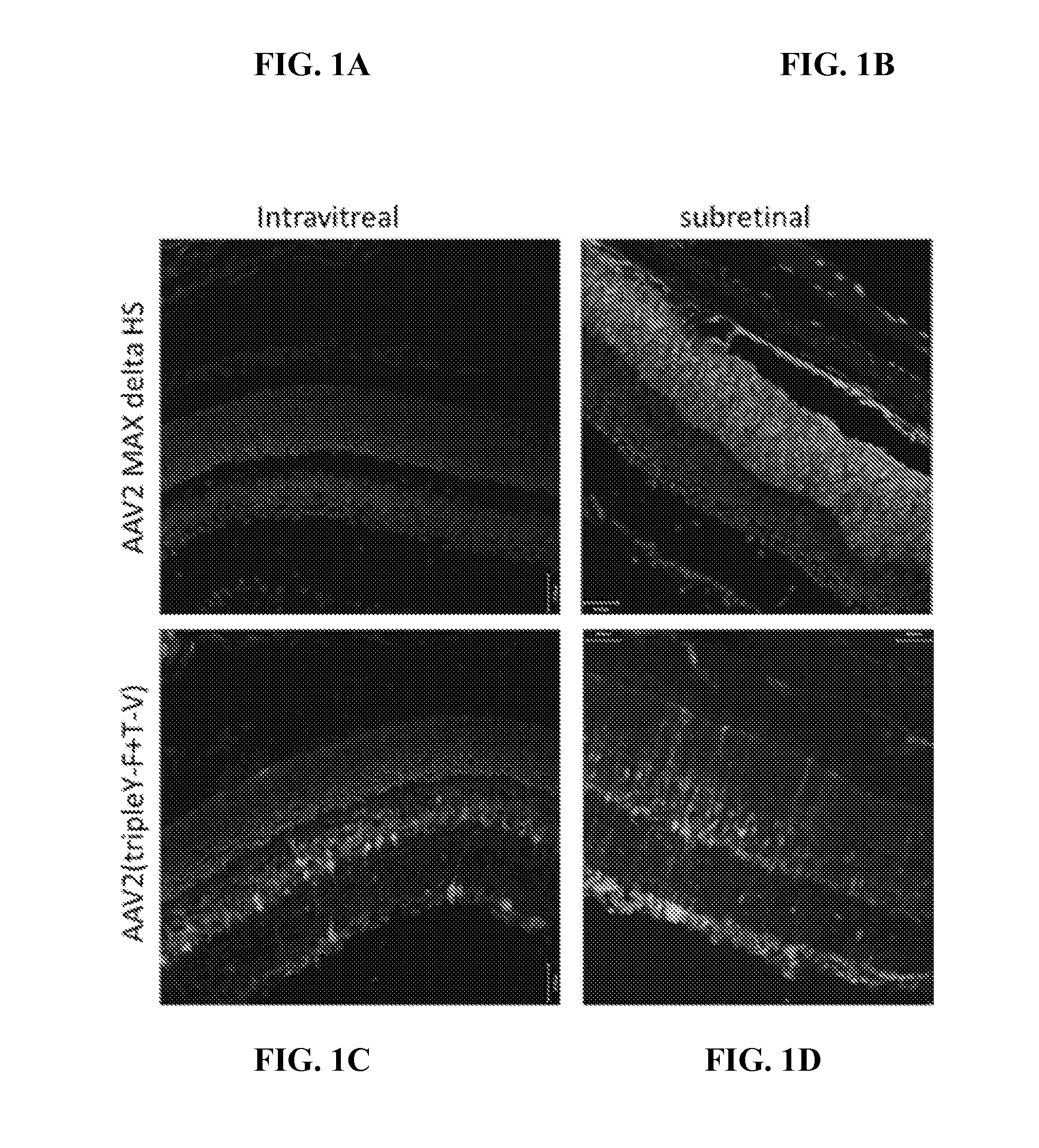
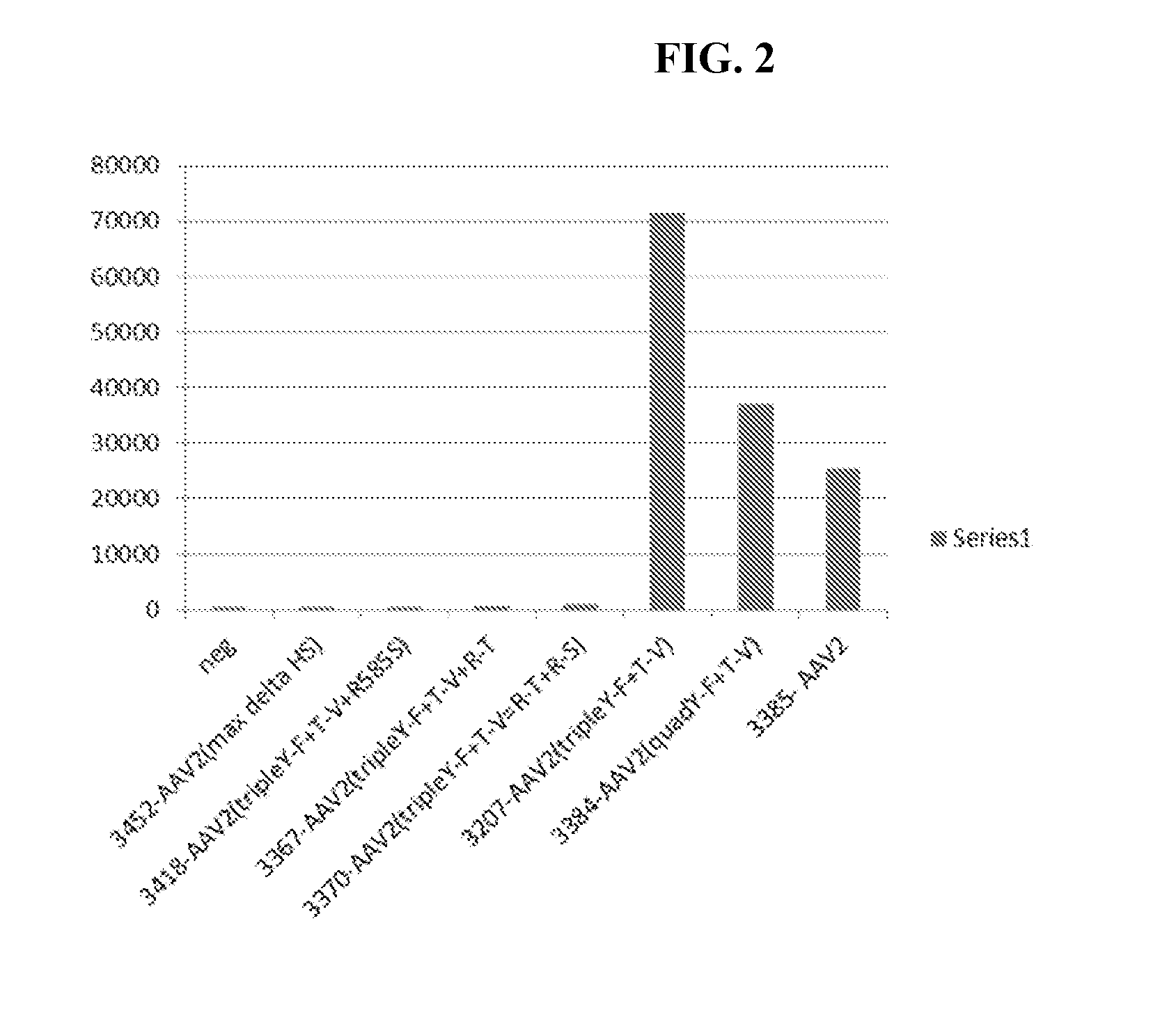
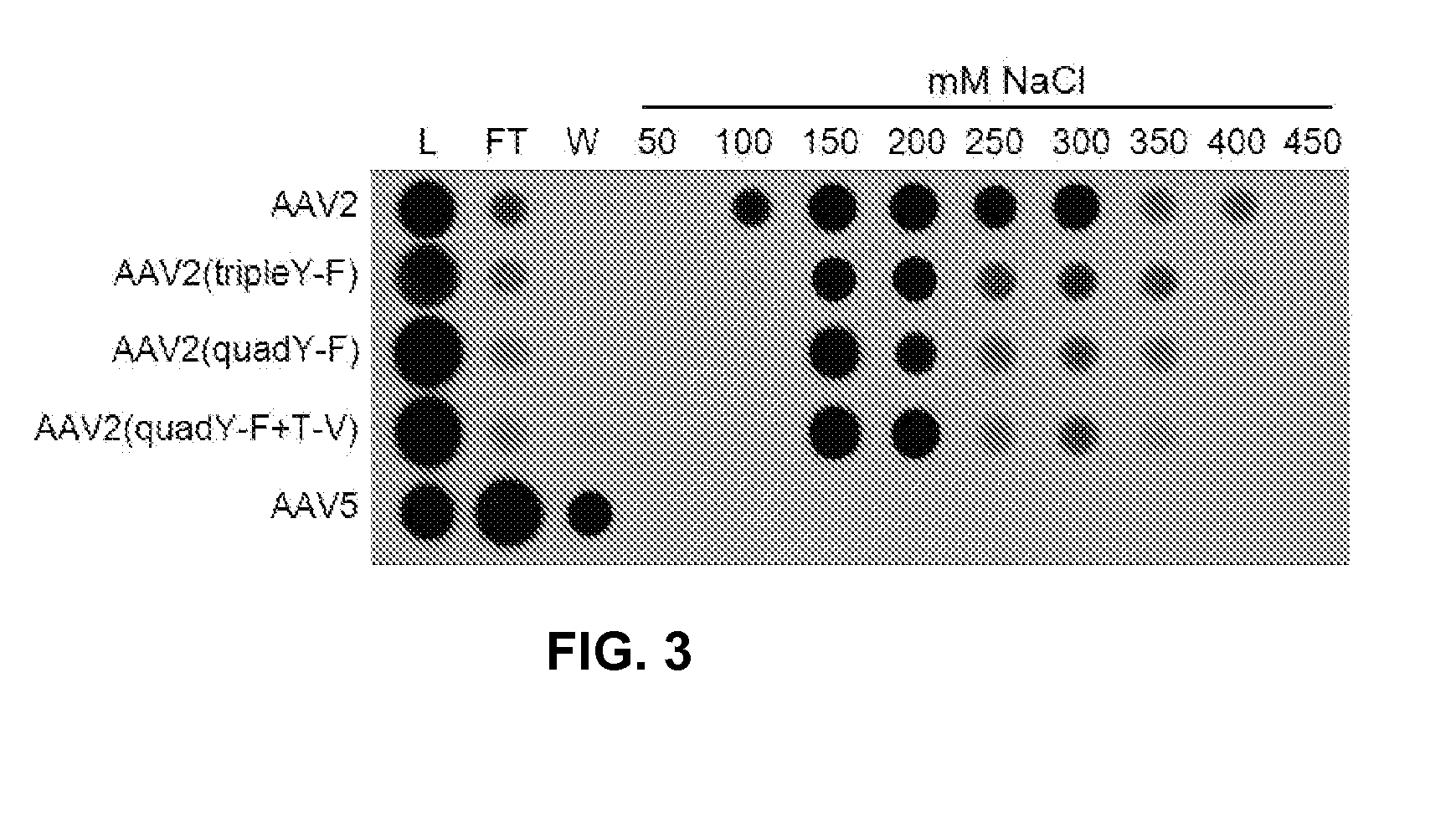
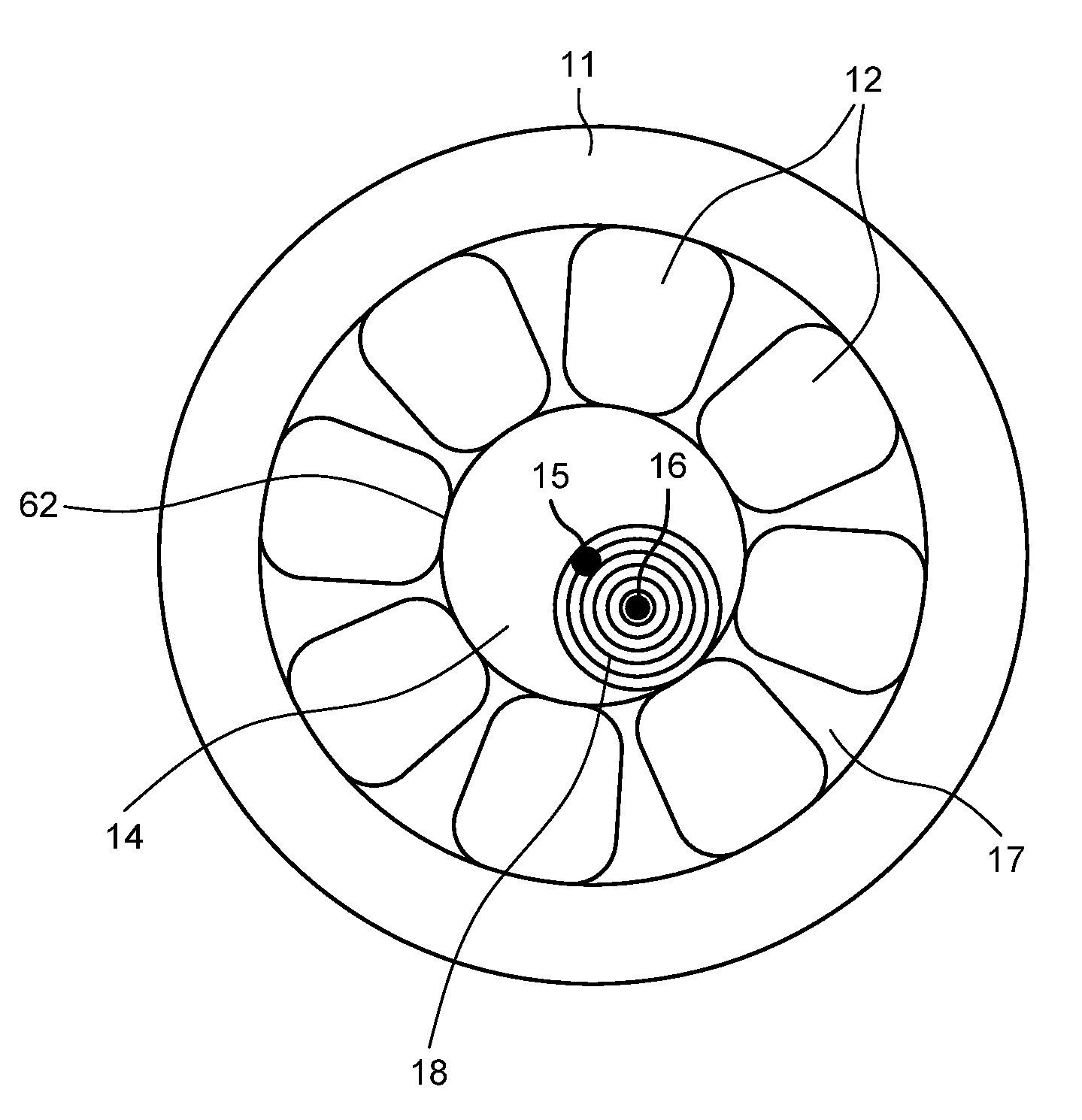
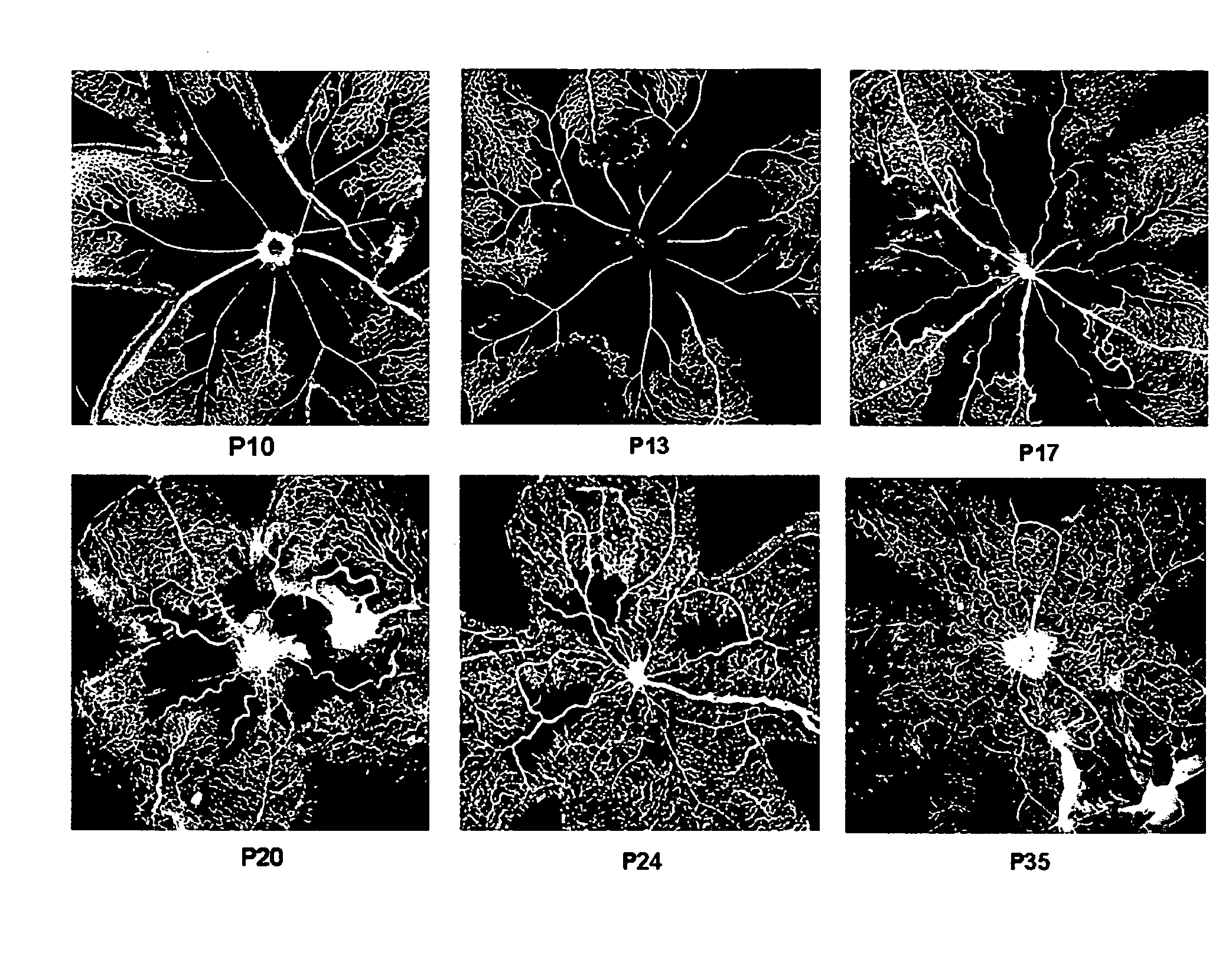
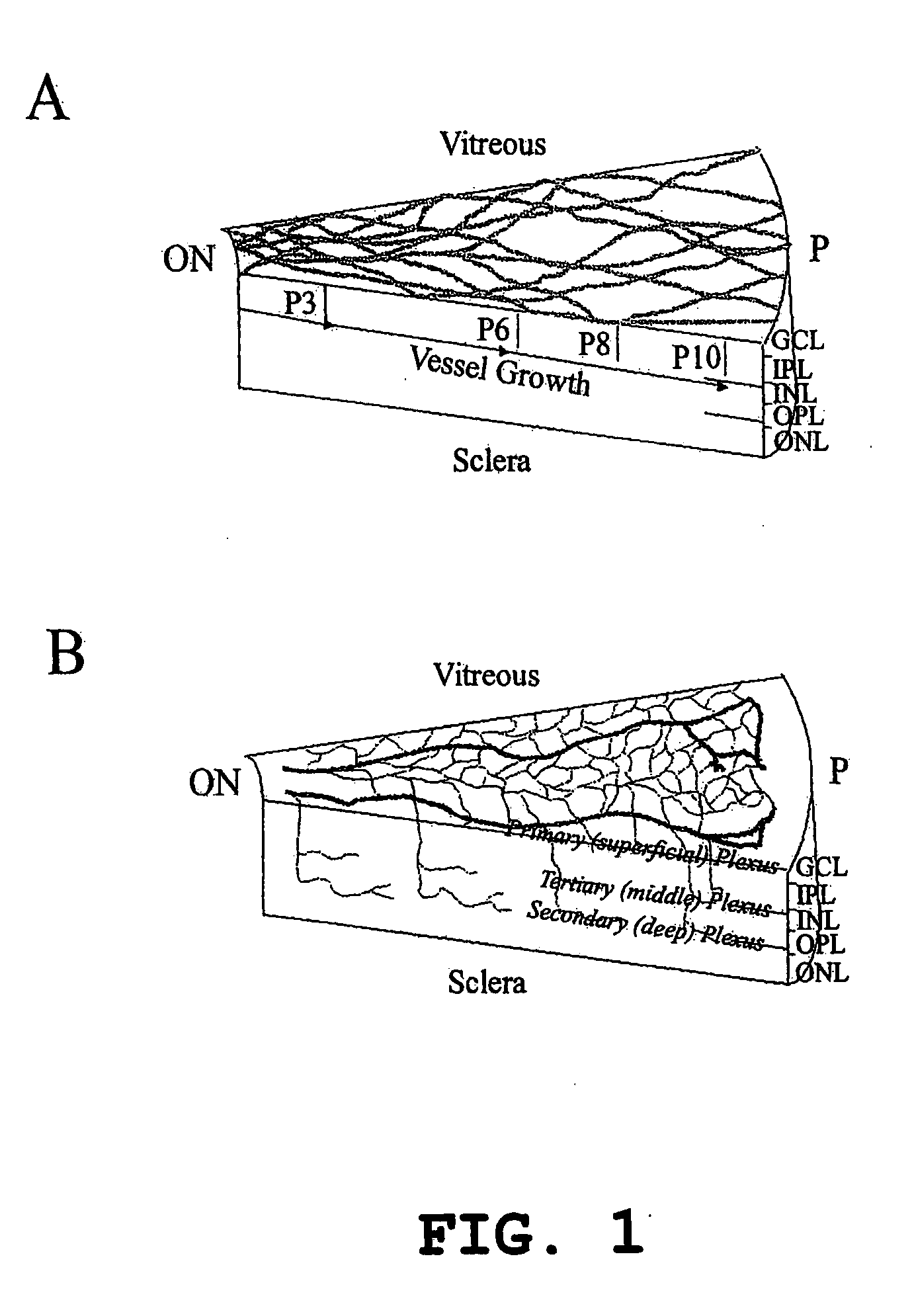
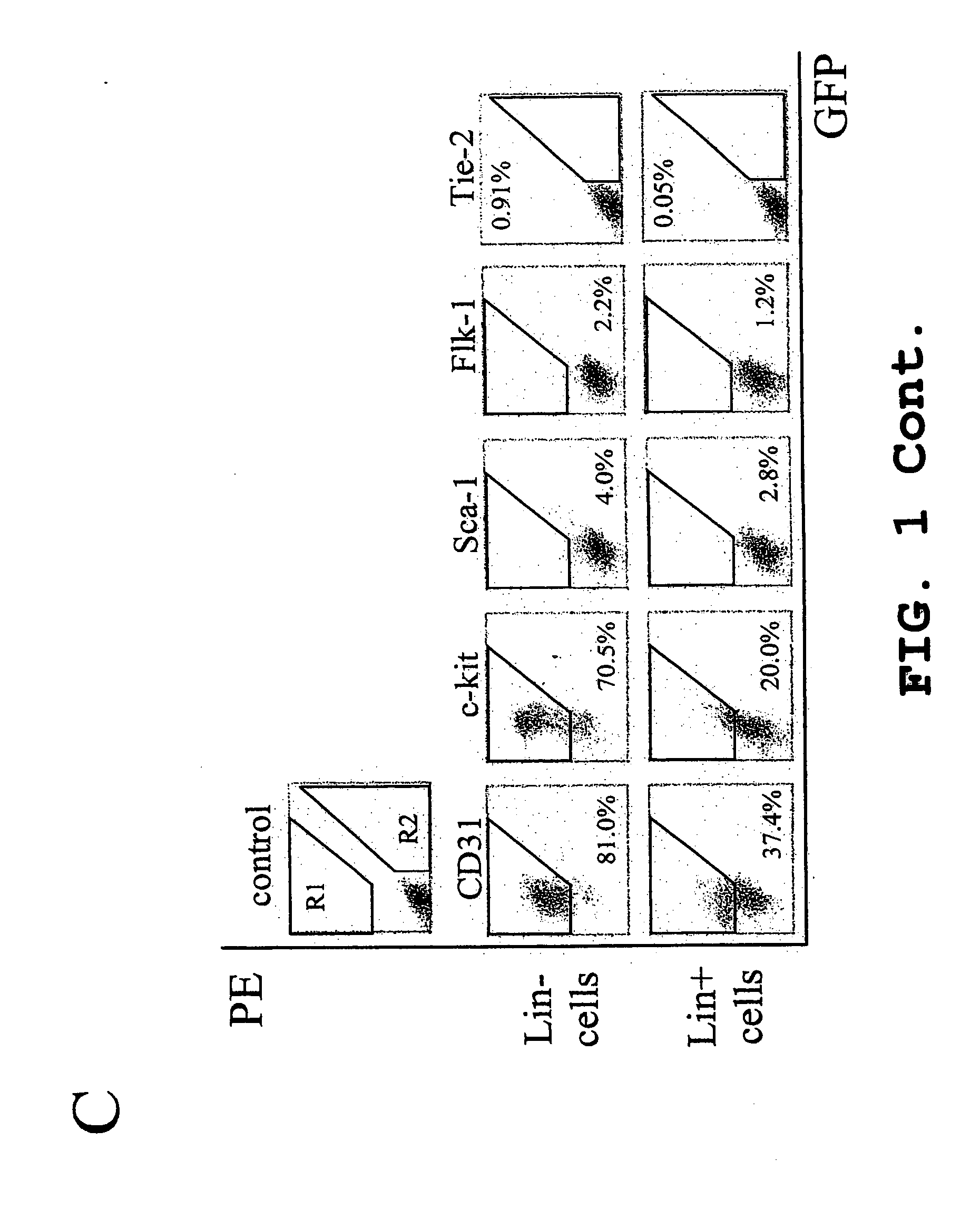
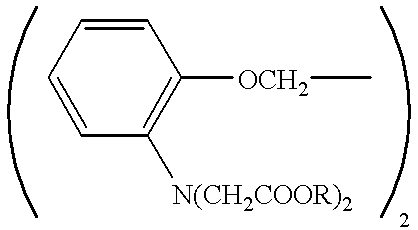
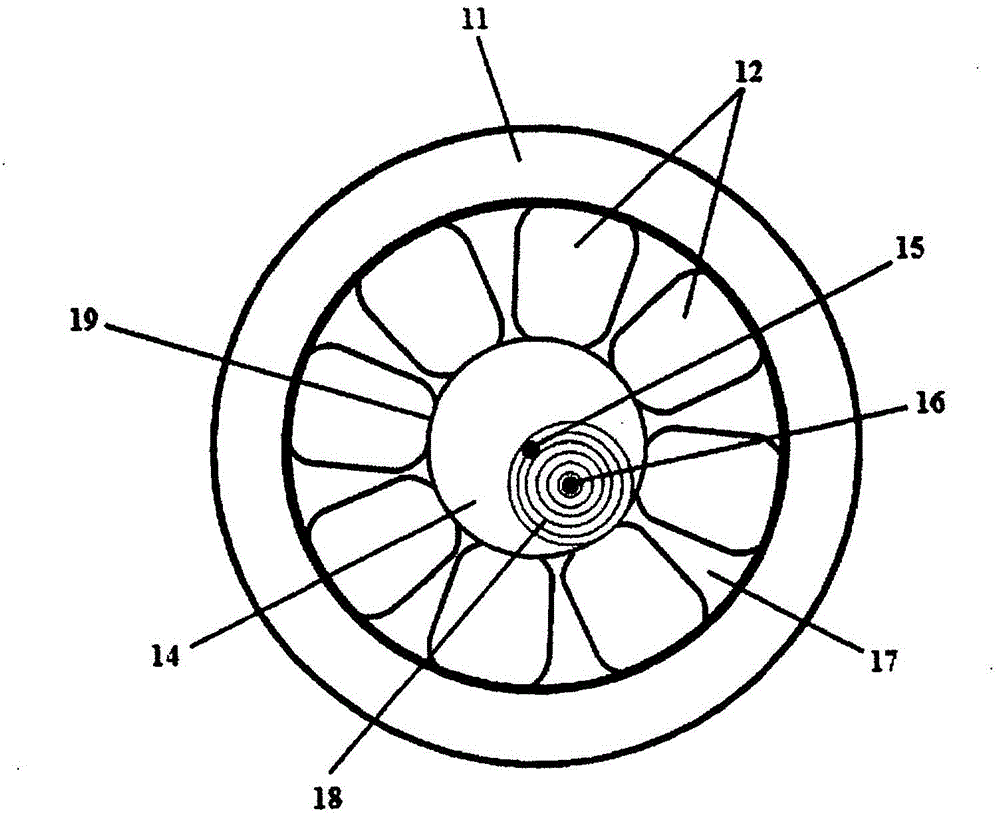
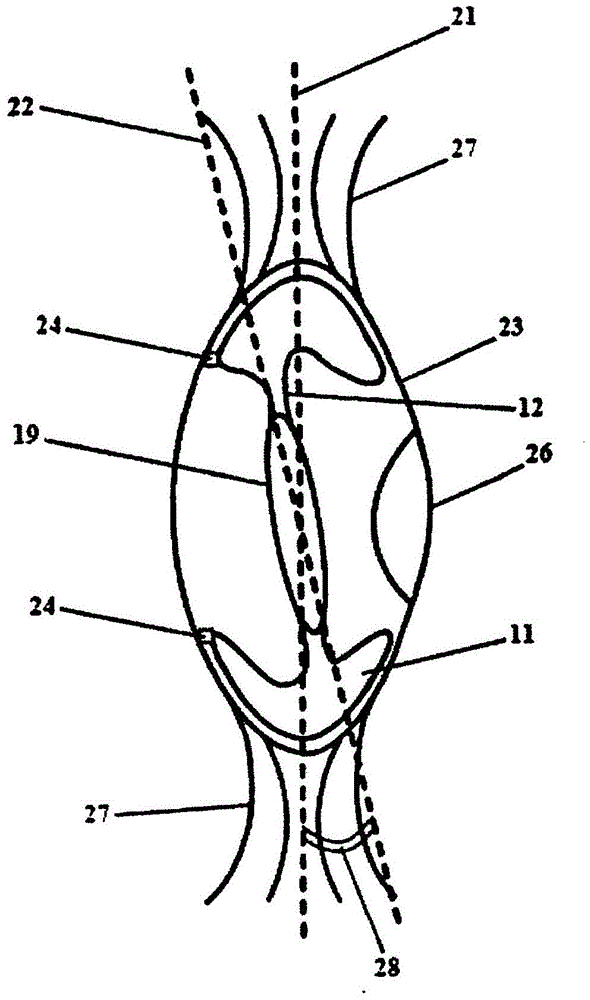
