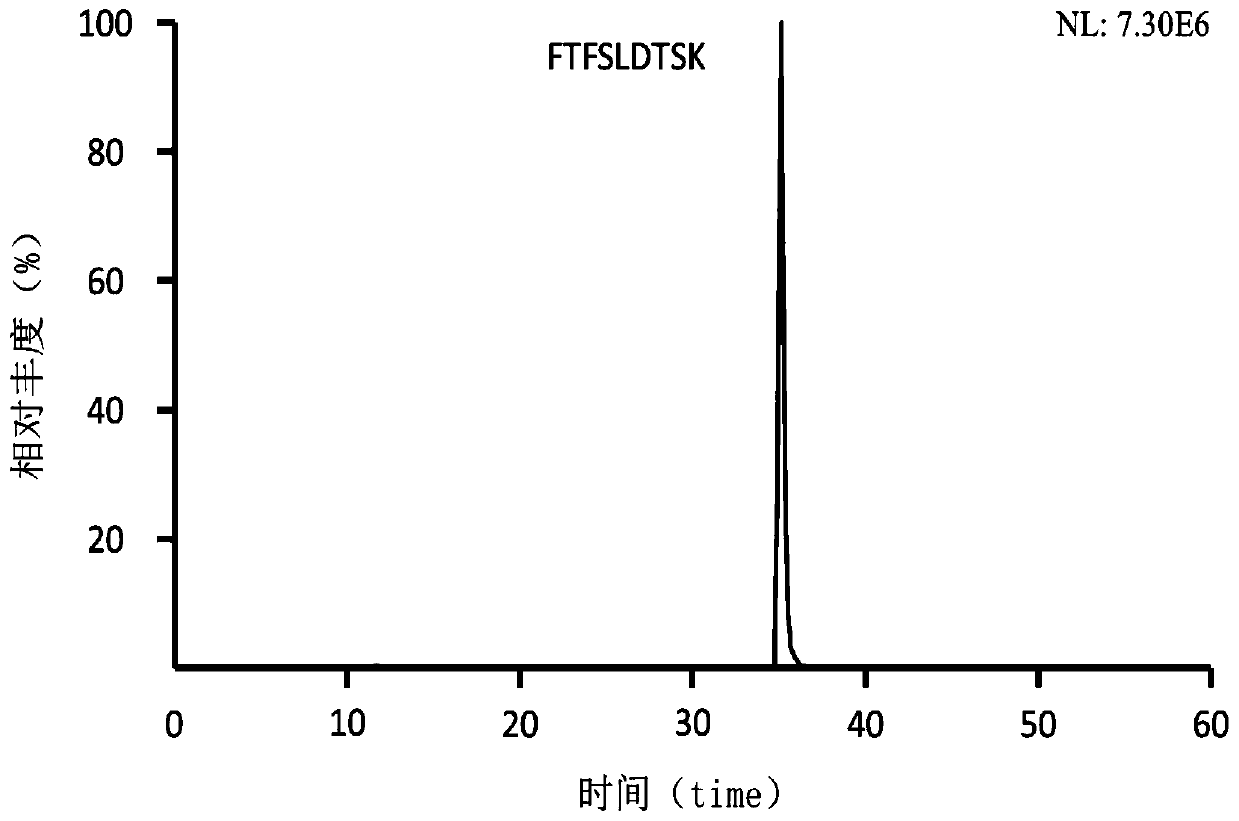
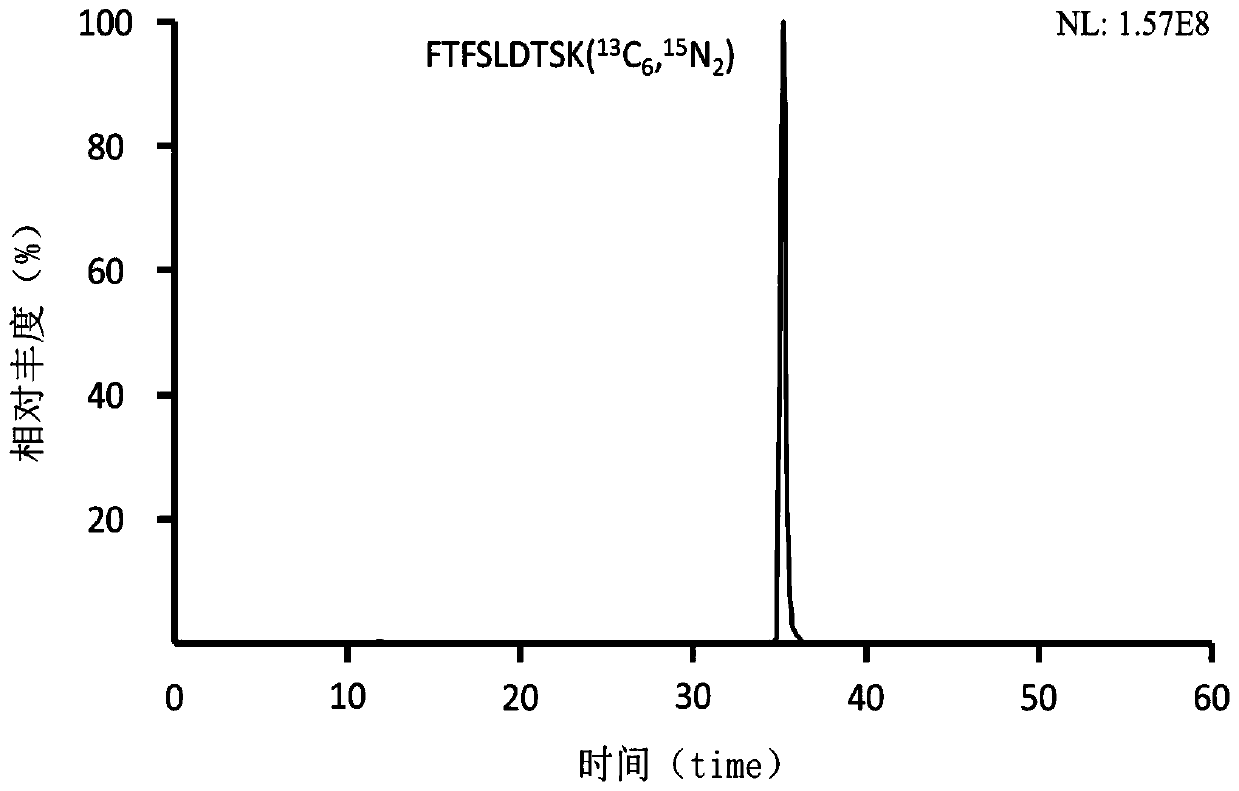
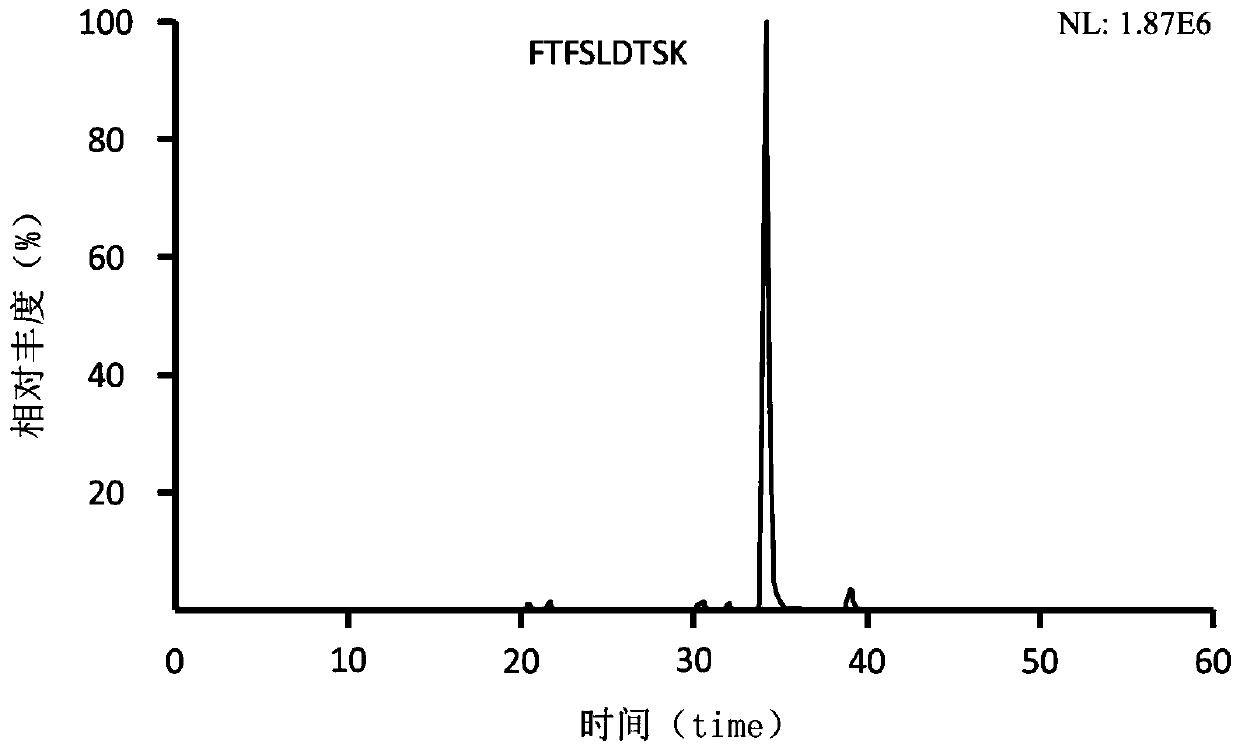
Preclinical pharmacokinetic study mass spectrometry analysis method of bevacizumab
A technology of pharmacokinetics and mass spectrometry, applied in the analysis of materials, scientific instruments, material separation, etc., can solve the problems of inability to distinguish endogenous protein interference, long development cycle, narrow quantitative range, etc., to achieve sample processing and The quantitative method is simple and easy to implement, which is conducive to the promotion of the method and the effect of the simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Analysis of monoclonal antibody drugs in rat plasma samples
[0042] Part 1: intravenous administration in rats
[0043] A. Rat intravenous administration process
[0044] (1) Choose a male rat with a body weight of 200 g±10 g and eat freely;
