Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
84 results about "Serum biomarkers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Serum biomarker is used to detect the irritable bowel syndrome (IBS). No single serum biomarker can reliably differentiate irritable bowel syndrome (IBS) from other functional gastrointestinal disorders or organic diseases of the gastrointestinal tract. Sepsis, an innate immunological response of systemic inflammation to infection,...
Serum biomarkers in hepatocellular carcinoma
InactiveUS20060084059A1Microbiological testing/measurementMaterial analysis by electric/magnetic meansHepatocellular carcinomaChronic liver disease
Certain biomarkers and biomarker combinations are useful in a qualifying hepatocellular carcinoma status in a patient. A diagnostic methodology employing these biomarkers and combinations can distinguish between hepatocellular carcinoma and chronic liver disease, for example.
Owner:CHINESE UNIV OF HONK KONG THE +1
Maternal serum biomarkers for detection of pre-eclampsia
InactiveUS20100016173A1Microbiological testing/measurementLibrary screeningProteomics methodsPlacental insufficiency
The present invention concerns the identification and detection of maternal serum biomarkers of pre-eclampsia and associated complications, gestational hypertension and placental insufficiency using global proteomic approaches. The invention further concerns the identification of maternal serum biomarkers for detection of pre-eclampsia and associated complications, gestational hypertension and placental insufficiency during early gestation.
Owner:HOLOGIC INC
Serum biomarkers in ischaemic heart disease
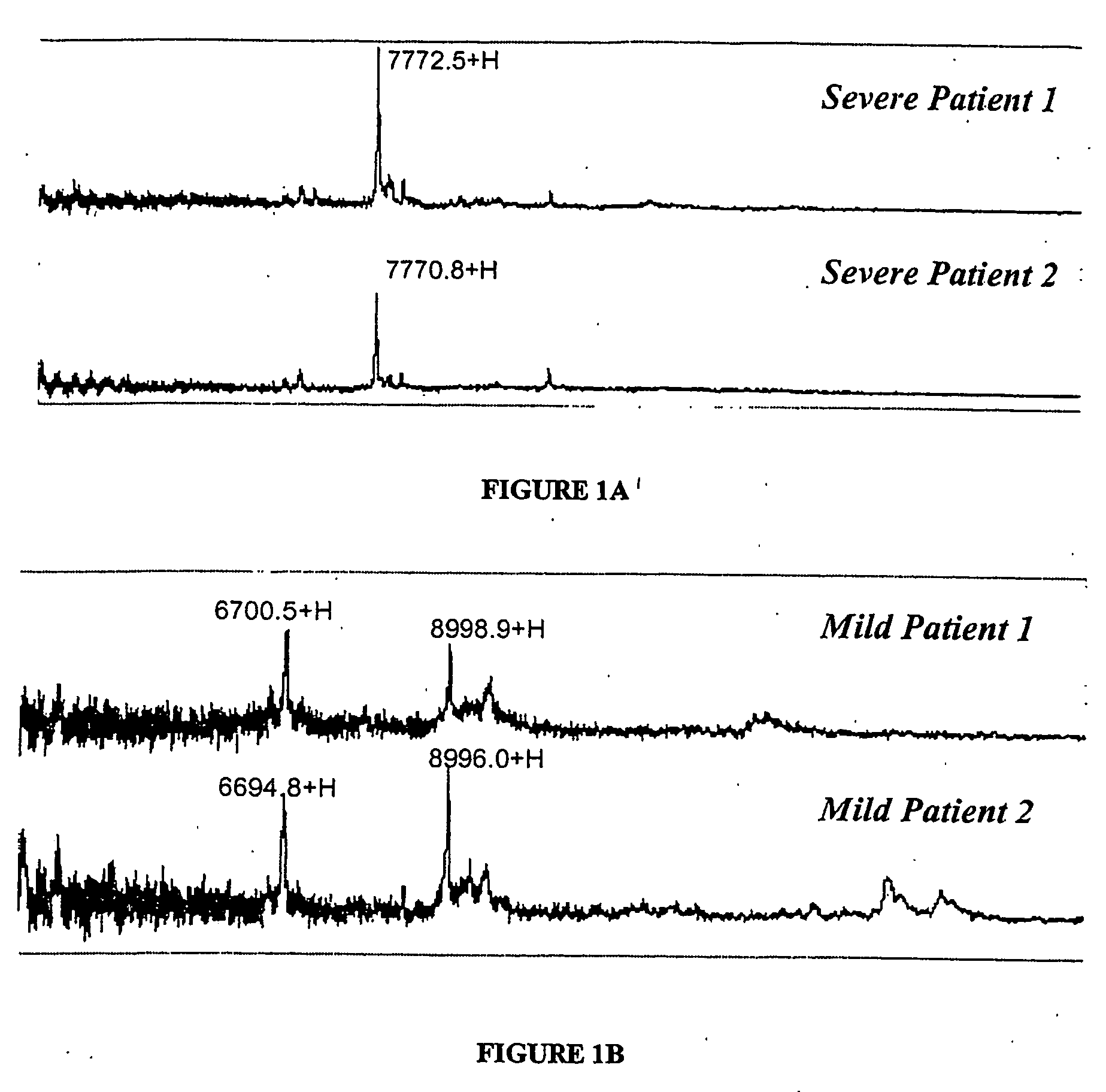
Certain biomarkers and biomarker combinations are useful in a qualifying ischaemic heart disease status in a patient. A diagnostic methodology employing these biomarkers and combinations can distinguish between ischaemic heart disease and normal, as well as between cases of severe myocardial infarction versus mild myocardial infarction.
Owner:VERMILLION INC
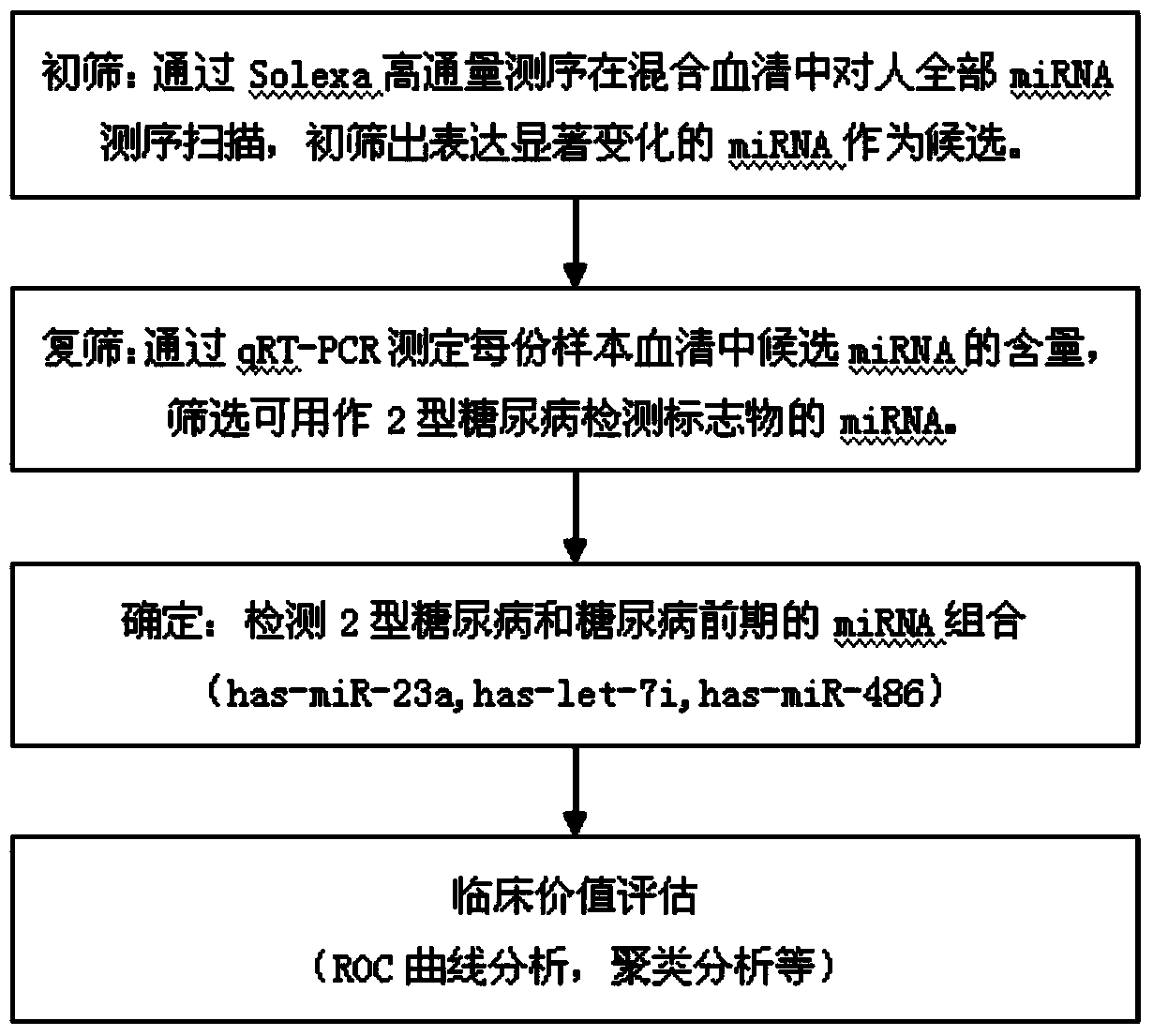
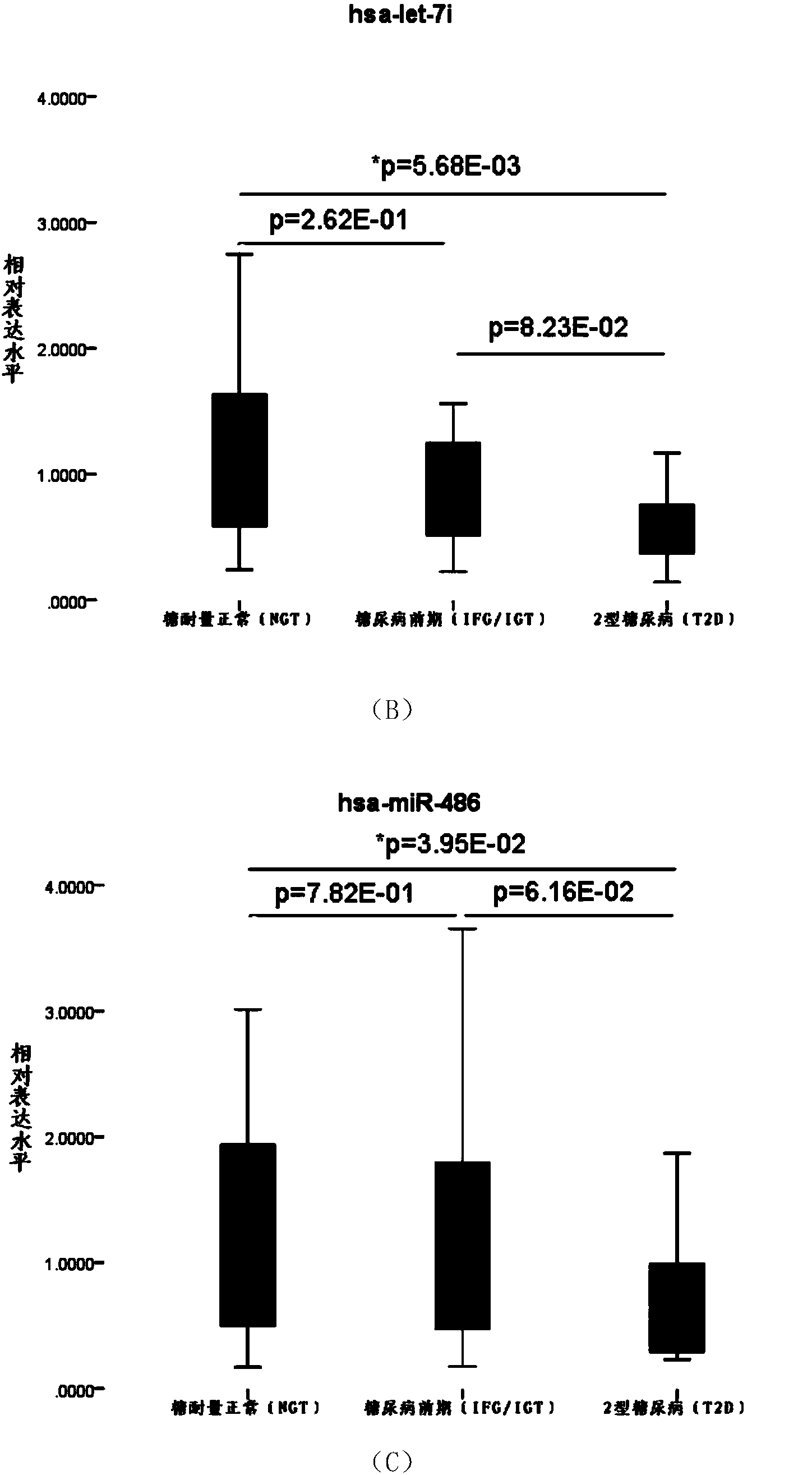
Serum miRNA biomarker of type 2 diabetes mellitus and application thereof
The invention discloses a serum miRNA composition, which is at least one of the following serum miRNAs: hsa-miR-23a, hsa-let-7i, and hsa-miR-486. The invention also simultaneously provides a fluorescent quantitative detection primer composition for detection of type 2 diabetes mellitus. The composition comprises an RT primer, an FW primer and an RV primer of each miRNA in hsa-miR-23a, hsa-let-7i, and hsa-miR-486. By adopting the serum miRNA marker for early diagnosis of type 2 diabetes mellitus and cancer risk evaluation provided by the invention, a serum miRNA biomarker can be used for diagnosing whether a subject has the type 2 diabetes mellitus or early diagnosis of the type 2 diabetes mellitus and cancer risk evaluation.
Owner:ZHEJIANG SCI-TECH UNIV +1
Preparation method and application of magnetic MIL-100 composite material
InactiveCN105203380ANo need to removeAvoid cumbersomePreparing sample for investigationAnalysis by material excitationCarbon layerSynthesis methods
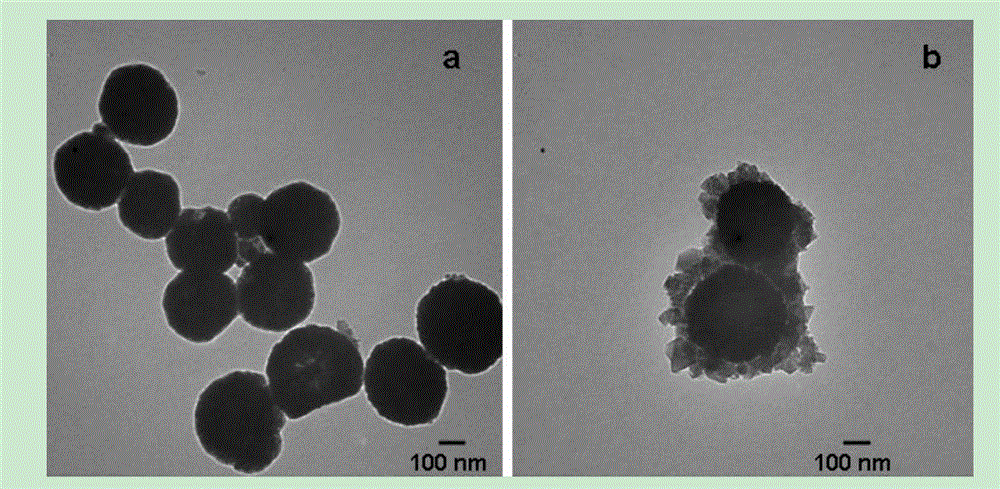
The invention provides a preparation method and application of a magnetic MIL-100 composite material. The magnetic MIL-100 composite material is Fe3O4 / C@MIL-100, wherein the MIL-100 is a metal-organic framework material constructed by iron and trimesic acid. A synthesis method comprises the following steps: firstly, preparing a carbon layer clad magnetic microsphere and preparing the Fe3O4 / C@MIL-100 composite material. The prepared magnetic MIL-100 composite material is used for finding and detecting a blood serum biomarker. The preparation method and the application, provided by the invention, have the advantages that a carbon layer is ingeniously and uniformly clad on the surface of a high-quality Fe3O4 microsphere by the preparation method, and the Fe3O4 / C@MIL-100 composite material is prepared in situ by a one-pot method; the composite material has a magnetic separation property of magnetic nano particles and a size exclusion effect of MIL-101; and high-abundant protein does not need to be removed, and the composite material can be rapidly and efficiently suitable for finding and detecting the blood serum biomarker.