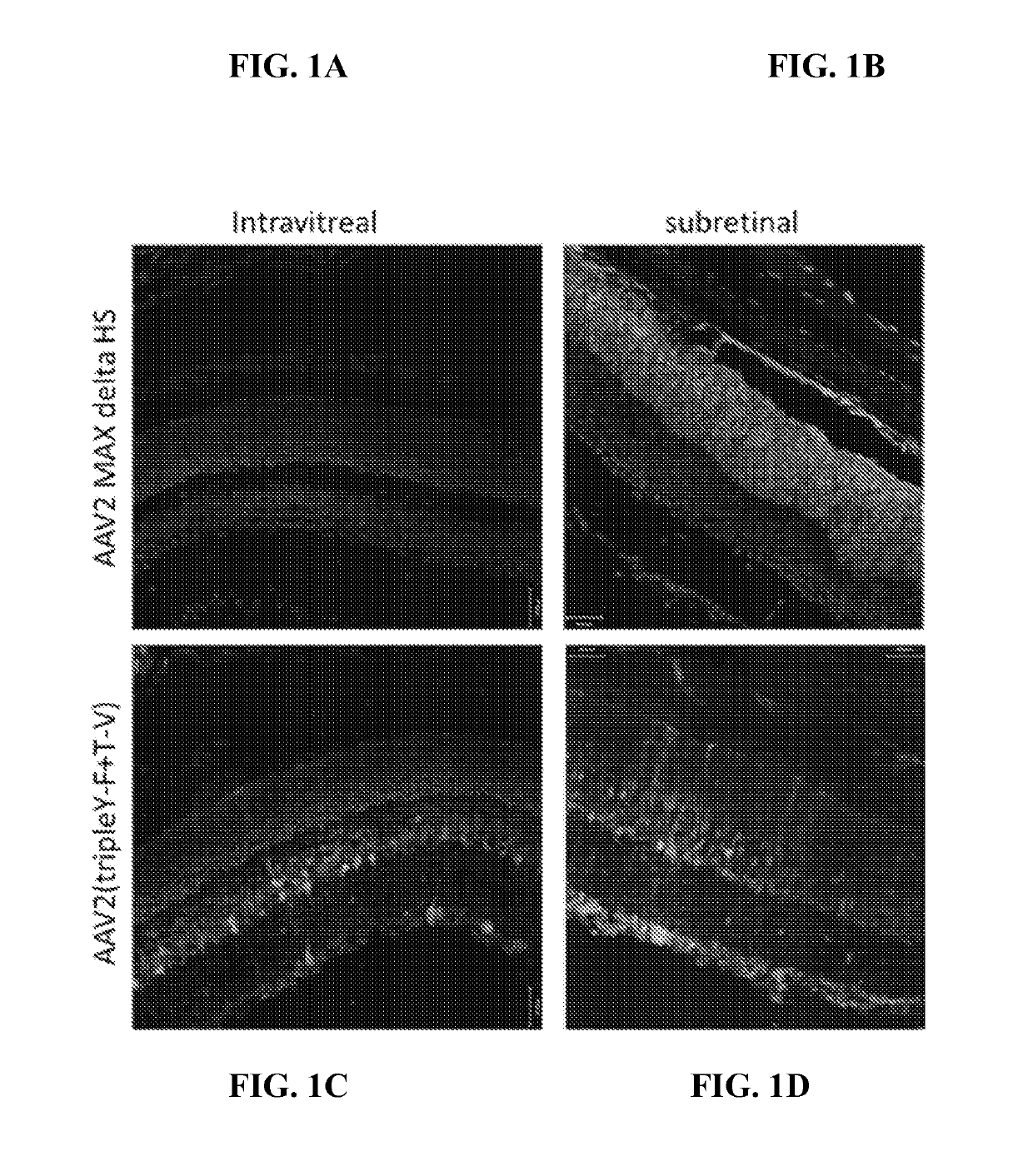
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "Mammalian eye" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mammals normally have a pair of eyes. Although mammalian vision is not so excellent as bird vision, it is at least dichromatic for most of mammalian species, with certain families (such as Hominidae) possessing a trichromatic color perception.
