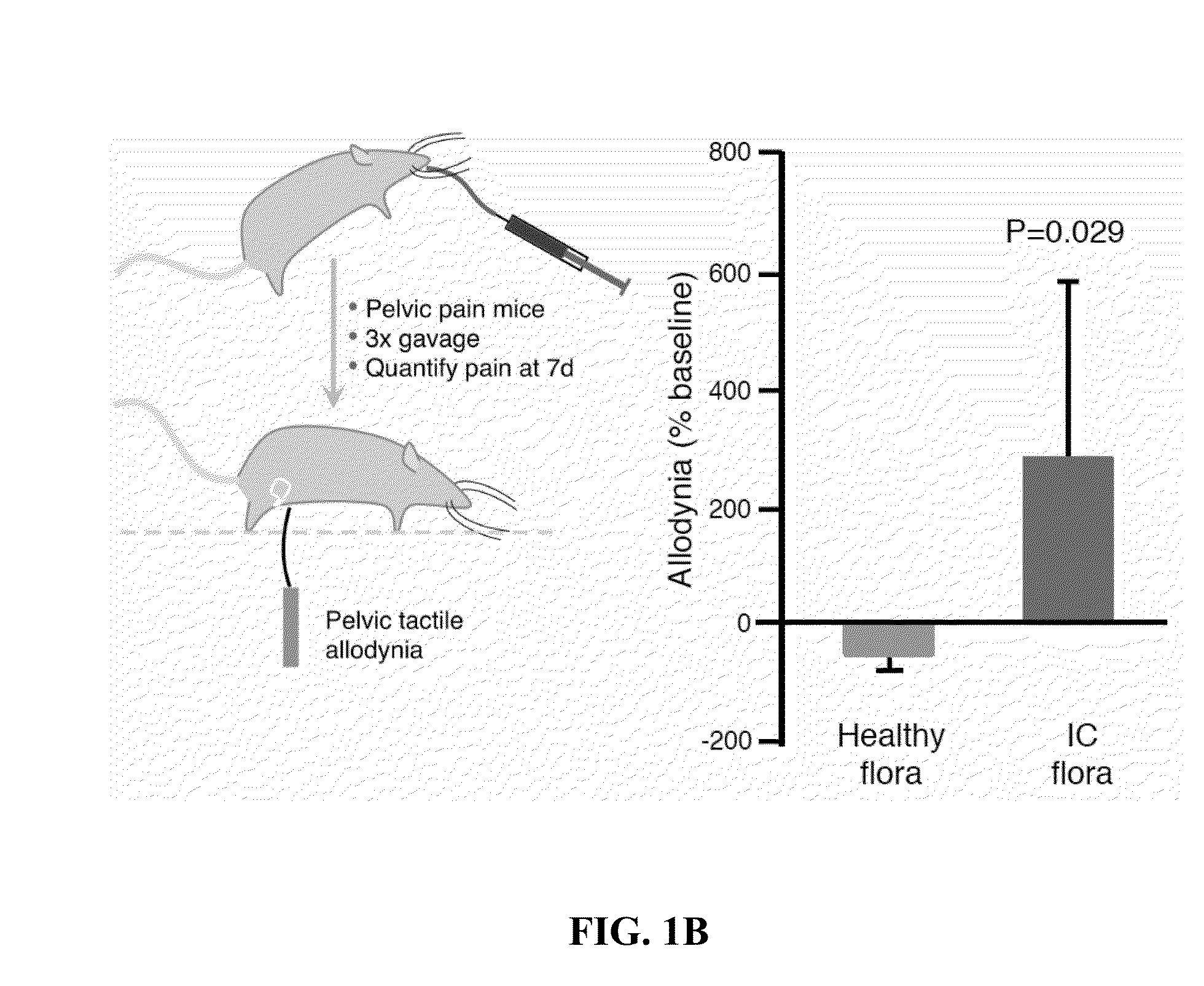
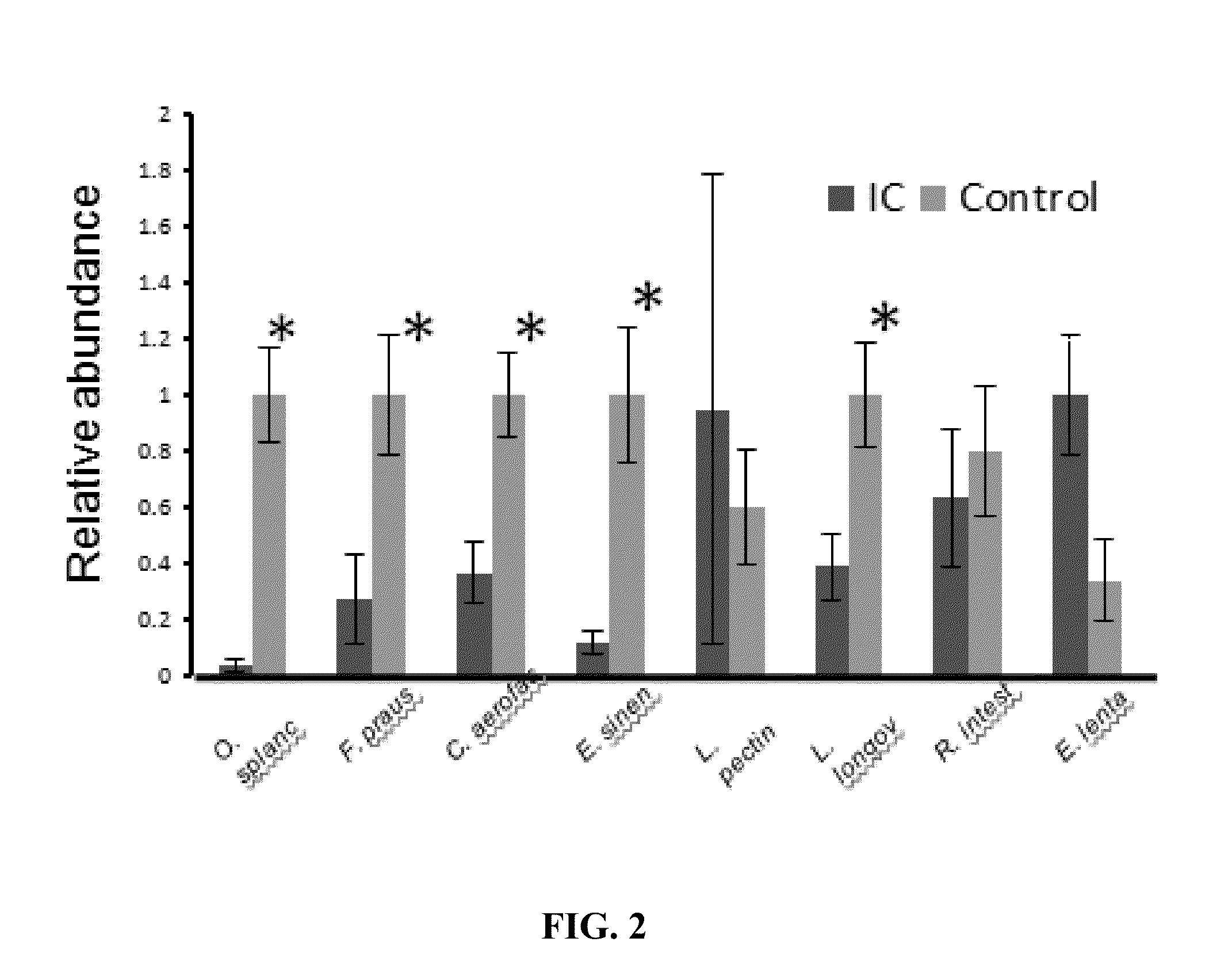
Altered microbiome of chronic pelvic pain
Provided herein are kits, compositions, and methods for diagnosing and treating interstitial cystitis (IC) and / or interstitial cystitis / bladder pain syndrome (IC / BPS) based on finding lower levels of certain bacteria in a subject's stool sample (e.g., O. splanchnicus, F. prausnitzii, C. aerofaciens, E. sinensis, L. longoviformis, and R. intestinalis). In certain embodiments, then present invention provides probiotic formulations containing live bacteria (e.g., from O. splanchnicus, F. prausnitzii, C. aerofaciens, E. sinensis, L. longoviformis, and R. intestinalis).
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
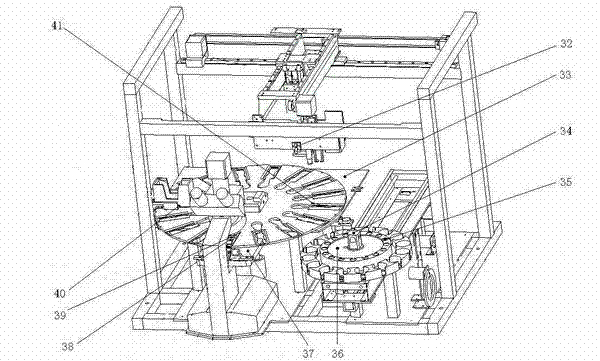
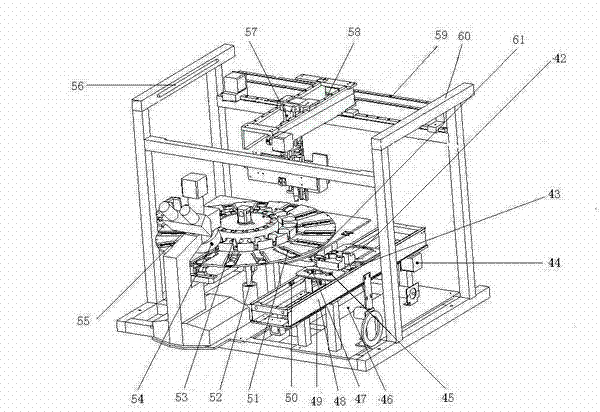
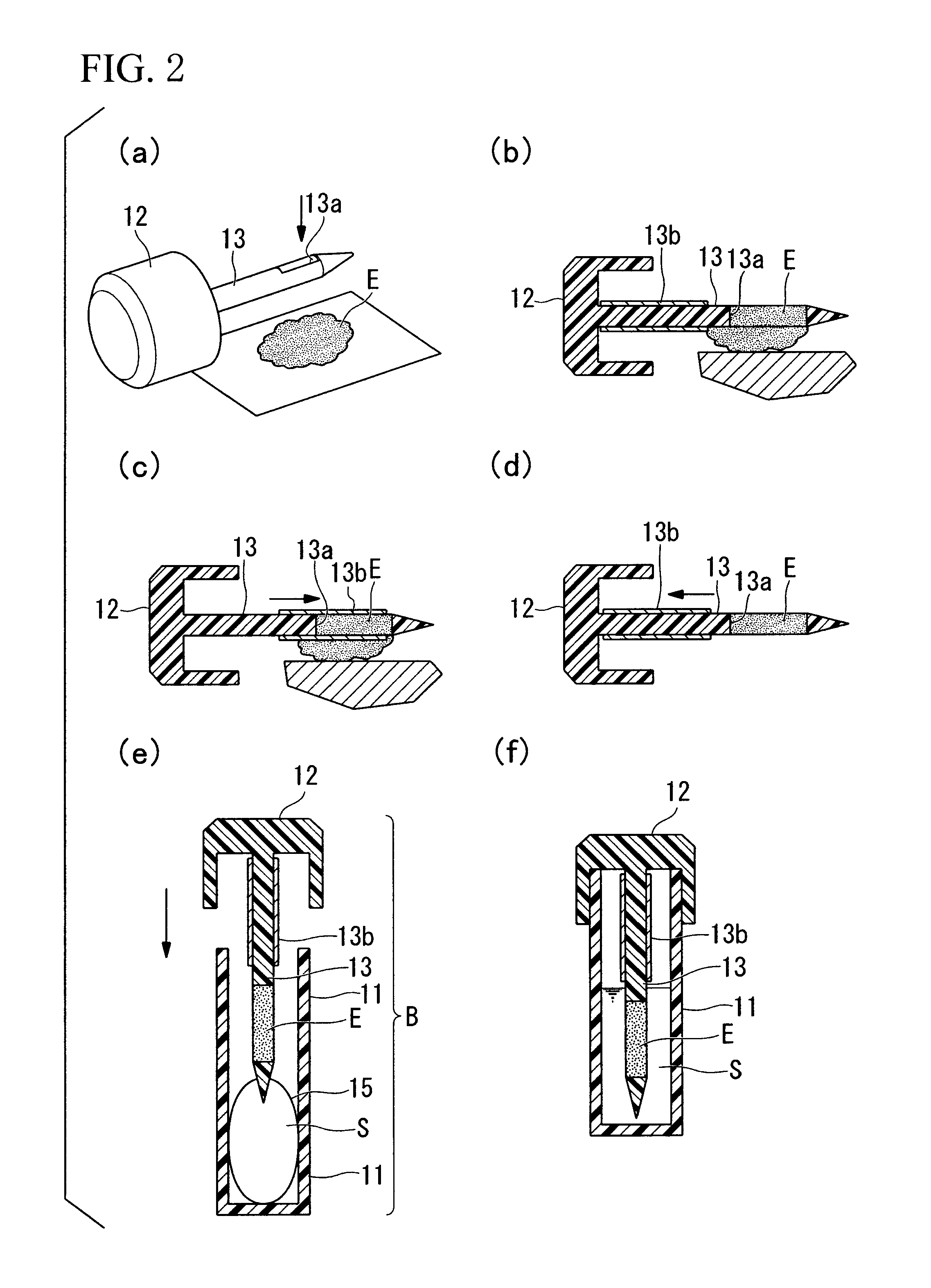
Automatic operating stool tester
ActiveCN102252968AClear viewUnobscuredMaterial analysis by optical meansNumerical controlSensory organ
The invention provides an automatic operating stool tester. The tester is used for carrying out automatic smear and microscopy on the stool and other human secretions and selectively preserving the image-text data. The tester is characterized in that a power-driven three-dimensional Cartesian coordinate manipulator is formed by X, Y and Z axes; an operating bottom plate is provided with an automatic sample injection slider device; an operating disk spindle core rod is movably connected with the operating bottom plate; an automatic slide adding device is fixed on a rack; a microscope is installed in the front of the rack; and an automatic slide push-out device is fixed on the microscope. The tester can automatically open the stool sample box, automatically put the slide in the box, automatically feel about the image via the liquid crystal display, move the slide for microscopy through numerical control, automatically print the locked image, automatically close the stool box, automatically recycle the waste articles, automatically carry out ultraviolet disinfection, completely prevent the operators from closely contacting the stool, greatly improve the sensory organ comfort in the testing process, greatly enhance the operation safety and repeatability, introduce the test report with images and texts to the stool routine and provide most direct basis for clinical diagnosis.
