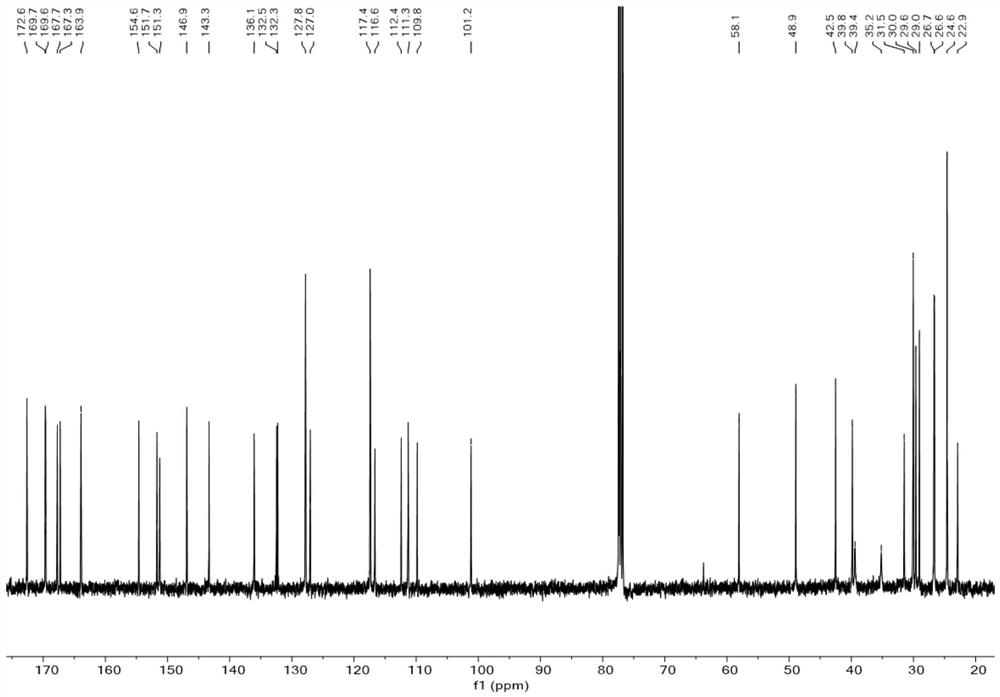
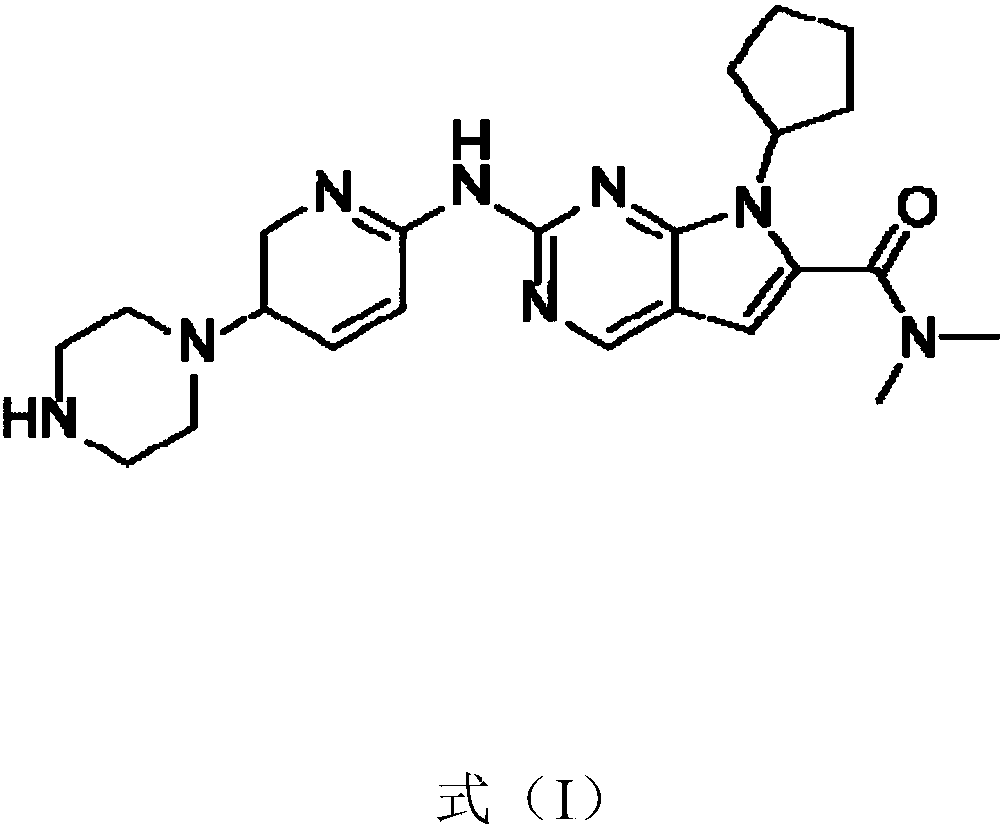
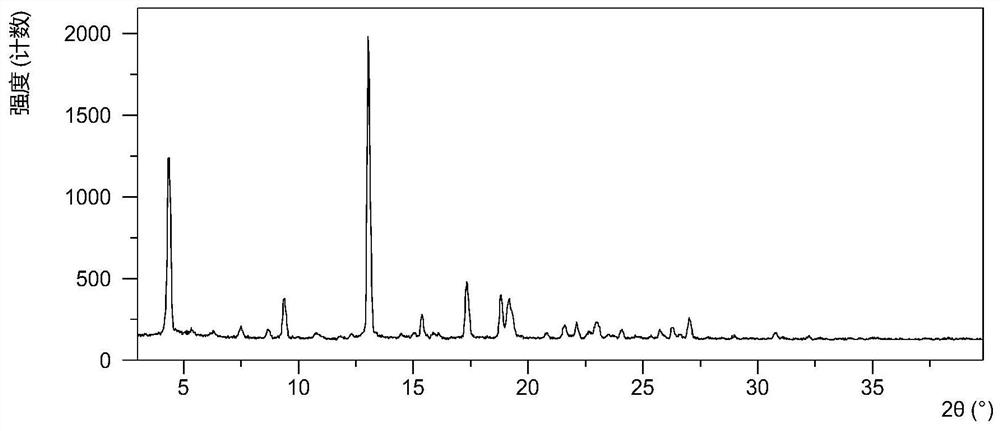
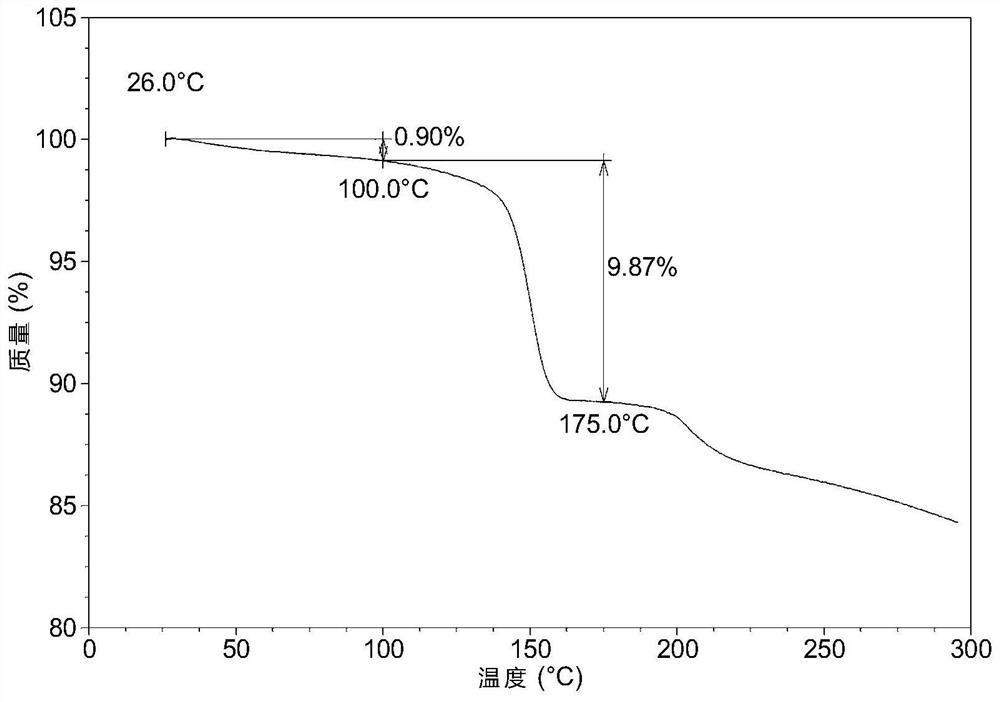
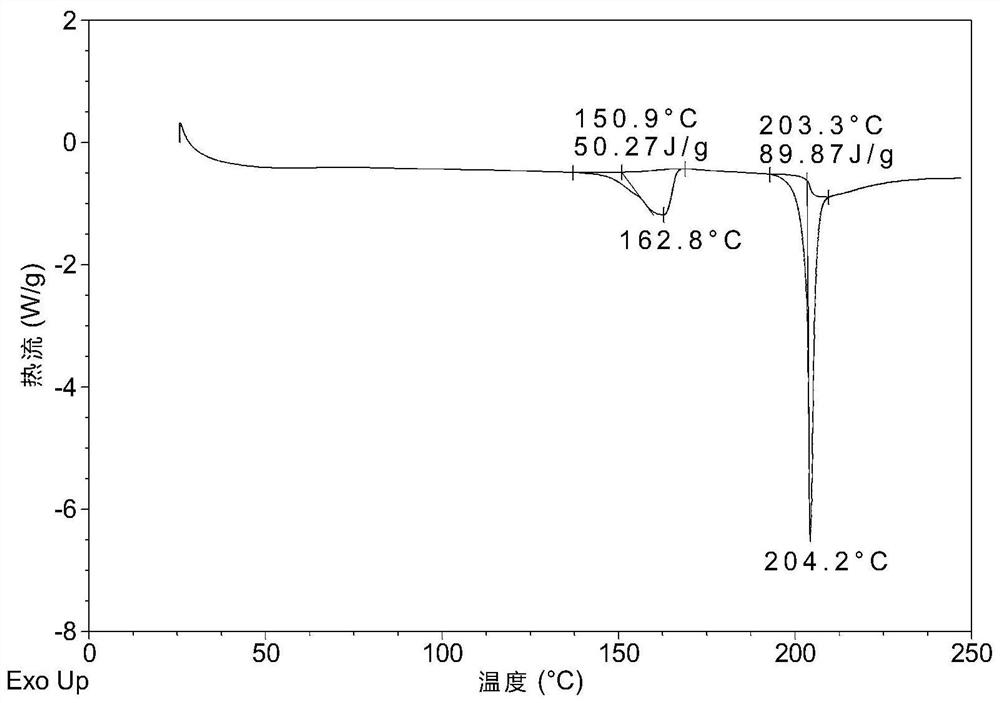
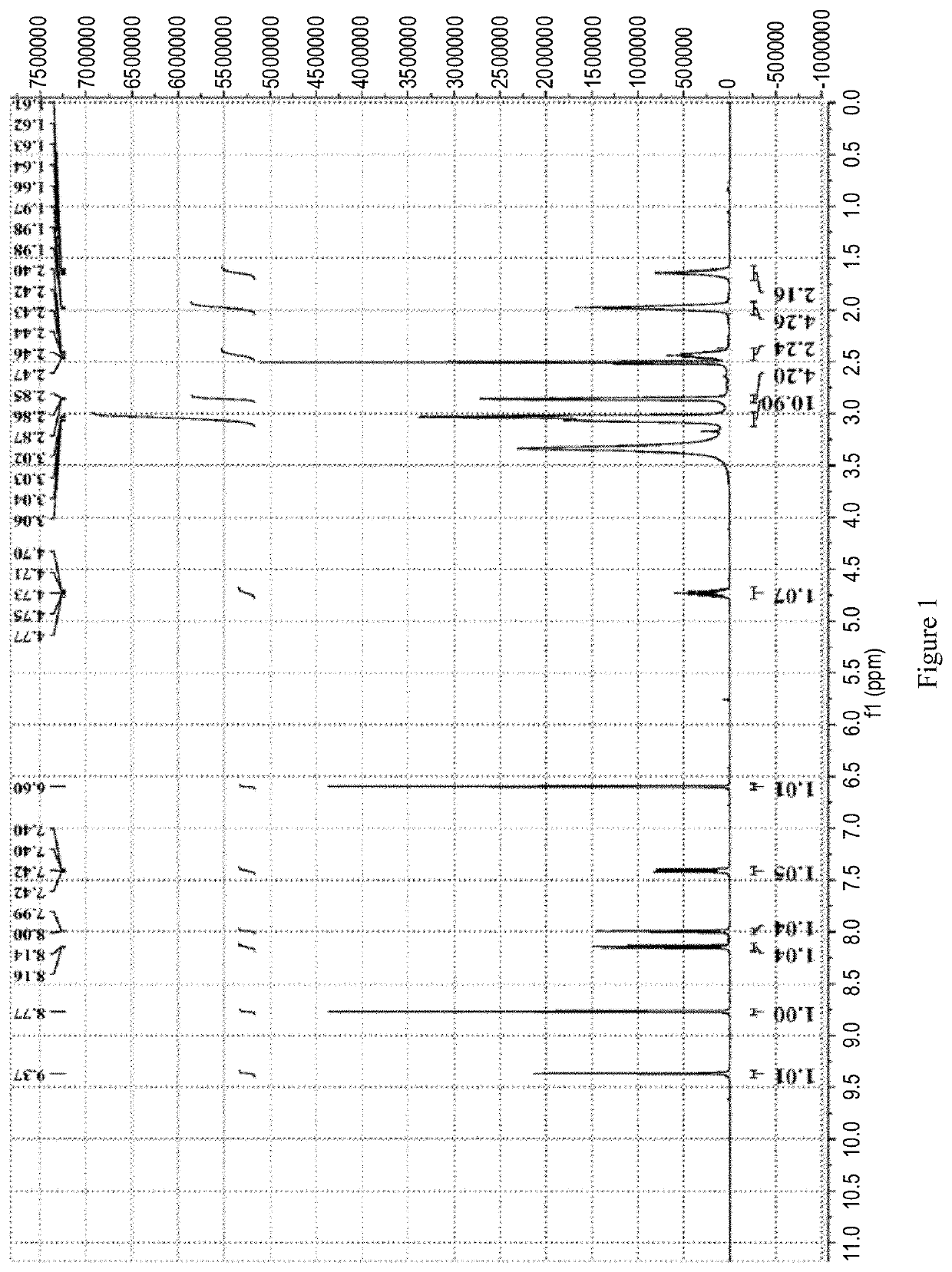
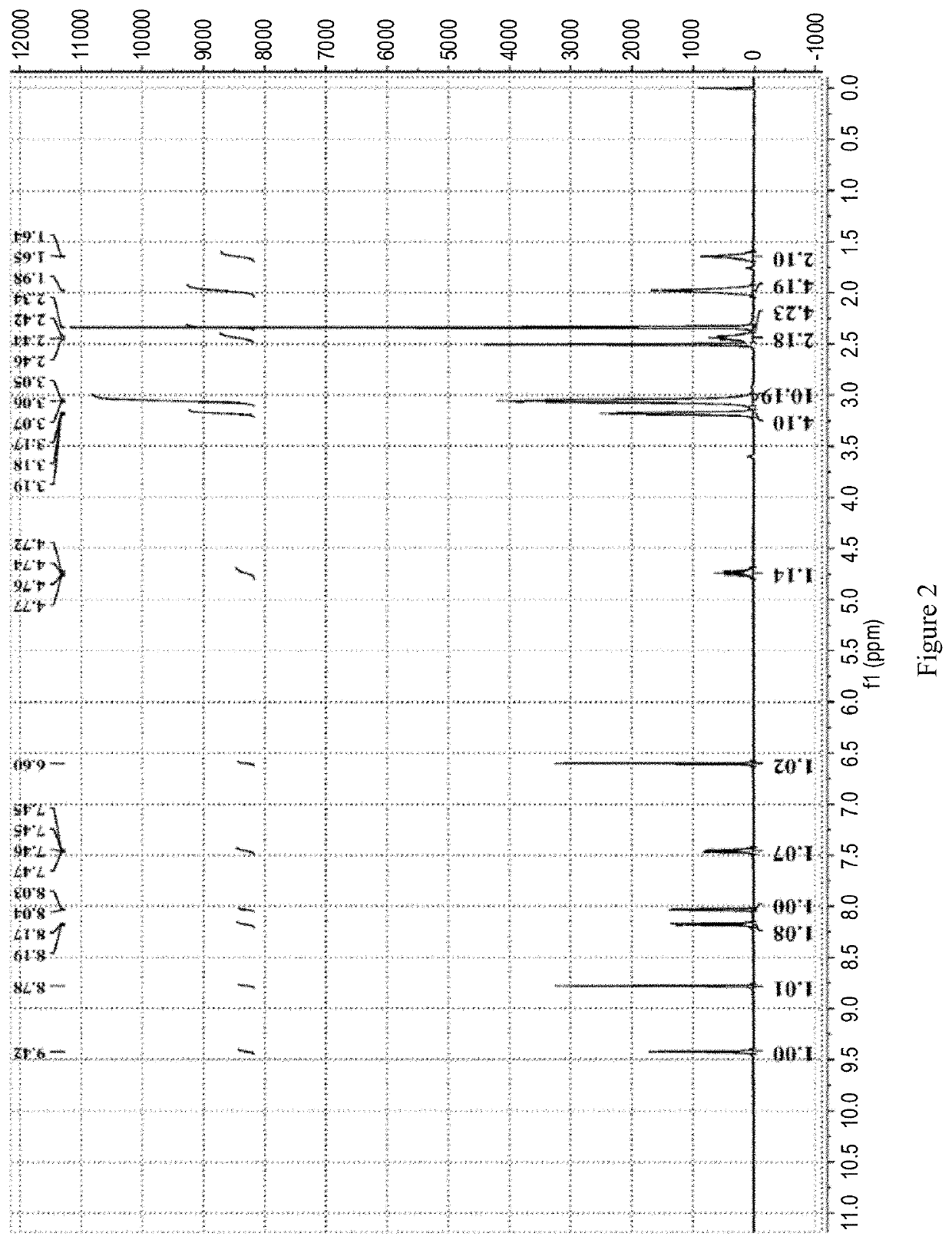
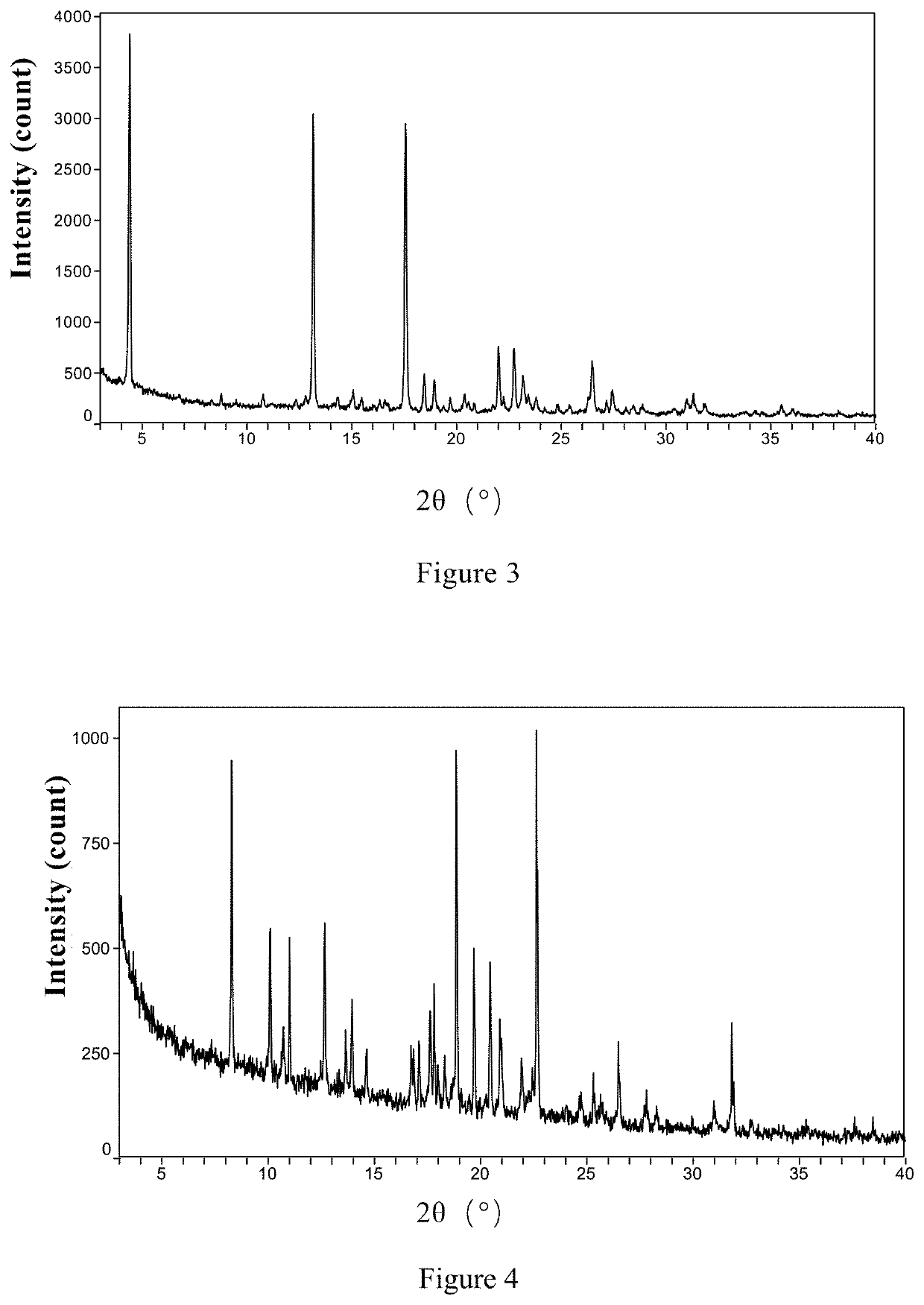
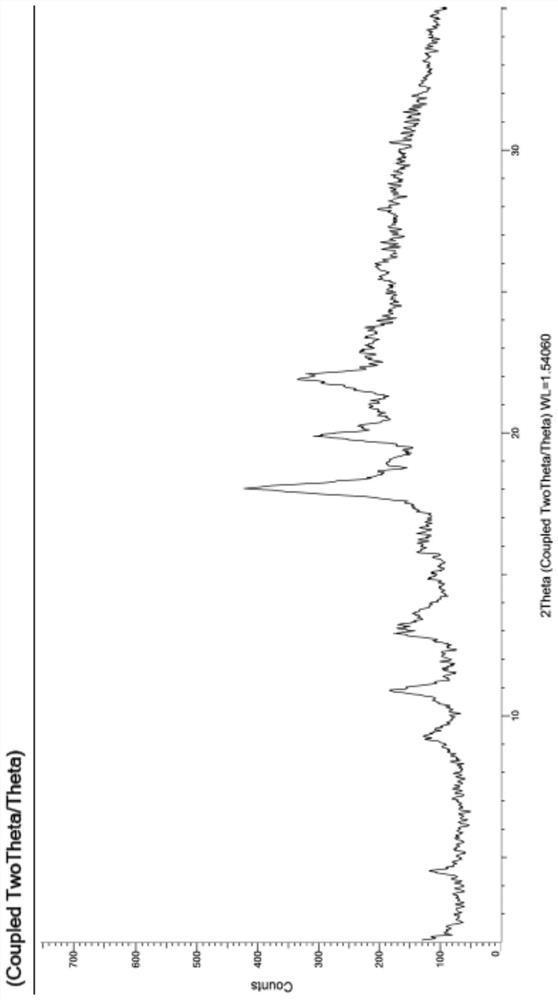
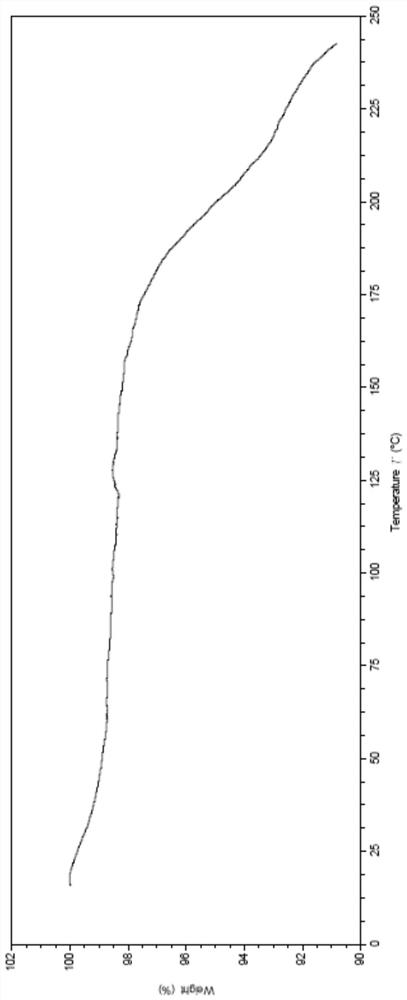
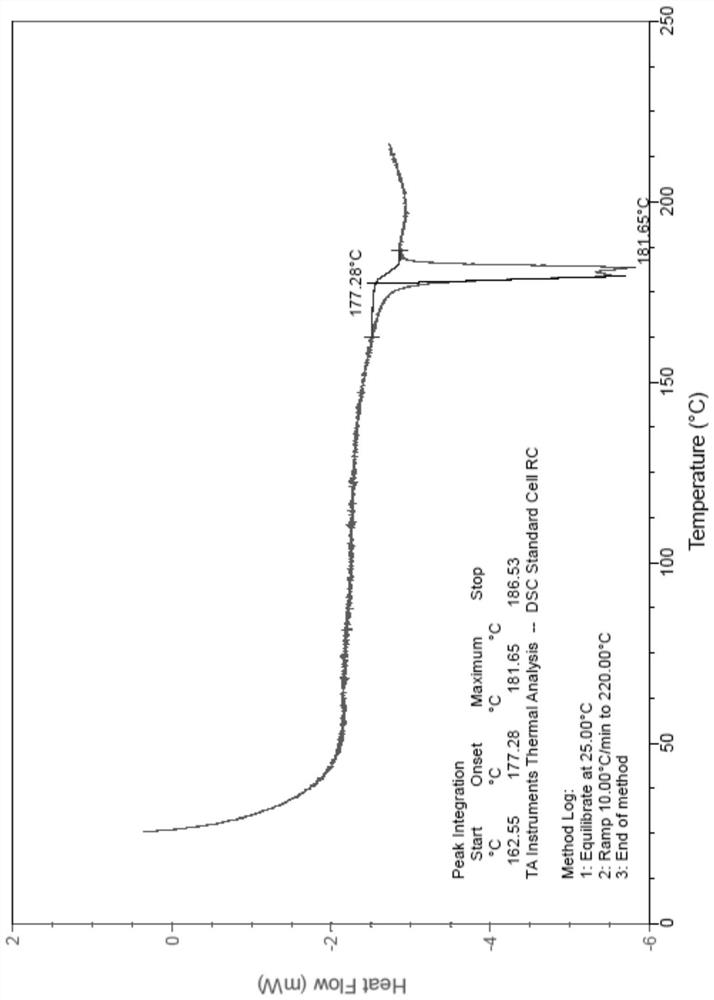
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "Ribociclib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
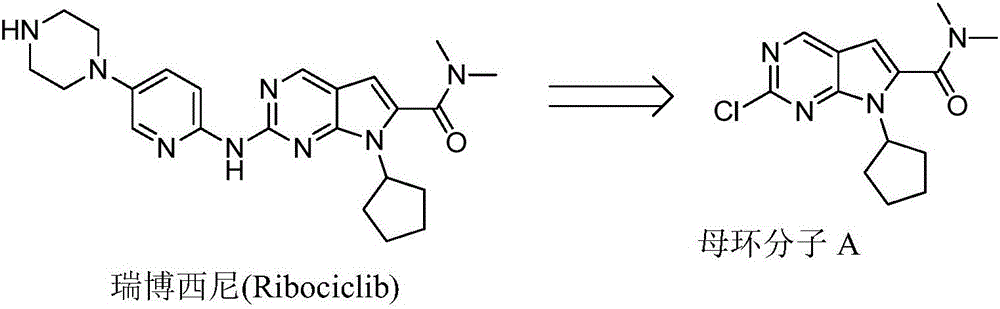
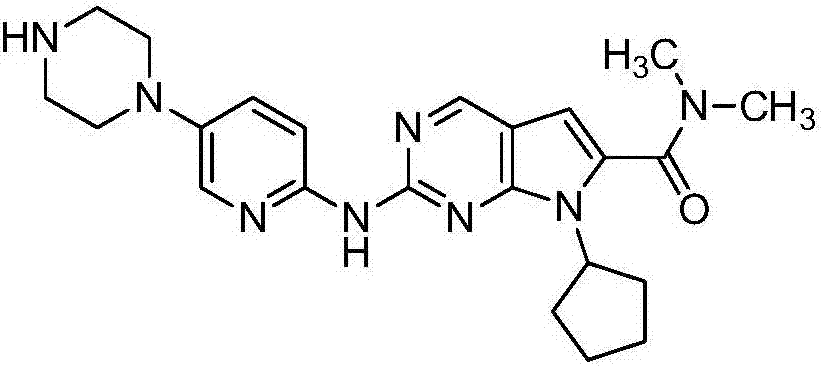
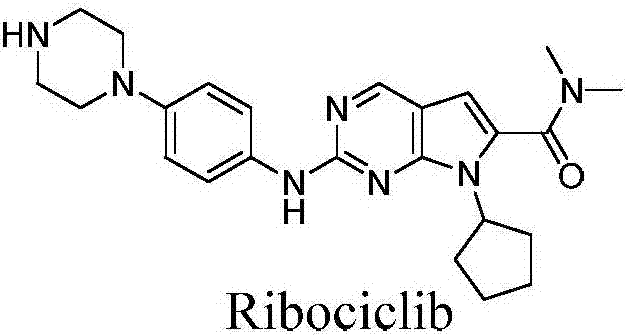
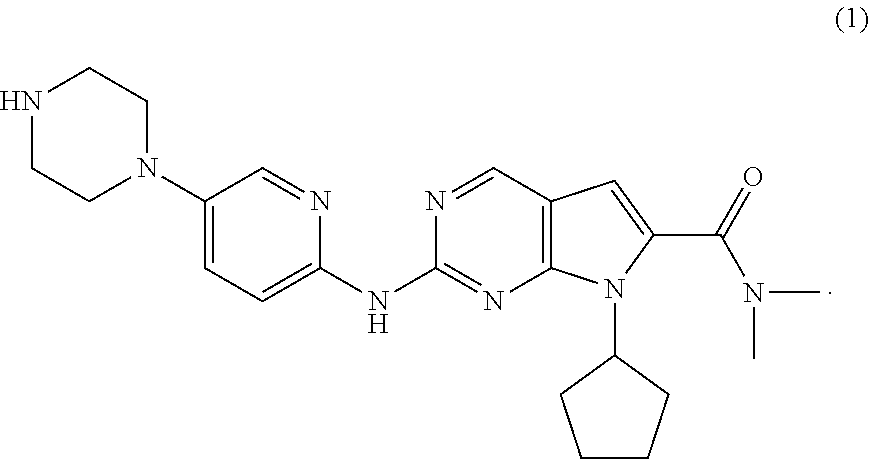
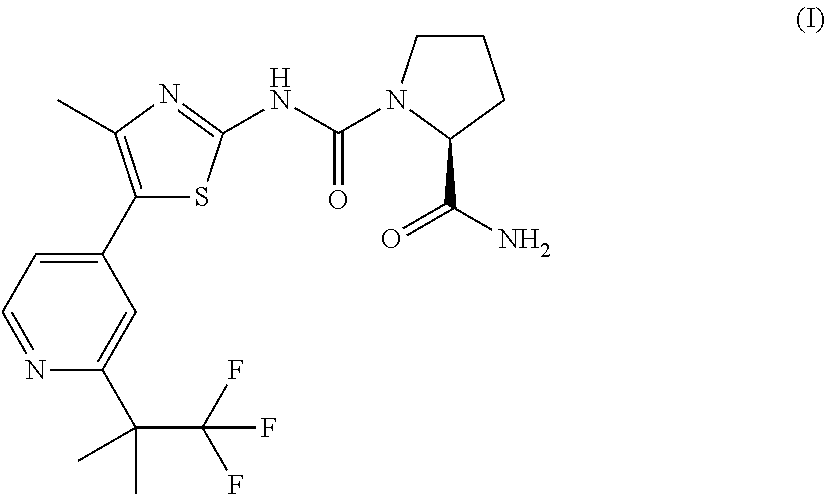
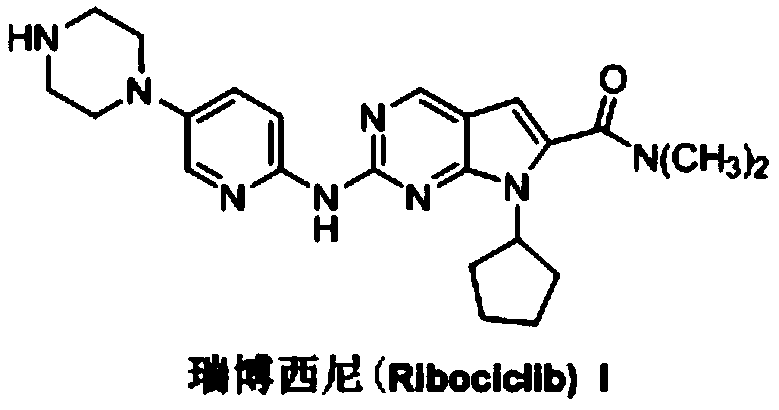
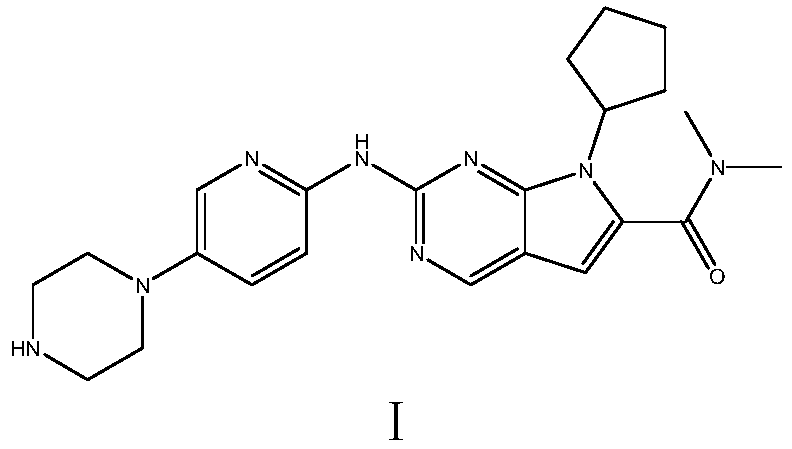
Ribociclib (trade name Kisqali) is an inhibitor of cyclin D1/CDK4 and CDK6, and is used for the treatment of certain kinds of breast cancer. It is also being studied as a treatment for other drug-resistant cancers. It was developed by Novartis and Astex Pharmaceuticals.
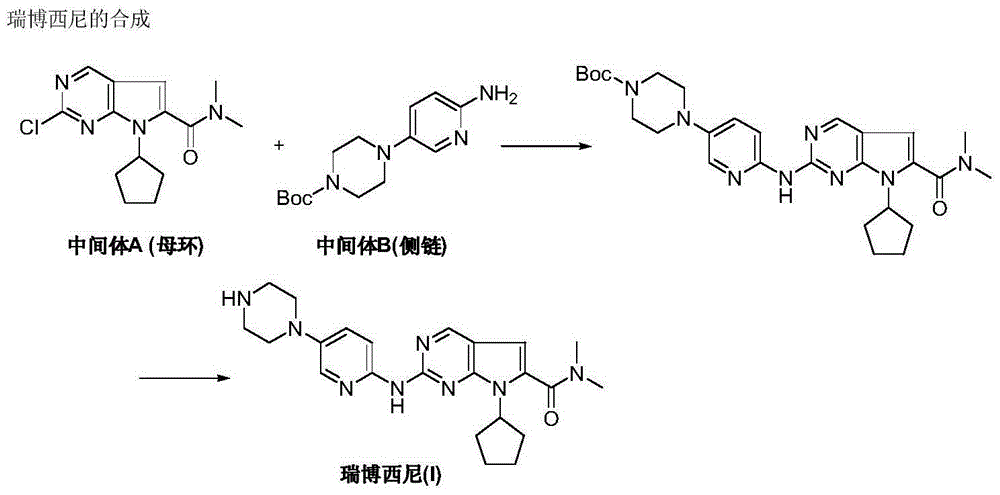
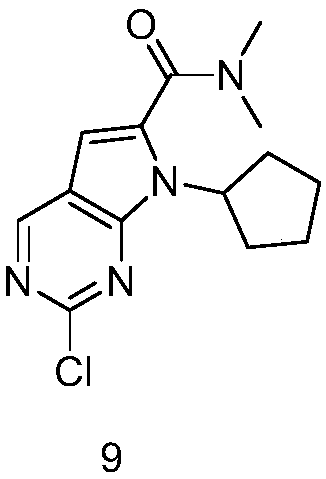
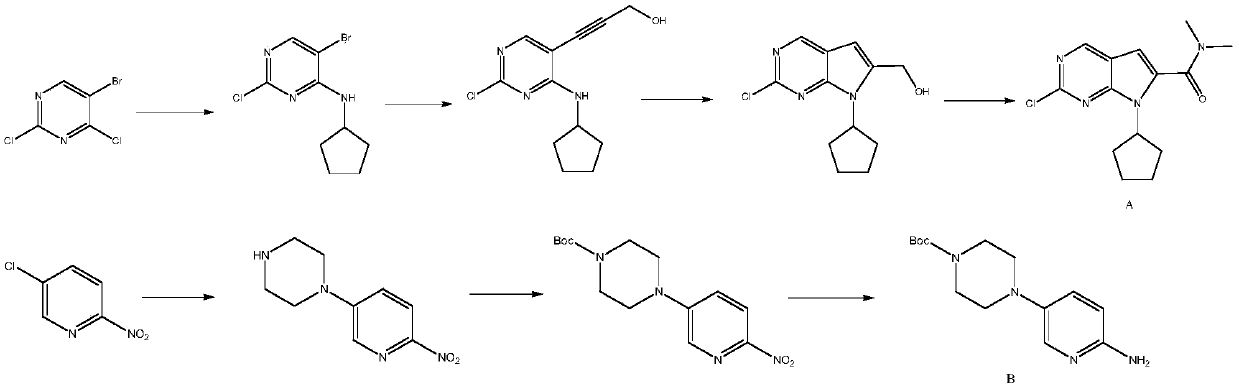
New synthesis method of ribociclib intermediate
ActiveCN106478641AEasy to operateImprove coupling conditionsOrganic chemistrySynthesis methodsSide chain
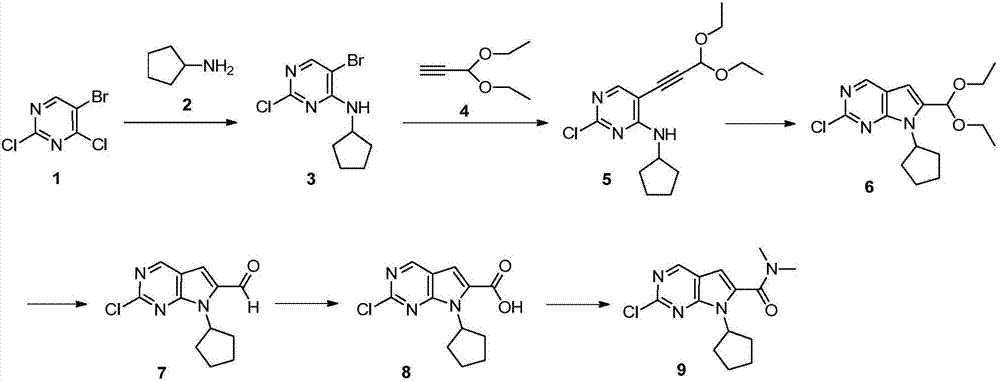
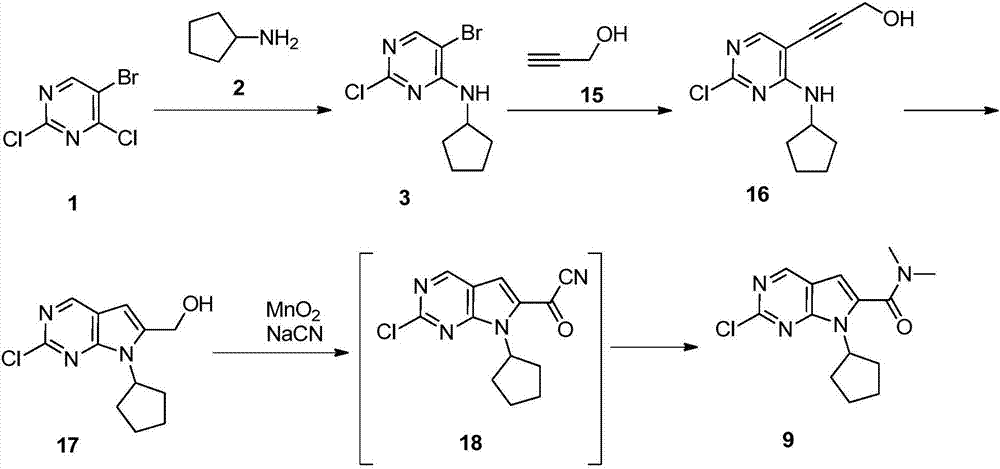
The invention discloses preparation of a ribociclib key intermediate A; propiolic acid ester or amide 2 is directly used as a Sonogashira coupling side chain, coupling conditions are optimized, an intermediate 3 is obtained with relatively high yield, the intermediate 3 is directly subjected to ring closing under simple conditions to complete construction of a mother ring molecule, to obtain a structural formula A or a precursor ester 4 of the structural formula A, and the precursor ester 4 is subjected to hydrolysis and condensation to obtain the structural formula A. The route has simple operation, reaction steps are shorted, the yield is relatively high, and the purity of the obtained product is relatively high, and the method is suitable for enlarged production, wherein the reaction route is described in the specification.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
Preparation method of ribociclib intermediate
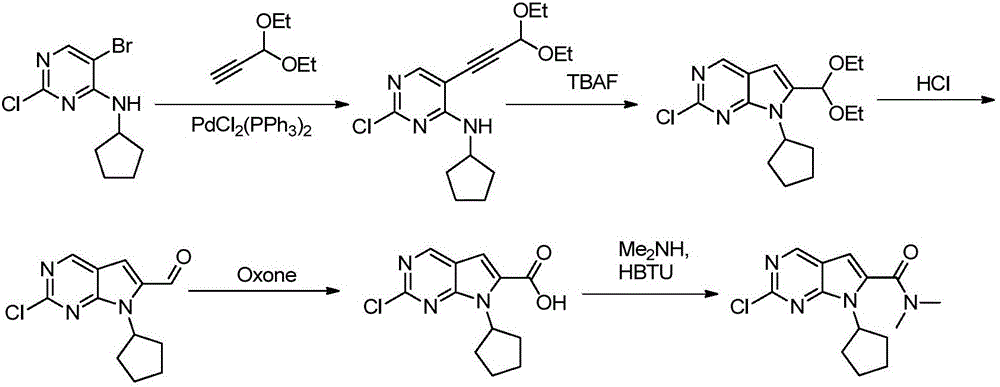
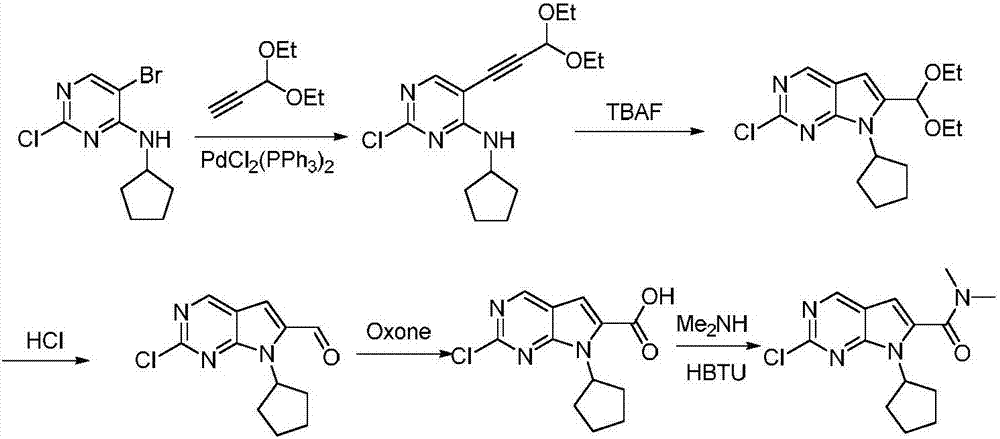
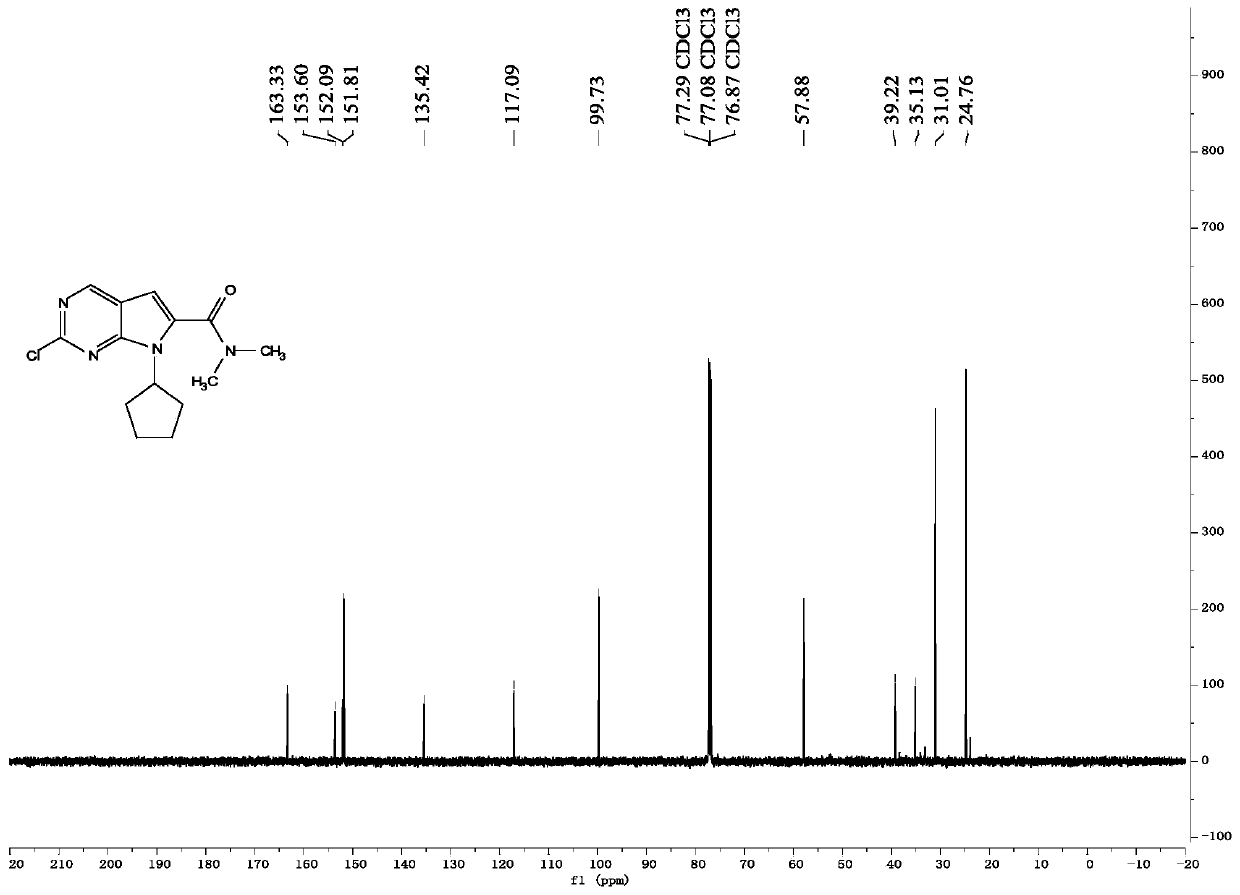
