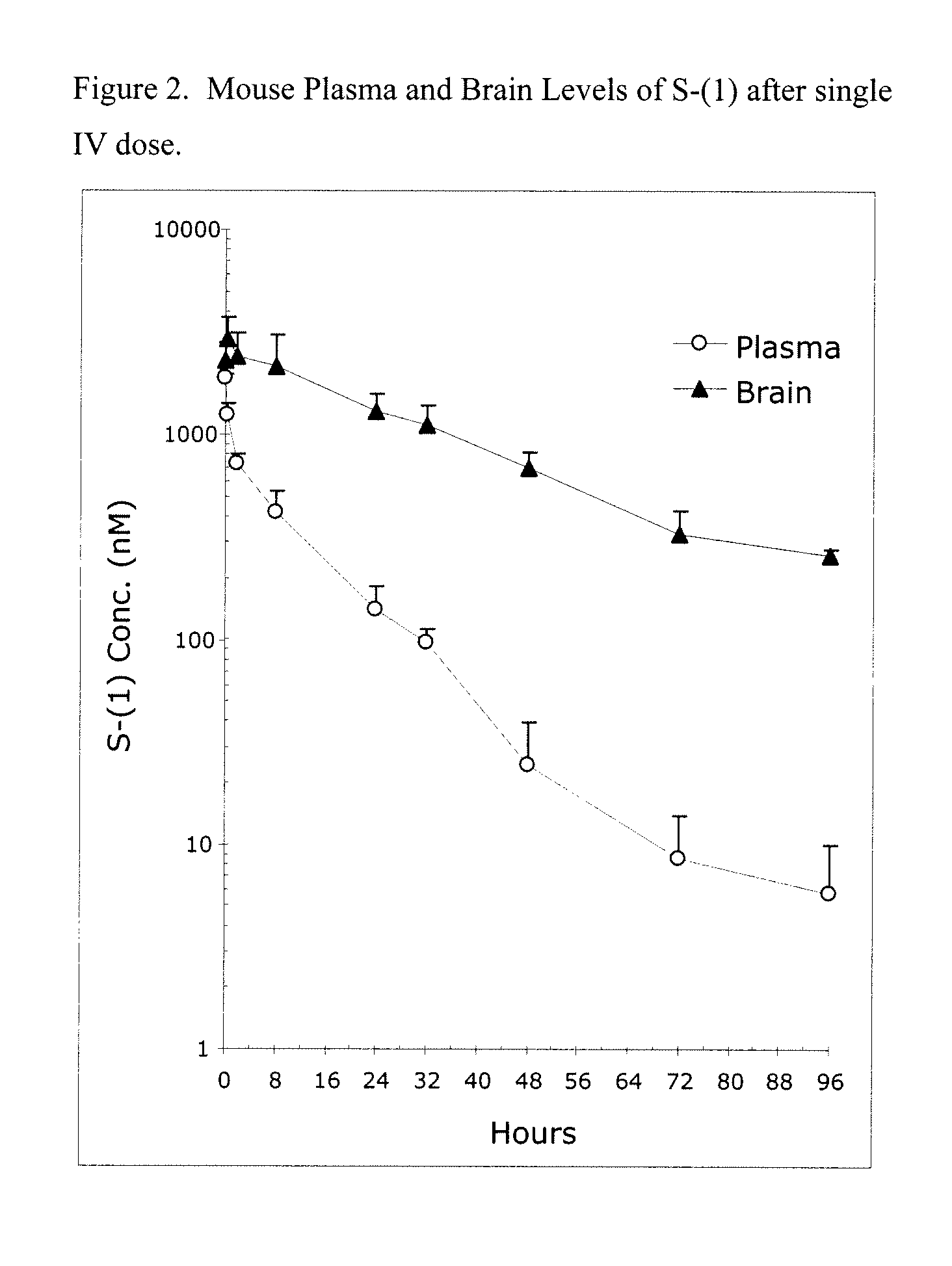
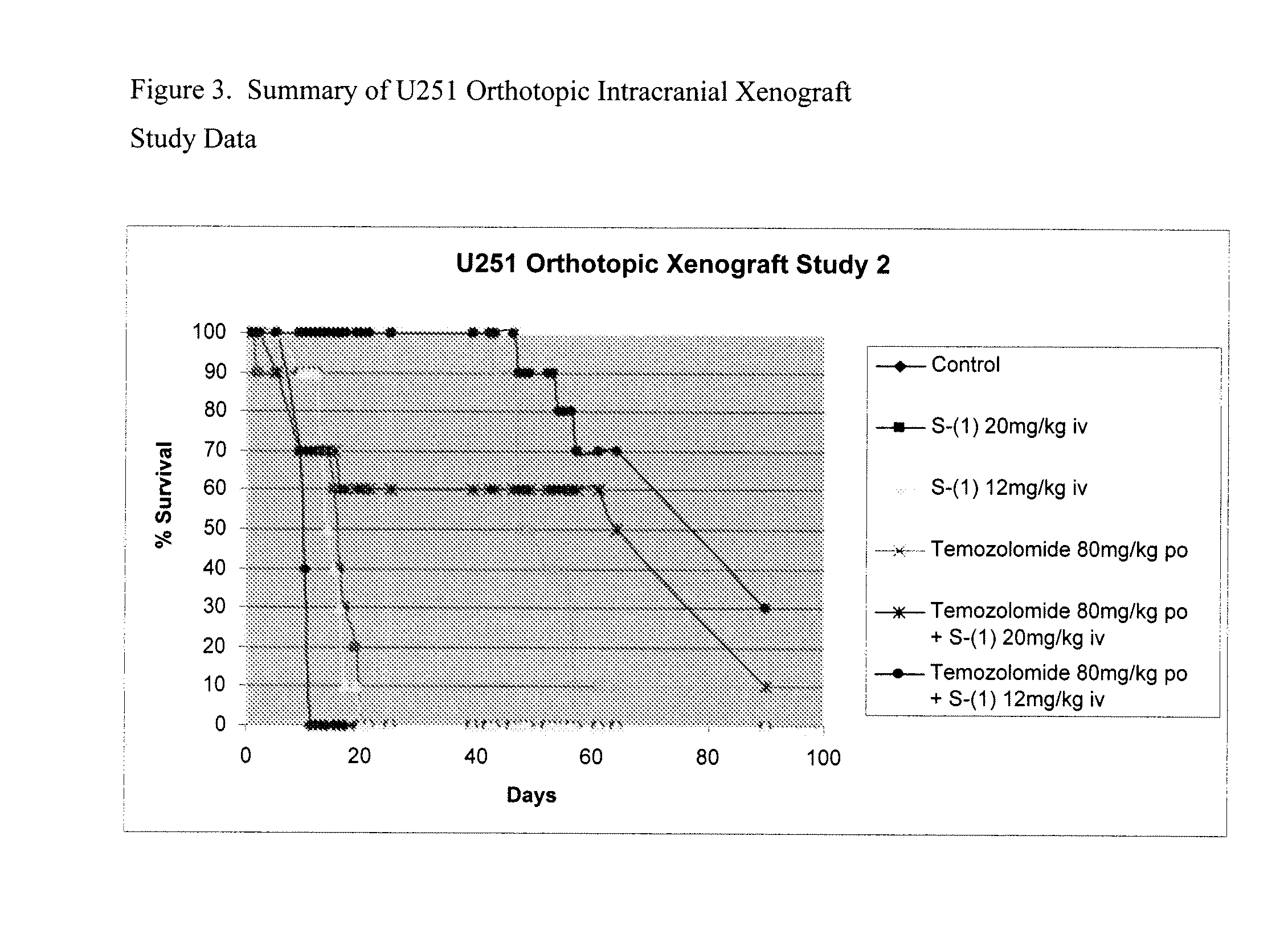
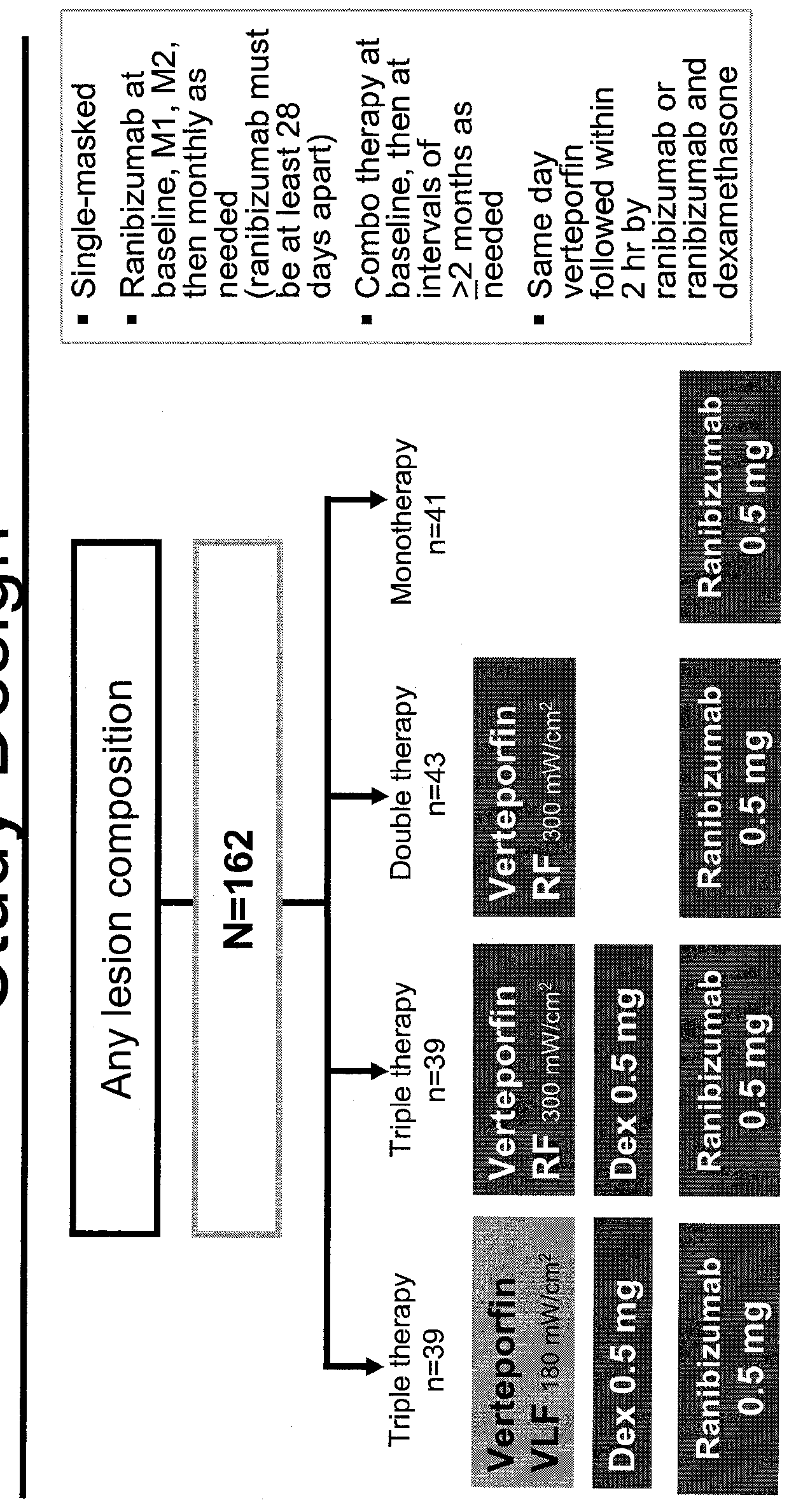
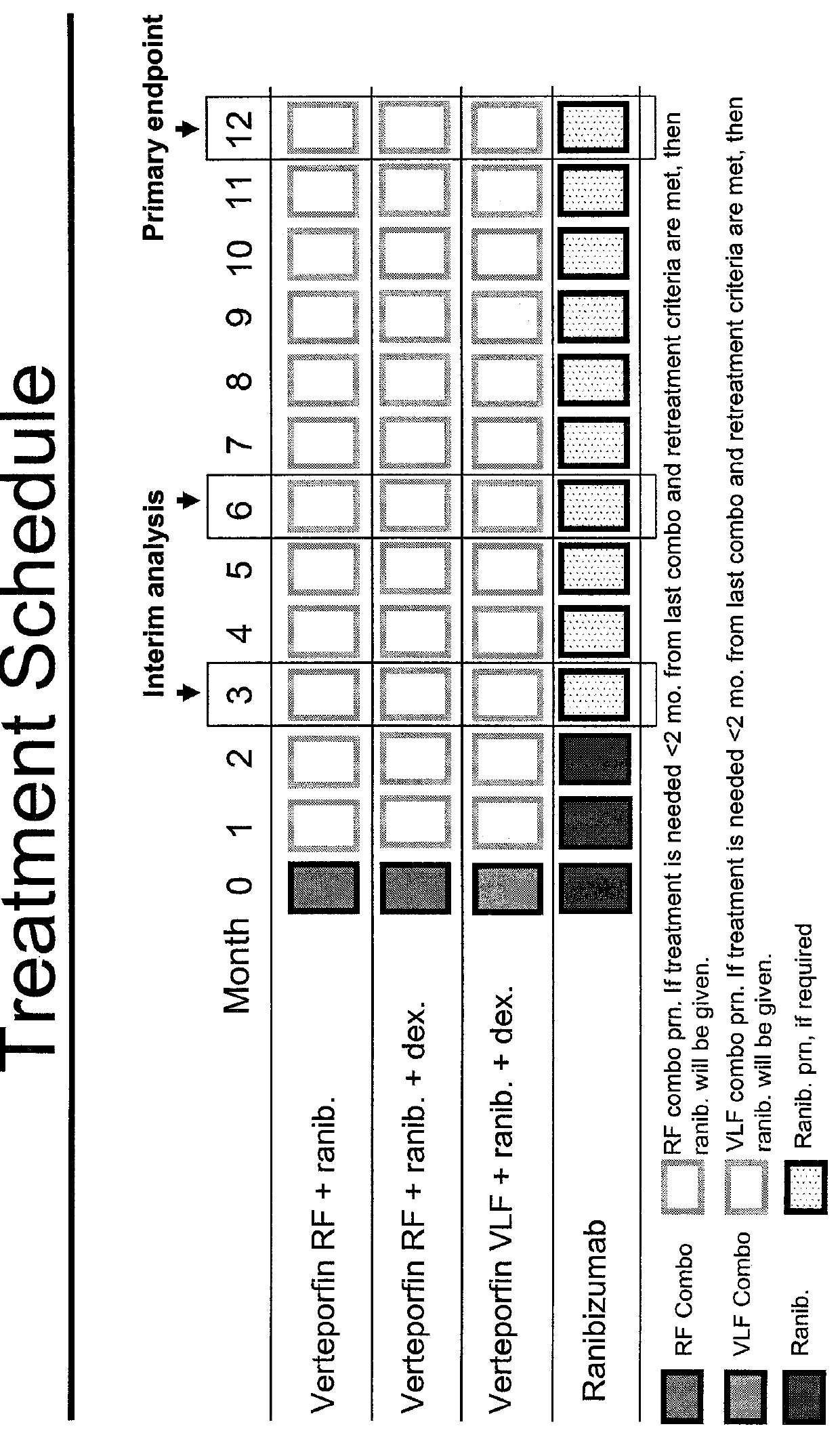
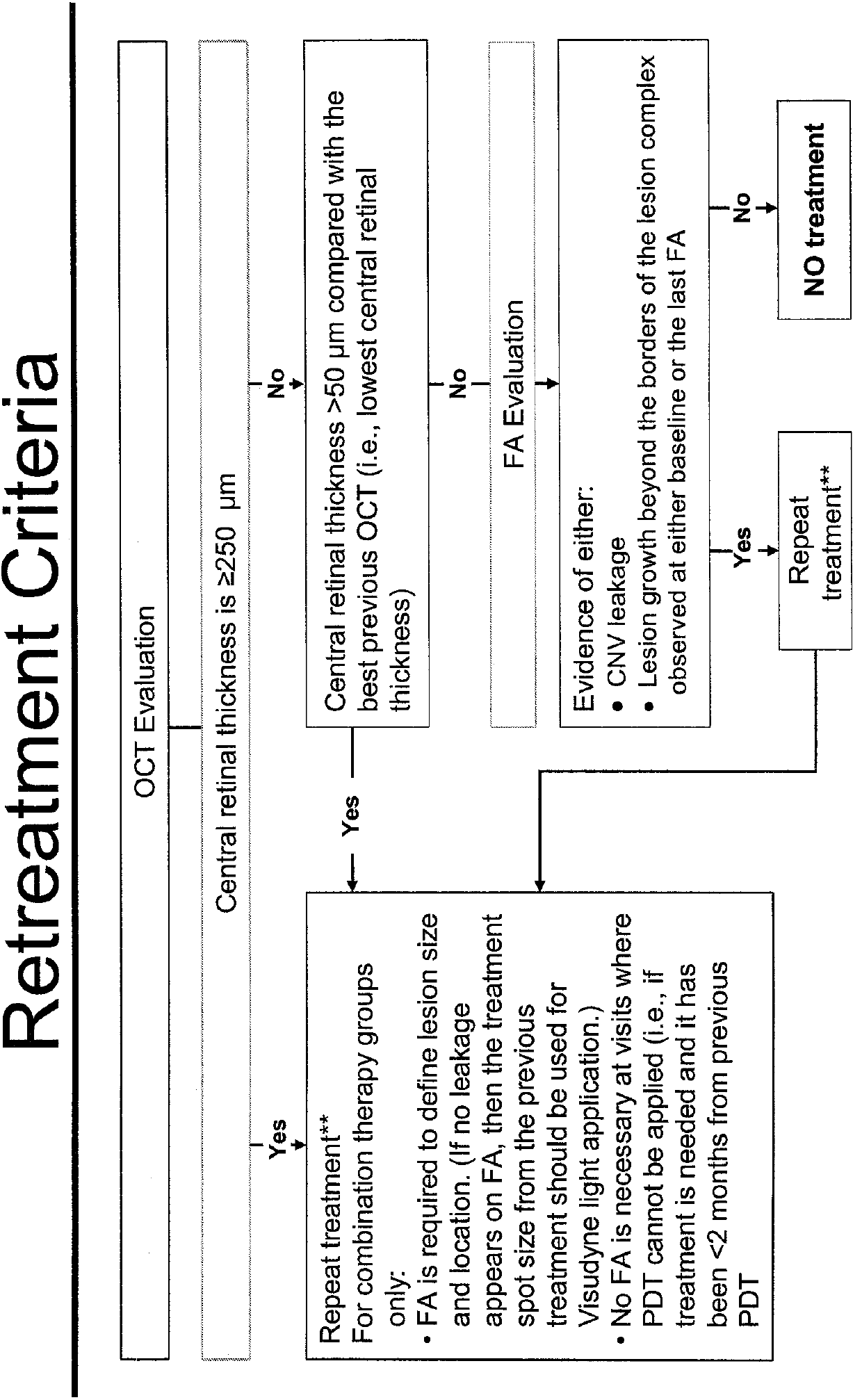
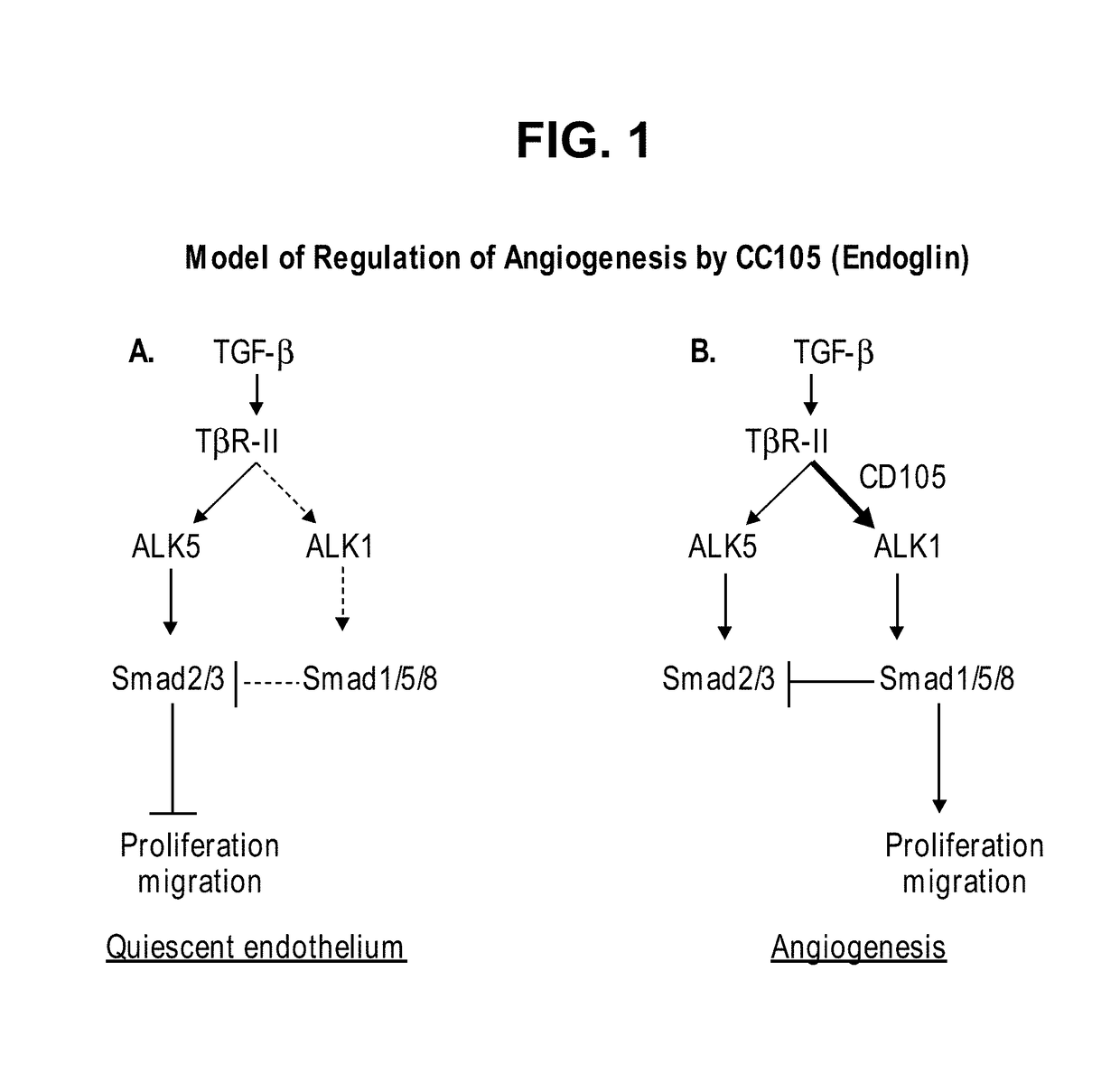
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
83 results about "Bevacizumab Injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bevacizumab, sold under the trade name Avastin, is medication used to treat a number of types of cancers and a specific eye disease. For cancer it is given by slow injection into a vein and used for colon cancer, lung cancer, glioblastoma, and renal-cell carcinoma.
