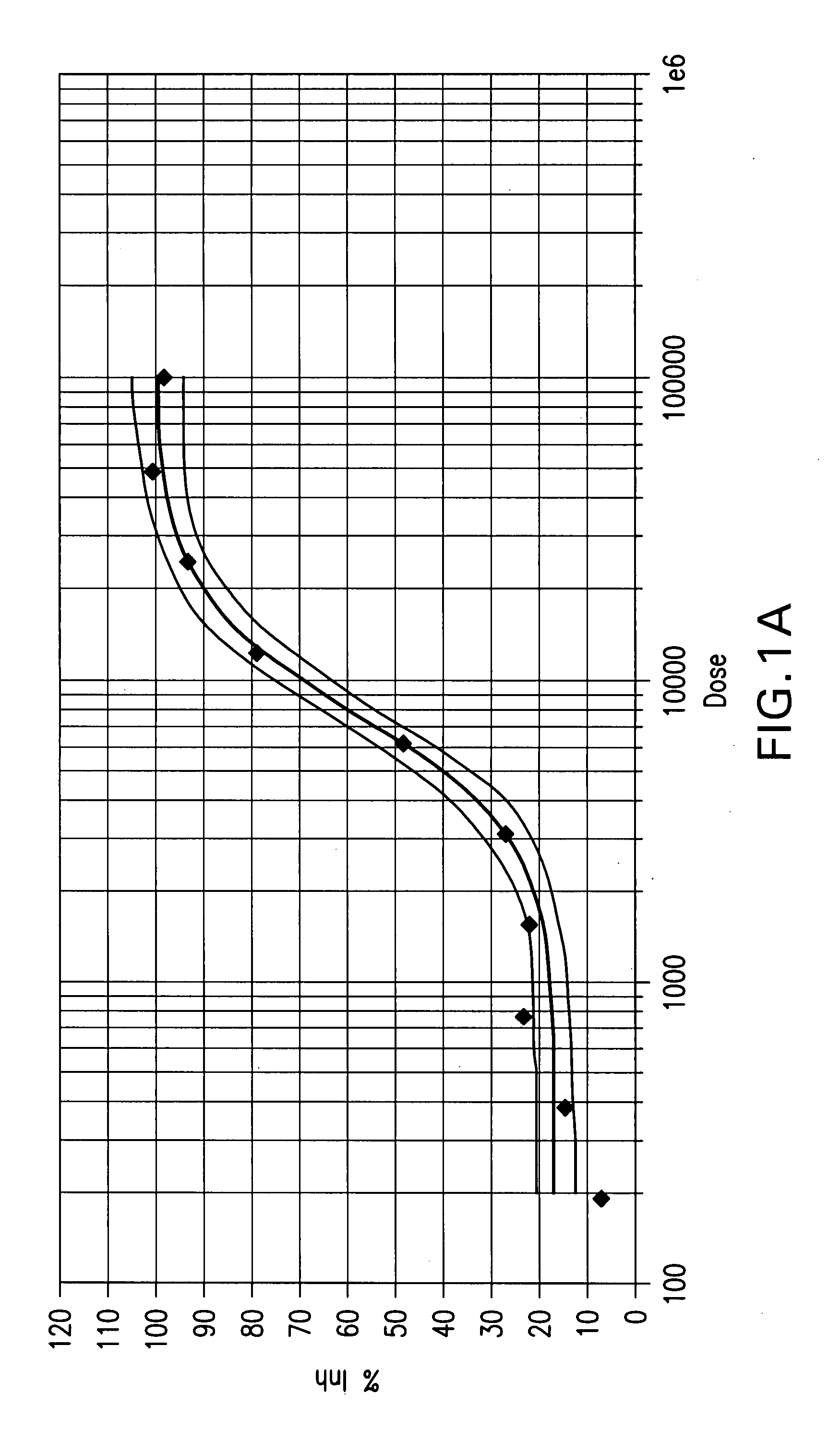
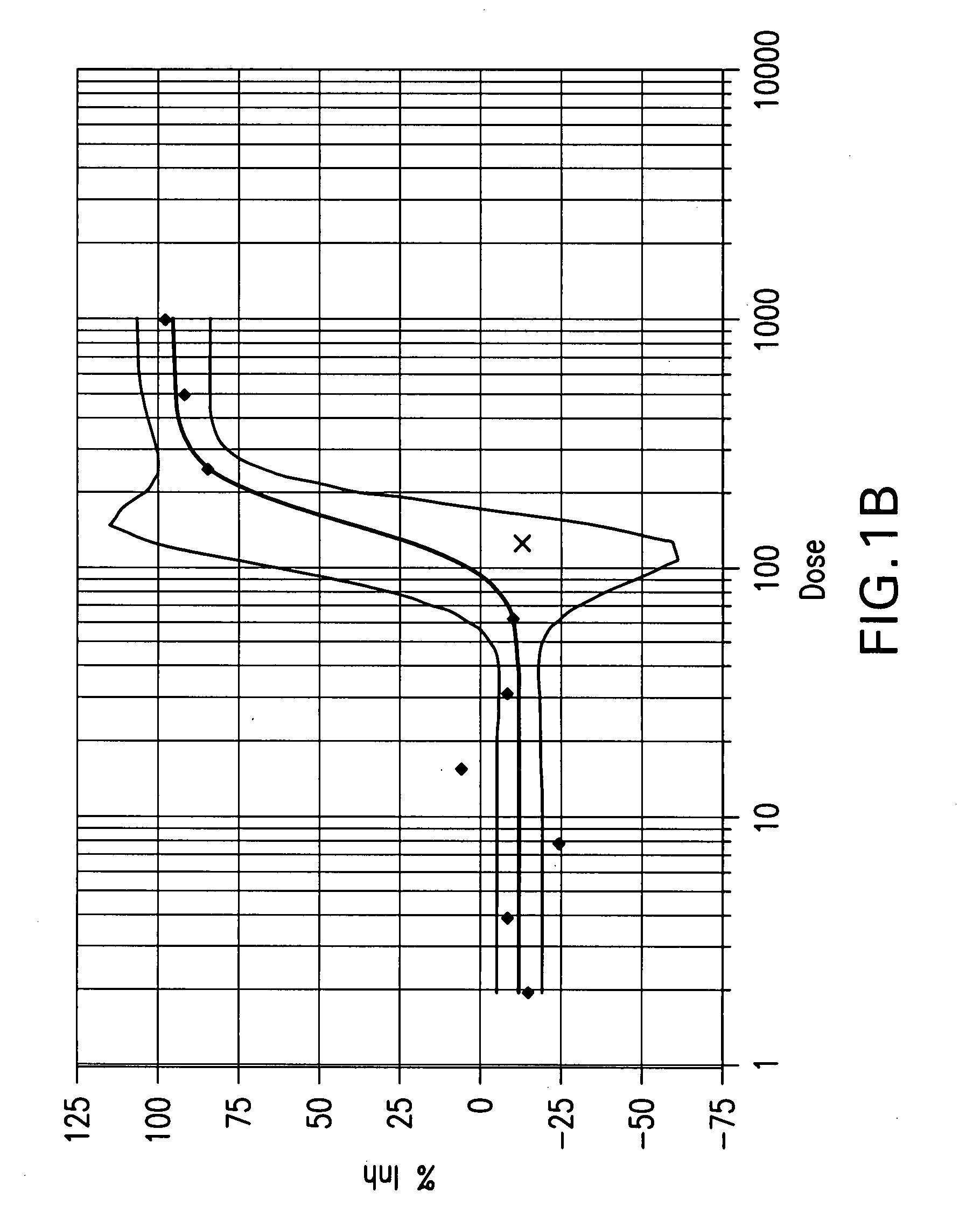
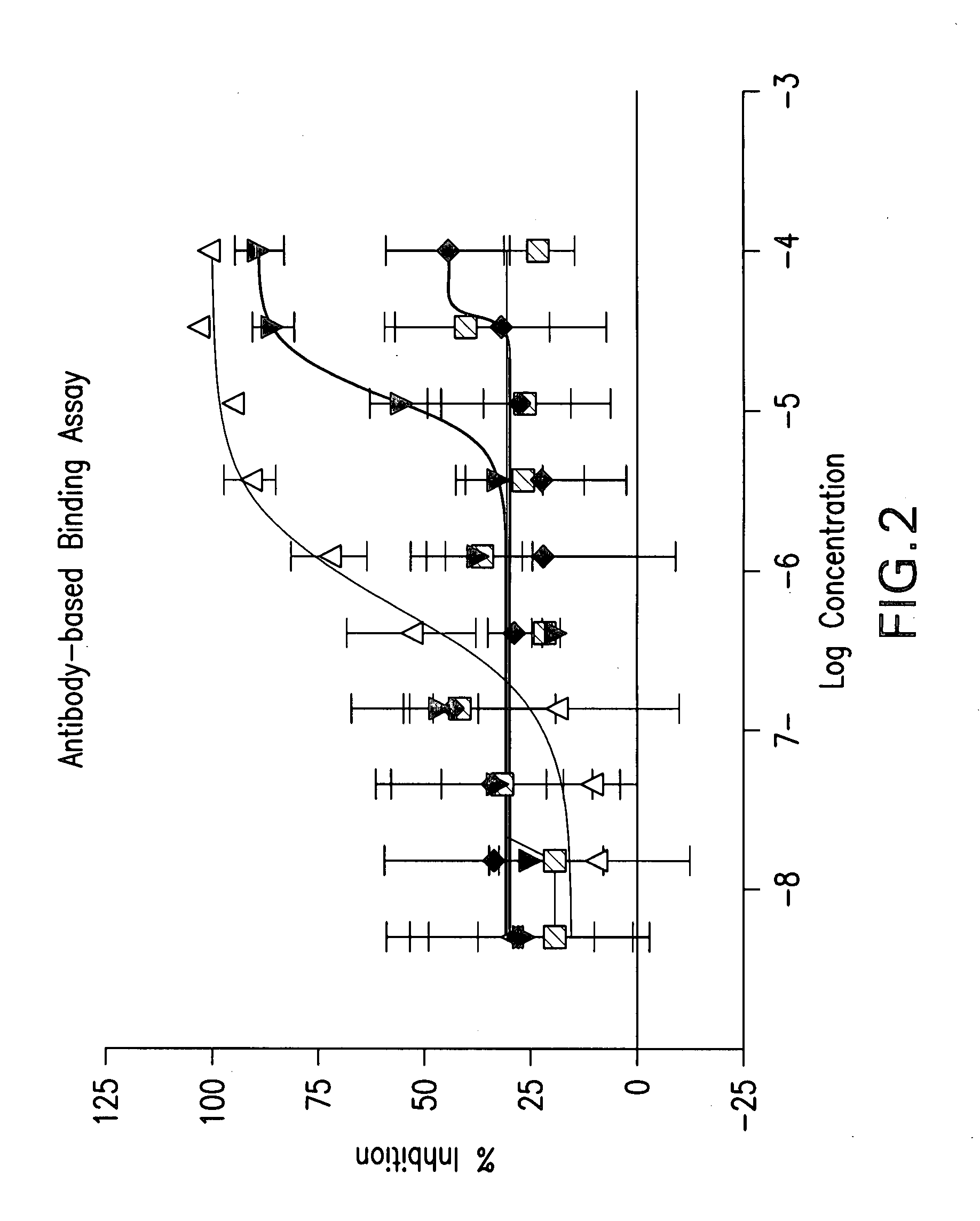
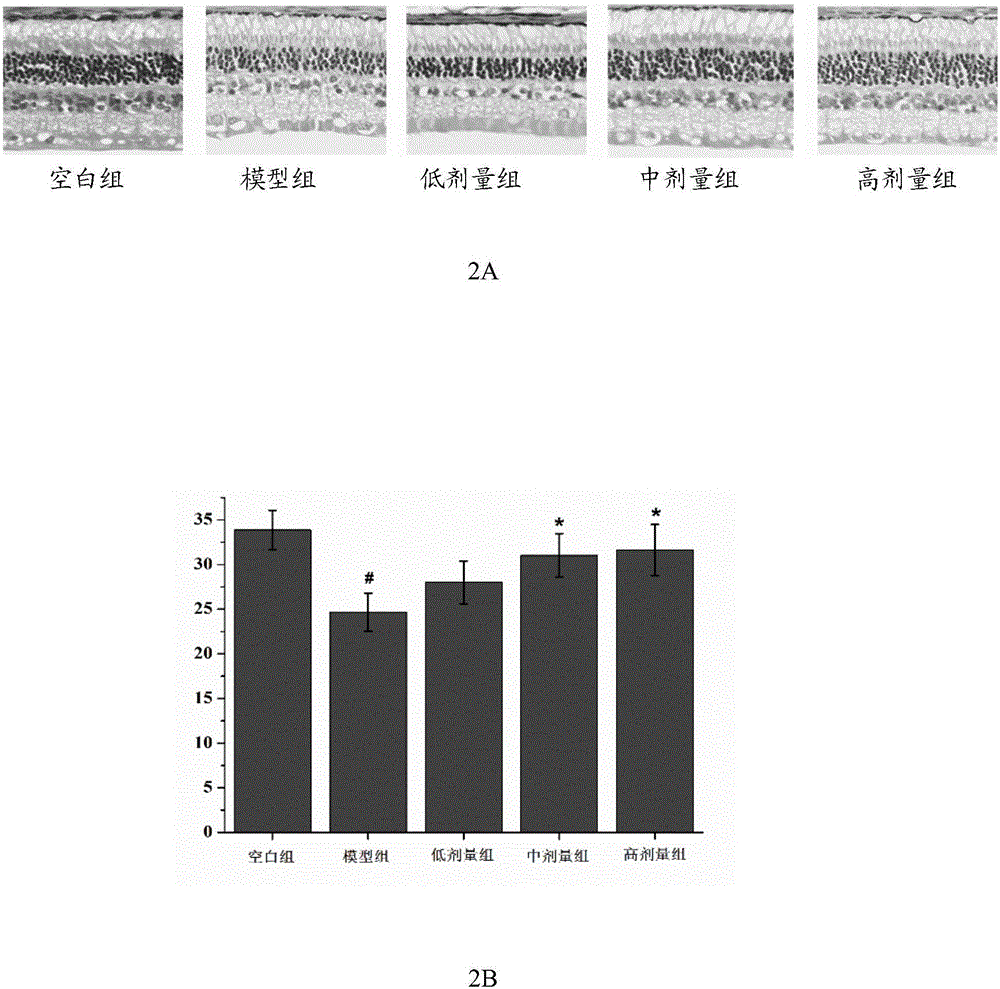
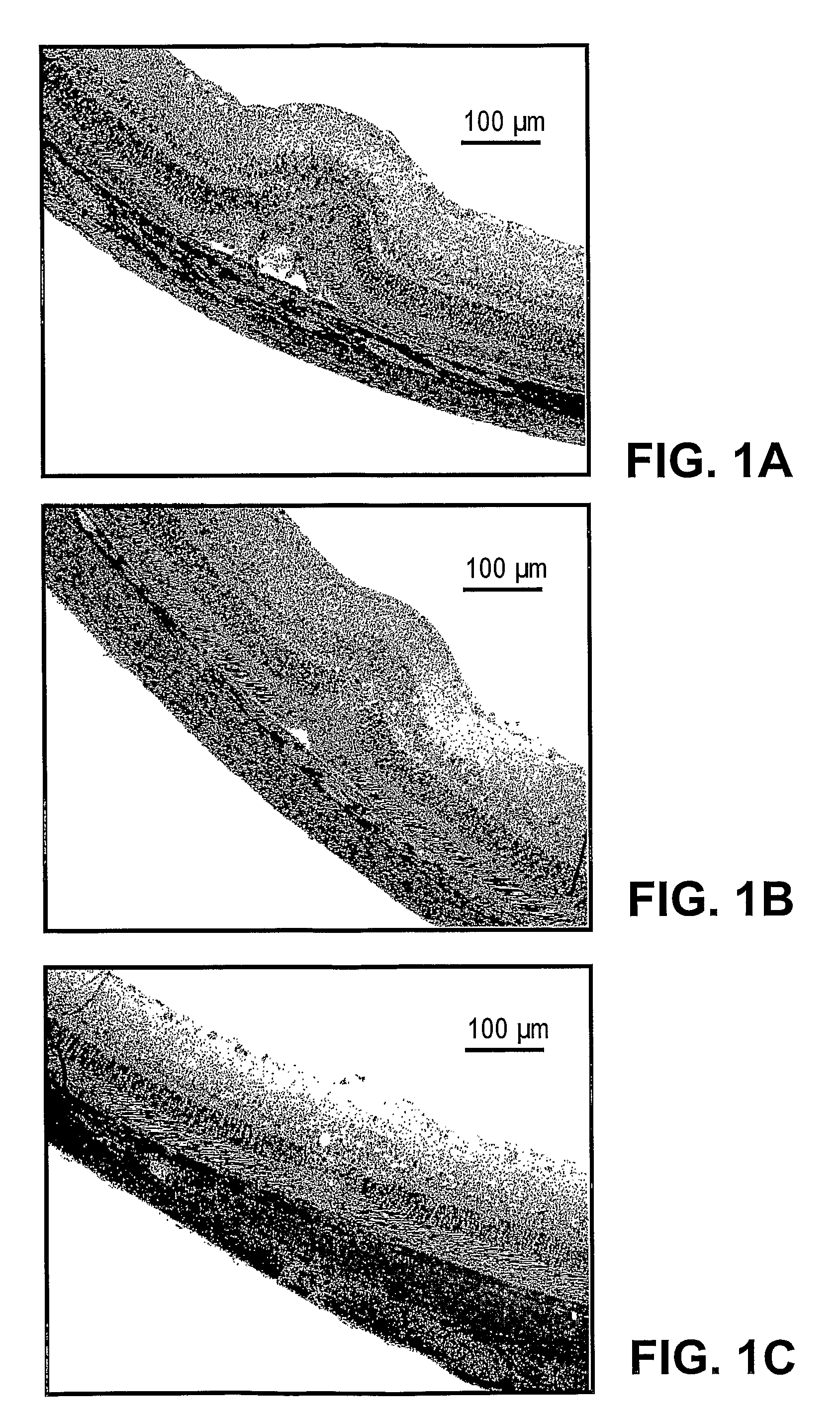
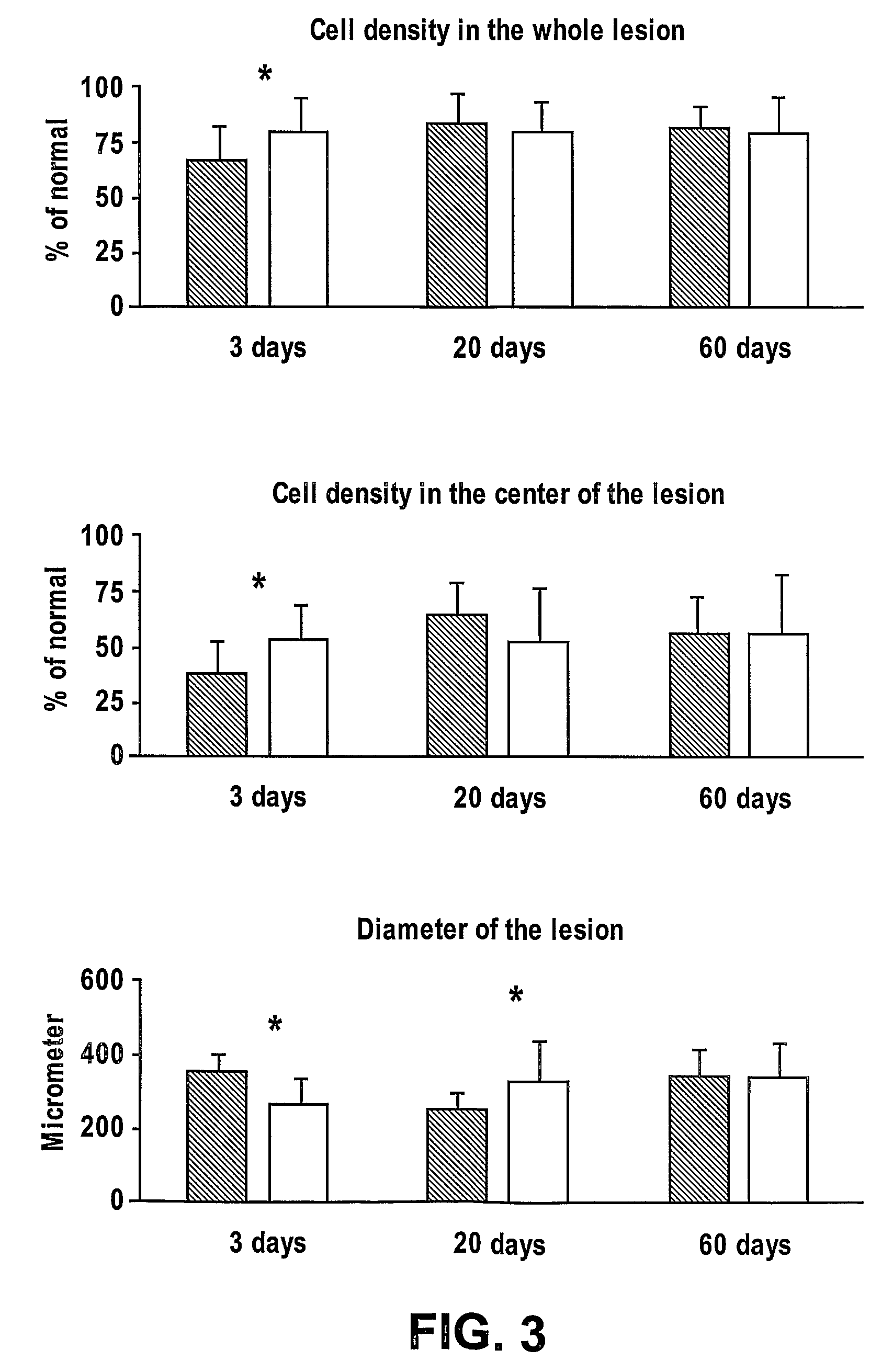
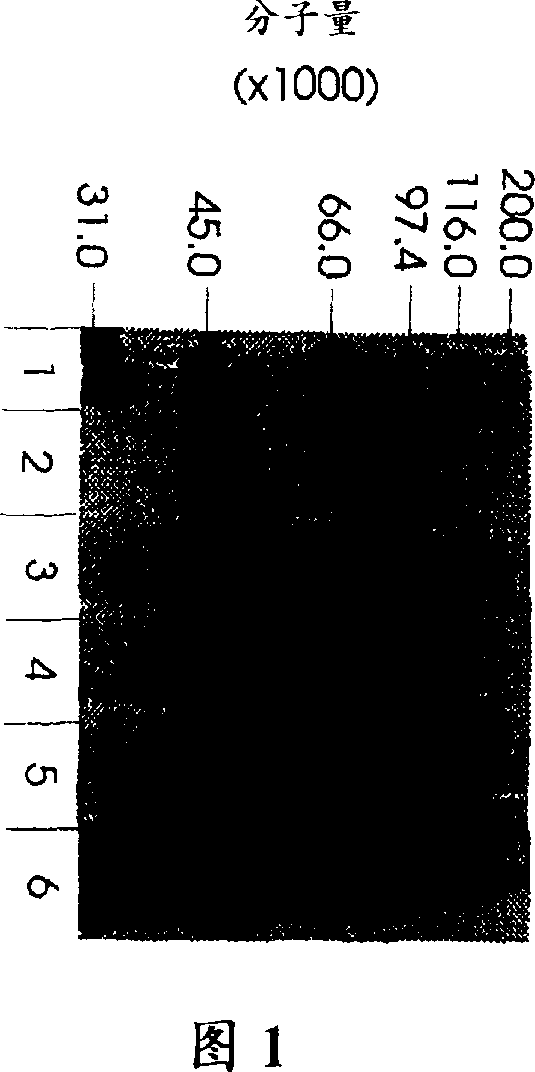
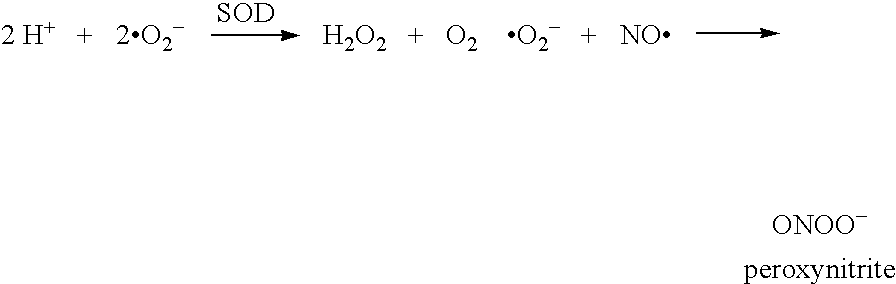Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
60 results about "Retinal damage" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vitamin deficiency can cause retina damage. Laser pointers often come with warnings about possible retina damages from pointing the beam at someone's eyes. The retina is in the back of the eye and can be damaged in numerous ways. The lasers used in barcode scanners could damage the retina if shined in the eye.
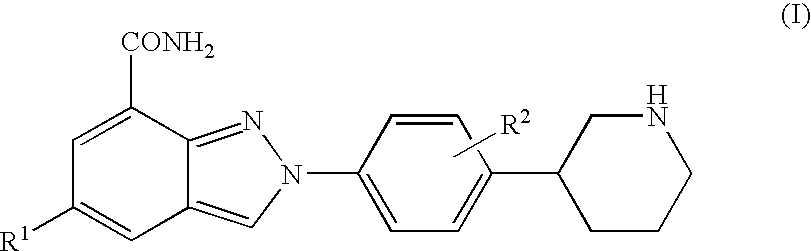
