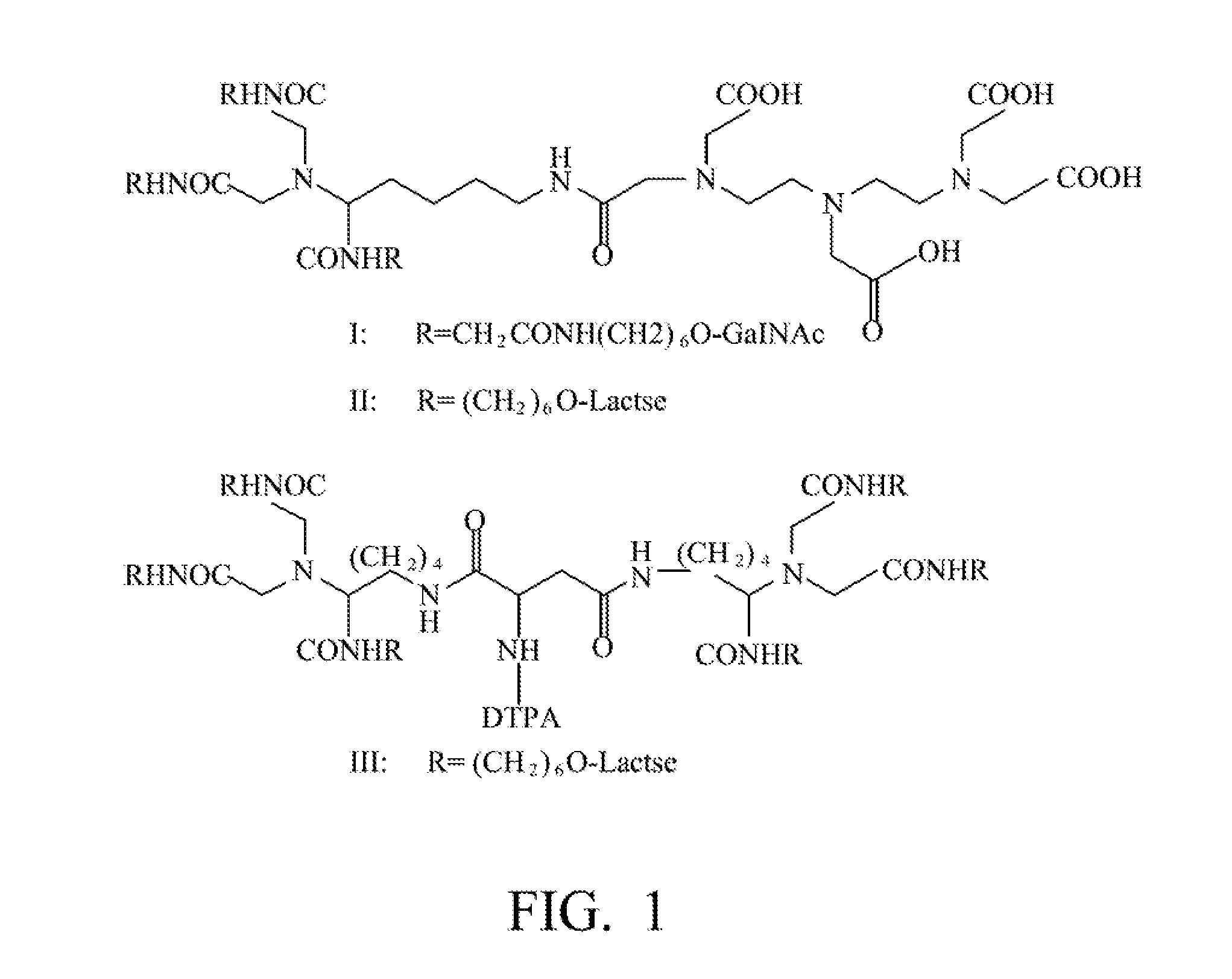
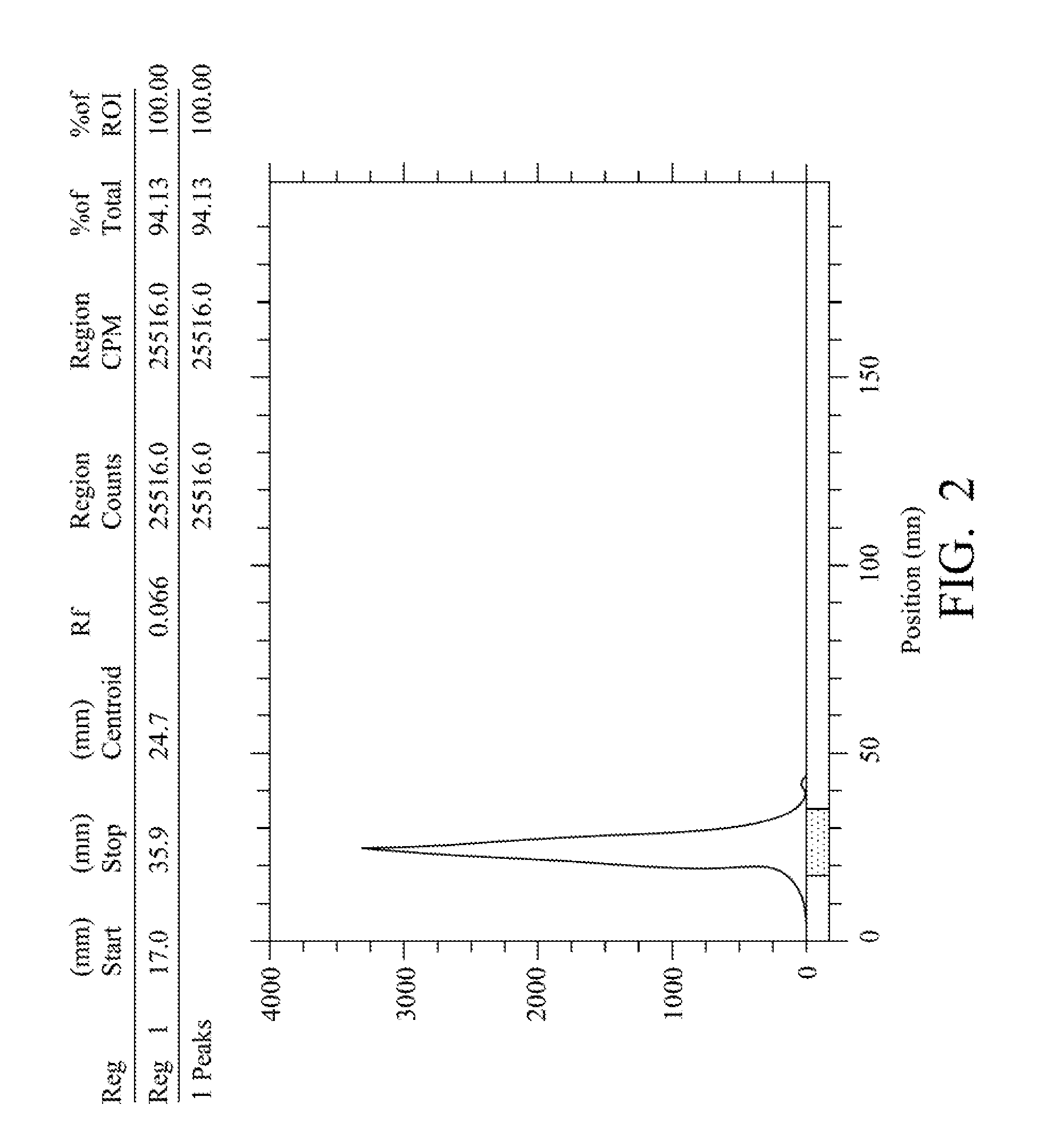
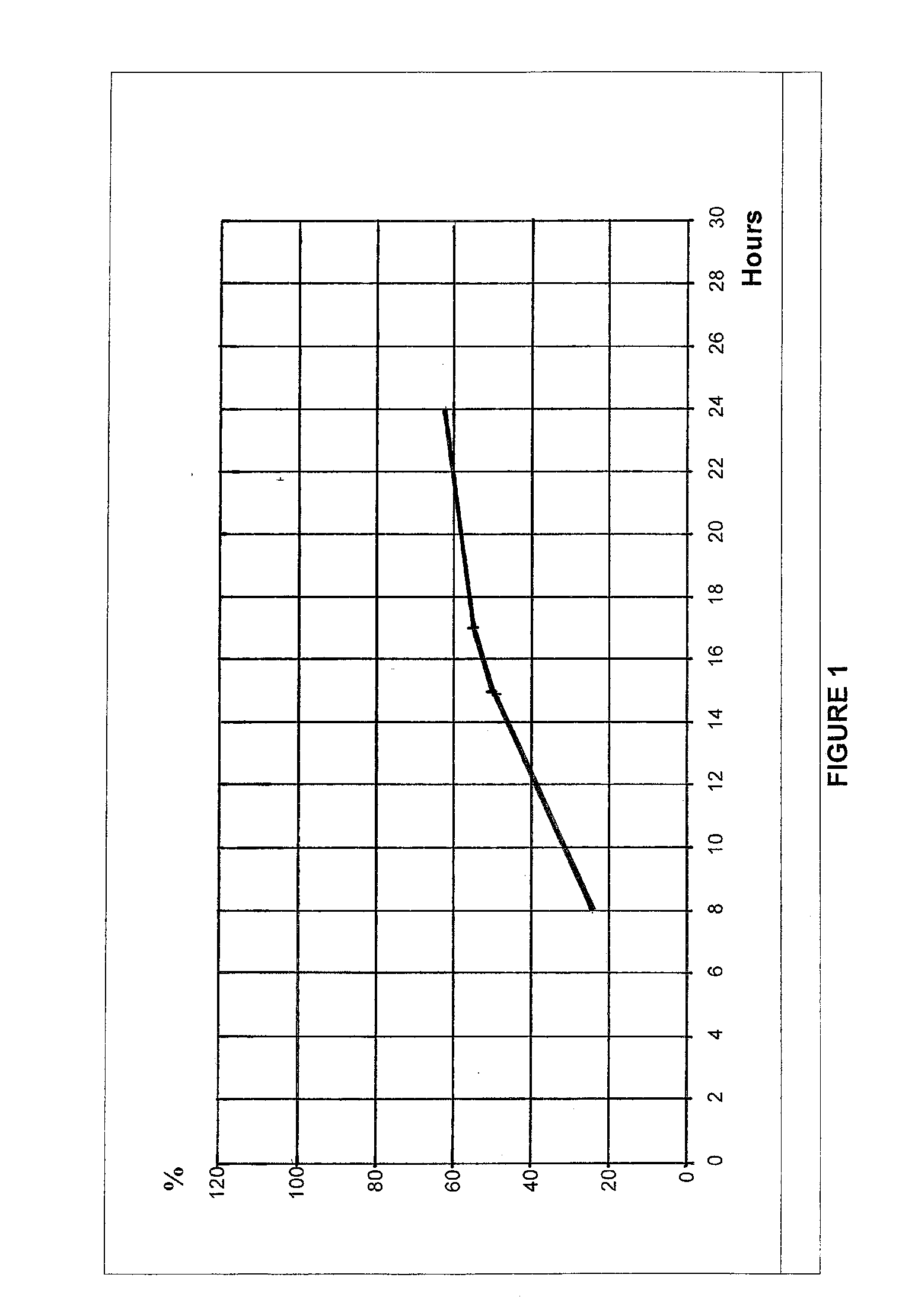
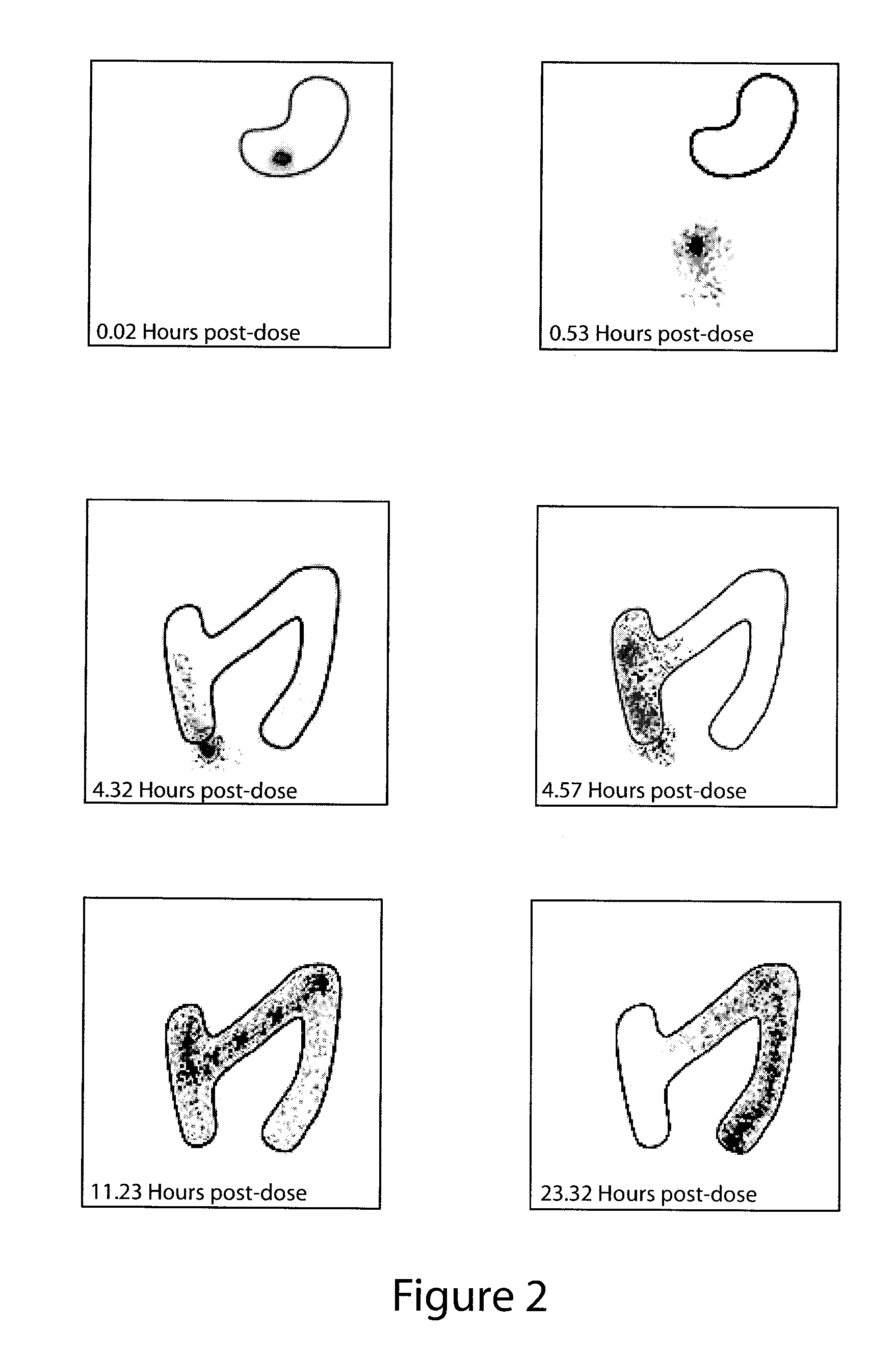
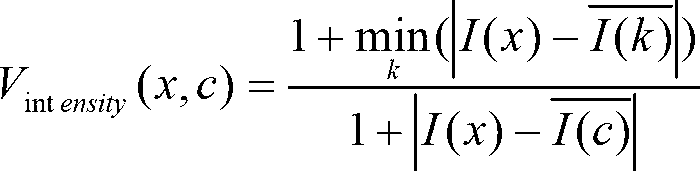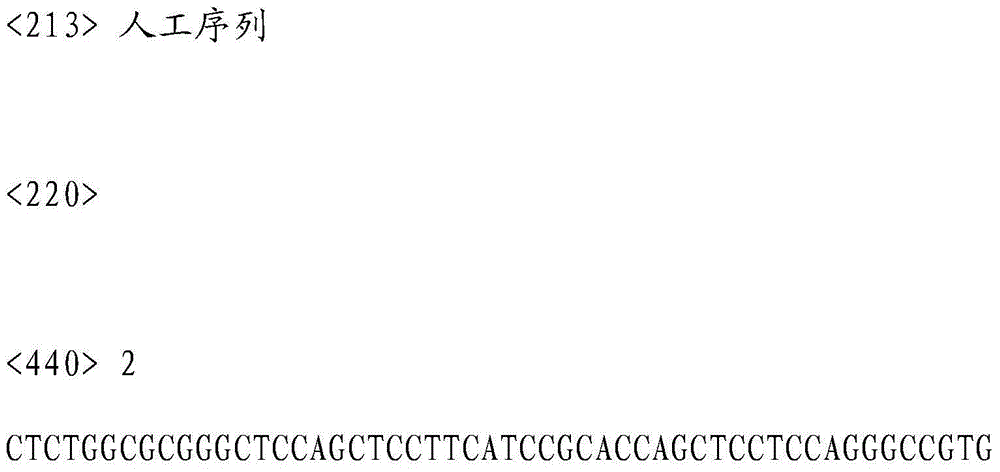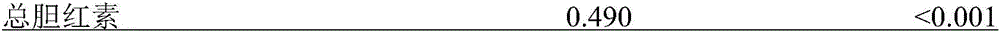Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
112 results about "Liver transplantation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
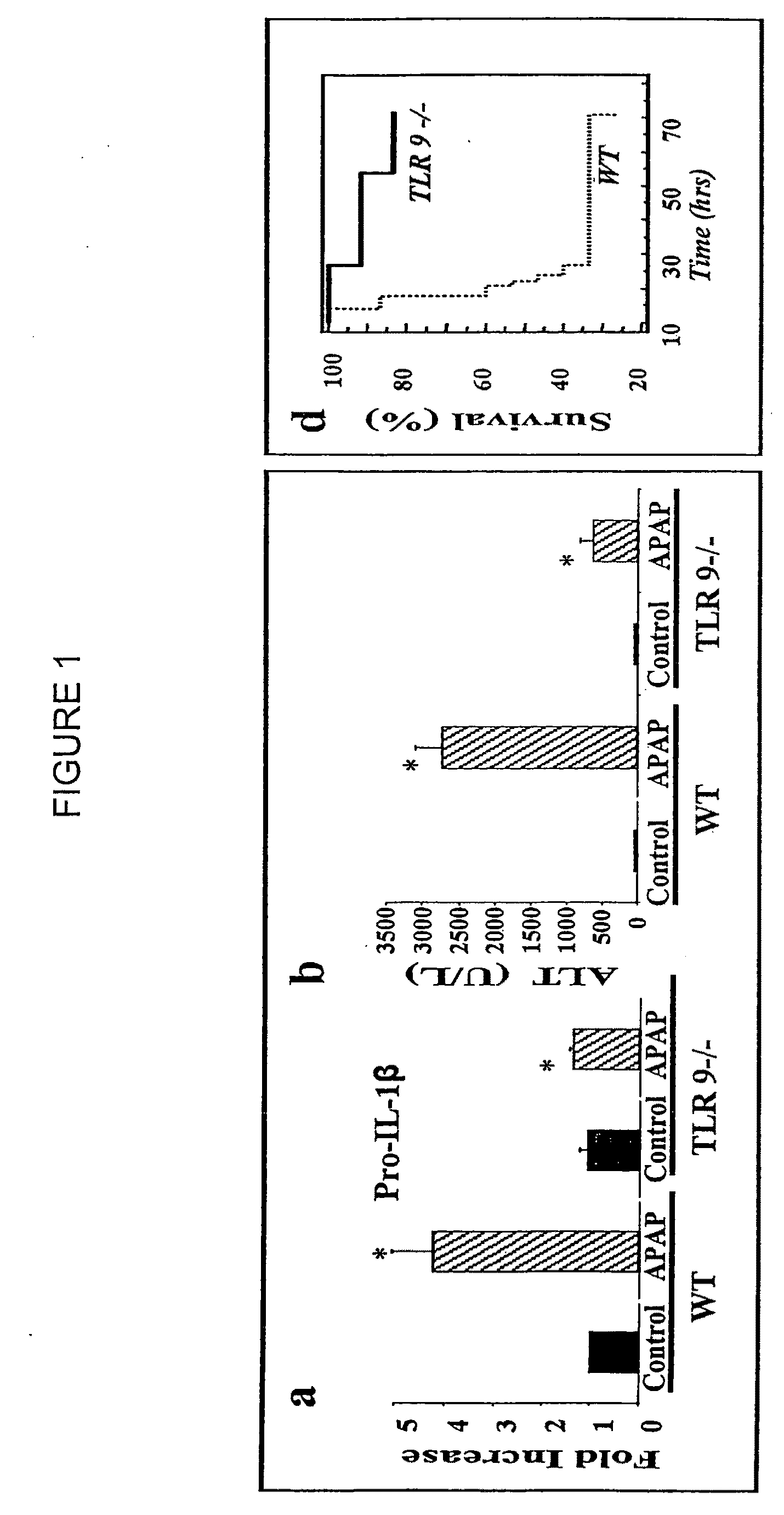
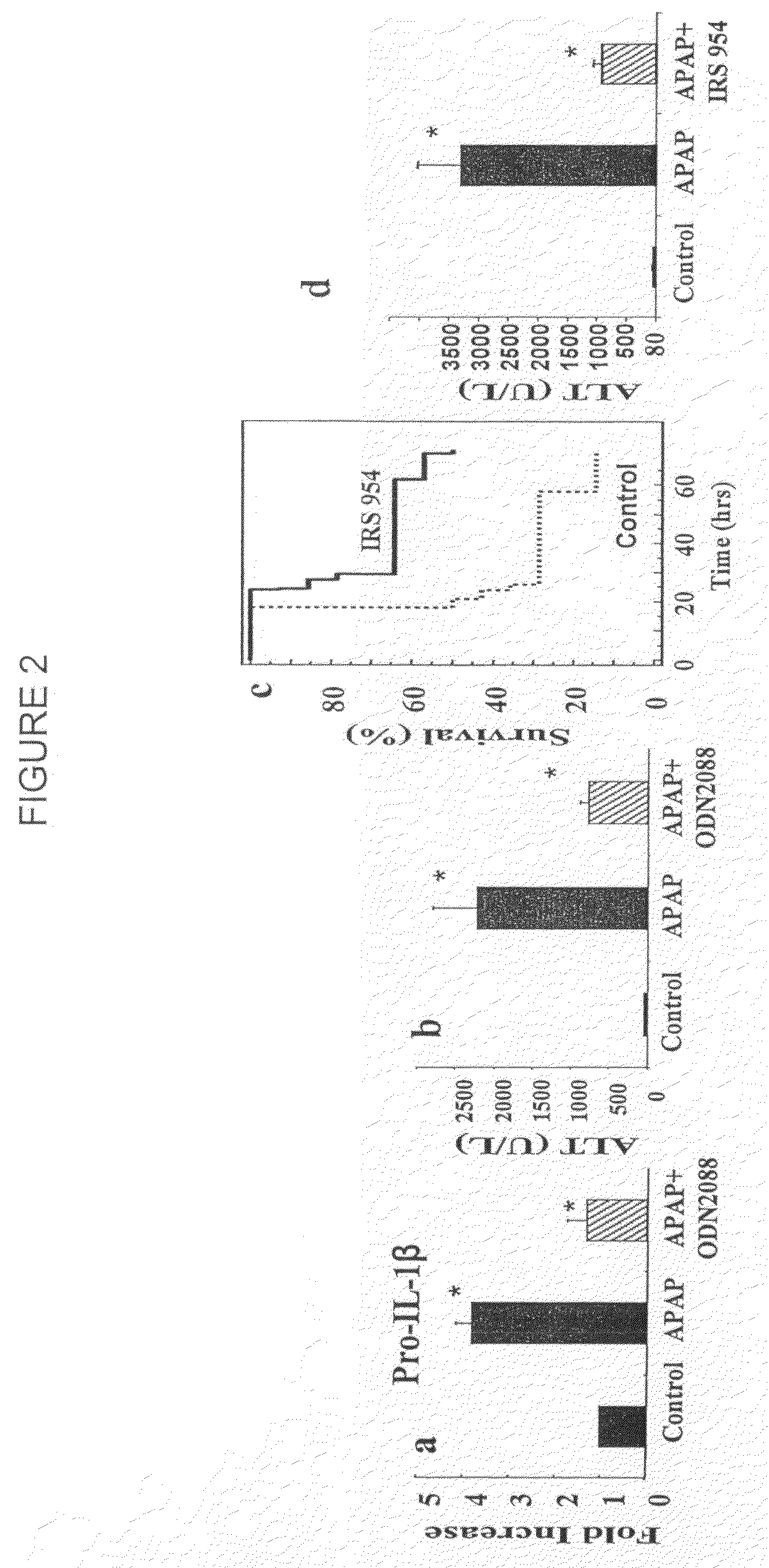
Liver transplantation or hepatic transplantation is the replacement of a diseased liver with the healthy liver from another person (allograft). Liver transplantation is a treatment option for end-stage liver disease and acute liver failure, although availability of donor organs is a major limitation. The most common technique is orthotopic transplantation, in which the native liver is removed and replaced by the donor organ in the same anatomic position as the original liver. The surgical procedure is complex, requiring careful harvest of the donor organ and meticulous implantation into the recipient. Liver transplantation is highly regulated, and only performed at designated transplant medical centers by highly trained transplant physicians and supporting medical team. The duration of the surgery ranges from 4 to 18 hours depending on outcome. Favorable outcomes require careful screening for eligible recipient, as well as a well-calibrated live or cadaveric donor match.
