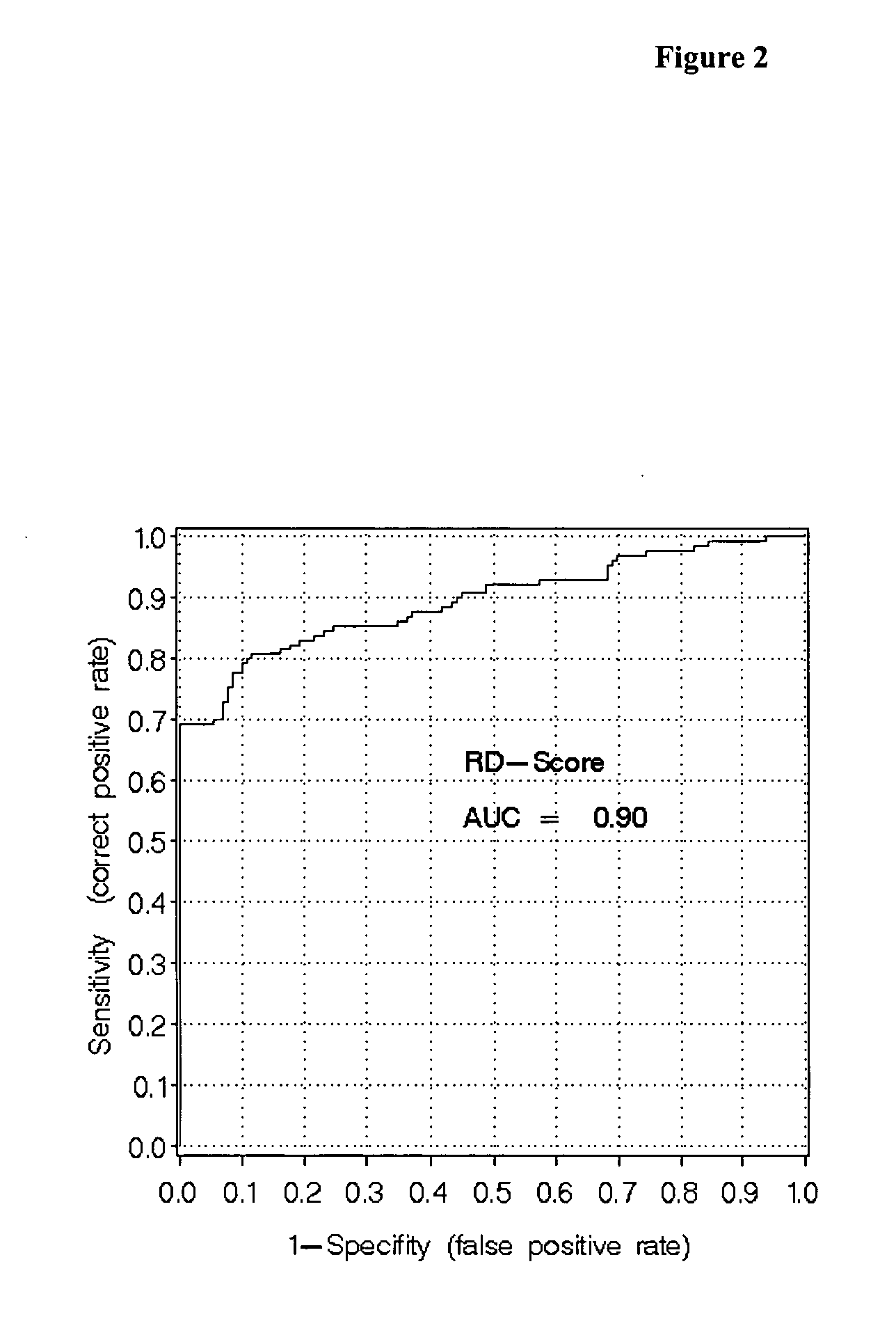
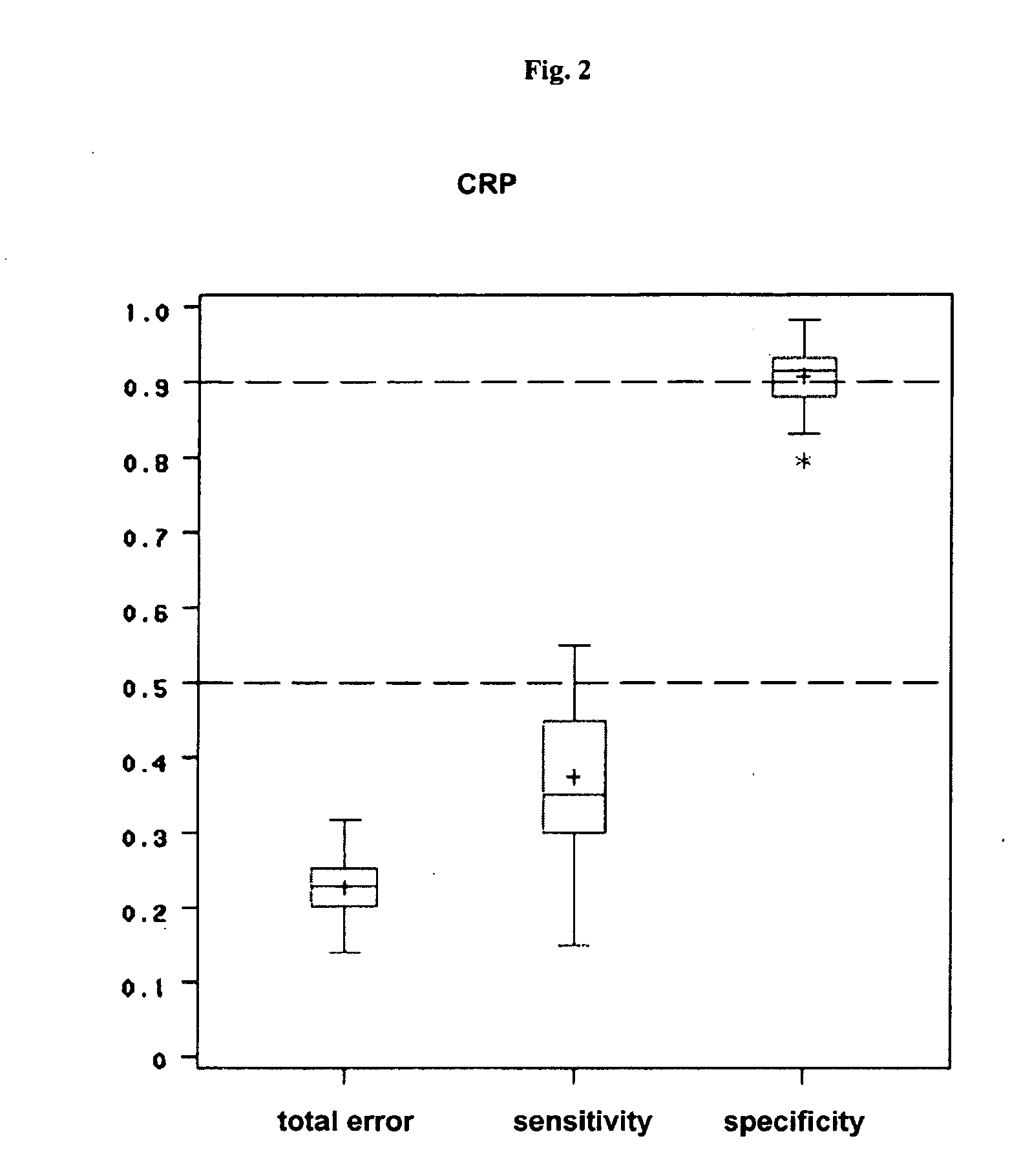
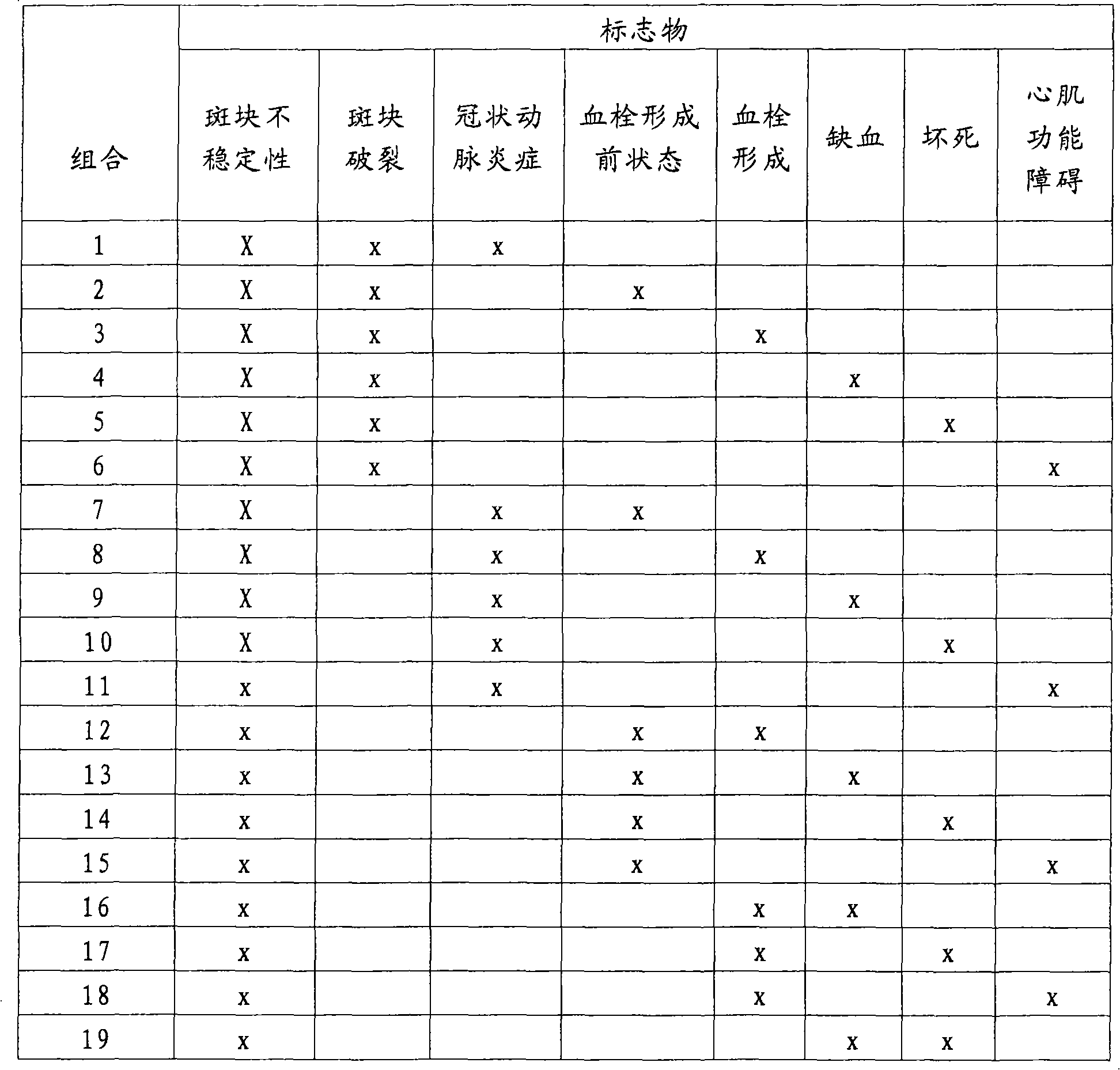
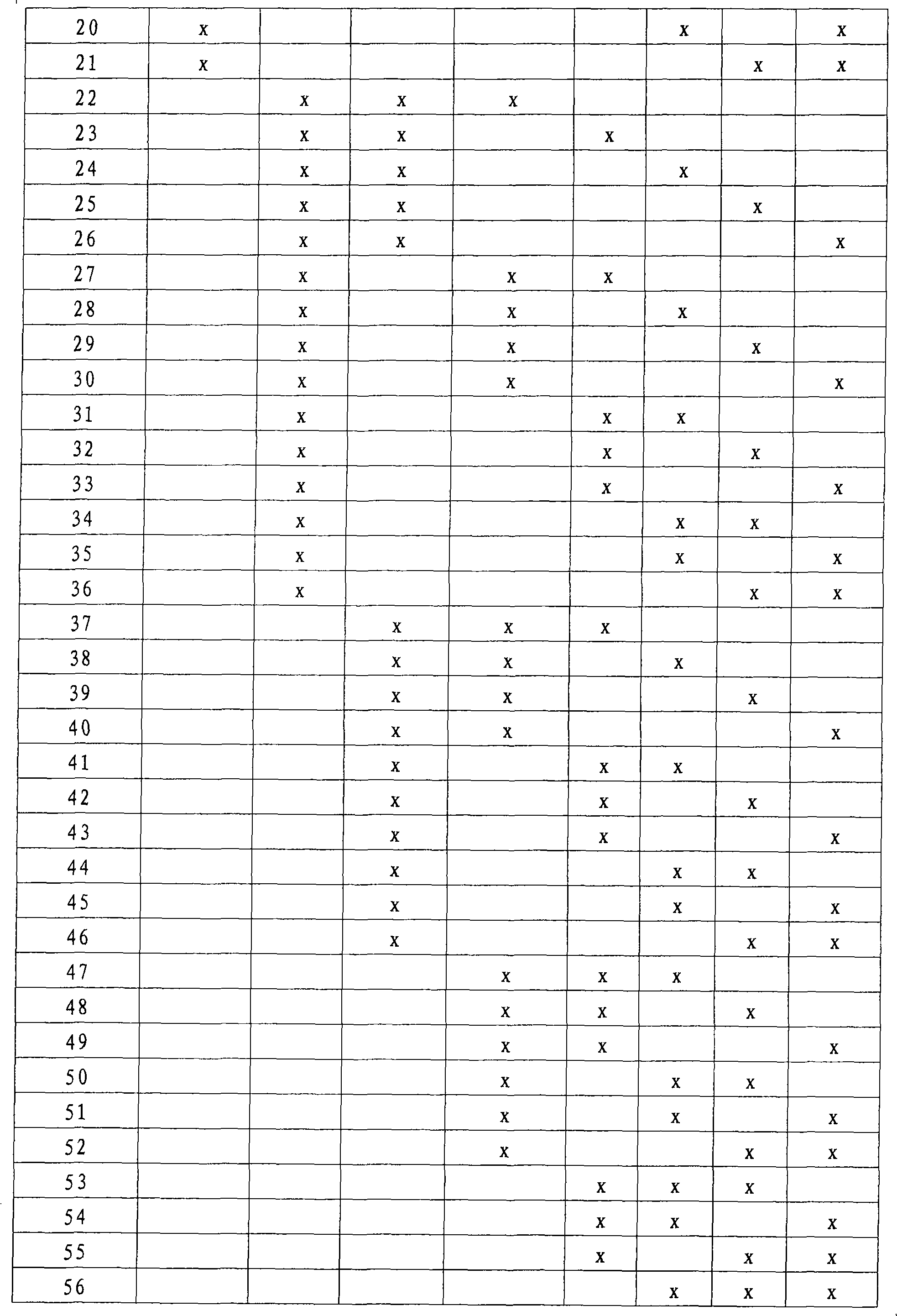
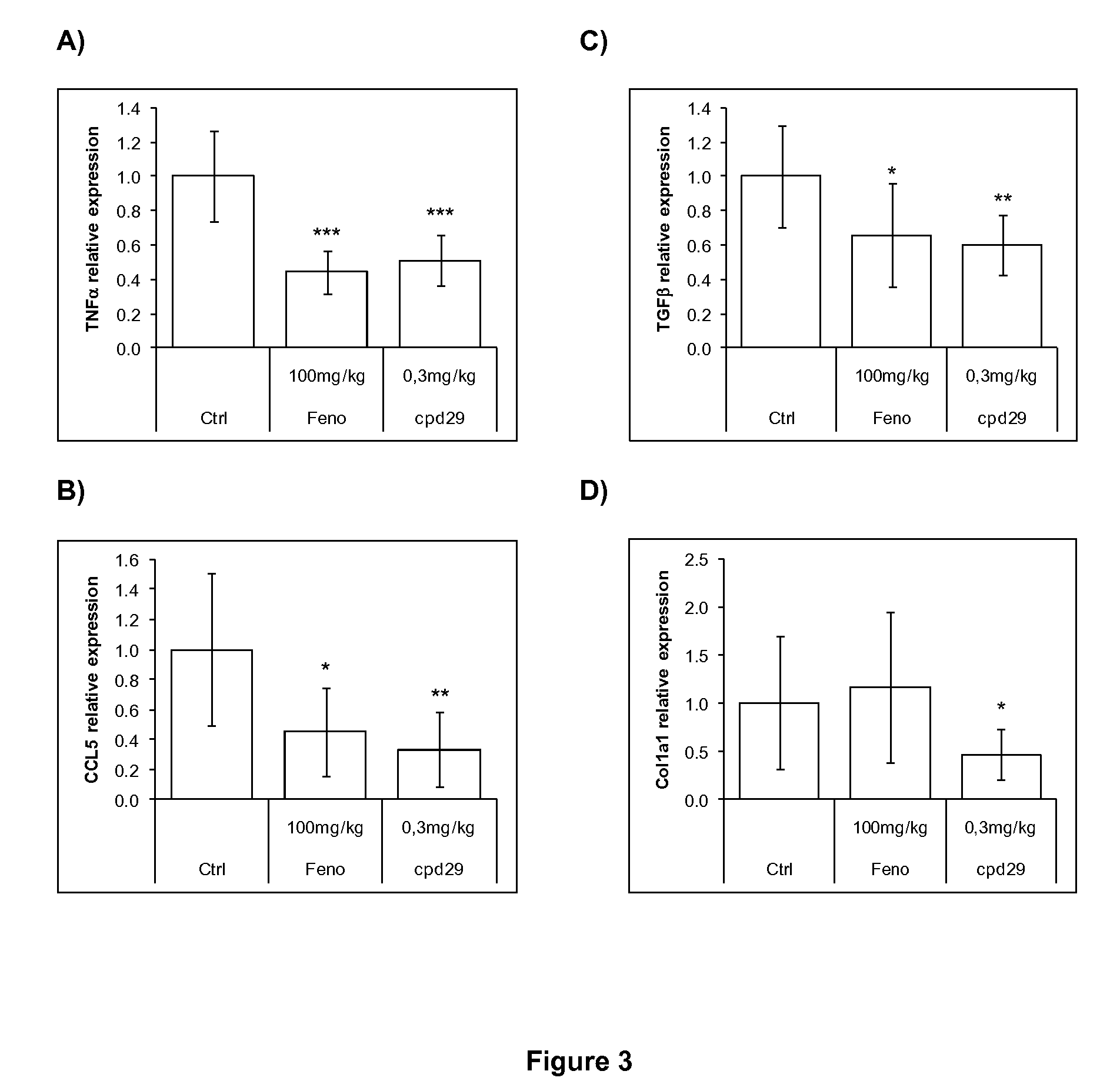
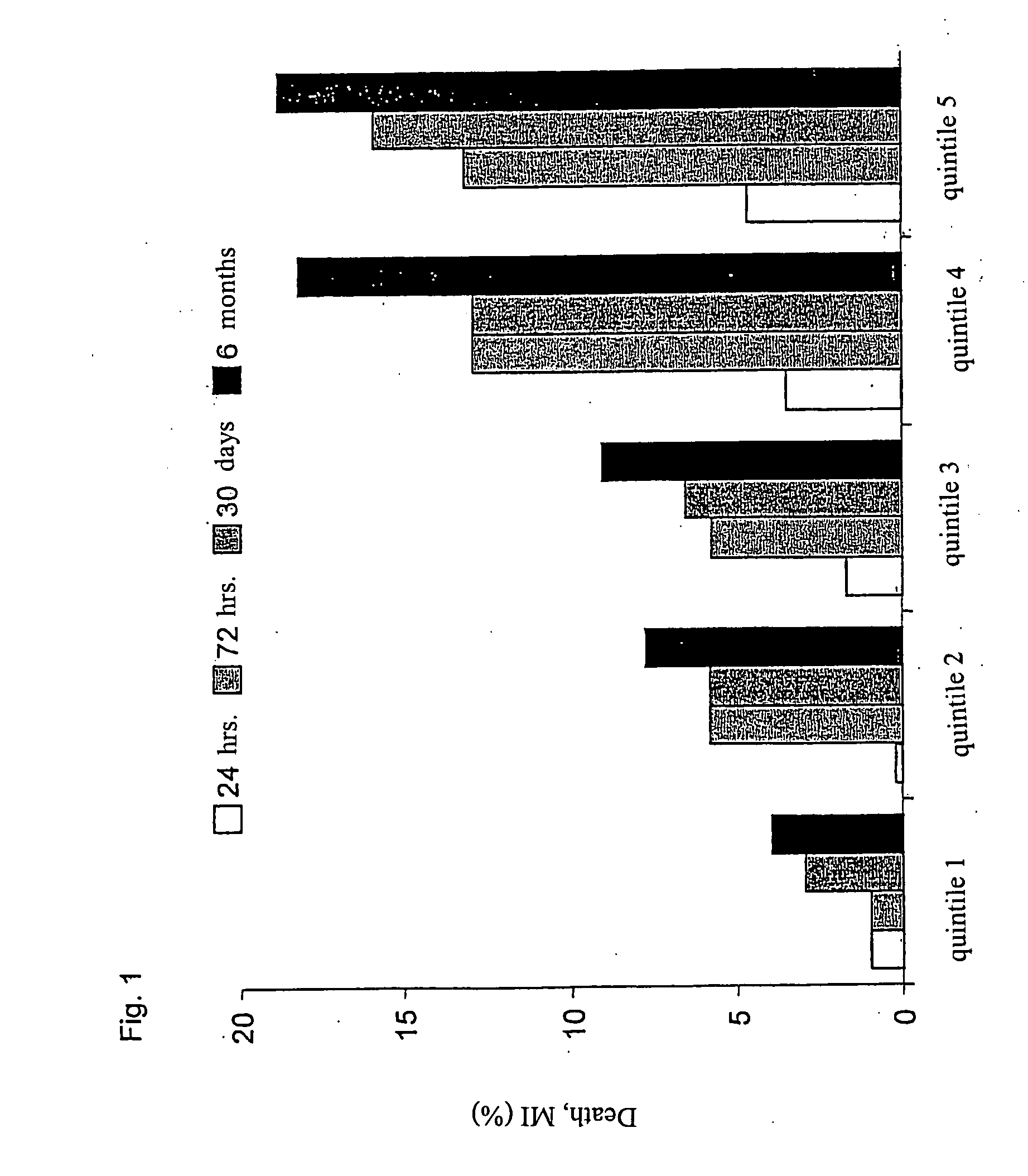
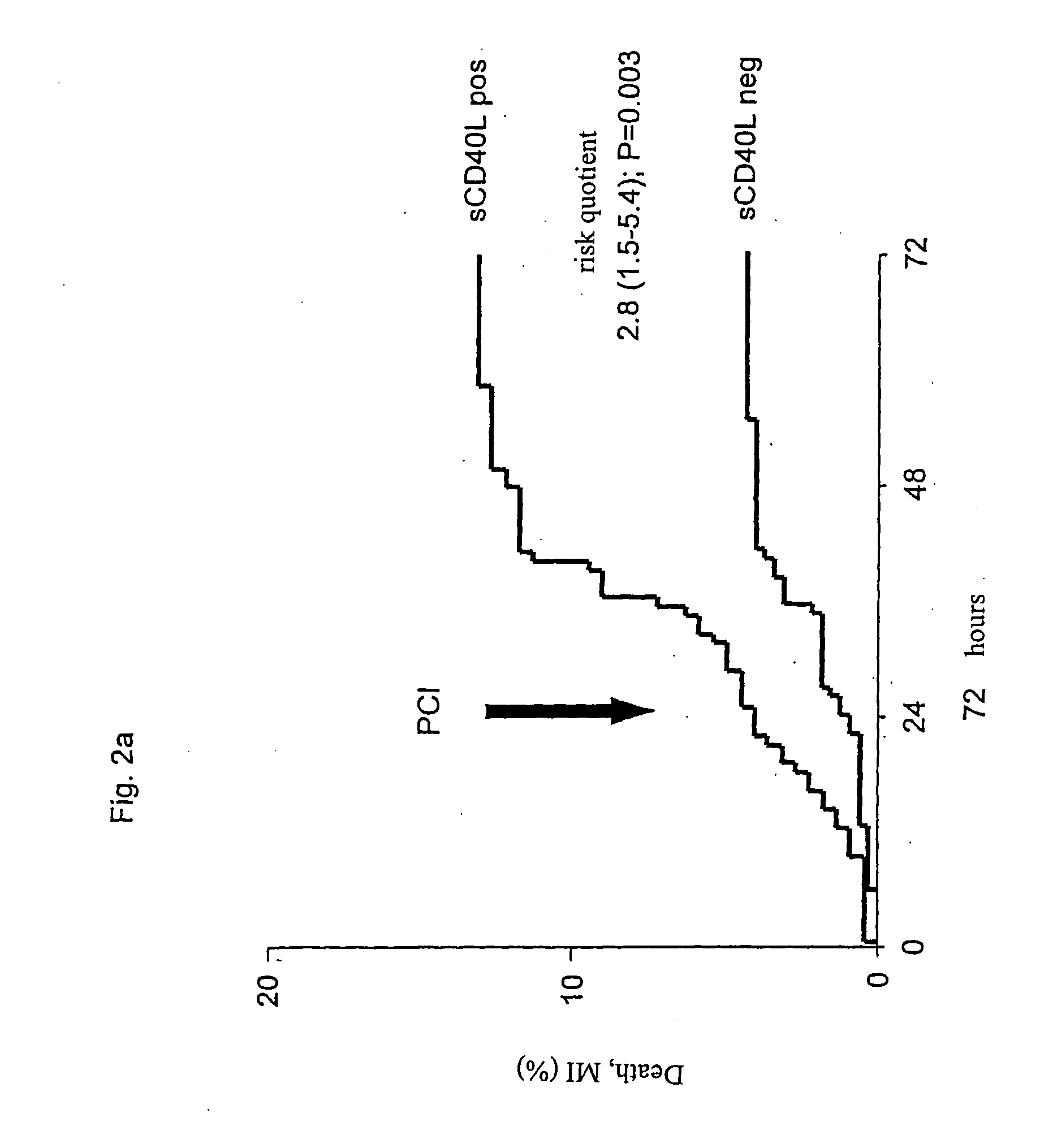
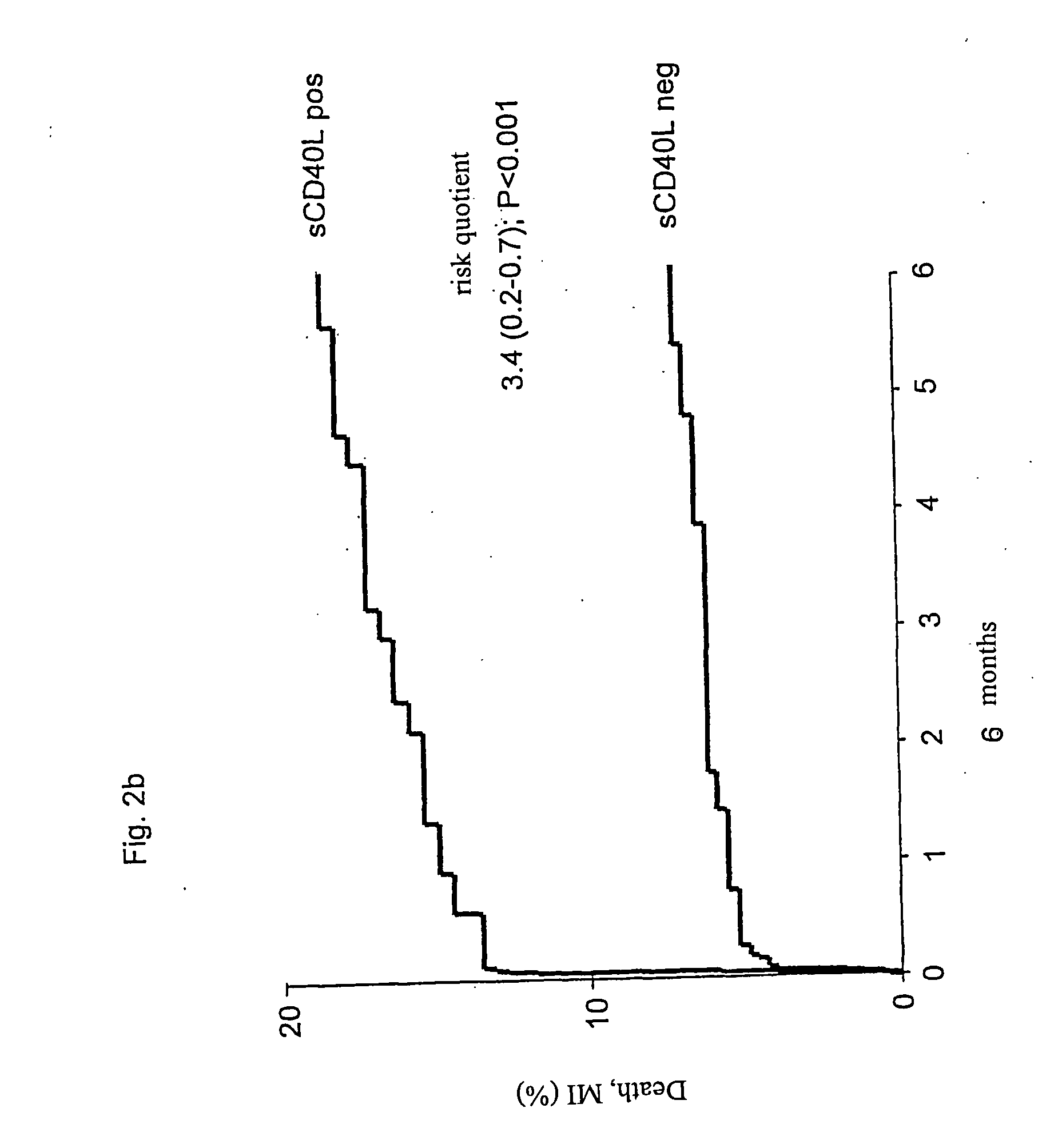
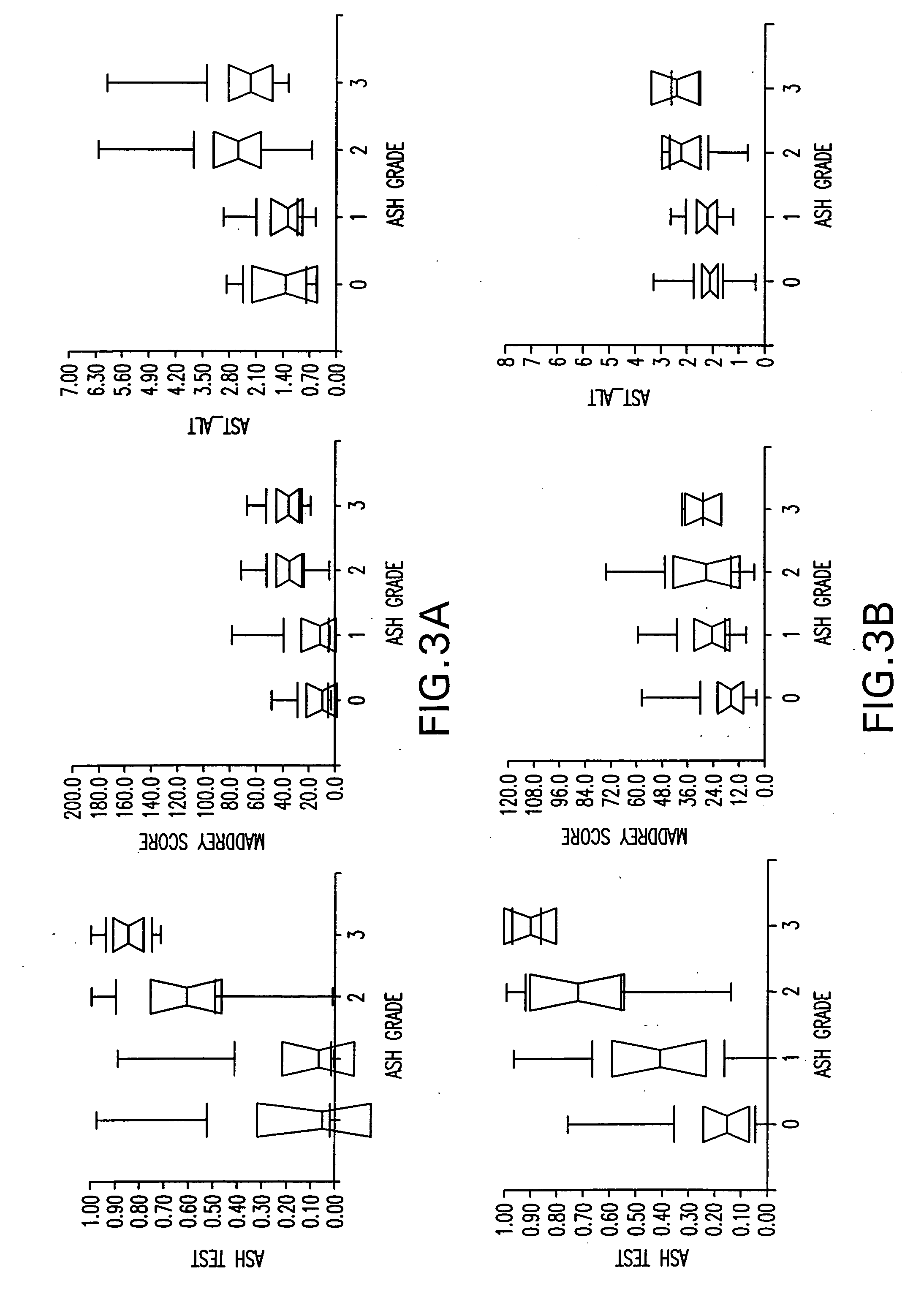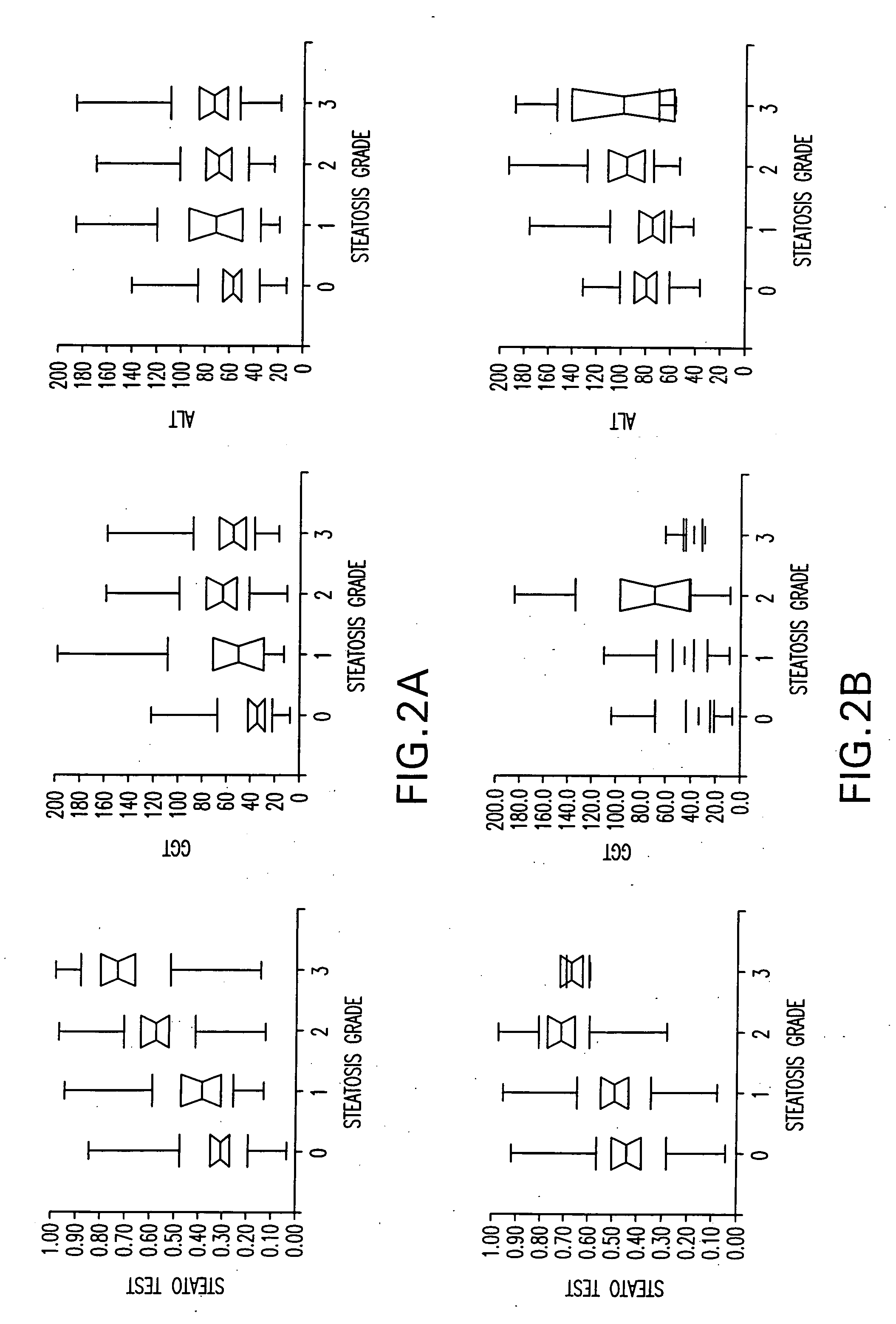Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
125 results about "Biochemical markers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Biochemical marker. Any biochemical compound such as an antigen, antibody, abnormal enzyme, or hormone that is sufficiently altered in a disease to serve as an aid in diagnosing or in predicting susceptibility to the disease.
