Slow/controlled release pellet composition containing ginkgo leaf extracts and preparation method thereof
A technology of extract and ginkgo biloba, which is applied in the field of sustained/controlled release pellets containing ginkgo biloba extract and its preparation, can solve the problems of low bioavailability, poor multi-component absorption, frequent administration, etc., and achieve Good reproducibility, stable drug properties, and the effect of reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
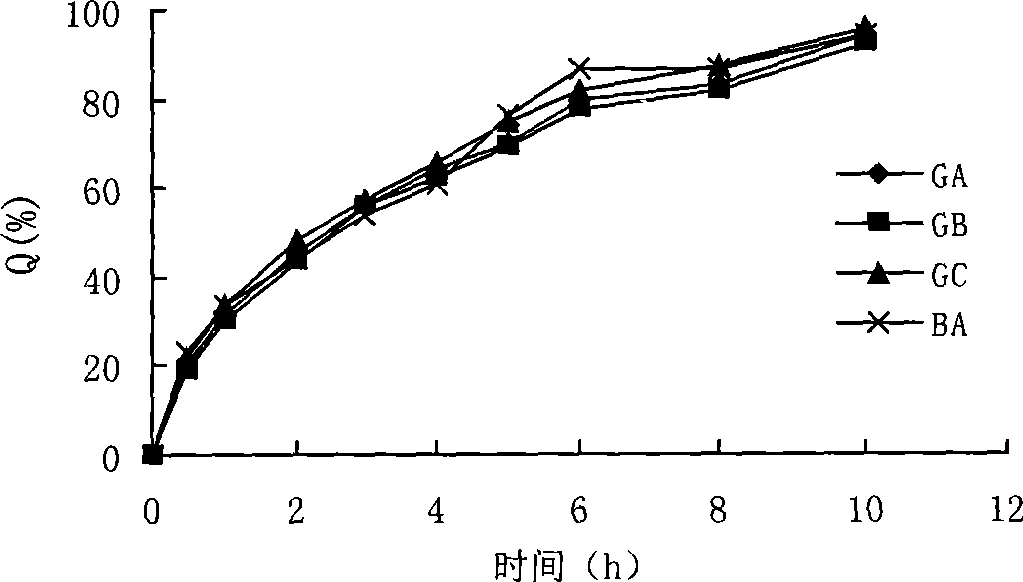
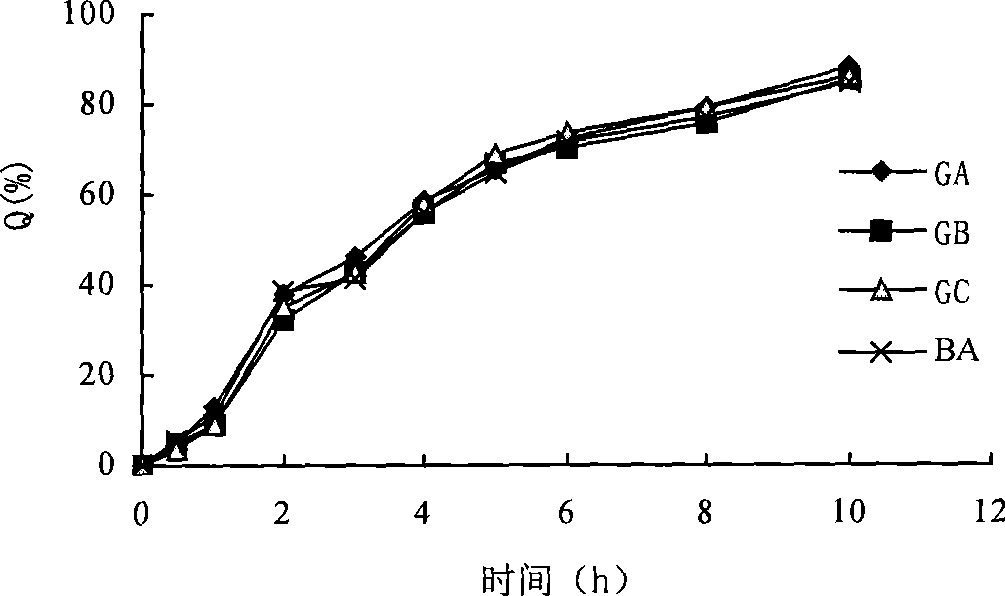
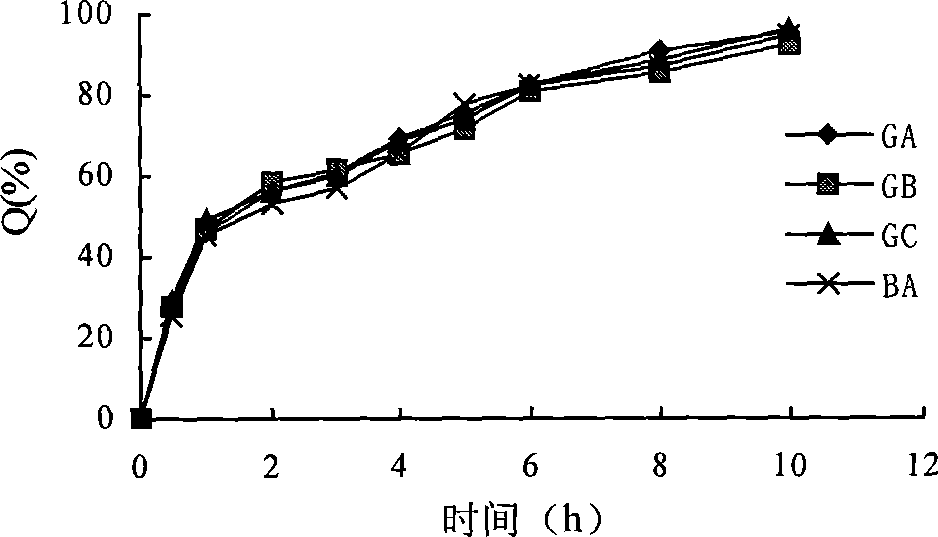
Image
Examples
Embodiment 1
[0048] Prescription 1 for immediate-release pellets:
[0049] Ginkgo Biloba Extract 1000g
[0050] Microcrystalline Cellulose 1600g
[0051] Starch 550g
[0052] Sodium carboxymethyl starch 350g
[0053]Weigh 1000g ginkgo leaf extract, 1600g microcrystalline cellulose, 550g starch, and 350g sodium carboxymethyl starch, mix in a small V-shaped mixer for 15 minutes, add 1500g of purified water in portions, and place a small V-shaped mixing mechanism soft material, Transfer to the extrusion spheronizer, set the speed of 25Hz to pass through the 1mm aperture screen to form thin strips, turn on the turntable, put the thin strips into the turntable, round at the speed of 10Hz for 15 minutes, adjust the reverse, roll the ball for 10 minutes, at 20Hz The speed is forward, and the high-speed rolling is formed for 30 minutes. Turn on the fan to slow down, collect the pellet cores, and dry them in an oven at 45°C for 2 hours to obtain the pellet cores containing medicine. Weighed, t...
Embodiment 2
[0057] Isolation layer coating prescription 1 Eudragit E 100 250g
[0058] Triethyl citrate 50.0g
[0059] Simethicone 10.0g