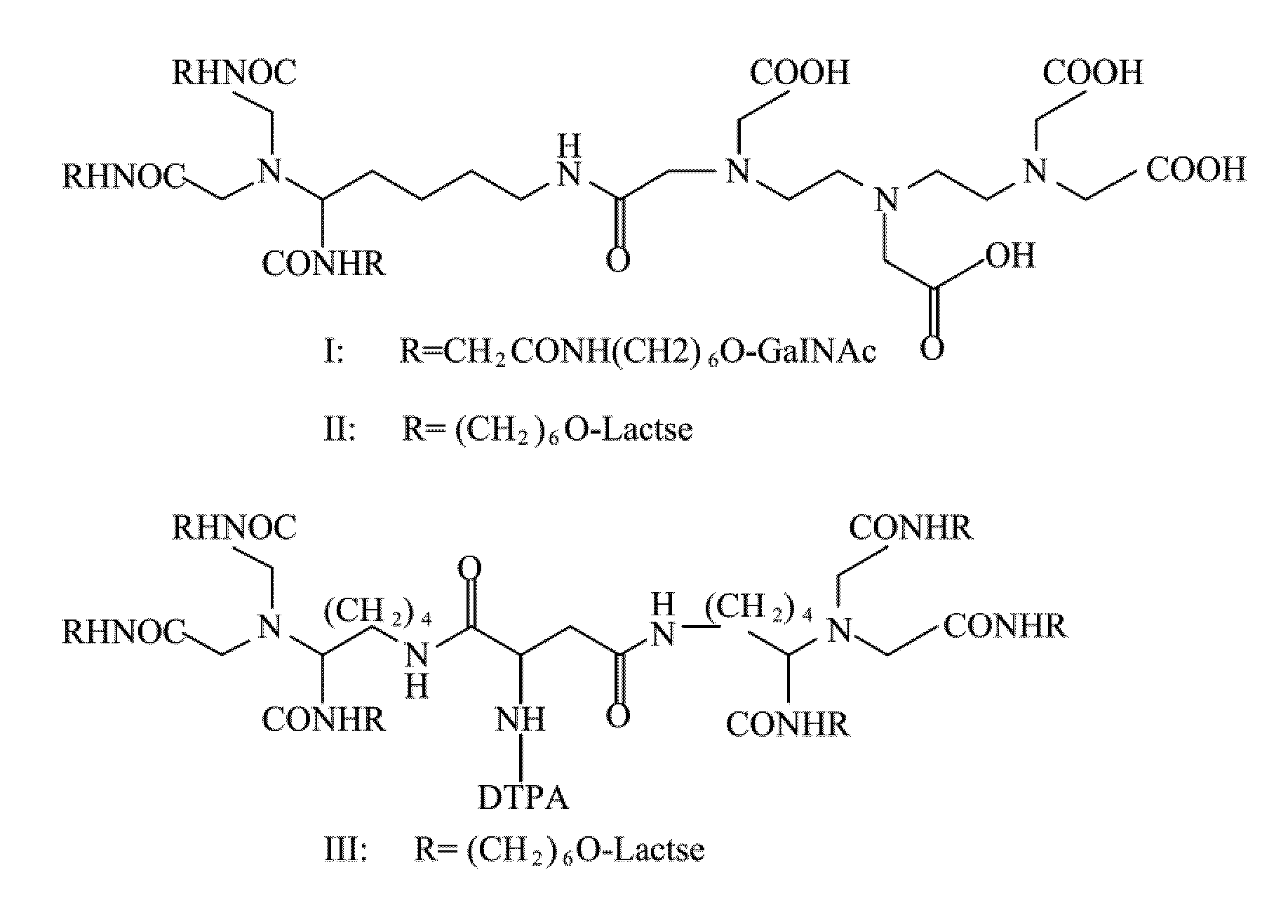
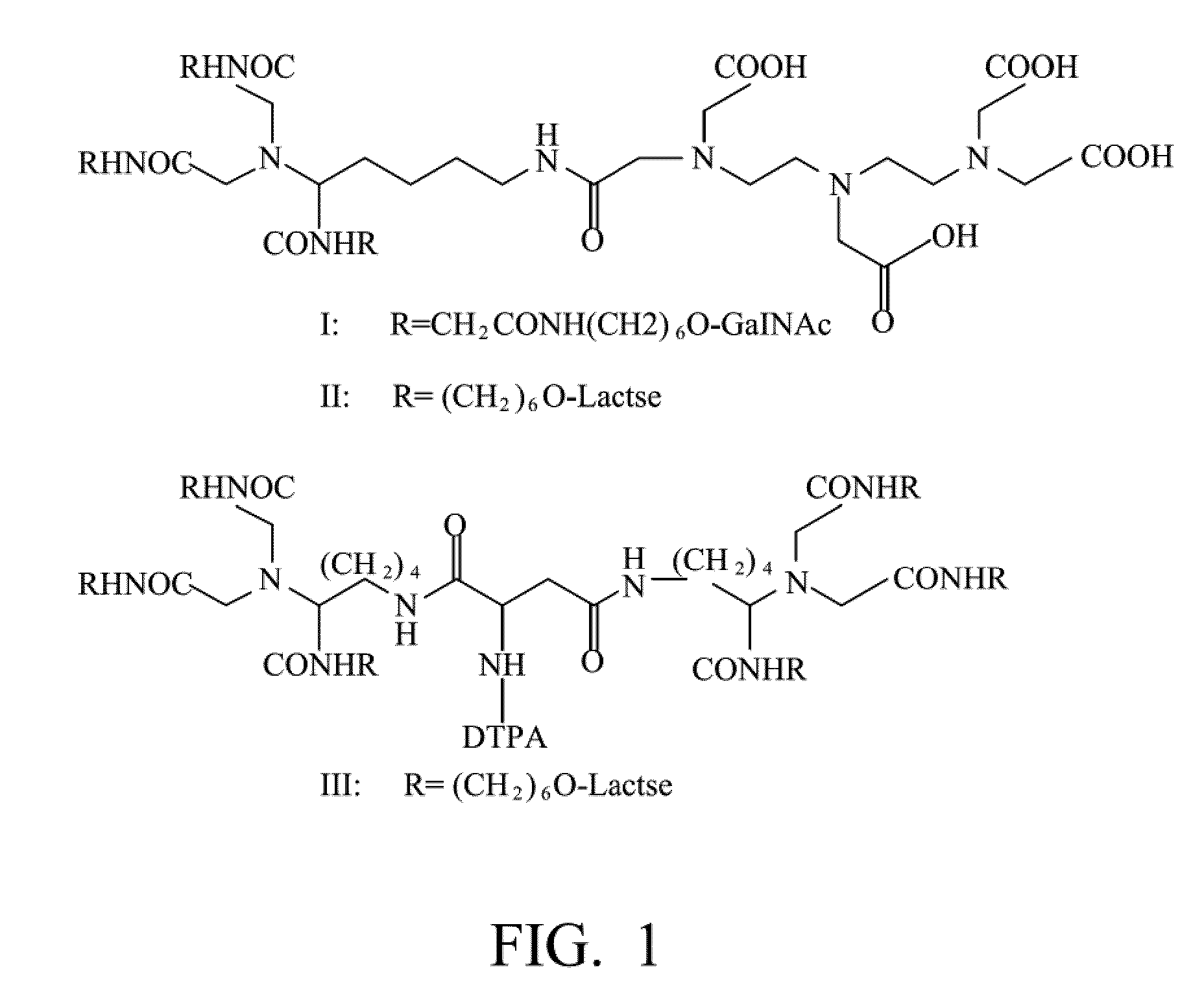
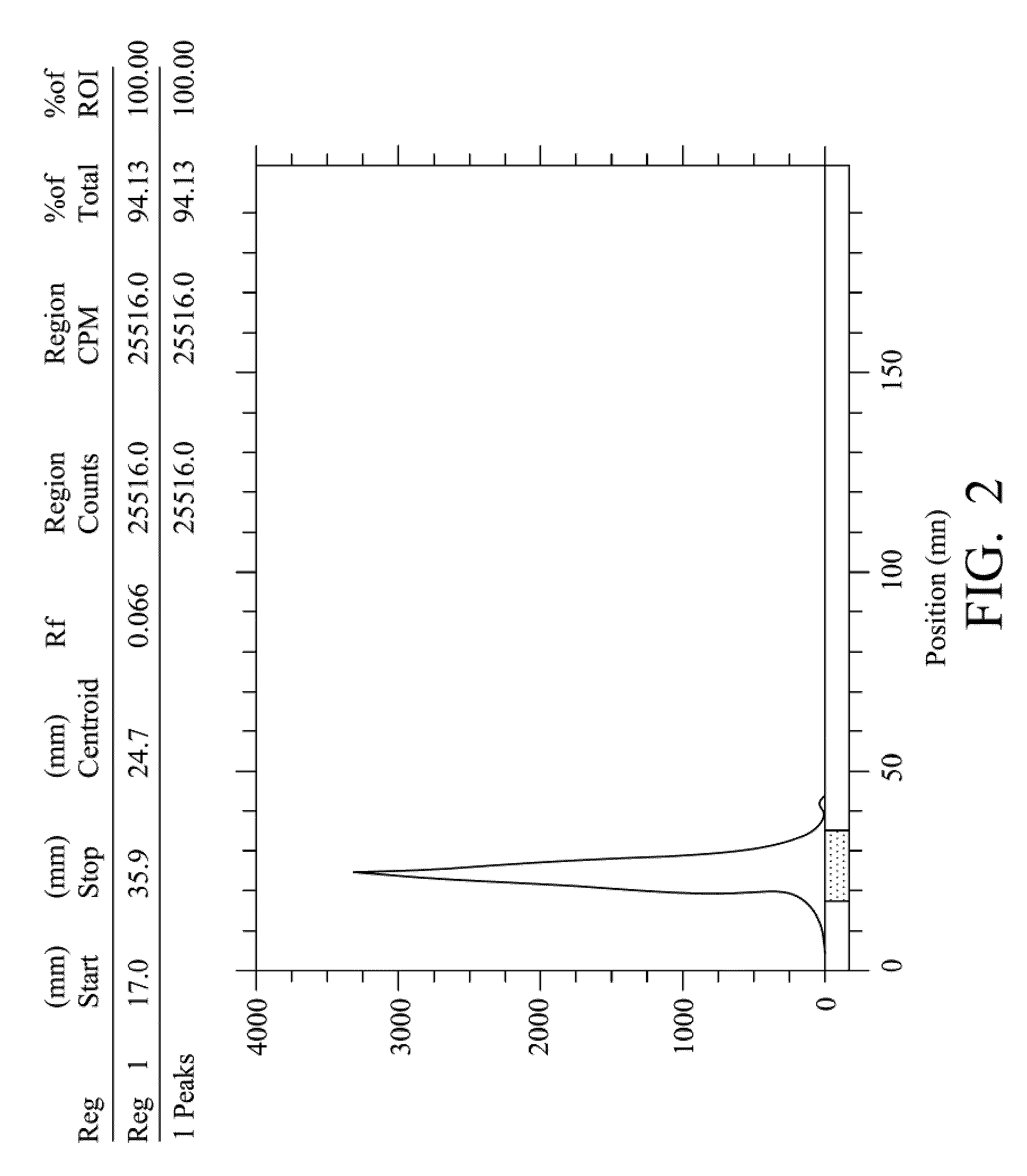
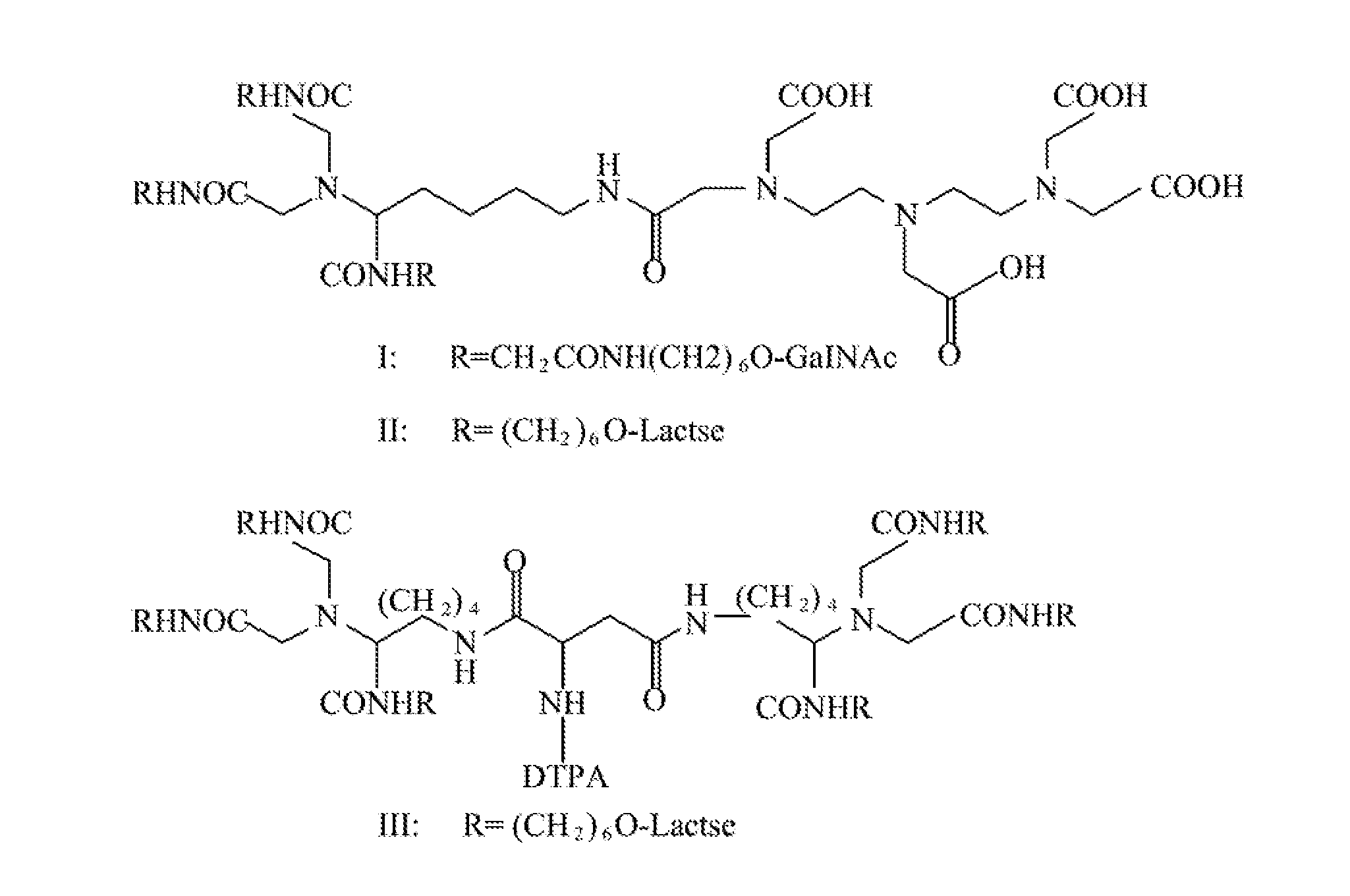
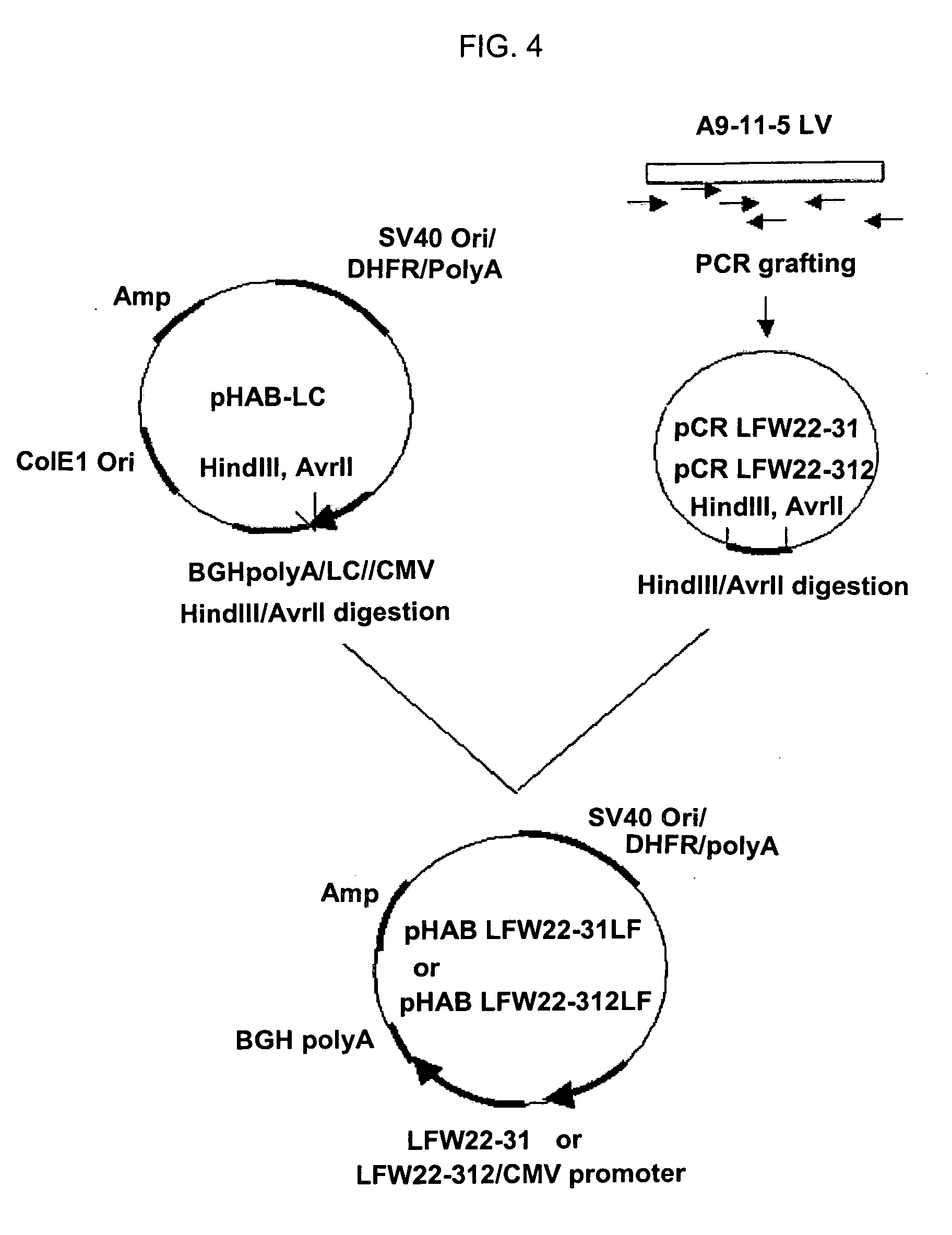
Quantification method for remaining liver function and novel liver receptor imaging agent
ActiveUS20110097265A1High measurement accuracyImprove accuracySugar derivativesSaccharide peptide ingredientsDiseaseReceptor
A test indicator for quantifying remaining liver function is provided. A novel liver receptor imaging agent with liver targeting property is utilized to develop a method for quantifying remaining liver function to serve as test indicator for judging the liver failure outcome in clinic, particularly for judging the necessity of liver transplantation for patients with liver failure or liver disease. The radioactivity uptake of the test indicator was negatively correlated with the extent of liver reserve. The cutoff value of liver reserve for liver transplantation is also disclosed.
Owner:INST NUCLEAR ENERGY RES ROCAEC
Quantification method for remaining liver function and novel liver receptor imaging agent
ActiveUS8435491B2High measurement accuracyImprove accuracySugar derivativesRadioactive preparation carriersDiseaseLiver function
A test indicator for quantifying remaining liver function is provided. A novel liver receptor imaging agent with liver targeting property is utilized to develop a method for quantifying remaining liver function to serve as test indicator for judging the liver failure outcome in clinic, particularly for judging the necessity of liver transplantation for patients with liver failure or liver disease. The radioactivity uptake of the test indicator was negatively correlated with the extent of liver reserve. The cutoff value of liver reserve for liver transplantation is also disclosed.
Owner:INST NUCLEAR ENERGY RES ROCAEC
Portable preserving device for liver external perfusing
InactiveCN1543785ASimple structurePortableDead animal preservationSuction devicesWater bathsPortal vein
The invention relates to a portable liver extracorporal charging preservation apparatus for liver transplantation comprising a constant temperature water bath tank for liver storage, an oxygenator, a hepatic artery charging circulation loop and a portal vein charging circulation loop, and an intelligent control device for controlling bath tank temperature and flow capacity of charging circulation loop. The liver dual perfusion model created by the invention can carry out oxygen cycle charging to the exsomatized liver at normothermia.
Owner:鞠烽炽 +2
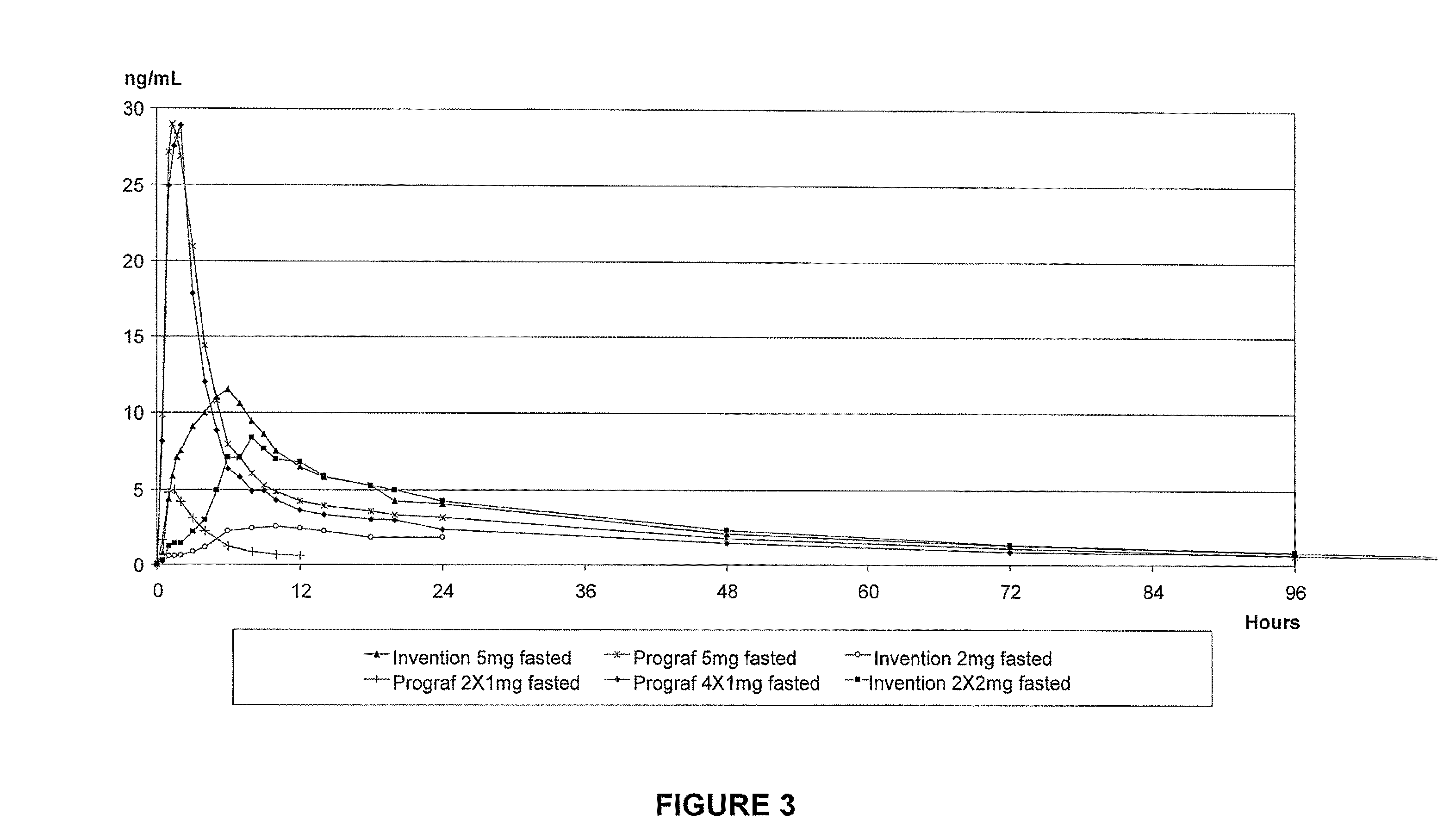
Tacrolimus for improved treatment of transplant patients
ActiveUS20100105717A1Improve bioavailabilityReduce riskBiocideOrganic chemistryTherapeutic effectIn vivo
An extended release oral dosage form comprising as active substance tacrolimus or a pharmaceutically active analogue thereof for a once daily immunosuppressive treatment of a patient in need thereof, preferable a kidney or liver transplant patient. The dosage form releases the active substance over an extended period of time. It also provides improved pharmacokinetic parameters due to an extended and constant in vivo release including substantial decreased peak concentrations, despite increased bioavailability, substantial extended times for maximal concentration, and higher minimal concentrations when compared with conventional immediate release dosage forms and a recent modified release tacrolimus dosage form.
