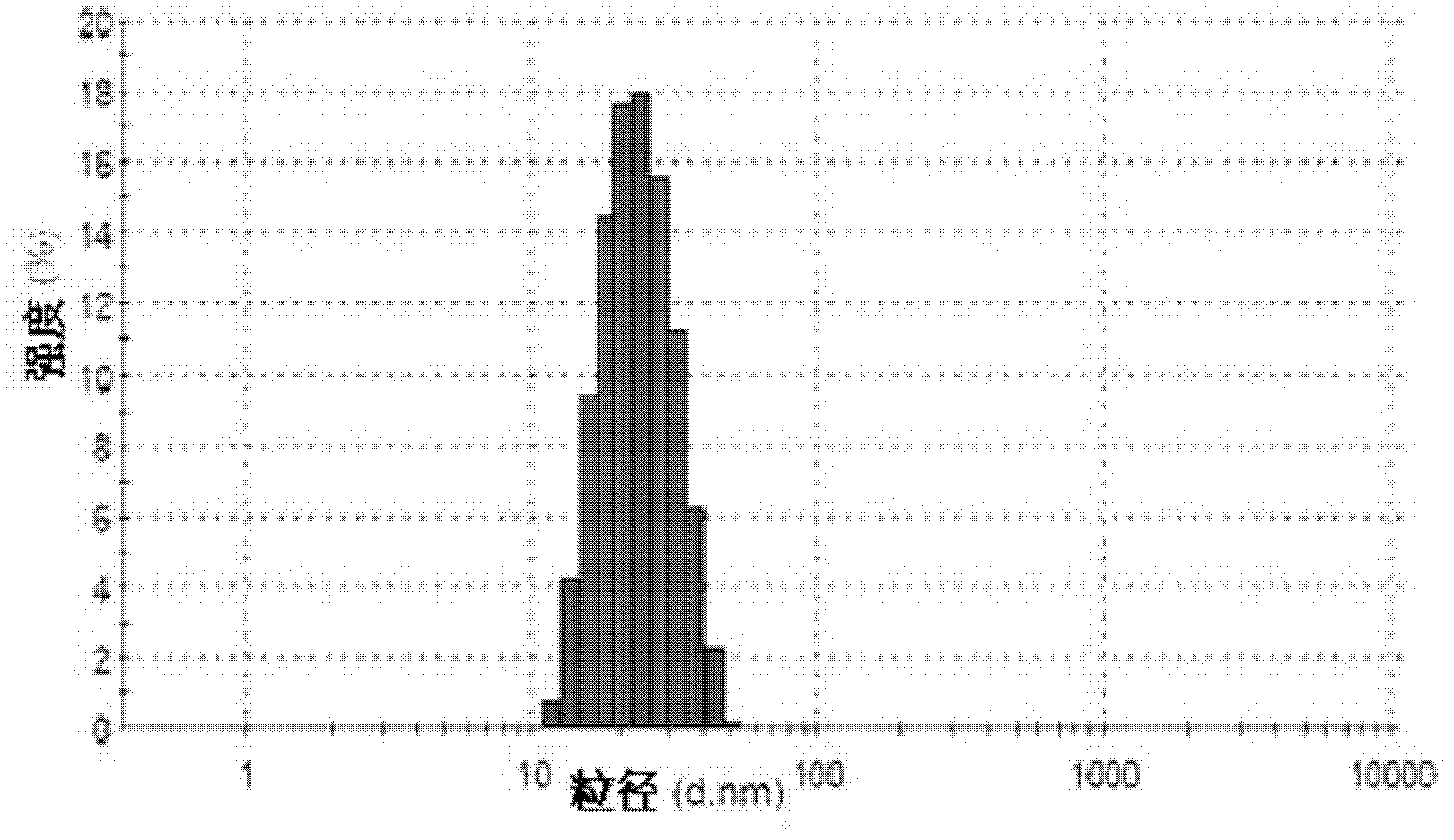
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
84 results about "Corneal transplantation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Corneal transplantation, also known as corneal grafting, is a surgical procedure where a damaged or diseased cornea is replaced by donated corneal tissue (the graft). When the entire cornea is replaced it is known as penetrating keratoplasty and when only part of the cornea is replaced it is known as lamellar keratoplasty. Keratoplasty simply means surgery to the cornea. The graft is taken from a recently dead individual with no known diseases or other factors that may affect the chance of survival of the donated tissue or the health of the recipient.
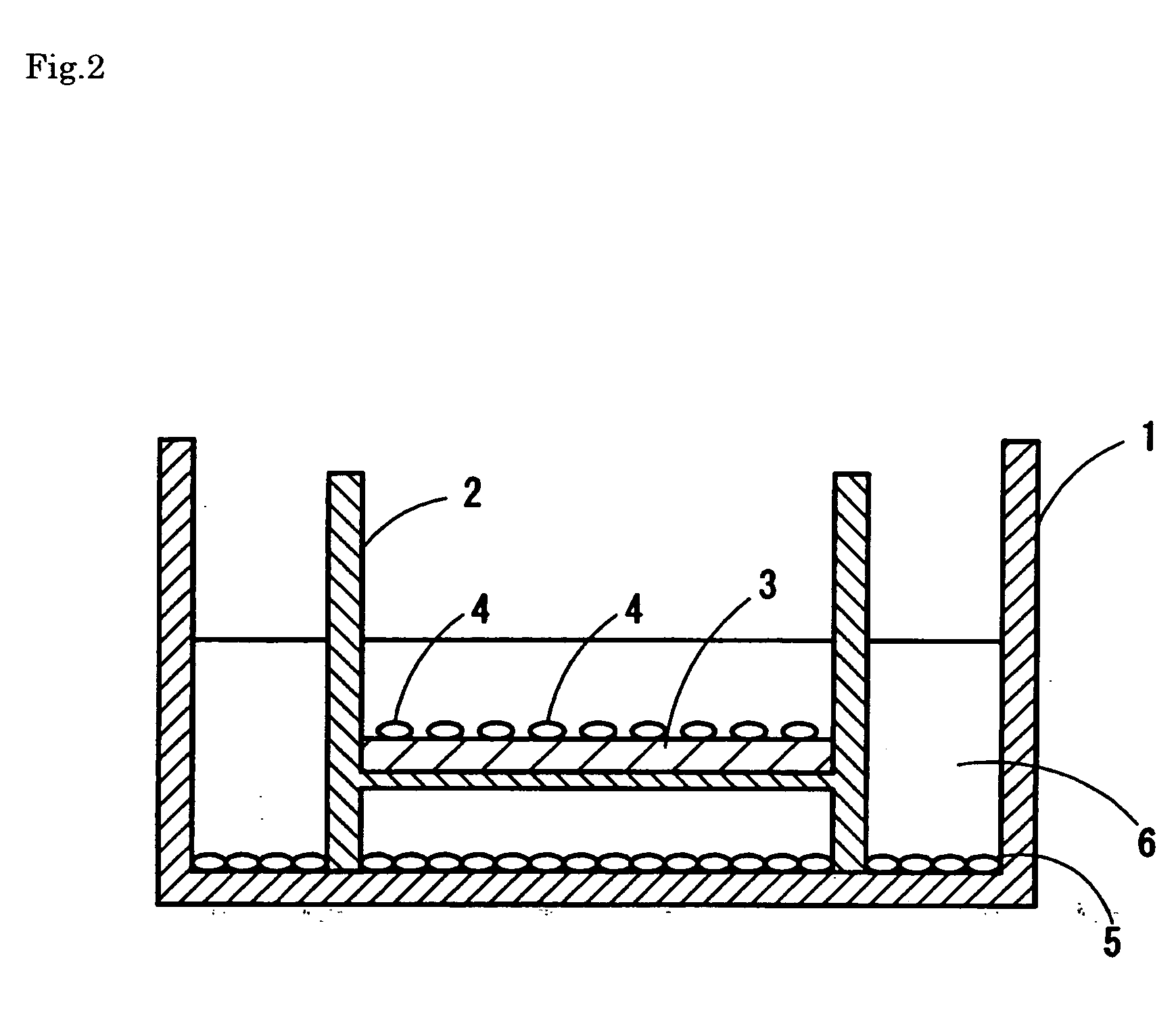
Acellular cornea or acellular corneal stroma, preparation method and application thereof
InactiveCN101985051ARetain toughnessLow immunogenicityProsthesisFreeze thawingVaccine Immunogenicity
The invention discloses acellular cornea or acellular corneal stroma, a preparation method and application thereof. The method comprises the following steps of: (1) obtaining fresh animal full-thickness cornea or corneal stroma; (2) removing corneal epithelium, corneal endothelium and stroma cells, namely 1, soaking the full-thickness cornea or the corneal stroma in pure water at room temperature; 2, placing the soaked full-thickness cornea or corneal stroma into enzyme solution, digesting with oscillating, and washing with balanced salt solution with oscillating; and 3, repeating freeze-thaw processes of the full-thickness cornea or the corneal stroma for 4 to 8 times and washing with balanced salt solution with oscillating to obtain the acellular cornea or the acellular corneal stroma; (3) dehydrating; and (4) sterilizing and storing. In the method, the decellularization processing time of the cornea is short; the influence on the structure and the physiological property of the cornea is small; and the processed cornea has very low immunogenicity which is similar to the property of natural cornea. The acellular cornea or the acellular corneal stroma can be applied to artificial cornea construction of tissue engineering and also can serve as a medical material applied to corneal transplantation and refraction surgery.
Owner:JINAN UNIVERSITY
Whole layer biological cornea as well as construction method and use thereof
The invention aims at providing a novel full-thickness biological cornea used for transplantation. The full-thickness biological cornea takes an animal cornea acellular matrix as a carrier, and the acellular matrix comprises animal cornea matrix cells, epithelial cells and endothelial cells which are cultured and augmented in vitro. The cornea is characterized in that the xenogenic corneal acellular matrix prepared by a biochemical method can be used as a good carrier for in vitro constructing the biological cornea in the aspects of shape, structure and biological compatibility, the matrix cells, epithelial cells and endothelial cells of the cornea are planted respectively, and dynamically cultured in the simulated in vivo environment of a biological reactor, so that the full-thickness biological cornea with nearly normal tissue structure and characteristics can be constructed in vitro, and the biological cornea can be used to simulate the physiological cornea for fundamental research on physiology, pathology and pharmacology; moreover, the biological cornea can also be directly used as the donator for corneal transplantation.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Stem cell regenerating surface cornea and its application in corneal transplantation
InactiveCN1398644AGuaranteed long-term effectivenessRich sourcesEye implantsArtificial cell constructsDiseaseCorneal disease
In the present invention an embryo or adult cornea epithelium is cut, digested with digesting liquid and centrifugated to prepare single cell suspension, and the cell suspension is cultured in culture dish or bottle with proper amount of culture medium and CO2 in 5% at 37 deg.c. The cultured cell is passed after cell converges to 80-90% and the second and sixth generation of cell is passed directly to amnion for culture for another 8-20 days to obtain the stem cell regenerating surface cornea of the present invention. The stem cell regenerating surface cornea may be used as material for treating corneal disease. The present invention provides a new material for treating corneal disease with rich material source, no danger of mouse-originated pollution, no or slight immunological rejection.