[0061] Weigh 250g of Eudragit E, add 220g of purified water in portions, keep stirring to prevent caking, add 50g of triethyl citrate, stir until the material is completely dissolved, pass through an 80-mesh sieve, and obtain the coating solution for the isolation layer. Preheat the above coating solution to 35°C, turn on the fluidized bed coating machine, weigh 250g of the pill core of Example 1, set the air inlet temperature to 45°C, and the relative humidity of the air inlet airflow to be 55% spray gun atomization pressure is 0.35MPa, and the air intake is 100m 3 / h. The coating liquid is connected to the liquid inlet through the infusion pump, and the flow rate is 1.5g / min, and the pellets are coated by continuous spraying, and the slow-release film liquid is continuou...
Embodiment 3
[0068] Immediate-release pellet core prescription 2:
[0069] Ginkgo Biloba Extract 1000g
[0070] Microcrystalline Cellulose 1500g
[0071] Dextrin 600g
[0072] Subeng King 300g
[0073] Weigh 1000g ginkgo biloba extract, 1500g microcrystalline cellulose, 600g dextrin, 300g Subengwang, mix in a small V-shaped mixer for 15 minutes, add 1800g of purified water in portions, put soft materials in a small V-shaped mixing mechanism, and turn Enter the extrusion spheronizer, set the speed of 25Hz to pass through the 1.5mm aperture screen to form thin strip materials, turn on the turntable, put the thin strip materials into the turntable, spheronize at the speed of 10Hz for 15 minutes, adjust the reverse, and roll the pellets for 10 minutes at 20Hz The speed is forward, and the high-speed rolling is formed for 30 minutes. Turn on the fan to slow down, collect the pellet cores, and dry them in an oven at 45°C for 2 hours to obtain the pellet cores containing medicine. Weighed, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com