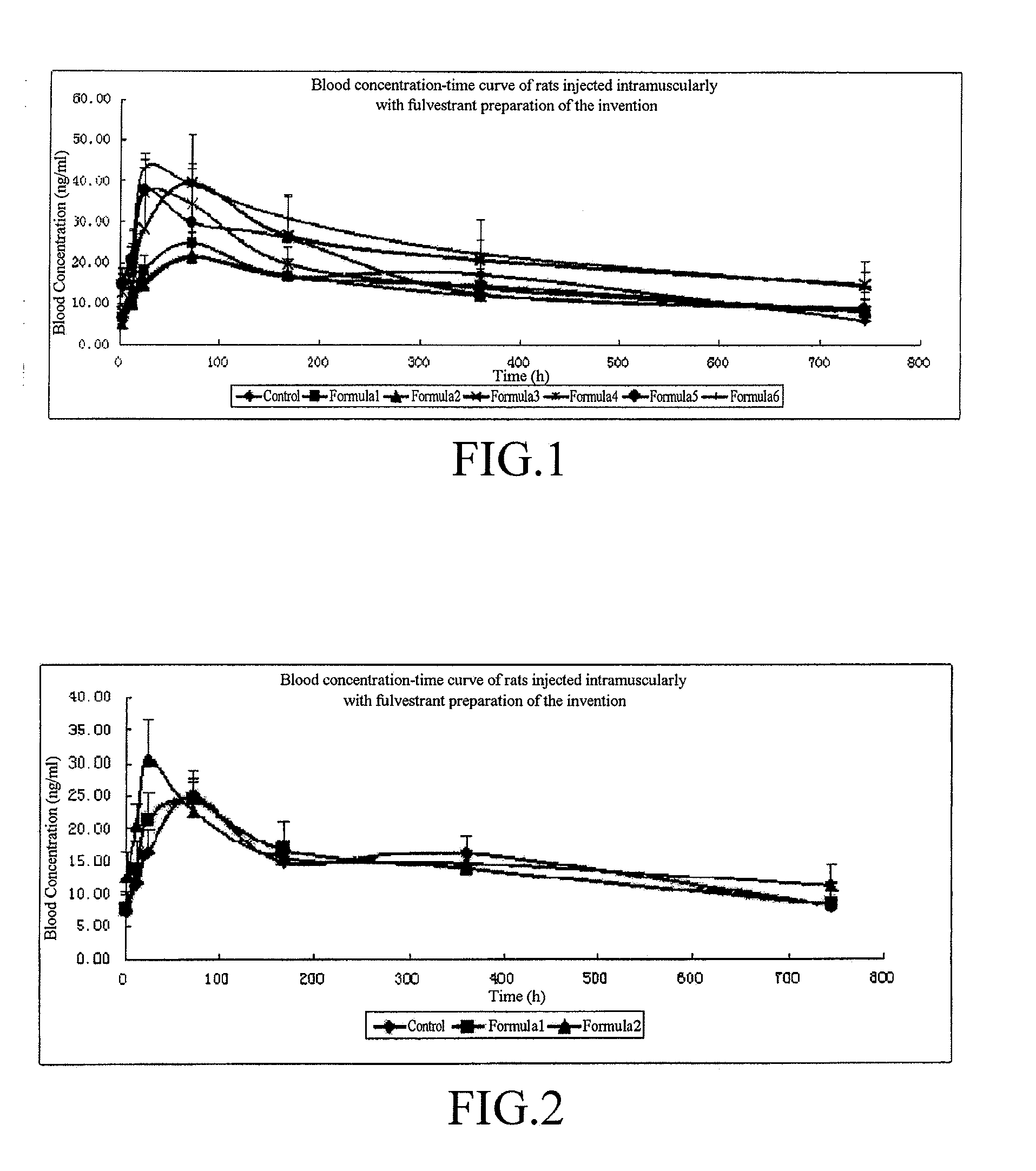
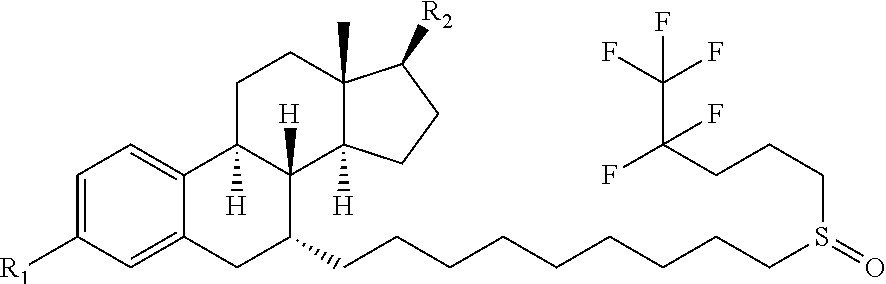
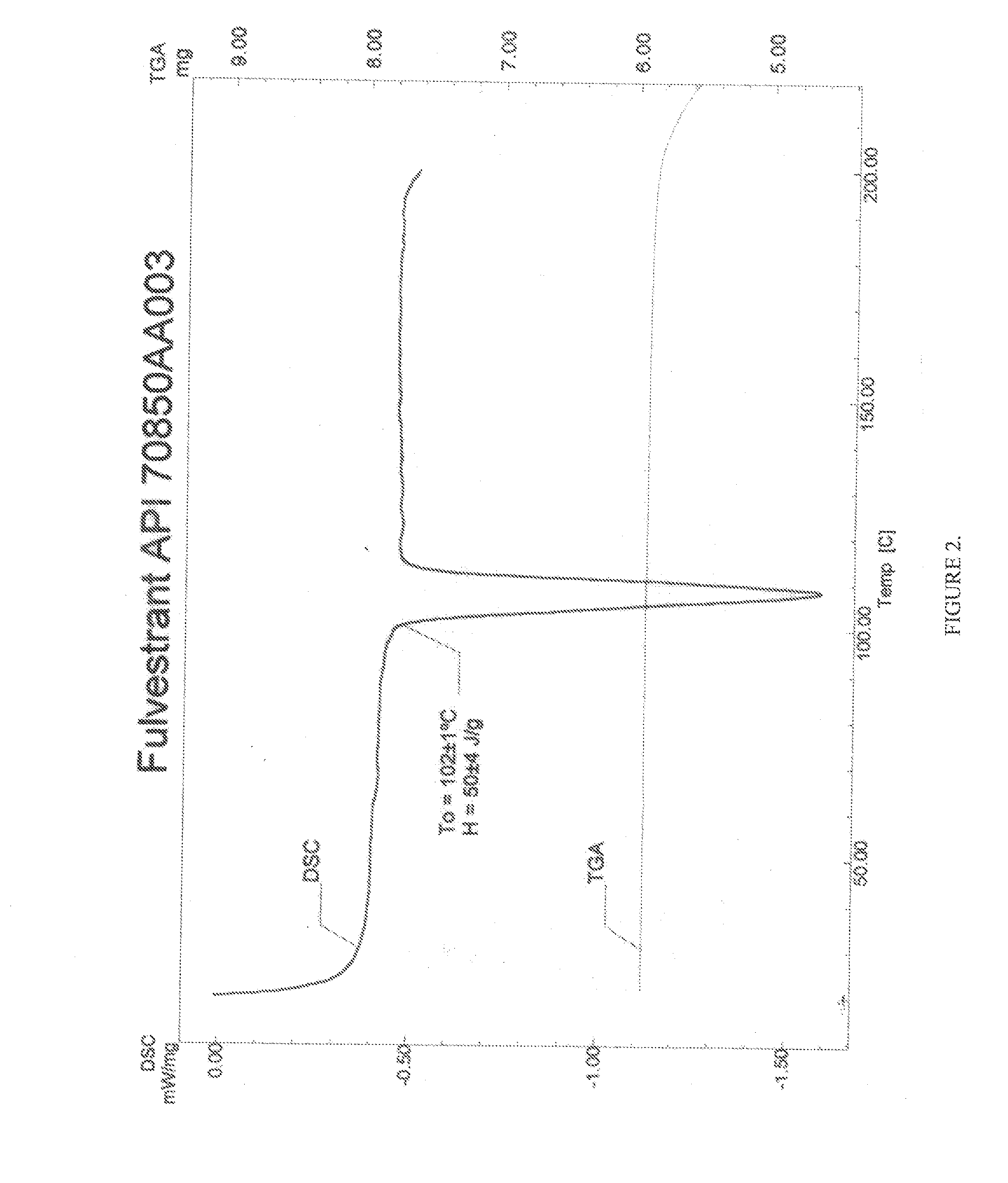
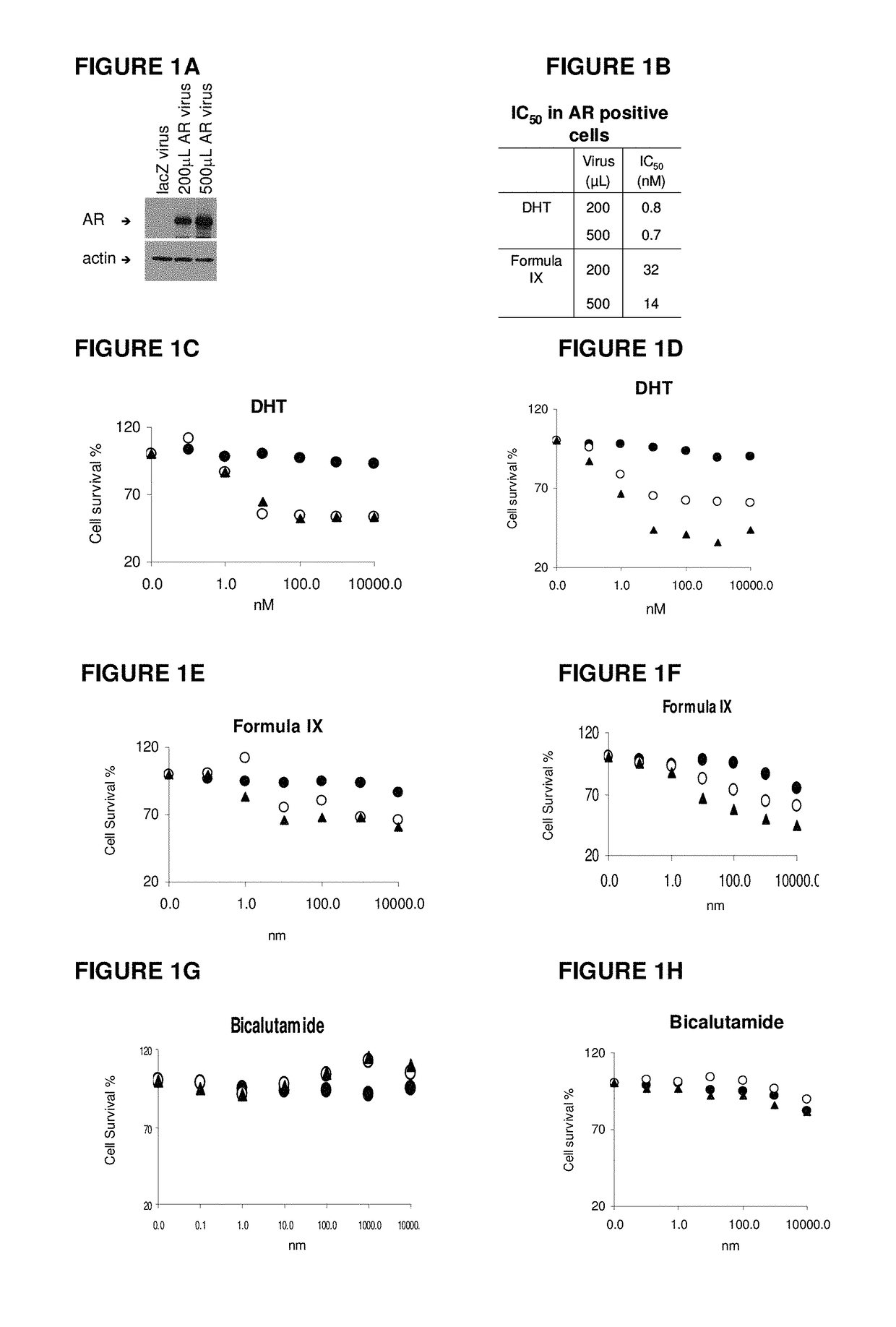
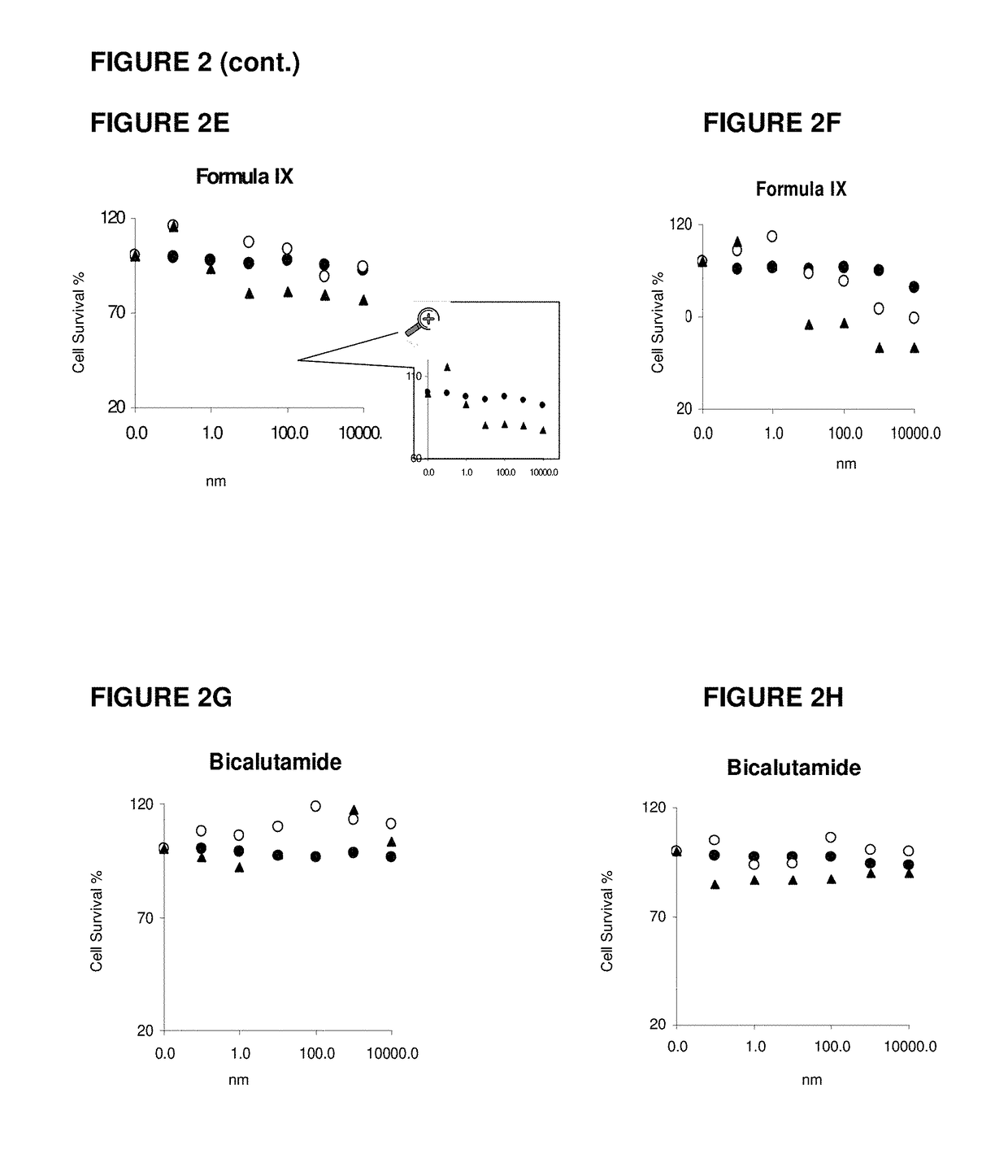
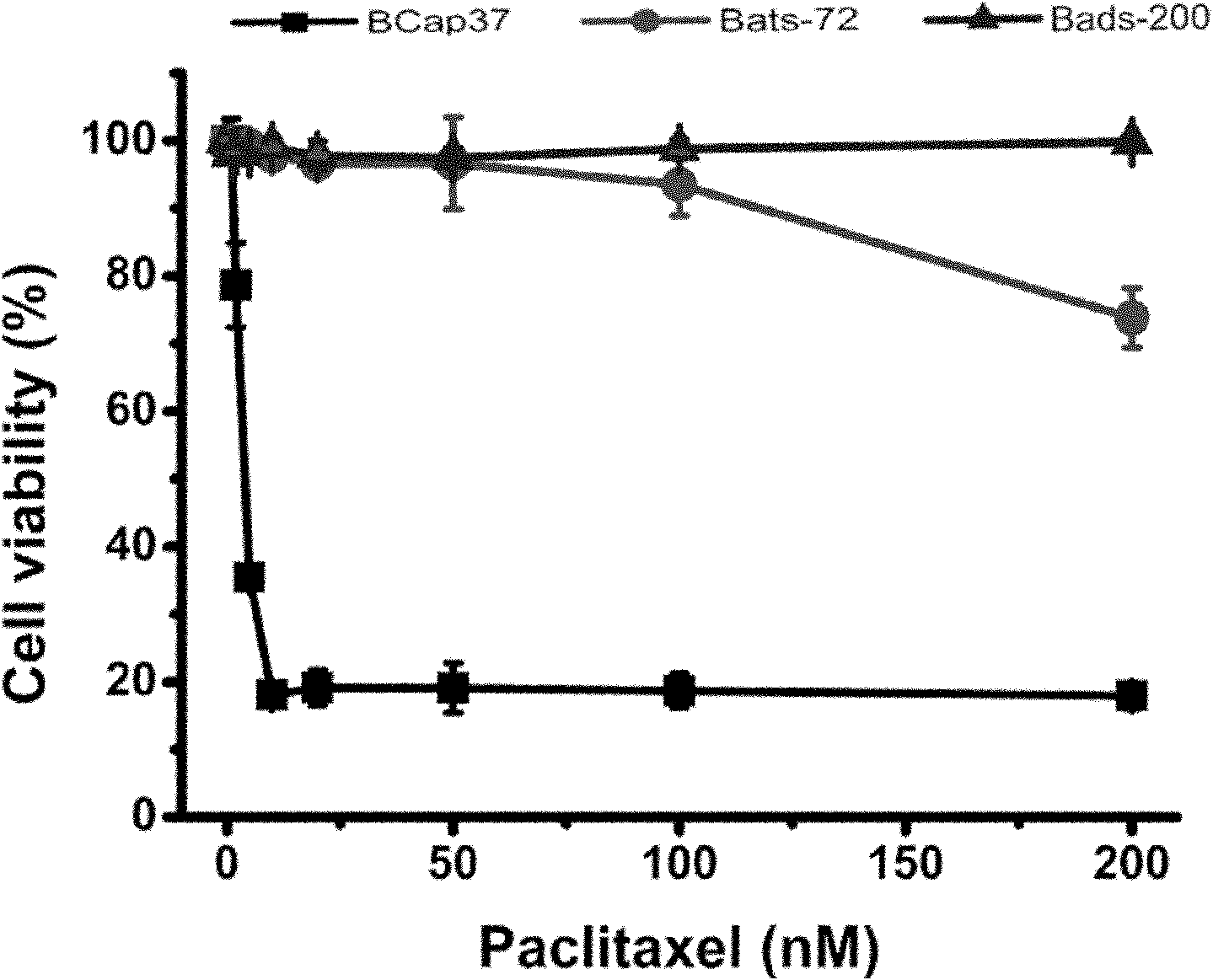
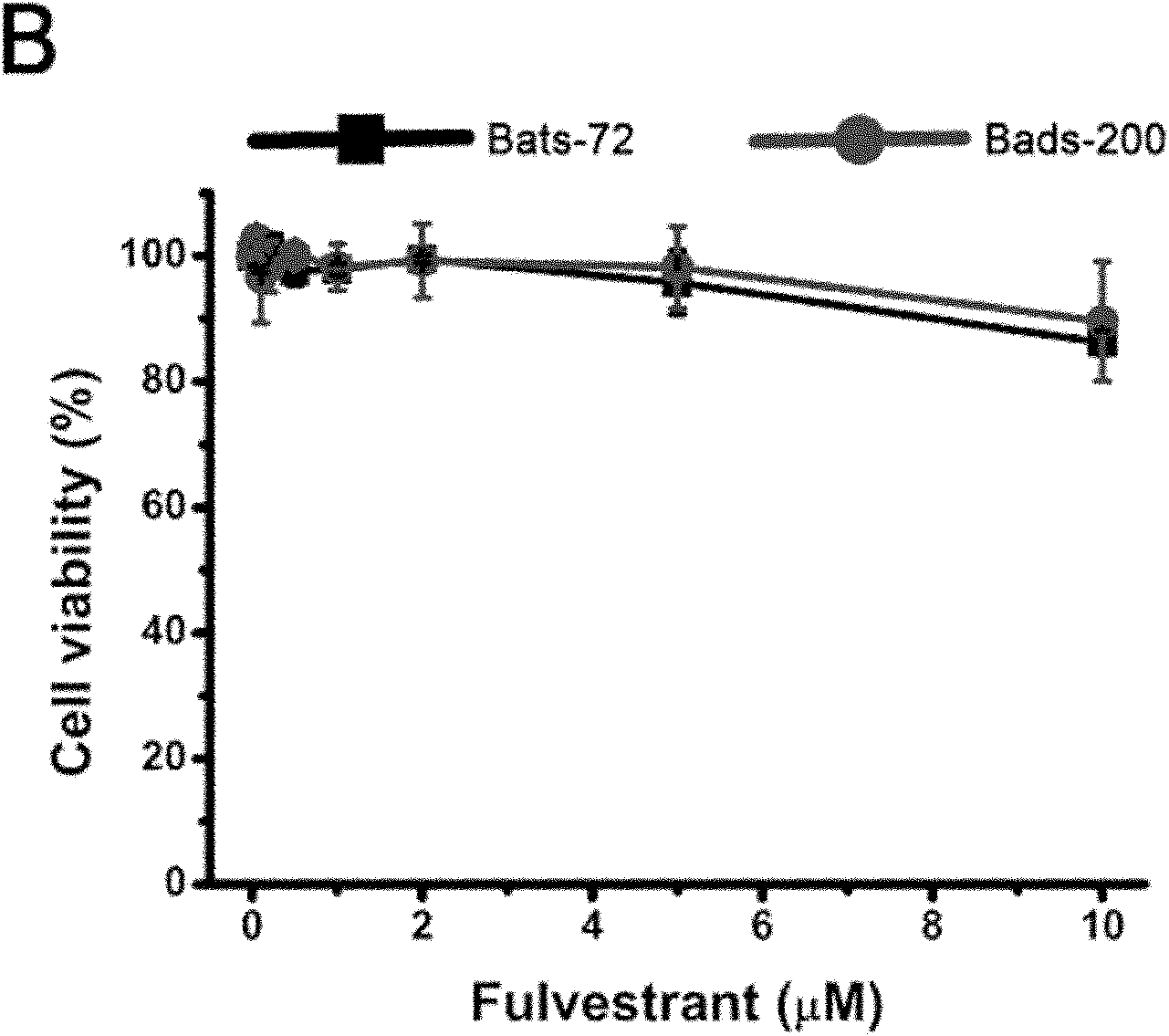
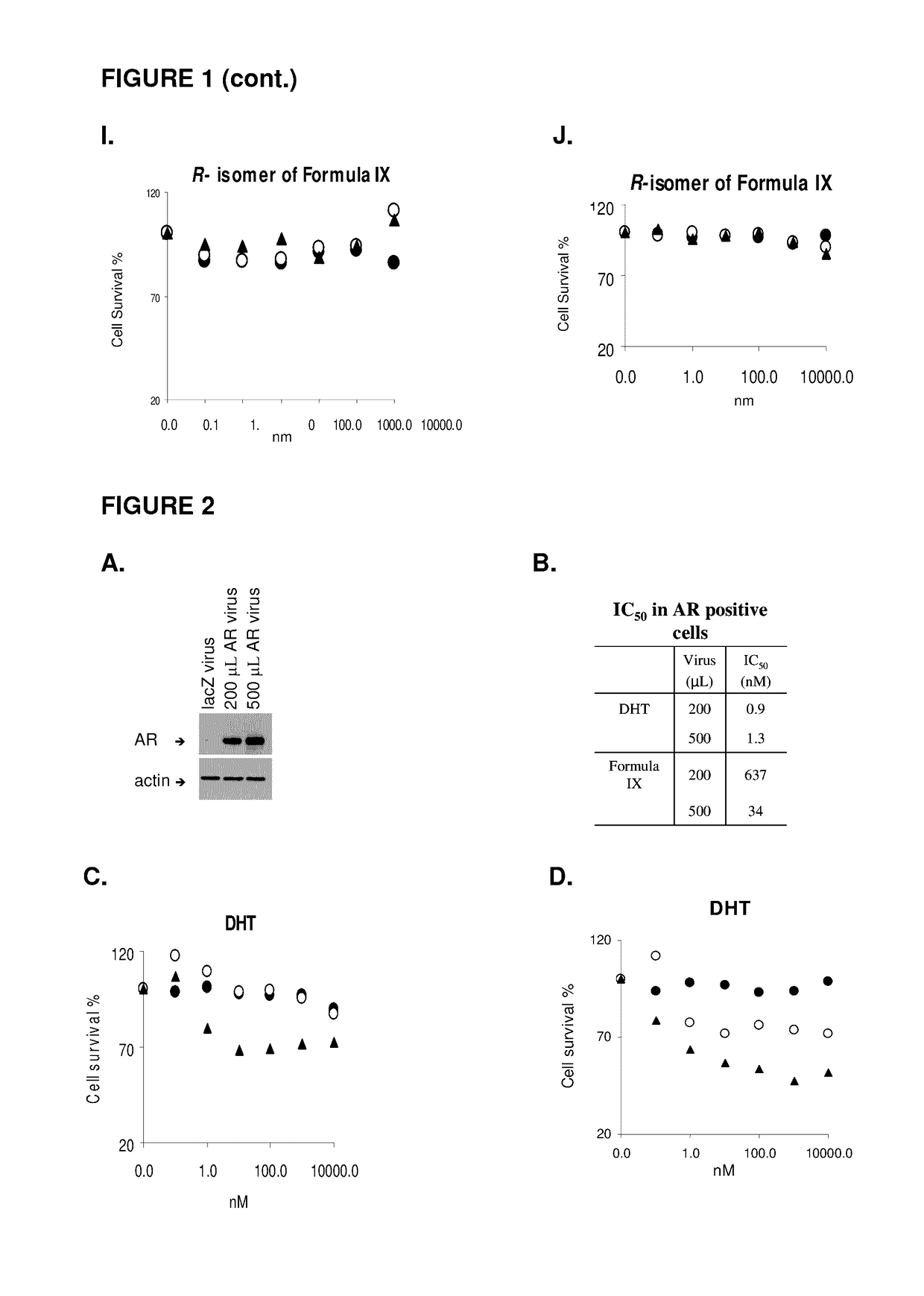
