Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
165 results about "Full thickness" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
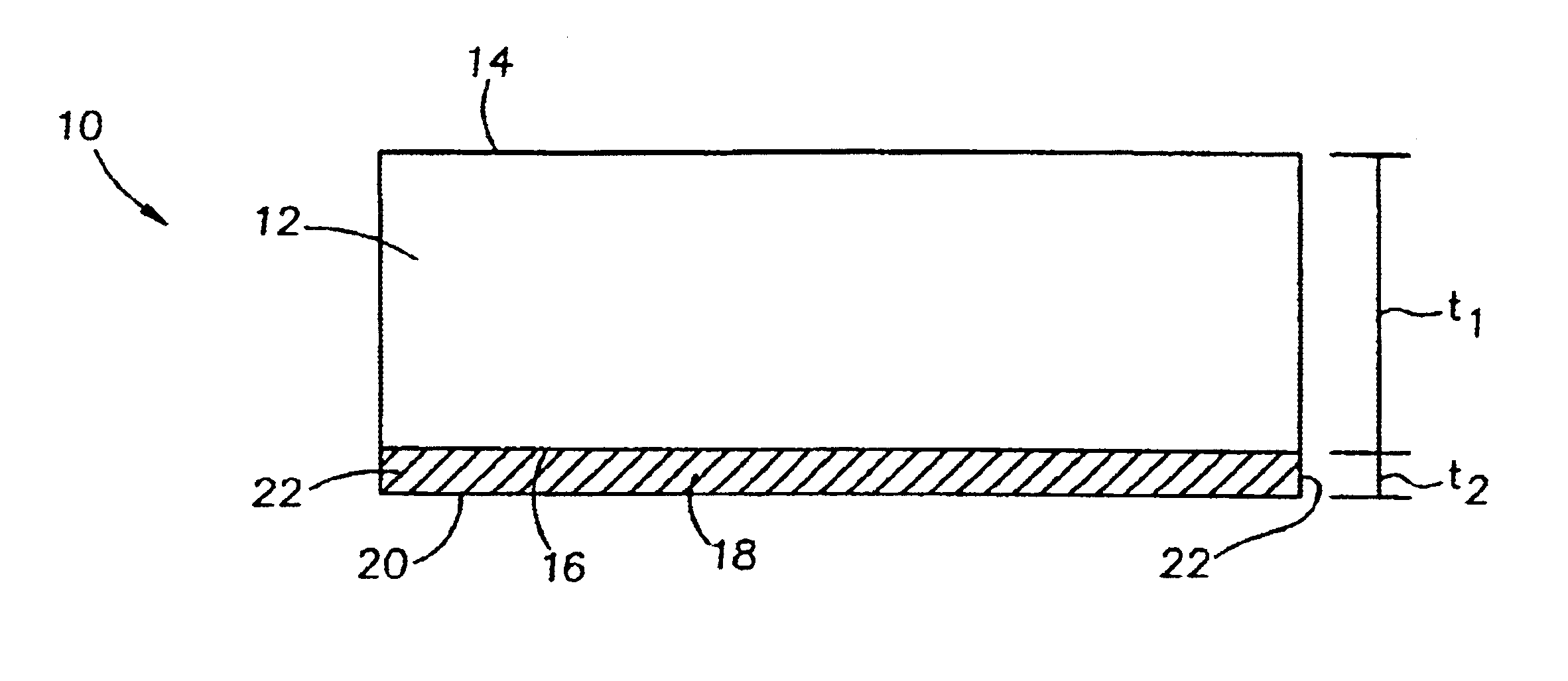
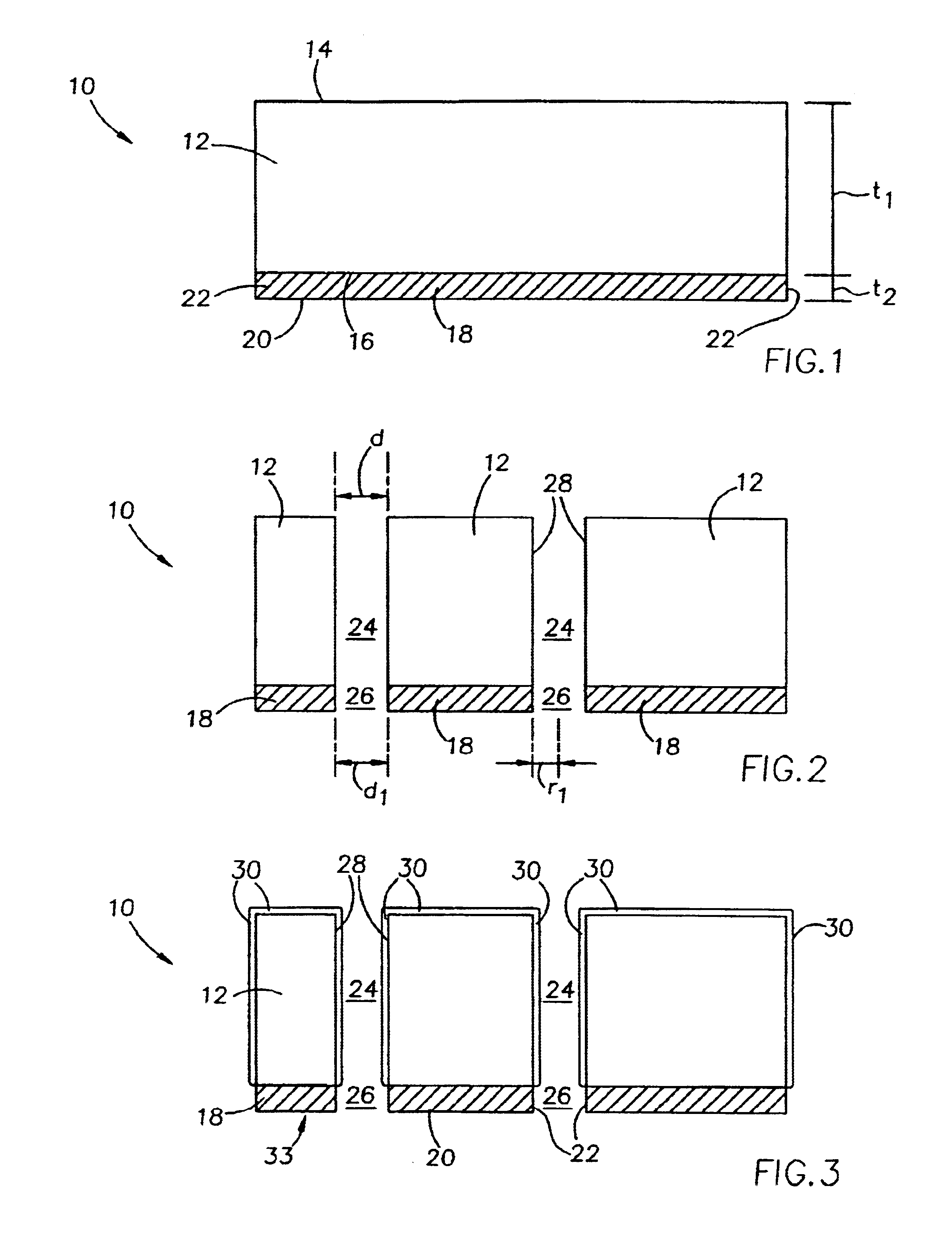
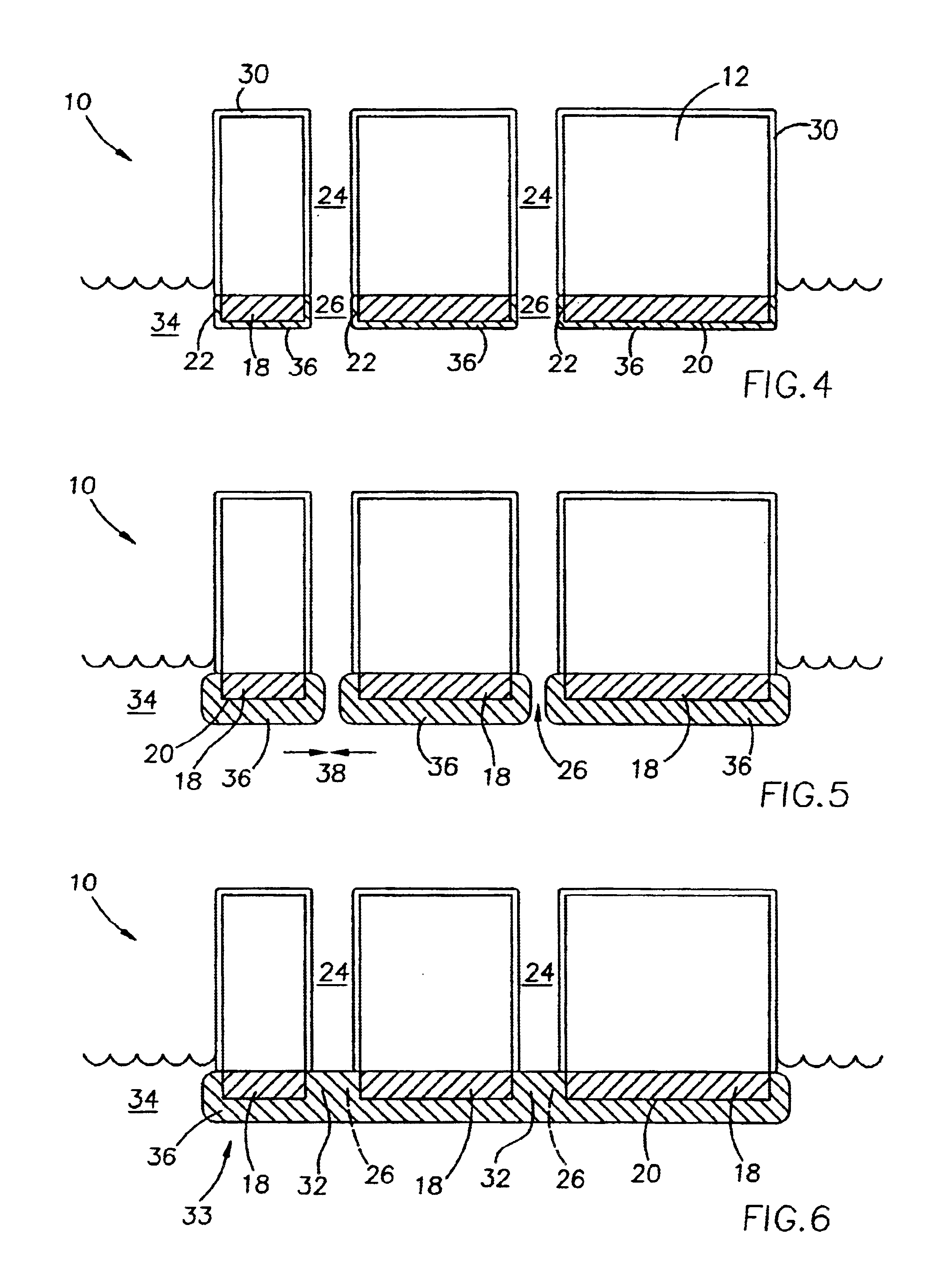
Full thickness wounds are wounds that extend past the two layers of skin (dermis and epidermis) and extend into the subcutaneous tissue (fat and muscle). Some full thickness wounds may even extend all the way to the bone.
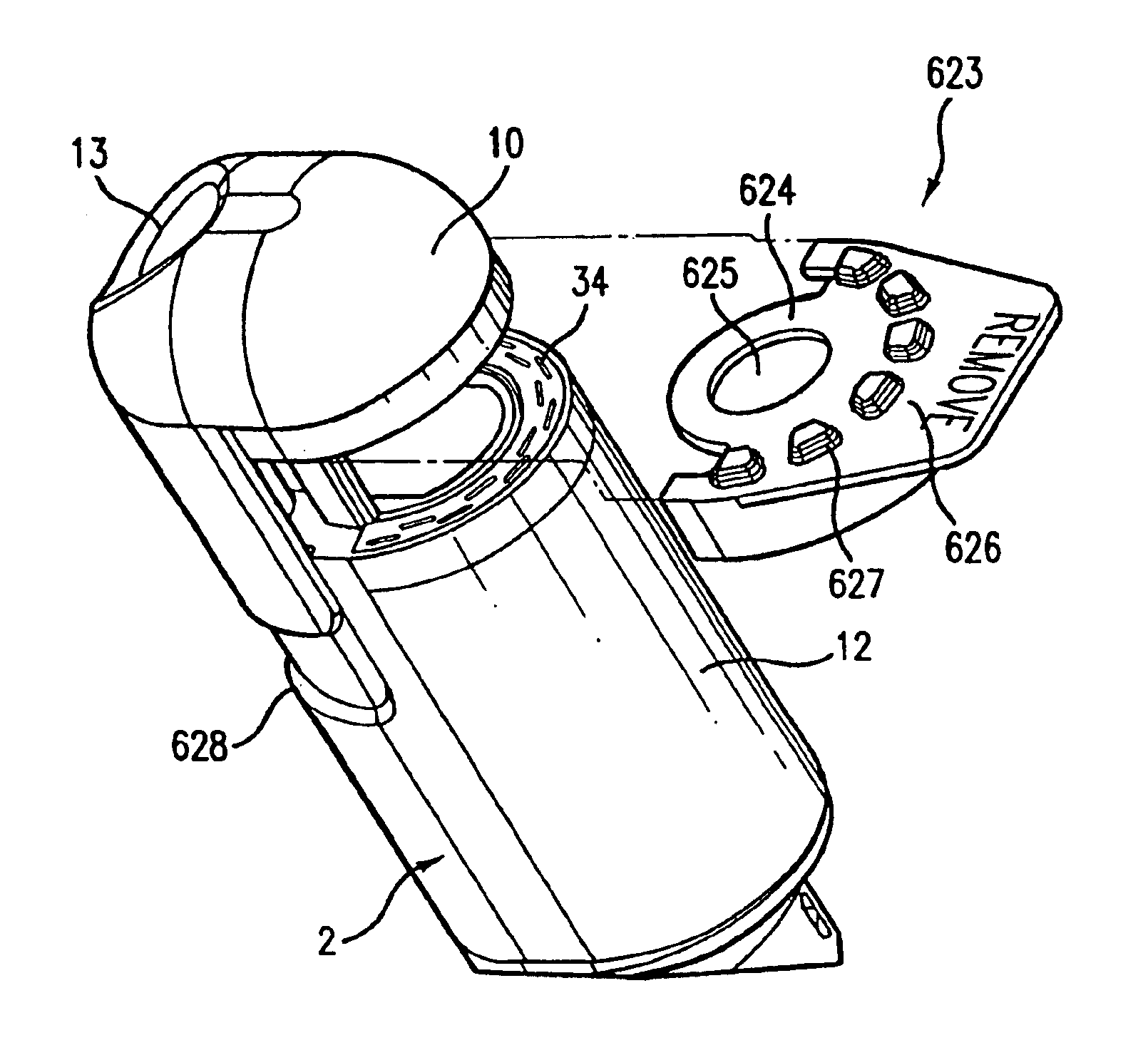
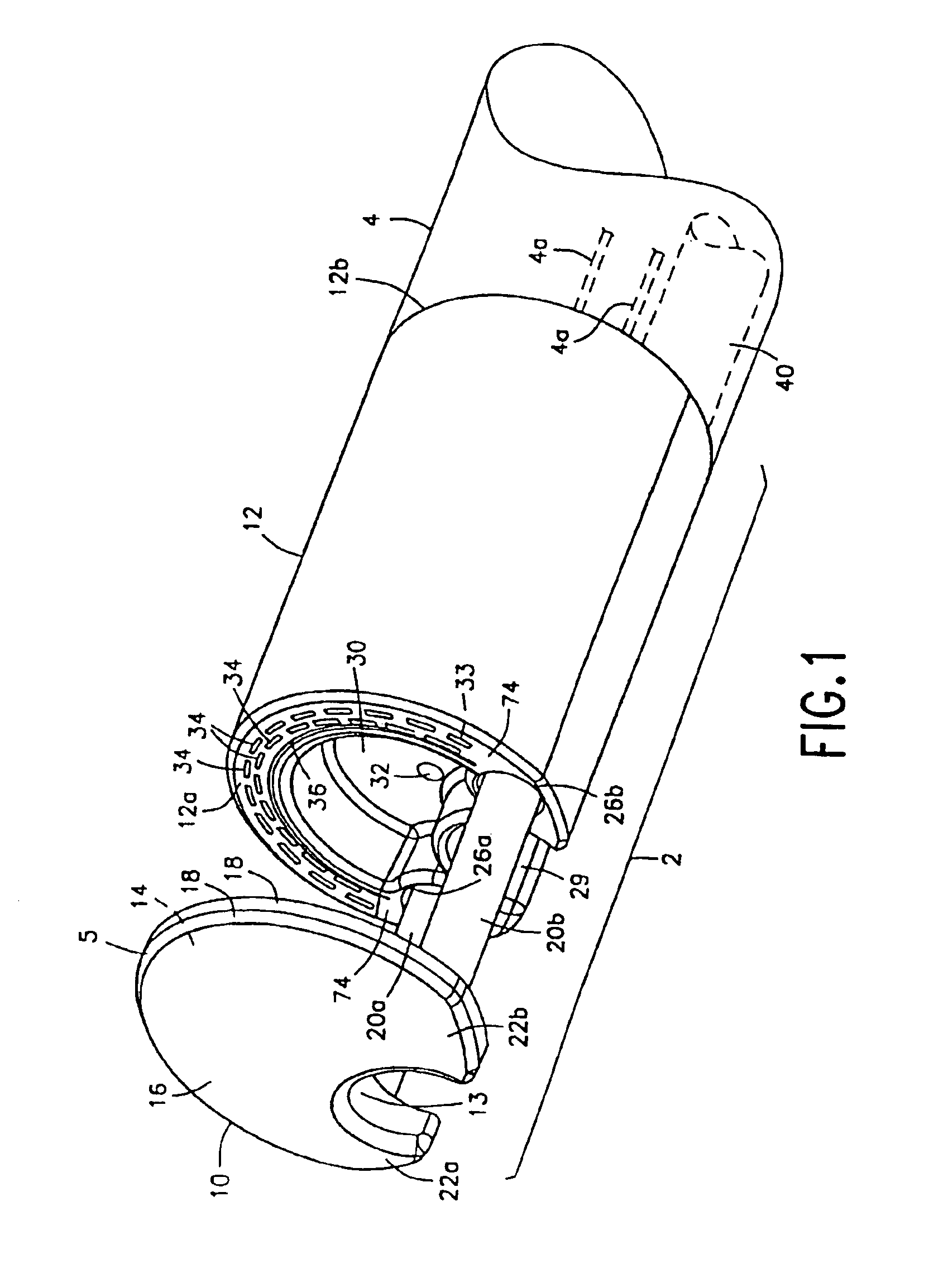
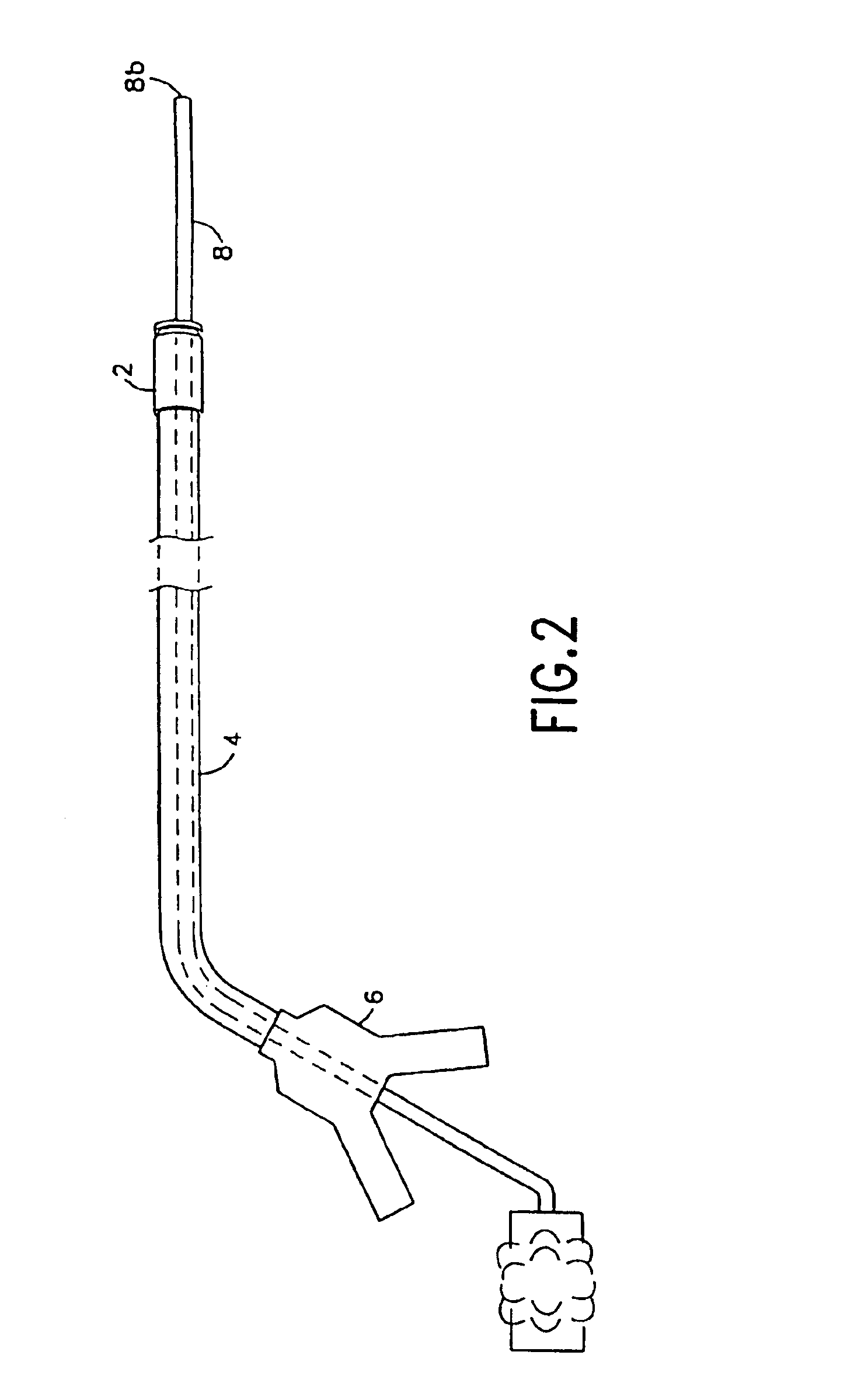
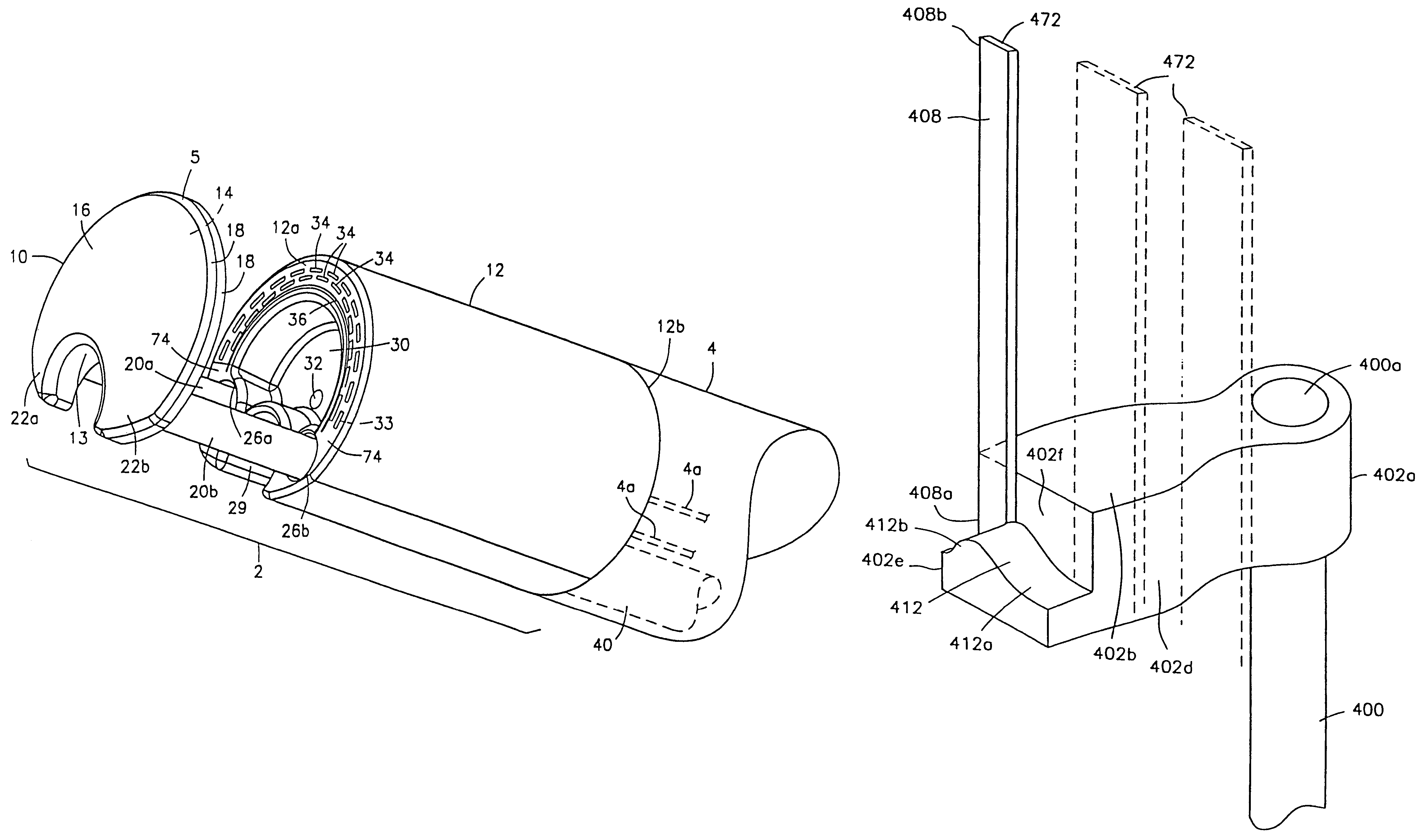
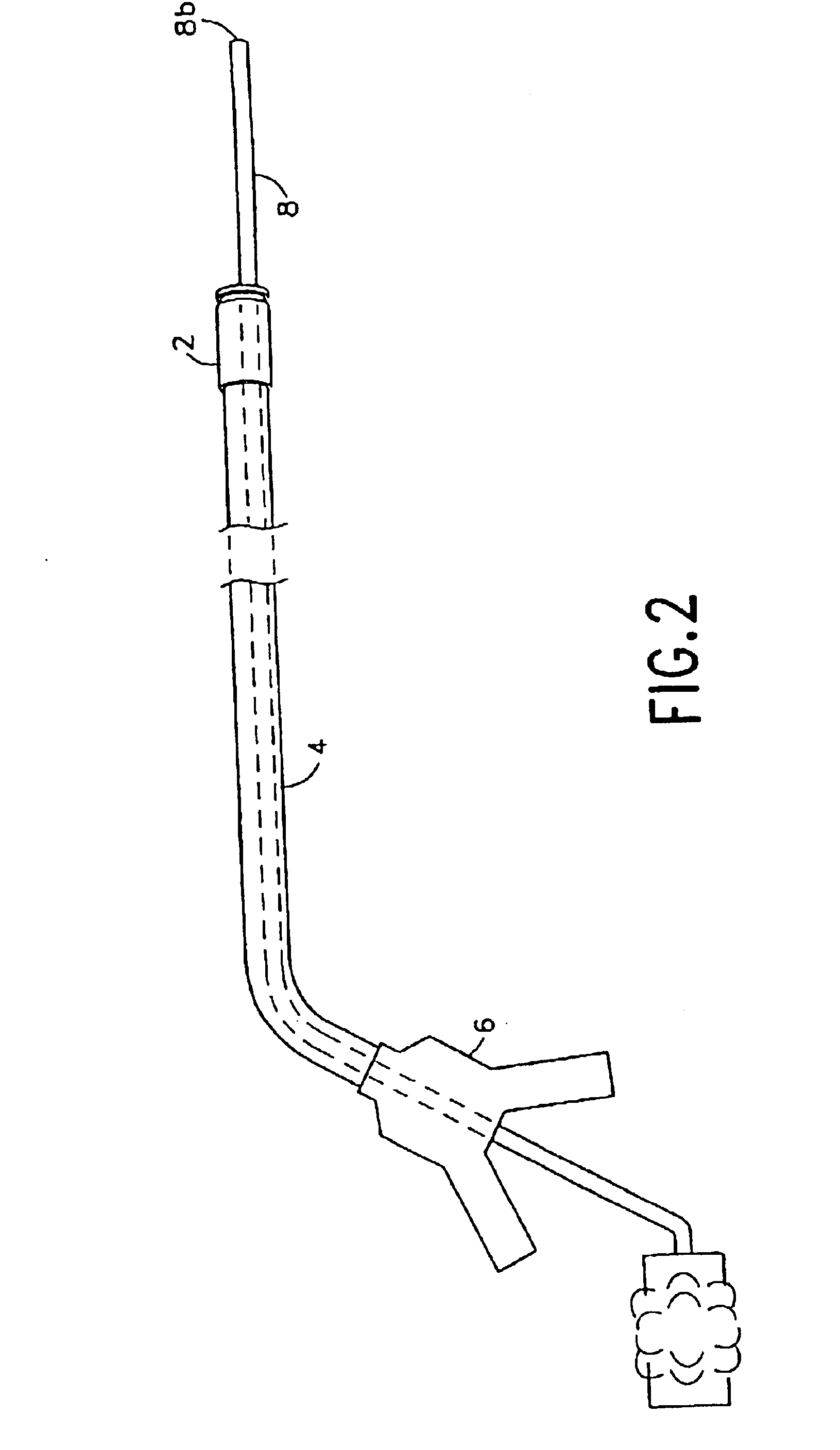
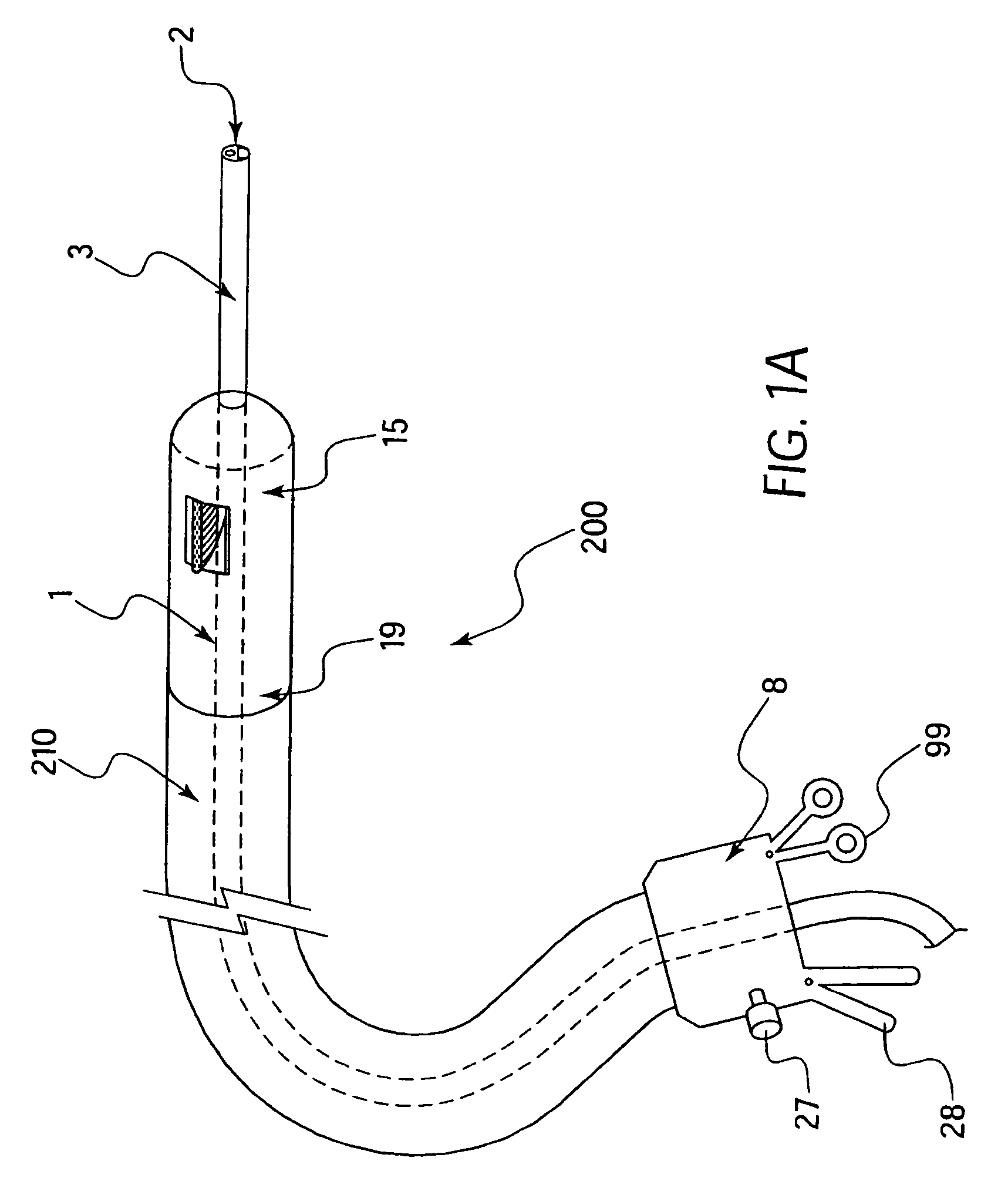
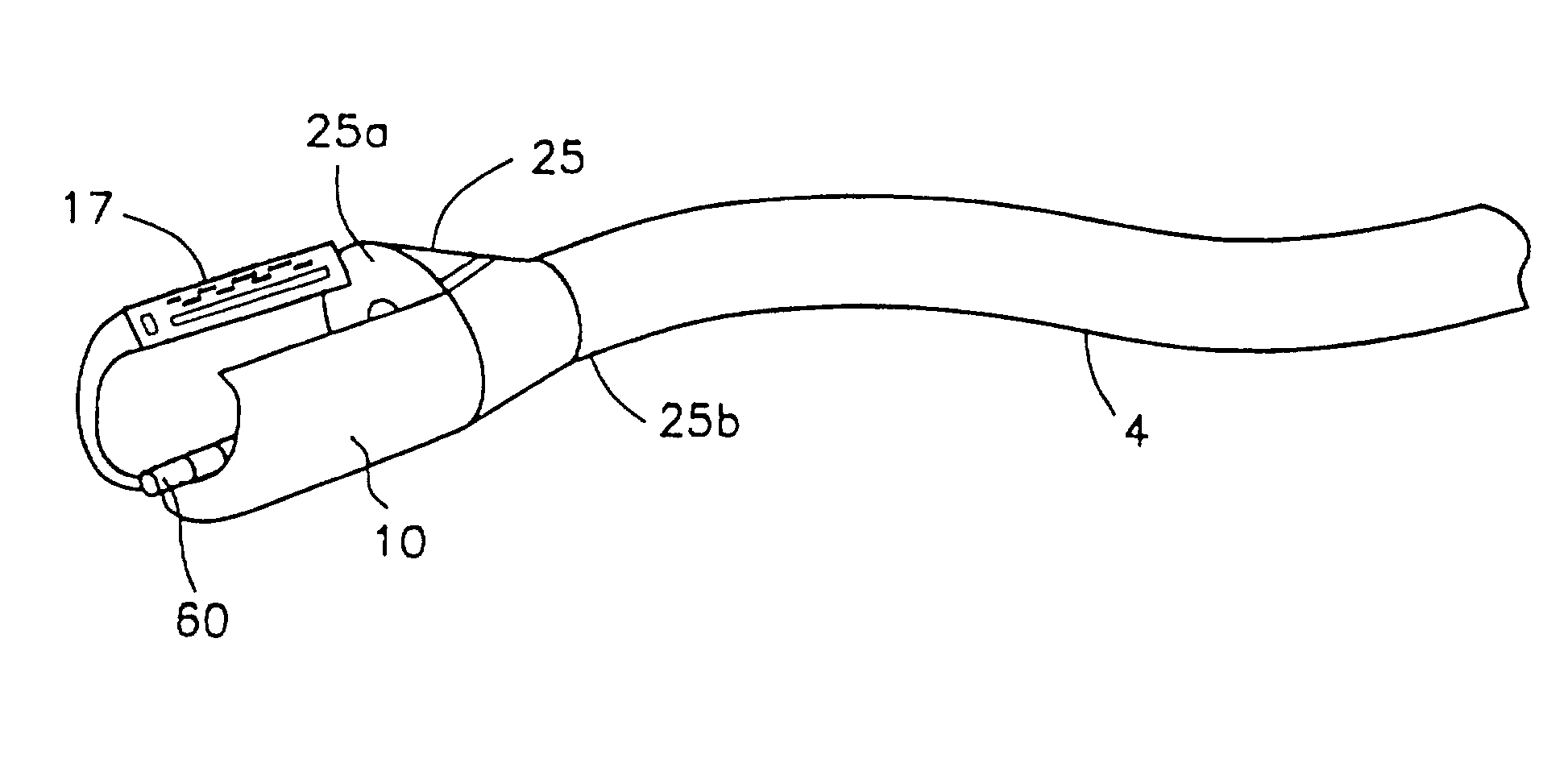
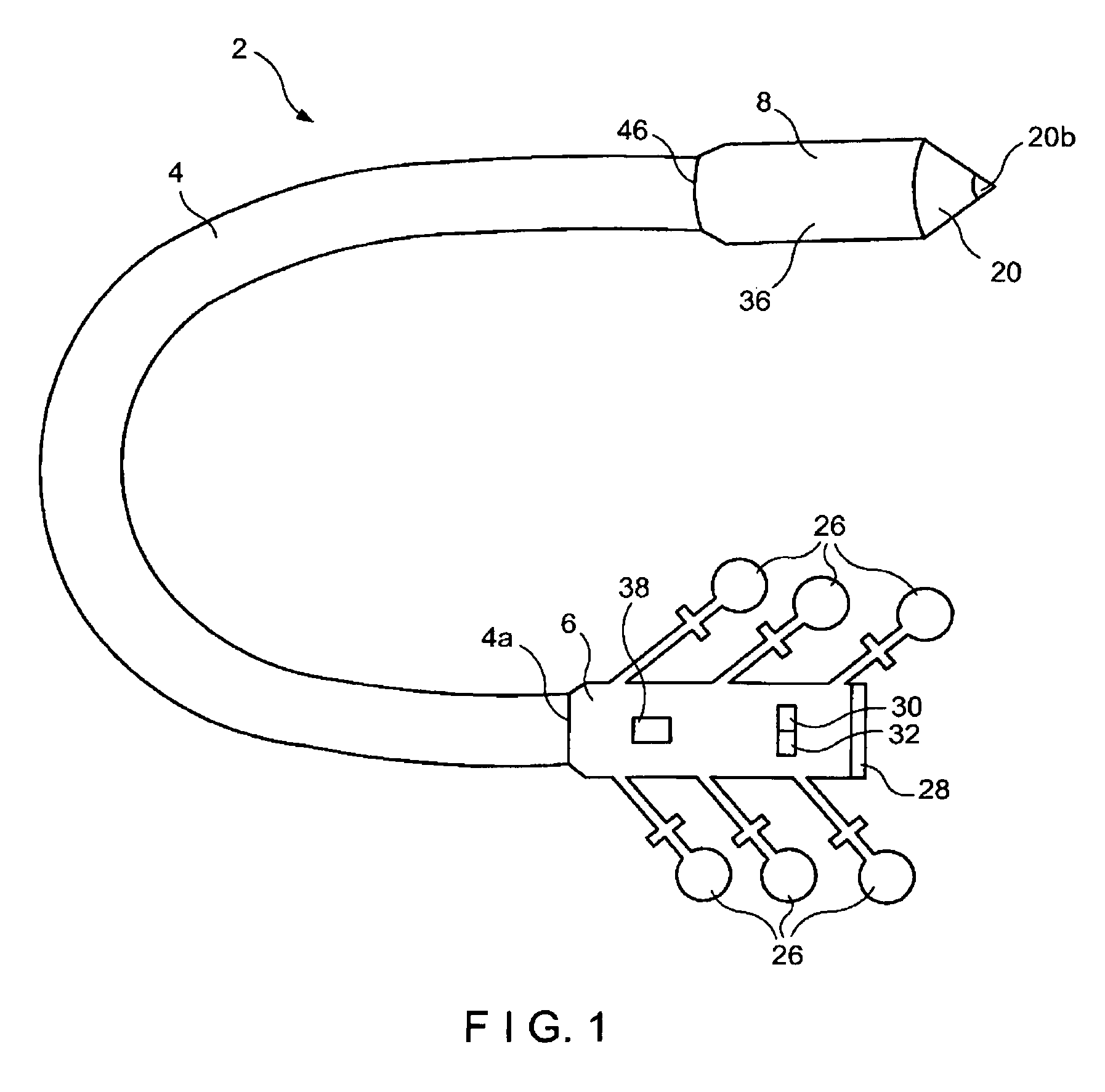
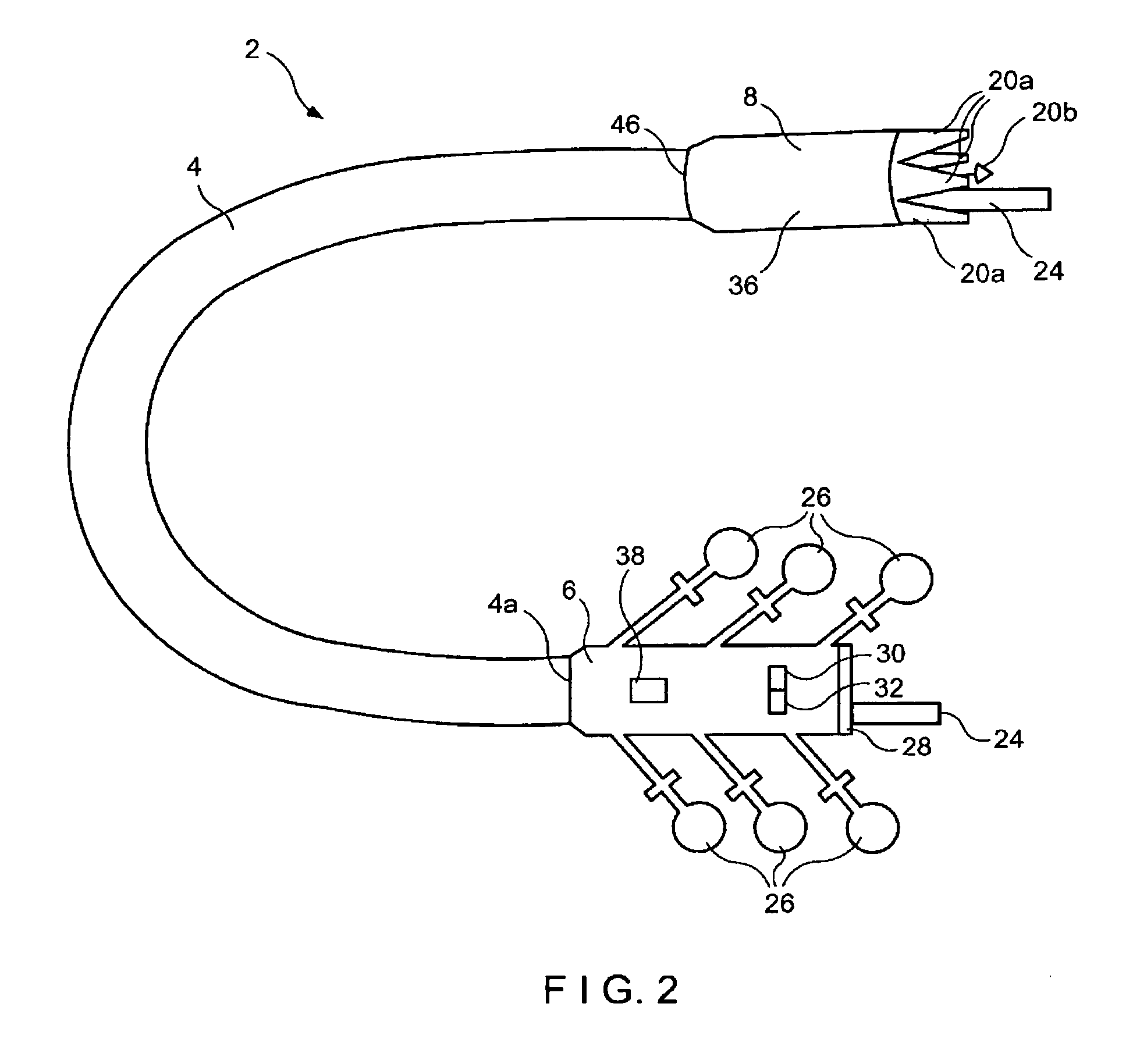
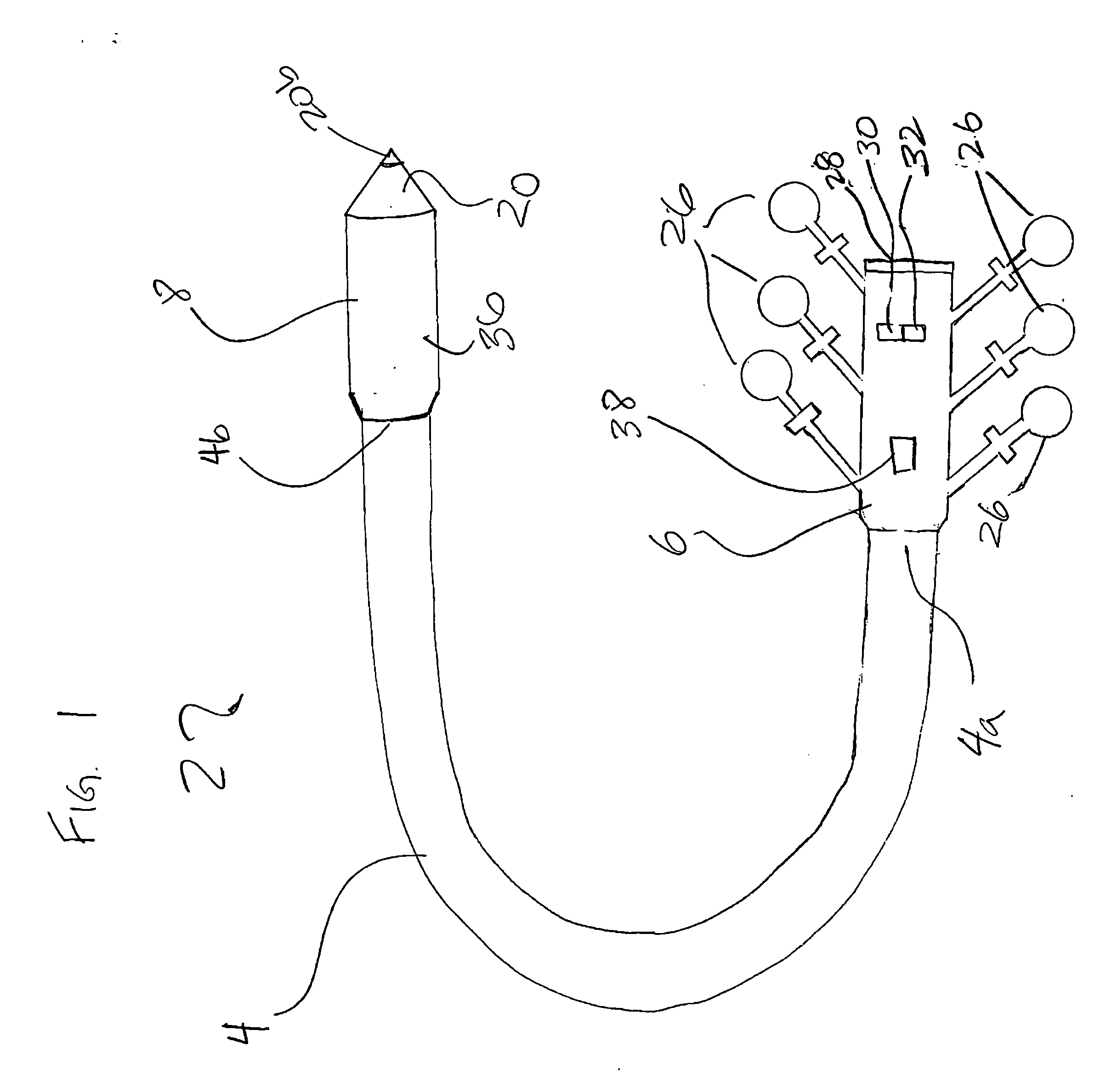
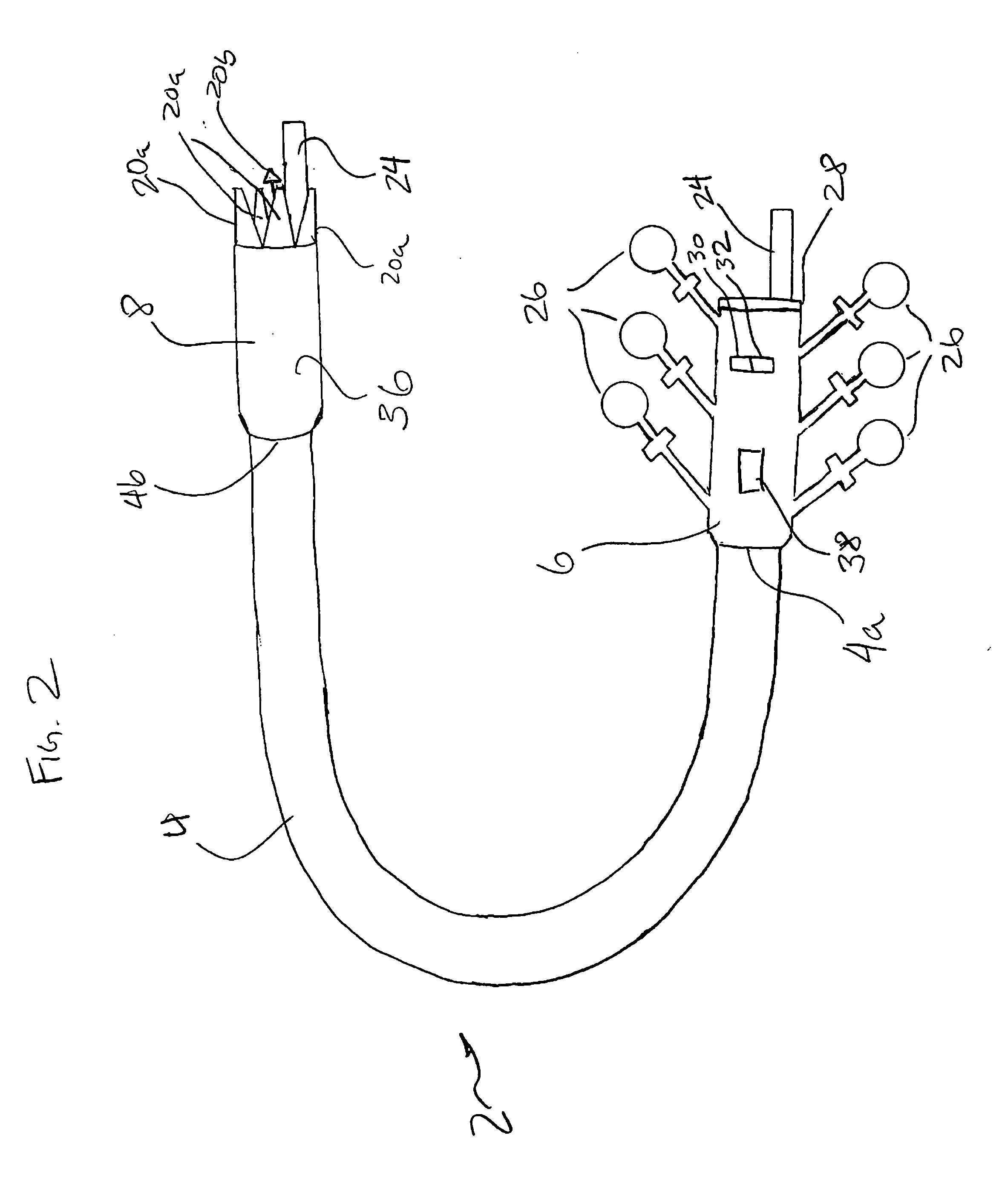
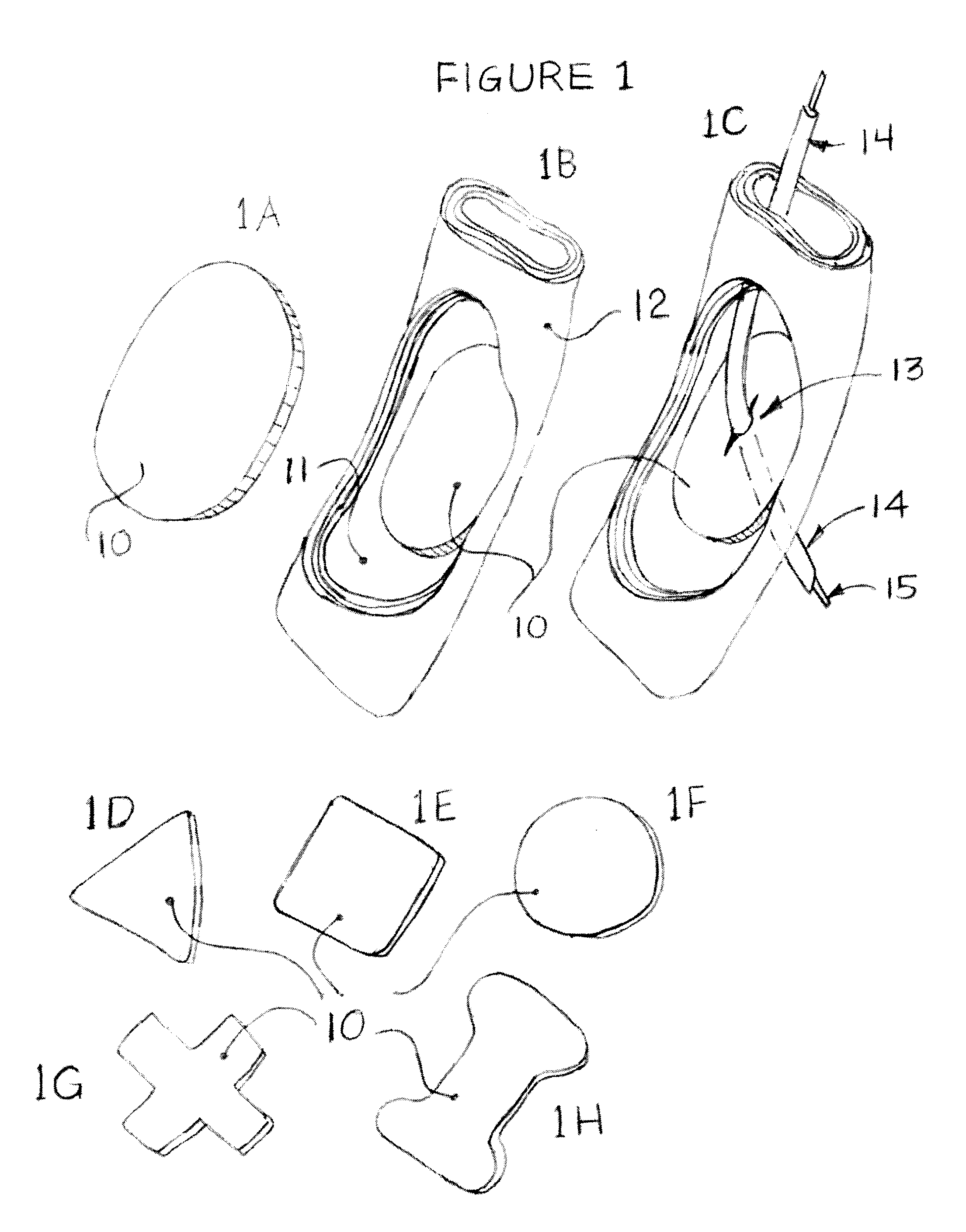
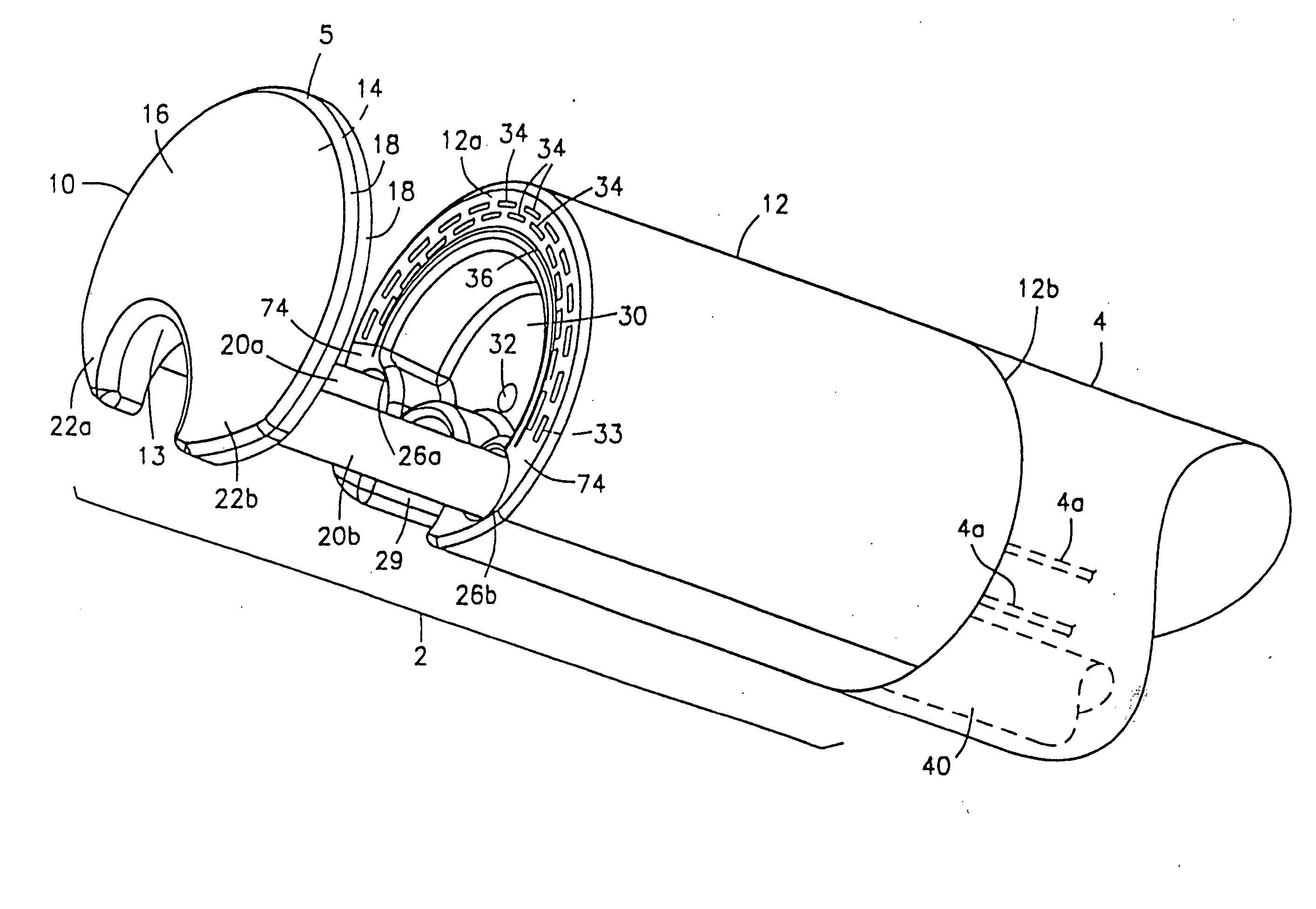
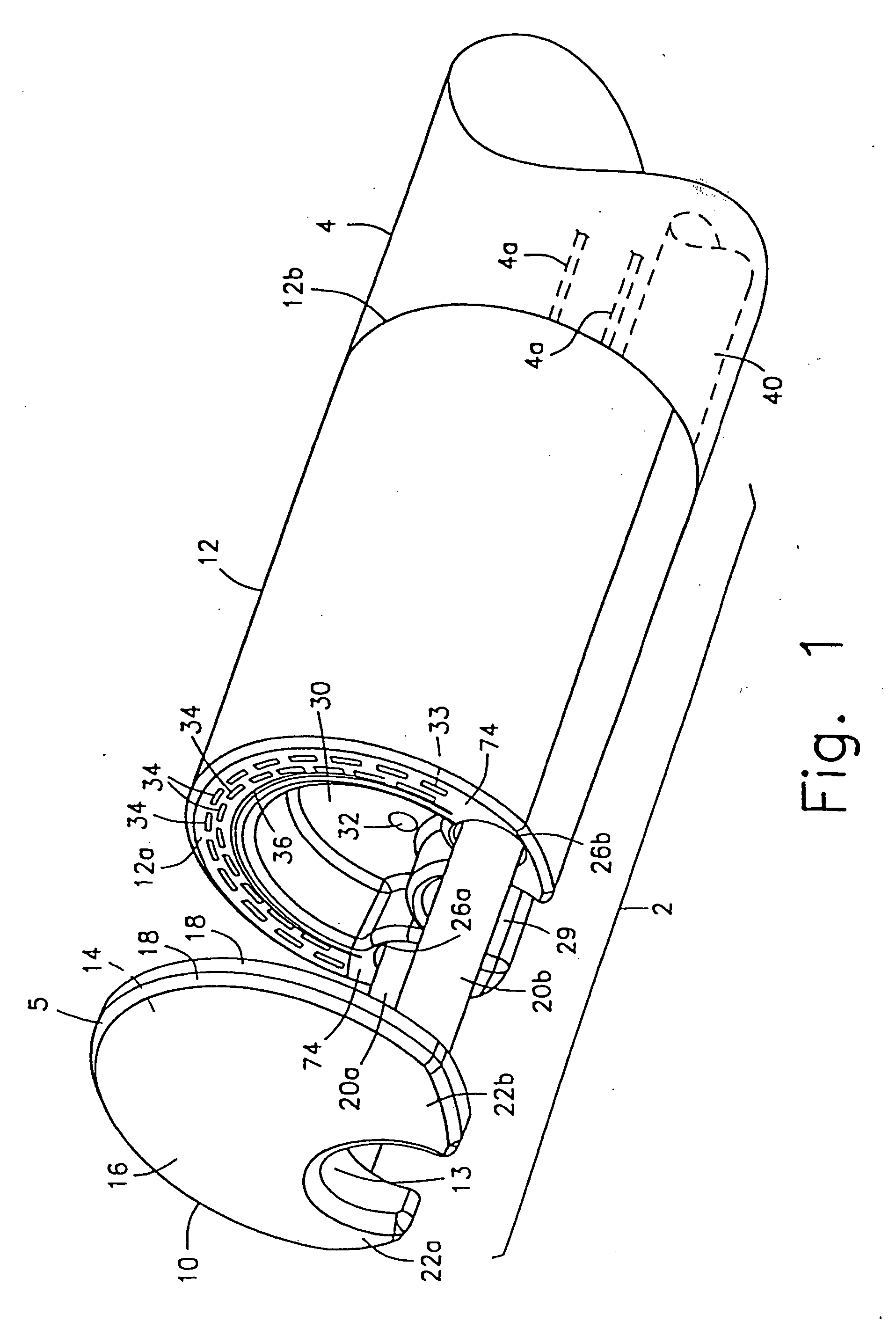
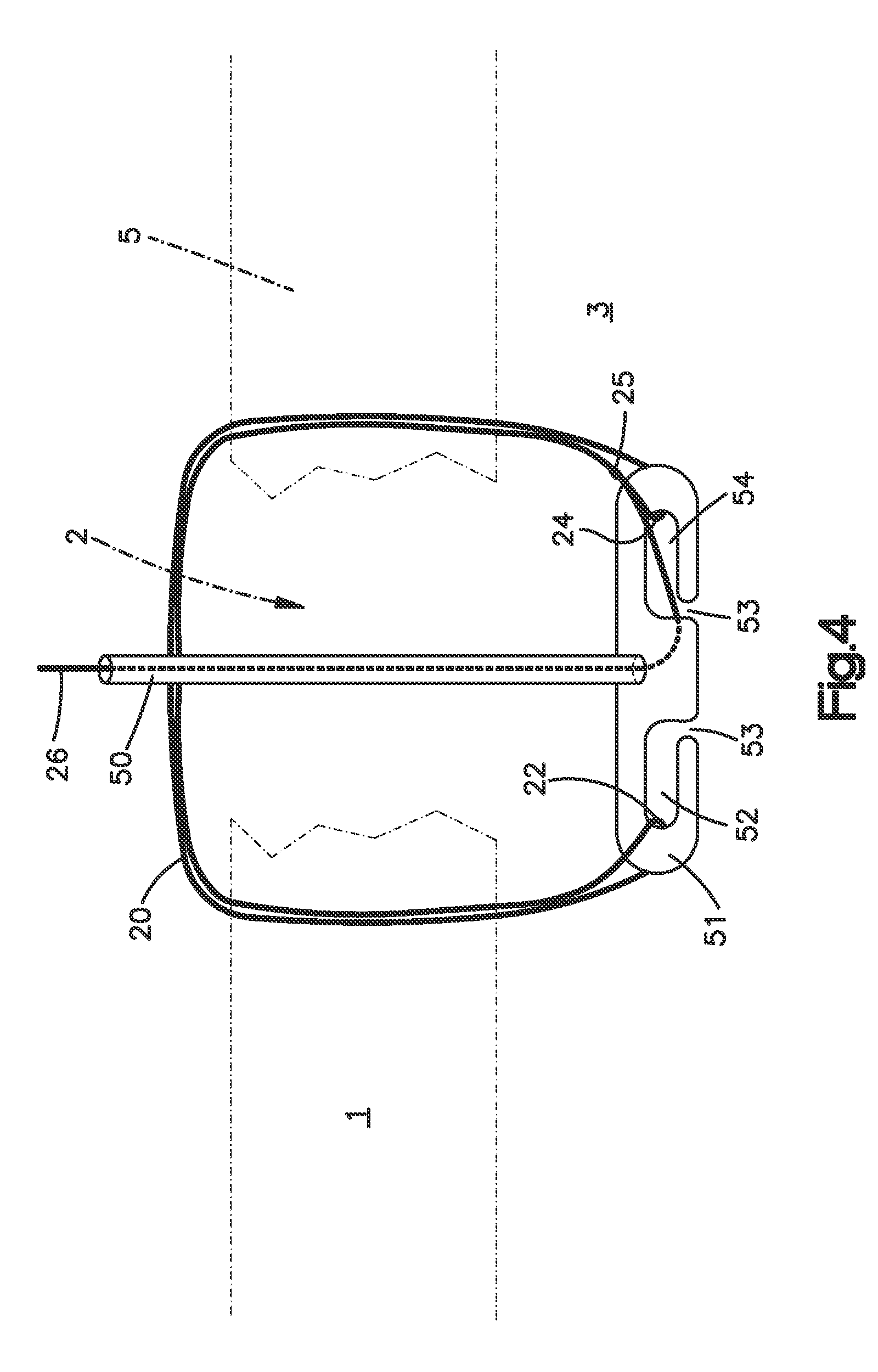
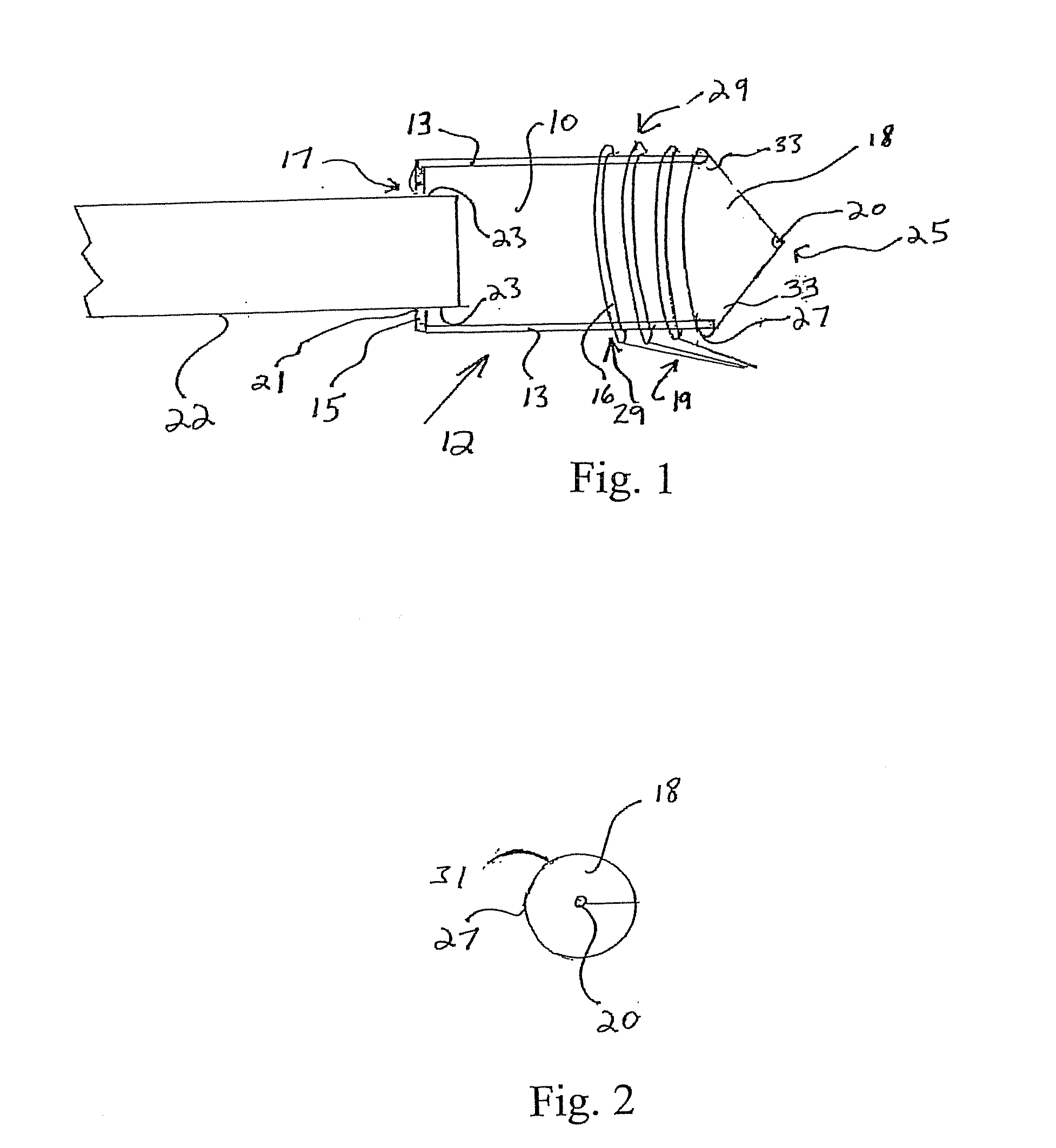
Integrated surgical staple retainer for a full thickness resectioning device
The present invention is directed to a full-thickness resection system comprising a flexible endoscope and a stapling mechanism, wherein the endoscope is slidably received through at least a portion of the stapling mechanism. The stapling mechanism includes an anvil and a stapling head mounted to the anvil so that the anvil and the stapling head are moveable with respect to one another. A position adjusting mechanism is provided for moving the anvil and the stapling head relative to one another.An integrated surgical retainer for use in the full thickness resectioning device is described. The integrated surgical staple retainer comprises a calibrating portion, a retaining