Owner:VELOXIS PHARM INC
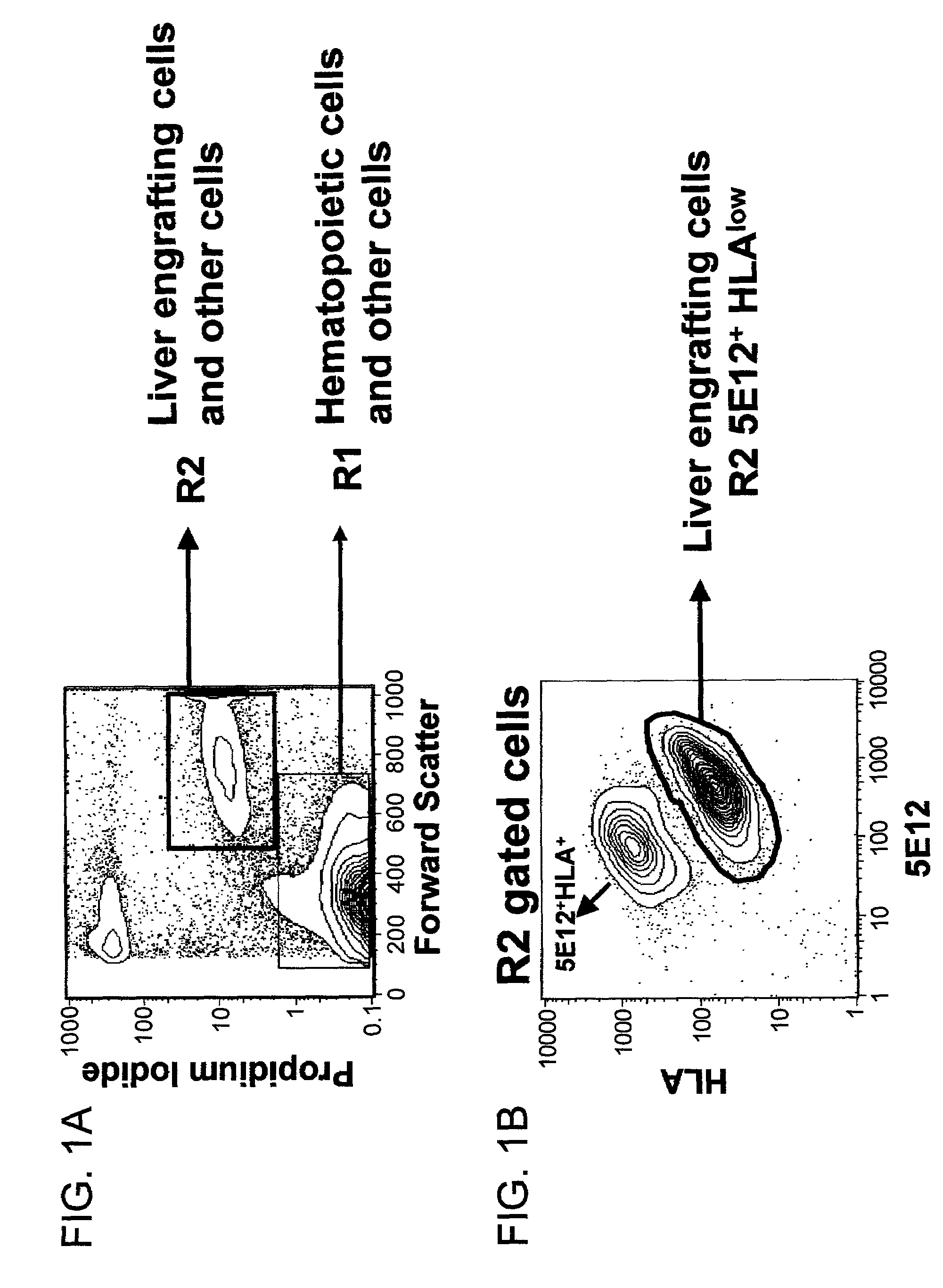
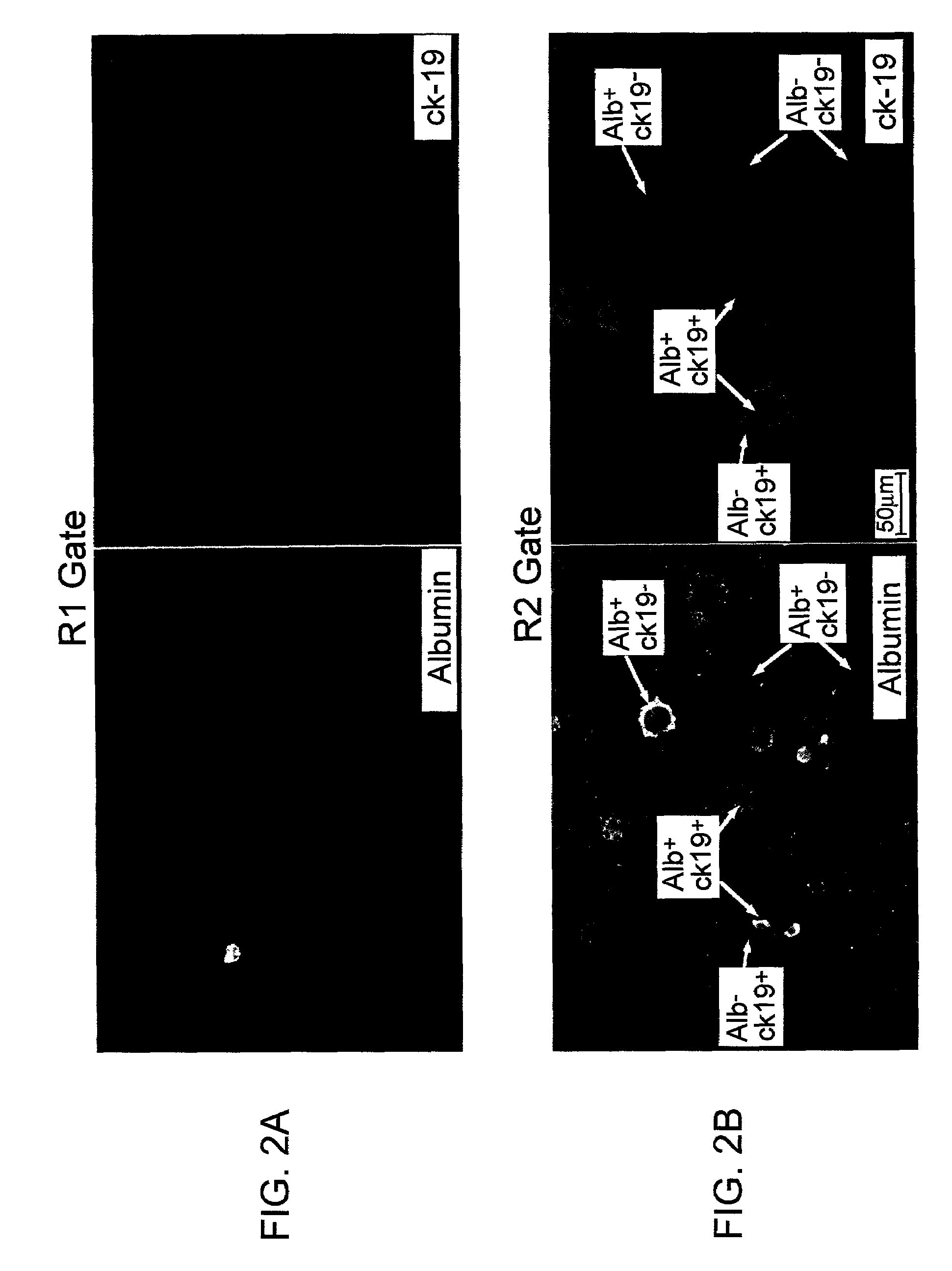
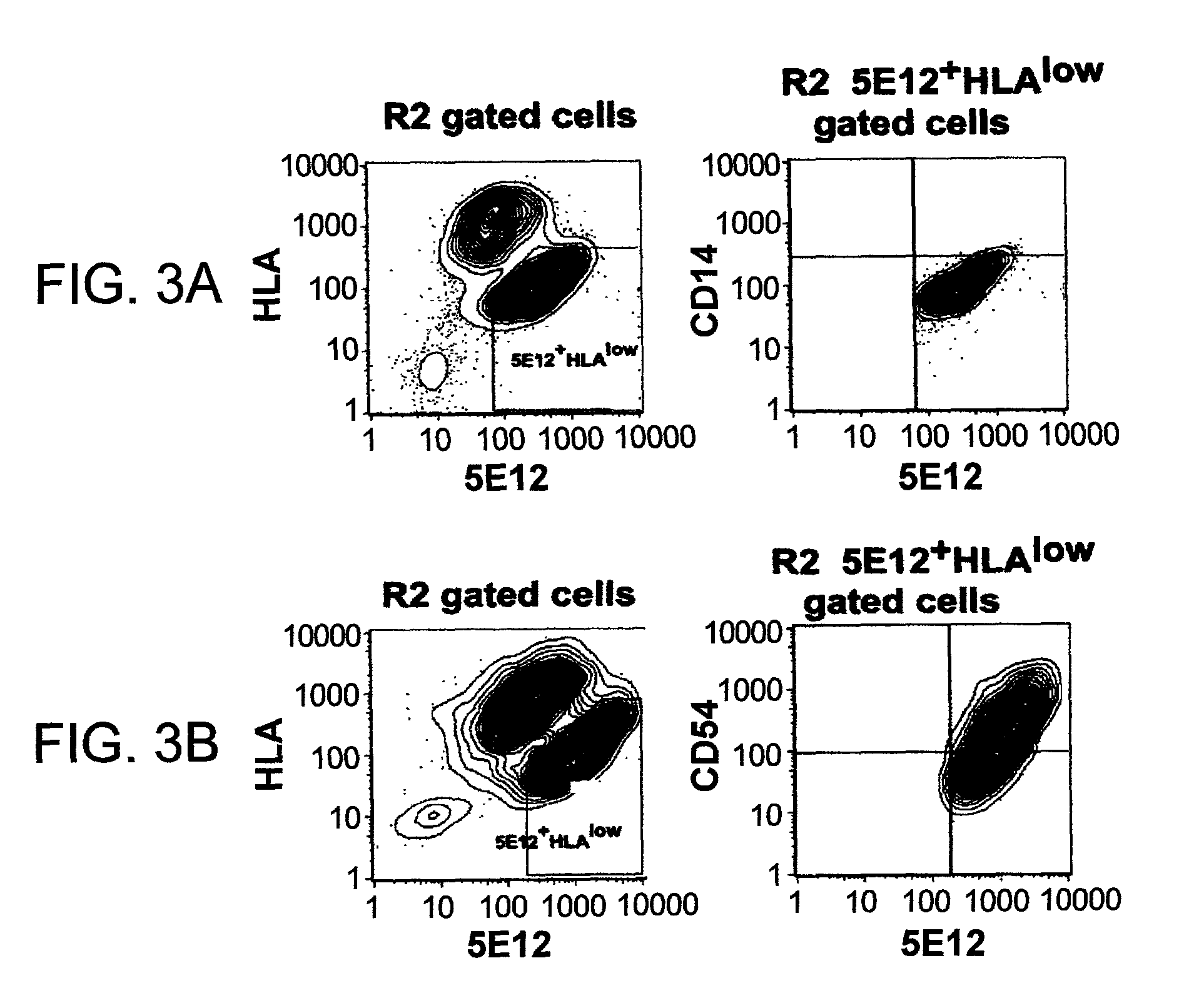
Liver engrafting cells, assays, and uses thereof
A substantially enriched mammalian hepatic liver engrafting cell population is provided. Methods are provided for the isolation and culture of this liver engrafting cell. The progenitor cells are obtained from a variety of sources, including fetal and adult tissues. The cells are useful in transplantation, for experimental evaluation, and as a source of lineage and cell specific products, including mRNA species useful in identifying genes specifically expressed in these cells, and as targets for the discovery of factors or molecules that can affect them.
Owner:BOCO SILICON VALLEY INC
Realization method of computer-assisted liver transplantation operation planning system
InactiveCN102938027AStable and efficient shape priorsEffective adaptabilityImage enhancementImage analysisVeinLiver parenchyma
The invention relates to a realization method of a computer-assisted liver transplantation operation planning system, comprising the following steps: step 1, establishing a normal person liver shape library; step 2, for the liver to be operated, modeling the initial partition result of the liver area by using a method based on sparse representation, and eliminating disturbance of singular point and noise data, so as to obtain a prior shape having a liver area with patient adaptability; steps 3, precisely partitioning the liver by utilizing the prior shape, and simultaneously separating out liver parenchyma, portal vein, liver vein and tumour; step 4, dividing the liver into eight liver segments independent in functions by utilizing the liver vein partition result, and calculating the volume of each liver segment and the tumour; and step 5, carrying out 3D visualization on the partitioning and segmenting result. Compared with the prior art, the realization method has the advantages of high precision and robustness, convenience in realization and capability of treating complicated liver shapes.
Owner:HEBEI UNIVERSITY
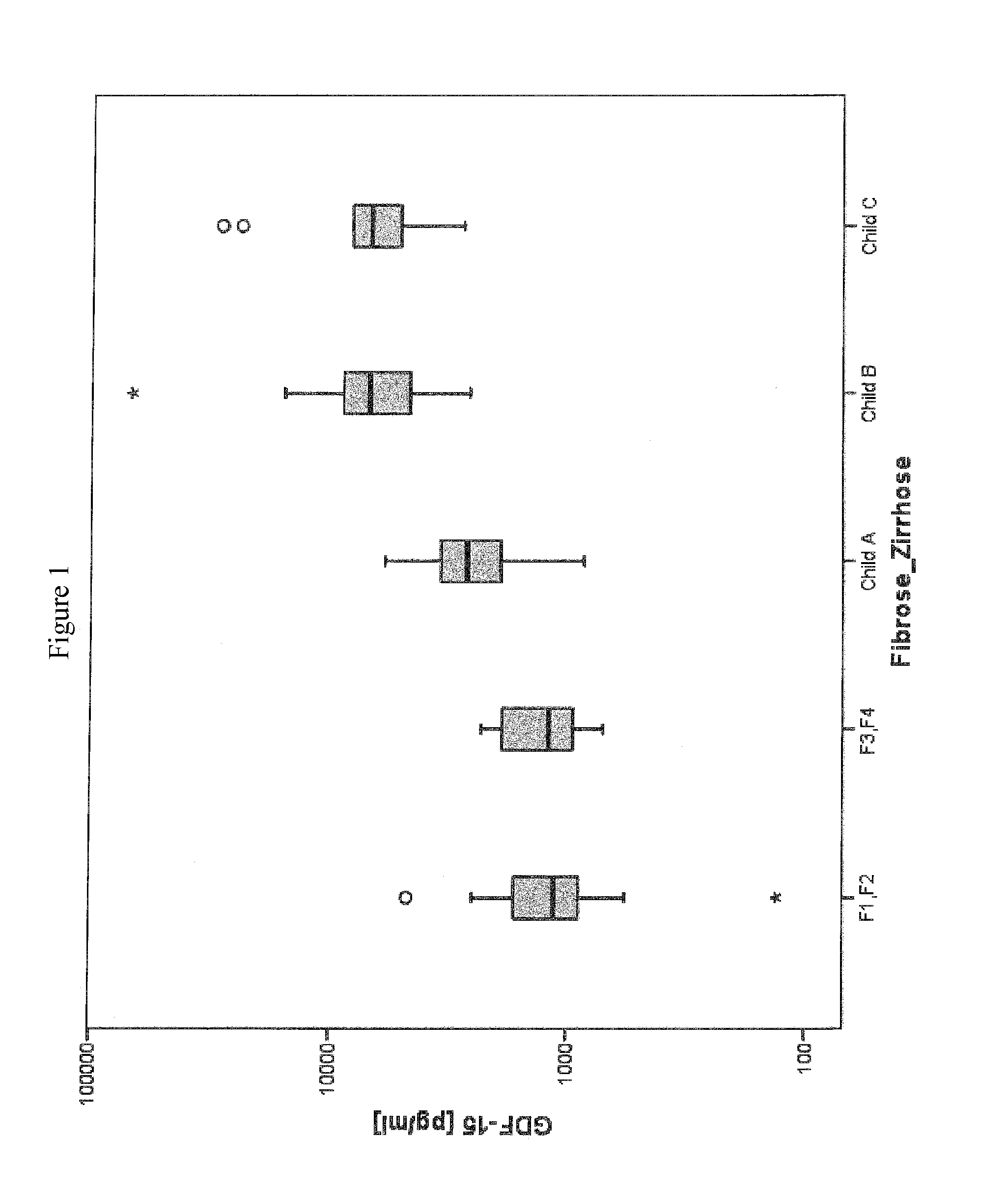
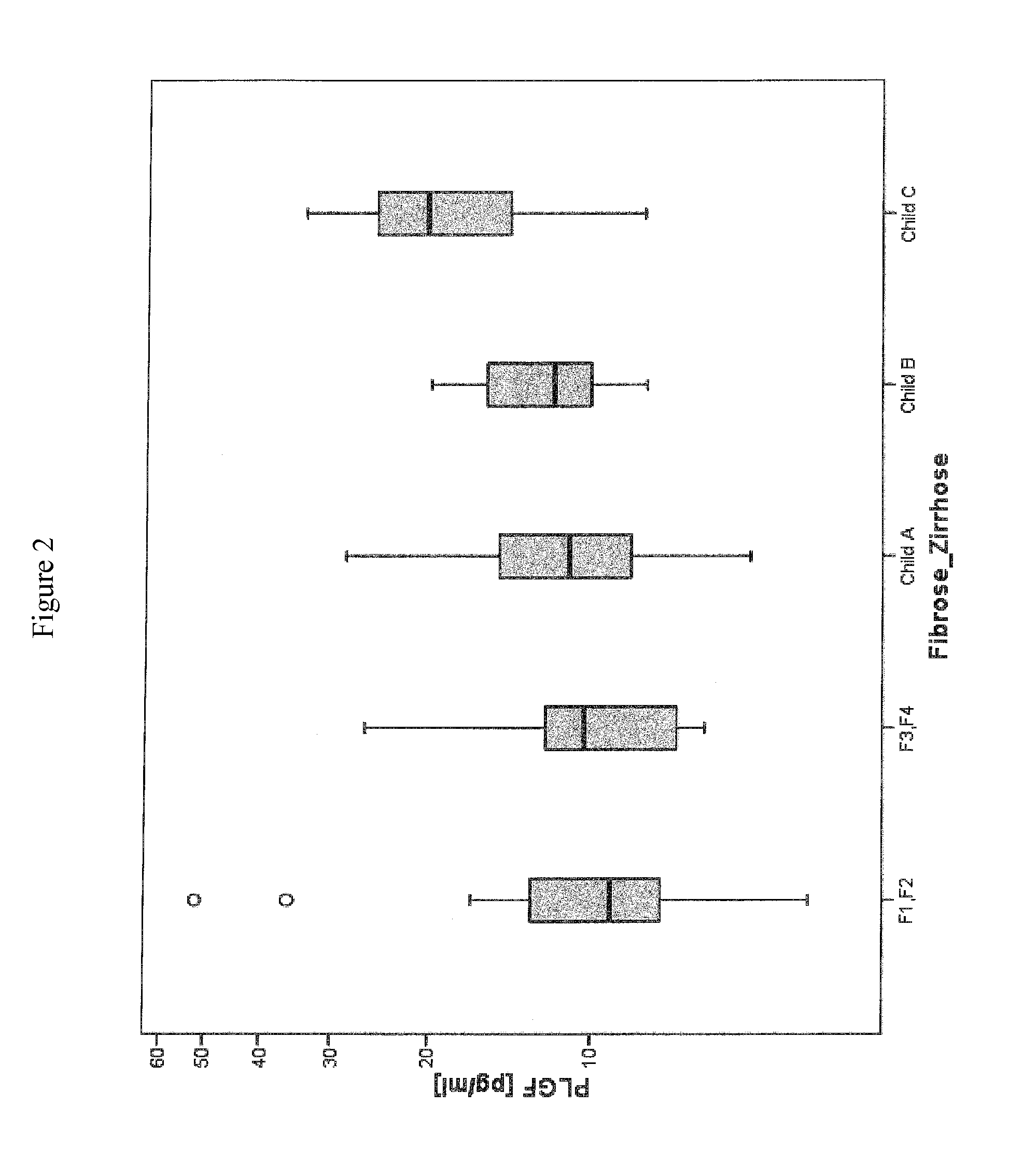
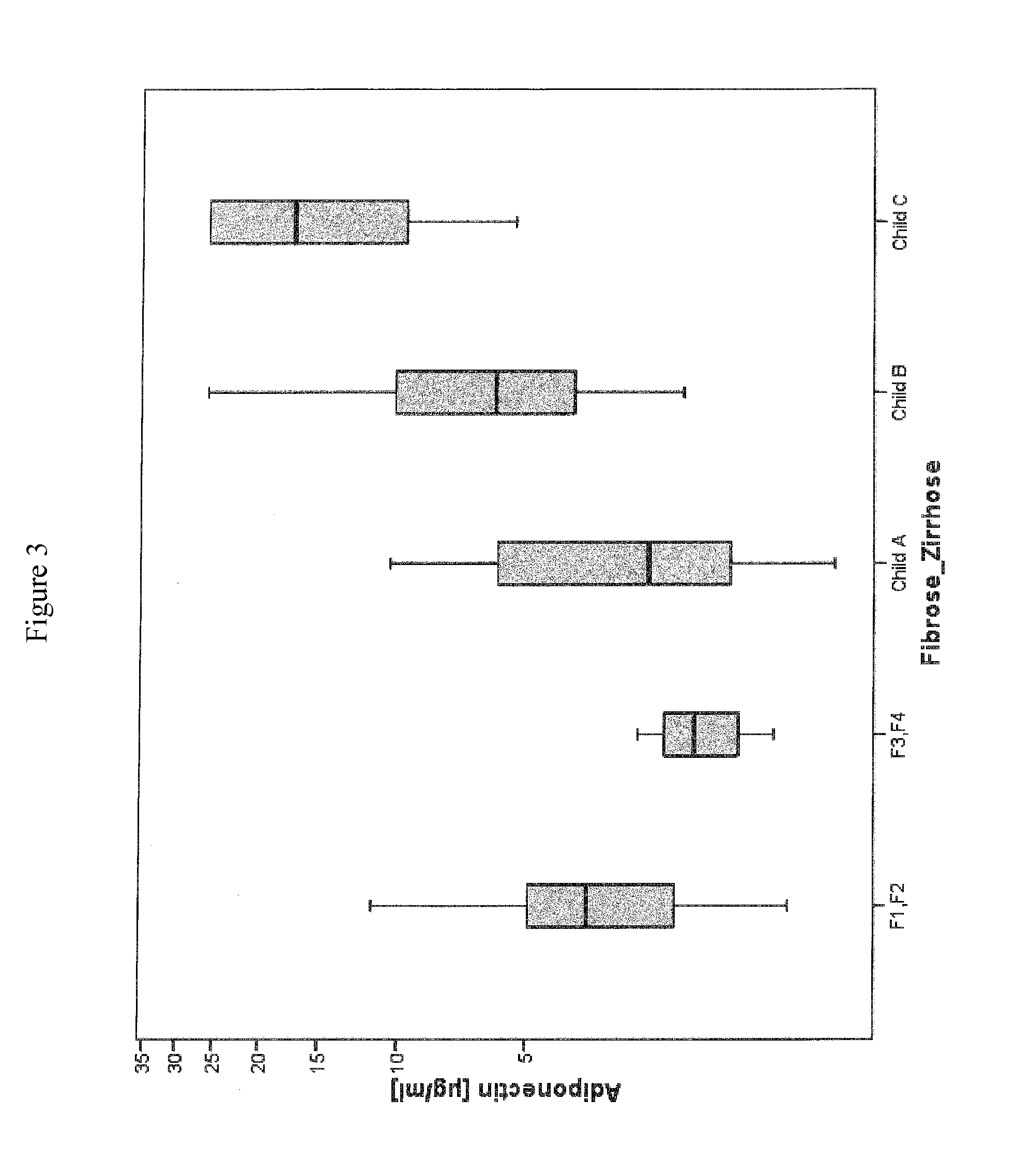
Method for assessment of severity of liver cirrhosis
InactiveUS20110257022A1Peptide librariesLibrary screeningHepatocyte growth factorLiver transplantation
Disclosed is a method for diagnosing whether a subject suffers from a mild or severe form of liver cirrhosis based on determining the amount of GDF-15 (growth differentiation factor 15), PlGF (placental growth factor), and / or hepatocyte growth factor (HGF) in a sample from the subject and comparing the thus determined amount(s) with a reference amount (reference amounts). The method may further include determining the amount of adiponectin in a sample from the subject, and comparing the amount to a reference amount for adiponectin. Also described is a method to identifying a subject being susceptible to liver transplantation including determining the amount of GDF-15, PlGF, and / or HGF in a sample from the subject and comparing the thus determined amount(s) with a reference amount (reference amounts).
