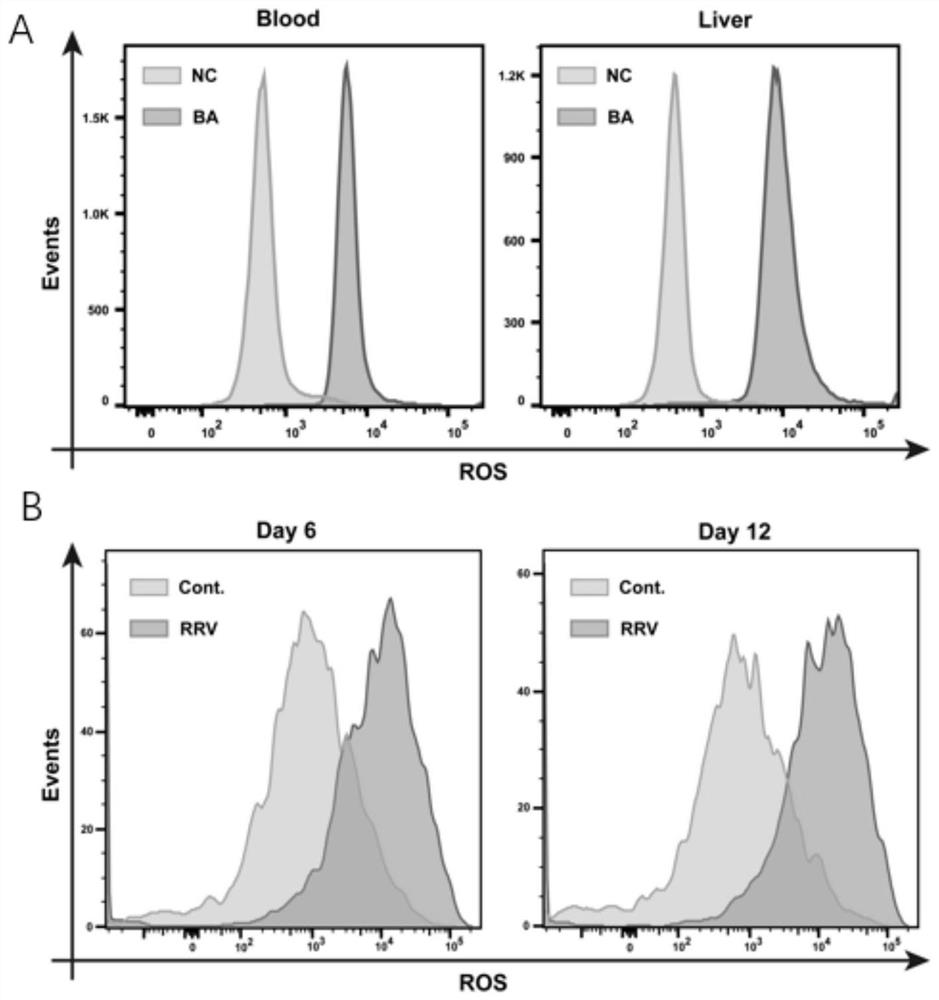
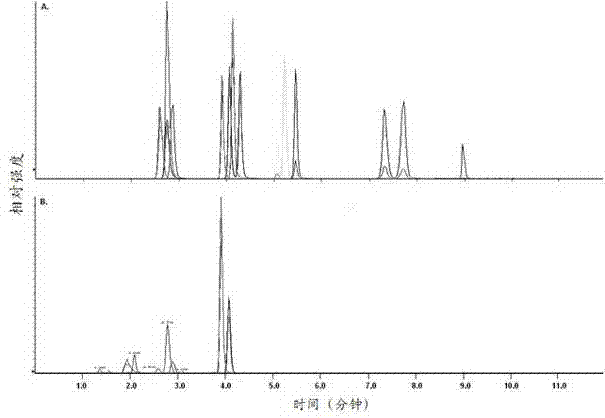
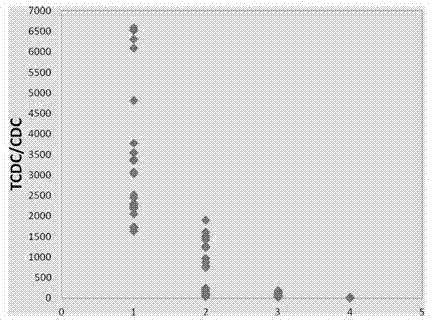
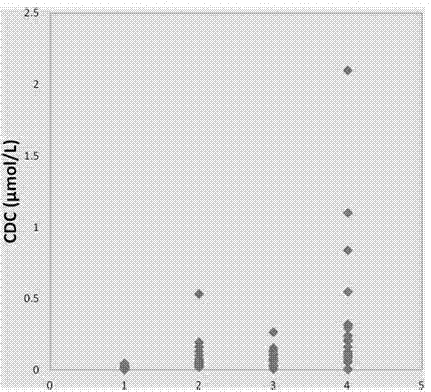
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Biliary atresia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Biliary atresia, also known as extrahepatic ductopenia and progressive obliterative cholangiopathy, is a childhood disease of the liver in which one or more bile ducts are abnormally narrow, blocked, or absent. It can be congenital or acquired. It has an incidence of one in 10,000–15,000 live births in the United States, and a prevalence of one in 16,700 in the British Isles. Biliary atresia is most common in East Asia, with a frequency of one in 5,000.
Serum marker MMP-7-based biliary atresia diagnosis kit
InactiveCN108267585AEasy to operateAid in early diagnosisDisease diagnosisPositive controlPRIMARY BILIARY ATRESIA
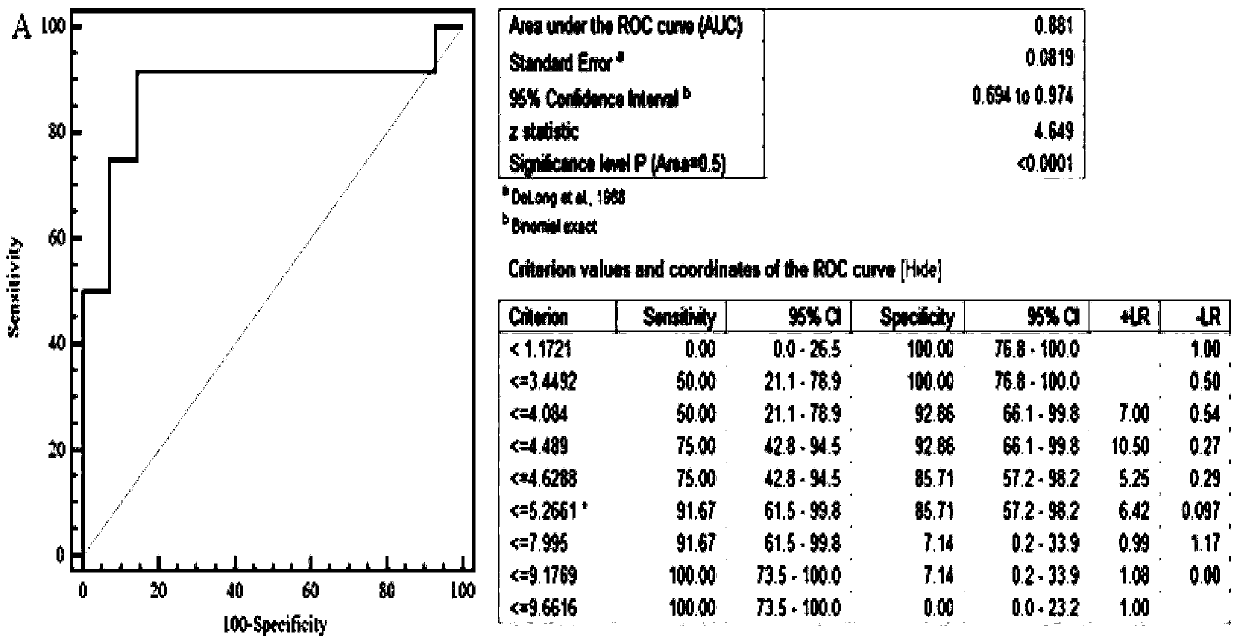
The invention relates to a serum marker MMP-7-based biliary atresia diagnosis kit. The diagnosis kit comprises an anti-human MMP-7 monoclonal antibody coated ELISA plate, a negative control solution,a positive control solution, an enzyme labeling reagent, an enzyme substrate solution, a blocking solution, a sample diluent, a washing solution and a stopping solution. The diagnosis kit is a new sensitive, safe, reliable and easily-operated commercial kit. Quantitative detection of the level of MMP-7 in human serum is helpful for early diagnosis of BA; the BA diagnosis sensitivity of the serum biomarker protein MMP-7 is 100%, and the BA diagnosis specificity is 95.6%, so the kit has the characteristics of high specificity and high sensitivity; and the kit improves the early diagnosis rate ofBA, reduces misdiagnosis, and improves the self liver survival rate of the BA.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Applications of diagnostic markers in biliary atresia of newborns
InactiveCN102818866AOptimal operation timeImprove the curative effect of surgeryComponent separationCholic acidChenodeoxycholic acid
The invention relates to applications of serum taurochenodeoxycholic acid and chenodeocycholic acid as diagnostic markers in the preparation of medical instruments for diagnosing biliary atresia of newborns. The diagnostic markers of the medical instruments are content ratio of the taurochenodeoxycholic acid and the chenodeocycholic axid in serum. The invention also provides the application of chenodeocycholic acid. The application of diagnostic markers in biliary atresia of the newborns has the advantages that the application of the taurochenodeoxycholic acid and the chenodeocycholic acid as diagnostic markers of biliary atresia is proposed for the firstly time, so that the biliary atresia can be distinguished from other cholestasis diseases of newborns, the best operation time of children can be strived for, and therefore, the operation effect can be improved.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Alpha crystal form of obeticholic acid as well as preparation method, medicine composition and application thereof
InactiveCN105859818AEasy to operateGood reproducibilityOrganic active ingredientsOrganic chemistry methodsSolventPRIMARY BILIARY ATRESIA
The invention relates to an alpha crystal form of obeticholic acid and a preparation method thereof. The preparation method comprises the following steps: (a) fully dissolving obeticholic acid under a backflow state in a mixed solvent of toluene or toluene and another kind of solvent forming a homogeneous system; (b) cooling the obtained obeticholic acid solution, so the alpha crystal form of the obeticholic acid precipitates out from the solution; (c) separating the alpha crystal form of the obeticholic acid out from the solution; and (d) placing the alpha crystal form of the obeticholic acid under 30-70 DEG C and drying for 3-30 hours at reduced pressure or ordinary pressure. The prepared alpha crystal form of obeticholic acid has the characteristics of better stability, higher purity, stable moisture content and the like. Furthermore, the invention also relates to a medicine composition of the alpha crystal form and the application of the alpha crystal form in preparing medicines for treating hepatic diseases such as biliary atresia, cholestatic liver disease, chronic liver disease, primary biliary cirrhosis, alcoholic fatty liver, non-alcoholic fatty liver disease and the like.