portion, and a grasping portion. The calibrating portion defines a circular opening which has a diameter substantially equal to a diameter of a working channel of the full thickness resectioning device. The retaining portion has a lower surface adapted to limit movement of the surgical staples in the full thickness resectioning device and is adjacent to the calibrating portion of the integrated surgical staple retainer.
Owner:BOSTON SCI SCIMED INC
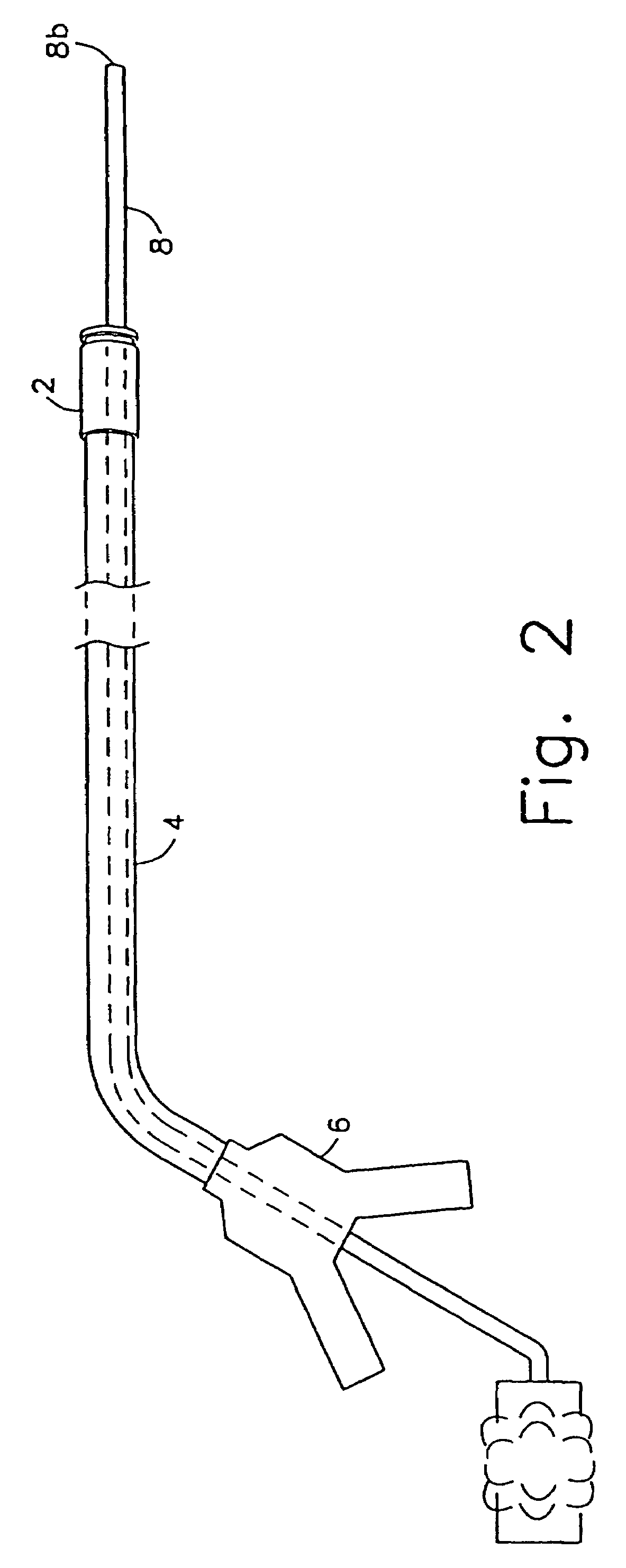
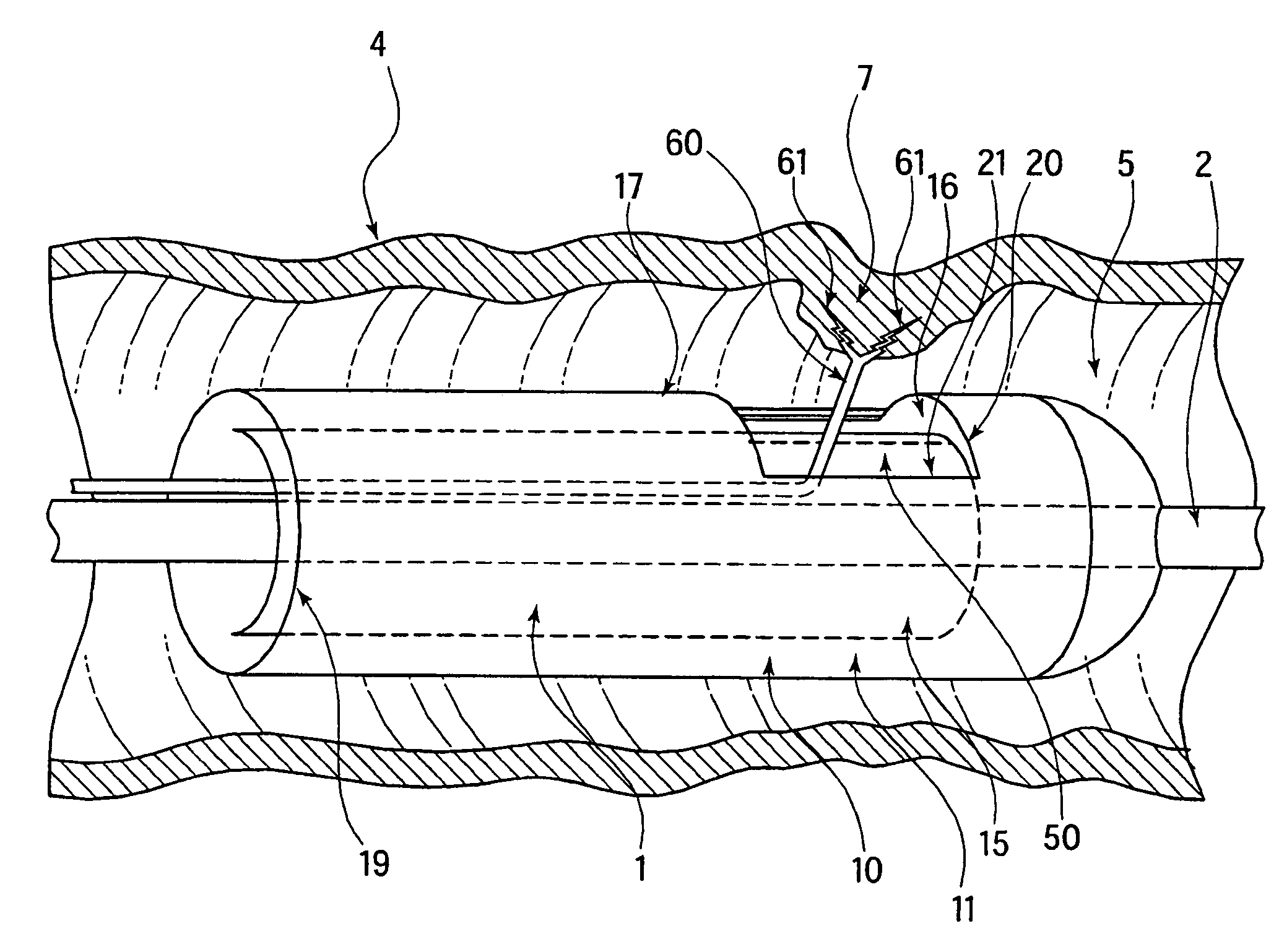
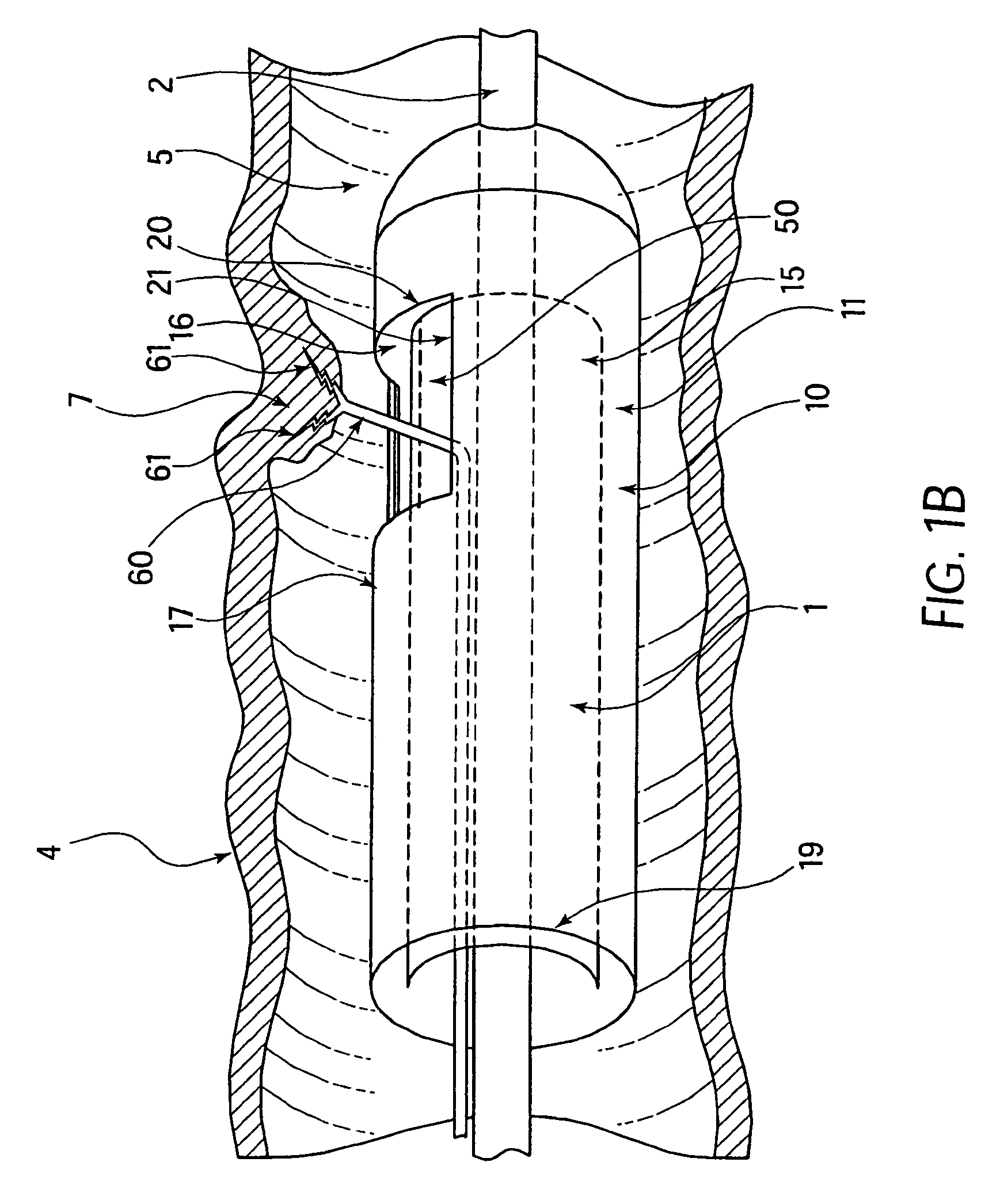
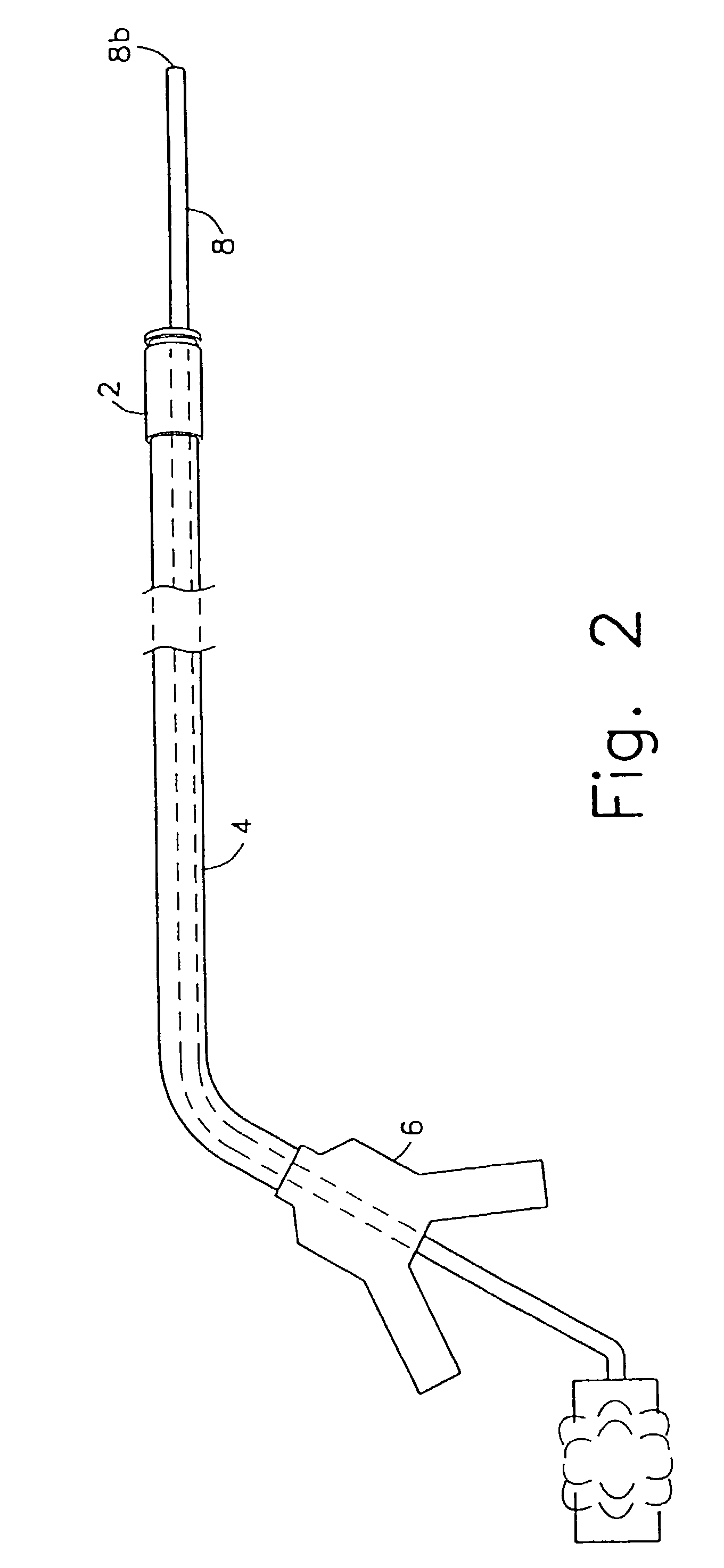
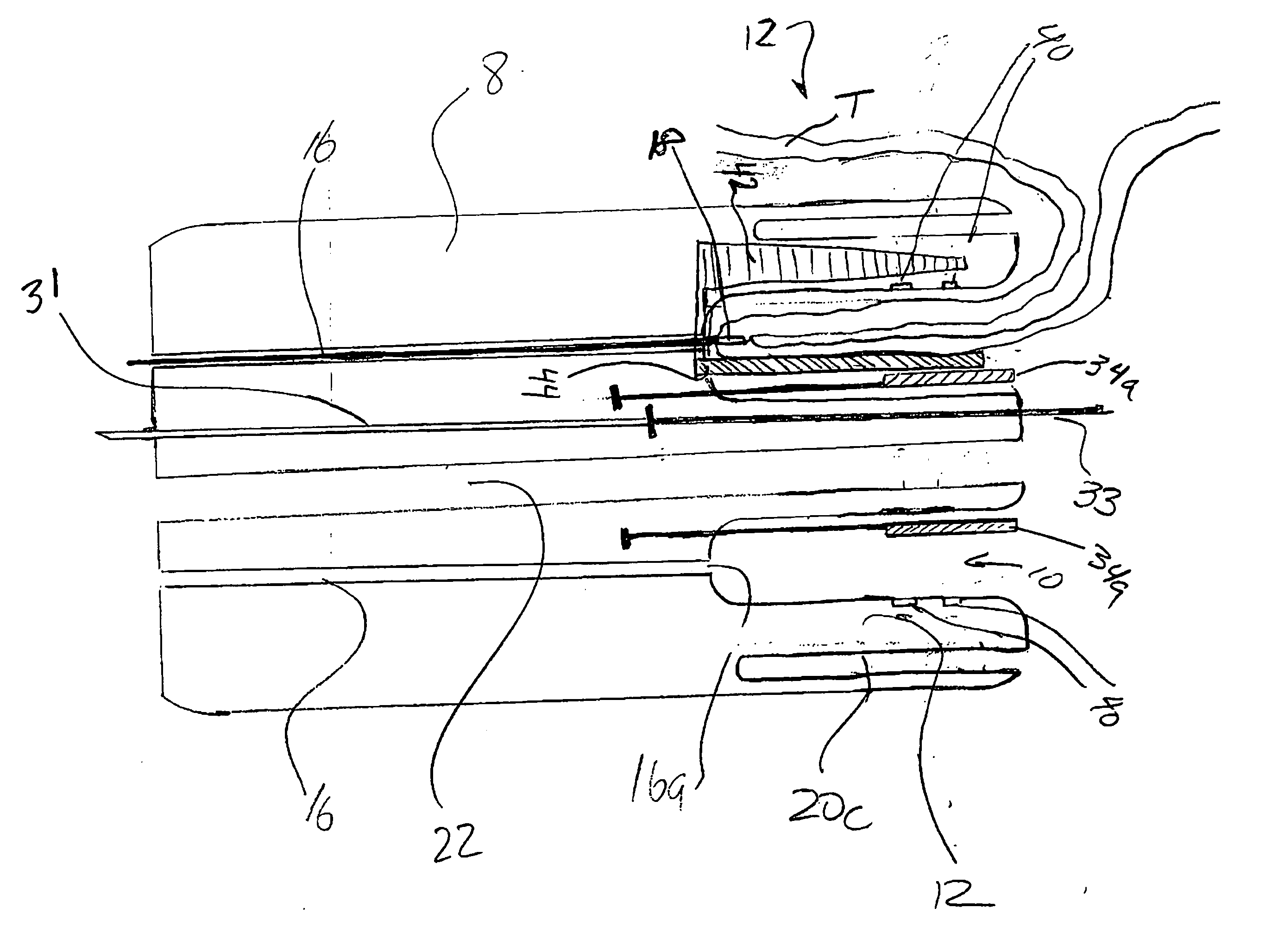
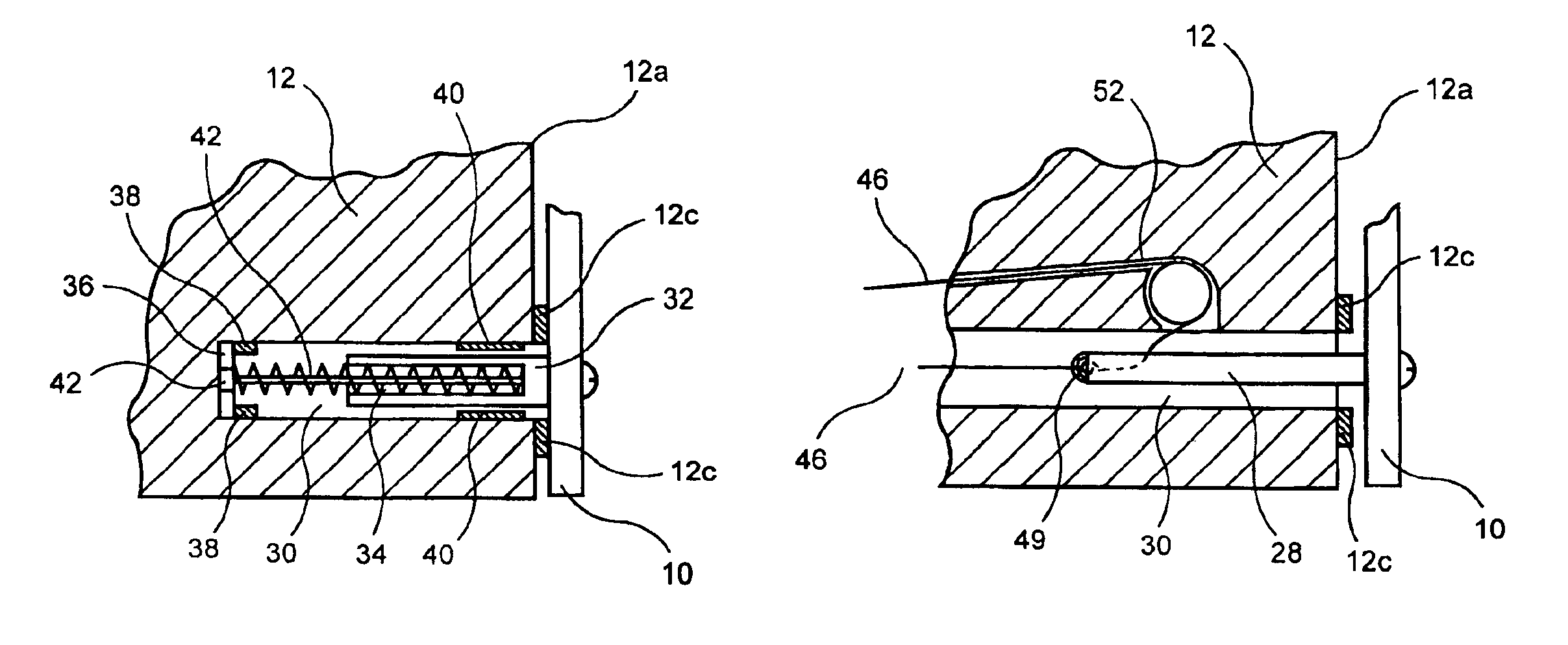
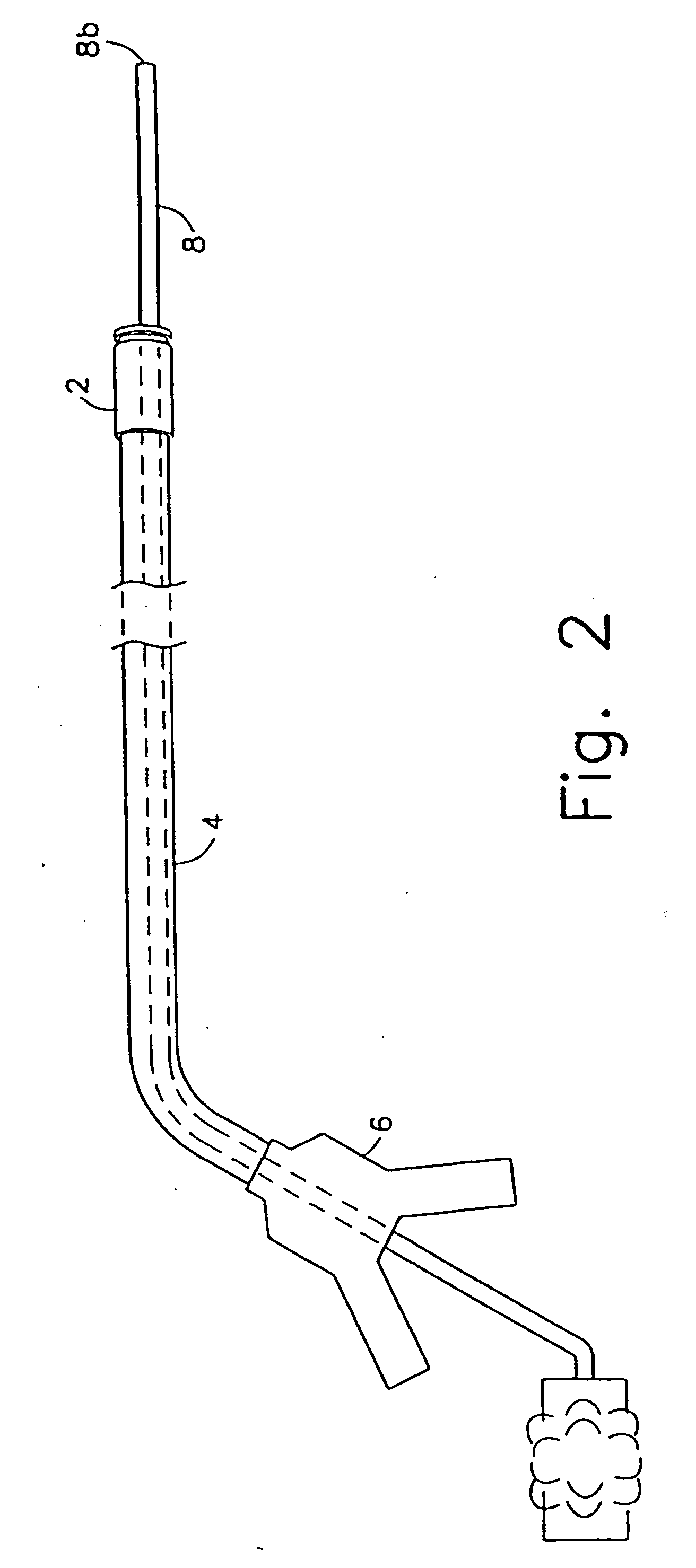
Method and device for full thickness resectioning of an organ
Described is a full-thickness resection system which includes a control unit coupled to a proximal end of a flexible endoscope. The control unit remains outside of a body when the stapling head is in an operative position within a body lumen. The control unit includes (i) an anvil actuator coupled to an anvil in the stapling head, actuation of the anvil actuator moves the anvil axially relative to a stapling mechanism in the stapling head to compress a folded full-thickness portion of lumenal tissue between the anvil and the stapling mechanism. In addition, the control unit includes (ii) a stapler actuator coupled to the stapling mechanism in the stapling head, actuation of the stapler actuator causing the stapling mechanism to drive staples through the folded lumenal tissue against the anvil. Also, the control unit includes (iii) a tissue cutter actuator coupled to a tissue cutter in the stapling head, actuation of the tissue cutter actuator causing the tissue cutter to resect portions of the folded lumenal tissue.
Owner:BOSTON SCI SCIMED INC
Integrated surgical staple retainer for a full thickness resectioning device
