Methods for treating ER+, HER2-, HRG+ breast cancer using combination therapies comprising an anti-ERBB3 antibody
A breast cancer, stop treatment technology, applied in the direction of antibody medical components, antibodies, chemical instruments and methods, can solve the problems of accelerated cytotoxic chemotherapy, short duration of treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Example 1: Phenotypically distinct HRG-positive cancer cells impact standard of care treatment in metastatic breast cancer models.
[0140] ErbB3 is a member of the human epidermal growth factor receptor (ErbB or HER) family, which consists of four receptors (ErbB1-4). A typical feature of ErbB networks is that the two members of the family, ErbB2 and ErbB3, are nonautonomous. ErbB2 lacks the ability to interact with growth factor ligands, while ErbB3 is defective in its kinase activity. Heregulin (HRG) is an ErbB 3 ligand that has been identified as a potent driver of proliferation and enhanced survival. HRG expression results in a distinct tumor cell phenotype characterized by an inability to respond to the effects of a variety of standard-of-care (SOC) therapies, including chemotherapy, antihormonal agents, and other targeted therapies.
[0141] In surveys of HRG expression, HRG+ cells are present in about 50% of cases in most solid tumor types. It is hypothesiz...
Embodiment 2
[0144] Example 2: Heregulin mRNA is prevalent in patients with ER+, HER2- breast cancer.
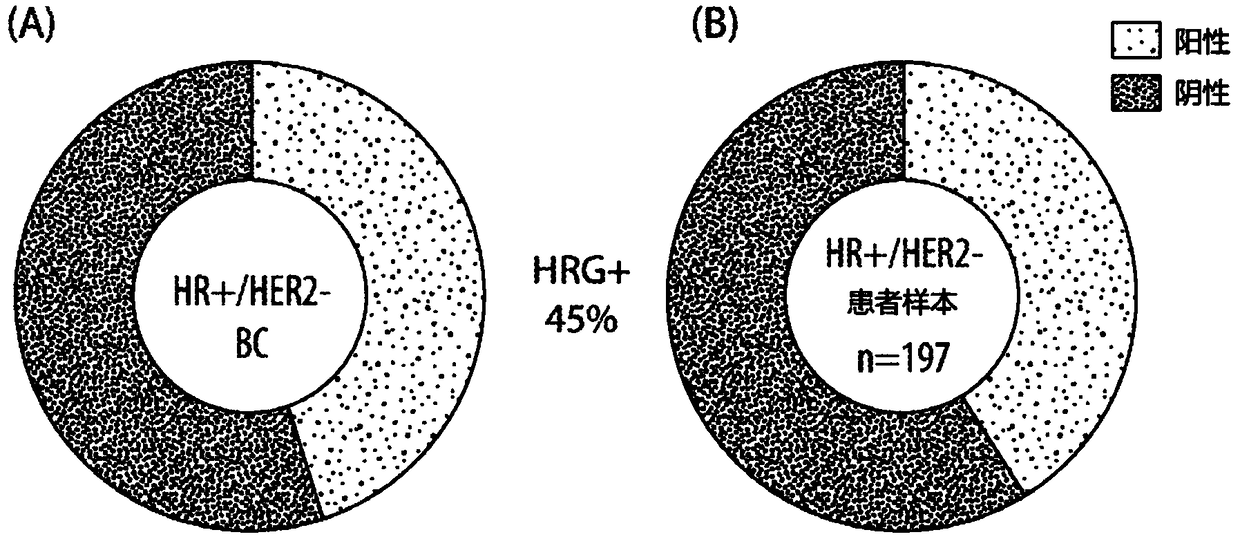
[0145] HRG expression in breast cancer cells can promote cancer progression and resistance to therapy by activating HER3 signaling. To elaborate on this finding, the prevalence of HRG mRNA was examined in the TCGA public database and by direct measurement of HRG mRNA in 197 ER-positive, HER2-negative breast cancer tumors using a clinically relevant HRG RNA-ISH assay. Both the TCGA database and patient samples were found to exhibit a prevalence of HRG mRNA with 45% ( figure 1 ).
