Biomarkers and assays for myocardial infarction
a biomarker and myocardial infarction technology, applied in the field of new and novel blood plasma/serum biomarkers, can solve the problems of imperfect prediction of conventional mi markers, and achieve the effect of improving the prediction accuracy of existing mi markers and improving the accuracy of analyzing myoglobin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preliminary Screening and Biomarker Identification
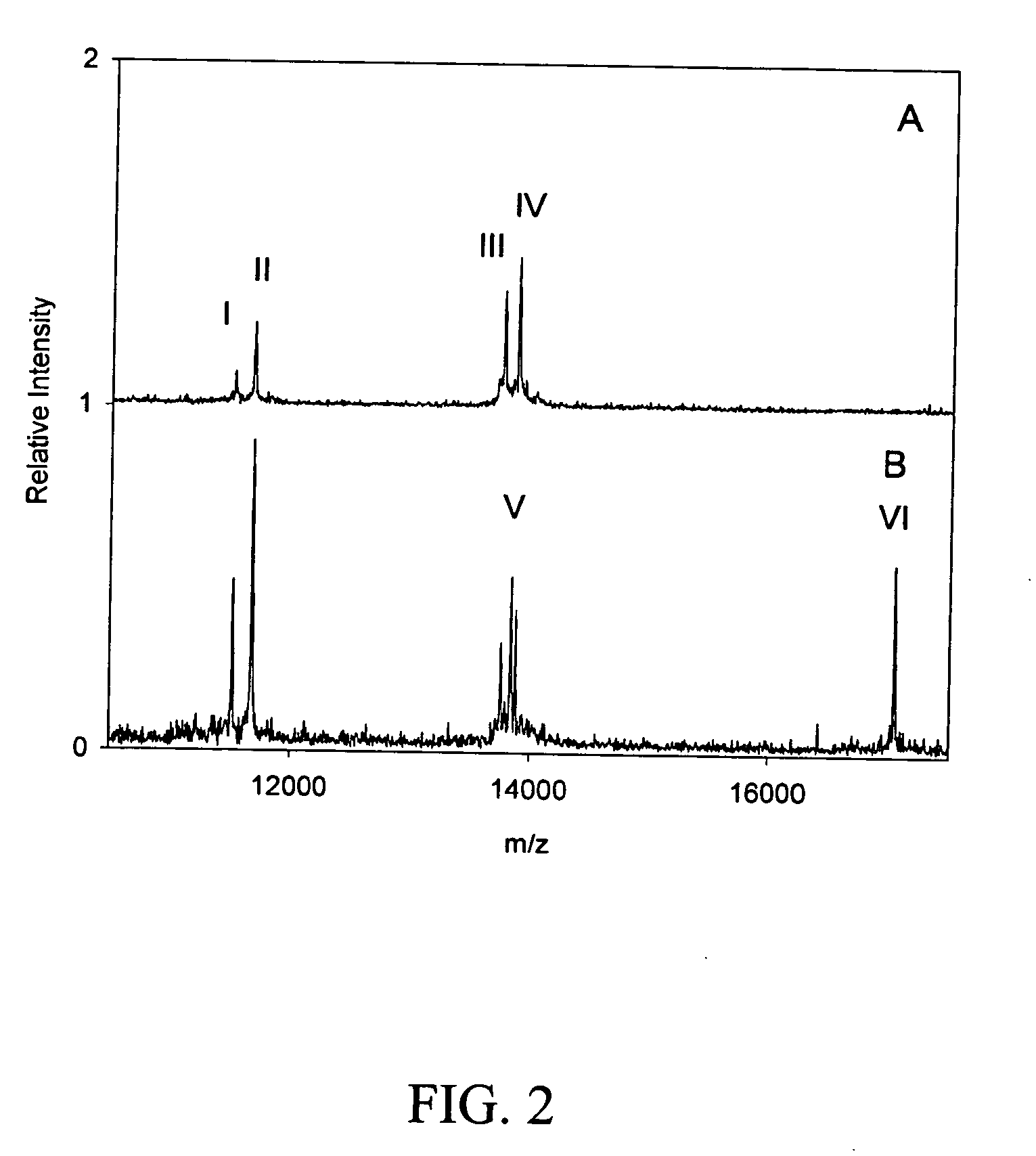
[0029] Previously, Nedelkov et al. reported on technologies and methodologies able to characterize full-length plasma proteins for the purpose of determining differences (e.g., identifying variants and quantitative modulations) found among the general population (PNAS 2005). In furthering this approach, we have developed 25 individual assays that have been incorporated into a high-throughput screening platform and used in creating a data foundation (in the healthy population) against which results from disease cohorts can be compared. The same panel of assays was applied to a small number of MI patients for the purpose of screening for putative markers. Essentially, MI samples were screened on a per protein basis to determine qualitative and / or quantitative differences away from foundational data. Noticeable qualitative (i.e., variants) and semi-quantitative differences were observed in two profiles resulting from the targeting of ...