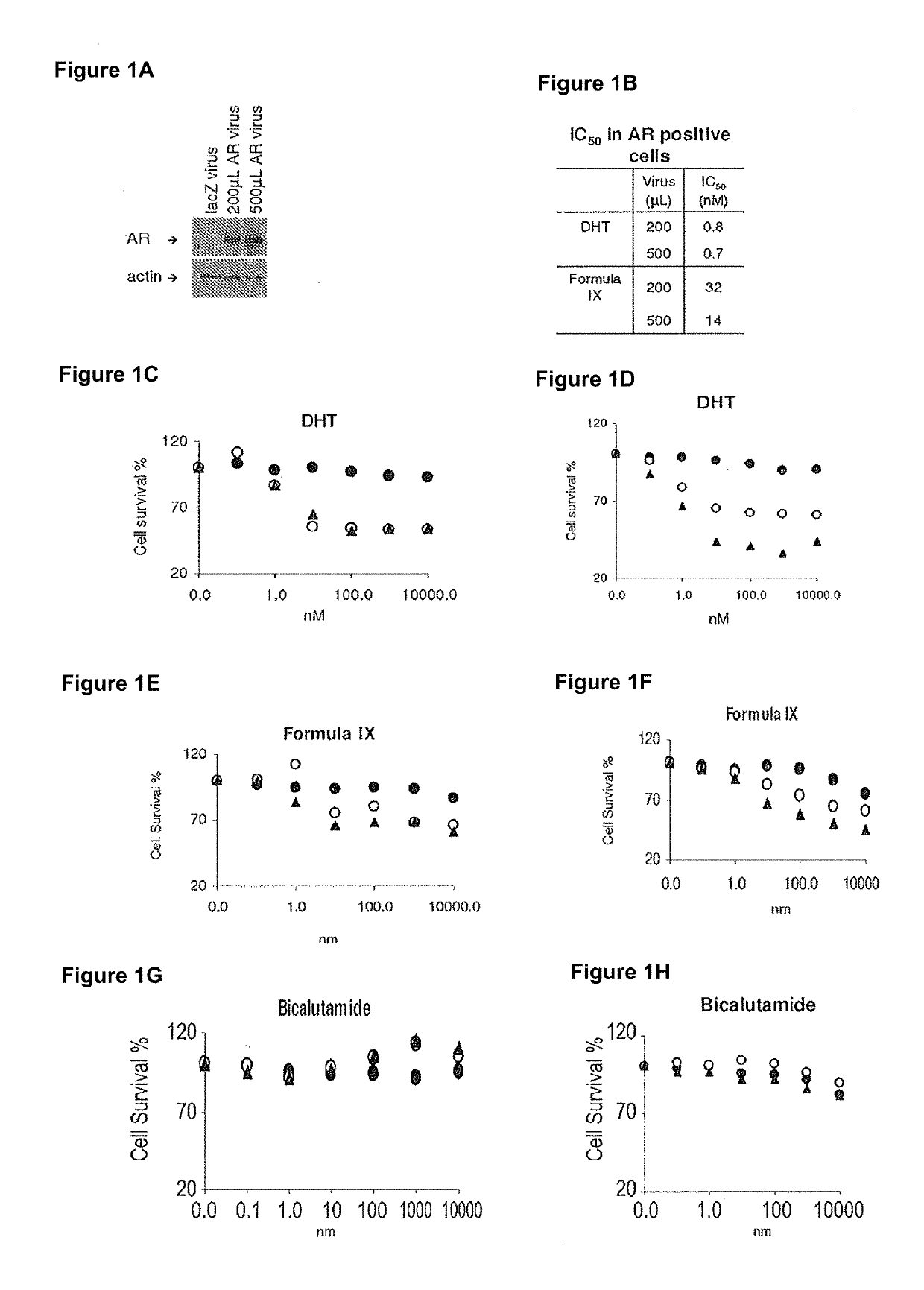
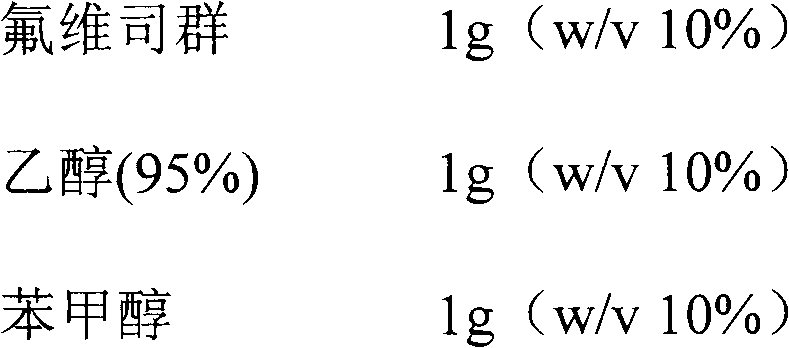Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
78 results about "Fulvestrant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
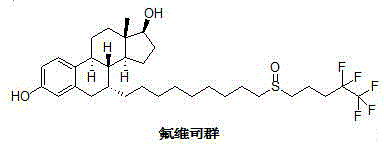
Fulvestrant is used to treat certain types of breast cancer.