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
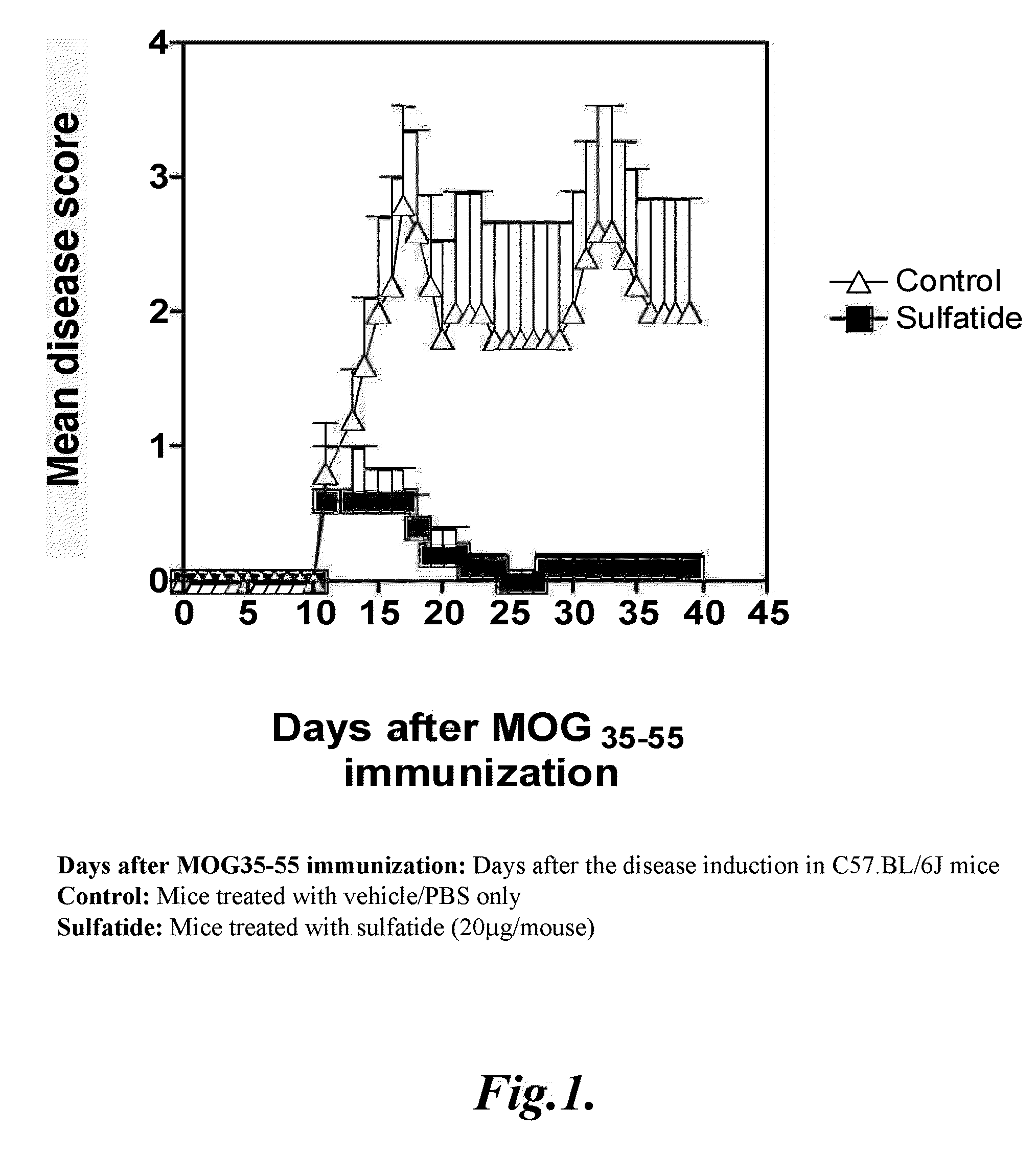
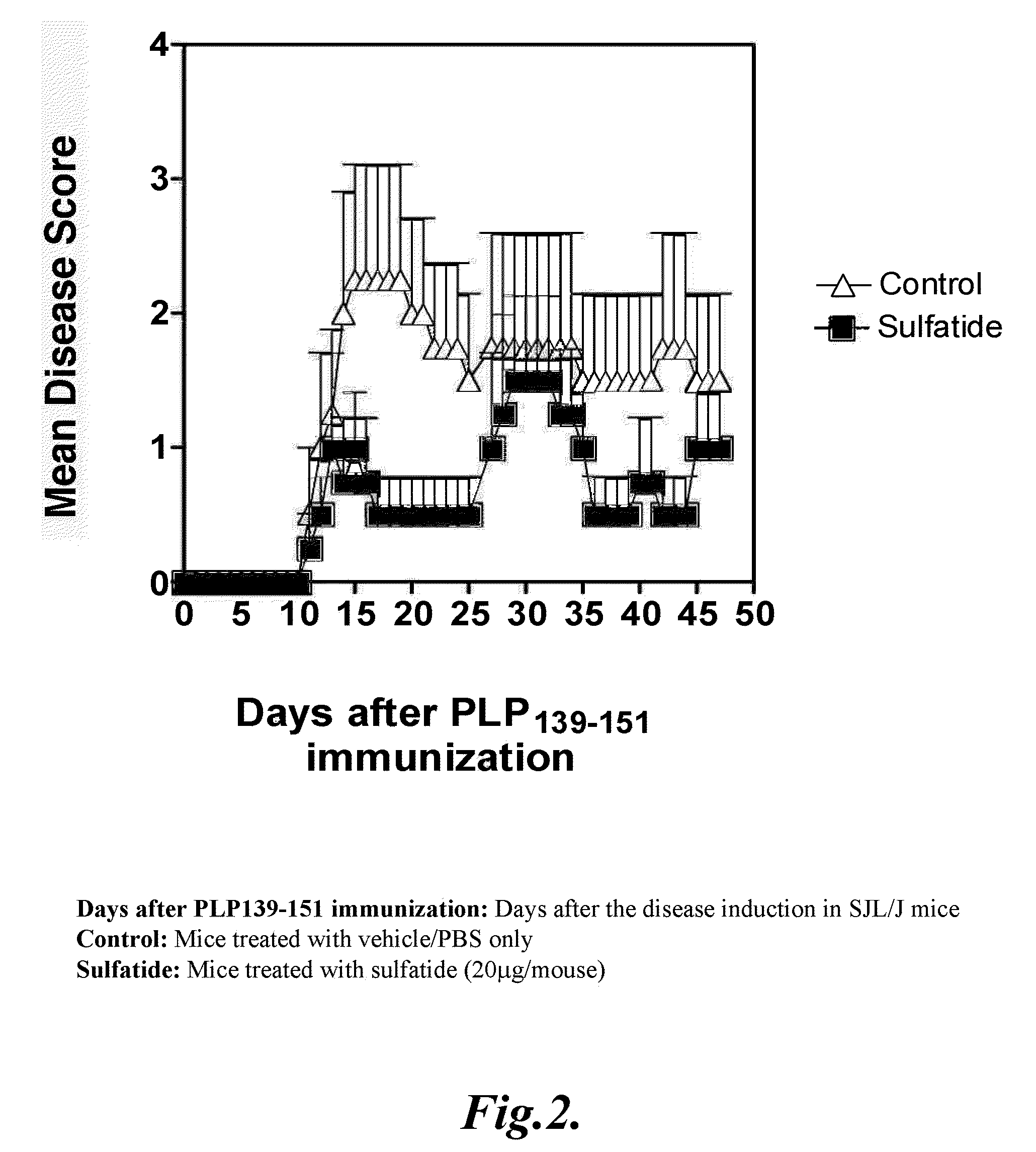
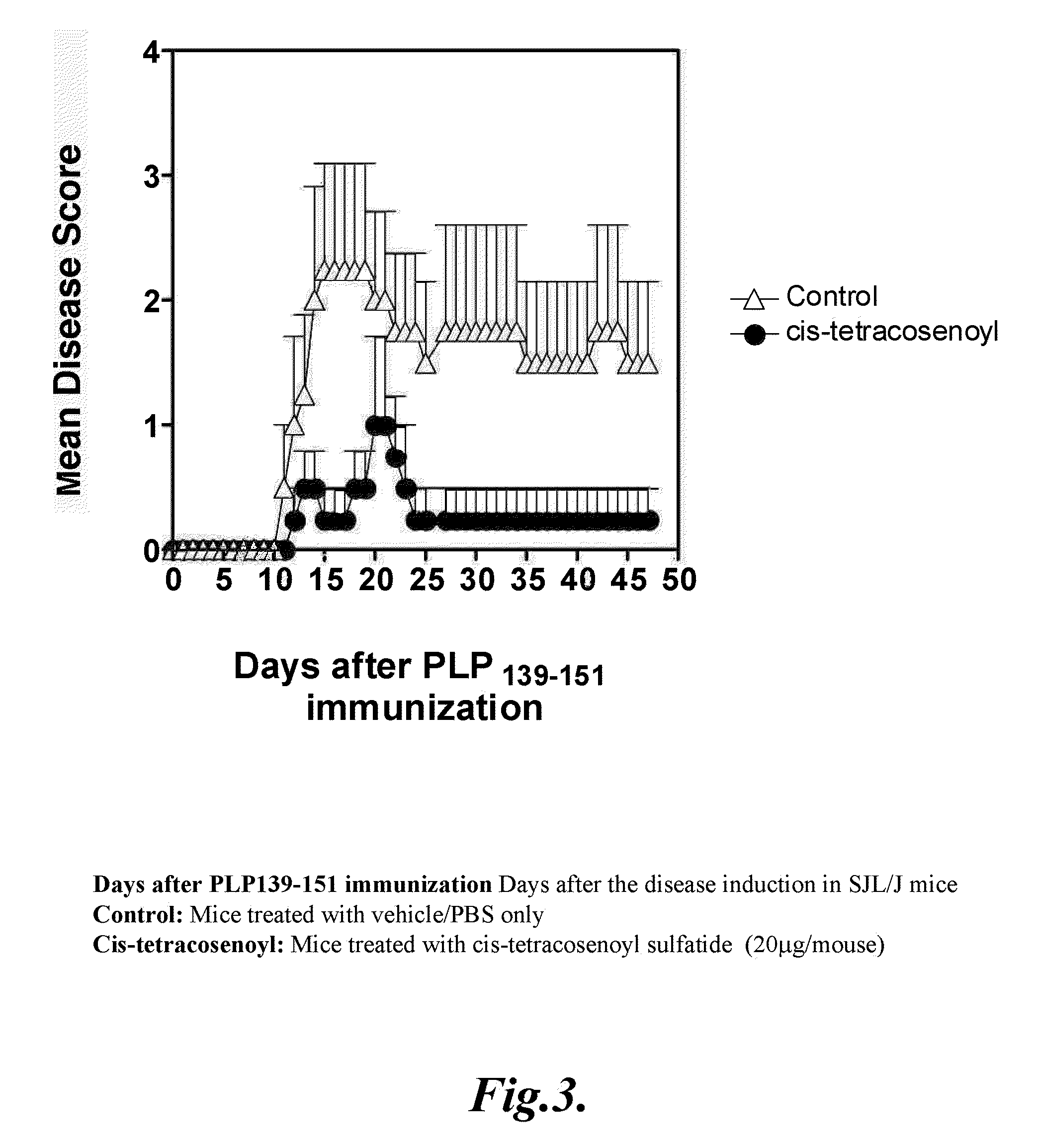
Prevention of hepatic ischemic reperfusion injury by administration of sulfatides
Hepatic ischemic reperfusion injury is a major complication of liver transplantation, resectional hepatic surgeries, trauma surgery and shock. Disclosed herein are methods for the prevention and treatment of ischemia and reperfusion injury with the administration of sulfatides. Also disclosed herein are methods of preventing and treating hepatic reperfusion injury by administering an amount of a sulfatide to the body of a patient effective to reduce or prevent the symptoms of the injury.
Owner:CHATURVEDI VIPIN KUMAR
Humanized antibody against S-surface antigen of hepatitis B virus
A humanized antibody of the present invention shows similar antigen-binding affinity to a mouse monoclonal antibody and significantly low immunogenecity. Therefore, the humanized antibody of the present invention can be effectively used for treating chronic hepatitis B and preventing HBV infection of a patient received liver transplantation and vertical transmission from a mother infected with HBV to a fetus.
Owner:YUHAN
Compositions and methods for reducing hepatotoxicity associated with drug administration and treating non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and associated cirrhosis
InactiveUS20090239831A1Improve efficiencyEnhancing armamentariumBiocideSalicyclic acid active ingredientsActive agentHigh doses
The present invention relates to the discovery that acetylsalicylic acid (ASA or aspirin), salicylic acid (SA) and related salicylate esters and their pharmaceutically acceptable salts, when coadministered in effective amounts with a drug or other bioactive agent which typically (in the absence of the salicylate compound) produces significant hepatotoxicity as a secondary indication, will substantially reduce or even eliminate such hepatotoxicity. Favorable therapeutic intervention results from the use of the present invention having the effect of reducing hepatotoxicity associated with the administration of certain drugs and other bioactive agents and in certain instances of allowing the administration of higher doses of a compound which, without the coadministration, would produce hepatotoxicity which limits or even negates the therapeutic value of the compound. The invention also relates to methods of reducing the likelihood of a patient at risk for non-alcoholic fatty liver diseases (NAFLD), including non-alcoholic steatohepatitis (NASH), or treating NAFLD or NASH including primary NASH, NASH secondary to liver transplantation (NASH post-liver transplantation) or cirrhosis represent alternative aspects of the present invention.
Owner:YALE UNIV
Intravenous injection employ persons hepatitis B immune globulin and method of preparing the same
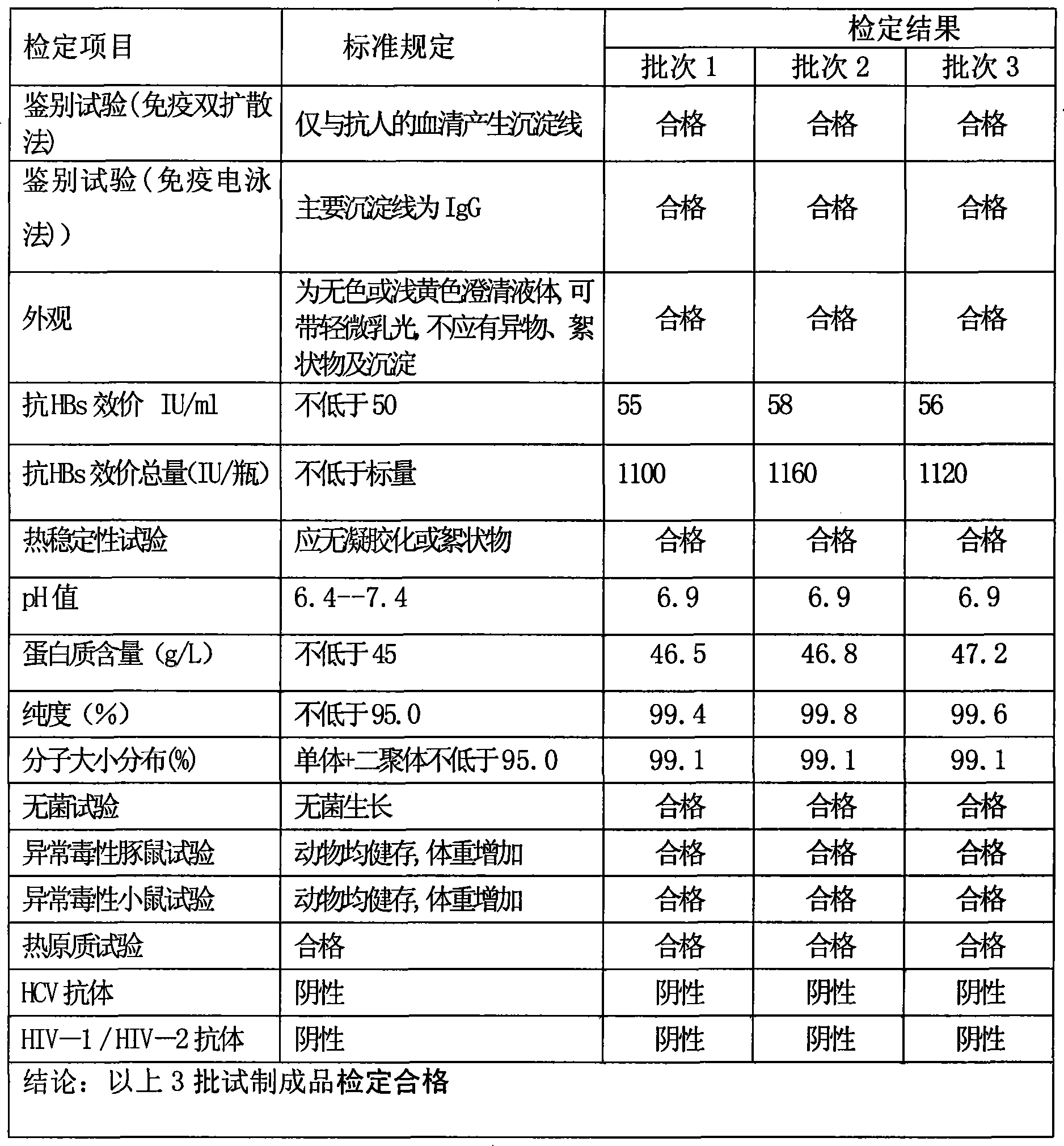
ActiveCN101249265AReduce infection rateImprove survival ratePowder deliverySerum immunoglobulinsInfection rateFiltration
The invention discloses hepatitis B immunoglobulin used in intravenous injection and a preparation method thereof. The hepatitis B immunoglobulin used in intravenous injection comprises a high-titer hepatitis B virus antibody, and the content of the immunoglobulin is not less than 95.0 percent of the total protein content; the titer of the hepatitis B antibody is more than 50IU / ml, and the sum of an IgG monomer and a dimmer is not less than 95 percent; the hepatitis B immunoglobulin is prepared through the multi-step separation, virus inactivation, ultra-filtration and dealcoholization and the filling of the raw plasma. The hepatitis B immunoglobulin used in intravenous injection is an effective drug used for preventing the patients with liver transplantation from being re-infected by the hepatitis B virus, and can obviously reduce the hepatitis B virus infection rate of the patients with liver transplantation and improve the survival rate of the patients; By overcoming the present technical bottleneck and adopting the unique process parameter control, the preparation process can produce immunoglobulin with anti-hepatitis B specificity with a conventional device of cold ethanol precipitation and pressure filtration and separation, and is suitable for scale industrial production. The products have good anti-hepatitis B activity, and are safe and reliable.
Owner:广东双林生物制药有限公司
Organ preserving liquid
PendingCN109769797APrevent subsequent damageEnsure stabilityDead animal preservationVascular endotheliumLiver transplantation
The invention provides a method for maintaining the survival and / or function of vascular endothelial cells in the in vitro preservation process and used preserving fluid. The method and the preservingfluid are suitable for storing various organs. The in vitro liver can survive to exceed 24h during the preservation by the organ preserving liquid and in the mechanical filling process; the liver microcirculation structure is complete; no obvious damage exists; the choleresis function is good; the liver synthesis and metabolism functions are good; the in vitro preservation time of the liver is greatly prolonged; the postoperative survival rate of later-period liver transplantation patients is improved.
Owner:HEFEI HUAQI BIOLOGICAL ENG CO LTD
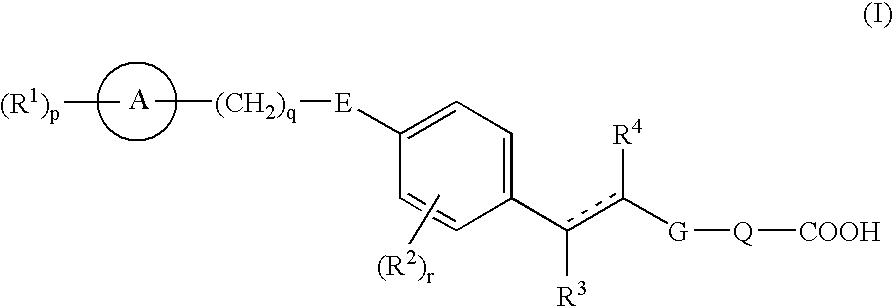
Carboxylic acid derivatives and drugs containing the same as the active ingredient
Compounds represented by formula (I), prodrugs thereof and salts thereof, and pharmaceutical compositions comprising the same as an active ingredient (wherein each symbol has the meaning as defined in the specification.).Because of having an EDG-1 agonism, the compounds represented by formula (I) are useful in preventing and / or treating peripheral arterial disease such as arteriosclerosis obliterans, thromboangiitis obliterans, Buerger's disease or diabetic neuropathy, sepsis, angiitis, nephritis, pneumonia, stroke, myocardial infarction, edematous state, atherosclerosis, varicosity such as hemorrhoid, anal fissure or fistula ani, dissecting aneurysm of the aorta, angina, DIC, pleuritis, congestive heart failure, multiple organ failure, bedsore, burn, chronic ulcerative colitis, Crohn's disease, heart transplantation, renal transplantation, dermal graft, liver transplantation, osteoporosis, pulmonary fibrosis, interstitial pneumonia, chronic hepatitis, liver cirrhosis, chronic renal failure, or glomerular sclerosis.