Owner:北京科宇联合干细胞生物技术有限公司
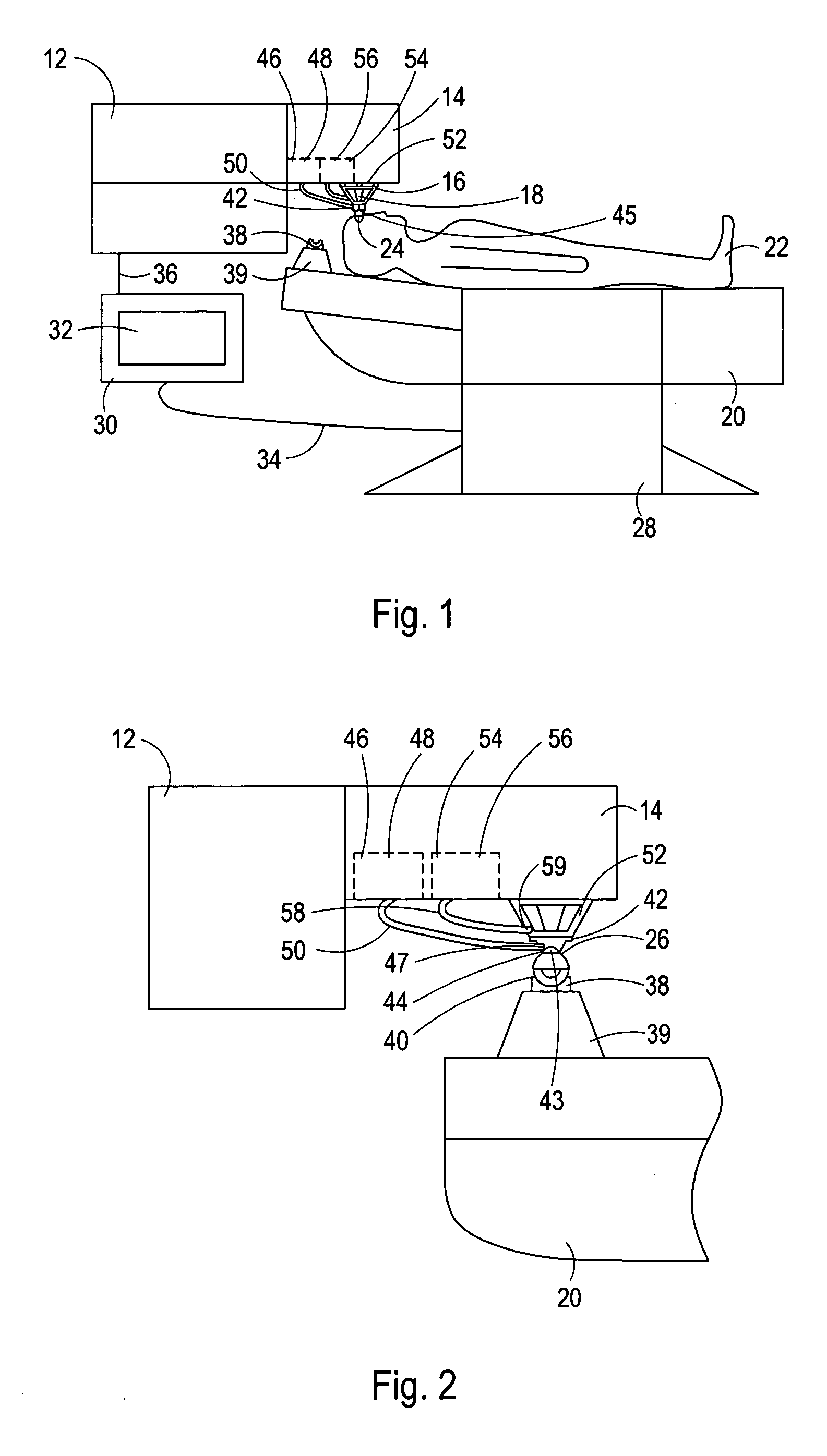
System for performing a corneal transplantation
A system for performing a corneal transplantation includes a laser source for generating a laser beam and a chair for positioning a patient relative to the laser source. A stabilizing element, engageable with the laser source, is fixated on the anterior surface of the patient's cornea to hold the cornea in alignment with the laser source. The laser source is then used to remove diseased tissue from the cornea of the patient, thereby creating a corneal cavity of known dimensions. In a subsequent step, a donor graft that was previously photoaltered to have substantially the same dimensions as the corneal cavity, is transplanted into the corneal cavity.
Owner:TECHNOLAS PERFECT VISION
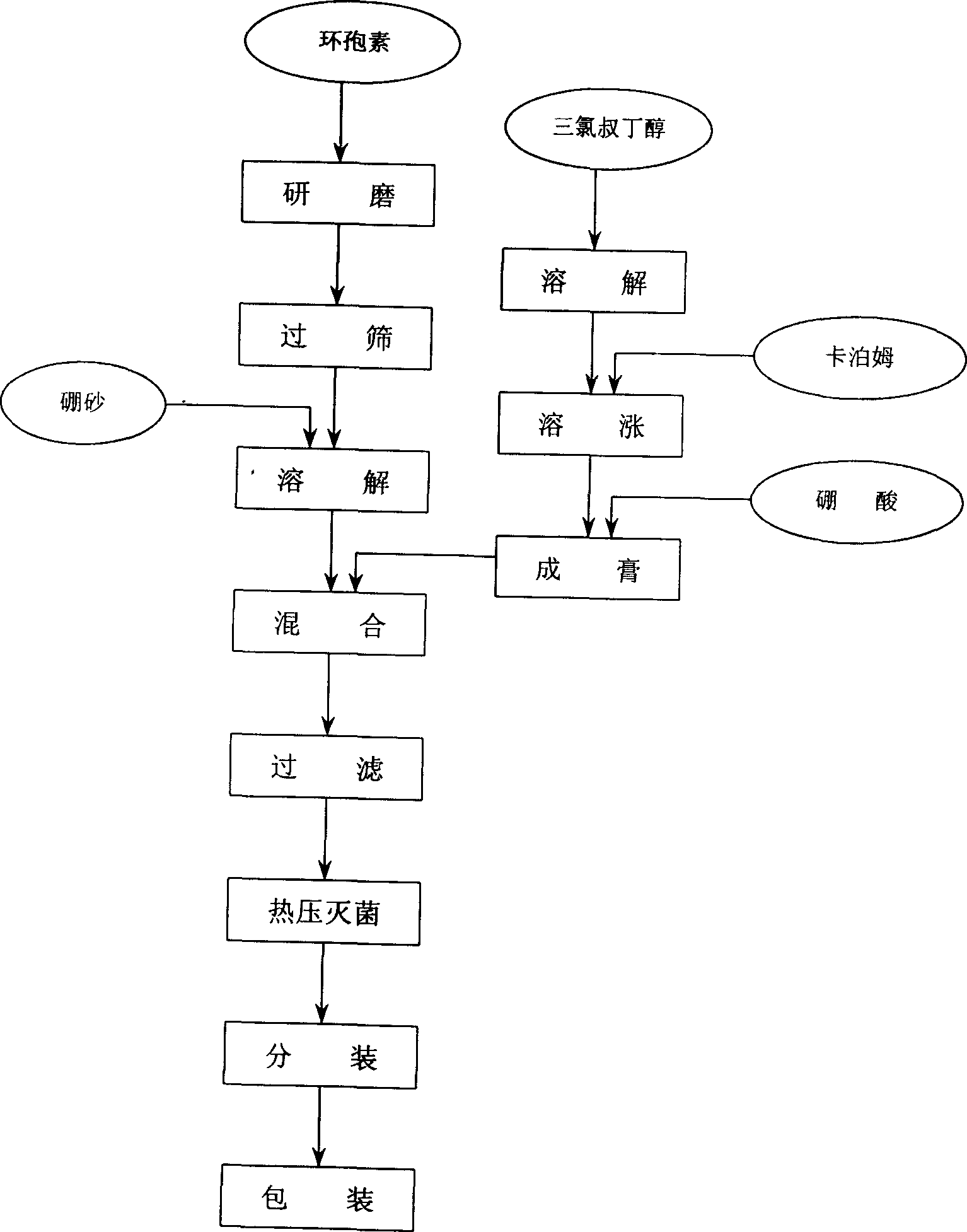
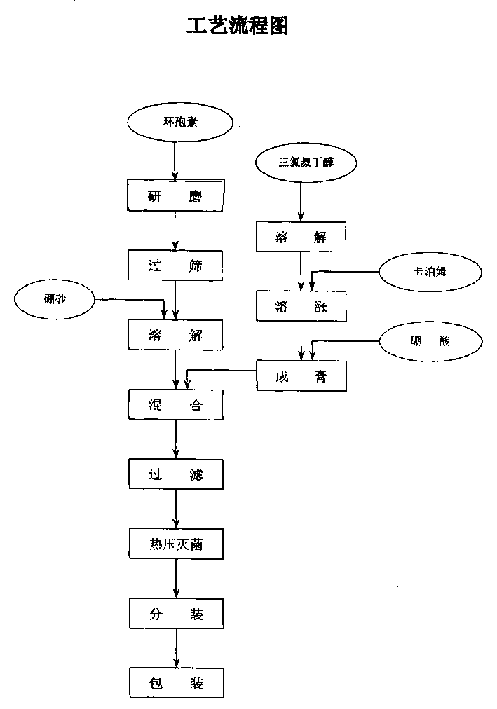
Eye cyclosporin gel
InactiveCN1456350ANot easy to diluteGood water solubilitySenses disorderPharmaceutical delivery mechanismCyclosporinsWhole body
A cyclosporin eye gel for treating the rejection reaction of corneal transplantation, keratoconjunctival xerosis, catarrhal conjunctivitis, and chemical burn of eye is prepared from cyclosporin, boric acid, borax, trichloro-tert-butanol, etc. Its advantages are long stay time in eye and high curative effect.