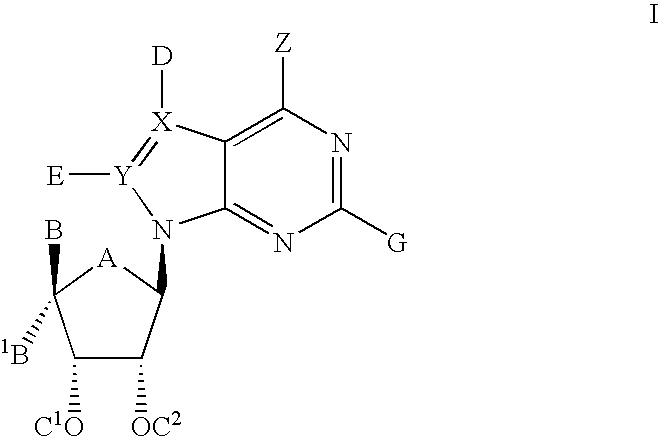
Amide substituted indazoles as poly(ADP-ribose)polymerase(PARP) inhibitors
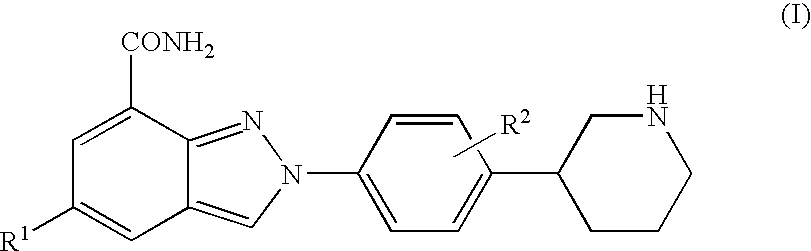
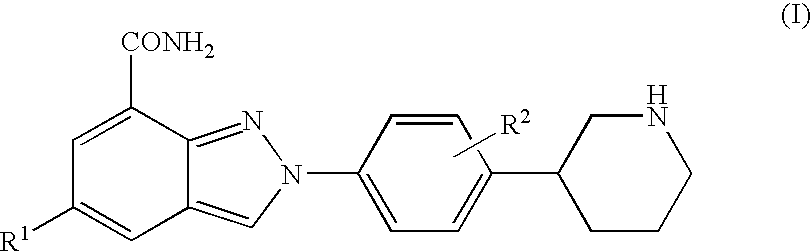
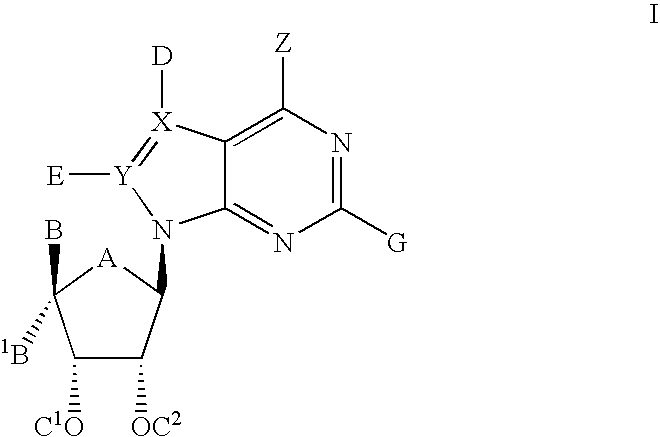
The present invention relates to compounds of formula I:and pharmaceutically acceptable salts, stereoisomers or tautomers thereof which are inhibitors of poly (ADP-ribose) polymerase (PARP) and thus useful for the treatment of cancer, inflammatory diseases, reperfusion injuries, ischemic conditions, stroke, renal failure, cardiovascular diseases, vascular diseases other than cardiovascular diseases, diabetes, neurodegenerative diseases, retroviral infection, retinal damage or skin senescence and UV-induced skin damage, and as chemo- and / or radiosensitizers for cancer treatment.
