Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
81 results about "Letrozole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
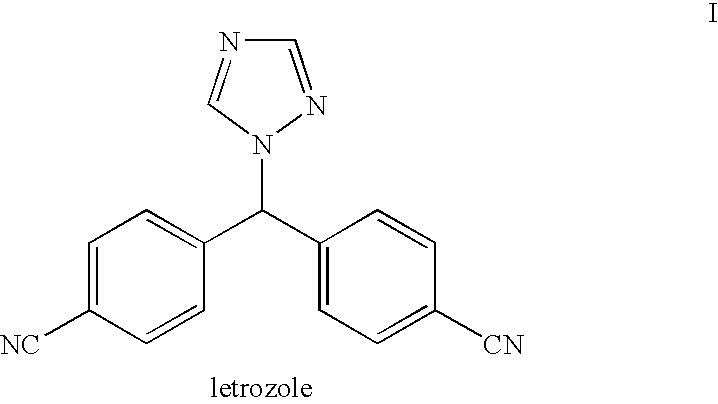
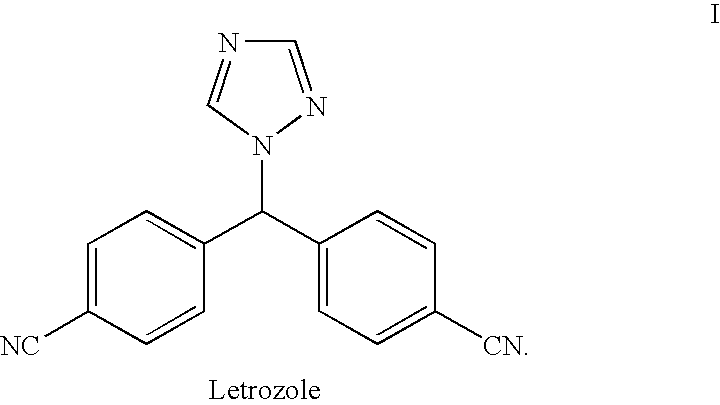
This medication is used to treat certain types of breast cancer (such as hormone-receptor-positive breast cancer) in women after menopause. Letrozole is also used to help prevent the cancer from returning.
Injectable depot compositions and its process of preparation
Novel injectable compositions are provided comprising an active agent which is tamsulosin or letrozole or its pharmaceutically acceptable salts, derivatives, isomers, polymorphs, solvates, hydrates, analogues, enantiomers, tautomeric forms or mixtures thereof and one or more pharmaceutically acceptable excipient(s) wherein the compositions are preferably formulated as biodegradable microparticles or nanoparticles which can optionally be reconstituted with an aqueous, hydro-alcoholic or oily liquid vehicle prior to administration. The novel injectable compositions of the present invention preferably form a depot upon administration in vivo and are in the form of an in situ gelling composition or an implant composition which provides a prolonged release of tamsulosin or letrozole for extended periods of time. Also described are process for preparation of such novel compositions and method of using them.