Owner:TIANJIN POLYTECHNIC UNIV
Serum marker MMP-7-based biliary atresia diagnosis kit
InactiveCN108267585AEasy to operateAid in early diagnosisDisease diagnosisPositive controlPRIMARY BILIARY ATRESIA
The invention relates to a serum marker MMP-7-based biliary atresia diagnosis kit. The diagnosis kit comprises an anti-human MMP-7 monoclonal antibody coated ELISA plate, a negative control solution,a positive control solution, an enzyme labeling reagent, an enzyme substrate solution, a blocking solution, a sample diluent, a washing solution and a stopping solution. The diagnosis kit is a new sensitive, safe, reliable and easily-operated commercial kit. Quantitative detection of the level of MMP-7 in human serum is helpful for early diagnosis of BA; the BA diagnosis sensitivity of the serum biomarker protein MMP-7 is 100%, and the BA diagnosis specificity is 95.6%, so the kit has the characteristics of high specificity and high sensitivity; and the kit improves the early diagnosis rate ofBA, reduces misdiagnosis, and improves the self liver survival rate of the BA.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
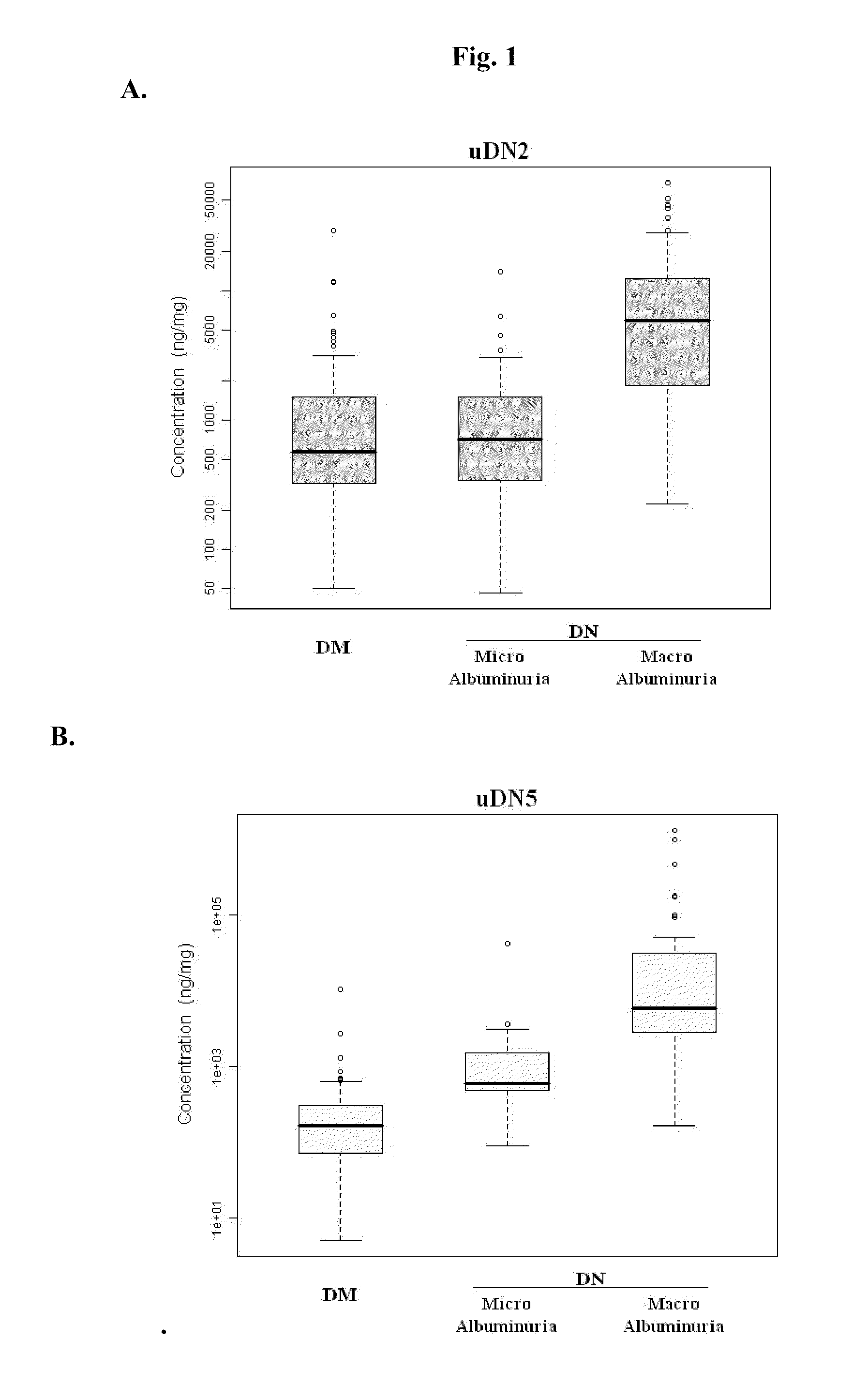
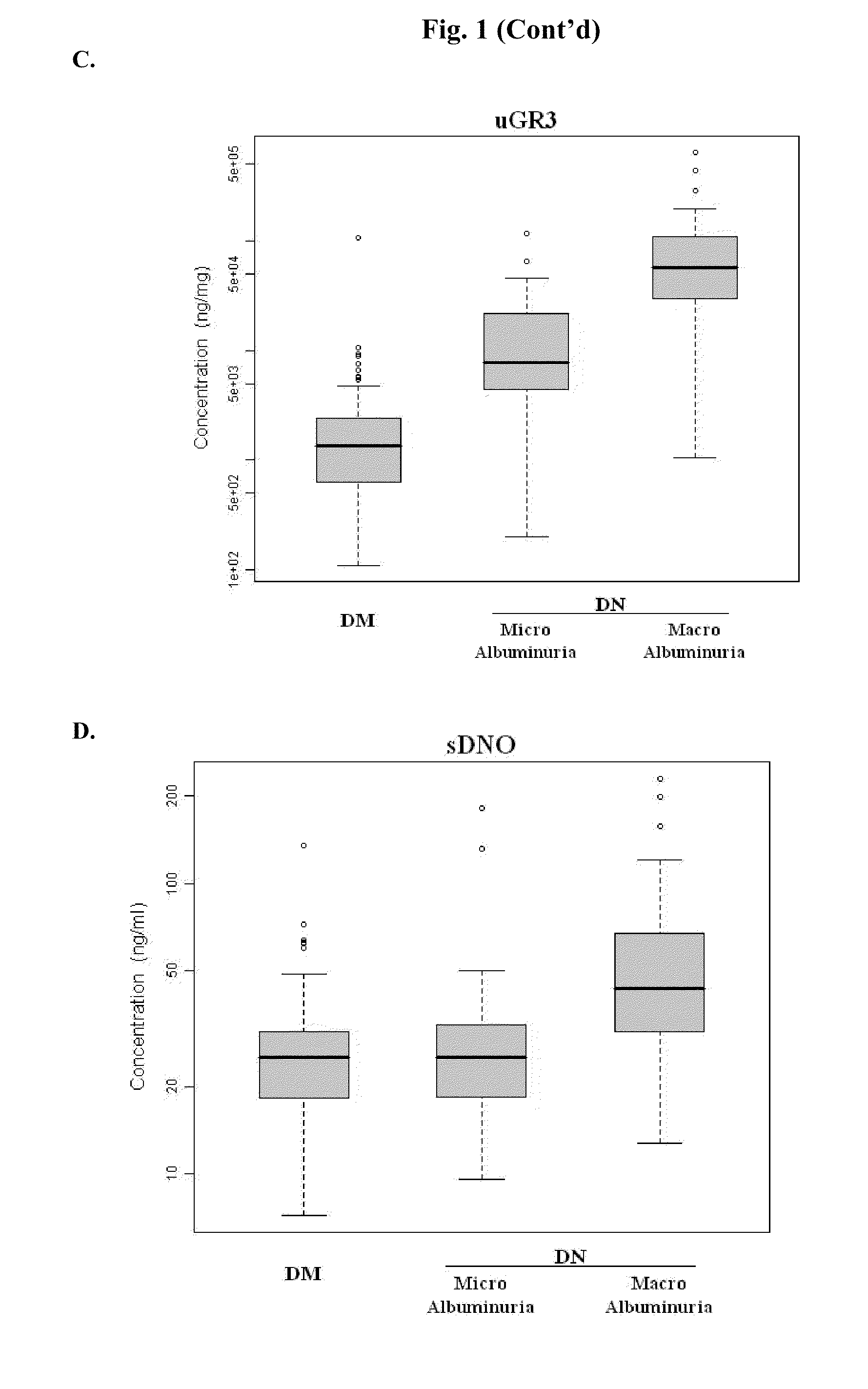
Urine and Serum Biomarkers Associated with Diabetic Nephropathy
Use of urine and serum biomarkers in diagnosing diabetic nephropathy, staging diabetic nephropathy, monitoring diabetic nephropathy progress, and assessing efficacy of diabetic nephropathy treatments. These biomarkers include urine precursor alpha-2-HS-glycoprotein, urine alpha-1 antitrypsin, urine alpha-1 acid glycoprotein, urine osteopontin, serum osteopontin, their fragments, and combinations thereof.
Owner:BIO PREVENTIVE MEDICINE CORP
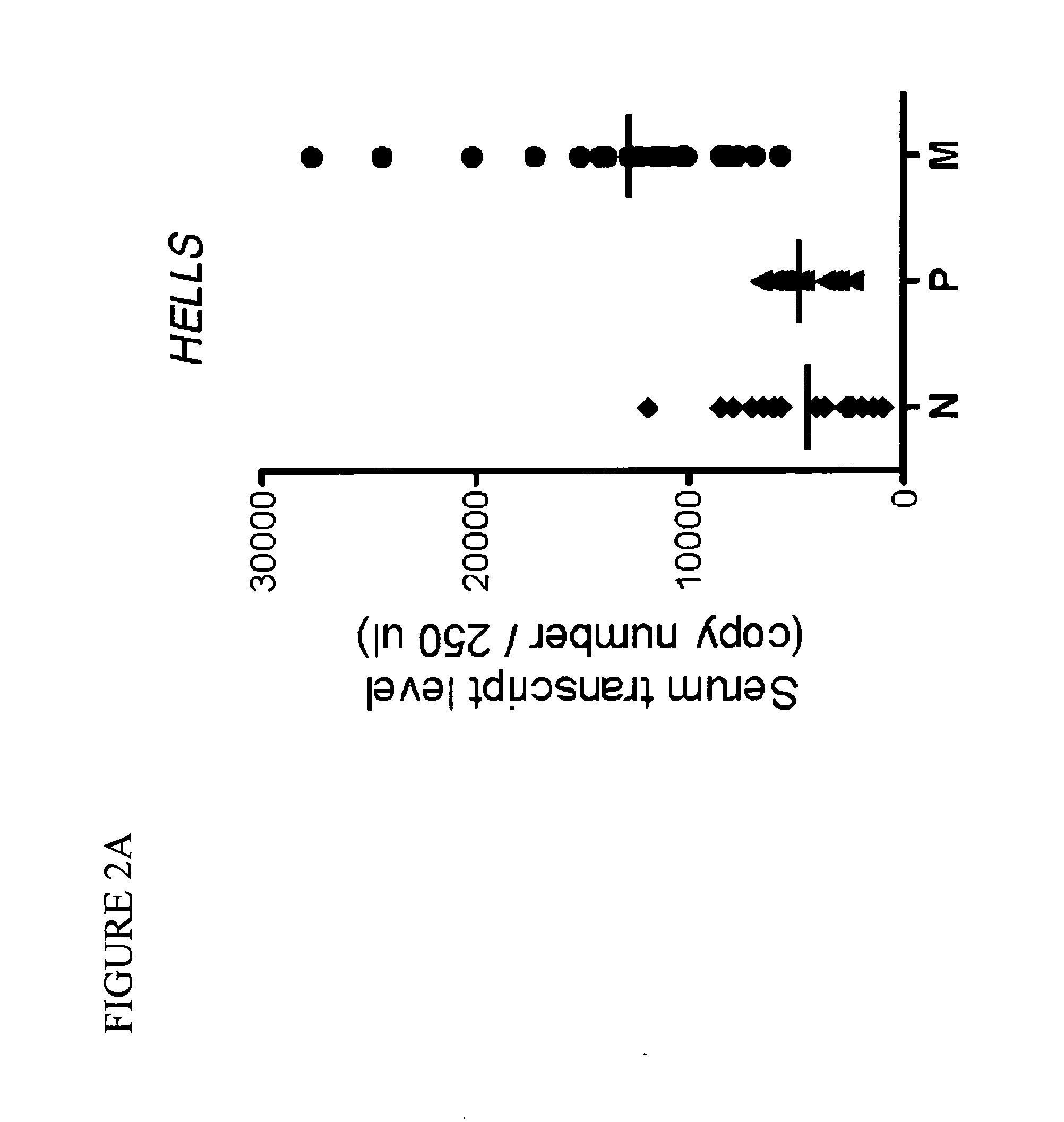
Serum biomarkers for melanoma metastasis
InactiveUS20120201750A1Organic active ingredientsIn-vivo radioactive preparationsMetastatic melanomaLymphatic Spread
The present invention relates to methods for predicting and evaluating metastasis of solid cancers, such as melanoma, in a subject by measuring serum biomarkers associated with a metastatic phenotype. In particular, the present invention provides a serum gene expression signature that is different between highly aggressive and more metastatic versus less aggressive and less metastatic melanomas by quantitatively measuring the levels of, inter alia, lymphoid-specific helicase (HELLS) and condensing complex subunit 2 (NCAPH) transcripts in a subject.
Owner:NEVADA CANCER INST
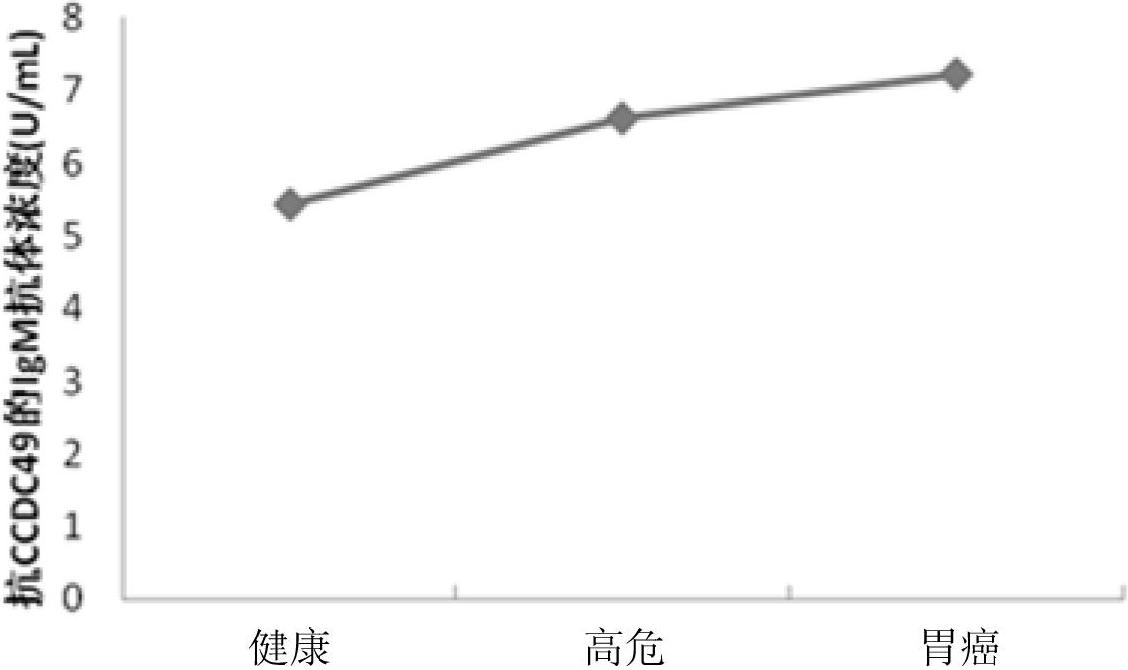
Diagnostic kit and application of coiled-coil domain containing protein 49 (CCDC49) in preparing gastric cancer early-stage diagnostic reagent
The invention relates to the field of the biological science, in particular to a diagnostic kit and an application of coiled-coil domain containing protein 49 (CCDC49) in preparing a gastric cancer early-stage diagnostic reagent. The diagnostic kit is used for diagnosing the early-stage gastric cancer. The diagnostic kit comprises an elisa plate, human protein CCDC49, standard serum, an enzyme labeled reagent, enzyme substrate solution, confining liquid, sample diluent, detergent liquid and termination liquid, wherein the human protein CCDC49 is wrapped on the elisa plate. Compared with the prior art, the diagnostic kit has advantages that 1, a commodity diagnostic kit which is sensitive, safe, reliable and easy to operate is provided, the immunoglobulin m (IgM) antibody for resisting the protein CCDC49 in the human serum can be qualitatively determined, and the early diagnosis of the gastric cancer can be assisted; and 2, the specificity of the provided serum biological labeled protein CCDC49 is 81 percent, the sensitivity is 82 percent, and the characteristics of high specificity and high sensitivity can be realized.
