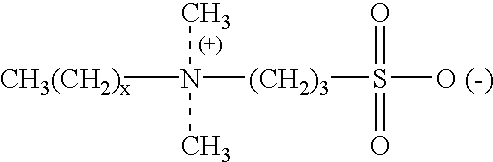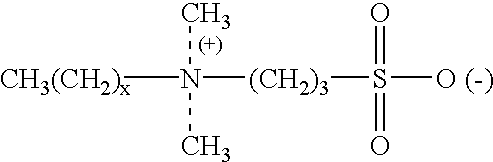Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
80 results about "Radioimmunoassay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A radioimmunoassay (RIA) is an immunoassay that uses radiolabeled molecules in a stepwise formation of immune complexes. A RIA is a very sensitive in vitro assay technique used to measure concentrations of substances, usually measuring antigen concentrations (for example, hormone levels in blood) by use of antibodies.
Methods and reagents to detect and characterize norwalk and related viruses
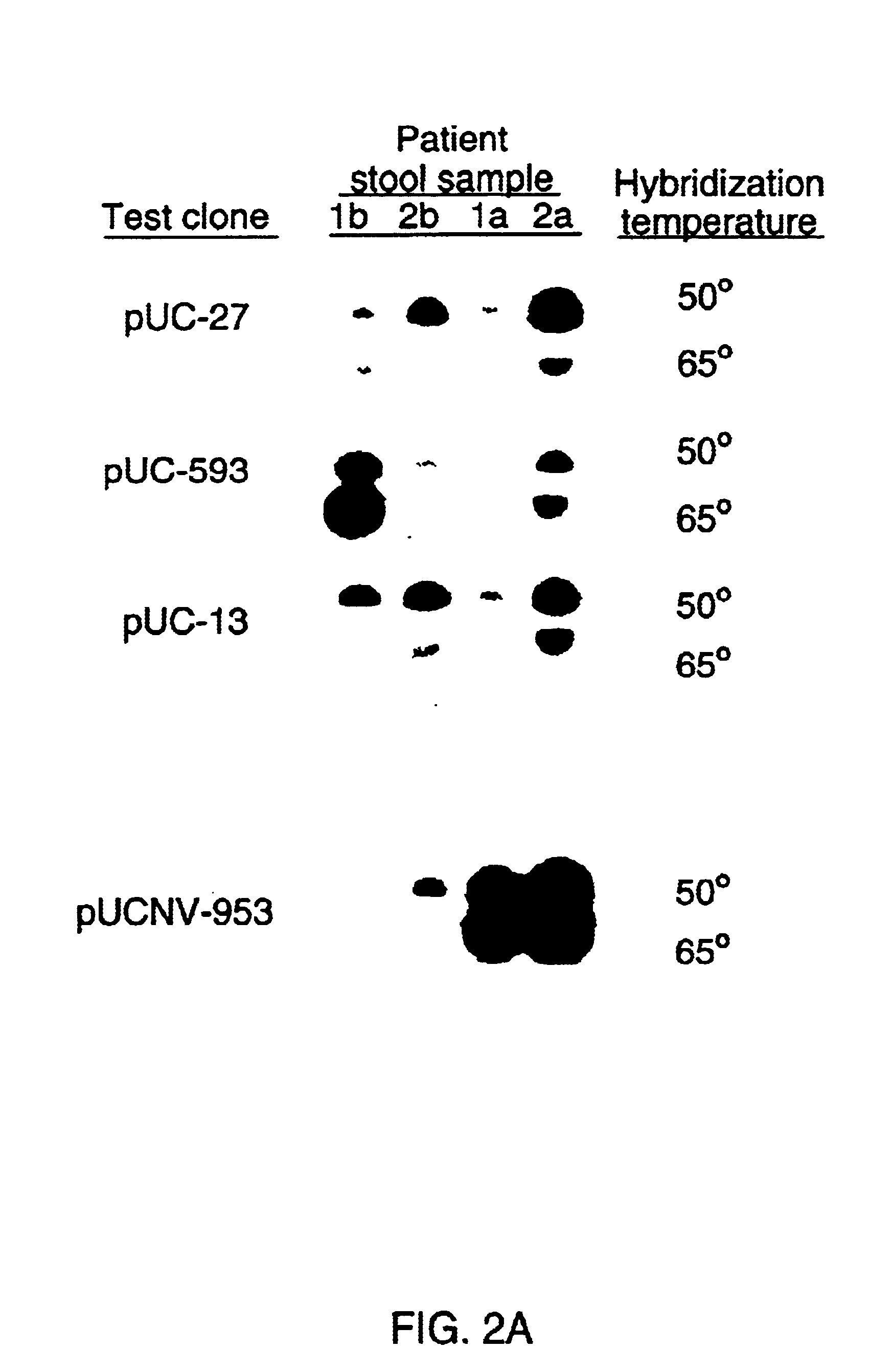
Double-stranded cDNA was synthesized from nucleic acid extracted from Norwalk virus purified from stool specimens of volunteers. One clone was isolated from a cDNA library constructed in a pUC-13 vector after amplification of the cDNA. The specificity of this cDNA (pUCNV-953) was shown by hybridization assays. The cDNA reacted with post (but not pre-) infection stool samples from Norwalk volunteers and with highly purified Norwalk virus, but not with other common enteric viruses such as hepatitis A virus and rotavirus. Finally, the probe detected virus in the same fractions of CsCl gradients in which viral antigen was detected using a specific Norwalk virus radioimmunoassay, and particles were detected by immune electron microscopy. Single-stranded RNA probes derived from the DNA clone after subcloning into an in vitro transcription vector were also used to show that the Norwalk virus contains a ssRNA genome of about 8 kb in size. The original clone was also used to detect additional cDNAs which represent at least 7 kb of nucleic acid of the Norwalk genome. The availability of a Norwalk-specific cDNA and the first partial genome sequence information allow rapid cloning of the entire genome and of establishment of sensitive diagnostic assays. Such assays can be based on detection of Norwalk virus nucleic acid or Norwalk viral antigen using polyclonal or monoclonal antibodies to proteins expressed from the cDNA or to synthetic peptides made based on the knowledge of the genome sequence. Assays using proteins deduced from the Norwlk virus genome and produced in expression systmes can measure antibody responses. Vaccines made by recombinant DNA technology are now feasible.
Owner:BAYLOR COLLEGE OF MEDICINE
Plant drug for preventing cancer II
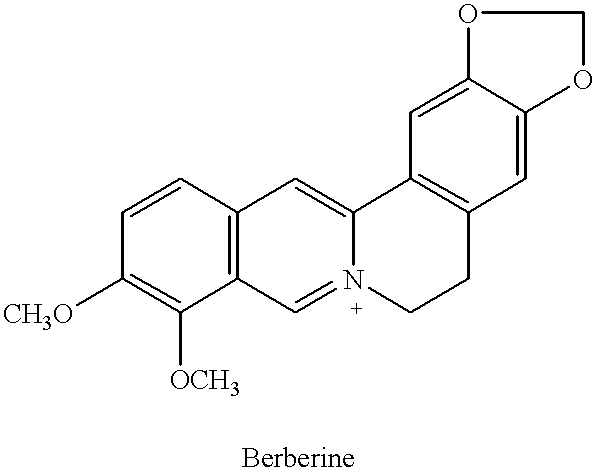
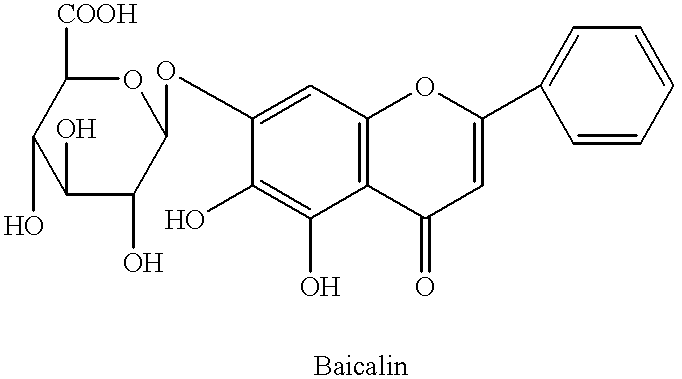
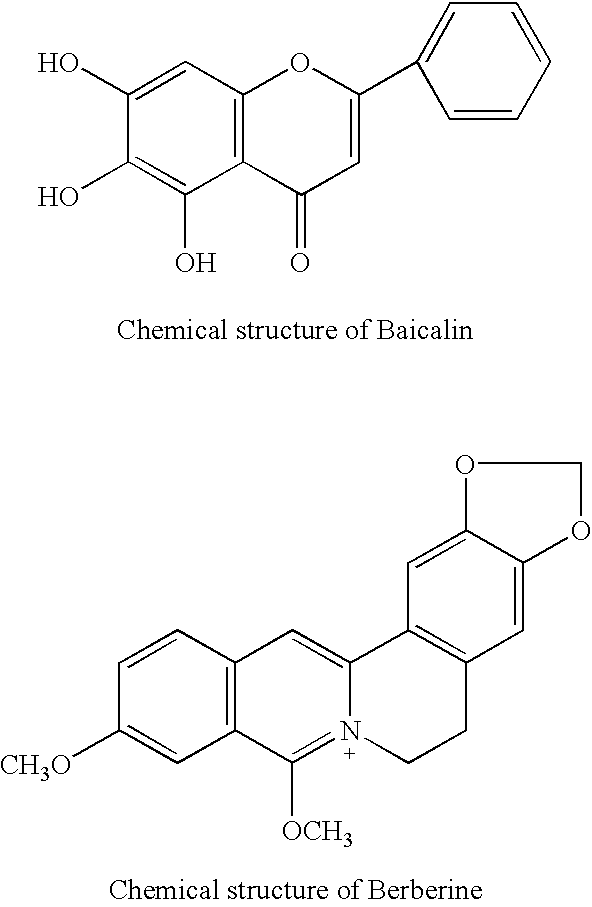
InactiveUS6290995B1Efficient analysisAccurate measurementBiocideUnknown materialsRadioimmunoassayBerberine
This invention relates to new safe natural drug, which is prevention of cancer and control of cancer cells. Specifically, this invention provides methods for producing of Berberine and Baicalin.Also, the present invention proved a new radioimmunoassay (RIA) method for precise determination of Berberine and Baicalin. The RIA is an efficient analytical method for large clinical programs including double blind analysis (DBA), and good clinical practice (GCP).
Owner:XINXIAN ZHAO
Hair analysis method
InactiveUS6949344B1Reliable solubilizationAccurate methodPre-tanning chemical treatmentMicrobiological testing/measurementBetaineDigestion
A method the direct analysis of an analyte in keratinized structures, e.g., hair and fingernails, which comprises preparing a mixture containing a low redox potential activator compound such as dithiothreitol or dithioerythritol, an enzyme suitable for the digestion of the keratin structure, a sample of the keratin structure and a biological detergent that aids the digestion of the keratinized structure at a relatively low pH, e.g., between about 6.2 and 8; permitting the enzyme to at least substantially digest the sample of keratin structure, and subjecting the digest solution to analysis, preferably by radioimmunoassay, to determine the identity and amount of analyte in the keratin structure sample. To accelerate the method, cupric sulfate may be added to the mixture after degradation of the keratin sample. The enzyme may be a peptidase, endopeptidase or proteinase, with papain, chymopapain, and proteinase K being preferred for use in the invention. The preferred biological detergents include betaine, sulfo-betaine, alkylglucosides and bile acids.
