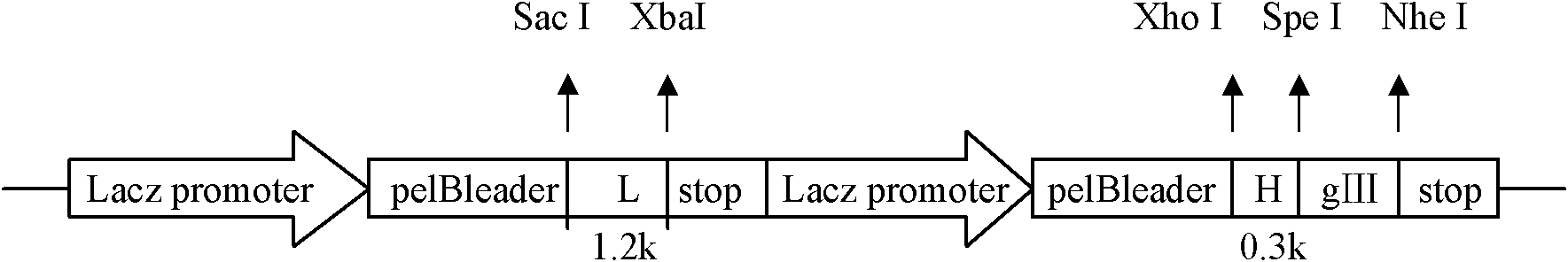
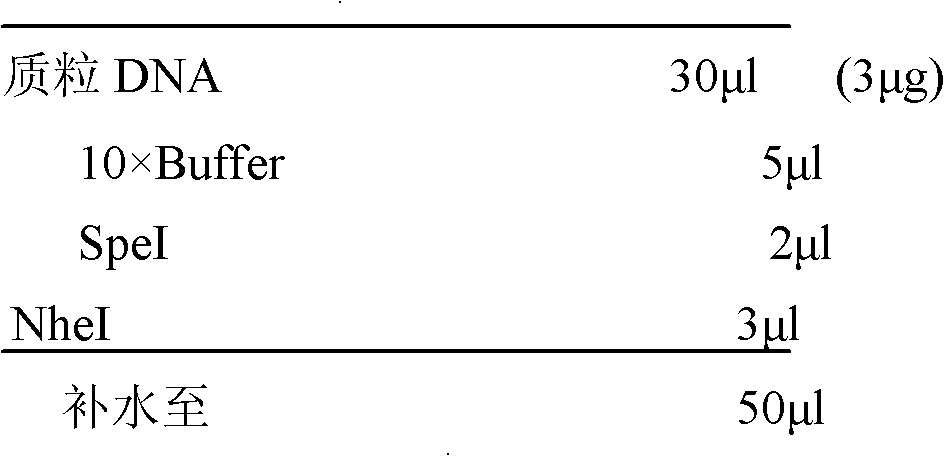
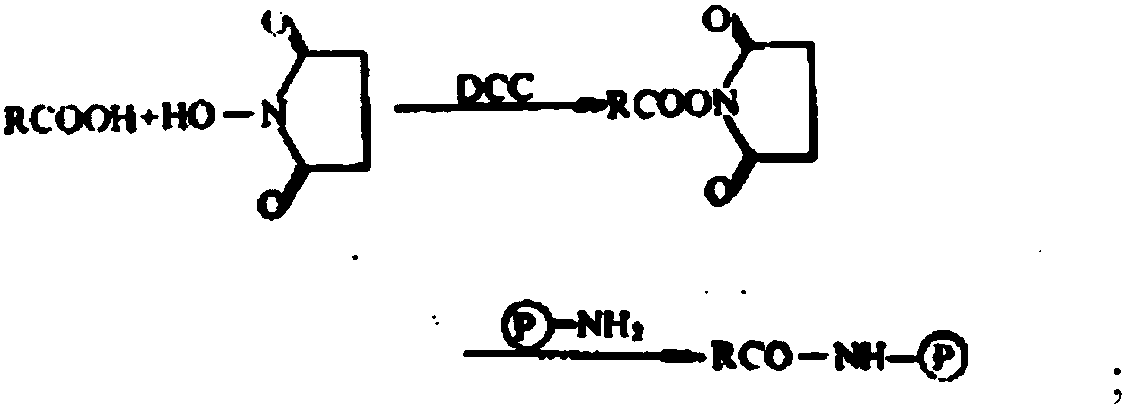
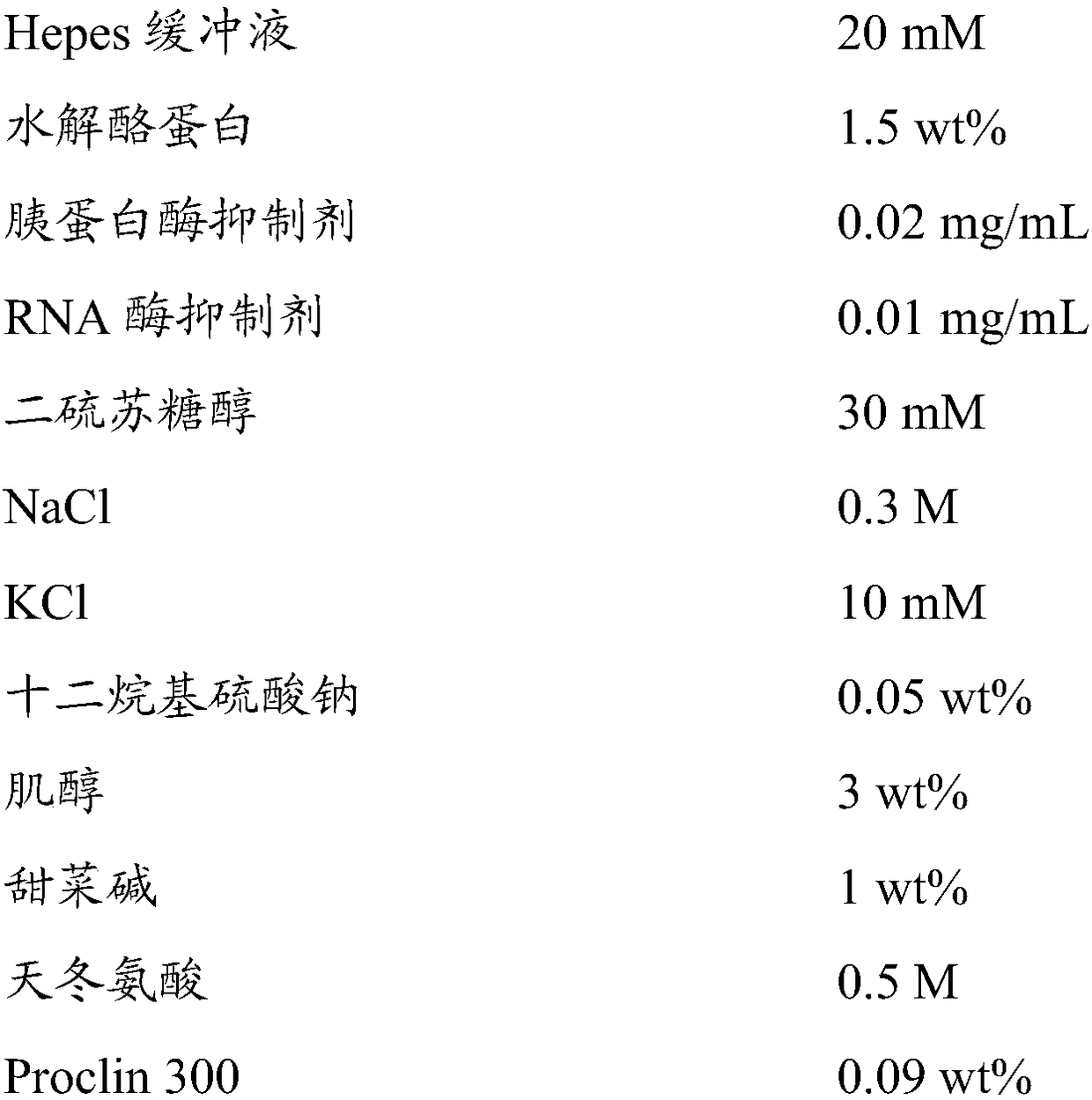
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Anti-nuclear antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antinuclear antibodies (ANAs, also known as antinuclear factor or ANF) are autoantibodies that bind to contents of the cell nucleus. In normal individuals, the immune system produces antibodies to foreign proteins (antigens) but not to human proteins (autoantigens). In some individuals, antibodies to human antigens are produced.
Anti-CCPand antinuclear antibodies in diagnosis of rheumatoid arthritis
The present invention relates to a method aiding in the assessment of rheumatoid arthritis. The method especially is used in the differential diagnosis of rheumatoid arthritis in vitro. The method is for example practiced by analyzing biochemical markers, comprising measuring in a sample both the concentration of anti-CCP and of antinuclear antibodies (ANA) correlating the concentrations determined to the diagnosis of rheumatoid arthritis. To further improve the assessment of RA in a method of this invention the level of one or more additional marker may be determined together with anti-CCP and ANA and be correlated to the absence or presence of RA. The invention also relates to the use of a marker panel comprising anti-CCP and ANA in the diagnosis of rheumatoid arthritis and it teaches a kit for performing the method of the invention.
Owner:KLAUSE URSULA +4
Blood-purifying adsorbing agent for cleaning antibody
InactiveCN101279242AHigh adsorption selectivityImprove stabilityOther blood circulation devicesOther chemical processesImmune complex depositionSorbent
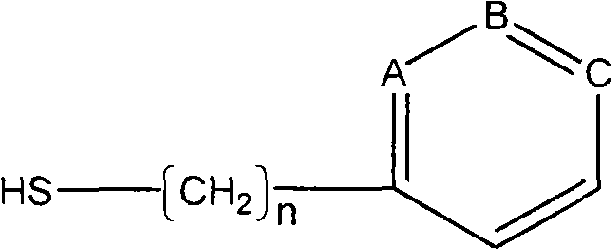