[0045] (2) Purchase the commercially available monoclonal antibody drug Bevacizumab injection with a concentration of 25 mg / mL;
[0046] (3) According to the clinical dosage conversion, the rat monoclonal antibody drug was given by intravenous injection at a dose of 50 mg / kg;
[0047] B. Preparation of rat plasma samples
[0048] Select different time points (0, 30 min, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h, 32 h), collect rat whole blood from vein, centrifuge at 13000 g for 5 min , Store in a refrigerator at -80℃.
[0049] Part 2: Determination of the monoclonal antibody Bevacizumab in rat plasma samples
[0050] A. Plasma sample pretreatment
[0051] (1) Reduction of plasma sample, add 2 mL rat plasma sample into polyethylene tube, add 9...
Embodiment 2
[0073] Example 2 Preparation of rat plasma standard curve sample
[0074] First configure the monoclonal antibody standard series solution: Dilute the monoclonal antibody standard solution stock solution (25 mg / mL) to 5, 10, 40, 100, 500, 1000, 1200 ng / mL with deionized water.
[0075] Then prepare the rat plasma standard curve sample and proceed in the following 7 steps:
[0076] (1) Reduction of rat plasma standard curve samples. Add 2 mL of monoclonal antibody standard series solution to polyethylene tube, add 2 mL of rat blank plasma, vortex and mix, and add 94 mL of pH buffer containing denaturant , Vortex and mix, add 2 mL reducing agent, vortex and mix, and incubate at 60°C for 1 h to obtain a reaction solution;
[0077] (2) For cysteine blocking, add 0.75 mg of solid cysteine blocking agent to the reaction solution, vortex and mix; incubate at 25°C in the dark for 40 min;
[0078] (3) Trypsin digestion of rat plasma standard curve samples, add 700 mL digestion buffer to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com