Ribociclib monosuccinate crystal form and its preparation method and use
A technology of succinate and succinic acid, which is applied in the field of drug crystals, can solve the problems of reducing tablet and filling production efficiency in preparations, poor physical stability, and difficult drug quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0205] Example 1: Preparation of seed crystals of Form X
[0206] 100.8 mg of compound (I) and 32.5 mg of succinic acid were added to 5.5 mL of a mixed solvent of acetonitrile and methanol (V:V=10:1), suspended and stirred for crystallization at 50°C for 24 hours, and then seed crystals (CN105085533B) were added thereto. Form I), suspended and stirred for crystallization at 50°C for 72 hours, centrifuged to separate the solid, and vacuum-dried at room temperature to obtain white solid crystals.
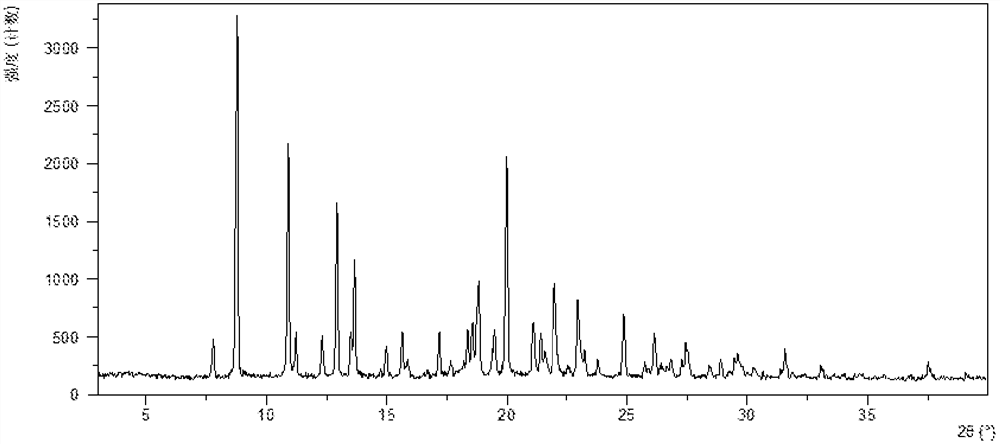
[0207] After testing, the obtained crystalline solid is the crystal form X of the present invention, and its X-ray powder diffraction data are as follows: figure 1 , as shown in Table 1.
[0208] Table 1
[0209]
[0210]
[0211]
Embodiment 2
[0212] Example 2: Preparation of Form X
[0213] 401.4 mg of compound (I) and 129.3 mg of succinic acid were added to 19.8 mL of a mixed solvent of acetonitrile and methanol (V:V=10:1), suspended and stirred for crystallization at 50°C for 24 hours, and 9 mL of the suspension was taken in another Into a glass bottle, the seed crystal of crystal form X was added, suspended and stirred for crystallization at 50° C. for 68 hours, the solid was separated by centrifugation, and vacuum-dried at room temperature to obtain a white crystalline solid.
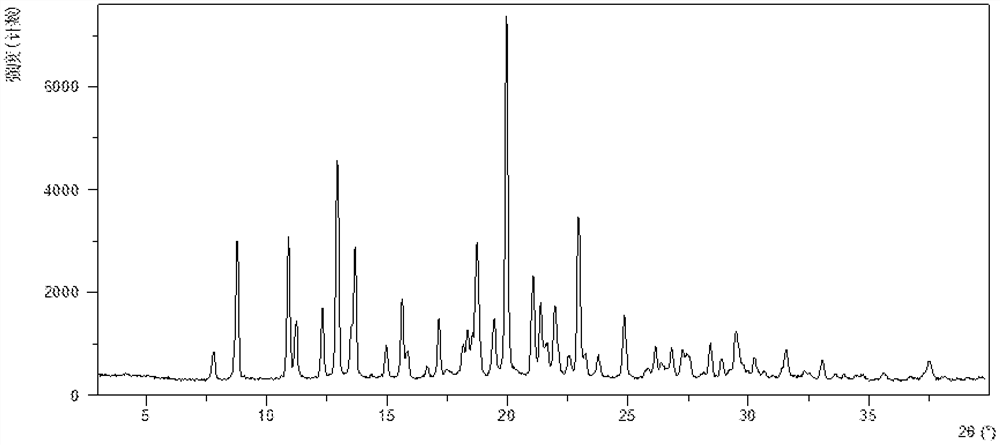
[0214] After testing, the obtained crystalline solid is the crystal form X of the present invention, and its X-ray powder diffraction data are as follows: figure 2 , as shown in Table 2.
