Hemi-succinate crystal form CSI of Ribociclib as well as preparation method and application thereof
A crystal form and application technology, applied in the hemisuccinate crystal form of ribociclib and its preparation, can solve problems affecting the clinical efficacy and safety of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Preparation method of crystal form CSI
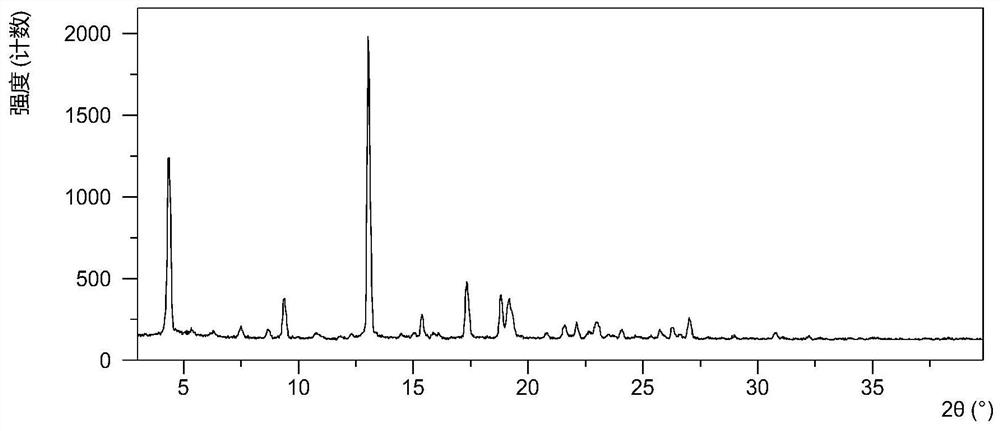
[0055] Add 1.0095g of compound (I) and 0.055g of succinic acid into 30mL of n-butanol / anisole (1:1, v / v) solvent, dissolve at 80°C, let stand at room temperature until the solid precipitates, centrifuge at 25°C Drying under vacuum for 13 hours, and vacuum drying at 40°C for 10 hours gave white solid crystals. After testing, the obtained crystalline solid is the crystal form CSI described in the present invention, and its XRPD pattern is as follows figure 1 , XRPD data are shown in Table 1.
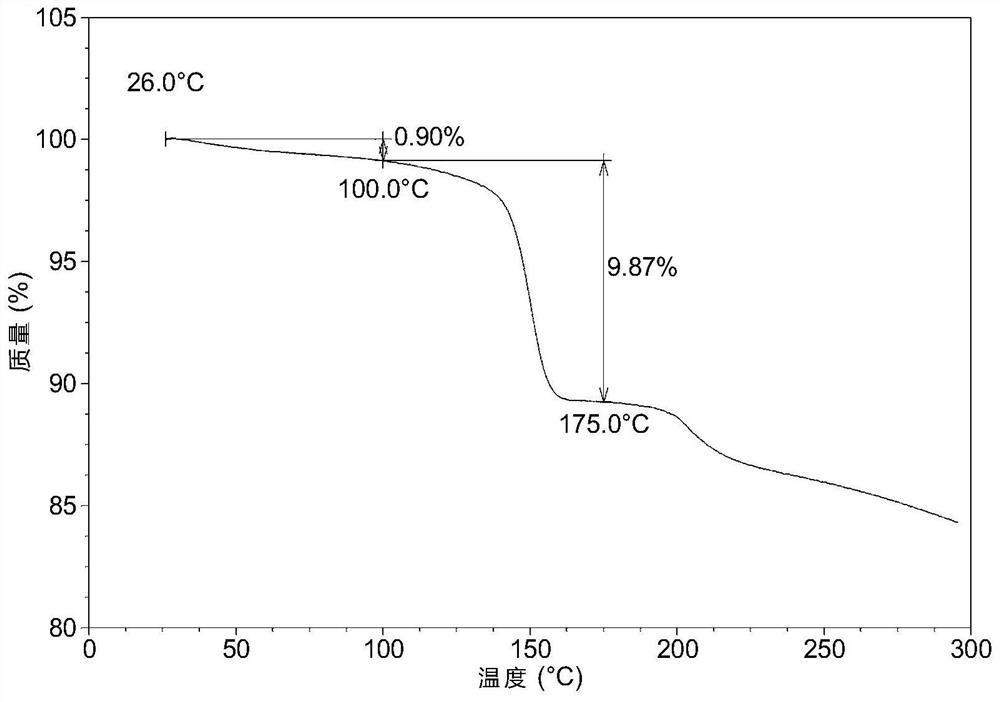
[0056] TGA as figure 2 As shown, when it is heated to 100°C, it has a mass loss of 0.9%, and from 100°C to 175°C, there is about 9.9% of the mass loss due to the removal of the solvent in the solvate.