Owner:PSYCHEMEDICS CORPORATION
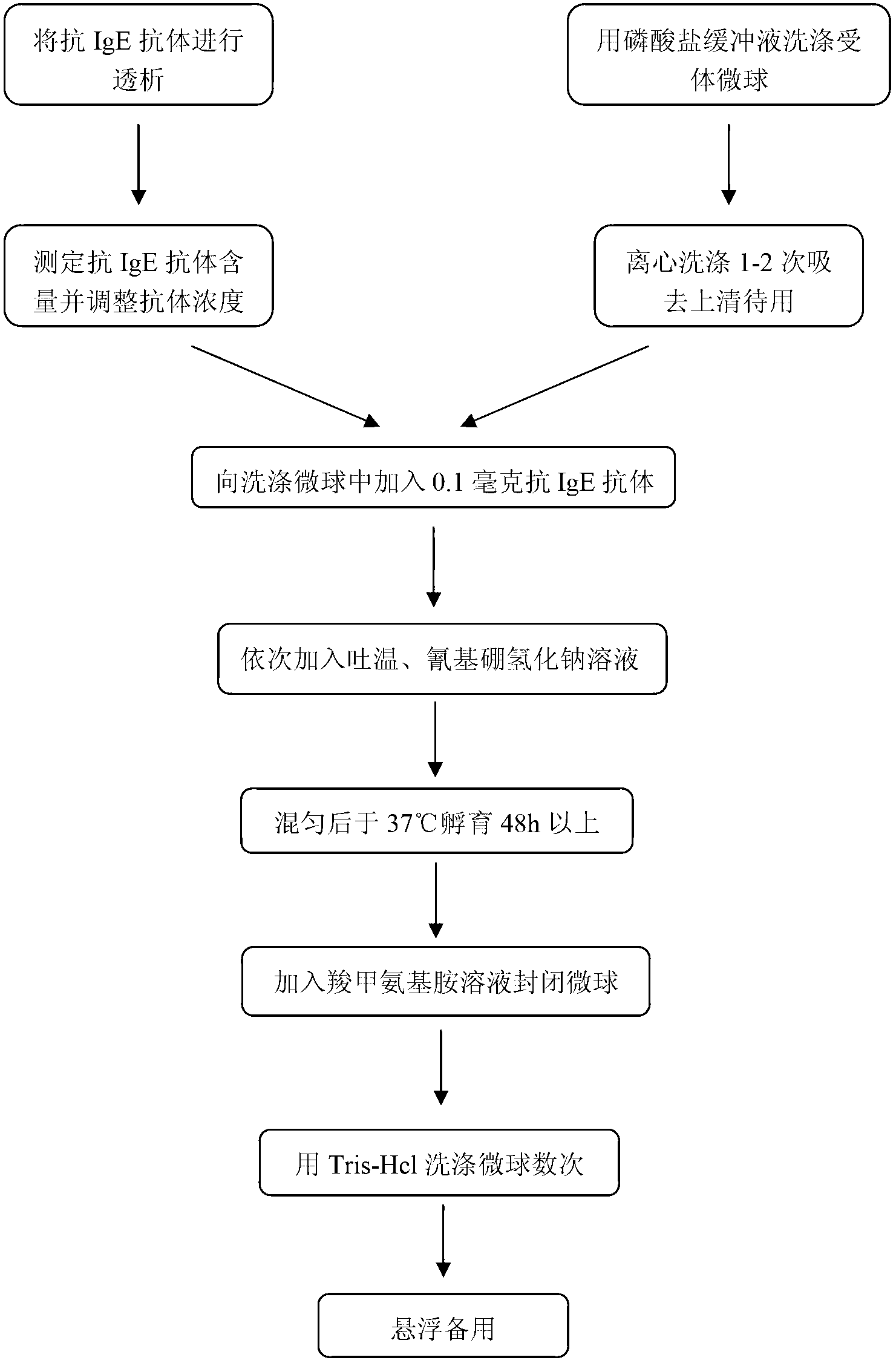
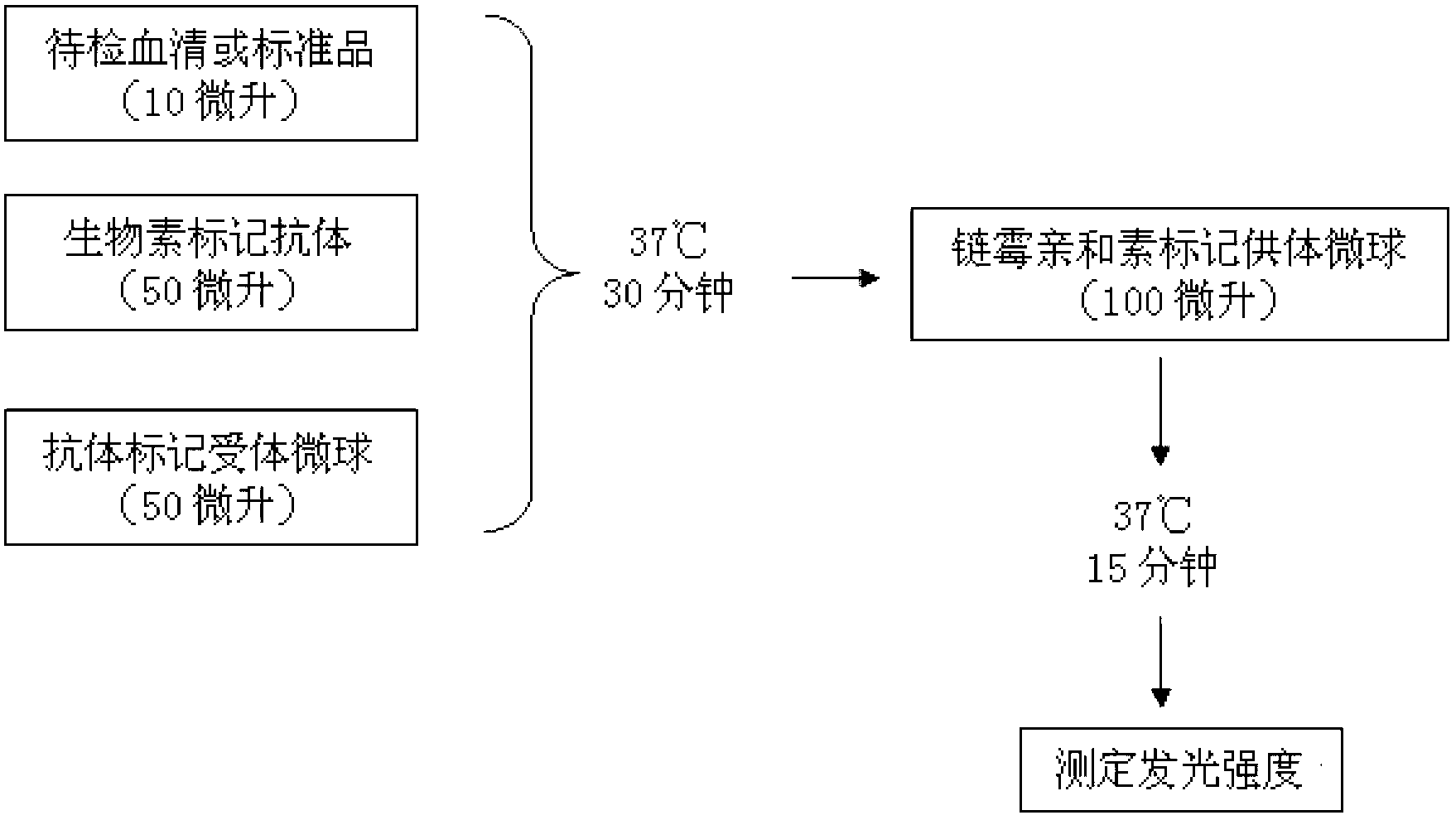
Diagnostic kit for determination of serum total IgE, preparation method and application method
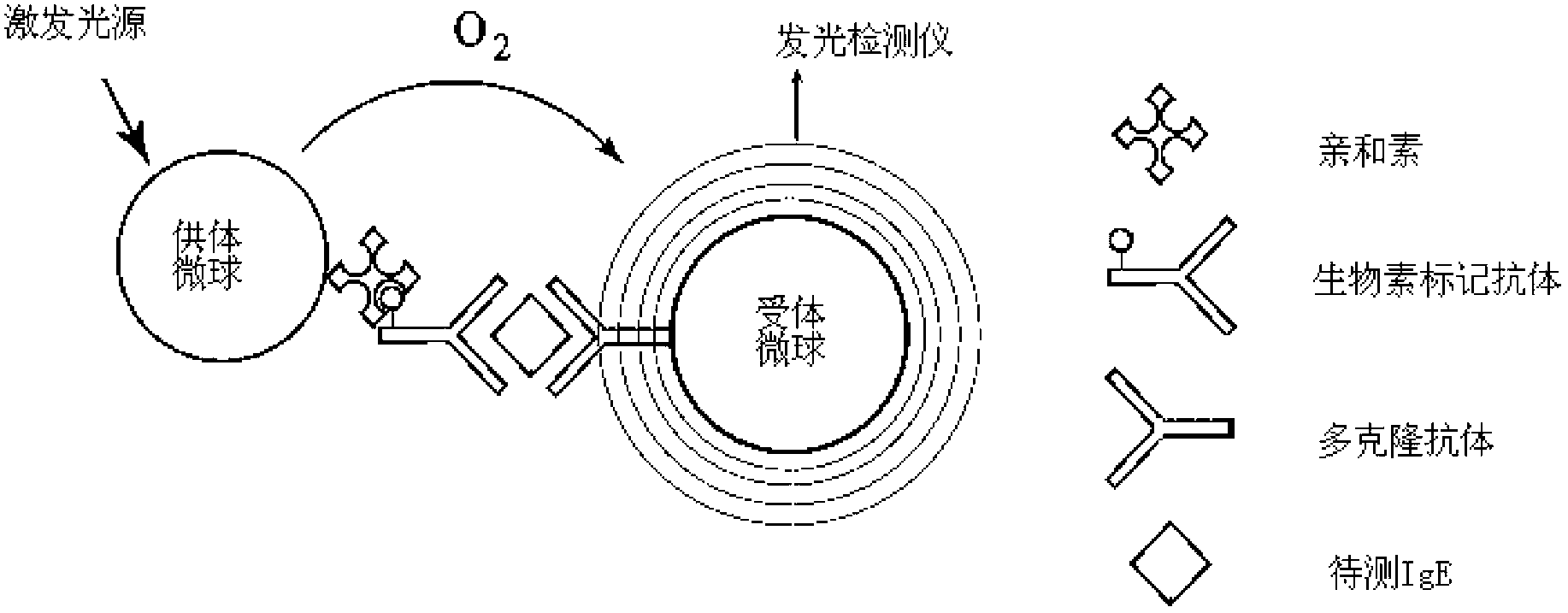
InactiveCN102798725AShorten detection timeReduce biasBiological testingFluorescence/phosphorescenceBiotin-streptavidin complexMicrosphere
The invention provides a diagnostic kit for determination of serum total IgE, a preparation method and an application method. The kit comprises an IgE standard substance, a biotin labeled rate anti-human IgE monoclonal antibody solution, a rabbit anti-human IgE polyclonal antibody coated acceptor microspheres solution and a streptavidin donor microspheres solution. The kit provided by the present invention solves a tedious washing step of current heterogeneous immunoassay kits, overcomes the defects of environmental pollution caused by radioimmunoassay and short shelf life, and overcomes the defects of poor repeatability of enzyme immunoassay analysis and easy hook effect generation, and the detection precision is higher than that of immuno-turbidimetric analysis.