The invention belongs to the field of organic synthesis and pharmaceutical synthesis and particularly relates to a preparation method of a ribociclib intermediate. According to the preparation method, after 2-halo-7-cyclopentyl-6-(((tetrahydro-2H-pyran-2-yl)oxy)methyl)-7H-pyrrolo[2,3-d] pyrimidine is obtained, the ribociclib intermediate, namely, 2-halo-7-cyclopentyl-N,N-dimethyl-7H-pyrrolo[2,3-d]pyrimidine-6-formamide is obtained through three steps of reactions, and high yield and high purity are realized in each reaction step, so that the total yield of the overall route is high and is remarkably better than that in the prior art; besides, raw materials are easy to obtain, the production cost is low, the preparation is simple and easy to operate, reaction reagents are environmentally friendly, and the preparation method of the ribociclib intermediate is particularly suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Synthesis technology of ribociclib
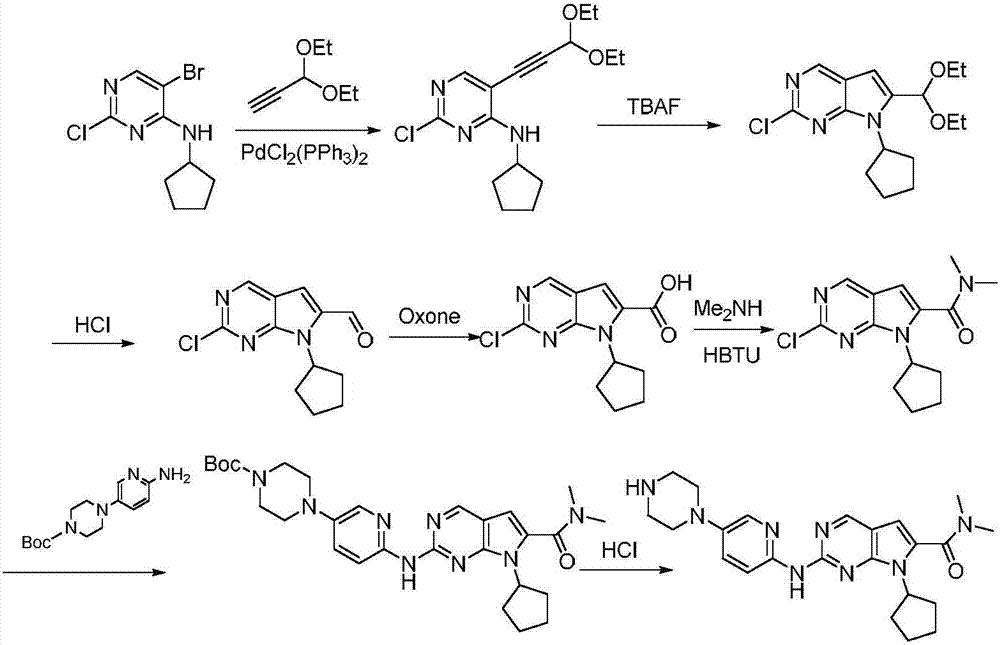
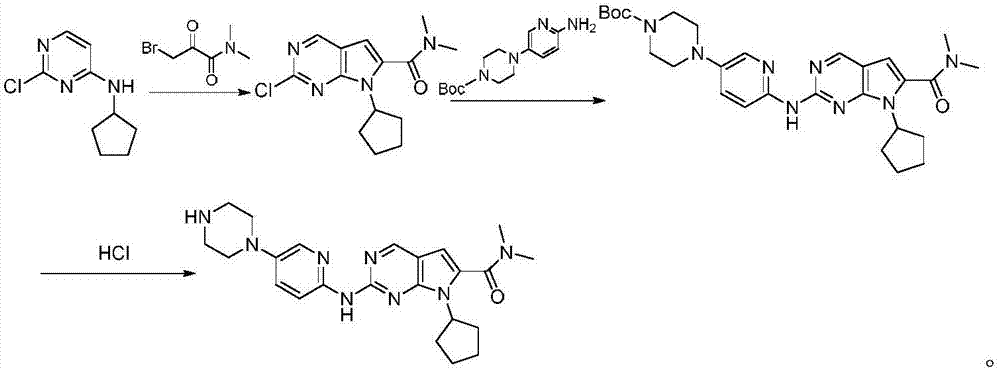
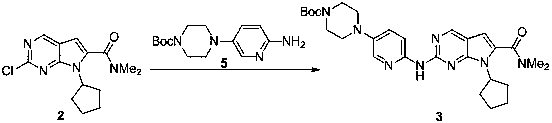
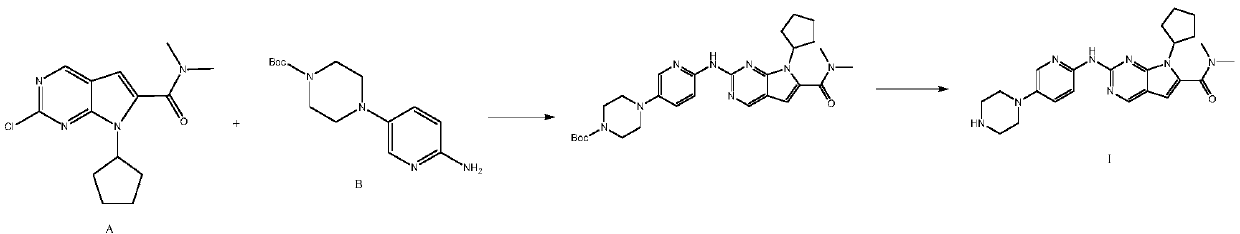
The invention discloses a synthesis technology of ribociclib. The synthesis technology comprises the following steps: 1) in the presence of cesium carbonate, 2-chloro-4-cyclopentylaminopyrimidine and 3-bromo-2-oxo-N,N-dimethylpropionamide react under the co-catalysis of cuprous iodide and L-proline to obtain 2-chloro-7-cyclopentyl-N,N-dimethyl-7H-pyrrole[2,3-d]pyrimindine-6-methanamide; and 2) a product obtained in the step 1) and 4-(6-aminopyridine-3-yl)piperazine-1-carboxylic acid tert-butyl ester are subjected to a nucleophilic reaction, and then formic acid-tert-butyl ester is removed under the acidic condition so as to obtain ribociclib. The synthesis technology of the medicine ribociclib for treating breast cancer has advantages of less reaction steps, mild condition and high yield, and is suitable for industrial production.
Owner:山东君瑞医药科技有限公司
Ribociclib synthesizing method
ActiveCN107936029AMild reaction conditionsFew synthetic stepsOrganic chemistryRibociclibSodium cyanide
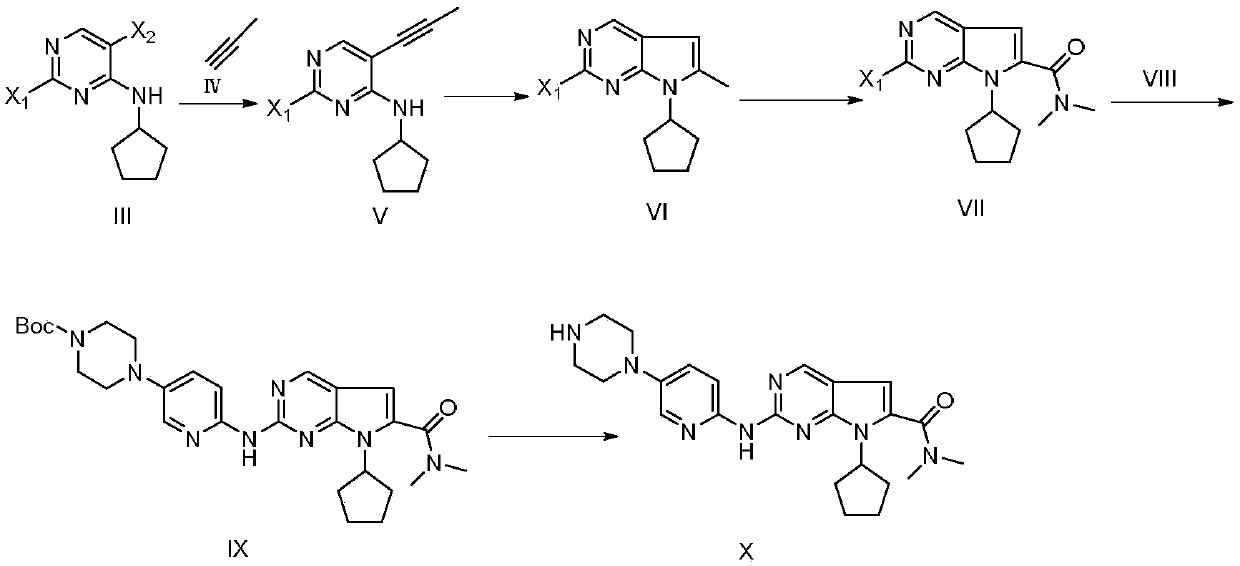
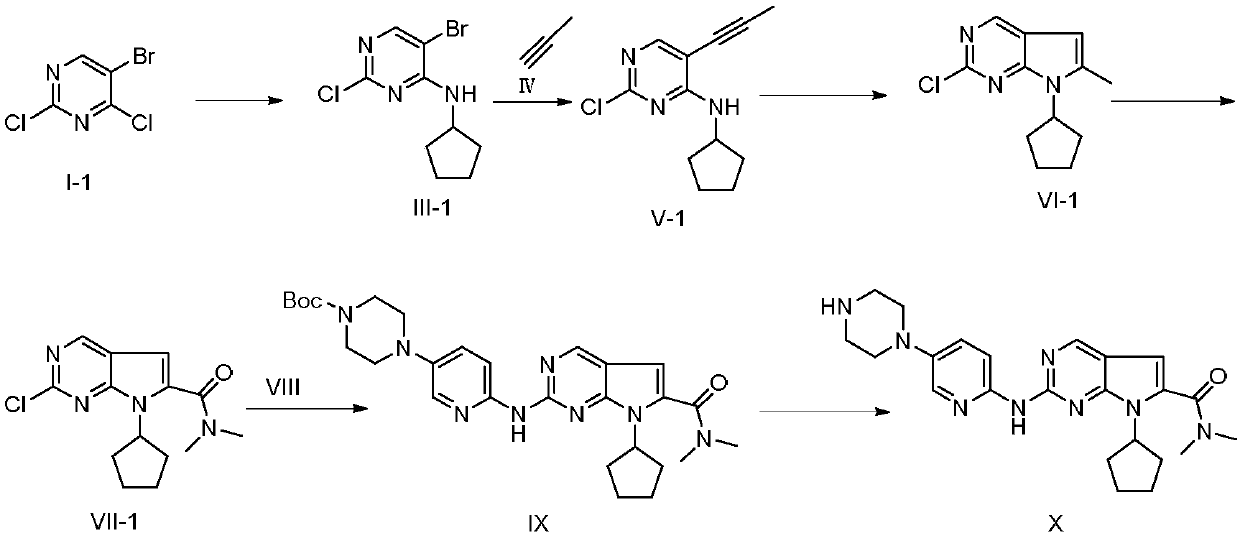
The invention discloses a ribociclib synthesizing method which comprises the following steps: (1) performing coupling reaction between compound in a formula III and compound in a formula IV under theaction of a first metal catalyst to obtain compound in a formula V; (2) performing self cyclization reaction on the compound in the formula V to obtain compound in a formula VI; (3) performing oxidization amidation between the compound in the formula VI and dimethylamine under the action of a second metal catalyst to obtain compound in a formula VII; (4) performing substitution reaction between the compound in the formula VII and compound in a formula VIII to obtain compound in a formula IX; (5) removing protection groups of the compound in the formula IX under the acid condition to obtain compound in a formula X, namely ribociclib. Compared with the prior art, the method disclosed by the invention avoids using noble metal catalysts, poisonous sodium cyanide reagents or the like; reactionconditions are moderate, synthesizing steps are small, reaction selectivity is good, total yield is higher, product liquid phase purity is high, production cost is greatly reduced, and the method is more suitable for industrial production. The formulas are shown in the description.
