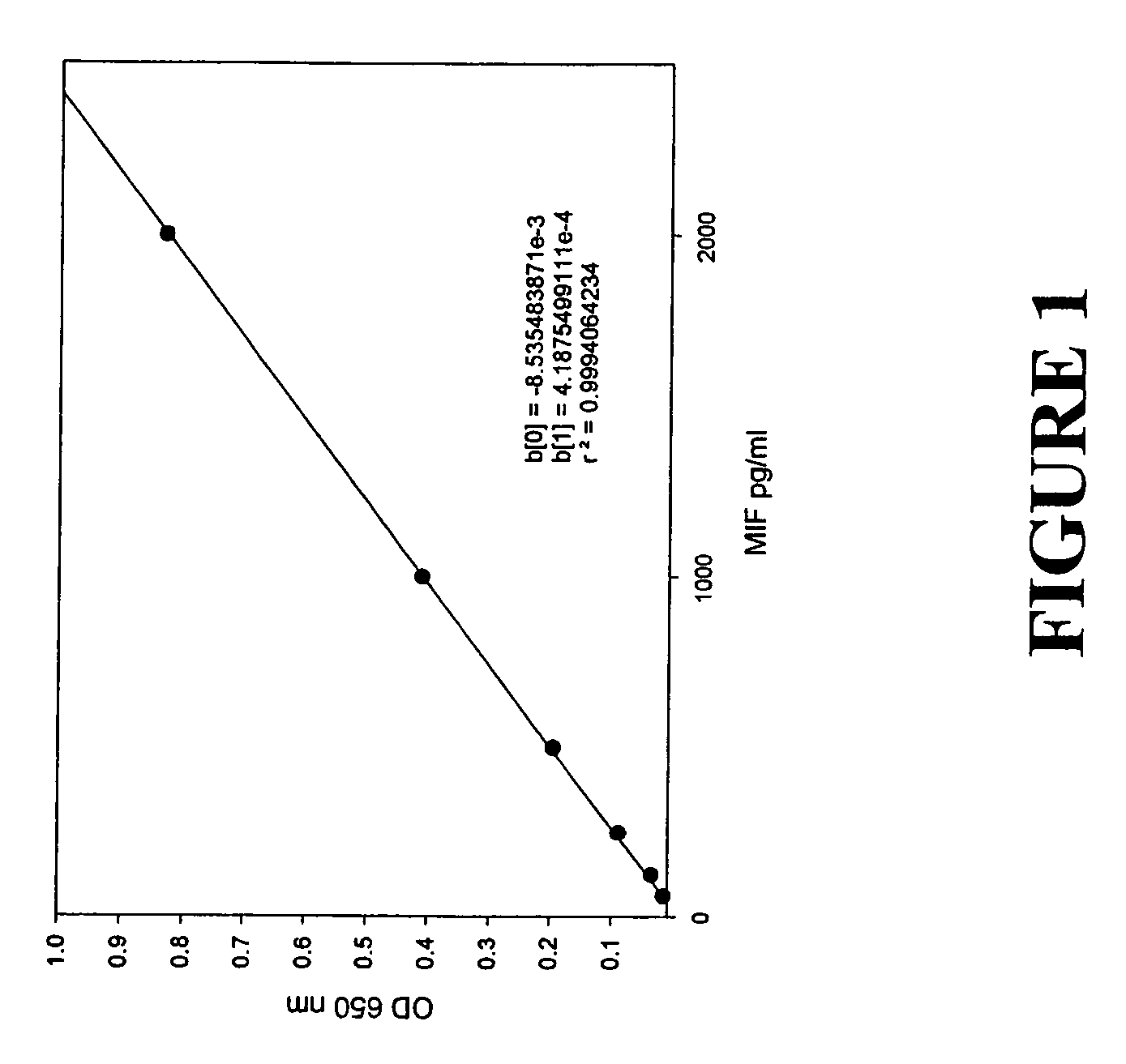
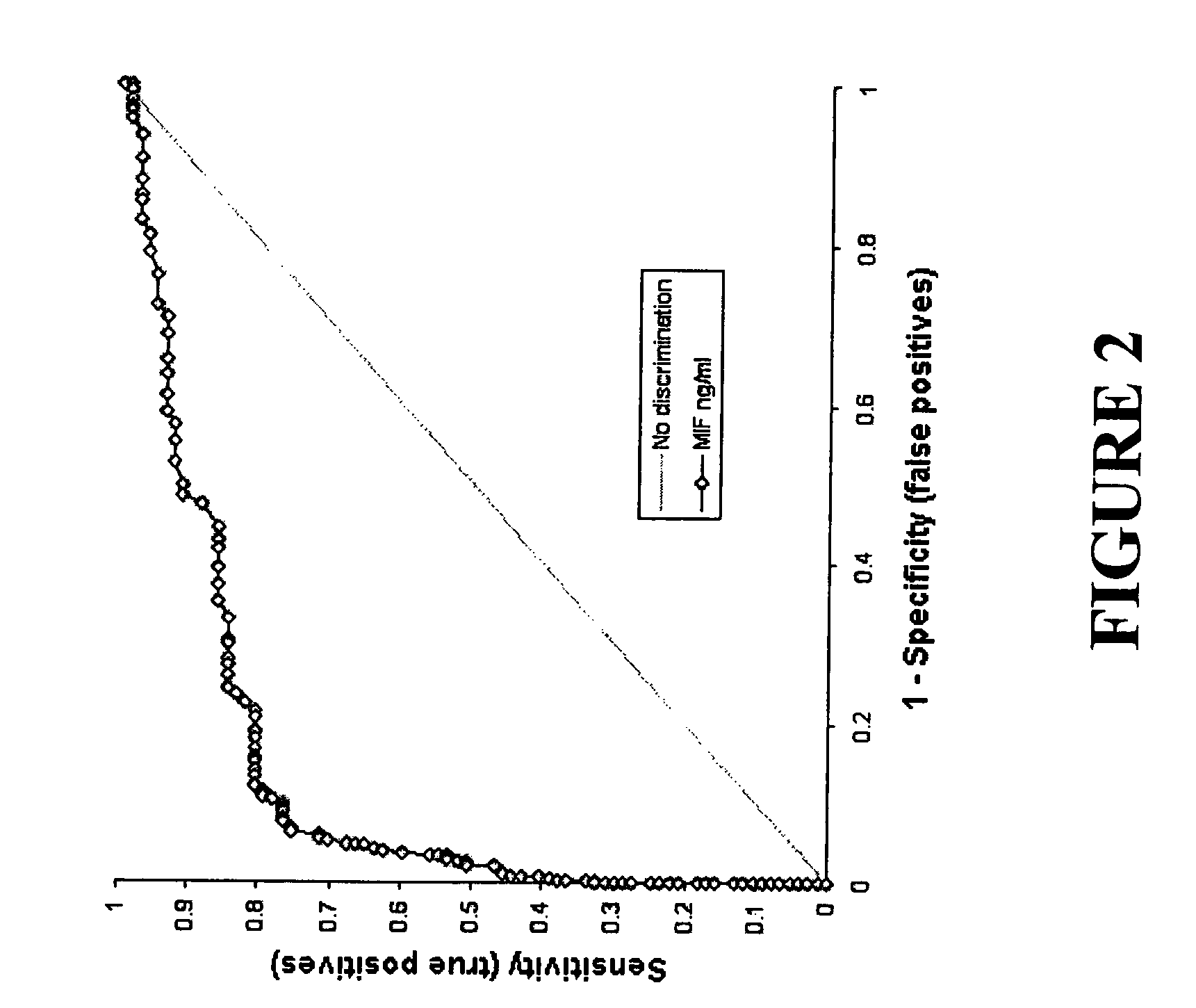
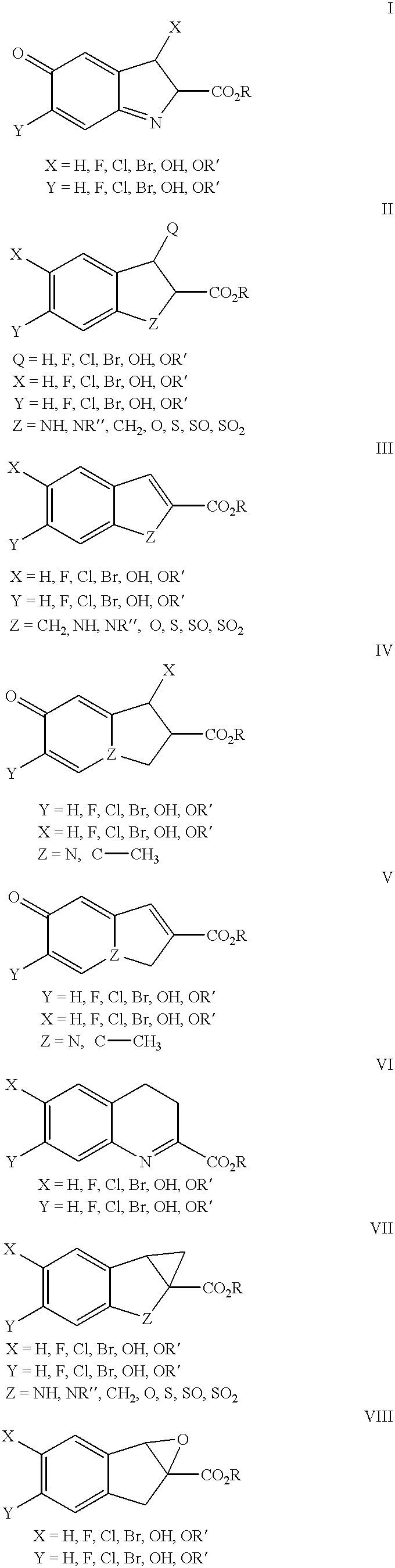
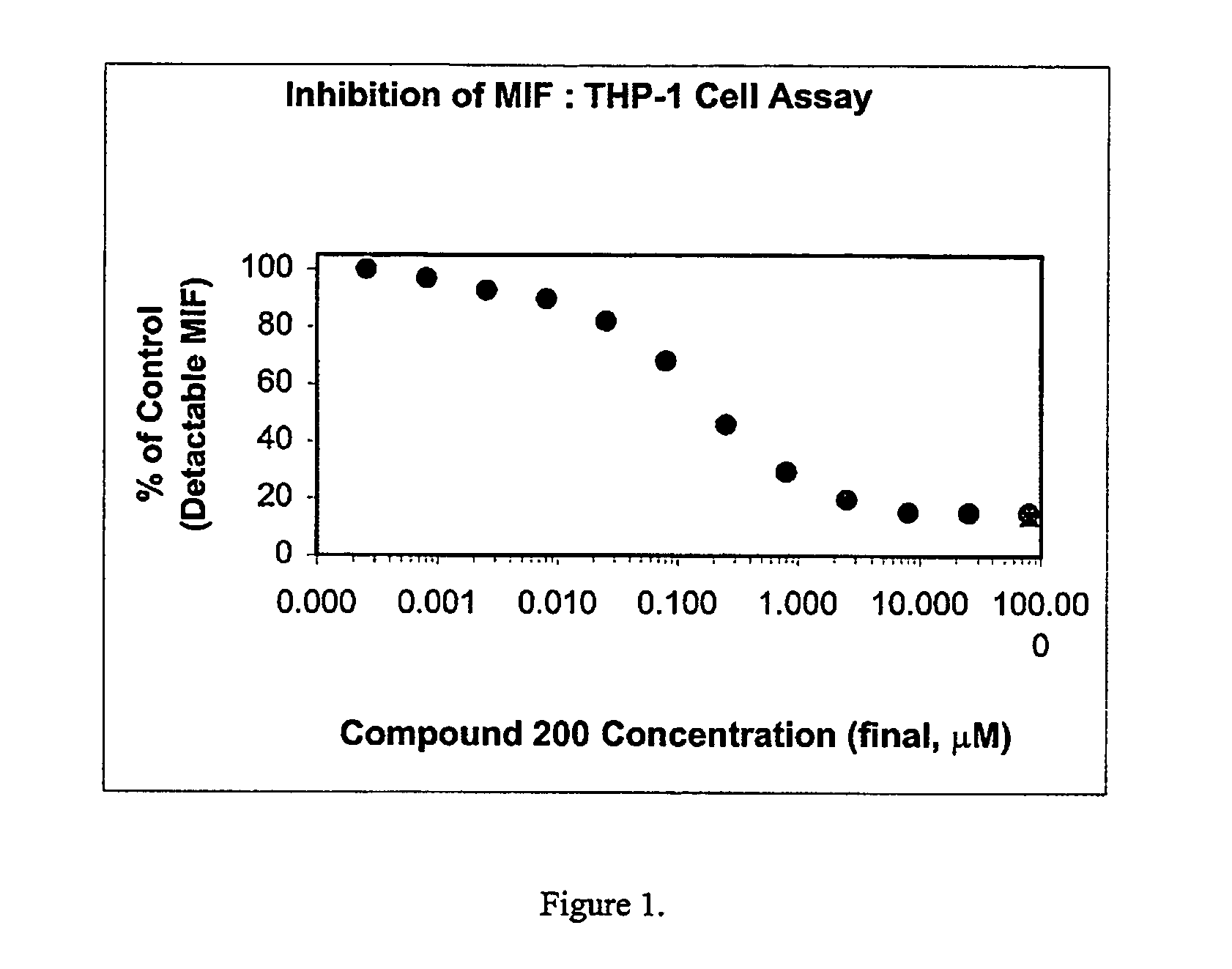
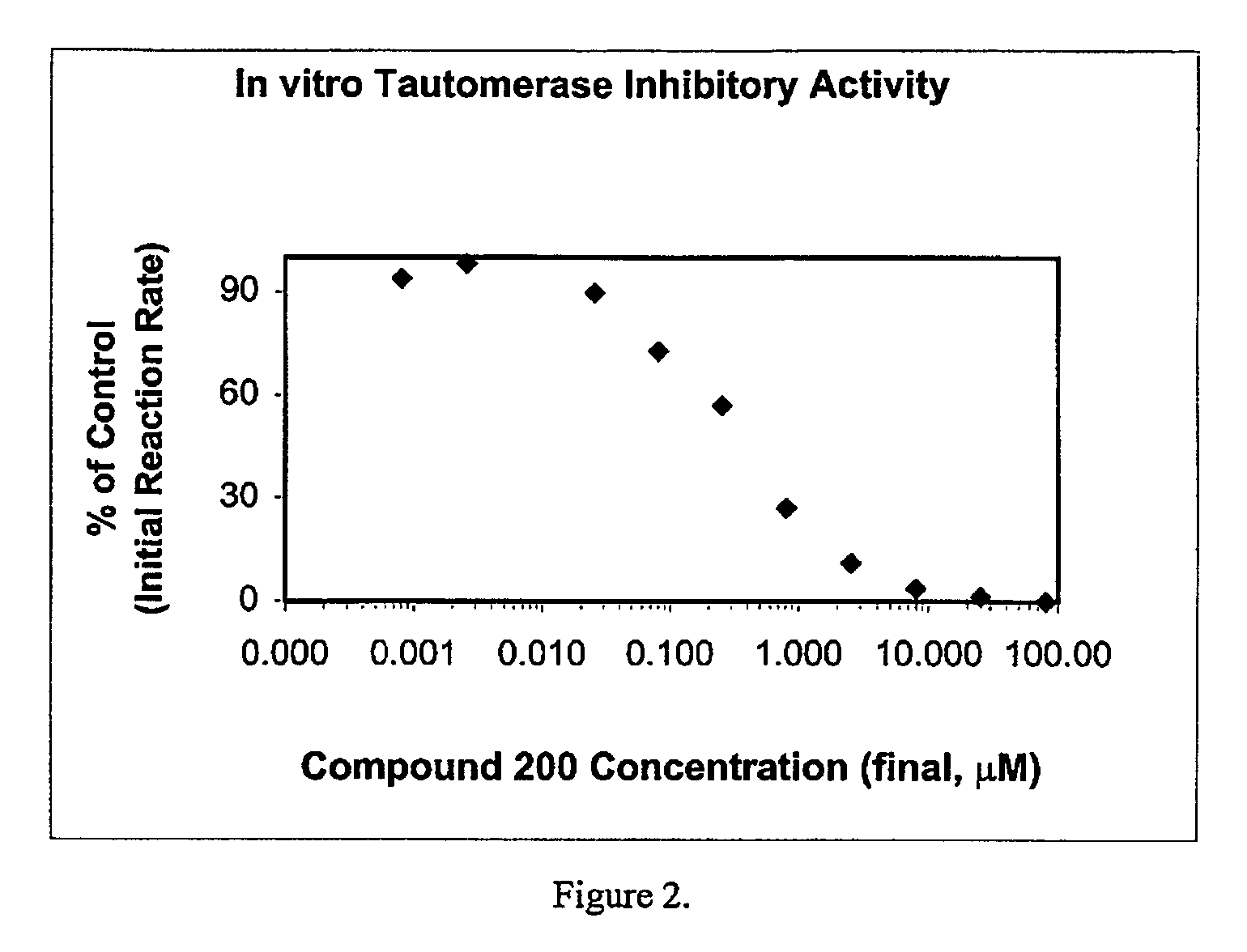
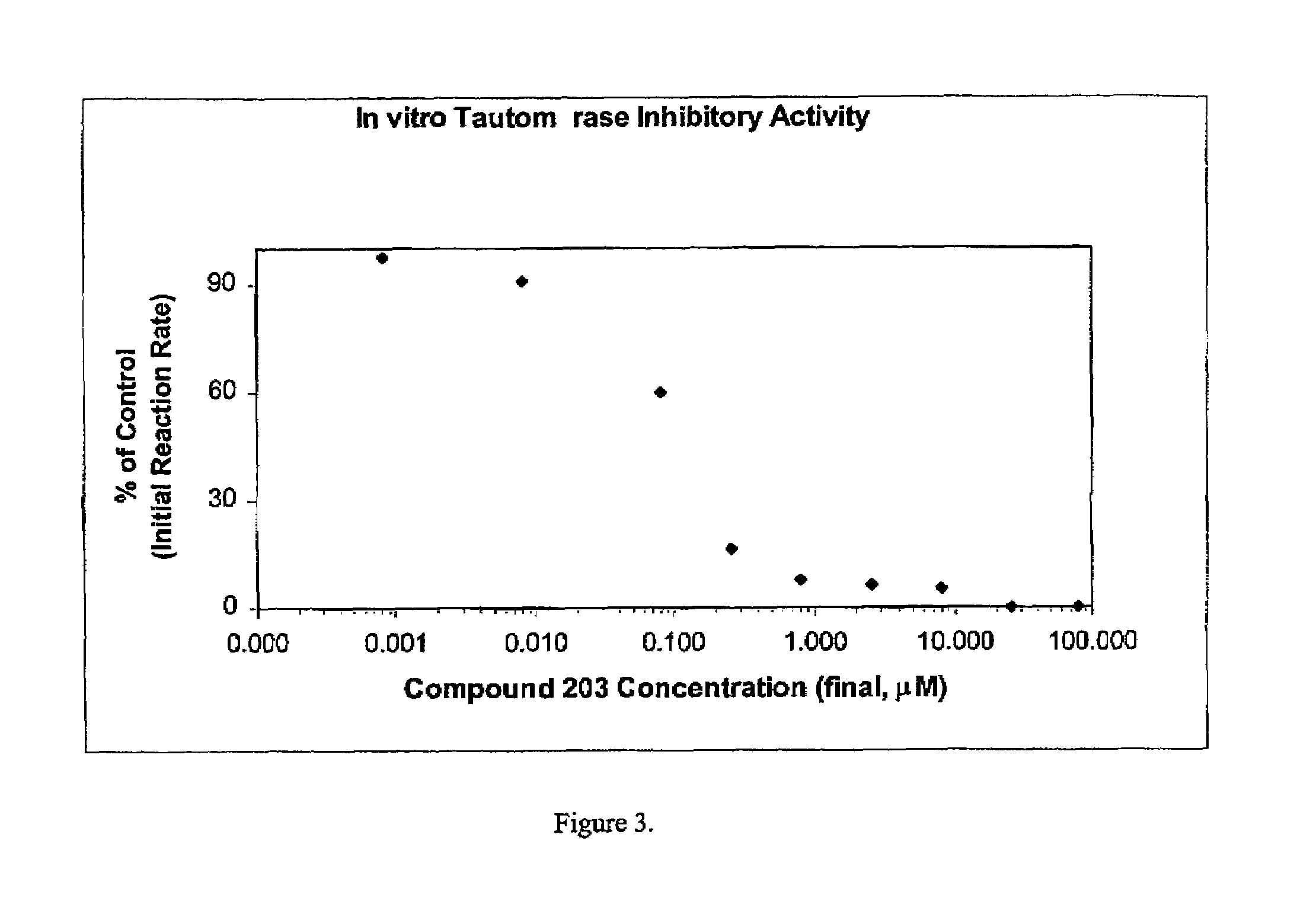
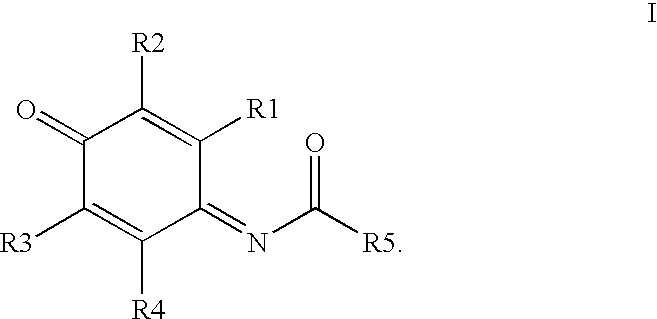
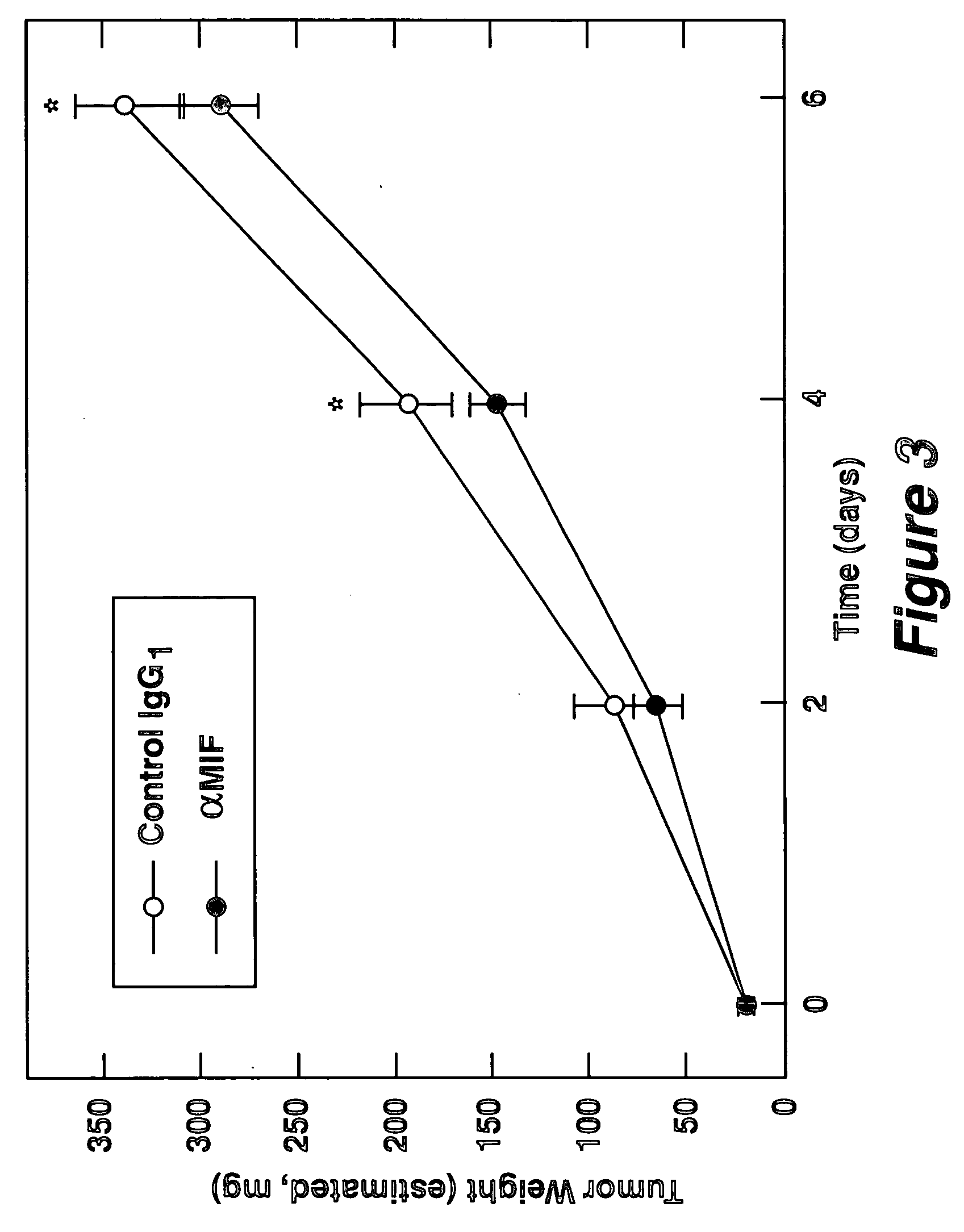
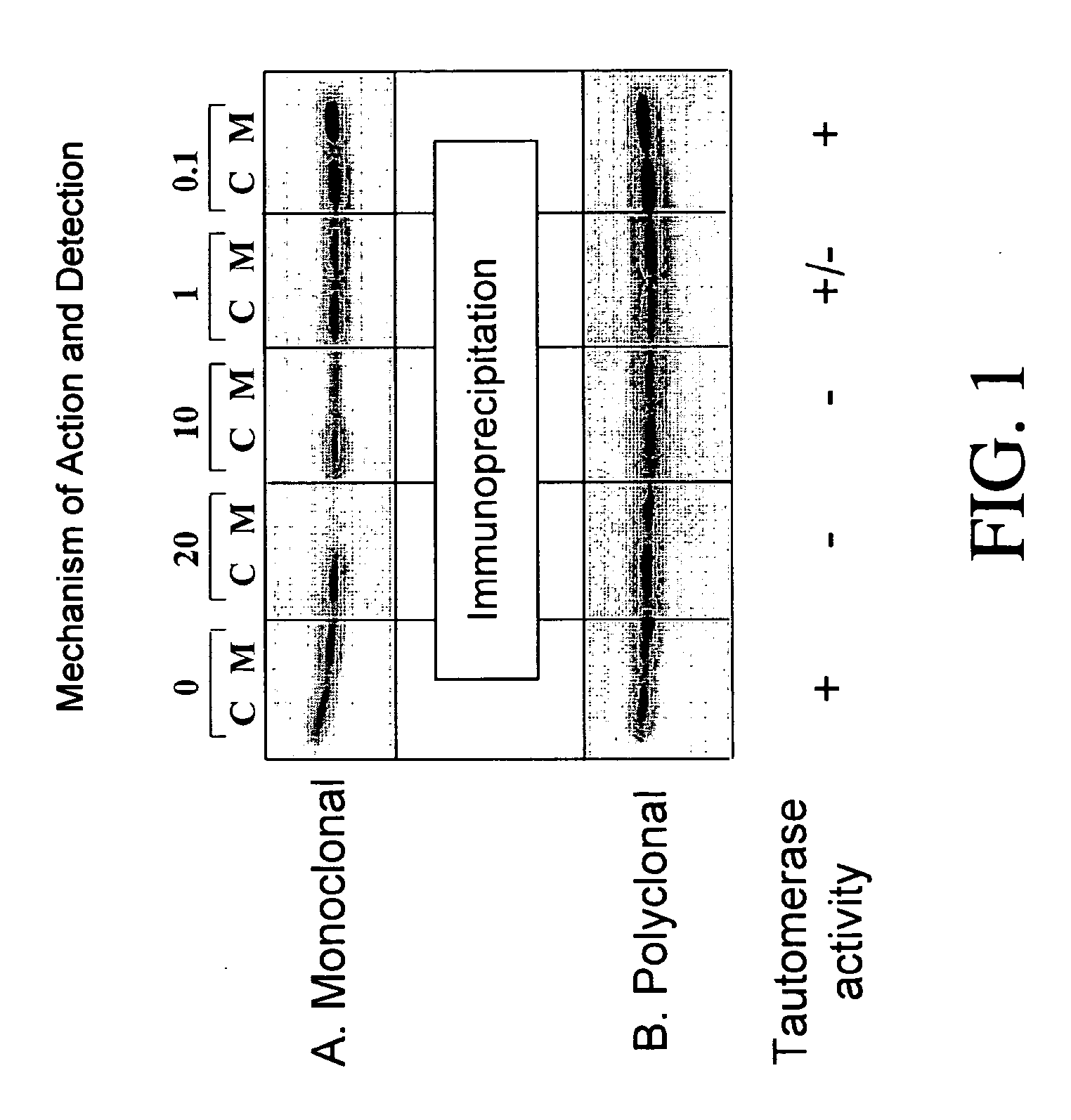
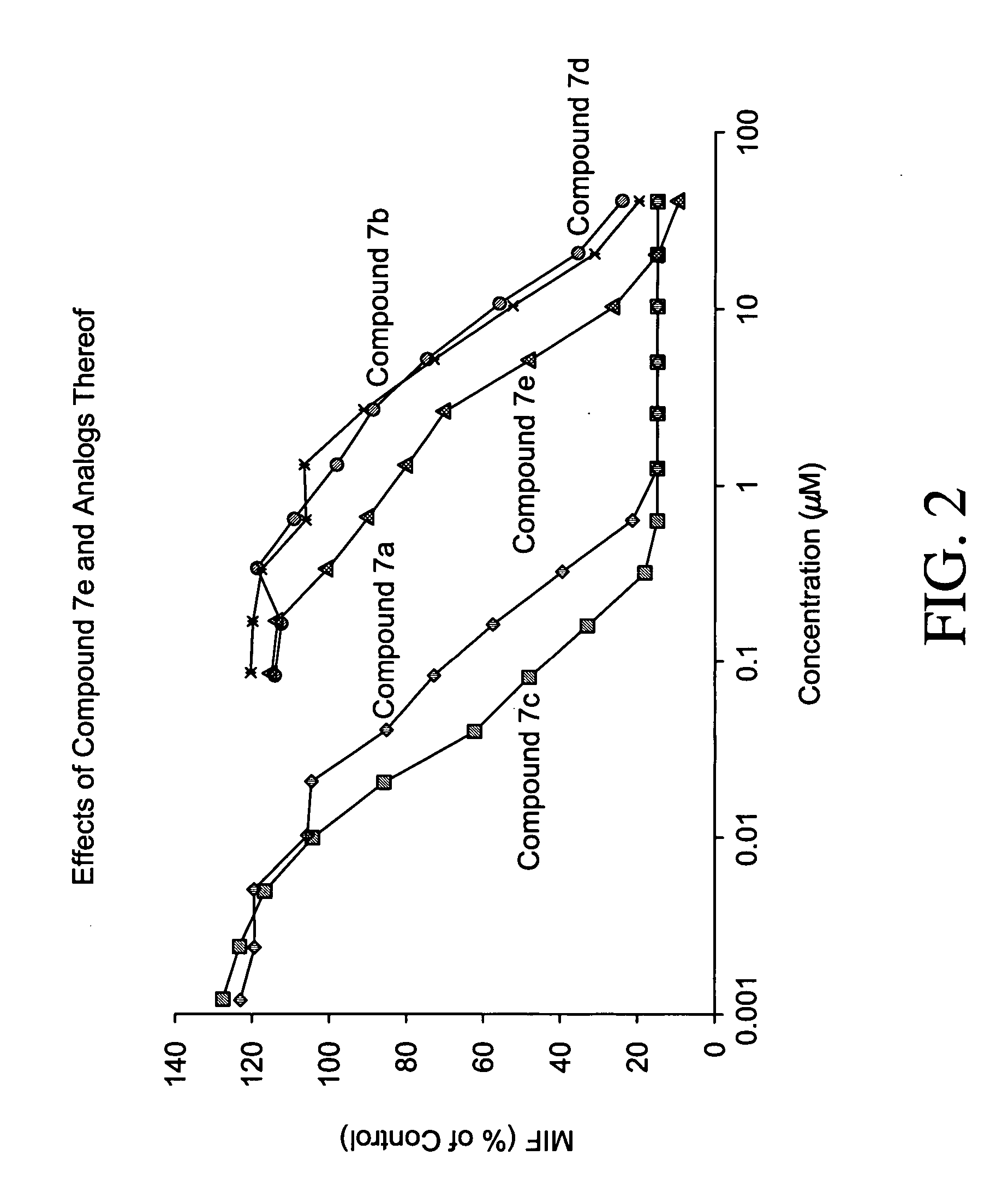
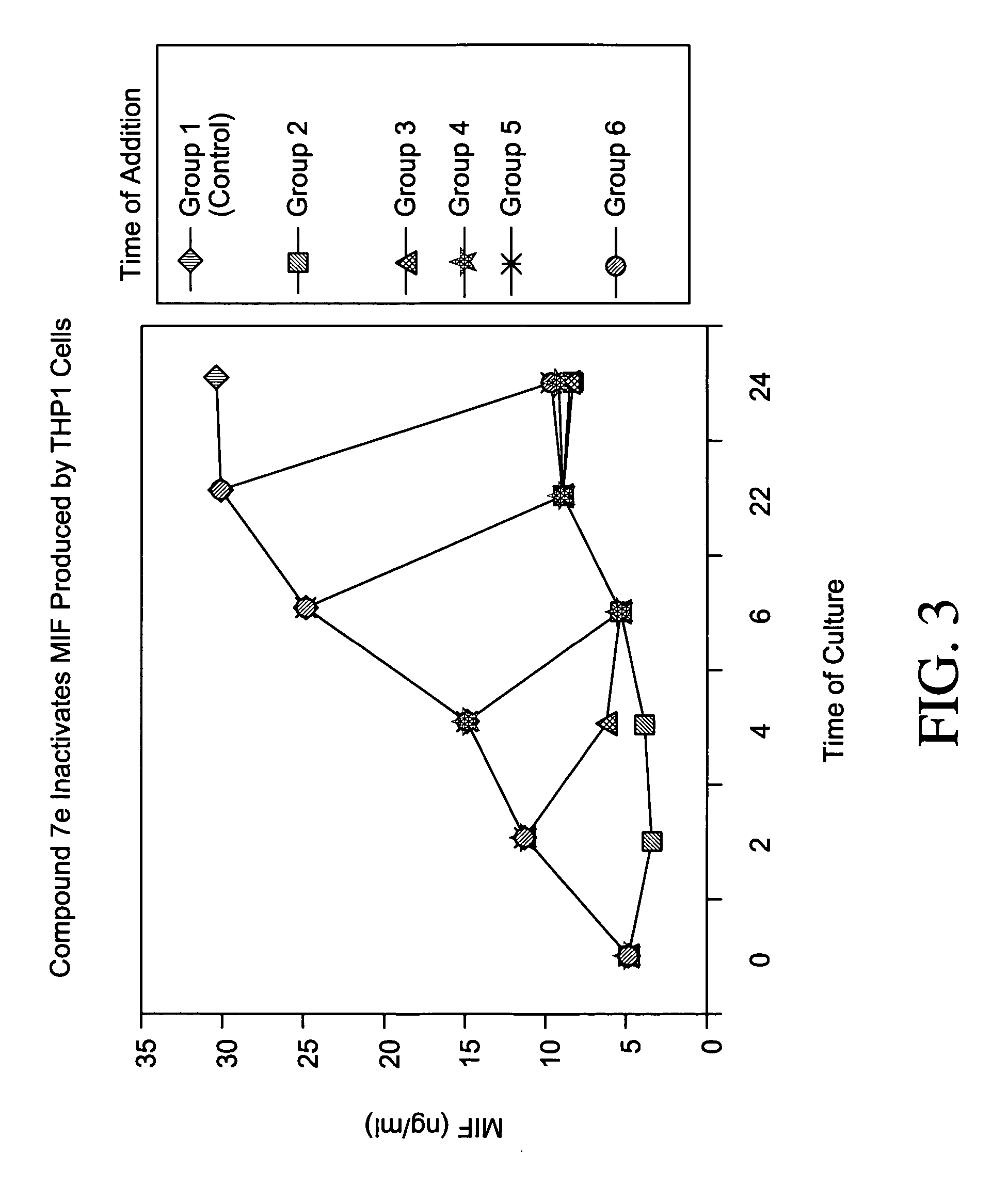
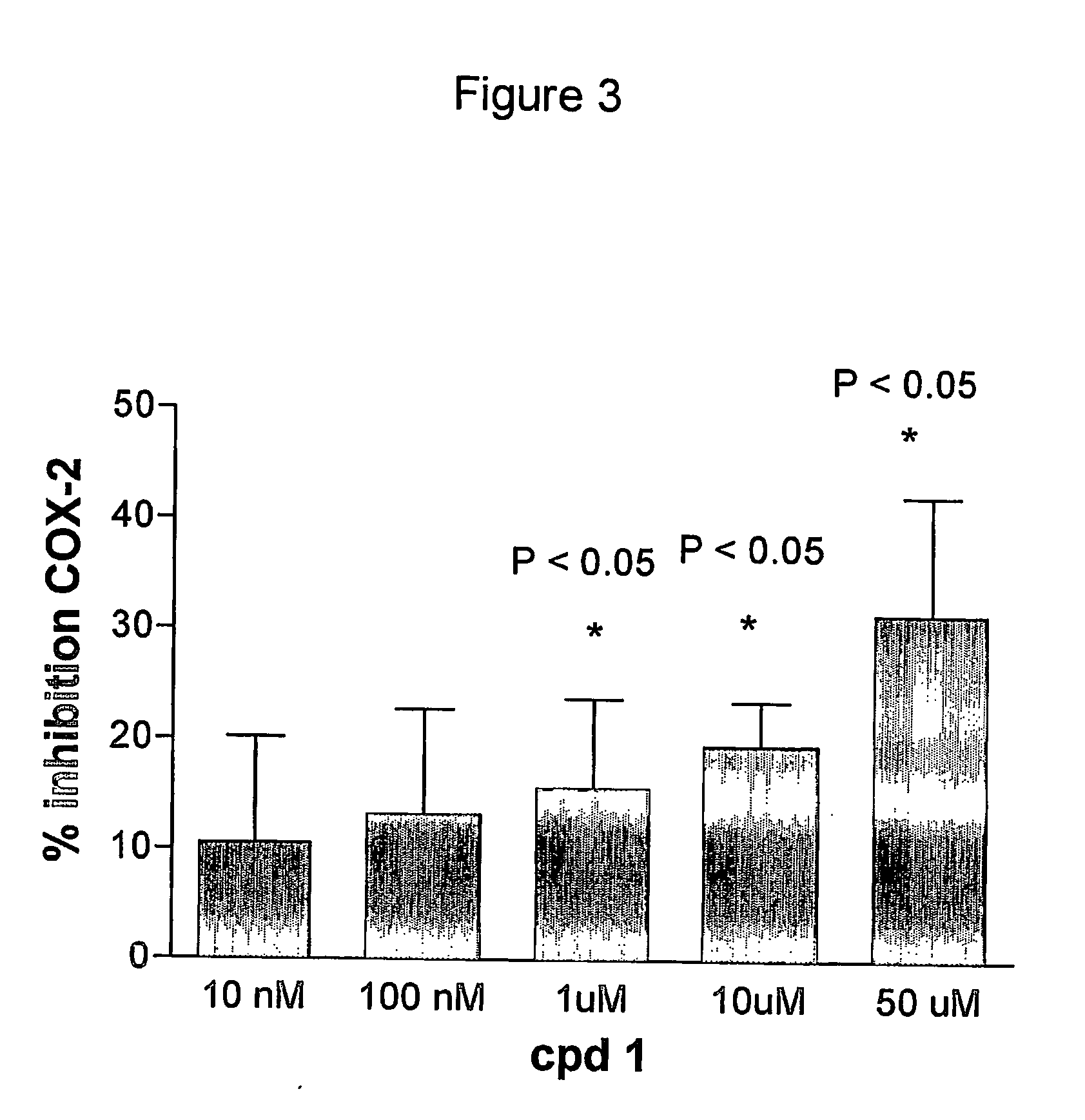
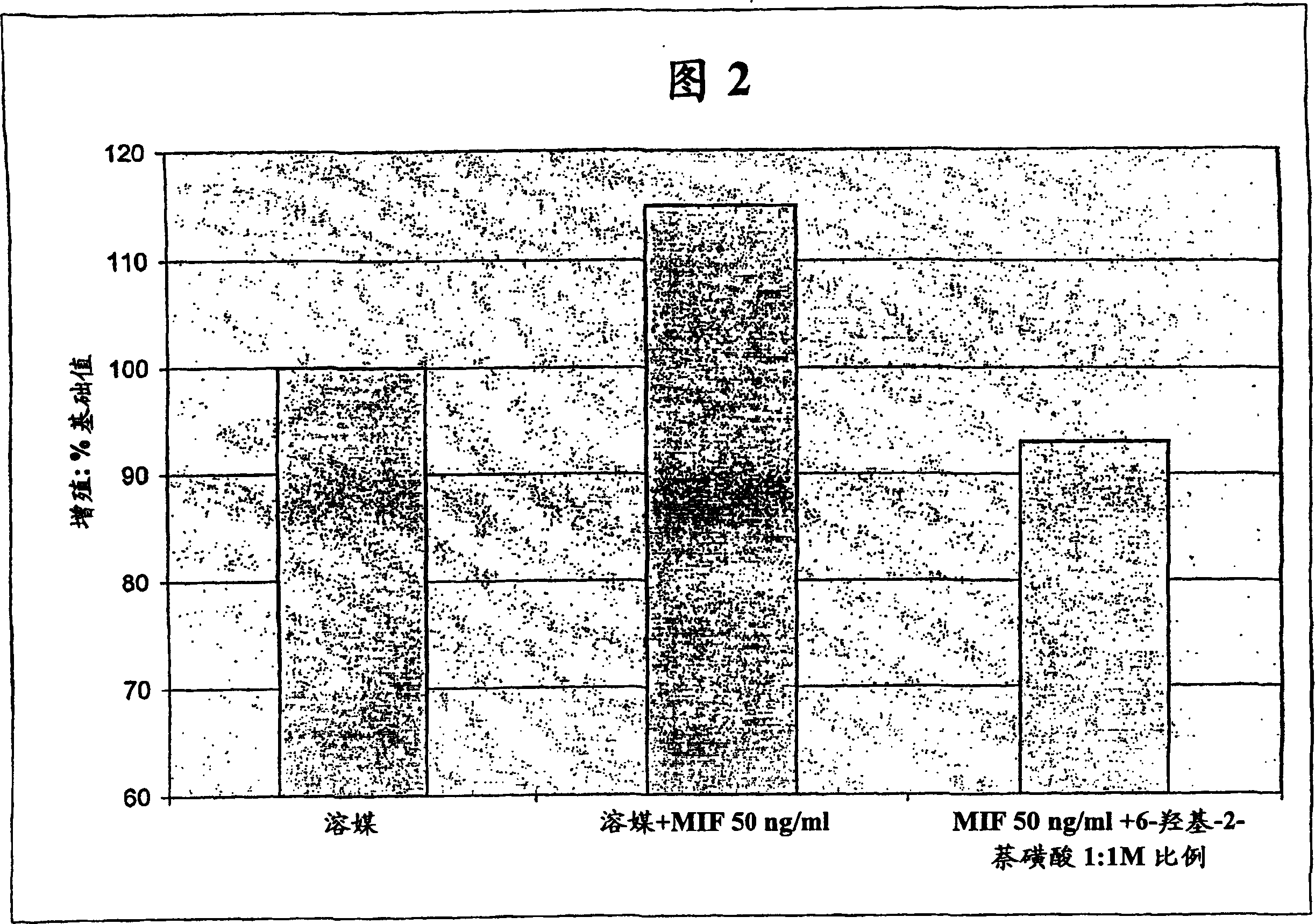
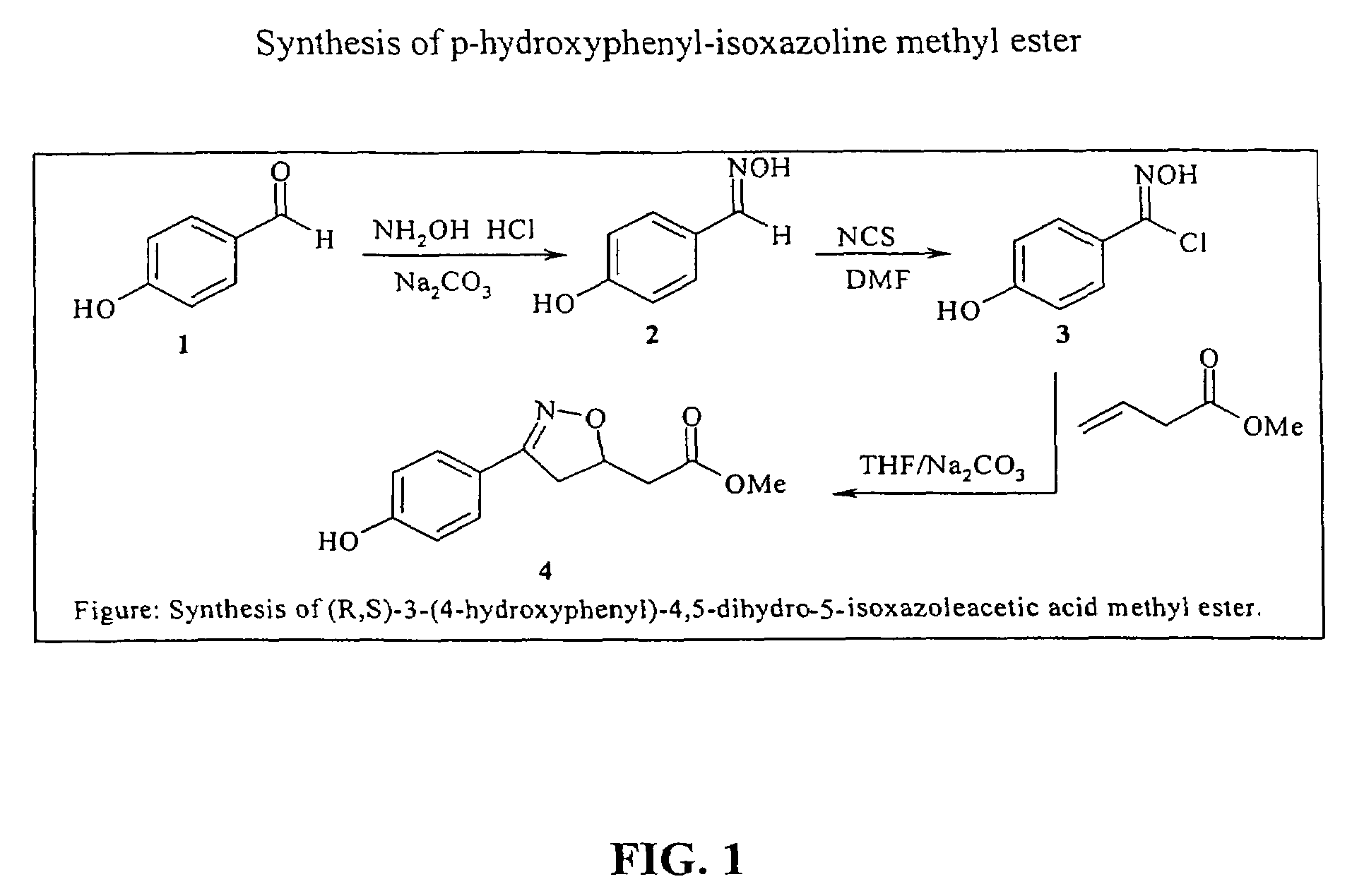
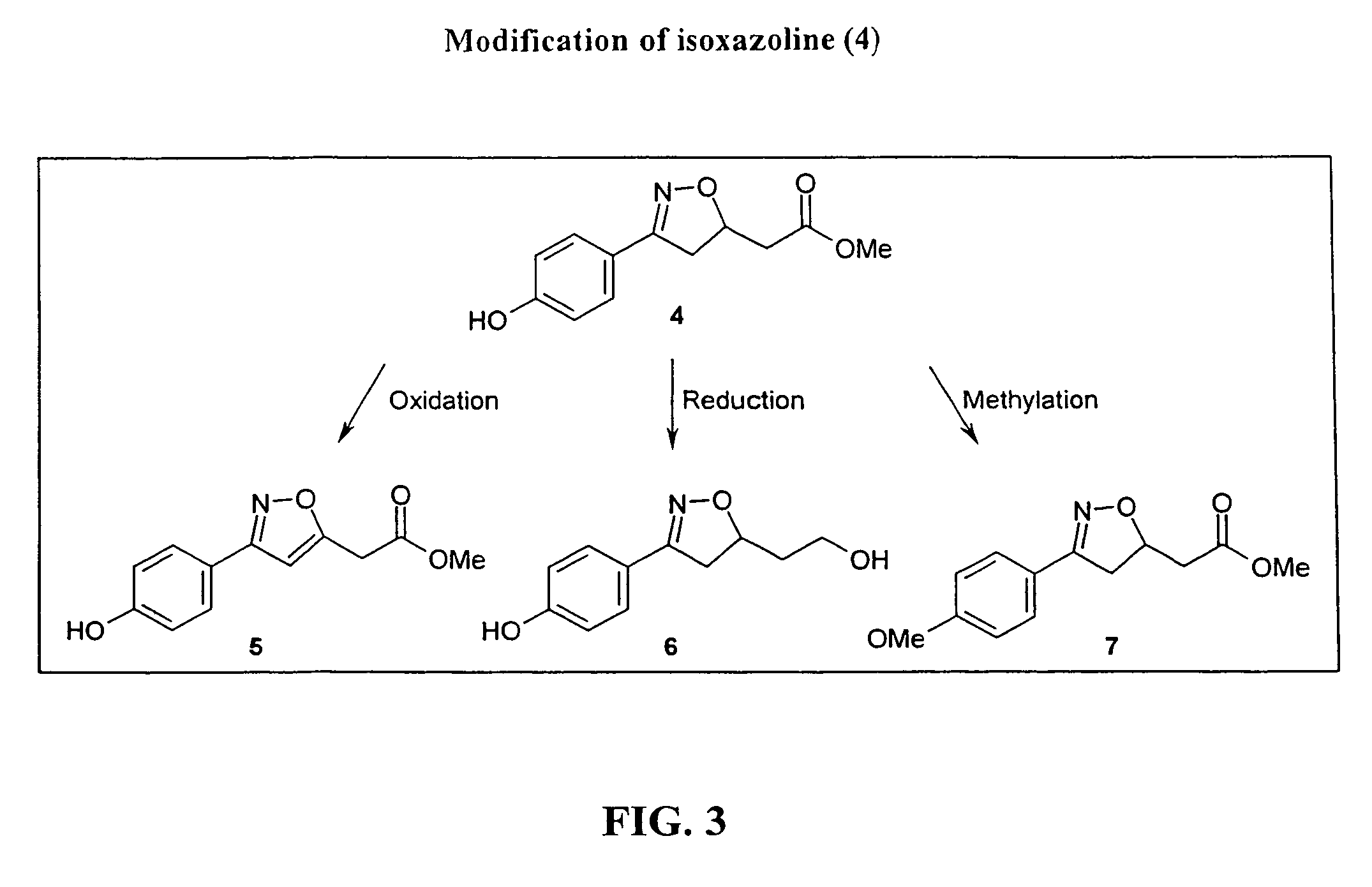
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