Owner:PANACEA BIOTEC
Process for the preparation of letrozole
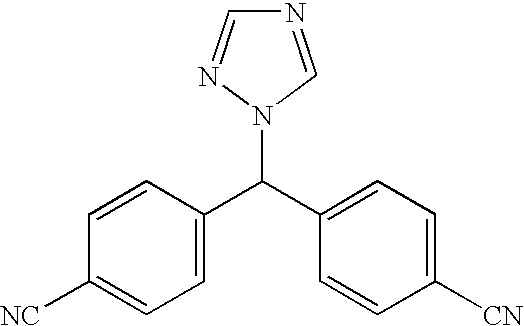
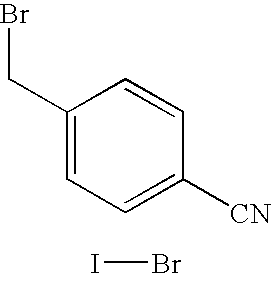
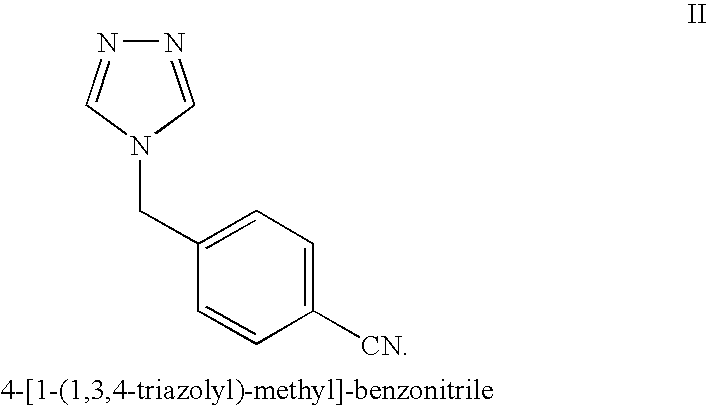
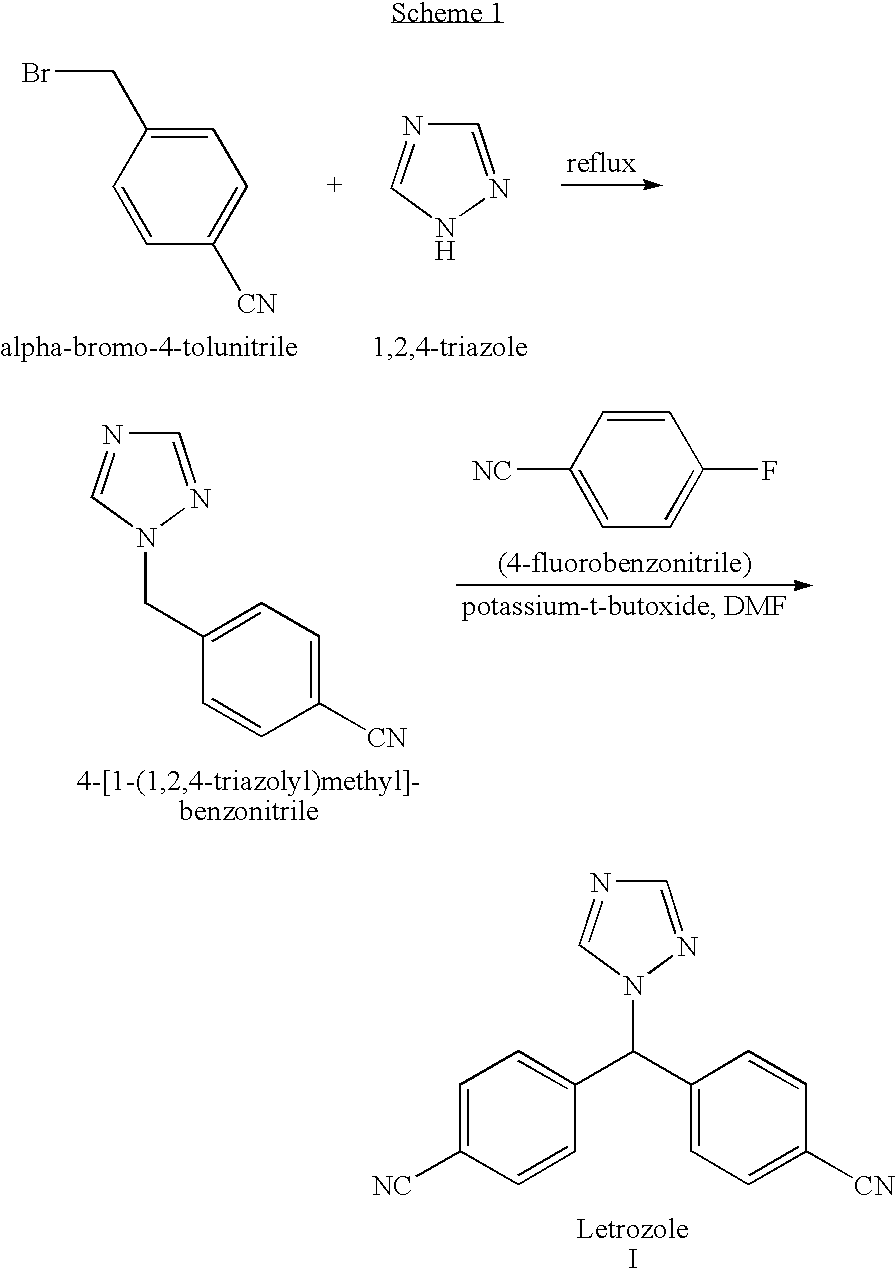
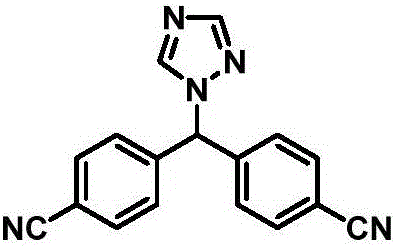
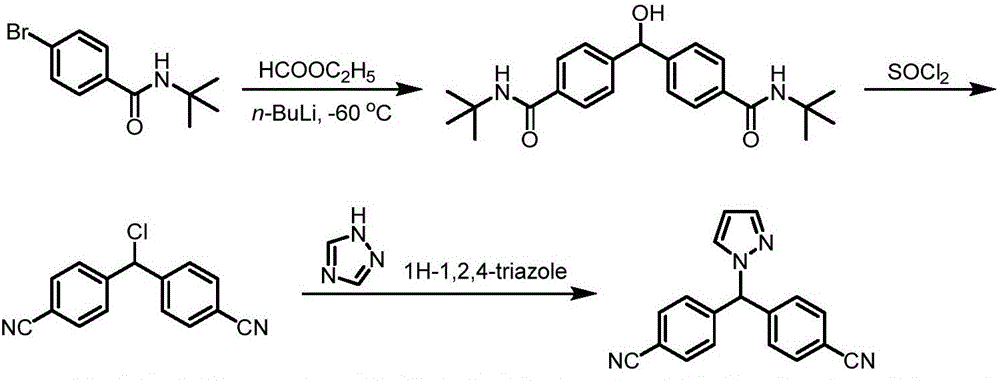
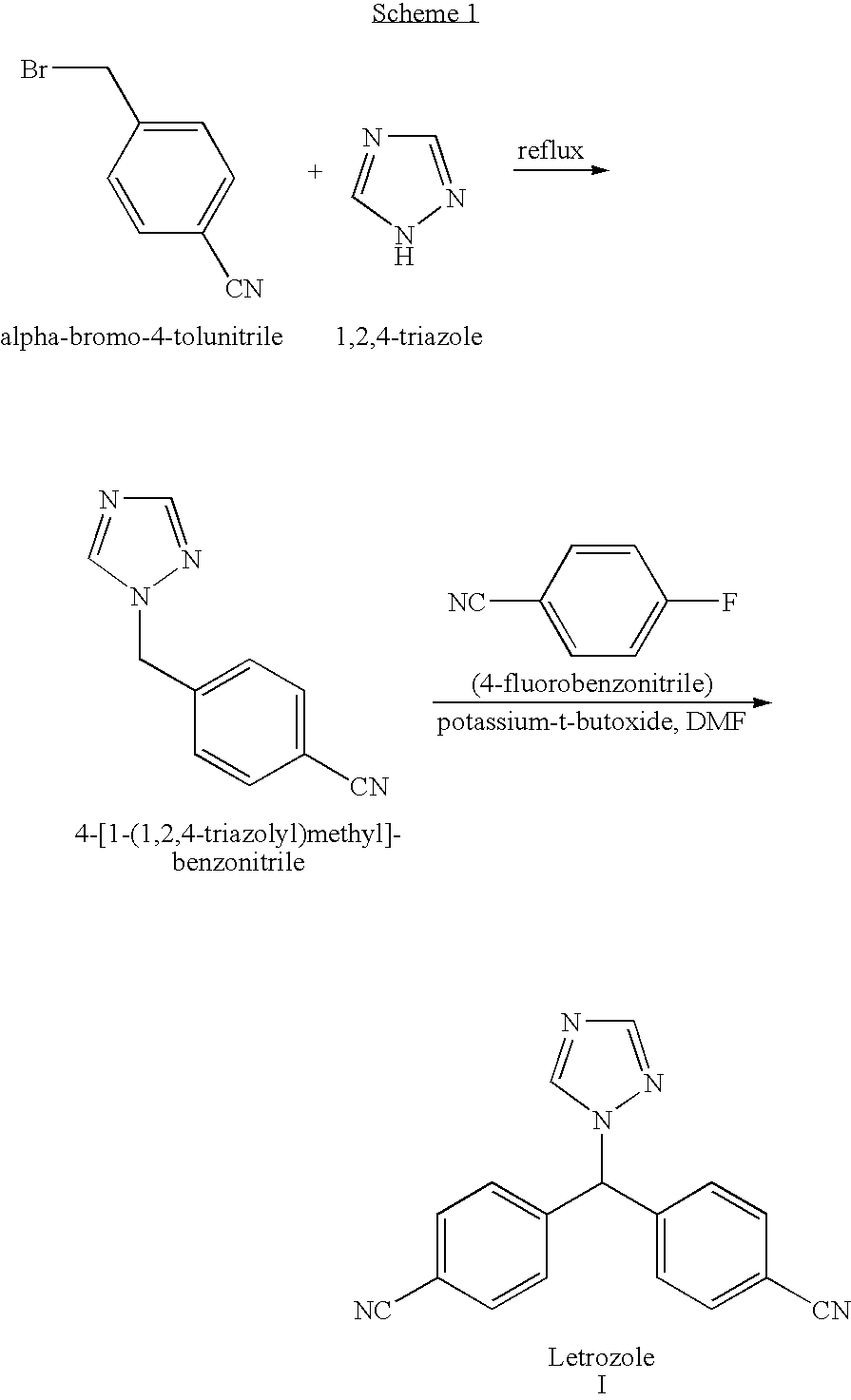
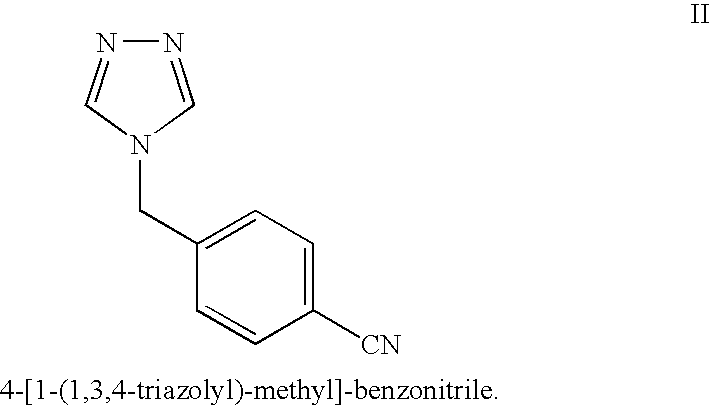
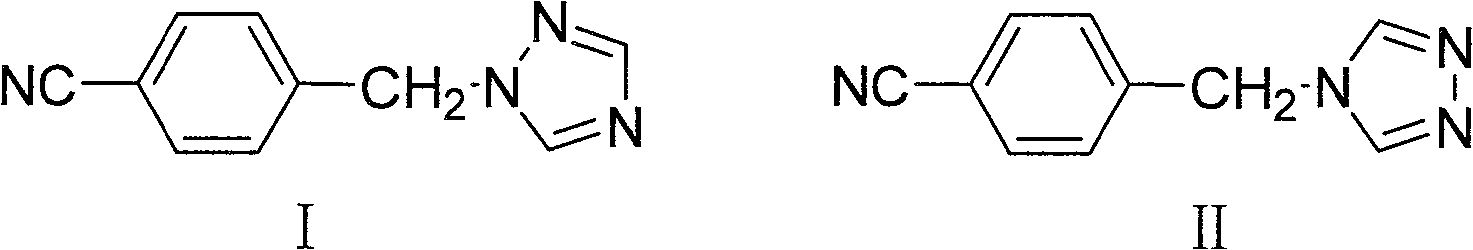
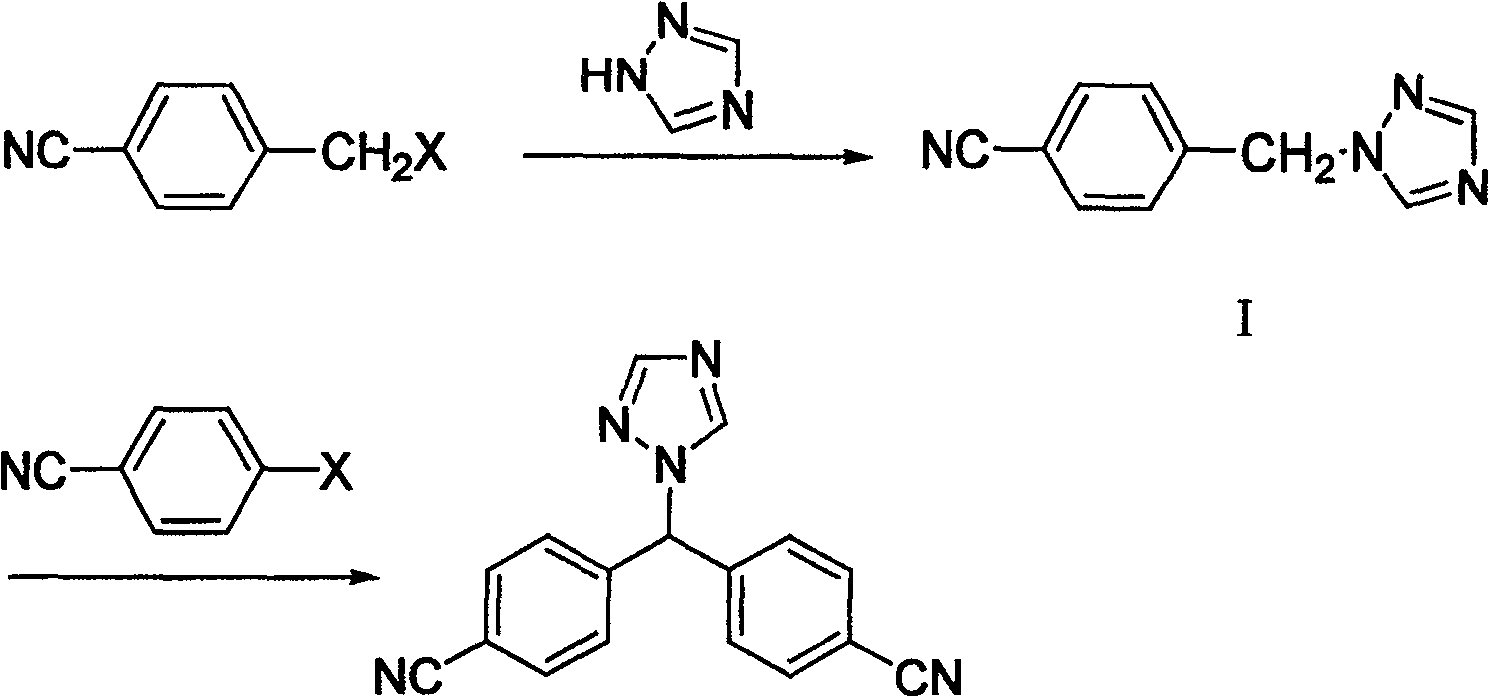
The invention provides a high-yield process for the preparation of letrozole having a high purity, without the need for removal of the 4-[1-(1,3,4-triazolyl)methyl]benzonitrile impurity at the intermediate stage. The invention also provides a process for the synthesis of letrozole in which formation of the impurity 4-[1-(1,3,4-triazolyl)methyl]benzonitrile during the first stage is minimized. In the process, a 4-(halomethyl)benzonitrile is reacted with a salt of 1H-1,2,4-triazole, reducing the formation of the impurity. Preferably, the preparation is conducted as a one-pot process.