A blood purification sorbent for antibody removal belongs to the technical field of biomedicine, which consists of the two parts of solid-phase carrier material and a petunidin fixed on the carrier by chemical coupling. The molecular structure of the petunidin is shown as above, wherein, one atom among A, B and C is N, the others are C; n is 0-2. The blood purification sorbent can adsorb the antibody component in plasma and autoantibodies such as rheumatoid factor, antinuclear antibody, etc. in heavy load, has limited nonspecific adsorption to other plasma components such as seralbumin, etc., and also has low preparation cost and stable physicochemical property. The material can be used as adsorption filler of a blood purification device for removing the autoantibody and immune complex in the plasma.
Owner:DALIAN UNIV OF TECH
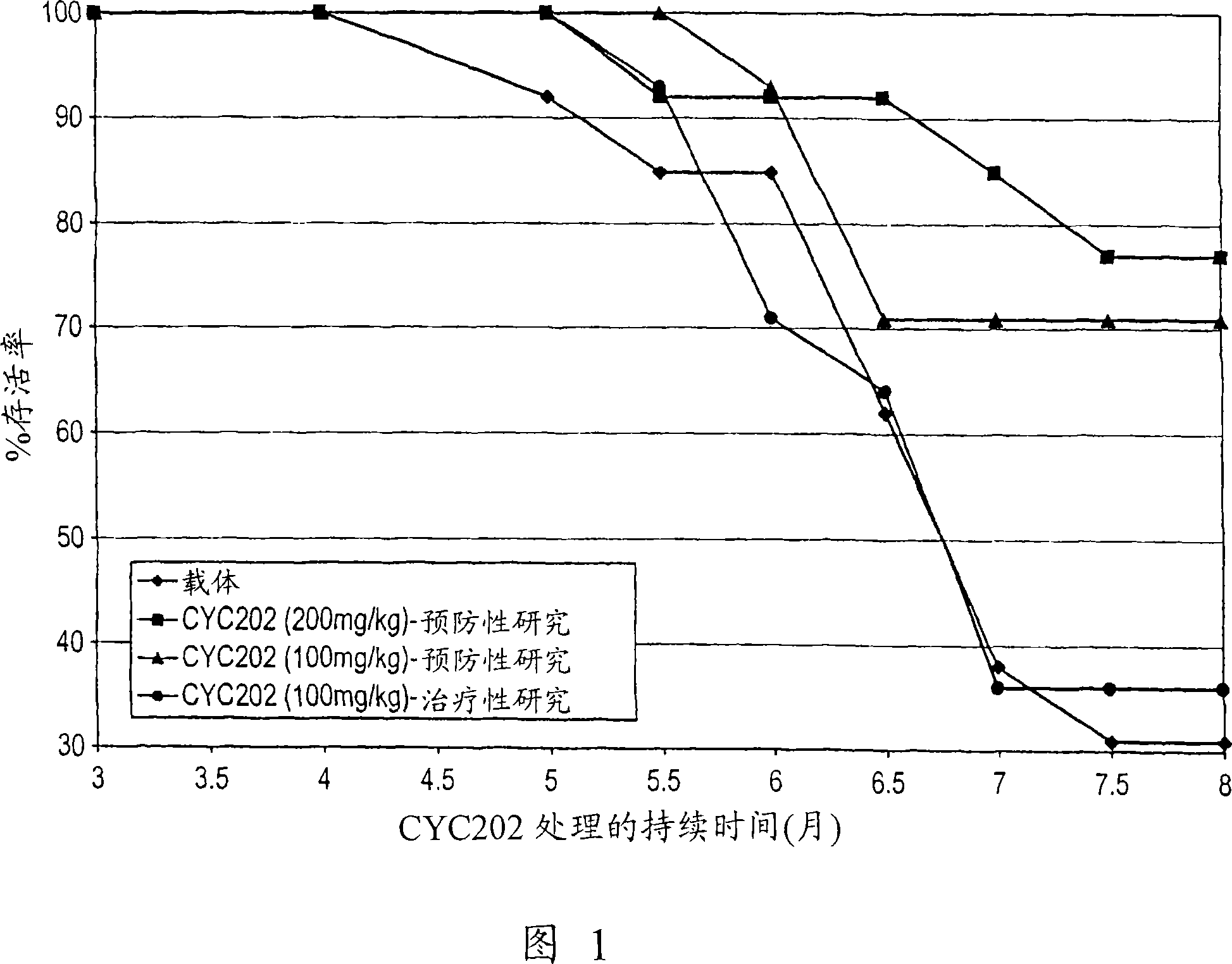
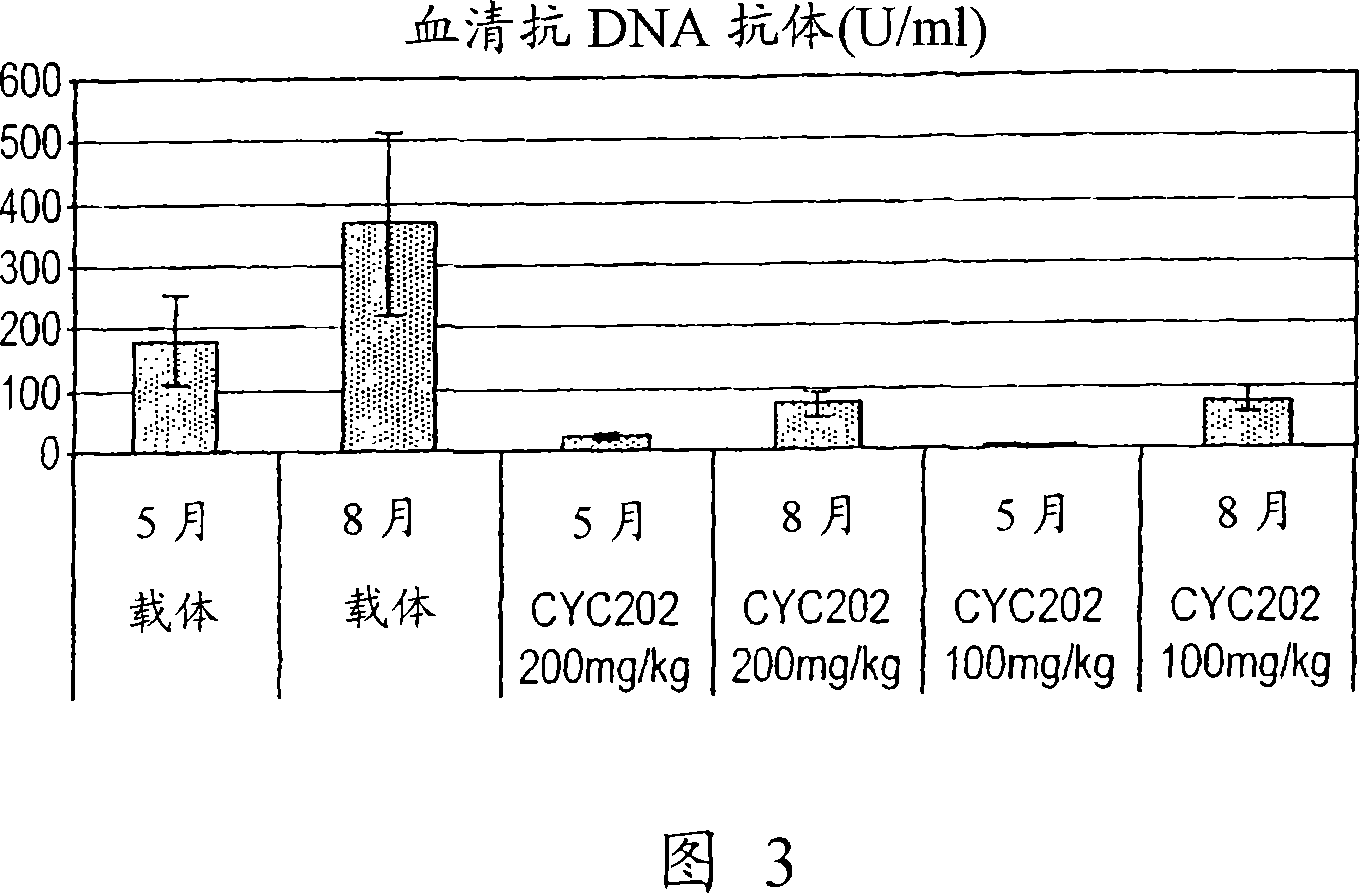
Purine and Pyrimidine Cdk Inhitbitors and Their use for The Treatment of Autoimmune Diseases
InactiveUS20080125404A1Inhibition formationEase of preparation and detectabilityBiocideAntipyreticDiseaseImmunologic disorders
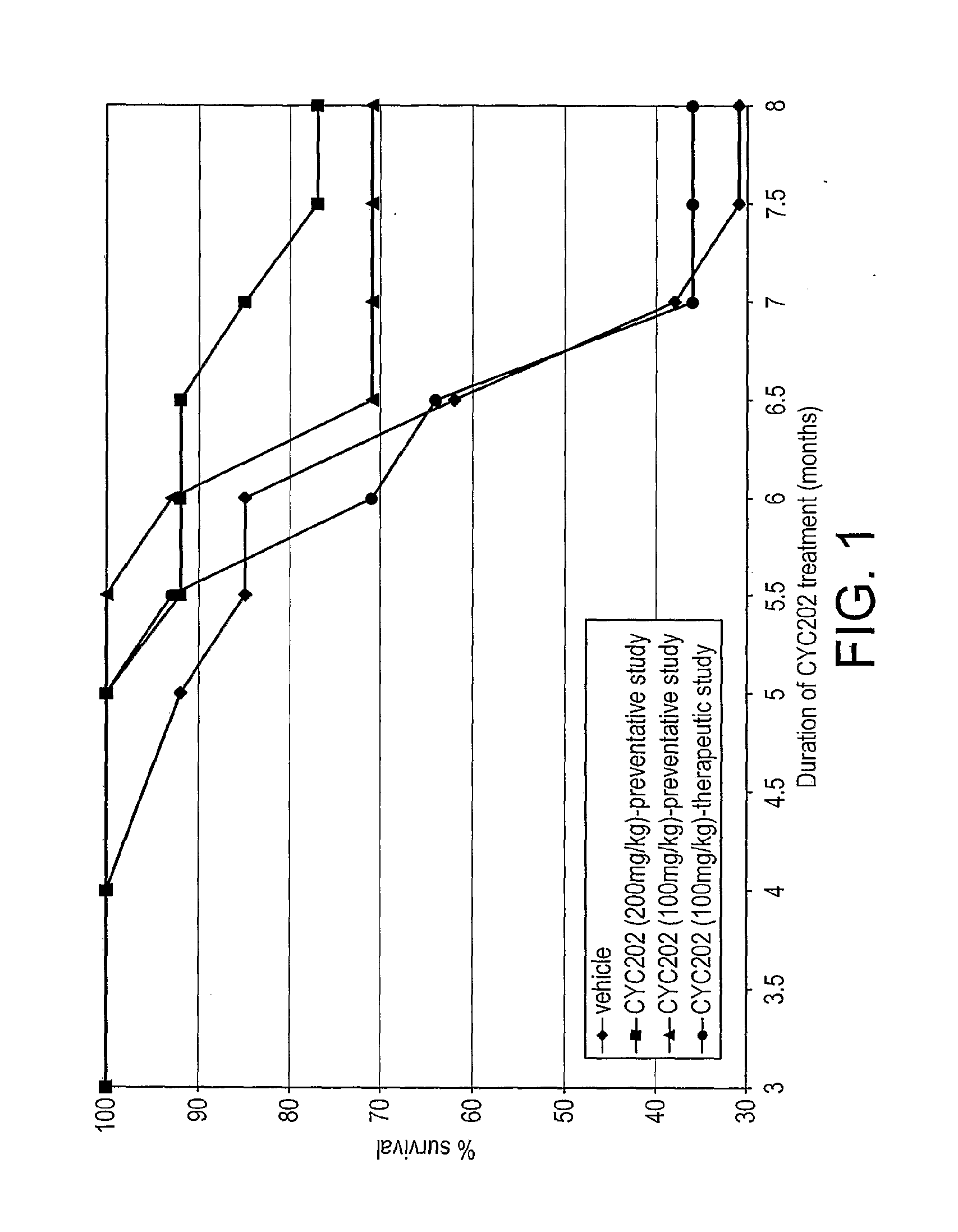
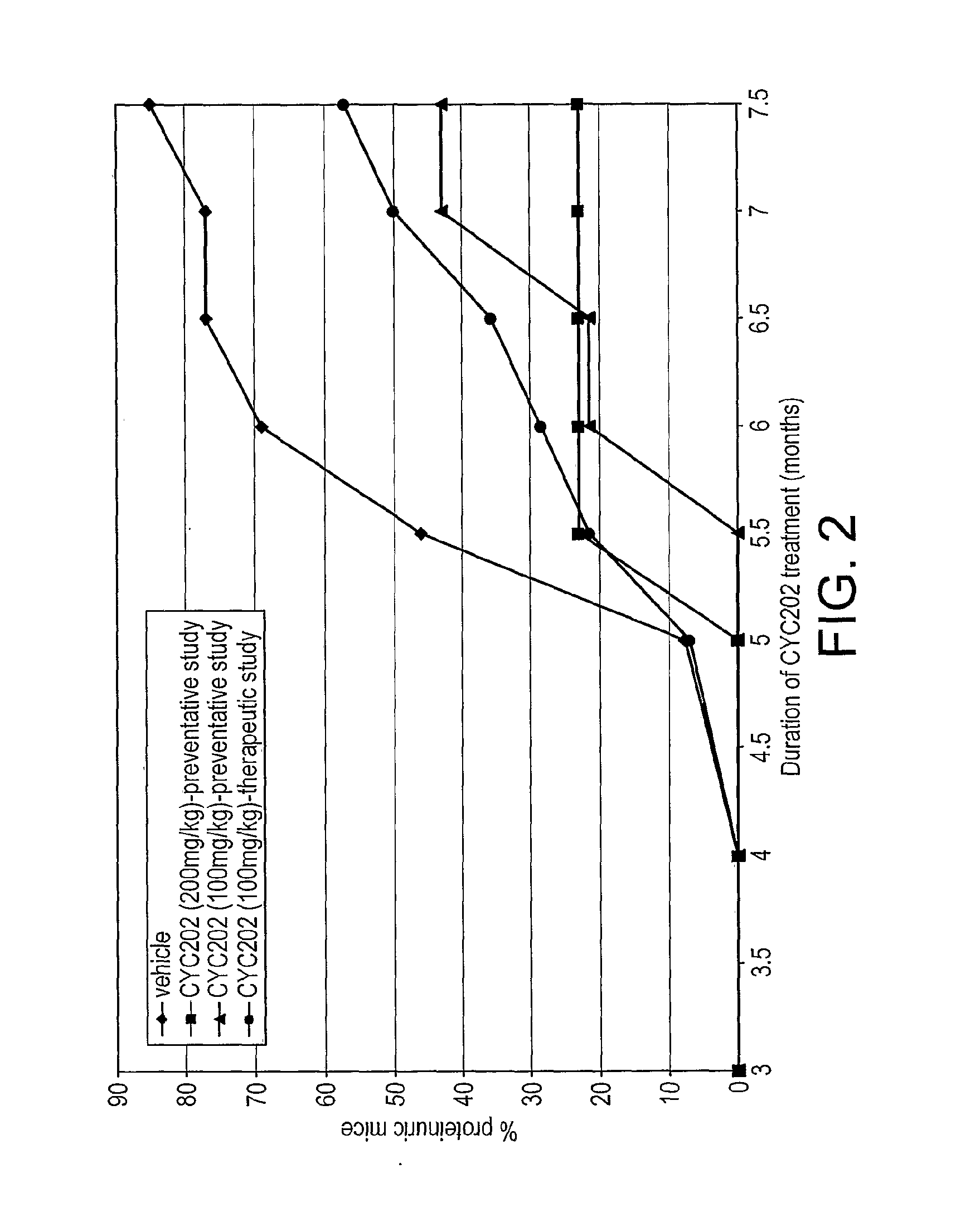
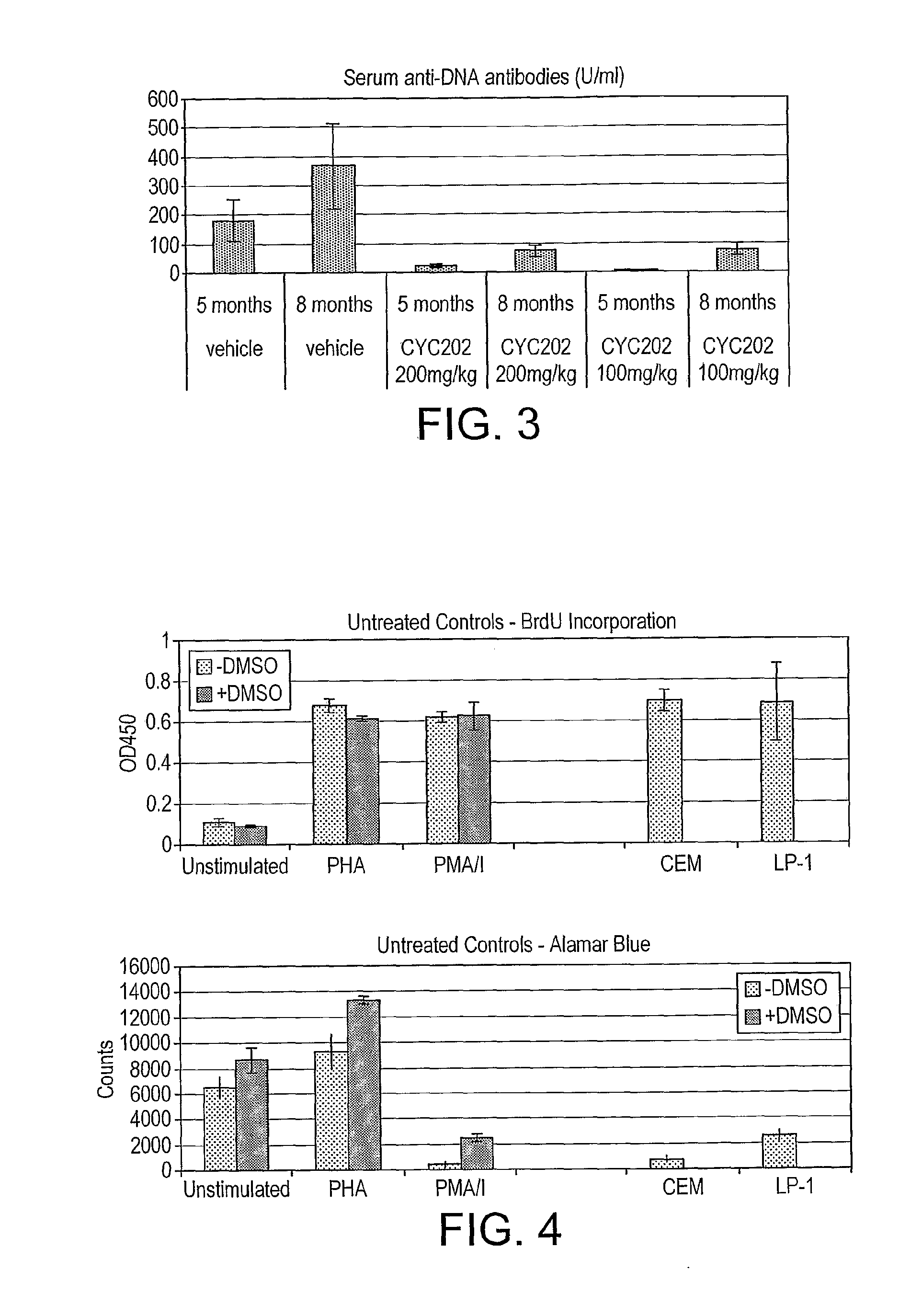
The present invention relates to the use of an inhibitor of CDK2 and / or CDK7 and / or CDK9, or a pharmaceutically acceptable salt thereof, in the preparation of a medicament for treating a disease associated with antinuclear antibodies, wherein the inhibitor of CDK2 and / or CDK7 and / or CDK9 or pharmaceutically acceptable salt thereof is administered in an amount sufficient to down-regulate the levels of antinuclear antibodies. A further aspect of the invention relates to a combination comprising an inhibitor of CDK2 and / or CDK7 and / or CDK9, or a pharmaceutically acceptable salt thereof, and methylprednisolone, and its use in the treatment of diseases associated with antinuclear antibodies, such as SLE.