Owner:刘继东
Methods and compositions for growing corneal endothelial and related cells on biopolymers and creation of artifical corneal transplants
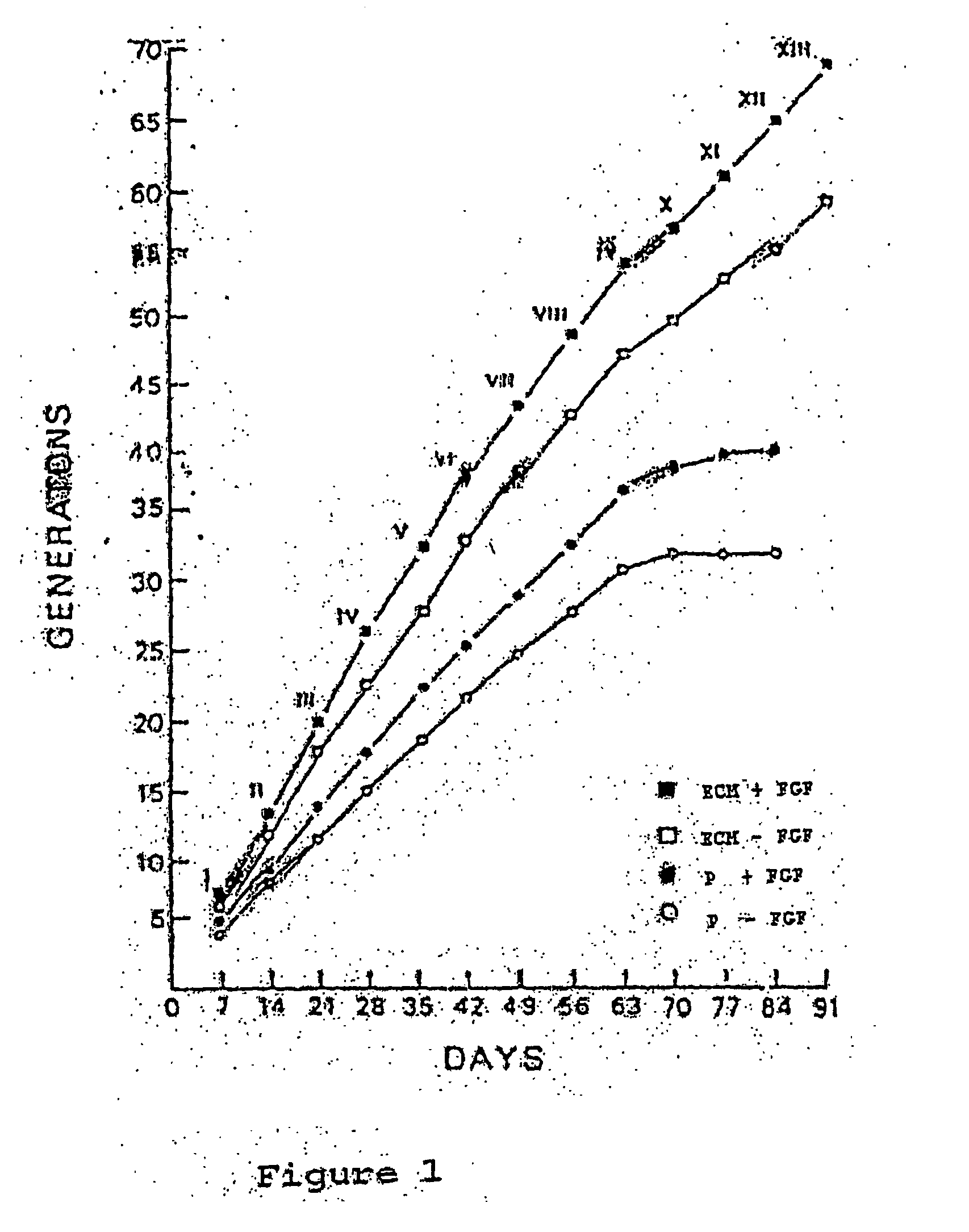
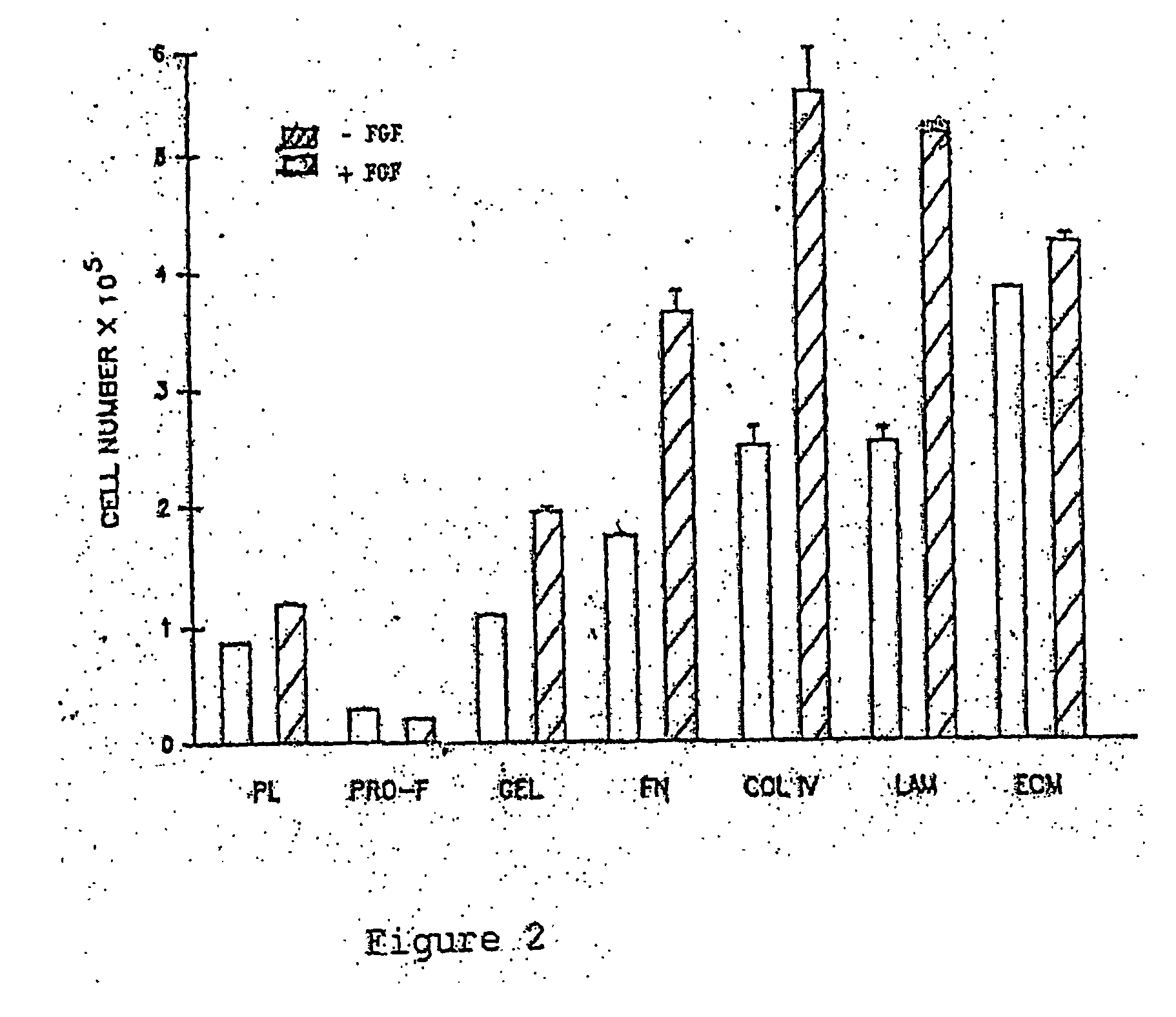
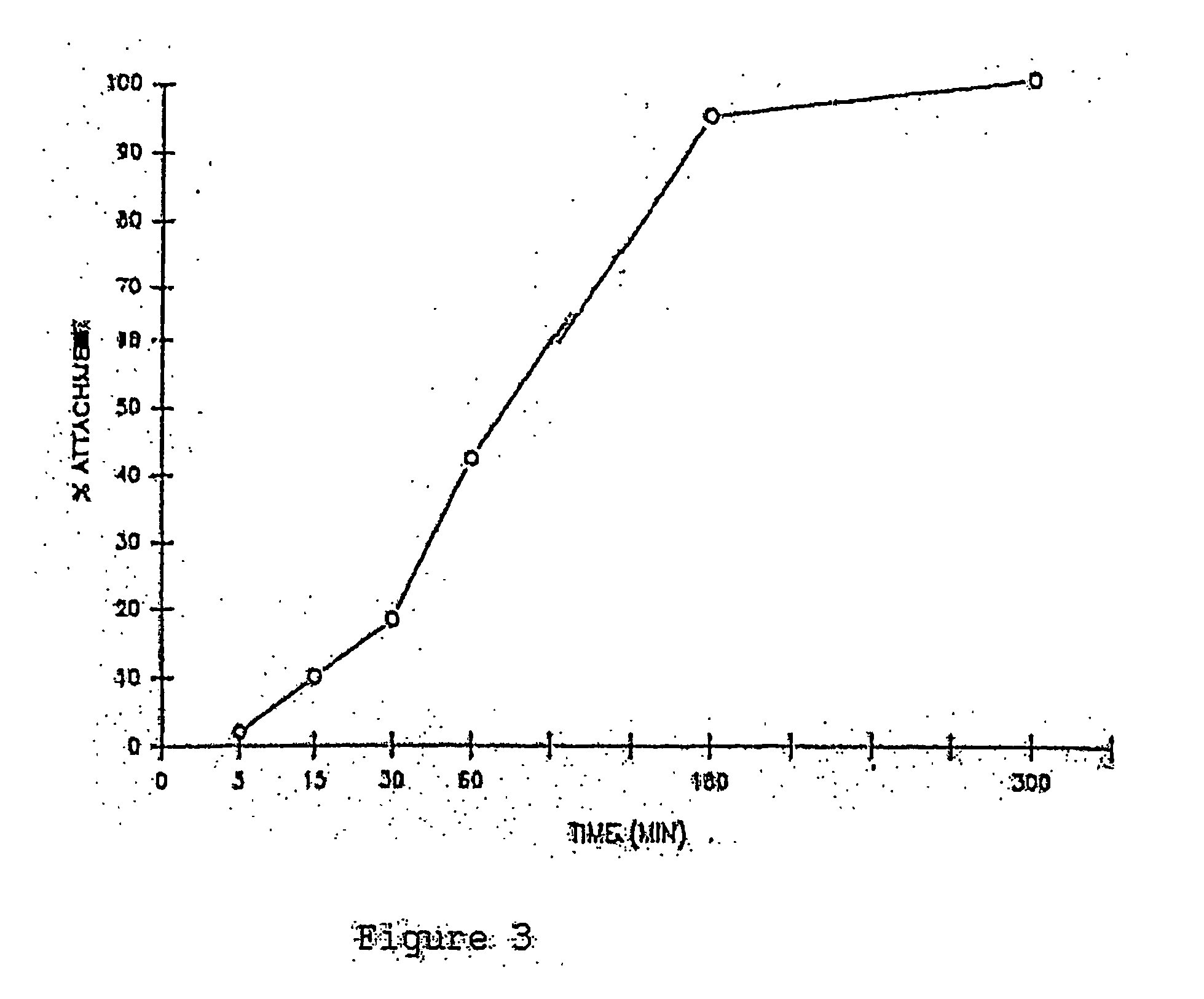
This invention discloses methods to attach and grow a monolayer of cultured human corneal endothelial cells onto the endothelial side of the stroma synthesized from biopolymer to generate a more bio-equivalent artificial cornea. The approaches will include the use of attachment and growth promoting agents such as fibronectin, laminin, RGDS, collagen type IV, bFGF conjugated with polycarbophil, and EGF conjugated with polycarbophil. The patent also describes a method to create a self-sustaining polymer containing adhesive molecules and growth factors to support the attachment and proliferation of cultured human corneal endothelial cells for corneal transplantation either as a half-thickness device or full-thickness button replacement. An approach for the implantation of cultured retinal pigment epithelial (RPE) cells into the sub-retinal space for treatment of age-related macular degeneration (ARMD) is disclosed in this invention. This method will enable the delivery of the transplanted RPE in a sheet of monolayer cells and will be better suited to perform their physiological function.
Owner:CELLULAR BIOENG
Cornea transplantation
ActiveUS20100087802A1Great freedomAllow useLaser surgeryEye implantsControl dataCorneal transplantation
A planning device generating control data for a treatment apparatus for cornea transplantation using a laser device to separate a corneal volume by at least one cut surface in the cornea and to separate a transplant, from a surrounding transplantation material by at least one cut surface wherein the planning device includes an interface supplying measurement data relating to parameters of the cornea. A computer defines a corneal cut surface which confines the corneal volume to be removed, and determines a transplant cut surface by using the transplantation material data and depending on the defined corneal cut surface. The transplant cut surface confines the transplant, and the computer generates one control data for each cut surface to control the laser, wherein the respective cut surfaces can be produced by the laser to isolate the corneal volume and the transplant and to make them removable.
Owner:CARL ZEISS MEDITEC AG
Reconstruction method of tissue engineering human corneal epithelium
The invention relates to a reconstruction method of tissue engineering human corneal epithelium. The method comprises the following steps of: adopting a DMEM / F12 culture medium containing 20% calf serum to carry out the in vitro culture on human corneal epithelium cells to a logarithmic growth phase, and adopting trypsin and a trypsin-EDTA digestion method to obtain a digestive amniotic carrier bracket of which the epithelium is completely removed; and after the digestive amniotic carrier bracket of which the epithelium is removed is flatly laid in a plug-in Petri dish and is firmly and pasted in a drying way, inoculating the human corneal epithelium cells at the logarithmic growth phase suspended in the DMEM / F12 culture medium containing IV type collagen and 20% calf serum to the plug-inPetri dish flatly laid with the digestive amniotic carrier bracket of which the epithelium is removed, and carrying out the in vitro reconstruction on the tissue engineering human corneal epithelium by a gas-liquid interface culture method. The invention has scientific and reasonable process, the reconstructed tissue engineering human corneal epithelium can be used for mass production, a lot of demands of vast blind patients with corneal epithelium diseases for the tissue engineering human corneal epithelium in clinical corneal transplantation treatment can be met, and the costs of the in vitro reconstruction and clinical treatment of the tissue engineering human corneal epithelium are low.