Owner:襄阳市科瑞杰医疗器械有限公司
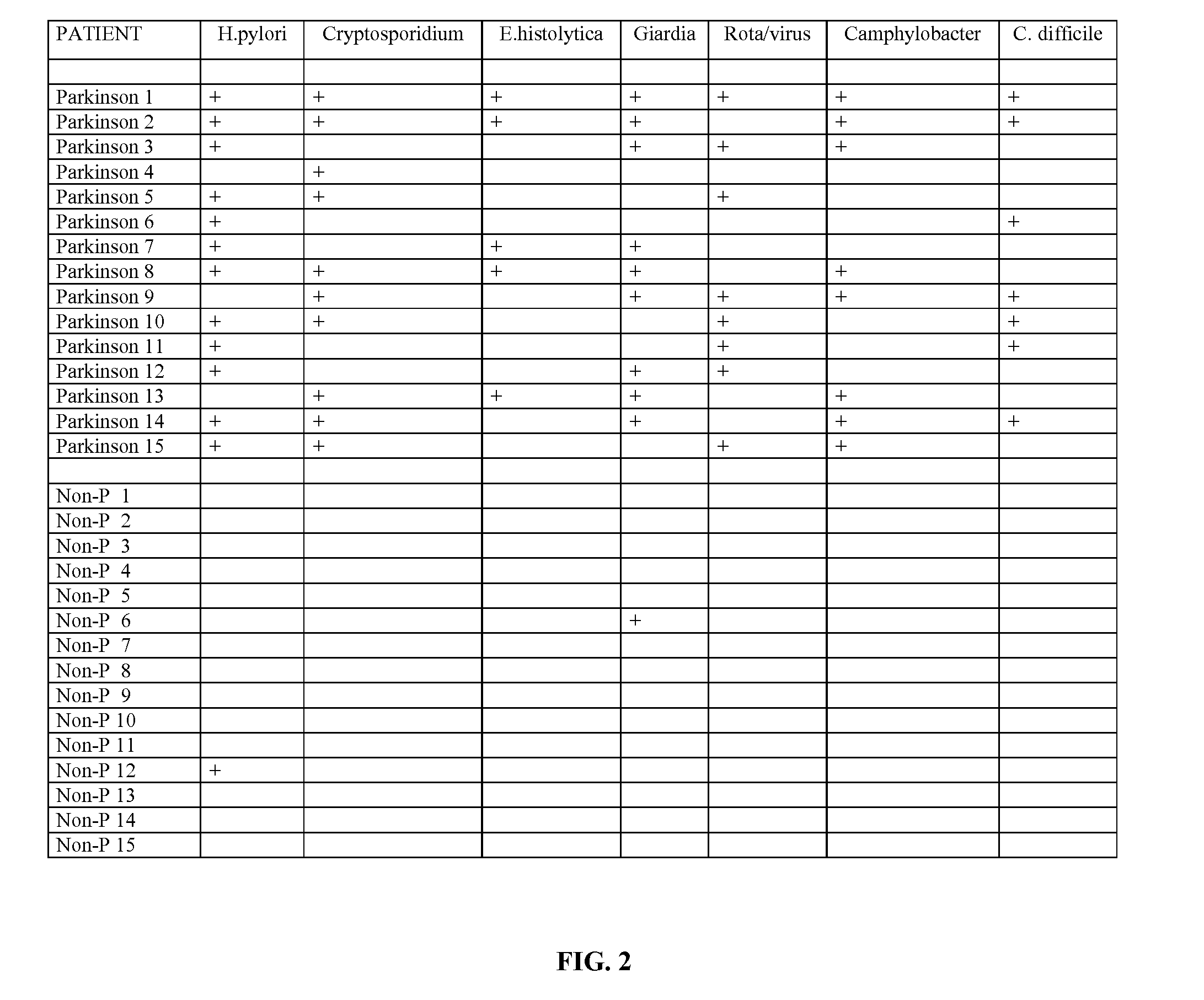
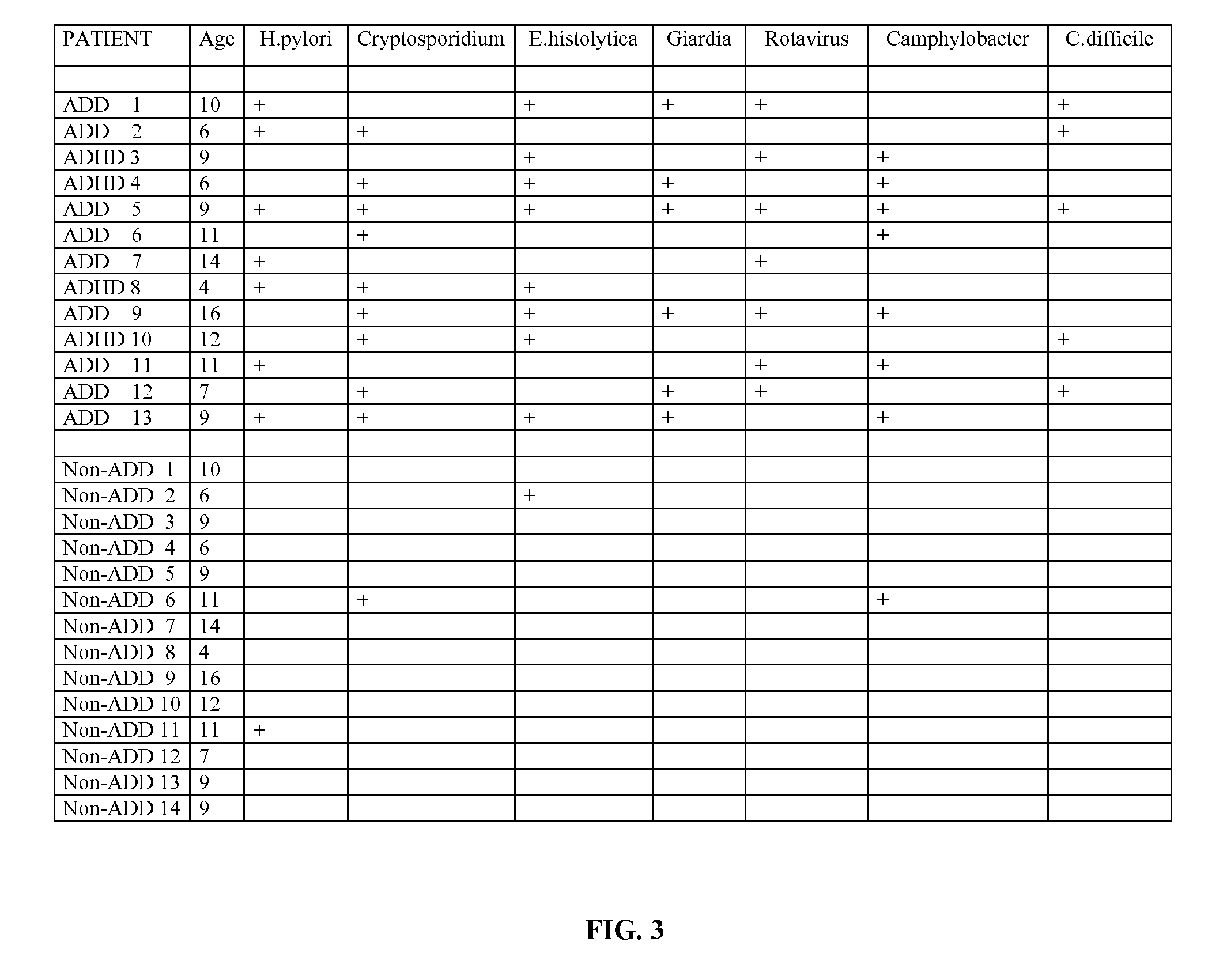
Methods for Diagnosing Pervasive Development Disorders, Dysautonomia and Other Neurological Conditions
Methods for aiding in the diagnosis of disorders including, but not limited to, PDDs (Pervasive Development Disorders), Dysautonomic disorders, Parkinson's disease and SIDS (Sudden Infant Death Syndrome). In one aspect, a diagnosis method comprises analyzing a stool sample of an individual for the presence of a biological marker (or marker compound) comprising one or more pathogens, which provides an indication of whether the individual has, or can develop, a disorder including, but not limited to, a PDD, Dysautonomia, Parkinsons disease and SIDS. Preferably, the presence of one or more pathogens is determined using a stool immunoassay to determine the presence of antigens in a stool sample, wherein such antigens are associated with one or more pathogens including, but not limited to, Giardia, Cryptosporidium, E. histolytica, C. difficile, Adenovirus, Rotavirus or H. pylori.
Owner:CUREMARK
Devices and methods for sample collection and analysis
InactiveUS20070092401A1Easy transferImprove fluid flowAnalysis using chemical indicatorsSamplingAnalyteSemi solid
The present invention provides devices, methods, and kits for the collection of a solid or semi-solid sample and analysis for the presence, absence, or quantity of an analyte. The invention provides an assay device having a housing containing a test element, a results window, and a docking area for receiving and engaging a sample collection slide. The docking area has a sample receiving orifice with one or more fluid transfer structures. In one embodiment the collection slide and device can be used to detect the presence of fecal occult blood (human hemoglobin) in a stool sample. Many other embodiments are described herein.
Owner:ACON BIOTECH (HANGZHOU) CO LTD
Device for stool sampling and occult blood self-detection
ActiveCN102798542ANo leakageWill not cause leakageWithdrawing sample devicesBiological testingStool occult bloodEmergency medicine
The invention provides a device for stool sampling and occult blood self-detection. The device comprises a cover, a main container, a stool sampling rod, a stool sampling rod guide channel, a stool dissolution tank, a stool liquid filter pipe, a communication pipe and a test strip detection tank. The stool sampling rod is fixed to the cover or is not fixed to the cover. A top end of the stool sampling rod is fixed to a top wall of the interior of the cover. The stool sampling rod is inserted into the stool sampling rod guide channel. The bottom of the stool liquid filter pipe is connected to the bottom of the test strip detection tank by the communication pipe. The device realizes quantitative acquisition, dissolution, filtration and immune test strip detection of a stool sample, does not produce liquid leakage in storage, conveying and detection, realizes stool sampling and occult blood self-detection in a single cycle, avoids stool direct viewing, is convenient for carrying, operation and cleaning, and is suitable for stool occult blood self-detection carried out by an average adult at any time or place. The device has a reasonable design, a simple structure and a low production cost, is convenient for operation, avoids stool liquid opening, realizes one-step detection after stool sampling, and is clean and safe.