Owner:CYCLACEL
Cyclohexenone compounds from antrodia camphorata to treat autoimmune diseases
ActiveUS20080312334A1Avoid complicationsAvoid side effectsBiocideOrganic chemistryDiseaseImmunologic disorders
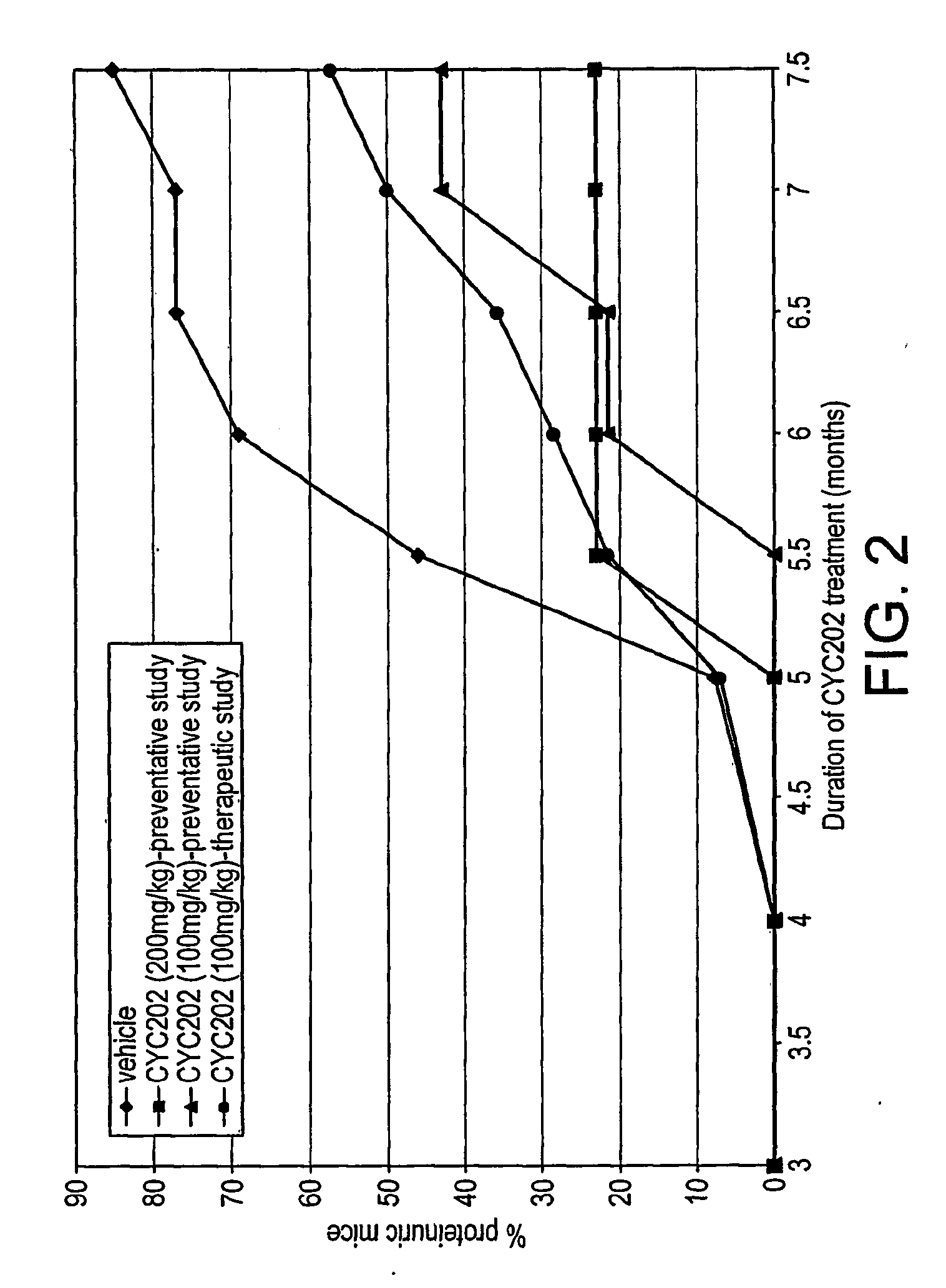
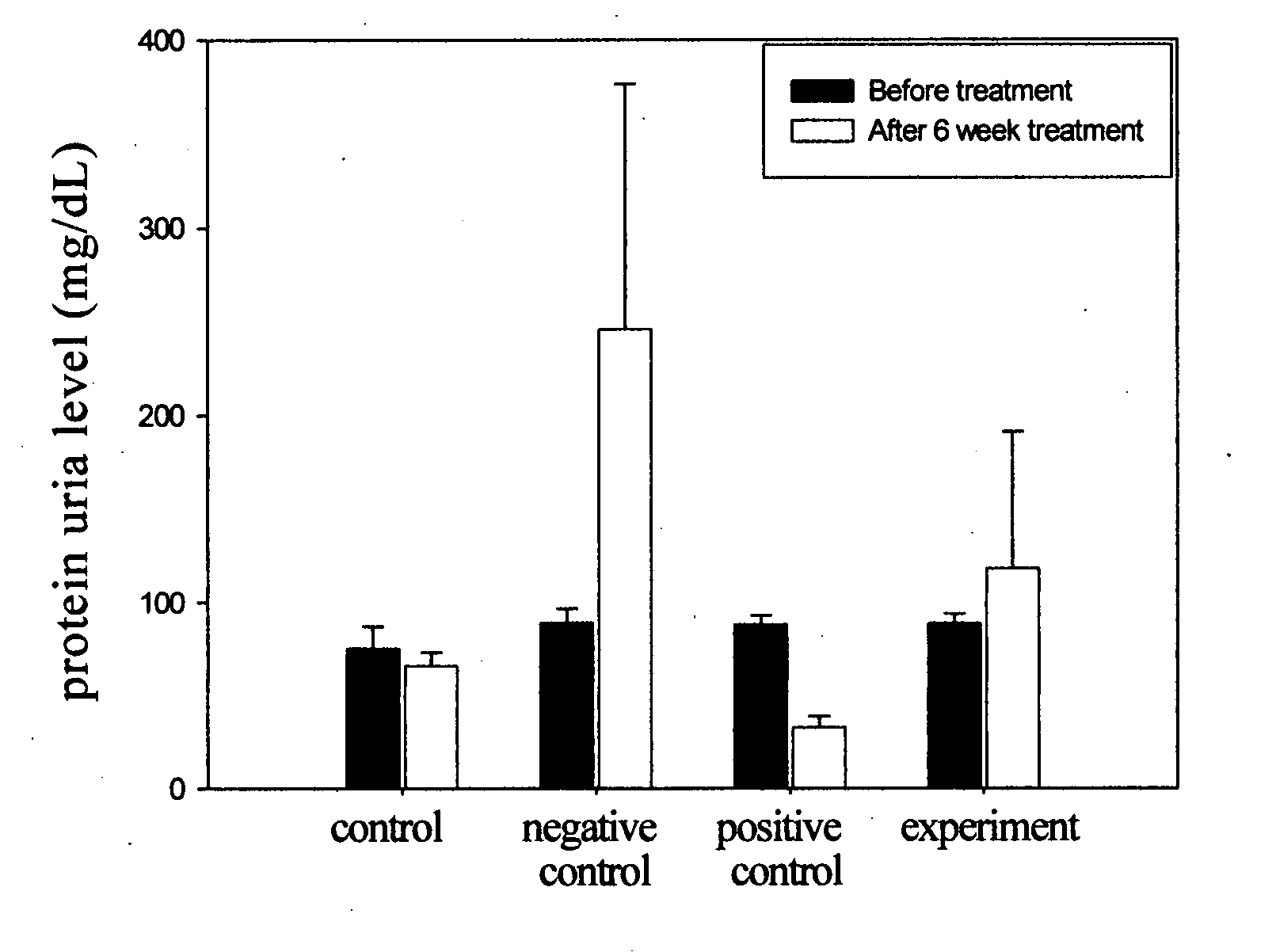
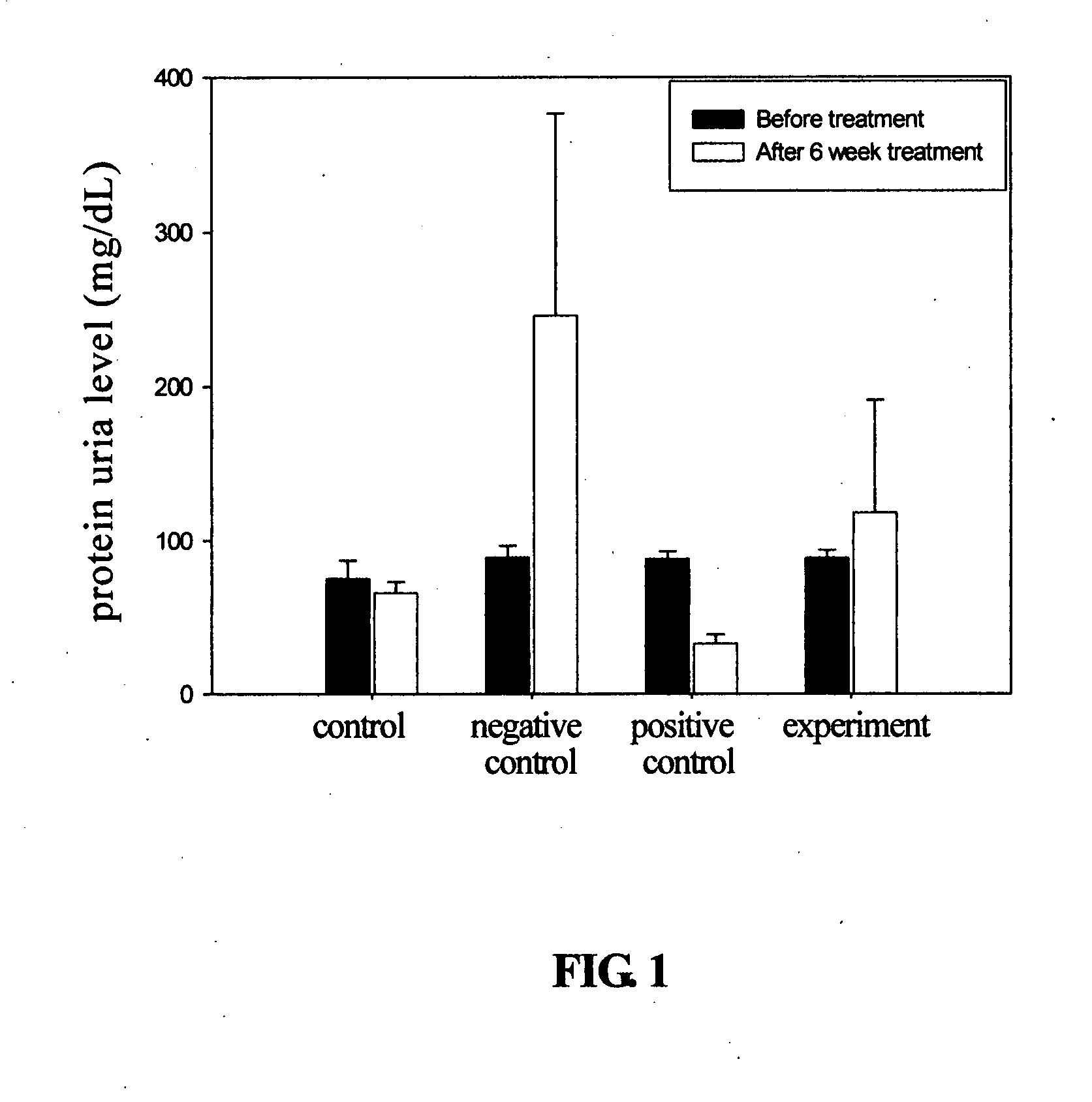
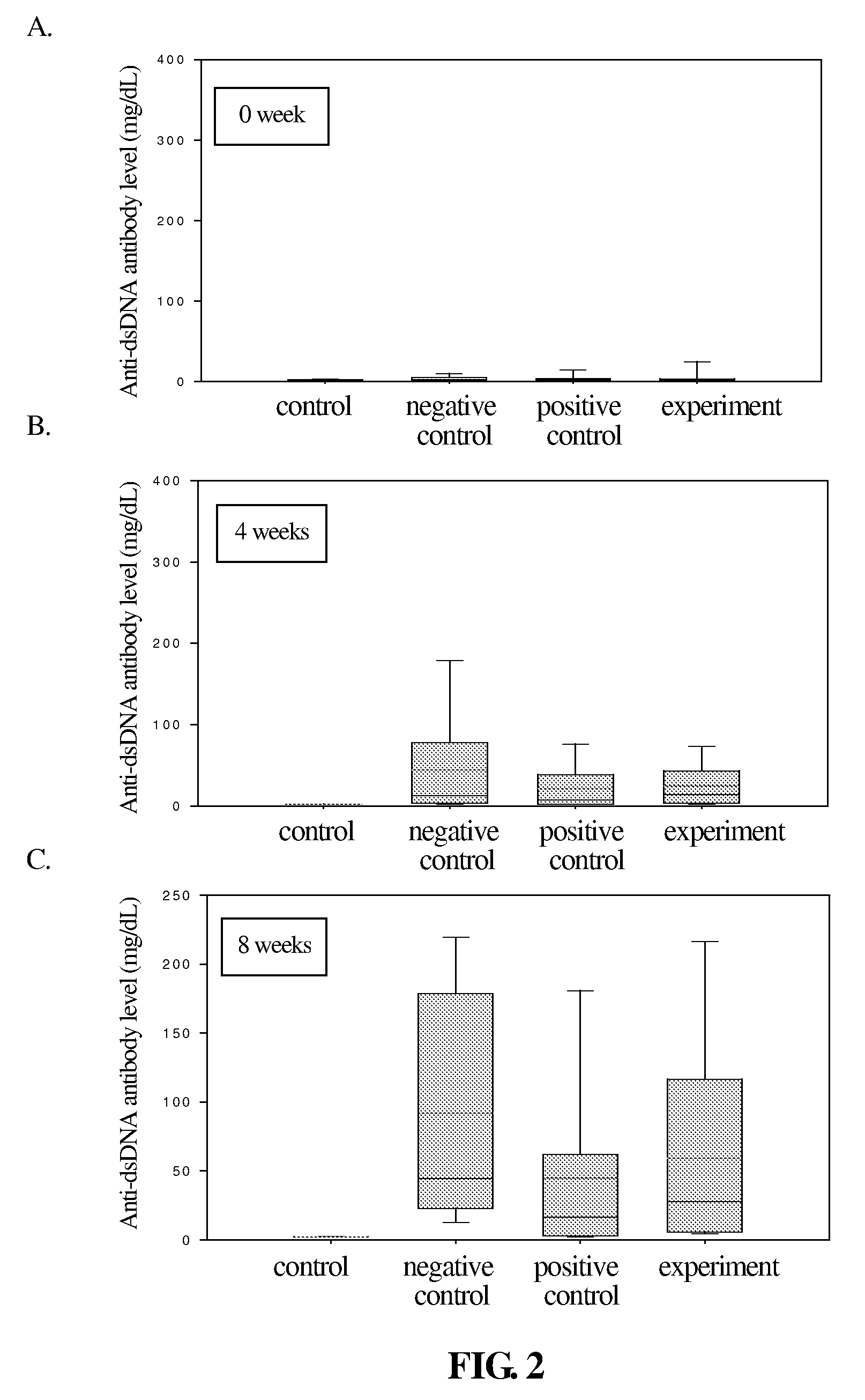
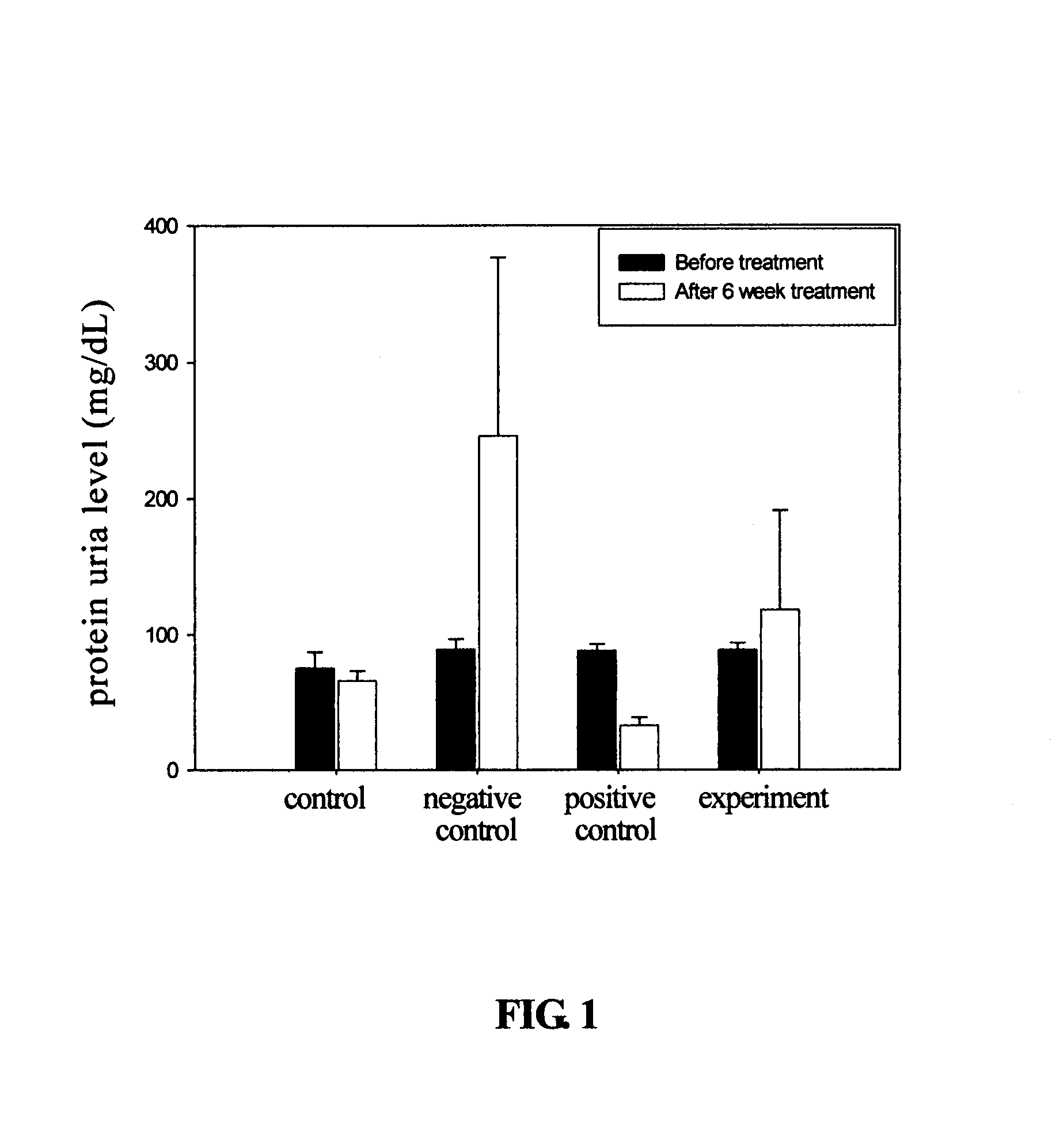
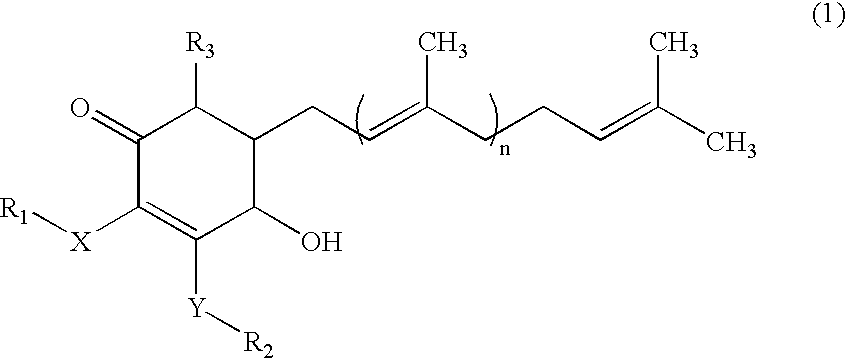
The present invention relates to a compound of Antrodia camphorata used to treat autoimmune diseases, in particular to an extract, 4-hydroxy-2,3-dimethoxy-6-methyl-5(3,7,11-trimethyl-dodeca-2,6,10-trienyl)-cyclohex-2-enone, isolated from Antrodia camphorata, and its use in alleviating symptoms of autoimmune diseases such as systemic lupus erythematosus (SLE). The cyclohexenone compound according to the present invention helps to decrease proteinuria levels and antinuclear antibody titers in SLE mammals in order to alleviate kidney inflammation and disease, as well as the self-damage caused by antinuclear antibodies. The purpose for prevention and treatment of autoimmune diseases and kidney diseases by the natural, side-effect free substance can then be accomplished.
Owner:GOLDEN BIOTECH
Cyclohexenone compounds from Antrodia camphorata to treat autoimmune diseases
ActiveUS7501454B2Lower Level RequirementsDelay progressBiocideOrganic chemistryImmunologic disordersCyclohexenone