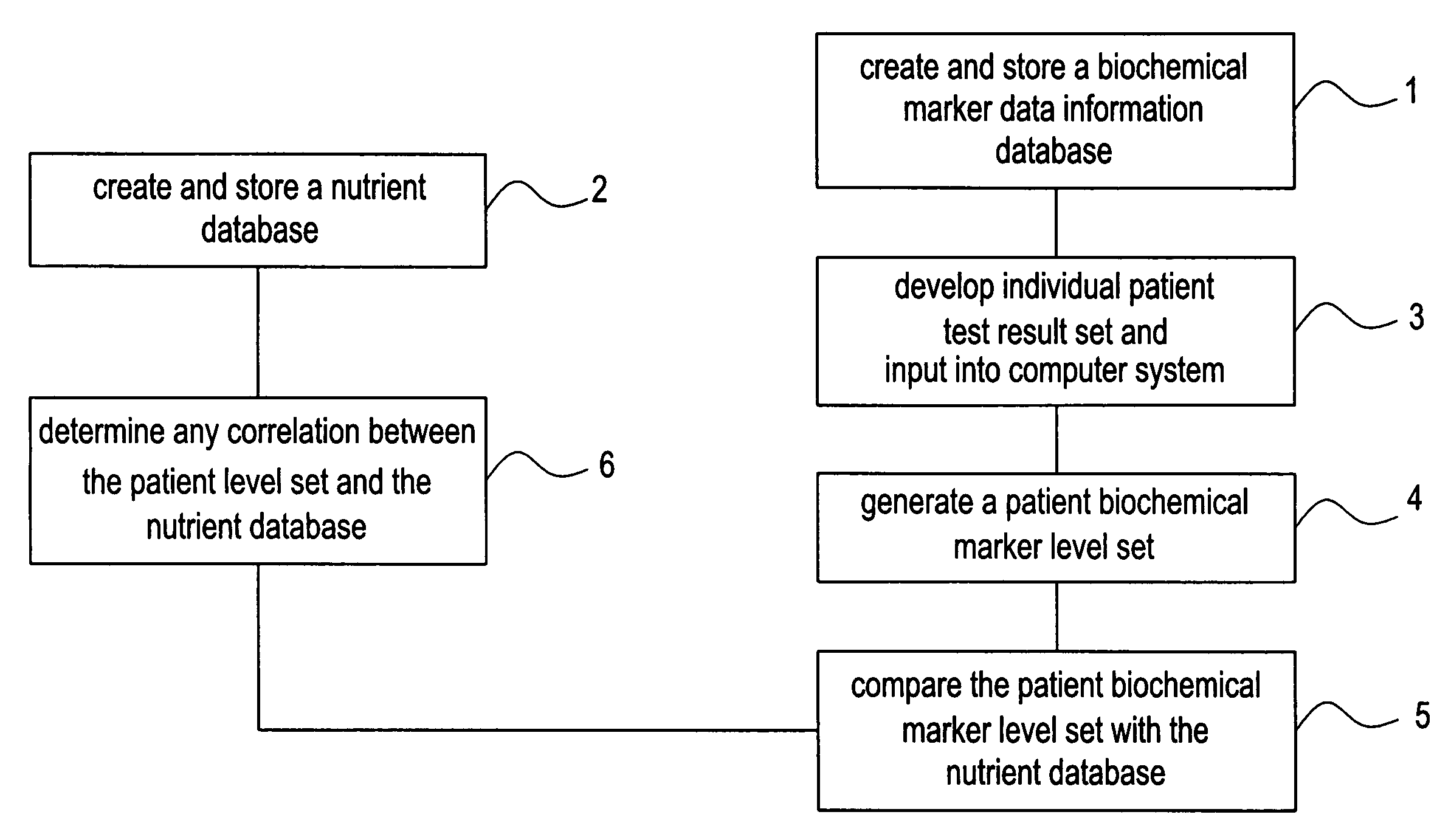
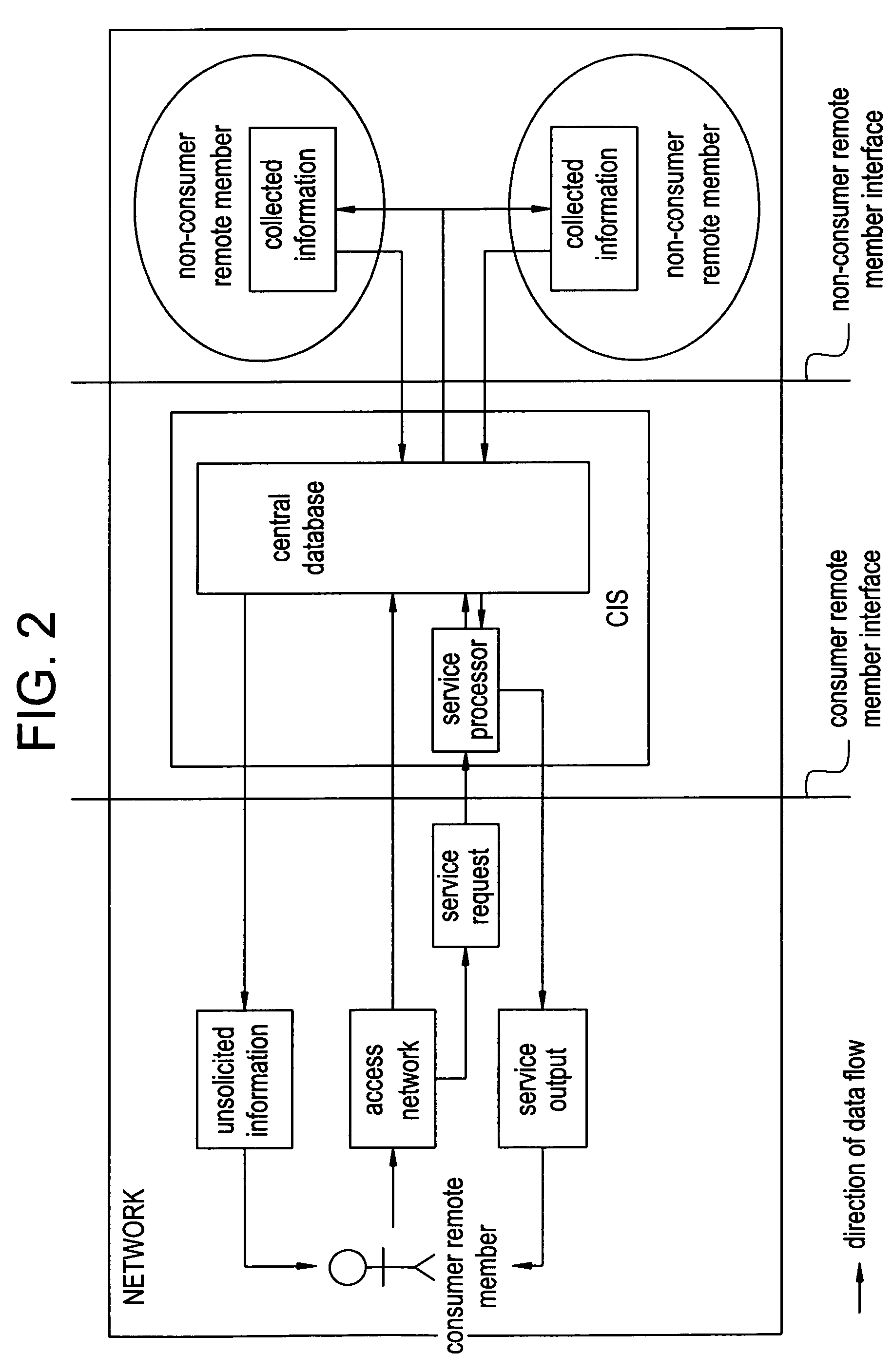
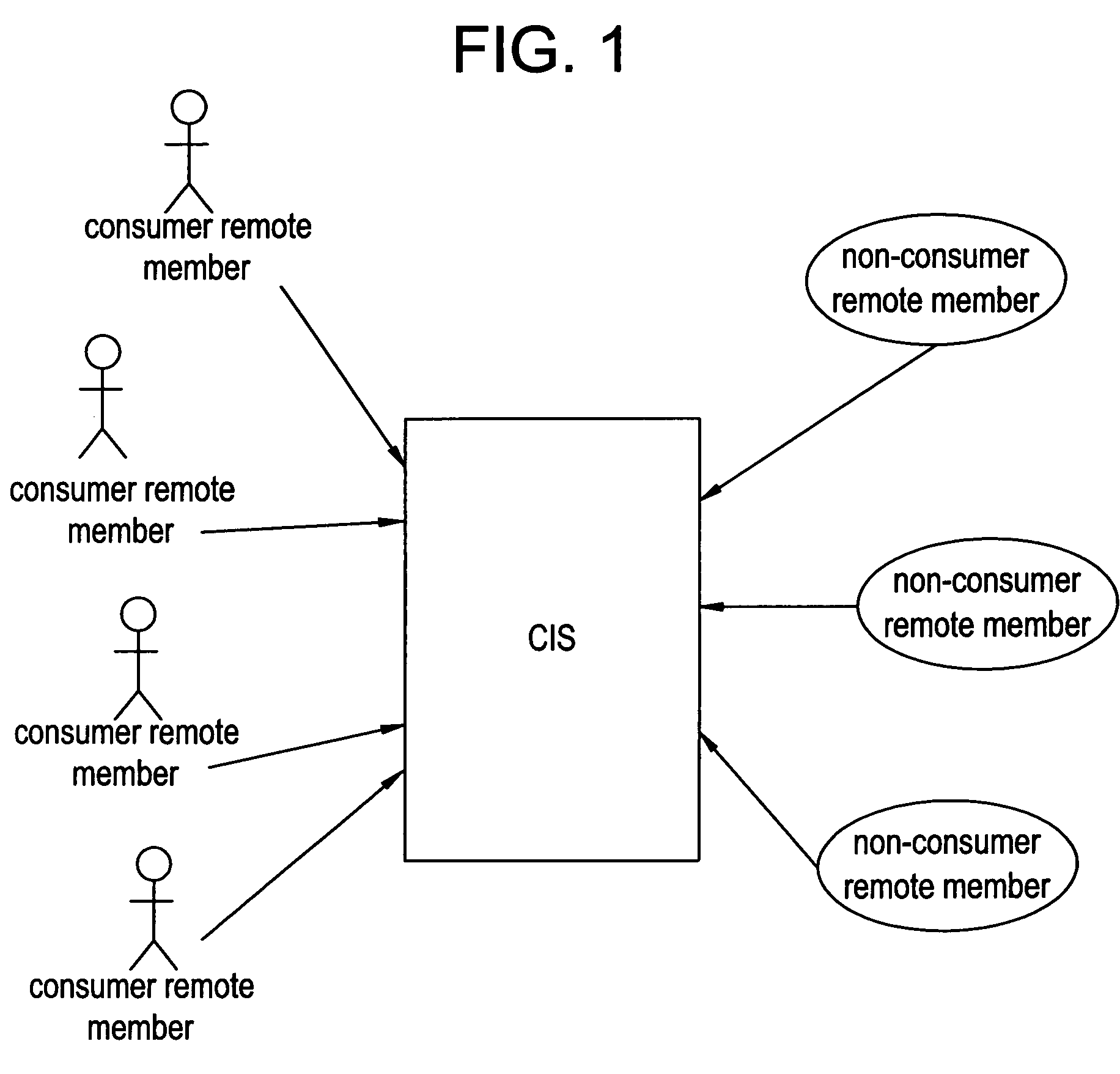
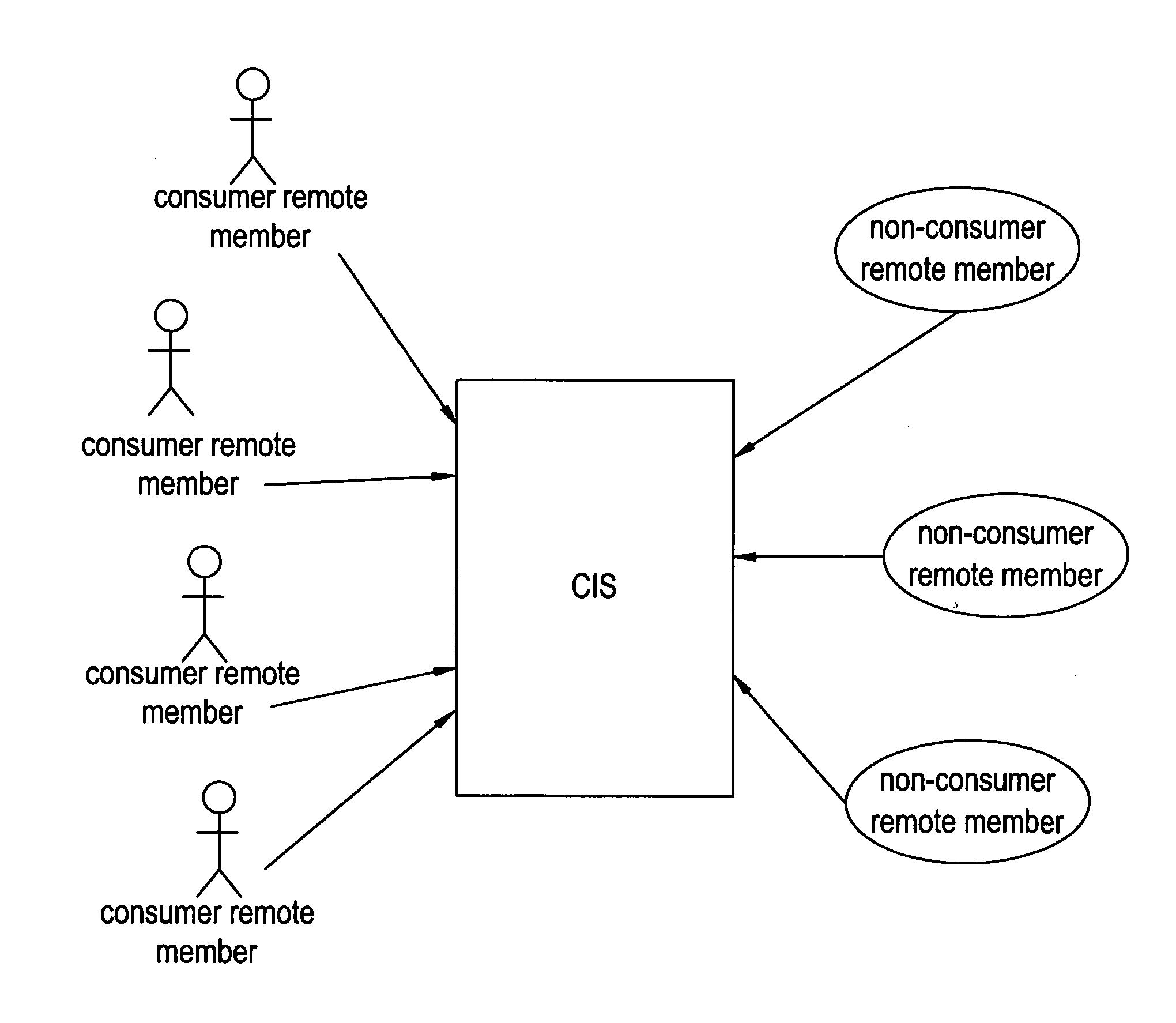
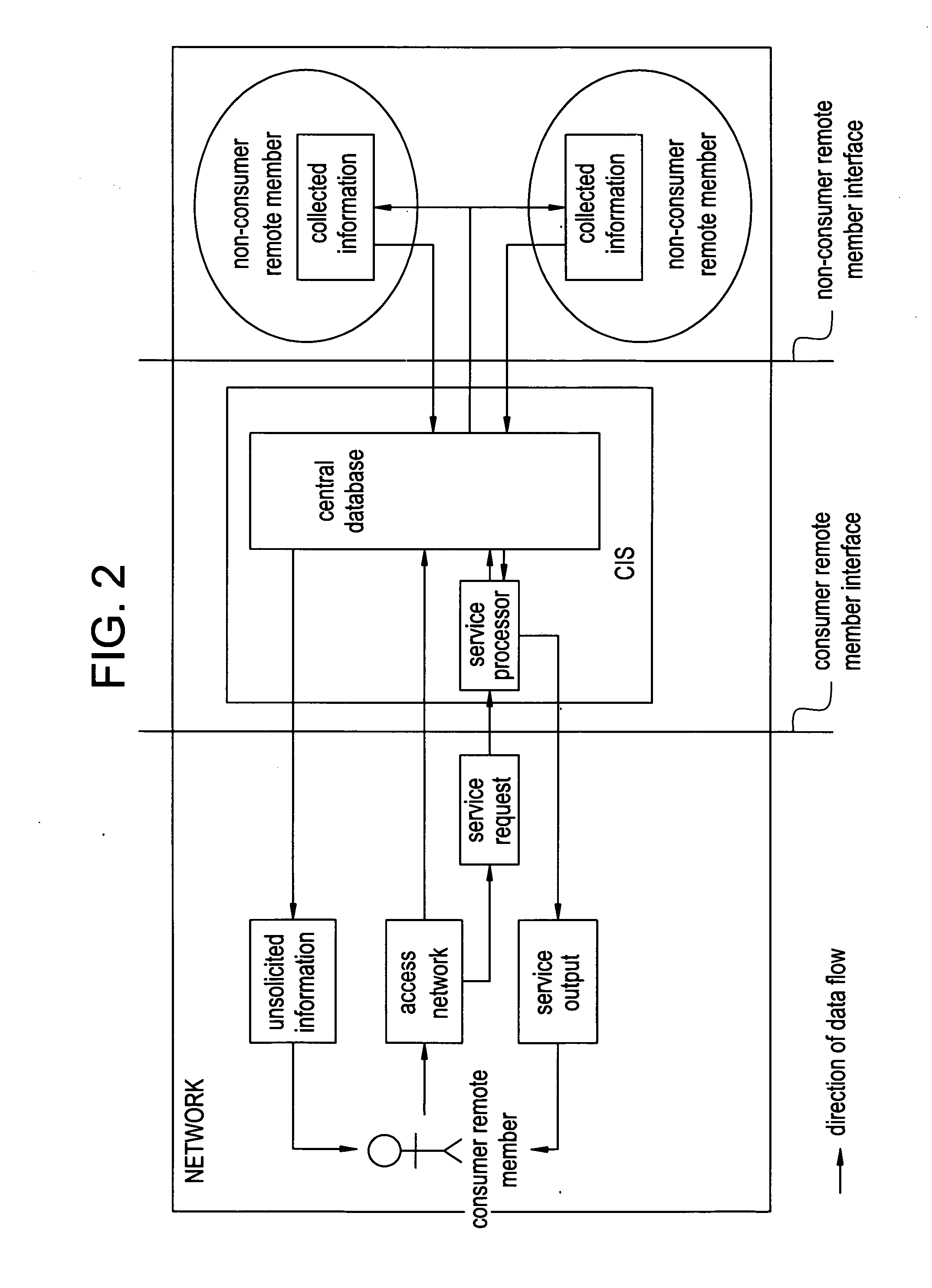
Network and methods for integrating individualized clinical test results and nutritional treatment
Owner:BODYBIO INC
Assessing colorectal cancer by measuring hemoglobin and m2-pk in a stool sample
InactiveUS20090075311A1Microbiological testing/measurementBiological material analysisMedicineBiochemical markers
The present invention relates to a method aiding in the assessment of colorectal cancer. The method especially is used in assessing the absence or presence of colorectal cancer in vitro. The method is, for example, practiced by analyzing biochemical markers, comprising measuring in a stool sample the concentration of hemoglobin and M2-PK and correlating the concentrations determined to the absence or presence of colorectal cancer. To further improve the assessment of colorectal cancer in a method of this invention the level of one or more additional marker may be determined together with hemoglobin and M2-PK in a stool sample and be correlated to the absence or presence of colorectal cancer. The invention also relates to the use of a marker panel comprising hemoglobin and M2-PK in the early diagnosis of colorectal cancer, and it teaches a kit for performing the method of the invention.
