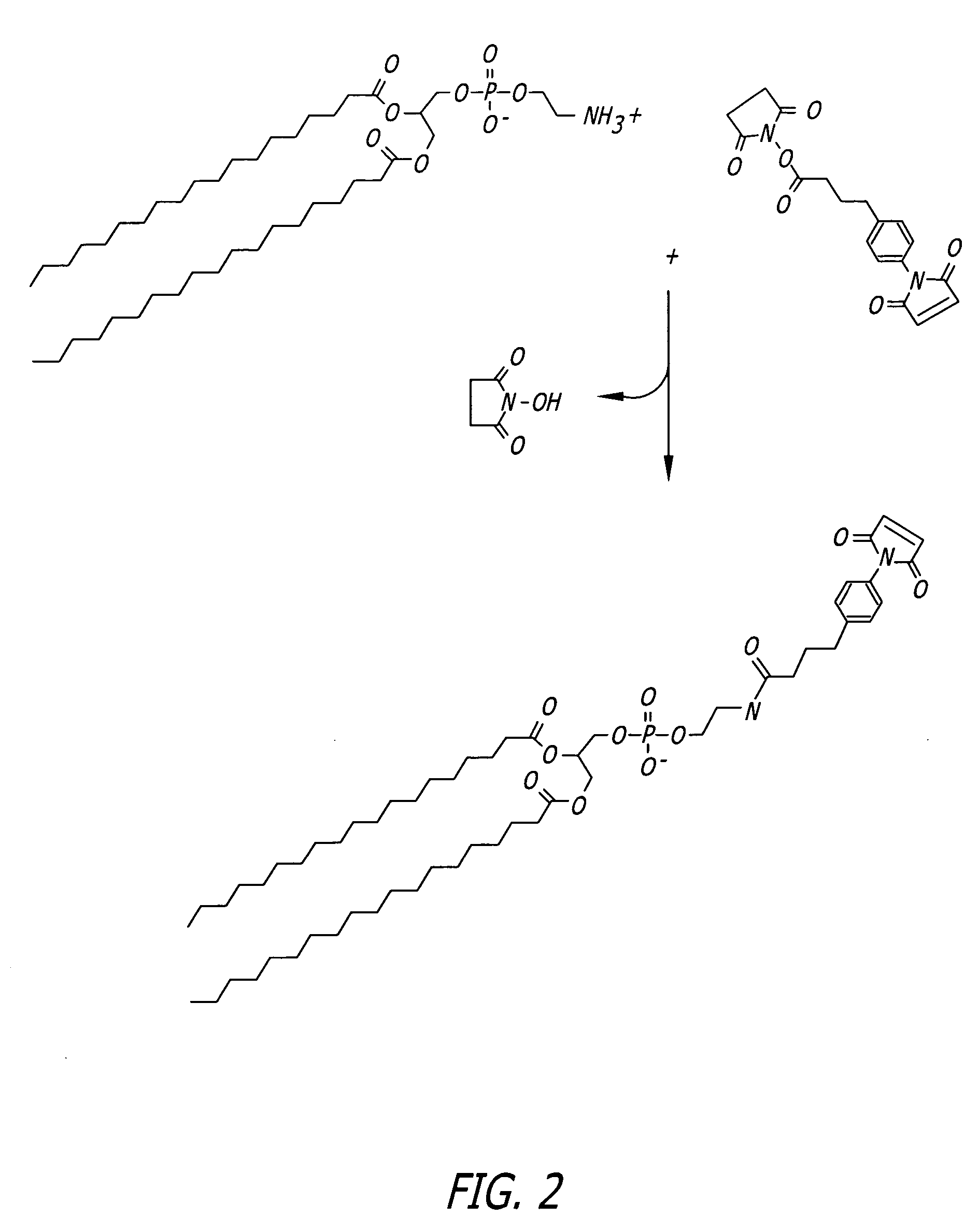
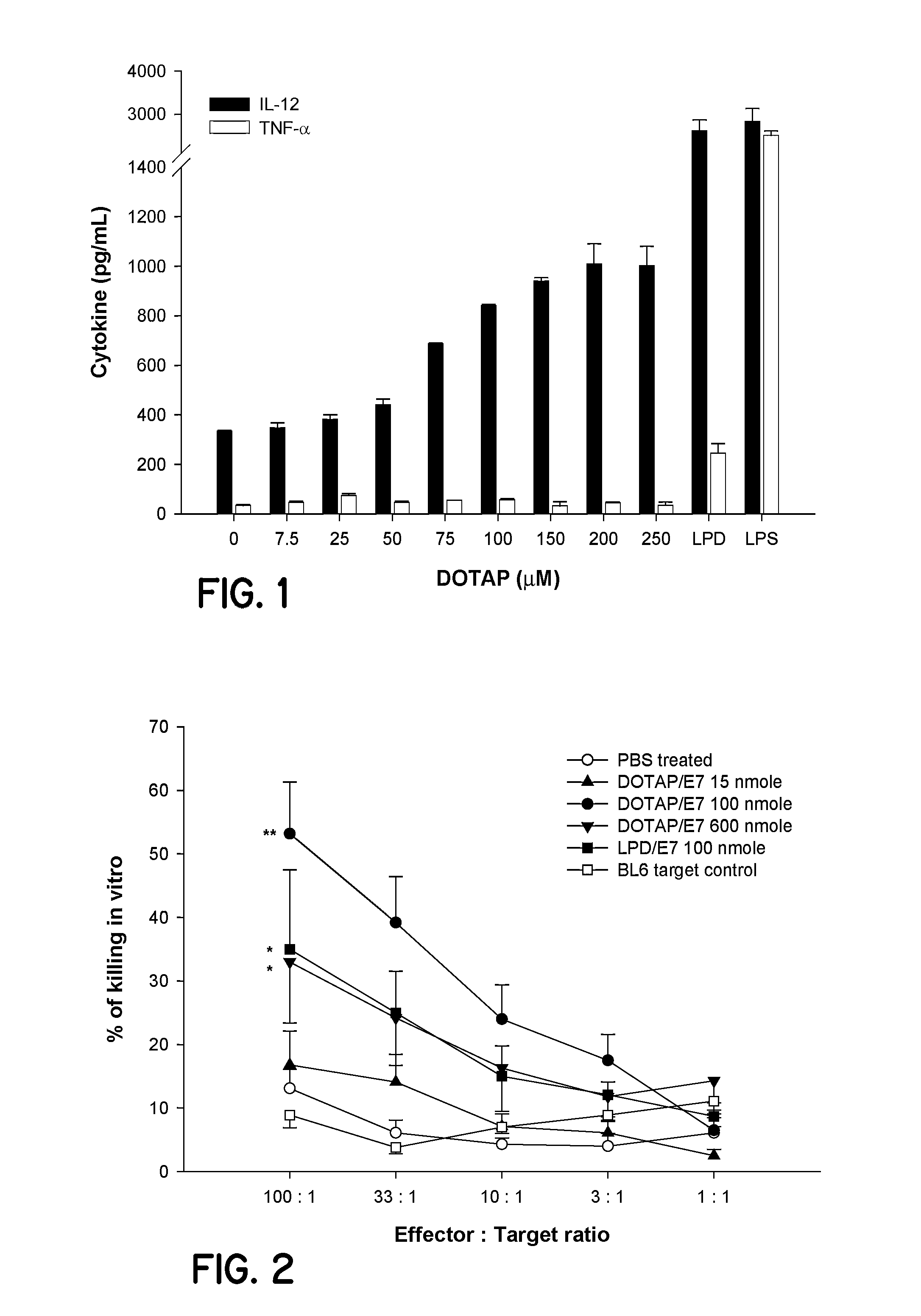
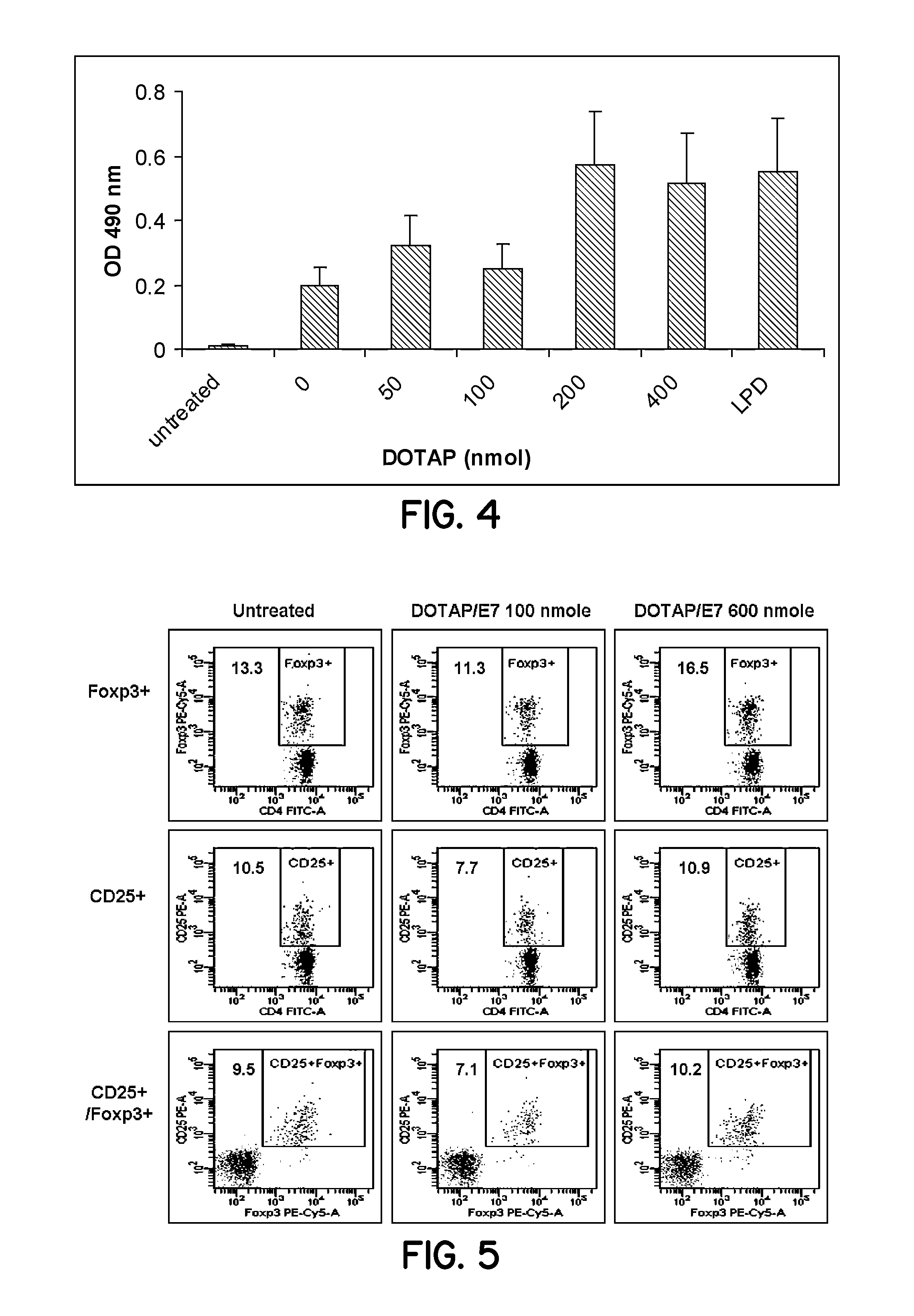
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
153results about How to "Suppress immune response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Engineered anti-IL-23 antibodies
ActiveUS20070048315A1Suppress immune responseInhibit biological activityNervous disorderAntipyreticAnti-CEA AntibodyAntibody
Owner:MERCK SHARP & DOHME LLC
Engineered anti-TSLP antibody
Owner:MERCK SHARP & DOHME CORP
Programmed immune responses using a vaccination node
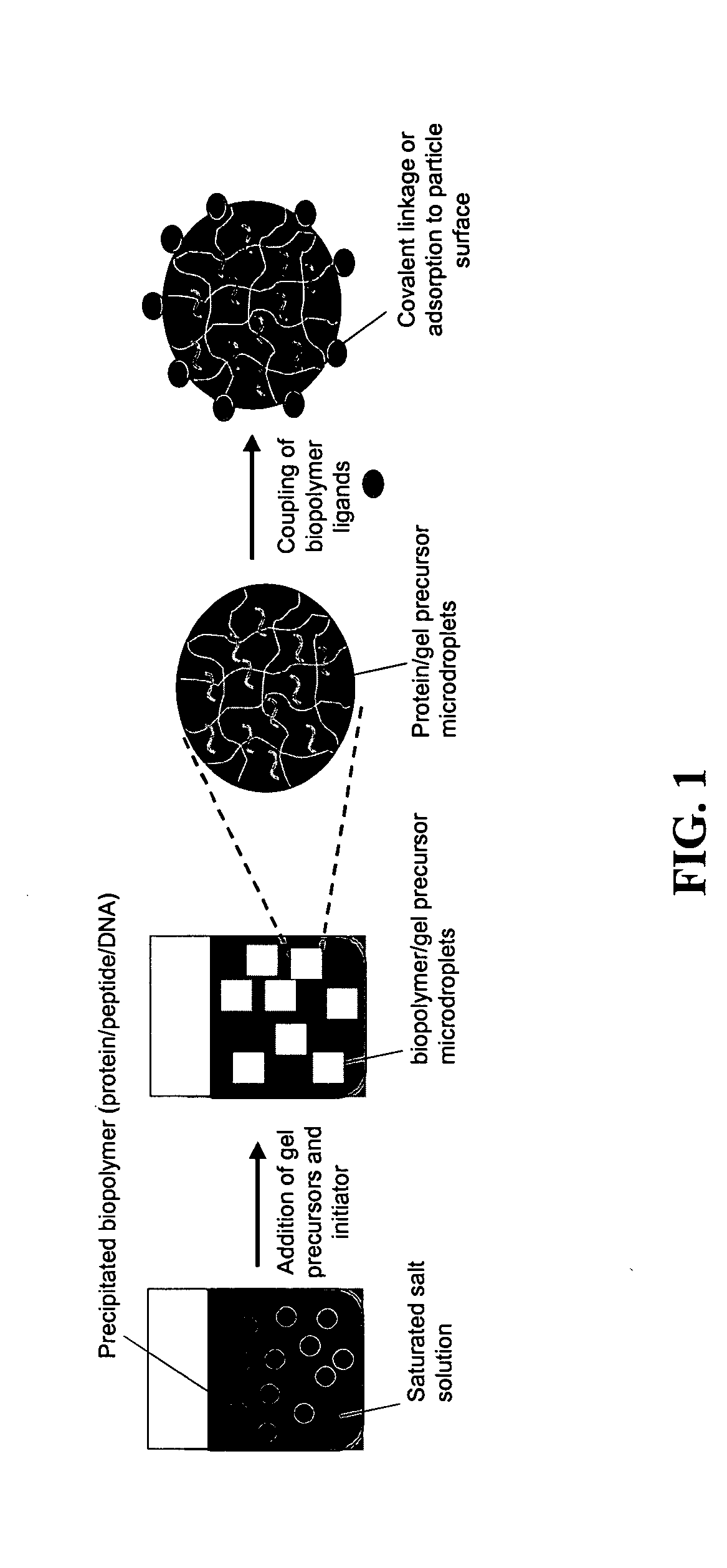
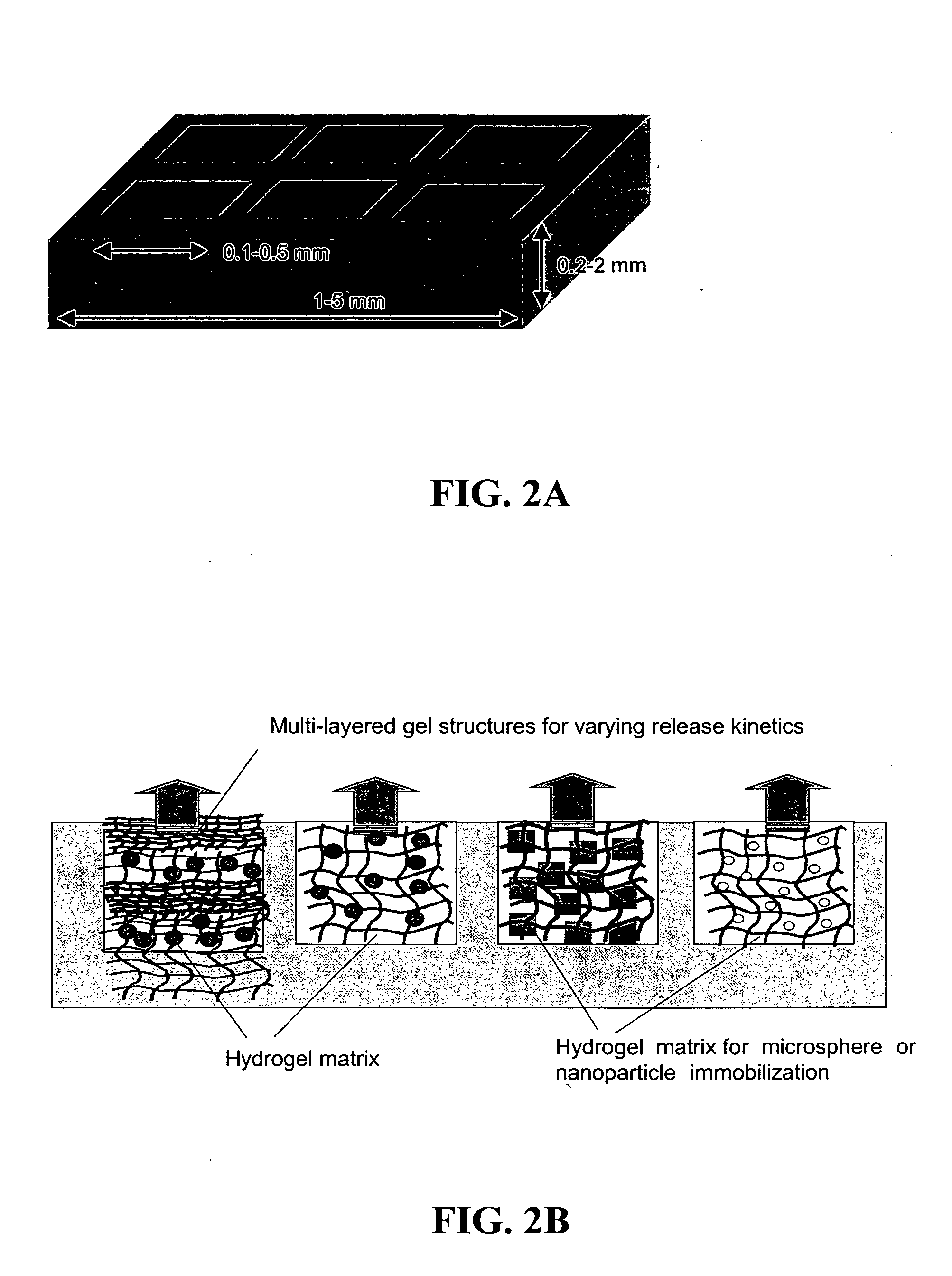
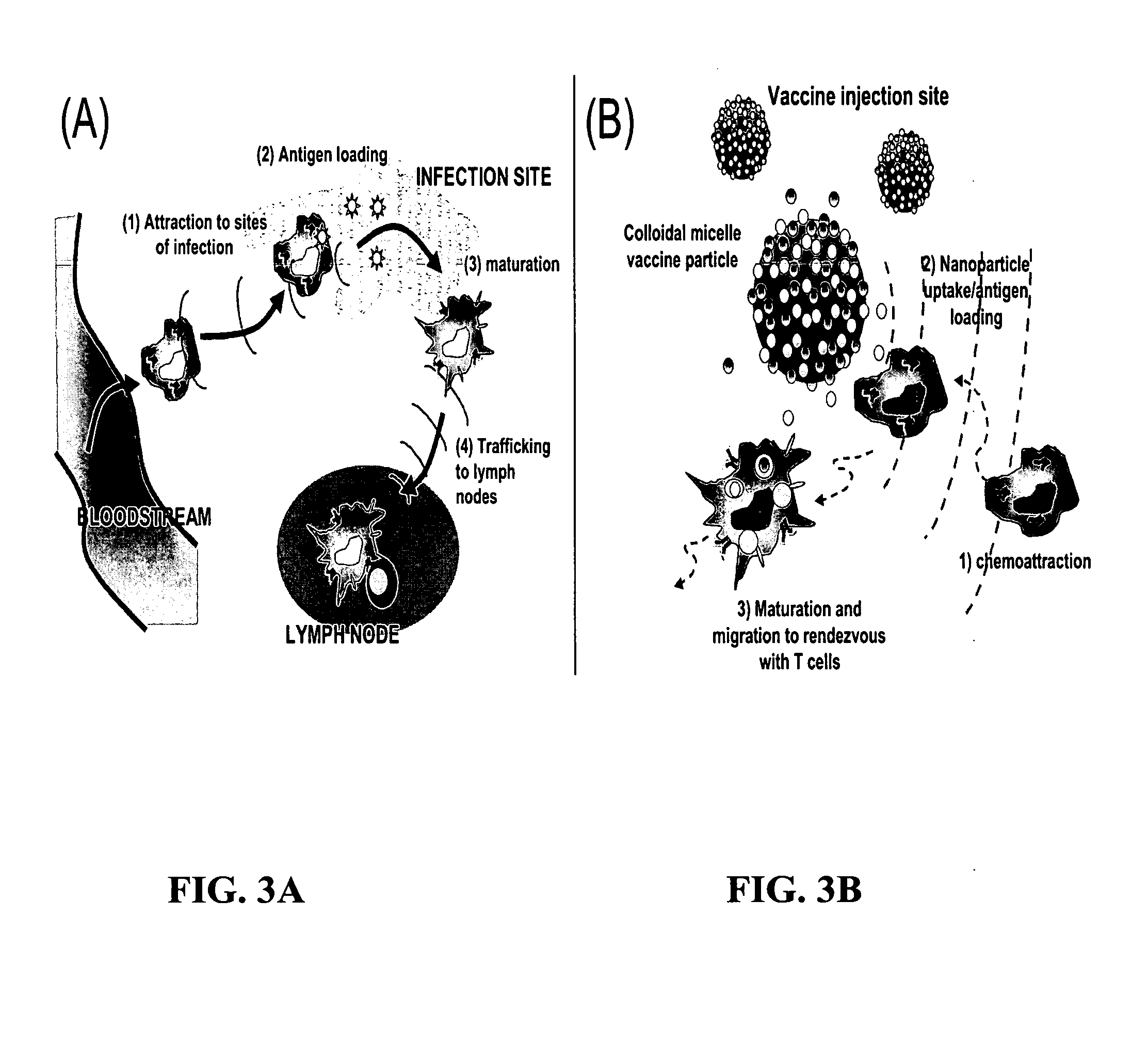
The present invention provides compositions and methods for modulating immune responses to antigens. One aspect of the present invention relates to a particle-based antigen delivery system (vaccination node) that comprises a hydrogel particle capable of both antigen presentation and DC activation. The VN may further comprise a chemoattractant-loaded microsphere capable of attracting