Owner:ONO PHARMA CO LTD
SNP in KRT8 gene exon area and determination method thereof
InactiveCN103602671ALiver cirrhosis controlLiver transplantation success rate controlMicrobiological testing/measurementDNA/RNA fragmentationPrimary biliary cirrhosisNucleotide
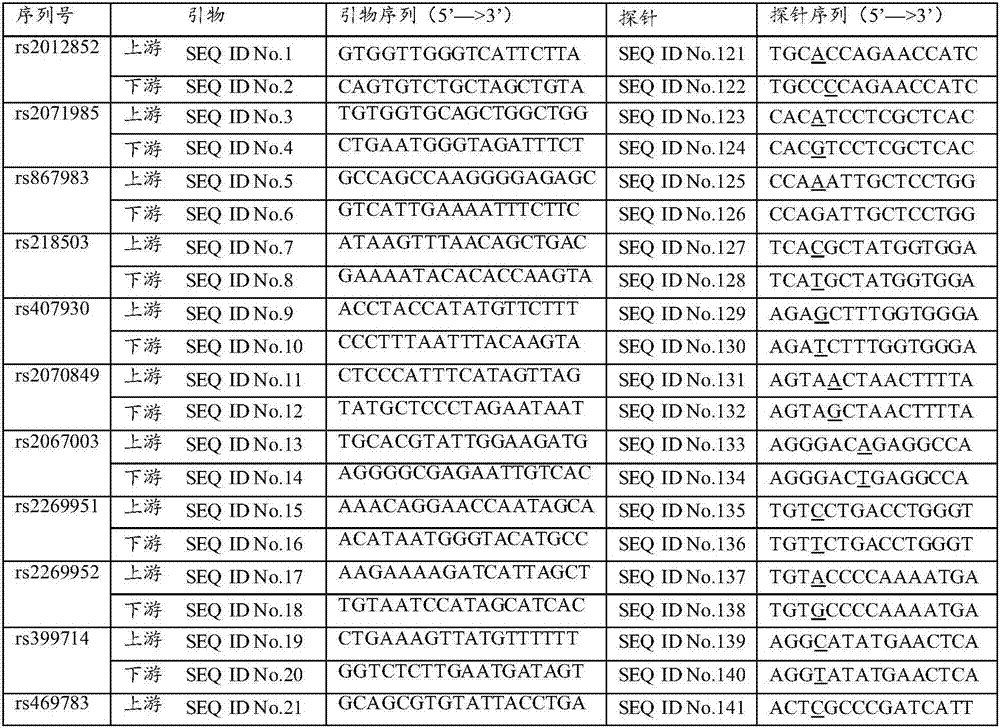
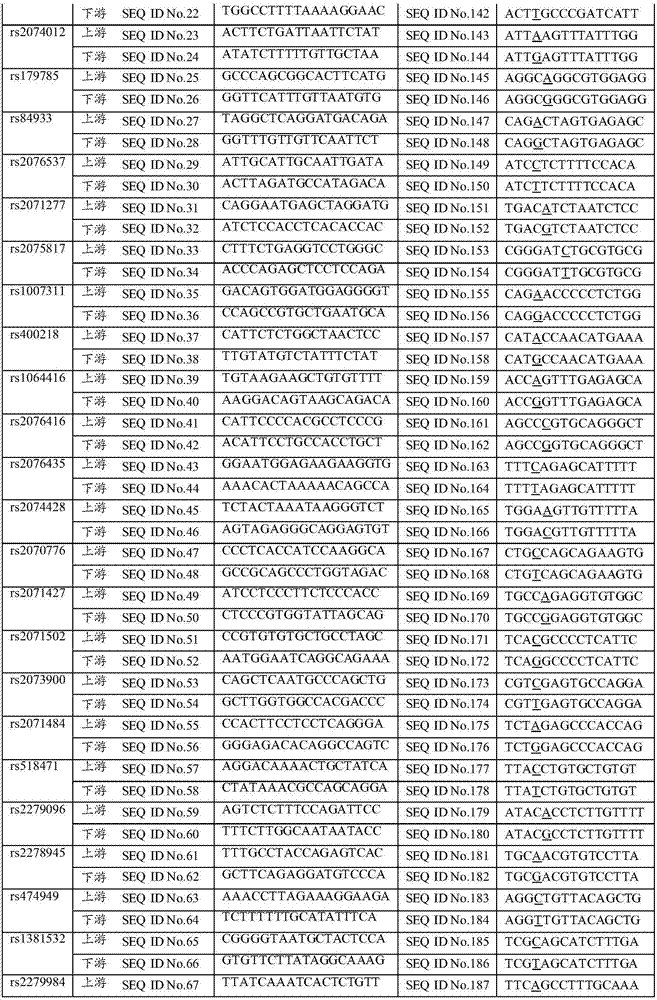
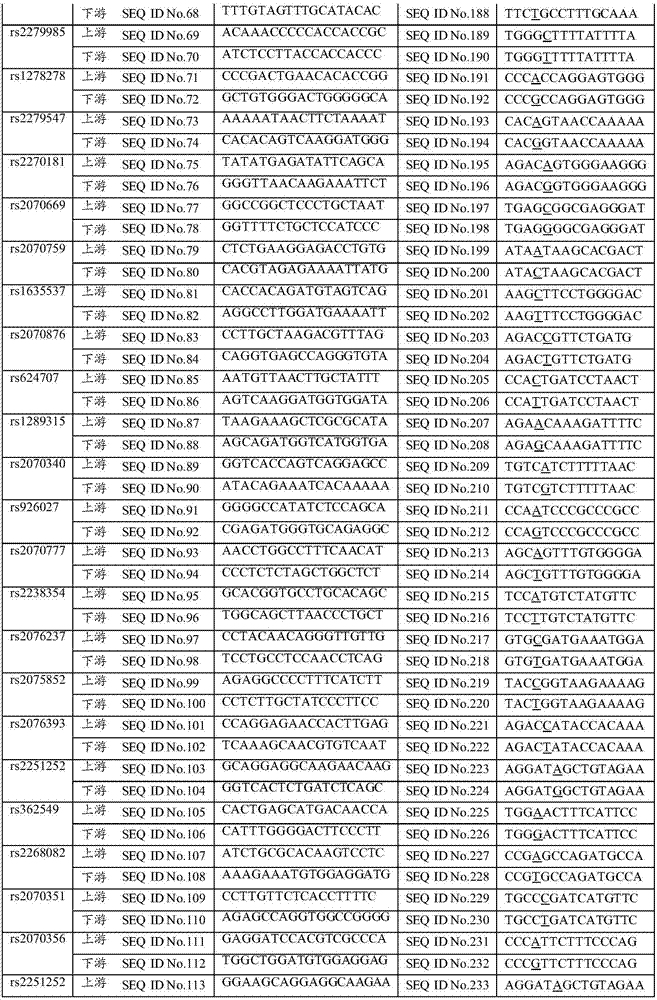
The invention discloses a SNP in KRT8 gene exon area and determination method thereof, and the SNP locus with G / T polymorphisms (GG) is at 12563 site of the eighth exon area of the KRT8 gene and has nucleotide sequences SEQ ID NO:1 and SEQ ID NO:2. The invention also provides a determination method of the SNP locus, and research and development of the polymorphisms can be applied to researches in the aspect of primary biliary cirrhosis, for example, control of primary biliary cirrhosis attack and hepatic cirrhosis degree or improvement of liver transplantation success rate.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
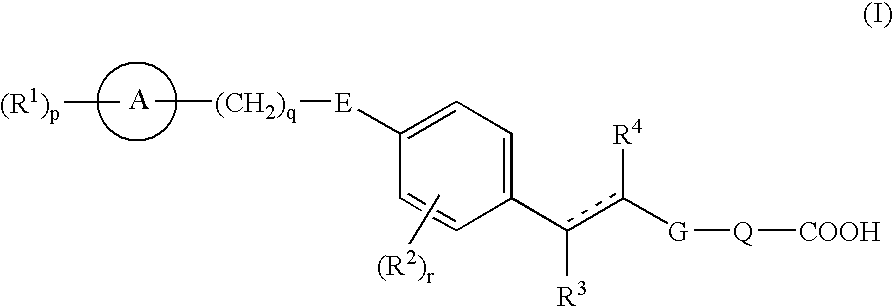
Carboxylic acid derivatives and pharmaceutical compositions comprising the same as an active ingredient
Compounds represented by formula (I), prodrugs thereof and salts thereof, and pharmaceutical compositions comprising the same as an active ingredient (wherein each symbol has the meaning as defined in the specification.). Because of having an EDG-1 agonism, the compounds represented by formula (I) are useful in preventing and / or treating peripheral arterial disease such as arteriosclerosis obliterans, thromboangiitis obliterans, Buerger's disease or diabetic neuropathy, sepsis, angiitis, nephritis, pneumonia, stroke, myocardial infarction, edematous state, atherosclerosis, varicosity such as hemorrhoid, anal fissure or fistula ani, dissecting aneurysm of the aorta, angina, DIC, pleuritis, congestive heart failure, multiple organ failure, bedsore, burn, chronic ulcerative colitis, Crohn's disease, heart transplantation, renal transplantation, dermal graft, liver transplantation, osteoporosis, pulmonary fibrosis, interstitial pneumonia, chronic hepatitis, liver cirrhosis, chronic renal failure, or glomerular sclerosis.
Owner:ONO PHARMA CO LTD
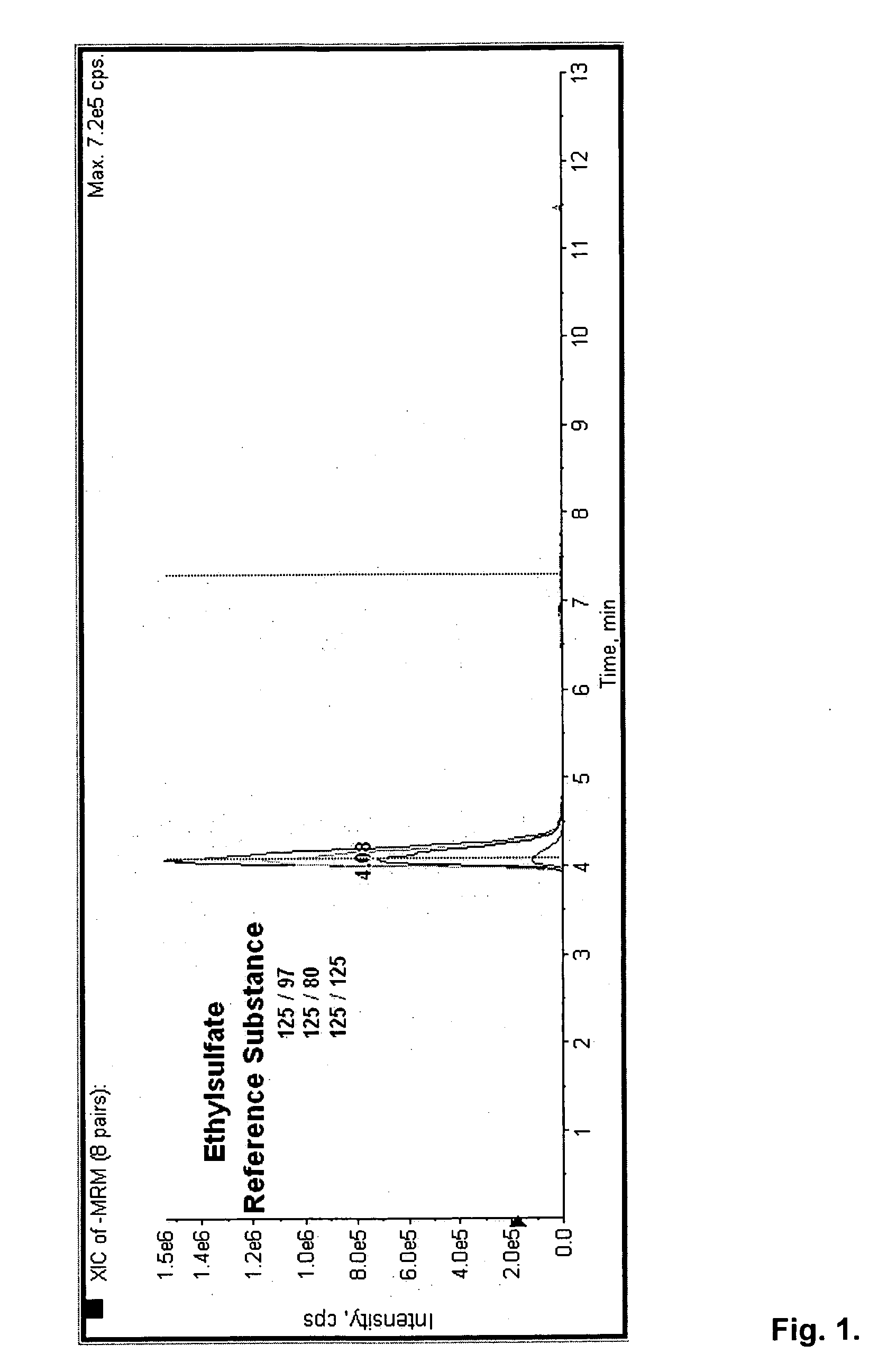
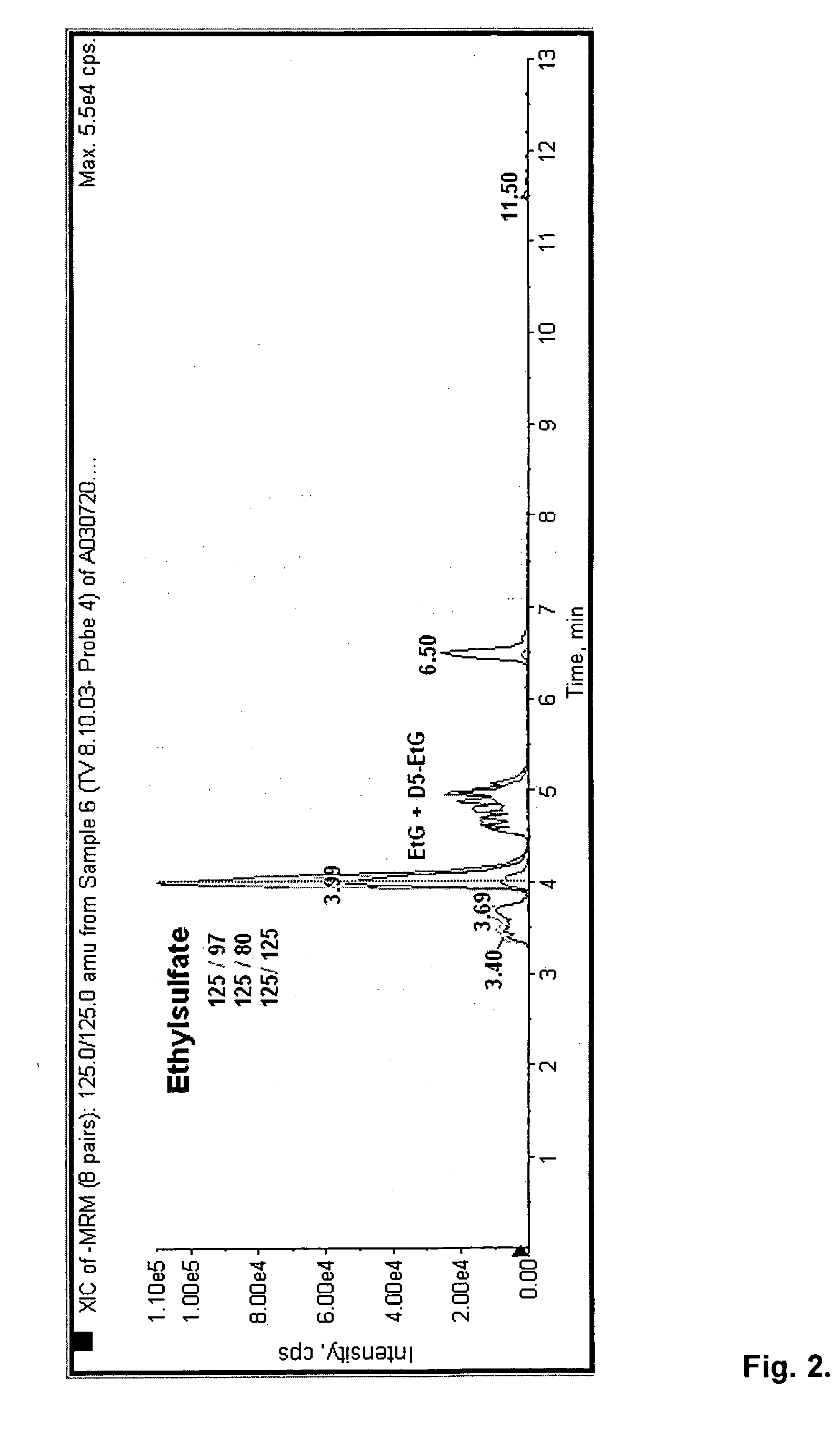
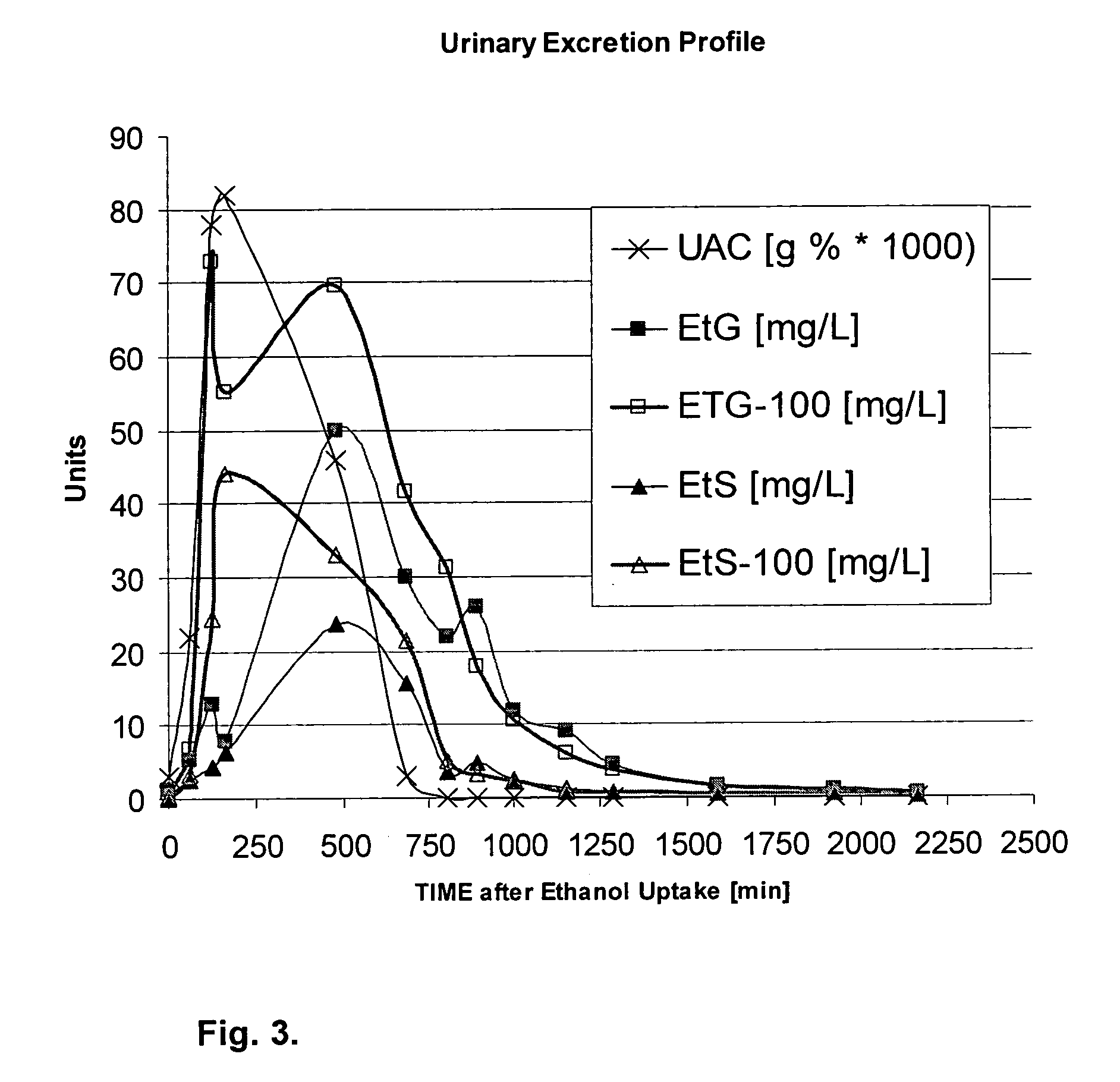
Direct ethanol metabolite ethyl sulfate as an useful diagnostic and therapeutic marker of alcohol consumption
InactiveUS20060084134A1Microbiological testing/measurementDisease diagnosisTime spectrumAlcohol intoxication
Accurate self report strategies, biological state markers, combinations of alternative biomarkers, and combinations of biomarkers and self reports capable of monitoring alcohol consumption with a high sensitivity and specificity over a broad time spectrum are disclosed. In particular, the use of ethyl sulfate (monoethylsulfate, molecular weight 126 g / mol, C2H5SO4H) to elucidate ethanol intake is described in the context of screening monitoring in various settings, e.g. a) after liver transplantation b) methadone maintenance patients with hepatitis C and comorbid excessive alcohol use c) underage drinking d) rehabilitation programs for alcoholics motivational feedback to improve knowledge on drinking patterns differentiating moderate / social drinking from problematic / harmful drinking differential diagnosis (e.g. elevated transaminases) evaluating treatment programs and drug trials elucidating the role of neuropsychological impairment following alcoholisation (i.e. hangover state) which plays a major role in accidents, disclosing recent drinking in social drinkers in risky situations (driving, workplaces, pregnancy (fetal alcohol syndrome (FAS)), and general monitoring.