Owner:MERCK SHARP & DOHME LLC
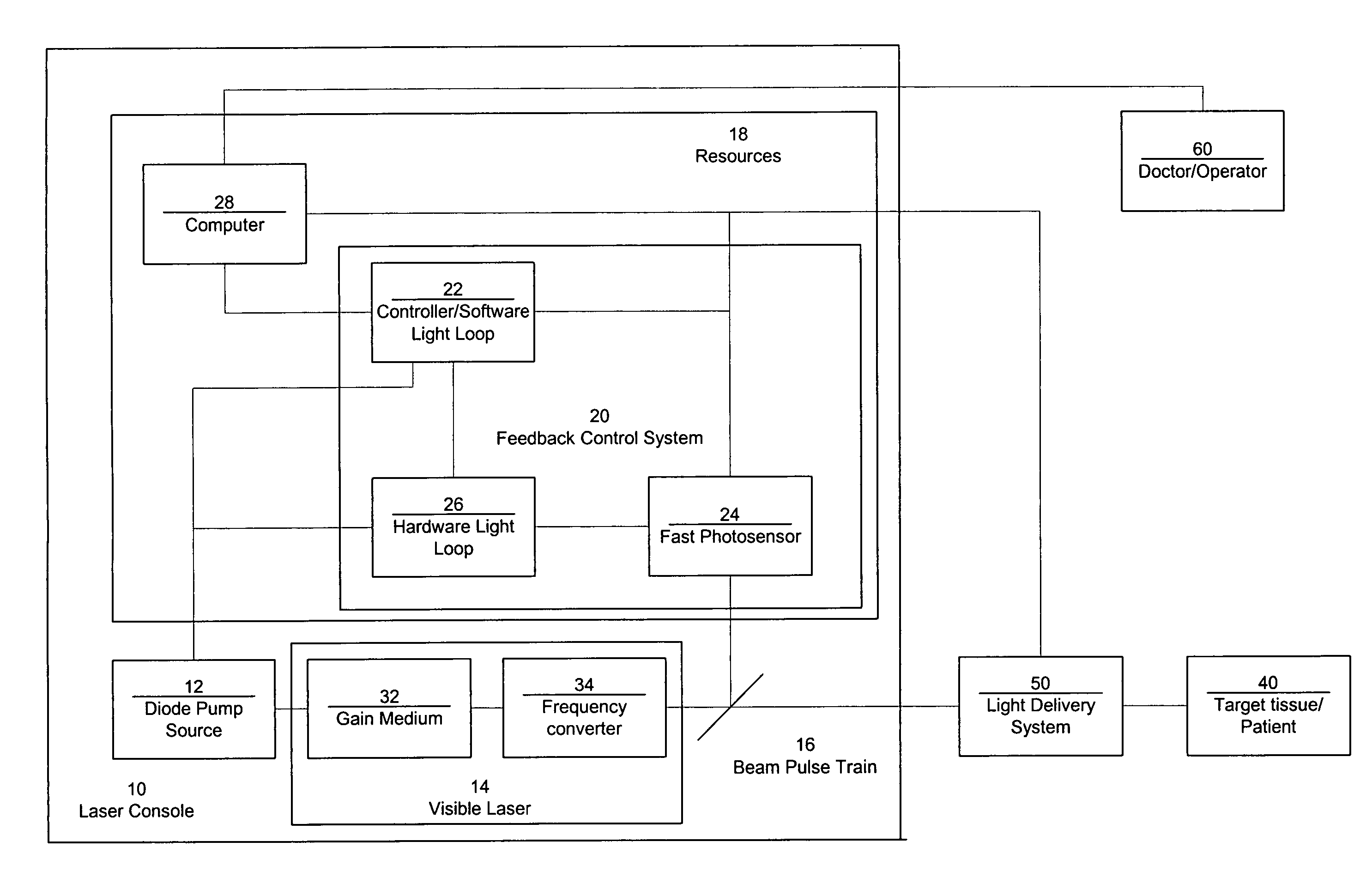
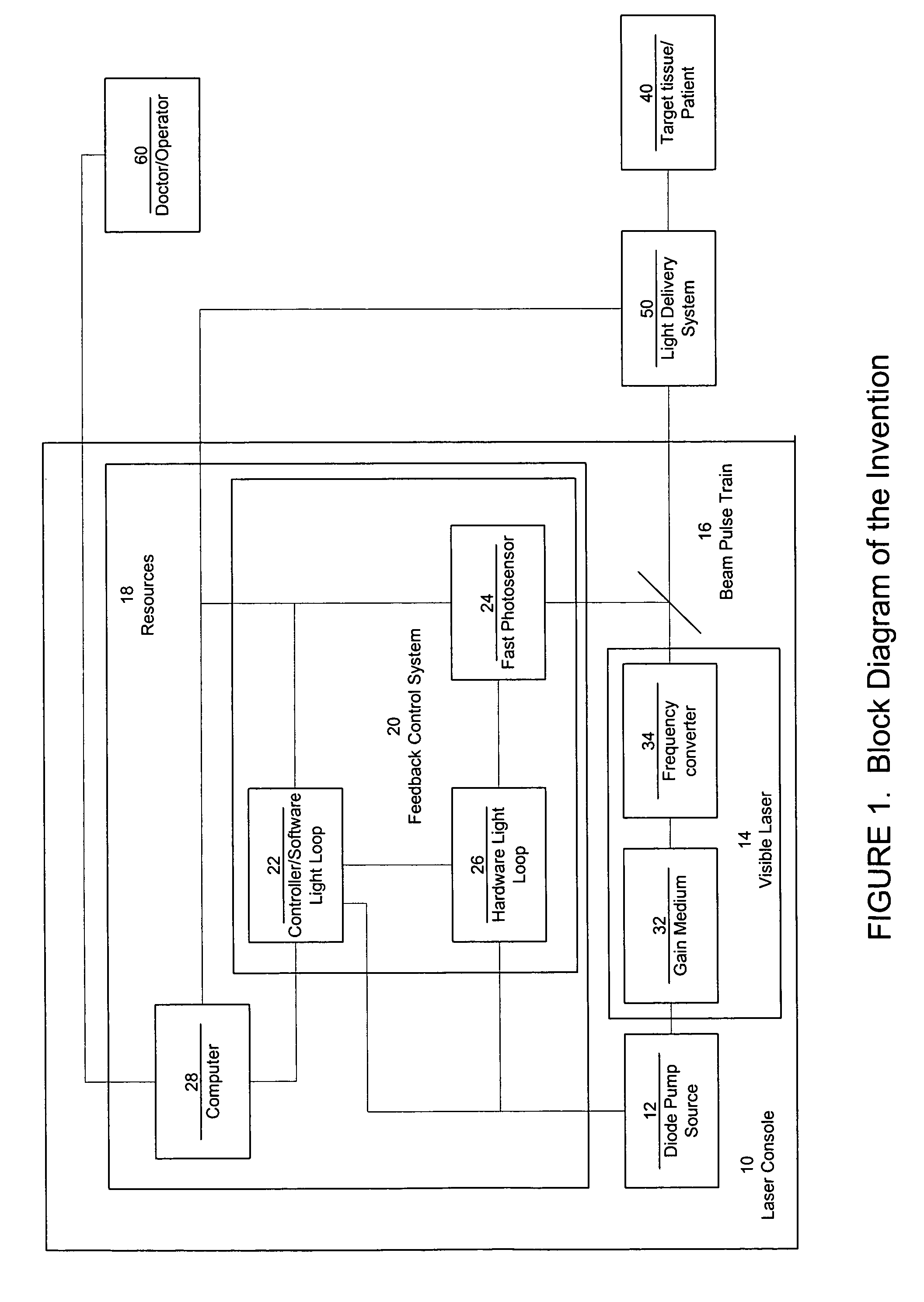
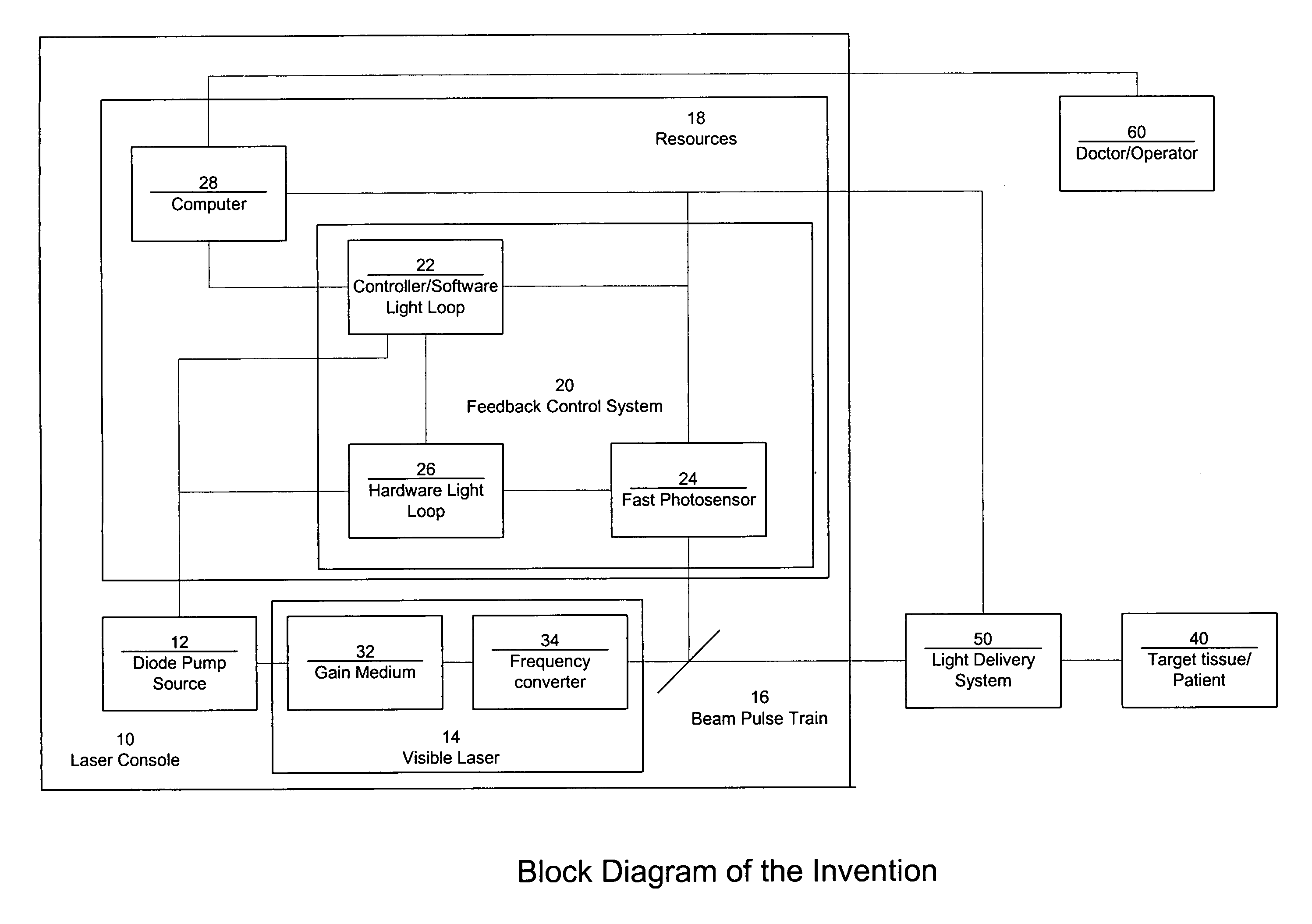
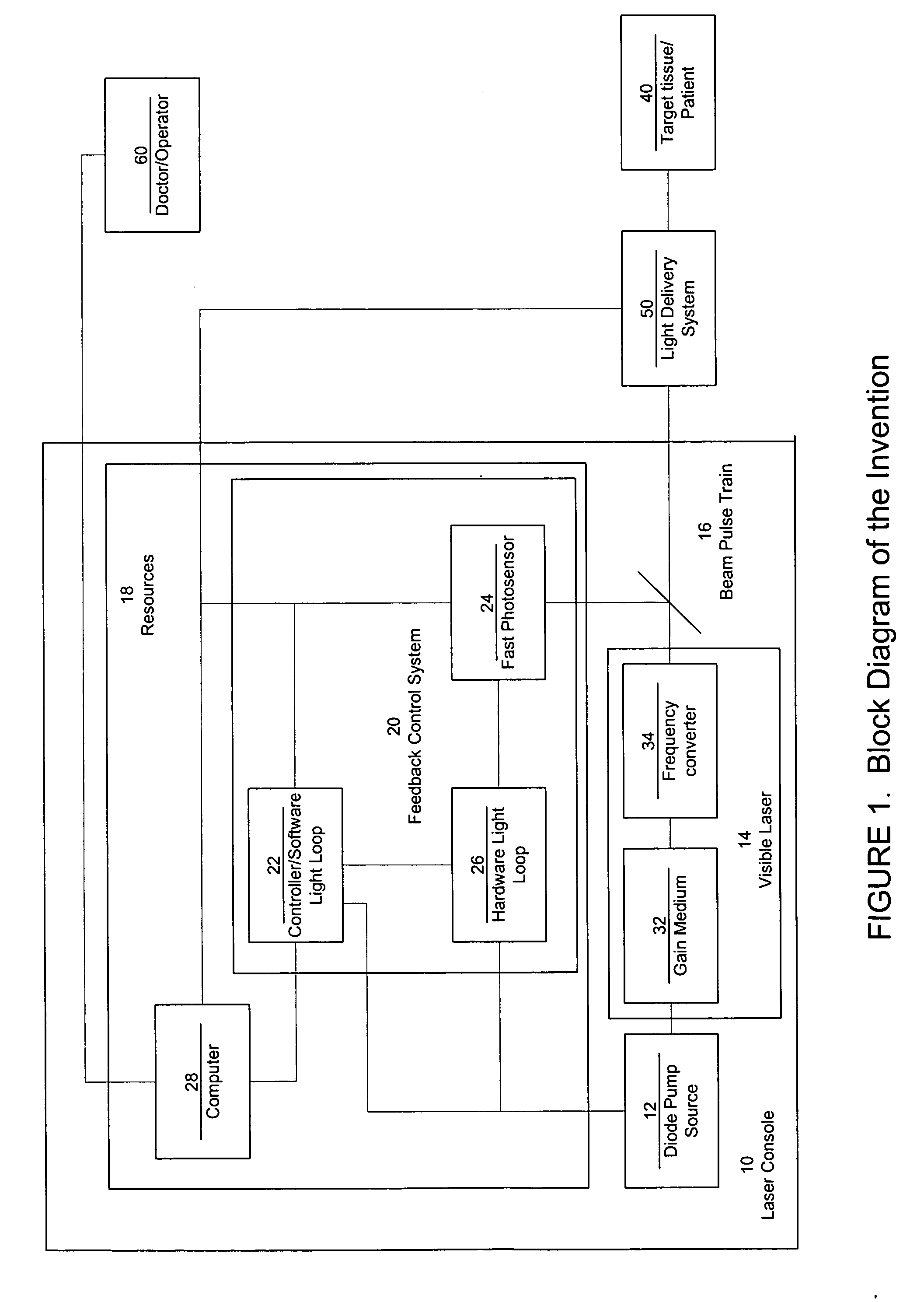
Laser system with short pulse characteristics and its methods of use
ActiveUS7771417B2Short and controlled pulse width trainsReduce power fluctuationsLaser surgeryLaser detailsPulse characteristicsRetinal damage
A laser system that includes a diode pump source. A frequency doubled solid state visible laser is pumped by the diode pump source and produces a pulsed laser output with a train of pulses. Resources provide instructions for the creation of the pulsed output, with on and off times that provide for substantial confinement of thermal effects at a target site. This laser system results in tissue specific photoactivation (or TSP) without photocoagulation damage to any of the adjacent tissues and without causing full thickness retinal damage and the associated vision loss.