Owner:SICOR SOC ITAL CORTICOSTEROIDI SPA
Micronization femara and its composition
The present invention discloses a kind of micronized letrozole and its composite. Said invention utilizes a micronization process to micronize said letrozole and make the micronized letrozole be combined with a proper carrier so as to obtain a micronized letrozol preparation with proper dissolubility. Said preparation can be used for effectively curing mammary cancer.
Owner:上海复旦复华药业有限公司
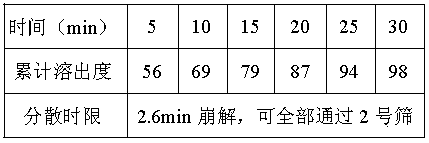
Dispersible tablet containing letrozole
ActiveCN101467971APromote dissolutionEasy to takeOrganic active ingredientsPill deliveryPost menopausalAdditive ingredient
The invention discloses a dispersible tablet containing letrozole which has letrozole action ingredient and pharmaceutical accessories. The tablet is applied to the treatment of post-menopausal breast cancer.
Owner:AVENTIS PHARMA HAINAN
Insoluble pharmaceutical composition
InactiveCN101669942AHigh dissolution rateSimple preparation processOrganic active ingredientsPharmaceutical non-active ingredientsMedicineDissolution
Owner:北京华禧联合科技发展有限公司
Letrozole production process
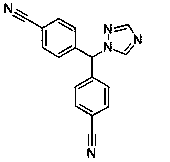
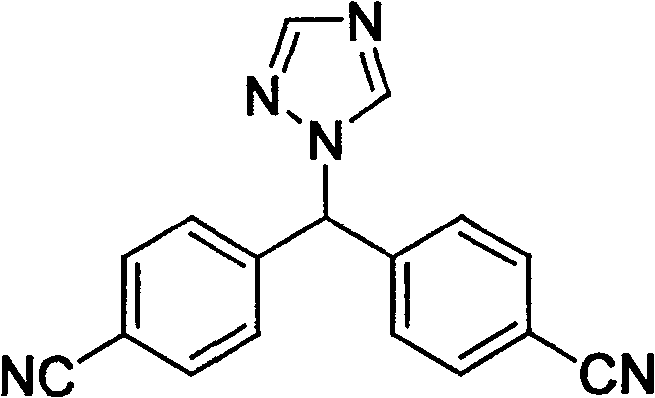
Provided is a method for preparing letrozole, which includes reacting an activated bis-(4-cyanophenyl)-methane with a triazole to produce letrozole, and, optionally, purifying the letrozole. Also provided are highly pure letrozole, and a method of purifying letrozole, which method includes precipitating letrozole, e.g., by selective precipitation from a reaction mixture and / or by subjecting the letrozole to one or more crystallizations.
Owner:CHEMAGIS
Process for preparing letrozole
Owner:DR REDDYS LAB LTD +1
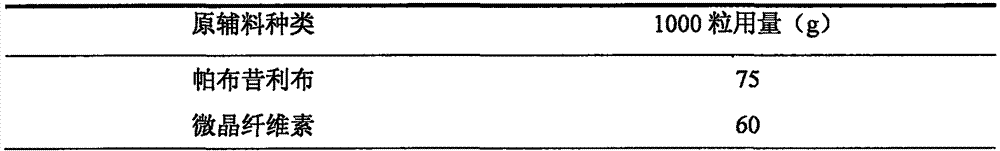
Capsule containing palbociclib and preparation method of capsule
The invention relates to a capsule containing palbociclib and a preparation method thereof, belonging to the technical field of pharmaceutical preparations. This product can be used in combination with letrozole for patients with advanced breast cancer. The capsule contains palbociclib, diluent, disintegrant and lubricant, wherein the mass ratio of palbociclib is 10% to 40%, and the mass ratio of diluent is 30% to 80%, The mass proportion of the disintegrating agent is 1%-10%, and the consumption amount of the lubricant is 0.5-5%. In addition, the present invention also discloses a method for preparing palbociclib capsules. The palbociclib capsules prepared by the method of the present invention have simple process and operation, and are particularly suitable for industrial production. The prepared The palbociclib capsule has good stability and good dissolution rate.
Owner:BEIJING XINLINGXIAN MEDICAL TECH DEV CO LTD
Large-scale cultivation method of all-female Nibea albiflora
ActiveCN110771536AReduce pollutionEasy to operateClimate change adaptationAccessory food factorsAnimal scienceAromatase inhibitor
The invention provides a large-scale cultivation method of all-female Nibea albiflora. The method mainly includes the steps of firstly, inducing the gynogenesis diploid of Nibea albiflora; inducing and identifying pseudo-male Nibea albiflora; thirdly, allowing the pseudo-male Nibea albiflora to mate with normal female Nibea albiflora to reproduce all-female Nibea albiflora. The method has the advantages that aromatase inhibitor letrozole is used to induce pseudo-male Nibea albiflora reproduction on the basis that the Nibea albiflora gynogenesis technology is built, the mating of the pseudo-male Nibea albiflora and the normal female Nibea albiflora is utilized to obtain the all-female Nibea albiflora for the first time; the built large-scale all-female Nibea albiflora cultivation method issimple to operate, safe, efficient and the like, the cultivated all-female Nibea albiflora is fast in growth, stable in character and the like, and strong technical support is provided for the realizing of Nibea albiflora unisexual cultivation.
Owner:MARINE FISHERIES RES INST OF ZHEJIANG
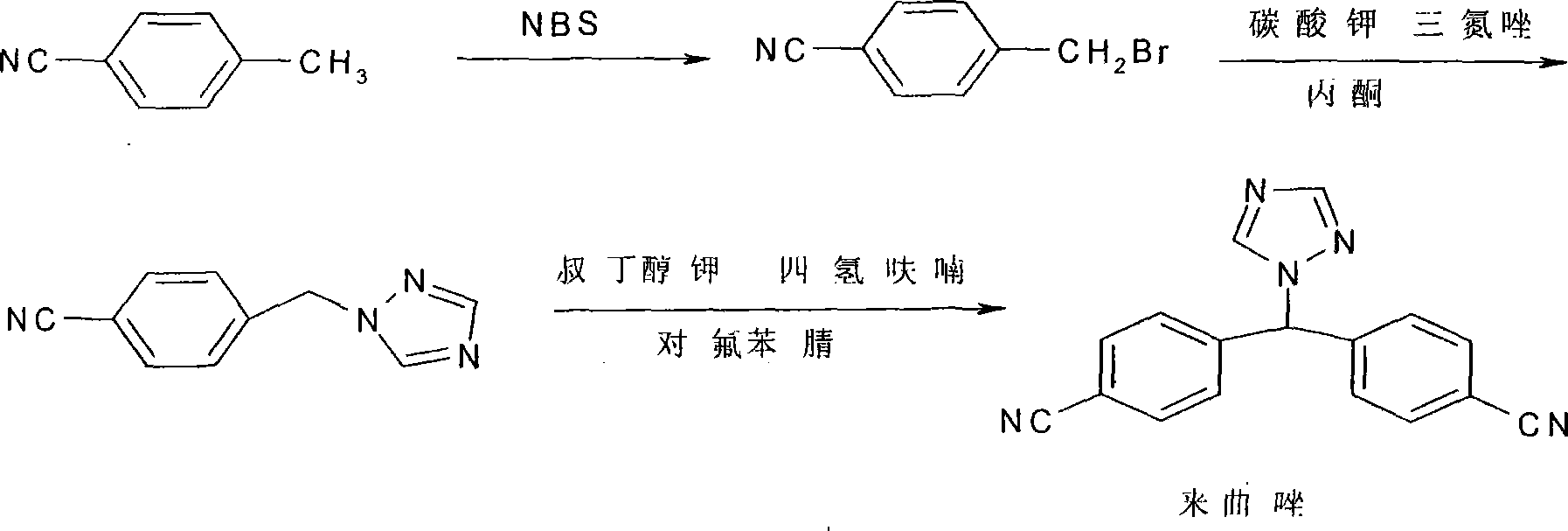
Prepn process of letrozole
The process of synthesizing letrozole includes the first reducing 4, 4'-dicyan diphenyl ketone with sodium borohydride in alcohol solvent to produce 4, 4'-dicyan diphenyl methanol; the subsequent halogenating 4, 4'-dicyan diphenyl methanol in non-polar solvent to produce 4, 4'-dicyan diphenyl methane halide; and the final condensation reaction between 4, 4'-dicyan diphenyl methane halide and 1, 2, 4-triazole in non-polar solvent to produce letrozole. The process has simple operation, high safety and low material cost, and is suitable for industrial production of letrozole.