85 results about "Macrophage migration inhibitory factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
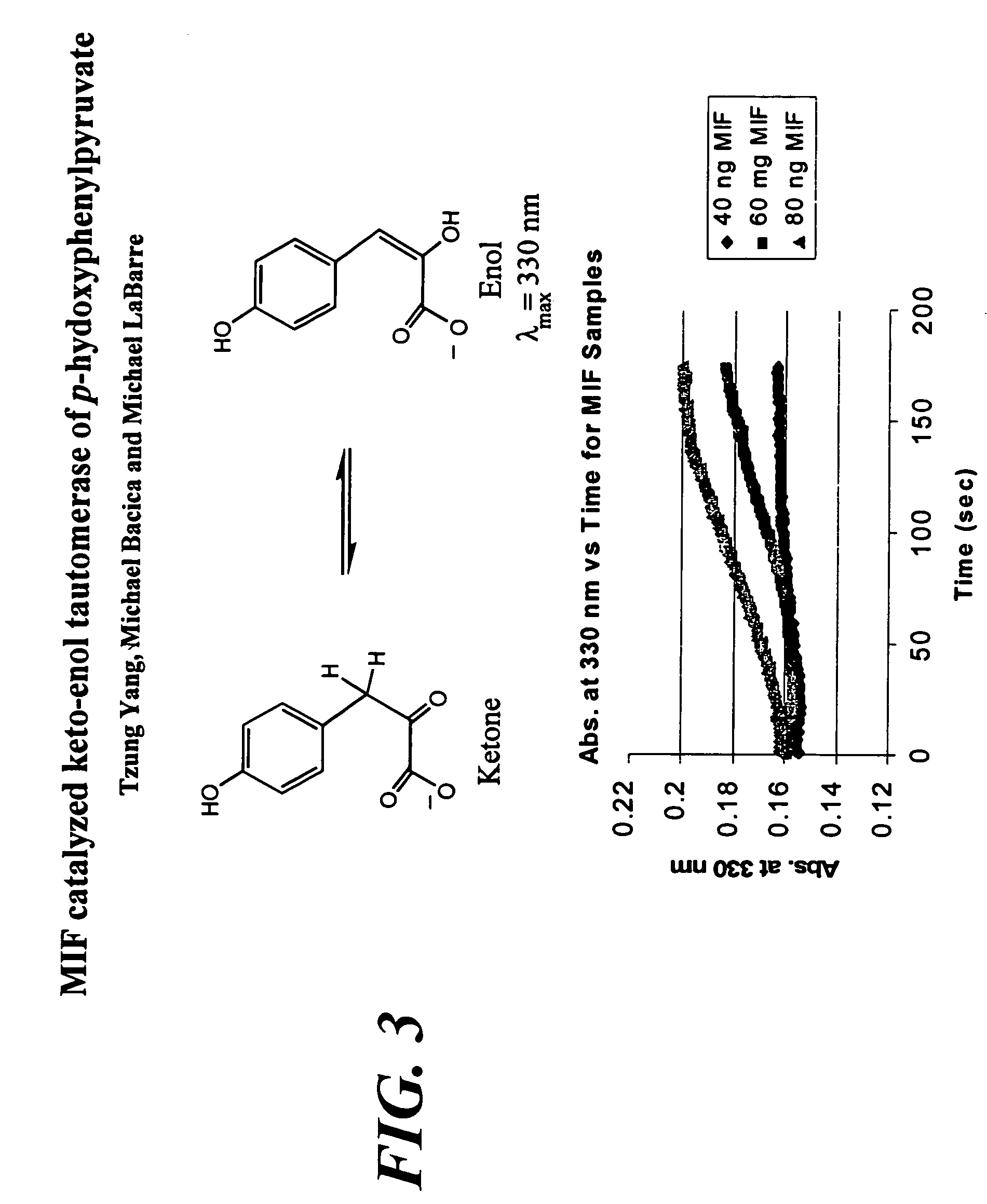
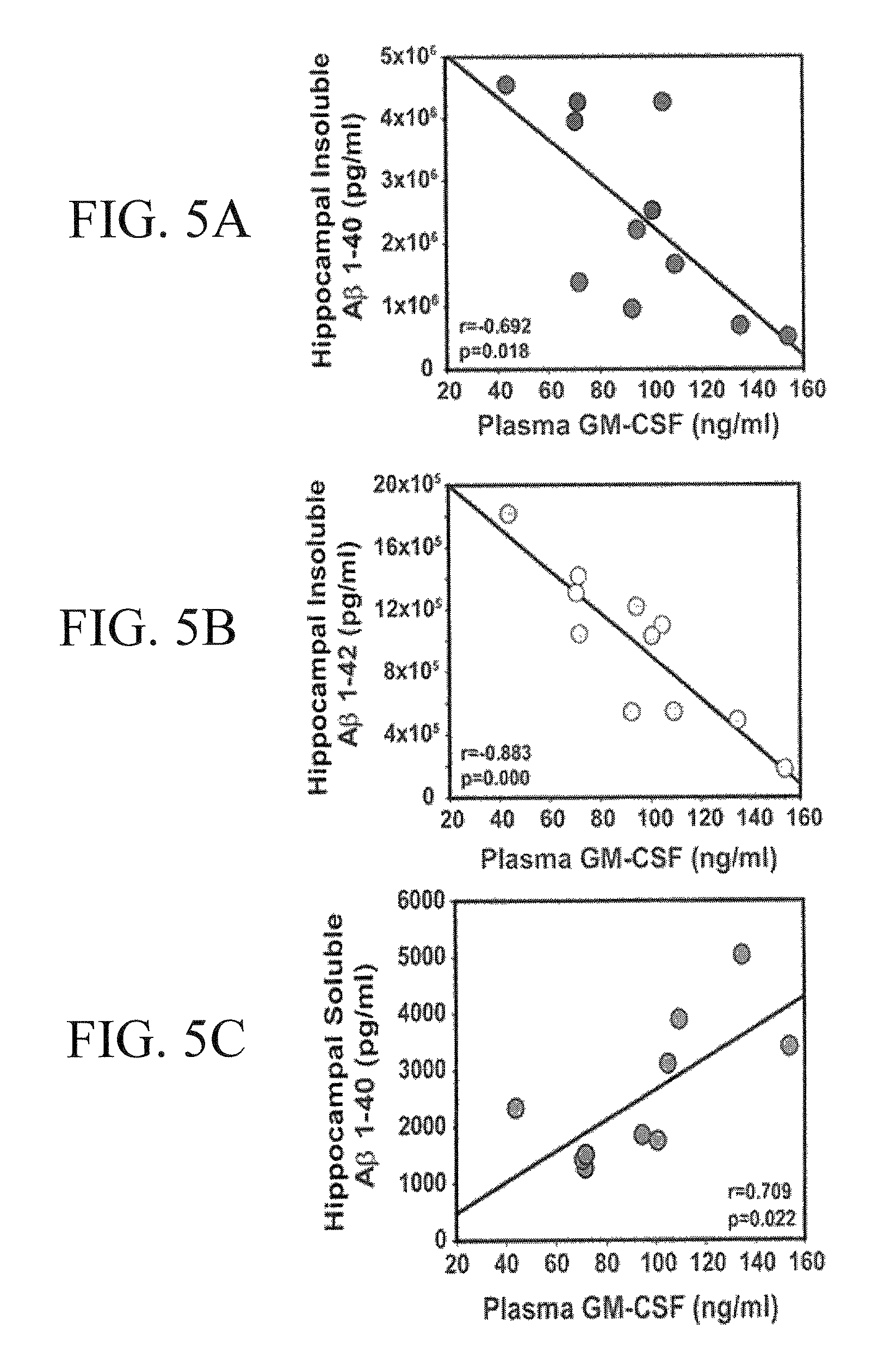
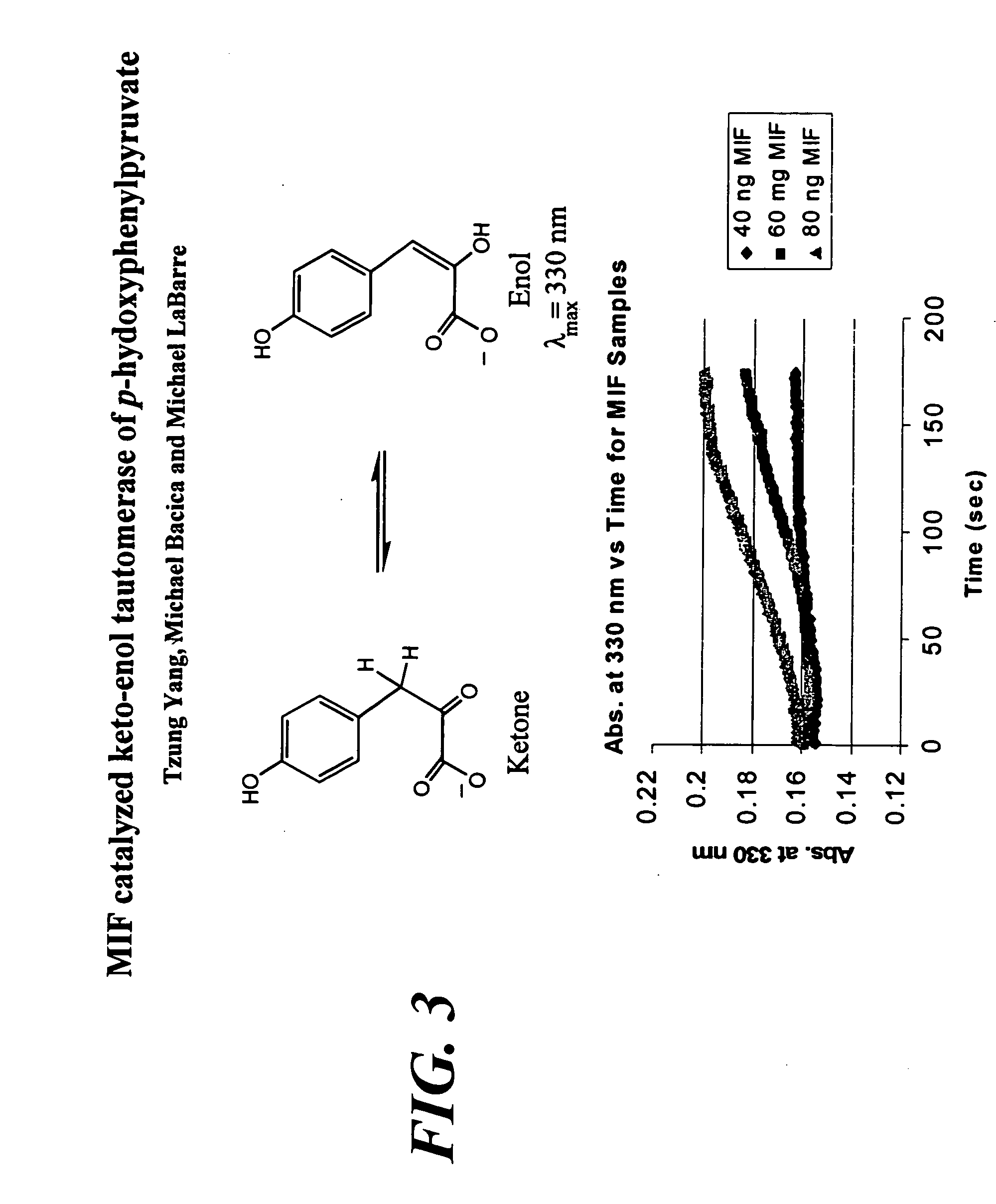
Macrophage migration inhibitory factor (MIF or MMIF), also known as glycosylation-inhibiting factor (GIF), L-dopachrome isomerase, or phenylpyruvate tautomerase is a protein that in humans is encoded by the MIF gene. MIF is an important regulator of innate immunity. The MIF protein superfamily also includes a second member with functionally related properties, the D-dopachrome tautomerase (D-DT).
Serum macrophage migration inhibitory factor (MIF) as marker for prostate cancer