Owner:IRIDEX CORP
Laser system with short pulse characteristics and its methods of use
ActiveUS20060187978A1Reduce thermal effectsShort and controlled pulse width trainsLaser surgeryLaser detailsPulse characteristicsRetinal damage
A laser system that includes a diode pump source. A frequency doubled solid state visible laser is pumped by the diode pump source and produces a pulsed laser output with a train of pulses. Resources provide instructions for the creation of the pulsed output, with on and off times that provide for substantial confinement of thermal effects at a target site. This laser system results in tissue specific photoactivation (or TSP) without photocoagulation damage to any of the adjacent tissues and without causing full thickness retinal damage and the associated vision loss.
Owner:IRIDEX CORP
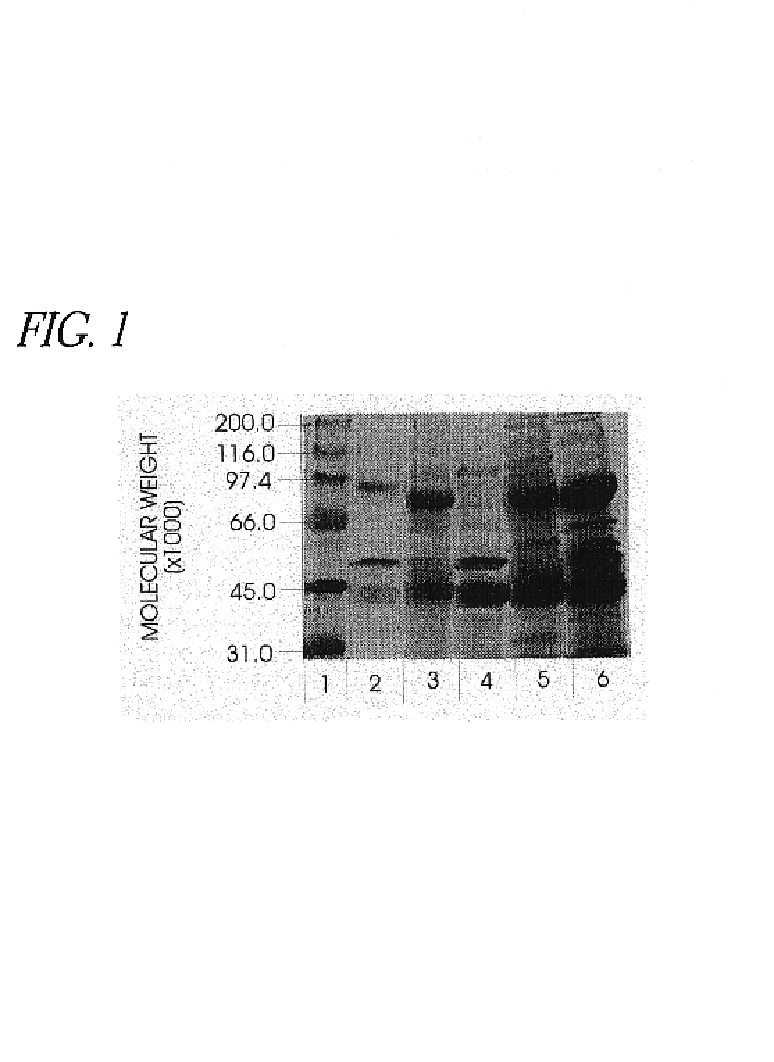
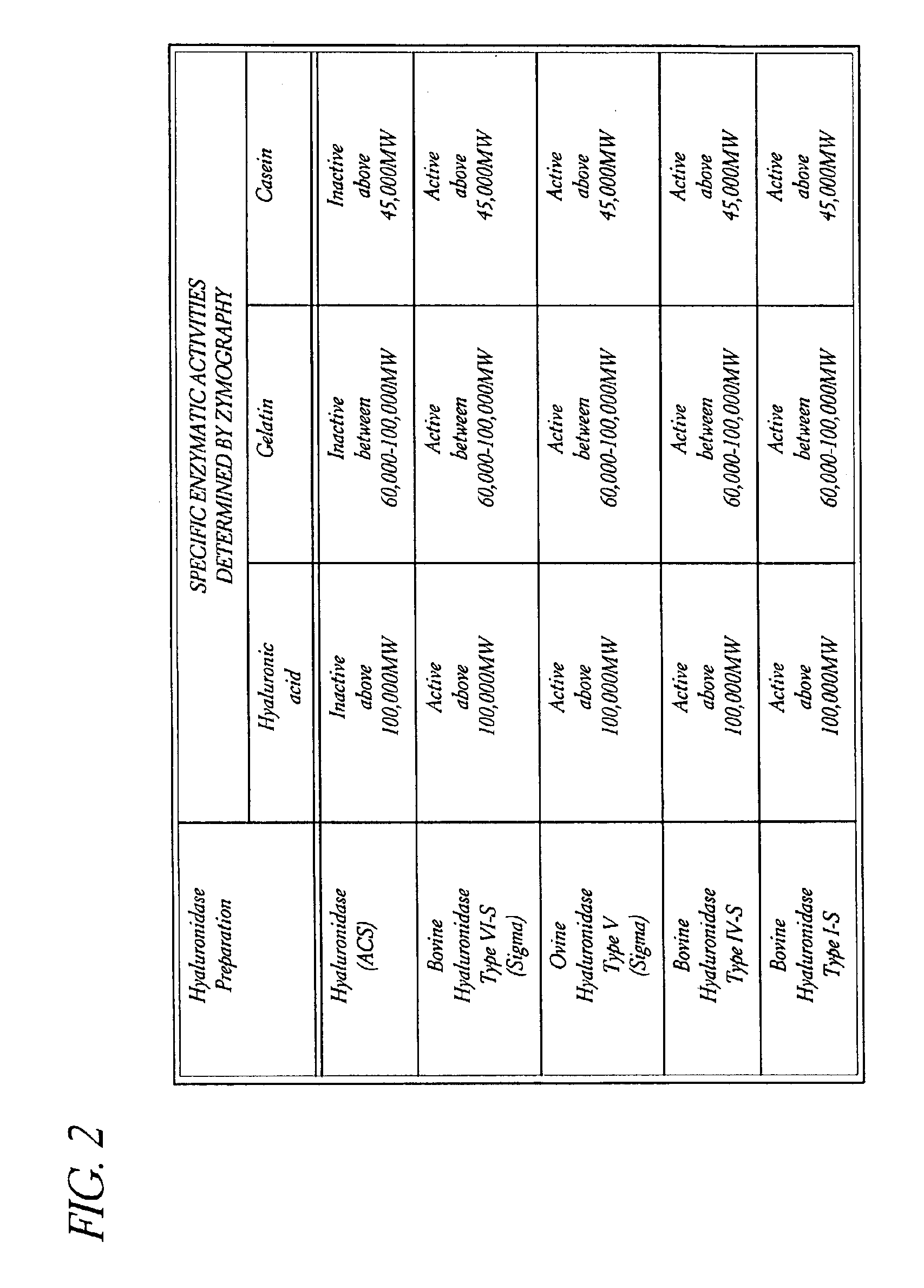
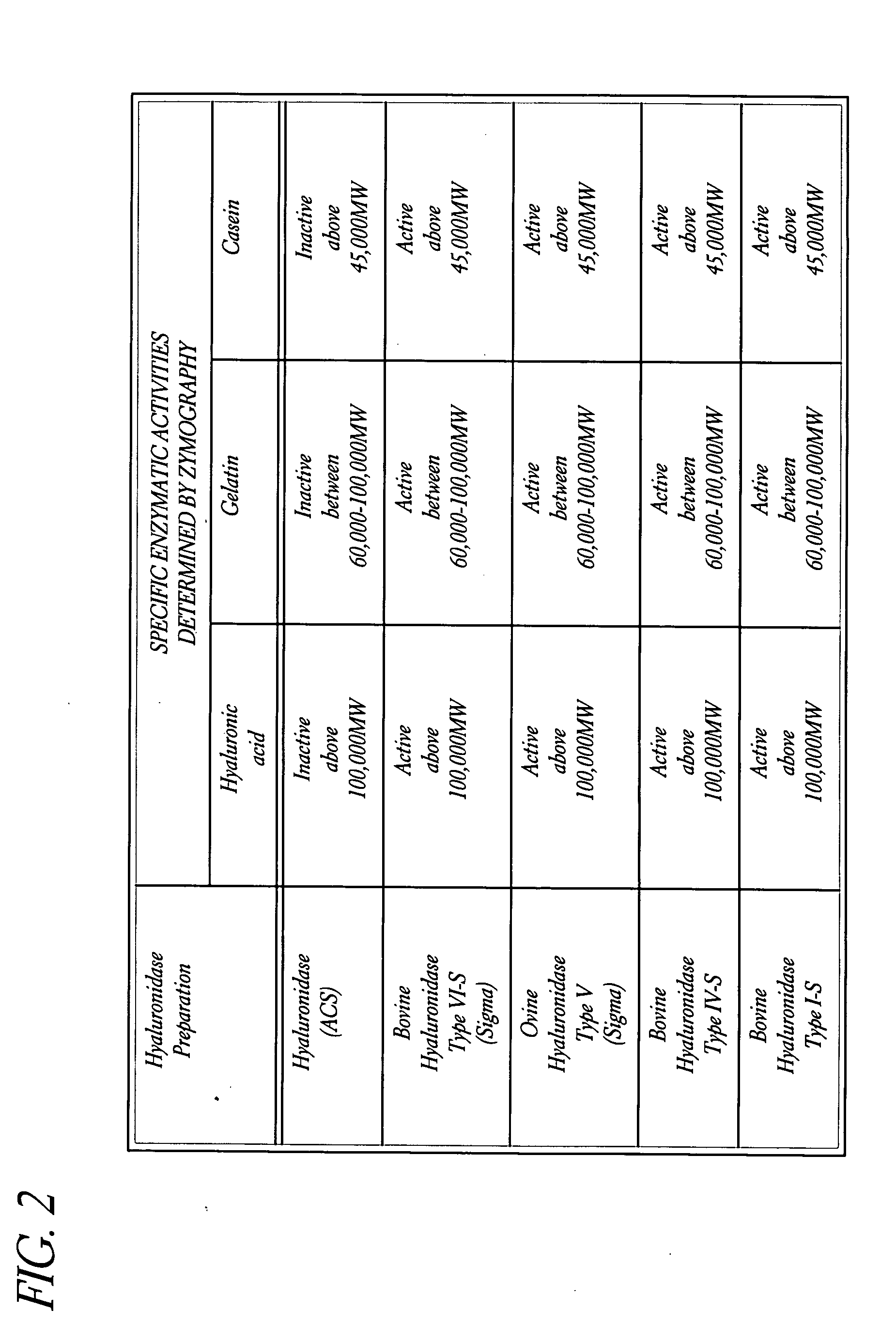
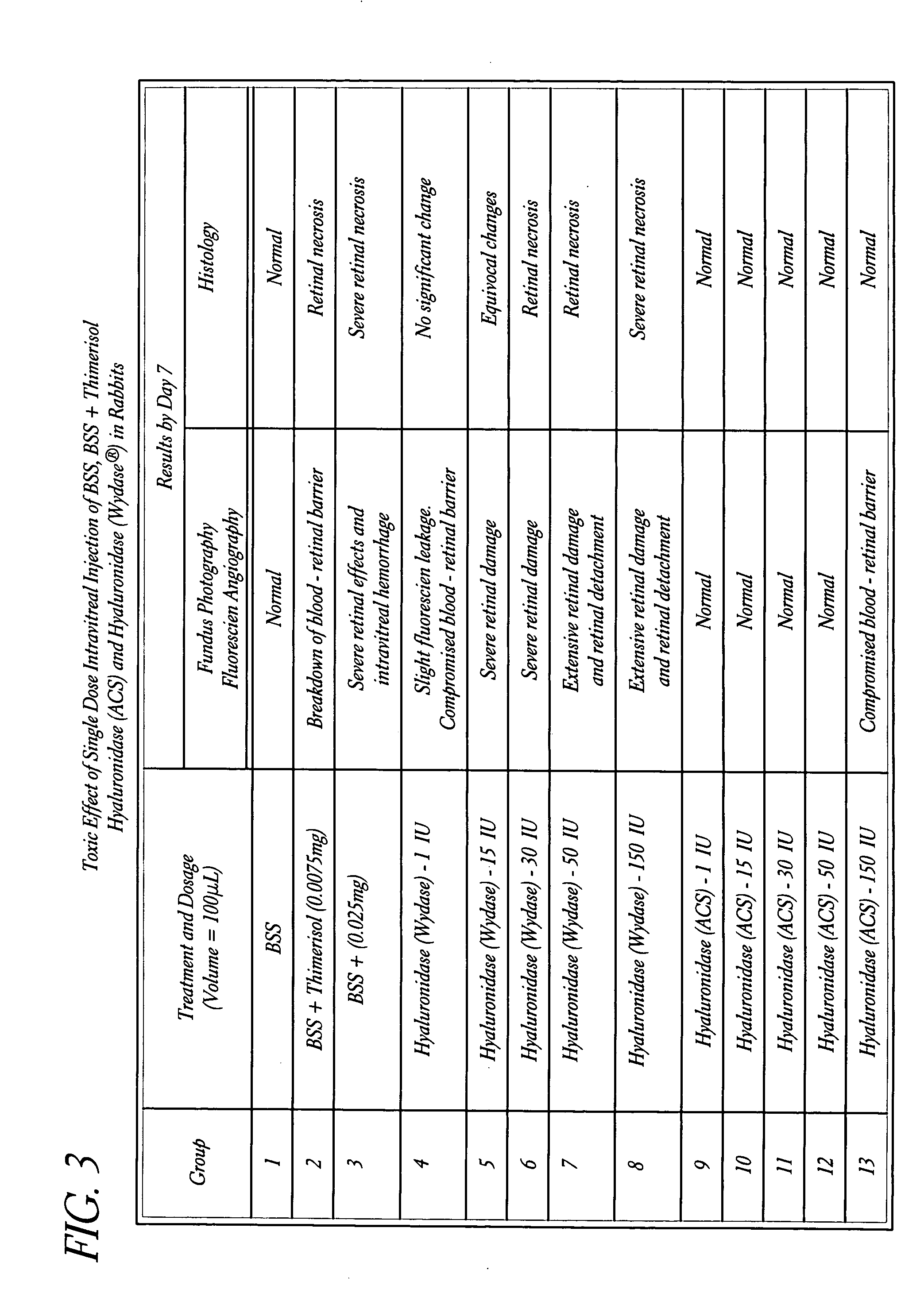
Use of hyaluronidase in the manufacture of an ophthalmic preparation for liquefying vitreous humor in the treatment of eye disorders
InactiveUS6863886B2Improve clearance rateIncreases rate of liquid exchangeBiocidePeptide/protein ingredientsDiseaseHyaluronidase
An enzymatic method is provided for treating ophthalmic disorders of the mammalian eye. Prevention of neovascularization and the increased rate of clearance from the vitreous of materials toxic to retina is accomplished by administering an amount of hyaluronidase effective to liquefy the vitreous humor of the treated eye without causing toxic damage to the eye. Liquefaction of the vitreous humor increases the rate of liquid exchange from the vitreal chamber. This increase in exchange removes those materials and conditions whose presence causes ophthalmological and retinal damage.