Owner:天津中企华科生物科技发展有限公司
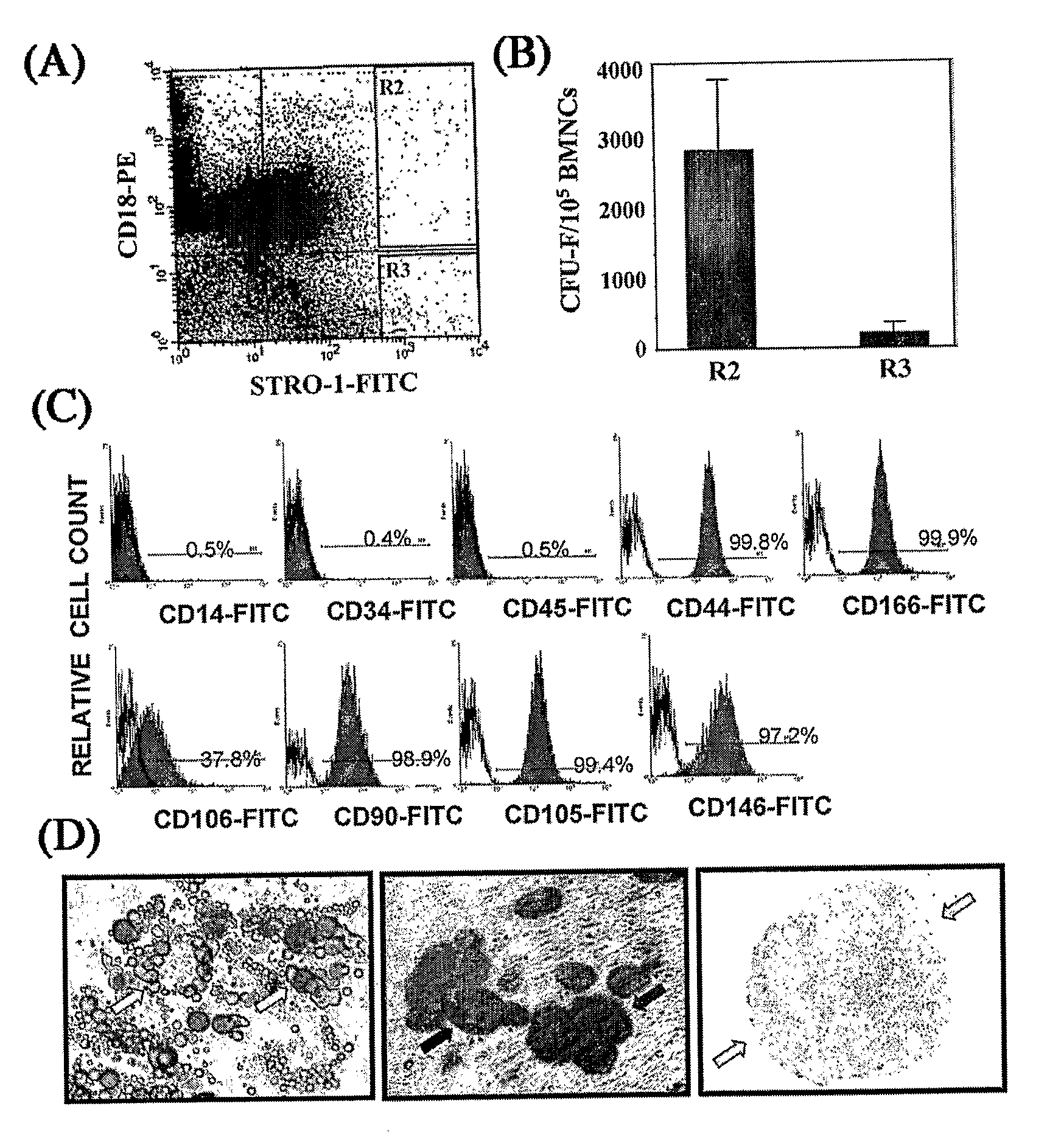
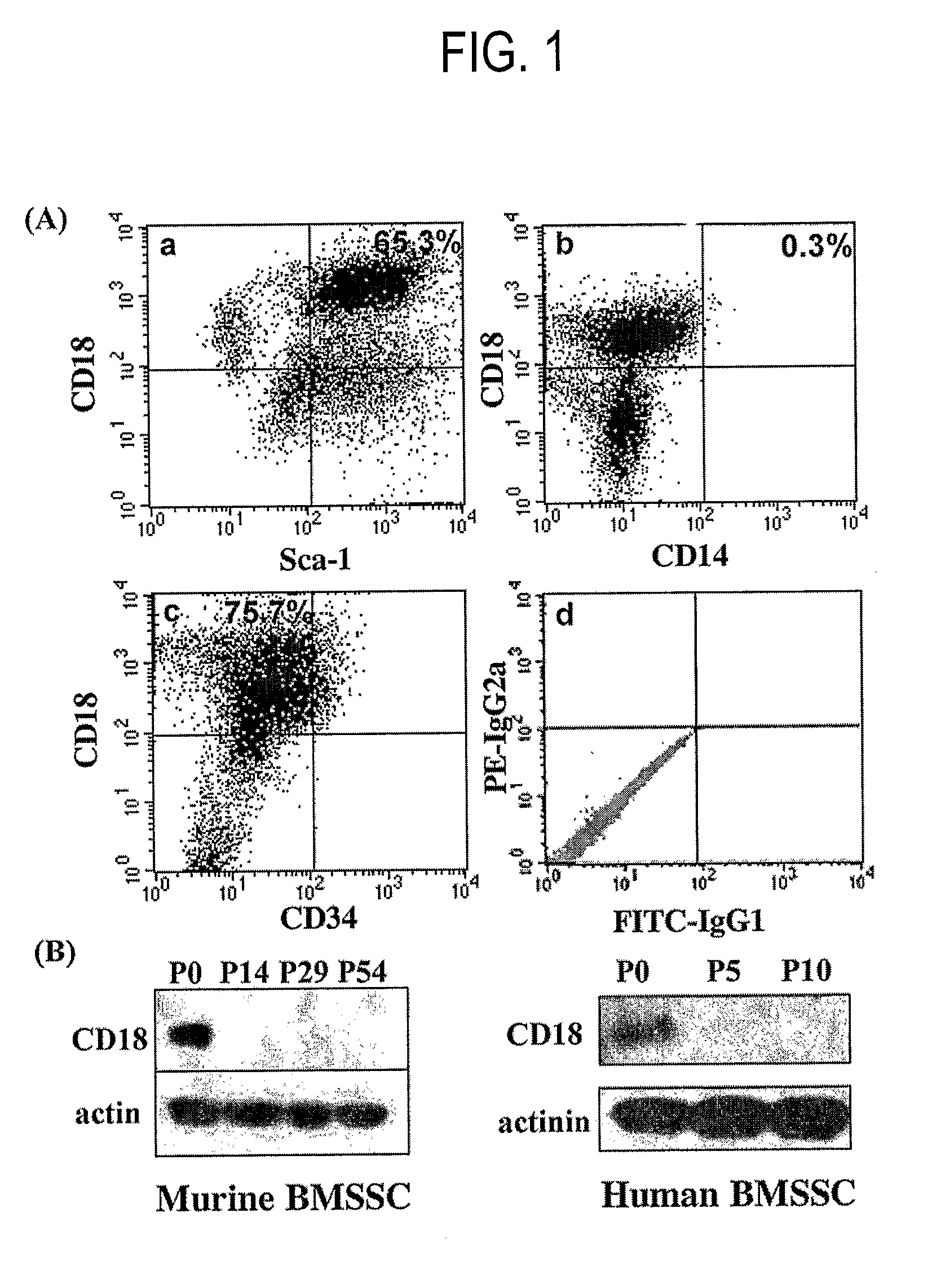
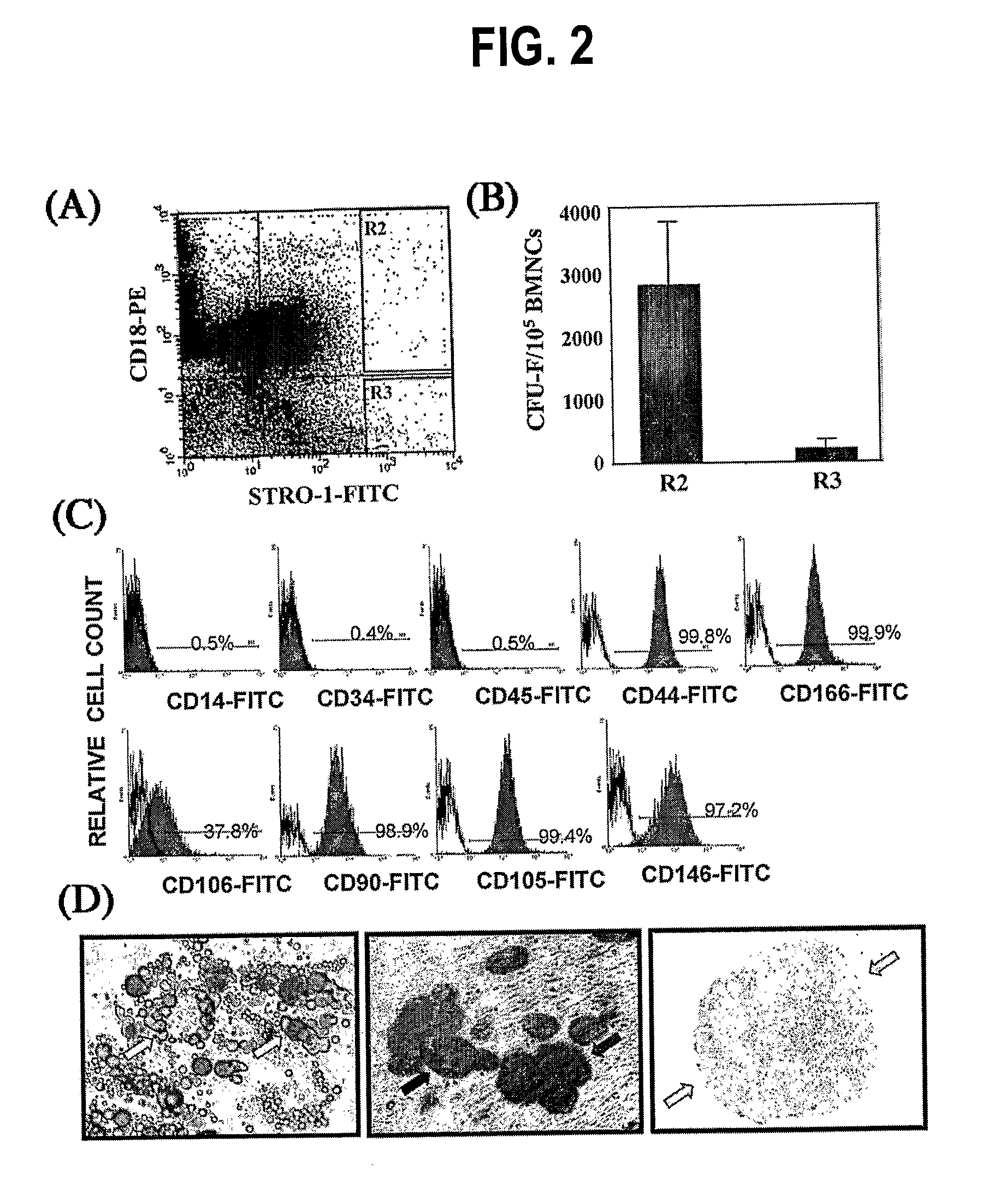
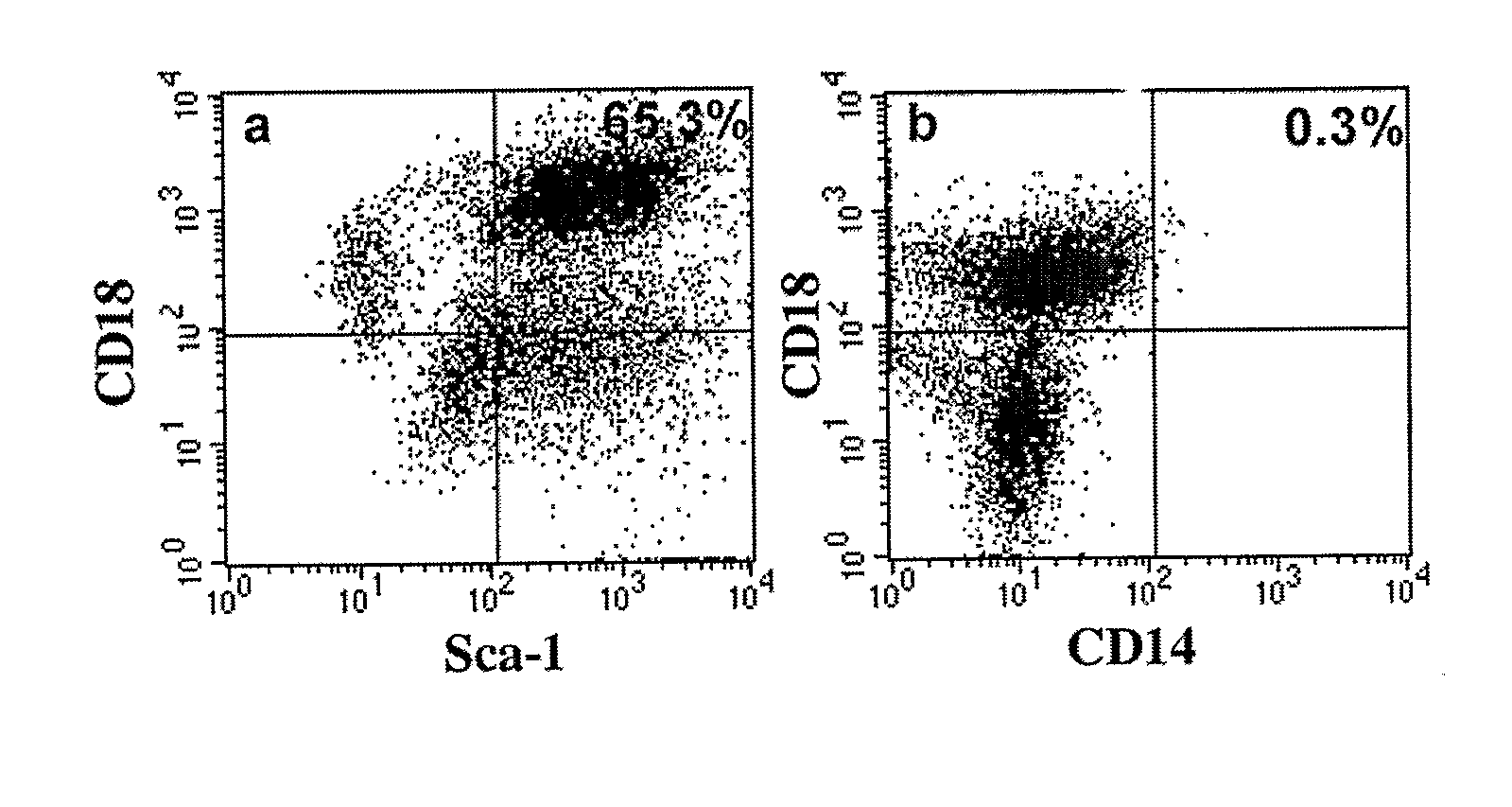
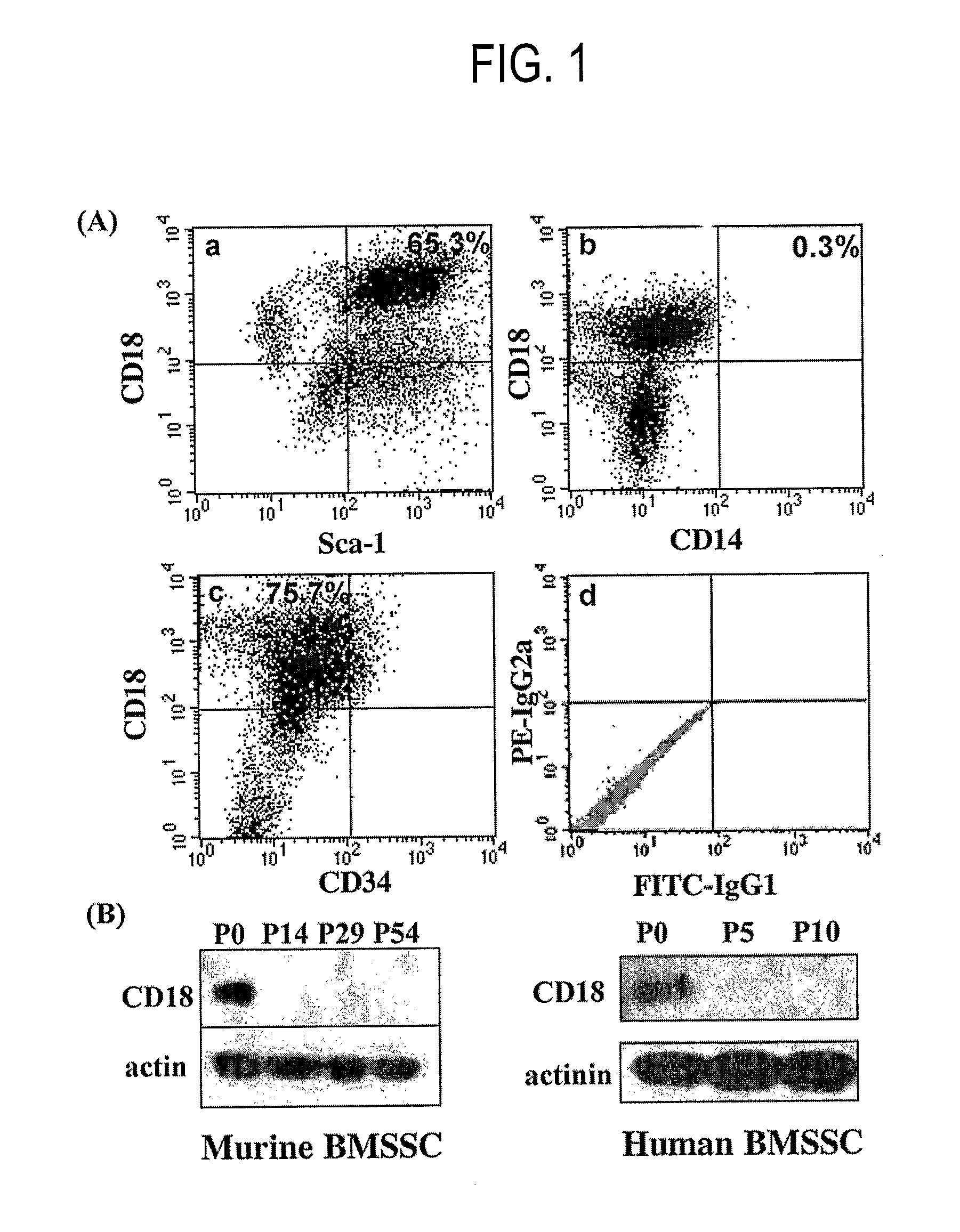
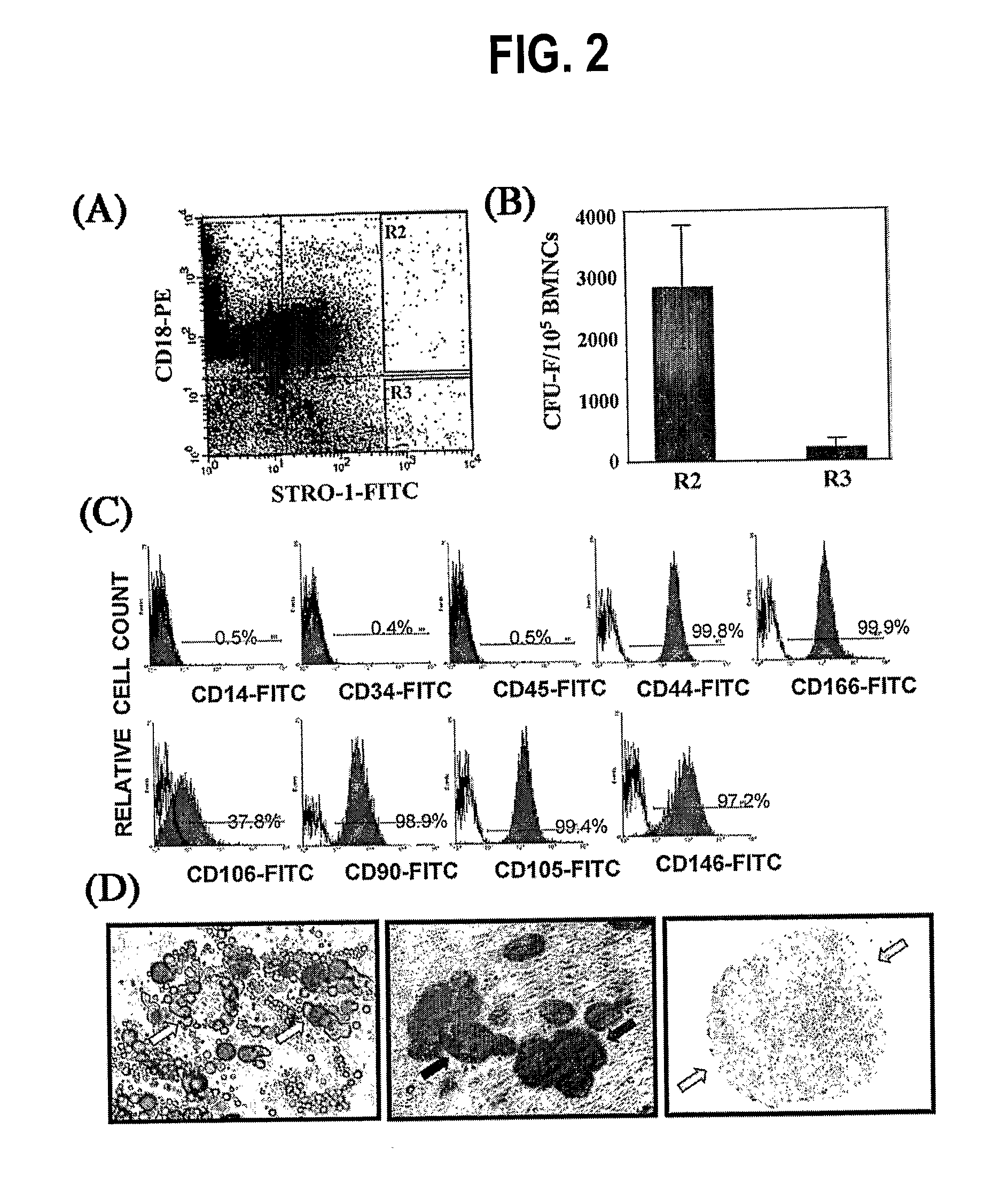
Integrin CD18 is a novel stromal stem cell marker and functions to promote osteogenesis
InactiveUS7732126B2Bioreactor/fermenter combinationsBiological substance pretreatmentsImmunoperoxidase StainingCancer research
The present invention is directed to a new bone marrow stromal stem cell (BMSSC) marker, CD18, for use in selecting a population of cells enriched in BMSSCs, from bone marrow cells, adipose cells, or peripheral blood. The invention is further directed to methods for selecting a population of cells enriched in BMSSCs based on the selective expression of CD18 on their surface, using techniques known in the art such as fluorescent assisted cell sorting, an immunomagnetic method, flow microfluorimetry, immunofluorescence, immunoperoxidase staining, radioimmunoassay and immunoaffinity chromatography. The invention is further directed to the BMSSCs isolated based on CD18 expression, and their use to treat various diseases. In one aspect, the HMSSCs are transformed with a vector having a normal gene for CD18, and the transformed BMSSCs are administered to treat bone degenerative diseases and diseases of bone involving abnormal expression of CD18 expression of CD18.
Owner:UNITED STATES OF AMERICA +1
One-step chemiluminiscence quantitative detection kit for hyaluronic acid
The invention relates to a chemiluminiscence kit for detecting content of hyaluronic acid in human serum, body fluid or tissue homogenate by one-step process, and belongs to the medical field of immunoassay. By solid coating and indirect enzyme labeling technology, the problem that a hyaluronic acid kit is unavailable for one-step detection is solved substantially, operation is more convenient, detection is more accurate, and reagents are more stable. The chemiluminiscence kit for detecting content of the hyaluronic acid in human serum, body fluid or tissue homogenate by one-step process comprises a calibrator, a coated board, an enzyme combination, chemiluminiscence substrate solution A and chemiluminiscence substrate solution B. The sample amount of each of the HA (hyaluronic acid) calibrator and the enzyme combination is 50 microliters in the kit, and reaction time of the one-step process is one hour. Scientific and reasonable range of normal values is determined by strict methodological appraisal, clinical measurement results are compared with a radioimmunoassay kit statistically, and accordingly the chemiluminiscence kit has the technical advantages of high sensitivity, accurate measurement, high repeatability, regent stability and the like and is worthy of vigorous popularization and application to market.
Owner:BEIJING NORTH INST OF BIOLOGICAL TECH
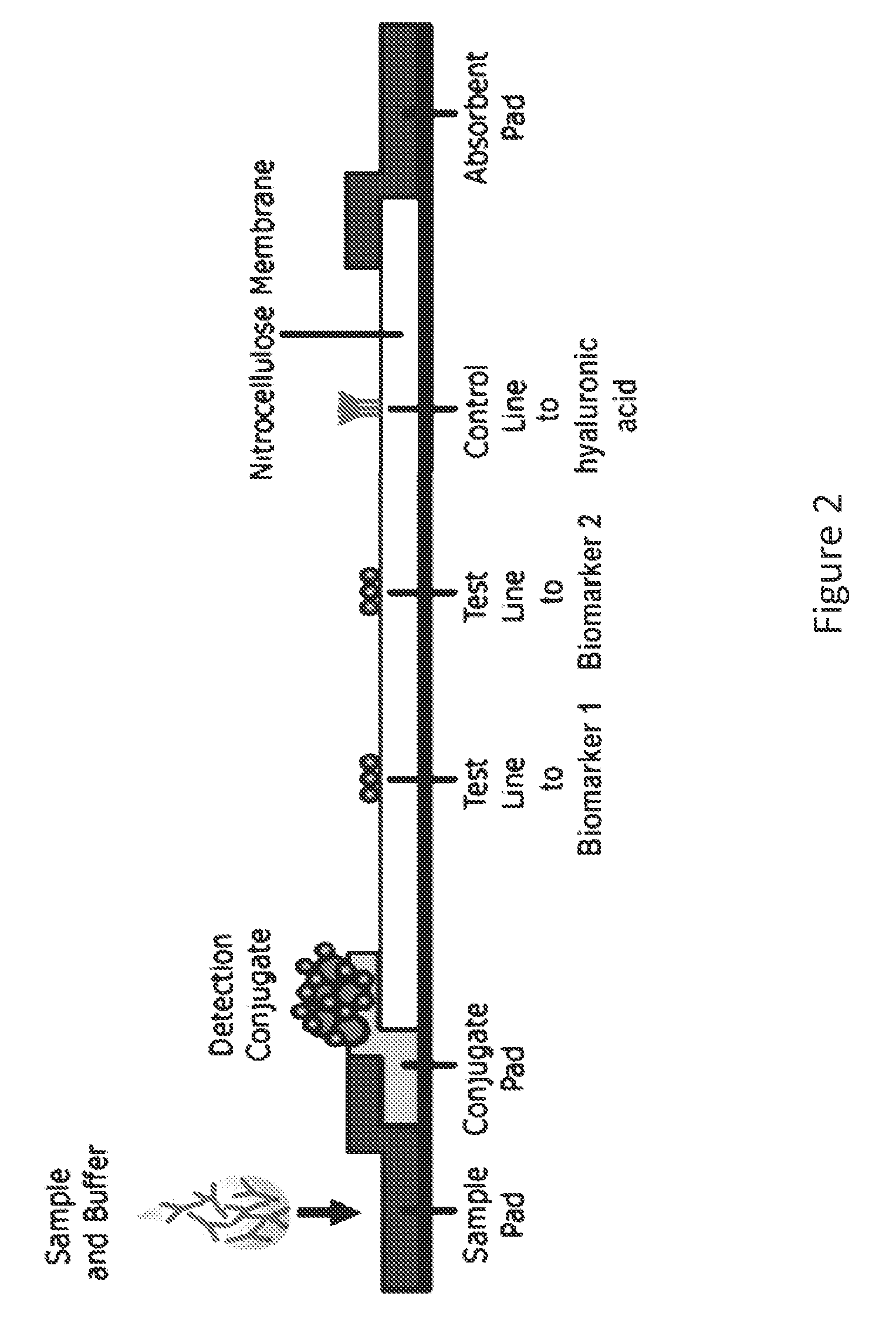
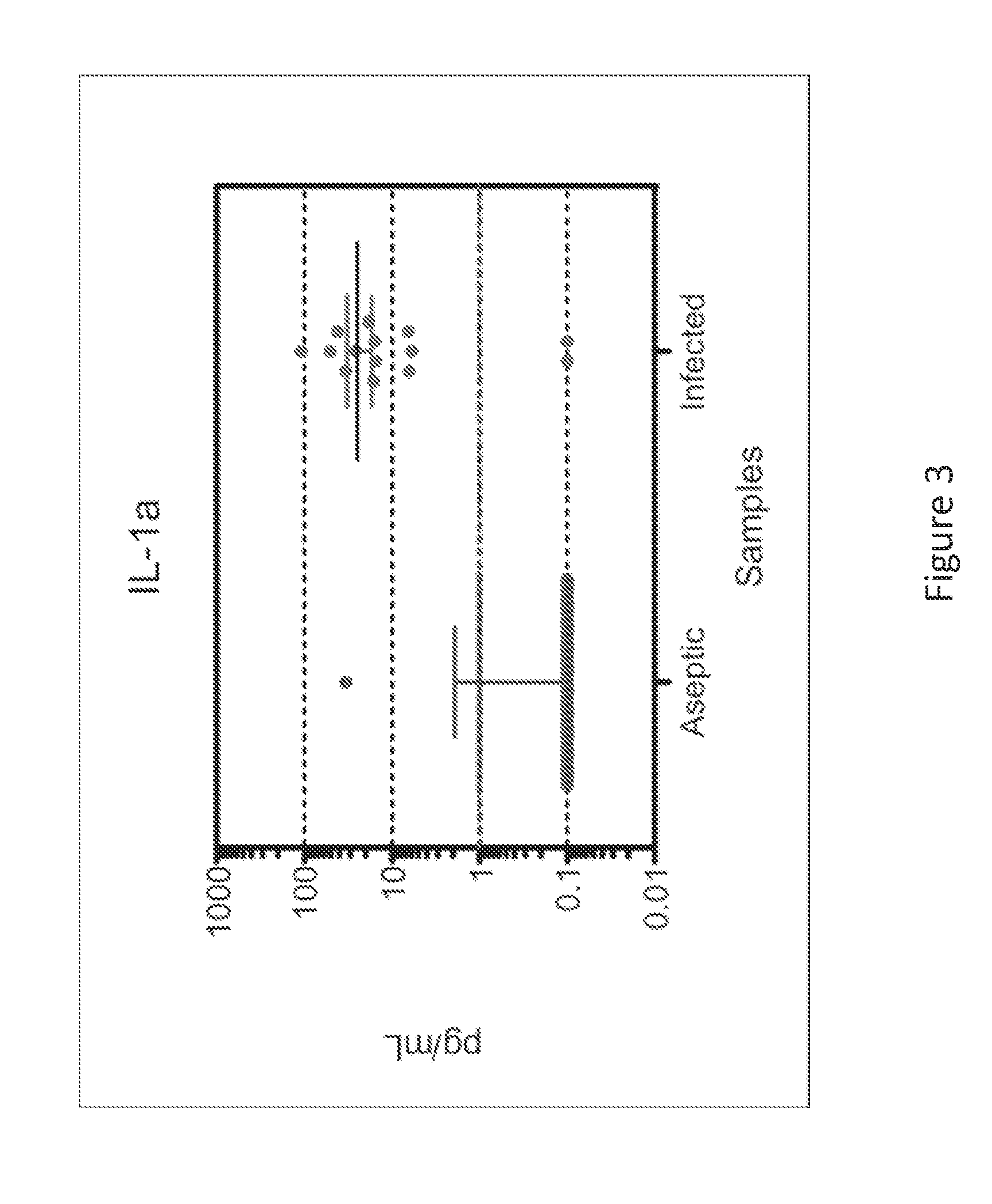
System for detecting infection in synovial fluid
ActiveUS20150011412A1Improve abilitiesBioreactor/fermenter combinationsPeptide librariesProteomics methodsRadioimmunoassay