DCs to the site of administration. Another aspect of the present invention relates to the use of the VN to modulate antigen presenting cells activation for the prevention and / treatment of various diseases, such as infectious diseases, cancers and autoimmune diseases.
Owner:VAXDESIGN
Self-assembling nanoparticle drug delivery system
InactiveUS20060292174A1Facilitate membrane transductionFacilitate transductionAntibacterial agentsAntimycoticsLipid formationMedicine
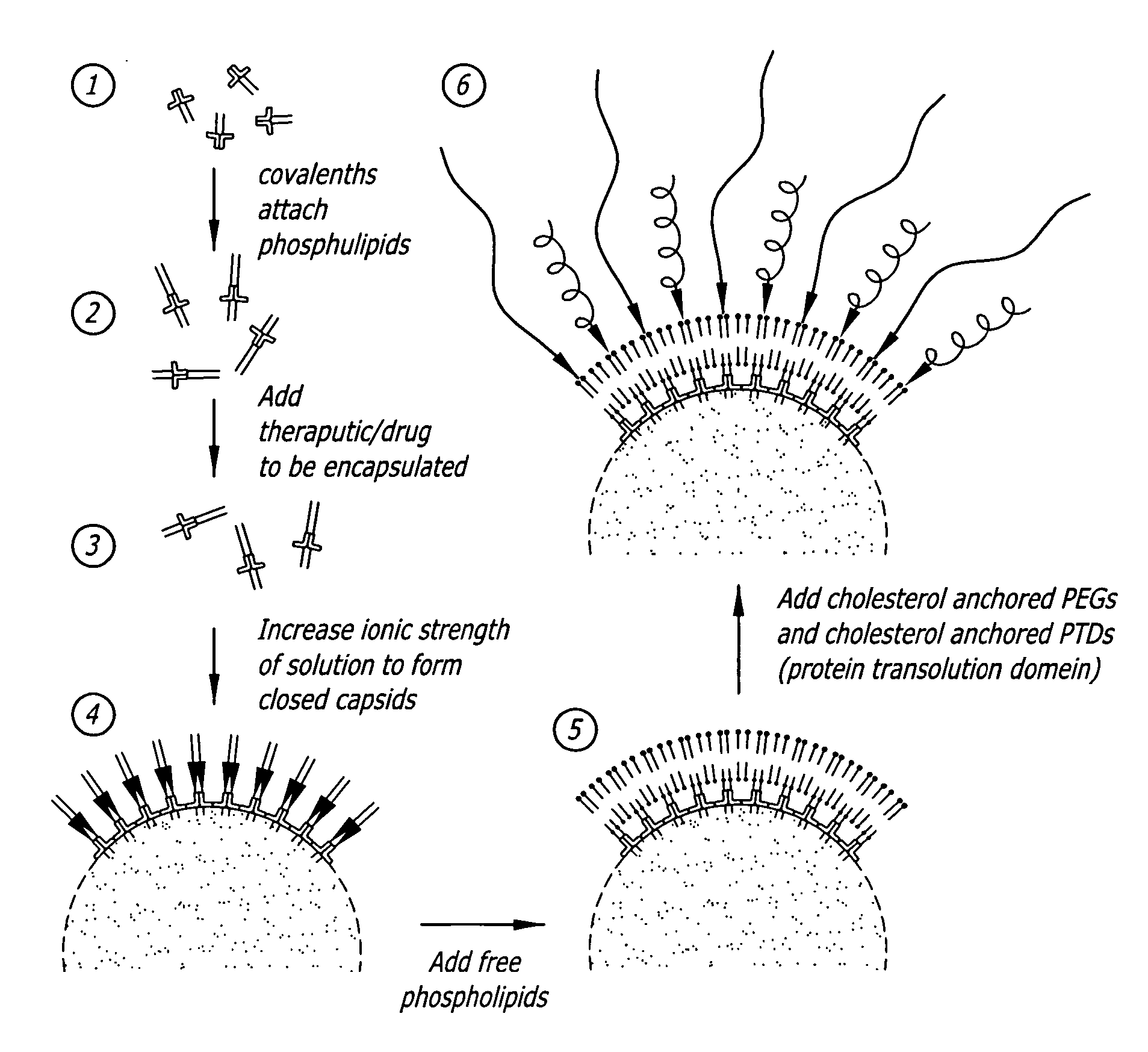
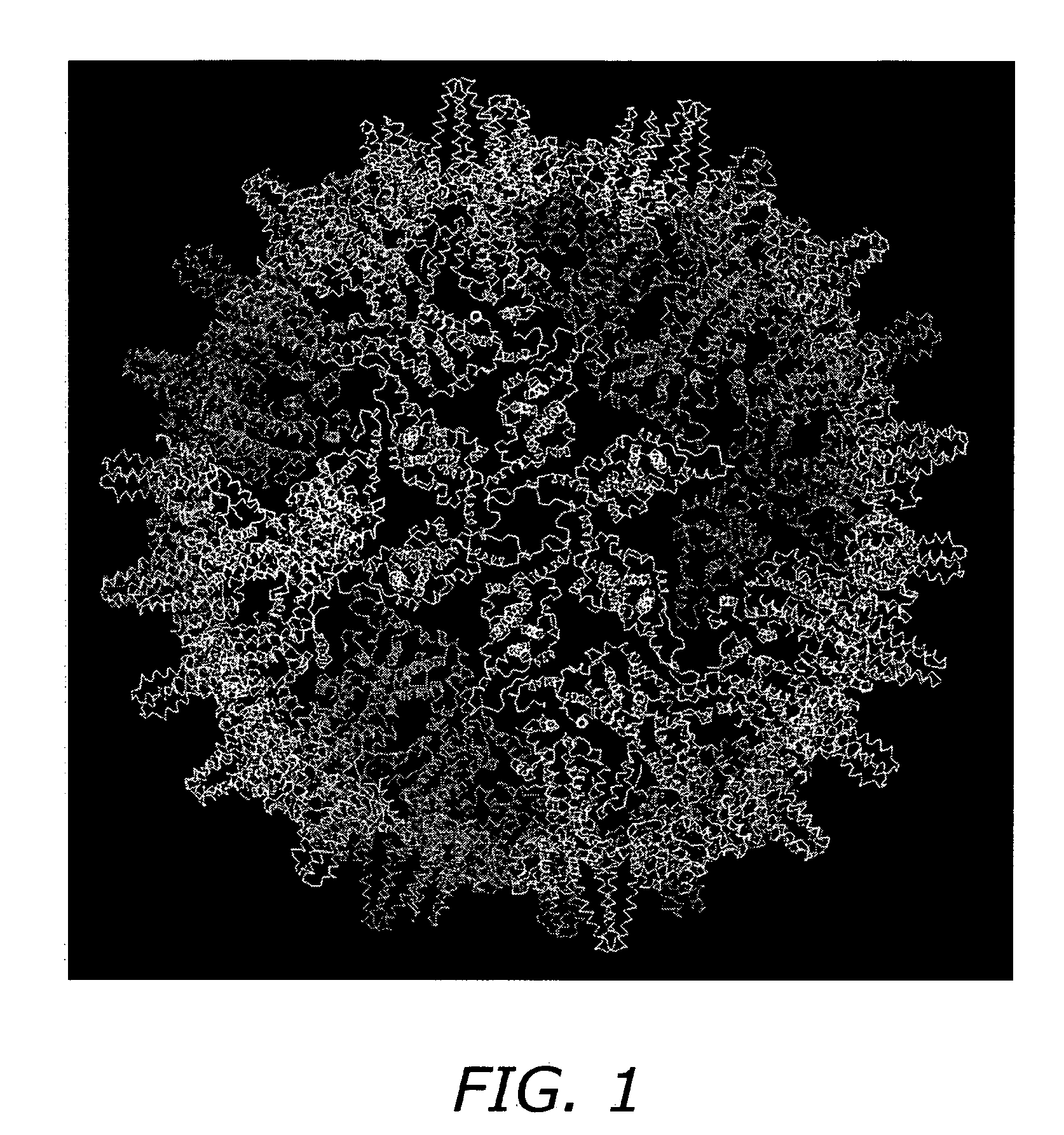
A self-assembling nanoparticle drug delivery system for the delivery of drugs including peptides, proteins, nucleic acids or synthetic chemical drugs is provided. The self-assembling nanoparticle drug delivery system described herein includes viral capsid proteins, such as Hepatitis B Virus core protein, encapsulating the drug, a lipid bi-layer envelope and targeting or facilitating molecules anchored in the lipid bilayer. A method for construction of the self-assembling nanocparticle drug delivery system is also provided.