Owner:HANGZHOU NEW HORIZON HEALTH TECH CO LTD
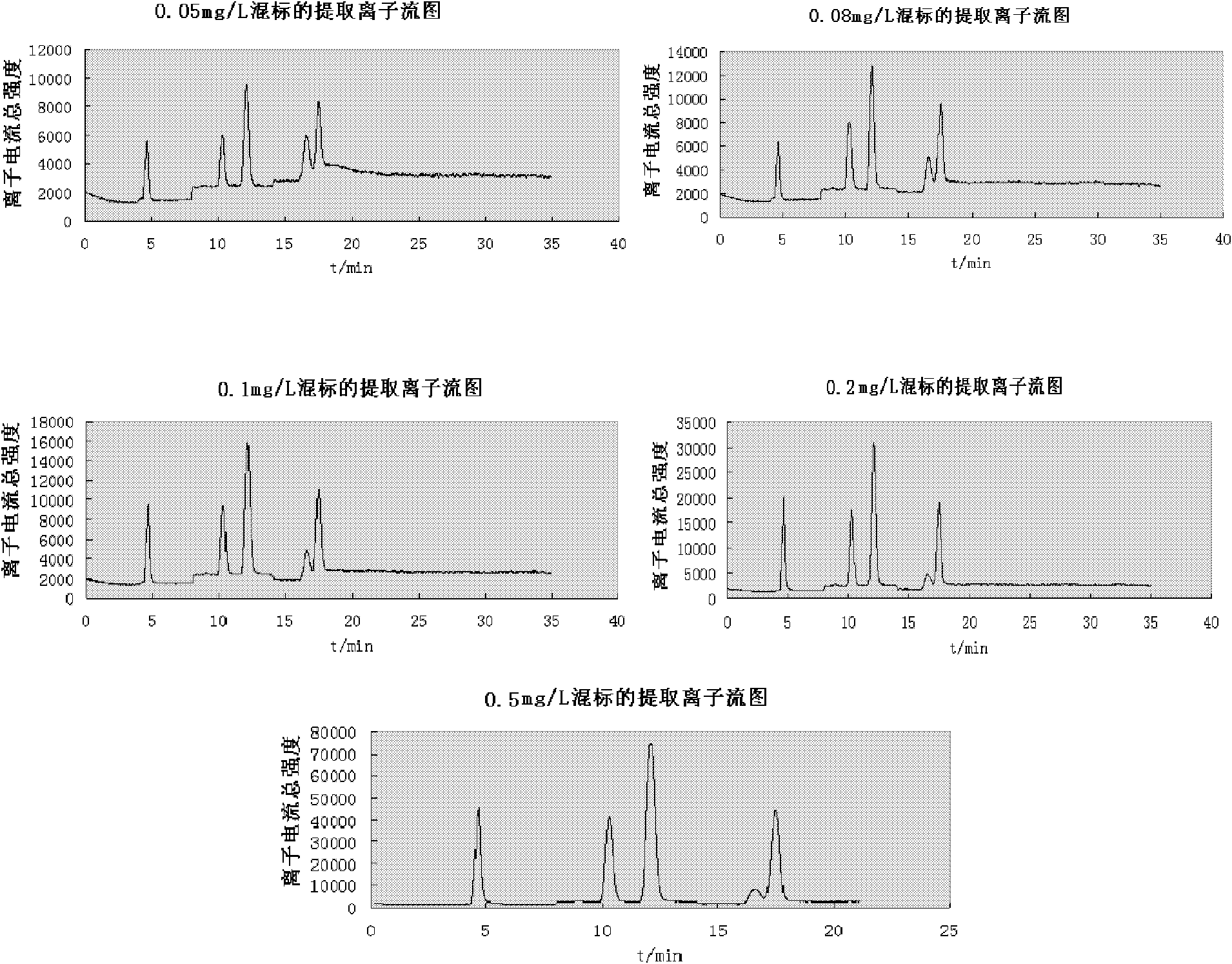
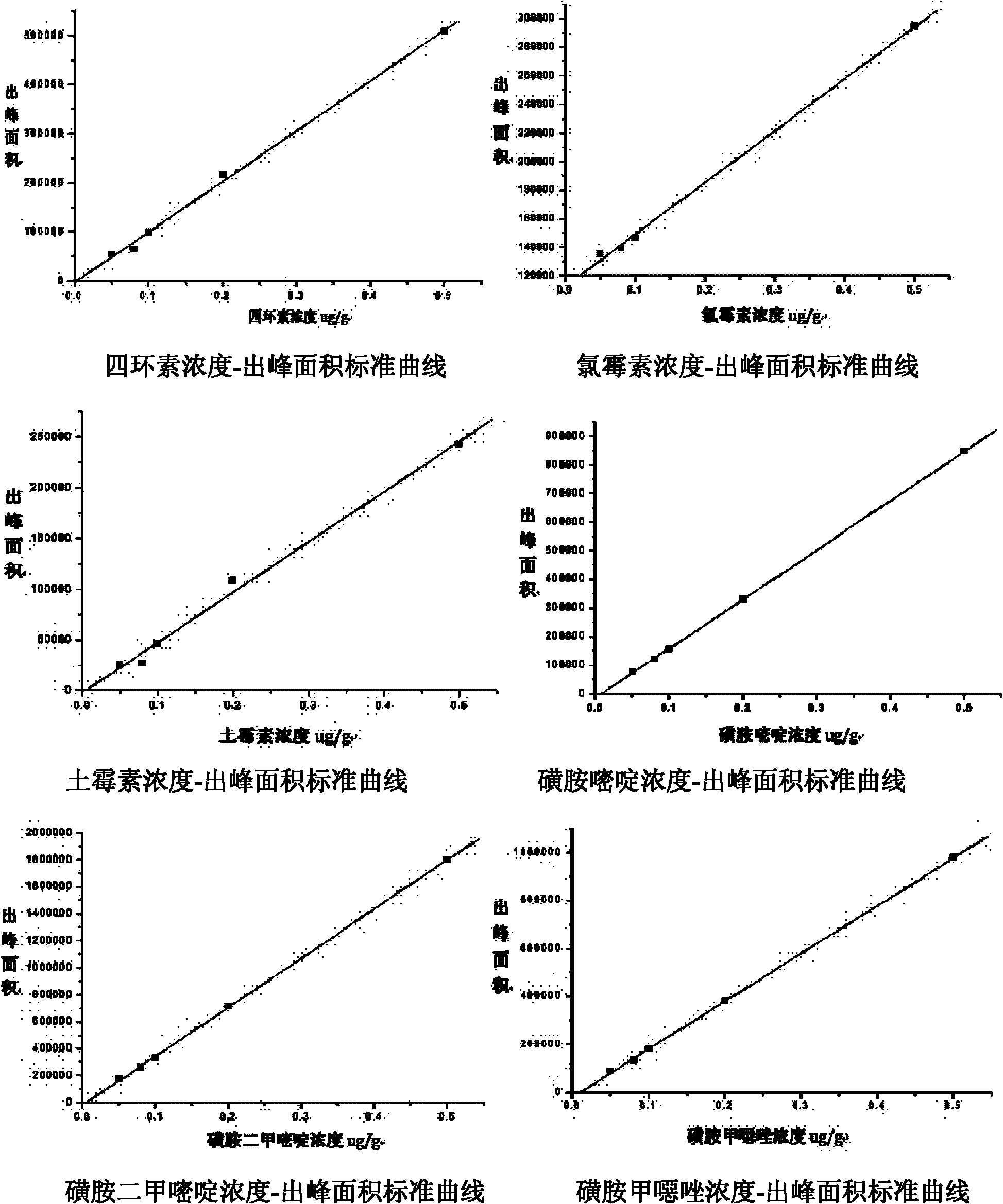
Simultaneous Quantitative Determination of Multiple Antibiotics at One Time
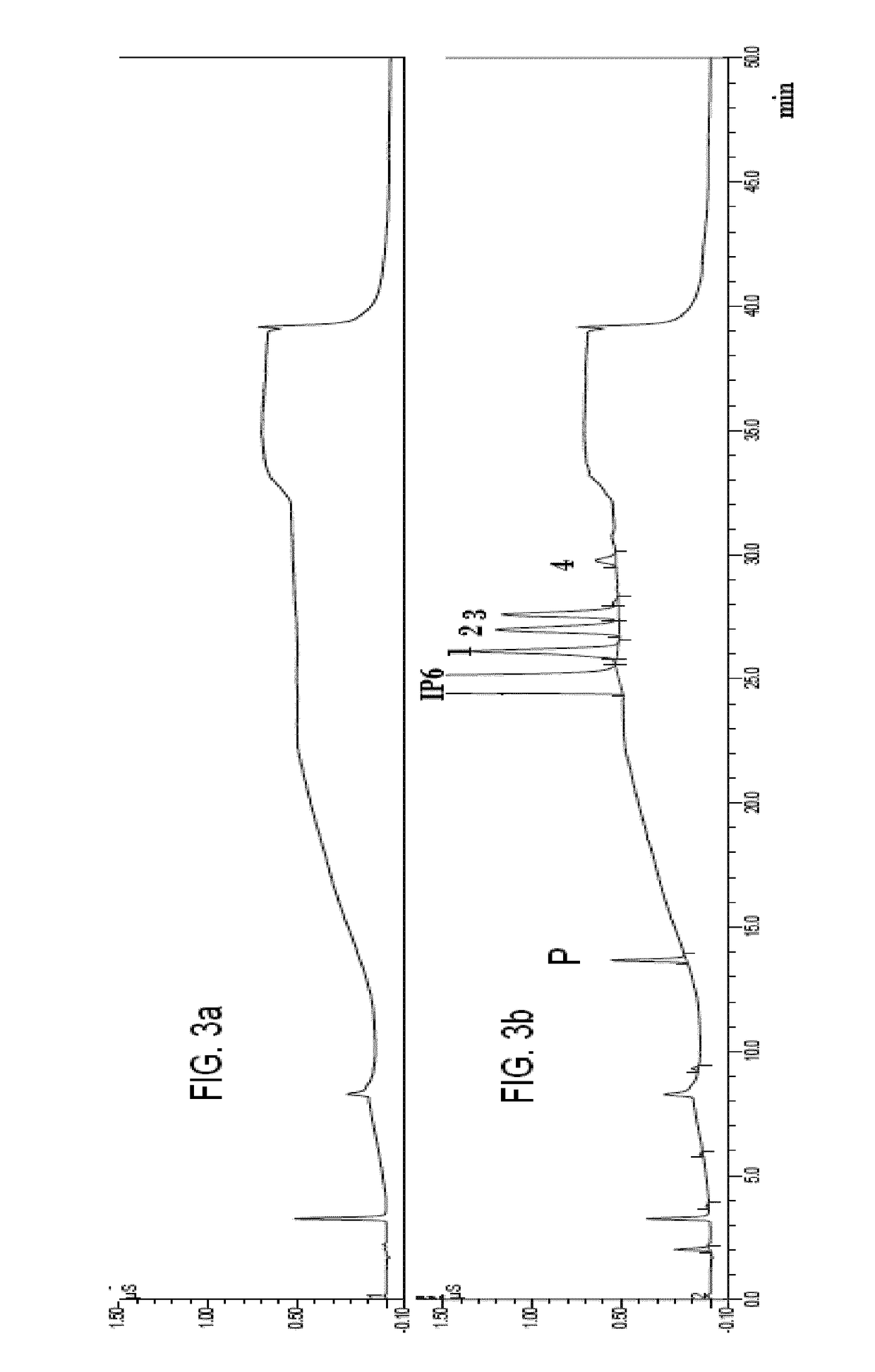
InactiveCN102269747ASolve the problem of difficult separation of peaks with similar peak timesAvoid errorsComponent separationAnalysis dataLow ionic strength
The present invention relates to a method for one-time simultaneous quantitative determination of multiple antibiotics, comprising: (1) preparing a sample filtrate; (2) setting liquid chromatography conditions and mass spectrometry conditions; (3) using the above liquid chromatography conditions and mass spectrometry conditions to determine For the sample filtrate prepared in step (1), analyze the data to determine the type and content of antibiotics in the analyzed sample. The present invention can simultaneously detect multiple antibiotics in one experiment and quantify the detected antibiotics at the same time. The combination of liquid chromatography-mass spectrometry not only solves the problem of difficult separation of peaks with similar peak times in liquid chromatography, but also overcomes the The error caused by the inconspicuous peak of low ion current intensity in the mass spectrum is eliminated; the invention can be widely used in the detection and quantitative analysis of antibiotics in various samples such as feces, soil, water, food, and the like.