Owner:SICHUAN RUIXIKANG BIOLOGICAL MEDICINE CO LTD
Efficient amplifying and culturing method for biliary epithelial cells
InactiveCN103031270AHigh purityPromote proliferationVertebrate cellsArtificial cell constructsEnzyme Inhibitor AgentBiliary tract
The invention discloses an efficient amplifying and culturing method for biliary epithelial cells in rabbit livers and belongs to the technical field of biological medicines. The efficient amplifying and culturing method comprises the following steps of: (1) separating, purifying and culturing the epithelial cells; (2) detecting cell growth lines; and (3) identifying the epithelial cells. The efficient amplifying and culturing method for the biliary epithelial cells, disclosed by the invention, has the advantages as follows: through a multi-step separating method, biliary epithelial cells with higher purities are obtained and a long-time multiplication culturing system for the biliary epithelial cells is established; a fact that the multiplication of the biliary epithelial cells in the livers can be obviously promoted through an Rho kinase inhibitor Y-27632 is first found; and the efficient amplifying and culturing method for the biliary epithelial cells, disclosed by the invention, has the advantages of simpler operation and the like, is suitable for promotion and application and can provide a platform for researches on a plurality of disease mechanisms including biliary atresia, primary biliary cirrhosis and the like.
Owner:SHAOXING UNIVERSITY
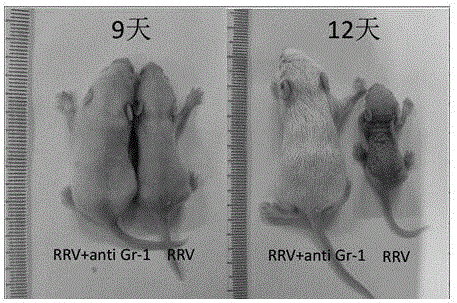
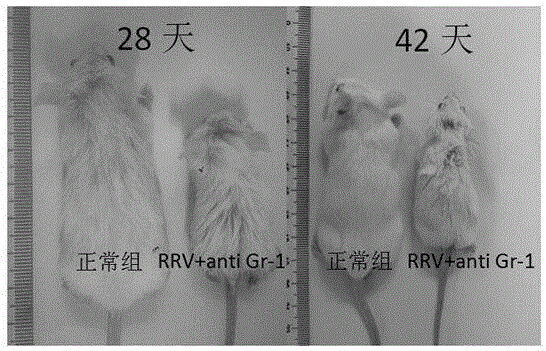
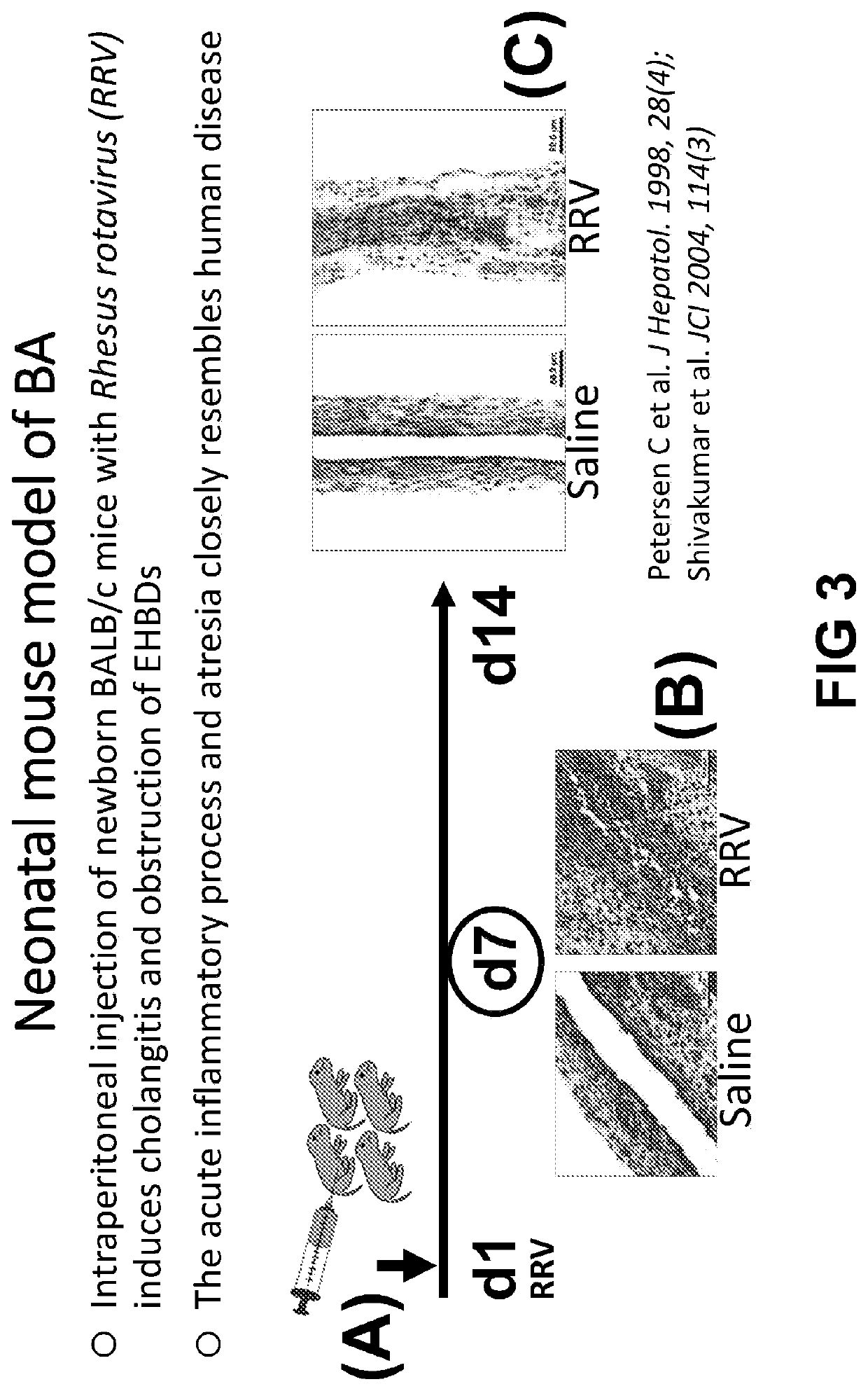
Construction method of animal model for biliary atresia of chronic liver disease
The invention relates to a construction method of an animal model for biliary atresia of chronic liver disease firstly utilizing a Gr-1 antibody to construct the construction method of the animal model for biliary atresia of chronic liver disease. The method comprises following steps: injecting rhesus monkey rotavirus within the 24 hours of neonatal mouse to induce a classic animal model for biliary atresia, firstly injecting the Gr-1 antibodies before rhesus monkey rotavirus is injected; injecting the Gr-1 antibodies multiple times after mouse are born on the 14th day to maintain the effective concentration range of the Gr-1 antibodies in the mouse; and injecting 5-20ng / 1.6g of Gr-1 antibodies to the mouse each time. The construction method of an animal model for biliary atresia of chronic liver disease has following beneficial effects: the model is capable of simulating the process of biliary atresia, development and prognosis, especially in the end-stage hepatocirrhosis phase.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
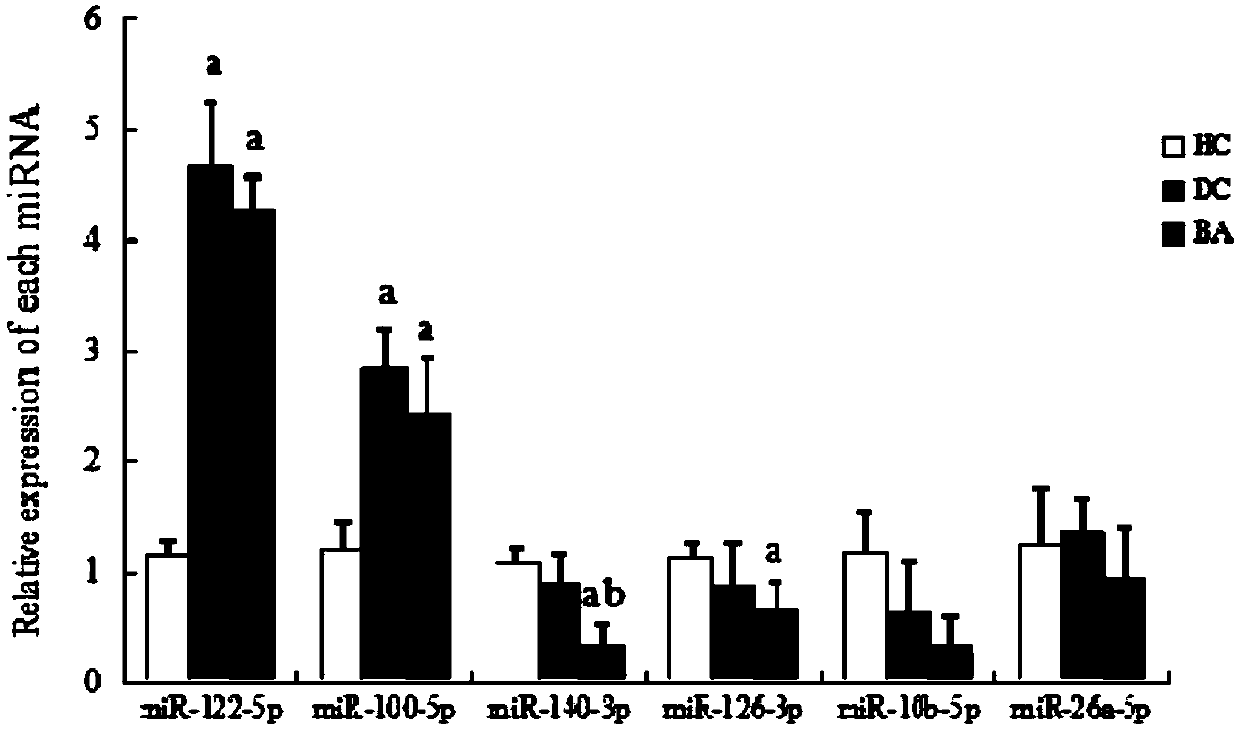
Primer set for detecting plasma miR-140-3p of child patient with biliary atresia and detection kit containing same
InactiveCN107904303AImprove featuresIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceSpecific detection
The invention discloses a primer set for detecting plasma miR-140-3p of a child patient with biliary atresia. The primer set comprises a reverse transcription primer shown in SEQ ID NO:1, a forward upstream primer as shown in SEQ ID NO:2, and a reverse primer as shown in SEQ ID NO:2. The invention further provides a detection kit containing the primer set. The primer set and the kit of fluorescentquantitative PCR for detecting the miR-140-3p, provided by the invention, have high specificity and sensitivity, and can be applied to rapid and specific detection of the miR-140-3p content in plasmain the fluorescent quantitative PCR.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