The present invention relates to a compound of Antrodia camphorata used to treat autoimmune diseases, in particular to an extract, 4-hydroxy-2,3-dimethoxy-6-methyl-5(3,7,11-trimethyl-dodeca-2,6,10-trienyl)-cyclohex-2-enone, isolated from Antrodia camphorata, and its use in alleviating symptoms of autoimmune diseases such as systemic lupus erythematosus (SLE). The cyclohexenone compound according to the present invention helps to decrease proteinuria levels and antinuclear antibody titers in SLE mammals in order to alleviate kidney inflammation and disease, as well as the self-damage caused by antinuclear antibodies. The purpose for prevention and treatment of autoimmune diseases and kidney diseases by the natural, side-effect free substance can then be accomplished.
Owner:GOLDEN BIOTECH
Methods for treating and diagnosing systemic lupus erythematosus
ActiveCN105229470ADisease diagnosisAntibody medical ingredientsAnti-nuclear antibodyLupus erythematosus
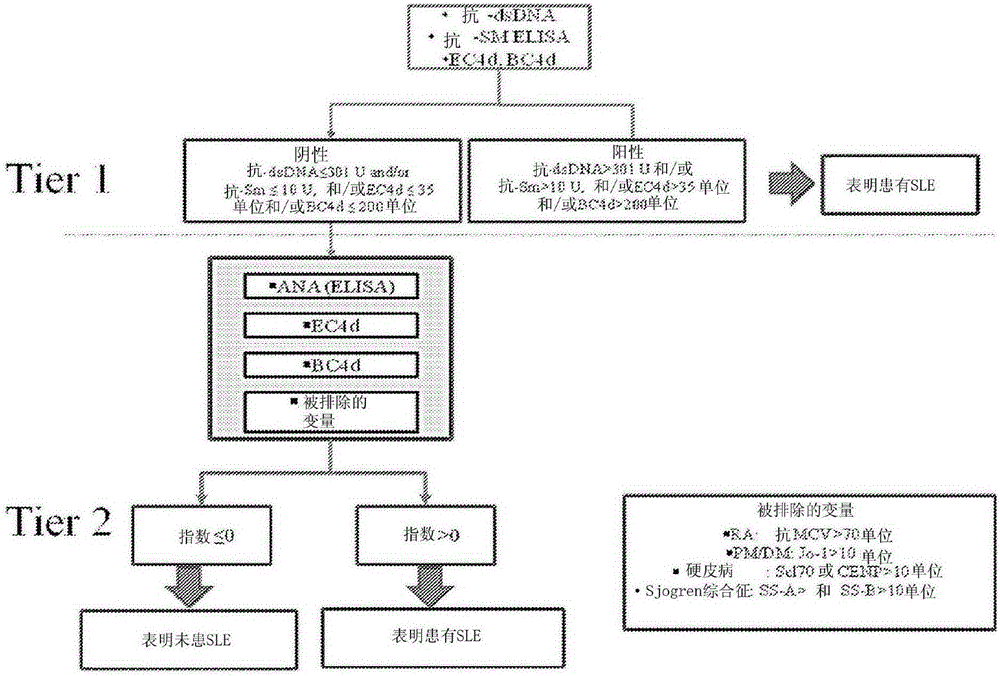
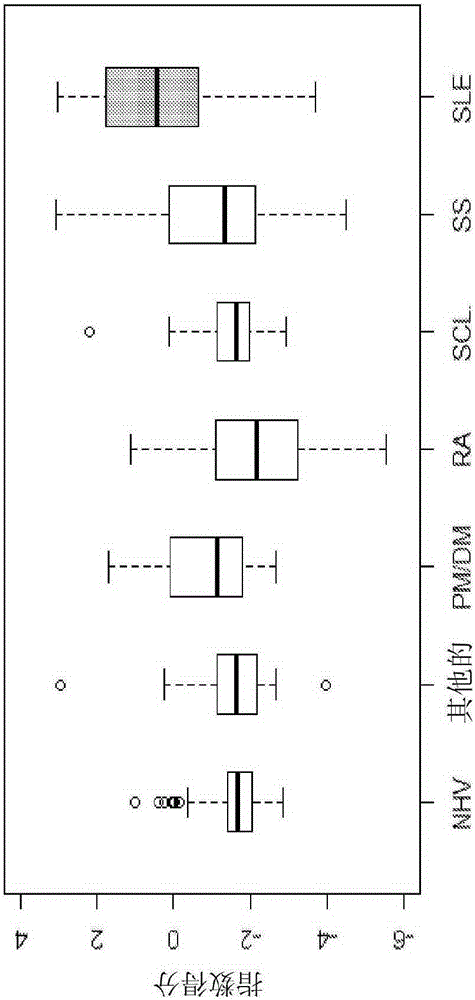
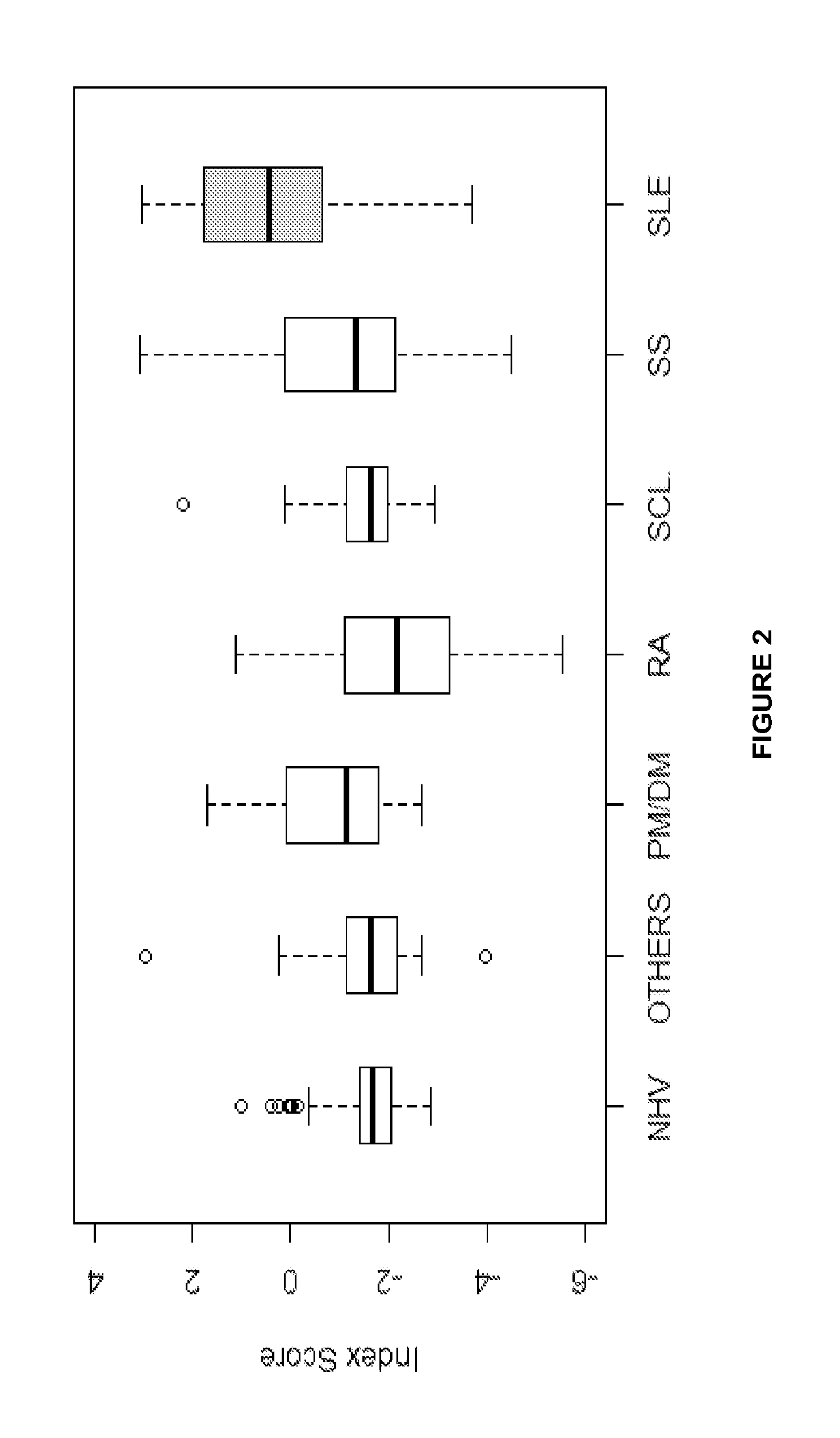
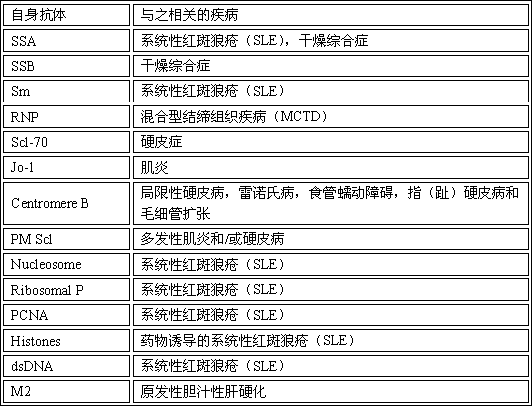
Methods and reagents for diagnosing, prognosing, and treating systemic lupus erythematosus (SLE) are disclosed, involving calculating an SLE risk score for a subject based on a level of each of an erythrocyte C4d (EC4d) marker, a B-cell C4d (BC4d) marker, antinuclear antibodies (ANA), anti-Smith antibodies (anti-Sm) and optional rule-out markers (SS-B / La, Scl-70, Jo-1, CENP, MCV).