Owner:杜焕达
Letrozole purification process
InactiveUS20070112203A1Easy to separatePromotes rapid oxidationBiocideOrganic chemistryLetrozoleImpurity
Owner:CHEMAGIS
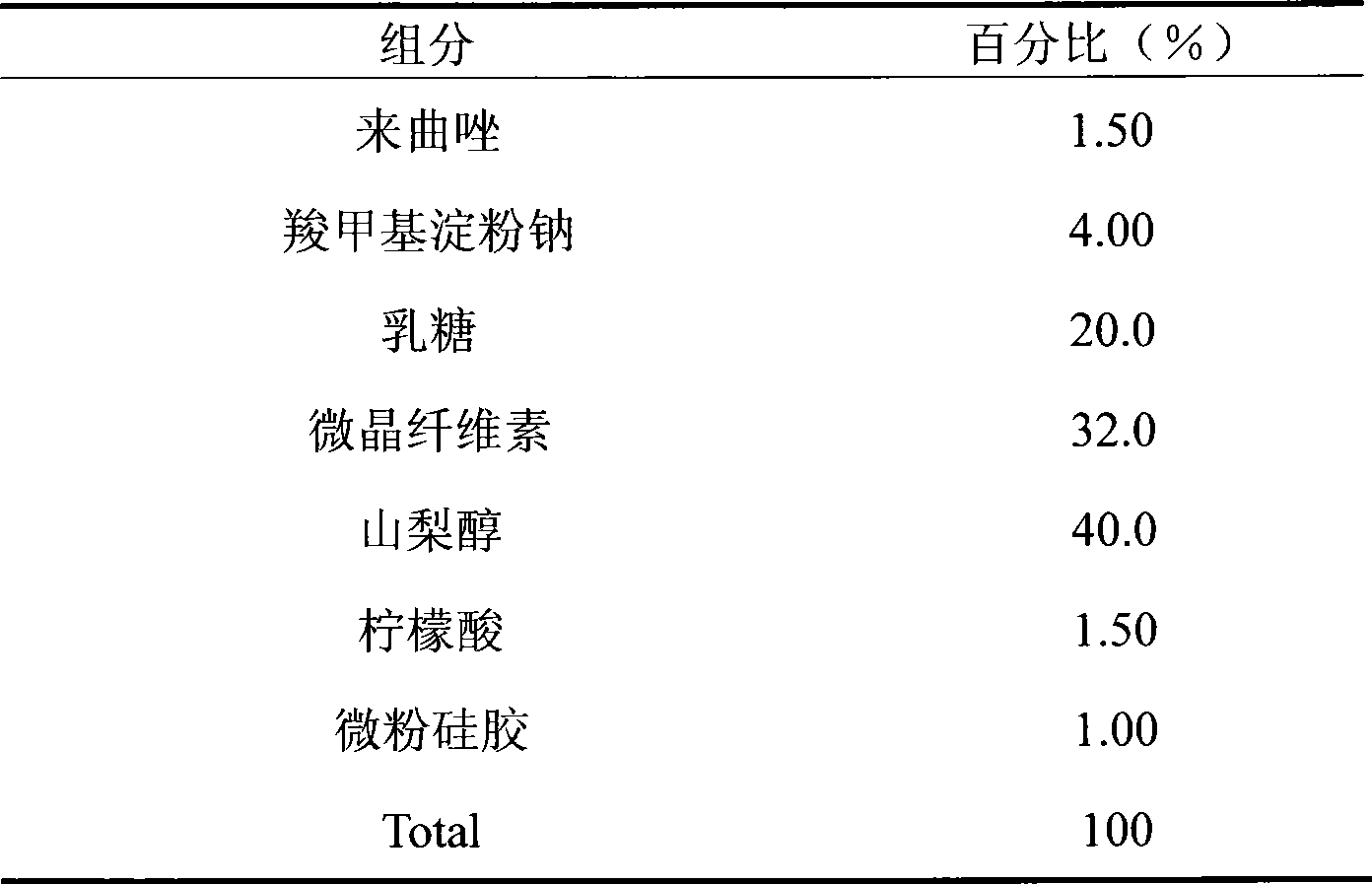
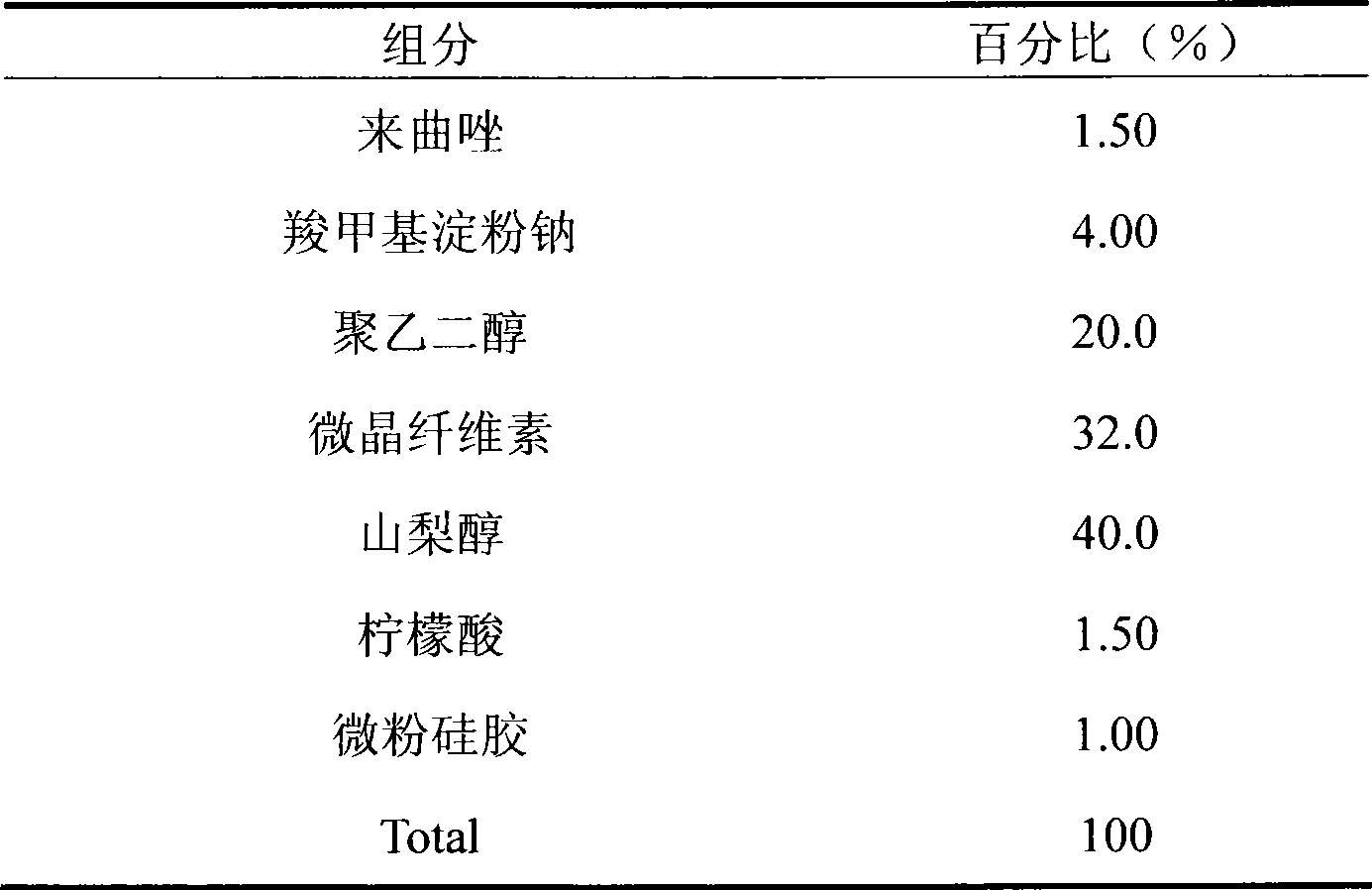
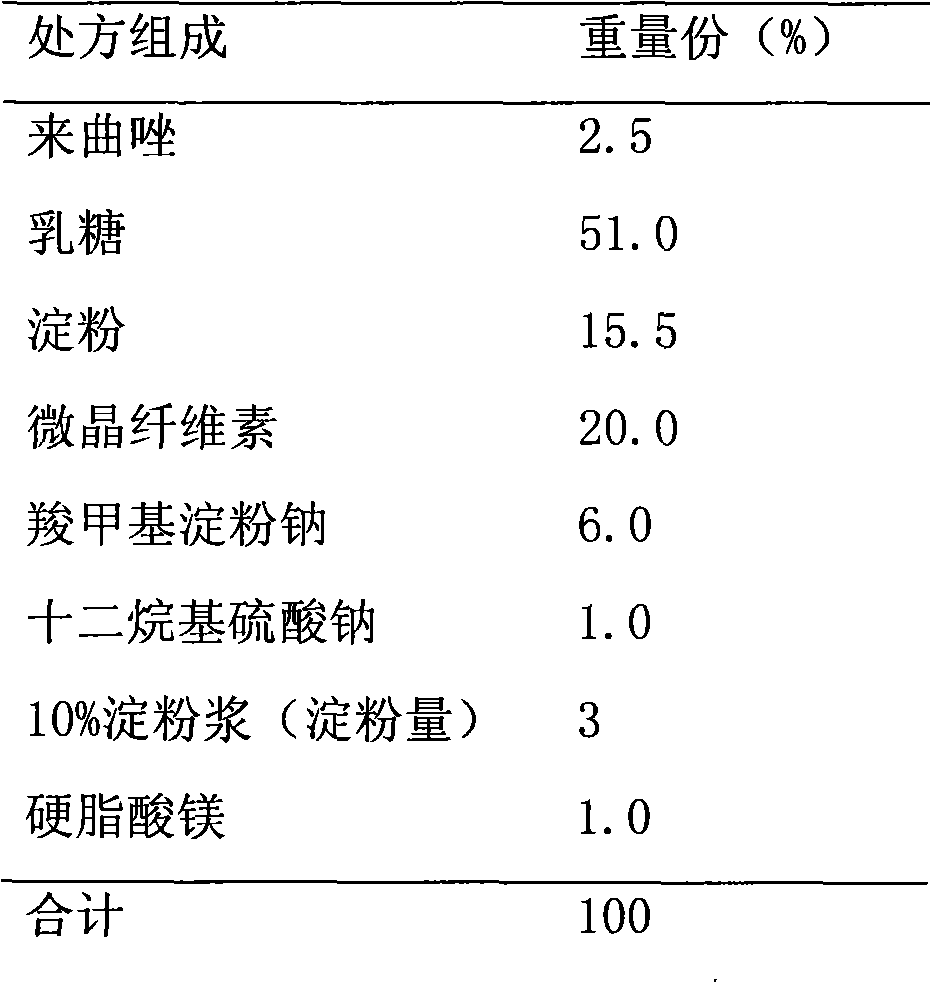
Preparation method for letrozole tablets with good stability
ActiveCN104586804ASolve the sticking problemImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsSilica gelLetrozole
The invention relates to a preparation method for letrozole tablets with good stability, and belongs to the technical field of pharmaceutical preparations. The preparation method comprises the following steps: dissolving 20-30 parts of letrozole in 120-180 parts of alcohol; adding 5-10 parts of titanium oxide, 3-6 parts of heavy magnesium carbonate and 10-20 parts of micro powder silica gel; reducing pressure and evaporating the alcohol to dryness; taking out the dried composition; screening the composition; adding 70-90 parts of a filling agent, 5-10 parts of a disintegrating agent and 4-8 parts of a lubricant; mixing the materials uniformly; directly conducting tabletting to obtain the letrozole tablets. The prepared letrozole tablets are good in medicine stability, and dissolution rate, and avoid sticking of the tablets.