Application of CD177 in preparation of product for diagnosing biliary atresia
PendingCN112080560ACheck age no limitGuaranteed accuracyMicrobiological testing/measurementDigestive systemLiver tissueMolecular diagnostic techniques
The invention provides application of CD177 in preparation of a product for diagnosing biliary atresia, and relates to the technical field of molecular diagnosis. According to the invention, by detecting a large number of biliary atresia child patient samples and combining animal experiments, it is proved that the number of CD177+ neutrophile granulocytes in biliary atresia peripheral blood and liver tissue can serve as a powerful index for evaluating the liver injury degree at first; and therefore, detection of CD177 genes, CD177 protein or the CD177+ neutrophile granulocytes can serve as a more convenient, direct and accurate method for clinically evaluating the illness state of biliary atresia child patients. The invention also proves that CD177 has a pro-inflammatory effect in biliaryatresia; and the inhibitor of CD177 can be used for research and development of drugs for treating biliary atresia.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
Biliary atresia evaluation system, method for detecting MMP-7, kit and application
PendingCN114563493AImprove diagnostic accuracySimple sampling methodComponent separationBiological material analysisBiliary tractBile acid
The invention provides a biliary atresia evaluation system, a method for detecting MMP-7, a kit and application. The biliary atresia assessment system is a biliary atresia risk assessment system based on MMP-7 and bile acid spectra, and the biliary atresia assessment system takes the concentration of MMP-7 in a subject sample and the concentration of bile acid in the subject sample as input and takes a risk assessment result as output. By adopting the technical scheme, the infantile biliary atresia can be conveniently, efficiently and accurately evaluated, screened and / or diagnosed, the diagnosis accuracy of the biliary atresia is remarkably improved, and non-invasive accurate diagnosis is realized.
Owner:WUXI DIAGNOSTICS LAB SHANGHAI CO LTD +2
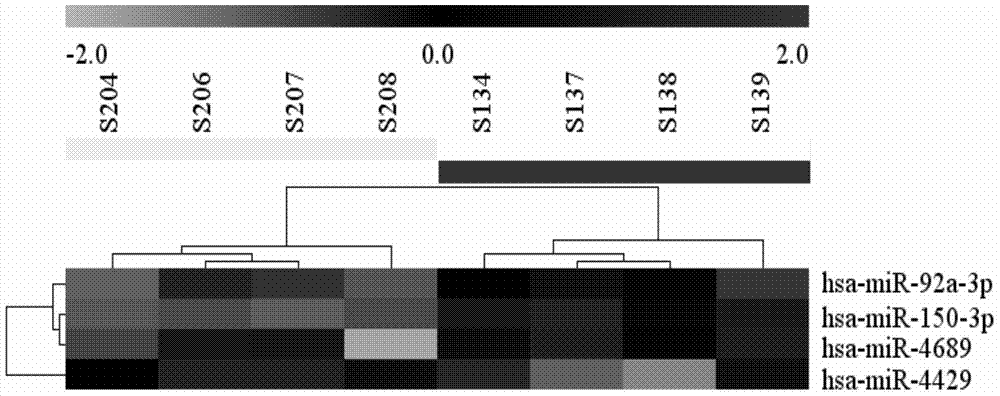
Detection and applications of microRNA serum marker of biliary atresia and cholestatic infant hepatitis syndrome
ActiveCN104774914AImproving the level of diagnosisHigh sensitivityMicrobiological testing/measurementBiliary tractPRIMARY BILIARY ATRESIA
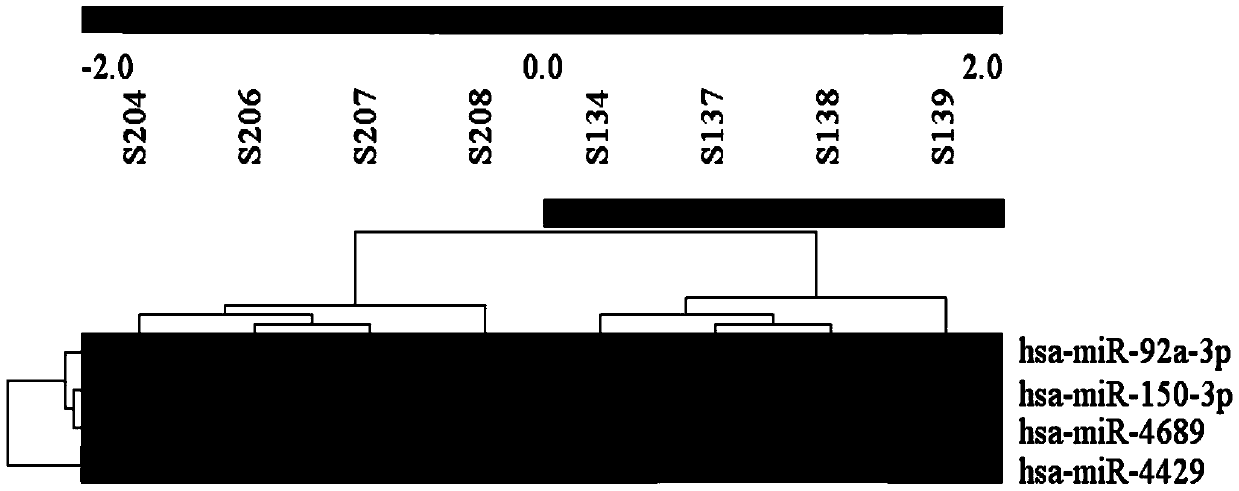
The invention belongs to the field of biological medicine and molecular biology, relates to a novel biliary atresia diagnostic marker, and more specifically provides applications of a set of microRNAs in preparing biliary atresia diagnostic preparations. The microRNA is composed of hsa-miR-150, hsa-miR-4689, hsa-miR-92a-3p, and hsa-miR-4429. It is confirmed by experiments that, in biliary atresia serum, expression amount of the miRNA possesses significant difference on the statistical significance with expression amount of a corresponding miRNA of cholestatic infant hepatitis syndrome. The invention also provides a detection method of the set of miRNAs. Compared with the prior art, the detection method is capable of saving time, and is high in sensitivity and accuracy, low in cost, and / or high in efficiency. And in addition, the invention also provides kits and chips relative to the applications.