Owner:KARL JOHANN
Network and methods for integrating individualized clinical test results and nutritional treatment
The present invention provides networks and method for linking consumers and nutritional pharmacologists offering personalized nutritional information through a central network site. The network includes a central integration site through which network members communicate with each other. The central integration site stores two or more databases in the storage medium. The databases store biochemical marker data information, nutritional and / or drug data information including a record for association and effect of nutrients with a particular biochemical marker, and / or drug. The network of the invention provides individualized nutritional diagnostic and treatment to consumers on the basis of their clinical test results.
Owner:BODYBIO INC
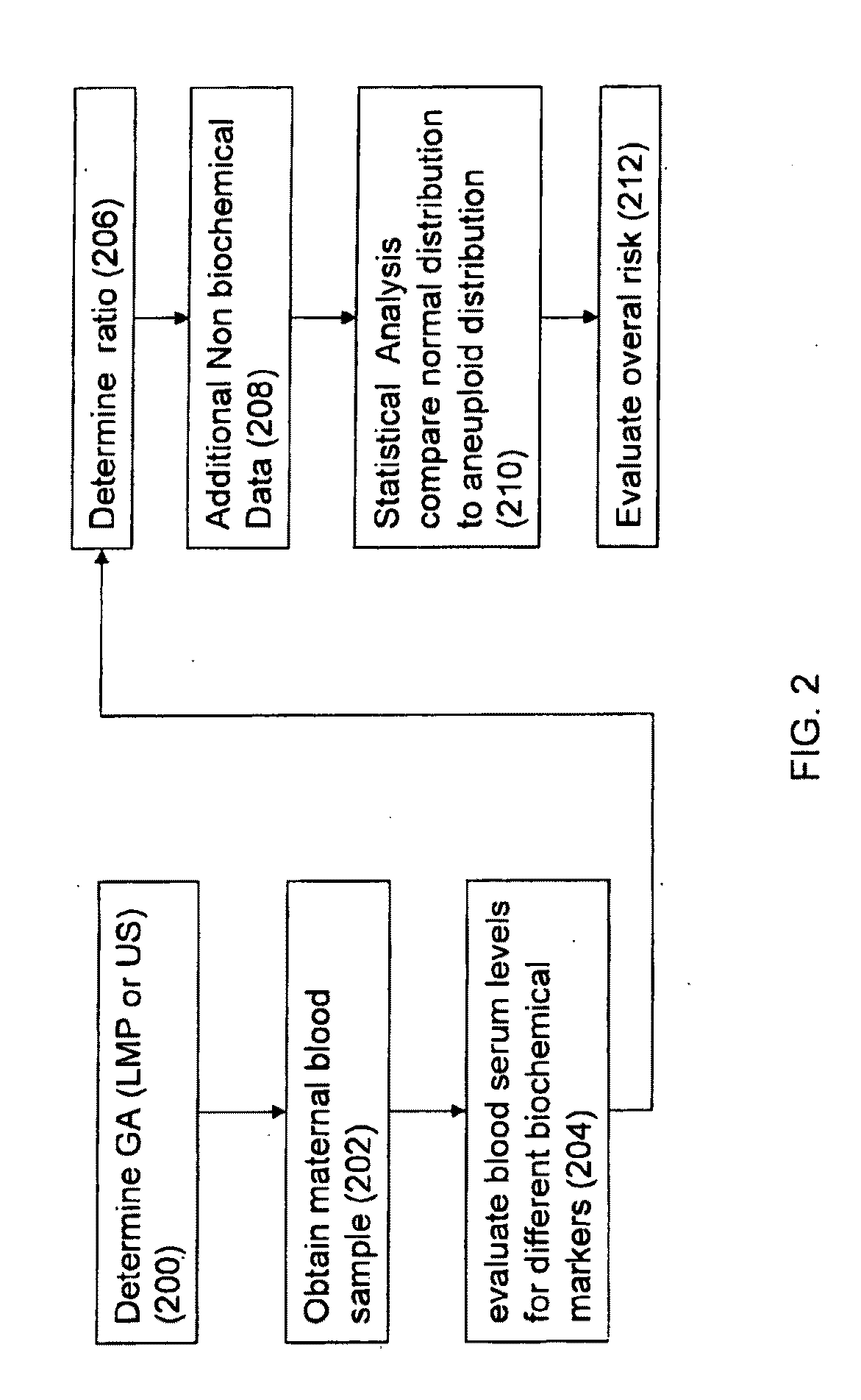
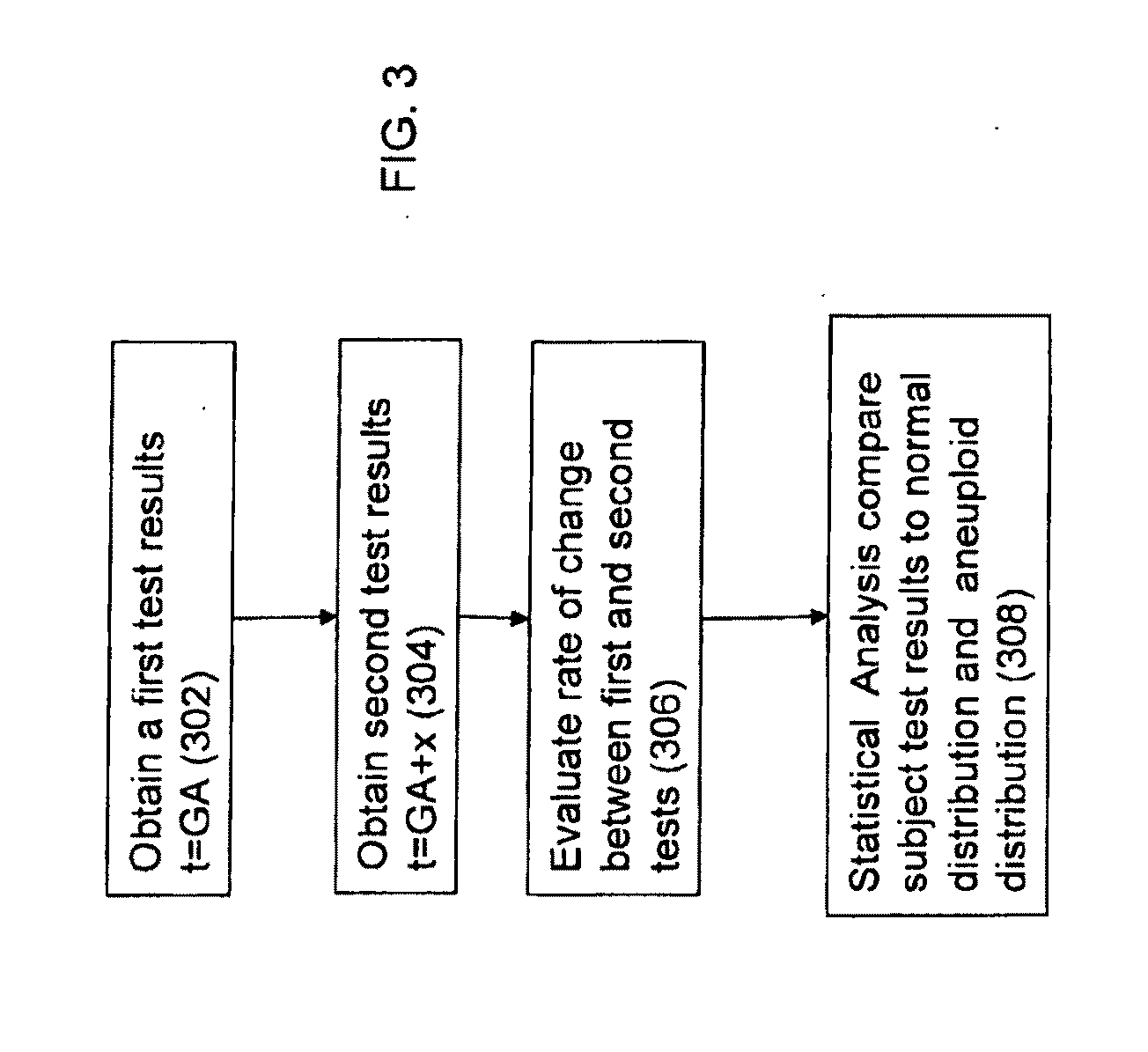
Method for antenatal estimation of risk of aneuploidy
InactiveUS20100120076A1Reducing false positive detectionImprove the detection rateMicrobiological testing/measurementBiological material analysisGenetic AnomalyTrisomy
The present invention relates to a system and a method for evaluating the risk of carrying a fetus with genetic anomalies such as aneuploidy and in particular, to such a system and method where a screening system and method is provided to identify fetus' having trisomy-21 (Down's syndrome) with the use of biochemical marker concentrations evaluated from the maternal blood serum.
Owner:BRAUN GUR +1
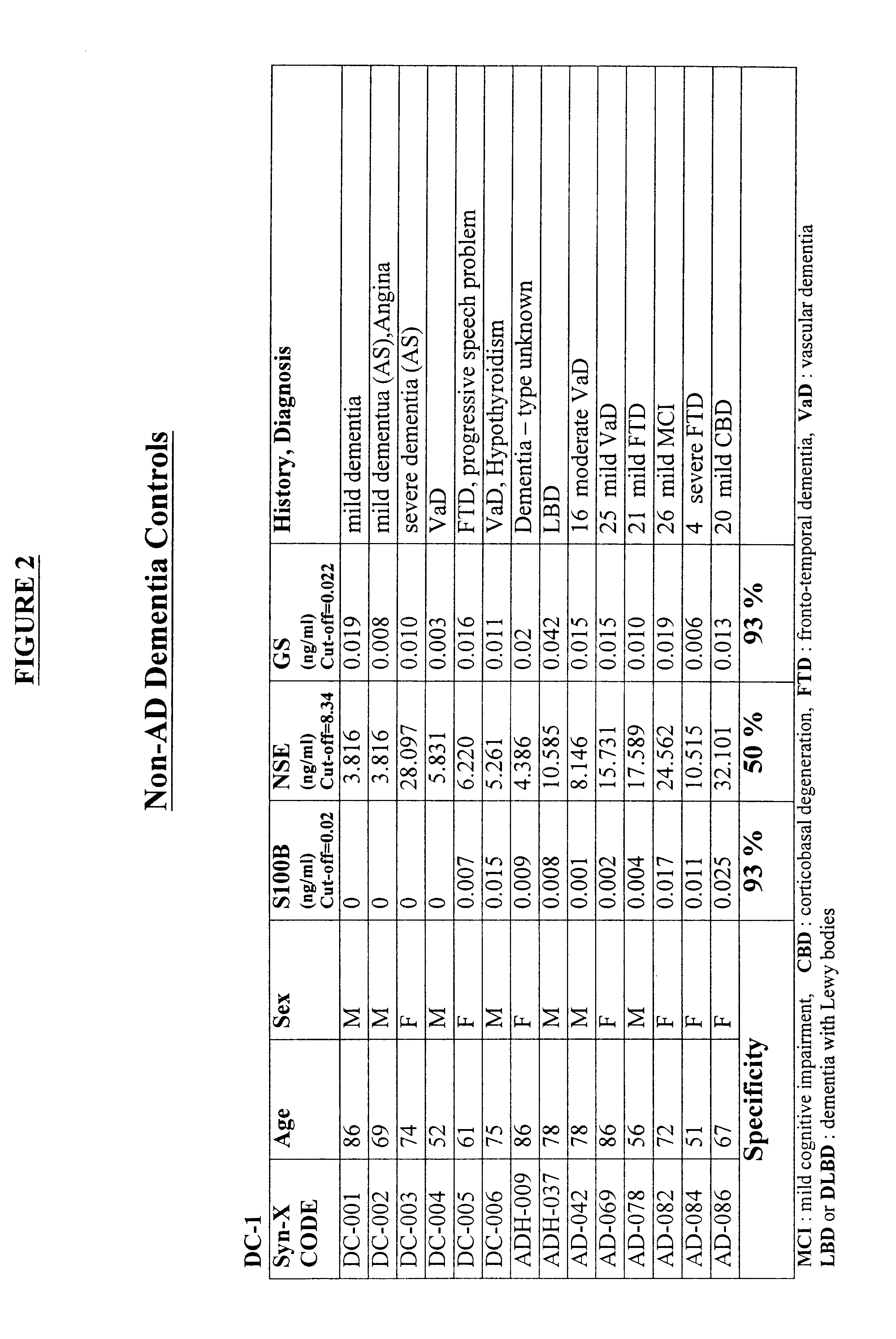
Process for differential diagnosis of Alzheimer's dementia and device therefor
InactiveUS6451547B1Efficient identificationImmunoglobulins against animals/humansDisease diagnosisWhole blood productBiochemical markers
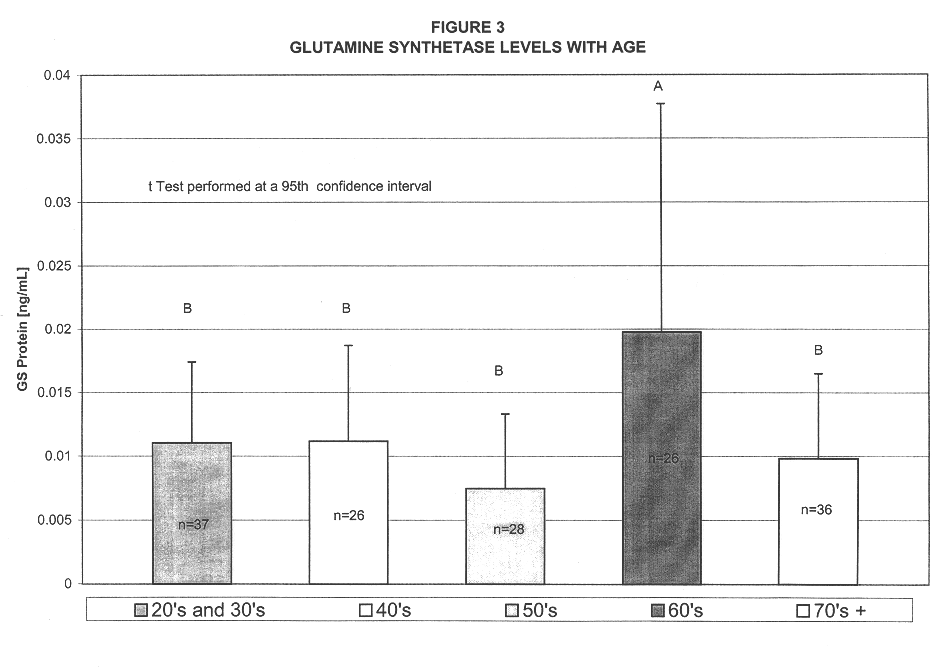
A method for diagnosing Alzheimer's disease(AD) is disclosed. The method involves directly detecting the presence of a biochemical marker, specifically human glutamine synthetase, in bodily fluid, preferably blood or a blood product. The detection is by an immunoassay incorporating an antibody specific to human glutamine synthetase. In addition, a method for distinguishing between AD and non-AD dementia is disclosed.