Owner:安庆奇创药业有限公司
Proteolysis targeting chimeric body, prodrug molecule for improving oral bioavailability of protein hydrolysis targeting chimeric body and application thereof
ActiveCN111909155ASignificant induction periodSignificantly induce apoptosisOrganic chemistryAntineoplastic agentsStage melanomaCancer cell
The invention provides a proteolytic targeting chimeric body, a prodrug molecule for improving oral bioavailability of the proteolytic targeting chimeric body and application thereof. According to thetechnical scheme, a novel PROTAC degradation agent compound is developed on the basis of a ribociclib derivative and a CRBN ligand. Small molecules can effectively degrade CDK2 / 4 / 6 and compounds thereof in malignant melanoma at the same time; the cell cycle can be quickly reset, and apoptosis of various cancer cells, especially melanoma cells, can be induced. A mechanism should be explained as that CDK 2 / 4 / 6 deficiency may lead to synthetic lethal effects of malignant melanoma in the presence of a compound. The results show that the combination of CDK2 / 4 / 6 is expected to become a kinase target for treating solid tumors. In addition, the invention also develops a prodrug with high oral bioavailability for the first time, and is convenient for oral administration in animal tests. A universal solution is provided for oral administration of PROTAC molecules with CRBN ligands.
Owner:DONGGUAN UNIV OF TECH
Ribociclib intermediate and preparation method thereof
ActiveCN105037236AEase of industrial productionRaw materials are easy to getOrganic chemistryHalogenBromocyclopentane
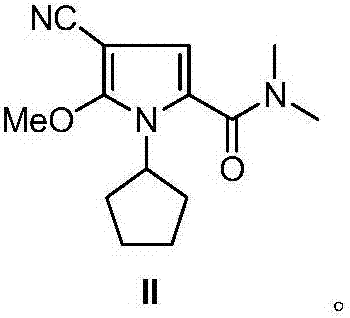
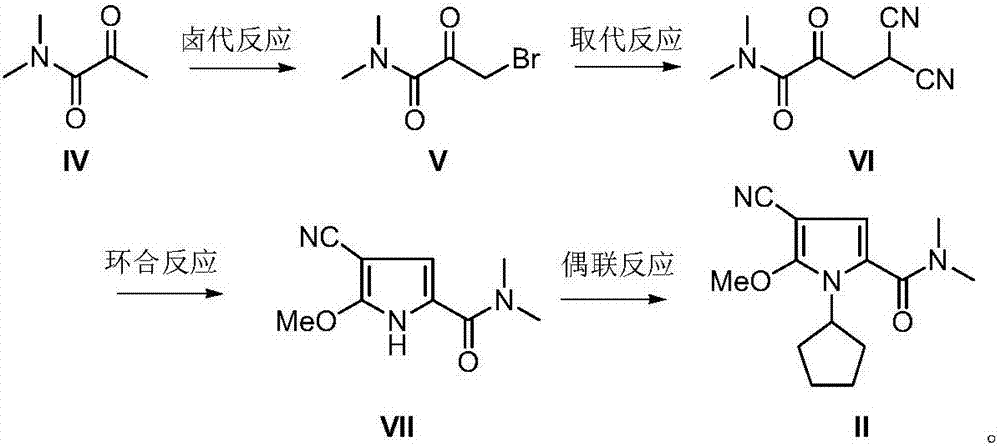
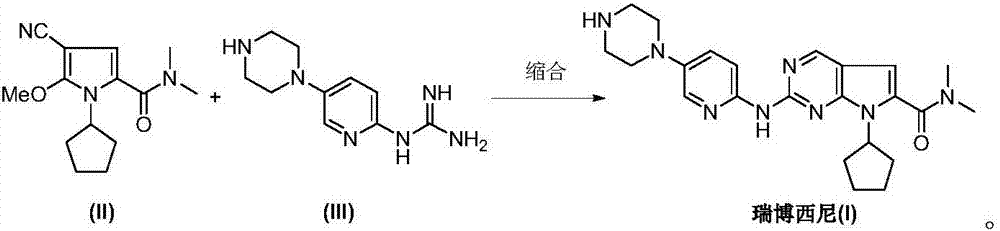
The invention discloses an intermediate N-cyclopentyl-2-methoxy-5-(N,N-dimethyl-formamido)-3-pyrrylformonitrile (II) for preparing ribociclib and a preparation method thereof. The preparation method comprises the following steps: carrying out halogenating reaction on N,N-dimethyl-2-carbonyl-propanamide (IV) to obtain N,N-dimethyl-1-halo-2-carbonyl-propanamide (V); carrying out substitution reaction on the intermediate (V) and malononitrile to obtain N,N-dimethyl-1,1-dicyano-3-carbonyl-butyramide (VI); carrying out cyclization reaction on the intermediate (VI) to obtain 2-methoxy-5-(N,N-dimethyl-formamido)-3-pyrrylformonitrile (VII); and carrying out coupling reaction on the intermediate (VII) and bromocyclopentane to obtain the ribociclib intermediate N-cyclopentyl-2-methoxy-5-(N,N-dimethyl-formamido)-3-pyrrylformonitrile (II). The intermediate (II) and N-[5-(1-piperazino)-2-piperidyl]guanidine (III) are subjected to condensation reaction to obtain the ribociclib. The preparation method has the advantages of accessible raw materials, simple technique, high economy and environment friendliness, and is suitable for industrial production.