Owner:SHANGHAI JIAO TONG UNIV
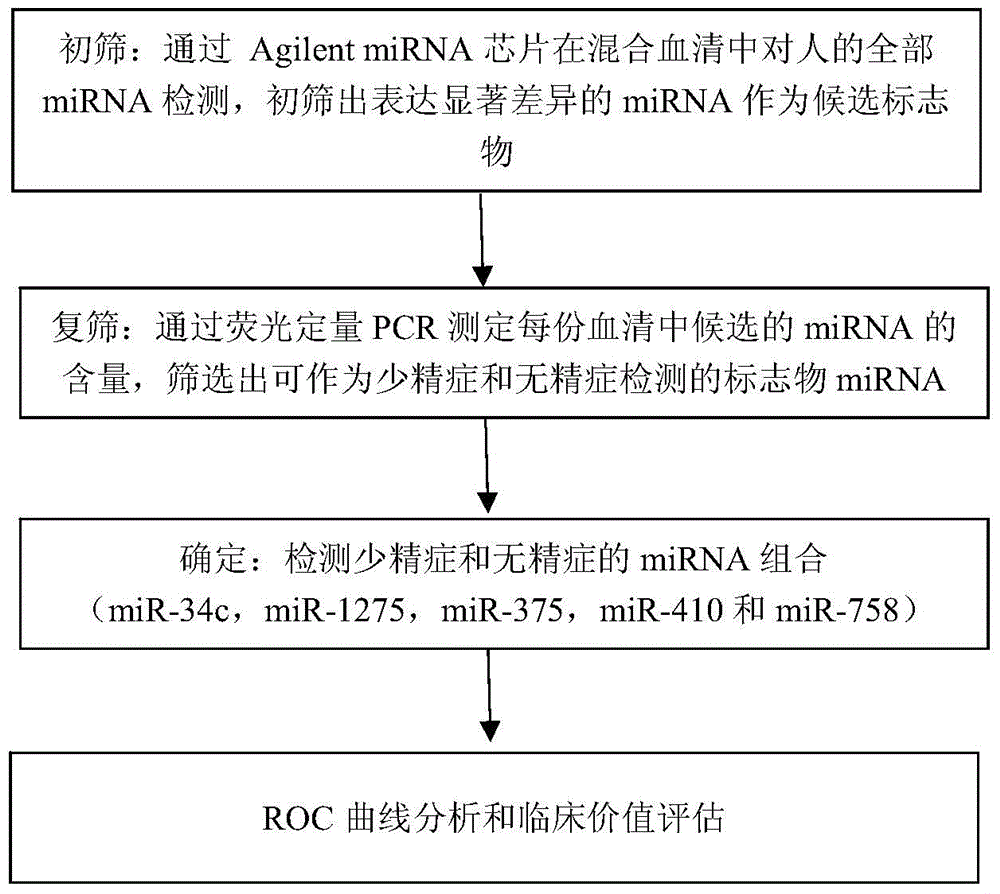
Application of serum miRNA biomarker
ActiveCN104450707AEasy to useRelieve painMicrobiological testing/measurementDNA/RNA fragmentationForward primerGenetics
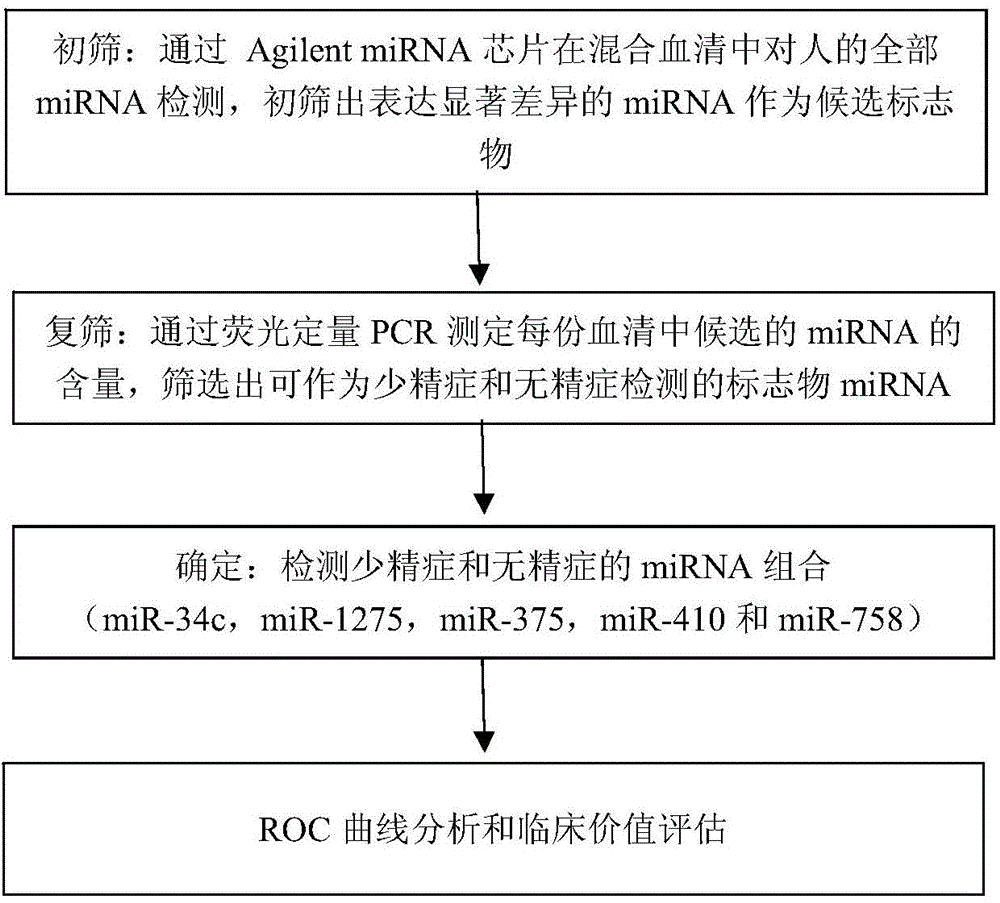
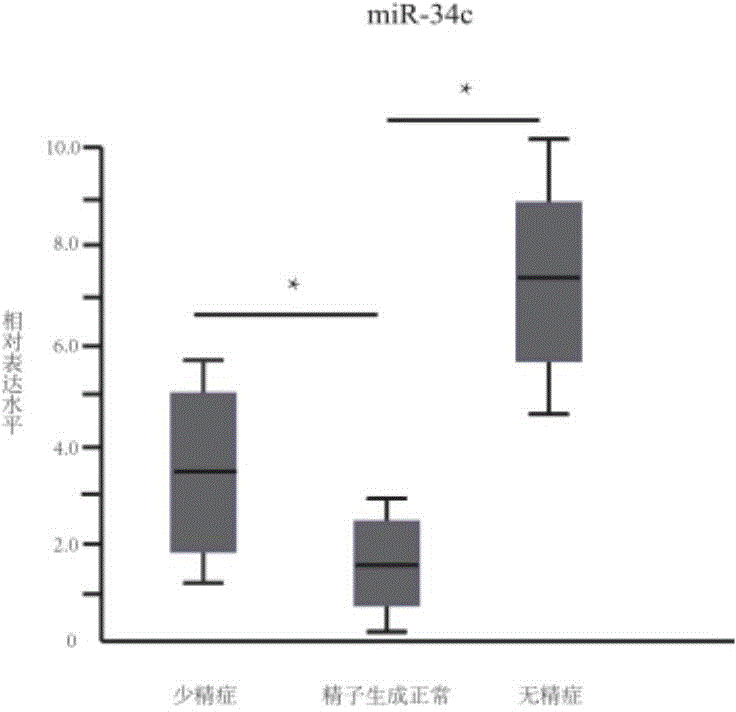
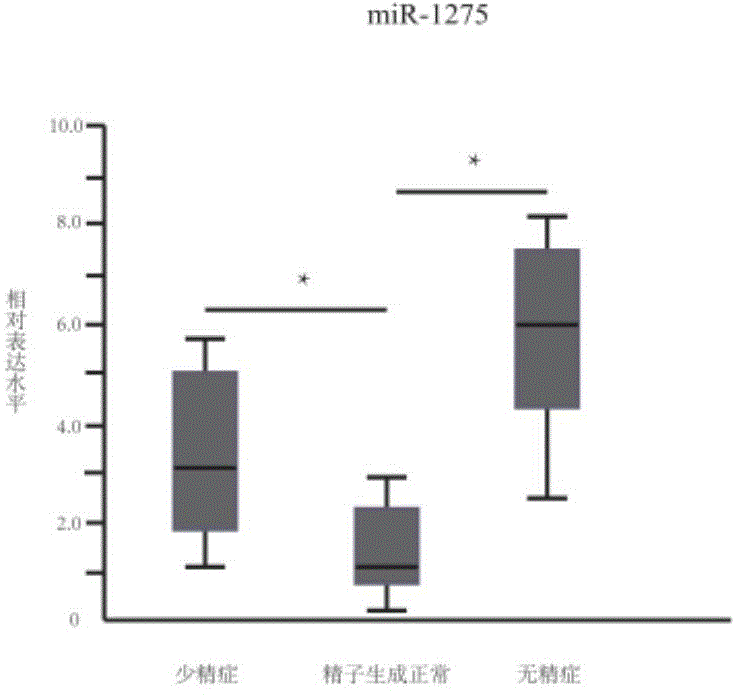
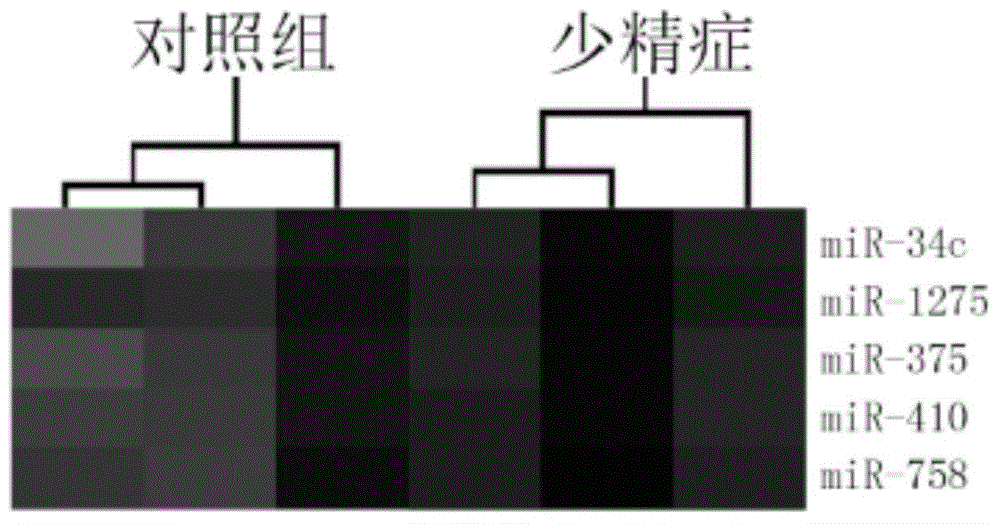
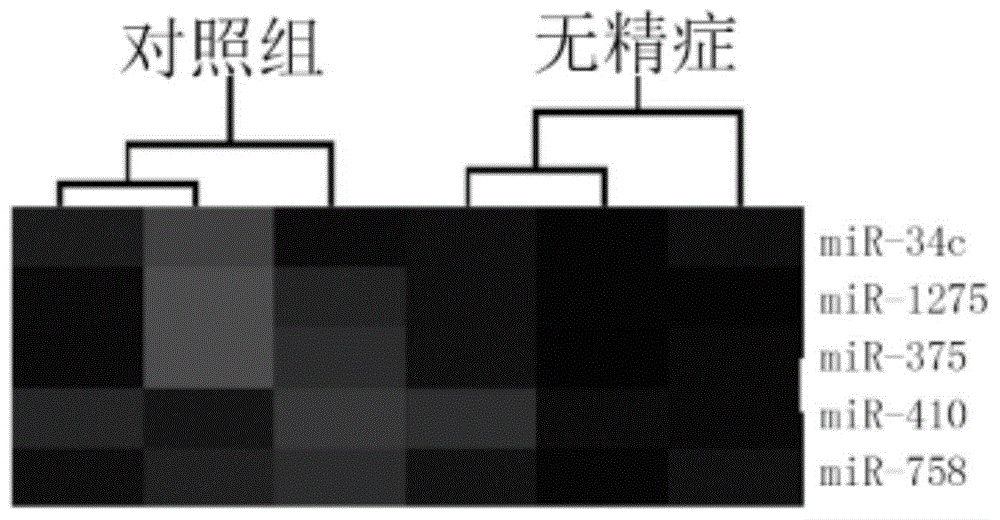
The invention provides application of a serum miRNA biomarker, and relates to biomarkers. The serum miRNA biomarker comprises at least one of miR-34c, miR-1275, miR-375, miR-410 and miR-758. The serum miRNA biomarker can be applied to preparation of a reagent for detecting oligospermia and azoospermia. A serum miRNA biomarker composition comprises at least one of the miRNA and sequences of reverse transcription primers, forward primers and reverse primers of each miRNA, and can be applied to preparation of a reagent for detecting oligospermia and azoospermia. The determined close correlation between the miRNA combination and indexes of oligospermia and azoospermia cases can be used as the biomarker to screen and diagnose patients with oligospermia and azoospermia, and an efficient basis is provided to clinical individual intervention treatment.
Owner:XIAMEN UNIV
Serum biomarkers of Hepatitis B Virus infected liver and methods for detection thereof
ActiveUS7257365B2Cosmonautic condition simulationsWave based measurement systemsHepatitis B virusBiomarker (petroleum)
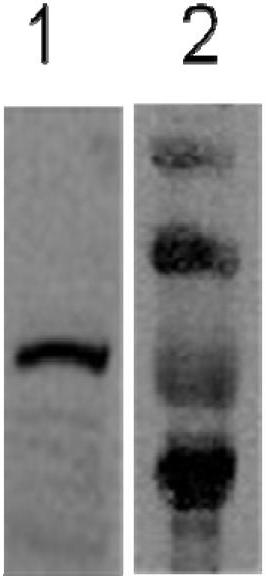
The invention provides a method for detecting the presence of altered serum proteins in an Hepatitis B Virus (HBV)-infected patient with liver inflammation, comprising: obtaining a sample of serum from the patient; subjecting the sample to protein gel electrophoresis to separate proteins contained therein; staining proteins separated on the electrophoresis gel with silver nitrate solution; scanning the images of stained proteins into an image analysis scanner to obtain gel images; comparing the gel images to control samples of electrophoresis gels prepared from serum of normal patient and serum of HBV-infected patient with liver inflammation to determine whether the sample of serum from the patient contains specific serum proteins. This invention also provides serum protein biomarkers for the diagnosis of patients with HBV infection and liver inflammation.
Owner:THE UNIVERSITY OF HONG KONG
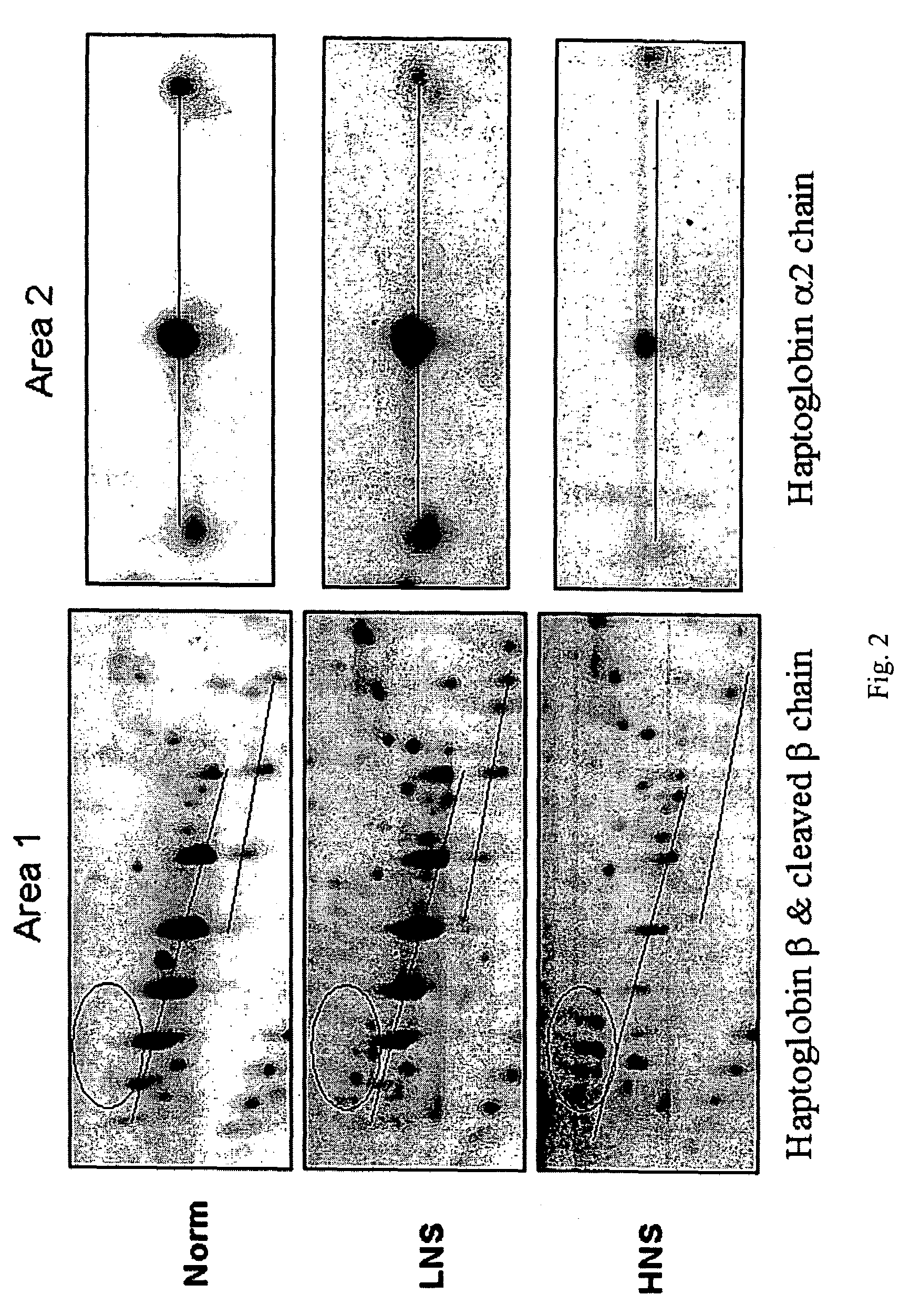
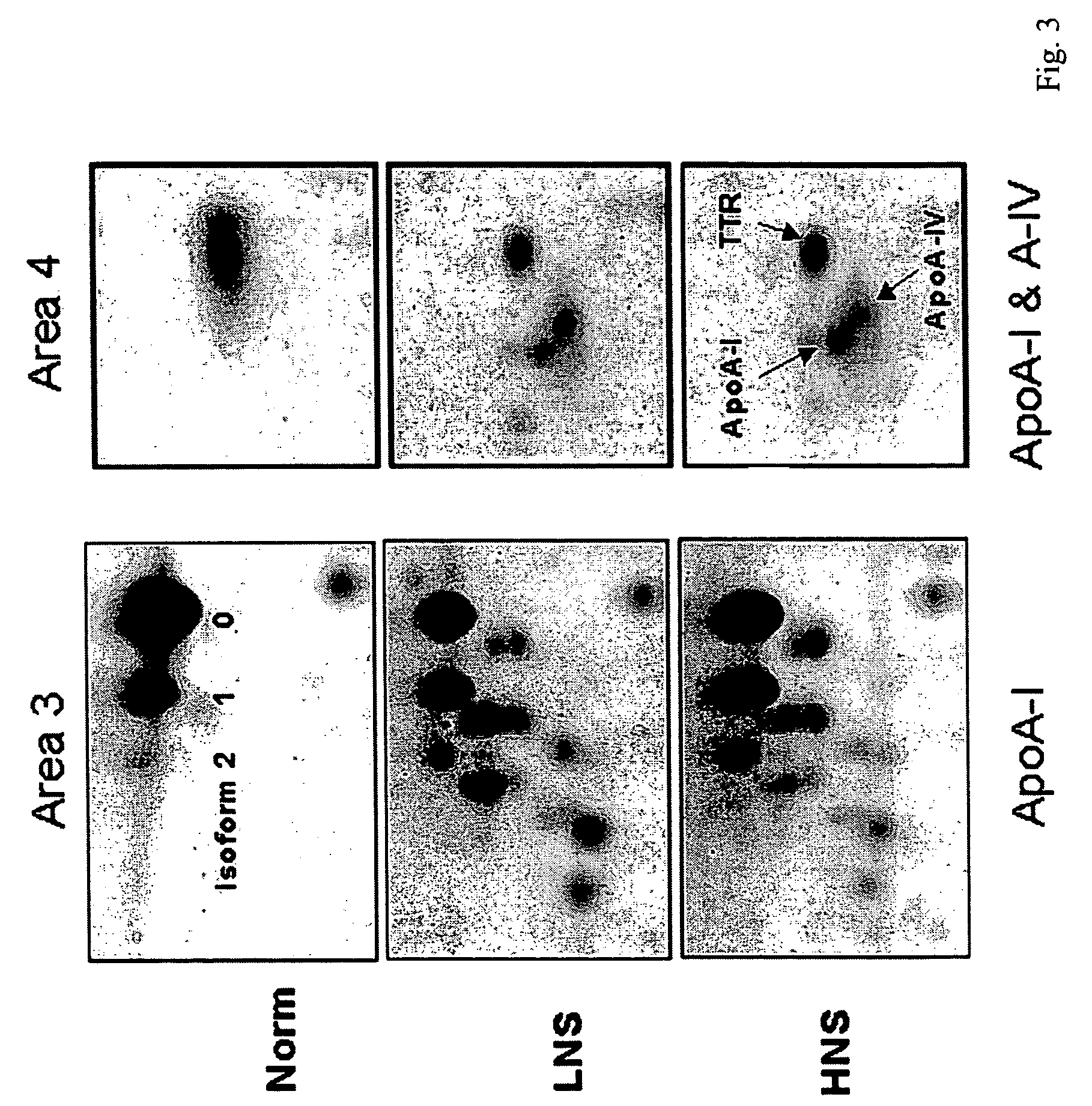
Serum Biomarkers for Diagnosing Liver Fibrosis and Method for Measuring the Same
InactiveUS20100323374A1Measured safely and accuratelyDepsipeptidesPeptide preparation methodsDiseaseMulti analyte
Disclosed are serum biomarkers for diagnosing liver fibrosis and methods for measuring the same. The serum biomarkers obtained from human serum include alpha2-macroglobulin (“A2M”), vitamin D binding protein (“VDBP”), apolipoprotein AI (“ApoAI”). The methods involve with immunoassay using specific antibodies to detect the biomarkers, including enzyme-linked immunosorbent assay (“ELISA”), radio immune assay (“RIA”) and flexible multi-analyte profiling (“xMAP”). ELISA, RIA or xMAP is used to measure changes of protein concentration for the specific protein biomarkers in serum for diagnosing liver fibrosis with suffering hepatitis B or C or other liver diseases. The measurement is safe and accurate. The method can be used before and after treatment of liver fibrosis. Thus, it is possible to achieve early diagnosis and treatment advocated in the preventive medicine.