Owner:CHILDRENS HOSPITAL OF FUDAN UNIV
Application of diagnostic marker for newborn biliary atresia
PendingCN112748249AOptimal operation timeImprove surgical prognosisComponent separationDisease diagnosisFull Term NeonateBiliary tract
The invention discloses application of a newborn biliary atresia diagnostic marker in preparation of a tool for evaluating and diagnosing newborn biliary atresia. The diagnostic product can be a medical instrument, a kit, test paper, a medical device and the like. The invention also provides a kit for detecting the biomarker and a use method of the kit. The invention provides an effective means for early screening of the risk of neonatal biliary atresia and diagnosis different from cholestasis caused by other reasons, improves the diagnosis efficiency and diagnosis accuracy of the neonatal biliary atresia, strives for time for taking treatment measures in time, and is beneficial to improving the treatment effect and prognosis of the neonatal biliary atresia.
Owner:HUMAN METABOLOMICS INST INC
Composition comprising butyric acid compound and application of composition
InactiveCN108283632AEffective occurrenceEfficient developmentMilk preparationDigestive systemIntestinal structureBiliary tract
The invention provides a composition comprising a butyric acid compound and an application of the composition. The butyric acid compound is used for preventing and curing biliary atresia and the complications. The composition comprising the butyric acid compound comprises the butyric acid compound and a nutrition agent or a pharmaceutical aid, and the concentration of the butyric acid compound is5-70 mM. The intervention technique generally acting on the intestine is creatively used to prevent and cure the biliary atresia and the complications. As a safe effective preventing and curing method, the butyric acid compound is directly used or added in the nutrition agent and used for pregnant and newborn individuals having no symptoms, so that the occurrence and development of the biliary atresia is prevented and cured fundamentally. The composition solves the technical problems of prevention and cure of complications of the biliary atresia and hepatic fibrosis at the same time, and a safe economical effective method is provided for the prevention and cure of biliary atresia and hepatic fibrosis.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE +1
Biliary atresia diagnostic kit
The invention relates to the field of medical diagnostics, in particular to a biliary atresia diagnostic kit, which comprises a CD177 + neutrophil sorting reagent and a reagent for observing mitochondrial morphology and / or judging whether mitochondria has dysfunction or not. According to the kit provided by the invention, a specimen required for detection is obtained during normal examination, sothat a patient does not need to be additionally subjected to traumatic detection, and the kit is beneficial to popularization and application in the field of biliary atresia detection.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
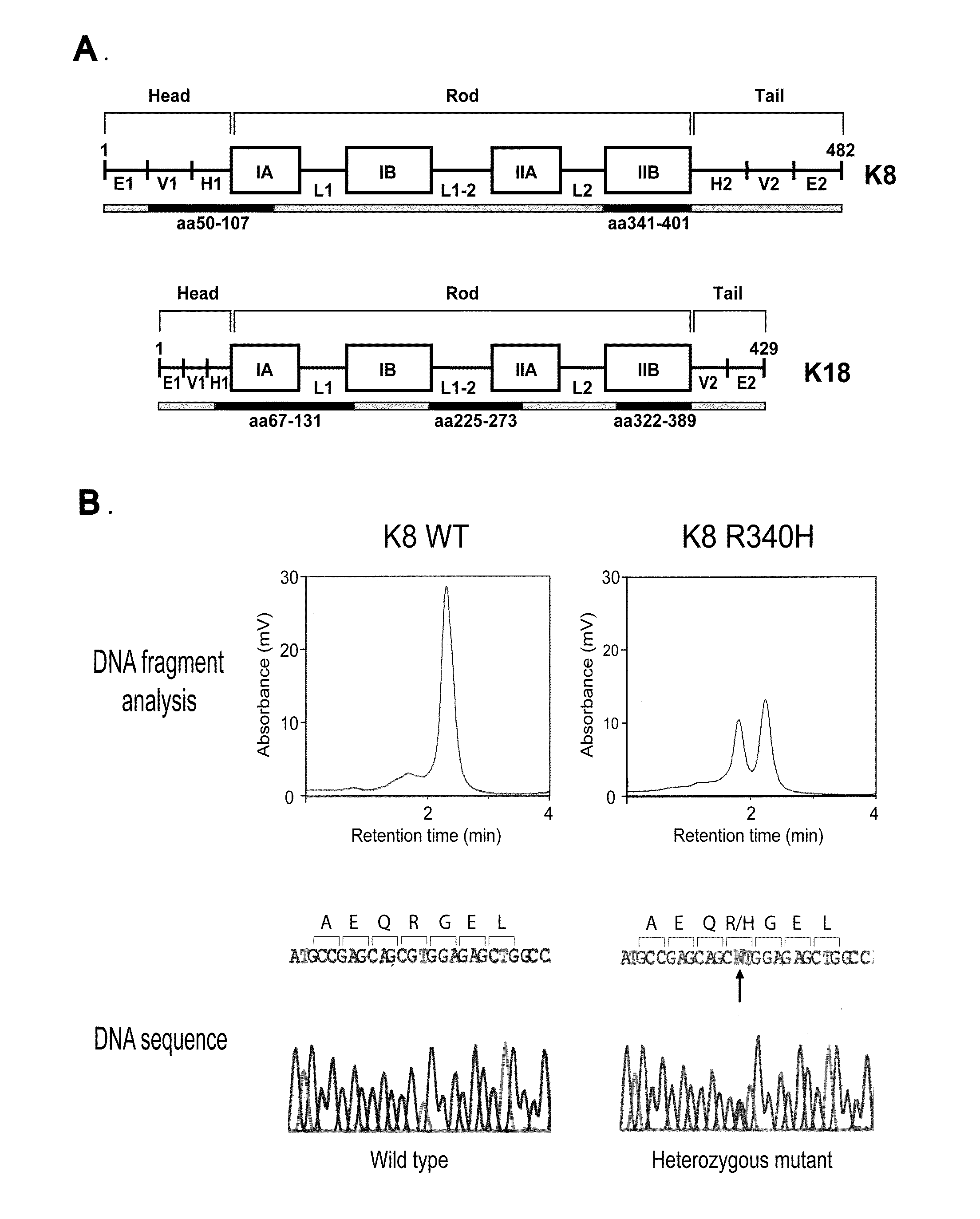
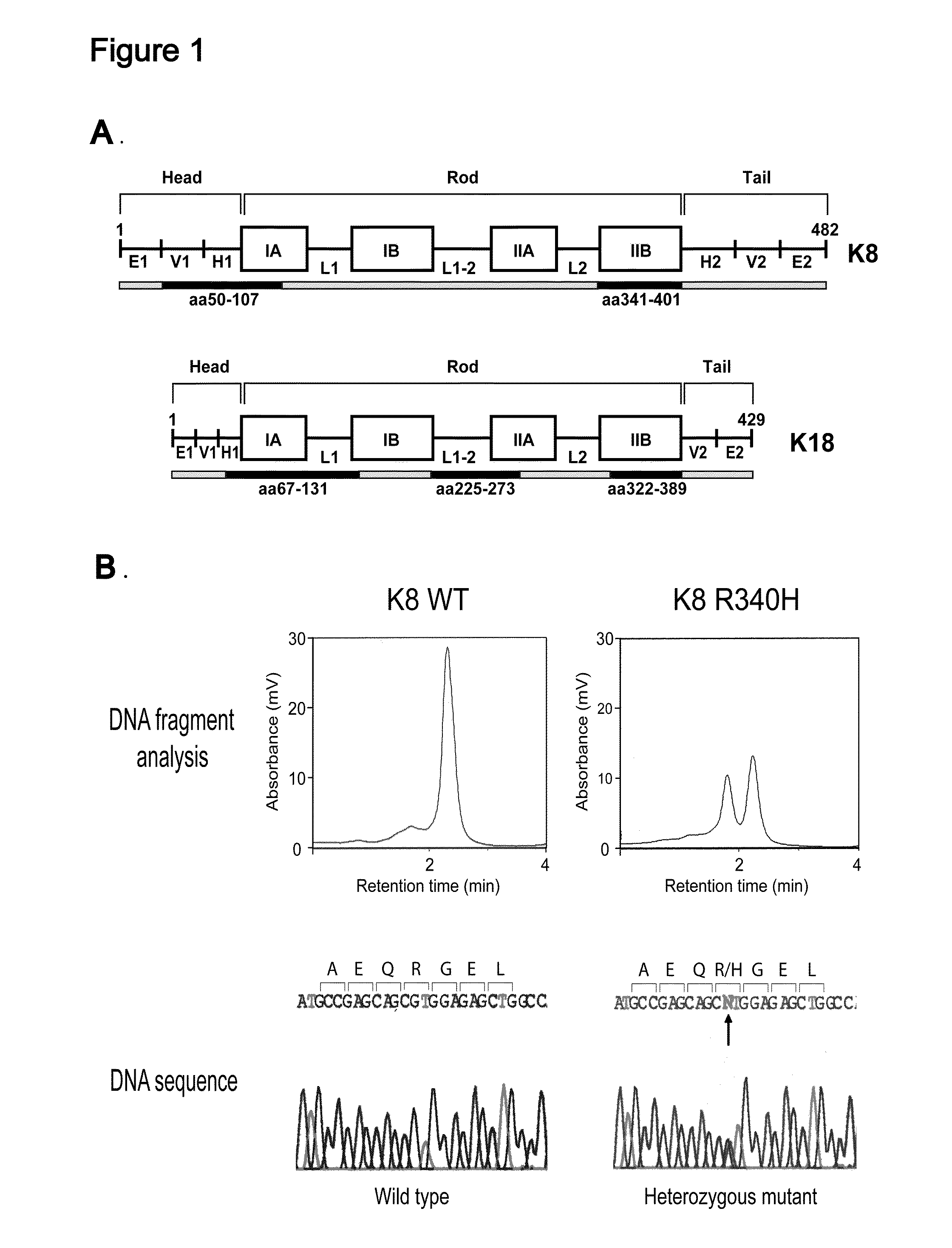
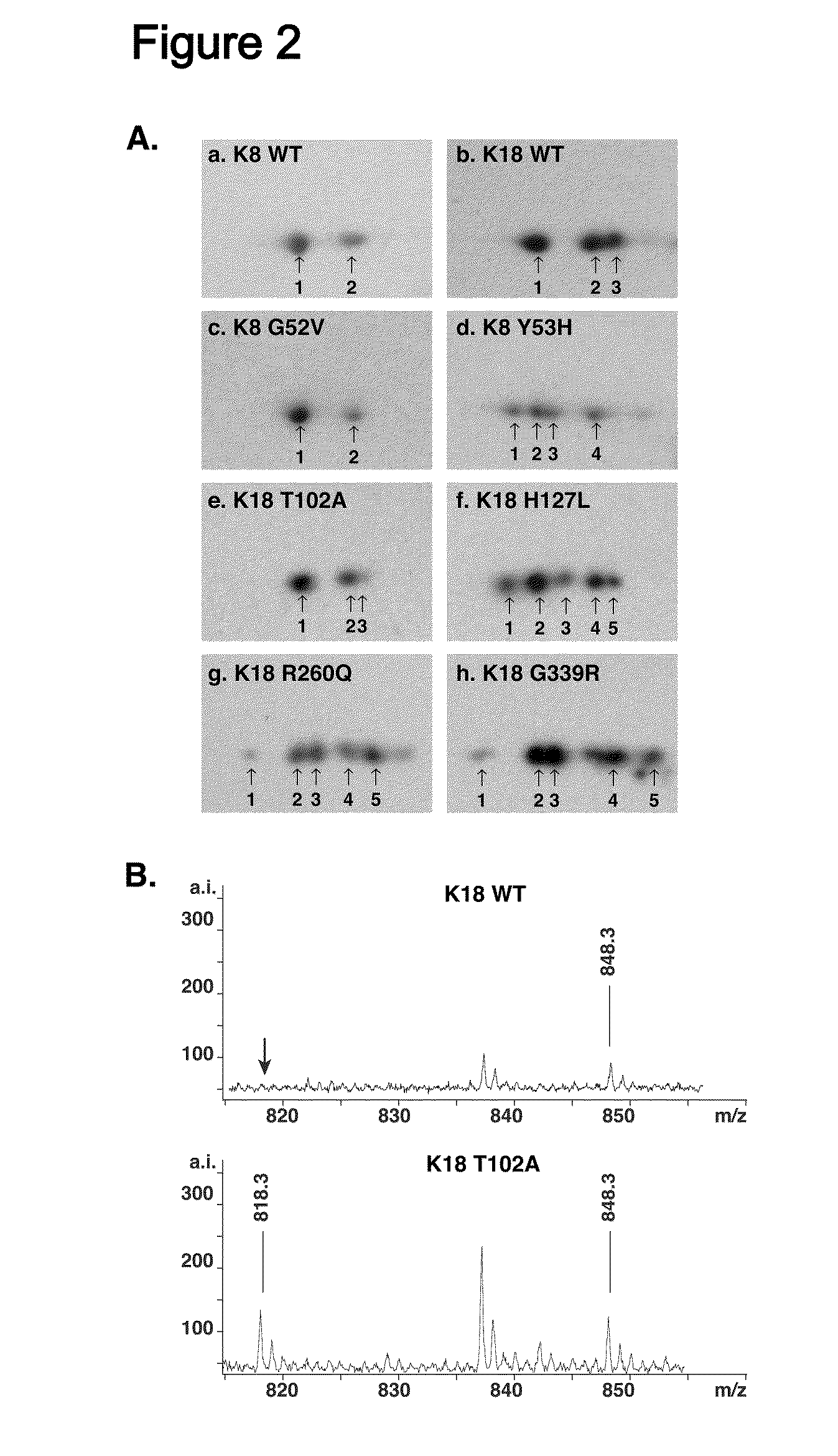
Keratin 8 mutations are risk factors for developing liver disease of multiple etiologies
ActiveUS7838217B1High incidenceModulate expressionSugar derivativesPeptide/protein ingredientsEtiologyHepatobiliary disease
Keratin 8 and 18 (K8 / K18) mutations are shown to be associated with a predisposition to liver or biliary tract disease, particularly noncryptogenic hepatobiliary disease. Unique K8 / K18 mutations are shown in patients with diseases including but without limitation to viral hepatitis, biliary atresia, alcoholic cirrhosis and other acute or chronic toxic liver injury, cryptogenic cirrhosis, acute fulminant hepatitis, autoimmune liver disease, cystic fibrosis, primary biliary cirrhosis, primary sclerosing cholangitis, diseases that are linked with cryptogenic cirrhosis, such as nonalcoholic steatohepatitis, and the like. Livers with keratin mutations had increased incidence of cytoplasmic filamentous deposits. Therefore, K8 / K18 are susceptibility genes for developing cryptogenic and noncryptogenic forms of liver disease. Mutant alleles are associated with disease susceptibility, and their detection is used in the diagnosis of a predisposition to these conditions.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Applications of diagnostic markers in biliary atresia of newborns
InactiveCN102818866BOptimal operation timeImprove the curative effect of surgeryComponent separationCholic acidChenodeoxycholic acid