Owner:嘉善创越知识产权服务有限公司
Method for breeding full-feminization rainbow trout
InactiveCN102783445AGood for sex controlReasonable settingClimate change adaptationPisciculture and aquariaAnimal scienceMale Phenotype
The invention aims at providing a method for breeding full-feminization rainbow trout. The method comprises the steps of first feeding a mixed feed containing a feed for common fishes and letrozole and then feeding the feed for common fishes in the breeding of first upward floating fries obtained in first fertilized ovum incubation; taking sperms of false milter with female genotype and male phenotype from adult fish to perform second fertilization together with ovum of the rainbow trout, and finally obtaining second adult fish, namely the full-feminization rainbow trout. The mixed feed containing the letrozole is used in the first feeding process, thereby facilitating control of gender of larval fish. Parameters of the method are reasonably set in two-time incubation and feeding steps, and various factors produce synergistic effect, thereby facilitating the incubation of the fertilized ovum to obtain upward floating fries and enabling the upward floating fries to grow to be adult fish. Various operation methods in the method are simple, convenient and easy to perform, and the method is good in adaptability and suitable for being popularized and used in vast numbers of farmers.
Owner:北京卧佛山庄养殖有限公司
Letrozole transdermal drug administration plaster and its preparation method
InactiveCN1927190AEasy to prepareEasy to operateOrganic active ingredientsAntineoplastic agentsPatch matrixAdhesive
Letrozole cutaneous penetration patch and its preparation method, it involves a cutaneous penetration patch and the patch preparation methods. The invention solves problems of the present Letrozole available dosage form drugs in the treatment of postmenopausal women with advanced aspects in short effective time, frequent administration, a more dverse reactions. It consists of matrix layer, back lining and protective film. The matrix layer of letrozole cutaneous penetration patch includes letrozole, adhesives and organic solvents. Their preparation methods: (a) spraid the mixture of letrozole, adhesives and organic solvents evenly on the back lining; (b) after they are dried, cover the protective membrane on the other surface of the patch matrix layer, then the letrozole cutaneous penetration patch is obtained. The patches matrix layer of the invention has good compatibility and adhesiveness with skin, good stability. The invention of the patches maintains constant speed, uniform release, obvious treatment results and long effective time, long recurrence interval, low recurrence rate. Preparation of the present invention patch method is simple and easy to operate.
Owner:哈尔滨健迪医药技术有限公司
Process for the preparation of letrozole
The invention provides a high-yield process for the preparation of letrozole having a high purity, without the need for removal of the 4-[1-(1,3,4-triazolyl)methyl]benzonitrile impurity at the intermediate stage. The invention also provides a process for the synthesis of letrozole in which formation of the impurity 4-[1-(1,3,4-triazolyl)methyl]benzonitrile during the first stage is minimized. In the process, a 4-(halomethyl)benzonitrile is reacted with a salt of 1H-1,2,4-triazole, reducing the formation of the impurity. Preferably, the preparation is conducted as a one-pot process.
Owner:SICOR SOC ITAL CORTICOSTEROIDI SPA
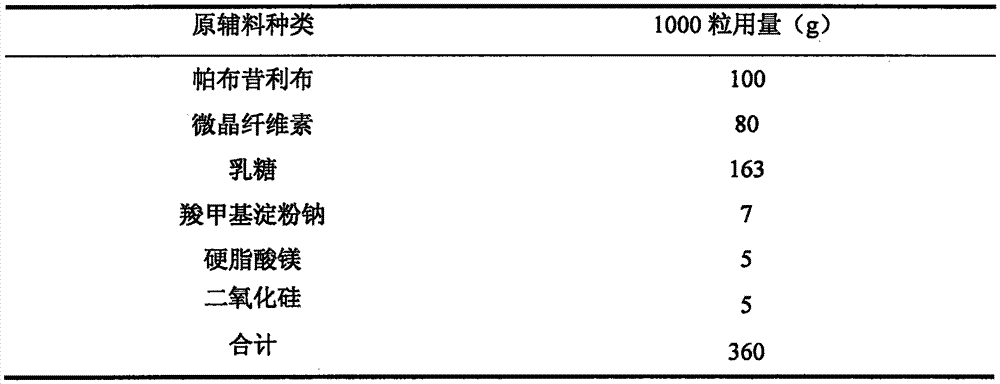
Letrozole tablet and preparation method thereof
ActiveCN103356495AImprove stabilityHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsMagnesium stearateLactose
The invention relates to a letrozole tablet and a preparation method thereof. The letrozole tablet comprises the following main auxiliary materials: microcrystalline cellulose, lactose, sodium starch glycolate, L-HPC (low-substituted hydroxypropyl cellulose), sodium lauryl sulfate, Tween 80, magnesium stearate and starch; the preferable sodium lauryl sulfate:Tween 80 ratio is 2:1, and the sodium starch glycolate:L-HPC ratio is 5:1. The tablet has stable product quality, obviously enhances the letrozole dissolution, and effectively enhances the bioavailability; and the tablet preparation technique is simple, does not need special equipment, and is convenient to operate and suitable for industrial production.
Owner:HAINAN LINHENG PHARMA
Method for controlling hoplobatrachus tigerinus sex ratio and application of method
InactiveCN105409873AMetamorphosis has no effectIncrease perversion rateClimate change adaptationPisciculture and aquariaGramZoology
The invention discloses a method for controlling hoplobatrachus tigerinus sex ratio and application of the method and belongs to the technical field of hoplobatrachus tigerinus artificial culture. The method includes: placing hatched tadpoles into a cultivation pond according to cultivation density of 3-4 / liter, using feed containing 0.08-0.12 milligram of testosterone or letrozole in each gram to feed the tadpoles under 29-34 DEG C for two weeks, and then using feed which does not contain the testosterone or letrozole to feed the tadpoles until metamorphosis is completed. By the method, the sex ratio after the metamorphosis can be adjusted effectively, and the cultivation efficiency of hoplobatrachus tigerinus can be increased greatly. Compared with feeding under natural conditions, the method has the advantages that a metamorphosis cycle can be shortened greatly, and metamorphosis rate can be increased.
Owner:LISHUI UNIV
Letrozole tablet with high dissolubility and preparation method thereof
ActiveCN102485217AEasy to prepareEasy to operateOrganic active ingredientsPharmaceutical non-active ingredientsCross-linkMANNITOL/SORBITOL
The invention discloses a Letrozole tablet with high dissolubility and a preparation method thereof. An effective component of the tablet is Letrozole, and polyvinylpyrrolidone K30, tween 80, mannitol, lactose, microcrystalline cellulose, cross-linked polyvinylpolypyrrolidone and magnesium stearate are used as accessories. The product of the tablet has stable quality and high dissolubility, enables absorption of Letrozole in a human body to be increased and bioavailability to be effectively improved and is easy to prepare and applicable to industrial production.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Preparation method of letrozole
InactiveCN105801501AThe method route is simpleSimple reaction conditionsOrganic chemistryEconomic benefitsLetrozole
The invention provides a preparation method of letrozole (6). The preparation method comprises the following steps: reacting a compound (1) with a compound (3) under the effect of an alkaline matter; performing a bromination reaction of the compound (3) to obtain a compound (4); condensing the compound (4) and 4-amino-1,2,4-triazole (5); and performing diazotization to remove the amino to obtain letrozole (6). According to the method provided by the invention, the route is simple; the cheap and easily available p-chlorobenzonitrile and p-tolunitrile are adopted as starting raw materials, and the target product letrozole is obtained through 3 steps of reactions in total; and the preparation method has the advantages of mild reaction conditions, simple and convenient operation, high yield, good chemical selectivity and low production cost, is suitable for industrial production and brings relatively great practical application value and social economic benefits.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of letrozole
The invention discloses a preparation method of letrozole. In the method, a process reaction post-processing operation process does not contain a reduced pressure distillation process, so that the reaction time is greatly shortened, the industrial cost is reduced, and the product yield is improved. According to the invention, the use of low-polarity highly-flammable reagents such as n-heptane and the like in the prior art is avoided, the operation risk is reduced, and the production safety is improved; reagents adopted in the invention are all common reagents, therefore, the method is more environment-friendly and economic; and the method is suitable for industrialized mass production.