Owner:DONGHUA UNIV
Methods and materials for noninvasive detection of colorectal neoplasia associated with inflammatory bowel disease
InactiveUS20130244235A1Improve discriminationSensitive highMicrobiological testing/measurementDNA methylationMammal
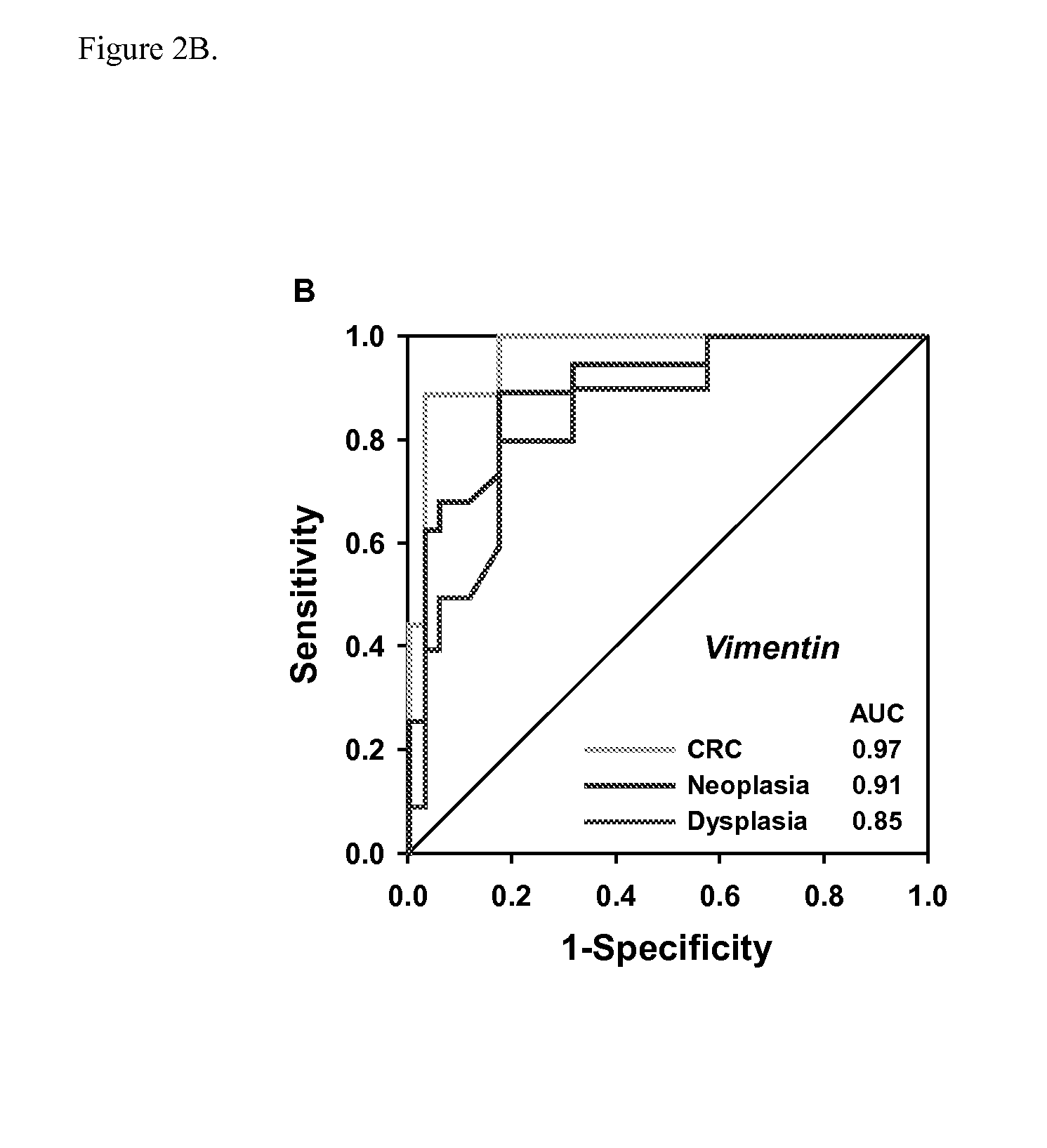
The present invention provides methods and materials related to the detection of colorectal neoplasia (CRN) associated with inflammatory bowel disease (IBD). The present invention provides markers specific for colorectal neoplasia associated with inflammatory bowel disease in or associated with a subject's stool sample. In particular, the present invention provides methods and materials for identifying mammals (e.g., humans) having colorectal neoplasia associated with inflammatory bowel disease by detecting the presence and level of indicators of colorectal neoplasia such as, for example, epigenetic alterations (e.g., DNA methylation) (e.g., CpG methylation) (e.g., CpG methylation in coding or regulatory regions of BMP3, NDRG4, vimentin, EYA4) in DNA from a stool sample obtained from the mammal.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
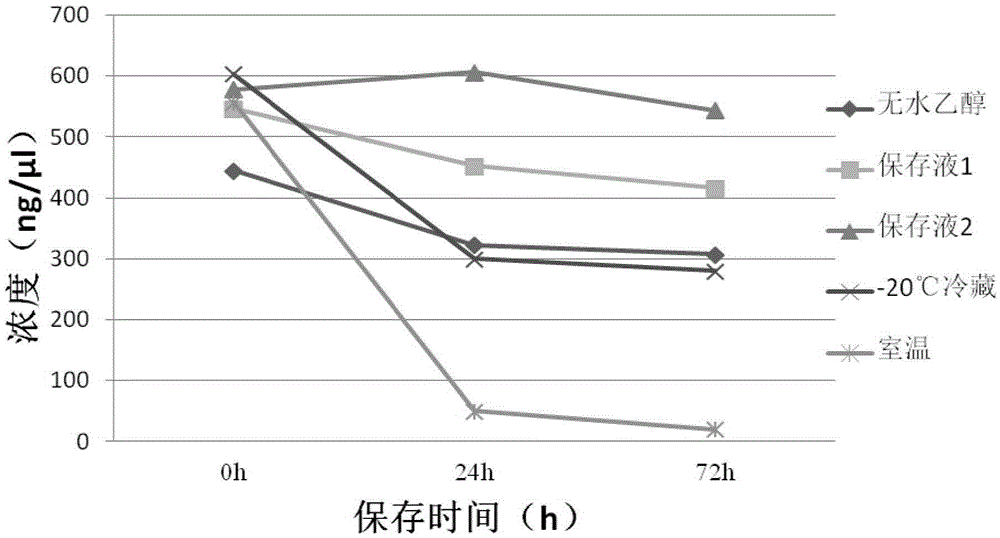
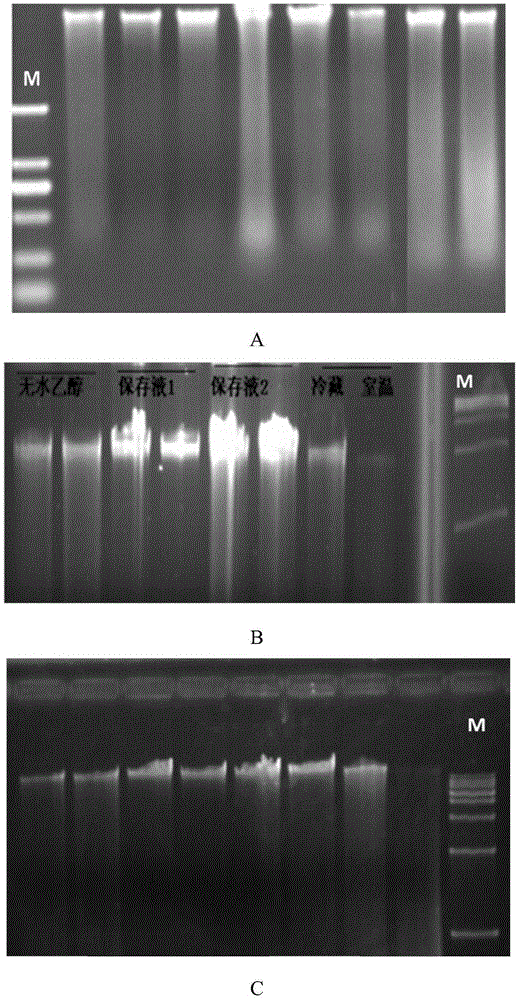
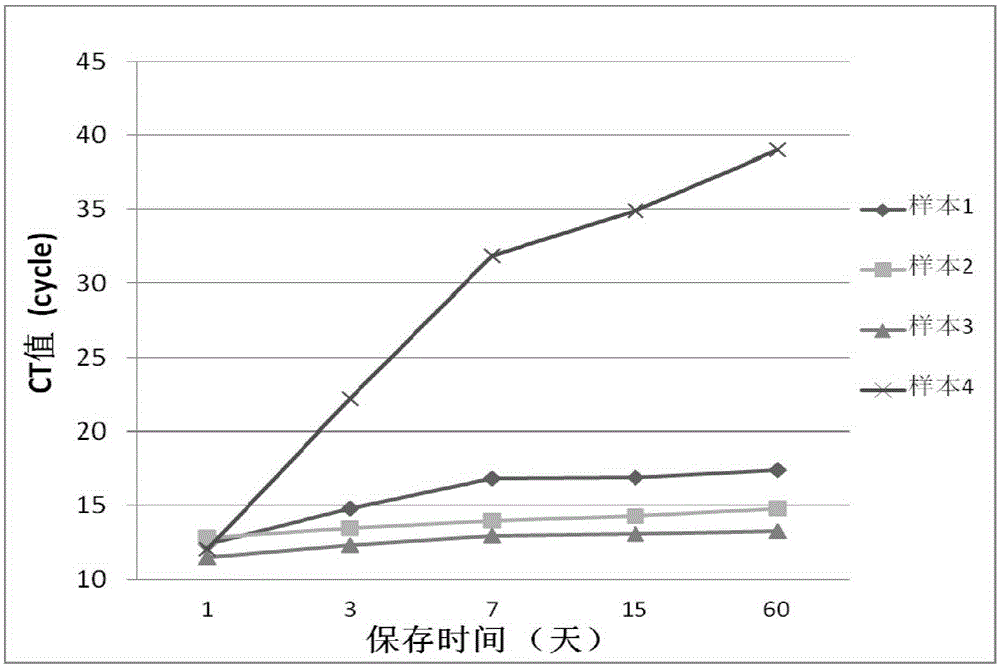
Stool sample preservation liquid, preparation method and application thereof
InactiveCN106596211AEasy to manufactureLow costPreparing sample for investigationIonic strengthAdjuvant
The invention discloses a stool sample preservation liquid, a preparation method and application thereof. Specifically, the stool sample preservation liquid contains: 45 vol%-60 vol% of a fixing agent; 0.1 mass%-15 mass% of a fixing agent adjuvant; 0.01 mass%-1.5 mass% of an anticoagulant; 10 vol%-15 vol% of a buffer solution; 0.01 mass%-1 mass% of an ionic strength maintenance agent; 0.01 vol%-0.5 vol% of a nonionic detergent; and the balance water. The stool sample preservation liquid provided by the invention has the characteristics of easy preparation and low cost, can preserve stool samples at room temperature, also can effectively inhibit DNA degradation and cell damage of stool samples, maintain cell shape, and can preserve samples for a long time, and the preserved samples have high qualification rate during reutilization.
Owner:SHENZHEN HUADA GENE INST
Devices and methods for sample collection and analysis
InactiveUS20090117660A1Reduce surface tensionRaise transfer toAnalysis using chemical indicatorsVaccination/ovulation diagnosticsAnalyteSemi solid
The present invention provides devices, methods, and kits for the collection of a solid or semi-solid sample and analysis for the presence, absence, or quantity of an analyte. The invention provides a collection slide having a first card and a second card. The first card has a sample collection area. The first and second cards have orifices allowing the passage of fluid through the sample collection area, and the cards are hingeably connected to each other. The invention also provides an assay device having a housing with a test element, a results window, and a docking area for receiving and engaging the collection slide. In one embodiment the collection slide and device can be used to detect the presence of fecal occult blood (human hemoglobin) in a stool sample. Many other embodiments are described herein.