Owner:NANOGEN INC
Use of 1,3-diphenylprop-2-en-1-one derivatives for treating liver disorders
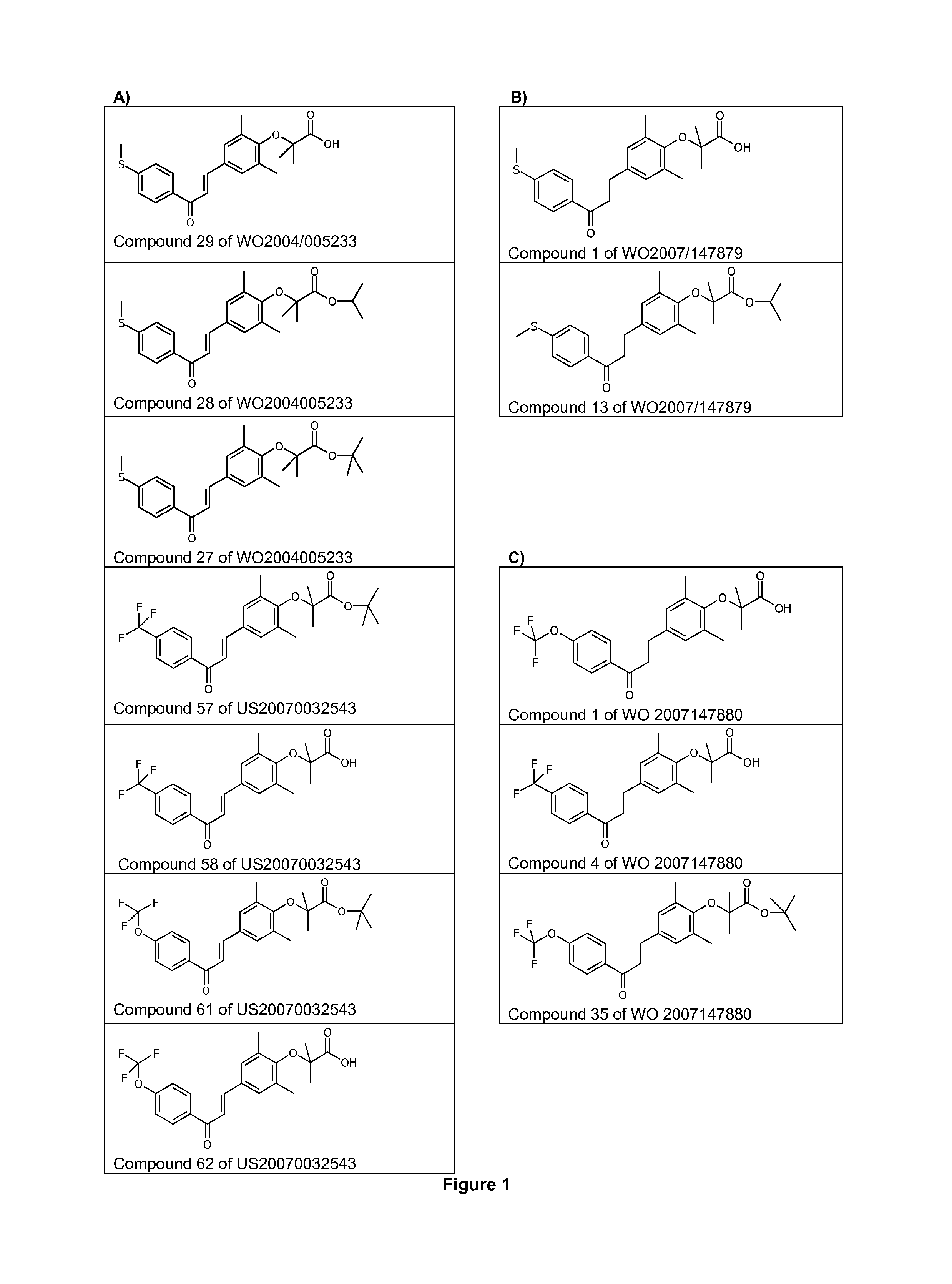
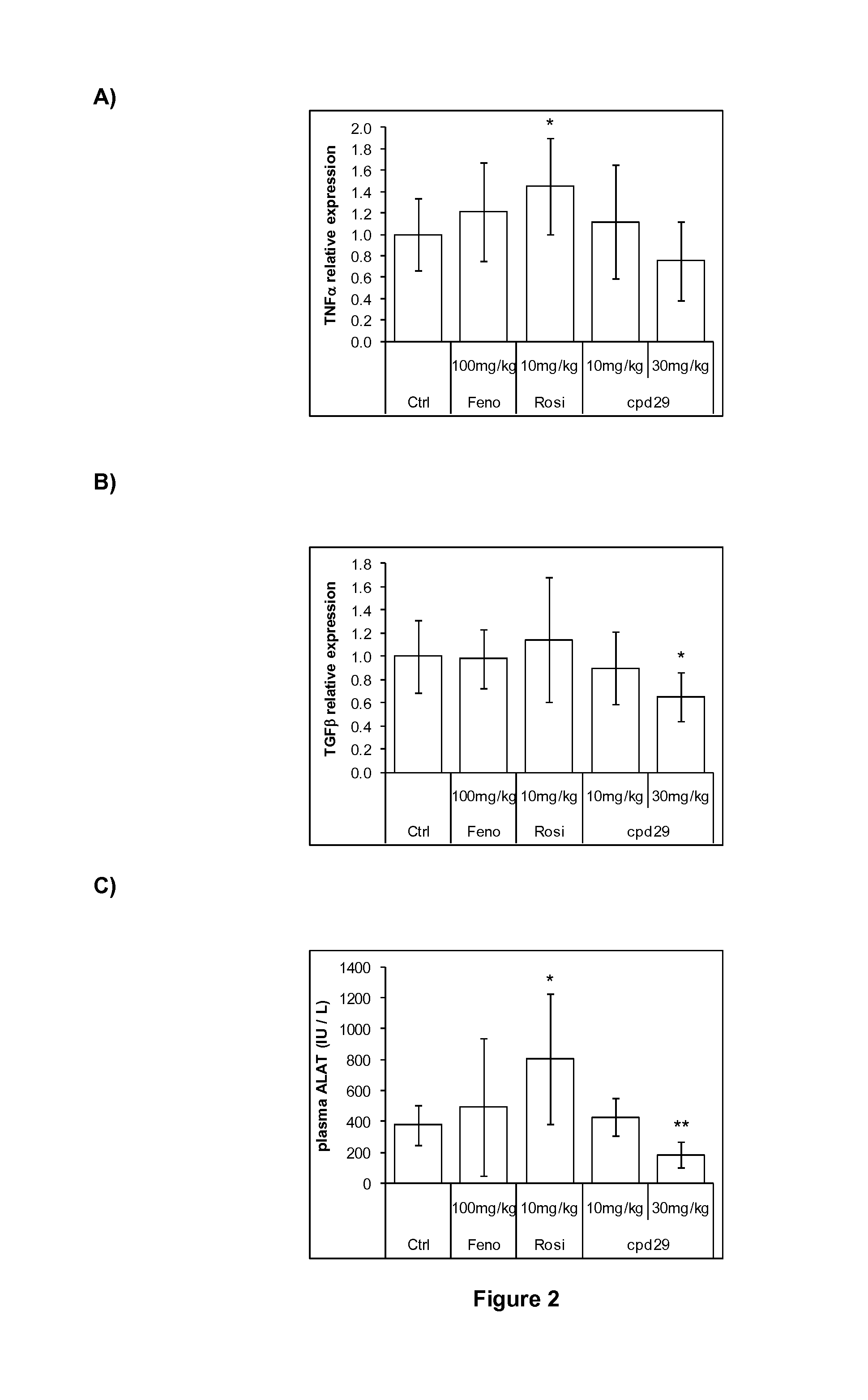
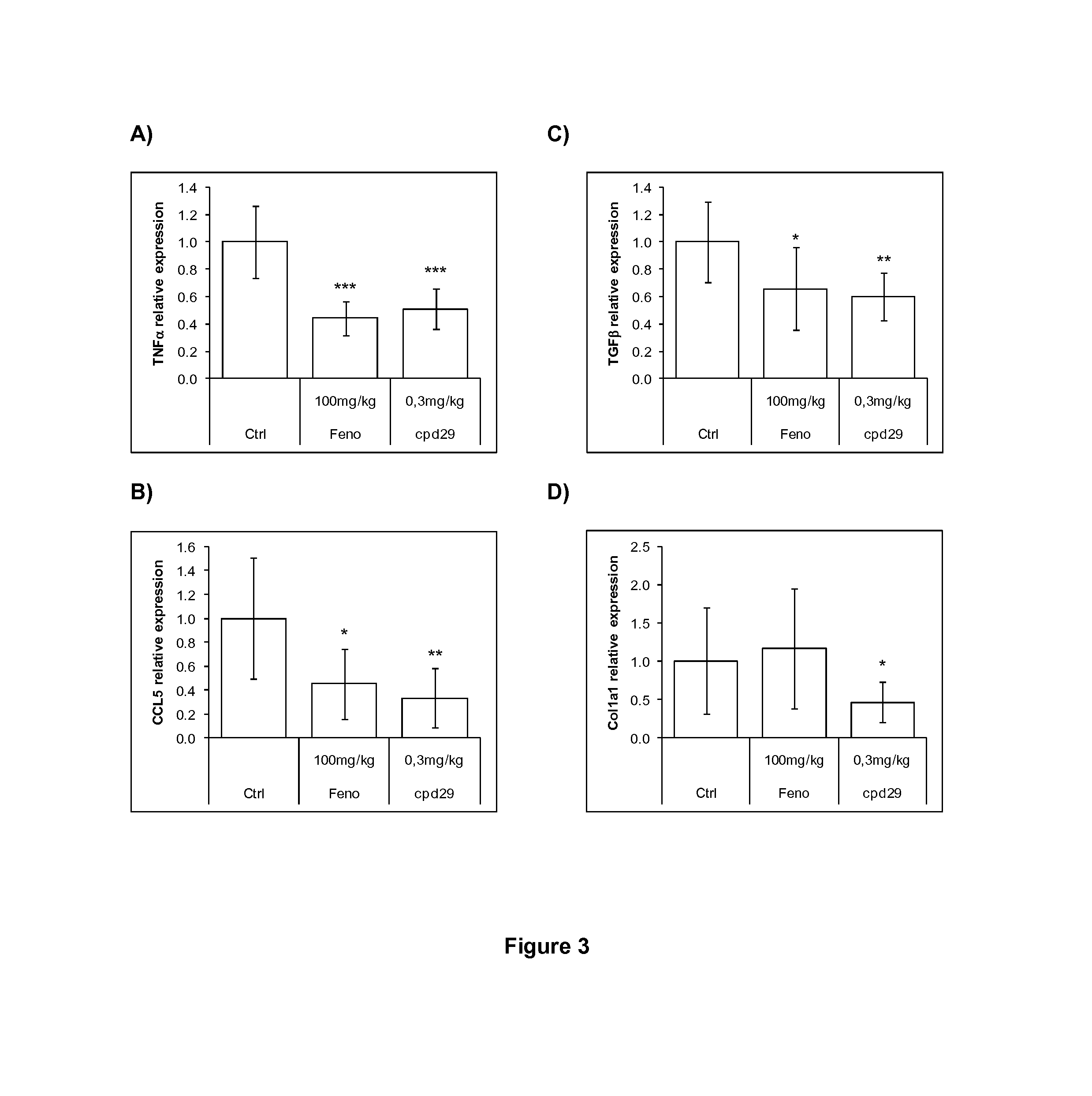
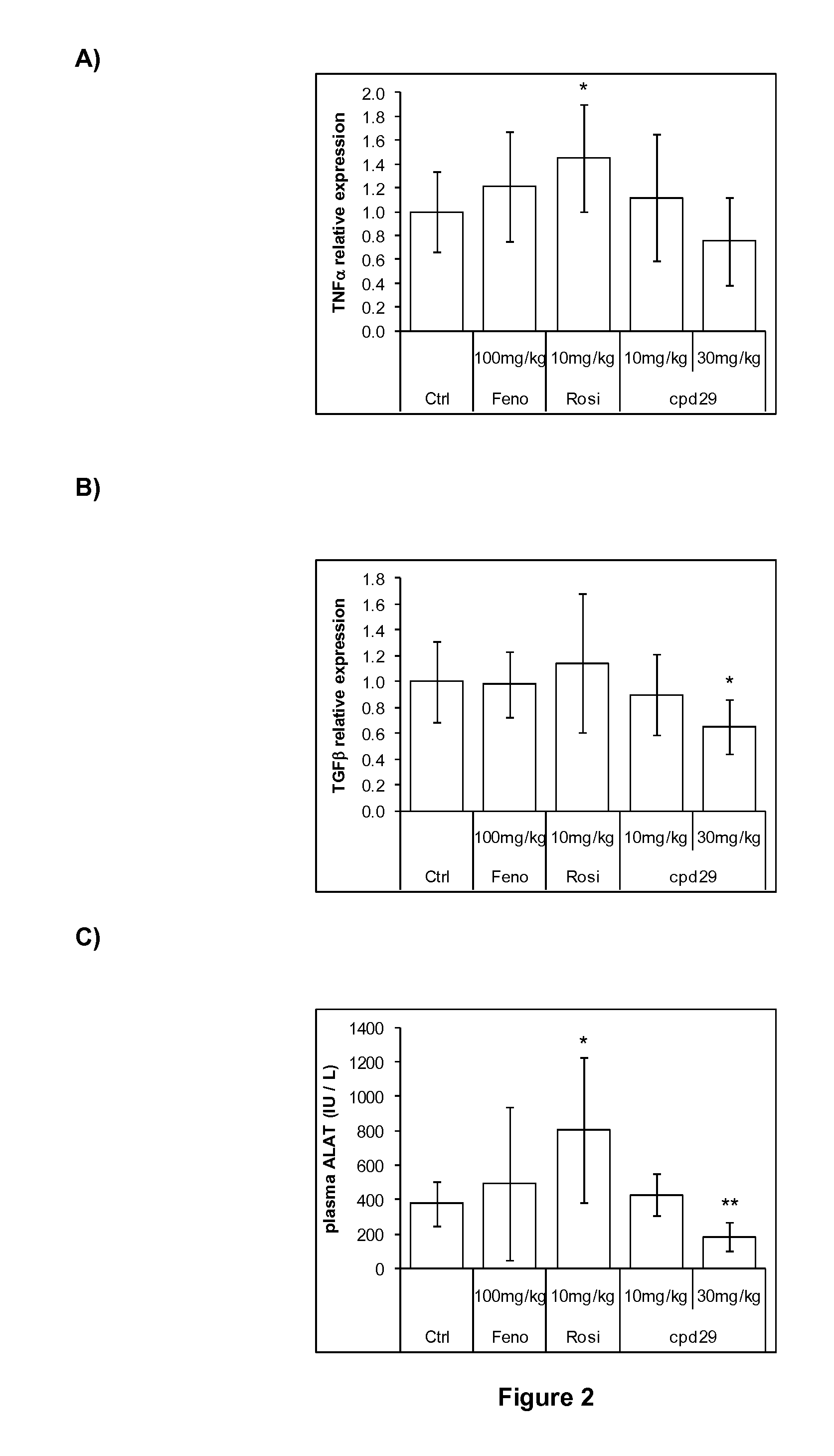
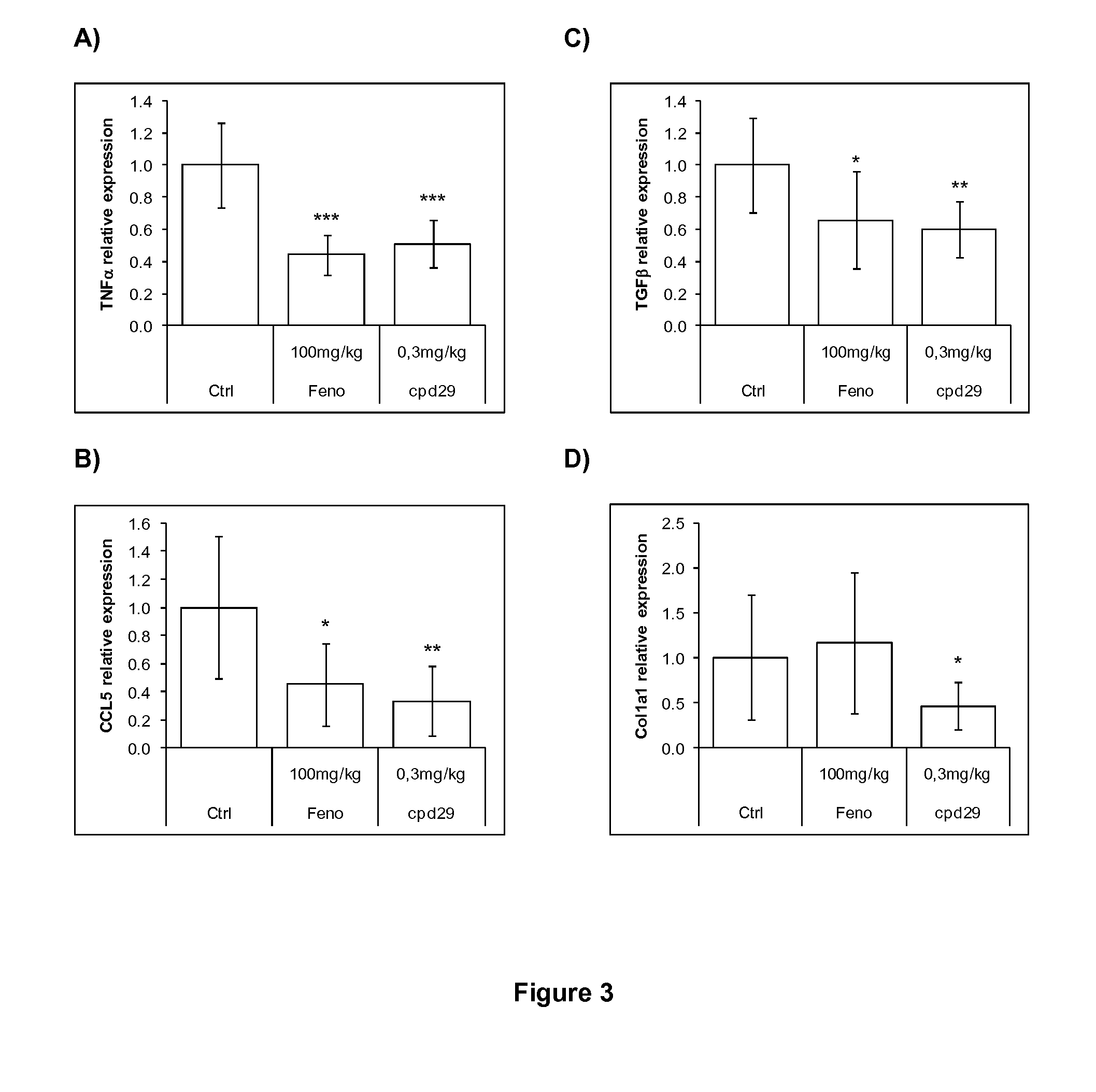
The invention provides 1,3-diphenylprop-2-en-1-one derivatives and pharmaceutical compositions comprising the same for treating liver disorders, in particular those requiring the reduction of plasma level of biochemical markers such as aminotransferases. The 1,3-diphenylprop-2-en-1-one derivatives of General Formula (I) have hepatoprotective properties and can be used in methods for treating liver disorders involving the pathological disruption, inflammation, degeneration, and / or proliferation of liver cells, such as liver fibrosis or fatty liver disease.
