Biliary atresia diagnostic kit
A diagnostic kit and biliary atresia technology, applied in the field of medical diagnostics, can solve the problems of lack of early diagnostic indicators and low cure rate in children with BA, and achieve the effect of promoting and using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
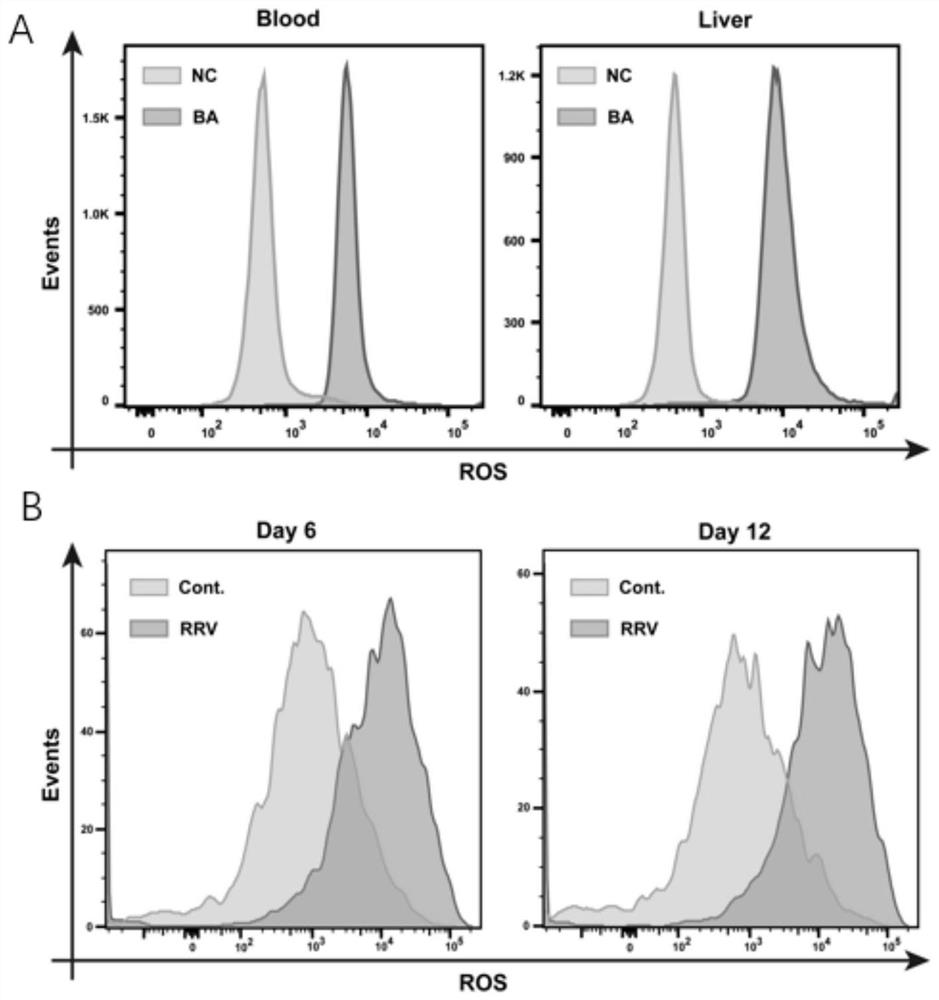
[0043] 1) Sorting of CD177+ cells in peripheral blood
[0044]The erythrocytes in peripheral blood of children in NC group and BA group were lysed with erythrocyte lysate to make single cell suspension for later use. Add FcR blocking to the cell suspension and incubate at room temperature for 10min, add anti-human PE conjugated CD177 antibody and incubate at 4°C for 30min, wash with flow staining buffer twice, add anti-PE Microbeads and incubate at 4°C for 15min, wash with flow staining buffer Twice, LD magnetic separation column was used to carry out the sorting of CD177+ cells on a magnetic cell sorter (MiltenyiBiotec, Germany), and the sorted CD177+ cells were tested for cell purity on a BD FACS Aria II (BD Biosciences, USA), And use cells with cell purity>95% for the next experiment.
[0045] 2) Electron microscope observation and analysis of mitochondria in CD177+ cells
[0046] Take 1.5×10 respectively 6 A CD177+ cell was selected from the peripheral blood samples of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com