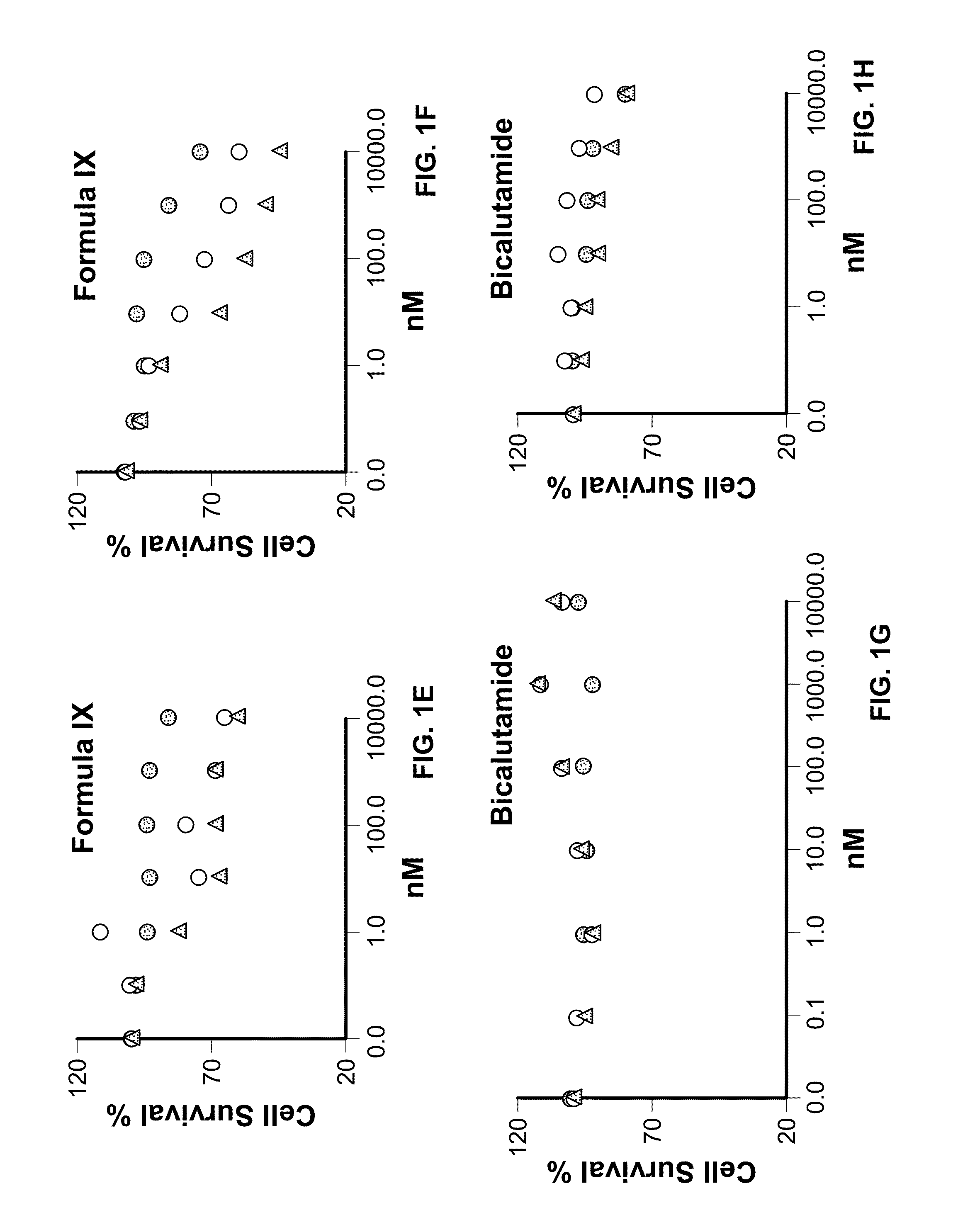
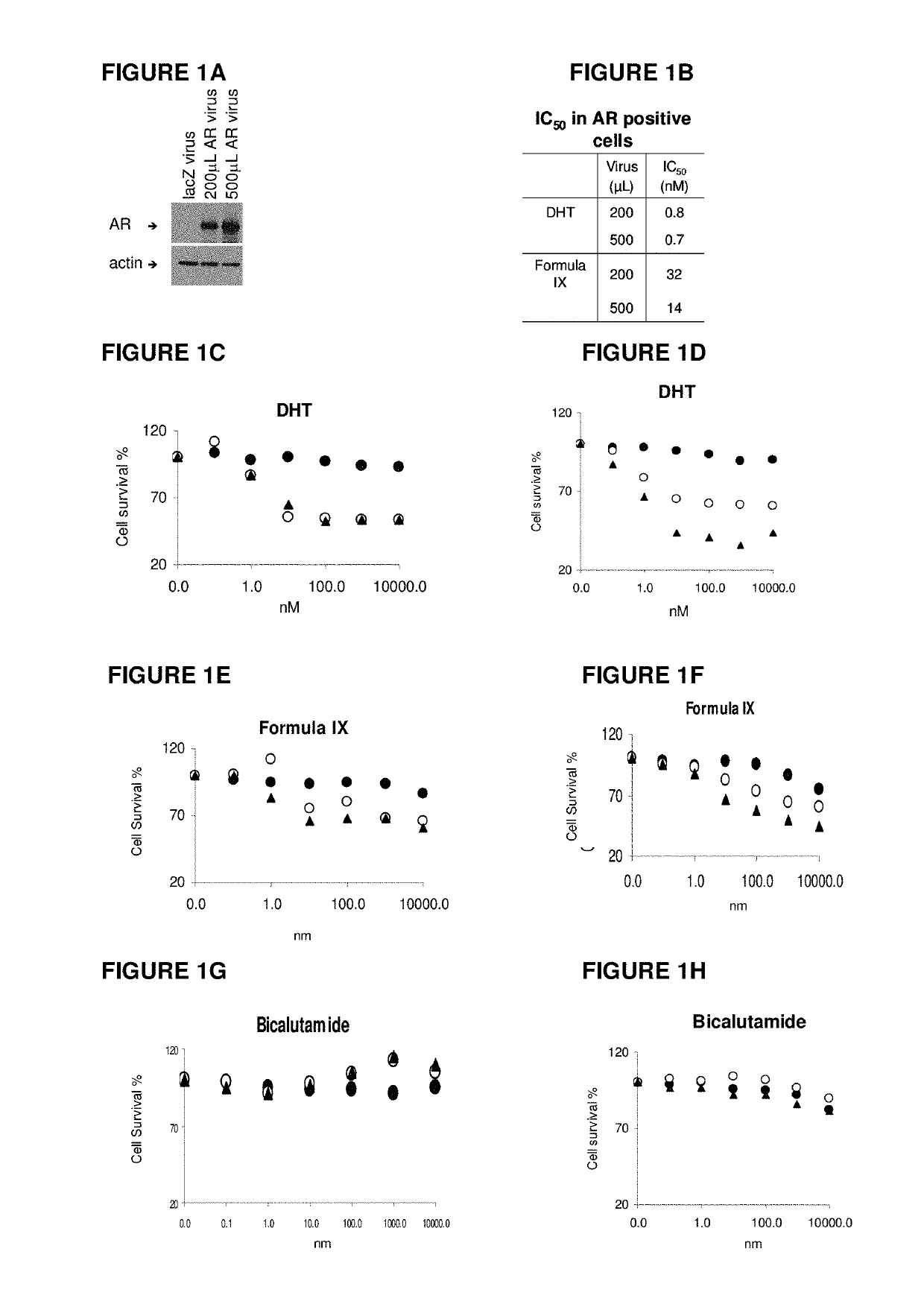
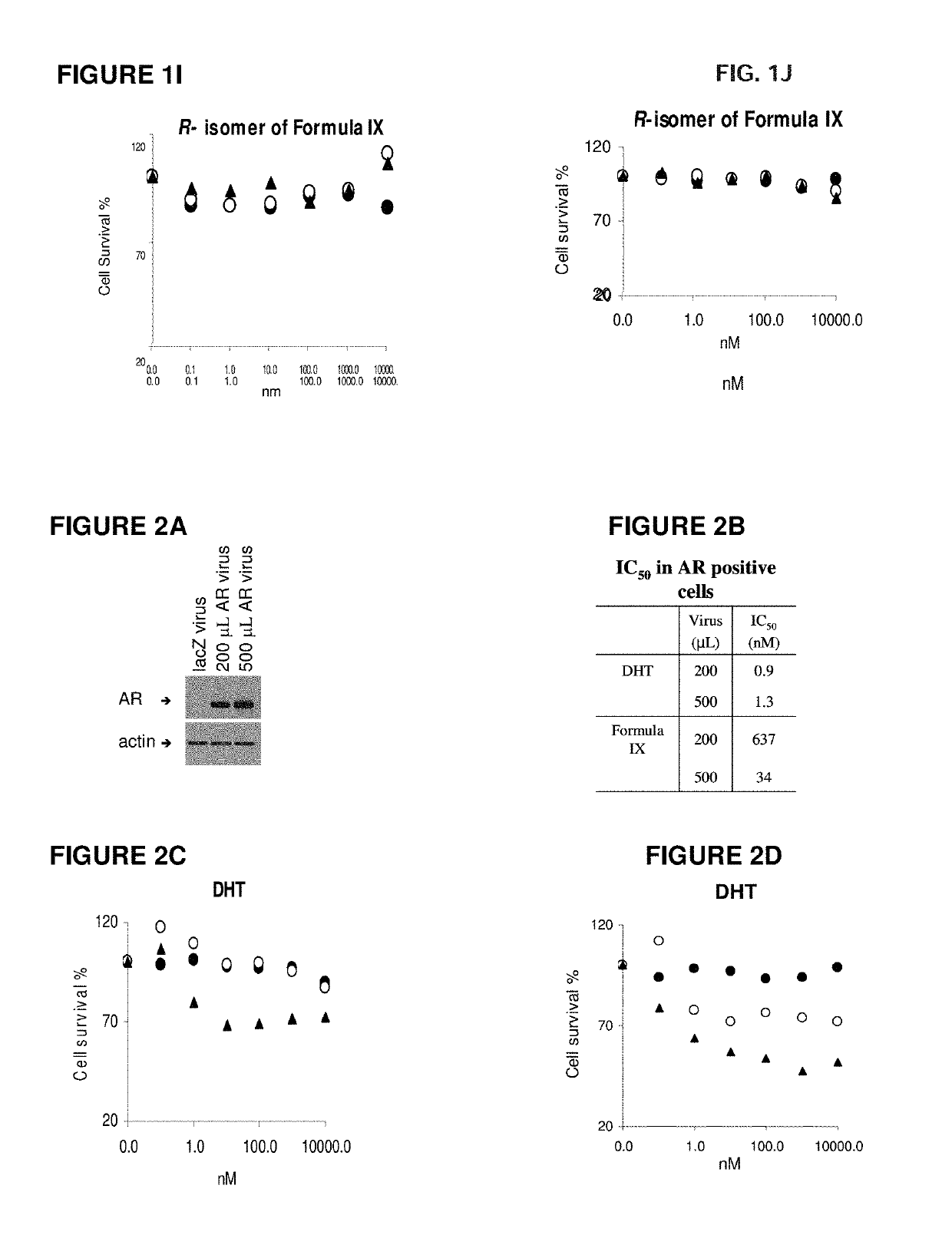
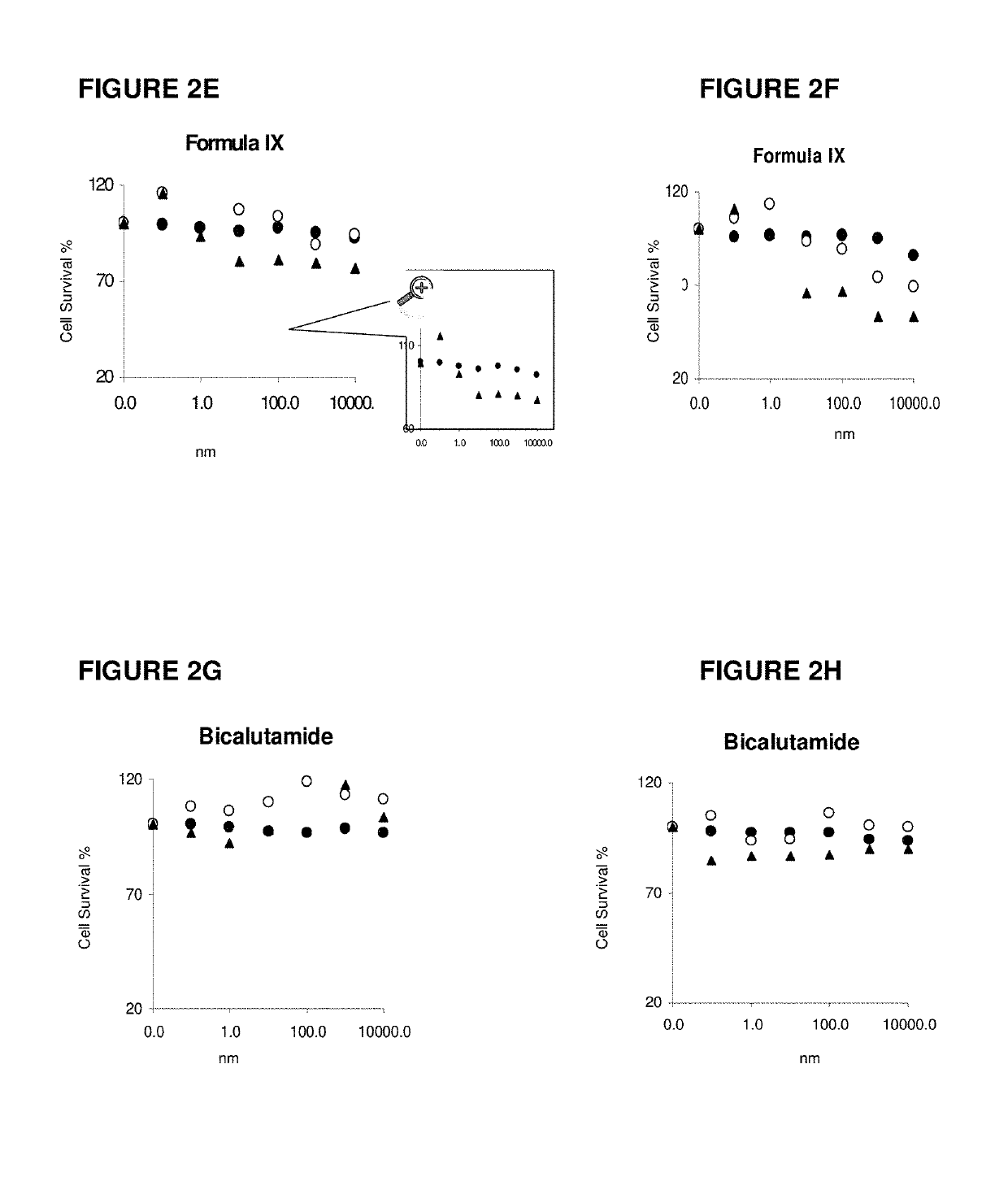
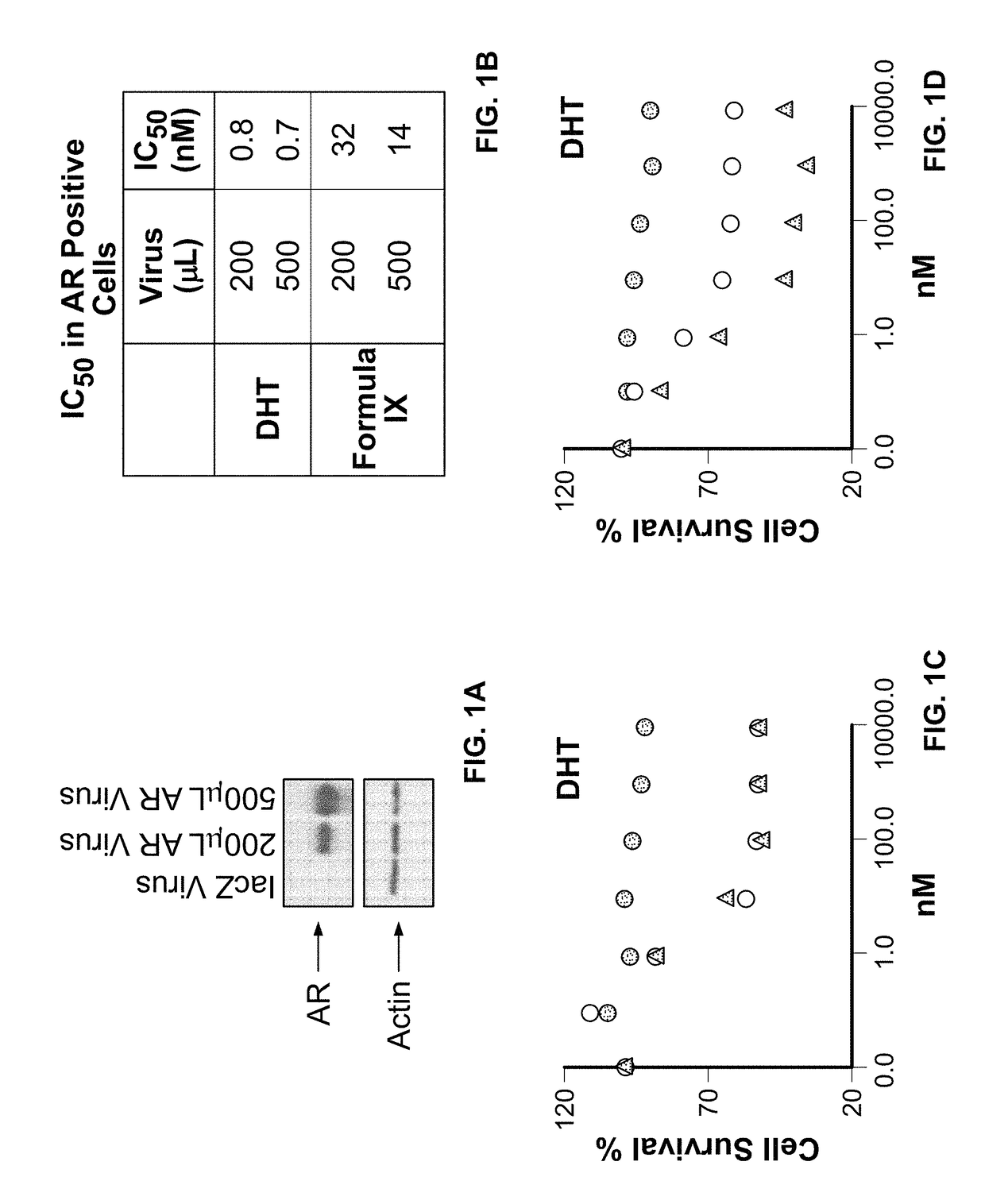
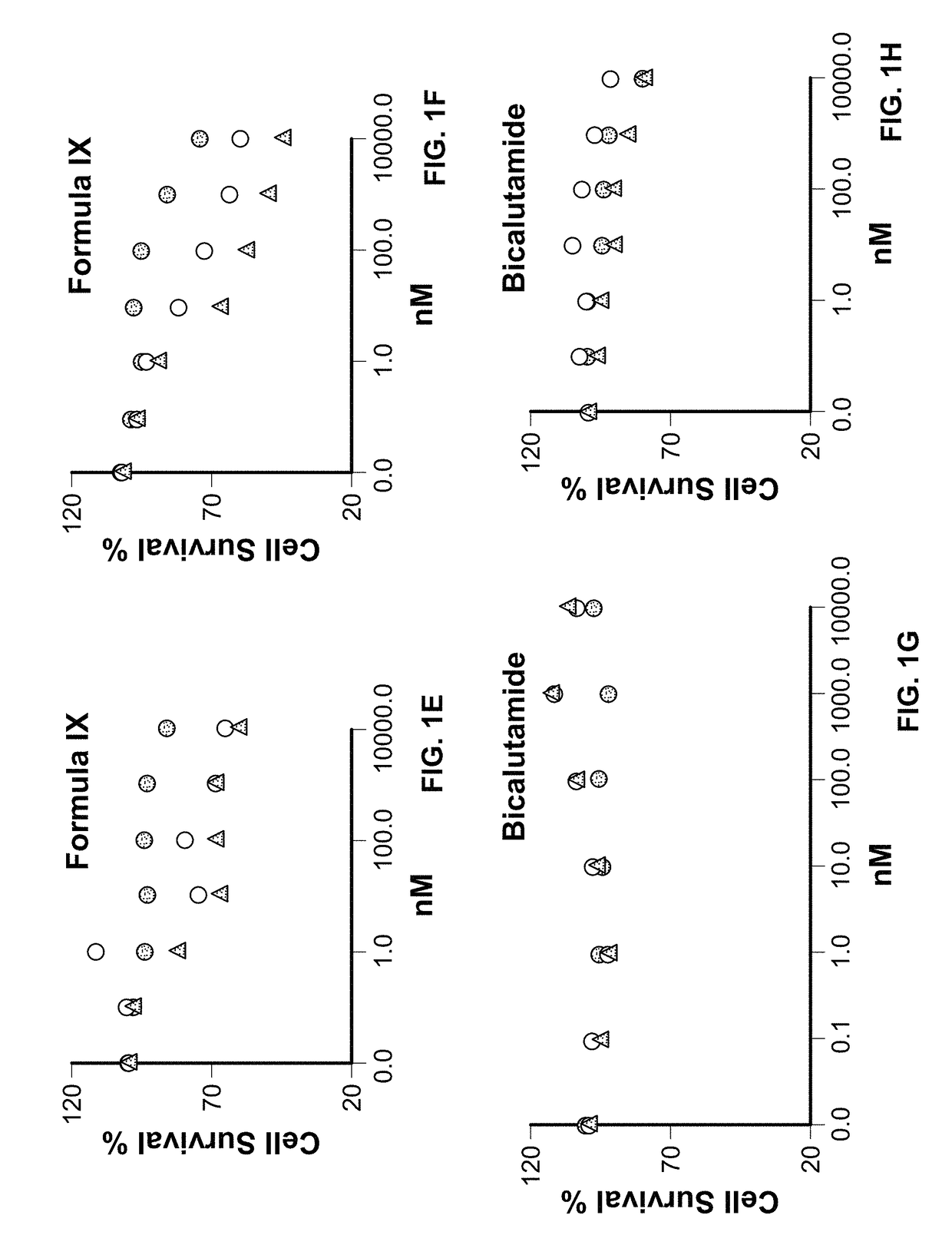
Method of treating estrogen receptor (ER) -positive breast cancers with selective androgen receptor modulator (SARMS)
This invention relates to the treatment of androgen receptor-positive breast cancer in a subject, for example a female subject. Accordingly, this invention provides methods of: a) treating a subject suffering from breast cancer; b) treating a subject suffering from metastatic breast cancer; c) treating a subject suffering from refractory breast cancer; d) treating a subject suffering from AR-positive breast cancer; e) treating a subject suffering from AR-positive refractory breast cancer; f) treating a subject suffering from AR-positive metastatic breast cancer; g) treating a subject suffering from AR-positive and ER-positive breast cancer; h) treating a subject suffering from triple negative breast cancer; i) treating a subject suffering from advanced breast cancer; j) treating a subject suffering from breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; k) treating, preventing, suppressing or inhibiting metastasis in a subject suffering from breast cancer; l) prolonging survival of a subject with breast cancer, and / or m) prolonging the progression-free survival of a subject with breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound, comprising administering to the subject a therapeutically effective amount of a SARM compound of this invention.
Owner:UNIV OF TENNESSEE RES FOUND
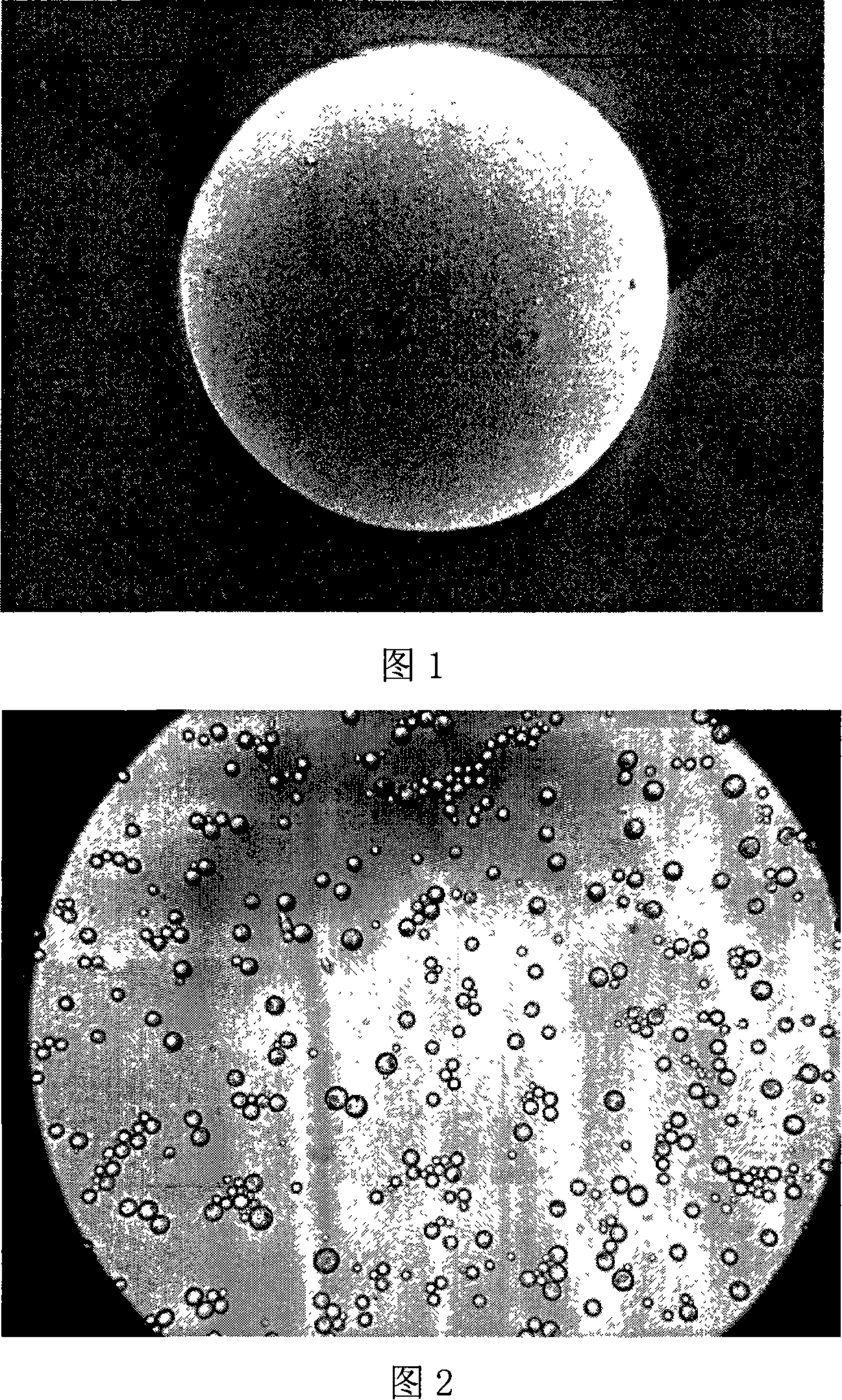
Method of manufacturing fulvestrant sustained-release microspheres
InactiveCN101108168ARound shapeUniform particle size distributionPowder deliveryOrganic active ingredientsMicrospherePolyvinyl alcohol