Owner:WURST FRIEDRICH MARTIN
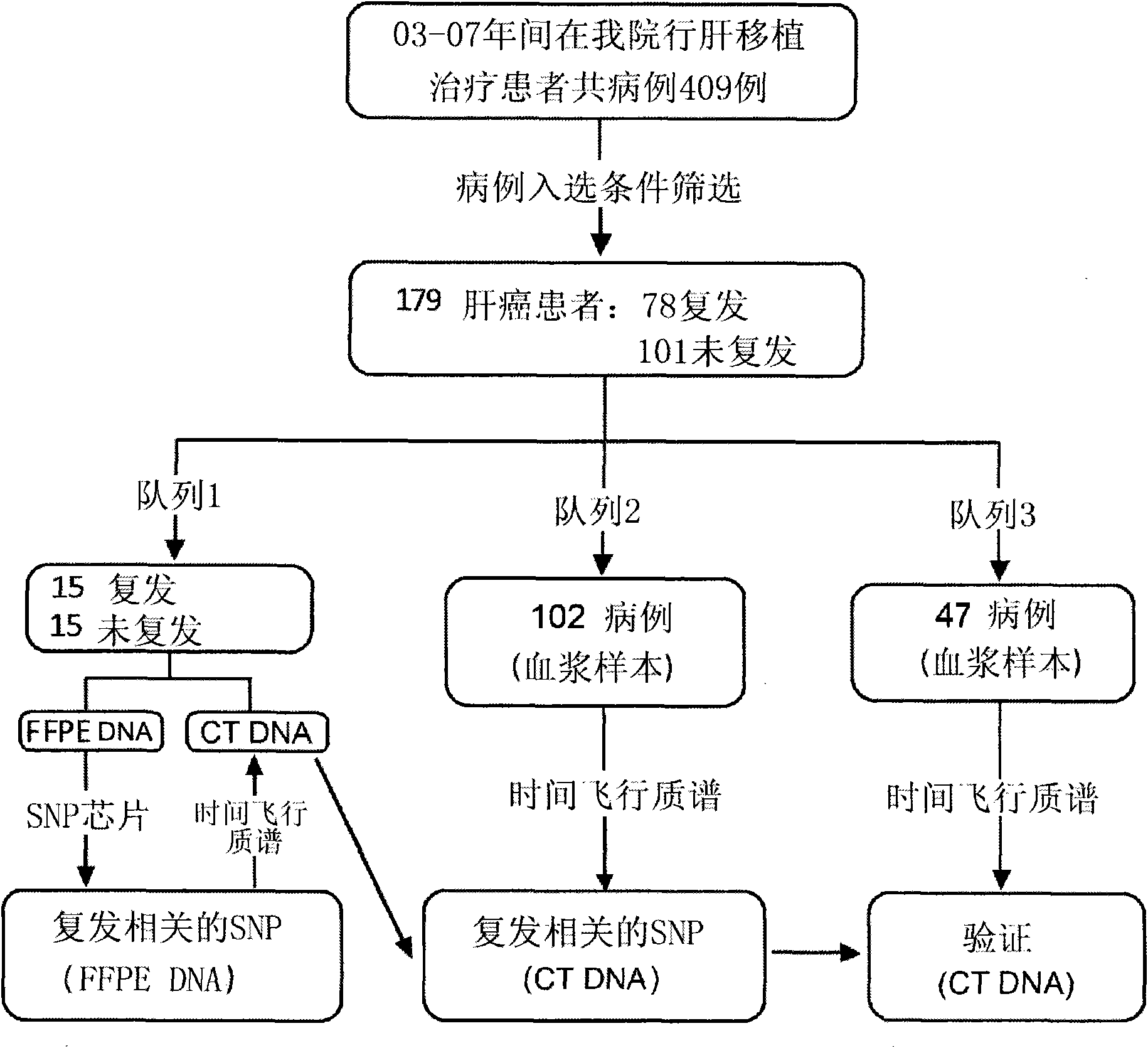
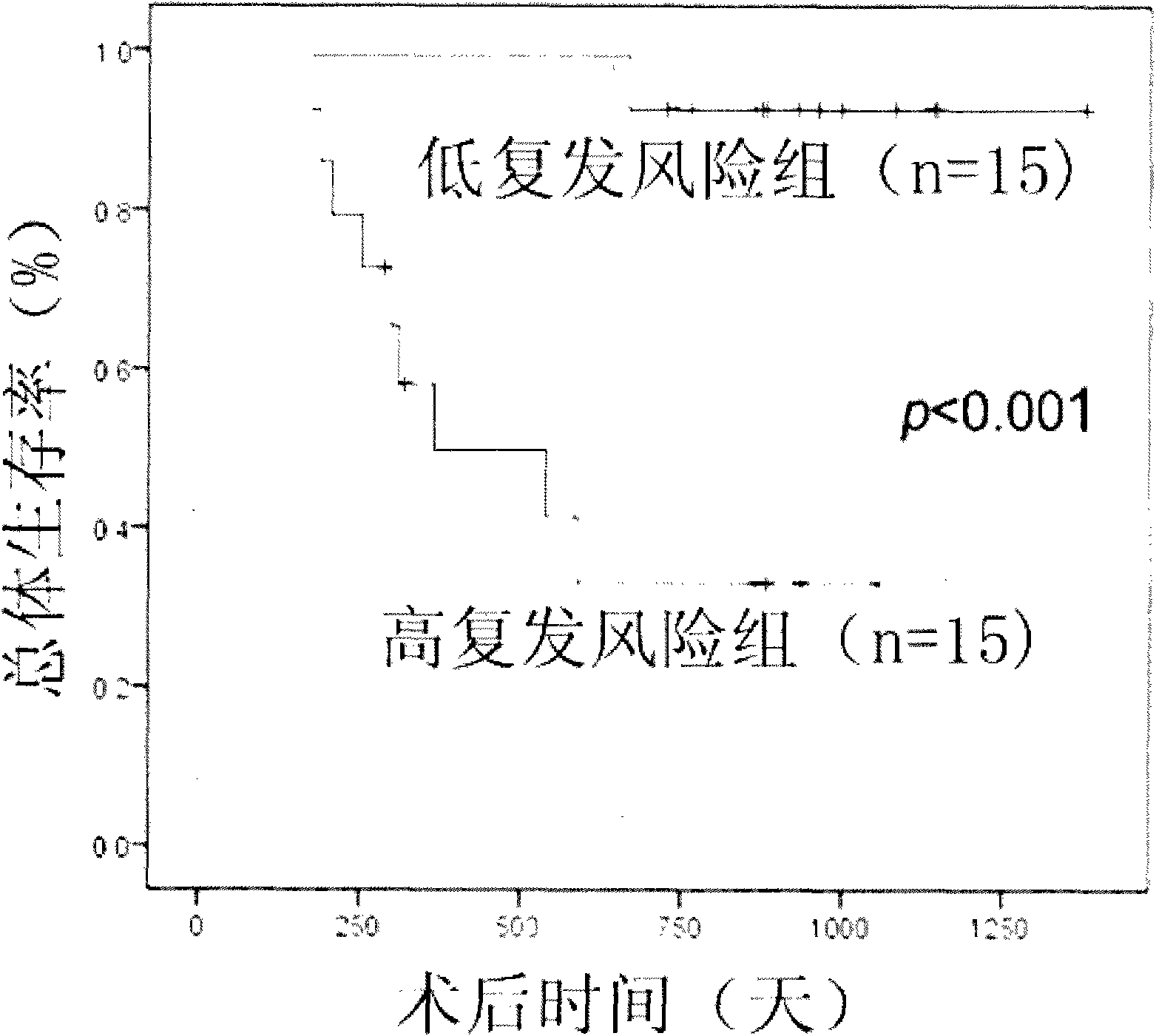
Recurrence predicting reagent kit for liver cancer liver transplantation postoperation
InactiveCN101880715AEffective predictionTraumaMicrobiological testing/measurementCvd riskGastroenterology
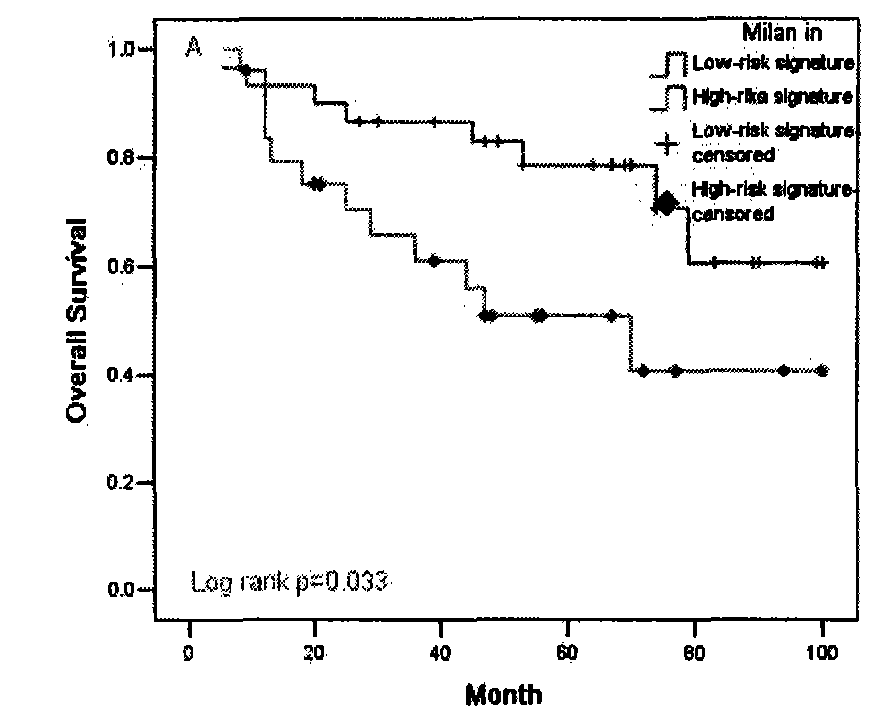
The invention provides a recurrence predicting reagent kit for liver cancer liver transplantation postoperation, which is characterized in that the reagent kit uses two SNPrs rs894151 and rs12438080 in a plasma circulation DNA as detection sites, uses upstream sequences and downstream sequences of the rs894151 and the rs12438080 as amplimers, and uses fluorescent probes as genotyping probes. The invention can evaluate the recurrence risk of the liver transplantation operation carried out by liver cancer patients before the operation, and in addition, the invention uses peripheral blood as detection samples, and has the characteristics of high speed, accuracy, convenience, simplicity, easy popularization and application and the like. The invention can be used for sieving the clinical liver cancer liver transplantation patients, can guide the auxiliary treatment after the liver cancer liver transplantation operation, and can reduce the postoperation recurrence rate of high-risk patients, so the prognosis of the liver cancer liver transplantation is improved, and the limited donor livers can be effectively utilized. The reagent kit has important clinical significance to the prognosis judgment on patients exceeding the Milan standard.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Tacrolimus metabotropic diagnostic marker and application thereof
InactiveCN106399528AImprove accuracyMicrobiological testing/measurementNucleotideDrug administration
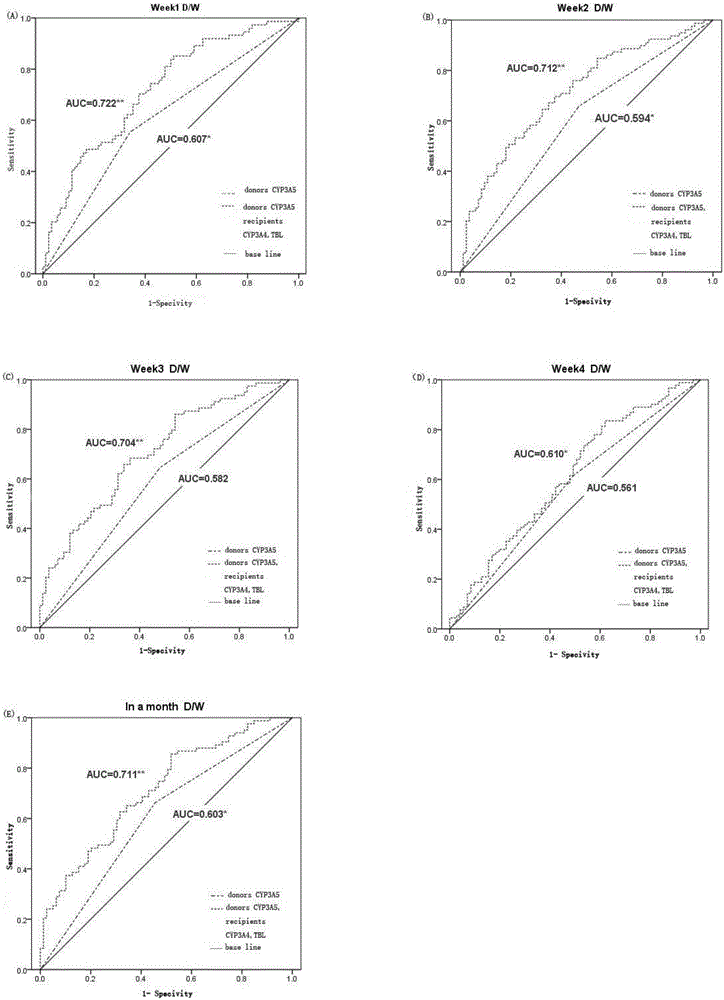
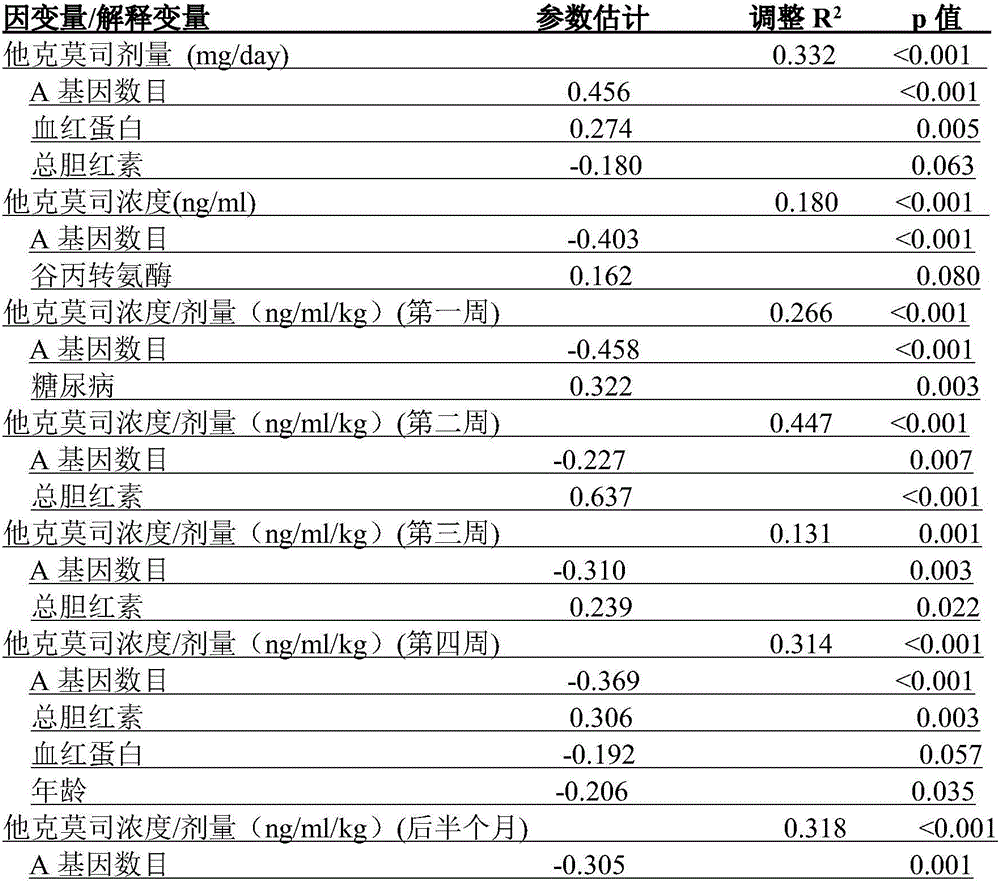
The invention relates to a metabotropic diagnosis kit used for detecting tacrolimus. The diagnosis kit comprises a reagent for detecting gene CYP3A4 rs2242480 SNP loci, a reagent for detecting gene CYP3A5 rs776746 SNP loci, and a reagent for detecting the total bilirubin content. The current clinical dosage of tacrolimus is determined according to the weight of patients, and different dosages of tacrolimus are needed for treatment so that different genotype patients can have the same target plasma concentration. By testing single nucleotide polymorphism (SNP) and clinical biochemical indicators of 170 pairs of liver transplantation donor CYP3A5 rs776746 loci and liver transplantation receptor CYP3A4 rs2242480 loci, the relation with tacrolimus metabolism is discussed, and the basis is provided for individualized drug administration of clinical liver transplantation patients.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
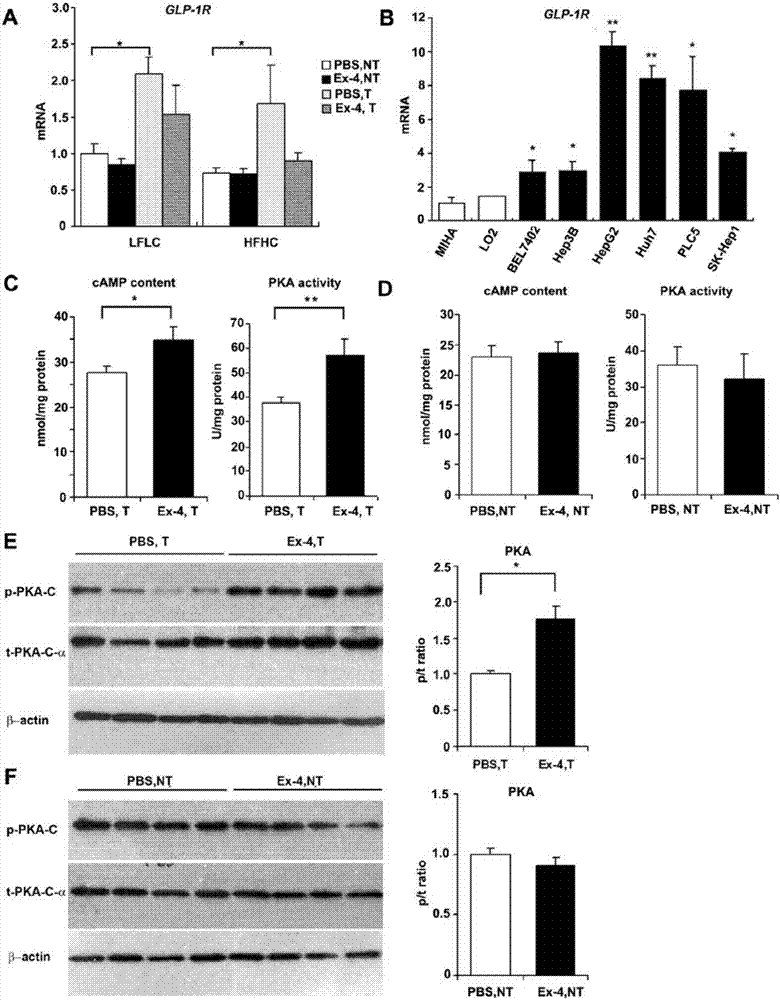
Drug for treating hepatocellular carcinoma and application of glucagon-like peptide-1 receptor stimulant and/or dipeptidyl peptidase 4 inhibitor
InactiveCN107050459APrevent proliferationPromote apoptosisOrganic active ingredientsPeptide/protein ingredientsDifferential effectsDipeptidyl peptidase
The invention provides a drug for treating hepatocellular carcinoma. The drug comprises a glucagon-like peptide-1 receptor stimulant and / or a dipeptidyl peptidase 4 inhibitor. The invention further provides application of the glucagon-like peptide-1 receptor stimulant and / or dipeptidyl peptidase 4 inhibitor to preparation of a drug for preventing or treating hepatocellular carcinoma, to preparation of a drug for liver function recovery after liver transplantation, and to preparation of a drug for preventing liver cancer relapse after liver transplantation. The glucagon-like peptide-1 receptor stimulant and / or dipeptidyl peptidase 4 inhibitor generates a differential effect in a tissue specificity way, so as to inhibit cancer cell proliferation and promote cancer cell apoptosis, meanwhile, liver cells are protected against apoptosis, so that obesity-dependent liver cancer or non-dependent liver cancer can be treated or weakened.