Owner:EXAGEN DIAGNOSTICS
Antrodia camphoratea pimelie kelone compound for treating autoimmune disease and medicine composition
ActiveCN101357883AOrganic active ingredientsOrganic compound preparationSide effectAnti-nuclear antibody
The invention relates to an antrodia camphorate cyclohexanedione compound used for treating autoimmune diseases and a medical composition thereof. The antrodia camphorate cyclohexanedione compound is about 4-hydroxyl-2, 3-dimethoxy-6-methyl-5(3, 7, 11-trimethyl-2, 6, 10-dodecatrien)-2-cyclohexanedione which is separated from antrodia camphorate extract and can relieve systemic erythromelalgia lupus and other symptoms caused by autoimmune diseases. Antrodia camphorate cyclohexanedione in the invention can reduce the content of urine protein of the mammalians with systemic erythromelalgia lupus and the thickness of the antinuclear antibody in blood, relieves kidney inflammation and lesion, lowers the harm of antinuclear antibody on autogenous tissues, and achieves the effect of treating autoimmune diseases such as systemic erythromelalgia lupus, and the like, and the diseases related to kidney by using natural materials without side effects.
Owner:GOLDEN BIOTECH
Kit for detecting antinuclear antibody spectrum related to autoimmune diseases (AIDs)
ActiveCN102937648AOriginalityPlay the role of interpretationMaterial analysisAntigenAutoimmune condition
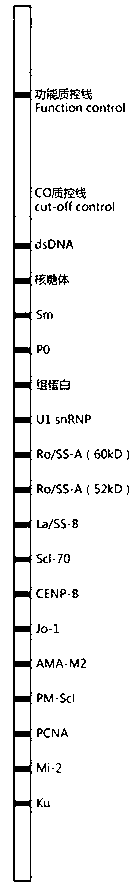
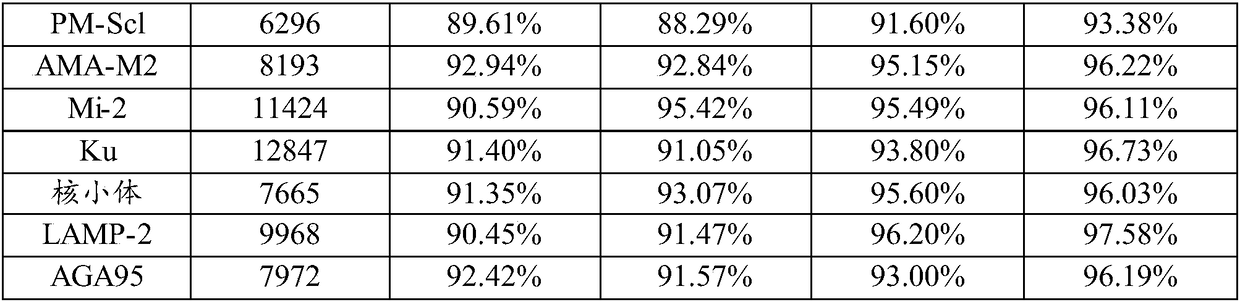
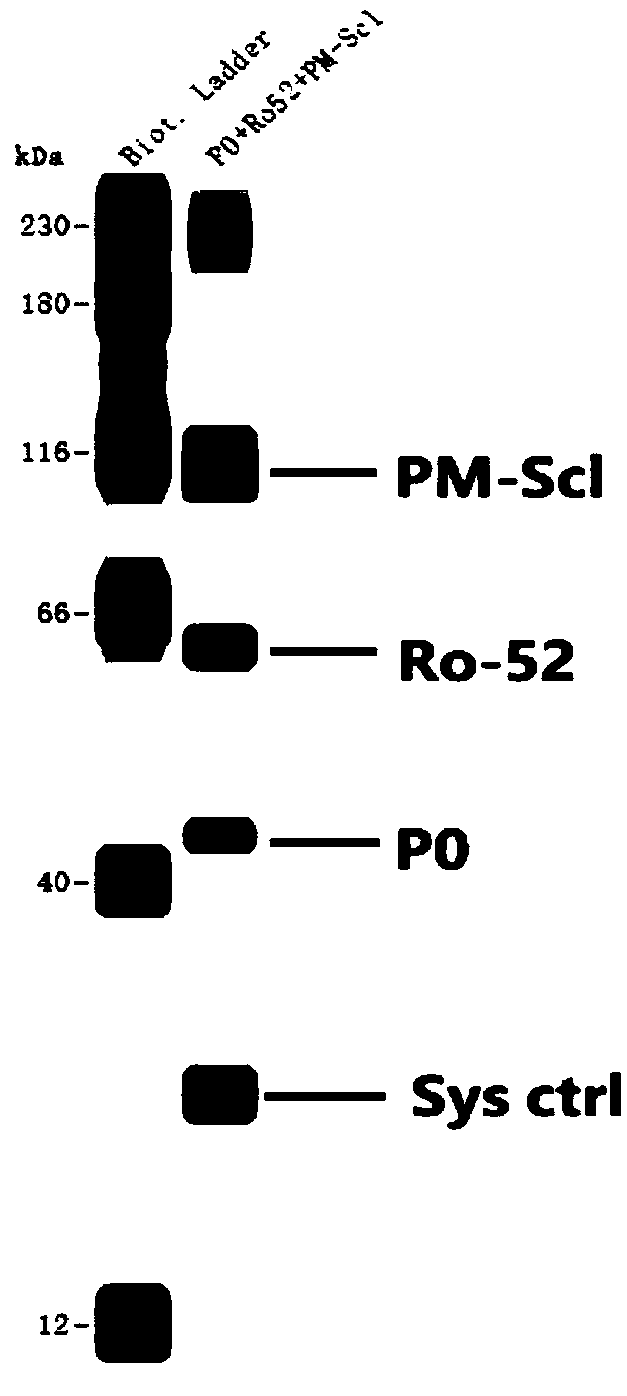
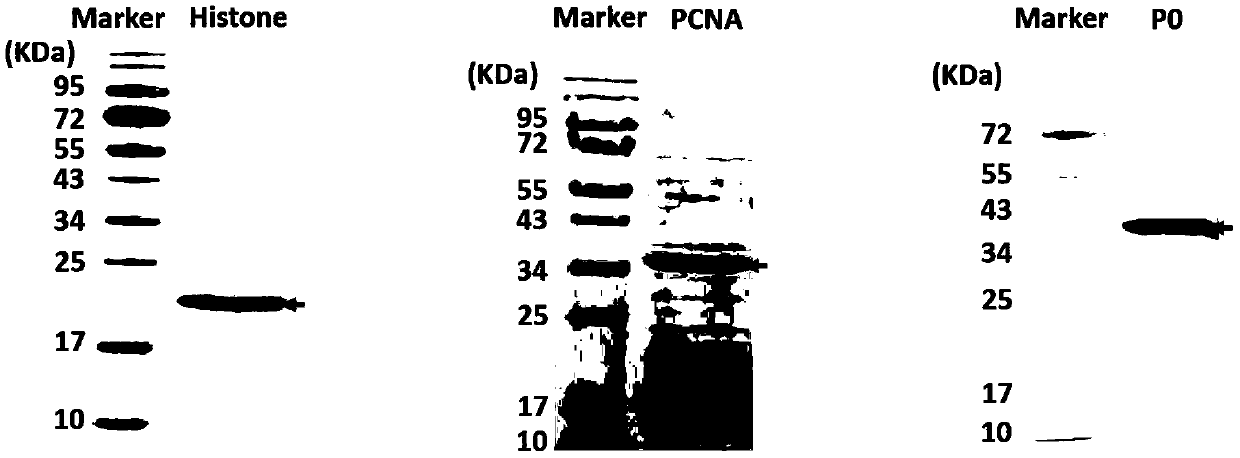
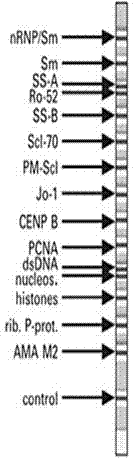
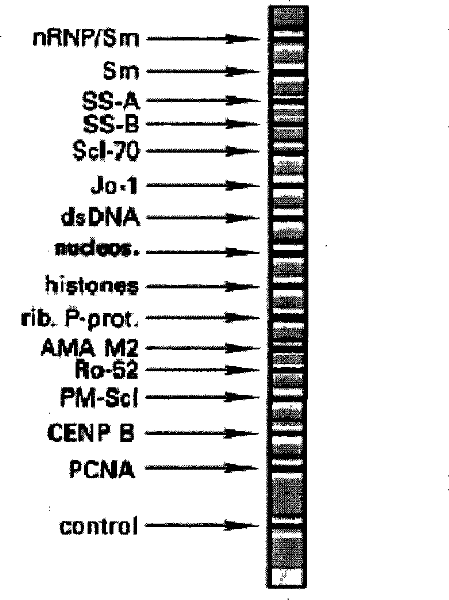
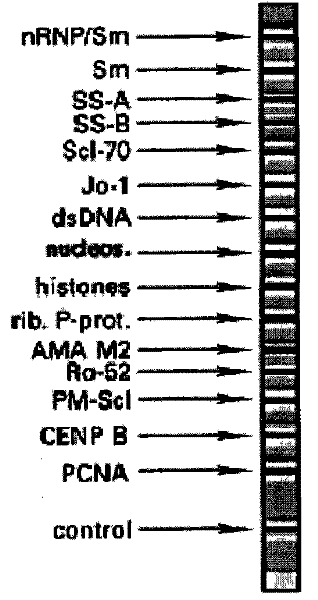
The invention relates to a kit for detecting antinuclear antibody spectrum related to AIDs. The kit comprises a membrane strip, enzyme-labeled liquid, a substrate and concentrating and washing incubation liquid, wherein the membrane strip is composed of a carrier piece and an antigen band, a critical quality control band and a functional quality control line which are sequentially fixed on the carrier piece. The antigen band is composed of at least two of dsDNA, nucleosomes, SmD1, ribosome P0proteins, histones, U1snRNP, Ro / SS-A(52KD), Ro / SS-A(60KD), La / SS-B, Sc1-70, CENP-B, Jo-1, AMA-M2, PM-Sc1, PCNA, Mi-2 and Ku through independent marking to a nitrocellulose membrane or a nylon membrane. The kit is provided with the ingenious critical quality control band, one critical quality control band can play a role in interpreting two or even more detection bands (antigen bands), and result determination is simpler and more reliable.
Owner:SICHUAN XINCHENG BIOLOGICAL CO LTD
Composition for enzyme linked immunosorbent assay kit, anti-nuclear antibody spectrum detection kit and preparation method thereof
ActiveCN108398551AGuaranteed stabilityImprove stabilityElectrophoretic profilingDisease diagnosisElisa kitAnti-nuclear antibody
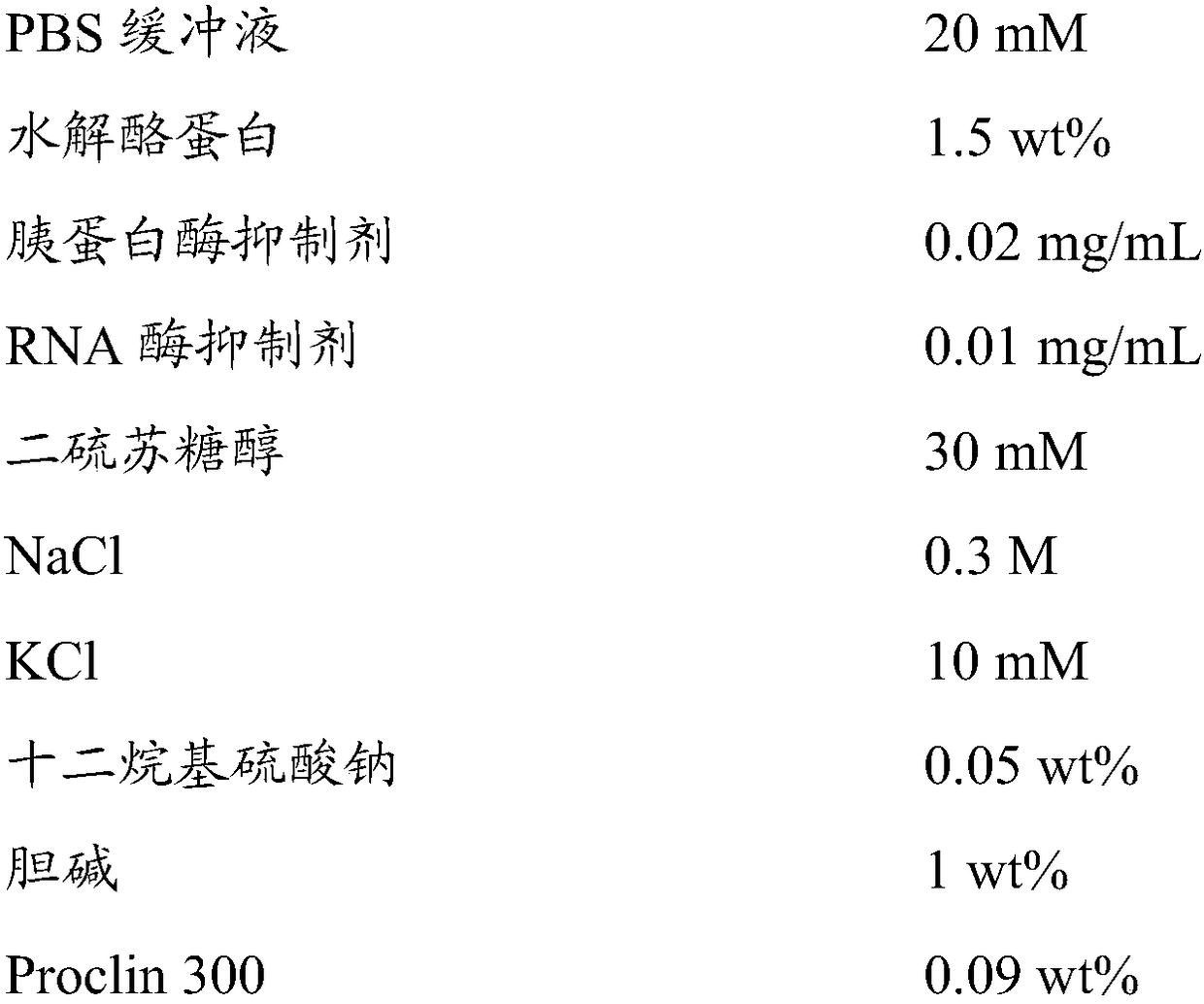
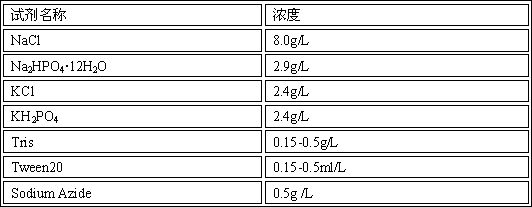
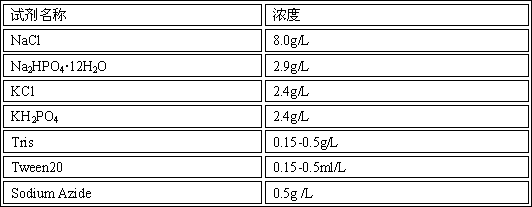
The invention relates to the technical field of enzyme linked immune, and discloses a composition for an enzyme linked immunosorbent assay kit, an anti-nuclear antibody spectrum detection kit and a preparation method thereof. The composition comprises sealing liquid and ELISA diluents; the sealing liquid contains casein, saccharose, PBS, mannitol, Tween20, lycine and sodium azide; the ELISA diluents contain PBS, casein, sodium p-hydroxybenzoate, PEG, lycine and Proclin300. By starting from the sealing liquid and the ELISA diluents of the enzyme linked immunosorbent assay kit, the proper ingredients are selected, so that the enzyme linked immunosorbent assay kit can maintain the detection stability for a long time. Meanwhile, the anti-nuclear antibody spectrum detection kit prepared from the composition has higher stability; the guarantee period is 2 years or more.
