A kind of industrial preparation method of Ribociclib
A compound and catalyst technology, applied in the field of medicinal chemistry, can solve the problems of large safety hazards, cumbersome process operation, and large amount of three wastes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
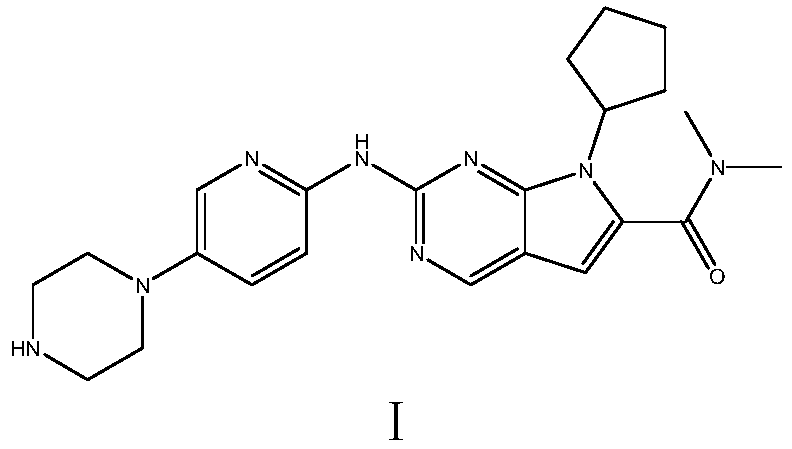
[0087] Example 1: Preparation of Ribociclib (I)
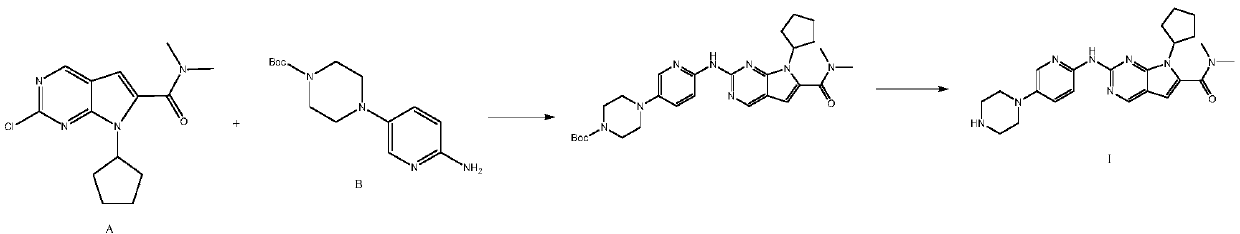
[0088] Step (1): N,N-Dimethyl-2-{5-[(4-tert-butoxycarbonylpiperazin-1-yl)pyridin-2-yl]}amino-7H-pyrrole[2,3- d] pyrimidine-6-carboxamide (Ⅵ 1 , molecular weight 466.5) preparation
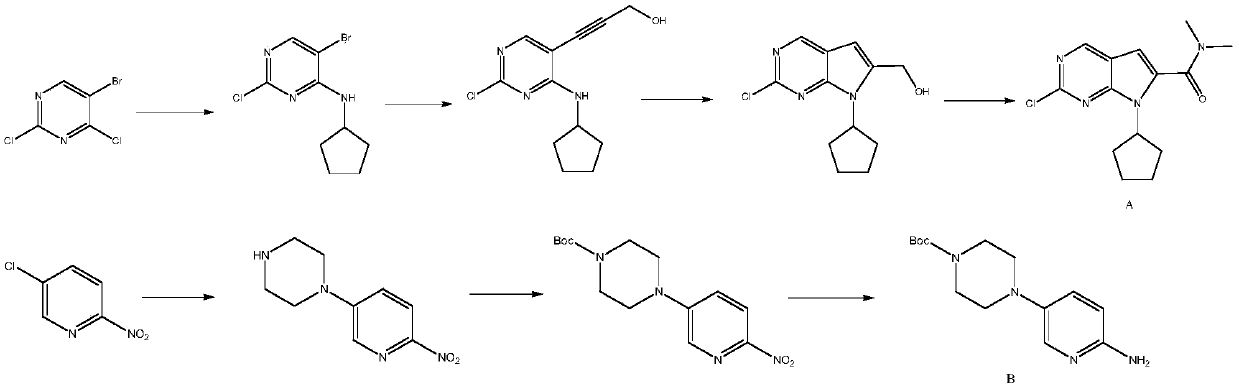
[0089] In a 500 ml four-necked flask, add 300 g of n-butanol, 41.8 g (0.2 moles) of N,N-dimethyl-2,2-dichloro-4-cyano n-butyramide (II), 35.5 g ( 0.3 mol) N,N-dimethylformamide dimethyl acetal (Ⅲ 1 ), 0.5 gram of piperidine, reacted for 4 hours at 115°C to obtain the compound of formula IV, and the gas phase detection reaction was completed (without separation); down to 25-30°C, add 70.5 grams (0.22 moles) of N-[5-(4- tert-butoxycarbonylpiperazin-1-yl)pyridin-2-yl]guanidine (V), react at 90-95°C for 12 hours, and the reaction is completed by liquid phase detection. Cool down to room temperature, filter, filter cake was washed once with 40 grams of ethanol, and dried to obtain 86.3 grams of light yellow solid N,N-dimethyl-2-{5-[(4-tert-butoxycarb...
Embodiment 2
[0096] Example 2: Preparation of Ribociclib (I)
[0097] Step (1): N,N-Dimethyl-2-{5-[(4-tert-butoxycarbonylpiperazin-1-yl)pyridin-2-yl]}amino-7H-pyrrole[2,3- d] pyrimidine-6-carboxamide (Ⅵ 1 ) preparation