The present invention is directed to a full-thickness resection system comprising a flexible endoscope and a stapling mechanism, wherein the endoscope is slidably received through at least a portion of the stapling mechanism. The stapling mechanism includes an anvil and a stapling head mounted to the anvil so that the anvil and the stapling head are moveable with respect to one another. A position adjusting mechanism is provided for moving the anvil and the stapling head relative to one another.An integrated surgical retainer for use in the full thickness resectioning device is described. The integrated surgical staple retainer comprises a calibrating portion, a retaining portion, and a grasping portion. The calibrating portion defines a circular opening which has a diameter substantially equal to a diameter of a working channel of the full thickness resectioning device. The retaining portion has a lower surface adapted to limit movement of the surgical staples in the full thickness resectioning device and is adjacent to the calibrating portion of the integrated surgical staple retainer.
Owner:BOSTON SCI SCIMED INC
Stapling and cutting in resectioning for full thickness resection devices
A stapling unit for use with an endoscopic stapling system adapted to be advanced along an endoscope to a predetermined location within a body lumen to staple the portion of tissue, as part of an occlusal or full thickness resectioning procedure. The stapling unit comprises a first casing having a distal end, a proximal end and a stapling device mounted thereto adjacent to a first window extending through a periphery of the first casing. The invention includes methods for the stapling, severing and removal of tissue by using the device.
Owner:BOSTON SCI SCIMED INC
Method and device for full thickness resectioning of an organ