Owner:INVERNESS SWITZERLAND GMBH
Method for determining location of gastrointestinal bleeding
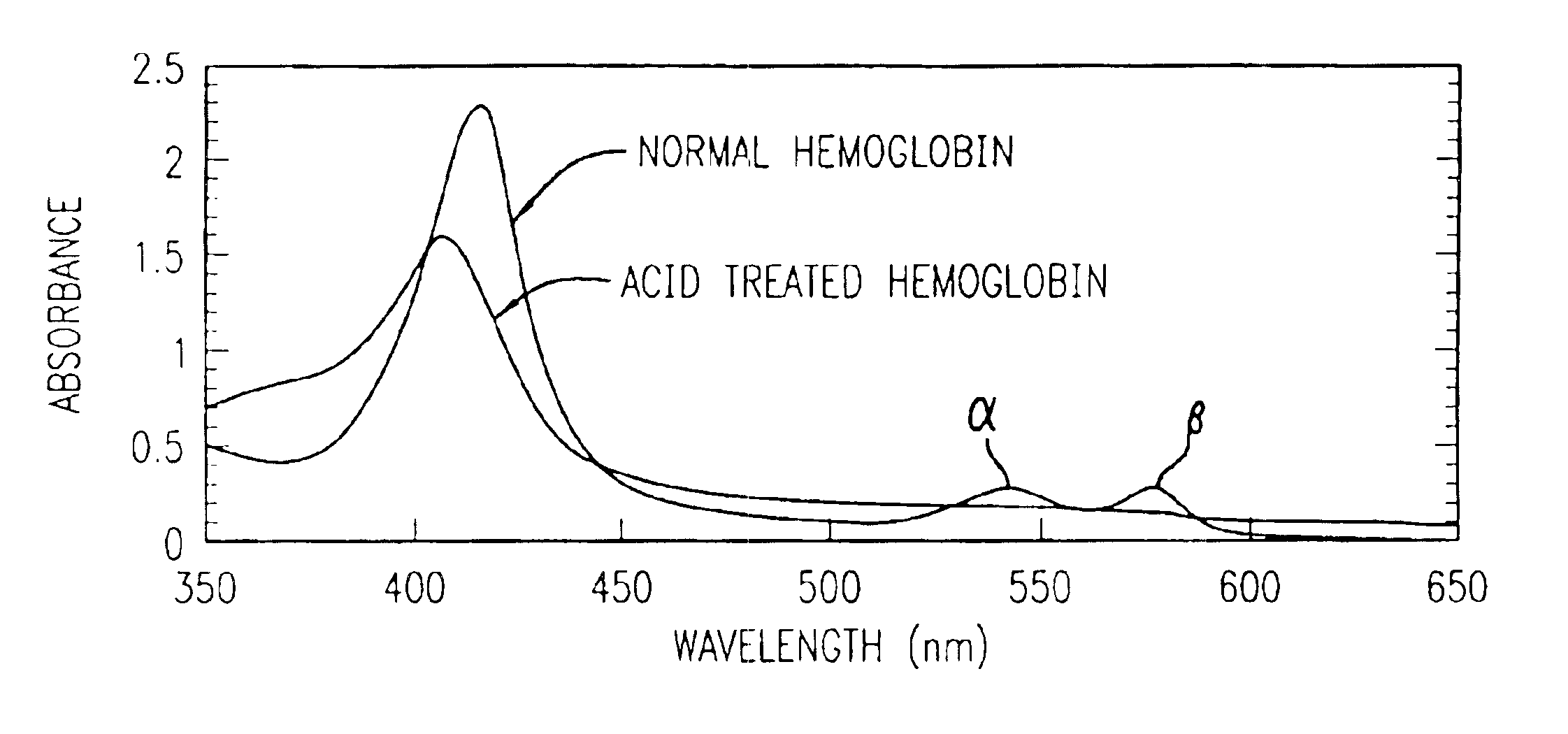
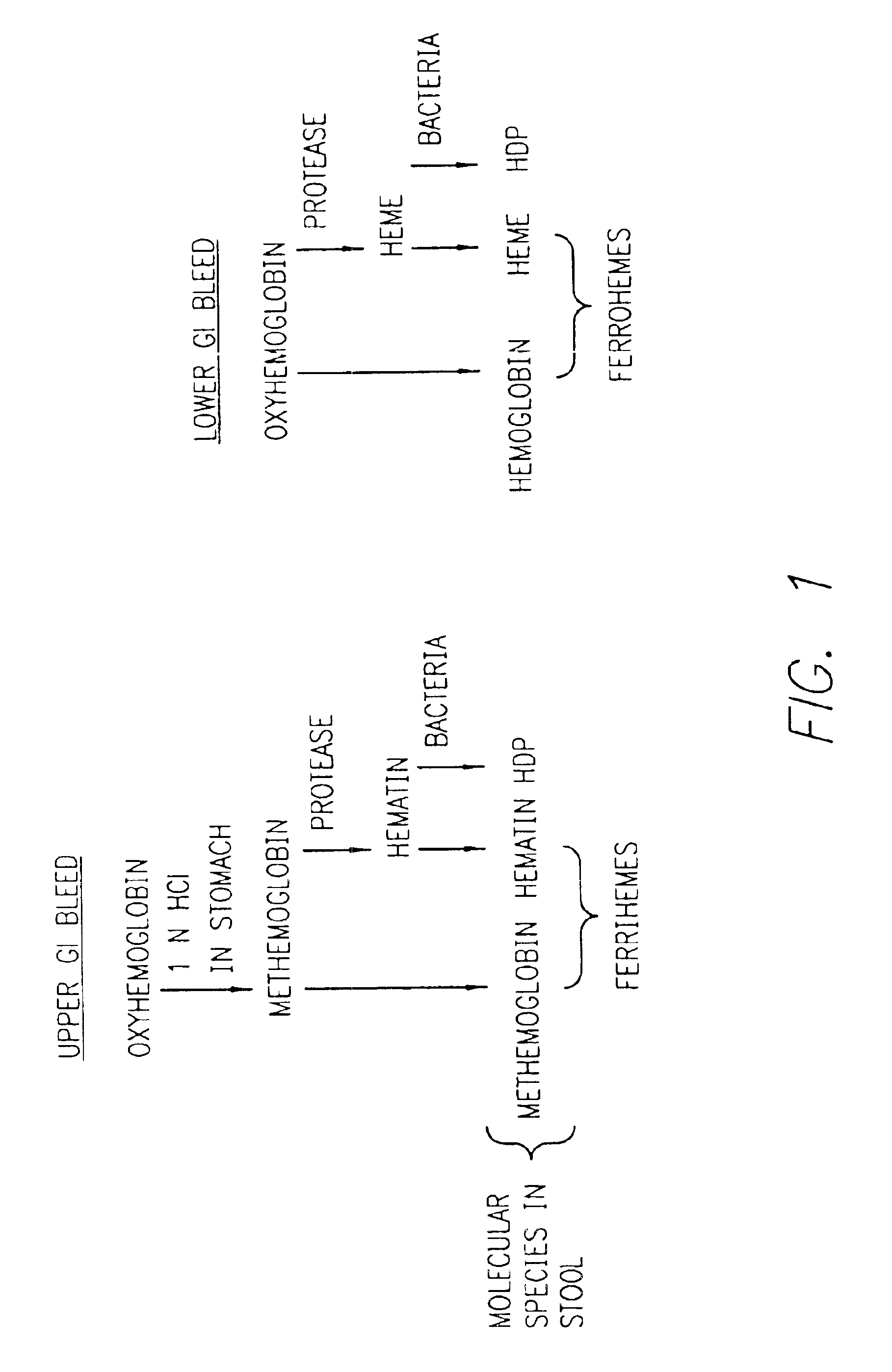
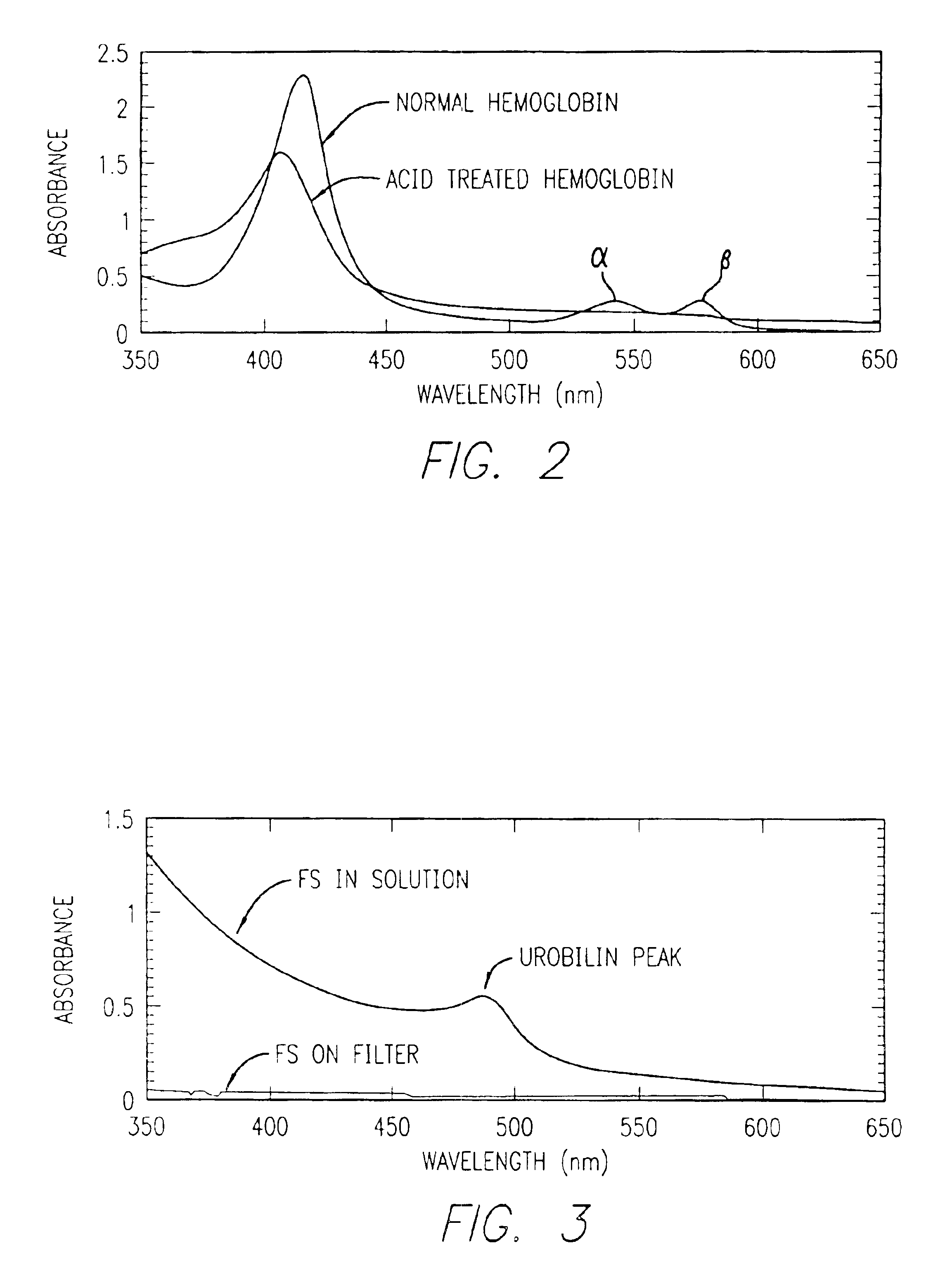
InactiveUS6844195B2Rapid and economicalPerformance requirementAnalysis using chemical indicatorsPreparing sample for investigationFecesLower Gastrointestinal Tract
A method for determining if blood in a stool sample originated from the upper or lower gastrointestinal tract. This includes a method for purifying and concentrating hemoglobin and its products from a stool sample to allow a simple and sensitive spectrophotometric analysis. A rapid, noninvasive determination of whether the blood originated from an upper gastrointestinal or lower gastrointestinal site is made on the basis of changes in the absorption spectra of hemoglobin that occur when hemoglobin is exposed to a highly acidic environment.
Owner:WESTERN RES
Nucleic acid extraction from complex matrices
InactiveUS8574890B2Good reproducibilityHigh sensitivityHydrolasesOther chemical processesSlurryDextran
The present disclosure describes an adsorbent and exemplary protocols for extracting nucleic acids, such as DNA and RNA, from complex matrices, such as stool samples and water samples. The adsorbent is activated charcoal coated with a material such as polyvinylpyrrolidone, dextran, or coconut flours. The adsorbent may be used in microcentrifuge spin columns, where it may be present as a slurry in a storage solution. The sample may be prepared by vortexing in a buffer solution, centrifuging, adding a protease to the supernatant, and passing the supernatant through a microcentrifuge spin column containing coated activated charcoal. The key components, including buffer, protease, and spin columns, may be packaged in a kit.