Owner:CHIMEROS
Stimulation of an immune response by cationic lipids
ActiveUS8877206B2Suppress immune responsePeptide/protein ingredientsOrganic compound preparationAntigen
Owner:PDS BIOTECH
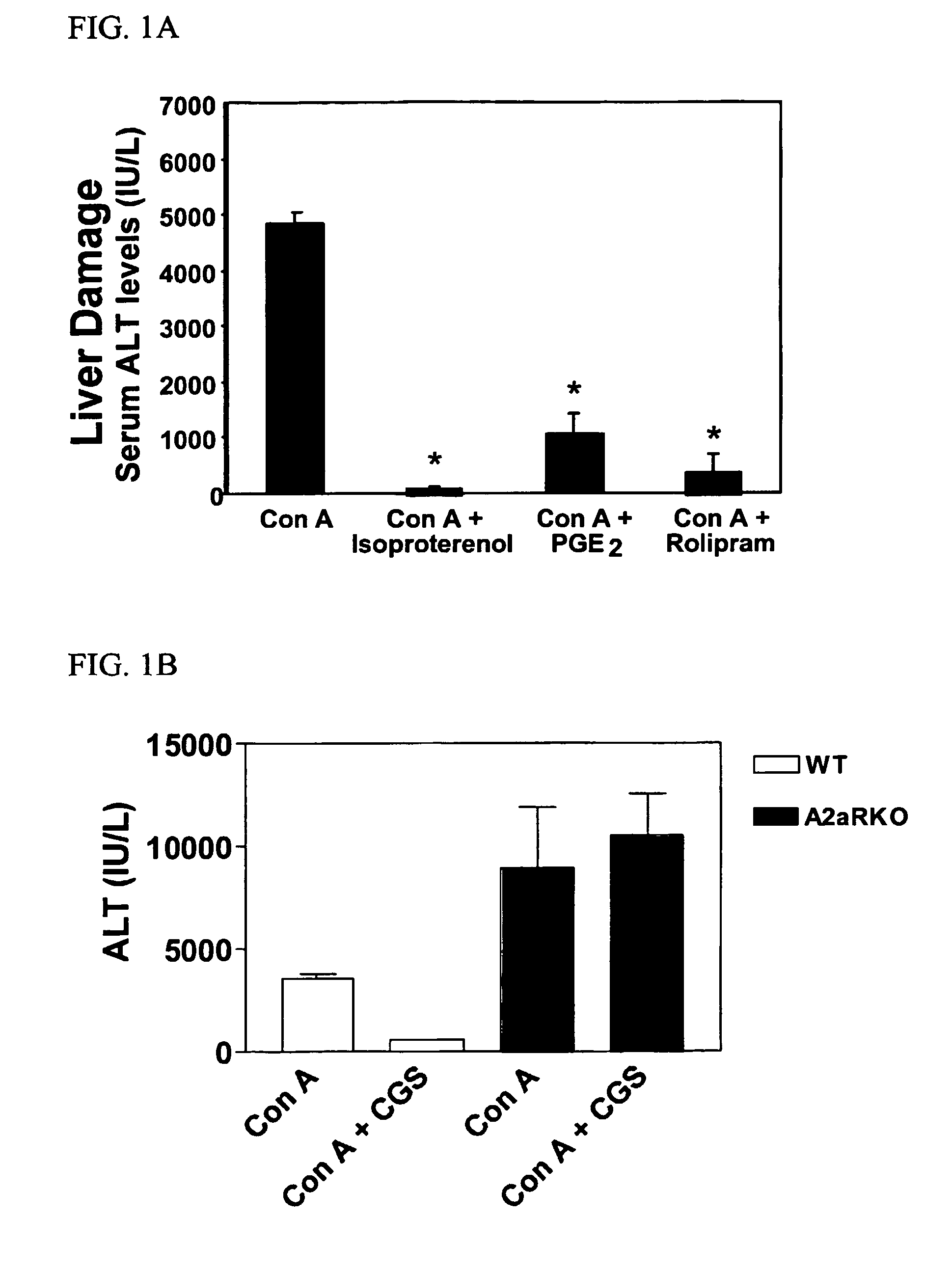
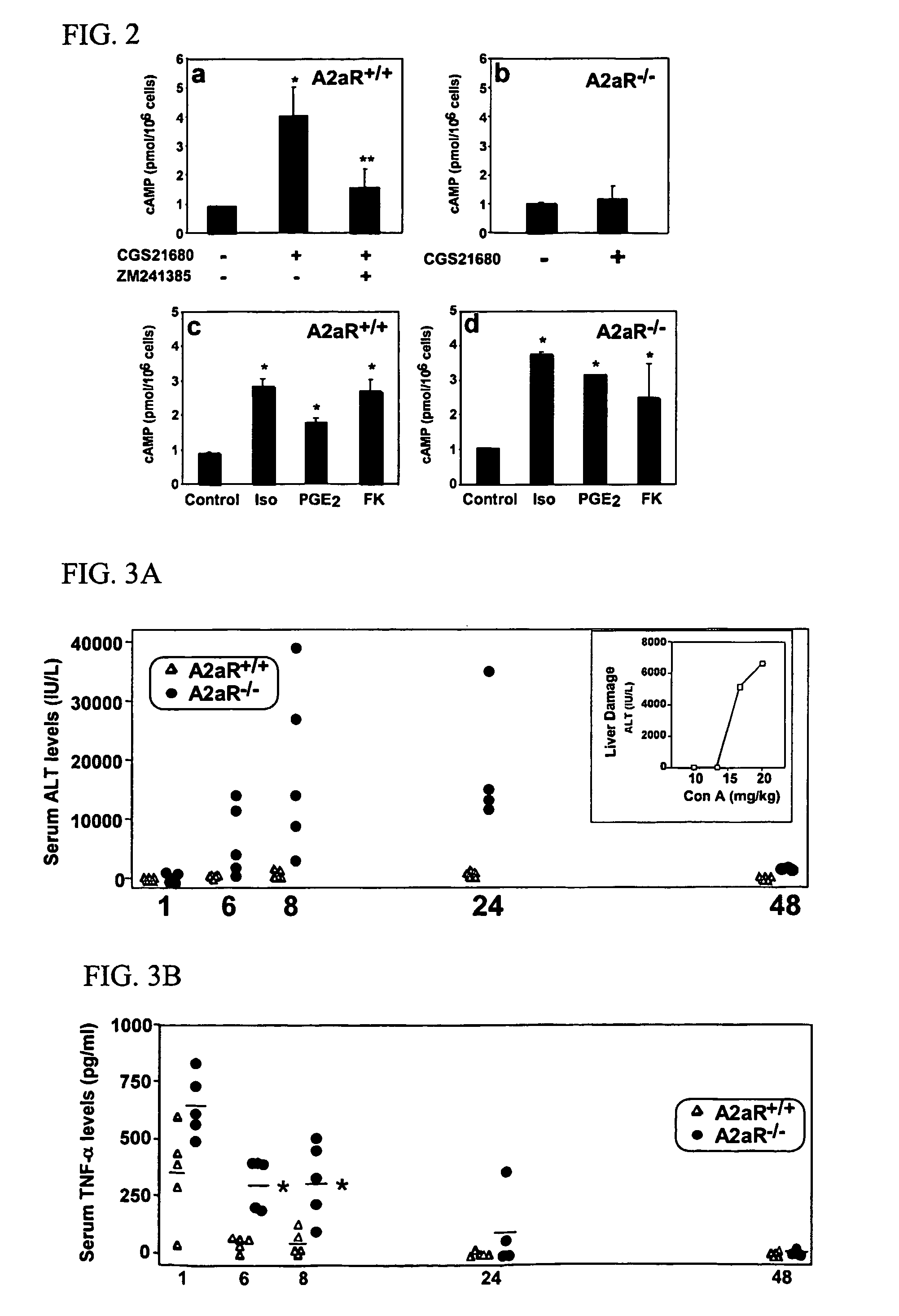
Methods for using extracellular adenosine inhibitors and adenosine receptor inhibitors to enhance immune response and inflammation
ActiveUS8080554B2Suppress immune responseEnhance immune responseAntibacterial agentsBiocideVaccine PotencyTumor destruction
A method is provided herein to increase an immune response to an antigen. The method includes administering an agent that inhibits extracellular adenosine or inhibits adenosine receptors. Also disclosed are methods to increase the efficacy of a vaccine and to increase an immune response to a tumor antigen or immune cell-mediated tumor destruction.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE DEPT OF
Stimulation of an immune response by cationic lipids
ActiveUS20090017057A1Suppress immune responseBacterial antigen ingredientsViral antigen ingredientsAntigenLipid formation
Owner:PDS BIOTECH
Method of treating rheumatoid arthritis and multiple sclerosis diseases of the human immune system
InactiveUS7098184B2Suppress immune responseAntibacterial agentsFungiExtracellular StructureCD4 antigen
The invention provides ligands and fragments thereof to a receptor on the surface of activated CD4+ T-cells. An exemplary ligand is designated ACT-4-L-h-1. Preferred fragments include purified extracellular domains of ligands. The invention also provides humanized and human antibodies to the ligand. The invention further provides methods of using the ligand and the antibodies in treatment of diseases and conditions of the immune system. The invention also provides methods of monitoring activated CD4+ T-cells using the ligands or fragments thereof.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
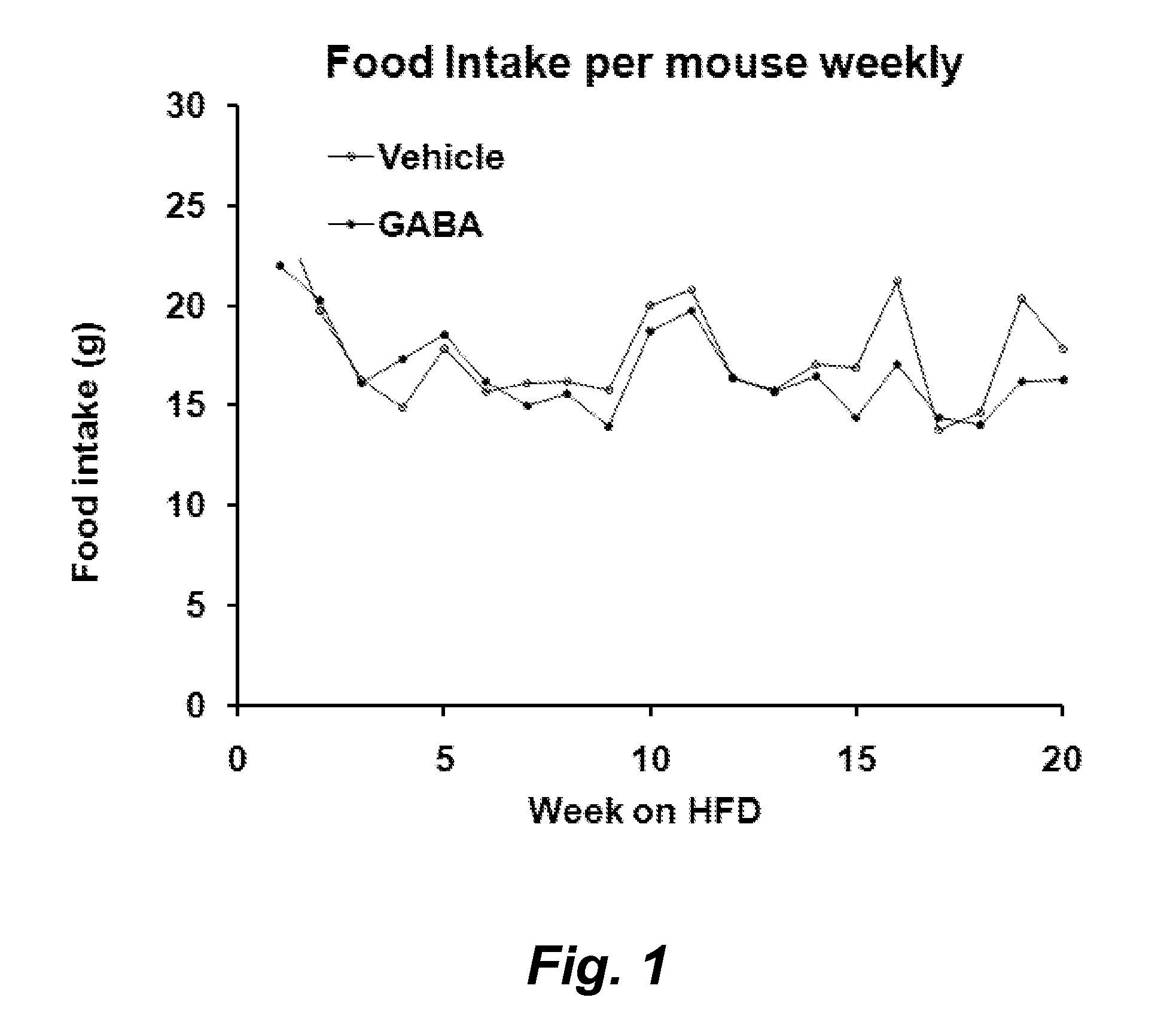
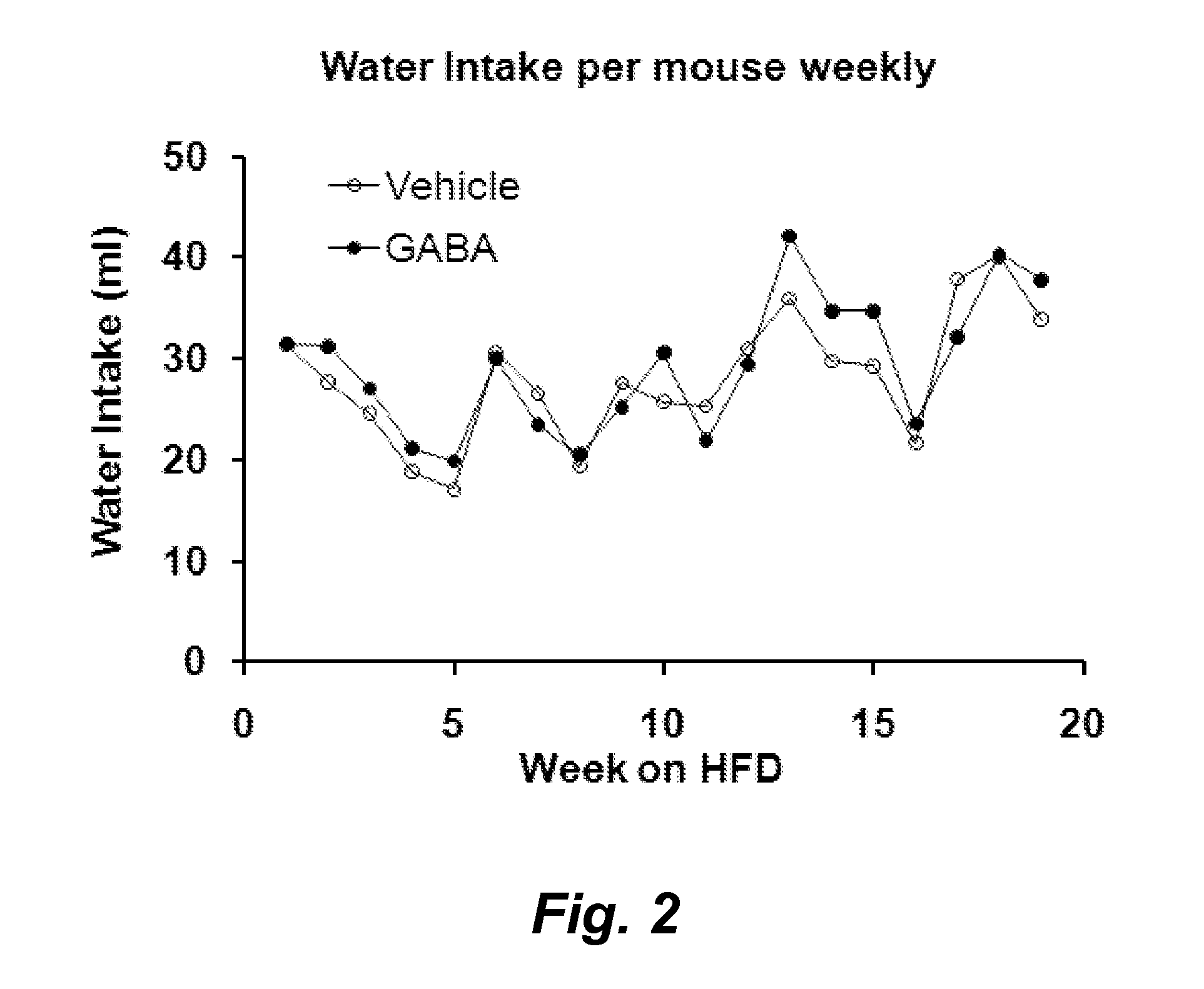
Gaba agonists in the treatment of disorders associated with metabolic syndrome and gaba combinations in treatment or prophylaxis of type i diabetes
In certain embodiments methods are provided for the therapeutic or prophylactic amelioration of one or more symptoms or disorders associated with metabolic syndrome. In various embodiments the methods involve administering to a subject in need thereof, a GABA receptor agonist, in an amount sufficient to ameliorate said one or more symptoms. In certain embodiments methods are provided for the prophylaxis or treatment of type I diabetes and related pathologies that involve the use of GABA or GABA agonists in combination with certain other compounds (e.g., one more antigens (e.g., GAD) that have a therapeutic effect in type I diabetes and / or an anti-CD3 antibody, an anti-CD20 antibody, exendin-4, and / or or a pro-insulin therapeutic).
Owner:RGT UNIV OF CALIFORNIA
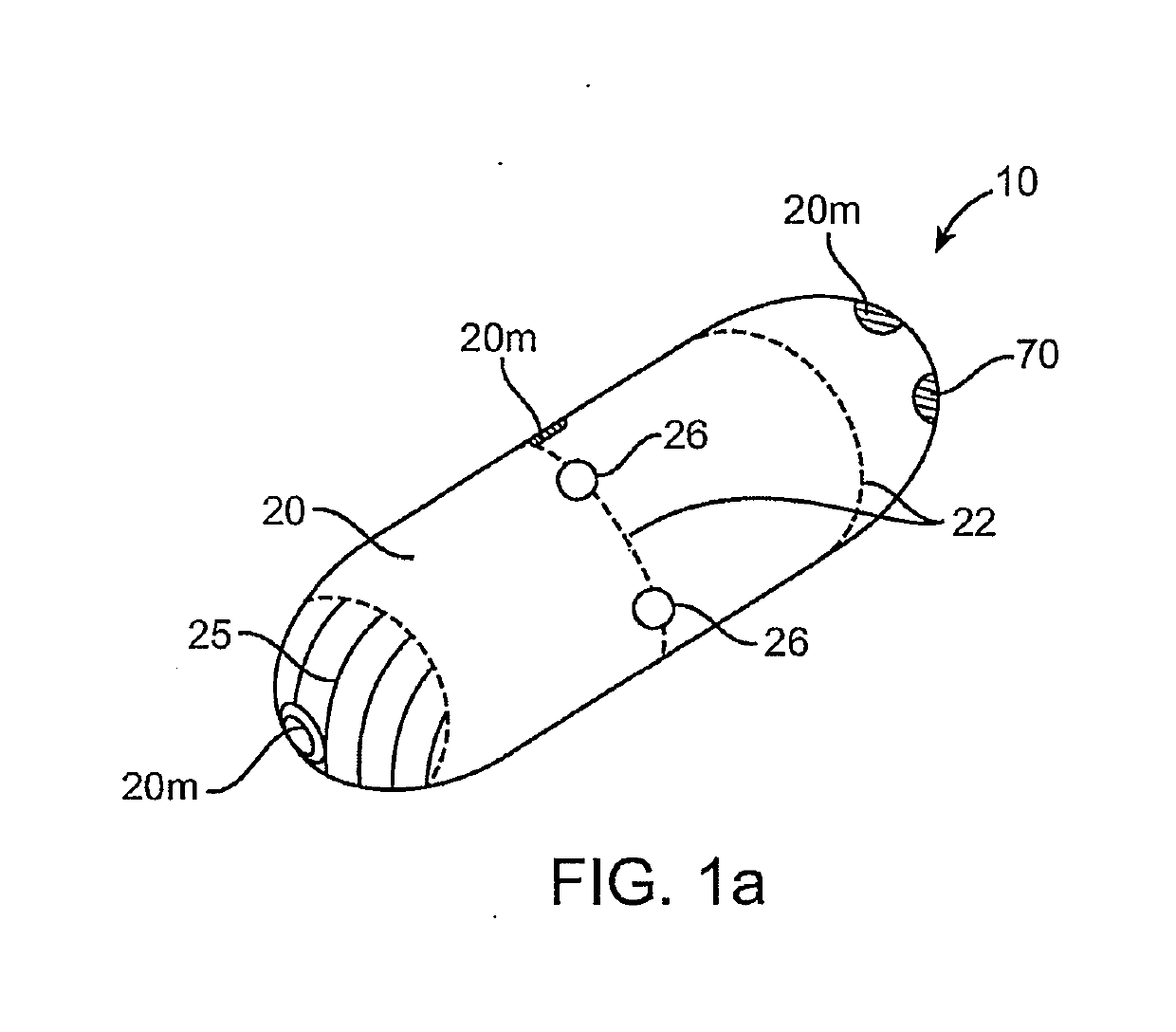
Clotting factor preparations for delivery into tissue of the intestinal tract using a swallowable drug delivery device
ActiveUS20190133937A1Improved long-term tolerance to and efficacyProcess controlPeptide/protein ingredientsPressure infusionIntestinal wallsTherapeutic effect
Embodiments provide devices, preparations and methods for delivering therapeutic agents (TAs) such as clotting factors (CFs, e.g., Factor 8) within the GI tract. Many embodiments provide a swallowable device e.g., a capsule for delivering TAs into the intestinal wall (IW). Embodiments also provide TA preparations configured to be contained within the capsule, advanced from the capsule into the IW and / or surrounding tissue (ST) and degrade to release the TA into the bloodstream to produce a therapeutic effect (e.g., improved clotting). The preparation can be operably coupled to delivery means having a first configuration where the preparation is contained in the capsule and a second configuration where the preparation is advanced out of the capsule into the IW or ST (e.g., the peritoneal cavity). Embodiments are particularly useful for delivery of CFs for treatment of clotting disorders (e.g., hemophilia) where such CFs are poorly absorbed and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
Self-assembling nanoparticle drug delivery system
InactiveUS7964196B2Facilitate transductionPromote degradationAntibacterial agentsAntimycoticsLipid formationNanoparticle
A self-assembling nanoparticle drug delivery system for the delivery of drugs including peptides, proteins, nucleic acids or synthetic chemical drugs is provided. The self-assembling nanoparticle drug delivery system described herein includes viral capsid proteins, such as Hepatitis B Virus core protein, encapsulating the drug, a lipid bi-layer envelope and targeting or facilitating molecules anchored in the lipid bilayer. A method for construction of the self-assembling nanocparticle drug delivery system is also provided.