Owner:SHENZHEN BLOT BIOTECH
Method for detecting core fucosylation level of serum IgG
The invention discloses a method for detecting the core fucosylation level of serum IgG. The method comprises a step of specifically identifying core fucosyl groups in the serum IgG by using lectin, but not comprises a step of sampling from an organism, and a serum sample is an in vitro sample. The lectin is an Aspergillus oryzae lectin AOL protein (domain) which can specifically identify the corefucosyl groups, and is obtained by prokaryotic expression, and the amino acid sequence of the lectin is represented by SEQ ID NO. 1; and the lectin is encoded by an FleA gene fragment represented bySEQ ID NO. 2. The method has potential application values in the screening of autoimmune diseases and the detection of the autoimmune diseases and provides a potential therapeutic way for possible immune regulation intervention after being combined with clinical anti-nuclear antibody titer detection.
Owner:DALIAN MEDICAL UNIVERSITY
Antinuclear antibody combined detection kit and detection method thereof
InactiveCN105021811AExcellent detection timeMeet the needs of useMaterial analysisFiltrationMembrane surface
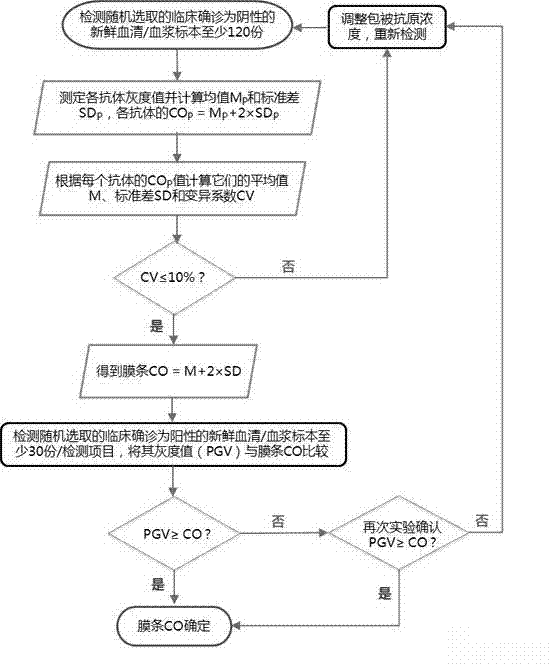
The invention discloses an antinuclear antibody combined detection kit which comprises a sample diluent, an enzyme conjugate, a washing fluid and a substrate. The invention also discloses a detection method using the antinuclear antibody combined detection kit. The detection method comprises balancing the detection kit to room temperature, processing a sample, rinsing a membrane surface in a card window, adding the sample and the washing fluid, adding the enzyme conjugate, developing, terminating, and the like. By using the kit and the method, multichannel multi-sample detection can be performed at the same time (that is, 16 antinuclear antibody detection projects can be performed at the same time, and 16 independent detection results can be provided), and the sensitivity can reach the detection level of an immunoblotting product and is far higher than the detection level of a dot immunogold filtration product; and the detection time of the kit is close to the detection time of dot immunogold filtration method and is far better than the detection time of an immunoblotting method, does not need reagent concentrate processing, warm bath, repeated cleaning and the like, and extremely well satisfies clinical usage.
Owner:厦门拜尔杰生物科技有限公司
Preparation method of human antinuclear antibody
InactiveCN102464717ASmall toxicityStrong against randomnessImmunoglobulins against animals/humansVector-based foreign material introductionCDNA libraryEscherichia coli
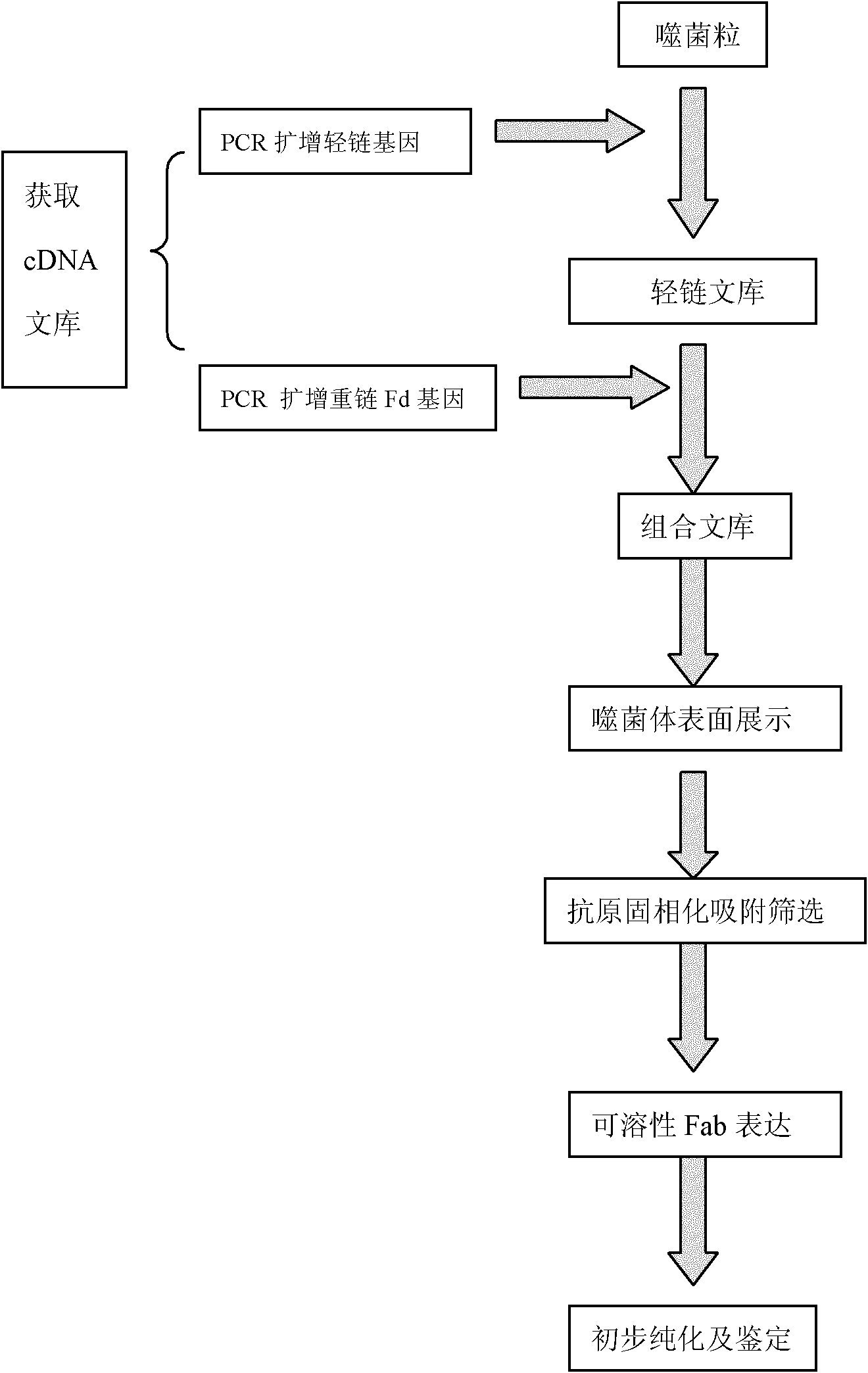
The invention discloses a preparation method of a human antinuclear antibody, comprising the following steps: collecting single nuclear cells of peripheral blood of a patient with the autoimmune disease, extracting the total RNA (Ribonucleic Acid), and carrying out reverse transcription to obtain a cDNA (Complementary Deoxyribonucleic Acid) library; amplifying a light chain k,1 gene and an immunoglobulin molecule heavy chain Fd gene by taking the acquired cDNA library as a template through a PCR (Polymerase Chain Reaction) method; cloning the light chain k,1 gene into a pComb3Hss carrier to construct a light chain library; loading the heavy chain Fd gene into a carrier to construct and finish a combinatorial library; transforming the obtained recombinant plasmid into escherichia coli XL1-Blue, infecting by using a helper phage M12KO7, and expressing the random combinatorial library on the surface of a filamentous phage to finish phage surface display; and purifying the Fab segment of the human antinuclear antibody by utilizing affinity chromatography. By using the preparation method, the defects that the random combinatorial antibody library has strong randomness, the screening workload is large and a specific antibody is not easy to obtain are overcome, and the specific antibody can be directly screened from a phage antibody library; and in addition, the method is simple and feasible, and the experimental period is shortened.
Owner:杭州博林生物技术有限公司
Stable preservation diluent and anti-nuclear antibody detection reagent, and preparation method and application thereof
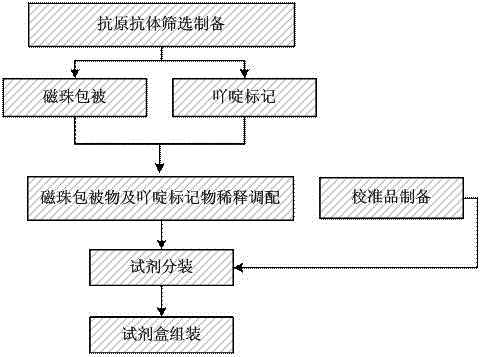
The invention discloses stable preservation diluent, anti-nuclear antibody detection reagent for the stable preservation diluent, a preparation method thereof and an anti-nuclear antibody detection kit of the anti-nuclear antibody detection reagent. The pH of the stable preservation diluent is 5-8, and the stable preservation diluent includes a buffer solution, salt, osmotic substances and a preservative; the osmotic substances are selected from at least one substance of polyol, methylamine and amino acid. The anti-nuclear antibody detection reagent suspends a coupling matter of anti-nuclear antibody target antigens and magnetic microspheres in the stable preservation diluent for storage to stably store the diluent including the buffer solution, the salt, the osmotic substances and the preservative, thereby providing the coupling matter of the anti-nuclear antibody target antigens and the magnetic microspheres with a stable liquid storage environment.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
Methods for treating and diagnosing Systemic Lupus Erythematosus
Methods and reagents for diagnosing, prognosing, and treating systemic lupus erythematosus (SLE) are disclosed, involving calculating an SLE risk score for a subject based on a level of each of an erythrocyte C4d (EC4d) marker, a B-cell C4d (BC4d) marker, antinuclear antibodies (ANA), anti-Smith antibodies (anti-Sm) and optional rule-out markers (SS-B / La, Scl-70, Jo-1, CENP, MCV).
Owner:EXAGEN INC
Methods for diagnosing and for monitoring the treatment of recurrent spontaneous abortion
ActiveUS20070160997A1Decrease in levelAvoid abortionAnalysis using chemical indicatorsMicrobiological testing/measurementAntigenAnti-nuclear antibody
The present invention discloses a method of diagnosis of immunological recurrent spontaneous abortion, comprising in vitro determining the level of antinuclear antibody in a body fluid of the patient and comparing the result with the level of corresponding antinuclear antibody of normal control. Particularly, isolated chromosome No. 2 or fragment thereof containing fibronectin encoding gene derived from male(s) is used as antigen in the method of the present invention for determining the level of corresponding antinuclear antibody in a body fluid sample of the patient. The present invention also discloses a diagnostic kit for immunological recurrent spontaneous abortion, and methods and kits for monitoring the therapeutic effect for immunological recurrent spontaneous abortion.
Owner:BEIJING XINJING ANTAI MEDICAL & TECH SERVICE
Detection method for rapidly and quantitatively detecting content of antinuclear antibody
PendingCN109324192APredict prognosisRapid Quantitative DetectionBiological testingAnti-nuclear antibodyAutoimmune disease
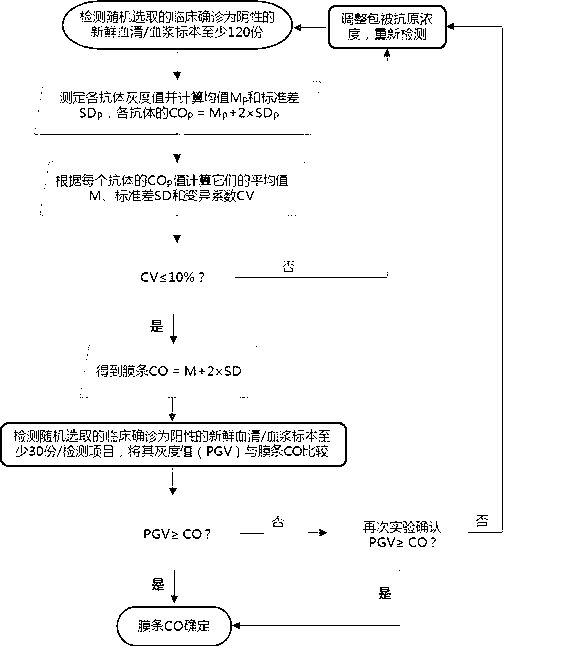
The invention belongs to the technical field of biological detection analysis, and relates to a detection method for rapidly and quantitatively detecting content of an antinuclear antibody. A method which employs an ultramicro full-automatic protein expression quantitative analysis system and is simultaneously used for rapidly detecting content of each type of a plurality of antibodies in a detection sample is proposed. The method can have the advantages of rapid and accurate quantitative detection, high specificity, high sensitivity and the like, the content of the antinuclear antibody in patient serum can be quantitatively analyzed, and automation is more easily achieved. By the detection method, the quantitative grading standard for evaluating an antibody value of the sample and optimalscreening positive critical value are further built, and the detection method is used for forecasting or estimating whether a human body is probably developed with or already developed with various autoimmune diseases.