The invention provides methods and systems for detecting a biomarker in a synovial fluid wherein the system also includes a control to ensure that the test sample is indeed synovial fluid. The biomarkers and the control for synovial fluid can be identified using proteomic methods, including but not limited to antibody based methods, such as an enzyme-linked immunosorbant assay (ELISA), a radioimmunoassay (RIA), or a lateral flow immunoassay.
Owner:CD DIAGNOSTICS
Kit and method for detection of thyroid peroxidase antibody
InactiveCN105467122AImprove accuracyHigh precisionChemiluminescene/bioluminescenceAntigenBiotin-streptavidin complex
The present invention discloses a kit and method for detection of thyroid peroxidase antibody. The kit includes thyroid peroxidase antibody series standards, a magnetic separation reagent of a magnetic particle suspension conjugated with streptavidin and pigment, a first reagent of an antigen solution containing biotin N-hydroxysuccinimide ester labeled thyroid peroxidase antigen, a second reagent of a solution containing alkaline phosphatase labeled mouse-anti-human IgG monoclonal antibody. The kit is used for detection of thyroid peroxidase antibody. By the above-described manner, the invention uses a biotin-streptavidin signal amplification system and alkaline phosphatase labeling to solve the traditional problem of low ELISA sensitivity; the nanometer magnetic particle separation system achieves high sensitivity for radioimmunoassay (RIA), but has no radioactive contamination, and has greatly improved period of validity, security and environmental performance.
Owner:SUZHOU HAOOUBO BIOPHARML
Bt crystallin CrylAc radio-immunity test reagent box, preparing and detecting method thereof
InactiveCN1501082AIncrease reaction rateReduce sampling timeMicrobiological testing/measurementMaterial analysis by optical meansAntigenMicroparticle
The present invention provides one kind of Bt crystallin Cry lAc radioimmunoassay kit and its preparation process and detection method. Magnetic particle is prepared with glutaraldehyde, Aerosol 604 and ferroferric oxide, and connected to Bt crystallin Cry lAc antibody. Under the action of magnetic field, Bt crystallin Cry lAc and Bt crystallin Cry lAc antibody react specifically in fast speed and the sample testing period is reduced to 40 min. The present invention also connect magnetic particle to IgG to prepare immunological separating agent, and under the action of magnetic field, the antigen antibody composition deposits automatically for separation without needing centrifugation.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
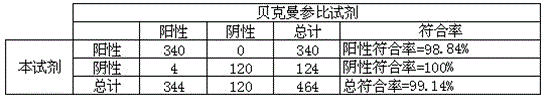
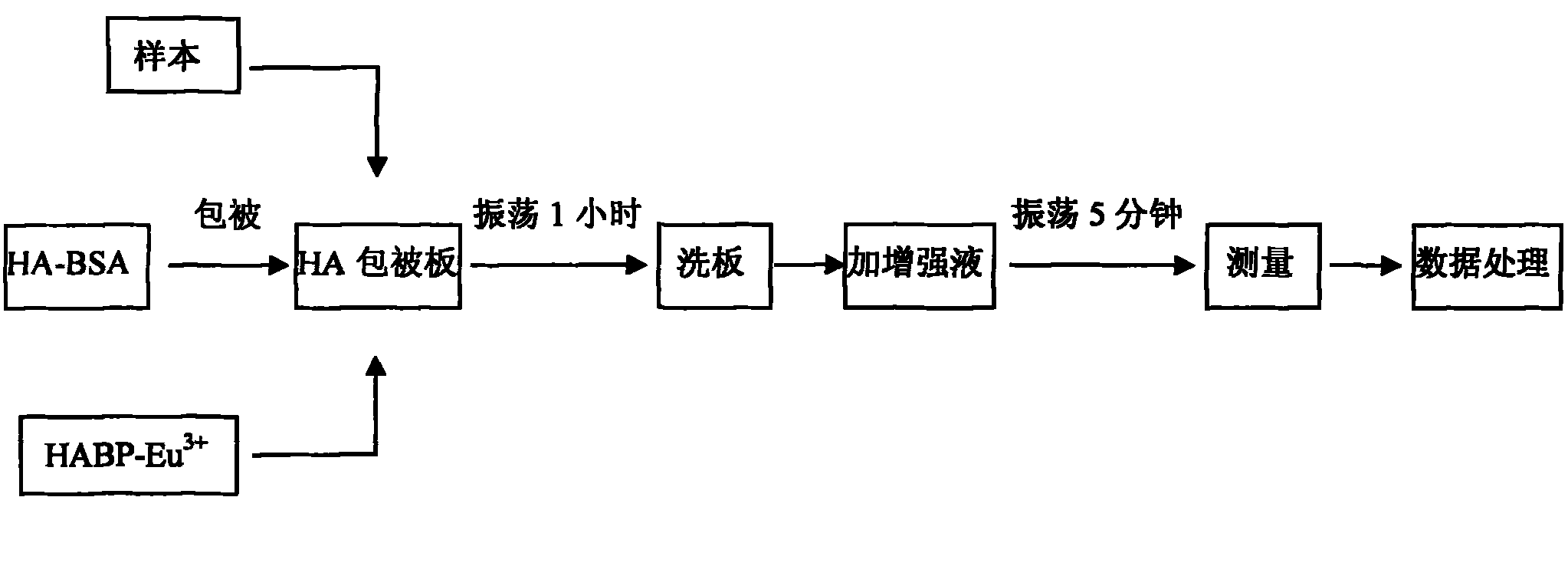
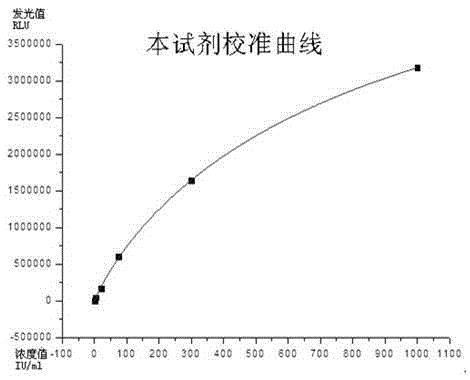
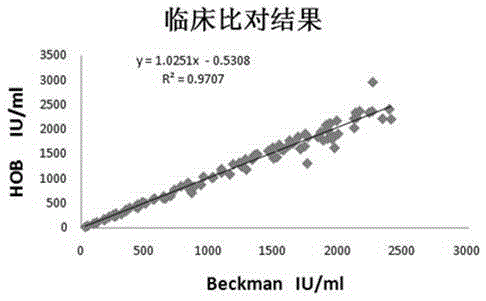
Method for detecting content of hyaluronic acid in human serum
InactiveCN102095847ADecreased fluorescence intensityImprove bindingMaterial analysisEuropiumPollution