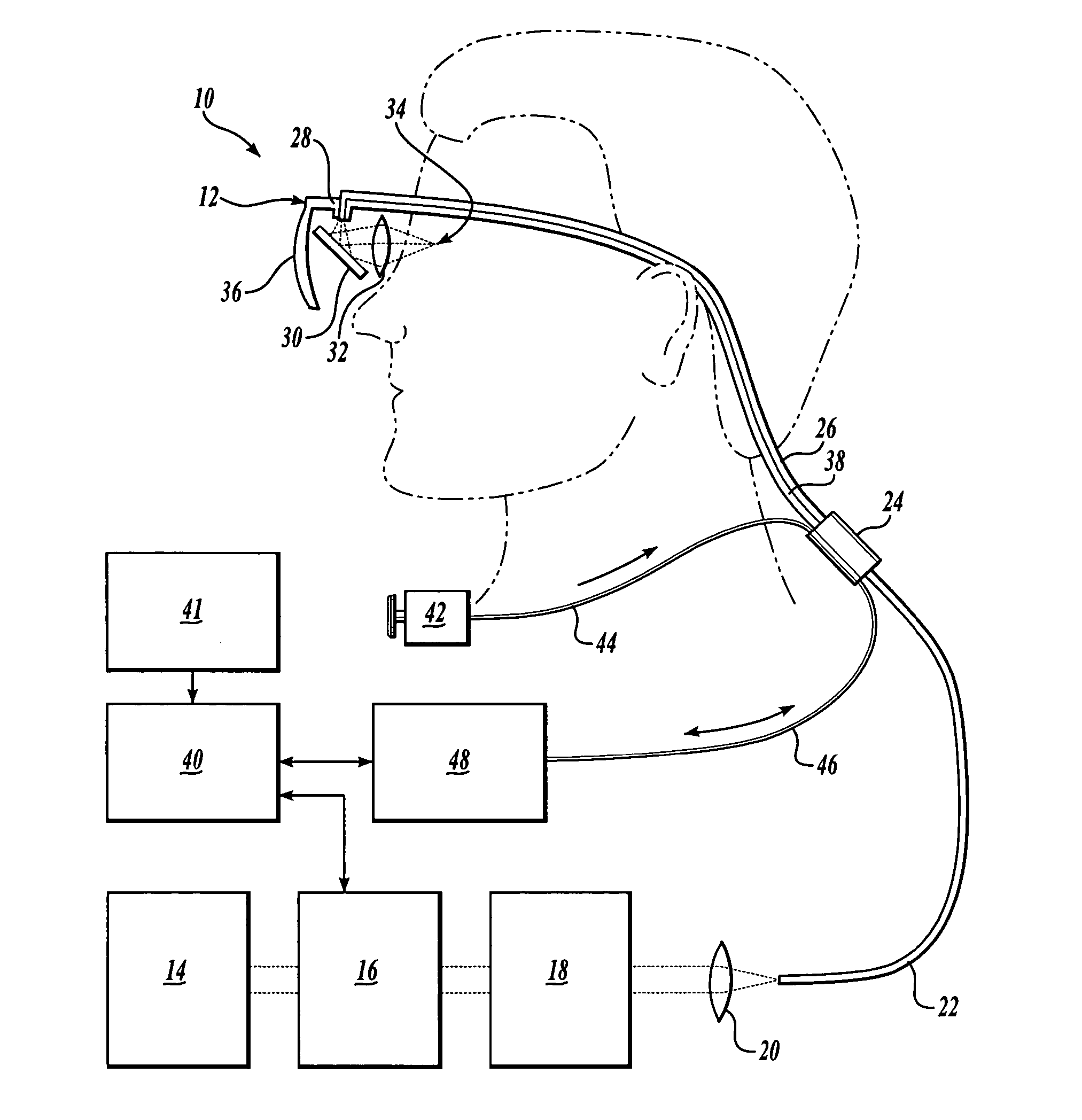
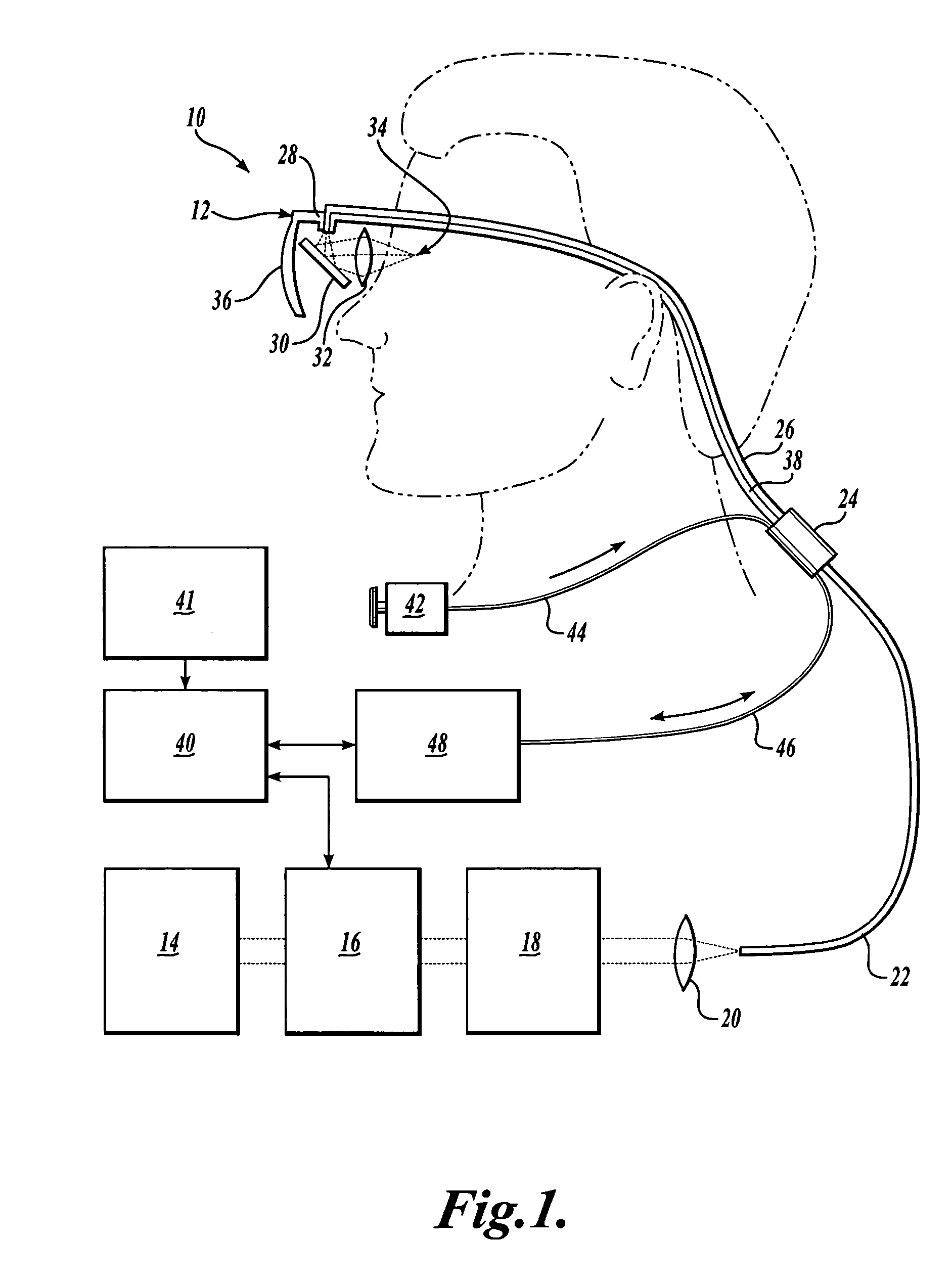
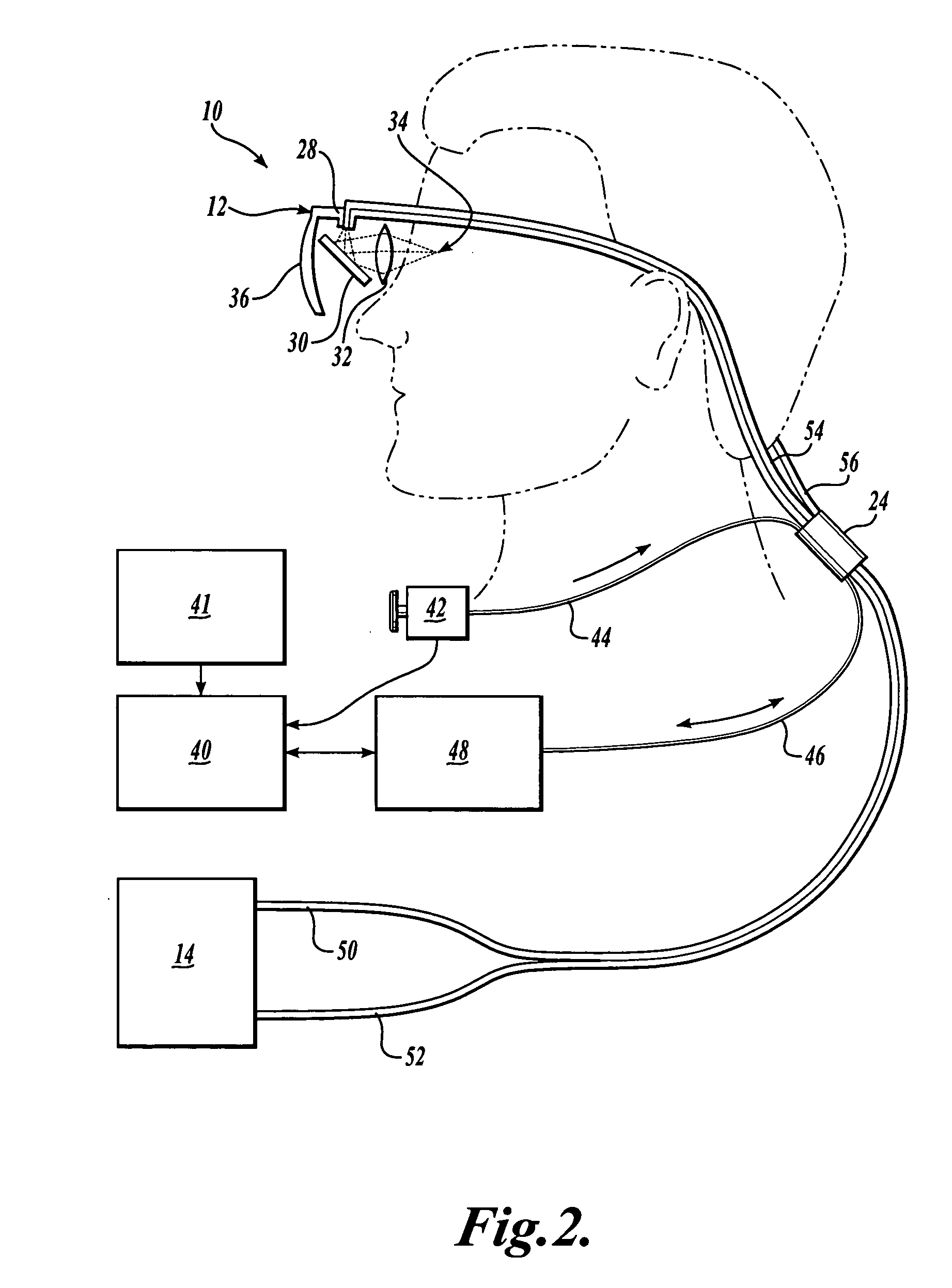
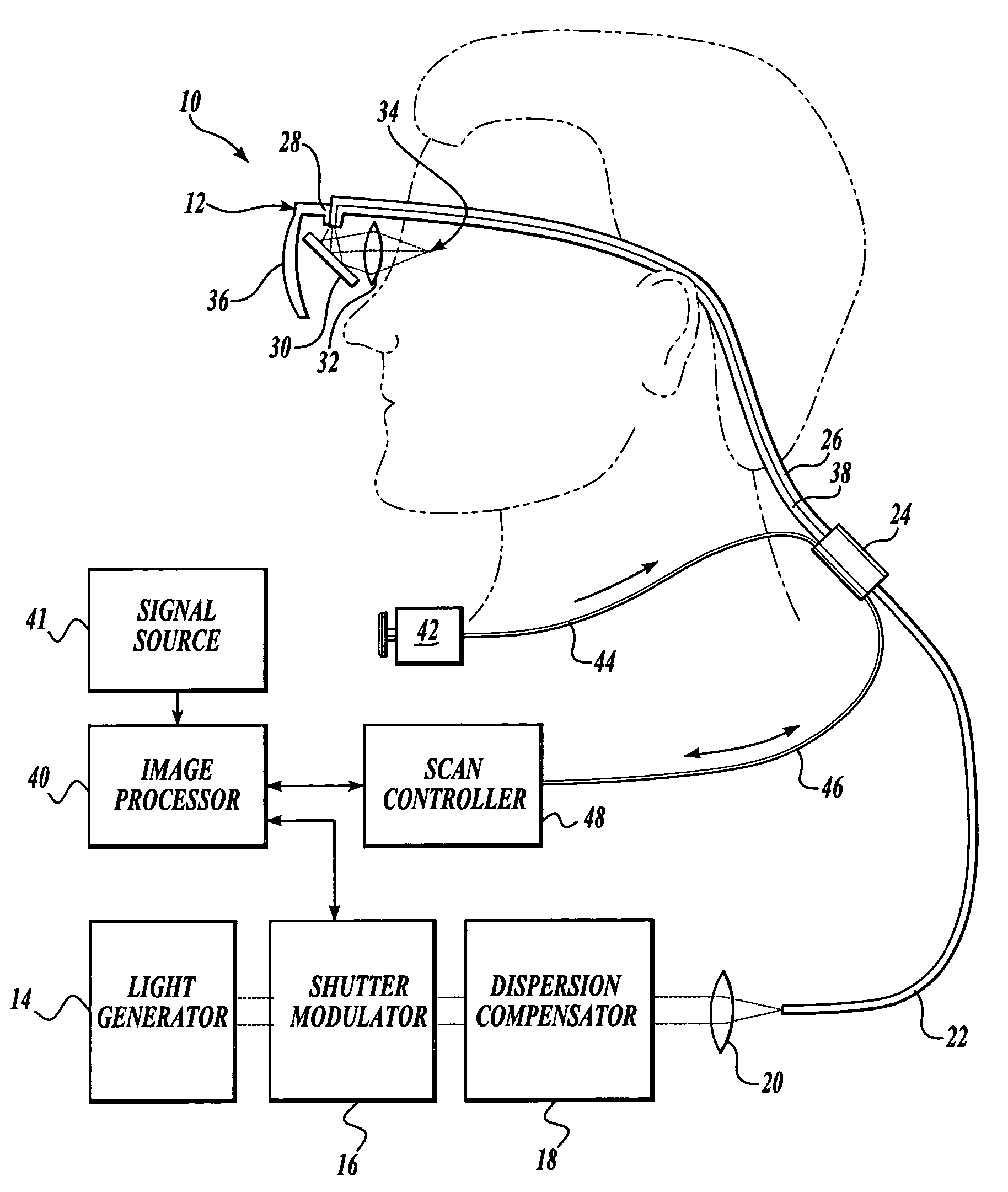
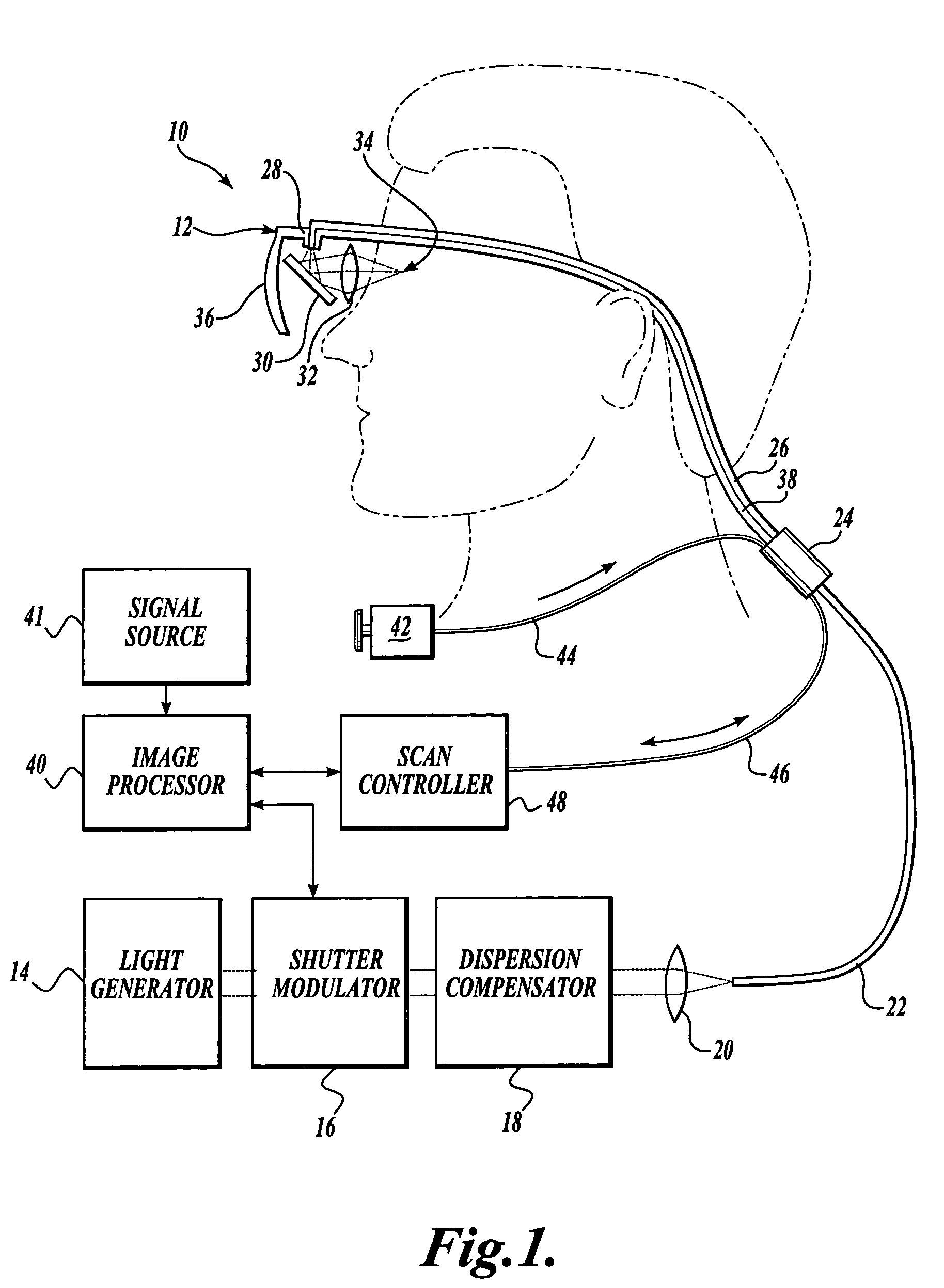
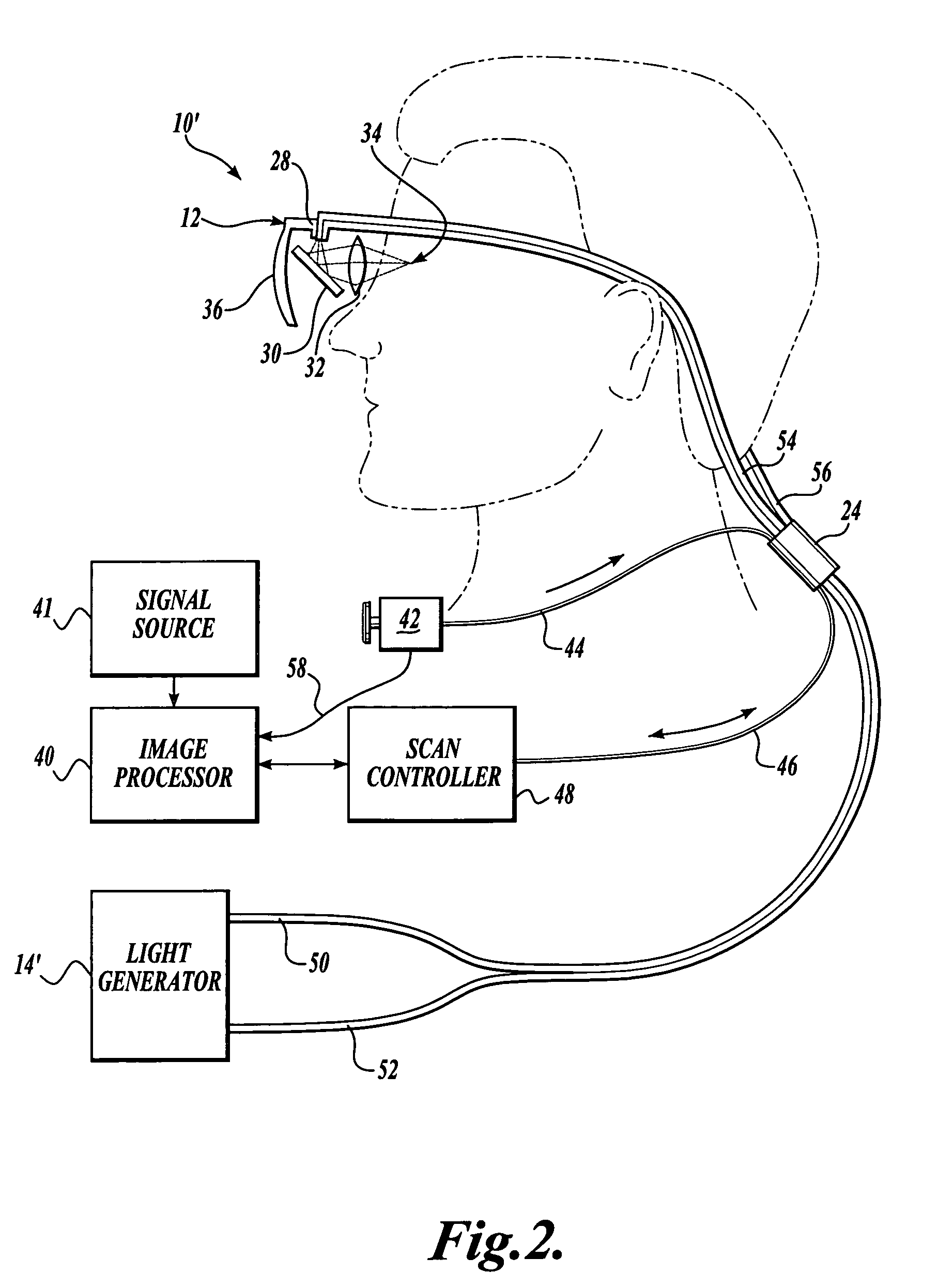
Scanning laser device and methods of use
InactiveUS20050015120A1Promote healthEasy SurvivalElectrotherapyDiagnosticsLight energyRetinal Neuron
In one aspect, the invention provides vision prosthesis systems. Exemplary vision prosthesis systems of the invention comprise a light energy generator operably connected to a wearable head piece comprising a device for directing light energy produced by the light energy generator onto a mammalian retina, wherein the light energy generator is tuned to emit light energy of sufficient power to modulate neural activity in the retina. In another aspect, the invention provides methods for irradiating neurons in the retina of the mammalian eye by directing light energy produced by a light energy generator onto a mammalian retina. The methods of the invention may be used to directly modulate the activity of retinal neurons or to introduce molecules into retinal cells.