Owner:GENFIT SA
Anti-CCPand antinuclear antibodies in diagnosis of rheumatoid arthritis
The present invention relates to a method aiding in the assessment of rheumatoid arthritis. The method especially is used in the differential diagnosis of rheumatoid arthritis in vitro. The method is for example practiced by analyzing biochemical markers, comprising measuring in a sample both the concentration of anti-CCP and of antinuclear antibodies (ANA) correlating the concentrations determined to the diagnosis of rheumatoid arthritis. To further improve the assessment of RA in a method of this invention the level of one or more additional marker may be determined together with anti-CCP and ANA and be correlated to the absence or presence of RA. The invention also relates to the use of a marker panel comprising anti-CCP and ANA in the diagnosis of rheumatoid arthritis and it teaches a kit for performing the method of the invention.
Owner:KLAUSE URSULA +4
Diagnostic assay
The present invention relates generally to a diagnostic device including a prognostic assay for parameters which are indicative of a condition or event associated with the systemic vasculature. More particularly, the present invention provides an assay to detect parameters associated with a vascular disease including cardiovascular, stroke, pulmonary, renovascular, cerebrovascular, thrombotic or generalized arterial or venous condition or event including acute coronary syndrome such as but not limited to acute myocardial infarction, heart failure, atheromoma or a thrombotic condition. The identification of these parameters or more particularly a pattern of parameters enables the diagnosis of a condition or event or the determination of the risk of development of a condition or event associated to the systemic vasculature. Still more particularly, the present invention is directed to a diagnostic device comprising a set of members wherein one or more of said members has or have specific or generic binding partners in a biological sample from an animal including human subject wherein the pattern of binding of the members to the binding partners is indicative, predictive or otherwise associated with a likelihood of a condition or event within the systemic vasculature. The absence of detection of specific or generic binding partners is also of indicative or predictive value. This is particularly important in cases where patients are unable to communicate advice to a physician on their own condition, such as during surgery or for patients in a coma. It is also useful in determining the risk of a vascular disease including cardiovascular, stroke, pulmonary, renovascular, cerebrovascular, thrombotic or generalized arterial or venous conditions or events in a healthy subject or a subject entering into an exposure to risk such as surgery or chemotherapy. The present invention is useful inter alia for the identification and / or quantitation of biochemical markers of conditions or events in the systemic vasculature such as heart disease, heart disorders, infections of the heart, stroke and thrombosis as well as the determination of a risk of development of these conditions including the absence of disorders or absence of risk of the development of a disorder. The assessment of such conditions may be made in a clinical setting, as part of triage, as part of a routine testing protocol and / or as a laboratory procedure.
Owner:SYDNEY UNIV OF
Macrophage migration inhibitory factor as a marker for cardiovascular risk
InactiveUS7445886B2Microbiological testing/measurementPeptide preparation methodsAssayHematological test
Macrophage migration inhibitory factor (MIF) is a clinically useful biochemical marker of cardiovascular risk. Risk assessment includes the step of detecting in the blood of a person MIF concentration as a marker of cardiovascular risk for the person. The method may further comprise the step of assigning to the person a cardiovascular risk metric proportional to the MIF concentration, and / or prescribing for the person a cardiovascular treatment modality in accordance with the MIF concentration. The method is useful as a primary screen, and may be used in conjunction with or as a substitute for additional tests, such as a stress test, CRP assay, LDL assay, etc. The detecting step may be repeated over time intervals and / or treatment to monitor change in cardiovascular risk for the person over time and / or treatment.
Owner:COOPER INST THE +1
Diagnosis method of alcholic or non-alcoholic steato-hepatitis using biochemical markers
ActiveUS20060172286A1Reduce in quantityLow costMicrobiological testing/measurementDiagnostic recording/measuringLiver fibrosisBiochemical markers
The present invention is drawn to a new diagnosis method for detecting the extent of alcoholic or non-alcoholic steato-hepatitis in a patient, in particular in a patient suffering from a disease involving alcoholic or non-alcoholic steato-hepatitis or who already had a positive diagnosis test of liver fibrosis and / or presence of liver necroinflammatory lesions, by using the serum concentration of easily detectable biological markers. The invention is also drawn to diagnosis kits for the implementation of the method.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS
Network and methods for integrating individualized clinical test results and nutritional treatment
The present invention provides networks and method for linking consumers and nutritional pharmacogeneticists offering personalized nutritional information through a central network site. The network includes a central integration site through which network members communicate with each other. The central integration site stores two or more databases in the storage medium. The databases store biochemical marker data information, nutritional and / or drug data information including a record for association and effect of nutrients with a particular biochemical marker, and / or drug. The network of the invention provides individualized nutritional diagnostic and treatment to consumers on the basis of their genetic test results.
Owner:BODYBIO INC
Use of HE4 and other biochemical markers for assessment of ovarian cancers
InactiveUS20070286865A1Improve the level ofRaise the possibilityAntibody ingredientsImmunoglobulinsPhysiologyBiochemical markers
Owner:MOORE RICHARD +1
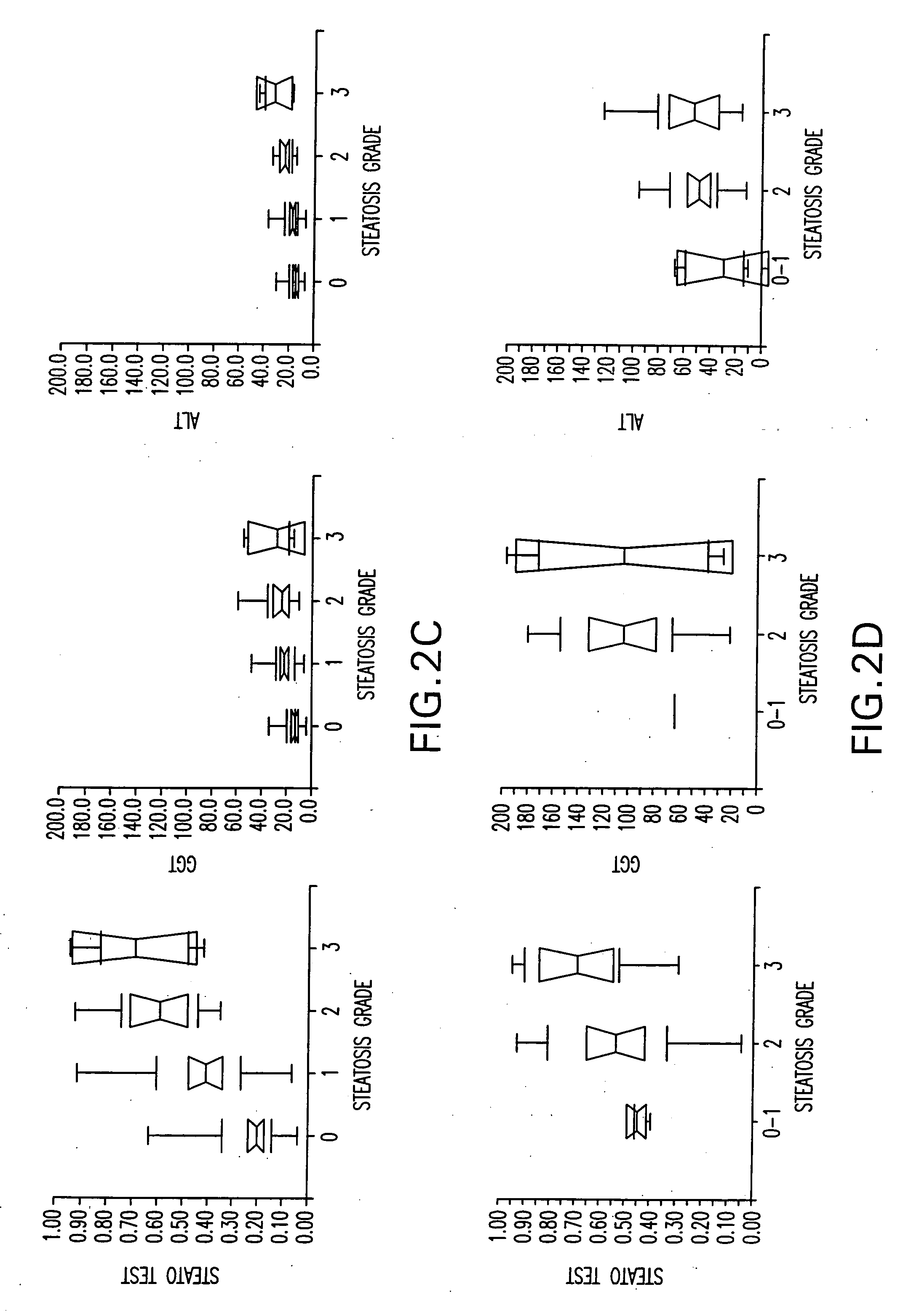
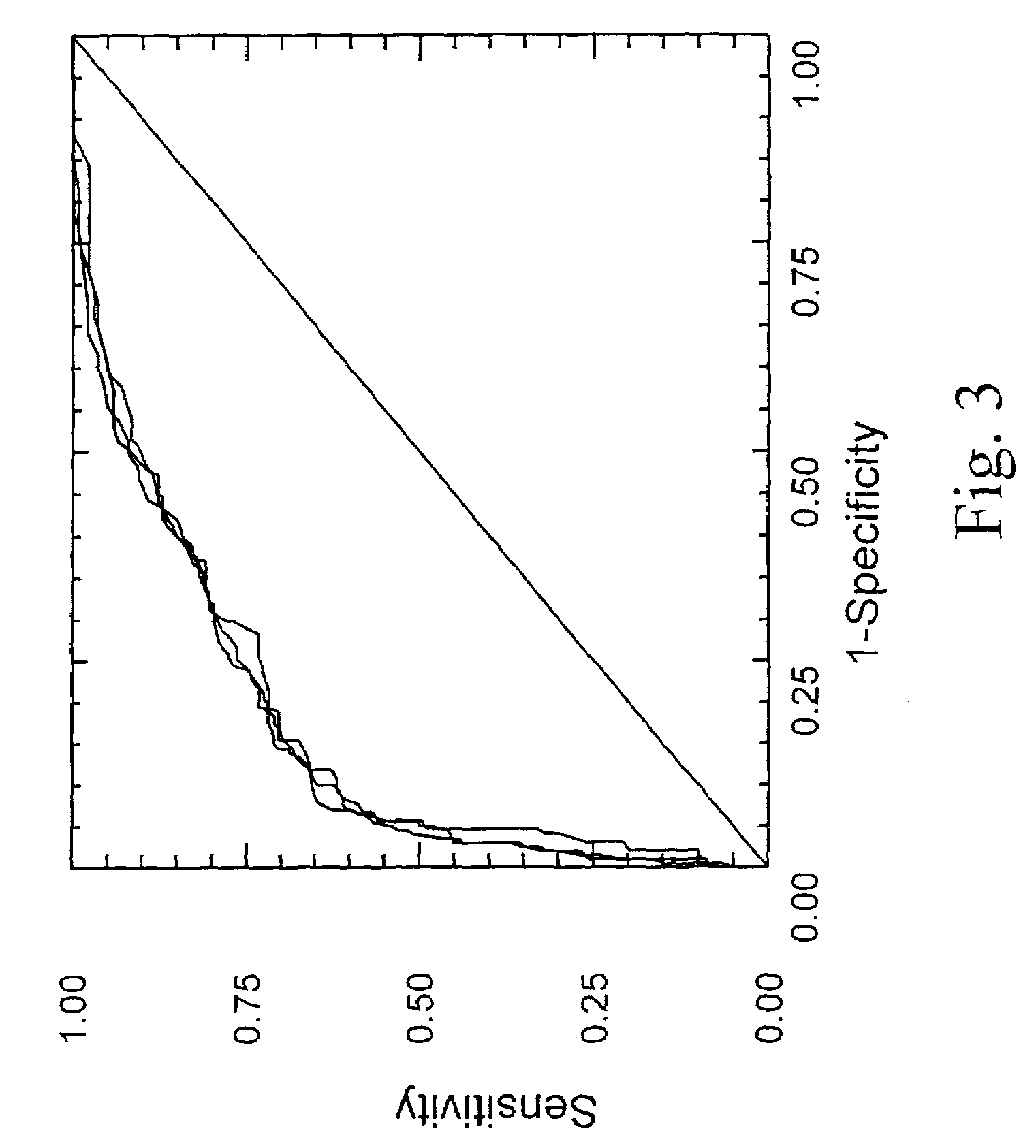
Diagnosis method of hepatic steatosis using biochemical markers
ActiveUS20060173629A1Low costReduce riskData processing applicationsMicrobiological testing/measurementLiver fibrosisBiochemical markers
The present invention is drawn to a new diagnosis method for detecting the extent of hepatic steatosis in a patient, in particular in a patient who suffers from a disease involving hepatic steatosis, or who already had a positive diagnosis test of liver fibrosis and / or presence of liver necroinflammatory lesions, by using the serum concentration of easily detectable biological markers. The invention is also drawn to diagnosis kits for the implementation of the method.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS
Diagnosis method of inflammatory, fibrotic or cancerous disease using biochemical markers
InactiveUS7225080B2Reduce in quantityLow costAmplifier modifications to reduce noise influenceDigital computer detailsLiver fibrosisBiochemical markers
The present invention is drawn to a new diagnosis method for detecting the extend of a inflammatory, fibrotic or cancerous disease in a patient, in particular liver fibrosis, in particular in a patient infected with hepatitis C virus, by using the serum concentration of easily detectable biological markers. The invention is also drawn to diagnosis kits for the implementation of the method.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS
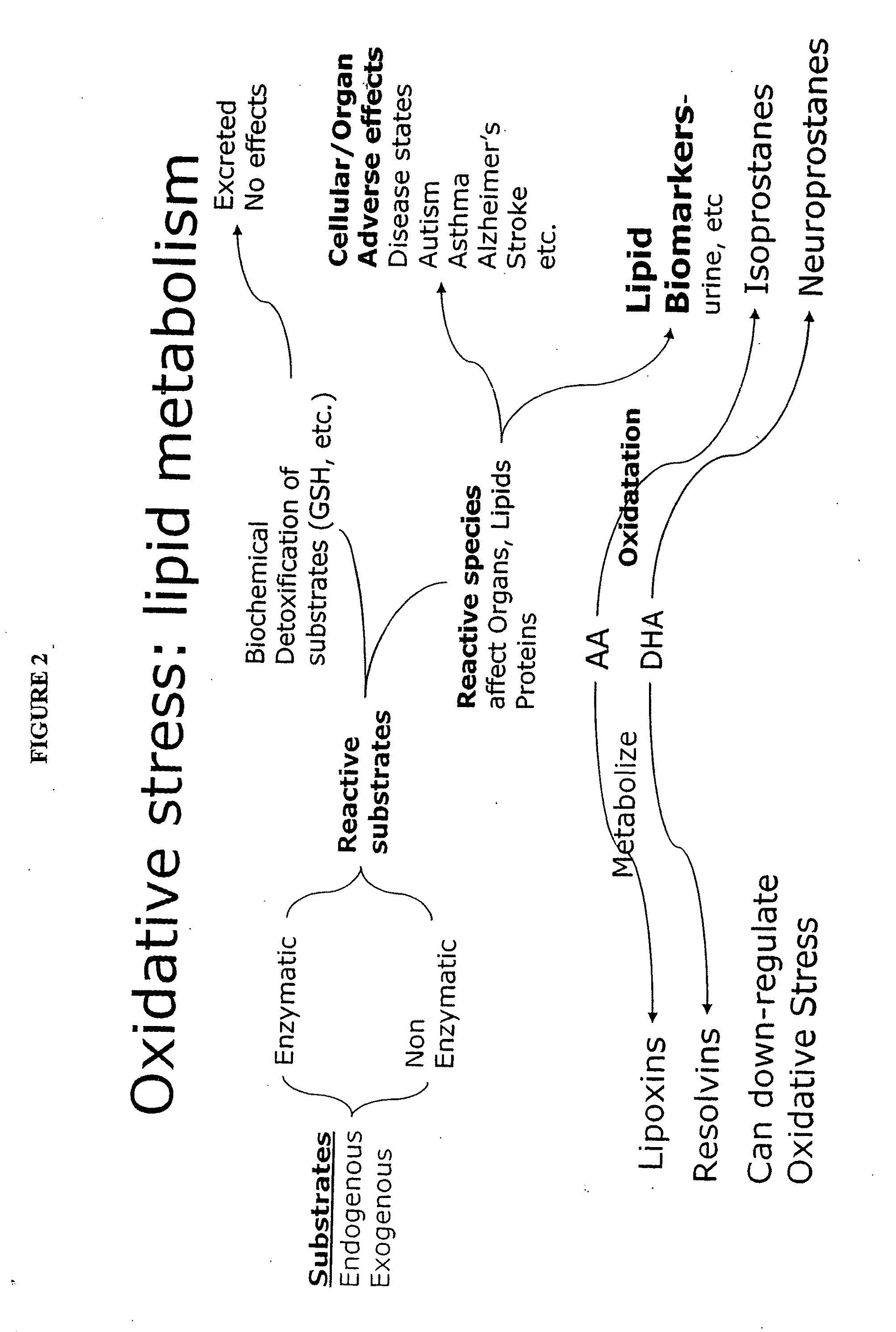
Biochemical Markers for Disease States and Genes for Identification of Biochemical Defects
InactiveUS20110229883A1Microbiological testing/measurementMaterial analysisMetaboliteOxidative stress
The present invention relates to a system utilizing biochemical markers and genetic markers to diagnose, predict, and / or monitor intervention of a number of diseases and conditions that have unresolved oxidative stress as an important component. The present invention relates generally to markers and assays for diagnosing, predicting, and monitoring disease, particularly disease-relevant oxidative stress and lipid metabolites and mediators. The oxidative stress, lipid metabolite and lipid mediator biochemical and genetic markers may be further combined with other disease associated or disease relevant markers in methods and assays for diagnosis, monitoring, and assessment of disease, particularly of complex diseases with multi-component factors. The system, methods and assays are applicable to various diseases, including autism, asthma, and Alzheimer's disease.