A full-thickness resection system comprises a flexible endoscope and a stapling mechanism, wherein the endoscope is slidably received through at least a portion of the stapling mechanism. The stapling mechanism comprises an anvil and a stapling head mounted to the anvil so that the anvil and the stapling head are moveable with respect to one another between a tissue receiving position and a stapling position and wherein a gap formed between the stapling head and the anvil is larger in the tissue receiving position than it is in the stapling position. A position adjusting mechanism is provided for moving the anvil and the stapling head between the tissue receiving and stapling positions and a staple firing mechanism sequentially fires a plurality of staples from the stapling head across the gap against the anvil and through any tissue received in the gap and a knife cuts a portion of tissue received within the gap. A control unit which remains outside the body is coupled to the stapling mechanism for controlling operation of the position adjusting mechanism and the staple firing mechanism.
Owner:BOSTON SCI SCIMED INC
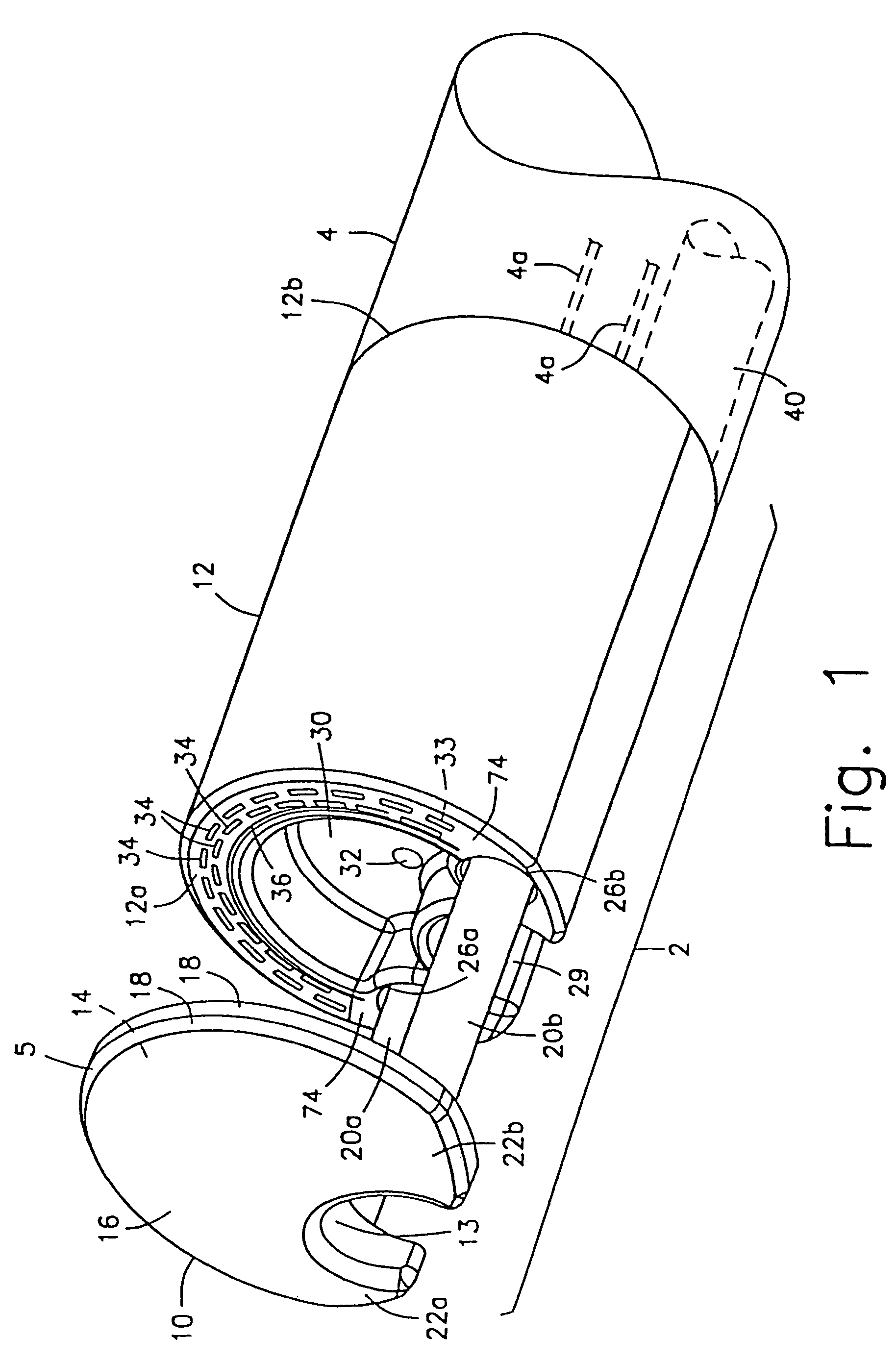
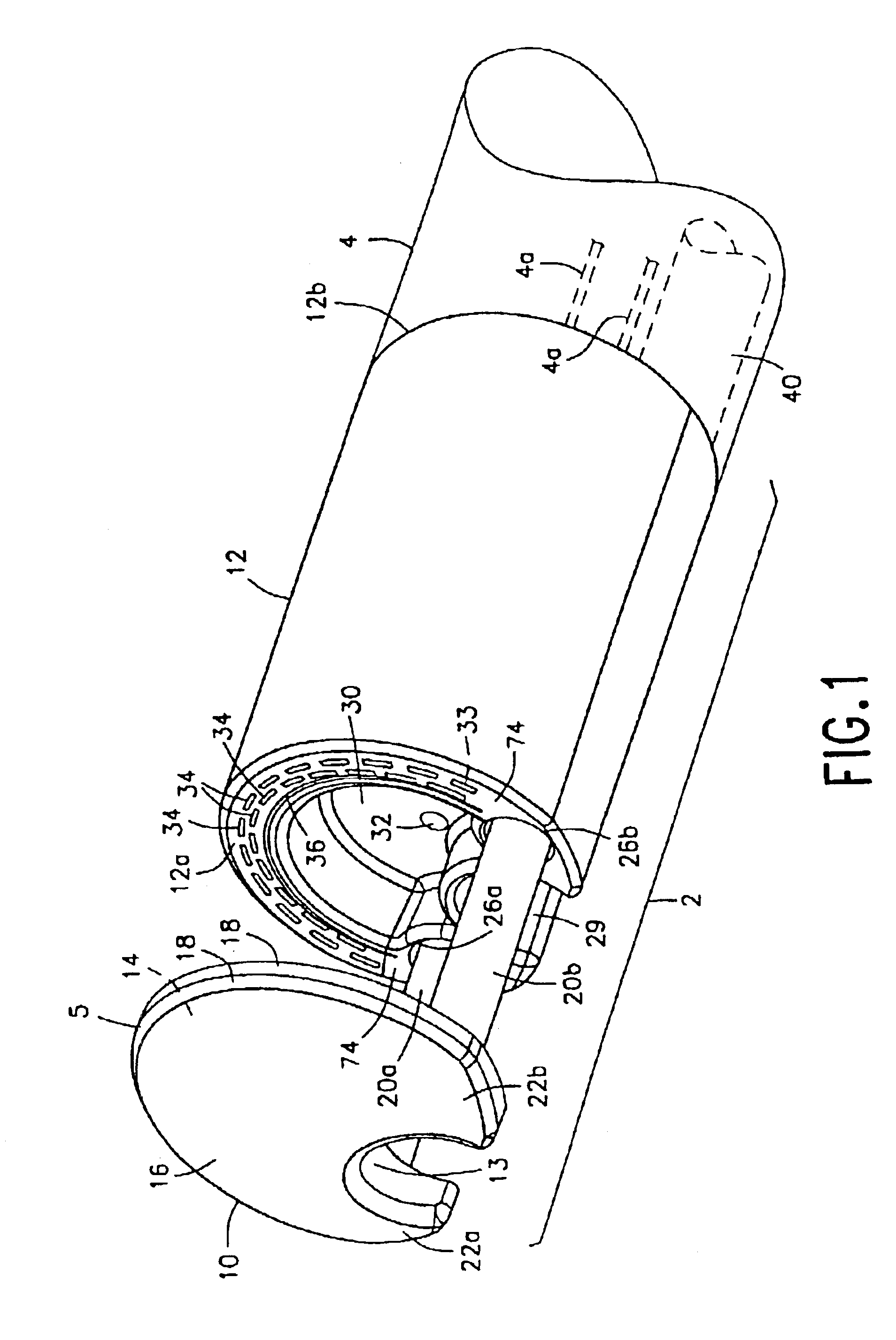
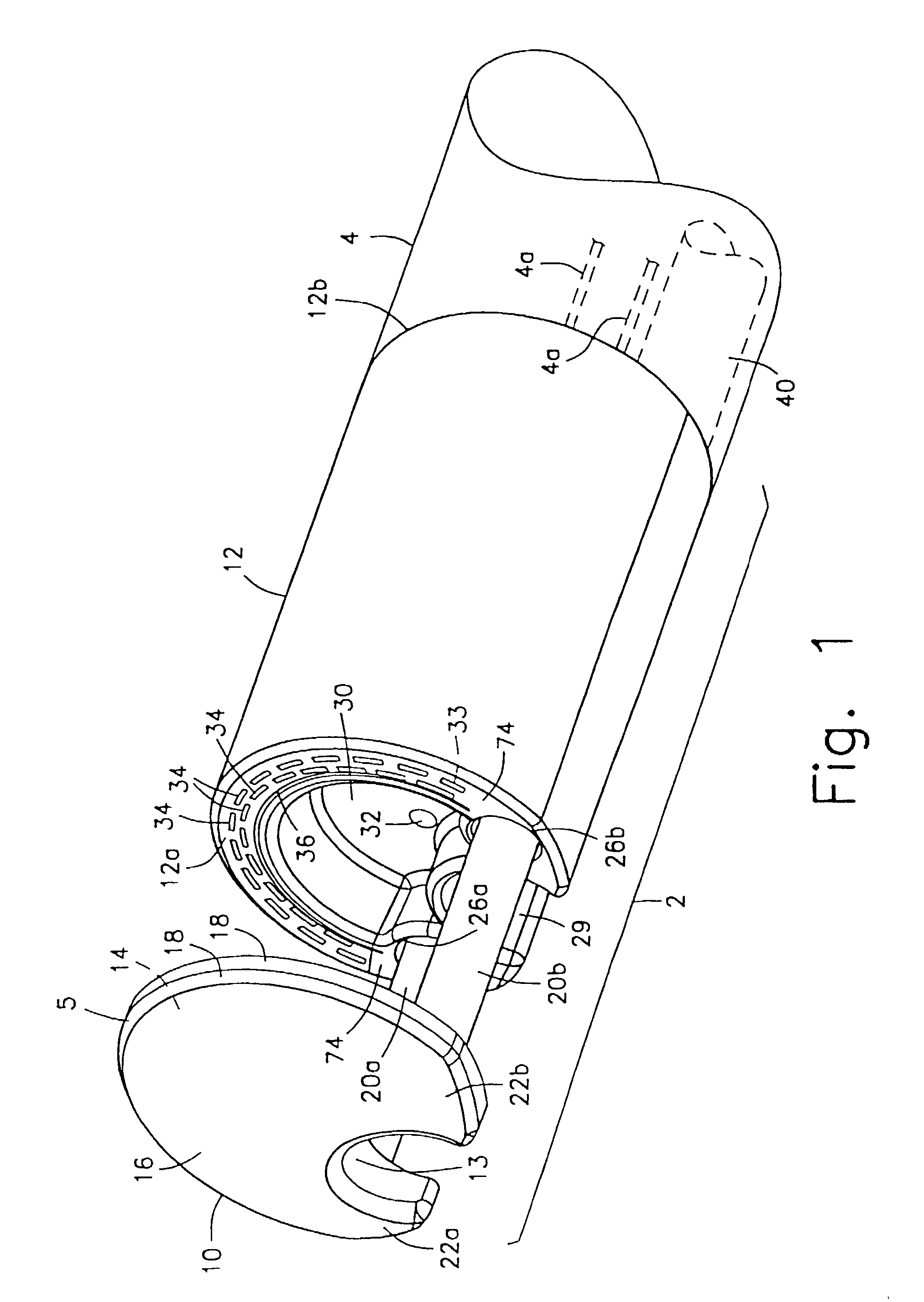
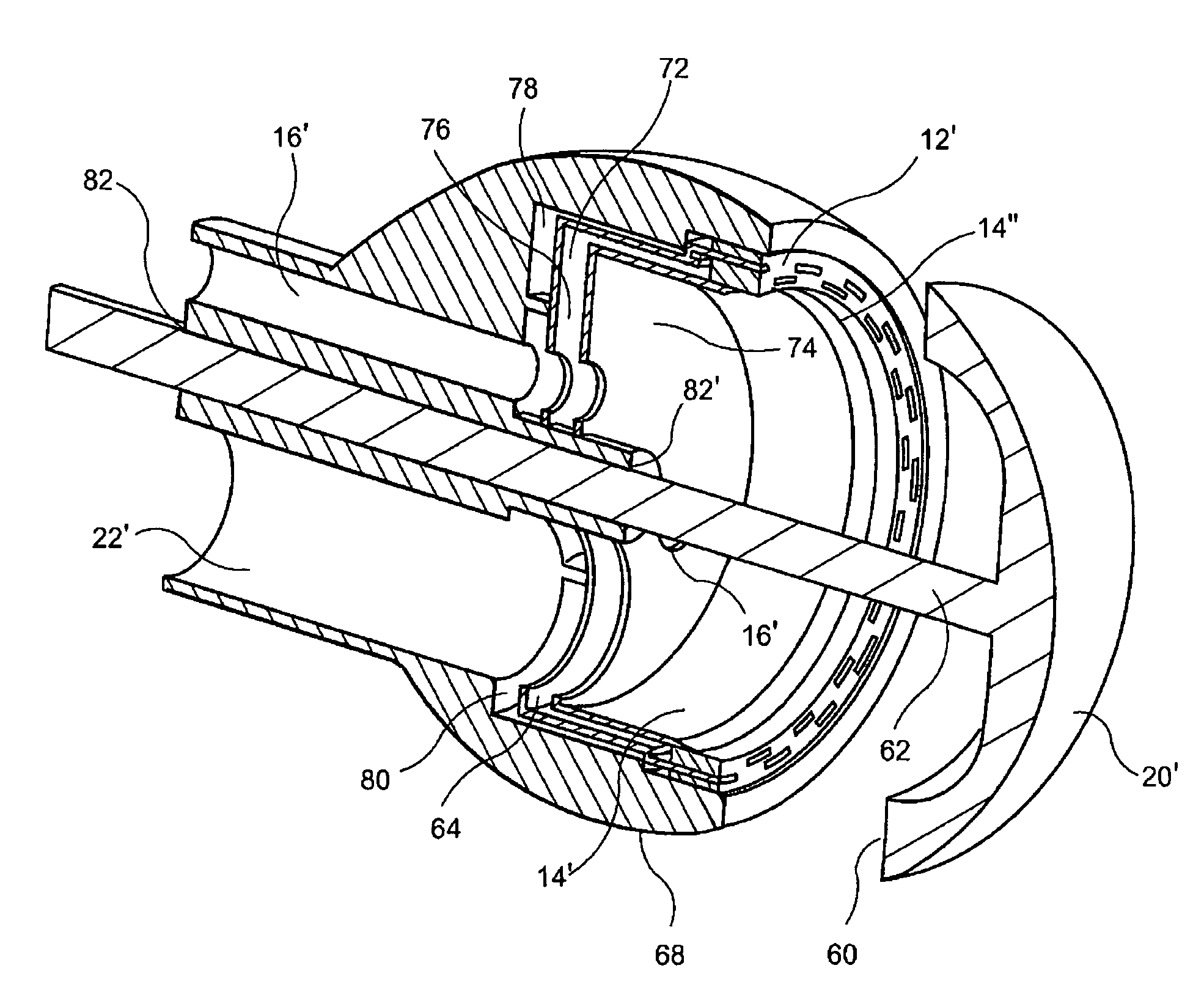
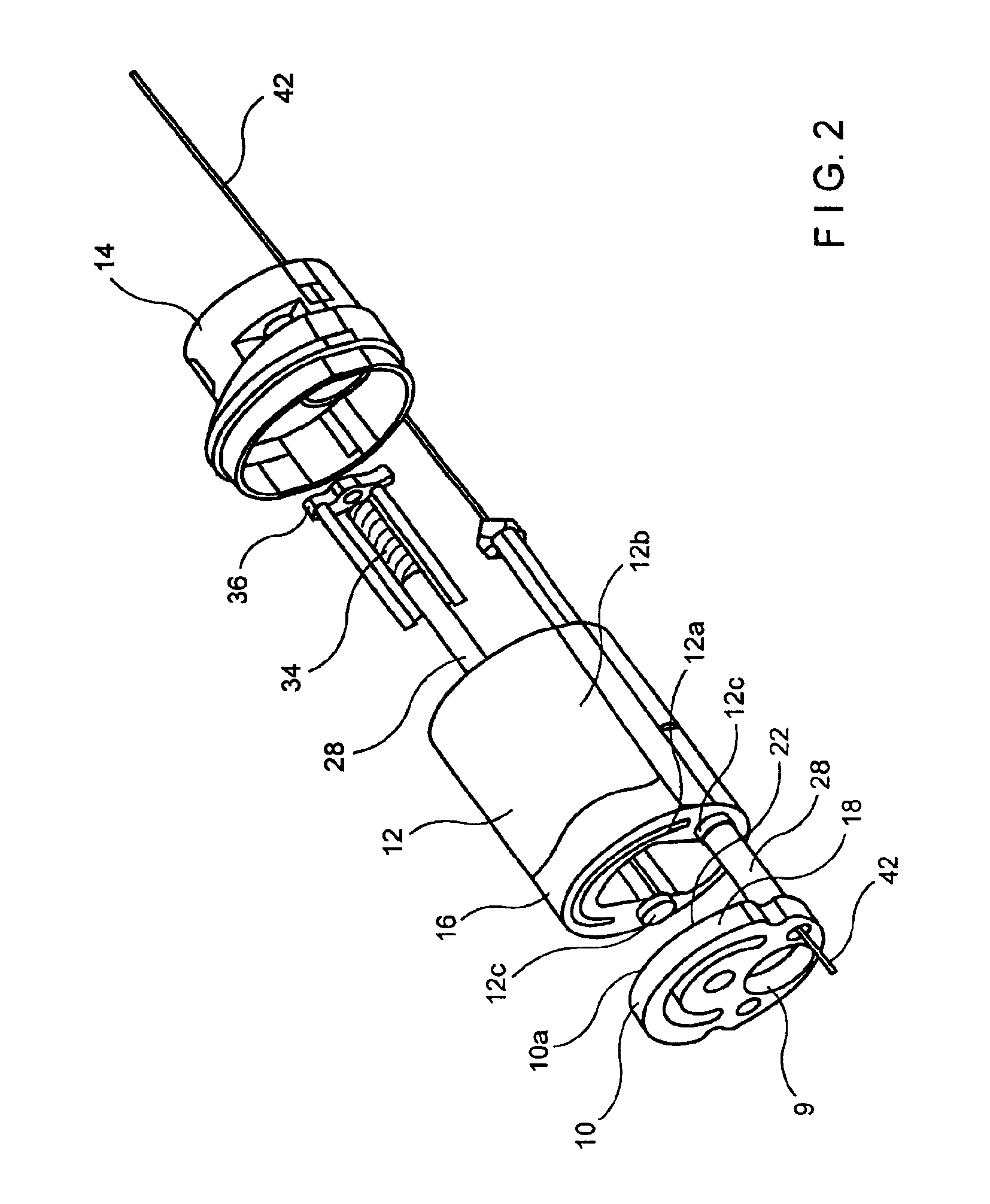
Circumferential full thickness resectioning device
An apparatus for performing endoluminal anastomosis of an organ comprises an operative head including an endoscope receiving lumen for slidably receiving a flexible endoscope therein, the operative head including an annular tissue receiving space extending around a circumference of a distal end thereof and a stapling mechanism for firing staples around an entire circumference of the tissue receiving space and a tissue gripping mechanism for drawing into the tissue receiving space a portion of tissue extending around an entire circumference of the organ.