Owner:北京华众恩康医药技术有限公司
Preparation method for intermediate of Ribociclib for treating breast cancers
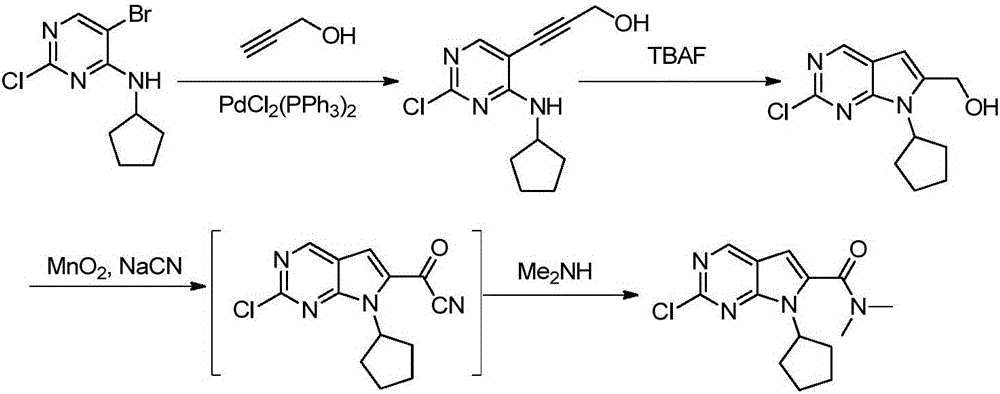
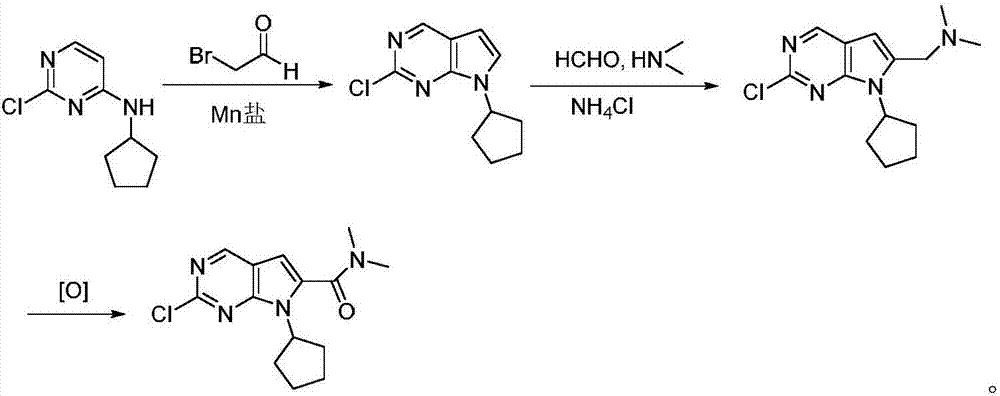
The invention discloses a preparation method of an intermediate of Ribociclib, wherein the preparation method comprises the following steps: 1) in the presence of manganese salt, making 2-chloro-4-cyclopentyl aminopyrimidine and 2-bromoacetaldehyde in a contact reaction to obtain 2-chloro-7-cyclopentyl-7H-pyrrole[2,3-d]pyrimindine; 2) making the product obtained in the step 1) react with formaldehyde and dimethylamine in an ammonium chloride aqueous solution to obtain 2-chloro-7-cyclopentyl-6-(N,N-dimethyl-aminomethyl)-7H-pyrrole[2,3-d] pyrimindine; 3) oxidizing the product obtained in the step 2) to obtain the Ribociclib intermediate, namely 2-chloro-7-cyclopentyl-N,N-dimethyl-7H-pyrrole[2,3-d]pyrimindine-6-formamide. The method is readily available in raw materials, and does not use precious metal catalysts and the like, and is further greatly improved in yield and suitable for industrial production, and ensures the supply of the raw materials for Ribociclib preparation and has great application prospects.
Owner:上海耀大生物科技有限公司
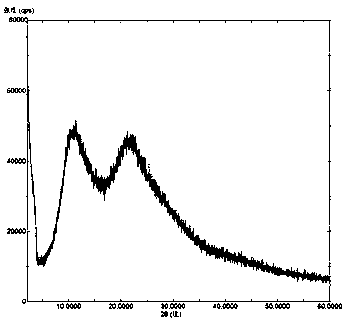
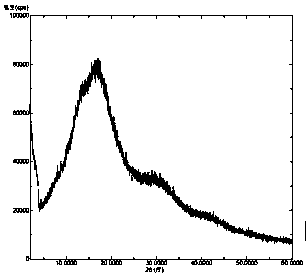
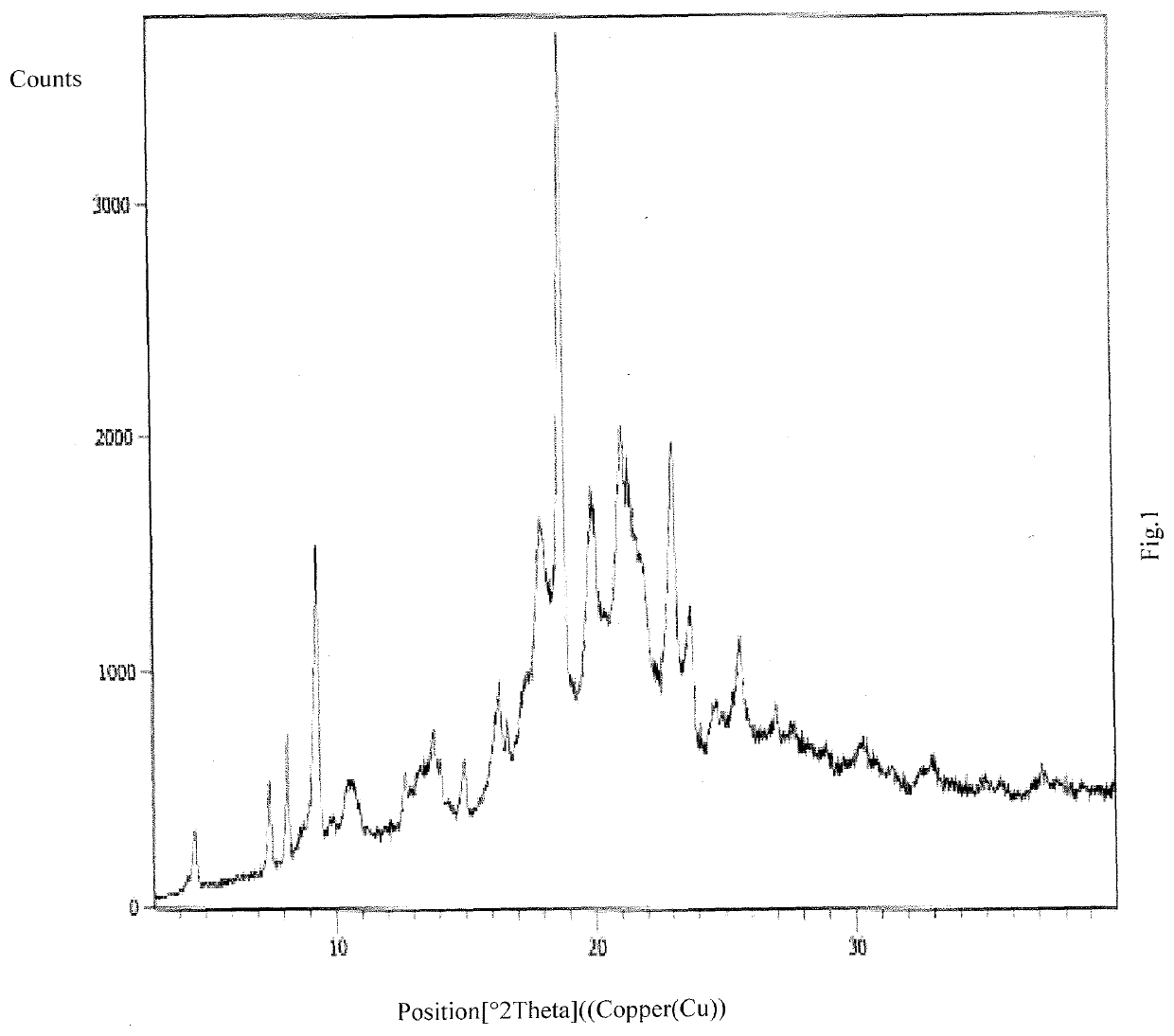
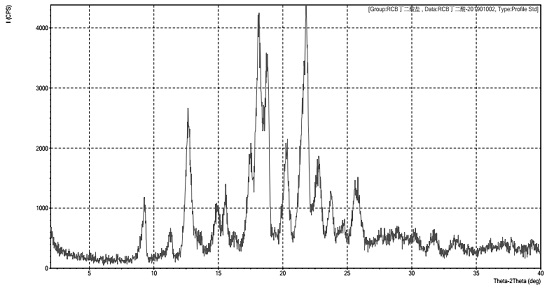
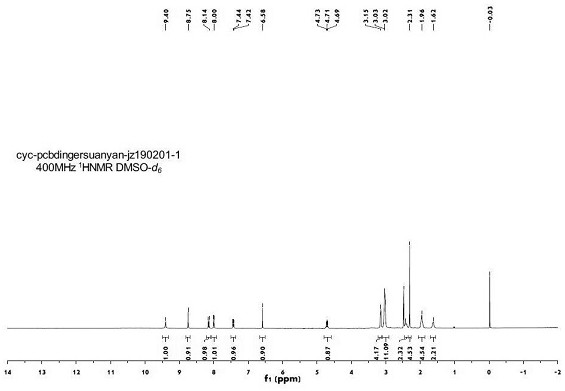
Solid dispersion of amorphous ribociclib or pharmaceutically acceptable salt thereof and pharmaceutical adjuvant, and preparation method thereof
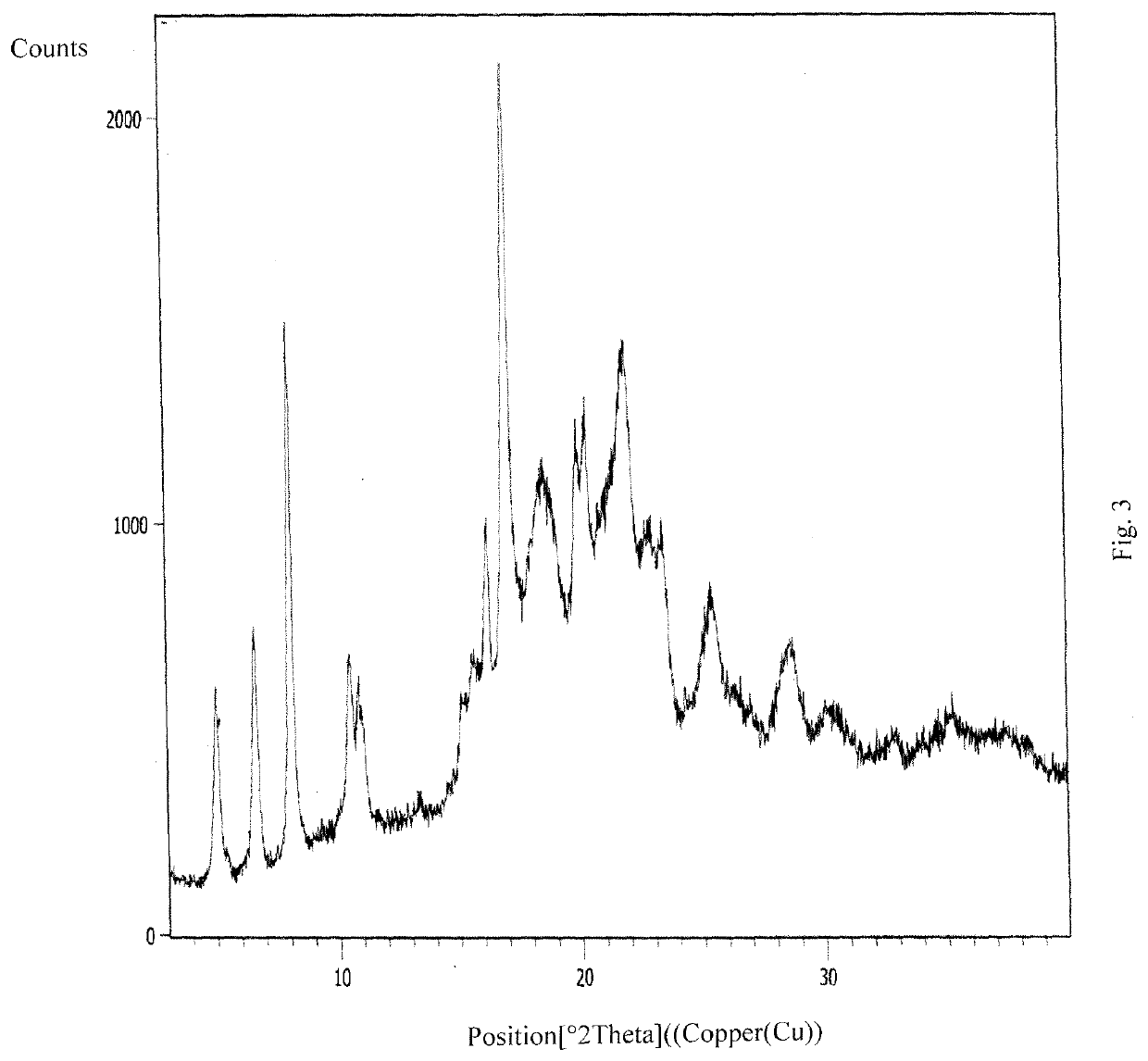
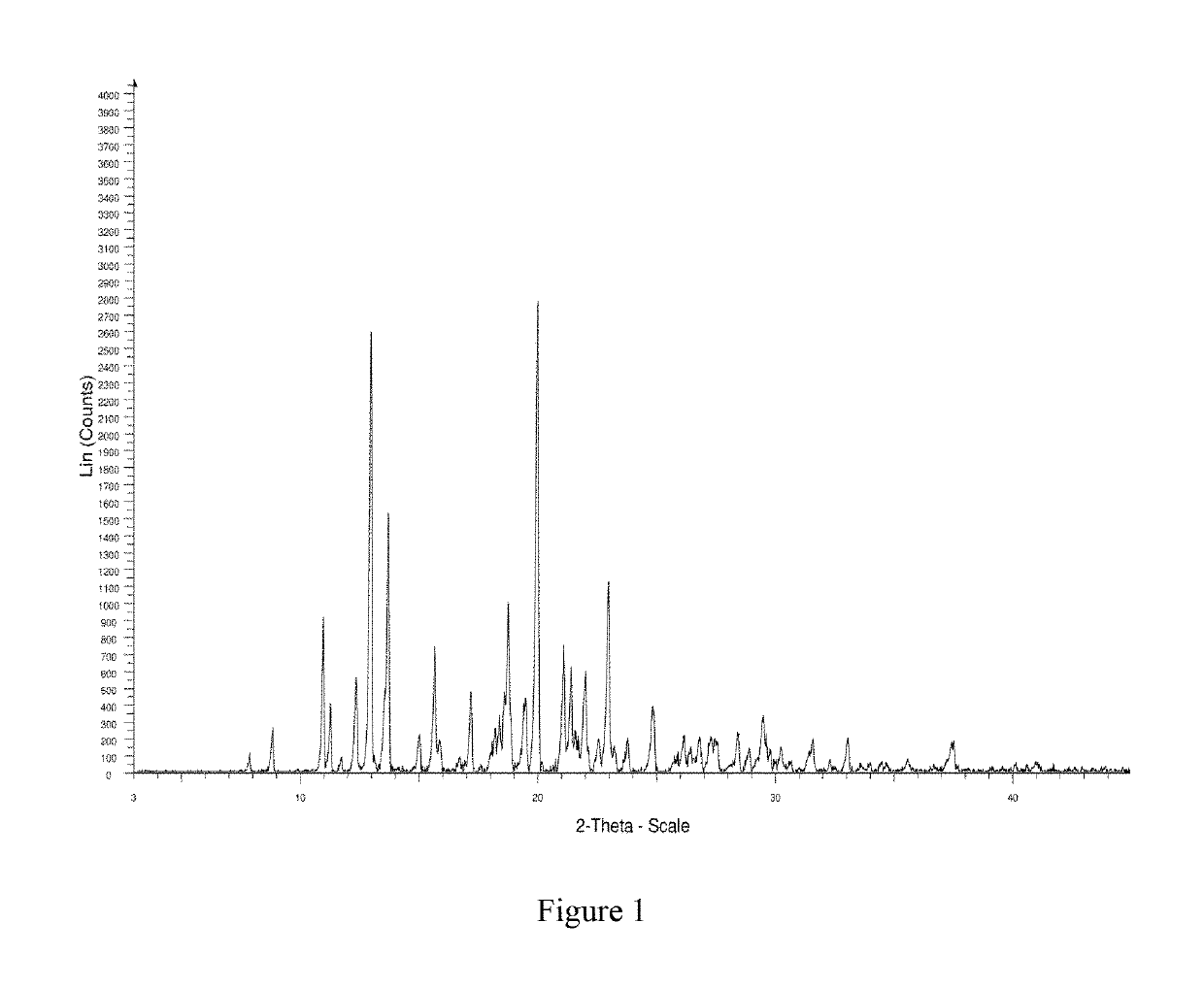
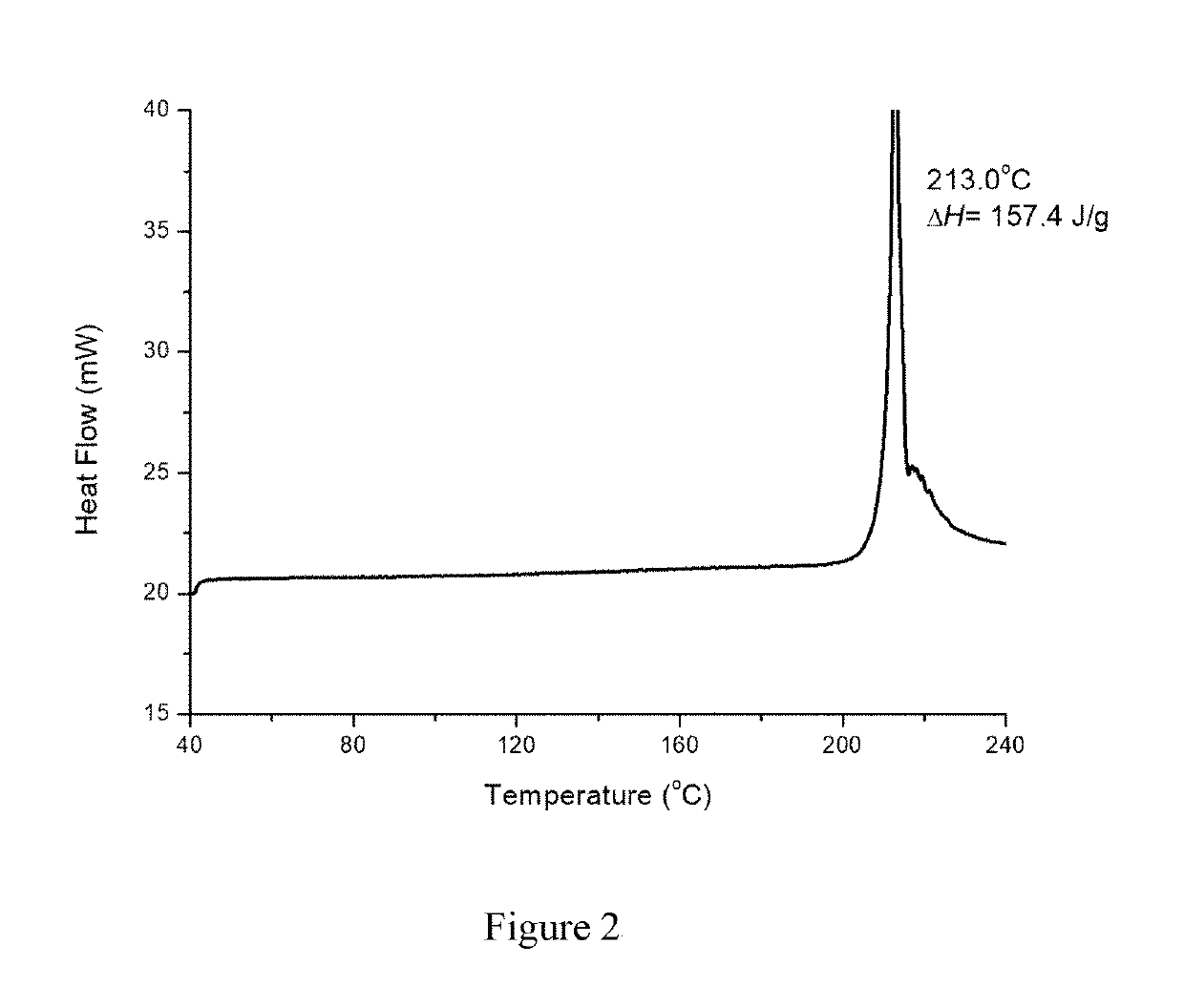
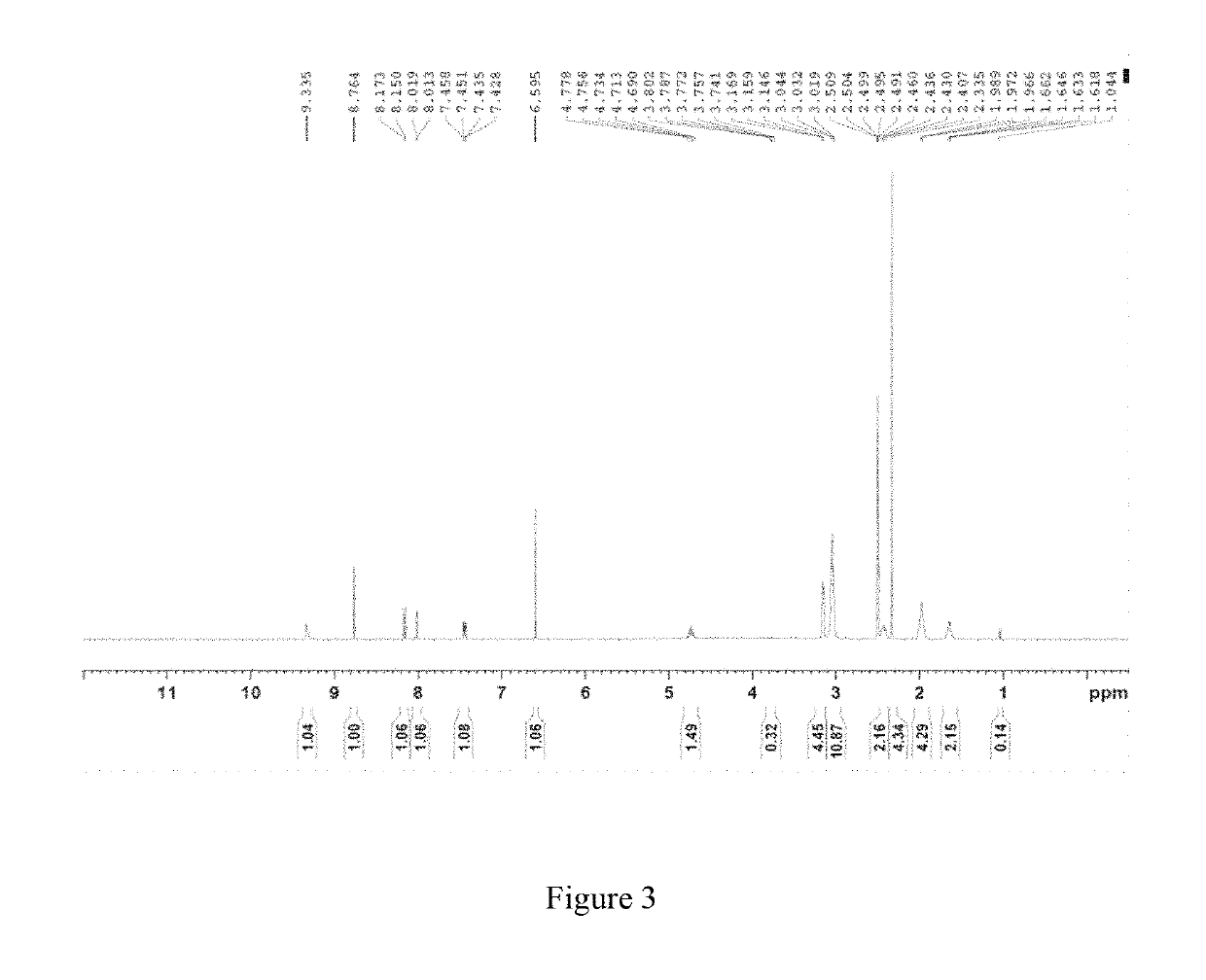
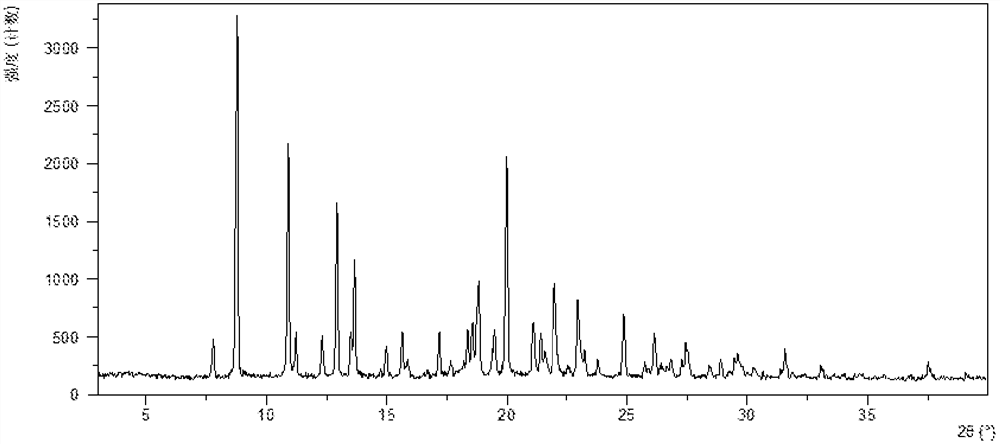
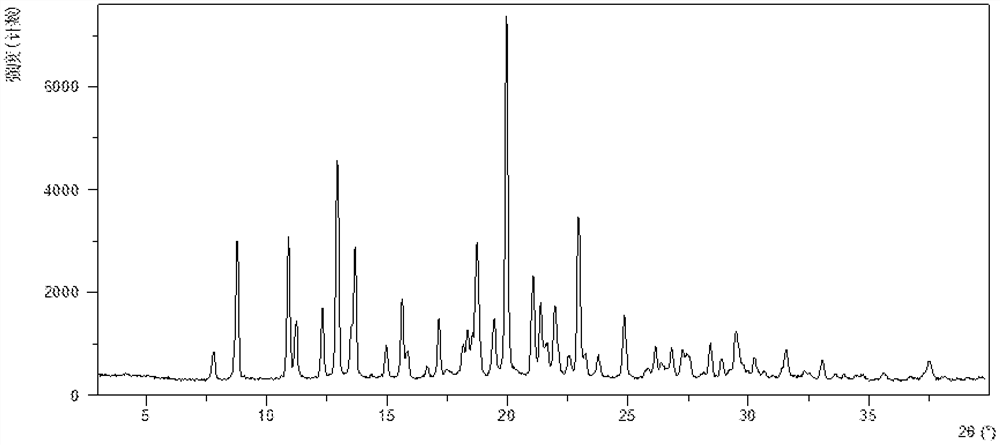
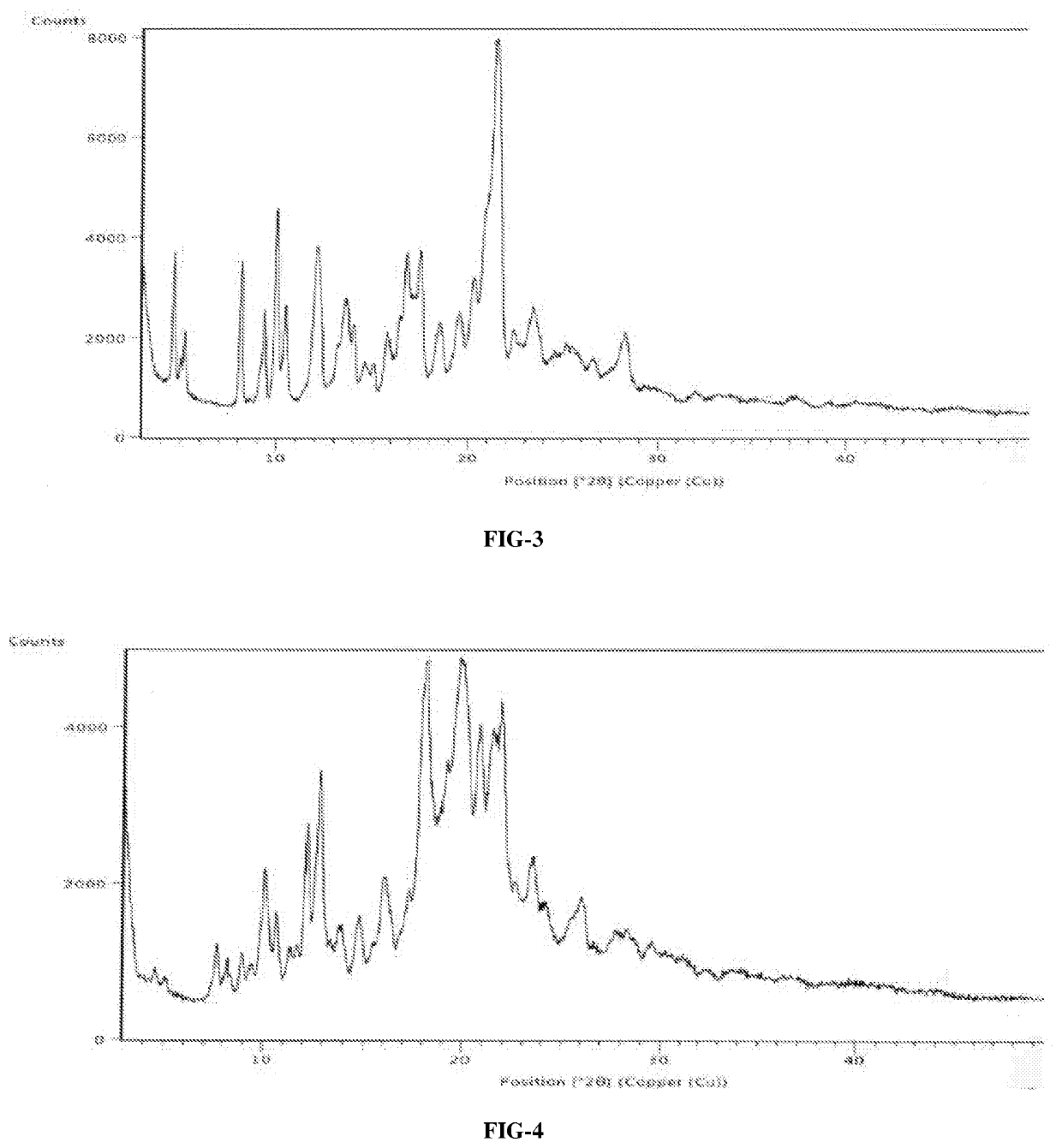
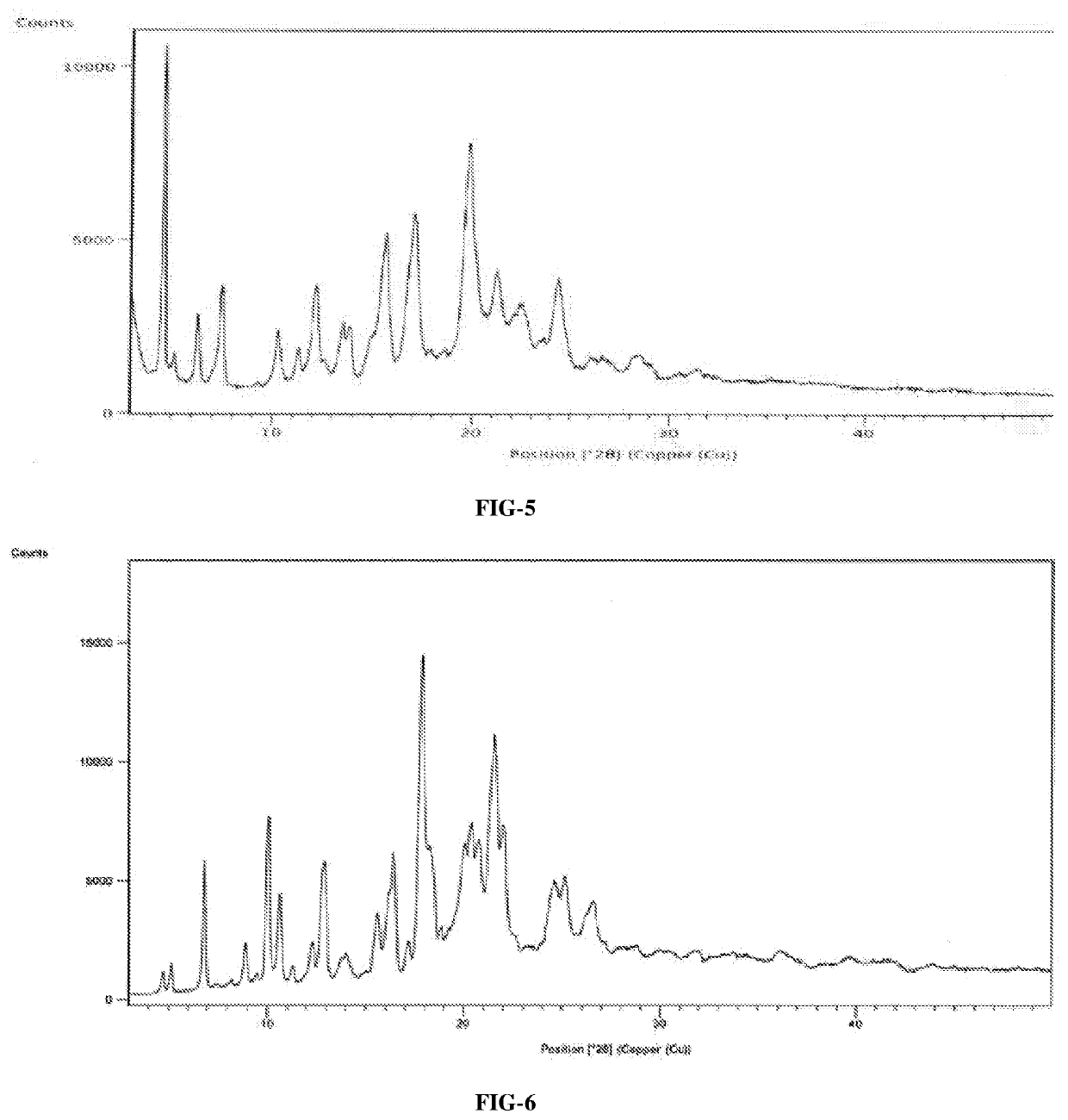
InactiveCN108245486AImprove stabilityLow pricePowder deliveryOrganic active ingredientsX-rayRibociclib
The invention provides a solid dispersion of amorphous ribociclib or a pharmaceutically acceptable salt thereof and a pharmaceutical adjuvant, and a preparation method thereof. The solid dispersion contains ribociclib or the pharmaceutically acceptable salt thereof and the pharmaceutical adjuvant, wherein a weight ratio of ribociclib or the pharmaceutically acceptable salt thereof to the pharmaceutical adjuvant is 1: (0.1-100), ribociclib or the pharmaceutically acceptable salt thereof is amorphous, and the X-ray powder diffraction spectrum of the solid dispersion does not contain the characteristic peaks of crystals of ribociclib or the pharmaceutically acceptable salt thereof after deduction of the background peaks of the pharmaceutical adjuvant. The solid dispersion of ribociclib or thepharmaceutically acceptable salt thereof and the pharmaceutical adjuvant has good stability and dispersibility; the dissolution rate of ribociclib or the pharmaceutically acceptable salt thereof is improved; the bioavailability of the solid dispersion and body absorption of ribociclib are improved; and under accelerated test conditions, the solid dispersion can maintain good physical stability and chemical stability. The preparation method for the amorphous solid dispersion of the invention has the advantages of simple operation, low cost, good reproducibility, easy realization and suitability for industrial production.
Owner:宁波爱诺医药科技有限公司
Preparation method of ribociclib and product and use thereof
InactiveCN109400612AAchieve primary separationMild responseOrganic chemistryCarboxylic acidRibociclib