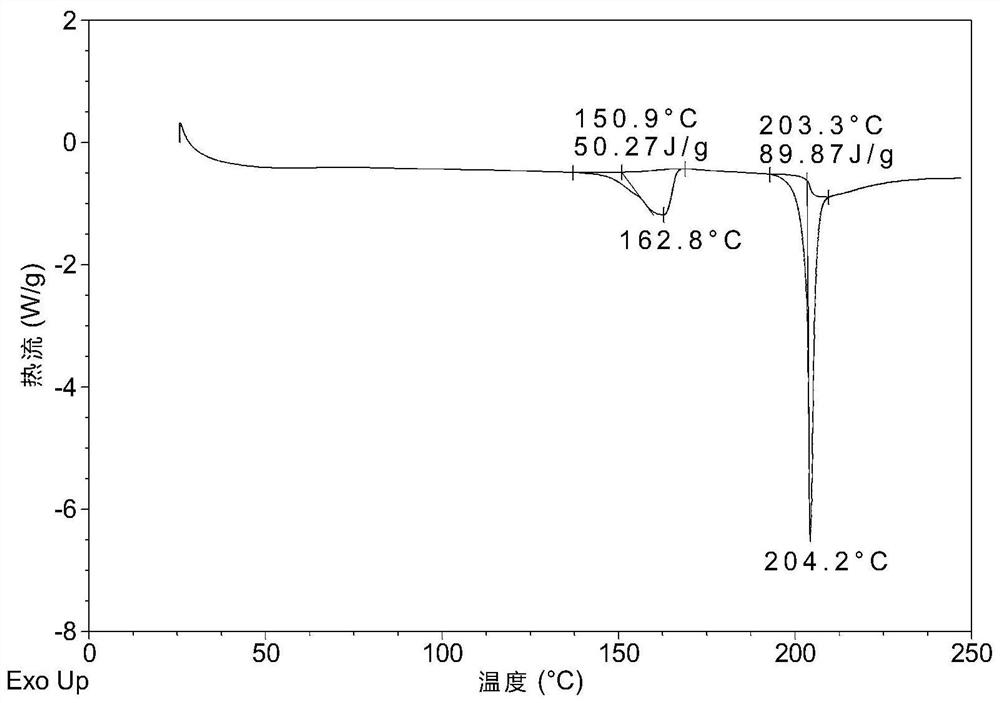
[0057] DSC such as image 3 As shown, it has two endothermic peaks, the endothermic peak around 151°C is the desolvation endothermic peak, and the endothermic peak around 203°C is the melting endothermic peak.
[0058] Table...
Embodiment 2
[0061] Example 2: Dynamic solubility of crystalline form CSI
[0062] Simulated gastrointestinal fluids such as SGF (simulated gastric fluid), FaSSIF (simulated fasting state intestinal fluid), FeSSIF (simulated fed state intestinal fluid) are biologically relevant media, which can better reflect the effect of gastrointestinal physiological environment on drug release. The solubility tested in such media is closer to the solubility in the human environment.
[0063] Take about 20 mg of the crystal form CSI of the present invention and suspend in 1.5 mL of SGF, 1.5 mL of FaSSIF, 1.5 mL of FeSSIF and 1.5 mL of water to prepare a suspension, and equilibrate for 1 hour, 4 hours and 24 hours respectively with high-efficiency liquid The content (mg / mL) of the sample in the solution was tested by phase chromatography.
Embodiment 3
[0064] Example 3: Intrinsic dissolution rate of crystalline form CSI
[0065] Weigh about 100mg of crystal form CSI, pour it into the inherent dissolution mold, and keep it under 5kN pressure for 1min to make a surface area of 0.5cm 2 For the flakes, the mold with the flakes was transferred to the dissolution apparatus to test the intrinsic dissolution rate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com