The invention relates to a method for detecting hyaluronic acid in human serum, body fluid or tissue homogenate by a radioimmunoassay method. By a method of using solid phase-coated and europium-marked hyaluronic acid binding protein (HABP-Eu<3+>), reagents such as a first antibody, a second antibody and the like are not needed, a sample is easy to add, reaction is quick, the reagents have long shelf life and are environmental-friendly, europium is stably combined with HABP, storage time can be more than 12 months, and radioactive pollution is avoided.
Owner:BEIJING NORTH INST OF BIOLOGICAL TECH
Reagent kit and method for detecting thyroglobulin antibody
InactiveCN105334316AOvercoming pollutionOvercome effectivenessDisease diagnosisBiological testingAntigenBiotin
The invention discloses a reagent kit and method for detecting a thyroglobulin antibody. The reagent kit contains a first reagent, a second reagent, a magnetic separation reagent, a chemical light-emitting substrate, a calibration article, a quality control article and cleaning liquid. The first reagent is a biotin reagent marked by thyroglobulin. The second reagent is a thyroglobulin enzyme combination reagent. The magnetic separation reagent is a superparamagnetic nanometer magnetic particle reagent marked by streptavidine, and the reagent kit is adopted for detecting the thyroglobulin antibody. Due to the mode, the defects that radioimmunoassay is high in pollution, the period of validity is short, common microwell plate enzyme immunoassay precision is poor, and sensitivity is poor are overcome, an alkaline phosphatase enzyme catalysis chemiluminescence system, a magnetic particle separation system and an unique antigen and antibody coupling technology are used, and the sensitivity, the precision, the stability, the validity period, the safety and the environment friendliness of the method are greatly improved.
Owner:SUZHOU HAOOUBO BIOPHARML
Chromogranin A chemiluminescence immunoassay reagent kit and preparation method thereof
ActiveCN102520195ANo radioactive contaminationStrong specificityChemiluminescene/bioluminescenceBiological testingChromogranin AAdditive ingredient
The invention discloses a chromogranin A chemiluminescence immunoassay reagent kit and a preparation method of the reagent kit. The reagent kit comprises the following ingredients: 1) chromogranin A standard products; 2) horseradish peroxidase labeled chromogranin A monoclonal antibodies; 3) chromogranin A monoclonal antibody covered magnetic particles; 4) white non-transparent micro porous plate; 5) cleaning solution and 6) chemiluminescence substrate A and B work solution. The chemiluminescence immunoassay and the nanometer magnetic particle technology are combined, and the chromogranin A chemiluminescence immunoassay reagent kit is provided. The defects of low sensitivity and poor accuracy of the traditional enzyme-linked immunosorbent assay (ELISA) method are overcome, the radioactive pollution of the radioimmunoassay (RIA) technology is avoided, and the chromogranin A chemiluminescence immunoassay reagent kit and the preparation method thereof have the advantages that safety is realized, the environment is protected, the specificity is high, the sensitivity and the accuracy are high, and the like.