Owner:BAUSCH & LOMB PHARMA HLDG
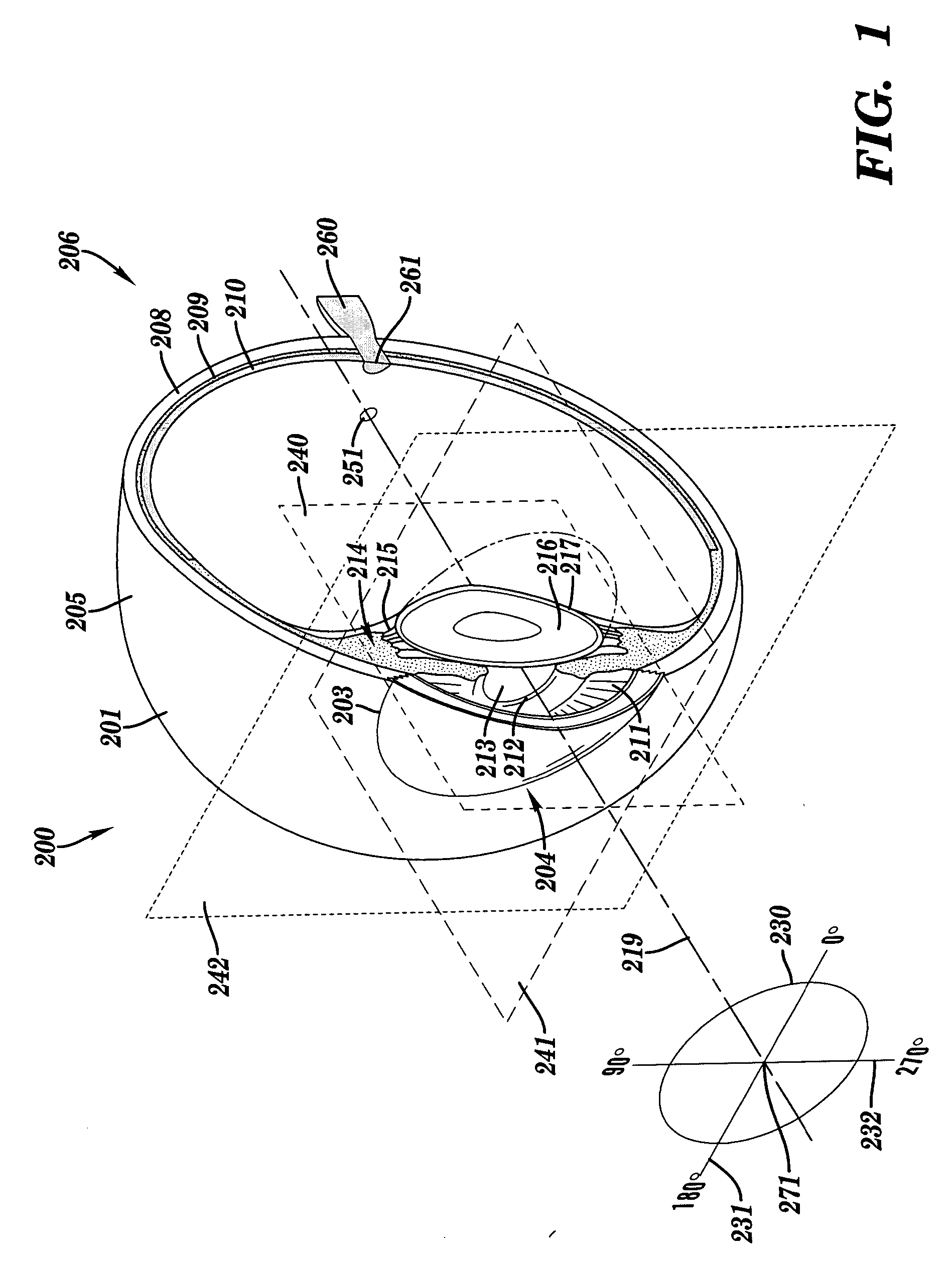
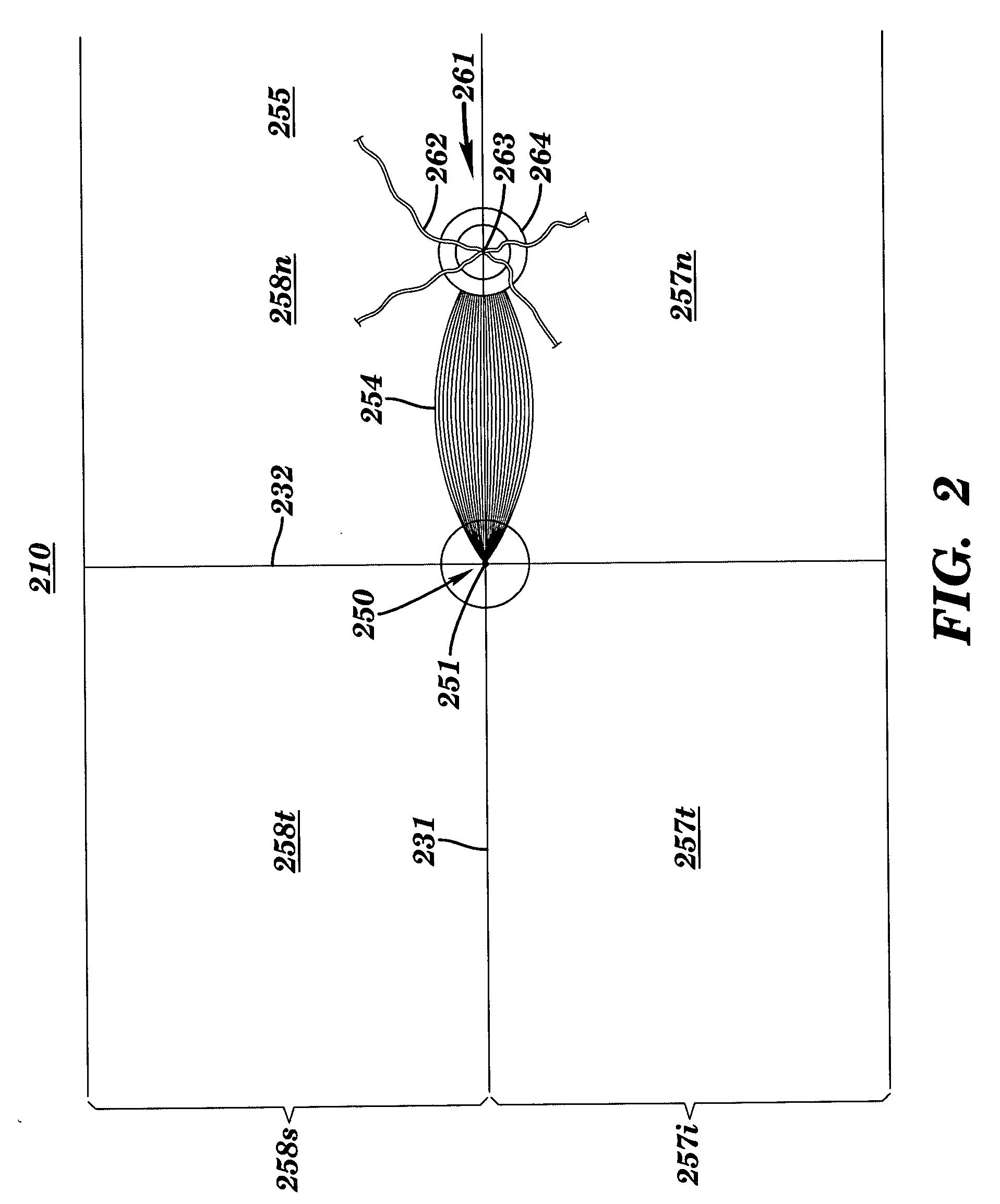
Apparatus and method for assessing retinal damage
InactiveUS20050200808A1Excessive duration of testShorten the construction periodEye diagnosticsVisual field lossSigmoid function
The invention administers an objective clinical test to an eye that measures the visual sensitivity of the superior retina and the inferior retina, by alternately presenting a stimulus pair comprising a shaped superior light stimulus and a shaped inferior light stimulus that are horizontal mirror images of one another and have shapes encompassing visual field defects. The shaped superior and inferior light stimuli stimulate pupillary responses whose amplitudes are measured. A cycle-averaged pupillary response balance and a luminance ratio are computed for each presentation of a stimulus pair. A stimulus pair response curve is computed by fitting cycle-averaged pupillary response balances to a sigmoid function of the luminance ratios. A balanced luminance ratio at which the cycle-averaged pupillary response balance is equal to about zero is computed from the sigmoid function. The balanced luminance ratio is indicative of the presence and location of retinal nerve damage.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
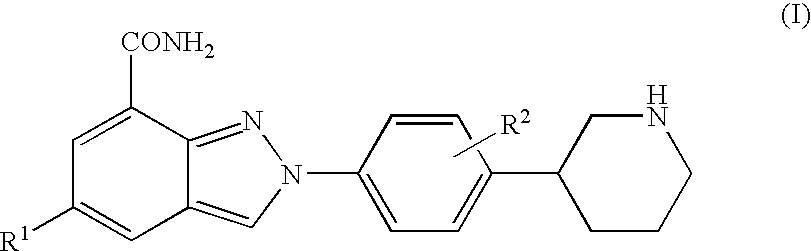
Amide substituted indazoles as poly(ADP-ribose)polymerase(PARP) inhibitors
The present invention relates to compounds of formula I:and pharmaceutically acceptable salts, stereoisomers or tautomers thereof which are inhibitors of poly (ADP-ribose) polymerase (PARP) and thus useful for the treatment of cancer, inflammatory diseases, reperfusion injuries, ischemic conditions, stroke, renal failure, cardiovascular diseases, vascular diseases other than cardiovascular diseases, diabetes, neurodegenerative diseases, retroviral infection, retinal damage or skin senescence and UV-induced skin damage, and as chemo- and / or radiosensitizers for cancer treatment.
Owner:MERCK SHARP & DOHME LLC
Synergistic composition and method of retarding and ameliorating photo induced retinal damage and cataracts while ameliorating dry eye syndrome
InactiveUS20110021465A1Improve performanceReducing and preventing symptomBiocideSenses disorderCns degenerative diseasesAstaxanthin
A composition and method of retarding and ameliorating eye diseases and injuries is disclosed. The method comprises administering a synergistic mixture of certain carotenoids, including the carotenoid astaxanthin, with phospholipid and triglyceride bound EPA and DHA derived from Krill oil, in which said krill oil contains at least 30% total phospholipids, in a therapeutically effective amount to prevent, retard or treat eye and central nervous system diseases or injuries, such as age-related macular degeneration, cataract, dry eye syndrome due to glandular inflammation and other central nervous system degenerative diseases, photic injury, ischemic diseases, and inflammatory diseases.