Owner:BOSTON SCI SCIMED INC
Circumferential full thickness resectioning device
ActiveUS20050145675A1Suture equipmentsStapling toolsFlexible endoscopeFull thickness resection device
An apparatus for performing endoluminal anastomosis of an organ comprises an operative head including an endoscope receiving lumen for slidably receiving a flexible endoscope therein, the operative head including an annular tissue receiving space extending around a circumference of a distal end thereof and a stapling mechanism for firing staples around an entire circumference of the tissue receiving space and a tissue gripping mechanism for drawing into the tissue receiving space a portion of tissue extending around an entire circumference of the organ.
Owner:BOSTON SCI SCIMED INC
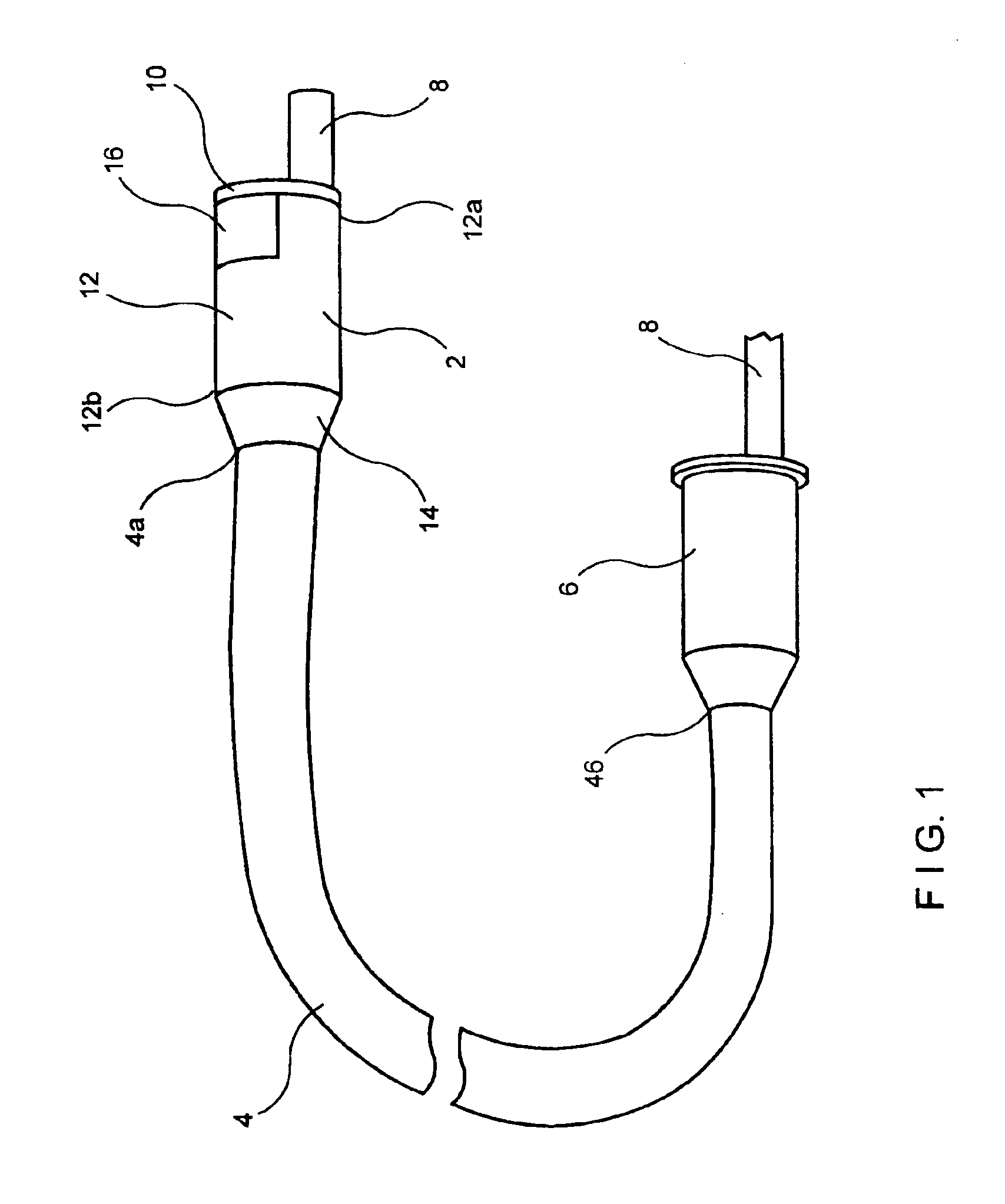
Full thickness resectioning device
A full thickness resection device comprises a control handle including an actuator wherein, when the device is in an operative position within a body lumen of a patient, the control handle remains outside the patient's body and a working head assembly coupled to a control handle by a flexible sheath, wherein, when the device is in the operative position; the working head assembly is located within a body lumen of the patient adjacent to a portion of tissue to be treated, the working head assembly including a tissue stapling mechanism including first and second tissue stapling members moveable relative to one another in combination with a first cable extending from the actuator through the flexible sheath to the first tissue stapling member so that, when the actuator is operated to draw the first cable proximally from the sheath, the first tissue stapling member is moved in a first direction relative to the second tissue stapling member.
Owner:BOSTON SCI SCIMED INC
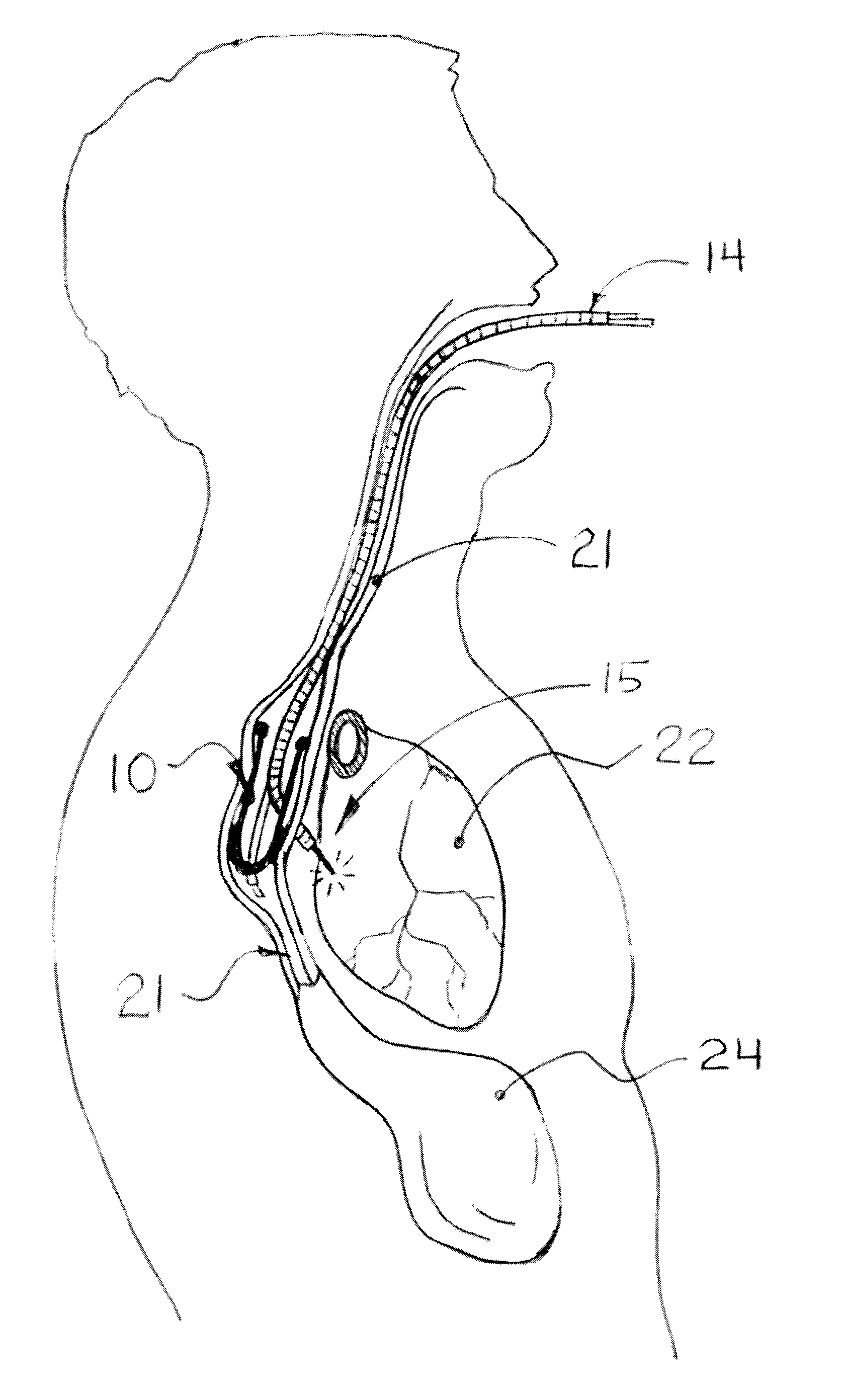
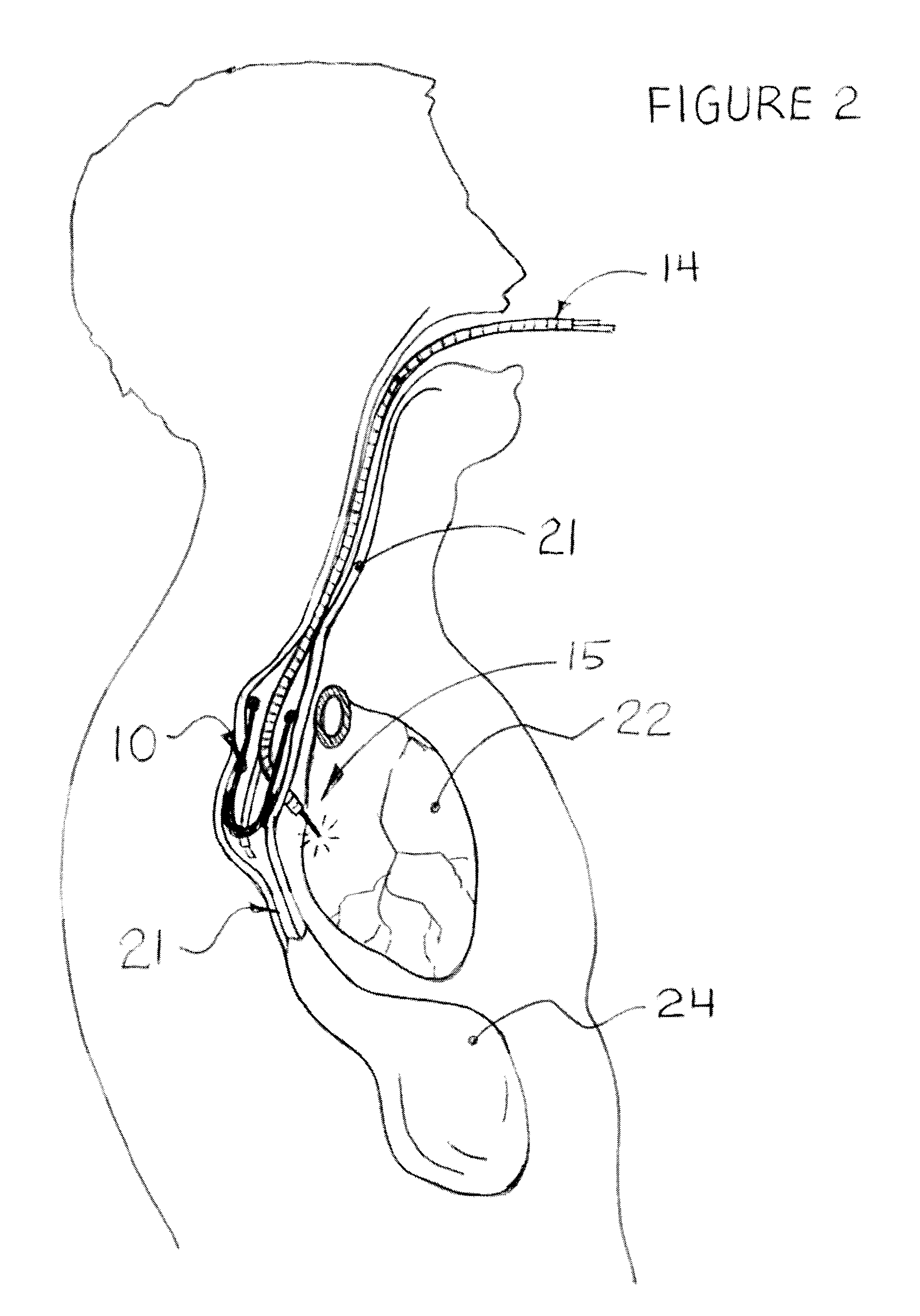
Methods and apparatus for transesophageal microaccess surgery
The current invention describes methods of transesophageal access to the neck and thorax to perform surgical interventions on structures outside the esophagus in both the cervical and the thoracic cavity. It describes a liner device made of a complete or partial tubular structure, or a flat plate, the liner having means to facilitate creation of a side opening, which may include a valve. The liner with its side opening form a port structure inside the esophageal lumen. The port structure allows elongated surgical devices to pass through a perforation across the full thickness of the esophageal wall to outside location, in a controlled way. The elongated surgical devices can be diagnostic scopes, therapeutic scopes, manual elongated surgical devices, robotic arms or the like. After being deployed outside the esophagus, the surgical devices can access structures outside the esophagus, in the neck and thorax in 360 degrees of freedom around the esophageal circumference. These structures can be bony, cartilaginous, spinal, vascular, soft tissue, deep tissues, lymph nodal, cardiac, pulmonary, tracheal, nervous, muscular or diaphragmatic, skin and subcutaneous tissues of the neck, skin and subcutaneous tissues of the anterior chest wall, skin and subcutaneous tissues of the skin of the back, and skin and layers of the breast.
Owner:MICROACCESS
Method and device for full thickness resectioning of an organ
Describeed is a full-thickness resection system which includes a control unit coupled to a proximal end of a flexible endoscope. The control unit remains outside of a body when the stapling head is in an operative position within a body lumen. The control unit includes (i) an anvil actuator coupled to an anvil in the stapling head, actuation of the anvil actuator moves the anvil axially relative to a stapling mechanism in the stapling head to compress a folded full-thickness portion of lumenal tissue between the anvil and the stapling mechanism. In addition, the control unit includes (ii) a stapler actuator coupled to the stapling mechanism in the stapling head, actuation of the stapler actuator causing the stapling mechanism to drive staples through the folded lumenal tissue against the anvil. Also, the control unit includes (iii) a tissue cutter actuator coupled to a tissue cutter in the stapling head, actuation of the tissue cutter actuator causing the tissue cutter to resect portions of the folded lumenal tissue.
Owner:BOSTON SCI SCIMED INC
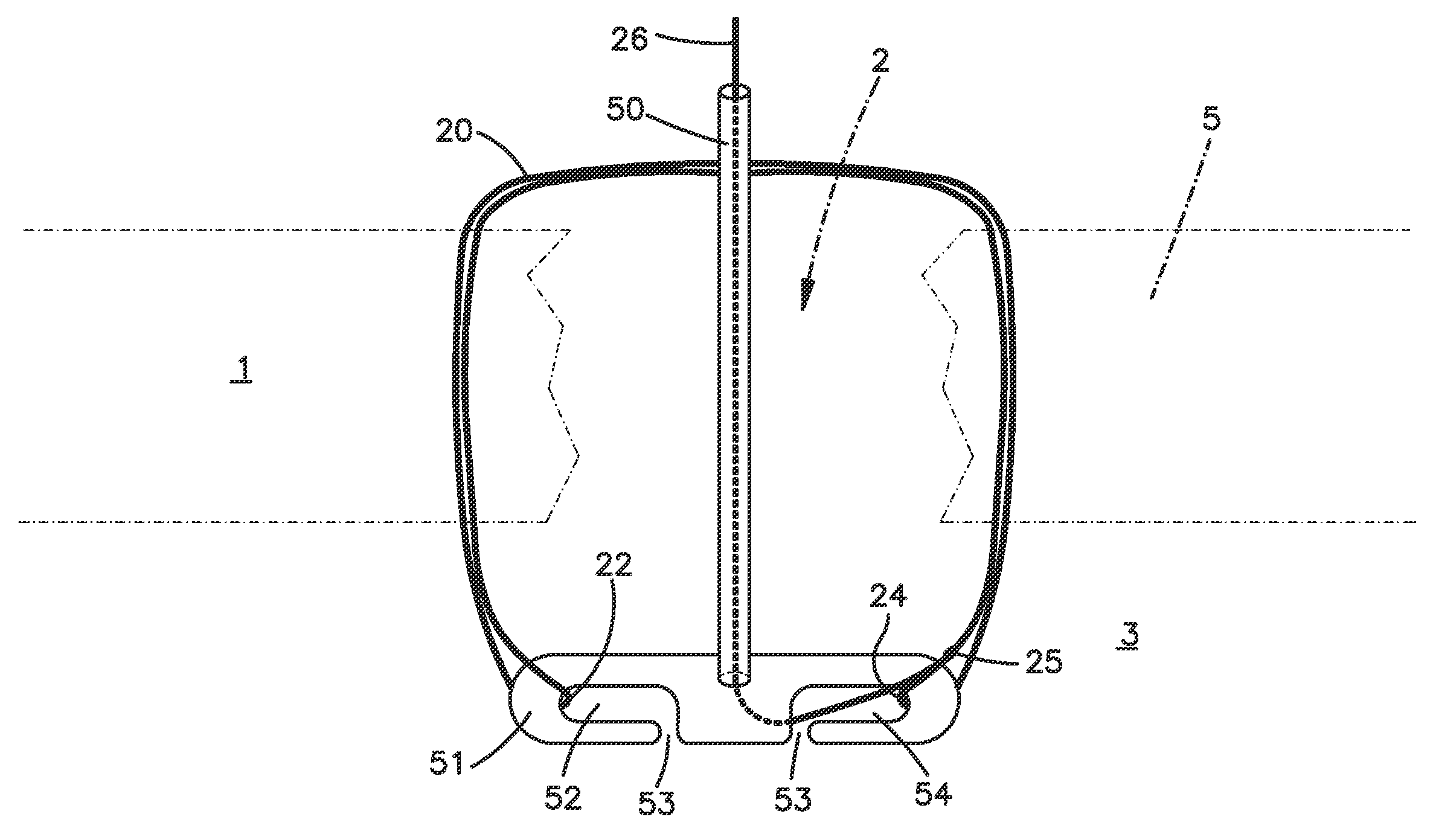
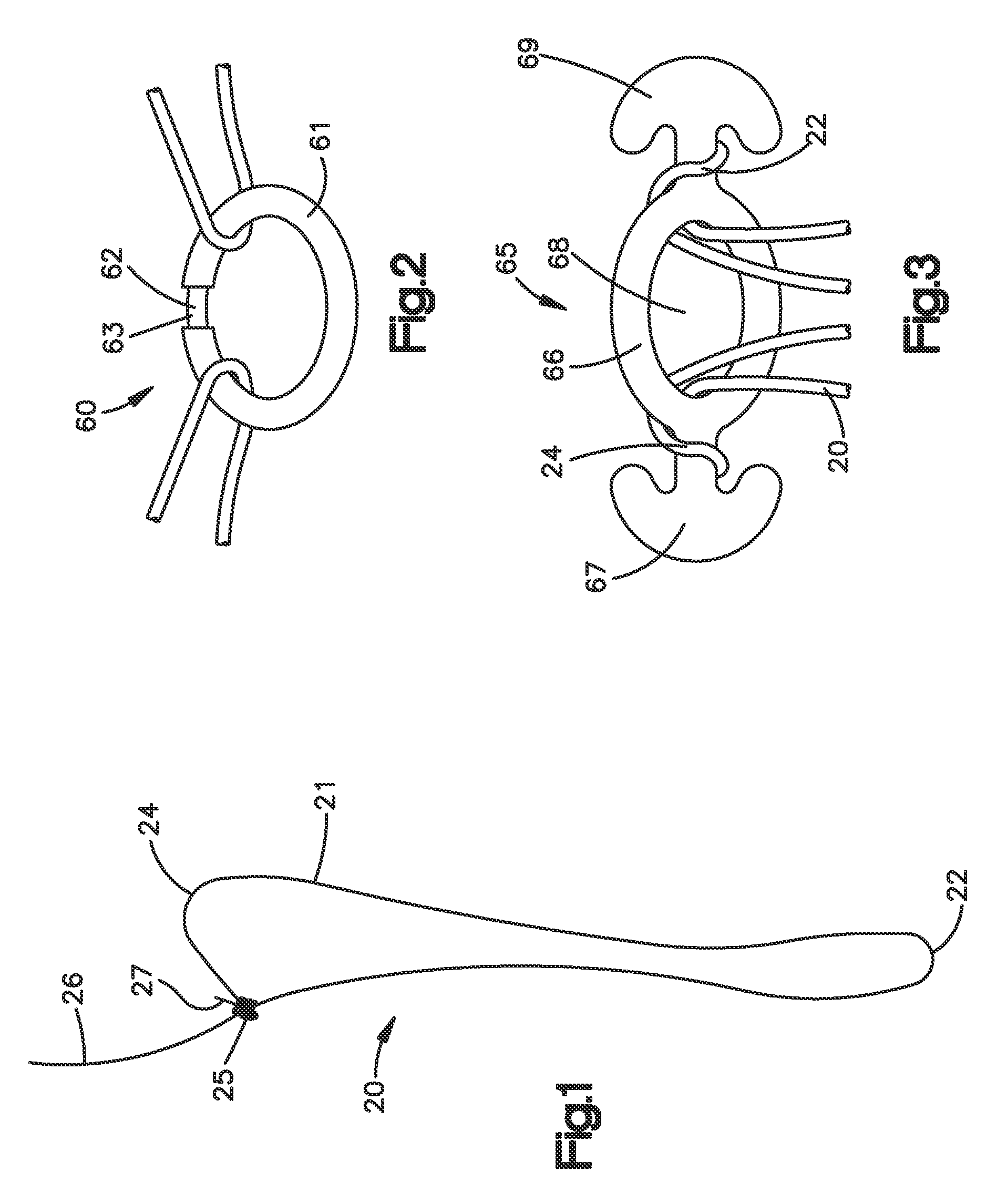
Suture based tissue repair
The present invention provides an apparatus for suture-based tissue repair, preferably for the annulus of a spinal disc, that includes a suture loop preferably pre-tied with a sliding knot, a clasp-type component that captures the ends of the suture loop, and an optional plug member that fills the tissue defect. Also disclosed is a method that places the suture loop in a full-thickness stitch encircling the tissue defect, secures the ends of the suture loop to the clasp, and cinches the suture loop to approximate the tissue without the need to tie knots. Also disclosed is a suture passer that enables a suture strand or loop to be passed through the tissue wall, captured, and retrieved. The suture passer may optionally incorporate a clasp in such an arrangement that enables a suture loop passed through the tissue wall to be captured directly by the clasp.
Owner:SYNTHES GMBH
Apparatus and method for laser treatment
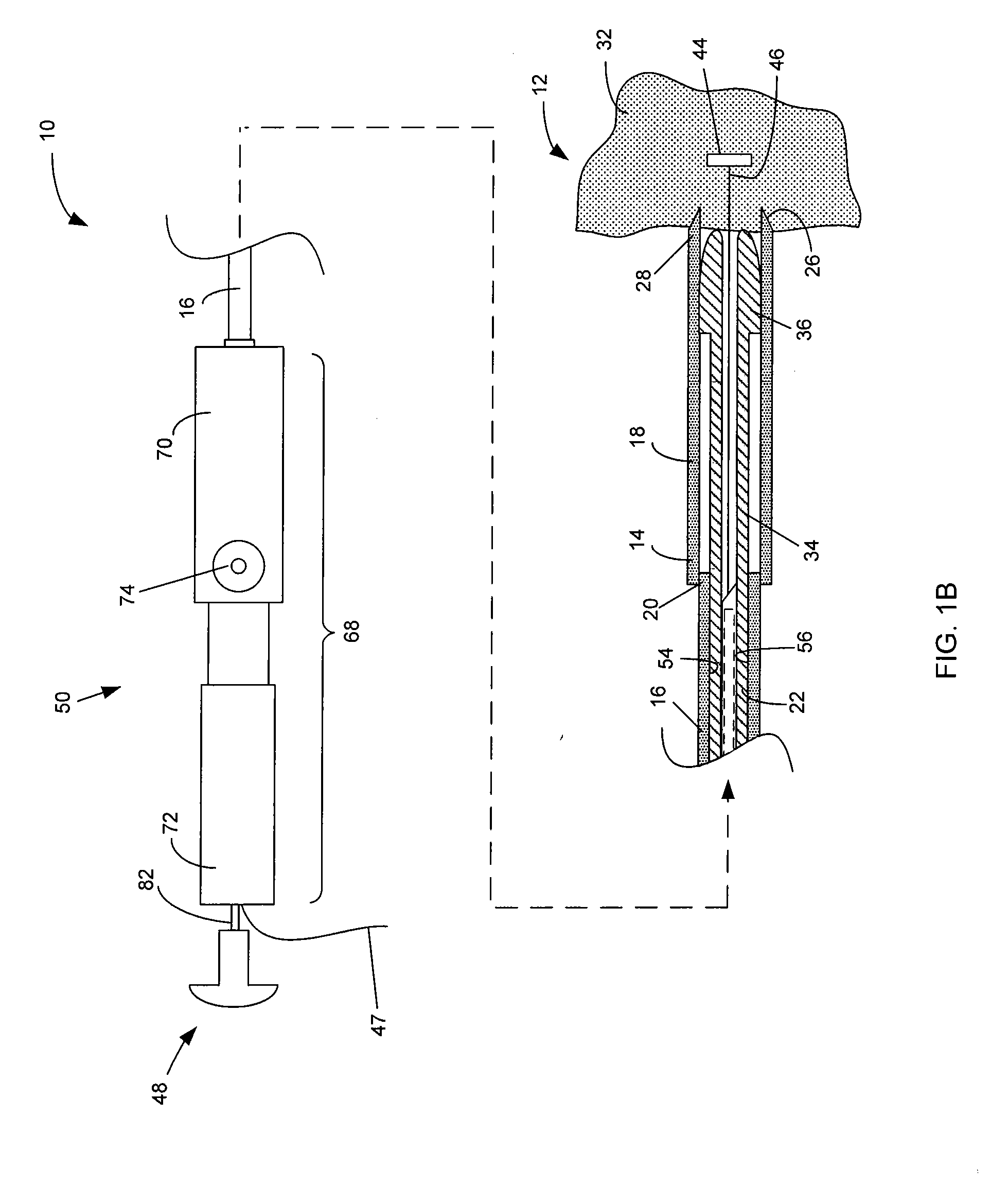
InactiveUS20050096643A1Optimize achievementEasy to moveControlling energy of instrumentCatheterLight energyLength wave
A method and apparatus for treating in situ biologic tissue include identifying a patient with a condition susceptible to treatment by forming a lesion in the tissue and accessing a surface of the tissue. A lesion formation tool is positioned against the accessed surface. The tool includes an optical fiber for guiding a coherent waveform of a selected wavelength to a fiber tip for discharge of light energy from the fiber tip. The wavelength is selected for the light energy to penetrate a full thickness of the tissue to form a volume of necrosed tissue through the thickness of the tissue. The tool further includes a guide tip coupled to the fiber tip. The guide tip is adapted to have a discharge bore aligned with the fiber tip to define an unobstructed light pathway from the fiber tip to the tissue surface. The guide tip is further adapted to be placed against the tissue surface with the guide tip slidable along the tissue surface in atraumatic sliding engagement with the discharge bore opposing the tissue surface. The lesion formation tool is manipulated to draw the guide tip over the tissue surface in a pathway while maintaining the discharge bore opposing the tissue surface to form a transmural lesion in the tissue extending a length of the pathway.