Owner:CHIMEROS
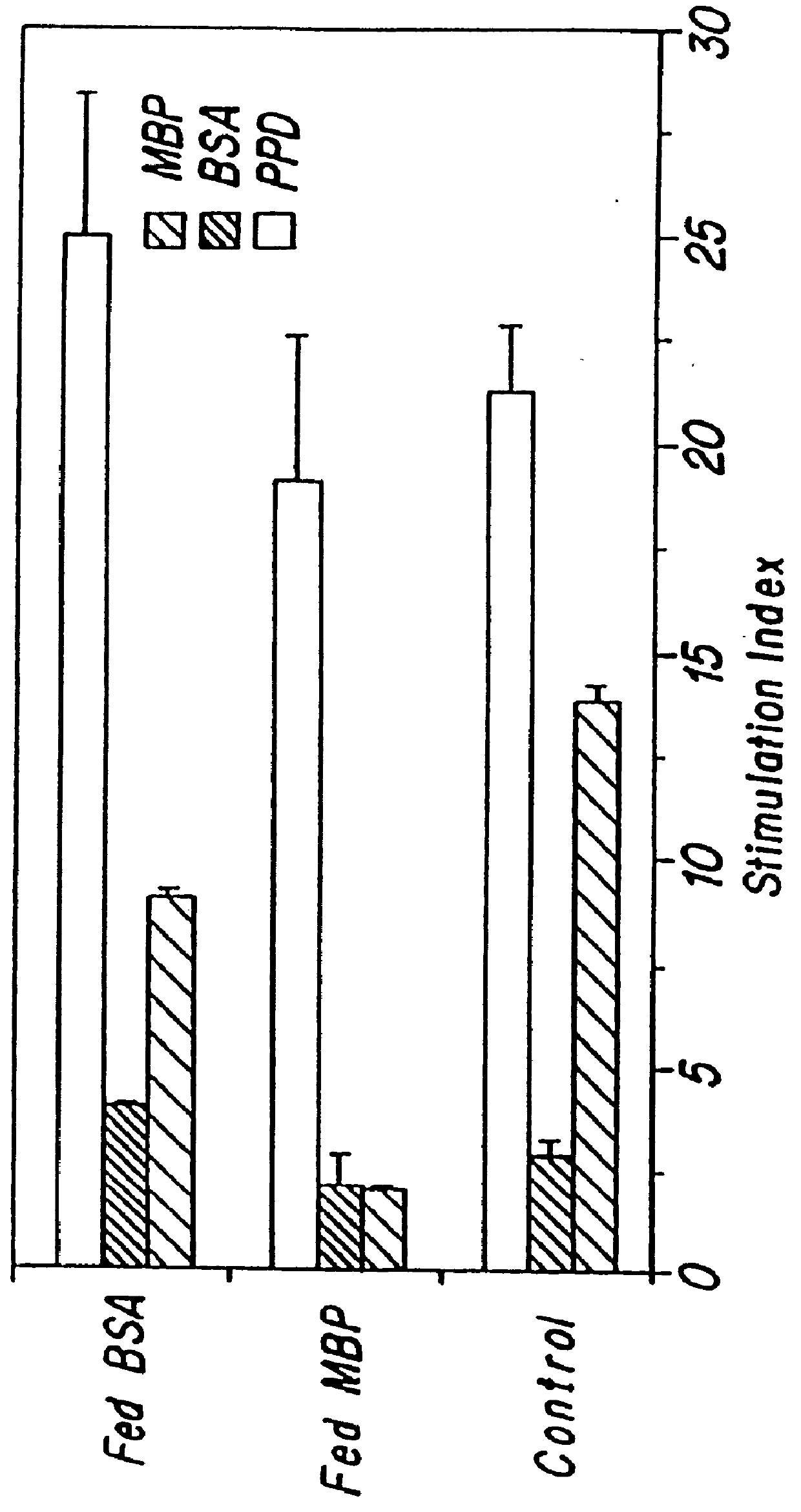
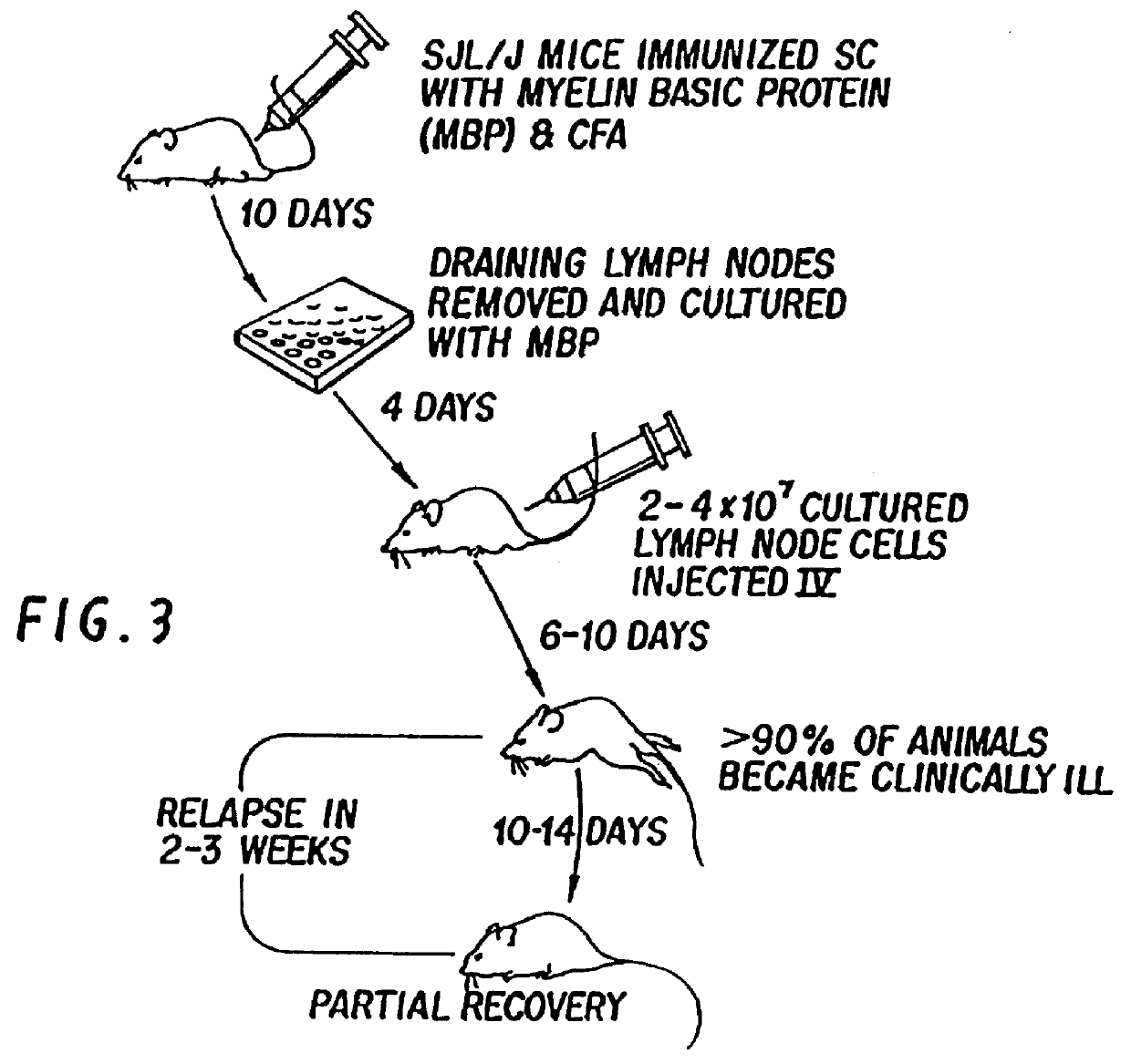
Treatment of autoimmune arthritis by oral administration of collagen
InactiveUS6019971ASuppresses development of diseaseSuppress immune responseBiocidePeptide/protein ingredientsAntigenDisease
The invention is directed to a method of treating a T cell-mediated autoimmune disease in animals, including humans, by the oral administration of autoantigens, fragments of autoantigens, or analogs structurally related to those autoantigens, which are specific for the particular autoimmune disease. The method of the invention includes both prophylactic and therapeutic measures.
Owner:BRONSON NUTRITIONALS
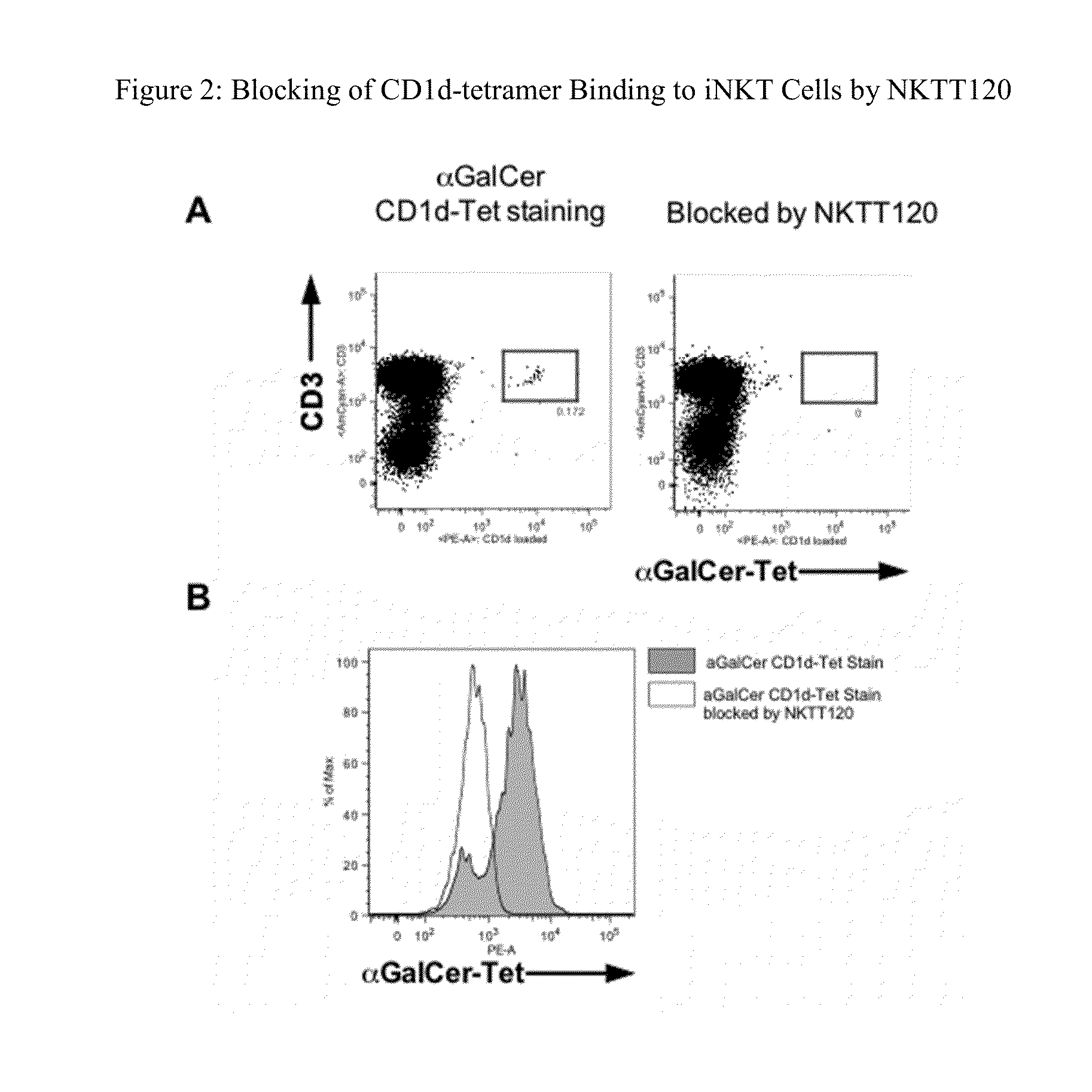
HUMANIZED ANTIBODIES TO iNKT
InactiveUS20130136735A1Suppress immune responseSufficiently suppressAntibacterial agentsAntipyreticBlocking antibodyHumanized antibody
Methods of treatment to suppress an immune response are provided. The method comprises administering to a subject in need of treatment a naked blocking antibody that binds selectively iNKT cells in an amount effective to suppress the subject's iNKT cell function. Compositions comprising, an isolated, humanized antibody that binds selectively iNKT cells are also provided.
Owner:NKT THERAPEUTICS
Methods for producing antibody
InactiveUS7750204B2Easy to getEfficient productionPeptide/protein ingredientsVirus peptidesImmune toleranceIndividual animal
This invention provides methods for producing antibodies, wherein the methods comprise the step of administering an immunogen comprising both a target antigen and a background antigen to transgenic animals, into which a gene coding for the background antigen has been introduced. Since immunotolerance to the background antigens have thus been induced in the transgenic animals, the animals efficiently produce antibodies to target antigens.
