Preparation method of human antinuclear antibody
An anti-nuclear antibody, human-derived technology, applied in the field of biochemistry, can solve the problems of complicated preparation technology, large molecular weight of monoclonal antibody, and poor tissue penetration, and achieve strong randomness, simple method, and tissue penetration powerful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
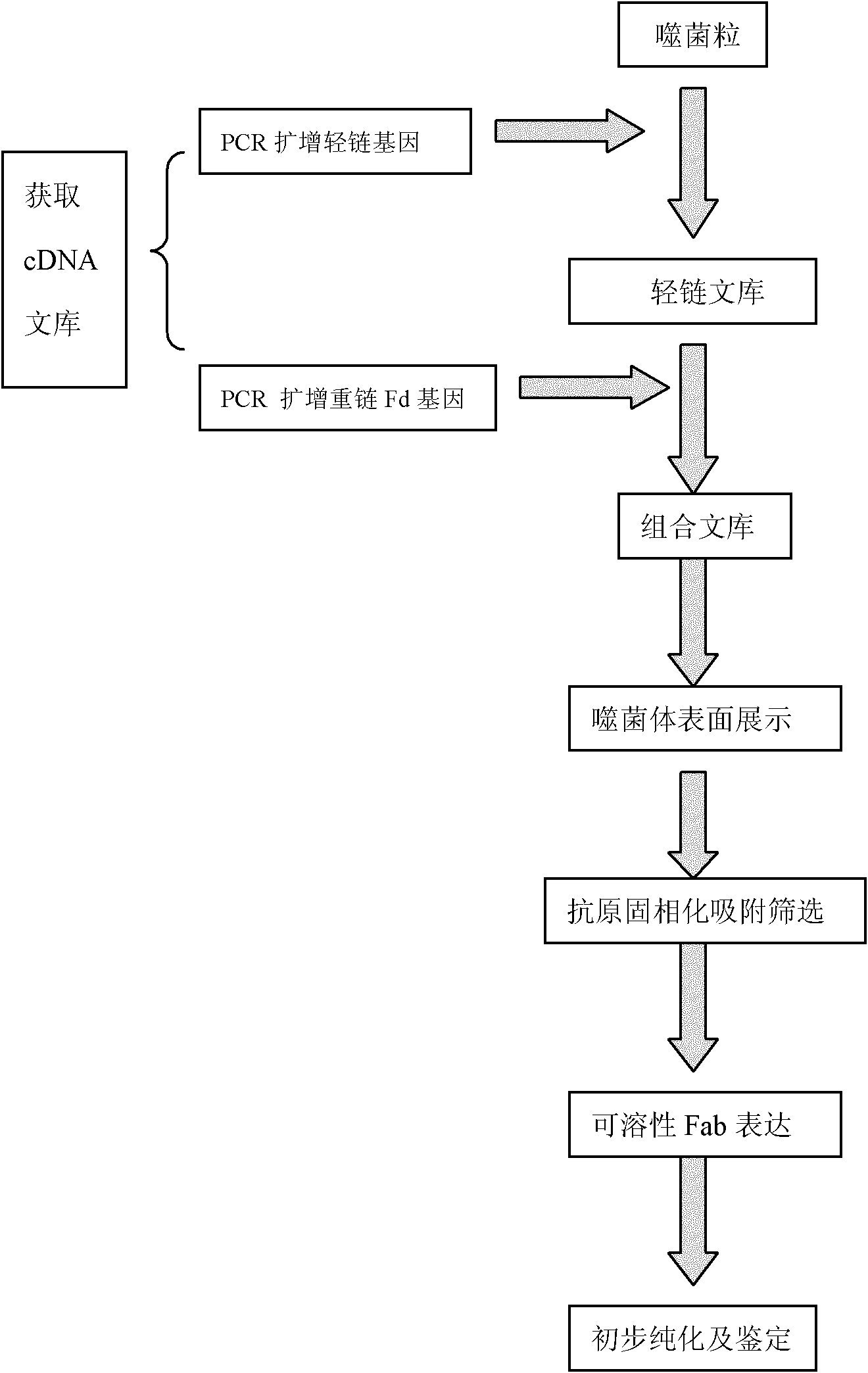
[0034] figure 1 Shown is the flow chart of the preparation method of the present invention.
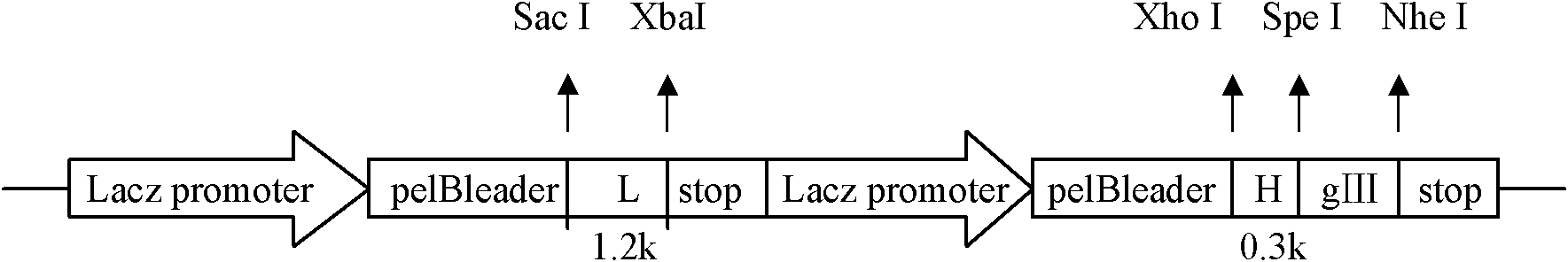
[0035] (1) Construction of phage antibody library
[0036] 1. Isolation of peripheral blood mononuclear cells
[0037] (1) Take 3ml of peripheral blood from patients with positive antinuclear antibodies and place it in a heparin anticoagulant tube;
[0038] (2) Dilute whole blood with normal saline 1:1;
[0039] (3) Add 3ml of lymphocyte separation medium into a clean 10ml centrifuge tube, and carefully add the diluted whole blood to the surface of the separation medium;
[0040] (4) centrifugal, 2000rpm, 20 minutes;
[0041] (5) Absorb the lymphocyte layer;
[0042] (6) Washing with PBS solution twice;
[0043] (7) Add PBS solution to resuspend, count the cells under a microscope and store in a -80°C refrigerator.
[0044] 2. Extraction of total RNA
[0045] (1) Extract total RNA from peripheral blood mononuclear cells with a small amount of column centrifugal total RNA extra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com