Owner:UNIV OF WASHINGTON
Medical uses of in situ formed gels
Balanced pH, hyperosmotic, hypoosmotic, or isoosmotic gels are ideal vehicles for drug delivery. They are especially suited for topical body cavity or injection application of drugs or diagnostic agents; for drug or diagnostic agent delivery to the eye of a mammal; as protective corneal shields; or as ablatable corneal masks useful in laser reprofiling of the cornea. The compositions without the addition of a drug or diagnostic agent are useful as medical devices, for instance, in separating surgically or otherwise injured tissue as a means of preventing adhesions.
Owner:VIEGAS TACEY X +2
Scanning laser device and methods of use
In one aspect, the invention provides vision prosthesis systems. Exemplary vision prosthesis systems of the invention comprise a light energy generator operably connected to a wearable head piece comprising a device for directing light energy produced by the light energy generator onto a mammalian retina, wherein the light energy generator is tuned to emit light energy of sufficient power to modulate neural activity in the retina. In another aspect, the invention provides methods for irradiating neurons in the retina of the mammalian eye by directing light energy produced by a light energy generator onto a mammalian retina. The methods of the invention may be used to directly modulate the activity of retinal neurons or to introduce molecules into retinal cells.
Owner:UNIV OF WASHINGTON
Artificial tear replacement solution
InactiveUS7001607B1Reduce wearGood film formingHalogenated hydrocarbon active ingredientsSenses disorderConjunctivaConjunctival sac
A tear replacement solution that contains at least one water-soluble fluorosurfactant, water and a non-polar component, preferably in gel form, and a method for the external treatment for the eye of an mammal by applying the tear replacement solution to the eye, preferably by placing in the conjunctival sac.
Owner:PHARMPUR
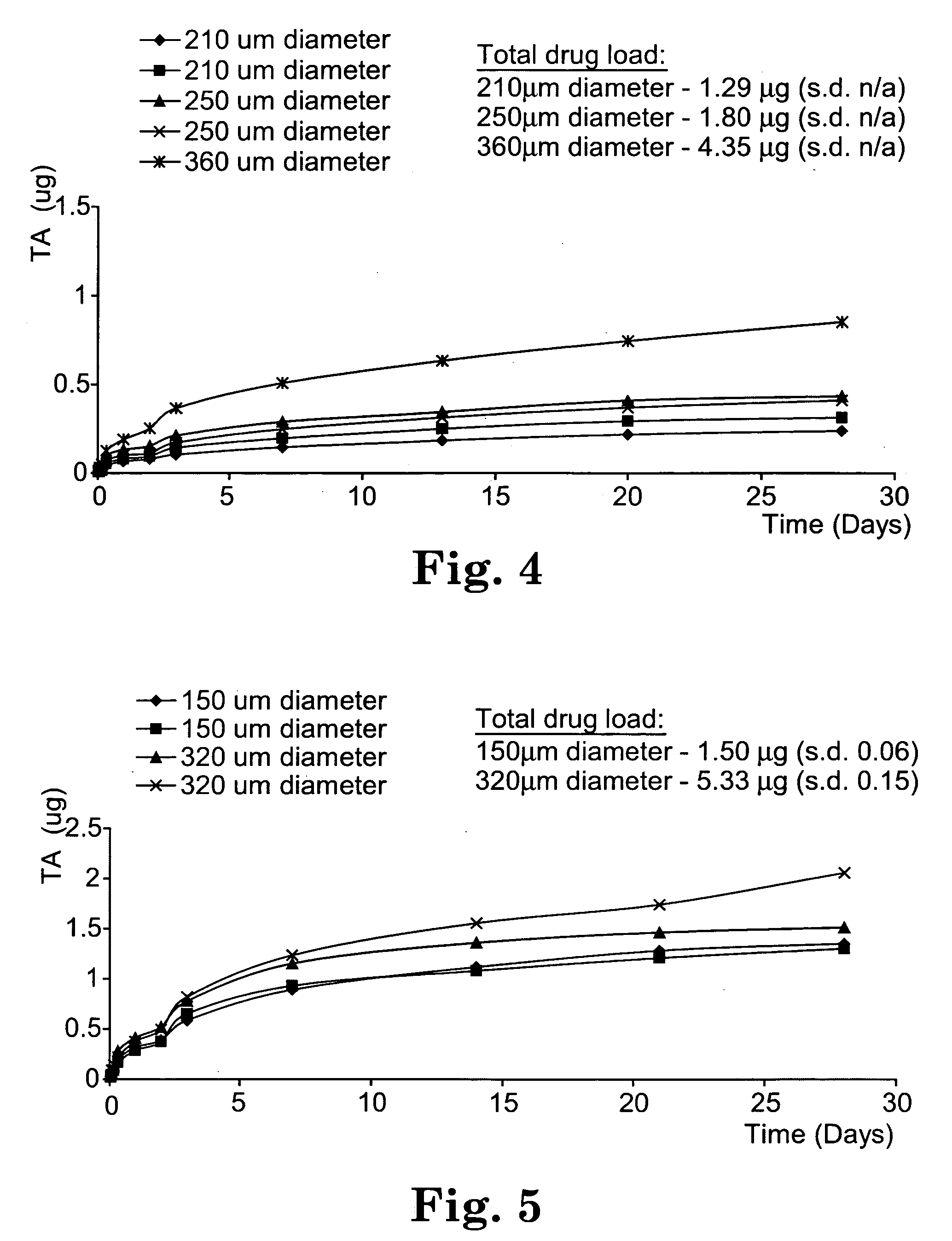
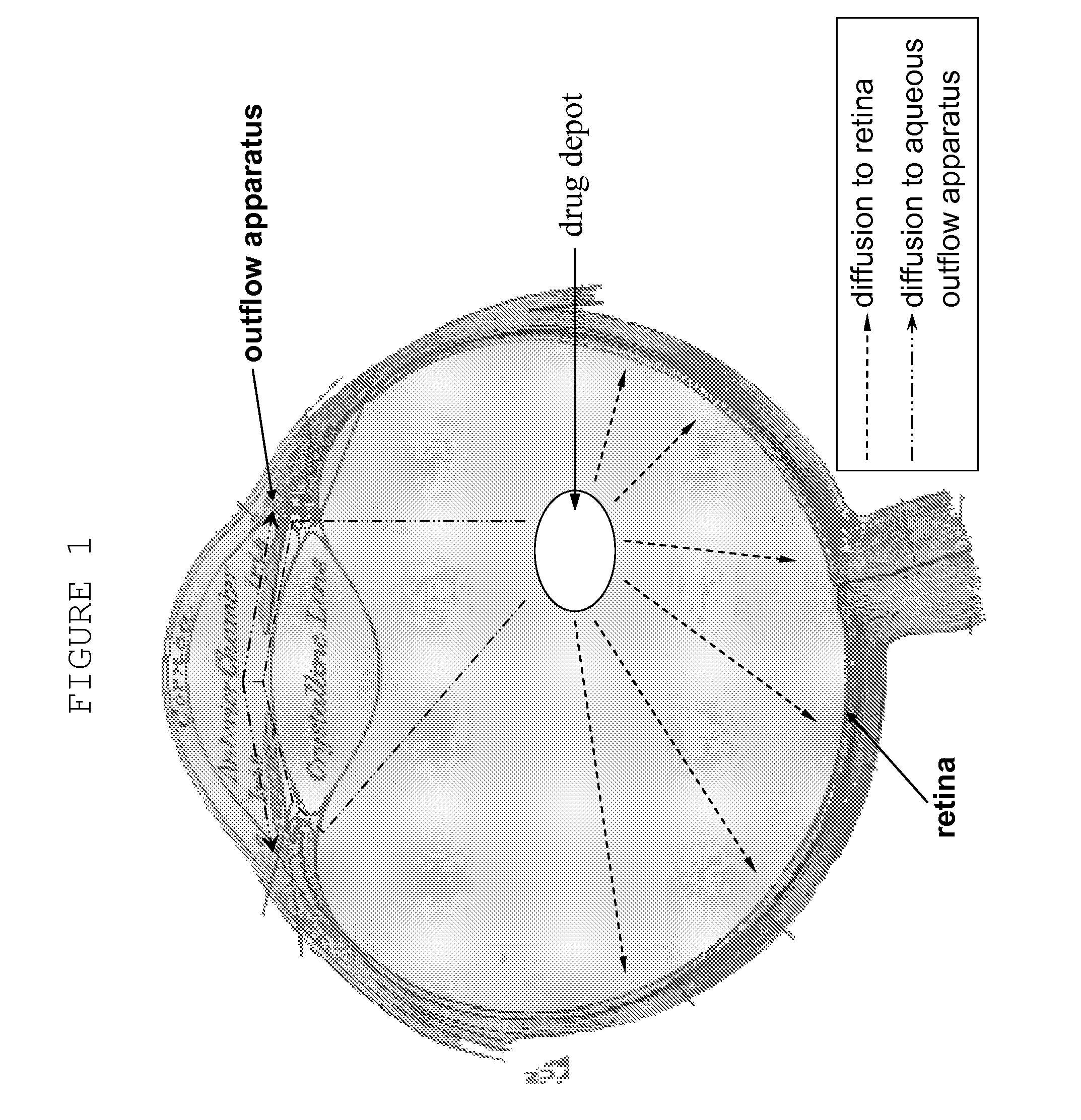
Sustained release implants and methods for subretinal delivery of bioactive agents to treat or prevent retinal disease
InactiveUS20060257451A1Inhibit progressQuantity maximizationSenses disorderEye implantsChoroid membraneOphthalmology
The invention relates to sustained release implants and to methods for treating eyes, particularly the eyes of mammals having eye disorders or diseases. By using the implants and methods described herein, the delivery of the one or more bioactive agents can be localized at a desired treatment site, particularly the choroid and the retina.
Owner:SURMODICS INC
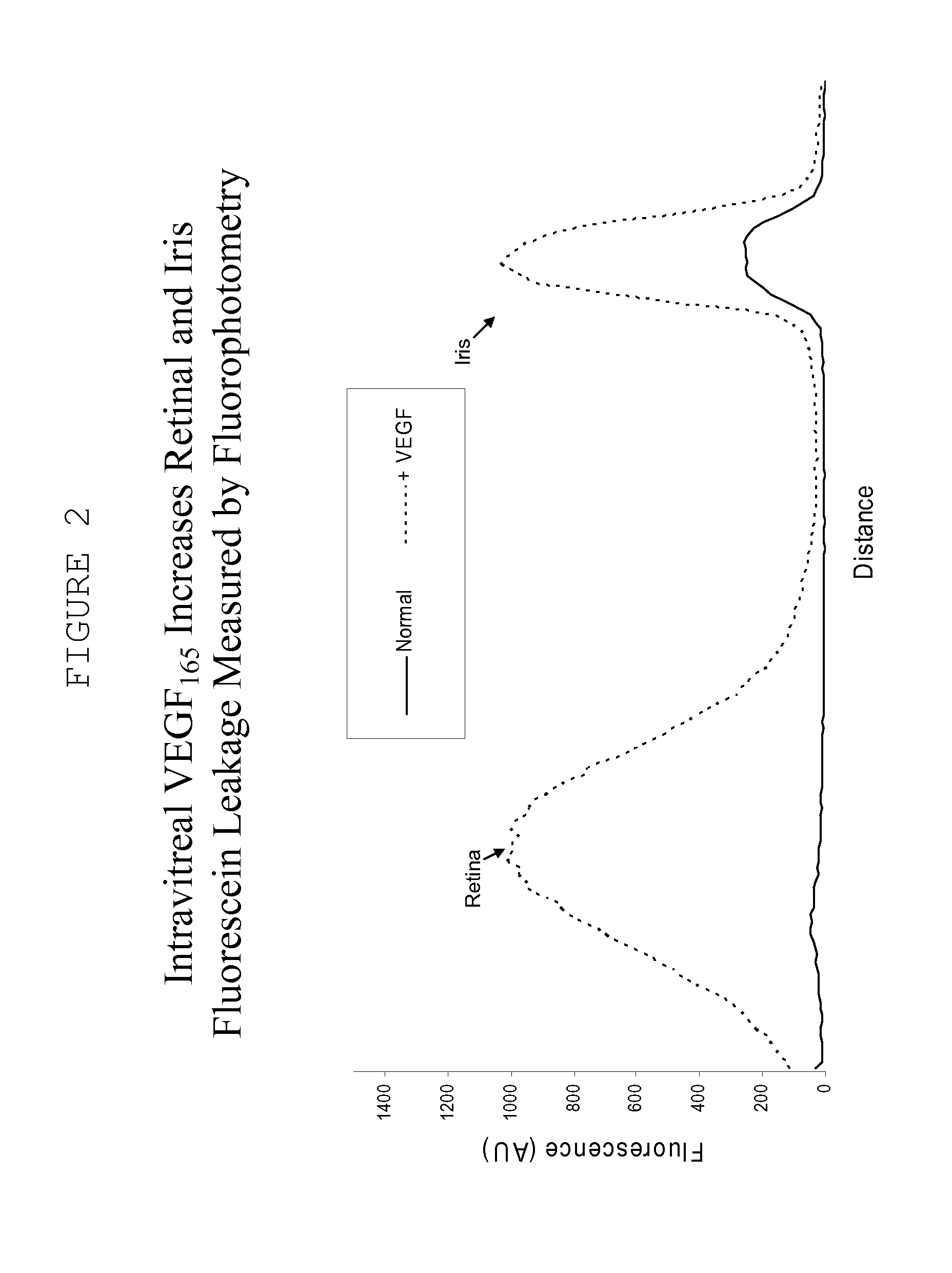
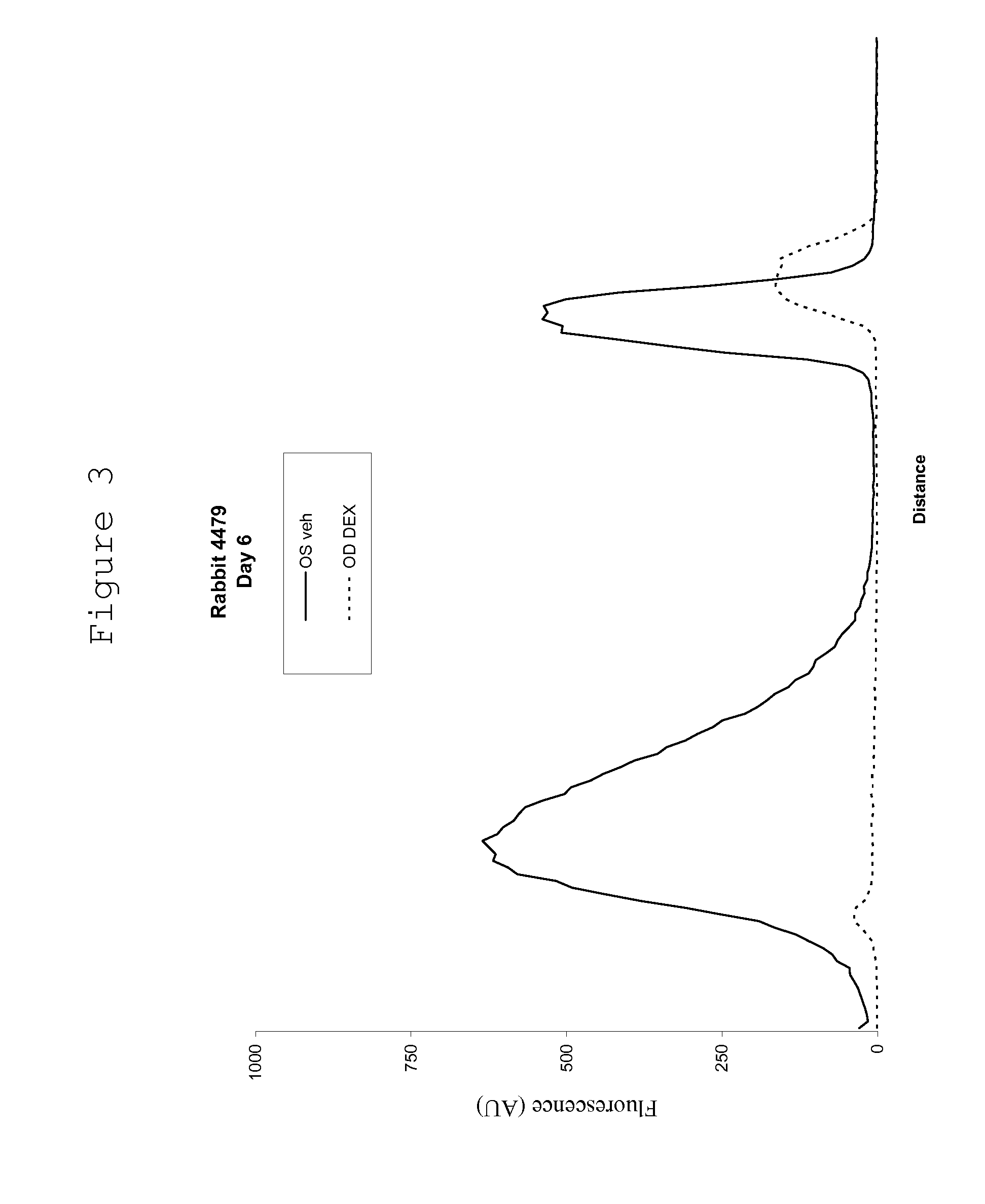
Ocular therapy using glucocorticoid derivatives selectively penetrating posterior segment tissues
Ophthalmically therapeutic materials, such as liquid-containing compositions and polymeric drug delivery systems, include a therapeutic component that includes an Glucocorticoid Derivative which, upon delivery to the posterior segment of a mammalian eye, does not significantly diffuse to the anterior segment of said eye. Methods of making and using the present materials are also described.