Embodiment 3
[0146] Example 3: Heregulin induces proliferation of ER+, HER2- breast cancer cell lines
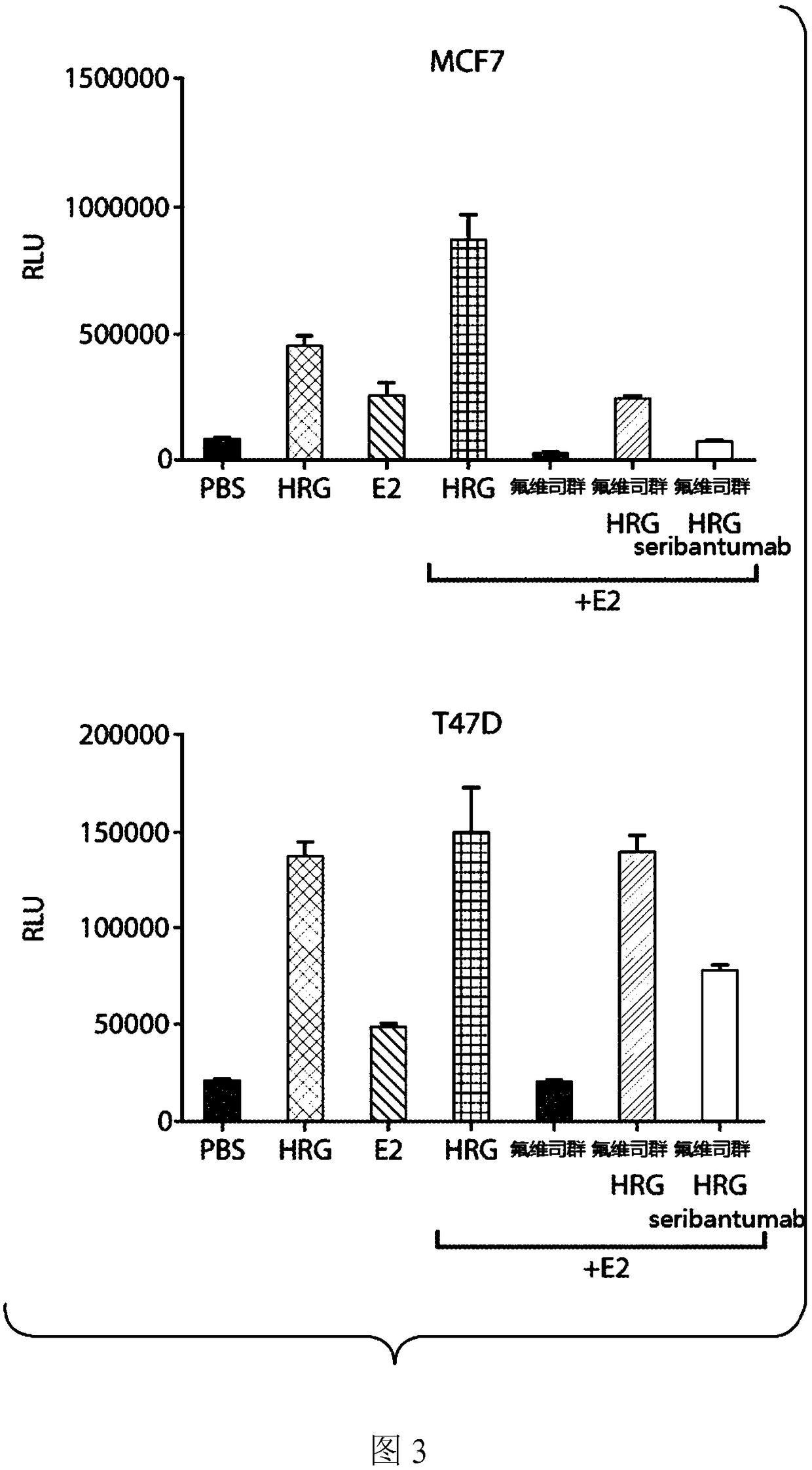
[0147] ER+, HER2- breast cancer cell lines with HRG were stimulated for 6 days and proliferation was measured in vitro. result( figure 2 ) showed that HRG induces the proliferation of MCF7, T47D and HCC1428 cell lines, all of which are ER+, HER2- cell models. These data support the conclusion that the presence of HRG drives cancer cell proliferation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com