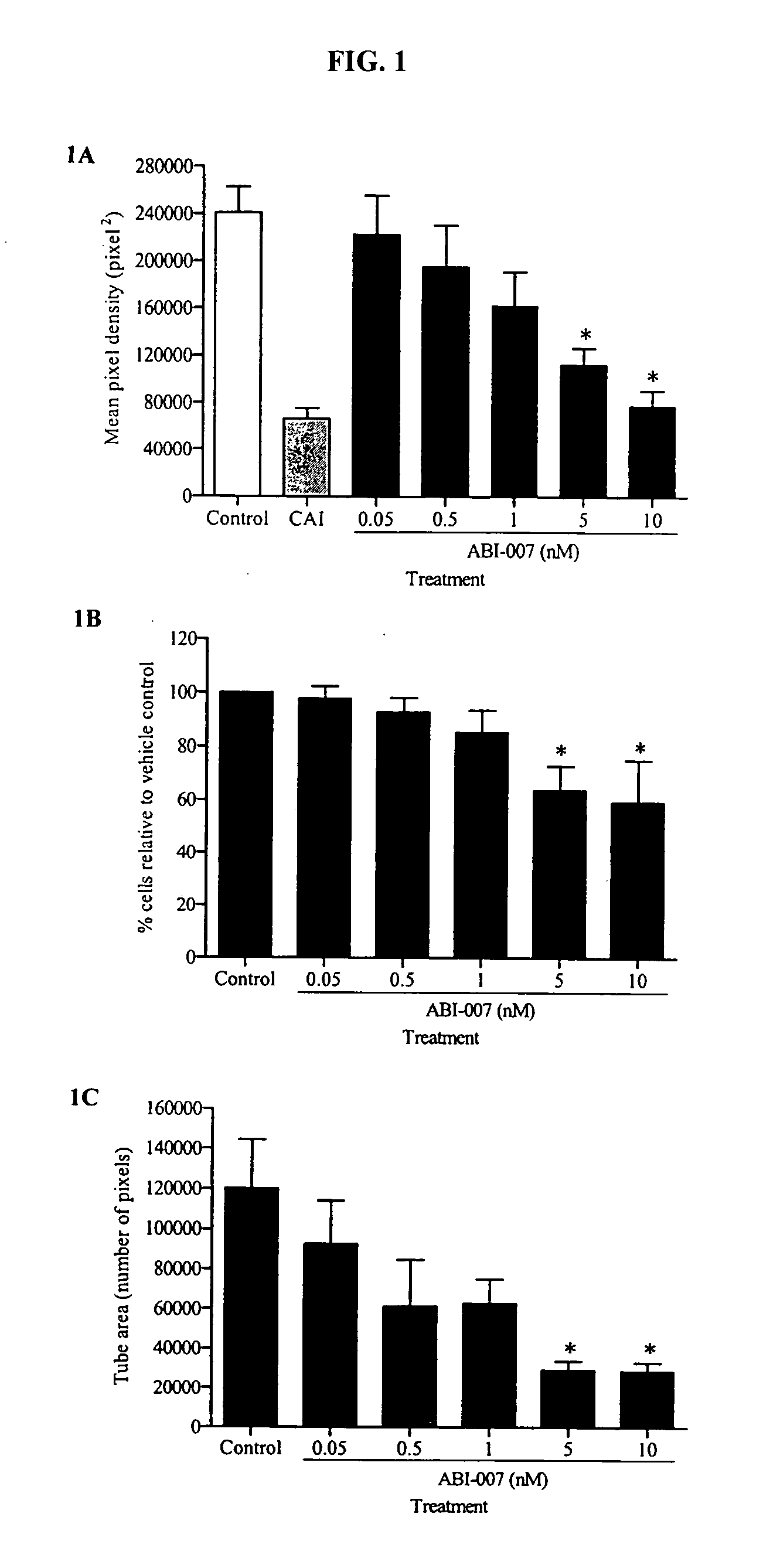
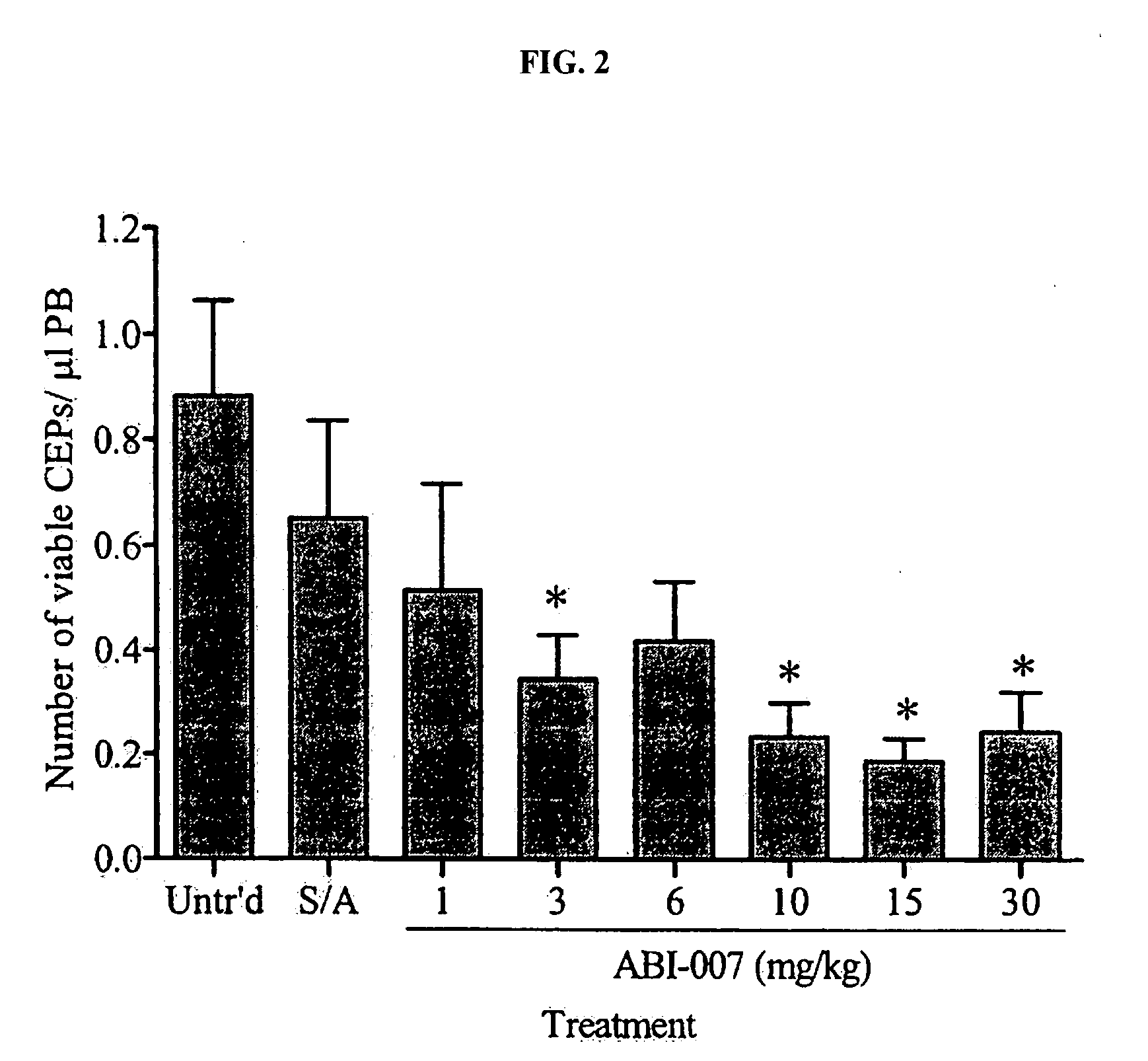
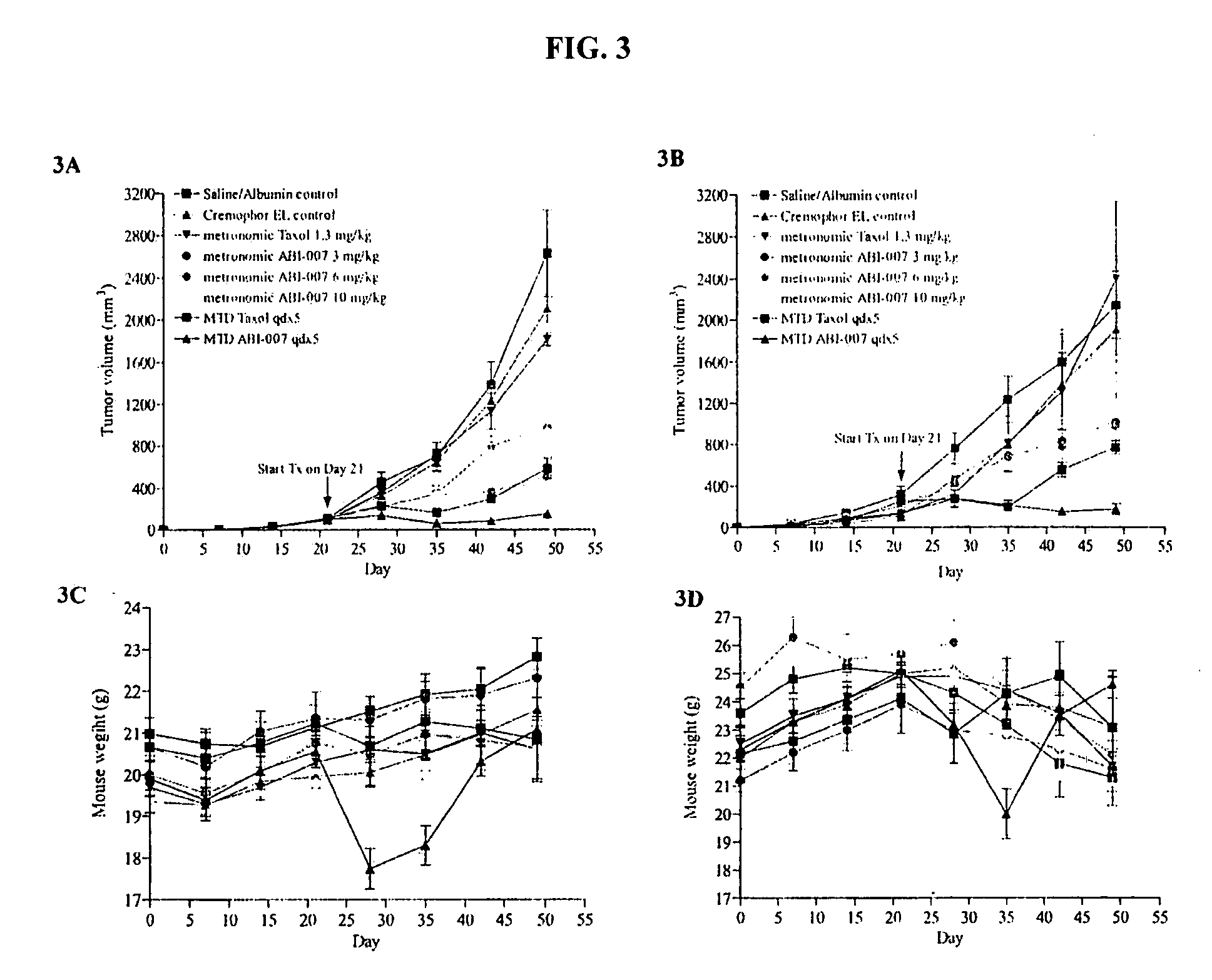
Nanoparticles of paclitaxel and albumin in combination with bevacizumab against cancer
InactiveUS20100112077A1Organic active ingredientsHeavy metal active ingredientsBevacizumab InjectionAnti vegf antibody
The present invention provides combination therapy methods of treating proliferative diseases (such as cancer) comprising a first therapy comprising administering to an individual an effective amount of a taxane in a nanoparticle composition, and a second therapy which may include, for example, radiation, surgery, administration of chemotherapeutic agents (such as an anti-VEGF antibody), or combinations thereof. Also provided are methods of administering to an individual a drug taxane in a nanoparticle composition based on a metronomic dosing regime.
Owner:ABRAXIS BIOSCI LLC
Method of treating cancer, especially soft tissue sarcoma utilizing gemcitabine in combination with docetaxel and anti-VEGF therapy (bevacizumab)
InactiveUS20070065449A1Great likelihoodLong median survivalBiocideGenetic material ingredientsAbnormal tissue growthLymphatic Spread
The present invention relates to a pharmaceutical cocktail, in particular, effective amounts of gemcitabine, in combination with effective amounts of docetaxel and angiogenesis inhibitor, especially a vascular endothelial growth factor (VEGF) inhibitor, such as bevacizumab for the treatment of cancer, in particular sarcoma, especially soft tissue sarcoma. Pharmaceutical compositions and methods of treating cancer, including sarcoma, especially soft tissue sarcoma (prolonging the patient's life, eliminating the tumor, improving the patient's quality of life, shrinking the tumor, prolonging survival and / or preventing the tumor's metastases) are additional aspects of the present invention.
Owner:STC UNM
Combination methods and compositions
InactiveUS20110223241A1BiocideCarbohydrate active ingredientsBevacizumab InjectionTherapeutic effect
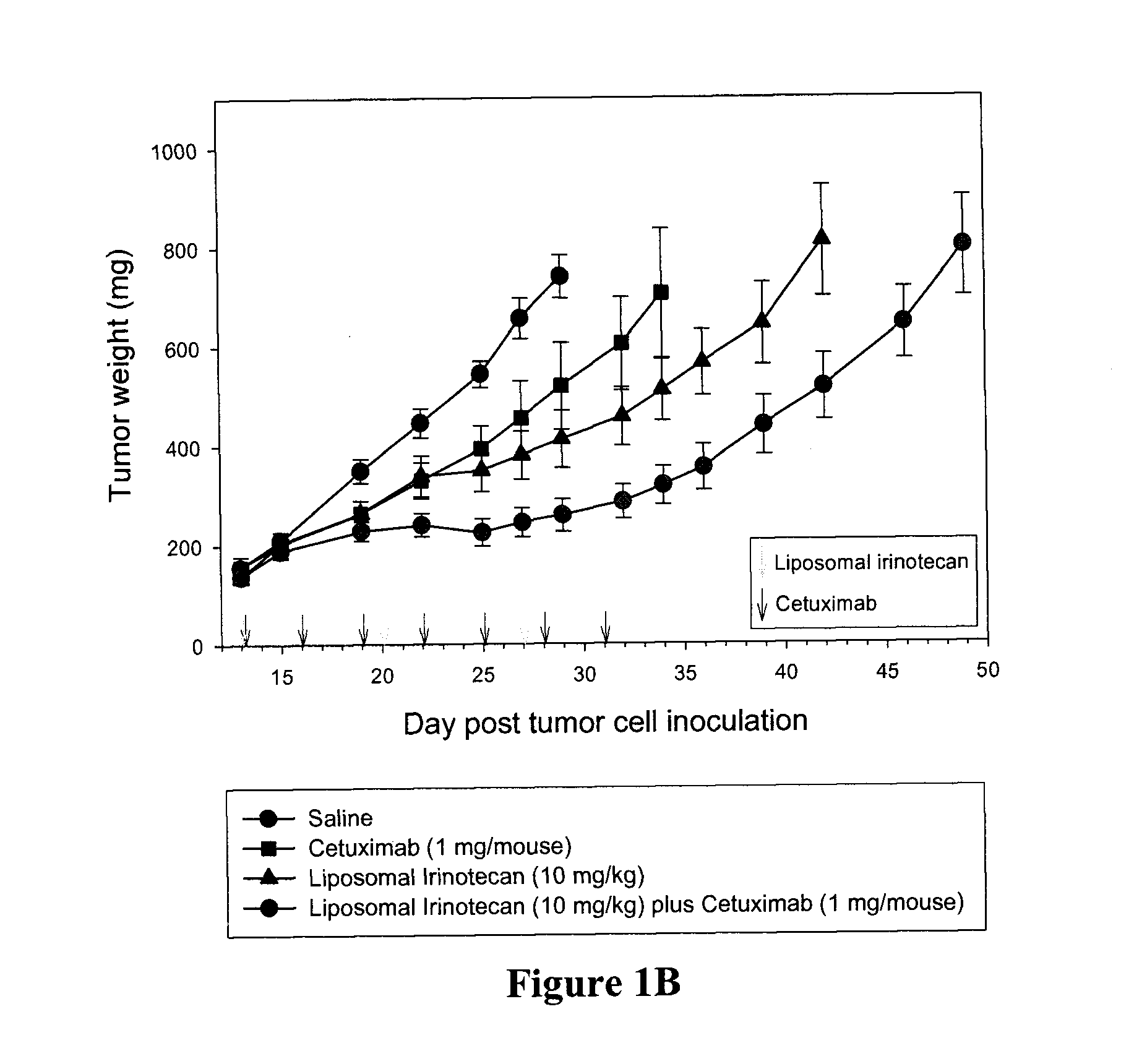
Compositions which comprise a liposomal water-soluble camptothecin and optionally a liposomal fluoropyrimidine in combination with a vascular epithelial growth factor (VEGF) inhibitor such as cetuximab or an epidermal growth factor receptor (EGFR) inhibitor such as bevacizumab are useful in achieving enhanced therapeutic effects for the treatment of cancer.
Owner:CELATOR PHARMA INC
Combination treatment for pathologic ocular angiogenesis
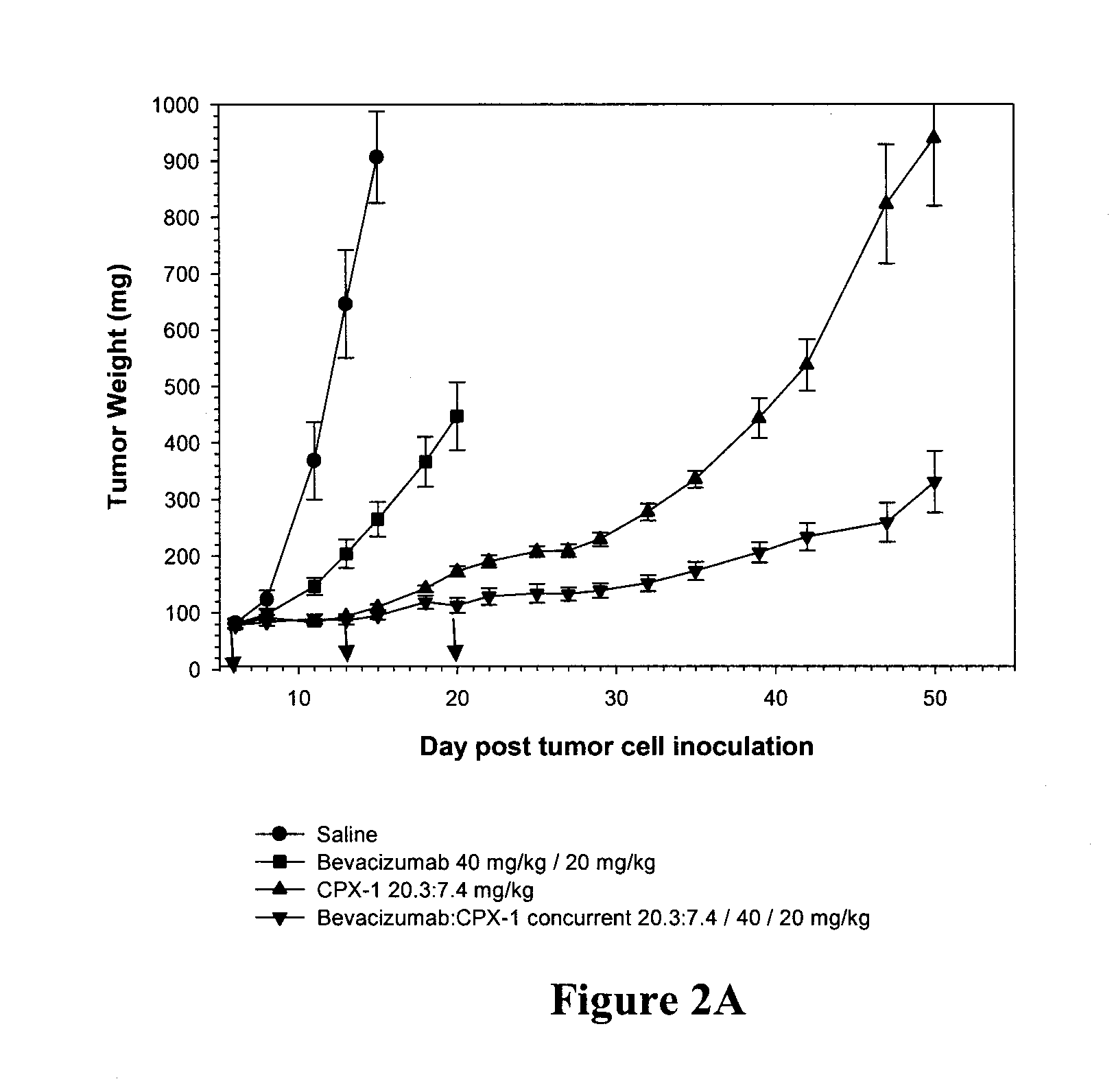
The present invention provides a combination therapy for the treatment of pathologic ocular disorders, such as age-related macular degeneration and choroidal neovascularization. The combination therapy of the invention includes administration of anecortave acetate and bevacizumab or ranibizumab.
Owner:ALCON INC
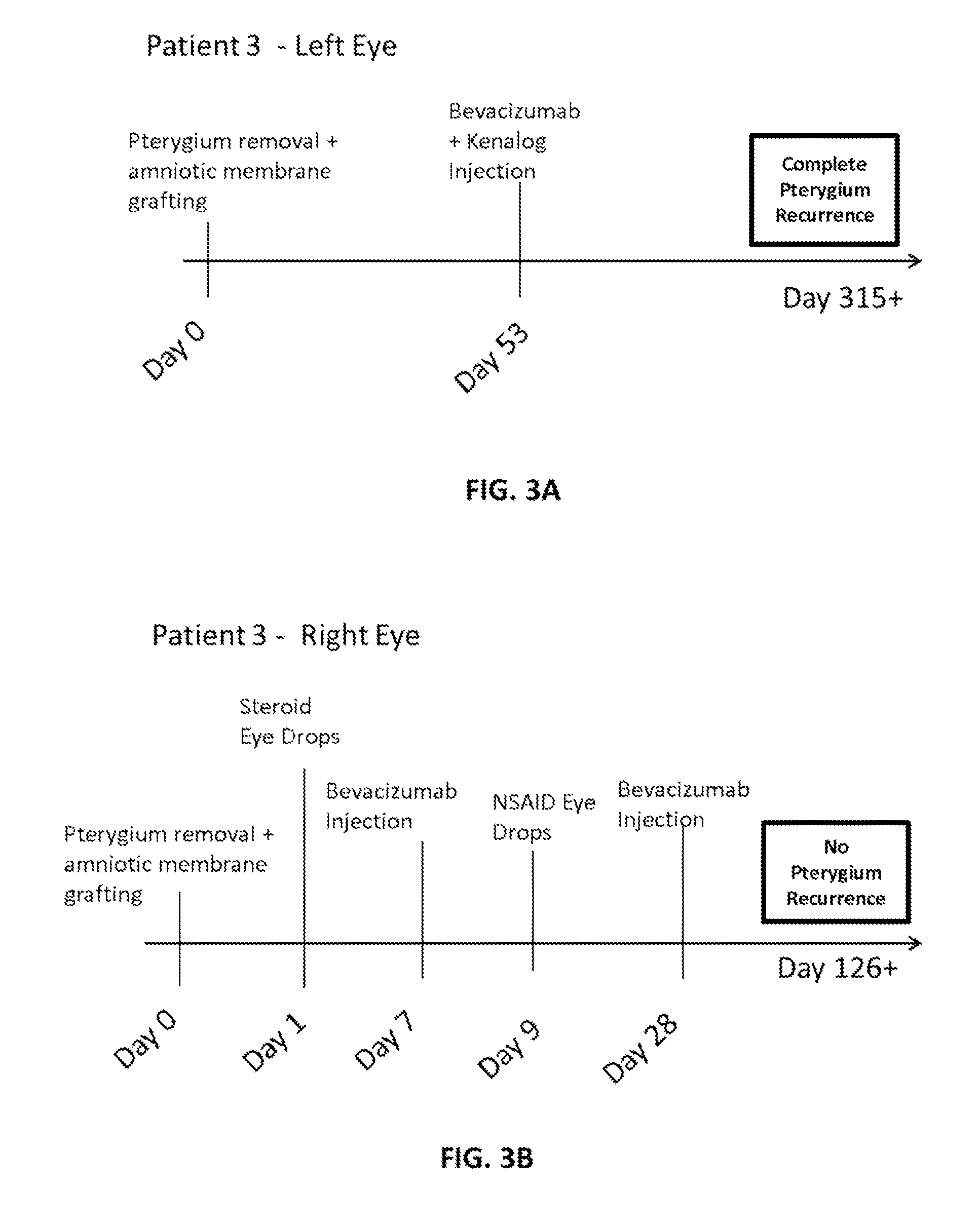
Methods of treating pterygium
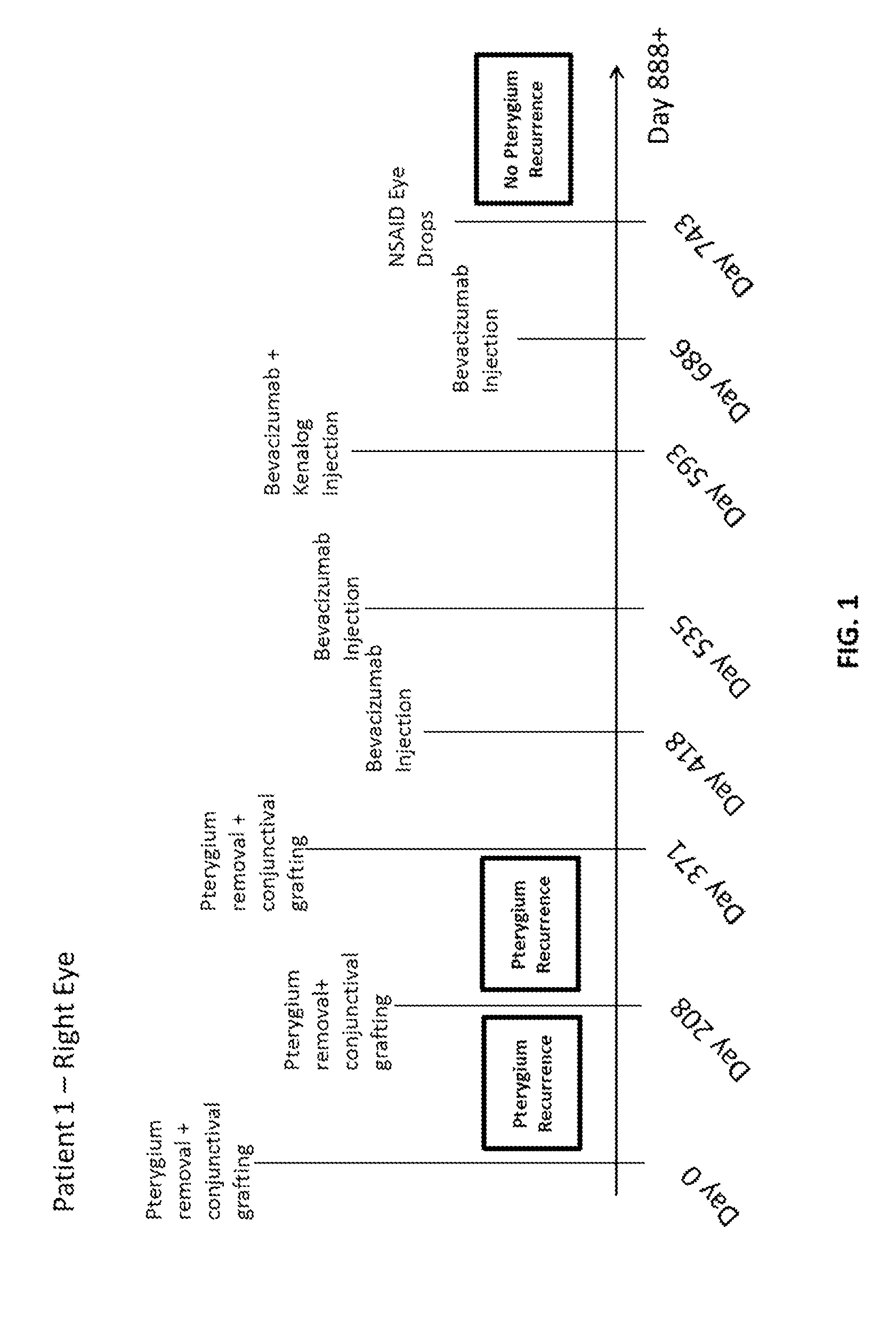
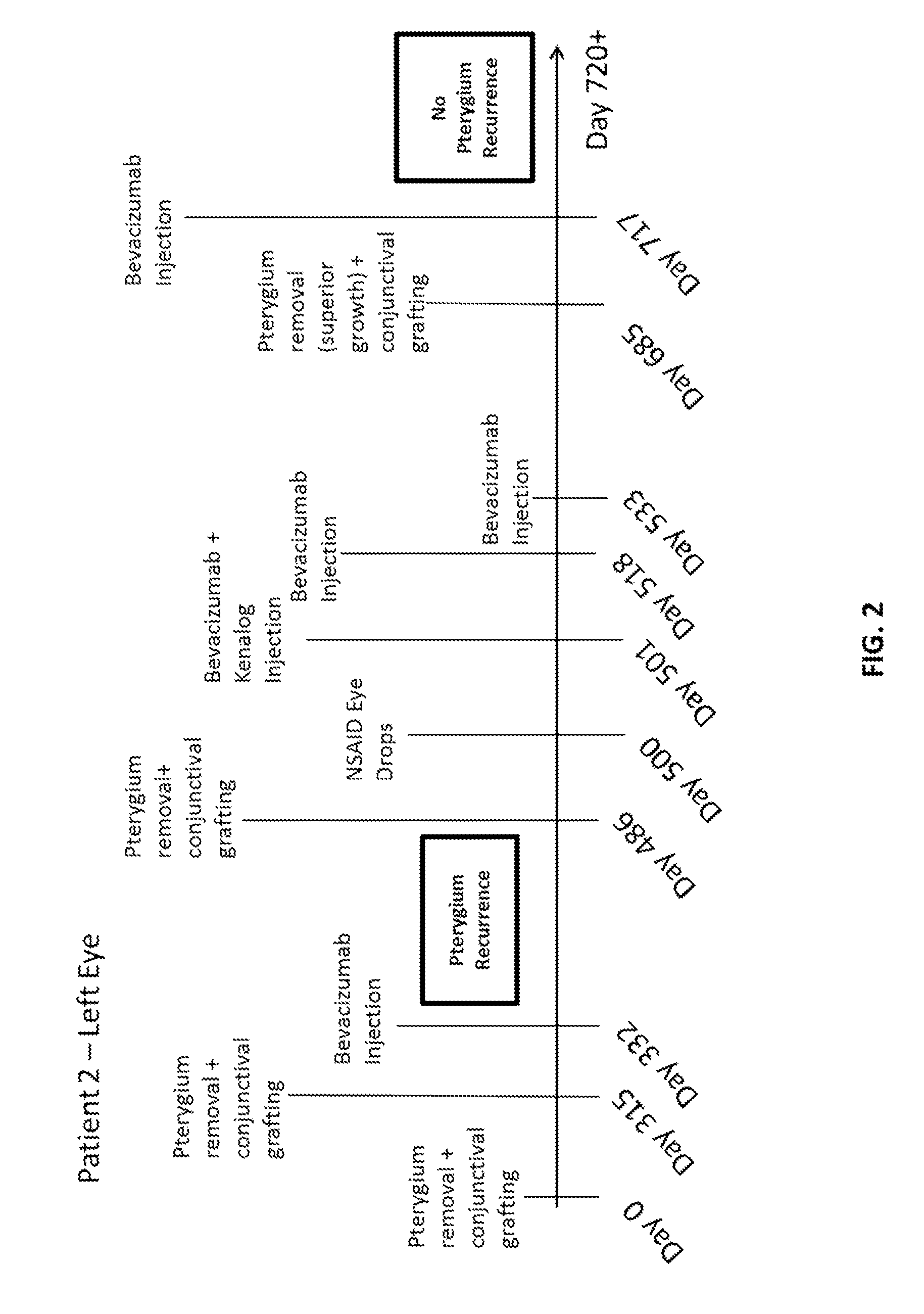
Methods for treating pterygium recurrence following pterygiectomy, and for treating keloid recurrence, following surgical removal of the keloid, are disclosed. The methods include administering an anti-VEGF agent (e.g., antibody (e.g., bevacizumab) or small molecule inhibitor of VEGF signaling), or a combination therapy that includes co-administering an anti-VEGF agent, with an anti-inflammatory steroid and / or a non-steroidal anti-inflammatory drug (NSAID) to a subject.