Owner:OCEAN UNIV OF CHINA +1
Method for preparing corneal endothelial cell
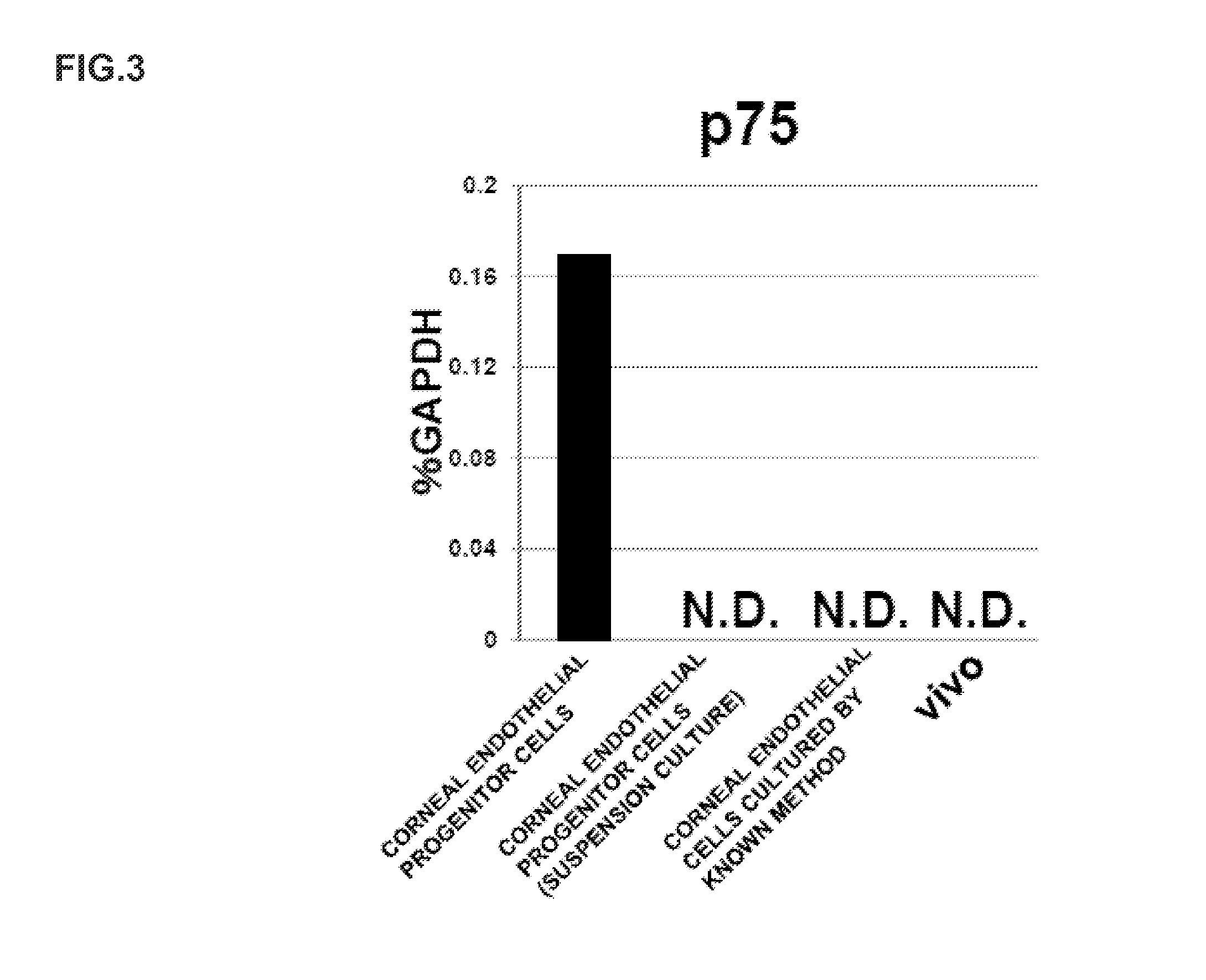
ActiveUS20140170751A1Improve proliferative abilityIncrease the number ofNervous system cellsUnknown materialsDiseaseProgenitor
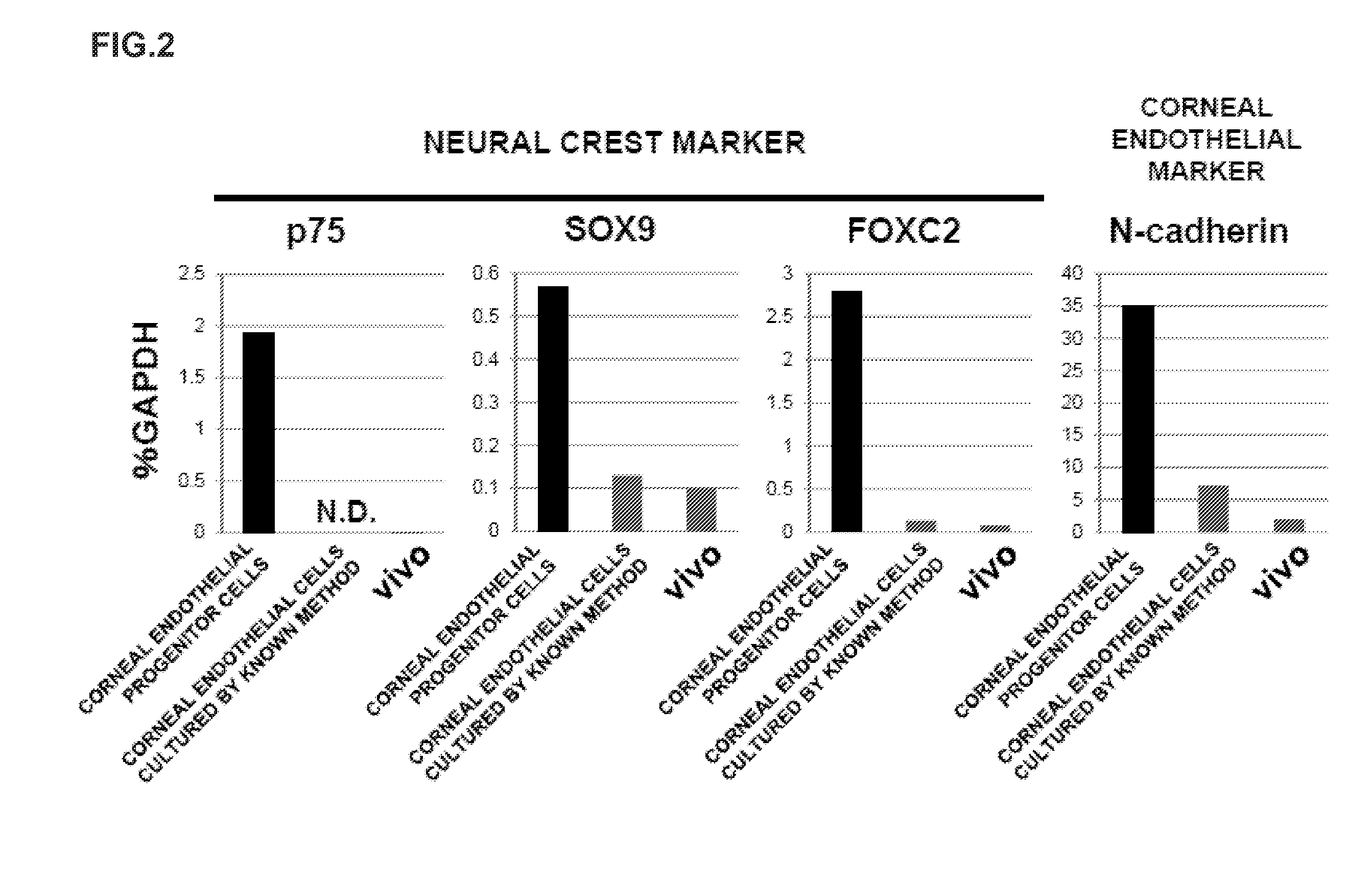
The present invention relates to a method for preparing corneal endothelial progenitor cells by adherent culturing a cell population isolated from corneal endothelial cell tissue at a low density by using a serum-free medium. The present invention also relates to a method for preparing corneal endothelial cells by differentiation-inducing the corneal endothelial progenitor cells obtained by the aforementioned method. According to the present invention, corneal endothelial progenitor cells can be selectively grown from a corneal tissue-derived cell population, and corneal endothelial cells obtained by inducing the corneal endothelial progenitor cells can be applied to treatment of corneal endothelial diseases. As a result, problems of corneal transplantation such as shortage of donors and occurrence of rejection can be solved.
Owner:OSAKA UNIV
Tacrolimus ophthalmic preparation and preparation method thereof
ActiveCN107929235AMinimal eye irritationImprove complianceOrganic active ingredientsSenses disorderConjunctival xerosisPatient compliance
The invention provides a tacrolimus ophthalmic preparation and a preparation method thereof, and belongs to the technical field of pharmaceutical preparations. According to the present invention, thetacrolimus ophthalmic preparation has a micro-emulsion-like structure, is clarified and transparent, has good patient compliance, is prepared from tacrolimus, oil for injection, a surfactant, a stabilizer, an osmotic pressure regulator, a preservative, a pH value regulator, a tackifier and water for injection, and is used for preventing and treating immunological rejection after corneal transplantation or immunological corneal xerosis and conjunctival xerosis. According to the present invention, with the tacrolimus ophthalmic preparation, the problem of the water insolubilization of tacrolimusis solved, the tacrolimus is firstly prepared into the clarified and transparent eye drops with the micro-emulsion-like structure, the irritation on the eyes by the existing tacrolimus-suspended ophthalmic eye drops can be substantially reduced, and the patient compliance can be improved, such that the tacrolimus ophthalmic preparation of the present invention is suitable for the long-term use byimmunological rejection after corneal transplantation, corneal xerosis and conjunctival xerosis, and other patients with indications.
Owner:SHENYANG XINGQI PHARM CO LTD
Preparation method of plantago seed polysaccharide extractive and applications thereof
The invention relates to a preparation method of an immunosuppressive activity plantago seed extractive and applications thereof. The preparation method comprises the following steps of: degreasing raw materials and obtaining crude extractive through water extraction and alcohol precipitation; deproteinizing and depigmenting the crude extractie through dialysis and ion exchange chromatography; and removing micromolecular chemical compositions to prepare the plantago seed extractive with higher purity. A total polysaccharide content in the inventive immunosuppressive activity plantago seed extractive accounts more than 72% of the extractive by weight. Shown by a great amount of pharmacological experiments, the plantago seed extractive has immunosuppressive activity, can be used for preparing immune inhibitors in various preparation forms or used as a raw material medicine for preparing other immunosuppressive medicines and used for medical purposes in treating diseases related to immune inflammatory injuries, such as nephritis, cystitis, prostatitis, urethritis, edema and eye diseases, such as uveitis, interstitial keratitis, corneal transplantation rejection, and the like.
