Antinuclear antibody chemiluminescence immunoassay kit and preparation method thereof
A chemiluminescence immunoassay and detection kit technology, applied in measurement devices, scientific instruments, instruments, etc., can solve the problems of high background in fluorescence determination, affecting the accuracy of detection results, and high background
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
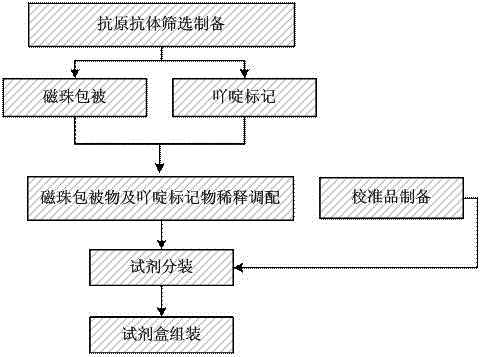
[0059] Such as figure 1 The preparation method of the above-mentioned antinuclear antibody chemiluminescent immunoassay kit shown comprises the following steps:
[0060] Take the suspension of carboxylated magnetic particles, remove the supernatant by magnetic separation, resuspend with MES buffer, then add EDC aqueous solution to activate the carboxyl groups on the surface of carboxylated magnetic particles, then add anti-nuclear antibody antigen, and suspend at room temperature for 2 hours After ~10h, remove the supernatant by magnetic separation and resuspend in Tris buffer to obtain carboxylated magnetic particles of antinuclear antibody antigen.
[0061] MES (2-(N-morpholine)ethanesulfonic acid) buffer had a concentration of 0.02M and a pH of 5.5.
[0062] Tris buffer has a concentration of 0.1 M and contains 2% BSA, pH 8.0.
[0063] The concentration of EDC (1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide) aqueous solution is 10mg / mL~20mg / mL, and the ratio of EDC to car...
Embodiment 1
[0079] Example 1: Preparation of antinuclear antibody chemiluminescent immunoassay kit
[0080] (1) Preparation of carboxylated magnetic particles coated with antinuclear antibody monoclonal antibody:
[0081] Take a suspension containing 50 mg of carboxylated magnetic particles (MagnaBind™, product number 21353) with a particle size of 0.05 μm ~ 1 μm, magnetically separate to remove the supernatant, resuspend with 0.02 M, pH 5.5 MES buffer, add 1 mL of newly prepared 10mg / mL EDC aqueous solution, activate the carboxyl group on the surface of the magnetic beads, add 4mg antinuclear antibody antigen, suspend at room temperature for 6h, magnetically separate, remove the supernatant, and reconstitute with 0.1M Tris buffer solution containing 2% BSA, pH 8.0 Suspend to 1mg / mL to obtain carboxylated magnetic particles of anti-nuclear antibody antigen, and store in 5mL bottles at 4°C for future use.
[0082] (2) Preparation of acridinium ester labeled with inhibin monoclonal antibod...
Embodiment 2
[0086] Embodiment 2: Antinuclear antibody chemiluminescent immunoassay method
[0087] The automatic chemiluminescent immunoassay analyzer (YHLO, catalog number iFlash3000) is used as the detection tool, and the methodological mode is the double antibody sandwich method, that is, 5 μL of sample, 50 μL of carboxylated magnetic particles coated with antinuclear antibody antigen, and 50 μL of antinuclear antibody treatment solution, reacted for 10 minutes, then added 100 μL of antinuclear antibody-coated acridinium ester, reacted for 10 minutes, carried out magnetic separation, the instrument sent the reaction mixture into the dark room, and added luminescent substrate A in turn Liquid (H 2 o 2 ) and solution B (NaOH) for luminescence reaction, and finally record the luminescence value.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com