Owner:PHAM RANDAL TANH HOANG
Method of treating androgen receptor (AR) -positive breast cancers with selective androgen receptor modulator (SARMS)
ActiveUS20140350102A1Treating and preventing and suppressing and inhibiting metastasisProlonged progression-free survivalBiocideOrganic chemistryToremifeneLymphatic Spread
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer advanced breast cancer; breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
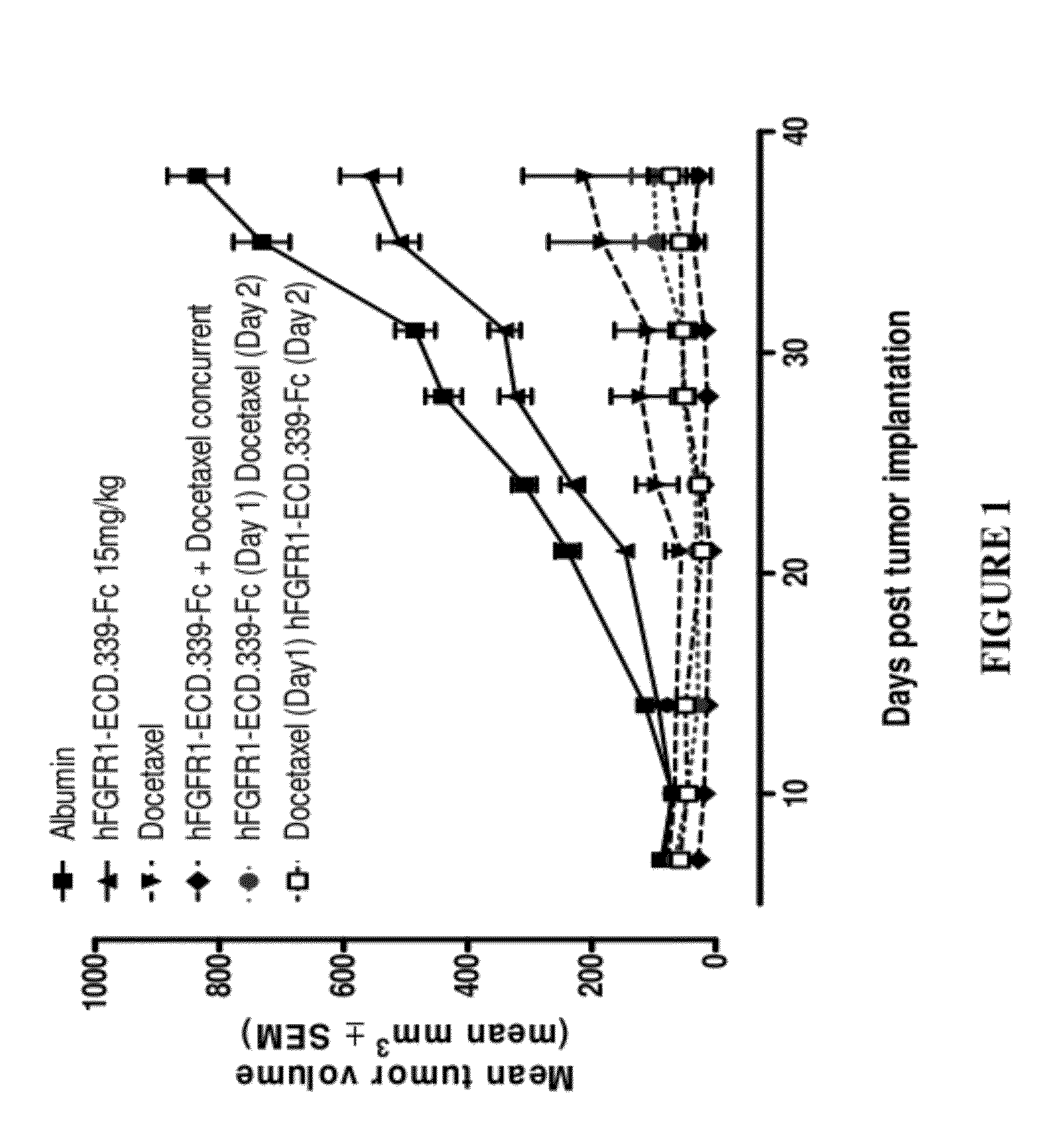
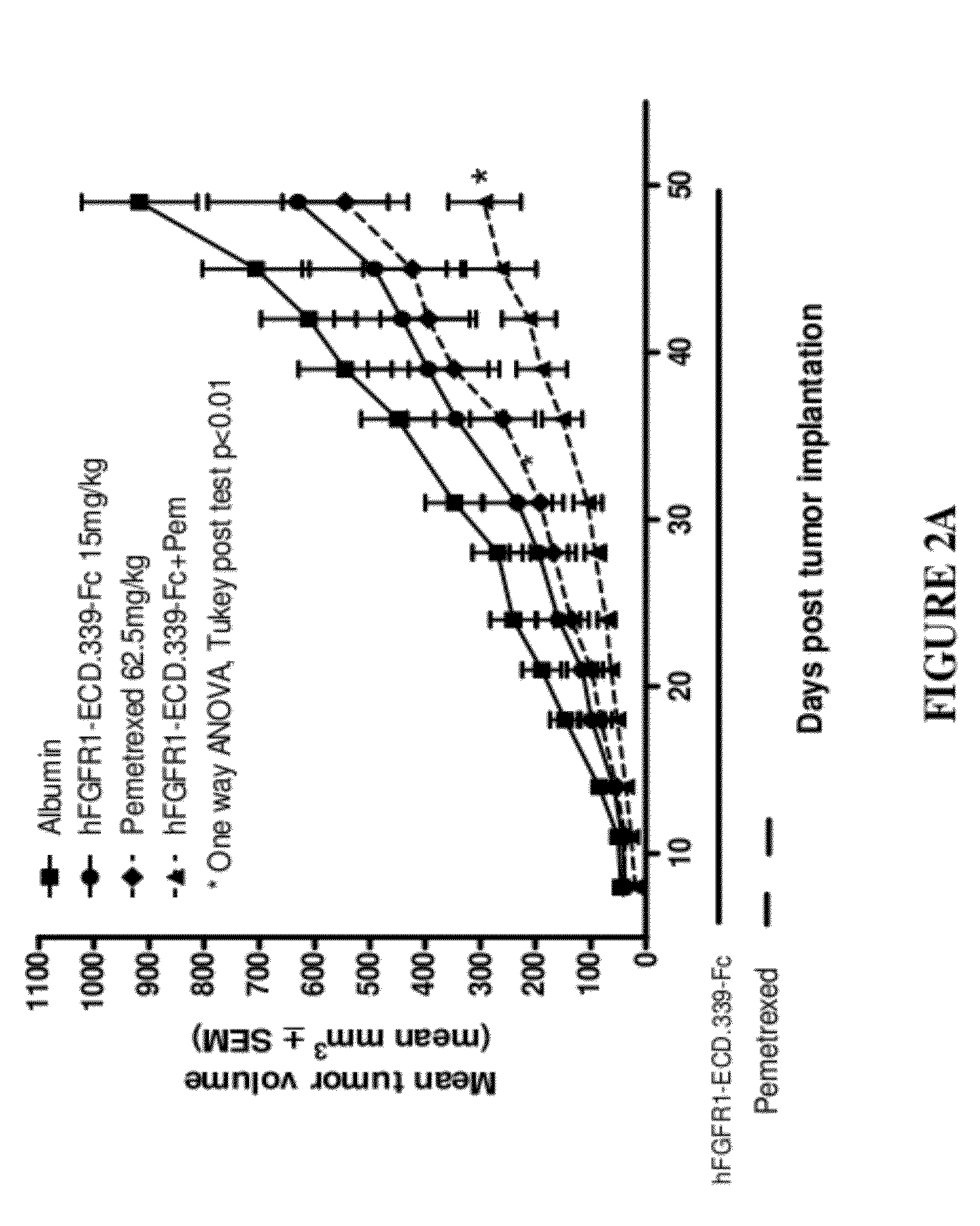
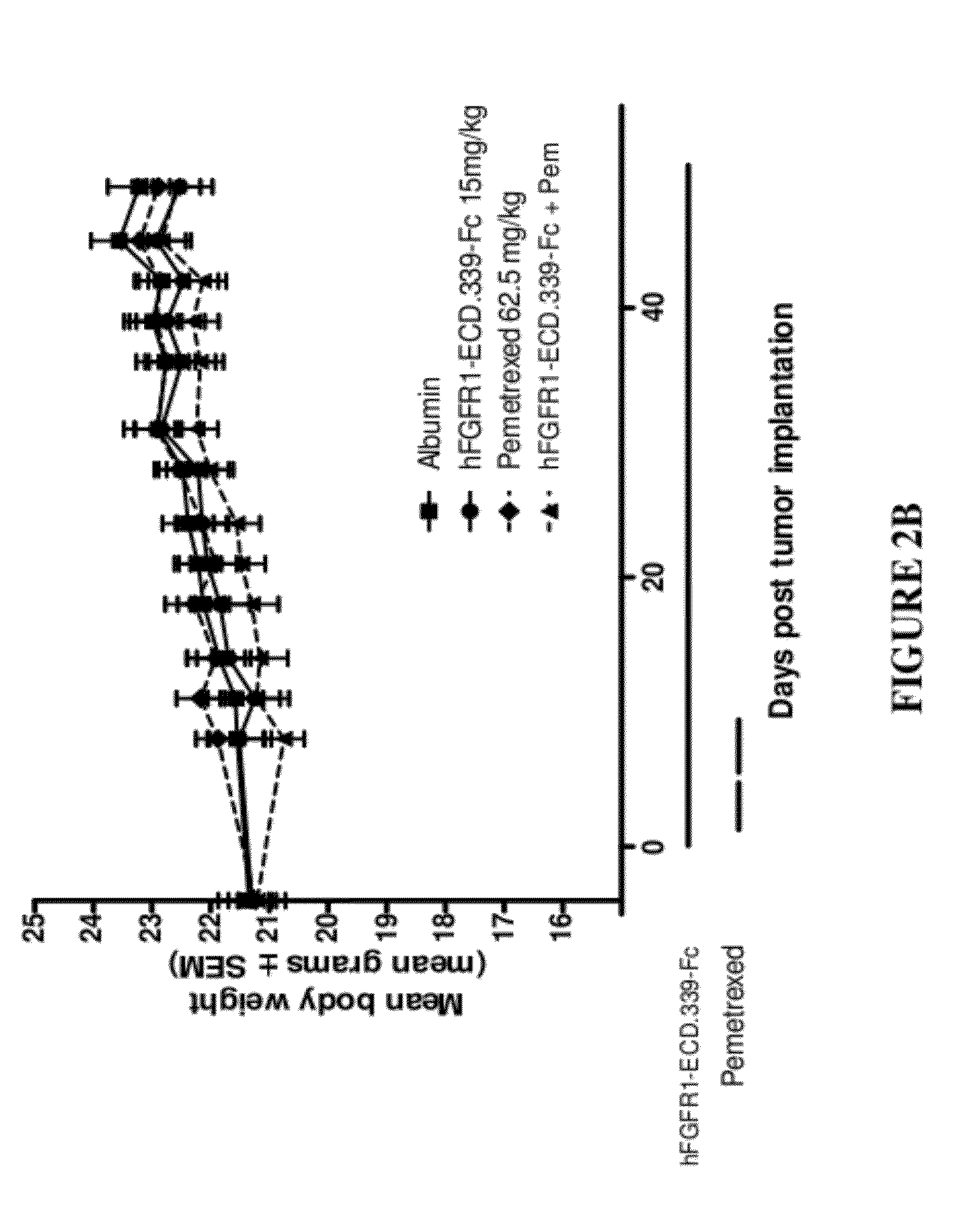
Fgfr1 extracellular domain combination therapies
InactiveUS20120237511A1Inhibit tumor cell growthOrganic active ingredientsHeavy metal active ingredientsCarboplatinDocetaxel
Methods of treating cancer comprising administering a fibroblast growth factor receptor 1 (FGFR1) extracellular domain (ECD) and / or an FGFR1 ECD fusion molecule in combination with at least one additional therapeutic agent selected from docetaxel, paclitaxel, vincristine, carboplatin, cisplatin, oxaliplatin, doxorubicin, 5-fluorouracil (5-FU), leucovorin, pemetrexed, and bevacizumab are provided. Dosage packs comprising an FGFR1 ECD and / or an FGFR1 ECD fusion molecule and / or at least one additional therapeutic agent selected from docetaxel, paclitaxel, vincristine, carboplatin, cisplatin, oxaliplatin, doxorubicin, 5-fluorouracil (5-FU), leucovorin, pemetrexed, and bevacizumab are also provided. In some embodiments, a dosage pack comprises instructions for administering FGFR1 ECD and / or FGFR1 ECD fusion molecule with at least one additional therapeutic agent.
Owner:FIVE PRIME THERAPEUTICS
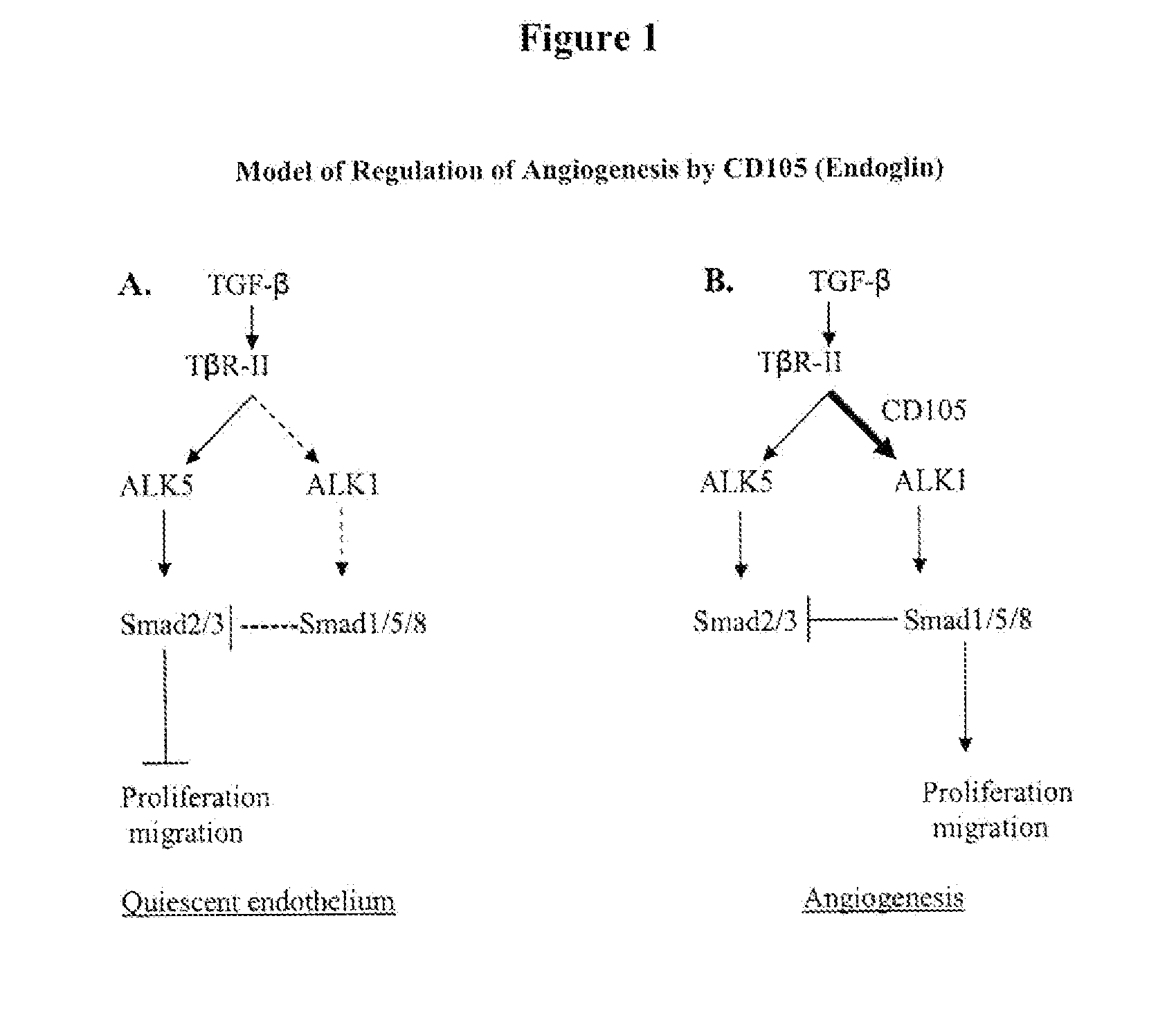
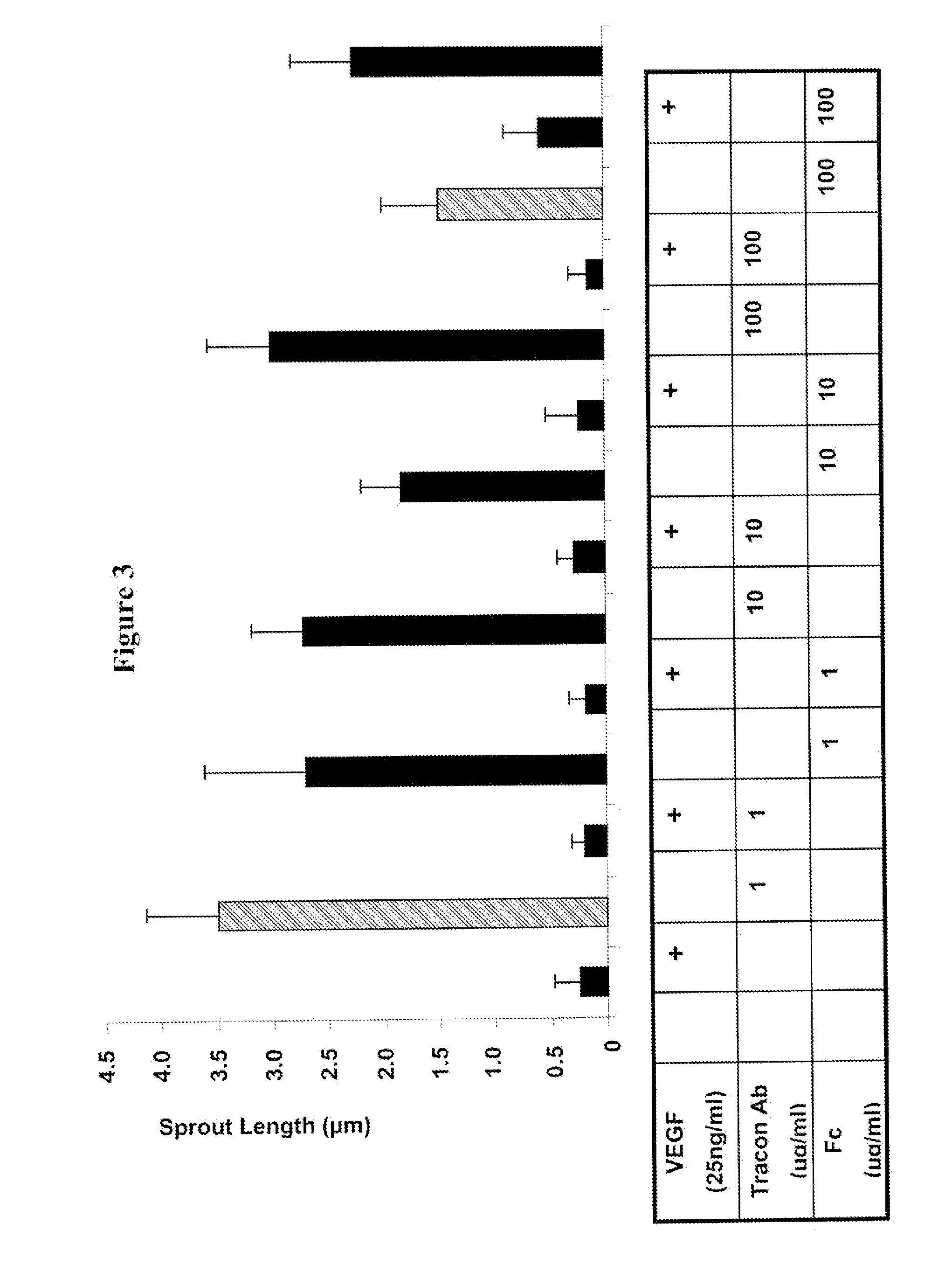
Combination therapy of cancer with Anti-endoglin antibodies and Anti-vegf agents
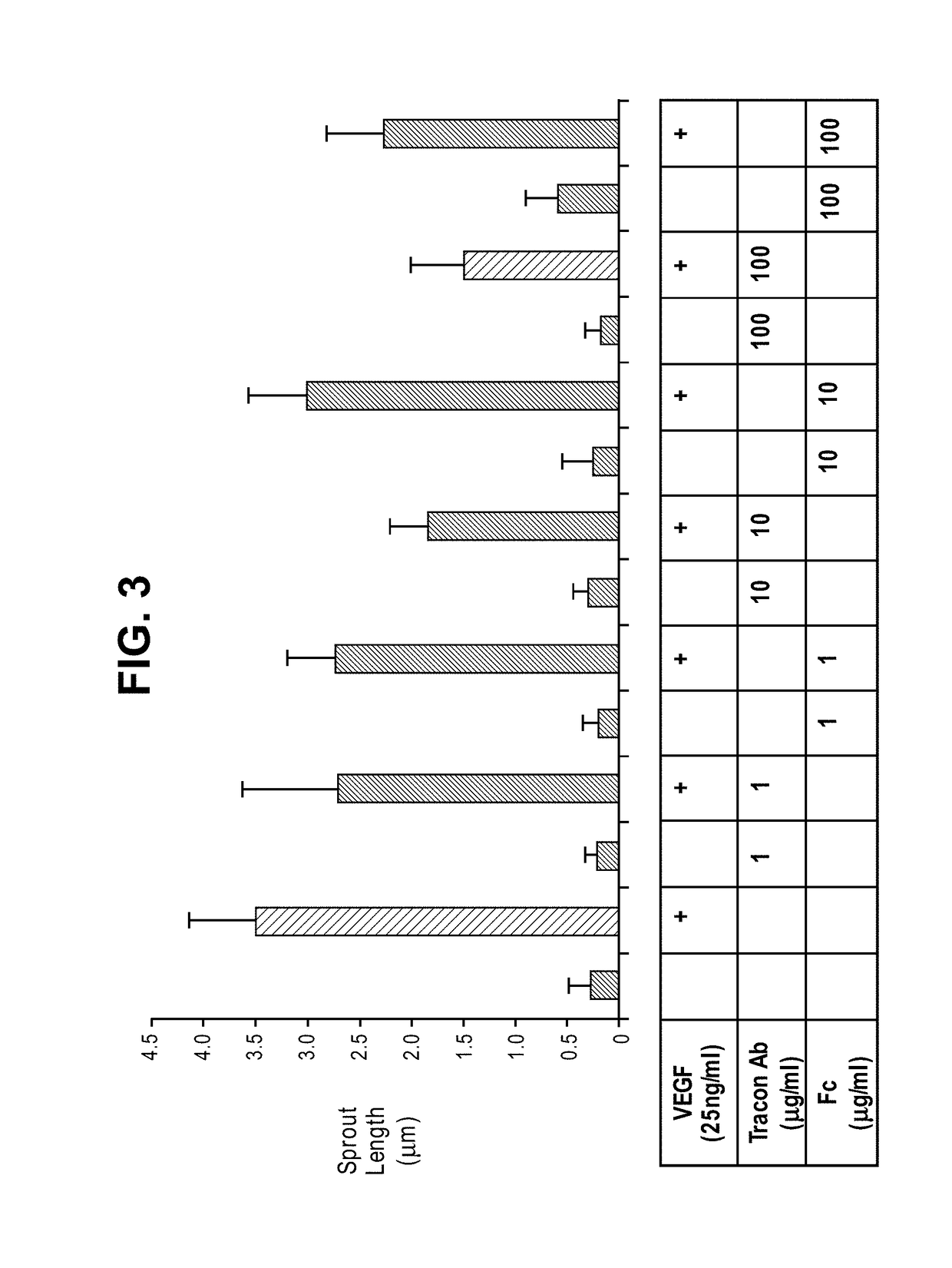
InactiveUS20120244147A1Improve the situationImprove survivalSenses disorderAntipyreticEndoglinBevacizumab Injection
The present application relates to compositions of chimeric anti-endoglin antibodies and anti-VEGF agents. Another aspect relates to the use of chimeric anti-endoglin antibodies and Bevacizumab. Another aspect relates to the use of the compositions to inhibit VEGF induced sprouting. Another aspect relates to the use of the compositions to inhibit angiogenesis.