Owner:匡海学
Corneal posterior lamella and use thereof
InactiveCN101745152AImprove proliferative abilityStrong self-renewal abilityEye implantsCorneal endothelial cellCorneal endothelium
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Sheet for corneal transplants
InactiveUS20120282318A1Stable transplantation resultAppropriate strengthBiocideSenses disorderCorneal endothelial cellBiocompatibility Testing
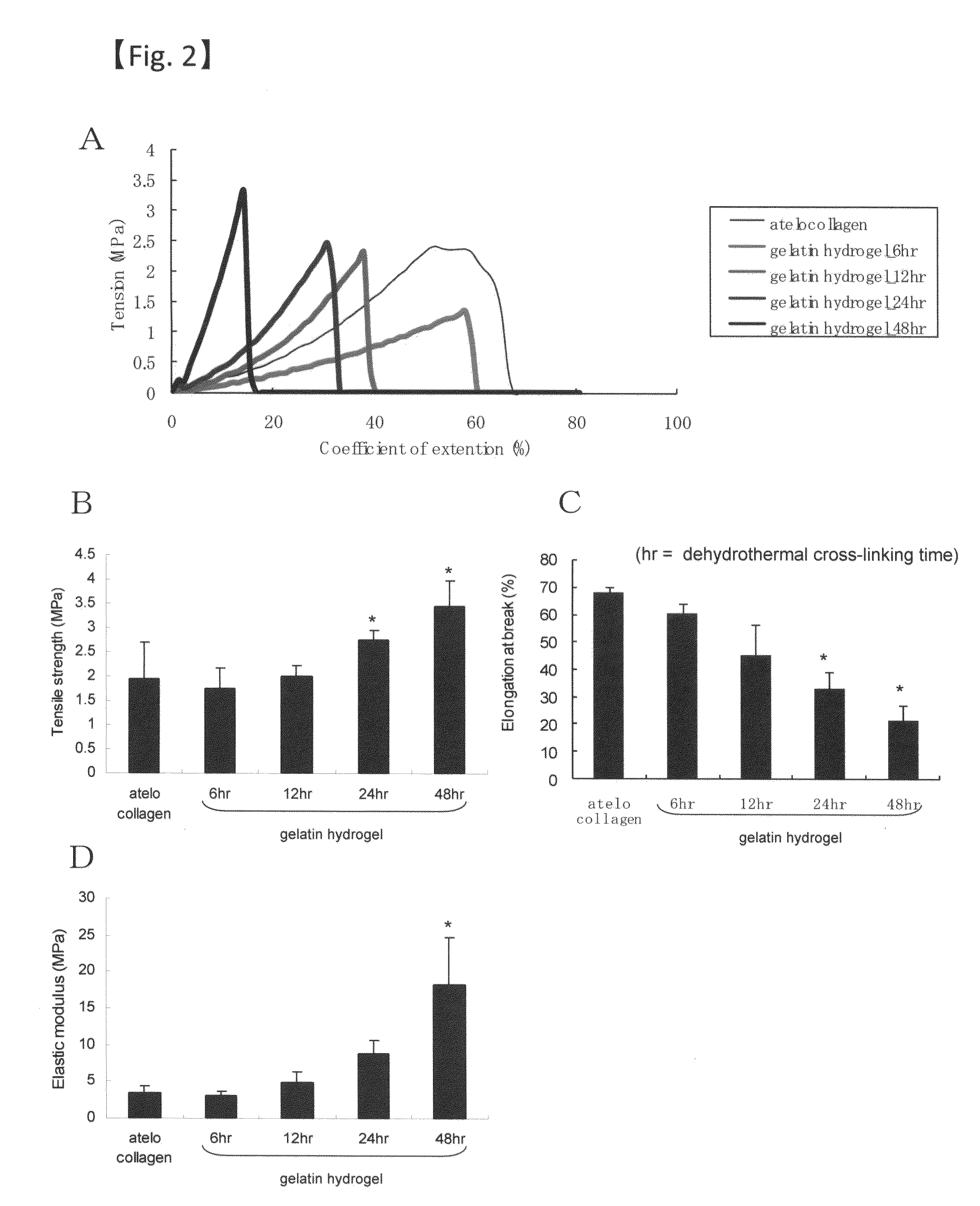
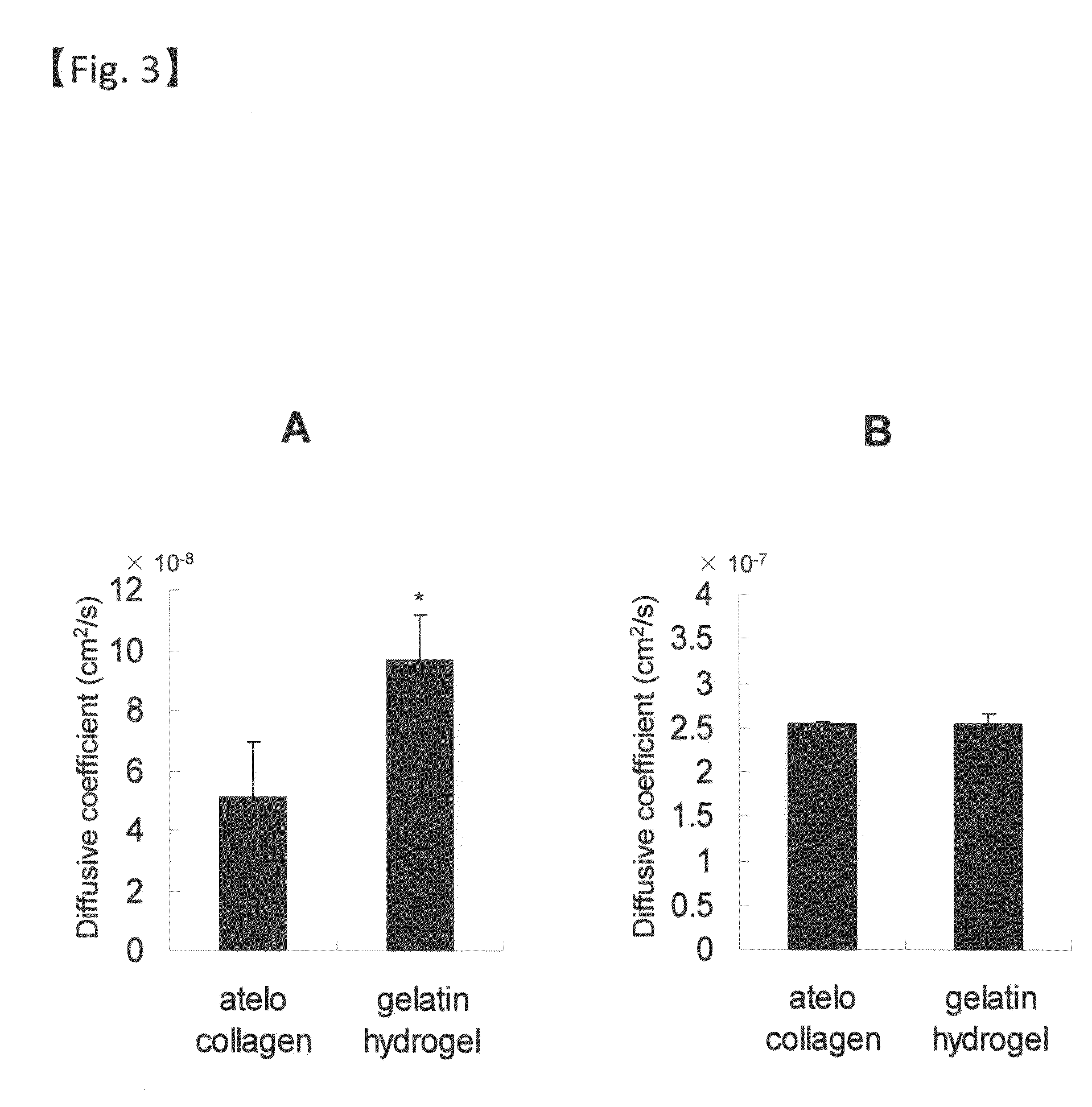
The present invention relates to a sheet for corneal transplants comprising corneal endothelial cells on a gelatin hydrogel, which is obtainable by seeding and culturing corneal endothelial cells on a gelatin hydrogel coated with collagen. The sheet of the present invention is extremely useful as a sheet for corneal transplants not only for its biocompatibility and biodegradability, but also for its high transparency.
Owner:OSAKA UNIV
Method for preparing carrier bracket of tissue engineering artificial corneal endothelium by using fresh amniotic membrane
The invention relates to a method for preparing a carrier bracket of tissue engineering artificial corneal endothelium by using a fresh amniotic membrane. The method comprises the following steps: soaking and disinfecting the fresh amniotic membrane by tobramycin sulfate injection according to the mass ratio of 1:1000; after reverse digestion using trypsin-EDTA digestive juice, lightly scraping the epithelial surface of the amniotic membrane by a cell scraper to completely remove residual epithelial cells to obtain the denuded amniotic membrane which is tiled in culture plate holes for fixingand dry-posting; coating by special coating liquid for the denuded amniotic membrane; and sucking and then drying the coating liquid to obtain the carrier bracket of the tissue engineering artificialcorneal endothelium. The method has scientific and reasonable process, the prepared carrier bracket can be mass produced to meet the heavy demand of scale reconstruction of the tissue engineering artificial corneal endothelium and create conditions for sight rehabilitation of corneal endothelium blindness through clinical corneal transplantation, and the preparation method of the carrier bracket has low cost in in-vitro reconstruction and clinical treatment of the tissue engineering artificial corneal endothelium.
Owner:青岛宇明生物技术有限公司
Collagen scaffold material for cornea
The invention discloses a scaffold material for clinical cornea reconstruction and a preparation method of the material, belongs to the field of medical biomaterials, and mainly aims at solving the present problems that the clinical supply of cornea donors is difficult and the success rate of operations is low. The cornea is reconstructed by providing a keratocyte growth matrix for a cornea receptor. The material prepared by the method is prepared by adjusting pH values of polyose and a collagen solution to be within a certain range at a certain environmental temperature and then conducting crosslinking membrane forming. The material is stable, uniform, transparent, water-proof and pressure-resistant, and is good in biocompatibility. The cornea reconstruction of the corneal transplantation receptor is facilitated.
Owner:TIANJIN SAIRUI BIOLOGICAL TECH
Corneal trephine
The invention provides a corneal trephine. The corneal trephine comprises a body part in a hollow cylindrical shape, a knife part in an approximate hollow cylindrical shape, and a finger holding part, wherein the thickness of a column wall of the knife part is gradually reduced from top to bottom; a knife edge for annular cutting is formed at the bottommost end of the column wall; an upper end of the knife edge is fixedly connected with a lower end of the body part; a central shaft line of the knife edge is overlapped with that of the body part; finger holding part comprises a hollow cylindrical body and lug bodies symmetrically fixed to an outer side surface of the hollow cylindrical body, the hollow cylindrical body of the finger holding part can be rotationally sleeved onto the body part; and an alignment device is also arranged on the finger holding part. When being used for corneal transplantation, the corneal trephine can be easily and accurately fixed to a cornea of a patient so as to avoid shifting or separation of the corneal trephine in the corneal transplantation, a proper and uniform pressure can be easily applied to the corneal trephine, and a knife feeding situation can be conveniently observed, thereby enabling the corneal transplantation to be safer and accurate.
Owner:苏州碧利医疗科技有限公司
Fiber-enhanced drug-loading hydrogel keratoprosthesis skirt stent and preparation method thereof
The invention belongs to the field of keratoprosthesis stent materials and provides a fiber-enhanced drug-loading hydrogel keratoprosthesis skirt stent and a preparation method thereof. The skirt stent comprises the following components in percentage by weight: 6wt%-10wt% of fibers, 8wt%-15wt% of biodegradable drug-loading microspheres and 75wt%-86wt% of hydrophilic hydrogel, wherein the fibers are disorderedly distributed in hydrophilic hydrogel. The fiber-enhanced drug-loading hydrogel keratoprosthesis skirt stent has special excellent biocompatibility and permeability of hydrogel and very high tensile strength; the inflammations and complications occurring after the corneal transplantation can be relieved by virtue of drugs released by the biodegradable drug-loading microspheres.
Owner:SICHUAN UNIV
Ectocornea-like sheet and method of constructing the same
InactiveUS20050003532A1Reduce differentiationHigh divisional potentialEye implantsArtificial cell constructsDiseaseOral mucosal epithelial cell
It is intended to provide a transplantation material applicable to ocular surface diseases with a need for ectocornea transplantation (i.e., an ectocornea-like sheet). Oral mucosal epithelial cells are inoculated onto an amnion and then cultured in the coexistence of supporter cells. When a layered structure of the oral mucosal epithelial cells is formed, the outermost layer is brought into contact with air, thereby inducing differentiation. Thus, an ectocornea-like sheet having an oral mucosal epithelial cell layer on the amnion is obtained.
Owner:AMNIOTEC +1
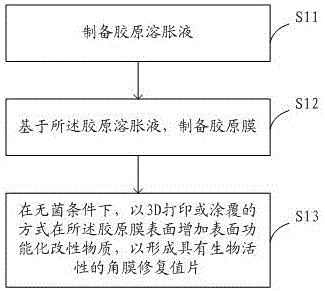
Preparation method of cornea repair graft with biological activity and cornea repair graft
ActiveCN106039403AHigh strengthImprove water absorptionPharmaceutical delivery mechanismTissue regenerationActive cellDrug biological activity
The invention discloses a preparation method of a cornea repair graft with biological activity and the cornea repair graft, and solves the technical problem of deficiency of cornea donors. The preparation method comprises the following steps: preparing collagen dispersion; preparing a collagen membrane on the basis of the collagen dispersion; adding surface functionalization modified substances on the surface of the collagen membrane in a 3D printing or coating mode under the sterile condition to form the cornea repair graft with biological activity. The surface functionalization modified substances are fine particles which are prepared from a cornea stroma, which does not contain a lesion part and contains active cells, of a patient, the biology and the biological stability of the high-strength collagen membrane can be improved, the physiological function of cornea is improved, after the modified collagen membrane is implanted into an eye of the patient, transparency and moisture content are good, the transparency can be recovered quickly clinically, the biological compatibility is good, and a good stent is provided for cornea cell culture of a cornea transplantation receptor.
