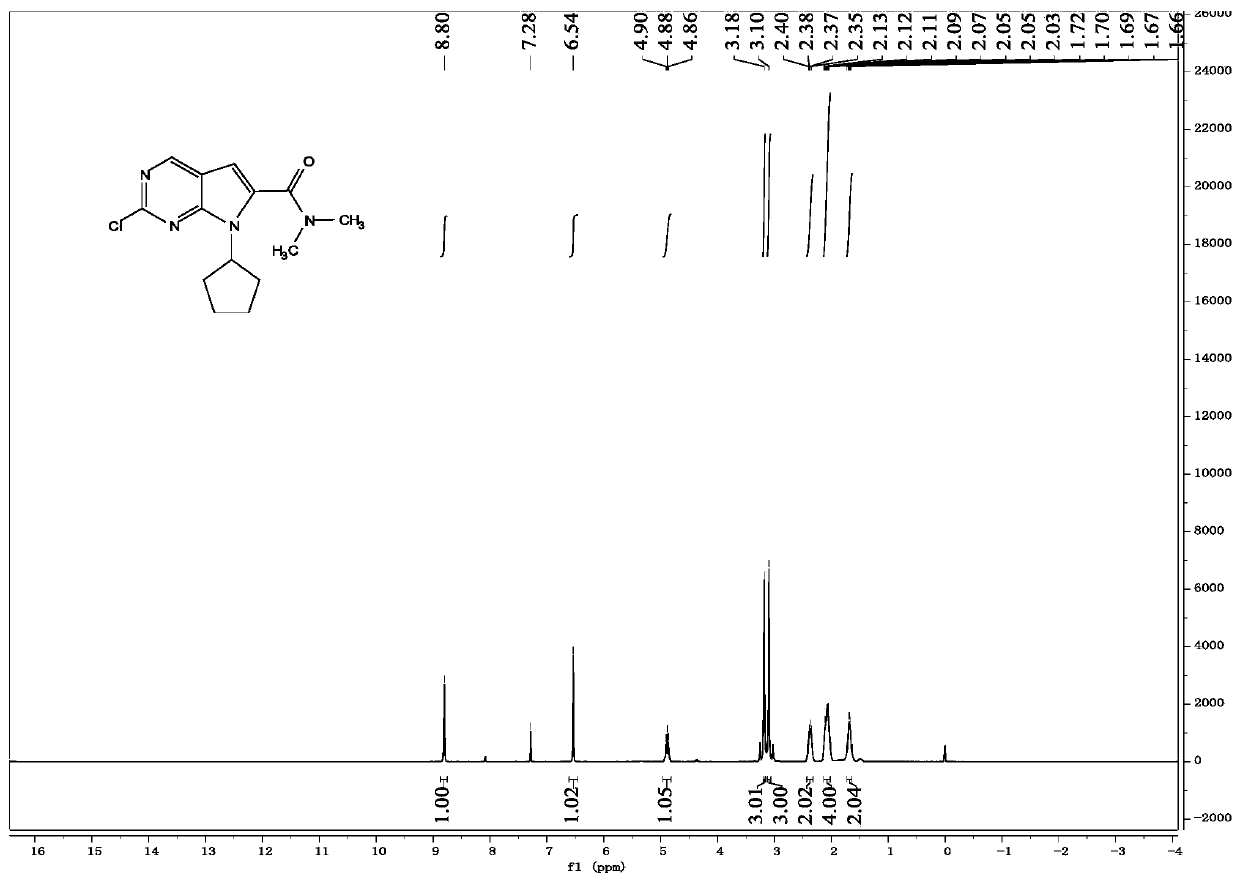
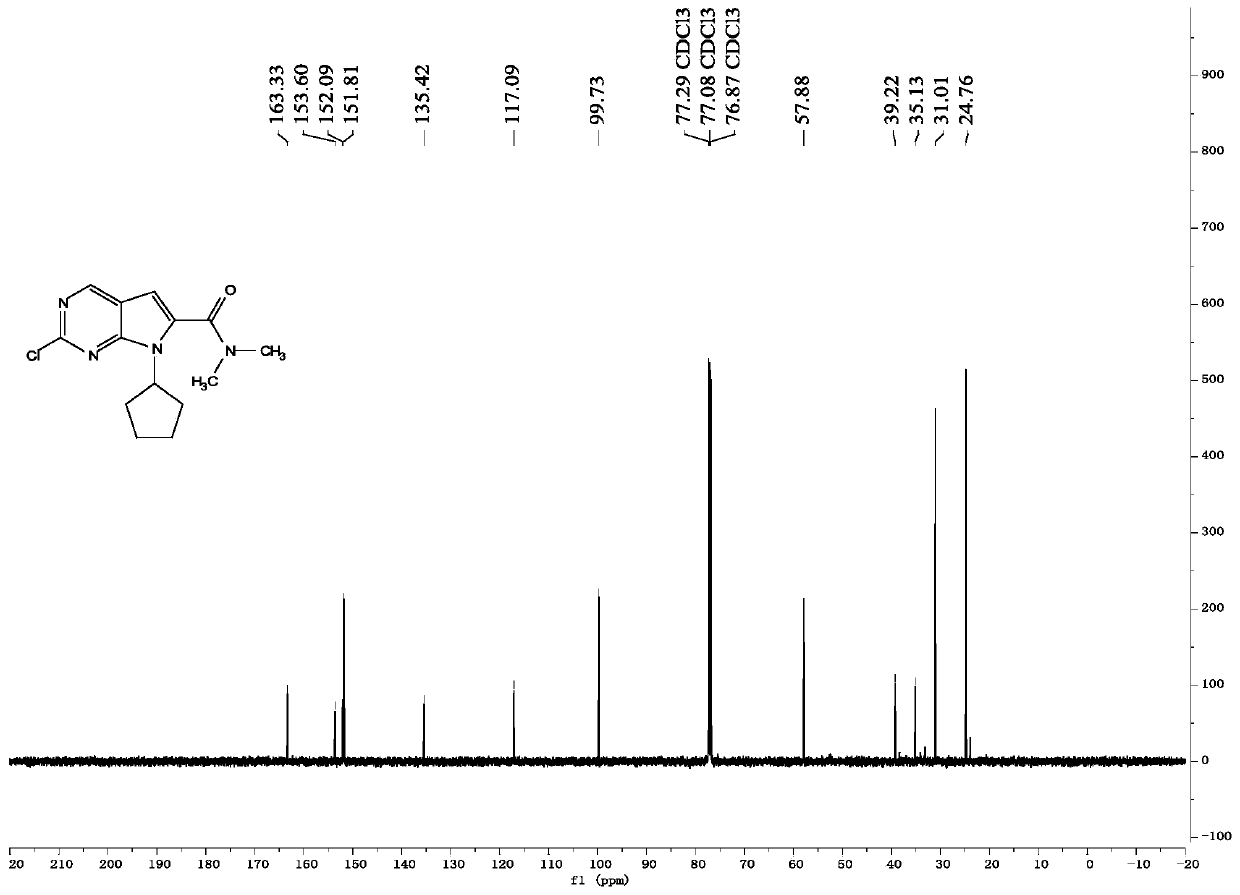
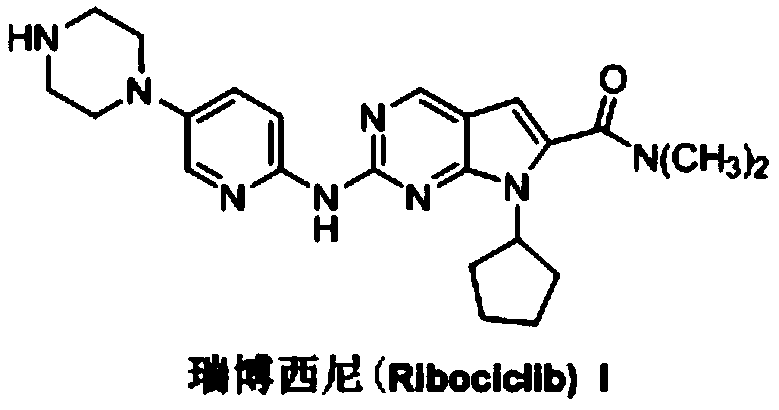
Synthesis method of antineoplastic drug ribociclib intermediate
A technology of ribociclib and a synthesis method, which is applied in the field of synthesis of antitumor drug intermediates, can solve the problems of long reaction steps and high production costs, and achieve the effects of shortening reaction steps, reducing production costs, facilitating three-waste treatment and industrialized production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of N,N-Dimethyl-2,3-dibromopropionamide
[0045] Dissolve N,N-dimethylacrylamide (30.0g, 0.30mol) in 200ml of toluene, add bromine (50.4g, 0.30mol) dropwise at room temperature, drop it over in 30 minutes, continue to stir at room temperature for 4h, and the reaction ends The latter toluene layer was washed with water (100 mL×2), dried over anhydrous sodium sulfate, and the toluene was evaporated under reduced pressure to obtain 73.5 g of white solid, yield: 95%.
Embodiment 2
[0047] Synthesis of N,N-Dimethyl-2,3-dibromopropionamide
[0048] Dissolve N,N-dimethylacrylamide (45.0g, 0.45mol) in 250ml of chloroform, add bromine (108.0g, 0.68mol) dropwise at room temperature, drop it over in 30 minutes, continue stirring at room temperature for 2h, and the reaction is complete The latter toluene layer was washed with water (100 mL×2), dried over anhydrous sodium sulfate, and the chloroform was distilled off under reduced pressure to obtain 106.0 g of white solid, yield: 92%.
Embodiment 3
[0050] Synthesis of N,N-Dimethylpropynamide
[0051] Dissolve the N,N-dimethyl-2,3-dibromopropionamide (40.0 g, 0.16 mol) prepared in Example 1 in 200 mL of tetrahydrofuran, cool in an ice bath to 0°C, and add potassium tert-butoxide in batches (18.0g, 0.16mol), add in 30 minutes, rise to room temperature and continue to stir the reaction for 2h, after the reaction is complete, distill off the tetrahydrofuran under reduced pressure, add 100ml of dichloromethane and 100ml of water, stir for 5min, separate the organic layer, Dry over sodium sulfate, evaporate dichloromethane under reduced pressure to obtain 10.6 g of white solid, yield: 68%, mp: 67-70°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com