A preparation method of ribociclib comprises the following steps: 1) utilizing 4-(6-aminopyridine-3-yl)piperazine-1-carboxylic acid tert-butyl ester and 2-chloro-4-cyclopentyl-N,N-dimethyl-7H-pyrrole[2,3-d]pyrimidine-6-formamide as raw materials and conducting reaction in a protective atmosphere with palladium acetate / BINAP as a catalyst, cesium carbonate as an acid absorber and 4-methyl-2-pentanone as a solvent to obtain 4-(6-(7-cyclopentyl-6-(dimethylaminoformyl)-7H-pyrrolo[2,3-d]pyrimidine-2-yl)aminopyridine-3-yl)piperazine-1-carboxylic acid tert-butyl ester); 2) dissolving the4-(6-(7-cyclopentyl-6-(dimethylaminoformyl)-7H-pyrrolo[2,3-d]pyrimidine-2-yl)aminopyridine-3-yl)piperazine-1-carboxylic acid tert-butyl ester) obtained in step 1) in an organic solvent, adding acid dropwise at the room temperature to remove tert-butyl formate, conducting liquid separation, adding a water-soluble organic solvent to an aqueous layer, separating solids and filtering to obtain ribociclib acid salt; 3) adding water to the ribociclib acid salt for dissolving, adding an adsorbent, filtering and adding alkali to the filtrate to obtain the ribociclib.
Owner:CHONGQING SANSHENG IND CO LTD
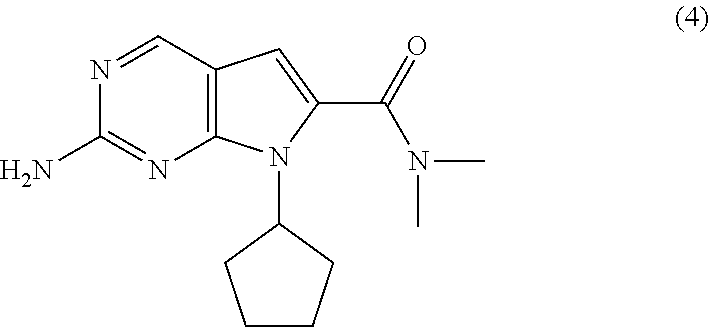
Processes for the Preparation of Ribociclib and Intermediates Thereof
The present invention provides processes for the preparation of Ribociclib, as well as intermediates useful in the preparation thereof. In particular, processes are provided for the preparation of a compound of Formula (4), and its conversion to Ribociclib (1).
Owner:APOTEX INC
An improved process for the preparation of ribociclib and its salts
ActiveUS20200277295A1Avoid tedious and long work-up procedureOrganic active ingredientsOrganic chemistry methodsPerfluoroacetic AcidPharmaceutical drug
The invention relates to a process for the preparation of ribociclib of formula V or its salts. The invention provides novel crystalline forms of ribociclib succinate and ribociclib trifluoroacetate. The present invention also relates to pharmaceutical compositions comprising a crystalline form of ribociclib succinate and at least a pharmaceutically acceptable carrier. It further relates to the use of such compositions in the treatment of cancer.
Owner:FRESENIUS KABI ONCOLOGY LTD
Synthesis method of ribociclib intermediate product and intermediate compound thereof
ActiveCN111100128AReduce usageSimple process routeOrganic chemistryChemical recyclingCombinatorial chemistryRibociclib
The invention discloses a synthesis method of a ribociclib intermediate product, which comprises the following steps: by using barbituric acid as a starting material, carrying out chlorination and formylation to obtain a compound 2; and performing condensation, cyclization, dechlorination, elimination and other reactions to obtain the rebociclib intermediate 2-chloro-7-cyclopentyl-N, N-dimethyl-7H-pyrrolo [2, 3-d] pyrimidine-6-formamide. In addition, the intermediate compound is also disclosed. The synthesis method can avoid the use of noble metal catalysis, has the advantages of simple process route, accessible raw materials and mild conditions, effectively lowers the production cost, and is suitable for large-scale production.
Owner:广安凯特制药有限公司
Crystal form B of ribociclib succinate
ActiveUS10336763B1Residue reductionLow production costOrganic active ingredientsOrganic chemistry methodsRibociclibSuccinic acid
The present invention relates to crystal forms B, C and D of Ribociclib succinate salt and derivatives thereof, and their preparation method and composition. The crystal forms B, C and D of Ribociclib succinate salts are obtained by adding 7-cyclopentyl-N,N-dimethyl-2-(5-(piperain-1-yl)pyridin-2-ylamino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide solution into succinic acid solution for reaction, stirring the solution under high temperature, filtering the solution after cooling off.
Owner:CHUNGHWA CHEM SYNTHESIS & BIOTECH
Pharmaceutical combination comprising the PI3K inhibitor alpelisib and the CDK4/6 inhibitor ribociclib, and the use thereof in the treatment/prevention of cancer
InactiveUS10328066B2Inhibit progressAvoid symptomsAntineoplastic agentsHeterocyclic compound active ingredientsCancer preventionRibociclib
The present disclosure pertains to a pharmaceutical combination comprising (a) an alpha-isoform specific PI3K inhibitor, (b) a cyclin dependent kinase 4 / 6 (CDK4 / 6) inhibitor, and (c) an antimetabolite antineoplastic agent; combined preparations and pharmaceutical compositions thereof; the uses of such a combination in the treatment or prevention of cancer; and methods of treating or preventing cancer in a subject comprising administering a therapeutically effective amount of such combination.