Owner:US NUTRACEUTICALS LLC
Use of hyaluronidase in the manufacture of an ophthalmic preparation for liquefying vitreous humor in the treatment of eye disorders
InactiveUS20050069531A1Improve clearance rateIncreases rate of liquid exchangePeptide/protein ingredientsDrug compositionsDiseaseHyaluronidase
An enzymatic method is provided for treating ophthalmic disorders of the mammalian eye. Prevention of neovascularization and the increased rate of clearance from the vitreous of materials toxic to retina is accomplished by administering an amount of hyaluronidase effective to liquefy the vitreous humor of the treated eye without causing toxic damage to the eye. Liquefaction of the vitreous humor increases the rate of liquid exchange from the vitreal chamber. This increase in exchange removes those materials and conditions whose presence causes ophthalmological and retinal damage.
Owner:KARAGEOZIAN HAMPAR +5
Display device measuring device simulating structure of human eyes and measuring method
ActiveCN105865755AData processing is accurateSpectrum investigationPhotometry using electric radiation detectorsMatching testSoftware system
The invention discloses a display device measuring device simulating the structure of human eyes and a measuring method. By means of an optical system and a software processing system, retinal imaging simulation and optical safe measurement are achieved. The optical system comprises one or two optical imaging devices, a light radiation measuring device and a dynamic measuring device. The software processing system comprises a human eye smooth tracking model, a subjective matching test model, a Photopic deviation model and other human eye visual models. A part of the optical system can finally generate images in human eye retinas by means of the optical imaging device of a simulation human eye structure and the software system simulating the human eye visual models. A light radiation measuring part of the optical system analyzes spectrum distribution needing light measurement through a photoelectric sensor array, and retinal damage can be accurately evaluated according to a retinal damage weight function curve. The measuring method simulating the structure of the human eyes is disclosed and can omnidirectionally evaluate color, brightness, motion blur and other characteristics of a display device in real time.
Owner:SOUTHEAST UNIV
Long-acting sustained release preparation for preventing or treating retinal damage, and preparation method thereof
ActiveCN102233129ARelieve painSolve the problem of long-term frequent injectionsSenses disorderPeptide/protein ingredientsMicrosphereRetinal ganglion
The invention belongs to the field of pharmaceutical preparations, and relates to a long-acting sustained release preparation for preventing or treating retinal damage, and a preparation method thereof. The long-acting sustained release preparation takes erythropoietin (EPO) as an active component, takes dextran as a protective agent for the active component, and takes poly(lactic-co-glycolic acid), polylactic acid or polycaprolactone as a coating component to prepare sustained release microspheres, wherein the erythropoietin is coated by the poly(lactic-co-glycolic acid), polylactic acid or polycaprolactone. Proven by animal experiments, the sustained release microspheres of the long-acting sustained release preparation provided by the invention have the same protective actions on the ganglionic cells of damaged retinas by single vitreous chamber injection and repeated EPO protein vitreous chamber injection, and the sustained release microspheres are capable of avoiding a series of complications caused by many times of injection and overcoming the defects of repeated intraocular injection administration and gene therapy. The long-acting sustained release preparation provided by the invention adopts intraocular local administration, can reduce the dosage and treatment cost, and can not generate adverse effects on other organs or tissues in vivo.
Owner:SHANGHAI JIAO TONG UNIV +1
Apparatus and method for assessing retinal damage
The invention administers an objective clinical test to an eye that measures the visual sensitivity of the superior retina and the inferior retina, by alternately presenting a stimulus pair comprising a shaped superior light stimulus and a shaped inferior light stimulus that are horizontal mirror images of one another and have shapes encompassing visual field defects. The shaped superior and inferior light stimuli stimulate pupillary responses whose amplitudes are measured. A cycle-averaged pupillary response balance and a luminance ratio are computed for each presentation of a stimulus pair. A stimulus pair response curve is computed by fitting cycle-averaged pupillary response balances to a sigmoid function of the luminance ratios. A balanced luminance ratio at which the cycle-averaged pupillary response balance is equal to about zero is computed from the sigmoid function. The balanced luminance ratio is indicative of the presence and location of retinal nerve damage.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Long-acting sustained release preparation for preventing or treating retinal damage, and preparation method thereof
ActiveCN102233129BImprove survival rateLong-term protectionSenses disorderPeptide/protein ingredientsMicrosphereRetinal ganglion
Owner:SHANGHAI JIAOTONG UNIV +1
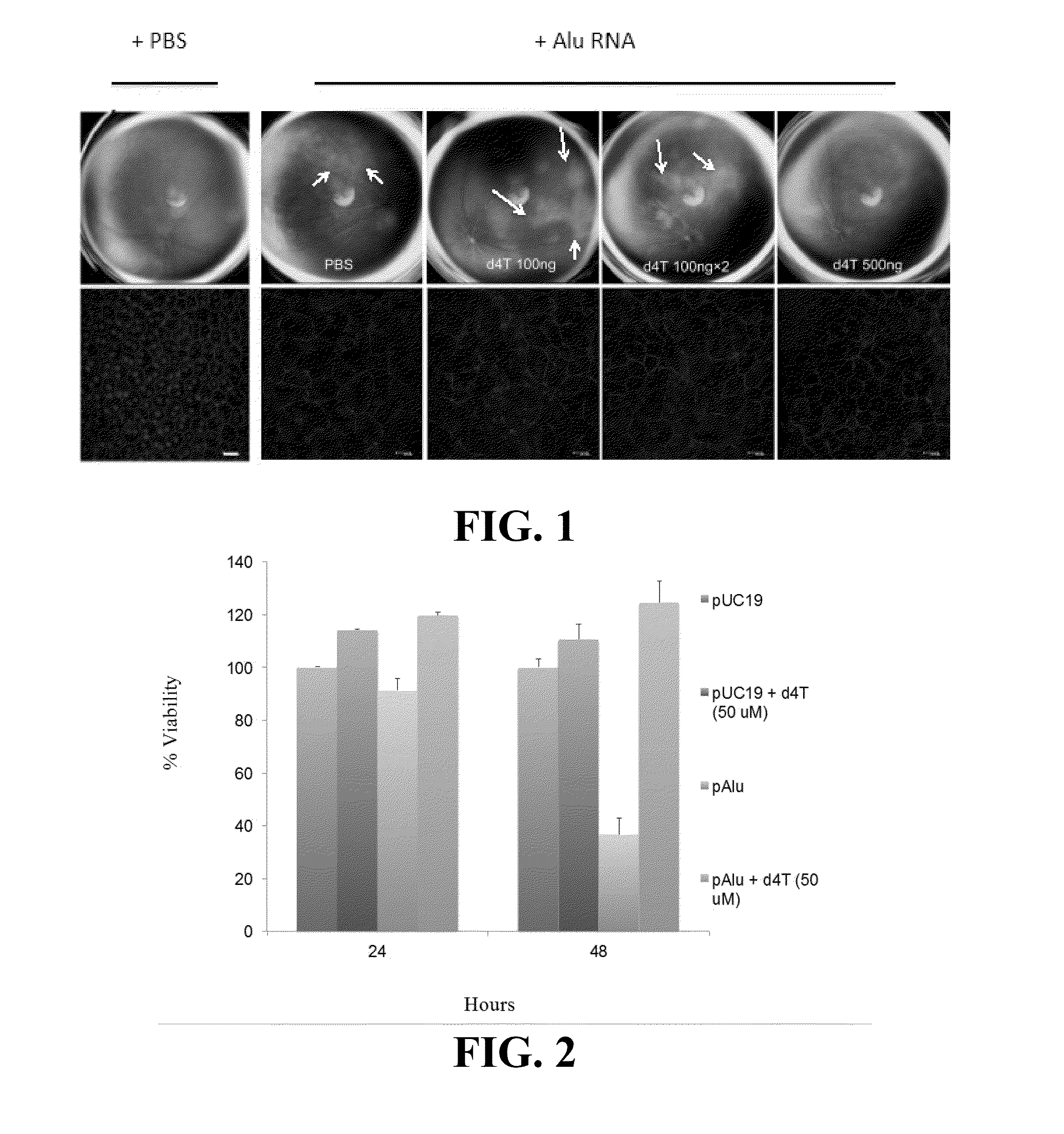
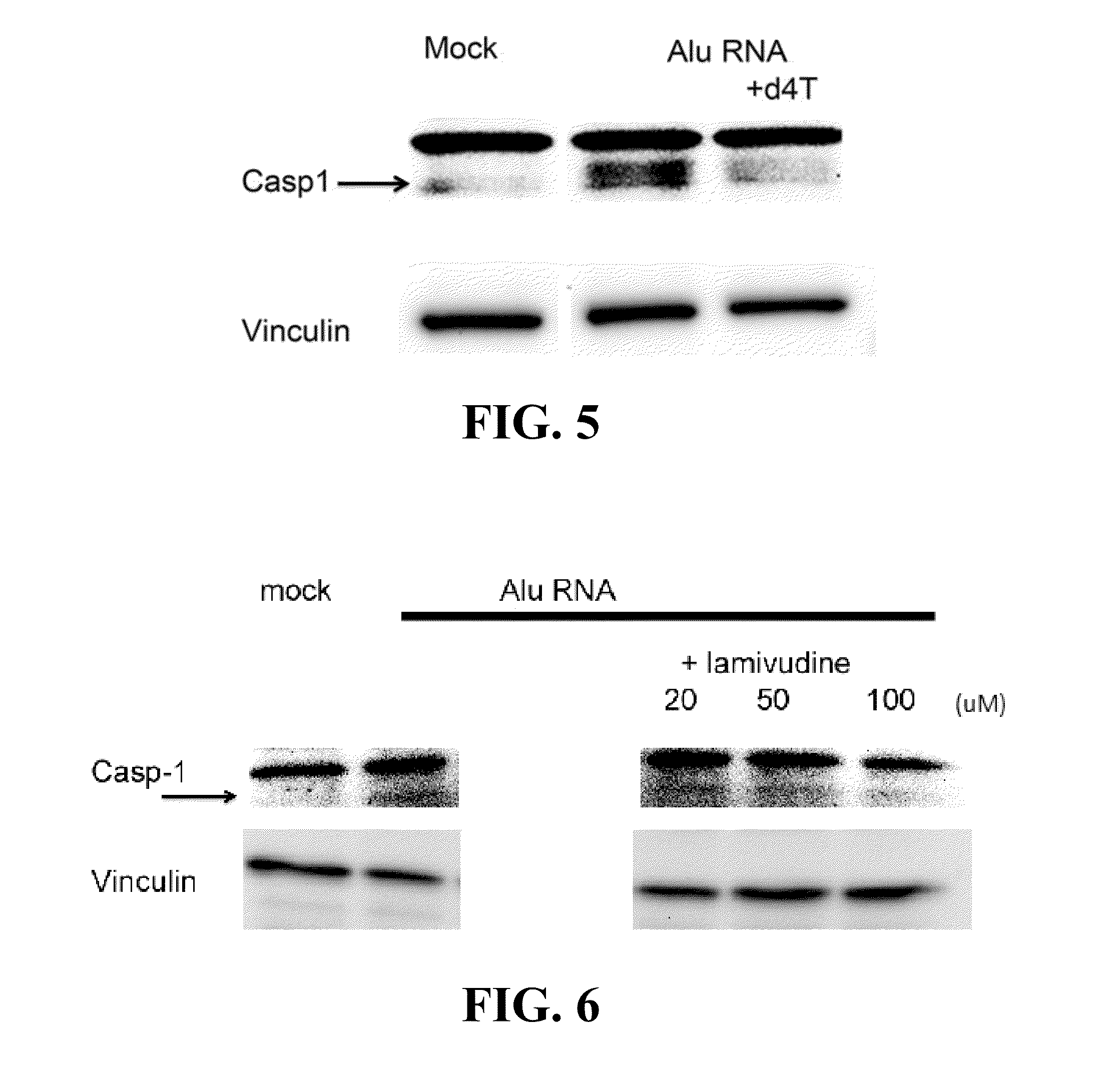
Compositions and methods for treating retinal degradation
The present disclosure relates to compositions and methods for treating retinal damage and / or retinal degradation. More specifically, this disclosure relates to methods for treating degradation of the retinal pigment epithelium by administering compositions comprising a nucleoside and / or a nucleoside or nucleotide reverse transcriptase inhibitor.
Owner:UNIV OF KENTUCKY RES FOUND
Bucky paper as a support membrane in retinal cell transplantation
A method for repairing a retinal system of an eye, using bucky paper on which a plurality of retina pigment epithelial cells and / or iris pigment epithelial cells and / or stem cells is deposited, either randomly or in a selected cell pattern. The cell-covered bucky paper is positioned in a sub-retinal space to transfer cells to this space and thereby restore the retina to its normal functioning, where retinal damage or degeneration, such as macular degeneration, has occurred.
Owner:USA AS REPRENTED BY THE ADMINISTATOR OF NASA +1
Synergistic composition and method of retarding and ameliorating photo induced retinal damage and cataracts while ameliorating dry eye syndrome using omega choline
InactiveUS20110237548A1Improve performanceReducing and preventing symptomBiocideSenses disorderTG - TriglycerideAstaxanthin
A composition and method of retarding and ameliorating eye diseases and injuries is disclosed. The method comprises administering a synergistic mixture of certain carotenoids, including the carotenoid astaxanthin, with phospholipid and triglyceride bound EPA and DHA derived from omega choline, in which said omega choline contains at least 30% total phospholipids, in a therapeutically effective amount to prevent, retard or treat eye central nervous system diseases or injuries, such as age-related macular degeneration, cataract, dry eye syndrome due to glandular inflammation and other central nervous system degenerative diseases, photic injury, ischemic diseases, and inflammatory diseases.