Owner:ENDOPHOTONIX
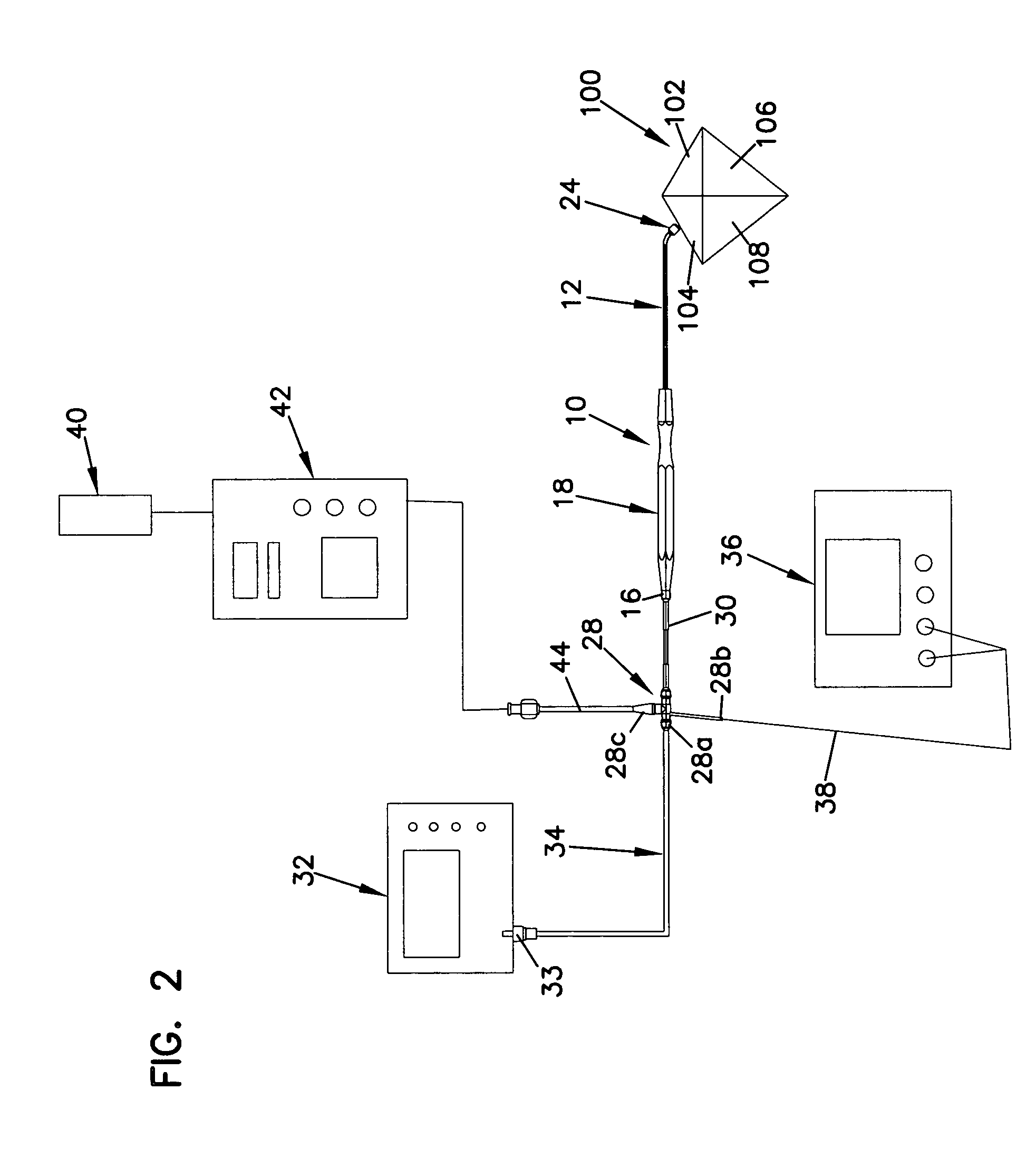
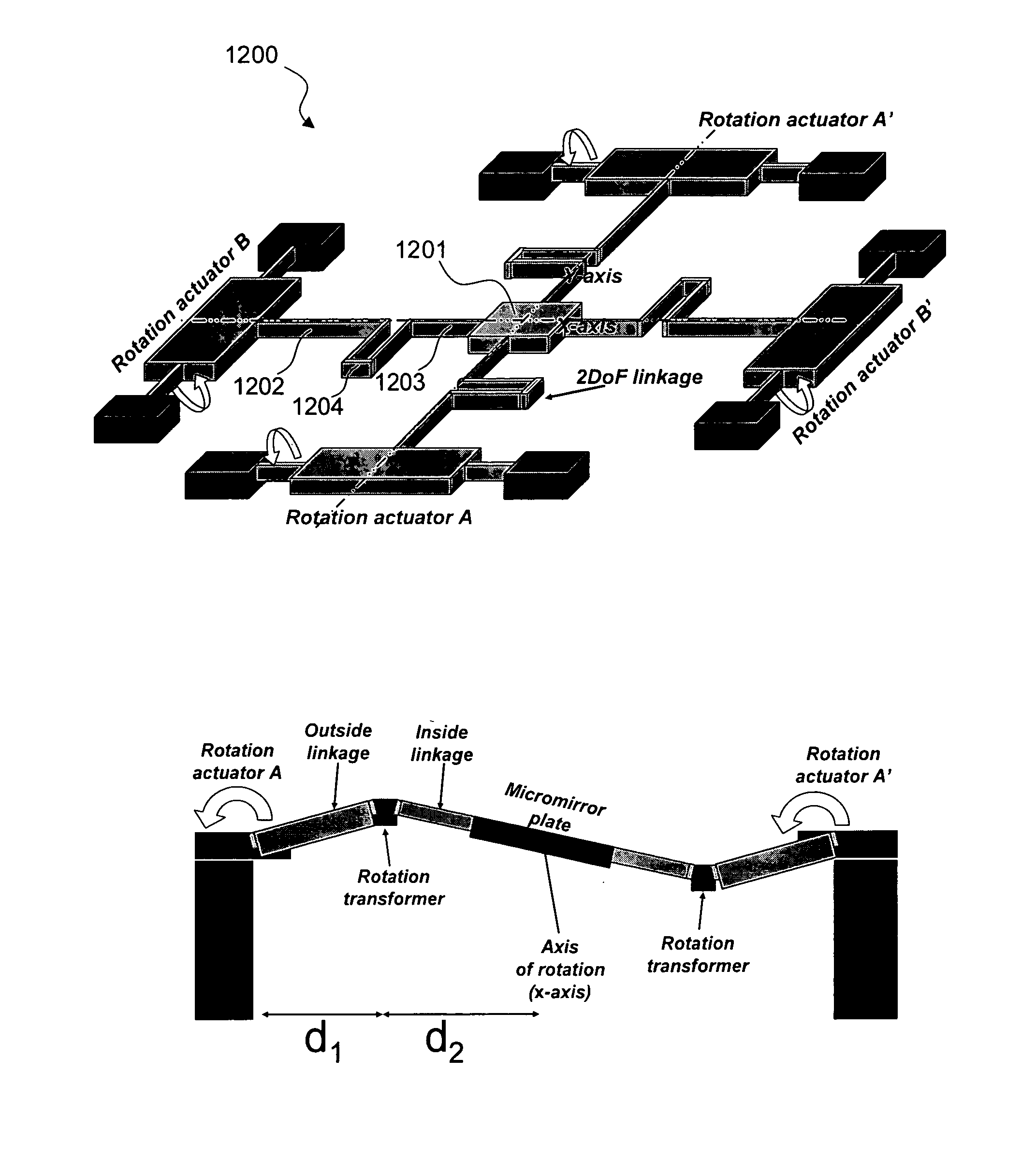
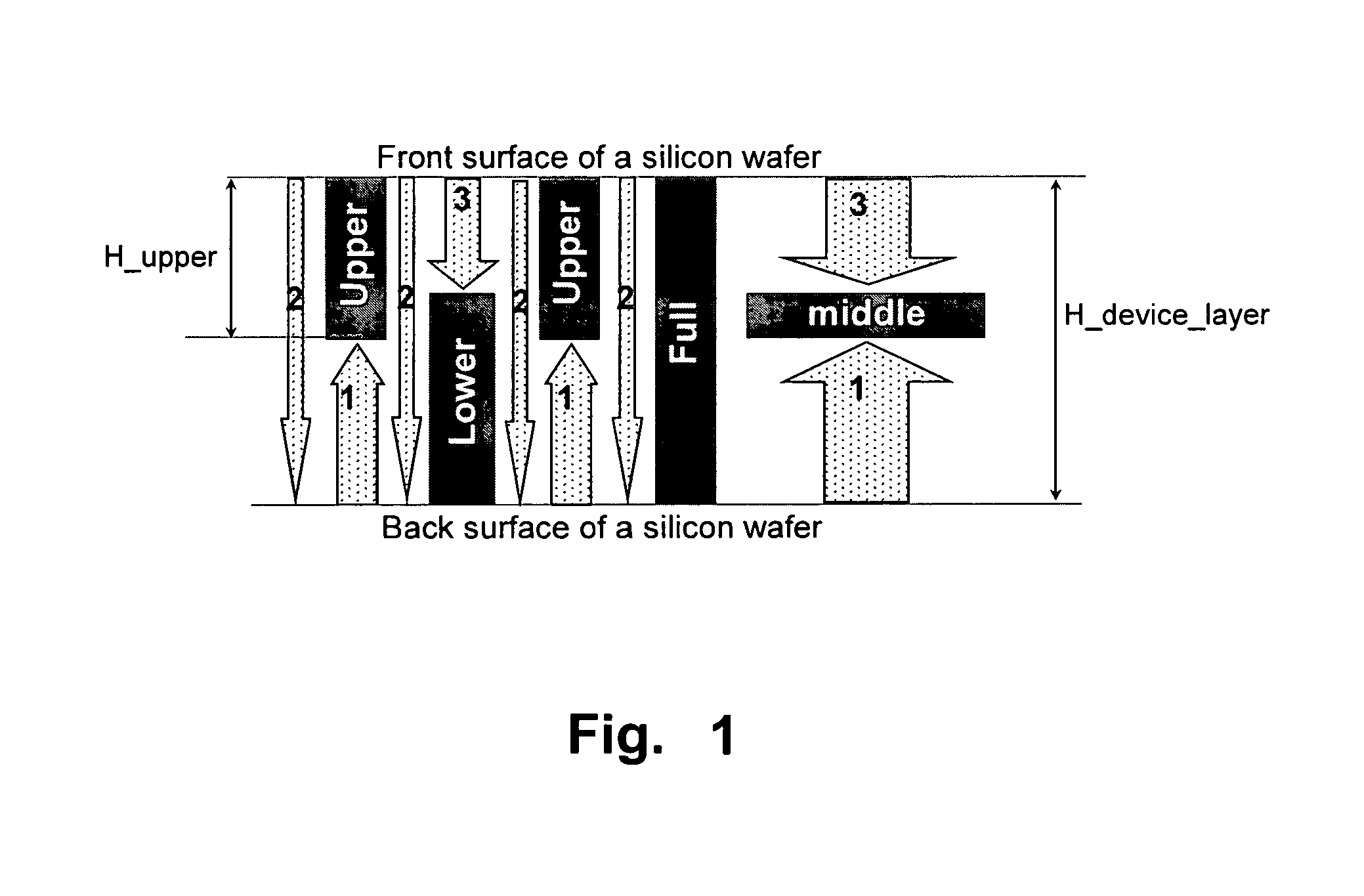
Gimbal-less micro-electro-mechanical-system tip-tilt and tip-tilt-piston actuators and a method for forming the same
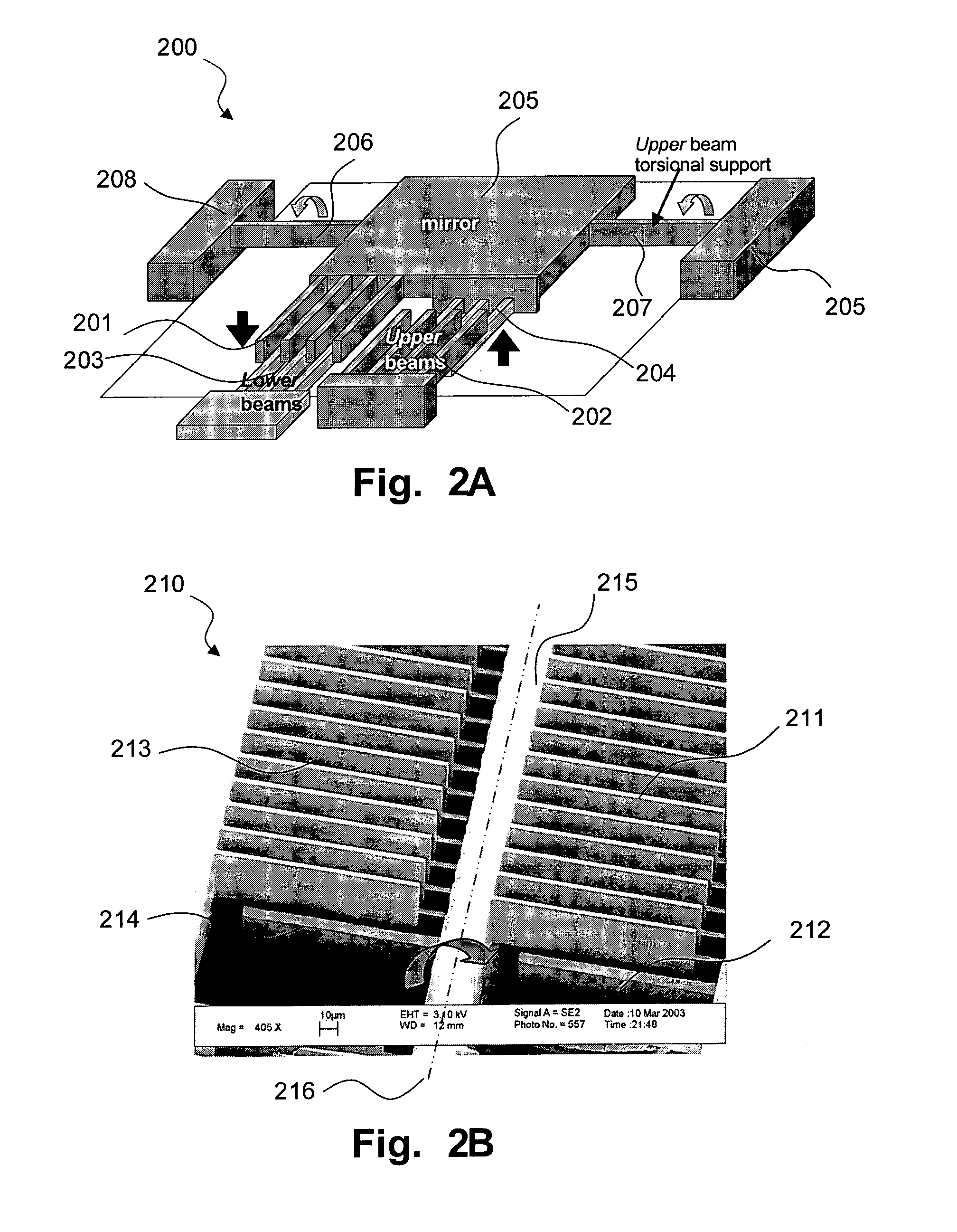
Fully monolithic gimbal-less micro-electro-mechanical-system (MEMS) devices with large static optical beam deflection and fabrications methods are disclosed. The devices can achieve high speed of operation for both axes. Actuators are connected to a device, or device mount by linkages that allow static two-axis rotation in addition to pistoning without the need for gimbals, or specialized isolation technologies. The device may be actuated by vertical comb-drive actuators, which are coupled by bi-axial flexures to a central micromirror or device mount. Devices may be fabricated by etching an upper layer both from the top side and from the bottom side to form beams at different levels, The beams include a plurality of lower beams, a plurality of full-thickness beams, and a plurality of upper beams, the lower, full-thickness and upper beams That form vertical combdrive actuators, suspension beams, flexures, and a device mount.
Owner:ADRIATIC RES INST
Silicon on insulator field effect transistors having shared body contact
InactiveUS6624459B1Improve memory cell stabilityImprove stabilityTransistorSolid-state devicesBit lineBody contact
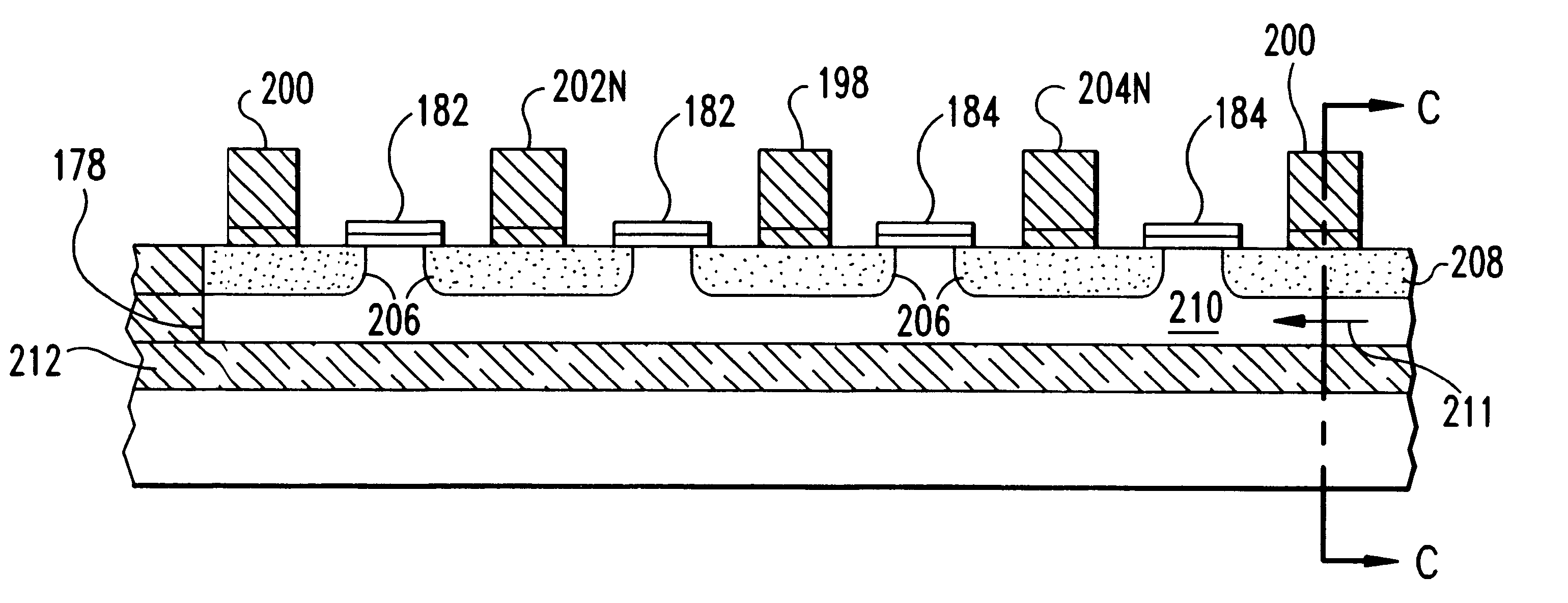
Silicon on insulator (SOI) field effect transistors (FET) with a shared body contact, a SRAM cell and array including the SOI FETs and the method of forming the SOI FETs. The SRAM cell has a hybrid SOI / bulk structure wherein the source / drain diffusions do not penetrate to the underlying insulator layer, resulting in a FET in the surface of an SOI layer with a body or substrate contact formed at a shared contact. FETs are formed on SOI silicon islands located on a BOX layer and isolated by shallow trench isolation (STI). NFET islands in the SRAM cells include a body contact to a P-type diffusion in the NFET island. Each NFET in the SRAM cells include at least one shallow source / drain diffusion that is shallower than the island thickness. A path remains under the shallow diffusions between NFET channels and the body contact. The P-type body contact diffusion is a deep diffusion, the full thickness of the island. Bit line diffusions shared by SRAM cells on adjacent wordlines may be deep diffusions.
Owner:INT BUSIENSS MACHINES IBM +1
Conductive through wafer vias
InactiveUS6852627B2Low costWell formedSemiconductor/solid-state device detailsVolume/mass flow measurementSemiconductorElectroplating
Methods for fabricating a conductive contact (through-via) through a full thickness of a substrate such as a semiconductor wafer or interposer substrate, and semiconductor devices and systems incorporating the conductive through-via are provided. The conductive contact is fabricated by applying a metal layer onto a backside of a substrate, forming a through-hole through the substrate and the metal layer, sealing the hole in the metal layer by an electroless plating process, and filling the hole by an electroplating or an electroless plating process.
Owner:MICRON TECH INC
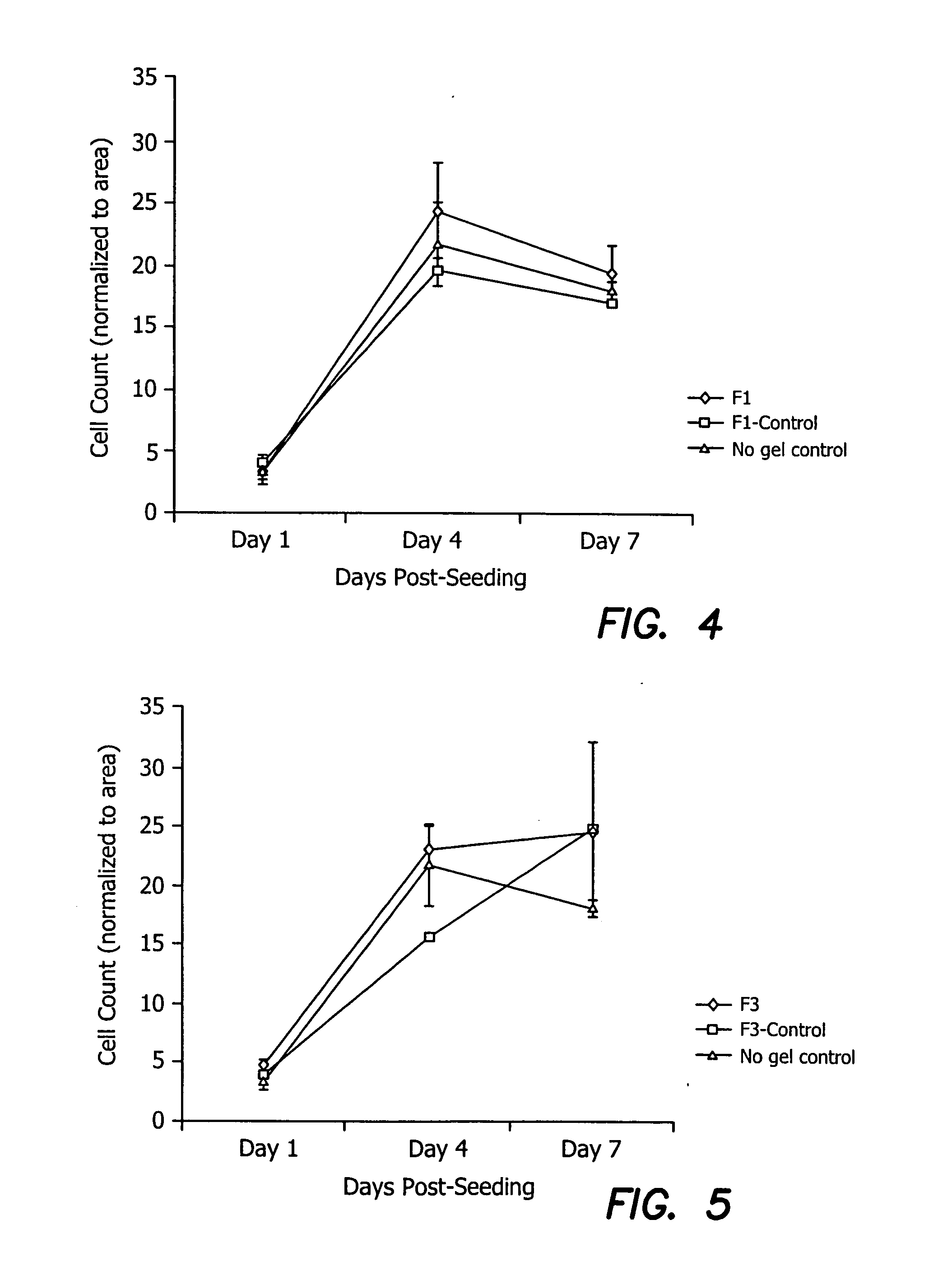
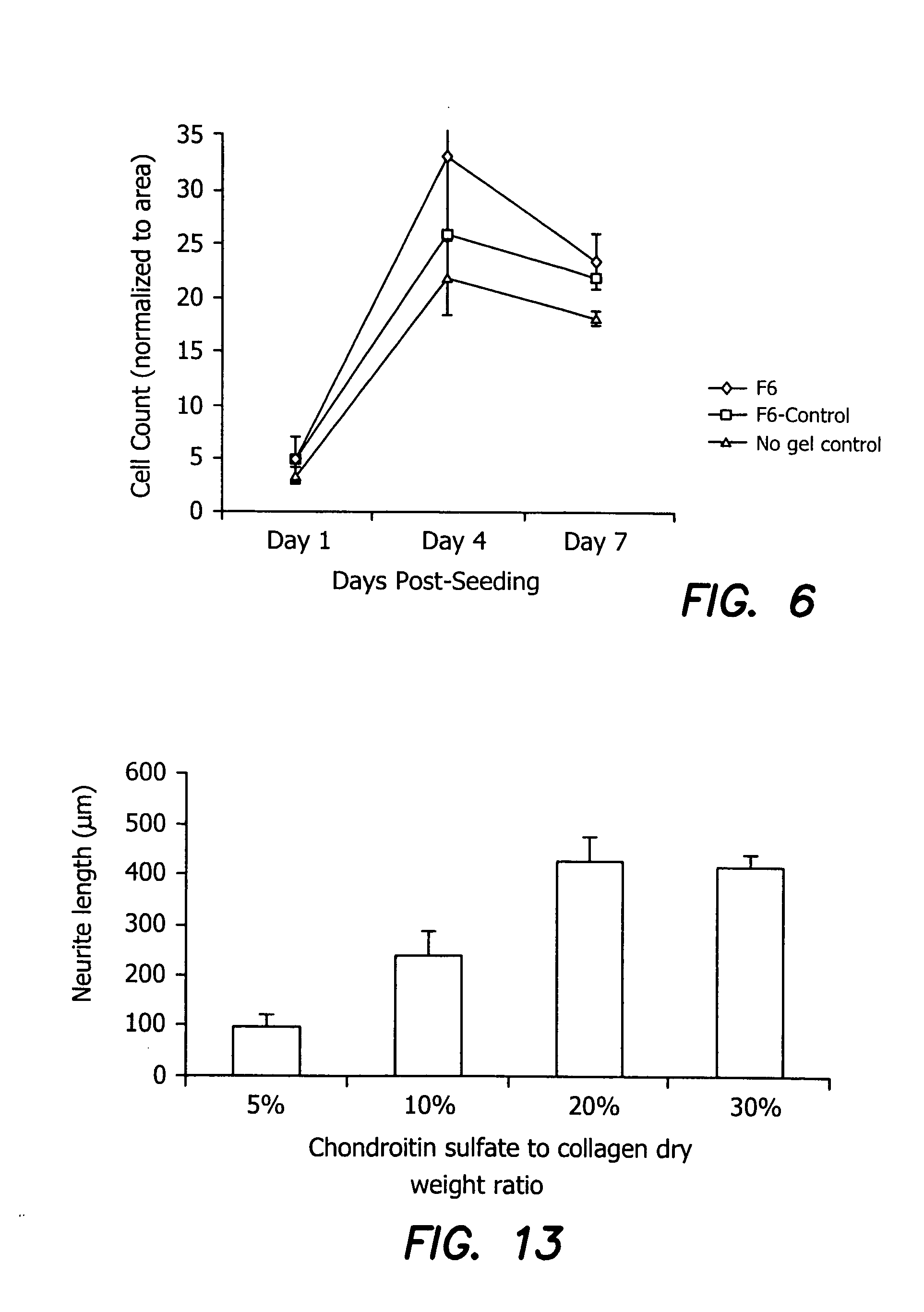
Vision enhancing ophthalmic devices and related methods and compositions
Devices, methods, and compositions for improving vision or treating diseases, disorders or injury of the eye are described. Ophthalmic devices, such as corneal onlays, corneal inlays, and full-thickness corneal implants, are made of a material that is effective in facilitating nerve growth through or over the device. The material may include an amount of collagen greater than 1% (w / w), such as between about 10% (w / w) and about 30% (w / w). The material may include collagen polymers and / or a second biopolymer or water-soluble synthetic polymer cross-linked using EDC / NHS chemistry. The material may additionally comprise a synthetic polymer. The devices are placed into an eye to correct or improve the vision of an individual or to treat a disease, disorder or injury of an eye of an individual.