Owner:HEALTH RES INC +1
Particles of paclitaxel and albumin in combination with bevacizumab against cancer
InactiveCN101573108AOrganic active ingredientsHeavy metal active ingredientsBevacizumab InjectionAnti vegf antibody
The present invention provides combination therapy methods of treating proliferative diseases (such as cancer) comprising a first therapy comprising administering to an individual an effective amount of a taxane in a nanoparticle composition, and a second therapy which may include, for example, radiation, surgery, administration of chemotherapeutic agents (such as an anti-VEGF antibody), or combinations thereof. Also provided are methods of administering to an individual a drug taxane in a nanoparticle composition based on a metronomic dosing regime.
Owner:ABRAXIS BIOSCI LLC
Age related macular degeneration treatment
InactiveUS20120156202A1Reduce edemaReducing blood cholesterolSenses disorderPharmaceutical delivery mechanismBlood vesselInsulin humulin
A method for treating age related macular degeneration (AMD) using an insulin preparation applied topically to the conjunctival sac of the affected eye. Another aspect of this invention is using antiangiogenic adjuvant therapeutic agents such as bevacizumab, ranibizumab, pegaptanib, etanercept, instilled in to the afflicted eye conjunctival sac with insulin to prevent further formation of new blood vessels, and shrink the existing pathologically formed blood vessels and reduce the edema in wet AMD. This method incorporates putting the patients on low fat diet, aerobic exercise, ketamine-a NMDA blocker, reducing the blood cholesterol using adjuvant therapeutic agents selected from Statins, that are inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A, (i.e. HMG-Co A) reductase which in turn reduce drusen formation that leads to AMD, combined with insulin ophthalmic drops.
Owner:SHANTHA TOTADA R +2
Novel Use of Sulfonamide Compound in Combination with Angiogenesis Inhibitor
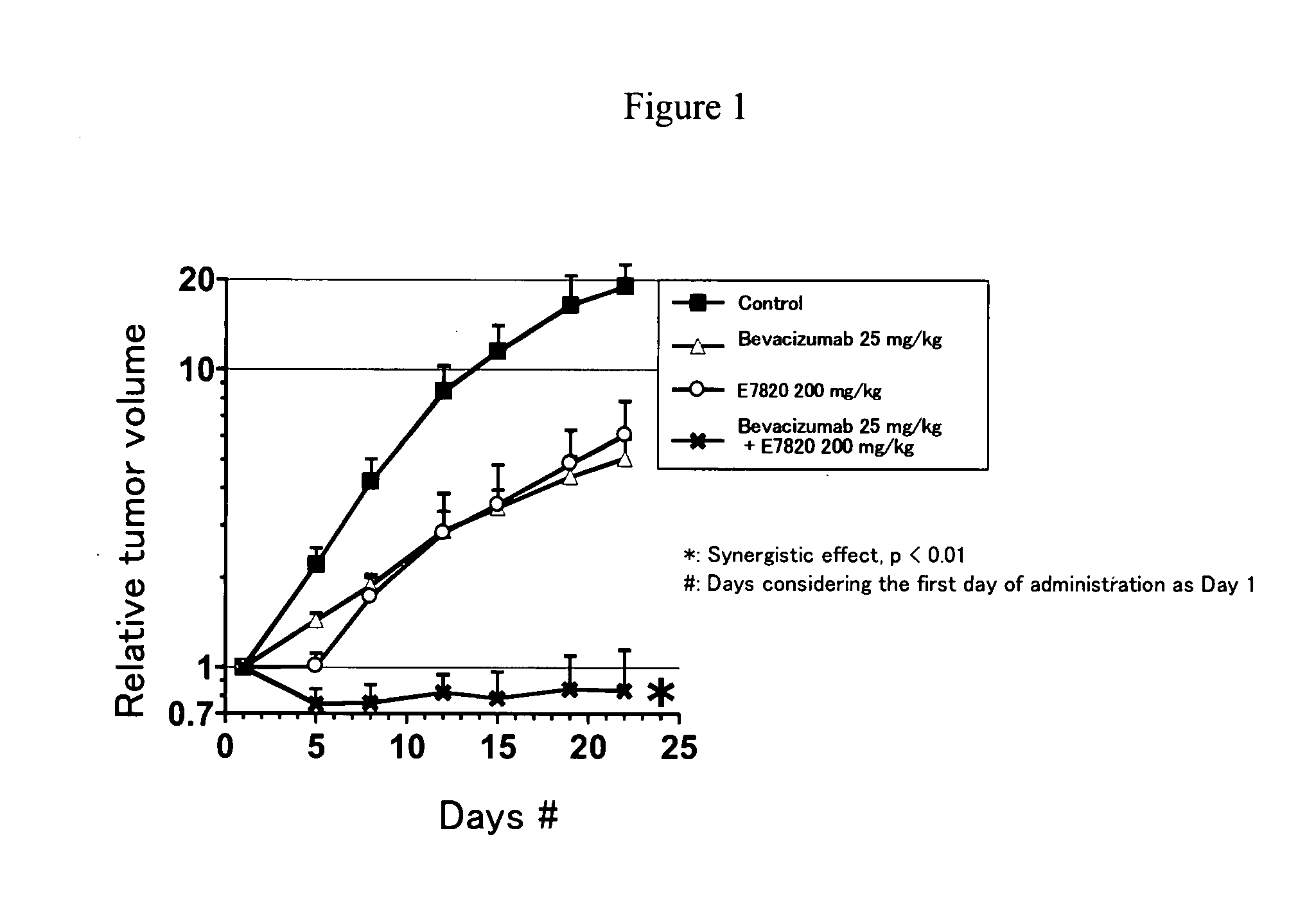
InactiveUS20080286282A1Remarkable anti-tumor effectSynergistic effectBiocideImmunoglobulins against growth factorsBevacizumab InjectionAngiogenesis growth factor
The present invention relates to a pharmaceutical composition, a kit and a method for treating cancer and / or a method for inhibiting angiogenesis, comprising a sulfonamide compound in combination with Bevacizumab.
Owner:EISIA R&D MANAGEMENT CO LTD
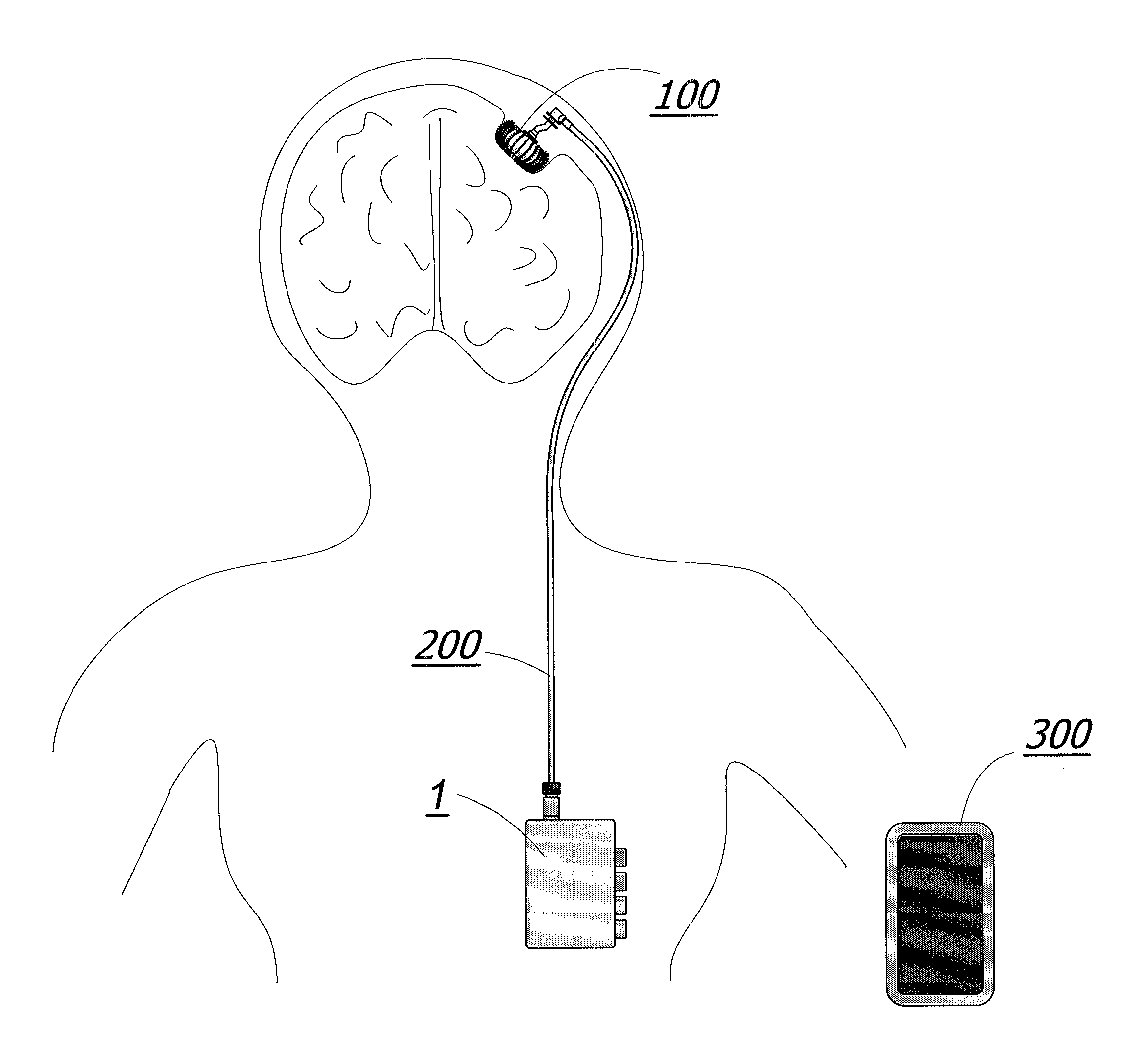
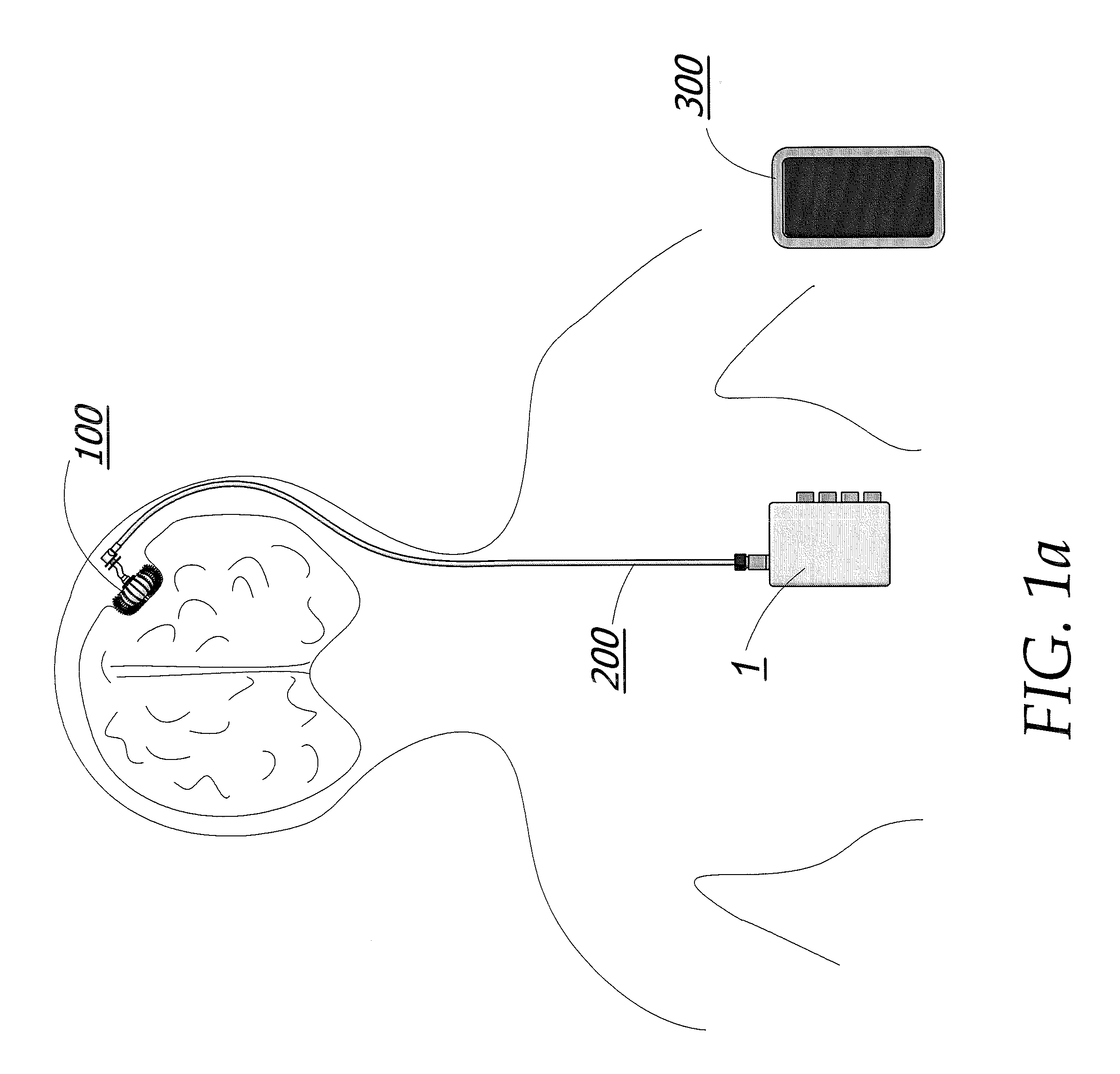
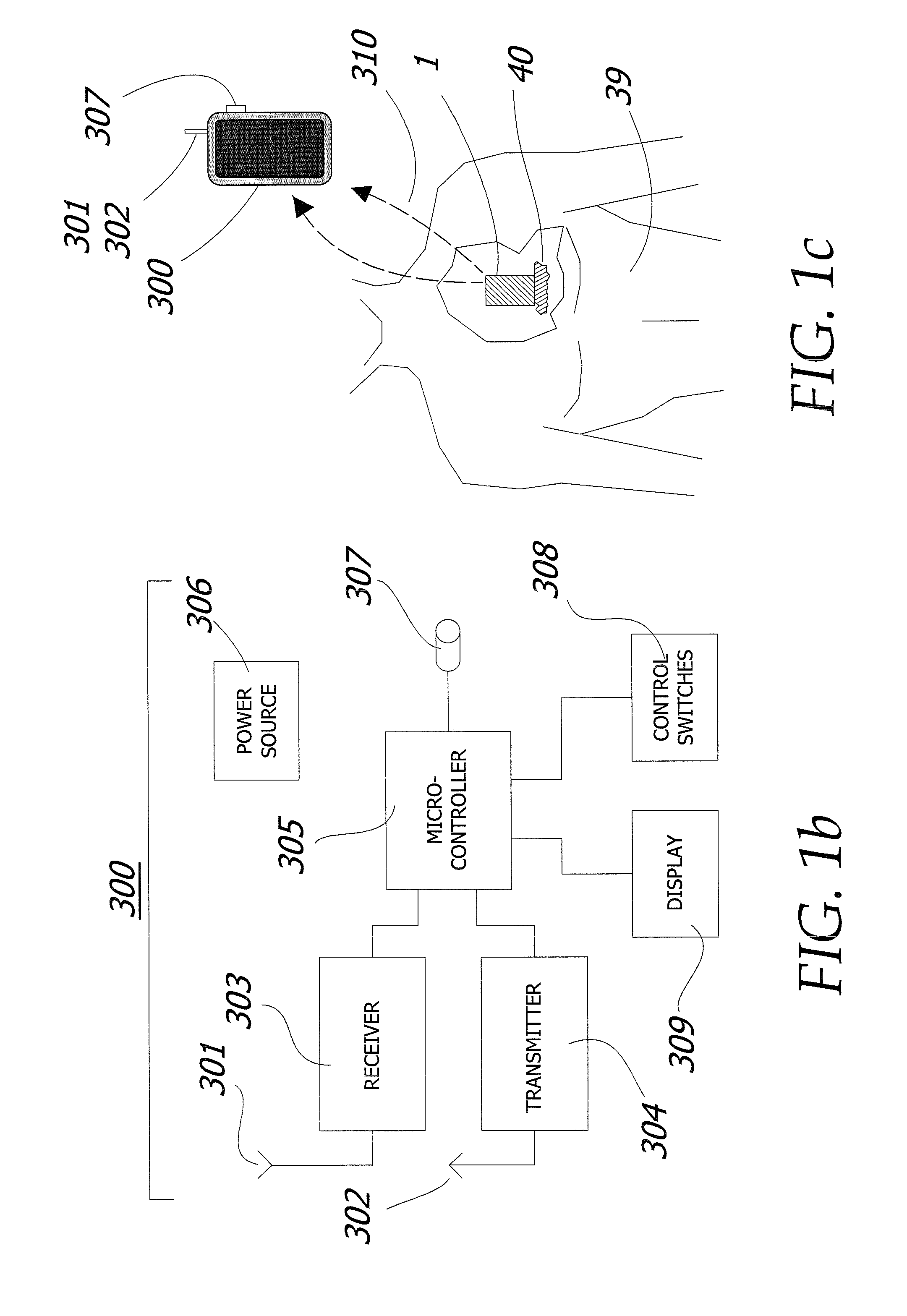
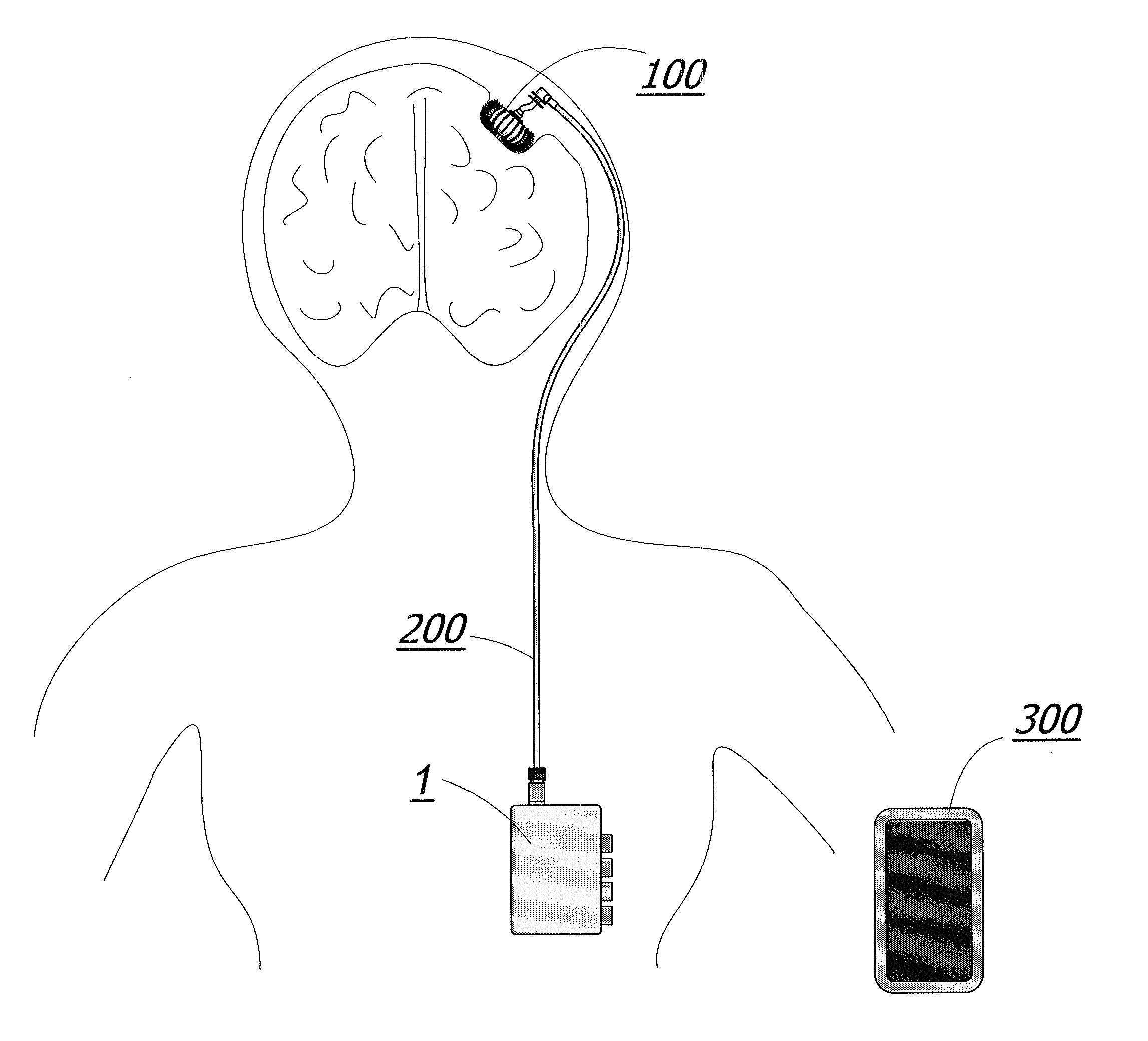
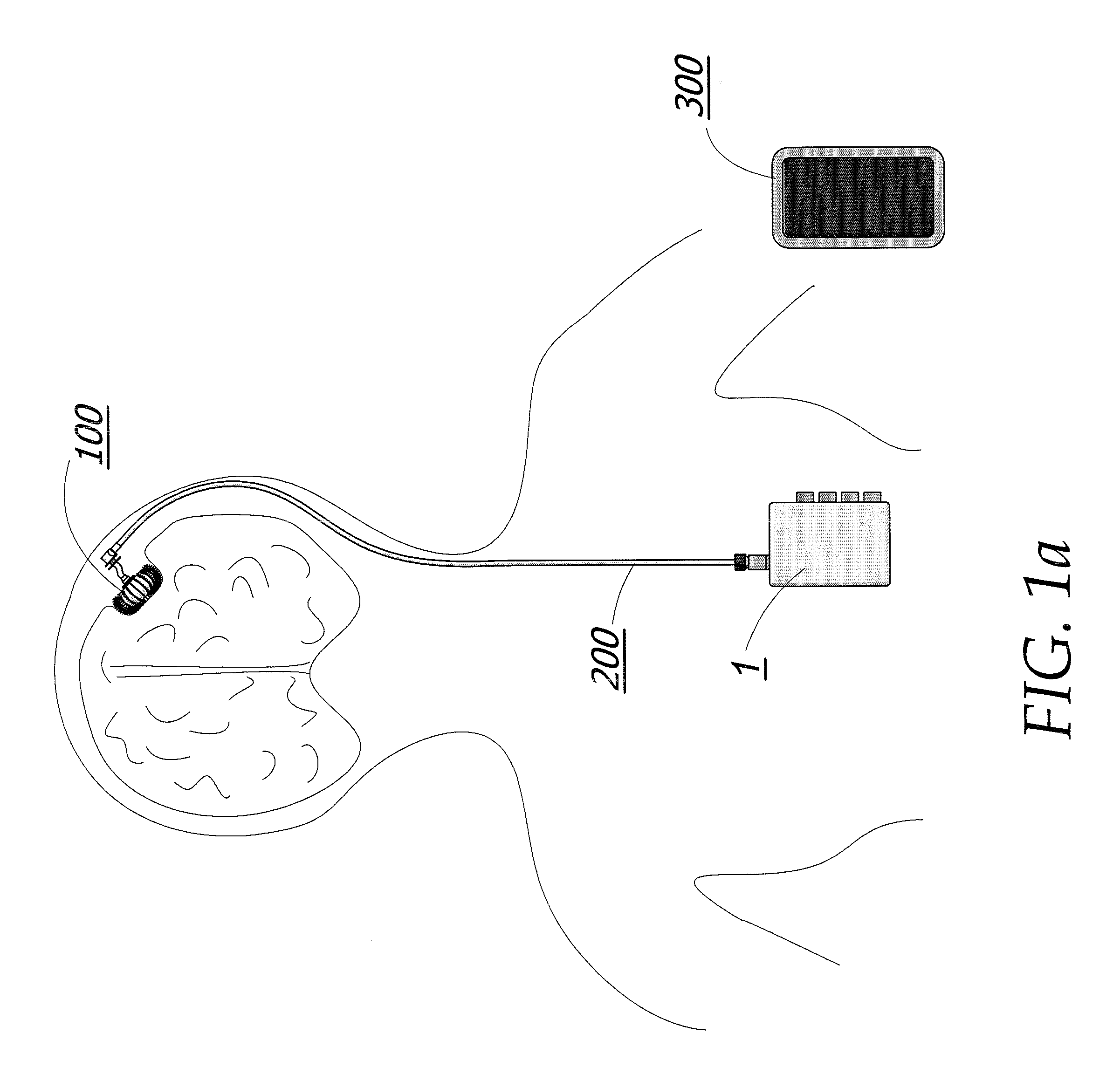
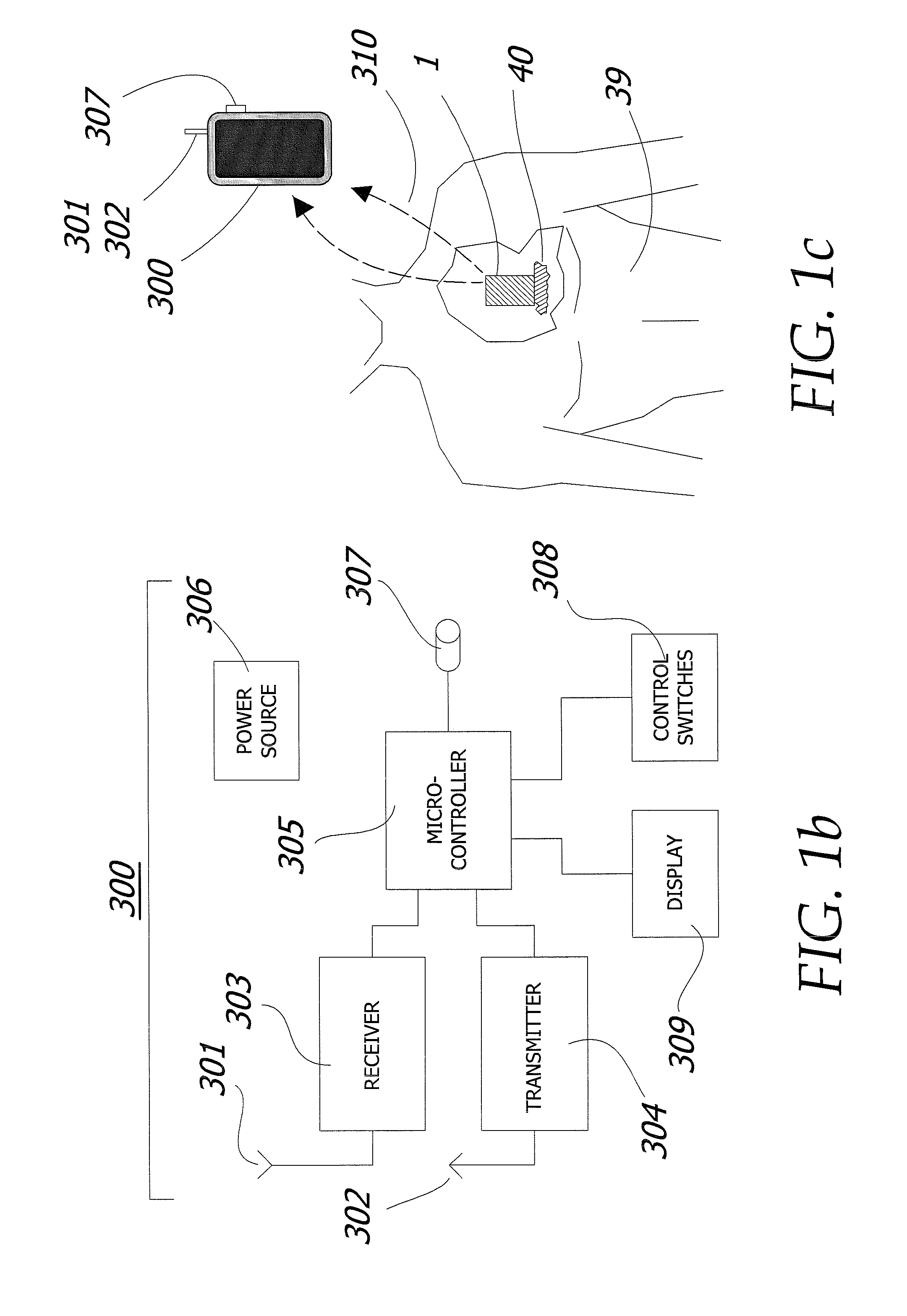
Enhanced method for delivering bevacizumab (avastin) into a brain tumor using an implanted magnetic breather pump
ActiveUS20110092960A1Optimization mechanismReduce eliminatePharmaceutical delivery mechanismMedical devicesBevacizumab InjectionDouble wall
A magnetically controlled pump is implanted into the brain of a patient and delivers a plurality of medicating agents mixed with Avastin at a controlled rate corresponding to the specific needs of the patient. The current invention comprises a flexible double walled pouch that is formed from two layers of polymer. The pouch is alternately expanded and contracting by magnetic solenoid. When contracted, the medicating agent Avastin is pushed out of the pouch through a plurality of needles. When the pouch is expanded, surrounding cerebral fluid is drawn into the space between the double walls of the pouch from which it is drawn through a catheter to an analyzer. In cases where a tumor resection is not performed, an intratumoral catheter will be implanted. Cerebral fluid drawn from the patient is analyzed. The operation of the apparatus and hence the treatment is remotely controlled based on these measurements and displayed through an external controller.