Owner:US NUTRACEUTICALS LLC
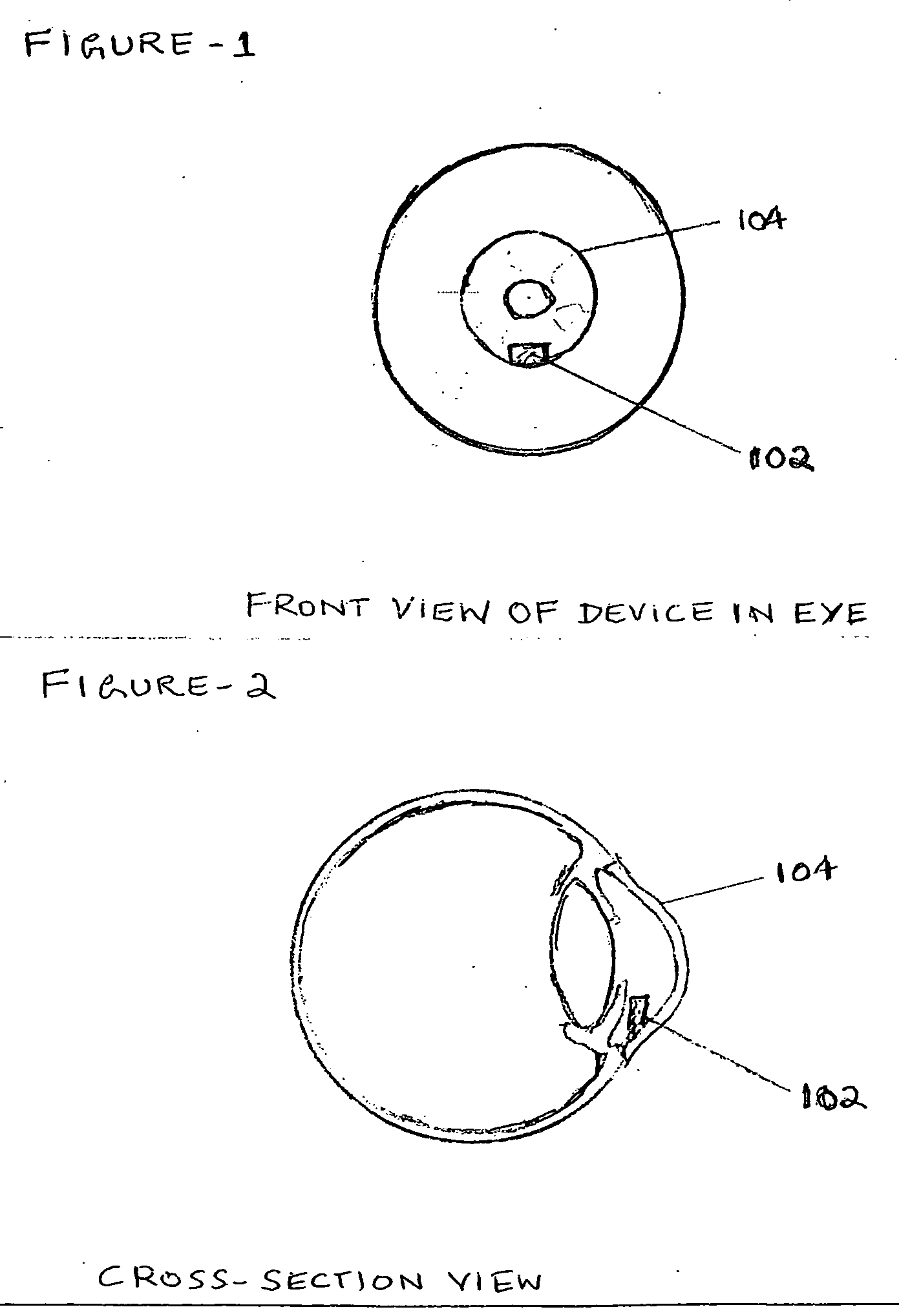
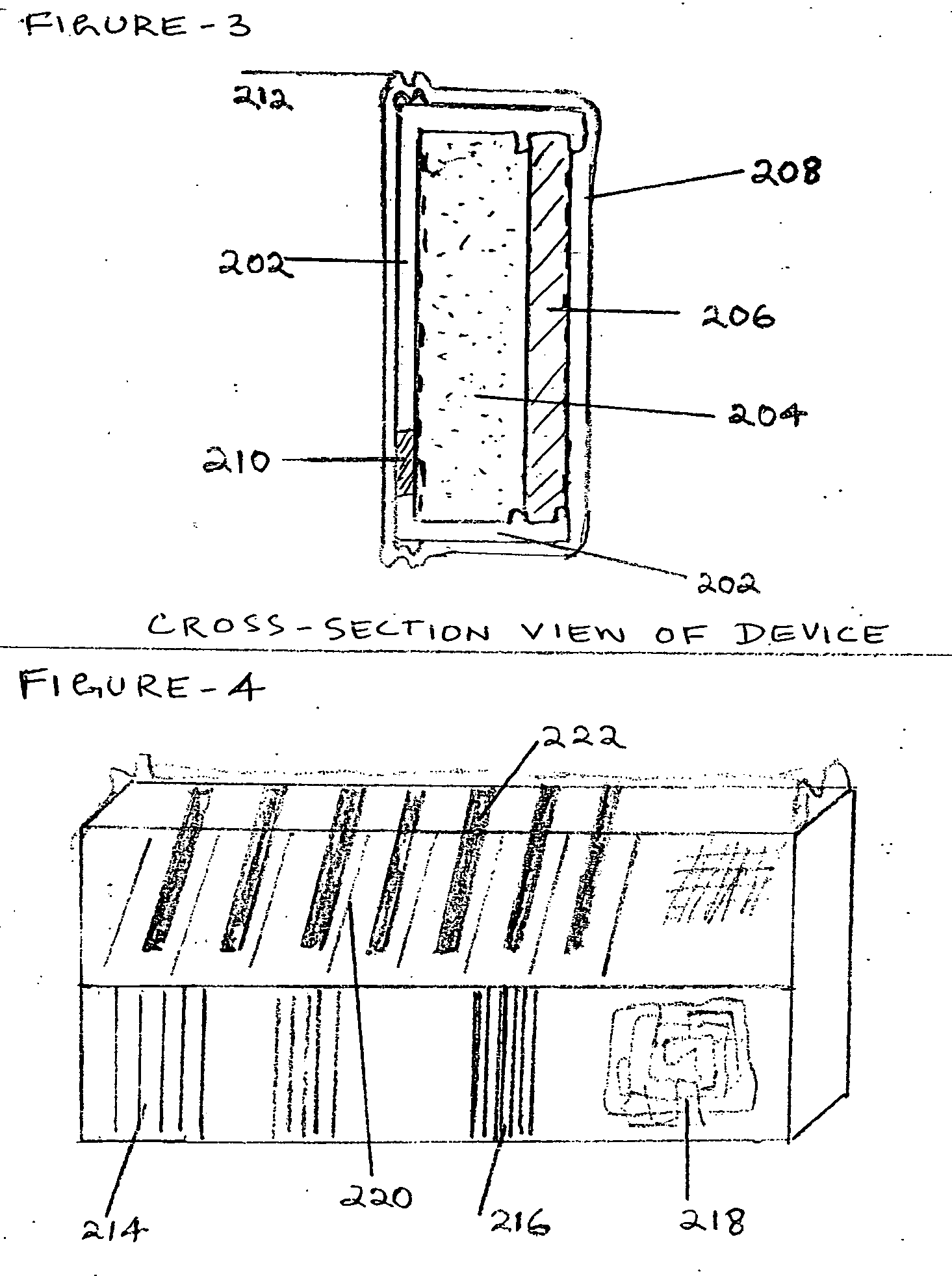
Device to directly monitor intra ocular pressure by a person based on pattern and colour changes
InactiveUS20110288396A1Reduce stressNecessary to changeEye surgeryPharmaceutical delivery mechanismIntra ocular pressureCrowds
A Device which enables a person to see for himself changes in Intra Ocular Pressure when he looks at his eye in the Mirror, in the routine course of everyday, as every one likes to see his Own face and eyes in the Mirror everyday. The device being Tiny and inserted surgically with minimum incision between Iris and Cornea, enables early indication of IOP in people with high risk of Glaucoma due to high blood pressure and genetic reasons and old age, the early indication enabling timely treatment before retinal damage. The device works on pattern changes due to pressure change which is compared with fixed background pattern and changes in spacing between lines of Diffraction grating and Hologram leading to colour changes due to interference effect of light. Mirror with an area of concave surface and Magnifying device along with comparison Chart enables proper monitoring of drug efficacy.
Owner:IYENGAR SUNDARAJA SITARAM +3
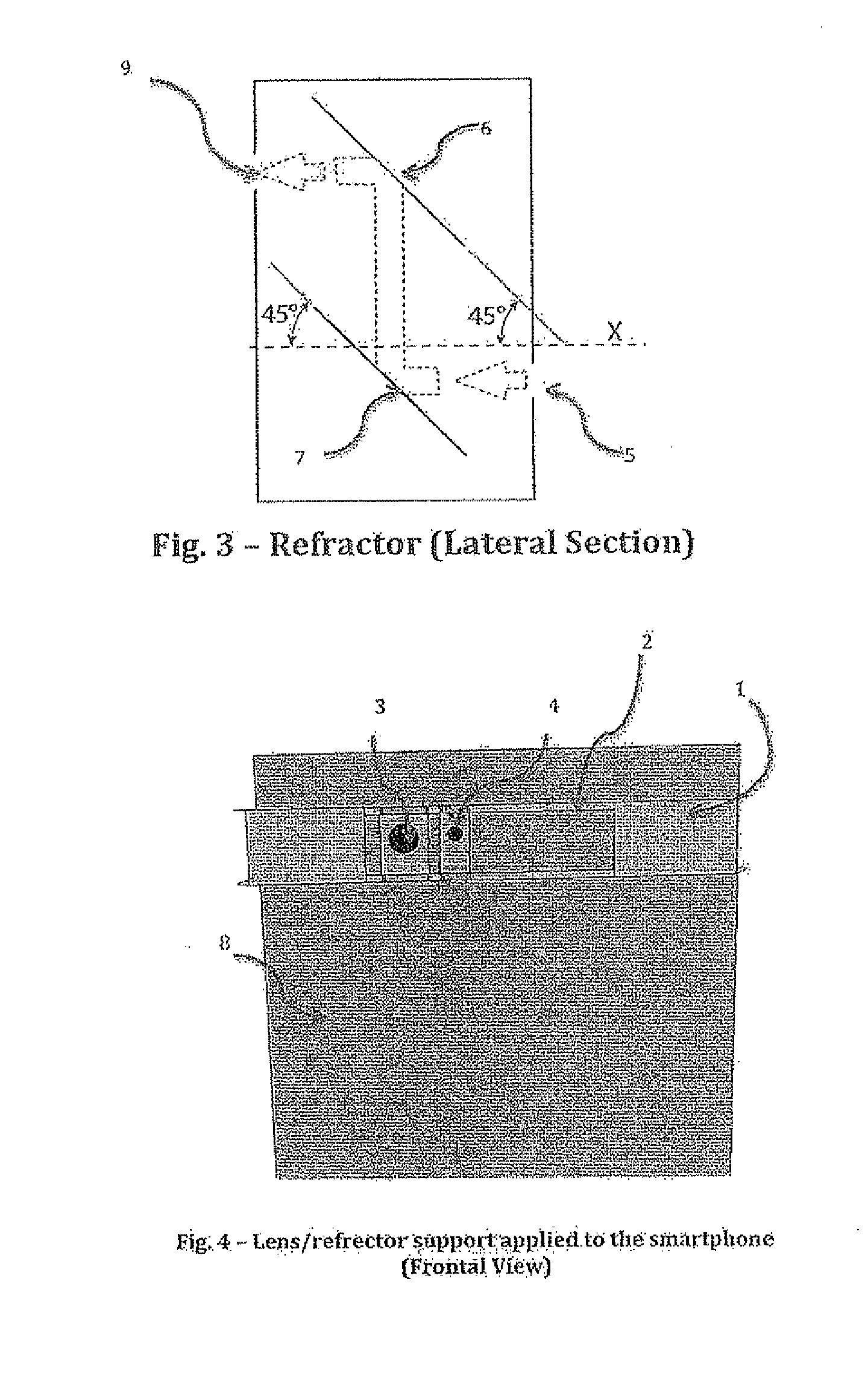
Portable medical device and method for quantitative retinal image analysis through a smartphone
ActiveUS20150320313A1Portable and inexpensiveImage enhancementImage analysisCloud baseHigh resolution image
The present invention essentially consists of an integrated system that allows acquisition and quantitative analysis of high-resolution images of the retina. The integrated system includes an optical device and a software app. The optical device is a smartphone-adapted lens for acquisition of high-resolution images of the retina. The software app is a smartphone app for quantitative analysis of the retinal images acquired through the hardware component of the device. The computation is carried out by means of cloud-based technologies. Quantitative indices of retinal damage are obtained. These indices are proven for being relevant for both clinical and research purposes.
Owner:UNIV DELLA CALABRIA
Method for identifying modulators of the NRF2-KEAP1-AREP pathway
InactiveUS20100029012A1Shorten the timeFluorescence enhancementBiological testingHuntingtons choreaAntioxidative stress
A method for identifying modulators of the Keap1-NrG-ARE pathway is described. In particular, an assay is described that identifies molecules that inhibit the binding of a labeled Nrf2 peptide with the kelch domain of the Keap1 protein. Molecules that inhibit the binding are activators of the Keap 1-Nrf2-ARE pathway. Activation of the Keap 1-Nrf2-ARE pathway may result in an increased accumulation of Nrf2 and the subsequent induction of protective enzymes, for example, the phase 2 detoxification enzymes. Activators of the Keap1-NrG-ARE pathway are useful for combating oxidative stress-related disorders, such as those associated with cancer, emphysema, Huntington's disease, light-induced retinal damage, and stroke.
Owner:MERCK SHARP & DOHME CORP
Composition with effects of protecting liver and improving eyesight and preparation method thereof
InactiveCN105125736APrevent dark adaptationInhibition formationSenses disorderHydroxy compound active ingredientsLuteinSide effect
The invention relates to the technical field of medical care, in particular to a composition with effects of protecting the liver and improving eyesight and a preparation method thereof. The composition mainly comprises mulberry, Chinese wolfberry fruit, chrysanthemum, mulberry leaf and lutein and / or lutein derivative. The composition has the advantages that medicinal and edible raw materials are carefully selected and reasonably combined to achieve a compound synergic effect, retinal damage can be effectively prevented by inhibiting oxidative stress reaction, an evident visual fatigue relieving effect is achieved, and immunity is increased; meanwhile, a definite liver damage preventing effect is achieved, the definite curative effects of protecting the liver and improving the eyesight are achieved, and the composition is free of toxic and side effects on human bodies, suitable for being eaten for a long time and capable of achieving beneficial effect of treatment and healthcare two-way regulation.