The invention relates to applications of serum taurochenodeoxycholic acid and chenodeocycholic acid as diagnostic markers in the preparation of medical instruments for diagnosing biliary atresia of newborns. The diagnostic markers of the medical instruments are content ratio of the taurochenodeoxycholic acid and the chenodeocycholic axid in serum. The invention also provides the application of chenodeocycholic acid. The application of diagnostic markers in biliary atresia of the newborns has the advantages that the application of the taurochenodeoxycholic acid and the chenodeocycholic acid as diagnostic markers of biliary atresia is proposed for the firstly time, so that the biliary atresia can be distinguished from other cholestasis diseases of newborns, the best operation time of children can be strived for, and therefore, the operation effect can be improved.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Compositions and methods for treating neonatal biliary atresia
ActiveUS20180147267A1Peptide/protein ingredientsDigestive systemPrimary biliary cirrhosisSerine Protease Inhibitors
Disclosed are methods and compositions for treatment of a subject having a biliary disorder. The methods include administering a therapeutically effective amount of a serine protease inhibitor to a subject in need thereof. The biliary disorder include biliary atresia, a biliopathy, Primary Biliary Cirrhosis (PBC), Primary Sclerosing Cholangitis (PSC), and combinations thereof. In certain aspects, the serine protease inhibitor may be a protease inhibitor rC1 Inhibitor.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Detection and application of microRNA serum markers in biliary atresia and cholestatic infantile hepatitis syndrome
The invention belongs to the fields of biomedicine and molecular biology, and relates to a new diagnostic marker for biliary atresia. Specifically, the application of a group of microRNAs in the preparation of biliary atresia diagnostic preparations is provided, and the microRNAs are composed of hsa-miR-150, hsa-miR-4689, hsa-miR-92a-3p and hsa-miR-4429. Experiments have shown that in the serum of biliary atresia, there is a statistically significant difference in the expression levels of the above miRNAs and the corresponding miRNAs of cholestatic infantile hepatitis syndrome. The present invention also provides a detection method for this group of miRNAs, which saves time, has higher sensitivity and accuracy, lower cost, and / or is more efficient than prior art detection. In addition, the present invention also provides kits and chips related to these applications.
Owner:CHILDRENS HOSPITAL OF FUDAN UNIV
Compositions and methods for treating neonatal biliary atresia
Disclosed are methods and compositions for treatment of a subject having a biliary disorder. The methods include administering a therapeutically effective amount of a serine protease inhibitor to a subject in need thereof. The biliary disorder include biliary atresia, a biliopathy, Primary Biliary Cirrhosis (PBC), Primary Sclerosing Cholangitis (PSC), and combinations thereof. In certain aspects, the serine protease inhibitor may be a protease inhibitor rC1 Inhibitor.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Application of N-acetylcysteine in preparation of product for treating biliary atresia and medicine for treating biliary atresia
InactiveCN113209070ADecreased direct bilirubinDecreased total bilirubinOrganic active ingredientsDigestive systemDiseaseBiliary tract
The invention provides application of N-acetylcysteine in preparation of a product for treating biliary atresia and a medicine for treating biliary atresia, and relates to the technical field of disease treatment. According to the application of N-acetylcysteine in preparation of the product for treating the biliary atresia, the research of the inventor finds that after N-acetylcysteine is administered to treat children with biliary atresia, the number of CD177<+> neutrophils and cell viability (ROS level, mitochondrial level and extracellular trap level) of the children with biliary atresia are obviously reduced, direct bilirubin and total bilirubin are obviously reduced, N-acetylcysteine has an improvement effect on treatment of biliary atresia, and the N-acetylcysteine is reliable in safety and can be used for preparing products for treating biliary atresia. The medicine for treating the biliary atresia comprises N-acetylcysteine, and has a treatment effect on the biliary atresia.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
Fluorinated bile acid derivatives
ActiveCN112771063AHas FXR agonist activityIncrease valueSulfonylurea active ingredientsDigestive systemBile JuiceBiliary tract
Compounds of general formula (I): wherein R2a, R2b, R3a, R3b, R5, Y and R7 are as defined herein are selective agonists at the FXR receptor and are useful for the treatment or prevention of diseases and conditions including nonalcoholic steatohepatitis (NASH); primary biliary cirrhosis; primary sclerosing cholangitis; biliary atresia; cholestatic liver disease; hepatitis C infection; alcoholic liver disease; fibrosis; or liver damage arising from fibrosis.