Owner:NAT RES COUNCIL OF CANADA +3
System and method for performing a full thickness tissue biopsy
A medical system for performing a tissue biopsy at a remote location within a patient is disclosed. The medical system comprises an elongate outer cutting member, an elongate inner member movably disposed within the outer member, and a tissue traction member for anchoring bodily tissue and pulling a sample of the tissue within the outer cutting member.
Owner:COOK MEDICAL TECH LLC
Laser system with short pulse characteristics and its methods of use
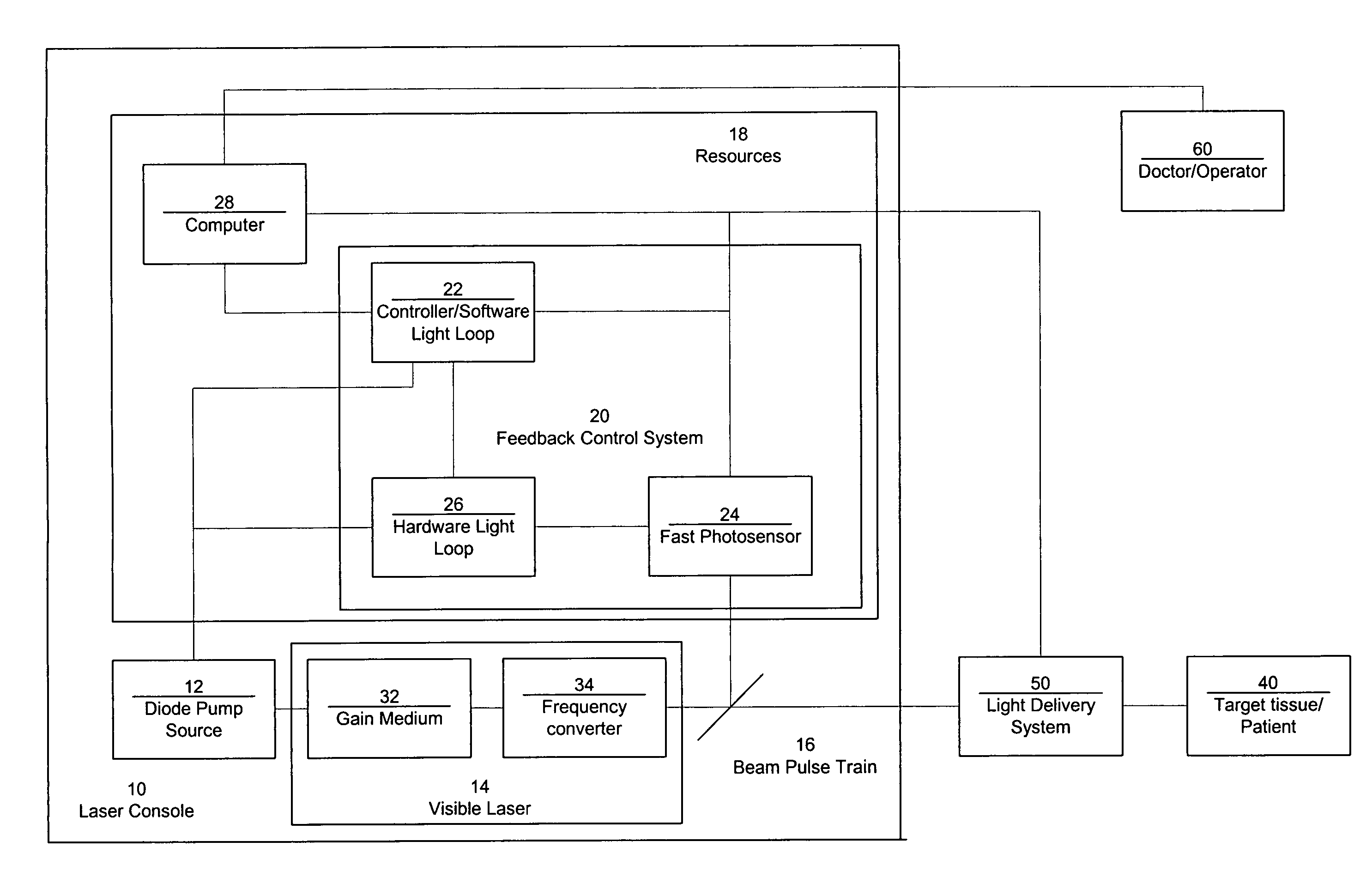
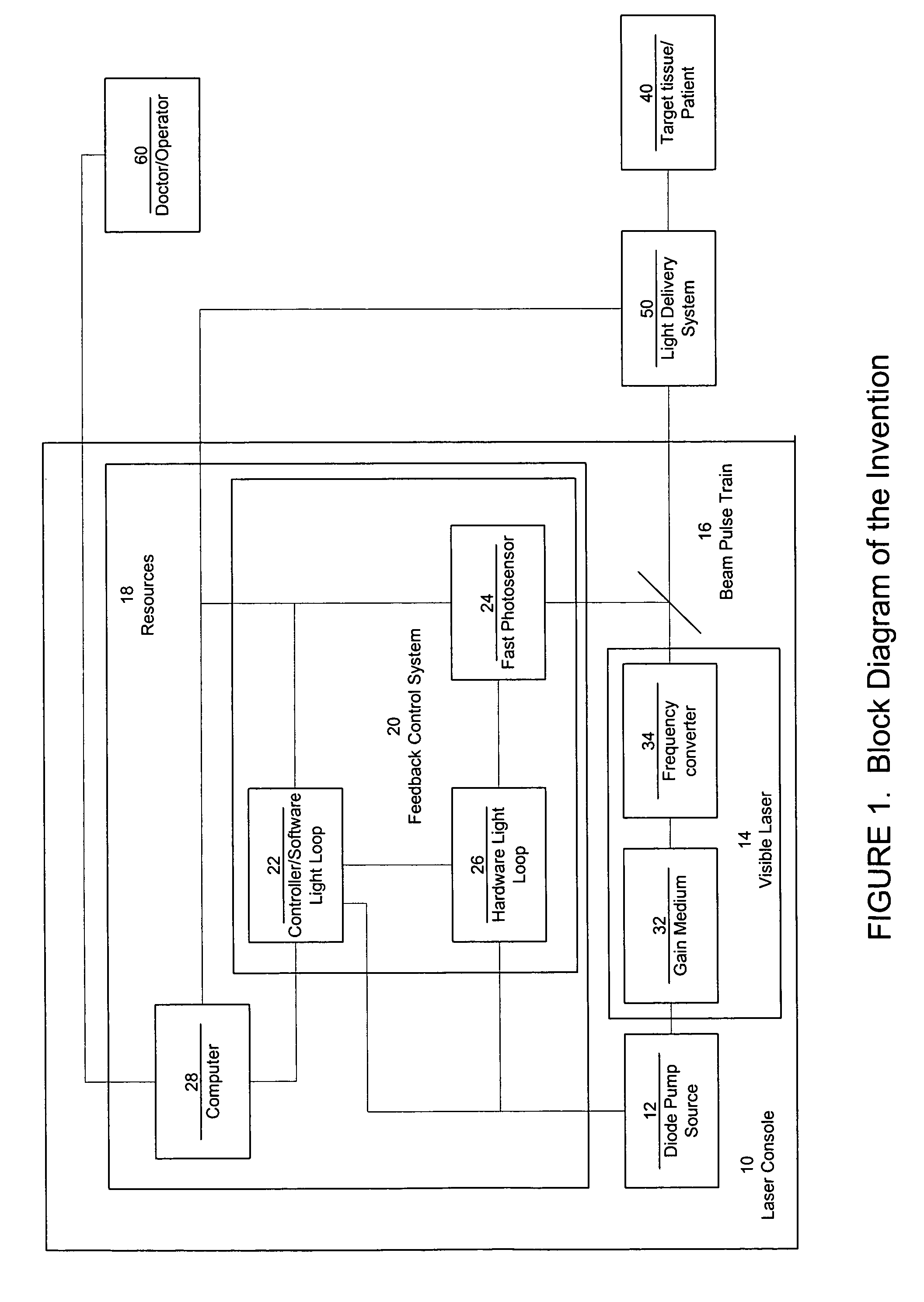
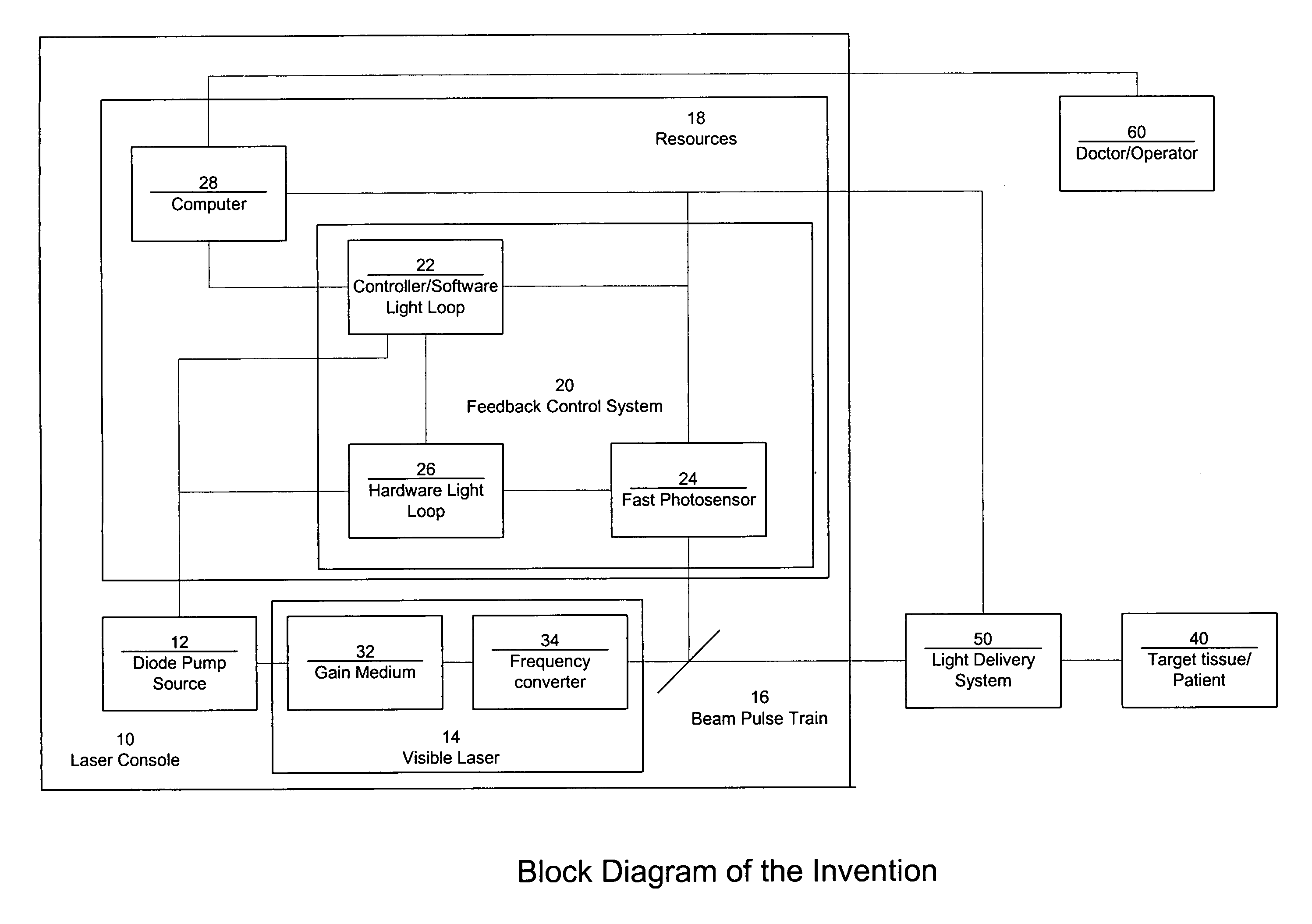
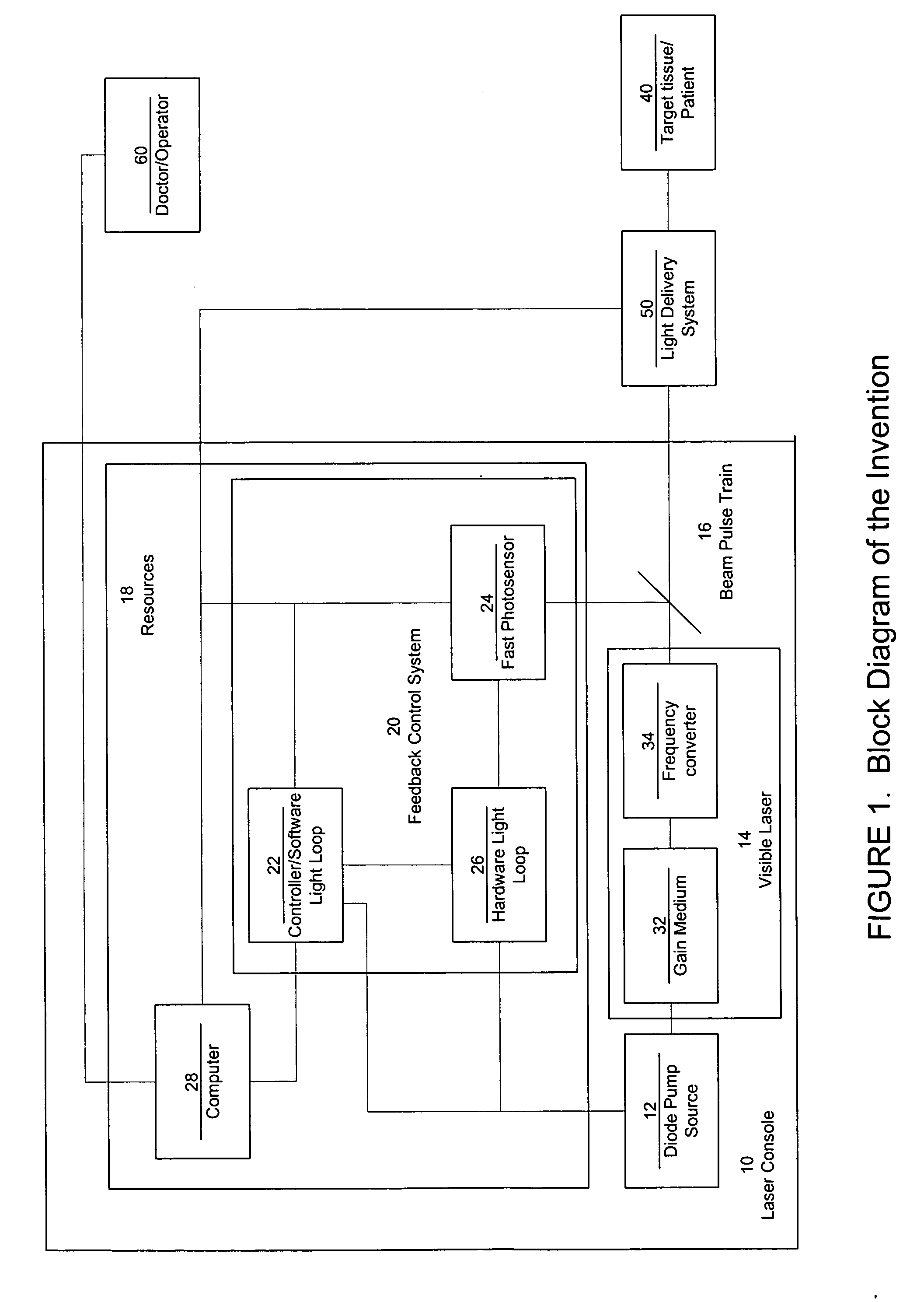
ActiveUS7771417B2Short and controlled pulse width trainsReduce power fluctuationsLaser surgeryLaser detailsPulse characteristicsRetinal damage
A laser system that includes a diode pump source. A frequency doubled solid state visible laser is pumped by the diode pump source and produces a pulsed laser output with a train of pulses. Resources provide instructions for the creation of the pulsed output, with on and off times that provide for substantial confinement of thermal effects at a target site. This laser system results in tissue specific photoactivation (or TSP) without photocoagulation damage to any of the adjacent tissues and without causing full thickness retinal damage and the associated vision loss.
Owner:IRIDEX CORP
Laser system with short pulse characteristics and its methods of use
ActiveUS20060187978A1Reduce thermal effectsShort and controlled pulse width trainsLaser surgeryLaser detailsPulse characteristicsRetinal damage
A laser system that includes a diode pump source. A frequency doubled solid state visible laser is pumped by the diode pump source and produces a pulsed laser output with a train of pulses. Resources provide instructions for the creation of the pulsed output, with on and off times that provide for substantial confinement of thermal effects at a target site. This laser system results in tissue specific photoactivation (or TSP) without photocoagulation damage to any of the adjacent tissues and without causing full thickness retinal damage and the associated vision loss.
Owner:IRIDEX CORP
Mechanical continuous mining method for gently inclined thin ore body
ActiveCN101975064ASolve the mining disasterResolutionUnderground miningSurface miningEngineeringDrill
The invention discloses a mechanical continuous mining method for a gently inclined thin ore body. Based on the principle of lengthening the slope distance to reduce the angle, ore blocks are arranged in an inclined way, so that an angle smaller than the original inclination angle can be formed between a working plane and the horizontal plane, thus creating an operating space for a drill jumbo and a scraper; a return air shaft is arranged at the corner of one ore room so as to be communicated with an upper horizontal return airway; each ore room is provided with an orepass used for the transmission of ores in the ore room; and a tunnel is respectively excavated along the strike direction above and below each ore room, the upper tunnel is used for ventilation connection, and the lower tunnel is used for ore removal connection. The invention is especially applicable to the mining of the gently inclined thin ore body of an underground mine, mining preparation has no requirements for undercutting projects, and the drill jumbos and scrapers are used for the operation, thereby achieving the characteristics of high degree of mechanization, high production efficiency, full-thickness continuous mining and low ore loss rate.
Owner:CENT SOUTH UNIV
Acellular cornea or acellular corneal stroma, preparation method and application thereof
InactiveCN101985051ARetain toughnessLow immunogenicityProsthesisFreeze thawingVaccine Immunogenicity
The invention discloses acellular cornea or acellular corneal stroma, a preparation method and application thereof. The method comprises the following steps of: (1) obtaining fresh animal full-thickness cornea or corneal stroma; (2) removing corneal epithelium, corneal endothelium and stroma cells, namely 1, soaking the full-thickness cornea or the corneal stroma in pure water at room temperature; 2, placing the soaked full-thickness cornea or corneal stroma into enzyme solution, digesting with oscillating, and washing with balanced salt solution with oscillating; and 3, repeating freeze-thaw processes of the full-thickness cornea or the corneal stroma for 4 to 8 times and washing with balanced salt solution with oscillating to obtain the acellular cornea or the acellular corneal stroma; (3) dehydrating; and (4) sterilizing and storing. In the method, the decellularization processing time of the cornea is short; the influence on the structure and the physiological property of the cornea is small; and the processed cornea has very low immunogenicity which is similar to the property of natural cornea. The acellular cornea or the acellular corneal stroma can be applied to artificial cornea construction of tissue engineering and also can serve as a medical material applied to corneal transplantation and refraction surgery.
Owner:JINAN UNIVERSITY
Endoscopic full thickness gastric reduction apparatus and method
InactiveUS20120239061A1Inhibition of contractionSmall sizeCannulasSurgical needlesGastric bandEndoscope
A device, adapted to receive one or more gastric bands and adapted to be coupled to a surgical instrument such as an endoscope, comprises a tubular main body and a cap comprising an opening through which a guidewire may be threaded. A triggering wire may be detachably coupled to one or more bands to permit remote removal of said bands. The bands may be improved bands comprising gripping points, an inner reinforcement ring, or an expanded surface area portion.A method for performing band ligation comprises the steps of: providing a device comprising a main body portion, a removably coupled cap, one or more gastric bands, and a band triggering wire; introducing the device through the mouth into the stomach; dislodging the cap; targeting and drying specific locations of the gastric wall; withdrawing a portion of the gastric wall into the device; and displacing one or more of the bands such that the band circumscribes said wall portion; and the administration of medication to prevent gastric contractions.