InactiveUS7361474B2Aggressive diseaseAggressive treatmentMicrobiological testing/measurementPeptide preparation methodsSerum igeGenetic Change
The present invention provides methods for detecting, diagnosing or prognosticating prostate cancer by measuring the levels of macrophage migration inhibitory factor (MIF) in the serum of an individual. The assay for MIF can be an immunoassay, such as ELISA, or a nucleic assay, such as Nouthern blot. Genetic changes within MIF gene can predict patients that express high levels of MIF.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Screening assay for the identification of inhibitors for macrophage migration inhibitory factor
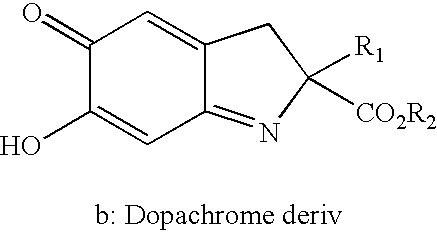
InactiveUS6420188B1Inhibit tautomerase activityPeptide/protein ingredientsAntipyreticAssayDopachrome
The present invention encompasses assays to identify compounds that inhibit the enzymatic activity of MIF which catalyzes the tautomerization of MIF-substrates, such as D-dopachrome to DHICA. In general, the assay is conducted in vitro by adding, mixing or combining MIF and a suitable substrate in the presence or absence of a test compound, and measuring the tautomerization of the substrate. The test compounds that inhibit tautomerization in the assay are identified as MIF inhibitors.