The invention discloses a faslodex controlled-release microballoon preparation method. The faslodex controlled-releasemicroballoon in the invention envelopes fulvestrant takes PLA or PLGA or PCL or other biodegradable materials as the carrier materials. Besides, the invention takes PVA or the miscible liquids of PCL and Tween 80 as the disperse medium. Then, with the emulsion solvent evaporation method, finish the preparation of fulvestrant controlled-release microballoon under the action of mechanical mixing or high-speed shearing. The microballoon has spherical shape and even distribution of grain size that is controllable within the scope of 15 to 125Mu m as well as reaches a drug-loading rate of over 7 per cent and encapsulation rate of over 80 per cent.
Owner:XIAN LIBANG PHARMA TECH
Pharmaceutical composition of fulvestrant
ActiveCN103070871AImprove solubilityMedication convenienceOrganic active ingredientsPharmaceutical non-active ingredientsAlcoholMedium-chain triglyceride
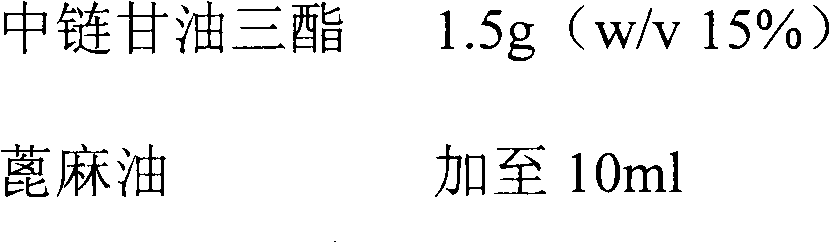
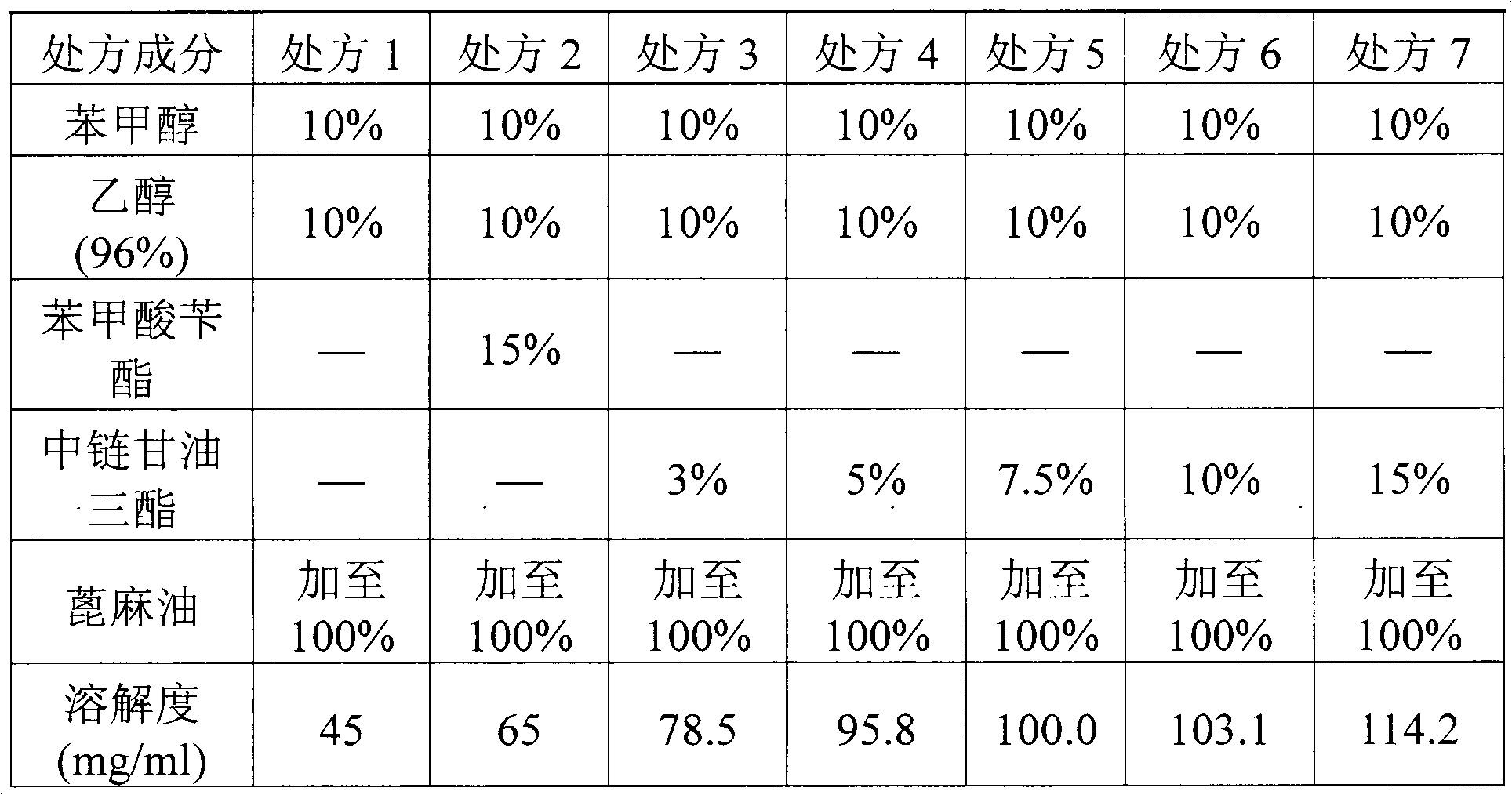
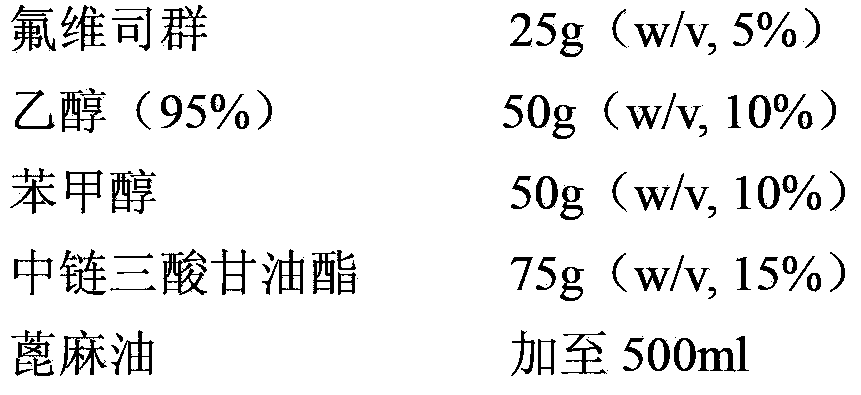
The invention relates to a pharmaceutical composition of fulvestrant. The pharmaceutical composition comprises: (1) fulvestrant; (2) at least one pharmaceutically acceptable alcohol; (3) medium-chain triglyceride; and (4) a castor oil matrix.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Fulvestrant compositions
InactiveUS20170027958A1Improve solubilityImprove stabilityOrganic active ingredientsOintment deliverySubject matterVitamin
The inventive subject matter provides ready to inject fulvestrant compositions with improved solubility and stability, and methods for preparing the same. Contemplated compositions include fulvestrant at a concentration of greater than 100 mg / ml, and maintain degradation of the fulvestrant at a level of less than 5 wt % when stored over at least three months at 25° C.