Owner:INST NUCLEAR ENERGY RES ROCAEC
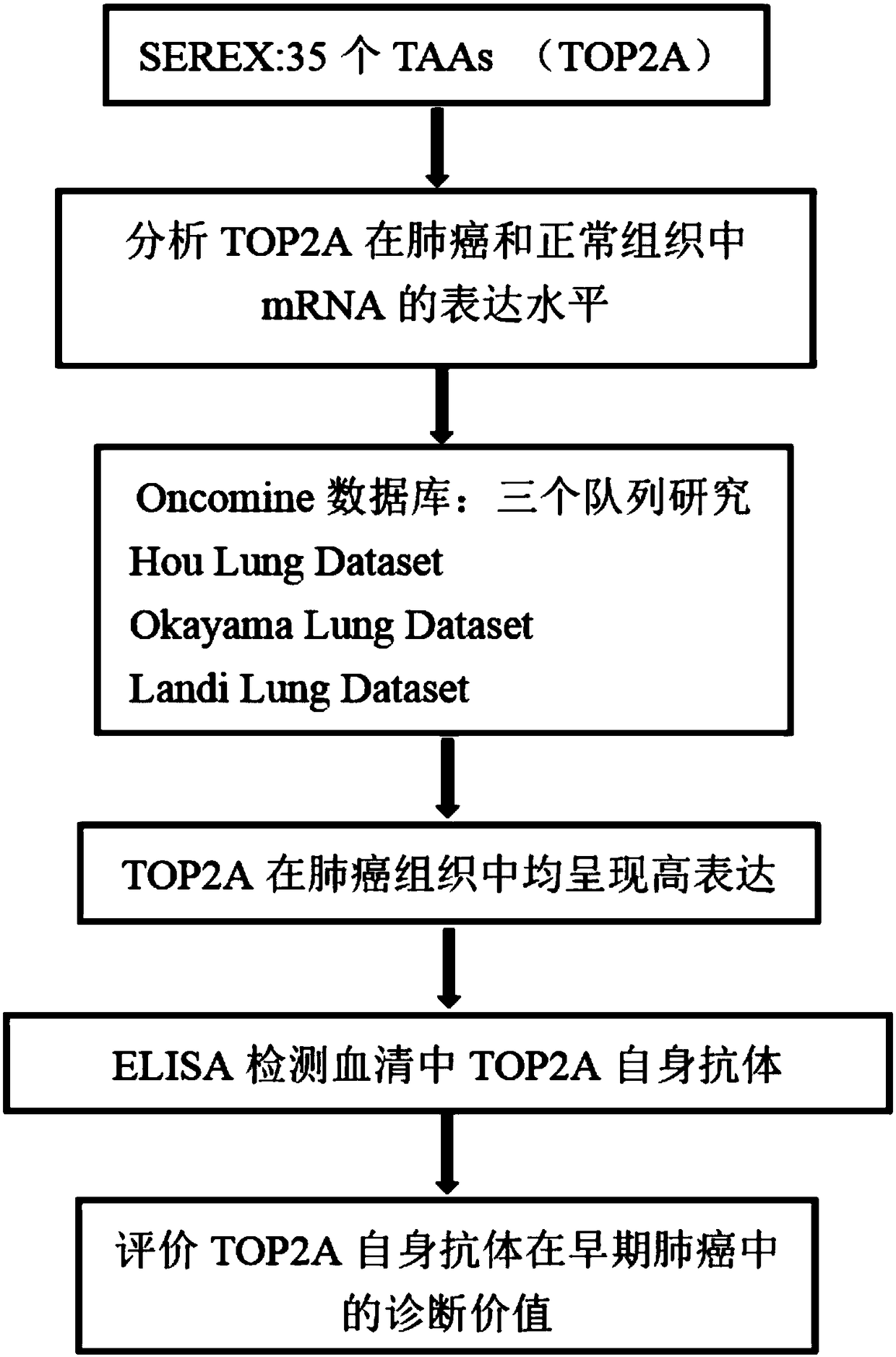
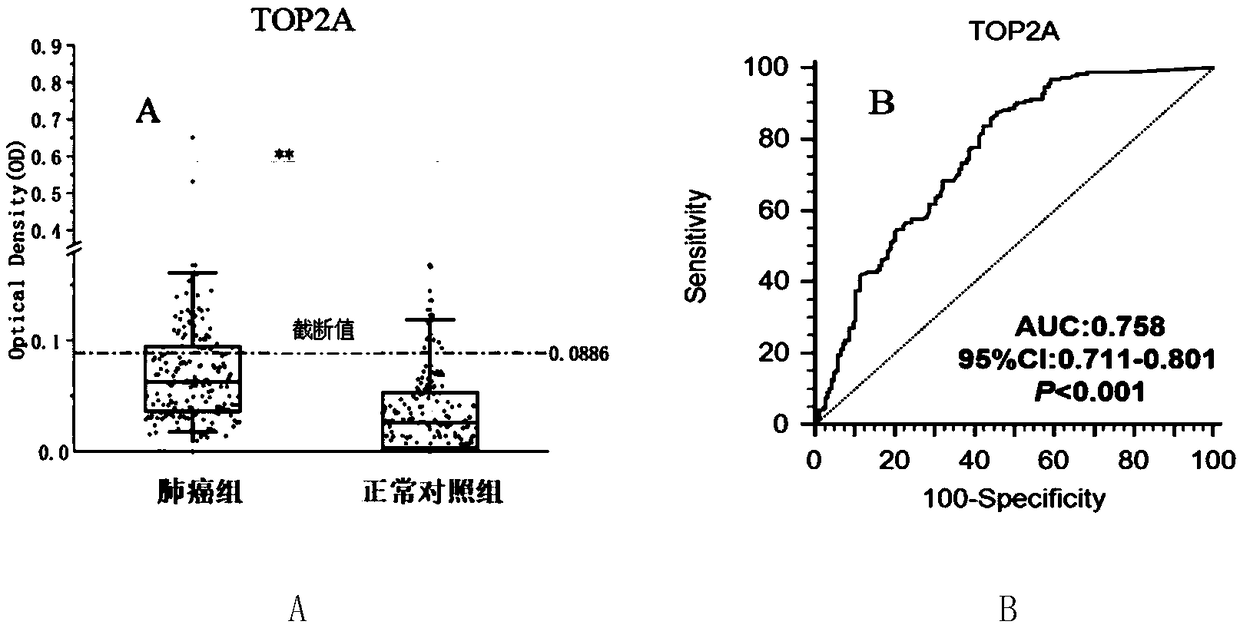
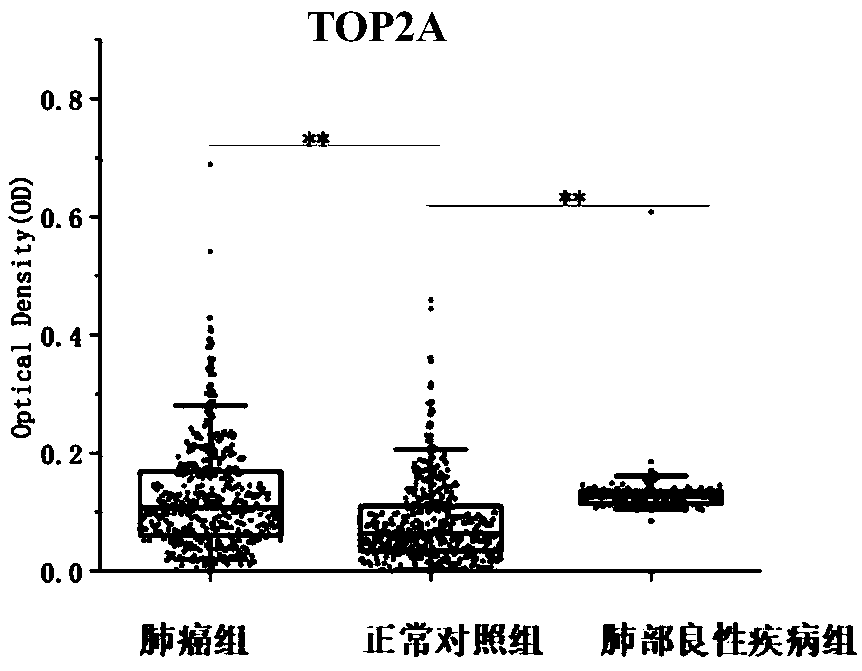
Application of TOP2A (Topoisomerase(DNA)II Alpha) autoantibody as lung cancer diagnostic marker
The invention discloses an application of TOP2A (Topoisomerase(DNA)II Alpha) autoantibody as a lung cancer diagnostic marker, and further discloses a screening and identification method for the TOP2Aautoantibody as the lung cancer diagnostic marker. A serum biomarker which is used for clinical lung cancer diagnosis and is simple to operate, low in cost, high in accuracy and noninvasive is provided. Research shows that with application of an ELISA (enzyme-linked immunosorbent assay) method in detection of the serum TOP2A autoantibody, lung cancer patients, normal people and patients with chronic lung diseases can be more accurately distinguished, and the TOP2A autoantibody can be used for early diagnosis of lung cancer. Under the background, the lung cancer patients are detected conveniently, quickly and effectively, and the TOP2A autoantibody can be used for clinical early diagnosis of lung cancer.