Owner:CHUGAI PHARMA CO LTD
Methods For Detecting Th1 Cells
InactiveUS20080248502A1Suppress immune responseDisease controlMicrobiological testing/measurementBiological material analysisActivated LymphocyteCell biology
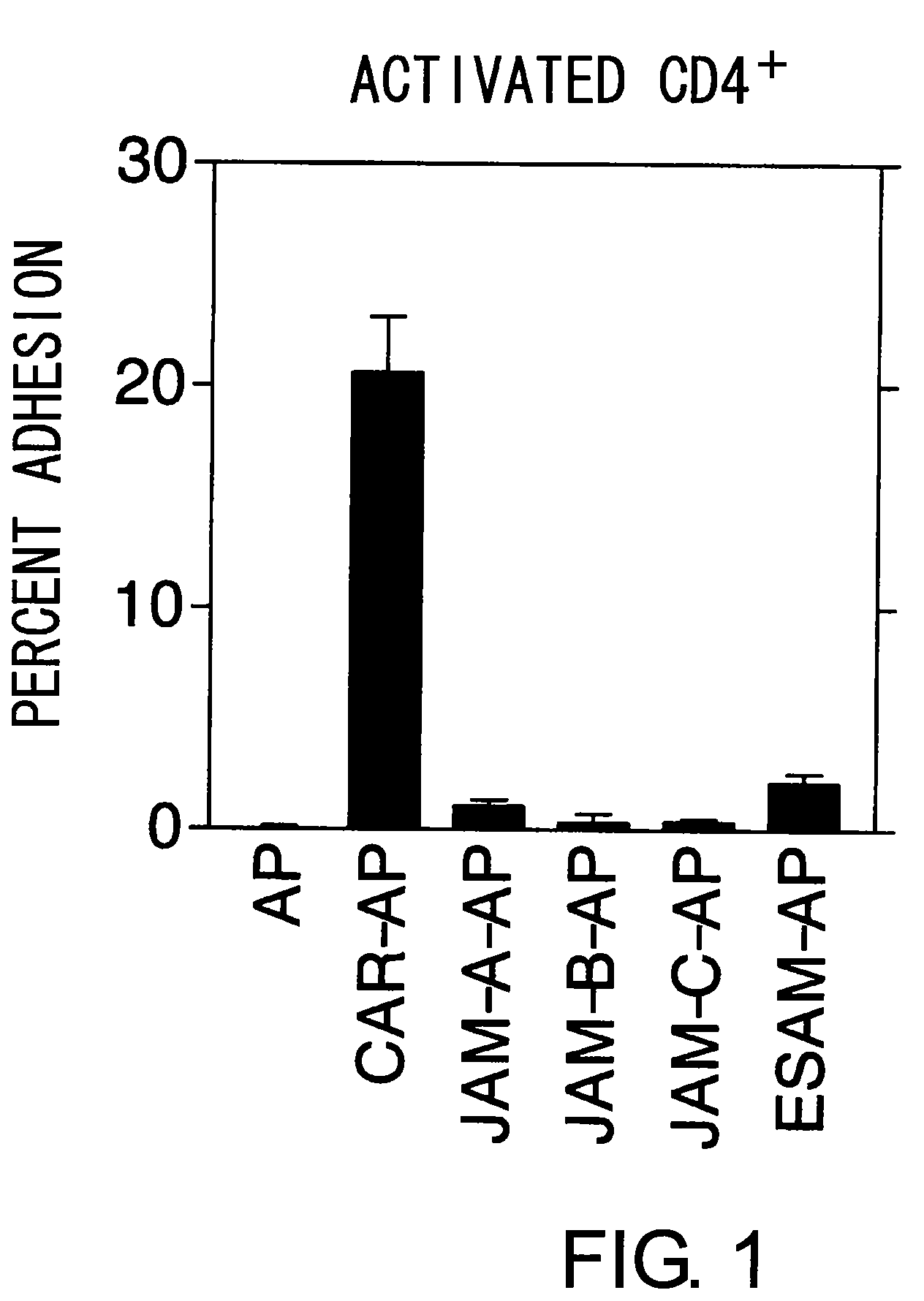
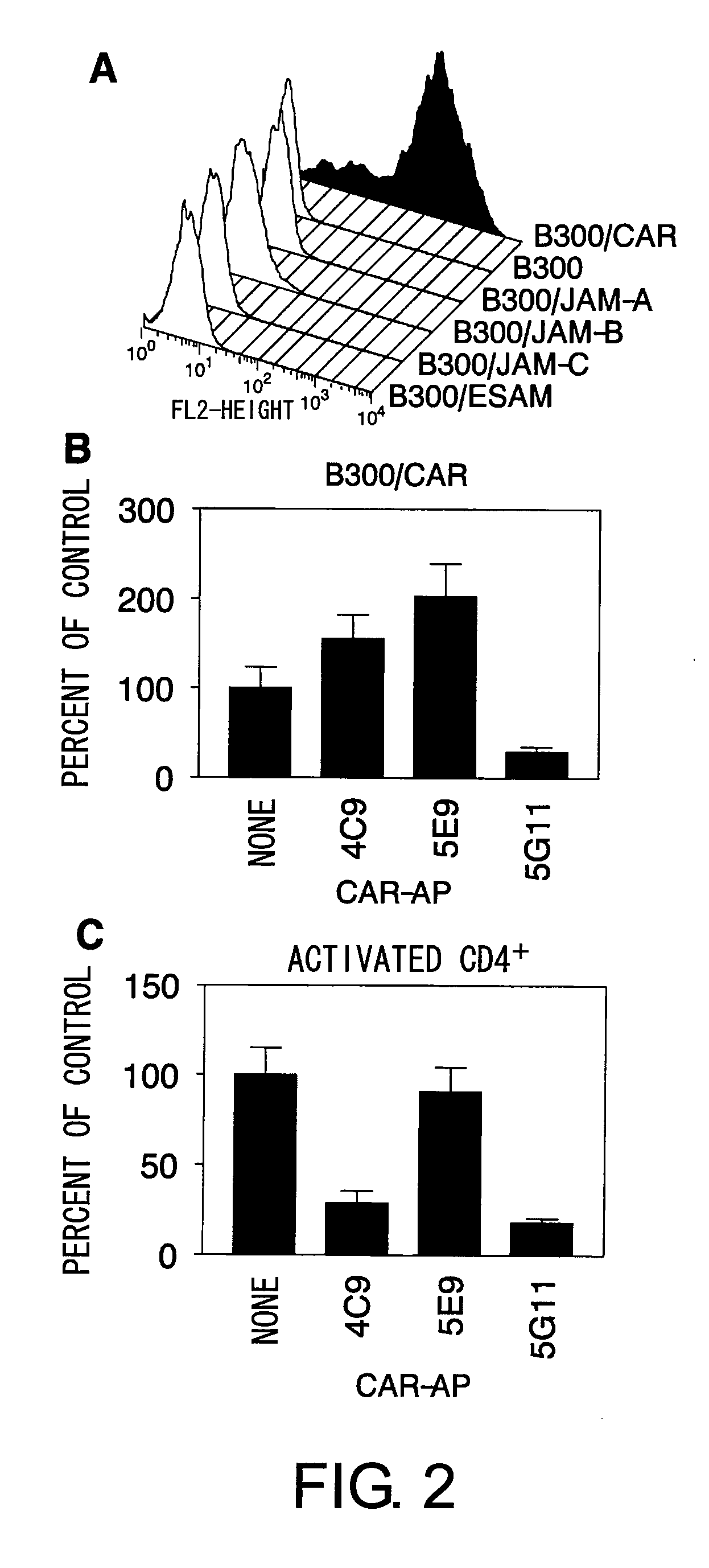
The inventors discovered that the adhesion molecule CAR, known to be localized in intracellular adhesion sites, functioned as an adhesion molecule for activated lymphocytes. Further, the inventors identified CARL, a novel CAR ligand expressed in lymphocytes, and clarified that the ligand was expressed selectively in Th1 cells. In addition, they found that anti-CAR antibodies could inhibit the adhesion of activated lymphocytes to CAR molecules. Thus, the present invention provides methods for detecting Th1 cells using CAR or anti-CARL antibodies, and methods of screening for inhibitors suppressing the adhesion of Th1 cells using the binding between CAR and CARL as an index. Furthermore, the present invention relates to methods of screening for inhibitors of the binding between CAR and CARL, antibodies that inhibit the binding between CAR and CARL, and therapeutic compositions comprising these antibodies. These are expected to be useful in diagnosing diseases, such as inflammation, in which infiltration of Th1 cells is involved, and in providing pharmaceutical agents for alleviating such diseases.
Owner:EISIA R&D MANAGEMENT CO LTD
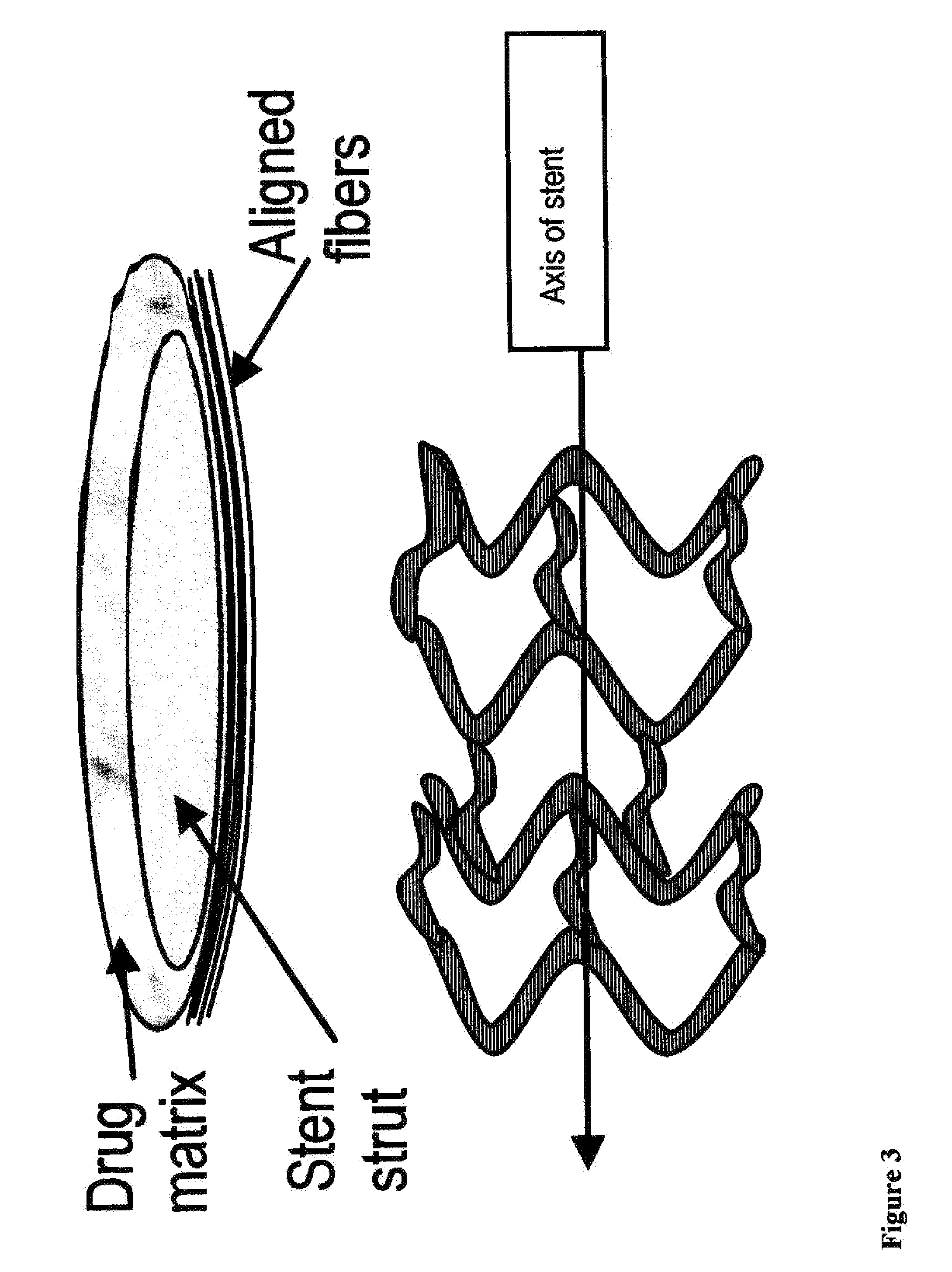
Extracellular matrix modulating coatings for medical devices
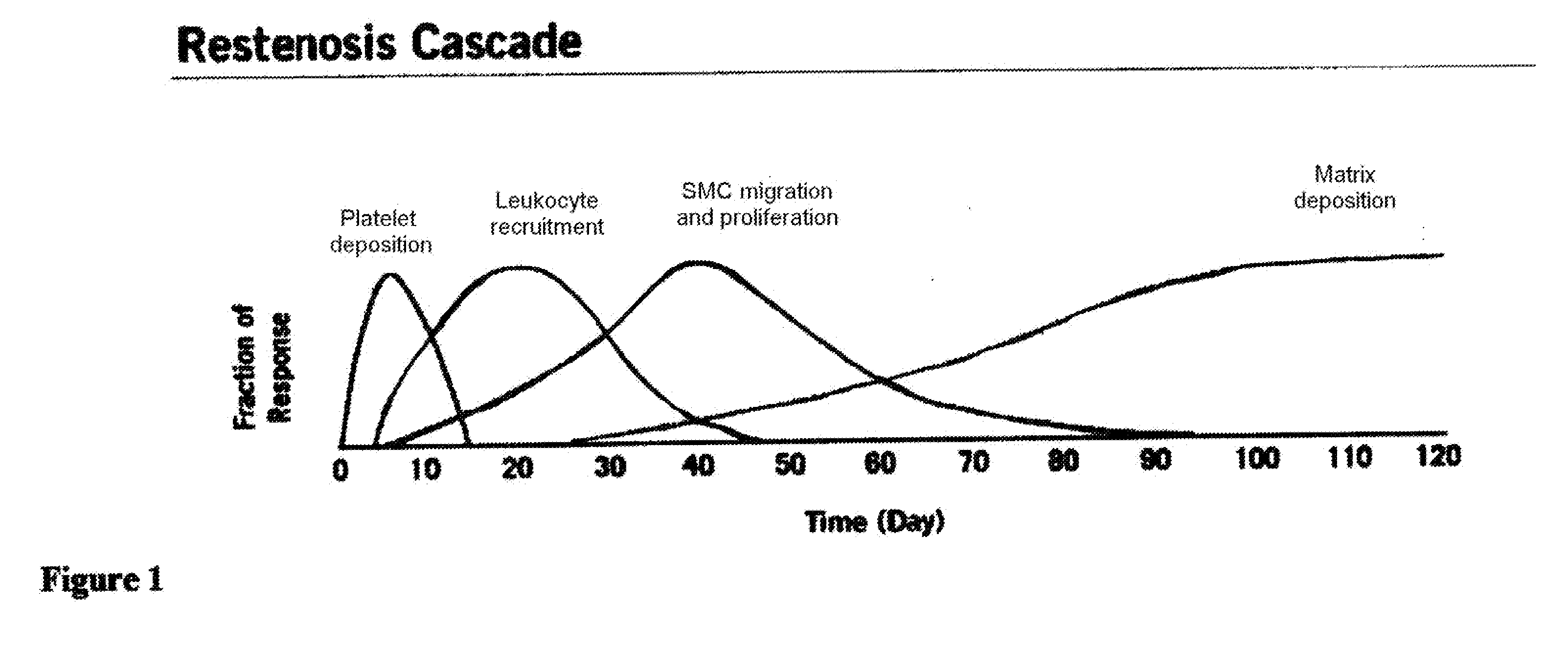
InactiveUS20100119578A1Preventing excessive buildSuppress immune responseBiocideStentsCell-Extracellular MatrixMedical device
A unique method and coatings are provided to promote / allow early stage tissue encapsulation / endothelization of medical devices while effectively controlling excessive tissue buildup by eluting antiproliferative therapeutic agent within a body of a patient. The method involves using a therapeutic agent that suppresses excessive extracellular matrix proliferation and allows / promotes thin tissue healing / encapsulation / endothelization of the device. Optimized timing of onset of elution to match onset of excessive extracellular matrix proliferation for maximum effectiveness is achieved by delay barrier with biochemical switch.
Owner:SPECIALIZED VASCULAR TECH
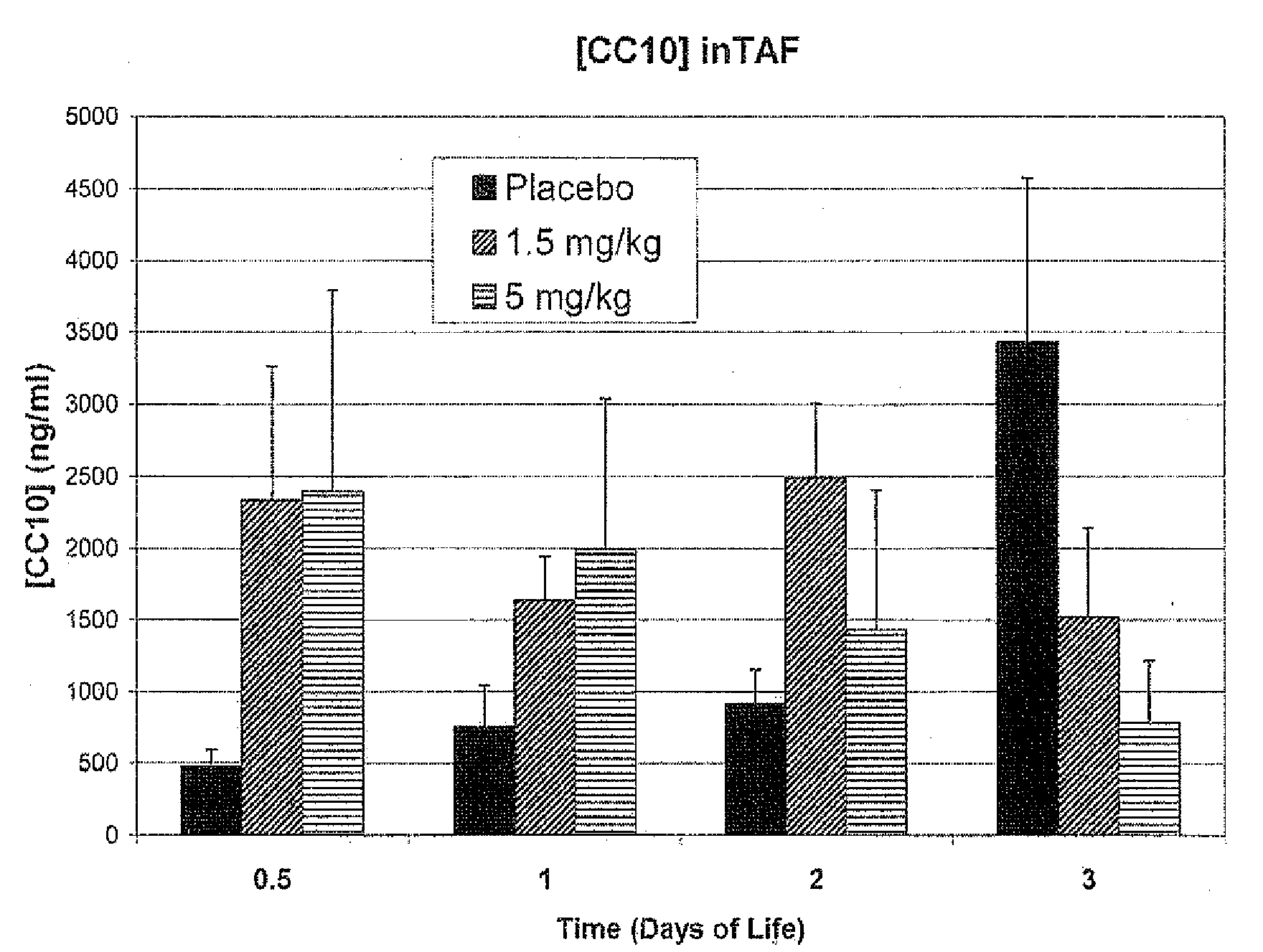
Methods and compositions for the reduction of neutrophil influx and for the treatment of bronchpulmonary dysplasia, respiratory distress syndrome, chronic lung disease, pulmonary fibrosis, asthma and chronic obstructive pulmonary disease
InactiveUS20090197808A1Inhibits platelet aggregationSuppress immune responsePeptide/protein ingredientsMicrobiological testing/measurementObstructive Pulmonary DiseasesNeutrophil granulocyte
The present invention relates generally to the use of recombinant human CC10 (rhCC10), also known as recombinant human uteroglobin, for use as a therapeutic in the treatment of Respiratory Distress Syndrome (RDS), Bronchopulmonary dysplasia (BPD), chronic lung disease and / or pulmonary fibrosis, Asthma and Chronic Obstructive Pulmonary Disease (COPD). More particularly, the invention provides methods, including broadly the critical dosage ranges of rhCC10, which may be administered to safely and effectively treat the aforementioned conditions. The invention further provides a composition useful in administering rhCC10 to humans.