Owner:SUN YAT SEN UNIV
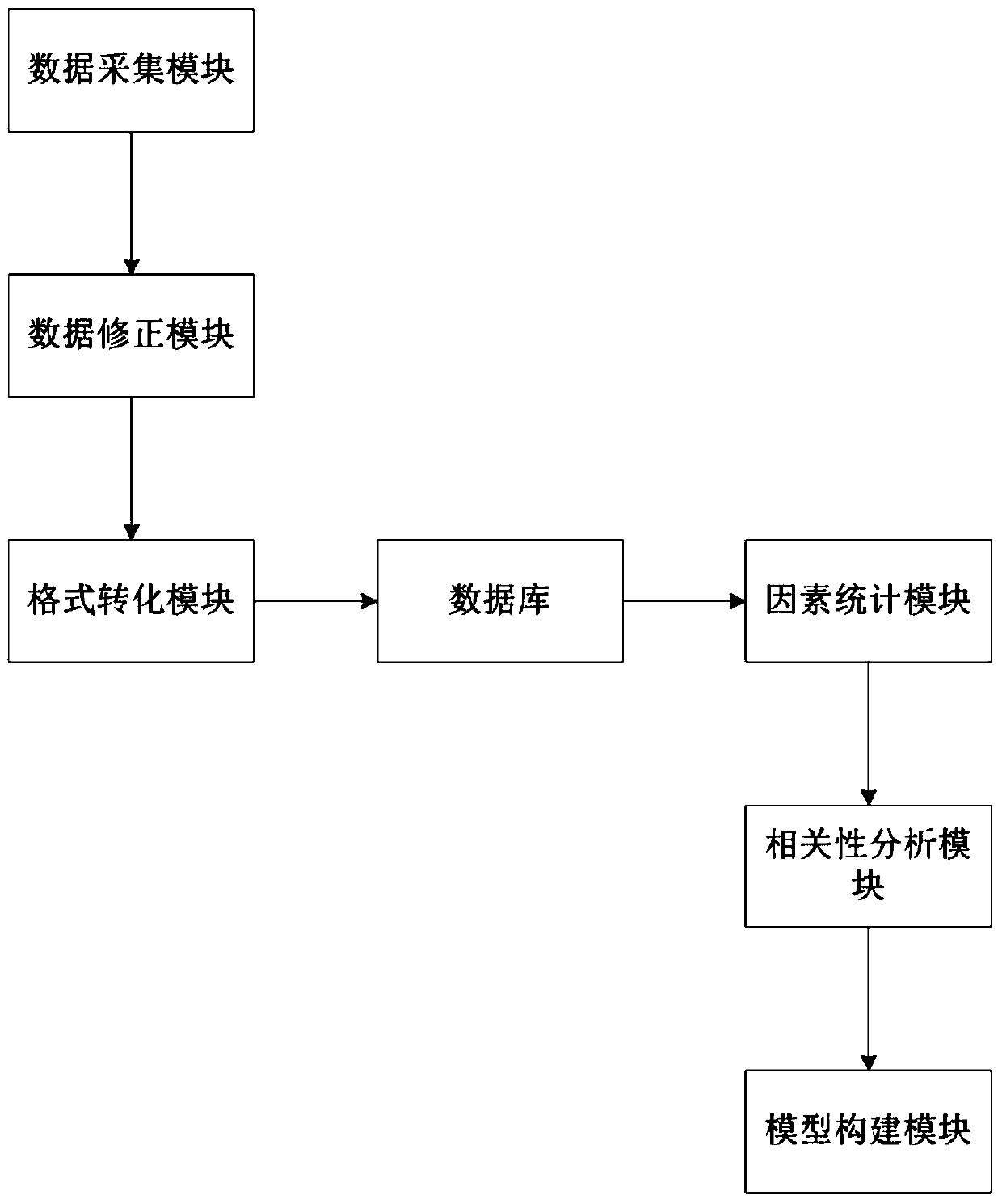
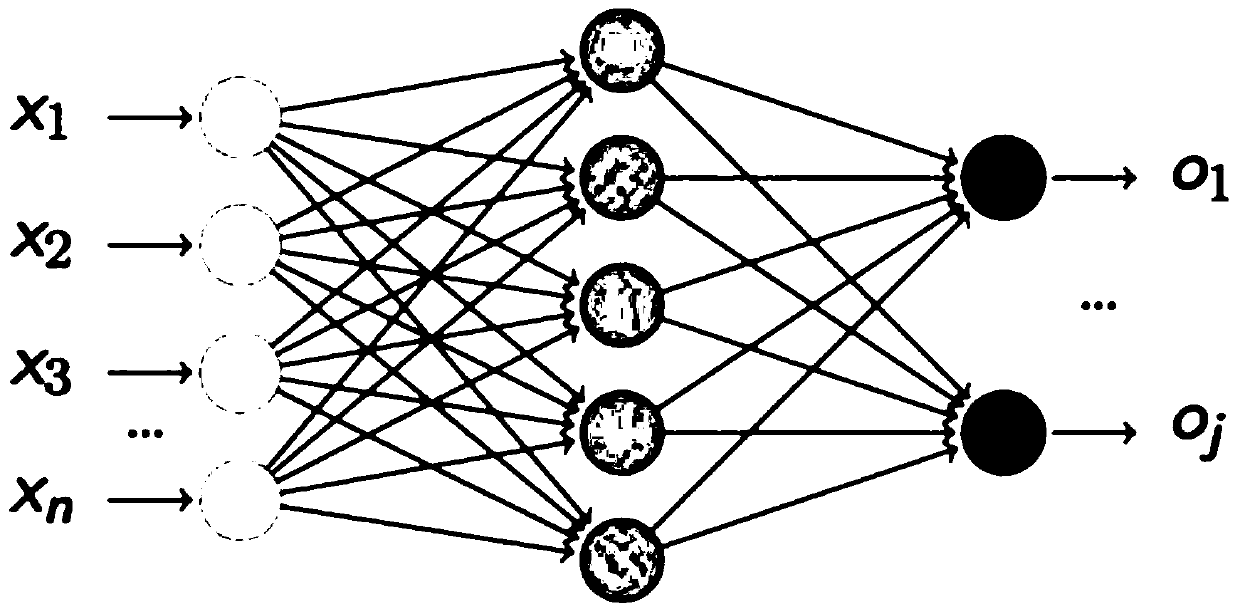
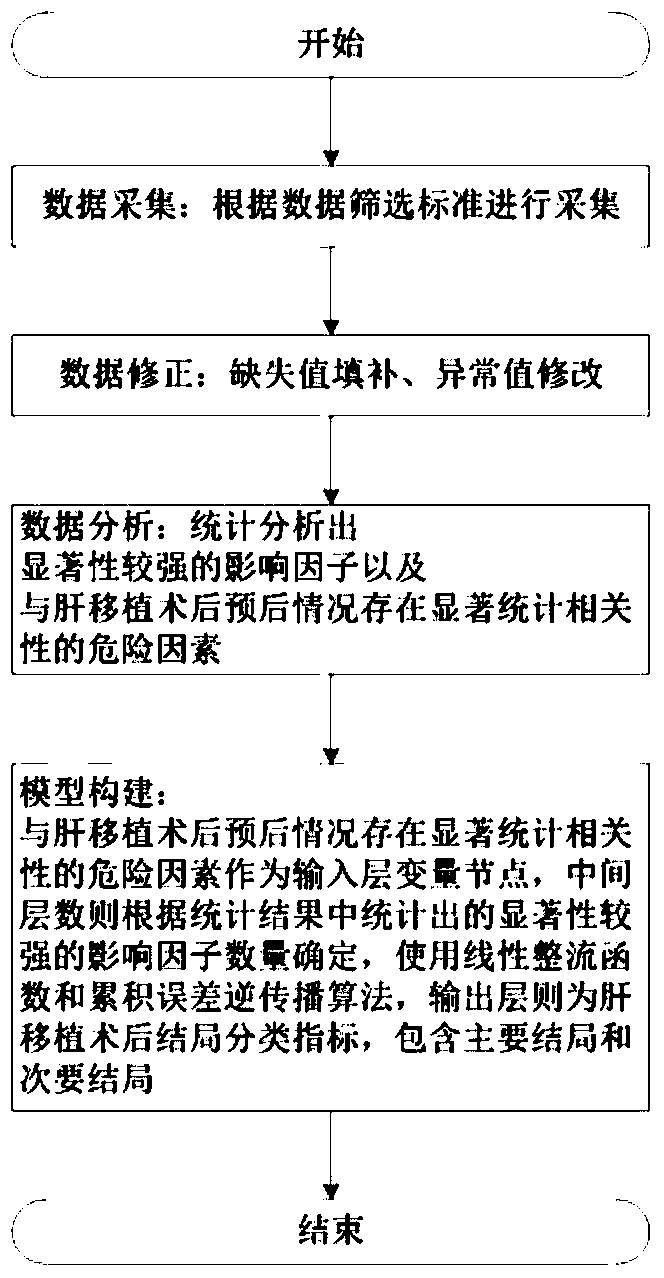
Prognosis prediction system after liver transplantation
InactiveCN110634571ATake advantage ofImprove accuracyMedical simulationHealth-index calculationStatistical correlationData acquisition
The invention relates to an artificial intelligence technology and the intelligent medical treatment technology field, aims at solving a problem of an inaccurate research result caused by research onthe premise of assumption of one or more influence factors in the prior art, and provides a prognosis prediction system after liver transplantation. The system comprises a data acquisition module foracquiring clinical data, a database for storing the clinical data, a factor statistics module and a correlation analysis module, wherein the clinical data includes basic information of a patient, clinical indexes, intraoperative processing measures and prognosis conditions after the liver transplantation; the factor statistics module is used for carrying out statistical testing on the clinical data and obtaining an influence factor with relatively strong significance; and the correlation analysis module is used for analyzing and detecting correlation between the influence factor and the prognosis conditions after the liver transplantation so as to obtain a risk factor having significant statistical correlation with the prognosis conditions after the liver transplantation. The method is applied to prediction of the prognosis conditions after the liver transplantation of the patient.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
Application of hsa_circRNA_102032 to liver cancer diagnosis, treatment and prognosis
ActiveCN107151702AMicrobiological testing/measurementDNA/RNA fragmentationOncologyLiver transplantation
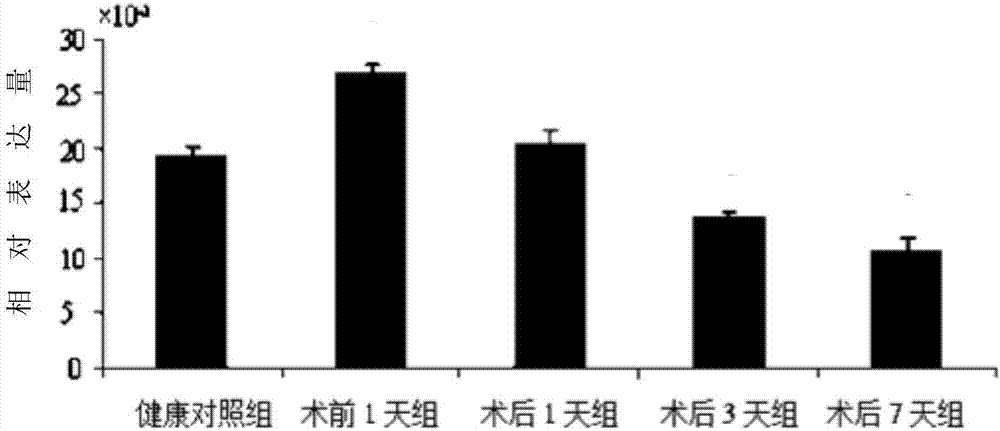
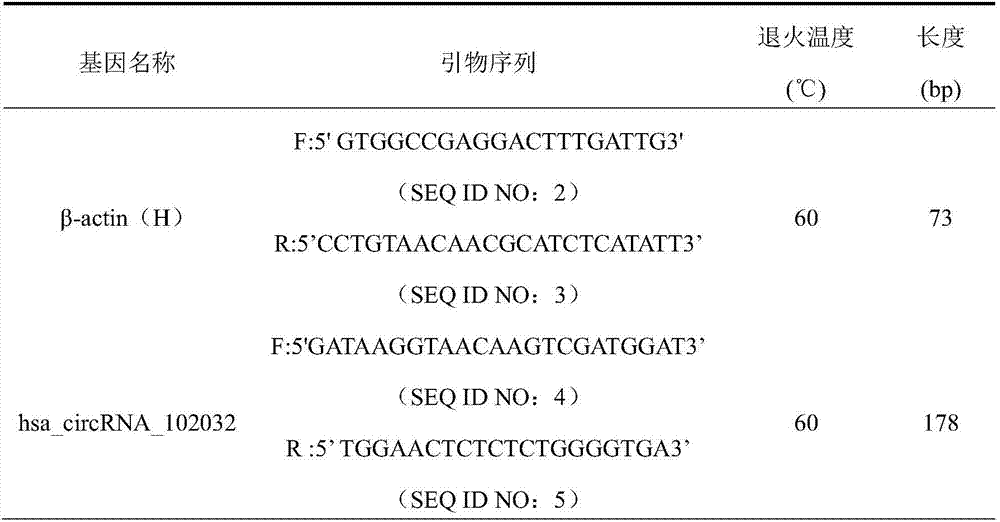
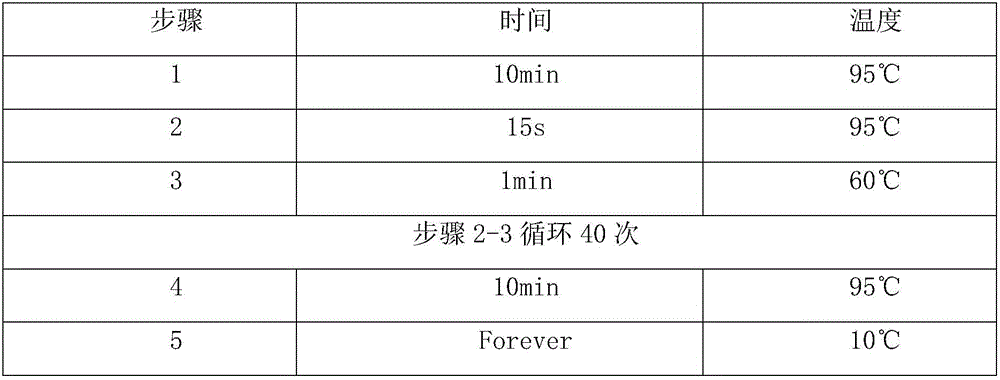
The invention discloses CircRNA with the sequence shown as SEQ ID NO:1, and further discloses an RT-PCR primer for detecting the CircRNA. The CircRNA has the obvious expression differences before and after the liver cancer operation; the expression quantity at one day before the lung cancer operation is obviously raised through being compared with that in a healthy control group; the obvious reduction trend exists on the 1st day, the 3rd day and the 7th day after the liver transplantation operation. The existing clinic auxiliary diagnosis technology used for the liver cancer is not complete, so that the CircRNA has the potential used as a biomarker relevant to the liver cancer.
Owner:SHENZHEN DONGDA BIOTECH
MiRNA-122 detection kit based on digital PCR platform and application thereof
InactiveCN106191293ASimplified sample loading workloadReduce mistakesMicrobiological testing/measurementSequence designWorkload
The invention discloses an miRNA-122 detection kit based on a digital PCR (Polymerase Chain Reaction) platform and application of the miRNA-122 to preparation of a kit for evaluating liver transplantation injury. Ingredients of a reverse transcription buffer solution and a PCR buffer solution of the kit provided by the invention are optimized, so that the long-period stable storage is facilitated; meanwhile, the sample loading workload can be simplified to the minimum, so that mistakes or errors possibly occurring during the sample loading can be reduced; and the obtaining of the stable and reliable detection result is facilitated. Compared with conventional common-use miRNA reverse transcription stem-loop primers and detection primers, the kit provided by the invention has the advantages that the basic group modification and optimization is performed on the reverse transcription stem-loop primer and detection primer sequence design; when the kit is used for digital PCR platform detection, the result is better and more stable; the miRNA-122 is used as an independent molecular marker; and through the clinic verification test, the application of the miRNA-122 detection kit as a molecular marker to the preparation of the kit for evaluating liver transplantation injury is realized through the development of the miRNA-122 detection kit.
Owner:成都仕康美生物科技有限公司
MicroRNA (ribonucleic acid) specific expression profile and application thereof
InactiveCN102586412AReduce healingReduce medical costsMicrobiological testing/measurementTumor recurrenceIntervention treatment
The invention belongs to the fields of biological and medical examination, and relates to microRNA (ribonucleic acid) specific expression profile and clinical application thereof. Tumor recurrence after liver transplantation for liver cancer can be judged by five miRNAs or comprehensive indexes of the microRNA specific expression profile, accuracy rate reaches 77%, further, tumor recurrence and prognosis conditions of an integral case group can be judged, tumor recurrence and prognosis conditions can also be judged for patients after Milan delamination of the patients by the aid of the 5 miRNAs or the comprehensive indexes, accordingly, the microRNA specific expression profile can be used for screening patients with high tumor recurrence risks after clinical liver transplantation for liver cancer, assists in judging prognosis and realizing early diagnosis of recurrence, and provides a basis for early intervention treatment and implementation of individualized treatment.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Primer and method for predicating acute rejection occurrence risk in recipient after liver transplantation
InactiveCN103014162AStrong specificityEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationGenotypeHepatitis B virus
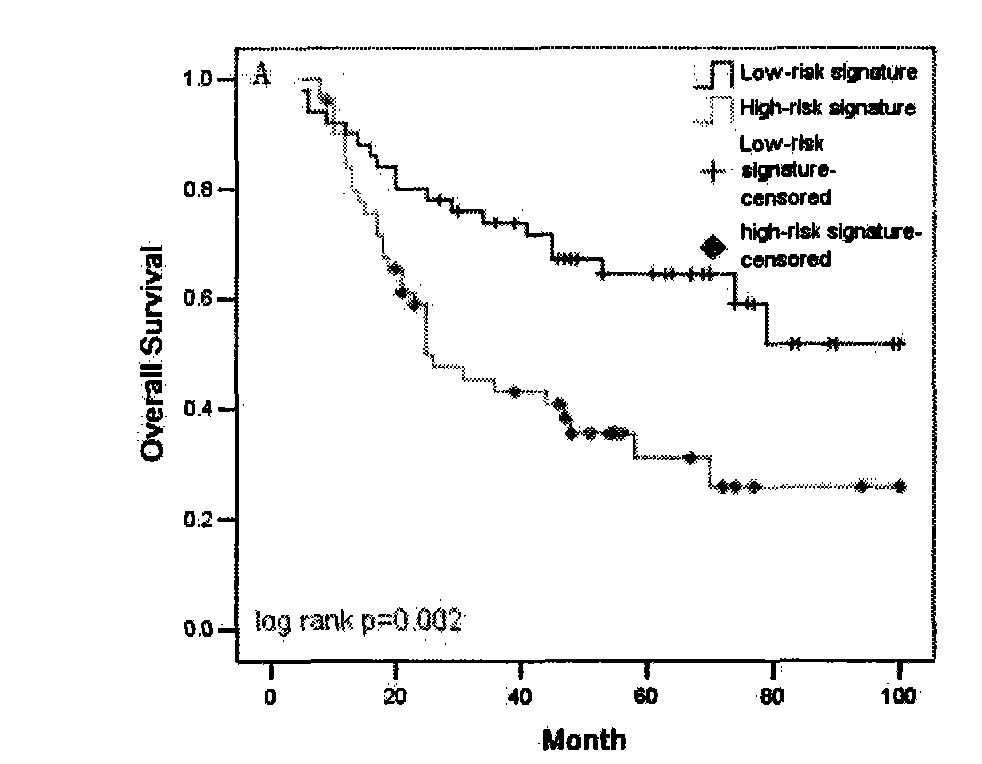
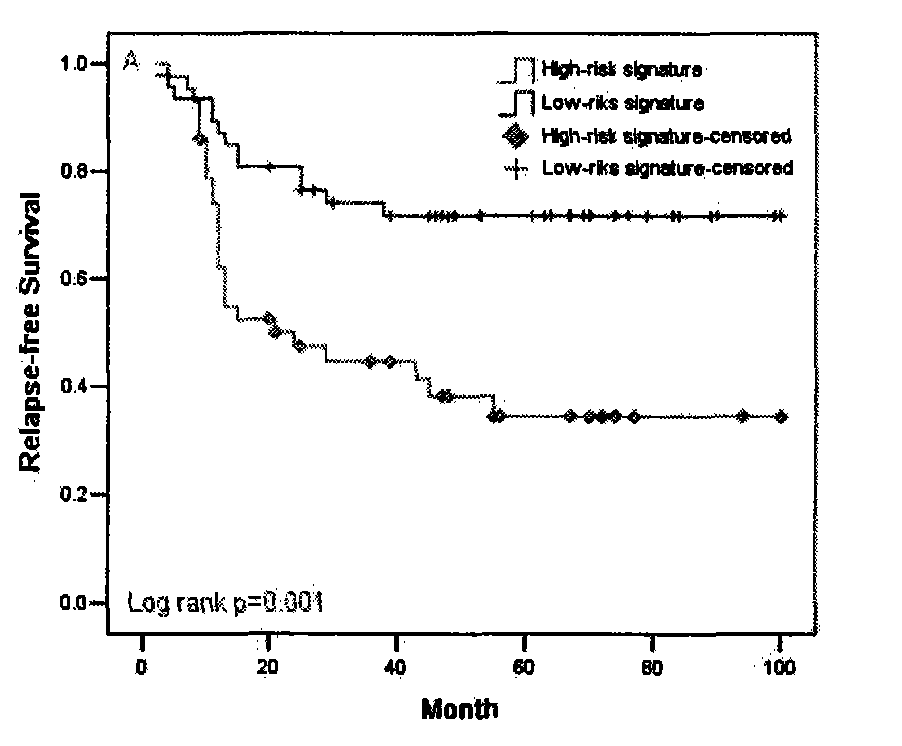
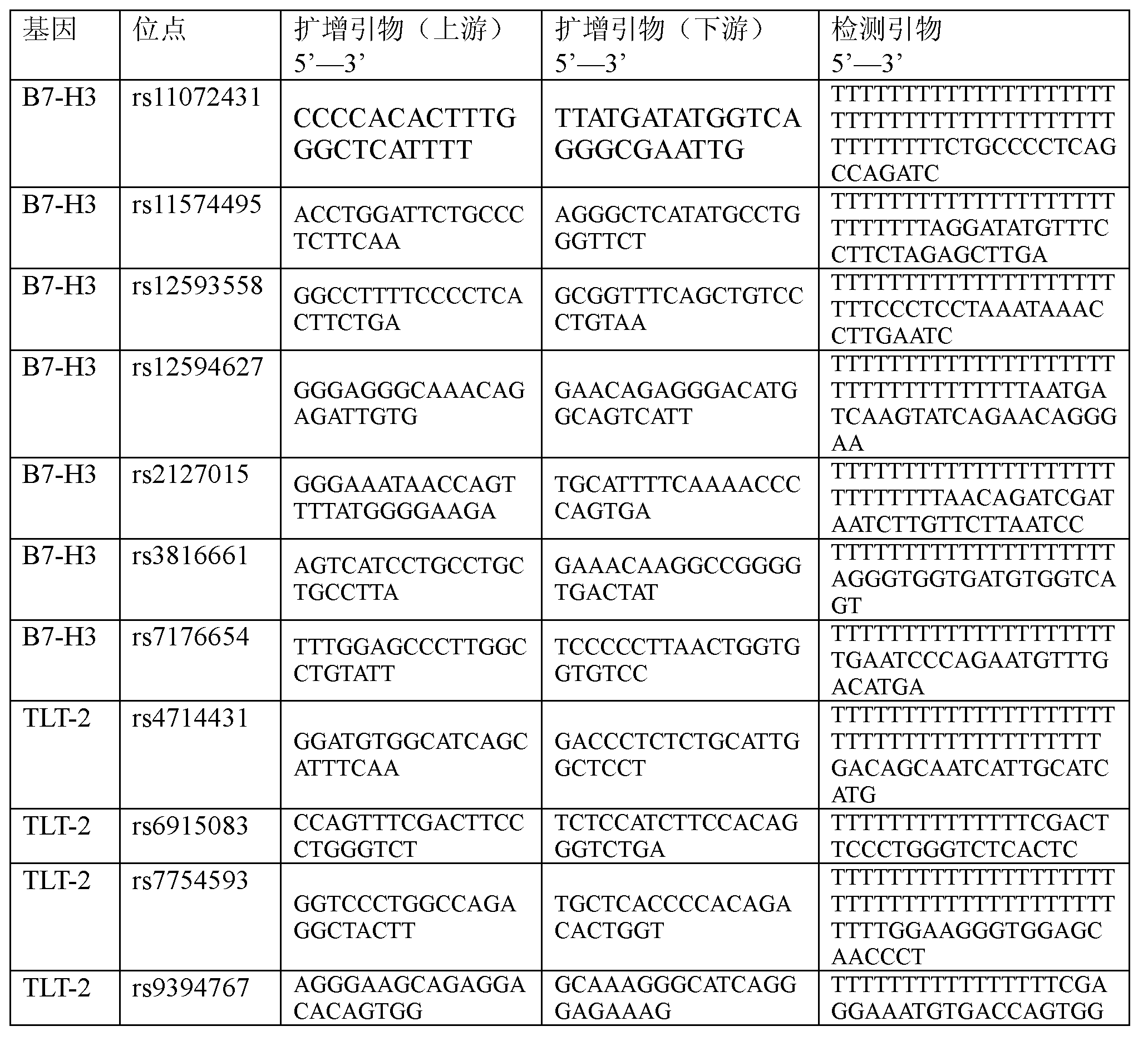
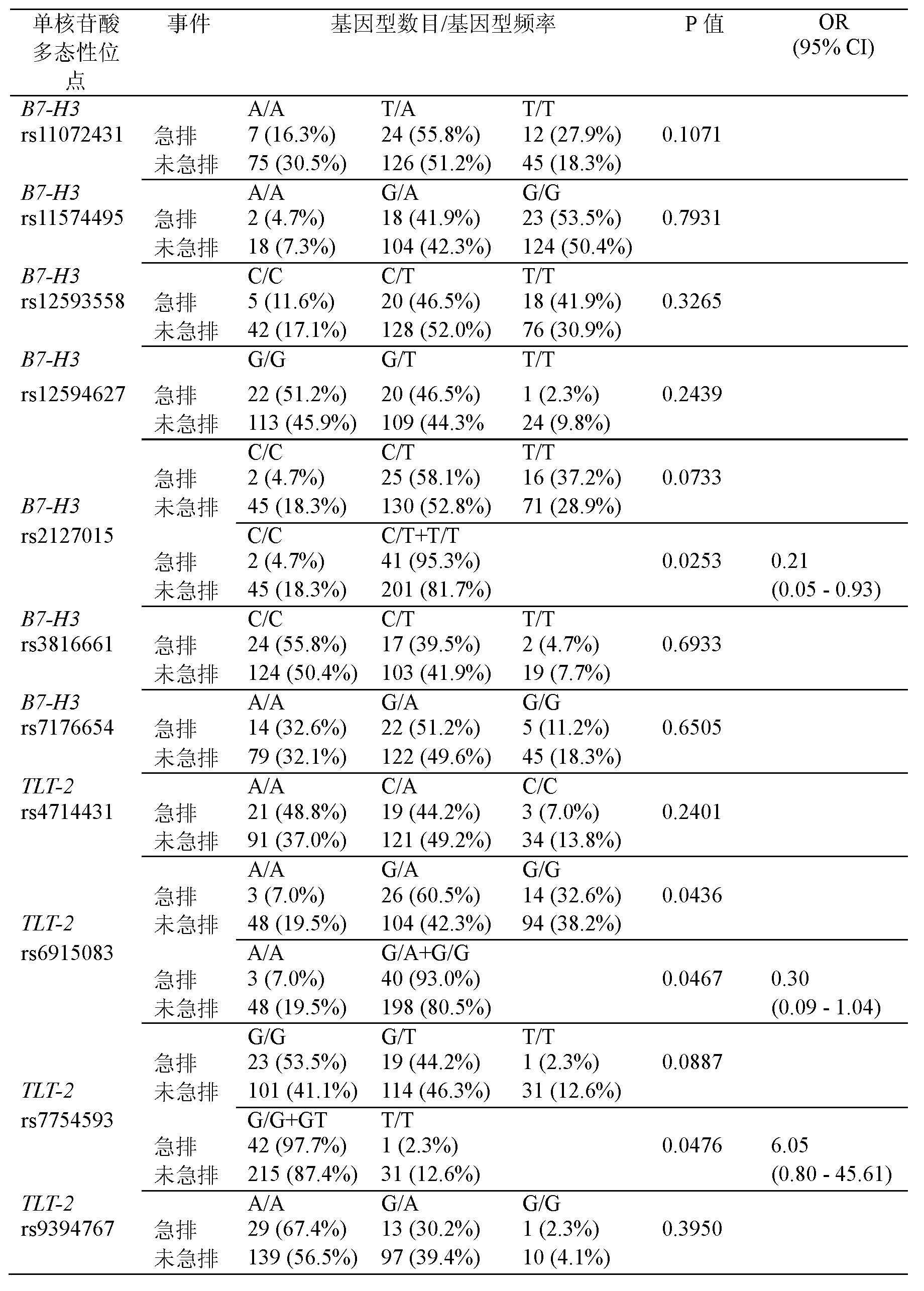
The invention discloses a primer and a method for predicating acute rejection occurrence risk in a recipient after liver transplantation. The primer comprises a pair of PCR (Polymerase Chain Reaction) amplification primers and an extension primer for sequencing amplified segments, which are respectively shown in SEQ ID NO.13-15. The method comprises the steps of: (1) detecting genotype of a single base mutation lotus rs2127015 of B7-H3 genes of the recipient; (2) detecting whether the recipient is infected by HBV (Hepatitis B Virus); and (3) calculating acute rejection occurrence risk index A of the recipient, wherein A=-1.122+1.493*B+0.817*C. The method of the invention is simple to operate, the sensitivity in predicating the acute rejection occurrence in the recipient reaches 69.8%, and the specificity is 54.7%.