Owner:BAXTER INT INC +1
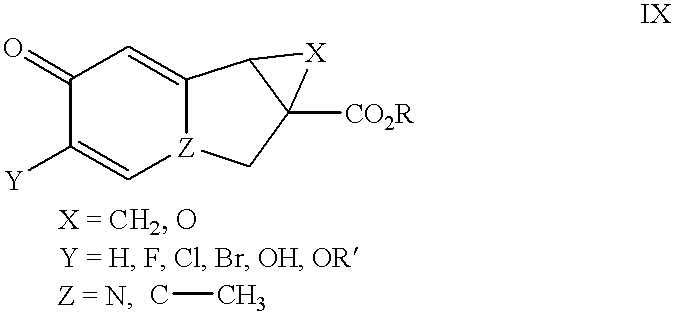
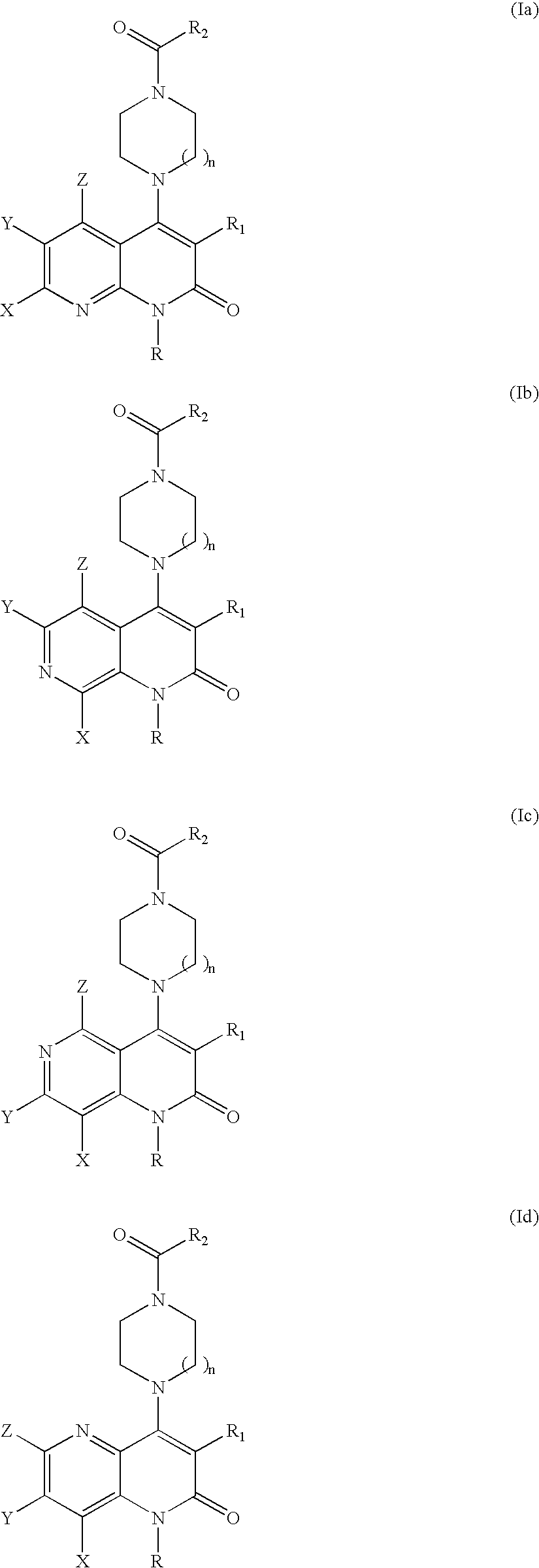
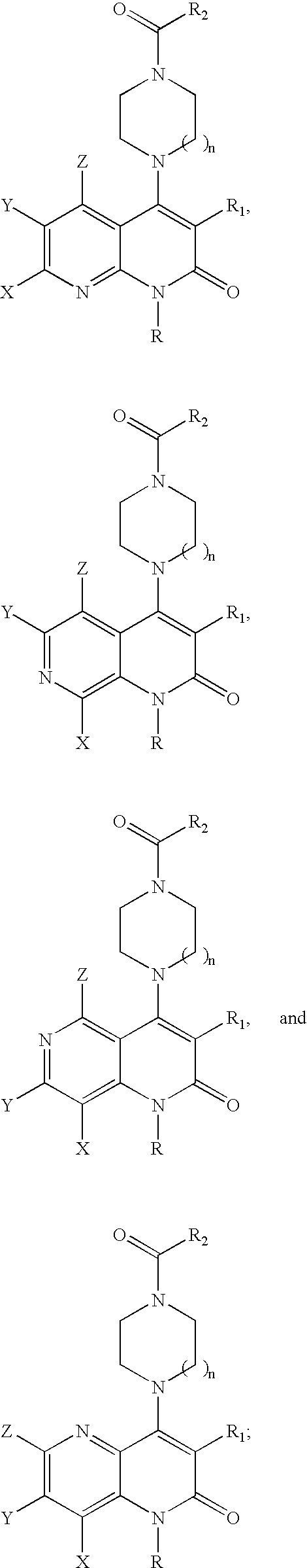
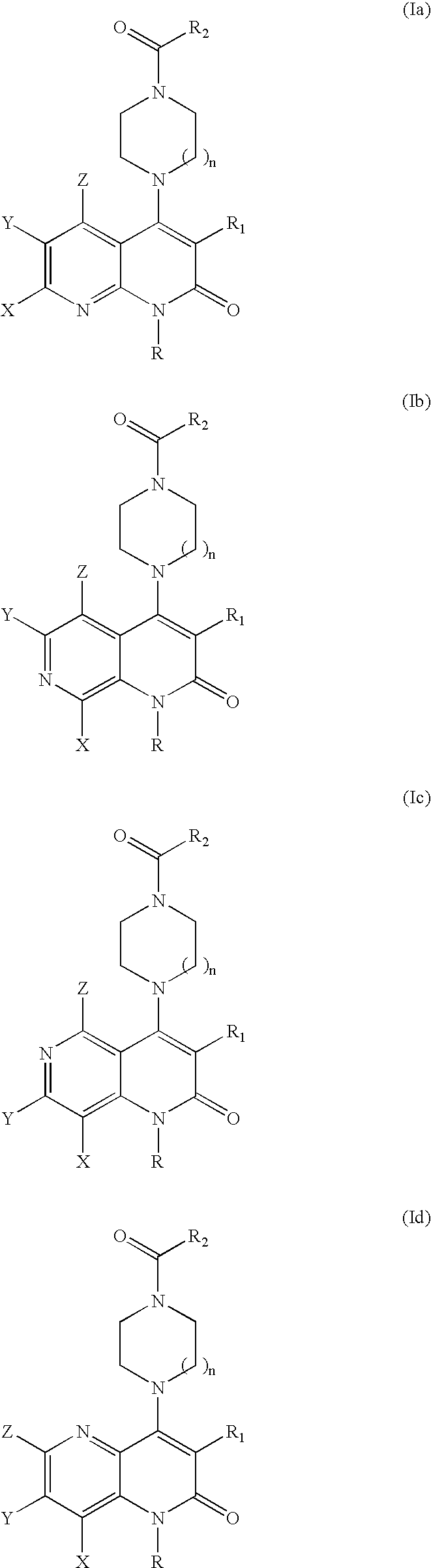
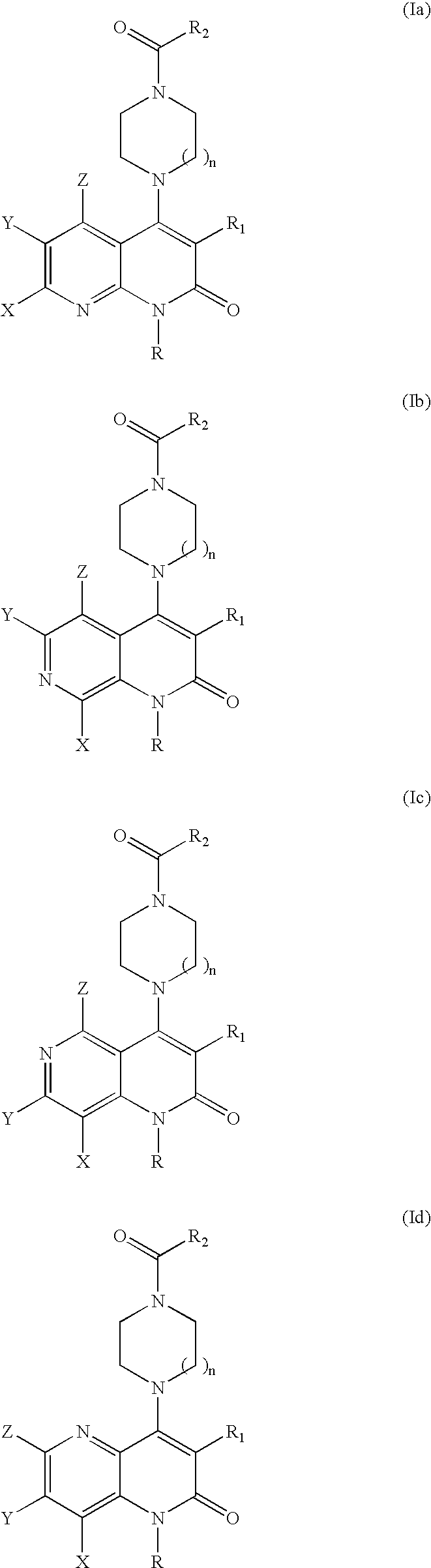
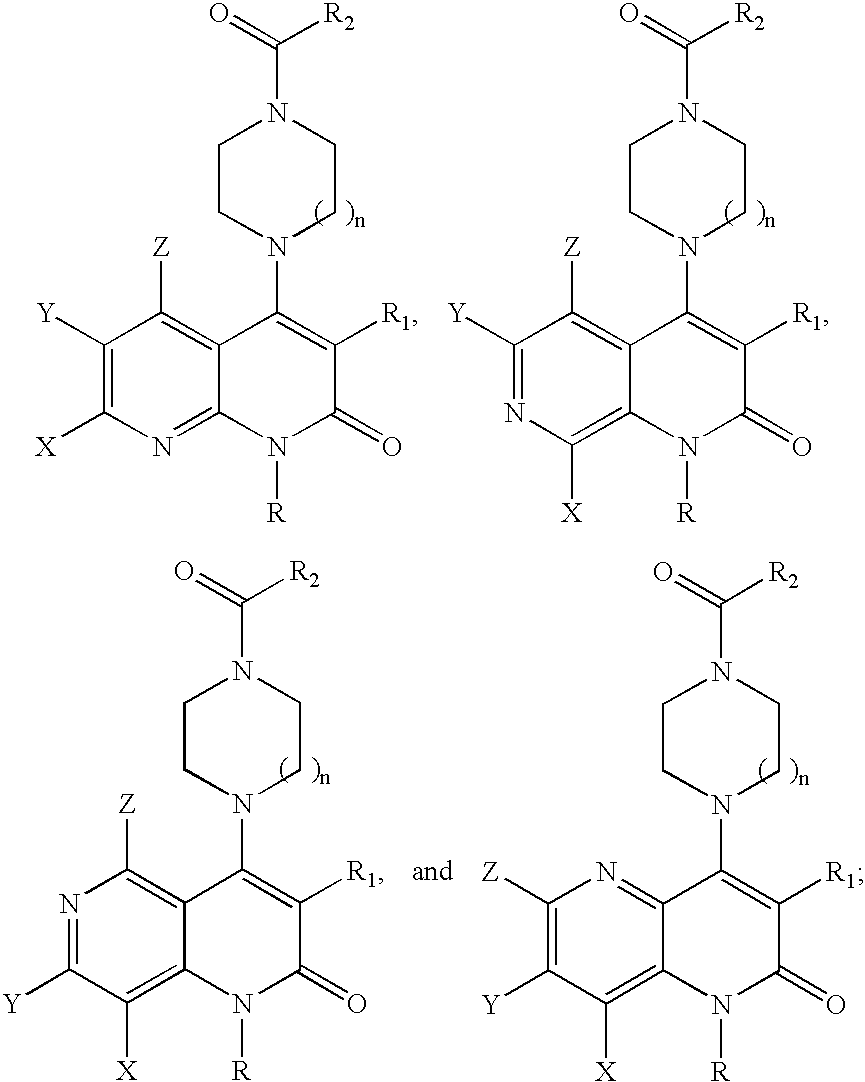
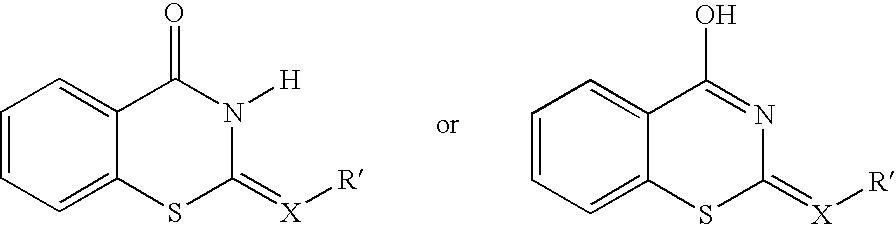
Substituted naphthyridine derivatives as inhibitors of macrophage migration inhibitory factor and their use in the treatment of human diseases
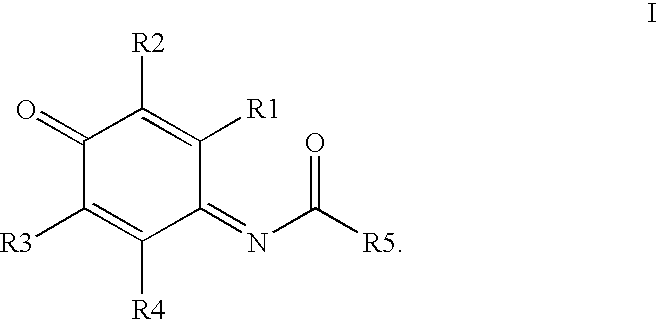
Inhibitors of MIF having a naphthyridine backbone are provided which have utility in the treatment of a variety of disorders, including the treatment of pathological conditions associated with MIF activity. The inhibitors of MIF have the following structures: including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof, wherein n, R, R1, R2, X, Y and Z are as defined herein. Compositions containing an inhibitor of MIF in combination with a pharmaceutically acceptable carrier are also provided, as well as methods for use of the same.
Owner:AVANIR PHARMA
Inhibitors of macrophage migration inhibitory factor and methods for identifying the same
InactiveUS7173036B2Reducing MIF activityReduced activityAntibacterial agentsBiocideDiseaseAbnormal macrophage
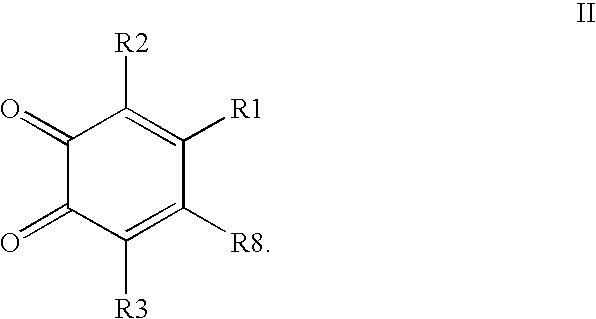
Inhibitors of MIF are provided which have utility in the treatment of a variety of disorders, including the treatment of pathological conditions associated with MIF activity. The inhibitors of MIF have the following structures:including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof, wherein n, R1, R2, R3, R4, X, and Z are as defined herein. Compositions containing an inhibitor of MIF in combination with a pharmaceutically acceptable carrier are also provided, as well as methods for use of the same.
Owner:AVANIR PHARMA
Compounds having MIF antagonist activity
There are disclosed methods of use and pharmaceutical compositions for two related genera of low molecular weight compounds comprising optionally substituted iminoquinone or orthoquinone ring systems. The compounds have MIF (macrophage migration inhibitory factor) antagonist activity and find utility as such. For example, the compounds are useful for treating a variety of diseases involving inflammatory activity or pro-inflammatory cytokine responses, such as autoimmune diseases (including rheumatiod arthritis, insulin-dependent diabetes, multiple sclerosis, graft versus host disease, lupus syndromes), asthma, arthritis, EAE, ARDS, psoriasis, interleukin-2 toxicity, proliferative vascular disease, and various forms of sepsis and septic shock, and other conditions characterized by underlying MIF responses including, for instance, tumor growth and neovascularization (angiogenesis).
Owner:CYTOKINE PHARMASCI
Protein markers for cardiovascular events
InactiveCN101889205AUse to reduce or preventReduce or prevent the risk of cardiovascular eventsDisease diagnosisBiological testingCathepsin SMacrophage migration inhibitory factor
The present invention relates to a method for predicting the risk of a subject developing a cardiovascular event comprising detecting at least one biomarker in (a sample of) the cardiovascular system from said subject, wherein said biomarker comprises at least one protein selected from the group consisting of Tumor Necrosis Factor Alpha Precursor; Lysosomal-associated Membrane Protein (1); Interleukin-5 Precursor; Interleukin-6 Precursor; C-C Motif Chemokine (2) Precursor; C-C Motif Chemokine (5) Precursor RANTES; Cathepsin L1 Precursor; Adenylate Kinase (1); Leukotriene B4 Receptor (1); Complement Factor D; Secreted Phosphoprotein (1); Small Inducible Cytokine A17 Precursor; C-X-C Motif Chemokine (10) Precursor; Tumor Necrosis Factor Ligand Superfamily Member (11)(RANKL); C-C Motif Chemokine (18) Precursor; 72 kDa Type IVCollagenase Precursor; Neutrophil Collagenase Precursor; fatty acid binding protein (4); calpain (2), (m / II) large subunit; Macrophage Migration Inhibitory Factor; Cathepsin S Precursor; Interleukin (13) Precursor; and soluble ICAM-1.