Owner:哈药集团股份有限公司 +1
Compound medicine containing letrozole and preparation method of compound medicine
InactiveCN106580906AIncreased dissolution rate of letrozoleImprove bioavailabilityPill deliveryAntineoplastic agentsCellulosePolyethylene glycol
The invention discloses a compound medicine containing letrozole and a preparation method of the compound medicine. The medicine contains the letrozole, copovidone, mannitol, cellulose, crosslinked povidone, magnesium stearate, polyethylene glycol, lactose and starch. The formula of the medicine consists of 8-20 parts of the letrozole, 80-150 parts of the copovidone, 1-3 parts of the mannitol, 4-6 parts of the cellulose, 1-2 parts of the crosslinked povidone, 1-2 parts of the magnesium stearate, 1-3 parts of the polyethylene glycol, 0.5-1 part of the lactose and 1-2 parts of the starch. The compound medicine provided by the invention can significantly improve a letrozole dissolution rate and can further improve bioavailability and clinical treatment effect of medicinal active ingredients; and in addition, a release speed can be reduced, so that the purpose of uniform administration is achieved; therefore, drug tolerance is enhanced.
Owner:JIANGSU SUNAN PHARMA IND CO LTD
Percutaneous drug administration cream ,ointment of letrozole and preparation method thereof
InactiveCN101172107AGood curative effectLittle side effectsOrganic active ingredientsAerosol deliverySide effectSkin penetration
The invention relates to the cream and the ointment of Letrozole tablet percutaneous administration and the preparation method of the cream and the ointment thereof, and relates to the cream and the ointment of the percutaneous administration and the preparation method of the cream and the ointment thereof. The invention solves the problems that the prior Letrozole tablet oral preparation cannot avoid the first pass effect of liver and the bad reaction is numerous. The cream and the ointment of the percutaneous administration are characterized in that the cream and the ointment of the percutaneous administration comprise raw materials and penetrating agent with the following percentages: 0.01 to 20 percent of Letrozole tablet and 0.1 to 20 percent of percutaneous penetrating agent. The invention has the method that through vitro and a skin penetration test, the Letrozole tablet only with oral preparation is filtered out a formula with good non-steady state / steady state distribution by a skin release curve, and then the cream and the ointment of the percutaneous administration are prepared, so as to lead principal medicine to avoid the liver first pass effect of oral administration to directly reach mammary gland target tissue, and to lead the concentration of target tissue internal medicine to be improved. Compared with the prior preparation at home and at abroad, the invention technically has the advantages that the prepared external preparation can reduce the side effect of Letrozole tablet and improve the curative effect of the Letrozole tablet, thereby the invention has good promotion and application value.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
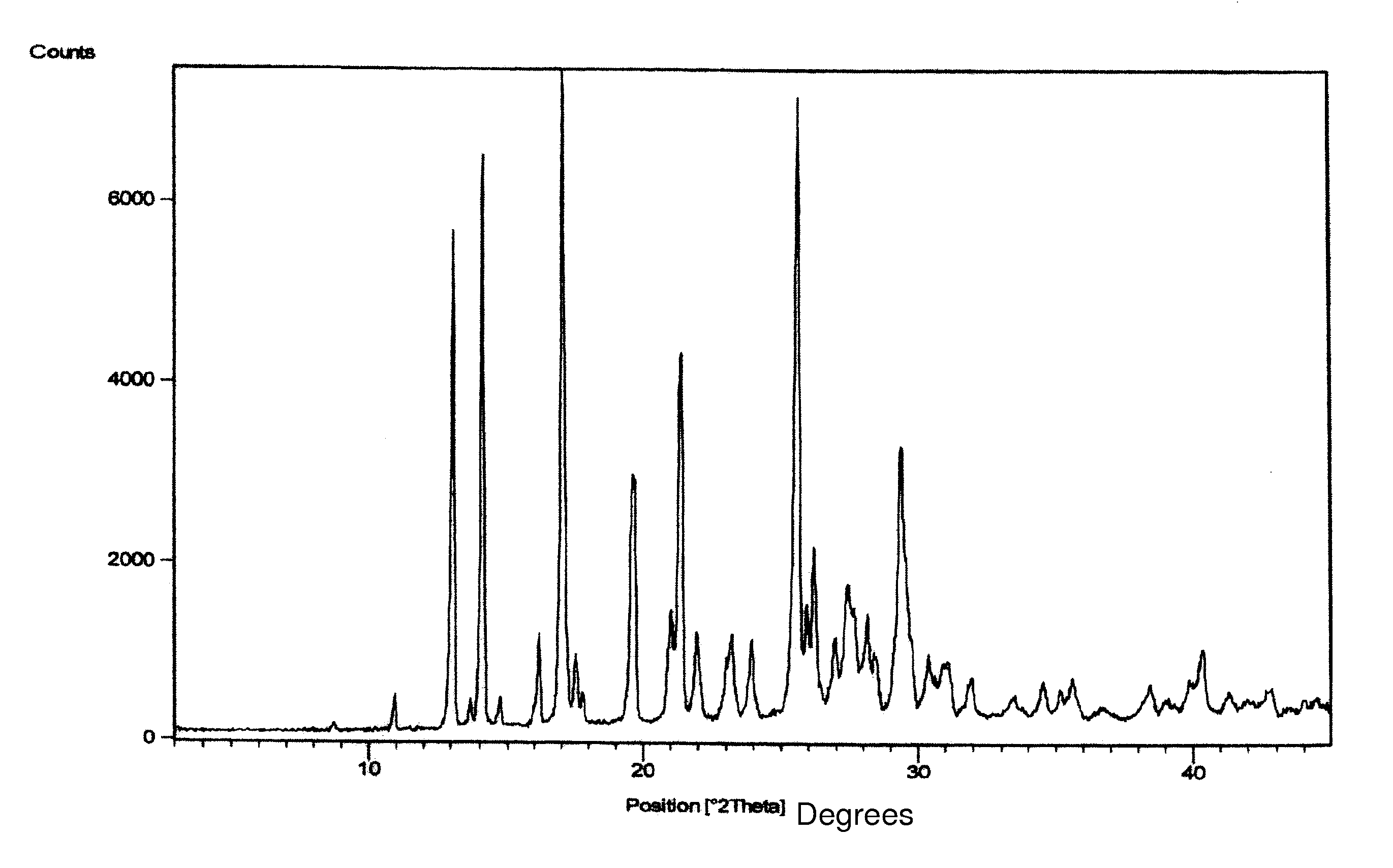
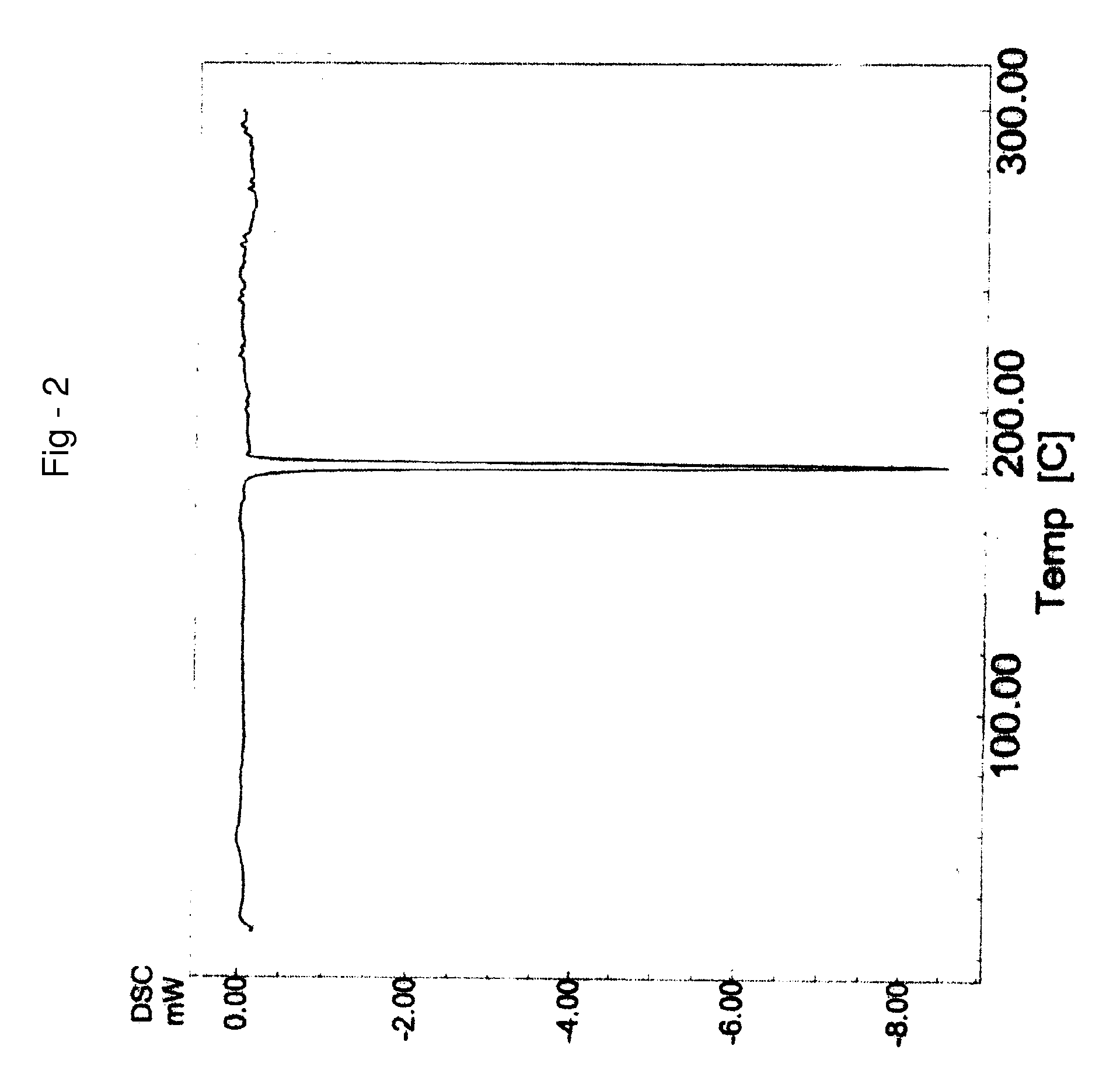
Crystalline forms of letrozole and processes for making them
Crystalline forms of letrozole can be made by precipitation and are useful in making pharmaceutical compositions.