Owner:QINGDAO UNIV
Antigen-immobilized matrix membrane, kit comprising the same for detecting antinuclear antibody spectrum related to autoimmune diseases and purpose thereof
PendingCN110346576AStrong specificityIncreased sensitivityDisease diagnosisBiological testingDiseaseAntigen testing
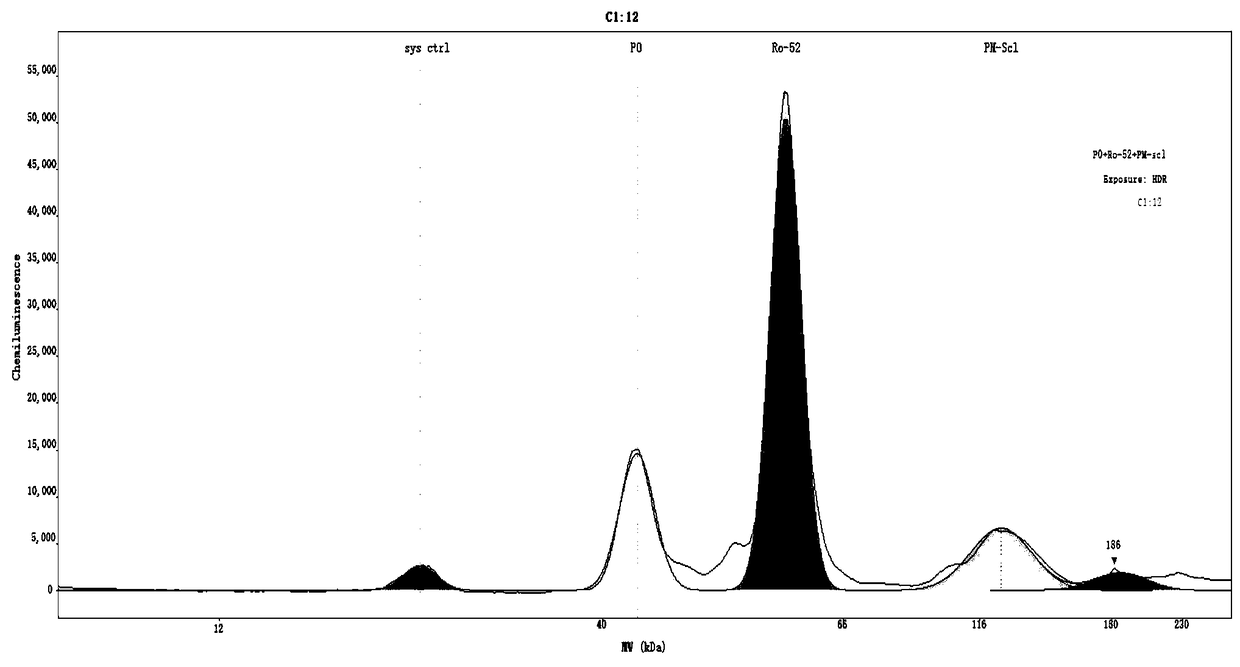
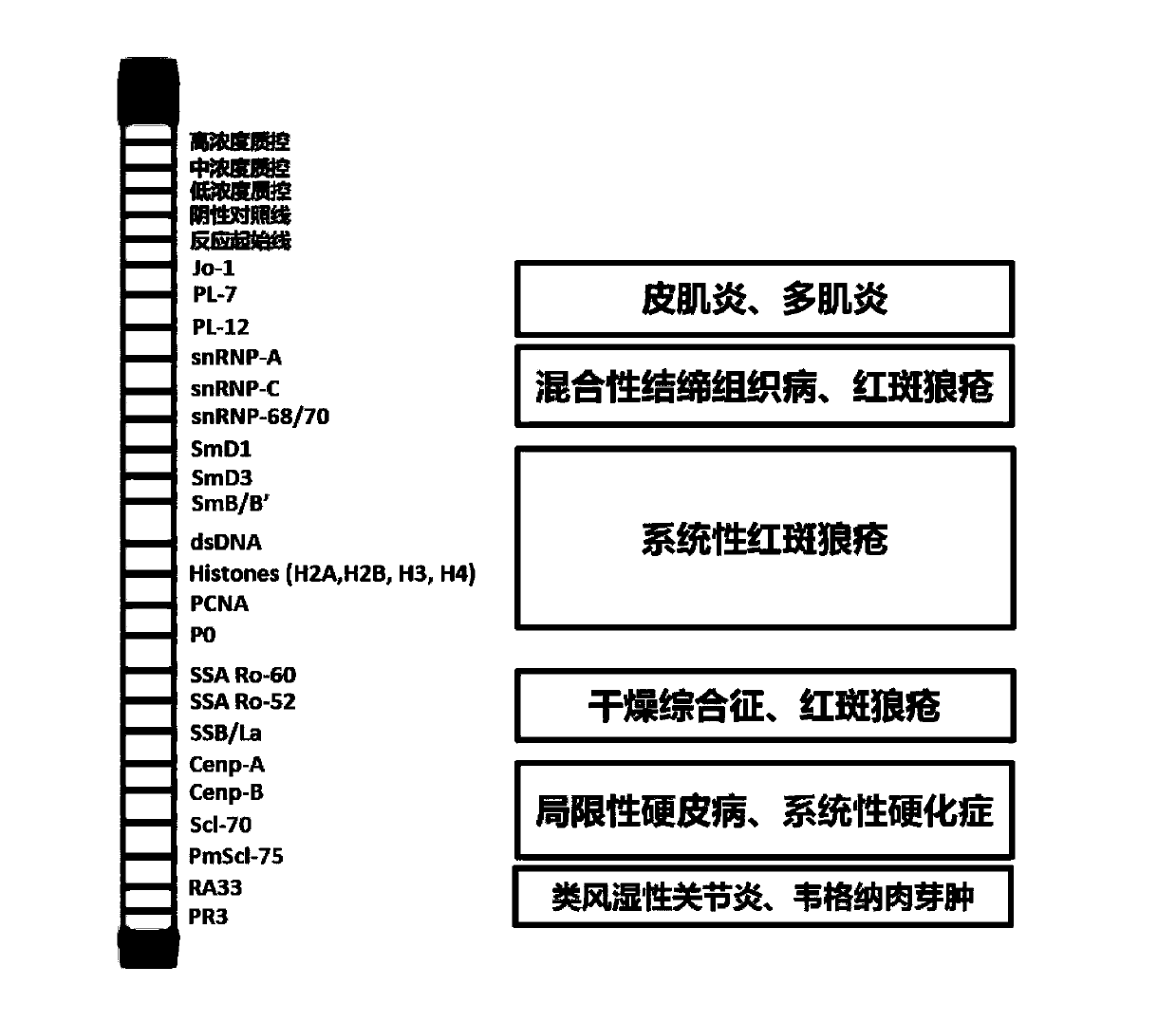
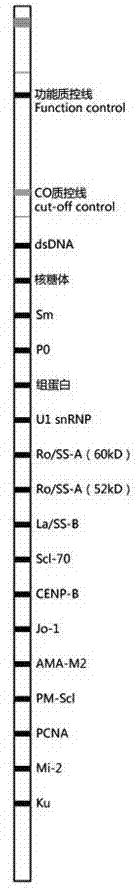
The invention discloses an antigen-immobilized matrix membrane, a kit comprising the same for detecting an antinuclear antibody spectrum related to autoimmune diseases and purpose thereof. The antigen-immobilized matrix membrane comprises but not only comprises following 22 mutually independent antigen detection lines including double-stranded DNA, histone, ribosome P0 protein, PCNA, SmD1, SmD3, SmB / B', snRNP-A, snRNP-C, snRNP68 / 70, SSA / Ro60, SSA / Ro52, SSB / La, Jo-1, PL-7, PL-12, PmScl, Scl-70, CENP-A, CENP-B, RA33 and PR3. When the kit containing the antigen-immobilized matrix membrane is usedfor detection, 22 kinds of autoimmune antibodies can be detected simultaneously; and thus diagnosis of eight kinds of common diffuse connective tissue diseases is assisted.
Owner:英诺诊断有限公司
Antinuclear antibody chemiluminescence immunoassay kit and preparation method thereof
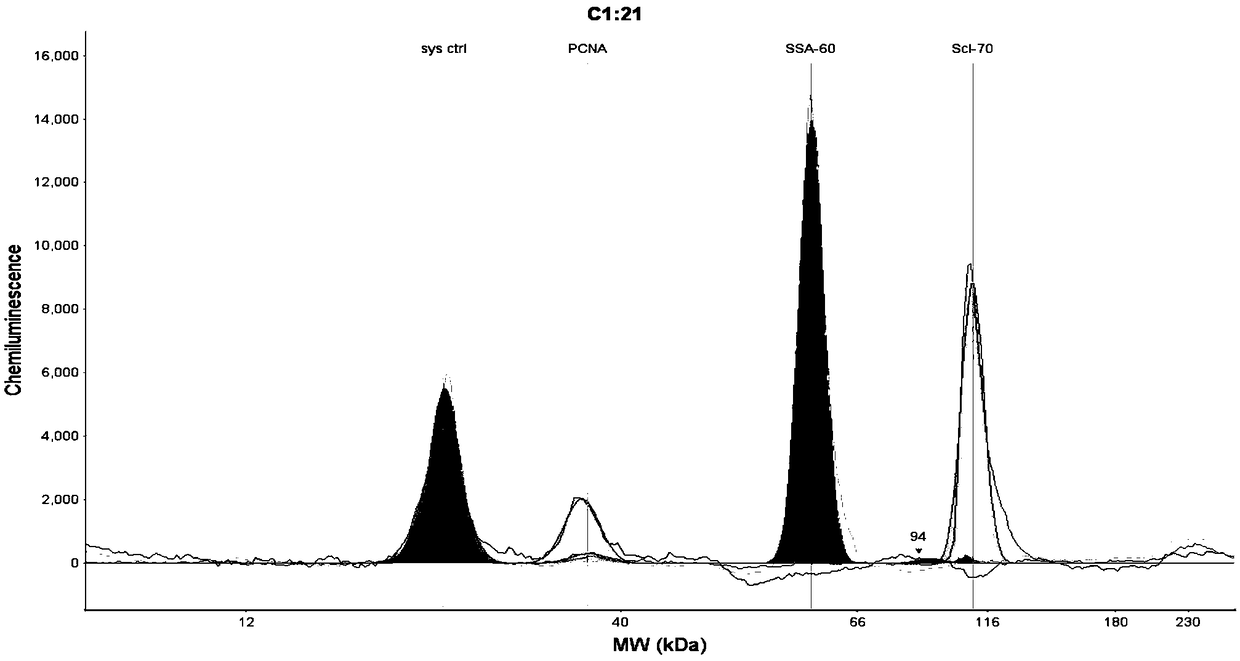
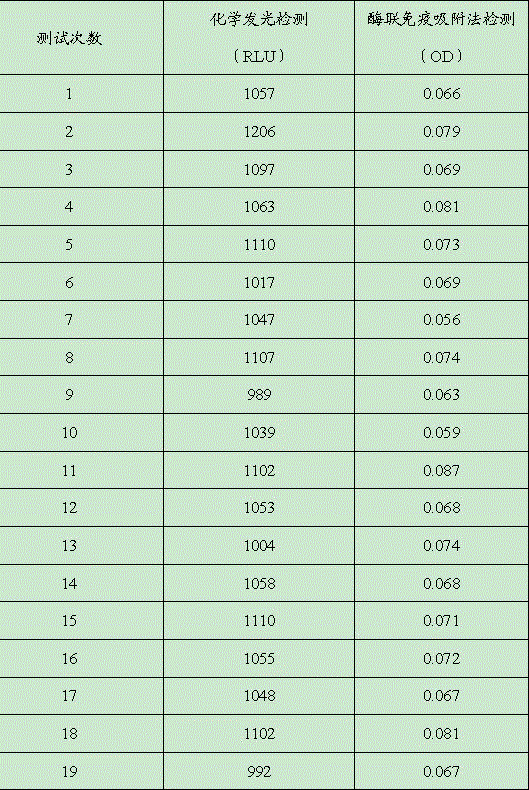
InactiveCN106248943AImprove detection accuracyHigh detection sensitivityMaterial analysisMonoclonal antibodyAnti-nuclear antibody
The invention discloses an antinuclear antibody chemiluminescence immunoassay kit and a preparation method thereof. The antinuclear antibody chemiluminescence immunoassay kit comprises antinuclear antibody monoclonal antibody-coated chloracetylated magnetic particles and an inhibin monoclonal antibody-labelled chemiluminescent marker. With a full-automatic chemiluminescence immunity analyzer as a testing tool, the antinuclear antibody chemiluminescence immunoassay kit can complete detection of antinuclear antibodies. Through experiments, detection sensitivity of the antinuclear antibody chemiluminescence immunoassay kit reaches 4 AU / mL. In comparison with sensitivity of a traditional antinuclear antibody detection method, the sensitivity of the invention is raised by at least 10 times. Detection precision of the antinuclear antibody chemiluminescence immunoassay kit is high.