Owner:卡瓦迪斯有限责任公司
Therapeutic uses of factors which inhibit or neutralize MIF activity
InactiveUS6774227B1Organic active ingredientsPeptide/protein ingredientsAntisense RNAMonoclonal antibody
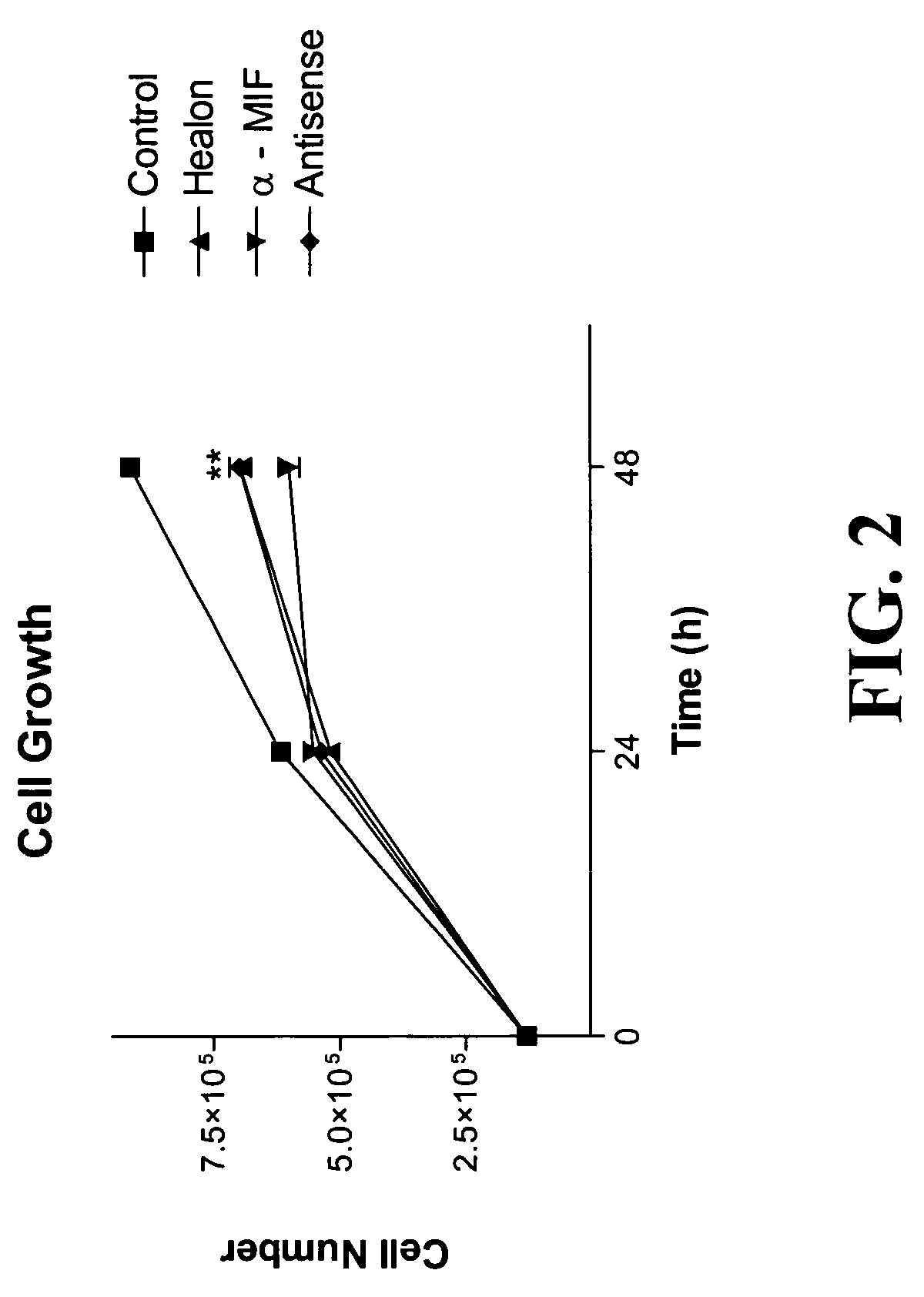
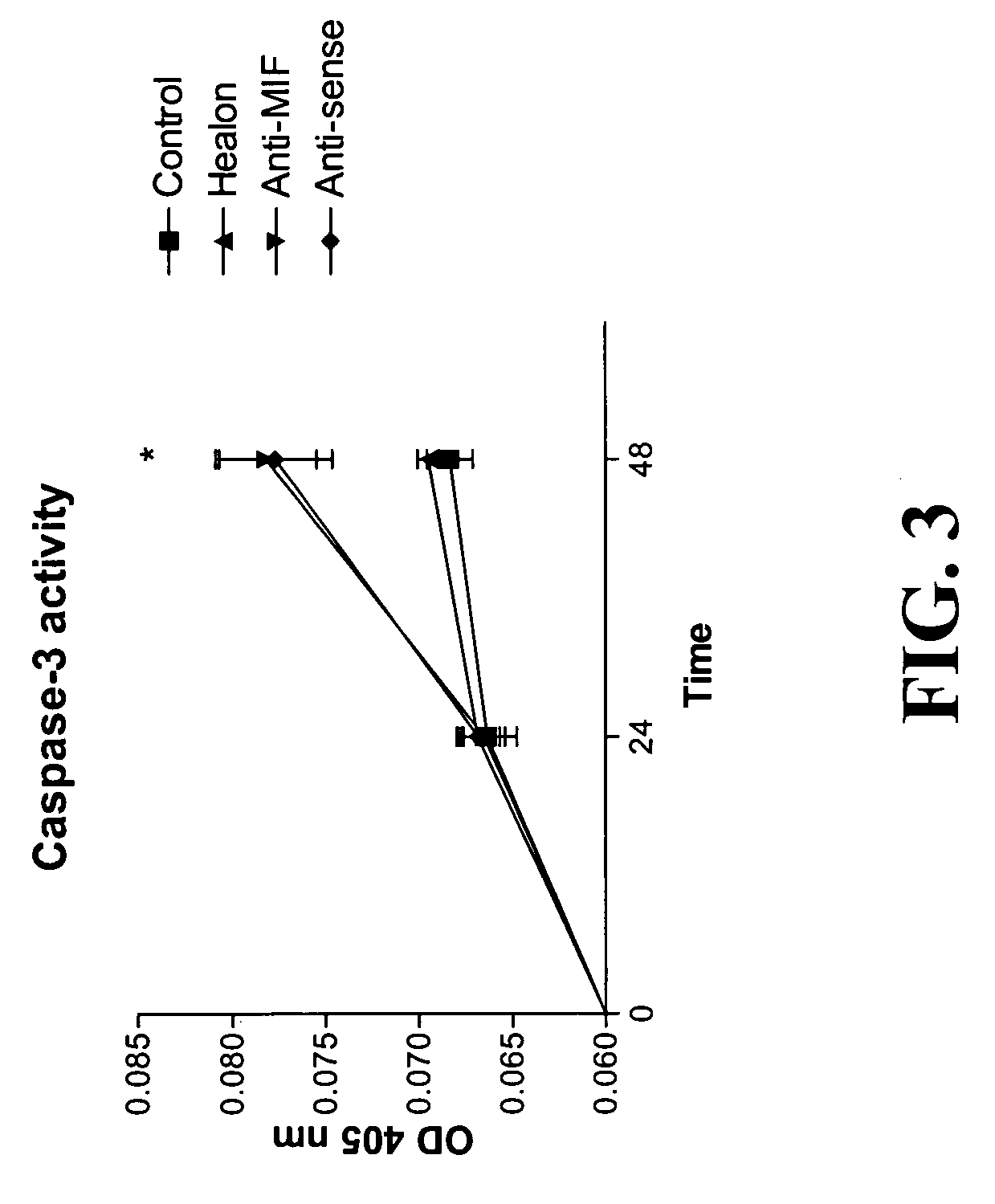
The present invention relates to methods of treating disorders related to cellular overproliferation comprising neutralizing the production or activity of macrophage migration inhibitory factor (MIF). The invention also relates to therapeutic compositions comprising factors which inhibit or neutralize MIF activity, such as, MIF antisense RNA molecules and MIF monoclonal antibodies and derivatives or analogs thereof. The invention further relates to the uses of such compositions and methods for the treatment of malignancies, including, but not limited to, B and T cell lymphomas.
Owner:BAXTER INT INC +1
Macrophage migration inhibitory factor as a marker for cardiovascular risk
InactiveUS7445886B2Microbiological testing/measurementPeptide preparation methodsAssayHematological test
Macrophage migration inhibitory factor (MIF) is a clinically useful biochemical marker of cardiovascular risk. Risk assessment includes the step of detecting in the blood of a person MIF concentration as a marker of cardiovascular risk for the person. The method may further comprise the step of assigning to the person a cardiovascular risk metric proportional to the MIF concentration, and / or prescribing for the person a cardiovascular treatment modality in accordance with the MIF concentration. The method is useful as a primary screen, and may be used in conjunction with or as a substitute for additional tests, such as a stress test, CRP assay, LDL assay, etc. The detecting step may be repeated over time intervals and / or treatment to monitor change in cardiovascular risk for the person over time and / or treatment.
Owner:COOPER INST THE +1
Method for preparing anti-MIF antibodies
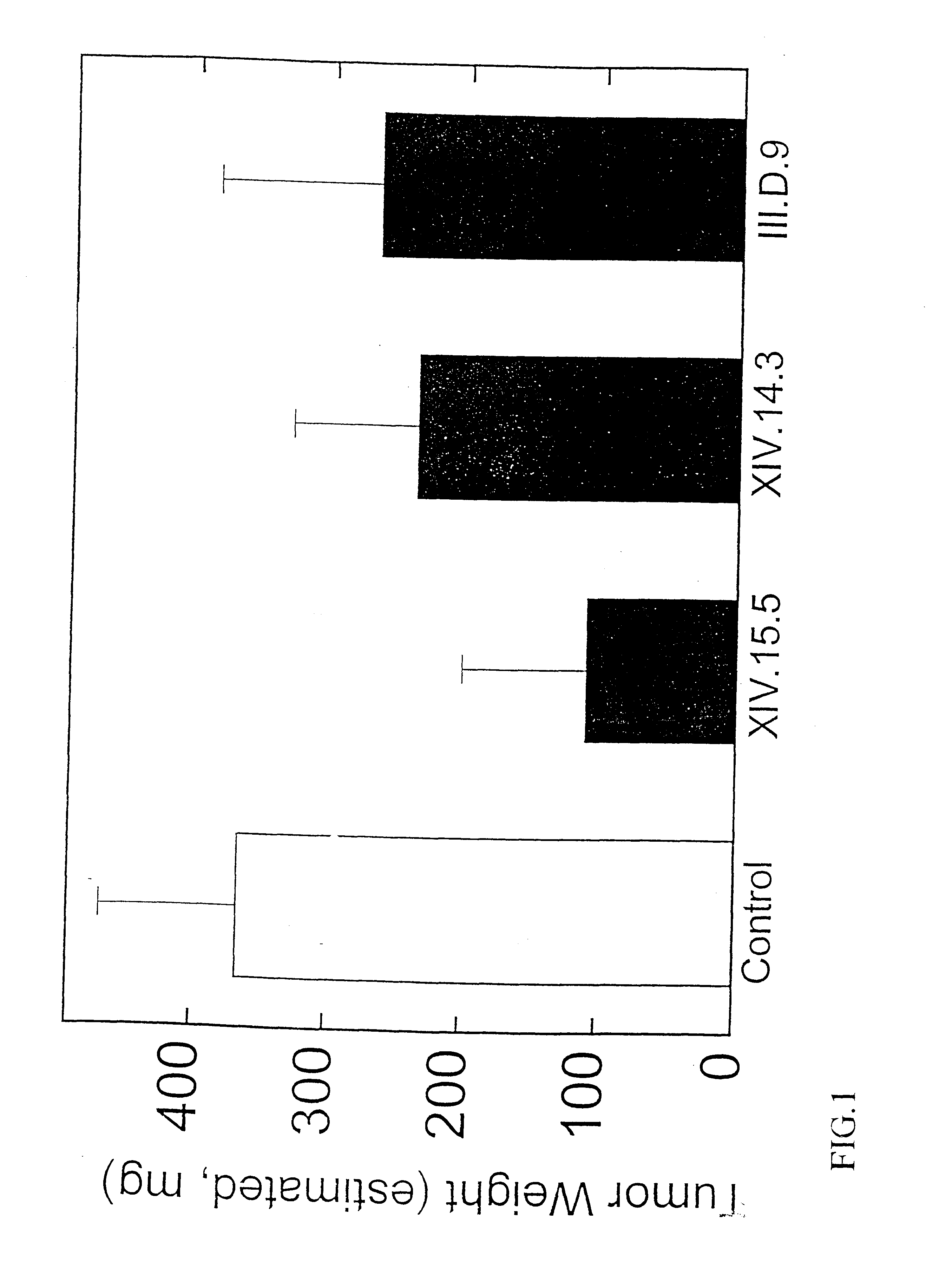
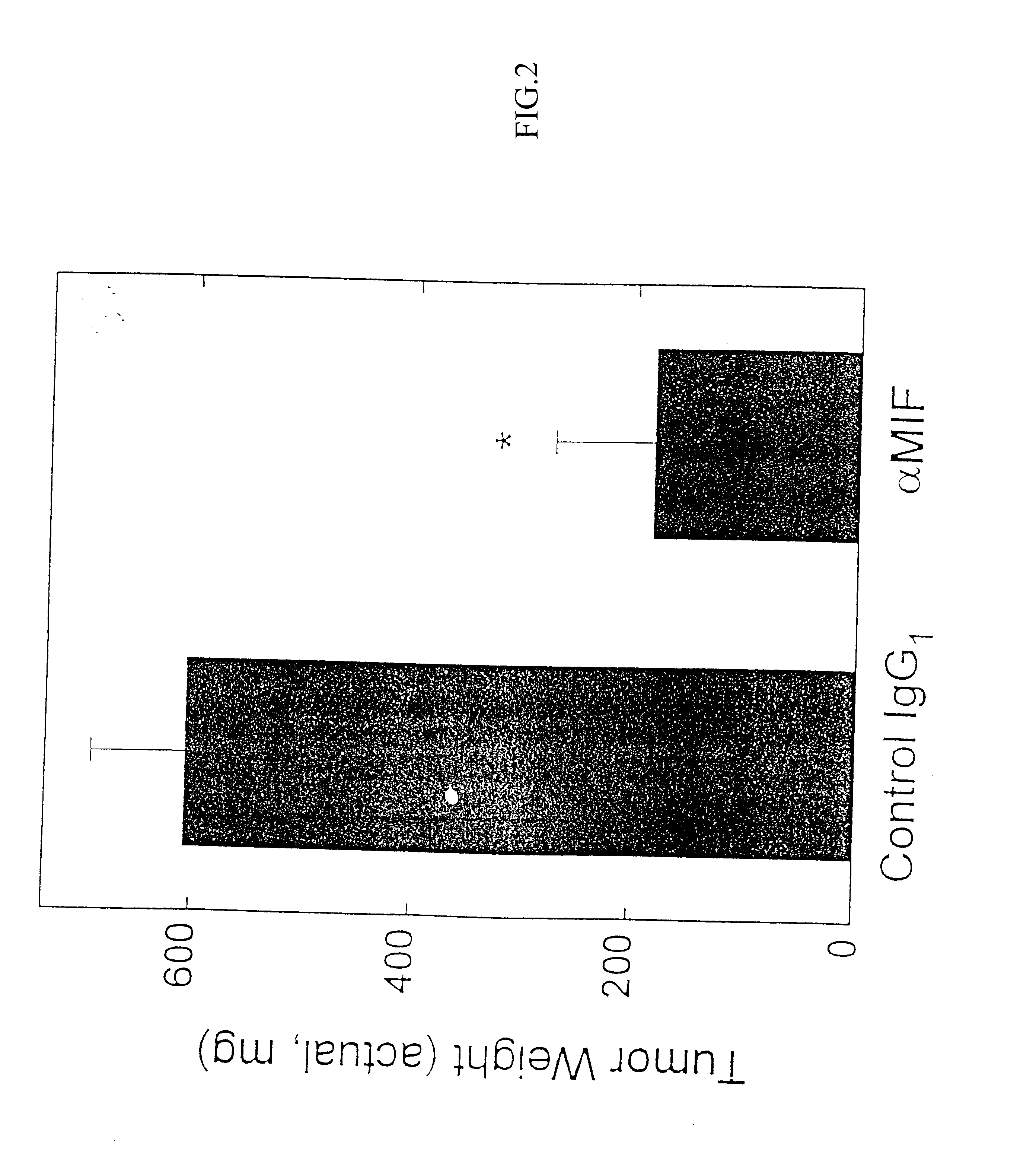
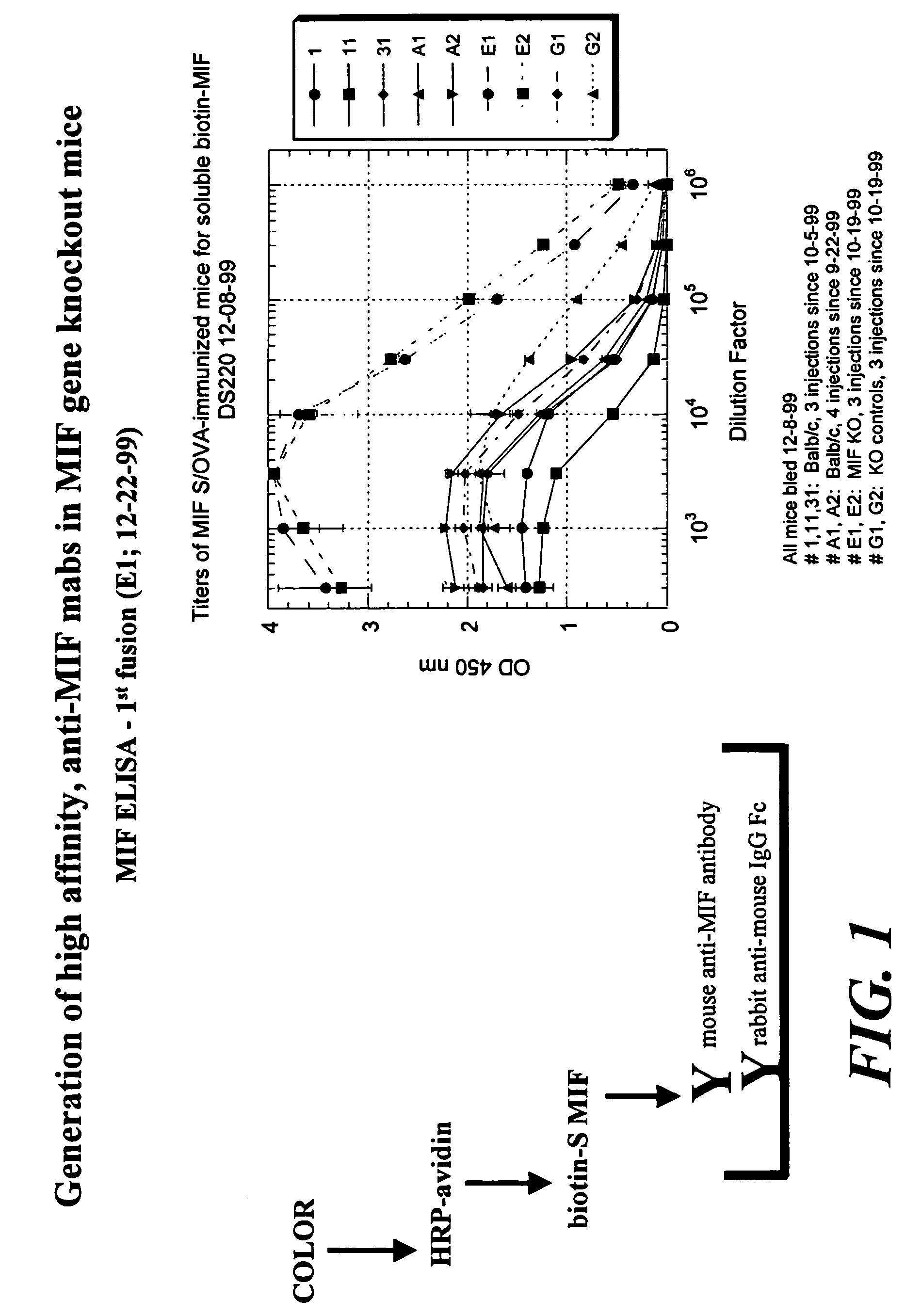
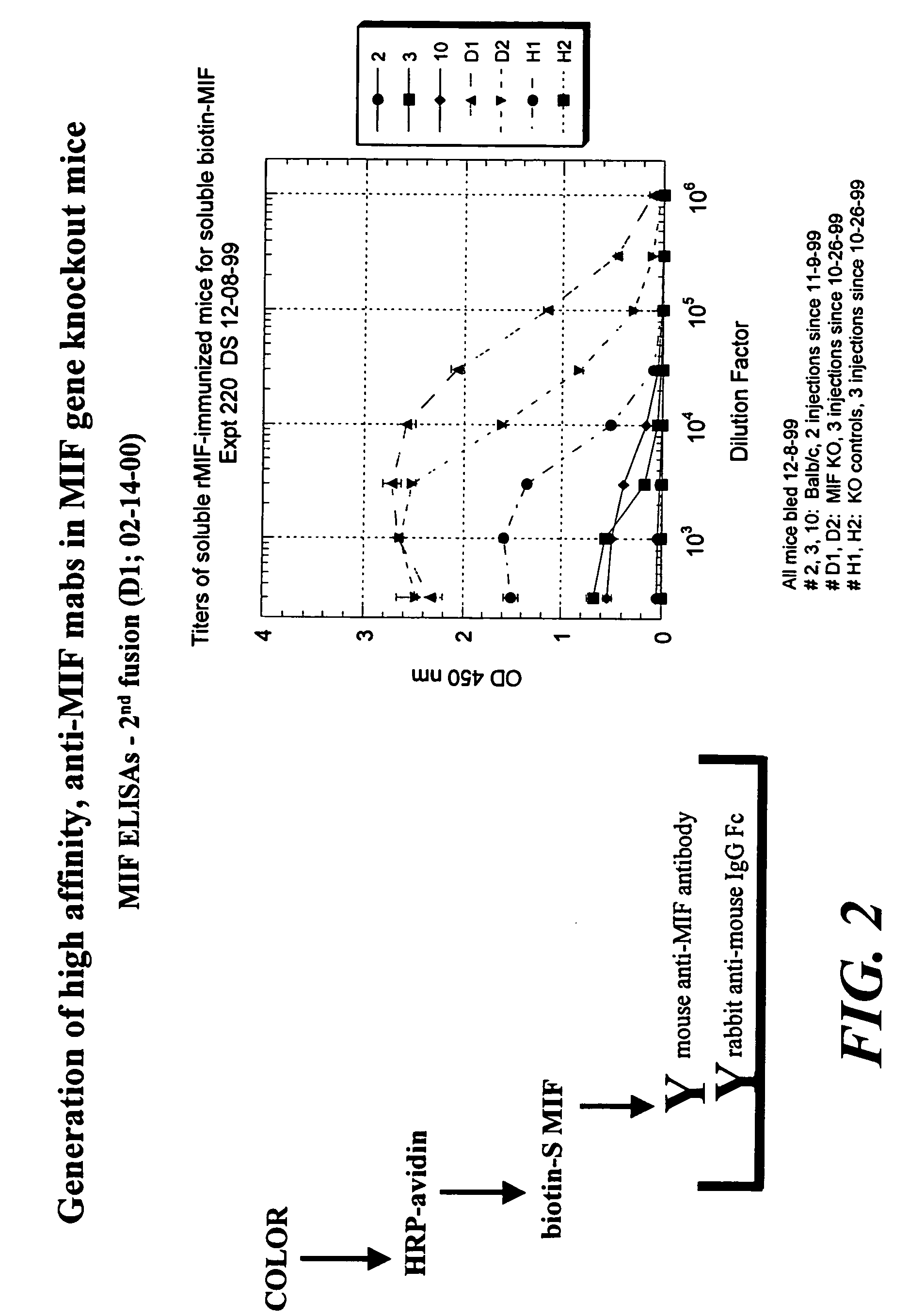
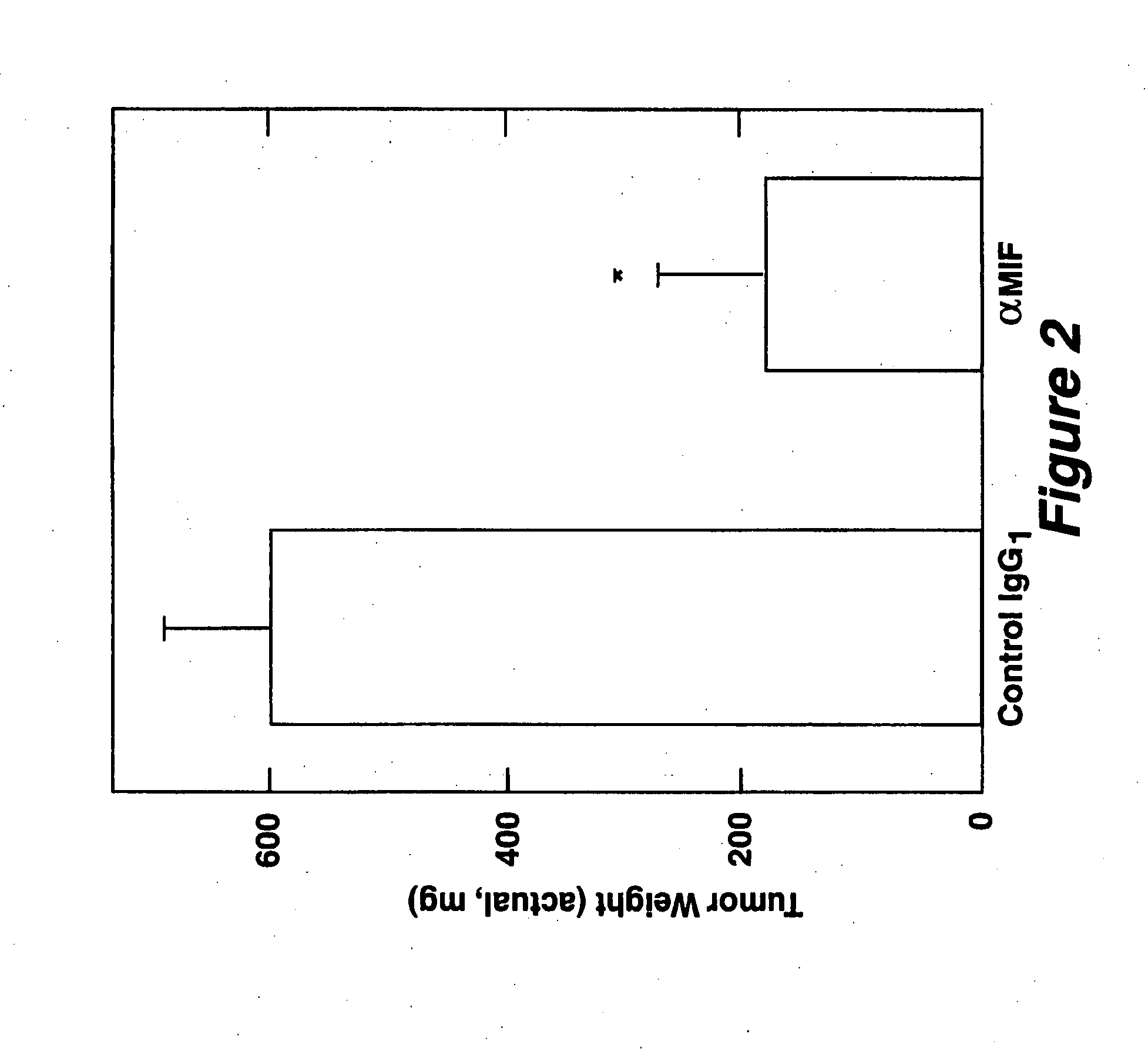
The specification provides methods of preparing high-affinity antibodies to a macrophage migration inhibitory factor (MIF) in animals in which the MIF gene has been homozygously knocked-out (MIF− / −). Also provided are methods of preparing hybridomas which produce the anti-MIF antibodies, methods of administering the antibodies to treat inflammatory or cancerous conditions and / or diseases modulated by MIF, as well as compositions comprising said high-affinity anti-MIF antibodies.
Owner:BAXALTA GMBH
Substituted naphthyridine derivatives as inhibitors of macrophage migration inhibitory factor and their use in the treatment of human diseases
InactiveUS7361760B2Reducing MIF activityAntibacterial agentsOrganic active ingredientsMedicineStereoisomerism
Inhibitors of MIF having a naphthyridine backbone are provided which have utility in the treatment of a variety of disorders, including the treatment of pathological conditions associated with MIF activity. The inhibitors of MIF have the following structures:including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof, wherein n, R, R1, R2, X, Y and Z are as defined herein. Compositions containing an inhibitor of MIF in combination with a pharmaceutically acceptable carrier are also provided, as well as methods for use of the same.
Owner:AVANIR PHARMA INC
Therapeutic uses of factors which inhibit or neutralize MIF activity
InactiveUS20040156848A1Organic active ingredientsPeptide/protein ingredientsAntisense RNAMonoclonal antibody
The present invention relates to methods of treating disorders related to cellular overproliferation comprising neutralizing the production or activity of macrophage migration inhibitory factor (MIF). The invention also relates to therapeutic compositions comprising factors which inhibit or neutralize MIF activity, such as, MIF antisense RNA molecules and MIF monoclonal antibodies and derivatives or analogs thereof. The invention further relates to the uses of such compositions and methods for the treatment of malignancies, including, but not limited to, B and T cell lymphomas.