Owner:ZHENGZHOU UNIV
Serum biomarkers for early detection of acute cellular rejection
The present invention provides an improved method of diagnosing a subject having received an organ transplant with Acute Cellular Rejection (ACR). The method comprises obtaining a biological sample from the subject, detecting an amount of at least one protein indicative of ACR in the sample, and comparing the amount of the protein in the sample to a control, wherein a difference between the amount of the protein in the sample relative to the control indicates the subject has or is developing ACR. The difference can be an increase or a decrease. In one version the biological sample comprises a serum sample, and the transplanted organ is selected from a heart, kidney, liver, bone marrow, pancreas, eye, lung or skin. A kit and methods of treating a subject having an organ transplant for ACR and treating an immune suppressed subject are also provided.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
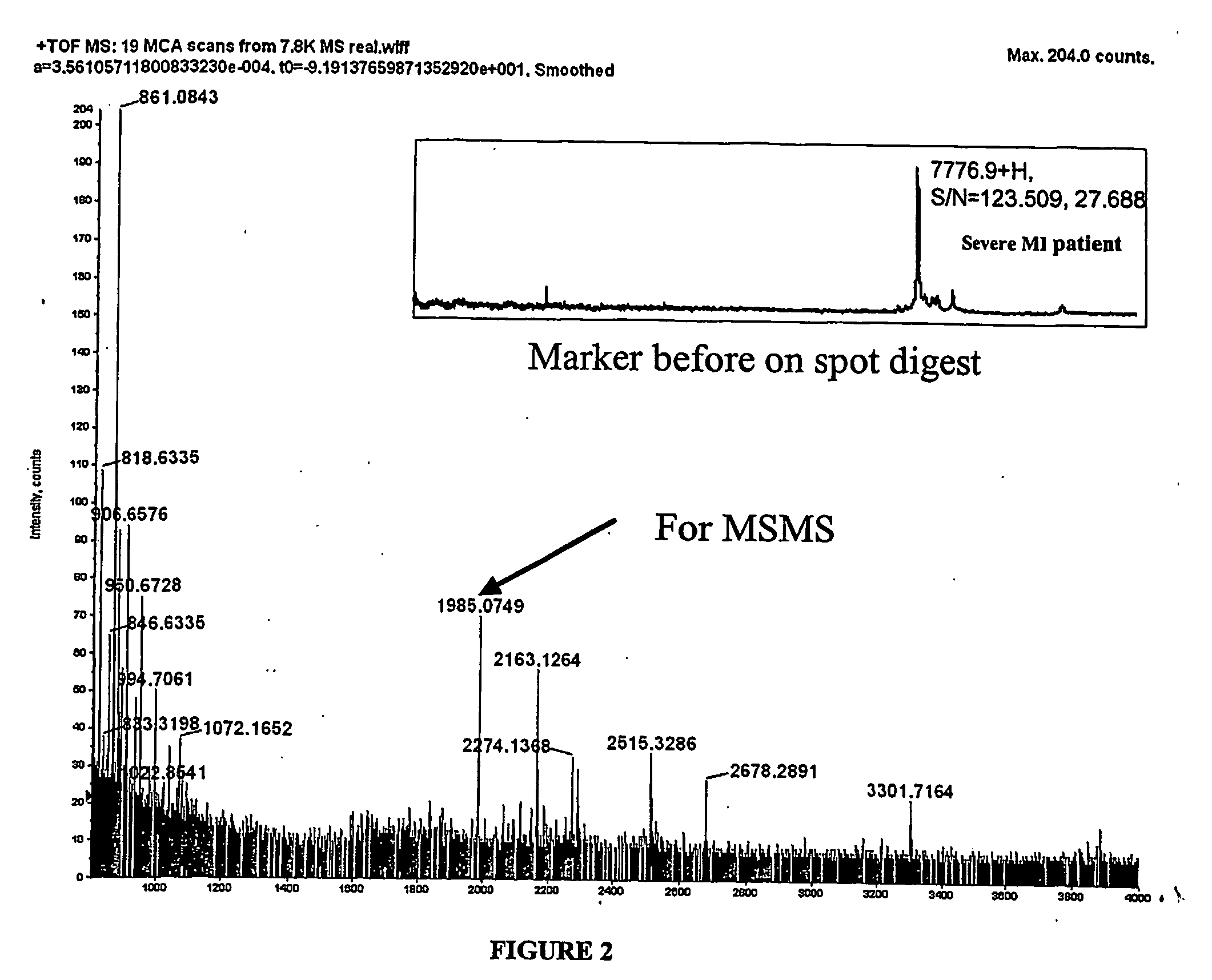
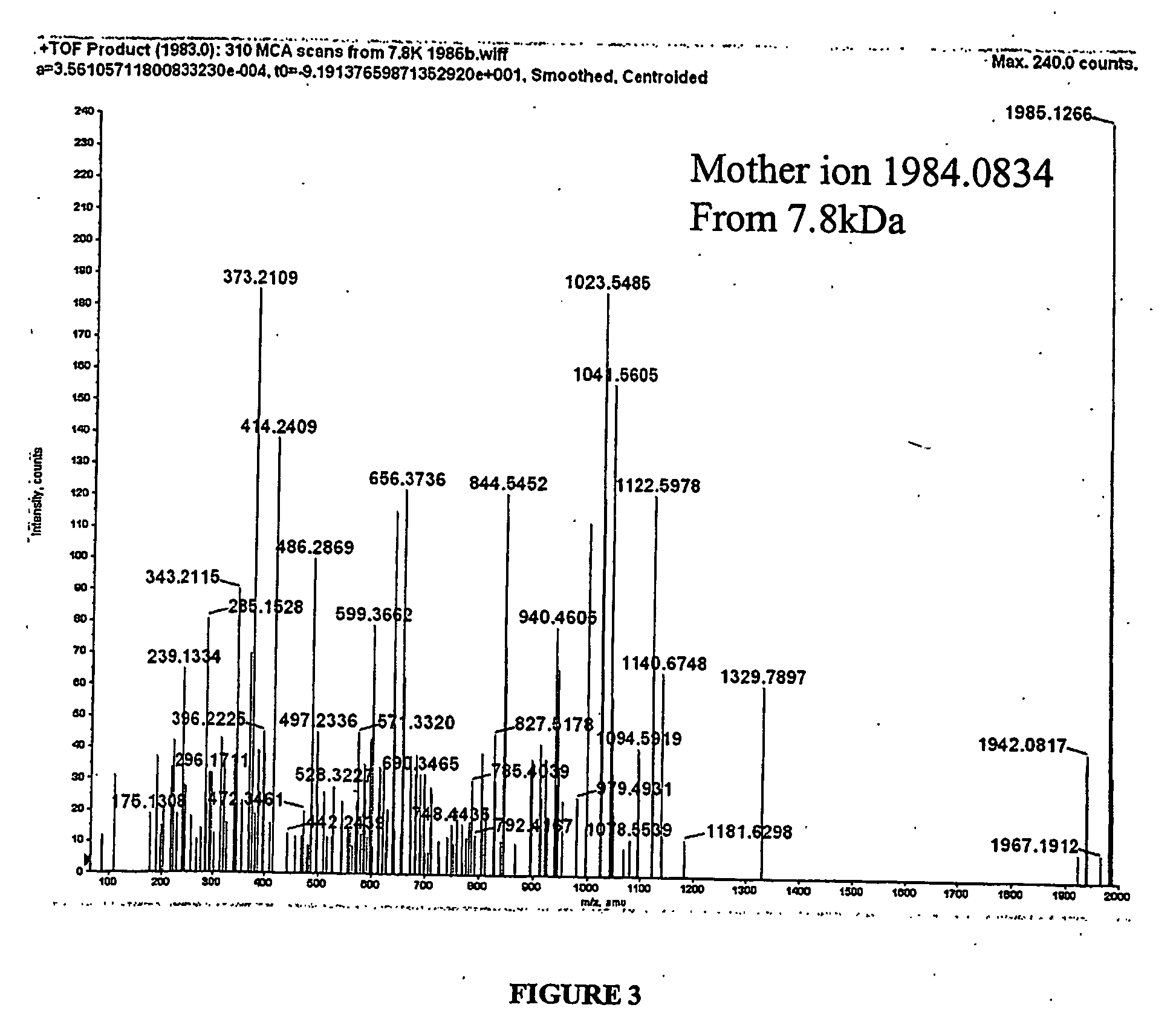
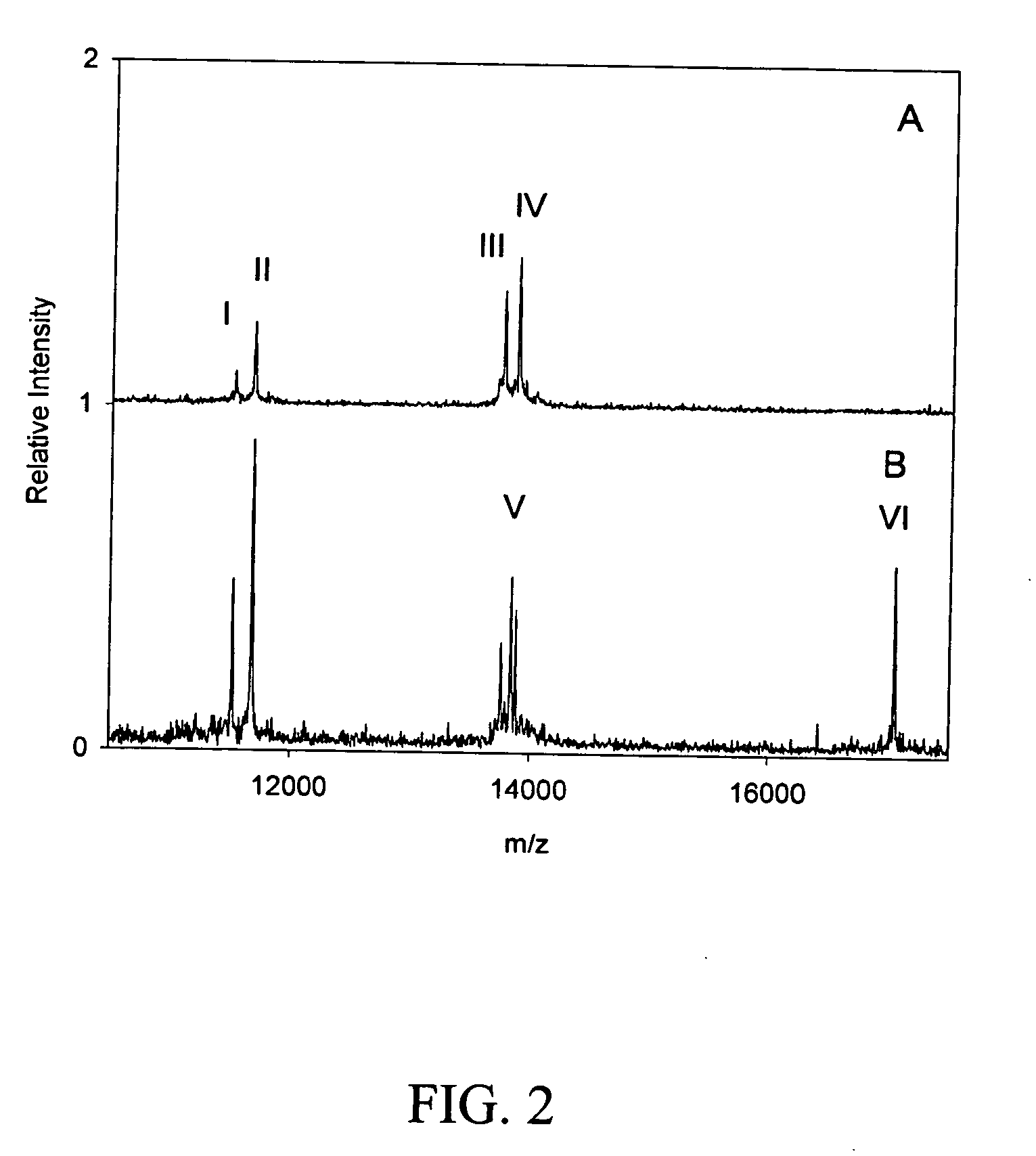
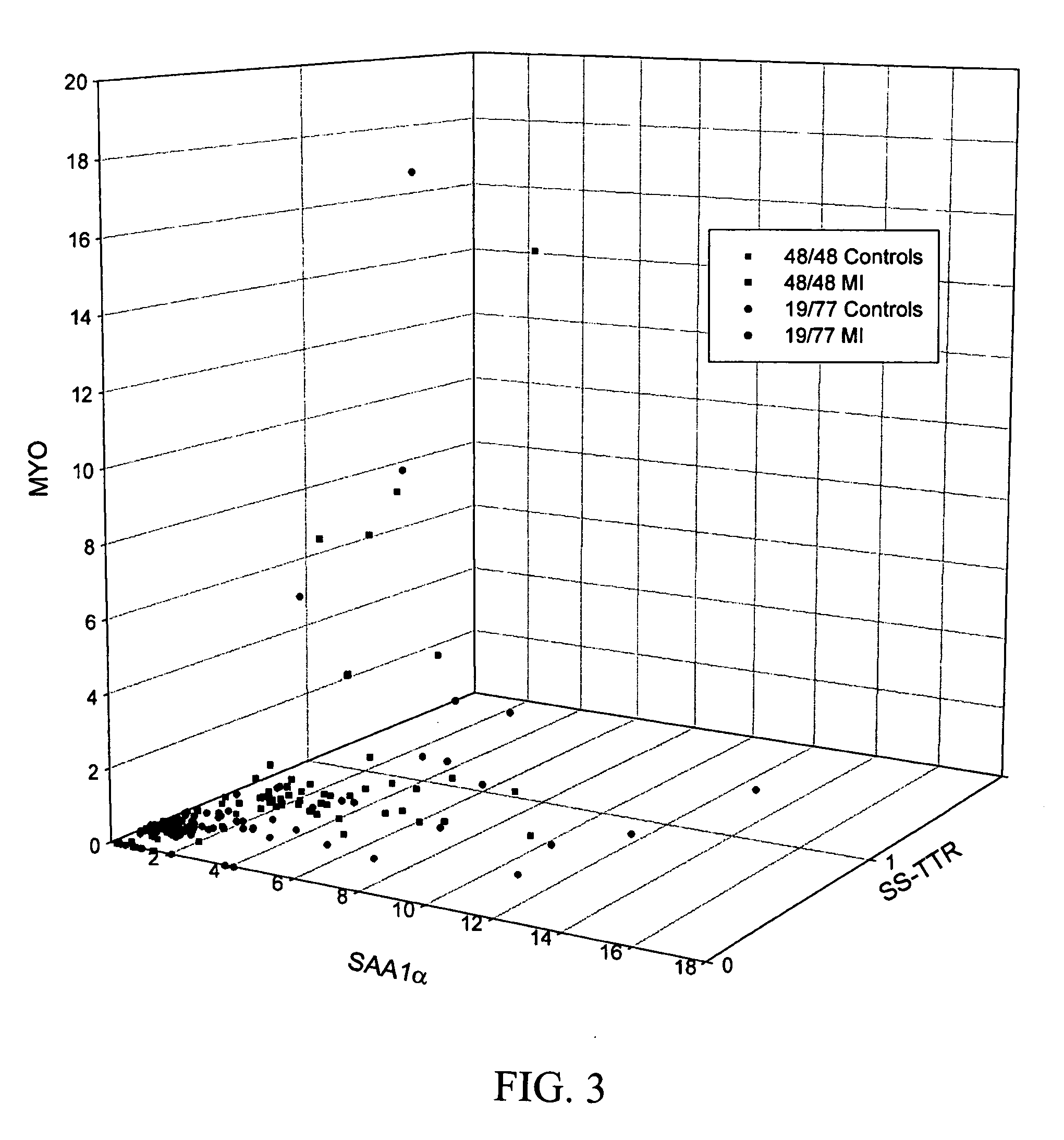
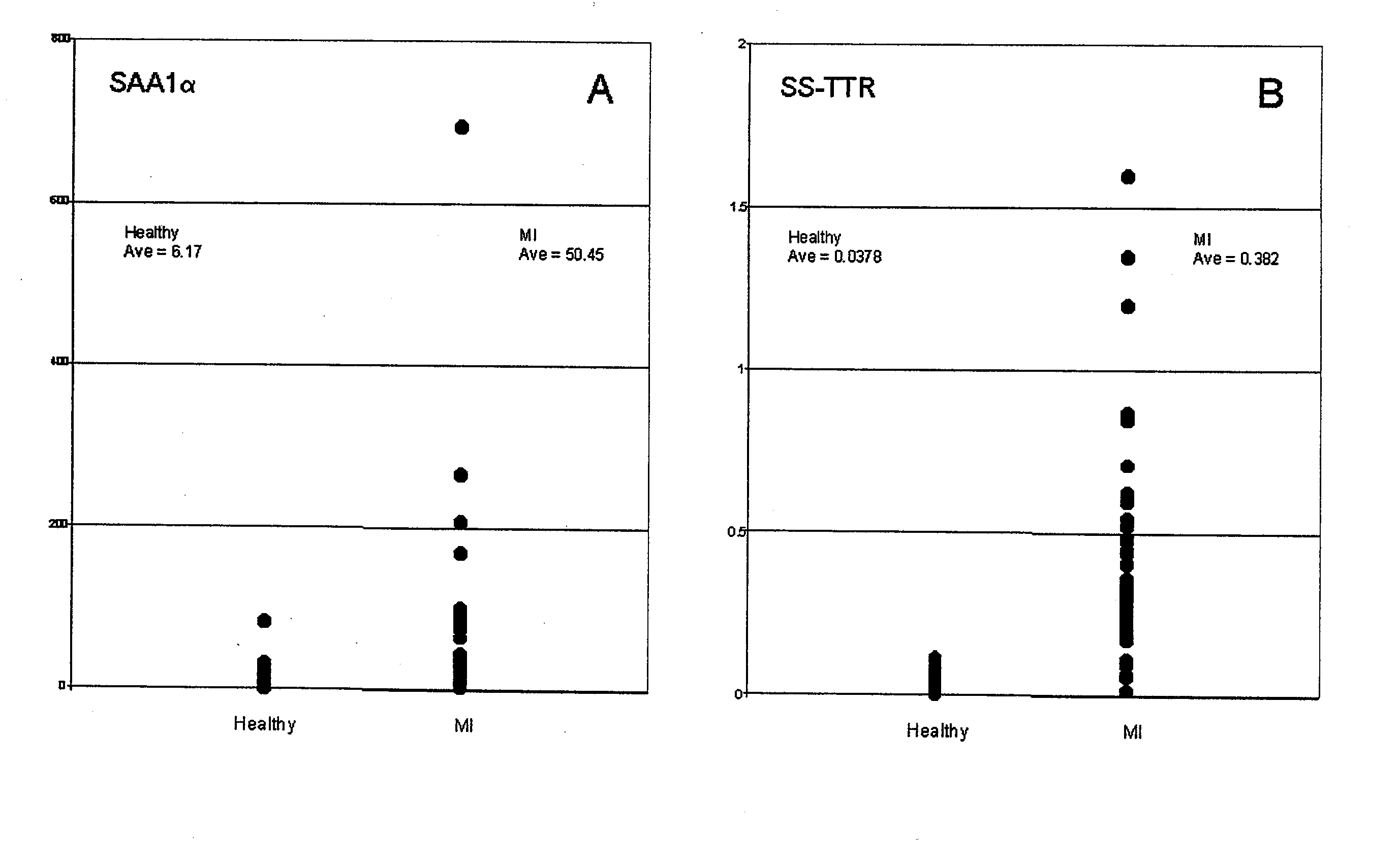
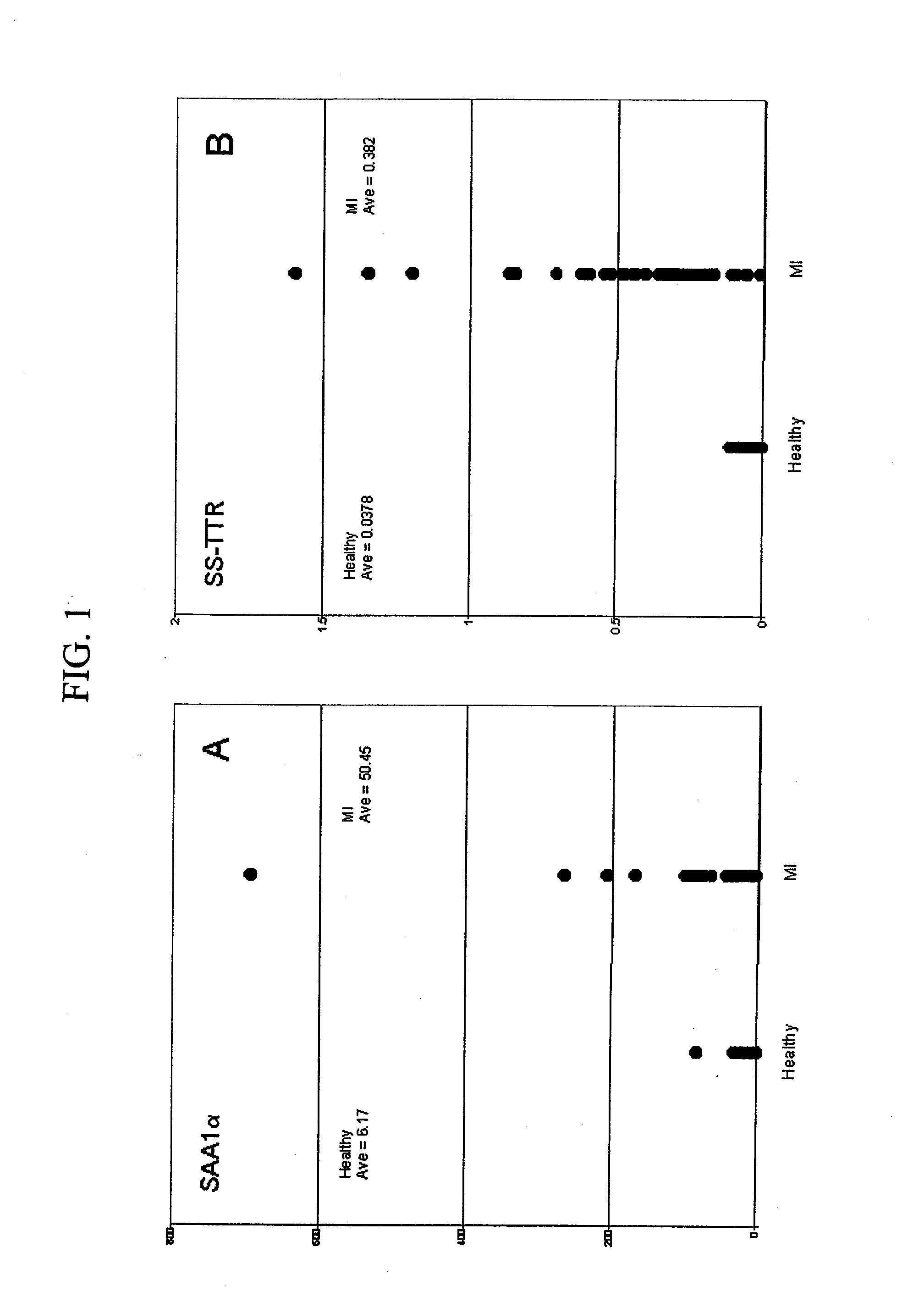
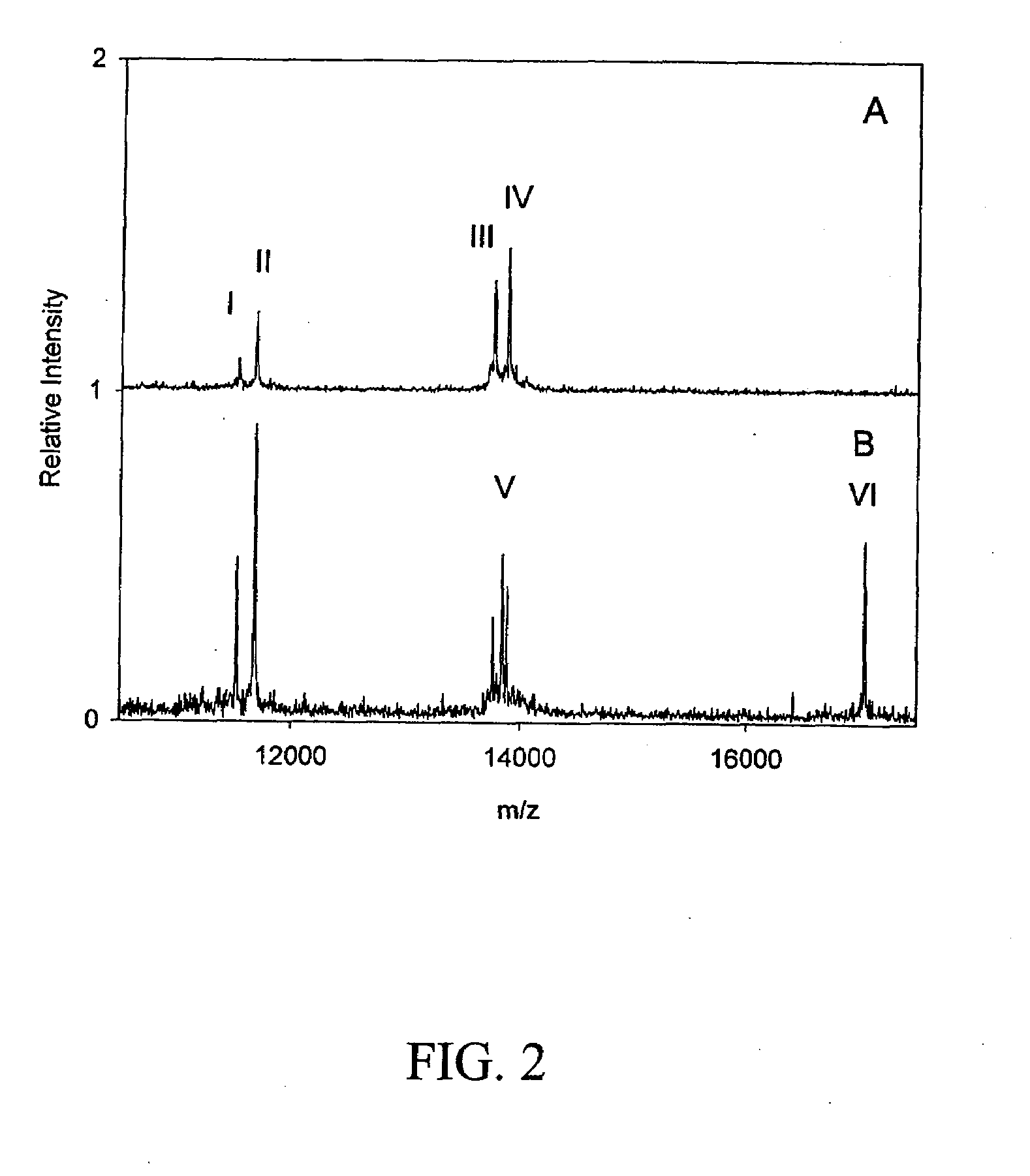
Biomarkers and assays for myocardial infarction
InactiveUS20070042426A1Improve forecast accuracyImprove analysis accuracyDisease diagnosisBiological testingMedicineTrue positive rate
Presented herein are novel blood plasma / serum biomarkers related to cardiovascular disease. These newly identified biomarkers create the basis for multiple (single) assays using traditional bioassay technologies and when used in combination yield exceptional clinical sensitivity and specificity in the determination of myocardial infarction (MI). A multiplexed, mass spectrometric immunoassay (MSIA) able to simultaneously assay for the new / novel biomarkers as well other MI markers is also presented. Means and methods for evaluating data generated using multiple biomarkers in order to validate findings and further the use of the multiplexed MI assay in clinical, diagnostic and therapeuatic uses is also included.
Owner:INTRINSIC BIOPROBES
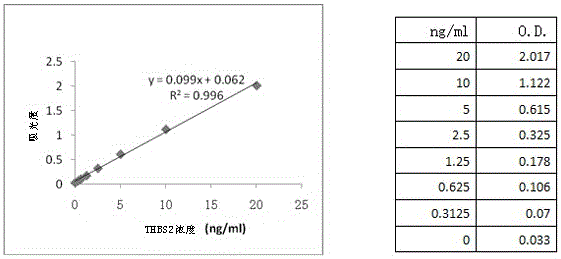
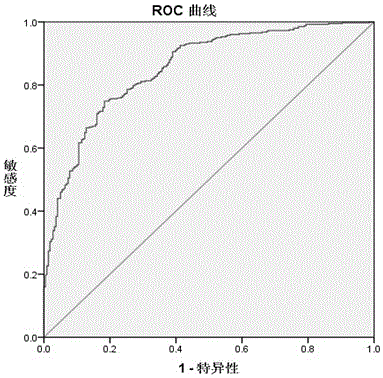
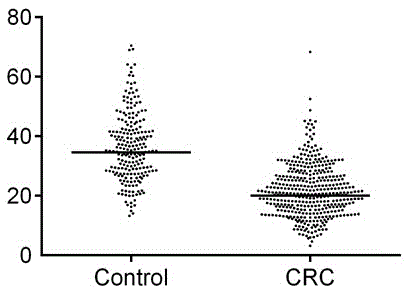
Application of THBS2(Thrombospondin-2) as rectal cancer serum marker and diagnostic kit
InactiveCN105092846AImprove featuresIncreased sensitivityMaterial analysisSerum markersQuantitative determination
Application of THBS2(Thrombospondin-2) as a rectal cancer serum marker and a diagnostic kit are disclosed, and belong to the technical field of biology. On the one hand, the invention provides application of THBS2 as the rectal cancer serum marker, and on the other hand, the invention provides the colorectal cancer diagnostic kit. The beneficial effects comprise that the provided serum biological marker has the specificity of 87.2% and the sensitivity of 65.0%, and possesses relatively high specificity and sensitivity; and the technical scheme provides a sensitive reliable operable method for quantitative determination on human serum THBS2 antigen level, and is beneficial for auxiliary early-stage diagnosis on colorectal cancer.
Owner:THE AFFILIATED SIR RUN RUN SHAW HOSPITAL OF SCHOOL OF MEDICINE ZHEJIANG UNIV
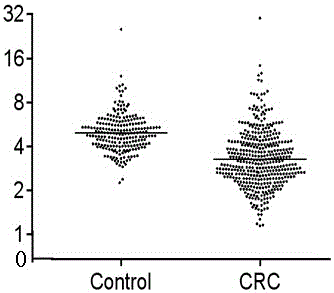
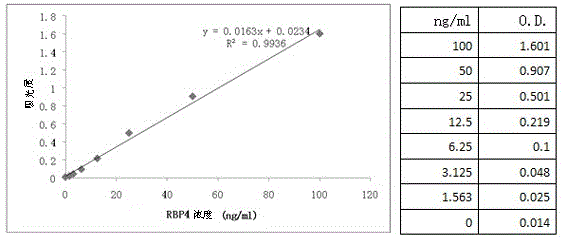
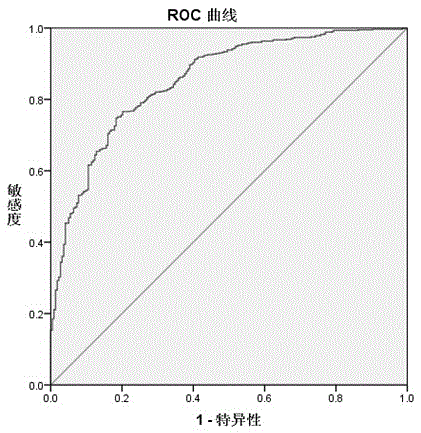
Application of RBP4 as colorectal cancer blood serum marker and diagnostic kit
InactiveCN105044360AImprove featuresIncreased sensitivityBiological material analysisBiological testingSerum biomarkersBiological organism