Owner:THERABRON THERAPEUTICS INC
CD106-positive cells, and identification and preparation method and application thereof
ActiveCN102539736APrevent rejectionSuppress immune responseSkeletal/connective tissue cellsEmbryonic cellsAdipogenesisAutoimmune disease
The invention discloses an identification and preparation method and application CD106-positive mesenchymal stem cells. The method comprises the following steps: identifying and isolating CD106-positive cells from mononuclear cells isolated from bone marrow, placenta or other tissues through an immunological method, carrying out adherent culture, and collecting adherent CD106-positive cells; or carrying out adherent culture of mononuclear cells isolated from bone marrow, placenta or other tissues, collecting adherent mesenchymal cells, and isolating the CD106-positive mesenchymal stem cells when the count of the obtained cells is large enough. The CD106-positive cells are mesenchymal stem cells with adherent growth, express mesenchymal stem cell-related immunological phenotype, and have osteogenesis, adipogenesis and other differentiation potencies. Compared with CD106-negative mesenchymal stem cells, the CD106-positive cells have higher immunosuppression capacity, can better inhibit proliferation of IFN-gamma (interferon-gamma) secretion of lymphocytes, and can be used for treating graft versus host disease (GVHD) and autoimmune diseases.
Owner:北京汉氏干细胞科技有限公司
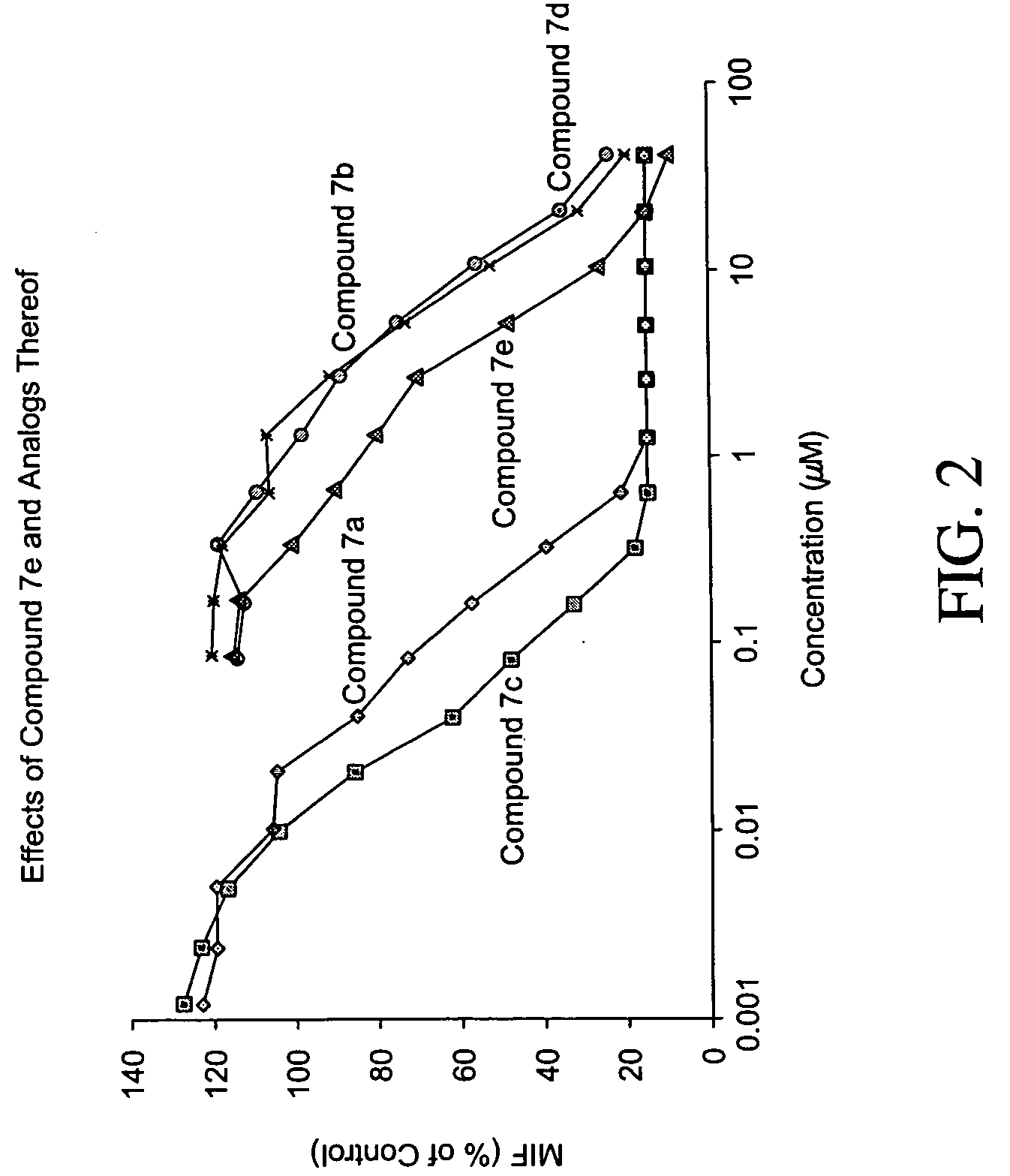
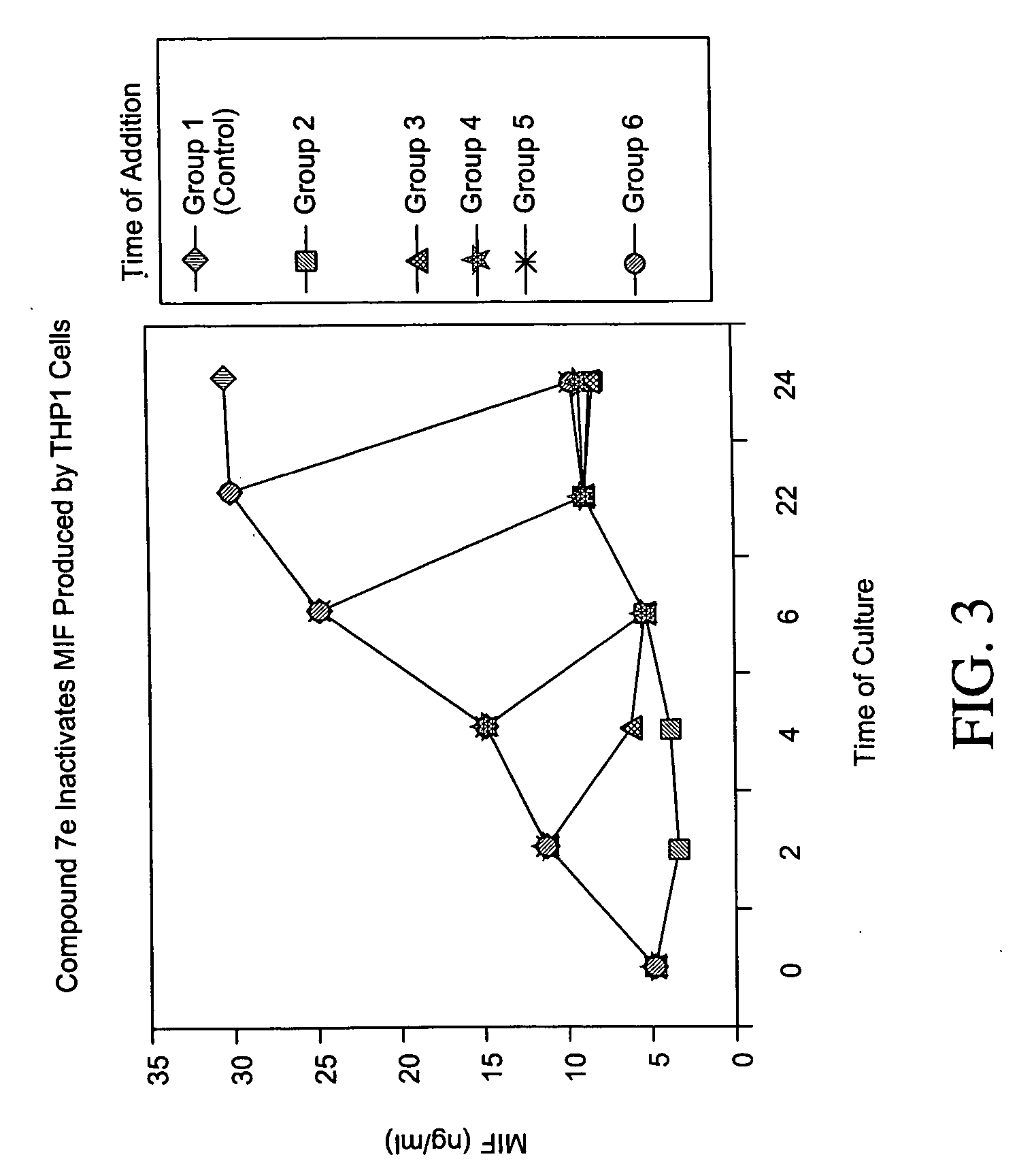
Inhibitors of macrophage migration inhibitory factor and methods for identifying the same
InactiveUS20050287617A1Suppress immune responseNervous disorderOrganic chemistryDiseaseStereoisomerism
Inhibitors of MIF are provided which have utility in the treatment of a variety of disorders, including the treatment of pathological conditions associated with MIF activity. The inhibitors of MIF have the following structures: including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof, wherein n, R1, R2, R3, R4, X, Y and Z are as defined herein. Compositions containing an inhibitor of MIF in combination with a pharmaceutically acceptable carrier are also provided, as well as methods for use of the same.
Owner:AVANIR PHARMA
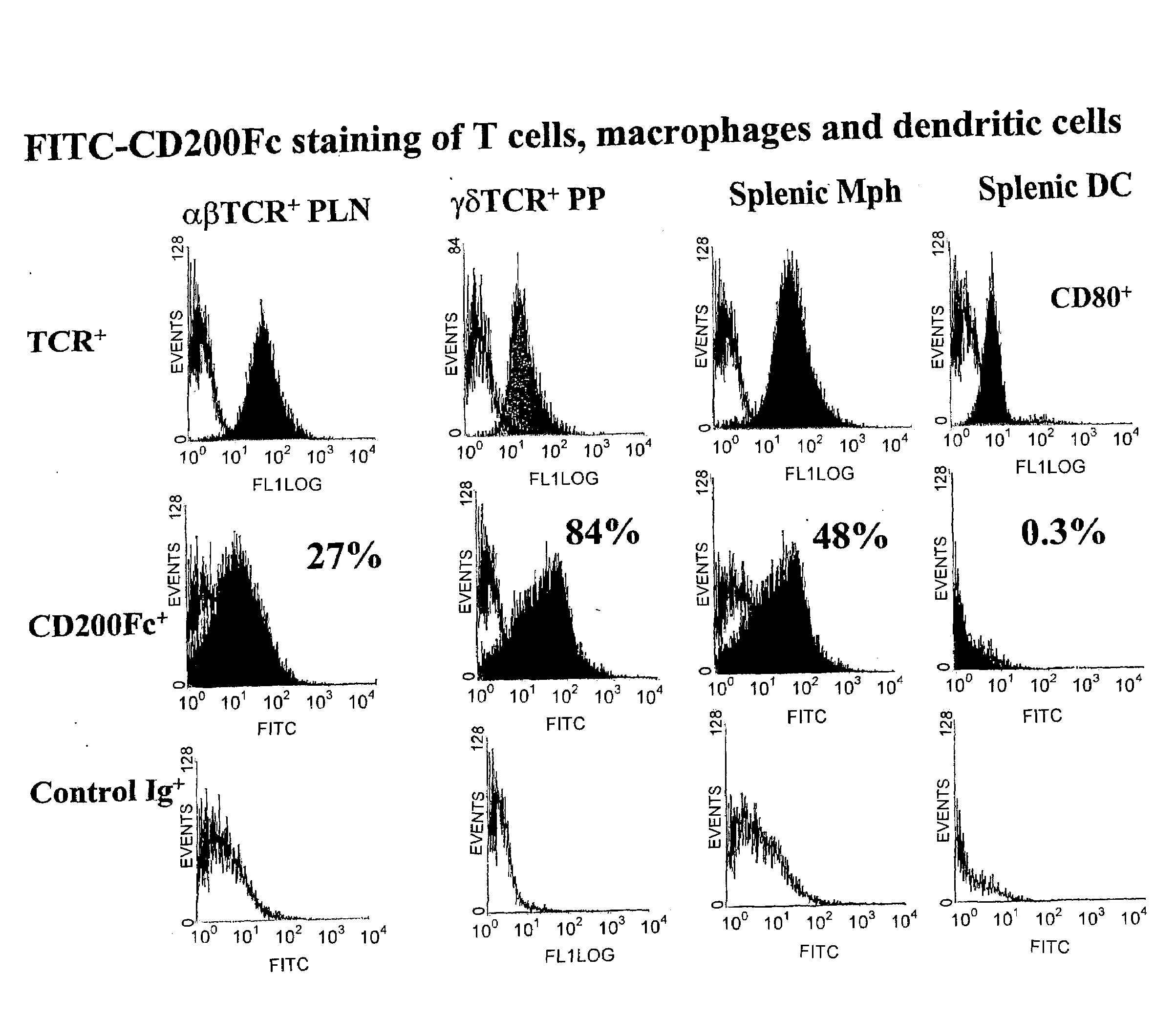
Modulation of CD200 receptors
InactiveUS20080267967A1Increasing immune suppressionSuppress immune responsePeptide/protein ingredientsAntibody mimetics/scaffoldsReceptor for activated C kinase 1Immune modulation
The present invention relates to CD200 receptor isoforms and modulators thereof and their use in methods of immune modulation and pharmaceutical compositions.
Owner:TRILLIUM THERAPEUTICS INC
Inhibitors of macrophage migration inhibitory factor and methods for identifying the same
InactiveUS20050282236A1Suppress immune responseNervous disorderAntipyreticMacrophage migration inhibitory factorDisease
Inhibitors of MIF are provided which have utility in the treatment of a variety of disorders, including the treatment of pathological conditions associated with MIF activity. The inhibitors of MIF have the following structures: including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof, wherein n, R1, R2, R3, R4, X, Y and Z are as defined herein. Compositions containing an inhibitor of MIF in combination with a pharmaceutically acceptable carrier are also provided, as well as methods for use of the same.
Owner:AVANIR PHARMA
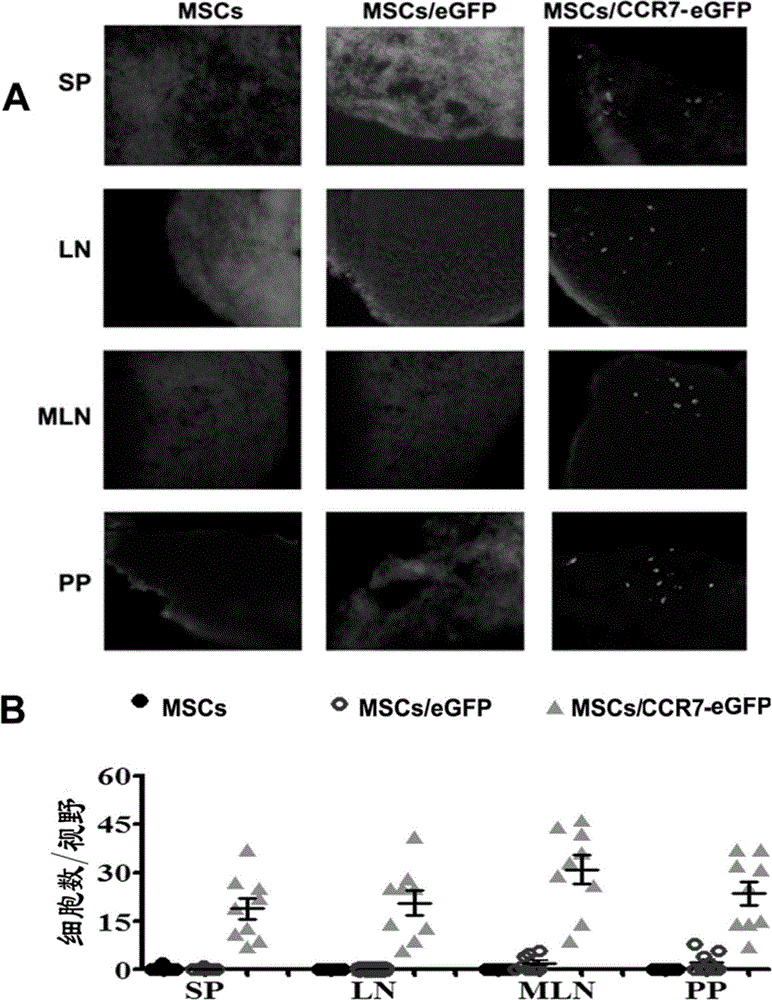
Use of recombinant mesenchymal stem cell in preparation of immunosuppressant
ActiveCN104800243ASuppress immune responseRetain anti-tumor abilityUnknown materialsOther foreign material introduction processesAbnormal tissue growthImmunologic disorders
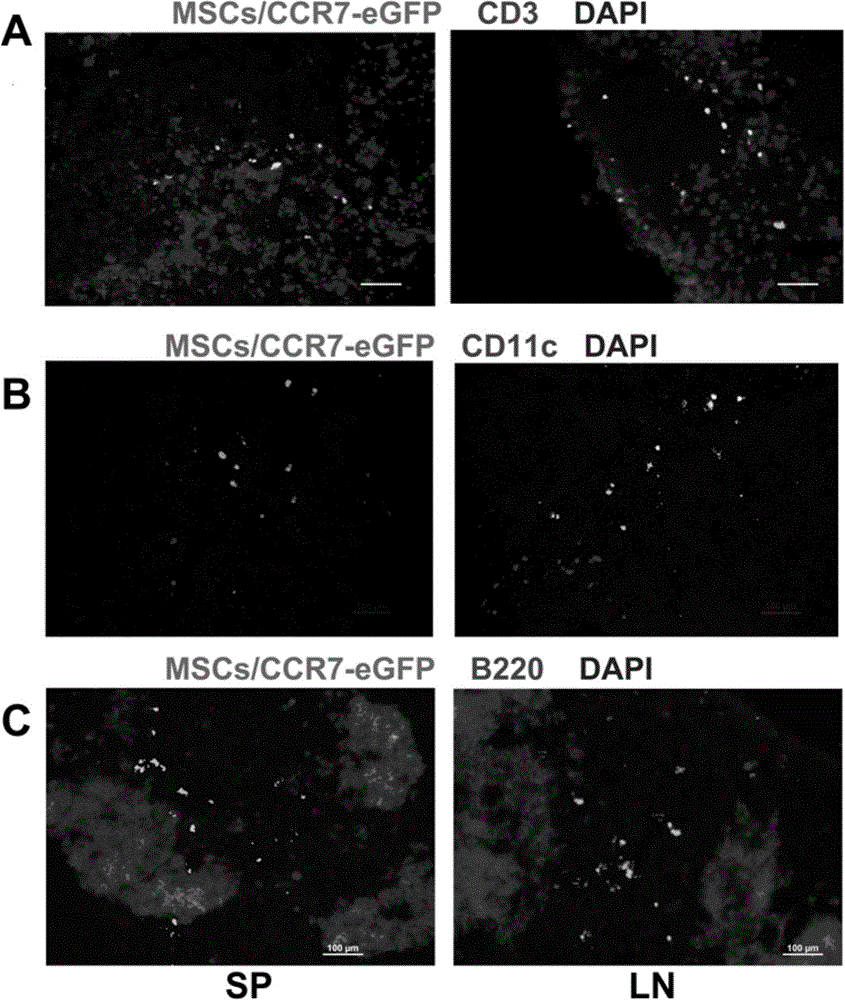
The invention discloses a use of a recombinant mesenchymal stem cell in preparation of an immunosuppressant. The invention relates to the recombinant mesenchymal stem cell capable of expressing a chemokine receptor CCR7 on a cytomembrane. After infusion, the recombinant mesenchymal stem cells can be specifically transferred to a secondary lymphatic organ, and a large amount of the recombinant mesenchymal stem cells are gathered in a T cell enrichment region in the secondary lymphatic organ and can efficiently inhibit T cell immunoreaction. Through use of the recombinant mesenchymal stem cell in treatment on graft-versus-host disease after bone marrow transplantation, immunological rejection after organ transplantation and autoimmune diseases, a cost is reduced, a cell use amount is reduced and side-effects are relieved. In treatment, the recombinant mesenchymal stem cell retains tumor cell killing effects and provides a high efficiency and beneficial novel approach for clinical treatment on graft-versus-host disease after bone marrow transplantation, immunological rejection after organ transplantation and autoimmune diseases of tumor high-risk groups.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Chinese medicine paste for curing osteoarthropathy and preparing method
InactiveCN1391943AGood Xin Wen DivergenceGood analgesic and anti-inflammatory effectAnthropod material medical ingredientsAerosol deliveryCotton clothBlood circulation
The Chinese medicine ointment for treating acute and chronic osteoarthropathy is prepared with Sichuan aconite root, wild aconite, cassia twig, achranthes root, dahurian angelica root and other over 30 kinds of Chinese medicinal materials and through soaking in bean oil, decoction, mixing, spreading to cotton cloth, folding to seal and other steps. It has the functions of warming meridian, expelling cold and wetness, promoting blood circulation to disperse blood clots, dredging merdian, etc. and has obvious curative effect on acute and chronic rheumatoid arthritis.