Owner:CYTOKINE PHARMASCI
Inhibitors of macrophage migration inhibitory factor and methods for identifying the same
InactiveUS20050287617A1Suppress immune responseNervous disorderOrganic chemistryDiseaseStereoisomerism
Inhibitors of MIF are provided which have utility in the treatment of a variety of disorders, including the treatment of pathological conditions associated with MIF activity. The inhibitors of MIF have the following structures: including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof, wherein n, R1, R2, R3, R4, X, Y and Z are as defined herein. Compositions containing an inhibitor of MIF in combination with a pharmaceutically acceptable carrier are also provided, as well as methods for use of the same.
Owner:AVANIR PHARMA
Therapeutic agents for multiple sclerosis
InactiveUS20050025767A1Nervous disorderImmunoglobulins against cytokines/lymphokines/interferonsSpinal cordActive ingredient
The present invention provides therapeutic agents for multiple sclerosis containing an antibody binding to macrophage migration inhibitory factor (MIF) as an active ingredient, and the therapeutic agents for multiple sclerosis of the present invention are effective for the conventional form of multiple sclerosis (C-MS) or the optic-spinal form of multiple sclerosis (OpS-Ms).
Owner:JUN NISHIHIRA
Methods for diagnosing and treating bladder cancer
InactiveUS20050196795A1Slow tumor growthMicrobiological testing/measurementBiological material analysisBladder cancerCarcinoma
The present invention relates to the diagnosis and treatment of bladder cancer. More specifically, this invention uses the levels of macrophage migration inhibitory factor (MIF) produced by the bladder epithelia (urothelia) as a marker for bladder cancer. Moreover, the present invention also provides a method for attenuating bladder carcinoma by inhibiting of macrophage MIF.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Cell death inhibitor
InactiveUS20060034832A1Prevent serum depletion-induced cell deathIncrease gene expressionCompound screeningNervous disorderAutoimmune conditionAutoimmune disease
The cell death inhibitor comprising a substance capable of binding to macrophage migration inhibitory factor is useful as a preventive / therapeutic agent for, e.g., heart diseases, neurodegenerative diseases, cerebrovascular diseases, central nervous infections, traumatic diseases, demyelinating diseases, bone / joint diseases, kidney diseases, liver diseases, myelodysplastic diseases, arteriosclerosis, diabetes, pulmonary hypertension, sepsis, inflammatory bowel diseases, autoimmune diseases, failure accompanying rejection in organ transplantation, AIDS, cancer, etc.
Owner:TAKEDA PHARMA CO LTD
Macrophage migration inhibitory factor as a marker for cardiovascular risk
InactiveUS20050054117A1Microbiological testing/measurementDisease diagnosisAbnormal macrophageTreatment modality
Macrophage migration inhibitory factor (MIF) is a clinically useful biochemical marker of cardiovascular risk. Risk assessment includes the step of detecting in the blood of a person MIF concentration as a marker of cardiovascular risk for the person. The method may further comprise the step of assigning to the person a cardiovascular risk metric proportional to the MIF concentration, and / or prescribing for the person a cardiovascular treatment modality in accordance with the MIF concentration. The method is useful as a primary screen, and may be used in conjunction with or as a substitute for additional tests, such as a stress test, CRP assay, LDL assay, etc. The detecting step may be repeated over time intervals and / or treatment to monitor change in cardiovascular risk for the person over time and / or treatment.