Owner:广州尤尼智康生物科技有限公司
Novel biological artificial cornea capable of realizing cellularization through in-vivo induction as well as realizing quick transparency
InactiveCN105688282ACurvature maintenanceQuick fitEye implantsTissue regenerationDepyrogenationBiocompatibility
The invention relates to a novel biological artificial cornea capable of realizing cellularization through in-vivo induction as well as realizing quick transparency. The novel biological artificial cornea is prepared with a method in the steps as follows: 1, obtaining of a cornea raw material; 2, preparation of a cornea scaffold; 3, deep modification of the cornea scaffold; 4, deep inactivation of the cornea for removal of pyrogen; 5, irradiation sterilization of the cornea; 6, cultivation and implantation of corneal endothelial cells. Deep and light lamellar biological artificial corneas with different thicknesses and a full-thickness biological artificial cornea can be established. After being implanted, the novel biological artificial cornea can be quickly healed, becomes transparent quickly and keeps good refraction, the eyesight can be recovered and the eyeball is beautified. The obtained biological artificial cornea has good mechanical performance and biocompatibility, a full-thickness cornea graft is formed after endothelial cells are implanted on a full-thickness cornea stroma scaffold and can be rebuilt in vivo, perform in-vivo induction to promote growth of corneal limbal stem cells and growth of corneal epithelial cells and become transparent quickly on the basis, the treatment efficiency and effects are improved, and an application of lamellar keratoplasty and an application of penetrating keratoplasty are both considered.
Owner:广州宏畅生物科技有限公司
Modified acellular corneal stroma and modification method thereof
ActiveCN105497985ARestore moisture contentImprove water absorptionPharmaceutical delivery mechanismTissue regenerationBiocompatibility TestingPre treatment
The invention belongs to the field of tissue engineering, and particularly relates to modified acellular corneal stroma and a modification method thereof. The modification method comprises the following steps: (1) carrying out pretreatment on acellular corneal stroma; (2) reacting with the pretreated acellular corneal stroma by amino acid, and carrying out graft modification on the acellular corneal stroma; and (3) carrying out post treatment on the acellular corneal stroma subjected to graft modification, and removing the residual material in the graft modification process to obtain the modified acellular corneal stroma. The modified acellular corneal stroma has good mechanical property and biocompatibility, also has excellent rehydration water-retaining properties, can quickly restore the transparency after being implanted, and can be applied to tissue engineering research, clinical corneal transplantation and the like.
Owner:欧亚科(广东)生命科学有限公司
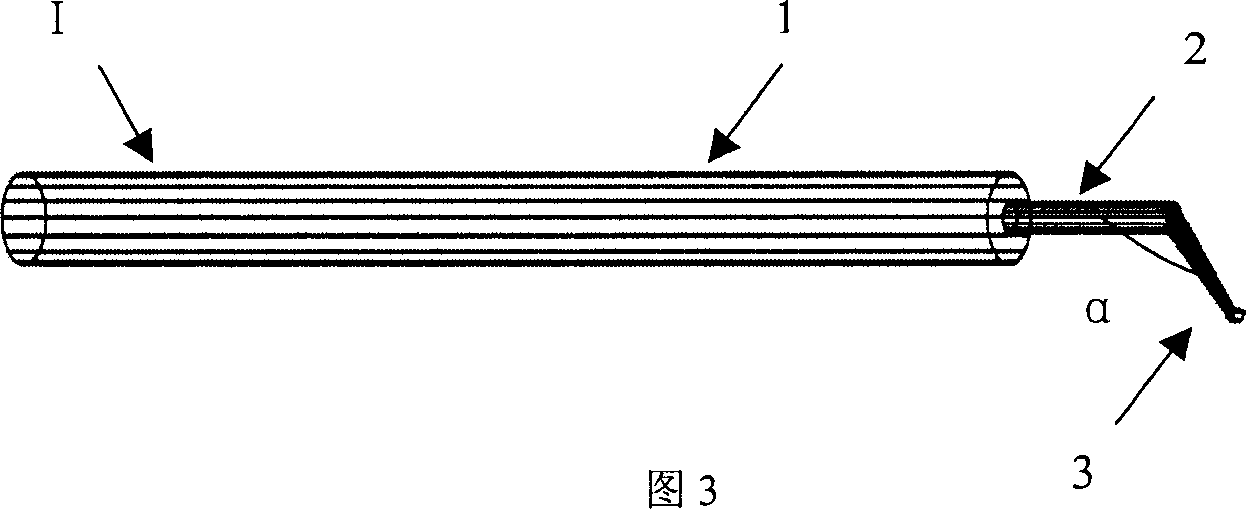
Full embedding bed deep lamellar cornea grafting composite instrument
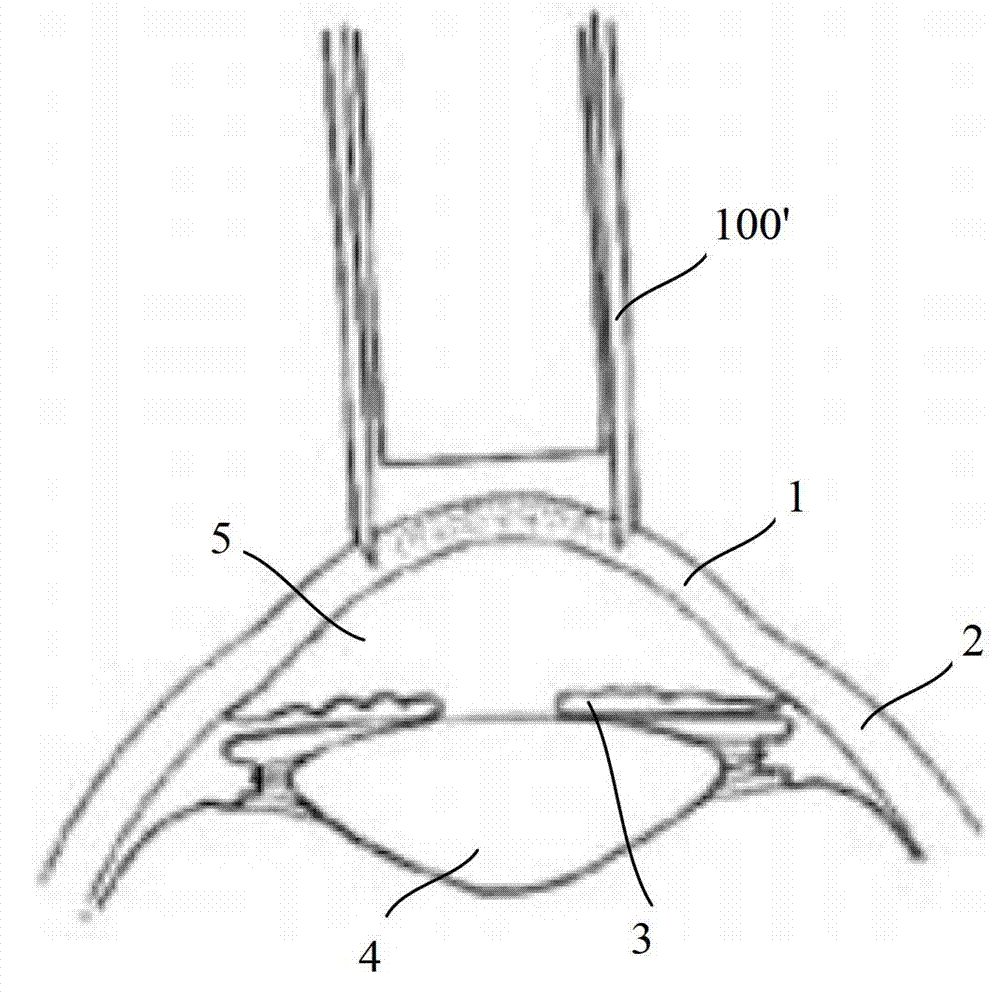
A combined apparatus for the deep corneal transplantation is composed of two deep corneal matrix hooks consisting of handle and the extended rod with hook head, and a viscoelastic agent injector consisting of needle seat and tube. Its advantages are short operation time and high safety and effect.
Owner:THE AFFILIATED SIR RUN RUN SHAW HOSPITAL OF SCHOOL OF MEDICINE ZHEJIANG UNIV
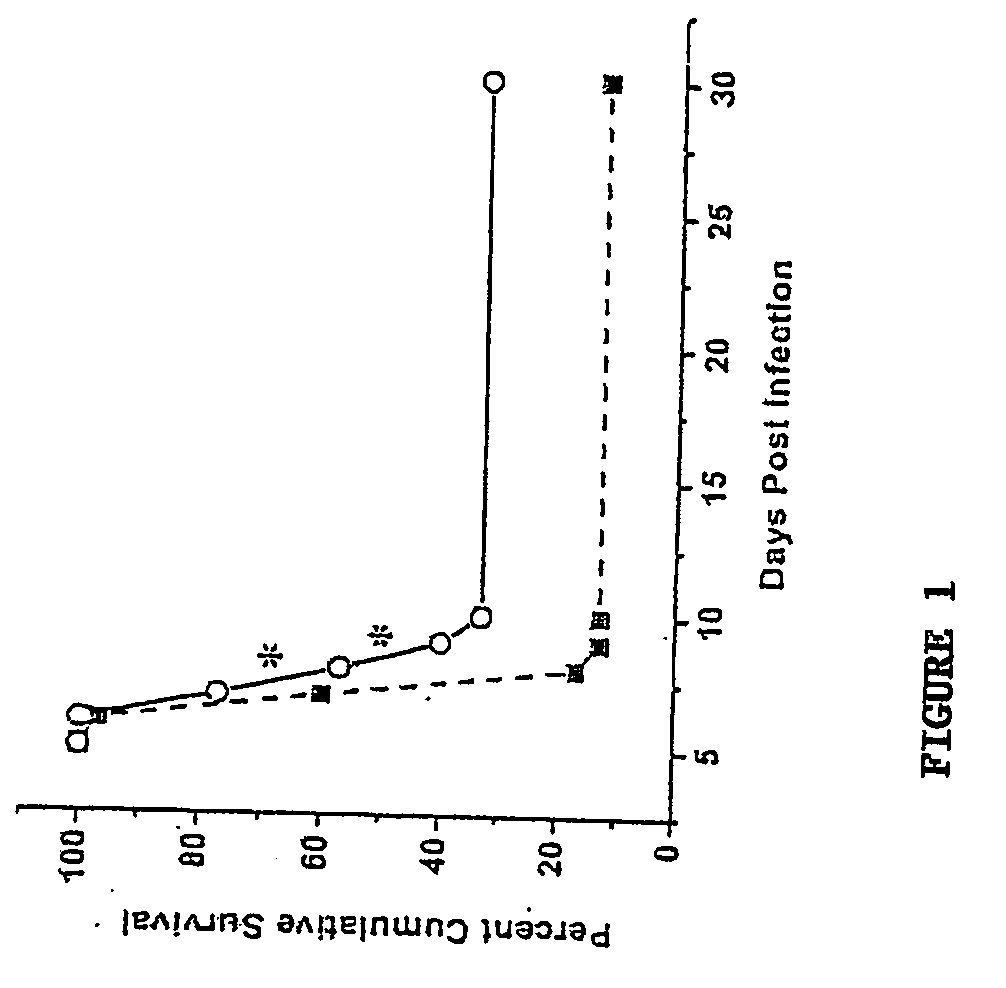
Methods for Treating Ocular Inflammation by Neutralizing Cxcl10 Activity
InactiveUS20080019973A1Reduce inflammationReduce corneal inflammationCompound screeningApoptosis detectionCXCL10Ocular inflammation
The invention provides a method of reducing ocular inflammation in an individual susceptible to ocular inflammation. The method involves administering to the individual an effective amount of a neutralizing agent specific for CXCL10. Also provided is a method for reducing spread of viral infection within ocular tissues of an individual susceptible to ocular viral infection, which involves administering to the individual an effective amount of a neutralizing agent specific for CXCL10. Further provided is a method of extending corneal graft survival following corneal transplantation in an individual, which involves administering to said individual an effective amount of a neutralizing agent specific for CXCL10. Additionally, the invention provides a method for identifying a compound for reducing ocular inflammation in an animal.