Owner:ALLERGAN INC
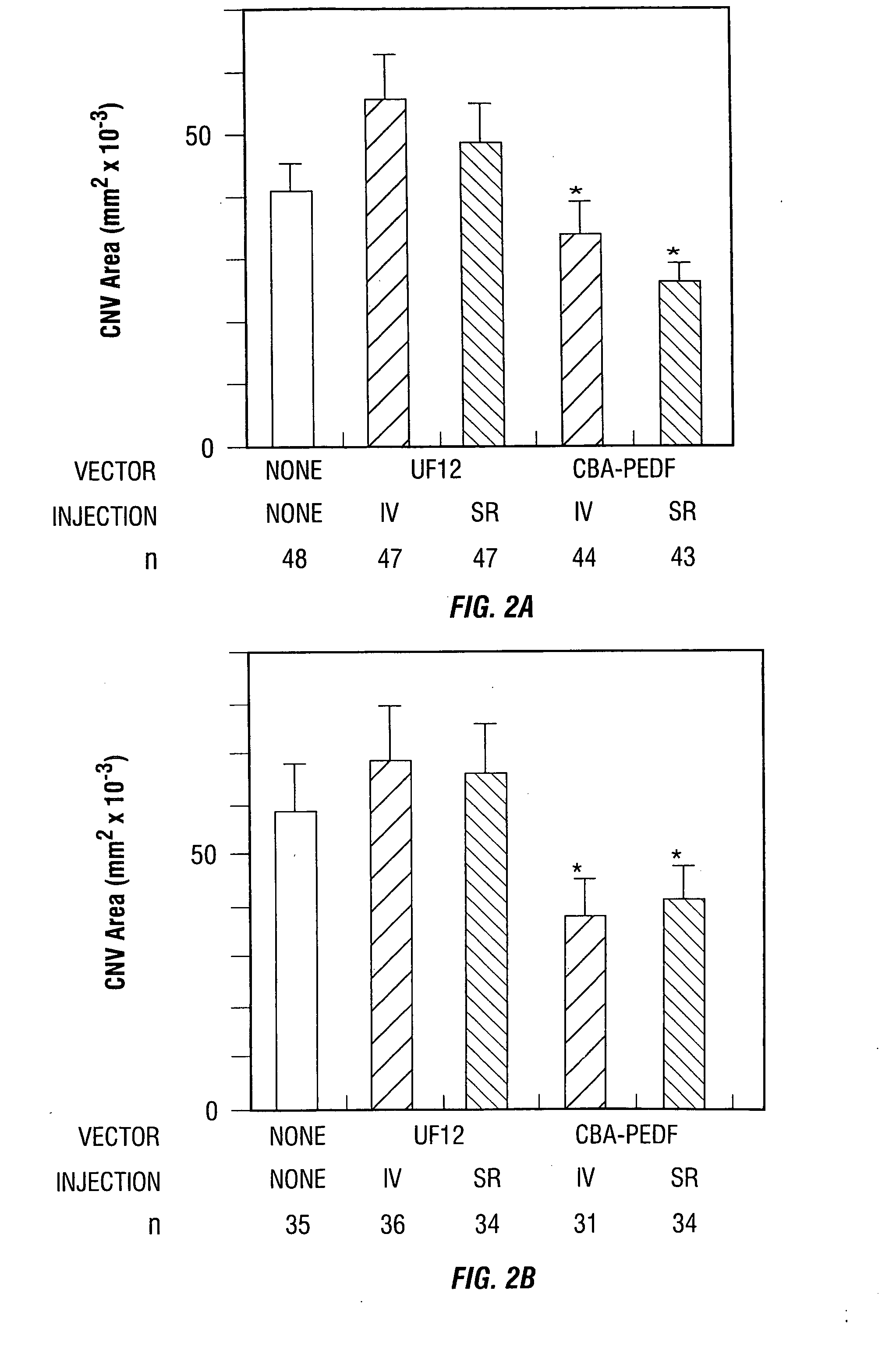
Raav vector compositions and methods for the treatment of choroidal neovascularization
InactiveUS20060193830A1Prevention of variousTreatment of variousBiocideSenses disorderPIGMENT EPITHELIUM-DERIVED FACTORDisease
Disclosed are methods for the use of therapeutic polypeptide-encoding polynucleotides in the creation of transformed host cells and transgenic animals is disclosed. In particular, the use of recombinant adeno-associated viral (rAAV) vector compositions comprising polynucleotide sequences that express one or more mammalian PEDF or anti-angiogenesis polypeptides is described. In particular, the invention provides gene therapy methods for the prevention, long-term treatment and / or amelioration of symptoms of a variety of conditions and disorders in a mammalian eye, including, for example blindness, loss of vision, retinal degeneration, macular degeneration, and related disorders resulting from retinal or choroidal neovascularization in affected individuals.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
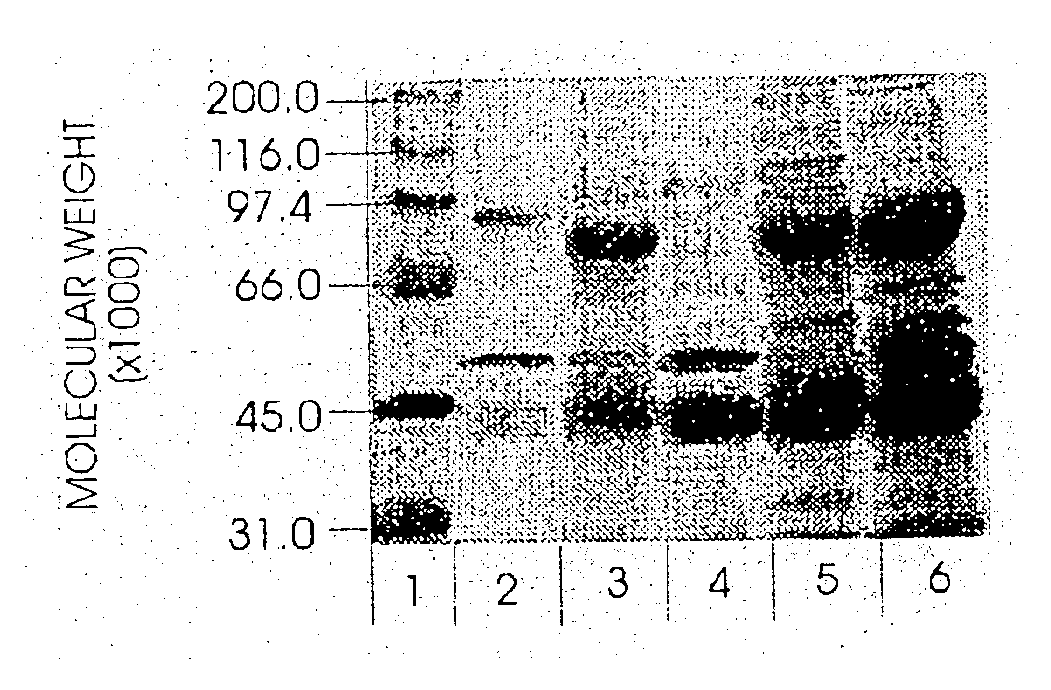
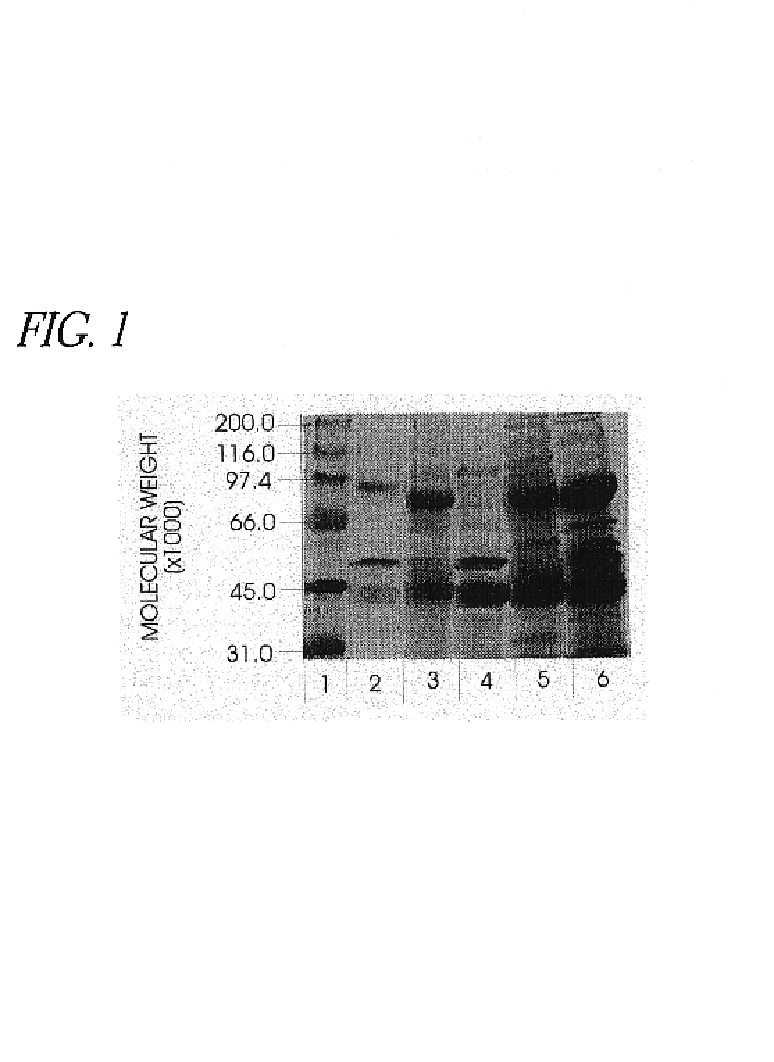
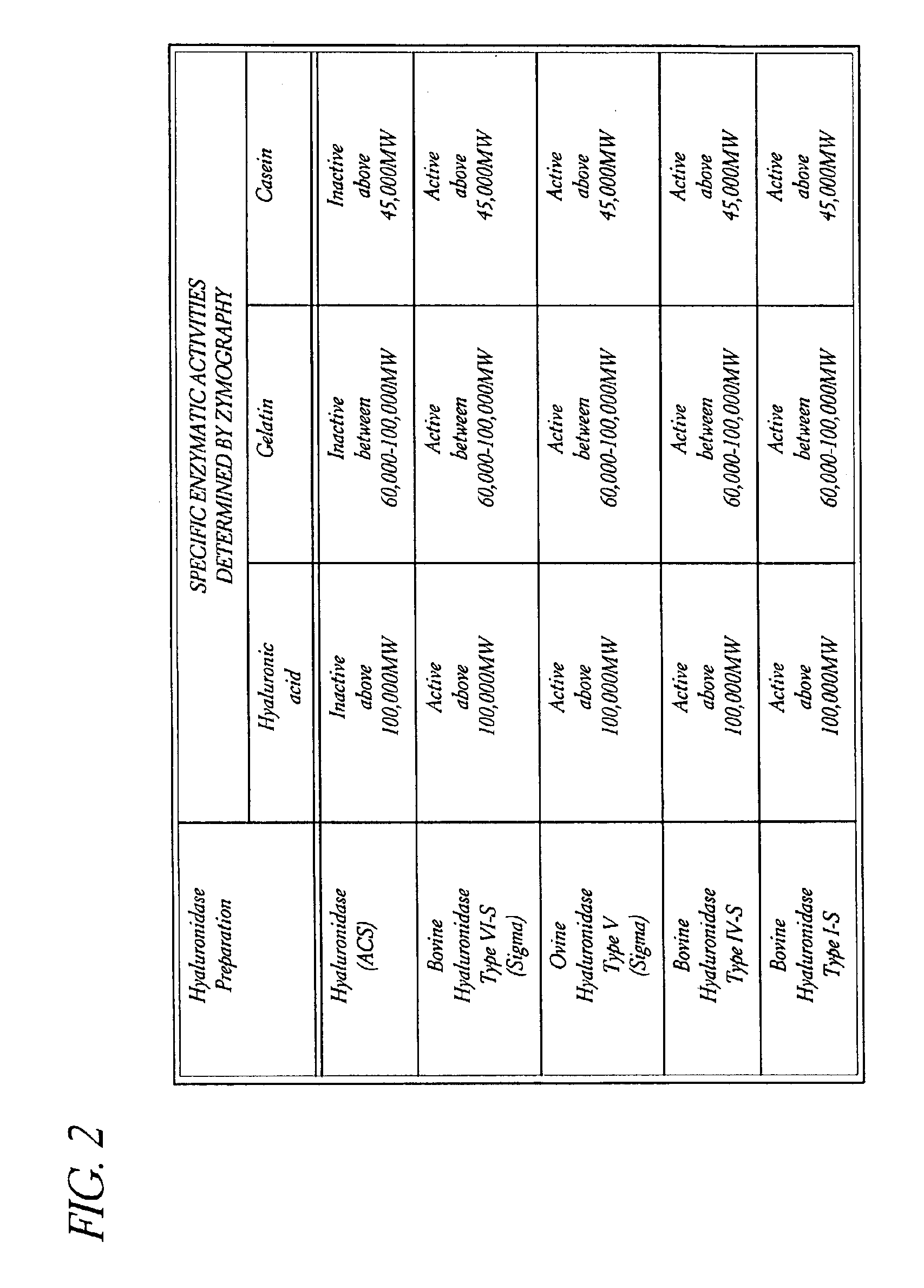
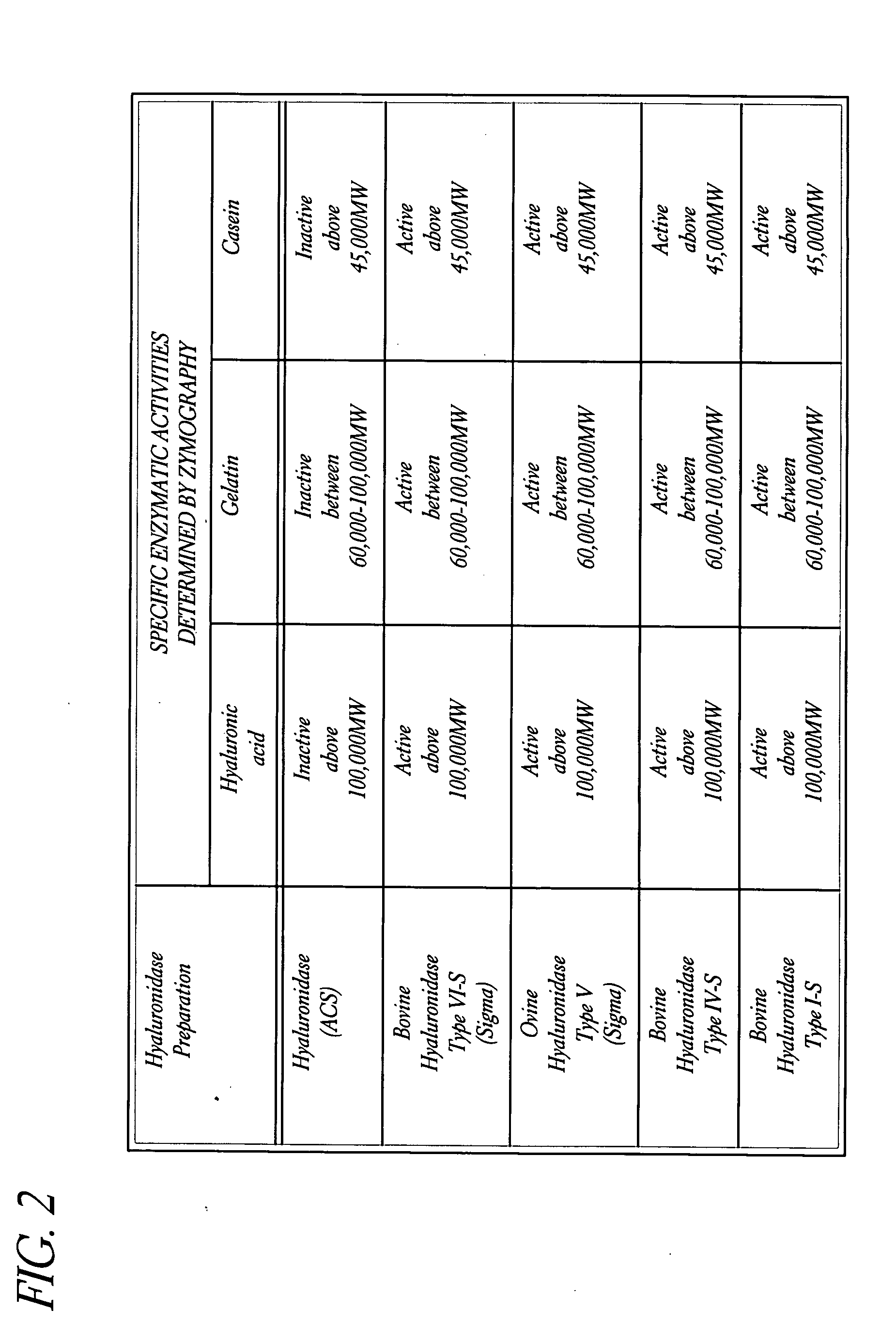
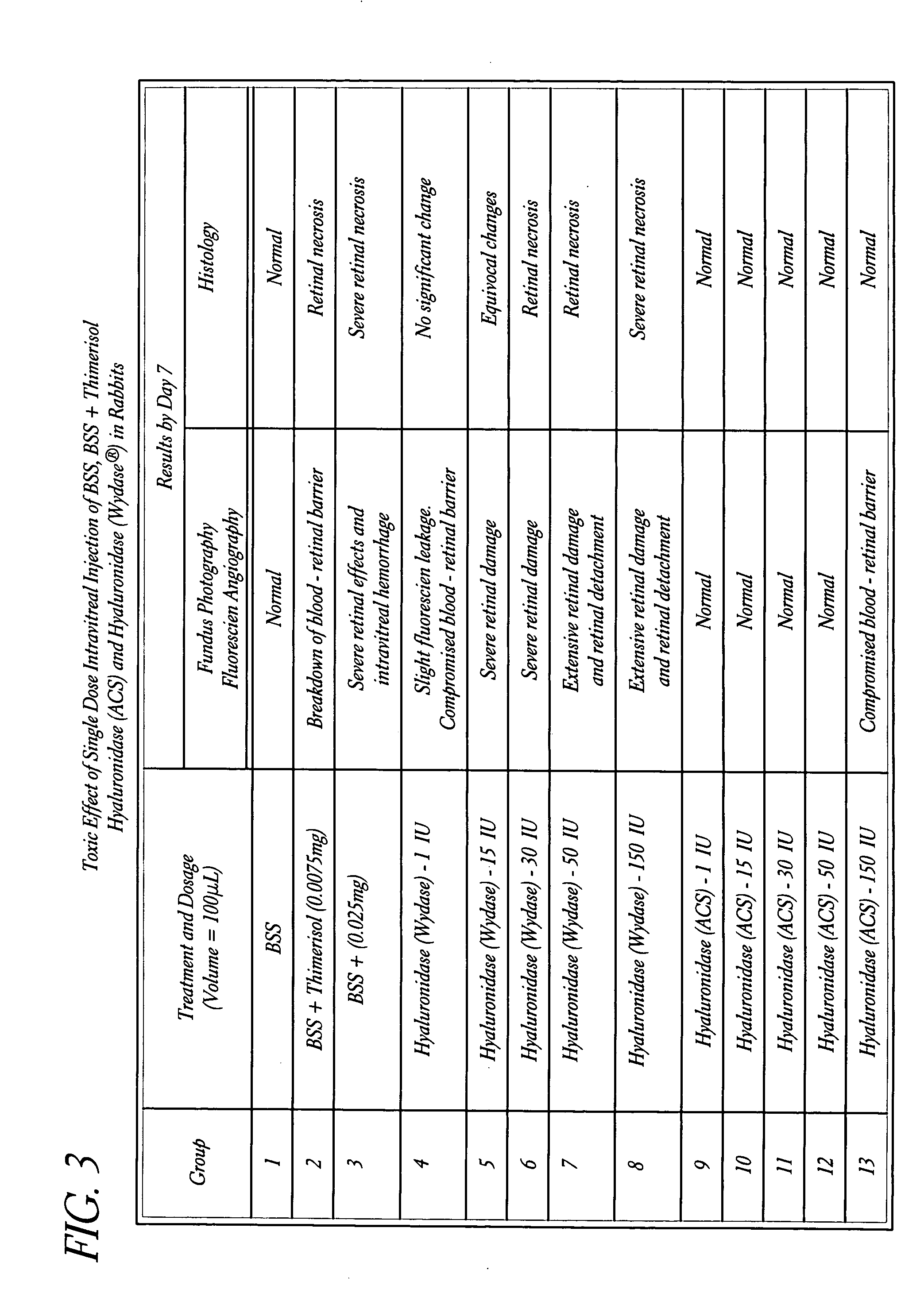
Use of hyaluronidase in the manufacture of an ophthalmic preparation for liquefying vitreous humor in the treatment of eye disorders
InactiveUS6863886B2Improve clearance rateIncreases rate of liquid exchangeBiocidePeptide/protein ingredientsDiseaseHyaluronidase
An enzymatic method is provided for treating ophthalmic disorders of the mammalian eye. Prevention of neovascularization and the increased rate of clearance from the vitreous of materials toxic to retina is accomplished by administering an amount of hyaluronidase effective to liquefy the vitreous humor of the treated eye without causing toxic damage to the eye. Liquefaction of the vitreous humor increases the rate of liquid exchange from the vitreal chamber. This increase in exchange removes those materials and conditions whose presence causes ophthalmological and retinal damage.