example 2
Biomarker Verification
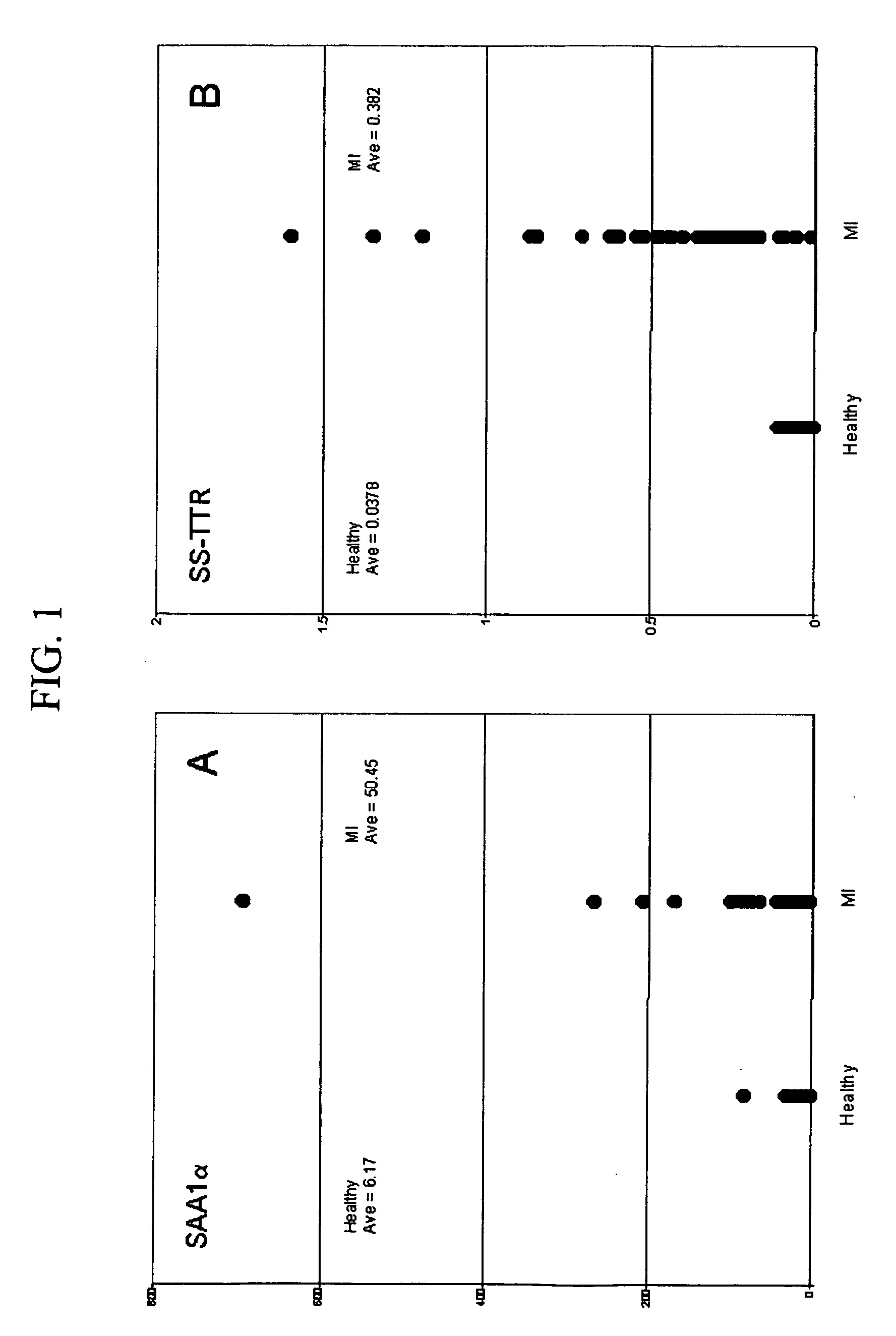
[0032] Verification assays for SAA1α and SS-TTR were performed on 48 MI samples versus 48 healthy controls. SS-TTR assays were performed as described in Examples 3 and 4, with the exception of adding 30 μL of equine serum to each sample, containing equine SAA (eSAA; Mr=12,289), which was co-extracted and analyzed simultaneous to the human SAA in order to serve as an internal reference standard for relative quantification. Two data sets were produced, and ion signals from each set—sulfonated (SS) -TTR and cysteinylated (cys) -TTR, and, hSAA1α and eSAA—were baseline integrated over a mass range of 0.15% of the Mr of each species using Proteome Analyzer Software (Intrinsic Bioprobes, Tempe, Ariz.). For comparison, the integrals for SS-TTR and hSAA1α were normalized to their respective internal references and the values plotted with respect to the health state of the individual. Results of this operation are shown in FIG. 1A and FIG. 1B, which illustrate elevated...