Owner:PHTHISIS DIAGNOSTICS
Method for the direct detection and/or quantification of at least one compound with a molecular weight of at least 200
ActiveUS9612250B2Enhanced advantageGood reproducibilitySugar derivativesMass spectrometric analysisCulture cellRetention time
Owner:SANIFIT THERAPEUTICS SA
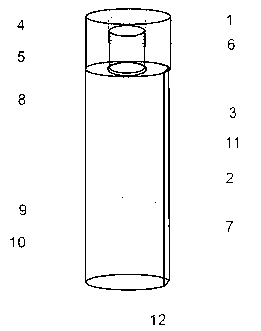
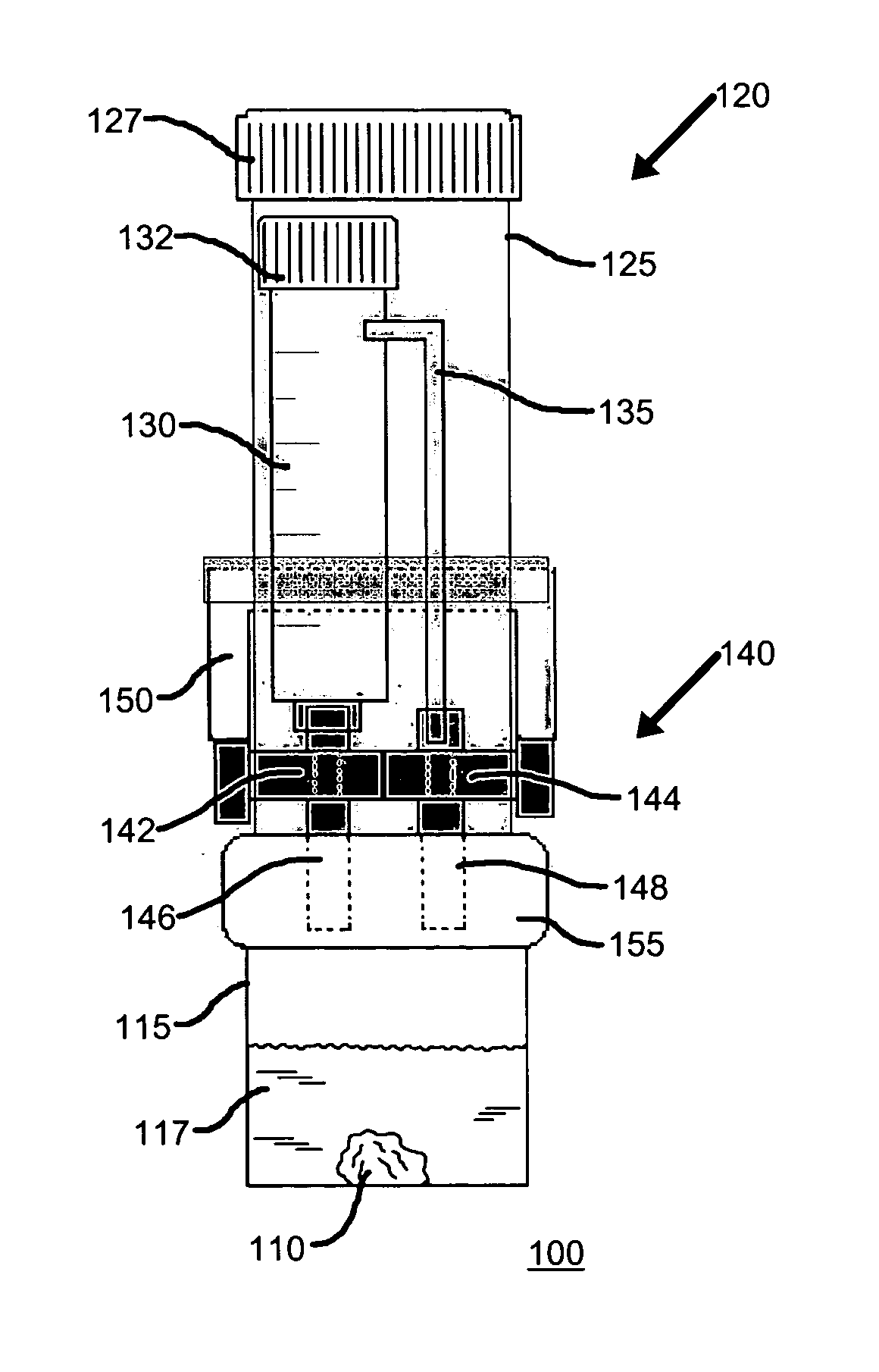
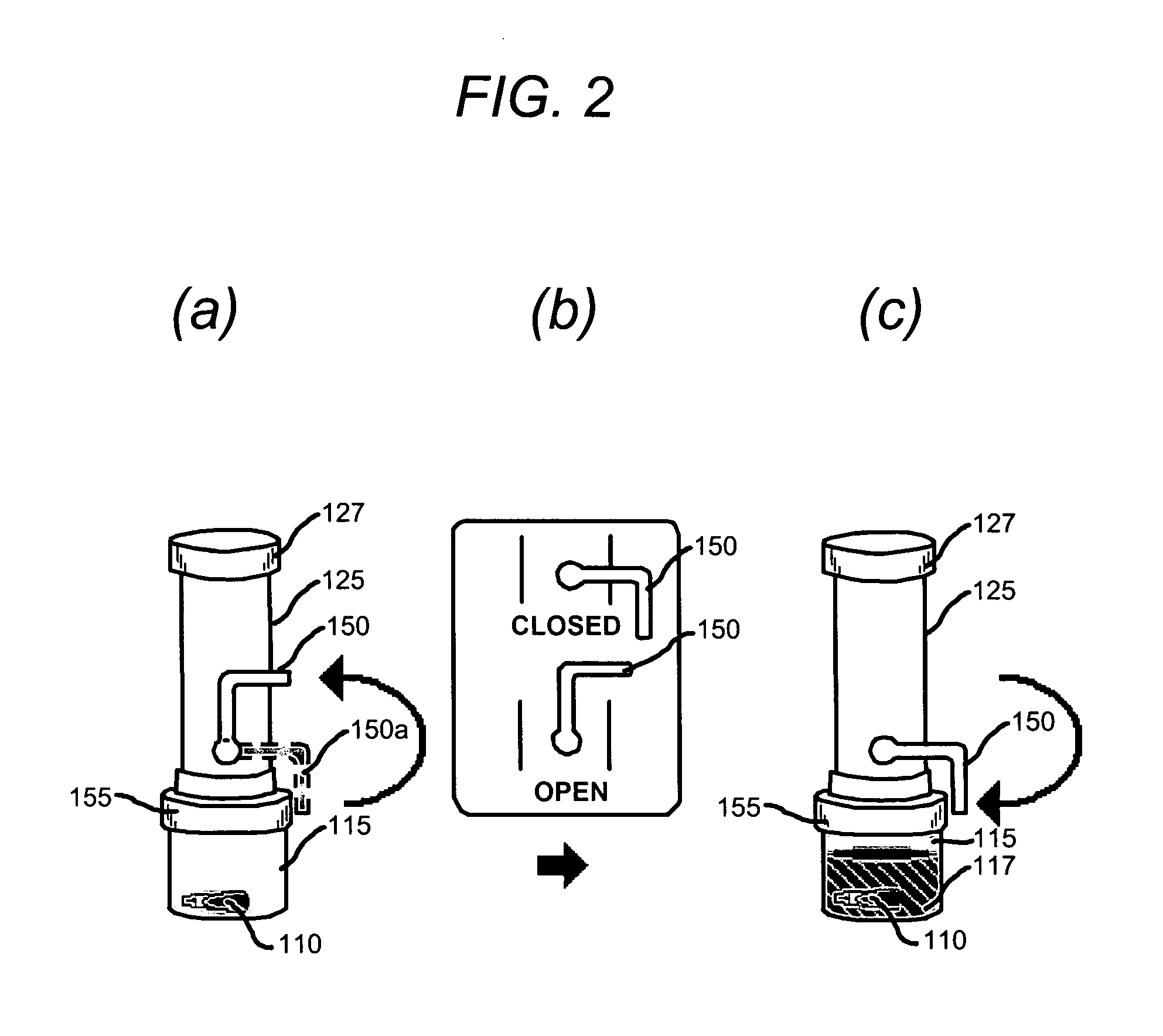
Device for collection and preservation of tissue or stool samples
InactiveUS20100311165A1Bioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringVALVE PORT
An arrangement for collecting a tissue or stool sample has a sample vial and a source of preservative fluid. A fluid valve controls a flow of the preservative fluid from the source of preservative fluid to the sample vial. An air equalization vent tube is coupled to the source of preservative fluid and an air valve controls a flow of air through the air equalization vent tube, the air valve and the fluid valve being actuated simultaneously. A scoop for collecting the sample is coupled to the sample vial coupler and extends into the sample vial. The preservative fluid flows from the source of preservative fluid to the sample vial by force of gravity upon actuation of the fluid valve. The air equalization vent tube transfers air from the sample vial to the source of preservative fluid upon actuation of the air valve.
Owner:WAYNE STATE UNIV
Devices and methods for sample collection and analysis
ActiveUS8062901B2Reduce surface tensionRaise transfer toAnalysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorAnalyteFecal occult blood
The present invention provides devices, methods, and kits for the collection of a solid or semi-solid sample and analysis for the presence, absence, or quantity of an analyte. The invention provides a collection slide having a first card and a second card. The first card has a sample collection area. The first and second cards have orifices allowing the passage of fluid through the sample collection area, and the cards are hingeably connected to each other. The invention also provides an assay device having a housing with a test element, a results window, and a docking area for receiving and engaging the collection slide. In one embodiment the collection slide and device can be used to detect the presence of fecal occult blood (human hemoglobin) in a stool sample. Many other embodiments are described herein.
Owner:ABBOTT RAPID DIAGNOSTICS INT UNLTD
Methods and materials for detecting colorectal neoplasm
InactiveUS20110236916A1Microbiological testing/measurementDisease diagnosisAbnormal tissue growthTumor-Related Protein
The present invention provides methods and materials related to the detection of colorectal neoplasm-specific markers in or associated with a subject's stool sample. In particular, the present invention provides methods and materials for identifying mammals having a colorectal neoplasm by detecting the presence of exfoliated epithelial markers (e.g., human DNA, tumor associated gene alterations, tumor associated proteins) and blood markers (e.g., homoglobin, serum proteins) in a stool sample obtained from the mammal.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Method for preparing stool sample, solution for preparing stool sample, and kit for collecting stool
ActiveUS20100248250A1Effective recoverySimple and easy mannerMicrobiological testing/measurementPreparing sample for investigationOrganic solventEnteric bacterium
Provided in the present invention are a method for preparing a stool sample without any need for complicated operations which is capable of efficiently recovering a nucleic acid or the like originating from mammalian cells, such as the cells exfoliated from the large intestine, in the stool; a solution for preparing a stool sample and a kit for stool collection which are used in the above method; and a method for recovering and analyzing the nucleic acid in stool using the stool sample prepared by the above method. More specifically, provided are a method for preparing a stool sample which is a method for preparing a stool sample in order to efficiently recover a nucleic acid from the stool sample, the method characterized in that the collected stool is mixed with a solution for preparing a stool sample which has a water-soluble organic solvent as an active ingredient; a solution for preparing a stool sample which is used in the method; a kit for collecting stool; a method for recovering a nucleic acid characterized by recovering a nucleic acid originating from indigenous enteric bacterium and a nucleic acid originating from an organism other than indigenous enteric bacterium at the same time from the stool sample prepared by the preparation method; and a method for analyzing a nucleic acid that analyzes the nucleic acid recovered by the method for recovering a nucleic acid.
Owner:OLYMPUS CORP
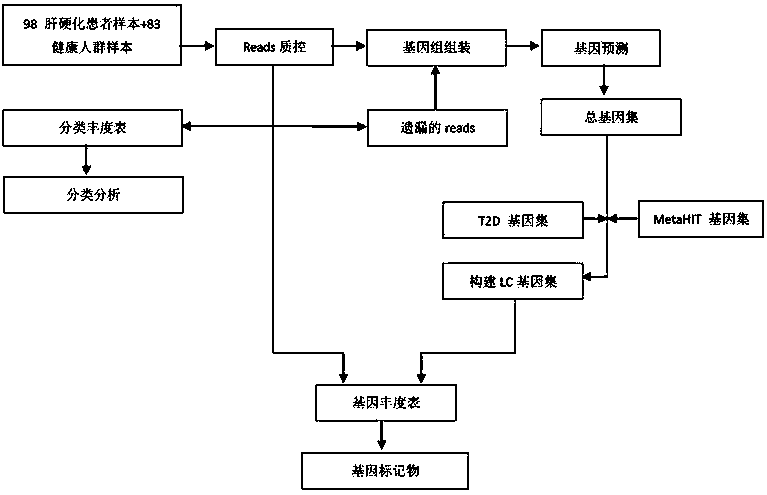
Biomarker of liver cirrhosis, and application thereof
InactiveCN104195145AEffective monitoring of susceptible populationsSurveillance of susceptible populationsMicrobiological testing/measurementDigestive systemMicroorganismFeces
The invention discloses a biomarker of liver cirrhosis, and an application thereof. Association analysis of the whole intestinal flora microorganisms is developed from stool samples of 98 patients suffering from liver cirrhosis and 83 healthy references to study and describe stool microflora and functional component characteristics. 15 genes form the biomarker as a highly accurate index for distinguishing the patients. The discovery is examined in other independent population to confirm the accuracy of the biomarker; and associated robustness between the detection flora and the liver cirrhosis are confirmed. The biomarker is at least 10 genes selected from the following group of 15 genes. 15 genes are enriched in the intestinal flora of the patients suffering from the liver cirrhosis. The invention also relates to a drug for treating the liver cirrhosis. The drug can promote or increase the number or expression of the genes. The invention also relates to a method for producing or screening the drug. The drug can promote or increase the number or expression of the biomarker. The invention also relates to a kit for detecting the liver cirrhosis, monitoring a treatment process, or producing and screening the drug. The kit is used for detecting the biomarker.