Owner:THEMIS MEDICARE LTD +1
Preparation method of fulvestrant
InactiveCN103788164AHigh yieldEasy to prepareSteroidsBulk chemical productionProtecting groupFulvestrant
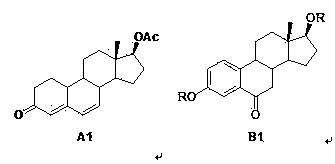
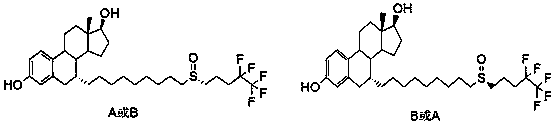
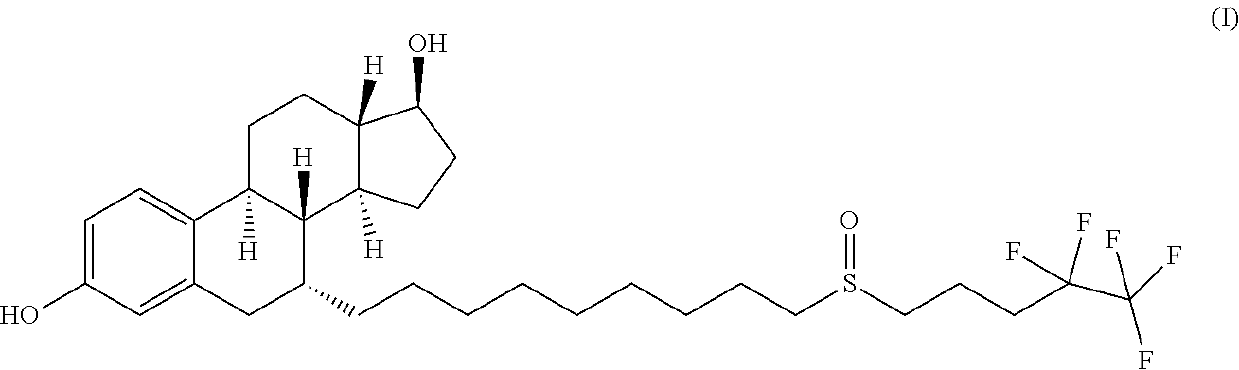
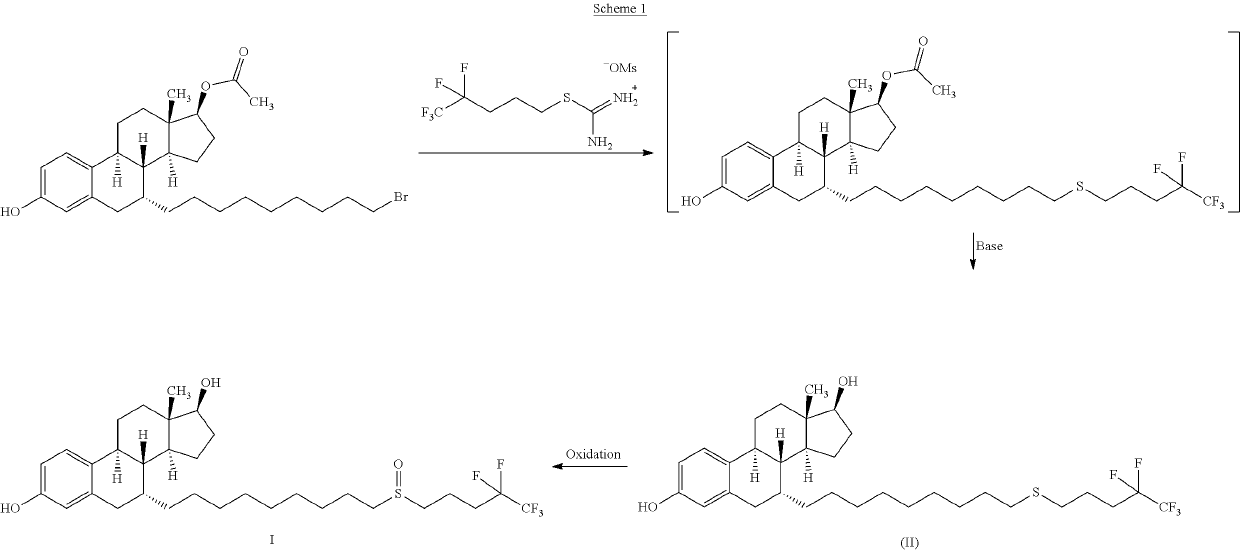
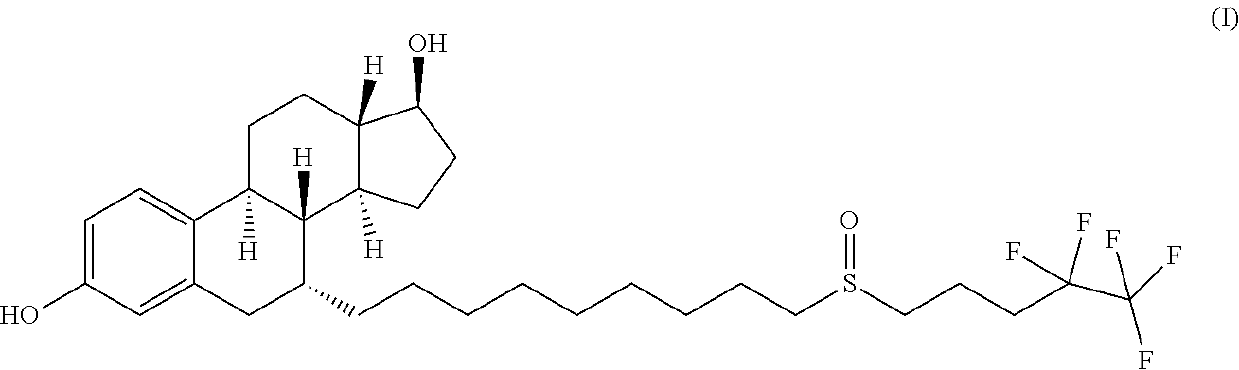
The invention relates to a preparation method of fulvestrant. The preparation method comprises a step of implementing reaction on a fulvestrant intermediate body (represented as formula II) and S-(4,4,5,5,5-pentafluoro amyl) isothiourea mesylate to introduce a pentafluoro amyl sulfenyl group, and further comprises a step (ii) of removing a protecting group of hydroxyl and / or a step iii) of oxidizing thioether into sulfoxide according to demand. According to the preparation method disclosed by the invention, raw materials are convenient to prepare and the post-processing is relatively simple, the yield of a target product is increased, the production cost is reduced, and the fulvestrant is applicable to large-scale industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Preparation method of fulvestrant intermediate
The invention provides a preparation method of a fulvestrant intermediate. The preparation method of the fulvestrant intermediate is characterized in that a water extraction starting material 1,9-nonanediol is adopted for controlling the content of 1,8-octylene glycol, an antioxidant is added for reducing byproducts which are generated by Michael addition and difficult to purify and pH value is regulated for reducing production of aromatized impurities difficult to remove, so that a route for preparing fulvestrant by virtue of the fulvestrant intermediate has the advantages that a final product is difficult to purify, the cost is low and industrialization can be easily realized. The prepared fulvestrant intermediate lays a key foundation for obtaining a route for synthesizing fulvestrant which is easy to purify and easy for industrialization.
Owner:天津孚音生物科技发展有限公司
Fulvestrant pharmaceutical composition
ActiveCN104337761AImprove stabilityImprove bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismGlycerolPharmaceutical medicine
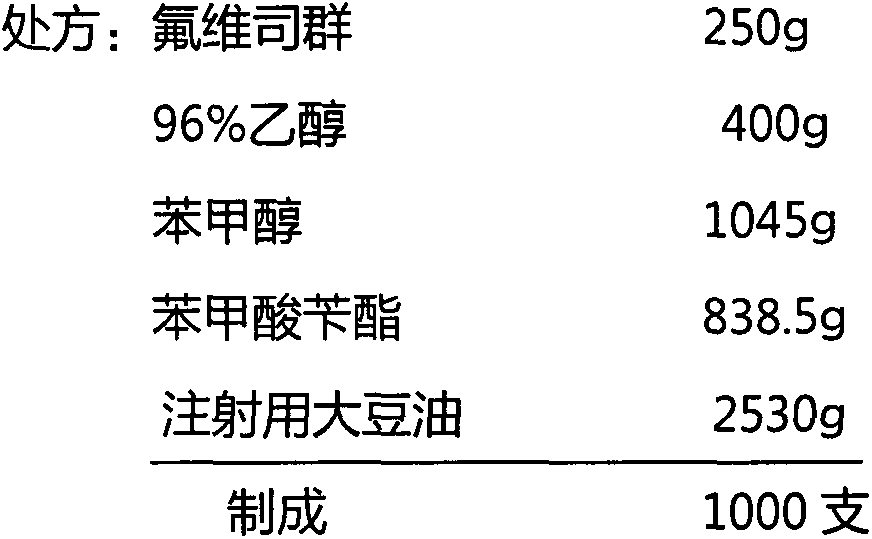
The invention relates to a fulvestrant pharmaceutical composition. Specifically, the invention relates to the fulvestrant-containing pharmaceutical composition for intramuscular injection, wherein the pharmaceutical composition comprises 1) fulvestrant, 2) a castor oil matrix; 3) at least one pharmaceutically acceptable alcohol, and 4) one or any mixture selected from the group consisting of medium-chain triglyceride, triglycerol caprylate-caprate, glyceryl triacetate, ethyl acetate and isopropyl myristate. The pharmaceutical composition is excellent in pharmaceutical safety and stability performance, and better in bioavailability.
Owner:JIANGSU HANSOH PHARMA CO LTD
New fulvestrant synthesis method
The invention discloses a new fulvestrant synthesis method, which comprises the following steps: by taking an intermediate X and pentafluoropentanol as starting materials, carrying out four-step reaction to obtain fulvestrant; the column chromatography purification is not required in the route, the fulvestrant meeting the pharmacopoeia criterion can be obtained only by recrystallizing the crude final product, the total yield achieves 50-60%, raw materials are all industrial products and easily available, and the quality is easily controlled.
Owner:天津孚音生物科技发展有限公司
Lactate-Based Fulvestrant or Fulvestrant Derivative Oily Preparation and Preparation Method Thereof
ActiveUS20150105357A1Highly exploitation value and application prospectEasy to optimizeBiocideHydroxy compound active ingredientsVegetable oilAntioxidant
An oily formulation of fulvestrant or derivatives thereof and a method for producing the same. The oily formulation comprises: fulvestrant or derivatives thereof in an amount of 10 mg / ml to 170 mg / ml; a lactate compound in an amount of 5 to 80% of the total weight of the formulation; a vegetable oil or synthetic oil (ester); an analgesic; and an optional antioxidant.
Owner:XIAN LIBANG PHARMA
Fulvestrant formulations
InactiveUS20090227552A1Organic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolPropylene glycol
The present invention relates to novel fulvestrant formulations comprising one or more of a propylene glycol or a pharmaceutically acceptable polyethylene glycol.
Owner:HOSPIRA AUSTRALIA PTY LTD
Pharmaceutical composition
The invention relates to a solid pharmaceutical composition comprising solid amorphous fulvestrant, said composition being formulated in combination with a solubilizing composition. The invention also relates to a method for preparing said composition and a kit including the composition.