Owner:LANE THOMAS E +1
Eye bonding sealant glue and preparation method thereof
ActiveCN110384822AShort operating timeStrong adhesionSurgical adhesivesPharmaceutical delivery mechanismAfter treatmentPenetrating Keratoplasties
The invention relates to eye bonding sealant glue and a preparation method thereof. Hydrogel is prepared from a first solution and a second solution, the first solution contains a first component which is formylation hyaluronic acid, and the second solution contains a second component which is amination hyaluronic acid. The preparation method comprises the step that the first solution and the second solution are mixed. The hydrogel can be used for sealant bonding treatment after eye operation treatment, for example, suture of penetrating keratoplasty or sealant of cornea trauma, repairing of retina with eye ground frozen or laser treatment and the like. Compared with a suture line, according to the eye bonding sealant glue, the needed operation time is shorter, the skill requirements of doctors are lower, the wound tension is more uniform, and astigmatism is not prone to occurring; and in addition, the anchoring strength of the hydrogel is high, and the implementation effect is good.
Owner:JIANGSU DISSUETECH MEDICAL TECH CO LTD
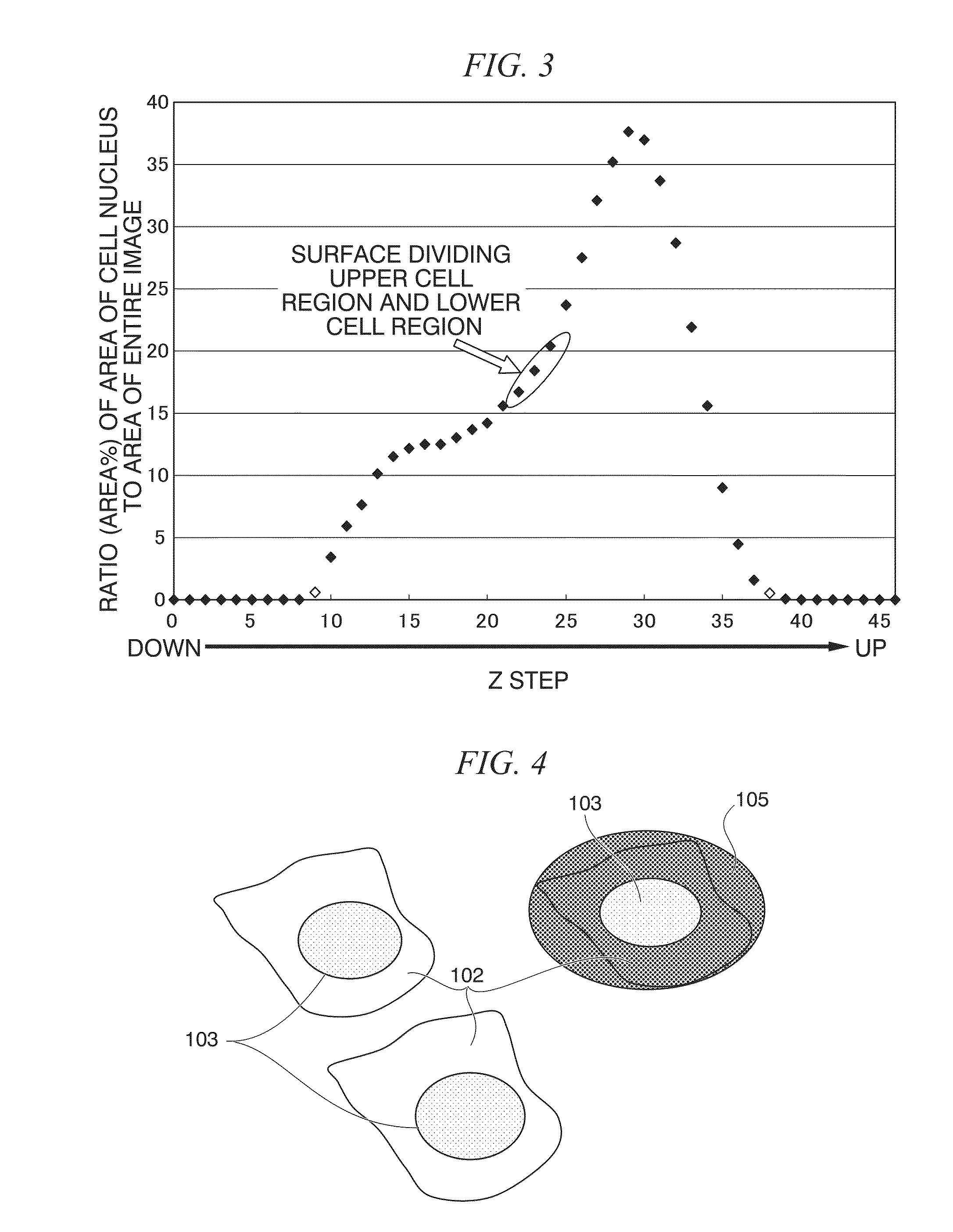
Method of analyzing image of cell in laminated structure and method of evaluating laminated structure for corneal transplantation
ActiveUS20140065639A1Efficient analysisEffective evaluationBiological testingFluorescence/phosphorescenceFluorescenceCell layer
A method of analyzing an image of a cell in a laminated structure may include the steps of: (a) fluorescently labeling a cell nucleus in the laminated structure having at least one cell layer and one or more other types of biomolecules; (b) acquiring a plurality of planar tomographic fluorescent labeled images in different height directions from the laminated structure for each type of fluorescently labeled biomolecules after the step (a); (c) superimposing a planar tomographic fluorescent labeled image group acquired in the step (b) to construct a three-dimensional tomographic image; (d) dividing the three-dimensional tomographic image constructed in the step (c) into one or two or more cell regions; (e) producing one planar stacked image for each divided cell region after the step (d); and (f) performing image analysis on each planar stacked image produced in the step (e) to analyze cells in the laminated structure.
Owner:EVIDENT CORP
Micelle loaded with Tacrolimus or pharmaceutical salts thereof, lyophilized preparation as well as preparation methods and applications
InactiveCN102198082ASimple preparation processEasy to operateOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolLung transplantation
The invention belongs to the technical field of pharmaceutical dosage forms and nano medicine preparation, and more particularly relates to a micelle loaded with Tacrolimus or pharmaceutical salts thereof, a lyophilized preparation as well as preparation methods and applications thereof. In order to achieve the clinical application of the micelle provided by the invention, the drug loading, the encapsulation rate and the stability of the preparation need to be ensured. The micelle is prepared from biodegradable polymer materials and Tacrolimus or pharmaceutical salts thereof in parts by weight: 1 part of Tacrolimus or pharmaceutical salts thereof and 1.5-99 parts of degradable polymer materials, wherein the degradable polymer materials are diblock polymer methoxy polyethylene glycol-polycaprolactone (the molecular weight ratio of MPEG to PCL is 0.5-2), and triblock polymer polycaprolactone-polyethylene glycol-polycaprolactone (the molecular weight ratio of PCL to PEG is 0.5-3). The micelle using Tacrolimus or pharmaceutical salts thereof or the lyophilized preparation of the micelle can be used in medicines for anti-rejection in liver transplantation, kidney transplantation, lung transplantation, corneal transplantation and other organ transplantations.
Owner:SICHUAN UNIV
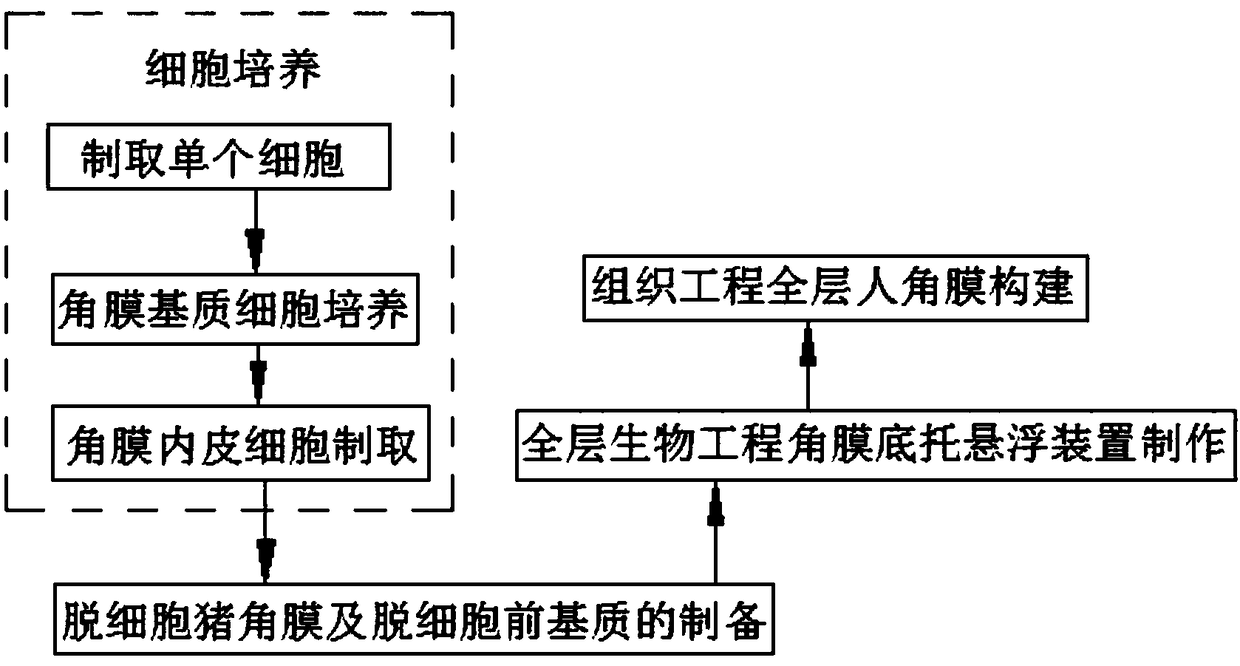