Owner:BAUSCH & LOMB PHARMA HLDG
Sustained release implants and methods for subretinal delivery of bioactive agents to treat or prevent retinal disease
InactiveUS8003124B2Quantity maximizationDesired flexibilitySenses disorderEye implantsDiseaseActive agent
The invention relates to sustained release implants and to methods for treating eyes, particularly the eyes of mammals having eye disorders or diseases. By using the implants and methods described herein, the delivery of the one or more bioactive agents can be localized at a desired treatment site, particularly the choroid and the retina.
Owner:SURMODICS INC
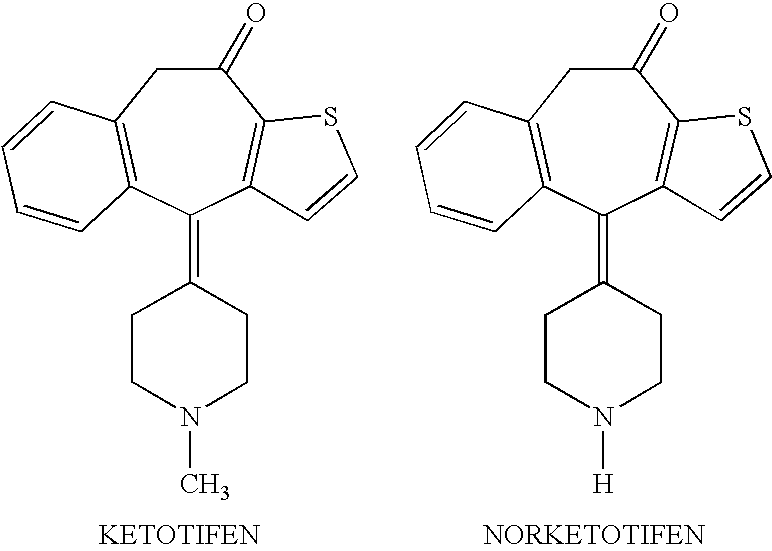
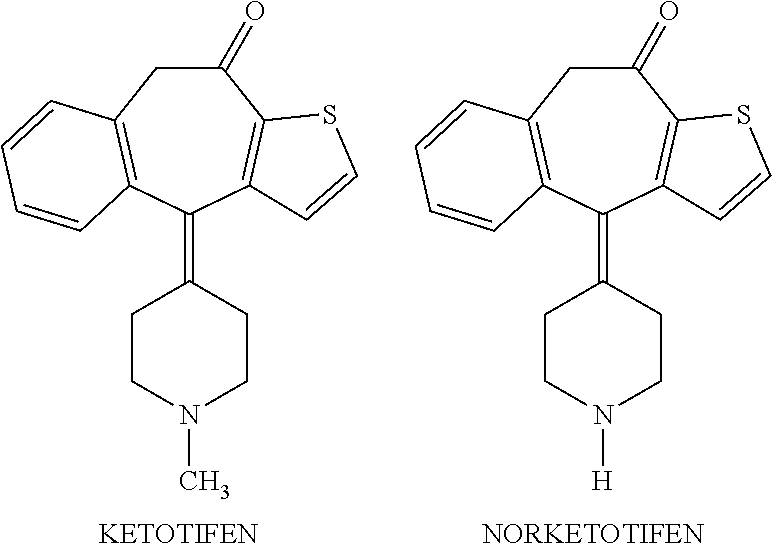
Ocular formulations of norketotifen
Ophthalmic compositions containing norketotifen and methods of making the same and the use thereof are disclosed. The methods also comprise administering to the eyes of a mammal in need thereof topical ophthalmic compositions containing norketotifen.
Owner:BRIDGE PHARMA INC
Improved raav vectors and methods for transduction of photoreceptors and rpe cells
ActiveUS20160369299A1More efficiencyImprove efficiencyVectorsGenetic material ingredientsDiseaseIn vivo
Disclosed are capsid-modified rAAV particles and expression vectors, as well as compositions and pharmaceutical formulations that comprise them. Also disclosed are methods of preparing and using novel capsid-protein-mutated particle or rAAV vector constructs in a variety of diagnostic and therapeutic applications including, inter alia, as delivery agents for diagnosis, treatment, or amelioration of one or more diseases, disorders, or dysfunctions of the mammalian eye. Also disclosed are methods for subretinal delivery of therapeutic gene constructs to mammalian photoreceptors and retinal pigment epithelial cells, as well as use of the disclosed compositions in the manufacture of medicaments for a variety of in vitro and / or in vivo applications including the treatment of a variety of inherited retinal diseases.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
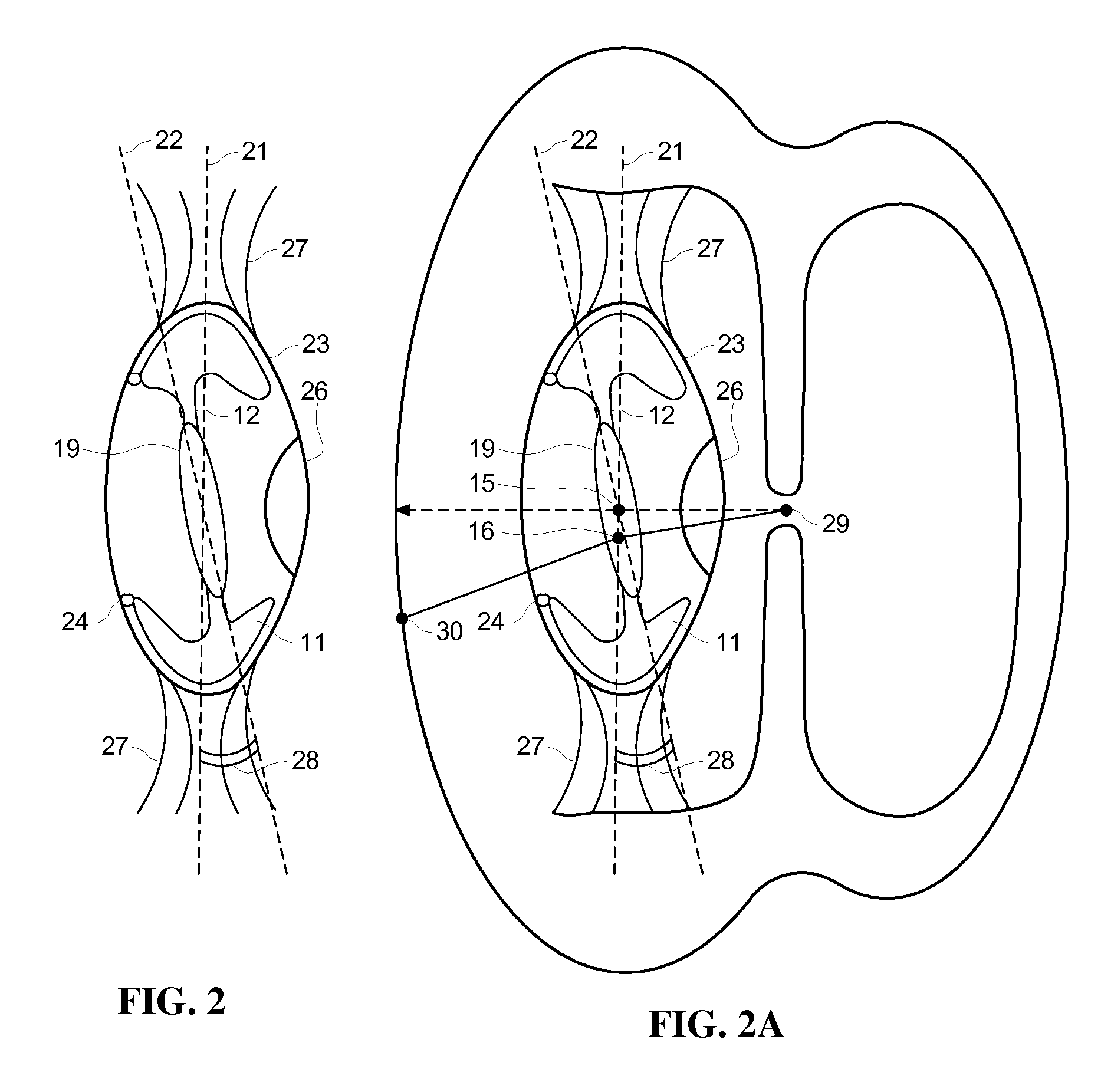
Pupil centered fovea focused optics assembly for intraocular lens
InactiveUS20130073039A1Reduce posterior capsular opacificationRequires some rigidityEye treatmentIntraocular lensOptical axisQuality of vision
The embodiments herein provide a pupil centered and fovea focused optics assembly comprising a ring platform provided inside a capsular bag of a mammalian eye to support an intraocular lens. The optical center of the intraocular lens is decentered with respect to the geometric center of the intraocular lens to align the optical centre of the lens with a visual axis of a pupil of the mammalian eye to improve the visual quality and to prevent an aberration. A plane of the intraocular lens is turned and tilted through a preset angle to point an optic axis of the intraocular lens to the Fovea.
Owner:MIRLAY RAM SRIKANTH
Buffered ophthalmic compositions and methods of use thereof
InactiveUS20120264681A1Accelerating and promoting healingPrevent or inhibit ocular tissue injury or woundingBiocideOrganic active ingredientsDiseaseActive agent
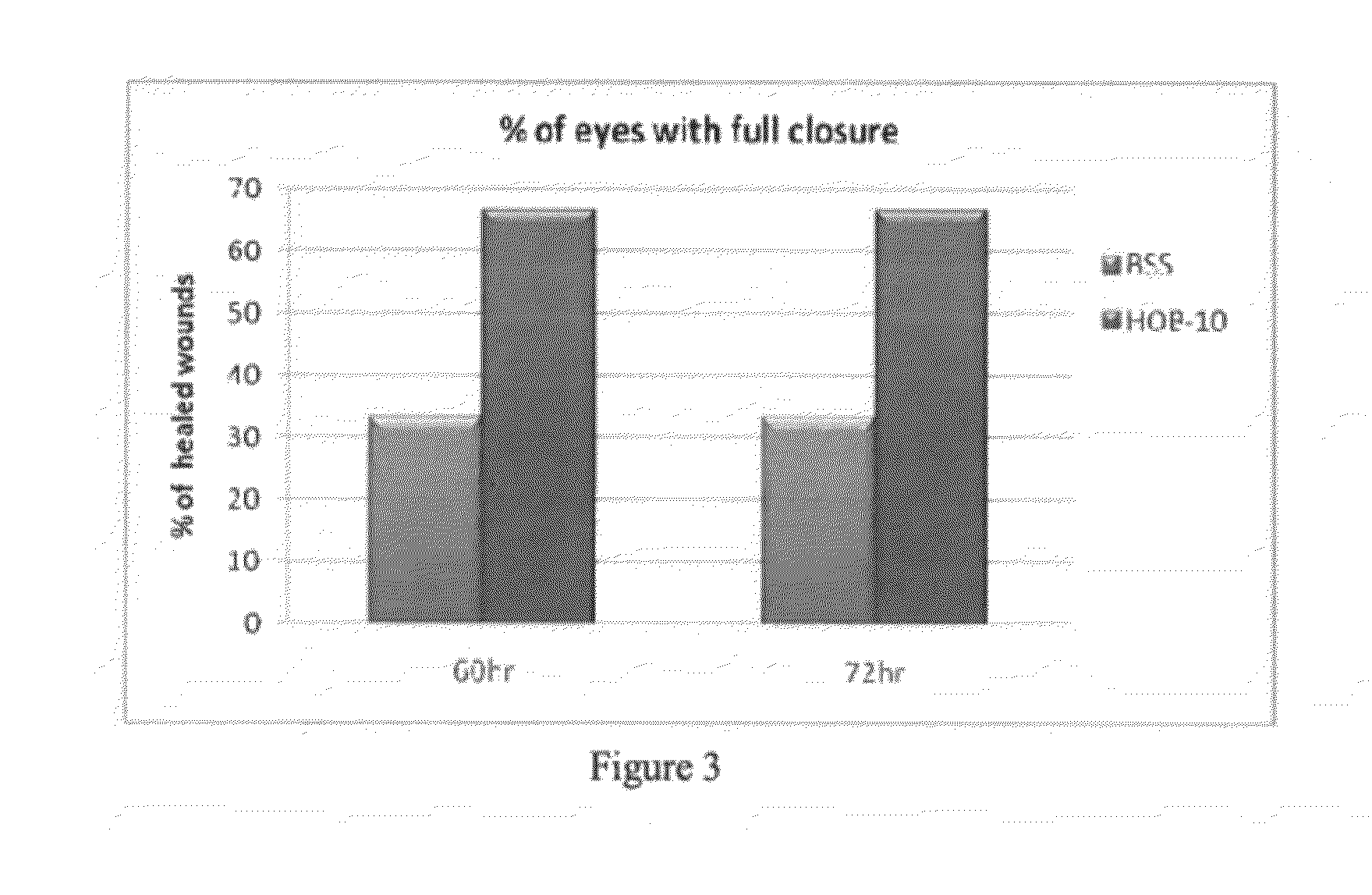
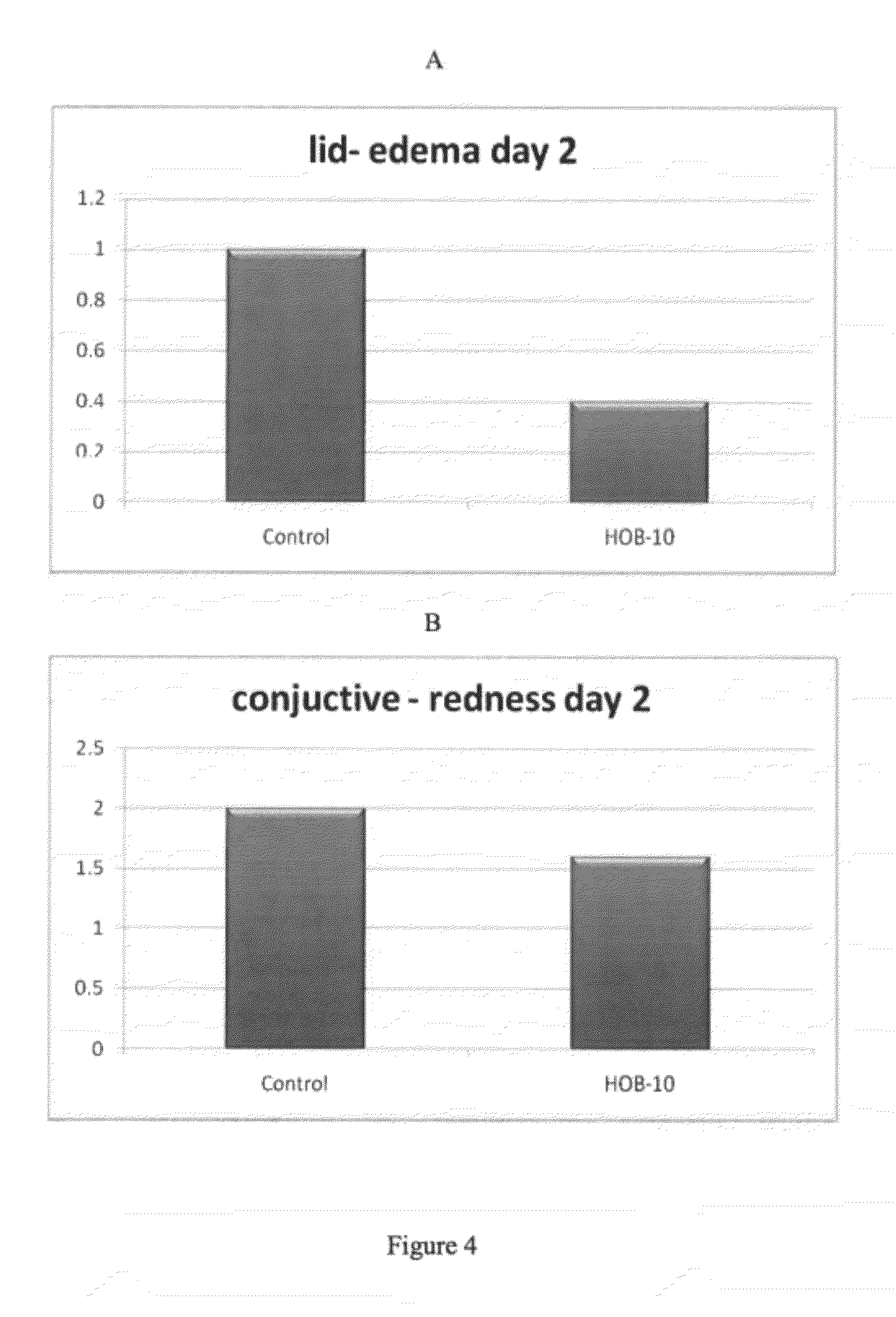
The present disclosure provides a buffered ophthalmic composition for formulation of topically administrable suspensions useful for treating eye disorders by promoting wound healing, delivery of pharmaceutically active agents, and lubricating the eye. In particular the ophthalmic composition includes a buffer solution compatible with application to a mammalian eye, wherein the buffer provides increased mechanism of action of pharmaceutically active agents as well as therapeutic qualities. The ophthalmic composition exhibits dual therapeutic action to alleviate various eye disorders as it concomitantly treats corneal ulcerations and excessive inflammation which results from various eye injuries.