Owner:COGNOS THERAPEUTICS INC
Enhanced method for delivering bevacizumab (avastin) into a brain tumor using an implanted magnetic breather pump
ActiveUS8323270B2Easy to useMaximize efficiencyPharmaceutical delivery mechanismMedical devicesBevacizumab InjectionDouble wall
A magnetically controlled pump is implanted into the brain of a patient and delivers a plurality of medicating agents mixed with Avastin at a controlled rate corresponding to the specific needs of the patient. The current invention comprises a flexible double walled pouch that is formed from two layers of polymer. The pouch is alternately expanded and contracting by magnetic solenoid. When contracted, the medicating agent Avastin is pushed out of the pouch through a plurality of needles. When the pouch is expanded, surrounding cerebral fluid is drawn into the space between the double walls of the pouch from which it is drawn through a catheter to an analyzer. In cases where a tumor resection is not performed, an intratumoral catheter will be implanted. Cerebral fluid drawn from the patient is analyzed. The operation of the apparatus and hence the treatment is remotely controlled based on these measurements and displayed through an external controller.
Owner:COGNOS THERAPEUTICS INC
Method for treating atrophic age related macular degeneration
InactiveCN102159246AOrganic active ingredientsSenses disorderBevacizumab InjectionDry age-related macular degeneration
The present invention relates to compositions and methods for treating dry age related macular degeneration (dry AMD) by administration to an intraocular location of an anti-neovascular agent (such as bevacizumab) in either a liquid or solid polymeric vehicle (or both), such as a biodegradable hyaluronic acid or PLGA (or PLA).
Owner:ALLERGAN INC
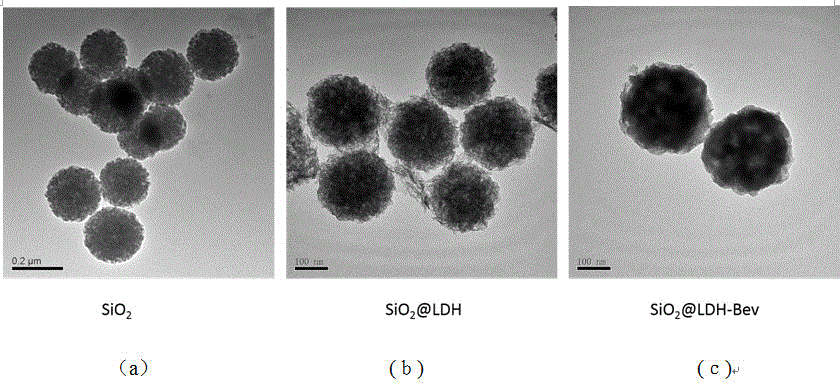
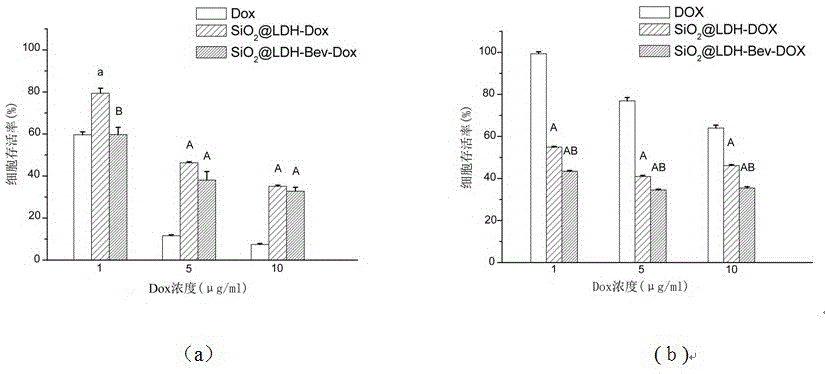
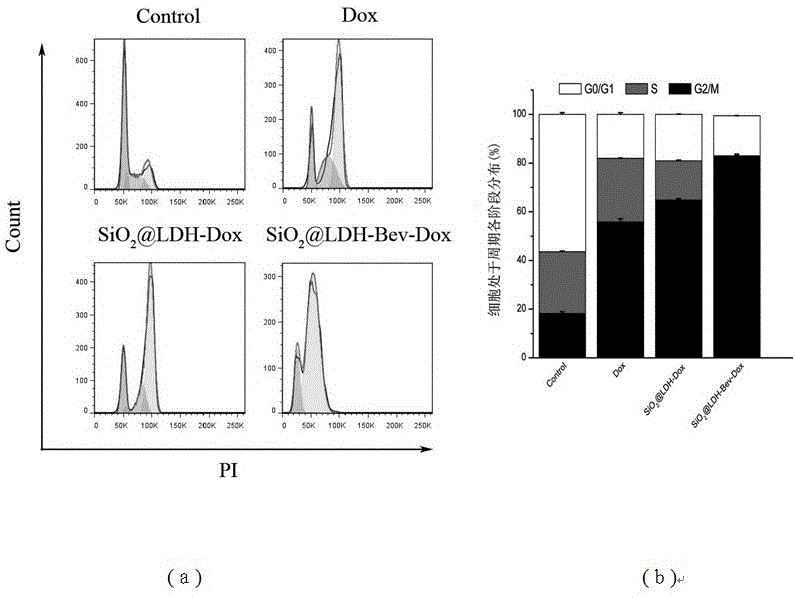
Nanometer drug carrier (bevacizumab medicated-SiO2@LDH (sodium dioxide @ double hydroxide)) with active tumor targeting function, preparation method and application
InactiveCN105999262AInorganic non-active ingredientsAntibody ingredientsSynthesis methodsTumor targeting
The invention relates to a nanometer drug carrier (bevacizumab medicated-SiO2@LDH (sodium dioxide @ double hydroxide)) with an active tumor-targeting function, a preparation method and an application. The nanometer drug carrier uses mesoporous silica as a core, an outer layer is coated with magnesium-aluminum LDH, and the surface is connected with bevacizumab to form a nanometer compound. The nanometer drug carrier can be carried with a treatment drug; the purpose of tumor-targeting treatment is realized by recognizing the bevacizumab specificity and combining with the characteristics of VEGF (vascular endothelial growth factor) which is excessively secreted by tumors. The nanometer drug carrier has the advantages that the safety, active targeting property and drug carrying effect are higher; the synthesis method is simple, the cost is economic, and the application prospect in tumor-targeting treatment is broad.
Owner:TONGJI UNIV
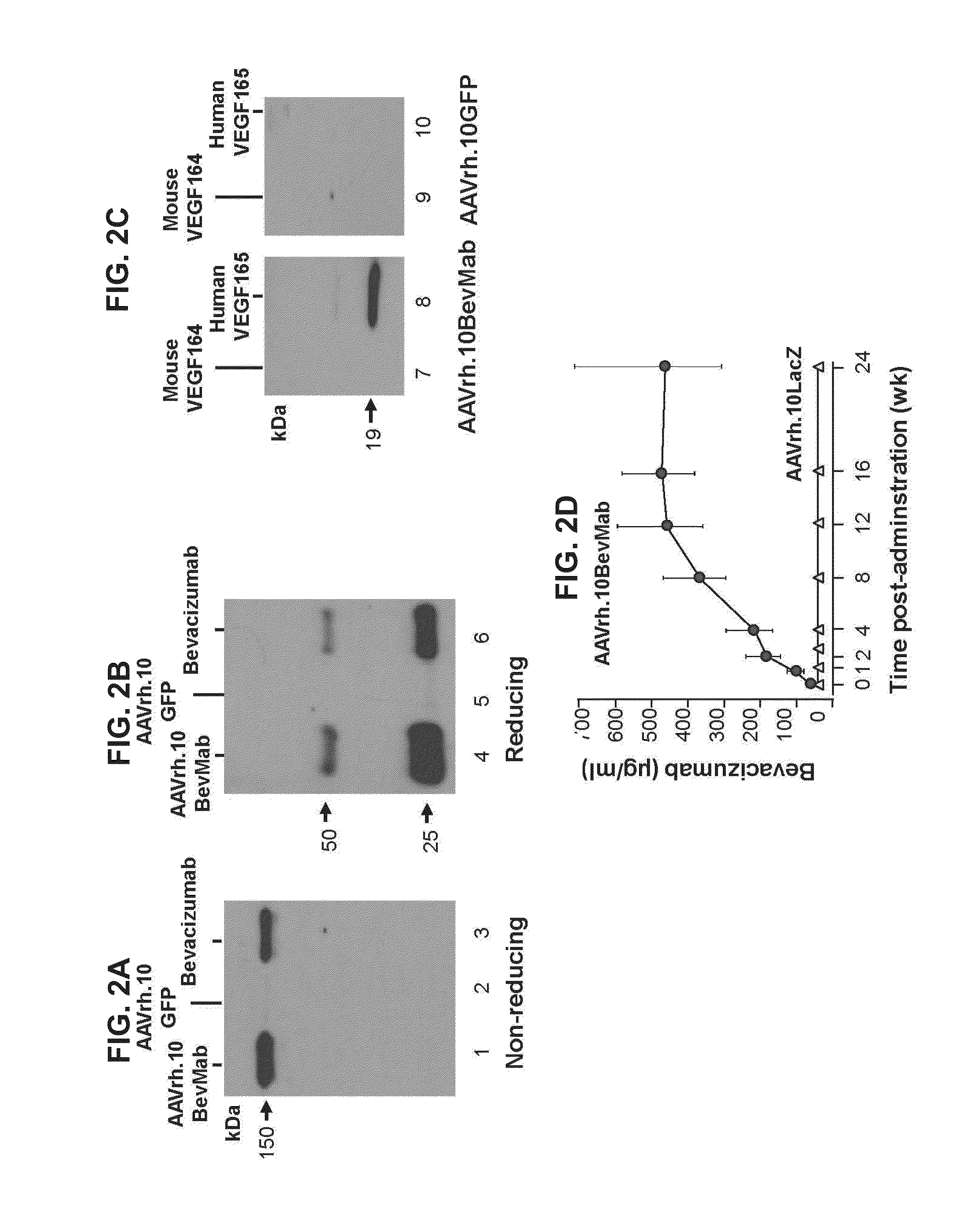
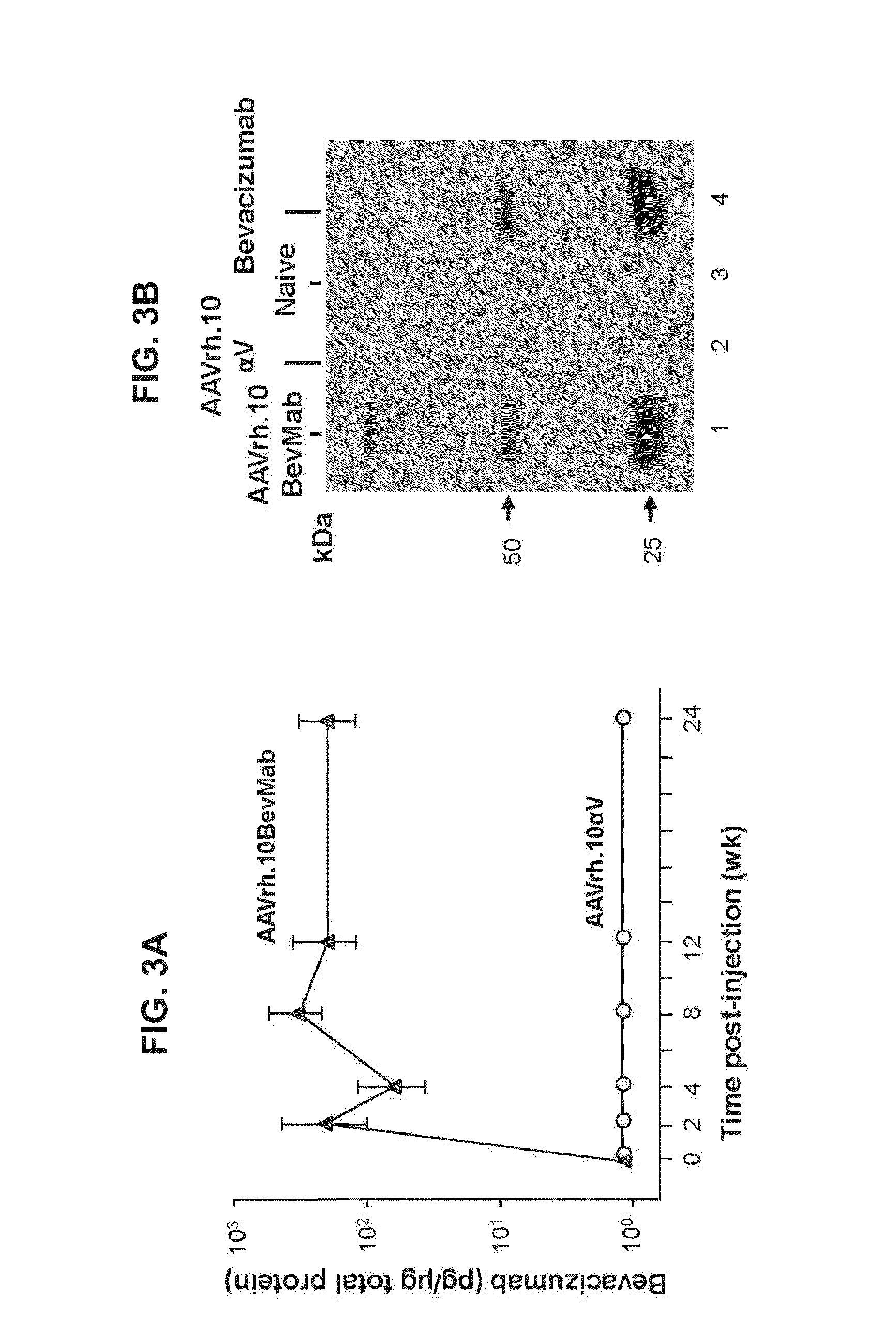
Virus-mediated delivery of bevacizumab for therapeutic applications
InactiveUS20130090375A1Reduction in total area of neovascularizationReduce neovascularizationOrganic active ingredientsSenses disorderBevacizumab InjectionOphthalmology
The invention provides a method of inhibiting ocular neovascularization in a mammal by administering a composition comprising a bevacizumab-encoding adeno-associated virus (AAV) vector directly to the eye of the mammal.