Owner:NZP UK LTD +1
Porta hepatis retractor for biliary atresia porta hepatis intestinal anastomosis
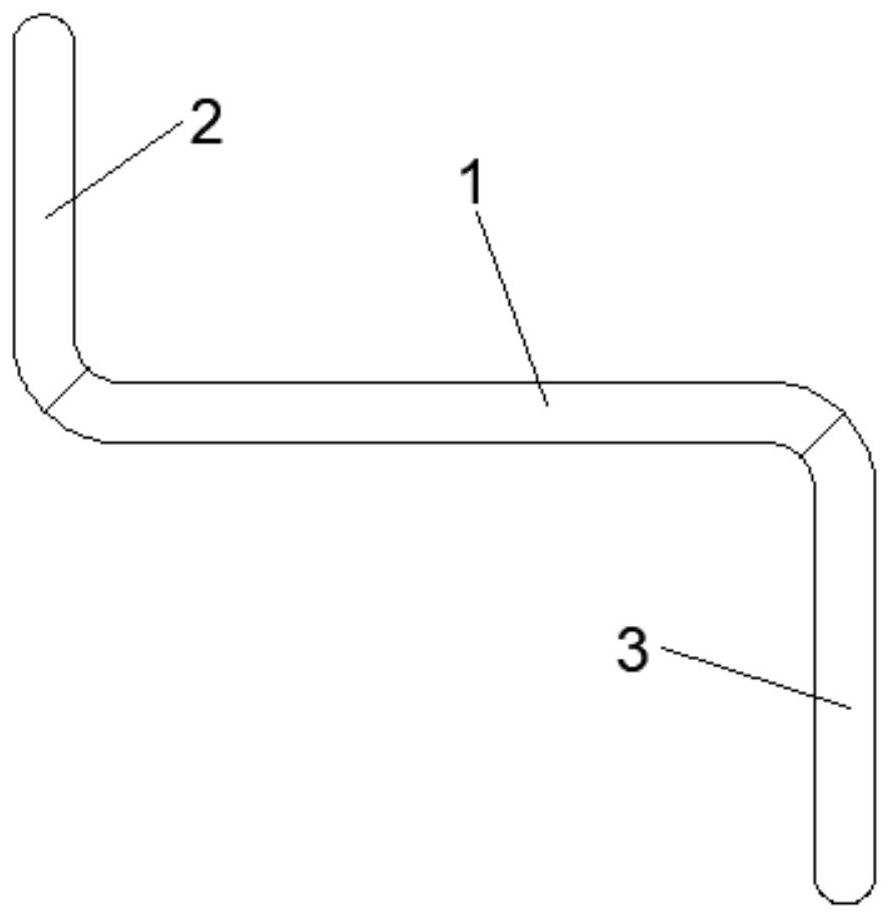
The invention discloses a porta hepatis retractor for biliary atresia porta hepatis intestinal anastomosis. The porta hepatis retractor comprises a retractor handle, a front retractor plate and a rearretractor plate, wherein the front retractor plate is arranged on the upper portion of one end of the retractor handle; the front retractor plate is perpendicular to the retractor handle; the rear retractor plate is arranged on the lower portion of the other end of the retractor handle; the rear retractor plate is perpendicular to the retractor handle; and the retractor handle, the front retractor plate and the rear retractor plate are of an integrated structure. The shape of the retractor is designed according to the form of the biliary atresia hypertrophic liver quadrate lope, hollow partsare arranged in the front retractor plate and the rear retractor plate, or the top end of the front retractor plate is provided with a protrusion bent towards one side of the retractor handle, and thebottom of the rear retractor plate is provided with a protrusion bent towards one side of the retractor handle, so that the stability of liver tissue traction is improved, and the optimal surgical exposure effect is achieved.
Owner:首都儿科研究所
Application of reagent for inhibiting MHC-I and/or II signaling pathway in treatment of biliary atresia
PendingCN114432449ARaise the ratioDelay drug resistancePeptide/protein ingredientsDigestive systemDiseaseBiliary tract
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
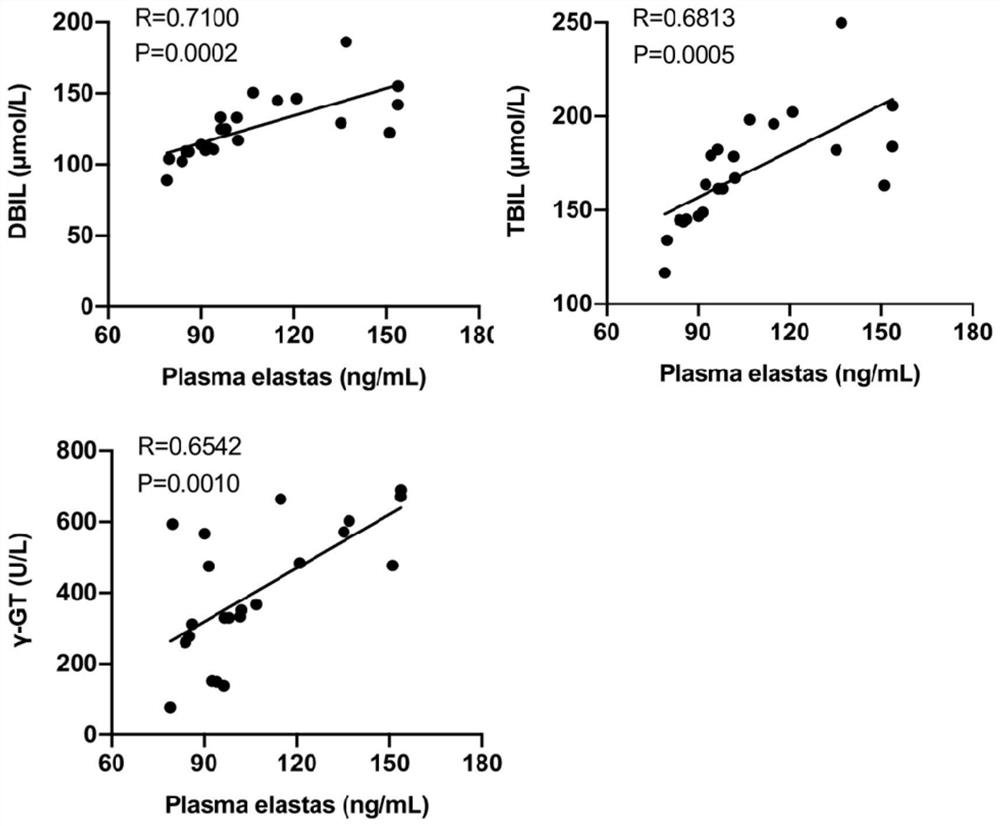
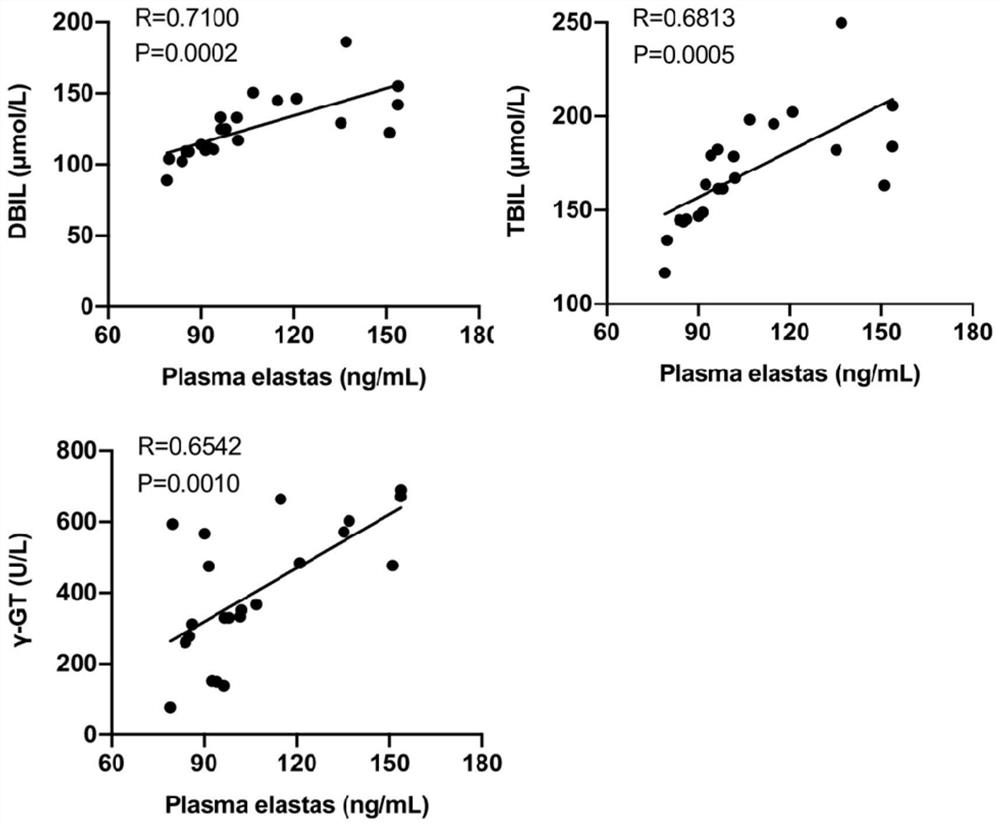
Application of neutrophil elastase in the preparation of products for diagnosing biliary atresia
ActiveCN112557658BEasy diagnosisEase of evaluationDigestive systemDisease diagnosisMolecular diagnostic techniquesLiver necrosis
The invention provides an application of neutrophil elastase in the preparation of a product for diagnosing biliary atresia, and relates to the technical field of molecular diagnosis. The invention firstly confirms that by detecting a large number of BA patient samples and combining in vivo and in vitro experiments The expression of NE in liver tissue and peripheral blood of BA can be used as a powerful indicator to improve the diagnosis of BA and evaluate the degree of liver injury. Therefore, detecting the expression of NE can provide a more convenient, direct and accurate method for clinical diagnosis and evaluation of the condition of children with BA. method. In addition, the present invention also confirms that the NE inhibitor has a therapeutic effect in animal experiments and can be used in the development of drugs for the treatment of BA.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
Keratin 8 Mutations are Risk Factors for Developing Liver Disease of Multiple Etiologies