Owner:郭好建
Method of constructing antibody
InactiveUS20050222391A1Easy to getEfficient productionPeptide/protein ingredientsVirus peptidesImmune toleranceTarget antigen
This invention provides methods for producing antibodies, wherein the methods comprise the step of administering an immunogen comprising both a target antigen and a background antigen to transgenic animals, into which a gene coding for the background antigen has been introduced. Since immunotolerance to the background antigens have thus been induced in the transgenic animals, the animals efficiently produce antibodies to target antigens.
Owner:CHUGAI PHARMA CO LTD
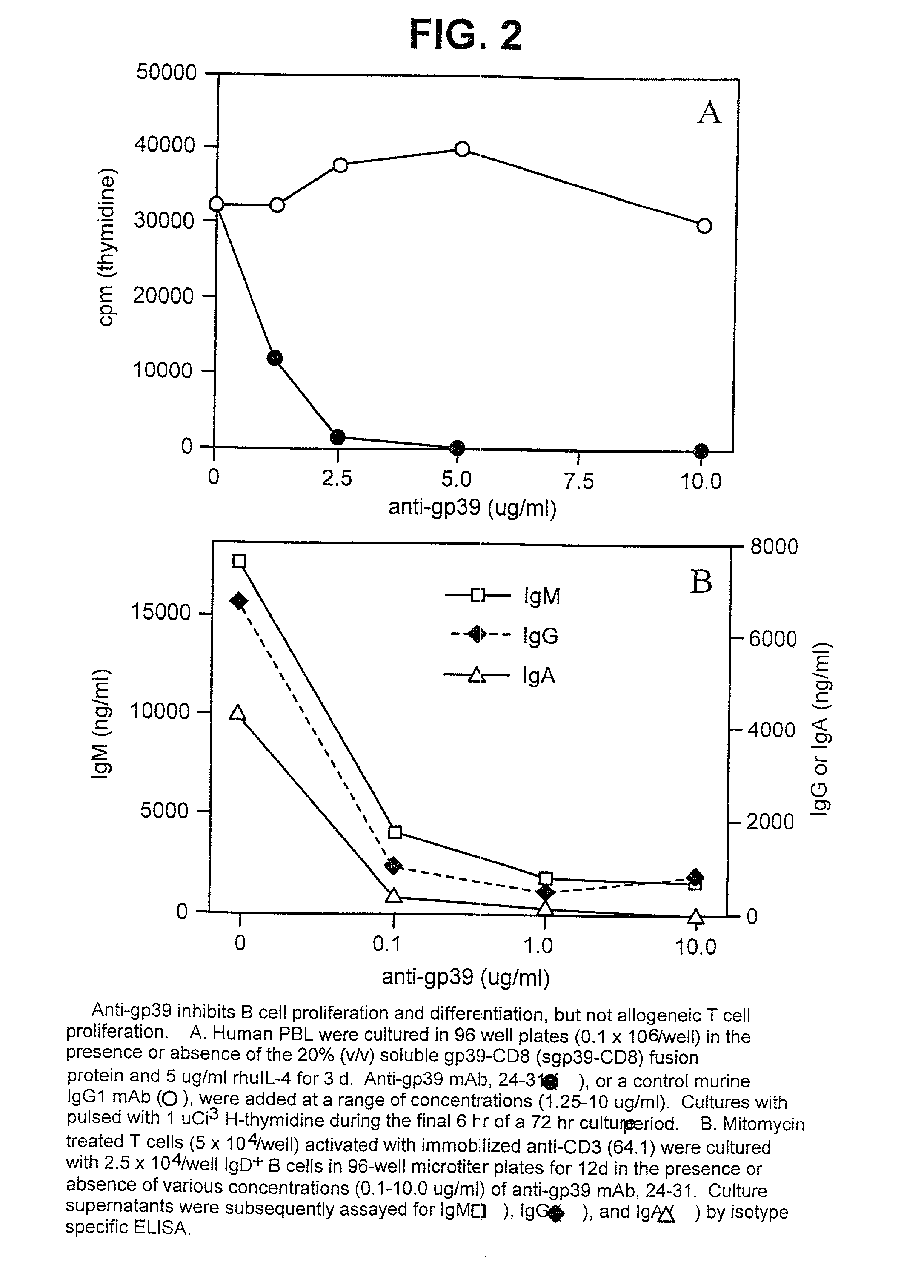
Non-agonistic antibodies to human gp39, compositions containing, and therapeutic use thereof
InactiveUS20030012781A1Suppress immune responseSimple methodBiocideNervous disorderDiseaseActivation cells
The present invention is directed to antibodies which bind human gp39, are antagonistic of the CD40 / CD40L interaction, but are non-agonistic of T-cell activation. The present invention is further directed to the use of these antibodies as therapeutic agents. These antibodies are especially useful for treatment of autoimmune diseases; and an immunosuppressant during transplantation of heterologous cells, tissues or organs, cell therapy, and gene therapy.
Owner:BIOGEN INC
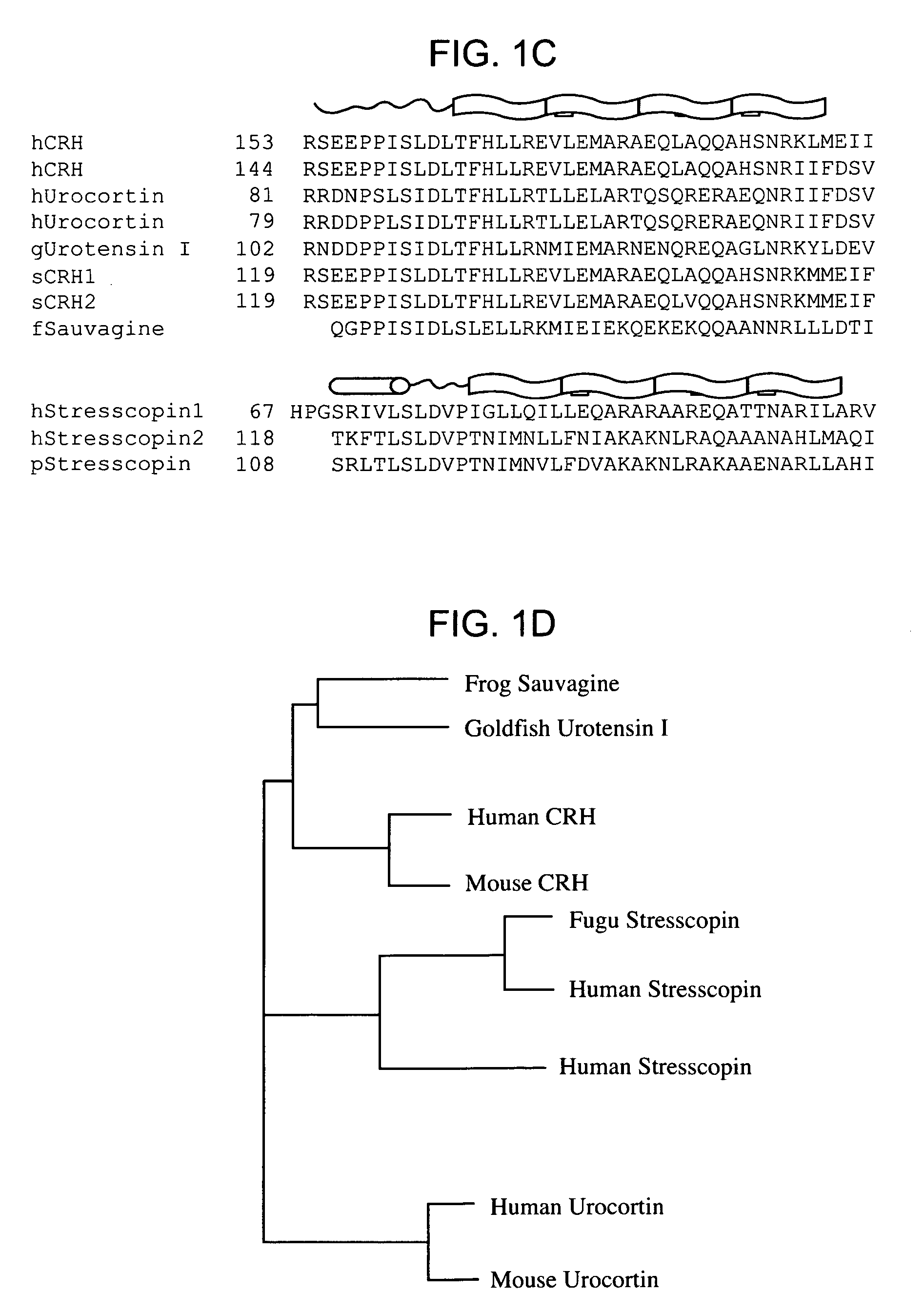
Stresscopins and their uses
InactiveUS7291341B2Suppress food intakeSuppress heat-induced edemaCompound screeningNervous disorderHypotensive agentsHeat induced
The invention provides novel nucleic acids and polypeptides, referred to herein as stresscopin 1 and stresscopin 2, which preferentially activate the CRH-R2 receptor over the R1 receptor. Stresscopins, analogs and mimetics, and related CRH-R2 agonists suppress food intake and heat-induced edema; but do not induce substantial release of ACTH. Stresscopin also finds use in the recovery phase of stress responses, as an anti-inflammatory agent, as a hypotensive agent, as a cardioprotective agent, and in the treatment of psychiatric and anxiolytic disorders. Stresscopin nucleic acid compositions find use in identifying homologous or related proteins and the DNA sequences encoding such proteins; in producing compositions that modulate the expression or function of the protein; and in studying associated physiological pathways.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
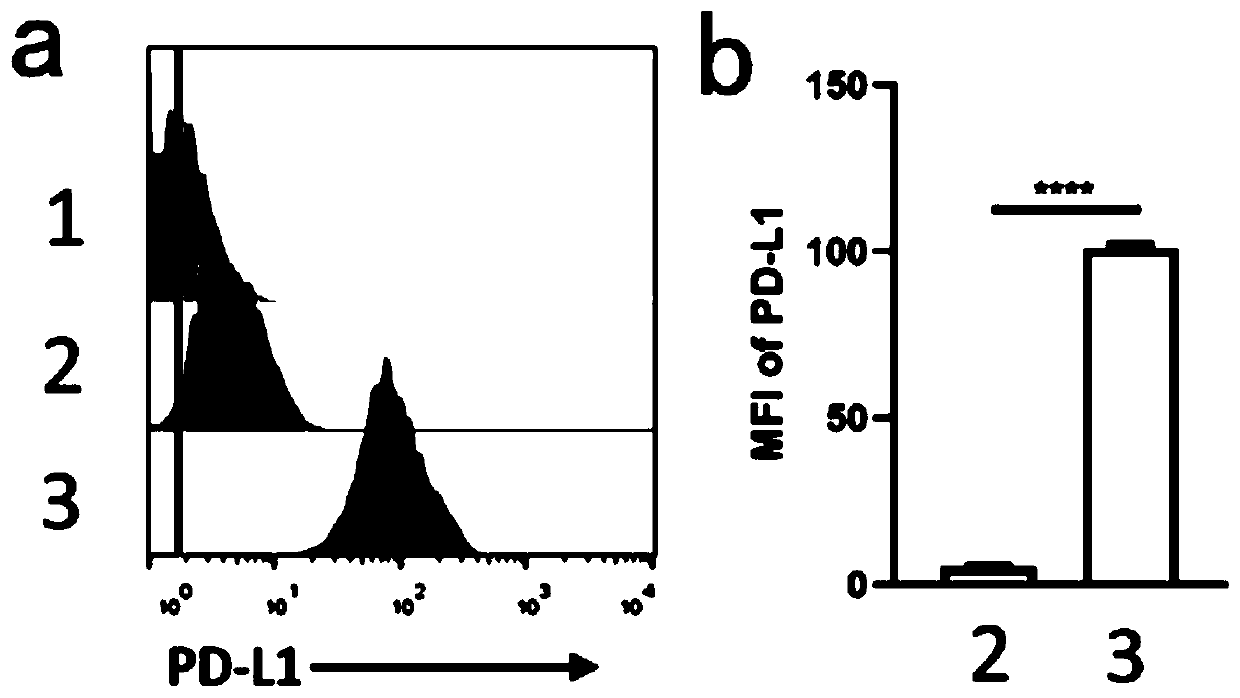
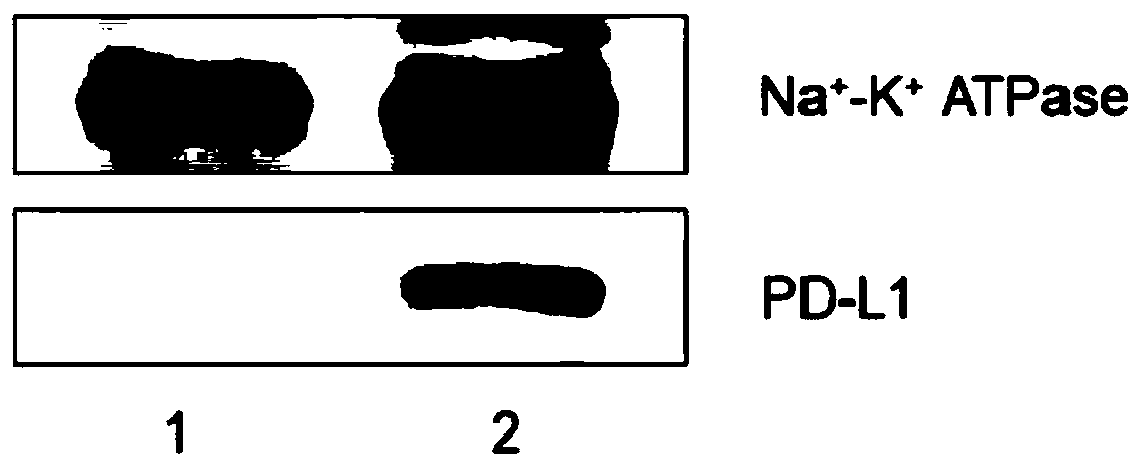
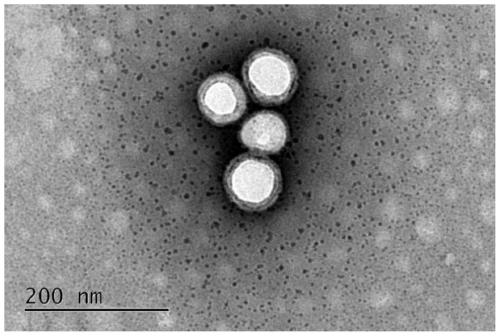
Mesenchymal stem cell membrane-coated bionic nanoparticles with overexpressed PD-L1 molecules on surface as well as preparation and application of mesenchymal stem cell membrane-coated bionic nanoparticles
ActiveCN111265549AThe preparation process is simple and matureHigh biosecurityAntibacterial agentsAntimycoticsCell membraneImmunity response
The invention relates to mesenchymal stem cell membrane-coated bionic nanoparticles with overexpressed PD-L1 molecules on the surface as well as preparation and application of the mesenchymal stem cell membrane-coated bionic nanoparticles. The mesenchymal stem cell membrane-coated bionic nanoparticle with the overexpressed PD-L1 molecules on the surface comprises a nano-core and a mesenchymal stemcell membrane wrapping the nano-core, the nano core comprises a polymer with biocompatibility, and PD-L1 molecules are overexpressed on the membrane surface of the mesenchymal stem cell membrane. Theinvention also discloses an application of the mesenchymal stem cell membrane-coated bionic nano-particle with over-expressed PD-L1 molecules on the surface in preparation of an inflammation treatment preparation. The bionic nanoparticle has a remarkable immunosuppression effect, is high in biocompatibility and simple and mature in preparation process, can be used for preparing an inflammation treatment preparation, can be effectively enriched in an inflammation part, and shows an excellent curative effect in inflammation treatment by inhibiting excessively activated immune response and cytokine storm of the inflammation part.