Owner:COOPER INST THE +1
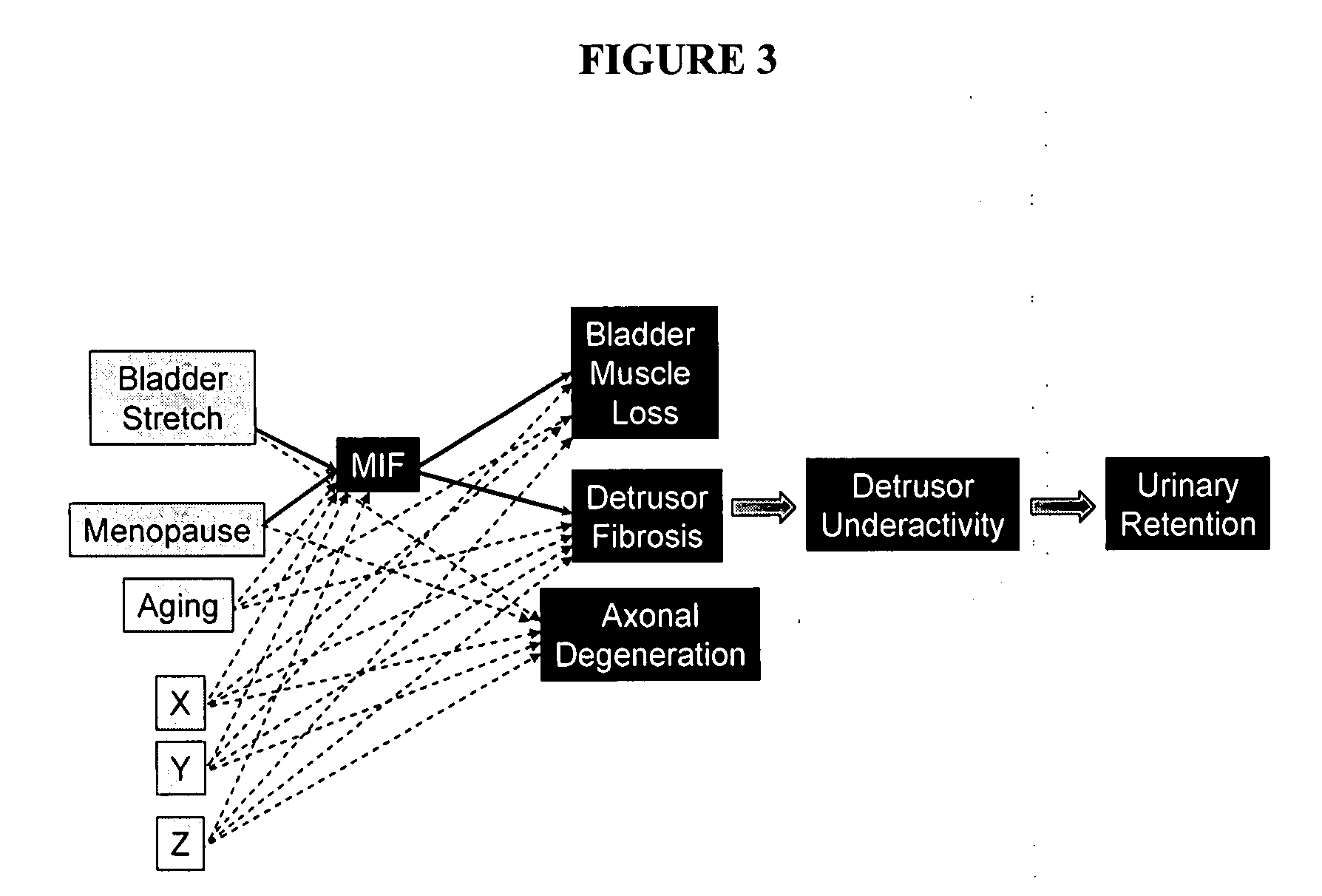
Compositions and methods for the modulation of detrusor activity
InactiveUS20080089884A1BiocideSsRNA viruses positive-senseMIF receptorMacrophage migration inhibition
The invention relates generally to methods for treating and / or preventing bladder disorders. In certain embodiments the invention comprises methods for the treatment and / or prevention of a bladder disorder comprising administering an effective amount of a therapeutic agent that decreases the expression, release or biological activity of macrophage migration inhibition factor (MIF) to a subject in need thereof. In other aspects the invention relates to a method for diagnosing bladder disease and / or bladder disease severity comprising screening for a MIF gene or MIF receptor gene polymorphism or expression level.
Owner:YALE UNIV +1
Macrophage migration inhibitory factor (MIF) as marker for urological inflammatory disease
The present invention relates to the association between detecting and quantifying the presence of macrophage migration inhibitory factor (MIF) in urine, bladder and prostate tissues for the purpose of diagnosis and prognosis of urological inflammatory. In addition, methods to inactivate MIF activity by use of antibodies or specific MIF inhibitors can be used to treat these diseases. For instance, such diseases as chronic pelvic pain syndrome, non-bacterial prostatitis, and interstitial cystitis may be mediated by MIF release. Periodic assays for MIF could be conducted for a patient to determine if the patient's MIF urine levels are high or increasing. In addition, intravesical MIF antibodies or other MIF-specific inhibitors would reduce or ameliorate these pelvic diseases.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Therapeutic uses of factors which inhibit or neutralize MIF activity
InactiveUS20080317759A1Organic active ingredientsPeptide/protein ingredientsAntisense RNAMonoclonal antibody
The present invention relates to methods of treating disorders related to cellular overproliferation comprising neutralizing the production or activity of macrophage migration inhibitory factor (MIF). The invention also relates to therapeutic compositions comprising factors which inhibit or neutralize MIF activity, such as, MIF antisense RNA molecules and MIF monoclonal antibodies and derivatives or analogs thereof. The invention further relates to the uses of such compositions and methods for the treatment of malignancies, including, but not limited to, B and T cell lymphomas.
Owner:BAXTER INT INC +1
Screening assay for the identification of inhibitors of macrophage migration inhibitory factor
The present invention encompasses assays to identify compounds that inhibit the enzymatic activity of MIF which catalyzes the tautomerization of MIF-substrates, such as D-dopachrome to DHICA. In general, the assay is conducted in vitro by adding, mixing or combining MIF and a suitable substrate in the presence or absence of a test compound, and measuring the tautomerization of the substrate. The test compounds that inhibit tautomerization in the assay are identified as MIF inhibitors.
Owner:CYTOKINE PHARMASCI
Methods of treating cognitive impairment
InactiveUS20110142795A1Decreasing and inhibiting progressionOrganic active ingredientsNervous disorderInsulin-like growth factorInterleukin 6
The subject invention concerns materials and methods for treating a person or animal having cognitive impairment. In one embodiment, the method comprises administering an effective amount of one or more inflammatory mediator(s), for example, fms-related tyrosine kinase 3 (Flt3) ligand, interleukin-6 (IL-6), macrophage migration inhibitory factor (MIF), interleukin-1 (IL-1), interleukin-3 (IL-3), erythropoietin (EPO), vascular endothelial growth factor A (VEGF-A), hypoxia-inducible transcription factor (HIF-1alpha), insulin like growth factor-1 (IGF-1), tumor necrosis factor (TNF), granulocyte colony-stimulating factor (G-CSF), granulocyte / macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), Stem Cell Factor (SCF), Darbepoetin (ARANESP), and metalloproteinases, to an animal or person in need of treatment.
Owner:UNIV OF SOUTH FLORIDA
Novel methods for the treatment of inflammatory diseases
Methods of inhibiting the cytokine or biological activity of Macrophage Migration Inhibitory Factor (MIF) comprising contacting MIF with a compound of formula (I) are provided. The invention also relates to methods of treating diseases or conditions where MIF cytokine or biological activity is implicated comprising administration of compounds of formula (I), either alone or as a part of combination therapy. Novel compounds of formula (I) are also provided for.
Owner:CORTICAL PTY LTD
Napththalene derivatives which inhibit the cytokine or biological activity of macrophage migration inhibitory factor (MIF)
Owner:CORTICAL PTY LTD
Isoxazoline compounds having MIF antagonist activity
InactiveUS7662843B2Reduce needReduce the amount requiredBiocideNervous disorderAutoimmune responsesAutoimmune disease
Methods of use and pharmaceutical compositions for a genus of low molecular weight compounds comprising optionally substituted isoxazoline ring systems that act as inhibitors of MIF (macrophage migration inhibitory factor) are disclosed. Specifically, the compounds are useful for treating a variety of diseases involving inflammatory activity or pro-inflammatory cytokine responses, such as autoimmune diseases (including rheumatiod arthritis, insulin-dependent diabetes, multiple sclerosis, graft versus host disease, lupus syndromes), asthma, arthritis, ARDS, psoriasis, interleukin-2 toxicity, proliferative vascular disease, and various forms of sepsis and septic shock, and other conditions characterized by underlying MIF responses including, for instance, tumor growth and neovascularization (angiogenesis).
Owner:CPSI STOCKHOLDER TRUST
Gold-based nanocrystals for medical treatments and electrochemical manufacturing processes therefor
The present invention relates to novel gold nanocrystals and nanocrystal shape distributions that have surfaces that are substantially free from organic impurities or films. Specifically, the surfaces are “clean” relative to the surfaces of gold nanoparticles made using chemical reduction processes that require organic reductants and / or surfactants to grow gold nanoparticles from gold ions in solution. The invention includes novel electrochemical manufacturing apparatuses and techniques for making the gold-based nanocrystals. The invention further includes pharmaceutical compositions thereof and the use of the gold nanocrystals or suspensions or colloids thereof for the treatment or prevention of diseases or conditions for which gold therapy is already known and more generally for conditions resulting from pathological cellular activation, such as inflammatory (including chronic inflammatory) conditions, autoimmune conditions, hypersensitivity reactions and / or cancerous diseases or conditions In one embodiment, the condition is mediated by MIF (macrophage migration inhibiting factor).
Owner:CLENE NANOMEDICINE INC A NEVADA CORP
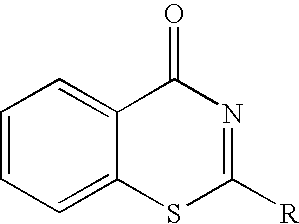
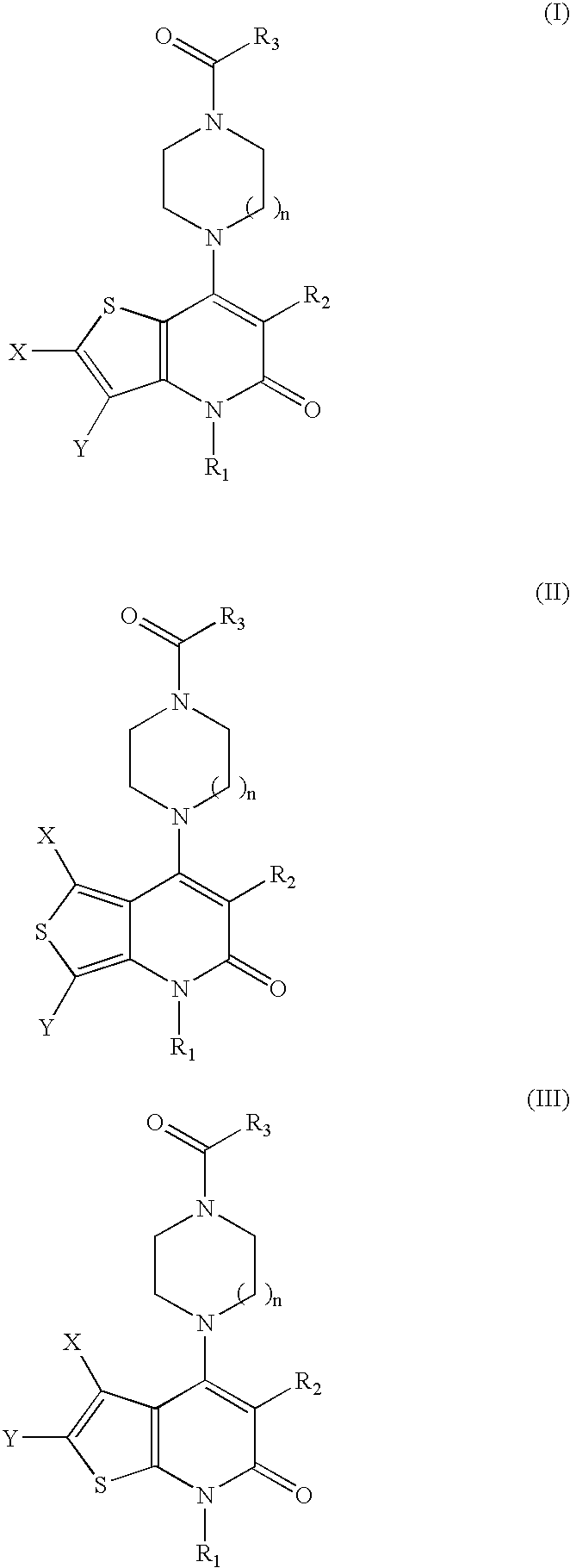
Thienopyridinone derivatives as macrophage migration inhibitory factor inhibitors
InactiveUS7365200B2Inhibitory activityAntibacterial agentsOrganic active ingredientsFree formStereoisomerism
Inhibitors of macrophage migration inhibitory factor having a thienopyridinone backbone are provided which have utility in the treatment of a variety of disorders, including the treatment of pathological conditions associated with macrophage migration inhibitory factor activity. The inhibitors of macrophage migration inhibitory factor have the following structures:including forms such as stereoisomers, free forms, pharmaceutically acceptable salts or esters thereof, solvates, or combinations of such forms, wherein n, R1, R2, R3, X, and Y are as defined herein. Compositions comprising an inhibitor of macrophage migration inhibitory factor in combination with a pharmaceutically acceptable carrier are also provided, as well as methods for use of the same.
Owner:AVANIR PHARMA INC
Methods of treating cognitive impairment
The subject invention concerns materials and methods for treating a person or animal having cognitive impairment. In one embodiment, the method comprises administering an effective amount of one or more inflammatory mediator(s), for example, fms-related tyrosine kinase 3 (Flt3) ligand, interleukin-6 (IL-6), macrophage migration inhibitory factor (MIF), interleukin-1 (IL-1), interleukin-3 (IL-3), erythropoietin (EPO), vascular endothelial growth factor A (VEGF-A), hypoxia-inducible transcription factor (HIF-1alpha), insulin like growth factor-1 (IGF-1), tumor necrosis factor (TNF), granulocyte colony-stimulating factor (G-CSF), granulocyte / macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), Stem Cell Factor (SCF), Darbepoetin (ARANESP), and metalloproteinases, to an animal or person in need of treatment.
Owner:UNIV OF SOUTH FLORIDA +1
Treatment Of Type 1 Diabetes With Inhibitors Of Macrophage Migration Inhibitory Factor
Methods of treating a mammal having type 1 diabetes or a risk for type 1 diabetes are provided. The methods comprise administering to the mammal a pharmaceutical composition comprising an agent that inhibits MIF in the mammal. Also provided are methods of evaluating whether a compound is useful for preventing or treating type 1 diabetes. The methods comprise determining whether the compound inhibits a macrophage migration inhibitory factor (MIF) in a mammal, then, if the compound inhibits the MIF, determining whether the compound inhibits development of type 1 diabetes.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Novel Methods for the Treatment of Inflammatory Diseases
Methods of inhibiting the cytokine or biological activity of Macrophage Migration Inhibitory Factor (MIF) comprising contacting MIF with a compound of formula (I) are provided. The invention also relates to methods of treating diseases or conditions where MIF cytokine or biological activity is implicated comprising administration of compounds of formula (I), either alone or as a part of combination therapy. Novel compounds of formula (I) are also provided for.
Owner:CORTICAL PTY LTD
Method for preparing anti-MIF antibodies
InactiveUS20060191024A1Antibacterial agentsOrganic active ingredientsHigh affinity antibodyAnti-CEA Antibody
The specification provides methods of preparing high-affinity antibodies to a macrophage migration inhibitory factor (MIF) in animals in which the MIF gene has been homozygously knocked-out (MIF− / −). Also provided are methods of preparing hybridomas which produce the anti-MIF antibodies, methods of administering the antibodies to treat inflammatory or cancerous conditions and / or diseases modulated by MIF, as well as compositions comprising said high-affinity anti-MIF antibodies.
Owner:BAXALTA GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com