Owner:MATHUR SANDIP V
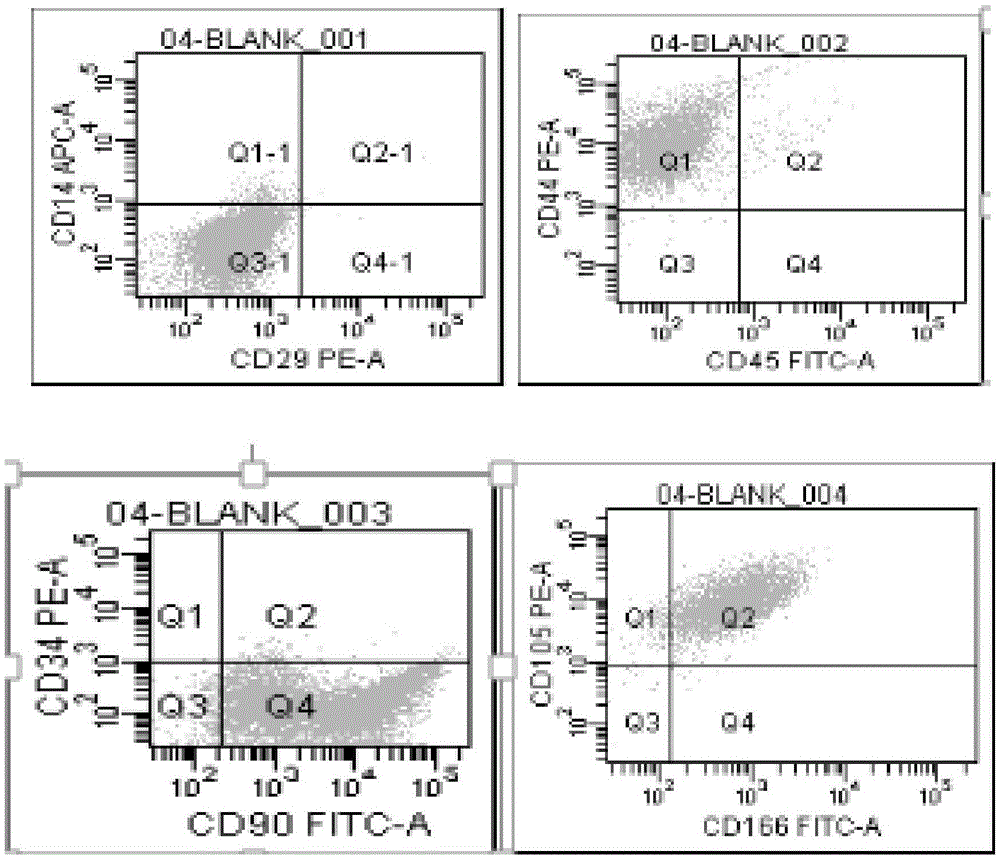
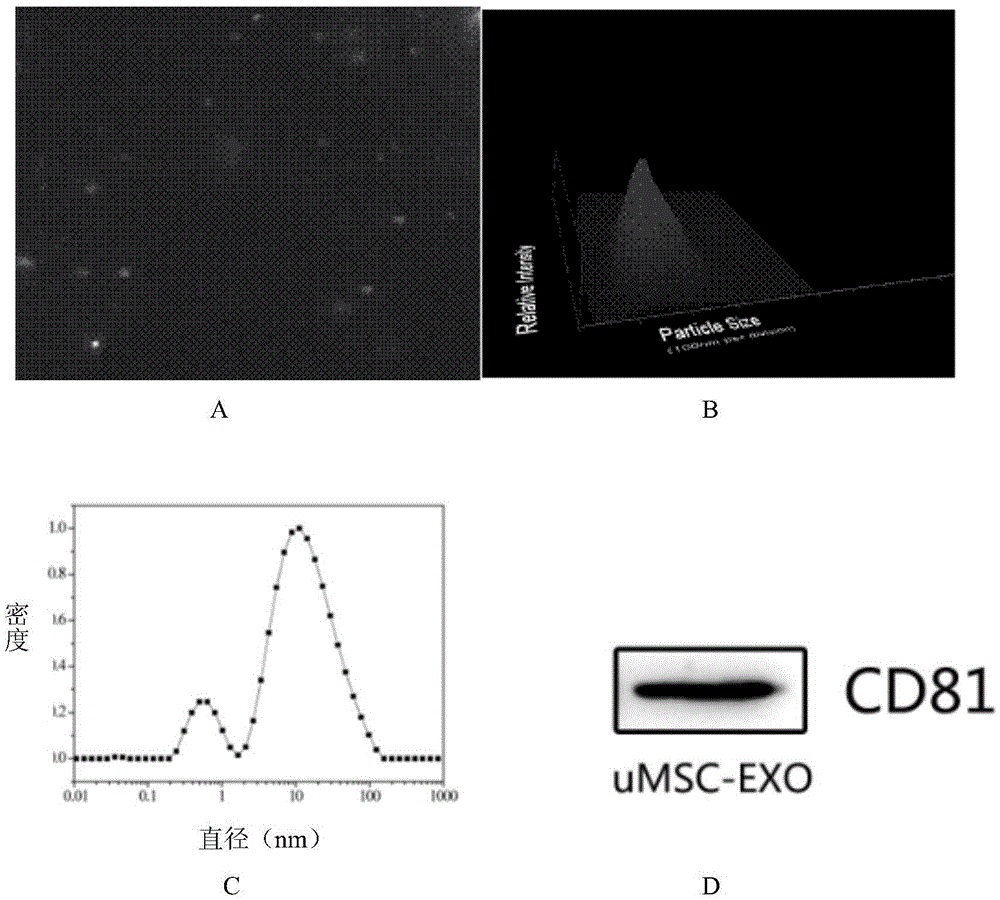
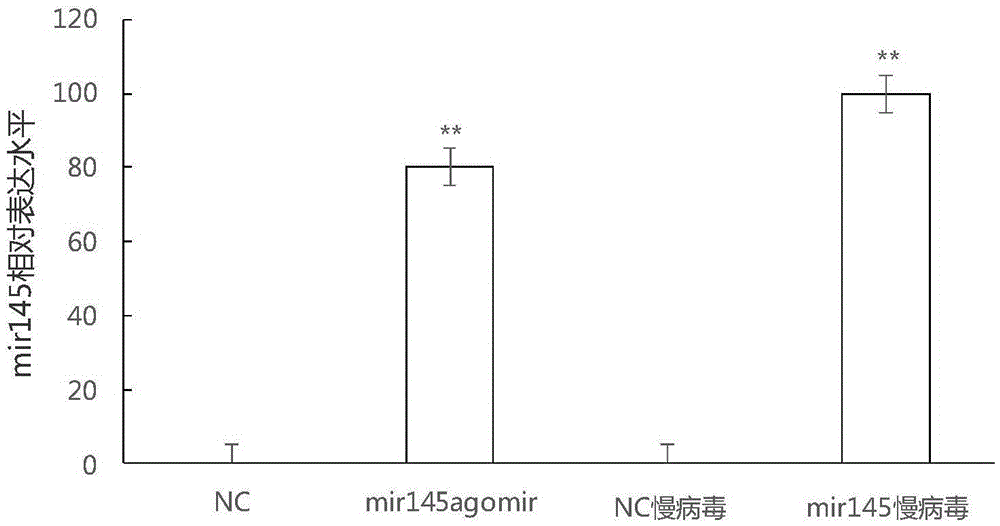
MiRNA145-5p-modified umbilical cord mesenchymal stem cell exosome and preparation and application of miRNA145-5p-modified umbilical cord mesenchymal stem cell exosome
ActiveCN105483081APowerfulTargetedSkeletal/connective tissue cellsUnknown materialsDefect healingMesenchymal stem cell
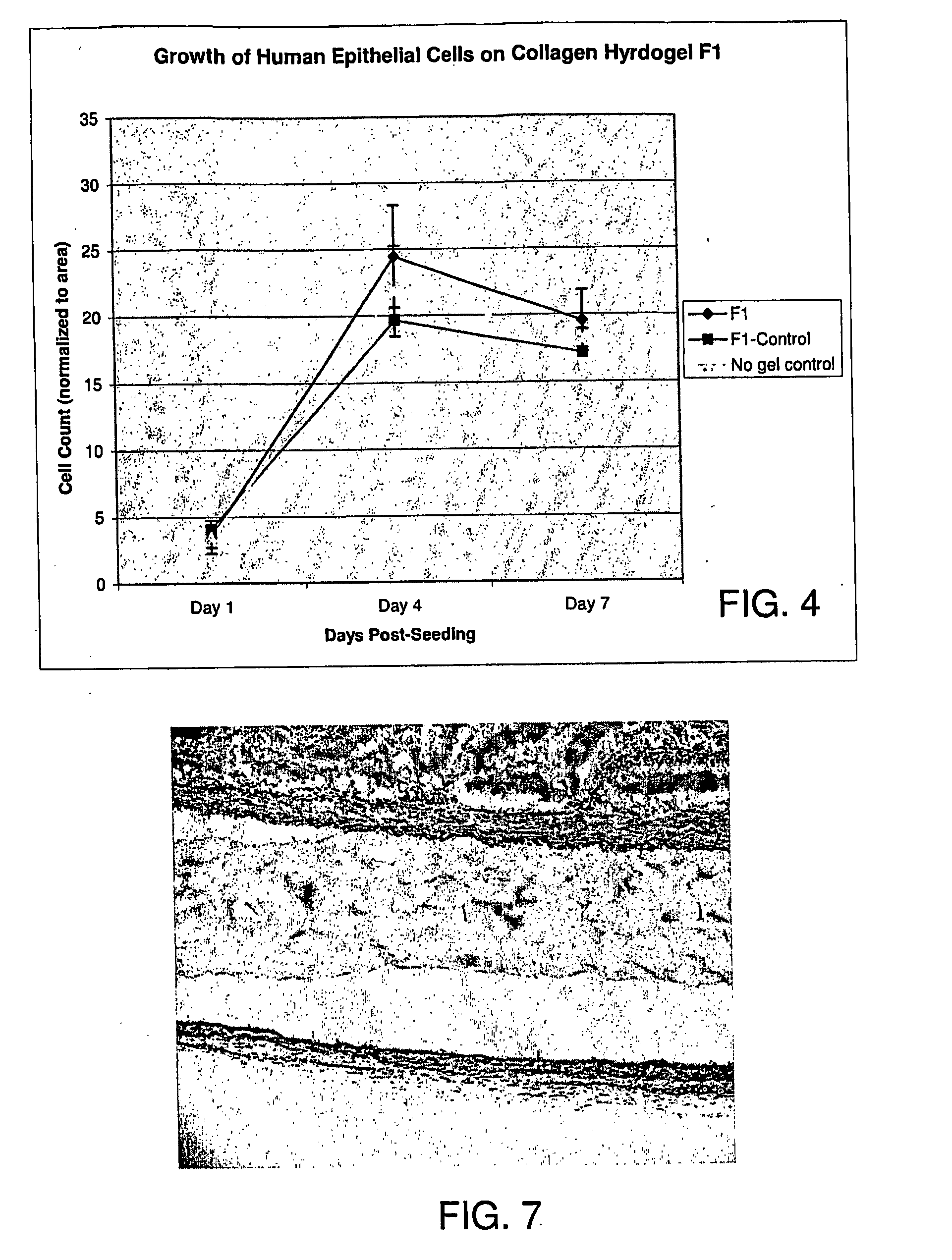
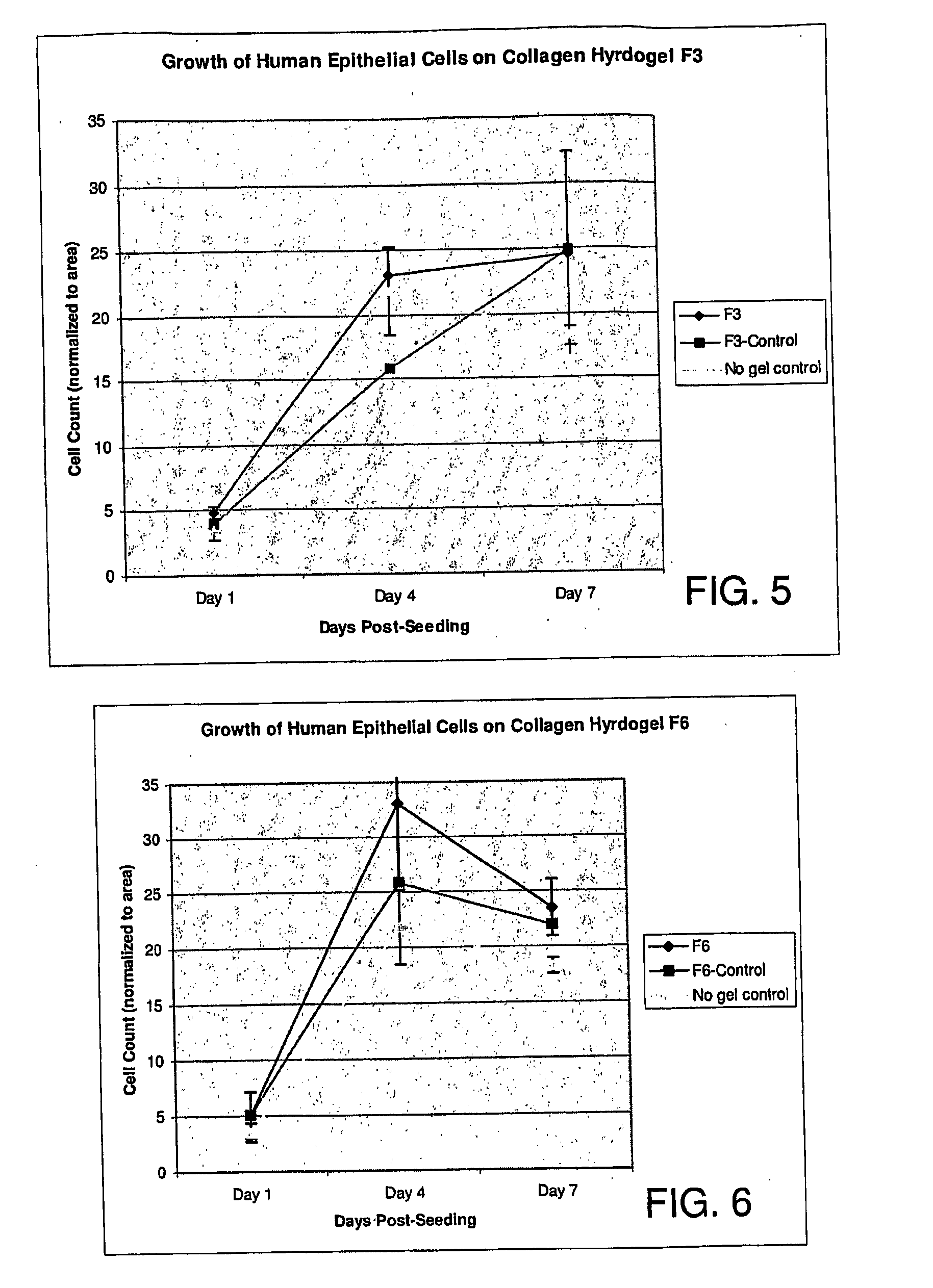
The invention belongs to the technical field of biology and particularly relates to a miRNA145-5p-modified umbilical cord mesenchymal stem cell exosome and preparation and application of the miRNA145-5p-modified umbilical cord mesenchymal stem cell exosome. The invention provides a preparation method of the highly-miRNA145-5p-expressed umsc-exosome (umbilical cord mesenchymal stem cell exosome) and application of various biological preparations for accelerating full-thickness skin defect healing and antagonizing scar contracture. The preparation method of the exosome includes (1), adopting fresh umbilical cords to culture human umbilical cord mesenchymal stem cells; (2) preparing mesenchymal stem cells highly expressed in miRNA145-5p; (3), storing conditional media; (4) performing exosome extraction and purification. The highly-miRNA145-5p-expressed umbilical cord mesenchymal stem cell exosome is transferred into a full-thickness wound model of rat backs by serving as a biological preparation, so that skin granulation tissue proliferation can be promoted effectively, wound healing is accelerated and scar contracture can be antagonized; novel approaches and novel methods are provided for wound treatment.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Compositions for topical application and other products from fresh beeswaxes
InactiveUS6099866AGood moisturizing effectHigh cure rateEdible oils/fats ingredientsCosmetic preparationsWrinkle skinBurned skin
The present invention is concerned with novel compositions containing fresh or virgin beeswaxes and oil, with and without water, and the utilization of such compositions for the treatment of skin in animals including man, for treatment for first, second, and third degree (partial and full thickness) burns due to sunburn, windburn, scalds, flash flame, electrical contact, chemical contact, cold, and the like, for use as a preventive aid for sunburn, windburns, skin chapping, chafing and the like, for promoting and accelerating the healing of burns, abrasions, lacerations, cuts, scratches, dry skin, chapped skin, wind burned skin, friction-type burns, chafing and the like, for use as a softening and moisturizing agent, for use in the reduction of wrinkles, for use as a topical application to irritations of the skin, including dry lips, chapped skin, abrasions, and the like, for use as an analgesic to be applied topically for the treatment and alleviation of pain in the skin and underlying tissues, for use as a protective barrier between the skin and other irritants, eg, saliva, urine, fecal material, various chemical irritants and the like, and for use as a base to which may be added other agents also promoting treatment, moisturization, protective or preventive measures for the skin.
Owner:SLIMAK K M
Methods and apparatus for transesophageal microaccess surgery
InactiveUS20100036197A1Avoid pollutionPrecise positioningBronchoscopesLaryngoscopesNODALOesophageal tube
The current invention describes methods of transesophageal access to the neck and thorax to perform surgical interventions on structures outside the esophagus in both the cervical and the thoracic cavity. It describes a liner device made of a complete or partial tubular structure, or a flat plate, the liner having means to facilitate creation of a side opening, which may include a valve. The liner with its side opening form a port structure inside the esophageal lumen. The port structure allows elongated surgical devices to pass through a perforation across the full thickness of the esophageal wall to outside location, in a controlled way. The elongated surgical devices can be diagnostic scopes, therapeutic scopes, manual elongated surgical devices, robotic arms or the like. After being deployed outside the esophagus, the surgical devices can access structures outside the esophagus, in the neck and thorax in 360 degrees of freedom around the esophageal circumference. These structures can be bony, cartilaginous, spinal, vascular, soft tissue, deep tissues, lymph nodal, cardiac, pulmonary, tracheal, nervous, muscular or diaphragmatic, skin and subcutaneous tissues of the neck, skin and subcutaneous tissues of the anterior chest wall, skin and subcutaneous tissues of the skin of the back, and skin and layers of the breast.
Owner:MICROACCESS
Disposable absorbent articles with skin health and odor control additives
The present invention relates to disposable absorbent articles comprising skin health and odor control additives for the treatment and prevention of skin irritation. The skin health treatment additive comprises an oil soluble wax that forms a tight lattice to protect the skin, and vitamins, lipids and triglycerides to protect and replenish the skin. Such disposable absorbent articles of the present invention are especially advantageous for the treatment and skin breakdown leading to partial full thickness wounds or pressure ulcers.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Coaxial via structure for optimizing signal transmission in multiple layer electronic device carriers
InactiveUS7091424B2Semiconductor/solid-state device detailsPrinted circuit aspectsHigh intensityEngineering
A coaxial via structure is adapted to transmit high speed signals or high intensity current through conductive layers of an electronic device carrier. The coaxial via structure comprises a central conductive track and an external conductive track separated by a dielectric material and is positioned in a core of the electronic device carrier or in the full thickness of the electronic device. The coaxial via structure can be combined with a stacked via structure so as allow efficient transmission of high speed signals across the electronic device carrier when a manufacturing process limits the creation of a full coaxial via structure across the entire electronic device carrier.
Owner:IBM CORP
Whole layer biological cornea as well as construction method and use thereof
The invention aims at providing a novel full-thickness biological cornea used for transplantation. The full-thickness biological cornea takes an animal cornea acellular matrix as a carrier, and the acellular matrix comprises animal cornea matrix cells, epithelial cells and endothelial cells which are cultured and augmented in vitro. The cornea is characterized in that the xenogenic corneal acellular matrix prepared by a biochemical method can be used as a good carrier for in vitro constructing the biological cornea in the aspects of shape, structure and biological compatibility, the matrix cells, epithelial cells and endothelial cells of the cornea are planted respectively, and dynamically cultured in the simulated in vivo environment of a biological reactor, so that the full-thickness biological cornea with nearly normal tissue structure and characteristics can be constructed in vitro, and the biological cornea can be used to simulate the physiological cornea for fundamental research on physiology, pathology and pharmacology; moreover, the biological cornea can also be directly used as the donator for corneal transplantation.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Display apparatus having a full-thickness through hole
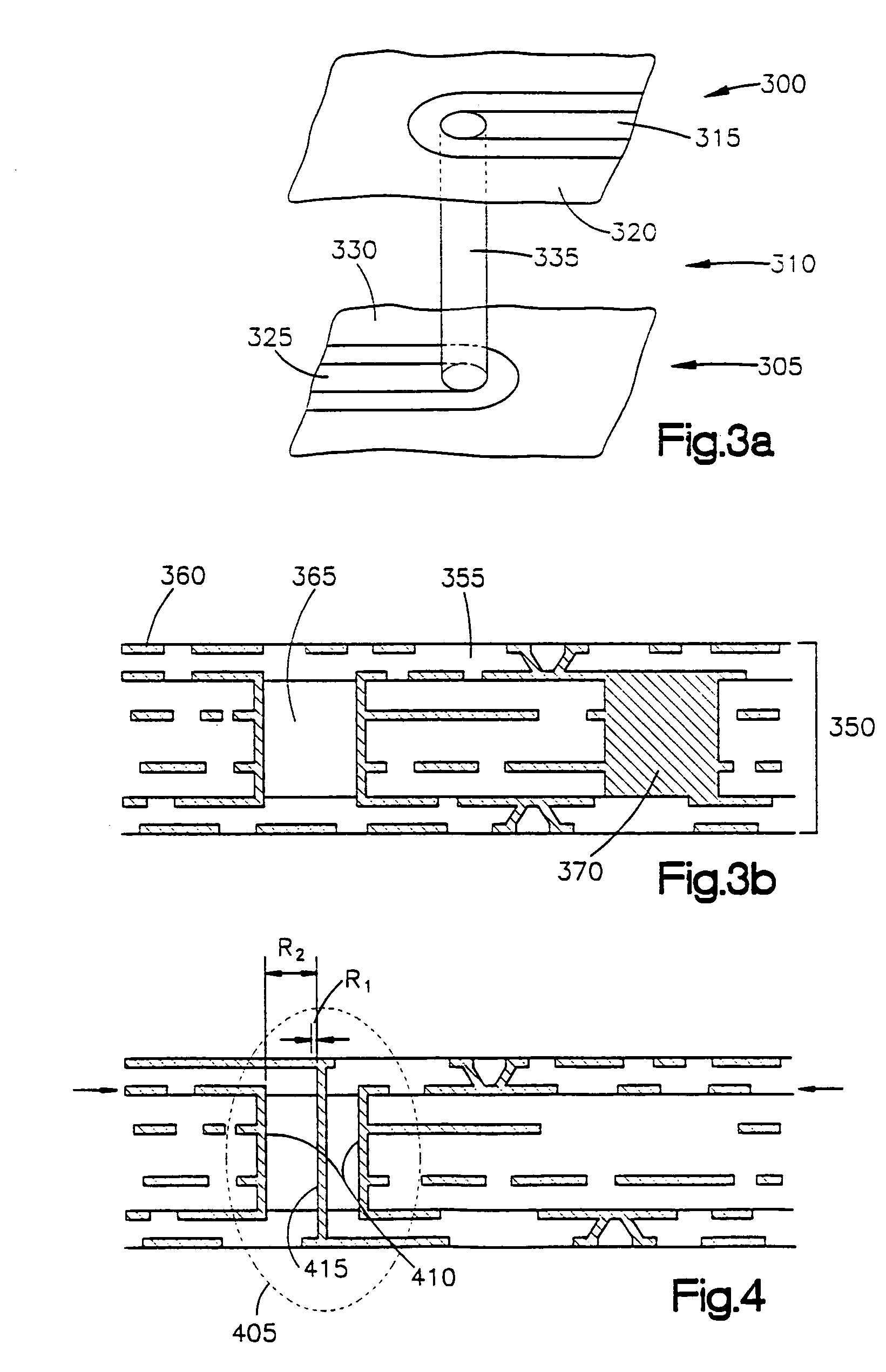
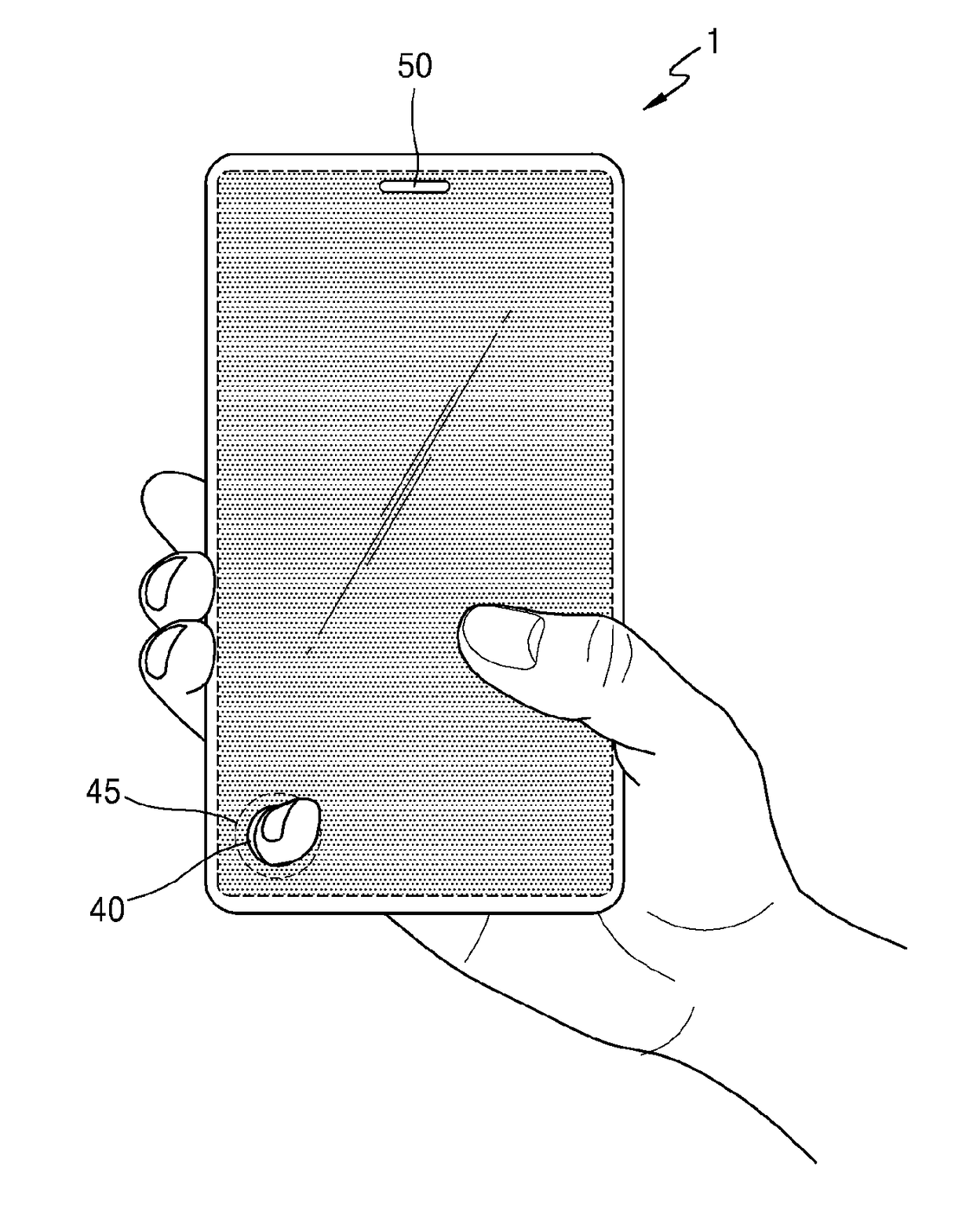
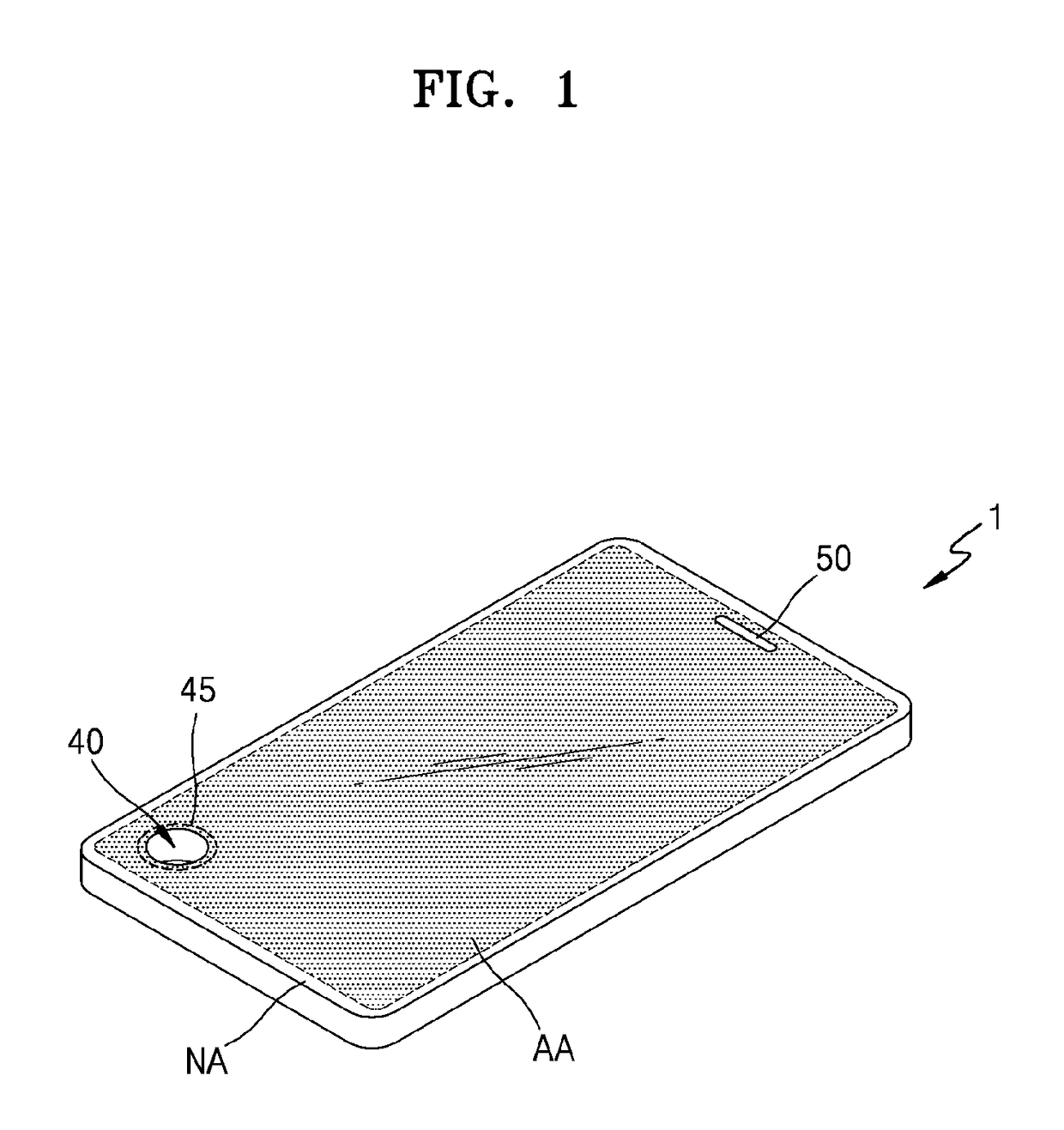
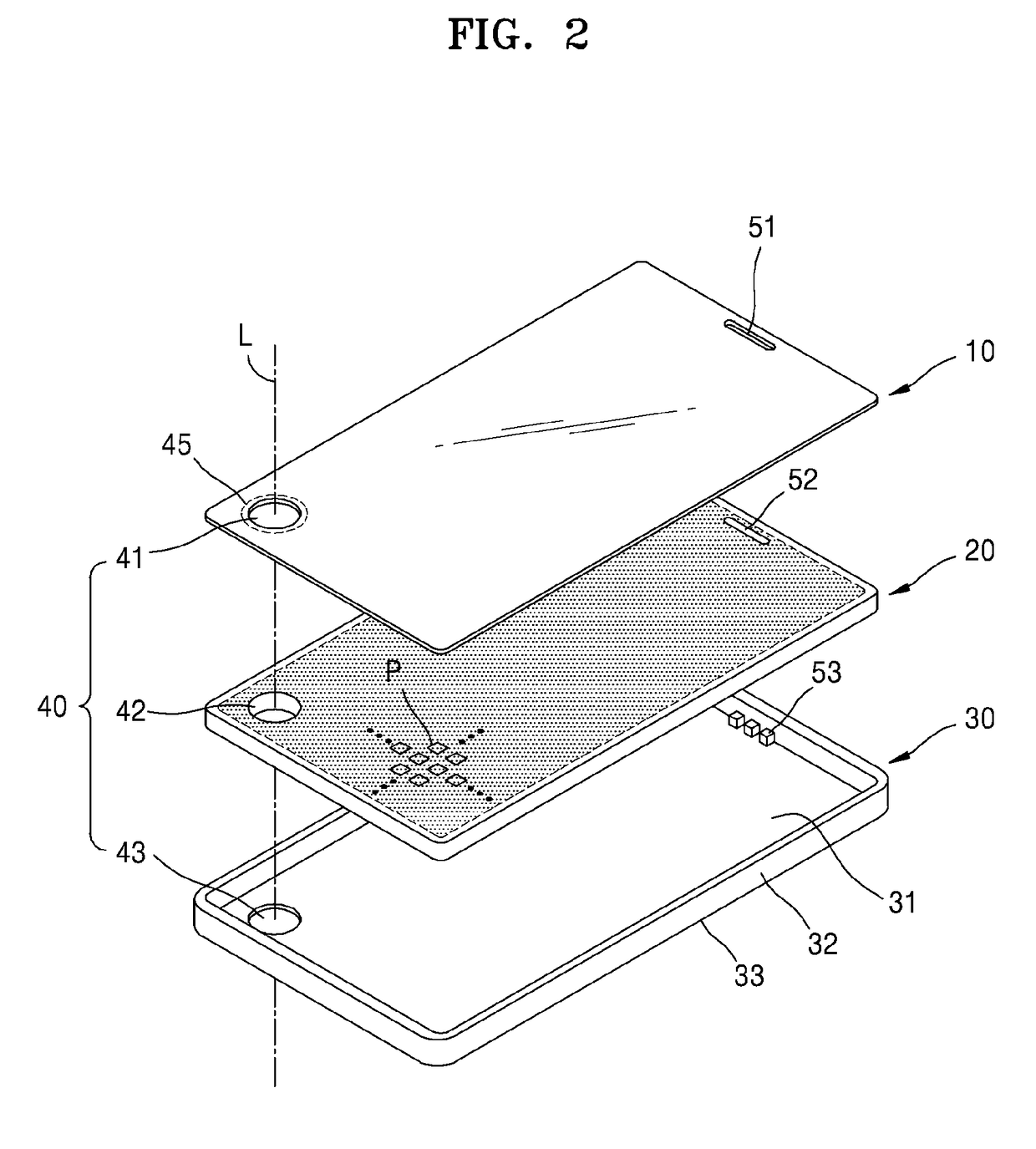
ActiveUS20170150618A1Function increaseInput/output for user-computer interactionBatteries circuit arrangementsFull thicknessComputer engineering
Owner:SAMSUNG DISPLAY CO LTD
Ophthalmic Device and Related Methods and Compositions
Devices, methods, and compositions for improving vision or treating diseases, disorders or injury of the eye are described. Ophthalmic devices, such as corneal onlays, corneal inlays, and full-thickness corneal implants, are made of a material that is effective in facilitating nerve growth through or over the device. The material may include an amount of collagen greater than 1% (w / w), such as between about 10% (w / w) and about 30% (w / w). The material may include collagen polymers and / or a second biopolymer or water-soluble synthetic polymer cross-linked using EDC / NHS chemistry. The material may additionally comprise a synthetic polymer. The devices are placed into an eye to correct or improve the vision of an individual or to treat a disease, disorder or injury of an eye of an individual.
Owner:OTTAWA HOSPITAL RES INST +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com