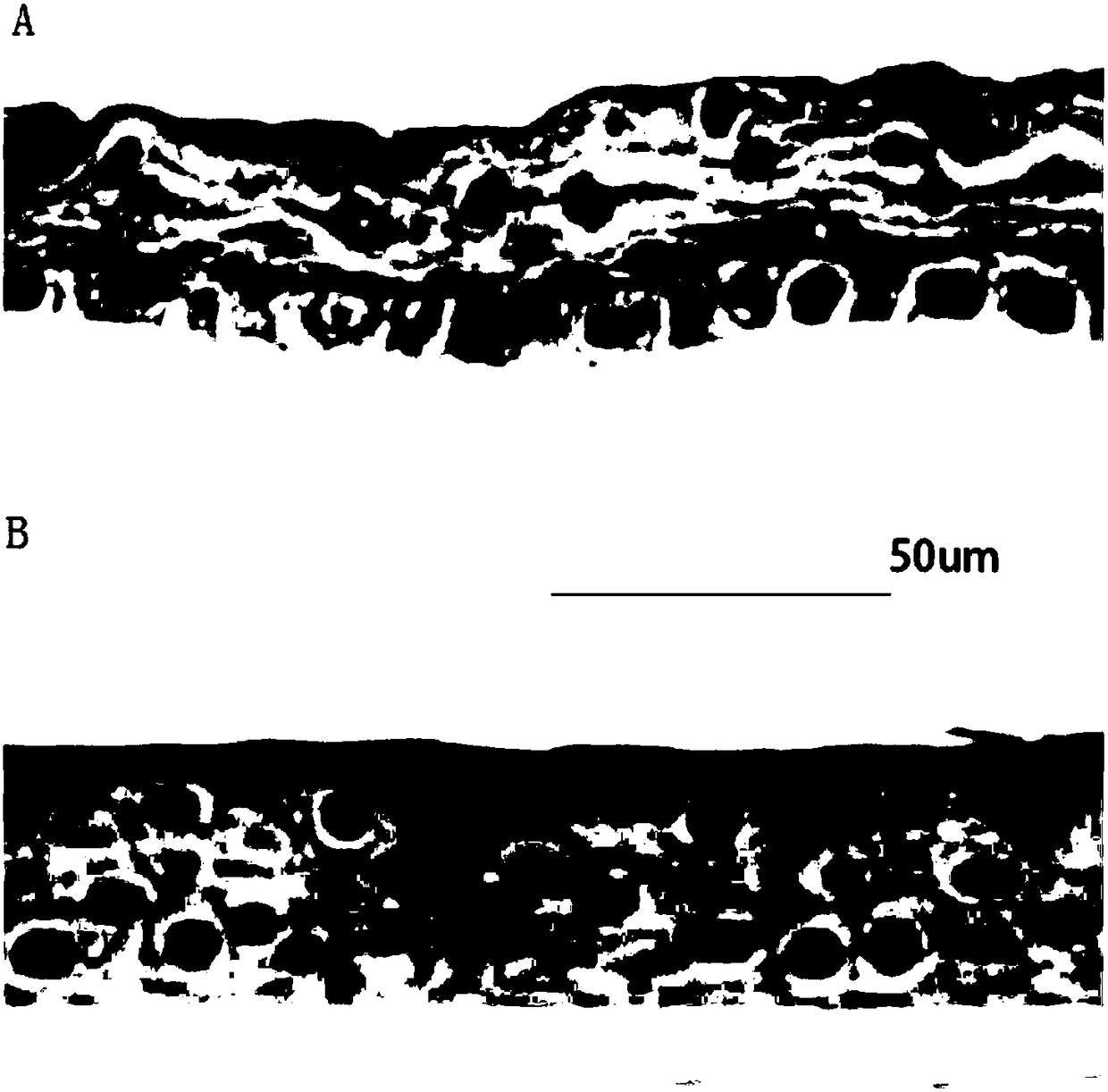
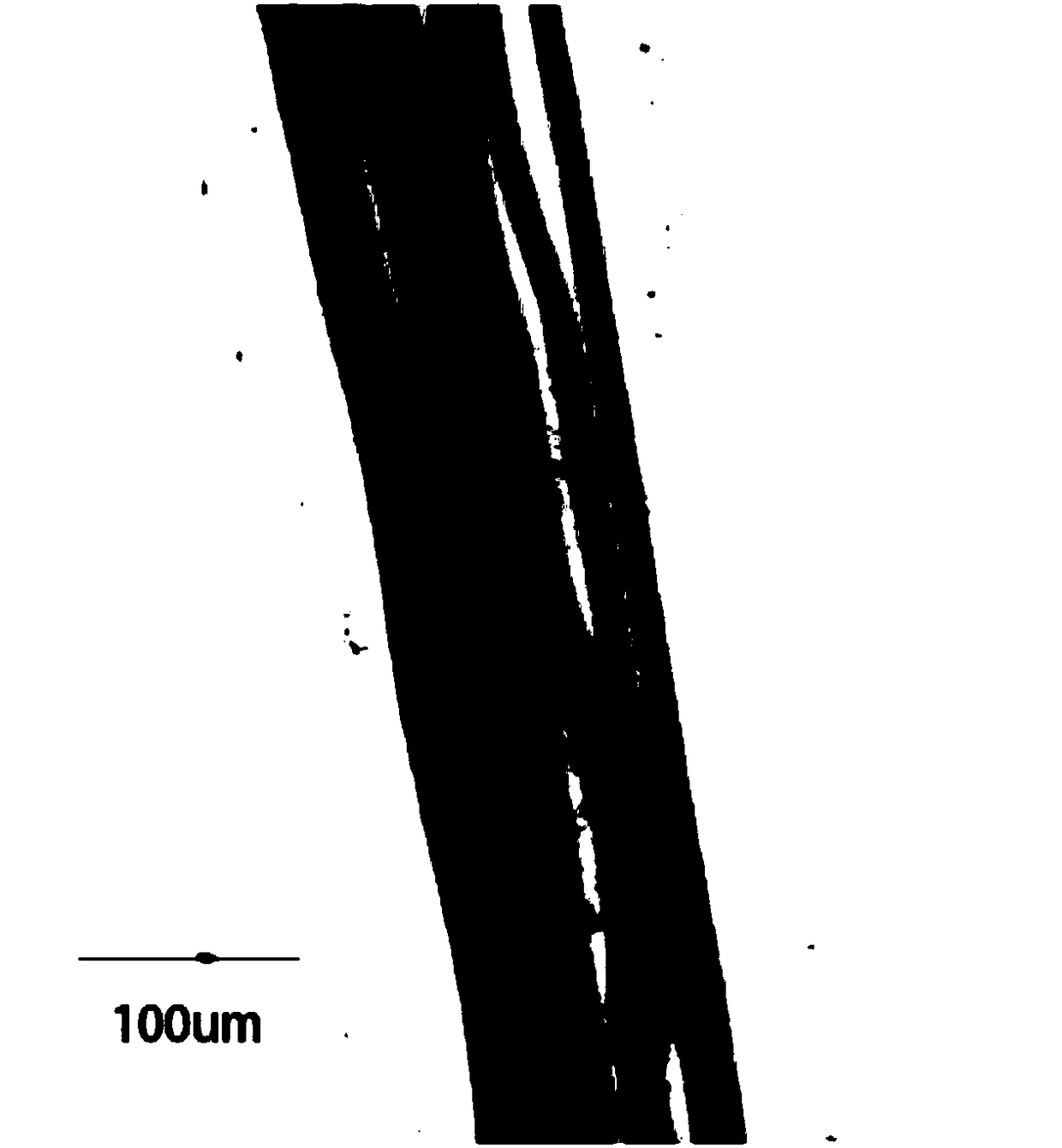
In-vitro construction method of bioengineering full-layer human cornea
InactiveCN108126240AAdequate biomechanical propertiesGood biocompatibilityNervous system cellsArtificial cell constructsPenicillinPenetrating Keratoplasties
The invention discloses an in-vitro construction method of bioengineering full-layer human cornea. Cell culture comprises preparation of single cell, cultivation of corneal stroma cells and preparation of corneal endothelial cells. The method comprises the following steps: washing a corneosclera ring of a donor with 100 U / ml penicillin and 80 U / ml streptomycin solution wash for three times, soaking into a 2.4 U / ml Dispase 2 solution, performing incubation in an incubator for 1 hour, lightly stripping limbal epithelium through a dissecting microscope and microscopic tweezers, soaking the stripped epithelial sheet into a 0.25 percent pancreatin-0.02 percent EDTA solution, and digesting in the incubator for 5 minutes again to obtain single cell. The in-vitro construction method of the bioengineering full-layer human cornea can be applied to an in-vitro medicine toxicity experiment, more importantly, sufficient biomechanical property is achieved, traction of a suture in penetrating keratoplasty and pressure in eyeballs after operation can be tolerated, and high biocompatibility and biological security are achieved, so the bioengineering full-layer human cornea can replace the donor's cornea to be applied to corneal transplantation.
Owner:SHANDONG PROVINCIAL HOSPITAL
Method for preparing biocompatible cornea and decellularization composition for biocompatible tissue
InactiveUS20160303288A1Promote regenerationMinimize immune rejection responseAdditive manufacturing apparatusTissue regenerationCell-Extracellular MatrixECM Protein
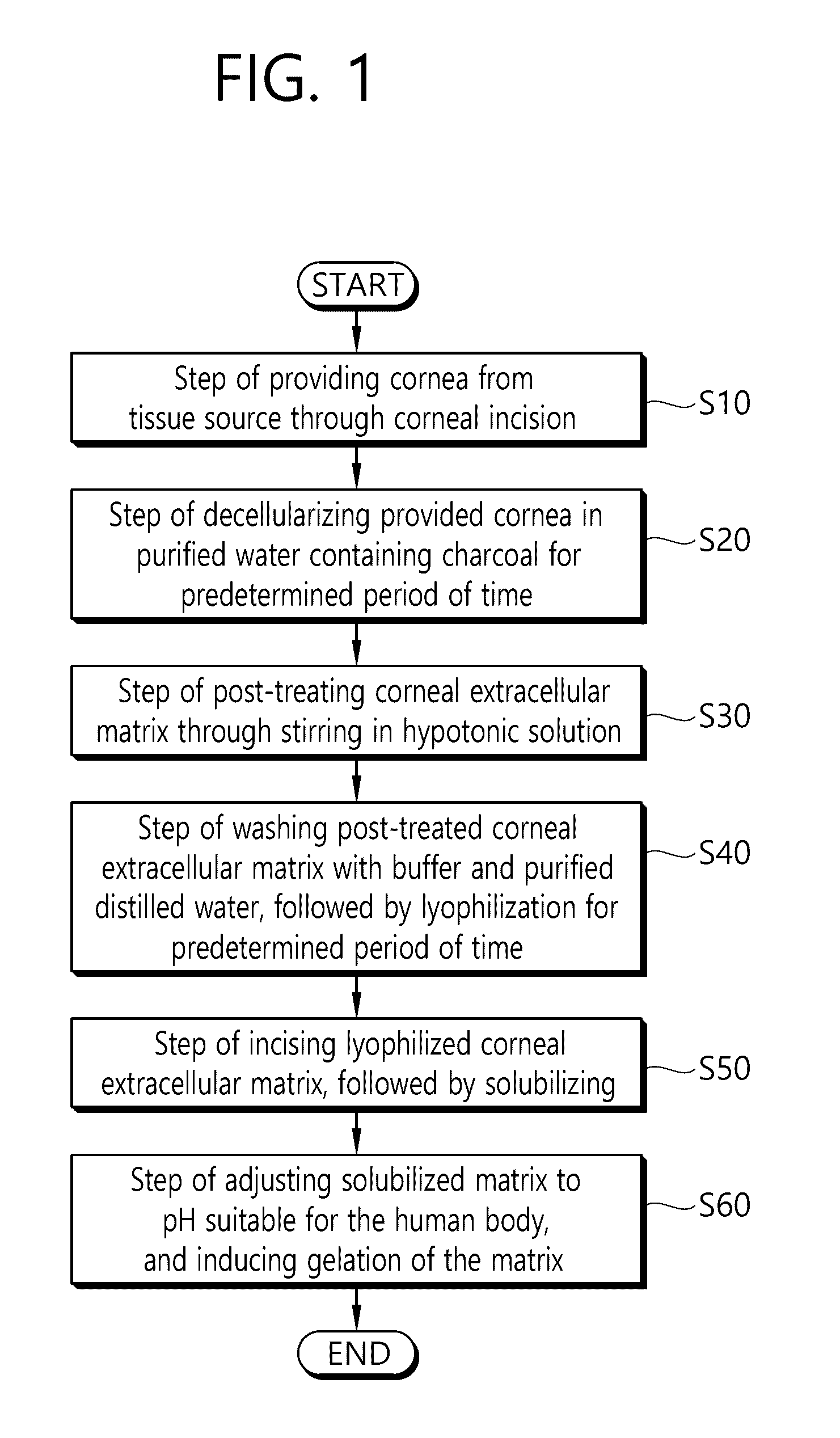
Disclosed is a method for preparing a biocompatible tissue, the method including: providing a cornea from a tissue source through corneal incision; decellularizing the provided cornea in purified water containing charcoal for a predetermined period of time; and post-treating the decellularized corneal extracellular matrix through stirring in a hypotonic solution, so that the charcoal is used as a decellularizing agent for the cornea, thereby allowing the regeneration of a corneal tissue like in the original corneal extracellular matrix, and the preparation of a cornea without an immune rejection response and a fast regeneration effect of a patient through transplantation of the cornea can be expected.
Owner:POSTECH ACAD IND FOUND
Whole eye cornea bioengineering cultivation method
InactiveCN108277204AAvoid rejectionAvoid finitenessNervous system cellsCell culture supports/coatingBiocompatibility TestingCornea layer
The invention relates to the field of cell culture and particularly relates to a whole eye cornea bioengineering cultivation method. The various layers of the cornea are respectively cultivated, and the advantage is that only a cornea layer needed by a patient is cultivated when the patient does not need the transplantation of the whole cornea. According to the provided preparation method of the whole cornea, a seed cell between the cornea epidermal layer and a mesenchymal layer is an autologous cell, a corneal endothelial cell is from a donator, a large quantity of endothelial cells are obtained through cultivation, and the limit of the source of the donator is avoided. A collagen culture medium is taken as a gluing medium between the epidermal layer and the mesenchymal layer, and the intraneural growth of the human bioengineering cornea is promoted. The provided whole cornea and partial cornea both have an excellent optical property, the patients all recovers the eyesight after the operation, the cornea has a stable biochemical characteristic, the dissolution of the cornea in the later period does not exist, the biocompatibility is excellent, and no rejection reaction is caused.
Owner:山东麦德克斯生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com