Keratin 8 and 18 (K8 / K18) mutations are shown to be associated with a predisposition to liver or biliary tract disease, particularly noncryptogenic hepatobiliary disease. Unique K8 / K18 mutations are shown in patients with diseases including but without limitation to viral hepatitis, biliary atresia, alcoholic cirrhosis and other acute or chronic toxic liver injury, cryptogenic cirrhosis, acute fulminant hepatitis, autoimmune liver disease, cystic fibrosis, primary biliary cirrhosis, primary sclerosing cholangitis, diseases that are linked with cryptogenic cirrhosis, such as nonalcoholic steatohepatitis, and the like. Livers with keratin mutations had increased incidence of cytoplasmic filamentous deposits. Therefore, K8 / K18 are susceptibility genes for developing cryptogenic and noncryptogenic forms of liver disease. Mutant alleles are associated with disease susceptibility, and their detection is used in the diagnosis of a predisposition to these conditions.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Application of neutrophil elastase in preparation of product for diagnosing biliary atresia
ActiveCN112557658AEasy diagnosisEase of evaluationDigestive systemDisease diagnosisMolecular diagnostic techniquesBiliary tract
The invention provides an application of neutrophil elastase in preparation of product for diagnosing biliary atresia and relates to the technical field of molecular diagnosis; by detecting a large number of BA child patient samples and combining in-vivo and in-vitro experiments, firstly, it is proved that expression of NE in BA liver tissue and peripheral blood can be used as a powerful index forimproving BA diagnosis and evaluating the liver injury degree, and therefore detection of expression of NE can be used as a more convenient, direct and accurate method for clinically diagnosing and evaluating the illness state of BA children patients; in addition, the invention also proves that the NE inhibitor plays a treatment role in animal experiments, and can be used for research and development of medicines for treating BA.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
Amyloid precursor protein as a diagnostic marker for biliary atresia
Provided are novel diagnostic and prognostic methods for biliary atresia using amyloid precursor protein.
Owner:VERSITECH LTD
Early screening marker for biliary atresia based on neonatal blood spot metabolite and application of early screening marker
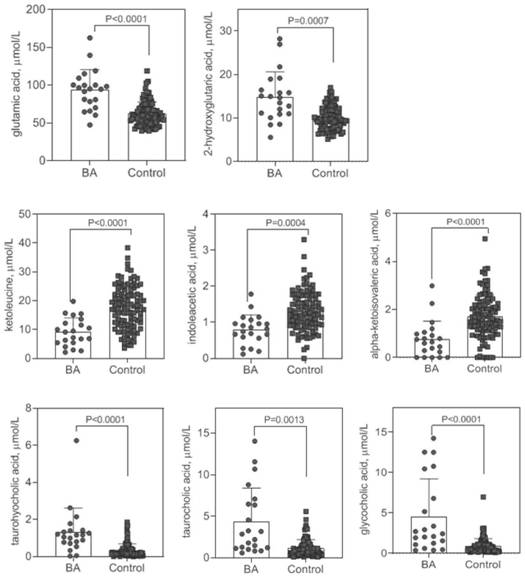
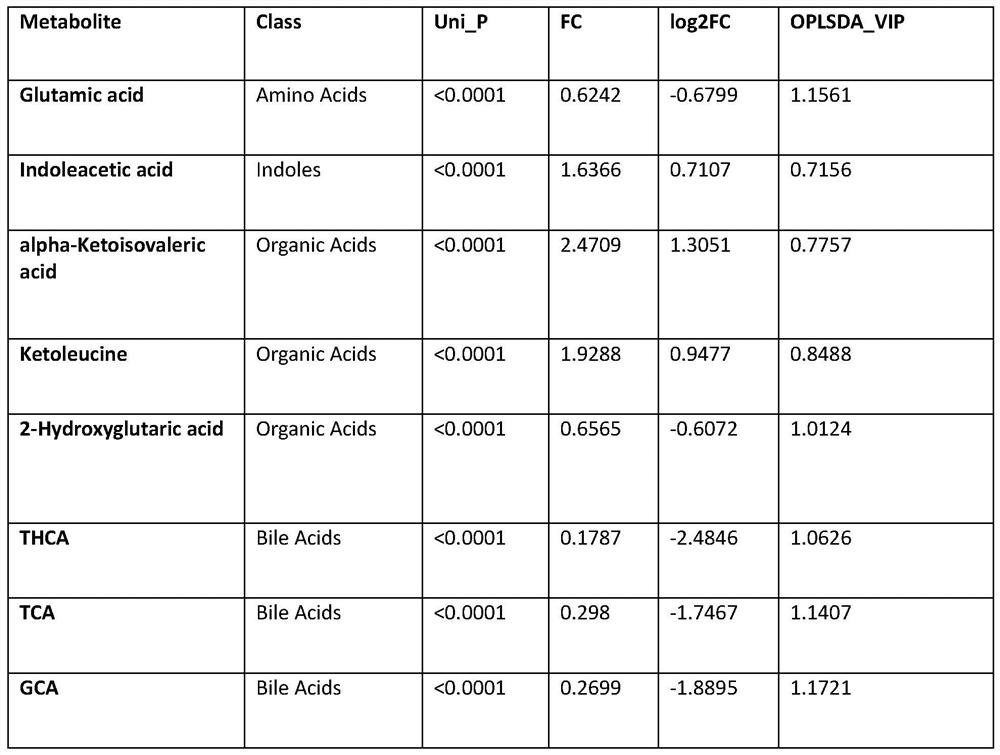
The invention provides application of a biomarker. The biomarker is characterized in that the biomarker is selected from one or more of the following specific biomarkers: A. Glutamic acid; B, indoleacetic acid (Indoleaceticacid); C, [alpha]-ketoisovalerate (alpha-ketoisovalerate), and C, [alpha]-ketoisovalerate ([alpha]-ketoisovalerate); D, ketoleucine (Ketoleucine) is used as a raw material; E. 2-hydroxyglutaric acid (2-hydroxyglutaric acid), and E. 2-hydroxyglutaric acid (2-hydroxyglutaric acid); F, taurohyocholic acid (THCA); G, taurocholic acid (TCA); and H, glycocholic acid (GCA). The application is used for preparing a screening kit for detecting early risk of neonatal biliary atresia.