Owner:ZHEJIANG UNIV
Stool sample preparation method, solution for preparing stool sample and stool collection kit
InactiveUS20110189673A1Easy to storeAvoid changeSugar derivativesMicrobiological testing/measurementOrganic acidFeces
The present invention relates to the providing of a method for preparing a stool sample that enables nucleic acids in a stool to be stably preserved without requiring a complex procedure, a stool sample preparation solution and stool collection kit used in that method, and a method for recovering and analyzing nucleic acids in a stool using a stool sample prepared according to the preparation method of the present invention, and a stool sample having superior preservation of nucleic acids contained in the stool sample is prepared by mixing a collected stool with the stool sample preparation solution having for an active ingredient thereof a water-soluble organic solvent containing an organic acid.
Owner:OLYMPUS CORP
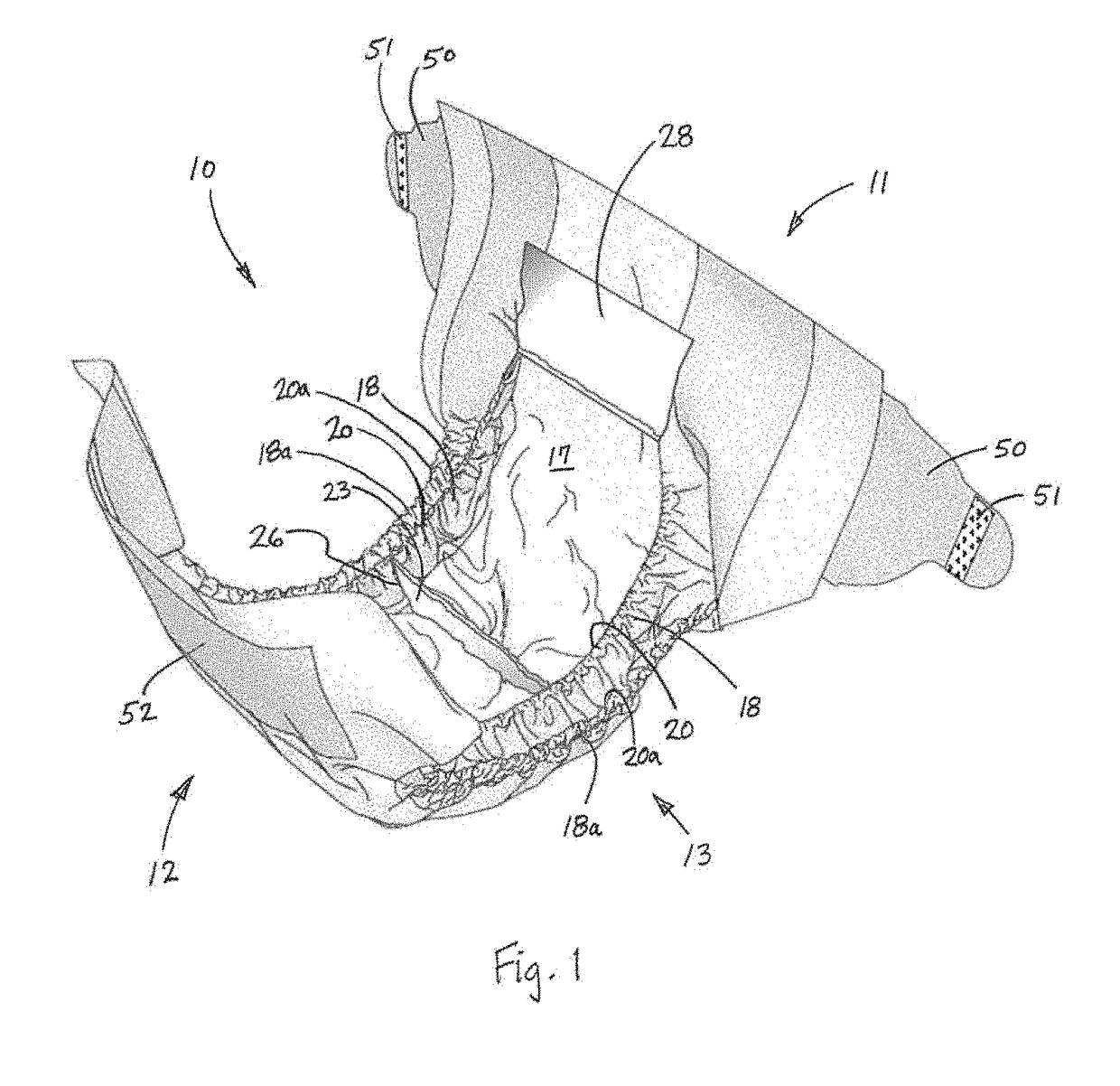
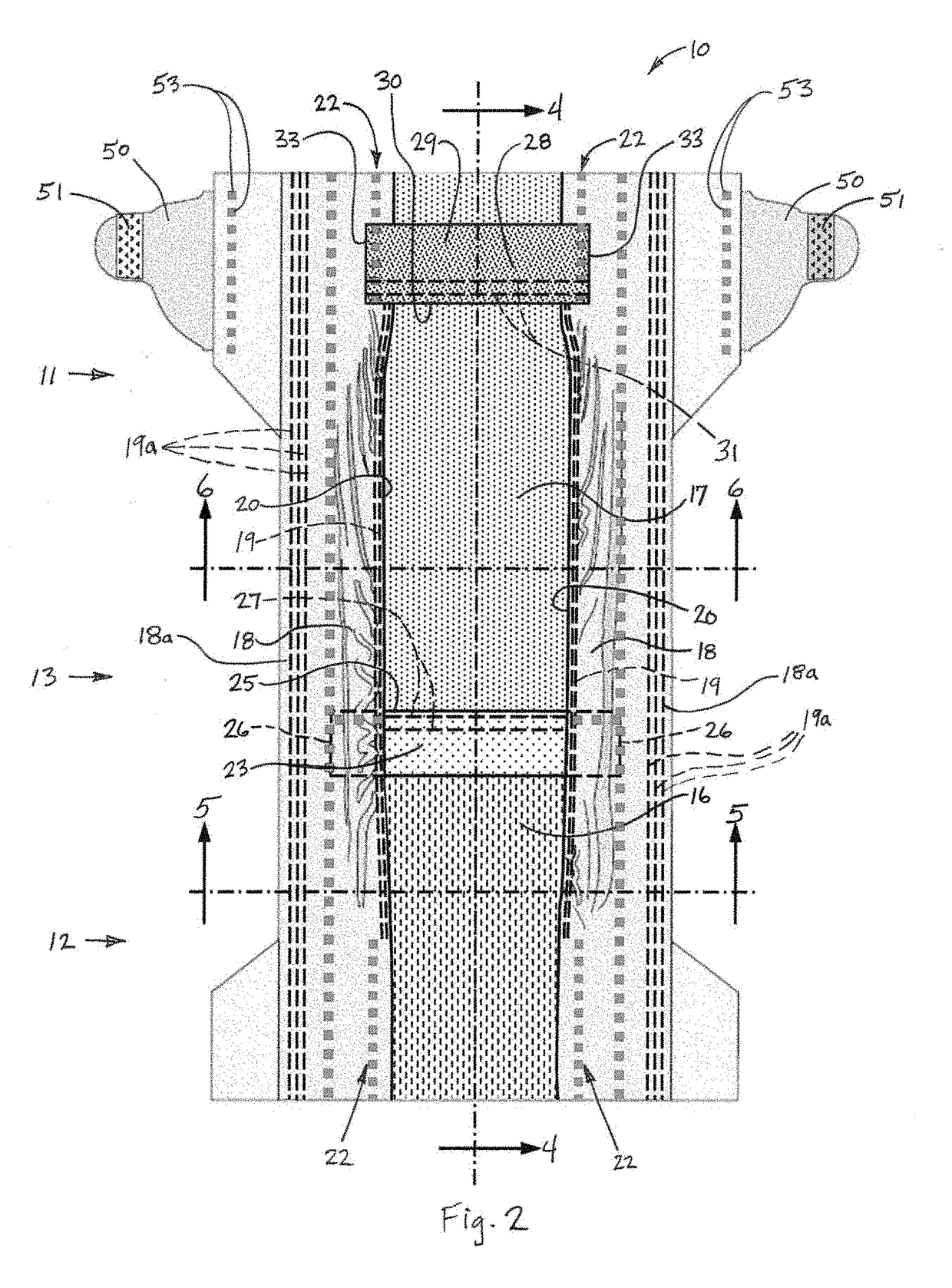
Diaper adapted for collection of uncontaminated and intact stool sample from an infant
A diaper configured for collection of a stool sample for an infant, and an associated method for collection, are disclosed. The diaper may include a backsheet including an effectively urine- and feces-impermeable material; a transverse perineal barrier including an effectively urine- and feces-impermeable material and having a proximal portion overlying the backsheet and sealingly connected to the diaper, and a free distal edge; and a liquid control structure disposed over the backsheet. The diaper may include additional features configured to receive a discharge of urine, isolate fecal material from urine, and facilitate collection of an intact stool sample following elimination.
Owner:THE PROCTER & GAMBLE COMPANY
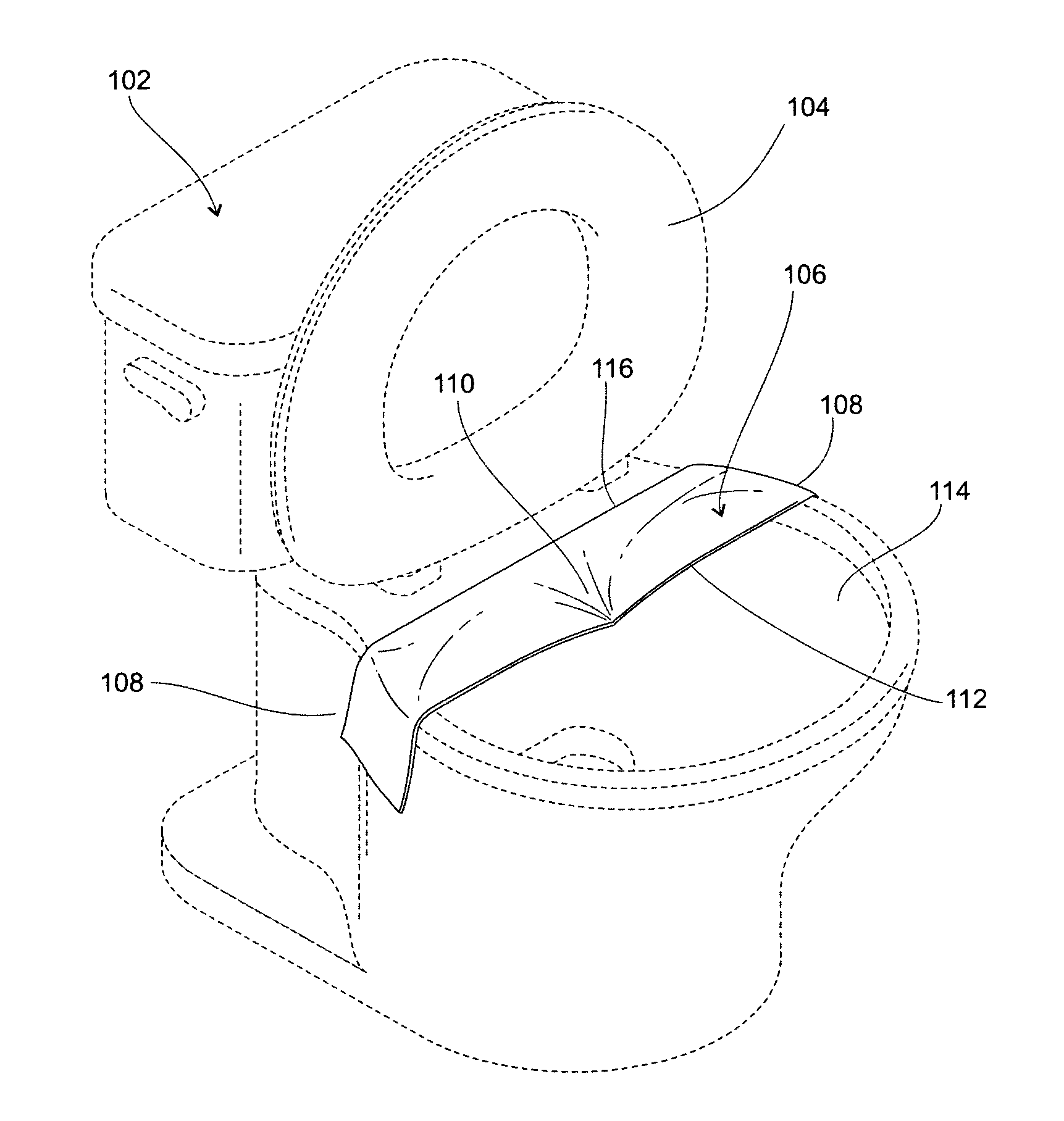
Stool sample collector
ActiveUS8613711B2Easy to keepAvoid insufficient lengthSurgeryBathroom accessoriesPolyvinyl alcoholEngineering
A stool sample collector is comprised of a sheet of water-soluble material such as heavy-weight polyvinyl alcohol film, six to nine inches by 20 to 26 inches. In use, the strip is positioned across the toilet bowl against the bowl's back rim, the front edge of the strip drooping down to a few inches above the toilet water. The strip is affixed across the bowl by moistening at each end and pressing against the sides of the bowl. Positioning of the strip is facilitated by markings on the strip. Retention of stool samples is enhanced by dimples or riffles in the strip. After use, the strip may be removed and flushed in a domestic toilet.