The invention discloses application of an RBP4 as a colorectal cancer blood serum marker and a diagnostic kit, and belongs to the technical field of biology. On one hand, the invention provides the technical scheme of the application of the RBP4 as the colorectal cancer blood serum marker, and on the other hand, the invention provides the technical scheme of the colorectal cancer diagnostic kit. The advantages that the blood serum biological marker has the specificity of 81.7% and the sensibility of 74.9% and has high specificity and sensibility, a flexible, reliable and operable method is provided, the antigen level of the RBP4 in human blood serum is quantitatively measured and the RBP4 can easily assist in colorectal cancer early diagnosis are achieved.
Owner:THE AFFILIATED SIR RUN RUN SHAW HOSPITAL OF SCHOOL OF MEDICINE ZHEJIANG UNIV
Biomarkers and assays for myocardial infarction
ActiveUS20090209047A1Improve forecast accuracyImprove analysis accuracyParticle separator tubesIsotope separationMedicineTrue positive rate
Owner:INTRINSIC BIOPROBES
Serum miRNA biomarker composition and application thereof
ActiveCN104450702AEasy to useRelieve painMicrobiological testing/measurementDNA/RNA fragmentationForward primerAzoospermia
The invention relates to a biomarker, and particularly relates to a serum miRNA biomarker composition and an application thereof. The serum miRNA biomarker composition comprises at least one miRNA and sequences of reverse transcription primers, forward primers and reverse primers of all miRNAs. The serum miRNA biomarker composition can be used for preparing oligozoospermia and azoospermia detection reagents. An oligozoospermia and azoospermia detection kit comprises the serum miRNA biomarker composition, Taq enzyme, dNTP, MgCl2 and a PCR buffer solution. The serum miRNA biomarker composition provided by the invention has the advantages of high sensitivity, low sample consumption, wide detection range and wide linear quantitative range.
Owner:XIAMEN UNIV
Lung cancer marker anti-ACTR3 autoantibody and applications thereof
The invention belongs to the technical field of biomedical detection, particularly relates to a lung cancer marker anti-ACTR3 autoantibody and applications thereof, and discloses a lung cancer marker,which is an anti-ACTR3 autoantibody. The invention further provides applications of the lung cancer marker in preparation of lung cancer detection kits. According to the present invention, the serumbiomarker with characteristics of simple operation, low cost, high accuracy and non-invasive application in clinic is provided; the research results show that the detection of the serum anti-ACTR3 autoantibody with the ELISA method can accurately distinguish lung cancer patients from normal people and patients with benign lung diseases, and can be used for early detection of lung cancer; and in the context, the marker for conveniently, rapidly and effectively detecting lung cancer patients and the lung cancer detection kit prepared by using the marker can be used for clinical early diagnosis of lung cancer.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Diagnostic kit and application of RNF19 (ring finger protein 19) in preparation of reagent for early diagnosis of gastric cancers
The invention relates to the field of bioscience, in particular to a diagnostic kit and application of an RNF19 (ring finger protein 19) in preparation of a reagent for early diagnosis of gastric cancers. The diagnostic kit is used for early diagnosis of gastric cancers and comprises an ELISA (enzyme-linked immunosorbent assay) plate, a human protein RNF19, standard serums, an ELISA reagent, an enzyme substrate solution, confining liquid, sample diluent, washing liquid and stop liquid, wherein the human protein RNF19 is enveloped on the ELISA plate. Compared with the prior art, the diagnostic kit has the following advantages: 1. a sensitive, safe, reliable and easy-to-operate commercial kit is provided, is used for qualitatively determining the level of the IgA antibody against RNF19 in the human serums, and is conductive to early diagnosis of gastric cancers in an aided manner; and 2. the provided serum biomarker RNF19 has specificity of 86% and sensitivity of 86% and has the characteristics of high specificity and high sensitivity.