Owner:SHENZHEN YHLO BIOTECH
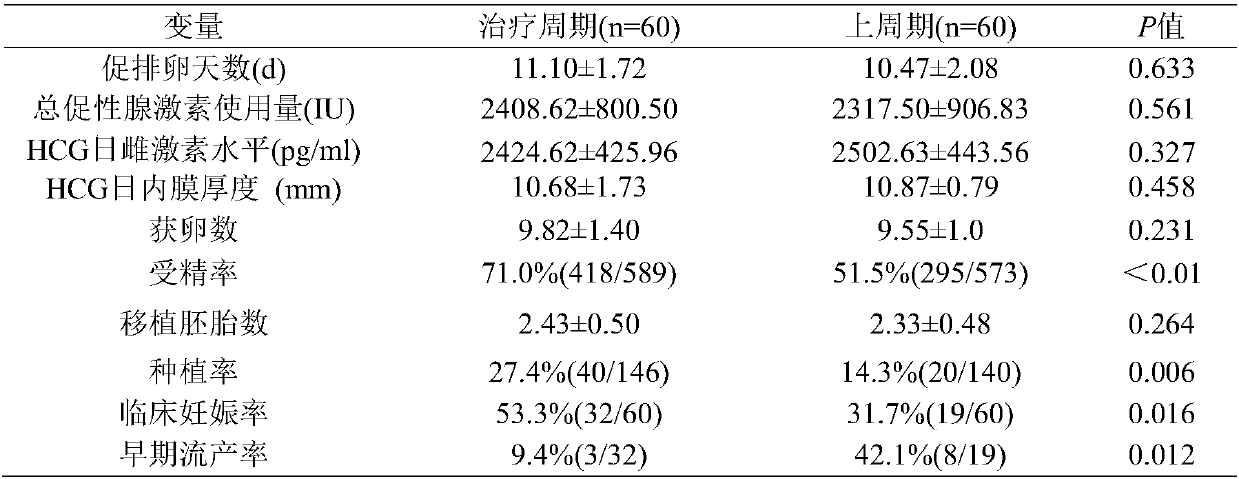
Combined medication method of prednisone and aspirin and application of method to preparation of combined medicine for treating anti-nuclear antibody positive infertility
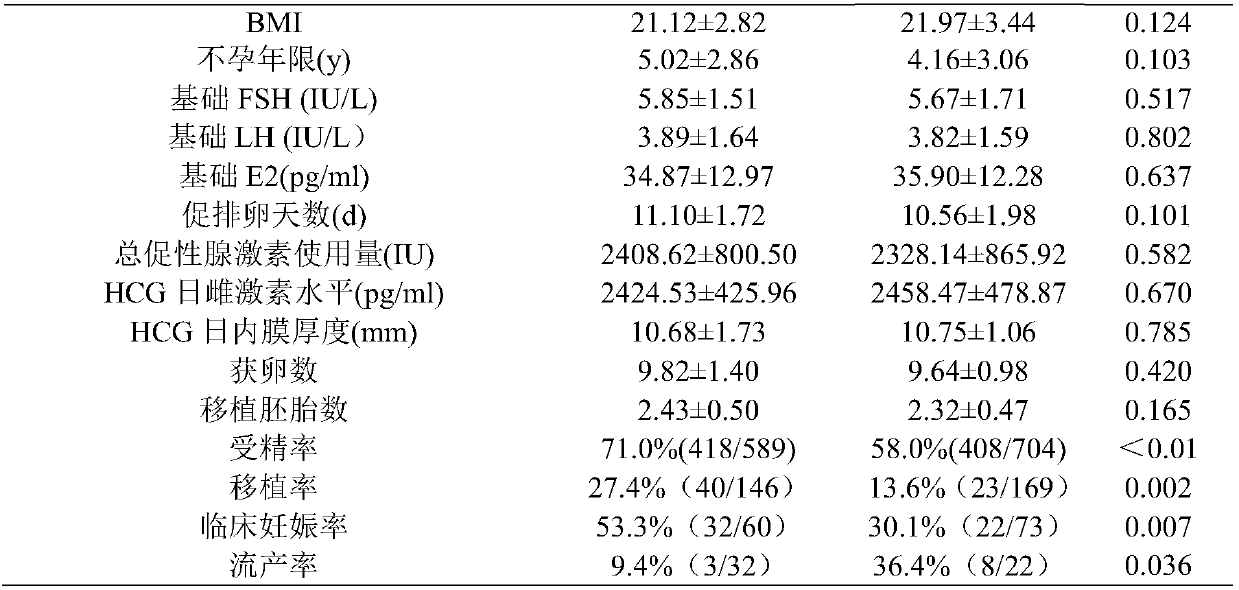
InactiveCN107802628AImprove fertilization rateImprove pregnancy rateOrganic active ingredientsSexual disorderAfter treatmentAnti-nuclear antibody
The invention discloses a combined medication method of prednisone and aspirin and application of the method to preparation of a combined medicine for treating anti-nuclear antibody positive infertility. According to the scheme of the invention, prednisone and aspirin are used to perform combined medication to treat the anti-nuclear antibody positive infertility in three months before ovulation induction. Through comparison treatment, the fertility rate, the planting rate and the pregnancy rate in a treatment group are higher than those in a non-treatment group, and the abortion rate in the non-treatment group is higher. Through comparison on IVF-ET between the cycle and the last cycle in the treatment group, the outcome indicates that the fertility rate, the planting rate and the pregnancy rate in the treatment cycle are higher than those in the last non-treatment cycle, and the abortion rate in the cycle after treatment is lower. By adoption of the scheme of the invention, the prednisone serving as immunosuppression is used in three months before ovulation induction to serve as anticoagulation aspirin treatment, so that the reproductive outcome of the anti-nuclear antibody positive women can be improved.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Screening process for atopic dermatitis
The present invention provides a process of screening patients for atopic dermatitis. The process includes the step of determining, in sera of the patient, the presence of antibodies against nuclear antigens such as transcription co-activator p75, wherein the presence of such antibodies indicates atopic dermatitis. The screening process can be used to detect atopic dermatitis in patients suffering from other conditions such as asthma or interstitial cystitis.
Owner:THE SCRIPPS RES INST
Purine and pyrimidine cdk inhibitors and their use for the treatment of autoimmune diseases
The present invention relates to the use of an inhibitor of CDK2 and / or CDK7 and / or CDK9, or a pharmaceutically acceptable salt thereof, in the preparation of a medicament for treating a disease associated with antinuclear antibodies, wherein the inhibitor of CDK2 and / or CDK7 and / or CDK9 or pharmaceutically acceptable salt thereof is administered in an amount sufficient to down-regulate the levels of antinuclear antibodies. A further aspect of the invention relates to a combination comprising an inhibitor of CDK2 and / or CDK7 and / or CDK9, or a pharmaceutically acceptable salt thereof, and methylprednisolone, and its use in the treatment of diseases associated with antinuclear antibodies, such as SLE.
Owner:CYCLACEL
Improved anti-nuclear antibody detection and diagnostics for systemic and non-systemic autoimmune disorders
ActiveCN107074927AVector-based foreign material introductionGrowth factors/regulatorsAutoimmune responsesAnti-nuclear antibody
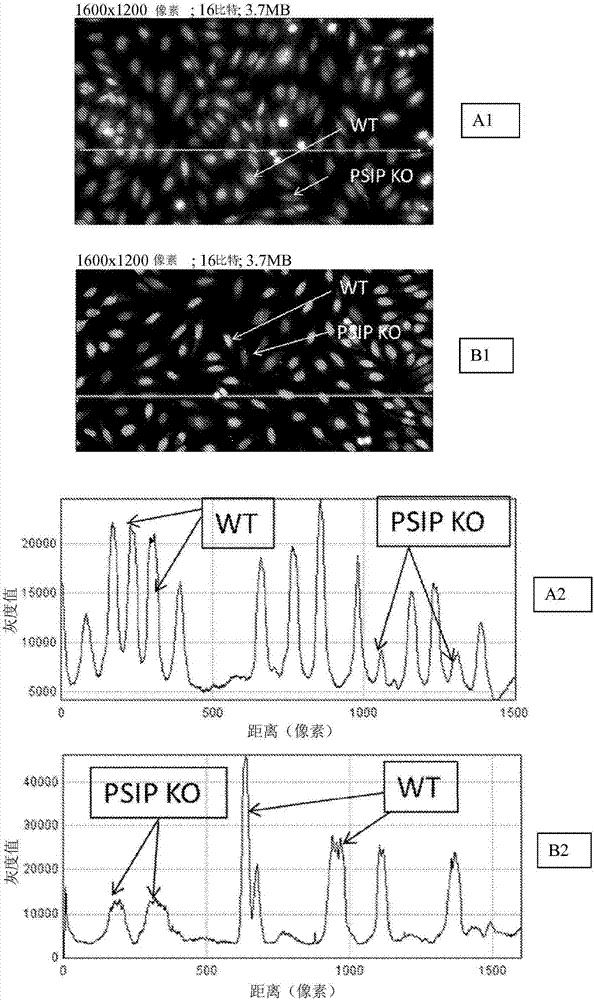
Provided are compositions that contain mammalian cells for use in detecting antibodies. The mammalian cells are modified such that they do not contain LEDGF protein. The mammalian cells are immobilized on a solid substrate. The compositions can also contain mammalian cells that contain the LEDGF protein. Methods for using the cell compositions in diagnostic approaches are included, such as kits for performing diagnostic tests.
Owner:IMMCO DIAGNOSTICS
Kit for detecting antinuclear antibody spectrum related to autoimmune diseases (AIDs)
The invention relates to a kit for detecting antinuclear antibody spectrum related to AIDs. The kit comprises a membrane strip, enzyme-labeled liquid, a substrate and concentrating and washing incubation liquid, wherein the membrane strip is composed of a carrier piece and an antigen band, a critical quality control band and a functional quality control line which are sequentially fixed on the carrier piece. The antigen band is composed of at least two of dsDNA, nucleosomes, SmD1, ribosome P0proteins, histones, U1snRNP, Ro / SS-A(52KD), Ro / SS-A(60KD), La / SS-B, Sc1-70, CENP-B, Jo-1, AMA-M2, PM-Sc1, PCNA, Mi-2 and Ku through independent marking to a nitrocellulose membrane or a nylon membrane. The kit is provided with the ingenious critical quality control band, one critical quality control band can play a role in interpreting two or even more detection bands (antigen bands), and result determination is simpler and more reliable.
Owner:SICHUAN XINCHENG BIOLOGICAL CO LTD
Detection kit for antinuclear antibody repertoires
The invention discloses a detection kit for antinuclear antibody repertoires, and belongs to the technical field of detection kits. The detection kit comprises a composite micro bead suspension, a fluorescence indicator and a sample diluent, wherein a plurality of micro beads are contained in the composite micro bead suspension; a part of micro beads are coated with different types of autologous antigens respectively, a part of micro beads are coated with antigens prepared from HEP-2 cells, a part of micro beads are coated with antigens capable of detecting non-specific antibodies in patient samples, and the remaining micro beads are four types of standard control micro beads; the fluorescence indicator is phycoerythrin labeled goat-anti-human IgG (specific gamma chain); the sample diluentis a scrubbing solution. The detection for a plurality of autologous antibodies can be performed for one patient sample inside the same hole, so that auxiliary diagnosis is provided for various systemic autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis, scleroderma, polymyositis, mixed connective tissue disease (MCTD), drug-induced SLE and sjogren syndrome.
Owner:宙斯生命科技(常州)有限公司
Purine and pyrimidine cdk inhibitors and their use for the treatment of autoimmune diseases
InactiveUS20120309723A1Inhibition formationEase of preparation and detectabilityBiocideAntipyreticAutoimmune diseaseAnti-nuclear antibody
The present invention relates to the use of an inhibitor of CDK2 and / or CDK7 and / or CDK9, or a pharmaceutically acceptable salt thereof, in the preparation of a medicament for treating a disease associated with antinuclear antibodies, wherein the inhibitor of CDK2 and / or CDK7 and / or CDK9 or pharmaceutically acceptable salt thereof is administered in an amount sufficient to down-regulate the levels of antinuclear antibodies. A further aspect of the invention relates to a combination comprising an inhibitor of CDK2 and / or CDK7 and / or CDK9, or a pharmaceutically acceptable salt thereof, and methylprednisolone, and its use in the treatment of diseases associated with antinuclear antibodies, such as SLE.
Owner:CYCLACEL
Antinuclear antibody utilized as a targeting agent for pharmaceutical compounds used in the treatment of cancer and other diseases
InactiveUS20130330402A1Improved profileImprove securityPowder deliveryAntibody ingredientsAnti-nuclear antibodyNeoplasm
Owner:SMITH HENRY J +1
Medicament and method for treating recurrent spontaneous abortion
ActiveUS20090131345A1Decrease in levelAvoid abortionOrganic active ingredientsGenetic material ingredientsMedicineAnti-nuclear antibody
The present invention discloses a pharmaceutical composition for treating a subject with immunological recurrent spontaneous abortion which comprises a therapeutically effective amount of a substance capable of lowering the in vivo level of antinuclear antibody. Particularly, the substance is chromosome No. 2 or fragment thereof containing fibronectin encoding gene derived from the spouse of said subject, or a mixture of chromosome No. 2 or fragment thereof containing fibronectin encoding gene derived from a plurality of males. The present invention also discloses a method for treating immunological recurrent spontaneous abortion.
Owner:BEIJING XINJING ANTAI MEDICAL & TECH SERVICE
Reagent kit for detecting autoimmunity disease related antinuclear antibodies spectrum and preparation method thereof
ActiveCN101329341BImprove detection efficiencyHigh sensitivityMaterial analysisDiseaseAutoimmune responses
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
Antrodia camphoratea pimelie kelone compound for treating autoimmune disease and medicine composition
ActiveCN101357883BOrganic active ingredientsOrganic compound preparationSide effectAutoimmune disease
Owner:GOLDEN BIOTECH
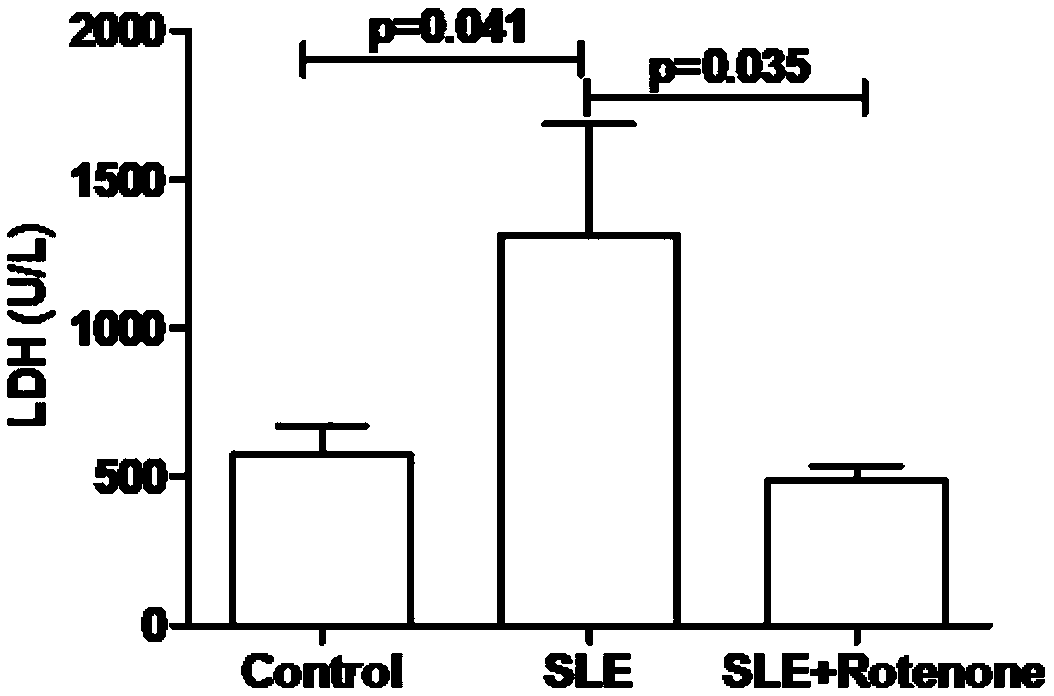
Application of rotenone in preparation of medicine for treating systemic lupus erythematosus
InactiveCN108815155AAlleviate myocardial damageInhibitory activityOrganic active ingredientsImmunological disordersAnti-nuclear antibodySystemic lupus erythematosus
The invention relates to application of rotenone in the preparation of a medicine for treating systemic lupus erythematosus and belongs to the field of Chinese medicine production. Rotenone can inhibit generation of antinuclear antibodies for lupus mice, alleviate myocardial damage of lupus mice, inhibit abnormal activation of B lymphocyte and splenomegaly for lupus mice and inhibit formation of lymph node tumor for lupus mice. Therefore, the invention provides a new candidate drug for treating systemic lupus erythematosus.
Owner:NANJING CHILDRENS HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com