Owner:天津市协和医药科技集团有限公司
Method and kit for detection of Anti-beta amyloid antibodies
InactiveUS20150094218A1Easy to disassembleHigh sensitivityPeptide librariesLibrary screeningCrystallographyCerebrospinal fluid
An in vitro method and a kit for the quantification of antibodies anti-beta amyloid protein in a sample of cerebrospinal fluid comprising the following steps a) concentrating the quantity of said antibodies of said sample with magnetic micro beads coated with macromolecules capable of binding said antibodies b) analysing the concentrated sample obtained in step a) by immunoenzyme assay or by radioimmunoassay; c) analysing a sample comprising antibodies anti-beta amyloid protein having a known titre with the same assay used in step b) and elaborating the related calibration curve, wherein said antibody having a known titre is a murine antibody belonging to the IgG2a or IgG2b class or a human or humanized antibody belonging to the IgG2b or IgGi class.
Owner:UNIV DEGLI STUDI DI MILANO BICOCCA
Integrin CD18 is a Novel Stromal Stem Cell Marker and Functions to Promote Osteogenesis
InactiveUS20070248998A1Easy to detectBioreactor/fermenter combinationsBiological substance pretreatmentsImmunoperoxidase StainingCancer research
The present invention is directed to a new bone marrow stromal stem cell (BMSSC) marker, CD18, for use in selecting a population of cells enriched in BMSSCs, from bone marrow cells, adipose cells, or peripheral blood. The invention is further directed to methods for selecting a population of cells enriched in BMSSCs based on the selective expression of CD18 on their surface, using techniques known in the art such as fluorescent assisted cell sorting, an immunomagnetic method, flow microfluorimetry, immunofluorescence, immunoperoxidase staining, radioimmunoassay and immunoaffinity chromatography. The invention is further directed to the BMSSCs isolated based on CD18 expression, and their use to treat various diseases. In one aspect, the HMSSCs are transformed with a vector having a normal gene for CD18, and the transformed BMSSCs are administered to treat bone degenerative diseases and diseases of bone involving abnormal expression of CD18 expression of CD18.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
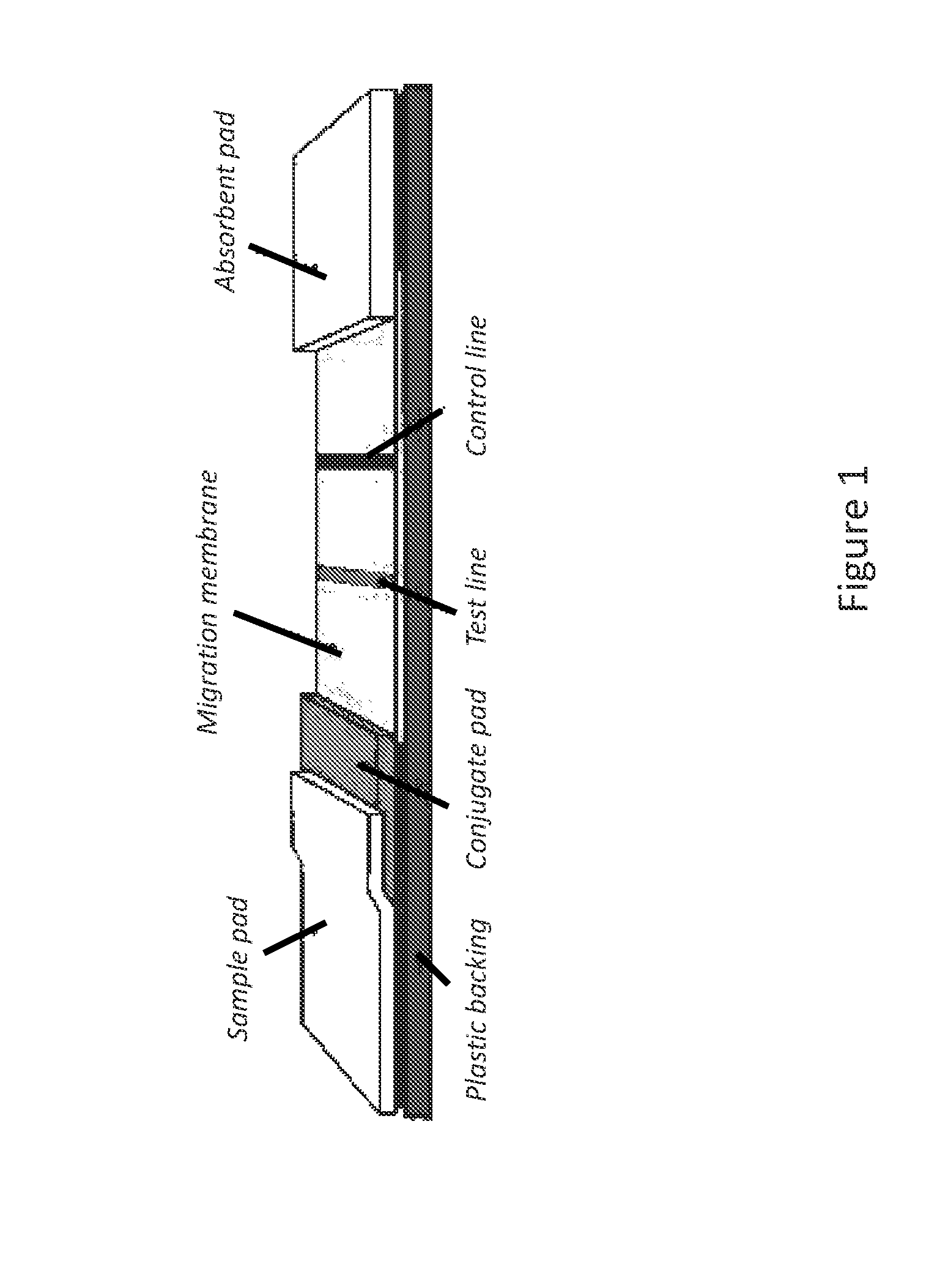
Test strip for fast detecting premature rupture of fetal membranes
InactiveCN102053157AImprove accuracyHigh detection sensitivityMaterial analysisLatex particleInsulin-like growth factor-binding protein
The invention discloses a test strip for fast detecting premature rupture of fetal membranes, which is formed in a way that a sample pad, a glass fiber membrane, a coating membrane and absorbent paper are sequentially overlapped and pasted on a substrate, wherein the glass fiber membrane is coated with a high-specificity monoclonal antibody of latex particle-labeled insulin-like growth factor binding protein-1 (IGFBP-1); and the coating membrane comprises a detection area coating an anti-IGFBP-1 specific monoclonal antibody and a control area coating an anti-mouse antibody. Compared with the radioimmunoassay method and the enzyme-linked immunosorbent assay (ELISA) method, the test strip disclosed by the invention has the advantages of operational safety, fast detection and the like, is simple and convenient and is applicable to individual detection. The test strip disclosed by the invention can be conveniently and fast operated by people without professional skills, can not cause damages and can realize timely on-site and household self detection of premature rupture of fetal membranes; and the result can be read easily.
Owner:GUANGZHOU WONDFO BIOTECH
Effect study method and application of melatonin in prediction of ovarian reserve and IVF-ET (in-vitro fertilization and embryo transfer) outcome
InactiveCN106168621AIncrease success rateFacilitates personalized treatment plansDiagnostic recording/measuringSensorsPhysiologyOvary
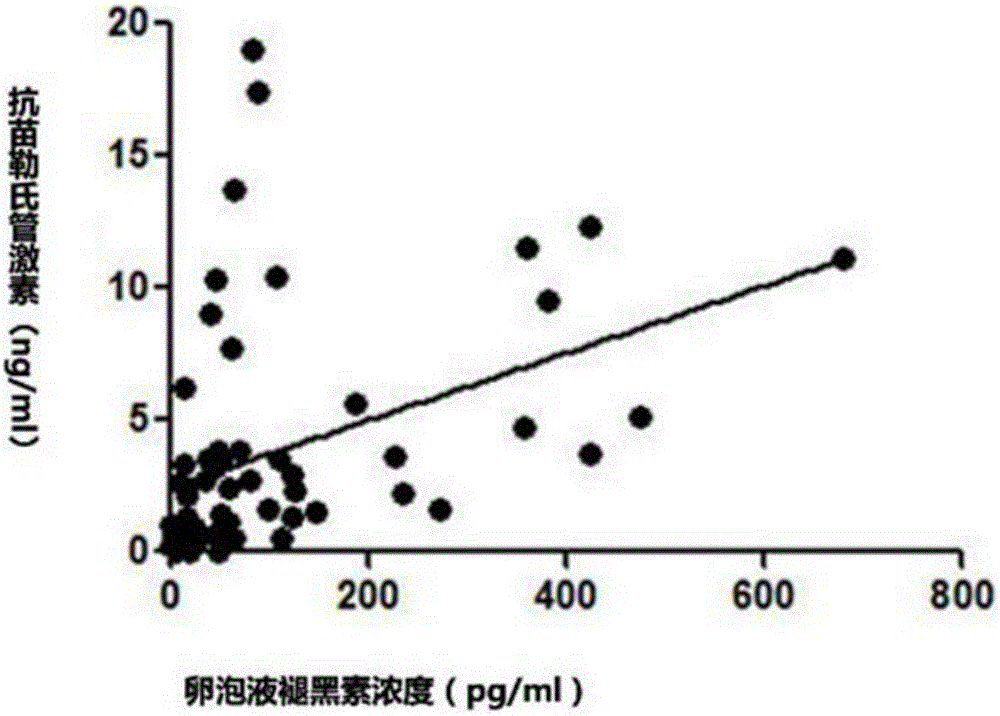
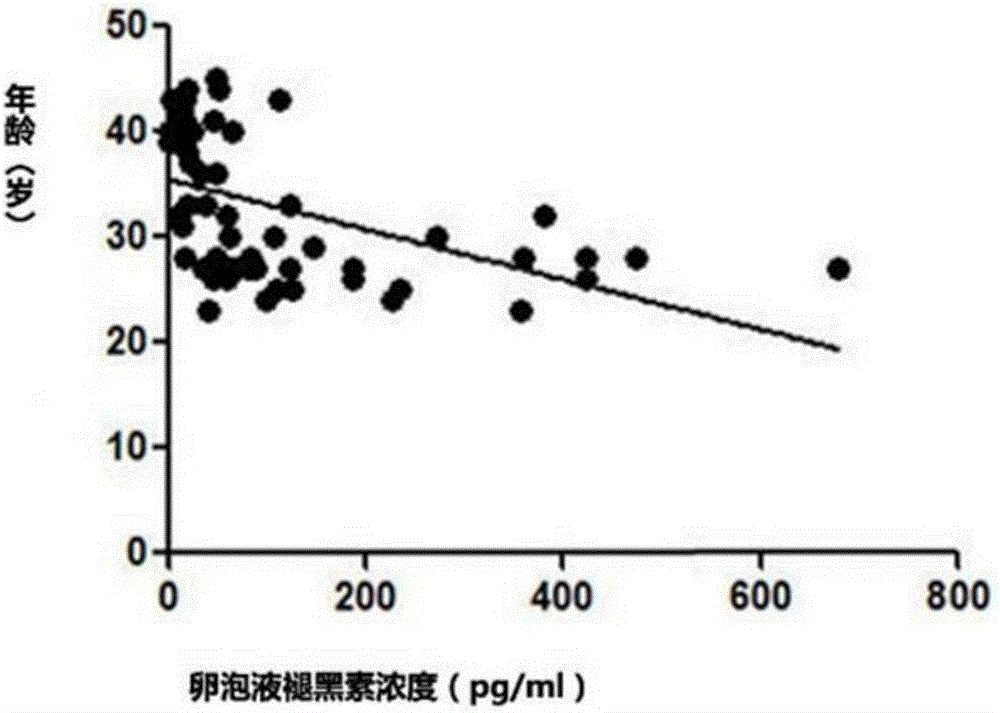
The invention discloses an effect study method of melatonin in prediction of ovarian reserve and IVF-ET (in-vitro fertilization and embryo transfer) outcome. The method includes: collecting basic clinical data of 61 females, and dividing the 61 females into three groups, namely a poor ovarian response group, a normal ovarian response group and a high ovarian response group mainly through age, retrieved oocyte number and anti-mullerian hormone value according to the stimulus response of the ovaries; collecting follicular fluid of the 61 females, adopting melatonin radioimmunoassay to determine melatonin concentration in the follicular fluid of the 61 females, and analyzing the levels of the melatonin concentration and other clinical data in the poor ovarian response group, the normal ovarian response group and the high ovarian response group; analyzing the levels of relevant data of in-vitro fertilization outcome in the poor ovarian response group, the normal ovarian response group and the high ovarian response group, and verifying relevance of the melatonin concentration and IVF-ET outcome; verifying relevance of the melatonin level and ovarian reserve. Through detection of melatonin concentration in the follicular fluid, ovarian reserve and IVF-ET outcome are predicted.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Method for extracting and detecting cortisol from pig hair
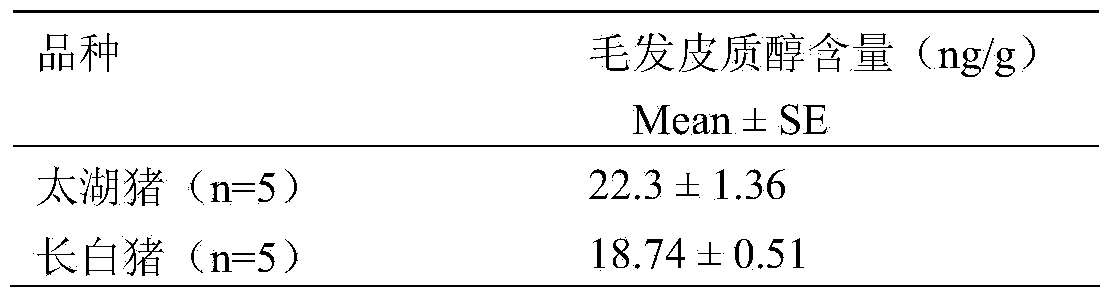
InactiveCN103776997AEasy to collectLow costDisease diagnosisBiological testingRadioimmunoassayOrganosolv
The invention discloses a method for extracting and detecting cortisol from pig hair, belonging o the field of analysis and detection. The method comprises the steps: preparing pig hair, washing the pig hair by using an organic solvent, air-drying, putting the air-dried pig hair into a centrifuge tube filled with the organic solvent, and rotating the centrifuge tube on a rotator; putting the centrifuge tube into a centrifugal machine for centrifugation after the treatment, and collecting a supernatant liquid; and concentrating and blow-drying the supernatant liquid by using a nitrogen flow, dissolving the blow-dried supernatant liquid in a PBS (Phosphate Buffered Solution), and then measuring the content of the cortisol in the solution by adopting a radioimmunoassay method. The method is a way for detecting the physiological status of a pig without causing abuse and stress to the pig, is simple and easy to implement and low in cost, and plays a quite positive effect for monitoring the health and welfare conditions of the pig.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for preparing a sterile transgenic fish
InactiveUS20050257281A1Easy to operateSolve the real problemPisciculture and aquariaFermentationCommon carpGonadotropin-releasing hormone-III
The present application disclosed a method for preparing a sterile transgenic fish, comprising constructing antisense RNA expression vector of salmon-type gonadotropin-releasing hormone, introducing the recombinant DNA fragment into carp oosperm by microinjection, and screening the sterile transgenic fish by Polymerase Chain Reaction and radioimmunoassay, wherein the expression vector comprising a promoter of carp beta actin (β-actin) gene, a complementary DNA fragment of antisense salmon-type gonadotropin-releasing hormone (sGnRH) gene from carp with 323 bp as a target gene comprising sGnRH decapeptide, the coding region of gonadotropin-releasing hormone associated peptide and 3′ non-coding sequence, and 3′ flanking sequence of grass carp growth hormone gene as a stop sequence. The method of the present invention is easy and convenient for operation which provides a basis for providing a technical platform of general applicability for solving the hereditary and ecological safety problems of transgenic fishes.