Owner:CAPITAL BUSINESS Y TION DE FINANZAS
Oxo-bridged bicyclo-heptylene sulfonamides compound containing different alkyl chain lengths, as well as preparation method and application thereof
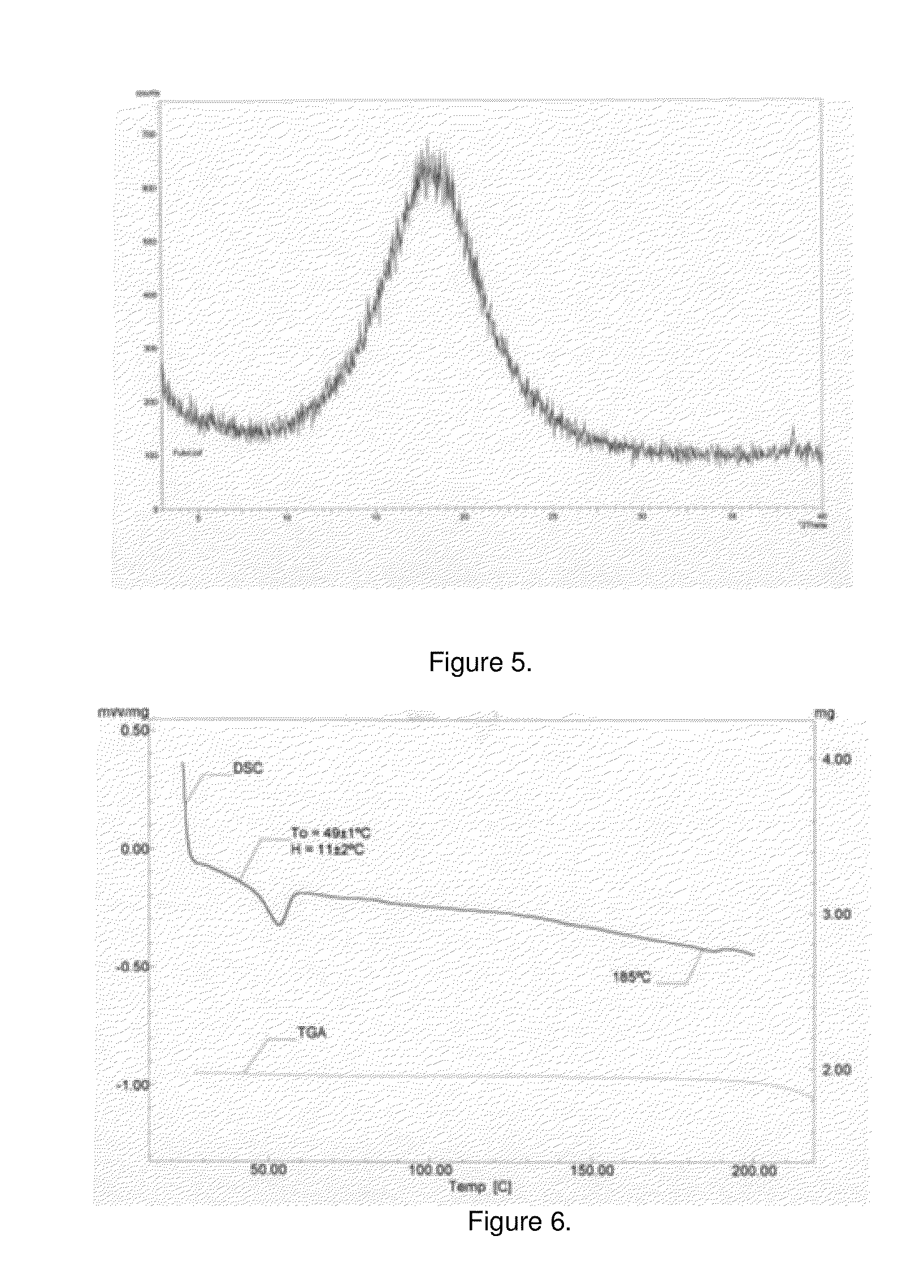
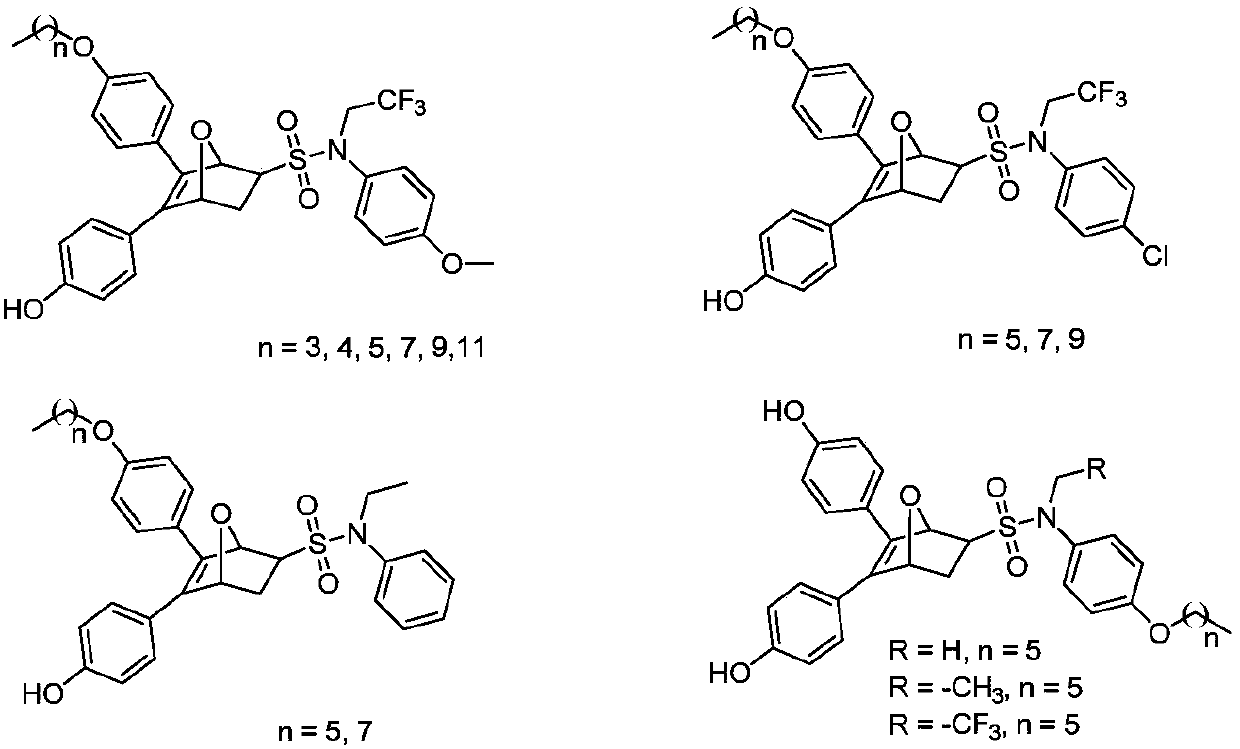
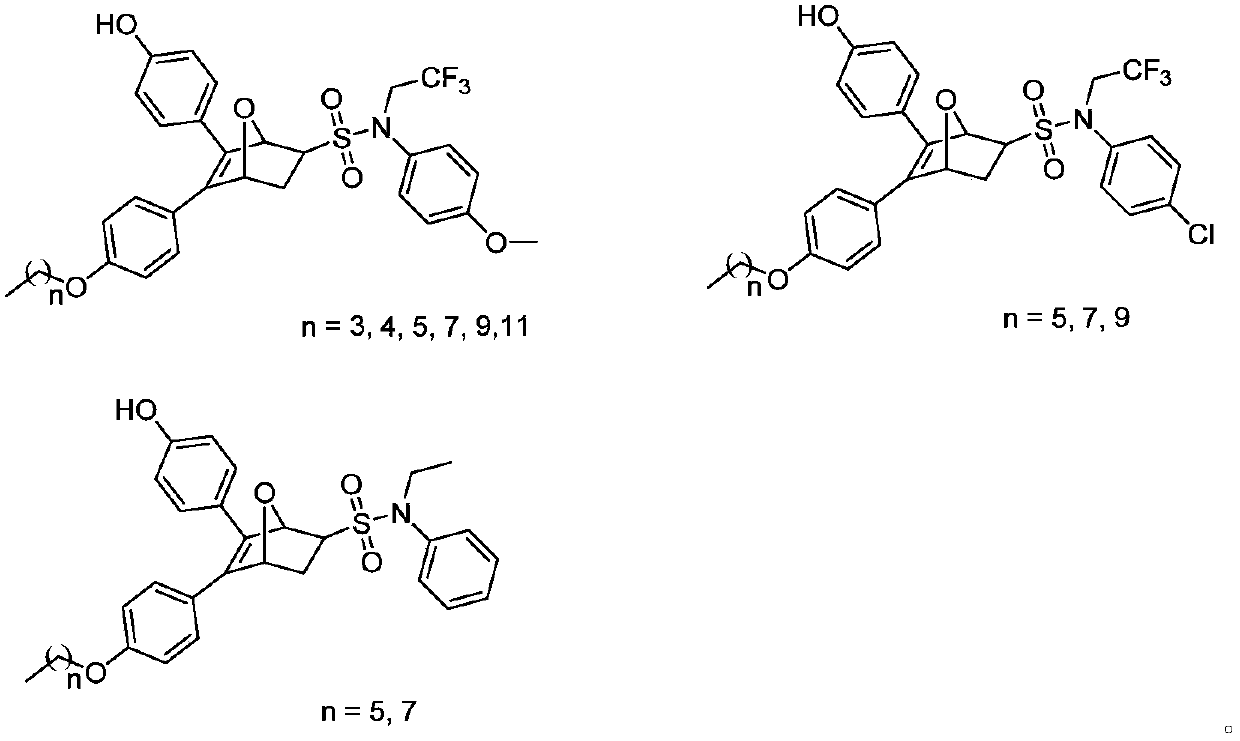
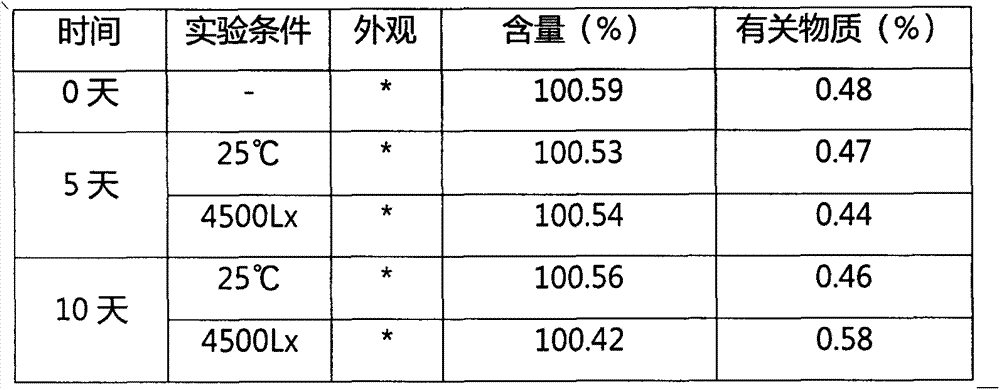
The invention discloses an oxo-bridged bicyclo-heptylene sulfonamides compound containing different alkyl chain lengths, as well as a preparation method and application thereof, and belongs to the technical field of medicines. 3-(4-hydroxycyclohexyl phenyl)-4-(4-alkoxy phenyl)-furan and a phenylethylene sulfonamide derivative are adopted as raw materials, no catalyst is needed, the raw materials are reacted at 90 DEG C for 8 hours, and the oxo-bridged bicyclo-heptylene sulfonamides compound containing the different alkyl chain lengths is obtained through one-step preparation. The action mode of the oxo-bridged bicyclo-heptylene sulfonamides compound is different from the action mode of existing anti-breast cancer drug tamoxifen, and the compound not only can be used for effectively inhibiting the growth of a breast cancer cell MCF-7, but also has favorable estrogen receptor alpha down-regulation activity equivalent to fulvestrant, and shows an application prospect of the compound in breast cancer therapy.
Owner:WUHAN UNIV
Fulvestrant containing oil needle preparation
The invention relates to a fulvestrant containing oil needle preparation for intramuscular injection. An excipient of the oil needle preparation is corn oil for injection. The oil needle preparation is filled in a glass ampoule bottle under an aseptic condition. The fulvestrant containing oil needle preparation has the advantages that as the excipient, the corn oil for injection is considered as a totally non-toxic and non-irritating substance; and relevant side effects caused by long-term contacts of the excipient, pharmaceutically acceptable alcohols and non-aqueous ester solvents with plastic products are avoided by selecting the glass ampoule bottle to contain the oil needle preparation.
Owner:南京承创医药科技有限公司
Fulvestrant containing oil needle preparation
InactiveCN103083231AOrganic active ingredientsPharmaceutical delivery mechanismSide effectIntramuscular injection
The invention relates to a fulvestrant containing oil needle preparation for intramuscular injection. An excipient of the oil needle preparation is soybean oil for injection. The oil needle preparation is filled in a glass ampoule bottle under an aseptic condition. The fulvestrant containing oil needle preparation has the advantages that as the excipient, the soybean oil for injection is considered as a totally non-toxic and non-irritating substance; and relevant side effects caused by long-term contacts of the excipient, pharmaceutically acceptable alcohols and non-aqueous ester solvents with plastic products are avoided by selecting the glass ampoule bottle to contain the oil needle preparation.
Owner:南京承创医药科技有限公司
Fulvestrant nanosphere/microsphere and preparative method and use thereof
ActiveUS8586092B2Improve thermoplasticityRate of releaseOrganic active ingredientsPowder deliveryMicrospherePolyethylene glycol
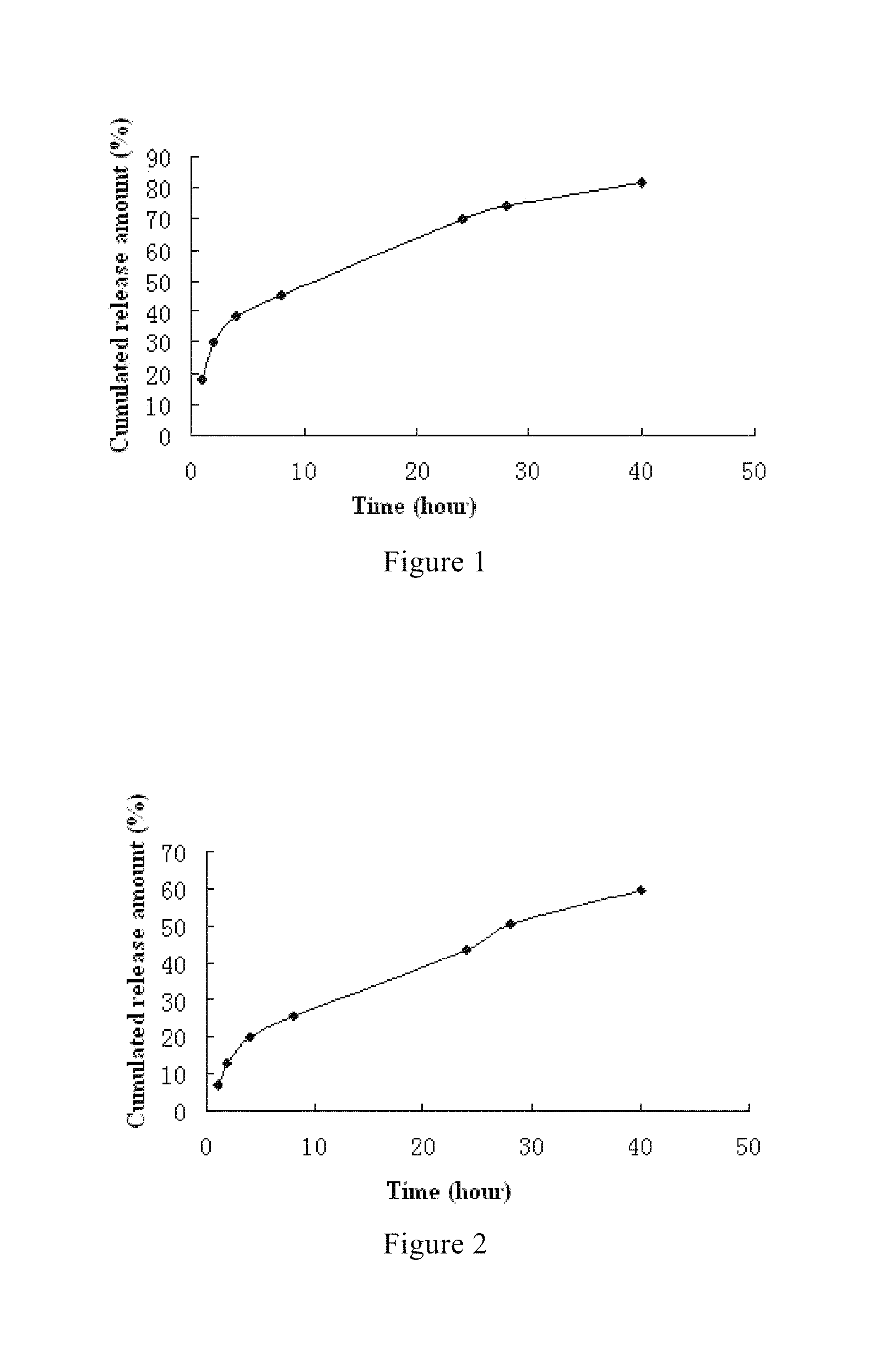
Fulvestrant nanosphere / microsphere and preparation method and use thereof are provided in the present invention. The carrier material of the fulvestrant nanosphere / microsphere is methoxy ended polyethylene glycol-polylactic acid block copolymer. The nanosphere / microsphere is prepared by solvent-nonsolvent method, in-liquid drying method and / or spray drying method, and has the features of high drug loading and high encapsulation efficiency, controllable release of medicine and no irritant to application site or blood vessel. The fulvestrant nanosphere / microsphere can be used to treat metastatic advanced breast cancer in post-menopausal woman.
Owner:XIAN LIBANG PHARMA TECH
Pharmaceutical composition
The invention relates to a solid pharmaceutical composition comprising solid amorphous fulvestrant, said composition being formulated in combination with a solubilizing composition. The invention also relates to a method for preparing said composition and a kit including the composition.