Owner:NOVARTIS AG
Compound and application thereof in synthesis of ribociclib
InactiveCN109553621AReduce operations to remove heavy metal residuesThe substitution reaction is completeOrganic chemistryBulk chemical productionQuality controlNitrogen
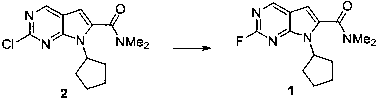
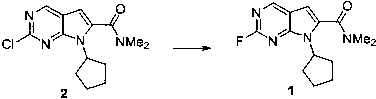
The invention discloses a compound and application thereof in synthesis of ribociclib, and relates to a preparation method and application of a pharmaceutical ribociclib intermediate compound I (6- fluoro - 5,7 - diazindole derivative). The F-substituted compound I avoids the use of a noble metal catalyst in the production process of synthesizing the ribociclib, so that the operation of removing heavy metal residues in the experiment operation is reduced, compared with the substitution reaction of A chlorine element, the method disclosed by the invention is more thorough, efficient and mild, has an important value for the production and purification and quality control of a medicine.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
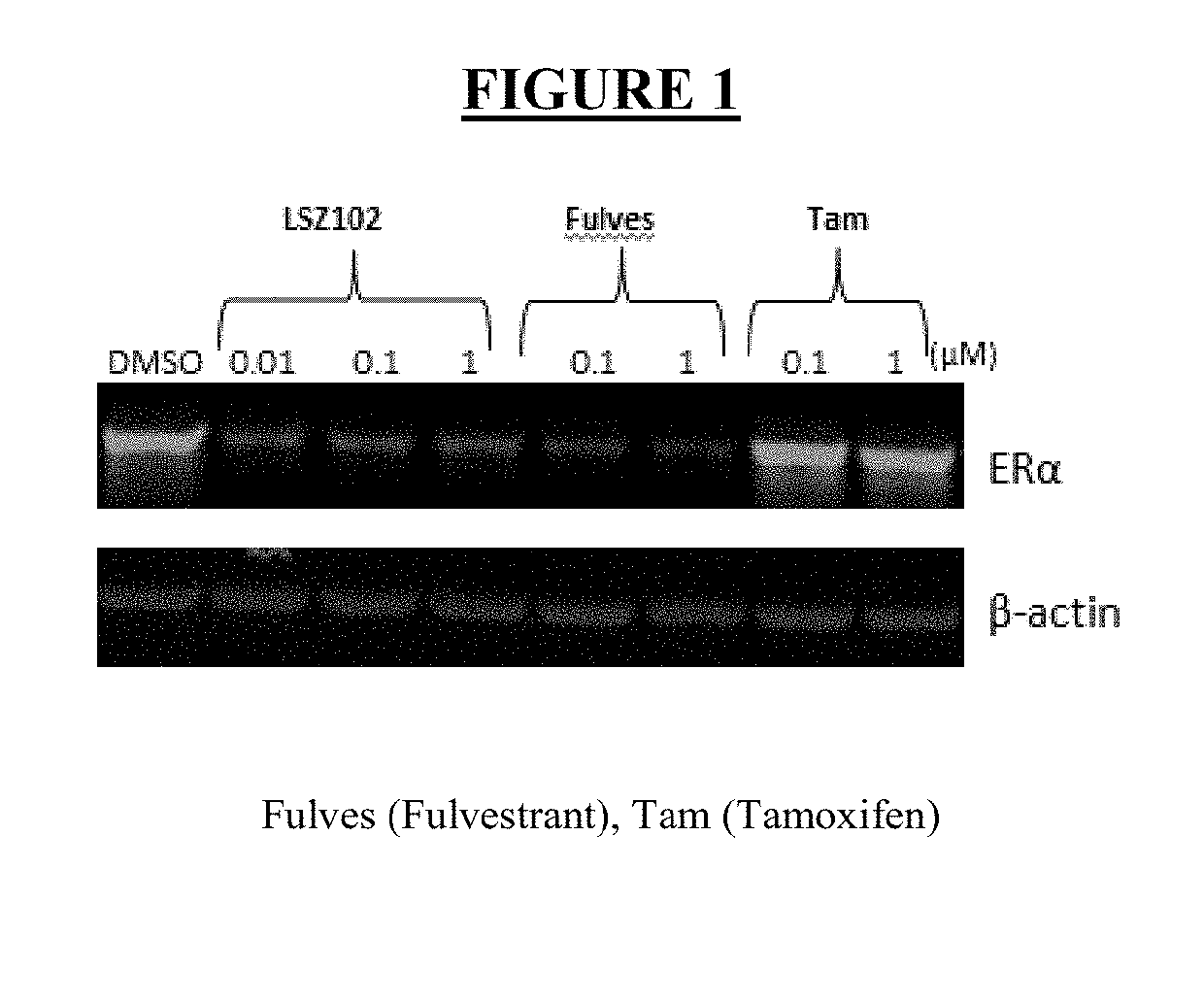
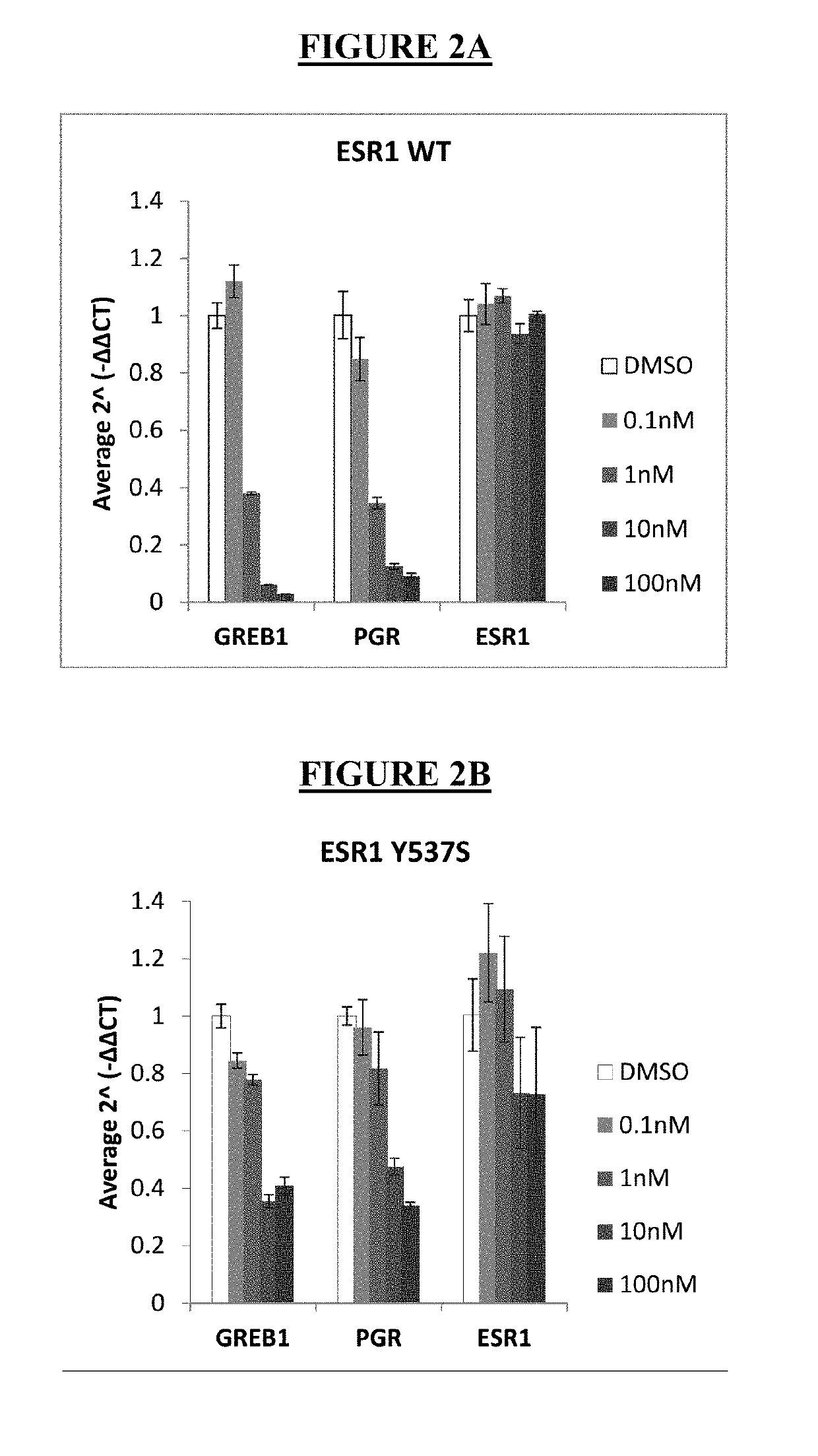
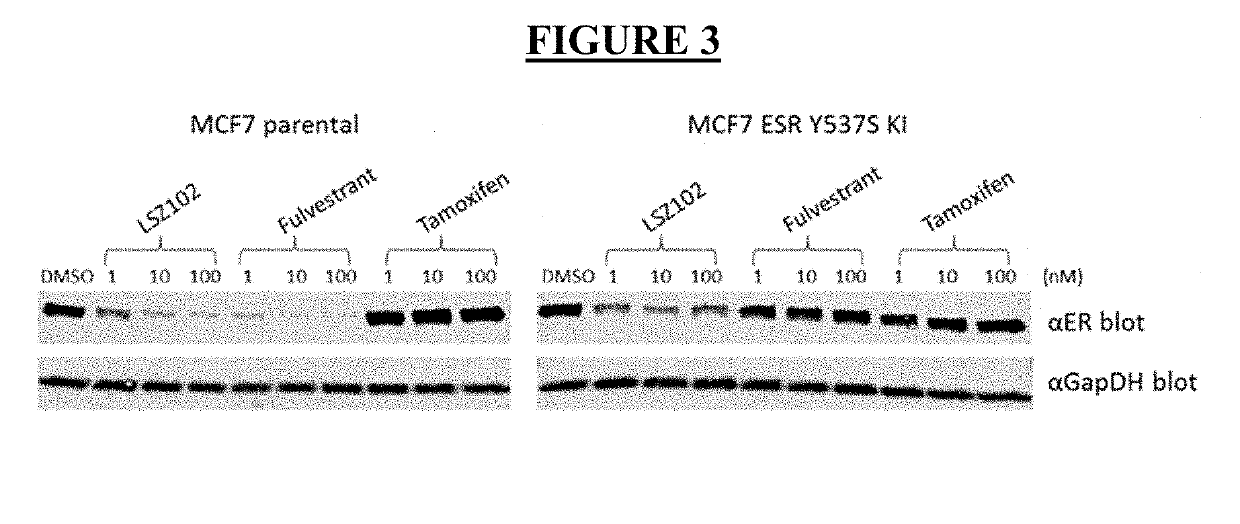
Pharmaceutical combination comprising lsz102 and ribociclib
ActiveUS20190142796A1Organic active ingredientsPharmaceutical delivery mechanismRibociclibEstrogen receptor
The present invention relates to a pharmaceutical combination comprising LSZ102 and ribociclib; pharmaceutical compositions comprising the same; and methods of using such combinations and compositions in the treatment or prevention of conditions in which degradation of estrogen receptors combined with CDK4 / 6 inhibition is beneficial in, for example, the treatment of cancers.
Owner:NOVARTIS AG
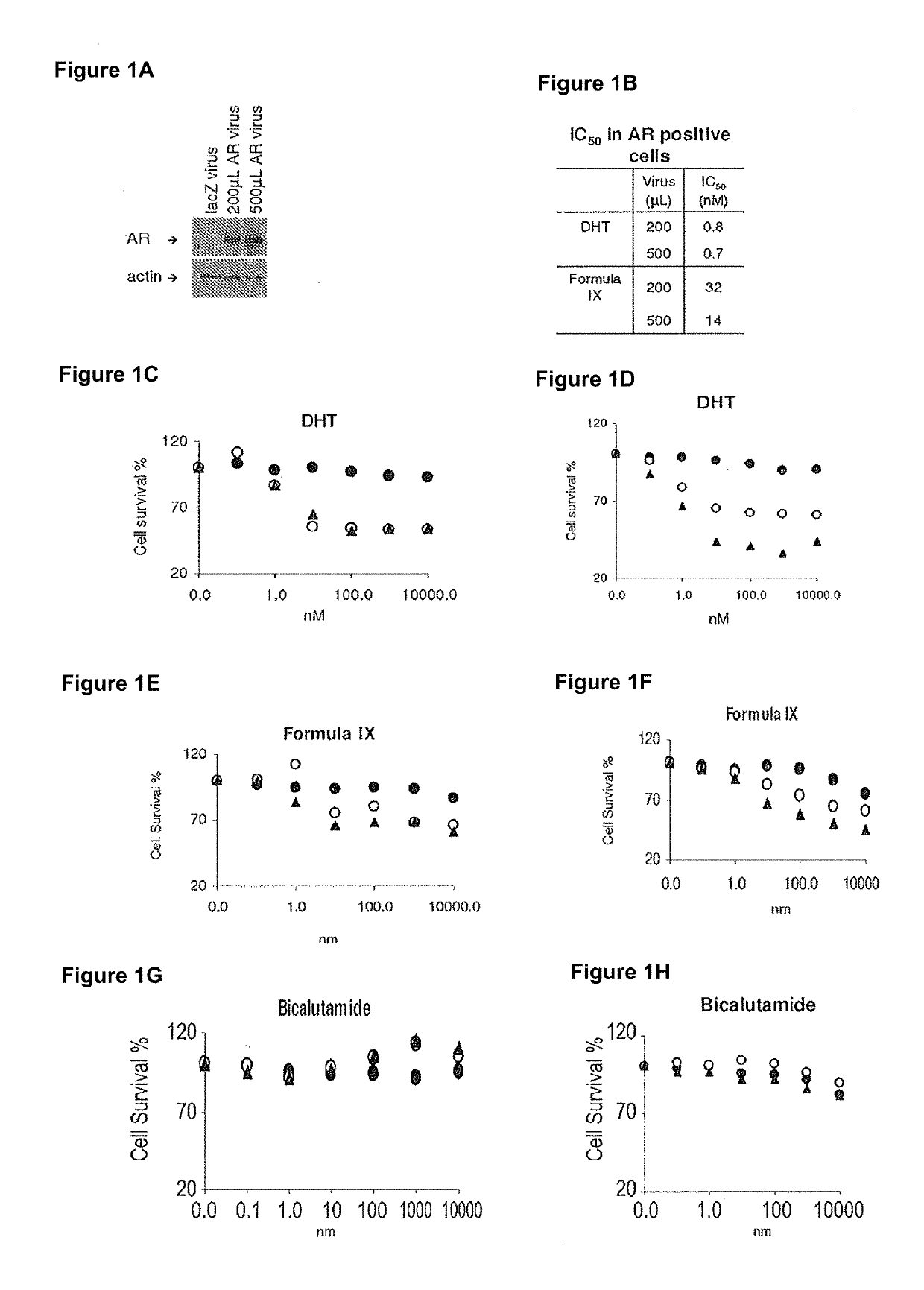
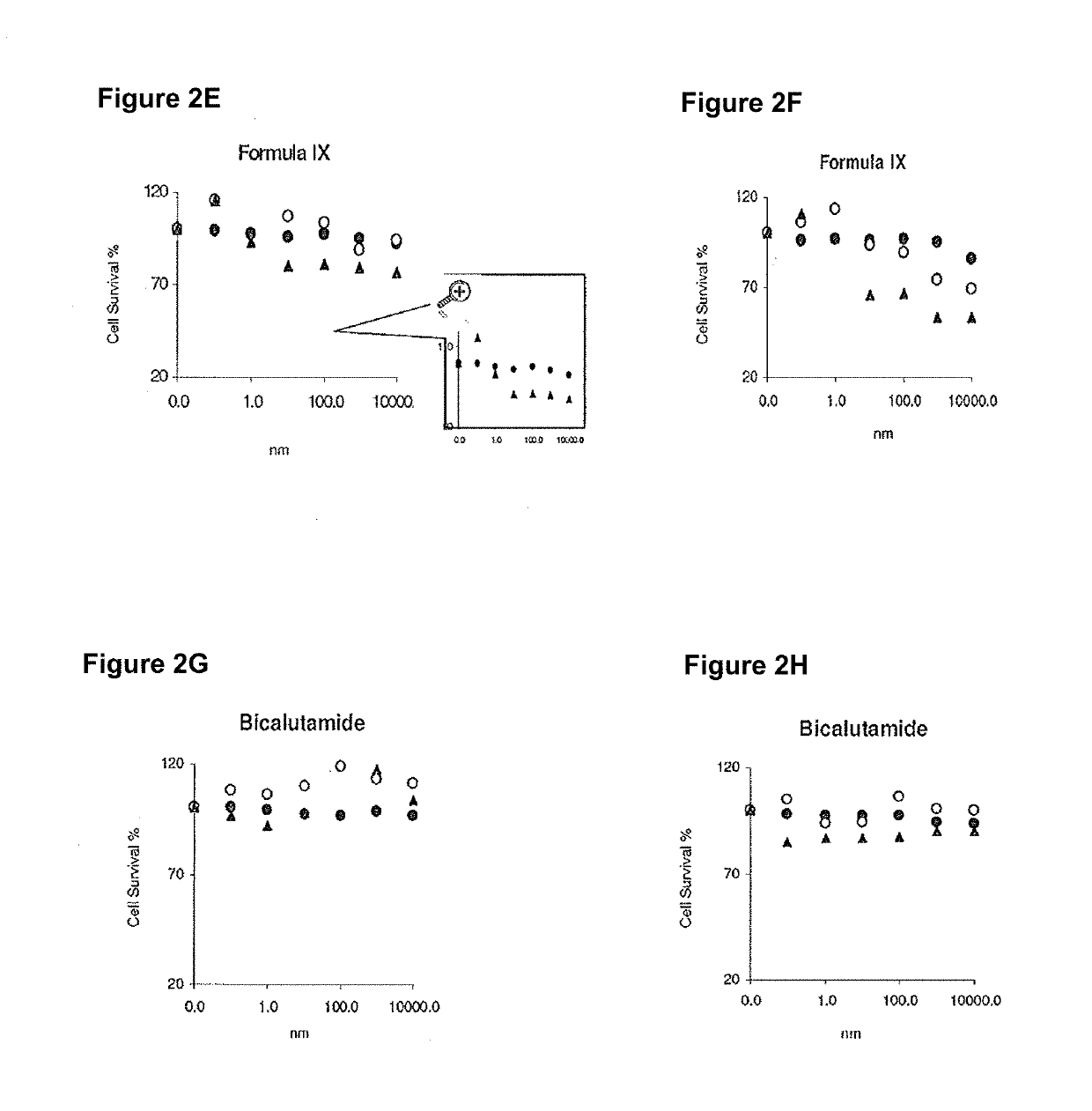
Method of treating er mutant expressing breast cancers with selective androgen receptor modulators (SARMS)
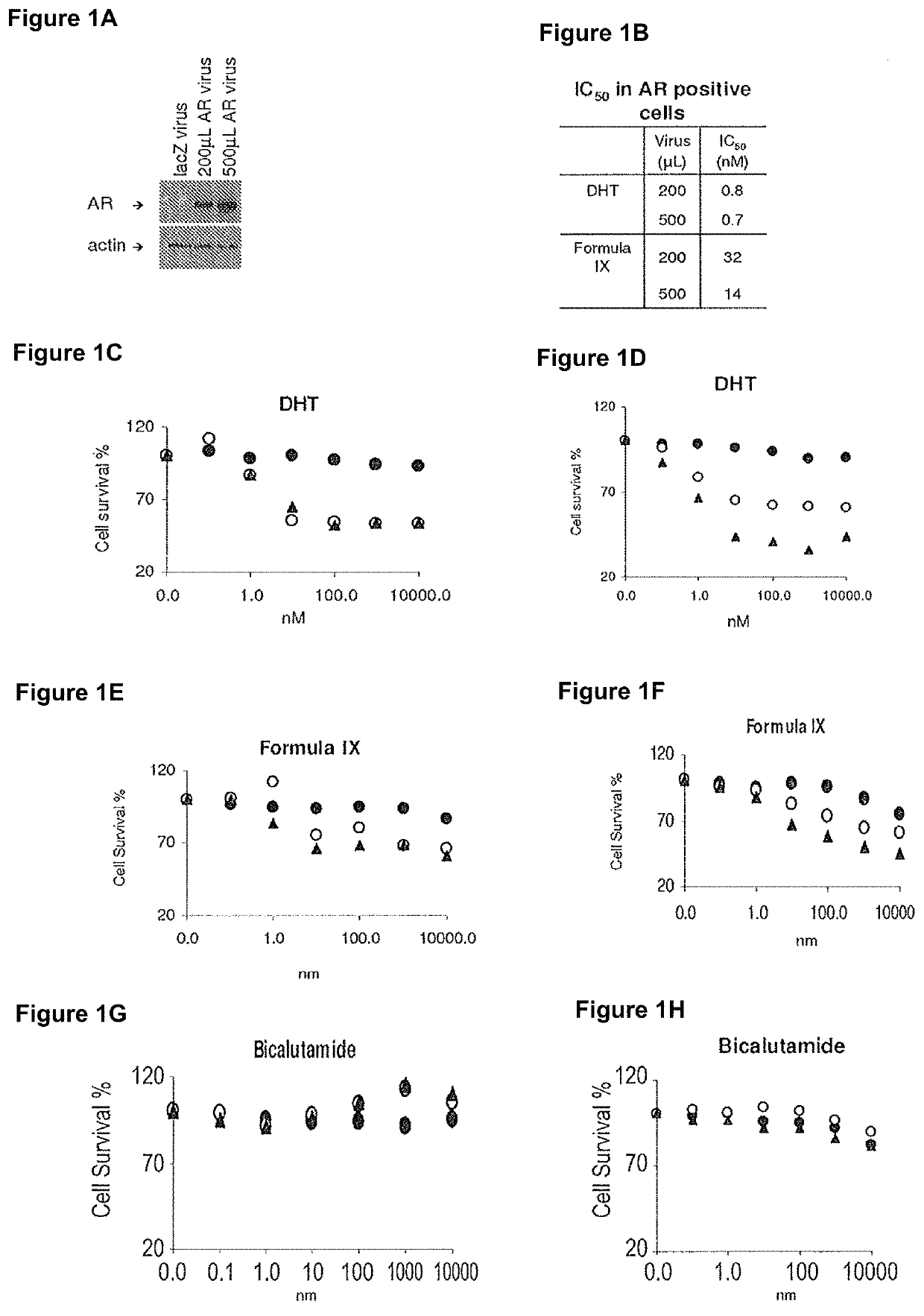
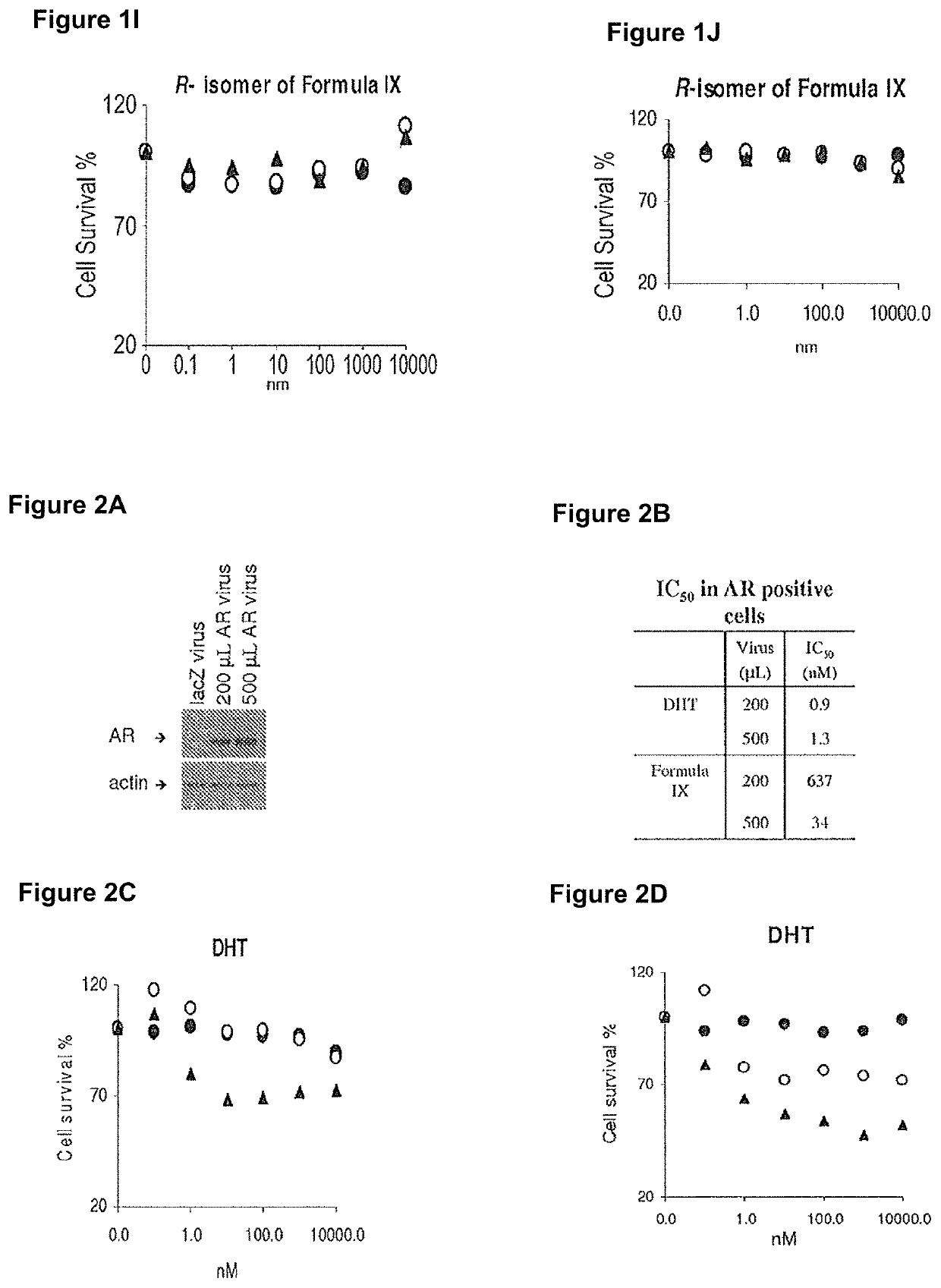
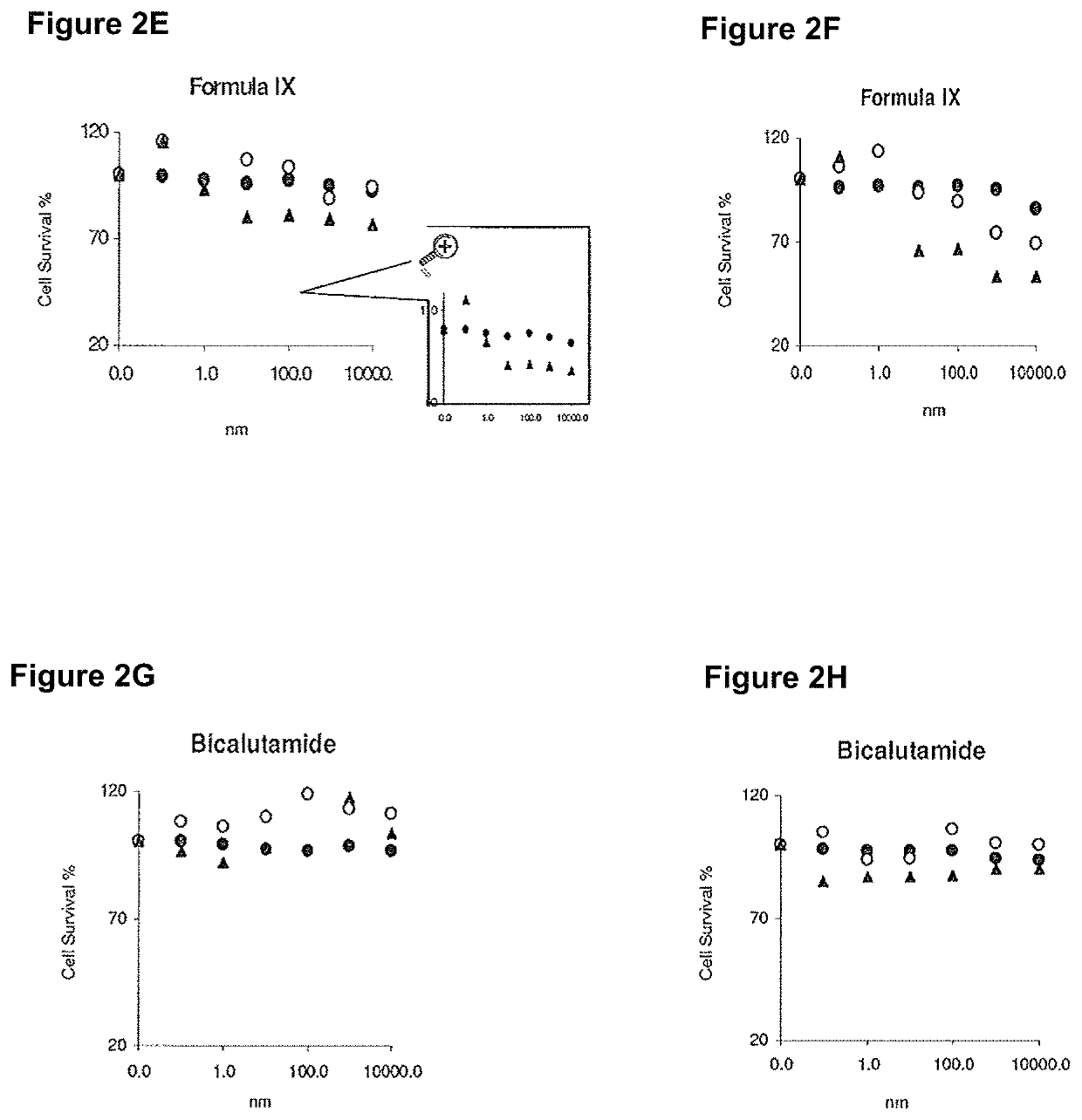
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; and / or treating a subject suffering from ER mutant expressing breast cancer, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Hemi-succinate crystal form CSI of Ribociclib as well as preparation method and application thereof
InactiveCN112794854AOrganic active ingredientsOrganic chemistry methodsPharmaceutical drugRibociclib
The invention relates to a crystal form of hemisuccinic acid Ribociclib, a preparation method and application thereof, a pharmaceutical composition containing the crystal form, and application of the crystal form in preparation of a cyclin-dependent kinase 4 / 6 inhibitor and a pharmaceutical preparation for treating breast cancer. Compared with the prior art, the Ribociclib crystal form CSI provided by the invention has one or more improved properties, and has important value for optimization and development of the medicine in the future.
Owner:CRYSTAL PHARMA CO LTD
Non-invasive method of evaluating breast cancers for selective androgen receptor modulator (SARM) therapy
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; treating a subject suffering from ER mutant expressing breast cancer and / or treating breast cancer in a subject, by first determining the 18F-16β-fluoro-5α-dihydrotestosterone (18F-DHT) tumor uptake and identifying said subject as having AR-positive breast cancer based on 18F-DHT tumor uptake, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Co-crystals of ribociclib and co-crystals of ribociclib monosuccinate, preparation method therefor, compositions thereof, and uses thereof
ActiveUS11286259B2Maintain good propertiesSuitable for processingOrganic active ingredientsOrganic chemistry methodsCholic acidAutoimmune condition
The present invention relates to co-crystals of ribociclib and co-crystals of ribociclib monosuccinate, comprising the co-crystal of ribociclib and saccharin, the co-crystal of ribociclib and cholic acid, the co-crystal of ribociclib and orotic acid, and the co-crystal of ribociclib monosuccinate and citric acid. Compared with the prior art, the co-crystals have one or more improved properties. The present invention also relates to methods of preparing the co-crystal of ribociclib and saccharin, the co-crystal of ribociclib and cholic acid, the co-crystal of ribociclib and orotic acid, and the co-crystal of ribociclib monosuccinate and citric acid, pharmaceutical compositions, and uses thereof in the preparation of medicines for treating and / or preventing diseases involving one or more symptoms of protein kinase related dysfunctions, cancers, transplant rejection and autoimmune diseases.
Owner:SOLIPHARMA
Crystal form of ribociclib succinate as well as preparation method and application thereof
InactiveCN111718347AImprove solubilityImprove stabilityOrganic active ingredientsOrganic compound preparationRibociclibSuccinic acid
The invention relates to a crystal form of ribociclib succinate as well as a preparation method and application thereof. Specifically, the invention provides a plurality of new crystal forms of ribociclib succinate and a preparation method thereof, and the new crystal forms have excellent solubility and other properties.