example 3
Mulitplexed Mass Spectrometric Immunoassay
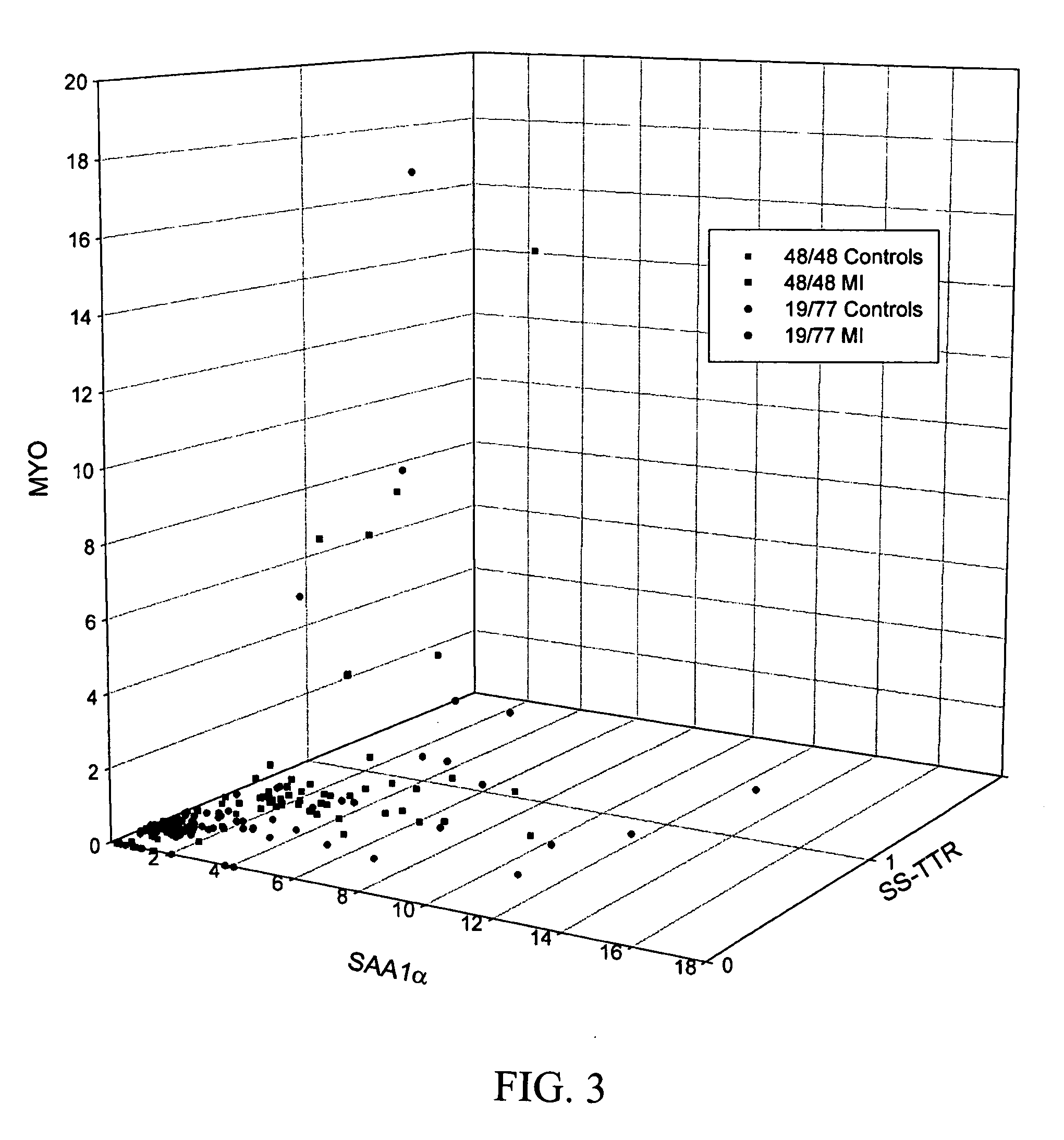
[0033] Based on the findings described above, a multiplexed MSIA was designed to target the two new putative biomarkers, SAA1α and SS-TTR, as well as the established MI marker myoglobin. Extraction devices for a multiplexed mass spectrometric immunoassay were prepared by coupling a mixture of mouse anti-human monoclonal antibody to serum amyloid A (MO-C40028A; Anogen, Mississauga, ON, Canada), rabbit anti-human polyclonal antibody to transthyretin (A0002; DakoCytomation, Carpinteria, Calif., USA) and goat anti-human polyclonal antibody to myoglobin (70-MG60; Fitzgerald, Concord, Mass., USA), to CDI (1,1′-Carbonyldiimidazole)—activated affinity-pipettes. Through side experiments, it was determined that a mixture of antibodies at the ratio of 0.08:0.04:0.10 mg / mL (SAA:TTR:MYO) was able to detect SAA and TTR at basal concentrations, and MYO at concentrations above 100 ng / mL. Using the extraction devices, each sample set was processed in paral...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com