Owner:CORNELL UNIVERSITY
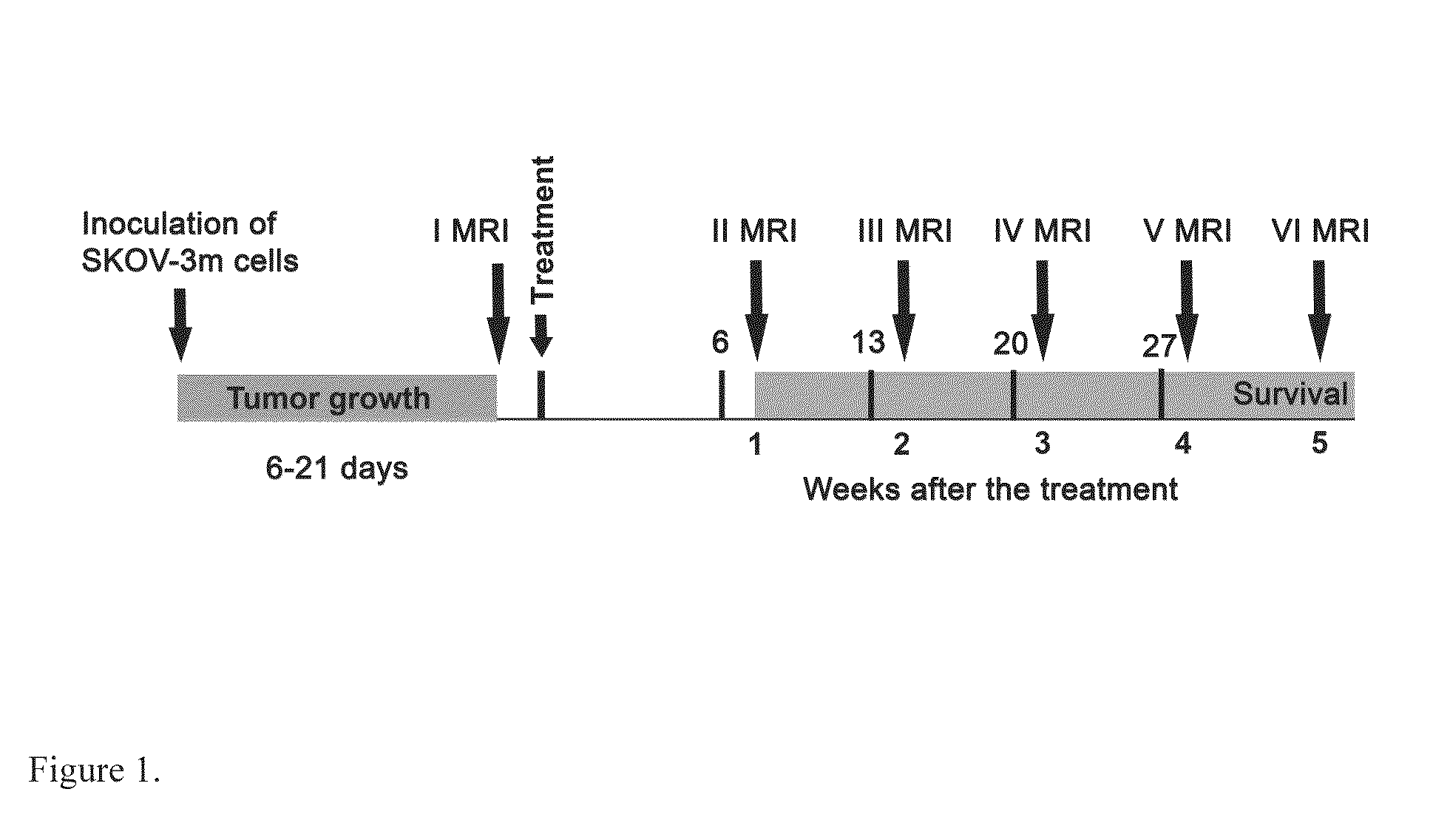
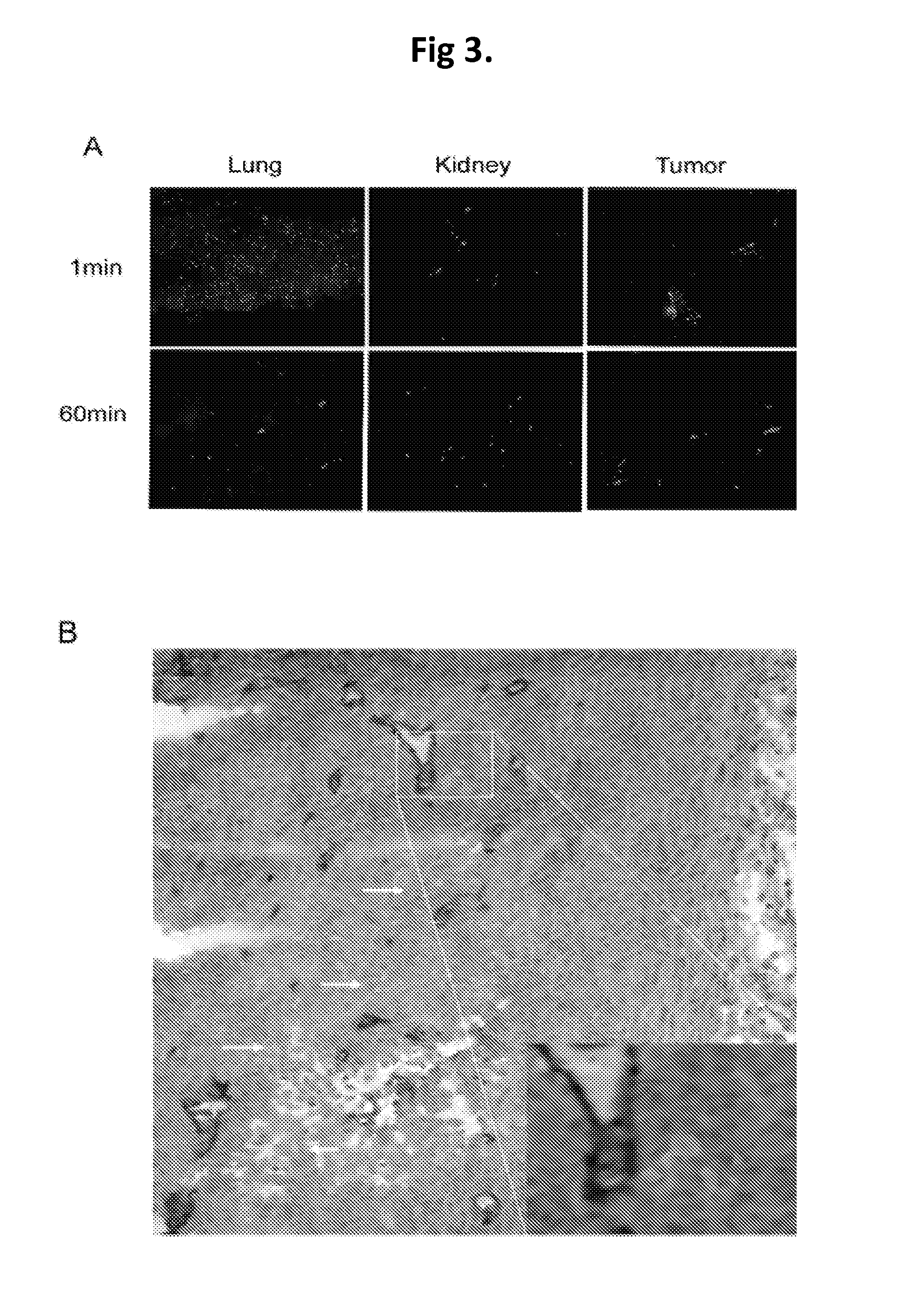
Anti-Angiogenic Gene Therapy With Soluble VEGF Receptors -1, -2 and -3 Together With Paclitaxel Prolongs Survival Of Mice With Human Ovarian Carcinoma
InactiveUS20140057851A1Good treatment effectPoor prognosisOrganic active ingredientsPeptide/protein ingredientsCarboplatinWilms' tumor
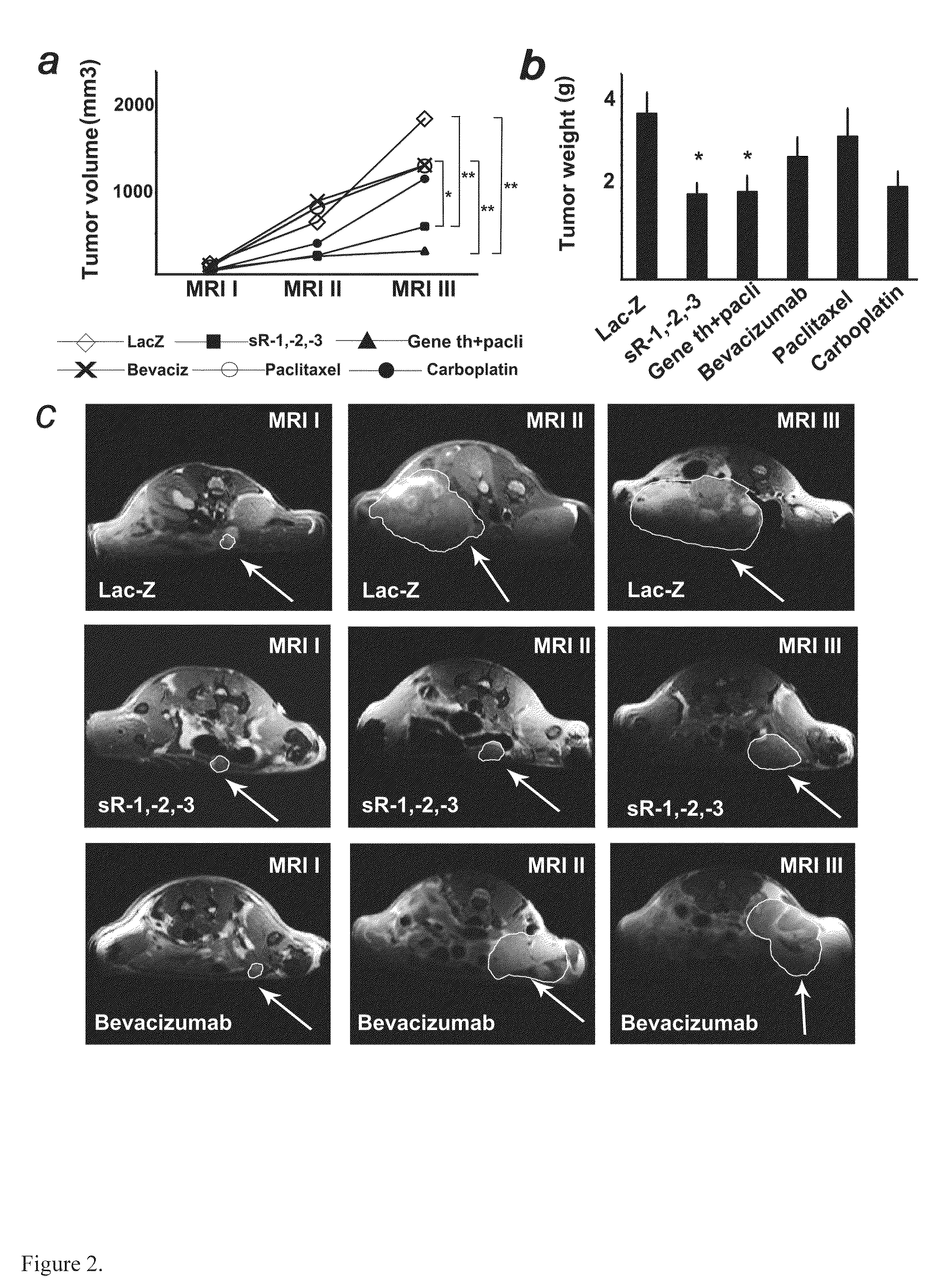
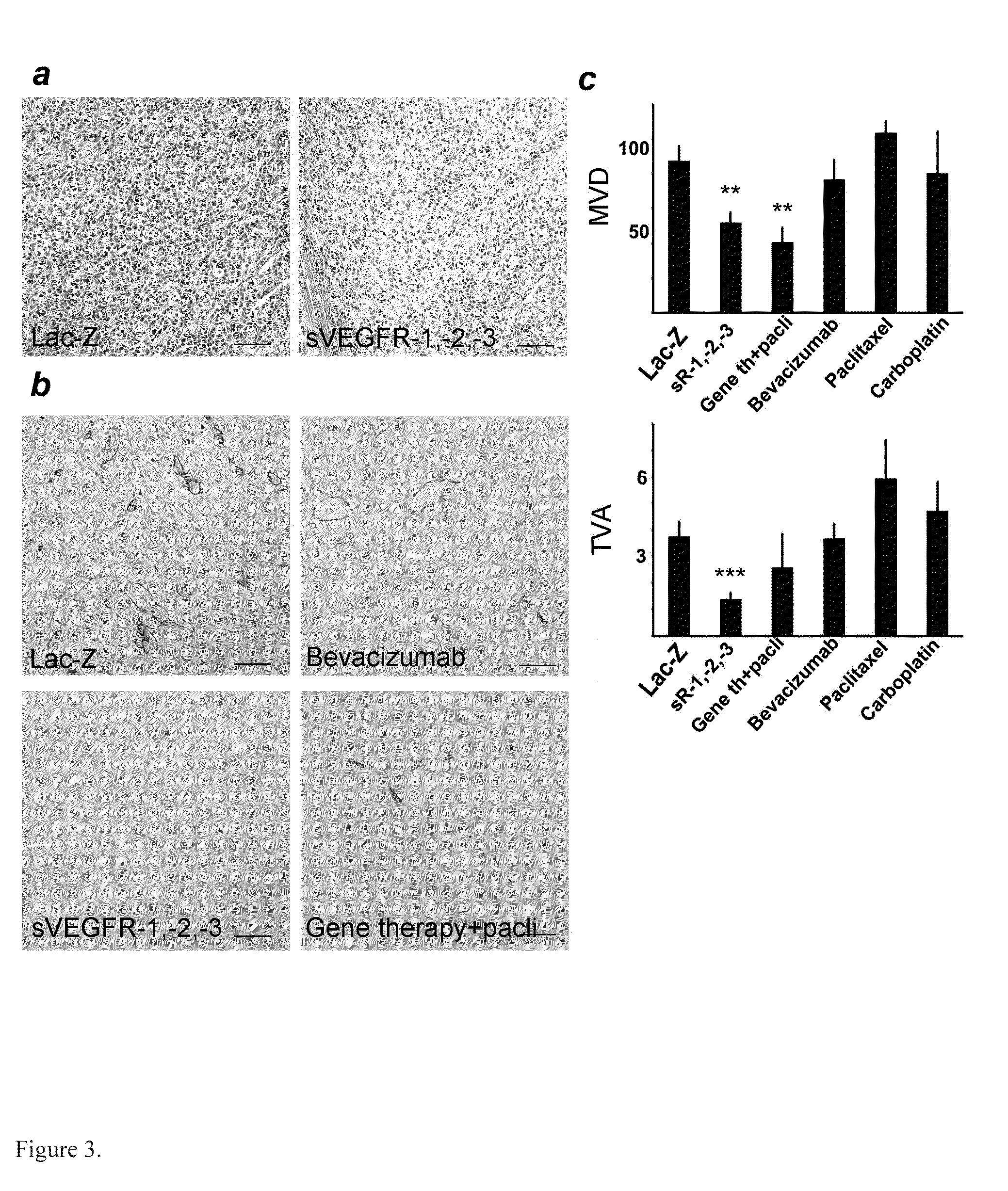
Anti-angiogenic gene therapy with a combination of soluble Vascular Endothelial Growth Factors (sVEGFR) improves the efficacy of chemotherapy with paclitaxel for reducing ovarian cancer mean tumor volume (in cubic millimetres) as measured using magnetic resonance imaging. The study groups were: AdLacZ control, combination of AdsVEGFR-1, -2 and -3, combination of AdsVEGFR-1, -2, -3 and paclitaxel, bevacizumab monotherapy, paclitaxel monotherapy and carboplatin monotherapy. Effectiveness was assessed by survival time and surrogate measures such as sequential MRI, immunohistochemistry, microvessel density and tumor growth. Antiangiogenic gene therapy combined with paclitaxel significantly prolonged the mean survival compared to the controls and all other treatment groups (p=0.001). Tumors of the mice treated by gene therapy were significantly smaller than in the control group (p=0.021). The mean vascular density and total vascular area were also significantly smaller in the tumors of the gene therapy group (p=0.01).
Owner:TRIZELL LTD
Methods of treating colon cancer using nanoparticle mtor inhibitor combination therapy
The present application provides methods of treating a colon cancer (such as advanced and / or metastatic colon cancer) in an individual, comprising administering to the individual: a) an effective amount of a composition comprising nanoparticles comprising an mTOR inhibitor (such as a limus drug, such as sirolimus or a derivative thereof) and an albumin, b) an effective amount of anti-VEGF antibody (such as bevacizumab), and c) a therapeutically effective FOLFOX regimen (such as FOLFOX4 or a modified FOLFOX6).
Owner:ABRAXIS BIOSCI LLC
Virus-mediated delivery of bevacizumab for therapeutic applications
InactiveUS20150182638A1Senses disorderPharmaceutical delivery mechanismBevacizumab InjectionOphthalmology
The invention provides a method of inhibiting ocular neovascularization in a mammal by administering a composition comprising a bevacizumab-encoding adeno-associated virus (AAV) vector directly to the eye of the mammal.
Owner:CORNELL UNIVERSITY
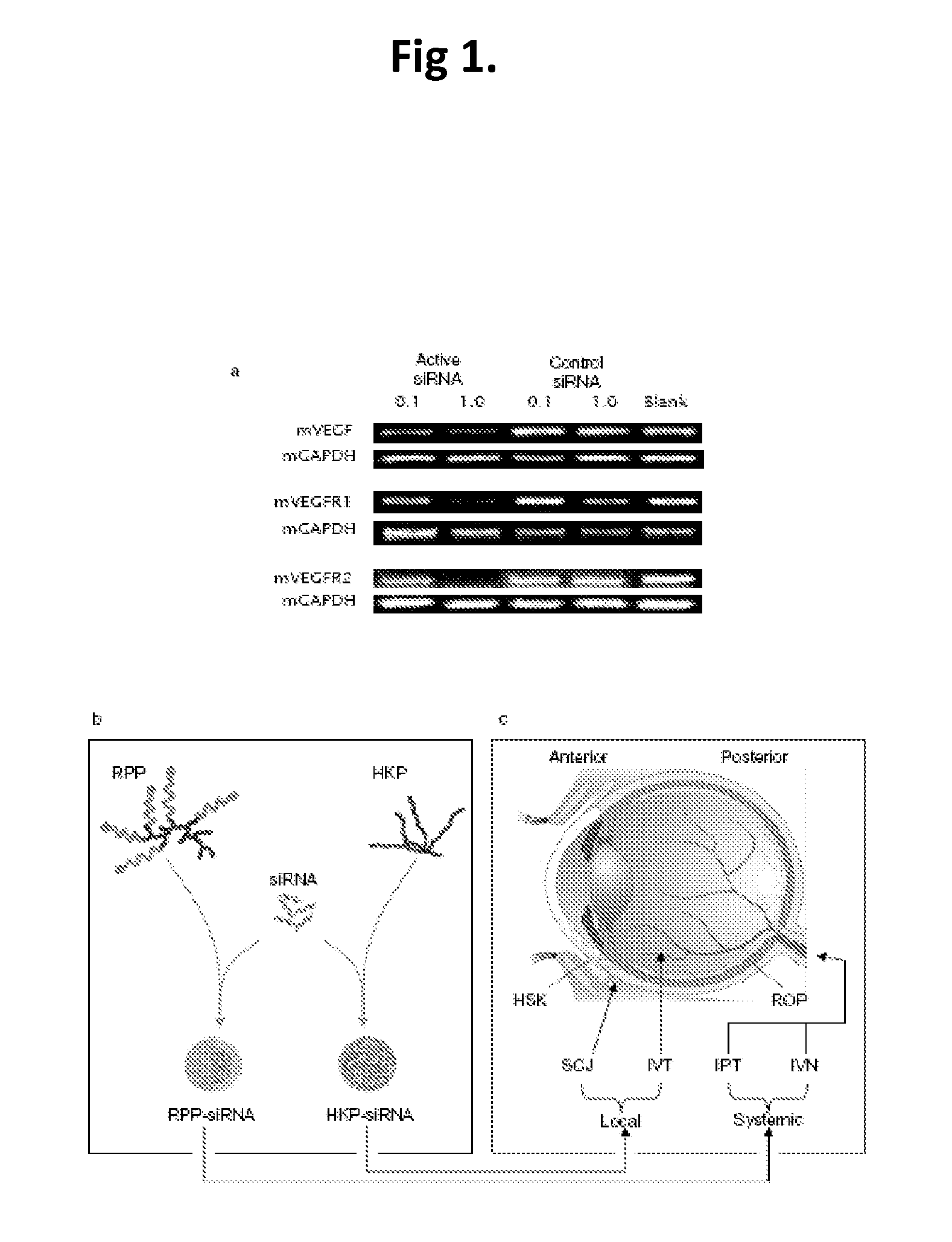
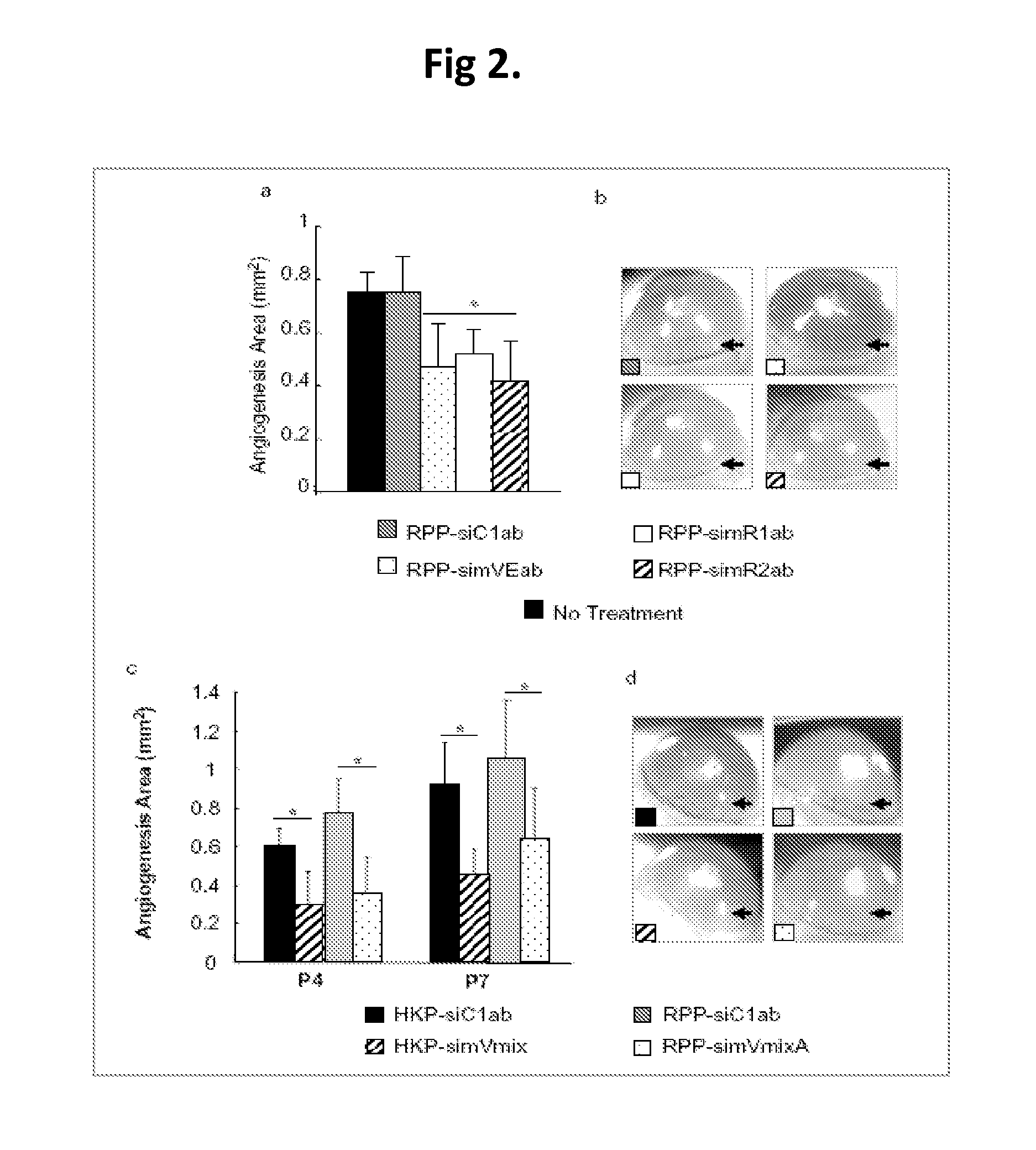
Compositions and methods using siRNA molecules and siRNA cocktails for the treatment of breast cancer
The present invention provides small interfering RNA (siRNA) molecules, compositions containing the molecules, and methods of using the molecules and compositions to treat breast cancer. In one aspect, a multi-targeted siRNAi cocktail is disclosed. The siRNA molecules may be encapsulated in nanoparticles to further enhance their anti-cancer activity. The compositions may also be used in combination with other anti-cancer agents, such as bevacizumab.
Owner:SIRNAOMICS INC
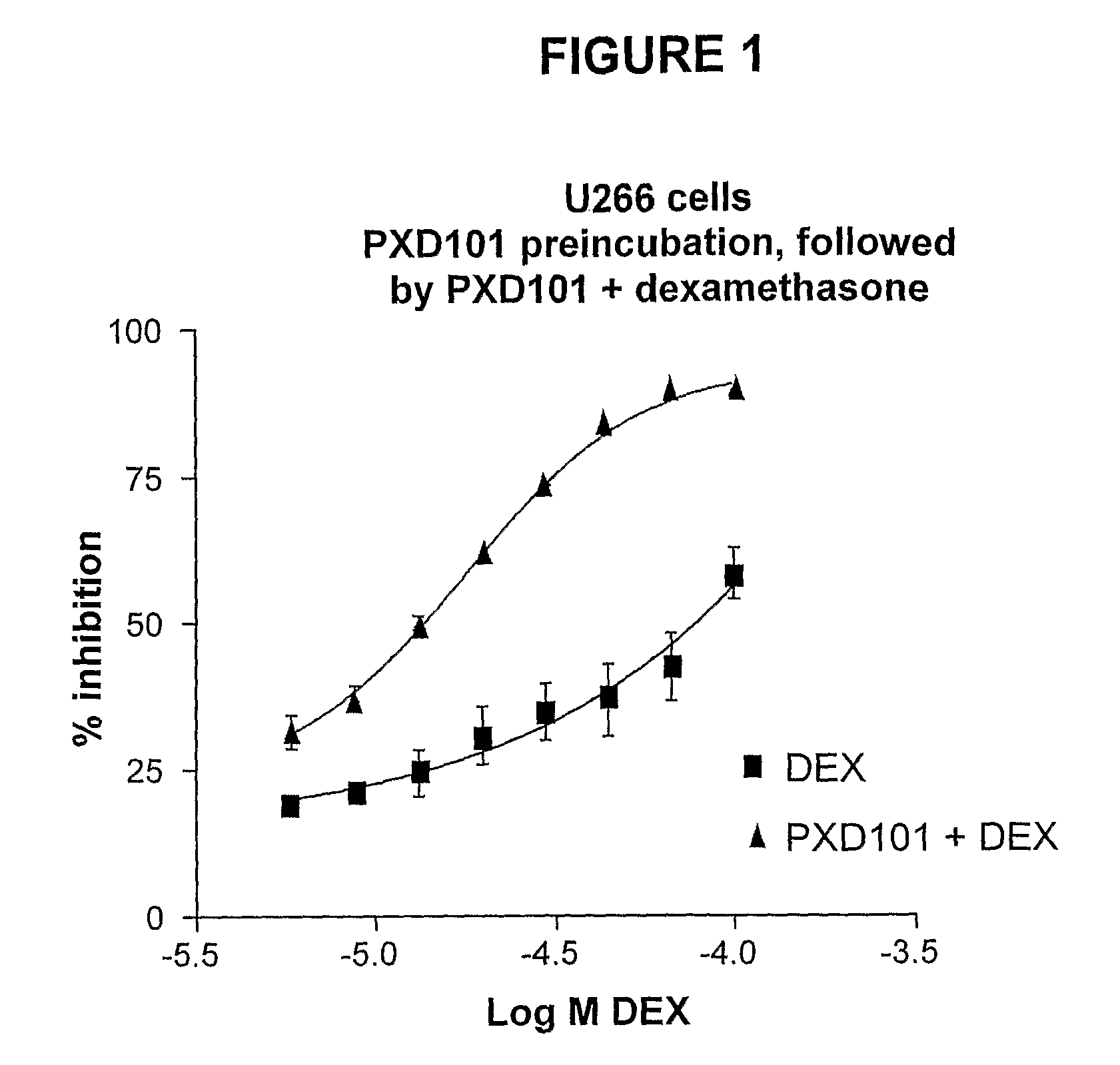
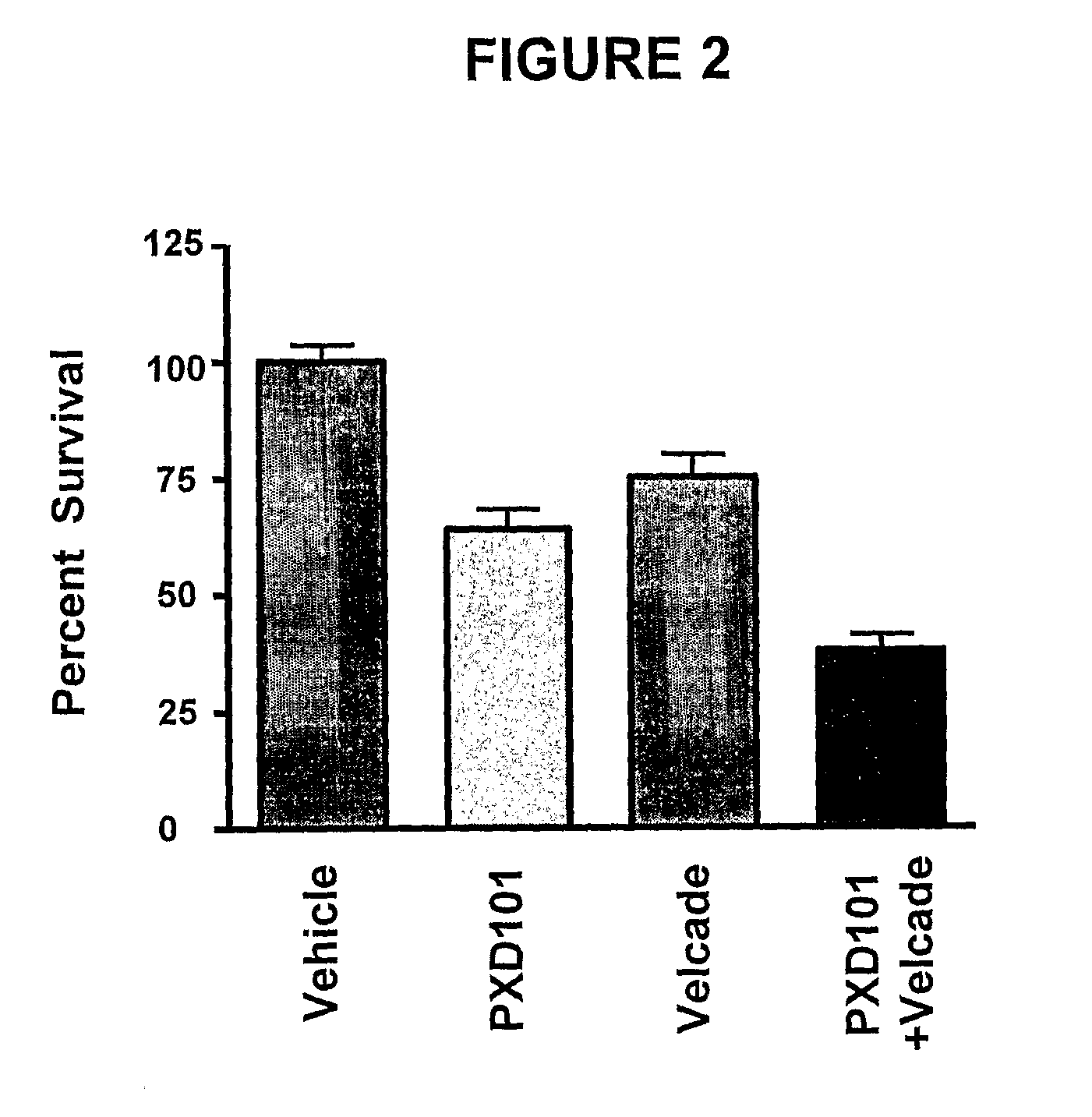
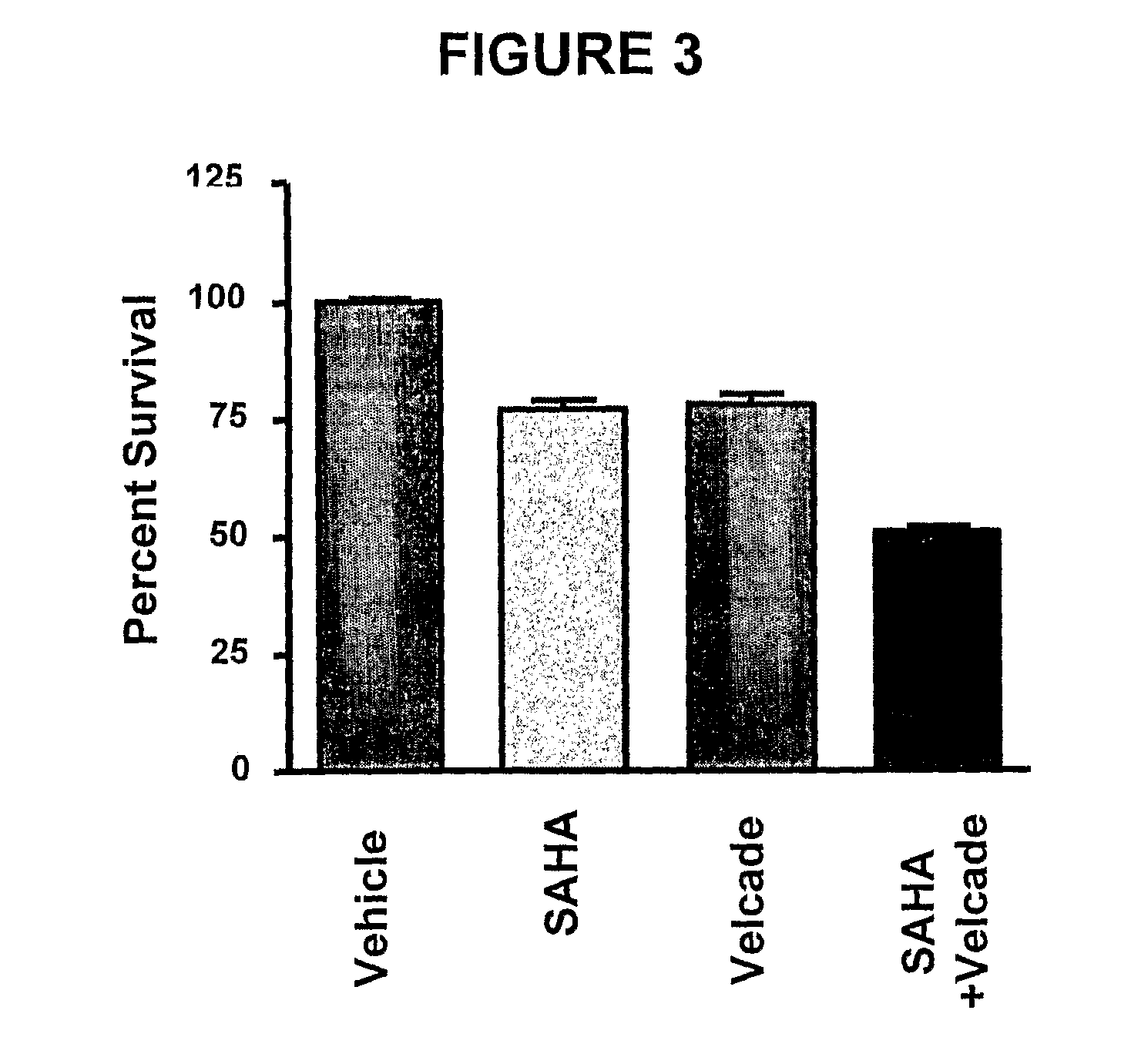
Histone deacetylase (HDAC) inhibitors (PXD101) for the treatment of cancer alone or in combination with chemotherapeutic agent
The present invention relates generally to methods for treating cancer. In one respect, the present invention relates to a method of treating a hematological cancer (e.g., multiple myeloma, leukemia, lymphoma) comprising administering to a patient in need thereof a therapeutically effective amount of a histone deacetylase inhibitor, for example, a histone deacetylase (HDAC) inhibitor as described herein, for example, PXD-101. In another respect, the present invention relates to a method of treating cancer (e.g., solid tumor cancer, e.g., rectal cancer, colon cancer, ovarian cancer; hematological cancer, e.g., multiple myeloma, leukemia, lymphoma) comprising administering to a patient in need thereof, a first amount of a histone deacetylase (HDAC) inhibitor, for example, a histone deacetylase inhibitor as described herein, for example, PXD-101, and a second amount of an other chemotherapeutic agent, for example, an other chemotherapeutic agent selected from: an antibody against VEGF, AVASTIN® (bevacizumab), an antibody against CD20, rituximab, bortezomib, thalidomide, dexamethasone, vincristine, doxorubicin, and melphalan, wherein the first and second amounts together comprise a therapeutically effective amount.