Owner:ROWAN UNIVERSITY
Use of HE4 and other biochemical markers for assessment of endometrial and uterine cancers
InactiveUS20080020473A1Microbiological testing/measurementImmunoglobulinsAbnormal tissue growthGynecology
The disclosure relates to use of the HE4 / HE4a marker(s) to assess endometrial and uterine cancer in a patient. The disclosure also relates to using tumors marks for diagnosis, grading and staging of endometrial and uterine cancers. The disclosure also relates to determining prognosis and treatment effectiveness of a patient who has been diagnosed with endometrial or uterine cancer.
Owner:FUJIREBIO AMERICA
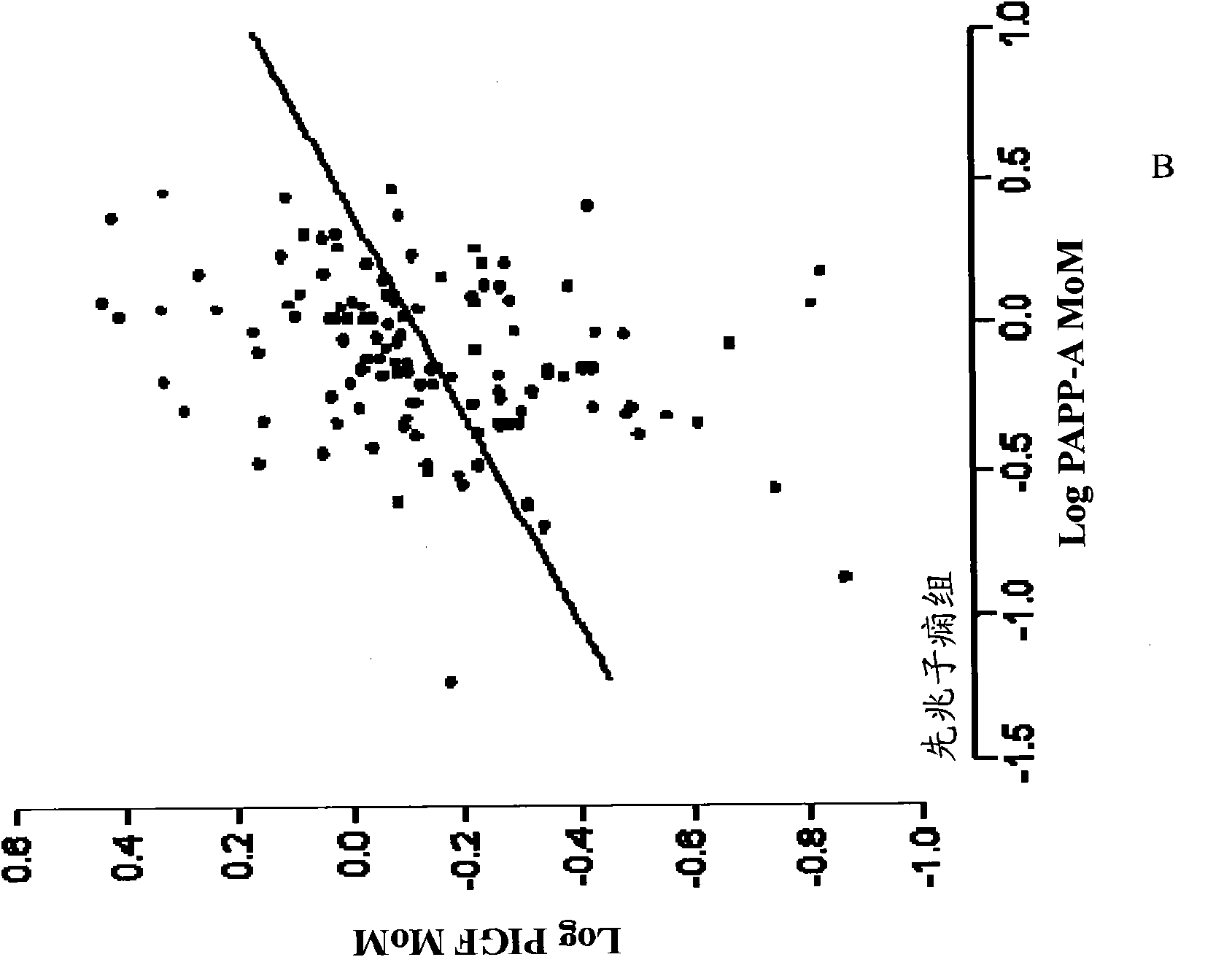
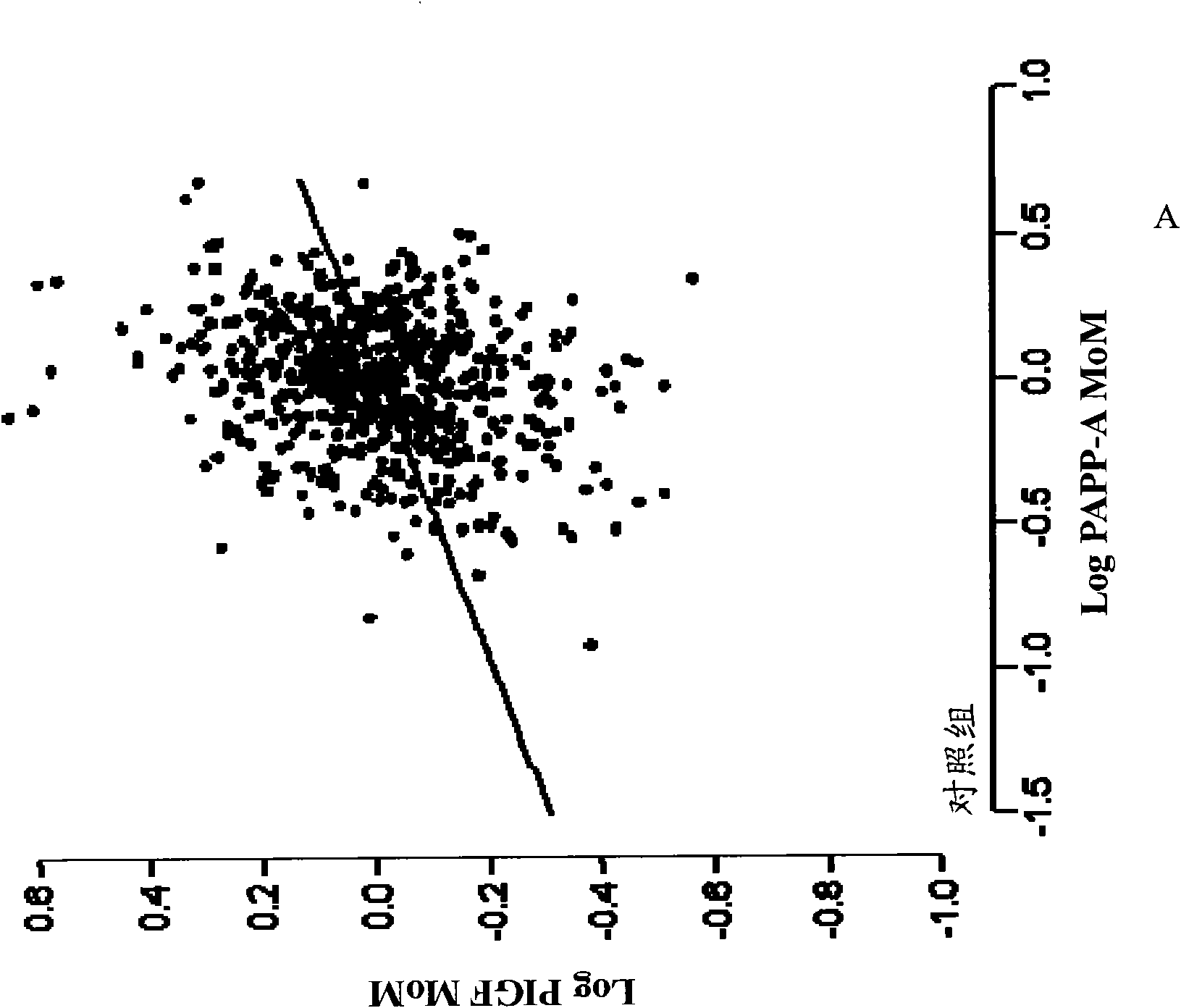
Methods for determining the risk of prenatal complications
The disclosure relates to methods, medical profiles, kits and apparatus for use in determining the risk that a pregnant individual has for developing pre-eclampsia based on amounts of certain biochemical markers in a biological sample from the individual and biophysical markers. The disclosure also relates to methods, medical profiles, kits and apparatus for use in determining the risk that a pregnant individual is carrying a fetus having a chromosomal abnormality based on amounts of certain biochemical markers in a biological sample from the individual and biophysical markers.
Owner:WALLAC
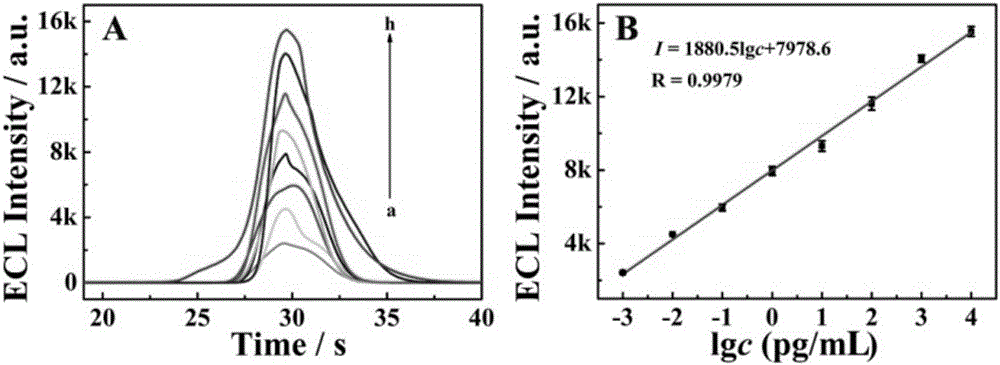
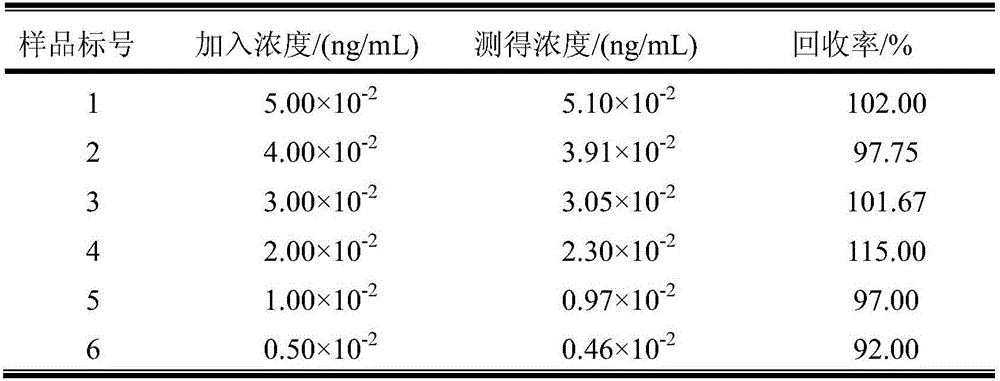
Production method, product, detection method and application of electrochemiluminescence immunosensor
InactiveCN106596969ARealize detectionStrong specificityChemiluminescene/bioluminescenceBiological testingAntigenMetal-organic framework
The invention discloses a production method, product, detection method and application of an electrochemiluminescence immunosensor based on CdTe@MOFs / CdTeQDs compound, and belongs to the field of electrochemiluminescence sensors. A metal-organic framework material is adopted to immobilize quantum dots, the special structure of the metal-organic framework material is used to immobilize the quantum dots in the metal-organic framework material and on the surface of the metal-organic framework material, the obtained composite material is used to immobilize a second antibody, a second antibody is immobilized under the assistance of a nano-gold functionalized titanium dioxide compound, and the obtained metal-organic framework material / quantum dot / antibody coupled compound and an antigen form a double-antibody sandwich immune reaction mode, so the identification and detection process of a target substance is realized, and the electrochemiluminescence signal is greatly enhanced. Effective detection of a biochemical marker is realized according to the intensity difference of the electrochemiluminescence signal of a substance-to-be detected with different concentrations.
Owner:SOUTHWEST UNIVERSITY
Pancreatic insulin-producing beta-cell lines derived from human pluripotent stem cells
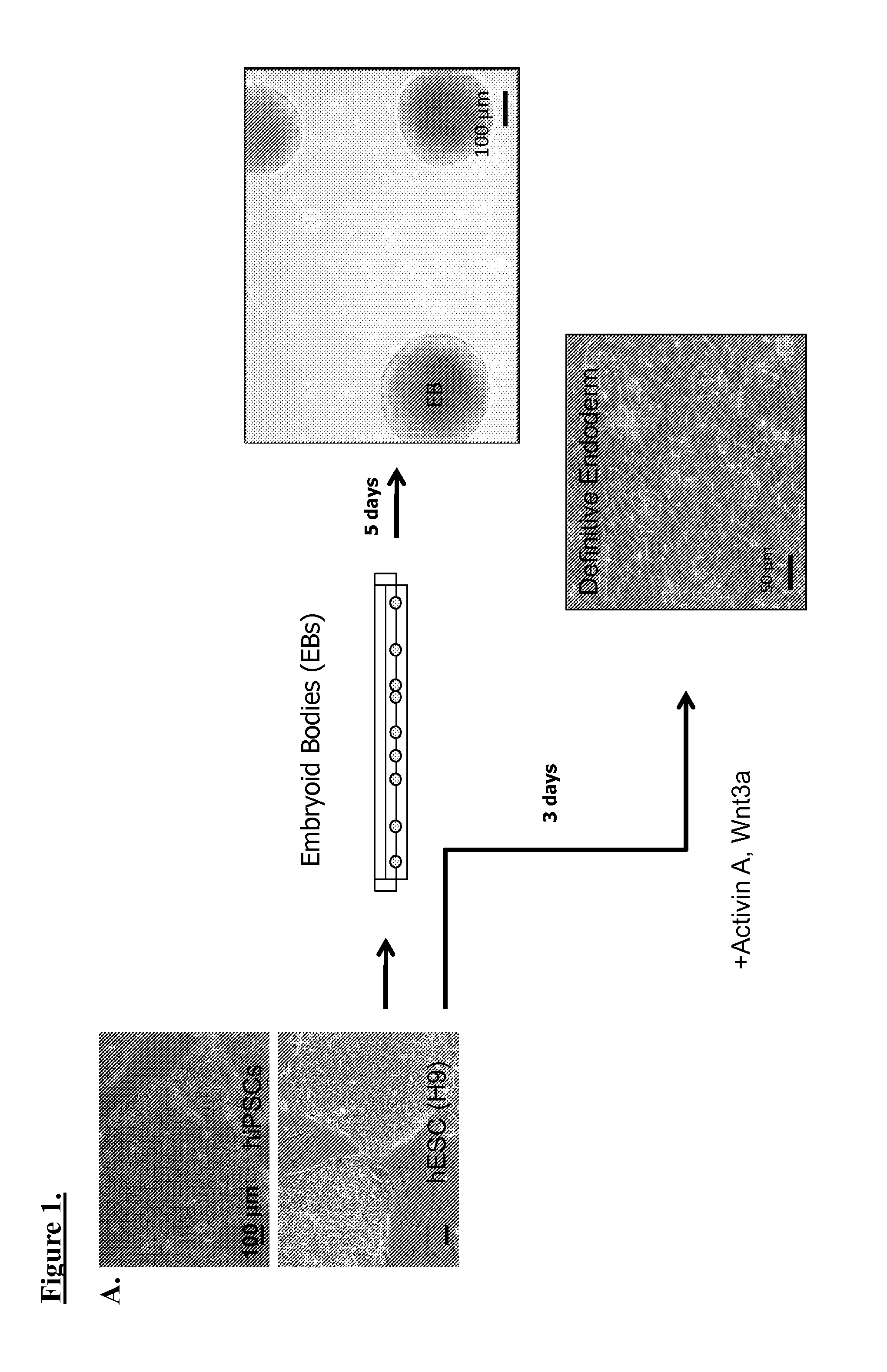
Production of beta-cells from stem cells from pluripotent stem cells have always been significantly lacking in at least one of the following properties: 1) functional properties related to insulin-production and glucose signaling response, 2) mature phenotype such as biochemical markers or cell structures, 3) efficiency in production of differentiated cells. Described herein is multistep differentiation protocol which substantially overcomes all of the existing limitations. Pluripotent stem cells, including induced pluripotent stem cells (iPSCs), and embryonic stem cells (ESCs) can be differentiated using an embryoid body (EB) formation step, followed by B maturation via endothelial cells (EC) co-culturing and incubation with a sequential series of bone morphogenic protein (BMP)-related growth factor cocktails. The resulting cells displayed functional properties, including insulin-production and glucose signaling response, and mature phenotype of C-peptide expression.