Owner:ANLITE SHANGHAI PHARMA TECH CO LTD +2
Combination cancer therapies
PendingUS20220047546A1Induce p5 activityOrganic active ingredientsAntineoplastic agentsDepressantRibociclib
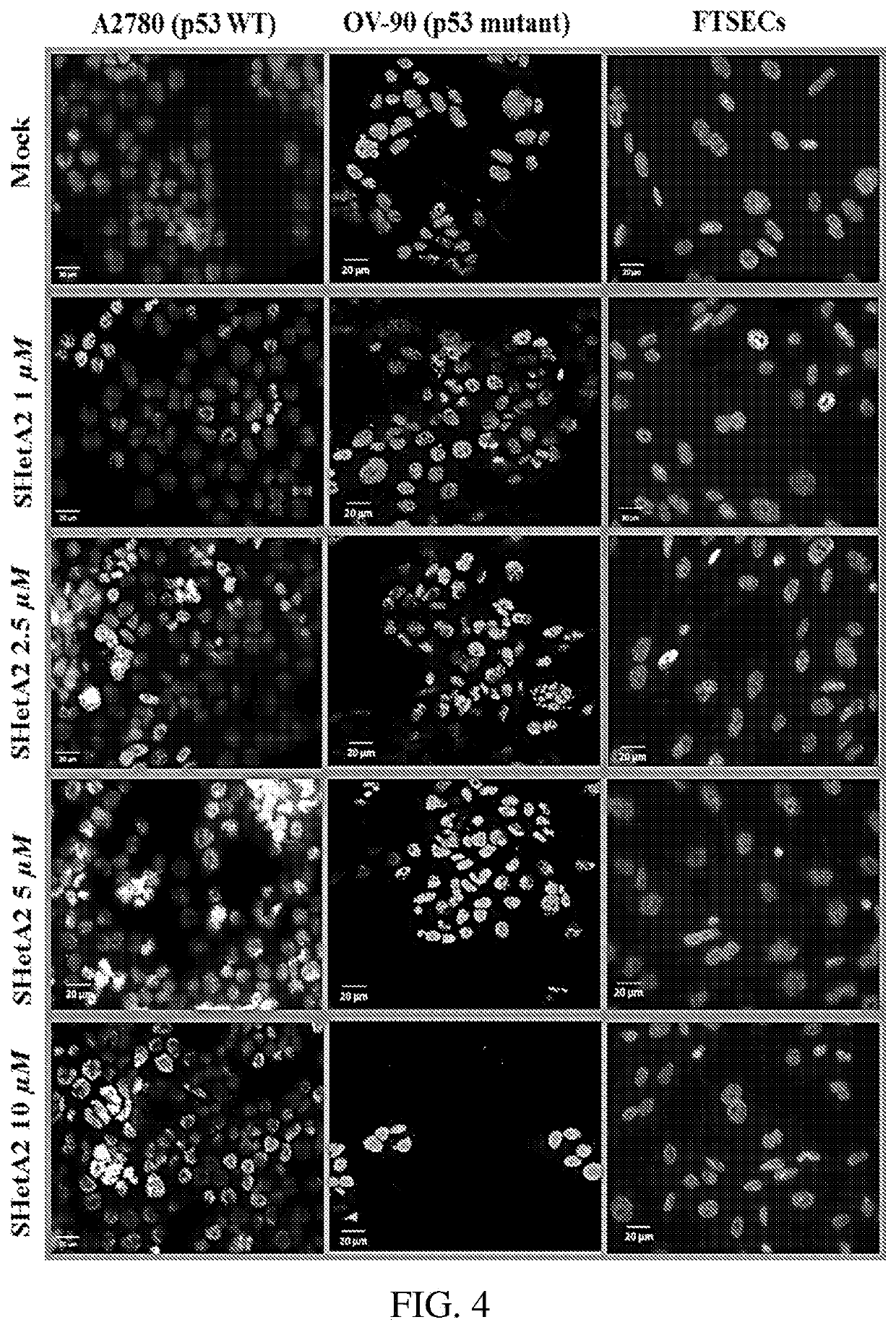
Drug combinations of a heteroarotinoid (e.g., SHetA2), and an Azabicyclooctan-3-one derivative (e.g., PRIMA-1 or PRIMAMET) and / or, a CDK4 / 6 inhibitor (e.g., Palbociclib, Abemaciclib, or Ribociclib), which are synergistically-effective as anti-cancer treatments, and kits and methods of use of such drug combinations.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Method of treating ER mutant expressing breast cancers with selective androgen receptor modulators (SARMs)
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; and / or treating a subject suffering from ER mutant expressing breast cancer, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Ribociclib monosuccinate crystal form and its preparation method and use
ActiveCN111094290BReduce humidityOvercome the disadvantages of high humidityOrganic active ingredientsOrganic chemistryPharmaceutical drugDrugs preparations
Crystal forms X, III and V of the monosuccinate salt of Ribociclib represented by the formula (1), and a preparation method thereof. A pharmaceutical composition containing the crystal form. Use of the crystal form in preparing cyclin-dependent kinase 4 / 6 inhibitors and pharmaceutical preparations for treating breast cancer.
Owner:CRYSTAL PHARMATECH CO LTD
An improved process for the preparation of ribociclib succinate and its novel crystalline forms thereof
The present invention relates to an improved process for the preparation of 7-cyclopentyl-N,N-dimethyl-2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide succinate (1 / 1) compound of formula-1a and its novel crystalline forms. The said compound of formula-1a is represented by the following structural formula: Formula-1a
Owner:NATCO PHARMA LTD
Synthesis method of antineoplastic drug ribociclib intermediate
The invention relates to a synthesis method of an antineoplastic drug ribociclib intermediate. The synthesis method includes the following steps that the electrophilic addition reaction is conducted on N,N-dimethylacrylamide and bromine to obtain N,N-dimethyl-2,3-dibromopropanamide, then under alkaline conditions, debromination is conducted to prepare N,N-dimethylpropiolamide, and at last the antineoplastic drug ribociclib intermediate is prepared by the reaction with N-cyclopentadienyl-2-chlorine-5-bromine-4-amidepyrimidinyl under the action of a catalyst. According to the synthesis method ofthe antineoplastic drug ribociclib intermediate, the preparation process is easy to operate, yield is high, the reaction route is short, three wastes are less, and industrial production is facilitated.
Owner:DONGHUA UNIV
A kind of industrial preparation method of Ribociclib
ActiveCN109928975BRaw materials are cheap and easy to getOptimize process flowOrganic chemistryBulk chemical productionRibociclibPyrrole
The invention relates to an industrial preparation method for ribociclib. The method comprises the following steps: carrying out a condensation reaction on N,N-dimethyl-2,2-dihalo-4-cyano-n-butanamide(II) and a methylenenation reagent (III) to prepare a compound of formula IV; condensing the compound of formula IV and a compound of formula V to obtain a compound of formula VI; carrying out an N-substitution reaction on the compound VI and cyclopentane halide to obtain N,N-dimethyl-7-cyclopentyl-2-{5-[(4-PG-substituted)piperazin-1-yl)pyridin-2-yl]}amino-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide (VIII), and removing the PG substituent from the compound VIII to obtain the ribociclib. The method of the invention has the advantages of cheap and easily available raw materials, simple process flow, safety in operation, and low cost.
Owner:XINFA PHARMA
Ribociclib intermediate and preparation method thereof
ActiveCN105037236BEase of industrial productionRaw materials are easy to getOrganic chemistryRainbowBromocyclopentane
The invention discloses an intermediate N-cyclopentyl-2-methoxy-5-(N,N-dimethyl-formamido)-3-pyrrolecarbonitrile for preparing Ribociclib (II) and its preparation method, the preparation steps include: N,N-dimethyl-2-carbonyl-propionamide (IV) is subjected to halogenation reaction to obtain N,N-dimethyl-1-halogen-2-carbonyl -Propionamide (V); N,N-dimethyl-1,1-dinitrile-3-carbonyl-butanamide (VI) is prepared by substitution reaction between intermediate V and malononitrile; intermediate VI is cyclically 2-methoxy-5-(N,N-dimethyl-formamide)-3-pyrrolecarbonitrile (VII) is prepared by the coupling reaction; the intermediate VII is prepared by the coupling reaction with bromocyclopentane Ribociclib intermediate N-cyclopentyl-2-methoxy-5-(N,N-dimethyl-formamido)-3-pyrrolecarbonitrile (II). The condensation reaction between intermediate II and N-[5-(1-piperazinyl)-2-piperidinyl]guanidine (III) produces ribociclib. The preparation method has easy-to-obtain raw materials, simple process, economical and environmental protection, and is suitable for industrial production.