Owner:TOPOTARGET UK LTD
Taxane analogs for the treatment of brain cancer
ActiveUS20110318334A1High activityImprove solubilityBiocideOrganic active ingredientsBevacizumab InjectionMammal
Provided herein are compounds and methods for the treatment of brain cancer in a mammal, wherein the method comprises the administration to the mammal a compound that stabilizes tubulin dimers or microtubles at G2-M interface during mitosis but is not a substrate for MDR protein. In particular, the present application relates to the use of an orally effective abeo-taxane, alone or in combination with temozolomide or bevacizumab, for the treatment of brain cancer.
Owner:TAPESTRY PHARMACEUTICALS INC
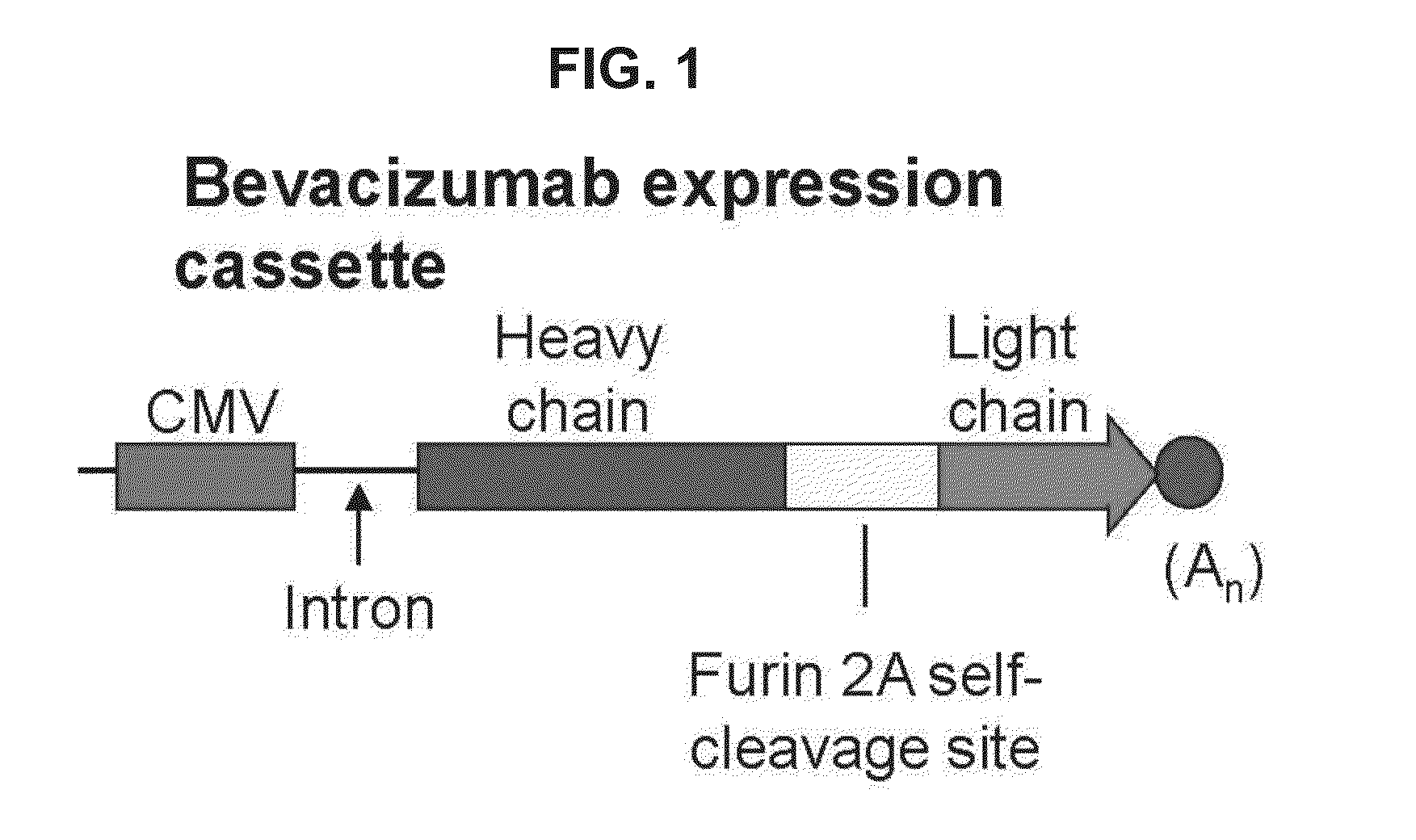
Preparing method and medical application of optimized plant source recombination humanized bevacizumab
InactiveCN106222194AIncrease productionReduce plant-specific glycosylationVaccinesBiological material analysisPlant SourcesLung cancer
The invention belongs to the technical field of biological medicine, and particularly relates to a preparing method and medical application of optimized plant source recombination humanized bevacizumab. Heavy-chain and light-chain fusion protein of the recombinant antibody bevacizumab is expressed in plants, heavy chain and light chain are expressed in an proportion appropriate to 1:1 by adding 2A sequence to fusion protein, the proportion promotes assembly of a complete antibody obviously, and results show that the yield of antibodies expressed with the system is high. Meanwhile, due to the fact that a stable tetramer structure is formed through equal-proportion assembly of the heavy chain and light chain of the recombinant antibody, the number of unassembled polypeptides easy to degrade is reduced, and then pure and consistent monoclonal antibodies are obtained. The bevacizumab developed with a new method is applied to medicine to be used for treating breast cancer, lung cancer, spongioblastoma, kidney cancer, cervix uterus cancer, ovarian cancer, colon cancer and rectal cancer.
Owner:深圳麦客思鱼生物科技发展有限公司
Photodynamic therapy for conditions of the eye
ActiveUS9226917B2Acceptable visual acuity outcomesAcceptable safety profileOrganic active ingredientsSenses disorderDiseasePorphyrin
Owner:BAUSCH LOMB IRELAND LTD
Combination therapy of cancer with Anti-endoglin antibodies and Anti-vegf agents
InactiveUS20180057602A1Relieve symptomsIncreased apoptosisSenses disorderBoron compound active ingredientsBevacizumab InjectionAngiogenesis growth factor
The present application relates to compositions of chimeric anti-endoglin antibodies and anti-VEGF agents. Another aspect relates to the use of chimeric anti-endoglin antibodies and Bevacizumab. Another aspect relates to the use of the compositions to inhibit VEGF induced sprouting. Another aspect relates to the use of the compositions to inhibit angiogenesis.
Owner:TRACON PHARMA +1
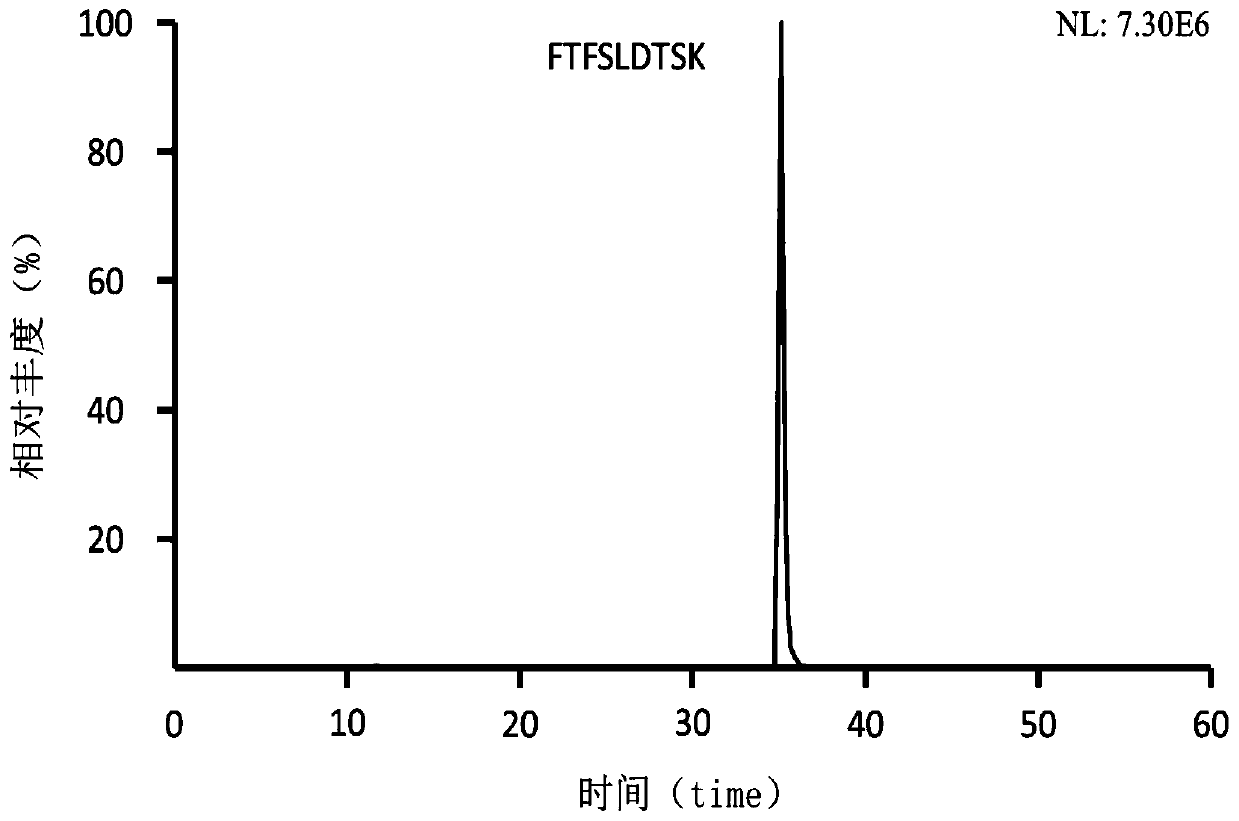
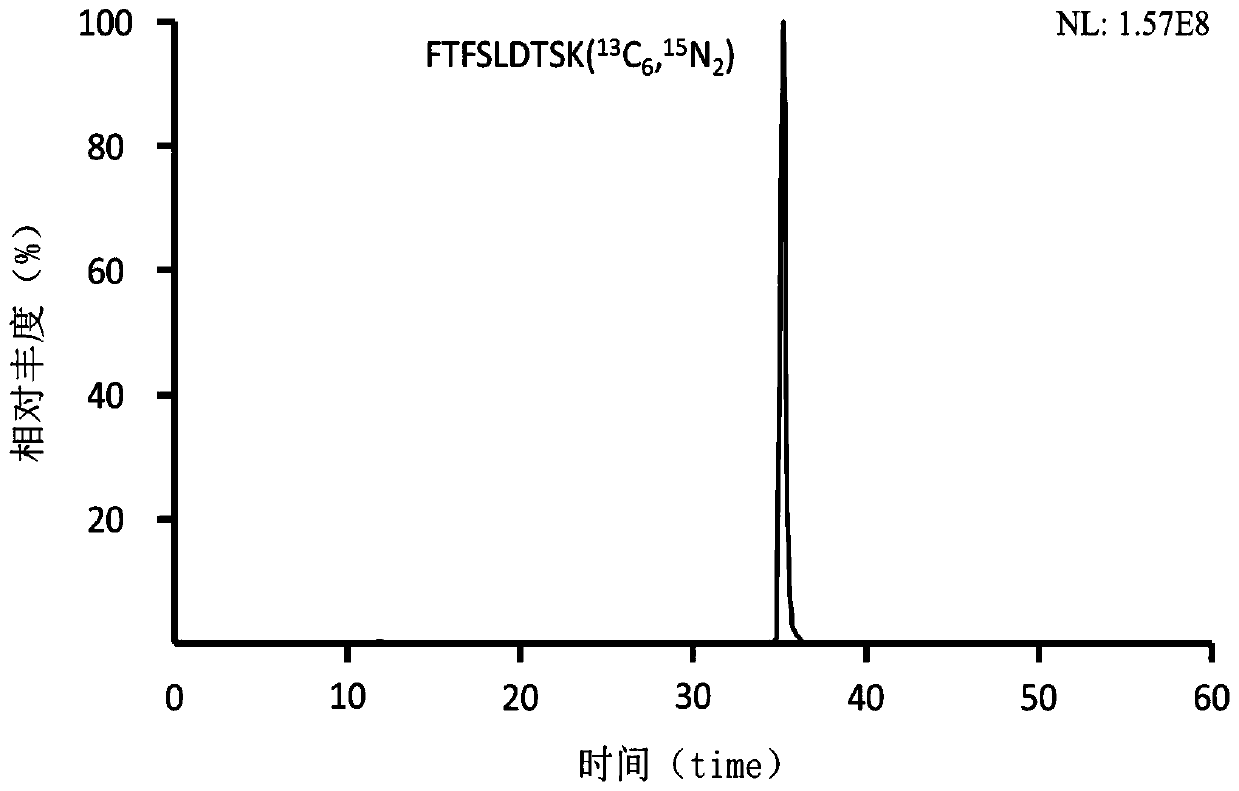
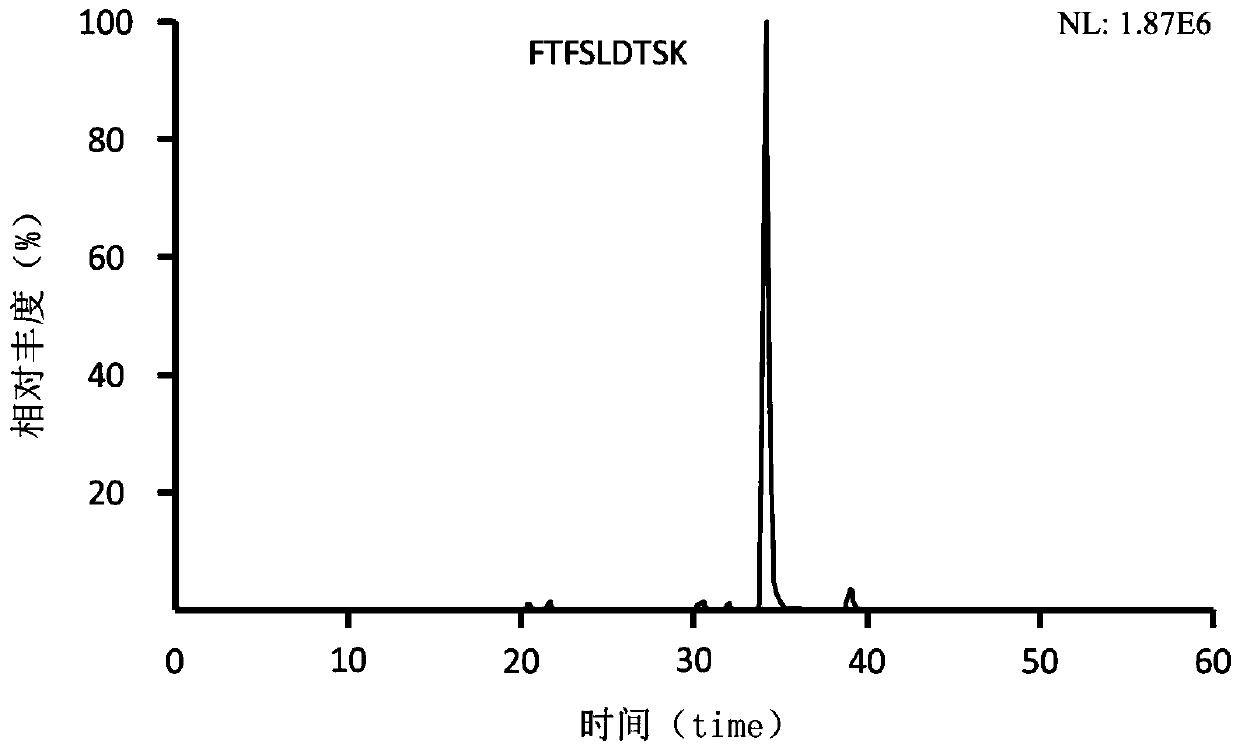
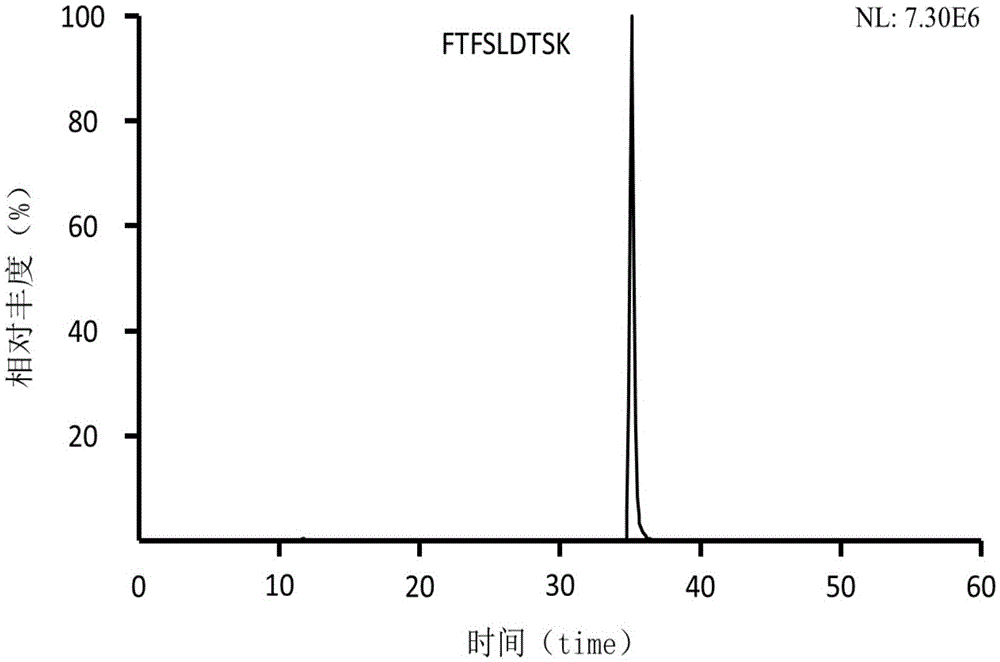
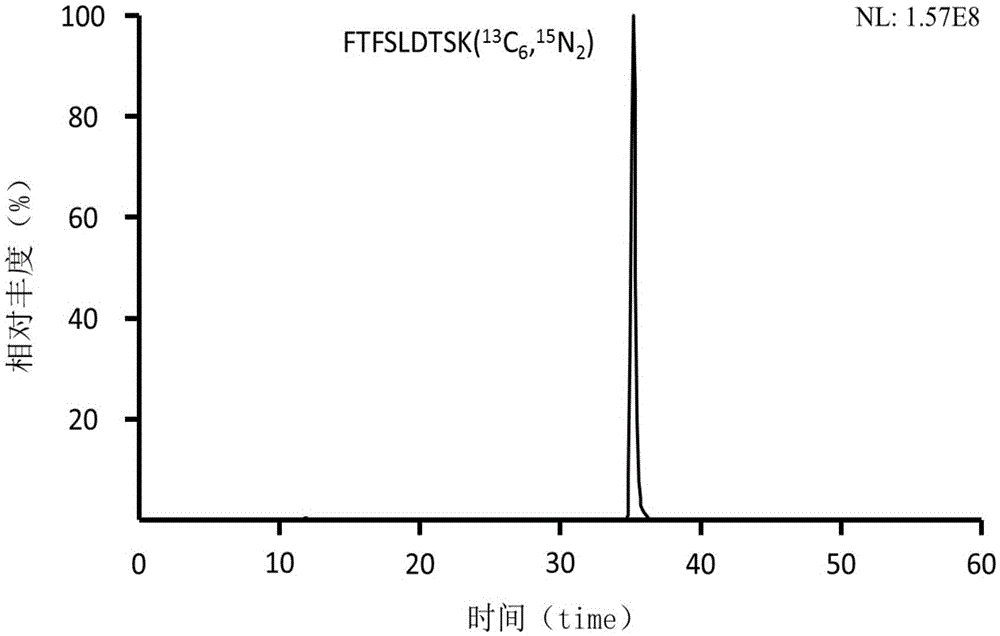
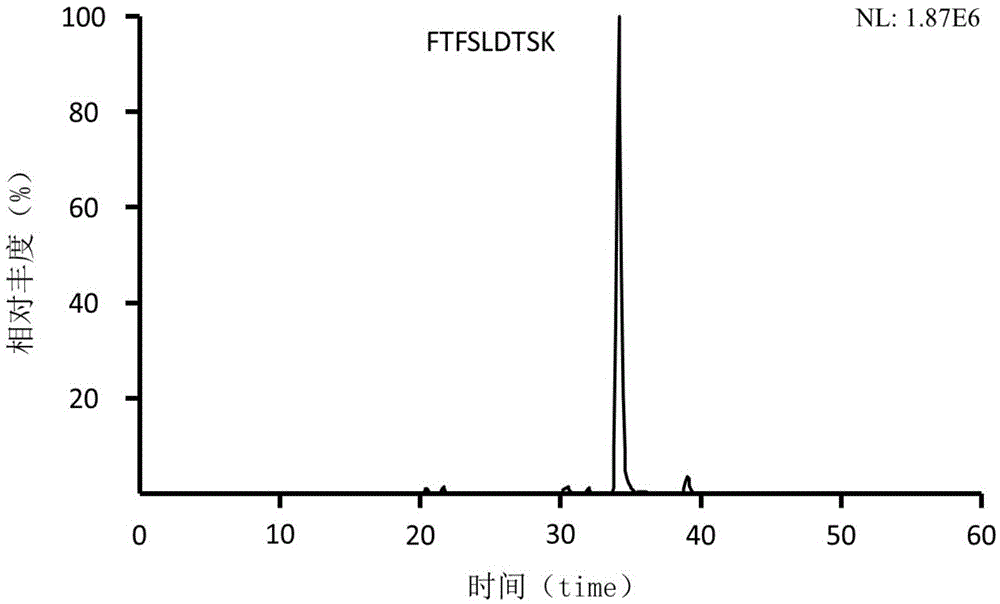
Preclinical pharmacokinetic study mass spectrometry analysis method of bevacizumab
InactiveCN110045028AEase of handlingSimple methodComponent separationLiquid chromatography mass spectroscopyHydrolysis
The invention discloses a pharmacokinetic mass spectrometry analysis method of monoclonal antibody drugs, and belongs to the technical field of proteomics. According to the technical scheme, a specific peptide fragment obtained by pancreatin hydrolysis of Bevacizumab is selected, wherein specificity means that the peptide fragment is only generated by pancreatin hydrolysis of Bevacizumab, and other proteins and protein drugs cannot generate the peptide fragment by pancreatin hydrolysis; and based on liquid chromatography-tandem mass spectrometry technology, a parallel reaction monitoring modeis adopted to scan and analyze the specific peptide fragment to establish a stable and reliable LC / MS / MS quantitative method for the specific peptide fragment. The pharmacokinetic mass spectrometry analysis method of the monoclonal antibody drugs has the characteristics of high flux, high sensitivity, good reproducibility and the like. Reagents and drugs used are cheaper and easier to obtain, thepreparation method is simple, and the method is beneficial to popularization. A sample treatment process and quantitative method are simple and easy to implement, and guiding significance for qualitative and quantitative research of other protein drugs and polypeptide drugs is achieved.