Owner:HEALOR LTD
Use of injectable dyes for staining an anterior lens capsule and vitreo-retinal interface
InactiveUS7014991B2Preparing sample for investigationMedical devicesOcular structureIndigotindisulfonate
A method of staining an ocular structure, the structure being a human or other mammalian eye or portion thereof, the method comprising staining the ocular structure with either indigotindisulfonate, Patent Blue V, Sulphan Blue, tolonium chloride, or Evans Blue. Ocular structures of particular interest are the anterior lens capsule and the vitreo-retinal interface.
Owner:INFINITE VISION
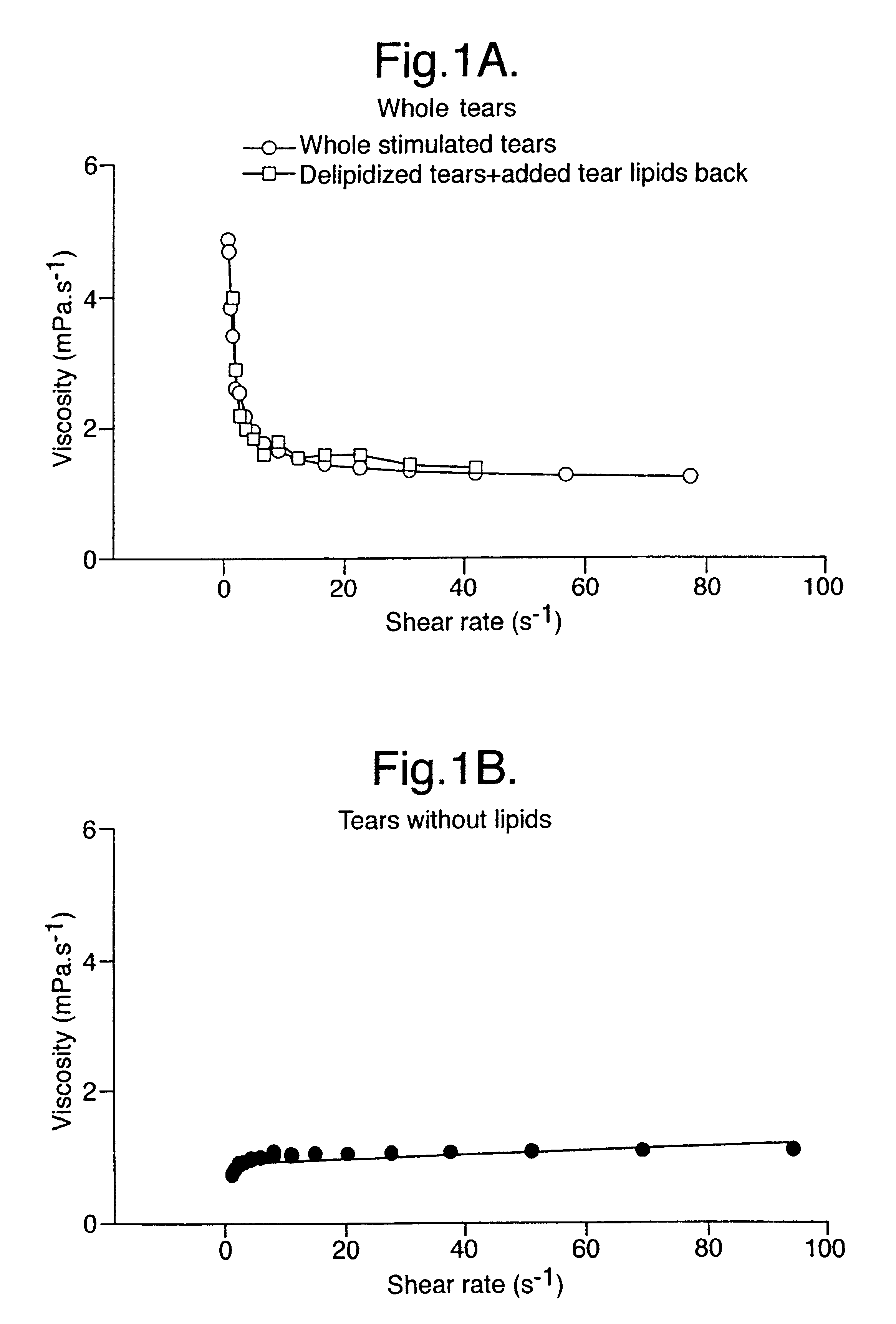
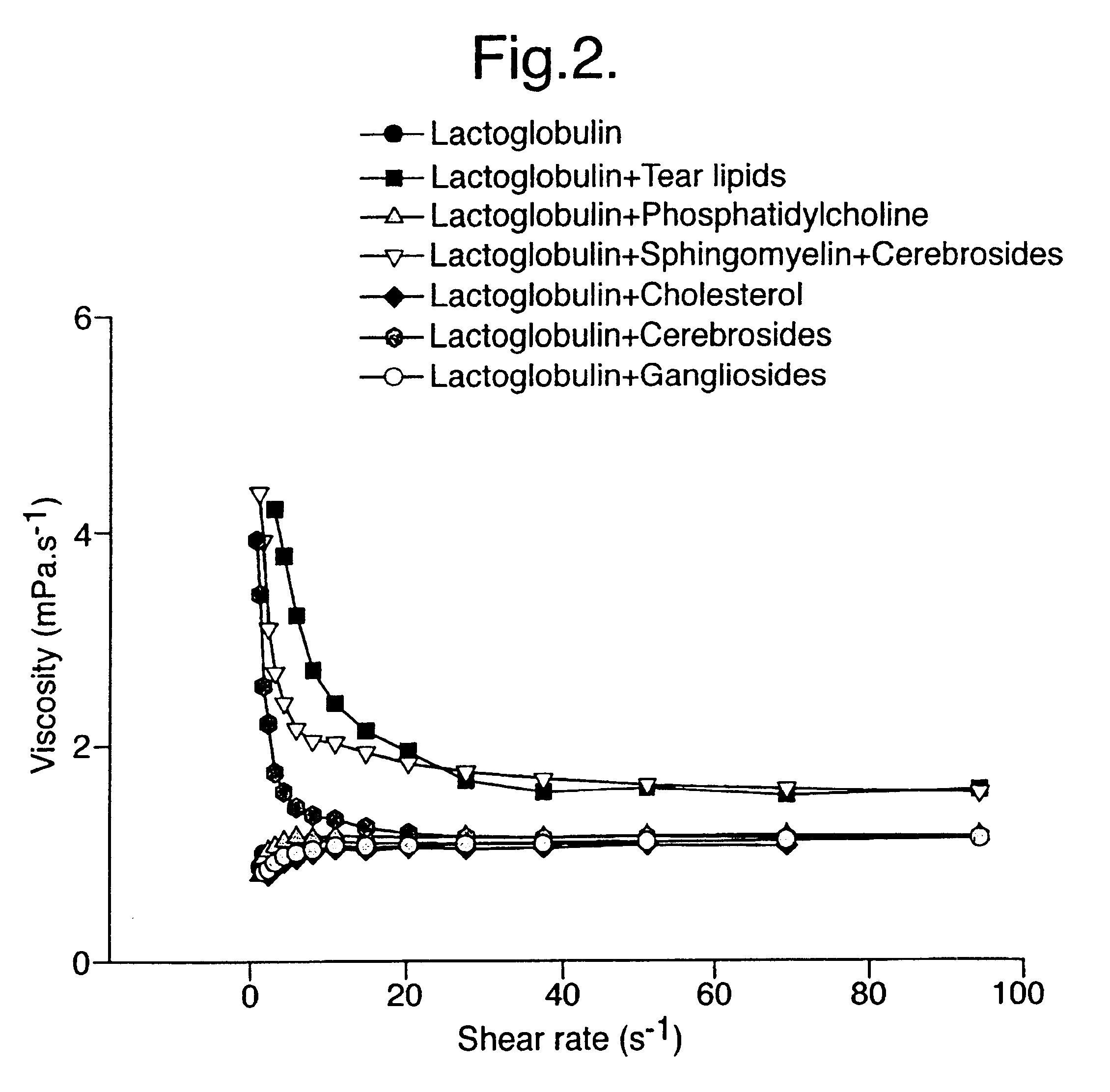
Artificial tear formulation
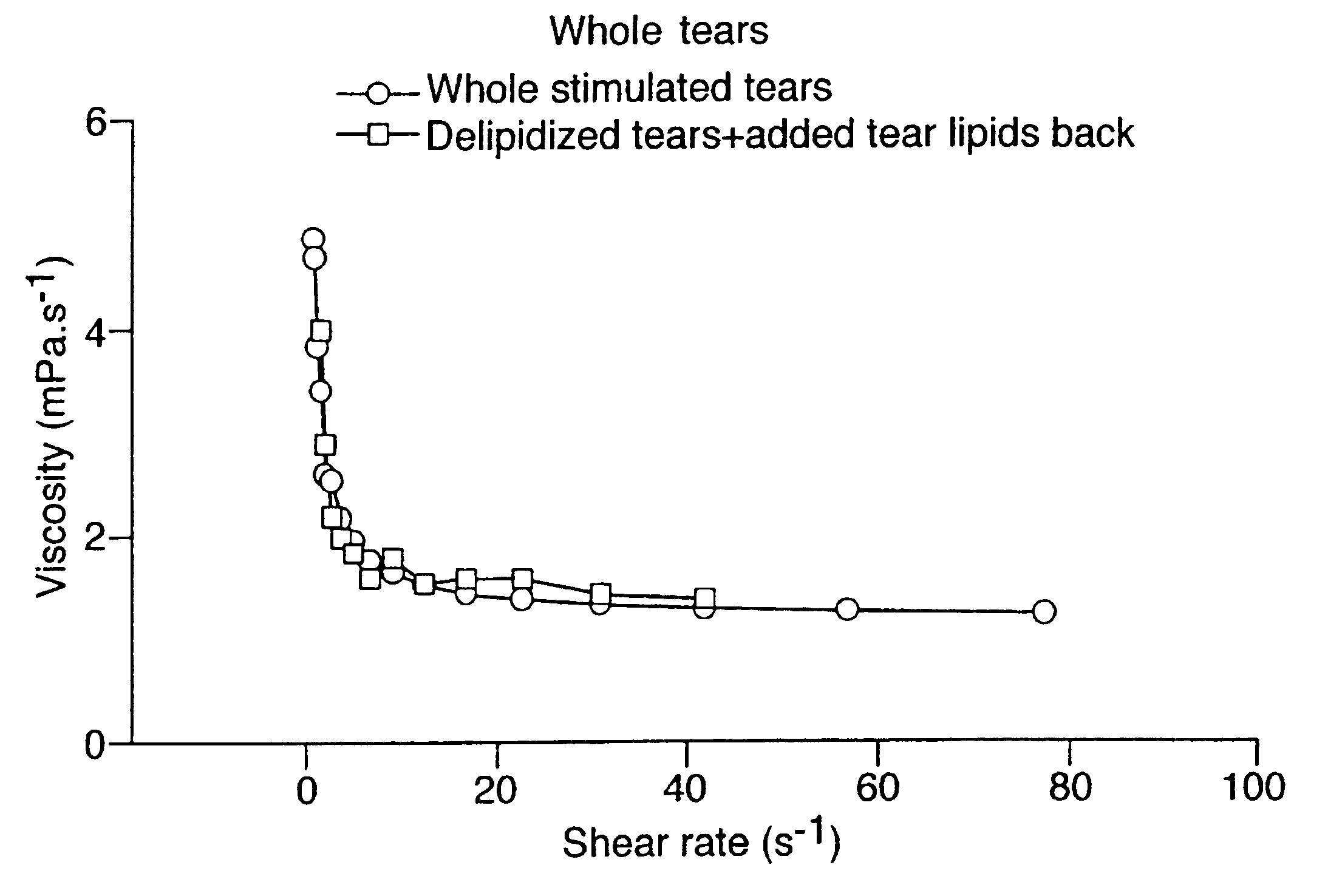
Provided by the present invention are formulations suitable for application to mammalian eyes which contain a lipid binding protein and a polar lipid, present as a soluble complex in an aqueous electrolyte. The formulations described have shear-thinning (non-Newtonian viscosity) and surface tension properties to natural tears and are therefore useful as artificial tear substitutes for the treatment of dry eyes (e.g. keratoconjunctivitis sicca) and useful in ophthalmic applications in general.
Owner:ISIS INNOVATION LTD
Methods and compositions for gene delivery to on bipolar cells
InactiveUS20170007720A1Overcome limitationsImprove efficiencyVectorsPeptide/protein ingredientsGene deliveryRetinitis pigmentosa
Disclosed are capsid-modified rAAV expression vectors, as well as infectious virions, compositions, and pharmaceutical formulations that include them. Also disclosed are methods of preparing and using novel capsid-protein-mutated rAAV vector constructs in a variety of diagnostic and therapeutic applications including, inter alia, as delivery agents for diagnosis, treatment, or amelioration of one or more diseases, disorders, or dysfunctions of the mammalian eye. Also disclosed are methods for intravitreal delivery of therapeutic gene constructs to retinal neuron cells, and specifically to ON bipolar cells, of the mammalian eye, as well as use of the disclosed compositions in the manufacture of medicaments for a variety of in vitro and / or in vivo applications including the treatment of retinitis pigmentosa, melanoma-associated retinopathy, and congenital stationary night blindness.
Owner:THE UNIV OF BRITISH COLUMBIA +1
Non-irritating compositions
The present invention provides a base composition that allows for the formulation of non-irritating cosmetic and / or dermatological compositions. The base composition includes one or more of electrolyte, buffer, mild preservative, lubricant, or any combinations thereof. It is preferred that one or more of the above components are eye-safe and / or eye-compatible. The present invention also provides photoprotective cosmetic and / or dermatological compositions that include the base composition and one or more sunscreen active components and are non-irritating to mammalian eyes.
Owner:EDGEWELL PERSONAL CARE BRANDS LLC
Non-irritating compositions
The present invention provides a base composition that allows for the formulation of non-irritating cosmetic and / or dermatological compositions. The base composition includes one or more of electrolyte, buffer, mild preservative, lubricant, or any combinations thereof. It is preferred that one or more of the above components are eye-safe and / or eye-compatible. The present invention also provides photoprotective cosmetic and / or dermatological compositions that include the base composition and one or more sunscreen active components and are non-irritating to mammalian eyes.
Owner:EDGEWELL PERSONAL CARE BRANDS LLC
Use of hyaluronidase in the manufacture of an ophthalmic preparation for liquefying vitreous humor in the treatment of eye disorders
InactiveUS20050069531A1Improve clearance rateIncreases rate of liquid exchangePeptide/protein ingredientsDrug compositionsDiseaseHyaluronidase
An enzymatic method is provided for treating ophthalmic disorders of the mammalian eye. Prevention of neovascularization and the increased rate of clearance from the vitreous of materials toxic to retina is accomplished by administering an amount of hyaluronidase effective to liquefy the vitreous humor of the treated eye without causing toxic damage to the eye. Liquefaction of the vitreous humor increases the rate of liquid exchange from the vitreal chamber. This increase in exchange removes those materials and conditions whose presence causes ophthalmological and retinal damage.