Owner:CAPITAL BUSINESS Y TION DE FINANZAS
Activators of the unfolded protein response
ActiveUS20200190029A1Increase lethalityIncreased therapeutic potentialOrganic active ingredientsOrganic chemistry methodsCancer cellOncology
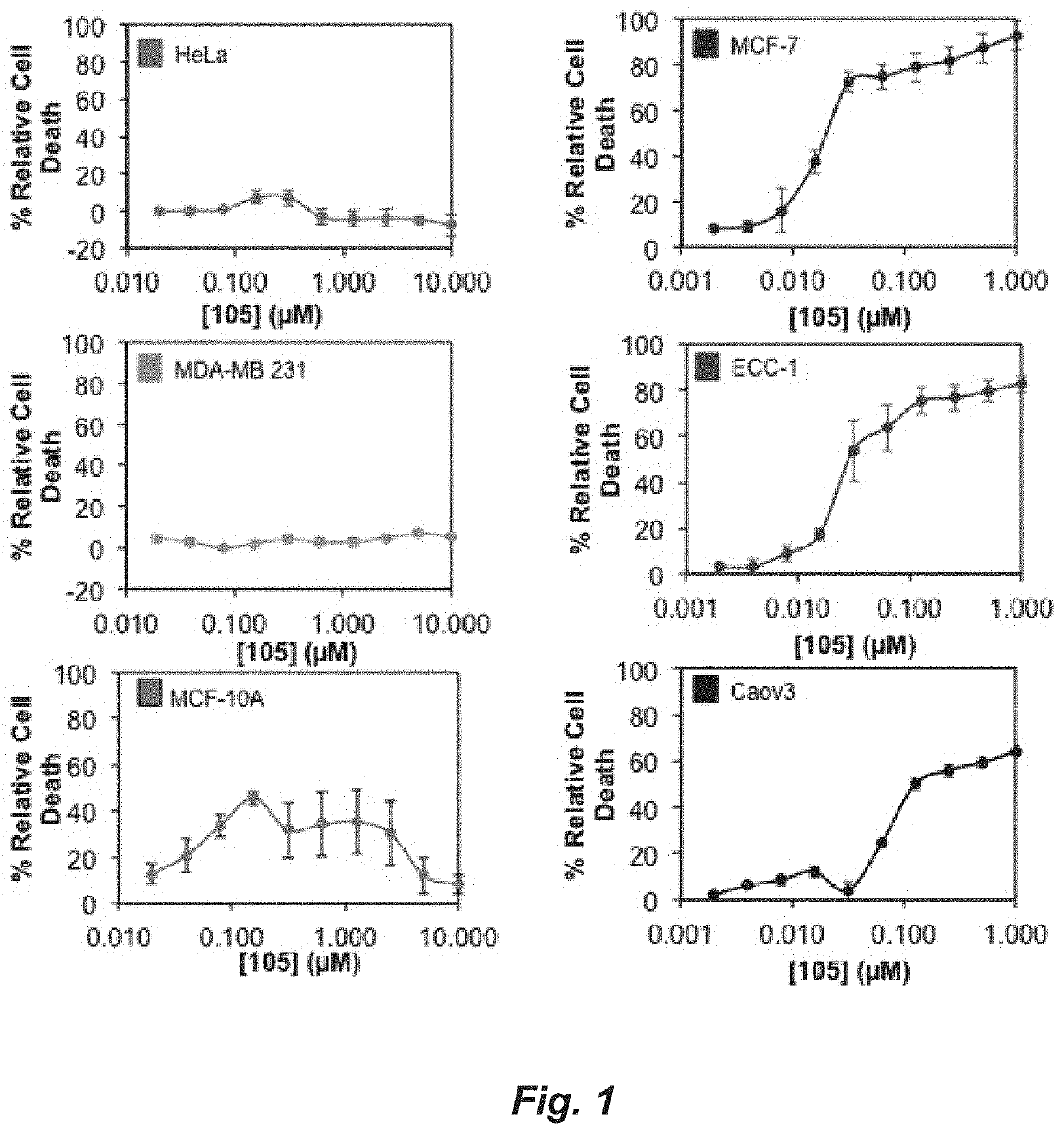
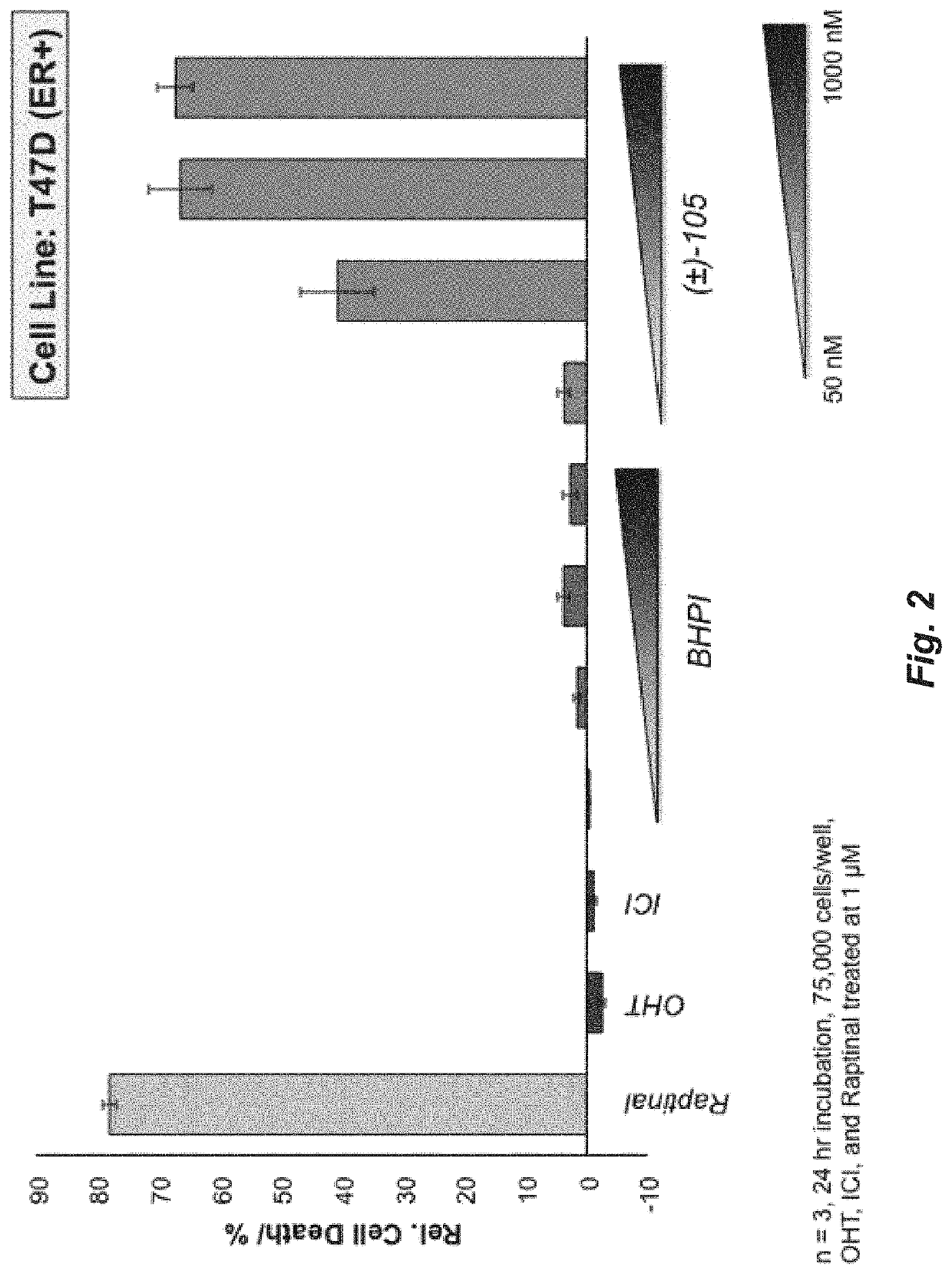
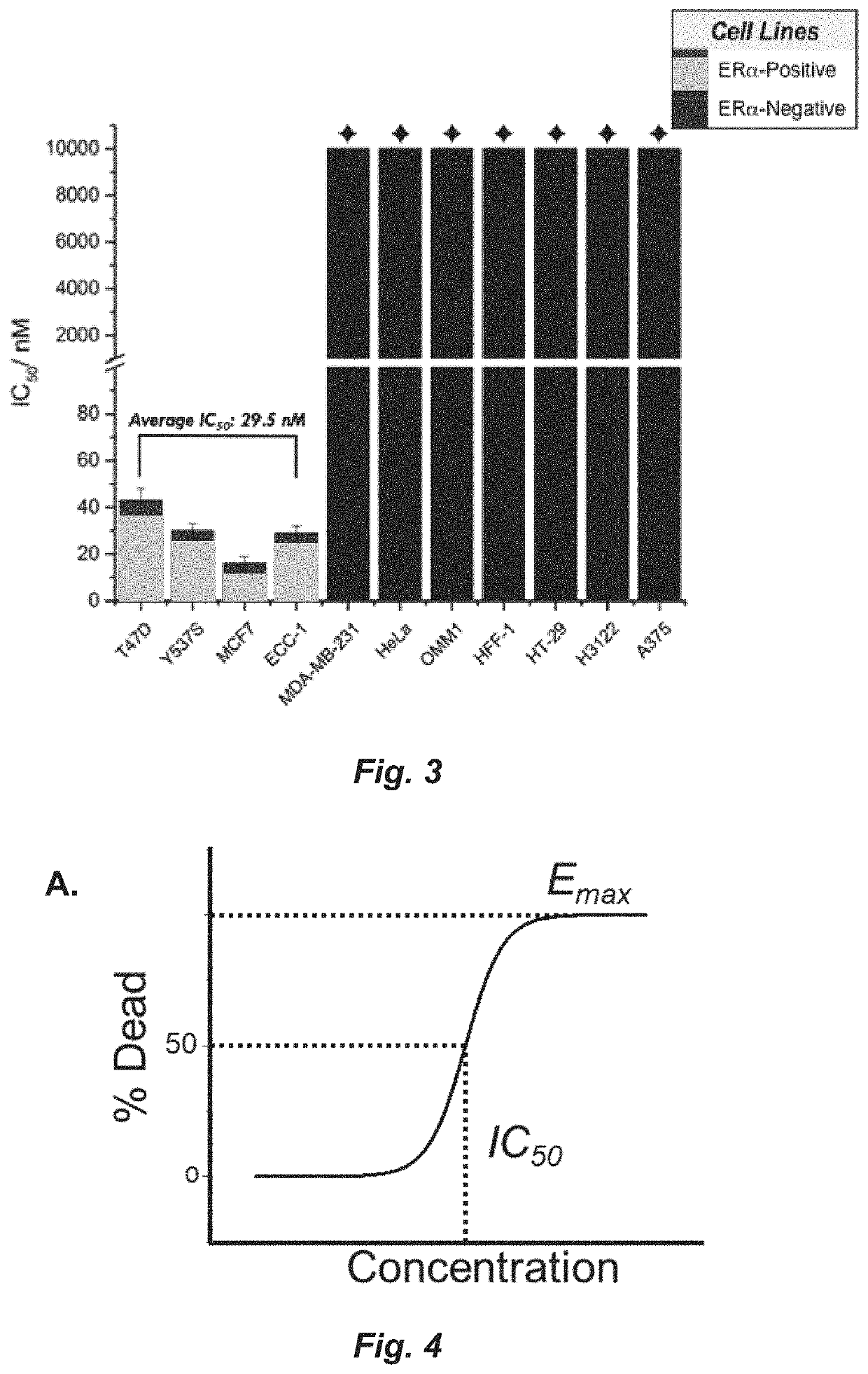
A set of small molecules ERα biomodulators that kill therapy-resistant ERα positive breast, ovarian, and endometrial cancer cells. These small molecules have increased therapeutic potential because of an increased ability to kill therapy-resistant breast cancer cells compared to BHPI and other conventional therapies (endocrine therapies, tamoxifen and fulvestrant / ICI). The new compounds do not only inhibit proliferation of the cancer cells but actually kills them, which prevents reactivation of tumors years later.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Method of treating HER2-positive breast cancers with selective androgen receptor modulators (SARMS)
ActiveUS10258596B2MicrocapsulesNitrile/isonitrile active ingredientsToremifeneHER2 Positive Breast Cancer
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, palbociclib (Ibrance), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; and / or HER2-positive; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Pharmaceutical composition of fulvestrant
ActiveCN103070871BImprove solubilityMedication convenienceOrganic active ingredientsPharmaceutical non-active ingredientsAlcoholMedium-chain triglyceride
The invention relates to a pharmaceutical composition of fulvestrant. The pharmaceutical composition comprises: (1) fulvestrant; (2) at least one pharmaceutically acceptable alcohol; (3) medium-chain triglyceride; and (4) a castor oil matrix.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Pharmaceutical composition
The invention relates to a solid pharmaceutical composition comprising solid amorphous fulvestrant, said composition being formulated in combination with a solubilizing composition. The invention also relates to a method for preparing said composition and a kit including the composition.
Owner:CAPITAL BUSINESS Y TION DE FINANZAS
Method of treating androgen receptor (AR)-positive breast cancers with selective androgen receptor modulator (SARMs)
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Application of fulvestrant in preparing antimicrotubular chemotherapeutic drug resistance reversal agent
InactiveCN102151277ALittle side effectsImprove toleranceOrganic active ingredientsAntineoplastic agentsSide effectApoptosis
The invention provides application of fulvestrant in preparing an antimicrotubular chemotherapeutic drug resistance reversal agent. The fulvestrant reverts antimicrotubular chemotherapeutic drug resistance of breast cancer cells without relying whether estrogen receptors are expressed or not and also without relying whether the function of restricting P-glycoprotein is achieved or not. The fulvestrant and the antitumor drugs which are used together can accelerate the antitumor drugs to induce apoptosis of drug-resistance cells of breast cancer with negative estrogen receptors. The fulvestrant has been approved to be used for clinics and has the advantages of less side effects, good tolerance of a patient, less cross drug resistance with other estrogen receptor regulators or chemotherapeutic drugs, and the like, has clinical operability while being collaboratively used with common chemotherapeutic drugs for clinically treating the breast cancer, such as taxol, adriamycin, vinorelbine, and the like and has favorable clinical application prospect as the antimicrotubular chemotherapeutic drug resistance reversal agent for treating the drug-resistance breast cancer.
Owner:ZHEJIANG UNIV
Method of treating androgen receptor (AR)-positive breast cancers with selective androgen receptor modulator (SARMs)
This invention relates to the treatment of androgen receptor-positive breast cancer in a subject, for example a female subject. Accordingly, this invention provides methods of: a) treating a subject suffering from breast cancer; b) treating a subject suffering from metastatic breast cancer; c) treating a subject suffering from refractory breast cancer; d) treating a subject suffering from AR-positive breast cancer; e) treating a subject suffering from AR-positive refractory breast cancer; f) treating a subject suffering from AR-positive metastatic breast cancer; g) treating a subject suffering from AR-positive and ER-positive breast cancer; h) treating a subject suffering from triple negative breast cancer; i) treating a subject suffering from advanced breast cancer; j) treating a subject suffering from breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; k) treating, preventing, suppressing or inhibiting metastasis in a subject suffering from breast cancer; l) prolonging survival of a subject with breast cancer, and / or m) prolonging the progression-free survival of a subject with breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound, comprising administering to the subject a therapeutically effective amount of a SARM compound of this invention.