Owner:南京智谱分子医学技术研究院有限公司
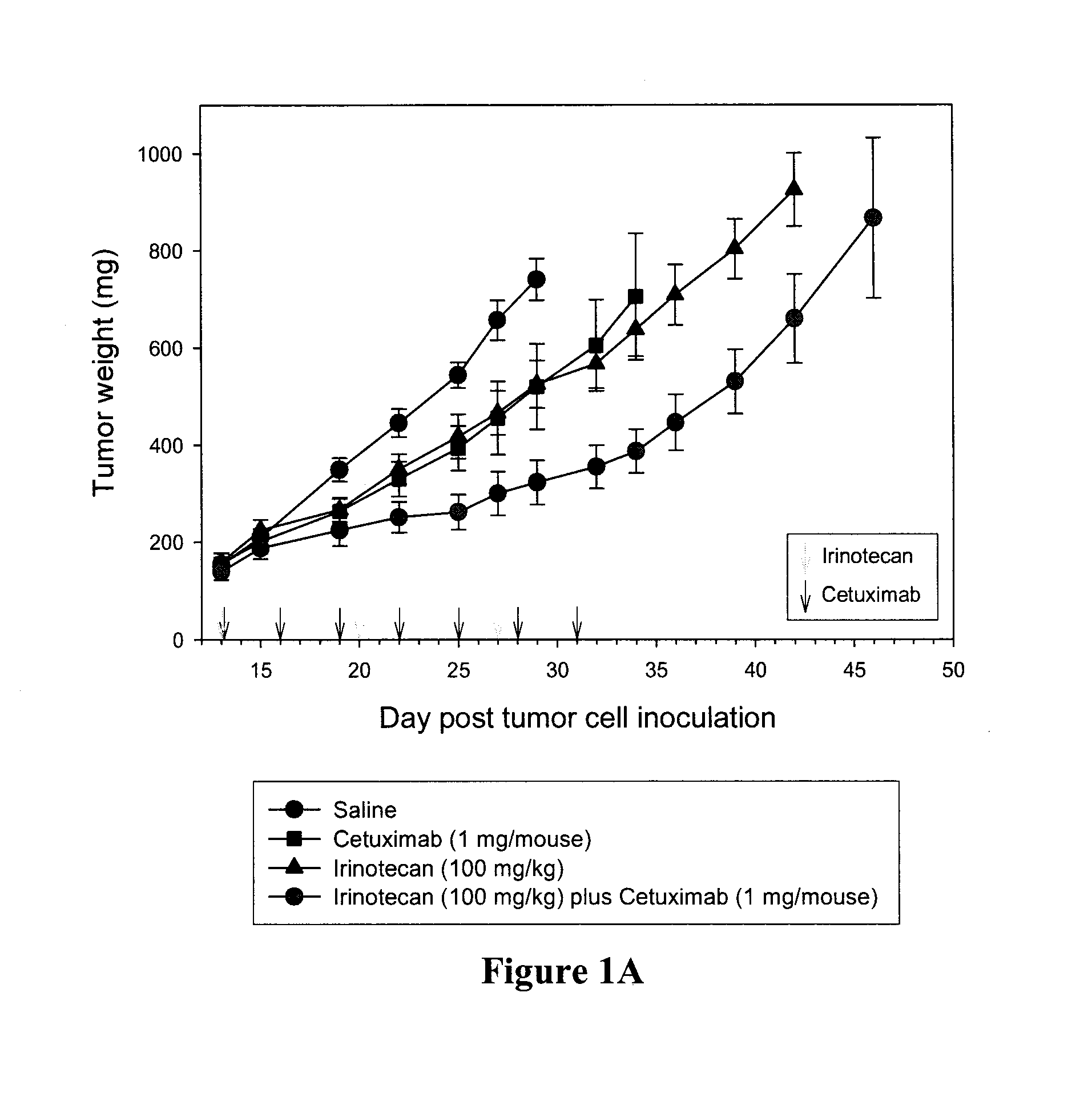
Combination therapies using agents that target tumor-associated stroma or tumor cells and tumor vasculature
ActiveUS20170101464A1Treating and ameliorating effectOrganic active ingredientsImmunoglobulins against growth factorsAbnormal tissue growthAntigen
The present invention provides, inter alia, methods for treating or ameliorating the effects of a disease, such as cancer, in a subject. The methods include: administering to a subject in need thereof (a) a therapeutically effective amount of a monoclonal antibody or antigen binding fragment of the present invention, and (b) a therapeutically effective amount of a combination therapy including bevacizumab and at least one additional therapeutic agent; or a therapeutically effective amount of at least one additional therapeutic agent selected from the group consisting of a COX-2 inhibitor (COXIB), a non-steroidal anti-inflammatory drug (NSAID), a prostaglandin E2 (PGE2) synthase inhibitor, and combinations thereof. Compositions, including pharmaceutical compositions, and kits for treating diseases, such as cancer, are also provided herein.
Owner:BIOMED VALLEY DISCOVERIES INC +1
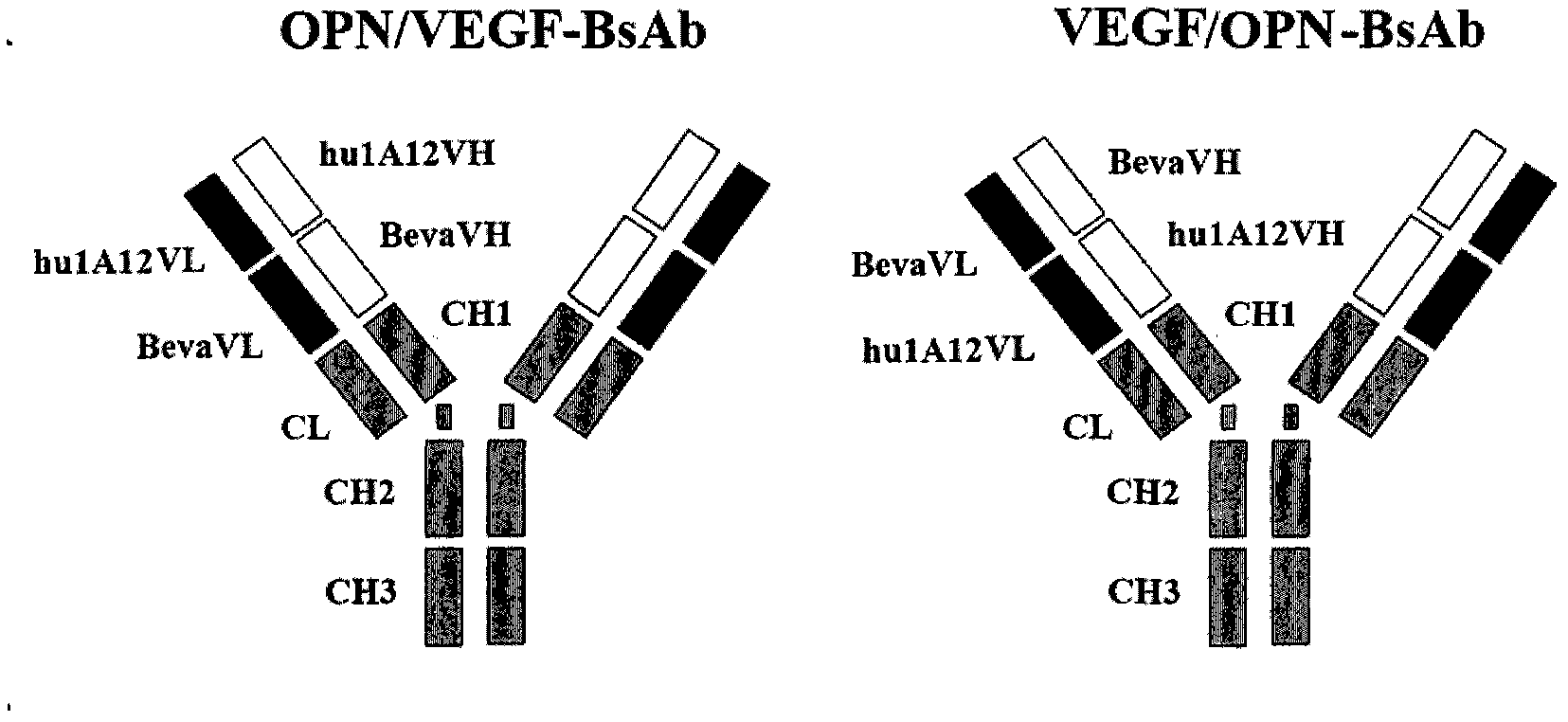
Anti-human VEGF/anti-OPN bispecific antibody, its preparation method and application
ActiveCN102372778AInhibit synthesisImmunoglobulins against growth factorsAntibody ingredientsTherapeutic effectWilms' tumor
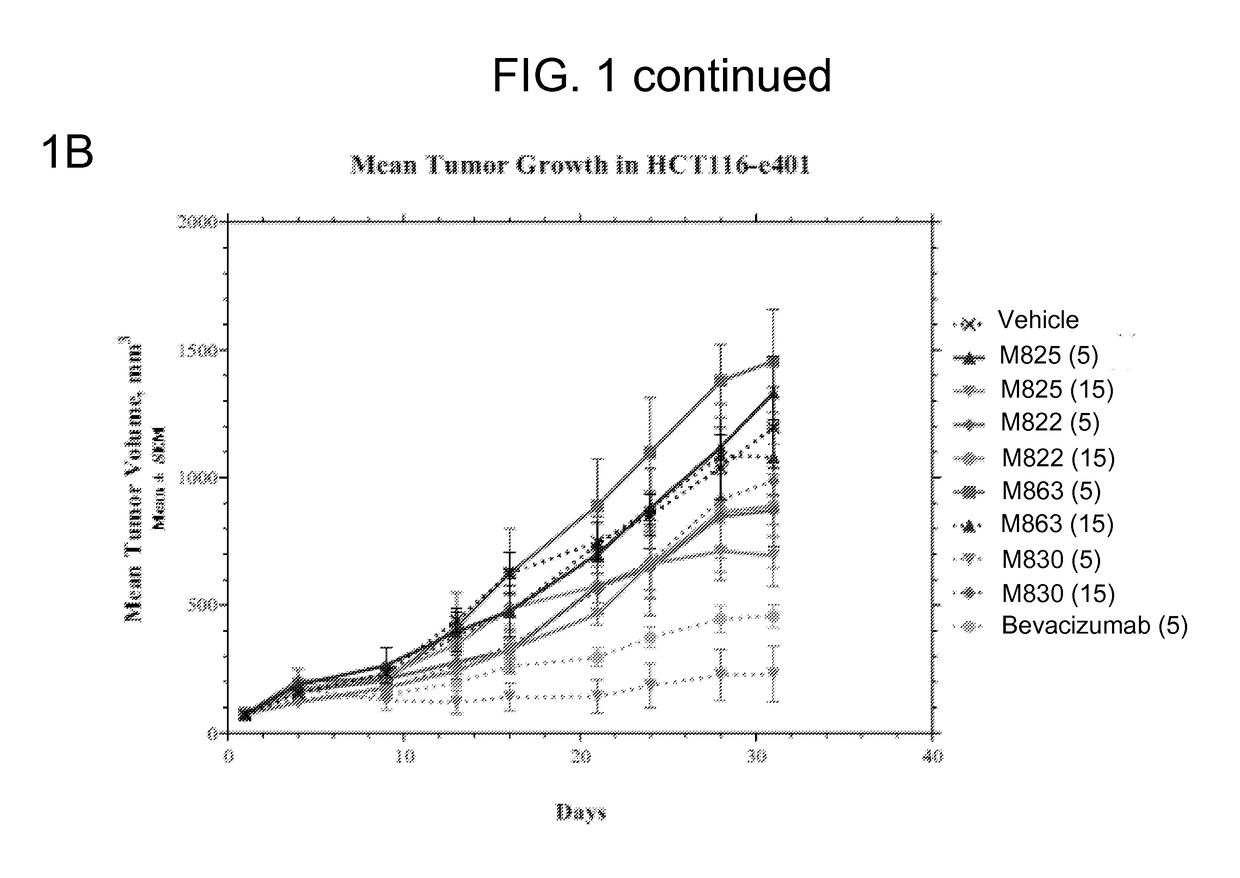
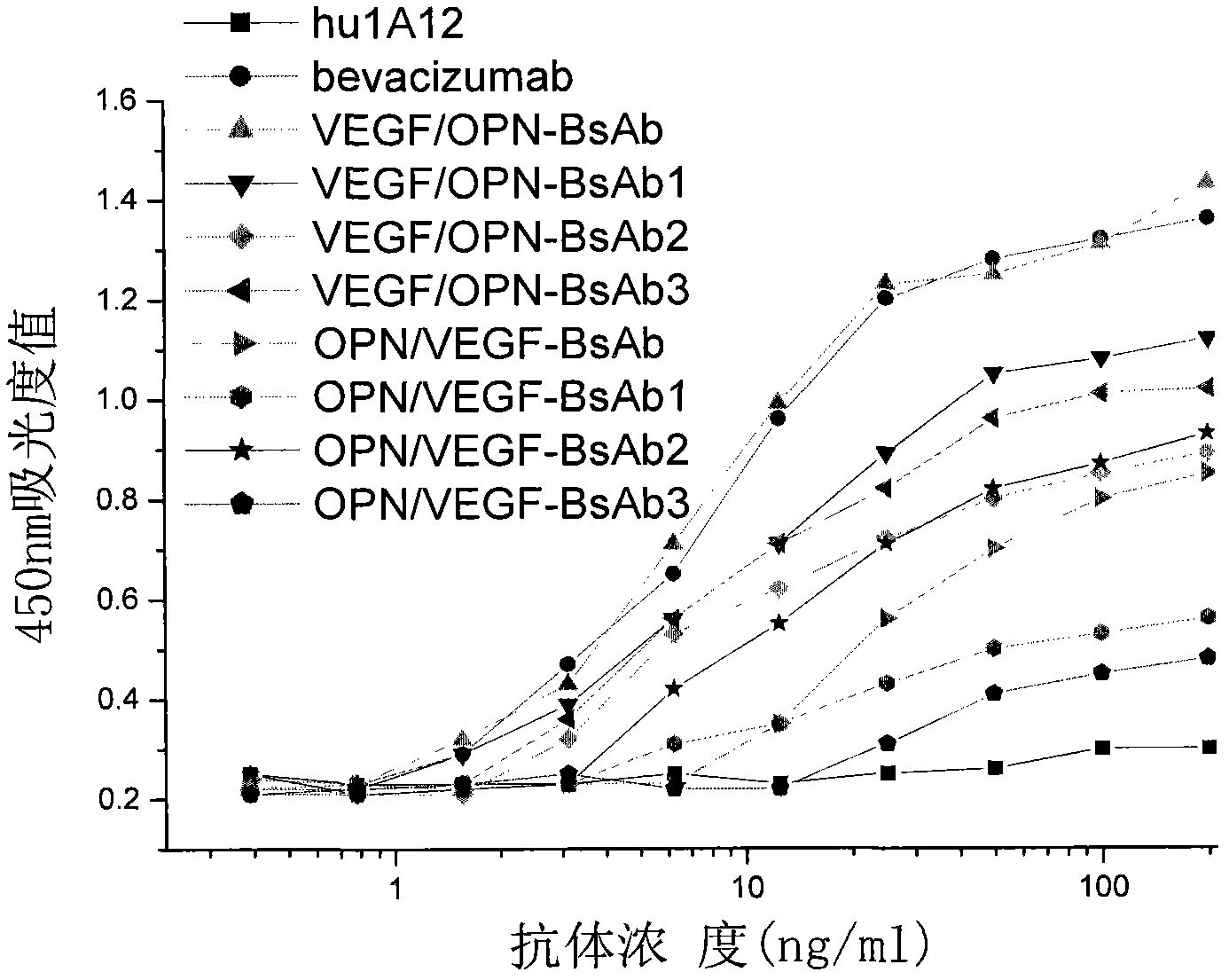
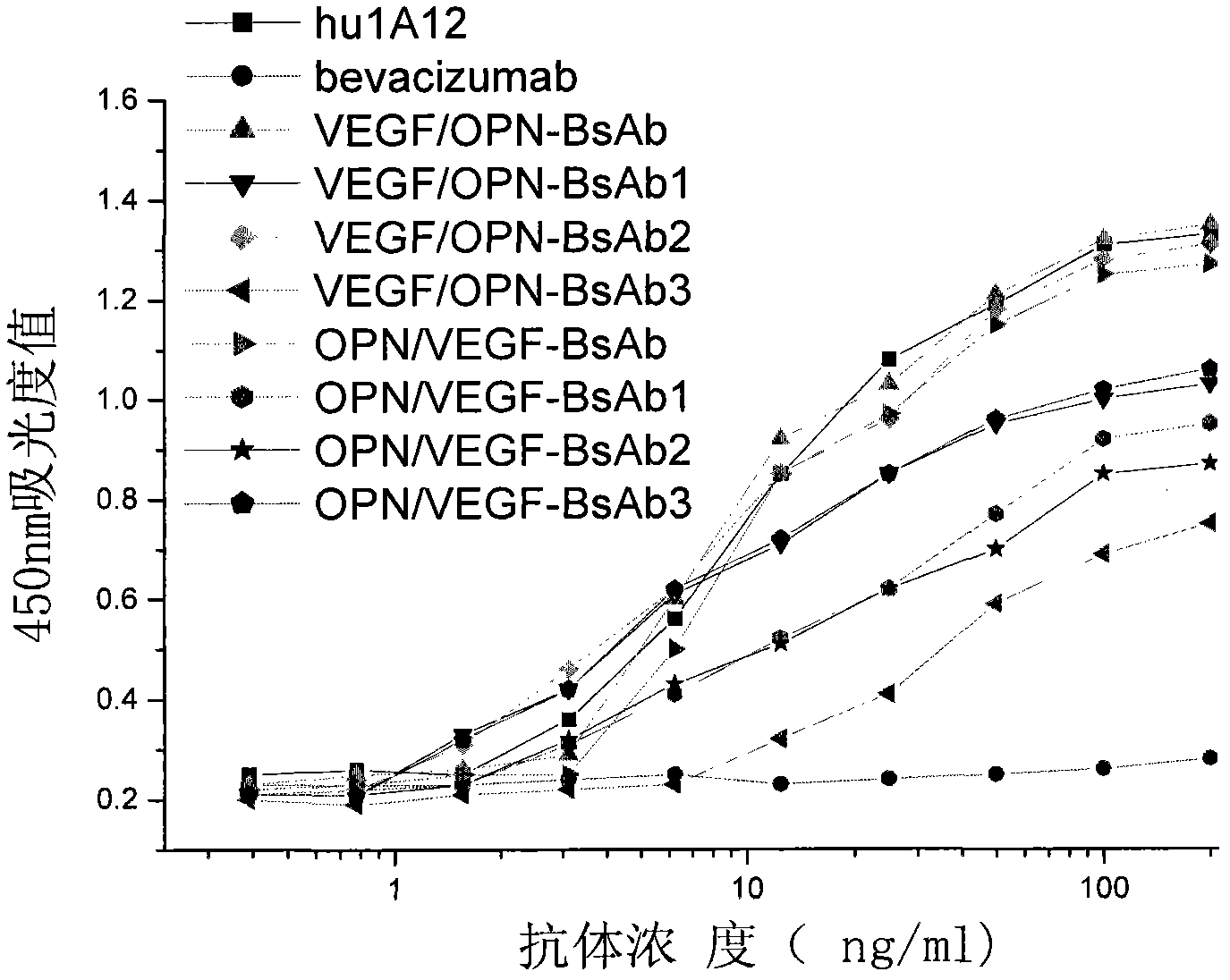
The invention, belonging to the technical field of biology, particularly discloses an anti-VEGF / anti-OPN double variable region IgG molecule like bispecific antibody VEGF / OPN-BsAb, its preparation method and application in preparing antitumor drugs. The antibody disclosed herein has amino acid sequences represented by SEQ ID No:10 and SED ID No:12, can be combined with VEGF and OPN, and has anti-neoplastic treatment effect similar with combing with Bevacizumab and hu1A12.
Owner:SHANGHAI NAT ENG RES CENT OF ANTIBODY MEDICINE
Diagnostic and therapeutic methods for cancer
ActiveUS20190369098A1Organic active ingredientsMicrobiological testing/measurementTyrosine kinaseKidney cancer
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer (e.g., kidney cancer (e.g., renal cell carcinoma (RCC)), lung cancer (e.g., non-small cell lung cancer (NSCLC)), bladder cancer (e.g., urothelial bladder cancer (UBC)), liver cancer (e.g., hepatocellular carcinoma (HCC)), ovarian cancer, or breast cancer (e.g., triple-negative breast cancer (TNBC))). The invention is based, at least in part, on the discovery that expression levels of one or more biomarkers described herein in a sample from an individual having cancer can be used in methods of predicting the therapeutic efficacy of treatment with a VEGF antagonist (e.g., an anti-VEGF antibody, (e.g., bevacizumab) or a VEGFR inhibitor (e.g., a multi-targeted tyrosine kinase inhibitor (e.g., sunitinib, axitinib, pazopanib, or cabozantinib))) and a PD-L1 axis binding antagonist (e.g., a PD-L1 binding antagonist (e.g., anti-PD-L1 antibody, e.g., atezolizumab (MPDL3280A)) or a PD-1 binding antagonist (e.g., anti-PD-1 antibody)), or with an angiogenesis inhibitor (e.g., a VEGF antagonist (e.g., a VEGFR inhibitor, (e.g., a multi-targeted tyrosine kinase inhibitor (e.g., sunitinib, axitinib, pazopanib, or cabozantinib)))).
Owner:GENENTECH INC
Pharmacokinetic mass spectrometry method of monoclonal antibody medicine
InactiveCN105572265AEase of handlingSimple methodComponent separationLiquid chromatography mass spectroscopyTandem mass spectrometry
The invention provides a pharmacokinetic mass spectrometry method of a monoclonal antibody medicine and belongs to the technical field of proteomics. With the adoption of the technical scheme, the pharmacokinetic mass spectrometry method comprises the following steps: selecting a specific peptide fragment obtained by hydrolyzing Bevacizumab with a pancreatic enzyme, wherein the specificity means that the peptide fragment is generated by only hydrolyzing the Bevacizumab with the pancreatic enzyme, and the peptide fragment cannot be generated by hydrolyzing other proteins and protein type medicines through the pancreatic enzyme; based on a liquid chromatograph-tandem mass spectrometry technology, scanning and analyzing the specific peptide fragment by using a parallel reaction monitoring mode, and establishing a stable and reliable LC / MS / MS quantifying method of the specific peptide fragment. The pharmacokinetic mass spectrometry method of the monoclonal antibody medicine has the characteristics of high flux, high sensitivity, good repeatability and the like; used reagents and medicines are low in price and easy to obtain and a preparation method is simple, so that the popularization of the method is facilitated; a sample treatment process and a quantifying method are simple and feasible, and the method has guide and reference meanings on qualitative and quantitative researches on the other protein type medicines and polypeptide medicines.
Owner:JILIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com