Owner:INST OF AQUATIC LIFE ACAD SINICA
Serum Biomarkers for Diagnosing Liver Fibrosis and Method for Measuring the Same
InactiveUS20100323374A1Measured safely and accuratelyDepsipeptidesPeptide preparation methodsDiseaseMulti analyte
Disclosed are serum biomarkers for diagnosing liver fibrosis and methods for measuring the same. The serum biomarkers obtained from human serum include alpha2-macroglobulin (“A2M”), vitamin D binding protein (“VDBP”), apolipoprotein AI (“ApoAI”). The methods involve with immunoassay using specific antibodies to detect the biomarkers, including enzyme-linked immunosorbent assay (“ELISA”), radio immune assay (“RIA”) and flexible multi-analyte profiling (“xMAP”). ELISA, RIA or xMAP is used to measure changes of protein concentration for the specific protein biomarkers in serum for diagnosing liver fibrosis with suffering hepatitis B or C or other liver diseases. The measurement is safe and accurate. The method can be used before and after treatment of liver fibrosis. Thus, it is possible to achieve early diagnosis and treatment advocated in the preventive medicine.
Owner:INST NUCLEAR ENERGY RES ROCAEC
Method for diagnosing cardiac troponin I (cTn I) in semi-quantitative mode by employing double-indicatrix immunochromatography
The invention discloses a detection method in the technical field of biological engineering, in particular a method for diagnosing cardiac troponin I (cTn I) in a semi-quantitative mode by employing double-indicatrix immunochromatography. Acute myocardial infarction (AMI) is a serious disease in a coronary heart disease, is a common emergency among middle-aged and elderly people, and is high in death rate. According to statistics, one third to a half of patients die before being sent to a hospital. In the next 20 years, the AMI is a primary cause for death of the patients around the world. The cTn I is a reliable index which is currently acknowledged and has the highest specificity and the longest duration for myocardial infarction diagnosis. At present, cTn I detection methods which are commonly used in clinic comprise an enzyme-linked immunosorbent assay method and a radioimmunoassay method, which are time-saving, labor-saving, difficult to popularize and high in cost. An immune colloidal gold technology is quickly developed in recent years and can be widely applied to detection of communicable diseases, early pregnancy and cancers. By employing colloidal gold immunochromatography, a quick detection method for the cTn I is established. By adoption of the method, a cTn I gray area of 1 to 5 ng / ml can be subjected to semi-quantitative detection.
Owner:正元盛邦(天津)生物科技有限公司
Immunochromatographic test strip for rapidly detecting acute pancreatitis and preparation method thereof
The invention discloses an immunochromatographic test strip for rapidly detecting acute pancreatitis and a preparation method thereof. The test strip is formed by sticking a sample pad, a glass fiber membrane labeling pad, a nitrocellulose coating membrane and absorbent paper to a substrate in sequence through lap joint, wherein a highly specific monoclonal antibody of trypsinogen-2 labeled by a biotin-avidin-colored latex complex is coated on the glass fiber membrane labeling pad; the nitrocellulose coating membrane comprises a detection region and a control region; the detection region is coated by another specific monoclonal antibody of trypsinogen-2 with epitope different from that of the labeled monoclonal antibody on the glass fiber membrane labeling pad; and the control region is coated by an anti-mouse antibody. Compared with radioimmunoassay and enzyme linked immunosorbent assay (ELISA), the test strip has the advantages of safety, simpleness and convenience in operation, suitability for single human / sample detection, rapidness and the like, dispenses with special skills, is free of trauma, is easy in result reading and can realize timely in-situ and family self-detectionof acute pancreatitis.
Owner:GUANGZHOU WONDFO BIOTECH
Radioimmunoassay using nanoparticle-antibody conjugates
Owner:CLEMSON UNIV RESEACRH FOUND
Human ICAM-1 extramembranous I-III domain gene expression product ,its prep. and application in radioimmunological technology
InactiveCN1448509AEasy to purifySimple and fast operationCell receptors/surface-antigens/surface-determinantsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseImmune profiling
The present invention relates to gene engineering technology. The I-III functional encoding gene outside intercellular adhesion molecule-1 (ICAM-1) membrane is recombined to expression plasmid pET42a, transferred into prokaryotic expression bacteria, and purified to obtain expression product GST-ICAM-1. The expression product is used as antigen in immunizing rabbit to obtain rabbit anti-human polyclonal antibody. Thus prepared expression product of human I-III functional encoding gene outside ICAM-1 membrane in prokaryotic system is easy to purify, low in cost and high in yield. The human serum sICAM-1 radioimmunoassay technology is developed and used in clinical test and has important significance in diagnosing the treating effect of Graves' disease.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
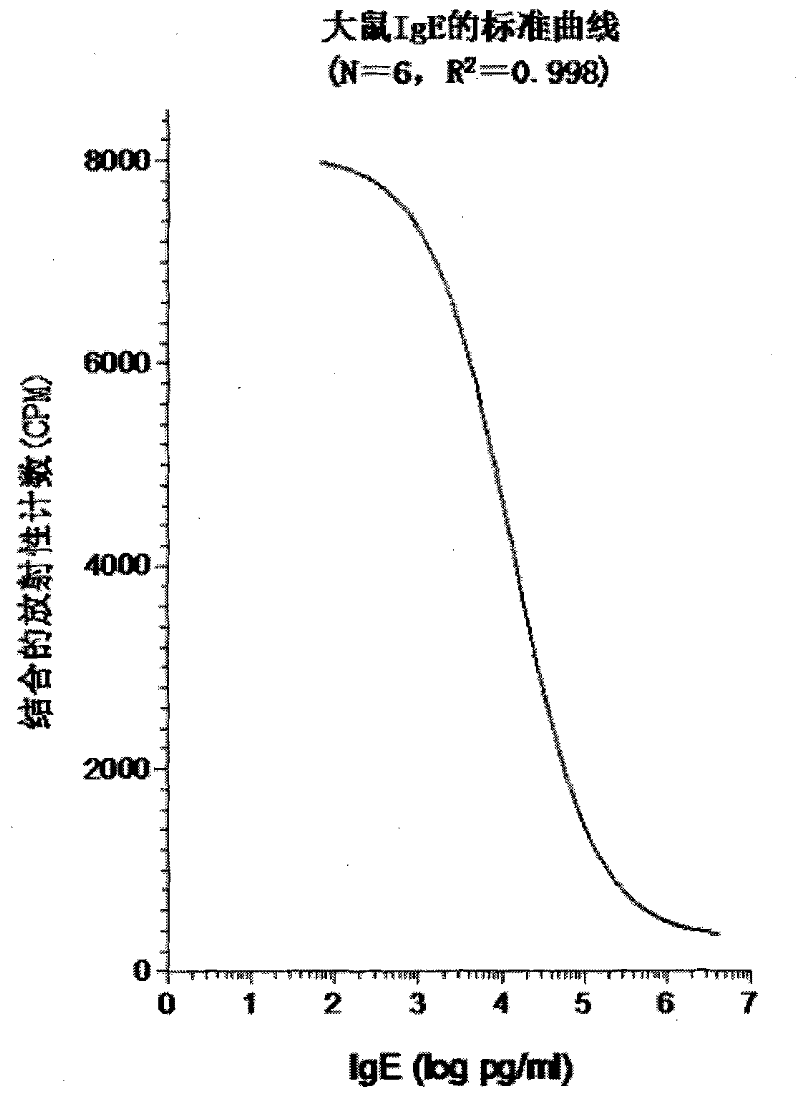
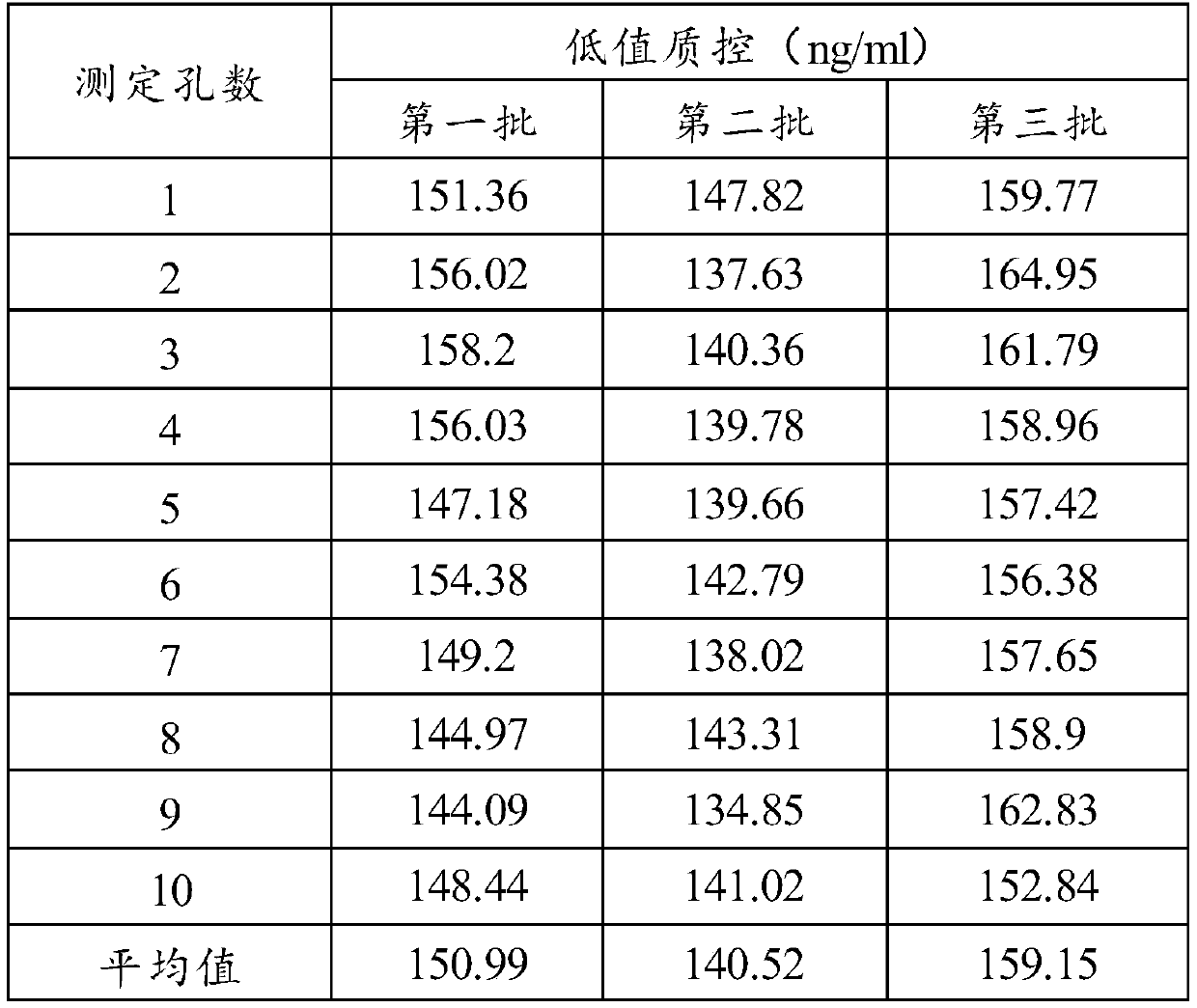
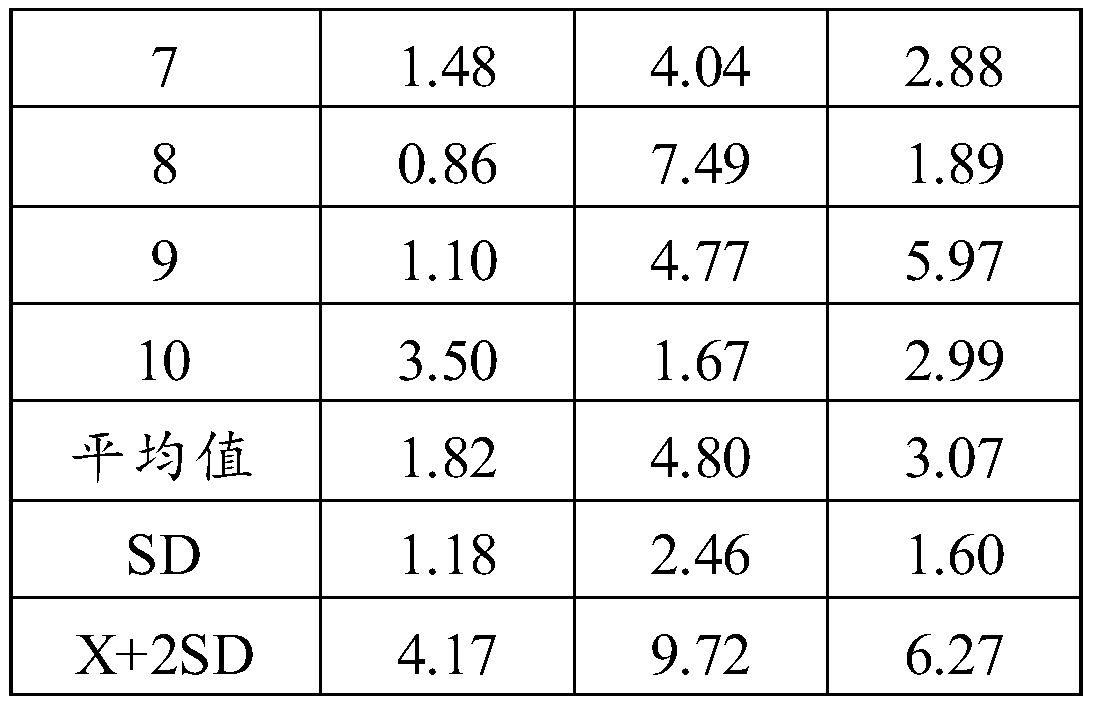
Radioimmunoassay kit for measuring rat serum total IgE, and detection method thereof
The invention discloses a magnetic separating radioimmunoassay kit for measuring rat serum total Ig. The radioimmunoassay kit comprises IgG magnetic nano particles and corresponding reagent. The analysis kit is characterized by having measurement sensitivity reaching 0.5ng / ml, good precision and accuracy, and can be used in measurement of microscale IgE in rat serum. The invention also discloses a method utilizing the radioimmunoassay kit for detection rat serum total IgE.
Owner:SHANGHAI UNIV OF T C M
Serological assay for neospora caninum