Owner:UNIV OF TENNESSEE RES FOUND
Method of treating er mutant expressing breast cancers with selective androgen receptor modulators (SARMS)
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; and / or treating a subject suffering from ER mutant expressing breast cancer, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
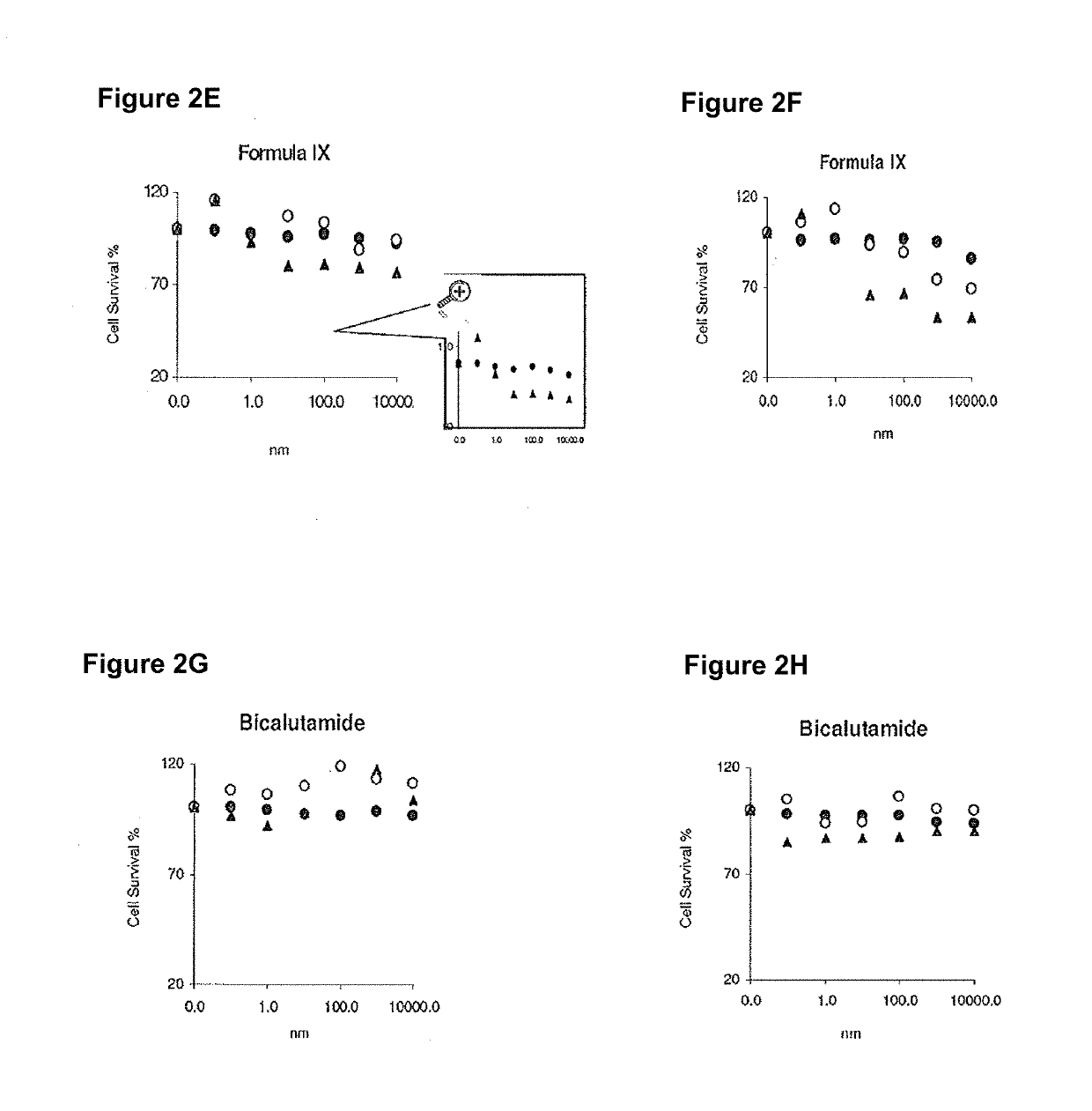
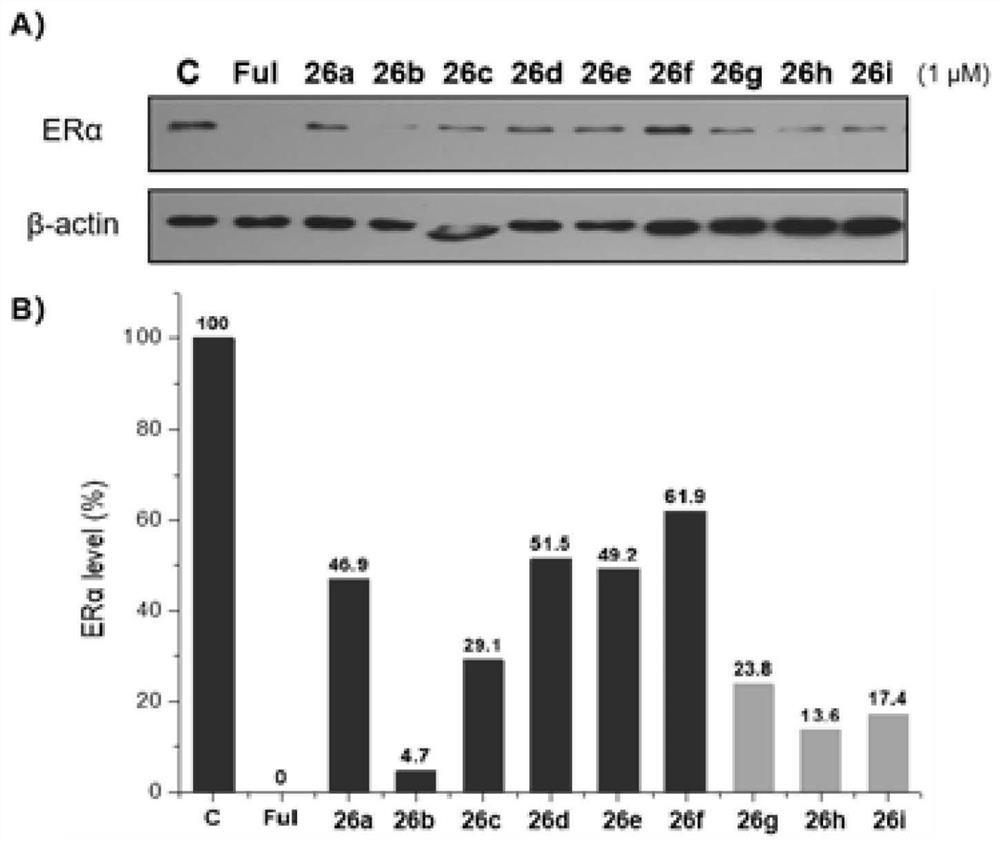
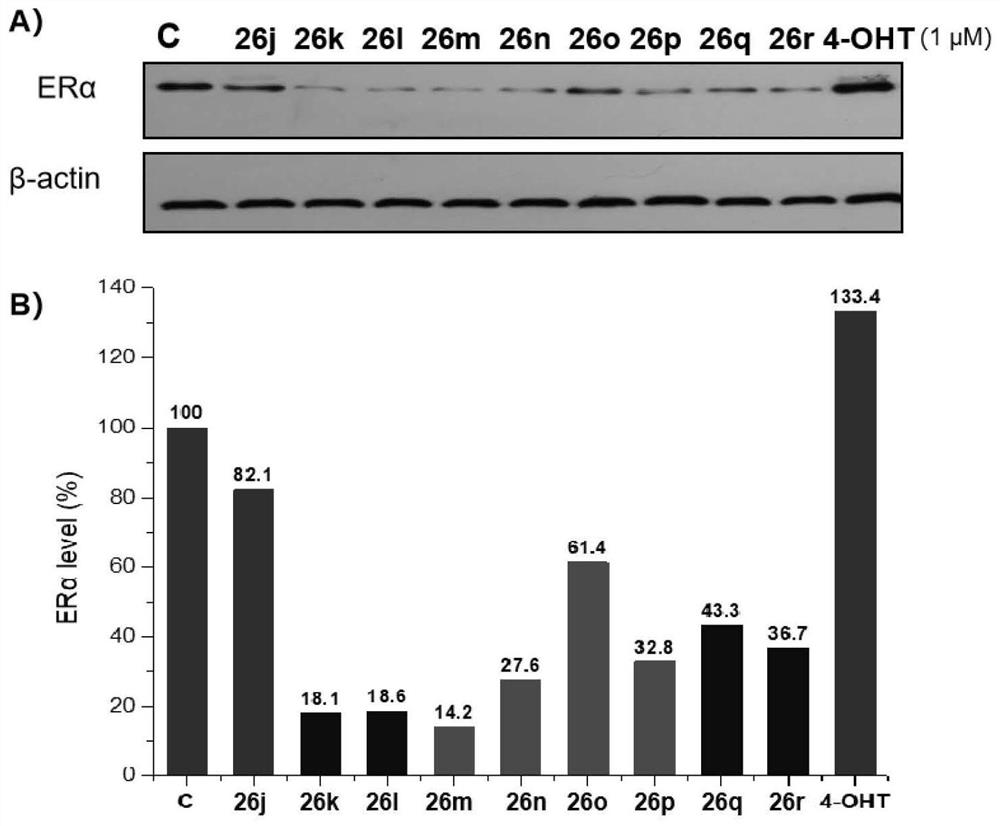
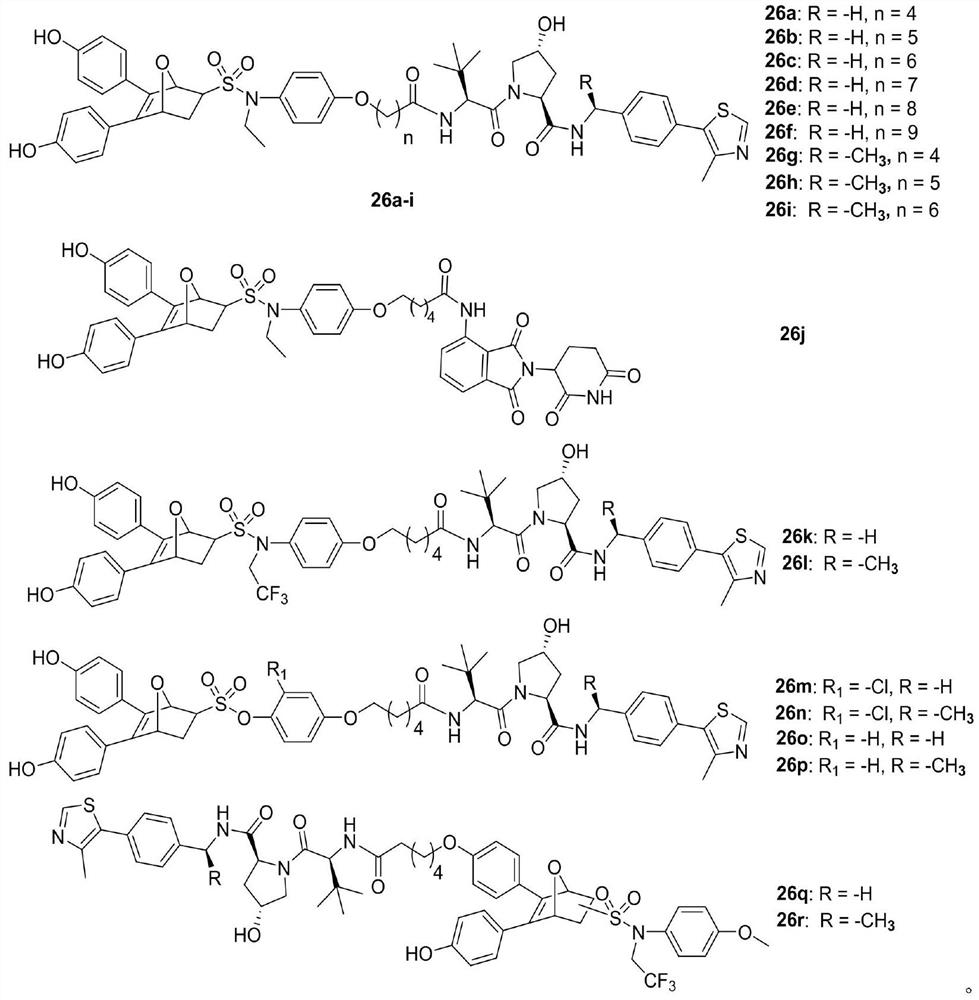
Proteolytic targeted chimeric compound taking oxygen bridge bicycloheptene compound as estrogen receptor ligand as well as preparation method and application
The invention discloses a proteolytic targeted chimeric compound taking an oxygen bridge bicycloheptene compound as an estrogen receptor ligand as well as a preparation method and an application. Tworeasonable synthesis modes are adopted, a VHL ligand or a CRBN ligand serves as an E3 ligase ligand part, oxygen bridge bicycloheptene sulfonate or sulfonamide estrogen receptor ligands are connectedthrough alkyl side chains with different lengths, and a series of target product Protac molecules are obtained through synthesis. An action mode of the Protac molecules is different from that of an existing anti-breast cancer drug tamoxifen, and the Protac molecules are targeted estrogen down-regulators. The compound not only retains a certain estrogen receptor binding capacity, but also has goodestrogen receptor alpha down-regulation activity equivalent to that of fulvestrant, can realize event-driven targeted estrogen receptor degradation, and is expected to overcome drug resistance causedby traditional endocrine treatment of the ER positive breast cancer through the method.
Owner:WUHAN UNIV
Method of treating estrogen receptor (ER)-positive breast cancers with selective androgen receptor modulator (SARMS)
Owner:UNIV OF TENNESSEE RES FOUND
Improved method for recovering fulvestrant with unqualified isomer ratio
ActiveCN107698647AImprove securityHigh recovery rateSteroidsEnvironmental engineeringImproved method
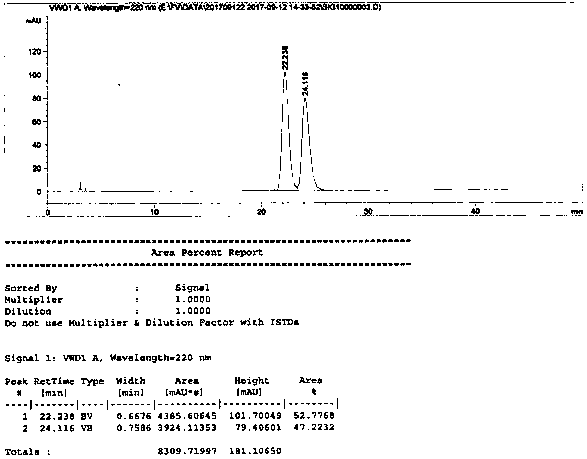
The invention relates to an improved method for recovering fulvestrant with an unqualified isomer ratio. A halide is adopted as a reduction catalyst for replacing reductants with potential safety hazard and environmental pollution in the prior art, so that the discharge of three wastes (waste gas, waste water and industrial residue) is reduced, the recovery rate of the fulvestrant is greatly improved, the recovery rate of the unqualified fulvestrant can reach about 70%, and thus the improved method is beneficial for industrial popularization.
Owner:HANGZHOU ALLSINO CHEM
Suppression and prevention of tumors and treatment of viruses
ActiveUS20140275240A1Risk minimizationShorten the progressBiocideAnimal repellantsVitamin CArtemisinins
Combinations of betaine and vitamin C are used to suppress or prevent malignant tumors or to treat viruses, e.g., by combining the two ingredients in a product consumed by a human, dog, or cat, such as an aqueous liquid such as grape juice, the ingredients being provided in containers with instructions for use, or in finished products, especially with support of tests demonstrating the effectiveness of the treatment for, e.g., preventing tumors in populations known to be at risk of developing tumors, or, treating existing cancers in combination with other cancer drugs such as anastrozole and / or fulvestrant and / or artemisinin either concurrently or sequentially to prevent the cancer from growing when the cancer drug is not being used, or in the treatment of viruses.
Owner:AYLOR ROBERT BENSON +2
Method of manufacturing fulvestrant sustained-release microspheres
InactiveCN101108168BRound shapeUniform particle size distributionOrganic active ingredientsPowder deliveryMicrospherePolyvinyl alcohol
The invention discloses a faslodex controlled-release microballoon preparation method. The faslodex controlled-releasemicroballoon in the invention envelopes fulvestrant takes PLA or PLGA or PCL or other biodegradable materials as the carrier materials. Besides, the invention takes PVA or the miscible liquids of PCL and Tween 80 as the disperse medium. Then, with the emulsion solvent evaporation method, finish the preparation of fulvestrant controlled-release microballoon under the action of mechanical mixing or high-speed shearing. The microballoon has spherical shape and even distribution of grain size that is controllable within the scope of 15 to 125Mu m as well as reaches a drug-loading rate of over 7 per cent and encapsulation rate of over 80 per cent.
Owner:XIAN LIBANG PHARMA TECH
Process for the preparation of fulvestrant
The present invention relates to a process for the preparation of Fulvestrant which includes recovering the by-products deriving from the oxidation of the corresponding sulfide and then subjecting the by-products to a reduction reaction in the presence of specific reducing agents.
Owner:FARMABIOS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com