Owner:KARAGEOZIAN HAMPAR +5
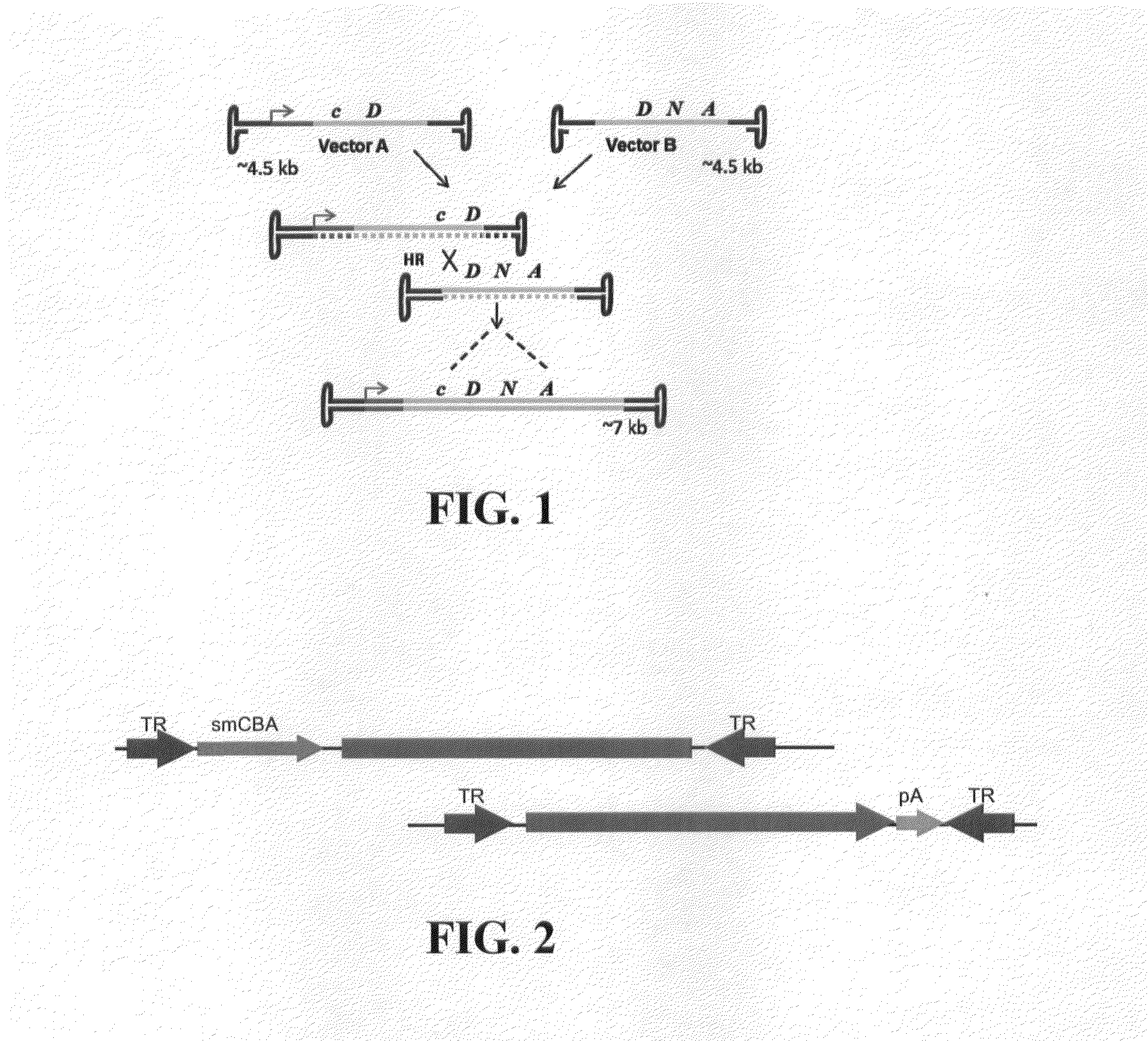
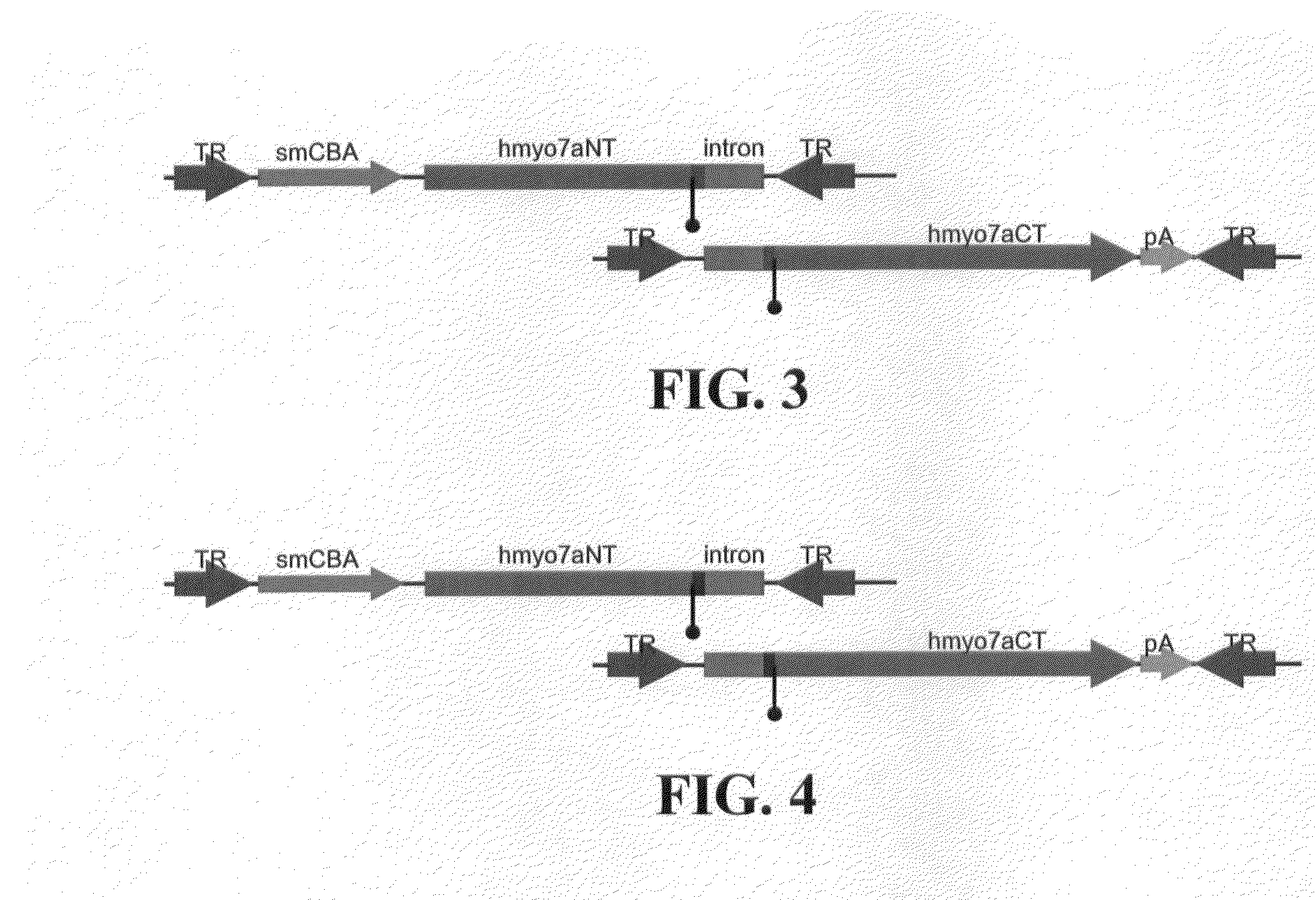
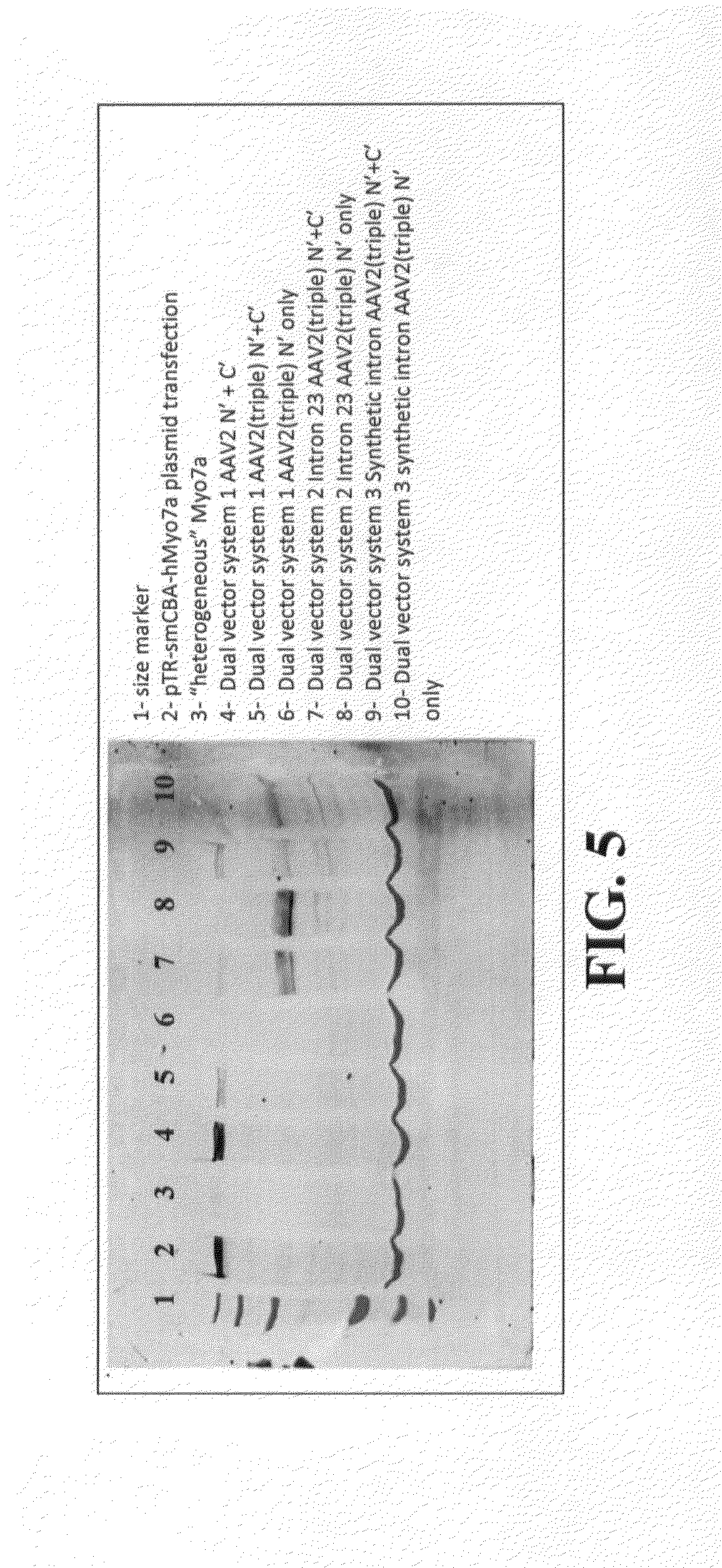
Dual-AAV Vector-Based Systems and Methods for Delivering Oversized Genes to Mammalian Cells
Disclosed are materials and methods for treating diseases of the mammalian eye, and in particular, Usher syndrome 1B (USH1B). The invention provides AAV-based, dual-vector systems that facilitate the expression of full-length proteins whose coding sequences exceed that of the polynucleotide packaging capacity of an individual AAV vector. In one embodiment, vector systems are provided that include i) a first AAV vector polynucleotide that includes an inverted terminal repeat at each end of the polynucleotide and a suitable promoter followed by a partial coding sequence that encodes an N-terminal portion of a full-length polypeptide; and ii) a second AAV vector polynucleotide that includes an inverted terminal repeat at each end of the polynucleotide and a partial coding sequence that encodes a C-terminal portion of a full-length polypeptide, optionally followed by a polyadenylation (pA) signal sequence. In another embodiment, the vector system includes i) a first AAV vector polynucleotide comprising an inverted terminal repeat at each end, a suitable promoter followed by a partial coding sequence that encodes an N-terminal portion of a full-length polypeptide followed by a splice donor site and intron and ii) a second AAV vector polynucleotide comprising an inverted terminal repeat at each end, followed by an intron and a splice-acceptor site for the intron, followed by a partial coding sequence that encodes a C-terminal portion of a full-length polypeptide, optionally followed by a polyadenylation (pA) signal sequence. The coding sequence or the intron sequence in the first and second AAV vectors preferably includes a sequence region that overlaps.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Isolated myeloid-like cell populations and methods of treatment therewith
The present invention provides an isolated myeloid-like cell population comprising a majority of cells that are lineage negative, and which express both CD44 antigen, CD11b antigen, and hypoxia inducible factor 1α (HIF-1α). These cells have beneficial vasculotrophic and neurotrophic activity when intraocularly administered to the eye of a mammal, particularly a mammal suffering from an ocular degenerative disease. The myeloid-like cells are isolated by treating bone marrow cells, peripheral blood cells or umbilical cord cells with an antibody against CD44 (hyaluronic acid receptor), against CD11b, CD14, CD33, or against a combination thereof and using flow cytometry to positively select CD44 and / or CD11b expressing cells therefrom. The isolated myeloid-like bone marrow cells of the invention can be transfected with a gene encoding a therapeutically useful protein, for delivering the gene to the retina.
Owner:THE SCRIPPS RES INST
Treatment of cone cell degeneration with transfected lineage negative hematopoietic stem cells
A method of preserving cone cells in the eye of a mammal suffering from a retinal degenerative disease comprises isolating from the bone marrow of the mammal a lineage negative hematopoietic stem cell population that includes endothelial progenitor cells, transfecting cells from the stem cell population with a gene that operably encodes an antiangiogenic fragment of human tryptophanyl tRNA synthetase (TrpRS), and subsequently intravitreally injecting the transfected cells into the eye of the mammal in an amount sufficient to inhibit the degeneration of cone cells in the retina of the eye. The treatment may be enhanced by stimulating proliferation of activated astrocytes in the retina using a laser.
Owner:THE SCRIPPS RES INST
Method and system with contact lens product for treating and preventing adverse eye conditions
ActiveUS8440217B1Effective controlEffectively controlling distribution and dosagePowder deliveryEye implantsDrug releaseContinuous release
A contact lens product, a method and system for forming the contact lens product, and a method of using the contact lens product. The contact lens product includes a soft disposable contact lens loaded with a drug and the carriers which carry the drug. The lens has a mechanical and optical structure formed by the core polymer included within the lens. The contact lens product is configured to have the drug released from its carrier continuously into an eye of a mammal while the contact lens product is adhered to the eye of the mammal during a continuous period of time, the drug being configured to treat or prevent at least one adverse condition of the eye of the mammal during the continuous period of time. The mammal may be a human being or a veterinary animal.
Owner:EL NAGGAR MAWAHEB M +1
Methods and compositions for drug delivery
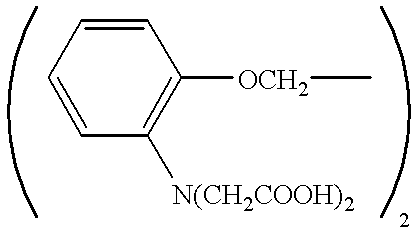
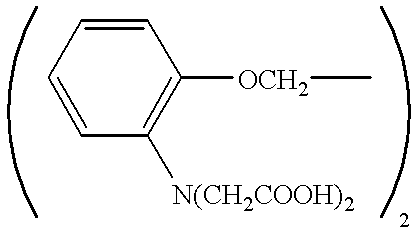
Pharmaceutical compositions and a method are disclosed for treating glaucoma and / or ocular hypertension in the mammalian eye by administering to the mammalian eye the pharmaceutical composition of the invention which contains as the active ingredient one or more compounds having calcium chelating activity. Examples of calcium chelating agents utilized in the pharmaceutical composition and method of treatment are:and lower alkyl and alkoxyalkyl esters thereof. Also disclosed are methods and compositions for delivering a drug within the cytoplasm of a cell having cell surface acetylcholinesterase or acetylcholinesterase receptors, wherein the compositions comprise an apolar prodrug joined to an acetylcholine ester or psuedoacetylcholine group.
Owner:ALLERGAN INC
Pupil centered fovea focused optics assembly for intraocular lens
The embodiments herein provide a pupil centered and fovea focused optics assembly comprising a ring platform provided inside a capsular bag of a mammalian eye to support an intraocular lens. The optical center of the intraocular lens is decentered with respect to the geometric center of the intraocular lens to align the optical centre of the lens with a visual axis of a pupil of the mammalian eye to improve the visual quality and to prevent an aberration. A plane of the intraocular lens is turned and tilted through a preset angle to point an optic axis of the intraocular lens to the Fovea. The preset angle is calculated based on an overall diameter of the capsular bag, a thickness of the ring platform, a decentering direction and a decentering distance of the optical axis of the intraocular lens using a three dimensional scanning of the mammalian eye.
Owner:拉姆·斯里坎茨·米拉伊
rAAV vectors and methods for transduction of photoreceptors and RPE cells
ActiveUS10308957B2Improve efficiencyRestore visual behaviorVectorsGenetic material ingredientsDiseaseIn vivo
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Methods for regeneration of a mammalian lens
The invention relates to methods for transdifferentiation of body tissues which can be used to generate specific cell types needed for regenerating a lens in the mammalian eye, following loss or removal of the original lens.
Owner:BARANOWITZ STEVEN
Methods of treatment of xerophthalmia with self-preserving ocular formulations of norketotifen
Self-preserving ophthalmic formulations containing norketotifen and methods of making the same and the use thereof in patients suffering from xerophthalmia are disclosed. The methods also comprise administering to the eyes of a mammal in need thereof, self-preserving topical ophthalmic formulations containing norketotifen, free from any added preservative.
Owner:BRIDGE PHARMA INC
Cyclosporin a compositions
InactiveUS20070219127A1Stable and long-term shelf-livesLong shelf-livesPowder deliveryBiocideDiseaseCiclosporin
A composition comprising cyclosporin A and a nonaqueous, physiologically acceptable liquid carrier, said composition being suitable for topical administration to an eye of a mammal is disclosed herein. Methods of treating disease related thereto are also disclosed.
Owner:ALLERGAN INC
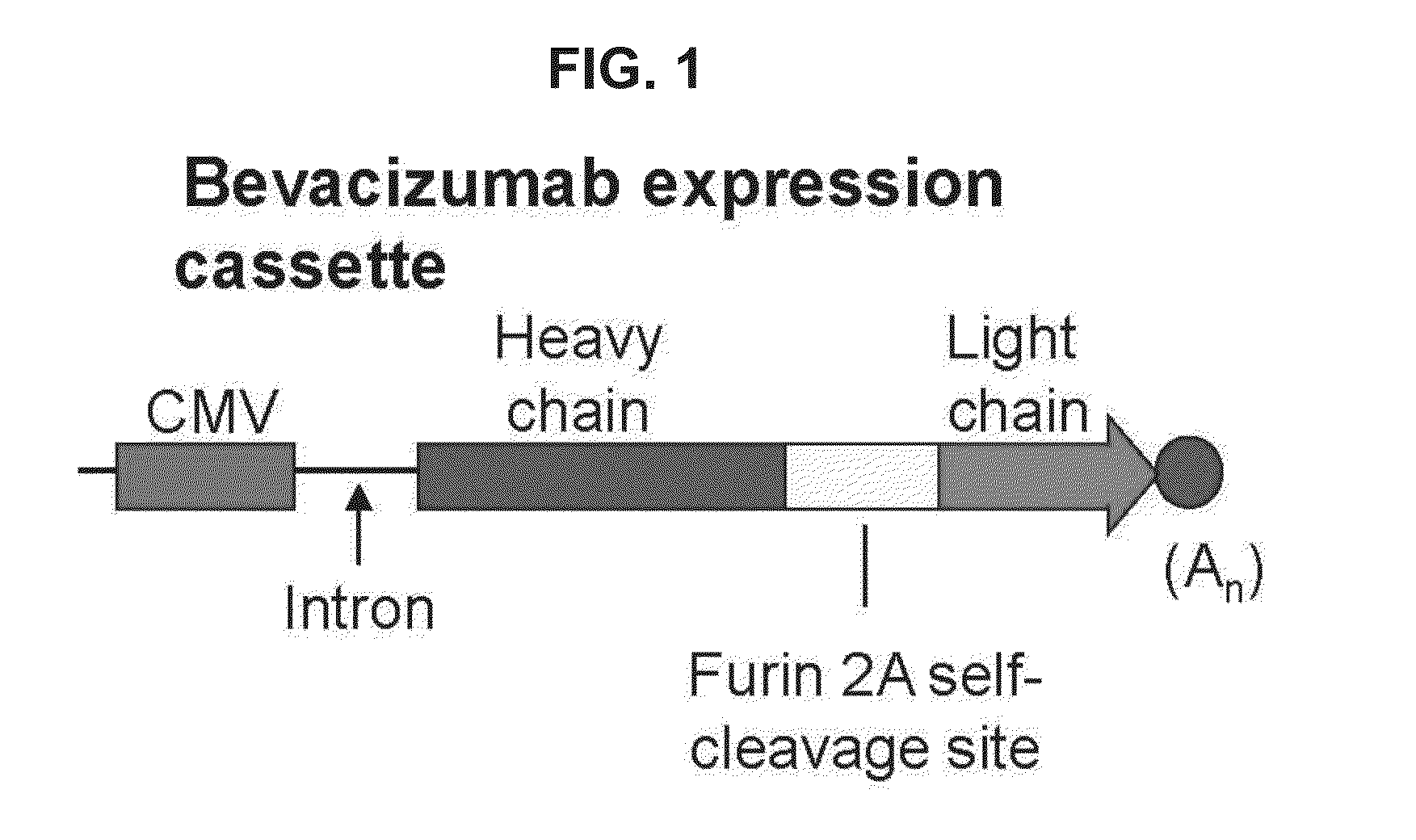
Virus-mediated delivery of bevacizumab for therapeutic applications
InactiveUS20130090375A1Reduction in total area of neovascularizationReduce neovascularizationOrganic active ingredientsSenses disorderBevacizumab InjectionOphthalmology
The invention provides a method of inhibiting ocular neovascularization in a mammal by administering a composition comprising a bevacizumab-encoding adeno-associated virus (AAV) vector directly to the eye of the mammal.
Owner:CORNELL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com