Owner:北京华众恩康医药技术有限公司
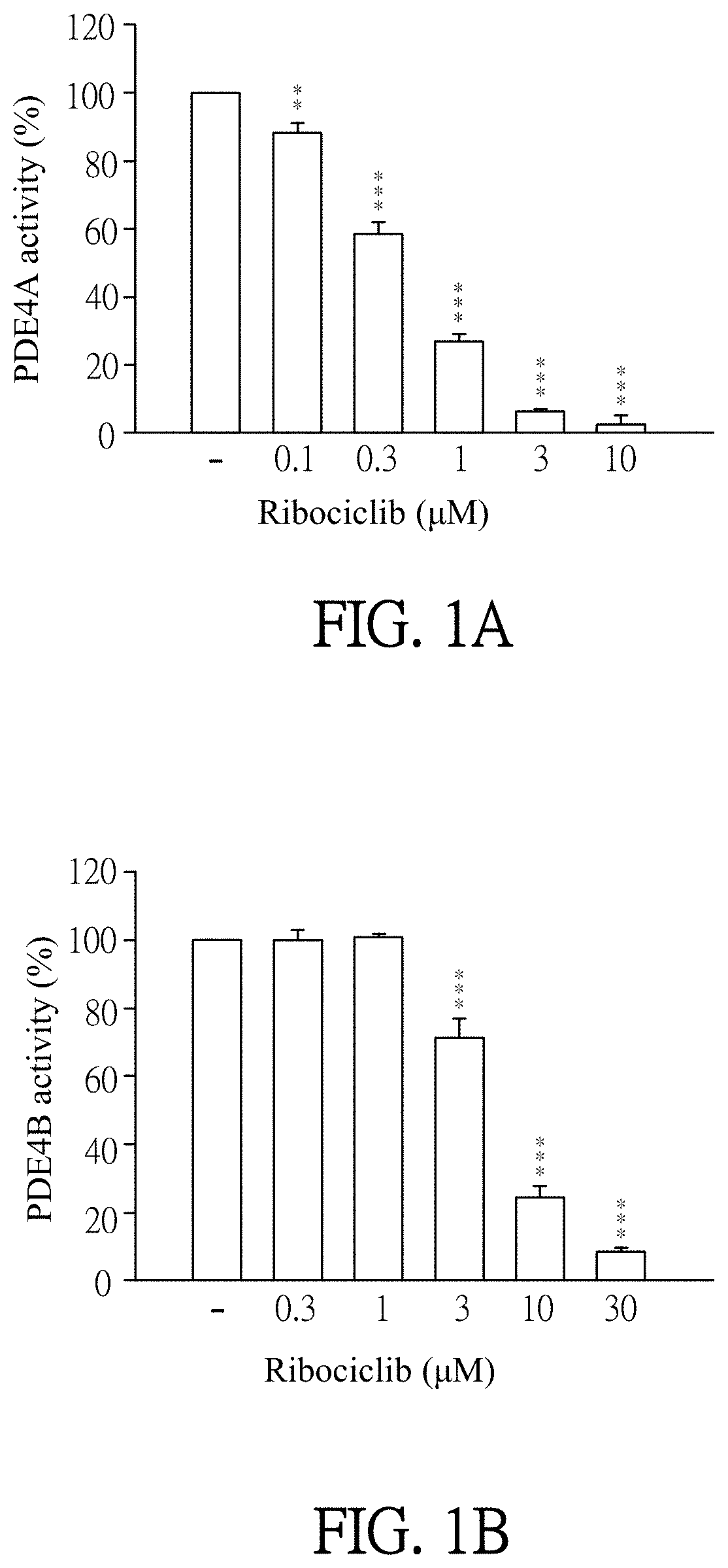
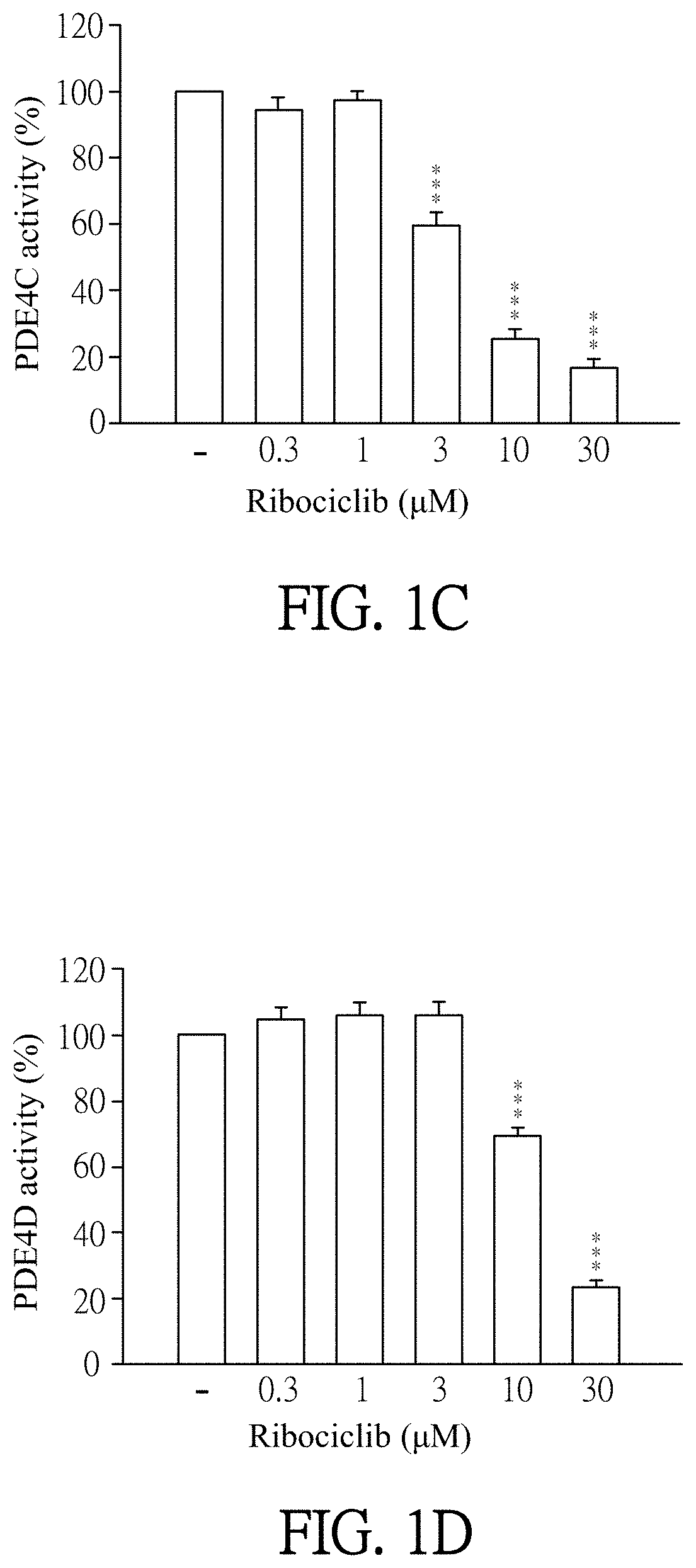
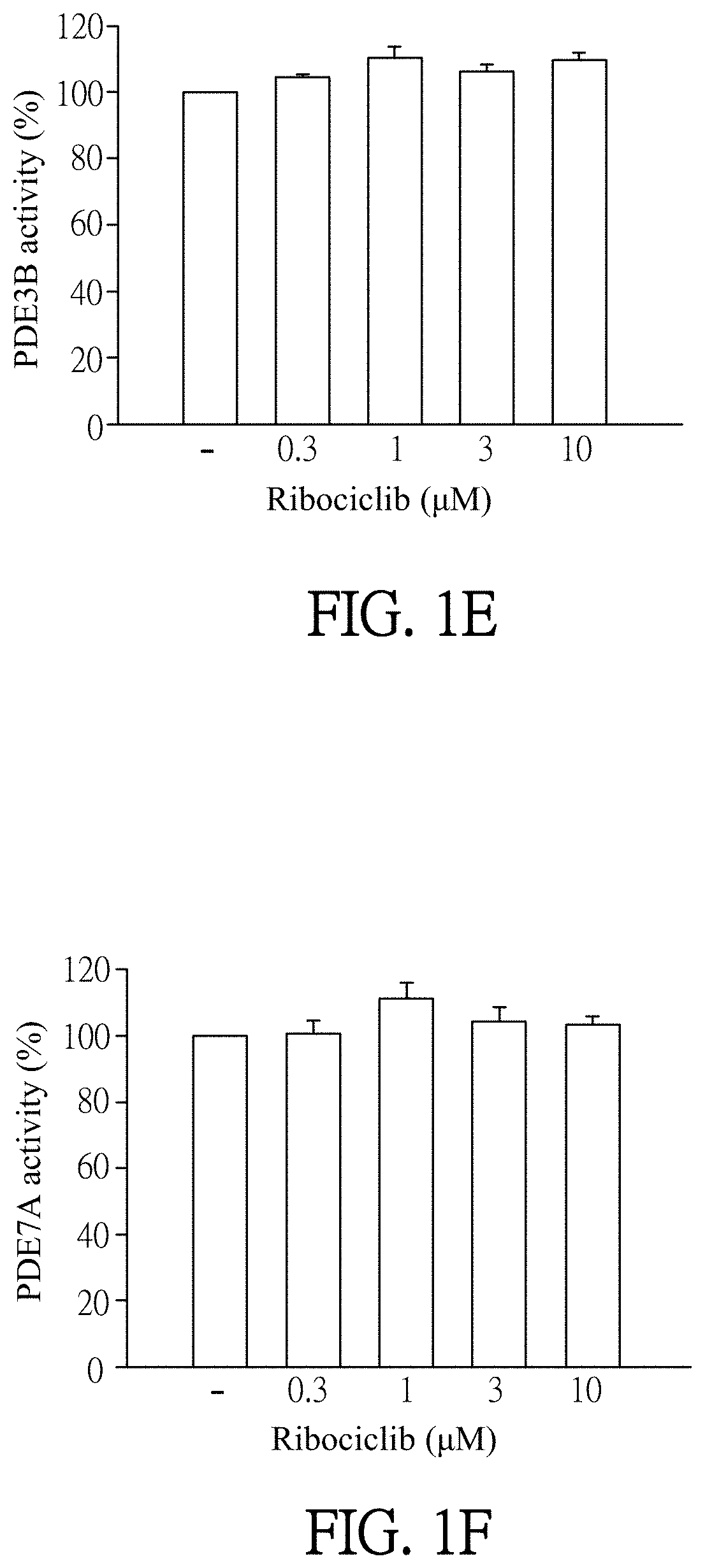
Pharmaceutical composition and use for applying ribociclib in phosphodiesterase 4-mediated disease treatment of patient and inhibition of phosphodiesterase 4 activity
ActiveUS11351172B2Reduced activityReduce usageAntibacterial agentsOrganic active ingredientsPhosphodiesteraseDisease
The invention provides a pharmaceutical composition for treating phosphodiesterase 4-mediated (PDE4-mediated) disease in a patient and inhibition of phosphodiesterase 4 (PDE4) activity. The pharmaceutical composition includes an effective amount of Ribociclib and a pharmaceutically acceptable carrier. The invention further provides a use of the pharmaceutical composition for treating PDE4-mediated disease in a patient. The application of the pharmaceutical composition of the present invention and use thereof are advantageous for inhibiting of PDE4 activity and thus treating a PDE4-mediated disease.
Owner:CHANG GUNG UNIVERSITY
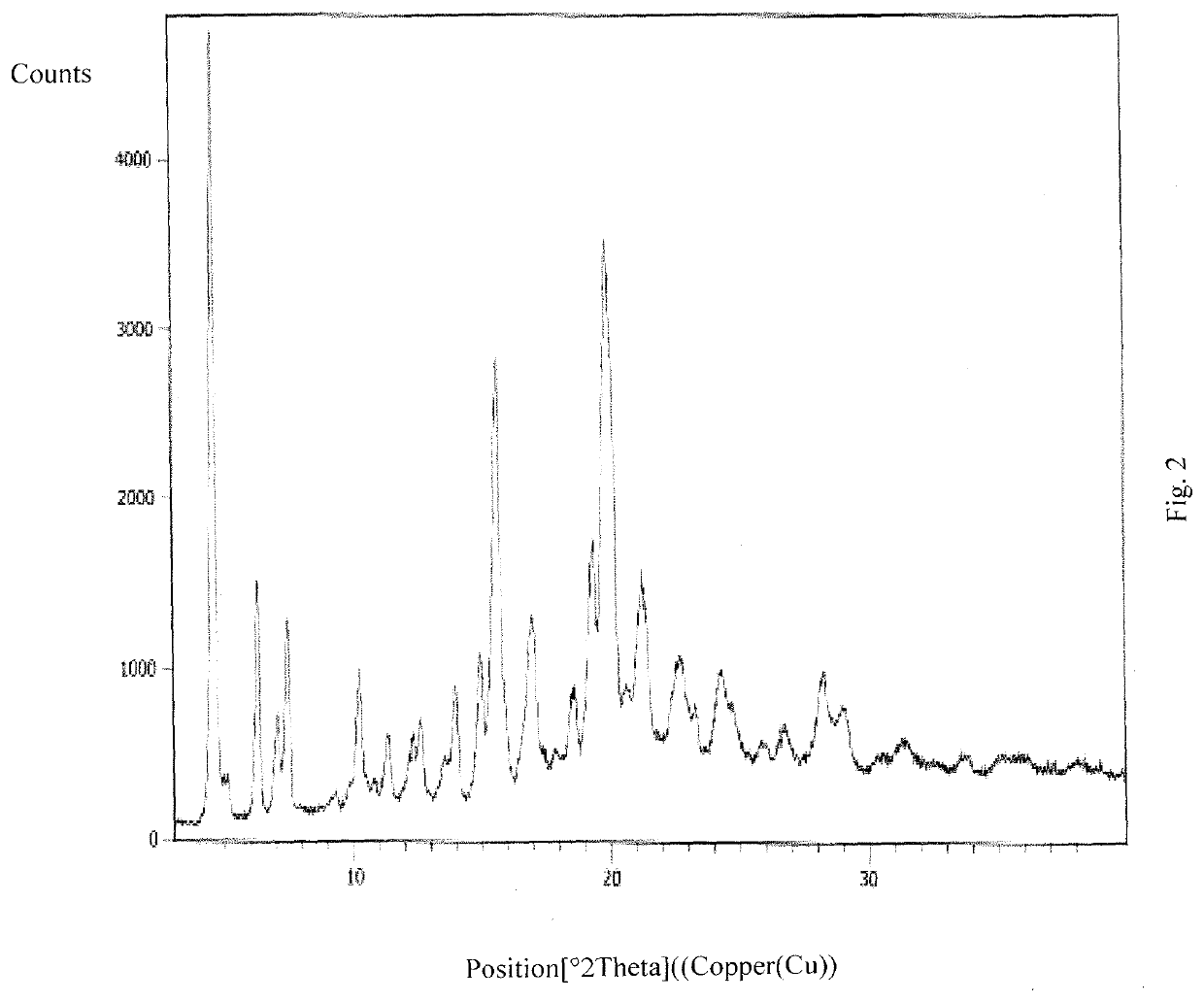
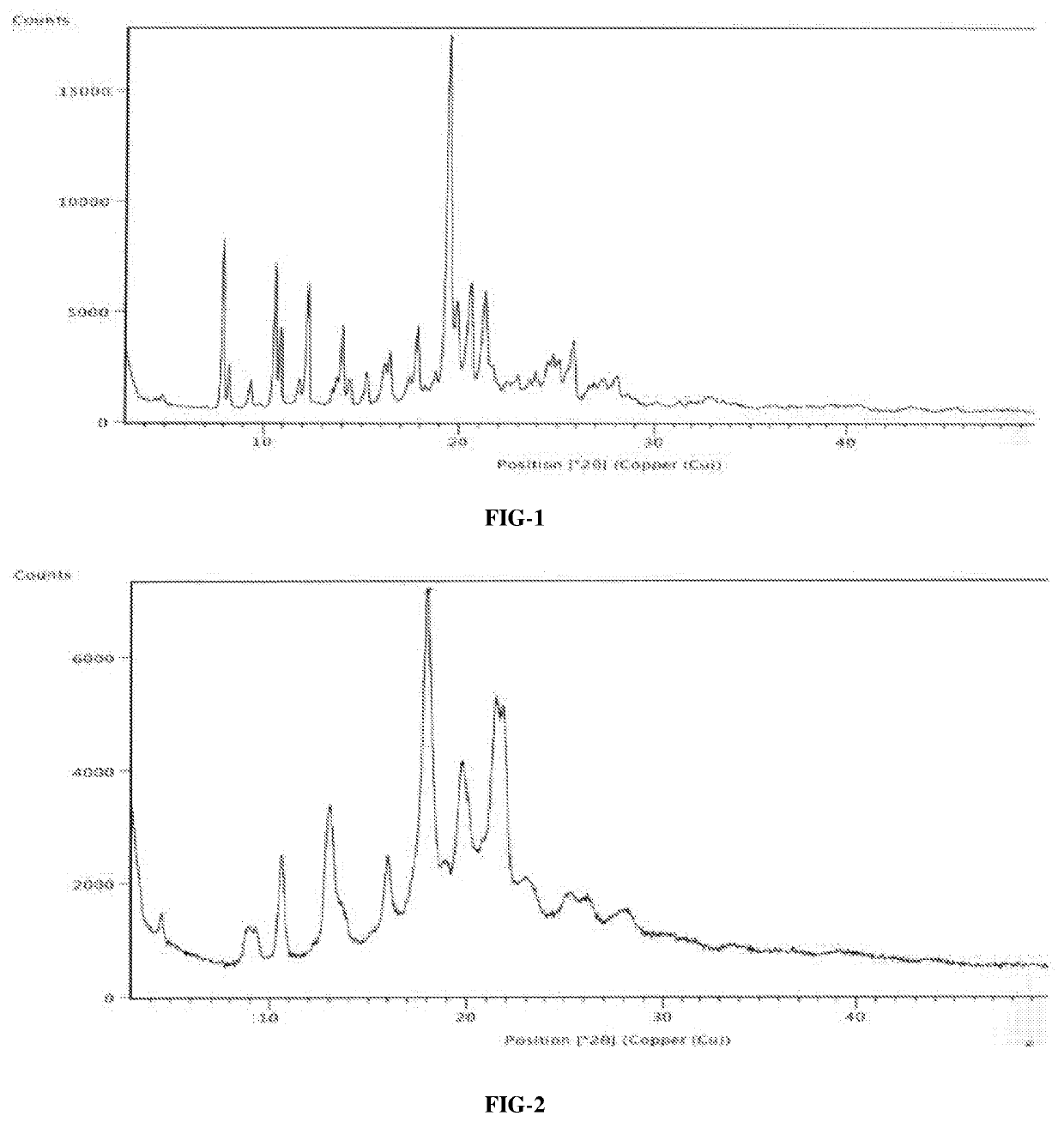
A crystal form of ribociclib monosuccinate
ActiveCN112010857BReduce humidityImprove stabilityOrganic active ingredientsOrganic chemistry methodsButanedioic acidRibociclib
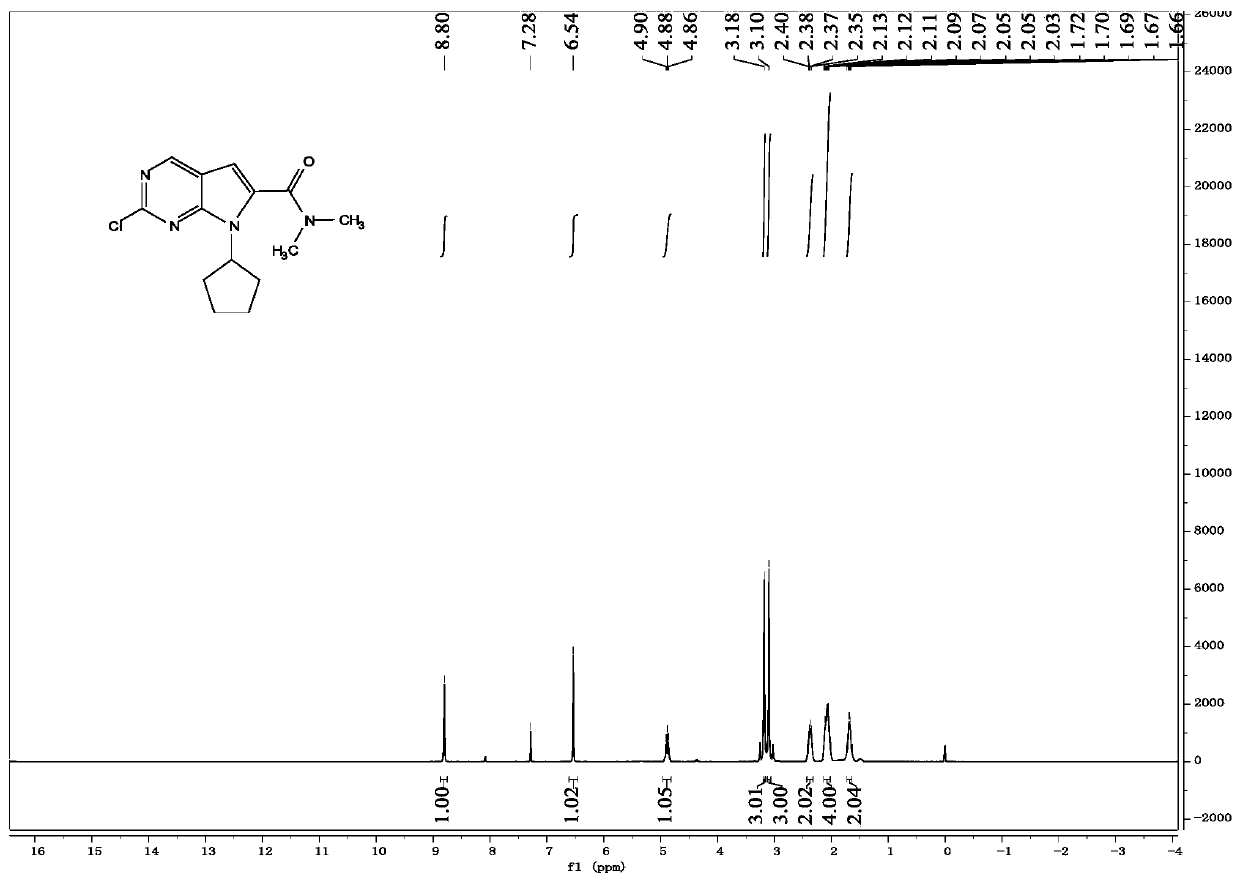
The present invention relates to a crystal form of Ribociclib monosuccinate, whose X-ray powder diffraction pattern at 25°C has a 2theta value of 9.0°±0.2°, 9.5°±0.2°, 11.5±0.2°, 12.9±0.2°, 13.9±0.2°, 15.1±0.2°, 15.8±0.2°, 17.7±0.2°, 18.3±0.2°, 19.0±0.2°, 20.5±0.2°, 21.9±0.2°, 23.0±0.2°, 23.9±0.2°, 24.9±0.2°, 25.9±0.2°, 27.9±0.2°, 29.2±0.2°, 29.9±0.2°, 30.5±0.2°, 32.0±0.2°, 33.7±0.2°, 36.2±0.2°, There are characteristic peaks at 37.4±0.2°, 37.7±0.2°, 39.5±0.2°, 41.0±0.2°, 41.6±0.2°, 44.0±0.2°. The crystal form I provided by the invention has low hygroscopicity, simplifies the preparation and post-treatment process of medicines, and is easy for industrial production. Compared with the existing crystal forms, the crystal form I has better stability, and it is not easy to turn crystals during storage, so as to avoid changes in bioavailability and drug efficacy. At the same time, it has better powder properties and has a strong Economic Value.
Owner:CHANGZHOU PHARMA FACTORY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com