Owner:THE FIRST AFFILIATED HOSPITAL ZHEJIANG UNIV COLLEGE OF MEDICINE
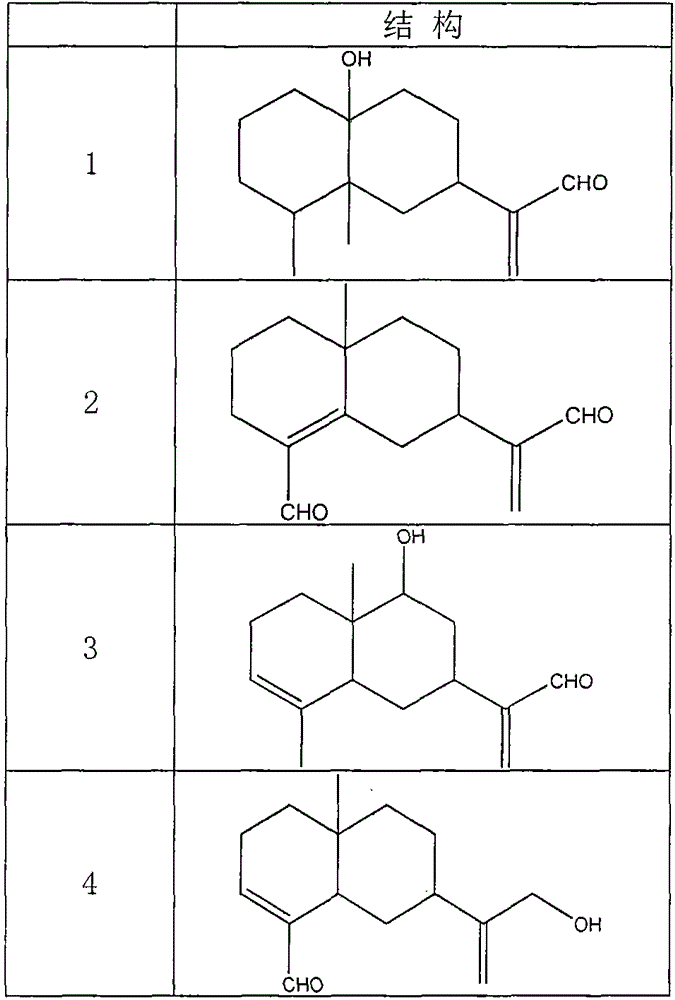
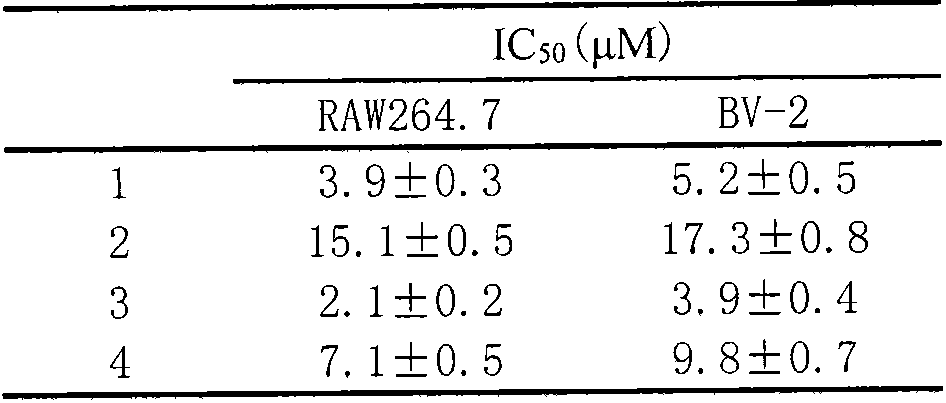
Use of sesquiterpene derivatives in aquilaria wood and pharmaceutical compositions thereof
The invention discloses application of sesquiterpene derivatives extracted and separated from aquilaria wood and pharmaceutical compositions thereof in prevention or treatment of inflammatory diseases, autoimmune diseases and organ transplant rejection, especially in prevention or treatment of pneumonia, bronchitis, hepatitis, enteritis, systemic informatory response, rheumatoid arthritis, systemic lupus erythematosus, renal transplantation rejection, liver transplantation rejection and hematopoietic stem cell transplantation rejection.
Owner:BEIJING UNIV OF CHINESE MEDICINE
Application and detection kit of SNP sites
ActiveCN107058562ASimple yet effective aidsHigh precisionMicrobiological testing/measurementOrgan transplantationCvd risk
The invention relates to the technical field of biology, in particular to application and a detection kit of SNP sites. The invention provides the application of the 60 SNP sites to assessment on post-liver transplantation graft injury and rejection risk; through detection on the specific 60 SNP sites of total receptor free DNA after transplantation and combination with a specific calculating method, the content of donor free DNA in the receptor free DNA, namely GcfDNA donor and receptor ratio is finally obtained; the degree of graft rejection is quickly and accurately judged according to the GcfDNA donor and receptor ratio without any intrusive detection, and a simple and effective auxiliary means is provided for clinical detection on the post-organ transplantation graft injury and the rejection risk.
Owner:成都仕康美生物科技有限公司
Expandable stent with variable-diameter sacculus for children
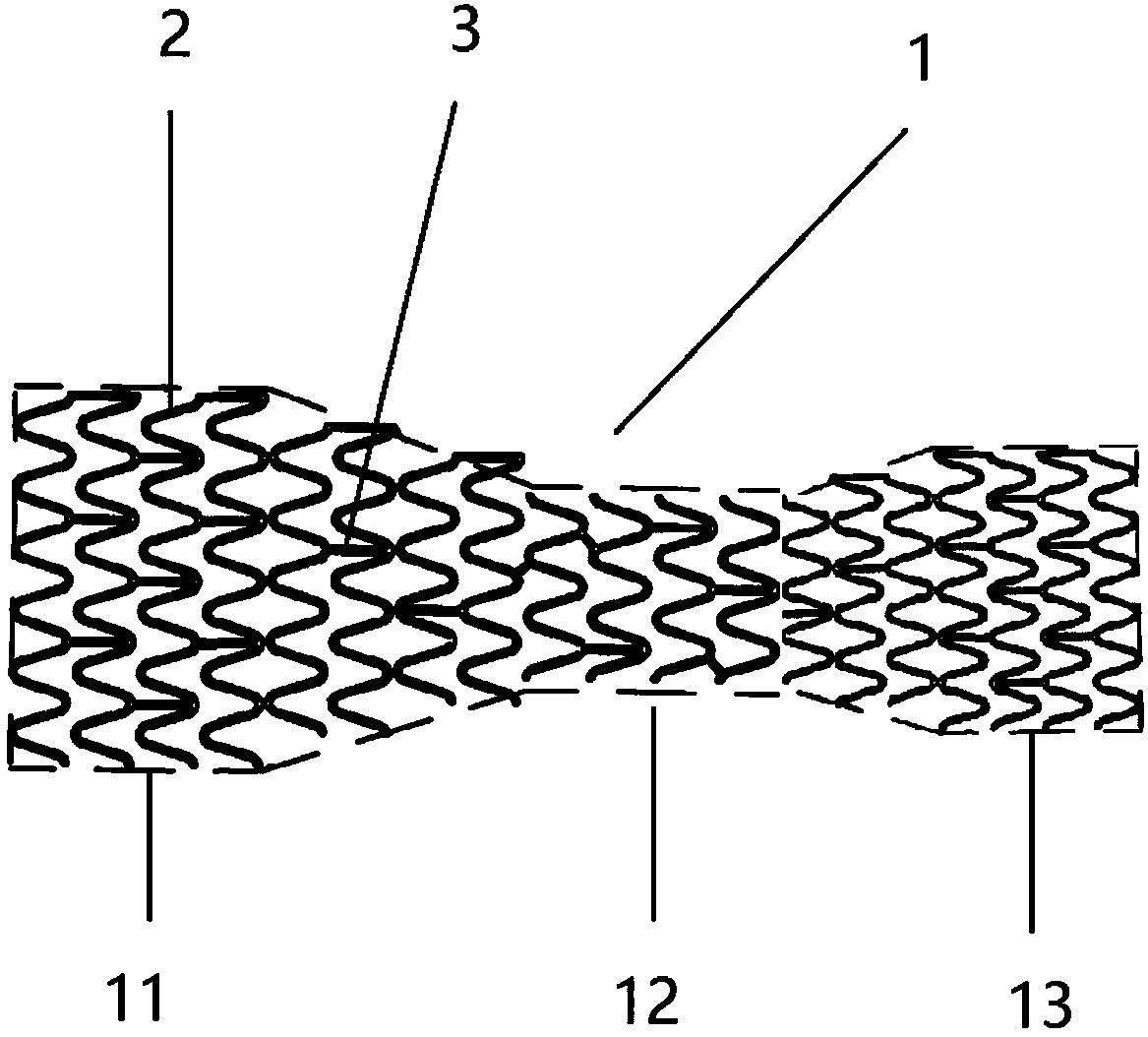
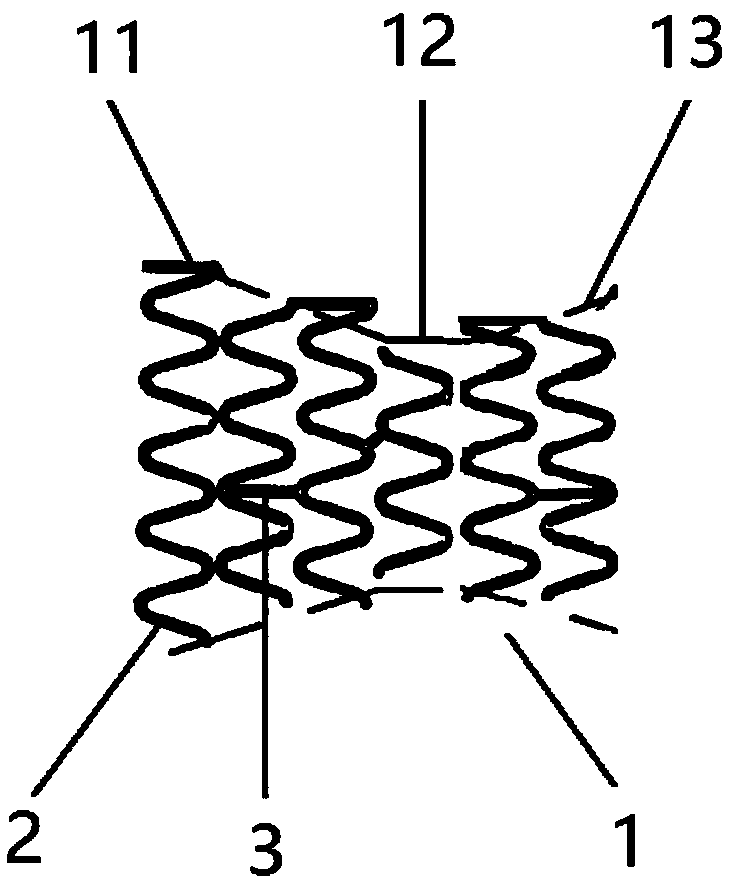
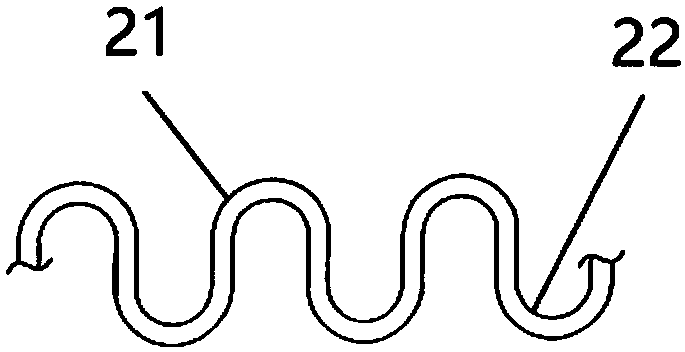
PendingCN109966021AGood compatibilityImprove distal malappositionStentsBlood vesselsPortal veinPercent Diameter Stenosis
The invention discloses a stent used for portal vein stenosis after liver transplantation in children, and belongs to the field of medical devices. A stent main body consists of a plurality of annularsupports distributed along an axial direction, and two adjacent annular supports are connected by at least one connector. The stent main body is divided into three parts: a proximal end, a middle part and a distal end, the pipe diameter of the middle part is the smallest, and the pipe diameter of the distal end is the largest. According to the present invention, the stent has the advantages thatthe stenosis of diseased vessels which is caused by pipe diameter mismatch and mainly concentrates on diameter changes is treated, the adhesion property of the vessel distal end and the expansion property of the vessel proximal end can be improved, and the physiological adaptability of the stent can be improved; the combined area and friction between the stent and a portal vein stent can be increased, the stability of the stent can be increased, and the incidence of stent displacement can be reduced; and the change of the portal vein intracavitary hemodynamics can be decreased, the risk of restenosis after stent implantation can be reduced, and the occurrence of adverse events can be decreased.
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
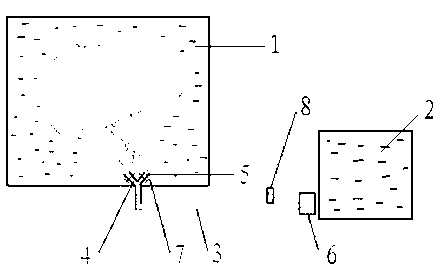
In vitro continuous filling device used for autologous liver transplantation
ActiveCN102986649ACorrect bad factorsImprove the function of remnant liverDead animal preservationMedicinePortal vein
The invention relates to an in vitro continuous filling device used for autologous liver transplantation. The in vitro continuous filling device comprises a liver removing platform (1) used for storing and removing liver, a liquid storage tank (2) and a Y-shaped pipe (3), wherein one end of the Y-shaped pipe (3) comprises a hepatic artery filling pipe (4) and a portal vein filling pipe (5) which are arranged in the liver removing platform (1), and the other end of the Y-shaped pipe (3) is communicated with the liquid storage tank (2); and the Y-shaped pipe (3) is also provided with a constant flow pump (6). The in vitro continuous filling device provided by the invention has the advantages that continuous low temperature filling is carried out on a machine by utilizing the in vitro continuous filling device, basic nutrients required by low temperature metabolism of the liver can be provided, and wastes produced during metabolism of the liver also can be eliminated; and various required medicines can be added into filling solution at any time, adverse factors caused by a pure static low temperature filling method can be corrected, and function of a disabled liver can be obviously improved, so that storage time is prolonged.
Owner:WUHAN UNIV
Micelle loaded with Tacrolimus or pharmaceutical salts thereof, lyophilized preparation as well as preparation methods and applications
InactiveCN102198082ASimple preparation processEasy to operateOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolLung transplantation
The invention belongs to the technical field of pharmaceutical dosage forms and nano medicine preparation, and more particularly relates to a micelle loaded with Tacrolimus or pharmaceutical salts thereof, a lyophilized preparation as well as preparation methods and applications thereof. In order to achieve the clinical application of the micelle provided by the invention, the drug loading, the encapsulation rate and the stability of the preparation need to be ensured. The micelle is prepared from biodegradable polymer materials and Tacrolimus or pharmaceutical salts thereof in parts by weight: 1 part of Tacrolimus or pharmaceutical salts thereof and 1.5-99 parts of degradable polymer materials, wherein the degradable polymer materials are diblock polymer methoxy polyethylene glycol-polycaprolactone (the molecular weight ratio of MPEG to PCL is 0.5-2), and triblock polymer polycaprolactone-polyethylene glycol-polycaprolactone (the molecular weight ratio of PCL to PEG is 0.5-3). The micelle using Tacrolimus or pharmaceutical salts thereof or the lyophilized preparation of the micelle can be used in medicines for anti-rejection in liver transplantation, kidney transplantation, lung transplantation, corneal transplantation and other organ transplantations.
Owner:SICHUAN UNIV
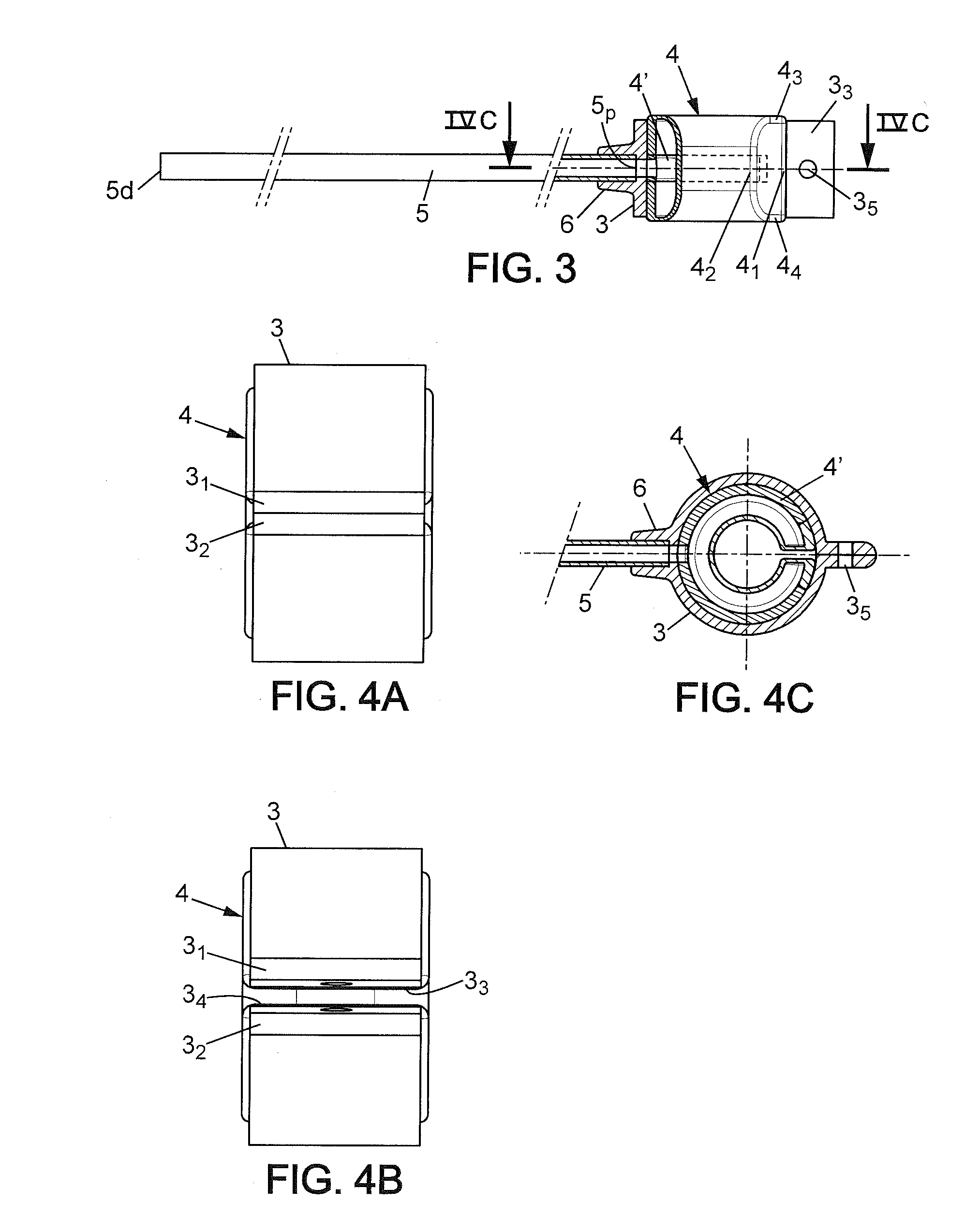
Adjustable vascular ring, means for treating sfs syndrome and implantable kit comprising such a ring, mould and method for obtaining such a ring
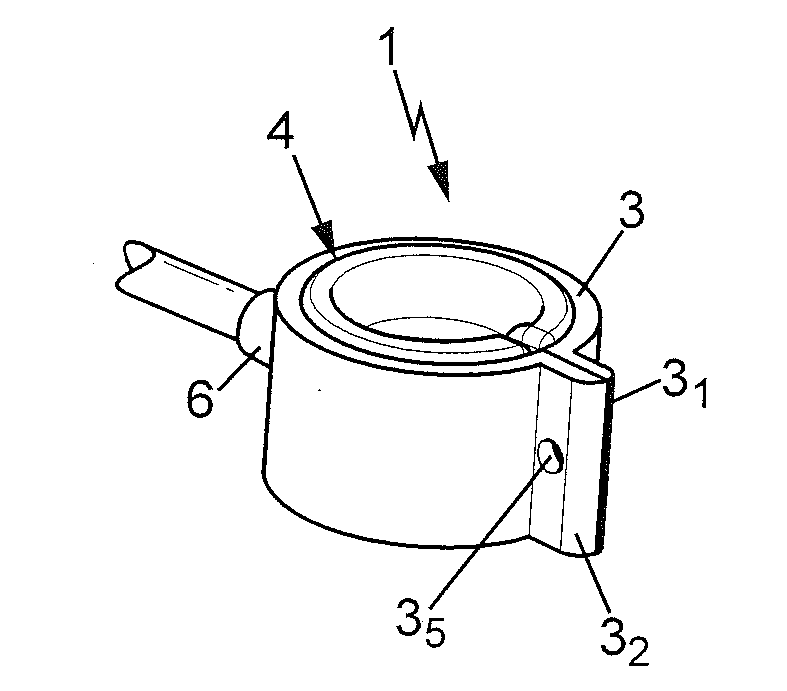
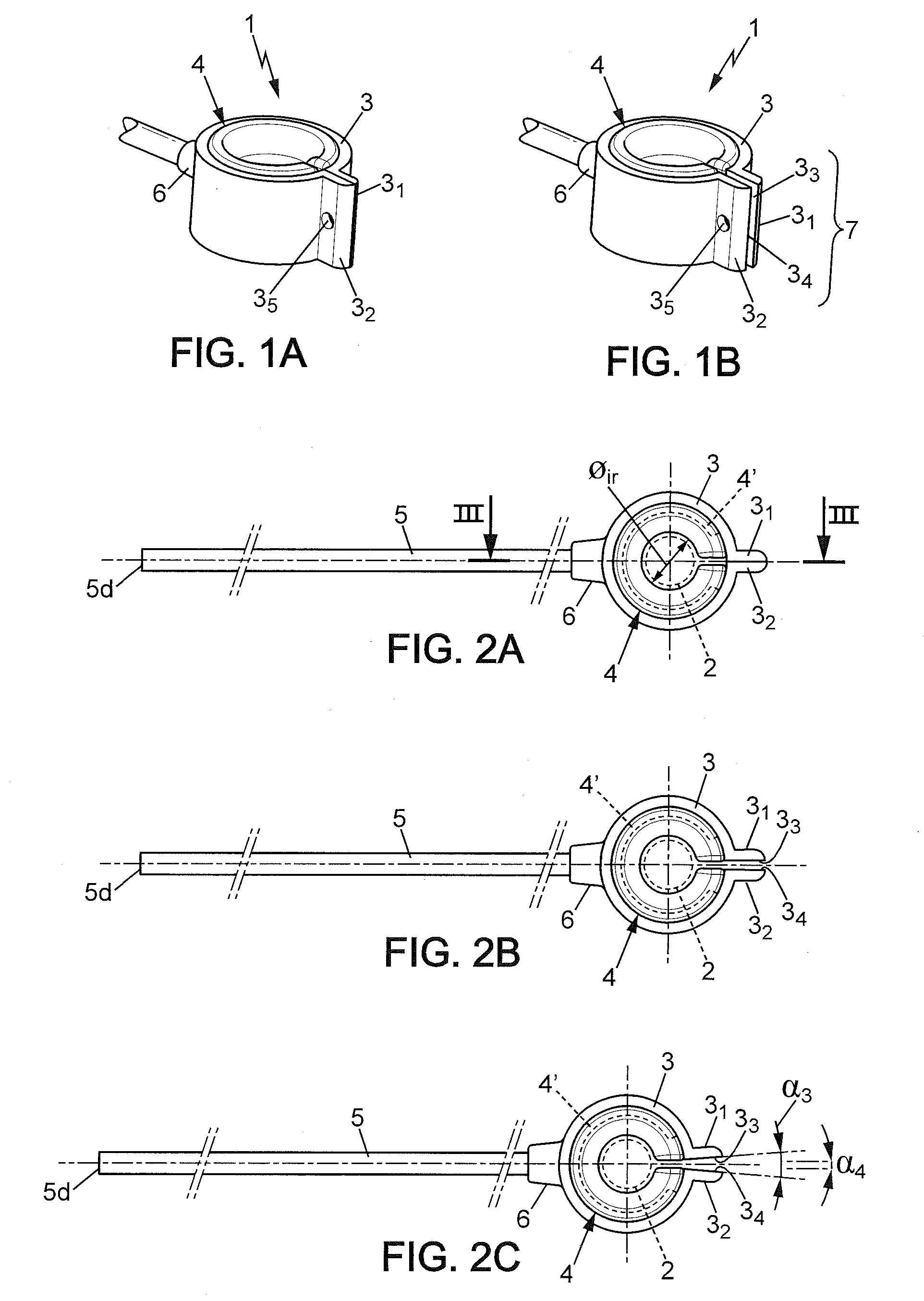
The invention relates to a perivascular ring having an inner diameter adjustable by inflation / deflation and adapted to be implanted and closed about a vessel for controlling the inner diameter of said vessel and thus the flow and / or pressure of a fluid flowing in said vessel. The ring of the invention is capable of regulating blood flow to the liver, in particular after an hepatectomy or hepatic transplant, and to substantially improve the survival chances of the patient. The ring according the invention has an adjustable inner diameter (φi) for implantation and locking about a vessel.
Owner:MEDICAL INNOVATION DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com