Owner:SUZHOU UNIV
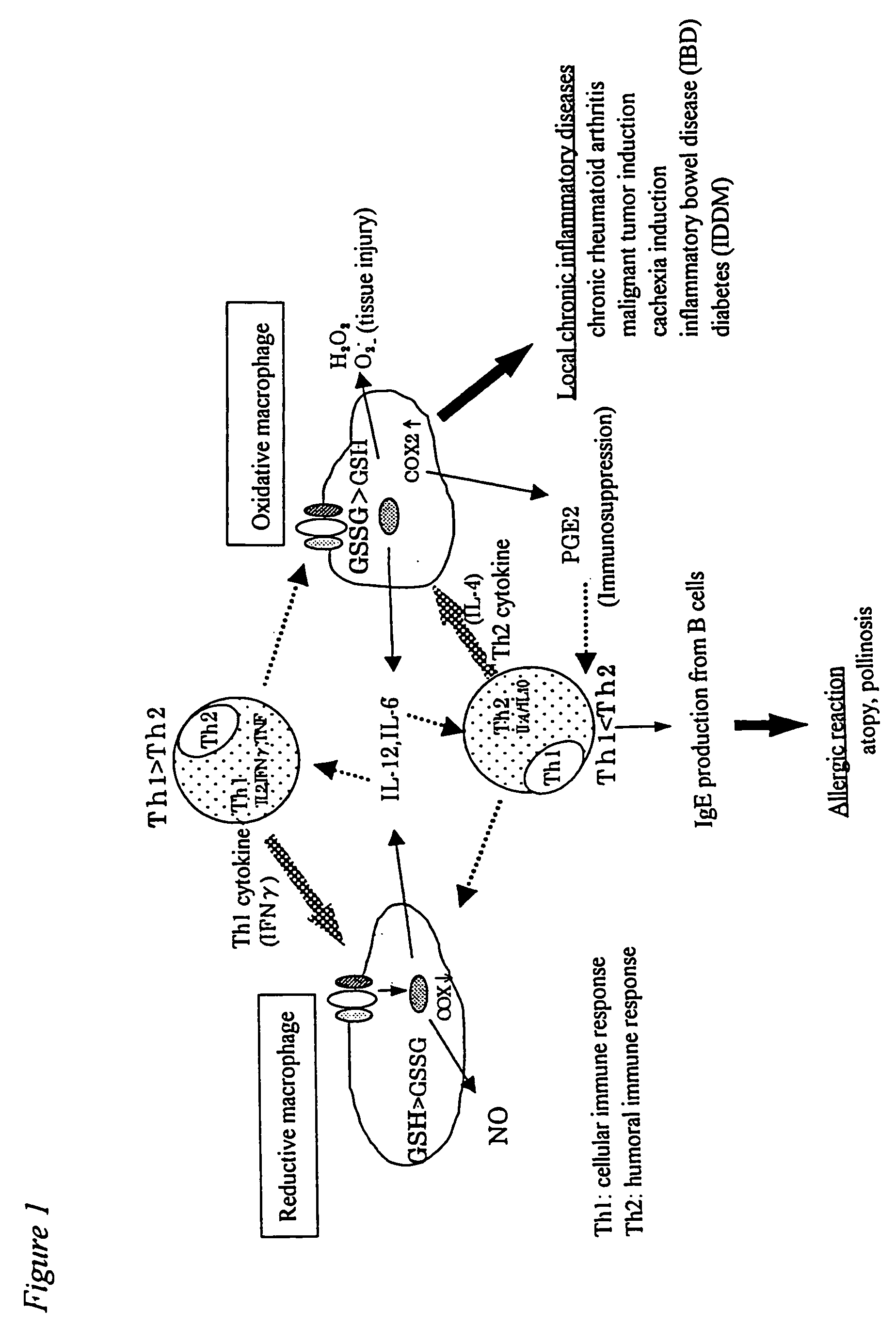
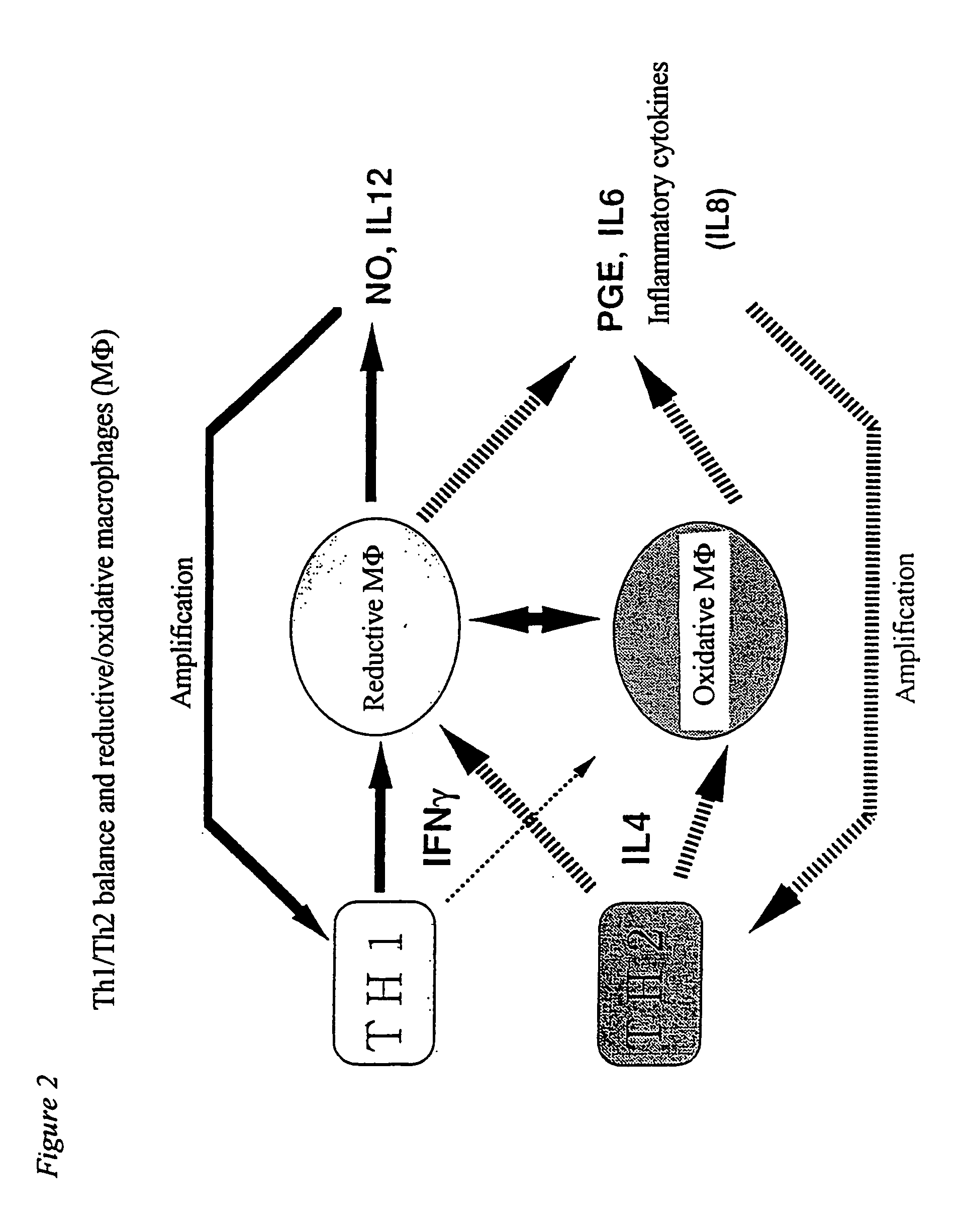
Method of suppressing immune response by reducing intracellular content of glutathione in macrophages and monocytes
InactiveUS20050239886A1Suppress immune responseReduce contentBiocideDipeptide ingredientsIntracellularMonocyte
Owner:AJINOMOTO CO INC
Traditional Chinese medicine for treating rheumatism and hyperosteogeny
InactiveCN101120982ASmooth bloodInhibit and reduce biological activityHeavy metal active ingredientsAnthropod material medical ingredientsSalvia miltiorrhizaRheumatism
The present invention relates to the field of traditional Chinese medicine techniques, which concretely relates to a Chinese medicine used for treating Rheumatism and hyperosteogeny. The present invention provides the Chinese medicine used for treating Rheumatism and hyperosteogeny, in order to overcome the problems in existing technology such as long period of treatment and imperfect results. The present invention comprises the nux vomica plant, the ground beetle insect, the earthworm, the dried whole scorpion, the seahorse, the Sichuan Niu Xi, the pangolin, the zaocys dhumnades, the centipede, the salvia miltiorrhiza, the musk, the Spanish red, the blood of dragon, the saffron crocus, the ledeboruiella root, the earth insect, the medicine, the frankincense 10-20g, crab bone, herb of garden balsam, the Chinese flowering quince, the root of herbaceous peony and so on. The present invention has accurately curative and remarkable effect on each kind of rheumatism, ossein proliferation illness, with non-toxic negative impact. The treatment effects are not only on general type rheumatism, ossein proliferation illness but also on various difficulties uniquely.
Owner:李金山
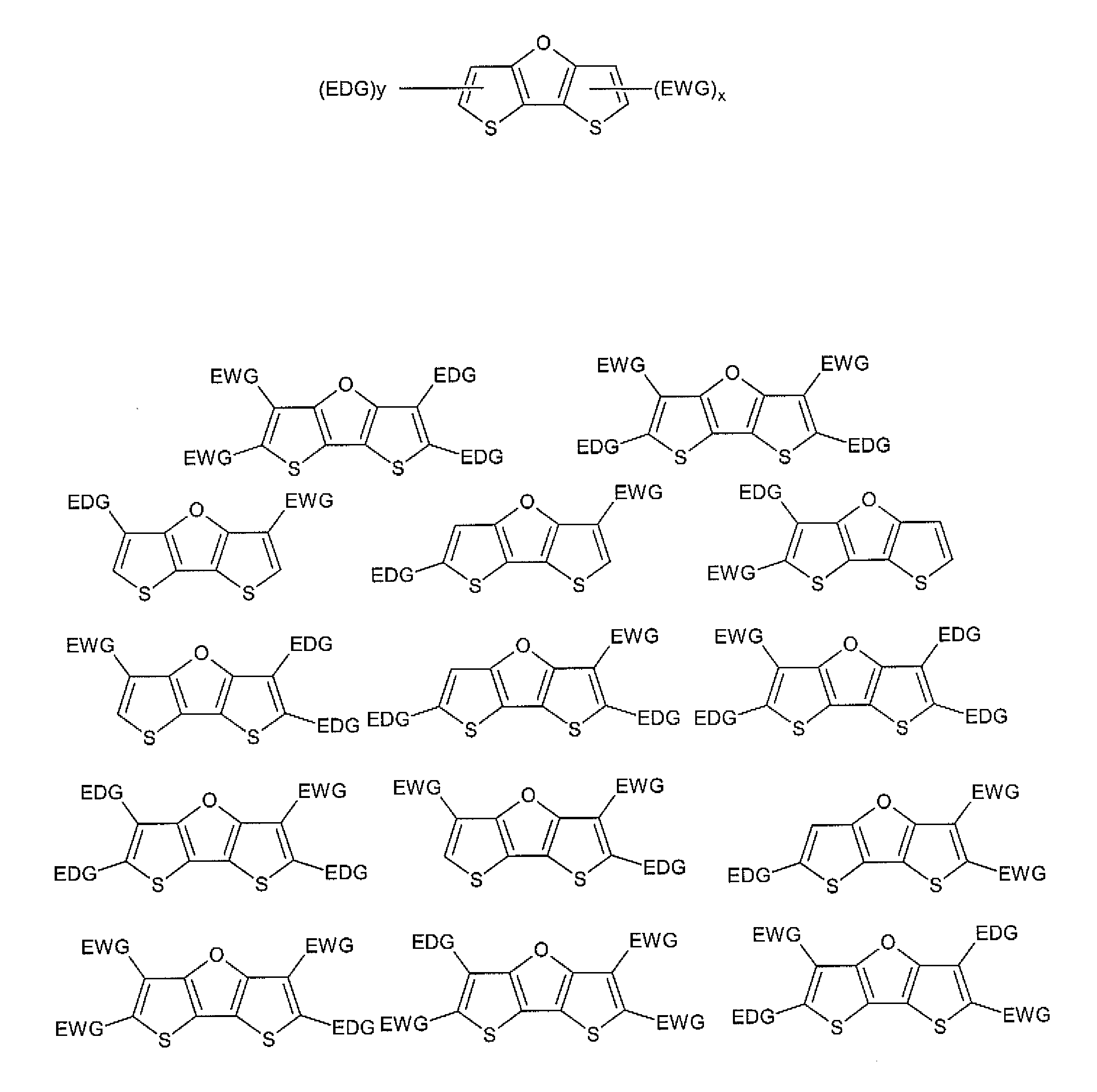
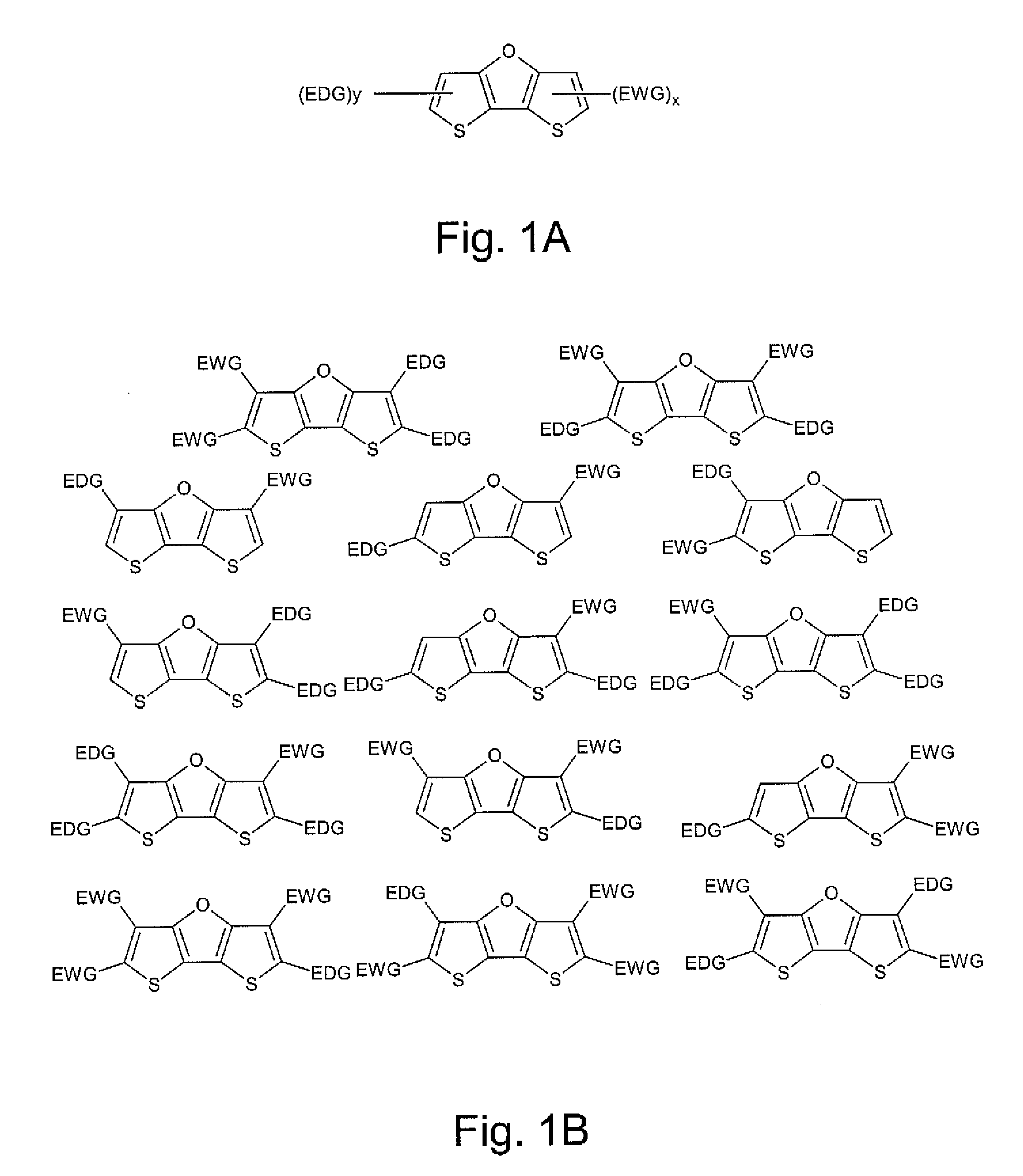
Dithienofuran Dyes for Imaging and Therapy
InactiveUS20110177006A1Convenient treatmentGood biocompatibilityUltrasonic/sonic/infrasonic diagnosticsMonoazo dyesPhysical propertyPhotochemistry
Owner:MALLINCKRODT INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com