Owner:广州博翀生物科技有限公司
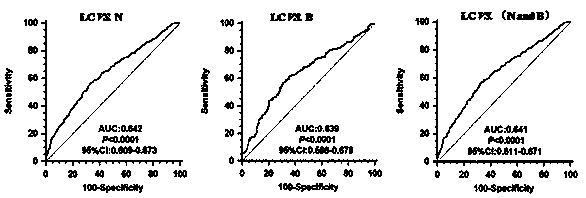
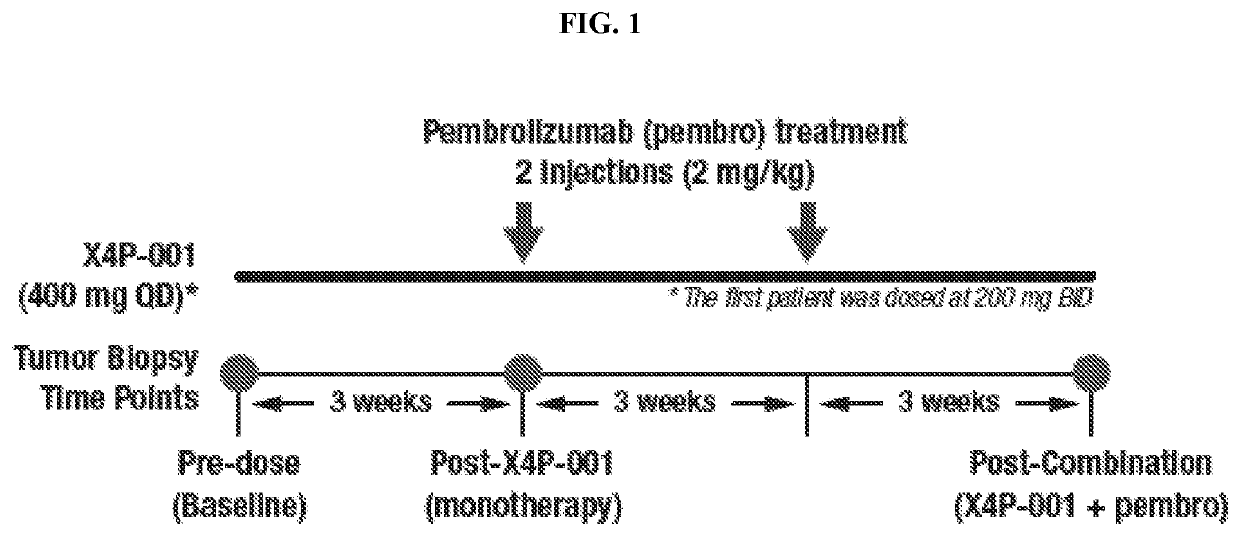
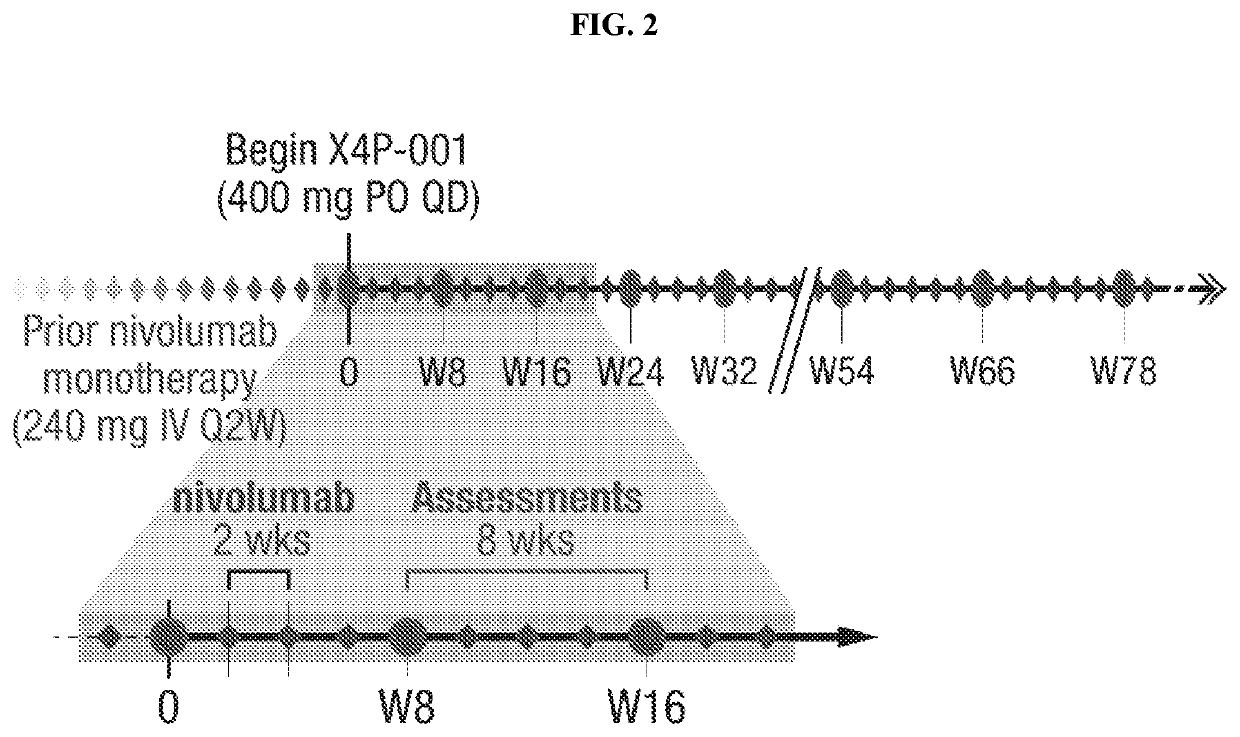
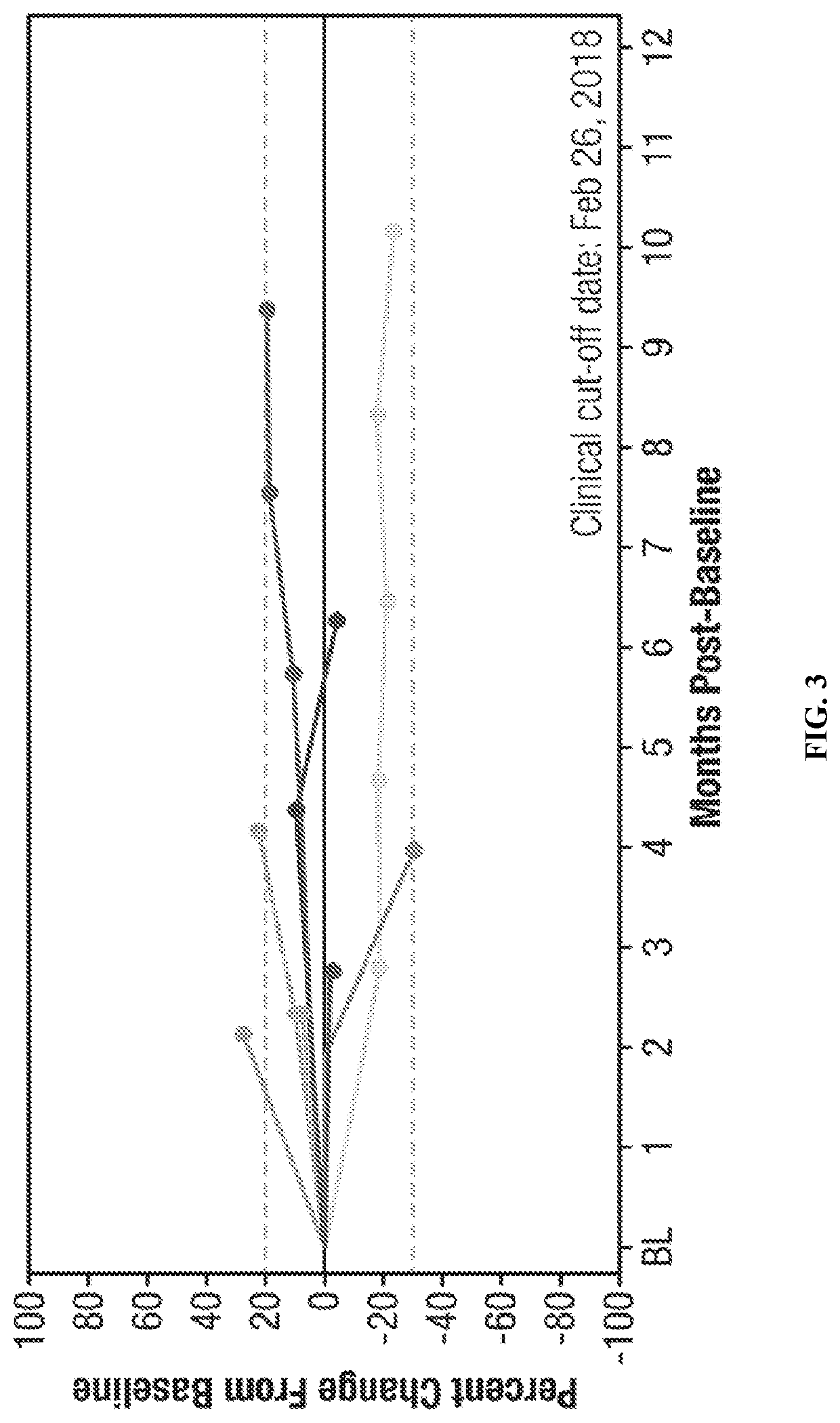
Cancer serum biomarkers and methods of use thereof
The present invention relates, in part, to certain serum biomarkers and use thereof in methods for treating cancer, such as in evaluating and / or in additional methods such as predicting patient responses to treatment with a CXCR4 inhibitor optionally in combination with a immunotherapeutic agent, in patients with a cancer such as melanoma, including resectable and unresectable melanoma.
Owner:X4 PHARMA INC
Application of karyosome protein SP110 and kit containing karyosome protein SP110 to preparation of early diagnosis reagent for alcoholic cardiomyopathy
ActiveCN108982868AEasy to operateStrong specificityDisease diagnosisBiological testingIgm antibodyBiomarker (petroleum)
Owner:HARBIN MEDICAL UNIVERSITY
Serum biomarkers for Chagas disease
InactiveUS20050260691A1Microbiological testing/measurementBiological material analysisMedicineChagas disease
Owner:MCGILL UNIV
Method to measure serum biomarkers for the diagnosis of liver fibrosis
InactiveUS20090275061A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSerum igeSerum samples
This invention discloses using SPR technology to simultaneously and quantitatively measure the concentrations of different liver fibrosis-associated serum biomarkers in a serum sample, which can be used for the diagnosis of liver fibrosis. It also discloses an efficient formula to make a mixed SAM that can greatly enhance the immobilization ability of the metal surface in SPR based techniques, which is good for the immobilization of relevant antibodies used for the detection of liver fibrosis-associated serum biomarkers for the diagnosis of liver fibrosis.
Owner:CMED TECH
Serum biomarker for early diagnosis of schistosomiasis japonica and screening method and application
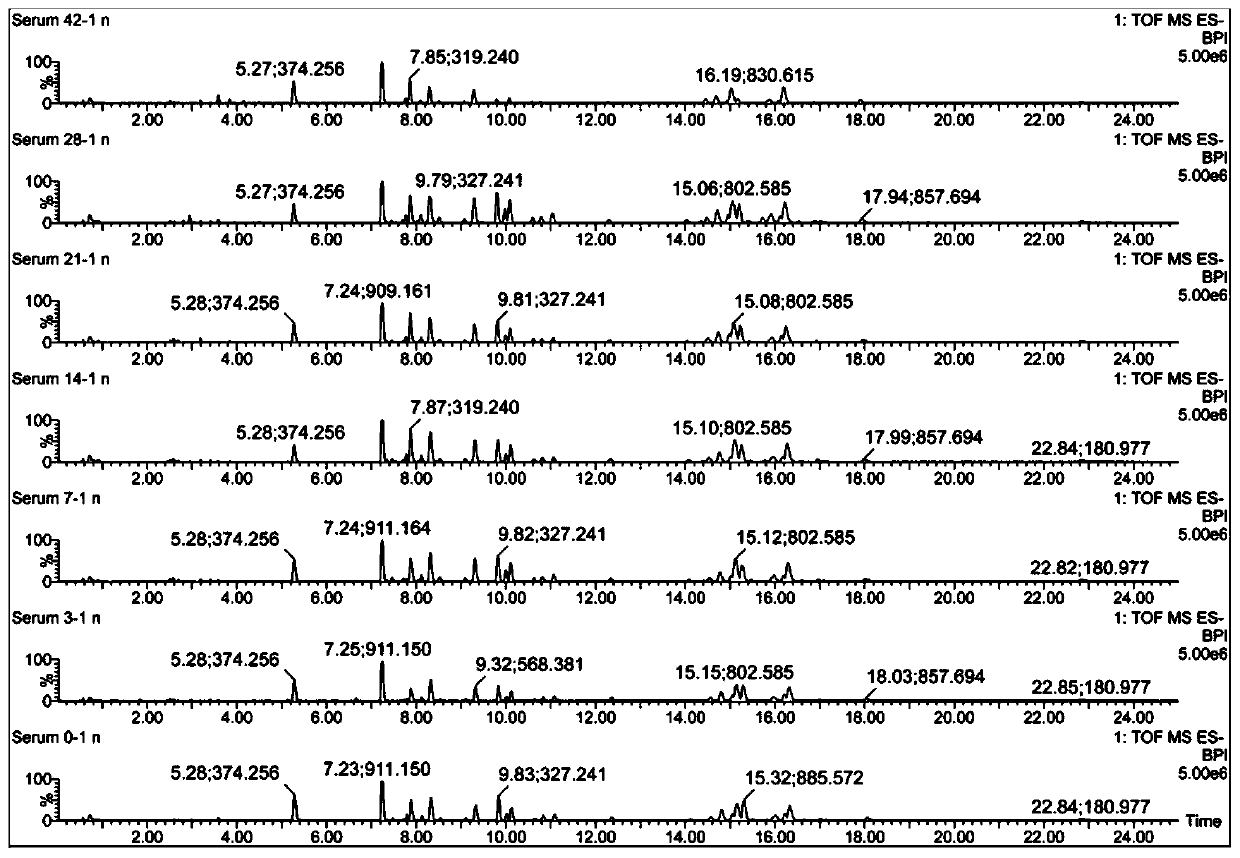
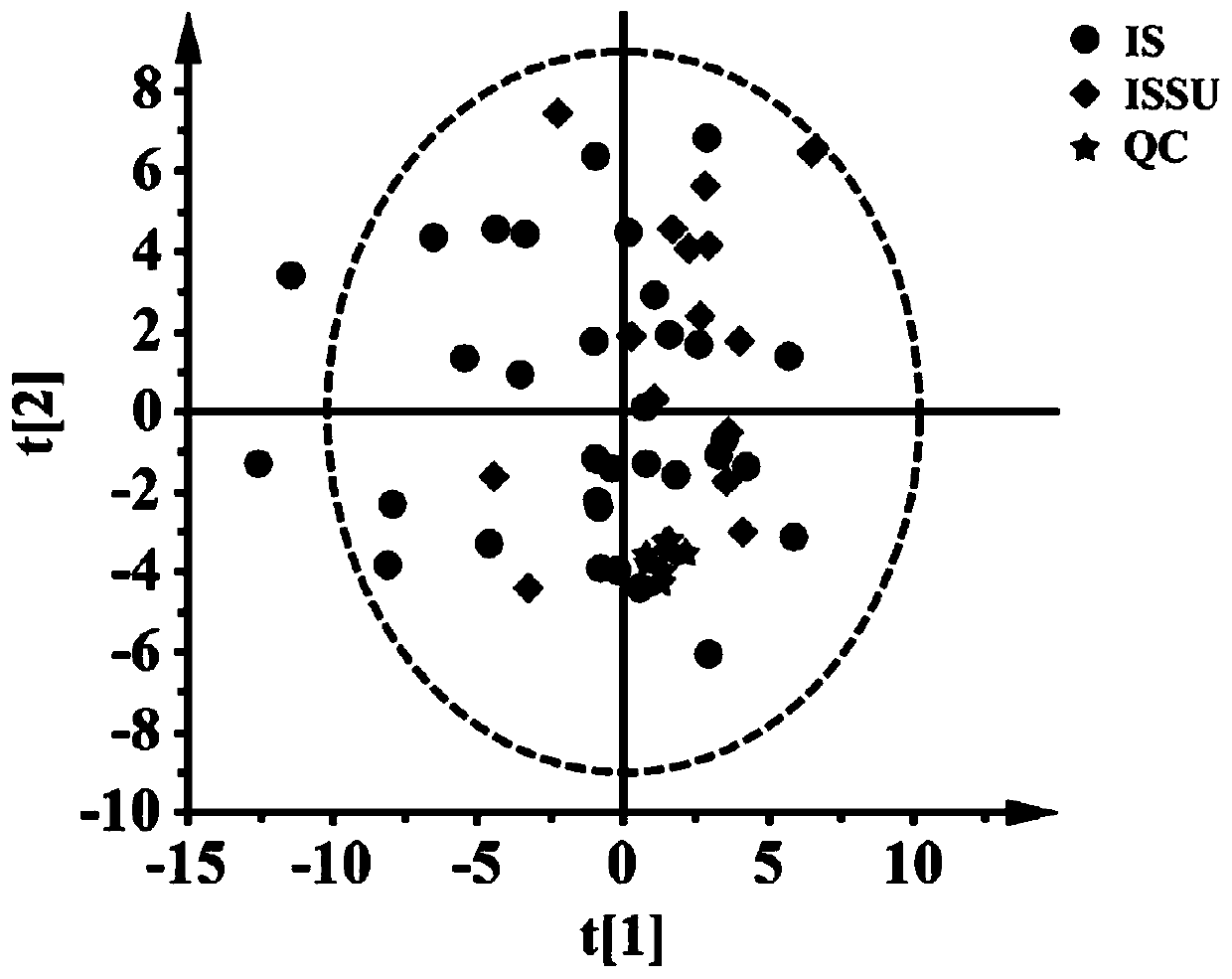
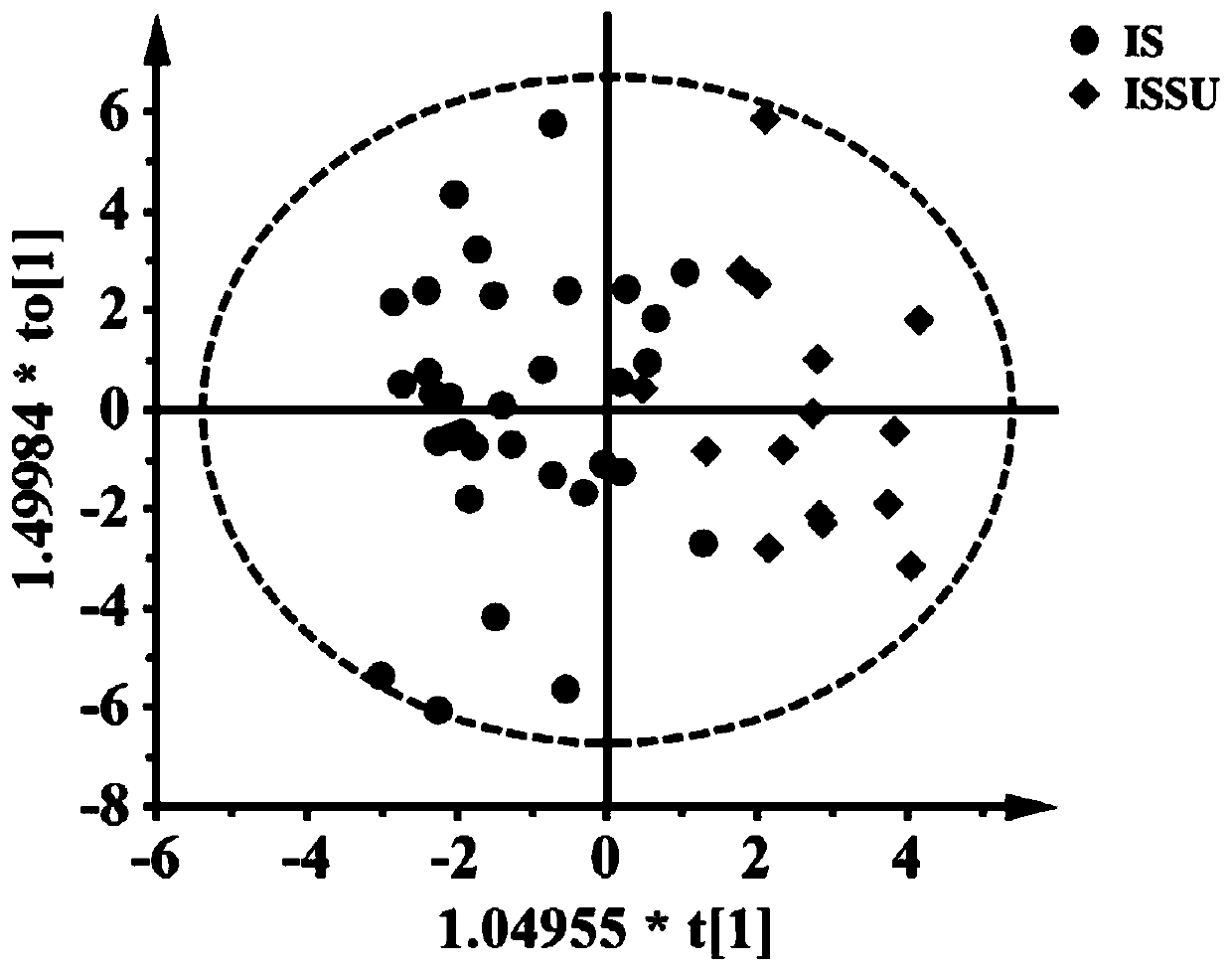
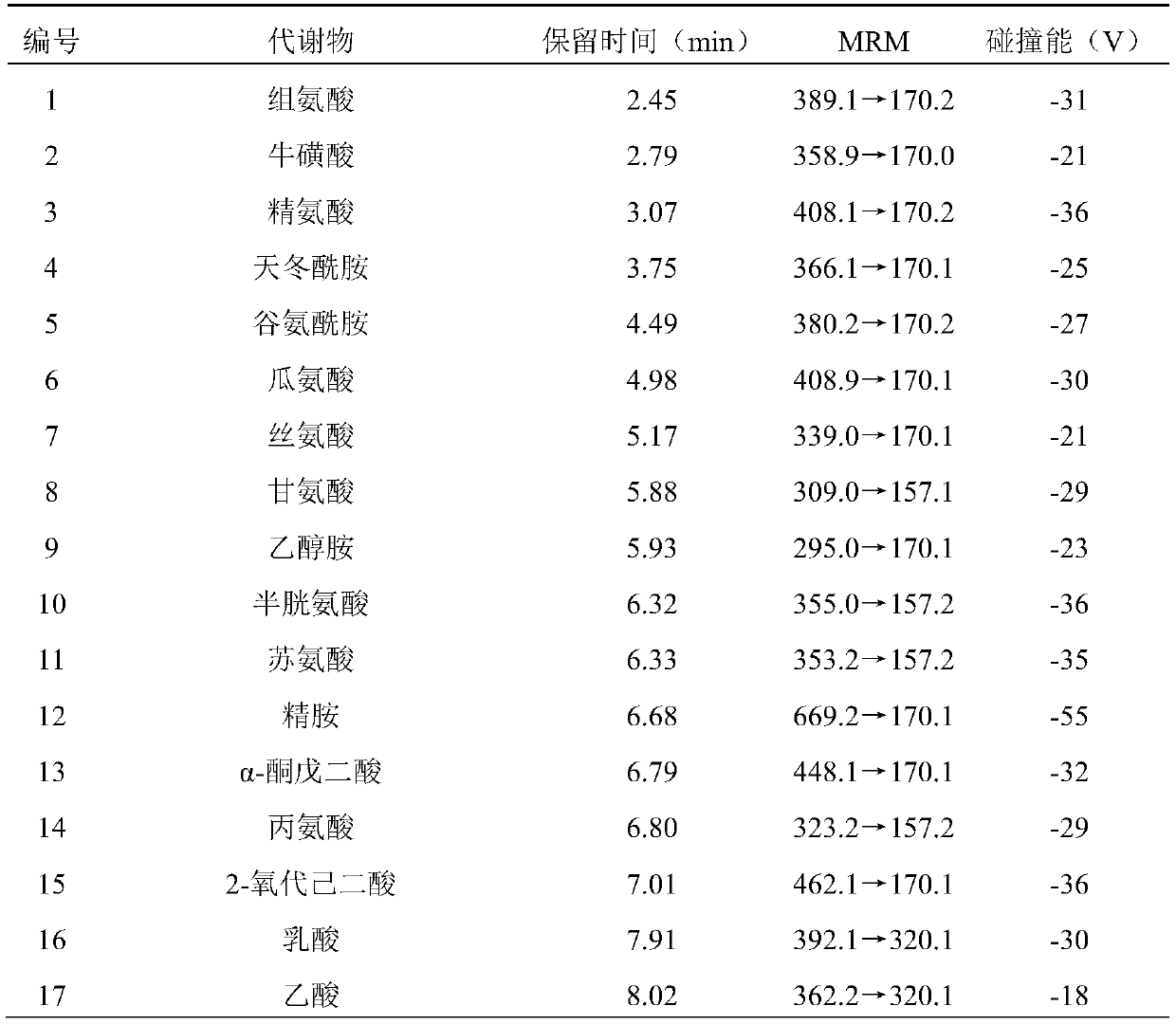
ActiveCN110763795AIndicators are predictiveGood forecastComponent separationSchistosomiasesMetabolite
The invention discloses a serum biomarker for early diagnosis of schistosomiasis japonica. The serum biomarker is phosphatidylcholine and / or palmitoyl choline. The sensitivity and specificity of the marker are both greater than 0.9, and the under-curve area (AUC) is greater than 0.9, which indicates that the two indexes have favorable predictability and can be used for early diagnosis of schistosomiasis japonicum katsurada. The invention further discloses a screening method of the serum biomarker for early diagnosis of schistosomiasis japonica based on metabonomics. A mouse schistosomiasis japonica model is constructed, and the metabonomics analysis is carried out on mouse serum by utilizing an ultra-high performance liquid chromatography-tandem mass spectrometry technology, and characteristic differential metabolites between a normal mouse and a schistosomiasis japonica infected mouse are found and analyzed, namely, the schistosomiasis japonica early diagnosis biomarker is obtained. The screening method has the characteristics of convenience and quickness, can accurately reflect the metabolic spectrum difference between a schistosomiasis japonicum infected mouse and a normal mouse, and is high in specificity.
Owner:SUN YAT SEN UNIV
Serum biomarker group for diagnosis of ischemic stroke complicated with stress ulcer and application thereof