[0098] In a 500 ml four-necked flask, add 300 g of n-butanol, 41.8 g (0.2 moles) of N,N-dimethyl-2,2-dichloro-4-cyano n-butyramide (II), 26.5 g ( 0.25 mol) of trimethyl orthoformate, 0.8 g of zinc chloride, reacted at 80-85° C. for 4 hours, and the reaction was completed by gas phase detection. Drop to 25-30°C, add 80.0 g (0.25 moles) of N-[5-(4-tert-butoxycarbonylpiperazin-1-yl)pyridin-2-yl]guanidine, react at 90-95°C for 12 hours, liquid The phase detection reaction is complete. Lowered to room temperature, filtered, and the filter cake was washed with 40 grams of ethanol to obtain 85.7 grams of light yellow solid N,N-dimethyl-2-{5-[(4-tert-butoxycarbonylpiperazin-1-yl)pyridine- 2-yl]}amino-7H-pyrrole[2,3-d]pyrimidine-6-carboxamide, molecular weight 466.5, liquid ph...
Embodiment 3
[0103] Example 3: Preparation of Ribociclib (I)
[0104] Step (1): N,N-Dimethyl-2-{5-[(4-benzylpiperazin-1-yl)pyridin-2-yl]}amino-7H-pyrrole[2,3-d] Pyrimidine-6-carboxamide (Ⅵ 2 , molecular weight 456.5) preparation
[0105] In a 500 ml four-necked flask, add 200 g of N,N-dimethylformamide, 41.8 g (0.2 moles) of N,N-dimethyl-2,2-dichloro-4-cyano n-butyramide ( Ⅱ), 35.5 g (0.3 moles) of N,N-dimethylformamide dimethyl acetal, 0.4 g of DBU, reacted at 110° C. for 4 hours, and the reaction was completed by gas phase detection. Decrease to 25-30°C, add 77.5 g (0.25 moles) N-[5-(4-benzylpiperazin-1-yl)pyridin-2-yl]guanidine, react at 100-105°C for 10 hours, liquid phase detection The reaction is complete. Recover N,N-dimethylformamide by distillation under reduced pressure, then add 50 grams of water and 50 grams of ethanol to the residue, filter, and wash the filter cake with 40 grams of ethanol to obtain 85.6 grams of light yellow solid N,N-dimethylformamide Base-2-{5-[(4-ben...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com