Owner:SYNTHON BV
Methods of Treating Primary Brain Tumors by Administering Letrozole
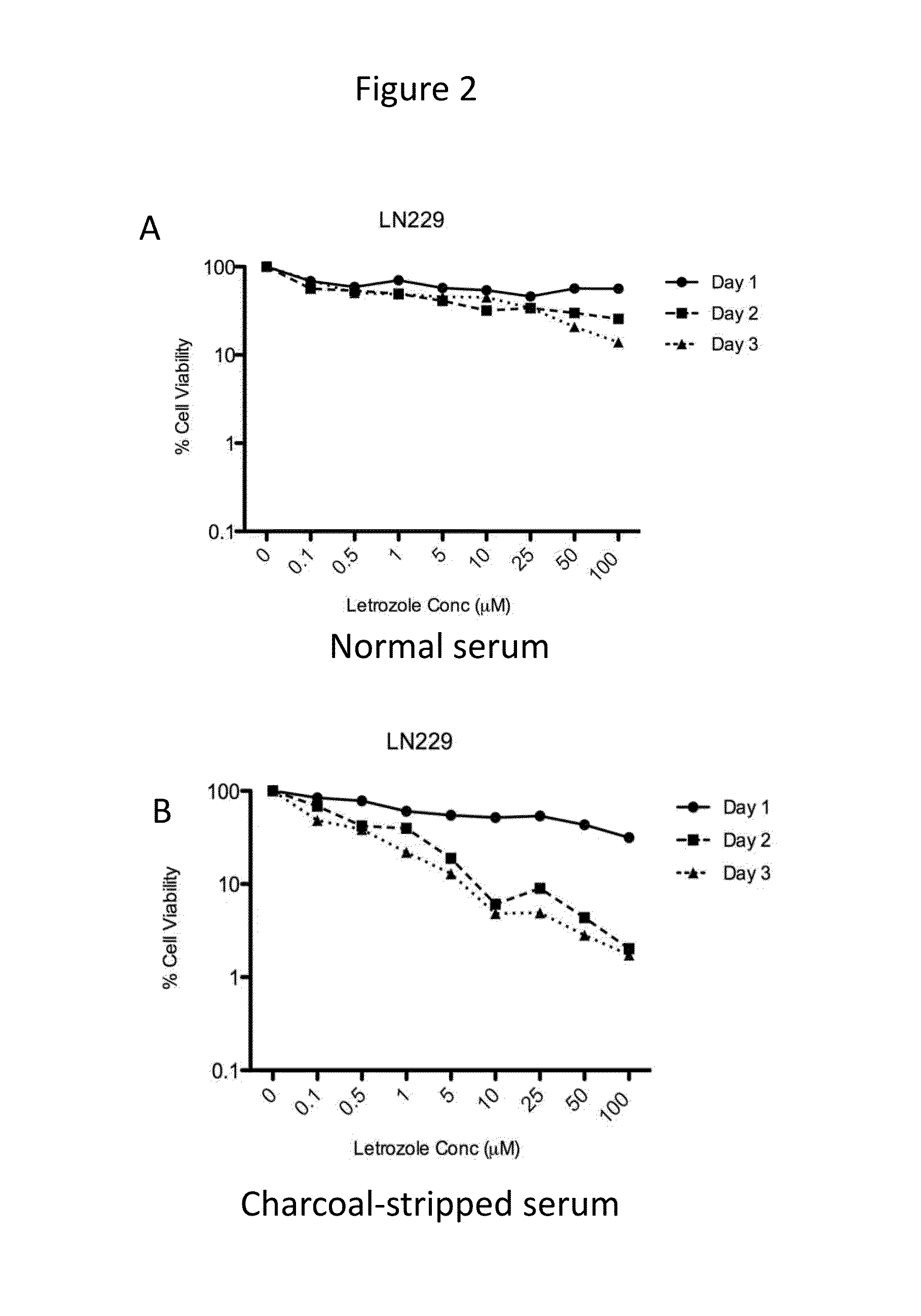
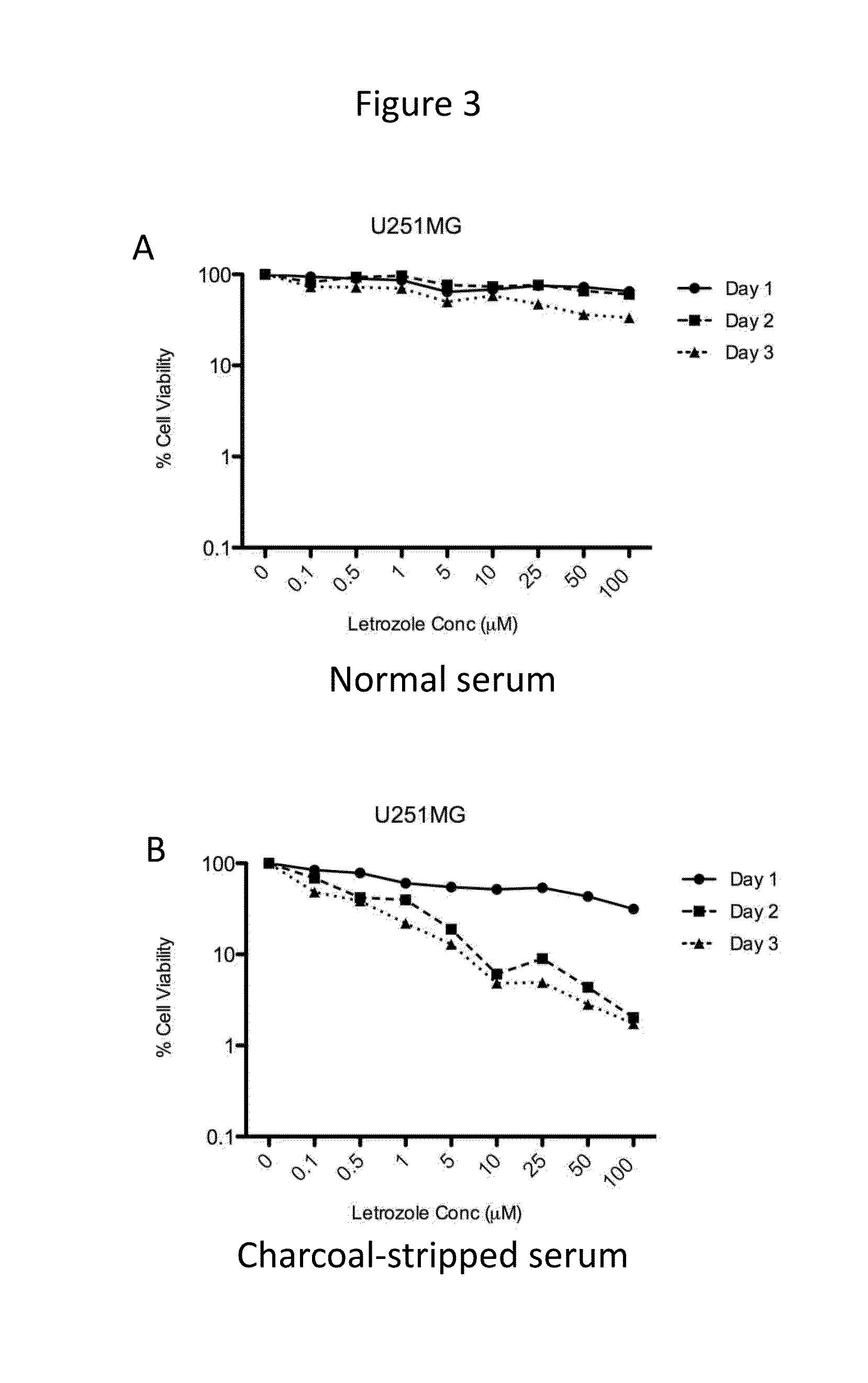
The present disclosure relates to the field of cancer treatment, and more specifically to the field of treatment of primary malignant brain tumors. Provided herein are methods of treating primary brain tumors, including gliomas, by administering to a patient in need thereof a therapeutically effective amount of the aromatase inhibitor letrozole.
Owner:UNIVERSITY OF CINCINNATI
Letrozole production process
Provided is a method for preparing letrozole, which includes reacting an activated bis-(4-cyanophenyl)-methane with a triazole to produce letrozole, and, optionally, purifying the letrozole. Also provided are highly pure letrozole, and a method of purifying letrozole, which method includes precipitating letrozole, e.g., by selective precipitation from a reaction mixture and / or by subjecting the letrozole to one or more crystallizations.