The invention discloses a group of serum metabolite micromolecule markers related to the diagnosis of ischemic stroke complicated with stress ulcer. The biomarkers include one or more of glycine, phenylalanine, ornithine, palmitoleic acid, arachidonic acid, and oleic acid. The analysis and detection method based on the biomarker group has advantages of high sensitivity, high throughput and simpleoperation; the accurate diagnosis of ischemic stroke complicated with stress ulcer can be realized; and a diagnostic reagent for the ischemic stroke complicated with stress ulcer can be prepared.
Owner:CHINA PHARM UNIV
Method for detecting serum biomarker of liver cancer patient
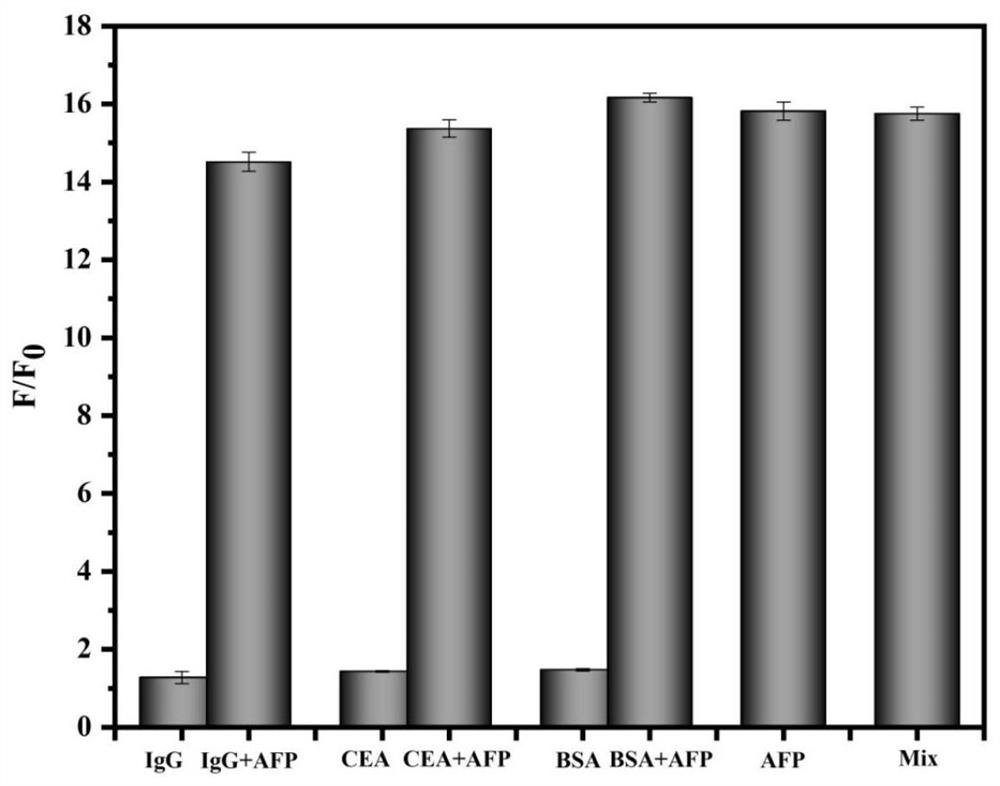
ActiveCN112098648ARealize highly sensitive detectionGood physical and chemical propertiesNanosensorsBiological testingFetuin bPeroxidase
The invention relates to a method for detecting a serum biomarker of a liver cancer patient. Alpha fetoprotein is closely related to generation and development of various tumors, is mainly used as a serum marker of liver cancer clinically, and is used for diagnosis and curative effect monitoring of primary liver cancer. Therefore, the high-sensitivity detection of the alpha fetoprotein is of greatsignificance to the early diagnosis and treatment of liver cancer patients. The invention provides a method for detecting alpha fetoprotein in human serum by using a novel signal controlled-release nano material for enzyme-free immunoassay based on alpha fetoprotein and primary and secondary antibodies thereof. The method not only overcomes the difficulties that the traditional method depends onperoxidase to generate signals and amplify the signals, but also remarkably improves the detection sensitivity, simplifies the detection process, reduces the cost, widens the application field, and solves the problems of complex enzyme-linked immunosorbent assay technology, high price, insufficient sensitivity and the like; the method has a wide application prospect in the fields of early diagnosis and treatment of tumors and the like, and a novel method with high sensitivity and high specificity is provided for enzyme-free immunodetection.
Owner:QINGDAO UNIV OF SCI & TECH
Pulmonary nodule auxiliary diagnosis method based on three-dimensional multi-resolution attention capsule network
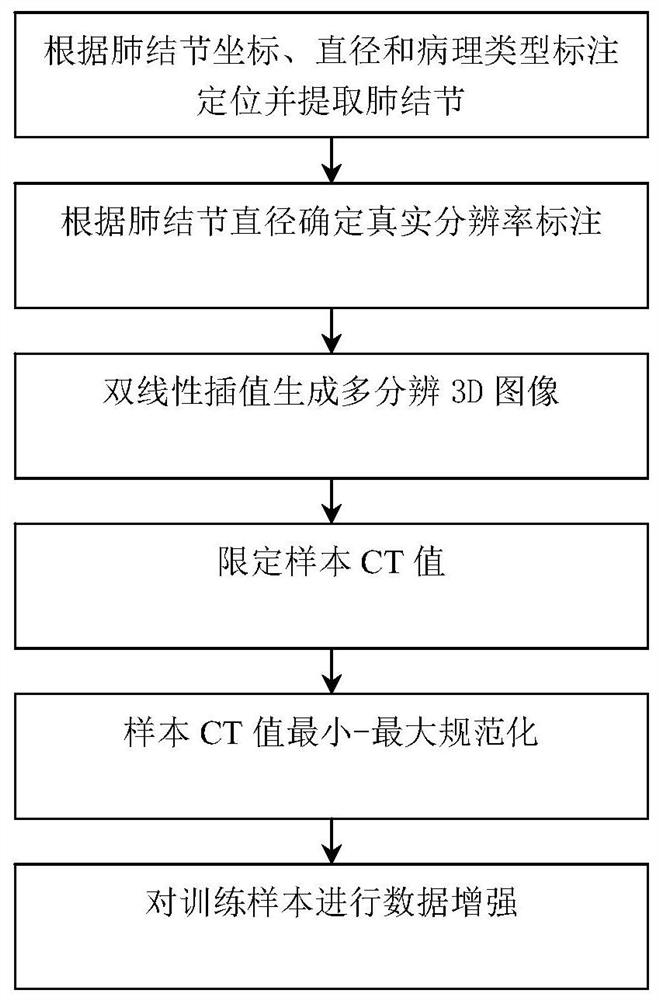
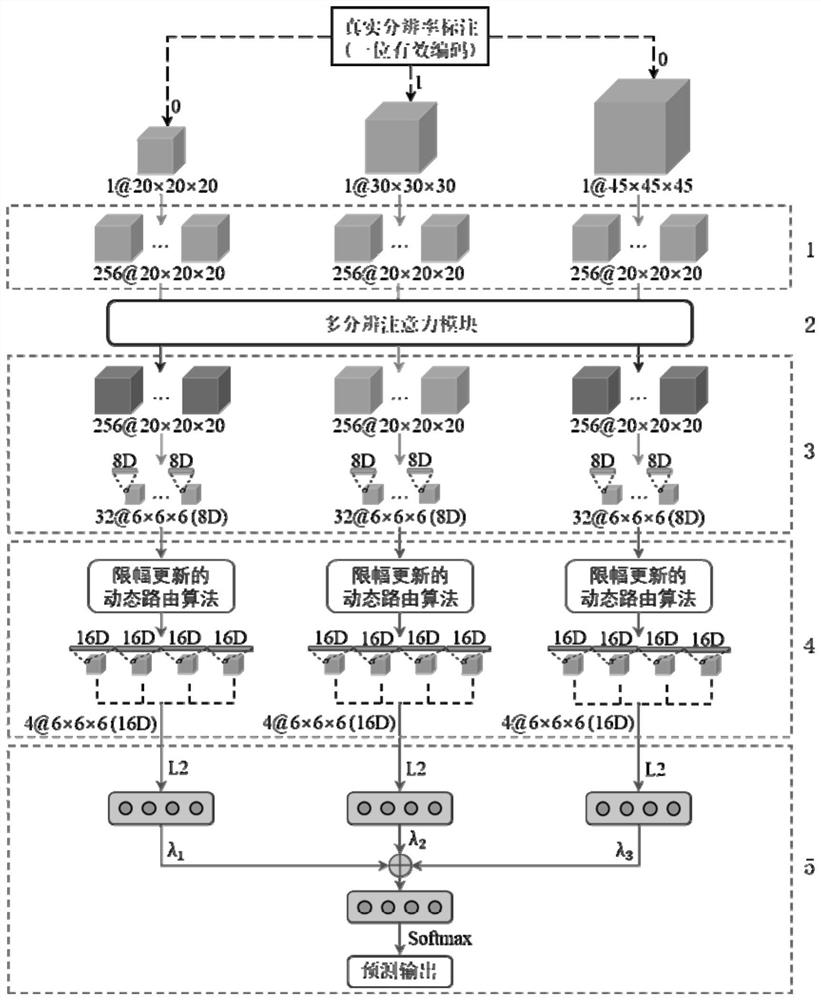
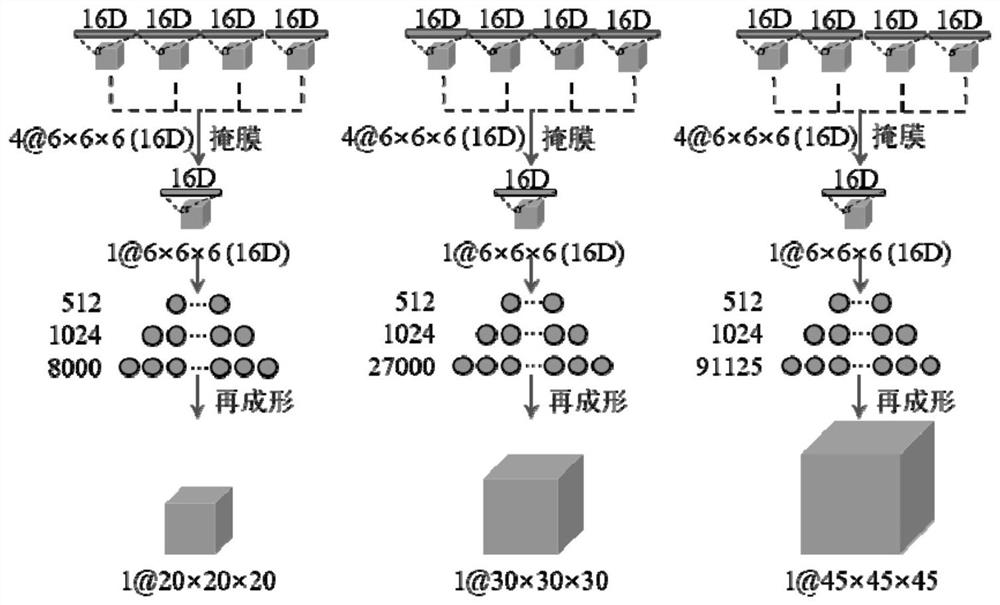
ActiveCN113208641AFully extractedFully learnComputerised tomographsTomographyPulmonary noduleData set
The invention discloses a pulmonary nodule auxiliary diagnosis method based on a three-dimensional multi-resolution attention capsule network, and belongs to the technical field of medical image processing. The method comprises the following steps: obtaining a pulmonary nodule CT image data set containing a pathological type label; preprocessing samples in the data set; constructing a three-dimensional multi-resolution attention capsule network; and inputting the preprocessed data sample into the three-dimensional multi-resolution attention capsule network for training, and improving the prediction capability of the three-dimensional multi-resolution attention capsule network on multiple pathological types of pulmonary nodules by learning sample distribution. According to the method of the invention, manual features do not need to be designed or auxiliary information such as serum biomarkers does not need to be utilized, and high prediction precision and high robustness can still be kept for small samples difficult to classify and unbalanced and multi-label clinical data sets.
Owner:SHANDONG UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com