Owner:BABCOCK LEE L
Methods of marking and testing pharmaceutical products
InactiveUS20060018832A1Quick identificationSimple technologyChemiluminescene/bioluminescencePharmaceutical delivery mechanismBiotechnologySporopollenin
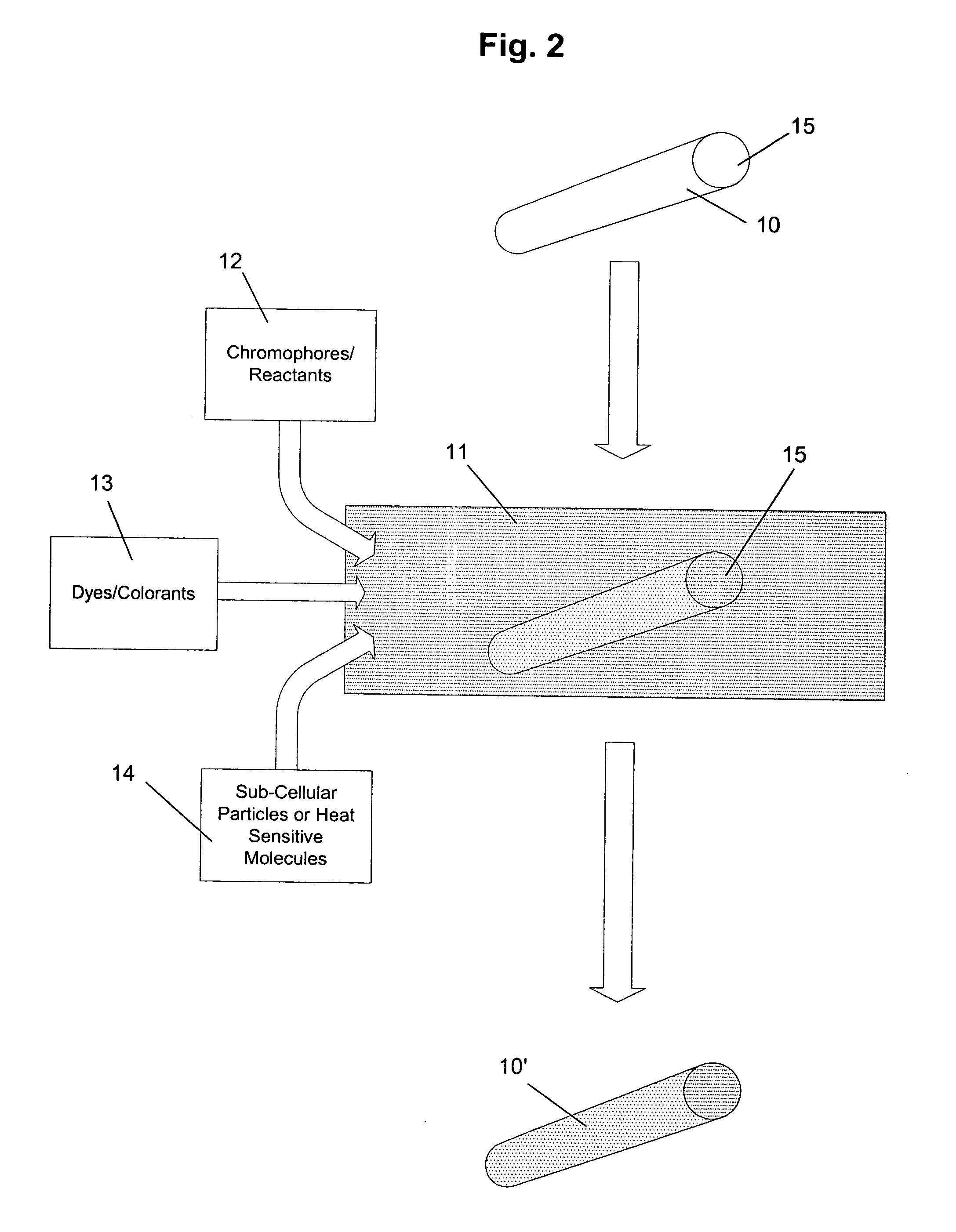
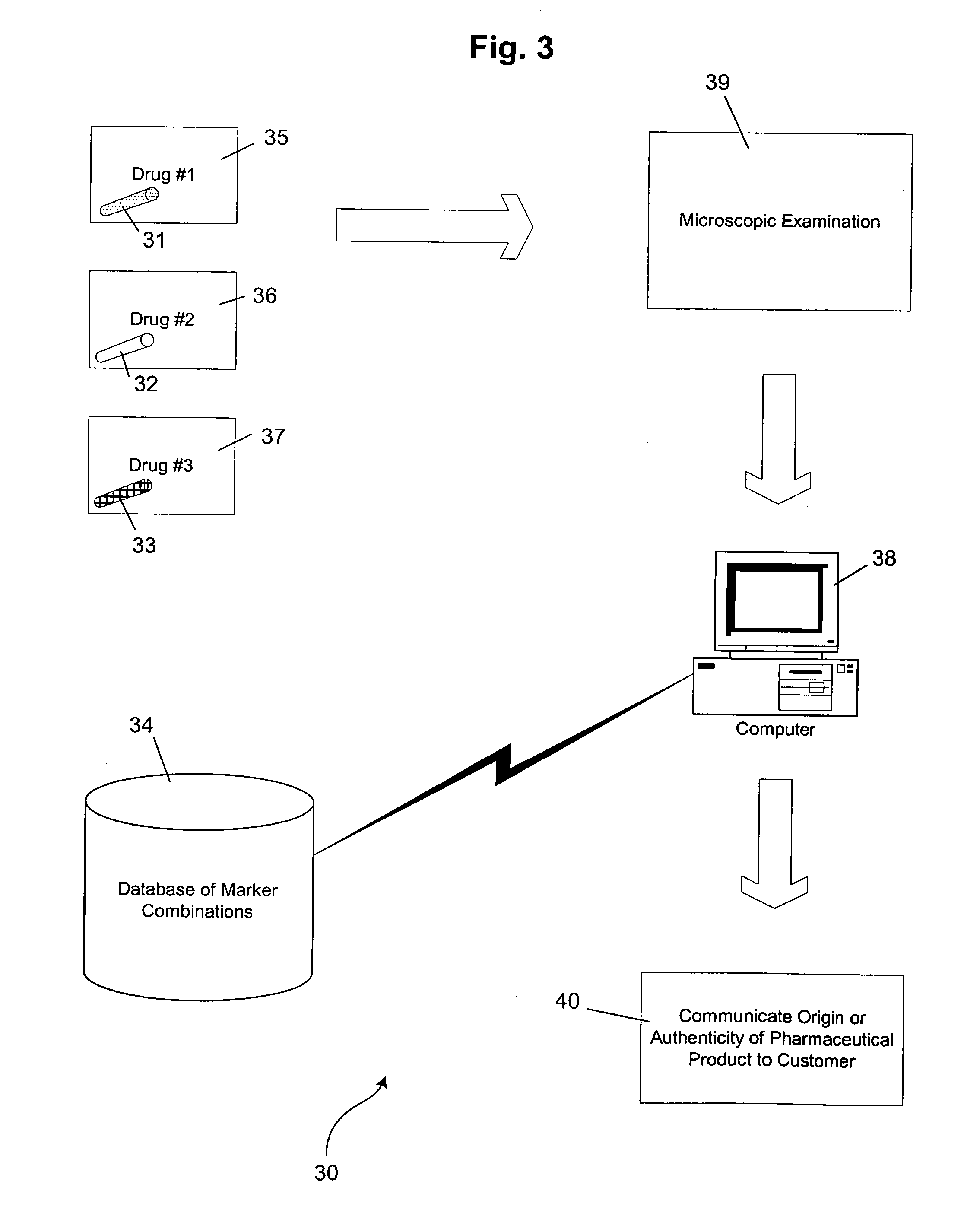
Methods of marking pharmaceutical products for use in clinical trials and for determining the origin or authenticity of the marked products are provided that use a variety of natural materials as markers. The natural materials have unique genetically controlled micromorphological structures that can be identified using enhanced visualization techniques. For example, cellulosic plant materials, sporopollenin and diatoms can be used as the natural materials. The natural materials are added to pharmaceutical products at sufficiently low levels so as not to have any significant effect on the products other than serving as markers. Dyes and reactants can be added to the natural materials to provide secondary markers. The markers can be identified in stool samples collected during clinical trials to prove that a particular pharmaceutical product has been ingested by a test subject.
Owner:BATES LYNN S
Method for monitoring micro-ecologic maturity of extracted stool sample 16s rRNA in intestinal tract of infant
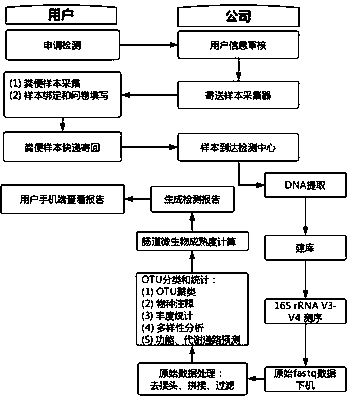
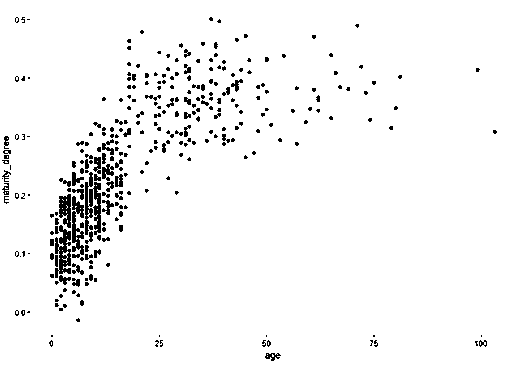
InactiveCN108841974ARealize evaluationEasy to sampleMicrobiological testing/measurementNutrition controlExperimental researchEvaluation result
The invention discloses a method for monitoring micro-ecologic maturity of extracted stool sample 16s rRNA in intestinal tract of the infant. The method comprises the processes of sample collecting, 16s-rRNA gene sequencing, sequencing data analyzing, intestinal tract microorganism maturity and intestinal tract cell age computing, and use report pushing; the monitoring index of the intestinal tract microorganism maturity is proposed at the first time, the intestinal tract microorganism maturity is the similarity of the infant intestinal tract microorganism and the mother intestinal tract microorganism on the constitution, and the higher the maturity is, the better the flora development is; and the index can reflect the immunity level of the infant. Through the experimental research and thedata analysis, the infant intestinal tract microorganism evaluation can be realized through a method for detecting the hyper-variable region of the stool sample 16s rRNA V3-V4, the method is convenient for sampling, fast in analysis speed, and low in cost; the evaluation result is consistent with the existing research, and the consumption-level intestinal tract microorganism detection becomes possible.
Owner:北京水母科技有限公司
Method of assessing colorectal cancer by measuring hemoglobin and M2-PK in a stool sample
The present invention relates to a method aiding in the assessment of colorectal cancer. The method especially is used in assessing the absence or presence of colorectal cancer in vitro. The method is for example practiced by analyzing biochemical markers, comprising measuring in a stool sample the concentration of hemoglobin and M2-PK and correlating the concentrations determined to the absence or presence of colorectal cancer. To further improve the assessment of colorectal cancer in a method of this invention the level of one or more additional marker may be determined together with hemoglobin and M2-PK in a stool sample and be correlated to the absence or presence of colorectal cancer. The invention also relates to the use of a marker panel comprising hemoglobin and M2-PK in the early diagnosis of colorectal cancer and it teaches a kit for performing the method of the invention.
Owner:F HOFFMANN LA ROCHE & CO AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com