Owner:CHEMAGIS
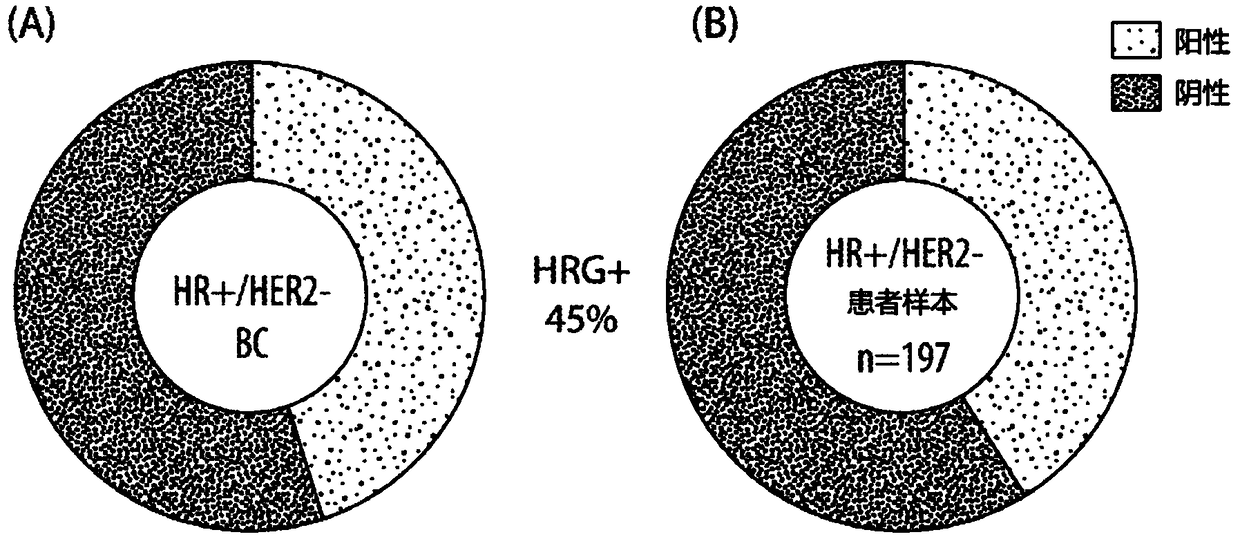
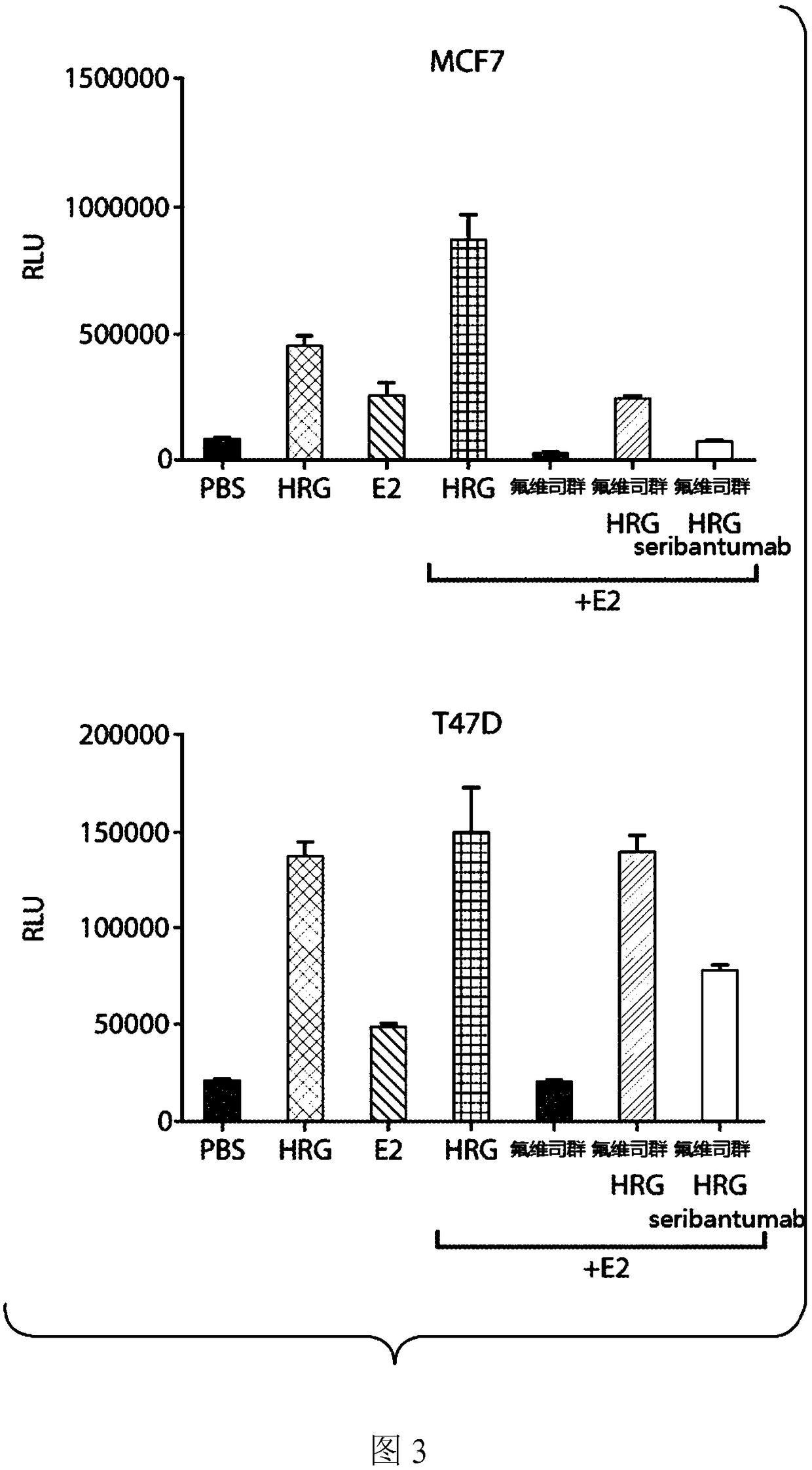
Methods for treating ER+, HER2-, HRG+ breast cancer using combination therapies comprising an anti-ERBB3 antibody
InactiveCN109310754AOrganic active ingredientsPharmaceutical delivery mechanismRegimenCombined Modality Therapy
Provided herein are compositions and methods for treating ER+, HER2- HRG+ breast cancer (e.g., metastatic ER+, HER2- breast cancer) in a patient by administering to the patient an anti-ErbB3 antibody(e.g., seribantumab), a CDK4 / 6 inhibitor (e.g., palbociclib), and an endocrine based therapy (e.g., letrozole or fulvestrant) according to a particular clinical dosage regimen (i.e., at a particular dose amount and according to a specific dosing schedule). Also provided herein are compositions and methods for treating ER+, HER2- HRG+ breast cancer (e.g., metastatic ER+, HER2- breast cancer) in a patient by administering to the patient an anti-ErbB3 antibody (e.g., seribantumab) and an endocrine based therapy (e.g., letrozole or fulvestrant) according to a particular clinical dosage regimen (i.e., at a particular dose amount and according to a specific dosing schedule).
Owner:MERRIMACK PHARMACEUTICALS INC
Letrozole dispersing tablet dosage form
InactiveCN103860490AReduce the burden onEasy to takeOrganic active ingredientsPill deliveryPatient complianceDrug product
The invention belongs to the technical field of medicine and relates to a letrozole dispersing tablet dosage form. A lot of clinical surveys prove that necessity of the letrozole dispersing tablet dosage form is confirmed and the letrozole dispersing tablet dosage form improves patient compliance and medicine bioavailability. The letrozole dispersing tablet dosage form can be used for treatment on breast cancer of postmenopausal women.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Process for preparing high purity letrozole
ActiveCN100560573CEfficient large-scale productionEconomies of Scale ProductionOrganic chemistryPolymer scienceLetrozole
Owner:AVENTIS PHARMA HAINAN
Injectable depot compositions and process of preparation of such compositions
InactiveCN101541316APromote ease of injectabilitySulfur/selenium/tellurium active ingredientsOintment deliveryActive agentProlonged release
Novel injectable compositions are provided comprising an active agent which is tarnsulosin or letrozole or its pharmaceutically acceptable salts, derivatives, isomers, polymorphs, solvates, hydrates, analogues, enantiomers, tautomeric forms or mixtures thereof and one or more pharmaceutically acceptable excipient(s) wherein the compositions are preferably formulated as biodegradable microparticles or nanoparticles which can optionally be reconstituted with an aqueous, hydro-alcoholic or oily liquid vehicle prior to administration. The novel injectable compositions of the present invention preferably form a depot upon administration in vivo and are in the form of an in situ gelling composition or an implant composition which provides a prolonged release of tamsulosin or letrozole for extended periods of time.; Also described are process for preparation of such novel compositions and method of using them.
Owner:PANACEA BIOTEC
Method for preparing letrozole impurity
InactiveCN103242198ACarboxylic acid nitrile preparationOrganic compound preparationDrug utilisationLetrozole
The invention relates to a method for preparing a letrozole impurity. A reference substance is provided for quantitative and qualitative analysis of letrozole impurity detection by synthesizing the letrozole impurity. Thus, the quality standard of letrozole is improved; guidance is provided for safe medication of letrozole; and meanwhile, an effective detection foundation is provided for obtaining the letrozole drug which accords with the quality standard of USP (United States Pharmacopoeia).
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com