Owner:INFINITUS (CHINA) CO LTD
Compositions and methods for treating retinal degradation
The present disclosure relates to compositions and methods for treating retinal damage and / or retinal degradation. More specifically, this disclosure relates to methods for treating degradation of the retinal pigment epithelium by administering compositions comprising a nucleoside and / or a nucleoside or nucleotide reverse transcriptase inhibitor.
Owner:UNIV OF KENTUCKY RES FOUND
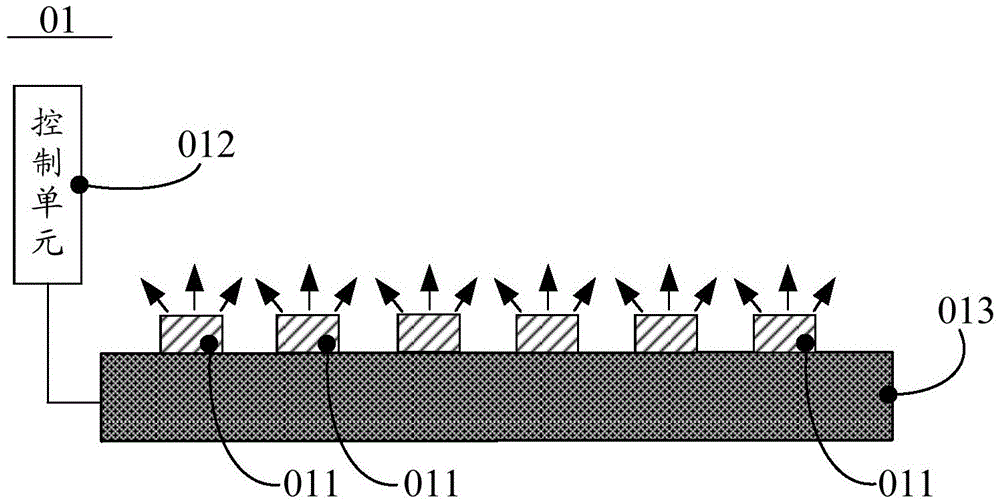
Light source, control method thereof, backlight module and liquid crystal display device
ActiveCN104879681AEvenly distributedFix damageMechanical apparatusElectrical apparatusLiquid-crystal displayPeak value
The invention discloses a light source, a control method thereof, a backlight module and a liquid crystal display device, and belongs to the technical field of liquid crystal display. The light source comprises a plurality of luminous chips and a control unit, wherein the luminous chips are connected with the control unit; the ratio of spectral energy of first visible light to spectral energy of standard sun visible light can be confirmed by the control unit according to a wavelength corresponding to a peak value of a partial wave peak of the first visible light; luminescence of the first luminous chip is controlled; and the ratio of luminous flux of light emitted from the first luminous chip to luminous flux of light emitted from the other luminous chips is equal to the ratio of the spectral energy of the first visible light to the spectral energy of the standard sun visible light. In the light source, light in various colors is distributed uniformly, the problems that the proportion of blue light in the LED (light emitting diode) light source is high and visual fatigue and even retina injury are caused are solved, and an eye protecting effect is achieved.
Owner:BOE TECH GRP CO LTD +1
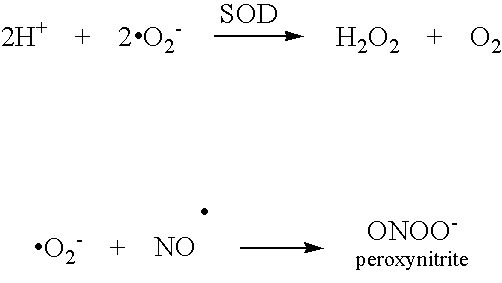
Superoxide dismutase mimics for the treatment of optic nerve and retinal damage
InactiveUS20050130951A1Avoid tissue damageOvercomes drawbackBiocideOrganic active ingredientsManganeseOptic nerve
Methods for preventing and treating damage to the optic nerve and / or retina by the use of SOD mimics, particularly pentaazacycle Mn(II) complex SOD mimics, are disclosed.
Owner:ALCON INC
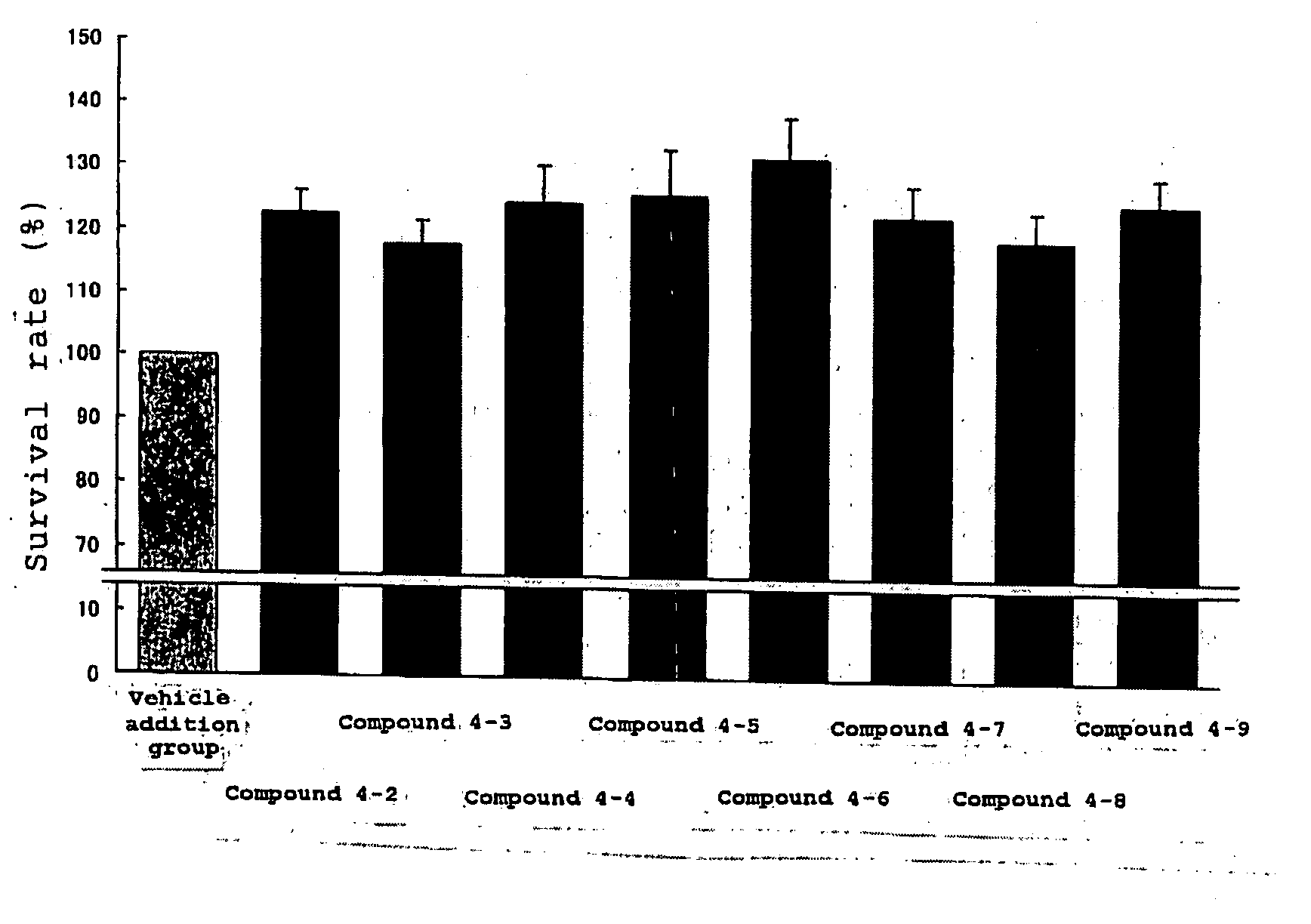
Protective Agent for Retinal Neuronal Cell Comprising Indazole Derivative as Active Ingredient
InactiveUS20090012123A1Prevention or treatment of an eye diseaseReduce usageBiocideSenses disorderCell damageRetinal damage
As a result of intensive studies for the purpose of finding a new medicinal use of an indazole derivative, it was found that an indazole derivative inhibits glutamate-induced retinal neuronal cell death in rat fetal retinal neuronal cells, in other words, the indazole derivative acts directly on the retinal neuronal cells and exhibits an effect of protecting retinal neuronal cells. Accordingly, the indazole derivative is useful for the prevention or treatment of an eye disease associated with retinal neuronal cell damage or retinal damage.
Owner:UBE IND LTD +1
Superoxide dismutase mimics for the treatment of optic nerve and retinal damage
InactiveUS20070060557A1Avoid tissue damageOvercomes drawbackOrganic active ingredientsBiocideManganeseOptic nerve
Methods for preventing and treating damage to the optic nerve and / or retina by the use of SOD mimics, particularly pentaazacycle Mn(II) complex SOD mimics, are disclosed.
Owner:ALCON INC
Inhibitors of adenosine kinase for the treatment of optic nerve and retinal damage
Methods for preventing and treating damage to the optic nerve and / or retina with adenosine kinase inhibitors are disclosed.
Owner:ALCON INC
Compositions and methods for treating retinal degradation
Owner:UNIV OF KENTUCKY RES FOUND
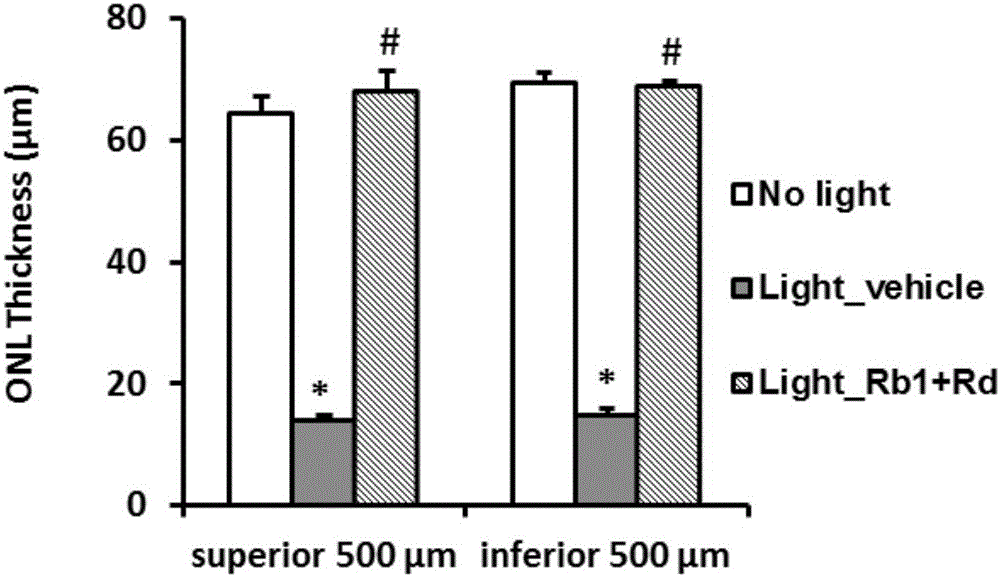
Ginsenoside Rb1 and Rd combination and application thereof to preparation of medicine for treating photoreceptor cell death related diseases
ActiveCN106074585ADegenerative disease treatment and improvementEnhanced inhibitory effectOrganic active ingredientsSenses disorderDiseaseDiabetes retinopathy
The invention relates to application of a ginsenoside Rb1 and Rd combination to preparation of a medicine for treating photoreceptor cell death related diseases. A mouse model suffering from retinal light damage is adopted to simulate the common key pathological links in the process of various degenerative retinal diseases so as to research the intervention effects of the ginsenoside Rb1 and Rd combination on the mouse model suffering from retinal light damage, and the results show that the medicinal combination can maintain the format of the retina outer nuclear layer, effectively prevent the retina outer nuclear layer from degenerative damage and thickness reduction, inhibit death of photoreceptor cells, block oxidative stress of retinal pigment epitheliums and photoreceptor cells, effectively inhibit retinal damage related immuno-inflammatory responses and remarkably improve the degenerative retinal diseases. Therefore, the ginsenoside Rb1 and Rd combination can be used for preparing the medicine for treating various degenerative retinal diseases including age-related macular degeneration, retinal pigment degeneration, Stargardt disease, cone-rod cell dystrophy, diabetic retinopathy and the like.
Owner:YUEYANG INTEGRATED TRADITIONAL CHINESE & WESTERN MEDICINE HOSPITAL SHANGHAI UNIV OF CHINESE TRADITIONAL MEDICINE
Health-care food having function of relieving visual fatigue
InactiveCN104957616AAvoid damageRelieve eye fatigueNatural extract food ingredientsFood ingredient functionsLingonberry extractSide effect
The invention discloses a health-care food having the function of relieving visual fatigue. The health-care food comprises, by weight, 1-35 parts of bilberry extracts, 1-35 parts of wolfberry extracts, 1-35 parts of chrysanthemum extracts, 1-35 parts of radix rehmanniae extracts and 0.5-35 parts of xanthophylls. The health-care food having the function of relieving the visual fatigue can directly or indirectly clear away oxygen radicals, supplement nutrient substances of visual cells, reduce damage to the retina, bring a comprehensive and systematic protection effect on the eyes and achieve the functions of protecting the vision and relieving the visual fatigue. The health-care food having the function of relieving the visual fatigue is safe and free of toxic and side effects because the natural and green plant extracts and modern functional substances are carefully selected to be raw materials based on the modern nutriology theory, the modern medicine pharmacology theory and the traditional Chinese medicine and herb theory.
Owner:HUNAN YANDI BIOLOGICAL ENG
Protection of the retina against laser injury by NAP and related peptides
Owner:RAMOT AT TEL AVIV UNIV LTD
Use of hyaluronidase in the manufacture of an ophthalmic preparation for liquefying vitreous humor in the treatment of eye disorders
An enzymatic method is provided for treating ophthalmic disorders of the mammalian eye. Prevention of neovascularization and the increased rate of clearance from the vitreous of materials toxic to retina is accomplished by administering an amount of hyaluronidase effective to liquefy the vitreous humor of the treated eye without causing toxic damage to the eye. Liquefaction of the vitreous humor increases the rate of liquid exchange from the vitreal chamber. This increase in exchange removes those materials and conditions whose presence causes ophthalmological and retinal damage.
Owner:EASTA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com