[0215] Compound (I) monosuccinate crystal form X prepared by the above method, which 1 H NMR spectrum as Figure 5 As shown, the identification data is as follows:
[0216] 1 H NMR (400MHz, D 2 O)δ8.63(s, 1H), 7.83(d, J=1.2Hz, 1H), 7.65-7.53(m...
Embodiment 3
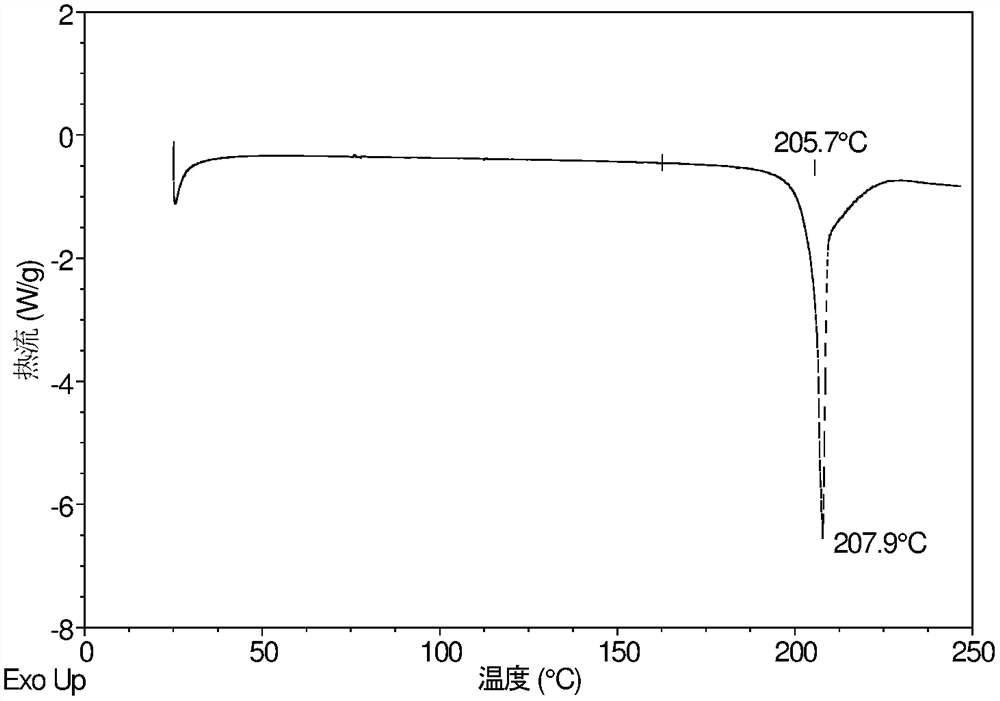
[0222] Example 3 Hygroscopicity of Form X
[0223] About 10 mg of the crystal form X of the present invention was taken for dynamic moisture adsorption (DVS) test. The results are shown in Table 3, and the DVS diagram of Form X is attached Image 6 , the XRPD overlays of Form X before and after the DVS test are shown in the attached Figure 7 .
[0224] table 3
[0225]
[0226] Note: The hygroscopicity data for the non-hydrated form is from CN103201275A [Page 16 / 17, Table 1, Cycle 1]
[0227] About the description of hygroscopicity characteristics and the definition of hygroscopicity weight gain (Chinese Pharmacopoeia 2015 edition general rule 9103 Drug hygroscopicity test guidelines, experimental conditions: 25℃±1℃, 80% relative humidity):
[0228] Deliquescence: Absorbs sufficient water to form a liquid
[0229] Extremely hygroscopic: wet weight gain is not less than 15.0%
[0230] Moisture: Moisture gain is less than 15.0% but not less than 2.0%
[0231] Slight...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com