Owner:SHANGHAI INST OF PEDIATRIC RES
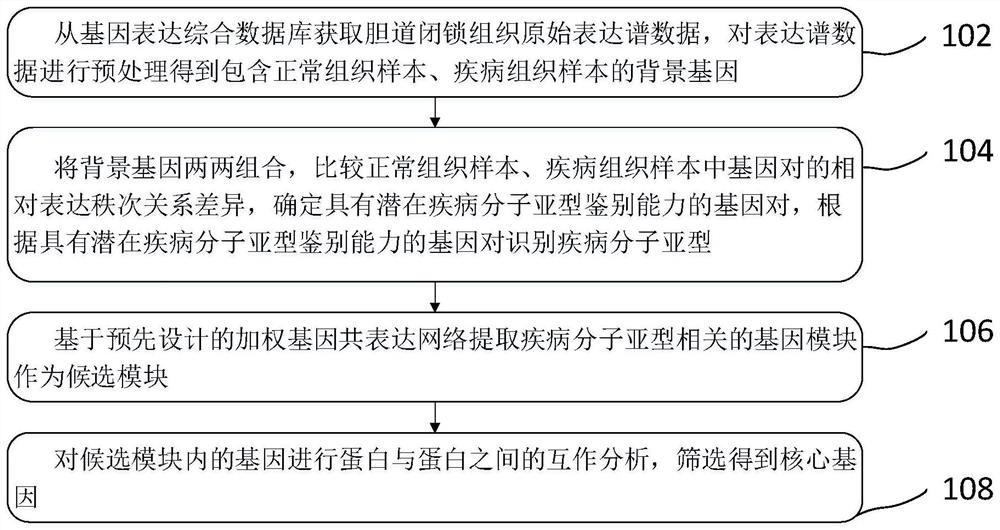
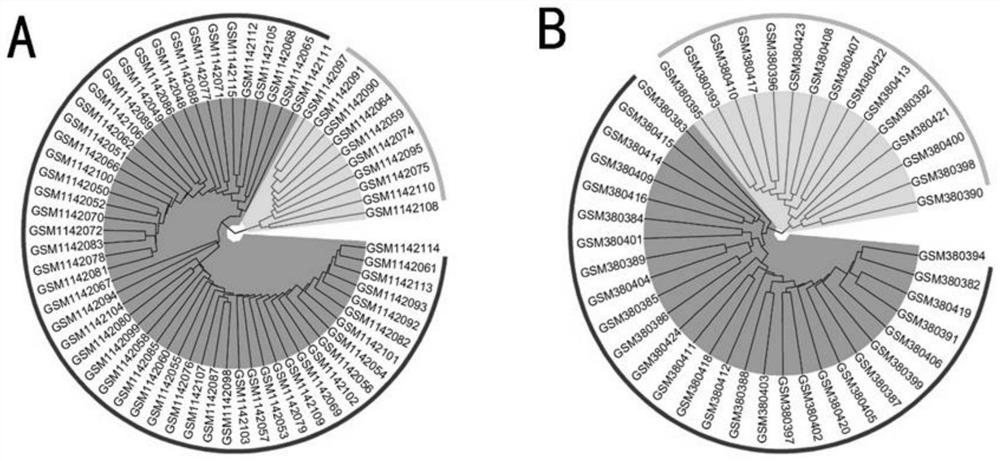
Biliary atresia potential molecular subtype and recognition method of core gene of biliary atresia potential molecular subtype
PendingCN114783515AOvercome the weakness of incomparable expression valuesOvercome incomparable weaknessesCharacter and pattern recognitionMedical automated diagnosisCore geneMedicine
The invention relates to a biliary atresia potential molecular subtype and core gene identification method, which comprises the following steps: based on a relative expression rank relationship among genes in a sample, mining potential gene pairs with subtype identification capability, and identifying and verifying the molecular subtype through clustering analysis. And then analyzing and identifying gene modules related to BA subtypes by using a weighted gene co-expression network, and screening core genes related to the subtypes. The biliary tract atresia subtype key gene identification method gets rid of limitation of gene expression absolute values, overcomes the disadvantage that expression values among different laboratories are incomparable, eliminates the influence of group difference on results to the greatest extent, and can be applied to single individual samples by the biliary tract atresia subtype key genes identified by the biliary tract atresia subtype key gene identification method.
Owner:GANNAN MEDICAL UNIV
A method for constructing an animal model of chronic liver cirrhosis with biliary atresia
ActiveCN106172229BAvoid deathReduce immunosuppressionImmunoglobulins against animals/humansAnimal husbandryDiseaseRotavirus RNA
The invention relates to a construction method of an animal model for biliary atresia of chronic liver disease firstly utilizing a Gr-1 antibody to construct the construction method of the animal model for biliary atresia of chronic liver disease. The method comprises following steps: injecting rhesus monkey rotavirus within the 24 hours of neonatal mouse to induce a classic animal model for biliary atresia, firstly injecting the Gr-1 antibodies before rhesus monkey rotavirus is injected; injecting the Gr-1 antibodies multiple times after mouse are born on the 14th day to maintain the effective concentration range of the Gr-1 antibodies in the mouse; and injecting 5-20ng / 1.6g of Gr-1 antibodies to the mouse each time. The construction method of an animal model for biliary atresia of chronic liver disease has following beneficial effects: the model is capable of simulating the process of biliary atresia, development and prognosis, especially in the end-stage hepatocirrhosis phase.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
Method based on gene/protein as diagnostic marker and therapeutic target for biliary atresia and application thereof
PendingCN111778325APerfect clinical theory mechanismImprove hypoxiaMicrobiological testing/measurementDigestive systemBiliary tractFibrosis
The invention discloses a method based on a gene / protein as a diagnostic marker and a therapeutic target for biliary atresia and application thereof. Notch3 signaling pathway and remodeling of hepaticartery system mediated thereby are first proposed in the context of biliary atresia (BA), and constitute a perfection to the existing theoretical mechanism. The Notch3-Hey1 gene / protein, which is highly expressed in liver tissue, can be converted into a BA diagnostic marker which is helpful to prediction of prognosis in children and selection of surgical surgical methods as an auxiliary means. Incurrent clinical practice, anti-inflammatory and anti-fibrosis treatment cannot achieve ideal effect, and Kasai operation can not completely stop the progression of BA; however, a new adjuvant therapy strategy is provided by Notch3 signaling pathway inhibitors, thereby beneficially improving hypoxia and bile duct injury in portal area, slowing down disease progression and raising survival rates of patients with BA liver
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Compositions and methods for treating liver disease
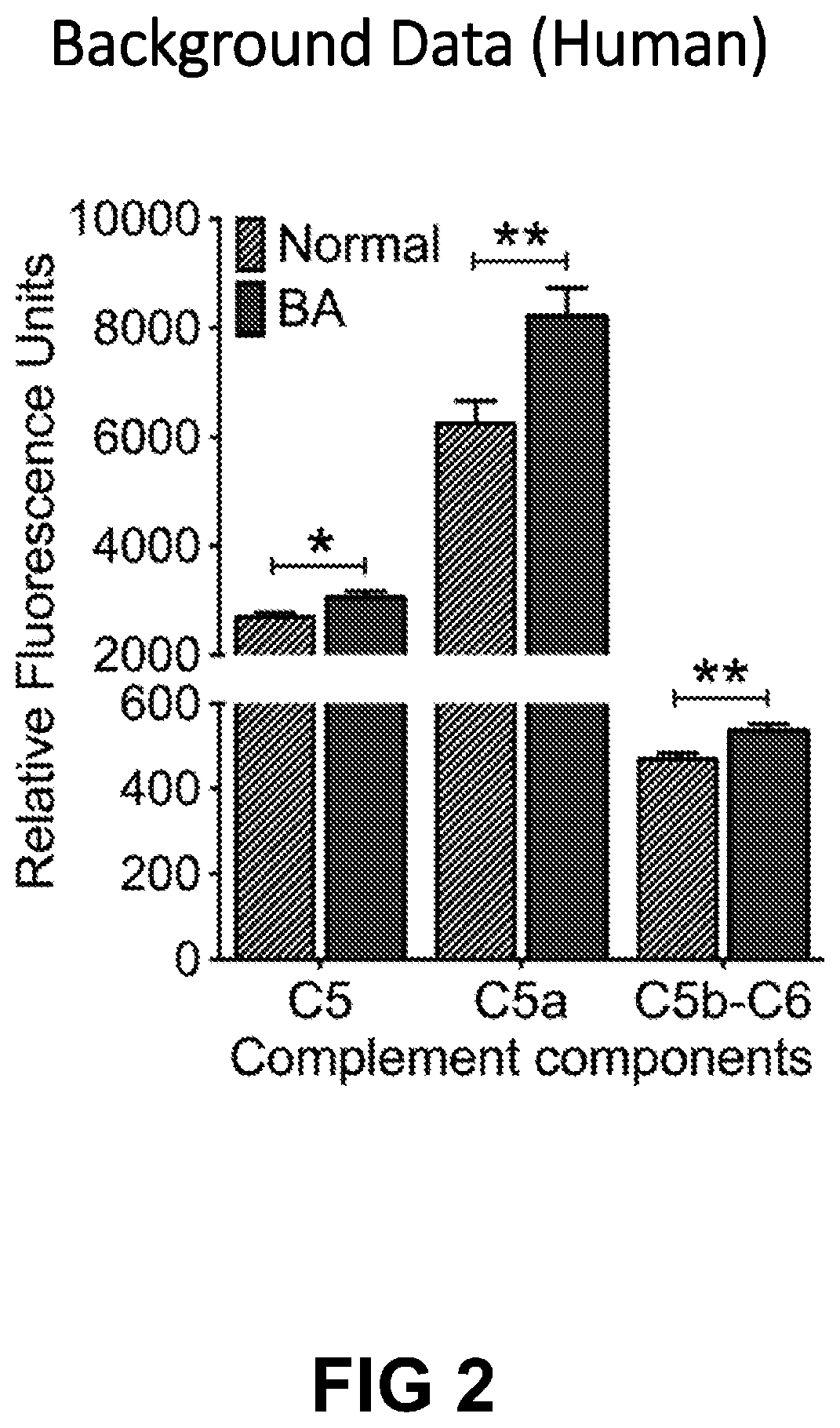
Disclosed are methods of treating a subject, particularly a human individual, more particularly a pediatric individual, having a biliary disorder, via administration of a therapeutically effective amount of a C5 inhibitor. The biliary disorder may include biliary atresia and post-Kasai biliary atresia.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com