Owner:CEDARS SINAI MEDICAL CENT
Use of 1,3-diphenylprop-2-en-1-one derivatives for treating liver disorders
The invention provides 1,3-diphenylprop-2-en-1-one derivatives and pharmaceutical compositions comprising the same for treating liver disorders, in particular those requiring the reduction of plasma level of biochemical markers such as amino-transferases. The 1,3-diphenylprop-2-en-1-one derivatives of General Formula (I) have hepatoprotective properties and can be used in methods for treating liver disorders involving the pathological disruption, inflammation, degeneration, and / or proliferation of liver cells, such as liver fibrosis or fatty liver disease.
Owner:GENFIT SA
Assessing rheumatoid arthritis by measuring anti-CCP and interleukin 6
The present invention relates to a method aiding in the assessment of rheumatoid arthritis. The method especially is used in assessing the absence or presence of rheumatoid arthritis in vitro. The method is for example practiced by analyzing biochemical markers, comprising measuring in a sample the concentration of anti-CCP and interleukin 6 and correlating the concentrations determined to the absence or presence of rheumatoid arthritis. To further improve the assessment of RA in a method of this invention the level of one or more additional marker may be determined together with anti-CCP and interleukin 6 and be correlated to the absence or presence of RA. The invention also relates to the use of a marker panel comprising anti-CCP and interleukin 6 in the diagnosis of rheumatoid arthritis and it teaches a kit for performing the method of the invention.
Owner:ROCHE DIAGNOSTICS OPERATIONS
Assessing risk of disease progression in rheumatoid arthritis patients
Disclosed is an in vitro method aiding in the further assessment of patients suffering from rheumatoid arthritis. The method especially is used in assessing whether an RA patient is at risk of disease progression. The method is for example practiced by analyzing biochemical markers, comprising measuring in a sample the concentration of at least C-reactive protein (CRP) and interleukin-6 and correlating the concentrations determined to the likelihood of an underlying rapidly progressing form of RA. A patient at high risk of a rapidly progressing disease might be a patient in need for treatment or if already treated in need for a different and more effective treatment. The invention also relates to the use of a marker panel comprising C-reactive protein and interleukin-6 in the assessment of a patient with rheumatoid arthritis and it teaches a protein array device and kit, respectively, for performing the method of the invention.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Method for the prediction of vascular events and the diagnosis of acute coronary syndrome
The present invention relates to a method for the prognosis of a vascular event in a patient suspected of being at risk for a vascular event, said patient presenting: - no elevation of the ST segment as seen on an electrocardiogram, and / or - a normal level of at least one myocardial necrosis marker, wherein the presence and / or levels of at least two different biochemical markers are measured in a biological sample of said patient, whereby the probability that the patient will experience a vascular event is deduced from the measured presence and / or levels of the biochemical markers such as MPO, sCD40L, IL-6, PaPP-A, CRP, D-dimer, troponin, and proBNP.
Owner:CENT NAT DE LA RECHERCHE SCI
Scd4ol and placental growth factor (plgf) used as a biochemical marker combination in cardiovascular diseases
ActiveUS20070042438A1Peptide/protein ingredientsNGF/TNF-superfamilyVascular inflammationBiochemical markers
The invention relates to novel markers of vascular inflammation and combinations thereof as diagnostic and prognostic tools in patients with cardiovascular diseases. The markers also act as tools that facilitate the selection of active ingredients for the treatment of such diseases, and finally act as starting points for the treatment of cardiovascular diseases. Furthermore, the invention relates to the creation of an individual risk profile of negative events that are associated with the progression of arteriosclerosis.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS PRODS
Immunoassay and kit for an early and simultaneous detection of biochemical markers in a patient's sample
The present invention comprises an immunochemical assay for determination of at least two antigens in a sample. The immunochemical assay comprises contacting a sample from a patient with a carrier molecule that contains at least two capture antibodies, each of which specifically binds to a binding moiety of an antigen in the sample. The assay further contains a detection antibody that specifically binds the same antigens on different binding moieties than binding moieties used by the capture antibodies. The detection agent is further attached to one or more detection probes to facilitate the detection of antigens in the sample. The immunochemical assay of the present invention is specifically designed to detect biochemical markers that are released at different time intervals in a patient's sample.
Owner:DIAGENICS INT CORP
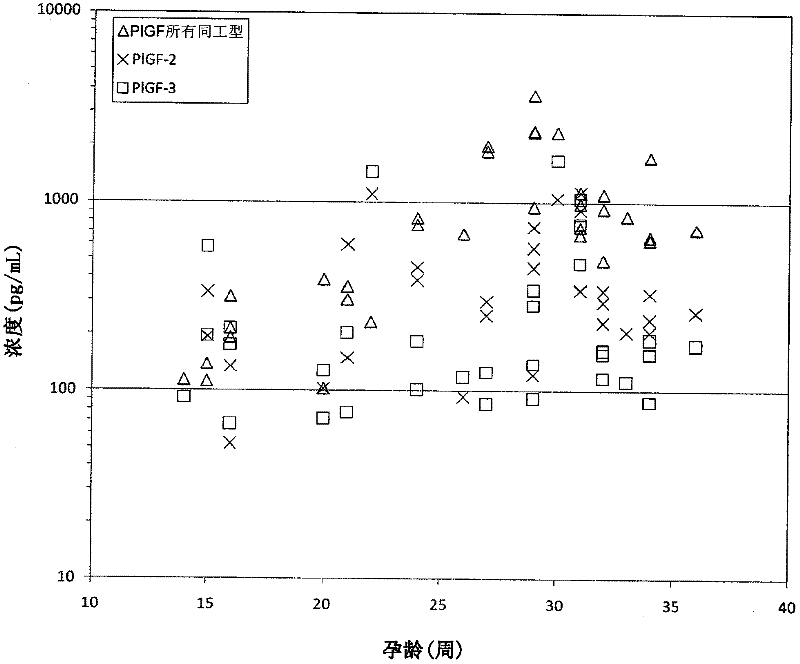
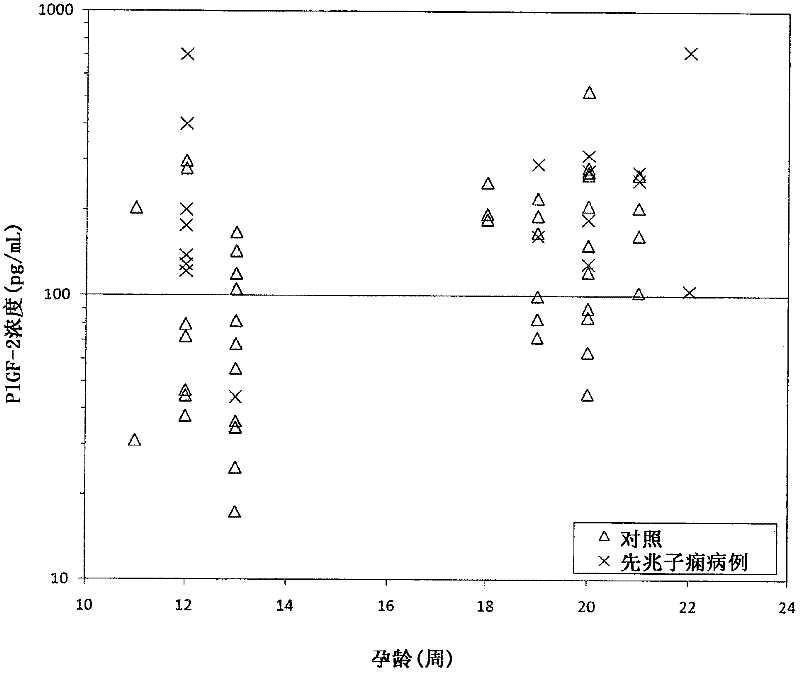
Method for determining the risk of preeclampsia using pigf-2 and pigf-3 markers
The present invention relates to a method for determining the risk of a pregnant woman developing pre-eclampsia. The method comprises i) determining the level of one or more biochemical markers in a sample obtained from a pregnant woman, and ii) comparing the level of the at least one biochemical marker in the sample with the level of the same biochemical marker in a control sample. A difference in the level of the biochemical marker in the sample relative to the control sample is indicative of an increased risk of developing pre-eclampsia. The isoform biochemical markers are preferably PlGF-2 and PlGF-3. The present invention relates also to a method for determining whether a pregnant woman has pre-eclampsia and as well as a kit for assessing the risk or presence of pre-eclampsia. In addition, the invention relates also to a computer program used in these determinations.
Owner:PERKINELMER HEALTH SCIENCES INC +2
Prognostic osteoarthritis biomarkers
InactiveUS20090299769A1Improve discriminationIncrease valueData processing applicationsComputer-assisted medical data acquisitionDisease freeBiochemical markers
A computer based calculation of a prognostic index I of osteoarthritis based on biochemical and imaging based biomarkers a mathematical combination of said values, wherein a first imaging based biomarker is a measure of the quantity of a cartilage in a joint compartment, a second imaging based biomarker relating to the quality of said cartilage in said joint compartment, and wherein a value of said first biomarker indicative of a larger quantity of cartilage affects the index to make it predictive of more disease progression, and a value of said second biochemical marker indicative of a greater departure from the quality of disease free cartilage affects the index to make it predictive of more risk of disease progression, exemplified byI=yHom+zVol+∑n=1NanOthernwhere y and z are numerical coefficients, Hom is the measured homogeneity, Vol is the measured cartilage volume, and where Othern represents N further biomarkers each having a respective numerical coefficient an, N being zero or an integer.
Owner:BIOCLINICA
Use of 1,3-diphenylprop-2-en-1-one derivatives for treating liver disorders
The invention provides 1,3-diphenylprop-2-en-1-one derivatives and pharmaceutical compositions comprising the same for treating liver disorders, in particular those requiring the reduction of plasma level of biochemical markers such as aminotransferases. The 1,3-diphenylprop-2-en-1-one derivatives of General Formula (I) have hepatoprotective properties and can be used in methods for treating liver disorders involving the pathological disruption, inflammation, degeneration, and / or proliferation of liver cells, such as liver fibrosis or fatty liver disease.
Owner:GENFIT SA
Method and apparatus for analyzing amniotic fluid
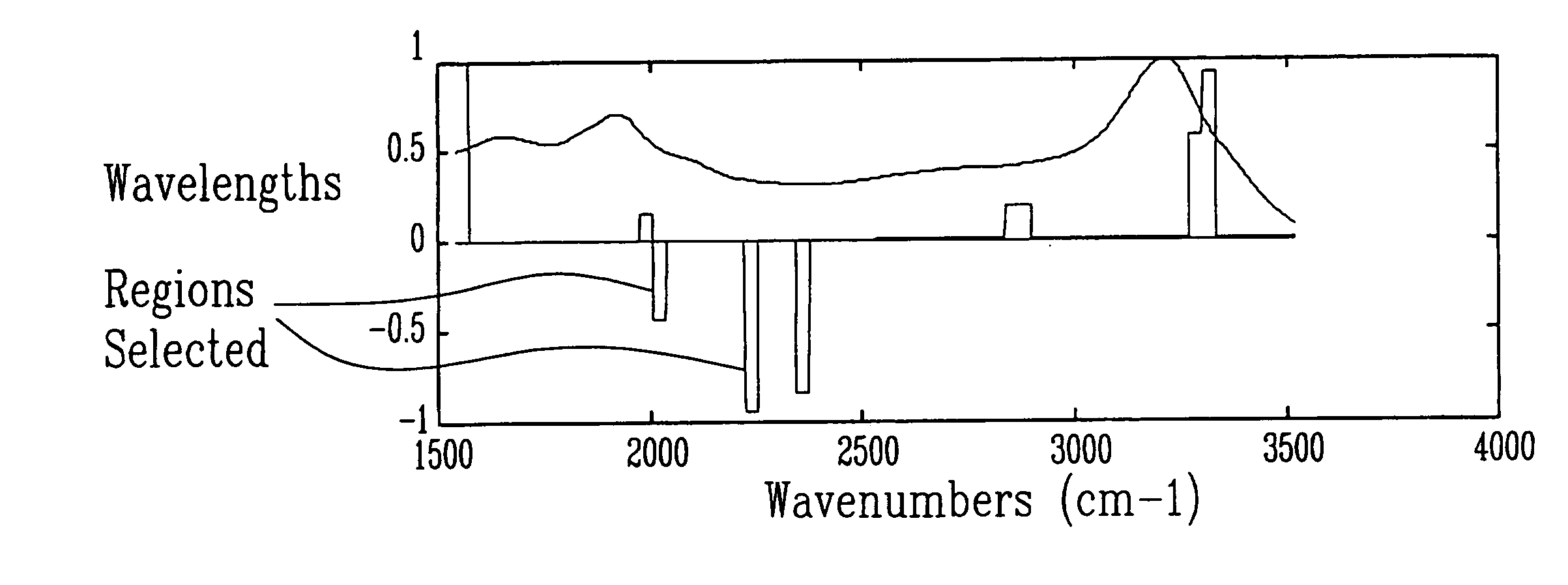
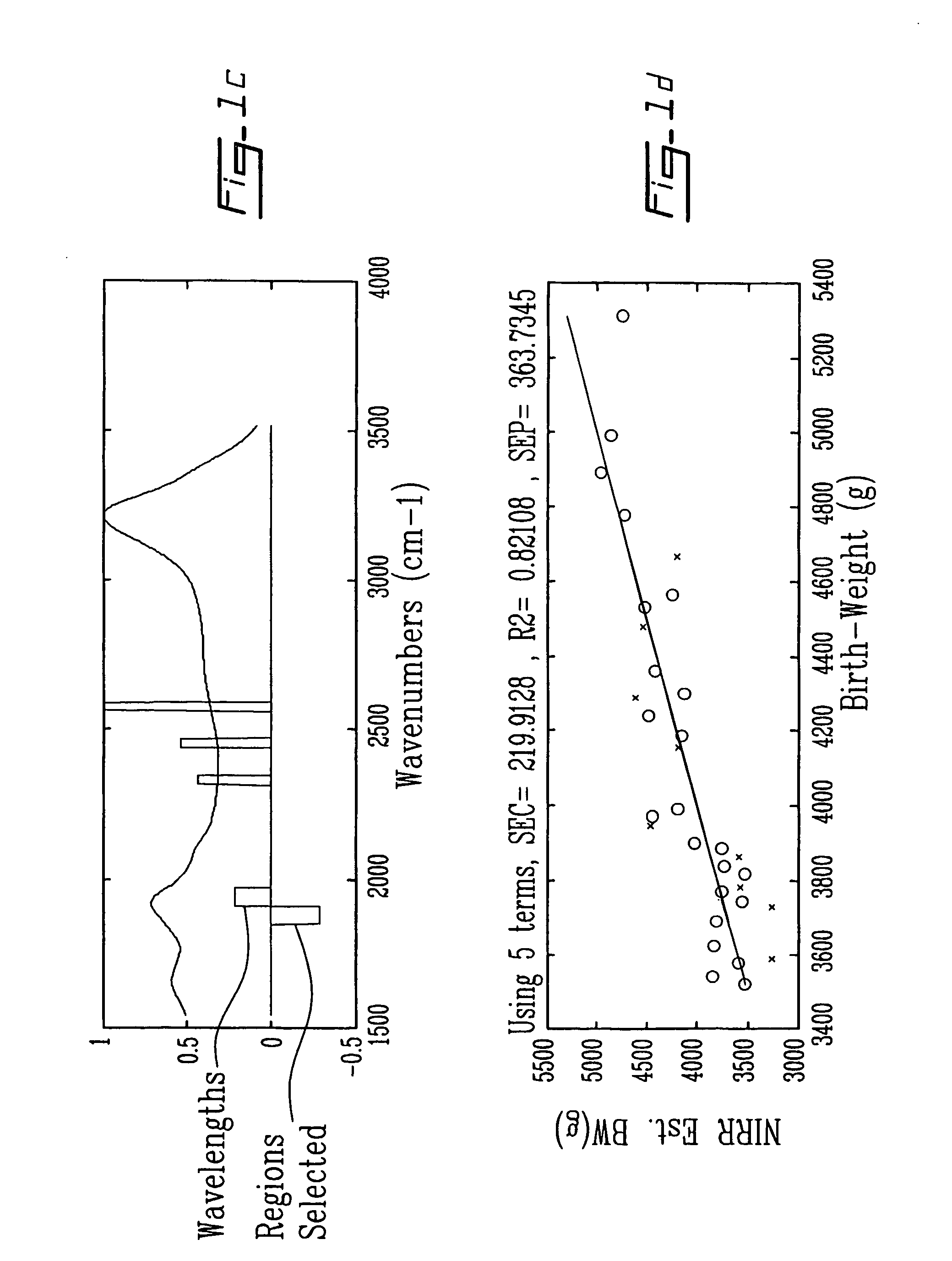
InactiveUS20060247536A1Easy to analyzeImprove accuracyHealth-index calculationDiagnostics using spectroscopyDiseaseObstetrics
Methods and spectra for monitoring fetal growth and predicting birth weight of an infant prior to birth are provided wherein one or more selected biological markers are measured in a sample of amniotic fluid obtained from a pregnant woman. Levels of the selected biochemical markers and / or spectra correlate with one or more medical conditions, such as fetal growth and birth weight of the infant, and gestational diabetes. A measurement probe for in situ measurement can be used safely and repeatedly. Monitoring and / or treatment of maternal and fetal health is also provided.
Owner:MCGILL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com