The present invention relates to the serological detection of animals inoculated with Neospora by reacting the test serum with a protein reactive with Neospora. The protein may be a full-length natural or recombinant Neospora or T. gondii bradyzoite fusion protein or a truncated fusion protein or a fragment thereof. The protein can be used in any of a number of assays including enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA), Western blot and other suitable forms of immunoassay.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
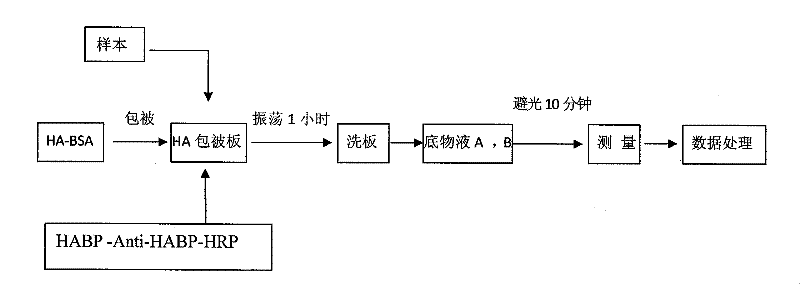
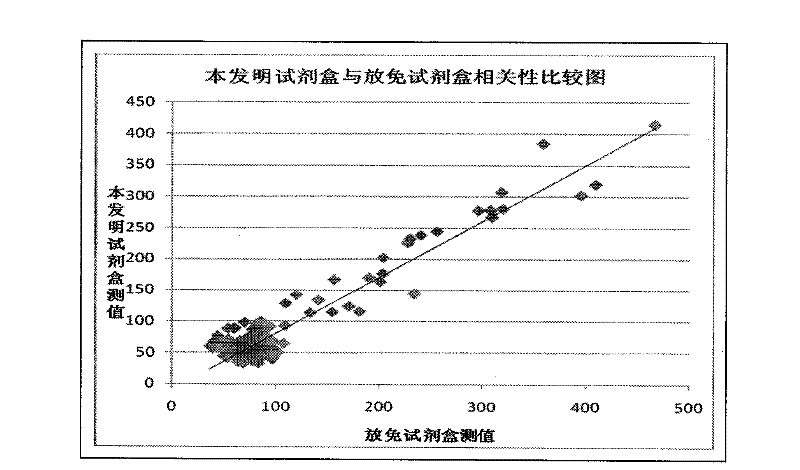
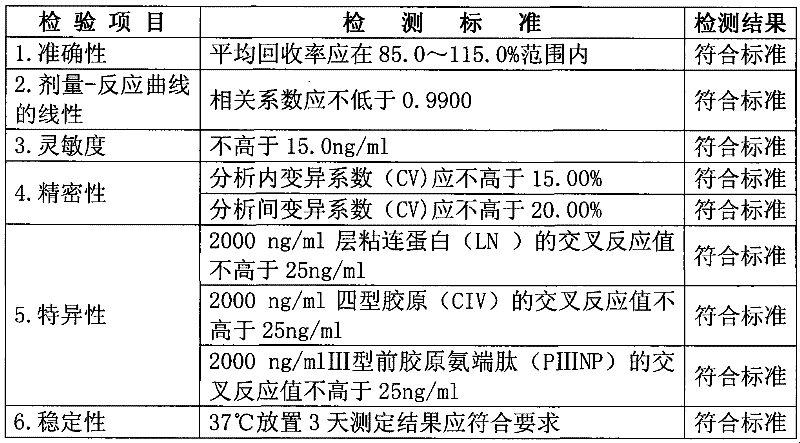
Hyaluronic acid detection kit and detection method
ActiveCN110907641AGuaranteed accuracyMeet testing needsMaterial analysis by observing effect on chemical indicatorBiological material analysisRadioimmunoassayAntiendomysial antibodies
The invention relates to the technical field of medical analysis, in particular to a hyaluronic acid detection kit and a detection method. The kit comprises a solid-phase carrier combined with an HA derivative, an HA binding protein and an enzyme-labeled HA binding protein antibody, wherein the HA derivative is formed by coupling the tail end of HA with a coupling protein. The kit disclosed by theinvention is good in accuracy, high in sensitivity, good in specificity and high in detection speed, and a result can be obtained within 40 minutes. The kit provided by the invention has extremely high conformity with liver biopsy results, overcomes many defects of a radioimmunoassay reagent and an enzyme immunoassay reagent, is suitable for the effective popularization and application in industry, has good stability, and can be stably stored for one year at 2-8 DEG C.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Active carrier with fixed amino-compound content and fixing method thereof
InactiveCN101776686ASave raw materialsBiologically activeMaterial analysisSolid-phase synthesisChemiluminescence immunoassay
The invention discloses an active carrier with fixed amino-compound content and a fixing method thereof and in particular relates to a method for fixing amino acid or protein on the surface of the solid-phase material. The method comprises the following steps: high polymer with aromatic nucleus is adopted as a solid material; through chemical method, the high polymer is provided with functional group sulfonic acid chloride, the sulfonic acid chloride is reacted with a target substance containing amino, so the target substance is fixed on the surface of the solid polymer in the form of a chemical bond. The active carrier has simple production process and low cost. The fixing method saves raw material, has good stability and homogeneity, and can be used for the fields such as the radioimmunoassay, enzyme immnunoassay, chemiluminescence Immunoassay, time resolution immunity analysis, polymerase chain reaction, gene engineering, solid-phase synthesis of polypeptide, and the like.
Owner:沙栩正 +1
Lincomycin detection kit
InactiveCN106526189AWide linear dynamic rangeLight signal lasts for a long timeMaterial analysisFreeze-dryingCarrier protein
The invention discloses a lincomycin detection kit. The lincomycin detection kit comprises test paper and a micro-porous reagent. The test paper comprises a reaction film, a sample absorption pad, a water absorption pad, a protection film and a bottom board and is formed by adhering the ample absorption pad, the reaction film, the water absorption pad and the protection film on the bottom borad sequentially, wherein the reaction film is provided with a detection area and a quality control area, a lincomycin hapten-carrier protein conjugate wraps the detection area, and an antiantibody wraps the quality control area. Lincomycin specificity antibody-colloidal gold marker is freeze-dried on the micro-porous reagent, and the micro-porous reagent is provided with micro-porous plugs. The lincomycin detection kit has the advantages of wide linear dynamics range, long optical signal duration time, simplicity and rapidity in analysis method, stable results, small errors, high safety and long service life. Through chemiluminescence immunoassay, the lincomycin detection kit succeeds in detecting substances which cannot be detected by the methods such as radioimmunoassay and enzyme-linked immunoassay, thereby being of great significance to early clinical diagnosis.
Owner:BEOSON JIANGSU FOOD SAFETY TECH CO LTD
Cell slide for detecting acetylcholine receptor autoantibody in human body fluid as well as preparation method and application of cell slide
ActiveCN113651881AHigh sensitivityStrong specificityNucleic acid vectorDisease diagnosisDiseaseHuman body
The invention discloses a cell slide for detecting an acetylcholine receptor autoantibody in human body fluid as well as a preparation method and application of the cell slide, and belongs to the technical field of biology. According to the scheme, the method comprises the following steps: co-transfecting each subunit of an acetylcholine receptor and a no-load plasmid according to a certain proportion, and then fixing, washing, terminating, washing and drying to prepare the cell slide for detecting the autoantibody of the acetylcholine receptor in human body fluid. Compared with a radioimmunoassay and an enzyme-linked immunosorbent assay, the method has higher sensitivity; compared with an immunofluorescence method reported in literatures in the prior art, the method has the advantage that the detection specificity is improved while the background is reduced. Therefore, from the perspective of research or as an auxiliary diagnosis means of the myasthenia gravis disease, the method has a good application prospect.
Owner:SHAANXI MYBIOTECH CO LTD
Plant drug for preventing cancer
This invention relates to new safe plant drug, which contains Berberine and Baicalin (BB), inhibits carcinogenic and mutagenic action, lowers tyrosine kinase and decreases synthesis of DNA, RNA and protein of cancer cells. Also, the present invention proved a new radioimmunoassay (RIA) method for precise determination of Berberine and Baicalin. The RIA is an efficient analytical method for large clinical programs including double blind analysis (DBA), and good clinical practice (GCP).
Owner:ZHAO XINXIAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com